
Be Part of the Ultimate Safety & Compliance Community
Trending news, knowledge-building content, and more – all personalized to you!
The U.S. Environmental Protection Agency (EPA) is finalizing changes to its test procedures required to be used by industries and municipalities when analyzing the chemical, physical, and biological properties of wastewater and other samples for reporting under the EPA's National Pollutant Discharge Elimination System permit program. The Clean Water Act requires the EPA to promulgate these test procedures (analytical methods) for analysis of pollutants. The EPA anticipates that these changes will provide increased flexibility for the regulated community in meeting monitoring requirements while improving data quality. In addition, this update to the CWA methods will incorporate technological advances in analytical technology and make a series of minor changes and corrections to existing approved methods. As such, the EPA expects that these changes will not result in any negative economic impacts.
DATES: This final rule is effective on June 17, 2024, published in the Federal Register April 16, 2024, page 27288.
View final rule.
| §136.3 Identification of test procedures. | ||
| (a), tables IA, IB, IC, ID, and IH | Revised | View text |
| (b) | Revised | View text |
| (e), table II, Footnote “5” | Revised | View text |
New Text
§136.3 Identification of test procedures.
(a)
* * * *
| Parameter and units | Method 1 | EPA | Standard methods | AOAC, ASTM, USGS | Other |
|---|---|---|---|---|---|
| Table IA notes: | |||||
| 1 The method must be specified when results are reported. | |||||
| 2 A 0.45-µm membrane filter (MF) or other pore size certified by the manufacturer to fully retain organisms to be cultivated and to be free of extractables which could interfere with their growth. | |||||
| 3 Microbiological Methods for Monitoring the Environment, Water and Wastes, EPA/600/8-78/017. 1978. US EPA. | |||||
| 4 U.S. Geological Survey Techniques of Water-Resource Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. USGS. | |||||
| 5 Because the MF technique usually yields low and variable recovery from chlorinated wastewaters, the Most Probable Number method will be required to resolve any controversies. | |||||
| 6 Tests must be conducted to provide organism enumeration (density). Select the appropriate configuration of tubes/filtrations and dilutions/volumes to account for the quality, character, consistency, and anticipated organism density of the water sample. | |||||
| 7 When the MF method has been used previously to test waters with high turbidity, large numbers of noncoliform bacteria, or samples that may contain organisms stressed by chlorine, a parallel test should be conducted with a multiple-tube technique to demonstrate applicability and comparability of results. | |||||
| 8 To assess the comparability of results obtained with individual methods, it is suggested that side-by-side tests be conducted across seasons of the year with the water samples routinely tested in accordance with the most current Standard Methods for the Examination of Water and Wastewater or EPA alternate test procedure (ATP) guidelines. | |||||
| 9 Annual Book of ASTM Standards—Water and Environmental Technology, Section 11.02. 2000, 1999, 1996. ASTM International. | |||||
| 10 Official Methods of Analysis of AOAC International. 16th Edition, 4th Revision, 1998. AOAC International. | |||||
| 11 Recommended for enumeration of target organism in sewage sludge. | |||||
| 12 The multiple-tube fermentation test is used in 9221B.2-2014. Lactose broth may be used in lieu of lauryl tryptose broth (LTB), if at least 25 parallel tests are conducted between this broth and LTB using the water samples normally tested, and this comparison demonstrates that the false-positive rate and false-negative rate for total coliform using lactose broth is less than 10 percent. No requirement exists to run the completed phase on 10 percent of all total coliform-positive tubes on a seasonal basis. | |||||
| 13 These tests are collectively known as defined enzyme substrate tests. | |||||
| 14 After prior enrichment in a presumptive medium for total coliform using 9221B.2-2014, all presumptive tubes or bottles showing any amount of gas, growth or acidity within 48 h ± 3 h of incubation shall be submitted to 9221F-2014. Commercially available EC-MUG media or EC media supplemented in the laboratory with 50 µg/mL of MUG may be used. | |||||
| 15 Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation Using Lauryl-Tryptose Broth (LTB) and EC Medium, EPA-821-R-14-009. September 2014. U.S. EPA. | |||||
| 16 Samples shall be enumerated by the multiple-tube or multiple-well procedure. Using multiple-tube procedures, employ an appropriate tube and dilution configuration of the sample as needed and report the Most Probable Number (MPN). Samples tested with Colilert® may be enumerated with the multiple-well procedures, Quanti-Tray® or Quanti-Tray®/2000 and the MPN calculated from the table provided by the manufacturer. | |||||
| 17 Colilert-18® is an optimized formulation of the Colilert® for the determination of total coliforms and E. coli that provides results within 18 h of incubation at 35°C rather than the 24 h required for the Colilert® test and is recommended for marine water samples. | |||||
| 18 Descriptions of the Colilert®, Colilert-18®, Quanti-Tray®, and Quanti-Tray®/2000 may be obtained from IDEXX Laboratories, Inc. | |||||
| 19 A description of the mColiBlue24® test is available from Hach Company. | |||||
| 20 Method 1681: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation Using A-1 Medium, EPA-821-R-06-013. July 2006. U.S. EPA. | |||||
| 21 Method 1603.1: Escherichia coli ( E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008. September 2023. U.S. EPA. | |||||
| 22 Method 1682: Salmonella in Sewage Sludge (Biosolids) by Modified Semisolid Rappaport-Vassiliadis (MSRV) Medium, EPA-821-R-14-012. September 2014. U.S. EPA. | |||||
| 23 A description of the Enterolert® test may be obtained from IDEXX Laboratories Inc. | |||||
| 24 Method 1600.1: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-23-006. September 2023. U.S. EPA. | |||||
| 25 Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, EPA-821-R-02-012. Fifth Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 26 Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, EPA-821-R-02-013. Fourth Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 27 Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, EPA-821-R-02-014. Third Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 28 To use Colilert-18® to assay for fecal coliforms, the incubation temperature is 44.5 ± 0.2 °C, and a water bath incubator is used. | |||||
| 29 On a monthly basis, at least ten blue colonies from positive samples must be verified using Lauryl Tryptose Broth and EC broth, followed by count adjustment based on these results; and representative non-blue colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. | |||||
| 30 On a monthly basis, at least ten sheen colonies from positive samples must be verified using lauryl tryptose broth and brilliant green lactose bile broth, followed by count adjustment based on these results; and representative non-sheen colonies should be verified using lauryl tryptose broth. Where possible, verifications should be done from randomized sample sources. | |||||
| 31 Subject coliform positive samples determined by 9222 B-2015 or other membrane filter procedure to 9222 I-2015 using NA-MUG media. | |||||
| 32 Verification of colonies by incubation of BHI agar at 10 ± 0.5 °C for 48 ± 3 h is optional. As per the Errata to the 23rd Edition of Standard Methods for the Examination of Water and Wastewater “Growth on a BHI agar plate incubated at 10 ± 0.5 °C for 48 ± 3 h is further verification that the colony belongs to the genus Enterococcus.” | |||||
| 33 9221F. 2-2014 allows for simultaneous detection of E. coli and thermotolerant fecal coliforms by adding inverted vials to EC-MUG; the inverted vials collect gas produced by thermotolerant fecal coliforms. | |||||
| Bacteria | |||||
| 1. Coliform (fecal), number per gram dry weight | Most Probable Number (MPN), 5 tube, 3 dilution, or | p. 132, 3 1680, 1115 1681 1120 | 9221 E-2014. | ||
| Membrane filter (MF), 25 single step | p. 124 3 | 9222 D-2015. 29 | |||
| 2. Coliform (fecal), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 132 3 | 9221 E-2014, 9221 F-2014. 33 | ||
| Multiple tube/multiple well, or | Colilert-18®. 131828 | ||||
| MF, 25 single step 5 | p. 124 3 | 9222 D-2015 29 | B-0050-85. 4 | ||
| 3. Coliform (total), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 114 3 | 9221 B-2014. | ||
| MF, 25 single step or | p. 108 3 | 9222 B-2015 30 | B-0025-85. 4 | ||
| MF, 25 two step with enrichment | p. 111 3 | 9222 B-2015. 30 | |||
| 4. E. coli, number per 100 mL | MPN 6816 multiple tube, or | 9221 B2014/9221 F-2014. 121433 | |||
| multiple tube/multiple well, or | 9223 B-2016 13 | 991.15 10 | Colilert®. 1318 Colilert-18®. 131718 | ||
| MF, 25678 two step, or | 9222 B-2015/9222 I-2015. 31 | ||||
| Single step | 1603.1 21 | m-ColiBlue24®. 19 | |||
| 5. Fecal streptococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MF, 2 or | p. 136 3 | 9230 C-2013 32 | B-0055-85. 4 | ||
| Plate count | p. 143. 3 | ||||
| 6. Enterococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MPN, 68 multiple tube/multiple well, or | 9230 D-2013 | D6503-99 9 | Enterolert®. 1323 | ||
| MF 25678 single step or | 1600.1 24 | 9230 C-2013. 32 | |||
| Plate count | p. 143. 3 | ||||
| 7. Salmonella , number per gram dry weight 11 | MPN multiple tube | 1682. 22 | |||
| Aquatic Toxicity | |||||
| 8. Toxicity, acute, fresh water organisms, LC 50 , percent effluent | Water flea, Cladoceran, Ceriodaphnia dubia acute | 2002.0. 25 | |||
| Water flea, Cladocerans, Daphnia pulex and Daphnia magna acute | 2021.0. 25 | ||||
| Fish, Fathead minnow, Pimephales promelas, and Bannerfin shiner, Cyprinella leedsi, acute | 2000.0. 25 | ||||
| Fish, Rainbow trout, Oncorhynchus mykiss, and brook trout, Salvelinus fontinalis, acute | 2019.0. 25 | ||||
| 9. Toxicity, acute, estuarine and marine organisms of the Atlantic Ocean and Gulf of Mexico, LC 50 , percent effluent | Mysid, Mysidopsis bahia, acute | 2007.0. 25 | |||
| Fish, Sheepshead minnow, Cyprinodon variegatus, acute | 2004.0. 25 | ||||
| Fish, Silverside, Menidia beryllina, Menidia menidia, and Menidia peninsulae, acute | 2006.0. 25 | ||||
| 10. Toxicity, chronic, fresh water organisms, NOEC or IC 25 , percent effluent | Fish, Fathead minnow, Pimephales promelas, larval survival and growth | 1000.0. 26 | |||
| Fish, Fathead minnow, Pimephales promelas, embryo-larval survival and teratogenicity | 1001.0. 26 | ||||
| Water flea, Cladoceran, Ceriodaphnia dubia, survival and reproduction | 1002.0. 26 | ||||
| Green alga, Selenastrum capricornutum, growth | 1003.0. 26 | ||||
| 11. Toxicity, chronic, estuarine and marine organisms of the Atlantic Ocean and Gulf of Mexico, NOEC or IC 25 , percent effluent | Fish, Sheepshead minnow, Cyprinodon variegatus, larval survival and growth. | 1004.0. 27 | |||
| Fish, Sheepshead minnow, Cyprinodon variegatus, embryo-larval survival and teratogenicity | 1005.0. 27 | ||||
| Fish, Inland silverside, Menidia beryllina, larval survival and growth | 1006.0. 27 | ||||
| Mysid, Mysidopsis bahia, survival, growth, and fecundity | 1007.0. 27 | ||||
| Sea urchin, Arbacia punctulata, fertilization | 1008.0. 27 | ||||
| Parameter | Methodology 58 | EPA 52 | Standard methods 84 | ASTM | USGS/AOAC/Other |
|---|---|---|---|---|---|
| Table IB Notes: | |||||
| 1 Methods for Chemical Analysis of Water and Wastes, EPA-600/4-79-020. Revised March 1983 and 1979, where applicable. U.S. EPA. | |||||
| 2 Methods for Analysis of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resource Investigations of the U.S. Geological Survey, Book 5, Chapter A1., unless otherwise stated. 1989. USGS. | |||||
| 3 Official Methods of Analysis of the Association of Official Analytical Chemists, Methods Manual, Sixteenth Edition, 4th Revision, 1998. AOAC International. | |||||
| 4 For the determination of total metals (which are equivalent to total recoverable metals) the sample is not filtered before processing. A digestion procedure is required to solubilize analytes in suspended material and to break down organic-metal complexes (to convert the analyte to a detectable form for colorimetric analysis). For non-platform graphite furnace atomic absorption determinations, a digestion using nitric acid (as specified in Section 4.1.3 of Methods for Chemical Analysis of Water and Wastes) is required prior to analysis. The procedure used should subject the sample to gentle acid refluxing, and at no time should the sample be taken to dryness. For direct aspiration flame atomic absorption (FLAA) determinations, a combination acid (nitric and hydrochloric acids) digestion is preferred, prior to analysis. The approved total recoverable digestion is described as Method 200.2 in Supplement I of “Methods for the Determination of Metals in Environmental Samples” EPA/600R-94/111, May 1994, and is reproduced in EPA Methods 200.7, 200.8, and 200.9 from the same Supplement. However, when using the gaseous hydride technique or for the determination of certain elements such as antimony, arsenic, selenium, silver, and tin by non-EPA graphite furnace atomic absorption methods, mercury by cold vapor atomic absorption, the noble metals and titanium by FLAA, a specific or modified sample digestion procedure may be required, and, in all cases the referenced method write-up should be consulted for specific instruction and/or cautions. For analyses using inductively coupled plasma-atomic emission spectrometry (ICP-AES), the direct current plasma (DCP) technique or EPA spectrochemical techniques (platform furnace AA, ICP-AES, and ICP-MS), use EPA Method 200.2 or an approved alternate procedure ( e.g., CEM microwave digestion, which may be used with certain analytes as indicated in this table IB); the total recoverable digestion procedures in EPA Methods 200.7, 200.8, and 200.9 may be used for those respective methods. Regardless of the digestion procedure, the results of the analysis after digestion procedure are reported as “total” metals. | |||||
| 5 Copper sulfate or other catalysts that have been found suitable may be used in place of mercuric sulfate. | |||||
| 6 Manual distillation is not required if comparability data on representative effluent samples are on file to show that this preliminary distillation step is not necessary; however, manual distillation will be required to resolve any controversies. In general, the analytical method should be consulted regarding the need for distillation. If the method is not clear, the laboratory may compare a minimum of 9 different sample matrices to evaluate the need for distillation. For each matrix, a matrix spike and matrix spike duplicate are analyzed both with and without the distillation step (for a total of 36 samples, assuming 9 matrices). If results are comparable, the laboratory may dispense with the distillation step for future analysis. Comparable is defined as <20% RPD for all tested matrices). Alternatively, the two populations of spike recovery percentages may be compared using a recognized statistical test. | |||||
| 7 Industrial Method Number 379-75 WE Ammonia, Automated Electrode Method, Technicon Auto Analyzer II. February 19, 1976. Bran & Luebbe Analyzing Technologies Inc. | |||||
| 8 The approved method is that cited in Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1979. USGS. | |||||
| 9 American National Standard on Photographic Processing Effluents. April 2, 1975. American National Standards Institute. | |||||
| 10 In-Situ Method 1003-8-2009, Biochemical Oxygen Demand (BOD) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 11 The use of normal and differential pulse voltage ramps to increase sensitivity and resolution is acceptable. | |||||
| 12 Carbonaceous biochemical oxygen demand (CBOD 5) must not be confused with the traditional BOD 5 test method which measures “total 5-day BOD.” The addition of the nitrification inhibitor is not a procedural option but must be included to report the CBOD 5 parameter. A discharger whose permit requires reporting the traditional BOD 5 may not use a nitrification inhibitor in the procedure for reporting the results. Only when a discharger's permit specifically states CBOD 5 is required can the permittee report data using a nitrification inhibitor. | |||||
| 13 OIC Chemical Oxygen Demand Method. 1978. Oceanography International Corporation. | |||||
| 14 Method 8000, Chemical Oxygen Demand, Hach Handbook of Water Analysis, 1979. Hach Company. | |||||
| 15 The back-titration method will be used to resolve controversy. | |||||
| 16 Orion Research Instruction Manual, Residual Chlorine Electrode Model 97-70. 1977. Orion Research Incorporated. The calibration graph for the Orion residual chlorine method must be derived using a reagent blank and three standard solutions, containing 0.2, 1.0, and 5.0 mL 0.00281 N potassium iodate/100 mL solution, respectively. | |||||
| 17 Method 245.7, Mercury in Water by Cold Vapor Atomic Fluorescence Spectrometry, EPA-821-R-05-001. Revision 2.0, February 2005. US EPA. | |||||
| 18 National Council of the Paper Industry for Air and Stream Improvement (NCASI) Technical Bulletin 253 (1971) and Technical Bulletin 803, May 2000. | |||||
| 19 Method 8506, Bicinchoninate Method for Copper, Hach Handbook of Water Analysis. 1979. Hach Company. | |||||
| 20 When using a method with block digestion, this treatment is not required. | |||||
| 21 Industrial Method Number 378-75WA, Hydrogen ion (pH) Automated Electrode Method, Bran & Luebbe (Technicon) Autoanalyzer II. October 1976. Bran & Luebbe Analyzing Technologies. | |||||
| 22 Method 8008, 1,10-Phenanthroline Method using FerroVer Iron Reagent for Water. 1980. Hach Company. | |||||
| 23 Method 8034, Periodate Oxidation Method for Manganese, Hach Handbook of Wastewater Analysis. 1979. Hach Company. | |||||
| 24 Methods for Analysis of Organic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3, (1972 Revised 1987). 1987. USGS. | |||||
| 25 Method 8507, Nitrogen, Nitrite-Low Range, Diazotization Method for Water and Wastewater. 1979. Hach Company. | |||||
| 26 Just prior to distillation, adjust the sulfuric-acid-preserved sample to pH 4 with 1 + 9 NaOH. | |||||
| 27 The colorimetric reaction must be conducted at a pH of 10.0 ± 0.2. | |||||
| 28 Addison, R.F., and R.G. Ackman. 1970. Direct Determination of Elemental Phosphorus by Gas-Liquid Chromatography, Journal of Chromatograph y, 47(3):421-426. | |||||
| 29 Approved methods for the analysis of silver in industrial wastewaters at concentrations of 1 mg/L and above are inadequate where silver exists as an inorganic halide. Silver halides such as the bromide and chloride are relatively insoluble in reagents such as nitric acid but are readily soluble in an aqueous buffer of sodium thiosulfate and sodium hydroxide to pH of 12. Therefore, for levels of silver above 1 mg/L, 20 mL of sample should be diluted to 100 mL by adding 40 mL each of 2 M Na 2 S 2 O 3 and NaOH. Standards should be prepared in the same manner. For levels of silver below 1 mg/L the approved method is satisfactory. | |||||
| 30 The use of EDTA decreases method sensitivity. Analysts may omit EDTA or replace with another suitable complexing reagent provided that all method-specified quality control acceptance criteria are met. | |||||
| 31 For samples known or suspected to contain high levels of silver ( e.g., in excess of 4 mg/L), cyanogen iodide should be used to keep the silver in solution for analysis. Prepare a cyanogen iodide solution by adding 4.0 mL of concentrated NH 4 OH, 6.5 g of KCN, and 5.0 mL of a 1.0 N solution of I 2 to 50 mL of reagent water in a volumetric flask and dilute to 100.0 mL. After digestion of the sample, adjust the pH of the digestate to <7 to prevent the formation of HCN under acidic conditions. Add 1 mL of the cyanogen iodide solution to the sample digestate and adjust the volume to 100 mL with reagent water (NOT acid). If cyanogen iodide is added to sample digestates, then silver standards must be prepared that contain cyanogen iodide as well. Prepare working standards by diluting a small volume of a silver stock solution with water and adjusting the pH>7 with NH 4 OH. Add 1 mL of the cyanogen iodide solution and let stand 1 hour. Transfer to a 100-mL volumetric flask and dilute to volume with water. | |||||
| 32 “Water Temperature-Influential Factors, Field Measurement and Data Presentation,” Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 1, Chapter D1. 1975. USGS. | |||||
| 33 Method 8009, Zincon Method for Zinc, Hach Handbook of Water Analysis, 1979. Hach Company. | |||||
| 34 Method AES0029, Direct Current Plasma (DCP) Optical Emission Spectrometric Method for Trace Elemental Analysis of Water and Wastes. 1986—Revised 1991. Thermo Jarrell Ash Corporation. | |||||
| 35 In-Situ Method 1004-8-2009, Carbonaceous Biochemical Oxygen Demand (CBOD) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 36 Microwave-assisted digestion may be employed for this metal, when analyzed by this methodology. Closed Vessel Microwave Digestion of Wastewater Samples for Determination of Metals. April 16, 1992. CEM Corporation. | |||||
| 37 When determining boron and silica, only plastic, PTFE, or quartz laboratory ware may be used from start until completion of analysis. | |||||
| 38 Only use n -hexane ( n -Hexane—85% minimum purity, 99.0% min. saturated C6 isomers, residue less than 1 mg/L) extraction solvent when determining Oil and Grease parameters—Hexane Extractable Material (HEM), or Silica Gel Treated HEM (analogous to EPA Methods 1664 Rev. A and 1664 Rev. B). Use of other extraction solvents is prohibited. | |||||
| 39 Method PAI-DK01, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Titrimetric Detection. Revised December 22, 1994. OI Analytical. | |||||
| 40 Method PAI-DK02, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Colorimetric Detection. Revised December 22, 1994. OI Analytical. | |||||
| 41 Method PAI-DK03, Nitrogen, Total Kjeldahl, Block Digestion, Automated FIA Gas Diffusion. Revised December 22, 1994. OI Analytical. | |||||
| 42 Method 1664 Rev. B is the revised version of EPA Method 1664 Rev. A. U.S. EPA. February 1999, Revision A. Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Non-polar Material) by Extraction and Gravimetry. EPA-821-R-98-002. U.S. EPA. February 2010, Revision B. Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Non-polar Material) by Extraction and Gravimetry. EPA-821-R-10-001. | |||||
| 43 Method 1631, Revision E, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, EPA-821-R-02-019. Revision E. August 2002, U.S. EPA. The application of clean techniques described in EPA's Method 1669: Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels, EPA-821-R-96-011, are recommended to preclude contamination at low-level, trace metal determinations. | |||||
| 44 Method OIA-1677-09, Available Cyanide by Ligand Exchange and Flow Injection Analysis (FIA). 2010. OI Analytical. | |||||
| 45 Open File Report 00-170, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Ammonium Plus Organic Nitrogen by a Kjeldahl Digestion Method and an Automated Photometric Finish that Includes Digest Cleanup by Gas Diffusion. 2000. USGS. | |||||
| 46 Open File Report 93-449, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Chromium in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1993. USGS. | |||||
| 47 Open File Report 97-198, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Molybdenum by Graphite Furnace Atomic Absorption Spectrophotometry. 1997. USGS. | |||||
| 48 Open File Report 92-146, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Total Phosphorus by Kjeldahl Digestion Method and an Automated Colorimetric Finish That Includes Dialysis. 1992. USGS. | |||||
| 49 Open File Report 98-639, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Arsenic and Selenium in Water and Sediment by Graphite Furnace-Atomic Absorption Spectrometry. 1999. USGS. | |||||
| 50 Open File Report 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Elements in Whole-water Digests Using Inductively Coupled Plasma-Optical Emission Spectrometry and Inductively Coupled Plasma-Mass Spectrometry. 1998. USGS. | |||||
| 51 Open File Report 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. USGS. | |||||
| 52 Unless otherwise indicated, all EPA methods, excluding EPA Method 300.1, are published in U.S. EPA. May 1994. Methods for the Determination of Metals in Environmental Samples, Supplement I, EPA/600/R-94/111; or U.S. EPA. August 1993. Methods for the Determination of Inorganic Substances in Environmental Samples, EPA/600/R-93/100. EPA Method 300.1 is U.S. EPA. Revision 1.0, 1997, including errata cover sheet April 27, 1999. Determination of Inorganic Ions in Drinking Water by Ion Chromatography. | |||||
| 53 Styrene divinyl benzene beads ( e.g., AMCO-AEPA-1 or equivalent) and stabilized formazin ( e.g., Hach StablCal TM or equivalent) are acceptable substitutes for formazin. | |||||
| 54 Waters Corp. Now included in ASTM D6508-15, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. 2015. | |||||
| 55 Kelada-01, Kelada Automated Test Methods for Total Cyanide, Acid Dissociable Cyanide, and Thiocyanate, EPA 821-B-01-009, Revision 1.2, August 2001. US EPA. Note: A 450-W UV lamp may be used in this method instead of the 550-W lamp specified if it provides performance within the quality control (QC) acceptance criteria of the method in a given instrument. Similarly, modified flow cell configurations and flow conditions may be used in the method, provided that the QC acceptance criteria are met. | |||||
| 56 QuikChem Method 10-204-00-1-X, Digestion and Distillation of Total Cyanide in Drinking and Wastewaters using MICRO DIST and Determination of Cyanide by Flow Injection Analysis. Revision 2.2, March 2005. Lachat Instruments. | |||||
| 57 When using sulfide removal test procedures described in EPA Method 335.4, reconstitute particulate that is filtered with the sample prior to distillation. | |||||
| 58 Unless otherwise stated, if the language of this table specifies a sample digestion and/or distillation “followed by” analysis with a method, approved digestion and/or distillation are required prior to analysis. | |||||
| 59 Samples analyzed for available cyanide using OI Analytical method OIA-1677-09 or ASTM method D6888-16 that contain particulate matter may be filtered only after the ligand exchange reagents have been added to the samples, because the ligand exchange process converts complexes containing available cyanide to free cyanide, which is not removed by filtration. Analysts are further cautioned to limit the time between the addition of the ligand exchange reagents and sample filtration to no more than 30 minutes to preclude settling of materials in samples. | |||||
| 60 Analysts should be aware that pH optima and chromophore absorption maxima might differ when phenol is replaced by a substituted phenol as the color reagent in Berthelot Reaction (“phenol-hypochlorite reaction”) colorimetric ammonium determination methods. For example, when phenol is used as the color reagent, pH optimum and wavelength of maximum absorbance are about 11.5 and 635 nm, respectively—see, Patton, C.J. and S.R. Crouch. March 1977. Anal. Chem. 49:464-469. These reaction parameters increase to pH > 12.6 and 665 nm when salicylate is used as the color reagent—see, Krom, M.D. April 1980. The Analyst 105:305-316. | |||||
| 61 If atomic absorption or ICP instrumentation is not available, the aluminon colorimetric method detailed in the 19th Edition of Standard Methods for the Examination of Water and Wastewater may be used. This method has poorer precision and bias than the methods of choice. | |||||
| 62 Easy (1-Reagent) Nitrate Method, Revision November 12, 2011. Craig Chinchilla. | |||||
| 63 Hach Method 10360, Luminescence Measurement of Dissolved Oxygen in Water and Wastewater and for Use in the Determination of BOD 5 and CBOD 5 . Revision 1.2, October 2011. Hach Company. This method may be used to measure dissolved oxygen when performing the methods approved in this table IB for measurement of biochemical oxygen demand (BOD) and carbonaceous biochemical oxygen demand (CBOD). | |||||
| 64 In-Situ Method 1002-8-2009, Dissolved Oxygen (DO) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 65 Mitchell Method M5331, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Leck Mitchell. | |||||
| 66 Mitchell Method M5271, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Leck Mitchell. | |||||
| 67 Orion Method AQ4500, Determination of Turbidity by Nephelometry. Revision 5, March 12, 2009. Thermo Scientific. | |||||
| 68 EPA Method 200.5, Determination of Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma-Atomic Emission Spectrometry, EPA/600/R-06/115. Revision 4.2, October 2003. US EPA. | |||||
| 69 Method 1627, Kinetic Test Method for the Prediction of Mine Drainage Quality, EPA-821-R-09-002. December 2011. US EPA. | |||||
| 70 Techniques and Methods Book 5-B1, Determination of Elements in Natural-Water, Biota, Sediment and Soil Samples Using Collision/Reaction Cell Inductively Coupled Plasma-Mass Spectrometry, Chapter 1, Section B, Methods of the National Water Quality Laboratory, Book 5, Laboratory Analysis, 2006. USGS. | |||||
| 71 Water-Resources Investigations Report 01-4132, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organic Plus Inorganic Mercury in Filtered and Unfiltered Natural Water with Cold Vapor-Atomic Fluorescence Spectrometry, 2001. USGS. | |||||
| 72 USGS Techniques and Methods 5-B8, Chapter 8, Section B, Methods of the National Water Quality Laboratory Book 5, Laboratory Analysis, 2011 USGS. | |||||
| 73 NECi Method N07-0003, “Nitrate Reductase Nitrate-Nitrogen Analysis,” Revision 9.0, March 2014, The Nitrate Elimination Co., Inc. | |||||
| 74 Timberline Instruments, LLC Method Ammonia-001, “Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Conductivity Cell Analysis,” June 2011, Timberline Instruments, LLC. | |||||
| 75 Hach Company Method 10206, “Spectrophotometric Measurement of Nitrate in Water and Wastewater,” Revision 2.1, January 2013, Hach Company. | |||||
| 76 Hach Company Method 10242, “Simplified Spectrophotometric Measurement of Total Kjeldahl Nitrogen in Water and Wastewater,” Revision 1.1, January 2013, Hach Company. | |||||
| 77 National Council for Air and Stream Improvement (NCASI) Method TNTP-W10900, “Total (Kjeldahl) Nitrogen and Total Phosphorus in Pulp and Paper Biologically Treated Effluent by Alkaline Persulfate Digestion,” June 2011, National Council for Air and Stream Improvement, Inc. | |||||
| 78 The pH adjusted sample is to be adjusted to 7.6 for NPDES reporting purposes. | |||||
| 79 I-2057-85 in U.S. Geological Survey Techniques of Water-Resources Investigations, Book 5, Chap. A1, Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, 1989. | |||||
| 80 Methods I-2522-90, I-2540-90, and I-2601-90 in U.S. Geological Survey Open-File Report 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments, 1993. | |||||
| 81 Method I-4472-97 in U.S. Geological Survey Open-File Report 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments, 1998. | |||||
| 82 FIAlab 100, “Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Fluorescence Detector Analysis”, April 4, 2018, FIAlab Instruments, Inc. | |||||
| 83 MACHEREY-NAGEL GmbH and Co. Method 036/038 NANOCOLOR® COD LR/HR, “Spectrophotometric Measurement of Chemical Oxygen Demand in Water and Wastewater”, Revision 1.5, May 2018, MACHEREY-NAGEL GmbH and Co. KG. | |||||
| 84 Please refer to the following applicable Quality Control Sections: Part 2000 Methods, Physical and Aggregate Properties 2020 (2021); Part 3000 Methods, Metals, 3020 (2021); Part 4000 Methods, Inorganic Nonmetallic Constituents, 4020 (2022); Part 5000 Methods, and Aggregate Organic Constituents, 5020 (2022). These Quality Control Standards are available for download at www.standardmethods.org at no charge. | |||||
| 85 Each laboratory may establish its own control limits by performing at least 25 glucose-glutamic acid (GGA) checks over several weeks or months and calculating the mean and standard deviation. The laboratory may then use the mean ± 3 standard deviations as the control limit for future GGA checks. However, GGA acceptance criteria can be no wider than 198 ± 30.5 mg/L for BOD 5 . GGA acceptance criteria for CBOD must be either 198 ± 30.5 mg/L, or the lab may develop control charts under the following conditions: dissolved oxygen uptake from the seed contribution is between 0.6-1.0 mg/L; control charts are performed on at least 25 GGA checks with three standard deviations from the derived mean; the RSD must not exceed 7.5%; and any single GGA value cannot be less than 150 mg/L or higher than 250 mg/L. | |||||
| 86 The approved method is that cited in Standard Methods for the Examination of Water and Wastewater, 14th Edition, 1976. | |||||
| 1. Acidity (as CaCO 3), mg/L | Electrometric endpoint or phenolphthalein endpoint | 2310 B-2020 | D1067-16 | I-1020-85. 2 | |
| 2. Alkalinity (as CaCO 3), mg/L | Electrometric or Colorimetric titration to pH 4.5, Manual | 2320 B-2021 | D1067-16 | 973.43, 3 I-1030-85. 2 | |
| Automatic | 310.2 (Rev. 1974) 1 | I-2030-85. 2 | |||
| 3. Aluminum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 D-2019 or 3111 E-2019 | I-3051-85. 2 | |||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97, 81 | |
| Direct Current Plasma (DCP) 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Eriochrome cyanine R) | 3500-Al B-2020. | ||||
| 4. Ammonia (as N), mg/L | Manual distillation 6 or gas diffusion (pH > 11), followed by any of the following: | 350.1 Rev. 2.0 (1993) | 4500-NH 3 B-2021 | 973.49. 3 | |
| Nesslerization | D1426-15 (A) | 973.49, 3 I-3520-85. 2 | |||
| Titration | 4500-NH 3 C-2021. | ||||
| Electrode | 4500-NH 3 D-2021 or E-2021 | D1426-15 (B) | |||
| Manual phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods | 4500-NH 3 F-2021 | See footnote. 60 | |||
| Automated phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods | 350.1, 30 Rev. 2.0 (1993) | 4500-NH 3 G-2021, 4500-NH 3 H-2021 | I-4523-85, 2 I-2522-90. 80 | ||
| Automated electrode | See footnote. 7 | ||||
| Ion Chromatography | D6919-17. | ||||
| Automated gas diffusion, followed by conductivity cell analysis | Timberline Ammonia-001. 74 | ||||
| Automated gas diffusion followed by fluorescence detector analysis | FIAlab100. 82 | ||||
| 5. Antimony—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019. | ||||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| 6. Arsenic—Total, 4 mg/L | Digestion, 4 followed by any of the following: | 206.5 (Issued 1978). 1 | |||
| AA gaseous hydride | 3114 B-2020 or 3114 C-2020 | D2972-15 (B) | I-3062-85. 2 | ||
| AA furnace | 3113 B-2020 | D2972-15 (C) | I-4063-98. 49 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5, Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05. 70 | |
| Colorimetric (SDDC) | 3500-As B-2020 | D2972-15 (A) | I-3060-85. 2 | ||
| 7. Barium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 D-2019 | I-3084-85. 2 | |||
| AA furnace | 3113 B-2020 | D4382-18. | |||
| ICP/AES 36 | 200.5, Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | See footnote. 34 | ||||
| 8. Beryllium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019 or 3111 E-2019 | D3645-15 (A) | I-3095-85. 2 | ||
| AA furnace | 3113 B-2020 | D3645-15 (B). | |||
| STGFAA | 200.9, Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | D4190-15 | See footnote. 34 | |||
| Colorimetric (aluminon) | See footnote 61 | ||||
| 9. Biochemical oxygen demand (BOD 5), mg/L | Dissolved Oxygen Depletion | 5210 B-2016 85 | 973.44 3 p. 17, 9 I-1578-78, 8 see footnote. 1063 | ||
| 10. Boron—Total, 37 mg/L | Colorimetric (curcumin) | 4500-B B-2011 | I-3112-85. 2 | ||
| ICP/AES | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | D4190-15 | See footnote. 34 | |||
| 11. Bromide, mg/L | Electrode | D1246-16 | I-1125-85. 2 | ||
| Ion Chromatography | 300.0 Rev 2.1 (1993), and 300.1 Rev 1.0 (1997) | 4110 B-2020, C-2020 or D-2020 | D4327-17 | 993.30, 3 I-2057-85. 79 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508 Rev. 2. 54 | ||
| 12. Cadmium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D3557-17 (A or B) | 974.27 3 p. 37, 9 I-3135-85 2 or I-3136-85. 2 | ||
| AA furnace | 3113 B-2020 | D3557-17 (D) | I-4138-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-1472-85 2 or I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Voltammetry 11 | D3557-17 (C). | ||||
| Colorimetric (Dithizone) | 3500-Cd D-1990. | ||||
| 13. Calcium—Total, 4 mg/L | Digestion 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 D-2019 | D511-14 (B) | I-3152-85. 2 | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Titrimetric (EDTA) | 3500-Ca B-2020 | D511-14 (A). | |||
| Ion Chromatography | D6919-17. | ||||
| 14. Carbonaceous biochemical oxygen demand (CBOD 5), mg/L 12 | Dissolved Oxygen Depletion with nitrification inhibitor | 5210 B-2016 85 | See footnotes. 35 63 | ||
| 15. Chemical oxygen demand (COD), mg/L | Titrimetric | 410.3 (Rev. 1978) 1 | 5220 B-2011 or C-2011 | D1252-06(12) (A) | 973.46 3 p. 17, 9 I-3560-85. 2 |
| Spectrophotometric, manual or automatic | 410.4 Rev. 2.0 (1993) | 5220 D-2011 | D1252-06(12) (B) | See footnotes, 131483 I-3561-85. 2 | |
| 16. Chloride, mg/L | Titrimetric: (silver nitrate) | 4500-Cl B-2021 | D512-12 (B) | I-1183-85. 2 | |
| (Mercuric nitrate) | 4500-Cl C-2021 | D512-12 (A) | 973.51, 3 I-1184-85. 2 | ||
| Colorimetric: manual | I-1187-85. 2 | ||||
| Automated (ferricyanide) | 4500-Cl E-2021 | I-2187-85. 2 | |||
| Potentiometric Titration | 4500-Cl D-2021. | ||||
| Ion Selective Electrode | D512-12 (C). | ||||
| Ion Chromatography | 300.0 Rev 2.1 (1993), and 300.1 Rev 1.0 (1997) | 4110 B-2020 or 4110 C-2020 | D4327-17 | 993.30, 3 I-2057-90. 51 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 17. Chlorine—Total residual, mg/L | Amperometric direct | 4500-Cl D-2011 | D1253-14. | ||
| Amperometric direct (low level) | 4500-Cl E-2011. | ||||
| Iodometric direct | 4500-Cl B-2011. | ||||
| Back titration ether end-point 15 | 4500-Cl C-2011. | ||||
| DPD-FAS | 4500-Cl F-2011. | ||||
| Spectrophotometric, DPD | 4500-Cl G-2011. | ||||
| Electrode | See footnote. 16 | ||||
| 17A. Chlorine—Free Available, mg/L | Amperometric direct | 4500-Cl D-2011 | D1253-14. | ||
| Amperometric direct (low level) | 4500-Cl E-2011. | ||||
| DPD-FAS | 4500-Cl F-2011. | ||||
| Spectrophotometric, DPD | 4500-Cl G-2011. | ||||
| 18. Chromium VI dissolved, mg/L | 0.45-micron filtration followed by any of the following: | ||||
| AA chelation-extraction | 3111 C-2019 | I-1232-85. 2 | |||
| Colorimetric (diphenyl-carbazide) | 3500-Cr B-2020 | D1687-17 (A) | I-1230-85. 2 | ||
| 19. Chromium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 | D1687-17 (B) | 974.27, 3 I-3236-85. 2 | ||
| AA chelation-extraction | 3111 C-2019. | ||||
| AA furnace | 3113 B-2020 | D1687-17 (C) | I-3233-93. 46 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (diphenyl-carbazide) | 3500-Cr B-2020. | ||||
| 20. Cobalt—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 C-2019 | D3558-15 (A or B) | p. 37, 9 I-323985. 2 | ||
| AA furnace | 3113 B-2020 | D3558-15 (C) | I-4243-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| DCP | D4190-15 | See footnote. 34 | |||
| 21. Color, platinum cobalt units or dominant wavelength, hue, luminance purity | Colorimetric (ADMI) | 2120 F-2021. 78 | |||
| Platinum cobalt visual comparison | 2120 B-2021 | I-1250-85. 2 | |||
| Spectrophotometric | See footnote. 18 | ||||
| 22. Copper—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1688-17 (A or B) | 974.27, 3 p. 37, 9 I-3270-85 2 or I-3271-85. 2 | ||
| AA furnace | 3113 B-2020 | D1688-17 (C) | I-4274-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Neocuproine) | 3500-Cu B-2020. | ||||
| Colorimetric (Bathocuproine) | 3500-Cu C-2020 | See footnote. 19 | |||
| 23. Cyanide—Total, mg/L | Automated UV digestion/distillation and Colorimetry | Kelada-01. 55 | |||
| Segmented Flow Injection, In-Line Ultraviolet Digestion, followed by gas diffusion amperometry | 4500-CN P-2021 | D7511-12 (17). | |||
| Manual distillation with MgCl 2 , followed by any of the following: | 335.4 Rev. 1.0 (1993) 57 | 4500-CN B-2021 and C-2021 | D2036-09(15)(A), D7284-20 | 10-204-00-1-X. 56 | |
| Flow Injection, gas diffusion amperometry | D2036-09(15)(A) D7284-20. | ||||
| Titrimetric | 4500-CN D-2021 | D2036-09(15)(A) | See footnote 9 p. 22. | ||
| Spectrophotometric, manual | 4500-CN E-2021 | D2036-09(15)(A) | I-3300-85. 2 | ||
| Semi-Automated 20 | 335.4 Rev. 1.0 (1993) 57 | 4500-CN N-2021 | 10-204-00-1-X, 56 I-4302-85. 2 | ||
| Ion Chromatography | D2036-09(15)(A). | ||||
| Ion Selective Electrode | 4500-CN F-2021 | D2036-09(15)(A). | |||
| 24. Cyanide—Available, mg/L | Cyanide Amenable to Chlorination (CATC); Manual distillation with MgCl 2 , followed by Titrimetric or Spectrophotometric | 4500-CN G-2021 | D2036-09(15)(B). | ||
| Flow injection and ligand exchange, followed by gas diffusion amperometry 59 | 4500-CN Q-2021 | D6888-16 | OIA-1677-09. 44 | ||
| Automated Distillation and Colorimetry (no UV digestion) | Kelada-01. 55 | ||||
| 24A. Cyanide—Free, mg/L | Flow Injection, followed by gas diffusion amperometry | 4500-CN R-2021 | D7237-18 (A) | OIA-1677-09. 44 | |
| Manual micro-diffusion and colorimetry | D4282-15. | ||||
| 25. Fluoride—Total, mg/L | Manual distillation, 6 followed by any of the following: | 4500-F B-2021 | D1179-16 (A). | ||
| Electrode, manual | 4500-F C-2021 | D1179-16 (B). | |||
| Electrode, automated | 4500-F G-2021 | I-4327-85. 2 | |||
| Colorimetric, (SPADNS) | 4500-F D-2021. | ||||
| Automated complexone | 4500-F E-2021. | ||||
| Ion Chromatography | 300.0 Rev 2.1 (1993) and 300.1 Rev 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 26. Gold—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 231.2 (Issued 1978) 1 | 3113 B-2020. | |||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| 27. Hardness—Total (as CaCO (3) , mg/L | Automated colorimetric | 130.1 (Issued 1971). 1 | |||
| Titrimetric (EDTA) | 2340 C-2021 | D1126-17 | 973.52B, 3 I-1338-85. 2 | ||
| Ca plus Mg as their carbonates, by any approved method for Ca and Mg (See Parameters 13 and 33), provided that the sum of the lowest point of quantitation for Ca and Mg is below the NPDES permit requirement for Hardness. | 2340 B-2021. | ||||
| 28. Hydrogen ion (pH), pH units | Electrometric measurement | 4500-H + B-2021 | D1293-18 (A or B) | 973.41, 3 I-1586-85. 2 | |
| Automated electrode | 150.2 (Dec. 1982) 1 | See footnote 21 I-2587-85. 2 | |||
| 29. Iridium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 235.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 30. Iron—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1068-15 (A) | 974.27, 3 I-3381-85. 2 | ||
| AA furnace | 3113 B-2020 | D1068-15 (B). | |||
| STGFAA | 200.9, Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Phenanthroline) | 3500-Fe B-2011 | D1068-15 (C) | See footnote. 22 | ||
| 31. Kjeldahl Nitrogen 5 —Total (as N), mg/L | Manual digestion 20 and distillation or gas diffusion, followed by any of the following: | 4500-N org B-2021 or C-2021 and 4500-NH 3 B-2021 | D3590-17 (A) | I-4515-91. 45 | |
| Titration | 4500-NH 3 C-2021 | 973.48. 3 | |||
| Nesslerization | D1426-15 (A). | ||||
| Electrode | 4500-NH 3 D-2021 or E-2021 | D1426-15 (B). | |||
| Semi-automated phenate | 350.1 Rev. 2.0 (1993) | 4500-NH 3 G-2021 or 4500-NH 3 H-2021. | |||
| Manual phenate, salicylate, or other substituted phenols in Berthelot reaction based methods | 4500-NH 3 F-2021 | See footnote. 60 | |||
| Automated gas diffusion, followed by conductivity cell analysis | Timberline Ammonia-001. 74 | ||||
| Automated gas diffusion followed by fluorescence detector analysis | FIAlab 100. 82 | ||||
| Automated Methods for TKN that do not require manual distillation. | |||||
| Automated phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods colorimetric (auto digestion and distillation) | 351.1 (Rev. 1978) 1 | I-4551-78. 8 | |||
| Semi-automated block digestor colorimetric (distillation not required) | 351.2 Rev. 2.0 (1993) | 4500-N org D-2021 | D3590-17 (B) | I-4515-91. 45 | |
| Block digester, followed by Auto distillation and Titration | See footnote. 39 | ||||
| Block digester, followed by Auto distillation and Nesslerization | See footnote. 40 | ||||
| Block Digester, followed by Flow injection gas diffusion (distillation not required) | See footnote. 41 | ||||
| Digestion with peroxdisulfate, followed by Spectrophotometric (2,6-dimethyl phenol) | Hach 10242. 76 | ||||
| Digestion with persulfate, followed by Colorimetric | NCASI TNTP W10900. 77 | ||||
| 32. Lead—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D3559-15 (A or B) | 974.27, 3 I-3399-85. 2 | ||
| AA furnace | 3113 B-2020 | D3559-15 (D) | I-4403-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Voltammetry 11 | D3559-15 (C). | ||||
| Colorimetric (Dithizone) | 3500-Pb B-2020. | ||||
| 33. Magnesium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | D511-14 (B) | 974.27, 3 I-3447-85. 2 | ||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Ion Chromatography | D6919-17. | ||||
| 34. Manganese—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D858-17 (A or B) | 974.27, 3 I-3454-85. 2 | ||
| AA furnace | 3113 B-2020 | D858-17 (C). | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5, Rev. 4.2 (2003); 68 200.7, Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Persulfate) | 3500-Mn B-2020 | 920.203. 3 | |||
| Colorimetric (Periodate) | See footnote. 23 | ||||
| 35. Mercury—Total, mg/L | Cold vapor, Manual | 245.1 Rev. 3.0 (1994) | 3112 B-2020 | D3223-17 | 977.22, 3 I-3462-85. 2 |
| Cold vapor, Automated | 245.2 (Issued 1974). 1 | ||||
| Cold vapor atomic fluorescence spectrometry (CVAFS) | 245.7 Rev. 2.0 (2005) 17 | I-4464-01. 71 | |||
| Purge and Trap CVAFS | 1631E. 43 | ||||
| 36. Molybdenum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019 | I-3490-85. 2 | |||
| AA furnace | 3113 B-2020 | I-3492-96. 47 | |||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | See footnote. 34 | ||||
| 37. Nickel—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1886-14 (A or B) | I-3499-85. 2 | ||
| AA furnace | 3113 B-2020 | D1886-14 (C) | I-4503-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| 38. Nitrate (as N), mg/L | Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Ion Selective Electrode | 4500-NO 3 D-2019. | ||||
| Colorimetric (Brucine sulfate) | 352.1 (Issued 1971) 1 | 973.50, 3 419D, 86 p. 28. 9 | |||
| Spectrophotometric (2,6-dimethylphenol) | Hach 10206. 75 | ||||
| Nitrate-nitrite N minus Nitrite N (see parameters 39 and 40). | |||||
| 39. Nitrate-nitrite (as N), mg/L | Cadmium reduction, Manual | 4500-NO 3 E-2019 | D3867-16 (B). | ||
| Cadmium reduction, Automated | 353.2 Rev. 2.0 (1993) | 4500-NO 3 F-2019 or 4500-NO 3 I-2019 | D3867-16 (A) | I-2545-90. 51 | |
| Automated hydrazine | 4500-NO 3 H-2019. | ||||
| Reduction/Colorimetric | See footnote. 62 | ||||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Enzymatic reduction, followed by automated colorimetric determination | D7781-14 | I-2547-11, 72 I-2548-11, 72 N07-0003. 73 | |||
| Enzymatic reduction, followed by manual colorimetric determination | 4500-NO 3 J-2018. | ||||
| Spectrophotometric (2,6-dimethylphenol) | Hach 10206. 75 | ||||
| 40. Nitrite (as N), mg/L | Spectrophotometric: Manual | 4500-NO 2 B-2021 | See footnote. 25 | ||
| Automated (Diazotization) | I-4540-85 2 see footnote, 62 I-2540-90. 80 | ||||
| Automated (*bypass cadmium reduction) | 353.2 Rev. 2.0 (1993) | 4500-NO 3 F-2019, 4500-NO 3 I-2019 | D3867-16 (A) | I-4545-85. 2 | |
| Manual (*bypass cadmium or enzymatic reduction) | 4500-NO 3 E-2019, 4500-NO 3 J-2018 | D3867-16 (B). | |||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Automated (*bypass Enzymatic reduction) | D7781-14 | I-2547-11, 72 I-2548-11, 72 N07-0003. 73 | |||
| 41. Oil and grease—Total recoverable, mg/L | Hexane extractable material (HEM): n -Hexane extraction and gravimetry | 1664 Rev. A 1664 Rev. B 42 | 5520 B or G-2021. 38 | ||
| Silica gel treated HEM (SGT-HEM): Silica gel treatment and gravimetry | 1664 Rev. A, 1664 Rev. B 42 | 5520 B or G-2021 38 and 5520 F-2021. 38 | |||
| 42. Organic carbon—Total (TOC), mg/L | Combustion | 5310 B-2014 | D7573-18a e1 | 973.47, 3 p. 14. 24 | |
| Heated persulfate or UV persulfate oxidation | 5310 C-2014, 5310 D-2011 | D4839-03(17) | 973.47, 3, p. 14. 24 | ||
| 43. Organic nitrogen (as N), mg/L | Total Kjeldahl N (Parameter 31) minus ammonia N (Parameter 4). | ||||
| 44. Ortho-phosphate (as P), mg/L | Ascorbic acid method: | ||||
| Automated | 365.1 Rev. 2.0 (1993) | 4500-P F-2021 or G-2021 | 973.56, 3 I-4601-85, 2 I-2601-90. 80 | ||
| Manual, single-reagent | 4500-P E-2021 | D515-88 (A) | 973.55. 3 | ||
| Manual, two-reagent | 365.3 (Issued 1978). 1 | ||||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 45. Osmium—Total 4 , mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 252.2 (Issued 1978). 1 | ||||
| 46. Oxygen, dissolved, mg/L | Winkler (Azide modification) | 4500-O (B-F)-2021 | D888-18 (A) | 973.45B, 3 I-1575-78. 8 | |
| Electrode | 4500-O G-2021 | D888-18 (B) | I-1576-78. 8 | ||
| Luminescence-Based Sensor | 4500-O H-2021 | D888-18 (C) | See footnotes. | ||
| 47. Palladium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 253.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| DCP | See footnote. 34 | ||||
| 48. Phenols, mg/L | Manual distillation, 26 followed by any of the following: | 420.1 (Rev. 1978) 1 | 5530 B-2021 | D1783-01(12) | |
| Colorimetric (4AAP) manual | 420.1 (Rev. 1978) 1 | 5530 D-2021 27 | D1783-01(12) (A or B). | ||
| Automated colorimetric (4AAP) | 420.4 Rev. 1.0 (1993). | ||||
| 49. Phosphorus (elemental), mg/L | Gas-liquid chromatography | See footnote. 28 | |||
| 50. Phosphorus—Total, mg/L | Digestion, 20 followed by any of the following: | 4500-P B (5)-2021 | 973.55. 3 | ||
| Manual | 365.3 (Issued 1978) 1 | 4500-P E-2021 | D515-88 (A). | ||
| Automated ascorbic acid reduction | 365.1 Rev. 2.0 (1993) | 4500-P (F-H)-2021 | 973.56, 3 I-4600-85. 2 | ||
| ICP/AES 436 | 200.7Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| Semi-automated block digestor (TKP digestion) | 365.4 (Issued 1974) 1 | D515-88 (B) | I-4610-91. 48 | ||
| Digestion with persulfate, followed by Colorimetric | NCASI TNTP W10900. 77 | ||||
| 51. Platinum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 255.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| DCP | See footnote. 34 | ||||
| 52. Potassium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | 973.5, 3 I-3630-85. 2 | |||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020. | |||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| Flame photometric | 3500-K B-2020. | ||||
| Electrode | 3500-K C-2020. | ||||
| Ion Chromatography | D6919-17. | ||||
| 53. Residue—Total, mg/L | Gravimetric, 103-105° | 2540 B-2020 | I-3750-85. 2 | ||
| 54. Residue—filterable, mg/L | Gravimetric, 180° | 2540 C-2020 | D5907-18 (B) | I-1750-85. 2 | |
| 55. Residue—non-filterable (TSS), mg/L | Gravimetric, 103-105° post-washing of residue | 2540 D-2020 | D5907-18 (A) | I-3765-85. 2 | |
| 56. Residue—settleable, mg/L | Volumetric (Imhoff cone), or gravimetric | 2540 F-2020. | |||
| 57. Residue—Volatile, mg/L | Gravimetric, 550° | 160.4 (Issued 1971) 1 | 2540 E-2020 | I-3753-85. 2 | |
| 58. Rhodium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration, or | 3111 B-2019. | ||||
| AA furnace | 265.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 59. Ruthenium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration, or | 3111 B-2019. | ||||
| AA furnace | 267.2. 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 60. Selenium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA furnace | 3113 B-2020 | D3859-15 (B) | I-4668-98. 49 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| AA gaseous hydride | 3114 B-2020, or 3114 C-2020 | D3859-15 (A) | I-3667-85. 2 | ||
| 61. Silica—Dissolved, 37 mg/L | 0.45-micron filtration followed by any of the following: | ||||
| Colorimetric, Manual | 4500-SiO 2 C-2021 | D859-16 | I-1700-85. 2 | ||
| Automated (Molybdosilicate) | 4500-SiO 2 E-2021 or F-2021 | I-2700-85. 2 | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| 62. Silver—Total, 4 31 mg/L | Digestion, 4 29 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 C-2019 | 974.27, 3 p. 37, 9 I-3720-85. 2 | |||
| AA furnace | 3113 B-2020 | I-4724-89. 51 | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | See footnote. 34 | ||||
| 63. Sodium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | 973.54, 3 I-3735-85. 2 | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Flame photometric | 3500-Na B-2020. | ||||
| Ion Chromatography | D6919-17. | ||||
| 64. Specific conductance, micromhos/cm at 25 °C | Wheatstone bridge | 120.1 (Rev. 1982) 1 | 2510 B-2021 | D1125-95(99) (A) | 973.40, 3 I-2781-85. 2 |
| 65. Sulfate (as SO 4), mg/L | Automated colorimetric | 375.2 Rev. 2.0 (1993) | 4500-SO 42 F-2021 or G-2021. | ||
| Gravimetric | 4500-SO 42 C-2021 or D-2021 | 925.54. 3 | |||
| Turbidimetric | 4500-SO 42 E-2021 | D516-16. | |||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30, 3 I-4020-05. 70 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508 Rev. 2. 54 | ||
| 66. Sulfide (as S), mg/L | Sample Pretreatment | 4500-S 2 B, C-2021. | |||
| Titrimetric (iodine) | 4500-S 2 F-2021 | I-3840-85. 2 | |||
| Colorimetric (methylene blue) | 4500-S 2 D-2021. | ||||
| Ion Selective Electrode | 4500-S 2 G-2021 | D4658-15. | |||
| 67. Sulfite (as SO 3), mg/L | Titrimetric (iodine-iodate) | 4500-SO 32 B-2021. | |||
| 68. Surfactants, mg/L | Colorimetric (methylene blue) | 5540 C-2021 | D2330-20. | ||
| 69. Temperature, °C | Thermometric | 2550 B-2010 | See footnote. 32 | ||
| 70. Thallium-Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 279.2 (Issued 1978) 1 | 3113 B-2020. | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4471-97 50 I-4472-97. 81 | |
| 71. Tin—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | I-3850-78. 8 | |||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994). | ||||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| 72. Titanium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 283.2 (Issued 1978). 1 | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994). | ||||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| 73. Turbidity, NTU 53 | Nephelometric | 180.1, Rev. 2.0 (1993) | 2130 B-2020 | D1889-00 | I-3860-85, 2 see footnotes. 656667 |
| 74. Vanadium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 3113 B-2020 | D3373-17. | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05. 70 | |
| DCP | D4190-15 | See footnote. 34 | |||
| Colorimetric (Gallic Acid) | 3500-V B-2011. | ||||
| 75. Zinc—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1691-17 (A or B) | 974.27 3 p. 37, 9 I-3900-85. 2 | ||
| AA furnace | 289.2 (Issued 1978). 1 | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7, Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Zincon) | 3500 Zn B-2020 | See footnote. 33 | |||
| 76. Acid Mine Drainage | 1627. 69 | ||||
| Parameter 1 | Method | EPA 27 | Standard methods 17 | ASTM | Other |
| 1. Acenaphthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 2. Acenaphthylene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 3. Acrolein | GC | 603 | |||
| GC/MS | 624.1 4 , 1624B. | ||||
| 4. Acrylonitrile | GC | 603 | |||
| GC/MS | 624.1 4 , 1624B | O-4127-96. 13 | |||
| 5. Anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 6. Benzene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 7. Benzidine | Spectro-photometric | See footnote 3 p.1. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020. | |||
| HPLC | 605 | ||||
| 8. Benzo(a)anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 9. Benzo(a)pyrene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 10. Benzo(b)fluoranthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 11. Benzo(g,h,i)perylene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 12. Benzo(k)fluoranthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 13. Benzyl chloride | GC | See footnote 3 p. 130. | |||
| GC/MS | See footnote 6 p. S102. | ||||
| 14. Butyl benzyl phthalate | GC | 606 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 15. bis(2-Chloroethoxy) methane | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 16. bis(2-Chloroethyl) ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 17. bis(2-Ethylhexyl) phthalate | GC | 606 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 18. Bromodichloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 19. Bromoform | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 20. Bromomethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 21. 4-Bromophenyl phenyl ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 22. Carbon tetrachloride | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 23. 4-Chloro-3-methyl phenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 24. Chlorobenzene | GC | 601, 602 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 O-4436-16. 14 | ||
| 25. Chloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96. 13 | ||
| 26. 2-Chloroethylvinyl ether | GC | 601 | |||
| GC/MS | 624.1, 1624B. | ||||
| 27. Chloroform | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 28. Chloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 29. 2-Chloronaphthalene | GC | 612 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 30. 2-Chlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 31. 4-Chlorophenyl phenyl ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 32. Chrysene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 33. Dibenzo(a,h)anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 34. Dibromochloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 35. 1,2-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 36. 1,3-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 37. 1,4-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 38. 3,3′-Dichlorobenzidine | GC/MS | 625.1, 1625B | 6410 B-2020. | ||
| HPLC | 605. | ||||
| 39. Dichlorodifluoromethane | GC | 601. | |||
| GC/MS | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | |||
| 40. 1,1-Dichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 41. 1,2-Dichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 42. 1,1-Dichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 43. trans -1,2-Dichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 44. 2,4-Dichlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 45. 1,2-Dichloropropane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 O-4436-16. 14 | ||
| 46. cis -1,3-Dichloropropene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 47. trans -1,3-Dichloropropene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 48. Diethyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 49. 2,4-Dimethylphenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 50. Dimethyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 51. Di- n -butyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 52. Di- n -octyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 53. 2, 4-Dinitrophenol | GC | 604 | 6420 B-2021 | See footnote 9 p. 27. | |
| GC/MS | 625.1, 1625B | 6410 B-2020. | |||
| 54. 2,4-Dinitrotoluene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 55. 2,6-Dinitrotoluene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 56. Epichlorohydrin | GC | See footnote 3 p. 130. | |||
| GC/MS | See footnote 6 p. S102. | ||||
| 57. Ethylbenzene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 58. Fluoranthene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 59. Fluorene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 60. 1,2,3,4,6,7,8-Heptachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 61. 1,2,3,4,7,8,9-Heptachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 62. 1,2,3,4,6,7,8- Heptachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 63. Hexachlorobenzene | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 64. Hexachlorobutadiene | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 65. Hexachlorocyclopentadiene | GC | 612. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 , p. 27, O-4127-96. 13 | ||
| 66. 1,2,3,4,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 67. 1,2,3,6,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 68. 1,2,3,7,8,9-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 69. 2,3,4,6,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 70. 1,2,3,4,7,8-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 71. 1,2,3,6,7,8-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 72. 1,2,3,7,8,9-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 73. Hexachloroethane | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 74. Indeno(1,2,3-c,d) pyrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 75. Isophorone | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 76. Methylene chloride | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 77. 2-Methyl-4,6-dinitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 78. Naphthalene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021. | |||
| 79. Nitrobenzene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | D4657-92 (98). | ||||
| 80. 2-Nitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 81. 4-Nitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 82. N-Nitrosodimethylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 83. N-Nitrosodi- n -propylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 84. N-Nitrosodiphenylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 85. Octachlorodibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 86. Octachlorodibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 87. 2,2′-oxybis(1-chloropropane) 12 [also known as bis(2-Chloro-1-methylethyl) ether] | GC | 611. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 88. PCB-1016 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 89. PCB-1221 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 90. PCB-1232 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 91. PCB-1242 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 92. PCB-1248 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 93. PCB-1254 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 94. PCB-1260 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 95. 1,2,3,7,8-Pentachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 96. 2,3,4,7,8-Pentachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 97. 1,2,3,7,8-Pentachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 98. Pentachlorophenol | GC | 604 | 6420 B-2021 | See footnote 3 p. 140. | |
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 99. Phenanthrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 100. Phenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 101. Pyrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 102. 2,3,7,8-Tetrachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 103. 2,3,7,8-Tetrachloro-dibenzo- p -dioxin | GC/MS | 613, 625.1 5 , 1613B | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 104. 1,1,2,2-Tetrachloroethane | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96. 13 | ||
| 105. Tetrachloroethene | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 106. Toluene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 107. 1,2,4-Trichlorobenzene | GC | 612 | See footnote 3 p. 130. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 108. 1,1,1-Trichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 109. 1,1,2-Trichloroethane | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 110. Trichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 111. Trichlorofluoromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1 | 6200 B-2020 | O-4127-96. 13 | ||
| 112. 2,4,6-Trichlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 113. Vinyl chloride | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 114. Nonylphenol | GC/MS | D7065-17. | |||
| 115. Bisphenol A (BPA) | GC/MS | D7065-17. | |||
| 116. p-tert -Octylphenol (OP) | GC/MS | D7065-17. | |||
| 117. Nonylphenol Monoethoxylate (NP1EO) | GC/MS | D7065-17. | |||
| 118. Nonylphenol Diethoxylate (NP2EO) | GC/MS | D7065-17. | |||
| 119. Adsorbable Organic Halides (AOX) | Adsorption and Coulometric Titration | 1650. 11 | |||
| 120. Chlorinated Phenolics | In Situ Acetylation and GC/MS | 1653. 11 | |||
| Table IC notes: |
| 1 All parameters are expressed in micrograms per liter (µg/L) except for Method 1613B, in which the parameters are expressed in picograms per liter (pg/L). |
| 2 The full text of Methods 601-613, 1613B, 1624B, and 1625B are provided at appendix A, Test Procedures for Analysis of Organic Pollutants. The standardized test procedure to be used to determine the method detection limit (MDL) for these test procedures is given at appendix B of this part, Definition and Procedure for the Determination of the Method Detection Limit. These methods are available at: https://www.epa.gov/cwa-methods as individual PDF files. |
| 3 Methods for Benzidine: Chlorinated Organic Compounds, Pentachlorophenol and Pesticides in Water and Wastewater. September 1978. U.S. EPA. |
| 4 Method 624.1 may be used for quantitative determination of acrolein and acrylonitrile, provided that the laboratory has documentation to substantiate the ability to detect and quantify these analytes at levels necessary to comply with any associated regulations. In addition, the use of sample introduction techniques other than simple purge-and-trap may be required. QC acceptance criteria from Method 603 should be used when analyzing samples for acrolein and acrylonitrile in the absence of such criteria in Method 624.1. |
| 5 Method 625.1 may be extended to include benzidine, hexachlorocyclopentadiene, N-nitrosodimethylamine, N-nitrosodi- n -propylamine, and N-nitrosodiphenylamine. However, when they are known to be present, Methods 605, 607, and 612, or Method 1625B, are preferred methods for these compounds. Method 625.1 may be applied to 2,3,7,8-Tetrachloro-dibenzo- p -dioxin for screening purposes only. |
| 6 Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. American Public Health Association (APHA). |
| 7 Each analyst must make an initial, one-time demonstration of their ability to generate acceptable precision and accuracy with Methods 601-603, 1624B, and 1625B in accordance with procedures in Section 8.2 of each of these methods. Additionally, each laboratory, on an on-going basis must spike and analyze 10% (5% for Methods 624.1 and 625.1 and 100% for methods 1624B and 1625B) of all samples to monitor and evaluate laboratory data quality in accordance with Sections 8.3 and 8.4 of these methods. When the recovery of any parameter falls outside the quality control (QC) acceptance criteria in the pertinent method, analytical results for that parameter in the unspiked sample are suspect. The results should be reported but cannot be used to demonstrate regulatory compliance. If the method does not contain QC acceptance criteria, control limits of ±three standard deviations around the mean of a minimum of five replicate measurements must be used. These quality control requirements also apply to the Standard Methods, ASTM Methods, and other methods cited. |
| 8 Organochlorine Pesticides and PCBs in Wastewater Using Empore TM Disk. Revised October 28, 1994. 3M Corporation. |
| 9 Method O-3116-87 is in Open File Report 93-125, Methods of Analysis by U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. USGS. |
| 10 Analysts may use Fluid Management Systems, Inc. Power-Prep system in place of manual cleanup provided the analyst meets the requirements of Method 1613B (as specified in Section 9 of the method) and permitting authorities. Method 1613, Revision B, Tetra- through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS. Revision B, 1994. U.S. EPA. The full text of this method is provided in appendix A to this part and at https://www.epa.gov/cwa-methods/approved-cwa-test-methods-organic-compounds. |
| 11 Method 1650, Adsorbable Organic Halides by Adsorption and Coulometric Titration. Revision C, 1997 U.S. EPA. Method 1653, Chlorinated Phenolics in Wastewater by In Situ Acetylation and GCMS. Revision A, 1997 U.S. EPA. The full text for both of these methods is provided at appendix A in part 430 of this chapter, The Pulp, Paper, and Paperboard Point Source Category. |
| 12 The compound was formerly inaccurately labeled as 2,2′-oxybis(2-chloropropane) and bis(2-chloroisopropyl) ether. Some versions of Methods 611, and 1625 inaccurately list the analyte as “bis(2-chloroisopropyl) ether,” but use the correct CAS number of 108-60-1. |
| 13 Method O-4127-96, U.S. Geological Survey Open-File Report 97-829, Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of 86 volatile organic compounds in water by gas chromatography/mass spectrometry, including detections less than reporting limits,1998, USGS. |
| 14 Method O-4436-16 U.S. Geological Survey Techniques and Methods, book 5, chap. B12, Determination of heat purgeable and ambient purgeable volatile organic compounds in water by gas chromatography/mass spectrometry, 2016, USGS. |
| 15 SGS AXYS Method 16130, “Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Waters and Agilent Gas Chromatography-Tandem-Mass Spectrometry (GC/MS/MS), Revision 1.0” is available at: https://www.sgsaxys.com/wp-content/uploads/2022/09/SGS-AXYS-Method-16130-Rev-1.0.pdf. |
| 16 Pace Analytical Method PAM-16130-SSI, “Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Shimadzu Gas Chromatography Mass Spectrometry (GC-MS/MS), Revision 1.1,” is available at: pacelabs.com. |
| 17 Please refer to the following applicable Quality Control Section: Part 6000 Individual Organic Compounds, 6020 (2019). The Quality Control Standards are available for download at standardmethods.org at no charge. |
| Parameter | Method | EPA | Standard methods 15 | ASTM | Other |
| 1. Aldrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96 (02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 2. Ametryn | GC | 507, 619 | See footnote 3 p. 83, see footnote 9 O-3106-93, see footnote 6 p. S68. | ||
| GC/MS | 525.2, 625.1 | See footnote 14 O-1121-91. | |||
| 3. Aminocarb | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| 4. Atraton | GC | 619 | See footnote 3 p. 83, see footnote 6 p. S68. | ||
| GC/MS | 625.1. | ||||
| 5. Atrazine | GC | 507, 619, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 6. Azinphos methyl | GC | 614, 622, 1657 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC-MS | 625.1 | See footnote 11 O-1126-95. | |||
| 7. Barban | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 8. α-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 9. β-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 10. δ-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 11. γ-BHC (Lindane) | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 , O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 11 , O-1126-95. | ||
| 12. Captan | GC | 617, 608.3 | 6630 B-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7. |
| 13. Carbaryl | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 531.1, 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 14. Carbophenothion | GC | 617, 608.3 | 6630 B-2021 | See footnote 4 page 27, see footnote 6 p. S73. | |
| GC/MS | 625.1. | ||||
| 15. Chlordane | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 16. Chloropropham | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 17. 2,4-D | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 18. 4,4′-DDD | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3105-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 19. 4,4′-DDE | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 , O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 20. 4,4′-DDT | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 21. Demeton-O | GC | 614, 622 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 625.1 | ||||
| 22. Demeton-S. | GC | 614, 622 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 625.1. | ||||
| 23. Diazinon | GC | 507, 614, 622, 1657 | See footnote 3 p. 25, see footnote 4 O-3104-83, see footnote 6 p. S51. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 24. Dicamba | GC | 615 | See footnote 3 p. 115. | ||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 25. Dichlofenthion | GC | 622.1 | See footnote 4 page 27, see footnote 6 p. S73. | ||
| 26. Dichloran | GC | 608.2, 617, 608.3 | 6630 B-2021 | See footnote 3 p. 7. | |
| 27. Dicofol | GC | 617, 608.3 | See footnote 4 O-3104-83. | ||
| 28. Dieldrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 29. Dioxathion | GC | 614.1, 1657 | See footnote 4 page 27, see footnote 6 p. S73. | ||
| 30. Disulfoton | GC | 507, 614, 622, 1657 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 31. Diuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| 32. Endosulfan I | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 13 O-2002-01. | ||
| 33. Endosulfan II | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 13 O-2002-01. | ||
| 34. Endosulfan Sulfate | GC | 617, 608.3 | 6630 C-2021 | See footnote 8 3M0222. | |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 35. Endrin | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 5 | 6410 B-2020. | |||
| 36. Endrin aldehyde | GC | 617, 608.3 | 6630 C-2021 | See footnote 8 3M0222. | |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 37. Ethion | GC | 614, 614.1, 1657 | See footnote 4 page 27, see footnote 6 , p. S73. | ||
| GC/MS | 625.1 | See footnote 13 O-2002-01. | |||
| 38. Fenuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 39. Fenuron-TCA | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 40. Heptachlor | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 | 6410 B-2020. | |||
| 41. Heptachlor epoxide | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 6 p. S73, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 42. Isodrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | See footnote 4 O-3104-83, see footnote 6 p. S73. | |
| GC/MS | 625.1. | ||||
| 43. Linuron | GC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| GC/MS | See footnote 11 O-1126-95. | ||||
| 44. Malathion | GC | 614, 1657 | 6630 B-2021 | See footnote 3 p. 25, see footnote 6 p. S51. | |
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 45. Methiocarb | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 46. Methoxychlor | GC | 505, 508, 608.2, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 47. Mexacarbate | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 48. Mirex | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83. |
| GC/MS | 625.1. | ||||
| 49. Monuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 50. Monuron-TCA | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 51. Neburon | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 52. Parathion methyl | GC | 614, 622, 1657 | 6630 B-2021 | See footnote 4 page 27, see footnote 3 p. 25. | |
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 53. Parathion ethyl | GC | 614 | 6630 B-2021 | See footnote 4 page 27, see footnote 3 p. 25. | |
| GC/MS | See footnote 11 O-1126-95. | ||||
| 54. PCNB | GC | 608.1, 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90 , D5812-96(02) | See footnote 3 p. 7. |
| 55. Perthane | GC | 617, 608.3 | D3086-90, D5812-96(02) | See footnote 4 O-3104-83. | |
| 56. Prometon | GC | 507, 619 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 57. Prometryn | GC | 507, 619 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 13 O-2002-01. | |||
| 58. Propazine | GC | 507, 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | ||||
| 59. Propham | TLC | See footnote 3 p. 10, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 60. Propoxur | TLC | See footnote 3 p. 94, see footnote 6 , p. S60. | |||
| HPLC | 632. | ||||
| 61. Secbumeton | TLC | See footnote 3 p. 83, see footnote 6 p. S68. | |||
| GC | 619. | ||||
| 62. Siduron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 63. Simazine | GC | 505, 507, 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 64. Strobane | GC | 617, 608.3 | 6630 B-2021 & C-2021 | See footnote 3 p. 7. | |
| 65. Swep | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 66. 2,4,5-T | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| 67. 2,4,5-TP (Silvex) | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| 68. Terbuthylazine | GC | 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68. | ||
| GC/MS | See footnote 13 O-2002-01. | ||||
| 69. Toxaphene | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 , see footnote 4 O-3105-83. |
| GC/MS | 525.1, 525.2, 625.1 | 6410 B-2020. | |||
| 70. Trifluralin | GC | 508, 617, 627, 1656, 608.3 | 6630 B-2021 | See footnote 3 p. 7, see footnote 9 O-3106-93. | |
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. |
| Table ID notes: |
| 1 Pesticides are listed in this table by common name for the convenience of the reader. Additional pesticides may be found under table IC of this section, where entries are listed by chemical name. |
| 2 The standardized test procedure to be used to determine the method detection limit (MDL) for these test procedures is given at appendix B to this part, Definition and Procedure for the Determination of the Method Detection Limit. |
| 3 Methods for Benzidine, Chlorinated Organic Compounds, Pentachlorophenol and Pesticides in Water and Wastewater. September 1978. U.S. EPA. This EPA publication includes thin-layer chromatography (TLC) methods. |
| 4 Methods for the Determination of Organic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3. 1987. USGS. |
| 5 The method may be extended to include α-BHC, γ-BHC, endosulfan I, endosulfan II, and endrin. However, when they are known to exist, Method 608 is the preferred method. |
| 6 Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. American Public Health Association (APHA). |
| 7 Each analyst must make an initial, one-time, demonstration of their ability to generate acceptable precision and accuracy with Methods 608.3 and 625.1 in accordance with procedures given in Section 8.2 of each of these methods. Additionally, each laboratory, on an on-going basis, must spike and analyze 10% of all samples analyzed with Method 608.3 or 5% of all samples analyzed with Method 625.1 to monitor and evaluate laboratory data quality in accordance with Sections 8.3 and 8.4 of these methods. When the recovery of any parameter falls outside the warning limits, the analytical results for that parameter in the unspiked sample are suspect. The results should be reported, but cannot be used to demonstrate regulatory compliance. These quality control requirements also apply to the Standard Methods, ASTM Methods, and other methods cited. |
| 8 Organochlorine Pesticides and PCBs in Wastewater Using Empore TM Disk. Revised October 28, 1994. 3M Corporation. |
| 9 Method O-3106-93 is in Open File Report 94-37, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Triazine and Other Nitrogen-Containing Compounds by Gas Chromatography with Nitrogen Phosphorus Detectors. 1994. USGS. |
| 10 EPA Methods 608.1, 608.2, 614, 614.1, 615, 617, 619, 622, 622.1, 627, and 632 are found in Methods for the Determination of Nonconventional Pesticides in Municipal and Industrial Wastewater, EPA 821-R-92-002, April 1992, U.S. EPA. EPA Methods 505, 507, 508, 525.1, 531.1 and 553 are in Methods for the Determination of Nonconventional Pesticides in Municipal and Industrial Wastewater, Volume II, EPA 821-R-93-010B, 1993, U.S. EPA. EPA Method 525.2 is in Determination of Organic Compounds in Drinking Water by Liquid-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry, Revision 2.0, 1995, U.S. EPA. EPA methods 1656 and 1657 are in Methods for The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume I, EPA 821-R-93-010A, 1993, U.S. EPA. Methods 608.3 and 625.1 are available at: cwa-methods/approved-cwa-test-methods-organic-compounds. |
| 11 Method O-1126-95 is in Open-File Report 95-181, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring. 1995. USGS. |
| 12 Method O-2060-01 is in Water-Resources Investigations Report 01-4134, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by Graphitized Carbon-Based Solid-Phase Extraction and High-Performance Liquid Chromatography/Mass Spectrometry. 2001. USGS. |
| 13 Method O-2002-01 is in Water-Resources Investigations Report 01-4098, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of moderate-use pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry. 2001. USGS. |
| 14 Method O-1121-91 is in Open-File Report 91-519, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of organonitrogen herbicides in water by solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring. 1992. USGS. |
| 15 Please refer to the following applicable Quality Control Section: Part 6000 Methods, Individual Organic Compounds 6020 (2019). These Quality Control Standards are available for download at www.standardmethods.org at no charge. |
* * * * *
| Parameter and units | Method 1 | EPA | Standard methods | AOAC, ASTM, USGS | Other |
| Bacteria | |||||
| 1. Coliform (fecal), number per 100 mL | Most Probable Number (MPN), 5 tube, 3 dilution, or | p. 132 3 | 9221 E-2014, 9221 F-2014. 32 | ||
| Membrane filter (MF) 2 , single step | p. 124 3 | 9222 D-2015 26 | B-0050-85. 4 | ||
| 2. Coliform (total), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 114 3 | 9221 B-2014. | ||
| MF 2 , single step or | p. 108 3 | 9222 B-2015 27 | B-0025-85. 4 | ||
| MF 2 , two step with enrichment | p. 111 3 | 9222 B-2015. 27 | |||
| 3. E. coli, number per 100 mL | MPN 5713 , multiple tube, or | 9221 B.3-2014/9221 F-2014. 101232 | |||
| Multiple tube/multiple well, or | 9223 B-2016 11 | 991.15 9 | Colilert® 1115 , Colilert-18®. 111415 | ||
| MF 2567 , two step, or | 1103.2 18 | 9222 B-2015/9222 I-2015 17 , 9213 D-2007 | D5392-93. 8 | ||
| Single step | 1603.1 19 , 1604 20 | m-ColiBlue24® 16 , KwikCount EC. 2829 | |||
| 4. Fecal streptococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MF 2 , or | p. 136 3 | 9230 C-2013 30 | B-0055-85. 4 | ||
| Plate count | p. 143. 3 | ||||
| 5. Enterococci, number per 100 mL | MPN 57 , multiple tube/multiple well, or | 9230 D-2013 | D6503-99 8 | Enterolert®. 1121 | |
| MF 2567 two step, or | 1106.2 22 | 9230 C-2013 30 | D5259-92. 8 | ||
| Single step, or | 1600.1 23 | 9230 C-2013. 30 | |||
| Plate count | p. 143. 3 | ||||
| Protozoa | |||||
| 6. Cryptosporidium | Filtration/IMS/FA | 1622 24 , 1623 25 , 1623.1. 2531 | |||
| 7. Giardia | Filtration/IMS/FA | 1623 25 , 1623.1. 2531 | |||
| Table 1H notes: |
| 1 The method must be specified when results are reported. |
| 2 A 0.45-µm membrane filter (MF) or other pore size certified by the manufacturer to fully retain organisms to be cultivated and to be free of extractables which could interfere with their growth. |
| 3 Microbiological Methods for Monitoring the Environment, Water and Wastes. EPA/600/8-78/017. 1978. US EPA. |
| 4 U.S. Geological Survey Techniques of Water-Resource Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. USGS. |
| 5 Tests must be conducted to provide organism enumeration (density). Select the appropriate configuration of tubes/filtrations and dilutions/volumes to account for the quality, character, consistency, and anticipated organism density of the water sample. |
| 6 When the MF method has not been used previously to test waters with high turbidity, large numbers of noncoliform bacteria, or samples that may contain organisms stressed by chlorine, a parallel test should be conducted with a multiple-tube technique to demonstrate applicability and comparability of results. |
| 7 To assess the comparability of results obtained with individual methods, it is suggested that side-by-side tests be conducted across seasons of the year with the water samples routinely tested in accordance with the most current Standard Methods for the Examination of Water and Wastewater or EPA alternate test procedure (ATP) guidelines. |
| 8 Annual Book of ASTM Standards—Water and Environmental Technology. Section 11.02. 2000, 1999, 1996. ASTM International. |
| 9 Official Methods of Analysis of AOAC International, 16th Edition, Volume I, Chapter 17. 1995. AOAC International. |
| 10 The multiple-tube fermentation test is used in 9221B.3-2014. Lactose broth may be used in lieu of lauryl tryptose broth (LTB), if at least 25 parallel tests are conducted between this broth and LTB using the water samples normally tested, and this comparison demonstrates that the false-positive rate and false-negative rate for total coliform using lactose broth is less than 10 percent. No requirement exists to run the completed phase on 10 percent of all total coliform-positive tubes on a seasonal basis. |
| 11 These tests are collectively known as defined enzyme substrate tests. |
| 12 After prior enrichment in a presumptive medium for total coliform using 9221B.3-2014, all presumptive tubes or bottles showing any amount of gas, growth or acidity within 48 h ± 3 h of incubation shall be submitted to 9221F-2014. Commercially available EC-MUG media or EC media supplemented in the laboratory with 50 µg/mL of MUG may be used. |
| 13 Samples shall be enumerated by the multiple-tube or multiple-well procedure. Using multiple-tube procedures, employ an appropriate tube and dilution configuration of the sample as needed and report the Most Probable Number (MPN). Samples tested with Colilert® may be enumerated with the multiple-well procedures, Quanti-Tray® or Quanti-Tray®/2000, and the MPN calculated from the table provided by the manufacturer. |
| 14 Colilert-18® is an optimized formulation of the Colilert® for the determination of total coliforms and E. coli that provides results within 18 h of incubation at 35 °C, rather than the 24 h required for the Colilert® test and is recommended for marine water samples. |
| 15 Descriptions of the Colilert®, Colilert-18®, Quanti-Tray ®, and Quanti-Tray®/2000 may be obtained from IDEXX Laboratories Inc. |
| 16 A description of the mColiBlue24® test may be obtained from Hach Company. |
| 17 Subject coliform positive samples determined by 9222B-2015 or other membrane filter procedure to 9222I-2015 using NA-MUG media. |
| 18 Method 1103.2: Escherichia coli ( E. coli) in Water by Membrane Filtration Using membrane-Thermotolerant Escherichia coli Agar (mTEC), EPA-821-R-23-009. September 2023. US EPA. |
| 19 Method 1603.1: Escherichia coli ( E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008. September 2023 . US EPA. |
| 20 Method 1604: Total Coliforms and Escherichia coli ( E. coli) in Water by Membrane Filtration by Using a Simultaneous Detection Technique (MI Medium), EPA 821-R-02-024. September 2002. US EPA. |
| 21 A description of the Enterolert® test may be obtained from IDEXX Laboratories Inc. |
| 22 Method 1106.2: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus -Esculin Iron Agar (mE-EIA), EPA-821-R-23-007. September 2023. US EPA. |
| 23 Method 1600.1: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-21-006. September 2023. US EPA. |
| 24 Method 1622 uses a filtration, concentration, immunomagnetic separation of oocysts from captured material, immunofluorescence assay to determine concentrations, and confirmation through vital dye staining and differential interference contrast microscopy for the detection of Cryptosporidium. Method 1622: Cryptosporidium in Water by Filtration/IMS/FA, EPA-821-R-05-001. December 2005. US EPA. |
| 25 Methods 1623 and 1623.1 use a filtration, concentration, immunomagnetic separation of oocysts and cysts from captured material, immunofluorescence assay to determine concentrations, and confirmation through vital dye staining and differential interference contrast microscopy for the simultaneous detection of Cryptosporidium and Giardia oocysts and cysts. Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA-821-R-05-002. December 2005. US EPA. Method 1623.1: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA 816-R-12-001. January 2012. US EPA. |
| 26 On a monthly basis, at least ten blue colonies from positive samples must be verified using Lauryl Tryptose Broth and EC broth, followed by count adjustment based on these results; and representative non-blue colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. |
| 27 On a monthly basis, at least ten sheen colonies from positive samples must be verified using Lauryl Tryptose Broth and brilliant green lactose bile broth, followed by count adjustment based on these results; and representative non-sheen colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. |
| 28 A description of KwikCount EC may be obtained from Roth Bioscience, LLC. |
| 29 Approved for the analyses of E. coli in freshwater only. |
| 30 Verification of colonies by incubation of BHI agar at 10 ± 0.5 °C for 48 ± 3 h is optional. As per the Errata to the 23rd Edition of Standard Methods for the Examination of Water and Wastewater “Growth on a BHI agar plate incubated at 10 ± 0.5 °C for 48 ± 3 h is further verification that the colony belongs to the genus Enterococcus.” |
| 31 Method 1623.1 includes updated acceptance criteria for IPR, OPR, and MS/MSD and clarifications and revisions based on the use of Method 1623 for years and technical support questions. |
| 32 9221 F.2-2014 allows for simultaneous detection of E. coli and thermotolerant fecal coliforms by adding inverted vials to EC-MUG; the inverted vials collect gas produced by thermotolerant fecal coliforms. |
* * * *
(b) The material listed in this paragraph (b) is incorporated by reference into this section with the approval of the Director of the Federal Register under 5 U.S.C. 552(a) and 1 CFR part 51. All approved incorporation by reference (IBR) material is available for inspection at the EPA and at the National Archives and Records Administration (NARA). Contact the EPA at: EPA's Water Docket, EPA West, 1301 Constitution Avenue NW, Room 3334, Washington, DC 20004; telephone: 202-566-2426; email: docket-customerservice@epa.gov. For information on the availability of this material at NARA, visit www.archives.gov/federal-register/cfr/ibr-locations or email fr.inspection@nara.gov. The material may be obtained from the following sources in this paragraph (b).
