
Be Part of the Ultimate Safety & Compliance Community
Trending news, knowledge-building content, and more – all personalized to you!
SUMMARY: The Environmental Protection Agency (EPA or the Agency) is amending the requirements in Subpart J of the National Oil and Hazardous Substances Pollution Contingency Plan (NCP) that govern the use of dispersants, other chemicals and other spill mitigating substances when responding to oil discharges into jurisdictional waters of the United States. This action addresses the efficacy and toxicity of dispersants and other chemical and biological agents, as well as public, state, local, and federal officials' concerns regarding their use. Specifically, the Agency is amending the Subpart J regulatory requirements for the NCP Product Schedule in two distinct ways. First, the Agency is adding new listing criteria, revising the efficacy and toxicity testing protocols, and clarifying the evaluation criteria for removing products from the NCP Product Schedule. Second, the Agency is amending requirements for the authorities, notifications, and data reporting when using chemical or biological agents in response to oil discharges to Clean Water Act (CWA) section 311 jurisdictional waters and adjoining shorelines. These requirements are anticipated to encourage the development of safer and more effective spill mitigating products and better target the use of these products to reduce the risks of oil discharges and response technologies to human health and the environment. Further, the amendments are intended to ensure that On-Scene Coordinators (OSCs), Regional Response Teams (RRTs), and Area Committees (ACs) have sufficient information to support agent authorization of use decisions.
DATES: This final rule is effective on December 11, 2023, published in the Federal Register June 12, 2023, page 38280.
View final rule.
| Subpart J—Use of Dispersants and Other Chemicals | ||
| Heading | Revised | View text |
| §300.900 General. | ||
| (a), (c) | Revised | View text |
| (d) | Added | View text |
| §300.905 NCP Product Schedule. | ||
| Entire section | Removed | View text |
| §300.910 Authorization for agent use. | ||
| Entire section | Revised | View text |
| §300.915 Data and information requirements for listing on the NCP Product Schedule or Sorbent Product List. | ||
| Entire section | Revised | View text |
| §300.920 Addition of products to Schedule. | ||
| Entire section | Removed | View text |
| §300.950 Submission of Proprietary Business Information (PBI). | ||
| Entire section | Added | View text |
| §300.955 Addition of a product to the NCP Product Schedule or Sorbent ProductLlist. | ||
| Entire section | Added | View text |
| §300.965 Mandatory Product Disclaimer. | ||
| Entire section | Added | View text |
| §300.970 Removal of a product from the NCP Product Schedule or Sorbent Product List. | ||
| Entire section | Added | View text |
| Appendix C to Part 300—Requirements for Product Testing Protocols and Summary Test Data: Dispersant Baffled Flask Efficacy and Toxicity Tests; Standard Acute Toxicity Test for Bioremediation Agents, Surface Washing Agents, Herding Agents, and Solidifiers; and Bioremediation Agent Efficacy Test | ||
| Entire appendix | Revised | View text |
| Appendix E to Part 300 | ||
| Entire appendix | Removed | View text |
New Text
§300.5 Definitions.
Subpart J—Use of Dispersants, and Other Chemical and Biological Agents
§300.900 General.
(a) Section 311(d)(2)(G) of the Clean Water Act (CWA) requires EPA to prepare a schedule identifying dispersants, other chemicals, other spill mitigating devices and substances, if any, that may be used in carrying out the NCP; and the waters and quantities in which they may be used safely. This subpart establishes a schedule that includes the NCP Product Schedule identifying chemical and biological agents, the Sorbents Product List, and the authorization of use procedures that, when taken together, identify the waters and quantities in which such dispersants, other chemicals, or other spill mitigating devices and substances may be used safely.
* * * * *
(c) This subpart applies to the use of chemical and biological agents as defined in Subpart A of this part, or other substances that may be used to remove, control, or otherwise mitigate oil discharges.
§300.910 Authorization for agent use.
Use of chemical or biological agents in response to oil discharges must be authorized by the OSC in accordance with the provisions of this section.
(a) Use of agents identified on the NCP Product Schedule or use of burning agents on oil discharges addressed by a preauthorization plan. Area Committees and RRTs shall address, as part of their planning activities, whether preauthorization of the use of chemical and biological agents listed on the NCP Product Schedule or the use of burning agents on certain oil discharges is appropriate. Area Committees and RRTs shall, as appropriate, include applicable approved preauthorization plans in ACPs and RCPs. When a preauthorization plan is approved in advance for the use of certain agents under specified discharge situations, then the OSC may authorize the use of agents listed on the NCP Product Schedule, or the use of burning agents, for the purpose for which they were specifically listed without obtaining the incident-specific concurrences and without the natural resource trustees consultations described in paragraph (b) of this section.
(1) Preauthorization plan development. For discharge situations identified where such agents may be used, the preauthorization plan must, at a minimum, specify limits for the quantities and the duration of use, and use parameters for water depth, distance to shoreline, and proximity to populated areas. In meeting the provisions of this paragraph, preauthorization plans should document how regional factors are addressed including likely sources and types of oil that might be discharged, various potential discharge scenarios, the existence and location of environmentally sensitive resources or restricted areas that might be impacted by discharged oil, and logistical factors including inventory, storage locations and manufacturing capability of available agents, availability of equipment needed for agent use, availability of adequately trained operators, and means to monitor agent use in the environment. Preauthorization plans are to be developed by the Area Committees or the RRT in consultation with the Area Committee(s).
(2) Preauthorization plan approval. The EPA representative to the RRT, the Department of Commerce and the Department of the Interior natural resource trustees and, as appropriate the RRT representative from the state(s) with jurisdiction over waters and adjoining shorelines within the preauthorization plan area shall review and either approve, approve with modification, or disapprove the preauthorization plans. The Area Committees and RRTs shall address the withdrawal of approval from a preauthorization plan, and the RRT shall notify the NRT of the status of the preauthorization plan within 30 days from any such withdrawal.
(3) Preauthorization plan reviews. The RRT in consultation with the Area Committee(s) must review, and revise, as needed, approved preauthorization plans. These reviews must be conducted following a regular timeframe, established by the RRT and documented in the plan, to address changes that may impact the conditions under which the use of chemical and biological agents have been preauthorized. Reviews must also be conducted in any affected region, at a minimum, after a major discharge or after a Spill of National Significance (SONS) relevant to the preauthorization plan area; to address revisions of the NCP Product Schedule impacting chemical or biological agents that may be individually listed within a preauthorization plan; and to reflect new listings of threatened and/or endangered species applicable to the preauthorization plan area. The EPA RRT representative, the Department of Commerce and Department of the Interior natural resource trustees, and the RRT representative from the state(s) with jurisdiction over the waters of the area to which a preauthorization plan applies shall review and either approve, approve with modification, or disapprove any revisions to the preauthorization plans.
(b) Use of agents identified on the NCP Product Schedule or use of burning agents on oil discharges not addressed by a preauthorization plan. For discharge situations that are not addressed by a preauthorization plan developed pursuant to paragraph (a) of this section, the OSC may authorize the use of chemical or biological agents identified on the NCP Product Schedule on an oil discharge, or the use of burning agents, for the specific purpose for which they were listed with the concurrence of the EPA RRT representative and, as appropriate, the concurrence of the RRT representatives from the state(s) with jurisdiction over the waters and adjoining shorelines threatened by the release or discharge, and in consultation with the Department of Commerce and Department of the Interior natural resource trustees. In meeting the provisions of this paragraph, the OSC must consider and document for their authorization request to the RRT, at a minimum, the parameters for the use of agents including the quantities requested to be authorized, the duration of use, the depth of water, the distance to shoreline and proximity to populated areas, and should consider and document factors such as environmentally sensitive resources or restricted areas that might be impacted, agent inventory and storage locations, agent manufacturing capability, availability of equipment needed for agent use, availability of adequately trained operators and appropriate means to monitor agent use in the environment.
(c) [Reserved]
(d) Temporary exception. In circumstances to prevent or substantially reduce an imminent threat to human life that cannot be immediately addressed by other procedures or provisions of the NCP, the OSC may authorize the provisional use of any chemical or biological agent, whether it is identified or not on the NCP Product Schedule, without obtaining the concurrence of the EPA RRT representative and, as appropriate, the RRT representatives from the state(s) with jurisdiction over the waters and adjoining shorelines threatened by the release or discharge, and without consultation with the Department of Commerce and the Department of the Interior natural resource trustees. This exception shall not be used as a substitute for compliance with §300.150 of this part, including the use of personal protective equipment, or when there is sufficient time to seek authorization in accordance with paragraphs (a) or (b) of this section. If an agent is authorized for use pursuant to this paragraph, the OSC shall notify as soon as possible the EPA RRT representative and as appropriate, the RRT representatives from the affected state(s) and the Department of Commerce and Department of the Interior natural resource trustees. The OSC shall document the circumstances and the reasons for use of the agent authorized pursuant to this paragraph. Agent use for individual circumstances under this exception shall be in accordance with paragraphs (a) or (b) of this section no later than 24 hours after initial application.
(e) Prohibited agents or substances. The OSC may not authorize the use of the following:
(1) Sinking agents, or any other chemical agent, biological agent, or any substance that is used to directly sink the oil to the bottom of a water body.
(2) [Reserved]
(f) Storage and use of agents listed on the NCP Product Schedule. (1) The OSC may authorize for use only products listed on the NCP Product Schedule that are documented and certified by the responsible party or its representative to have been stored under the conditions provided by the submitter under §300.915(a)(6), and whose date of use does not exceed the expiration date listed on the container's label unless otherwise specified for expired products as provided in §300.910(f)(2), at the time of the incident.
(2) The OSC may authorize for use products listed on the NCP Product Schedule that exceed their expiration date after the responsible party or its representative documents and certifies that the expired product has been stored under the conditions provided by the submitter under §300.915(a)(6) and still meets the applicable efficacy and toxicity listing provisions under §300.915, based on testing of representative samples within the previous 12 months.
(g) Supplemental testing, monitoring, and information. The RRT may require, for both planning and response, including authorization of use, supplemental toxicity and efficacy testing, or submission of available data and information that addresses site, area, and ecosystem-specific concerns relative to the use of any chemical or biological agent. The product manufacturer or responsible party shall provide, upon request of the RRT or OSC, additional monitoring or testing data and information to inform chemical or biological agent use decisions specific to a response.
(h) Recovery of chemical agents and other substances from the environment. The responsible party shall ensure that removal actions adequately contain, collect, store, and dispose of chemical agents and other substances that are to be recovered from the environment, unless otherwise directed by the OSC. Chemical agents and other substances to be recovered include solidifiers, surface washing agents, and sorbents. The OSC should, at a minimum, consider factors such as the safety of response personnel and harm to the environment in making determinations pursuant to this paragraph.
(i) Reporting of agent use. (1) The authorizing OSC shall provide the RRT the following information on chemical and biological agents used in response to an oil discharge: product name, product category, quantity and concentrations used, duration of use, location(s) of use, any available data collected, and any available analyses of efficacy and environmental effects. This information must be provided within 30 days of completion of agent use. This information may be submitted in accordance with the OSC reporting provisions under §300.165 of this part, as applicable, subject to the 30-day timing requirement.
(2) In support of sections 300.135(n) and 300.155(a) and (b) of this part, the authorizing OSC shall provide for notification to the public, updated during a response as appropriate, the following information on chemical and biological agents used in response to an oil discharge: product name, product category, quantity and concentrations used, duration of use, and location(s) of use.
§300.915 Data and information requirements for listing on the NCP Product Schedule or Sorbent Product List.
If you are submitting an application for listing a product to the NCP Product Schedule or Sorbent Product List, you must provide EPA the information required under §300.955. Technical product data submissions are not required for burning agents. Your submission for each product must contain:
(a) General information for any product category. (1) Your name, physical address, email, and telephone number;
(2) Your identity and documentation of that identity, as the manufacturer of the product, vendor, importer, distributor of the product, and/or a designated agent acting on behalf of the manufacturer.
(3) All name(s), brand(s), and/or trademark(s) under which the product is to be sold;
(4) Names, physical addresses, emails , and telephone numbers of the primary distributors, vendors, importers and/or designated agent acting on behalf of the manufacturer;
(5) The Safety Data Sheet (SDS) for the product;
(6) The maximum, minimum, and optimum temperature, humidity, and other relevant conditions for product storage and a brief description of the consequences to performance if the product is not stored within these limits;
(7) The anticipated shelf life of the product at the storage conditions noted in paragraph (a)(6) of this section and documentation for this determination;
(8) A sample product label for all name(s), brand(s), and/or trademark(s) under which the product is to be sold that includes manufacture and expiration dates, and conditions for storage. You may use an existing label provided it already contains the required dates and storage information;
(9) The chemical or biological agent category under which you want the product to be considered for listing on the NCP Product Schedule, including detailed information on the specific process(es) through which the product affects the oil, and the specific environment(s) on which it is intended to be used ( e.g., waters and/or adjoining shorelines). If your product meets the definition of more than one chemical or biological agent category, you must identify all applicable categories and provide the test data to meet the listing criteria appropriate to each;
(10) Recommended product use procedures, including product concentrations, use ratios, types of application equipment, conditions for use, any application restrictions; and, as applicable, procedures for product and oil containment, collection, recovery, and disposal. These procedures must address, as appropriate, variables such as weather, water salinity, water temperature, types and weathering states of oils or other pollutants. The procedures must include supporting documentation and current applicable standard methods used to determine them;
(11) Available information on environmental fate, including any known measured data, methodologies, and supporting documentation, on the persistence, bioconcentration factor, bioaccumulation factor, and biodegradability of the product and all of its components in the environment;
(12) The physical and chemical properties of the product, as appropriate, and a citation for the current applicable standard methods used to determine them, including:
(i) Physical state and appearance;
(ii) Vapor pressure;
(iii) Flash point;
(iv) Pour point;
(v) Viscosity;
(vi) Specific gravity;
(vii) Particle size for solid components; and
(viii) pH;
(13) The identity and concentration of all components in the product, including each specific component name; corresponding Chemical Abstract Service (CAS) Registry Number; the maximum, minimum, and average weight percent of each component in the product; and the intended function of each component ( e.g., solvent, surfactant);
(14) For products that also contain microorganisms, enzymes, and/or nutrients, provide the following along with a citation or a description of the methodology used to determine:
(i) The name of all microorganisms by current genus and species, including any reclassifications, and any physical, chemical, or biological manipulation of the genetic composition and the weight percent of each genus in the product;
(ii) The name of all enzymes and their International Union of Biochemistry (I.U.B.) number(s); Enzyme Classification (EC) code numbers; the source of each enzyme; units; and specific oil-degrading activity;
(iii) The name(s), maximum, minimum, and average weight percent of the nutrients contained in the product; and
(iv) Data, methodology, and supporting documentation, for the levels of bacterial, fungal, or viral pathogens or opportunistic pathogens including, but not limited to: enteric bacteria such as Salmonella, fecal coliforms, Shigella, coagulase positive Staphylococci, and beta hemolytic Streptococci and enterococci;
(15) Data, methodology, and supporting documentation for the levels of the following:
(i) Arsenic, cadmium, chromium, copper, lead, mercury, nickel, vanadium, zinc, and any other heavy metal reasonably expected to be in the product;
(ii) Cyanide;
(iii) Chlorinated hydrocarbons;
(iv) Pesticides;
(v) Polychlorinated Biphenyls (PCBs); and
(vi) Polycyclic aromatic hydrocarbons (PAHs).
(16) Certification, including data, methodology, and supporting documentation, indicating that the product does not contain any of the prohibited agents or substances identified in §300.910(e);
(17) Information about the accredited laboratory that conducted the required tests, including:
(i) Name of the laboratory, address, contact name, email, and phone number; and
(ii) The national and/or international accreditations held by the laboratory that are applicable to the test(s) performed;
(18) All test data and calculations, including:
(i) Raw data and replicates, including positive controls;
(ii) Notes and observations collected during tests;
(iii) Calculated mean values and standard deviations;
(iv) Reports, including a summary of stock solution preparation;
(v) Source and preparation of test organisms;
(vi) Test conditions; and
(vii) Chain of custody forms;
(19) An estimate of the annual product production volume, the average and maximum amount that could be produced per day, and the time frame needed to reach that maximum production rate in days;
(20) Recognition received from EPA's Design for the Environment (DfE) or Safer Choice programs, as applicable; and
(21) International product testing or use data or certifications, if available, informing the performance capabilities or environmental impacts of the product.
(b) Dispersant testing and listing requirements —(1) Dispersant efficacy test and listing criteria. Test the dispersant product for efficacy using the Baffled Flask Test (BFT) method in Appendix C to part 300. To be listed on the NCP Product Schedule, the dispersant must demonstrate for each temperature a Dispersant Effectiveness (DE) at the 95% lower confidence level (LCL 95 ) greater than or equal to:
(i) ≥70% for Strategic Petroleum Reserve Bryan Mound at 5 °C;
(ii) ≥75% for Strategic Petroleum Reserve Bryan Mound at 25 °C;
(2) Dispersant toxicity tests and listing criteria. Use the methods specified in Appendix C to part 300 to test the dispersant alone, and the dispersant mixed with Strategic Petroleum Reserve Bryan Mound for acute toxicity, using Americamysis bahia and Menidia beryllina. Use the methods specified in Appendix C to part 300 to test the dispersant alone for developmental toxicity using Strongylocentrotus purpuratus or Arbacia punctulata and for subchronic effects using Americamysis bahia and Menidia beryllina. To be listed on the NCP Product Schedule, the dispersant alone must demonstrate:
(i) A median lethal concentration (LC 50 ) at the lower 95% confidence interval greater than 10 ppm;
(ii) An inhibition concentration for 50% of the test species (IC 50 ) at the lower 95% confidence interval greater than 1 ppm; and
(iii) A subchronic No Observed Effect Concentration (NOEC) greater than 1 ppm.
(3) Limitations. A dispersant may only be listed on the NCP Product Schedule for use in saltwater environments for which it meets the efficacy and toxicity listing criteria.
(c) Surface washing agent testing and listing requirements —(1) Surface washing agent efficacy test and listing criteria. To be listed on the NCP Product Schedule, using an applicable standard methodology, the surface washing agent must meet an efficacy of greater than or equal to 30% in either freshwater or saltwater, or both, depending on the intended product use.
(2) Surface washing agent toxicity test and listing criteria. Using the toxicity test methodology in Appendix C to part 300, test the surface washing agent for acute toxicity against freshwater species Ceriodaphnia dubia and Pimephales promelas, or saltwater species Americamysis bahia and Menidia beryllina, or both, depending on the intended product use. To be listed on the NCP Product Schedule, the surface washing agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(3) Limitations. Surface washing agent listing would be for use only in freshwater and/or saltwater environments for which it was tested and for which it met the efficacy and toxicity listing criteria.
(d) Bioremediation agent testing and listing requirements —(1) Bioremediation agent efficacy test and listing criteria. To be listed on the NCP Product Schedule, a bioremediation agent must successfully degrade both alkanes and aromatics as determined by gas chromatography/mass spectrometry (GC/MS) in freshwater or saltwater, or both, depending on the intended product use, following the test method specified in Appendix C to part 300. The percentage reduction of total alkanes (aliphatic fraction) from the GC/MS analysis must be greater than or equal to 85% at day 28, based on the ninety-fifth (95th) percentile Upper Confidence Limit (UCL 95 ) for both freshwater and saltwater. The percentage reduction of total aromatics (aromatic fraction) must be greater than or equal to 35% at day 28 for both saltwater and freshwater based on the UCL95.
(2) Bioremediation agent toxicity test and listing criteria. The bioremediation agent must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the bioremediation agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(3) Limitations. Bioremediation agent listing would be for use only in the freshwater and/or saltwater environments for which it was tested and for which it met the efficacy and toxicity listing criteria.
(4) Generic listing. If the product consists solely of: ammonium nitrate, ammonium phosphate, ammonium sulfate, calcium ammonium nitrate, sodium nitrate, potassium nitrate, synthetically-derived urea, sodium triphosphate (or tripolyphosphate), sodium phosphate, potassium phosphate (mono- or dibasic), triple super phosphate, potassium sulphate, or any combination thereof, no technical product data are required. The product will be generically listed as non-proprietary nutrients on the NCP Product Schedule, and no further action is necessary.
(e) Solidifier testing and listing requirements. (1) Solidifiers must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the solidifier must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(2) Limitations. Solidifier listing would be for use only in the freshwater and/or saltwater environments for which it was tested and for which it met the toxicity listing criteria.
(f) Herding agent testing and listing requirements. (1) Herding agents must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the herding agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(2) Limitations. Herding agent listing would be for use only in freshwater and/or saltwater environments for which it was tested and for which it met the toxicity listing criteria.
(g) Sorbent requirements. Known sorbent materials and products will be identified on a publicly available Sorbent Product List for the use of such products when responding to an oil discharge as follows:
(1) For sorbent products that consist solely of the following materials, or any combination thereof, no technical data are required to be submitted for listing on the Sorbent Product List, and no further action is necessary for use as a sorbent:
(i) Feathers, cork, peat moss, and cellulose fibers such as bagasse, corncobs, and straw;
(ii) Volcanic ash, perlite, vermiculite, zeolite, and clay; and
(iii) Polypropylene, polyethylene, polyurethane, and polyester.
(2) If the product consists of one or more natural organic substances, inorganic/mineral compounds, and/or synthetic compounds not specifically identified in paragraph (g)(1) of this section but you believe the product meets the definition of a sorbent then, as applicable under §300.955(a) and (b), you must submit the following information for consideration for listing it as a sorbent on the Sorbent Product List:
(i) The information required under paragraphs (a)(1) through (a)(8), and paragraph (a)(13) through (a)(15) of this section;
(ii) The certification required under paragraph (a)(16) of this section; and
(iii) Information, including data, to support the claim your product meets the sorbent definition under §300.5.
Appendix C to Part 300—Requirements for Product Testing Protocols and Summary Test Data: Dispersant Baffled Flask Efficacy and Toxicity Tests; Standard Acute Toxicity Test for Bioremediation Agents, Surface Washing Agents, Herding Agents, and Solidifiers; and Bioremediation Agent Efficacy Test
Table of Contents
1.0 Applicability and Scope
2.0 Baffled Flask Dispersant Efficacy Test (BFT)
3.0 Dispersant Toxicity Testing
4.0 Standard Acute Toxicity Testing for Surface Washing Agents, Bioremediation Agents, Herding Agents, and Solidifiers
5.0 Bioremediation Agent Efficacy Test Protocol
Illustrations
Figure Number
1. A Baffled Trypsinizing Flask
Tables
Table Number
1. Constituent Concentrations for GP2 Artificial Seawater
2. Test Oil Characteristics
3. Stock Standard Solution Preparation
4. Dispersant Calibration Example for Test Oil
5. Sample Calculation With ANS
6. Toxicity Testing Requirements for Dispersants
7. Summary of Test Conditions—Dispersant Toxicity
8. Toxicity Testing Requirements for Surface Washing Agents, Herding Agents, Bioremediation Agents and Solidifiers
9. Summary of Test Conditions—Surface Washing Agents, Herding Agents, Bioremediation Agents and Solidifiers Toxicity
10. Artificial Seawater Nutrient Concentrations
11. Artificial Seawater Nutrient Concentrations for Bioremediation Agents Having No Nutrients Included
12. Constituent Concentrations for Artificial Freshwater (Bushnell-Haas)
13. Freshwater Nutrient Concentrations
14. Artificial Freshwater Nutrient Concentration for Bioremediation Agents Having No Nutrients Included
15. Bioremediation Efficacy Test—Summary of Experimental Setup
16. Bioremediation Efficacy—Summary of Analytical Procedures
17. QA/QC Checks
Standard Operating Procedures Tables
SOP 3–1 Amount of Stock Solutions Required To Make the Working Standards
SOP 4–1 Ions Associated With Retention Time Groups
SOP 4–2 Instrumental Conditions for Crude Oil Analysis
SOP 4–3 Ion Abundance Criteria for DFTPP
SOP 4–4 Target Compound List
1.0 Applicability and Scope. This Appendix establishes laboratory protocols required under Subpart J (Use of Dispersants and Other Chemical and Biological Agents) of 40 CFR part 300 (National Oil and Hazardous Substances Pollution Contingency Plan) to make listing determinations for the Product Schedule. The protocols apply, based on product type, to dispersants, bioremediation agents, surface washing agents, herding agents, and solidifiers as defined in Subpart A (Introduction) of 40 CFR part 300.
2.0 Baffled Flask Dispersant Efficacy Test (BFT)
2.1 Summary. This laboratory protocol establishes procedures to evaluate the degree to which a product effectively disperses oil spilled on the surface of seawater, using a modified 150-mL screw-cap trypsinizing flask (an Erlenmeyer flask with baffles) with a glass and Teflon® stopcock near the bottom to allow removal of subsurface water samples without disturbing the surface oil layer. The efficacy of a dispersant is measured using one reference oil, Strategic Petroleum Oil Reserve Bryan Mound at two temperatures (5 °C and 25 °C). Six replicates and one method blank are required at each temperature. A layer of oil is placed on the surface of artificial seawater, and the dispersant is added to the slick at a dispersant:oil ratio (DOR) of 1:25 (4%) by volume. A standard orbital shaker table provides turbulent mixing at a speed of 250 revolutions per minute (rpm) for 10 minutes, immediately after which it is maintained stationary for 10 minutes to allow non-dispersed oil to rise to the water's surface. An undisturbed water sample is removed from the bottom of the flask through the stopcock, extracted with dichloromethane (DCM), and analyzed for oil content by UV-visible absorption spectrophotometry at wavelengths ranging between 340 and 400 nm.
2.2 Apparatus. All equipment must be maintained and calibrated per standard laboratory procedures.
2.2.1 Modified Trypsinizing Flask. A modified 150 mL glass screw-capped Erlenmeyer flasks with baffles ( e.g., Wheaton No. 355394 or equivalent) fitted with a 2 mm bore Teflon® stopcock and glass tubing, the center of which is no more than 1.3 cm from the bottom, as shown in Figure 1.

Figure 1. A Baffled Trypsinizing Flask
2.2.2 Orbital Shaker Table. An orbital shaker table with a variable speed control unit capable of maintaining 250 rpm. The orbital diameter must be approximately 1.0 inch (2.5 cm) +/−0.1 inch (0.25 cm).
2.2.3 Spectrophotometer. A UV-visible spectrophotometer capable of measuring absorbance between 340 and 400 nm ( e.g., Shimadzu UV–1800, Agilent 8453, or equivalent). Use standard transmission-matched quartz 10-mm path length rectangular cells with PTFE cover for absorbance measurements.
2.2.4 Glassware. Including: 25-ml graduated mixing cylinders (a graduated cylinder with a ground glass stopper); 50- and 100-ml graduated cylinders; 125-mL separatory funnels with Teflon stopcocks; 10-ml volumetric flasks; 30-ml crimp style glass serum bottles; 1-, 2-, 5-mL pipettes; other miscellaneous laboratory items.
2.2.5 Micropipettor. Use a micropipettor capable of dispensing 4 µL of dispersant and 100 µL of oil ( e.g., Brinkmann Eppendorf repeater pipettor with 100 µL and 5 mL syringe tip attachments or equivalent).
2.2.6 Syringes. 25-, 100-, 250-, 1,000-, 2,500-, 5,000-µl gas-tight syringes.
2.2.7 Constant temperature rooms or incubators to hold the shaker at 5 °C and 25 °C.
2.2.8 Analytical Balance.
2.2.9 Chemical fume hood.
2.3 Reagents.
2.3.1 Artificial seawater. Use the artificial seawater GP2 formulation shown in Table 1 of this Appendix.
2.3.2 Test oil. Use the EPA standard reference oil Strategic Petroleum Reserve Bryan Mound. To obtain this oil at no charge (except for a minimal shipping fee), see the instructions at http://www.epa.gov/emergencies/content/ncp/index.htm. Selected properties are summarized in Table 2 of this Appendix.
2.3.3 Dichloromethane (DCM) (also known as methylene chloride), pesticide quality.
2.4 Container Handling and Storage.
2.4.1 Glassware. If the glassware has been used with oil before, rinse with DCM to remove as much of the oil adhering to the sides of the flask as possible; waste DCM may be used. Soak in warm water with detergent and individually wash with bristled brushes. First rinse with tap water, then follow with two de-ionized water rinses. Dry either on a rack or in a 110 °C drying oven. After drying, rinse with fresh DCM (use sparingly).
2.4.2 Serum bottles and other non-volumetric glassware. Bake for at least 4 hours in a muffle furnace at 450 °C.
2.5 Calibration Curve for the UV-visible spectrophotometer.
2.5.1 Stock Standard Solution Preparation. Stock standard solution concentrations are based on the mass measurements after each addition and density determinations of the oil/dispersant/DCM solution using a density bottle or a 1-mL gas tight syringe. An example calculation is given in Table 3 of this Appendix according to the following equation:
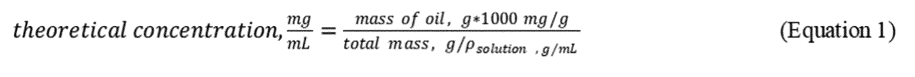
Use the reference oil and the specific dispersant being tested for a particular set of experimental test runs. Prepare the stock standard solution of dispersant-oil mixture in DCM, starting with 2 ml of the oil, then adding 80 µl of the dispersant followed by 18 ml of DCM.
2.5.2 Six -point Calibration Curve. For the reference oil, add specific volumes of its stock standard solution (given in Table 4 of this Appendix) to 30 ml of artificial seawater in a 125 ml separatory funnel. Extract the oil/dispersant water mixture with triplicate 5 ml volumes of DCM. Follow each DCM addition by 15 seconds of vigorous shaking, carefully releasing the initial pressure inside the separatory funnel by partially removing the glass stopper inside a fume hood after the first few shakes. Then, allow a 2-minute stationary period for phase separation for each extraction. Drain the extracts into a 25-mL graduated mixing cylinder. Release any entrained bubbles of DCM from the water layer by sideways shaking of the funnel. Use precaution not to drain water into the DCM extract as it can affect the absorbance readings. Adjust the final volume of the collected extracts to 25 mL in the mixing cylinder using DCM. Determine specific masses for oil concentrations in the standards as volumes of oil/dispersant solution multiplied by the concentration of the stock solution. An example calculation is given in Table 4 of this Appendix. One calibration curve is needed for the reference oil and dispersant combination.
2.6 Sample Preparation and Testing. See section 2.7 of this Appendix for a detailed description of the spectrophotometer's linear calibration procedure.
2.6.1 Six replicates of the oil and test dispersant are required at each temperature plus two additional tests of method blanks (artificial seawater without oil and dispersant), one at each temperature. A completed test consists of 14 baffled flask tests (a total of six replicates for the reference oil/test dispersant combination at two temperatures (5 °C and 25 °C), plus two method blanks).
2.6.2 Attach a 3-inch length of Teflon tubing to the stopcock of each of the 150-mL baffled flasks. Add 120 mL of artificial seawater to each flask. Put screw cap on flasks and place them at the appropriate temperature (either 5 °C or 25 °C) for equilibration.
2.6.3 Calibrate and adjust the shaker table to 250 ± 10 rpm.
2.6.4 Prepare and time separately each baffled flask. Sequentially add 100 µL of oil and 4 µL of dispersant to the flask layering them onto the center of the seawater to give a dispersant-to-oil ratio (DOR) of 1:25. Avoid any oil or dispersant splashing on the flask walls, as it may reduce efficacy or cause errors in the calculated results. Discard the sample and repeat the setup if: (1) any oil or dispersant splashing occurs during the additions, or (2) the dispersant contacts the water first rather than the oil. This is especially important for 5 °C work because of increased oil viscosity.
2.6.5 For the oil, fill the tip of the pipettor, using a wipe to remove any oil from the sides of the tip. Holding the pipettor vertically, dispense several times back into the reservoir to ensure that the oil flows smoothly. Insert the syringe tip vertically into the baffled flask and let the bottom of the pipettor rest on the neck of the flask. Slowly and carefully dispense the oil one time onto the center of the water's surface. The remainder of the oil can either be returned to the oil bottle or set aside for use in the next test flask.
Note to 2.6.5: If a Brinkmann Eppendorf repeater pipettor is used for dispensing the oil, attach a 5-mL syringe tip, and set the dial to 1.
2.6.6 For the dispersant, use the same procedure as for the oil to dispense onto the center of the oil slick surface. As the dispersant first contacts the oil, it will usually push the oil to the sides of the flask. Replace the screw cap onto the flask.
Note to 2.6.6: If a Brinkmann Eppendorf repeater pipettor is used for dispensing the dispersant, attach a 100-µL syringe tip, and set the dial to 2.
2.6.7 Carefully place flask securely onto the shaker and agitate for 10 ± 0.25 minutes at 250 ± 10 rpm.
2.6.8 Remove the flask from the shaker table and allow a stationary, quiescent period of 10 ± 0.25 minutes to allow undispersed and/or recoalesced oil droplets to refloat to the surface.
2.6.9 Carefully open the screw cap, then the stopcock at the bottom, and discard the first several mL of seawater into a waste beaker to remove non-mixed water-oil initially trapped in the stopcock tubing. Collect a volume slightly greater than 30-mL into a 50-mL graduated cylinder. Adjust the collected volume to the 30-mL mark by removing excess with a disposable glass Pasteur pipette. A web-like emulsion may form at the solvent/water interface during the water sample extraction. Avoid pulling any emulsion phase into the DCM extract as it may cloud the DCM-extract, leading to error.
2.6.10 Transfer the water-oil sample from the graduated cylinder into a 125-mL glass separatory funnel fitted with a Teflon stopcock.
2.6.11 Add 5 mL DCM to the separatory funnel. Start shaking, releasing pressure into the fume hood by loosening the glass stopper. Shake vigorously at least 20 times for 15 seconds.
2.6.12 Allow the funnel to remain in a stationary position for 2 minutes to allow phase separation of the water and DCM.
2.6.13 Drain the DCM layer from the separatory funnel into a 25 mL mixing cylinder. Avoid pulling any emulsion phase into the DCM extract as it may cloud the DCM extract.
2.6.14 Repeat the DCM-extraction process two or three additional times until the DCM is clear. Collect each extract in the graduated cylinder. After the final extraction, lightly shake the separatory funnel sideways once or twice to dislodge entrained bubbles of DCM and drain.
2.6.15 Adjust the final volume to a known quantity, 25 mL, in the mixing cylinder. Using a syringe, dispense 2.5 mL or 5.0 mL of a reference oil sample into a 10-mL volumetric flask, and fill with DCM to make either a 1:4 or 1:2 dilution, respectively.
2.6.16 If analysis cannot be conducted immediately, store the extracted DCM samples at 4 ± 2 °C until time of analysis. Glass-stoppered mixing cylinders may be used for short-term storage or prior to bringing the extracts up to volume. After bringing to volume, transfer the DCM extracts to 25–30 ml crimp-style serum vials with aluminum/Teflon seals.
2.6.17 Complete all analysis within 10 consecutive days from when the sample was collected.
2.7 UV-Visible Spectrophotometer Linear Stability Calibration
2.7.1 A six-point calibration of the UV-visible spectrophotometer is required at least once per day for each oil. The stability calibration criterion is determined with the six oil standards identified in Table 4 of this Appendix.
2.7.2 Turn on spectrophotometer and allow it to warm up for at least 30 minutes before beginning analysis. Blank the instrument for the wavelengths between 340 and 400 nm with DCM.
2.7.3 If refrigerated, allow all extracts, standards, and samples to warm to room temperature.
2.7.4 Determine the absorbance of the six standards between the wavelengths of 340 and 400 nm. This can be done by either one of the following methods:
2.7.4.1 Trapezoidal Rule. Program the spectrophotometer to take readings every 5λ or 10λ and calculate the area under the curve using the Trapezoidal rule:
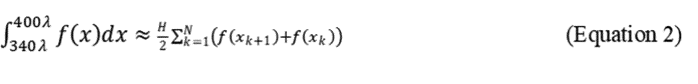
where N + 1 = number of absorbance measurements to delineate N equally spaced sections of the curve, and H = the distance (λ) between each reading. For H = 5, N + 1 = 13 measurements, for H = 10, N + 1 = 7. The following formula illustrates readings taken every 10λ.
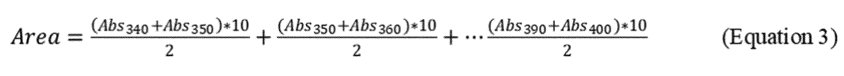
When using readings taken every 5λ, each absorbance sum is multiplied by 5.
2.7.4.2 Automatic Integration. Program the spectrophotometer to automatically integrate the area under the curve between 340 nm and 400 nm.
2.7.4.3 If the wavelengths must be manually set on the spectrophotometer, the older method of only measuring at 340λ, 370λ, and 400λ may be used. Then calculate using the trapezoidal rule for N + 1 = 3, H = 30. While the resulting area count with the older method is less accurate, the final results are similar since the inaccuracy is systematic.
2.7.5 After determining the area count for each standard, determine the response factor (RF) for the oil at each concentration using the following equation:
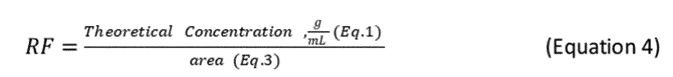
2.7.6 Spectrophotometer stability for the initial calibration is acceptable when the RFs of the six standard extracts are less than 10% different from the overall mean value for the six standards, as calculated in Equation 5 of this Appendix and depicted in the example in Table 4 of this Appendix.
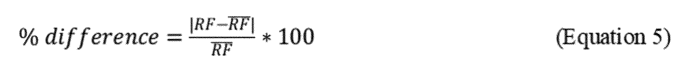
2.7.7 If this criterion is satisfied, begin analysis of sample extracts. Absorbances greater than or equal to 3.5 are not included because absorbance saturation occurs at and above this value. If any of the standard oil extracts fails to satisfy the initial-stability criterion, the source of the problem ( e.g., preparation protocol for the oil standards, spectrophotometer stability, etc.) must be corrected before analysis of the sample extracts begins.
2.7.8 Determine the slope of the calibration points by using linear regression forced zero intercept:
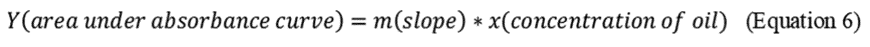
2.8 Spectrophotometric Analysis and Calculations
2.8.1 Once a successful calibration curve for the reference oil has been created and verified, measure experimental replicates for the reference oil at each temperature followed by a standard check sample.
2.8.2 Determine the area for the absorbance values obtained for the experimental samples by using Equation 2 of this Appendix and illustrated by Equation 3 of this Appendix.
2.8.3 Calculate the Total Oil dispersed and the percentage of oil dispersed (%OD) based on the ratio of oil dispersed in the test system to the total oil added to the system, as follows:
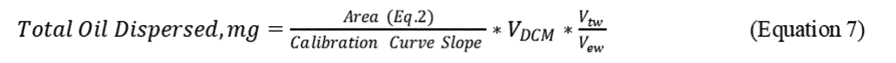
where:
V DCM = final volume of the DCM extract (mL)
V tw = total seawater in Baffled Flask (120 mL)
V ew = volume seawater extracted (30 mL)
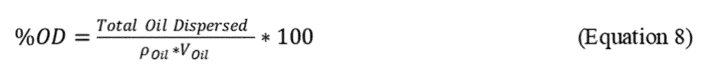
where:
r Oil = density of the specific test oil, mg/mL and
V Oil = Volume (mL of oil added to test flask (100 µL = 0.1 mL))
2.8.4 The %ODs for the six replicates within a particular treatment are then subjected to an outlier test, the Grubb's Test or Maximum Normal Residual test (6). A convenient internet-based calculator of a Grubbs outlier may be found at: http://www.graphpad.com/quickcalcs/Grubbs1.cfm. If an outlier is detected (p < 0.05), analyze an additional replicate to obtain the required six replicates.
2.8.5 Report the Dispersion Efficacy value for each oil and each temperature, which is the lower 95% confidence level of the 6 independent replicates (DE LCL95 ) for each oil/temperature combination. Error bars are not needed as reporting the lower confidence level computationally takes the variability of the replicates into account as shown in Equation 9 of this Appendix.

where = mean percentage oil dispersed for the n = 6 replicates, S = standard deviation, and t (n-1,1-) = 100 * (1-α)th percentile from the t-distribution with n-1 degrees of freedom. For 6 replicates, t n-1,1- = 2.015, where α = 0.05. An example of the calculations is given in Table 5 of this Appendix.
2.9 Performance Criterion
The dispersant product tested will remain in consideration for listing on the NCP Product Schedule if the dispersant efficacy (DE LCL95 ), as calculated in section 2.8.6 of this Appendix, is:
| Oil | Temp (°C) | DE LCL95 (%) |
|---|---|---|
| Bryan Mound | 5 | ≥70 |
| Bryan Mound | 25 | ≥75 |
2.10 Quality Control (QC) Procedures for Oil Concentration Measurements
2.10.1 Absorbance readings. Perform at least 5% of all UV-visible spectrophotometric measurements in duplicate as a QC check on the analytical measurement method. The absorbance values for the duplicates must agree within ±5% of their mean value.
2.10.2 Method blanks. Analytical method blanks involve an analysis of artificial seawater blanks (artificial seawater without oil or dispersant in a baffled flask) through testing and analytical procedures. Analyze method blanks with a frequency of at least two per completed test. Oil concentrations in method blanks must be less than detectable limits.
2.10.3 Accuracy. Determine accuracy by using a mid-point standard calibration check after each set of replicate samples analyzed. The acceptance criterion is based on a percent recovery of 90–110% using the following equation:

2.10.4 Calibration QC checks. Before analyzing samples, the spectrophotometer must meet an instrument stability calibration criterion using the oil standards. The instrument stability for initial calibration is acceptable when the RFs (Equation 5 of this Appendix) for each of the six standard concentration levels are less than 10% different from the overall mean value.
| Constituent | Concentration (g/L) |
|---|---|
| * Use Stock Solution, 1 mL/L GP2 for 100X stock solution for Bromide, Borate, and Strontium. 10 mL/L GP2 for bicarbonate—10X stock solution as it is not soluble in a 100X solution. Adjust to pH 8.0 prior to autoclaving. | |
| NaCl | 21.03 |
| Na 2 SO 4 | 3.52 |
| KCl | 0.61 |
| KBr * | 0.088 |
| Na 2 B 4 O 7 × 10H 2 O * | 0.034 |
| MgCl 2 × 6H 2 O | 9.50 |
| CaCl 2 × 2H 2 O | 1.32 |
| SrCl 2 × 6H 2 O * | 0.02 |
| NaHCO 2 * | 0.17 |
| Oil | Density, mg/mL @15 °C | API gravity @15 °C | Viscosity @25 °C, (cSt) | Category by API gravity |
|---|---|---|---|---|
| SPR Bryan Mound | 0.8320 | 38.6 | 4.721 | Light Oil. |
| Item | Identifier | Amount |
|---|---|---|
| Mass of Bottle, g | A | 29.498 |
| Mass of Bottle + oil, g | B | 31.225 |
| Mass of bottle + disp + oil + DCM, g | C | 54.380 |
| Mass of oil, g ( derived ) | F = B−A | 1.727 |
| Mass of disp + oil + DCM, g ( derived ) | G = C−A | 24.882 |
| Mass of 1 mL syringe, g | D | 14.556 |
| Mass of 1 mL syringe + solution, g | E | 15.820 |
| Density of solution, g/mL ( derived ) | H = E−D | 1.264 |
| Volume of solution, mL ( derived ) | I = G/H | 19.687 |
| Conc. of stock solution, mg/mL ( derived ) | J = F*1000/I | 87.704 |
| Oil + Dispersant Stock Standard Solution Concentration = 87.7 mg/mL ( Table 3 ) | ||||||
|---|---|---|---|---|---|---|
| Standard—stock vol. (uL) | Theoretical conc., mg/mL | Area (340–400 nm) | RF | Avg. RF | Dev. from avg. RF | Slope |
| 25 | 0.088 | 4.126 | 0.021 | 0.021 | 2.931 | 48.759 |
| 50 | 0.175 | 8.757 | 0.020 | 3.017 | ||
| 100 | 0.351 | 16.559 | 0.021 | 2.577 | ||
| 150 | 0.526 | 25.666 | 0.021 | 0.731 | ||
| 200 | 0.702 | 34.142 | 0.021 | 0.500 | ||
| 250 | 0.877 | 43.006 | 0.020 | 1.260 | ||
| Rep | Area (340–400 nm) | Dilution factor | Extract volume (ml) * | Conc, mg/mL. | Mass in 30 mL, mg | Total oil dispersed, mg | Efficiency, % | Average | Std. dev. | Variance | Coef. of variation | LCL95 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * = 25 ml of DCM extract captured oil from 30 ml of aqueous DE test. | ||||||||||||
| 1 | 32.197 | 1 | 25 | 0.66 | 16.51 | 66.03 | 79.76 | 81.30 | 4.46 | 19.85 | 5.48 | 81.30 |
| 2 | 35.470 | 1 | 25 | 0.73 | 18.19 | 72.75 | 87.87 | |||||
| 3 | 30.260 | 1 | 25 | 0.62 | 15.52 | 62.06 | 74.96 | |||||
| 4 | 31.831 | 1 | 25 | 0.65 | 16.32 | 65.28 | 78.85 | |||||
| 5 | 33.355 | 1 | 25 | 0.68 | 17.10 | 68.41 | 82.63 | |||||
| 6 | 33.791 | 1 | 25 | 0.69 | 17.33 | 69.30 | 83.71 | |||||
2.11 References for Section 2.0
(1) U.S. Environmental Protection Agency (1994), “Swirling Flask Dispersant Effectiveness Test,” Title 40 Code of Federal Regulations, Pt. 300, Appendix C, pp 47458–47461.