(1) Environmental Monitoring and Support Laboratory, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from: National Technical Information Service, 5285 Port Royal Road, Springfield, Virginia 22161
(i) Microbiological Methods for Monitoring the Environment, Water, and Wastes. 1978. EPA/600/8-78/017, Pub. No. PB-290329/A.S.
(A) Part III Analytical Methodology, Section B Total Coliform Methods, page 108. Table IA, Note 3; Table IH, Note 3.
(B) Part III Analytical Methodology, Section B Total Coliform Methods, 2.6.2 Two-Step Enrichment Procedure, page 111. Table IA, Note 3; Table IH, Note 3.
(C) Part III Analytical Methodology, Section B Total Coliform Methods, 4 Most Probable Number (MPN) Method, page 114. Table IA, Note 3; Table IH, Note 3.
(D) Part III Analytical Methodology, Section C Fecal Coliform Methods, 2 Direct Membrane Filter (MF) Method, page 124. Table IA, Note 3; Table IH, Note 3.
(E) Part III, Analytical Methodology, Section C Fecal Coliform Methods, 5 Most Probable Number (MPN) Method, page 132. Table IA, Note 3; Table IH, Note 3.
(F) Part III Analytical Methodology, Section D Fecal Streptococci, 2 Membrane Filter (MF) Method, page 136. Table IA, Note 3; Table IH, Note 3.
(G) Part III Analytical Methodology, Section D Fecal Streptococci, 4 Most Probable Number Method, page 139. Table IA, Note 3; Table IH, Note 3.
(H) Part III Analytical Methodology, Section D Fecal Streptococci, 5 Pour Plate Method, page 143. Table IA, Note 3; Table IH, Note 3.
(ii) [Reserved]
(2) Environmental Monitoring and Support Laboratory, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(3) National Exposure Risk Laboratory-Cincinnati, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available from http://water.epa.gov/scitech/methods/cwa/index.cfm or from the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161. Telephone: 800-553-6847.
(i) Methods for the Determination of Inorganic Substances in Environmental Samples. August 1993. EPA/600/R-93/100, Pub. No. PB 94120821. Table IB, Note 52.
(A) Method 180.1, Determination of Turbidity by Nephelometry. Revision 2.0. Table IB, Note 52.
(B) Method 300.0, Determination of Inorganic Anions by Ion Chromatography. Revision 2.1. Table IB, Note 52.
(C) Method 335.4, Determination of Total Cyanide by Semi-Automated Colorimetry. Revision 1.0. Table IB, Notes 52 and 57.
(D) Method 350.1, Determination of Ammonium Nitrogen by Semi-Automated Colorimetry. Revision 2.0. Table IB, Notes 30 and 52.
(E) Method 351.2, Determination of Total Kjeldahl Nitrogen by Semi-Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(F) Method 353.2, Determination of Nitrate-Nitrite Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(G) Method 365.1, Determination of Phosphorus by Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(H) Method 375.2, Determination of Sulfate by Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(I) Method 410.4, Determination of Chemical Oxygen Demand by Semi-Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(ii) Methods for the Determination of Metals in Environmental Samples, Supplement I. May 1994. EPA/600/R-94/111, Pub. No. PB 95125472. Table IB, Note 52.
(A) Method 200.7, Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 4.4. Table IB, Note 52.
(B) Method 200.8, Determination of Trace Elements in Water and Wastes by Inductively Coupled Plasma Mass Spectrometry. Revision 5.3. Table IB, Note 52.
(C) Method 200.9, Determination of Trace Elements by Stabilized Temperature Graphite Furnace Atomic Absorption Spectrometry. Revision 2.2. Table IB, Note 52.
(D) Method 218.6, Determination of Dissolved Hexavalent Chromium in Drinking Water, Groundwater, and Industrial Wastewater Effluents by Ion Chromatography. Revision 3.3. Table IB, Note 52.
(E) Method 245.1, Determination of Mercury in Water by Cold Vapor Atomic Absorption Spectrometry. Revision 3.0. Table IB, Note 52.
(4) National Exposure Risk Laboratory-Cincinnati, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(i) EPA Method 200.5, Determination of Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 4.2, October 2003. EPA/600/R-06/115. Table IB, Note 68.
(ii) EPA Method 525.2, Determination of Organic Compounds in Drinking Water by Liquid-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry. Revision 2.0, 1995. Table ID, Note 10.
(5) Office of Research and Development, Cincinnati OH. U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from ORD Publications, CERI, U.S. Environmental Protection Agency, Cincinnati OH 45268.
(i) Methods for Benzidine, Chlorinated Organic Compounds, Pentachlorophenol, and Pesticides in Water and Wastewater. 1978. Table IC, Note 3; Table ID, Note 3.
(ii) Methods for Chemical Analysis of Water and Wastes. March 1979. EPA-600/4-79-020. Table IB, Note 1.
(iii) Methods for Chemical Analysis of Water and Wastes. Revised March 1983. EPA-600/4-79-020. Table IB, Note 1.
(A) Method 120.1, Conductance, Specific Conductance, μmhos at 25°C. Revision 1982. Table IB, Note 1.
(B) Method 130.1, Hardness, Total (mg/L as CaCO3), Colorimetric, Automated EDTA. Issued 1971. Table IB, Note 1.
(C) Method 150.2, pH, Continuous Monitoring (Electrometric). December 1982. Table IB, Note 1.
(D) Method 160.4, Residue, Volatile, Gravimetric, Ignition at 550°C. Issued 1971. Table IB, Note 1.
(E) Method 206.5, Arsenic, Sample Digestion Prior to Total Arsenic Analysis by Silver Diethyldithiocarbamate or Hydride Procedures. Issued 1978. Table IB, Note 1.
(F) Method 231.2, Gold, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(G) Method 245.2, Mercury, Automated Cold Vapor Technique. Issued 1974. Table IB, Note 1.
(H) Method 252.2, Osmium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(I) Method 253.2, Palladium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(J) Method 255.2, Platinum, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(K) Method 265.2, Rhodium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(L) Method 279.2, Thallium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(M) Method 283.2, Titanium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(N) Method 289.2, Zinc, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(O) Method 310.2, Alkalinity, Colorimetric, Automated, Methyl Orange. Revision 1974. Table IB, Note 1.
(P) Method 351.1, Nitrogen, Kjeldahl, Total, Colorimetric, Automated Phenate. Revision 1978. Table IB, Note 1.
(Q) Method 352.1, Nitrogen, Nitrate, Colorimetric, Brucine. Issued 1971. Table IB, Note 1.
(R) Method 365.3, Phosphorus, All Forms, Colorimetric, Ascorbic Acid, Two Reagent. Issued 1978. Table IB, Note 1.
(S) Method 365.4, Phosphorus, Total, Colorimetric, Automated, Block Digestor AA II. Issued 1974. Table IB, Note 1.
(T) Method 410.3, Chemical Oxygen Demand, Titrimetric, High Level for Saline Waters. Revision 1978. Table IB, Note 1.
(U) Method 420.1, Phenolics, Total Recoverable, Spectrophotometric, Manual 4-AAP With Distillation. Revision 1978. Table IB, Note 1.
(iv) Prescribed Procedures for Measurement of Radioactivity in Drinking Water. 1980. EPA-600/4-80-032. Table IE.
(A) Method 900.0, Gross Alpha and Gross Beta Radioactivity. Table IE.
(B) Method 903.0, Alpha-Emitting iRadio Isotopes. Table IE.
(C) Method 903.1, Radium-226, Radon Emanation Technique. Table IE.
(D) Appendix B, Error and Statistical Calculations. Table IE.
(6) Office of Science and Technology, U.S. Environmental Protection Agency, Washington DC (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(i) Method 1625C, Semivolatile Organic Compounds by Isotope Dilution GCMS. 1989. Table IF.
(ii) [Reserved]
(7) Office of Water, U.S. Environmental Protection Agency, Washington DC (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from National Technical Information Service, 5285 Port Royal Road, Springfield, Virginia 22161.
(i) Method 1631, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. Revision E, August 2002. EPA-821-R-02-019, Pub. No. PB2002-108220. Table IB, Note 43.
(ii) Kelada-01, Kelada Automated Test Methods for Total Cyanide, Acid Dissociable Cyanide, and Thiocyanate. Revision 1.2, August 2001. EPA 821-B-01-009, Pub. No. PB 2001-108275. Table IB, Note 55.
(iii) In the compendium Analytical Methods for the Determination of Pollutants in Pharmaceutical Manufacturing Industry Wastewaters. July 1998. EPA 821-B-98-016, Pub. No. PB95201679. Table IF, Note 1.
(A) EPA Method 1666, Volatile Organic Compounds Specific to the Pharmaceutical Industry by Isotope Dilution GC/MS. Table IF, Note 1.
(B) EPA Method 1667, Formaldehyde, Isobutyraldehyde, and Furfural by Derivatization Followed by High Performance Liquid Chromatography. Table IF.
(C) Method 1671, Volatile Organic Compounds Specific to the Pharmaceutical Manufacturing Industry by GC/FID. Table IF.
(iv) Methods For The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume I. Revision I, August 1993. EPA 821-R-93-010A, Pub. No. PB 94121654. Tables ID, IG.
(A) Method 608.1, Organochlorine Pesticides. Table ID, Note 10; Table IG, Note 3.
(B) Method 608.2, Certain Organochlorine Pesticides. Table ID, Note 10; Table IG, Note 3.
(C) Method 614, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(D) Method 614.1, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(E) Method 615, Chlorinated Herbicides. Table ID, Note 10; Table IG, Note 3.
(F) Method 617, Organohalide Pesticides and PCBs. Table ID, Note 10; Table IG, Note 3.
(G) Method 619, Triazine Pesticides. Table ID, Note 10; Table IG, Note 3.
(H) Method 622, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(I) Method 622.1, Thiophosphate Pesticides. Table ID, Note 10; Table IG, Note 3.
(J) Method 627, Dinitroaniline Pesticides. Table ID, Note 10; Table IG, Notes 1 and 3.
(K) Method 629, Cyanazine. Table IG, Note 3.
(L) Method 630, Dithiocarbamate Pesticides. Table IG, Note 3.
(M) Method 630.1, Dithiocarbamate Pesticides. Table IG, Note 3.
(N) Method 631, Benomyl and Carbendazim. Table IG, Note 3.
(O) Method 632, Carbamate and Urea Pesticides. Table ID, Note 10; Table IG, Note 3.
(P) Method 632.1, Carbamate and Amide Pesticides. Table IG, Note 3.
(Q) Method 633, Organonitrogen Pesticides. Table IG, Note 3.
(R) Method 633.1, Neutral Nitrogen-Containing Pesticides. Table IG, Note 3.
(S) Method 637, MBTS and TCMTB. Table IG, Note 3.
(T) Method 644, Picloram. Table IG, Note 3.
(U) Method 645, Certain Amine Pesticides and Lethane. Table IG, Note 3.
(V) Method 1656, Organohalide Pesticides. Table ID, Note 10; Table IG, Notes 1 and 3.
(W) Method 1657, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(X) Method 1658, Phenoxy-Acid Herbicides. Table IG, Note 3.
(Y) Method 1659, Dazomet. Table IG, Note 3.
(Z) Method 1660, Pyrethrins and Pyrethroids. Table IG, Note 3.
(AA) Method 1661, Bromoxynil. Table IG, Note 3.
(BB) Ind-01. Methods EV-024 and EV-025, Analytical Procedures for Determining Total Tin and Triorganotin in Wastewater. Table IG, Note 3.
(v) Methods For The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume II. August 1993. EPA 821-R-93-010B, Pub. No. PB 94166311. Table IG.
(A) Method 200.9, Determination of Trace Elements by Stabilized Temperature Graphite Furnace Atomic Absorption Spectrometry. Table IG, Note 3.
(B) Method 505, Analysis of Organohalide Pesticides and Commercial Polychlorinated Biphenyl (PCB) Products in Water by Microextraction and Gas Chromatography. Table ID, Note 10; Table IG, Note 3.
(C) Method 507, The Determination of Nitrogen- and Phosphorus-Containing Pesticides in Water by Gas Chromatography with a Nitrogen-Phosphorus Detector. Table ID, Note 10; Table IG, Note 3.
(D) Method 508, Determination of Chlorinated Pesticides in Water by Gas Chromatography with an Electron Capture Detector. Table ID, Note 10; Table IG, Note 3.
(E) Method 515.1, Determination of Chlorinated Acids in Water by Gas Chromatography with an Electron Capture Detector. Table IG, Notes 2 and 3.
(F) Method 515.2, Determination of Chlorinated Acids in Water Using Liquid-Solid Extraction and Gas Chromatography with an Electron Capture Detector. Table IG, Notes 2 and 3.
(G) Method 525.1, Determination of Organic Compounds in Drinking Water by Liquids-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry. Table ID, Note 10; Table IG, Note 3.
(H) Method 531.1, Measurement of N-Methylcarbamoyloximes and N-Methylcarbamates in Water by Direct Aqueous Injection HPLC with Post-Column Derivatization. Table ID, Note 10; Table IG, Note 3.
(I) Method 547, Determination of Glyphosate in Drinking Water by Direct-Aqueous-Injection HPLC, Post-Column Derivatization, and Fluorescence Detection. Table IG, Note 3.
(J) Method 548, Determination of Endothall in Drinking Water by Aqueous Derivatization, Liquid-Solid Extraction, and Gas Chromatography with Electron-Capture Detector. Table IG, Note 3.
(K) Method 548.1, Determination of Endothall in Drinking Water by Ion-Exchange Extraction, Acidic Methanol Methylation and Gas Chromatography/Mass Spectrometry. Table IG, Note 3.
(L) Method 553, Determination of Benzidines and Nitrogen-Containing Pesticides in Water by Liquid-Liquid Extraction or Liquid-Solid Extraction and Reverse Phase High Performance Liquid Chromatography/Particle Beam/Mass Spectrometry Table ID, Note 10; Table IG, Note 3.
(M) Method 555, Determination of Chlorinated Acids in Water by High Performance Liquid Chromatography With a Photodiode Array Ultraviolet Detector. Table IG, Note 3.
(vi) In the compendium Methods for the Determination of Organic Compounds in Drinking Water. Revised July 1991, December 1998. EPA-600/4-88-039, Pub. No. PB92-207703. Table IF.
(A) EPA Method 502.2, Volatile Organic Compounds in Water by Purge and Trap Capillary Column Gas Chromatography with Photoionization and Electrolytic Conductivity Detectors in Series. Table IF.
(B) [Reserved]
(vii) In the compendium Methods for the Determination of Organic Compounds in Drinking Water-Supplement II. August 1992. EPA-600/R-92-129, Pub. No. PB92-207703. Table IF.
(A) EPA Method 524.2, Measurement of Purgeable Organic Compounds in Water by Capillary Column Gas Chromatography/Mass Spectrometry. Table IF.
(B) [Reserved]
(viii) Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, Fifth Edition. October 2002. EPA 821-R-02-012, Pub. No. PB2002-108488. Table IA, Note 26.
(ix) Short-Term Methods for Measuring the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, Fourth Edition. October 2002. EPA 821-R-02-013, Pub. No. PB2002-108489. Table IA, Note 27.
(x) Short-Term Methods for Measuring the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, Third Edition. October 2002. EPA 821-R-02-014, Pub. No. PB2002-108490. Table IA, Note 28.
(8) Office of Water, U.S. Environmental Protection Agency (U.S. EPA), mail code 4303T, 1301 Constitution Avenue NW, Washington, DC 20460; website: www.epa.gov/cwa-methods.
(i) Method 245.7, Mercury in Water by Cold Vapor Atomic Fluorescence Spectrometry. Revision 2.0, February 2005. EPA-821-R-05-001. Table IB, Note 17.
(ii) Method 1103.2: Escherichia coli (E. coli) in Water by Membrane Filtration Using membrane-Thermotolerant Escherichia coli Agar (mTEC), EPA-821-R-23-009. September 2023. Table IH, Note 18.
(iii) Method 1106.2: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus -Esculin Iron Agar (mE-EIA), EPA-821-R-23-007. September 2023. Table IH, Note 22.
(iv) Method 1600.1: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-23-006, September 2023. Table 1A, Note 24; Table IH, Note 23.
(v) Method 1603.1: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008, September 2023. Table IA, Note 21; Table IH, Note 19.
(vi) Method 1604: Total Coliforms and Escherichia coli ( E. coli) in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium). September 2002. EPA-821-R-02-024. Table IH, Note 21.
(vii) Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016, Table IA, Notes 25, 26, and 27.
(viii) Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. December 2005. EPA-821-R-05-002. Table IH, Note 26.
(ix) Method 1623.1: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA 816-R-12-001. January 2012. U.S. EPA, Table IH, Notes 25 and 31.
(x) Method 1627, Kinetic Test Method for the Prediction of Mine Drainage Quality. December 2011. EPA-821-R-09-002. Table IB, Note 69.
(xi) Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Nonpolar Material) by Extraction and Gravimetry. Revision A, February 1999. EPA-821-R-98-002. Table IB, Notes 38 and 42.
(xii) Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Nonpolar Material) by Extraction and Gravimetry, Revision B, February 2010. EPA-821-R-10-001. Table IB, Notes 38 and 42.
(xiii) Method 1669, Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels. July 1996. Table IB, Note 43.
(xiv) Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation using Lauryl Tryptose Broth (LTB) and EC Medium. September 2014. EPA-821-R-14-009.Table IA, Note 15.
(xv) Method 1681: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation using A-1 Medium. July 2006. EPA 821-R-06-013. Table IA, Note 20.
(xvi) Method 1682: Salmonella in Sewage Sludge (Biosolids) by Modified Semisolid Rappaport-Vassiliadis (MSRV) Medium. September 2014. EPA 821-R-14-012. Table IA, Note 23.
(9) American National Standards Institute, 1430 Broadway, New York NY 10018.
(i) ANSI. American National Standard on Photographic Processing Effluents. April 2, 1975. Table IB, Note 9.
(ii) [Reserved]
(10) American Public Health Association, 800 I Street, NW, Washington, DC 20001; phone: (202)777-2742, website: www.standardmethods.org.
(i) Standard Methods for the Examination of Water and Wastewater. 14th Edition, 1975. Table IB, Notes 27 and 86.
(ii) Standard Methods for the Examination of Water and Wastewater. 15th Edition, 1980, Table IB, Note 30; Table ID.
(iii) Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. Table IC, Note 6; Table ID, Note 6.
(iv) Standard Methods for the Examination of Water and Wastewater. 18th Edition, 1992. Tables IA, IB, IC, ID, IE, and IH.
(v) Standard Methods for the Examination of Water and Wastewater. 19th Edition, 1995. Tables IA, IB, IC, ID, IE, and IH.
(vi) Standard Methods for the Examination of Water and Wastewater. 20th Edition, 1998. Tables IA, IB, IC, ID, IE, and IH.
(vii) Standard Methods for the Examination of Water and Wastewater. 21st Edition, 2005. Table IB, Notes 17 and 27.
(viii) 2120, Color. Revised September 4, 2021. Table IB.
(ix) 2130, Turbidity. Revised 2020. Table IB.
(x) 2310, Acidity. Revised 2020. Table IB.
(xi) 2320, Alkalinity. Revised 2021. Table IB.
(xii) 2340, Hardness. Revised 2021. Table IB.
(xiii) 2510, Conductivity. Revised 2021. Table IB.
(xiv) 2540, Solids. Revised 2020. Table IB.
(xv) 2550, Temperature. 2010. Table IB.
(xvi) 3111, Metals by Flame Atomic Absorption Spectrometry. Revised 2019. Table IB.
(xvii) 3112, Metals by Cold-Vapor Atomic Absorption Spectrometry. Revised 2020. Table IB.
(xviii) 3113, Metals by Electrothermal Atomic Absorption Spectrometry. Revised 2020. Table IB.
(xix) 3114, Arsenic and Selenium by Hydride Generation/Atomic Absorption Spectrometry. Revised 2020, Table IB.
(xx) 3120, Metals by Plasma Emission Spectroscopy. Revised 2020. Table IB.
(xxi) 3125, Metals by Inductively Coupled Plasma-Mass Spectrometry. Revised 2020. Table IB.
(xxii) 3500-Al, Aluminum. Revised 2020. Table IB.
(xxiii) 3500-As, Arsenic. Revised 2020. Table IB.
(xxiv) 3500-Ca, Calcium. Revised 2020. Table IB.
(xxv) 3500-Cr, Chromium. Revised 2020. Table IB.
(xxvi) 3500-Cu, Copper. Revised 2020. Table IB.
(xxvii) 3500-Fe, Iron. 2011. Table IB.
(xxviii) 3500-Pb, Lead. Revised 2020. Table IB.
(xxix) 3500-Mn, Manganese. Revised 2020. Table IB.
(xxx) 3500-K, Potassium. Revised 2020. Table IB.
(xxxi) 3500-Na, Sodium. Revised 2020. Table IB.
(xxxii) 3500-V, Vanadium. 2011. Table IB.
(xxxiii) 3500-Zn, Zinc. Revised 2020. Table IB.
(xxxiv) 4110, Determination of Anions by Ion Chromatography. Revised 2020. Table IB.
(xxxv) 4140, Inorganic Anions by Capillary Ion Electrophoresis. Revised 2020. Table IB.
(xxxvi) 4500-B, Boron. 2011. Table IB.
(xxxvii) 4500 Cl − , Chloride. Revised 2021. Table IB.
(xxxviii) 4500-Cl, Chlorine (Residual). 2011. Table IB.
(xxxix) 4500-CN − , Cyanide. Revised 2021. Table IB.
(xl) 4500-F − , Fluoride. Revised 2021. Table IB.
(xli) 4500-H + , pH. 2021. Table IB.
(xlii) 4500-NH 3 , Nitrogen (Ammonia). Revised 2021. Table IB.
(xliii) 4500-NO 2− , Nitrogen (Nitrite). Revised 2021. Table IB.
(xliv) 4500-NO 3− , Nitrogen (Nitrate). Revised 2019. Table IB.
(xlv) 4500-N (org) , Nitrogen (Organic). Revised 2021. Table IB.
(xlvi) 4500-O, Oxygen (Dissolved). Revised 2021. Table IB.
(xlvii) 4500-P, Phosphorus. Revised 2021. Table IB.
(xlviii) 4500-SiO 2 , Silica. Revised 2021. Table IB.
(xlix) 4500-S 2− , Sulfide. Revised 2021. Table IB.
(l) 4500-SO 32− , Sulfite. Revised 2021. Table IB.
(li) 4500-SO 42− , Sulfate. Revised 2021. Table IB.
(lii) 5210, Biochemical Oxygen Demand (BOD). Revised 2016. Table IB.
(liii) 5220, Chemical Oxygen Demand (COD). 2011. Table IB.
(liv) 5310, Total Organic Carbon (TOC). Revised 2014. Table IB.
(lv) 5520, Oil and Grease. Revised 2021. Table IB.
(lvi) 5530, Phenols. Revised 2021. Table IB.
(lvii) 5540, Surfactants. Revised 2021. Table IB.
(lviii) 6200, Volatile Organic Compounds. Revised 2020. Table IC.
(lix) 6410, Extractable Base/Neutrals and Acids. Revised 2020. Tables IC and ID.
(lx) 6420, Phenols. Revised 2021. Table IC.
(lxi) 6440, Polynuclear Aromatic Hydrocarbons. Revised 2021. Table IC.
(lxii) 6630, Organochlorine Pesticides. Revised 2021. Table ID.
(lxiii) 6640, Acidic Herbicide Compounds. Revised 2021. Table ID.
(lxiv) 7110, Gross Alpha and Gross Beta Radioactivity (Total, Suspended, and Dissolved). 2000. Table IE.
(lxv) 7500, Radium. 2001. Table IE.
(lxvi) 9213, Recreational Waters. 2007. Table IH.
(lxvii) 9221, Multiple-Tube Fermentation Technique for Members of the Coliform Group. Approved 2014. Table IA, Notes 12, 14; and 33; Table IH, Notes 10, 12, and 32.
(lxviii) 9222, Membrane Filter Technique for Members of the Coliform Group. 2015. Table IA, Note 31; Table IH, Note 17.
(lxix) 9223 Enzyme Substrate Coliform Test. 2016. Table IA; Table IH.
(lxx) 9230 Fecal Enterococcus/Streptococcus Groups. 2013. Table IA, Note 32; Table IH.
(11) The Analyst, The Royal Society of Chemistry, RSC Publishing, Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, United Kingdom. (Also available from most public libraries.)
(i) Spectrophotometric Determination of Ammonia: A Study of a Modified Berthelot Reaction Using Salicylate and Dichloroisocyanurate. Krom, M.D. 105:305-316, April 1980. Table IB, Note 60.
(ii) [Reserved]
(12) Analytical Chemistry, ACS Publications, 1155 Sixteenth St. NW., Washington DC 20036. (Also available from most public libraries.)
(i) Spectrophotometric and Kinetics Investigation of the Berthelot Reaction for the Determination of Ammonia. Patton, C.J. and S.R. Crouch. 49(3):464-469, March 1977. Table IB, Note 60.
(ii) [Reserved]
(13) AOAC International, 481 North Frederick Avenue, Suite 500, Gaithersburg, MD 20877-2417.
(i) Official Methods of Analysis of AOAC International. 16th Edition, 4th Revision, 1998.
(A) 920.203, Manganese in Water, Persulfate Method. Table IB, Note 3.
(B) 925.54, Sulfate in Water, Gravimetric Method. Table IB, Note 3.
(C) 973.40, Specific Conductance of Water. Table IB, Note 3.
(D) 973.41, pH of Water. Table IB, Note 3.
(E) 973.43, Alkalinity of Water, Titrimetric Method. Table IB, Note 3.
(F) 973.44, Biochemical Oxygen Demand (BOD) of Water, Incubation Method. Table IB, Note 3.
(G) 973.45, Oxygen (Dissolved) in Water, Titrimetric Methods. Table IB, Note 3.
(H) 973.46, Chemical Oxygen Demand (COD) of Water, Titrimetric Methods. Table IB, Note 3.
(I) 973.47, Organic Carbon in Water, Infrared Analyzer Method. Table IB, Note 3.
(J) 973.48, Nitrogen (Total) in Water, Kjeldahl Method. Table IB, Note 3.
(K) 973.49, Nitrogen (Ammonia) in Water, Colorimetric Method. Table IB, Note 3.
(L) 973.50, Nitrogen (Nitrate) in Water, Brucine Colorimetric Method. Table IB, Note 3.
(M) 973.51, Chloride in Water, Mercuric Nitrate Method. Table IB, Note 3.
(N) 973.52, Hardness of Water. Table IB, Note 3.
(O) 973.53, Potassium in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(P) 973.54, Sodium in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(Q) 973.55, Phosphorus in Water, Photometric Method. Table IB, Note 3.
(R) 973.56, Phosphorus in Water, Automated Method. Table IB, Note 3.
(S) 974.27, Cadmium, Chromium, Copper, Iron, Lead, Magnesium, Manganese, Silver, Zinc in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(T) 977.22, Mercury in Water, Flameless Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(U) 991.15. Total Coliforms and Escherichia coli in Water Defined Substrate Technology (Colilert) Method. Table IA, Note 10; Table IH, Note 10.
(V) 993.14, Trace Elements in Waters and Wastewaters, Inductively Coupled Plasma-Mass Spectrometric Method. Table IB, Note 3.
(W) 993.23, Dissolved Hexavalent Chromium in Drinking Water, Ground Water, and Industrial Wastewater Effluents, Ion Chromatographic Method. Table IB, Note 3.
(X) 993.30, Inorganic Anions in Water, Ion Chromatographic Method. Table IB, Note 3.
(ii) [Reserved]
(14) Applied and Environmental Microbiology, American Society for Microbiology, 1752 N Street NW., Washington DC 20036. (Also available from most public libraries.)
(i) New Medium for the Simultaneous Detection of Total Coliforms and Escherichia coli in Water. Brenner, K.P., C.C. Rankin, Y.R. Roybal, G.N. Stelma, Jr., P.V. Scarpino, and A.P. Dufour. 59:3534-3544, November 1993. Table IH, Note 21.
(ii) [Reserved]
(15) ASTM International, 100 Barr Harbor Drive, P.O. Box C700, West Conshohocken, PA 19428-2959; phone: (877)909-2786; website: www.astm.org.
(i) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1994. Tables IA, IB, IC, ID, IE, and IH.
(ii) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1996. Tables IA, IB, IC, ID, IE, and IH.
(iii) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1999. Tables IA, IB, IC, ID, IE, and IH.
(iv) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 2000. Tables IA, IB, IC, ID, IE, and IH.
(v) ASTM D511-14, Standard Test Methods for Calcium and Magnesium in Water. Approved October 1, 2014. Table IB.
(vi) ASTM D512-12, Standard Test Methods for Chloride Ion in Water. Approved June 15, 2012. Table IB.
(vii) ASTM D515-88, Test Methods for Phosphorus in Water, March 1989. Table IB.
(viii) ASTM D516-16, Standard Test Method for Sulfate Ion in Water. Approved June 1, 2016. Table IB.
(ix) ASTM D858-17, Standard Test Methods for Manganese in Water. Approved June 1, 2017. Table IB.
(x) ASTM D859-16, Standard Test Method for Silica in Water. Approved June 15, 2016. Table IB.
(xi) ASTM D888-18, Standard Test Methods for Dissolved Oxygen in Water. Approved May 1, 2018. Table IB.
(xii) ASTM D1067-16, Standard Test Methods for Acidity or Alkalinity of Water. Approved June 15, 2016. Table IB.
(xiii) ASTM D1068-15, Standard Test Methods for Iron in Water. Approved October 1, 2015. Table IB.
(xiv) ASTM D1125-95 (Reapproved 1999), Standard Test Methods for Electrical Conductivity and Resistivity of Water. December 1995. Table IB.
(xv) ASTM D1126-17, Standard Test Method for Hardness in Water. Approved December 1, 2017. Table IB.
(xvi) ASTM D1179-16, Standard Test Methods for Fluoride Ion in Water. Approved June 15, 2016. Table IB.
(xvii) ASTM D1246-16, Standard Test Method for Bromide Ion in Water. June 15, 2016. Table IB.
(xviii) ASTM D1252-06 (Reapproved 2012), Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. Approved June 15, 2012. Table IB.
(xix) ASTM D1253-14, Standard Test Method for Residual Chlorine in Water. Approved January 15, 2014. Table IB.
(xx) ASTM D1293-18, Standard Test Methods for pH of Water. Approved January 15, 2018. Table IB.
(xxi) ASTM D1426-15, Standard Test Methods for Ammonia Nitrogen in Water. Approved March 15, 2015. Table IB.
(xxii) ASTM D1687-17, Standard Test Methods for Chromium in Water. Approved June 1, 2017. Table IB.
(xxiii) ASTM D1688-17, Standard Test Methods for Copper in Water. Approved June 1, 2017. Table IB.
(xxiv) ASTM D1691-17, Standard Test Methods for Zinc in Water. Approved June 1, 2017. Table IB.
(xxv) ASTM D1783-01 (Reapproved 2012), Standard Test Methods for Phenolic Compounds in Water. Approved June 15, 2012. Table IB.
(xxvi) ASTM D1886-14, Standard Test Methods for Nickel in Water. Approved October 1, 2014. Table IB.
(xxvii) ASTM D1889-00, Standard Test Method for Turbidity of Water. October 2000. Table IB.
(xxviii) ASTM D1890-96, Standard Test Method for Beta Particle Radioactivity of Water. April 1996. Table IE.
(xxix) ASTM D1943-96, Standard Test Method for Alpha Particle Radioactivity of Water. April 1996. Table IE.
(xxx) ASTM D1976-20, Standard Test Method for Elements in Water by Inductively-Coupled Argon Plasma Atomic Emission Spectroscopy. Approved May 1, 2020. Table IB.
(xxxi) ASTM D2036-09 (Reapproved 2015), Standard Test Methods for Cyanides in Water. Approved July 15, 2015. Table IB.
(xxxii) ASTM D2330-20, Standard Test Method for Methylene Blue Active Substances. Approved January 1, 2020. Table 1B.
(xxxiii) ASTM D2460-97, Standard Test Method for Alpha-Particle-Emitting Isotopes of Radium in Water. October 1997. Table IE.
(xxxiv) ASTM D2972-15, Standard Tests Method for Arsenic in Water. Approved February 1, 2015. Table IB.
(xxxv) ASTM D3223-17, Standard Test Method for Total Mercury in Water. Approved June 1, 2017. Table IB.
(xxxvi) ASTM D3371-95, Standard Test Method for Nitriles in Aqueous Solution by Gas-Liquid Chromatography, February 1996. Table IF.
(xxxvii) ASTM D3373-17, Standard Test Method for Vanadium in Water. Approved June 1, 2017. Table IB.
(xxxviii) ASTM D3454-97, Standard Test Method for Radium-226 in Water. February 1998. Table IE.
(xxxix) ASTM D3557-17, Standard Test Method for Cadmium in Water. Approved June 1, 2017. Table IB.
(xl) ASTM D3558-15, Standard Test Method for Cobalt in Water. Approved February 1, 2015. Table IB.
(xli) ASTM D3559-15, Standard Test Methods for Lead in Water. Approved June 1, 2015. Table IB.
(xlii) ASTM D3590-17, Standard Test Methods for Total Kjeldahl Nitrogen in Water. Approved June 1, 2017. Table IB.
(xliii) ASTM D3645-15, Standard Test Methods for Beryllium in Water. Approved February 1, 2015. Table IB.
(xliv) ASTM D3695-95, Standard Test Method for Volatile Alcohols in Water by Direct Aqueous-Injection Gas Chromatography. April 1995. Table IF.
(xlv) ASTM D3859-15, Standard Test Methods for Selenium in Water. Approved March 15, 2015. Table IB.
(xlvi) ASTM D3867-16, Standard Test Method for Nitrite-Nitrate in Water. Approved June 1, 2016. Table IB.
(xlvii) ASTM D4190-15, Standard Test Method for Elements in Water by Direct- Current Plasma Atomic Emission Spectroscopy. Approved February 1, 2015. Table IB.
(xlviii) ASTM D4282-15, Standard Test Method for Determination of Free Cyanide in Water and Wastewater by Microdiffusion. Approved July 15, 2015. Table IB.
(xlix) ASTM D4327-17, Standard Test Method for Anions in Water by Suppressed Ion Chromatography. Approved December 1, 2017. Table IB.
(l) ASTM D4382-18, Standard Test Method for Barium in Water, Atomic Absorption Spectrophotometry, Graphite Furnace. Approved February 1, 2018. Table IB.
(li) ASTM D4657-92 (Reapproved 1998), Standard Test Method for Polynuclear Aromatic Hydrocarbons in Water. January 1993. Table IC.
(lii) ASTM D4658-15, Standard Test Method for Sulfide Ion in Water. Approved March 15, 2015. Table IB.
(liii) ASTM D4763-88 (Reapproved 2001), Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy. September 1988. Table IF.
(liv) ASTM D4839-03 (Reapproved 2017), Standard Test Method for Total Carbon and Organic Carbon in Water by Ultraviolet, or Persulfate Oxidation, or Both, and Infrared Detection. Approved December 15, 2017. Table IB.
(lv) ASTM D5257-17, Standard Test Method for Dissolved Hexavalent Chromium in Water by Ion Chromatography. Approved December 1, 2017. Table IB.
(lvi) ASTM D5259-92, Standard Test Method for Isolation and Enumeration of Enterococci from Water by the Membrane Filter Procedure. October 1992. Table IH, Note 9.
(lvii) ASTM D5392-93, Standard Test Method for Isolation and Enumeration of Escherichia coli in Water by the Two-Step Membrane Filter Procedure. September 1993. Table IH, Note 9.
(lviii) ASTM D5673-16, Standard Test Method for Elements in Water by Inductively Coupled Plasma—Mass Spectrometry. Approved February 1, 2016. Table IB.
(lix) ASTM D5907-18, Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water. Approved May 1, 2018. Table IB.
(lx) ASTM D6503-99, Standard Test Method for Enterococci in Water Using Enterolert. April 2000. Table IA Note 9, Table IH, Note 9.
(lxi) ASTM. D6508-15, Standard Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Approved October 1, 2015. Table IB, Note 54.
(lxii) ASTM. D6888-16, Standard Test Method for Available Cyanides with Ligand Displacement and Flow Injection Analysis (FIA) Utilizing Gas Diffusion Separation and Amperometric Detection. Approved February 1, 2016. Table IB, Note 59.
(lxiii) ASTM. D6919-17, Standard Test Method for Determination of Dissolved Alkali and Alkaline Earth Cations and Ammonium in Water and Wastewater by Ion Chromatography. Approved June 1, 2017. Table IB.
(lxiv) ASTM. D7065-17, Standard Test Method for Determination of Nonylphenol, Bisphenol A, p-tert -Octylphenol, Nonylphenol Monoethoxylate and Nonylphenol Diethoxylate in Environmental Waters by Gas Chromatography Mass Spectrometry. Approved December 15, 2017. Table IC.
(lxv) ASTM D7237-18, Standard Test Method for Free Cyanide with Flow Injection Analysis (FIA) Utilizing Gas Diffusion Separation and Amperometric Detection. Approved December 1, 2018. Table IB.
(lxvi) ASTM D7284-20, Standard Test Method for Total Cyanide in Water by Micro Distillation followed by Flow Injection Analysis with Gas Diffusion Separation and Amperometric Detection. Approved August 1, 2020. Table IB.
(lxvii) ASTM D7365-09a (Reapproved 2015), Standard Practice for Sampling, Preservation and Mitigating Interferences in Water Samples for Analysis of Cyanide. Approved July 15, 2015. Table II, Notes 5 and 6.
(lxviii) ASTM. D7511-12 (Reapproved 2017) e1 , Standard Test Method for Total Cyanide by Segmented Flow Injection Analysis, In-Line Ultraviolet Digestion and Amperometric Detection. Approved July 1, 2017. Table IB.
(lxix) ASTM D7573-18a e1 , Standard Test Method for Total Carbon and Organic Carbon in Water by High Temperature Catalytic Combustion and Infrared Detection. Approved December 15, 2018. Table IB.
(lxx) ASTM D7781-14, Standard Test Method for Nitrite-Nitrate in Water by Nitrate Reductase, Approved April 1, 2014. Table IB.
(16) Bran & Luebbe Analyzing Technologies, Inc., Elmsford NY 10523.
(i) Industrial Method Number 378-75WA, Hydrogen Ion (pH) Automated Electrode Method, Bran & Luebbe (Technicon) Auto Analyzer II. October 1976. Table IB, Note 21.
(ii) [Reserved]
(17) CEM Corporation, P.O. Box 200, Matthews NC 28106-0200.
(i) Closed Vessel Microwave Digestion of Wastewater Samples for Determination of Metals. April 16, 1992. Table IB, Note 36.
(ii) [Reserved]
(18) Craig R. Chinchilla, 900 Jorie Blvd., Suite 35, Oak Brook IL 60523. Telephone: 630-645-0600.
(i) Nitrate by Discrete Analysis Easy (1-Reagent) Nitrate Method, (Colorimetric, Automated, 1 Reagent). Revision 1, November 12, 2011. Table IB, Note 62.
(ii) [Reserved]
(19) FIAlab Instruments, Inc., 334 2151 N. Northlake Way, Seattle, WA 98103; phone: (425)376-0450; website: www.flowinjection.com/app-notes/epafialab100.
(i) FIAlab 100, Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Fluorescence Detector Analysis, April 4, 2018. Table IB, Note 82.
(ii) [Reserved]
(20) Hach Company, P.O. Box 389, Loveland CO 80537.
(i) Method 8000, Chemical Oxygen Demand. Hach Handbook of Water Analysis. 1979. Table IB, Note 14.
(ii) Method 8008, 1,10-Phenanthroline Method using FerroVer Iron Reagent for Water. 1980. Table IB, Note 22.
(iii) Method 8009, Zincon Method for Zinc. Hach Handbook for Water Analysis. 1979. Table IB, Note 33.
(iv) Method 8034, Periodate Oxidation Method for Manganese. Hach Handbook for Water Analysis. 1979. Table IB, Note 23.
(v) Method 8506, Bicinchoninate Method for Copper. Hach Handbook of Water Analysis. 1979. Table IB, Note 19.
(vi) Method 8507, Nitrogen, Nitrite-Low Range, Diazotization Method for Water and Wastewater. 1979. Table IB, Note 25.
(vii) Method 10206, Hach Company TNTplus 835/836 Nitrate Method 10206, Spectrophotometric Measurement of Nitrate in Water and Wastewater. Revision 2.1, January 10, 2013. Table IB, Note 75.
(viii) Method 10242, Hach Company TNTplus 880 Total Kjeldahl Nitrogen Method 10242, Simplified Spectrophotometric Measurement of Total Kjeldahl Nitrogen in Water and Wastewater. Revision 1.1, January 10, 2013. Table IB, Note 76.
(ix) Hach Method 10360, Luminescence Measurement of Dissolved Oxygen in Water and Wastewater and for Use in the Determination of BOD5and cBOD5. Revision 1.2, October 2011. Table IB, Note 63.
(x) m-ColiBlue24® Method, for total Coliforms and E. coli. Revision 2, 1999. Table IA, Note 18; Table IH, Note 17.
(21) IDEXX Laboratories Inc., One Idexx Drive, Westbrook ME 04092.
(i) Colilert. 2013. Table IA, Notes 17 and 18; Table IH, Notes 14, 15 and 16.
(ii) Colilert-18. 2013. Table IA, Notes 17 and 18; Table IH, Notes 14, 15 and 16.
(iii) Enterolert. 2013. Table IA, Note 24; Table IH, Note 12.
(iv) Quanti-Tray Insert and Most Probable Number (MPN) Table. 2013. Table IA, Note 18; Table IH, Notes 14 and 16.
(22) In-Situ Incorporated, 221 E. Lincoln Ave., Ft. Collins CO 80524. Telephone: 970-498-1500.
(i) In-Situ Inc. Method 1002-8-2009, Dissolved Oxygen Measurement by Optical Probe. 2009. Table IB, Note 64.
(ii) In-Situ Inc. Method 1003-8-2009, Biochemical Oxygen Demand (BOD) Measurement by Optical Probe. 2009. Table IB, Note 10.
(iii) In-Situ Inc. Method 1004-8-2009, Carbonaceous Biochemical Oxygen Demand (CBOD) Measurement by Optical Probe. 2009. Table IB, Note 35.
(23) Journal of Chromatography, Elsevier/North-Holland, Inc., Journal Information Centre, 52 Vanderbilt Avenue, New York NY 10164. (Also available from most public libraries.
(i) Direct Determination of Elemental Phosphorus by Gas-Liquid Chromatography. Addison, R.F. and R.G. Ackman. 47(3): 421-426, 1970. Table IB, Note 28.
(ii) [Reserved]
(24) Lachat Instruments, 6645 W. Mill Road, Milwaukee WI 53218, Telephone: 414-358-4200.
(i) QuikChem Method 10-204-00-1-X, Digestion and Distillation of Total Cyanide in Drinking and Wastewaters using MICRO DIST and Determination of Cyanide by Flow Injection Analysis. Revision 2.2, March 2005. Table IB, Note 56.
(ii) [Reserved]
(25) Leck Mitchell, Ph.D., P.E., 656 Independence Valley Dr., Grand Junction CO 81507. Telephone: 970-244-8661.
(i) Mitchell Method M5271, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Table IB, Note 66.
(ii) Mitchell Method M5331, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Table IB, Note 65.
(26) MACHEREY-NAGEL GmbH and Co., 2850 Emrick Blvd., Bethlehem, PA 18020; Phone: (888)321-6224.
(i) Method 036/038 NANOCOLOR® COD LR/HR, Spectrophotometric Measurement of Chemical Oxygen Demand in Water and Wastewater, Revision 1.5, May 2018. Table IB, Note 83.
(ii) [Reserved]
(27) Micrology Laboratories, LLC (now known as Roth Bioscience, LLC), 1303 Eisenhower Drive, Goshen, IN 46526; phone: (574)533-3351.
(i) KwikCount TM EC Medium E. coli enzyme substrate test, Rapid Detection of E. coli in Beach Water By KwikCount TM EC Membrane Filtration. 2014. Table IH, Notes 28 and 29.
(ii) [Reserved]
(28) National Council of the Paper Industry for Air and Stream Improvements, Inc. (NCASI), 260 Madison Avenue, New York NY 10016.
(i) NCASI Method TNTP-W10900, Total Nitrogen and Total Phophorus in Pulp and Paper Biologically Treated Effluent by Alkaline Persulfate Digestion. June 2011. Table IB, Note 77.
(ii) NCASI Technical Bulletin No. 253, An Investigation of Improved Procedures for Measurement of Mill Effluent and Receiving Water Color. December 1971. Table IB, Note 18.
(iii) NCASI Technical Bulletin No. 803, An Update of Procedures for the Measurement of Color in Pulp Mill Wastewaters. May 2000. Table IB, Note 18.
(29) The Nitrate Elimination Co., Inc. (NECi), 334 Hecla St., Lake Linden NI 49945.
(i) NECi Method N07-0003, Method for Nitrate Reductase Nitrate-Nitrogen Analysis. Revision 9.0. March 2014. Table IB, Note 73.
(ii) [Reserved]
(30) Oceanography International Corporation, 512 West Loop, P.O. Box 2980, College Station TX 77840.
(i) OIC Chemical Oxygen Demand Method. 1978. Table IB, Note 13.
(ii) [Reserved]
(31) OI Analytical, Box 9010, College Station TX 77820-9010.
(i) Method OIA-1677-09, Available Cyanide by Ligand Exchange and Flow Injection Analysis (FIA). Copyright 2010. Table IB, Note 59.
(ii) Method PAI-DK01, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Titrimetric Detection. Revised December 22, 1994. Table IB, Note 39.
(iii) Method PAI-DK02, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Colorimetric Detection. Revised December 22, 1994. Table IB, Note 40.
(iv) Method PAI-DK03, Nitrogen, Total Kjeldahl, Block Digestion, Automated FIA Gas Diffusion. Revised December 22, 1994. Table IB, Note 41.
(32) ORION Research Corporation, 840 Memorial Drive, Cambridge, Massachusetts 02138.
(i) ORION Research Instruction Manual, Residual Chlorine Electrode Model 97-70. 1977. Table IB, Note 16.
(ii) [Reserved]
(33) Pace Analytical Services, LLC, 1800 Elm Street, SE, Minneapolis, MN 55414; phone: (612)656-2240.
(i) PAM-16130-SSI, Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Shimadzu Gas Chromatography Mass Spectrometry (GC-MS/MS), Revision 1.1, May 20, 2022. Table IC, Note 17.
(ii) [Reserved]
(34) SGS AXYS Analytical Services, Ltd., 2045 Mills Road, Sidney, British Columbia, Canada, V8L 5X2; phone: (888)373-0881.
(i) SGS AXYS Method 16130, Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Waters and Agilent Gas Chromatography-Mass Spectrometry (GC/MS/MS)., Revision 1.0, revised August 2020. Table IC, Note 16.
(ii) [Reserved]
(35) Technicon Industrial Systems, Tarrytown NY 10591.
(i) Industrial Method Number 379-75WE Ammonia, Automated Electrode Method, Technicon Auto Analyzer II. February 19, 1976. Table IB, Note 7.
(ii) [Reserved]
(36) Thermo Jarrell Ash Corporation, 27 Forge Parkway, Franklin MA 02038.
(i) Method AES0029. Direct Current Plasma (DCP) Optical Emission Spectrometric Method for Trace Elemental Analysis of Water and Wastes. 1986, Revised 1991. Table IB, Note 34.
(ii) [Reserved]
(37) Thermo Scientific, 166 Cummings Center, Beverly MA 01915. Telephone: 1-800-225-1480. www.thermoscientific.com.
(i) Thermo Scientific Orion Method AQ4500, Determination of Turbidity by Nephelometry. Revision 5, March 12, 2009. Table IB, Note 67.
(ii) [Reserved]
(38) 3M Corporation, 3M Center Building 220-9E-10, St. Paul MN 55144-1000.
(i) Organochlorine Pesticides and PCBs in Wastewater Using EmporeTMDisk” Test Method 3M 0222. Revised October 28, 1994. Table IC, Note 8; Table ID, Note 8.
(ii) [Reserved]
(39) Timberline Instruments, LLC, 1880 South Flatiron Ct., Unit I, Boulder CO 80301.
(i) Timberline Amonia-001, Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Conductivity Cell Analysis. June 24, 2011. Table IB, Note 74.
(ii) [Reserved]
(40) U.S. Geological Survey (USGS), U.S. Department of the Interior, Reston, Virginia. Available from USGS Books and Open-File Reports (OFR) Section, Federal Center, Box 25425, Denver, CO 80225; phone: (703)648-5953; website: ww.usgs.gov.
(i) Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods. U.S. Geological Survey Techniques and Methods, Book 5—Laboratory Analysis, Section B—Methods of the National Water Quality Laboratory, Chapter 8. 2011. Table IB, Note 72.
(ii) Techniques and Methods—Book 5, Laboratory Analysis—Section B, Methods of the National Water Quality Laboratory—Chapter 12, Determination of Heat Purgeable and Ambient Purgeable Volatile Organic Compounds in Water by Gas Chromatography/Mass Spectrometry 2016.
(iii) Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, editors, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1979. Table IB, Note 8.
(iv) Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1989. Table IB, Notes 2 and 79.
(v) Methods for the Determination of Organic Substances in Water and Fluvial Sediments. Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3. 1987. Table IB, Note 24; Table ID, Note 4.
(vi) OFR 76-177, Selected Methods of the U.S. Geological Survey of Analysis of Wastewaters. 1976. Table IE, Note 2.
(vii) OFR 91-519, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organonitrogen Herbicides in Water by Solid-Phase Extraction and Capillary-Column Gas Chromatography/Mass Spectrometry With Selected-Ion Monitoring. 1992. Table ID, Note 14.
(viii) OFR 92-146, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Total Phosphorus by a Kjeldahl Digestion Method and an Automated Colorimetric Finish That Includes Dialysis. 1992. Table IB, Note 48.
(ix) OFR 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. Table IB, Notes 51 and 80; Table IC, Note 9.
(x) OFR 93-449, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Chromium in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1993. Table IB, Note 46.
(xi) OFR 94-37, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Triazine and Other Nitrogen-containing Compounds by Gas Chromatography with Nitrogen Phosphorus Detectors. 1994. Table ID, Note 9.
(xii) OFR 95-181, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by C-18 Solid-Phase Extraction and Capillary-Column Gas Chromatography/Mass Spectrometry With Selected-Ion Monitoring. 1995. Table ID, Note 11.
(xiii) OFR 97-198, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Molybdenum in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1997. Table IB, Note 47.
(xiv) OFR 97-829, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of 86 Volatile Organic Compounds in Water by Gas Chromatography/Mass Spectrometry, Including Detections Less Than Reporting Limits. 1998. Table IC, Note 13.
(xv) OFR 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Elements in Whole-Water Digests Using Inductively Coupled Plasma-Optical Emission Spectrometry and Inductively Coupled Plasma-Mass Spectrometry. 1998. Table IB, Notes 50 and 81.
(xvi) OFR 98-639, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Arsenic and Selenium in Water and Sediment by Graphite Furnace—Atomic Absorption Spectrometry. 1999. Table IB, Note 49.
(xvii) OFR 00-170, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Ammonium Plus Organic Nitrogen by a Kjeldahl Digestion Method and an Automated Photometric Finish that Includes Digest Cleanup by Gas Diffusion. 2000. Table IB, Note 45.
(xviii) Techniques and Methods Book 5-B1, Determination of Elements in Natural-Water, Biota, Sediment and Soil Samples Using Collision/Reaction Cell Inductively Coupled Plasma-Mass Spectrometry. Chapter 1, Section B, Methods of the National Water Quality Laboratory, Book 5, Laboratory Analysis. 2006. Table IB, Note 70.
(xix) U.S. Geological Survey Techniques of Water-Resources Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. Table IA, Note 4; Table IH, Note 4.
(xx) Water-Resources Investigation Report 01-4098, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Moderate-Use Pesticides and Selected Degradates in Water by C-18 Solid-Phase Extraction and Gas Chromatography/Mass Spectrometry. 2001. Table ID, Note 13.
(xxi) Water-Resources Investigations Report 01-4132, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organic Plus Inorganic Mercury in Filtered and Unfiltered Natural Water With Cold Vapor-Atomic Fluorescence Spectrometry. 2001. Table IB, Note 71.
(xxii) Water-Resources Investigation Report 01-4134, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by Graphitized Carbon-Based Solid-Phase Extraction and High-Performance Liquid Chromatography/Mass Spectrometry. 2001. Table ID, Note 12.
(xxiii) Water Temperature—Influential Factors, Field Measurement and Data Presentation, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 1, Chapter D1. 1975. Table IB, Note 32.
(41) Waters Corporation, 34 Maple Street, Milford MA 01757, Telephone: 508-482-2131, Fax: 508-482-3625. (i) Method D6508, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Revision 2, December 2000. Table IB, Note 54. (ii) [Reserved]
(i) Method D6508, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Revision 2, December 2000. Table IB, Note 54.
(ii) [Reserved]
* * * *
(e)
* * * *
Table II—Required Containers, Preservation Techniques, and Holding Times
* * * *
5 ASTM D7365-09a (15) specifies treatment options for samples containing oxidants (e.g ., chlorine) for cyanide analyses. Also, Section 9060A of Standard Methods for the Examination of Water and Wastewater (23rd edition) addresses dechlorination procedures for microbiological analyses.
The U.S. Environmental Protection Agency (EPA) is finalizing changes to its test procedures required to be used by industries and municipalities when analyzing the chemical, physical, and biological properties of wastewater and other samples for reporting under the EPA's National Pollutant Discharge Elimination System permit program. The Clean Water Act requires the EPA to promulgate these test procedures (analytical methods) for analysis of pollutants. The EPA anticipates that these changes will provide increased flexibility for the regulated community in meeting monitoring requirements while improving data quality. In addition, this update to the CWA methods will incorporate technological advances in analytical technology and make a series of minor changes and corrections to existing approved methods. As such, the EPA expects that these changes will not result in any negative economic impacts.
DATES: This final rule is effective on June 17, 2024, published in the Federal Register April 16, 2024, page 27288.
View final rule.
| §136.3 Identification of test procedures. | ||
| (a), tables IA, IB, IC, ID, and IH | Revised | View text |
| (b) | Revised | View text |
| (e), table II, Footnote “5” | Revised | View text |
New Text
§136.3 Identification of test procedures.
(a)
* * * *
| Parameter and units | Method 1 | EPA | Standard methods | AOAC, ASTM, USGS | Other |
|---|---|---|---|---|---|
| Table IA notes: | |||||
| 1 The method must be specified when results are reported. | |||||
| 2 A 0.45-µm membrane filter (MF) or other pore size certified by the manufacturer to fully retain organisms to be cultivated and to be free of extractables which could interfere with their growth. | |||||
| 3 Microbiological Methods for Monitoring the Environment, Water and Wastes, EPA/600/8-78/017. 1978. US EPA. | |||||
| 4 U.S. Geological Survey Techniques of Water-Resource Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. USGS. | |||||
| 5 Because the MF technique usually yields low and variable recovery from chlorinated wastewaters, the Most Probable Number method will be required to resolve any controversies. | |||||
| 6 Tests must be conducted to provide organism enumeration (density). Select the appropriate configuration of tubes/filtrations and dilutions/volumes to account for the quality, character, consistency, and anticipated organism density of the water sample. | |||||
| 7 When the MF method has been used previously to test waters with high turbidity, large numbers of noncoliform bacteria, or samples that may contain organisms stressed by chlorine, a parallel test should be conducted with a multiple-tube technique to demonstrate applicability and comparability of results. | |||||
| 8 To assess the comparability of results obtained with individual methods, it is suggested that side-by-side tests be conducted across seasons of the year with the water samples routinely tested in accordance with the most current Standard Methods for the Examination of Water and Wastewater or EPA alternate test procedure (ATP) guidelines. | |||||
| 9 Annual Book of ASTM Standards—Water and Environmental Technology, Section 11.02. 2000, 1999, 1996. ASTM International. | |||||
| 10 Official Methods of Analysis of AOAC International. 16th Edition, 4th Revision, 1998. AOAC International. | |||||
| 11 Recommended for enumeration of target organism in sewage sludge. | |||||
| 12 The multiple-tube fermentation test is used in 9221B.2-2014. Lactose broth may be used in lieu of lauryl tryptose broth (LTB), if at least 25 parallel tests are conducted between this broth and LTB using the water samples normally tested, and this comparison demonstrates that the false-positive rate and false-negative rate for total coliform using lactose broth is less than 10 percent. No requirement exists to run the completed phase on 10 percent of all total coliform-positive tubes on a seasonal basis. | |||||
| 13 These tests are collectively known as defined enzyme substrate tests. | |||||
| 14 After prior enrichment in a presumptive medium for total coliform using 9221B.2-2014, all presumptive tubes or bottles showing any amount of gas, growth or acidity within 48 h ± 3 h of incubation shall be submitted to 9221F-2014. Commercially available EC-MUG media or EC media supplemented in the laboratory with 50 µg/mL of MUG may be used. | |||||
| 15 Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation Using Lauryl-Tryptose Broth (LTB) and EC Medium, EPA-821-R-14-009. September 2014. U.S. EPA. | |||||
| 16 Samples shall be enumerated by the multiple-tube or multiple-well procedure. Using multiple-tube procedures, employ an appropriate tube and dilution configuration of the sample as needed and report the Most Probable Number (MPN). Samples tested with Colilert® may be enumerated with the multiple-well procedures, Quanti-Tray® or Quanti-Tray®/2000 and the MPN calculated from the table provided by the manufacturer. | |||||
| 17 Colilert-18® is an optimized formulation of the Colilert® for the determination of total coliforms and E. coli that provides results within 18 h of incubation at 35°C rather than the 24 h required for the Colilert® test and is recommended for marine water samples. | |||||
| 18 Descriptions of the Colilert®, Colilert-18®, Quanti-Tray®, and Quanti-Tray®/2000 may be obtained from IDEXX Laboratories, Inc. | |||||
| 19 A description of the mColiBlue24® test is available from Hach Company. | |||||
| 20 Method 1681: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation Using A-1 Medium, EPA-821-R-06-013. July 2006. U.S. EPA. | |||||
| 21 Method 1603.1: Escherichia coli ( E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008. September 2023. U.S. EPA. | |||||
| 22 Method 1682: Salmonella in Sewage Sludge (Biosolids) by Modified Semisolid Rappaport-Vassiliadis (MSRV) Medium, EPA-821-R-14-012. September 2014. U.S. EPA. | |||||
| 23 A description of the Enterolert® test may be obtained from IDEXX Laboratories Inc. | |||||
| 24 Method 1600.1: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-23-006. September 2023. U.S. EPA. | |||||
| 25 Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, EPA-821-R-02-012. Fifth Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 26 Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, EPA-821-R-02-013. Fourth Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 27 Short-term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, EPA-821-R-02-014. Third Edition, October 2002. U.S. EPA; and U.S. EPA Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016. | |||||
| 28 To use Colilert-18® to assay for fecal coliforms, the incubation temperature is 44.5 ± 0.2 °C, and a water bath incubator is used. | |||||
| 29 On a monthly basis, at least ten blue colonies from positive samples must be verified using Lauryl Tryptose Broth and EC broth, followed by count adjustment based on these results; and representative non-blue colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. | |||||
| 30 On a monthly basis, at least ten sheen colonies from positive samples must be verified using lauryl tryptose broth and brilliant green lactose bile broth, followed by count adjustment based on these results; and representative non-sheen colonies should be verified using lauryl tryptose broth. Where possible, verifications should be done from randomized sample sources. | |||||
| 31 Subject coliform positive samples determined by 9222 B-2015 or other membrane filter procedure to 9222 I-2015 using NA-MUG media. | |||||
| 32 Verification of colonies by incubation of BHI agar at 10 ± 0.5 °C for 48 ± 3 h is optional. As per the Errata to the 23rd Edition of Standard Methods for the Examination of Water and Wastewater “Growth on a BHI agar plate incubated at 10 ± 0.5 °C for 48 ± 3 h is further verification that the colony belongs to the genus Enterococcus.” | |||||
| 33 9221F. 2-2014 allows for simultaneous detection of E. coli and thermotolerant fecal coliforms by adding inverted vials to EC-MUG; the inverted vials collect gas produced by thermotolerant fecal coliforms. | |||||
| Bacteria | |||||
| 1. Coliform (fecal), number per gram dry weight | Most Probable Number (MPN), 5 tube, 3 dilution, or | p. 132, 3 1680, 1115 1681 1120 | 9221 E-2014. | ||
| Membrane filter (MF), 25 single step | p. 124 3 | 9222 D-2015. 29 | |||
| 2. Coliform (fecal), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 132 3 | 9221 E-2014, 9221 F-2014. 33 | ||
| Multiple tube/multiple well, or | Colilert-18®. 131828 | ||||
| MF, 25 single step 5 | p. 124 3 | 9222 D-2015 29 | B-0050-85. 4 | ||
| 3. Coliform (total), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 114 3 | 9221 B-2014. | ||
| MF, 25 single step or | p. 108 3 | 9222 B-2015 30 | B-0025-85. 4 | ||
| MF, 25 two step with enrichment | p. 111 3 | 9222 B-2015. 30 | |||
| 4. E. coli, number per 100 mL | MPN 6816 multiple tube, or | 9221 B2014/9221 F-2014. 121433 | |||
| multiple tube/multiple well, or | 9223 B-2016 13 | 991.15 10 | Colilert®. 1318 Colilert-18®. 131718 | ||
| MF, 25678 two step, or | 9222 B-2015/9222 I-2015. 31 | ||||
| Single step | 1603.1 21 | m-ColiBlue24®. 19 | |||
| 5. Fecal streptococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MF, 2 or | p. 136 3 | 9230 C-2013 32 | B-0055-85. 4 | ||
| Plate count | p. 143. 3 | ||||
| 6. Enterococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MPN, 68 multiple tube/multiple well, or | 9230 D-2013 | D6503-99 9 | Enterolert®. 1323 | ||
| MF 25678 single step or | 1600.1 24 | 9230 C-2013. 32 | |||
| Plate count | p. 143. 3 | ||||
| 7. Salmonella , number per gram dry weight 11 | MPN multiple tube | 1682. 22 | |||
| Aquatic Toxicity | |||||
| 8. Toxicity, acute, fresh water organisms, LC 50 , percent effluent | Water flea, Cladoceran, Ceriodaphnia dubia acute | 2002.0. 25 | |||
| Water flea, Cladocerans, Daphnia pulex and Daphnia magna acute | 2021.0. 25 | ||||
| Fish, Fathead minnow, Pimephales promelas, and Bannerfin shiner, Cyprinella leedsi, acute | 2000.0. 25 | ||||
| Fish, Rainbow trout, Oncorhynchus mykiss, and brook trout, Salvelinus fontinalis, acute | 2019.0. 25 | ||||
| 9. Toxicity, acute, estuarine and marine organisms of the Atlantic Ocean and Gulf of Mexico, LC 50 , percent effluent | Mysid, Mysidopsis bahia, acute | 2007.0. 25 | |||
| Fish, Sheepshead minnow, Cyprinodon variegatus, acute | 2004.0. 25 | ||||
| Fish, Silverside, Menidia beryllina, Menidia menidia, and Menidia peninsulae, acute | 2006.0. 25 | ||||
| 10. Toxicity, chronic, fresh water organisms, NOEC or IC 25 , percent effluent | Fish, Fathead minnow, Pimephales promelas, larval survival and growth | 1000.0. 26 | |||
| Fish, Fathead minnow, Pimephales promelas, embryo-larval survival and teratogenicity | 1001.0. 26 | ||||
| Water flea, Cladoceran, Ceriodaphnia dubia, survival and reproduction | 1002.0. 26 | ||||
| Green alga, Selenastrum capricornutum, growth | 1003.0. 26 | ||||
| 11. Toxicity, chronic, estuarine and marine organisms of the Atlantic Ocean and Gulf of Mexico, NOEC or IC 25 , percent effluent | Fish, Sheepshead minnow, Cyprinodon variegatus, larval survival and growth. | 1004.0. 27 | |||
| Fish, Sheepshead minnow, Cyprinodon variegatus, embryo-larval survival and teratogenicity | 1005.0. 27 | ||||
| Fish, Inland silverside, Menidia beryllina, larval survival and growth | 1006.0. 27 | ||||
| Mysid, Mysidopsis bahia, survival, growth, and fecundity | 1007.0. 27 | ||||
| Sea urchin, Arbacia punctulata, fertilization | 1008.0. 27 | ||||
| Parameter | Methodology 58 | EPA 52 | Standard methods 84 | ASTM | USGS/AOAC/Other |
|---|---|---|---|---|---|
| Table IB Notes: | |||||
| 1 Methods for Chemical Analysis of Water and Wastes, EPA-600/4-79-020. Revised March 1983 and 1979, where applicable. U.S. EPA. | |||||
| 2 Methods for Analysis of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resource Investigations of the U.S. Geological Survey, Book 5, Chapter A1., unless otherwise stated. 1989. USGS. | |||||
| 3 Official Methods of Analysis of the Association of Official Analytical Chemists, Methods Manual, Sixteenth Edition, 4th Revision, 1998. AOAC International. | |||||
| 4 For the determination of total metals (which are equivalent to total recoverable metals) the sample is not filtered before processing. A digestion procedure is required to solubilize analytes in suspended material and to break down organic-metal complexes (to convert the analyte to a detectable form for colorimetric analysis). For non-platform graphite furnace atomic absorption determinations, a digestion using nitric acid (as specified in Section 4.1.3 of Methods for Chemical Analysis of Water and Wastes) is required prior to analysis. The procedure used should subject the sample to gentle acid refluxing, and at no time should the sample be taken to dryness. For direct aspiration flame atomic absorption (FLAA) determinations, a combination acid (nitric and hydrochloric acids) digestion is preferred, prior to analysis. The approved total recoverable digestion is described as Method 200.2 in Supplement I of “Methods for the Determination of Metals in Environmental Samples” EPA/600R-94/111, May 1994, and is reproduced in EPA Methods 200.7, 200.8, and 200.9 from the same Supplement. However, when using the gaseous hydride technique or for the determination of certain elements such as antimony, arsenic, selenium, silver, and tin by non-EPA graphite furnace atomic absorption methods, mercury by cold vapor atomic absorption, the noble metals and titanium by FLAA, a specific or modified sample digestion procedure may be required, and, in all cases the referenced method write-up should be consulted for specific instruction and/or cautions. For analyses using inductively coupled plasma-atomic emission spectrometry (ICP-AES), the direct current plasma (DCP) technique or EPA spectrochemical techniques (platform furnace AA, ICP-AES, and ICP-MS), use EPA Method 200.2 or an approved alternate procedure ( e.g., CEM microwave digestion, which may be used with certain analytes as indicated in this table IB); the total recoverable digestion procedures in EPA Methods 200.7, 200.8, and 200.9 may be used for those respective methods. Regardless of the digestion procedure, the results of the analysis after digestion procedure are reported as “total” metals. | |||||
| 5 Copper sulfate or other catalysts that have been found suitable may be used in place of mercuric sulfate. | |||||
| 6 Manual distillation is not required if comparability data on representative effluent samples are on file to show that this preliminary distillation step is not necessary; however, manual distillation will be required to resolve any controversies. In general, the analytical method should be consulted regarding the need for distillation. If the method is not clear, the laboratory may compare a minimum of 9 different sample matrices to evaluate the need for distillation. For each matrix, a matrix spike and matrix spike duplicate are analyzed both with and without the distillation step (for a total of 36 samples, assuming 9 matrices). If results are comparable, the laboratory may dispense with the distillation step for future analysis. Comparable is defined as <20% RPD for all tested matrices). Alternatively, the two populations of spike recovery percentages may be compared using a recognized statistical test. | |||||
| 7 Industrial Method Number 379-75 WE Ammonia, Automated Electrode Method, Technicon Auto Analyzer II. February 19, 1976. Bran & Luebbe Analyzing Technologies Inc. | |||||
| 8 The approved method is that cited in Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1979. USGS. | |||||
| 9 American National Standard on Photographic Processing Effluents. April 2, 1975. American National Standards Institute. | |||||
| 10 In-Situ Method 1003-8-2009, Biochemical Oxygen Demand (BOD) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 11 The use of normal and differential pulse voltage ramps to increase sensitivity and resolution is acceptable. | |||||
| 12 Carbonaceous biochemical oxygen demand (CBOD 5) must not be confused with the traditional BOD 5 test method which measures “total 5-day BOD.” The addition of the nitrification inhibitor is not a procedural option but must be included to report the CBOD 5 parameter. A discharger whose permit requires reporting the traditional BOD 5 may not use a nitrification inhibitor in the procedure for reporting the results. Only when a discharger's permit specifically states CBOD 5 is required can the permittee report data using a nitrification inhibitor. | |||||
| 13 OIC Chemical Oxygen Demand Method. 1978. Oceanography International Corporation. | |||||
| 14 Method 8000, Chemical Oxygen Demand, Hach Handbook of Water Analysis, 1979. Hach Company. | |||||
| 15 The back-titration method will be used to resolve controversy. | |||||
| 16 Orion Research Instruction Manual, Residual Chlorine Electrode Model 97-70. 1977. Orion Research Incorporated. The calibration graph for the Orion residual chlorine method must be derived using a reagent blank and three standard solutions, containing 0.2, 1.0, and 5.0 mL 0.00281 N potassium iodate/100 mL solution, respectively. | |||||
| 17 Method 245.7, Mercury in Water by Cold Vapor Atomic Fluorescence Spectrometry, EPA-821-R-05-001. Revision 2.0, February 2005. US EPA. | |||||
| 18 National Council of the Paper Industry for Air and Stream Improvement (NCASI) Technical Bulletin 253 (1971) and Technical Bulletin 803, May 2000. | |||||
| 19 Method 8506, Bicinchoninate Method for Copper, Hach Handbook of Water Analysis. 1979. Hach Company. | |||||
| 20 When using a method with block digestion, this treatment is not required. | |||||
| 21 Industrial Method Number 378-75WA, Hydrogen ion (pH) Automated Electrode Method, Bran & Luebbe (Technicon) Autoanalyzer II. October 1976. Bran & Luebbe Analyzing Technologies. | |||||
| 22 Method 8008, 1,10-Phenanthroline Method using FerroVer Iron Reagent for Water. 1980. Hach Company. | |||||
| 23 Method 8034, Periodate Oxidation Method for Manganese, Hach Handbook of Wastewater Analysis. 1979. Hach Company. | |||||
| 24 Methods for Analysis of Organic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3, (1972 Revised 1987). 1987. USGS. | |||||
| 25 Method 8507, Nitrogen, Nitrite-Low Range, Diazotization Method for Water and Wastewater. 1979. Hach Company. | |||||
| 26 Just prior to distillation, adjust the sulfuric-acid-preserved sample to pH 4 with 1 + 9 NaOH. | |||||
| 27 The colorimetric reaction must be conducted at a pH of 10.0 ± 0.2. | |||||
| 28 Addison, R.F., and R.G. Ackman. 1970. Direct Determination of Elemental Phosphorus by Gas-Liquid Chromatography, Journal of Chromatograph y, 47(3):421-426. | |||||
| 29 Approved methods for the analysis of silver in industrial wastewaters at concentrations of 1 mg/L and above are inadequate where silver exists as an inorganic halide. Silver halides such as the bromide and chloride are relatively insoluble in reagents such as nitric acid but are readily soluble in an aqueous buffer of sodium thiosulfate and sodium hydroxide to pH of 12. Therefore, for levels of silver above 1 mg/L, 20 mL of sample should be diluted to 100 mL by adding 40 mL each of 2 M Na 2 S 2 O 3 and NaOH. Standards should be prepared in the same manner. For levels of silver below 1 mg/L the approved method is satisfactory. | |||||
| 30 The use of EDTA decreases method sensitivity. Analysts may omit EDTA or replace with another suitable complexing reagent provided that all method-specified quality control acceptance criteria are met. | |||||
| 31 For samples known or suspected to contain high levels of silver ( e.g., in excess of 4 mg/L), cyanogen iodide should be used to keep the silver in solution for analysis. Prepare a cyanogen iodide solution by adding 4.0 mL of concentrated NH 4 OH, 6.5 g of KCN, and 5.0 mL of a 1.0 N solution of I 2 to 50 mL of reagent water in a volumetric flask and dilute to 100.0 mL. After digestion of the sample, adjust the pH of the digestate to <7 to prevent the formation of HCN under acidic conditions. Add 1 mL of the cyanogen iodide solution to the sample digestate and adjust the volume to 100 mL with reagent water (NOT acid). If cyanogen iodide is added to sample digestates, then silver standards must be prepared that contain cyanogen iodide as well. Prepare working standards by diluting a small volume of a silver stock solution with water and adjusting the pH>7 with NH 4 OH. Add 1 mL of the cyanogen iodide solution and let stand 1 hour. Transfer to a 100-mL volumetric flask and dilute to volume with water. | |||||
| 32 “Water Temperature-Influential Factors, Field Measurement and Data Presentation,” Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 1, Chapter D1. 1975. USGS. | |||||
| 33 Method 8009, Zincon Method for Zinc, Hach Handbook of Water Analysis, 1979. Hach Company. | |||||
| 34 Method AES0029, Direct Current Plasma (DCP) Optical Emission Spectrometric Method for Trace Elemental Analysis of Water and Wastes. 1986—Revised 1991. Thermo Jarrell Ash Corporation. | |||||
| 35 In-Situ Method 1004-8-2009, Carbonaceous Biochemical Oxygen Demand (CBOD) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 36 Microwave-assisted digestion may be employed for this metal, when analyzed by this methodology. Closed Vessel Microwave Digestion of Wastewater Samples for Determination of Metals. April 16, 1992. CEM Corporation. | |||||
| 37 When determining boron and silica, only plastic, PTFE, or quartz laboratory ware may be used from start until completion of analysis. | |||||
| 38 Only use n -hexane ( n -Hexane—85% minimum purity, 99.0% min. saturated C6 isomers, residue less than 1 mg/L) extraction solvent when determining Oil and Grease parameters—Hexane Extractable Material (HEM), or Silica Gel Treated HEM (analogous to EPA Methods 1664 Rev. A and 1664 Rev. B). Use of other extraction solvents is prohibited. | |||||
| 39 Method PAI-DK01, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Titrimetric Detection. Revised December 22, 1994. OI Analytical. | |||||
| 40 Method PAI-DK02, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Colorimetric Detection. Revised December 22, 1994. OI Analytical. | |||||
| 41 Method PAI-DK03, Nitrogen, Total Kjeldahl, Block Digestion, Automated FIA Gas Diffusion. Revised December 22, 1994. OI Analytical. | |||||
| 42 Method 1664 Rev. B is the revised version of EPA Method 1664 Rev. A. U.S. EPA. February 1999, Revision A. Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Non-polar Material) by Extraction and Gravimetry. EPA-821-R-98-002. U.S. EPA. February 2010, Revision B. Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Non-polar Material) by Extraction and Gravimetry. EPA-821-R-10-001. | |||||
| 43 Method 1631, Revision E, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry, EPA-821-R-02-019. Revision E. August 2002, U.S. EPA. The application of clean techniques described in EPA's Method 1669: Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels, EPA-821-R-96-011, are recommended to preclude contamination at low-level, trace metal determinations. | |||||
| 44 Method OIA-1677-09, Available Cyanide by Ligand Exchange and Flow Injection Analysis (FIA). 2010. OI Analytical. | |||||
| 45 Open File Report 00-170, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Ammonium Plus Organic Nitrogen by a Kjeldahl Digestion Method and an Automated Photometric Finish that Includes Digest Cleanup by Gas Diffusion. 2000. USGS. | |||||
| 46 Open File Report 93-449, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Chromium in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1993. USGS. | |||||
| 47 Open File Report 97-198, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Molybdenum by Graphite Furnace Atomic Absorption Spectrophotometry. 1997. USGS. | |||||
| 48 Open File Report 92-146, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Total Phosphorus by Kjeldahl Digestion Method and an Automated Colorimetric Finish That Includes Dialysis. 1992. USGS. | |||||
| 49 Open File Report 98-639, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Arsenic and Selenium in Water and Sediment by Graphite Furnace-Atomic Absorption Spectrometry. 1999. USGS. | |||||
| 50 Open File Report 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Elements in Whole-water Digests Using Inductively Coupled Plasma-Optical Emission Spectrometry and Inductively Coupled Plasma-Mass Spectrometry. 1998. USGS. | |||||
| 51 Open File Report 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. USGS. | |||||
| 52 Unless otherwise indicated, all EPA methods, excluding EPA Method 300.1, are published in U.S. EPA. May 1994. Methods for the Determination of Metals in Environmental Samples, Supplement I, EPA/600/R-94/111; or U.S. EPA. August 1993. Methods for the Determination of Inorganic Substances in Environmental Samples, EPA/600/R-93/100. EPA Method 300.1 is U.S. EPA. Revision 1.0, 1997, including errata cover sheet April 27, 1999. Determination of Inorganic Ions in Drinking Water by Ion Chromatography. | |||||
| 53 Styrene divinyl benzene beads ( e.g., AMCO-AEPA-1 or equivalent) and stabilized formazin ( e.g., Hach StablCal TM or equivalent) are acceptable substitutes for formazin. | |||||
| 54 Waters Corp. Now included in ASTM D6508-15, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. 2015. | |||||
| 55 Kelada-01, Kelada Automated Test Methods for Total Cyanide, Acid Dissociable Cyanide, and Thiocyanate, EPA 821-B-01-009, Revision 1.2, August 2001. US EPA. Note: A 450-W UV lamp may be used in this method instead of the 550-W lamp specified if it provides performance within the quality control (QC) acceptance criteria of the method in a given instrument. Similarly, modified flow cell configurations and flow conditions may be used in the method, provided that the QC acceptance criteria are met. | |||||
| 56 QuikChem Method 10-204-00-1-X, Digestion and Distillation of Total Cyanide in Drinking and Wastewaters using MICRO DIST and Determination of Cyanide by Flow Injection Analysis. Revision 2.2, March 2005. Lachat Instruments. | |||||
| 57 When using sulfide removal test procedures described in EPA Method 335.4, reconstitute particulate that is filtered with the sample prior to distillation. | |||||
| 58 Unless otherwise stated, if the language of this table specifies a sample digestion and/or distillation “followed by” analysis with a method, approved digestion and/or distillation are required prior to analysis. | |||||
| 59 Samples analyzed for available cyanide using OI Analytical method OIA-1677-09 or ASTM method D6888-16 that contain particulate matter may be filtered only after the ligand exchange reagents have been added to the samples, because the ligand exchange process converts complexes containing available cyanide to free cyanide, which is not removed by filtration. Analysts are further cautioned to limit the time between the addition of the ligand exchange reagents and sample filtration to no more than 30 minutes to preclude settling of materials in samples. | |||||
| 60 Analysts should be aware that pH optima and chromophore absorption maxima might differ when phenol is replaced by a substituted phenol as the color reagent in Berthelot Reaction (“phenol-hypochlorite reaction”) colorimetric ammonium determination methods. For example, when phenol is used as the color reagent, pH optimum and wavelength of maximum absorbance are about 11.5 and 635 nm, respectively—see, Patton, C.J. and S.R. Crouch. March 1977. Anal. Chem. 49:464-469. These reaction parameters increase to pH > 12.6 and 665 nm when salicylate is used as the color reagent—see, Krom, M.D. April 1980. The Analyst 105:305-316. | |||||
| 61 If atomic absorption or ICP instrumentation is not available, the aluminon colorimetric method detailed in the 19th Edition of Standard Methods for the Examination of Water and Wastewater may be used. This method has poorer precision and bias than the methods of choice. | |||||
| 62 Easy (1-Reagent) Nitrate Method, Revision November 12, 2011. Craig Chinchilla. | |||||
| 63 Hach Method 10360, Luminescence Measurement of Dissolved Oxygen in Water and Wastewater and for Use in the Determination of BOD 5 and CBOD 5 . Revision 1.2, October 2011. Hach Company. This method may be used to measure dissolved oxygen when performing the methods approved in this table IB for measurement of biochemical oxygen demand (BOD) and carbonaceous biochemical oxygen demand (CBOD). | |||||
| 64 In-Situ Method 1002-8-2009, Dissolved Oxygen (DO) Measurement by Optical Probe. 2009. In-Situ Incorporated. | |||||
| 65 Mitchell Method M5331, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Leck Mitchell. | |||||
| 66 Mitchell Method M5271, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Leck Mitchell. | |||||
| 67 Orion Method AQ4500, Determination of Turbidity by Nephelometry. Revision 5, March 12, 2009. Thermo Scientific. | |||||
| 68 EPA Method 200.5, Determination of Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma-Atomic Emission Spectrometry, EPA/600/R-06/115. Revision 4.2, October 2003. US EPA. | |||||
| 69 Method 1627, Kinetic Test Method for the Prediction of Mine Drainage Quality, EPA-821-R-09-002. December 2011. US EPA. | |||||
| 70 Techniques and Methods Book 5-B1, Determination of Elements in Natural-Water, Biota, Sediment and Soil Samples Using Collision/Reaction Cell Inductively Coupled Plasma-Mass Spectrometry, Chapter 1, Section B, Methods of the National Water Quality Laboratory, Book 5, Laboratory Analysis, 2006. USGS. | |||||
| 71 Water-Resources Investigations Report 01-4132, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organic Plus Inorganic Mercury in Filtered and Unfiltered Natural Water with Cold Vapor-Atomic Fluorescence Spectrometry, 2001. USGS. | |||||
| 72 USGS Techniques and Methods 5-B8, Chapter 8, Section B, Methods of the National Water Quality Laboratory Book 5, Laboratory Analysis, 2011 USGS. | |||||
| 73 NECi Method N07-0003, “Nitrate Reductase Nitrate-Nitrogen Analysis,” Revision 9.0, March 2014, The Nitrate Elimination Co., Inc. | |||||
| 74 Timberline Instruments, LLC Method Ammonia-001, “Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Conductivity Cell Analysis,” June 2011, Timberline Instruments, LLC. | |||||
| 75 Hach Company Method 10206, “Spectrophotometric Measurement of Nitrate in Water and Wastewater,” Revision 2.1, January 2013, Hach Company. | |||||
| 76 Hach Company Method 10242, “Simplified Spectrophotometric Measurement of Total Kjeldahl Nitrogen in Water and Wastewater,” Revision 1.1, January 2013, Hach Company. | |||||
| 77 National Council for Air and Stream Improvement (NCASI) Method TNTP-W10900, “Total (Kjeldahl) Nitrogen and Total Phosphorus in Pulp and Paper Biologically Treated Effluent by Alkaline Persulfate Digestion,” June 2011, National Council for Air and Stream Improvement, Inc. | |||||
| 78 The pH adjusted sample is to be adjusted to 7.6 for NPDES reporting purposes. | |||||
| 79 I-2057-85 in U.S. Geological Survey Techniques of Water-Resources Investigations, Book 5, Chap. A1, Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, 1989. | |||||
| 80 Methods I-2522-90, I-2540-90, and I-2601-90 in U.S. Geological Survey Open-File Report 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments, 1993. | |||||
| 81 Method I-4472-97 in U.S. Geological Survey Open-File Report 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments, 1998. | |||||
| 82 FIAlab 100, “Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Fluorescence Detector Analysis”, April 4, 2018, FIAlab Instruments, Inc. | |||||
| 83 MACHEREY-NAGEL GmbH and Co. Method 036/038 NANOCOLOR® COD LR/HR, “Spectrophotometric Measurement of Chemical Oxygen Demand in Water and Wastewater”, Revision 1.5, May 2018, MACHEREY-NAGEL GmbH and Co. KG. | |||||
| 84 Please refer to the following applicable Quality Control Sections: Part 2000 Methods, Physical and Aggregate Properties 2020 (2021); Part 3000 Methods, Metals, 3020 (2021); Part 4000 Methods, Inorganic Nonmetallic Constituents, 4020 (2022); Part 5000 Methods, and Aggregate Organic Constituents, 5020 (2022). These Quality Control Standards are available for download at www.standardmethods.org at no charge. | |||||
| 85 Each laboratory may establish its own control limits by performing at least 25 glucose-glutamic acid (GGA) checks over several weeks or months and calculating the mean and standard deviation. The laboratory may then use the mean ± 3 standard deviations as the control limit for future GGA checks. However, GGA acceptance criteria can be no wider than 198 ± 30.5 mg/L for BOD 5 . GGA acceptance criteria for CBOD must be either 198 ± 30.5 mg/L, or the lab may develop control charts under the following conditions: dissolved oxygen uptake from the seed contribution is between 0.6-1.0 mg/L; control charts are performed on at least 25 GGA checks with three standard deviations from the derived mean; the RSD must not exceed 7.5%; and any single GGA value cannot be less than 150 mg/L or higher than 250 mg/L. | |||||
| 86 The approved method is that cited in Standard Methods for the Examination of Water and Wastewater, 14th Edition, 1976. | |||||
| 1. Acidity (as CaCO 3), mg/L | Electrometric endpoint or phenolphthalein endpoint | 2310 B-2020 | D1067-16 | I-1020-85. 2 | |
| 2. Alkalinity (as CaCO 3), mg/L | Electrometric or Colorimetric titration to pH 4.5, Manual | 2320 B-2021 | D1067-16 | 973.43, 3 I-1030-85. 2 | |
| Automatic | 310.2 (Rev. 1974) 1 | I-2030-85. 2 | |||
| 3. Aluminum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 D-2019 or 3111 E-2019 | I-3051-85. 2 | |||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97, 81 | |
| Direct Current Plasma (DCP) 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Eriochrome cyanine R) | 3500-Al B-2020. | ||||
| 4. Ammonia (as N), mg/L | Manual distillation 6 or gas diffusion (pH > 11), followed by any of the following: | 350.1 Rev. 2.0 (1993) | 4500-NH 3 B-2021 | 973.49. 3 | |
| Nesslerization | D1426-15 (A) | 973.49, 3 I-3520-85. 2 | |||
| Titration | 4500-NH 3 C-2021. | ||||
| Electrode | 4500-NH 3 D-2021 or E-2021 | D1426-15 (B) | |||
| Manual phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods | 4500-NH 3 F-2021 | See footnote. 60 | |||
| Automated phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods | 350.1, 30 Rev. 2.0 (1993) | 4500-NH 3 G-2021, 4500-NH 3 H-2021 | I-4523-85, 2 I-2522-90. 80 | ||
| Automated electrode | See footnote. 7 | ||||
| Ion Chromatography | D6919-17. | ||||
| Automated gas diffusion, followed by conductivity cell analysis | Timberline Ammonia-001. 74 | ||||
| Automated gas diffusion followed by fluorescence detector analysis | FIAlab100. 82 | ||||
| 5. Antimony—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019. | ||||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| 6. Arsenic—Total, 4 mg/L | Digestion, 4 followed by any of the following: | 206.5 (Issued 1978). 1 | |||
| AA gaseous hydride | 3114 B-2020 or 3114 C-2020 | D2972-15 (B) | I-3062-85. 2 | ||
| AA furnace | 3113 B-2020 | D2972-15 (C) | I-4063-98. 49 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5, Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05. 70 | |
| Colorimetric (SDDC) | 3500-As B-2020 | D2972-15 (A) | I-3060-85. 2 | ||
| 7. Barium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 D-2019 | I-3084-85. 2 | |||
| AA furnace | 3113 B-2020 | D4382-18. | |||
| ICP/AES 36 | 200.5, Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | See footnote. 34 | ||||
| 8. Beryllium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019 or 3111 E-2019 | D3645-15 (A) | I-3095-85. 2 | ||
| AA furnace | 3113 B-2020 | D3645-15 (B). | |||
| STGFAA | 200.9, Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | D4190-15 | See footnote. 34 | |||
| Colorimetric (aluminon) | See footnote 61 | ||||
| 9. Biochemical oxygen demand (BOD 5), mg/L | Dissolved Oxygen Depletion | 5210 B-2016 85 | 973.44 3 p. 17, 9 I-1578-78, 8 see footnote. 1063 | ||
| 10. Boron—Total, 37 mg/L | Colorimetric (curcumin) | 4500-B B-2011 | I-3112-85. 2 | ||
| ICP/AES | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | D4190-15 | See footnote. 34 | |||
| 11. Bromide, mg/L | Electrode | D1246-16 | I-1125-85. 2 | ||
| Ion Chromatography | 300.0 Rev 2.1 (1993), and 300.1 Rev 1.0 (1997) | 4110 B-2020, C-2020 or D-2020 | D4327-17 | 993.30, 3 I-2057-85. 79 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508 Rev. 2. 54 | ||
| 12. Cadmium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D3557-17 (A or B) | 974.27 3 p. 37, 9 I-3135-85 2 or I-3136-85. 2 | ||
| AA furnace | 3113 B-2020 | D3557-17 (D) | I-4138-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-1472-85 2 or I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Voltammetry 11 | D3557-17 (C). | ||||
| Colorimetric (Dithizone) | 3500-Cd D-1990. | ||||
| 13. Calcium—Total, 4 mg/L | Digestion 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 D-2019 | D511-14 (B) | I-3152-85. 2 | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Titrimetric (EDTA) | 3500-Ca B-2020 | D511-14 (A). | |||
| Ion Chromatography | D6919-17. | ||||
| 14. Carbonaceous biochemical oxygen demand (CBOD 5), mg/L 12 | Dissolved Oxygen Depletion with nitrification inhibitor | 5210 B-2016 85 | See footnotes. 35 63 | ||
| 15. Chemical oxygen demand (COD), mg/L | Titrimetric | 410.3 (Rev. 1978) 1 | 5220 B-2011 or C-2011 | D1252-06(12) (A) | 973.46 3 p. 17, 9 I-3560-85. 2 |
| Spectrophotometric, manual or automatic | 410.4 Rev. 2.0 (1993) | 5220 D-2011 | D1252-06(12) (B) | See footnotes, 131483 I-3561-85. 2 | |
| 16. Chloride, mg/L | Titrimetric: (silver nitrate) | 4500-Cl B-2021 | D512-12 (B) | I-1183-85. 2 | |
| (Mercuric nitrate) | 4500-Cl C-2021 | D512-12 (A) | 973.51, 3 I-1184-85. 2 | ||
| Colorimetric: manual | I-1187-85. 2 | ||||
| Automated (ferricyanide) | 4500-Cl E-2021 | I-2187-85. 2 | |||
| Potentiometric Titration | 4500-Cl D-2021. | ||||
| Ion Selective Electrode | D512-12 (C). | ||||
| Ion Chromatography | 300.0 Rev 2.1 (1993), and 300.1 Rev 1.0 (1997) | 4110 B-2020 or 4110 C-2020 | D4327-17 | 993.30, 3 I-2057-90. 51 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 17. Chlorine—Total residual, mg/L | Amperometric direct | 4500-Cl D-2011 | D1253-14. | ||
| Amperometric direct (low level) | 4500-Cl E-2011. | ||||
| Iodometric direct | 4500-Cl B-2011. | ||||
| Back titration ether end-point 15 | 4500-Cl C-2011. | ||||
| DPD-FAS | 4500-Cl F-2011. | ||||
| Spectrophotometric, DPD | 4500-Cl G-2011. | ||||
| Electrode | See footnote. 16 | ||||
| 17A. Chlorine—Free Available, mg/L | Amperometric direct | 4500-Cl D-2011 | D1253-14. | ||
| Amperometric direct (low level) | 4500-Cl E-2011. | ||||
| DPD-FAS | 4500-Cl F-2011. | ||||
| Spectrophotometric, DPD | 4500-Cl G-2011. | ||||
| 18. Chromium VI dissolved, mg/L | 0.45-micron filtration followed by any of the following: | ||||
| AA chelation-extraction | 3111 C-2019 | I-1232-85. 2 | |||
| Colorimetric (diphenyl-carbazide) | 3500-Cr B-2020 | D1687-17 (A) | I-1230-85. 2 | ||
| 19. Chromium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 | D1687-17 (B) | 974.27, 3 I-3236-85. 2 | ||
| AA chelation-extraction | 3111 C-2019. | ||||
| AA furnace | 3113 B-2020 | D1687-17 (C) | I-3233-93. 46 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (diphenyl-carbazide) | 3500-Cr B-2020. | ||||
| 20. Cobalt—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 C-2019 | D3558-15 (A or B) | p. 37, 9 I-323985. 2 | ||
| AA furnace | 3113 B-2020 | D3558-15 (C) | I-4243-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| DCP | D4190-15 | See footnote. 34 | |||
| 21. Color, platinum cobalt units or dominant wavelength, hue, luminance purity | Colorimetric (ADMI) | 2120 F-2021. 78 | |||
| Platinum cobalt visual comparison | 2120 B-2021 | I-1250-85. 2 | |||
| Spectrophotometric | See footnote. 18 | ||||
| 22. Copper—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1688-17 (A or B) | 974.27, 3 p. 37, 9 I-3270-85 2 or I-3271-85. 2 | ||
| AA furnace | 3113 B-2020 | D1688-17 (C) | I-4274-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Neocuproine) | 3500-Cu B-2020. | ||||
| Colorimetric (Bathocuproine) | 3500-Cu C-2020 | See footnote. 19 | |||
| 23. Cyanide—Total, mg/L | Automated UV digestion/distillation and Colorimetry | Kelada-01. 55 | |||
| Segmented Flow Injection, In-Line Ultraviolet Digestion, followed by gas diffusion amperometry | 4500-CN P-2021 | D7511-12 (17). | |||
| Manual distillation with MgCl 2 , followed by any of the following: | 335.4 Rev. 1.0 (1993) 57 | 4500-CN B-2021 and C-2021 | D2036-09(15)(A), D7284-20 | 10-204-00-1-X. 56 | |
| Flow Injection, gas diffusion amperometry | D2036-09(15)(A) D7284-20. | ||||
| Titrimetric | 4500-CN D-2021 | D2036-09(15)(A) | See footnote 9 p. 22. | ||
| Spectrophotometric, manual | 4500-CN E-2021 | D2036-09(15)(A) | I-3300-85. 2 | ||
| Semi-Automated 20 | 335.4 Rev. 1.0 (1993) 57 | 4500-CN N-2021 | 10-204-00-1-X, 56 I-4302-85. 2 | ||
| Ion Chromatography | D2036-09(15)(A). | ||||
| Ion Selective Electrode | 4500-CN F-2021 | D2036-09(15)(A). | |||
| 24. Cyanide—Available, mg/L | Cyanide Amenable to Chlorination (CATC); Manual distillation with MgCl 2 , followed by Titrimetric or Spectrophotometric | 4500-CN G-2021 | D2036-09(15)(B). | ||
| Flow injection and ligand exchange, followed by gas diffusion amperometry 59 | 4500-CN Q-2021 | D6888-16 | OIA-1677-09. 44 | ||
| Automated Distillation and Colorimetry (no UV digestion) | Kelada-01. 55 | ||||
| 24A. Cyanide—Free, mg/L | Flow Injection, followed by gas diffusion amperometry | 4500-CN R-2021 | D7237-18 (A) | OIA-1677-09. 44 | |
| Manual micro-diffusion and colorimetry | D4282-15. | ||||
| 25. Fluoride—Total, mg/L | Manual distillation, 6 followed by any of the following: | 4500-F B-2021 | D1179-16 (A). | ||
| Electrode, manual | 4500-F C-2021 | D1179-16 (B). | |||
| Electrode, automated | 4500-F G-2021 | I-4327-85. 2 | |||
| Colorimetric, (SPADNS) | 4500-F D-2021. | ||||
| Automated complexone | 4500-F E-2021. | ||||
| Ion Chromatography | 300.0 Rev 2.1 (1993) and 300.1 Rev 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 26. Gold—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 231.2 (Issued 1978) 1 | 3113 B-2020. | |||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| 27. Hardness—Total (as CaCO (3) , mg/L | Automated colorimetric | 130.1 (Issued 1971). 1 | |||
| Titrimetric (EDTA) | 2340 C-2021 | D1126-17 | 973.52B, 3 I-1338-85. 2 | ||
| Ca plus Mg as their carbonates, by any approved method for Ca and Mg (See Parameters 13 and 33), provided that the sum of the lowest point of quantitation for Ca and Mg is below the NPDES permit requirement for Hardness. | 2340 B-2021. | ||||
| 28. Hydrogen ion (pH), pH units | Electrometric measurement | 4500-H + B-2021 | D1293-18 (A or B) | 973.41, 3 I-1586-85. 2 | |
| Automated electrode | 150.2 (Dec. 1982) 1 | See footnote 21 I-2587-85. 2 | |||
| 29. Iridium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 235.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 30. Iron—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1068-15 (A) | 974.27, 3 I-3381-85. 2 | ||
| AA furnace | 3113 B-2020 | D1068-15 (B). | |||
| STGFAA | 200.9, Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Phenanthroline) | 3500-Fe B-2011 | D1068-15 (C) | See footnote. 22 | ||
| 31. Kjeldahl Nitrogen 5 —Total (as N), mg/L | Manual digestion 20 and distillation or gas diffusion, followed by any of the following: | 4500-N org B-2021 or C-2021 and 4500-NH 3 B-2021 | D3590-17 (A) | I-4515-91. 45 | |
| Titration | 4500-NH 3 C-2021 | 973.48. 3 | |||
| Nesslerization | D1426-15 (A). | ||||
| Electrode | 4500-NH 3 D-2021 or E-2021 | D1426-15 (B). | |||
| Semi-automated phenate | 350.1 Rev. 2.0 (1993) | 4500-NH 3 G-2021 or 4500-NH 3 H-2021. | |||
| Manual phenate, salicylate, or other substituted phenols in Berthelot reaction based methods | 4500-NH 3 F-2021 | See footnote. 60 | |||
| Automated gas diffusion, followed by conductivity cell analysis | Timberline Ammonia-001. 74 | ||||
| Automated gas diffusion followed by fluorescence detector analysis | FIAlab 100. 82 | ||||
| Automated Methods for TKN that do not require manual distillation. | |||||
| Automated phenate, salicylate, or other substituted phenols in Berthelot reaction-based methods colorimetric (auto digestion and distillation) | 351.1 (Rev. 1978) 1 | I-4551-78. 8 | |||
| Semi-automated block digestor colorimetric (distillation not required) | 351.2 Rev. 2.0 (1993) | 4500-N org D-2021 | D3590-17 (B) | I-4515-91. 45 | |
| Block digester, followed by Auto distillation and Titration | See footnote. 39 | ||||
| Block digester, followed by Auto distillation and Nesslerization | See footnote. 40 | ||||
| Block Digester, followed by Flow injection gas diffusion (distillation not required) | See footnote. 41 | ||||
| Digestion with peroxdisulfate, followed by Spectrophotometric (2,6-dimethyl phenol) | Hach 10242. 76 | ||||
| Digestion with persulfate, followed by Colorimetric | NCASI TNTP W10900. 77 | ||||
| 32. Lead—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D3559-15 (A or B) | 974.27, 3 I-3399-85. 2 | ||
| AA furnace | 3113 B-2020 | D3559-15 (D) | I-4403-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Voltammetry 11 | D3559-15 (C). | ||||
| Colorimetric (Dithizone) | 3500-Pb B-2020. | ||||
| 33. Magnesium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | D511-14 (B) | 974.27, 3 I-3447-85. 2 | ||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Ion Chromatography | D6919-17. | ||||
| 34. Manganese—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D858-17 (A or B) | 974.27, 3 I-3454-85. 2 | ||
| AA furnace | 3113 B-2020 | D858-17 (C). | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5, Rev. 4.2 (2003); 68 200.7, Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Persulfate) | 3500-Mn B-2020 | 920.203. 3 | |||
| Colorimetric (Periodate) | See footnote. 23 | ||||
| 35. Mercury—Total, mg/L | Cold vapor, Manual | 245.1 Rev. 3.0 (1994) | 3112 B-2020 | D3223-17 | 977.22, 3 I-3462-85. 2 |
| Cold vapor, Automated | 245.2 (Issued 1974). 1 | ||||
| Cold vapor atomic fluorescence spectrometry (CVAFS) | 245.7 Rev. 2.0 (2005) 17 | I-4464-01. 71 | |||
| Purge and Trap CVAFS | 1631E. 43 | ||||
| 36. Molybdenum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019 | I-3490-85. 2 | |||
| AA furnace | 3113 B-2020 | I-3492-96. 47 | |||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | See footnote. 34 | ||||
| 37. Nickel—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1886-14 (A or B) | I-3499-85. 2 | ||
| AA furnace | 3113 B-2020 | D1886-14 (C) | I-4503-89. 51 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| 38. Nitrate (as N), mg/L | Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Ion Selective Electrode | 4500-NO 3 D-2019. | ||||
| Colorimetric (Brucine sulfate) | 352.1 (Issued 1971) 1 | 973.50, 3 419D, 86 p. 28. 9 | |||
| Spectrophotometric (2,6-dimethylphenol) | Hach 10206. 75 | ||||
| Nitrate-nitrite N minus Nitrite N (see parameters 39 and 40). | |||||
| 39. Nitrate-nitrite (as N), mg/L | Cadmium reduction, Manual | 4500-NO 3 E-2019 | D3867-16 (B). | ||
| Cadmium reduction, Automated | 353.2 Rev. 2.0 (1993) | 4500-NO 3 F-2019 or 4500-NO 3 I-2019 | D3867-16 (A) | I-2545-90. 51 | |
| Automated hydrazine | 4500-NO 3 H-2019. | ||||
| Reduction/Colorimetric | See footnote. 62 | ||||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Enzymatic reduction, followed by automated colorimetric determination | D7781-14 | I-2547-11, 72 I-2548-11, 72 N07-0003. 73 | |||
| Enzymatic reduction, followed by manual colorimetric determination | 4500-NO 3 J-2018. | ||||
| Spectrophotometric (2,6-dimethylphenol) | Hach 10206. 75 | ||||
| 40. Nitrite (as N), mg/L | Spectrophotometric: Manual | 4500-NO 2 B-2021 | See footnote. 25 | ||
| Automated (Diazotization) | I-4540-85 2 see footnote, 62 I-2540-90. 80 | ||||
| Automated (*bypass cadmium reduction) | 353.2 Rev. 2.0 (1993) | 4500-NO 3 F-2019, 4500-NO 3 I-2019 | D3867-16 (A) | I-4545-85. 2 | |
| Manual (*bypass cadmium or enzymatic reduction) | 4500-NO 3 E-2019, 4500-NO 3 J-2018 | D3867-16 (B). | |||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| Automated (*bypass Enzymatic reduction) | D7781-14 | I-2547-11, 72 I-2548-11, 72 N07-0003. 73 | |||
| 41. Oil and grease—Total recoverable, mg/L | Hexane extractable material (HEM): n -Hexane extraction and gravimetry | 1664 Rev. A 1664 Rev. B 42 | 5520 B or G-2021. 38 | ||
| Silica gel treated HEM (SGT-HEM): Silica gel treatment and gravimetry | 1664 Rev. A, 1664 Rev. B 42 | 5520 B or G-2021 38 and 5520 F-2021. 38 | |||
| 42. Organic carbon—Total (TOC), mg/L | Combustion | 5310 B-2014 | D7573-18a e1 | 973.47, 3 p. 14. 24 | |
| Heated persulfate or UV persulfate oxidation | 5310 C-2014, 5310 D-2011 | D4839-03(17) | 973.47, 3, p. 14. 24 | ||
| 43. Organic nitrogen (as N), mg/L | Total Kjeldahl N (Parameter 31) minus ammonia N (Parameter 4). | ||||
| 44. Ortho-phosphate (as P), mg/L | Ascorbic acid method: | ||||
| Automated | 365.1 Rev. 2.0 (1993) | 4500-P F-2021 or G-2021 | 973.56, 3 I-4601-85, 2 I-2601-90. 80 | ||
| Manual, single-reagent | 4500-P E-2021 | D515-88 (A) | 973.55. 3 | ||
| Manual, two-reagent | 365.3 (Issued 1978). 1 | ||||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30. 3 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508, Rev. 2. 54 | ||
| 45. Osmium—Total 4 , mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 252.2 (Issued 1978). 1 | ||||
| 46. Oxygen, dissolved, mg/L | Winkler (Azide modification) | 4500-O (B-F)-2021 | D888-18 (A) | 973.45B, 3 I-1575-78. 8 | |
| Electrode | 4500-O G-2021 | D888-18 (B) | I-1576-78. 8 | ||
| Luminescence-Based Sensor | 4500-O H-2021 | D888-18 (C) | See footnotes. | ||
| 47. Palladium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 253.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| DCP | See footnote. 34 | ||||
| 48. Phenols, mg/L | Manual distillation, 26 followed by any of the following: | 420.1 (Rev. 1978) 1 | 5530 B-2021 | D1783-01(12) | |
| Colorimetric (4AAP) manual | 420.1 (Rev. 1978) 1 | 5530 D-2021 27 | D1783-01(12) (A or B). | ||
| Automated colorimetric (4AAP) | 420.4 Rev. 1.0 (1993). | ||||
| 49. Phosphorus (elemental), mg/L | Gas-liquid chromatography | See footnote. 28 | |||
| 50. Phosphorus—Total, mg/L | Digestion, 20 followed by any of the following: | 4500-P B (5)-2021 | 973.55. 3 | ||
| Manual | 365.3 (Issued 1978) 1 | 4500-P E-2021 | D515-88 (A). | ||
| Automated ascorbic acid reduction | 365.1 Rev. 2.0 (1993) | 4500-P (F-H)-2021 | 973.56, 3 I-4600-85. 2 | ||
| ICP/AES 436 | 200.7Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| Semi-automated block digestor (TKP digestion) | 365.4 (Issued 1974) 1 | D515-88 (B) | I-4610-91. 48 | ||
| Digestion with persulfate, followed by Colorimetric | NCASI TNTP W10900. 77 | ||||
| 51. Platinum—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 255.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| DCP | See footnote. 34 | ||||
| 52. Potassium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | 973.5, 3 I-3630-85. 2 | |||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020. | |||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| Flame photometric | 3500-K B-2020. | ||||
| Electrode | 3500-K C-2020. | ||||
| Ion Chromatography | D6919-17. | ||||
| 53. Residue—Total, mg/L | Gravimetric, 103-105° | 2540 B-2020 | I-3750-85. 2 | ||
| 54. Residue—filterable, mg/L | Gravimetric, 180° | 2540 C-2020 | D5907-18 (B) | I-1750-85. 2 | |
| 55. Residue—non-filterable (TSS), mg/L | Gravimetric, 103-105° post-washing of residue | 2540 D-2020 | D5907-18 (A) | I-3765-85. 2 | |
| 56. Residue—settleable, mg/L | Volumetric (Imhoff cone), or gravimetric | 2540 F-2020. | |||
| 57. Residue—Volatile, mg/L | Gravimetric, 550° | 160.4 (Issued 1971) 1 | 2540 E-2020 | I-3753-85. 2 | |
| 58. Rhodium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration, or | 3111 B-2019. | ||||
| AA furnace | 265.2 (Issued 1978). 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 59. Ruthenium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration, or | 3111 B-2019. | ||||
| AA furnace | 267.2. 1 | ||||
| ICP/MS | 3125 B-2020. | ||||
| 60. Selenium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA furnace | 3113 B-2020 | D3859-15 (B) | I-4668-98. 49 | ||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES 36 | 200.5 Rev 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05 70 I-4472-97. 81 | |
| AA gaseous hydride | 3114 B-2020, or 3114 C-2020 | D3859-15 (A) | I-3667-85. 2 | ||
| 61. Silica—Dissolved, 37 mg/L | 0.45-micron filtration followed by any of the following: | ||||
| Colorimetric, Manual | 4500-SiO 2 C-2021 | D859-16 | I-1700-85. 2 | ||
| Automated (Molybdosilicate) | 4500-SiO 2 E-2021 or F-2021 | I-2700-85. 2 | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| 62. Silver—Total, 4 31 mg/L | Digestion, 4 29 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 or 3111 C-2019 | 974.27, 3 p. 37, 9 I-3720-85. 2 | |||
| AA furnace | 3113 B-2020 | I-4724-89. 51 | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4472-97. 81 | |
| DCP | See footnote. 34 | ||||
| 63. Sodium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | 973.54, 3 I-3735-85. 2 | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | I-4471-97. 50 | ||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| Flame photometric | 3500-Na B-2020. | ||||
| Ion Chromatography | D6919-17. | ||||
| 64. Specific conductance, micromhos/cm at 25 °C | Wheatstone bridge | 120.1 (Rev. 1982) 1 | 2510 B-2021 | D1125-95(99) (A) | 973.40, 3 I-2781-85. 2 |
| 65. Sulfate (as SO 4), mg/L | Automated colorimetric | 375.2 Rev. 2.0 (1993) | 4500-SO 42 F-2021 or G-2021. | ||
| Gravimetric | 4500-SO 42 C-2021 or D-2021 | 925.54. 3 | |||
| Turbidimetric | 4500-SO 42 E-2021 | D516-16. | |||
| Ion Chromatography | 300.0 Rev. 2.1 (1993) and 300.1 Rev. 1.0 (1997) | 4110 B-2020 or C-2020 | D4327-17 | 993.30, 3 I-4020-05. 70 | |
| CIE/UV | 4140 B-2020 | D6508-15 | D6508 Rev. 2. 54 | ||
| 66. Sulfide (as S), mg/L | Sample Pretreatment | 4500-S 2 B, C-2021. | |||
| Titrimetric (iodine) | 4500-S 2 F-2021 | I-3840-85. 2 | |||
| Colorimetric (methylene blue) | 4500-S 2 D-2021. | ||||
| Ion Selective Electrode | 4500-S 2 G-2021 | D4658-15. | |||
| 67. Sulfite (as SO 3), mg/L | Titrimetric (iodine-iodate) | 4500-SO 32 B-2021. | |||
| 68. Surfactants, mg/L | Colorimetric (methylene blue) | 5540 C-2021 | D2330-20. | ||
| 69. Temperature, °C | Thermometric | 2550 B-2010 | See footnote. 32 | ||
| 70. Thallium-Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019. | ||||
| AA furnace | 279.2 (Issued 1978) 1 | 3113 B-2020. | |||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20. | ||
| ICP/MS | 200.8, Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4471-97 50 I-4472-97. 81 | |
| 71. Tin—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 B-2019 | I-3850-78. 8 | |||
| AA furnace | 3113 B-2020. | ||||
| STGFAA | 200.9 Rev. 2.2 (1994). | ||||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994). | ||||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| 72. Titanium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 283.2 (Issued 1978). 1 | ||||
| ICP/AES | 200.7 Rev. 4.4 (1994). | ||||
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14. 3 | |
| DCP | See footnote. 34 | ||||
| 73. Turbidity, NTU 53 | Nephelometric | 180.1, Rev. 2.0 (1993) | 2130 B-2020 | D1889-00 | I-3860-85, 2 see footnotes. 656667 |
| 74. Vanadium—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration | 3111 D-2019. | ||||
| AA furnace | 3113 B-2020 | D3373-17. | |||
| ICP/AES | 200.5 Rev. 4.2 (2003), 68 200.7 Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05. 70 | |
| DCP | D4190-15 | See footnote. 34 | |||
| Colorimetric (Gallic Acid) | 3500-V B-2011. | ||||
| 75. Zinc—Total, 4 mg/L | Digestion, 4 followed by any of the following: | ||||
| AA direct aspiration 36 | 3111 B-2019 or 3111 C-2019 | D1691-17 (A or B) | 974.27 3 p. 37, 9 I-3900-85. 2 | ||
| AA furnace | 289.2 (Issued 1978). 1 | ||||
| ICP/AES 36 | 200.5 Rev. 4.2 (2003), 68 200.7, Rev. 4.4 (1994) | 3120 B-2020 | D1976-20 | I-4471-97. 50 | |
| ICP/MS | 200.8 Rev. 5.4 (1994) | 3125 B-2020 | D5673-16 | 993.14, 3 I-4020-05, 70 I-4472-97. 81 | |
| DCP 36 | D4190-15 | See footnote. 34 | |||
| Colorimetric (Zincon) | 3500 Zn B-2020 | See footnote. 33 | |||
| 76. Acid Mine Drainage | 1627. 69 | ||||
| Parameter 1 | Method | EPA 27 | Standard methods 17 | ASTM | Other |
| 1. Acenaphthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 2. Acenaphthylene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 3. Acrolein | GC | 603 | |||
| GC/MS | 624.1 4 , 1624B. | ||||
| 4. Acrylonitrile | GC | 603 | |||
| GC/MS | 624.1 4 , 1624B | O-4127-96. 13 | |||
| 5. Anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 6. Benzene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 7. Benzidine | Spectro-photometric | See footnote 3 p.1. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020. | |||
| HPLC | 605 | ||||
| 8. Benzo(a)anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 9. Benzo(a)pyrene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 10. Benzo(b)fluoranthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 11. Benzo(g,h,i)perylene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 12. Benzo(k)fluoranthene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 13. Benzyl chloride | GC | See footnote 3 p. 130. | |||
| GC/MS | See footnote 6 p. S102. | ||||
| 14. Butyl benzyl phthalate | GC | 606 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 15. bis(2-Chloroethoxy) methane | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 16. bis(2-Chloroethyl) ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 17. bis(2-Ethylhexyl) phthalate | GC | 606 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 18. Bromodichloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 19. Bromoform | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 20. Bromomethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 21. 4-Bromophenyl phenyl ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 22. Carbon tetrachloride | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 23. 4-Chloro-3-methyl phenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 24. Chlorobenzene | GC | 601, 602 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 O-4436-16. 14 | ||
| 25. Chloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96. 13 | ||
| 26. 2-Chloroethylvinyl ether | GC | 601 | |||
| GC/MS | 624.1, 1624B. | ||||
| 27. Chloroform | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 28. Chloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 29. 2-Chloronaphthalene | GC | 612 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 30. 2-Chlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 31. 4-Chlorophenyl phenyl ether | GC | 611 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 32. Chrysene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 33. Dibenzo(a,h)anthracene | GC | 610 | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 34. Dibromochloromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 35. 1,2-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 36. 1,3-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 37. 1,4-Dichlorobenzene | GC | 601, 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1625B | 6200 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 38. 3,3′-Dichlorobenzidine | GC/MS | 625.1, 1625B | 6410 B-2020. | ||
| HPLC | 605. | ||||
| 39. Dichlorodifluoromethane | GC | 601. | |||
| GC/MS | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | |||
| 40. 1,1-Dichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 41. 1,2-Dichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 42. 1,1-Dichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 43. trans -1,2-Dichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 44. 2,4-Dichlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 45. 1,2-Dichloropropane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 O-4436-16. 14 | ||
| 46. cis -1,3-Dichloropropene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 47. trans -1,3-Dichloropropene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 48. Diethyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 49. 2,4-Dimethylphenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 50. Dimethyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 51. Di- n -butyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 52. Di- n -octyl phthalate | GC | 606. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 53. 2, 4-Dinitrophenol | GC | 604 | 6420 B-2021 | See footnote 9 p. 27. | |
| GC/MS | 625.1, 1625B | 6410 B-2020. | |||
| 54. 2,4-Dinitrotoluene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 55. 2,6-Dinitrotoluene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 56. Epichlorohydrin | GC | See footnote 3 p. 130. | |||
| GC/MS | See footnote 6 p. S102. | ||||
| 57. Ethylbenzene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 58. Fluoranthene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 59. Fluorene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 60. 1,2,3,4,6,7,8-Heptachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 61. 1,2,3,4,7,8,9-Heptachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 62. 1,2,3,4,6,7,8- Heptachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 63. Hexachlorobenzene | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 64. Hexachlorobutadiene | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 65. Hexachlorocyclopentadiene | GC | 612. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 , p. 27, O-4127-96. 13 | ||
| 66. 1,2,3,4,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 67. 1,2,3,6,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 68. 1,2,3,7,8,9-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 69. 2,3,4,6,7,8-Hexachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 70. 1,2,3,4,7,8-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 71. 1,2,3,6,7,8-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 72. 1,2,3,7,8,9-Hexachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 73. Hexachloroethane | GC | 612. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96. 13 | ||
| 74. Indeno(1,2,3-c,d) pyrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 75. Isophorone | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 76. Methylene chloride | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 77. 2-Methyl-4,6-dinitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 78. Naphthalene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021. | |||
| 79. Nitrobenzene | GC | 609. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | D4657-92 (98). | ||||
| 80. 2-Nitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 81. 4-Nitrophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 82. N-Nitrosodimethylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 83. N-Nitrosodi- n -propylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 84. N-Nitrosodiphenylamine | GC | 607. | |||
| GC/MS | 625.1 5 , 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 85. Octachlorodibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 86. Octachlorodibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 87. 2,2′-oxybis(1-chloropropane) 12 [also known as bis(2-Chloro-1-methylethyl) ether] | GC | 611. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 88. PCB-1016 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 89. PCB-1221 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 90. PCB-1232 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 91. PCB-1242 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 92. PCB-1248 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 93. PCB-1254 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 94. PCB-1260 | GC | 608.3 | See footnote 3 p. 43, see footnote. 8 | ||
| GC/MS | 625.1 | 6410 B-2020. | |||
| 95. 1,2,3,7,8-Pentachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 96. 2,3,4,7,8-Pentachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 97. 1,2,3,7,8-Pentachloro-dibenzo- p -dioxin | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 98. Pentachlorophenol | GC | 604 | 6420 B-2021 | See footnote 3 p. 140. | |
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 99. Phenanthrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 100. Phenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 101. Pyrene | GC | 610. | |||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| HPLC | 610 | 6440 B-2021 | D4657-92 (98). | ||
| 102. 2,3,7,8-Tetrachloro-dibenzofuran | GC/MS | 1613B 10 | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 103. 2,3,7,8-Tetrachloro-dibenzo- p -dioxin | GC/MS | 613, 625.1 5 , 1613B | SGS AXYS 16130 15 , PAM 16130-SSI. 16 | ||
| 104. 1,1,2,2-Tetrachloroethane | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96. 13 | ||
| 105. Tetrachloroethene | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 106. Toluene | GC | 602 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 107. 1,2,4-Trichlorobenzene | GC | 612 | See footnote 3 p. 130. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27, O-4127-96 13 , O-4436-16. 14 | ||
| 108. 1,1,1-Trichloroethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 109. 1,1,2-Trichloroethane | GC | 601 | 6200 C-2020 | See footnote 3 p. 130. | |
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 110. Trichloroethene | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 111. Trichlorofluoromethane | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1 | 6200 B-2020 | O-4127-96. 13 | ||
| 112. 2,4,6-Trichlorophenol | GC | 604 | 6420 B-2021. | ||
| GC/MS | 625.1, 1625B | 6410 B-2020 | See footnote 9 p. 27. | ||
| 113. Vinyl chloride | GC | 601 | 6200 C-2020. | ||
| GC/MS | 624.1, 1624B | 6200 B-2020 | O-4127-96 13 , O-4436-16. 14 | ||
| 114. Nonylphenol | GC/MS | D7065-17. | |||
| 115. Bisphenol A (BPA) | GC/MS | D7065-17. | |||
| 116. p-tert -Octylphenol (OP) | GC/MS | D7065-17. | |||
| 117. Nonylphenol Monoethoxylate (NP1EO) | GC/MS | D7065-17. | |||
| 118. Nonylphenol Diethoxylate (NP2EO) | GC/MS | D7065-17. | |||
| 119. Adsorbable Organic Halides (AOX) | Adsorption and Coulometric Titration | 1650. 11 | |||
| 120. Chlorinated Phenolics | In Situ Acetylation and GC/MS | 1653. 11 | |||
| Table IC notes: |
| 1 All parameters are expressed in micrograms per liter (µg/L) except for Method 1613B, in which the parameters are expressed in picograms per liter (pg/L). |
| 2 The full text of Methods 601-613, 1613B, 1624B, and 1625B are provided at appendix A, Test Procedures for Analysis of Organic Pollutants. The standardized test procedure to be used to determine the method detection limit (MDL) for these test procedures is given at appendix B of this part, Definition and Procedure for the Determination of the Method Detection Limit. These methods are available at: https://www.epa.gov/cwa-methods as individual PDF files. |
| 3 Methods for Benzidine: Chlorinated Organic Compounds, Pentachlorophenol and Pesticides in Water and Wastewater. September 1978. U.S. EPA. |
| 4 Method 624.1 may be used for quantitative determination of acrolein and acrylonitrile, provided that the laboratory has documentation to substantiate the ability to detect and quantify these analytes at levels necessary to comply with any associated regulations. In addition, the use of sample introduction techniques other than simple purge-and-trap may be required. QC acceptance criteria from Method 603 should be used when analyzing samples for acrolein and acrylonitrile in the absence of such criteria in Method 624.1. |
| 5 Method 625.1 may be extended to include benzidine, hexachlorocyclopentadiene, N-nitrosodimethylamine, N-nitrosodi- n -propylamine, and N-nitrosodiphenylamine. However, when they are known to be present, Methods 605, 607, and 612, or Method 1625B, are preferred methods for these compounds. Method 625.1 may be applied to 2,3,7,8-Tetrachloro-dibenzo- p -dioxin for screening purposes only. |
| 6 Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. American Public Health Association (APHA). |
| 7 Each analyst must make an initial, one-time demonstration of their ability to generate acceptable precision and accuracy with Methods 601-603, 1624B, and 1625B in accordance with procedures in Section 8.2 of each of these methods. Additionally, each laboratory, on an on-going basis must spike and analyze 10% (5% for Methods 624.1 and 625.1 and 100% for methods 1624B and 1625B) of all samples to monitor and evaluate laboratory data quality in accordance with Sections 8.3 and 8.4 of these methods. When the recovery of any parameter falls outside the quality control (QC) acceptance criteria in the pertinent method, analytical results for that parameter in the unspiked sample are suspect. The results should be reported but cannot be used to demonstrate regulatory compliance. If the method does not contain QC acceptance criteria, control limits of ±three standard deviations around the mean of a minimum of five replicate measurements must be used. These quality control requirements also apply to the Standard Methods, ASTM Methods, and other methods cited. |
| 8 Organochlorine Pesticides and PCBs in Wastewater Using Empore TM Disk. Revised October 28, 1994. 3M Corporation. |
| 9 Method O-3116-87 is in Open File Report 93-125, Methods of Analysis by U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. USGS. |
| 10 Analysts may use Fluid Management Systems, Inc. Power-Prep system in place of manual cleanup provided the analyst meets the requirements of Method 1613B (as specified in Section 9 of the method) and permitting authorities. Method 1613, Revision B, Tetra- through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS. Revision B, 1994. U.S. EPA. The full text of this method is provided in appendix A to this part and at https://www.epa.gov/cwa-methods/approved-cwa-test-methods-organic-compounds. |
| 11 Method 1650, Adsorbable Organic Halides by Adsorption and Coulometric Titration. Revision C, 1997 U.S. EPA. Method 1653, Chlorinated Phenolics in Wastewater by In Situ Acetylation and GCMS. Revision A, 1997 U.S. EPA. The full text for both of these methods is provided at appendix A in part 430 of this chapter, The Pulp, Paper, and Paperboard Point Source Category. |
| 12 The compound was formerly inaccurately labeled as 2,2′-oxybis(2-chloropropane) and bis(2-chloroisopropyl) ether. Some versions of Methods 611, and 1625 inaccurately list the analyte as “bis(2-chloroisopropyl) ether,” but use the correct CAS number of 108-60-1. |
| 13 Method O-4127-96, U.S. Geological Survey Open-File Report 97-829, Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of 86 volatile organic compounds in water by gas chromatography/mass spectrometry, including detections less than reporting limits,1998, USGS. |
| 14 Method O-4436-16 U.S. Geological Survey Techniques and Methods, book 5, chap. B12, Determination of heat purgeable and ambient purgeable volatile organic compounds in water by gas chromatography/mass spectrometry, 2016, USGS. |
| 15 SGS AXYS Method 16130, “Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Waters and Agilent Gas Chromatography-Tandem-Mass Spectrometry (GC/MS/MS), Revision 1.0” is available at: https://www.sgsaxys.com/wp-content/uploads/2022/09/SGS-AXYS-Method-16130-Rev-1.0.pdf. |
| 16 Pace Analytical Method PAM-16130-SSI, “Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Shimadzu Gas Chromatography Mass Spectrometry (GC-MS/MS), Revision 1.1,” is available at: pacelabs.com. |
| 17 Please refer to the following applicable Quality Control Section: Part 6000 Individual Organic Compounds, 6020 (2019). The Quality Control Standards are available for download at standardmethods.org at no charge. |
| Parameter | Method | EPA | Standard methods 15 | ASTM | Other |
| 1. Aldrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96 (02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 2. Ametryn | GC | 507, 619 | See footnote 3 p. 83, see footnote 9 O-3106-93, see footnote 6 p. S68. | ||
| GC/MS | 525.2, 625.1 | See footnote 14 O-1121-91. | |||
| 3. Aminocarb | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| 4. Atraton | GC | 619 | See footnote 3 p. 83, see footnote 6 p. S68. | ||
| GC/MS | 625.1. | ||||
| 5. Atrazine | GC | 507, 619, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 6. Azinphos methyl | GC | 614, 622, 1657 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC-MS | 625.1 | See footnote 11 O-1126-95. | |||
| 7. Barban | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 8. α-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 9. β-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 10. δ-BHC | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 11. γ-BHC (Lindane) | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 , O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 11 , O-1126-95. | ||
| 12. Captan | GC | 617, 608.3 | 6630 B-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7. |
| 13. Carbaryl | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 531.1, 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 14. Carbophenothion | GC | 617, 608.3 | 6630 B-2021 | See footnote 4 page 27, see footnote 6 p. S73. | |
| GC/MS | 625.1. | ||||
| 15. Chlordane | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 16. Chloropropham | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 17. 2,4-D | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 18. 4,4′-DDD | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3105-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 19. 4,4′-DDE | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 , O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 20. 4,4′-DDT | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 21. Demeton-O | GC | 614, 622 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 625.1 | ||||
| 22. Demeton-S. | GC | 614, 622 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 625.1. | ||||
| 23. Diazinon | GC | 507, 614, 622, 1657 | See footnote 3 p. 25, see footnote 4 O-3104-83, see footnote 6 p. S51. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 24. Dicamba | GC | 615 | See footnote 3 p. 115. | ||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 25. Dichlofenthion | GC | 622.1 | See footnote 4 page 27, see footnote 6 p. S73. | ||
| 26. Dichloran | GC | 608.2, 617, 608.3 | 6630 B-2021 | See footnote 3 p. 7. | |
| 27. Dicofol | GC | 617, 608.3 | See footnote 4 O-3104-83. | ||
| 28. Dieldrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020 | See footnote 11 O-1126-95. | ||
| 29. Dioxathion | GC | 614.1, 1657 | See footnote 4 page 27, see footnote 6 p. S73. | ||
| 30. Disulfoton | GC | 507, 614, 622, 1657 | See footnote 3 p. 25, see footnote 6 p. S51. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 31. Diuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| 32. Endosulfan I | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 13 O-2002-01. | ||
| 33. Endosulfan II | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 3M0222. |
| GC/MS | 625.1 5 | 6410 B-2020 | See footnote 13 O-2002-01. | ||
| 34. Endosulfan Sulfate | GC | 617, 608.3 | 6630 C-2021 | See footnote 8 3M0222. | |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 35. Endrin | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 5 | 6410 B-2020. | |||
| 36. Endrin aldehyde | GC | 617, 608.3 | 6630 C-2021 | See footnote 8 3M0222. | |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 37. Ethion | GC | 614, 614.1, 1657 | See footnote 4 page 27, see footnote 6 , p. S73. | ||
| GC/MS | 625.1 | See footnote 13 O-2002-01. | |||
| 38. Fenuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 39. Fenuron-TCA | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 40. Heptachlor | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 | 6410 B-2020. | |||
| 41. Heptachlor epoxide | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 6 p. S73, see footnote 8 3M0222. |
| GC/MS | 625.1 | 6410 B-2020. | |||
| 42. Isodrin | GC | 617, 608.3 | 6630 B-2021 & C-2021 | See footnote 4 O-3104-83, see footnote 6 p. S73. | |
| GC/MS | 625.1. | ||||
| 43. Linuron | GC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | 553 | See footnote 12 O-2060-01. | |||
| GC/MS | See footnote 11 O-1126-95. | ||||
| 44. Malathion | GC | 614, 1657 | 6630 B-2021 | See footnote 3 p. 25, see footnote 6 p. S51. | |
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 45. Methiocarb | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 46. Methoxychlor | GC | 505, 508, 608.2, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83, see footnote 8 3M0222. |
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 47. Mexacarbate | TLC | See footnote 3 p. 94, see footnote 6 p. S60. | |||
| HPLC | 632. | ||||
| GC/MS | 625.1. | ||||
| 48. Mirex | GC | 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 4 O-3104-83. |
| GC/MS | 625.1. | ||||
| 49. Monuron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 50. Monuron-TCA | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 51. Neburon | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 52. Parathion methyl | GC | 614, 622, 1657 | 6630 B-2021 | See footnote 4 page 27, see footnote 3 p. 25. | |
| GC/MS | 625.1 | See footnote 11 O-1126-95. | |||
| 53. Parathion ethyl | GC | 614 | 6630 B-2021 | See footnote 4 page 27, see footnote 3 p. 25. | |
| GC/MS | See footnote 11 O-1126-95. | ||||
| 54. PCNB | GC | 608.1, 617, 608.3 | 6630 B-2021 & C-2021 | D3086-90 , D5812-96(02) | See footnote 3 p. 7. |
| 55. Perthane | GC | 617, 608.3 | D3086-90, D5812-96(02) | See footnote 4 O-3104-83. | |
| 56. Prometon | GC | 507, 619 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 57. Prometryn | GC | 507, 619 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 13 O-2002-01. | |||
| 58. Propazine | GC | 507, 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | ||||
| 59. Propham | TLC | See footnote 3 p. 10, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 60. Propoxur | TLC | See footnote 3 p. 94, see footnote 6 , p. S60. | |||
| HPLC | 632. | ||||
| 61. Secbumeton | TLC | See footnote 3 p. 83, see footnote 6 p. S68. | |||
| GC | 619. | ||||
| 62. Siduron | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| HPLC/MS | See footnote 12 O-2060-01. | ||||
| 63. Simazine | GC | 505, 507, 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68, see footnote 9 O-3106-93. | ||
| GC/MS | 525.1, 525.2, 625.1 | See footnote 11 O-1126-95. | |||
| 64. Strobane | GC | 617, 608.3 | 6630 B-2021 & C-2021 | See footnote 3 p. 7. | |
| 65. Swep | TLC | See footnote 3 p. 104, see footnote 6 p. S64. | |||
| HPLC | 632. | ||||
| 66. 2,4,5-T | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| 67. 2,4,5-TP (Silvex) | GC | 615 | 6640 B-2021 | See footnote 3 p. 115, see footnote 4 O-3105-83. | |
| 68. Terbuthylazine | GC | 619, 1656, 608.3 | See footnote 3 p. 83, see footnote 6 p. S68. | ||
| GC/MS | See footnote 13 O-2002-01. | ||||
| 69. Toxaphene | GC | 505, 508, 617, 1656, 608.3 | 6630 B-2021 & C-2021 | D3086-90, D5812-96(02) | See footnote 3 p. 7, see footnote 8 , see footnote 4 O-3105-83. |
| GC/MS | 525.1, 525.2, 625.1 | 6410 B-2020. | |||
| 70. Trifluralin | GC | 508, 617, 627, 1656, 608.3 | 6630 B-2021 | See footnote 3 p. 7, see footnote 9 O-3106-93. | |
| GC/MS | 525.2, 625.1 | See footnote 11 O-1126-95. |
| Table ID notes: |
| 1 Pesticides are listed in this table by common name for the convenience of the reader. Additional pesticides may be found under table IC of this section, where entries are listed by chemical name. |
| 2 The standardized test procedure to be used to determine the method detection limit (MDL) for these test procedures is given at appendix B to this part, Definition and Procedure for the Determination of the Method Detection Limit. |
| 3 Methods for Benzidine, Chlorinated Organic Compounds, Pentachlorophenol and Pesticides in Water and Wastewater. September 1978. U.S. EPA. This EPA publication includes thin-layer chromatography (TLC) methods. |
| 4 Methods for the Determination of Organic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3. 1987. USGS. |
| 5 The method may be extended to include α-BHC, γ-BHC, endosulfan I, endosulfan II, and endrin. However, when they are known to exist, Method 608 is the preferred method. |
| 6 Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. American Public Health Association (APHA). |
| 7 Each analyst must make an initial, one-time, demonstration of their ability to generate acceptable precision and accuracy with Methods 608.3 and 625.1 in accordance with procedures given in Section 8.2 of each of these methods. Additionally, each laboratory, on an on-going basis, must spike and analyze 10% of all samples analyzed with Method 608.3 or 5% of all samples analyzed with Method 625.1 to monitor and evaluate laboratory data quality in accordance with Sections 8.3 and 8.4 of these methods. When the recovery of any parameter falls outside the warning limits, the analytical results for that parameter in the unspiked sample are suspect. The results should be reported, but cannot be used to demonstrate regulatory compliance. These quality control requirements also apply to the Standard Methods, ASTM Methods, and other methods cited. |
| 8 Organochlorine Pesticides and PCBs in Wastewater Using Empore TM Disk. Revised October 28, 1994. 3M Corporation. |
| 9 Method O-3106-93 is in Open File Report 94-37, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Triazine and Other Nitrogen-Containing Compounds by Gas Chromatography with Nitrogen Phosphorus Detectors. 1994. USGS. |
| 10 EPA Methods 608.1, 608.2, 614, 614.1, 615, 617, 619, 622, 622.1, 627, and 632 are found in Methods for the Determination of Nonconventional Pesticides in Municipal and Industrial Wastewater, EPA 821-R-92-002, April 1992, U.S. EPA. EPA Methods 505, 507, 508, 525.1, 531.1 and 553 are in Methods for the Determination of Nonconventional Pesticides in Municipal and Industrial Wastewater, Volume II, EPA 821-R-93-010B, 1993, U.S. EPA. EPA Method 525.2 is in Determination of Organic Compounds in Drinking Water by Liquid-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry, Revision 2.0, 1995, U.S. EPA. EPA methods 1656 and 1657 are in Methods for The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume I, EPA 821-R-93-010A, 1993, U.S. EPA. Methods 608.3 and 625.1 are available at: cwa-methods/approved-cwa-test-methods-organic-compounds. |
| 11 Method O-1126-95 is in Open-File Report 95-181, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring. 1995. USGS. |
| 12 Method O-2060-01 is in Water-Resources Investigations Report 01-4134, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by Graphitized Carbon-Based Solid-Phase Extraction and High-Performance Liquid Chromatography/Mass Spectrometry. 2001. USGS. |
| 13 Method O-2002-01 is in Water-Resources Investigations Report 01-4098, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of moderate-use pesticides in water by C-18 solid-phase extraction and capillary-column gas chromatography/mass spectrometry. 2001. USGS. |
| 14 Method O-1121-91 is in Open-File Report 91-519, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of organonitrogen herbicides in water by solid-phase extraction and capillary-column gas chromatography/mass spectrometry with selected-ion monitoring. 1992. USGS. |
| 15 Please refer to the following applicable Quality Control Section: Part 6000 Methods, Individual Organic Compounds 6020 (2019). These Quality Control Standards are available for download at www.standardmethods.org at no charge. |
* * * * *
| Parameter and units | Method 1 | EPA | Standard methods | AOAC, ASTM, USGS | Other |
| Bacteria | |||||
| 1. Coliform (fecal), number per 100 mL | Most Probable Number (MPN), 5 tube, 3 dilution, or | p. 132 3 | 9221 E-2014, 9221 F-2014. 32 | ||
| Membrane filter (MF) 2 , single step | p. 124 3 | 9222 D-2015 26 | B-0050-85. 4 | ||
| 2. Coliform (total), number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 114 3 | 9221 B-2014. | ||
| MF 2 , single step or | p. 108 3 | 9222 B-2015 27 | B-0025-85. 4 | ||
| MF 2 , two step with enrichment | p. 111 3 | 9222 B-2015. 27 | |||
| 3. E. coli, number per 100 mL | MPN 5713 , multiple tube, or | 9221 B.3-2014/9221 F-2014. 101232 | |||
| Multiple tube/multiple well, or | 9223 B-2016 11 | 991.15 9 | Colilert® 1115 , Colilert-18®. 111415 | ||
| MF 2567 , two step, or | 1103.2 18 | 9222 B-2015/9222 I-2015 17 , 9213 D-2007 | D5392-93. 8 | ||
| Single step | 1603.1 19 , 1604 20 | m-ColiBlue24® 16 , KwikCount EC. 2829 | |||
| 4. Fecal streptococci, number per 100 mL | MPN, 5 tube, 3 dilution, or | p. 139 3 | 9230 B-2013. | ||
| MF 2 , or | p. 136 3 | 9230 C-2013 30 | B-0055-85. 4 | ||
| Plate count | p. 143. 3 | ||||
| 5. Enterococci, number per 100 mL | MPN 57 , multiple tube/multiple well, or | 9230 D-2013 | D6503-99 8 | Enterolert®. 1121 | |
| MF 2567 two step, or | 1106.2 22 | 9230 C-2013 30 | D5259-92. 8 | ||
| Single step, or | 1600.1 23 | 9230 C-2013. 30 | |||
| Plate count | p. 143. 3 | ||||
| Protozoa | |||||
| 6. Cryptosporidium | Filtration/IMS/FA | 1622 24 , 1623 25 , 1623.1. 2531 | |||
| 7. Giardia | Filtration/IMS/FA | 1623 25 , 1623.1. 2531 | |||
| Table 1H notes: |
| 1 The method must be specified when results are reported. |
| 2 A 0.45-µm membrane filter (MF) or other pore size certified by the manufacturer to fully retain organisms to be cultivated and to be free of extractables which could interfere with their growth. |
| 3 Microbiological Methods for Monitoring the Environment, Water and Wastes. EPA/600/8-78/017. 1978. US EPA. |
| 4 U.S. Geological Survey Techniques of Water-Resource Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. USGS. |
| 5 Tests must be conducted to provide organism enumeration (density). Select the appropriate configuration of tubes/filtrations and dilutions/volumes to account for the quality, character, consistency, and anticipated organism density of the water sample. |
| 6 When the MF method has not been used previously to test waters with high turbidity, large numbers of noncoliform bacteria, or samples that may contain organisms stressed by chlorine, a parallel test should be conducted with a multiple-tube technique to demonstrate applicability and comparability of results. |
| 7 To assess the comparability of results obtained with individual methods, it is suggested that side-by-side tests be conducted across seasons of the year with the water samples routinely tested in accordance with the most current Standard Methods for the Examination of Water and Wastewater or EPA alternate test procedure (ATP) guidelines. |
| 8 Annual Book of ASTM Standards—Water and Environmental Technology. Section 11.02. 2000, 1999, 1996. ASTM International. |
| 9 Official Methods of Analysis of AOAC International, 16th Edition, Volume I, Chapter 17. 1995. AOAC International. |
| 10 The multiple-tube fermentation test is used in 9221B.3-2014. Lactose broth may be used in lieu of lauryl tryptose broth (LTB), if at least 25 parallel tests are conducted between this broth and LTB using the water samples normally tested, and this comparison demonstrates that the false-positive rate and false-negative rate for total coliform using lactose broth is less than 10 percent. No requirement exists to run the completed phase on 10 percent of all total coliform-positive tubes on a seasonal basis. |
| 11 These tests are collectively known as defined enzyme substrate tests. |
| 12 After prior enrichment in a presumptive medium for total coliform using 9221B.3-2014, all presumptive tubes or bottles showing any amount of gas, growth or acidity within 48 h ± 3 h of incubation shall be submitted to 9221F-2014. Commercially available EC-MUG media or EC media supplemented in the laboratory with 50 µg/mL of MUG may be used. |
| 13 Samples shall be enumerated by the multiple-tube or multiple-well procedure. Using multiple-tube procedures, employ an appropriate tube and dilution configuration of the sample as needed and report the Most Probable Number (MPN). Samples tested with Colilert® may be enumerated with the multiple-well procedures, Quanti-Tray® or Quanti-Tray®/2000, and the MPN calculated from the table provided by the manufacturer. |
| 14 Colilert-18® is an optimized formulation of the Colilert® for the determination of total coliforms and E. coli that provides results within 18 h of incubation at 35 °C, rather than the 24 h required for the Colilert® test and is recommended for marine water samples. |
| 15 Descriptions of the Colilert®, Colilert-18®, Quanti-Tray ®, and Quanti-Tray®/2000 may be obtained from IDEXX Laboratories Inc. |
| 16 A description of the mColiBlue24® test may be obtained from Hach Company. |
| 17 Subject coliform positive samples determined by 9222B-2015 or other membrane filter procedure to 9222I-2015 using NA-MUG media. |
| 18 Method 1103.2: Escherichia coli ( E. coli) in Water by Membrane Filtration Using membrane-Thermotolerant Escherichia coli Agar (mTEC), EPA-821-R-23-009. September 2023. US EPA. |
| 19 Method 1603.1: Escherichia coli ( E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008. September 2023 . US EPA. |
| 20 Method 1604: Total Coliforms and Escherichia coli ( E. coli) in Water by Membrane Filtration by Using a Simultaneous Detection Technique (MI Medium), EPA 821-R-02-024. September 2002. US EPA. |
| 21 A description of the Enterolert® test may be obtained from IDEXX Laboratories Inc. |
| 22 Method 1106.2: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus -Esculin Iron Agar (mE-EIA), EPA-821-R-23-007. September 2023. US EPA. |
| 23 Method 1600.1: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-21-006. September 2023. US EPA. |
| 24 Method 1622 uses a filtration, concentration, immunomagnetic separation of oocysts from captured material, immunofluorescence assay to determine concentrations, and confirmation through vital dye staining and differential interference contrast microscopy for the detection of Cryptosporidium. Method 1622: Cryptosporidium in Water by Filtration/IMS/FA, EPA-821-R-05-001. December 2005. US EPA. |
| 25 Methods 1623 and 1623.1 use a filtration, concentration, immunomagnetic separation of oocysts and cysts from captured material, immunofluorescence assay to determine concentrations, and confirmation through vital dye staining and differential interference contrast microscopy for the simultaneous detection of Cryptosporidium and Giardia oocysts and cysts. Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA-821-R-05-002. December 2005. US EPA. Method 1623.1: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA 816-R-12-001. January 2012. US EPA. |
| 26 On a monthly basis, at least ten blue colonies from positive samples must be verified using Lauryl Tryptose Broth and EC broth, followed by count adjustment based on these results; and representative non-blue colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. |
| 27 On a monthly basis, at least ten sheen colonies from positive samples must be verified using Lauryl Tryptose Broth and brilliant green lactose bile broth, followed by count adjustment based on these results; and representative non-sheen colonies should be verified using Lauryl Tryptose Broth. Where possible, verifications should be done from randomized sample sources. |
| 28 A description of KwikCount EC may be obtained from Roth Bioscience, LLC. |
| 29 Approved for the analyses of E. coli in freshwater only. |
| 30 Verification of colonies by incubation of BHI agar at 10 ± 0.5 °C for 48 ± 3 h is optional. As per the Errata to the 23rd Edition of Standard Methods for the Examination of Water and Wastewater “Growth on a BHI agar plate incubated at 10 ± 0.5 °C for 48 ± 3 h is further verification that the colony belongs to the genus Enterococcus.” |
| 31 Method 1623.1 includes updated acceptance criteria for IPR, OPR, and MS/MSD and clarifications and revisions based on the use of Method 1623 for years and technical support questions. |
| 32 9221 F.2-2014 allows for simultaneous detection of E. coli and thermotolerant fecal coliforms by adding inverted vials to EC-MUG; the inverted vials collect gas produced by thermotolerant fecal coliforms. |
* * * *
(b) The material listed in this paragraph (b) is incorporated by reference into this section with the approval of the Director of the Federal Register under 5 U.S.C. 552(a) and 1 CFR part 51. All approved incorporation by reference (IBR) material is available for inspection at the EPA and at the National Archives and Records Administration (NARA). Contact the EPA at: EPA's Water Docket, EPA West, 1301 Constitution Avenue NW, Room 3334, Washington, DC 20004; telephone: 202-566-2426; email: docket-customerservice@epa.gov. For information on the availability of this material at NARA, visit www.archives.gov/federal-register/cfr/ibr-locations or email fr.inspection@nara.gov. The material may be obtained from the following sources in this paragraph (b).