(2) Sorial, G.A., A.D. Venosa, K.M, Koran, E. Holder, and D.W. King. 2004. “Oil spill dispersant effectiveness protocol: I. Impact of operational variables.” ASCE J. Env. Eng. 130(10):1073–1084.
(3) Sorial, G.A., A.D. Venosa, K.M, Koran, E. Holder, and D.W. King. 2004. “Oil spill dispersant effectiveness protocol: II. Performance of revised protocol.” ASCE J. Env. Eng. 130(10):1085–1093.
(4) Venosa, A.D., D.W. King, and G.A. Sorial. 2002. “The baffled flask test for dispersant effectiveness: a round robin evaluation of reproducibility and repeatability.” Spill Sci. & Technol. Bulletin 7(5–6):299–308.
(5) Spotte, S., G. Adams, and P.M. Bubucis. 1984. “GP2 medium is an synthetic seawater for culture or maintenance of marine organisms,” Zoo Biol, 3:229–240.
(6) Grubbs, F. 1969. “Sample Criteria for Testing Outlying Observations,” Annals of Mathematical Statistics, pp. 27–58.
3.0 Dispersant Toxicity Testing
3.1 Summary. This laboratory protocol includes testing for: (1) dispersant standard static acute toxicity tests for the mysid shrimp, Americamysis bahia (48-hr duration) and the inland silverside, Menidia beryllina (96-hr duration); (2) dispersant-oil mixture static acute toxicity tests for Americamysis bahia and Menidia beryllina (48-hr and 96-hr duration, respectively); (3) dispersant developmental assay for Strongylocentrotus purpuratus or Arbacia punctulata, (72-hr duration); and (4) dispersant 7-day static subchronic tests with Americamysis bahia and Menidia beryllina (Table 6 of this Appendix).
| Test procedure | ||||
|---|---|---|---|---|
| Test substance | 96-Hr static acute: Menidia beryllina | 48-Hr static acute: AmericamysisBahia | 72-Hr sea urchin developmentalassay | 7-Day subchronic: M. beryllina &A. bahia |
| Dispersant only | yes | yes | yes | yes . |
| Dispersant—Reference Oil Mixture | yes | yes | no | no . |
3.2 Preparation of Stock Solutions
3.2.1 Dispersant. Prepare a 1000 μL/L primary stock solution prior to test initiation by adding 1.1 mL of dispersant to 1100 mL of dilution water consisting of salinity adjusted uncontaminated natural or artificial seawater, in a glass vessel. Using a laboratory top stirrer equipped with a stainless-steel blade, center the stirrer blade in the mixing vessel one inch off the bottom. Initially mix the resulting stock solution for approximately five seconds at speeds of <10,000 rpm to avoid foaming. Thereafter, set the speed to provide a 70% vortex. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Suspend mixing of the stock solution after the removal of each aliquot. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 3.5 and 3.6 of this Appendix.
3.2.2 Dispersant-Reference Oil(s) Mixtures. Use Strategic Petroleum Reserve Bryan Mound reference oil. To obtain this oil at no charge (except for a minimal shipping fee) see https://www.epa.gov/emergency-response/national-contingency-plan-subpart-j#howto. Assessment of dispersant-reference oil mixture (DOM) toxicity is determined for each reference oil using the aqueous phase of a chemically enhanced-water accommodated fraction (CE–WAF). Fit a glass aspirator bottle (approximately 23 L) equipped with a hose bib at the base with a length of silicon tubing containing a hose clamp. Fill the bottle with 19L of seawater leaving a 20% headspace above the liquid, place on a magnetic stir plate then add and center a stir bar. Add the reference oil at 25 g/L using a silicon tube attached to a glass funnel that reaches just below the water surface. Using this method reduces the production of air bubbles on the oil surface slick. Adjust the stir plate to obtain an oil vortex of 25% of the total volume of the seawater, then add the dispersant to be tested at a ratio of 1:10 dispersant:oil (2.5 g/L). Securely seal the bottle to reduce the loss of volatiles using a silicon stopper and wraps of Parafilm and stir for 18 hours, then allow the solution to settle for 6 hours. Maintain the temperature at 25 °C during stirring and settling. Purge the hose at the base of the bottle of any material followed by removal of the CE–WAF (aqueous phase) into a clean glass container without disturbing the surface oil slick. The CE–WAF should be remixed and 1 to 2 L removed for chemical analysis of total petroleum hydrocarbons (TPH) following the procedures outlined in section 3.4 of this Appendix. The remaining volume will be used for the preparation of exposure solutions following procedures outlined in section 3.3 of this Appendix. To reduce time and cost, mix sufficient amounts of dispersant product-reference oil mixture CE–WAF to allow preparation of exposure solutions for conducting simultaneous acute tests with both Americamysis bahia and Menidia beryllina.
3.3 Preparation of Exposure Concentrations.
3.3.1 Concentration Selection. Preliminary rangefinder tests may be necessary using a series of logarithmic concentrations ( e.g. 0.1, 1, 10, 100 µl dispersant product/L or mg TPH/L) to determine the appropriate exposure concentration range necessary to determine LC 50 values and 95% confidence intervals. For definitive tests, conduct a minimum of five test concentrations using a geometric ratio between 1.5 and 2.0 ( e.g. 2, 4, 8, 16, and 32). Note that when testing only the dispersant product, the highest test concentration must not exceed the dispersant's self-dispersibility limit.
3.3.2 Exposure Concentrations. Exposure solutions are prepared by adding the appropriate amount of stock solution directly to dilution water in each test chamber. Mix each exposure solution using five rotations in one direction followed by five rotations in the opposite direction using a solid glass stir rod.
3.3.3 Reference Toxicants. Separate toxicity tests must be performed with a reference toxicant for each species tested. Conduct additional reference toxicity tests any time a change in the population or source of a test species occurs. Use sodium dodecyl sulfate (SDS), also known as dodecyl sodium sulfate (DSS), and sodium lauryl sulfate (SLS) as the reference toxicant for exposures conducted with Menidia beryllina and Americamysis bahia. Use copper chloride as the reference toxicant for exposures conducted with the sea urchin developmental test. Use reagent grade quality SDS and copper chloride for tests. Information on procedures for conducting reference toxicant tests with these species can be found in the specific EPA methods documents cited in sections 3.5.1, 3.6.1, and 3.7.1 of this Appendix.
3.4 Chemical Analysis of Stock Solutions. Add the 1 L sample of CE–WAF (Section 3.2.2 of this Appendix) solutions directly to amber glass bottles with Teflon®-lined cap. Collect a replicate sample in the event of accidental loss or if reanalysis of the stock solution becomes necessary. Adjust sample to a pH=2 using 50% hydrochloric acid, immediately refrigerate and analyze within 48 hours of collection. Analyze samples for C9–C32 TPH by gas chromatography-flame ionization detection (GC–FID) following EPA SW–846, Method 8015B–DRO (4). Report TPH concentration of stock solutions as milligrams TPH/L and use in the calculation of exposure concentrations for all toxicity tests conducted with CE–WAF.
3.5 Static Acute Tests with M. beryllina and A. bahia
3.5.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with dispersant product or a mixture of dispersant product and reference oil (DOM).
3.5.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2 of this Appendix and exposure concentrations following section 3.3 of this Appendix.
3.5.3 Number of Treatments, Replicates and Organisms. Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment.
3.5.4 Exposure Period. Test duration is 48-hr for Americamysis bahia and 96-hr for Menidia beryllina. Mortality must be recorded at each 24-hour period of each test.
3.5.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 μl/L or is determined to be greater than the limits of water solubility of dispersibility.
3.5.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 7 of this Appendix.
3.6 Sea Urchin Developmental Test with Dispersant Product
3.6.1 General. Use Section 15, “Purple Urchin, Strongylocentrotus purpuratus and Sand Dollar, Dendraster excentricus Larval Development Test Method” of EPA's Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to West Coast Marine and Estuarine Organisms (EPA/600/R–95–136) (2). Alternatively, the development of the urchin Arbacia punctulata may be tested (see Table 7).
3.6.2 Test Organism. Tests of dispersant products are to follow methods for the purple urchin only. Tests with the sand dollar are not required.
3.6.3 Test Solutions. Modify procedures in EPA/600/R–95–136, Section 15 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2.1 of this Appendix and exposure concentrations following section 3.3 of this Appendix.
3.6.4 Number of Treatments and Replicates. Conduct a minimum of four replicates of five exposure treatments plus a minimum of four replicate dilution water controls.
3.6.5 Exposure Duration and Test Endpoint. Examine the effects of the dispersant product on normal development of sea urchin embryos over a period of 72 hours. An IC 50 (the exposure concentration at which normal development is inhibited in 50% of the embryos) with 95% confidence intervals are to be determined in place of an IC 25. The concentration of dispersant causing inhibition of development in 50% of exposed embryos (IC 50 ) with the lower and upper 95% confidence intervals (LCI 95 and ULCI 95 ) must be calculated at the end of the exposure period. Mortality determinations are not required.
3.6.6 Test Acceptability. Requirements of the assay are: (i) ≥80% normal larval development in the control treatment, (ii) the minimum significant difference (MSD) that can be statically detected relative to the control is ≤25%, iii) test results which support the determination of a statistically valid IC 50 and 95% confidence interval unless the LC 50 is >1000 μl/L or is greater than the limits of water solubility of dispersibility.
3.6.7 Urchin Developmental Test Summary. A summary of required test conditions is provided in Table 7 of this Appendix.
3.7 Seven-day Subchronic Tests with M. beryllina and A. bahia
3.7.1 General. Use Section 13, Method 1006.0, “Inland Silverside ( Menidia beryllina ) Larval Survival and Growth Method,” and Section 14, Method 1007.0, “Mysid ( Mysidopsis [renamed Americamysis ] bahia ) Survival, Growth, and Fecundity Method” of EPA's Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms (EPA–821–R–02–014) (3) for testing of dispersant product.
3.7.2 Test Solutions. Modify procedures in EPA–821–R–02–014, sections 13 and 14 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2.1 of this Appendix and exposure concentrations following section 3.3 of this Appendix. Exposure solutions should be renewed every 24 hours for the duration of the test.
3.7.3 Number of Treatments, Replicates and Organisms. (i) Menidia beryllina: Conduct a minimum of four replicates of at least five exposure treatments plus a minimum of four replicate dilution water controls. Expose ten M. beryllina per replicate treatment. (ii) Americamysis bahia: Conduct a minimum of eight replicates of at least five exposure treatments plus a minimum of eight replicate dilution water controls. Expose five A. bahia per replicate treatment.
3.7.4 Exposure Duration and Test Endpoint. The test duration is seven days for both species. Test endpoints for Menidia beryllina are survival and growth (dry weight) and for Americamysis bahia is survival, growth (dry weight) and fecundity. Calculate an LC 50 and 95% confidence interval for survival and IC 25 and IC 50 with 95% confidence intervals for growth (and fecundity for A. bahia only). Report the lowest observed effect concentration (LOEC) and no observed effect concentration (NOEC) for each endpoint.
3.7.5 Test Acceptability. Requirements of the assay are: (i) ≥80% survival in the control treatment for each species, (ii) dry weights must meet the specific requirements as stipulated in Method 1006.0 for Menidia beryllina and Method 1007.0 for Americamysis bahia.
3.7.6 Subchronic Test Summary. A summary of required test conditions for each species is provided in Table 7 of this Appendix.
3.8 Laboratory Report. The laboratory must include, for each toxicity test report, all applicable information, data and analyses as follows:
3.8.1 Test Objective: protocol title and source, endpoint(s);
3.8.2 Product Information: product name, manufacturer contact information, lot number, production date, date received/chain of custody;
3.8.3 Contract Facility: contact information;
3.8.4 Dilution Water: source, pretreatment, physical and chemical characteristics (pH, salinity);
3.8.5 Test Conditions: date and time of test (start and end), test chambers type and volume, volume of solution per chamber, number of organisms per chamber, number of replicate chambers per treatment, feeding frequency, amount and type of food, test concentrations, test temperature (mean and range), test salinity (mean and range);
3.8.6 Test Organisms: common and scientific name, source contact information, age and date purchased, acclimation conditions ( e.g., temperature, salinity, both mean and range), age at test start;
3.8.7 Reference toxicant: date received, lot number, date of most recent test, results and current Cumulative Sum Chart, dilution water used, physical and chemical methods used;
3.8.8 Quality Assurance: verification of laboratory accreditation, including subcontractor facilities;
3.8.9 Test Results: raw data in tabular and graphical form, daily records of affected organisms in each concentration replicate and controls, table of required endpoints ( i.e., LC 50 with 95% confidence interval (CI), IC 25 and IC 50 with 95% CI, LOEC and NOEC), statistical methods used to calculate endpoints, summary tables of test conditions and QA data;
3.8.10 Analytical Results: method summary including Limit of Detection (LOD)/Limit of Quantitation (LOQ), deviations and reasons if any, sample summary, results including chromatograms and data qualifiers, QA summary including calibration curves, method blank and surrogate recovery, analytical results summary; and
3.8.11 Conclusions: Relationship between test endpoints and threshold limit.
| Acute M. beryllina | Acute A. bahia | Subchronic M. beryllina | Subchronic A. bahia | Development S. purpuratus/A. punctulata | |
|---|---|---|---|---|---|
| 1 Recommended minimum value. | |||||
| 2 Less than or equal to 24-hr range in age. | |||||
| Test type | Static non-renewal | Static non-renewal | Static renewal (daily) | Static renewal (daily) | Static non-renewal. |
| Test duration | 96 hours | 48 hours | 7 days | 7 days | 72 ± 2 hours. |
| Salinity | 20 ± 2‰ | 20 ± 2‰ | 20 ± 2‰ | 20 ± 2‰ | 34 ± 2‰. |
| Temperature | 25 ± 1 °C. Test temperatures must not deviate (maximum minus minimum temperature) by for than 3 °C during the test. | 15 ± 1 °C. | |||
| Light quality | Ambient laboratory illumination. 10–20 μE/m 2 /s. 16 h light, 8 h darkness, with phase in/out period recommended. | ||||
| Light intensity | |||||
| Photoperiod | |||||
| Test chamber size 1 | 250 mL | 250 mL | 600 mL–1 L | 400 mL | 30 mL. |
| Test solution volume 1 | 200 mL | 200 mL | 500–750 mL | 150 mL | 10 mL. |
| Age of test organism 2 | 9–14 days | 1–5 days | 7–11 days | 7 days | 1 hr old fertilized eggs. |
| No. organisms per test chamber | 10 | 10 | 10 | 5 | 25 embryos per mL. |
| No. of replicate chambers per concentration | 3 | 3 | 4 | 8 | 4. |
| Feeding regime | Refer to specific feeding procedures provided in each test method. | None. | |||
| Aeration | None, unless DO falls below 4.0 mg/L, then aerate all chambers. Rate: <100 bubbles/minute. 5 exposure concentrations and a control (minimum required). | ||||
| Test concentrations | |||||
| Test acceptability (required) | ≥90% survival in controls | ≥90% survival in controls | For controls: ≥80% survival; average dry weight ≥0.5mg where test starts with 7 day old larvae, or ≥0.43 mg for larvae preserved for ≤7days | For controls: ≥80% survival; average dry weight ≥0.20 mg | ≥80% normal shell development in controls. |
3.9 References for Section 3.0
(1) U.S. EPA. 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fifth Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–012).
(2) U.S. EPA. 1995. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to West Coast Marine and Estuarine Organisms. First Edition. U.S. Environmental Protection Agency, Washington, DC (EPA/600/R–95–136)
(3) U.S. EPA. 2002. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms. Third Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–014).
(4) U.S. EPA. 2008. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods U.S. Environmental Protection Agency, Washington, DC (SW–846) http://www.epa.gov/osw/hazard/testmethods/sw846/online/index.htm .
4.0 Standard Acute Toxicity Testing of Surface Washing Agents, Bioremediation Agents, Herding Agents, and Solidifiers.
4.1 Summary. This laboratory protocol includes testing for: (1) saltwater standard static acute toxicity tests for test products with the mysid shrimp, Americamysis bahia (48-hr duration) and the inland silverside, Menidia beryllina (96-hr duration); and (2) freshwater standard static acute toxicity tests for test products with the daphnid, Ceriodaphnia dubia (48-hr duration) and the fathead minnow, Pimephales promelas (96-hr duration) (see Table 8 of this Appendix).
| Application environment | Test procedure | |||
| 96-hr Static acute: Menidia beryllina | 48-hr Static acute: Americamysis bahia | 96-hr Static acute: Pimephales promelas | 48-hr Static acute: Ceriodaphnia dubia | |
| Saltwater only | yes | yes | no | no. |
| Freshwater only | no | no | yes | yes. |
| Freshwater and saltwater use | yes | yes | yes | yes. |
4.2 Dilution Water. Use Section 7 of EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) [1] for preparation of the appropriate dilution water for each species tested. Use of clean natural or synthetic seawater for tests conducted with saltwater species is acceptable.
4.3 Preparation of Stock Solutions.
4.3.1 Liquid Surface Washing Agents and/or Herding Agents. Prepare a 1000 µL/L stock solution prior to test initiation by adding 1.1 mL of test product to 1100 mL of dilution water in a glass vessel. Place on a magnetic stir plate then add and center a stir bar and adjust the stir plate to obtain a vortex of 25% of the total volume of the liquid. Mix the resulting stock solution for approximately five minutes at room temperature. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 4.6 and/or 4.7 of this Appendix, as appropriate.
4.3.2 Bioremediation Agents. For products consisting of two or more liquid and/or solid components, prepare the product following the manufacturers recommended procedure and ensure the test product mixture is completely blended. Prepare a 1000 µL/L stock solution prior to test initiation by adding 1.1 mL of the test product mixture to 1100 mL of dilution water in a glass vessel. Place on a magnetic stir plate then add and center a stir bar and adjust the stir plate to obtain a vortex of 25% of the total volume of the liquid. Mix the resulting stock solution for approximately five minutes at room temperature. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 4.5 and/or 4.6 of this Appendix, as appropriate.
4.3.3 Solid Phase Products. Assessment of the toxicity of solidifiers and other solid phase products are determined using the aqueous phase of water-accommodated fractions (WAFs) of the test product. Fit a glass aspirator bottle (approximately 23L) equipped with a hose bib at the base with a length of silicon tubing containing a hose clamp. Fill the bottle with 19L of dilution water leaving a 20% headspace above the liquid, place on a magnetic stir plate then add and center a stir bar. Add the test product at 25 g/L and securely seal the bottle using a silicon stopper and wraps of parafilm. Adjust the stir plate to obtain a vortex of 25% of the total fluid volume, stir for 18 hours then settle for 6 hours. Maintain the temperature at 25 °C during stirring and settling. Purge the hose at the base of the bottle of any material followed by removal of the WAF (aqueous phase) into a clean glass container without disturbing the product on the surface. The WAF should be remixed and used for the preparation of exposure solutions following procedures outlined in section 4.4 of this Appendix.
4.4 Preparation of Exposure Concentrations.
4.4.1 Concentration Selection. Preliminary rangefinder tests may be necessary using a series of logarithmic concentrations ( e.g. 0.1, 1, 10, 100 µl test product/L) to determine the appropriate exposure concentration range necessary to determine LC 50 values and 95% confidence intervals. For definitive tests, conduct a minimum of five test concentrations using a geometric ratio between 1.5 and 2.0 ( e.g. 2, 4, 8, 16, and 32). Note that when testing the product, the highest test concentration should not exceed the test product's self-dispersibility limit.
4.4.2 Exposure Concentrations. Exposure solutions are prepared by adding the appropriate amount of stock solution directly to dilution water in each test chamber. Mix each exposure solution using five rotations in one direction followed by five rotations in the opposite direction using a solid glass stir rod.
4.4.3 Reference Toxicants. Separate toxicity tests must be performed with a reference toxicant for each species tested. Conduct additional reference toxicity tests any time a change in the culture population or source of a test species occurs. Use reagent grade quality sodium dodecyl sulfate (SDS), also known as dodecyl sodium sulfate (DSS), and sodium lauryl sulfate (SLS) as the reference toxicant. Information on procedures for conducting reference toxicant tests with these species can be found in section 4 of EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (3).
4.5 Saltwater Static Acute Tests with Menidia beryllina and Americamysis bahia
4.5.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with the test product.
4.5.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following the appropriate sections (4.3.1, 4.3.2, or 4.3.3) of this Appendix and exposure concentrations following section 4.4 of this Appendix.
4.5.3 Number of Treatments, Replicates and Organisms. Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment.
4.5.4 Exposure Period. Test duration is 48-hr for A. bahia and 96-hr for M. beryllina. Mortality must be recorded at each 24 hour period of each test.
4.5.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 µl/L or is determined to be greater than the limits of water solubility or dispersibility.
4.5.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 9 of this Appendix.
4.6 Freshwater Static Acute Tests with Pimephales promelas and Ceriodaphnia dubia
4.6.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with the test product.
4.6.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following the appropriate sections (4.3.1, 4.3.2, or 4.3.3) of this Appendix and exposure concentrations following section 4.4 of this Appendix.
4.6.3 Number of Treatments, Replicates and Organisms. P. promelas: Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment. C. dubia: Conduct a minimum of four replicates of at least five exposure treatments plus a minimum of four replicate dilution water controls. Expose five organisms per replicate treatment.
4.6.4 Exposure Period. Test duration is 48-hr for C. dubia and 96-hr for P. promelas. Mortality must be recorded at each 24 hour period of each test.
4.6.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 µl/L or is determined to be greater than the limits of water solubility of dispersibility.
4.6.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 9 of this Appendix.
4.7 Laboratory Report. The laboratory must include, for each toxicity test report, all applicable information, data and analyses as follows:
4.7.1 Test Objective: protocol title and source, endpoint(s);
4.7.2 Product Information: product name, manufacturer contact information, lot number, production date, date received/chain of custody;
4.7.3 Contract Facility: contact information;
4.7.4 Dilution Water: source, pretreatment, physical and chemical characteristics (pH, salinity);
4.7.5 Test Conditions: date and time of test (start and end), test chambers type and volume, volume of solution per chamber, number of organisms per chamber, number of replicate chambers per treatment, feeding frequency, amount and type of food, test concentrations, test temperature (mean and range), test salinity (mean and range);
4.7.6 Test Organisms: common and scientific name, source contact information, age and date purchased, acclimation conditions ( e.g., temperature, salinity, both mean and range), age at test start;
4.7.7 Reference toxicant: date received, lot number, date of most recent test, results and current Cumulative Sum Chart, dilution water used, physical and chemical methods used;
4.7.8 Quality Assurance: verification of laboratory accreditation, including subcontractor facilities;
4.7.9 Test Results: raw data in tabular and graphical form, daily records of affected organisms in each concentration replicate and controls, table of required endpoints ( i.e., LC 50 , 95% CI, inhibited concentration for 50% of the species (IC 50 ), lower observed effect concentration (LOEC) and no observed effect concentration (NOEC)), statistical methods used to calculate endpoints, summary tables of test conditions and QA data; and
4.7.10 Conclusions: Relationship between test endpoints and threshold limit.
| Saltwater acute M. beryllina | Saltwater acute A. bahia | Freshwater acute P. promelas | Freshwater acute C. dubia | |
|---|---|---|---|---|
| 1 Recommended minimum value. | ||||
| 2 Less than or equal to 24-hr range in age. | ||||
| Test type | Static non-renewal | Static non-renewal | Static non-renewal | Static non-renewal. |
| Test duration | 96 hours | 48 hours | 96 hours | 48 hours. |
| Salinity | 20 ± 2‰ | 20 ± 2‰ | NA | NA. |
| Temperature | 25 ± 1 °C. Test temperatures must not deviate (maximum minus minimum temperature) by more than 3 °C during the test. Ambient laboratory illumination. 10–20 µE/m 2 /s. 16 h light, 8 h darkness, with phase in/out period recommended. | |||
| Light quality | ||||
| Light intensity | ||||
| Photoperiod | ||||
| Test chamber size 1 | 250 mL | 250 mL | 250 mL | 30 mL. |
| Test solution volume 1 | 200 mL | 200 mL | 200 mL | 15 mL. |
| Age of test organism 2 | 9–14 days | 1–5 days | 1–14 days | <24 hours. |
| No. organisms per test chamber | 10 | 10 | 10 | 5. |
| No. of replicate chambers per concentration (minimum) | 3 | 3 | 3 | 4. |
| Feeding regime | Refer to specific feeding procedures provided in each test method. None, unless DO falls below 4.0 mg/L, then aerate all chambers. Rate: <100 bubbles/minute. 5 exposure concentrations and a control (minimum required). ≥90% survival in controls. | |||
| Aeration | ||||
| Test concentrations | ||||
| Test acceptability (required) | ||||
4.8 References for Section 4
(1) U.S. EPA. 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fifth Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–012).
5.0 Bioremediation Agent Efficacy Test Protocol
5.1 Summary. This protocol quantifies changes in weathered Alaska North Slope (ANS) crude oil composition of alkanes and aromatics resulting from the use of a bioremediation agent in either artificial seawater or freshwater. The manufacturer may test either one or both freshwater or saltwater, depending on the product's intended use. Biodegradation of the alkanes and aromatics is monitored for 28 days at 20–23 °C. Product flasks at Day 28 are compared to Day 0 flasks to determine reductions in alkanes and aromatics. A positive control of a known oil-degrading bacterial consortium supplied by EPA is tested. A negative, sterile control is also set up containing exposure water, weathered crude oil, product, and a sterilant, sodium azide. The purpose of the negative, killed control is to make sure the disappearance of the oil constituents at day 28 is due to biodegradation and not some physical loss such as volatilization. The day 28 GC/MS results from the killed control must not be less than 90% of the day 0 results. The sample preparation procedure extracts the oil phase into the solvent dichloromethane (DCM) (also known as methylene chloride) with a subsequent solvent exchange into hexane. The hexane extracts are analyzed by a high-resolution gas chromatograph/mass spectrometer (GC/MS) operated in the selected ion monitoring mode (SIM) at a scan rate of >5 scans per second.
Note to 5.1: Alaska North Slope (ANS) crude oil is artificially weathered by distillation at 521 °F (272 °C) to remove the low molecular weight hydrocarbons to approximate natural weathering processes that occur after a spill.
5.2 Apparatus. All equipment must be maintained and calibrated per standard laboratory procedures.
5.2.1 Assorted flasks and other glassware;
5.2.2 Graduated cylinders (100 mL);
5.2.3 Deionized water;
5.2.4 250 mL borosilicate glass Erlenmeyer flasks;
5.2.5 250 mL separatory funnels with stopcocks
5.2.6 Pasteur pipettes;
5.2.7 Multichannel pipettor (5–50 mL and 50–200 mL);
5.2.8 Autoclave; environmental room or incubator;
5.2.9 Balance accurate to 0.1 mg;
5.2.10 Orbital shaker table with clamps sized to hold flasks securely;
5.2.11 GC/MS instrument equipped with a DB–5 capillary column (30 m, 0.25 mm ID, and 0.25 mm film thickness) or equivalent, and a split/splitless injection port operating in the splitless mode, such as an Agilent 6890 GC/5973 MS (or equivalent) equipped with an auto-sampler for testing multiple samples; and
5.2.12 Fixed Rotor Centrifuge.
5.3 Reagents and consortium medium.
5.3.1 Stock Seawater Preparation. Prepare the artificial seawater GP2 (modified from Spotte et al., 1984) following the procedures in section 2.3 of this Appendix, to obtain the final concentration of the salts listed in Table 1 of this Appendix, except for the sodium bicarbonate (NaHCO 3 ) which is prepared separately. Autoclave the artificial seawater. Filter sterilize the concentrated solution of sodium bicarbonate through a 0.45 μm membrane filter and add to the autoclaved and cooled artificial seawater GP2 to obtain the final concentration listed in Table 1 of this Appendix.
5.3.2 Seawater for the positive control flasks. Prepare sodium triphosphate (a.k.a., sodium tripolyphosphate) (Na 5 P 3 O 10 ), potassium nitrate (KNO 3 ), and ferric chloride hexahydrate (FeCl 3 · 6H 2 O) as a concentrated solution. Filter sterilize through a 0.45 μm membrane filter and add to autoclaved artificial seawater to obtain the final nutrient concentrations listed in Table 10 of this Appendix. Calibrate the pH meter at room temperature (approximately 20–23 °C) using commercial buffers of pH 4.0, 7.0, and 10.0, as appropriate, prior to use. Adjust the pH of the artificial seawater with concentrated hydrochloric acid (HCl) or 10 normality sodium hydroxide (10 N NaOH), as appropriate.
| Constituent | Final concentration, g/L |
|---|---|
| * Added aseptically after the GP2 has been autoclaved to limit phosphorus and iron precipitation. | |
| * FeCl 3 · 6H 2 O | 0.050 |
| KNO 3 | 2.890 |
| * Na 5 P 3 O 10 | 0.297 |
5.3.3 Seawater for bioremediation agents that do not include nutrients. If a bioremediation agent contains living microorganisms but not nutrients (or limiting concentrations of nutrients), then nutrients may be added by the manufacturer. However, the total concentration of the nutrients added to the bioremediation agent must not exceed the final concentrations listed in Table 11 of this Appendix.
| Constituent | Final concentration, g/L |
|---|---|
| as Iron (Fe) | 0.010 |
| as Nitrogen (N) | 0.400 |
| as Phosphorus (P) | 0.075 |
If nutrients are supplied by the product manufacturer, the specific composition and concentration used in the efficacy testing must be submitted.
5.3.4 Freshwater Preparation. The artificial freshwater, which is a modification of Bushnell-Haas medium (Haines et al., 2005), is prepared following the concentrations listed in Table 12 of this Appendix and then autoclaved. The pH is adjusted to 7.4 before autoclaving. Constituents removed from the original formulation are KNO 3 , K 2 HPO4 and KH 2 PO 4 .
| Constituent | Final concentration (mg/L) |
|---|---|
| MgSO 4 · 7H 2 O | 200 |
| CaCl 2 · 2H 2 O | 20 |
| FeCl 3 · 6H 2 O | 50 |
| MnSO 4 × H 2 O | 0.0302 |
| H 3 BO 3 | 0.0572 |
| ZnSO 4 × 7H 2 O | 0.0428 |
| (NH 4 ) 6 Mo 7 O 2 | 0.0347 |
5.3.5 Freshwater for the positive control. To prepare the freshwater for the positive controls, prepare the nutrients potassium phosphate monobasic (KH2PO4), potassium phosphate dibasic (K2HPO4) and potassium nitrate (KNO3) as a concentrated solution. Filter sterilize and add to autoclaved artificial freshwater to obtain the final concentrations given in Table 13 of this Appendix. Calibrate the pH meter at room temperature (approximately 20–23 °C) using commercial buffers of pH 4.0, 7.0, and 10.0, as appropriate, prior to use. Adjust the pH of the artificial freshwater to 7.4 with 1 N HCl or 1 N NaOH, as appropriate.
| Constituent | Final concentration (g/L) 1 |
|---|---|
| 1 Adjust pH to 7.4 prior to autoclaving. | |
| KNO 3 | 2.89 |
| KH 2 PO 4 | 1.00 |
| K 2 HPO 4 | 1.00 |
5.3.6 Freshwater for bioremediation agents that contain living microorganisms but not nutrients or limiting concentrations of nutrients. If a bioremediation agent does not include nutrients, then nutrients may be added. However, the total concentration of the nutrients added to the bioremediation agent must not exceed the final concentrations provided in Table 14 of this Appendix.
| Constituent | Final concentration, g/L 1 |
|---|---|
| 1 Adjust to pH 7.4 prior to autoclaving. | |
| as Iron (Fe) | not added since iron is already in the freshwater solution. |
| as Nitrogen (N) | 0.400. |
| as Phosphorus (P) | 0.400. |
If nutrients are supplied by the product vendor, the specific composition and concentration used in the efficacy testing must be submitted.
5.3.7 Oil Preparation. The test oil, weathered ANS521 crude oil, can be obtained from EPA at no charge (except for a minimal shipping fee). See https://www.epa.gov/emergency-response/national-contingency-plan-subpart-j#howto for more information.
5.3.8 Sodium azide sterilant. Prepare a stock solution of NaN 3 for addition to the negative killed control. The final concentration in the killed controls will be 0.5 g/L.
5.4 Experimental Setup and Procedure
5.4.1 Autoclave clean borosilicate glass Erlenmeyer flasks (250 mL) for 20 minutes at 121 °C at 15 psig.
5.4.2 Label flasks with the appropriate code (negative control, positive control, or product; day to be sampled (0 or 28); letter indicating replicate number) to reflect the following treatment design in Table 15 of this Appendix:
| * The laboratory must report positive control test results conducted within the year of any test results for bioremediation products, for one or both types of water as applicable. | |||
| Treatment | Number of replicates at sampling times | Analysis | |
| Day 0 | Day 28 | ||
| Negative (killed) Control (oil + exposure water + product + EPA consortium + NaN 3 sterilant) | 0 | 3 | GC/MS |
| * Positive control (oil + exposure water + nutrients + EPA consortium) | 6 | 6 | GC/MS |
| Test Type 1: Product containing living microorganisms (oil + exposure water + living product + supplemented nutrients (if necessary)) | 6 | 6 | GC/MS |
| Test Type 2: Product containing proprietary nutrients but no live microorganisms (oil + exposure water + product + EPA consortium) | 6 | 6 | GC/MS |
| Test Type 3: Product (such as an enzyme) containing no live microorganisms and no nutrients (oil + exposure water + product) | 6 | 6 | GC/MS |
5.4.3 Aseptically dispense 100 mL of pre-sterilized artificial exposure water (seawater or freshwater) into each sterile flask. For the positive control flasks, use exposure water containing nutrients.
5.4.4 Tare the labeled flasks containing exposure water and other additions, as necessary, on the balance with a minimum accuracy of 0.01 g. Add drop-wise 0.50 g oil (this results in a final oil concentration of 5 g/L) using a sterile Pasteur pipette to the center of the flask taking care to avoid splashing the oil onto the sides of the flasks. Record the precise weight. ANS521 may be previously warmed in a hot water bath at 60 °C for 40–60 minutes to facilitate its flow. Take precautions when handling and charging the flasks to minimize the likelihood of contamination by exogenous microbes, including using a new sterile pipette for each series of flasks.
5.4.5 Preparation of the EPA consortium for both the positive control flasks and the flasks containing non-living bio-stimulation products. Use the supplied vials containing approximately 5 mL of the known EPA consortium frozen in glycerol. Thaw the supplied vials at room temperature ( do not allow cultures preserved in glycerol to sit at room temperature past thawing ), transfer the contents of the thawed vials to a single sterile centrifuge tube, rinse tubes with two volumes each of sterile exposure water, centrifuge at between 6,000- and 7,000-times gravity (6,000–7,000 × g ) for 15 minutes using a fixed rotor to fully pellet the cells. Carefully resuspend the cell pellet in sterile exposure water using the appropriate volume to achieve the desired seeding density, which will be provided by EPA upon shipment of the consortium.
5.4.6 Positive control flasks contain exposure water, oil, nutrients, and the EPA consortium.
5.4.7 Negative killed control flasks for all products shall contain exposure water, oil, product, the EPA consortium for products not containing a living culture, and the sodium azide sterilant at a final concentration of 0.5 g/L. Add the sodium azide sterilant prior to adding any product or EPA consortium. For the negative killed control flasks and product flasks, prepare and add the product to the flasks in a concentration specified by the manufacturer or vendor.
5.4.8 For non-living products that contain nutrient only, use the EPA consortium as the inoculum.
5.4.9 For other non-living products ( e.g., enzymes), do not add nutrients or the EPA consortium as the inoculum as they are not needed.
5.4.10 For products containing living microorganisms, prepare 6 flasks the same way as in Steps a–d, but without the EPA consortium. A product that contains its own nutrients must not be amended with nutrients, unless the product contains insufficient nutrients. Since this is a closed flask test, nutrients could be limiting if they are at the same concentration as used in the field. This could cause the product to fail the test. Thus, the manufacturer has the option to supplement its product with a higher concentration of nutrients than that contained in the product. Any nutrient supplements to a product must be reported and must not exceed the concentration limits in Table 10 (for seawater) and 13 (for freshwater) of this Appendix, as applicable.
5.4.11 Cap all flasks either with sterile cotton stoppers or loosely applied aluminum foil to allow gas exchange with the atmosphere. Set aside the T = 0 flasks for immediate extraction and analysis. Place the rest of the flasks onto the orbital shaker table. Do not tip the flasks excessively to avoid stranding oil above the mixing area of the flask. Set the orbital shaker to 200 rpm and shake the flasks for 28 days at 20–23 °C in the dark.
5.4.12 Submit all information on added microorganisms and nutrients for testing in the data report.
5.5 Sampling and Chemical Analysis.
5.5.1 Summary. At each sampling event (Days 0 and 28), product and control flasks are sacrificed for analysis of residual oil concentrations (SOP 4 of this Appendix). Record all physical observations for each flask (such as degree of emulsification, whether the oil has congealed into tar balls, wall growth, color, etc.) at each sampling. The analytical procedure is summarized in Table 16 of this Appendix. Dichloromethane (DCM) is the solvent used for the initial extraction. Solvent-exchange the extract into hexane prior to injection into the gas chromatograph. The solvent exchange is done to prevent asphaltenes from contaminating the column.
| Matrix | Measurement | Sampling/ measurement method | Analysis method | Sample container/quantity of sample | Preservation/ storage (°C) | Holding times (months) |
|---|---|---|---|---|---|---|
| DCM | N/A | Solvent Exchange to Hexane | N/A | Capped Vial with Teflon septa, 30 mL | 4 | 6 |
| Hexane | Hydrocarbon Concentration | SOP 4 | GC/MS | Capped Vial with Teflon septa, 10 mL | 4 | 6 |
5.5.2 Hydrocarbon Extraction. To measure extraction efficiency, 200 µL of the 400 mg/L surrogate recovery standard (compounds and concentrations described in SOP 1 in this Appendix) is added to each flask. Add 50 mL DCM to each flask. Transfer the contents to a 250 mL separatory funnel and shake for 2 minutes; allow the phases to separate for 2 minutes. If an emulsion remains after 2 minutes, centrifuge the emulsion in Teflon® centrifuge tubes for at least ten minutes in a low-speed centrifuge at 3,000 times gravity (3,000 × g ) to break the emulsion and recover the DCM phase. Pass the DCM extract through a funnel plugged with glass wool and containing approximately 20 g anhydrous, granular sodium sulfate (Na 2 SO 4 ) to remove water. Repeat the steps above two more times with 25 mL DCM each (100 mL DCM used in total). Add 10 mL DCM on to the sodium sulfate after the third extraction to rinse off any oil residue. Collect the extract in 125 mL serum vials, capped with Teflon lined septa and aluminum crimp seals, and store at 4 °C for up to 6 months.
5.5.3 Solvent Exchange. Perform a solvent exchange (DCM to hexane) prior to GC/MS analysis to prevent injection of asphaltenes into the GC/MS column. Transfer the DCM extract to concentration tubes. Place the tubes in a 29 °C water bath under a stream of dry nitrogen gas. Reduce the sample to 1 mL and transfer the extract to a 10 mL volumetric flask. Rinse the concentration tube with hexane and add it to the volumetric flask 2 times. Adjust the final volume with hexane to 10 mL.
5.5.4 Hydrocarbon Analysis. Quantify the concentrations of 25 alkanes, 32 aromatics and hopane (SOP 4, Table SOP 4.4 of this Appendix) using an Agilent 6890 GC/5973 MS or equivalent equipped with a 30-m × 0.25-mm ID × 0.25-μm film thickness DB–5 or equivalent fused silica column. To prepare the samples, transfer 1.0 mL of the hexane extract into a 2 mL autosampler vial with Teflon lined cap. Add 20 μL of internal standard solution to each vial with a syringe or positive displacement pipettor. SOP 2 of this Appendix outlines the procedure for preparing the internal standard solution. Load vials onto the autosampler tray and analyze in selected ion monitoring mode (SIM). Sum the individual alkane concentrations for the total alkane concentration and the individual aromatic concentrations for total aromatic concentrations in each flask.
5.6 Quality Assurance/Quality Control (QA/QC).
5.6.1 Objectives. The critical variables to be analyzed for each set of experimental conditions are the individual petroleum hydrocarbons, i.e., the alkanes ranging in carbon number from nC–14 to nC–35, plus pristane and phytane, and the 2- to 4-ring polycyclic aromatic hydrocarbons (PAHs) and their alkylated homologs as listed in SOP 4 of this Appendix. The quality assurance objectives for precision, accuracy, and detection limits are ±20%, 75–125% recovery, and 22.5 µg/L on average for the 58 compounds, respectively. For more details, refer to the SOPs of this Appendix.
5.6.2 Precision Objectives. Precision is presented as relative percent difference (RPD) for duplicate measurements and as relative standard deviation (RSD, or coefficient of variance) for triplicate measurements, applicable to replication of treatments as separate samples.
5.6.3 Accuracy Objectives. These are based on the check standards and standard oil samples run concurrently with the sample analyses for GC/MS analysis of critical compounds. Critical compounds in the check standards and in the oil standards must fall within 75–125% of expected values for the analysis to be valid. Six surrogate compounds (SOP 1 of this Appendix) added to each sample before extraction can also serve as a surrogate for determining accuracy. The measured surrogate concentrations must fall within 75–125% of expected values.
5.6.4 Calibration Range. Conduct all measurements within the linear calibration range of the instrument. The calibrated concentration range for GC/MS analysis is 0.1 mg/L to 30 mg/L. If the measured concentration of any critical compound is above the calibration range, dilute the sample and re-analyze to quantify that particular compound within the linear calibration range.