(1) Environmental Monitoring and Support Laboratory, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from: National Technical Information Service, 5285 Port Royal Road, Springfield, Virginia 22161
(i) Microbiological Methods for Monitoring the Environment, Water, and Wastes. 1978. EPA/600/8-78/017, Pub. No. PB-290329/A.S.
(A) Part III Analytical Methodology, Section B Total Coliform Methods, page 108. Table IA, Note 3; Table IH, Note 3.
(B) Part III Analytical Methodology, Section B Total Coliform Methods, 2.6.2 Two-Step Enrichment Procedure, page 111. Table IA, Note 3; Table IH, Note 3.
(C) Part III Analytical Methodology, Section B Total Coliform Methods, 4 Most Probable Number (MPN) Method, page 114. Table IA, Note 3; Table IH, Note 3.
(D) Part III Analytical Methodology, Section C Fecal Coliform Methods, 2 Direct Membrane Filter (MF) Method, page 124. Table IA, Note 3; Table IH, Note 3.
(E) Part III, Analytical Methodology, Section C Fecal Coliform Methods, 5 Most Probable Number (MPN) Method, page 132. Table IA, Note 3; Table IH, Note 3.
(F) Part III Analytical Methodology, Section D Fecal Streptococci, 2 Membrane Filter (MF) Method, page 136. Table IA, Note 3; Table IH, Note 3.
(G) Part III Analytical Methodology, Section D Fecal Streptococci, 4 Most Probable Number Method, page 139. Table IA, Note 3; Table IH, Note 3.
(H) Part III Analytical Methodology, Section D Fecal Streptococci, 5 Pour Plate Method, page 143. Table IA, Note 3; Table IH, Note 3.
(ii) [Reserved]
(2) Environmental Monitoring and Support Laboratory, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(3) National Exposure Risk Laboratory-Cincinnati, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available from http://water.epa.gov/scitech/methods/cwa/index.cfm or from the National Technical Information Service (NTIS), 5285 Port Royal Road, Springfield, VA 22161. Telephone: 800-553-6847.
(i) Methods for the Determination of Inorganic Substances in Environmental Samples. August 1993. EPA/600/R-93/100, Pub. No. PB 94120821. Table IB, Note 52.
(A) Method 180.1, Determination of Turbidity by Nephelometry. Revision 2.0. Table IB, Note 52.
(B) Method 300.0, Determination of Inorganic Anions by Ion Chromatography. Revision 2.1. Table IB, Note 52.
(C) Method 335.4, Determination of Total Cyanide by Semi-Automated Colorimetry. Revision 1.0. Table IB, Notes 52 and 57.
(D) Method 350.1, Determination of Ammonium Nitrogen by Semi-Automated Colorimetry. Revision 2.0. Table IB, Notes 30 and 52.
(E) Method 351.2, Determination of Total Kjeldahl Nitrogen by Semi-Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(F) Method 353.2, Determination of Nitrate-Nitrite Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(G) Method 365.1, Determination of Phosphorus by Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(H) Method 375.2, Determination of Sulfate by Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(I) Method 410.4, Determination of Chemical Oxygen Demand by Semi-Automated Colorimetry. Revision 2.0. Table IB, Note 52.
(ii) Methods for the Determination of Metals in Environmental Samples, Supplement I. May 1994. EPA/600/R-94/111, Pub. No. PB 95125472. Table IB, Note 52.
(A) Method 200.7, Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 4.4. Table IB, Note 52.
(B) Method 200.8, Determination of Trace Elements in Water and Wastes by Inductively Coupled Plasma Mass Spectrometry. Revision 5.3. Table IB, Note 52.
(C) Method 200.9, Determination of Trace Elements by Stabilized Temperature Graphite Furnace Atomic Absorption Spectrometry. Revision 2.2. Table IB, Note 52.
(D) Method 218.6, Determination of Dissolved Hexavalent Chromium in Drinking Water, Groundwater, and Industrial Wastewater Effluents by Ion Chromatography. Revision 3.3. Table IB, Note 52.
(E) Method 245.1, Determination of Mercury in Water by Cold Vapor Atomic Absorption Spectrometry. Revision 3.0. Table IB, Note 52.
(4) National Exposure Risk Laboratory-Cincinnati, U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(i) EPA Method 200.5, Determination of Trace Elements in Drinking Water by Axially Viewed Inductively Coupled Plasma-Atomic Emission Spectrometry. Revision 4.2, October 2003. EPA/600/R-06/115. Table IB, Note 68.
(ii) EPA Method 525.2, Determination of Organic Compounds in Drinking Water by Liquid-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry. Revision 2.0, 1995. Table ID, Note 10.
(5) Office of Research and Development, Cincinnati OH. U.S. Environmental Protection Agency, Cincinnati OH (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from ORD Publications, CERI, U.S. Environmental Protection Agency, Cincinnati OH 45268.
(i) Methods for Benzidine, Chlorinated Organic Compounds, Pentachlorophenol, and Pesticides in Water and Wastewater. 1978. Table IC, Note 3; Table ID, Note 3.
(ii) Methods for Chemical Analysis of Water and Wastes. March 1979. EPA-600/4-79-020. Table IB, Note 1.
(iii) Methods for Chemical Analysis of Water and Wastes. Revised March 1983. EPA-600/4-79-020. Table IB, Note 1.
(A) Method 120.1, Conductance, Specific Conductance, μmhos at 25°C. Revision 1982. Table IB, Note 1.
(B) Method 130.1, Hardness, Total (mg/L as CaCO3), Colorimetric, Automated EDTA. Issued 1971. Table IB, Note 1.
(C) Method 150.2, pH, Continuous Monitoring (Electrometric). December 1982. Table IB, Note 1.
(D) Method 160.4, Residue, Volatile, Gravimetric, Ignition at 550°C. Issued 1971. Table IB, Note 1.
(E) Method 206.5, Arsenic, Sample Digestion Prior to Total Arsenic Analysis by Silver Diethyldithiocarbamate or Hydride Procedures. Issued 1978. Table IB, Note 1.
(F) Method 231.2, Gold, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(G) Method 245.2, Mercury, Automated Cold Vapor Technique. Issued 1974. Table IB, Note 1.
(H) Method 252.2, Osmium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(I) Method 253.2, Palladium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(J) Method 255.2, Platinum, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(K) Method 265.2, Rhodium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(L) Method 279.2, Thallium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(M) Method 283.2, Titanium, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(N) Method 289.2, Zinc, Atomic Absorption, Furnace Technique. Issued 1978. Table IB, Note 1.
(O) Method 310.2, Alkalinity, Colorimetric, Automated, Methyl Orange. Revision 1974. Table IB, Note 1.
(P) Method 351.1, Nitrogen, Kjeldahl, Total, Colorimetric, Automated Phenate. Revision 1978. Table IB, Note 1.
(Q) Method 352.1, Nitrogen, Nitrate, Colorimetric, Brucine. Issued 1971. Table IB, Note 1.
(R) Method 365.3, Phosphorus, All Forms, Colorimetric, Ascorbic Acid, Two Reagent. Issued 1978. Table IB, Note 1.
(S) Method 365.4, Phosphorus, Total, Colorimetric, Automated, Block Digestor AA II. Issued 1974. Table IB, Note 1.
(T) Method 410.3, Chemical Oxygen Demand, Titrimetric, High Level for Saline Waters. Revision 1978. Table IB, Note 1.
(U) Method 420.1, Phenolics, Total Recoverable, Spectrophotometric, Manual 4-AAP With Distillation. Revision 1978. Table IB, Note 1.
(iv) Prescribed Procedures for Measurement of Radioactivity in Drinking Water. 1980. EPA-600/4-80-032. Table IE.
(A) Method 900.0, Gross Alpha and Gross Beta Radioactivity. Table IE.
(B) Method 903.0, Alpha-Emitting iRadio Isotopes. Table IE.
(C) Method 903.1, Radium-226, Radon Emanation Technique. Table IE.
(D) Appendix B, Error and Statistical Calculations. Table IE.
(6) Office of Science and Technology, U.S. Environmental Protection Agency, Washington DC (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm.
(i) Method 1625C, Semivolatile Organic Compounds by Isotope Dilution GCMS. 1989. Table IF.
(ii) [Reserved]
(7) Office of Water, U.S. Environmental Protection Agency, Washington DC (US EPA). Available at http://water.epa.gov/scitech/methods/cwa/index.cfm or from National Technical Information Service, 5285 Port Royal Road, Springfield, Virginia 22161.
(i) Method 1631, Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. Revision E, August 2002. EPA-821-R-02-019, Pub. No. PB2002-108220. Table IB, Note 43.
(ii) Kelada-01, Kelada Automated Test Methods for Total Cyanide, Acid Dissociable Cyanide, and Thiocyanate. Revision 1.2, August 2001. EPA 821-B-01-009, Pub. No. PB 2001-108275. Table IB, Note 55.
(iii) In the compendium Analytical Methods for the Determination of Pollutants in Pharmaceutical Manufacturing Industry Wastewaters. July 1998. EPA 821-B-98-016, Pub. No. PB95201679. Table IF, Note 1.
(A) EPA Method 1666, Volatile Organic Compounds Specific to the Pharmaceutical Industry by Isotope Dilution GC/MS. Table IF, Note 1.
(B) EPA Method 1667, Formaldehyde, Isobutyraldehyde, and Furfural by Derivatization Followed by High Performance Liquid Chromatography. Table IF.
(C) Method 1671, Volatile Organic Compounds Specific to the Pharmaceutical Manufacturing Industry by GC/FID. Table IF.
(iv) Methods For The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume I. Revision I, August 1993. EPA 821-R-93-010A, Pub. No. PB 94121654. Tables ID, IG.
(A) Method 608.1, Organochlorine Pesticides. Table ID, Note 10; Table IG, Note 3.
(B) Method 608.2, Certain Organochlorine Pesticides. Table ID, Note 10; Table IG, Note 3.
(C) Method 614, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(D) Method 614.1, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(E) Method 615, Chlorinated Herbicides. Table ID, Note 10; Table IG, Note 3.
(F) Method 617, Organohalide Pesticides and PCBs. Table ID, Note 10; Table IG, Note 3.
(G) Method 619, Triazine Pesticides. Table ID, Note 10; Table IG, Note 3.
(H) Method 622, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(I) Method 622.1, Thiophosphate Pesticides. Table ID, Note 10; Table IG, Note 3.
(J) Method 627, Dinitroaniline Pesticides. Table ID, Note 10; Table IG, Notes 1 and 3.
(K) Method 629, Cyanazine. Table IG, Note 3.
(L) Method 630, Dithiocarbamate Pesticides. Table IG, Note 3.
(M) Method 630.1, Dithiocarbamate Pesticides. Table IG, Note 3.
(N) Method 631, Benomyl and Carbendazim. Table IG, Note 3.
(O) Method 632, Carbamate and Urea Pesticides. Table ID, Note 10; Table IG, Note 3.
(P) Method 632.1, Carbamate and Amide Pesticides. Table IG, Note 3.
(Q) Method 633, Organonitrogen Pesticides. Table IG, Note 3.
(R) Method 633.1, Neutral Nitrogen-Containing Pesticides. Table IG, Note 3.
(S) Method 637, MBTS and TCMTB. Table IG, Note 3.
(T) Method 644, Picloram. Table IG, Note 3.
(U) Method 645, Certain Amine Pesticides and Lethane. Table IG, Note 3.
(V) Method 1656, Organohalide Pesticides. Table ID, Note 10; Table IG, Notes 1 and 3.
(W) Method 1657, Organophosphorus Pesticides. Table ID, Note 10; Table IG, Note 3.
(X) Method 1658, Phenoxy-Acid Herbicides. Table IG, Note 3.
(Y) Method 1659, Dazomet. Table IG, Note 3.
(Z) Method 1660, Pyrethrins and Pyrethroids. Table IG, Note 3.
(AA) Method 1661, Bromoxynil. Table IG, Note 3.
(BB) Ind-01. Methods EV-024 and EV-025, Analytical Procedures for Determining Total Tin and Triorganotin in Wastewater. Table IG, Note 3.
(v) Methods For The Determination of Nonconventional Pesticides In Municipal and Industrial Wastewater, Volume II. August 1993. EPA 821-R-93-010B, Pub. No. PB 94166311. Table IG.
(A) Method 200.9, Determination of Trace Elements by Stabilized Temperature Graphite Furnace Atomic Absorption Spectrometry. Table IG, Note 3.
(B) Method 505, Analysis of Organohalide Pesticides and Commercial Polychlorinated Biphenyl (PCB) Products in Water by Microextraction and Gas Chromatography. Table ID, Note 10; Table IG, Note 3.
(C) Method 507, The Determination of Nitrogen- and Phosphorus-Containing Pesticides in Water by Gas Chromatography with a Nitrogen-Phosphorus Detector. Table ID, Note 10; Table IG, Note 3.
(D) Method 508, Determination of Chlorinated Pesticides in Water by Gas Chromatography with an Electron Capture Detector. Table ID, Note 10; Table IG, Note 3.
(E) Method 515.1, Determination of Chlorinated Acids in Water by Gas Chromatography with an Electron Capture Detector. Table IG, Notes 2 and 3.
(F) Method 515.2, Determination of Chlorinated Acids in Water Using Liquid-Solid Extraction and Gas Chromatography with an Electron Capture Detector. Table IG, Notes 2 and 3.
(G) Method 525.1, Determination of Organic Compounds in Drinking Water by Liquids-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry. Table ID, Note 10; Table IG, Note 3.
(H) Method 531.1, Measurement of N-Methylcarbamoyloximes and N-Methylcarbamates in Water by Direct Aqueous Injection HPLC with Post-Column Derivatization. Table ID, Note 10; Table IG, Note 3.
(I) Method 547, Determination of Glyphosate in Drinking Water by Direct-Aqueous-Injection HPLC, Post-Column Derivatization, and Fluorescence Detection. Table IG, Note 3.
(J) Method 548, Determination of Endothall in Drinking Water by Aqueous Derivatization, Liquid-Solid Extraction, and Gas Chromatography with Electron-Capture Detector. Table IG, Note 3.
(K) Method 548.1, Determination of Endothall in Drinking Water by Ion-Exchange Extraction, Acidic Methanol Methylation and Gas Chromatography/Mass Spectrometry. Table IG, Note 3.
(L) Method 553, Determination of Benzidines and Nitrogen-Containing Pesticides in Water by Liquid-Liquid Extraction or Liquid-Solid Extraction and Reverse Phase High Performance Liquid Chromatography/Particle Beam/Mass Spectrometry Table ID, Note 10; Table IG, Note 3.
(M) Method 555, Determination of Chlorinated Acids in Water by High Performance Liquid Chromatography With a Photodiode Array Ultraviolet Detector. Table IG, Note 3.
(vi) In the compendium Methods for the Determination of Organic Compounds in Drinking Water. Revised July 1991, December 1998. EPA-600/4-88-039, Pub. No. PB92-207703. Table IF.
(A) EPA Method 502.2, Volatile Organic Compounds in Water by Purge and Trap Capillary Column Gas Chromatography with Photoionization and Electrolytic Conductivity Detectors in Series. Table IF.
(B) [Reserved]
(vii) In the compendium Methods for the Determination of Organic Compounds in Drinking Water-Supplement II. August 1992. EPA-600/R-92-129, Pub. No. PB92-207703. Table IF.
(A) EPA Method 524.2, Measurement of Purgeable Organic Compounds in Water by Capillary Column Gas Chromatography/Mass Spectrometry. Table IF.
(B) [Reserved]
(viii) Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, Fifth Edition. October 2002. EPA 821-R-02-012, Pub. No. PB2002-108488. Table IA, Note 26.
(ix) Short-Term Methods for Measuring the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, Fourth Edition. October 2002. EPA 821-R-02-013, Pub. No. PB2002-108489. Table IA, Note 27.
(x) Short-Term Methods for Measuring the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, Third Edition. October 2002. EPA 821-R-02-014, Pub. No. PB2002-108490. Table IA, Note 28.
(8) Office of Water, U.S. Environmental Protection Agency (U.S. EPA), mail code 4303T, 1301 Constitution Avenue NW, Washington, DC 20460; website: www.epa.gov/cwa-methods.
(i) Method 245.7, Mercury in Water by Cold Vapor Atomic Fluorescence Spectrometry. Revision 2.0, February 2005. EPA-821-R-05-001. Table IB, Note 17.
(ii) Method 1103.2: Escherichia coli (E. coli) in Water by Membrane Filtration Using membrane-Thermotolerant Escherichia coli Agar (mTEC), EPA-821-R-23-009. September 2023. Table IH, Note 18.
(iii) Method 1106.2: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus -Esculin Iron Agar (mE-EIA), EPA-821-R-23-007. September 2023. Table IH, Note 22.
(iv) Method 1600.1: Enterococci in Water by Membrane Filtration Using membrane- Enterococcus Indoxyl-β-D-Glucoside Agar (mEI), EPA-821-R-23-006, September 2023. Table 1A, Note 24; Table IH, Note 23.
(v) Method 1603.1: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified membrane-Thermotolerant Escherichia coli Agar (Modified mTEC), EPA-821-R-23-008, September 2023. Table IA, Note 21; Table IH, Note 19.
(vi) Method 1604: Total Coliforms and Escherichia coli ( E. coli) in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium). September 2002. EPA-821-R-02-024. Table IH, Note 21.
(vii) Whole Effluent Toxicity Methods Errata Sheet, EPA 821-R-02-012-ES. December 2016, Table IA, Notes 25, 26, and 27.
(viii) Method 1623: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. December 2005. EPA-821-R-05-002. Table IH, Note 26.
(ix) Method 1623.1: Cryptosporidium and Giardia in Water by Filtration/IMS/FA. EPA 816-R-12-001. January 2012. U.S. EPA, Table IH, Notes 25 and 31.
(x) Method 1627, Kinetic Test Method for the Prediction of Mine Drainage Quality. December 2011. EPA-821-R-09-002. Table IB, Note 69.
(xi) Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Nonpolar Material) by Extraction and Gravimetry. Revision A, February 1999. EPA-821-R-98-002. Table IB, Notes 38 and 42.
(xii) Method 1664, n -Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n -Hexane Extractable Material (SGT-HEM; Nonpolar Material) by Extraction and Gravimetry, Revision B, February 2010. EPA-821-R-10-001. Table IB, Notes 38 and 42.
(xiii) Method 1669, Sampling Ambient Water for Trace Metals at EPA Water Quality Criteria Levels. July 1996. Table IB, Note 43.
(xiv) Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation using Lauryl Tryptose Broth (LTB) and EC Medium. September 2014. EPA-821-R-14-009.Table IA, Note 15.
(xv) Method 1681: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation using A-1 Medium. July 2006. EPA 821-R-06-013. Table IA, Note 20.
(xvi) Method 1682: Salmonella in Sewage Sludge (Biosolids) by Modified Semisolid Rappaport-Vassiliadis (MSRV) Medium. September 2014. EPA 821-R-14-012. Table IA, Note 23.
(9) American National Standards Institute, 1430 Broadway, New York NY 10018.
(i) ANSI. American National Standard on Photographic Processing Effluents. April 2, 1975. Table IB, Note 9.
(ii) [Reserved]
(10) American Public Health Association, 800 I Street, NW, Washington, DC 20001; phone: (202)777-2742, website: www.standardmethods.org.
(i) Standard Methods for the Examination of Water and Wastewater. 14th Edition, 1975. Table IB, Notes 27 and 86.
(ii) Standard Methods for the Examination of Water and Wastewater. 15th Edition, 1980, Table IB, Note 30; Table ID.
(iii) Selected Analytical Methods Approved and Cited by the United States Environmental Protection Agency, Supplement to the 15th Edition of Standard Methods for the Examination of Water and Wastewater. 1981. Table IC, Note 6; Table ID, Note 6.
(iv) Standard Methods for the Examination of Water and Wastewater. 18th Edition, 1992. Tables IA, IB, IC, ID, IE, and IH.
(v) Standard Methods for the Examination of Water and Wastewater. 19th Edition, 1995. Tables IA, IB, IC, ID, IE, and IH.
(vi) Standard Methods for the Examination of Water and Wastewater. 20th Edition, 1998. Tables IA, IB, IC, ID, IE, and IH.
(vii) Standard Methods for the Examination of Water and Wastewater. 21st Edition, 2005. Table IB, Notes 17 and 27.
(viii) 2120, Color. Revised September 4, 2021. Table IB.
(ix) 2130, Turbidity. Revised 2020. Table IB.
(x) 2310, Acidity. Revised 2020. Table IB.
(xi) 2320, Alkalinity. Revised 2021. Table IB.
(xii) 2340, Hardness. Revised 2021. Table IB.
(xiii) 2510, Conductivity. Revised 2021. Table IB.
(xiv) 2540, Solids. Revised 2020. Table IB.
(xv) 2550, Temperature. 2010. Table IB.
(xvi) 3111, Metals by Flame Atomic Absorption Spectrometry. Revised 2019. Table IB.
(xvii) 3112, Metals by Cold-Vapor Atomic Absorption Spectrometry. Revised 2020. Table IB.
(xviii) 3113, Metals by Electrothermal Atomic Absorption Spectrometry. Revised 2020. Table IB.
(xix) 3114, Arsenic and Selenium by Hydride Generation/Atomic Absorption Spectrometry. Revised 2020, Table IB.
(xx) 3120, Metals by Plasma Emission Spectroscopy. Revised 2020. Table IB.
(xxi) 3125, Metals by Inductively Coupled Plasma-Mass Spectrometry. Revised 2020. Table IB.
(xxii) 3500-Al, Aluminum. Revised 2020. Table IB.
(xxiii) 3500-As, Arsenic. Revised 2020. Table IB.
(xxiv) 3500-Ca, Calcium. Revised 2020. Table IB.
(xxv) 3500-Cr, Chromium. Revised 2020. Table IB.
(xxvi) 3500-Cu, Copper. Revised 2020. Table IB.
(xxvii) 3500-Fe, Iron. 2011. Table IB.
(xxviii) 3500-Pb, Lead. Revised 2020. Table IB.
(xxix) 3500-Mn, Manganese. Revised 2020. Table IB.
(xxx) 3500-K, Potassium. Revised 2020. Table IB.
(xxxi) 3500-Na, Sodium. Revised 2020. Table IB.
(xxxii) 3500-V, Vanadium. 2011. Table IB.
(xxxiii) 3500-Zn, Zinc. Revised 2020. Table IB.
(xxxiv) 4110, Determination of Anions by Ion Chromatography. Revised 2020. Table IB.
(xxxv) 4140, Inorganic Anions by Capillary Ion Electrophoresis. Revised 2020. Table IB.
(xxxvi) 4500-B, Boron. 2011. Table IB.
(xxxvii) 4500 Cl − , Chloride. Revised 2021. Table IB.
(xxxviii) 4500-Cl, Chlorine (Residual). 2011. Table IB.
(xxxix) 4500-CN − , Cyanide. Revised 2021. Table IB.
(xl) 4500-F − , Fluoride. Revised 2021. Table IB.
(xli) 4500-H + , pH. 2021. Table IB.
(xlii) 4500-NH 3 , Nitrogen (Ammonia). Revised 2021. Table IB.
(xliii) 4500-NO 2− , Nitrogen (Nitrite). Revised 2021. Table IB.
(xliv) 4500-NO 3− , Nitrogen (Nitrate). Revised 2019. Table IB.
(xlv) 4500-N (org) , Nitrogen (Organic). Revised 2021. Table IB.
(xlvi) 4500-O, Oxygen (Dissolved). Revised 2021. Table IB.
(xlvii) 4500-P, Phosphorus. Revised 2021. Table IB.
(xlviii) 4500-SiO 2 , Silica. Revised 2021. Table IB.
(xlix) 4500-S 2− , Sulfide. Revised 2021. Table IB.
(l) 4500-SO 32− , Sulfite. Revised 2021. Table IB.
(li) 4500-SO 42− , Sulfate. Revised 2021. Table IB.
(lii) 5210, Biochemical Oxygen Demand (BOD). Revised 2016. Table IB.
(liii) 5220, Chemical Oxygen Demand (COD). 2011. Table IB.
(liv) 5310, Total Organic Carbon (TOC). Revised 2014. Table IB.
(lv) 5520, Oil and Grease. Revised 2021. Table IB.
(lvi) 5530, Phenols. Revised 2021. Table IB.
(lvii) 5540, Surfactants. Revised 2021. Table IB.
(lviii) 6200, Volatile Organic Compounds. Revised 2020. Table IC.
(lix) 6410, Extractable Base/Neutrals and Acids. Revised 2020. Tables IC and ID.
(lx) 6420, Phenols. Revised 2021. Table IC.
(lxi) 6440, Polynuclear Aromatic Hydrocarbons. Revised 2021. Table IC.
(lxii) 6630, Organochlorine Pesticides. Revised 2021. Table ID.
(lxiii) 6640, Acidic Herbicide Compounds. Revised 2021. Table ID.
(lxiv) 7110, Gross Alpha and Gross Beta Radioactivity (Total, Suspended, and Dissolved). 2000. Table IE.
(lxv) 7500, Radium. 2001. Table IE.
(lxvi) 9213, Recreational Waters. 2007. Table IH.
(lxvii) 9221, Multiple-Tube Fermentation Technique for Members of the Coliform Group. Approved 2014. Table IA, Notes 12, 14; and 33; Table IH, Notes 10, 12, and 32.
(lxviii) 9222, Membrane Filter Technique for Members of the Coliform Group. 2015. Table IA, Note 31; Table IH, Note 17.
(lxix) 9223 Enzyme Substrate Coliform Test. 2016. Table IA; Table IH.
(lxx) 9230 Fecal Enterococcus/Streptococcus Groups. 2013. Table IA, Note 32; Table IH.
(11) The Analyst, The Royal Society of Chemistry, RSC Publishing, Royal Society of Chemistry, Thomas Graham House, Science Park, Milton Road, Cambridge CB4 0WF, United Kingdom. (Also available from most public libraries.)
(i) Spectrophotometric Determination of Ammonia: A Study of a Modified Berthelot Reaction Using Salicylate and Dichloroisocyanurate. Krom, M.D. 105:305-316, April 1980. Table IB, Note 60.
(ii) [Reserved]
(12) Analytical Chemistry, ACS Publications, 1155 Sixteenth St. NW., Washington DC 20036. (Also available from most public libraries.)
(i) Spectrophotometric and Kinetics Investigation of the Berthelot Reaction for the Determination of Ammonia. Patton, C.J. and S.R. Crouch. 49(3):464-469, March 1977. Table IB, Note 60.
(ii) [Reserved]
(13) AOAC International, 481 North Frederick Avenue, Suite 500, Gaithersburg, MD 20877-2417.
(i) Official Methods of Analysis of AOAC International. 16th Edition, 4th Revision, 1998.
(A) 920.203, Manganese in Water, Persulfate Method. Table IB, Note 3.
(B) 925.54, Sulfate in Water, Gravimetric Method. Table IB, Note 3.
(C) 973.40, Specific Conductance of Water. Table IB, Note 3.
(D) 973.41, pH of Water. Table IB, Note 3.
(E) 973.43, Alkalinity of Water, Titrimetric Method. Table IB, Note 3.
(F) 973.44, Biochemical Oxygen Demand (BOD) of Water, Incubation Method. Table IB, Note 3.
(G) 973.45, Oxygen (Dissolved) in Water, Titrimetric Methods. Table IB, Note 3.
(H) 973.46, Chemical Oxygen Demand (COD) of Water, Titrimetric Methods. Table IB, Note 3.
(I) 973.47, Organic Carbon in Water, Infrared Analyzer Method. Table IB, Note 3.
(J) 973.48, Nitrogen (Total) in Water, Kjeldahl Method. Table IB, Note 3.
(K) 973.49, Nitrogen (Ammonia) in Water, Colorimetric Method. Table IB, Note 3.
(L) 973.50, Nitrogen (Nitrate) in Water, Brucine Colorimetric Method. Table IB, Note 3.
(M) 973.51, Chloride in Water, Mercuric Nitrate Method. Table IB, Note 3.
(N) 973.52, Hardness of Water. Table IB, Note 3.
(O) 973.53, Potassium in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(P) 973.54, Sodium in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(Q) 973.55, Phosphorus in Water, Photometric Method. Table IB, Note 3.
(R) 973.56, Phosphorus in Water, Automated Method. Table IB, Note 3.
(S) 974.27, Cadmium, Chromium, Copper, Iron, Lead, Magnesium, Manganese, Silver, Zinc in Water, Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(T) 977.22, Mercury in Water, Flameless Atomic Absorption Spectrophotometric Method. Table IB, Note 3.
(U) 991.15. Total Coliforms and Escherichia coli in Water Defined Substrate Technology (Colilert) Method. Table IA, Note 10; Table IH, Note 10.
(V) 993.14, Trace Elements in Waters and Wastewaters, Inductively Coupled Plasma-Mass Spectrometric Method. Table IB, Note 3.
(W) 993.23, Dissolved Hexavalent Chromium in Drinking Water, Ground Water, and Industrial Wastewater Effluents, Ion Chromatographic Method. Table IB, Note 3.
(X) 993.30, Inorganic Anions in Water, Ion Chromatographic Method. Table IB, Note 3.
(ii) [Reserved]
(14) Applied and Environmental Microbiology, American Society for Microbiology, 1752 N Street NW., Washington DC 20036. (Also available from most public libraries.)
(i) New Medium for the Simultaneous Detection of Total Coliforms and Escherichia coli in Water. Brenner, K.P., C.C. Rankin, Y.R. Roybal, G.N. Stelma, Jr., P.V. Scarpino, and A.P. Dufour. 59:3534-3544, November 1993. Table IH, Note 21.
(ii) [Reserved]
(15) ASTM International, 100 Barr Harbor Drive, P.O. Box C700, West Conshohocken, PA 19428-2959; phone: (877)909-2786; website: www.astm.org.
(i) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1994. Tables IA, IB, IC, ID, IE, and IH.
(ii) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1996. Tables IA, IB, IC, ID, IE, and IH.
(iii) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 1999. Tables IA, IB, IC, ID, IE, and IH.
(iv) Annual Book of ASTM Standards, Water, and Environmental Technology, Section 11, Volumes 11.01 and 11.02. 2000. Tables IA, IB, IC, ID, IE, and IH.
(v) ASTM D511-14, Standard Test Methods for Calcium and Magnesium in Water. Approved October 1, 2014. Table IB.
(vi) ASTM D512-12, Standard Test Methods for Chloride Ion in Water. Approved June 15, 2012. Table IB.
(vii) ASTM D515-88, Test Methods for Phosphorus in Water, March 1989. Table IB.
(viii) ASTM D516-16, Standard Test Method for Sulfate Ion in Water. Approved June 1, 2016. Table IB.
(ix) ASTM D858-17, Standard Test Methods for Manganese in Water. Approved June 1, 2017. Table IB.
(x) ASTM D859-16, Standard Test Method for Silica in Water. Approved June 15, 2016. Table IB.
(xi) ASTM D888-18, Standard Test Methods for Dissolved Oxygen in Water. Approved May 1, 2018. Table IB.
(xii) ASTM D1067-16, Standard Test Methods for Acidity or Alkalinity of Water. Approved June 15, 2016. Table IB.
(xiii) ASTM D1068-15, Standard Test Methods for Iron in Water. Approved October 1, 2015. Table IB.
(xiv) ASTM D1125-95 (Reapproved 1999), Standard Test Methods for Electrical Conductivity and Resistivity of Water. December 1995. Table IB.
(xv) ASTM D1126-17, Standard Test Method for Hardness in Water. Approved December 1, 2017. Table IB.
(xvi) ASTM D1179-16, Standard Test Methods for Fluoride Ion in Water. Approved June 15, 2016. Table IB.
(xvii) ASTM D1246-16, Standard Test Method for Bromide Ion in Water. June 15, 2016. Table IB.
(xviii) ASTM D1252-06 (Reapproved 2012), Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. Approved June 15, 2012. Table IB.
(xix) ASTM D1253-14, Standard Test Method for Residual Chlorine in Water. Approved January 15, 2014. Table IB.
(xx) ASTM D1293-18, Standard Test Methods for pH of Water. Approved January 15, 2018. Table IB.
(xxi) ASTM D1426-15, Standard Test Methods for Ammonia Nitrogen in Water. Approved March 15, 2015. Table IB.
(xxii) ASTM D1687-17, Standard Test Methods for Chromium in Water. Approved June 1, 2017. Table IB.
(xxiii) ASTM D1688-17, Standard Test Methods for Copper in Water. Approved June 1, 2017. Table IB.
(xxiv) ASTM D1691-17, Standard Test Methods for Zinc in Water. Approved June 1, 2017. Table IB.
(xxv) ASTM D1783-01 (Reapproved 2012), Standard Test Methods for Phenolic Compounds in Water. Approved June 15, 2012. Table IB.
(xxvi) ASTM D1886-14, Standard Test Methods for Nickel in Water. Approved October 1, 2014. Table IB.
(xxvii) ASTM D1889-00, Standard Test Method for Turbidity of Water. October 2000. Table IB.
(xxviii) ASTM D1890-96, Standard Test Method for Beta Particle Radioactivity of Water. April 1996. Table IE.
(xxix) ASTM D1943-96, Standard Test Method for Alpha Particle Radioactivity of Water. April 1996. Table IE.
(xxx) ASTM D1976-20, Standard Test Method for Elements in Water by Inductively-Coupled Argon Plasma Atomic Emission Spectroscopy. Approved May 1, 2020. Table IB.
(xxxi) ASTM D2036-09 (Reapproved 2015), Standard Test Methods for Cyanides in Water. Approved July 15, 2015. Table IB.
(xxxii) ASTM D2330-20, Standard Test Method for Methylene Blue Active Substances. Approved January 1, 2020. Table 1B.
(xxxiii) ASTM D2460-97, Standard Test Method for Alpha-Particle-Emitting Isotopes of Radium in Water. October 1997. Table IE.
(xxxiv) ASTM D2972-15, Standard Tests Method for Arsenic in Water. Approved February 1, 2015. Table IB.
(xxxv) ASTM D3223-17, Standard Test Method for Total Mercury in Water. Approved June 1, 2017. Table IB.
(xxxvi) ASTM D3371-95, Standard Test Method for Nitriles in Aqueous Solution by Gas-Liquid Chromatography, February 1996. Table IF.
(xxxvii) ASTM D3373-17, Standard Test Method for Vanadium in Water. Approved June 1, 2017. Table IB.
(xxxviii) ASTM D3454-97, Standard Test Method for Radium-226 in Water. February 1998. Table IE.
(xxxix) ASTM D3557-17, Standard Test Method for Cadmium in Water. Approved June 1, 2017. Table IB.
(xl) ASTM D3558-15, Standard Test Method for Cobalt in Water. Approved February 1, 2015. Table IB.
(xli) ASTM D3559-15, Standard Test Methods for Lead in Water. Approved June 1, 2015. Table IB.
(xlii) ASTM D3590-17, Standard Test Methods for Total Kjeldahl Nitrogen in Water. Approved June 1, 2017. Table IB.
(xliii) ASTM D3645-15, Standard Test Methods for Beryllium in Water. Approved February 1, 2015. Table IB.
(xliv) ASTM D3695-95, Standard Test Method for Volatile Alcohols in Water by Direct Aqueous-Injection Gas Chromatography. April 1995. Table IF.
(xlv) ASTM D3859-15, Standard Test Methods for Selenium in Water. Approved March 15, 2015. Table IB.
(xlvi) ASTM D3867-16, Standard Test Method for Nitrite-Nitrate in Water. Approved June 1, 2016. Table IB.
(xlvii) ASTM D4190-15, Standard Test Method for Elements in Water by Direct- Current Plasma Atomic Emission Spectroscopy. Approved February 1, 2015. Table IB.
(xlviii) ASTM D4282-15, Standard Test Method for Determination of Free Cyanide in Water and Wastewater by Microdiffusion. Approved July 15, 2015. Table IB.
(xlix) ASTM D4327-17, Standard Test Method for Anions in Water by Suppressed Ion Chromatography. Approved December 1, 2017. Table IB.
(l) ASTM D4382-18, Standard Test Method for Barium in Water, Atomic Absorption Spectrophotometry, Graphite Furnace. Approved February 1, 2018. Table IB.
(li) ASTM D4657-92 (Reapproved 1998), Standard Test Method for Polynuclear Aromatic Hydrocarbons in Water. January 1993. Table IC.
(lii) ASTM D4658-15, Standard Test Method for Sulfide Ion in Water. Approved March 15, 2015. Table IB.
(liii) ASTM D4763-88 (Reapproved 2001), Standard Practice for Identification of Chemicals in Water by Fluorescence Spectroscopy. September 1988. Table IF.
(liv) ASTM D4839-03 (Reapproved 2017), Standard Test Method for Total Carbon and Organic Carbon in Water by Ultraviolet, or Persulfate Oxidation, or Both, and Infrared Detection. Approved December 15, 2017. Table IB.
(lv) ASTM D5257-17, Standard Test Method for Dissolved Hexavalent Chromium in Water by Ion Chromatography. Approved December 1, 2017. Table IB.
(lvi) ASTM D5259-92, Standard Test Method for Isolation and Enumeration of Enterococci from Water by the Membrane Filter Procedure. October 1992. Table IH, Note 9.
(lvii) ASTM D5392-93, Standard Test Method for Isolation and Enumeration of Escherichia coli in Water by the Two-Step Membrane Filter Procedure. September 1993. Table IH, Note 9.
(lviii) ASTM D5673-16, Standard Test Method for Elements in Water by Inductively Coupled Plasma—Mass Spectrometry. Approved February 1, 2016. Table IB.
(lix) ASTM D5907-18, Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water. Approved May 1, 2018. Table IB.
(lx) ASTM D6503-99, Standard Test Method for Enterococci in Water Using Enterolert. April 2000. Table IA Note 9, Table IH, Note 9.
(lxi) ASTM. D6508-15, Standard Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Approved October 1, 2015. Table IB, Note 54.
(lxii) ASTM. D6888-16, Standard Test Method for Available Cyanides with Ligand Displacement and Flow Injection Analysis (FIA) Utilizing Gas Diffusion Separation and Amperometric Detection. Approved February 1, 2016. Table IB, Note 59.
(lxiii) ASTM. D6919-17, Standard Test Method for Determination of Dissolved Alkali and Alkaline Earth Cations and Ammonium in Water and Wastewater by Ion Chromatography. Approved June 1, 2017. Table IB.
(lxiv) ASTM. D7065-17, Standard Test Method for Determination of Nonylphenol, Bisphenol A, p-tert -Octylphenol, Nonylphenol Monoethoxylate and Nonylphenol Diethoxylate in Environmental Waters by Gas Chromatography Mass Spectrometry. Approved December 15, 2017. Table IC.
(lxv) ASTM D7237-18, Standard Test Method for Free Cyanide with Flow Injection Analysis (FIA) Utilizing Gas Diffusion Separation and Amperometric Detection. Approved December 1, 2018. Table IB.
(lxvi) ASTM D7284-20, Standard Test Method for Total Cyanide in Water by Micro Distillation followed by Flow Injection Analysis with Gas Diffusion Separation and Amperometric Detection. Approved August 1, 2020. Table IB.
(lxvii) ASTM D7365-09a (Reapproved 2015), Standard Practice for Sampling, Preservation and Mitigating Interferences in Water Samples for Analysis of Cyanide. Approved July 15, 2015. Table II, Notes 5 and 6.
(lxviii) ASTM. D7511-12 (Reapproved 2017) e1 , Standard Test Method for Total Cyanide by Segmented Flow Injection Analysis, In-Line Ultraviolet Digestion and Amperometric Detection. Approved July 1, 2017. Table IB.
(lxix) ASTM D7573-18a e1 , Standard Test Method for Total Carbon and Organic Carbon in Water by High Temperature Catalytic Combustion and Infrared Detection. Approved December 15, 2018. Table IB.
(lxx) ASTM D7781-14, Standard Test Method for Nitrite-Nitrate in Water by Nitrate Reductase, Approved April 1, 2014. Table IB.
(16) Bran & Luebbe Analyzing Technologies, Inc., Elmsford NY 10523.
(i) Industrial Method Number 378-75WA, Hydrogen Ion (pH) Automated Electrode Method, Bran & Luebbe (Technicon) Auto Analyzer II. October 1976. Table IB, Note 21.
(ii) [Reserved]
(17) CEM Corporation, P.O. Box 200, Matthews NC 28106-0200.
(i) Closed Vessel Microwave Digestion of Wastewater Samples for Determination of Metals. April 16, 1992. Table IB, Note 36.
(ii) [Reserved]
(18) Craig R. Chinchilla, 900 Jorie Blvd., Suite 35, Oak Brook IL 60523. Telephone: 630-645-0600.
(i) Nitrate by Discrete Analysis Easy (1-Reagent) Nitrate Method, (Colorimetric, Automated, 1 Reagent). Revision 1, November 12, 2011. Table IB, Note 62.
(ii) [Reserved]
(19) FIAlab Instruments, Inc., 334 2151 N. Northlake Way, Seattle, WA 98103; phone: (425)376-0450; website: www.flowinjection.com/app-notes/epafialab100.
(i) FIAlab 100, Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Fluorescence Detector Analysis, April 4, 2018. Table IB, Note 82.
(ii) [Reserved]
(20) Hach Company, P.O. Box 389, Loveland CO 80537.
(i) Method 8000, Chemical Oxygen Demand. Hach Handbook of Water Analysis. 1979. Table IB, Note 14.
(ii) Method 8008, 1,10-Phenanthroline Method using FerroVer Iron Reagent for Water. 1980. Table IB, Note 22.
(iii) Method 8009, Zincon Method for Zinc. Hach Handbook for Water Analysis. 1979. Table IB, Note 33.
(iv) Method 8034, Periodate Oxidation Method for Manganese. Hach Handbook for Water Analysis. 1979. Table IB, Note 23.
(v) Method 8506, Bicinchoninate Method for Copper. Hach Handbook of Water Analysis. 1979. Table IB, Note 19.
(vi) Method 8507, Nitrogen, Nitrite-Low Range, Diazotization Method for Water and Wastewater. 1979. Table IB, Note 25.
(vii) Method 10206, Hach Company TNTplus 835/836 Nitrate Method 10206, Spectrophotometric Measurement of Nitrate in Water and Wastewater. Revision 2.1, January 10, 2013. Table IB, Note 75.
(viii) Method 10242, Hach Company TNTplus 880 Total Kjeldahl Nitrogen Method 10242, Simplified Spectrophotometric Measurement of Total Kjeldahl Nitrogen in Water and Wastewater. Revision 1.1, January 10, 2013. Table IB, Note 76.
(ix) Hach Method 10360, Luminescence Measurement of Dissolved Oxygen in Water and Wastewater and for Use in the Determination of BOD5and cBOD5. Revision 1.2, October 2011. Table IB, Note 63.
(x) m-ColiBlue24® Method, for total Coliforms and E. coli. Revision 2, 1999. Table IA, Note 18; Table IH, Note 17.
(21) IDEXX Laboratories Inc., One Idexx Drive, Westbrook ME 04092.
(i) Colilert. 2013. Table IA, Notes 17 and 18; Table IH, Notes 14, 15 and 16.
(ii) Colilert-18. 2013. Table IA, Notes 17 and 18; Table IH, Notes 14, 15 and 16.
(iii) Enterolert. 2013. Table IA, Note 24; Table IH, Note 12.
(iv) Quanti-Tray Insert and Most Probable Number (MPN) Table. 2013. Table IA, Note 18; Table IH, Notes 14 and 16.
(22) In-Situ Incorporated, 221 E. Lincoln Ave., Ft. Collins CO 80524. Telephone: 970-498-1500.
(i) In-Situ Inc. Method 1002-8-2009, Dissolved Oxygen Measurement by Optical Probe. 2009. Table IB, Note 64.
(ii) In-Situ Inc. Method 1003-8-2009, Biochemical Oxygen Demand (BOD) Measurement by Optical Probe. 2009. Table IB, Note 10.
(iii) In-Situ Inc. Method 1004-8-2009, Carbonaceous Biochemical Oxygen Demand (CBOD) Measurement by Optical Probe. 2009. Table IB, Note 35.
(23) Journal of Chromatography, Elsevier/North-Holland, Inc., Journal Information Centre, 52 Vanderbilt Avenue, New York NY 10164. (Also available from most public libraries.
(i) Direct Determination of Elemental Phosphorus by Gas-Liquid Chromatography. Addison, R.F. and R.G. Ackman. 47(3): 421-426, 1970. Table IB, Note 28.
(ii) [Reserved]
(24) Lachat Instruments, 6645 W. Mill Road, Milwaukee WI 53218, Telephone: 414-358-4200.
(i) QuikChem Method 10-204-00-1-X, Digestion and Distillation of Total Cyanide in Drinking and Wastewaters using MICRO DIST and Determination of Cyanide by Flow Injection Analysis. Revision 2.2, March 2005. Table IB, Note 56.
(ii) [Reserved]
(25) Leck Mitchell, Ph.D., P.E., 656 Independence Valley Dr., Grand Junction CO 81507. Telephone: 970-244-8661.
(i) Mitchell Method M5271, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Table IB, Note 66.
(ii) Mitchell Method M5331, Determination of Turbidity by Nephelometry. Revision 1.0, July 31, 2008. Table IB, Note 65.
(26) MACHEREY-NAGEL GmbH and Co., 2850 Emrick Blvd., Bethlehem, PA 18020; Phone: (888)321-6224.
(i) Method 036/038 NANOCOLOR® COD LR/HR, Spectrophotometric Measurement of Chemical Oxygen Demand in Water and Wastewater, Revision 1.5, May 2018. Table IB, Note 83.
(ii) [Reserved]
(27) Micrology Laboratories, LLC (now known as Roth Bioscience, LLC), 1303 Eisenhower Drive, Goshen, IN 46526; phone: (574)533-3351.
(i) KwikCount TM EC Medium E. coli enzyme substrate test, Rapid Detection of E. coli in Beach Water By KwikCount TM EC Membrane Filtration. 2014. Table IH, Notes 28 and 29.
(ii) [Reserved]
(28) National Council of the Paper Industry for Air and Stream Improvements, Inc. (NCASI), 260 Madison Avenue, New York NY 10016.
(i) NCASI Method TNTP-W10900, Total Nitrogen and Total Phophorus in Pulp and Paper Biologically Treated Effluent by Alkaline Persulfate Digestion. June 2011. Table IB, Note 77.
(ii) NCASI Technical Bulletin No. 253, An Investigation of Improved Procedures for Measurement of Mill Effluent and Receiving Water Color. December 1971. Table IB, Note 18.
(iii) NCASI Technical Bulletin No. 803, An Update of Procedures for the Measurement of Color in Pulp Mill Wastewaters. May 2000. Table IB, Note 18.
(29) The Nitrate Elimination Co., Inc. (NECi), 334 Hecla St., Lake Linden NI 49945.
(i) NECi Method N07-0003, Method for Nitrate Reductase Nitrate-Nitrogen Analysis. Revision 9.0. March 2014. Table IB, Note 73.
(ii) [Reserved]
(30) Oceanography International Corporation, 512 West Loop, P.O. Box 2980, College Station TX 77840.
(i) OIC Chemical Oxygen Demand Method. 1978. Table IB, Note 13.
(ii) [Reserved]
(31) OI Analytical, Box 9010, College Station TX 77820-9010.
(i) Method OIA-1677-09, Available Cyanide by Ligand Exchange and Flow Injection Analysis (FIA). Copyright 2010. Table IB, Note 59.
(ii) Method PAI-DK01, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Titrimetric Detection. Revised December 22, 1994. Table IB, Note 39.
(iii) Method PAI-DK02, Nitrogen, Total Kjeldahl, Block Digestion, Steam Distillation, Colorimetric Detection. Revised December 22, 1994. Table IB, Note 40.
(iv) Method PAI-DK03, Nitrogen, Total Kjeldahl, Block Digestion, Automated FIA Gas Diffusion. Revised December 22, 1994. Table IB, Note 41.
(32) ORION Research Corporation, 840 Memorial Drive, Cambridge, Massachusetts 02138.
(i) ORION Research Instruction Manual, Residual Chlorine Electrode Model 97-70. 1977. Table IB, Note 16.
(ii) [Reserved]
(33) Pace Analytical Services, LLC, 1800 Elm Street, SE, Minneapolis, MN 55414; phone: (612)656-2240.
(i) PAM-16130-SSI, Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Shimadzu Gas Chromatography Mass Spectrometry (GC-MS/MS), Revision 1.1, May 20, 2022. Table IC, Note 17.
(ii) [Reserved]
(34) SGS AXYS Analytical Services, Ltd., 2045 Mills Road, Sidney, British Columbia, Canada, V8L 5X2; phone: (888)373-0881.
(i) SGS AXYS Method 16130, Determination of 2,3,7,8-Substituted Tetra- through Octa-Chlorinated Dibenzo- p -Dioxins and Dibenzofurans (CDDs/CDFs) Using Waters and Agilent Gas Chromatography-Mass Spectrometry (GC/MS/MS)., Revision 1.0, revised August 2020. Table IC, Note 16.
(ii) [Reserved]
(35) Technicon Industrial Systems, Tarrytown NY 10591.
(i) Industrial Method Number 379-75WE Ammonia, Automated Electrode Method, Technicon Auto Analyzer II. February 19, 1976. Table IB, Note 7.
(ii) [Reserved]
(36) Thermo Jarrell Ash Corporation, 27 Forge Parkway, Franklin MA 02038.
(i) Method AES0029. Direct Current Plasma (DCP) Optical Emission Spectrometric Method for Trace Elemental Analysis of Water and Wastes. 1986, Revised 1991. Table IB, Note 34.
(ii) [Reserved]
(37) Thermo Scientific, 166 Cummings Center, Beverly MA 01915. Telephone: 1-800-225-1480. www.thermoscientific.com.
(i) Thermo Scientific Orion Method AQ4500, Determination of Turbidity by Nephelometry. Revision 5, March 12, 2009. Table IB, Note 67.
(ii) [Reserved]
(38) 3M Corporation, 3M Center Building 220-9E-10, St. Paul MN 55144-1000.
(i) Organochlorine Pesticides and PCBs in Wastewater Using EmporeTMDisk” Test Method 3M 0222. Revised October 28, 1994. Table IC, Note 8; Table ID, Note 8.
(ii) [Reserved]
(39) Timberline Instruments, LLC, 1880 South Flatiron Ct., Unit I, Boulder CO 80301.
(i) Timberline Amonia-001, Determination of Inorganic Ammonia by Continuous Flow Gas Diffusion and Conductivity Cell Analysis. June 24, 2011. Table IB, Note 74.
(ii) [Reserved]
(40) U.S. Geological Survey (USGS), U.S. Department of the Interior, Reston, Virginia. Available from USGS Books and Open-File Reports (OFR) Section, Federal Center, Box 25425, Denver, CO 80225; phone: (703)648-5953; website: ww.usgs.gov.
(i) Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods. U.S. Geological Survey Techniques and Methods, Book 5—Laboratory Analysis, Section B—Methods of the National Water Quality Laboratory, Chapter 8. 2011. Table IB, Note 72.
(ii) Techniques and Methods—Book 5, Laboratory Analysis—Section B, Methods of the National Water Quality Laboratory—Chapter 12, Determination of Heat Purgeable and Ambient Purgeable Volatile Organic Compounds in Water by Gas Chromatography/Mass Spectrometry 2016.
(iii) Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, editors, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1979. Table IB, Note 8.
(iv) Methods for Determination of Inorganic Substances in Water and Fluvial Sediments, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A1. 1989. Table IB, Notes 2 and 79.
(v) Methods for the Determination of Organic Substances in Water and Fluvial Sediments. Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 5, Chapter A3. 1987. Table IB, Note 24; Table ID, Note 4.
(vi) OFR 76-177, Selected Methods of the U.S. Geological Survey of Analysis of Wastewaters. 1976. Table IE, Note 2.
(vii) OFR 91-519, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organonitrogen Herbicides in Water by Solid-Phase Extraction and Capillary-Column Gas Chromatography/Mass Spectrometry With Selected-Ion Monitoring. 1992. Table ID, Note 14.
(viii) OFR 92-146, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Total Phosphorus by a Kjeldahl Digestion Method and an Automated Colorimetric Finish That Includes Dialysis. 1992. Table IB, Note 48.
(ix) OFR 93-125, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Inorganic and Organic Constituents in Water and Fluvial Sediments. 1993. Table IB, Notes 51 and 80; Table IC, Note 9.
(x) OFR 93-449, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Chromium in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1993. Table IB, Note 46.
(xi) OFR 94-37, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Triazine and Other Nitrogen-containing Compounds by Gas Chromatography with Nitrogen Phosphorus Detectors. 1994. Table ID, Note 9.
(xii) OFR 95-181, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by C-18 Solid-Phase Extraction and Capillary-Column Gas Chromatography/Mass Spectrometry With Selected-Ion Monitoring. 1995. Table ID, Note 11.
(xiii) OFR 97-198, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Molybdenum in Water by Graphite Furnace Atomic Absorption Spectrophotometry. 1997. Table IB, Note 47.
(xiv) OFR 97-829, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of 86 Volatile Organic Compounds in Water by Gas Chromatography/Mass Spectrometry, Including Detections Less Than Reporting Limits. 1998. Table IC, Note 13.
(xv) OFR 98-165, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Elements in Whole-Water Digests Using Inductively Coupled Plasma-Optical Emission Spectrometry and Inductively Coupled Plasma-Mass Spectrometry. 1998. Table IB, Notes 50 and 81.
(xvi) OFR 98-639, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Arsenic and Selenium in Water and Sediment by Graphite Furnace—Atomic Absorption Spectrometry. 1999. Table IB, Note 49.
(xvii) OFR 00-170, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Ammonium Plus Organic Nitrogen by a Kjeldahl Digestion Method and an Automated Photometric Finish that Includes Digest Cleanup by Gas Diffusion. 2000. Table IB, Note 45.
(xviii) Techniques and Methods Book 5-B1, Determination of Elements in Natural-Water, Biota, Sediment and Soil Samples Using Collision/Reaction Cell Inductively Coupled Plasma-Mass Spectrometry. Chapter 1, Section B, Methods of the National Water Quality Laboratory, Book 5, Laboratory Analysis. 2006. Table IB, Note 70.
(xix) U.S. Geological Survey Techniques of Water-Resources Investigations, Book 5, Laboratory Analysis, Chapter A4, Methods for Collection and Analysis of Aquatic Biological and Microbiological Samples. 1989. Table IA, Note 4; Table IH, Note 4.
(xx) Water-Resources Investigation Report 01-4098, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Moderate-Use Pesticides and Selected Degradates in Water by C-18 Solid-Phase Extraction and Gas Chromatography/Mass Spectrometry. 2001. Table ID, Note 13.
(xxi) Water-Resources Investigations Report 01-4132, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Organic Plus Inorganic Mercury in Filtered and Unfiltered Natural Water With Cold Vapor-Atomic Fluorescence Spectrometry. 2001. Table IB, Note 71.
(xxii) Water-Resources Investigation Report 01-4134, Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Determination of Pesticides in Water by Graphitized Carbon-Based Solid-Phase Extraction and High-Performance Liquid Chromatography/Mass Spectrometry. 2001. Table ID, Note 12.
(xxiii) Water Temperature—Influential Factors, Field Measurement and Data Presentation, Techniques of Water-Resources Investigations of the U.S. Geological Survey, Book 1, Chapter D1. 1975. Table IB, Note 32.
(41) Waters Corporation, 34 Maple Street, Milford MA 01757, Telephone: 508-482-2131, Fax: 508-482-3625. (i) Method D6508, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Revision 2, December 2000. Table IB, Note 54. (ii) [Reserved]
(i) Method D6508, Test Method for Determination of Dissolved Inorganic Anions in Aqueous Matrices Using Capillary Ion Electrophoresis and Chromate Electrolyte. Revision 2, December 2000. Table IB, Note 54.
(ii) [Reserved]
* * * *
(e)
* * * *
Table II—Required Containers, Preservation Techniques, and Holding Times
* * * *
5 ASTM D7365-09a (15) specifies treatment options for samples containing oxidants (e.g ., chlorine) for cyanide analyses. Also, Section 9060A of Standard Methods for the Examination of Water and Wastewater (23rd edition) addresses dechlorination procedures for microbiological analyses.

Specialized Industries
Go beyond the regulations! Visit the Institute for in-depth guidance on a wide range of compliance subjects in safety and health, transportation, environment, and human resources.
J. J. Keller® COMPLIANCE NETWORK is a premier online safety and compliance community, offering members exclusive access to timely regulatory content in workplace safety (OSHA), transportation (DOT), environment (EPA), and human resources (DOL).

Interact With Our Compliance Experts
Puzzled by a regulatory question or issue? Let our renowned experts provide the answers and get your business on track to full compliance!

Upcoming Events
Reference the Compliance Network Safety Calendar to keep track of upcoming safety and compliance events. Browse by industry or search by keyword to see relevant dates and observances, including national safety months, compliance deadlines, and more.
In March 2023, the U.S. Environmental Protection Agency (EPA) proposed and requested comment on the National Primary Drinking Water Regulation (NPDWR) and health-based Maximum Contaminant Level Goals (MCLGs) for six per- and polyfluoroalkyl substances (PFAS): perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), hexafluoropropylene oxide dimer acid (HFPO-DA, commonly known as GenX Chemicals), and perfluorobutane sulfonic acid (PFBS). After consideration of public comment and consistent with the provisions set forth under the Safe Drinking Water Act (SDWA), the EPA is finalizing NPDWRs for these six PFAS. Through this action, the EPA is finalizing MCLGs for PFOA and PFOS at zero. Considering feasibility, the EPA is promulgating individual Maximum Contaminant Levels (MCLs) for PFOA and PFOS at 4.0 nanograms per liter (ng/L) or parts per trillion (ppt). The EPA is also finalizing individual MCLGs and is promulgating individual MCLs for PFHxS, PFNA, and HFPO-DA at 10 ng/L. In addition to the individual MCLs for PFHxS, PFNA, and HFPO-DA, in consideration of the known toxic effects, dose additive health concerns and occurrence and likely co-occurrence in drinking water of these three PFAS, as well as PFBS, the EPA is finalizing a Hazard Index (HI) of 1 (unitless) as the MCLG and MCL for any mixture containing two or more of PFHxS, PFNA, HFPO-DA, and PFBS. Once fully implemented, the EPA estimates that the rule will prevent thousands of deaths and reduce tens of thousands of serious PFAS-attributable illnesses.
DATES: This final rule is effective on June 25, 2024, published in the Federal Register April 26, 2024, page 32532.
View final rule.
The EPA is amending specific provisions in the Greenhouse Gas Reporting Rule to improve data quality and consistency. This action updates the General Provisions to reflect revised global warming potentials; expands reporting to additional sectors; improves the calculation, recordkeeping, and reporting requirements by updating existing methodologies; improves data verifications; and provides for collection of additional data to better inform and be relevant to a wide variety of Clean Air Act provisions that the EPA carries out. This action adds greenhouse gas monitoring and reporting for five source categories including coke calcining; ceramics manufacturing; calcium carbide production; caprolactam, glyoxal, and glyoxylic acid production; and facilities conducting geologic sequestration of carbon dioxide with enhanced oil recovery. These revisions also include changes that will improve implementation of the rule such as updates to applicability estimation methodologies, simplifying calculation and monitoring methodologies, streamlining recordkeeping and reporting, and other minor technical corrections or clarifications. This action also establishes and amends confidentiality determinations for the reporting of certain data elements to be added or substantially revised in these amendments.
DATES: This rule is effective January 1, 2025, published in the Federal Register April 25, 2024, page 31802.
View final rule.
Hi everyone! Welcome to the monthly news roundup video, where we’ll go over the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. Let’s get started! The Office of Management and Budget completed its review of OSHA’s worker walkaround final rule on March 20. The next step is publication in the Federal Register. The rule expands the criteria for who employees can authorize to act as their representative during an OSHA inspection.
Stand Up 4 Grain Safety Week was held the week of March 25. This annual event brings attention to hazards in the grain handling and storage industry and encourages employers to focus on safe work practices.
Over 100 people die in ladder-related deaths each year, and thousands more suffer disabling injuries. During Ladder Safety Month, which is held each March, the American Ladder Institute promotes ladder safety to decrease the number of injuries and fatalities.
Between 2010 and 2023, 11 miners drowned in incidents involving submerged mobile equipment. In response, the Mine Safety and Health Administration issued a safety alert. It recommends measures miners should take when operating equipment near water.
And finally, turning to environmental news, EPA finalized amendments to its Risk Management Program in an effort to improve safety at facilities that use and distribute hazardous chemicals. The rule seeks to improve chemical process safety; assist in planning for, preparing for, and responding to accidents; and increase public awareness of chemical hazards at regulated sites.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
The Environmental Protection Agency (EPA) is promulgating new greenhouse gas (GHG) emissions standards for model year (MY) 2032 and later heavy-duty highway vehicles that phase in starting as early MY 2027 for certain vehicle categories. The phase in revises certain MY 2027 GHG standards that were established previously under EPA's Greenhouse Gas Emissions and Fuel Efficiency Standards for Medium- and Heavy-Duty Engines and Vehicles—Phase 2 rule (“HD GHG Phase 2”). This document also updates discrete elements of the Averaging Banking and Trading program, including providing additional flexibilities for manufacturers to support the implementation of the Phase 3 program balanced by limiting the availability of certain advanced technology credits initially established under the HD GHG Phase 2 rule. EPA is also adding warranty requirements for batteries and other components of zero-emission vehicles and requiring customer-facing battery state-of-health monitors for plug-in hybrid and battery electric vehicles. In this action, we are also finalizing additional revisions, including clarifying and editorial amendments to certain highway heavy-duty vehicle provisions and certain test procedures for heavy-duty engines.
DATES: This final rule is effective on June 21, 2024, published in the Federal Register April 22, 2024, page 29440.
View final rule.
The Environmental Protection Agency (EPA) finalized a rule to designate two widely used PFAS — perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), including their salts and structural isomers — as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA).
The rule requires entities to immediately report releases of PFOA and PFOS that meet or exceed the reportable quantity (1 pound) to the:
Further, it gives EPA the authority to hold polluters responsible for paying for or conducting investigations and cleanup of PFOA and PFOS releases. In a memorandum, EPA clarified that it will focus enforcement efforts on significant contributors to PFAS releases.
Additionally:
The rule takes effect 60 days after it’s published in the Federal Register.
Key to remember: EPA has designated PFOA and PFOS as CERCLA hazardous substances, requiring immediate release notifications for the two PFAS and expanding the agency’s authority to hold parties responsible for contamination accountable.
It’s time for that special event that happens once every four years, often testing the endurance of the participants who’ve spent the prior years preparing for this very moment. No, it’s not the Summer Olympics, though that’s a great guess. It’s the Chemical Data Report!
Under the Toxic Substances Control ACT (TSCA), the Environmental Protection Agency’s (EPA’s) Chemical Data Reporting (CDR) rule requires manufacturers (including importers) to report information on the production and use of chemicals in commerce if they meet certain production volume thresholds at any one site. The submission period for the 2024 report runs from June 1 to September 30, 2024.
Use these tips to help you complete a Chemical Data Report worthy of a gold medal.
The TSCA Chemical Substance Inventory (TSCA Inventory) lists the covered chemical substances. Generally, the production volume threshold is 25,000 pounds or more of a chemical substance at a site. However, a reduced reporting threshold (2,500 pounds) applies to chemical substances subject to:
Further, certain full and partial exemptions apply to facilities based on the:
To confirm whether your facility must report:
The CDR rule requires facilities to report the total annual production volume of covered chemical substances for each calendar year since the last principal reporting year.
In other words, if a chemical substance at your facility meets or exceeds the corresponding reporting threshold during any calendar year covered by the report, you must include the total annual production volume of that chemical for every covered calendar year.
For example, you must list on the 2024 report the production volumes of every reportable chemical substance for 2020, 2021, 2022, and 2023.
All CDR data must be reported electronically on Form U (EPA Form 7740-8) through e-CDRweb on EPA’s Central Data Exchange (CDX) system. Reporting is site-specific, so if your organization has multiple sites with reportable chemicals, you must submit a Form U for each site.
Keep in mind that you submit only one form per site, so all reportable chemical substances at a specific site are listed on the same Form U. You may have to submit multiple forms only if you have more than one site covered by the CDR rule.
To submit a Chemical Data Report, you must first register with the CDX system and be approved by EPA. Plus, you must register the name of the organization on whose behalf you’re submitting a Form U. If you’re already registered on CDX, you can add the CDR reporting flow to your current registration.
Because each type of user role has varying permissions, it’s essential to register for the right one. User roles include:
Only Primary Authorized Officials may submit initial Chemical Data Reports. So, if you’re the one who will submit Form U, confirm that you’re registered as a Primary Authorized Official.
The CDR rule requires organizations to keep records of all CDR information reported on Form U to EPA for at least five years (711.25). The five-year timeline begins on the last day of the submission period.
Additionally, you may have to amend Form U after submitting the initial report. This can apply if:
Key to remember: The Chemical Data Report can be a major undertaking, but with these tips, you can cross the finish line with a report worthy of a gold medal.
Under the Clean Air Act, the Environmental Protection Agency (EPA) is establishing new, more protective emissions standards for criteria pollutants and greenhouse gases (GHG) for light-duty vehicles and Class 2b and 3 (‘‘medium-duty’’) vehicles that will phase-in over model years 2027 through 2032. In addition, EPA is finalizing GHG program revisions in several areas, including off-cycle and air conditioning credits, the treatment of upstream emissions associated with zero-emission vehicles and plug-in hybrid electric vehicles in compliance calculations, medium-duty vehicle incentive multipliers, and vehicle certification and compliance. EPA is also establishing new standards to control refueling emissions from incomplete medium-duty vehicles, and battery durability and warranty requirements for light-duty and medium-duty electric and plug-in hybrid electric vehicles. EPA is also finalizing minor amendments to update program requirements related to aftermarket fuel conversions, importing vehicles and engines, evaporative emission test procedures, and test fuel specifications for measuring fuel economy.
DATES: This final rule is effective on June 17, 2024, published in the Federal Register April 18, 2024, page 27842.
View final rule.
On August 31, 2020, in accordance with requirements under the Clean Air Act (CAA), the U.S. Environmental Protection Agency (EPA) performed a 5-year review of the Standards of Performance for New Stationary Sources and Emissions Guidelines for Existing Sources: Other Solid Waste Incineration (OSWI) Units, which includes certain very small municipal waste combustion (VSMWC) and institutional waste incineration (IWI) units. In the same action, the EPA proposed to remove the title V permitting requirements for air curtain incinerators (ACI) that burn only wood waste, clean lumber, yard waste, or a mixture of these three types of waste. In response to supportive comments received on the August 2020 proposal, this action is finalizing, as proposed, to remove the title V permitting requirements for ACIs that only burn wood waste, clean lumber, yard waste, or a mixture of those, and are not located at title V major sources or subject to title V for other reasons. The EPA is finalizing this proposed action now to simplify the compliance obligations for owners and operators of these types of units.
DATES: The effective date of this rule is April 17, 2024, published in the Federal Register April 17, 2024, page 27392.
View final rule.
| §60.2966 Am I required to apply for and obtain a title V operating permit for my unit? | ||
| Entire section | Revised | View text |
| §60.2967 When must I submit a title V permit application for my new unit? | ||
| Entire section | Revised | View text |
| §60.2969 What are the requirements for temporary-use incinerators and air curtain incinerators used in disaster recovery? | ||
| Entire section | Removed and reserved | View text |
| §60.2974 Am I required to apply for and obtain a title V operating permit for my air curtain incinerator that burns only wood waste, clean lumber, and yard waste? | ||
| Entire section | Removed and reserved | View text |
| Subpart FFFF - Emission Guidelines and Compliance Times for Other Solid Waste Incineration Units That Commenced Construction On or Before December 9, 2004 | ||
| Heading | Revised | View text |
| §60.3059 Am I required to apply for and obtain a title V operating permit for my unit? | ||
| Entire section | Revised | View text |
| §60.3060 When must I submit a title V permit application for my existing unit? | ||
| Entire section | Removed and reserved | View text |
Previous Text
§60.2966 Am I required to apply for and obtain a title V operating permit for my unit?
Yes, if you are subject to this subpart, you are required to apply for and obtain a title V operating permit unless you meet the relevant requirements for an exemption specified in §60.2887.
§60.2967 When must I submit a title V permit application for my new unit?
(a) If your new unit subject to this subpart is not subject to an earlier permit application deadline, a complete title V permit application must be submitted on or before one of the dates specified in paragraphs (a)(1) or (2) of this section. (See section 503(c) of the Clean Air Act and 40 CFR 70.5(a)(1)(i) and 40 CFR 71.5(a)(1)(i).)
(1) For a unit that commenced operation as a new source as of December 16, 2005, then a complete title V permit application must be submitted not later than December 18, 2006.
(2) For a unit that does not commence operation as a new source until after December 16, 2005, then a complete title V permit application must be submitted not later than 12 months after the date the unit commences operation as a new source.
(b) If your new unit subject to this subpart is subject to title V as a result of some triggering requirement(s) other than this subpart (for example, a unit subject to this subpart may be a major source or part of a major source), then your unit may be required to apply for a title V permit prior to the deadlines specified in paragraph (a) of this section. If more than one requirement triggers a source's obligation to apply for a title V permit, the 12-month timeframe for filing a title V permit application is triggered by the requirement that first causes the source to be subject to title V. (See section 503(c) of the Clean Air Act and 40 CFR 70.3(a) and (b), 40 CFR 70.5(a)(1)(i), 40 CFR 71.3(a) and (b), and 40 CFR 71.5(a)(1)(i).)
(c) A “complete” title V permit application is one that has been determined or deemed complete by the relevant permitting authority under section 503(d) of the Clean Air Act and 40 CFR 70.5(a)(2) or 40 CFR 71.5(a)(2). You must submit a complete permit application by the relevant application deadline in order to operate after this date in compliance with Federal law. (See sections 503(d) and 502(a) of the Clean Air Act and 40 CFR 70.7(b) and 40 CFR 71.7(b).)
Subpart FFFF - Emission Guidelines and Compliance Times for Other Solid Waste Incineration Units That Commenced Construction On or Before December 9, 2004
§60.3059 Am I required to apply for and obtain a title V operating permit for my unit?
Yes, if you are subject to an applicable EPA-approved and effective Clean Air Act section 111(d)/129 State or Tribal plan or an applicable and effective Federal plan, you are required to apply for and obtain a title V operating permit unless you meet the relevant requirements for an exemption specified in §60.2993.
EPA is proposing significant new use rules (SNURs) under the Toxic Substances Control Act (TSCA) for chemical substances that were the subject of premanufacture notices (PMNs). The chemical substances received “not likely to present an unreasonable risk” determinations pursuant to TSCA. The SNURs require persons who intend to manufacture (defined by statute to include import) or process any of these chemical substances for an activity that is proposed as a significant new use by this rulemaking to notify EPA at least 90 days before commencing that activity. The required notification initiates EPA's evaluation of the use, under the conditions of use for that chemical substance. In addition, the manufacture or processing for the significant new use may not commence until EPA has conducted a review of the required notification, made an appropriate determination regarding that notification, and taken such actions as required by that determination.
DATES: This proposed rule is published in the Federal Register April 8, 2024, page 24398.
View proposed rule.
This action finalizes the residual risk and technology review (RTR) conducted for the Commercial Sterilization Facilities source category regulated under national emission standards for hazardous air pollutants (NESHAP) under the Clean Air Act. The EPA is finalizing decisions concerning the RTR, including definitions for affected sources, emission standards for previously unregulated sources, amendments pursuant to the risk review to address ethylene oxide (EtO) emissions from certain sterilization chamber vents (SCVs), aeration room vents (ARVs), chamber exhaust vents (CEVs), and room air emissions, and amendments pursuant to the technology review for certain SCVs and ARVs. In addition, we are taking final action to correct and clarify regulatory provisions related to emissions during periods of startup, shutdown, and malfunction (SSM), including removing exemptions for periods of SSM. We are also taking final action to require owners and operators to demonstrate compliance through the use of EtO continuous emissions monitoring systems (CEMS), with exceptions for very small users of EtO; add provisions for electronic reporting of performance test results and other reports; and include other technical revisions to improve consistency and clarity. We estimate that these final amendments will reduce EtO emissions from this source category by approximately 21 tons per year (tpy).
DATES: This final rule is effective on April 5, 2024, published in the Federal Register April 5, 2024, page 24090.
View final rule.
| Appendix B to Part 60—Performance Specifications | ||
| Performance Specification 19 | Added | View text |
| Appendix F to Part 60—Quality Assurance Procedures | ||
| Procedure 7 | Added | View text |
| §63.14 Incorporations by reference. | ||
| (a), (f), (i) introductory text | Revised | View text |
| (i)(88)-(120) | Redesignated | View text |
| Subpart O—Ethylene Oxide Emissions Standards for Sterilization Facilities | ||
| Entire subpart | Revised | View text |
Previous Text
§63.14 Incorporations by reference.
(a) The materials listed in this section are incorporated by reference into this part with the approval of the Director of the Federal Register under 5 U.S.C. 552(a) and 1 CFR part 51. To enforce any edition other than that specified in this section, a document must be published in the Federal Register and the material must be available to the public. All approved materials are available for inspection at the Air and Radiation Docket and Information Center (Air Docket) in the EPA Docket Center (EPA/DC) at Rm. 3334, EPA West Bldg., 1301 Constitution Ave. NW, Washington, DC. The EPA/DC Public Reading Room hours of operation are 8:30 a.m. to 4:30 p.m., Monday through Friday, excluding legal holidays. The telephone number of the EPA/DC Public Reading Room is (202) 566-1744, and the telephone number for the Air Docket is (202) 566-1742. These approved materials are also available for inspection at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, email fedreg.legal@nara.gov or go to www.archives.gov/federal-register/cfr/ibr-locations.html. In addition, these materials are available from the following sources:
* * * *
(f) American Society of Mechanical Engineers (ASME), Three Park Avenue, New York, NY 10016-5990, Telephone (800) 843-2763, http://www.asme.org; also available from HIS, Incorporated, 15 Inverness Way East, Englewood, CO 80112, Telephone (877) 413-5184, http://global.ihs.com.
(1) ANSI/ASME PTC 19.10-1981, Flue and Exhaust Gas Analyses [Part 10, Instruments and Apparatus], issued August 31, 1981, IBR approved for §§63.309(k), 63.457(k), 63.772(e) and (h), 63.865(b), 63.997(e), 63.1282(d) and (g), and 63.1625(b), table 5 to subpart EEEE, §§63.3166(a), 63.3360(e), 63.3545(a), 63.3555(a), 63.4166(a), 63.4362(a), 63.4766(a), 63.4965(a), and 63.5160(d), table 4 to subpart UUUU, table 3 to subpart YYYY, §§63.7822(b), 63.7824(e), 63.7825(b), 63.8000(d), 63.9307(c), 63.9323(a), 63.9621(b) and (c), 63.11148(e), 63.11155(e), 63.11162(f), 63.11163(g), 63.11410(j), 63.11551(a), 63.11646(a), and 63.11945, and table 4 to subpart AAAAA, table 5 to subpart DDDDD, table 4 to subpart JJJJJ, table 4 to subpart KKKKK, table 4 to subpart SSSSS, tables 4 and 5 of subpart UUUUU, table 1 to subpart ZZZZZ, and table 4 to subpart JJJJJJ.
(2) [Reserved]
* * * *
(i) ASTM International, 100 Barr Harbor Drive, Post Office Box C700, West Conshohocken, PA 19428-2959, Telephone (610) 832-9585, http://www.astm.org; also available from ProQuest, 789 East Eisenhower Parkway, Ann Arbor, MI 48106-1346, Telephone (734) 761-4700, http://www.proquest.com.
Subpart O—Ethylene Oxide Emissions Standards for Sterilization Facilities
§63.360 Applicability.
(a) All sterilization sources using 1 ton (see definition) in sterilization or fumigation operations are subject to the emissions standards in §63.362, except as specified in paragraphs (b) through (e) of this section. Owners or operators of sources using 1 ton (see definition) subject to the provisions of this subpart must comply with the requirements of subpart A, of this part according to the applicability of subpart A of this part to such sources in Table 1 of this section.
| Reference | Applies to sources using 10 tons in subpart O a | Applies to sources using 1 to 10 tons in subpart O a | Comment |
|---|---|---|---|
| a See definition. | |||
| 63.1(a)(1) | Yes | Additional terms defined in §63.361; when overlap between subparts A and O occurs, subpart O takes precedence. | |
| 63.1(a)(2) | Yes | ||
| 63.1(a)(3) | Yes | ||
| 63.1(a)(4) | Yes | Subpart O clarifies the applicability of each paragraph in subpart A to sources subject to subpart O. | |
| 63.1(a)(5) | No | Reserved. | |
| 63.1(a)(6) | Yes | ||
| 63.1(a)(7) | Yes | ||
| 63.1.1(a)(8) | Yes | ||
| 63.1(a)(9) | No | Reserved. | |
| 63.1(a)(10) | Yes | ||
| 63.1(a)(11) | Yes | §63.366(a) of subpart O also allows report submissions via fax and on electronic media. | |
| 63.1(a)(12)-(14) | Yes | ||
| 63.1(b)(1)-(2) | Yes | ||
| 63.1(b)(3) | No | §63.367 clarifies the applicability of recordkeeping requirements for sources that determine they are not subject to the emissions standards. | |
| 63.1(c)(1) | Yes | Subpart O clarifies the applicability of each paragraph in subpart A to sources subject to subpart O in this table. | |
| 63.1(c)(2) | Yes | §63.360(f) exempts area sources subject to this subpart from the obligation to obtain Title V operating permits. | |
| 63.1(c)(3) | No | Reserved. | |
| 63.1(c)(4) | Yes | ||
| 63.1(c)(5) | No | §63.360 specifies applicability. | |
| 63.1(d) | No | Reserved. | |
| 63.1(e) | Yes | ||
| 63.2 | Yes | Additional terms defined in §63.361; when overlap between subparts A and O occurs, subpart O takes precedence. | |
| 63.3 | Yes | Other units used in subpart O are defined in the text of subpart O. | |
| 63.4(a)(1)-(3) | Yes | ||
| 63.4(a)(4) | No | Reserved. | |
| 63.4(a)(5) | Yes | ||
| 63.4(b) | Yes | ||
| 63.4(c) | Yes | ||
| 63.5(a) | No | §63.366(b)(1) contains applicability requirements for constructed or reconstructed sources. | |
| 63.5(b)(1) | Yes | No | |
| 63.5(b)(2) | No | Reserved. | |
| 63.5(b)(3) | No | See §63.366(b)(2). | |
| 63.5(b)(4) | Yes | No | |
| 63.5(b)(5) | Yes | No | |
| 63.5(b)(6) | Yes | No | |
| 63.5(c) | No | Reserved. | |
| 63.5(d)(1)-(2) | No | See §63.366(b)(3). | |
| 63.5(d)(3)-(4) | Yes | No | |
| 63.5(e) | Yes | No | |
| 63.5(f)(1)-(2) | No | See §63.366(b)(4). | |
| 63.6(a)(1) | Yes | ||
| 63.6(a)(2) | No | §63.360 specifies applicability. | |
| 63.6(b)-(c) | No | §63.360(g) specifies compliance dates for sources. | |
| 63.6(d) | No | Reserved. | |
| 63.6(e) | No | Subpart O does not contain any operation and maintenance plan requirements. | |
| 63.6(f)(1) | No | §63.362(b) specifies when the standards apply. | |
| 63.6(f)(2)(i) | Yes | ||
| 63.6(f)(2)(ii) | No | §63.363 specifies parameters for determining compliance. | |
| 63.6(f)(2)(iii)-(iv) | Yes | ||
| 63.6(f)(2)(v) | No | ||
| 63.6(f)(3) | Yes | ||
| 63.6(g) | Yes | ||
| 63.6(h) | No | Subpart O does not contain any opacity or visible emission standards. | |
| 63.6(i)(1)-(14) | Yes | ||
| 63.6(i)(15) | No | Reserved | |
| 63.6(i)(16) | Yes | ||
| 63.6(j) | Yes | ||
| 63.7(a)(1) | Yes | ||
| 63.7(a)(2) | Yes | ||
| 63.7(a)(3) | Yes | ||
| 63.7(b) | Yes | ||
| 63.7(c) | Yes | No | |
| 63.7(d) | Yes | No | |
| 63.7(e) | Yes | §63.365 also contains test methods specific to sources subject to the emissions standards. | |
| 63.7(f) | Yes | ||
| 63.7(g)(1) | Yes | ||
| 63.7(g)(2) | No | Reserved | |
| 63.7(g)(3) | Yes | ||
| 63.7(h) | Yes | ||
| 63.8(a)(1) | Yes | ||
| 63.8(a)(2) | Yes | ||
| 63.8(a)(3) | No | Reserved | |
| 63.8(a)(4) | Yes | ||
| 63.8(b)(1) | Yes | ||
| 63.8(b)(2) | Yes | ||
| 63.8(b)(3) | No | ||
| 63.8(c)(1) (i)-(ii) | No | A startup, shutdown, and malfunction plan is not required for these standards. | |
| 63.8(c)(1)(iii) | Yes | ||
| 63.8(c)(2)-(3) | Yes | ||
| 63.8(c)(4)-(5) | No | Frequency of monitoring measurements is provided in §63.364; opacity monitors are not required for these standards. | |
| 63.8(c)(6) | No | Performance specifications for gas chromatographs and temperature monitors are contained in §63.365. | |
| 63.8(c)(7)(i)(A)-(B) | No | Performance specifications for gas chromatographs and temperature monitors are contained in §63.365. | |
| 63.8(c)(7)(i)(C) | No | Opacity monitors are not required for these standards. | |
| 63.8(c)(7)(ii) | No | Performance specifications for gas chromatographs and temperature monitors are contained in §63.365. | |
| 63.8(c)(8) | No | ||
| 63.8(d) | Yes | No | |
| 63.8(e)(1) | Yes | ||
| 63.8(e)(2) | Yes | ||
| 63.8(e)(3) | Yes | No | |
| 63.8(e)(4) | Yes | ||
| 63.8(e)(5)(i) | Yes | ||
| 63.8(e)(5)(ii) | No | Opacity monitors are not required for these standards. | |
| 63.8(f)(1)-(5) | Yes | ||
| 63.8(f)(6) | No | ||
| 63.8(g)(1) | Yes | ||
| 63.8(g)(2) | No | ||
| 63.8(g)(3)-(5) | Yes | ||
| 63.9(a) | Yes | ||
| 63.9(b)(1)-(i) | Yes | ||
| 63.9(b)(1)(ii)-(iii) | No | §63.366(c)(1)(i) contains language for sources that increase usage such that the source becomes subject to the emissions standards. | |
| 63.9(b)(2)-(3) | Yes | §63.366(c)(3) contains additional information to be included in the initial report for existing and new sources. | |
| 63.9(b)(4)-(5) | No | §63.366(c)(1)(ii) and (iii) contains requirements for new or reconstructed sources subject to the emissions standards. | |
| 63.9(c) | Yes | ||
| 63.9(d) | No | ||
| 63.9(e) | Yes | ||
| 63.9(f) | No | Opacity monitors are not required for these standards. | |
| 63.9(g)(1) | Yes | ||
| 63.9(g)(2)-(3) | No | Opacity monitors and relative accuracy testing are not required for these standards. | |
| 63.9(h)(1)-(3) | Yes | ||
| 63.9(h)(4) | No | Reserved. | |
| 63.9(h)(5) | No | §63.366(c)(2) instructs sources to submit actual data. | |
| 63.9(h)(6) | Yes | ||
| 63.9(i) | Yes | ||
| 63.9(j) | Yes | ||
| 63.10(a) | Yes | ||
| 63.10(b)(1) | Yes | ||
| 63.10(b)(2)(i) | No | Not applicable due to batch nature of the industry. | |
| 63.10(b)(2)(ii) | Yes | ||
| 63.10(b)(2)(iii) | No | ||
| 63.10(b)(2)(iv)-(v) | No | A startup, shutdown, and malfunction plan is not required for these standards. | |
| 63.10(b)(2)(vi)-(xii) | Yes | ||
| 63.10(b)(2)(xiii) | No | ||
| 63.10(b)(2)(xiv) | Yes | ||
| 63.10(b)(3) | No | §63.367 (b) and (c) contains applicability determination requirements. | |
| 63.10(c)(1) | Yes | ||
| 63.10(c)(2)-(4) | No | Reserved. | |
| 63.10(c)(5) | Yes | ||
| 63.10(c)(6) | No | ||
| 63.10(c)(7) | No | Not applicable due to batch nature of the industry. | |
| 63.10(c)(8) | Yes | ||
| 63.10(c)(9) | No | ||
| 63.10(c)(10)-(13) | Yes | ||
| 63.10(c)(14) | Yes | No | |
| 63.10(c)(15) | No | A startup, shutdown, and malfunction plan is not required for these standards. | |
| 63.10(d)(1) | Yes | ||
| 63.10(d)(2) | Yes | ||
| 63.10(d)(3) | No | Subpart O does not contain opacity or visible emissions standards. | |
| 63.10(d)(4) | Yes | ||
| 63.10(d)(5) | No | A startup, shutdown, and malfunction plan is not required for these standards. | |
| 63.10(e)(1) | Yes | ||
| 63.10(e)(2)(i) | Yes | ||
| 63.10(e)(2)(ii) | No | Opacity monitors are not required for these standards. | |
| 63.10(e)(3)(i)-(iv) | Yes | ||
| 63.10(e)(3)(v) | No | §63.366(a)(3) specifies contents and submittal dates for excess emissions and monitoring system performance reports. | |
| 63.10(e)(3)(vi)-(viii) | Yes | ||
| 63.10(e)(4) | No | Opacity monitors are not required for these standards. | |
| 63.10(f) | Yes | ||
| 63.11 | Yes | ||
| 63.12-63.15 | Yes | ||
(b) Sterilization sources using less than 1 ton (see definition) are not subject to the emissions standards in §63.362. The recordkeeping requirements of §63.367(c) apply.
(c) This subpart does not apply to beehive fumigators.
(d) This subpart does not apply to research or laboratory facilities as defined in section 112(c)(7) of title III of the Clean Air Act Amendment of 1990.
(e) This subpart does not apply to ethylene oxide sterilization operations at stationary sources such as hospitals, doctors offices, clinics, or other facilities whose primary purpose is to provide medical services to humans or animals.
(f) If you are an owner or operator of an area source subject to this subpart, you are exempt from the obligation to obtain a permit under 40 CFR part 70 or 71, provided you are not required to obtain a permit under 40 CFR 70.3(a) or 71.3(a) for a reason other than your status as an area source under this subpart. Notwithstanding the previous sentence, you must continue to comply with the provisions of this subpart applicable to area sources.
(g) The owner or operator shall comply with the provisions of this subpart as follows:
(1) All sterilization chamber vents subject to the emissions standards in §63.362 with an initial startup date before December 6, 1998, no later than December 6, 1998.
(2) All sterilization chamber vents subject to the emissions standards in §63.362 with an initial startup date on or after December 6, 1998, immediately upon initial startup of the source.
(3) All sterilization chamber vents at sources using less than 1 ton of ethylene oxide that increase their ethylene oxide usage after December 6, 1998 such that the sterilization chamber vent becomes subject to the emissions standards in §63.362(c), immediately upon becoming subject to the emission standards.
(4) All aeration room vents subject to the emissions standards in §63.362 with an initial startup date before December 6, 2000, no later than December 6, 2000.
(5) All aeration room vents subject to the emissions standards in §63.362 with an initial startup date on or after December 6, 2000, immediately upon initial startup of the source.
(6) All aeration room vents at sources using less than 10 tons that increase their ethylene oxide usage after December 6, 2000, such that the aeration room vents become subject to the emissions standards in §63.362, immediately upon becoming subject to the emission standards.
(7)-(10) [Reserved]
§63.361 Definitions.
Terms and nomenclature used in this subpart are defined in the Clean Air Act (the Act) as amended in 1990, §§63.2 and 63.3 of subpart A of this part, or in this section. For the purposes of subpart O, if the same term is defined in subpart A and in this section, it shall have the meaning given in this section.
Aeration room means any vessel or room that is used to facilitate off-gassing of ethylene oxide at a sterilization facility.
Aeration room vent means the point(s) through which the evacuation of ethylene oxide-laden air from an aeration room occurs.
Baseline temperature means a minimum temperature at the outlet from the catalyst bed of a catalytic oxidation control device or at the exhaust point from the combustion chamber of a thermal oxidation control device.
Chamber exhaust vent means the point(s) through which ethylene oxide-laden air is removed from the sterilization chamber during chamber unloading following the completion of sterilization and associated air washes.
Compliance date means the date by which a source subject to the emissions standards in §63.362 is required to be in compliance with the standard.
Deviation means any instance in which an affected source, subject to this subpart, or an owner or operator of such a source:
(1) Fails to meet any requirement or obligation established by this subpart including, but not limited to, any emission limitation (including any operating limit) or work practice standard;
(2) Fails to meet any term or condition that is adopted to implement an applicable requirement in this subpart and that is included in the operating permit for any affected source required to obtain such a permit; or
(3) Fails to meet any emission limitation (including any operating limit) or work practice standard in this subpart during startup, shutdown, or malfunction, regardless of whether or not such failure is permitted by this subpart.
Effective date means the date of promulgation in the Federal Register notice.
Initial startup date means the date when a source subject to the emissions standards in §63.362 first begins operation of a sterilization process.
Manifolding emissions means combining ethylene oxide emissions from two or more different vent types for the purpose of controlling these emissions with a single control device.
Maximum ethylene glycol concentration means any concentration of ethylene glycol in the scrubber liquor of an acid-water scrubber control device established during a performance test when the scrubber achieves at least 99-percent control of ethylene oxide emissions.
Maximum liquor tank level means any level of scrubber liquor in the acid-water scrubber liquor recirculation tank established during a performance test when the scrubber achieves at least 99-percent control of ethylene oxide emissions.
Oxidation temperature means the temperature at the outlet point of a catalytic oxidation unit control device or at the exhaust point from the combustion chamber for a thermal oxidation unit control device.
Source(s) using less than 1 ton means source(s) using less than 907 kg (1 ton) of ethylene oxide within all consecutive 12-month periods after December 6, 1996.
Source(s) using 1 ton means source(s) using 907 kg (1 ton) or more of ethylene oxide within any consecutive 12-month period after December 6, 1996.
Source(s) using 1 to 10 tons means source(s) using 907 kg (1 ton) or more of ethylene oxide in any consecutive 12-month period but less than 9,070 kg (10 tons) of ethylene oxide in all consecutive 12-month periods after December 6, 1996.
Source(s) using less than 10 tons means source(s) using less than 9,070 kg (10 tons) of ethylene oxide in all consecutive 12-month periods after December 6, 1996.
Source(s) using 10 tons means source(s) using 9,070 kg (10 tons) or more of ethylene oxide in any consecutive 12-month period after December 6, 1996.
Sterilization chamber means any enclosed vessel or room that is filled with ethylene oxide gas, or an ethylene oxide/inert gas mixture, for the purpose of sterilizing and/or fumigating at a sterilization facility.
Sterilization chamber vent means the point (prior to the vacuum pump) through which the evacuation of ethylene oxide from the sterilization chamber occurs following sterilization or fumigation, including any subsequent air washes.
Sterilization facility means any stationary source where ethylene oxide is used in the sterilization or fumigation of materials.
Sterilization operation means any time when ethylene oxide is removed from the sterilization chamber through the sterilization chamber vent or the chamber exhaust vent or when ethylene oxide is removed from the aeration room through the aeration room vent.
Thermal oxidizer means all combustion devices except flares.
§63.362 Standards.
(a) Each owner or operator of a source subject to the provisions of this subpart shall comply with these requirements on and after the compliance date specified in §63.360(g). The standards of this section are summarized in Table 1 of this section.
| Existing and new sources | Source type | Sterilization chamber vent | Aeration room vent | Chamber exhaust vent |
|---|---|---|---|---|
| Source size | <907 kg (<1 ton) | No control required; minimal recordkeeping requirements apply (see §63.367(c)). | ||
| ≥907 kg and <9,070 kg (≥1 ton and <10 tons) | 99% emission reduction (see §63.362(c)) | No control | No control. | |
| ≥9,070 kg (≥10 tons) | 99% emission reduction (see §63.362(c)) | 1 ppm maximum outlet concentration or 99% emission reduction (see §63.362(d)) | No control. | |
(b) Applicability of emission limits. The emission limitations of paragraphs (c), (d), and (e) of this section apply during sterilization operation. The emission limitations do not apply during periods of malfunction.
(c) Sterilization chamber vent at sources using 1 ton. Each owner or operator of a sterilization source using 1 ton shall reduce ethylene oxide emissions to the atmosphere by at least 99 percent from each sterilization chamber vent.
(d) Aeration room vent at sources using 10 tons. Each owner or operator of a sterilization source using 10 tons shall reduce ethylene oxide emissions to the atmosphere from each aeration room vent to a maximum concentration of 1 ppmv or by at least 99 percent, whichever is less stringent, from each aeration room vent.
(e) [Reserved]
§63.363 Compliance and performance provisions.
(a)(1) The owner or operator of a source subject to emissions standards in §63.362 shall conduct an initial performance test using the procedures listed in §63.7 according to the applicability in Table 1 of §63.360, the procedures listed in this section, and the test methods listed in §63.365.
(2) The owner or operator of all sources subject to these emissions standards shall complete the performance test within 180 days after the compliance date for the specific source as determined in §63.360(g).
(b) The procedures in paragraphs (b)(1) through (3) of this section shall be used to determine initial compliance with the emission limits under §63.362(c), the sterilization chamber vent standard and to establish operating limits for the control devices:
(1) The owner or operator shall determine the efficiency of control devices used to comply with §63.362(c) using the test methods and procedures in §63.365(b).
(2) For facilities with acid-water scrubbers, the owner or operator shall establish as an operating limit either:
(i) The maximum ethylene glycol concentration using the procedures described in §63.365(e)(1); or
(ii) The maximum liquor tank level using the procedures described in §63.365(e)(2).
(3) For facilities with catalytic oxidizers or thermal oxidizers, the operating limit consists of the recommended minimum oxidation temperature provided by the oxidation unit manufacturer for an operating limit.
(4) Facilities with catalytic oxidizers shall comply with one of the following work practices:
(i) Once per year after the initial compliance test, conduct a performance test during routine operations, i.e., with product in the chamber using the procedures described in §63.365(b) or (d) as appropriate. If the percent efficiency is less than 99 percent, restore the catalyst as soon as practicable but no later than 180 days after conducting the performance test; or
(ii) Once per year after the initial compliance test, analyze ethylene oxide concentration data from §63.364(e) or a continuous emission monitoring system (CEMS) and restore the catalyst as soon as practicable but no later than 180 days after data analysis; or,
(iii) Every 5 years, beginning 5 years after the initial compliance test (or by December 6, 2002, whichever is later), replace the catalyst bed with new catalyst material.
(c) The procedures in paragraphs (c)(1) through (3) of this section shall be used to determine initial compliance with the emission limits under §63.362(d), the aeration room vent standard:
(1) The owner or operator shall comply with either paragraph (b)(2) or (3) of this section.
(2) Determine the concentration of ethylene oxide emitted from the aeration room into the atmosphere (after any control device used to comply with §63.362(d)) using the methods in §63.365(c)(1); or
(3) Determine the efficiency of the control device used to comply with §63.362(d) using the test methods and procedures in §63.365(d)(2).
(d) [Reserved]
(e) For facilities complying with the emissions limits under §63.362 with a control technology other than acid-water scrubbers or catalytic or thermal oxidizers, the owner or operator of the facility shall provide to the Administrator or delegated authority information describing the design and operation of the air pollution control system, including recommendations for the operating parameters to be monitored to demonstrate continuous compliance. Based on this information, the Administrator will determine the operating parameter(s) to be measured during the performance test. During the performance test required in paragraph (a) of this section, using the methods approved in §63.365(g), the owner or operator shall determine the site-specific operating limit(s)for the operating parameters approved by the Administrator.
(f) A facility must demonstrate continuous compliance with each operating limit and work practice standard required under this section, except during periods of startup, shutdown, and malfunction, according to the methods specified in §63.364.
§63.364 Monitoring requirements.
(a)(1) The owner or operator of a source subject to emissions standards in §63.362 shall comply with the monitoring requirements in §63.8 of subpart A of this part, according to the applicability in Table 1 of §63.360, and in this section.
(2) Each owner or operator of an ethylene oxide sterilization facility subject to these emissions standards shall monitor the parameters specified in this section. All monitoring equipment shall be installed such that representative measurements of emissions or process parameters from the source are obtained. For monitoring equipment purchased from a vendor, verification of the operational status of the monitoring equipment shall include completion of the manufacturer's written specifications or recommendations for installation, operation, and calibration of the system.
(b) For sterilization facilities complying with §63.363(b) or (d) through the use of an acid-water scrubber, the owner or operator shall either:
(1) Sample the scrubber liquor and analyze and record once per week the ethylene glycol concentration of the scrubber liquor using the test methods and procedures in §63.365(e)(1). Monitoring is required during a week only if the scrubber unit has been operated; or
(2) Measure and record once per week the level of the scrubber liquor in the recirculation tank. The owner or operator shall install, maintain, and use a liquid level indicator to measure the scrubber liquor tank level (i.e., a marker on the tank wall, a dipstick, a magnetic indicator, etc.). Monitoring is required during a week only if the scrubber unit has been operated.
(c) For sterilization facilities complying with §63.363(b) or (c) through the use of catalytic oxidation or thermal oxidation, the owner or operator shall either comply with §63.364(e) or continuously monitor and record the oxidation temperature at the outlet to the catalyst bed or at the exhaust point from the thermal combustion chamber using the temperature monitor described in paragraph (c)(4) of this section. Monitoring is required only when the oxidation unit is operated. From 15-minute or shorter period temperature values, a data acquisition system for the temperature monitor shall compute and record a daily average oxidation temperature. Strip chart data shall be converted to record a daily average oxidation temperature each day any instantaneous temperature recording falls below the minimum temperature.
(1)-(3) [Reserved]
(4) The owner or operator shall install, calibrate, operate, and maintain a temperature monitor accurate to within ±5.6°C (±10°F) to measure the oxidation temperature. The owner or operator shall verify the accuracy of the temperature monitor twice each calendar year with a reference temperature monitor (traceable to National Institute of Standards and Technology (NIST) standards or an independent temperature measurement device dedicated for this purpose). During accuracy checking, the probe of the reference device shall be at the same location as that of the temperature monitor being tested. As an alternative, the accuracy temperature monitor may be verified in a calibrated oven (traceable to NIST standards).
(d) For sterilization facilities complying with §63.363(b) or (c) through the use of a control device other than acid-water scrubbers or catalytic or thermal oxidizers, the owner or operator shall monitor the parameters as approved by the Administrator using the methods and procedures in §63.365(g).
(e) Measure and record once per hour the ethylene oxide concentration at the outlet to the atmosphere after any control device according to the procedures specified in §63.365(c)(1). The owner or operator shall compute and record a 24-hour average daily. The owner or operator will install, calibrate, operate, and maintain a monitor consistent with the requirements of performance specification (PS) 8 or 9 in 40 CFR part 60, appendix B, to measure ethylene oxide. The daily calibration requirements of section 7.2 of PS-9 or Section 13.1 of PS-8 are required only on days when ethylene oxide emissions are vented to the control device.
(f) [Reserved]
§63.365 Test methods and procedures.
(a) Performance testing. The owner or operator of a source subject to the emissions standards in §63.362 shall comply with the performance testing requirements in §63.7 of subpart A of this part, according to the applicability in Table 1 of §63.360, and in this section.
(b) Efficiency at the sterilization chamber vent. California Air Resources Board (CARB) Method 431 or the following procedures shall be used to determine the efficiency of all types of control devices used to comply with §63.362(c), sterilization chamber vent standard.
(1) First evacuation of the sterilization chamber. These procedures shall be performed on an empty sterilization chamber, charged with a typical amount of ethylene oxide, for the duration of the first evacuation under normal operating conditions (i.e., sterilization pressure and temperature).
(i) The amount of ethylene oxide loaded into the sterilizer (Wc) shall be determined by either:
(A) Weighing the ethylene oxide gas cylinder(s) used to charge the sterilizer before and after charging. Record these weights to the nearest 45 g (0.1 lb). Multiply the total mass of gas charged by the weight percent ethylene oxide present in the gas.
(B) Installing calibrated rotameters at the sterilizer inlet and measuring flow rate and duration of sterilizer charge. Use the following equation to convert flow rate to weight of ethylene oxide:
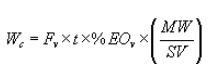
where:
Wc = weight of ethylene oxide charged, g (lb)
Fv = volumetric flow rate, liters per minute (L/min) corrected to 20°C and 101.325 kilopascals (kPa) (scf per minute (scfm) corrected to 68°F and 1 atmosphere of pressure (atm)); the flowrate must be constant during time (t)
t = time, min
%EOV = volume fraction ethylene oxide
SV = standard volume, 24.05 liters per mole (L/mole) = 22.414 L/mole ideal gas law constant corrected to 20°C and 101.325 kPa (385.32 scf per mole (scf/mole) = 359 scf/mole ideal gas law constant corrected to 68°F and 1 atm).
MW = molecular weight of ethylene oxide, 44.05 grams per gram-mole (g/g-mole) (44.05 pounds per pound-mole (lb/lb-mole)), or
(C) Calculating the mass based on the conditions of the chamber immediately after it has been charged using the following equation:
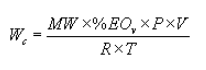
where:
P = chamber pressure, kPa (psia)
V = chamber volume, liters (L) (ft 3)
R = gas constant, 8.313 L·kPa/g-mole·(10.73 psia·ft 3/mole°R)
T = temperature, K (°R)
Note:
If the ethylene oxide concentration is in weight percent, use the following equation to calculate mole fraction:
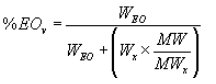
where:
WEO = weight percent of ethylene oxide
Wx = weight percent of compound in the balance of the mixture
MWx = molecular weight of compound in the balance gas mixture
(ii) The residual mass of ethylene oxide in the sterilizer shall be determined by recording the chamber temperature, pressure, and volume after the completion of the first evacuation and using the following equation:
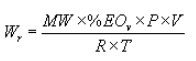
where:
Wr = weight of ethylene oxide remaining in chamber (after the first evacuation), in g (lb)
(iii) Calculate the total mass of ethylene oxide at the inlet to the control device (Wi) by subtracting the residual mass (Wr) calculated in paragraph (b)(1)(ii) of this section from the charged weight (Wc) calculated in paragraph (b)(1)(i) of this section.
(iv) The mass of ethylene oxide emitted from the control device outlet (Wo) shall be calculated by continuously monitoring the flow rate and concentration using the following procedure.
(A) Measure the flow rate through the control device exhaust continuously during the first evacuation using the procedure found in 40 CFR part 60, appendix A, Test Methods 2, 2A, 2C, or 2D, as appropriate. (Method 2D (using orifice plates or Rootstype meters) is recommended for measuring flow rates from sterilizer control devices.) Record the flow rate at 1-minute intervals throughout the test cycle, taking the first reading within 15 seconds after time zero. Time zero is defined as the moment when the pressure in the sterilizer is released. Correct the flow to standard conditions (20°C and 101.325 kPa (68°F and 1 atm)) and determine the flow rate for the run as outlined in the test methods listed in paragraph (b) of this section.
(B) Test Method 18 or 25A, 40 CFR part 60, appendix A (hereafter referred to as Method 18 or 25A, respectively), shall be used to measure the concentration of ethylene oxide.
(1) Prepare a graph of volumetric flow rate versus time corresponding to the period of the run cycle. Integrate the area under the curve to determine the volume.
(2) Calculate the mass of ethylene oxide by using the following equation:
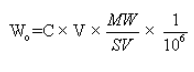
Where:
Wo = Mass of ethylene oxide, g (lb)
C = concentration of ethylene oxide in ppmv
V = volume of gas exiting the control device corrected to standard conditions, L (ft 3)
1/10 6 = correction factor LEO/10 6 LTOTAL GAS (ft 3EO/10 6 ft 3TOTAL GAS)
(3) Calculate the efficiency by the equation in paragraph (b)(1)(v) of this section.
(C) [Reserved]
(v) Determine control device efficiency (% Eff) using the following equation:
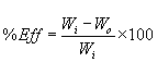
where:
% Eff = percent efficiency
Wi = mass flow rate into the control device
Wo = mass flow rate out of the control device
(vi) Repeat the procedures in paragraphs (b)(1) (i) through (v) of this section three times. The arithmetic average percent efficiency of the three runs shall determine the overall efficiency of the control device.
(2) [Reserved]
(c) Concentration determination. The following procedures shall be used to determine the ethylene oxide concentration.
(1) Parameter monitoring. For determining the ethylene oxide concentration required in §63.364(e), follow the procedures in PS 8 or PS 9 in 40 CFR part 60, appendix B. Sources complying with PS 8 are exempt from the relative accuracy procedures in sections 2.4 and 3 of PS-8.
(2) Initial compliance. For determining the ethylene oxide concentration required in §63.363(c)(2), the procedures outlined in Method 18 or Method 25 A (40 CFR part 60, appendix A) shall be used. A Method 18 or Method 25A test consists of three 1-hour runs. If using Method 25A to determine concentration, calibrate and report Method 25A instrument results using ethylene oxide as the calibration gas. The arithmetic average of the ethylene oxide concentration of the three test runs shall determine the overall outlet ethylene oxide concentration from the control device.
(d) Efficiency determination at the aeration room vent (not manifolded). The following procedures shall be used to determine the efficiency of a control device used to comply with §63.362(d), the aeration room vent standard.
(1) Determine the concentration of ethylene oxide at the inlet and outlet of the control device using the procedures in Method 18 or 25A in 40 CFR part 60, appendix A. A test is comprised of three 1-hour runs.
(2) Determine control device efficiency (% Eff) using the following equation:
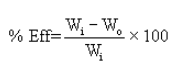
Where:
% Eff = percent efficiency
Wi = mass flow rate into the control device
WO = mass flow rate out of the control device
(3) Repeat the procedures in paragraphs (d)(1) and (2) of this section three times. The arithmetic average percent efficiency of the three runs shall determine the overall efficiency of the control device.
(e) Determination of baseline parameters for acid-water scrubbers. The procedures in this paragraph shall be used to determine the monitored parameters established in §63.363(b), (d), or (e) for acid-water scrubbers and to monitor the parameters as established in §63.364(b).
(1) Ethylene glycol concentration. For determining the ethylene glycol concentration, the facility owner or operator shall establish the maximum ethylene glycol concentration as the ethylene glycol concentration averaged over three test runs; the sampling and analysis procedures in ASTM D 3695-88, Standard Test Method for Volatile Alcohols in Water By Direct Aqueous-Injection Gas Chromatography, (incorporated by reference - see §63.14) shall be used to determine the ethylene glycol concentration.
(2) Scrubber liquor tank level. For determining the scrubber liquor tank level, the sterilization facility owner or operator shall establish the maximum liquor tank level based on a single measurement of the liquor tank level during one test run.
(f) [Reserved]
(g) An owner or operator of a sterilization facility seeking to demonstrate compliance with the standards found at §63.362(c), (d), or (e) with a control device other than an acid-water scrubber or catalytic or thermal oxidation unit shall provide to the Administrator the information requested under §63.363(f). The owner or operator shall submit: a description of the device; test results collected in accordance with §63.363(f) verifying the performance of the device for controlling ethylene oxide emissions to the atmosphere to the levels required by the applicable standards; the appropriate operating parameters that will be monitored; and the frequency of measuring and recording to establish continuous compliance with the standards. The monitoring plan submitted identifying the compliance monitoring is subject to the Administrator's approval. The owner or operator of the sterilization facility shall install, calibrate, operate, and maintain the monitor(s) approved by the Administrator based on the information submitted by the owner or operator. The owner or operator shall include in the information submitted to the Administrator proposed performance specifications and quality assurance procedures for their monitors. The Administrator may request further information and shall approve appropriate test methods and procedures.
(h) An owner or operator of a sterilization facility seeking to demonstrate compliance with the requirements of §63.363 or §">63.364, with a monitoring device or procedure other than a gas chromatograph or a flame ionization analyzer, shall provide to the Administrator information describing the operation of the monitoring device or procedure and the parameter(s) that would demonstrate continuous compliance with each operating limit. The Administrator may request further information and will specify appropriate test methods and procedures.
§63.366 Reporting requirements.
(a) The owner or operator of a source subject to the emissions standards in §63.362 shall fulfill all reporting requirements in §§63.10(a), (d), (e), and (f) of subpart A, according to the applicability in Table 1 of §63.360. These reports will be made to the Administrator at the appropriate address identified in §63.13 of subpart A of this part.
(1) Reports required by subpart A and this section may be sent by U.S. mail, fax, or by another courier.
(i) Submittals sent by U.S. mail shall be postmarked on or before the specified date.
(ii) Submittals sent by other methods shall be received by the Administrator on or before the specified date.
(2) If acceptable to both the Administrator and the owner or operator of a source, reports may be submitted on electronic media.
(3) Content and submittal dates for deviations and monitoring system performance reports. All deviations and monitoring system performance reports and all summary reports, if required per §63.10(e)(3)(vii) and (viii), shall be delivered or postmarked within 30 days following the end of each calendar half or quarter as appropriate (see §63.10(e)(3)(i) through (iv) for applicability). Written reports of deviations from an operating limit shall include all information required in §63.10(c)(5) through (13), as applicable in Table 1 of §63.360, and information from any calibration tests in which the monitoring equipment is not in compliance with PS 9 or the method used for temperature calibration. The written report shall also include the name, title, and signature of the responsible official who is certifying the accuracy of the report. When no deviations have occurred or monitoring equipment has not been inoperative, repaired, or adjusted, such information shall be stated in the report.
(b) Construction and reconstruction. The owner or operator of each source using 10 tons shall fulfill all requirements for construction or reconstruction of a source in §63.5 of subpart A of this part, according to the applicability in Table 1 of §63.360, and in this paragraph.
(1) Applicability.(i) This paragraph and §63.5 of subpart A of this part implement the preconstruction review requirements of section 112(i)(1) for sources subject to these emissions standards. In addition, this paragraph and §63.5 of subpart A of this part include other requirements for constructed and reconstructed sources that are or become subject to these emissions standards.
(ii) After the effective date, the requirements in this section and in §63.5 of subpart A of this part apply to owners or operators who construct a new source or reconstruct a source subject to these emissions standards after December 6, 1994. New or reconstructed sources subject to these emissions standards with an initial startup date before the effective date are not subject to the preconstruction review requirements specified in paragraphs (b) (2) and (3) of this section and §63.5(d) (3) and (4) and (e) of subpart A of this part.
(2) After the effective date, whether or not an approved permit program is effective in the State in which a source is (or would be) located, no person may construct a new source or reconstruct a source subject to these emissions standards, or reconstruct a source such that the source becomes a source subject to these emissions standards, without obtaining advance written approval from the Administrator in accordance with the procedures specified in paragraph (b)(3) of this section and §63.5(d) (3) and (4) and (e) of subpart A of this part.
(3) Application for approval of construction or reconstruction. The provisions of paragraph (b)(3) of this section and §63.5(d) (3) and (4) of subpart A of this part implement section 112(i)(1) of the Act.
(i) General application requirements.(A) An owner or operator who is subject to the requirements of paragraph (b)(2) of this section shall submit to the Administrator an application for approval of the construction of a new source subject to these emissions standards, the reconstruction of a source subject to these emissions standards, or the reconstruction of a source such that the source becomes a source subject to these emissions standards. The application shall be submitted as soon as practicable before the construction or reconstruction is planned to commence (but not sooner than the effective date) if the construction or reconstruction commences after the effective date. The application shall be submitted as soon as practicable before the initial startup date but no later than 60 days after the effective date if the construction or reconstruction had commenced and the initial startup date had not occurred before the effective date. The application for approval of construction or reconstruction may be used to fulfill the initial notification requirements of paragraph (c)(1)(iii) of this section. The owner or operator may submit the application for approval well in advance of the date construction or reconstruction is planned to commence in order to ensure a timely review by the Administrator and that the planned commencement date will not be delayed.
(B) A separate application shall be submitted for each construction or reconstruction. Each application for approval of construction or reconstruction shall include at a minimum:
(1) The applicant's name and address.
(2) A notification of intention to construct a new source subject to these emissions standards or make any physical or operational change to a source subject to these emissions standards that may meet or has been determined to meet the criteria for a reconstruction, as defined in §63.2 of subpart A of this part.
(3) The address (i.e., physical location) or proposed address of the source.
(4) An identification of the relevant standard that is the basis of the application.
(5) The expected commencement date of the construction or reconstruction.
(6) The expected completion date of the construction or reconstruction.
(7) The anticipated date of (initial) startup of the source.
(8) The type and quantity of hazardous air pollutants emitted by the source, reported in units and averaging times and in accordance with the test methods specified in the standard, or if actual emissions data are not yet available, an estimate of the type and quantity of hazardous air pollutants expected to be emitted by the source reported in units and averaging times specified. The owner or operator may submit percent reduction information, if the standard is established in terms of percent reduction. However, operating parameters, such as flow rate, shall be included in the submission to the extent that they demonstrate performance and compliance.
(9) Other information as specified in paragraph (b)(3)(ii) of this section and §63.5(d)(3) of subpart A of this part.
(C) An owner or operator who submits estimates or preliminary information in place of the actual emissions data and analysis required in paragraphs (b)(3)(i)(B)(8) and (ii) of this section shall submit the actual, measured emissions data and other correct information as soon as available but no later than with the notification of compliance status required in paragraph (c)(2) of this section.
(ii) Application for approval of construction. Each application for approval of construction shall include, in addition to the information required in paragraph (b)(3)(i)(B) of this section, technical information describing the proposed nature, size, design, operating design capacity, and method of operation of the source subject to these emissions standards, including an identification of each point of emission for each hazardous air pollutant that is emitted (or could be emitted) and a description of the planned air pollution control system (equipment or method) for each emission point. The description of the equipment to be used for the control of emissions shall include each control device for each hazardous air pollutant and the estimated control efficiency (percent) for each control device. The description of the method to be used for the control of emissions shall include an estimated control efficiency (percent) for that method. Such technical information shall include calculations of emission estimates in sufficient detail to permit assessment of the validity of the calculations. An owner or operator who submits approximations of control efficiencies under paragraph (b)(3) of this section shall submit the actual control efficiencies as specified in paragraph (b)(3)(i)(C) of this section.
(4) Approval of construction or reconstruction based on prior State preconstruction review. (i) The Administrator may approve an application for construction or reconstruction specified in paragraphs (b)(2) and (3) of this section and §63.5(d)(3) and (4) of subpart A of this part if the owner or operator of a new or reconstructed source who is subject to such requirement demonstrates to the Administrator's satisfaction that the following conditions have been (or will be) met:
(A) The owner or operator of the new or reconstructed source subject to these emissions standards has undergone a preconstruction review and approval process in the State in which the source is (or would be) located before the effective date and has received a federally enforceable construction permit that contains a finding that the source will meet these emissions standards as proposed, if the source is properly built and operated;
(B) In making its finding, the State has considered factors substantially equivalent to those specified in §63.5(e)(1) of subpart A of this part.
(ii) The owner or operator shall submit to the Administrator the request for approval of construction or reconstruction no later than the application deadline specified in paragraph (b)(3)(i) of this section. The owner or operator shall include in the request information sufficient for the Administrator's determination. The Administrator will evaluate the owner or operator's request in accordance with the procedures specified in §63.5 of subpart A of this part. The Administrator may request additional relevant information after the submittal of a request for approval of construction or reconstruction.
(c) Notification requirements. The owner or operator of each source subject to the emissions standards in §63.362 shall fulfill all notification requirements in §63.9 of subpart A of this part, according to the applicability in Table 1 of §63.360, and in this paragraph.
(1) Initial notifications. (i)(A) If a source that otherwise would be subject to these emissions standards subsequently increases its use of ethylene oxide within any consecutive 12-month period after December 6, 1996, such that the source becomes subject to these emissions standards or other requirements, such source shall be subject to the notification requirements of §63.9 of subpart A of this part.
(B) Sources subject to these emissions standards may use the application for approval of construction or reconstruction under paragraph (b)(3)(ii) of this section and §63.5(d) (3) of subpart A of this part, respectively, if relevant to fulfill the initial notification requirements.
(ii) The owner or operator of a new or reconstructed source subject to these emissions standards that has an initial startup date after the effective date and for which an application for approval of construction or reconstruction is required under paragraph (b)(3) of this section and §63.5(d) (3) and (4) of subpart A of this part shall provide the following information in writing to the Administrator:
(A) A notification of intention to construct a new source subject to these emissions standards, reconstruct a source subject to these emissions standards, or reconstruct a source such that the source becomes a source subject to these emissions standards with the application for approval of construction or reconstruction as specified in paragraph (b)(3)(i)(A) of this section;
(B) A notification of the date when construction or reconstruction was commenced, submitted simultaneously with the application for approval of construction or reconstruction, if construction or reconstruction was commenced before the effective date of these standards;
(C) A notification of the date when construction or reconstruction was commenced, delivered or postmarked not later than 30 days after such date, if construction or reconstruction was commenced after the effective date of these standards;
(D) A notification of the anticipated date of startup of the source, delivered or postmarked not more than 60 days nor less than 30 days before such date; and
(E) A notification of the actual date of initial startup of the source, delivered or postmarked within 15 calendar days after that date.
(iii) After the effective date, whether or not an approved permit program is effective in the State in which a source subject to these emissions standards is (or would be) located, an owner or operator who intends to construct a new source subject to these emissions standards or reconstruct a source subject to these emissions standards, or reconstruct a source such that it becomes a source subject to these emissions standards, shall notify the Administrator in writing of the intended construction or reconstruction. The notification shall be submitted as soon as practicable before the construction or reconstruction is planned to commence (but no sooner than the effective date of these standards) if the construction or reconstruction commences after the effective date of the standard. The notification shall be submitted as soon as practicable before the initial startup date but no later than 60 days after the effective date of this standard if the construction or reconstruction had commenced and the initial startup date has not occurred before the standard's effective date. The notification shall include all the information required for an application for approval of construction or reconstruction as specified in paragraph (b)(3) of this section and §63.5(d)(3) and (4) of subpart A of this part. For sources subject to these emissions standards, the application for approval of construction or reconstruction may be used to fulfill the initial notification requirements of §63.9 of subpart A of this part.
(2) If an owner or operator of a source subject to these emissions standards submits estimates or preliminary information in the application for approval of construction or reconstruction required in paragraph (b)(3)(ii) of this section and §63.5(d)(3) of subpart A of this part, respectively, in place of the actual emissions data or control efficiencies required in paragraphs (b)(3)(i)(B)(8) and (ii) of this section, the owner or operator shall submit the actual emissions data and other correct information as soon as available but no later than with the initial notification of compliance status.
(3) The owner or operator of any existing sterilization facility subject to this subpart shall also include the amount of ethylene oxide used during the previous consecutive 12-month period in the initial notification report required by §63.9(b)(2) and (3) of subpart A of this part. For new sterilization facilities subject to this subpart, the amount of ethylene oxide used shall be an estimate of expected use during the first consecutive 12-month period of operation.
§63.367 Recordkeeping requirements.
(a) The owner or operator of a source subject to §63.362 shall comply with the recordkeeping requirements in §63.10(b) and (c), according to the applicability in Table 1 of §63.360, and in this section. All records required to be maintained by this subpart or a subpart referenced by this subpart shall be maintained in such a manner that they can be readily accessed and are suitable for inspection. The most recent 2 years of records shall be retained onsite or shall be accessible to an inspector while onsite. The records of the preceding 3 years, where required, may be retained offsite. Records may be maintained in hard copy or computer-readable form including, but not limited to, on paper, microfilm, computer, computer disk, magnetic tape, or microfiche.
(b) The owners or operators of a source using 1 to 10 tons not subject to §63.362 shall maintain records of ethylene oxide use on a 12-month rolling average basis (until the source changes its operations to become a source subject to §63.362).
(c) The owners or operators of a source using less than 1 ton shall maintain records of ethylene oxide use on a 12-month rolling average basis (until the source changes its operations to become a source subject to §63.362).
(d) The owners or operators complying with §63.363(b) (4) shall maintain records of the compliance test, data analysis, and if catalyst is replaced, proof of replacement.
§63.368 Implementation and enforcement.
(a) This subpart can be implemented and enforced by the U.S. EPA, or a delegated authority such as the applicable State, local, or Tribal agency. If the U.S. EPA Administrator has delegated authority to a State, local, or Tribal agency, then that agency, in addition to the U.S. EPA, has the authority to implement and enforce this subpart. Contact the applicable U.S. EPA Regional Office to find out if implementation and enforcement of this subpart is delegated to a State, local, or Tribal agency.
(b) In delegating implementation and enforcement authority of this subpart to a State, local, or Tribal agency under subpart E of this part, the authorities contained in paragraph (c) of this section are retained by the Administrator of U.S. EPA and cannot be transferred to the State, local, or Tribal agency.
(c) The authorities that cannot be delegated to State, local, or Tribal agencies are as specified in paragraphs (c)(1) through (4) of this section.
(1) Approval of alternatives to the requirements in §§63.360 and 63.362.
(2) Approval of major alternatives to test methods under §63.7(e)(2)(ii) and (f), as defined in §63.90, and as required in this subpart.
(3) Approval of major alternatives to monitoring under §63.8(f), as defined in §63.90, and as required in this subpart.
(4) Approval of major alternatives to recordkeeping and reporting under §63.10(f), as defined in §63.90, and as required in this subpart.
“What’s the worst that could happen?” You’ve likely heard and have even asked this rhetorical question. Under the Clean Water Act (CWA), it’s no longer rhetorical; it’s now a regulatory question that must be answered with formal, written plans by facilities that could discharge dangerous chemicals into nearby waterbodies.
The Environmental Protection Agency (EPA) finalized a rule (codified at 40 CFR Part 118) that requires certain facilities to develop and submit Facility Response Plans (FRPs) for worst-case discharges or the potential for worst-case discharges of CWA hazardous substances into navigable water.
Use this article to help you determine whether your facility is covered by the CWA hazardous substance FRP requirements.
The final rule covers “onshore, non-transportation-related facilities that could reasonably be expected to cause substantial harm to the environment by discharging a CWA hazardous substance into or on the navigable waters, adjoining shorelines, or exclusive economic zone.” As with all other regulations, the definitions of the terms determine what the rules mean and what they require.
A facility considered to pose substantial harm must meet three conditions. The CWA hazardous substance FRP requirements apply to facilities that:
The final rule requires facilities to model worst-case discharge scenarios that represent each covered CWA hazardous substance. Through these scenarios, facilities determine whether they meet the first three substantial harm criteria above.
Determine whether your facility qualifies for any exceptions or exemptions to the FRP requirements listed at 118.8.
Exceptions include:
Exemptions apply to:
If your facility meets the applicability criteria and doesn’t qualify for any exceptions or exemptions, it’s subject to the CWA hazardous substance FRP requirements. You must prepare, submit to EPA, and implement an FRP.
Covered facilities must submit FRPs to EPA within three years of May 28, 2024 (the effective date of the rule).
Key to remember: A final rule under the Clean Water Act requires certain facilities to develop, submit to EPA, and implement Facility Response Plans for worst-case discharges of hazardous substances into navigable waters.
On July 6, 2020, the U.S. Environmental Protection Agency (EPA or the Agency) finalized the residual risk and technology review (RTR) conducted for the Ethylene Production source category, which is part of the Generic Maximum Achievable Control Technology Standards National Emission Standards for Hazardous Air Pollutants (NESHAP); on July 7, 2020, the EPA finalized the RTR conducted for the Organic Liquids Distribution (Non-Gasoline) NESHAP; and on August 12, 2020, the EPA finalized the RTR conducted for the Miscellaneous Organic Chemical Manufacturing NESHAP. Amendments to the Petroleum Refinery Sector NESHAP were most recently finalized on February 4, 2020. Subsequently, the EPA received and granted various petitions for reconsideration on these NESHAP for, among other things, the provisions related to the work practice standards for pressure relief devices (PRDs), emergency flaring, and degassing of floating roof storage vessels. This action finalizes proposed amendments to remove the force majeure exemption for PRDs and emergency flaring, incorporate clarifications for the degassing requirements for floating roof storage vessels, and address other corrections and clarifications.
DATES: This final action is effective on April 4, 2024, published in the Federal Register April 4, 2024, page 23840.
View final rule.
| §63.641 Definitions. | ||
| Definition of “Flare” | Revised | View text |
| §63.643 Miscellaneous process vent provisions. | ||
| (c)(1)-(2) | Revised | View text |
| §63.648 Equipment leak standards. | ||
| (j)(3)(iv), (j)(3)(v)(B) and (C), (j)(6) introductory text, and (j)(6)(ii) | Revised | View text |
| §63.655 Reporting and recordkeeping requirements. | ||
| (g) | Revised | View text |
| (i)(9)-(12) | Revised | View text |
| §63.670 Requirements for flare control devices. | ||
| (b), (d) introductory text, (e), (l)(5) introductory text, (o)(4)(iv), (o)(6), (o)(7)(ii) through (o)(7)(v) | Revised | View text |
| (d)(3) | Added | View text |
| §63.671 Requirements for flare monitoring systems. | ||
| (e) introductory text | Revised | View text |
| (e)(4), (f) | Added | View text |
| Appendix to Subpart CC of Part 63—Tables | ||
| Table 13 | Revised | View text |
| §63.1100 Applicability. | ||
| (b), (g)(7)(iii) | Revised | View text |
| §63.1102 Compliance schedule. | ||
| (c)(11), (d)(2)(ii), (e)(2)(iii) | Revised | View text |
| §63.1103 Source category-specific applicability, definitions, and requirements. | ||
| (e) | Revised | View text |
| §63.1107 Equipment leaks. | ||
| (h)(3)(iv), (h)(3)(v)(B) and (C), (h)(6) introductory text, and (h)(6)(ii) | Revised | View text |
| §63.1109 Recordkeeping requirements. | ||
| (f)(2), (3), and (5), and (i)(2) | Revised | View text |
| §63.1110 Reporting requirements. | ||
| (a)(10), (e)(4)(iii), (e)(4)(iv)(A) and (B), (e)(5)(iii), and (e)(8)(iii) | Revised | View text |
| §63.2346 What emission limitations, operating limits, and work practice standards must I meet? | ||
| (a)(6) introductory text, (e) | Revised | View text |
| (a)(6)(iv) | Added | View text |
| §63.2378 How do I demonstrate continuous compliance with the emission limitations, operating limits, and work practice standards? | ||
| (e) | Revised | View text |
| §63.2382 What notifications must I submit and when and what information should be submitted? | ||
| (d) | Revised | View text |
| §63.2386 What reports must I submit and when and what information is to be submitted in each? | ||
| (f), (g), and (h) | Revised | View text |
| (i)-(j) | Removed | View text |
| §63.2406 What definitions apply to this subpart? | ||
| Definition of ”Force majeure event” | Removed | View text |
| Table 12 to Subpart EEEE of Part 63—Applicability of General Provisions to Subpart EEEE | ||
| “63.9(k)” | Added | View text |
| ”63.7(a)(4)”“63.9(k)” | Revised | View text |
| §63.2450 What are my general requirements for complying with this subpart? | ||
| (e)(1), (e)(5)(iv), (e)(5)(viii)(B), (e)(6)(i), (e)(7) introductory text, (v)(1)(i), (v)(1)(ii), and (v)(2) | Revised | View text |
| §63.2460 What requirements must I meet for batch process vents? | ||
| (c)(9) introductory text | View text | |
| §63.2470 What requirements must I meet for storage tanks? | ||
| (f) | Revised | View text |
| §63.2480 What requirements must I meet for equipment leaks? | ||
| (a), (e)(2)(ii), (e)(2)(iii), (e)(3)(iv), (e)(3)(v)(B), (e)(3)(v)(C), (e)(6)(ii), (f)(18)(iii), (f)(18)(vi), (f)(18)(x), and (f)(18)(xiii) | Revised | View text |
| §63.2490 What requirements must I meet for heat exchange systems? | ||
| (a), (d) introductory text, and (d)(4)(iii) introductory text; | Revised | View text |
| (e) | Added | View text |
| §63.2492 How do I determine whether my process vent, storage tank, or equipment is in ethylene oxide service? | ||
| (b) | Revised | View text |
| §63.2493 What requirements must I meet for process vents, storage tanks, or equipment that are in ethylene oxide service? | ||
| (a)(2)(vi) introductory text, (a)(2)(vi)(C), (a)(2)(viii), (b)(2), (b)(4) introductory text, (b)(4)(iv), (b)(6), (d)(1)(iii), (d)(2)(iii), (d)(3), (d)(4)(v), and (e) introductory text | Revised | View text |
| §63.2515 What notifications must I submit and when? | ||
| (d) | Revised | View text |
| §63.2520 What reports must I submit and when? | ||
| (d) introductory text, (e) introductory text, (e)(2), (e)(14)(iii), (e)(16), (f) and (g) | Revised | View text |
| (d)(6) | Added | View text |
| (h)-(i) | Removed | View text |
| §63.2525 What records must I keep? | ||
| (o), (p)(2), (p)(3), (p)(5), (q)(2), (r)(1), (r)(4)(iv) introductory text, (r)(4)(iv)(B) and (r)(4)(iv)(C) | Revised | View text |
| (r)(4)(iv)(D) | Added | View text |
| §63.2550 What definitions apply to this subpart? | ||
| “In ethylene oxide service’” | Revised | View text |
| Table 10 to Subpart FFFF of Part 63—Work Practice Standards for Heat Exchange Systems | ||
| Entire table | Revised | View text |
| Table 12 to Subpart FFFF of Part 63—Applicability of General Provisions to Subpart FFFF | ||
| Entry ”63.9(k)” | Revised | View text |
Previous Text
§63.641 Definitions.
* * * *
Flare means a combustion device lacking an enclosed combustion chamber that uses an uncontrolled volume of ambient air to burn gases. For the purposes of this rule, the definition of flare includes, but is not necessarily limited to, air-assisted flares, steam-assisted flares and non-assisted flares.
§63.643 Miscellaneous process vent provisions.
* * * *
(c)(1) Prior to venting to the atmosphere, process liquids are removed from the equipment as much as practical and the equipment is depressured to a control device meeting requirements in paragraphs (a)(1) or (2) of this section, a fuel gas system, or back to the process until one of the following conditions, as applicable, is met.
(i) The vapor in the equipment served by the maintenance vent has a lower explosive limit (LEL) of less than 10 percent.
(ii) If there is no ability to measure the LEL of the vapor in the equipment based on the design of the equipment, the pressure in the equipment served by the maintenance vent is reduced to 5 pounds per square inch gauge (psig) or less. Upon opening the maintenance vent, active purging of the equipment cannot be used until the LEL of the vapors in the maintenance vent (or inside the equipment if the maintenance is a hatch or similar type of opening) is less than 10 percent.
(iii) The equipment served by the maintenance vent contains less than 72 pounds of total volatile organic compounds (VOC).
(iv) If the maintenance vent is associated with equipment containing pyrophoric catalyst (e.g., hydrotreaters and hydrocrackers) and a pure hydrogen supply is not available at the equipment at the time of the startup, shutdown, maintenance, or inspection activity, the LEL of the vapor in the equipment must be less than 20 percent, except for one event per year not to exceed 35 percent.
(v) If, after applying best practices to isolate and purge equipment served by a maintenance vent, none of the applicable criterion in paragraphs (c)(1)(i) through (iv) of this section can be met prior to installing or removing a blind flange or similar equipment blind, the pressure in the equipment served by the maintenance vent is reduced to 2 psig or less. Active purging of the equipment may be used provided the equipment pressure at the location where purge gas is introduced remains at 2 psig or less.
(c)(2) Except for maintenance vents complying with the alternative in paragraph (c)(1)(iii) of this section, the owner or operator must determine the LEL or, if applicable, equipment pressure using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
§63.648 Equipment leak standards.
* * * *
(j)(3)(iv) The owner or operator shall determine the total number of release events occurred during the calendar year for each affected pressure relief device separately. The owner or operator shall also determine the total number of release events for each pressure relief device for which the root cause analysis concluded that the root cause was a force majeure event, as defined in this subpart.
* * * *
(j)(3)(v)(B) A second release event not including force majeure events from a single pressure relief device in a 3 calendar year period for the same root cause for the same equipment.
(j)(3)(v)(C) A third release event not including force majeure events from a single pressure relief device in a 3 calendar year period for any reason.
* * * *
(j)(6) Root cause analysis and corrective action analysis. A root cause analysis and corrective action analysis must be completed as soon as possible, but no later than 45 days after a release event. Special circumstances affecting the number of root cause analyses and/or corrective action analyses are provided in paragraphs (j)(6)(i) through (iv) of this section.
* * * *
(j)(6)(ii) You may conduct a single root cause analysis and corrective action analysis for a single emergency event that causes two or more pressure relief devices to release, regardless of the equipment served, if the root cause is reasonably expected to be a force majeure event, as defined in this subpart.
§63.655 Reporting and recordkeeping requirements.
* * * *
(g) The owner or operator of a source subject to this subpart shall submit Periodic Reports no later than 60 days after the end of each 6-month period when any of the information specified in paragraphs (g)(1) through (7) of this section or paragraphs (g)(9) through (14) of this section is collected. The first 6-month period shall begin on the date the Notification of Compliance Status report is required to be submitted. A Periodic Report is not required if none of the events identified in paragraphs (g)(1) through (7) of this section or paragraphs (g)(9) through (14) of this section occurred during the 6-month period unless emissions averaging is utilized. Quarterly reports must be submitted for emission points included in emission averages, as provided in paragraph (g)(8) of this section. An owner or operator may submit reports required by other regulations in place of or as part of the Periodic Report required by this paragraph (g) if the reports contain the information required by paragraphs (g)(1) through (14) of this section.
(1) For storage vessels, Periodic Reports shall include the information specified for Periodic Reports in paragraphs (g)(2) through (5) of this section. Information related to gaskets, slotted membranes, and sleeve seals is not required for storage vessels that are part of an existing source complying with §63.646.
(2) Internal floating roofs.(i) An owner or operator who elects to comply with §63.646 by using a fixed roof and an internal floating roof or by using an external floating roof converted to an internal floating roof shall submit the results of each inspection conducted in accordance with §63.120(a) of subpart G in which a failure is detected in the control equipment.
(A) For vessels for which annual inspections are required under §63.120(a)(2)(i) or (a)(3)(ii) of subpart G, the specifications and requirements listed in paragraphs (g)(2)(i)(A)(1) through (3) of this section apply.
(1) A failure is defined as any time in which the internal floating roof is not resting on the surface of the liquid inside the storage vessel and is not resting on the leg supports; or there is liquid on the floating roof; or the seal is detached from the internal floating roof; or there are holes, tears, or other openings in the seal or seal fabric; or there are visible gaps between the seal and the wall of the storage vessel.
(2) Except as provided in paragraph (g)(2)(i)(A)(3) of this section, each Periodic Report shall include the date of the inspection, identification of each storage vessel in which a failure was detected, and a description of the failure. The Periodic Report shall also describe the nature of and date the repair was made or the date the storage vessel was emptied.
(3) If an extension is utilized in accordance with §63.120(a)(4) of subpart G, the owner or operator shall, in the next Periodic Report, identify the vessel; include the documentation specified in §63.120(a)(4) of subpart G; and describe the date the storage vessel was emptied and the nature of and date the repair was made.
(B) For vessels for which inspections are required under §63.120(a)(2)(ii), (a)(3)(i), or (a)(3)(iii) of subpart G (i.e., internal inspections), the specifications and requirements listed in paragraphs (g)(2)(i)(B)(1) and (2) of this section apply.
(1) A failure is defined as any time in which the internal floating roof has defects; or the primary seal has holes, tears, or other openings in the seal or the seal fabric; or the secondary seal (if one has been installed) has holes, tears, or other openings in the seal or the seal fabric; or, for a storage vessel that is part of a new source, the gaskets no longer close off the liquid surface from the atmosphere; or, for a storage vessel that is part of a new source, the slotted membrane has more than a 10 percent open area.
(2) Each Periodic Report shall include the date of the inspection, identification of each storage vessel in which a failure was detected, and a description of the failure. The Periodic Report shall also describe the nature of and date the repair was made.
(ii) An owner or operator who elects to comply with §63.660 by using a fixed roof and an internal floating roof shall submit the results of each inspection conducted in accordance with §63.1063(c)(1), (d)(1), and (d)(2) of subpart WW in which a failure is detected in the control equipment. For vessels for which inspections are required under §63.1063(c) and (d), the specifications and requirements listed in paragraphs (g)(2)(ii)(A) through (C) of this section apply.
(A) A failure is defined in §63.1063(d)(1) of subpart WW.
(B) Each Periodic Report shall include a copy of the inspection record required by §63.1065(b) of subpart WW when a failure occurs.
(C) An owner or operator who elects to use an extension in accordance with §63.1063(e)(2) of subpart WW shall, in the next Periodic Report, submit the documentation required by §63.1063(e)(2).
(3) External floating roofs.(i) An owner or operator who elects to comply with §63.646 by using an external floating roof shall meet the periodic reporting requirements specified in paragraphs (g)(3)(i)(A) through (C) of this section.
(A) The owner or operator shall submit, as part of the Periodic Report, documentation of the results of each seal gap measurement made in accordance with §63.120(b) of subpart G in which the seal and seal gap requirements of §63.120(b)(3), (4), (5), or (6) of subpart G are not met. This documentation shall include the information specified in paragraphs (g)(3)(i)(A)(1) through (4) of this section.
(1) The date of the seal gap measurement.
(2) The raw data obtained in the seal gap measurement and the calculations described in §63.120(b)(3) and (4) of subpart G.
(3) A description of any seal condition specified in §63.120(b)(5) or (6) of subpart G that is not met.
(4) A description of the nature of and date the repair was made, or the date the storage vessel was emptied.
(B) If an extension is utilized in accordance with §63.120(b)(7)(ii) or (b)(8) of subpart G, the owner or operator shall, in the next Periodic Report, identify the vessel; include the documentation specified in §63.120(b)(7)(ii) or (b)(8) of subpart G, as applicable; and describe the date the vessel was emptied and the nature of and date the repair was made.
(C) The owner or operator shall submit, as part of the Periodic Report, documentation of any failures that are identified during visual inspections required by §63.120(b)(10) of subpart G. This documentation shall meet the specifications and requirements in paragraphs (g)(3)(i)(C)(1) and (2) of this section.
(1) A failure is defined as any time in which the external floating roof has defects; or the primary seal has holes or other openings in the seal or the seal fabric; or the secondary seal has holes, tears, or other openings in the seal or the seal fabric; or, for a storage vessel that is part of a new source, the gaskets no longer close off the liquid surface from the atmosphere; or, for a storage vessel that is part of a new source, the slotted membrane has more than 10 percent open area.
(2) Each Periodic Report shall include the date of the inspection, identification of each storage vessel in which a failure was detected, and a description of the failure. The Periodic Report shall also describe the nature of and date the repair was made.
(ii) An owner or operator who elects to comply with §63.660 by using an external floating roof shall meet the periodic reporting requirements specified in paragraphs (g)(3)(ii)(A) and (B) of this section.
(A) For vessels for which inspections are required under §63.1063(c)(2), (d)(1), and (d)(3) of subpart WW, the owner or operator shall submit, as part of the Periodic Report, a copy of the inspection record required by §63.1065(b) of subpart WW when a failure occurs. A failure is defined in §63.1063(d)(1).
(B) An owner or operator who elects to use an extension in accordance with §63.1063(e)(2) or (c)(2)(iv)(B) of subpart WW shall, in the next Periodic Report, submit the documentation required by those paragraphs.
(4) [Reserved]
(5) An owner or operator who elects to comply with §63.646 or §63.660 by installing a closed vent system and control device shall submit, as part of the next Periodic Report, the information specified in paragraphs (g)(5)(i) through (v) of this section, as applicable.
(i) The Periodic Report shall include the information specified in paragraphs (g)(5)(i)(A) and (B) of this section for those planned routine maintenance operations that would require the control device not to meet the requirements of either §63.119(e)(1) or (2) of subpart G, §63.985(a) and (b) of subpart SS or §63.670, as applicable.
(A) A description of the planned routine maintenance that is anticipated to be performed for the control device during the next 6 months. This description shall include the type of maintenance necessary, planned frequency of maintenance, and lengths of maintenance periods.
(B) A description of the planned routine maintenance that was performed for the control device during the previous 6 months. This description shall include the type of maintenance performed and the total number of hours during those 6 months that the control device did not meet the requirements of either §63.119(e)(1) or (2) of subpart G, §63.985(a) and (b) of subpart SS or §63.670, as applicable, due to planned routine maintenance.
(ii) If a control device other than a flare is used, the Periodic Report shall describe each occurrence when the monitored parameters were outside of the parameter ranges documented in the Notification of Compliance Status report. The description shall include: Identification of the control device for which the measured parameters were outside of the established ranges, and causes for the measured parameters to be outside of the established ranges.
(iii) If a flare is used prior to January 30, 2019 and prior to electing to comply with the requirements in §63.670, the Periodic Report shall describe each occurrence when the flare does not meet the general control device requirements specified in §63.11(b) of subpart A and shall include: Identification of the flare that does not meet the general requirements specified in §63.11(b) of subpart A, and reasons the flare did not meet the general requirements specified in §63.11(b) of subpart A.
(iv) If a flare is used on or after the date for which compliance with the requirements in §63.670 is elected, which can be no later than January 30, 2019, the Periodic Report shall include the items specified in paragraph (g)(11) of this section.
(v) An owner or operator who elects to comply with §63.660 by installing an alternate control device as described in §63.1064 of subpart WW shall submit, as part of the next Periodic Report, a written application as described in §63.1066(b)(3) of subpart WW.
(6) For miscellaneous process vents for which continuous parameter monitors are required by this subpart, periods of excess emissions shall be identified in the Periodic Reports and shall be used to determine compliance with the emission standards.
(i) Period of excess emission means any of the following conditions:
(A) An operating day when the daily average value of a monitored parameter, except presence of a flare pilot flame, is outside the range specified in the Notification of Compliance Status report. Monitoring data recorded during periods of monitoring system breakdown, repairs, calibration checks and zero (low-level) and high-level adjustments shall not be used in computing daily average values of monitored parameters.
(B) An operating day when all pilot flames of a flare are absent.