5.6.5 Quality Control. Table 17 of this Appendix summarizes the QC checks for each measurement. See the corresponding SOP in this Appendix for detailed descriptions of QC checks, frequency, acceptance criteria, and corrective actions.
| Sample matrix | Measurement | QA/QC check | Frequency | Acceptance criteria | Corrective action |
|---|---|---|---|---|---|
| DCM | GC/MS hydrocarbon analysis | Blanks | Once per calibrated run | Peak area of interfering peaks <10% of lowest standard peak area | Flush with solvent, clean injection port, and/or bake column. |
| DCM | GC/MS hydrocarbon analysis | DFTPP Check Standard | Once per calibrated run | Must pass all DFTPP criteria | If any criteria fail, retune and rerun DFTPP check standard. |
| DCM | GC/MS hydrocarbon analysis | Initial Calibration Samples | Once per calibrated run | Response Factor RSD ≤25% or R2 >0.99 | If RSD for any one compound >25%, recalibrate. |
| DCM | GC/MS hydrocarbon analysis | Calibration Check Standards | Every 10–15 samples | ±25% of expected values | If >5 compounds are out of range, recalibrate and rerun samples. |
| Hexane | GC/MS hydrocarbon analysis | Surrogates | Every Sample | ±30% of expected values | Re-inject. |
| Hexane | GC/MS hydrocarbon analysis | Biomarker Concentration | Every Sample | ±25% of average values | Re-inject. |
5.7 Pass/Fail Criteria.
5.7.1 Calculate the mean and standard deviation of the hopane-normalized total aromatics (sum of all resolved aromatics) and hopane-normalized total alkane concentrations (sum of all resolved alkanes) from the 6 independent replicates at days 0 and 28. To normalize, divide the sum of the alkane analytes and the sum of the aromatic analytes in each replicate by the hopane concentration in the corresponding replicate.
5.7.2 From those data, calculate the 95% Upper Confidence Level (UCL95) at days 0 and 28 using the following formula (Equation 11 of this Appendix):

where:
x (028) = total hopane-normalized alkane or total hopane-normalized aromatic mean of 6 replicates at days 0 and 28,
t95, 5 df = the 95% one-tailed t-value with 5 degrees of freedom (2.015),
s = the standard deviation of the 6 replicates at day 0 and 28, and
n = no. of replicates = 6.
5.7.3 Using Equation 12 of this Appendix, calculate the % reduction of each oil fraction from day 0 to day 28, using the day 0 and 28 UCL 95 hopane-normalized values for each fraction:
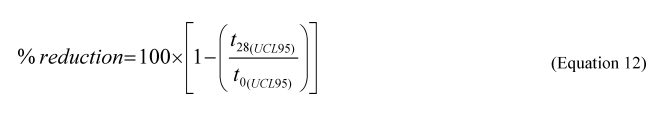
where:
t28(95) = UCL 95 of the hopane-normalized total alkane or total aromatic mean of 6 replicates on day 28, and
t0( = UCL 95 of the hopane-normalized total alkane or total aromatic mean of 6 replicates on day 0.
5.7.4 A product is successful in saltwater or freshwater if the % reduction of total alkanes (aliphatic fraction) from the GC/MS analysis is greater than or equal to 85% and the % reduction of total aromatics (aromatic fraction) is greater than or equal to 35% at day 28 based on the UCL 95 (Equation 12 of this Appendix). The benchmark reduction ranges in aliphatic and aromatic fractions for the positive control are the same as for the products specified above. The average concentration of the biomarker hopane at day 28 must not differ from the average concentration at day 0 by more than 12% in the positive control. If the conditions for the positive control are not met, the entire procedure must be repeated.
5.8 Data Verification and Reporting. GC/MS data files are generated by MS ChemStation software (the Agilent standard software for GC/MS) or equivalent for each injection. Data files contain summed ion chromatograms and selected ion chromatograms. Calibration curves are generated within MS ChemStation software, and all data files are calculated against the calibration curve by MS ChemStation. Data verification would be done by crosschecking between analysts for 10% of the raw data and its reduction process.
5.9 Laboratory Report. The summary of findings from a product test must include the data listings for each analyte that was analyzed ( i.e., all individual alkanes and aromatics in the list of required analytes), along with QA/QC checks (see Table 17) and instrument detection/reporting limits for each analyte. Express all concentrations as mg analyte/L exposure water.
5.10 Standard Operating Procedures (SOPs) 1–4
5.10.1 SOP 1. Preparation of Surrogate Recovery Standards
5.10.1.1 Preparation:
5.10.1.1.1 Solvents: Dichloromethane (DCM), Optima grade or equivalent.
5.10.1.1.2 Reagents:
D36-Heptadecane (C17)
D50-Tetracosane (C24)
D66-Dotriacontane (C32)
D10-1-Methylnaphthalene
D10-Phenanthrene
D10-Pyrene
5-beta-cholestane (coprostane)
Note: Deuterated reagents are available from Cambridge Isotope Laboratories, Andover, MA.
5.10.1.1.3 Equipment:
Micro-spatula
Small beakers
Glass funnel
Analytical balance (0.0001g)
Vials with Teflon-lined caps
Teflon wash bottle with Optima grade DCM
Volumetric flask (250 mL), class A
Pasteur pipettes
5.10.1.2 Procedure:
5.10.1.2.1 Using a calibrated analytical balance, weigh 100 mg (0.100 g) of each reagent into separate 10–25 mL beakers.
5.10.1.2.2 Dissolve the reagents in their beakers by adding 10 mL DCM. Use a Pasteur pipette to transfer the solutions to a single 250 mL volumetric flask.
5.10.1.2.3 Wash the beakers 3 or 4 times with DCM. Use a Pasteur pipette to transfer each of the washings to the 250 mL volumetric flask.
5.10.1.2.4 Dilute the solution to the 250 mL volume mark on the volumetric flask with DCM.
5.10.1.2.5 Use a glass stopper to seal the flask and homogenize the solution by inverting the flask 5 or more times. The final concentration of this solution is 400 mg/L for each of the reagents.
5.10.1.2.6 Transfer the solution into 40 mL storage vials and cap with Teflon-lined caps and label each with the date of preparation, operator, sample names, and concentrations.
5.10.1.2.7 Weigh each vial and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.1.2.8 Store these vials at 0 °C or lower.
5.10.1.2.9 Before using, allow the solution to come to room temperature, and then shake it well.
5.10.1.2.10 Weigh the vial before using it and compare the weight with the last weight recorded on the vial.
5.10.1.2.11 If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Prepare a new solution if the integrity has been compromised.
5.10.1.3 Quality Control: Inject 20 μL of the surrogate stock solution into 1 mL DCM. Add 20 μL of the internal standard solution (SOP 2 of this Appendix). Analyze this solution by GC/MS using a calibrated method (SOPs 3 and 4 of this Appendix). The expected concentration of each of the corresponding surrogate compounds is 8 ± 2 mg/L. If the measured value does not fall within this range, prepare and measure another independent surrogate solution. If the measured concentration of the second surrogate solution is within the allowable tolerance range, the calibration and instrument conditions are acceptable; properly discard the first surrogate solution. If the concentration of the second surrogate solution is also out of range, then clean and recalibrate the instrument until the problem is resolved.
5.10.2 SOP 2. Preparation of Internal Standard Solution
5.10.2.1 Preparation:
5.10.2.1.1 Solvents: Dichloromethane (DCM), Optima grade or equivalent
5.10.2.1.2 Reagents:
D34 n-Hexadecane (C16)
D42 n-Eicosane (C20)
D62 n-Triacontane (C30)
D8-Naphthalene
D10-Anthracene
D12-Chrysene
5-alpha-Androstane
Note: Deuterated reagents are available from Cambridge Isotope Laboratories, Andover, MA.
5.10.2.1.3 Equipment:
Micro-spatula
Small beakers
Glass funnel
Analytical balance (0.0001g), calibrated and checked for accuracy
Amber vials with Teflon-lined caps, labeled
Teflon wash bottle with DCM
Volumetric flask (200 mL), class A
Pasteur pipettes
5.10.2.2 Procedure:
5.10.2.2.1 Using a calibrated analytical balance, weigh 100 mg (0.100 g) of each of the reagents into separate small beakers.
5.10.2.2.2 Dissolve the reagents in their beakers by adding 10 mL DCM; using a Pasteur pipette, transfer the solutions to a single 200 mL volumetric flask.
5.10.2.2.3 Wash the beakers 3 or 4 times with DCM; use a Pasteur pipette to transfer each of the washings to the 200 mL volume mark on the volumetric flask.
5.10.2.2.4 Dilute the solution with DCM to the 200 mL volume.
5.10.2.2.5 Seal the flask with a glass stopper and homogenize the solution by inverting the flask a minimum of 5 times. The final concentration of this solution is 500 mg/L of each reagent.
5.10.2.2.6 Transfer the solution into 40 mL storage vials and cap with Teflon-lined caps. Label each vial with the date of preparation, operator, sample names, and concentrations.
5.10.2.2.7 Weigh each vial, and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.2.2.8 Store this solution at 0 °C or lower.
5.10.2.2.9 Before using, allow the solution to come to room temperature, and then shake it well.
5.10.2.2.10 Weigh the vial before using it, and compare the weight with the last weight recorded on the vial.
5.10.2.2.11 If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Prepare a new solution if the integrity has been compromised.
5.10.2.3 Quality Control: Inject 20 μL of the internal standard solution into 1 mL DCM. Analyze this solution by GC/MS. The only peaks corresponding to the internal standards must appear. If other peaks appear, particularly close to the internal standard peaks, discard the internal standard solution and prepare a new solution.
5.10.3 SOP 3. Preparation of Working Standards, Check Standards, and Oil Standards for GC/MS Consistency.
5.10.3.1 Preparation:
5.10.3.1.1 Solvent: Dichloromethane (DCM), Optima grade or equivalent
5.10.3.1.2 Stock solutions:
5.10.3.1.2.1 Oil analysis standard: 44 compounds, 100 mg/L in hexane/DCM (9:1), four, 1-mL vials required. Available from Absolute Standards, Inc., Hamden, CT, Part #90311.
5.10.3.1.2.2 Nine compound PAH standard: 1,000 mg/L in DCM, one vial. Available from Absolute Standards, Inc., Hamden, CT, Part #90822.
5.10.3.1.2.3 1,2-Benzodiphenylene sulfide, (synonym for naphthobenzothiophene). Prepare a 2 mg/mL stock solution. Available from Sigma-Aldrich Co., Part # 255122, purity 99%.
5.10.3.1.2.4 Hopane solution (17 α (H), 21β (H), 0.1 mg/mL in isooctane. Available from Sigma-Aldrich Co. Part #90656.
5.10.3.1.2.5 Surrogate solution: 400 mg/L of each reagent in DCM (see SOP 1 of this Appendix).
5.10.3.1.2.6 Internal standard solution, 500 mg/L in DCM (see SOP 2 of this Appendix).
5.10.3.1.3 Alaska North Slope Crude Oil 521 (ANS521).
5.10.3.1.4 Equipment:
5.10.3.1.4.1 Glass storage vials with Teflon-lined caps (2 mL and 40 mL capacity);
5.10.3.1.4.2 Volumetric flasks, Class A, 5 mL, 10 mL, and 100 mL
5.10.3.1.4.3 Glass syringes capable of dispensing 25–500 µL with an accuracy and precision of ± 1%, or equivalent
5.10.3.1.4.4 Wheaton repetitive dispenser, Model 411 STEP–PETTE or equivalent
5.10.3.1.4.5 Teflon wash bottle filled with Optima grade DCM or equivalent grade DCM
5.10.3.1.4.6 Pasteur pipettes
The volumes of stock solutions required to make the working standards are listed in Table SOP 3.1 of this Appendix.
| Stock standards | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| Working standards concentration, mg/L | Oil analysis mix (44 compounds, 100 mg/L) μL | Aromatics mix (9 compounds, 1,000 mg/L) μL | 1,2-Benzo- diphenylene sulfide (NBT) (2 mg/mL) μL | Surrogate solution (100 mg/L) μL | Hopane solution (100 mg/L) μL | Volumetric flask volume mL | ISTD (500 mg/L) μL |
| * Make extra STD 5 for use as check standard. | |||||||
| STD 30 (no hopane) | 1,500 | 150 | 75 | 375 | 0 | 5 | 100 |
| STD 20 (5 mg/L hopane) | 1,000 | 100 | 50 | 250 | 250 | 5 | 100 |
| STD 10 (2.5 mg/L hopane) | 500 | 50 | 25 | 125 | 125 | 5 | 100 |
| STD 5 * (1 mg/L hopane) | 500 | 50 | 25 | 125 | 100 | 10 | 200 |
| STD 5-Utility (1 mg/L hopane) | 500 | 50 | 25 | 125 | 100 | 10 (used for preparation of STD 2.5 & STD 1) | 0 |
| STD 2.5 (0.5 mg/L hopane) | Use 5 mL of STD 5-Utility and dilute to 10 mL. Use 2 mL of STD 5-Utility and dilute to 10 mL. Use 0.2 mL of STD 5-Utility and dilute to 10 mL. | 200 | |||||
| STD 1 (0.2 mg/L hopane) | 200 | ||||||
| STD 0.1 (0.2 mg/L hopane) | 200 | ||||||
5.10.3.2 Procedure for Working Standards and Check Standards:
5.10.3.2.1 Label three 5 mL volumetric flasks as STD30, STD20, STD10, and two 10 mL volumetric flasks as STD5, and STD5-utility.
5.10.3.2.2 Add 1–2 mL of DCM to each volumetric flask.
5.10.3.2.3 Using glass syringes, add the appropriate volume of stock solution A (as listed in Table SOP 3.1 of this Appendix) to the flasks labeled STD30, STD20, STD10, STD5, and STD5-utility.
5.10.3.2.4 Wash the walls of the inner neck of the flasks with several drops of DCM to rinse off the residue of the stock solution into the flasks.
5.10.3.2.5 Repeat Step 3 and Step 4 to dispense stock solutions B–E (do not add stock solution F, internal standard solution, at this step).
5.10.3.2.6 Dilute to volume with DCM for all the above flasks, seal with glass stoppers, and invert several times to homogenize the solutions.
5.10.3.2.7 Label three additional 10 mL volumetric flasks as STD2.5, STD1, and STD0.1. Wet with 1–2 mL DCM.
5.10.3.2.8 Dispense 5 mL of STD5-utility solution into flask STD2.5, 2 mL of STD5-utility solution into flask STD1, and 0.2 mL of STD5-utility solution into flask STD0.1.
5.10.3.2.9 Dilute to volume with DCM, seal with glass stoppers, and invert several times to homogenize the solutions.
5.10.3.2.10 Using a 100 μL glass syringe, dispense 100 μL of internal standard solution into flasks STD30, STD20, and STD10. Dispense 200 μL into flasks STD5, STD2.5, STD1, and STD0.1 to give a final concentration of 10 mg/L internal standard.
5.10.3.2.11 Seal with glass stoppers, and invert the flasks several times to homogenize the solutions.
5.10.3.2.12 Transfer the solutions into 2 mL storage vials, and cap with Teflon-lined caps.
5.10.3.1.13 Label each vial with date of preparation, analyst, sample names, and concentrations.
5.10.3.2.14 Weigh each storage vial and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.3.2.15 Store this solution at 0 °C or below.
5.10.3.2.16 Before using, allow the solution to come to room temperature, and shake it well.
5.10.3.2.17 Weigh the vial before opening, and compare the weight with the last weight recorded on the vial. If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Do not use the solution if the integrity has been compromised.
5.10.3.3 Procedure for Oil Standard. In a 100 mL volumetric flask, weigh 0.500 g of the standard ANS521 crude oil, add 2 mL of surrogate solution (see SOP 1 of this Appendix), and bring to volume with DCM. Add 2 mL of internal standard solution (see SOP 2 of this Appendix). Follow steps 5.10.3.2.11 through 5.10.3.2.17 of this SOP, substituting 40 mL storage vials for the 2 mL vials.
5.10.3.4 Quality Control/Quality Assurance:
5.10.3.4.1 Run the seven standard solutions using the GC/MS method (SOP 4) on a tuned GC/MS. Use the EnviroQuant software or equivalent to calculate the average Relative Response Factor (RRF) and the relative standard deviation (RSD) of the RRFs for each analyte over the six concentrations. The RRF is defined as:

5.10.3.4.2 The RSD of the RRFs for all analytes must be 25% or less. Alternatively, the coefficients of determination (R2) for the calibration curve for each target compounds and surrogate should be over 0.99.
5.10.4 SOP 4. GC/MS Method for the Analysis of Crude Oil Samples.
5.10.4.1 Instrument Specifications:
5.10.4.1.1 Use an Agilent 6890 GC coupled with an Agilent 5973 mass selective detector (MSD) and an Agilent 6890 series auto sampler or equivalent, equipped with a DB–5 capillary column (30 m, 0.25 mm I.D., and 0.25 μm film thickness) or equivalent, and a split/splitless injection port operating in the splitless mode. Data acquisition occurs in the SIM (selected ion monitoring) mode for quantitative analysis. In SIM mode, the dwell time of each ion is set to be 10 milliseconds and the ions are split up into groups by retention time. One way to divide the ions is by retention time grouping as shown in Table SOP 4.1 of this Appendix. The number of ions in each ion group must be constant, yielding the same scan rate for each group.
| Group | Ions |
|---|---|
| 1 | 57, 66, 128, 136, 142, 152, 156, 166, 170, 184. |
| 2 | 57, 66, 166, 170, 178, 180, 184, 188, 192, 194, 198, 208. |
| 3 | 57, 66, 178, 184, 188, 192, 194, 198, 202, 206, 208, 212, 220, 226. |
| 4 | 57, 66, 192, 198, 202, 206, 208, 212, 216, 220, 226, 230, 234, 245. |
| 5 | 57, 66, 191, 217, 228, 240, 242, 248, 256, 262, 264, 270, 276, 284. |
5.10.4.1.2 Table SOP 4.2 of this Appendix summarizes the instrumental conditions for crude oil analysis. Use only ultra-high purity helium (99.999% pure) as the carrier gas. In series, connect a moisture trap, an oxygen trap, and an organic trap to the carrier gas line before it enters the column.
| Instrument | Agilent 6890 Series II Gas Chromatograph (GC) with an Agilent 5973MSD and an Agilent 6890 auto sampler, or equivalent. |
| Column | DB–5 capillary column (30 m, 0.25 mm I.D., and 0.25-mm film thickness) or equivalent. |
| Carrier Gas | Helium, ultra-high purity grade (99.999%). |
| Inlet Temperature | 300 °C. |
| Transfer Line (detector) Temperature | 310 °C. |
| Oven Temperature Program | 50 °C for 4 minutes, then 7 °C/min to 310 °C, hold for 18 minutes. |
| Flow Rate | Constant flow at 1mL/min. Linear velocity: 36.2 cm/sec. |
| Injection Volume | 1 µL. |
| Split/Splitless Mode | Splitless. |
| Total Run Time | 59.18 minutes. |
5.10.4.2 Procedure for preparing the instrument:
5.10.4.2.1 Lower the injection port temperature and the oven temperature to 50 °C or less to avoid oxidation of the column.
5.10.4.2.2 Replace the liner with a clean, silanized liner. Do not touch the liner with bare fingers. A small piece of muffled glass wool may be inserted to protect the column.
5.10.4.2.3 Return the injection port and oven to the appropriate temperatures.
5.10.4.2.4 Wait five minutes after the temperature equilibrates before using the instrument.
5.10.4.3 Procedure for tuning the MSD:
5.10.4.3.1 Perform an air/water check. The value reported for the relative abundance of water (m/z 18), nitrogen (m/z 28), oxygen (m/z 32), or carbon dioxide (m/z 44) shall be less than 5% of the base peak for the system to be considered leak free and are expected to be closed to 1% for a stable system.
5.10.4.3.2 Tune the MSD using the Standard Autotune program and the decafluorotriphenylphosphine (DFTPP) Tune program to reduce instrument variability. The Autotune report file is referenced by the instrument when performing an air/water check and thus must be run at least once per month. Run standards and samples using DFTPP Tune parameters, and retune the instrument using DFTPP Tune at least once per week. The tune programs use three fragment ions of perfluorotributylamine (PFTBA) as a standard for tuning: m/z 69, 219, and 502. Tune reports must meet the following criteria:
5.10.4.3.2.1 Symmetrical peaks;
5.10.4.3.2.2 Mass assignments within ±0.2 amu's from 69, 219, and 502;
5.10.4.3.2.3 Peak widths within 0.5 ± 0.1 amu's;
5.10.4.3.2.4 Relative abundance is 100% for ion 69, at least 35% for ion 219, and at least 1% for ion 502;
5.10.4.3.2.5 Relative abundances for isotope masses 70, 220, and 503 ± 0.2 amu's are 0.5–1.5%, 2–8%, and 5–15%, respectively; and
5.10.4.3.2.6 Air and water peaks at m/z = 18, 28, 32, and 44 amu's must be very small and consistent with historical values.
5.10.4.4 Maintaining a log book. Maintain an instrument log book, and make entries for each use. Include the following information in the logbook: operator name, helium cylinder tank pressure and outlet pressure, vacuum gauge reading, any maintenance performed on the instrument (such as changing the injection port liner, gold seal, guard column, source cleaning), sequence name, data path, samples in order of injection, method information, GC column number, and the Standard Auto Tune report and DFTPP Tune report.
5.10.4.5 Running a Solvent Blank: Following a liner change or at the start of a new run, run an injection of a pure solvent to confirm that the system is free of excessive or interfering contamination. Analyze the solvent in SCAN mode using the same temperature program used for sample analysis. If contamination is present, analyze additional samples of fresh solvent until the interfering contamination is removed.
5.10.4.6 Checking the DFTPP Tune: Prior to running the first calibration standard, verify the instrument tune conditions by running a 10 ng/μL DFTPP check standard to check the mass measuring accuracy of the MS, the resolution sensitivity, the baseline threshold, and the ion abundance ranges. Run the standard using the DFTPP method provided with the instrument. Each of the criteria identified in Table SOP 4.2 of this Appendix must be met before using the instrument for analysis:
| Mass, M/z | Relative to mass | Relative abundance criteria | Purpose of checkpoint |
|---|---|---|---|
| 51 | 442 | 10–80% of the base peak | Low mass sensitivity. |
| 68 | 69 | <2% of mass 69 | Low mass resolution. |
| 70 | 69 | <2% of mass 69 | Low mass resolution. |
| 127 | 442 | 10–80% of the base peak | Low-mid mass sensitivity. |
| 197 | 198 | <2% of mass 198 | Mid mass resolution. |
| 198 | 442 | Base peak or >50% of 442 | Mid mass resolution and sensitivity. |
| 199 | 198 | 5–9% of mass 198 | Mid mass resolution and isotope ratio. |
| 275 | 442 | 10–60% of the base peak | Mid-high mass sensitivity. |
| 365 | 442 | >1% of the base peak | Baseline threshold. |
| 441 | 443 | Present and < mass 443 | High mass resolution. |
| 442 | 442 | Base peak or >50% of 198 | High mass resolution and sensitivity. |
| 443 | 442 | 15–24% of mass 442 | High mass resolution and isotopic ratio. |
5.10.4.7 Calibrating with a Multiple-Point Calibration Curve. A 5- or 6-point calibration curve is obtained by running 5 or 6 working standards (see SOP 3) on the tuned GC/MS instrument. Calculate the relative response factor (RRF) for each compound relative to its corresponding deuterated internal standard as indicated in Table SOP 4.3 of this Appendix. The relative standard deviation (RSD) of the RRFs for each compound must be less than 25%. Run an independently prepared check standard immediately after the calibration standards to validate the accuracy of the calibration curve.
5.10.4.8 Running Samples. Once the calibration curve has been validated, samples can be analyzed. Dispense 1,000 μL of sample extract into labeled auto-sampler vials. Add 20 μL of the internal standard solution (see SOP 2 of this Appendix) to the extract using a syringe or a positive displacement pipettor. Run a check standard every 10 samples to ensure the consistency of the instrument. The RRF for each compound in the check standard must be within 25% of the average RRF obtained in the initial calibration.
5.10.4.9 Quantification: Once a calibration table has been generated, quantify each data file using the “Calculate and Generate” function in the MS ChemStation software, or equivalent software. Review individual peak integration manually to ensure proper baseline integration. The quantification of a compound is based on the peak area of the primary ion (Q Ion) indicated in Table SOP 4.4 of this Appendix.
| Compound name | Quantitation ion | Reference compound for response factor | Internal standard for quantitation |
|---|---|---|---|
| * Summed compounds; draw an integration line underneath all peaks with selected ion. | |||
| N D34 C16 | 66 | N D34 C16 | D34 n C16 Q Ion 66. |
| n-C14 | 57 | n C14 | |
| n-C15 | 57 | n C15 | |
| n-C16 | 57 | n C16 | |
| N D34 C17 | 66 | N D34 C17 | |
| n-C17 | 57 | n C17 | |
| Pristane | 57 | Pristane | |
| n-C18 | 57 | n C18 | |
| Phytane | 57 | Phytane | |
| n C19 | 57 | n C19 | |
| N D42 C20 | 66 | N D42 C20 | D42 n C20 Q Ion 66. |
| n C20 | 57 | n C20 | |
| n C21 | 57 | n C21 | |
| n C22 | 57 | n C22 | |
| n C23 | 57 | n C23 | |
| N D50 C 24 | 66 | N D50 C 24 | |
| n C24 | 57 | n C24 | |
| n C25 | 57 | n C25 | |
| n C26 | 57 | n C26 | |
| n C27 | 57 | n C27 | |
| n C28 | 57 | n C28 | |
| n C29 | 57 | n C29 | |
| N D62 C30 | 66 | N D62 C30 | D62 n C30Q Ion 66. |
| n C30 | 57 | n C30 | |
| n C31 | 57 | n C31 | |
| N D66 C32 | 57 | N D66 C32 | |
| n C32 | 57 | n C32 | |
| n C33 | 57 | n C33 | |
| n C34 | 57 | n C34 | |
| n C35 | 57 | n C35 | |
| D8 Naphthalene | 136 | D8 Naphthalene | D8 Naphthalene Q Ion 136. |
| Naphthalene | 128 | Naphthalene | |
| D10 1-Methylnaphthalene | 152 | D10 1-Methylnaphthalene | |
| C1 Naphthalene * | 142 | C1 Naphthalene | |
| C2 Naphthalene * | 156 | C2 Naphthalene | |
| C3 Naphthalene * | 170 | C3 Naphthalene | |
| C4 Naphthalene * | 184 | C3 Naphthalene | |
| D10 Anthracene | 188 | D10 Anthracene | D10 Anthracene Q Ion 188. |
| D10 Phenanthrene | 188 | D10 Phenanthrene | |
| Phenanthrene | 178 | Phenanthrene | |
| C1 Phenanthrene * | 192 | C1 Phenanthrene | |
| C2 Phenanthrene * | 206 | C2 Phenanthrene | |
| C3 Phenanthrene * | 220 | C2 Phenanthrene | |
| C4 Phenanthrene * | 234 | C2 Phenanthrene | |
| Fluorene | 166 | Fluorene | |
| C1 Fluorene * | 180 | Fluorene | |
| C2 Fluorene * | 194 | Fluorene | |
| C3 Fluorene * | 208 | Fluorene | |
| Dibenzothiophene | 184 | Dibenzothiophene | |
| C1 Dibenzothiophene * | 198 | Dibenzothiophene | |
| C2 Dibenzothiophene * | 212 | Dibenzothiophene | |
| C3 Dibenzothiophene * | 226 | Dibenzothiophene | |
| Naphthobenzothiophene (NBT) | 234 | Naphthobenzothiophene | |
| C1 NBT * | 248 | Naphthobenzothiophene | |
| C2 NBT * | 262 | Naphthobenzothiophene | |
| C3 NBT * | 276 | Naphthobenzothiophene | |
| Fluoranthene | 202 | Fluoranthene | |
| D10 Pyrene | 212 | D10 Pyrene | |
| Pyrene | 202 | Pyrene | |
| C1 Pyrene * | 216 | Pyrene | |
| C2 Pyrene * | 230 | Pyrene | |
| D12 Chrysene | 240 | D12 Chrysene | D12 Chrysene Q Ion 240. |
| Benzo(a)anthracene/Chrysene * | 228 | Chrysene | |
| C1 Chrysene * | 242 | Chrysene | |
| C2 Chrysene * | 256 | Chrysene | |
| C3 Chrysene * | 270 | Chrysene | |
| C4 Chrysene * | 284 | Chrysene | |
| 5α-androstane | 245 | 5α-androstane | 5α-androstane Q Ion 245. |
| Coprostane | 219 | Coprostane | |
| Hopane | 191 | Hopane | |
5.10.4.10 Equation 14 of this Appendix is used to calculate the concentration of analytes in units of μg/g oil added:
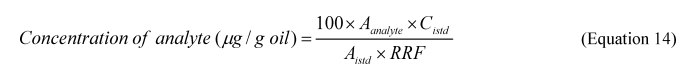
where:
A analyte = the peak area of the analyte,
C istd = the concentration of the internal standard,
A istd = the area of the internal standard,
RRF = the relative response factor, and
100 is the conversion factor to convert mg/L DCM to μg/g oil added.
5.10.4.11 If some analytes are not commercially available, the RRFs of other compounds (usually the parent compound) are used to quantify those analytes. For example, the RRF of C3-naphthalene may be used to calculate the concentrations of C3- and C4-naphthalenes. See Table SOP 4.4 of this Appendix for details. The quantification of these alkylated PAHs is relative because it is assumed that the molecular ions of the alkylated PAHs have the same RRFs as the parent compound ions. Nevertheless, these relative concentrations are useful for monitoring the fate of these compounds during the course of any analysis, as long as their concentrations are measured in a consistent way throughout the analysis.
5.10.4.12 Concentration calculations for all target compounds are performed using EnviroQuant software or equivalent. Data for each sample can be printed directly using a customized report template. Data can also be automatically entered into a spreadsheet within the EnviroQuant software.
5.10.5 Quality Assurance/Quality Control. The following criteria must be met before any samples are analyzed:
5.10.5.1 Air/water check to verify the system is leak free.
5.10.5.2 AutoTune and DFTPP Tune pass all criteria.
5.10.5.3 DFTPP check standard passes all criteria.
5.10.5.4 Solvent blank scan indicates the GC/MS system is free of interfering contamination.
5.10.5.5 Prepare and monitor a control chart of a standard oil analysis. Concentrations of the analytes in the control chart must be no more than 25% different from their historical averages.
5.10.5.6 Relative response factors for analytes in the check standards inserted between every 10 samples must be no more than 25 percent different from the average RRF of those same analytes in the calibration curve. Peak shapes must be symmetrical.
5.11 References for Section 5
(1) Haines, J.R., E.J. Kleiner, K.A. McClellan, K.M. Koran, E.L. Holder, D.W. King, and A.D. Venosa. 2005. “Laboratory evaluation of oil spill bioremediation products in salt and freshwater systems.” J. Ind. Microbiol. Biotech 32: 171–185.
SUMMARY: The Environmental Protection Agency (EPA or the Agency) is amending the requirements in Subpart J of the National Oil and Hazardous Substances Pollution Contingency Plan (NCP) that govern the use of dispersants, other chemicals and other spill mitigating substances when responding to oil discharges into jurisdictional waters of the United States. This action addresses the efficacy and toxicity of dispersants and other chemical and biological agents, as well as public, state, local, and federal officials' concerns regarding their use. Specifically, the Agency is amending the Subpart J regulatory requirements for the NCP Product Schedule in two distinct ways. First, the Agency is adding new listing criteria, revising the efficacy and toxicity testing protocols, and clarifying the evaluation criteria for removing products from the NCP Product Schedule. Second, the Agency is amending requirements for the authorities, notifications, and data reporting when using chemical or biological agents in response to oil discharges to Clean Water Act (CWA) section 311 jurisdictional waters and adjoining shorelines. These requirements are anticipated to encourage the development of safer and more effective spill mitigating products and better target the use of these products to reduce the risks of oil discharges and response technologies to human health and the environment. Further, the amendments are intended to ensure that On-Scene Coordinators (OSCs), Regional Response Teams (RRTs), and Area Committees (ACs) have sufficient information to support agent authorization of use decisions.
DATES: This final rule is effective on December 11, 2023, published in the Federal Register June 12, 2023, page 38280.
View final rule.
| Subpart J—Use of Dispersants and Other Chemicals | ||
| Heading | Revised | View text |
| §300.900 General. | ||
| (a), (c) | Revised | View text |
| (d) | Added | View text |
| §300.905 NCP Product Schedule. | ||
| Entire section | Removed | View text |
| §300.910 Authorization for agent use. | ||
| Entire section | Revised | View text |
| §300.915 Data and information requirements for listing on the NCP Product Schedule or Sorbent Product List. | ||
| Entire section | Revised | View text |
| §300.920 Addition of products to Schedule. | ||
| Entire section | Removed | View text |
| §300.950 Submission of Proprietary Business Information (PBI). | ||
| Entire section | Added | View text |
| §300.955 Addition of a product to the NCP Product Schedule or Sorbent ProductLlist. | ||
| Entire section | Added | View text |
| §300.965 Mandatory Product Disclaimer. | ||
| Entire section | Added | View text |
| §300.970 Removal of a product from the NCP Product Schedule or Sorbent Product List. | ||
| Entire section | Added | View text |
| Appendix C to Part 300—Requirements for Product Testing Protocols and Summary Test Data: Dispersant Baffled Flask Efficacy and Toxicity Tests; Standard Acute Toxicity Test for Bioremediation Agents, Surface Washing Agents, Herding Agents, and Solidifiers; and Bioremediation Agent Efficacy Test | ||
| Entire appendix | Revised | View text |
| Appendix E to Part 300 | ||
| Entire appendix | Removed | View text |
New Text
§300.5 Definitions.
Subpart J—Use of Dispersants, and Other Chemical and Biological Agents
§300.900 General.
(a) Section 311(d)(2)(G) of the Clean Water Act (CWA) requires EPA to prepare a schedule identifying dispersants, other chemicals, other spill mitigating devices and substances, if any, that may be used in carrying out the NCP; and the waters and quantities in which they may be used safely. This subpart establishes a schedule that includes the NCP Product Schedule identifying chemical and biological agents, the Sorbents Product List, and the authorization of use procedures that, when taken together, identify the waters and quantities in which such dispersants, other chemicals, or other spill mitigating devices and substances may be used safely.
* * * * *
(c) This subpart applies to the use of chemical and biological agents as defined in Subpart A of this part, or other substances that may be used to remove, control, or otherwise mitigate oil discharges.
§300.910 Authorization for agent use.
Use of chemical or biological agents in response to oil discharges must be authorized by the OSC in accordance with the provisions of this section.
(a) Use of agents identified on the NCP Product Schedule or use of burning agents on oil discharges addressed by a preauthorization plan. Area Committees and RRTs shall address, as part of their planning activities, whether preauthorization of the use of chemical and biological agents listed on the NCP Product Schedule or the use of burning agents on certain oil discharges is appropriate. Area Committees and RRTs shall, as appropriate, include applicable approved preauthorization plans in ACPs and RCPs. When a preauthorization plan is approved in advance for the use of certain agents under specified discharge situations, then the OSC may authorize the use of agents listed on the NCP Product Schedule, or the use of burning agents, for the purpose for which they were specifically listed without obtaining the incident-specific concurrences and without the natural resource trustees consultations described in paragraph (b) of this section.
(1) Preauthorization plan development. For discharge situations identified where such agents may be used, the preauthorization plan must, at a minimum, specify limits for the quantities and the duration of use, and use parameters for water depth, distance to shoreline, and proximity to populated areas. In meeting the provisions of this paragraph, preauthorization plans should document how regional factors are addressed including likely sources and types of oil that might be discharged, various potential discharge scenarios, the existence and location of environmentally sensitive resources or restricted areas that might be impacted by discharged oil, and logistical factors including inventory, storage locations and manufacturing capability of available agents, availability of equipment needed for agent use, availability of adequately trained operators, and means to monitor agent use in the environment. Preauthorization plans are to be developed by the Area Committees or the RRT in consultation with the Area Committee(s).
(2) Preauthorization plan approval. The EPA representative to the RRT, the Department of Commerce and the Department of the Interior natural resource trustees and, as appropriate the RRT representative from the state(s) with jurisdiction over waters and adjoining shorelines within the preauthorization plan area shall review and either approve, approve with modification, or disapprove the preauthorization plans. The Area Committees and RRTs shall address the withdrawal of approval from a preauthorization plan, and the RRT shall notify the NRT of the status of the preauthorization plan within 30 days from any such withdrawal.
(3) Preauthorization plan reviews. The RRT in consultation with the Area Committee(s) must review, and revise, as needed, approved preauthorization plans. These reviews must be conducted following a regular timeframe, established by the RRT and documented in the plan, to address changes that may impact the conditions under which the use of chemical and biological agents have been preauthorized. Reviews must also be conducted in any affected region, at a minimum, after a major discharge or after a Spill of National Significance (SONS) relevant to the preauthorization plan area; to address revisions of the NCP Product Schedule impacting chemical or biological agents that may be individually listed within a preauthorization plan; and to reflect new listings of threatened and/or endangered species applicable to the preauthorization plan area. The EPA RRT representative, the Department of Commerce and Department of the Interior natural resource trustees, and the RRT representative from the state(s) with jurisdiction over the waters of the area to which a preauthorization plan applies shall review and either approve, approve with modification, or disapprove any revisions to the preauthorization plans.
(b) Use of agents identified on the NCP Product Schedule or use of burning agents on oil discharges not addressed by a preauthorization plan. For discharge situations that are not addressed by a preauthorization plan developed pursuant to paragraph (a) of this section, the OSC may authorize the use of chemical or biological agents identified on the NCP Product Schedule on an oil discharge, or the use of burning agents, for the specific purpose for which they were listed with the concurrence of the EPA RRT representative and, as appropriate, the concurrence of the RRT representatives from the state(s) with jurisdiction over the waters and adjoining shorelines threatened by the release or discharge, and in consultation with the Department of Commerce and Department of the Interior natural resource trustees. In meeting the provisions of this paragraph, the OSC must consider and document for their authorization request to the RRT, at a minimum, the parameters for the use of agents including the quantities requested to be authorized, the duration of use, the depth of water, the distance to shoreline and proximity to populated areas, and should consider and document factors such as environmentally sensitive resources or restricted areas that might be impacted, agent inventory and storage locations, agent manufacturing capability, availability of equipment needed for agent use, availability of adequately trained operators and appropriate means to monitor agent use in the environment.
(c) [Reserved]
(d) Temporary exception. In circumstances to prevent or substantially reduce an imminent threat to human life that cannot be immediately addressed by other procedures or provisions of the NCP, the OSC may authorize the provisional use of any chemical or biological agent, whether it is identified or not on the NCP Product Schedule, without obtaining the concurrence of the EPA RRT representative and, as appropriate, the RRT representatives from the state(s) with jurisdiction over the waters and adjoining shorelines threatened by the release or discharge, and without consultation with the Department of Commerce and the Department of the Interior natural resource trustees. This exception shall not be used as a substitute for compliance with §300.150 of this part, including the use of personal protective equipment, or when there is sufficient time to seek authorization in accordance with paragraphs (a) or (b) of this section. If an agent is authorized for use pursuant to this paragraph, the OSC shall notify as soon as possible the EPA RRT representative and as appropriate, the RRT representatives from the affected state(s) and the Department of Commerce and Department of the Interior natural resource trustees. The OSC shall document the circumstances and the reasons for use of the agent authorized pursuant to this paragraph. Agent use for individual circumstances under this exception shall be in accordance with paragraphs (a) or (b) of this section no later than 24 hours after initial application.
(e) Prohibited agents or substances. The OSC may not authorize the use of the following:
(1) Sinking agents, or any other chemical agent, biological agent, or any substance that is used to directly sink the oil to the bottom of a water body.
(2) [Reserved]
(f) Storage and use of agents listed on the NCP Product Schedule. (1) The OSC may authorize for use only products listed on the NCP Product Schedule that are documented and certified by the responsible party or its representative to have been stored under the conditions provided by the submitter under §300.915(a)(6), and whose date of use does not exceed the expiration date listed on the container's label unless otherwise specified for expired products as provided in §300.910(f)(2), at the time of the incident.
(2) The OSC may authorize for use products listed on the NCP Product Schedule that exceed their expiration date after the responsible party or its representative documents and certifies that the expired product has been stored under the conditions provided by the submitter under §300.915(a)(6) and still meets the applicable efficacy and toxicity listing provisions under §300.915, based on testing of representative samples within the previous 12 months.
(g) Supplemental testing, monitoring, and information. The RRT may require, for both planning and response, including authorization of use, supplemental toxicity and efficacy testing, or submission of available data and information that addresses site, area, and ecosystem-specific concerns relative to the use of any chemical or biological agent. The product manufacturer or responsible party shall provide, upon request of the RRT or OSC, additional monitoring or testing data and information to inform chemical or biological agent use decisions specific to a response.
(h) Recovery of chemical agents and other substances from the environment. The responsible party shall ensure that removal actions adequately contain, collect, store, and dispose of chemical agents and other substances that are to be recovered from the environment, unless otherwise directed by the OSC. Chemical agents and other substances to be recovered include solidifiers, surface washing agents, and sorbents. The OSC should, at a minimum, consider factors such as the safety of response personnel and harm to the environment in making determinations pursuant to this paragraph.
(i) Reporting of agent use. (1) The authorizing OSC shall provide the RRT the following information on chemical and biological agents used in response to an oil discharge: product name, product category, quantity and concentrations used, duration of use, location(s) of use, any available data collected, and any available analyses of efficacy and environmental effects. This information must be provided within 30 days of completion of agent use. This information may be submitted in accordance with the OSC reporting provisions under §300.165 of this part, as applicable, subject to the 30-day timing requirement.
(2) In support of sections 300.135(n) and 300.155(a) and (b) of this part, the authorizing OSC shall provide for notification to the public, updated during a response as appropriate, the following information on chemical and biological agents used in response to an oil discharge: product name, product category, quantity and concentrations used, duration of use, and location(s) of use.
§300.915 Data and information requirements for listing on the NCP Product Schedule or Sorbent Product List.
If you are submitting an application for listing a product to the NCP Product Schedule or Sorbent Product List, you must provide EPA the information required under §300.955. Technical product data submissions are not required for burning agents. Your submission for each product must contain:
(a) General information for any product category. (1) Your name, physical address, email, and telephone number;
(2) Your identity and documentation of that identity, as the manufacturer of the product, vendor, importer, distributor of the product, and/or a designated agent acting on behalf of the manufacturer.
(3) All name(s), brand(s), and/or trademark(s) under which the product is to be sold;
(4) Names, physical addresses, emails , and telephone numbers of the primary distributors, vendors, importers and/or designated agent acting on behalf of the manufacturer;
(5) The Safety Data Sheet (SDS) for the product;
(6) The maximum, minimum, and optimum temperature, humidity, and other relevant conditions for product storage and a brief description of the consequences to performance if the product is not stored within these limits;
(7) The anticipated shelf life of the product at the storage conditions noted in paragraph (a)(6) of this section and documentation for this determination;
(8) A sample product label for all name(s), brand(s), and/or trademark(s) under which the product is to be sold that includes manufacture and expiration dates, and conditions for storage. You may use an existing label provided it already contains the required dates and storage information;
(9) The chemical or biological agent category under which you want the product to be considered for listing on the NCP Product Schedule, including detailed information on the specific process(es) through which the product affects the oil, and the specific environment(s) on which it is intended to be used ( e.g., waters and/or adjoining shorelines). If your product meets the definition of more than one chemical or biological agent category, you must identify all applicable categories and provide the test data to meet the listing criteria appropriate to each;
(10) Recommended product use procedures, including product concentrations, use ratios, types of application equipment, conditions for use, any application restrictions; and, as applicable, procedures for product and oil containment, collection, recovery, and disposal. These procedures must address, as appropriate, variables such as weather, water salinity, water temperature, types and weathering states of oils or other pollutants. The procedures must include supporting documentation and current applicable standard methods used to determine them;
(11) Available information on environmental fate, including any known measured data, methodologies, and supporting documentation, on the persistence, bioconcentration factor, bioaccumulation factor, and biodegradability of the product and all of its components in the environment;
(12) The physical and chemical properties of the product, as appropriate, and a citation for the current applicable standard methods used to determine them, including:
(i) Physical state and appearance;
(ii) Vapor pressure;
(iii) Flash point;
(iv) Pour point;
(v) Viscosity;
(vi) Specific gravity;
(vii) Particle size for solid components; and
(viii) pH;
(13) The identity and concentration of all components in the product, including each specific component name; corresponding Chemical Abstract Service (CAS) Registry Number; the maximum, minimum, and average weight percent of each component in the product; and the intended function of each component ( e.g., solvent, surfactant);
(14) For products that also contain microorganisms, enzymes, and/or nutrients, provide the following along with a citation or a description of the methodology used to determine:
(i) The name of all microorganisms by current genus and species, including any reclassifications, and any physical, chemical, or biological manipulation of the genetic composition and the weight percent of each genus in the product;
(ii) The name of all enzymes and their International Union of Biochemistry (I.U.B.) number(s); Enzyme Classification (EC) code numbers; the source of each enzyme; units; and specific oil-degrading activity;
(iii) The name(s), maximum, minimum, and average weight percent of the nutrients contained in the product; and
(iv) Data, methodology, and supporting documentation, for the levels of bacterial, fungal, or viral pathogens or opportunistic pathogens including, but not limited to: enteric bacteria such as Salmonella, fecal coliforms, Shigella, coagulase positive Staphylococci, and beta hemolytic Streptococci and enterococci;
(15) Data, methodology, and supporting documentation for the levels of the following:
(i) Arsenic, cadmium, chromium, copper, lead, mercury, nickel, vanadium, zinc, and any other heavy metal reasonably expected to be in the product;
(ii) Cyanide;
(iii) Chlorinated hydrocarbons;
(iv) Pesticides;
(v) Polychlorinated Biphenyls (PCBs); and
(vi) Polycyclic aromatic hydrocarbons (PAHs).
(16) Certification, including data, methodology, and supporting documentation, indicating that the product does not contain any of the prohibited agents or substances identified in §300.910(e);
(17) Information about the accredited laboratory that conducted the required tests, including:
(i) Name of the laboratory, address, contact name, email, and phone number; and
(ii) The national and/or international accreditations held by the laboratory that are applicable to the test(s) performed;
(18) All test data and calculations, including:
(i) Raw data and replicates, including positive controls;
(ii) Notes and observations collected during tests;
(iii) Calculated mean values and standard deviations;
(iv) Reports, including a summary of stock solution preparation;
(v) Source and preparation of test organisms;
(vi) Test conditions; and
(vii) Chain of custody forms;
(19) An estimate of the annual product production volume, the average and maximum amount that could be produced per day, and the time frame needed to reach that maximum production rate in days;
(20) Recognition received from EPA's Design for the Environment (DfE) or Safer Choice programs, as applicable; and
(21) International product testing or use data or certifications, if available, informing the performance capabilities or environmental impacts of the product.
(b) Dispersant testing and listing requirements —(1) Dispersant efficacy test and listing criteria. Test the dispersant product for efficacy using the Baffled Flask Test (BFT) method in Appendix C to part 300. To be listed on the NCP Product Schedule, the dispersant must demonstrate for each temperature a Dispersant Effectiveness (DE) at the 95% lower confidence level (LCL 95 ) greater than or equal to:
(i) ≥70% for Strategic Petroleum Reserve Bryan Mound at 5 °C;
(ii) ≥75% for Strategic Petroleum Reserve Bryan Mound at 25 °C;
(2) Dispersant toxicity tests and listing criteria. Use the methods specified in Appendix C to part 300 to test the dispersant alone, and the dispersant mixed with Strategic Petroleum Reserve Bryan Mound for acute toxicity, using Americamysis bahia and Menidia beryllina. Use the methods specified in Appendix C to part 300 to test the dispersant alone for developmental toxicity using Strongylocentrotus purpuratus or Arbacia punctulata and for subchronic effects using Americamysis bahia and Menidia beryllina. To be listed on the NCP Product Schedule, the dispersant alone must demonstrate:
(i) A median lethal concentration (LC 50 ) at the lower 95% confidence interval greater than 10 ppm;
(ii) An inhibition concentration for 50% of the test species (IC 50 ) at the lower 95% confidence interval greater than 1 ppm; and
(iii) A subchronic No Observed Effect Concentration (NOEC) greater than 1 ppm.
(3) Limitations. A dispersant may only be listed on the NCP Product Schedule for use in saltwater environments for which it meets the efficacy and toxicity listing criteria.
(c) Surface washing agent testing and listing requirements —(1) Surface washing agent efficacy test and listing criteria. To be listed on the NCP Product Schedule, using an applicable standard methodology, the surface washing agent must meet an efficacy of greater than or equal to 30% in either freshwater or saltwater, or both, depending on the intended product use.
(2) Surface washing agent toxicity test and listing criteria. Using the toxicity test methodology in Appendix C to part 300, test the surface washing agent for acute toxicity against freshwater species Ceriodaphnia dubia and Pimephales promelas, or saltwater species Americamysis bahia and Menidia beryllina, or both, depending on the intended product use. To be listed on the NCP Product Schedule, the surface washing agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(3) Limitations. Surface washing agent listing would be for use only in freshwater and/or saltwater environments for which it was tested and for which it met the efficacy and toxicity listing criteria.
(d) Bioremediation agent testing and listing requirements —(1) Bioremediation agent efficacy test and listing criteria. To be listed on the NCP Product Schedule, a bioremediation agent must successfully degrade both alkanes and aromatics as determined by gas chromatography/mass spectrometry (GC/MS) in freshwater or saltwater, or both, depending on the intended product use, following the test method specified in Appendix C to part 300. The percentage reduction of total alkanes (aliphatic fraction) from the GC/MS analysis must be greater than or equal to 85% at day 28, based on the ninety-fifth (95th) percentile Upper Confidence Limit (UCL 95 ) for both freshwater and saltwater. The percentage reduction of total aromatics (aromatic fraction) must be greater than or equal to 35% at day 28 for both saltwater and freshwater based on the UCL95.
(2) Bioremediation agent toxicity test and listing criteria. The bioremediation agent must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the bioremediation agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(3) Limitations. Bioremediation agent listing would be for use only in the freshwater and/or saltwater environments for which it was tested and for which it met the efficacy and toxicity listing criteria.
(4) Generic listing. If the product consists solely of: ammonium nitrate, ammonium phosphate, ammonium sulfate, calcium ammonium nitrate, sodium nitrate, potassium nitrate, synthetically-derived urea, sodium triphosphate (or tripolyphosphate), sodium phosphate, potassium phosphate (mono- or dibasic), triple super phosphate, potassium sulphate, or any combination thereof, no technical product data are required. The product will be generically listed as non-proprietary nutrients on the NCP Product Schedule, and no further action is necessary.
(e) Solidifier testing and listing requirements. (1) Solidifiers must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the solidifier must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(2) Limitations. Solidifier listing would be for use only in the freshwater and/or saltwater environments for which it was tested and for which it met the toxicity listing criteria.
(f) Herding agent testing and listing requirements. (1) Herding agents must be tested for acute toxicity in freshwater or saltwater, or both, depending on the intended product use, following the method specified in Appendix C to part 300. To be listed on the NCP Product Schedule, the herding agent must demonstrate an LC 50 at the lower 95% confidence interval greater than 10 ppm in either freshwater or saltwater for all tested species.
(2) Limitations. Herding agent listing would be for use only in freshwater and/or saltwater environments for which it was tested and for which it met the toxicity listing criteria.
(g) Sorbent requirements. Known sorbent materials and products will be identified on a publicly available Sorbent Product List for the use of such products when responding to an oil discharge as follows:
(1) For sorbent products that consist solely of the following materials, or any combination thereof, no technical data are required to be submitted for listing on the Sorbent Product List, and no further action is necessary for use as a sorbent:
(i) Feathers, cork, peat moss, and cellulose fibers such as bagasse, corncobs, and straw;
(ii) Volcanic ash, perlite, vermiculite, zeolite, and clay; and
(iii) Polypropylene, polyethylene, polyurethane, and polyester.
(2) If the product consists of one or more natural organic substances, inorganic/mineral compounds, and/or synthetic compounds not specifically identified in paragraph (g)(1) of this section but you believe the product meets the definition of a sorbent then, as applicable under §300.955(a) and (b), you must submit the following information for consideration for listing it as a sorbent on the Sorbent Product List:
(i) The information required under paragraphs (a)(1) through (a)(8), and paragraph (a)(13) through (a)(15) of this section;
(ii) The certification required under paragraph (a)(16) of this section; and
(iii) Information, including data, to support the claim your product meets the sorbent definition under §300.5.
Appendix C to Part 300—Requirements for Product Testing Protocols and Summary Test Data: Dispersant Baffled Flask Efficacy and Toxicity Tests; Standard Acute Toxicity Test for Bioremediation Agents, Surface Washing Agents, Herding Agents, and Solidifiers; and Bioremediation Agent Efficacy Test
Table of Contents
1.0 Applicability and Scope
2.0 Baffled Flask Dispersant Efficacy Test (BFT)
3.0 Dispersant Toxicity Testing
4.0 Standard Acute Toxicity Testing for Surface Washing Agents, Bioremediation Agents, Herding Agents, and Solidifiers
5.0 Bioremediation Agent Efficacy Test Protocol
Illustrations
Figure Number
1. A Baffled Trypsinizing Flask
Tables
Table Number
1. Constituent Concentrations for GP2 Artificial Seawater
2. Test Oil Characteristics
3. Stock Standard Solution Preparation
4. Dispersant Calibration Example for Test Oil
5. Sample Calculation With ANS
6. Toxicity Testing Requirements for Dispersants
7. Summary of Test Conditions—Dispersant Toxicity
8. Toxicity Testing Requirements for Surface Washing Agents, Herding Agents, Bioremediation Agents and Solidifiers
9. Summary of Test Conditions—Surface Washing Agents, Herding Agents, Bioremediation Agents and Solidifiers Toxicity
10. Artificial Seawater Nutrient Concentrations
11. Artificial Seawater Nutrient Concentrations for Bioremediation Agents Having No Nutrients Included
12. Constituent Concentrations for Artificial Freshwater (Bushnell-Haas)
13. Freshwater Nutrient Concentrations
14. Artificial Freshwater Nutrient Concentration for Bioremediation Agents Having No Nutrients Included
15. Bioremediation Efficacy Test—Summary of Experimental Setup
16. Bioremediation Efficacy—Summary of Analytical Procedures
17. QA/QC Checks
Standard Operating Procedures Tables
SOP 3–1 Amount of Stock Solutions Required To Make the Working Standards
SOP 4–1 Ions Associated With Retention Time Groups
SOP 4–2 Instrumental Conditions for Crude Oil Analysis
SOP 4–3 Ion Abundance Criteria for DFTPP
SOP 4–4 Target Compound List
1.0 Applicability and Scope. This Appendix establishes laboratory protocols required under Subpart J (Use of Dispersants and Other Chemical and Biological Agents) of 40 CFR part 300 (National Oil and Hazardous Substances Pollution Contingency Plan) to make listing determinations for the Product Schedule. The protocols apply, based on product type, to dispersants, bioremediation agents, surface washing agents, herding agents, and solidifiers as defined in Subpart A (Introduction) of 40 CFR part 300.
2.0 Baffled Flask Dispersant Efficacy Test (BFT)
2.1 Summary. This laboratory protocol establishes procedures to evaluate the degree to which a product effectively disperses oil spilled on the surface of seawater, using a modified 150-mL screw-cap trypsinizing flask (an Erlenmeyer flask with baffles) with a glass and Teflon® stopcock near the bottom to allow removal of subsurface water samples without disturbing the surface oil layer. The efficacy of a dispersant is measured using one reference oil, Strategic Petroleum Oil Reserve Bryan Mound at two temperatures (5 °C and 25 °C). Six replicates and one method blank are required at each temperature. A layer of oil is placed on the surface of artificial seawater, and the dispersant is added to the slick at a dispersant:oil ratio (DOR) of 1:25 (4%) by volume. A standard orbital shaker table provides turbulent mixing at a speed of 250 revolutions per minute (rpm) for 10 minutes, immediately after which it is maintained stationary for 10 minutes to allow non-dispersed oil to rise to the water's surface. An undisturbed water sample is removed from the bottom of the flask through the stopcock, extracted with dichloromethane (DCM), and analyzed for oil content by UV-visible absorption spectrophotometry at wavelengths ranging between 340 and 400 nm.
2.2 Apparatus. All equipment must be maintained and calibrated per standard laboratory procedures.
2.2.1 Modified Trypsinizing Flask. A modified 150 mL glass screw-capped Erlenmeyer flasks with baffles ( e.g., Wheaton No. 355394 or equivalent) fitted with a 2 mm bore Teflon® stopcock and glass tubing, the center of which is no more than 1.3 cm from the bottom, as shown in Figure 1.

Figure 1. A Baffled Trypsinizing Flask
2.2.2 Orbital Shaker Table. An orbital shaker table with a variable speed control unit capable of maintaining 250 rpm. The orbital diameter must be approximately 1.0 inch (2.5 cm) +/−0.1 inch (0.25 cm).
2.2.3 Spectrophotometer. A UV-visible spectrophotometer capable of measuring absorbance between 340 and 400 nm ( e.g., Shimadzu UV–1800, Agilent 8453, or equivalent). Use standard transmission-matched quartz 10-mm path length rectangular cells with PTFE cover for absorbance measurements.
2.2.4 Glassware. Including: 25-ml graduated mixing cylinders (a graduated cylinder with a ground glass stopper); 50- and 100-ml graduated cylinders; 125-mL separatory funnels with Teflon stopcocks; 10-ml volumetric flasks; 30-ml crimp style glass serum bottles; 1-, 2-, 5-mL pipettes; other miscellaneous laboratory items.
2.2.5 Micropipettor. Use a micropipettor capable of dispensing 4 µL of dispersant and 100 µL of oil ( e.g., Brinkmann Eppendorf repeater pipettor with 100 µL and 5 mL syringe tip attachments or equivalent).
2.2.6 Syringes. 25-, 100-, 250-, 1,000-, 2,500-, 5,000-µl gas-tight syringes.
2.2.7 Constant temperature rooms or incubators to hold the shaker at 5 °C and 25 °C.
2.2.8 Analytical Balance.
2.2.9 Chemical fume hood.
2.3 Reagents.
2.3.1 Artificial seawater. Use the artificial seawater GP2 formulation shown in Table 1 of this Appendix.
2.3.2 Test oil. Use the EPA standard reference oil Strategic Petroleum Reserve Bryan Mound. To obtain this oil at no charge (except for a minimal shipping fee), see the instructions at http://www.epa.gov/emergencies/content/ncp/index.htm. Selected properties are summarized in Table 2 of this Appendix.
2.3.3 Dichloromethane (DCM) (also known as methylene chloride), pesticide quality.
2.4 Container Handling and Storage.
2.4.1 Glassware. If the glassware has been used with oil before, rinse with DCM to remove as much of the oil adhering to the sides of the flask as possible; waste DCM may be used. Soak in warm water with detergent and individually wash with bristled brushes. First rinse with tap water, then follow with two de-ionized water rinses. Dry either on a rack or in a 110 °C drying oven. After drying, rinse with fresh DCM (use sparingly).
2.4.2 Serum bottles and other non-volumetric glassware. Bake for at least 4 hours in a muffle furnace at 450 °C.
2.5 Calibration Curve for the UV-visible spectrophotometer.
2.5.1 Stock Standard Solution Preparation. Stock standard solution concentrations are based on the mass measurements after each addition and density determinations of the oil/dispersant/DCM solution using a density bottle or a 1-mL gas tight syringe. An example calculation is given in Table 3 of this Appendix according to the following equation:
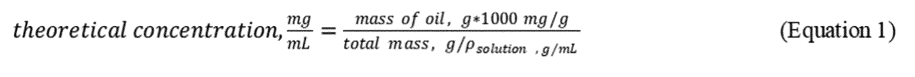
Use the reference oil and the specific dispersant being tested for a particular set of experimental test runs. Prepare the stock standard solution of dispersant-oil mixture in DCM, starting with 2 ml of the oil, then adding 80 µl of the dispersant followed by 18 ml of DCM.
2.5.2 Six -point Calibration Curve. For the reference oil, add specific volumes of its stock standard solution (given in Table 4 of this Appendix) to 30 ml of artificial seawater in a 125 ml separatory funnel. Extract the oil/dispersant water mixture with triplicate 5 ml volumes of DCM. Follow each DCM addition by 15 seconds of vigorous shaking, carefully releasing the initial pressure inside the separatory funnel by partially removing the glass stopper inside a fume hood after the first few shakes. Then, allow a 2-minute stationary period for phase separation for each extraction. Drain the extracts into a 25-mL graduated mixing cylinder. Release any entrained bubbles of DCM from the water layer by sideways shaking of the funnel. Use precaution not to drain water into the DCM extract as it can affect the absorbance readings. Adjust the final volume of the collected extracts to 25 mL in the mixing cylinder using DCM. Determine specific masses for oil concentrations in the standards as volumes of oil/dispersant solution multiplied by the concentration of the stock solution. An example calculation is given in Table 4 of this Appendix. One calibration curve is needed for the reference oil and dispersant combination.
2.6 Sample Preparation and Testing. See section 2.7 of this Appendix for a detailed description of the spectrophotometer's linear calibration procedure.
2.6.1 Six replicates of the oil and test dispersant are required at each temperature plus two additional tests of method blanks (artificial seawater without oil and dispersant), one at each temperature. A completed test consists of 14 baffled flask tests (a total of six replicates for the reference oil/test dispersant combination at two temperatures (5 °C and 25 °C), plus two method blanks).
2.6.2 Attach a 3-inch length of Teflon tubing to the stopcock of each of the 150-mL baffled flasks. Add 120 mL of artificial seawater to each flask. Put screw cap on flasks and place them at the appropriate temperature (either 5 °C or 25 °C) for equilibration.
2.6.3 Calibrate and adjust the shaker table to 250 ± 10 rpm.
2.6.4 Prepare and time separately each baffled flask. Sequentially add 100 µL of oil and 4 µL of dispersant to the flask layering them onto the center of the seawater to give a dispersant-to-oil ratio (DOR) of 1:25. Avoid any oil or dispersant splashing on the flask walls, as it may reduce efficacy or cause errors in the calculated results. Discard the sample and repeat the setup if: (1) any oil or dispersant splashing occurs during the additions, or (2) the dispersant contacts the water first rather than the oil. This is especially important for 5 °C work because of increased oil viscosity.
2.6.5 For the oil, fill the tip of the pipettor, using a wipe to remove any oil from the sides of the tip. Holding the pipettor vertically, dispense several times back into the reservoir to ensure that the oil flows smoothly. Insert the syringe tip vertically into the baffled flask and let the bottom of the pipettor rest on the neck of the flask. Slowly and carefully dispense the oil one time onto the center of the water's surface. The remainder of the oil can either be returned to the oil bottle or set aside for use in the next test flask.
Note to 2.6.5: If a Brinkmann Eppendorf repeater pipettor is used for dispensing the oil, attach a 5-mL syringe tip, and set the dial to 1.
2.6.6 For the dispersant, use the same procedure as for the oil to dispense onto the center of the oil slick surface. As the dispersant first contacts the oil, it will usually push the oil to the sides of the flask. Replace the screw cap onto the flask.
Note to 2.6.6: If a Brinkmann Eppendorf repeater pipettor is used for dispensing the dispersant, attach a 100-µL syringe tip, and set the dial to 2.
2.6.7 Carefully place flask securely onto the shaker and agitate for 10 ± 0.25 minutes at 250 ± 10 rpm.
2.6.8 Remove the flask from the shaker table and allow a stationary, quiescent period of 10 ± 0.25 minutes to allow undispersed and/or recoalesced oil droplets to refloat to the surface.
2.6.9 Carefully open the screw cap, then the stopcock at the bottom, and discard the first several mL of seawater into a waste beaker to remove non-mixed water-oil initially trapped in the stopcock tubing. Collect a volume slightly greater than 30-mL into a 50-mL graduated cylinder. Adjust the collected volume to the 30-mL mark by removing excess with a disposable glass Pasteur pipette. A web-like emulsion may form at the solvent/water interface during the water sample extraction. Avoid pulling any emulsion phase into the DCM extract as it may cloud the DCM-extract, leading to error.
2.6.10 Transfer the water-oil sample from the graduated cylinder into a 125-mL glass separatory funnel fitted with a Teflon stopcock.
2.6.11 Add 5 mL DCM to the separatory funnel. Start shaking, releasing pressure into the fume hood by loosening the glass stopper. Shake vigorously at least 20 times for 15 seconds.
2.6.12 Allow the funnel to remain in a stationary position for 2 minutes to allow phase separation of the water and DCM.
2.6.13 Drain the DCM layer from the separatory funnel into a 25 mL mixing cylinder. Avoid pulling any emulsion phase into the DCM extract as it may cloud the DCM extract.
2.6.14 Repeat the DCM-extraction process two or three additional times until the DCM is clear. Collect each extract in the graduated cylinder. After the final extraction, lightly shake the separatory funnel sideways once or twice to dislodge entrained bubbles of DCM and drain.
2.6.15 Adjust the final volume to a known quantity, 25 mL, in the mixing cylinder. Using a syringe, dispense 2.5 mL or 5.0 mL of a reference oil sample into a 10-mL volumetric flask, and fill with DCM to make either a 1:4 or 1:2 dilution, respectively.
2.6.16 If analysis cannot be conducted immediately, store the extracted DCM samples at 4 ± 2 °C until time of analysis. Glass-stoppered mixing cylinders may be used for short-term storage or prior to bringing the extracts up to volume. After bringing to volume, transfer the DCM extracts to 25–30 ml crimp-style serum vials with aluminum/Teflon seals.
2.6.17 Complete all analysis within 10 consecutive days from when the sample was collected.
2.7 UV-Visible Spectrophotometer Linear Stability Calibration
2.7.1 A six-point calibration of the UV-visible spectrophotometer is required at least once per day for each oil. The stability calibration criterion is determined with the six oil standards identified in Table 4 of this Appendix.
2.7.2 Turn on spectrophotometer and allow it to warm up for at least 30 minutes before beginning analysis. Blank the instrument for the wavelengths between 340 and 400 nm with DCM.
2.7.3 If refrigerated, allow all extracts, standards, and samples to warm to room temperature.
2.7.4 Determine the absorbance of the six standards between the wavelengths of 340 and 400 nm. This can be done by either one of the following methods:
2.7.4.1 Trapezoidal Rule. Program the spectrophotometer to take readings every 5λ or 10λ and calculate the area under the curve using the Trapezoidal rule:
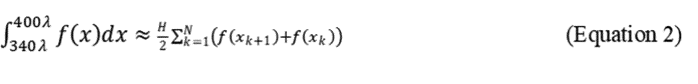
where N + 1 = number of absorbance measurements to delineate N equally spaced sections of the curve, and H = the distance (λ) between each reading. For H = 5, N + 1 = 13 measurements, for H = 10, N + 1 = 7. The following formula illustrates readings taken every 10λ.
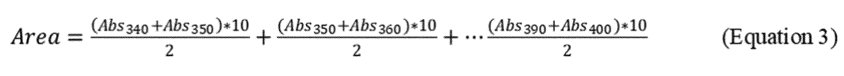
When using readings taken every 5λ, each absorbance sum is multiplied by 5.
2.7.4.2 Automatic Integration. Program the spectrophotometer to automatically integrate the area under the curve between 340 nm and 400 nm.
2.7.4.3 If the wavelengths must be manually set on the spectrophotometer, the older method of only measuring at 340λ, 370λ, and 400λ may be used. Then calculate using the trapezoidal rule for N + 1 = 3, H = 30. While the resulting area count with the older method is less accurate, the final results are similar since the inaccuracy is systematic.
2.7.5 After determining the area count for each standard, determine the response factor (RF) for the oil at each concentration using the following equation:
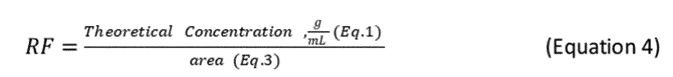
2.7.6 Spectrophotometer stability for the initial calibration is acceptable when the RFs of the six standard extracts are less than 10% different from the overall mean value for the six standards, as calculated in Equation 5 of this Appendix and depicted in the example in Table 4 of this Appendix.
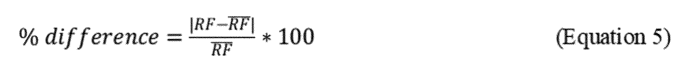
2.7.7 If this criterion is satisfied, begin analysis of sample extracts. Absorbances greater than or equal to 3.5 are not included because absorbance saturation occurs at and above this value. If any of the standard oil extracts fails to satisfy the initial-stability criterion, the source of the problem ( e.g., preparation protocol for the oil standards, spectrophotometer stability, etc.) must be corrected before analysis of the sample extracts begins.
2.7.8 Determine the slope of the calibration points by using linear regression forced zero intercept:
2.8 Spectrophotometric Analysis and Calculations
2.8.1 Once a successful calibration curve for the reference oil has been created and verified, measure experimental replicates for the reference oil at each temperature followed by a standard check sample.
2.8.2 Determine the area for the absorbance values obtained for the experimental samples by using Equation 2 of this Appendix and illustrated by Equation 3 of this Appendix.
2.8.3 Calculate the Total Oil dispersed and the percentage of oil dispersed (%OD) based on the ratio of oil dispersed in the test system to the total oil added to the system, as follows:
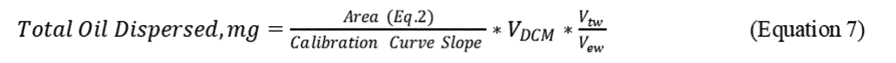
where:
V DCM = final volume of the DCM extract (mL)
V tw = total seawater in Baffled Flask (120 mL)
V ew = volume seawater extracted (30 mL)
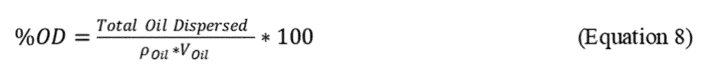
where:
r Oil = density of the specific test oil, mg/mL and
V Oil = Volume (mL of oil added to test flask (100 µL = 0.1 mL))
2.8.4 The %ODs for the six replicates within a particular treatment are then subjected to an outlier test, the Grubb's Test or Maximum Normal Residual test (6). A convenient internet-based calculator of a Grubbs outlier may be found at: http://www.graphpad.com/quickcalcs/Grubbs1.cfm. If an outlier is detected (p < 0.05), analyze an additional replicate to obtain the required six replicates.
2.8.5 Report the Dispersion Efficacy value for each oil and each temperature, which is the lower 95% confidence level of the 6 independent replicates (DE LCL95 ) for each oil/temperature combination. Error bars are not needed as reporting the lower confidence level computationally takes the variability of the replicates into account as shown in Equation 9 of this Appendix.

where = mean percentage oil dispersed for the n = 6 replicates, S = standard deviation, and t (n-1,1-) = 100 * (1-α)th percentile from the t-distribution with n-1 degrees of freedom. For 6 replicates, t n-1,1- = 2.015, where α = 0.05. An example of the calculations is given in Table 5 of this Appendix.
2.9 Performance Criterion
The dispersant product tested will remain in consideration for listing on the NCP Product Schedule if the dispersant efficacy (DE LCL95 ), as calculated in section 2.8.6 of this Appendix, is:
| Oil | Temp (°C) | DE LCL95 (%) |
|---|---|---|
| Bryan Mound | 5 | ≥70 |
| Bryan Mound | 25 | ≥75 |
2.10 Quality Control (QC) Procedures for Oil Concentration Measurements
2.10.1 Absorbance readings. Perform at least 5% of all UV-visible spectrophotometric measurements in duplicate as a QC check on the analytical measurement method. The absorbance values for the duplicates must agree within ±5% of their mean value.
2.10.2 Method blanks. Analytical method blanks involve an analysis of artificial seawater blanks (artificial seawater without oil or dispersant in a baffled flask) through testing and analytical procedures. Analyze method blanks with a frequency of at least two per completed test. Oil concentrations in method blanks must be less than detectable limits.
2.10.3 Accuracy. Determine accuracy by using a mid-point standard calibration check after each set of replicate samples analyzed. The acceptance criterion is based on a percent recovery of 90–110% using the following equation:

2.10.4 Calibration QC checks. Before analyzing samples, the spectrophotometer must meet an instrument stability calibration criterion using the oil standards. The instrument stability for initial calibration is acceptable when the RFs (Equation 5 of this Appendix) for each of the six standard concentration levels are less than 10% different from the overall mean value.
| Constituent | Concentration (g/L) |
|---|---|
| * Use Stock Solution, 1 mL/L GP2 for 100X stock solution for Bromide, Borate, and Strontium. 10 mL/L GP2 for bicarbonate—10X stock solution as it is not soluble in a 100X solution. Adjust to pH 8.0 prior to autoclaving. | |
| NaCl | 21.03 |
| Na 2 SO 4 | 3.52 |
| KCl | 0.61 |
| KBr * | 0.088 |
| Na 2 B 4 O 7 × 10H 2 O * | 0.034 |
| MgCl 2 × 6H 2 O | 9.50 |
| CaCl 2 × 2H 2 O | 1.32 |
| SrCl 2 × 6H 2 O * | 0.02 |
| NaHCO 2 * | 0.17 |
| Oil | Density, mg/mL @15 °C | API gravity @15 °C | Viscosity @25 °C, (cSt) | Category by API gravity |
|---|---|---|---|---|
| SPR Bryan Mound | 0.8320 | 38.6 | 4.721 | Light Oil. |
| Item | Identifier | Amount |
|---|---|---|
| Mass of Bottle, g | A | 29.498 |
| Mass of Bottle + oil, g | B | 31.225 |
| Mass of bottle + disp + oil + DCM, g | C | 54.380 |
| Mass of oil, g ( derived ) | F = B−A | 1.727 |
| Mass of disp + oil + DCM, g ( derived ) | G = C−A | 24.882 |
| Mass of 1 mL syringe, g | D | 14.556 |
| Mass of 1 mL syringe + solution, g | E | 15.820 |
| Density of solution, g/mL ( derived ) | H = E−D | 1.264 |
| Volume of solution, mL ( derived ) | I = G/H | 19.687 |
| Conc. of stock solution, mg/mL ( derived ) | J = F*1000/I | 87.704 |
| Oil + Dispersant Stock Standard Solution Concentration = 87.7 mg/mL ( Table 3 ) | ||||||
|---|---|---|---|---|---|---|
| Standard—stock vol. (uL) | Theoretical conc., mg/mL | Area (340–400 nm) | RF | Avg. RF | Dev. from avg. RF | Slope |
| 25 | 0.088 | 4.126 | 0.021 | 0.021 | 2.931 | 48.759 |
| 50 | 0.175 | 8.757 | 0.020 | 3.017 | ||
| 100 | 0.351 | 16.559 | 0.021 | 2.577 | ||
| 150 | 0.526 | 25.666 | 0.021 | 0.731 | ||
| 200 | 0.702 | 34.142 | 0.021 | 0.500 | ||
| 250 | 0.877 | 43.006 | 0.020 | 1.260 | ||
| Rep | Area (340–400 nm) | Dilution factor | Extract volume (ml) * | Conc, mg/mL. | Mass in 30 mL, mg | Total oil dispersed, mg | Efficiency, % | Average | Std. dev. | Variance | Coef. of variation | LCL95 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * = 25 ml of DCM extract captured oil from 30 ml of aqueous DE test. | ||||||||||||
| 1 | 32.197 | 1 | 25 | 0.66 | 16.51 | 66.03 | 79.76 | 81.30 | 4.46 | 19.85 | 5.48 | 81.30 |
| 2 | 35.470 | 1 | 25 | 0.73 | 18.19 | 72.75 | 87.87 | |||||
| 3 | 30.260 | 1 | 25 | 0.62 | 15.52 | 62.06 | 74.96 | |||||
| 4 | 31.831 | 1 | 25 | 0.65 | 16.32 | 65.28 | 78.85 | |||||
| 5 | 33.355 | 1 | 25 | 0.68 | 17.10 | 68.41 | 82.63 | |||||
| 6 | 33.791 | 1 | 25 | 0.69 | 17.33 | 69.30 | 83.71 | |||||
2.11 References for Section 2.0
(1) U.S. Environmental Protection Agency (1994), “Swirling Flask Dispersant Effectiveness Test,” Title 40 Code of Federal Regulations, Pt. 300, Appendix C, pp 47458–47461.
(2) Sorial, G.A., A.D. Venosa, K.M, Koran, E. Holder, and D.W. King. 2004. “Oil spill dispersant effectiveness protocol: I. Impact of operational variables.” ASCE J. Env. Eng. 130(10):1073–1084.
(3) Sorial, G.A., A.D. Venosa, K.M, Koran, E. Holder, and D.W. King. 2004. “Oil spill dispersant effectiveness protocol: II. Performance of revised protocol.” ASCE J. Env. Eng. 130(10):1085–1093.
(4) Venosa, A.D., D.W. King, and G.A. Sorial. 2002. “The baffled flask test for dispersant effectiveness: a round robin evaluation of reproducibility and repeatability.” Spill Sci. & Technol. Bulletin 7(5–6):299–308.
(5) Spotte, S., G. Adams, and P.M. Bubucis. 1984. “GP2 medium is an synthetic seawater for culture or maintenance of marine organisms,” Zoo Biol, 3:229–240.
(6) Grubbs, F. 1969. “Sample Criteria for Testing Outlying Observations,” Annals of Mathematical Statistics, pp. 27–58.
3.0 Dispersant Toxicity Testing
3.1 Summary. This laboratory protocol includes testing for: (1) dispersant standard static acute toxicity tests for the mysid shrimp, Americamysis bahia (48-hr duration) and the inland silverside, Menidia beryllina (96-hr duration); (2) dispersant-oil mixture static acute toxicity tests for Americamysis bahia and Menidia beryllina (48-hr and 96-hr duration, respectively); (3) dispersant developmental assay for Strongylocentrotus purpuratus or Arbacia punctulata, (72-hr duration); and (4) dispersant 7-day static subchronic tests with Americamysis bahia and Menidia beryllina (Table 6 of this Appendix).
| Test procedure | ||||
|---|---|---|---|---|
| Test substance | 96-Hr static acute: Menidia beryllina | 48-Hr static acute: AmericamysisBahia | 72-Hr sea urchin developmentalassay | 7-Day subchronic: M. beryllina &A. bahia |
| Dispersant only | yes | yes | yes | yes . |
| Dispersant—Reference Oil Mixture | yes | yes | no | no . |
3.2 Preparation of Stock Solutions
3.2.1 Dispersant. Prepare a 1000 μL/L primary stock solution prior to test initiation by adding 1.1 mL of dispersant to 1100 mL of dilution water consisting of salinity adjusted uncontaminated natural or artificial seawater, in a glass vessel. Using a laboratory top stirrer equipped with a stainless-steel blade, center the stirrer blade in the mixing vessel one inch off the bottom. Initially mix the resulting stock solution for approximately five seconds at speeds of <10,000 rpm to avoid foaming. Thereafter, set the speed to provide a 70% vortex. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Suspend mixing of the stock solution after the removal of each aliquot. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 3.5 and 3.6 of this Appendix.
3.2.2 Dispersant-Reference Oil(s) Mixtures. Use Strategic Petroleum Reserve Bryan Mound reference oil. To obtain this oil at no charge (except for a minimal shipping fee) see https://www.epa.gov/emergency-response/national-contingency-plan-subpart-j#howto. Assessment of dispersant-reference oil mixture (DOM) toxicity is determined for each reference oil using the aqueous phase of a chemically enhanced-water accommodated fraction (CE–WAF). Fit a glass aspirator bottle (approximately 23 L) equipped with a hose bib at the base with a length of silicon tubing containing a hose clamp. Fill the bottle with 19L of seawater leaving a 20% headspace above the liquid, place on a magnetic stir plate then add and center a stir bar. Add the reference oil at 25 g/L using a silicon tube attached to a glass funnel that reaches just below the water surface. Using this method reduces the production of air bubbles on the oil surface slick. Adjust the stir plate to obtain an oil vortex of 25% of the total volume of the seawater, then add the dispersant to be tested at a ratio of 1:10 dispersant:oil (2.5 g/L). Securely seal the bottle to reduce the loss of volatiles using a silicon stopper and wraps of Parafilm and stir for 18 hours, then allow the solution to settle for 6 hours. Maintain the temperature at 25 °C during stirring and settling. Purge the hose at the base of the bottle of any material followed by removal of the CE–WAF (aqueous phase) into a clean glass container without disturbing the surface oil slick. The CE–WAF should be remixed and 1 to 2 L removed for chemical analysis of total petroleum hydrocarbons (TPH) following the procedures outlined in section 3.4 of this Appendix. The remaining volume will be used for the preparation of exposure solutions following procedures outlined in section 3.3 of this Appendix. To reduce time and cost, mix sufficient amounts of dispersant product-reference oil mixture CE–WAF to allow preparation of exposure solutions for conducting simultaneous acute tests with both Americamysis bahia and Menidia beryllina.
3.3 Preparation of Exposure Concentrations.
3.3.1 Concentration Selection. Preliminary rangefinder tests may be necessary using a series of logarithmic concentrations ( e.g. 0.1, 1, 10, 100 µl dispersant product/L or mg TPH/L) to determine the appropriate exposure concentration range necessary to determine LC 50 values and 95% confidence intervals. For definitive tests, conduct a minimum of five test concentrations using a geometric ratio between 1.5 and 2.0 ( e.g. 2, 4, 8, 16, and 32). Note that when testing only the dispersant product, the highest test concentration must not exceed the dispersant's self-dispersibility limit.
3.3.2 Exposure Concentrations. Exposure solutions are prepared by adding the appropriate amount of stock solution directly to dilution water in each test chamber. Mix each exposure solution using five rotations in one direction followed by five rotations in the opposite direction using a solid glass stir rod.
3.3.3 Reference Toxicants. Separate toxicity tests must be performed with a reference toxicant for each species tested. Conduct additional reference toxicity tests any time a change in the population or source of a test species occurs. Use sodium dodecyl sulfate (SDS), also known as dodecyl sodium sulfate (DSS), and sodium lauryl sulfate (SLS) as the reference toxicant for exposures conducted with Menidia beryllina and Americamysis bahia. Use copper chloride as the reference toxicant for exposures conducted with the sea urchin developmental test. Use reagent grade quality SDS and copper chloride for tests. Information on procedures for conducting reference toxicant tests with these species can be found in the specific EPA methods documents cited in sections 3.5.1, 3.6.1, and 3.7.1 of this Appendix.
3.4 Chemical Analysis of Stock Solutions. Add the 1 L sample of CE–WAF (Section 3.2.2 of this Appendix) solutions directly to amber glass bottles with Teflon®-lined cap. Collect a replicate sample in the event of accidental loss or if reanalysis of the stock solution becomes necessary. Adjust sample to a pH=2 using 50% hydrochloric acid, immediately refrigerate and analyze within 48 hours of collection. Analyze samples for C9–C32 TPH by gas chromatography-flame ionization detection (GC–FID) following EPA SW–846, Method 8015B–DRO (4). Report TPH concentration of stock solutions as milligrams TPH/L and use in the calculation of exposure concentrations for all toxicity tests conducted with CE–WAF.
3.5 Static Acute Tests with M. beryllina and A. bahia
3.5.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with dispersant product or a mixture of dispersant product and reference oil (DOM).
3.5.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2 of this Appendix and exposure concentrations following section 3.3 of this Appendix.
3.5.3 Number of Treatments, Replicates and Organisms. Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment.
3.5.4 Exposure Period. Test duration is 48-hr for Americamysis bahia and 96-hr for Menidia beryllina. Mortality must be recorded at each 24-hour period of each test.
3.5.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 μl/L or is determined to be greater than the limits of water solubility of dispersibility.
3.5.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 7 of this Appendix.
3.6 Sea Urchin Developmental Test with Dispersant Product
3.6.1 General. Use Section 15, “Purple Urchin, Strongylocentrotus purpuratus and Sand Dollar, Dendraster excentricus Larval Development Test Method” of EPA's Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to West Coast Marine and Estuarine Organisms (EPA/600/R–95–136) (2). Alternatively, the development of the urchin Arbacia punctulata may be tested (see Table 7).
3.6.2 Test Organism. Tests of dispersant products are to follow methods for the purple urchin only. Tests with the sand dollar are not required.
3.6.3 Test Solutions. Modify procedures in EPA/600/R–95–136, Section 15 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2.1 of this Appendix and exposure concentrations following section 3.3 of this Appendix.
3.6.4 Number of Treatments and Replicates. Conduct a minimum of four replicates of five exposure treatments plus a minimum of four replicate dilution water controls.
3.6.5 Exposure Duration and Test Endpoint. Examine the effects of the dispersant product on normal development of sea urchin embryos over a period of 72 hours. An IC 50 (the exposure concentration at which normal development is inhibited in 50% of the embryos) with 95% confidence intervals are to be determined in place of an IC 25. The concentration of dispersant causing inhibition of development in 50% of exposed embryos (IC 50 ) with the lower and upper 95% confidence intervals (LCI 95 and ULCI 95 ) must be calculated at the end of the exposure period. Mortality determinations are not required.
3.6.6 Test Acceptability. Requirements of the assay are: (i) ≥80% normal larval development in the control treatment, (ii) the minimum significant difference (MSD) that can be statically detected relative to the control is ≤25%, iii) test results which support the determination of a statistically valid IC 50 and 95% confidence interval unless the LC 50 is >1000 μl/L or is greater than the limits of water solubility of dispersibility.
3.6.7 Urchin Developmental Test Summary. A summary of required test conditions is provided in Table 7 of this Appendix.
3.7 Seven-day Subchronic Tests with M. beryllina and A. bahia
3.7.1 General. Use Section 13, Method 1006.0, “Inland Silverside ( Menidia beryllina ) Larval Survival and Growth Method,” and Section 14, Method 1007.0, “Mysid ( Mysidopsis [renamed Americamysis ] bahia ) Survival, Growth, and Fecundity Method” of EPA's Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms (EPA–821–R–02–014) (3) for testing of dispersant product.
3.7.2 Test Solutions. Modify procedures in EPA–821–R–02–014, sections 13 and 14 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following section 3.2.1 of this Appendix and exposure concentrations following section 3.3 of this Appendix. Exposure solutions should be renewed every 24 hours for the duration of the test.
3.7.3 Number of Treatments, Replicates and Organisms. (i) Menidia beryllina: Conduct a minimum of four replicates of at least five exposure treatments plus a minimum of four replicate dilution water controls. Expose ten M. beryllina per replicate treatment. (ii) Americamysis bahia: Conduct a minimum of eight replicates of at least five exposure treatments plus a minimum of eight replicate dilution water controls. Expose five A. bahia per replicate treatment.
3.7.4 Exposure Duration and Test Endpoint. The test duration is seven days for both species. Test endpoints for Menidia beryllina are survival and growth (dry weight) and for Americamysis bahia is survival, growth (dry weight) and fecundity. Calculate an LC 50 and 95% confidence interval for survival and IC 25 and IC 50 with 95% confidence intervals for growth (and fecundity for A. bahia only). Report the lowest observed effect concentration (LOEC) and no observed effect concentration (NOEC) for each endpoint.
3.7.5 Test Acceptability. Requirements of the assay are: (i) ≥80% survival in the control treatment for each species, (ii) dry weights must meet the specific requirements as stipulated in Method 1006.0 for Menidia beryllina and Method 1007.0 for Americamysis bahia.
3.7.6 Subchronic Test Summary. A summary of required test conditions for each species is provided in Table 7 of this Appendix.