(C) An operating day when monitoring data required to be recorded in paragraphs (i)(3) (i) and (ii) of this section are available for less than 75 percent of the operating hours.
(D) For data compression systems under paragraph (h)(5)(iii) of this section, an operating day when the monitor operated for less than 75 percent of the operating hours or a day when less than 18 monitoring values were recorded.
(ii) For miscellaneous process vents, excess emissions shall be reported for the operating parameters specified in table 10 of this subpart unless other site-specific parameter(s) have been approved by the operating permit authority.
(iii) For periods in closed vent systems when a Group 1 miscellaneous process vent stream was detected in the bypass line or diverted from the control device and either directly to the atmosphere or to a control device that does not comply with the requirements in §63.643(a), report the date, time, duration, estimate of the volume of gas, the concentration of organic HAP in the gas and the resulting mass emissions of organic HAP that bypassed the control device. For periods when the flow indicator is not operating, report the date, time, and duration.
(7) If a performance test for determination of compliance for a new emission point subject to this subpart or for an emission point that has changed from Group 2 to Group 1 is conducted during the period covered by a Periodic Report, the results of the performance test shall be included in the Periodic Report.
(i) Results of the performance test shall include the identification of the source tested, the date of the test, the percentage of emissions reduction or outlet pollutant concentration reduction (whichever is needed to determine compliance) for each run and for the average of all runs, and the values of the monitored operating parameters.
(ii) The complete test report shall be maintained onsite.
(8) The owner or operator of a source shall submit quarterly reports for all emission points included in an emissions average.
(i) The quarterly reports shall be submitted no later than 60 calendar days after the end of each quarter. The first report shall be submitted with the Notification of Compliance Status report no later than 150 days after the compliance date specified in §63.640.
(ii) The quarterly reports shall include:
(A) The information specified in this paragraph and in paragraphs (g)(2) through (g)(7) of this section for all storage vessels and miscellaneous process vents included in an emissions average;
(B) The information required to be reported by §63.428 (h)(1), (h)(2), and (h)(3) for each gasoline loading rack included in an emissions average, unless this information has already been submitted in a separate report;
(C) The information required to be reported by §63.567(e)(4) and (j)(3) of subpart Y for each marine tank vessel loading operation included in an emissions average, unless the information has already been submitted in a separate report;
(D) Any information pertaining to each wastewater stream included in an emissions average that the source is required to report under the Implementation Plan for the source;
(E) The credits and debits calculated each month during the quarter;
(F) A demonstration that debits calculated for the quarter are not more than 1.30 times the credits calculated for the quarter, as required under §§63.652(e)(4);
(G) The values of any inputs to the credit and debit equations in §63.652 (g) and (h) that change from month to month during the quarter or that have changed since the previous quarter; and
(H) Any other information the source is required to report under the Implementation Plan for the source.
(iii) Every fourth quarterly report shall include the following:
(A) A demonstration that annual credits are greater than or equal to annual debits as required by §63.652(e)(3); and
(B) A certification of compliance with all the emissions averaging provisions in §63.652 of this subpart.
(9) For heat exchange systems, Periodic Reports must include the following information:
(i) The number of heat exchange systems at the plant site subject to the monitoring requirements in §63.654.
(ii) The number of heat exchange systems at the plant site found to be leaking.
(iii) For each monitoring location where the total strippable hydrocarbon concentration was determined to be equal to or greater than the applicable leak definitions specified in §63.654(c)(6), identification of the monitoring location (e.g., unique monitoring location or heat exchange system ID number), the measured total strippable hydrocarbon concentration, the date the leak was first identified, and, if applicable, the date the source of the leak was identified;
(iv) For leaks that were repaired during the reporting period (including delayed repairs), identification of the monitoring location associated with the repaired leak, the total strippable hydrocarbon concentration measured during re-monitoring to verify repair, and the re-monitoring date (i.e., the effective date of repair); and
(v) For each delayed repair, identification of the monitoring location associated with the leak for which repair is delayed, the date when the delay of repair began, the date the repair is expected to be completed (if the leak is not repaired during the reporting period), the total strippable hydrocarbon concentration and date of each monitoring event conducted on the delayed repair during the reporting period, and an estimate of the potential strippable hydrocarbon emissions over the reporting period associated with the delayed repair.
(10) For pressure relief devices subject to the requirements §63.648(j), Periodic Reports must include the information specified in paragraphs (g)(10)(i) through (iv) of this section.
(i) For pressure relief devices in organic HAP gas or vapor service, pursuant to §63.648(j)(1), report any instrument reading of 500 ppm or greater.
(ii) For pressure relief devices in organic HAP gas or vapor service subject to §63.648(j)(2), report confirmation that any monitoring required to be done during the reporting period to show compliance was conducted.
(iii) For pilot-operated pressure relief devices in organic HAP service, report each pressure release to the atmosphere through the pilot vent that equals or exceeds 72 pounds of VOC per day, including duration of the pressure release through the pilot vent and estimate of the mass quantity of each organic HAP released.
(iv) For pressure relief devices in organic HAP service subject to §63.648(j)(3), report each pressure release to the atmosphere, including duration of the pressure release and estimate of the mass quantity of each organic HAP released, and the results of any root cause analysis and corrective action analysis completed during the reporting period, including the corrective actions implemented during the reporting period and, if applicable, the implementation schedule for planned corrective actions to be implemented subsequent to the reporting period.
(11) For flares subject to §63.670, Periodic Reports must include the information specified in paragraphs (g)(11)(i) through (iv) of this section.
(i) Records as specified in paragraph (i)(9)(i) of this section for each 15-minute block during which there was at least one minute when regulated material is routed to a flare and no pilot flame is present.
(ii) Visible emission records as specified in paragraph (i)(9)(ii)(C) of this section for each period of 2 consecutive hours during which visible emissions exceeded a total of 5 minutes.
(iii) The 15-minute block periods for which the applicable operating limits specified in §63.670(d) through (f) are not met. Indicate the date and time for the period, the net heating value operating parameter(s) determined following the methods in §63.670(k) through (n) as applicable.
(iv) For flaring events meeting the criteria in §63.670(o)(3):
(A) The start and stop time and date of the flaring event.
(B) The length of time for which emissions were visible from the flare during the event.
(C) The periods of time that the flare tip velocity exceeds the maximum flare tip velocity determined using the methods in §63.670(d)(2) and the maximum 15-minute block average flare tip velocity recorded during the event.
(D) Results of the root cause and corrective actions analysis completed during the reporting period, including the corrective actions implemented during the reporting period and, if applicable, the implementation schedule for planned corrective actions to be implemented subsequent to the reporting period.
(12) For delayed coking units, the Periodic Report must include the information specified in paragraphs (g)(12)(i) through (iv) of this section.
(i) For existing source delayed coking units, any 60-cycle average exceeding the applicable limit in §63.657(a)(1).
(ii) For new source delayed coking units, any direct venting event exceeding the applicable limit in §63.657(a)(2).
(iii) The total number of double quenching events performed during the reporting period.
(iv) For each double quenching draining event when the drain water temperature exceeded 210°F, report the drum, date, time, the coke drum vessel pressure or temperature, as applicable, when pre-vent draining was initiated, and the maximum drain water temperature during the pre-vent draining period.
(13) For maintenance vents subject to the requirements in §63.643(c), Periodic Reports must include the information specified in paragraphs (g)(13)(i) through (iv) of this section for any release exceeding the applicable limits in §63.643(c)(1). For the purposes of this reporting requirement, owners or operators complying with §63.643(c)(1)(iv) must report each venting event for which the lower explosive limit is 20 percent or greater; owners or operators complying with §63.643(c)(1)(v) must report each venting event conducted under those provisions and include an explanation for each event as to why utilization of this alternative was required.
(i) Identification of the maintenance vent and the equipment served by the maintenance vent.
(ii) The date and time the maintenance vent was opened to the atmosphere.
(iii) The lower explosive limit, vessel pressure, or mass of VOC in the equipment, as applicable, at the start of atmospheric venting. If the 5 psig vessel pressure option in §63.643(c)(1)(ii) was used and active purging was initiated while the lower explosive limit was 10 percent or greater, also include the lower explosive limit of the vapors at the time active purging was initiated.
(iv) An estimate of the mass of organic HAP released during the entire atmospheric venting event.
(14) Any changes in the information provided in a previous Notification of Compliance Status report.
* * * *
(i)(9) For each flare subject to §63.670, each owner or operator shall keep the records specified in paragraphs (i)(9)(i) through (xii) of this section up-to-date and readily accessible, as applicable.
(i) Retain records of the output of the monitoring device used to detect the presence of a pilot flame as required in §63.670(b) for a minimum of 2 years. Retain records of each 15-minute block during which there was at least one minute that no pilot flame is present when regulated material is routed to a flare for a minimum of 5 years.
(ii) Retain records of daily visible emissions observations or video surveillance images required in §63.670(h) as specified in the paragraphs (i)(9)(ii)(A) through (C), as applicable, for a minimum of 3 years.
(A) If visible emissions observations are performed using Method 22 at 40 CFR part 60, appendix A-7, the record must identify whether the visible emissions observation was performed, the results of each observation, total duration of observed visible emissions, and whether it was a 5-minute or 2-hour observation. If the owner or operator performs visible emissions observations more than one time during a day, the record must also identify the date and time of day each visible emissions observation was performed.
(B) If video surveillance camera is used, the record must include all video surveillance images recorded, with time and date stamps.
(C) For each 2 hour period for which visible emissions are observed for more than 5 minutes in 2 consecutive hours, the record must include the date and time of the 2 hour period and an estimate of the cumulative number of minutes in the 2 hour period for which emissions were visible.
(iii) The 15-minute block average cumulative flows for flare vent gas and, if applicable, total steam, perimeter assist air, and premix assist air specified to be monitored under §63.670(i), along with the date and time interval for the 15-minute block. If multiple monitoring locations are used to determine cumulative vent gas flow, total steam, perimeter assist air, and premix assist air, retain records of the 15-minute block average flows for each monitoring location for a minimum of 2 years, and retain the 15-minute block average cumulative flows that are used in subsequent calculations for a minimum of 5 years. If pressure and temperature monitoring is used, retain records of the 15-minute block average temperature, pressure and molecular weight of the flare vent gas or assist gas stream for each measurement location used to determine the 15-minute block average cumulative flows for a minimum of 2 years, and retain the 15-minute block average cumulative flows that are used in subsequent calculations for a minimum of 5 years.
(iv) The flare vent gas compositions specified to be monitored under §63.670(j). Retain records of individual component concentrations from each compositional analyses for a minimum of 2 years. If NHVvg analyzer is used, retain records of the 15-minute block average values for a minimum of 5 years.
(v) Each 15-minute block average operating parameter calculated following the methods specified in §63.670(k) through (n), as applicable.
(vi) [Reserved]
(vii) All periods during which operating values are outside of the applicable operating limits specified in §63.670(d) through (f) when regulated material is being routed to the flare.
(viii) All periods during which the owner or operator does not perform flare monitoring according to the procedures in §63.670(g) through (j).
(ix) Records of periods when there is flow of vent gas to the flare, but when there is no flow of regulated material to the flare, including the start and stop time and dates of periods of no regulated material flow.
(x) Records when the flow of vent gas exceeds the smokeless capacity of the flare, including start and stop time and dates of the flaring event.
(xi) Records of the root cause analysis and corrective action analysis conducted as required in §63.670(o)(3), including an identification of the affected facility, the date and duration of the event, a statement noting whether the event resulted from the same root cause(s) identified in a previous analysis and either a description of the recommended corrective action(s) or an explanation of why corrective action is not necessary under §63.670(o)(5)(i).
(xii) For any corrective action analysis for which implementation of corrective actions are required in §63.670(o)(5), a description of the corrective action(s) completed within the first 45 days following the discharge and, for action(s) not already completed, a schedule for implementation, including proposed commencement and completion dates.
(10) [Reserved]
(11) For each pressure relief device subject to the pressure release management work practice standards in §63.648(j)(3), the owner or operator shall keep the records specified in paragraphs (i)(11)(i) through (iii) of this section. For each pilot-operated pressure relief device subject to the requirements at §63.648(j)(3), the owner or operator shall keep the records specified in paragraph (i)(11)(iv) of this section.
(i) Records of the prevention measures implemented as required in §63.648(j)(3)(ii), if applicable.
(ii) Records of the number of releases during each calendar year and the number of those releases for which the root cause was determined to be a force majeure event. Keep these records for the current calendar year and the past five calendar years.
(iii) For each release to the atmosphere, the owner or operator shall keep the records specified in paragraphs (i)(11)(iii)(A) through (D) of this section.
(A) The start and end time and date of each pressure release to the atmosphere.
(B) Records of any data, assumptions, and calculations used to estimate of the mass quantity of each organic HAP released during the event.
(C) Records of the root cause analysis and corrective action analysis conducted as required in §63.648(j)(3)(iii), including an identification of the affected facility, the date and duration of the event, a statement noting whether the event resulted from the same root cause(s) identified in a previous analysis and either a description of the recommended corrective action(s) or an explanation of why corrective action is not necessary under §63.648(j)(7)(i).
(D) For any corrective action analysis for which implementation of corrective actions are required in §63.648(j)(7), a description of the corrective action(s) completed within the first 45 days following the discharge and, for action(s) not already completed, a schedule for implementation, including proposed commencement and completion dates.
(iv) For pilot-operated pressure relief devices, general or release-specific records for estimating the quantity of VOC released from the pilot vent during a release event, and records of calculations used to determine the quantity of specific HAP released for any event or series of events in which 72 or more pounds of VOC are released in a day.
(12) For each maintenance vent opening subject to the requirements in §63.643(c), the owner or operator shall keep the applicable records specified in paragraphs (i)(12)(i) through (vi) of this section.
(i) The owner or operator shall maintain standard site procedures used to deinventory equipment for safety purposes (e.g., hot work or vessel entry procedures) to document the procedures used to meet the requirements in §63.643(c). The current copy of the procedures shall be retained and available on-site at all times. Previous versions of the standard site procedures, is applicable, shall be retained for five years.
(ii) If complying with the requirements of §63.643(c)(1)(i) and the lower explosive limit at the time of the vessel opening exceeds 10 percent, identification of the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, and the lower explosive limit at the time of the vessel opening.
(iii) If complying with the requirements of §63.643(c)(1)(ii) and either the vessel pressure at the time of the vessel opening exceeds 5 psig or the lower explosive limit at the time of the active purging was initiated exceeds 10 percent, identification of the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, the pressure of the vessel or equipment at the time of discharge to the atmosphere and, if applicable, the lower explosive limit of the vapors in the equipment when active purging was initiated.
(iv) If complying with the requirements of §63.643(c)(1)(iii), records used to estimate the total quantity of VOC in the equipment and the type and size limits of equipment that contain less than 72 pounds of VOC at the time of maintenance vent opening. For each maintenance vent opening for which the deinventory procedures specified in paragraph (i)(12)(i) of this section are not followed or for which the equipment opened exceeds the type and size limits established in the records specified in this paragraph, identification of the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, and records used to estimate the total quantity of VOC in the equipment at the time the maintenance vent was opened to the atmosphere.
(v) If complying with the requirements of §63.643(c)(1)(iv), identification of the maintenance vent, the process units or equipment associated with the maintenance vent, records documenting the lack of a pure hydrogen supply, the date of maintenance vent opening, and the lower explosive limit of the vapors in the equipment at the time of discharge to the atmosphere for each applicable maintenance vent opening.
(vi) If complying with the requirements of §63.643(c)(1)(v), identification of the maintenance vent, the process units or equipment associated with the maintenance vent, records documenting actions taken to comply with other applicable alternatives and why utilization of this alternative was required, the date of maintenance vent opening, the equipment pressure and lower explosive limit of the vapors in the equipment at the time of discharge, an indication of whether active purging was performed and the pressure of the equipment during the installation or removal of the blind if active purging was used, the duration the maintenance vent was open during the blind installation or removal process, and records used to estimate the total quantity of VOC in the equipment at the time the maintenance vent was opened to the atmosphere for each applicable maintenance vent opening.
§63.670 Requirements for flare control devices.
* * * *
(b) Pilot flame presence. The owner or operator shall operate each flare with a pilot flame present at all times when regulated material is routed to the flare. Each 15-minute block during which there is at least one minute where no pilot flame is present when regulated material is routed to the flare is a deviation of the standard. Deviations in different 15-minute blocks from the same event are considered separate deviations. The owner or operator shall monitor for the presence of a pilot flame as specified in paragraph (g) of this section.
* * * *
(d) Flare tip velocity. For each flare, the owner or operator shall comply with either paragraph (d)(1) or (2) of this section, provided the appropriate monitoring systems are in-place, whenever regulated material is routed to the flare for at least 15-minutes and the flare vent gas flow rate is less than the smokeless design capacity of the flare.
* * * *
(e) Combustion zone operating limits. For each flare, the owner or operator shall operate the flare to maintain the net heating value of flare combustion zone gas (NHVcz) at or above 270 British thermal units per standard cubic feet (Btu/scf) determined on a 15-minute block period basis when regulated material is routed to the flare for at least 15-minutes. The owner or operator shall monitor and calculate NHVcz as specified in paragraph (m) of this section.
* * * *
(5) When a continuous monitoring system is used as provided in paragraph (j)(1) or (3) of this section and, if applicable, paragraph (j)(4) of this section, the owner or operator may elect to determine the 15-minute block average NHVvg using either the calculation methods in paragraph (l)(5)(i) of this section or the calculation methods in paragraph (l)(5)(ii) of this section. The owner or operator may choose to comply using the calculation methods in paragraph (l)(5)(i) of this section for some flares at the petroleum refinery and comply using the calculation methods (l)(5)(ii) of this section for other flares. However, for each flare, the owner or operator must elect one calculation method that will apply at all times, and use that method for all continuously monitored flare vent streams associated with that flare. If the owner or operator intends to change the calculation method that applies to a flare, the owner or operator must notify the Administrator 30 days in advance of such a change.
(i) Feed-forward calculation method. When calculating NHVvg for a specific 15-minute block:
(A) Use the results from the first sample collected during an event, (for periodic flare vent gas flow events) for the first 15-minute block associated with that event.
(B) If the results from the first sample collected during an event (for periodic flare vent gas flow events) are not available until after the second 15-minute block starts, use the results from the first sample collected during an event for the second 15-minute block associated with that event.
(C) For all other cases, use the results that are available from the most recent sample prior to the 15-minute block period for that 15-minute block period for all flare vent gas steams. For the purpose of this requirement, use the time that the results become available rather than the time the sample was collected. For example, if a sample is collected at 12:25 a.m. and the analysis is completed at 12:38 a.m., the results are available at 12:38 a.m. and these results would be used to determine compliance during the 15-minute block period from 12:45 a.m. to 1:00 a.m.
(ii) Direct calculation method. When calculating NHVvg for a specific 15-minute block:
(A) If the results from the first sample collected during an event (for periodic flare vent gas flow events) are not available until after the second 15-minute block starts, use the results from the first sample collected during an event for the first 15-minute block associated with that event.
(B) For all other cases, use the arithmetic average of all NHVvg measurement data results that become available during a 15-minute block to calculate the 15-minute block average for that period. For the purpose of this requirement, use the time that the results become available rather than the time the sample was collected. For example, if a sample is collected at 12:25 a.m. and the analysis is completed at 12:38 a.m., the results are available at 12:38 a.m. and these results would be used to determine compliance during the 15-minute block period from 12:30 a.m. to 12:45 a.m.
* * * *
(o)(4)(iv) You may conduct a single root cause analysis and corrective action analysis for a single event that causes two or more flares to have a flow event meeting the criteria in paragraph (o)(3)(i) or (ii) of this section, regardless of the configuration of the flares, if the root cause is reasonably expected to be a force majeure event, as defined in this subpart.
* * * *
(o)(6) The owner or operator shall determine the total number of events for which a root cause and corrective action analyses was required during the calendar year for each affected flare separately for events meeting the criteria in paragraph (o)(3)(i) of this section and those meeting the criteria in paragraph (o)(3)(ii) of this section. For the purpose of this requirement, a single root cause analysis conducted for an event that met both of the criteria in paragraphs (o)(3)(i) and (ii) of this section would be counted as an event under each of the separate criteria counts for that flare. Additionally, if a single root cause analysis was conducted for an event that caused multiple flares to meet the criteria in paragraph (o)(3)(i) or (ii) of this section, that event would count as an event for each of the flares for each criteria in paragraph (o)(3) of this section that was met during that event. The owner or operator shall also determine the total number of events for which a root cause and correct action analyses was required and the analyses concluded that the root cause was a force majeure event, as defined in this subpart.
* * * *
(o)(7)(ii) Two visible emissions exceedance events meeting the criteria in paragraph (o)(3)(i) of this section that were not caused by a force majeure event from a single flare in a 3 calendar year period for the same root cause for the same equipment.
(iii) Two flare tip velocity exceedance events meeting the criteria in paragraph (o)(3)(ii) of this section that were not caused by a force majeure event from a single flare in a 3 calendar year period for the same root cause for the same equipment.
(iv) Three visible emissions exceedance events meeting the criteria in paragraph (o)(3)(i) of this section that were not caused by a force majeure event from a single flare in a 3 calendar year period for any reason.
(v) Three flare tip velocity exceedance events meeting the criteria in paragraph (o)(3)(ii) of this section that were not caused by a force majeure event from a single flare in a 3 calendar year period for any reason.
§63.671 Requirements for flare monitoring systems.
* * * *
(e) Additional requirements for gas chromatographs. For monitors used to determine compositional analysis for net heating value per §63.670(j)(1), the gas chromatograph must also meet the requirements of paragraphs (e)(1) through (3) of this section.
Appendix to Subpart CC of Part 63—Tables
* * * *
| Parameter | Minimum accuracy requirements | Calibration requirements |
|---|---|---|
| Temperature | ±1 percent over the normal range of temperature measured, expressed in degrees Celsius (C), or 2.8 degrees C, whichever is greater | Conduct calibration checks at least annually; conduct calibration checks following any period of more than 24 hours throughout which the temperature exceeded the manufacturer's specified maximum rated temperature or install a new temperature sensor.
At least quarterly, inspect all components for integrity and all electrical connections for continuity, oxidation, and galvanic corrosion, unless the CPMS has a redundant temperature sensor. |
| Record the results of each calibration check and inspection. | ||
| Locate the temperature sensor in a position that provides a representative temperature; shield the temperature sensor system from electromagnetic interference and chemical contaminants. | ||
| Flow Rate for All Flows Other Than Flare Vent Gas | ±5 percent over the normal range of flow measured or 1.9 liters per minute (0.5 gallons per minute), whichever is greater, for liquid flow | Conduct a flow sensor calibration check at least biennially (every two years); conduct a calibration check following any period of more than 24 hours throughout which the flow rate exceeded the manufacturer's specified maximum rated flow rate or install a new flow sensor. |
| ±5 percent over the normal range of flow measured or 280 liters per minute (10 cubic feet per minute), whichever is greater, for gas flow | At least quarterly, inspect all components for leakage, unless the CPMS has a redundant flow sensor. | |
| ±5 percent over the normal range measured for mass flow | Record the results of each calibration check and inspection.
Locate the flow sensor(s) and other necessary equipment (such as straightening vanes) in a position that provides representative flow; reduce swirling flow or abnormal velocity distributions due to upstream and downstream disturbances. | |
| Flare Vent Gas Flow Rate | ±20 percent of flow rate at velocities ranging from 0.03 to 0.3 meters per second (0.1 to 1 feet per second)
±5 percent of flow rate at velocities greater than 0.3 meters per second (1 feet per second) | Conduct a flow sensor calibration check at least biennially (every two years); conduct a calibration check following any period of more than 24 hours throughout which the flow rate exceeded the manufacturer's specified maximum rated flow rate or install a new flow sensor.
At least quarterly, inspect all components for leakage, unless the CPMS has a redundant flow sensor. |
| Record the results of each calibration check and inspection. | ||
| Locate the flow sensor(s) and other necessary equipment (such as straightening vanes) in a position that provides representative flow; reduce swirling flow or abnormal velocity distributions due to upstream and downstream disturbances. | ||
| Pressure | ±5 percent over the normal operating range or 0.12 kilopascals (0.5 inches of water column), whichever is greater | Review pressure sensor readings at least once a week for straightline (unchanging) pressure and perform corrective action to ensure proper pressure sensor operation if blockage is indicated.
Using an instrument recommended by the sensor's manufacturer, check gauge calibration and transducer calibration annually; conduct calibration checks following any period of more than 24 hours throughout which the pressure exceeded the manufacturer's specified maximum rated pressure or install a new pressure sensor. |
| At least quarterly, inspect all components for integrity, all electrical connections for continuity, and all mechanical connections for leakage, unless the CPMS has a redundant pressure sensor. | ||
| Record the results of each calibration check and inspection. | ||
| Locate the pressure sensor(s) in a position that provides a representative measurement of the pressure and minimizes or eliminates pulsating pressure, vibration, and internal and external corrosion. | ||
| Net Heating Value by Calorimeter | ±2 percent of span | Specify calibration requirements in your site specific CPMS monitoring plan. Calibration requirements should follow manufacturer's recommendations at a minimum.
Temperature control (heated and/or cooled as necessary) the sampling system to ensure proper year-round operation. |
| Where feasible, select a sampling location at least two equivalent diameters downstream from and 0.5 equivalent diameters upstream from the nearest disturbance. Select the sampling location at least two equivalent duct diameters from the nearest control device, point of pollutant generation, air in-leakages, or other point at which a change in the pollutant concentration or emission rate occurs. | ||
| Net Heating Value by Gas Chromatograph | As specified in Performance Specification 9 of 40 CFR part 60, appendix B | Follow the procedure in Performance Specification 9 of 40 CFR part 60,appendix B, except that a single daily mid-level calibration check can be used (rather than triplicate analysis), the multi-point calibration can be conducted quarterly (rather than monthly), and the sampling line temperature must be maintained at a minimum temperature of 60°C (rather than 120°C). |
| Hydrogen analyzer | ±2 percent over the concentration measured or 0.1 volume percent, whichever is greater | Specify calibration requirements in your site specific CPMS monitoring plan. Calibration requirements should follow manufacturer's recommendations at a minimum. |
| Where feasible, select the sampling location at least two equivalent duct diameters from the nearest control device, point of pollutant generation, air in-leakages, or other point at which a change in the pollutant concentration occurs. |
§63.1100 Applicability.
* * * *
(b) Subpart A requirements. The following provisions of subpart A of this part (General Provisions), §§63.1 through 63.5, and §§63.12 through 63.15, apply to owners or operators of affected sources subject to this subpart. For sources that reclassify from major source to area source status, the applicable provisions of §63.9(j) and (k) apply. Beginning no later than the compliance dates specified in §63.1102(c), for ethylene production affected sources, §§63.7(a)(4), (c), (e)(4), and (g)(2) and 63.10(b)(2)(vi) also apply.
* * * *
(iii) Beginning no later than the compliance dates specified in §63.1102(c), flares subject to the requirements in 40 CFR part 63, subpart CC and used as a control device for an emission point subject to the requirements in Table 7 to §63.1103(e) are only required to comply with the flare requirements in 40 CFR part 63, subpart CC. This paragraph does not apply to multi-point pressure assisted flares.
§63.1102 Compliance schedule.
* * * *
(c)(11) The requirements in §63.1108(a)(4)(i), (b)(1)(ii), (b)(2), and (b)(4)(ii)(B).
* * * *
(d)(2)(ii) The compliance requirements specified in §63.1108(a)(4)(i), (b)(1)(ii), (b)(2), and (b)(4)(ii)(B).
* * * *
(e)(2)(iii) The compliance requirements specified in §63.1108(a)(4)(i), (b)(1)(ii), (b)(2), and (b)(4)(ii)(B).
§63.1103 Source category-specific applicability, definitions, and requirements.
* * * *
(e) Ethylene production applicability, definitions, and requirements- (1) Applicability- (i) Affected source. For the ethylene production (as defined in paragraph (e)(2) of this section) source category, the affected source comprises all emission points listed in paragraphs (e)(1)(i)(A) through (G) of this section that are associated with an ethylene production unit that is located at a major source, as defined in section 112(a) of the Act.
(A) All storage vessels (as defined in §63.1101) that store liquids containing organic HAP.
(B) All ethylene process vents (as defined in paragraph (e)(2) of this section) from continuous unit operations.
(C) All transfer racks (as defined in paragraph (e)(2) of this section) that load HAP-containing material.
(D) Equipment (as defined in §63.1101) that contains or contacts organic HAP.
(E) All waste streams (as defined in paragraph (e)(2) of this section) associated with an ethylene production unit.
(F) All heat exchange systems (as defined in §63.1082(b)) associated with an ethylene production unit.
(G) All ethylene cracking furnaces and associated decoking operations.
(ii) Exceptions. The emission points listed in paragraphs (e)(1)(ii) (A) through (L) of this section are in the ethylene production source category but are not subject to the requirements of paragraph (e)(3) of this section.
(A) Equipment that is located within an ethylene production unit that is subject to this subpart but does not contain organic HAP.
(B) Stormwater from segregated sewers.
(C) Water from fire-fighting and deluge systems in segregated sewers.
(D) Spills.
(E) Water from safety showers.
(F) Water from testing of fire-fighting and deluge systems.
(G) Vessels storing organic liquids that contain organic HAP as impurities.
(H) Transfer racks, loading arms, or loading hoses that only transfer liquids containing organic HAP as impurities.
(I) Transfer racks, loading arms, or loading hoses that vapor balance during all transfer operations.
(J) Air emissions from all ethylene cracking furnaces.
(K) Pressure vessels designed to operate in excess of 204.9 kilopascals and without emissions to the atmosphere.
(L) Vessels permanently attached to motor vehicles such as trucks, railcars, barges, or ships.
(iii) Exclusions. The provisions of this subpart do not apply to process units and emission points subject to subparts F, G, H, I and CC of this part.
(iv) Compliance schedule. The compliance schedule for the ethylene production source category is specified in §63.1102.
(2) Definitions. Ethylene process vent means a gas stream with a flow rate greater than 0.005 standard cubic meters per minute containing greater than 20 parts per million by volume HAP that is continuously discharged during operation of an ethylene production unit, as defined in this section. Ethylene process vents are gas streams that are discharged to the atmosphere (or the point of entry into a control device, if any) either directly or after passing through one or more recovery devices. Ethylene process vents do not include relief valve discharges; gaseous streams routed to a fuel gas system; leaks from equipment regulated under this subpart; episodic or nonroutine releases such as those associated with startup, shutdown, and malfunction; and in situ sampling systems (online analyzers).
Decoking operation means the coke combustion activity that occurs inside the radiant tube(s) in the ethylene cracking furnace firebox. Coke combustion activities during decoking can also occur in other downstream equipment such as the process gas outlet piping and transfer line exchangers or quench points.
Ethylene process vent means a gas stream with a flow rate greater than 0.005 standard cubic meters per minute containing greater than 20 parts per million by volume HAP that is continuously discharged during operation of an ethylene production unit. On and after July 6, 2023, ethylene process vent means a gas stream with a flow rate greater than 0.005 standard cubic meters per minute containing greater than 20 parts per million by volume HAP that is continuously or periodically discharged during operation of an ethylene production unit. Ethylene process vents are gas streams that are discharged to the atmosphere (or the point of entry into a control device, if any) either directly or after passing through one or more recovery devices. Ethylene process vents do not include:
(A) Pressure relief device discharges;
(B) Gaseous streams routed to a fuel gas system, including any flares using fuel gas, of which less than 50 percent of the fuel gas is derived from an ethylene production unit;
(C) Gaseous streams routed to a fuel gas system whereby any flares using fuel gas, of which 50 percent or more of the fuel gas is derived from an ethylene production unit, comply with §63.1103(e)(4) beginning no later than the compliance dates specified in §63.1102(c);
(D) Leaks from equipment regulated under this subpart;
(E) Episodic or nonroutine releases such as those associated with startup, shutdown, and malfunction until July 6, 2023;
(F) In situ sampling systems (online analyzers) until July 6, 2023; and
(G) Coke combustion emissions from decoking operations beginning no later than the compliance dates specified in §63.1102(c).
Ethylene production or production unit means a chemical manufacturing process unit in which ethylene and/or propylene are produced by separation from petroleum refining process streams or by subjecting hydrocarbons to high temperatures in the presence of steam. The ethylene production unit includes the separation of ethylene and/or propylene from associated streams such as a C4 product, pyrolysis gasoline, and pyrolysis fuel oil. Ethylene production does not include the manufacture of SOCMI chemicals such as the production of butadiene from the C4 stream and aromatics from pyrolysis gasoline.
Force majeure event means a release of HAP, either directly to the atmosphere from a pressure relief device or discharged via a flare, that is demonstrated to the satisfaction of the Administrator to result from an event beyond the owner or operator's control, such as natural disasters; acts of war or terrorism; loss of a utility external to the ethylene production unit (e.g., external power curtailment), excluding power curtailment due to an interruptible service agreement; and fire or explosion originating at a near or adjoining facility outside of the ethylene production unit that impacts the ethylene production unit's ability to operate.Force majeure event means a release of HAP, either directly to the atmosphere from a pressure relief device or discharged via a flare, that is demonstrated to the satisfaction of the Administrator to result from an event beyond the owner or operator's control, such as natural disasters; acts of war or terrorism; loss of a utility external to the ethylene production unit (e.g., external power curtailment), excluding power curtailment due to an interruptible service agreement; and fire or explosion originating at a near or adjoining facility outside of the ethylene production unit that impacts the ethylene production unit's ability to operate.
Heat exchange system means any cooling tower system or once-through cooling water system (e.g., river or pond water). A heat exchange system can include an entire recirculating or once-through cooling system.
Organic HAP means the compounds listed in Table 1 to subpart XX of this part.
Pressure-assisted multi-point flare means a flare system consisting of multiple flare burners in staged arrays whereby the vent stream pressure is used to promote mixing and smokeless operation at the flare burner tips. Pressure-assisted multi-point flares are designed for smokeless operation at velocities up to Mach = 1 conditions (i.e., sonic conditions), can be elevated or at ground level, and typically use cross-lighting for flame propagation to combust any flare vent gases sent to a particular stage of flare burners.
Pressure relief device means a valve, rupture disk, or similar device used only to release an unplanned, nonroutine discharge of gas from process equipment in order to avoid safety hazards or equipment damage. A pressure relief device discharge can result from an operator error, a malfunction such as a power failure or equipment failure, or other unexpected cause. Such devices include conventional, spring-actuated relief valves, balanced bellows relief valves, pilot-operated relief valves, rupture disks, and breaking, buckling, or shearing pin devices. Devices that are actuated either by a pressure of less than or equal to 2.5 pounds per square inch gauge or by a vacuum are not pressure relief devices.
Periodically discharged means gas stream discharges that are intermittent for which the total organic HAP concentration is greater than 20 parts per million by volume and total volatile organic compound emissions are 50 pounds per day or more. These intermittent discharges are associated with routine operations, maintenance activities, startups, shutdowns, malfunctions, or process upsets and do not include pressure relief device discharges or discharges classified as maintenance vents.
Radiant tube(s) means any portion of the tube coil assembly located within the ethylene cracking furnace firebox whereby a thermal cracking reaction of hydrocarbons (in the presence of steam) occurs. Hydrocarbons and steam pass through the radiant tube(s) of the ethylene cracking furnace during normal operation and coke is removed from the inside of the radiant tube(s) during decoking operation.
Relief valve means a type of pressure relief device that is designed to re-close after the pressure relief.
Transfer rack means the collection of loading arms and loading hoses at a single loading rack that is used to fill tank trucks and/or railcars with organic HAP. Transfer rack includes the associated pumps, meters, shutoff valves, relief valves, and other piping and valves. Transfer rack does not include racks, arms, or hoses that contain organic HAP only as impurities; or racks, arms, or hoses that vapor balance during all loading operations.
Waste means any material resulting from industrial, commercial, mining, or agricultural operations, or from community activities, that is discarded or is being accumulated, stored, or physically, chemically, thermally, or biologically treated prior to being discarded, recycled, or discharged.
Waste stream means the waste generated by a particular process unit, product tank, or waste management unit. The characteristics of the waste stream (e.g., flow rate, HAP concentration, water content) are determined at the point of waste generation. Examples of a waste stream include process wastewater, product tank drawdown, sludge and slop oil removed from waste management units, and landfill leachate.
(3) Requirements. The owner or operator must control organic HAP emissions from each affected source emission point by meeting the applicable requirements specified in Table 7 to this section. An owner or operator must perform the applicability assessment procedures and methods for process vents specified in §63.1104, except for paragraphs (d), (g), (h) through (j), (l)(1), and (n). An owner or operator must perform the applicability assessment procedures and methods for equipment leaks specified in §63.1107. General compliance, recordkeeping, and reporting requirements are specified in §§63.1108 through 63.1112. Before July 6, 2023, minimization of emissions from startup, shutdown, and malfunctions must be addressed in the startup, shutdown, and malfunction plan required by §63.1111; the plan must also establish reporting and recordkeeping of such events. A startup, shutdown, and malfunction plan is not required on and after July 6, 2023 and the requirements specified in §63.1111 no longer apply; however, for historical compliance purposes, a copy of the plan must be retained and available on-site for five years after July 6, 2023. Except as specified in paragraph (e)(4)(i) of this section, procedures for approval of alternate means of emission limitations are specified in §63.1113.
(4) Flares. Beginning no later than the compliance dates specified in §63.1102(c), if a steam-assisted, air-assisted, non-assisted, or pressure-assisted multi-point flare is used as a control device for an emission point subject to the requirements in Table 7 to this section, then the owner or operator must meet the applicable requirements for flares as specified in §§63.670 and 63.671 of subpart CC, including the provisions in Tables 12 and 13 to subpart CC of this part, except as specified in paragraphs (e)(4)(i) through (xiv) of this section. This requirement also applies to any flare using fuel gas from a fuel gas system, of which 50 percent or more of the fuel gas is derived from an ethylene production unit, being used to control an emission point subject to the requirements in Table 7 of this section. For purposes of compliance with this paragraph, the following terms are defined in §63.641 of subpart CC: Assist air, assist steam, center steam, combustion zone, combustion zone gas, flare, flare purge gas, flare supplemental gas, flare sweep gas, flare vent gas, lower steam, net heating value, perimeter assist air, pilot gas, premix assist air, total steam, and upper steam.
(i) The owner or operator may elect to comply with the alternative means of emissions limitation requirements specified in of §63.670(r) of subpart CC in lieu of the requirements in §63.670(d) through (f) of subpart CC, as applicable. However, instead of complying with §63.670(r)(3) of subpart CC, the owner or operator must submit the alternative means of emissions limitation request following the requirements in §63.1113.
(ii) Instead of complying with §63.670(o)(2)(i) of subpart CC, the owner or operator must develop and implement the flare management plan no later than the compliance dates specified in §63.1102(c).
(iii) Instead of complying with §63.670(o)(2)(iii) of subpart CC, if required to develop a flare management plan and submit it to the Administrator, then the owner or operator must also submit all versions of the plan in portable document format (PDF) to the EPA via the Compliance and Emissions Data Reporting Interface (CEDRI), which can be accessed through the EPA's Central Data Exchange (CDX) (https://cdx.epa.gov/). If you claim some of the information in your flare management plan is confidential business information (CBI), submit a version with the CBI omitted via CEDRI. A complete plan, including information claimed to be CBI and clearly marked as CBI, must be mailed to the following address: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, U.S. EPA Mailroom (E143-01), Attention: Ethylene Production Sector Lead, 109 T.W. Alexander Drive, Research Triangle Park, NC 27711.
(iv) Section 63.670(o)(3)(ii) of subpart CC and all references to §63.670(o)(3)(ii) of subpart CC do not apply. Instead, the owner or operator must comply with the maximum flare tip velocity operating limit at all times.
(v) Substitute “ethylene production unit” for each occurrence of “petroleum refinery.”
(vi) Each occurrence of “refinery” does not apply.
(vii) Except as specified in paragraph (e)(4)(vii)(G) of this section, if a pressure-assisted multi-point flare is used as a control device for an emission point subject to the requirements in Table 7 to this section, then the owner or operator must comply with the requirements specified in paragraphs (e)(4)(vii)(A) through (F) of this section.
(A) The owner or operator is not required to comply with the flare tip velocity requirements in §63.670(d) and (k) of subpart CC;
(B) The owner or operator must substitute “800” for each occurrence of “270” in §63.670(e) of subpart CC;
(C) The owner or operator must determine the 15-minute block average NHVvg using only the direct calculation method specified in §63.670(l)(5)(ii) of subpart CC;
(D) Instead of complying with §63.670(b) and (g) of subpart CC, if a pressure-assisted multi-point flare uses cross-lighting on a stage of burners rather than having an individual pilot flame on each burner, the owner or operator must operate each stage of the pressure-assisted multi-point flare with a flame present at all times when regulated material is routed to that stage of burners. Each stage of burners that cross-lights in the pressure-assisted multi-point flare must have at least two pilots with at least one continuously lit and capable of igniting all regulated material that is routed to that stage of burners. Each 15-minute block during which there is at least one minute where no pilot flame is present on a stage of burners when regulated material is routed to that stage is a deviation of the standard. Deviations in different 15-minute blocks from the same event are considered separate deviations. The pilot flame(s) on each stage of burners that use cross-lighting must be continuously monitored by a thermocouple or any other equivalent device used to detect the presence of a flame;
(E) Unless the owner or operator of a pressure-assisted multi-point flare chooses to conduct a cross-light performance demonstration as specified in this paragraph, the owner or operator must ensure that if a stage of burners on the flare uses cross-lighting, that the distance between any two burners in series on that stage is no more than 6 feet when measured from the center of one burner to the next burner. A distance greater than 6 feet between any two burners in series may be used provided the owner or operator conducts a performance demonstration that confirms the pressure-assisted multi-point flare will cross-light a minimum of three burners and the spacing between the burners and location of the pilot flame must be representative of the projected installation. The compliance demonstration must be approved by the permitting authority and a copy of this approval must be maintained onsite. The compliance demonstration report must include: A protocol describing the test methodology used, associated test method QA/QC parameters, the waste gas composition and NHVcz of the gas tested, the velocity of the waste gas tested, the pressure-assisted multi-point flare burner tip pressure, the time, length, and duration of the test, records of whether a successful cross-light was observed over all of the burners and the length of time it took for the burners to cross-light, records of maintaining a stable flame after a successful cross-light and the duration for which this was observed, records of any smoking events during the cross-light, waste gas temperature, meteorological conditions (e.g., ambient temperature, barometric pressure, wind speed and direction, and relative humidity), and whether there were any observed flare flameouts; and
(F) The owner or operator of a pressure-assisted multi-point flare must install and operate pressure monitor(s) on the main flare header, as well as a valve position indicator monitoring system for each staging valve to ensure that the flare operates within the proper range of conditions as specified by the manufacturer. The pressure monitor must meet the requirements in Table 13 to subpart CC of this part.
(G) If a pressure-assisted multi-point flare is operating under the requirements of an approved alternative means of emission limitations, the owner or operator shall either continue to comply with the terms of the alternative means of emission limitations or comply with the provisions in paragraphs (e)(4)(vii)(A) through (F) of this section.
(viii) If an owner or operator chooses to determine compositional analysis for net heating value with a continuous process mass spectrometer, the owner or operator must comply with the requirements specified in paragraphs (e)(4)(viii)(A) through (G) of this section.
(A) The owner or operator must meet the requirements in §63.671(e)(2). The owner or operator may augment the minimum list of calibration gas components found in §63.671(e)(2) with compounds found during a pre-survey or known to be in the gas through process knowledge.
(B) Calibration gas cylinders must be certified to an accuracy of 2 percent and traceable to National Institute of Standards and Technology (NIST) standards.
(C) For unknown gas components that have similar analytical mass fragments to calibration compounds, the owner or operator may report the unknowns as an increase in the overlapped calibration gas compound. For unknown compounds that produce mass fragments that do not overlap calibration compounds, the owner or operator may use the response factor for the nearest molecular weight hydrocarbon in the calibration mix to quantify the unknown component's NHVvg.
(D) The owner or operator may use the response factor for n-pentane to quantify any unknown components detected with a higher molecular weight than n-pentane.
(E) The owner or operator must perform an initial calibration to identify mass fragment overlap and response factors for the target compounds.
(F) The owner or operator must meet applicable requirements in Performance Specification 9 of 40 CFR part 60, appendix B, for continuous monitoring system acceptance including, but not limited to, performing an initial multi-point calibration check at three concentrations following the procedure in Section 10.1 and performing the periodic calibration requirements listed for gas chromatographs in Table 13 to subpart CC of this part, for the process mass spectrometer. The owner or operator may use the alternative sampling line temperature allowed under Net Heating Value by Gas Chromatograph in Table 13 to subpart CC of this part.
(G) The average instrument calibration error (CE) for each calibration compound at any calibration concentration must not differ by more than 10 percent from the certified cylinder gas value. The CE for each component in the calibration blend must be calculated using the following equation:
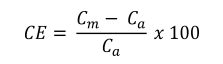
Where:
Cm = Average instrument response (ppm)
Ca = Certified cylinder gas value (ppm)
(ix) An owner or operator using a gas chromatograph or mass spectrometer for compositional analysis for net heating value may choose to use the CE of NHVmeasured versus the cylinder tag value NHV as the measure of agreement for daily calibration and quarterly audits in lieu of determining the compound-specific CE. The CE for NHV at any calibration level must not differ by more than 10 percent from the certified cylinder gas value. The CE for must be calculated using the following equation:
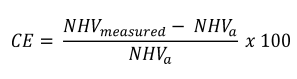
Where:
NHVmeasured = Average instrument response (Btu/scf)
NHVa = Certified cylinder gas value (Btu/scf)
(x) Instead of complying with §63.670(p) of subpart CC, the owner or operator must keep the flare monitoring records specified in §63.1109(e).
(xi) Instead of complying with §63.670(q) of subpart CC, the owner or operator must comply with the reporting requirements specified in §63.1110(d) and (e)(4).
(xii) When determining compliance with the pilot flame requirements specified in §63.670(b) and (g), substitute “pilot flame or flare flame” for each occurrence of “pilot flame.”
(xiii) When determining compliance with the flare tip velocity and combustion zone operating limits specified in §63.670(d) and (e), the requirement effectively applies starting with the 15-minute block that includes a full 15 minutes of the flaring event. The owner or operator is required to demonstrate compliance with the velocity and NHVcz requirements starting with the block that contains the fifteenth minute of a flaring event. The owner or operator is not required to demonstrate compliance for the previous 15-minute block in which the event started and contained only a fraction of flow.
(xiv) In lieu of meeting the requirements in §§63.670 and 63.671 of subpart CC, an owner or operator may submit a request to the Administrator for approval of an alternative test method in accordance with §63.7(f). The alternative test method must be able to demonstrate on an ongoing basis at least once every 15-minutes that the flare meets 96.5% combustion efficiency and provide a description of the alternative recordkeeping and reporting that would be associated with the alternative test method. The alternative test method request may also include a request to use the alternative test method in lieu of the pilot or flare flame monitoring requirements of 63.670(g).
(5) Maintenance vents. Unless an extension is requested in accordance with the provisions in §63.6(i) of subpart A, beginning no later than the compliance dates specified in §63.1102(c), an owner or operator may designate an ethylene process vent as a maintenance vent if the vent is only used as a result of startup, shutdown, maintenance, or inspection of equipment where equipment is emptied, depressurized, degassed, or placed into service. The owner or operator must comply with the applicable requirements in paragraphs (e)(5)(i) through (iii) of this section for each maintenance vent.
(i) Prior to venting to the atmosphere, remove process liquids from the equipment as much as practical and depressurize the equipment to either: A flare meeting the requirements specified in paragraph (e)(4) of this section, or a non-flare control device meeting the requirements specified in §63.982(c)(2) of subpart SS, until one of the following conditions, as applicable, is met.
(A) The vapor in the equipment served by the maintenance vent has a lower explosive limit (LEL) of less than 10 percent.
(B) If there is no ability to measure the LEL of the vapor in the equipment based on the design of the equipment, the pressure in the equipment served by the maintenance vent is reduced to 5 pounds per square inch gauge (psig) or less. Upon opening the maintenance vent, active purging of the equipment cannot be used until the LEL of the vapors in the maintenance vent (or inside the equipment if the maintenance is a hatch or similar type of opening) is less than 10 percent.
(C) The equipment served by the maintenance vent contains less than 50 pounds of total volatile organic compounds (VOC).
(D) If, after applying best practices to isolate and purge equipment served by a maintenance vent, none of the applicable criterion in paragraphs (e)(5)(i)(A) through (C) of this section can be met prior to installing or removing a blind flange or similar equipment blind, then the pressure in the equipment served by the maintenance vent must be reduced to 2 psig or less before installing or removing the equipment blind. During installation or removal of the equipment blind, active purging of the equipment may be used provided the equipment pressure at the location where purge gas is introduced remains at 2 psig or less.
(ii) Except for maintenance vents complying with the alternative in paragraph (e)(5)(i)(C) of this section, the owner or operator must determine the LEL or, if applicable, equipment pressure using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
(iii) For maintenance vents complying with the alternative in paragraph (e)(5)(i)(C) of this section, the owner or operator must determine mass of VOC in the equipment served by the maintenance vent based on the equipment size and contents after considering any contents drained or purged from the equipment. Equipment size may be determined from equipment design specifications. Equipment contents may be determined using process knowledge.
(6) Bypass lines. Beginning on the compliance dates specified in §63.1102(c), the use of a bypass line at any time on a closed vent system to divert emissions subject to the requirements in Table 7 to §63.1103(e) to the atmosphere or to a control device not meeting the requirements specified in Table 7 of this subpart is an emissions standards violation. If the owner or operator is subject to the bypass monitoring requirements of §63.983(a)(3) of subpart SS, then the owner or operator must continue to comply with the requirements in §63.983(a)(3) of subpart SS and the recordkeeping and reporting requirements in §§63.998(d)(1)(ii) and 63.999(c)(2) of subpart SS, in addition to paragraph (e)(9) of this section, the recordkeeping requirements specified in §63.1109(g), and the reporting requirements specified in §63.1110(e)(6). For purposes of compliance with this paragraph, the phrase “Except for equipment needed for safety purposes such as pressure relief devices, low leg drains, high point bleeds, analyzer vents, and open-ended valves or lines” in §63.983(a)(3) does not apply; instead, the exemptions specified in paragraph (e)(6)(i) and (ii) of this section apply.
(i) Except for pressure relief devices subject to 40 CFR 63.1107(h)(4), equipment such as low leg drains and equipment subject to the requirements specified in paragraph (f) of Table 7 to §63.1103(e) are not subject to this paragraph (e)(6) of this section.
(ii) Open-ended valves or lines that use a cap, blind flange, plug, or second valve and follow the requirements specified in §60.482-6(a)(2), (b), and (c) or follow requirements codified in another regulation that are the same as §60.482-6(a)(2), (b), and (c) are not subject to this paragraph (e)(6) of this section.
(7) Decoking operation standards for ethylene cracking furnaces. Beginning no later than the compliance dates specified in §63.1102(c), the owner or operator must comply with paragraph (e)(7)(i) of this section and also use at least two of the control measures specified in paragraphs (e)(7)(ii) through (v) of this section to minimize coke combustion emissions from the decoking of the radiant tube(s) in each ethylene cracking furnace.
(i) During normal operations, conduct daily inspections of the firebox burners and repair all burners that are impinging on the radiant tube(s) as soon as practical, but not later than 1 calendar day after the flame impingement is found. The owner or operator may delay burner repair beyond 1 calendar day using the procedures specified in paragraphs (e)(7)(i)(A) and (B) of this section provided the repair cannot be completed during normal operations, the burner cannot be shutdown without significantly impacting the furnace heat distribution and firing rate, and action is taken to reduce flame impingement as much as possible during continued operation. An inspection may include, but is not limited to: visual inspection of the radiant tube(s) for localized bright spots (this may be confirmed with a temperature gun), use of luminescent powders injected into the burner to illuminate the flame pattern, or identifying continued localized coke build-up that causes short runtimes between decoking cycles. A repair may include, but is not limited to: Taking the burner out of service, replacing the burner, adjusting the alignment of the burner, adjusting burner configuration, making burner air corrections, repairing a malfunction of the fuel liquid removal equipment, or adding insulation around the radiant tube(s).
(A) If a shutdown for repair would cause greater emissions than the potential emissions from delaying repair, repair must be completed following the next planned decoking operation (and before returning the ethylene cracking furnace back to normal operations) or during the next ethylene cracking furnace complete shutdown (when the ethylene cracking furnace firebox is taken completely off-line), whichever is earlier.
(B) If a shutdown for repair would cause lower emissions than the potential emissions from delaying repair, then shutdown of the ethylene cracking furnace must immediately commence and the repair must be completed before returning the ethylene cracking furnace back to normal operations.
(ii) During decoking operations, beginning before the expected end of the air-in decoke time, continuously monitor (or use a gas detection tube or equivalent sample technique every three hours to monitor) the CO2 concentration in the combined decoke effluent downstream of the last component being decoked for an indication that the coke combustion in the ethylene cracking furnace radiant tube(s) is complete. The owner or operator must immediately initiate procedures to stop the coke combustion once the CO2 concentration at the outlet consistently reaches a level that indicates combustion of coke is complete and site decoke completion assurance procedures have been concluded.
(iii) During decoking operations, continuously monitor the temperature at the radiant tube(s) outlet when air is being introduced to ensure the coke combustion occurring inside the radiant tube(s) is not so aggressive (i.e., too hot) that it damages either the radiant tube(s) or ethylene cracking furnace isolation valve(s). The owner or operator must immediately initiate procedures to reduce the temperature at the radiant tube(s) outlet once the temperature reaches a level that indicates combustion of coke inside the radiant tube(s) is too aggressive.
(iv) After decoking, but before returning the ethylene cracking furnace back to normal operations, verify that decoke air is no longer being added.
(v) After decoking, but before returning the ethylene cracking furnace back to normal operations and/or during normal operations, inject materials into the steam or feed to reduce coke formation inside the radiant tube(s) during normal operation.
(8) Ethylene cracking furnace isolation valve inspections. Beginning no later than the compliance dates specified in §63.1102(c), the owner or operator must conduct ethylene cracking furnace isolation valve inspections as specified in paragraphs (e)(8)(i) and (ii) of this section.
(i) Prior to decoking operation, inspect the applicable ethylene cracking furnace isolation valve(s) to confirm that the radiant tube(s) being decoked is completely isolated from the ethylene production process so that no emissions generated from decoking operations are sent to the ethylene production process. If poor isolation is identified, then the owner or operator must rectify the isolation issue prior to continuing decoking operations to prevent leaks into the ethylene production process.
(ii) Prior to returning the ethylene cracking furnace to normal operations after a decoking operation, inspect the applicable ethylene cracking furnace isolation valve(s) to confirm that the radiant tube(s) that was decoked is completely isolated from the decoking pot or furnace firebox such that no emissions are sent from the radiant tube(s) to the decoking pot or furnace firebox once the ethylene cracking furnace returns to normal operation. If poor isolation is identified, then the owner or operator must rectify the isolation issue prior to continuing normal operations to prevent product from escaping to the atmosphere through the decoking pot or furnace firebox.
(9) Startup, shutdown, and malfunction referenced provisions. Beginning no later than the compliance dates specified in §63.1102(c), the referenced provisions specified in paragraphs (e)(9)(i) through (xx) of this section do not apply when demonstrating compliance with paragraph (e)(3) of this section.
(i) The second sentence of §63.181(d)(5)(i) of subpart H.
(ii) The second sentence of §63.983(a)(5) of subpart SS.
(iii) The phrase “except during periods of start-up, shutdown and malfunction as specified in the referencing subpart” in §63.984(a) of subpart SS.
(iv) The phrase “except during periods of start-up, shutdown and malfunction as specified in the referencing subpart” in §63.985(a) of subpart SS.
(v) The phrase “other than start-ups, shutdowns, or malfunctions” in §63.994(c)(1)(ii)(D) of subpart SS.
(vi) Section 63.996(c)(2)(ii) of subpart SS.
(vii) The last sentence of §63.997(e)(1)(i) of subpart SS.
(viii) Section 63.998(b)(2)(iii) of subpart SS.
(ix) The phrase “other than periods of startups, shutdowns, and malfunctions” from §63.998(b)(5)(i)(A) of subpart SS.
(x) The phrase “other than a start-up, shutdown, or malfunction” from §63.998(b)(5)(i)(B)(3) of subpart SS.
(xi) The phrase “other than periods of startups, shutdowns, and malfunctions” from §63.998(b)(5)(i)(C) of subpart SS.
(xii) The phrase “other than a start-up, shutdown, or malfunction” from §63.998(b)(5)(ii)(C) of subpart SS.
(xiii) The phrase “except as provided in paragraphs (b)(6)(i)(A) and (B) of this section” from §63.998(b)(6)(i) of subpart SS.
(xiv) The second sentence of §63.998(b)(6)(ii) of subpart SS.
(xv) Section 63.998(c)(1)(ii)(D) through (G) of subpart SS.
(xvi) Section 63.998(d)(3) of subpart SS.
(xvii) The phrase “may be included as part of the startup, shutdown, and malfunction plan, as required by the referencing subpart for the source, or” from §63.1024(f)(4)(i) of subpart UU.
(xviii) The phrase “(except periods of startup, shutdown, or malfunction)” from §63.1026(e)(1)(ii)(A) of subpart UU.
(xix) The phrase “(except periods of startup, shutdown, or malfunction)” from §63.1028(e)(1)(i)(A) of subpart UU.
(xx) The phrase “(except periods of startup, shutdown, or malfunction)” from §63.1031(b)(1) of subpart UU.
(10) Storage vessel degassing. Beginning no later than the compliance dates specified in §63.1102(c), for each storage vessel subject to paragraph (b) or (c) of Table 7 to §63.1103(e), the owner or operator must comply with paragraphs (e)(10)(i) through (iii) of this section during storage vessel shutdown operations (i.e., emptying and degassing of a storage vessel) until the vapor space concentration in the storage vessel is less than 10 percent of the LEL. The owner or operator must determine the LEL using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
(i) Remove liquids from the storage vessel as much as practicable;
(ii) Comply with one of the following:
(A) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to a flare and meet the requirements of §63.983 and paragraphs (e)(4) and (9) of this section.
(B) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to any combination of non-flare control devices and meet the requirements specified in §63.982(c)(1) and paragraph (e)(9) of this section.
(C) Reduce emissions of total organic HAP by 98 weight-percent by routing emissions to a fuel gas system or process and meet the requirements specified in §63.982(d) and paragraph (e)(9) of this section.
(iii) Maintain records necessary to demonstrate compliance with the requirements in §63.1108(a)(4)(ii) including, if appropriate, records of existing standard site procedures used to empty and degas (deinventory) equipment for safety purposes.
| If you own or operate . . . | And if . . . | Then you must . . . |
|---|---|---|
| (a) A storage vessel (as defined in §63.1101) that stores liquid containing organic HAP | (1) The maximum true vapor pressure of total organic HAP is ≥3.4 kilopascals but <76.6 kilopascals; and the capacity of the vessel is ≥4 cubic meters but <95 cubic meters | (i) Fill the vessel through a submerged pipe; or (ii) Comply with the requirements for storage vessels with capacities ≥95 cubic meters. |
| (b) A storage vessel (as defined in §63.1101) that stores liquid containing organic HAP | (1) The maximum true vapor pressure of total organic HAP is ≥3.4 kilopascals but <76.6 kilopascals; and the capacity of the vessel is ≥95 cubic meters | (i) Except as specified in paragraph (b)(1)(iii) of this table, comply with the requirements of subpart WW of this part; or (ii) Except as specified in paragraph (b)(1)(iii) of this table, reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to any combination of control devices and meet the requirements of §63.982(a)(1). (iii) Beginning no later than the compliance dates specified in §63.1102(c), comply with paragraph (b)(1)(iii)(A), (B), (C), or (D) of this table, and (e)(10) of this section. (A) Comply with the requirements of subpart WW of this part; or (B) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to a flare and meet the requirements of §63.983 and paragraphs (e)(4) and (9) of this section; or (C) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to any combination of non-flare control devices and meet the requirements specified in §63.982(c)(1) and (e)(9) of this section; or (D) Reduce emissions of total organic HAP by 98 weight-percent by routing emissions to a fuel gas system(a) or process and meet the requirements specified in §63.982(d) and (e)(9) of this section. |
| (c) A storage vessel (as defined in §63.1101) that stores liquid containing organic HAP | (1) The maximum true vapor pressure of total organic HAP is ≥76.6 kilopascals | (i) Except as specified in paragraph (c)(1)(ii) of this table, reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to any combination of control devices and meet the requirements of §63.982(a)(1). (ii) Beginning no later than the compliance dates specified in §63.1102(c), comply with paragraph (c)(1)(ii)(A), (B), or (C) of this table, and (e)(10) of this section. (A) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to a flare and meet the requirements of §63.983 and paragraphs (e)(4) and (9) of this section; or (B) Reduce emissions of total organic HAP by 98 weight-percent by venting emissions through a closed vent system to any combination of non-flare control devices and meet the requirements specified in §63.982(c)(1) and (e)(9) of this section; or (C) Reduce emissions of total organic HAP by 98 weight-percent by routing emissions to a fuel gas system(a) or process and meet the requirements specified in §63.982(d) and (e)(9) of this section. |
| (d) An ethylene process vent (as defined in paragraph (e)(2) of this section) | (1) The process vent is at an existing source and the vent stream has a flow rate ≥0.011 scmm and a total organic HAP concentration ≥50 parts per million by volume on a dry basis; or the process vent is at a new source and the vent stream has a flow rate ≥0.008 scmm and a total organic HAP concentration ≥30 parts per million by volume on a dry basis | (i) Except as specified in paragraph (d)(1)(ii) of this table, reduce emissions of organic HAP by 98 weight-percent; or reduce organic HAP or TOC to a concentration of 20 parts per million by volume on a dry basis corrected to 3% oxygen; whichever is less stringent, by venting emissions through a closed vent system to any combination of control devices and meet the requirements specified in §63.982(b) and (c)(2). (ii) Beginning no later than the compliance dates specified in §63.1102(c), comply with the maintenance vent requirements specified in paragraph (e)(5) of this section and either paragraph (d)(1)(ii)(A) or (B) of this table. (A) Reduce emissions of organic HAP by 98 weight-percent; or reduce organic HAP or TOC to a concentration of 20 parts per million by volume on a dry basis corrected to 3-percent oxygen; whichever is less stringent, by venting emissions through a closed vent system to a flare and meet the requirements of §63.983 and paragraphs (e)(4) and (9) of this section; or (B) Reduce emissions of organic HAP by 98 weight-percent; or reduce organic HAP or TOC to a concentration of 20 parts per million by volume on a dry basis corrected to 3-percent oxygen; whichever is less stringent, by venting emissions through a closed vent system to any combination of non-flare control devices and meet the requirements specified in §63.982(c)(2) and (e)(9) of this section. |
| (e) A transfer rack (as defined in paragraph (e)(2) of this section) | (1) Materials loaded have a true vapor pressure of total organic HAP ≥3.4 kilopascals and ≥76 cubic meters per day (averaged over any consecutive 30-day period) of HAP-containing material is loaded | (i) Reduce emissions of organic HAP by 98 weight-percent; or reduce organic HAP or TOC to a concentration of 20 parts per million by volume on a dry basis corrected to 3-percent oxygen; whichever is less stringent, by venting emissions through a closed vent system to any combination of control devices as specified in §63.1105 and meet the requirements specified in paragraph (e)(9) of this section.; or |
| (ii) Install process piping designed to collect the HAP-containing vapors displaced from tank trucks or railcars during loading and to route it to a process, a fuel gas system, or a vapor balance system, as specified in §63.1105 and meet the requirements specified in paragraph (e)(9) of this section.(a) | ||
| (f) Equipment (as defined in §63.1101) that contains or contacts organic HAP | (1) The equipment contains or contacts ≥5 weight-percent organic HAP; and the equipment is not in vacuum service | (i) Except as specified in paragraph (f)(1)(ii) of this table, comply with the requirements of subpart UU of this part. (ii) Beginning no later than the compliance dates specified in §63.1102(c), comply with the requirements of paragraph (e)(9) of this section and subpart UU of this part, except instead of complying with the pressure relief device requirements of §63.1030 of subpart UU, meet the requirements of §63.1107(h), and in lieu of the flare requirement of §63.1034(b)(2)(iii), comply with the requirements specified in paragraph (e)(4) of this section.(a) |
| (g) Processes that generate waste (as defined in paragraph (e)(2) of this section | (1) The waste stream contains any of the following HAP: Benzene, cumene, ethyl benzene, hexane, naphthalene, styrene, toluene, o-xylene, m-xylene, p-xylene, or 1,3-butadiene | Comply with the waste requirements of subpart XX of this part. For ethylene production unit waste stream requirements, terms have the meanings specified in subpart XX. |
| (h) A heat exchange system (as defined in §63.1082(b)) | Comply with the heat exchange system requirements of subpart XX of this part. | |
| (i) A closed vent system that contains one or more bypass lines | (1) The bypass line could divert a vent stream directly to the atmosphere or to a control device not meeting the requirements in this table | Beginning no later than the compliance dates specified in §63.1102(c), comply with the requirements specified in paragraphs (e)(6) and (9) of this section. |
| (j) A decoking operation associated with an ethylene cracking furnace | Beginning no later than the compliance dates specified in §63.1102(c), comply with the requirements specified in paragraphs (e)(7) and (8) of this section. | |
| (a) Beginning no later than the compliance dates specified in §63.1102(c), any flare using fuel gas from a fuel gas system, of which 50 percent or more of the fuel gas is derived from an ethylene production unit as determined on an annual average basis, must be in compliance with paragraph (e)(4) of this section. | ||
§63.1107 Equipment leaks.
* * * *
(h)(3)(iv) The owner or operator must determine the total number of release events that occurred during the calendar year for each affected pressure relief device separately. The owner or operator must also determine the total number of release events for each pressure relief device for which the root cause analysis concluded that the root cause was a force majeure event, as defined in §63.1103(e)(2).
(h)(3)(v)(B) A second release event not including force majeure events from a single pressure relief device in a 3-calendar year period for the same root cause for the same equipment.
* * * *
(h)(6) Root cause analysis and corrective action analysis. A root cause analysis and corrective action analysis must be completed as soon as possible, but no later than 45 days after a release event. Special circumstances affecting the number of root cause analyses and/or corrective action analyses are provided in paragraphs (h)(6)(i) through (iv) of this section.
* * * *
(h)(6)(ii) Prior to June 3, 2024, you may conduct a single root cause analysis and corrective action analysis for a single emergency event that causes two or more pressure relief devices to release, regardless of the equipment served, if the root cause is reasonably expected to be a force majeure event, as defined in §63.1103(e)(2).
§63.1109 Recordkeeping requirements.
* * * *
(2) If complying with the requirements of §63.1103(e)(5)(i)(A) and the LEL at the time of the vessel opening exceeds 10 percent, records that identify the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, and the LEL at the time of the vessel opening.
(3) If complying with the requirements of §63.1103(e)(5)(i)(B) and either the vessel pressure at the time of the vessel opening exceeds 5 psig or the LEL at the time of the active purging was initiated exceeds 10 percent, records that identify the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, the pressure of the vessel or equipment at the time of discharge to the atmosphere and, if applicable, the LEL of the vapors in the equipment when active purging was initiated.
* * * *
(5) If complying with the requirements of §63.1103(e)(5)(i)(D), identification of the maintenance vent, the process units or equipment associated with the maintenance vent, records documenting actions taken to comply with other applicable alternatives and why utilization of this alternative was required, the date of maintenance vent opening, the equipment pressure and LEL of the vapors in the equipment at the time of discharge, an indication of whether active purging was performed and the pressure of the equipment during the installation or removal of the blind if active purging was used, the duration the maintenance vent was open during the blind installation or removal process, and records used to estimate the total quantity of VOC in the equipment at the time the maintenance vent was opened to the atmosphere for each applicable maintenance vent opening.
* * * *
(i)(2) Records of the number of releases during each calendar year and the number of those releases for which the root cause was determined to be a force majeure event. Keep these records for the current calendar year and the past five calendar years.
§63.1110 Reporting requirements.
* * * *
(a)(10) Beginning no later than the compliance dates specified in §63.1102(c), within 60 days after the date of completing each performance test required by this subpart, the owner or operator must submit the results of the performance test following the procedures specified in paragraphs (a)(10)(i)(A) through (C) of this section.
(i) Beginning no later than the compliance dates specified in §63.1102(c) for ethylene production affected sources, specified in §63.1102(d) for cyanide chemicals manufacturing affected sources, and specified in §63.1102(e) for carbon black production affected sources, within 60 days after the date of completing each performance test required by this subpart or applicability assessment required by §63.1103(f)(3)(iv), the owner or operator must submit the results of the performance test or applicability assessment following the procedures specified in paragraphs (a)(10)(i)(A) through (C) of this section.
(A) Data collected using test methods supported by the EPA's Electronic Reporting Tool (ERT) as listed on the EPA's ERT website (https://www.epa.gov/electronic-reporting-air-emissions/electronic-reporting-tool-ert) at the time of the test. Submit the results of the performance test or applicability assessment to the EPA via CEDRI, which can be accessed through the EPA's CDX ( https://cdx.epa.gov/ ). The data must be submitted in a file format generated through the use of the EPA's ERT. Alternatively, you may submit an electronic file consistent with the extensible markup language (XML) schema listed on the EPA's ERT website.
(B) Data collected using test methods that are not supported by the EPA's ERT as listed on the EPA's ERT website at the time of the test. The results of the performance test or applicability assessment must be included as an attachment in the ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the ERT generated package or alternative file to the EPA via CEDRI.
(C) CBI. Do not use CEDRI to submit information you claim as CBI. Anything submitted to CEDRI cannot later be claimed CBI. Although we do not expect persons to assert a claim of CBI, if an owner or operator wishes to assert a CBI claim for some of the information submitted under paragraph (a)(10)(i)(A) or (B) of this section, then the owner or operator must submit a complete file, including information claimed to be CBI, to the EPA. The file must be generated through the use of the EPA's ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. EPA/OAQPS/CORE CBI Office, Attention: Group Leader, Measurement Policy Group, MD C404-02, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via EPA's CDX as described in paragraphs (a)(10)(i)(A) and (B) of this section. All CBI claims must be asserted at the time of submission. Furthermore, under CAA section 114(c), emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available.
(ii) Beginning no later than the compliance dates specified in §63.1102(c) through (e), the owner or operator must submit all subsequent Notification of Compliance Status reports required under paragraph (a)(4) of this section in PDF format to the EPA via CEDRI, which can be accessed through EPA's CDX ( https://cdx.epa.gov/ ). All subsequent Periodic Reports required under paragraph (a)(5) of this section must be submitted to the EPA via CEDRI using the appropriate electronic report template on the CEDRI website ( https://www.epa.gov/electronic-reporting-air-emissions/compliance-and-emissions-data-reporting-interface-cedri ) for this subpart beginning no later than the compliance dates specified in §63.1102(c) through (e) or once the report template has been available on the CEDRI website for 1 year, whichever date is later. The date report templates become available will be listed on the CEDRI website. The report must be submitted by the deadline specified in this subpart, regardless of the method in which the report is submitted. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim, then submit a complete report, including information claimed to be CBI, to the EPA. Periodic Reports must be generated using the appropriate template on the CEDRI website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. EPA/OAQPS/CORE CBI Office, MD C404-02, 4930 Old Page Road, Durham NC 27703 to the attention of the applicable person specified in paragraphs (A) through (C) of this section. The same file with the CBI omitted must be submitted to the EPA via the EPA's CDX as described earlier in this paragraph. All CBI claims must be asserted at the time of submission. Furthermore, under CAA section 114(c), emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available.
(A) Ethylene Production Sector Lead
(B) Cyanide Chemicals Manufacturing Sector Lead
(C) Carbon Black Production Sector Lead
(iii) If you are required to electronically submit a report through CEDRI in the EPA's CDX, you may assert a claim of EPA system outage for failure to timely comply with the reporting requirement. To assert a claim of EPA system outage, the owner or operator must meet the requirements outlined in paragraphs (a)(10)(iii)(A) through (G) of this section.
(A) The owner or operator must have been or will be precluded from accessing CEDRI and submitting a required report within the time prescribed due to an outage of either the EPA's CEDRI or CDX systems.
(B) The outage must have occurred within the period of time beginning five business days prior to the date that the submission is due.
(C) The outage may be planned or unplanned.
(D) The owner or operator must submit notification to the Administrator in writing as soon as possible following the date you first knew, or through due diligence should have known, that the event may cause or has caused a delay in reporting.
(E) The owner or operator must provide to the Administrator a written description identifying:
(1) The date(s) and time(s) when CDX or CEDRI was accessed and the system was unavailable;
(2) A rationale for attributing the delay in reporting beyond the regulatory deadline to EPA system outage;
(3) Measures taken or to be taken to minimize the delay in reporting; and
(4) The date by which you propose to report, or if you have already met the reporting requirement at the time of the notification, the date you reported.
(F) The decision to accept the claim of EPA system outage and allow an extension to the reporting deadline is solely within the discretion of the Administrator.
(G) In any circumstance, the report must be submitted electronically as soon as possible after the outage is resolved.
(iv) If you are required to electronically submit a report through CEDRI in the EPA's CDX, you may assert a claim of force majeure for failure to timely comply with the reporting requirement. To assert a claim of force majeure, the owner or operator must meet the requirements outlined in paragraphs (a)(10)(iv)(A) through (E) of this section.
(A) You may submit a claim if a force majeure event is about to occur, occurs, or has occurred or there are lingering effects from such an event within the period of time beginning five business days prior to the date the submission is due. For the purposes of this paragraph, a force majeure event is defined as an event that will be or has been caused by circumstances beyond the control of the affected facility, its contractors, or any entity controlled by the affected facility that prevents you from complying with the requirement to submit a report electronically within the time period prescribed. Examples of such events are acts of nature (e.g., hurricanes, earthquakes, or floods), acts of war or terrorism, or equipment failure or safety hazard beyond the control of the affected facility (e.g., large scale power outage).
(B) The owner or operator must submit notification to the Administrator in writing as soon as possible following the date you first knew, or through due diligence should have known, that the event may cause or has caused a delay in reporting.
(C) The owner or operator must provide to the Administrator:
(1) A written description of the force majeure event;
(2) A rationale for attributing the delay in reporting beyond the regulatory deadline to the force majeure event;
(3) Measures taken or to be taken to minimize the delay in reporting; and
(4) The date by which you propose to report, or if you have already met the reporting requirement at the time of the notification, the date you reported.