3.8 Laboratory Report. The laboratory must include, for each toxicity test report, all applicable information, data and analyses as follows:
3.8.1 Test Objective: protocol title and source, endpoint(s);
3.8.2 Product Information: product name, manufacturer contact information, lot number, production date, date received/chain of custody;
3.8.3 Contract Facility: contact information;
3.8.4 Dilution Water: source, pretreatment, physical and chemical characteristics (pH, salinity);
3.8.5 Test Conditions: date and time of test (start and end), test chambers type and volume, volume of solution per chamber, number of organisms per chamber, number of replicate chambers per treatment, feeding frequency, amount and type of food, test concentrations, test temperature (mean and range), test salinity (mean and range);
3.8.6 Test Organisms: common and scientific name, source contact information, age and date purchased, acclimation conditions ( e.g., temperature, salinity, both mean and range), age at test start;
3.8.7 Reference toxicant: date received, lot number, date of most recent test, results and current Cumulative Sum Chart, dilution water used, physical and chemical methods used;
3.8.8 Quality Assurance: verification of laboratory accreditation, including subcontractor facilities;
3.8.9 Test Results: raw data in tabular and graphical form, daily records of affected organisms in each concentration replicate and controls, table of required endpoints ( i.e., LC 50 with 95% confidence interval (CI), IC 25 and IC 50 with 95% CI, LOEC and NOEC), statistical methods used to calculate endpoints, summary tables of test conditions and QA data;
3.8.10 Analytical Results: method summary including Limit of Detection (LOD)/Limit of Quantitation (LOQ), deviations and reasons if any, sample summary, results including chromatograms and data qualifiers, QA summary including calibration curves, method blank and surrogate recovery, analytical results summary; and
3.8.11 Conclusions: Relationship between test endpoints and threshold limit.
| Acute M. beryllina | Acute A. bahia | Subchronic M. beryllina | Subchronic A. bahia | Development S. purpuratus/A. punctulata | |
|---|---|---|---|---|---|
| 1 Recommended minimum value. | |||||
| 2 Less than or equal to 24-hr range in age. | |||||
| Test type | Static non-renewal | Static non-renewal | Static renewal (daily) | Static renewal (daily) | Static non-renewal. |
| Test duration | 96 hours | 48 hours | 7 days | 7 days | 72 ± 2 hours. |
| Salinity | 20 ± 2‰ | 20 ± 2‰ | 20 ± 2‰ | 20 ± 2‰ | 34 ± 2‰. |
| Temperature | 25 ± 1 °C. Test temperatures must not deviate (maximum minus minimum temperature) by for than 3 °C during the test. | 15 ± 1 °C. | |||
| Light quality | Ambient laboratory illumination. 10–20 μE/m 2 /s. 16 h light, 8 h darkness, with phase in/out period recommended. | ||||
| Light intensity | |||||
| Photoperiod | |||||
| Test chamber size 1 | 250 mL | 250 mL | 600 mL–1 L | 400 mL | 30 mL. |
| Test solution volume 1 | 200 mL | 200 mL | 500–750 mL | 150 mL | 10 mL. |
| Age of test organism 2 | 9–14 days | 1–5 days | 7–11 days | 7 days | 1 hr old fertilized eggs. |
| No. organisms per test chamber | 10 | 10 | 10 | 5 | 25 embryos per mL. |
| No. of replicate chambers per concentration | 3 | 3 | 4 | 8 | 4. |
| Feeding regime | Refer to specific feeding procedures provided in each test method. | None. | |||
| Aeration | None, unless DO falls below 4.0 mg/L, then aerate all chambers. Rate: <100 bubbles/minute. 5 exposure concentrations and a control (minimum required). | ||||
| Test concentrations | |||||
| Test acceptability (required) | ≥90% survival in controls | ≥90% survival in controls | For controls: ≥80% survival; average dry weight ≥0.5mg where test starts with 7 day old larvae, or ≥0.43 mg for larvae preserved for ≤7days | For controls: ≥80% survival; average dry weight ≥0.20 mg | ≥80% normal shell development in controls. |
3.9 References for Section 3.0
(1) U.S. EPA. 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fifth Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–012).
(2) U.S. EPA. 1995. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to West Coast Marine and Estuarine Organisms. First Edition. U.S. Environmental Protection Agency, Washington, DC (EPA/600/R–95–136)
(3) U.S. EPA. 2002. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms. Third Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–014).
(4) U.S. EPA. 2008. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods U.S. Environmental Protection Agency, Washington, DC (SW–846) http://www.epa.gov/osw/hazard/testmethods/sw846/online/index.htm .
4.0 Standard Acute Toxicity Testing of Surface Washing Agents, Bioremediation Agents, Herding Agents, and Solidifiers.
4.1 Summary. This laboratory protocol includes testing for: (1) saltwater standard static acute toxicity tests for test products with the mysid shrimp, Americamysis bahia (48-hr duration) and the inland silverside, Menidia beryllina (96-hr duration); and (2) freshwater standard static acute toxicity tests for test products with the daphnid, Ceriodaphnia dubia (48-hr duration) and the fathead minnow, Pimephales promelas (96-hr duration) (see Table 8 of this Appendix).
| Application environment | Test procedure | |||
| 96-hr Static acute: Menidia beryllina | 48-hr Static acute: Americamysis bahia | 96-hr Static acute: Pimephales promelas | 48-hr Static acute: Ceriodaphnia dubia | |
| Saltwater only | yes | yes | no | no. |
| Freshwater only | no | no | yes | yes. |
| Freshwater and saltwater use | yes | yes | yes | yes. |
4.2 Dilution Water. Use Section 7 of EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) [1] for preparation of the appropriate dilution water for each species tested. Use of clean natural or synthetic seawater for tests conducted with saltwater species is acceptable.
4.3 Preparation of Stock Solutions.
4.3.1 Liquid Surface Washing Agents and/or Herding Agents. Prepare a 1000 µL/L stock solution prior to test initiation by adding 1.1 mL of test product to 1100 mL of dilution water in a glass vessel. Place on a magnetic stir plate then add and center a stir bar and adjust the stir plate to obtain a vortex of 25% of the total volume of the liquid. Mix the resulting stock solution for approximately five minutes at room temperature. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 4.6 and/or 4.7 of this Appendix, as appropriate.
4.3.2 Bioremediation Agents. For products consisting of two or more liquid and/or solid components, prepare the product following the manufacturers recommended procedure and ensure the test product mixture is completely blended. Prepare a 1000 µL/L stock solution prior to test initiation by adding 1.1 mL of the test product mixture to 1100 mL of dilution water in a glass vessel. Place on a magnetic stir plate then add and center a stir bar and adjust the stir plate to obtain a vortex of 25% of the total volume of the liquid. Mix the resulting stock solution for approximately five minutes at room temperature. Using a glass pipette, remove appropriate aliquots of stock solution from between the mixing vessel wall and edge of the vortex and place directly into the dilution water within an exposure vessel. Base the preparation of exposure solutions on the nominal concentration of the stock solution and follow procedures outlined in sections 4.5 and/or 4.6 of this Appendix, as appropriate.
4.3.3 Solid Phase Products. Assessment of the toxicity of solidifiers and other solid phase products are determined using the aqueous phase of water-accommodated fractions (WAFs) of the test product. Fit a glass aspirator bottle (approximately 23L) equipped with a hose bib at the base with a length of silicon tubing containing a hose clamp. Fill the bottle with 19L of dilution water leaving a 20% headspace above the liquid, place on a magnetic stir plate then add and center a stir bar. Add the test product at 25 g/L and securely seal the bottle using a silicon stopper and wraps of parafilm. Adjust the stir plate to obtain a vortex of 25% of the total fluid volume, stir for 18 hours then settle for 6 hours. Maintain the temperature at 25 °C during stirring and settling. Purge the hose at the base of the bottle of any material followed by removal of the WAF (aqueous phase) into a clean glass container without disturbing the product on the surface. The WAF should be remixed and used for the preparation of exposure solutions following procedures outlined in section 4.4 of this Appendix.
4.4 Preparation of Exposure Concentrations.
4.4.1 Concentration Selection. Preliminary rangefinder tests may be necessary using a series of logarithmic concentrations ( e.g. 0.1, 1, 10, 100 µl test product/L) to determine the appropriate exposure concentration range necessary to determine LC 50 values and 95% confidence intervals. For definitive tests, conduct a minimum of five test concentrations using a geometric ratio between 1.5 and 2.0 ( e.g. 2, 4, 8, 16, and 32). Note that when testing the product, the highest test concentration should not exceed the test product's self-dispersibility limit.
4.4.2 Exposure Concentrations. Exposure solutions are prepared by adding the appropriate amount of stock solution directly to dilution water in each test chamber. Mix each exposure solution using five rotations in one direction followed by five rotations in the opposite direction using a solid glass stir rod.
4.4.3 Reference Toxicants. Separate toxicity tests must be performed with a reference toxicant for each species tested. Conduct additional reference toxicity tests any time a change in the culture population or source of a test species occurs. Use reagent grade quality sodium dodecyl sulfate (SDS), also known as dodecyl sodium sulfate (DSS), and sodium lauryl sulfate (SLS) as the reference toxicant. Information on procedures for conducting reference toxicant tests with these species can be found in section 4 of EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (3).
4.5 Saltwater Static Acute Tests with Menidia beryllina and Americamysis bahia
4.5.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with the test product.
4.5.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following the appropriate sections (4.3.1, 4.3.2, or 4.3.3) of this Appendix and exposure concentrations following section 4.4 of this Appendix.
4.5.3 Number of Treatments, Replicates and Organisms. Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment.
4.5.4 Exposure Period. Test duration is 48-hr for A. bahia and 96-hr for M. beryllina. Mortality must be recorded at each 24 hour period of each test.
4.5.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 µl/L or is determined to be greater than the limits of water solubility or dispersibility.
4.5.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 9 of this Appendix.
4.6 Freshwater Static Acute Tests with Pimephales promelas and Ceriodaphnia dubia
4.6.1 General. Use EPA's Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (EPA–821–R–02–012) (1) for testing each species separately with the test product.
4.6.2 Test Solutions. Modify procedures in EPA–821–R–02–012 specifically dealing with the handling and toxicity testing of effluents or receiving water samples as follows: Prepare stock solutions following the appropriate sections (4.3.1, 4.3.2, or 4.3.3) of this Appendix and exposure concentrations following section 4.4 of this Appendix.
4.6.3 Number of Treatments, Replicates and Organisms. P. promelas: Conduct a minimum of three replicates of at least five exposure treatments plus a minimum of three replicate dilution water controls. Expose ten organisms per replicate treatment. C. dubia: Conduct a minimum of four replicates of at least five exposure treatments plus a minimum of four replicate dilution water controls. Expose five organisms per replicate treatment.
4.6.4 Exposure Period. Test duration is 48-hr for C. dubia and 96-hr for P. promelas. Mortality must be recorded at each 24 hour period of each test.
4.6.5 Test Acceptability. For each test performed, survival of control animals must be >90% and test results must allow determination of statistically valid LC 50 and 95% confidence interval values except in cases where the LC 50 is >1000 µl/L or is determined to be greater than the limits of water solubility of dispersibility.
4.6.6 Static Acute Test Summary. A summary of required test conditions is provided in Table 9 of this Appendix.
4.7 Laboratory Report. The laboratory must include, for each toxicity test report, all applicable information, data and analyses as follows:
4.7.1 Test Objective: protocol title and source, endpoint(s);
4.7.2 Product Information: product name, manufacturer contact information, lot number, production date, date received/chain of custody;
4.7.3 Contract Facility: contact information;
4.7.4 Dilution Water: source, pretreatment, physical and chemical characteristics (pH, salinity);
4.7.5 Test Conditions: date and time of test (start and end), test chambers type and volume, volume of solution per chamber, number of organisms per chamber, number of replicate chambers per treatment, feeding frequency, amount and type of food, test concentrations, test temperature (mean and range), test salinity (mean and range);
4.7.6 Test Organisms: common and scientific name, source contact information, age and date purchased, acclimation conditions ( e.g., temperature, salinity, both mean and range), age at test start;
4.7.7 Reference toxicant: date received, lot number, date of most recent test, results and current Cumulative Sum Chart, dilution water used, physical and chemical methods used;
4.7.8 Quality Assurance: verification of laboratory accreditation, including subcontractor facilities;
4.7.9 Test Results: raw data in tabular and graphical form, daily records of affected organisms in each concentration replicate and controls, table of required endpoints ( i.e., LC 50 , 95% CI, inhibited concentration for 50% of the species (IC 50 ), lower observed effect concentration (LOEC) and no observed effect concentration (NOEC)), statistical methods used to calculate endpoints, summary tables of test conditions and QA data; and
4.7.10 Conclusions: Relationship between test endpoints and threshold limit.
| Saltwater acute M. beryllina | Saltwater acute A. bahia | Freshwater acute P. promelas | Freshwater acute C. dubia | |
|---|---|---|---|---|
| 1 Recommended minimum value. | ||||
| 2 Less than or equal to 24-hr range in age. | ||||
| Test type | Static non-renewal | Static non-renewal | Static non-renewal | Static non-renewal. |
| Test duration | 96 hours | 48 hours | 96 hours | 48 hours. |
| Salinity | 20 ± 2‰ | 20 ± 2‰ | NA | NA. |
| Temperature | 25 ± 1 °C. Test temperatures must not deviate (maximum minus minimum temperature) by more than 3 °C during the test. Ambient laboratory illumination. 10–20 µE/m 2 /s. 16 h light, 8 h darkness, with phase in/out period recommended. | |||
| Light quality | ||||
| Light intensity | ||||
| Photoperiod | ||||
| Test chamber size 1 | 250 mL | 250 mL | 250 mL | 30 mL. |
| Test solution volume 1 | 200 mL | 200 mL | 200 mL | 15 mL. |
| Age of test organism 2 | 9–14 days | 1–5 days | 1–14 days | <24 hours. |
| No. organisms per test chamber | 10 | 10 | 10 | 5. |
| No. of replicate chambers per concentration (minimum) | 3 | 3 | 3 | 4. |
| Feeding regime | Refer to specific feeding procedures provided in each test method. None, unless DO falls below 4.0 mg/L, then aerate all chambers. Rate: <100 bubbles/minute. 5 exposure concentrations and a control (minimum required). ≥90% survival in controls. | |||
| Aeration | ||||
| Test concentrations | ||||
| Test acceptability (required) | ||||
4.8 References for Section 4
(1) U.S. EPA. 2002. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fifth Edition. U.S. Environmental Protection Agency, Washington, DC (EPA–821–R–02–012).
5.0 Bioremediation Agent Efficacy Test Protocol
5.1 Summary. This protocol quantifies changes in weathered Alaska North Slope (ANS) crude oil composition of alkanes and aromatics resulting from the use of a bioremediation agent in either artificial seawater or freshwater. The manufacturer may test either one or both freshwater or saltwater, depending on the product's intended use. Biodegradation of the alkanes and aromatics is monitored for 28 days at 20–23 °C. Product flasks at Day 28 are compared to Day 0 flasks to determine reductions in alkanes and aromatics. A positive control of a known oil-degrading bacterial consortium supplied by EPA is tested. A negative, sterile control is also set up containing exposure water, weathered crude oil, product, and a sterilant, sodium azide. The purpose of the negative, killed control is to make sure the disappearance of the oil constituents at day 28 is due to biodegradation and not some physical loss such as volatilization. The day 28 GC/MS results from the killed control must not be less than 90% of the day 0 results. The sample preparation procedure extracts the oil phase into the solvent dichloromethane (DCM) (also known as methylene chloride) with a subsequent solvent exchange into hexane. The hexane extracts are analyzed by a high-resolution gas chromatograph/mass spectrometer (GC/MS) operated in the selected ion monitoring mode (SIM) at a scan rate of >5 scans per second.
Note to 5.1: Alaska North Slope (ANS) crude oil is artificially weathered by distillation at 521 °F (272 °C) to remove the low molecular weight hydrocarbons to approximate natural weathering processes that occur after a spill.
5.2 Apparatus. All equipment must be maintained and calibrated per standard laboratory procedures.
5.2.1 Assorted flasks and other glassware;
5.2.2 Graduated cylinders (100 mL);
5.2.3 Deionized water;
5.2.4 250 mL borosilicate glass Erlenmeyer flasks;
5.2.5 250 mL separatory funnels with stopcocks
5.2.6 Pasteur pipettes;
5.2.7 Multichannel pipettor (5–50 mL and 50–200 mL);
5.2.8 Autoclave; environmental room or incubator;
5.2.9 Balance accurate to 0.1 mg;
5.2.10 Orbital shaker table with clamps sized to hold flasks securely;
5.2.11 GC/MS instrument equipped with a DB–5 capillary column (30 m, 0.25 mm ID, and 0.25 mm film thickness) or equivalent, and a split/splitless injection port operating in the splitless mode, such as an Agilent 6890 GC/5973 MS (or equivalent) equipped with an auto-sampler for testing multiple samples; and
5.2.12 Fixed Rotor Centrifuge.
5.3 Reagents and consortium medium.
5.3.1 Stock Seawater Preparation. Prepare the artificial seawater GP2 (modified from Spotte et al., 1984) following the procedures in section 2.3 of this Appendix, to obtain the final concentration of the salts listed in Table 1 of this Appendix, except for the sodium bicarbonate (NaHCO 3 ) which is prepared separately. Autoclave the artificial seawater. Filter sterilize the concentrated solution of sodium bicarbonate through a 0.45 μm membrane filter and add to the autoclaved and cooled artificial seawater GP2 to obtain the final concentration listed in Table 1 of this Appendix.
5.3.2 Seawater for the positive control flasks. Prepare sodium triphosphate (a.k.a., sodium tripolyphosphate) (Na 5 P 3 O 10 ), potassium nitrate (KNO 3 ), and ferric chloride hexahydrate (FeCl 3 · 6H 2 O) as a concentrated solution. Filter sterilize through a 0.45 μm membrane filter and add to autoclaved artificial seawater to obtain the final nutrient concentrations listed in Table 10 of this Appendix. Calibrate the pH meter at room temperature (approximately 20–23 °C) using commercial buffers of pH 4.0, 7.0, and 10.0, as appropriate, prior to use. Adjust the pH of the artificial seawater with concentrated hydrochloric acid (HCl) or 10 normality sodium hydroxide (10 N NaOH), as appropriate.
| Constituent | Final concentration, g/L |
|---|---|
| * Added aseptically after the GP2 has been autoclaved to limit phosphorus and iron precipitation. | |
| * FeCl 3 · 6H 2 O | 0.050 |
| KNO 3 | 2.890 |
| * Na 5 P 3 O 10 | 0.297 |
5.3.3 Seawater for bioremediation agents that do not include nutrients. If a bioremediation agent contains living microorganisms but not nutrients (or limiting concentrations of nutrients), then nutrients may be added by the manufacturer. However, the total concentration of the nutrients added to the bioremediation agent must not exceed the final concentrations listed in Table 11 of this Appendix.
| Constituent | Final concentration, g/L |
|---|---|
| as Iron (Fe) | 0.010 |
| as Nitrogen (N) | 0.400 |
| as Phosphorus (P) | 0.075 |
If nutrients are supplied by the product manufacturer, the specific composition and concentration used in the efficacy testing must be submitted.
5.3.4 Freshwater Preparation. The artificial freshwater, which is a modification of Bushnell-Haas medium (Haines et al., 2005), is prepared following the concentrations listed in Table 12 of this Appendix and then autoclaved. The pH is adjusted to 7.4 before autoclaving. Constituents removed from the original formulation are KNO 3 , K 2 HPO4 and KH 2 PO 4 .
| Constituent | Final concentration (mg/L) |
|---|---|
| MgSO 4 · 7H 2 O | 200 |
| CaCl 2 · 2H 2 O | 20 |
| FeCl 3 · 6H 2 O | 50 |
| MnSO 4 × H 2 O | 0.0302 |
| H 3 BO 3 | 0.0572 |
| ZnSO 4 × 7H 2 O | 0.0428 |
| (NH 4 ) 6 Mo 7 O 2 | 0.0347 |
5.3.5 Freshwater for the positive control. To prepare the freshwater for the positive controls, prepare the nutrients potassium phosphate monobasic (KH2PO4), potassium phosphate dibasic (K2HPO4) and potassium nitrate (KNO3) as a concentrated solution. Filter sterilize and add to autoclaved artificial freshwater to obtain the final concentrations given in Table 13 of this Appendix. Calibrate the pH meter at room temperature (approximately 20–23 °C) using commercial buffers of pH 4.0, 7.0, and 10.0, as appropriate, prior to use. Adjust the pH of the artificial freshwater to 7.4 with 1 N HCl or 1 N NaOH, as appropriate.
| Constituent | Final concentration (g/L) 1 |
|---|---|
| 1 Adjust pH to 7.4 prior to autoclaving. | |
| KNO 3 | 2.89 |
| KH 2 PO 4 | 1.00 |
| K 2 HPO 4 | 1.00 |
5.3.6 Freshwater for bioremediation agents that contain living microorganisms but not nutrients or limiting concentrations of nutrients. If a bioremediation agent does not include nutrients, then nutrients may be added. However, the total concentration of the nutrients added to the bioremediation agent must not exceed the final concentrations provided in Table 14 of this Appendix.
| Constituent | Final concentration, g/L 1 |
|---|---|
| 1 Adjust to pH 7.4 prior to autoclaving. | |
| as Iron (Fe) | not added since iron is already in the freshwater solution. |
| as Nitrogen (N) | 0.400. |
| as Phosphorus (P) | 0.400. |
If nutrients are supplied by the product vendor, the specific composition and concentration used in the efficacy testing must be submitted.
5.3.7 Oil Preparation. The test oil, weathered ANS521 crude oil, can be obtained from EPA at no charge (except for a minimal shipping fee). See https://www.epa.gov/emergency-response/national-contingency-plan-subpart-j#howto for more information.
5.3.8 Sodium azide sterilant. Prepare a stock solution of NaN 3 for addition to the negative killed control. The final concentration in the killed controls will be 0.5 g/L.
5.4 Experimental Setup and Procedure
5.4.1 Autoclave clean borosilicate glass Erlenmeyer flasks (250 mL) for 20 minutes at 121 °C at 15 psig.
5.4.2 Label flasks with the appropriate code (negative control, positive control, or product; day to be sampled (0 or 28); letter indicating replicate number) to reflect the following treatment design in Table 15 of this Appendix:
| * The laboratory must report positive control test results conducted within the year of any test results for bioremediation products, for one or both types of water as applicable. | |||
| Treatment | Number of replicates at sampling times | Analysis | |
| Day 0 | Day 28 | ||
| Negative (killed) Control (oil + exposure water + product + EPA consortium + NaN 3 sterilant) | 0 | 3 | GC/MS |
| * Positive control (oil + exposure water + nutrients + EPA consortium) | 6 | 6 | GC/MS |
| Test Type 1: Product containing living microorganisms (oil + exposure water + living product + supplemented nutrients (if necessary)) | 6 | 6 | GC/MS |
| Test Type 2: Product containing proprietary nutrients but no live microorganisms (oil + exposure water + product + EPA consortium) | 6 | 6 | GC/MS |
| Test Type 3: Product (such as an enzyme) containing no live microorganisms and no nutrients (oil + exposure water + product) | 6 | 6 | GC/MS |
5.4.3 Aseptically dispense 100 mL of pre-sterilized artificial exposure water (seawater or freshwater) into each sterile flask. For the positive control flasks, use exposure water containing nutrients.
5.4.4 Tare the labeled flasks containing exposure water and other additions, as necessary, on the balance with a minimum accuracy of 0.01 g. Add drop-wise 0.50 g oil (this results in a final oil concentration of 5 g/L) using a sterile Pasteur pipette to the center of the flask taking care to avoid splashing the oil onto the sides of the flasks. Record the precise weight. ANS521 may be previously warmed in a hot water bath at 60 °C for 40–60 minutes to facilitate its flow. Take precautions when handling and charging the flasks to minimize the likelihood of contamination by exogenous microbes, including using a new sterile pipette for each series of flasks.
5.4.5 Preparation of the EPA consortium for both the positive control flasks and the flasks containing non-living bio-stimulation products. Use the supplied vials containing approximately 5 mL of the known EPA consortium frozen in glycerol. Thaw the supplied vials at room temperature ( do not allow cultures preserved in glycerol to sit at room temperature past thawing ), transfer the contents of the thawed vials to a single sterile centrifuge tube, rinse tubes with two volumes each of sterile exposure water, centrifuge at between 6,000- and 7,000-times gravity (6,000–7,000 × g ) for 15 minutes using a fixed rotor to fully pellet the cells. Carefully resuspend the cell pellet in sterile exposure water using the appropriate volume to achieve the desired seeding density, which will be provided by EPA upon shipment of the consortium.
5.4.6 Positive control flasks contain exposure water, oil, nutrients, and the EPA consortium.
5.4.7 Negative killed control flasks for all products shall contain exposure water, oil, product, the EPA consortium for products not containing a living culture, and the sodium azide sterilant at a final concentration of 0.5 g/L. Add the sodium azide sterilant prior to adding any product or EPA consortium. For the negative killed control flasks and product flasks, prepare and add the product to the flasks in a concentration specified by the manufacturer or vendor.
5.4.8 For non-living products that contain nutrient only, use the EPA consortium as the inoculum.
5.4.9 For other non-living products ( e.g., enzymes), do not add nutrients or the EPA consortium as the inoculum as they are not needed.
5.4.10 For products containing living microorganisms, prepare 6 flasks the same way as in Steps a–d, but without the EPA consortium. A product that contains its own nutrients must not be amended with nutrients, unless the product contains insufficient nutrients. Since this is a closed flask test, nutrients could be limiting if they are at the same concentration as used in the field. This could cause the product to fail the test. Thus, the manufacturer has the option to supplement its product with a higher concentration of nutrients than that contained in the product. Any nutrient supplements to a product must be reported and must not exceed the concentration limits in Table 10 (for seawater) and 13 (for freshwater) of this Appendix, as applicable.
5.4.11 Cap all flasks either with sterile cotton stoppers or loosely applied aluminum foil to allow gas exchange with the atmosphere. Set aside the T = 0 flasks for immediate extraction and analysis. Place the rest of the flasks onto the orbital shaker table. Do not tip the flasks excessively to avoid stranding oil above the mixing area of the flask. Set the orbital shaker to 200 rpm and shake the flasks for 28 days at 20–23 °C in the dark.
5.4.12 Submit all information on added microorganisms and nutrients for testing in the data report.
5.5 Sampling and Chemical Analysis.
5.5.1 Summary. At each sampling event (Days 0 and 28), product and control flasks are sacrificed for analysis of residual oil concentrations (SOP 4 of this Appendix). Record all physical observations for each flask (such as degree of emulsification, whether the oil has congealed into tar balls, wall growth, color, etc.) at each sampling. The analytical procedure is summarized in Table 16 of this Appendix. Dichloromethane (DCM) is the solvent used for the initial extraction. Solvent-exchange the extract into hexane prior to injection into the gas chromatograph. The solvent exchange is done to prevent asphaltenes from contaminating the column.
| Matrix | Measurement | Sampling/ measurement method | Analysis method | Sample container/quantity of sample | Preservation/ storage (°C) | Holding times (months) |
|---|---|---|---|---|---|---|
| DCM | N/A | Solvent Exchange to Hexane | N/A | Capped Vial with Teflon septa, 30 mL | 4 | 6 |
| Hexane | Hydrocarbon Concentration | SOP 4 | GC/MS | Capped Vial with Teflon septa, 10 mL | 4 | 6 |
5.5.2 Hydrocarbon Extraction. To measure extraction efficiency, 200 µL of the 400 mg/L surrogate recovery standard (compounds and concentrations described in SOP 1 in this Appendix) is added to each flask. Add 50 mL DCM to each flask. Transfer the contents to a 250 mL separatory funnel and shake for 2 minutes; allow the phases to separate for 2 minutes. If an emulsion remains after 2 minutes, centrifuge the emulsion in Teflon® centrifuge tubes for at least ten minutes in a low-speed centrifuge at 3,000 times gravity (3,000 × g ) to break the emulsion and recover the DCM phase. Pass the DCM extract through a funnel plugged with glass wool and containing approximately 20 g anhydrous, granular sodium sulfate (Na 2 SO 4 ) to remove water. Repeat the steps above two more times with 25 mL DCM each (100 mL DCM used in total). Add 10 mL DCM on to the sodium sulfate after the third extraction to rinse off any oil residue. Collect the extract in 125 mL serum vials, capped with Teflon lined septa and aluminum crimp seals, and store at 4 °C for up to 6 months.
5.5.3 Solvent Exchange. Perform a solvent exchange (DCM to hexane) prior to GC/MS analysis to prevent injection of asphaltenes into the GC/MS column. Transfer the DCM extract to concentration tubes. Place the tubes in a 29 °C water bath under a stream of dry nitrogen gas. Reduce the sample to 1 mL and transfer the extract to a 10 mL volumetric flask. Rinse the concentration tube with hexane and add it to the volumetric flask 2 times. Adjust the final volume with hexane to 10 mL.
5.5.4 Hydrocarbon Analysis. Quantify the concentrations of 25 alkanes, 32 aromatics and hopane (SOP 4, Table SOP 4.4 of this Appendix) using an Agilent 6890 GC/5973 MS or equivalent equipped with a 30-m × 0.25-mm ID × 0.25-μm film thickness DB–5 or equivalent fused silica column. To prepare the samples, transfer 1.0 mL of the hexane extract into a 2 mL autosampler vial with Teflon lined cap. Add 20 μL of internal standard solution to each vial with a syringe or positive displacement pipettor. SOP 2 of this Appendix outlines the procedure for preparing the internal standard solution. Load vials onto the autosampler tray and analyze in selected ion monitoring mode (SIM). Sum the individual alkane concentrations for the total alkane concentration and the individual aromatic concentrations for total aromatic concentrations in each flask.
5.6 Quality Assurance/Quality Control (QA/QC).
5.6.1 Objectives. The critical variables to be analyzed for each set of experimental conditions are the individual petroleum hydrocarbons, i.e., the alkanes ranging in carbon number from nC–14 to nC–35, plus pristane and phytane, and the 2- to 4-ring polycyclic aromatic hydrocarbons (PAHs) and their alkylated homologs as listed in SOP 4 of this Appendix. The quality assurance objectives for precision, accuracy, and detection limits are ±20%, 75–125% recovery, and 22.5 µg/L on average for the 58 compounds, respectively. For more details, refer to the SOPs of this Appendix.
5.6.2 Precision Objectives. Precision is presented as relative percent difference (RPD) for duplicate measurements and as relative standard deviation (RSD, or coefficient of variance) for triplicate measurements, applicable to replication of treatments as separate samples.
5.6.3 Accuracy Objectives. These are based on the check standards and standard oil samples run concurrently with the sample analyses for GC/MS analysis of critical compounds. Critical compounds in the check standards and in the oil standards must fall within 75–125% of expected values for the analysis to be valid. Six surrogate compounds (SOP 1 of this Appendix) added to each sample before extraction can also serve as a surrogate for determining accuracy. The measured surrogate concentrations must fall within 75–125% of expected values.
5.6.4 Calibration Range. Conduct all measurements within the linear calibration range of the instrument. The calibrated concentration range for GC/MS analysis is 0.1 mg/L to 30 mg/L. If the measured concentration of any critical compound is above the calibration range, dilute the sample and re-analyze to quantify that particular compound within the linear calibration range.
5.6.5 Quality Control. Table 17 of this Appendix summarizes the QC checks for each measurement. See the corresponding SOP in this Appendix for detailed descriptions of QC checks, frequency, acceptance criteria, and corrective actions.
| Sample matrix | Measurement | QA/QC check | Frequency | Acceptance criteria | Corrective action |
|---|---|---|---|---|---|
| DCM | GC/MS hydrocarbon analysis | Blanks | Once per calibrated run | Peak area of interfering peaks <10% of lowest standard peak area | Flush with solvent, clean injection port, and/or bake column. |
| DCM | GC/MS hydrocarbon analysis | DFTPP Check Standard | Once per calibrated run | Must pass all DFTPP criteria | If any criteria fail, retune and rerun DFTPP check standard. |
| DCM | GC/MS hydrocarbon analysis | Initial Calibration Samples | Once per calibrated run | Response Factor RSD ≤25% or R2 >0.99 | If RSD for any one compound >25%, recalibrate. |
| DCM | GC/MS hydrocarbon analysis | Calibration Check Standards | Every 10–15 samples | ±25% of expected values | If >5 compounds are out of range, recalibrate and rerun samples. |
| Hexane | GC/MS hydrocarbon analysis | Surrogates | Every Sample | ±30% of expected values | Re-inject. |
| Hexane | GC/MS hydrocarbon analysis | Biomarker Concentration | Every Sample | ±25% of average values | Re-inject. |
5.7 Pass/Fail Criteria.
5.7.1 Calculate the mean and standard deviation of the hopane-normalized total aromatics (sum of all resolved aromatics) and hopane-normalized total alkane concentrations (sum of all resolved alkanes) from the 6 independent replicates at days 0 and 28. To normalize, divide the sum of the alkane analytes and the sum of the aromatic analytes in each replicate by the hopane concentration in the corresponding replicate.
5.7.2 From those data, calculate the 95% Upper Confidence Level (UCL95) at days 0 and 28 using the following formula (Equation 11 of this Appendix):

where:
x (028) = total hopane-normalized alkane or total hopane-normalized aromatic mean of 6 replicates at days 0 and 28,
t95, 5 df = the 95% one-tailed t-value with 5 degrees of freedom (2.015),
s = the standard deviation of the 6 replicates at day 0 and 28, and
n = no. of replicates = 6.
5.7.3 Using Equation 12 of this Appendix, calculate the % reduction of each oil fraction from day 0 to day 28, using the day 0 and 28 UCL 95 hopane-normalized values for each fraction:
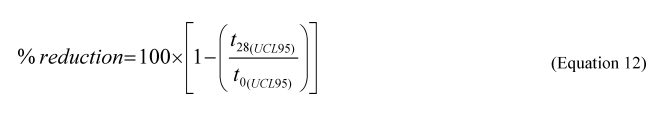
where:
t28(95) = UCL 95 of the hopane-normalized total alkane or total aromatic mean of 6 replicates on day 28, and
t0( = UCL 95 of the hopane-normalized total alkane or total aromatic mean of 6 replicates on day 0.
5.7.4 A product is successful in saltwater or freshwater if the % reduction of total alkanes (aliphatic fraction) from the GC/MS analysis is greater than or equal to 85% and the % reduction of total aromatics (aromatic fraction) is greater than or equal to 35% at day 28 based on the UCL 95 (Equation 12 of this Appendix). The benchmark reduction ranges in aliphatic and aromatic fractions for the positive control are the same as for the products specified above. The average concentration of the biomarker hopane at day 28 must not differ from the average concentration at day 0 by more than 12% in the positive control. If the conditions for the positive control are not met, the entire procedure must be repeated.
5.8 Data Verification and Reporting. GC/MS data files are generated by MS ChemStation software (the Agilent standard software for GC/MS) or equivalent for each injection. Data files contain summed ion chromatograms and selected ion chromatograms. Calibration curves are generated within MS ChemStation software, and all data files are calculated against the calibration curve by MS ChemStation. Data verification would be done by crosschecking between analysts for 10% of the raw data and its reduction process.
5.9 Laboratory Report. The summary of findings from a product test must include the data listings for each analyte that was analyzed ( i.e., all individual alkanes and aromatics in the list of required analytes), along with QA/QC checks (see Table 17) and instrument detection/reporting limits for each analyte. Express all concentrations as mg analyte/L exposure water.
5.10 Standard Operating Procedures (SOPs) 1–4
5.10.1 SOP 1. Preparation of Surrogate Recovery Standards
5.10.1.1 Preparation:
5.10.1.1.1 Solvents: Dichloromethane (DCM), Optima grade or equivalent.
5.10.1.1.2 Reagents:
D36-Heptadecane (C17)
D50-Tetracosane (C24)
D66-Dotriacontane (C32)
D10-1-Methylnaphthalene
D10-Phenanthrene
D10-Pyrene
5-beta-cholestane (coprostane)
Note: Deuterated reagents are available from Cambridge Isotope Laboratories, Andover, MA.
5.10.1.1.3 Equipment:
Micro-spatula
Small beakers
Glass funnel
Analytical balance (0.0001g)
Vials with Teflon-lined caps
Teflon wash bottle with Optima grade DCM
Volumetric flask (250 mL), class A
Pasteur pipettes
5.10.1.2 Procedure:
5.10.1.2.1 Using a calibrated analytical balance, weigh 100 mg (0.100 g) of each reagent into separate 10–25 mL beakers.
5.10.1.2.2 Dissolve the reagents in their beakers by adding 10 mL DCM. Use a Pasteur pipette to transfer the solutions to a single 250 mL volumetric flask.
5.10.1.2.3 Wash the beakers 3 or 4 times with DCM. Use a Pasteur pipette to transfer each of the washings to the 250 mL volumetric flask.
5.10.1.2.4 Dilute the solution to the 250 mL volume mark on the volumetric flask with DCM.
5.10.1.2.5 Use a glass stopper to seal the flask and homogenize the solution by inverting the flask 5 or more times. The final concentration of this solution is 400 mg/L for each of the reagents.
5.10.1.2.6 Transfer the solution into 40 mL storage vials and cap with Teflon-lined caps and label each with the date of preparation, operator, sample names, and concentrations.
5.10.1.2.7 Weigh each vial and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.1.2.8 Store these vials at 0 °C or lower.
5.10.1.2.9 Before using, allow the solution to come to room temperature, and then shake it well.
5.10.1.2.10 Weigh the vial before using it and compare the weight with the last weight recorded on the vial.
5.10.1.2.11 If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Prepare a new solution if the integrity has been compromised.
5.10.1.3 Quality Control: Inject 20 μL of the surrogate stock solution into 1 mL DCM. Add 20 μL of the internal standard solution (SOP 2 of this Appendix). Analyze this solution by GC/MS using a calibrated method (SOPs 3 and 4 of this Appendix). The expected concentration of each of the corresponding surrogate compounds is 8 ± 2 mg/L. If the measured value does not fall within this range, prepare and measure another independent surrogate solution. If the measured concentration of the second surrogate solution is within the allowable tolerance range, the calibration and instrument conditions are acceptable; properly discard the first surrogate solution. If the concentration of the second surrogate solution is also out of range, then clean and recalibrate the instrument until the problem is resolved.
5.10.2 SOP 2. Preparation of Internal Standard Solution
5.10.2.1 Preparation:
5.10.2.1.1 Solvents: Dichloromethane (DCM), Optima grade or equivalent
5.10.2.1.2 Reagents:
D34 n-Hexadecane (C16)
D42 n-Eicosane (C20)
D62 n-Triacontane (C30)
D8-Naphthalene
D10-Anthracene
D12-Chrysene
5-alpha-Androstane
Note: Deuterated reagents are available from Cambridge Isotope Laboratories, Andover, MA.
5.10.2.1.3 Equipment:
Micro-spatula
Small beakers
Glass funnel
Analytical balance (0.0001g), calibrated and checked for accuracy
Amber vials with Teflon-lined caps, labeled
Teflon wash bottle with DCM
Volumetric flask (200 mL), class A
Pasteur pipettes
5.10.2.2 Procedure:
5.10.2.2.1 Using a calibrated analytical balance, weigh 100 mg (0.100 g) of each of the reagents into separate small beakers.
5.10.2.2.2 Dissolve the reagents in their beakers by adding 10 mL DCM; using a Pasteur pipette, transfer the solutions to a single 200 mL volumetric flask.
5.10.2.2.3 Wash the beakers 3 or 4 times with DCM; use a Pasteur pipette to transfer each of the washings to the 200 mL volume mark on the volumetric flask.
5.10.2.2.4 Dilute the solution with DCM to the 200 mL volume.
5.10.2.2.5 Seal the flask with a glass stopper and homogenize the solution by inverting the flask a minimum of 5 times. The final concentration of this solution is 500 mg/L of each reagent.
5.10.2.2.6 Transfer the solution into 40 mL storage vials and cap with Teflon-lined caps. Label each vial with the date of preparation, operator, sample names, and concentrations.
5.10.2.2.7 Weigh each vial, and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.2.2.8 Store this solution at 0 °C or lower.
5.10.2.2.9 Before using, allow the solution to come to room temperature, and then shake it well.
5.10.2.2.10 Weigh the vial before using it, and compare the weight with the last weight recorded on the vial.
5.10.2.2.11 If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Prepare a new solution if the integrity has been compromised.
5.10.2.3 Quality Control: Inject 20 μL of the internal standard solution into 1 mL DCM. Analyze this solution by GC/MS. The only peaks corresponding to the internal standards must appear. If other peaks appear, particularly close to the internal standard peaks, discard the internal standard solution and prepare a new solution.
5.10.3 SOP 3. Preparation of Working Standards, Check Standards, and Oil Standards for GC/MS Consistency.
5.10.3.1 Preparation:
5.10.3.1.1 Solvent: Dichloromethane (DCM), Optima grade or equivalent
5.10.3.1.2 Stock solutions:
5.10.3.1.2.1 Oil analysis standard: 44 compounds, 100 mg/L in hexane/DCM (9:1), four, 1-mL vials required. Available from Absolute Standards, Inc., Hamden, CT, Part #90311.
5.10.3.1.2.2 Nine compound PAH standard: 1,000 mg/L in DCM, one vial. Available from Absolute Standards, Inc., Hamden, CT, Part #90822.
5.10.3.1.2.3 1,2-Benzodiphenylene sulfide, (synonym for naphthobenzothiophene). Prepare a 2 mg/mL stock solution. Available from Sigma-Aldrich Co., Part # 255122, purity 99%.
5.10.3.1.2.4 Hopane solution (17 α (H), 21β (H), 0.1 mg/mL in isooctane. Available from Sigma-Aldrich Co. Part #90656.
5.10.3.1.2.5 Surrogate solution: 400 mg/L of each reagent in DCM (see SOP 1 of this Appendix).
5.10.3.1.2.6 Internal standard solution, 500 mg/L in DCM (see SOP 2 of this Appendix).
5.10.3.1.3 Alaska North Slope Crude Oil 521 (ANS521).
5.10.3.1.4 Equipment:
5.10.3.1.4.1 Glass storage vials with Teflon-lined caps (2 mL and 40 mL capacity);
5.10.3.1.4.2 Volumetric flasks, Class A, 5 mL, 10 mL, and 100 mL
5.10.3.1.4.3 Glass syringes capable of dispensing 25–500 µL with an accuracy and precision of ± 1%, or equivalent
5.10.3.1.4.4 Wheaton repetitive dispenser, Model 411 STEP–PETTE or equivalent
5.10.3.1.4.5 Teflon wash bottle filled with Optima grade DCM or equivalent grade DCM
5.10.3.1.4.6 Pasteur pipettes
The volumes of stock solutions required to make the working standards are listed in Table SOP 3.1 of this Appendix.
| Stock standards | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| Working standards concentration, mg/L | Oil analysis mix (44 compounds, 100 mg/L) μL | Aromatics mix (9 compounds, 1,000 mg/L) μL | 1,2-Benzo- diphenylene sulfide (NBT) (2 mg/mL) μL | Surrogate solution (100 mg/L) μL | Hopane solution (100 mg/L) μL | Volumetric flask volume mL | ISTD (500 mg/L) μL |
| * Make extra STD 5 for use as check standard. | |||||||
| STD 30 (no hopane) | 1,500 | 150 | 75 | 375 | 0 | 5 | 100 |
| STD 20 (5 mg/L hopane) | 1,000 | 100 | 50 | 250 | 250 | 5 | 100 |
| STD 10 (2.5 mg/L hopane) | 500 | 50 | 25 | 125 | 125 | 5 | 100 |
| STD 5 * (1 mg/L hopane) | 500 | 50 | 25 | 125 | 100 | 10 | 200 |
| STD 5-Utility (1 mg/L hopane) | 500 | 50 | 25 | 125 | 100 | 10 (used for preparation of STD 2.5 & STD 1) | 0 |
| STD 2.5 (0.5 mg/L hopane) | Use 5 mL of STD 5-Utility and dilute to 10 mL. Use 2 mL of STD 5-Utility and dilute to 10 mL. Use 0.2 mL of STD 5-Utility and dilute to 10 mL. | 200 | |||||
| STD 1 (0.2 mg/L hopane) | 200 | ||||||
| STD 0.1 (0.2 mg/L hopane) | 200 | ||||||
5.10.3.2 Procedure for Working Standards and Check Standards:
5.10.3.2.1 Label three 5 mL volumetric flasks as STD30, STD20, STD10, and two 10 mL volumetric flasks as STD5, and STD5-utility.
5.10.3.2.2 Add 1–2 mL of DCM to each volumetric flask.
5.10.3.2.3 Using glass syringes, add the appropriate volume of stock solution A (as listed in Table SOP 3.1 of this Appendix) to the flasks labeled STD30, STD20, STD10, STD5, and STD5-utility.
5.10.3.2.4 Wash the walls of the inner neck of the flasks with several drops of DCM to rinse off the residue of the stock solution into the flasks.
5.10.3.2.5 Repeat Step 3 and Step 4 to dispense stock solutions B–E (do not add stock solution F, internal standard solution, at this step).
5.10.3.2.6 Dilute to volume with DCM for all the above flasks, seal with glass stoppers, and invert several times to homogenize the solutions.
5.10.3.2.7 Label three additional 10 mL volumetric flasks as STD2.5, STD1, and STD0.1. Wet with 1–2 mL DCM.
5.10.3.2.8 Dispense 5 mL of STD5-utility solution into flask STD2.5, 2 mL of STD5-utility solution into flask STD1, and 0.2 mL of STD5-utility solution into flask STD0.1.
5.10.3.2.9 Dilute to volume with DCM, seal with glass stoppers, and invert several times to homogenize the solutions.
5.10.3.2.10 Using a 100 μL glass syringe, dispense 100 μL of internal standard solution into flasks STD30, STD20, and STD10. Dispense 200 μL into flasks STD5, STD2.5, STD1, and STD0.1 to give a final concentration of 10 mg/L internal standard.
5.10.3.2.11 Seal with glass stoppers, and invert the flasks several times to homogenize the solutions.
5.10.3.2.12 Transfer the solutions into 2 mL storage vials, and cap with Teflon-lined caps.
5.10.3.1.13 Label each vial with date of preparation, analyst, sample names, and concentrations.
5.10.3.2.14 Weigh each storage vial and record its weight on the label. This weight is used to monitor possible evaporation during storage.
5.10.3.2.15 Store this solution at 0 °C or below.
5.10.3.2.16 Before using, allow the solution to come to room temperature, and shake it well.
5.10.3.2.17 Weigh the vial before opening, and compare the weight with the last weight recorded on the vial. If the weights are consistent, the integrity of the solution can be assumed. If not, investigate and resolve the cause. Do not use the solution if the integrity has been compromised.
5.10.3.3 Procedure for Oil Standard. In a 100 mL volumetric flask, weigh 0.500 g of the standard ANS521 crude oil, add 2 mL of surrogate solution (see SOP 1 of this Appendix), and bring to volume with DCM. Add 2 mL of internal standard solution (see SOP 2 of this Appendix). Follow steps 5.10.3.2.11 through 5.10.3.2.17 of this SOP, substituting 40 mL storage vials for the 2 mL vials.
5.10.3.4 Quality Control/Quality Assurance:
5.10.3.4.1 Run the seven standard solutions using the GC/MS method (SOP 4) on a tuned GC/MS. Use the EnviroQuant software or equivalent to calculate the average Relative Response Factor (RRF) and the relative standard deviation (RSD) of the RRFs for each analyte over the six concentrations. The RRF is defined as:

5.10.3.4.2 The RSD of the RRFs for all analytes must be 25% or less. Alternatively, the coefficients of determination (R2) for the calibration curve for each target compounds and surrogate should be over 0.99.
5.10.4 SOP 4. GC/MS Method for the Analysis of Crude Oil Samples.
5.10.4.1 Instrument Specifications:
5.10.4.1.1 Use an Agilent 6890 GC coupled with an Agilent 5973 mass selective detector (MSD) and an Agilent 6890 series auto sampler or equivalent, equipped with a DB–5 capillary column (30 m, 0.25 mm I.D., and 0.25 μm film thickness) or equivalent, and a split/splitless injection port operating in the splitless mode. Data acquisition occurs in the SIM (selected ion monitoring) mode for quantitative analysis. In SIM mode, the dwell time of each ion is set to be 10 milliseconds and the ions are split up into groups by retention time. One way to divide the ions is by retention time grouping as shown in Table SOP 4.1 of this Appendix. The number of ions in each ion group must be constant, yielding the same scan rate for each group.
| Group | Ions |
|---|---|
| 1 | 57, 66, 128, 136, 142, 152, 156, 166, 170, 184. |
| 2 | 57, 66, 166, 170, 178, 180, 184, 188, 192, 194, 198, 208. |
| 3 | 57, 66, 178, 184, 188, 192, 194, 198, 202, 206, 208, 212, 220, 226. |
| 4 | 57, 66, 192, 198, 202, 206, 208, 212, 216, 220, 226, 230, 234, 245. |
| 5 | 57, 66, 191, 217, 228, 240, 242, 248, 256, 262, 264, 270, 276, 284. |
5.10.4.1.2 Table SOP 4.2 of this Appendix summarizes the instrumental conditions for crude oil analysis. Use only ultra-high purity helium (99.999% pure) as the carrier gas. In series, connect a moisture trap, an oxygen trap, and an organic trap to the carrier gas line before it enters the column.
| Instrument | Agilent 6890 Series II Gas Chromatograph (GC) with an Agilent 5973MSD and an Agilent 6890 auto sampler, or equivalent. |
| Column | DB–5 capillary column (30 m, 0.25 mm I.D., and 0.25-mm film thickness) or equivalent. |
| Carrier Gas | Helium, ultra-high purity grade (99.999%). |
| Inlet Temperature | 300 °C. |
| Transfer Line (detector) Temperature | 310 °C. |
| Oven Temperature Program | 50 °C for 4 minutes, then 7 °C/min to 310 °C, hold for 18 minutes. |
| Flow Rate | Constant flow at 1mL/min. Linear velocity: 36.2 cm/sec. |
| Injection Volume | 1 µL. |
| Split/Splitless Mode | Splitless. |
| Total Run Time | 59.18 minutes. |
5.10.4.2 Procedure for preparing the instrument:
5.10.4.2.1 Lower the injection port temperature and the oven temperature to 50 °C or less to avoid oxidation of the column.
5.10.4.2.2 Replace the liner with a clean, silanized liner. Do not touch the liner with bare fingers. A small piece of muffled glass wool may be inserted to protect the column.
5.10.4.2.3 Return the injection port and oven to the appropriate temperatures.
5.10.4.2.4 Wait five minutes after the temperature equilibrates before using the instrument.
5.10.4.3 Procedure for tuning the MSD:
5.10.4.3.1 Perform an air/water check. The value reported for the relative abundance of water (m/z 18), nitrogen (m/z 28), oxygen (m/z 32), or carbon dioxide (m/z 44) shall be less than 5% of the base peak for the system to be considered leak free and are expected to be closed to 1% for a stable system.
5.10.4.3.2 Tune the MSD using the Standard Autotune program and the decafluorotriphenylphosphine (DFTPP) Tune program to reduce instrument variability. The Autotune report file is referenced by the instrument when performing an air/water check and thus must be run at least once per month. Run standards and samples using DFTPP Tune parameters, and retune the instrument using DFTPP Tune at least once per week. The tune programs use three fragment ions of perfluorotributylamine (PFTBA) as a standard for tuning: m/z 69, 219, and 502. Tune reports must meet the following criteria:
5.10.4.3.2.1 Symmetrical peaks;
5.10.4.3.2.2 Mass assignments within ±0.2 amu's from 69, 219, and 502;
5.10.4.3.2.3 Peak widths within 0.5 ± 0.1 amu's;
5.10.4.3.2.4 Relative abundance is 100% for ion 69, at least 35% for ion 219, and at least 1% for ion 502;
5.10.4.3.2.5 Relative abundances for isotope masses 70, 220, and 503 ± 0.2 amu's are 0.5–1.5%, 2–8%, and 5–15%, respectively; and
5.10.4.3.2.6 Air and water peaks at m/z = 18, 28, 32, and 44 amu's must be very small and consistent with historical values.
5.10.4.4 Maintaining a log book. Maintain an instrument log book, and make entries for each use. Include the following information in the logbook: operator name, helium cylinder tank pressure and outlet pressure, vacuum gauge reading, any maintenance performed on the instrument (such as changing the injection port liner, gold seal, guard column, source cleaning), sequence name, data path, samples in order of injection, method information, GC column number, and the Standard Auto Tune report and DFTPP Tune report.
5.10.4.5 Running a Solvent Blank: Following a liner change or at the start of a new run, run an injection of a pure solvent to confirm that the system is free of excessive or interfering contamination. Analyze the solvent in SCAN mode using the same temperature program used for sample analysis. If contamination is present, analyze additional samples of fresh solvent until the interfering contamination is removed.
5.10.4.6 Checking the DFTPP Tune: Prior to running the first calibration standard, verify the instrument tune conditions by running a 10 ng/μL DFTPP check standard to check the mass measuring accuracy of the MS, the resolution sensitivity, the baseline threshold, and the ion abundance ranges. Run the standard using the DFTPP method provided with the instrument. Each of the criteria identified in Table SOP 4.2 of this Appendix must be met before using the instrument for analysis:
| Mass, M/z | Relative to mass | Relative abundance criteria | Purpose of checkpoint |
|---|---|---|---|
| 51 | 442 | 10–80% of the base peak | Low mass sensitivity. |
| 68 | 69 | <2% of mass 69 | Low mass resolution. |
| 70 | 69 | <2% of mass 69 | Low mass resolution. |
| 127 | 442 | 10–80% of the base peak | Low-mid mass sensitivity. |
| 197 | 198 | <2% of mass 198 | Mid mass resolution. |
| 198 | 442 | Base peak or >50% of 442 | Mid mass resolution and sensitivity. |
| 199 | 198 | 5–9% of mass 198 | Mid mass resolution and isotope ratio. |
| 275 | 442 | 10–60% of the base peak | Mid-high mass sensitivity. |
| 365 | 442 | >1% of the base peak | Baseline threshold. |
| 441 | 443 | Present and < mass 443 | High mass resolution. |
| 442 | 442 | Base peak or >50% of 198 | High mass resolution and sensitivity. |
| 443 | 442 | 15–24% of mass 442 | High mass resolution and isotopic ratio. |
5.10.4.7 Calibrating with a Multiple-Point Calibration Curve. A 5- or 6-point calibration curve is obtained by running 5 or 6 working standards (see SOP 3) on the tuned GC/MS instrument. Calculate the relative response factor (RRF) for each compound relative to its corresponding deuterated internal standard as indicated in Table SOP 4.3 of this Appendix. The relative standard deviation (RSD) of the RRFs for each compound must be less than 25%. Run an independently prepared check standard immediately after the calibration standards to validate the accuracy of the calibration curve.
5.10.4.8 Running Samples. Once the calibration curve has been validated, samples can be analyzed. Dispense 1,000 μL of sample extract into labeled auto-sampler vials. Add 20 μL of the internal standard solution (see SOP 2 of this Appendix) to the extract using a syringe or a positive displacement pipettor. Run a check standard every 10 samples to ensure the consistency of the instrument. The RRF for each compound in the check standard must be within 25% of the average RRF obtained in the initial calibration.
5.10.4.9 Quantification: Once a calibration table has been generated, quantify each data file using the “Calculate and Generate” function in the MS ChemStation software, or equivalent software. Review individual peak integration manually to ensure proper baseline integration. The quantification of a compound is based on the peak area of the primary ion (Q Ion) indicated in Table SOP 4.4 of this Appendix.
| Compound name | Quantitation ion | Reference compound for response factor | Internal standard for quantitation |
|---|---|---|---|
| * Summed compounds; draw an integration line underneath all peaks with selected ion. | |||
| N D34 C16 | 66 | N D34 C16 | D34 n C16 Q Ion 66. |
| n-C14 | 57 | n C14 | |
| n-C15 | 57 | n C15 | |
| n-C16 | 57 | n C16 | |
| N D34 C17 | 66 | N D34 C17 | |
| n-C17 | 57 | n C17 | |
| Pristane | 57 | Pristane | |
| n-C18 | 57 | n C18 | |
| Phytane | 57 | Phytane | |
| n C19 | 57 | n C19 | |
| N D42 C20 | 66 | N D42 C20 | D42 n C20 Q Ion 66. |
| n C20 | 57 | n C20 | |
| n C21 | 57 | n C21 | |
| n C22 | 57 | n C22 | |
| n C23 | 57 | n C23 | |
| N D50 C 24 | 66 | N D50 C 24 | |
| n C24 | 57 | n C24 | |
| n C25 | 57 | n C25 | |
| n C26 | 57 | n C26 | |
| n C27 | 57 | n C27 | |
| n C28 | 57 | n C28 | |
| n C29 | 57 | n C29 | |
| N D62 C30 | 66 | N D62 C30 | D62 n C30Q Ion 66. |
| n C30 | 57 | n C30 | |
| n C31 | 57 | n C31 | |
| N D66 C32 | 57 | N D66 C32 | |
| n C32 | 57 | n C32 | |
| n C33 | 57 | n C33 | |
| n C34 | 57 | n C34 | |
| n C35 | 57 | n C35 | |
| D8 Naphthalene | 136 | D8 Naphthalene | D8 Naphthalene Q Ion 136. |
| Naphthalene | 128 | Naphthalene | |
| D10 1-Methylnaphthalene | 152 | D10 1-Methylnaphthalene | |
| C1 Naphthalene * | 142 | C1 Naphthalene | |
| C2 Naphthalene * | 156 | C2 Naphthalene | |
| C3 Naphthalene * | 170 | C3 Naphthalene | |
| C4 Naphthalene * | 184 | C3 Naphthalene | |
| D10 Anthracene | 188 | D10 Anthracene | D10 Anthracene Q Ion 188. |
| D10 Phenanthrene | 188 | D10 Phenanthrene | |
| Phenanthrene | 178 | Phenanthrene | |
| C1 Phenanthrene * | 192 | C1 Phenanthrene | |
| C2 Phenanthrene * | 206 | C2 Phenanthrene | |
| C3 Phenanthrene * | 220 | C2 Phenanthrene | |
| C4 Phenanthrene * | 234 | C2 Phenanthrene | |
| Fluorene | 166 | Fluorene | |
| C1 Fluorene * | 180 | Fluorene | |
| C2 Fluorene * | 194 | Fluorene | |
| C3 Fluorene * | 208 | Fluorene | |
| Dibenzothiophene | 184 | Dibenzothiophene | |
| C1 Dibenzothiophene * | 198 | Dibenzothiophene | |
| C2 Dibenzothiophene * | 212 | Dibenzothiophene | |
| C3 Dibenzothiophene * | 226 | Dibenzothiophene | |
| Naphthobenzothiophene (NBT) | 234 | Naphthobenzothiophene | |
| C1 NBT * | 248 | Naphthobenzothiophene | |
| C2 NBT * | 262 | Naphthobenzothiophene | |
| C3 NBT * | 276 | Naphthobenzothiophene | |
| Fluoranthene | 202 | Fluoranthene | |
| D10 Pyrene | 212 | D10 Pyrene | |
| Pyrene | 202 | Pyrene | |
| C1 Pyrene * | 216 | Pyrene | |
| C2 Pyrene * | 230 | Pyrene | |
| D12 Chrysene | 240 | D12 Chrysene | D12 Chrysene Q Ion 240. |
| Benzo(a)anthracene/Chrysene * | 228 | Chrysene | |
| C1 Chrysene * | 242 | Chrysene | |
| C2 Chrysene * | 256 | Chrysene | |
| C3 Chrysene * | 270 | Chrysene | |
| C4 Chrysene * | 284 | Chrysene | |
| 5α-androstane | 245 | 5α-androstane | 5α-androstane Q Ion 245. |
| Coprostane | 219 | Coprostane | |
| Hopane | 191 | Hopane | |
5.10.4.10 Equation 14 of this Appendix is used to calculate the concentration of analytes in units of μg/g oil added:
where:
A analyte = the peak area of the analyte,
C istd = the concentration of the internal standard,
A istd = the area of the internal standard,
RRF = the relative response factor, and
100 is the conversion factor to convert mg/L DCM to μg/g oil added.
5.10.4.11 If some analytes are not commercially available, the RRFs of other compounds (usually the parent compound) are used to quantify those analytes. For example, the RRF of C3-naphthalene may be used to calculate the concentrations of C3- and C4-naphthalenes. See Table SOP 4.4 of this Appendix for details. The quantification of these alkylated PAHs is relative because it is assumed that the molecular ions of the alkylated PAHs have the same RRFs as the parent compound ions. Nevertheless, these relative concentrations are useful for monitoring the fate of these compounds during the course of any analysis, as long as their concentrations are measured in a consistent way throughout the analysis.
5.10.4.12 Concentration calculations for all target compounds are performed using EnviroQuant software or equivalent. Data for each sample can be printed directly using a customized report template. Data can also be automatically entered into a spreadsheet within the EnviroQuant software.
5.10.5 Quality Assurance/Quality Control. The following criteria must be met before any samples are analyzed:
5.10.5.1 Air/water check to verify the system is leak free.
5.10.5.2 AutoTune and DFTPP Tune pass all criteria.
5.10.5.3 DFTPP check standard passes all criteria.
5.10.5.4 Solvent blank scan indicates the GC/MS system is free of interfering contamination.
5.10.5.5 Prepare and monitor a control chart of a standard oil analysis. Concentrations of the analytes in the control chart must be no more than 25% different from their historical averages.
5.10.5.6 Relative response factors for analytes in the check standards inserted between every 10 samples must be no more than 25 percent different from the average RRF of those same analytes in the calibration curve. Peak shapes must be symmetrical.
5.11 References for Section 5
(1) Haines, J.R., E.J. Kleiner, K.A. McClellan, K.M. Koran, E.L. Holder, D.W. King, and A.D. Venosa. 2005. “Laboratory evaluation of oil spill bioremediation products in salt and freshwater systems.” J. Ind. Microbiol. Biotech 32: 171–185.

Specialized Industries
Go beyond the regulations! Visit the Institute for in-depth guidance on a wide range of compliance subjects in safety and health, transportation, environment, and human resources.
J. J. Keller® COMPLIANCE NETWORK is a premier online safety and compliance community, offering members exclusive access to timely regulatory content in workplace safety (OSHA), transportation (DOT), environment (EPA), and human resources (DOL).

Interact With Our Compliance Experts
Puzzled by a regulatory question or issue? Let our renowned experts provide the answers and get your business on track to full compliance!

Upcoming Events
Reference the Compliance Network Safety Calendar to keep track of upcoming safety and compliance events. Browse by industry or search by keyword to see relevant dates and observances, including national safety months, compliance deadlines, and more.
The Environmental Protection Agency (EPA) is taking interim final action on corrections and clarifications to the new source performance standards (NSPS) for electric arc furnaces and argon-oxygen decarburization vessels in the steel industry. The corrections and clarifications are being made to address unintended and inadvertent errors in the recently finalized standards.
DATES: This interim final rule is effective on February 14, 2024, published in the Federal Register February 14, 2024, page 11198.
View final rule.
| §60.273 Emission monitoring. | ||
| (c) and (d)(2) | Revised | View text |
| §60.274 Monitoring of operations. | ||
| (b)(1), (b)(3), and (c) | Revised | View text |
| (i)(9) | Revised | View text |
| §60.276 Recordkeeping and reporting requirements. | ||
| (a) | Revised | View text |
| §60.273a Emission monitoring. | ||
| (c) and (d)(2) | Revised | View text |
| §60.274a Monitoring of operations. | ||
| (b)(1), (b)(3), and (c) | Revised | View text |
| (h)(9) | Revised | View text |
| §60.276a Recordkeeping and reporting requirements. | ||
| (c) | Revised | View text |
| §60.271b Definitions. | ||
| definition “Shop opacity” | Revised | View text |
| §60.272b Standard for particulate matter. | ||
| (a)(3) | Revised | View text |
| §60.273b Emission monitoring. | ||
| (c), (d)(2), (d)(3), and (e) introductory text | Revised | View text |
| §60.274b Monitoring of operations. | ||
| (b) and (c) | Revised | View text |
| (h)(9) | Revised | View text |
| §60.276b Recordkeeping and reporting requirements. | ||
| (c) | Revised | View text |
Previous Text
§60.273 Emission monitoring.
* * * *
(c) A continuous monitoring system for the measurement of the opacity of emissions discharged into the atmosphere from the control device(s) is not required on any modular, multi-stack, negative-pressure or positive- pressure fabric filter or on any single-stack fabric filter if observations of the opacity of the visible emissions from the control device are performed by a certified visible emission observer and the owner installs and operates a bag leak detection system according to paragraph (e) of this section whenever the control device is being used to remove particulate matter from the EAF. Visible emission observations shall be conducted at least once per day of the control device for at least three 6-minute periods when the furnace is operating in the melting and refining period. All visible emissions observations shall be conducted in accordance with EPA Method 9 of appendix A to this part, or, as an alternative, according to ASTM D7520–16 (incorporated by reference, see §60.17), with the caveats described under Shop opacity in §60.271. If visible emissions occur from more than one point, the opacity shall be recorded for any points where visible emissions are observed. Where it is possible to determine that a number of visible emission points relate to only one incident of the visible emission, only one set of three 6-minute observations will be required. In that case, the EPA Method 9 observations must be made for the point of highest opacity that directly relates to the cause (or location) of visible emissions observed during a single incident. Records shall be maintained of any 6-minute average that is in excess of the emission limit specified in §60.272(a)(2).
(d) * * *
(2) No less than once per week, commencing from the tap of one EAF heat cycle to the tap of the following heat cycle. A melt shop with more than one EAF shall conduct these readings while both EAFs are in operation. Both EAFs are not required to be on the same schedule for tapping.
* * * *
§60.274 Monitoring of operations.
* * * *
(b) * * *
(1) Monitor and record on a continuous basis the rolling 15-minute average furnace static pressure (if a DEC system is in use, and a furnace static pressure gauge is installed according to paragraph (f) of this section) and either:
(i) Install, calibrate, and maintain a monitoring device that continuously records the capture system fan motor amperes and damper position(s);
(ii) Install, calibrate, and maintain a monitoring device that continuously records on a rolling 15-minute average basis either the volumetric flow rate through each separately ducted hood; or
(iii) Install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet and continuously record damper position(s).
* * * *
(3) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(c) When the owner or operator of an affected facility is required to demonstrate compliance with the standards under §60.272(a)(3) and at any other time that the Administrator may require (under section 114 of the CAA, as amended), the owner or operator shall determine during periods in which a hood is operated for the purpose of capturing emissions from the affected facility subject to paragraph (b) of this section, either:
(1) Monitor and record the fan motor amperes at each damper position, and damper position consistent with paragraph (i)(5) of this section;
(2) install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate through each separately ducted hood; or
(3) install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet and monitor and record the damper position consistent with paragraph (i)(5) of this section.
(4) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(5) The owner or operator may petition the Administrator or delegated authority for reestablishment of these parameters whenever the owner or operator can demonstrate to the Administrator's or delegated authority's satisfaction that the EAF operating conditions upon which the parameters were previously established are no longer applicable. The values of the parameters as determined during the most recent demonstration of compliance shall be the appropriate operational range or control set point throughout each applicable period. Operation at values beyond the accepted operational range or control set point may be subject to the requirements of §60.276(a).
* * * *
(i) * * *
(9) Parameters monitored pursuant to paragraphs (i)(6)–(8) of this section shall be recorded on a rolling averaging period not to exceed 15 minutes.
* * * *
§60.276 Recordkeeping and reporting requirements.
(a) Continuous operation at a furnace static pressure that exceeds the operational range or control setting under §60.274(g), for owners and operators that elect to install a furnace static pressure monitoring device under §60.274(f) or operation at flow rates lower than those established under §60.274(c) may be considered by the Administrator or delegated authority to be unacceptable operation and maintenance of the affected facility. Operation at such values shall be reported to the Administrator or delegated authority semiannually.
* * * *
§60.273a Emission monitoring.
* * * *
(c) A continuous monitoring system for the measurement of the opacity of emissions discharged into the atmosphere from the control device(s) is not required on any modular, multi-stack, negative-pressure or positive-pressure fabric filter or on any single-stack fabric filter if observations of the opacity of the visible emissions from the control device are performed by a certified visible emission observer and the owner installs and operates a bag leak detection system according to paragraph (e) of this section whenever the control device is being used to remove particulate matter from the EAF or AOD. Visible emission observations shall be conducted at least once per day of the control device for at least three 6-minute periods when the furnace is operating in the melting and refining period. All visible emissions observations shall be conducted in accordance with EPA Method 9, or, as an alternative, according to ASTM D7520–16 (incorporated by reference, see §60.17), with the caveats described under Shop opacity in §60.271. If visible emissions occur from more than one point, the opacity shall be recorded for any points where visible emissions are observed. Where it is possible to determine that a number of visible emission points relate to only one incident of the visible emission, only one set of three 6-minute observations will be required. In that case, the EPA Method 9 observations must be made for the point of highest opacity that directly relates to the cause (or location) of visible emissions observed during a single incident. Records shall be maintained of any 6-minute average that is in excess of the emission limit specified in §60.272a(a)(2).
* * * *
(d)* * *
(2) No less than once per week, commencing from the tap of one EAF heat cycle to the tap of the following heat cycle. A melt shop with more than one EAF shall conduct these readings while both EAFs are in operation. Both EAFs are not required to be on the same schedule for tapping.
* * * *
§60.274a Monitoring of operations.
* * * *
(b) * * *
(1) Monitor and record on a continuous basis the rolling 15-minute average furnace static pressure (if a DEC system is in use, and a furnace static pressure gauge is installed according to paragraph (f) of this section) and either:
(i) Install, calibrate, and maintain a monitoring device that continuously records the capture system fan motor amperes and damper position(s);
(ii) Install, calibrate, and maintain a monitoring device that continuously records on a rolling 15-minute average basis either the volumetric flow rate through each separately ducted hood; or
(iii) Install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet continuously record damper positions(s).
* * * *
(3) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(c) When the owner or operator of an affected facility is required to demonstrate compliance with the standards under §60.272a(a)(3) and at any other time that the Administrator may require (under section 114 of the CAA, as amended), the owner or operator shall determine during periods in which a hood is operated for the purpose of capturing emissions from the affected facility subject to paragraph (b) of this section, all damper positions and either the:
(1) Monitor and record the fan motor amperes at each damper position, and damper position consistent with paragraph (h)(5) of this section;
(2) Install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate through each separately ducted hood; or
(3) Install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet and monitor and record the damper position consistent with paragraph (h)(5) of this section.
(4) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(5) The owner or operator may petition the Administrator or delegated authority for reestablishment of these parameters whenever the owner or operator can demonstrate to the Administrator's or delegated authority's satisfaction that the affected facility operating conditions upon which the parameters were previously established are no longer applicable. The values of the parameters as determined during the most recent demonstration of compliance shall be the appropriate operational range or control set point throughout each applicable period. Operation at values beyond the accepted operational range or control set point may be subject to the requirements of §60.276a(c).
* * * *
(h) * * *
(9) Parameters monitored pursuant to paragraphs (h)(6) through (8) of this section shall be recorded on a rolling averaging period not to exceed 15 minutes.
§60.276a Recordkeeping and reporting requirements.
* * * *
(c) Continuous operation at a furnace static pressure that exceeds the operational range or control setting under §60.274a(g), for owners and operators that elect to install a furnace static pressure monitoring device under §60.274a(f) or operation at flow rates lower than those established under §60.274a(c) may be considered by the Administrator or delegated authority to be unacceptable operation and maintenance of the affected facility. Operation at such values shall be reported to the Administrator or delegated authority semiannually.
* * * *
§60.271b Definitions.
* * * *
Shop opacity means the arithmetic average of 24 observations of the opacity of any EAF or AOD emissions emanating from, and not within, the shop, during melting and refining, and during tapping, taken in accordance with EPA Method 9 of appendix A of this part, and during charging, according to the procedures in section 2.5 of Method 9 in appendix A to part 60 of this chapter, with the modification to determine the 3-minute block average opacity from the average of 12 consecutive observations recorded at 15-second intervals. For the daily opacity observation during melting and refining, during charging, and during tapping, facilities may measure opacity by EPA Method 22 of appendix A of this part, modified to require the recording of the aggregate duration of visible emissions at 15 second intervals. Alternatively, ASTM D7520–16 (incorporated by reference, see §60.17), may be used with the following five conditions:
(1) During the digital camera opacity technique (DCOT) certification procedure outlined in Section 9.2 of ASTM D7520–16 (incorporated by reference, see §60.17), the owner or operator or the DCOT vendor must present the plumes in front of various backgrounds of color and contrast representing conditions anticipated during field use such as blue sky, trees, and mixed backgrounds (clouds and/or a sparse tree stand);
(2) The owner or operator must also have standard operating procedures in place including daily or other frequency quality checks to ensure the equipment is within manufacturing specifications as outlined in Section 8.1 of ASTM D7520–16 (incorporated by reference, see §60.17);
(3) The owner or operator must follow the recordkeeping procedures outlined in §60.7(f) for the DCOT certification, compliance report, data sheets, and all raw unaltered JPEGs used for opacity and certification determination;
(4) The owner or operator or the DCOT vendor must have a minimum of four independent technology users apply the software to determine the visible opacity of the 300 certification plumes. For each set of 25 plumes, the user may not exceed 15 percent opacity of anyone reading and the average error must not exceed 7.5 percent opacity;
(5) Use of this approved alternative does not provide or imply a certification or validation of any vendor's hardware or software. The onus to maintain and verify the certification and/or training of the DCOT camera, software, and operator in accordance with ASTM D7520–16 (incorporated by reference, see §60.17) and these requirements is on the facility, DCOT operator, and DCOT vendor.
* * * *
§60.272b Standard for particulate matter.
(a) * * *
(3) Exit from a shop and, due solely to the operations of any affected EAF(s) or AOD vessel(s) during melting and refining exhibit greater than 0 percent opacity, and during charging exhibit greater than 6 percent opacity, as measured in accordance with EPA Method 9 of appendix A of this part, and during charging, exhibit greater than 6 percent opacity, as measured according to the procedures in section 2.5 of Method 9 in appendix A to part 60 of this chapter, with the modification of this section of Method 9 to determine the 3-minute block average opacity from the average of 12 consecutive observations recorded at 15-second intervals; or, as an alternative, according to ASTM D7520–16 (incorporated by reference, see §60.17), with the caveats described under Shop opacity in §60.271 or, for the daily opacity observations, exhibit 0 seconds of visible emissions as measured by EPA Method 22 of appendix A of this part, modified to require the recording of the aggregate duration of visible emissions at 15 second intervals. Shop opacity shall be recorded for any point(s) during melting and refining, during charging, and during tapping where visible emissions are observed. Where it is possible to determine that a number of visible emission sites relate to only one incident of visible emissions during melting and refining, during charging, or during tapping, only one observation of shop opacity or visible emissions will be required during melting and refining, during charging, or during tapping. In this case, the shop opacity or visible emissions observations must be made for the point of highest emissions during melting and refining, during charging, or during tapping that directly relates to the cause (or location) of visible emissions observed during a single incident.
* * * *
§60.273b Emission monitoring.
* * * *
(c) A continuous monitoring system for the measurement of the opacity of emissions discharged into the atmosphere from the control device(s) is not required on any modular, multi-stack, negative-pressure or positive-pressure fabric filter or on any single-stack fabric filter if observations of the opacity of the visible emissions from the control device are performed by a certified visible emission observer and the owner installs and operates a bag leak detection system according to paragraph (e) of this section whenever the control device is being used to remove particulate matter from the EAF or AOD. Visible emission observations shall be conducted at least once per day on the control device for at least three 6-minute periods when the furnace is operating in the melting and refining period. All visible emissions observations shall be conducted in accordance with EPA Method 9, or, as an alternative, according to ASTM D7520–16 (incorporated by reference, see §60.17), with the caveats described under Shop opacity in §60.271. If visible emissions occur from more than one point, the opacity shall be recorded for any points where visible emissions are observed. Where it is possible to determine that a number of visible emission points relate to only one incident of the visible emission, only one set of three 6-minute observations will be required. In that case, the EPA Method 9 observations must be made for the point of highest opacity that directly relates to the cause (or location) of visible emissions observed during a single incident. Records shall be maintained of any 6-minute average that is in excess of the emission limit specified in §60.272b(a)(2).
(d)* * *
(2) No less than once per week, commencing from the tap of one EAF heat cycle to the tap of the following heat cycle. A melt shop with more than one EAF shall conduct these readings while both EAFs are in operation. Both EAFs are not required to be on the same schedule for tapping.
(3) Shop opacity shall be determined as the arithmetic average of 24 consecutive 15-second opacity observations of emissions from the shop taken in accordance with EPA Method 9 during melting and refining and during tapping; and during charging determined according to the procedures in section 2.5 of Method 9 in appendix A to part 60 of this chapter, with the modification to determine the 3-minute block average opacity from the average of 12 consecutive observations recorded at 15-second intervals; or, as an alternative, according to ASTM D7520–16 (incorporated by reference, see §60.17), with the caveats described under Shop opacity in §60.271, or as the total duration of visible emissions measured according to EPA Method 22 over a six minute period, modified to require the recording of the aggregate duration of visible emissions at 15 second intervals. Shop opacity shall be recorded for any point(s) where visible emissions are observed. Where it is possible to determine that a number of visible emission points relate to only one incident of visible emissions, only one observation of shop opacity will be required. In this case, the shop opacity observations must be made for the point of highest opacity that directly relates to the cause (or location) of visible emissions observed during a single incident. Shop opacity shall be determined daily during melting and refining, during charging, and during tapping.
(e) A bag leak detection system must be installed on all fabric filters and operated on all single-stack fabric filters whenever the control device is being used to remove particulate matter from the EAF or AOD vessel if the owner or operator elects not to install and operate a continuous opacity monitoring system as provided for under paragraph (c) of this section. In addition, the owner or operator shall meet the visible emissions observation requirements in paragraph (c) of this section. The bag leak detection system must meet the specifications and requirements of paragraphs (e)(1) through (8) of this section.
§60.274b Monitoring of operations.
* * * *
(b) Except as provided under paragraph (e) of this section, the owner or operator subject to the provisions of this subpart shall conduct the following monitoring of the capture system to demonstrate continuous compliance:
(1) If a DEC system is in use, according to paragraph (f) of this section, monitor and record on a continuous basis the furnace static pressure and any one of (2) through (4) in this paragraph:
(2) Monitor and record the fan motor amperes at each damper position, and damper position consistent with paragraph (h)(5) of this section;
(3) Install, calibrate, and maintain a monitoring device that continuously records the volumetric air flow rate or static pressure at each separately ducted hood; or
(4) Install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet and monitor and record the damper position consistent with paragraph (h)(5) of this section.
(5) The static pressure monitoring device(s) shall be installed in an EAF or DEC duct prior to combining with other ducts and prior to the introduction of ambient air, at a location that has no flow disturbance due to the junctions.
(6) The volumetric flow monitoring device(s) may be installed in any appropriate location in the capture system such that reproducible flow rate monitoring will result. The flow rate monitoring device(s) shall have an accuracy of ±10 percent over its normal operating range and shall be calibrated according to the manufacturer's instructions. The Administrator may require the owner or operator to demonstrate the accuracy of the monitoring device(s) relative to EPA Methods 1 and 2 of appendix A of this part.
(7) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(c) When the owner or operator of an affected facility is required to demonstrate compliance with the standards under §60.272b(a)(3) and at any other time that the Administrator may require (under section 114 of the CAA, as amended), the owner or operator shall determine during all periods in which a hood is operated for the purpose of capturing emissions from the affected facility subject to paragraph (b) of this section, either:
(1) Monitor and record the fan motor amperes at each damper position, and damper position consistent with paragraph (h)(5) of this section;
(2) install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate through each separately ducted hood; or
(3) install, calibrate, and maintain a monitoring device that continuously records the volumetric flow rate at the control device inlet and monitor and record the damper position consistent with paragraph (h)(5) of this section.
(4) Parameters monitored pursuant to this paragraph, excluding damper position, shall be recorded on a rolling averaging period not to exceed 15 minutes.
(5) The owner or operator may petition the Administrator or delegated authority for reestablishment of these parameters whenever the owner or operator can demonstrate to the Administrator's or delegated authority's satisfaction that the affected facility operating conditions upon which the parameters were previously established are no longer applicable. The values of the parameters as determined during the most recent demonstration of compliance shall be the appropriate operational range or control set point throughout each applicable period. Operation at values beyond the accepted operational range or control set point may be subject to the requirements of §60.276b(c).
* * * *
(h) * * *
(9) Parameters monitored pursuant to paragraphs (h)(6)–(8) of this section shall be recorded on a rolling averaging period not to exceed 15 minutes.
§60.276b Recordkeeping and reporting requirements.
* * * *
(c) Operation at a furnace static pressure that exceeds the operational range or control setting under §60.274b(g), for owners and operators that elect to install a furnace static pressure monitoring device under 60.274b(f) or operation ranges or control settings outside of those established under §60.274b(c) may be considered by the Administrator or delegated authority to be unacceptable operation and maintenance of the affected facility. Operation at such values shall be reported to the Administrator or delegated authority semiannually.
* * * *
On February 7, 2024, the Environmental Protection Agency (EPA) made a significant move to improve air quality by finalizing a rule that lowers the National Ambient Air Quality Standards (NAAQS) for fine particulate matter (PM2.5). This marks the first time in a decade that such a change has been implemented. The new rule reduces the primary annual PM2.5 standard from 12.0 micrograms per cubic meter to 9.0 micrograms per cubic meter.
It's important to note that this adjustment focuses on the primary annual PM2.5 standard. Other air quality standards for PM remain unchanged, including the primary 24-hour PM2.5 standard, the primary 24-hour coarse PM (PM10) standard, and the secondary PM2.5 and PM10 standards. This development underscores EPA's commitment to safeguarding public health and the environment.
The final rule:
EPA establishes standards for six harmful pollutants (called criteria pollutants), including carbon monoxide, lead, PM, ozone, nitrogen dioxide, and sulfur dioxide. These standards are based solely on protecting public health and welfare without considering the cost of making changes.
PM2.5, also known as "soot," comes from various sources, such as vehicles, industrial facilities, construction sites, and fires. It’s associated with serious health issues like heart attacks, respiratory illnesses, and premature death. EPA estimates the new standard will prevent around 4,500 premature deaths and 290,000 lost workdays annually by 2032. Additionally, it’s expected to generate approximately $46 billion in net health benefits each year.
The Clean Air Act requires that EPA review the NAAQS every five years to ensure their adequacy. The process is a multistage, robust review of the current science that requires significant expert input. If a standard is tightened, there’s a cascading effect on air quality policies and programs across the country. States and local regions must ensure that the sources of pollution in their area decrease their emissions so that the region can meet the new, more stringent national standard.
The effective date of the strengthened PM2.5 standard isn’t immediate. The rule becomes officially effective 60 days after it’s published in the Federal Register. This is expected to happen sometime in April 2024.
Implementation is a multiphase process driven by requirements in the Clean Air Act. In the first phase, EPA makes initial designations of whether areas meet the revised standards. States then develop and submit State Implementation Plans (or SIPs) outlining how to achieve the new standards. This phase, which must be done within three years, involves identifying emission sources, setting regulations, and implementing control measures.
An industrial source with high emissions must apply for a permit to build a facility or expand operations in a way that increases air pollution. Here’s how your facility may be affected:
Key to remember: EPA finalized a rule to tighten the NAAQS for fine particulate matter, lowering the primary annual standard from 12.0 to 9.0 micrograms per cubic meter.
Inside EPA, the Toxic Substances Control Act (TSCA) program may have, for years, looked much like a scene from an episode of “I Love Lucy,” where Lucy and Ethel take jobs at a chocolate factory. Instead of wrapping chocolates that whizzed by on a production line, however, EPA scientists faced an avalanche of new chemical submissions. A final rule, though, set to appear in the Federal Register may give these scientists some relief.
Prior to 2016, the agency had been reviewing just 20 percent of all new chemicals in 90 days before they entered commerce. The remaining 80 percent sailed through the new chemical submittal process without as much scrutiny and were stamped approved in 90 days.
Then, in 2016, a new law stepped things up. It mandated the review of 100 percent of new chemical submissions in the 90-day time frame. Despite the increase in duties, the agency suffered insufficient funding and staffing for the TSCA program. That meant many chemicals were stuck in review and could not go to market. Even those chemicals needed for modern technologies like semiconductors, batteries, and biotech faced the same bottleneck. Stakeholders on all sides were frustrated.
In a Congressional hearing on January 24 this year, EPA Assistant Administrator Michal Freedhoff, Ph.D., concluded: “The truth is we’re not able to achieve all that TSCA was expected to. The problem’s clear. TSCA’s underfunded … We don’t need to change the law. We need funding to implement the law we have.”
Now, though, EPA posted a pre-publication version of a final rule to increase TSCA fees, as authorized under the law. The rule updates how EPA will recover authorized costs and ensure that collected fees provide the agency with 25 percent of authorized costs. It takes effect 60 days after the rule is published in the Federal Register.
The rule would require payment of fees for eight categories of activities or events under TSCA sections 4, 5, and 6:
Most fee responsibilities under the rule are assigned to chemical manufacturers (including importers). In certain cases, fees may also apply to chemical processors. An example would be when a processor submits a SNUN under TSCA section 5 or is identified in a TSCA section 4 test order.
Entities that meet the definition of a “small business concern,” as defined, can receive a discount of approximately 80 percent.
Where multiple entities are subject to a fee, the final rule allows those entities to pay individually or through a consortium of payers. EPA will divide the total fee amongst responsible individual and joint payers per a formula and process described in the rule.
During fiscal years 2024-2026, EPA says it will work to track actual TSCA implementation costs and use that data to adjust future fees, if appropriate. As required by law, EPA will evaluate and re-adjust the fees, if necessary, every three years. Note that the TSCA program is also funded, in part, by the Congressional budget.
For TSCA funding and fees information, visit our TSCA Fees discussion and EPA’s “Toxic Substances Control Act (TSCA) Administration Fees” webpage.
Also check out the archived webcast, “Oversight of Toxic Substances Control Act Amendments Implementation,” a hearing held by the Senate Committee on Environment & Public Works on January 24. That hearing covered “all things TSCA related,” including the ever-controversial topic of worker protections.
In fact, when asked by one Senator why the TSCA Program is suffering “mission creep” into the OSHA arena, Freedhoff explained, “TSCA says that we have to consider the risks to ‘potentially exposed and susceptible subpopulations,’ and that term is explicitly defined to include workers.” She added that OSHA standards don’t protect everyone. By that she meant they don’t cover self-employed workers nor public workers that are not subject to a state OSHA plan. TSCA, then, can fill that gap.
The Senator replied that given the funding issues, “staying in the lane would probably be helpful.”
EPA posted a pre-publication version of a final rule to increase TSCA fees. It takes effect 60 days after publication in the Federal Register.
Hi everyone! Welcome to the monthly news roundup video, where we’ll go over the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. Let’s get started!
Effective January 15, OSHA penalties increased 3.2 percent for inflation. Most penalties increased to $16,131. Willful and serious violations, however, increased to $161,323.
Construction workers aged 45 and older suffer more severe injuries and higher associated costs than other age groups. Most injuries are due to slips, trips, and falls.
Washington State updated its process safety management rules to better protect workers in petroleum refineries from the hazards of volatile chemicals. The rules take effect December 27, 2024.
Bloodborne pathogens topped the list of OSHA violations for the healthcare industry in 2023. Hazard Communication was the second most cited standard, followed by respiratory protection.
OSHA Region 2 launched a regional emphasis program that targets tree trimming, tree removal, and land clearing operations. Region 2 includes New York, New Jersey, Puerto Rico, and the U.S. Virgin Islands.