(D) The decision to accept the claim of force majeure and allow an extension to the reporting deadline is solely within the discretion of the Administrator.
(E) In any circumstance, the reporting must occur as soon as possible after the force majeure event occurs.
* * * *
(e)(4)(iii) The periods specified in §63.1109(e)(7). Indicate the date and start time for the period, and the net heating value operating parameter(s) determined following the methods in §63.670(k) through (n) of subpart CC as applicable.
* * * *
(e)(4)(iv)(A) The start and stop time and date of the flaring event.
(B) The length of time that emissions were visible from the flare during the event.
* * * *
(e)(5)(iii) The LEL, vessel pressure, or mass of VOC in the equipment, as applicable, at the start of atmospheric venting. If the 5 psig vessel pressure option in §63.1103(e)(5)(i)(B) was used and active purging was initiated while the LEL was 10 percent or greater, also include the LEL of the vapors at the time active purging was initiated.
* * * *
(e)(8)(iii) For pressure relief devices in organic HAP service subject to §63.1107(h)(3), report each pressure release to the atmosphere, including duration of the pressure release and estimate of the mass quantity of each organic HAP released; the results of any root cause analysis and corrective action analysis completed during the reporting period, including the corrective actions implemented during the reporting period; and, if applicable, the implementation schedule for planned corrective actions to be implemented subsequent to the reporting period.
* * * *
§63.2346 What emission limitations, operating limits, and work practice standards must I meet?
* * * *
(a)(6) Beginning no later than the compliance dates specified in §63.2342(e), tank emissions during storage tank shutdown operations (i.e., emptying and degassing of a storage tank) for each storage tank at an affected source storing organic liquids that meets the tank capacity and liquid vapor pressure criteria for control in items 3 through 6 of Table 2 to this subpart, or items 1 through 3 of Table 2b to this subpart, you must comply with paragraphs (a)(6)(i) through (iii) of this section during tank emptying and degassing until the vapor space concentration in the tank is less than 10 percent of the lower explosive limit (LEL). The owner or operator must determine the LEL using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
* * * *
(e) Operating limits. For each high throughput transfer rack, you must meet each operating limit in Table 3 to this subpart for each control device used to comply with the provisions of this subpart whenever emissions from the loading of organic liquids are routed to the control device. Except as specified in paragraph (k) of this section, for each storage tank and low throughput transfer rack, you must comply with paragraph (l) of this section and the requirements for monitored parameters as specified in subpart SS of this part, for storage vessels and, during the loading of organic liquids, for low throughput transfer racks, respectively. Alternatively, you may comply with the operating limits in Table 3 to this subpart.
§63.2378 How do I demonstrate continuous compliance with the emission limitations, operating limits, and work practice standards?
* * * *
(e) Beginning no later than the compliance dates specified in §63.2342(e), paragraphs (b) through (d) of this section no longer apply. Instead, you must be in compliance with each emission limitation, operating limit, and work practice standard specified in paragraph (a) of this section at all times, except during periods of nonoperation of the affected source (or specific portion thereof) resulting in cessation of the emissions to which this subpart applies and must comply with the requirements specified in paragraphs (e)(1) through (5) of this section, as applicable. Equipment subject to the work practice standards for equipment leak components in Table 4 to this subpart, item 4 are not subject to this paragraph (e).
(1) Except as specified in paragraphs (e)(3) through (5) of this section, the use of a bypass line at any time on a closed vent system to divert a vent stream to the atmosphere or to a control device not meeting the requirements specified in paragraph (a) of this section is an emissions standards deviation.
(2) If you are subject to the bypass monitoring requirements of §63.983(a)(3), then you must continue to comply with the requirements in §63.983(a)(3) and the recordkeeping and reporting requirements in §§63.998(d)(1)(ii) and 63.999(c)(2), in addition to §63.2346(l), the recordkeeping requirements specified in §63.2390(g), and the reporting requirements specified in §63.2386(c)(12).
(3) Periods of planned routine maintenance of a control device used to control storage tank breathing loss emissions, during which the control device does not meet the emission limits in Table 2 or 2b to this subpart, must not exceed 240 hours per year. The level of material in the storage vessel shall not be increased during periods that the closed-vent system or control device is bypassed to perform routine maintenance.
(4) If you elect to route emissions from storage tanks to a fuel gas system or to a process, as allowed by §63.982(d), to comply with the emission limits in Table 2 or 2b to this subpart, the total aggregate amount of time during which the breathing loss emissions bypass the fuel gas system or process during the calendar year without being routed to a control device, for all reasons (except product changeovers of flexible operation units and periods when a storage tank has been emptied and degassed), must not exceed 240 hours. The level of material in the storage vessel shall not be increased during periods that the fuel gas system or process is bypassed to perform routine maintenance.
§63.2382 What notifications must I submit and when and what information should be submitted?
* * * *
(d)(3) Submitting Notification of Compliance Status. Beginning no later than the compliance dates specified in §63.2342(e), you must submit all subsequent Notification of Compliance Status reports to the EPA via CEDRI, which can be accessed through EPA's Central Data Exchange (CDX) (https://cdx.epa.gov/). If you claim some of the information required to be submitted via CEDRI is confidential business information (CBI), then submit a complete report, including information claimed to be CBI, to the EPA. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, U.S. EPA Mailroom (C404-02), Attention: Organic Liquids Distribution Sector Lead, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via EPA's CDX as described earlier in this paragraph. You may assert a claim of EPA system outage or force majeure for failure to timely comply with this reporting requirem
§63.2386 What reports must I submit and when and what information is to be submitted in each?
* * * *
(f) Beginning no later than the compliance dates specified in §63.2342(e), you must submit all Compliance reports to the EPA via CEDRI, which can be accessed through EPA's CDX (https://cdx.epa.gov/). You must use the appropriate electronic report template on the CEDRI website (https://www.epa.gov/electronic-reporting-air-emissions/compliance-and-emissions-data-reporting-interface-cedri) for this subpart. The date report templates become available will be listed on the CEDRI website. Unless the Administrator or delegated state agency or other authority has approved a different schedule for submission of reports under §§63.9(i) and 63.10(a), the report must be submitted by the deadline specified in this subpart, regardless of the method in which the report is submitted. If you claim some of the information required to be submitted via CEDRI is CBI, submit a complete report, including information claimed to be CBI, to the EPA. The report must be generated using the appropriate form on the CEDRI website or an alternate electronic file consistent with the extensible markup language (XML) schema listed on the CEDRI website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, U.S. EPA Mailroom (C404-02), Attention: Organic Liquids Distribution Sector Lead, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via EPA's CDX as described earlier in this paragraph. You may assert a claim of EPA system outage or force majeure for failure to timely comply with this reporting requirement provided you meet the requirements outlined in paragraph (i) or (j) of this section, as applicable.
(g) Beginning no later than the compliance dates specified in §63.2342(e), you must start submitting performance test reports in accordance with this paragraph. Unless otherwise specified in this subpart, within 60 days after the date of completing each performance test required by this subpart, you must submit the results of the performance test following the procedures specified in paragraphs (g)(1) through (3) of this section.
(1) Data collected using test methods supported by the EPA's Electronic Reporting Tool (ERT) as listed on the EPA's ERT website (https://www.epa.gov/electronic-reporting-air-emissions/electronic-reporting-tool-ert) at the time of the test. Submit the results of the performance test to the EPA via CEDRI, which can be accessed through the EPA's CDX (https://cdx.epa.gov/). The data must be submitted in a file format generated through the use of the EPA's ERT. Alternatively, you may submit an electronic file consistent with the XML schema listed on the EPA's ERT website.
(2) Data collected using test methods that are not supported by the EPA's ERT as listed on the EPA's ERT website at the time of the test. The results of the performance test must be included as an attachment in the ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the ERT generated package or alternative file to the EPA via CEDRI.
(3) CBI. If you claim some of the information submitted under paragraph (g)(1) or (2) of this section is CBI, then you must submit a complete file, including information claimed to be CBI, to the EPA. The file must be generated through the use of the EPA's ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. EPA/OAQPS/CORE CBI Office, Attention: Group Leader, Measurement Policy Group, MD C404-02, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via EPA's CDX as described in paragraphs (g)(1) and (2) of this section.
(h) Beginning no later than the compliance dates specified in §63.2342(e), you must start submitting performance evaluation reports in accordance with this paragraph. Unless otherwise specified in this subpart, within 60 days after the date of completing each CEMS performance evaluation (as defined in §63.2) , you must submit the results of the performance evaluation following the procedures specified in paragraphs (h)(1) through (3) of this section.
(1) Performance evaluations of CEMS measuring relative accuracy test audit (RATA) pollutants that are supported by the EPA's ERT as listed on the EPA's ERT website at the time of the evaluation. Submit the results of the performance evaluation to the EPA via CEDRI, which can be accessed through the EPA's CDX. The data must be submitted in a file format generated through the use of the EPA's ERT. Alternatively, you may submit an electronic file consistent with the XML schema listed on the EPA's ERT website.
(2) Performance evaluations of CEMS measuring RATA pollutants that are not supported by the EPA's ERT as listed on the EPA's ERT website at the time of the evaluation. The results of the performance evaluation must be included as an attachment in the ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the ERT generated package or alternative file to the EPA via CEDRI.
(3) CBI. If you claim some of the information submitted under paragraph (h)(1) or (2) of this section is CBI, then you must submit a complete file, including information claimed to be CBI, to the EPA. The file must be generated through the use of the EPA's ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. EPA/OAQPS/CORE CBI Office, Attention: Group Leader, Measurement Policy Group, MD C404-02, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via the EPA's CDX as described in paragraphs (h)(1) and (2) of this section.
Table 12 to Subpart EEEE of Part 63—Applicability of General Provisions to Subpart EEEE
* * * *
| §63.9(k) | Electronic reporting procedures | Procedure to report electronically for notification in §63.9(j) | Yes, only as specified in §63.9(j). |
§63.2450 What are my general requirements for complying with this subpart?
* * * *
(e)(1) Except when complying with §63.2485, if you reduce organic HAP emissions by venting emissions through a closed-vent system to any combination of control devices (except a flare) or recovery devices, you must meet the requirements of paragraph (e)(4) of this section, and the requirements of §63.982(c) and the requirements referenced therein.
* * * *
(e)(5)(iv) Instead of complying with paragraph (o)(2)(iii) of §63.670 of subpart CC, if required to develop a flare management plan and submit it to the Administrator, then you must also submit all versions of the plan in portable document format (PDF) to the EPA via the Compliance and Emissions Data Reporting Interface (CEDRI), which can be accessed through the EPA's Central Data Exchange (CDX) (https://cdx.epa.gov/). The EPA will make all the information submitted through CEDRI available to the public without further notice to you. Do not use CEDRI to submit information you claim as confidential business information (CBI). Anything submitted using CEDRI cannot later be claimed to be CBI. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim, submit a version with the CBI omitted via CEDRI. A complete plan, including information claimed to be CBI and clearly marked as CBI, must be mailed to the following address: U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, CORE CBI Office, U.S. EPA Mailroom (C404-02), Attention: Miscellaneous Organic Chemical Manufacturing Sector Lead, 4930 Old Page Rd., Durham, NC 27703. All CBI claims must be asserted at the time of submission. Furthermore, under CAA section 114(c) emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available.
* * * *
(e)(5)(viii)(B) You must substitute "800" for each occurrence of "270" in paragraph (e) of §63.670 of subpart CC;
* * * *
(e)(6)(i) If you are subject to the bypass monitoring requirements of §63.148(f) of subpart G, then you must continue to comply with the requirements in §63.148(f) of subpart G and the recordkeeping and reporting requirements in §§63.148(j)(2) and (3) of subpart G, and (h)(3) of subpart G, in addition to the applicable requirements specified in §63.2485(q), the recordkeeping requirements specified in §63.2525(n), and the reporting requirements specified in §63.2520(e)(12).
* * * *
(e)(7) Beginning no later than the compliance dates specified in §63.2445(g), if you reduce organic HAP emissions by venting emissions through a closed-vent system to an adsorber(s) that cannot be regenerated or a regenerative adsorber(s) that is regenerated offsite, then you must comply with paragraphs (e)(4) and (6) of this section and the requirements in §63.983, and you must install a system of two or more adsorber units in series and comply with the requirements specified in paragraphs (e)(7)(i) through (iii) of this section.
* * * *
(v)(1)(i) The vapor in the equipment served by the maintenance vent has a lower explosive limit (LEL) of less than 10 percent and has an outlet concentration less than or equal to 20 ppmv hydrogen halide and halogen HAP.
* * * *
(v)(1)(ii) If there is no ability to measure the LEL of the vapor in the equipment based on the design of the equipment, the pressure in the equipment served by the maintenance vent is reduced to 5 pounds per square inch gauge (psig) or less. Upon opening the maintenance vent, active purging of the equipment cannot be used until the LEL of the vapors in the maintenance vent (or inside the equipment if the maintenance is a hatch or similar type of opening) is less than 10 percent.
* * * *
(v)(2) Except for maintenance vents complying with the alternative in paragraph (v)(1)(iii) of this section, you must determine the LEL or, if applicable, equipment pressure using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
§63.2460 What requirements must I meet for batch process vents?
* * * *
(c)(9) Requirements for a biofilter. If you use a biofilter to meet either the95-percent reduction requirement or outlet concentration requirement specified in Table 2 to this subpart, you must meet the requirements specified in paragraphs (c)(9)(i) through (vi) of this section.
§63.2470 What requirements must I meet for storage tanks?
* * * *
(f) Storage tank degassing. Beginning no later than the compliance dates specified in §63.2445(g), for each storage tank subject to item 1 of Table 4 to this subpart, you must comply with paragraphs (f)(1) through (3) of this section during storage tank shutdown operations (i.e., emptying and degassing of a storage tank) until the vapor space concentration in the storage tank is less than 10 percent of the LEL. You must determine the LEL using process instrumentation or portable measurement devices and follow procedures for calibration and maintenance according to manufacturer's specifications.
§63.2480 What requirements must I meet for equipment leaks?
(a) You must meet each requirement in Table 6 to this subpart that applies to your equipment leaks, except as specified in paragraphs (b) through (f) of this section. For each light liquid pump, valve, and connector in ethylene oxide service as defined in §63.2550(i), you must also meet the applicable requirements specified in §§63.2492 and 63.2493(d) and (e).
* * * *
(e)(2)(i) If the pressure relief device does not consist of or include a rupture disk, conduct instrument monitoring, as specified in §63.1023(b) of subpart UU, §63.180(c) of subpart H, or §65.104(b) of this chapter, no later than 5 calendar days after the pressure relief device returns to organic HAP gas or vapor service following a pressure release to verify that the pressure relief device is operating with an instrument reading of less than 500 ppm.
* * * *
(e)(2)(iii) If the pressure relief device consists only of a rupture disk, install a replacement disk as soon as practicable after a pressure release, but no later than 5 calendar days after the pressure release. You must not initiate startup of the equipment served by the rupture disk until the rupture disc is replaced. You must conduct instrument monitoring, as specified in §63.1023(b) of subpart UU, §63.180(c) of subpart H, or §65.104(b) of this chapter, no later than 5 calendar days after the pressure relief device returns to organic HAP gas or vapor service following a pressure release to verify that the pressure relief device is operating with an instrument reading of less than 500 ppm.
* * * *
(e)(3)(iv) You must determine the total number of release events that occurred during the calendar year for each affected pressure relief device separately. You must also determine the total number of release events for each pressure relief device for which the root cause analysis concluded that the root cause was a force majeure event, as defined in §63.2550.
* * * *
(e)(3)(5)(B) A second release event not including force majeure events from a single pressure relief device in a 3 calendar year period for the same root cause for the same equipment.
(e)(3)(5)(C) A third release event not including force majeure events from a single pressure relief device in a 3 calendar year period for any reason.
* * * *
(e)(6)(ii) You may conduct a single root cause analysis and corrective action analysis for a single emergency event that causes two or more pressure relief devices to release, regardless of the equipment served, if the root cause is reasonably expected to be a force majeure event, as defined in §63.2550.
* * * *
(f)(18)(iii) In §63.181(b)(2)(i), replace the reference to §63.165(a) with §63.2480(e)(1).
* * * *
(f)(18)(vi) The information required to be reported under §63.182(d)(2)(xiv) is now required to be reported under §63.2520(e)(15)(i) through (iii).
* * * *
(f)(18)(x) The reference to §63.1030(c) in §63.1022(a)(1)(v) no longer applies. Instead comply with the §63.2480(e)(1) and (2).
* * * *
(f)(18)(xiii) The information required to be reported under §63.1039(b)(4) is now required to be reported under §63.2520(e)(15)(i) and (ii).
§63.2490 What requirements must I meet for heat exchange systems?
* * * *
(a) You must comply with each requirement in Table 10 to this subpart that applies to your heat exchange systems, except as specified in paragraphs (b) through (d) of this section.
* * * *
(d) Unless one or more of the conditions specified in §63.104(a)(1), (2), (5), and (6) are met, beginning no later than the compliance dates specified in §63.2445(g), the requirements of §63.104 as specified in Table 10 to this subpart and paragraphs (b) and (c) of this section no longer apply. Instead, you must monitor the cooling water for the presence of total strippable hydrocarbons that indicate a leak according to paragraph (d)(1) of this section, and if you detect a leak, then you must repair it according to paragraphs (d)(2) and (3) of this section, unless repair is delayed according to paragraph (d)(4) of this section. At any time before the compliance dates specified in §63.2445(g), you may choose to comply with the requirements in this paragraph (d) in lieu of the requirements of §63.104 as specified in Table 10 to this subpart and paragraphs (b) and (c) of this section. The requirements in this paragraph (d) do not apply to heat exchange systems that have a maximum cooling water flow rate of 10 gallons per minute or less.
* * * *
(d)(4)(iii) The delay of repair action level is a total strippable hydrocarbon concentration (as methane) in the stripping gas of 62 ppmv or, for heat exchange systems with a recirculation rate of 10,000 gallons per minute or less, the delay of repair action level is a total hydrocarbon mass emissions rate (as methane) or 1.8 kg/hr. The delay of repair action level is assessed as described in paragraph (d)(4)(iii)(A) or (B) of this section, as applicable.
§63.2492 How do I determine whether my process vent, storage tank, or equipment is in ethylene oxide service?
* * * *
(b) For storage tanks, you must measure the concentration of ethylene oxide of the fluid stored in the storage tanks using Method 624.1 of 40 CFR part 136, appendix A, or preparation by Method 5031 and analysis by Method 8260D (both incorporated by reference, see §63.14) in the SW-846 Compendium. In lieu of preparation by SW-846 Method 5031, you may use SW-846 Method 5030B (incorporated by reference, see §63.14), as long as: You do not use a preservative in the collected sample; you store the sample with minimal headspace as cold as possible and at least below 4 degrees C; and you analyze the sample as soon as possible, but in no case longer than 7 days from the time the sample was collected. If you are collecting a sample from a pressure vessel, you must maintain the sample under pressure both during and following sampling.
§63.2493 What requirements must I meet for process vents, storage tanks, or equipment that are in ethylene oxide service?
* * * *
(a)(2)(vi) If you vent emissions through a closed-vent system to a scrubber, then you must establish operating parameter limits by monitoring the operating parameters specified in paragraphs (a)(2)(vi)(A) through (C) of this section during the performance test.
* * * *
(a)(2)(vi)(C) Temperature of the water entering the scrubber column. The temperature may be measured at any point after the heat exchanger and prior to entering the top of the scrubber column. Determine the average inlet water temperature as the average of the test run averages.
* * * *
(a)(2)(viii) If you vent emissions through a closed-vent system to a control device other than a flare, scrubber, or thermal oxidizer, then you must notify the Administrator of the operating parameters that you plan to monitor during the performance test prior to establishing operating parameter limits for the control device.
* * * *
(b)(2) Continuously monitor the ethylene oxide concentration at the exit of the control device using an FTIR CEMS meeting the requirements of Performance Specification 15 of 40 CFR part 60, appendix B, and §63.2450(j). If you use an FTIR CEMS, you do not need to conduct the performance testing required in paragraph (b)(3) of this section or the operating parameter monitoring required in paragraphs (b)(4) through (6) of this section.
* * * *
(b)(4) If you vent emissions through a closed-vent system to a scrubber, then you must comply with §63.2450(e)(4) and (6) and the requirements in §63.983, and you must meet the operating parameter limits specified in paragraphs (b)(4)(i) through (v) of this section.
* * * *
(b)(4)(iv) Maximum temperature of the water entering the scrubber column, equal to the average temperature measured during the most recent performance test. Compliance with the inlet water temperature operating limit must be determined continuously on a 1-hour block basis. Use a temperature sensor with a minimum accuracy of ±1 percent over the normal range of the temperature measured, expressed in degrees Celsius, or 2.8 degrees Celsius, whichever is greater.
* * * *
(b)(6) If you vent emissions through a closed-vent system to a control device other than a flare, scrubber, or thermal oxidizer, then you must comply with §63.2450(e)(4) and (6) and the requirements in §63.983, and you must monitor the operating parameters identified in paragraph (a)(2)(viii) of this section and meet the established operating parameter limits to ensure continuous compliance. The frequency of monitoring and averaging time will be determined based upon the information provided to the Administrator.
* * * *
(d)(1)(iii) When a leak is detected, it must be repaired as soon as practicable, but not later than 15 calendar days after it is detected.
* * * *
(d)(2)(iii) When a leak is detected, it must be repaired as soon as practicable, but not later than 15 calendar days after it is detected.
* * * *
(d)(3) For each light liquid pump or connector in ethylene oxide service that is added to an affected source, and for each light liquid pump or connector in ethylene oxide service that replaces a light liquid pump or connector in ethylene oxide service, you must initially monitor for leaks within 5 days after initial startup of the equipment.
* * * *
(d)(4)(v) Replace all references to §63.2445(g) with §63.2445(h).
* * * *
(e) Non-applicable referenced provisions. The referenced provisions specified in paragraphs (e)(1) through (15) of this section do not apply when demonstrating compliance with this section.
* * * *
§63.2515 What notifications must I submit and when?
* * * *
(d) Supplement to Notification of Compliance Status. You must also submit supplements to the Notification of Compliance Status as specified in §63.2520(d)(3) through (5).
§63.2520 What reports must I submit and when?
* * * *
(d) Notification of compliance status report. You must submit a notification of compliance status report according to the schedule in paragraph (d)(1) of this section, and the notification of compliance status report must contain the information specified in paragraphs (d)(2) through (5) of this section.
* * * *
(e) Compliance report. The compliance report must contain the information specified in paragraphs (e)(1) through (17) of this section. On and after August 12, 2023 or once the reporting template for this subpart has been available on the CEDRI website for 1 year, whichever date is later, you must submit all subsequent reports to the EPA via the CEDRI, which can be accessed through the EPA's CDX (https://cdx.epa.gov/). The EPA will make all the information submitted through CEDRI available to the public without further notice to you. Do not use CEDRI to submit information you claim as CBI. Anything submitted using CEDRI cannot later be claimed to be CBI. You must use the appropriate electronic report template on the CEDRI website (https://www.epa.gov/electronic-reporting-air-emissions/compliance-and-emissions-data-reporting-interface-cedri) for this subpart. The date report templates become available will be listed on the CEDRI website. Unless the Administrator or delegated state agency or other authority has approved a different schedule for submission of reports under §§63.9(i) and 63.10(a) of subpart A, the report must be submitted by the deadline specified in this subpart, regardless of the method in which the report is submitted. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim, submit a complete report, including information claimed to be CBI, to the EPA. The report must be generated using the appropriate form on the CEDRI website or an alternate electronic file consistent with the extensible markup language (XML) schema listed on the CEDRI website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, CORE CBI Office, U.S. EPA Mailroom (C404-02), Attention: Miscellaneous Organic Chemical Manufacturing Sector Lead, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via the EPA's CDX as described in this paragraph (e). All CBI claims must be asserted at the time of submission. Furthermore under CAA section 114(c) emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available. You may assert a claim of EPA system outage or force majeure for failure to timely comply with the reporting requirement in this paragraph (e) provided you meet the requirements outlined in paragraph (i) or (j) of this section, as applicable.
* * * *
(e)(2) Statement by a responsible official with that official's name, title, and signature, certifying the accuracy of the content of the report. If your report is submitted via CEDRI, the certifier's electronic signature during the submission process replaces the requirement in this paragrpah (e)(2).
* * * *
(e)(14)(iii) The lower explosive limit in percent, vessel pressure in psig, or mass in pounds of VOC in the equipment, as applicable, at the start of atmospheric venting. If the 5 psig vessel pressure option in §63.2450(v)(1)(ii) was used and active purging was initiated while the lower explosive limit was 10 percent or greater, also include the lower explosive limit of the vapors at the time active purging was initiated.
* * * *
(e)(16) For each heat exchange system subject to §63.2490(d), beginning no later than the compliance dates specified in §63.2445(g), the reporting requirements of §63.104(f)(2) no longer apply; instead, the compliance report must include the information specified in paragraphs (e)(16)(i) through (v) of this section.
(i) The number of heat exchange systems at the plant site subject to the monitoring requirements in §63.2490(d) during the reporting period;
(ii) The number of heat exchange systems subject to the monitoring requirements in §63.2490(d) at the plant site found to be leaking during the reporting period;
(iii) For each monitoring location where the total strippable hydrocarbon concentration or total hydrocarbon mass emissions rate was determined to be equal to or greater than the applicable leak definitions specified in §63.2490(d)(1)(v) during the reporting period, identification of the monitoring location (e.g., unique monitoring location or heat exchange system ID number), the measured total strippable hydrocarbon concentration or total hydrocarbon mass emissions rate, the date the leak was first identified, and, if applicable, the date the source of the leak was identified;
(iv) For leaks that were repaired during the reporting period (including delayed repairs), identification of the monitoring location associated with the repaired leak, the total strippable hydrocarbon concentration or total hydrocarbon mass emissions rate measured during re-monitoring to verify repair, and the re-monitoring date (i.e., the effective date of repair); and
(v) For each delayed repair, identification of the monitoring location associated with the leak for which repair is delayed, the date when the delay of repair began, the date the repair is expected to be completed (if the leak is not repaired during the reporting period), the total strippable hydrocarbon concentration or total hydrocarbon mass emissions rate and date of each monitoring event conducted on the delayed repair during the reporting period, and an estimate in pounds of the potential total hydrocarbon emissions over the reporting period associated with the delayed repair.
* * * *
(f) Performance test reports. Beginning no later than October 13, 2020, you must submit performance test reports in accordance with this paragraph (f). Unless otherwise specified in this subpart, within 60 days after the date of completing each performance test required by this subpart, you must submit the results of the performance test following the procedures specified in paragraphs (f)(1) through (3) of this section.
(1) Data collected using test methods supported by the EPA's Electronic Reporting Tool (ERT) as listed on the EPA's ERT website (https://www.epa.gov/electronic-reporting-air-emissions/electronic-reporting-tool-ert) at the time of the test. Submit the results of the performance test to the EPA via CEDRI, which can be accessed through the EPA's CDX (https://cdx.epa.gov/). The data must be submitted in a file format generated through the use of the EPA's ERT. Alternatively, you may submit an electronic file consistent with the extensible markup language (XML) schema listed on the EPA's ERT website.
(2) Data collected using test methods that are not supported by the EPA's ERT as listed on the EPA's ERT website at the time of the test. The results of the performance test must be included as an attachment in the ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the ERT generated package or alternative file to the EPA via CEDRI.
(3) Confidential business information (CBI). The EPA will make all the information submitted through CEDRI available to the public without further notice to you. Do not use CEDRI to submit information you claim as CBI. Anything submitted using CEDRI cannot later be claimed to be CBI. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim, you must submit a complete file, including information claimed to be CBI, to the EPA. The file must be generated through the use of the EPA's ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, CORE CBI Office, U.S. EPA Mailroom (C404-02), Attention: Group Leader, Measurement Policy Group, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via the EPA's CDX as described in paragraph (f)(1) and (2) of this section. All CBI claims must be asserted at the time of submission. Furthermore, under CAA section 114(c) emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available.
(g) CEMS relative accuracy test audit (RATA) Performance evaluation reports. Beginning no later than October 13, 2020, you must start submitting CEMS RATA performance evaluation reports in accordance with this paragraph (g). Unless otherwise specified in this subpart, within 60 days after the date of completing each continuous monitoring system performance evaluation (as defined in §63.2), you must submit the results of the performance evaluation following the procedures specified in paragraphs (g)(1) through (3) of this section.
(1) Performance evaluations of CMS measuring RATA pollutants that are supported by the EPA's ERT as listed on the EPA's ERT website at the time of the evaluation. Submit the results of the performance evaluation to the EPA via CEDRI, which can be accessed through the EPA's CDX. The data must be submitted in a file format generated through the use of the EPA's ERT. Alternatively, you may submit an electronic file consistent with the XML schema listed on the EPA's ERT website.
(2) Performance evaluations of CMS measuring RATA pollutants that are not supported by the EPA's ERT as listed on the EPA's ERT website at the time of the evaluation. The results of the performance evaluation must be included as an attachment in the ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the ERT generated package or alternative file to the EPA via CEDRI.
(3) Confidential business information (CBI). The EPA will make all the information submitted through CEDRI available to the public without further notice to you. Do not use CEDRI to submit information you claim as CBI. Anything submitted using CEDRI cannot later be claimed to be CBI. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim, you must submit a complete file, including information claimed to be CBI, to the EPA. The file must be generated through the use of the EPA's ERT or an alternate electronic file consistent with the XML schema listed on the EPA's ERT website. Submit the file on a compact disc, flash drive, or other commonly used electronic storage medium and clearly mark the medium as CBI. Mail the electronic medium to U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Sector Policies and Programs Division, CORE CBI Office, U.S. EPA Mailroom (C404-02), Attention: Group Leader, Measurement Policy Group, 4930 Old Page Rd., Durham, NC 27703. The same file with the CBI omitted must be submitted to the EPA via the EPA's CDX as described in paragraphs (g)(1) and (2) of this section. All CBI claims must be asserted at the time of submission. Furthermore, under CAA section 114(c) emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available.
§63.2525 What records must I keep?
* * * *
(o) For each nonregenerative adsorber and regenerative adsorber that is regenerated offsite subject to the requirements in §63.2450(e)(7), you must keep the applicable records specified in paragraphs (o)(1) through (4) of this section.
(1) Outlet HAP or TOC concentration for each adsorber bed measured during each performance test conducted.
(2) Daily outlet HAP or TOC concentration.
(3) Date and time you last replaced the adsorbent.
(4) If you conduct monitoring less frequently than daily as specified in §63.2450(e)(7)(iii)(B), you must record the average life of the bed.
* * * *
(p)(2) If complying with the requirements of §63.2450(v)(1)(i) and the lower explosive limit at the time of the vessel opening exceeds 10 percent, identification of the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, and the lower explosive limit at the time of the vessel opening.
(p)(3) If complying with the requirements of §63.2450(v)(1)(ii) and either the vessel pressure at the time of the vessel opening exceeds 5 psig or the lower explosive limit at the time of the active purging was initiated exceeds 10 percent, identification of the maintenance vent, the process units or equipment associated with the maintenance vent, the date of maintenance vent opening, the pressure of the vessel or equipment at the time of discharge to the atmosphere and, if applicable, the lower explosive limit of the vapors in the equipment when active purging was initiated.
* * * *
(p)(5) If complying with the requirements of §63.2450(v)(1)(iv), identification of the maintenance vent, the process units or equipment associated with the maintenance vent, records documenting actions taken to comply with other applicable alternatives and why utilization of this alternative was required, the date of maintenance vent opening, the equipment pressure and lower explosive limit of the vapors in the equipment at the time of discharge, an indication of whether active purging was performed and the pressure of the equipment during the installation or removal of the blind if active purging was used, the duration the maintenance vent was open during the blind installation or removal process, and records used to estimate the total quantity of VOC in the equipment at the time the maintenance vent was opened to the atmosphere for each applicable maintenance vent opening.
* * * *
(q)(2) Records of the number of releases during each calendar year and the number of those releases for which the root cause was determined to be a force majeure event. Keep these records for the current calendar year and the past 5 calendar years.
* * * *
(r)(1) Monitoring data required by §63.2490(d) that indicate a leak, the date the leak was detected, or, if applicable, the basis for determining there is no leak.
* * * *
(r)(4)(iv) An estimate of the potential total hydrocarbon emissions from the leaking heat exchange system or heat exchanger for each required delay of repair monitoring interval following the procedures in paragraphs (r)(4)(iv)(A) through (C) of this section.
* * * *
(r)(4)(iv)(B) For delay of repair monitoring intervals prior to repair of the leak, calculate the potential total hydrocarbon emissions for the leaking heat exchange system or heat exchanger for the monitoring interval by multiplying the mass emissions rate, determined in §63.2490(d)(1)(iii)(B) or paragraph (r)(4)(iv)(A) of this section, by the duration of the delay of repair monitoring interval. The duration of the delay of repair monitoring interval is the time period starting at midnight on the day of the previous monitoring event or at midnight on the day the repair would have had to be completed if the repair had not been delayed, whichever is later, and ending at midnight of the day the of the current monitoring event.
(C) For delay of repair monitoring intervals ending with a repaired leak, calculate the potential total hydrocarbon emissions for the leaking heat exchange system or heat exchanger for the final delay of repair monitoring interval by multiplying the duration of the final delay of repair monitoring interval by the mass emissions rate determined for the last monitoring event prior to the re-monitoring event used to verify the leak was repaired. The duration of the final delay of repair monitoring interval is the time period starting at midnight of the day of the last monitoring event prior to re-monitoring to verify the leak was repaired and ending at the time of the re-monitoring event that verified that the leak was repaired.
§63.2550 What definitions apply to this subpart?
* * * *
In ethylene oxide service means the following:
(1) For equipment leaks, any equipment that contains or contacts a fluid (liquid or gas) that is at least 0.1 percent by weight of ethylene oxide. If information exists that suggests ethylene oxide could be present in equipment, the equipment is considered to be "in ethylene oxide service" unless sampling and analysis is performed as specified in §63.2492 to demonstrate that the equipment does not meet the definition of being "in ethylene oxide service". Examples of information that could suggest ethylene oxide could be present in equipment, include calculations based on safety data sheets, material balances, process stoichiometry, or previous test results provided the results are still relevant to the current operating conditions.
(2) For process vents, each batch and continuous process vent in a process that, when uncontrolled, contains a concentration of greater than or equal to 1 ppmv undiluted ethylene oxide, and when combined, the sum of all these process vents would emit uncontrolled ethylene oxide emissions greater than or equal to 5 lb/yr (2.27 kg/yr). If information exists that suggests ethylene oxide could be present in a batch or continuous process vent, then the batch or continuous process vent is considered to be "in ethylene oxide service" unless an analysis is performed as specified in §63.2492 to demonstrate that the batch or continuous process vent does not meet the definition of being "in ethylene oxide service". Examples of information that could suggest ethylene oxide could be present in a batch or continuous process vent, include calculations based on safety data sheets, material balances, process stoichiometry, or previous test results provided the results are still relevant to the current operating conditions.
(3) For storage tanks, storage tanks of any capacity and vapor pressure storing a liquid that is at least 0.1 percent by weight of ethylene oxide. If knowledge exists that suggests ethylene oxide could be present in a storage tank, then the storage tank is considered to be "in ethylene oxide service" unless sampling and analysis is performed as specified in §63.2492 to demonstrate that the storage tank does not meet the definition of being "in ethylene oxide service". The exemptions for "vessels storing organic liquids that contain HAP only as impurities" and "pressure vessels designed to operate in excess of 204.9 kilopascals and without emissions to the atmosphere" listed in the definition of "storage tank" in this section do not apply for storage tanks that may be in ethylene oxide service. Examples of information that could suggest ethylene oxide could be present in a storage tank, include calculations based on safety data sheets, material balances, process stoichiometry, or previous test results provided the results are still relevant to the current operating conditions.
Table 10 to Subpart FFFF of Part 63—Work Practice Standards for Heat Exchange Systems
| For each . . . | You must . . . |
|---|---|
| Heat exchange system, as defined in §63.101 | a. Comply with the requirements of §63.104 and the requirements referenced therein, except as specified in §63.2490(b) and (c); or |
| b. Comply with the requirements in §63.2490(d). |
Table 12 to Subpart FFFF of Part 63—Applicability of General Provisions to Subpart FFFF
* * * *
| §63.9(k) | Electronic reporting procedures | Yes, as specified in §63.9(j). |
The Environmental Protection Agency (EPA or the Agency) is requesting comments and information to assist in the potential development of regulations for the manufacture (including importing), processing (including recycling), and distribution in commerce of lead for wheel-balancing weights (“lead wheel weights”) under the Toxic Substances Control Act (TSCA). To inform this consideration, EPA is requesting comment and information from all stakeholders on the use and exposure to lead from the manufacture (including importing), processing (including recycling), distribution in commerce, use, and disposal of lead wheel weights, as well as information on their substitutes, to help determine if there is unreasonable risk to human health and the environment associated with this use. This action is relevant to a petition for a writ of mandamus filed in August 2023, by the Ecology Center, Center for Environmental Health, United Parents Against Lead & Other Environmental Hazards, and Sierra Club in the United States Court of Appeals for the Ninth Circuit requesting the court to direct EPA to conduct a rulemaking regulating lead wheel weights under TSCA.
DATES: Comments must be received on or before May 3, 2024, published in the Federal Register April 3, 2024, page 22972.
View proposed rule.
The U.S. Environmental Protection Agency (EPA or the Agency) is finalizing amendments to the National Emission Standards for Hazardous Air Pollutants (NESHAP) for Integrated Iron and Steel Manufacturing Facilities to regulate hazardous air pollutant (HAP) emissions. The amendments include: HAP from unmeasured fugitive and intermittent particulate (UFIP) sources previously not regulated by the NESHAP; previously unregulated HAP for sinter plants:; previously unregulated pollutants for blast furnace (BF) stoves and basic oxygen process furnaces (BOPFs) primary control devices; and previously unregulated pollutants for BF primary control devices. We are also finalizing an update to the technology review for this source category.
DATES: This final rule is effective June 3, 2024, published in the Federal Register April 3, 2024, page 23294 .
View final rule.
| §63.14 Incorporations by reference. | ||
| (i)(88), (i)(110), (o) | Revised | View text |
| (o)(3) | Added | View text |
| §63.7782 What parts of my plant does this subpart cover? | ||
| (c)-(e) | Revised | View text |
| §63.7783 When do I have to comply with this subpart? | ||
| (a) introductory text | Revised | View text |
| (g) | Added | View text |
| §63.7791 How do I comply with the requirements for the control of mercury from BOPF Groups? | ||
| Section heading | Revised | View text |
| §63.7792 What fenceline monitoring requirements must I meet? | ||
| Entire section | Added | View text |
| §63.7793 What work practice standards must I meet? | ||
| Entire section | Added | View text |
| §63.7800 What are my operation and maintenance requirements? | ||
| (b) introductory text | Revised | View text |
| (b)(8)-(9) | Added | View text |
| §63.7820 By what date must I conduct performance tests or other initial compliance demonstrations? | ||
| (e) | Revised | View text |
| §63.7821 When must I conduct subsequent performance tests? | ||
| Entire section | Revised | View text |
| §63.7823 What test methods and other procedures must I use to demonstrate initial compliance with the opacity limits? | ||
| (a) | Revised | View text |
| (c)(3), (d)(6), (f)-(h) | Added | View text |
| §63.7825 What test methods and other procedures must I use to demonstrate initial compliance with the emission limits for hazardous air pollutants? | ||
| Section heading | Revised | View text |
| (a) introductory text; (b)(1)(v), (b)(2), (c) | Revised | View text |
| (g)(-(k) | Added | View text |
| §63.7830 What are my monitoring requirements? | ||
| (e)(2) | Revised | View text |
| §63.7833 How do I demonstrate continuous compliance with the emission limitations that apply to me? | ||
| (j) | Added | View text |
| §63.7840 What notifications must I submit and when? | ||
| (g)(3), (h)(3) | Removed | View text |
| (i) | Added | View text |
| §63.7841 What reports must I submit and when? | ||
| (b)(14), (h) | Added | View text |
| (d) | Revised | View text |
| §63.7842 What records must I keep? | ||
| (d) | Revised | View text |
| (f), (g) | Added | View text |
| §63.7852 What definitions apply to this subpart? | ||
| Definitions for “Iron beaching operation”, Large blast furnace”, “Planned bleeder valve opening”, “Slip”, “Small blast furnace”, “Total hydrocarbons (THC)”, and “Unplanned bleeder valve opening” | Added | View text |
| Table 1 to Subpart FFFFF of Part 63 - Emission, Opacity, and Work Practice Limits | ||
| Entire table | Revised | View text |
| Table 2 to Subpart FFFFF of Part 63 - Initial Compliance With Emission and Opacity Limits | ||
| Entire table | Revised | View text |
| Table 3 to Subpart FFFFF of Part 63 - Continuous Compliance With Emission and Opacity Limits | ||
| Entire table | Revised | View text |
| Table 4 to Subpart FFFFF of Part 63 - Applicability of General Provisions to Subpart FFFFF | ||
| Entire table | Revised | View text |
| Table 5 to Subpart FFFFF of Part 63 - Toxic Equivalency Factors | ||
| Entire table | Added | View text |
| Table 6 to Subpart FFFFF of Part 63 - List of Polycyclic Aromatic Hydrocarbons | ||
| Entire table | Added | View text |
New Text
§63.14 Incorporations by reference.
* * * *
(i)(88) ASTM D6348-12 (Reapproved 2020), Determination of Gaseous Compounds by Extractive Direct Interface Fourier Transform (FTIR) Spectroscopy, including Annexes A1 through A8, Approved December 1; 2020, IBR approved for §§63.365(b); 63.7825(g) and (h) .
* * * * *
(i)(110) ASTM D7520-16, Standard Test Method for Determining the Opacity of a Plume in the Outdoor Ambient Atmosphere, approved April 1, 2016; IBR approved for §§63.1625(b); table 3 to subpart LLLLL; 63.7823(c) through (f), 63.7833(g); 63.11423(c).
* * * * *
(o) U.S. Environmental Protection Agency, 1200 Pennsylvania Avenue NW, Washington, DC 20460; phone: (202) 272-0167; website: www.epa.gov/aboutepa/forms/contact-epa .
§63.7782 What parts of my plant does this subpart cover?
* * * *
(c) This subpart covers emissions from the sinter plant windbox exhaust, discharge end, and sinter cooler; the blast furnace casthouse; the blast furnace stove; and the BOPF shop including each individual BOPF and shop ancillary operations (hot metal transfer, hot metal desulfurization, slag skimming, and ladle metallurgy). This subpart also covers fugitive and intermittent particulate emissions from blast furnace unplanned bleeder valve openings, blast furnace planned bleeder valve openings, blast furnace and BOPF slag processing, handling, and storage, blast furnace bell leaks, beaching of iron from blast furnaces, blast furnace casthouse fugitives, and BOPF shop fugitives.
(d) A sinter plant, blast furnace, blast furnace stove, or BOPF shop at your integrated iron and steel manufacturing facility is existing if you commenced construction or reconstruction of the affected source before July 13, 2001.
(e) A sinter plant, blast furnace, blast furnace stove, or BOPF shop at your integrated iron and steel manufacturing facility is new if you commence construction or reconstruction of the affected source on or after July 13, 2001. An affected source is reconstructed if it meets the definition of reconstruction in §63.2.
§63.7783 When do I have to comply with this subpart?
(a) If you have an existing affected source, you must comply with each emission limitation, standard, and operation and maintenance requirement in this subpart that applies to you by the dates specified in paragraphs (a)(1) and (2) of this section. This paragraph does not apply to the emission limitations for BOPF group: mercury (Hg); sinter plant windbox: Hg, hydrochloric acid (HCl), carbonyl sulfide (COS); Blast Furnace casthouse: HCl, total hydrocarbon (THC); Blast Furnace stove: HCl and total hydrocarbon (THC); primary emission control system for a BOPF: 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) toxic equivalent (TEQ), HCl, THC; fugitive and intermittent particulate sources.
* * * *
§63.7791 How do I comply with the requirements for the control of mercury from BOPF Groups?
* * * *
§63.7800 What are my operation and maintenance requirements?
* * * *
(b) You must prepare and operate at all times according to a written operation and maintenance plan for each capture system or control device subject to an operating limit in §63.7790(b). Each plan must address the elements in paragraphs (b)(1) through (9) of this section.
§63.7820 By what date must I conduct performance tests or other initial compliance demonstrations?
* * * *
(e) Notwithstanding the deadlines in this section, existing and new affected sources must comply with the deadlines for making the initial compliance demonstrations for the BOPF Group mercury emission limit set forth in paragraphs (e)(1) through (4) in this section.
§63.7821 When must I conduct subsequent performance tests?
(a) You must conduct subsequent performance tests to demonstrate compliance with all applicable emission and opacity limits in table 1 to this subpart at the frequencies specified in paragraphs (b) through (m) of this section.
(b) For each sinter cooler at an existing sinter plant and each emissions unit equipped with a control device other than a baghouse, you must conduct subsequent particulate matter and opacity performance tests no less frequently than twice (at mid-term and renewal) during each term of your title V operating permit.
(c) For each emissions unit equipped with a baghouse, you must conduct subsequent particulate matter and opacity performance tests no less frequently than once during each term of your title V operating permit.
(d) For sources without a title V operating permit, you must conduct subsequent particulate matter and opacity performance tests every 2.5 years.
(e) For each BOPF Group, if demonstrating compliance with the mercury emission limit in table 1 to this subpart through performance testing under §§63.7825 and 63.7833, you must conduct subsequent performance tests twice per permit cycle ( i.e., mid-term and initial/final) for sources with title V operating permits, and every 2.5 years for sources without a title V operating permit, at the outlet of the control devices for the BOPF Group.
(f) For each sinter plant windbox, you must conduct subsequent mercury, hydrogen chloride, carbonyl sulfide, dioxin/furan, and polycyclic aromatic hydrocarbon performance tests every 5 years.
(g) For each blast furnace stove and BOPF shop primary emission control device, you must conduct subsequent hydrogen chloride and total hydrocarbon testing every 5 years. For the BOPF shop primary emission control device, you must also conduct subsequent dioxin/furan testing every 5 years.
(h) For each blast furnace casthouse and BOPF shop, you must conduct subsequent opacity tests two times per month during a cast, or during a full heat cycle, as appropriate.
(i) For planned bleeder valve openings on each blast furnace, you must conduct opacity tests according to §63.7823(f) for each planned opening.
(j) For slag processing, handling, and storage operations for each blast furnace or BOPF, you must conduct subsequent opacity tests once per week for a minimum of 18 minutes for each: BF pit filling; BOPF slag pit filling; BF pit digging; BOPF slag pit digging; and one slag handling (either truck loading or dumping slag to slag piles).
(k) For large bells on each blast furnace, you must conduct visible emissions testing on the interbell relief valve according to EPA Method 22 in appendix A-7 to part 60 of this chapter, unless specified in paragraphs (k)(1) through (3) of this section. Testing must be conducted monthly, for 15 minutes.
(1) If visible emissions are detected for a large bell during the monthly visible emissions testing, you must conduct EPA Method 9 (in appendix A-4 to part 60 of this chapter) opacity tests in place of EPA Method 22 testing on that bell once per month, taking 3-minute averages for 15 minutes, until the large bell seal is repaired or replaced.
(2) If the average of 3 instantaneous visible emission readings taken while the interbell relief valve is exhausting exceeds 20 percent, you must initiate corrective action within five business days.
(3) Ten business days after the initial opacity exceedance of 20 percent, you must conduct an EPA Method 9 opacity test, taking 3-minute averages for 15 minutes. If the average of 3 instantaneous visible emissions readings from this test exceeds 20 percent, you must repair or replace that bell seal within 4 months.
(l) For small bells on each blast furnace, you must conduct visible emissions testing according to EPA Method 22 in appendix A-7 to part 60 of this chapter. Testing must be conducted monthly for 15 minutes. If visible emissions are observed, you must compare the period between the visible emissions being present and the most recent bell seal repair or replacement. If this time period or throughput is shorter or lower than the period or throughput stated in the O&M plan required by 63.7800, this new shorter period or lower limit shall be placed in the O&M plan as the work practice limit.
(m) For each blast furnace casthouse, you must conduct subsequent hydrogen chloride and total hydrocarbon testing every 5 years.
§63.7823 What test methods and other procedures must I use to demonstrate initial compliance with the opacity limits?
(a) For each discharge end of a sinter plant, sinter plant cooler, blast furnace casthouse, BOPF shop, and large bell on a blast furnace, you must conduct each performance test that applies to your affected source based on representative performance ( i.e., performance based on normal operating conditions) of the affected source for the period being tested, according to the conditions detailed in paragraphs (b) through (d) of this section. Representative conditions exclude periods of startup and shutdown. You shall not conduct performance tests during periods of malfunction. You must record the process information that is necessary to document operating conditions during the test and include in such record an explanation to support that such conditions represent normal operation. Upon request, you shall make available to the Administrator such records as may be necessary to determine the conditions of performance tests.
* * * *
§63.7825 What test methods and other procedures must I use to demonstrate initial compliance with the emission limits for hazardous air pollutants?
(a) If demonstrating compliance with the emission limits in Table 1 to this subpart through performance testing, you must conduct a performance test to demonstrate initial compliance with the emission limit. If demonstrating compliance with the emission limit through performance testing, you must conduct each performance test that applies to your affected source based on representative performance ( i.e., performance based on normal operating conditions) of the affected source for the period being tested, according to the conditions detailed in paragraphs (b) through (k) of this section. Representative conditions exclude periods of startup and shutdown. You shall not conduct performance tests during periods of malfunction. Initial compliance tests must be conducted by the deadlines in §63.7820(e).
* * * * *
(b) * * *
(1) * * *
(v) EPA Method 29 or 30B in appendix A-8 to part 60 of this chapter to determine the concentration of mercury from the exhaust stream stack of each unit. If performing measurements using EPA Method 29, you must collect a minimum sample volume of 1.7 dscm (60 dscf). Alternative test methods may be considered on a case-by-case basis per §63.7(f).
(2) Three valid test runs are needed to comprise a performance test of each unit in table 1 to this subpart as applicable. If the performance testing results for any of the emission points yields a non-detect value, then the method detection limit (MDL) must be used to calculate the mass emissions (lb) for that emission unit and, in turn, for calculating the sum of the emissions (in units of pounds of mercury per ton of steel scrap or pounds of mercury per ton of product sinter) for all units subject to the emission standard for determining compliance. If the resulting mercury emissions are greater than the MACT emission standard, the owner or operator may use procedures that produce lower MDL results and repeat the mercury performance testing one additional time for any emission point for which the measured result was below the MDL. If this additional testing is performed, the results from that testing must be used to determine compliance ( i.e., there are no additional opportunities allowed to lower the MDL).
* * * * *
(c) Calculate the mass emissions, based on the average of three test run values, for each BOPF Group unit (or combination of units that are ducted to a common stack and are tested when all affected sources are operating pursuant to paragraph (a) of this section) using equation 1 to this paragraph (c) as follows:
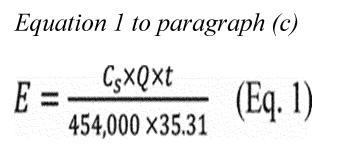
Where:
E = Mass emissions of pollutant, pounds (lb);
C s = Concentration of pollutant in stack gas, mg/dscm;
454,000 = Conversion factor (mg/lb);
Q = Volumetric flow rate of stack gas, dscf/min;
35.31 = Conversion factor (dscf/dscm); and
t = Duration of test, minutes.
* * * *
§63.7830 What are my monitoring requirements?
* * * *
(e)(2) Compute and record the 30-day rolling average of the volatile organic compound emissions (lbs/ton of sinter) for each operating day using the procedures in §63.7824(e).
§63.7841 What reports must I submit and when?
* * * *
(d) CEDRI submission. If you are required to submit reports following the procedure specified in this paragraph, you must submit reports to the EPA via CEDRI, which can be accessed through EPA's CDX ( https://cdx.epa.gov/ ). You must use the appropriate electronic report template on the CEDRI website ( https://www.epa.gov/electronic-reporting-air-emissions/compliance-and-emissions-data-reporting-interface-cedri ) for this subpart. The date report templates become available will be listed on the CEDRI website. The report must be submitted by the deadline specified in this subpart, regardless of the method in which the report is submitted. Do not use CEDRI to submit information you claim as CBI. Although we do not expect persons to assert a claim of CBI, if you wish to assert a CBI claim for some of the information in the report, you must submit a complete file, including information claimed to be CBI, to the EPA following the procedures in paragraphs (d)(1) and (2) of this section. Clearly mark the part or all of the informatioqn that you claim to be CBI. Information not marked as CBI may be authorized for public release without prior notice. Information marked as CBI will not be disclosed except in accordance with procedures set forth in 40 CFR part 2. All CBI claims must be asserted at the time of submission. Anything submitted using CEDRI cannot later be claimed CBI. Furthermore, under CAA section 114(c), emissions data is not entitled to confidential treatment, and the EPA is required to make emissions data available to the public. Thus, emissions data will not be protected as CBI and will be made publicly available. You must submit the same file submitted to the CBI office with the CBI omitted to the EPA via the EPA's CDX as described earlier in this paragraph.
(1) The preferred method to receive CBI is for it to be transmitted electronically using email attachments, File Transfer Protocol, or other online file sharing services. Electronic submissions must be transmitted directly to the OAQPS CBI Office at the email address oaqpscbi@epa.gov, and as described above, should include clear CBI markings and be flagged to the attention of the Integrated Iron and Steel Sector Lead. If assistance is needed with submitting large electronic files that exceed the file size limit for email attachments, and if you do not have your own file sharing service, please email oaqpscbi@epa.gov to request a file transfer link.
(2) If you cannot transmit the file electronically, you may send CBI information through the postal service to the following address: OAQPS Document Control Officer (C404-02), OAQPS, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27711, Attention Integrated Iron and Steel Sector Lead. The mailed CBI material should be double wrapped and clearly marked. Any CBI markings should not show through the outer envelope.
§63.7842 What records must I keep?
* * * *
(d) You must keep the records required in §§63.7823, 63.7833, and 63.7834 to show continuous compliance with each emission limitation and operation and maintenance requirement that applies to you. This includes a record of each large and small bell repair and replacement, a record of the date on which the large bell opacity has exceeded 20 percent, and the most current time period or throughput over which no opacity was observed from the small bell.
Table 1 to Subpart FFFFF of Part 63 - Emission, Opacity, and Work Practice Limits
As required in §63.7790(a), you must comply with each applicable emission, opacity, and work practice limit in the following table:
| For . . . | You must comply with each of the following . . . |
|---|---|
| 1 This limit applies if the cooler is vented to the same control device as the discharge end. | |
| 2 This concentration limit (gr/dscf) for a control device does not apply to discharges inside a building or structure housing the discharge end at an existing sinter plant, inside a casthouse at an existing blast furnace, or inside an existing BOPF shop if the control device was installed before August 30, 2005. | |
| 3 This limit applies to control devices operated in parallel for a single BOPF during the oxygen blow. | |
| 1. Each windbox exhaust stream at an existing sinter plant | a. You must not cause to be discharged to the atmosphere any gases that contain particulate matter in excess of 0.4 lb/ton of product sinter; |
| b. You must not cause to be discharged to the atmosphere any gases that contain mercury in excess of 0.000018 lb/ton of product sinter; | |
| c. You must not cause to be discharged to the atmosphere any gases that contain hydrogen chloride in excess of 0.025 lb/ton of product sinter; | |
| d. You must not cause to be discharged to the atmosphere any gases that contain carbonyl sulfide in excess of 0.064 lb/ton of product sinter; | |
| e. You must not cause to be discharged to the atmosphere any gases that contain D/F TEQs in excess of 1.1E-08 lb/ton of product sinter; and | |
| f. You must not cause to be discharged to the atmosphere any gases that contain polycyclic aromatic hydrocarbons in excess of 0.0018 lb/ton of product sinter. | |
| 2. Each windbox exhaust stream at a new sinter plant | a. You must not cause to be discharged to the atmosphere any gases that contain particulate matter in excess of 0.3 lb/ton of product sinter; |
| b. You must not cause to be discharged to the atmosphere any gases that contain mercury in excess of 0.000012 lb/ton of product sinter; | |
| c. You must not cause to be discharged to the atmosphere any gases that contain hydrogen chloride in excess of 0.0012 lb/ton of product sinter; | |
| d. You must not cause to be discharged to the atmosphere any gases that contain carbonyl sulfide in excess of 0.030 lb/ton of product sinter; | |
| e. You must not cause to be discharged to the atmosphere any gases that contain D/F TEQs in excess of 1.1E-08 lb/ton of product sinter; and | |
| f. You must not cause to be discharged to the atmosphere any gases that contain polycyclic aromatic hydrocarbons in excess of 0.0015 lb/ton of product sinter. | |
| 3. Each discharge end at an existing sinter plant | a. You must not cause to be discharged to the atmosphere any gases that exit from one or more control devices that contain, on a flow-weighted basis, particulate matter in excess of 0.02 gr/dscf; 12 and |
| b. You must not cause to be discharged to the atmosphere any secondary emissions that exit any opening in the building or structure housing the discharge end that exhibit opacity greater than 20 percent (6-minute average). | |
| 4. Each discharge end at a new sinter plant | a. You must not cause to be discharged to the atmosphere any gases that exit from one or more control devices that contain, on a flow weighted basis, particulate matter in excess of 0.01 gr/dscf; and |
| b. You must not cause to be discharged to the atmosphere any secondary emissions that exit any opening in the building or structure housing the discharge end that exhibit opacity greater than 10 percent (6-minute average). | |
| 5. Each sinter cooler at an existing sinter plant | You must not cause to be discharged to the atmosphere any emissions that exhibit opacity greater than 10 percent (6-minute average). |
| 6. Each sinter cooler at a new sinter plant | You must not cause to be discharged to the atmosphere any gases that contain particulate matter in excess of 0.01 gr/dscf. |
| 7. Each casthouse at an existing blast furnace | a. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain particulate matter in excess of 0.01 gr/dscf; 2 |
| b. You must not cause to be discharged to the atmosphere any secondary emissions that exit all openings in the casthouse or structure housing the blast furnace that exhibit opacity greater than 20 percent (6-minute average); | |
| c. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain hydrogen chloride in excess of 0.0056 lb/ton of iron; | |
| d. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain total hydrocarbons as propane in excess of 0.48 lb/ton of iron; and | |
| e. You must not cause unplanned bleeder valve openings in excess of 4 events per year for large blast furnaces or 15 events per year for small blast furnaces. | |
| 8. Each casthouse at a new blast furnace | a. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain particulate matter in excess of 0.003 gr/dscf; and |
| b. You must not cause to be discharged to the atmosphere any secondary emissions that exit all openings in the casthouse or structure housing the blast furnace that exhibit opacity greater than 15 percent (6-minute average); | |
| c. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain hydrogen chloride in excess of 0.00059 lb/ton of iron; | |
| d. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain total hydrocarbons as propane in excess of 0.035 lb/ton of iron; and | |
| e. You must not cause unplanned bleeder valve openings in excess of zero events per year. | |
| 9. Each BOPF at a new or existing shop | a. You must not cause to be discharged to the atmosphere any gases that exit from a primary emission control system for a BOPF with a closed hood system at a new or existing BOPF shop that contain, on a flow-weighted basis, particulate matter in excess of 0.03 gr/dscf during the primary oxygen blow; 23 |
| b. You must not cause to be discharged to the atmosphere any gases that exit from a primary emission control system for a BOPF with an open hood system that contain, on a flow-weighted basis, particulate matter in excess of 0.02 gr/dscf during the steel production cycle for an existing BOPF shop 23 or 0.01 gr/dscf during the steel production cycle for a new BOPF shop; 3 | |
| c. You must not cause to be discharged to the atmosphere any gases that exit from a control device used solely for the collection of secondary emissions from the BOPF that contain particulate matter in excess of 0.01 gr/dscf for an existing BOPF shop 2 or 0.0052 gr/dscf for a new BOPF shop; | |
| d. You must not cause to be discharged to the atmosphere any gases that exit from a primary emission control system for a BOPF that contain hydrogen chloride in excess of 0.058 lb/ton of steel for existing sources and 2.8E-04 lb/ton steel for new sources; | |
| e. You must not cause to be discharged to the atmosphere any gases that exit from a primary emission control system for a BOPF that contain THC as propane in excess of 0.04 lb/ton of steel for existing sources and 0.0017 lb/ton of steel for new sources; and | |
| f. You must not cause to be discharged to the atmosphere any gases that exit from a primary emission control system for a BOPF that contain D/F TEQs in excess of 9.2E-10 lb/ton of steel. | |
| 10. Each hot metal transfer, skimming, and desulfurization operation at a new or existing BOPF shop | You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain particulate matter in excess of 0.01 gr/dscf for an existing BOPF shop 2 or 0.003 gr/dscf for a new BOPF shop. |
| 11. Each ladle metallurgy operation at a new or existing BOPF shop | You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain particulate matter in excess of 0.01 gr/dscf for an existing BOPF shop 2 or 0.004 gr/dscf for a new BOPF shop. |
| 12. Each existing BOPF shop | You must not cause to be discharged to the atmosphere any secondary emissions that exit any opening in the BOPF shop or any other building housing the BOPF or BOPF shop operation that exhibit opacity greater than 20 percent (3-minute average). |
| 13. Each new BOPF shop | a. You must not cause to be discharged to the atmosphere any secondary emissions that exit any opening in the BOPF shop or other building housing a bottom-blown BOPF or BOPF shop operations that exhibit opacity (for any set of 6-minute averages) greater than 10 percent, except that one 6-minute period not to exceed 20 percent may occur once per steel production cycle; or |
| b. You must not cause to be discharged to the atmosphere any secondary emissions that exit any opening in the BOPF shop or other building housing a top-blown BOPF or BOPF shop operations that exhibit opacity (for any set of 3-minute averages) greater than 10 percent, except that one 3-minute period greater than 10 percent but less than 20 percent may occur once per steel production cycle. | |
| 14. Each BOPF Group at an existing BOPF shop | You must not cause to be discharged to the atmosphere any gases that exit from the collection of BOPF Group control devices that contain mercury in excess of 0.00026 lb/ton of steel scrap input to the BOPF. |
| 15. Each BOPF Group at a new BOPF shop | You must not cause to be discharged to the atmosphere any gases that exit from the collection of BOPF Group control devices that contain mercury in excess of 0.000081 lb/ton of steel scrap input to the BOPF. |
| 16. Each planned bleeder valve opening at a new or existing blast furnace | You must not cause to be discharged to the atmosphere any emissions that exhibit opacity greater than 8 percent (6-minute average). |
| 17. Each slag processing, handling and storage operation for a new or existing blast furnace or BOPF | You must not cause to be discharged to the atmosphere any emissions that exhibit opacity greater than 10 percent (6-minute average). |
| 18. Each existing blast furnace stove | a. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain HCl in excess of 0.0012 lb/MMBtu; and |
| b. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain THC in excess of 0.12 lb/MMBtu. | |
| 19. Each new blast furnace stove | a. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain HCl in excess of 4.2e-4 lb/MMBtu; and |
| b. You must not cause to be discharged to the atmosphere any gases that exit from a control device that contain THC in excess of 0.0054 lb/MMBtu. | |
Table 2 to Subpart FFFFF of Part 63 - Initial Compliance With Emission and Opacity Limits
As required in §63.7826(a)(1), you must demonstrate initial compliance with the emission and opacity limits according to the following table:
| For . . . | You have demonstrated initial compliance if . . . |
|---|---|
| 1. Each windbox exhaust stream at an existing sinter plant | a. The process-weighted mass rate of particulate matter from a windbox exhaust stream, measured according to the performance test procedures in §63.7822(c), did not exceed 0.4 lb/ton of product sinter; |
| b. The process-weighted mass rate of mercury from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.000018 lb/ton of product sinter; | |
| c. The process-weighted mass rate of hydrogen chloride from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.025 lb/ton of product sinter; | |
| d. The process-weighted mass rate of carbonyl sulfide from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.064 lb/ton of product sinter; | |
| e. The process-weighted mass rate of D/F TEQs from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 1.1E-08 lb/ton of product sinter; and | |
| f. The process-weighted mass rate of polycyclic aromatic hydrocarbons from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0018 lb/ton of product sinter. | |
| 2. Each windbox exhaust stream at a new sinter plant | a. The process-weighted mass rate of particulate matter from a windbox exhaust stream, measured according to the performance test procedures in §63.7822(c), did not exceed 0.3 lb/ton of product sinter; |
| b. The process-weighted mass rate of mercury from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.000012 lb/ton of product sinter; | |
| c. The process-weighted mass rate of hydrogen chloride from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0012 lb/ton of product sinter; | |
| d. The process-weighted mass rate of carbonyl sulfide from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.030 lb/ton of product sinter; | |
| e. The process-weighted mass rate of D/F TEQs from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 1.1E-08 lb/ton of product sinter; and | |
| f. The process-weighted mass rate of polycyclic aromatic hydrocarbons from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0015 lb/ton of product sinter. | |
| 3. Each discharge end at an existing sinter plant | a. The flow-weighted average concentration of particulate matter from one or more control devices applied to emissions from a discharge end, measured according to the performance test procedures in §63.7822(d), did not exceed 0.02 gr/dscf; and |
| b. The opacity of secondary emissions from each discharge end, determined according to the performance test procedures in §63.7823(c), did not exceed 20 percent (6-minute average). | |
| 4. Each discharge end at a new sinter plant | a. The flow-weighted average concentration of particulate matter from one or more control devices applied to emissions from a discharge end, measured according to the performance test procedures in §63.7822(d), did not exceed 0.01 gr/dscf; and |
| b. The opacity of secondary emissions from each discharge end, determined according to the performance test procedures in §63.7823(c), did not exceed 10 percent (6-minute average). | |
| 5. Each sinter cooler at an existing sinter plant | The opacity of emissions, determined according to the performance test procedures in §63.7823(e), did not exceed 10 percent (6-minute average). |
| 6. Each sinter cooler at a new sinter plant | The average concentration of particulate matter, measured according to the performance test procedures in §63.7822(b), did not exceed 0.01 gr/dscf. |
| 7. Each casthouse at an existing blast furnace | a. The average concentration of particulate matter from a control device applied to emissions from a casthouse, measured according to the performance test procedures in §63.7822(e), did not exceed 0.01 gr/dscf; |
| b. The opacity of secondary emissions from each casthouse, determined according to the performance test procedures in §63.7823(c), did not exceed 20 percent (6-minute average); | |
| c. The process-weighted mass rate of hydrogen chloride from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0056 lb/ton of iron; | |
| d. The process-weighted mass rate of total hydrocarbons from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.48 lb/ton of iron; and | |
| e. The number of unplanned bleeder valve openings in one year, as reported according to the specifications in §63.7841(b)(14), did not exceed 4 events for large blast furnaces or 15 events for small blast furnaces. | |
| 8. Each casthouse at a new blast furnace | a. The average concentration of particulate matter from a control device applied to emissions from a casthouse, measured according to the performance test procedures in §63.7822(e), did not exceed 0.003 gr/dscf; and |
| b. The opacity of secondary emissions from each casthouse, determined according to the performance test procedures in §63.7823(c), did not exceed 15 percent (6-minute average); | |
| c. The process-weighted mass rate of hydrogen chloride from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.00059 lb/ton of iron; | |
| d. The process-weighted mass rate of total hydrocarbons from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.035 lb/ton of iron; and | |
| e. The number of unplanned bleeder valve openings in one year, as reported according to the specifications in §63.7841(b)(14), did not exceed zero events. | |
| 9. Each BOPF at a new or existing BOPF shop | a. The average concentration of particulate matter from a primary emission control system applied to emissions from a BOPF with a closed hood system, measured according to the performance test procedures in §63.7822(f), did not exceed 0.03 gr/dscf for a new or existing BOPF shop; |
| b. The average concentration of particulate matter from a primary emission control system applied to emissions from a BOPF with an open hood system, measured according to the performance test procedures in §63.7822(g), did not exceed 0.02 gr/dscf for an existing BOPF shop or 0.01 gr/dscf for a new BOPF shop; | |
| c. The average concentration of particulate matter from a control device applied solely to secondary emissions from a BOPF, measured according to the performance test procedures in §63.7822(g), did not exceed 0.01 gr/dscf for an existing BOPF shop or 0.0052 gr/dscf for a new BOPF shop; | |
| d. The process-weighted mass rate of hydrogen chloride from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.058 lb/ton of steel for an existing BOPF shop or 0.00028 lb/ton of steel for a new BOPF shop; | |
| e. The process-weighted mass rate of total hydrocarbons from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.04 lb/ton of steel for an existing BOPF shop or 0.0017 lb/ton of steel for a new BOPF shop; and | |
| f. The process-weighted mass rate of D/F TEQs from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 9.2e-10 lb/ton of steel. | |
| 10. Each hot metal transfer skimming, and desulfurization at a new or existing BOPF shop | The average concentration of particulate matter from a control device applied to emissions from hot metal transfer, skimming, or desulfurization, measured according to the performance test procedures in §63.7822(h), did not exceed 0.01 gr/dscf for an existing BOPF shop or 0.003 gr/dscf for a new BOPF shop. |
| 11. Each ladle metallurgy operation at a new or existing BOPF shop | The average concentration of particulate matter from a control device applied to emissions from a ladle metallurgy operation, measured according to the performance test procedures in §63.7822(h), did not exceed 0.01 gr/dscf for an existing BOPF shop or 0.004 gr/dscf for a new BOPF shop. |
| 12. Each existing BOPF shop | The opacity of secondary emissions from each BOPF shop, determined according to the performance test procedures in §63.7823(d), did not exceed 20 percent (3-minute average). |
| 13. Each new BOPF shop | a. The opacity of the highest set of 6-minute averages from each BOPF shop housing a bottom-blown BOPF, determined according to the performance test procedures in §63.7823(d), did not exceed 20 percent and the second highest set of 6-minute averages did not exceed 10 percent; or |
| b. The opacity of the highest set of 3-minute averages from each BOPF shop housing a top-blown BOPF, determined according to the performance test procedures in §63.7823(d), did not exceed 20 percent and the second highest set of 3-minute averages did not exceed 10 percent. | |
| 14. Each BOPF Group at an existing BOPF shop | If demonstrating compliance through performance testing, the average emissions of mercury from the collection of BOPF Group control devices applied to the emissions from the BOPF Group, measured according to the performance test procedures in §63.7825, did not exceed 0.00026 lb/ton steel scrap input to the BOPF. |
| 15. Each BOPF Group at a new BOPF shop | If demonstrating compliance through performance testing, the average emissions of mercury from the collection of BOPF Group control devices applied to the emissions from the BOPF Group, measured according to the performance test procedures in §63.7825, did not exceed 0.000081 lb/ton steel scrap input to the BOPF. |
| 16. Each planned bleeder valve opening at a new or existing blast furnace | The opacity of emissions, determined according to the performance test procedures in §63.7823(f), did not exceed 8 percent (6-minute average). |
| 17. Each slag processing, handling and storage operation for a new or existing blast furnace or BOPF | The opacity of emissions, determined according to the performance test procedures in §63.7823(g), did not exceed 10 percent (6-minute average). |
| 18. Each existing blast furnace stove | a. The process-weighted mass rate of HCl from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0012 lb/MMBtu; and |
| b. The process-weighted mass rate of THC from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.12 lb/MMBtu. | |
| 19. Each new blast furnace stove | a. The process-weighted mass rate of HCl from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 4.2e-4 lb/MMBtu; and |
| b. The process-weighted mass rate of THC from a windbox exhaust stream, measured according to the performance test procedures in §63.7825, did not exceed 0.0054 lb/MMBtu. |
Table 3 to Subpart FFFFF of Part 63 - Continuous Compliance With Emission and Opacity Limits
As required in §63.7833(a), you must demonstrate continuous compliance with the emission and opacity limits according to the following table:
| For . . . | You must demonstrate continuous compliance by . . . |
|---|---|
| 1. Each windbox exhaust stream at an existing sinter plant | a. Maintaining emissions of particulate matter at or below 0.4 lb/ton of product sinter; b. Conducting subsequent performance tests at the frequencies specified in §63.7821; |
| c. Maintaining emissions of mercury at or below 0.000018 lb/ton of product sinter; | |
| d. Maintaining emissions of hydrogen chloride at or below 0.025 lb/ton of product sinter; | |
| e. Maintaining emissions of carbonyl sulfide at or below 0.064 lb/ton of product sinter; | |
| f. Maintaining emissions of D/F TEQs at or below 1.1E-08 lb/ton of product sinter; and | |
| g. Maintaining emissions of polycyclic aromatic hydrocarbons at or below 0.0018 lb/ton of product sinter. | |
| 2. Each windbox exhaust stream at a new sinter plant | a. Maintaining emissions of particulate matter at or below 0.3 lb/ton of product sinter; b. Conducting subsequent performance tests at the frequencies specified in §63.7821; |
| c. Maintaining emissions of mercury at or below 0.000012 lb/ton of product sinter; | |
| d. Maintaining emissions of hydrogen chloride at or below 0.0012 lb/ton of product sinter; | |
| e. Maintaining emissions of carbonyl sulfide at or below 0.030 lb/ton of product sinter; | |
| f. Maintaining emissions of D/F TEQs at or below 1.1E-08 lb/ton of product sinter; and | |
| g. Maintaining emissions of polycyclic aromatic hydrocarbons at or below 0.0015 lb/ton of product sinter. | |
| 3. Each discharge end at an existing sinter plant | a. Maintaining emissions of particulate matter from one or more control devices at or below 0.02 gr/dscf; and b. Maintaining the opacity of secondary emissions that exit any opening in the building or structure housing the discharge end at or below 20 percent (6-minute average); and |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 4. Each discharge end at a new sinter plant | a. Maintaining emissions of particulate matter from one or more control devices at or below 0.01 gr/dscf; and b. Maintaining the opacity of secondary emissions that exit any opening in the building or structure housing the discharge end at or below 10 percent (6-minute average); and |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 5. Each sinter cooler at an existing sinter plant | a. Maintaining the opacity of emissions that exit any sinter cooler at or below 10 percent (6-minute average); and b. Conducting subsequent performance tests at the frequencies specified in §63.7821. |
| 6. Each sinter cooler at a new sinter plant | a. Maintaining emissions of particulate matter at or below 0.1 gr/dscf; and |
| b. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 7. Each casthouse at an existing blast furnace | a. Maintaining emissions of particulate matter from a control device at or below 0.01 gr/dscf; b. Maintaining the opacity of secondary emissions that exit all openings in the casthouse or structure housing the casthouse at or below 20 percent (6-minute average); |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821; | |
| d. Maintaining emissions of hydrogen chloride at or below 0.0056 lb/ton of iron; | |
| e. Maintaining emissions of total hydrocarbons at or below 0.48 lb/ton of iron; and | |
| f. Maintaining unplanned bleeder valve openings at or below 4 events per year for large blast furnaces or 15 events per year for small blast furnaces. | |
| 8. Each casthouse at a new blast furnace | a. Maintaining emissions of particulate matter from a control device at or below 0.003 gr/dscf; b. Maintaining the opacity of secondary emissions that exit all openings in the casthouse or structure housing the casthouse at or below 15 percent (6-minute average); |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821; | |
| d. Maintaining emissions of hydrogen chloride at or below 0.00059 lb/ton of iron; | |
| e. Maintaining emissions of total hydrocarbons at or below 0.035 lb/ton of iron; and | |
| f. Maintaining unplanned bleeder valve openings at zero events per year. | |
| 9. Each BOPF at a new or existing BOPF shop | a. Maintaining emissions of particulate matter from the primary control system for a BOPF with a closed hood system at or below 0.03 gr/dscf; |
| b. Maintaining emissions of particulate matter from the primary control system for a BOPF with an open hood system at or below 0.02 gr/dscf for an existing BOPF shop or 0.01 gr/dscf for a new BOPF shop; | |
| c. Maintaining emissions of particulate matter from a control device applied solely to secondary emissions from a BOPF at or below 0.01 gr/dscf for an existing BOPF shop or 0.0052 gr/dscf for a new BOPF shop; | |
| d. Conducting subsequent performance tests at the frequencies specified in §63.7821; | |
| e. Maintaining emissions of hydrogen chloride from a primary emission control system for a BOPF at or below 0.058 lb/ton of steel for existing sources and 2.8E-04 lb/ton steel for new sources; | |
| f. Maintaining emissions of THC from a primary emission control system for a BOPF at or below 0.04 lb/ton of steel for existing sources and 0.0017 lb/ton of steel for new sources; and | |
| g. Maintaining emissions of D/F TEQs from a primary emission control system for a BOPF at or below 9.2E-10 lb/ton of steel. | |
| 10. Each hot metal transfer, skimming, and desulfurization operation at a new or existing BOPF shop | a. Maintaining emissions of particulate matter from a control device at or below 0.01 gr/dscf at an existing BOPF or 0.003 gr/dscf for a new BOPF; and b. Conducting subsequent performance tests at the frequencies specified in §63.7821. |
| 11. Each ladle metallurgy operation at a new or existing BOPF shop | a. Maintaining emissions of particulate matter from a control device at or below 0.01 gr/dscf at an existing BOPF shop or 0.004 gr/dscf for a new BOPF shop; and |
| b. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 12. Each existing BOPF shop | a. Maintaining the opacity of secondary emissions that exit any opening in the BOPF shop or other building housing the BOPF shop or shop operation at or below 20 percent (3-minute average); and |
| b. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 13. Each new BOPF shop | a. Maintaining the opacity (for any set of 6-minute averages) of secondary emissions that exit any opening in the BOPF shop or other building housing a bottom-blown BOPF or shop operation at or below 10 percent, except that one 6-minute period greater than 10 percent but no more than 20 percent may occur once per steel production cycle; |
| b. Maintaining the opacity (for any set of 3-minute averages) of secondary emissions that exit any opening in the BOPF shop or other building housing a top-blown BOPF or shop operation at or below 10 percent, except that one 3-minute period greater than 10 percent but less than 20 percent may occur once per steel production cycle; and | |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 14. Each BOPF Group at an existing BOPF shop | a. Maintaining emissions of mercury from the collection of BOPF Group control devices at or below 0.00026 lb/ton steel scrap input to the BOPF; and |
| b. If demonstrating compliance through performance testing, conducting subsequent performance tests at the frequencies specified in §63.7821; and | |
| c. If demonstrating compliance through §63.7791(c), (d), or (e), maintaining records pursuant to §63.7842(e). | |
| 15. Each BOPF Group at a new BOPF shop | a. Maintaining emissions of mercury from the collection of BOPF Group control devices at or below 0.000081 lb/ton steel scrap input to the BOPF; and |
| b. If demonstrating compliance through performance testing, conducting subsequent performance tests at the frequencies specified in §63.7821; and | |
| c. If demonstrating compliance through §63.7791(c), (d), or (e), maintaining records pursuant to §63.7842(e). | |
| 16. Each planned bleeder valve opening at a new or existing blast furnace | a. Maintaining the opacity of emissions that exit any bleeder valve as a result of a planned opening at or below 8 percent (6-minute average); and |
| b. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 17. Each slag processing, handling and storage operation for a new or existing blast furnace or BOPF | a. Maintaining the opacity of emissions that exit any slag processing, handling, or storage operation at or below 10 percent (6-minute average); and b. Conducting subsequent performance tests at the frequencies specified in §63.7821. |
| 18. Each existing blast furnace stove | a. Maintaining emissions of HCl at or below 0.0012 lb/MMBtu; |
| b. Maintaining emissions of THC at or below 0.12 lb/MMBtu; and | |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821. | |
| 19. Each new blast furnace stove | a. Maintaining emissions of HCl at or below 4.2e-4 lb/MMBtu; |
| b. Maintaining emissions of THC at or below 0.0054 lb/MMBtu; and | |
| c. Conducting subsequent performance tests at the frequencies specified in §63.7821. |
Table 4 to Subpart FFFFF of Part 63 - Applicability of General Provisions to Subpart FFFFF
As required in §63.7850, you must comply with the requirements of the NESHAP General Provisions (subpart A of this part) shown in the following table:
| Citation | Subject | Applies to subpart FFFFF | Explanation |
|---|---|---|---|
| §63.1 | Applicability | Yes | |
| §63.2 | Definitions | Yes | |
| §63.3 | Units and Abbreviations | Yes | |
| §63.4 | Prohibited Activities | Yes | |
| §63.5 | Construction/Reconstruction | Yes | |
| §63.6(a), (b), (c), (d), (e)(1)(iii), (f)(2)-(3), (g), (h)(2)(ii)-(h)(9) | Compliance with Standards and Maintenance Requirements | Yes | |
| §63.6(e)(1)(i) | General Duty to Minimize Emissions | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7810(d) for general duty requirement. |
| §63.6(e)(1)(ii) | Requirement to Correct Malfunctions ASAP | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes, on or before January 11, 2021, and No thereafter | |
| §63.6(e)(3) | SSM Plan Requirements | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7810(c). |
| §63.6(f)(1) | Compliance except during SSM | No | See §63.7810(a). |
| §63.6(h)(1) | Compliance except during SSM | No | See §63.7810(a). |
| §63.6(h)(2)(i) | Determining Compliance with Opacity and VE Standards | No | Subpart FFFFF specifies methods and procedures for determining compliance with opacity emission and operating limits. |
| §63.6(i) | Extension of Compliance with Emission Standards | Yes | |
| §63.6(j) | Exemption from Compliance with Emission Standards | Yes | |
| §63.7(a)(1)-(2) | Applicability and Performance Test Dates | No | Subpart FFFFF and specifies performance test applicability and dates. |
| §63.7(a)(3), (b)-(d), (e)(2)-(4), (f)-(h) | Performance Testing Requirements | Yes | |
| §63.7(e)(1) | Performance Testing | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §§63.7822(a), 63.7823(a), and 63.7825(a). |
| §63.8(a)(1)-(3), (b), (c)(1)(ii), (c)(2)-(3), (c)(4)(i)-(ii), (c)(5)-(6), (c)(7)-(8), (d)(1)-(2), (e), (f)(1)-(5), (g)(1)-(4) | Monitoring Requirements | Yes | CMS requirements in §63.8(c)(4)(i)-(ii), (c)(5)-(6), (d)(1)-(2), and (e) apply only to COMS. |
| §63.8(a)(4) | Additional Monitoring Requirements for Control Devices in §63.11 | No | Subpart FFFFF does not require flares. |
| §63.8(c)(1)(i) | General Duty to Minimize Emissions and CMS Operation | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | |
| §63.8(c)(1)(iii) | Requirement to Develop SSM Plan for CMS | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | |
| §63.8(c)(4) | Continuous Monitoring System Requirements | No | Subpart FFFFF specifies requirements for operation of CMS. |
| §63.8(d)(3) | Written procedures for CMS | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7842(b)(3). |
| §63.8(f)(6) | RATA Alternative | No | |
| §63.8(g)(5) | Data Reduction | No | Subpart FFFFF specifies data reduction requirements. |
| §63.9 | Notification Requirements | Yes | Additional notifications for CMS in §63.9(g) apply only to COMS. |
| §63.10(a), (b)(1), (b)(2)(x), (b)(2)(xiv), (b)(3), (c)(1)-(6), (c)(9)-(14), (d)(1)-(4), (e)(1)-(2), (e)(4), (f) | Recordkeeping and Reporting Requirements | Yes | Additional records for CMS in §63.10(c)(1)-(6), (9)-(14), and reports in §63.10(d)(1)-(2) apply only to COMS. |
| §63.10(b)(2)(i) | Recordkeeping of Occurrence and Duration of Startups and Shutdowns | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | |
| §63.10(b)(2)(ii) | Recordkeeping of Failures to Meet a Standard | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7842(a)(2)-(4) for recordkeeping of (1) date, time, and duration of failure to meet the standard; (2) listing of affected source or equipment, and an estimate of the quantity of each regulated pollutant emitted over the standard; and (3) actions to minimize emissions and correct the failure. |
| §63.10(b)(2)(iii) | Maintenance Records | Yes | |
| §63.10(b)(2)(iv) | Actions Taken to Minimize Emissions During SSM | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7842(a)(4) for records of actions taken to minimize emissions. |
| §63.10(b)(2)(v) | Actions Taken to Minimize Emissions During SSM | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7842(a)(4) for records of actions taken to minimize emissions. |
| §63.10(b)(2)(vi) | Recordkeeping for CMS Malfunctions | Yes | |
| §63.10(b)(2)(vii)-(ix) | Other CMS Requirements | Yes | |
| §63.10(b)(2)(xiii) | CMS Records for RATA Alternative | No | |
| §63.10(c)(7)-(8) | Records of Excess Emissions and Parameter Monitoring Exceedances for CMS | No | Subpart FFFFF specifies record requirements; see §63.7842. |
| §63.10(c)(15) | Use of SSM Plan | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | |
| §63.10(d)(5)(i) | Periodic SSM Reports | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | See §63.7841(b)(4) for malfunction reporting requirements. |
| §63.10(d)(5)(ii) | Immediate SSM Reports | No, for new or reconstructed sources which commenced construction or reconstruction after August 16, 2019. For all other affected sources, Yes on or before January 11, 2021, and No thereafter | |
| §63.10(e)(3) | Excess Emission Reports | No | Subpart FFFFF specifies reporting requirements; see §63.7841. |
| §63.11 | Control Device Requirements | No | Subpart FFFFF does not require flares. |
| §63.12 | State Authority and Delegations | Yes | |
| §63.13-§63.16 | Addresses, Incorporations by Reference, Availability of Information and Confidentiality, Performance Track Provisions | Yes |
Climate change is no longer an impending threat. It is here and it has tangible consequences. As industry professionals, we understand the scientific basis for climate change and its anticipated effects.