EPA continues to strengthen its regulation of per- and polyfluoroalkyl — or PFAS — substances. A new rule prevents facilities from using any of the 300+ inactive PFAS before EPA conducts a risk determination and, if necessary, regulates the activity.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
The Environmental Protection Agency (EPA) finalized its reconsideration of the National Ambient Air Quality Standards for Particulate Matter (NAAQS PM), strengthening the annual primary emissions limit of fine particulate matter (PM2.5), also known as soot.
What changed?
Under the Clean Air Act, EPA sets primary and secondary NAAQS. Primary standards focus on protecting human health, while secondary standards concentrate on protecting public welfare (e.g., preventing environmental damage).
In the finalized NAAQS, EPA strengthened the primary annual PM2.5 standard from 12 micrograms per cubic meter to 9 micrograms per cubic meter. The agency also:
What didn’t change?
The agency maintained:
Does this affect my facility?
Upon the effective date of the final rule, all applicants for permits to construct a new major source or make a major modification to an existing stationary source must conduct an air quality analysis that considers the revised PM2.5 NAAQS. Facilities with a Prevention of Significant Deterioration (PSD) permit in progress must show the new or modified source won’t violate or cause a violation of the new annual primary PM2.5 NAAQS.
When EPA establishes a new NAAQS or revises an existing one, it begins a years-long process to implement the new standards in states:
Now is the time to proactively consider ways your facility can further limit PM2.5 emissions. Your organization will be better prepared to comply with any future PM emissions control regulations.
Key to remember: EPA strengthened the annual primary standards for fine particulate matter, also known as soot.
The Environmental Protection Agency (EPA or the Agency) is proposing to amend its regulation under the Resource Conservation and Recovery Act (RCRA) by adding nine specific per-and polyfluoroalkyl substances (PFAS), their salts, and their structural isomers, to its list of hazardous constituents. These nine PFAS are perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorobutanesulfonic acid (PFBS), hexafluoropropylene oxide-dimer acid (HFPO–DA or GenX), perfluorononanoic acid (PFNA), perfluorohexanesulfonic acid (PFHxS), perfluorodecanoic acid (PFDA), perfluorohexanoic acid (PFHxA), and perfluorobutanoic acid (PFBA). EPA's criteria for listing substances as hazardous constituents under RCRA require that they have been shown in scientific studies to have toxic, carcinogenic, mutagenic, or teratogenic effects on humans or other life forms. EPA reviewed and evaluated key toxicity and epidemiological studies and assessments for the nine PFAS to determine whether the available data for these PFAS meet the Agency's criteria for listing substances as hazardous constituents under RCRA. Based on EPA's evaluation, the above nine PFAS, their salts, and their structural isomers meet the criteria for being listed as RCRA hazardous constituents. As a result of this proposed rule, if finalized, when corrective action requirements are imposed at a facility, these PFAS would be among the hazardous constituents expressly identified for consideration in RCRA facility assessments and, where necessary, further investigation and cleanup through the RCRA corrective action process at RCRA treatment, storage, and disposal facilities.
DATES: This proposed rule is published in the Federal Register February 8, 2024, page 8606.
View proposed rule.
This proposed rule would amend the definition of hazardous waste applicable to corrective action to address releases from solid waste management units at RCRA-permitted treatment, storage, and disposal facilities and make related conforming amendments, thereby providing clear regulatory authority to fully implement the Resource Conservation and Recovery Act (RCRA) statutory requirement that permitted facilities conduct corrective action to address releases not only of substances listed or identified as hazardous waste in the regulations but of any substance that meets the statutory definition of hazardous waste. The proposed rule would also provide notice of EPA's interpretation that the statutory definition of hazardous waste applies to corrective action for releases from solid waste management units at permitted and interim status facilities.
DATES: This proposed rule is published in the Federal Register February 8, 2024, page 8598.
View proposed rule.
The State of South Dakota Department of Agriculture and Natural Resources has applied to the Environmental Protection Agency (EPA) for final authorization of the changes to its hazardous waste program under the Resource Conservation and Recovery Act (RCRA). The EPA has determined that these changes satisfy all requirements needed to qualify for final authorization, and is authorizing the State's changes through this direct final action. The EPA uses the regulations entitled, “Approved State Hazardous Waste Management Programs” to provide notice of the authorization status of State programs and to incorporate by reference those provisions of State statutes and regulations that will be subject to the EPA's inspection and enforcement. This rule also codifies in the regulations the approval of South Dakota's hazardous waste management program and incorporates by reference authorized provisions of the State's regulations.
DATES: This direct final rule is effective on April 8, 2024 is published in the Federal Register February 8, 2024, page 8540.
| §272.2101 South Dakota State-administered program: Final authorization. | ||
| Entire section | Revised | View text |
| Appendix A to Part 272—State Requirements | ||
| Listing for “South Dakota” | Revised | View text |
New Text
§272.2101 South Dakota State-administered program: Final authorization.
(a) History of the State of South Dakota authorization. Pursuant to section 3006(b) of RCRA, 42 U.S.C. 6926(b), South Dakota has final authorization for the following elements as submitted to EPA in South Dakota's base program application for final authorization which was approved by EPA effective on November 2, 1984. Subsequent program revision applications were approved effective on June 17, 1991, November 8, 1993, March 11, 1994, September 23, 1996, June 8, 2000, May 24, 2004, March 8, 2006, August 8, 2012, August 23, 2016, and April 8, 2024.
(b) Enforcement authority. The State of South Dakota has primary responsibility for enforcing its hazardous waste management program. However, EPA retains the authority to exercise its inspection and enforcement authorities in accordance with sections 3007, 3008, 3013, and 7003 of RCRA, 42 U.S.C. 6927, 6928, 6934, and 6973, and any other applicable statutory and regulatory provisions, regardless of whether the State has taken its own actions, as well as in accordance with other statutory and regulatory provisions.
(c) State statutes and regulations —(1) Incorporation by reference. The South Dakota regulations cited in paragraph (c)(1)(i) of this section are incorporated by reference as part of the hazardous waste management program under Subtitle C of RCRA, 42 U.S.C. 6921 et seq. The Director of the Federal Register in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. This material is available for inspection at the EPA and at the National Archives and Records Administration (NARA). You may inspect a copy at EPA Region 8, 1595 Wynkoop Street, Denver, Colorado, phone number (303) 312–6667. For information on the availability of this material at NARA, email: fr.inspection@nara.gov, or go to: https://www.archives.gov/federal-register/cfr/ibr-locations.html. You may obtain copies of the South Dakota regulations that are incorporated by reference in this paragraph from South Dakota Legislative Research Council, 3rd Floor, State Capitol, 500 East Capitol Avenue, Pierre, SD 57501, phone number 605–773–3251.
(i) EPA-Approved South Dakota Regulatory Requirements Applicable to the Hazardous Waste Management Program, dated June 2022.
(ii) [Reserved]
(2) Legal basis. The following provisions provide the legal basis for the State's implementation of the hazardous waste program, but they are not being incorporated by reference and do not replace Federal authorities:
(i) South Dakota Codified Laws (SDCL), as amended, 2021 Revision, Title 1, State Affairs and Government: Chapter 1–26, Administrative Procedures and Rules, sections 1–26–1(1), 1–26–1(4), 1–26–1(8) introductory paragraph, 1–26–1(8)(a), 1–26–2, 1–26–6.6, 1–26–16 through 1–26–19, 1–26–19.1, 1–26–19.2, 1–26–21, 1–26–27, 1–26–29, 1–26–30, 1–26–30.1, 1–26–30.2, 1–26–30.4, 1–26–31, 1–26–31.1, 1–26–31.2, 1–26–31.4, 1–26–35 and 1–26–36; Chapter 1–27, Public Records and Files, sections 1–27–1, 1–27–3, 1–27–9(2) and 1–27–28, 1–27–31; Chapter 1–32, Executive Reorganization, section 1–32–1(1); Chapter 1–41, Department of Agriculture and Natural Resources, sections 1–41–3.4, 1–41–18, 1–41–24 and 1–41–25.1.
(ii) SDCL, as amended, 2021 Revision, Title 15, Civil Procedure: Chapter 15–6, Rules of Procedure in Circuit Courts, section 15–6–24(a)–(c).
(iii) SDCL, as amended, 2021 Revision, Title 19, Evidence: Chapter 19–13, Privileges, sections 19–19–502(1), 19–19–502(5), 19–19–502(b), 19–19–507 and 19–19–509.
(iv) SDCL, as amended, 2021 Revision, Title 21, Judicial Remedies: Chapter 21–8, Injunction, section 21–8–1.
(v) SDCL, as amended, 2021 Revision, Title 22, Crimes: Chapter 22–6, Authorized Punishments, sections 22–6–1 introductory paragraph and 22–6–1(7).
(vi) SDCL, as amended, 2021 Revision, Title 23, Law Enforcement: Chapter 23–5, Criminal Identification, sections 23–5–1, 23–5–10(1), 23–5–10(3), 23–5–10(4) and 23–5–11 first sentence; Chapter 23–6, Criminal Statistics, section 23–6–4.
(vii) SDCL, as amended, 2021 Revision, Title 34, Public Health and Safety: Chapter 34–21, Radiation and Uranium Resources Exposure Control, section 34–21–2(7).
(viii) SDCL, as amended, 2021 Revision, Title 34A, Environmental Protection: Chapter 34A–6, Solid Waste Disposal, section 34A–6–1.3(17); Chapter 34A–10, Remedies for Protection of Environment, sections 34A–10–1, 34A–10–2, 34A–10–2.5, 34A–10–5, 34A–10–11, 34A–10–14 and 34A–10–16, Chapter 34A–11, Hazardous Waste Management, sections 34A–11–1, 34A–11–2 through 34A–11–4, 34A–11–5, 34A–11–8 through 34A–11–12, 34A–11–13 through 34A–11–16, 34A–11–17 through 34A–11–19, 34A–11–21 and 34A–11–22; Chapter 34A–12, Regulated Substance Discharges, sections 34A–12–1(8), 34A–12–4, 34A–12–6, 34A–12–8 through 34A–12–13, 34A–12–13.1 and 34A–12–14.
(ix) SDCL, as amended, 2021 Revision, Title 37, Trade Regulation, Chapter 37–29, Uniform Trade Secrets Act, section 37–29–1(4).
(x) Administrative Rules of South Dakota (ARSD), Article 74:08, Administrative Fees, effective April 19, 2021: Chapter 74:08:01, Fees for Records Reproduction, sections 74:08:01:01, 74:08:01:03, 74:08:01:04, 74:08:01:05.
(3) Related legal provisions. The following statutory provisions are broader in scope than the Federal program, are not part of the authorized program, are not incorporated by reference, and are not federally enforceable:
(i) SDCL, as amended, 2021 Revision, Title 34A, Environmental Protection, Chapter 34A–11, Hazardous Waste Management, sections 34A–11–12.1, 34A–11–16.1, 34A–11–25 and 34A–11–26.
(ii) [Reserved]
(4) Unauthorized State amendments. South Dakota has adopted but is not authorized for the following Federal final rules:
(i) Hazardous Waste Management System; User Fees for the Electronic Waste Manifest System and Amendments to Manifest Regulations (Non-HSWA), published in the Federal Register of 1/3/18.
(ii) Management Standards for Hazardous Waste Pharmaceuticals and Amendment to the P075 Listing for Nicotine (HSWA/Non-HSWA), published in the Federal Register of 2/22/19.
(iii) Those Federal rules written under RCRA provisions that predate HSWA (non-HSWA) which the State has adopted, but for which it is not authorized, are not federally enforceable. In contrast, EPA will continue to enforce the Federal HSWA standards for which South Dakota is not authorized until the State receives specific authorization from EPA.
(5) Memorandum of Agreement. The Memorandum of Agreement between EPA Region 8 and the State of South Dakota, signed by the Secretary of the South Dakota Department of Agriculture and Natural Resources Secretary on March 20, 2023, and by the EPA Region 8 Regional Administrator on March 10, 2023, although not incorporated by reference, is referenced as part of the authorized hazardous waste management program under subtitle C of RCRA, 42 U.S.C. 6921 et seq.
(6) Statement of legal authority. “Attorney General's Statement for Final Authorization”, signed by the Attorney General of South Dakota on May 24, 1984, and revisions, supplements and addenda to that Statement dated January 14, 1991, September 11, 1992, September 25, 1992, April 1, 1993, September 24, 1993, December 29, 1994, September 5, 1995, October 23, 1997, October 27, 1997, October 28, 1997, November 5, 1999, June 26, 2000, June 18, 2002, October 19, 2004, May 11, 2009, May 5, 2015, and November 29, 2021, although not incorporated by reference, are referenced as part of the authorized hazardous waste management program under subtitle C of RCRA, 42 U.S.C. 6921 et seq.
(7) Program Description. The Program Description and any other materials submitted as supplements thereto, although not incorporated by reference, are referenced as part of the authorized hazardous waste management program under subtitle C of RCRA, 42 U.S.C. 6921 et seq.
Appendix A to Part 272—State Requirements
* * * * *
South Dakota
The regulatory provisions include:
Administrative Rules of South Dakota (ARSD), Article 74:28, Hazardous Waste, as amended effective April 19, 2021, adopting by reference the Federal regulations as of July 1, 2018, and 83 FR 61552 (November 30, 2018).
Sections 74:28:21:01 (except the reference to “260.4 and 260.5” at 74:28:21:01(3)(b)(xii), and (14)(f)), 74:28:21:02, 74:28:21:03, 74:28:22:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)”), 74:28:23:01, 74:28:24:01, 74:28:25:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)”), 74:28:25:02 through 74:28:25:05, 74:28:26:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)” in the introductory paragraph), 74:28:27:01 (except the phrase “; 84 FR 36, 5938–5950 (February 22, 2019)” in the introductory paragraph), 74:28:28:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)”), 74:28:28:02 through 74:28:28:05, 74:28:29:01, 74:28:30:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)”) and 74:28:33:01 (except the phrase “; and 84 FR 36, 5938–5950 (February 22, 2019)”); Article 74:36, Air Pollution Control Program, section 74:36:11:01.
Copies of the South Dakota regulations that are incorporated by reference are available from South Dakota Legislative Research Council, 3rd Floor, State Capitol, 500 East Capitol Avenue, Pierre, SD 57501, (Phone: 605–773–3251).
* * * * *
The Office of Management and Budget (OMB) recently completed its review of the proposed rule to expand the National Emission Standards for Hazardous Air Pollutants (NESHAP) for lime manufacturing plants. If the Environmental Protection Agency (EPA) finalizes the rule, it will regulate four additional hazardous air pollutants (HAPs). The OMB also has many other proposed NESHAP-strengthening rules queued for review.
This news likely brings up some questions. What exactly is a NESHAP? Is your facility subject to NESHAP regulations? Let’s take a closer look.
EPA limits the amount of HAPs a facility can release into the air (or emit) through NESHAPs. The regulations apply to stationary sources, which are any buildings, structures, facilities, or installations that emit or may emit a HAP.
The agency puts stationary sources into industrial groups called source categories. Examples of the categories include asbestos, lead acid battery manufacturing, and petroleum refineries. EPA provides on its website a complete list of the source categories and where to find each category in the regulations.
The first potential mishap a facility faces is not knowing whether it’s covered by a NESHAP.
EPA controls HAP emissions in two ways. The rules under 40 CFR Part 61 regulate emissions by the type of HAPs released through specific activities, like removing asbestos when renovating a building. Part 63 regulates emissions based on the source categories, such as integrated iron and steel manufacturing.
To determine whether any NESHAPs apply to your facility, answer these questions.
1. Does your facility conduct any of the activities regulated under Part 61?
If your facility conducts any activities covered by Part 61, you must comply with the NESHAP for that specific activity and HAP. For example, a plant that produces vinyl chloride by any process is subject to the NESHAP for vinyl chloride (Part 61 Subpart F).
If your facility does not conduct any activities covered under Part 61, it’s not subject to the NESHAP regulations under Part 61.
2. Does a source category under Part 63 cover your facility?
If your facility does not fall under any source category identified in Part 63, it’s not subject to a NESHAP. However, if your facility is covered by a source category, you must then determine whether your facility is a major source or an area source.
Note that most source categories are regulated under Part 63, but a few are regulated under Part 61 in Subparts BB, C, D, E, F, FF, L, M, N, O, and P.
3. Does your facility qualify as a major source or an area source under Part 63?
A major source is any facility or group of facilities located on the same property under common control that emits or has the potential to emit either:
An area source is any facility or group of facilities that emit HAPs below the major source thresholds.
It’s important to distinguish between major and area sources because both types have applicable NESHAPs based on different standards. Maximum Achievable Control Technology (MACT) standards apply to major sources and reflect the maximum degree of HAP emission reduction possible. Some area sources are subject to Generally Available Control Technologies standards, which are typically less stringent than MACT standards and require minimum management practices to reduce HAP emissions.
If your facility falls under a source category and is either a major source or an area source with an applicable NESHAP, it’s subject to the NESHAP regulations under Part 63.
Help keep your facility compliant with these tips:
Key to remember: The first step of compliance with the National Emission Standards for Hazardous Air Pollutants is to determine whether your facility is covered by the regulations.
This action announces the Environmental Protection Agency's (EPA's) approval of alternative testing methods for use in measuring the levels of contaminants in drinking water to determine compliance with national primary drinking water regulations. The Safe Drinking Water Act authorizes EPA to approve the use of alternative testing methods through publication in the Federal Register . EPA is using this streamlined authority to make 93 additional methods available for analyzing drinking water samples. This expedited approach provides public water systems, laboratories, and primacy agencies with more timely access to new measurement techniques and greater flexibility in the selection of analytical methods, thereby reducing monitoring costs while maintaining public health protection.
DATES: This action is effective January 30, 2024, published in the Federal Register January 30, 2024, page 5773.
View final rule.
| Appendix A to Subpart C of Part 141—Alternative Testing Methods Approved for Analyses Under the Safe Drinking Water Act | ||
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.23(k)(1) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.24(e)(1) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.25(a) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.74(a)(1) | Revised | View text |
| Table Alternative Testing Methods for Disinfectant Residuals Listed at 40 CFR 141.74(a)(2) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.131(b)(1) | Revised | View text |
| Table Alternative Testing Methods for Disinfectant Residuals Listed at 40 CFR 141.131(c)(1) | Revised | View text |
| Table Alternative Testing Methods for Parameters Listed at 40 CFR 141.131(d) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.402(c)(2) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 141.852(a)(5) | Revised | View text |
| Table Alternative Testing Methods for Contaminants Listed at 40 CFR 143.4(b) | Revised | View text |
Previous Text
Appendix A to Subpart C of Part 141—Alternative Testing Methods Approved for Analyses Under the Safe Drinking Water Act
* * * *
| Contaminant | Methodology | EPA method | SM 21st edition 1 | SM 22nd edition 28 | SM 23rd edition 49 | SM Online 3 | ASTM 4 | Other |
|---|---|---|---|---|---|---|---|---|
| Alkalinity | Titrimetric | 2320 B | 2320 B | 2320 B | D1067-06 B, 11 B, 16 B | |||
| Antimony | Hydride—Atomic Absorption | D 3697-07, -12, -17 | ||||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Arsenic | Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | D 2972-08 C, -15 C | ||
| Hydride Atomic Absorption | 3114 B | 3114 B | 3114 B | 3114 B-09 | D 2972-08 B, -15 B | |||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Barium | Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | ||||
| Atomic Absorption; Direct | 3111 D | 3111 D | 3111 D | |||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Beryllium | Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | D 3645-08 B, -15 B | |||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Cadmium | Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | |||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Calcium | EDTA titrimetric | 3500-Ca B | 3500-Ca B | 3500-Ca B | D 511-09, -14 A | |||
| Atomic Absorption; Direct Aspiration | 3111 B | 3111 B | 3111 B | D 511-09, -14 B | ||||
| Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | |||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Ion Chromatography | D 6919-09, -17 | |||||||
| Chromium | Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Copper | Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | D 1688-07, -12 C, -17 C | ||
| Atomic Absorption; Direct Aspiration | 3111 B | 3111 B | 3111 B | D 1688-07, -12 A, -17 A | ||||
| Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | |||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Colorimetry | Hach Method 8026. 35 Hach Method 10272 36 | |||||||
| Conductivity | Conductance | 2510 B | 2510 B | 2510 B | D 1125-14 A | |||
| Cyanide | Manual Distillation with MgCl 2 followed by: | 4500-CN C | 4500-CN C | 4500-CN C | 4500-CN C-99 | D 2036-06 A | ||
| Spectrophotometric, Amenable | 4500-CN G | 4500-CN G | 4500-CN G | D 2036-06 B | ||||
| Spectrophotometric Manual | 4500-CN E | 4500-CN E | 4500-CN E | D2036-06 A | ||||
| Selective Electrode | 4500-CN F | 4500-CN F | 4500-CN F | |||||
| Gas Chromatography/Mass Spectrometry Headspace | ME355.01. 7 | |||||||
| Fluoride | Ion Chromatography | 4110 B | 4110 B | 4110 B | D 4327-11, -17 | |||
| Manual Distillation; Colorimetric SPADNS | 4500-F B, D | 4500-F B, D | 4500-F B, D | |||||
| Manual Electrode | 4500-F C | 4500-F C | 4500-F C | D 1179-04, 10 B, 16 B | ||||
| Automated Alizarin | 4500-F E | 4500-F E | 4500-F E | |||||
| Arsenite-Free Colorimetric SPADNS | Hach SPADNS. 2 Method 10225. 22 | |||||||
| Lead | Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | D 3559-08 D, -15 D | ||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Differential Pulse Anodic Stripping Voltametry | Method 1001, Rev. 1.1 57 | |||||||
| Magnesium | Atomic Absorption | 3111 B | 3111 B | 3111 B | D 511-09, -14 B | |||
| Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | |||||
| Complexation Titrimetric Methods | 3500-Mg B | 3500-Mg B | 3500-Mg B | D 511-09, -14 A | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Ion Chromatography | D 6919-09, -17 | |||||||
| Mercury | Manual, Cold Vapor | 3112 B | 3112 B | 3112 B | 3112 B-09 | D 3223-12, -17 | ||
| Nickel | Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | ||||
| Atomic Absorption; Direct | 3111 B | 3111 B | 3111 B | |||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Nitrate | Ion Chromatography | 4110 B | 4110 B | 4110 B | D 4327-11, -17 | |||
| Automated Cadmium Reduction | 4500-NO 3 F | 4500-NO 3 F | 4500-NO 3 F | |||||
| Manual Cadmium Reduction | 4500-NO 3 E | 4500-NO 3 E | 4500-NO 3 E | |||||
| Ion Selective Electrode | 4500-NO 3 D | 4500-NO 3 D | 4500-NO 3 D | |||||
| Reduction/Colorimetric | Systea Easy (1-Reagent). 8 NECi Nitrate-Reductase. 40 | |||||||
| Colorimetric; Direct | Hach TNTplus TM 835/836 Method 10206. 23 | |||||||
| Capillary Ion Electrophoresis | D 6508-15 | |||||||
| Nitrite | Ion Chromatography | 4110 B | 4110 B | 4110 B | D 4327-11, -17 | |||
| Automated Cadmium Reduction | 4500-NO 3 F | 4500-NO 3 F | 4500-NO 3 F | |||||
| Manual Cadmium Reduction | 4500-NO 3 E | 4500-NO 3 E | 4500-NO 3 E | |||||
| Spectrophotometric | 4500-NO 2 B | 4500-NO 2 B | 4500-NO 2 B | |||||
| Reduction/Colorimetric | Systea Easy (1-Reagent). 8 NECi Nitrate-Reductase. 40 | |||||||
| Capillary Ion Electrophoresis | D 6508-15 | |||||||
| Ortho-phosphate | Ion Chromatography | 4110 B | 4110 B | 4110 B | D 4327-11, -17 | |||
| Colorimetric, ascorbic acid, single reagent | 4500-P E | 4500-P E | 4500-P E | 4500-P E-99 | ||||
| Colorimetric, Automated, Ascorbic Acid | 4500-P F | 4500-P F | 4500-P F | 4500-P F-99 | Thermo Fisher Discrete Analyzer. 41 | |||
| Capillary Ion Electrophoresis | D 6508-15 | |||||||
| pH | Electrometric | 150.3 48 | 4500-H + B | 4500-H + B | 4500-H + B | D 1293-12, -18 | ||
| Selenium | Hydride-Atomic Absorption | 3114 B | 3114 B | 3114 B | 3114 B-09 | D 3859-08 A, -15 A | ||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B | 3113 B-04, B-10 | D 3859-08 B, -15 B | |||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Silica | Colorimetric | D859-05, 10, 16 | ||||||
| Molybdosilicate | 4500-SiO 2 C | 4500-SiO 2 C | 4500-SiO 2 C | |||||
| Heteropoly blue | 4500-SiO 2 D | 4500-SiO 2 D | 4500-SiO 2 D | |||||
| Automated for Molybdate-reactive Silica | 4500-SiO 2 E | 4500-SiO 2 E | 4500-SiO 2 E | |||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Inductively Coupled Plasma | 3120 B | 3120 B | 3120 B | |||||
| Sodium | Atomic Absorption; Direct Aspiration | 3111 B | 3111 B | 3111 B | ||||
| Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2 2 | |||||||
| Ion Chromatography | D 6919-09, -17 | |||||||
| Temperature | Thermometric | 2550 | 2550 | 2550 | 2550-10 |
| Contaminant | Methodology | EPA method | SM 21st edition 1 | SM 22nd edition, 28 SM 23rd edition 49 | SM Online 3 | ASTM 4 | Other |
|---|---|---|---|---|---|---|---|
| Benzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Carbon tetrachloride | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Chlorobenzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,2-Dichlorobenzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,4-Dichlorobenzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,2-Dichloroethane | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| cis-Dichloroethylene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| trans-Dichloroethylene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Dichloromethane | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,2-Dichloropropane | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Ethylbenzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Styrene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Tetrachloroethylene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,1,1-Trichloroethane | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Trichloroethylene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Toluene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,2,4-Trichlorobenzene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,1-Dichloroethylene | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 1,1,2-Trichlorethane | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Vinyl chloride | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| Xylenes (total) | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. | |||||
| 2,4-D | Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06 | D 5317-20. | ||
| 2,4,5-TP (Silvex) | Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06 | D 5317-20. | ||
| Alachlor | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Atrazine | Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC/ESI-MS/MS) | 25 536. | |||||
| Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3, 26 523. | ||||||
| Benzo(a)pyrene | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Carbofuran | High-performance liquid chromatography (HPLC) with post-column derivatization and fluorescence detection | 6610 B | 6610 B | 6610 B-04. | |||
| Liquid Chromatography/Mass Spectrometry | 58 ME 531 | ||||||
| Chlordane | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3 | |||||
| Dalapon | Ion Chromatography Electrospray Ionization Tandem Mass Spectrometry (IC-ESI-MS/MS) | 14 557. | |||||
| Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06. | ||||
| Di(2-ethylhexyl)adipate | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Di(2-ethylhexyl)phthalate | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Dibromochloropropane (DBCP) | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3. | |||||
| Dinoseb | Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06. | |||
| Endrin | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Ethyl dibromide (EDB) | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3. | |||||
| Glyphosate | High-Performance Liquid Chromatography (HPLC) with Post-Column Derivatization and Fluorescence Detection | 6651 B | 6651 B | 6651 B-00, B-05. | |||
| Heptachlor | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Heptachlor Epoxide | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Hexachlorobenzene | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Hexachlorocyclo-pentadiene | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Lindane | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Methoxychlor | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Oxamyl | High-performance liquid chromatography (HPLC) with post-column derivatization and fluorescence detection | 6610 B | 6610 B | 6610 B-04. | |||
| Liquid Chromatography/Mass Spectrometry | 58 ME 531. | ||||||
| PCBs (as Aroclors) | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Pentachlorophenol | Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06 | D 5317-20. | ||
| Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | ||||||
| Picloram | Gas Chromatography/Electron Capture Detection (GC/ECD) | 6640 B | 6640 B | 6640 B-01, B-06 | D 5317-20. | ||
| Simazine | Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (LC/ESI-MS/MS) | 25 536. | |||||
| Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3, 26 523. | ||||||
| Toxaphene | Solid Phase Extraction/Gas Chromatography/Mass Spectrometry (GC/MS) | 24 525.3. | |||||
| Total Trihalomethanes | Purge &Trap/Gas Chromatography/Mass Spectrometry | 9 524.3, 29 524.4. |
| Contaminant | Methodology | EPA method | SM 21st edition 1 | SM 22nd edition, 28 SM 23rd edition 49 | ASTM 4 | SM Online 3 |
|---|---|---|---|---|---|---|
| Naturally Occurring: | ||||||
| Gross alpha and beta | Evaporation | 900.0, Rev. 1.0 50 | 7110 B | 7110 B | ||
| Liquid Scintillation | D 7283-17 | 7110 D-17. | ||||
| Gross alpha | Coprecipitation | 7110 C | 7110 C | |||
| Radium 226 | Radon emanation | 903.1, Rev. 1.0 53 | 7500-Ra C | 7500-Ra C | D 3454-05, -18 | |
| Radiochemical | 903.0, Rev. 1.0 54 | 7500-Ra B | 7500-Ra B | D 2460-07 | ||
| Gamma Spectrometry | 7500-Ra E | 7500-Ra E-07. | ||||
| Radium 228 | Radiochemical | 904.0, Rev. 1.0 62 | 7500-Ra D | 7500-Ra D. | ||
| Gamma Spectrometry | 7500-Ra E | 7500-Ra E-07. | ||||
| Uranium | Radiochemical | 7500-U B | 7500-U B | |||
| ICP-MS | 3125 | D 5673-05, 10, 16 | ||||
| Alpha spectrometry | 7500-U C | 7500-U C | D 3972-09 | |||
| Laser Phosphorimetry | D 5174-07 | |||||
| Alpha Liquid Scintillation Spectrometry | D 6239-09 | |||||
| Man-Made: | ||||||
| Radioactive Cesium | Radiochemical | 7500-Cs B | 7500-Cs B | |||
| Gamma Ray Spectrometry | 7120 | 7120 | D 3649-06 | |||
| Radioactive Iodine | Radiochemical | 7500-I B, 7500-I C, 7500-I D | 7500-I B, 7500-I C, 7500-I D | D 3649-06. | ||
| Gamma Ray Spectrometry | 7120 | 7120 | D 4785-08, -20. | |||
| Radioactive Strontium 89, 90 | Radiochemical | 7500-Sr B | 7500-Sr B | |||
| Tritium | Liquid Scintillation | 7500- 3 H B | 7500- 3 H B | D 4107-08, -20 | ||
| Gamma Emitters | Gamma Ray Spectrometry | 7120, 7500-Cs B, 7500-I B | 7120, 7500-Cs B, 7500-I B | D 3649-06, D 4785-08, -20 |
| Organism | Methodology | SM 21st edition 1 | SM 22nd edition 28 | SM 23rd edition 49 | SM Online 3 | Other |
|---|---|---|---|---|---|---|
| Total Coliform | Total Coliform Fermentation Technique | 9221 A, B, C | 9221 A, B, C | 9221 A, B, C | 9221 A,B,C-06 | |
| Total Coliform Membrane Filter Technique | 9222 A, B, C | 9222 A, B, C. | ||||
| ONPG-MUG Test | 9223 | 9223 B | 9223 B | 9223 B-04. | ||
| Fecal Coliforms | Fecal Coliform Procedure | 9221 E | 9221 E | 9221 E | 9221 E-06. | |
| Fecal Coliform Filter Procedure | 9222 D | 9222 D | 9222 D | 9222 D-06. | ||
| Heterotrophic bacteria | Pour Plate Method | 9215 B | 9215 B | 9215 B | 9215 B-04. | |
| Turbidity | Nephelometric Method | 2130 B | 2130 B | 2130 B | Hach Method 8195, Rev. 3.0. 52 | |
| Laser Nephelometry (on-line) | Mitchell M5271, 10 Mitchell M5331, Rev. 1.2, 42 Lovibond PTV 6000. 46 | |||||
| LED Nephelometry (on-line) | Mitchell M5331, 11 Mitchell M5331, Rev. 1.2 42 , Lovibond PTV 2000. 45 | |||||
| LED Nephelometry (on-line) | AMI Turbiwell, 15 Lovibond PTV 1000. 44 | |||||
| LED Nephelometry (portable) | Orion AQ4500, 12 Lovibond TB 3500, 64 Lovibond TB 5000. 65 | |||||
| Laser Nephelometry (portable) | Lovibond TB 6000 63. | |||||
| 360° Nephelometry | Hach Method 10258, Rev. 1.0, 39 Hach Method 10258, Rev. 2.0. 51 |
| Residual | Methodology | EPA methods | SM 21st edition 1 | SM 22nd edition 28 , SM 23rd edition 49 | ASTM 4 | Other |
| Free Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D | D 1253-08, -14 | ||
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F | ||||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G | Hach Method 10260. 31 | |||
| Indophenol Colorimetric | Hach Method 10241. 34 | |||||
| Syringaldazine (FACTS) | 4500-Cl H | 4500-Cl H | ||||
| On-line Chlorine Analyzer | 334.0 16 | |||||
| Amperometric Sensor | ChloroSense 17 , ChloroSense Rev. 1.1. 59 | |||||
| Total Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D | D 1253-08, -14 | ||
| Amperometric Titration (Low level measurement) | 4500-Cl E | 4500-Cl E | ||||
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F | ||||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G | Hach Method 10260. 31 | |||
| Iodometric Electrode | 4500-Cl I | 4500-Cl I | ||||
| On-line Chlorine Analyzer | 334.0 16 | |||||
| Amperometric Sensor | ChloroSense 17 , ChloroSense, Rev. 1.1. 59 | |||||
| Indophenol Colorimetric | 127. 55 | |||||
| Chlorine Dioxide | Amperometric Titration | 4500-ClO 2 C | 4500-ClO 2 C | |||
| Amperometric Titration | 4500-ClO 2 E | 4500-ClO 2 E | ||||
| Amperometric Sensor | ChlordioX Plus 32 , ChlordioX Plus, Rev. 1.1. 60 | |||||
| Ozone | Indigo Method | 4500-O 3 B | 4500-O 3 B |
| Contaminant | Methodology | EPA method | ASTM 4 | SM online 3 | SM 21st edition 1 | SM 22nd edition, 28 SM 23rd edition 49 | Other |
|---|---|---|---|---|---|---|---|
| TTHM | P&T/GC/MS | 524.3, 9 524.4. 29 | |||||
| HAA5 | LLE (diazomethane)/GC/ECD | 6251 B-07 | 6251 B | 6251 B. | |||
| Ion Chromatography Electrospray Ionization Tandem Mass Spectrometry (IC-ESI-MS/MS) | 557. 14 | ||||||
| Two-Dimensional Ion Chromatography (IC) with Suppressed Conductivity Detection | Thermo Fisher 557.1. 47 | ||||||
| Bromate | Two-Dimensional Ion Chromatography (IC) | 302.0. 18 | |||||
| Ion Chromatography Electrospray Ionization Tandem Mass Spectrometry (IC-ESI-MS/MS) | 557. 14 | ||||||
| Chemically Suppressed Ion Chromatography | D 6581-08 A. | ||||||
| Electrolytically Suppressed Ion Chromatography | D 6581-08 B. | ||||||
| Chlorite | Chemically Suppressed Ion Chromatography | D 6581-08 A. | |||||
| Electrolytically Suppressed Ion Chromatography | D 6581-08 B. | ||||||
| Chlorite—daily monitoring as prescribed in 40 CFR 141.132(b)(2)(i)(A) | Amperometric Titration Amperometric Sensor | 4500-ClO 2 E | 4500-ClO 2 E | ChlordioX Plus 32 , ChlordioX Plus, Rev. 1.1. 60 |
| Residual | Methodology | SM 21st edition 1 | SM 22nd edition, 28 SM 23rd edition 49 | ASTM 4 | Other |
|---|---|---|---|---|---|
| Free Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D | D 1253-08, -14 | |
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F | |||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G | Hach Method 10260. 31 | ||
| Indophenol Colorimetric | Hach Method 10241. 34 | ||||
| Syringaldazine (FACTS) | 4500-Cl H | 4500-Cl H | |||
| Amperometric Sensor | ChloroSense 17 , ChloroSense, Rev. 1.1. 59 | ||||
| On-line Chlorine Analyzer | EPA 334.0. 16 | ||||
| Combined Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D | D 1253-08, -14. | |
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F | |||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G | Hach Method 10260. 31 | ||
| Total Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D | D 1253-08, -14 | |
| Low level Amperometric Titration | 4500-Cl E | 4500-Cl E | |||
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F | |||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G | Hach Method 10260. 31 | ||
| Iodometric Electrode | 4500-Cl I | 4500-Cl I | |||
| Amperometric Sensor | ChloroSense, 17 ChloroSense, Rev. 1.1. 59 | ||||
| On-line Chlorine Analyzer | |||||
| On-line Chlorine Analyzer | EPA 334.0. 16 | ||||
| Chlorine Dioxide | Amperometric Method II | 4500-ClO 2 E | 4500-ClO 2 E | ||
| Amperometric Sensor | ChlordioX Plus, 32 ChlordioX Plus, Rev. 1.1. 60 |
| Parameter | Methodology | SM 21st edition 1 | SM 22nd edition 28 | SM 23rd edition 49 | SM online 3 | EPA | Other |
|---|---|---|---|---|---|---|---|
| Total Organic Carbon (TOC) | High Temperature Combustion | 5310 B | 5310 B | 5310 B | 415.3, Rev 1.2. 19 | ||
| Persulfate-Ultraviolet or Heated Persulfate Oxidation | 5310 C | 5310 C | 5310 C | 415.3, Rev 1.2. 19 | Hach Method 10267. 38 | ||
| Wet Oxidation | 5310 D | 5310 D | 415.3, Rev 1.2. 19 | ||||
| Ozone Oxidation | Hach Method 10261. 37 | ||||||
| Specific Ultraviolet Absorbance (SUVA) | Calculation using DOC and UV 254 data | 415.3, Rev 1.2. 19 | |||||
| Dissolved Organic Carbon (DOC) | High Temperature Combustion | 5310 B | 5310 B | 5310 B | 415.3, Rev 1.2. 19 | ||
| Persulfate-Ultraviolet or Heated Persulfate Oxidation | 5310 C | 5310 C | 5310 C | 415.3, Rev 1.2. 19 | |||
| Wet Oxidation | 5310 D | 5310 D | 415.3, Rev 1.2. 19 | ||||
| Ultraviolet absorption at 254 nm (UV 254) | Spectrophotometry | 5910 B | 5910 B | 5910 B | 5910 B-11 | 415.3, Rev 1.2. 19 |
| Organism | Methodology | SM 20th edition 6 | SM 21st edition 1 | SM 22nd edition 28 | SM 23rd edition 49 | SM online 3 | Other |
|---|---|---|---|---|---|---|---|
| E. coli | Colilert | 9223 B | 9223 B | 9223 B | 9223 B-97, B-04 | ||
| Colisure | 9223 B | 9223 B | 9223 B | 9223 B-97, B-04 | |||
| Colilert-18 | 9223 B | 9223 B | 9223 B | 9223 B | 9223 B-97, B-04 | ||
| Readycult® | Readycult®. 20 | ||||||
| Colitag | Modified Colitag 13 , Modified Colitag, Version 2.0. 61 | ||||||
| Chromocult® | Chromocult®. 21 | ||||||
| EC-MUG | 9221 F | 9221 F | 9221 F-06 | ||||
| NA-MUG | 9222 I | ||||||
| m-ColiBlue24 Test | 9222 J | ||||||
| Enterococci | Multiple-Tube Technique | 9230 B-04. | |||||
| Membrane Filter Techniques | 9230 C. | ||||||
| Fluorogenic Substrate Enterococcus Test (using Enterolert) | 9230 D. | ||||||
| Coliphage | Two-Step Enrichment Presence-Absence Procedure | Fast Phage. 30 |
| Organism | Methodology category | Method | SM 20th, 21st editions 1 6 | SM 22nd edition 28 | SM 23rd edition 49 | SM online 3 |
|---|---|---|---|---|---|---|
| Total Coliforms | Lactose Fermentation Methods | Standard Total Coliform Fermentation Technique | 9221 B.1, B.2 | 9221 B.1, B.2, B.3, B.4 | 9221 B.1, B.2-06. | |
| Presence-Absence (P-A) Coliform Test | 9221 D.1, D.2, D.3 | |||||
| Membrane Filtration Methods | Standard Total Coliform Membrane Filter Procedure using Endo Media | 9222 B, C. | ||||
| Simultaneous Detection of Total Coliforms and E. coli by Dual Chromogen Membrane Filter Procedure (using mColiBlue24 medium) | 9222 J. | |||||
| Simultaneous Detection of Total Coliform Bacteria and Escherichia coli Using RAPID' E.coli (REC2) in Drinking Water. 56 | ||||||
| Enzyme Substrate Methods | Colilert® | 9223 B | 9223 B | 9223 B-04 | ||
| Colisure® | 9223 B | 9223 B | 9223 B-04. | |||
| Colilert-18 | 9223 B | 9223 B | 9223 B | 9223 B-04. | ||
| Tecta EC/TC. 33 43 | ||||||
| Modified Colitag TM , Version 2.0. 61 | ||||||
| Escherichia coli | Escherichia coli Procedure (following Lactose Fermentation Methods) | EC-MUG medium | 9221 F.1 | 9221 F.1 | 9221 F.1-06. | |
| Escherichia coli Partitioning Methods (following Membrane Filtration Methods) | EC broth with MUG (EC-MUG) | 9222 H. | ||||
| NA-MUG medium | 9222 I. | |||||
| Simultaneous Detection of Total Coliforms and E. coli by Dual Chromogen Membrane Filter Procedure | mColiBlue24 medium | 9222 J. | ||||
| Membrane Filtration Method | Simultaneous Detection of Total Coliform Bacteria and Escherichia coli Using RAPID' E.coli (REC2) in Drinking Water. 56 | |||||
| Enzyme Substrate Methods | Colilert® | 9223 B | 9223 B | 9223 B-04. | ||
| Colisure® | 9223 B | 9223 B | 9223 B-04. | |||
| Colilert-18 | 9223 B | 9223 B | 9223 B | 9223 B-04. | ||
| Tecta EC/TC. 33 43 | ||||||
| Modified Colitag TM , Version 2.0. 61 |
| Contaminant | Methodology | EPA method | ASTM 4 | SM 21st edition 1 | SM 22nd edition, 28 SM 23rd edition 49 | SM online 3 |
|---|---|---|---|---|---|---|
| Aluminum | Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2. 2 | ||||
| Atomic Absorption; Direct | 3111 D | 3111 D. | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B-04, B-10. | |||
| Inductively Coupled Plasma | 3120 B | 3120 B. | ||||
| Chloride | Silver Nitrate Titration | D 512-04 B, 12 B | 4500-Cl - B | 4500-Cl - B | ||
| Ion Chromatography | D 4327-11, -17 | 4110 B | 4110 B | |||
| Potentiometric Titration | 4500-Cl - D | 4500-Cl - D | ||||
| Color | Visual Comparison | 2120 B | 2120 B. | |||
| Foaming Agents | Methylene Blue Active Substances (MBAS) | 5540 C | 5540 C. | |||
| Iron | Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2. 2 | ||||
| Atomic Absorption; Direct | 3111 B | 3111 B. | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B-04, B-10. | |||
| Inductively Coupled Plasma | 3120 B | 3120 B. | ||||
| Manganese | Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2. 2 | ||||
| Atomic Absorption; Direct | 3111 B | 3111 B. | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B-04, B-10. | |||
| Inductively Coupled Plasma | 3120 B | 3120 B. | ||||
| Odor | Threshold Odor Test | 2150 B | 2150 B. | |||
| Silver | Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2. 2 | ||||
| Atomic Absorption; Direct | 3111 B | 3111 B. | ||||
| Atomic Absorption; Furnace | 3113 B | 3113 B | 3113 B-04, B-10. | |||
| Inductively Coupled Plasma | 3120 B | 3120 B. | ||||
| Sulfate | Ion Chromatography | D 4327-11, -17 | 4110 B | 4110 B | ||
| Gravimetric with ignition of residue | 4500-SO 4- C | 4500-SO 4- C | 4500-SO 4- C-97. | |||
| Gravimetric with drying of residue | 4500-SO 4- D | 4500-SO 4- D | 4500-SO 4- D-97. | |||
| Turbidimetric method | D 516-07, 11, 16 | 4500-SO 4- E | 4500-SO 4- E | 4500-SO 4- E-97. | ||
| Automated methylthymol blue method | 4500-SO 4- F | 4500-SO 4- F | 4500-SO 4- F-97. | |||
| Total Dissolved Solids | Total Dissolved Solids Dried at 180 deg C | 2540 C | 2540 C. | |||
| Zinc | Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) | 200.5, Revision 4.2. 2 | ||||
| Atomic Absorption; Direct Aspiration | 3111 B | 3111 B. | ||||
| Inductively Coupled Plasma | 3120 B | 3120 B. |
In a recent settlement case, the EPA alleges that a construction company violated the Clean Water Act (CWA) and related state laws during the construction of solar farms in Alabama, Idaho, and Illinois between 2016 and 2022. In total the company will pay just over two million dollars to resolve the allegations. The states of Alabama and Illinois joined the EPA in the settlement. The amount to be paid will include civil penalties, mitigation, and restoration projects.