However, translating this knowledge into actionable strategies requires robust risk planning. A recent survey conducted by the J. J. Keller Center for Market Insights revealed that only 20 percent of facilities currently engage in risk planning related to climate change.
Related article: 5 easy ways to keep up with environmental regulatory changes
Several factors impact how and why a business plans for environmental risk.
Business continuity: The domino effect of climate disruption
Among facilities currently engaged in risk planning for climate change, 65 percent consider business continuity. Extreme weather events are becoming more frequent and severe due to climate change. Floods, droughts, wildfires, and heat waves can disrupt critical infrastructure, transportation networks, and power grids. This can have a cascading effect, impacting a company's ability to operate and deliver products or services.
Proactive risk planning involves identifying vulnerabilities in your operations — from reliance on suppliers in one area to outdated infrastructure vulnerable to flooding. By conducting vulnerability assessments and developing contingency plans, businesses can ensure a smoother transition during climate disruptions, minimizing downtime and financial losses.
Brand image and reputation: The price of inaction
More than ever before, consumers are holding organizations responsible for their environmental impact. Failure to act on climate change can significantly hurt an organization's brand image and reputation. Ignoring climate risks suggests a lack of foresight and responsibility, potentially leading to boycotts, negative press coverage, and difficulty attracting employees.
Conversely, demonstrating proactive risk planning by implementing sustainable practices and adapting to climate change can enhance a company's brand image. It can translate to increased customer support, greater investor trust, and an edge in the marketplace.
Physical damage to facilities and assets: Counting the cost of climate
Among facilities currently engaging in risk planning for climate change, 59 percent consider physical damage. Climate change is a physical threat to businesses, with increased and often extreme geological and meteorological events posing a risk to facilities and assets. Rising sea levels threaten coastal locations, while extreme weather events can damage infrastructure and equipment.
Risk planning helps identify these exposures and develops strategies to mitigate them, such as elevating critical infrastructure, investing in flood-protection measures, or expanding production facilities across different geographic areas. By proactively addressing physical risks, businesses can minimize the financial burden of repairs and potential reconstruction efforts.
Regulatory risk and noncompliance costs: Staying ahead of the curve
Governments are progressively implementing regulations to address climate change. These regulations may include stricter environmental standards for production processes and mandatory reporting of greenhouse gas emissions. Businesses that fail to adapt their operations to comply with necessary regulations risk hefty fines and legal penalties.
Risk planning involves staying informed about evolving regulations and developing a strategy for compliance. This can help businesses easily adapt and avoid costly fines or even operational shutdowns.
Revenue loss: The bottom line of climate change
Climate change can negatively impact an organization's revenue stream in several ways. For example, droughts and floods can disrupt farming operations and supply chains, leading to shortages and price increases that can discourage customers. Severe weather events can damage attractions and infrastructure, impacting the tourism and hospitality industries. Additionally, regulations intended to address climate change, such as carbon taxing, can impact production costs, potentially leading to price increases and reduced end user demand. Risk planning helps businesses identify these potential losses and create approaches to mitigate them. Examples include investing in climate-smart agriculture, developing drought-resistant crops, or exploring alternative production methods that are less susceptible to climate disruptions.
Key to remember: Climate change is a complex challenge. Risk planning can help businesses protect their operations, reputation, and financial well-being.
The U.S. Environmental Protection Agency (EPA or Agency) is finalizing facility response plan requirements for worst case discharges of Clean Water Act (CWA) hazardous substances for onshore non-transportation-related facilities that could reasonably be expected to cause substantial harm to the environment by discharging a CWA hazardous substance into or on the navigable waters, adjoining shorelines, or exclusive economic zone.
DATES: This final rule is effective on May 28, 2024, published in the Federal Register, page 21924.
View final rule.
| Part 118—Clean Water Act Hazardous Substances Facility Response Plans | ||
| Entire part | Added | View text |
| §300.185 Nongovernmental participation. | ||
| (a) | Revised | View text |
| §300.211 OPA facility and vessel response plans. | ||
| (c) | Revised | View text |
| §300.411 Response to CWA hazardous substance worst case discharges. | ||
| Entire section | Added | View text |
New Text
§300.185 Nongovernmental participation.
(a) Industry groups, academic organizations, and others are encouraged to commit resources for response operations. Specific commitments should be listed in the RCP and ACP. Those entities required to develop tank vessel and facility response plans under CWA section 311(j) must be able to respond to a worst case discharge to the maximum extent practicable, and shall commit sufficient resources to implement other aspects of those plans in accordance with the requirements of 30 CFR part 254, 33 CFR parts 150, 154, and 155; 40 CFR parts 112 and 118; and 49 CFR parts 171 and 194.
* * * * *
§300.211 OPA facility and vessel response plans.
* * * * *
(c) For non-transportation-related onshore facilities, these regulations are codified in 40 CFR 112.20 and 40 CFR part 118;
* * * * *
The Environmental Protection Agency (EPA or the Agency) is issuing this final rule under the Toxic Substances Control Act (TSCA) to address to the extent necessary the unreasonable risk of injury to health presented by chrysotile asbestos based on the risks posed by certain conditions of use. The injuries to human health include mesothelioma and lung, ovarian, and laryngeal cancers resulting from chronic inhalation exposure to chrysotile asbestos.
DATES: This final rule is effective on May 28, 2024, published in the Federal Register March 28, 2024, page 21970.
View final rule.
| Subpart F—Chrysotile Asbestos | ||
| Entire subpart | Added | View text |
The Environmental Protection Agency (EPA or the Agency) is proposing to require manufacturers (including importers) of 16 chemical substances to submit copies and lists of certain unpublished health and safety studies to EPA. Health and safety studies sought by this action will help inform EPA's responsibilities pursuant to TSCA, including prioritization, risk evaluation, and risk management.
DATES: This proposed rule is published in the Federal Register March 26, 2024, page 20918.
View proposed rule.
An EPA final rule to designate two PFAS as hazardous substances under CERCLA is expected this year! However, a recent “standing-room-only” Senate hearing evaluated potential consequences of the rule. Much of the discussion was over liability concerns for “passive receivers” like water utilities and landfills. The upcoming rule may not provide EPA with flexibility to exempt them.
During the March 20 hearing, Senator Tom Carper (D-DE), Chairperson of the Senate Environment and Public Works Committee, made it a point that PFAS has proved useful. It has even saved many lives as a firefighting foam, he asserted. PFAS is short for per- and polyfluoroalkyl substances.
It’s also a pervasive threat to human health, Carper concluded. A health advocate who testified at the hearing warned the Senators that failing to address PFAS in drinking water will result in thousands of deaths. Cause-of-death examples he said include cancer, cardiovascular disease, and infant low birth weights.
Carper also brought up the millions of dollars to cleanup PFAS. He said it costs only $50 to $1,000 per pound to manufacture PFAS products. Yet, it costs $3M to $18M per pound to remove the compounds from wastewater, the lawmaker remarked. Testimony suggested that legal defense costs and technology investments can also add up to several million dollars for a facility.
Everyone at the hearing agreed that EPA has never “directly” listed a substance as a CERCLA substance under 40 CFR 302. CERCLA is the Comprehensive Environmental Response, Compensation, and Liability Act.
Looking back, EPA has always defined CERCLA hazardous substances that were first regulated by other laws, like the Clean Water Act (CWA) or the Resource Conservation and Recovery Act (RCRA). In this case, PFAS compounds would be declared CERCLA hazardous substances. However, that would be without regulation under another law. This is called “CERCLA first.”
That means, for example, there are no federal water or waste permits for PFAS (yet) that could provide an exemption (or liability shield) from CERCLA. Moreover, CERCLA is retroactive. So, even if PFAS is covered by later permits, parties may be responsible for PFAS released to water, air, or land before the issuance of a permit.
The Cabinet Secretary for the New Mexico Environment Department testified that he favors listing discarded PFAS as a RCRA hazardous waste first.
Carper stated that he has heard mounting concerns about the potential unintended impacts of the impending designation rule. Specifically, he mentioned entities that do not use these chemicals. The lawmaker explained that they could be held responsible for downstream PFAS contamination simply because the contamination traveled through their facility.
Senator and Ranking Member Shelley Capito (R-WV) agreed. “If an entity meets the definition of a ‘potentially responsible party,’ that entity is liable for all cleanup costs, regardless of intent or exercise of due care ... These entities are known as ‘passive receivers.” [They] did not manufacture or generate PFAS and were unknowingly, or required by law, to catch or to receive these contaminants.”
A legal expert for the Congressional Research Service revealed that under the upcoming rule, “Entities that have been involved in releases of PFAS could be held liable if the other preconditions to liability are met and no exemptions apply.” Her examples included:
Some testified that EPA has promised to focus enforcement efforts on PFAS manufacturers and industries that release significant amounts of PFAS. However, someone clarified that EPA would not be bound by this policy. Plus, states, tribes, and private parties can still bring litigation. A landfill association representative added, “If EPA chooses not to take any action, the passive receiver has no protection from a suit brought by another potentially responsible party.”
Both Carper and Capito say they’re looking for a bipartisan legislative response to the concerns. Legislative options might include:
Congress could also go the other way. It could direct EPA to designate various PFAS as hazardous substances under CERCLA.
A recent Senate hearing on PFAS went over liability and a possible liability shield for passive receivers like water utilities and landfills.
Each year, spring ushers in the season for green — budding trees, blooming plants, and the Greenhouse Gas Reporting Program (GHGRP). Okay, so the last item isn’t typically associated with thoughts of springtime. However, the March 31 deadline for the annual GHGRP report always springs up.
The GHGRP requires covered facilities, suppliers, and sites to submit an annual report of greenhouse gas (GHG) emissions data and other relevant information from the previous calendar year to the Environmental Protection Agency (EPA).
Although the reporting years change, the best practices for submitting accurate and timely reports don’t. Use the following checklist to help you comply with the GHG annual report requirements.
The GHGRP generally applies to:
See 40 CFR 98.2 for the complete eligibility requirements that facilities, suppliers, and CO2 injectors must meet to be subject to the GHG annual reporting requirements.
The GHGRP groups reporters by specific industry types known as source categories. Each facility must report GHG emissions for all source categories that apply.
The subparts under Part 98 list the requirements for each source category, such as:
For example, Part 98 Subpart Q lists the reporting requirements for the Iron and Steel Production category, while Part 98 Subpart MM lists the requirements for the Suppliers of Petroleum Products category.
Tables A-3, A-4, and A-5 under Part 98 Subpart A list the source categories and any specific reporting thresholds.
Reporters must use specific methodologies established in the regulations to determine GHG emissions from each source category. The subparts under Part 98 contain the approved calculation methodologies for each source category and generally include several options.
If you meet the method’s requirements, you can change emission calculation methods from year to year and within the same year.
A designated representative is the individual responsible for submitting the GHG reports on behalf of the owners and operators of the facility or supplier. The regulations at 98.4 allow (but don’t require) the designated representative to appoint an alternate designated representative and one or more agents who can act on their behalf.
EPA will not accept annual GHG emissions reports from a facility or supplier without a Certificate of Representation for a designated representative. This certificate must be submitted at least 60 days before the initial annual GHG emissions report deadline.
The GHG annual report is submitted electronically through EPA’s electronic Greenhouse Gas Reporting Tool (e-GGRT). To submit the annual report, you must first register as an e-GGRT user and create an account. If you already have an active EPA Central Data Exchange (CDX) account, log into e-GGRT with your existing CDX username and password. If you don’t have a CDX account or cannot log in with those credentials, create a new user account on e-GGRT.
All e-GGRT users must have the Electronic Signature Agreement (ESA) on file with EPA. You can electronically sign the agreement or submit a signed hard copy. Once EPA approves the agreement, the agency will send you an account activation notice, and you can begin registering a facility and the designated representative.
The designated representative must register as an e-GGRT user and, once EPA approves the representative’s ESA, accept the appointment as the designated representative and electronically sign the Certificate of Representation. The certificate allows the representative to certify, sign, and submit the annual GHG report to EPA. Resubmission of the certificate is required only when there are updates to the facility profile or information about the designated representative changes (including replacing the existing representative).
Key to remember: Annual reports for the Greenhouse Gas Reporting Program are due by March 31 and require reporters to include emissions data from all applicable source categories.
Quick action using cardiopulmonary resuscitation (CPR) and automated external defibrillators(AEDs) can save the lives of the nearly 350,000 cardiac event victims each year outside of a hospital setting. But what does OSHA require for the workplace? What you didn’t know about OSHA regulations regarding AEDs may surprise you.
For every minute a patient is in cardiac arrest, their chances of survival decrease dramatically. When a patient doesn’t have a pulse and isn’t breathing, CPR should be performed until an AED is available. It’s important to note that CPR alone does not restart the heart. CPR is an oxygen circulation procedure. AEDs, on the other hand, are meant for lifesaving intervention.
CPR and early defibrillation are vital components of the emergency medical services (EMS) chain of survival that increases the odds of cardiac patient survival. However, according to the American Heart Association (AHA), even the best CPR can’t provide enough circulation of oxygen to the brain and heart for more than a few minutes. In fact, a patient whose brain is deprived of oxygen for 10 minutes or more seldom recovers.
Just like a reliable vehicle, the circulatory system is the human body’s blood transportation system, and the heart is the engine. Amazingly, the heart generates its own electrical impulses, pumping in a regular, rhythmic manner. As with any engine, the heart requires a certain amount of pressure to function and doesn’t work well when clogged with grease or debris. The most common causes of sudden cardiac arrest include a heart attack, electrocution, and asphyxiation — all of which could occur in the workplace. Common signs and symptoms include:
CPR provides the pressure for the body’s “engine” to oxygen circulating, while an AED provides the electrical impulses to keep the engine pumping.
OSHA 1910.151 requires first aid treatment be provided in the absence of an infirmary, clinic, or hospital in near proximity to the workplace used to treat injured employees. This may include assisting a victim of cardiac arrest using CPR or defibrillation.
OSHA requirements for CPR and defibrillation differ considerably. Standards requiring CPR include:
OSHA recommends basic adult CPR refresher training and retesting every year, and first aid training at least once every three years. CPR training include facilitated discussion along with ’hands-on’ skills training that uses mannequins and partner practice.
Though OSHA recognizes AEDs as important lifesaving technology that plays a role in treating cardiac arrest, the agency doesn’t currently require their use in the workplace. Instead, OSHA wants employers to assess their own requirements for AEDs as part of their first aid response.
AEDs are considered Class III medical devices which means the Food and Drug Administration (FDA) has some oversight on their use. Almost all AEDs require the purchaser to obtain a prescription from a physician under FDA regulations. The prescription process is meant as a quality control mechanism to ensure AEDs are properly maintained, that all designated responders are properly trained, and assist employers with establishing an emergency response plan for their workplace AED program.
The AHA requires AED operators to also receive CPR training as an “integral part of providing lifesaving aid to people suffering sudden cardiac arrest.” Though easy to use, each AED is slightly different, so training helps users understand the unique traits and supplies for the individual units at their workplace. Additionally, AED users must be trained to understand the signs of a sudden cardiac arrest, when to activate the EMS system, and how to perform CPR.
AEDs are light, portable, easy to use, and inexpensive. They’re best placed near high-hazard areas such as confined spaces, near electrical energy, or in remote work areas. Response time to reach AEDs should be kept within 3–5-minutes.
| Need more information on defibrillators in the workplace? See our ezExplanation on AEDs. |
Many states require or encourage CPR and AED training from nationally recognized organizations. Any AED training should include CPR training. OSHA doesn’t offer first aid or CPR training, nor certify trainers. Training by a nationally recognized organization, such as AHA, the American Red Cross, or National Safety Council is recommended.
While OSHA doesn’t currently require the use of AEDs in the workplace, they do expect employers to assess their own AED requirements as part of their first aid response. AED training is required by most states and should include CPR with a hands-on practical component.
OSHA requires employers to provide all workers with immediately available and sanitary restroom or toilet facilities. But does this include truckers and delivery drivers that stop at your facilities? The sanitation standards (1910.141, 1926.51, and 1928.110) are meant to protect all workers from adverse health effects from unsanitary toilets facilities, or the unavailability of facilities when needed.
Bipartisan legislation has recently been introduced in the House that would require businesses to provide restroom access to truckers who are loading or delivering cargo at their warehouses, manufacturers, distribution centers, retailers, and ports.
Supported by leading organizations in the trucking industry, the Trucker Bathroom Access Act (H.R. 9592) was introduced on Dec. 15, 2022. The bill requires retailers, warehouses, and other establishments with existing restrooms to provide access to drivers who are loading or delivering cargo. Additionally, operators of ports and marine terminals must provide access for drayage and parking while accessing such restrooms.
This amendment to Title 49 would exempt some employers from the bill including filling and service stations, and restaurants 800-square feet or smaller with restrooms intended for employee use only. The bill doesn’t require employers to construct new restrooms but to give truck drivers the same access as employees or customers.
Commercial truckers and delivery drivers are the lifeline of our supply chain of supplies, products, and consumables. Working tirelessly all hours, during holidays and weekends, and throughout the pandemic, they continue to deliver critical food and emergency supplies to companies everywhere. Employers have the privilege of demonstrating gratitude to truckers and delivery drivers with a positive work environment.
The benefits of allowing truckers and delivery drivers the convenience and safety of readily available, sanitary restroom facilities are plenty. They’re able to rest and reset when necessary, which keeps them and others safer on the roads. Equally important, restroom availability prevents drivers from having to search for available facilities elsewhere, allowing them to keep a timely delivery schedule, limit supply chain delays, and ultimately lower costs for employers and customers.
The proposed Trucker Bathroom Access Act will require retailers, warehouses, and other establishments with existing restrooms to provide access to truckers and delivery drivers who are loading or delivering cargo. Access to restrooms keeps them refreshed and ready to deliver essential supplies to companies across the country.
The Environmental Protection Agency (EPA) is amending the Toxic Substances Control Act (TSCA) regulations for polymers manufactured under the terms of the polymer exemption by extending the submission deadline for reporting. The regulations require that manufacturers (includes importers) of polymers manufactured under the terms of the exemption submit a report of manufacture or import by January 31 of the year subsequent to initial manufacture. On June 7, 2023, EPA amended the exemption reporting requirement to require that the exemption report and accompanying confidentiality claims be submitted electronically. Because EPA experienced technical difficulties with the launch of the new electronic reporting tool, EPA is extending the reporting period for 2024 from January 31 to March 31 to allow manufacturers additional time to submit their reports and accompanying claims to EPA using the electronic reporting tool.
DATES: This final rule is effective on February 16, 2024, published in the Federal Register February 16, 2024, page 12248.
View final rule.
| §723.250 Polymers. | ||
| (f) introductory text | Revised | View text |
Previous Text
§723.250 Polymers.
* * * * *
(f) Exemption report for polymers manufactured under the terms of this section. For substances exempt under paragraphs (e)(1) through (3) of this section a report of manufacture or import must be submitted by January 31 of the year subsequent to initial manufacture. The report and accompanying claims must be submitted via CDX (https://cdx.epa.gov/), using the TSCA Section 5 Notices and Supports—ePMN application. See §720.40(a)(2)(ii) of this subchapter for information on how to access e-PMN software. The notice must include:
* * * * *
Hi everyone! Welcome to the monthly news roundup video, where we’ll go over the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. Let’s get started!
Effective January 15, OSHA penalties increased 3.2 percent for inflation. Most penalties increased to $16,131. Willful and serious violations, however, increased to $161,323.
Construction workers aged 45 and older suffer more severe injuries and higher associated costs than other age groups. Most injuries are due to slips, trips, and falls.
Washington State updated its process safety management rules to better protect workers in petroleum refineries from the hazards of volatile chemicals. The rules take effect December 27, 2024.
Bloodborne pathogens topped the list of OSHA violations for the healthcare industry in 2023. Hazard Communication was the second most cited standard, followed by respiratory protection.
OSHA Region 2 launched a regional emphasis program that targets tree trimming, tree removal, and land clearing operations. Region 2 includes New York, New Jersey, Puerto Rico, and the U.S. Virgin Islands.
EPA continues to strengthen its regulation of per- and polyfluoroalkyl — or PFAS — substances. A new rule prevents facilities from using any of the 300+ inactive PFAS before EPA conducts a risk determination and, if necessary, regulates the activity.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
The Environmental Protection Agency (EPA) is proposing a regulation to implement the requirements of the Clean Air Act (CAA) as specified in the Methane Emissions Reduction Program of the Inflation Reduction Act. This program requires the EPA to impose and collect an annual charge on methane emissions that exceed specified waste emissions thresholds from an owner or operator of an applicable facility that reports more than 25,000 metric tons of carbon dioxide equivalent of greenhouse gases emitted per year pursuant to the petroleum and natural gas systems source category requirements of the Greenhouse Gas Reporting Rule. The proposal would implement calculation procedures, flexibilities, and exemptions related to the waste emissions charge and proposes to establish confidentiality determinations for data elements included in waste emissions charge filings.
DATES: This proposed rule is published in the Federal Register January 26, 2024, page 5318.
View proposed rule.
Safety has the workforce brimming with color. In fact, 29 CFR 1910.144 and 1910.145 tell us precisely what OSHA expects for safety color coding to identify hazards in the workplace. Signs, warning labels, symbols, and other color coding in your facilities should have your employees seeing red. But what if they can’t?
Though rare, color blindness is the inability to distinguish between colors as most people do. This makes it difficult for workers to see colors intended to protect them from harm. Color blindness can vary, making it difficult to distinguish between red and green or blue and yellow hues - the very shades of safety.
Some individuals can’t see any colors, which is called monochromacy. Workers with this type of color blindness may have trouble seeing clearly and may be more sensitive to light. Employers must collaborate with these employees to ensure alternative measures are taken to protect their eyes and clearly communicate warnings and hazards.
The color identifiers below differentiate the various levels of risk and hazards for workplace safety. Employers must ensure workers with color blindness are able to understand hazards in the workplace and the meaning of signs and warning labels.
RED - identifies fire and fire protective apparatus, danger, and emergency stops. It marks areas near open flames or flammable materials, fire extinguishers, and where workers are directed to stop an action.
ORANGE - warns workers of hazardous parts of equipment that could physically harm people or the facility. Typically used as labels on machinery, orange may also be used on signs, hard hats, safety vests, and other objects.
YELLOW - designates caution and is used for marking physical hazards, such as falling, pinch points, contact hazards, and other similar hazards.
GREEN - identifies directional safety information. This includes pointing workers to emergency egresses, safety showers or eyewash stations, first aid stations, and other safety equipment.
BLUE - not always safety-related, provides information regarding a particular location, process, or item. Employers may use blue signs to convey workplace policies, instructions, or locations, such as “Employees Only.”
PURPLE - often combined with yellow, alerts workers to radiation hazards.
BLACK/WHITE - provides instructional and directional information. This includes speed limits, one-way traffic, and aisle markings.
Having a standardized color-coding system for safety is effective for alerting employees of workplace hazards - if they can see the colors properly. For those who can’t, employers must ensure these workers understand the hazards and warning signs throughout the workplace.
| Interested in learning more? See our ezExplanation on Color Coding. |
Not only are employers required to ensure workers understand warning signs and colors, but they must also protect workers from becoming color blind. That’s right - color blindness can be acquired. Exposure to lead or carbon disulfide can cause color blindness, even at low levels. Terminal illness and alcohol consumption can also contribute to color blindness, so employers should promote health as part of their safety and health programs.
Color blindness is considered a disability according to the Americans with Disabilities Act (ADA). Employers are required to reasonably accommodate employees with disabilities.
Employers must ensure employees with color blindness are able to understand hazards in the workplace and the meaning of signs and warning labels. The ADA requires employers to make reasonable accommodations for workers with disabilities, including color blindness.
The 150 air-mile exemptions, which are in the regulations at 395.1(e)(1) and (2), allow a driver to use a time record in place of a log, provided that certain conditions are met. While this is possibly the most widely used hours-of-service exemption, it may be the most commonly misused exemption, as well.
To be able to use this logging exemption in 395.1(e)(1), the driver must:
The company must retain the time record and have it available for inspection for six months.
| Need more info? View our ezExplanation on the 150 air-mile exception. |
If the driver cannot meet the terms of the exemption (he or she goes too far or works too many hours), the driver must complete a regular driver’s log for the day as soon as the exemption no longer applies.
If the driver has had to complete a log 8 or fewer days out of the last 30 days, the driver can use a paper log for the day. If the driver had to complete a log more than 8 days out of the last 30 days, the driver needs to use an electronic log for the day (unless one of the ELD exemptions applies, such as operating a vehicle older than model year 2000).
When a property-carrying driver is operating under the 150 air-mile exemption, the driver is also exempt from having to take the required 30-minute break (see 395.3(a)(3)(ii)).
If the driver began the day as a 150 air-mile driver and has driven more than 8 consecutive hours without a break, and something unexpected happens and the driver can no longer use the 150 air-mile exemption, the driver must stop and immediately take the 30-minute break as well as start logging. If the driver went outside of the 150 air-mile area before the driver had 8 hours of driving without a break from driving, the driver would be expected to take the break at the appropriate time.
Here are some of the common myths and misunderstandings about the 150 air-mile exemption:
The 150 air-mile exemption at 395.1(e)(2) only applies to drivers that: Operate property-carrying vehicles that do not require a CDL to operate, and Stay within the 150 air-miles of their work reporting location.
If the driver stays within the 150 air-mile radius of the work reporting location, and returns to the work reporting location within 14 hours on 5 of the last 7 days, and 16 hours on 2 of the last seven days, the driver is allowed to use a time record in place of a log.
If the driver does not meet the terms of the exception, the driver will need to complete a log for the day. If the driver had to log more than 8 days out of the last 30 days, the driver will need to use an electronic log for the day. All of the other issues discussed above would apply to these drivers as well.
If you have drivers that use these exemptions, you will need to check time records to make sure they are complying with the appropriate time limits. You will also need to check movement records to verify that the drivers using these exemptions are staying within the mandated area (within 150 air-miles of the work reporting location for the day).
If a driver is over the hours limit, or has gone too far, you need to verify that the submitted a log for the day, either paper or electronic, depending on how many days the driver had to log out of the previous 30 days.
During an audit, if it is discovered that your drivers are using these exemptions incorrectly, you will be cited for not having drivers’ logs when required. Each day this occurred will be another violation, so the fine could be rather large if you are not managing the use of these exemptions!
When drivers fail a DOT test or engage in other prohibited drug or alcohol behavior, their commercial driving careers are stalled until specific steps in rehabilitation and treatment are completed.
| Read our FAQ: What happens if a DOT return-to-duty or follow-up test is canceled? |
A commercial driver is required to go through the DOT return-to-duty process if:
Actual knowledge occurs when information is provided to the motor carrier indicating a DOT testing violation. This might be learned through:
When a motor carrier learns of a testing violation on a new hire, it must obtain proof that the driver has completed the return-to-duty process. Otherwise, the motor carrier would have to begin the return-to-duty process or pick up where it left off.
To resume a safety-sensitive function, the driver must complete the following return-to-duty steps in Subpart O of Part 40.
When drivers engage in prohibited drug or alcohol behavior, they must be immediately removed from performing all safety-sensitive functions. If on a dispatch, a driver must be told of the test result and instructed to park the vehicle. This notification often involves making arrangements to get the driver home and continue the run with different driver.
The employer must present the driver with a list of substance abuse professionals (SAPs) who have the appropriate credentials and DOT training to perform driver evaluations. The list must be given without a fee and made available to the driver (or driver applicant) whether or not the carrier retains the driver.
If the motor carrier does not have another face-to-face meeting with the driver, this list may be mailed or emailed to the driver.
For drug test results, the medical review officer (MRO) will report the violation to the Clearinghouse. This includes shy bladder scenarios without a valid medical explanation.
Failed alcohol tests (.04 or greater BAC), actual knowledge, and certain refusal to test scenarios are reported by the motor carrier to the Clearinghouse.
After the SAP list is given to a driver, the motor carrier cannot force a driver to begin the process. Nevertheless, to resume safety-sensitive functions, the driver must seek a face-to-face evaluation from a qualified SAP as a first step.
Payment of the evaluation is not required of the employer. Instead, it is based on labor-management agreements and healthcare benefits.
The SAP’s referral to an education and/or treatment program is based on a clinical evaluation of the driver during the face-to-face meeting. The SAP should have a working knowledge of what programs and counselors are available.
The SAP may take into consideration the driver’s ability to pay and insurance coverage. Once a SAP-approved provider has been agreed upon with the driver, the SAP will facilitate the referral and provide the program with the diagnostic determinations that led to the treatment plan. Programs range from outpatient treatment to partial or full in-patient resources.
Once the treatment plan has ended, the SAP will determine if it was a success. This decision is based on information provided by the education and/or treatment program and another face-to-face evaluation with the driver.
This second evaluation will result in one of three determinations:
If the SAP is satisfied with the driver’s ability to return to driving, the SAP will issue a report to the designated employer representative (DER). This report will list any continuing treatment and education, if required, and the number of DOT follow-up drug and/or alcohol tests required in a given time frame. The driver will be required to have a minimum of six unannounced follow-up tests in the first 12 months following the return to a safety-sensitive function. The SAP may require follow-up testing for up to five years.
For all Part 382 violations occurring since January 6, 2020, the SAP is required to report the successful completion of the evaluation and treatment to the Clearinghouse, provided the driver has designated the SAP in the driver’s personal Clearinghouse account.
The DER must wait for the go-ahead in the SAP report before sending the driver for the return-to-duty drug and/or alcohol test. All return-to-duty drug tests are performed under direct observation. The motor carrier must report a negative return-to-duty test to the Clearinghouse. In order for the “prohibited” status to be lifted from the driver’s record, both the SAP and motor carrier submission must be entered onto the driver’s record.
Once the Clearinghouse is no longer showing an unresolved testing violation, the driver can return to a safety-sensitive function.
Editor’s Note: Violations occurring prior to January 6, 2020, are not tracked in the Clearinghouse. Instead, the motor carrier would use the SAP report as a green light to perform the return-to-duty test, and once the negative test result is received, the driver can resume a safety-sensitive function.
After the driver returns to safety-sensitive functions, the motor carrier must carry out the unannounced follow-up tests under direct observation as prescribed in the SAP report. The DER must ensure that the tests do not have any discernible pattern.
The follow-up tests are in addition to any other DOT-required tests (e.g., random, post-accident). For instance, you cannot use a follow-up test as a substitute for a random test or vice versa.
If the driver leaves the motor carrier prior to the completion of the very last follow-up test, the next employer(s) must pick up where the process left off.
When the follow-up program is complete, the motor carrier under whose program the last test was performed must report this to the Clearinghouse. If the violation predates the Clearinghouse, the employer does not report the completed follow-up program to anyone.
If the motor carrier fails to begin or continue with a driver’s DOT return-to-duty process and follow-up testing, it is an acute violation that could cost the company up to $15,876. Allowing this driver to operate a CMV puts the carrier at risk of negligent entrustment claims if there is a crash.
The FMCSA is planning to test the effects of letting commercial truck drivers “pause” their 14-hour on-duty limit by up to 3 hours per day.
The agency is hoping to enlist up to 400 drivers to participate in its three-year “Split Duty Period Pilot Program.” Participants would be allowed to use one off-duty break of between 30 minutes and 3 hours to pause the 14-hour driving window, as long as they take 10 consecutive hours off duty at the end of the day. The pause should enable drivers to reduce fatigue, avoid congestion, reduce the pressure to speed, and be more productive, the FMCSA says.
Normally, short breaks taken during a driver’s day must be subtracted from the driver’s 14-consecutive-hour window during which driving is allowed.
| For more information, see our ezExplanation on the 14-hour on-duty rule. |
Under new rules in effect on September 29, 2020, some truck drivers can pause their 14-hour limit with a break of 2 hours or more, but only if they also spend at least 7 hours in a sleeper berth (see below). Under the pilot program, drivers could pause the clock with off-duty time alone, without the need for a sleeper berth. This idea was proposed back in 2019 but didn’t find its way into the recent rule changes because the FMCSA didn’t have enough data to justify it.
As required by law, the FMCSA is gathering public input on the proposal until November 2nd. It will then decide whether to implement the program. After the program concludes, the agency will need to report to Congress on its findings before it could proceed with any changes to the hours-of-service regulations.
Participation in the pilot program would be limited to between 200 and 400 commercial driver’s license (CDL) holders from companies of all sizes, with each driver participating for up to one year. Motor carriers that want to enroll in the program will need to apply via an FMCSA website which could be available late this year. Comments on the proposal may be submitted online at www.regulations.gov under docket number FMCSA-2020-0098.
Truck drivers who fall under the federal hours-of-service rules can already pause their 14-hour clock with a short rest break, as of September 29, 2020 (see log image). This is known as the “split sleeper-berth” option, and it works like this:
Key to remember: The FMCSA plans to test the safety of allowing truck drivers to pause their 14-hour clock with a rest break of up to 3 hours, even if they don’t have a sleeper berth. The pilot program could open later this year.
As of January 1, 2023, heavy-duty trucks and buses with engines from model years 2007 to 2009 operating in California must be either:
This is the final step in the phase-in of the Truck and Bus regulation. Covered vehicles originally built with engines older than model year 2006 must have already been replaced or retrofitted.
| Need more on CMV maintenance? See our ezExplanation Inspection and Maintenance. |
One issue that has come up is new vehicles are not available for delivery before January 1, 2023. This means fleets that operate in California that cannot get a replacement vehicle will be faced with the choice of either retiring their 2007 to 2009 engine vehicles without a replacement or operating in violation as of January 1, 2023.
However, the California Air Resources Board (CARB), the agency that oversees the Truck and Bus program, is aware of the situation and has provided an exemption. If the company has a written contract to purchase a new vehicle to replace a vehicle with a 2007 to 2009 engine in place before September 1, 2023, the existing vehicle can be operated until the replacement is placed in service.
To use this exemption, the company must register in CARB’s Truck Regulation Upload, Compliance, and Reporting System (TRUCRS) and report the use of the exemption. This is especially important to California-based companies as vehicle registration is tied to compliance with the Truck and Bus regulations requirements.
As of January 1, 2023, California will be placing roadside emissions monitoring devices (REMD) at locations around the state. These devices check emissions on any vehicle that passes it. If the device determines a vehicle may be a high emitter, the owner will receive a Notice to Submit to Testing. This will require the vehicle to be brought in to a “referee” location where the emissions and emissions components can be inspected.
These Truck and Bus requirements go into effect on January 1, 2023, and only apply to vehicles operating in California.
Most motor carriers review their roadside inspection reports for the obvious reasons: fixing mechanical defects and identifying unsafe or noncompliant driver behavior.
Some violations are easy to decipher, such as a burned-out light bulb or exceeding the speed limit by a specific range. Others take a little more to figure out, such as doing the math to determine when and how a driver exceeded hours-of-service (HOS) limits. Then there are all those 392.2 violations with suffixes. Some count against a carrier’s Compliance, Safety, Accountability (CSA) scores, while others do not, depending on whether they contribute to causing a crash.
One that often baffles motor carriers is 392.2C.
Section 392.2C is enforcement’s code for “failure to obey traffic control device.” The C stands for control.
The citation appears in the severity table for the Unsafe Driving BASIC (Behavior Analysis and Safety Improvement Category). The violation has been assigned a value of 5 out 10, with 10 being the most severe. The violation is used when calculating both the carrier’s and driver’s Unsafe Driving BASIC scores.
In most instances, the traffic control device is not a signal light or stop or yield sign. Rather, it is the sign that instructs the driver to pull into a weigh station.
| View our Weigh Stations ezExplanation for additional information. |
The vehicles that must stop at scales and inspection locations vary from state to state and even from location to location within a state. The “weigh scale ahead” or similar sign should be the driver’s guide.
If the sign reads:
Often those who operate commercial vehicles not requiring a commercial driver’s license, such as a large pickup truck or small box truck, mistakenly believe weigh scale inspections are just for larger rigs.
If a driver goes past a weigh station without pulling in as directed by a traffic control device, enforcement will pursue and pull over the driver. The officer will then escort the driver back to the weigh station for a roadside inspection.
Even if the driver was honestly confused whether the sign applied to the vehicle, it is too late. And more than likely enforcement’s interest has been piqued. It is highly unlikely the driver will be waived through at this point, and 392.2C will be entered on the roadside inspection report.
CSA’s enforcement model suggests finding the root cause of roadside inspection violations to prevent future occurrences and ultimately improve BASIC scores.
A violation of 392.2C may have one of several root causes, such as:
Whatever the reason, it must be addressed with the driver. Corrective actions range from refresher training to termination. If the driver was trying to avoid enforcement for other reasons (drugs, alcohol, over HOS limits), these other violations need to be addressed accordingly.
Key to remember: Failing to obey a traffic control device will be used in calculation of the CSA Unsafe Driving BASIC scores. Motor carriers should address the root cause of the violation so it does not recur.
A workplace safety definition for “safety-sensitive position” may lead some motor carriers to mistakenly put employees who don’t qualify in their DOT drug and alcohol testing program.
The Federal Motor Carrier Safety Administration (FMCSA) clearly defines a safety-sensitive position.
It is one where the employee is expected to operate a commercial motor vehicle (CMV) requiring a commercial driver’s license (CDL). Only these drivers can be placed in the motor carrier’s DOT drug and alcohol testing program under 49 CFR Part 382.
As a result, a carrier would not classify a forklift operator, driver helper, and other positions as safety sensitive for purposes of testing under Part
A driver who operates an FMCSA-regulated vehicle that does not require a CDL fits within the scope of workplace safety-sensitive duties, but not FMCSA.
For property-carrying vehicles, a non-CDL CMV is one that is:
For passenger carriers, a non-CDL CMV is designed to transport 9-15 passengers, including the driver, for compensation.
Even though the above vehicles and drivers are subject to the bulk of FMCSA’s safety regulations, the vehicles (and subsequently the drivers) do not qualify for CDL licensing or FMCSA testing.
If the driver happens to hold CDL, it still does not qualify as a safety-sensitive position. Applicability is always based on whether the employee is assigned to operate a CDL CMV.
Non-CDL CMV drivers are prohibited from operating while impaired under 49 CFR 392.4 and 392.5, but there is no testing mechanism under DOT authority. Testing would be best practice (non-DOT) and managed under the workplace drug program.
If a motor carrier mistakenly uses the workplace criteria for its DOT testing, the number and types of positions placed in the random pool far exceed commercial drivers.
For the general workforce, the term “safety sensitive” has been tossed around, but never clearly defined by OSHA (Occupational Safety and Health Administration). Many safety professionals tie the term to OSHA’s General Duty Clause (GDC), which requires that employers provide all workers with a safe and healthful workplace.
Specifically, the GDC requires employers to recognize hazards that cause or likely will cause death or serious physical harm. Any job title that is likely to cause death or serious harm to someone — including the employee, coworkers, or the general public — is usually put on a list of safety-sensitive positions.
The employer must look at each job’s hazards and decide if the position is classified by its organization as safety sensitive. Examples may include:
Even someone who works as a roofer may be considered a safety-sensitive position because the employee could trip and fall from a high elevation, causing serious personal harm.
Key to remember: When assembling the list of names for your DOT testing program, only include those individuals who are expected to operate a CDL CMV.
With the labor market still tight, employers might choose to hang onto employees even if they’re underperforming. But what about when complaints are rolling in from different angles? Take, for example, a lackluster supervisor who’s annoying employees and disappointing customers.
An employer could be hesitant to let the supervisor go, especially if there’s no documentation backing up claims of misconduct. The employer must weigh their options to decide if putting the supervisor on a performance improvement plan (PIP) or moving right to termination is the ideal choice.
At-will employment
For starters, in most states employers may terminate an employee at-will, meaning they can fire employees for pretty much any reason as long as it doesn’t discriminate against someone in a protected class based on sex, age, race, religion, etc. Employers also cannot terminate in retaliation for an employee making a claim of harassment, discrimination, or safety concerns.
Aside from these limits, employers can terminate employees for good cause, bad cause, or no cause at all.
PIP or terminate
Deciding whether to put an employee on a PIP or terminate must be decided on a case-by-case basis.
A PIP is usually for job performance issues (hence, performance improvement plan). This could mean anything from not making enough sales to being inept at the job’s essential functions. If job performance doesn’t improve under the PIP, termination may be the end result depending on company policies and practices.
Even if an employee has job performance issues, the employer can terminate without going through the PIP process first, unless the usual process is to implement a PIP with employees who have had similar problems. In that case, not doing a PIP could be seen as discrimination against an employee, especially if the person falls into a protected class.
Workplace misconduct, however, is another situation altogether. This could be anything from a one-off poor joke to pervasive harassment. Snapping at customers or coworkers (or worse), for example, is a conduct issue. An employer could issue a warning or move right to termination if the behavior is clearly illegal or a serious threat to workplace safety.
| Read more: ezExplanation on discharging employees |
Termination tips
If an employer decides to terminate, they should treat the employee as respectfully as possible during the termination process. Also, an employer should carefully and clearly communicate the job-related reasons for the termination to avoid any hint of discrimination. Lastly, an employer should document the reasons and reiterate the steps taken leading up to the termination and keep those records handy in case the employee files a wrongful termination lawsuit.
Key to remember: Employers sometimes struggle when making termination decisions. Having a process in place and documenting steps along the way can help if a case lands in court.
Wage overpayment errors happen for many reasons — from clerical mistakes to payroll system snafus.
Regardless of the reason, employees are not necessarily entitled to keep the extra money, and employers need to know their obligations for recouping it.
Under the federal Fair Labor Standards Act (FLSA), employers don’t need an employee’s permission to recoup wage overpayments. The extra money is seen as a loan or a wage advancement to the employee.
Because of this, employers are generally free to recoup the overpayment from the next paycheck — even if such a deduction cuts into the minimum wage or overtime pay due the employee under the FLSA.
State laws, however, may have greater restrictions. For example, New York employers may only make deductions from an employee’s wages for “an overpayment of wages where such overpayment is due to a mathematical or other clerical error by the employer.”
There are also limitations on the timing and duration, frequency, and method and amount of recovery.
The bottom line is, employers should try to avoid getting themselves into an overpayment situation in the first place. Supervisors should closely review their direct reports’ timesheets to catch errors before paychecks are issued
Also, employees should be encouraged to review their pay stubs for accuracy to help catch mistakes sooner rather than later.
If a mistake happens, employers should do their due diligence to communicate with the affected employees and make a reasonable plan to recoup the funds (provided it’s allowed under state law) so as not to cause any unnecessary financial harm to employees.
Wage overpayment errors can and will occur. Employers need to know their obligations under both federal and state laws before recouping money.
Employers sometimes get tripped up on how to calculate the 1,250 hours worked eligibility criterion when employees need leave under the Family and Medical Leave Act (FMLA).
Does working overtime count toward the 1,250?
Recently, someone asked if overtime hours counted toward the 1,250 hours worked requirement (it does).
All hours actually worked apply to the 1,250, whether overtime or regular time, even if the overtime is not mandatory.
The 1,250 hours is calculated in relation to when the leave will begin, not when the employee puts an employer on notice of the need for leave.
Whether an employee is allowed to work overtime, however, is generally up to company policy. As far as pay goes, remember, if the employee is nonexempt (“hourly”) and works any overtime (mandatory or voluntary) the employee must be paid time and one-half for all hours worked over 40 within the workweek.
More about FMLA leave requirements
To be eligible to take FMLA leave, employees must:
Whether an employee has worked the minimum 1,250 hours is calculated based on determining compensable hours or work under the Fair Labor Standards Act (FLSA).
Calculating the 1,250 hours worked
When it comes to figuring out if an employee has worked at least 1,250 hours, it can get tricky. As was mentioned above, all hours worked, regular and overtime, must be counted.
Hours not worked should not be counted. The “not worked hours” include such time off as vacation time, sick leave, paid or unpaid holidays, or any other time in which an employee isn’t actually working — which can include disability, bereavement, FMLA and other forms of leave.
Once an employee meets the three eligibility criteria, including the 1,250 hours worked, for a particular leave reason, the employee remains eligible for the duration of the 12-month leave year period.
If the employee needs leave for another, different reason, eligibility would be recalculated.
Key to remember: All hours worked must be included in the 1,250 hours criterion when determining whether an employee is eligible for FMLA leave. Hours that aren’t worked (like vacation) are not included.
When an employee is on leave under the Family and Medical Leave Act (FMLA), the employer must maintain benefits under the company’s group health plan.
Thus, employees generally must continue paying their share of the health insurance premiums.
But how do employees pay their share of the premiums when FMLA leave is unpaid? Employers may offer three payment options:
Employers may allow a combination of these options, such as pre-pay for part of the leave and catch-up for the remainder. Below is a breakdown of the three available payment options.
When unpaid FMLA leave is foreseeable, employers may allow employees to pre-pay their premiums. For example, if an employee is adopting a child and requests several weeks for bonding time but does not have enough vacation to cover the entire absence, an employer could allow the employee to pre-pay his or her premiums for the portion of the leave that would be unpaid.
Employers may not require an employee to pre-pay, so this cannot be the only option offered.
If an employee chooses this option, however, employers may collect premiums on a pre-tax basis – with one exception. If the absence will extend into the next tax year (such as leave from December through January), only the premiums for the current tax year may be pre-paid with pre-tax income. The IRS does not allow employees to defer untaxed income from one year to the next.
In this example, the premiums for January could either be pre-paid with after-tax income, or the employee could elect one of the other options (pay-as-you-go or catch-up).
Under the pay-as-you-go option, employees pay their share of the premiums based upon the agreed terms made between the employer and employee. These payments are usually made on an after-tax basis.
For example, the employee might mail in a personal check every two weeks. If the employee fails to send in the checks, or otherwise fails to make payments using the agreed-upon system, the FMLA does allow employers to drop coverage after giving specified notices of non-payment.
Dropping coverage would likely cause some administrative headaches, and some insurers may refuse to do this because the employee would have to be reinstated to the health plan upon return from FMLA leave.
Therefore, employers may prefer to continue coverage by paying the employee’s share of the premiums, then use the catch-up option once the employee returns to work. Some insurance carriers recommend this as an alternative to dropping coverage.
Under the catch-up option, the employer and employee agree that the employee will not pay premiums until he or she returns from leave.
This option might be used when the need for FMLA leave was not foreseeable, such as having to care for a parent who was unexpectedly hospitalized.
To use this option, the employer and employee must agree in advance that:
When the employee returns, the employer collects the current premiums plus any catch-up payments, perhaps taking double premiums, until caught up. Contributions under the catch-up option may be taken on a pre-tax basis.
The IRS regulations indicate that, if the employee chooses the pay-as-you-go option, but fails to make the required payments, you may change to the catch-up option even without the employee’s prior agreement.
Employees on unpaid FMLA leave must still pay their share of health insurance premiums by either pre-paying, paying as they go, or making catch-up contributions upon returning to work.
A new year often begins a new round of employee performance reviews. Since the Family and Medical Leave Act (FMLA) allows eligible employees to take up to 12 (or 26) weeks of leave, many events can occur during an employee’s leave, including the employee’s pre-scheduled performance review. Such reviews might take place on an annual or other scheduled basis. How you treat the timing of those reviews should include some thought.
If, for example, Jo Employee takes 12 weeks of FMLA leave, during which her annual performance review is scheduled, here are some questions to ponder:
Delaying a review
An annual performance review generally takes into consideration a full years’ worth of work. Some employers think it’s best to delay the performance review by the same amount of time an employee took FMLA leave to capture an entire years’ work. This practice, however, might risk running afoul of one of the cornerstones of the FMLA: Returning the employee to his or her position, including the equivalent pay, benefits, and working conditions.
The issues can be particularly concerning if the performance review affects wage increases or other compensation.
What the regulations say
The FMLA regulations indicate that an equivalent position includes equivalent pay, which includes any unconditional pay increases that may have occurred during the FMLA leave period. Equivalent pay also includes bonuses or payments, whether discretionary or non-discretionary. FMLA leave cannot undermine the employee’s right to such pay.
Furthermore, “… employers cannot use the taking of FMLA leave as a negative factor in employment actions, such as hiring, promotions, or disciplinary actions; nor can FMLA leave be counted under no fault attendance policies.” [29 CFR 825.220(c)]
Avoiding a negative factor
Therefore, you would need to look at whether delaying an employee’s performance review could be seen as having a negative factor for the employee.
If, for example, Jo Employee took 12 weeks of leave from April through June, during which she would otherwise have obtained a pay increase in May, but you delayed this increase until September (so you could use a full 12 months of work), you may have violated the equivalent pay provision. If delaying a review creates a new review schedule going forward, the negative impacts could continue.
If, however, a pay increase is conditioned upon seniority, length of service, or work performed, you would grant it in accordance with your policy or practice as applied to other employees on an equivalent leave status for a reason that does not qualify as FMLA leave.
In other words, don’t treat an employee on FMLA leave differently than you would an employee on other forms of leave.
Key to remember: It might be less risky to keep the performance review on schedule and prorate wage increases to account for FMLA leave.
As of January 1, California and Washington have new marijuana laws that restrict testing for the drug.
Both states, which have legalized recreational and medical use, put limits on pre-employment testing for cannabis at the start of the year.
Washington’s rationale for the new law is that a pre-employment marijuana test limits job opportunities for those who use cannabis, because an applicant will test positive for up to 30 days after using it. The new law is designed to prevent the restriction of job opportunities based on an applicant’s past cannabis use.
California’s law notes that a test for marijuana detects past use of the drug, but does not prove whether or not an individual is impaired by it. Its protections are broader than Washington’s, and apply to employees as well as applicants. As a result, they also restrict reasonable suspicion, post-accident, and random marijuana tests.
Testing restrictions in California and Washington
The new state laws take aim at tests measuring nonpsychoactive cannabis metabolites. These metabolites are created by the body after cannabis is smoked or consumed in an edible.
Their presence indicates that cannabis has been used, but the fact that they are present does not prove at a person is impaired by the drug.
The laws do not allow employers to make employment decisions based on marijuana tests that show the presence of nonspychoactive cannabis metabolites. While tests that do not detect these metabolites are technically allowed, from a practical standpoint they are difficult to find.
Current drug tests that detect the presence of THC (the chemical in marijuana causing impairment) are likely to detect nonpsychactive metabolites as well. Other tests that rely on baseline performance to prove impairment would be difficult to use for applicants, as there would be no previous information to use for comparison.
Exceptions to state laws
Both states have exceptions that allow employers to conduct marijuana tests under certain circumstances.
Washington’s restrictions do not apply to:
California’s law allows marijuana tests for:
An employee in the building and construction trades.
Applicants or employees hired for positions that require a federal government background investigation or security clearance in accordance with regulations issued by the U.S. Department of Defense or equivalent regulations applying to other agencies.
Testing conducted under state or federal laws requiring applicants or employees to be tested for controlled substances.
3 steps for employers to take
To make sure workplace drug testing in California and Washington is conducted appropriately, employers should:
Update pre-employment drug testing policies in California and Washington so they comply with the new laws.
Identify positions that are exempt from testing restrictions. When a position qualifies for an exception, make applicants aware that that marijuana testing will be conducted.
Train hiring managers and supervisors about the new requirements. Make sure they understand when marijuana testing is not allowed.
Key to remember: California and Washington have new marijuana testing restrictions that are in place as of January 1. Workplace policies and practices need to be updated to comply with the new laws.
OSHA’s 1910.151(c) standard, Medical Services and First Aid, requires that employers provide emergency eyewashes when employees may be exposed to injurious corrosive materials during the course of their work. Employers have a wide range of eyewash types available to choose from on the market, including portable units (i.e., eyewash bottles). While many employers use bottles, OSHA says that they can’t be the only eyewash made available to employees, and their use should be limited.
The OSHA standard does not provide a great deal of detail on eyewashes for employers. However, where the regulation is silent, OSHA refers employers to the American National Standards Institute (ANSI) standard Z358.1-2009, “Emergency Eyewash and Shower Equipment,” regarding installation, operation, and maintenance of emergency eyewashes. This includes capacity and flushing requirements. The ANSI standard states that an eyewash must deliver 0.4 gallons of flushing fluid per minute for at least 15 minutes.
As such, ANSI says that an eyewash bottle does not meet these criteria; therefore, it can only be used to support eyewashes that do (i.e., plumbed and self-contained units), but cannot replace them.
The reason for this limitation is that eyewash bottles simply cannot provide the required 15 minutes of flushing. Eyewash bottles typically hold less than a gallon of water, which would supply the user with flushing fluid for approximately 1 minute. Even larger self-contained units (those with bladders) that have a capacity of 5 to 10 gallons would only provide maximum use of about 5 minutes.
In other words, eyewash bottles don’t provide an adequate amount of flushing fluid and cannot be considered a primary means of protection.
For this reason, OSHA warns that the use of eyewash bottles should be limited. In a 1986 memorandum to Regional Administrators, the agency states, “In general, squeeze bottles should not be used except where the hazard severity or distance from plumbed eyewash equipment requires personal equipment at work stations for immediate flushing prior to prolonged flushing at a plumbed or self-contained unit.”
In other words, employers can provide eyewash bottles in instances where plumbed or self-contained units can’t reasonably be provided (e.g., an outside yard) in the immediate work area, but only until they can reach a unit which can provide the amount of flushing fluid necessary to flush the eyes for at least 15 minutes.
OSHA expects the employer to determine the level of the potential risk to employees and provide eyewash (and/or shower) protection accordingly. The severity of the hazard(s) involved is a critical consideration when making this determination. In the past, OSHA has said that 1910.151(c)is meant to cover strong acids and alkalis, and the requirement to provide suitable facilities for quick drenching or flushing depends on the exposure and the strength of the hazardous chemical. Chemicals and materials such as household detergents or cleaners, sawdust, metal filings, etc. would not require emergency eyewash (or shower) under the standard.
If an employer determines that an eyewash is needed, then it must meet the provisions set forth in the American National Standards Institute (ANSI) standard Z358.1. The agency uses the ANSI standard as an enforcement tool. This is clarified in a November 1, 2002, Letter of Interpretation, which says, “If OSHA inspects a workplace and finds unsuitable facilities for quick drenching or flushing of the eyes and body, a citation under 29 CFR 1910.151(c)would be issued. When determining whether the eyewash or shower facilities are suitable given the circumstances of a particular worksite, OSHA may refer to the most recent consensus standard regarding eyewash or shower equipment…”
| Need information on eyewash inspections? See our Institute document on Inspections and Maintenance. |
Without the ANSI standard, employers would find it difficult to demonstrate to OSHA exactly how their eyewash and shower units were suitable exclusive to the regulatory language under 1910.151(c) since it’s limited and vague.
Eyewash bottles don’t meet the requirement under 1910.151(c) to provide “suitable” facilities for quick drenching or flushing of the eyes. They cannot be the only eyewash provided in the workplace.
The COVID-19 outbreak created a shortage of latex and nitrile gloves in many workplaces.
Latex and nitrile gloves are used extensively in health care, and their disposable (single use) nature meant that large quantities were consumed during the peak of the pandemic. The shortage was also worsened because of hoarding by some consumers. In addition, certain businesses and government agencies began using these gloves to protect employees, even if their workers didn’t normally require gloves on the job.
If you have trouble obtaining your staff’s usual gloves, be prepared to identify feasible alternatives. You don’t want to endanger them by having them wear any old gloves they find lying around.
To identify alternatives for workers who rely on latex or nitrile gloves as PPE, you must know which chemicals workers handle or come in contact with. That’s because all glove materials are not suitable for all hazards.
Evaluate which materials offer appropriate protection from the specific chemicals that workers handle to select appropriate alternative gloves.
Here’s a summary of glove types and the protection given to help evaluate alternatives.
Butyl gloves protect against a variety of chemicals such as peroxide, highly corrosive acids, strong bases, alcohols, aldehydes, ketones, esters and nitrocompounds. Butyl gloves also resist oxidation, ozone corrosion and abrasion, and remain flexible at low temperatures. However, they do not perform well with aliphatic and aromatic hydrocarbons and halogenated solvents.
Natural (latex) rubber gloves have good elasticity and temperature resistance, and resist abrasions well. They protect against most water solutions of acids, alkalis, salts, and ketones. Latex gloves may cause allergic reactions and may not be appropriate for all employees. Hypoallergenic gloves, glove liners, and powderless gloves are possible alternatives for employees who are allergic.
Neoprene gloves protect against hydraulic fluids, gasoline, alcohols, organic acids, and alkalis. Their chemical and wear resistance are generally better than gloves of natural rubber.
Nitrile gloves are intended for jobs requiring dexterity, and they stand up even after prolonged exposure to substances that cause other gloves to deteriorate. They offer protection when working with greases, oils, acids, caustics, and alcohols but are not recommended for use with strong oxidizing agents, aromatic solvents, ketones, and acetates.
Each year, the National Fire Protection Agency (NFPA) reminds employers not to prop open fire doors for convenience. Propping open doors has become a common violation of fire codes after the pandemic because workers didn’t want to become exposed to germs on common touchpoints.
I know firsthand this is an issue at construction jobsites and remember telling workers not to prop open fire doors in our clients’ facilities. Workers were doing this out of convenience because they carried things into and out of the existing facility. Propping open a fire door, or wedging it open, are serious fire and safety hazards. Keep fire doors closed to prevent smoke and fire from spreading into the fire evacuation route, like a stairwell. OSHA and NFPA don’t prohibit propping open a fire exit door but caution employers against doing this for safety and security reasons.
Fire doors must remain closed, although some may be designed to automatically close when fire and smoke are sensed by jobsite fire detection equipment. To reduce the need to disinfect frequently touched points, workers can push open fire doors using their sleeves by pushing against the push bar instead of using their hands. You can also increase housekeeping efforts and the frequency that doorknobs, handles, and push bars are cleaned throughout the shift.
One way employers try to handle skyrocketing inflation is to manage first aid supplies. But do OSHA regulations allow employers to lock first aid supplies as a way to control costs?
Our experts are often asked whether OSHA permits locking first aid supplies. In a January 23, 2007, OSHA letter of interpretation (LOI), OSHA confirmed that first aid cabinets can be locked. The LOI stated, however, that first aid supplies must be readily accessible in the event of an emergency. Additionally, 29 CFR 1910.151(b) states: “In the absence of an infirmary, clinic, or hospital in near proximity to the workplace which is used for the treatment of all injured employees, a person or persons shall be adequately trained to render first aid. Adequate first aid supplies shall be readily available.”
OSHA defines “readily available” as accessible within three to five minutes and warns that locking first aid supplies, whether kits or cabinets, may limit employee accessibility per the standard. The agency advises that if an employer was relying on first aid services not provided by a clinic, infirmary, or hospital and adequate first aid supplies were not available when needed, then the employer would be in violation of 1910.151(b).
If you’re concerned with supplies being used in a manner not intended by the company, there are ways to manage supplies. For example, employers could use vending machines that allow employees to scan their badges and get basic supplies or personal protective equipment free of charge. This can help employers manage their supply chain and evaluate by whom and for what supplies are being used.
If opting to lock your first aid supplies, remember to make supplies readily accessible (within three to five minutes). This may require that additional keys for locks be made available to multiple personnel at all times when workers are present.
Although their recommendations are non-mandatory, OSHA suggests using the American National Standards Institute (ANSI) for reference to determine what supplies you need to have. The contents for Class A kits listed in the ANSI standard should be adequate for small worksites. Class B kits are designed with a broader range and quantity of supplies to deal with injuries in more complex or high-risk environments (for example, larger operations or multiple operations conducted at the same location).
It’s important to note that although OSHA is still citing ANSI’s 1998 standard, an updated version of the standard, ANSI/International Safety Equipment Association (ISEA) Z308.1-2021, was approved on April 15, 2022, and went into effect on October 15, 2022. Major changes to the standard included:
Determining what first aid supplies should be accessible depends on the workplace hazards and potential injuries. A great place to begin is by assessing your Form 300 injury logs to see the types of injuries already reported. Most employers perform risk assessments, beginning with a review of the Form 300 logs, to drive their decisions. OSHA also provides guidance to employers in 1910.151 Appendix A.
Employers must understand the accessibility risks associated with locking first aid cabinets even though OSHA and ANSI do not prohibit this practice. First aid supplies must be readily accessible (within three to five minutes) in the event of an emergency.
Employers must select a North American Industry Classification System (NAICS) code for every establishment, which usually means a single business location. This code determines whether the establishment is exempt from keeping an OSHA 300 Log. For locations that must keep a 300 Log, the code determines whether the establishment must submit injury data to OSHA by March 2nd.
The NAICS codes get updated every five years, with 2022 as the most current. Adding confusion, different OSHA regulations use different versions of the codes. For example:
Searching codes online may default to the 2022 version, and some codes changed. For example, the 1904.41 appendix lists 4529 for “Other general merchandise stores” which covers retail outlets like dollar stores and variety stores. However, searching that code in the 2022 list shows “no result” since that number changed. The 2022 NAICS code for general merchandise stores is 4552, but that code does not appear in OSHA’s appendix. Employers using the 2022 NAICS codes may incorrectly believe their establishment is not on OSHA’s list.
Employers can search older versions of the NAICS codes at https://www.census.gov/naics/ which also indicates whether a particular code has changed in more recent versions.
In addition to using the NAICS list for the correct year, employers must choose the correct code for each establishment. If a location engages in more than one type of business activity, employers must choose only one NAICS code for OSHA recordkeeping. OSHA says to choose the code for the activity that generates the most revenue or has the most employees.
In some cases, employers can divide a single physical location into more than one “establishment” as defined in 1904.46. To split a single location into multiple establishments, all of the following must apply:
For example, OSHA noted that if an employer operates a construction company at the same physical location as a lumber yard, the employer may consider each business as a separate establishment.
For employers with multiple establishments, the NAICS code for each location should reflect the primary business activity at each establishment. For example, a manufacturing company might have a separate administration office. Using a manufacturing code for the office might not be appropriate, even though it supports the other manufacturing locations. However, NAICS codes starting 5511 for “Management of Companies and Enterprises” might apply.
For example, code 551114 gives examples as follows:
That might better describe a corporate administrative office, if the location does not have any warehousing or manufacturing operations. In fact, codes starting 5511 appear on OSHA’s list of establishments under 1904.2 that are exempt from keeping a 300 Log, so applying the correct code could mean that office doesn’t need a 300 Log at all.
Finally, counting employees gets confusing because some OSHA regulations require counting all employees in the company (combining all locations) and others require counting only the employees at each establishment.
Key to remember: NAICS codes update every five years, and employers must use the correct list, which may differ in various regulations.
Hi everyone! Welcome to the monthly news roundup video, where we’ll review the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. With that said, let’s get started!
For the 13th year in a row, fall protection for construction topped OSHA’s list of violations. In fiscal year 2023, there were over 7,000 recorded violations, up from 5,250 in fiscal year 2022.
Workers who are exposed to lead in industries such as painting, battery manufacturing, and building renovation risk bringing lead home on their clothing and personal items. Take-home lead can contaminate a worker’s car and home, posing an exposure risk to family members. A new NIOSH publication outlines steps workers can take to minimize this risk.
Private industry employers reported 2.8 million nonfatal workplace injuries and illnesses in 2022. This is a 7.5 percent increase over 2021. The increase is due to a rise in both illnesses, which were up 26.1 percent, and injuries, which were up 4.5 percent. Respiratory illness cases drove the spike in reported illnesses.
OSHA faces significant challenges in ensuring worker safety, particularly in high-risk industries. This is according to a report from the Office of Inspector General, or OIG. Among OSHA’s top challenges are verifying timely hazard abatement, employer reporting, completing inspections, workplace violence, and protecting workers from crystalline silica.
And finally, turning to environmental news, a recent EPA rule requires covered facilities to include all quantities of per- and polyfluoroalkyl substances, or PFAS, on their Toxics Release Inventory reports. The rule also mandates that suppliers notify product users of the presence of any chemicals of special concern contained in their mixtures and products.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