Case Study
Specifically, the company failed to comply with National Pollutant Discharge Elimination System (NPDES) permits, designed to regulate construction site runoff. Solar farm construction involves clearing and grading large sections of land, which can lead to significant erosion and major runoff of sediment into waterways if stormwater controls at the site are inadequate. A complaint filed with the settlement alleges that during its construction of solar farms near American Falls, Idaho, Lafayette, Alabama, and Perry and White Counties, Illinois, the construction company failed to use proper stormwater controls, did not conduct regular site inspections by qualified personnel and did not accurately report and address stormwater issues. These are all requirements of the NPDES Construction General Permit. These combined breaches put waterways at risk from sediment-laden runoff, potentially harming aquatic life and jeopardizing downstream drinking water quality.
Learn more about stormwater in our EzExplanation
What is an NPDES permit?
The CWA bans discharging any type of industrial, municipal, and agricultural waste through a point source into a water of the United States unless they have an NPDES permit. The permit contains limits on what you can discharge, monitoring and reporting requirements, and other provisions to ensure that the discharge does not impact water quality. The permit interprets general requirements of the CWA into specific provisions tailored to the operations related to discharging pollutants. NPDES permits make sure that a state's mandatory standards for clean water and the federal minimums are being met.
CGP requirements
When it rains, water washes over the soil and things stored outside on a construction site. This water can pick up dirt, trash, and chemicals from the soil and carry them to nearby sewers or bodies of water. The CGP ensures that site operators have measures in place to prevent this pollution and keep the water clean for the community and the environment. Typically, if your construction activities disturb one or more acres of land, or will disturb less than one acre of land but are part of a common plan of development you will need to file for coverage under the CGP. Before submitting your Notice of Intent (NOI), the form you file to obtain coverage under the CGP, you must develop a Stormwater Pollution Prevention Plan (SWPPP) outlines how you plan to implement erosion and sediment controls and meet other requirements of the permit on your construction site.
Additional CGP requirements include:
Key to remember
Solar farms are important for helping to reduce the impact of climate change. However, the companies that build these solar farms need to follow certain rules to protect the environment, just like any other construction project.
The Environmental Protection Agency (EPA) is proposing a regulation to implement the requirements of the Clean Air Act (CAA) as specified in the Methane Emissions Reduction Program of the Inflation Reduction Act. This program requires the EPA to impose and collect an annual charge on methane emissions that exceed specified waste emissions thresholds from an owner or operator of an applicable facility that reports more than 25,000 metric tons of carbon dioxide equivalent of greenhouse gases emitted per year pursuant to the petroleum and natural gas systems source category requirements of the Greenhouse Gas Reporting Rule. The proposal would implement calculation procedures, flexibilities, and exemptions related to the waste emissions charge and proposes to establish confidentiality determinations for data elements included in waste emissions charge filings.
DATES: This proposed rule is published in the Federal Register January 26, 2024, page 5318.
View proposed rule.
The Environmental Protection Agency (EPA or the Agency) is proposing a regulation to revise the technology-based effluent limitations guidelines and standards (ELGs) for the meat and poultry products (MPP) point source category. The proposed rule would improve water quality and protect human health and the environment by reducing the discharge of nutrients and other pollutants to the nation's surface waters. EPA is proposing several regulatory options, including the preferred option discussed in this notice. The preferred option is estimated to cost $232 million annually and reduce pollutant discharges by approximately 100 million pounds per year.
DATES: This proposed rule is published in the Federal Register January 23, 2024, page 4474.
View proposed rule.
The Environmental Protection Agency (EPA) continues to add regulations to per- and polyfluoroalkyl substances (PFAS). The new rule finalized in January 2024 prevents facilities from using inactive PFAS before EPA reviews the planned use and makes a risk determination.
This significant new use rule (SNUR) applies to the 329 PFAS designated as inactive on the Toxic Substances Control Act (TSCA) Chemical Substance Inventory and aren’t yet subject to an existing SNUR. An inactive PFAS hasn’t been manufactured (including imported) or processed in the U.S. since June 21, 2006.
What are PFAS?
Also called “forever chemicals,” PFAS are a group of thousands of manufactured chemicals that are used widely in industry and consumer products. Many take a long time to break down and build up over time. They’re found nearly everywhere in water, air, soil, and consumer products, such as nonstick cookware, food packaging, clothing, and shampoo.
Some studies suggest that exposure to PFAS may lead to health issues. However, research continues since much remains unknown about the chemical substances. EPA regulates PFAS to prevent them from entering air, land, and water at levels that may harm human health and the environment.
What are the new SNUR requirements?
Under TSCA, EPA can determine that a certain use of a chemical substance is a “significant new use” and require anyone who wants to use the chemical substance for that purpose to first notify the agency. EPA will then conduct a review and risk determination. The new SNUR requires EPA to conduct reviews and risk determinations of the inactive PFAS before they can be used again.
If your facility wants to manufacture (including import) or process an inactive PFAS, notify EPA at least 90 days through a significant new use notice before beginning use. Once EPA receives the notification, the agency will:
If the new use doesn’t present an unreasonable risk and EPA makes an affirmative determination, your facility can use the PFAS. However, if the new use presents an unreasonable risk, the agency must first regulate the activity before you can use the PFAS.
What are other recent regulations of PFAS?
Over the past three years, EPA has implemented the 2021–2024 PFAS Strategic Roadmap, the agency's comprehensive strategy to address the risks of PFAS exposure and contamination through specific regulatory actions. The agency focuses on three directives: researching the forever chemicals, restricting the release of them into the environment, and cleaning up PFAS-contaminated sites.
This final rule joins EPA’s other actions taken via the PFAS Strategic Roadmap. These include:
How does this affect you?
If your facility manufactures (including imports) or processes PFAS, you’ll likely face more regulations and reporting requirements. So, how can you remain compliant? Stay informed of EPA’s latest regulatory actions regarding PFAS. Consider these best practices:
Also, consider the proactive approach to search for safer alternative chemicals you can substitute for PFAS. It’s vital to choose chemicals already reviewed by EPA to ensure the alternative option doesn’t also present an unreasonable risk to human health and the environment.
Key to remember: EPA continues to strengthen its regulation of PFAS. A new rule now prevents facilities from using any of the 300+ inactive PFAS before EPA conducts a risk determination and, if necessary, regulates the activity.
The State of Mississippi (Mississippi or State) has applied to the Environmental Protection Agency (EPA) for final approval of revisions to its Underground Storage Tank Program (UST Program) under subtitle I of the Resource Conservation and Recovery Act (RCRA). Pursuant to RCRA, the EPA is taking direct final action, subject to public comment, to approve revisions to the UST Program. The EPA has reviewed Mississippi's revisions and has determined that these revisions satisfy all requirements needed for approval. In addition, this action also codifies the EPA's approval of Mississippi's revised UST Program and inCcorporates by reference those provisions of the State statutes and regulations that the EPA has determined meet the requirements for approval.
DATES:
This rule is effective March 18, 2024, published in the Federal Register January 18, 2024, page 3354.
View final rule.
| §282.2 Incorporation by reference. | ||
| (b)(4) | Revised | View text |
| §282.74 Mississippi State-Administered Program. | ||
| Entire section | Revised | View text |
| Appendix A to Part 282 - State Requirements Incorporated by Reference in Part 282 of the Code of Federal Regulations | ||
| Entry for Mississippi | Revised | View text |
New Text
§282.2 Incorporation by reference.
* * * *
(b)(4) Region 4 (Alabama, Florida, Georgia, Kentucky, Mississippi, North Carolina, South Carolina, Tennessee): 61 Forsyth Street, SW, Atlanta, Georgia 30303-8960; Phone Number: (404) 562-9900.
§282.74 Mississippi State-Administered Program.
(a) History of the approval of Mississippi's program. The State of Mississippi (Mississippi or State) is approved to administer and enforce an underground storage tank (UST) program in lieu of the Federal program under subtitle I of the Resource Conservation and Recovery Act of 1976 (RCRA), as amended, 42 U.S.C. 6991 et seq. The State's Underground Storage Tank Program (UST Program), as administered by the Mississippi Department of Environmental Quality (MDEQ), was approved by EPA pursuant to 42 U.S.C. 6991c and part 281 of this chapter. EPA approved the Mississippi UST Program on June 11, 1990, and it was effective on July 11, 1990. A subsequent program revision was approved by EPA and became effective March 18, 2024.
(b) Enforcement authority. Mississippi has primary responsibility for administering and enforcing its federally approved UST Program. However, EPA retains the authority to exercise its corrective action, inspection, and enforcement authorities under sections 9003(h), 9005, and 9006 of subtitle I of RCRA, 42 U.S.C. 6991b(h), 6991d, and 6991e, as well as under any other applicable statutory and regulatory provisions.
(c) Retention of program approval. To retain program approval, Mississippi must revise its approved UST Program to adopt new changes to the Federal subtitle I program which make it more stringent, in accordance with section 9004 of RCRA, 42 U.S.C. 6991c, and 40 CFR part 281, subpart E. If Mississippi obtains approval for revised requirements pursuant to section 9004 of RCRA, 42 U.S.C. 6991c, the newly approved statutory and regulatory provisions will be added to this subpart and notice of any change will be published in the Federal Register .
(d) Final approval. Mississippi has final approval for the following elements of its UST Program submitted to EPA and approved effective June 11, 1990, and the program revisions approved by EPA effective on March 18, 2024:
(1) State statutes and regulations— (i) Incorporation by reference. The Mississippi materials cited in this paragraph (d)(1)(i), and listed in appendix A to this part, are incorporated by reference as part of the UST Program under subtitle I of RCRA, 42 U.S.C. 6991 et seq. (See §282.2 for incorporation by reference approval and inspection information.) You may obtain copies of the Mississippi statutes and regulations that are incorporated by reference in this paragraph (d)(1)(i) from the Mississippi Department of Environmental Quality, P.O. Box 2261, Jackson, MS 29335; Phone number: (601) 961–5171; website: https://www.mdeq.ms.gov/water/groundwater-assessment-and-remediation/underground-storage-tanks/.
(A) “Mississippi Statutory Requirements Applicable to the Underground Storage Tank Program,” dated September 5, 2023.
(B) “Mississippi Regulatory Requirements Applicable to the Underground Storage Tank Program,” dated September 5, 2023.
(ii) Legal basis. EPA considered the following statutes and regulations which provide the legal basis for the State's implementation of the UST Program, but do not replace Federal authorities. Further, these provisions are not being incorporated by reference, unless the provisions place requirements on regulated entities.
(A) Mississippi Underground Storage Tank Act (the UST Act) of 1988, Miss. Code Ann. sections 49–17–401 to 49–17–435 (2022).
(1) Section 49–17–409, as to the first sentence, insofar as it provides for compliance monitoring and the promulgation of regulations for the reporting of releases.
(2) Section 49–17–413(1), insofar as it provides for compliance monitoring, and the promulgation of regulations for the implementation of the State UST Program.
(3) Section 49–17–415, insofar as it provides for compliance monitoring and establishes authority to conduct inspections, tests, and obtain information from owners.
(4) Section 49–17–419, insofar as it establishes authority over corrective action.
(5) Section 49–17–425, insofar as it provides for the sharing of information with EPA.
(6) Section 49–17–427, insofar as it provides for enforcement response, enforcement of orders, assessment of penalties under the UST Act, proceedings before the commission, and limitations on liability.
(7) Section 49–17–431, insofar as it provides for appeal of any decision by the commission or the director.
(B) Mississippi Air and Water Pollution Control Law, Miss. Code Ann. sections 49–17–27 and 49–17–31 to 49–17–41 (2020).
(1) Section 49–17–27, insofar as it provides for enforcement response and injunctive relief.
(2) Section 49–17–31, insofar as it provides for enforcement response, notice of violations, enforcement of regulations and orders, procedures for contested cases, and assessment of penalties.
(3) Section 49–17–33, insofar as it provides for hearing procedures, issuance of orders, and penalties.
(4) Section 49–17–35, insofar as it provides for enforcement response, public participation, and citizen intervention.
(5) Section 49–17–37, insofar as it provides for hearing procedures and transcripts.
(6) Section 49–17–39, insofar as it provides for the sharing of information with EPA.
(7) Section 49–17–41, insofar as it provides for appeal rights for aggrieved parties.
(C) Mississippi's Underground Storage Tank Regulations, 11 Miss. Admin. Code Pt. 5, Ch. 2 (2018).
(1) R. 2.3, 280.36, insofar as it provides for delivery prohibition and enforcement of the State UST Program.
(2) R. 2.6, 280.67, insofar as it provides for public participation in the corrective action process.
(D) Rule 24(a)(2) of the Mississippi Rules of Civil Procedure (1982), insofar as it provides for citizen intervention and public participation in the State enforcement process.
(iii) Other provisions not incorporated by reference. The following statutory and regulatory provisions applicable to the Mississippi UST Program are broader in scope than the Federal program, external to the State UST program approval requirements, or are being excluded for other reasons as noted below. Therefore, these provisions are not part of the approved UST Program and are not incorporated by reference in this section:
(A) Mississippi Underground Storage Tank Act (the UST Act) of 1988, Miss. Code Ann. sections 49–17–401 to 49–17–435 (2022).
(1) 49–17–403(b) is broader in scope as to the definition of “Bonded distributor,” insofar as it is associated with the regulation of entities other than owners and operators as these terms are defined in 40 CFR 280.12.
(2) Section 49–17–403(o) is broader in scope as to the definition of “Response action contractor,” insofar as it is associated with the regulation of entities other than owners and operators as these terms are defined in 40 CFR 280.12.
(3) Section 49–17–403(p) is broader in scope as to the definition of “Retailer,” insofar as it is associated with the regulation of entities other than owners and operators as these terms are defined in 40 CFR 280.12.
(4) Section 49–17–403(q) is broader in scope as to the definition of “Substantial compliance,” insofar as it relates to a State fund.
(5) Section 49–17–405 is broader in scope insofar as it provides for the creation of the Mississippi Groundwater Protection Trust Fund (State Fund), promulgation of regulations regarding the State Fund, criteria for qualified expenditure of funds, and liability of owners for fund expenditures.
(6) Section 49–17–407 is broader in scope insofar as it creates an environmental protection fee, provides limits on use of the State Fund, and addresses third party claims.
(7) Section 49–17–409 is broader in scope, all except for the first sentence, insofar as it provides for the eligibility requirements of the State Fund and reimbursement of costs from owners.
(8) Section 49–17–421 is broader in scope insofar as it establishes an annual tank regulatory fee.
(9) Section 49–17–422 is broader in scope insofar as it creates an Underground Storage Tank Advisory Council.
(10) Section 49–17–423 is broader in scope insofar as it pertains to the commission's administration of funds from the Leaking Underground Storage Tank Trust Fund.
(11) Section 49–17–429 is broader in scope insofar as it requires the certification of individuals to install, alter, or remove underground storage tanks and provides for the promulgation of regulations setting forth certification requirements.
(12) Section 49–17–433 is external insofar as it pertains to the severability of the State UST Act.
(13) Section 49–17–435 is external insofar as it contains reporting obligations on the State agency, not a regulated entity.
(B) Mississippi Air and Water Pollution Control Law, Miss. Code Ann. sections 49–17–27 and 49–17–31 to 49–17–41 (2020).
(1) Section 49–17–32 is external insofar as it does not pertain to the State UST Program.
(2) Section 49–17–34 is external insofar as it does not pertain to the State UST Program.
(3) Section 49–17–36 is external insofar as it does not pertain to the State UST Program.
(C) Mississippi's Groundwater Protection Trust Fund Regulations, 11 Miss. Admin. Code Pt. 5, Ch. 1 (2009) is broader in scope insofar as these provisions regulate Immediate Response Action Contractors, Environmental Response Action Contractors, and the State Fund.
(D) Mississippi's Underground Storage Tank Regulations, 11 Miss. Admin. Code Pt. 5, Ch. 2 (2018).
(1) R. 2.1, 280.12 is broader in scope as to the definition of “Ancillary equipment,” insofar as it pertains to dispensers.
(2) R. 2.1, 280.12 is broader in scope as to the definition of “Certificate of Operation,” insofar as it requires UST systems to be permitted by MDEQ and the payment of tank regulatory fees.
(3) R. 2.1, 280.12 is broader in scope as to the definition of “Motor fuel,” insofar as it includes 100% biodiesel or ethanol.
(4) R. 2.1, 280.12 is broader in scope as to the definition of “New tank system,” insofar as it includes dispensers as part of the new tank system.
(5) R. 2.1, 280.12 is broader in scope as to the definition of “Register,” insofar as it requires notification for installation, replacement, and change in operational status of a dispenser.
(6) R. 2.1, 280.12 is broader in scope as to the definition of “Replace,” insofar as it considers replacement of a dispenser to constitute a new UST system.
(7) R. 2.2, 280.20(j) is broader in scope insofar as it regulates shear valves.
(8) R. 2.2, 280.22(a) and (b) are broader in scope insofar as these provisions regulate dispensers.
(9) R. 2.3, 280.34(g) through (i) are broader in scope insofar as these provisions regulate dispensers.
(10) R. 2.3, 280.35(a)(4) is broader in scope insofar as it regulates dispensers.
(11) R. 2.3, 280.35(b)(1) is broader in scope insofar as it regulates shear valves.
(12) R. 2.3, 280.38(b)(1)(iii) is broader in scope insofar as it regulates shear valves.
(13) R. 2.8, 280.91(e) and (f), are excluded for other reasons. Paragraph (e) is excluded only insofar as it includes Indian tribes as a “local government entity,” and paragraph (f) is excluded insofar as EPA retains responsibility for implementing the Federal UST program in Indian country.
Note 1 to paragraph (d)(1)(iii)(D)(13).
MDEQ does not regulate any USTs on Indian lands and EPA retains responsibility for implementing the Federal UST program in Indian country. In a subsequent rulemaking, MDEQ will revise these provisions to remove references to the State's regulation of USTs in Indian country.
(14) R. 2.8, 280.92, is excluded for other reasons only insofar as the definition of “Local government” includes Indian tribes.
Note 2 to paragraph (d)(1)(iii)(D)(14).
MDEQ does not regulate any USTs on Indian lands and the EPA retains responsibility for implementing the Federal UST program in Indian country. In a subsequent rulemaking, MDEQ will revise the definition of “Local government” to exclude Indian tribes.
(15) R. 2.8, 280.100 is external insofar as it is not applicable in a State with an approved UST program.
(E) Mississippi's Underground Storage Tank Regulations for the Certification of Persons Who Install, Alter, and Remove Underground Storage Tanks, 11 Miss. Admin. Code Pt. 5, Ch. 3 (2018) is broader in scope insofar as these provisions provide for the certification and regulation of persons who install, alter, test, and permanently close underground storage tanks.
(2) Statement of legal authority. The Attorney General's Statement, signed by the Mississippi Attorney General on July 27, 2023, though not incorporated by reference, is referenced as part of the approved underground storage tank program under subtitle I of RCRA, 42 U.S.C. 6991 et seq.
(3) Demonstration of procedures for adequate enforcement. The “Demonstration of Adequate Enforcement Procedures” submitted in the application dated July 31, 2023, as amended on August 17, 2023, though not incorporated by reference, is referenced as part of the approved underground storage tank program under subtitle I of RCRA, 42 U.S.C. 6991 et seq.
(4) Program description. The program description submitted in the application dated July 31, 2023, as amended on August 17, 2023, though not incorporated by reference, is referenced as part of the approved underground storage tank program under subtitle I of RCRA, 42 U.S.C. 6991 et seq.
(5) Memorandum of Agreement. The Memorandum of Agreement between EPA Region 4 and the MDEQ, signed by the EPA Regional Administrator on October 12, 2018, though not incorporated by reference, is referenced as part of the approved underground storage tank program under subtitle I of RCRA, 42 U.S.C. 6991 et seq.
Appendix A to Part 282 - State Requirements Incorporated by Reference in Part 282 of the Code of Federal Regulations
* * * *
Mississippi
(a) The statutory provisions include:
Mississippi Underground Storage Tank Act (the UST Act) of 1988, Miss. Code Ann. sections 49–17–401 to 49–17–435 (2022):
49–17–401 Short Title.
49–17–403 Definitions, except (b), (o), (p), and (q).
49–17–411 Compliance with regulations.
49–17–413 Rules and regulations, except for (1).
49–17–417 Repealed.
Note to paragraph (a) of Appendix A to Part 282.
Miss. Code Ann. section 49–17–413(2) is approved as part of the State UST Program to the extent that Mississippi will not grant a variance that makes its UST Program less stringent than the Federal regulations. In practice, Mississippi does not grant variances for the UST Program. Mississippi has agreed to execute a revised Memorandum of Agreement with EPA stating that Mississippi will limit the scope of its variance authority to only those situations where the Federal regulations allow the implementing agency to approve flexibilities.
(b) The regulatory provisions include:
Mississippi's Underground Storage Tank Regulations, 11 Miss. Admin. Code Pt. 5, Ch. 2 (2018):
Rule 2.1 Program Scope and Interim Prohibition
280.10 Applicability.
280.11 Installation requirements for partially excluded UST systems.
280.12 Definitions, except for “dispensers” in the definition of “Ancillary equipment;” the definition of “Certificate of Operation;” “including 100% biodiesel or ethanol” from the definition of “Motor fuel;” “dispensers” and (c) from the definition of “New tank system;” “dispensers” from the definition of “Register;” “dispensers” and (c) from the definition of “Replace.”
280.13 Industry codes and recommended practices.
Rule 2.2 UST Systems: Design, Construction, Installation and Notification
280.20 Performance Standards for new UST systems, except for (j).
280.21 Upgrading of existing UST systems.
280.22 Notification requirements, except as applied to “dispensers” in (a) and (b).
Rule 2.3 General Operating Requirements
280.30 Operation and maintenance of spill and overfill prevention.
280.31 Operation and maintenance of secondary containment.
280.32 Operation and maintenance of corrosion protection.
280.33 Compatibility.
280.34 Repairs and replacements, except as applied to “dispenser(s)” in (g), (h), and (i).
280.35 Reporting recordkeeping, except as applied to “dispensers” in (a)(4); and except as applied to “shear valves” in (b)(1).
280.37 Operator training.
280.38 Operation and maintenance walkthrough inspections, except for (b)(1)(iii).
Rule 2.4 Leak Detection
280.40 General requirements for all UST systems.
280.41 Requirements for petroleum UST systems.
280.42 Requirements for hazardous substance UST systems.
280.43 Methods of leak detection for tanks.
280.44 Methods of leak detection for piping.
280.45 Leak detection recordkeeping.
Rule 2.5 Leak Reporting, Release Reporting, Investigation, and Confirmation
280.50 Reporting of leaks and suspected releases.
280.51 Investigation due to off-site impacts.
280.52 Release investigation and confirmation steps.
280.53 Reporting and cleanup of spills and overfills.
Rule 2.6 Release Response and Corrective Action for UST Systems Containing Petroleum or Hazardous Substances
280.60 General.
280.61 Initial response.
280.62 Initial abatement measures and site check.
280.63 Initial site characterization.
280.64 Free product removal.
280.65 Investigations for soil and ground-water cleanup.
280.66 Corrective action plan.
Rule 2.7 Out-of-Service UST Systems and Closure
280.70 Temporary closure.
280.71 Permanent closure and changes-in-service.
280.72 Assessing the site at closure or change-in-service.
280.73 Applicability to previously closed UST systems.
280.74 Closure records.
Rule 2.8 Financial Responsibility
280.90 Applicability.
280.91 Compliance dates, except for “including Indian tribes” in (e), and (f).
280.92 Definition of terms, except for “and includes Indian tribes” from the definition of “Local government.”
280.93 Amount and scope of required financial responsibility.
280.94 Allowable mechanisms and combinations of mechanisms.
280.95 Financial test of self-insurance.
280.96 Guarantee.
280.97 Insurance and risk retention group coverage.
280.98 Surety bond.
280.99 Letter of credit.
280.101 State fund or other State assurance.
280.102 Trust fund.
280.103 Standby trust fund.
280.104 Local government bond rating test.
280.105 Local government financial test.
280.106 Local government guarantee.
280.107 Local government fund.
280.108 Substitution of financial assurance mechanisms by owner or operator.
280.109 Cancellation or nonrenewal by a provider of financial assurance.
280.110 Reporting by owner or operator.
280.111 Recordkeeping.
280.112 Drawing on financial assurance mechanisms.
280.113 Release from the requirements.
280.114 Bankruptcy or other incapacity of owner or operator or provider of financial assurance.
280.115 Replenishment of guarantees, letters of credit, or surety bonds.
280.116 Suspension of enforcement. [Reserved]
Rule 2.9 Lender Liability
280.120 Definitions.
280.121 Participation in management.
280.122 Ownership of an underground storage tank or underground storage tank system or facility or property on which an underground storage tank or underground storage tank system is located.
280.123 Operating an underground storage tank or underground storage tank system.
Rule 2.10 UST Systems with Field-Constructed Tanks and Airport Hydrant Fuel Distribution Systems.
280.130 Definitions.
280.131 General requirements.
280.132 Additions, exceptions, and alternatives for UST systems with field-constructed tanks and airport hydrant systems.
Note to paragraph (b) of Appendix A to Part 282.
11 Miss. Admin. Code Pt. 5, Ch. 2, 280.42(b)(5) is approved as part of the UST Program only to the extent that Mississippi will not allow alternate release detection methods for hazardous substance UST systems installed on or after October 13, 2015. Sections 40 CFR 281.33(e) and 280.42(e) of the Federal regulations only allow alternate release detection methods for hazardous substance UST systems installed prior to October 13, 2015. Mississippi's section 280.42(b)(5) does not contain an analogous limitation on the use of alternative release detection methods. In practice, MDEQ does not allow alternative release detection methods for hazardous substance tanks installed after October 1, 2008. In a subsequent rulemaking, MDEQ will revise 11 Miss. Admin. Code Pt. 5, Ch. 2, R. 2.4, section 280.42(b)(5) to clarify this point.
(C) Copies of the Mississippi statutes and regulations that are incorporated by reference are available from the Mississippi Department of Environmental Quality, P.O. Box 2261, Jackson, MS 29335; Phone number: (601) 961–5171; website: https://www.mdeq.ms.gov/water/groundwater-assessment-and-remediation/underground-storage-tanks/.
The increase in unhealthy air pollution produced by wildfires has intensified the challenges associated with protecting workers from exposure to this health hazard. However, this exposure can be reduced with knowledge, safe work practices, and appropriate personal protective equipment (PPE). It’s important for employers to have a plan in place to protect workers by preventing or minimizing exposure to hazardous air quality.
Wildfire smoke is composed of harmful chemicals and tiny particles of partially burned material less than 2.5 micrometers in diameter, which present a significant health hazard for workers exposed to it. These particles can enter the lungs and even the bloodstream, and are linked to serious or even fatal health effects, such as:
There are currently three states with regulations that specifically address the air quality index (AQI) as it relates to wildfire smoke:
Absent any standard, all employers across the country have an obligation under the General Duty Clause to protect their workers from exposure to unhealthy levels of air pollutants due to wildfire smoke emissions.
Employers should take protective measures to reduce smoke exposure for outdoor workers including:
Air quality is a complex and evolving challenge. The EPA has established an AQI for five major air pollutants regulated by the Clean Air Act. Each of these pollutants has a national air quality standard set by EPA to protect public health:
Employers must create protective measures to reduce employee exposure to these major air pollutants. When AQI values are above 100, as listed on the EPA’s AirNow.gov website, air quality is unhealthy: first for certain sensitive groups of people, then for everyone as AQI values increase.
Employers should have a plan to prevent employee exposure to dangerous air quality levels.
Under the Toxic Substances Control Act (TSCA), EPA is finalizing a significant new use rule (SNUR) for 329 per- and poly-fluoroalkyl substances (PFAS) that are designated as inactive on the TSCA Chemical Substance Inventory. PFAS are a group of chemicals that have been used in industry and consumer products since the 1940s because of their useful properties, such as water and stain resistance. Many PFAS break down very slowly and can build up in people, animals, and the environment over time. Exposure at certain levels to specific PFAS can adversely impact human health and other living things. Persons subject to the final SNUR are required to notify EPA at least 90 days before commencing any manufacture (including import) or processing of the chemical substance for a significant new use. Once EPA receives a notification, EPA must review and make an affirmative determination on the notification, and take such action as is required by any such determination before the manufacture (including import) or processing for the significant new use can commence. Such a review will assess whether the new use may present unreasonable risk to health or the environment and ensure that EPA takes appropriate action as required to protect health or the environment.
DATES: This final rule is effective March 11, 2024, published in the Federal Register January 11, 2024, page 1822.
View final rule.
| §9.1 OMB approvals under the Paperwork Reduction Act. | ||
| Entry for §721.11777 | Added | View text |
| §721.11777 Per- and poly-fluoroalkyl chemical substances designated as inactive on the TSCA Inventory. | ||
| Entire section | Added | View text |
The Environmental Protection Agency (EPA) proposes to update its title V operating permit program regulations to more clearly reflect the EPA's existing interpretations and policies concerning when and whether “applicable requirements” established in other Clean Air Act (CAA or the Act) programs should be reviewed, modified, and/or implemented through the title V operating permits program. Specifically, this action clarifies the limited situations in which requirements under the New Source Review (NSR) preconstruction permitting program would be reviewed using the EPA's unique title V oversight authorities. Additionally, this action clarifies that requirements related to an owner or operator's general duty to prevent accidental releases of hazardous substances are not “applicable requirements” for title V purposes and are not implemented through title V.
DATES: This proposed rule is published in the Federal Register January 9, 2024, page 1150.
View proposed rule.
It’s a leap year in 2024, so facilities have an extra day to prepare the National Biennial Resource Conservation and Recovery Act (RCRA) Hazardous Waste Report, commonly known as the Biennial Report.
Due by March 1st of every even-numbered year, the Biennial Report covers the hazardous waste activities regulated under RCRA Subtitle C that occurred during the preceding odd-numbered year (or reporting year). It describes each generated hazardous waste, how it was generated, how much was generated, and how it was managed. The report itself contains four forms:
It can sometimes feel like taking a leap in the dark when trying to complete the report, especially if you’re unfamiliar with any of the forms. This article takes a closer look at each form to shed light on what you need to accurately complete the Biennial Report.
Facilities required to file a Biennial Report include those which, during any calendar month of a reporting year:
If your facility meets the definition of an LQG in any calendar month of a reporting year, you must complete a Biennial Report for the entire reporting year, not just the month(s) your facility was an LQG.
Check your facility’s state requirements! While very small quantity generators (VSQGs) and small quantity generators (SQGs) aren’t required to file the federal Biennial Report, states may have different reporting requirements and different generator categories. For example: California requires TSDFs to report annually, and Maine requires annual reporting and has different generator categories (SQG, SQG Plus, and LQG).
Once you confirm that your facility must complete a report, begin gathering the information to fill out the forms. Here’s a closer look at each form in the Biennial Report.
The Site ID Form (EPA Form 8700-12) is the same form used to obtain an EPA Identification Number (specific to each facility) and notify EPA of any changes to the information on the form, including generator status.
When using the Site ID Form for the Biennial Report:
A GM Form must be submitted for each hazardous waste used to determine your facility’s generator status. This includes hazardous waste:
Note that at the federal level, hazardous waste exported to a foreign country doesn’t require a GM Form; it requires the Annual Report (262.83(g)). However, your state may require you to submit the Annual Report with the Biennial Report.
If your facility received RCRA hazardous waste from off-site and managed the waste on-site (even with a subsequent transfer off-site) during the reporting year, you must submit a WR Form. It includes hazardous waste:
You must submit an OI form only if the state in which your facility operates requires it. The form includes the names and addresses of the sites identified in the Biennial Report (including generators, transporters, and receiving facilities).
EPA and states use the data from Biennial Reports to manage the nation’s RCRA-regulated hazardous waste. Unfiled, incomplete, and late reports result in inaccurate data that can ultimately affect federal and state regulations designed to protect human health and the environment.
Further, if you fail to submit a complete, accurate Biennial Report by the deadline, you can face enforcement actions such as compliance orders and civil and criminal lawsuits that may result in steep fines and even jail time.
By remaining compliant with the Biennial Reports, your facility:
Key to remember: RCRA’s Biennial Report, due by March 1st of this year, can contain up to four forms to complete based on a covered facility’s hazardous waste management activities and state requirements.
The U.S. Environmental Protection Agency is amending a provision of the recently finalized Technology Transitions Program under the American Innovation and Manufacturing Act (AIM Act). This action allows one additional year, until January 1, 2026, solely for the installation of new residential and light commercial air conditioning and heat pump systems using components manufactured or imported prior to January 1, 2025. The existing January 1, 2025, compliance date for the installation of certain residential and light commercial air conditioning and heat pump systems may result in significant stranded inventory that was intended for new residential construction. EPA is promulgating this action to mitigate the potential for significant stranded inventory in this subsector. In addition, EPA is clarifying that residential ice makers are not included in the household refrigerator and freezer subsector under the Technology Transitions Rule and are not subject to the restrictions for that subsector. EPA is requesting comments on all aspects of this rule.
DATES: This interim final rule is effective on December 26, 2023, published in the Federal Register December 26, 2023, page 88825.
View final rule.
| §84.54 Restrictions on the use of hydrofluorocarbons. | ||
| (c)(1) | Revised | View text |
Previous Text
§84.54 Restrictions on the use of hydrofluorocarbons.
* * * * *
(c) * * *
(1) Effective January 1, 2025, residential or light commercial air-conditioning or heat pump systems using a regulated substance, or a blend containing a regulated substance, with a global warming potential of 700 or greater, except for variable refrigerant flow air-conditioning and heat pump systems;
* * * * *
Quick action using cardiopulmonary resuscitation (CPR) and automated external defibrillators(AEDs) can save the lives of the nearly 350,000 cardiac event victims each year outside of a hospital setting. But what does OSHA require for the workplace? What you didn’t know about OSHA regulations regarding AEDs may surprise you.
For every minute a patient is in cardiac arrest, their chances of survival decrease dramatically. When a patient doesn’t have a pulse and isn’t breathing, CPR should be performed until an AED is available. It’s important to note that CPR alone does not restart the heart. CPR is an oxygen circulation procedure. AEDs, on the other hand, are meant for lifesaving intervention.
CPR and early defibrillation are vital components of the emergency medical services (EMS) chain of survival that increases the odds of cardiac patient survival. However, according to the American Heart Association (AHA), even the best CPR can’t provide enough circulation of oxygen to the brain and heart for more than a few minutes. In fact, a patient whose brain is deprived of oxygen for 10 minutes or more seldom recovers.
Just like a reliable vehicle, the circulatory system is the human body’s blood transportation system, and the heart is the engine. Amazingly, the heart generates its own electrical impulses, pumping in a regular, rhythmic manner. As with any engine, the heart requires a certain amount of pressure to function and doesn’t work well when clogged with grease or debris. The most common causes of sudden cardiac arrest include a heart attack, electrocution, and asphyxiation — all of which could occur in the workplace. Common signs and symptoms include:
CPR provides the pressure for the body’s “engine” to oxygen circulating, while an AED provides the electrical impulses to keep the engine pumping.
OSHA 1910.151 requires first aid treatment be provided in the absence of an infirmary, clinic, or hospital in near proximity to the workplace used to treat injured employees. This may include assisting a victim of cardiac arrest using CPR or defibrillation.
OSHA requirements for CPR and defibrillation differ considerably. Standards requiring CPR include:
OSHA recommends basic adult CPR refresher training and retesting every year, and first aid training at least once every three years. CPR training include facilitated discussion along with ’hands-on’ skills training that uses mannequins and partner practice.
Though OSHA recognizes AEDs as important lifesaving technology that plays a role in treating cardiac arrest, the agency doesn’t currently require their use in the workplace. Instead, OSHA wants employers to assess their own requirements for AEDs as part of their first aid response.
AEDs are considered Class III medical devices which means the Food and Drug Administration (FDA) has some oversight on their use. Almost all AEDs require the purchaser to obtain a prescription from a physician under FDA regulations. The prescription process is meant as a quality control mechanism to ensure AEDs are properly maintained, that all designated responders are properly trained, and assist employers with establishing an emergency response plan for their workplace AED program.
The AHA requires AED operators to also receive CPR training as an “integral part of providing lifesaving aid to people suffering sudden cardiac arrest.” Though easy to use, each AED is slightly different, so training helps users understand the unique traits and supplies for the individual units at their workplace. Additionally, AED users must be trained to understand the signs of a sudden cardiac arrest, when to activate the EMS system, and how to perform CPR.
AEDs are light, portable, easy to use, and inexpensive. They’re best placed near high-hazard areas such as confined spaces, near electrical energy, or in remote work areas. Response time to reach AEDs should be kept within 3–5-minutes.
| Need more information on defibrillators in the workplace? See our ezExplanation on AEDs. |
Many states require or encourage CPR and AED training from nationally recognized organizations. Any AED training should include CPR training. OSHA doesn’t offer first aid or CPR training, nor certify trainers. Training by a nationally recognized organization, such as AHA, the American Red Cross, or National Safety Council is recommended.
While OSHA doesn’t currently require the use of AEDs in the workplace, they do expect employers to assess their own AED requirements as part of their first aid response. AED training is required by most states and should include CPR with a hands-on practical component.
OSHA requires employers to provide all workers with immediately available and sanitary restroom or toilet facilities. But does this include truckers and delivery drivers that stop at your facilities? The sanitation standards (1910.141, 1926.51, and 1928.110) are meant to protect all workers from adverse health effects from unsanitary toilets facilities, or the unavailability of facilities when needed.
Bipartisan legislation has recently been introduced in the House that would require businesses to provide restroom access to truckers who are loading or delivering cargo at their warehouses, manufacturers, distribution centers, retailers, and ports.
Supported by leading organizations in the trucking industry, the Trucker Bathroom Access Act (H.R. 9592) was introduced on Dec. 15, 2022. The bill requires retailers, warehouses, and other establishments with existing restrooms to provide access to drivers who are loading or delivering cargo. Additionally, operators of ports and marine terminals must provide access for drayage and parking while accessing such restrooms.
This amendment to Title 49 would exempt some employers from the bill including filling and service stations, and restaurants 800-square feet or smaller with restrooms intended for employee use only. The bill doesn’t require employers to construct new restrooms but to give truck drivers the same access as employees or customers.
Commercial truckers and delivery drivers are the lifeline of our supply chain of supplies, products, and consumables. Working tirelessly all hours, during holidays and weekends, and throughout the pandemic, they continue to deliver critical food and emergency supplies to companies everywhere. Employers have the privilege of demonstrating gratitude to truckers and delivery drivers with a positive work environment.
The benefits of allowing truckers and delivery drivers the convenience and safety of readily available, sanitary restroom facilities are plenty. They’re able to rest and reset when necessary, which keeps them and others safer on the roads. Equally important, restroom availability prevents drivers from having to search for available facilities elsewhere, allowing them to keep a timely delivery schedule, limit supply chain delays, and ultimately lower costs for employers and customers.
The proposed Trucker Bathroom Access Act will require retailers, warehouses, and other establishments with existing restrooms to provide access to truckers and delivery drivers who are loading or delivering cargo. Access to restrooms keeps them refreshed and ready to deliver essential supplies to companies across the country.
SUMMARY: The U.S. Environmental Protection Agency (the EPA) is soliciting information and requesting comments to assist in the potential development of non-regulatory and regulatory options that would ensure the proper management of used industrial containers that held hazardous chemicals or hazardous waste, up to and including the drum reconditioning process. Options could include revising the Resource Conservation and Recovery Act (RCRA) regulations or other, non-regulatory options. This Advance Notice of Proposed Rulemaking (ANPRM) does not propose any regulatory requirements or change any existing regulatory requirements.
DATES: Comments must be received on or before November 22, 2023, published in the Federal Register August 11, 2023, page 54537.
View proposed rule.
Hi everyone! Welcome to the monthly news roundup video, where we’ll go over the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. Let’s get started!
Effective January 15, OSHA penalties increased 3.2 percent for inflation. Most penalties increased to $16,131. Willful and serious violations, however, increased to $161,323.
Construction workers aged 45 and older suffer more severe injuries and higher associated costs than other age groups. Most injuries are due to slips, trips, and falls.
Washington State updated its process safety management rules to better protect workers in petroleum refineries from the hazards of volatile chemicals. The rules take effect December 27, 2024.
Bloodborne pathogens topped the list of OSHA violations for the healthcare industry in 2023. Hazard Communication was the second most cited standard, followed by respiratory protection.
OSHA Region 2 launched a regional emphasis program that targets tree trimming, tree removal, and land clearing operations. Region 2 includes New York, New Jersey, Puerto Rico, and the U.S. Virgin Islands.
EPA continues to strengthen its regulation of per- and polyfluoroalkyl — or PFAS — substances. A new rule prevents facilities from using any of the 300+ inactive PFAS before EPA conducts a risk determination and, if necessary, regulates the activity.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
Safety has the workforce brimming with color. In fact, 29 CFR 1910.144 and 1910.145 tell us precisely what OSHA expects for safety color coding to identify hazards in the workplace. Signs, warning labels, symbols, and other color coding in your facilities should have your employees seeing red. But what if they can’t?
Though rare, color blindness is the inability to distinguish between colors as most people do. This makes it difficult for workers to see colors intended to protect them from harm. Color blindness can vary, making it difficult to distinguish between red and green or blue and yellow hues - the very shades of safety.
Some individuals can’t see any colors, which is called monochromacy. Workers with this type of color blindness may have trouble seeing clearly and may be more sensitive to light. Employers must collaborate with these employees to ensure alternative measures are taken to protect their eyes and clearly communicate warnings and hazards.
The color identifiers below differentiate the various levels of risk and hazards for workplace safety. Employers must ensure workers with color blindness are able to understand hazards in the workplace and the meaning of signs and warning labels.
RED - identifies fire and fire protective apparatus, danger, and emergency stops. It marks areas near open flames or flammable materials, fire extinguishers, and where workers are directed to stop an action.
ORANGE - warns workers of hazardous parts of equipment that could physically harm people or the facility. Typically used as labels on machinery, orange may also be used on signs, hard hats, safety vests, and other objects.
YELLOW - designates caution and is used for marking physical hazards, such as falling, pinch points, contact hazards, and other similar hazards.
GREEN - identifies directional safety information. This includes pointing workers to emergency egresses, safety showers or eyewash stations, first aid stations, and other safety equipment.
BLUE - not always safety-related, provides information regarding a particular location, process, or item. Employers may use blue signs to convey workplace policies, instructions, or locations, such as “Employees Only.”
PURPLE - often combined with yellow, alerts workers to radiation hazards.
BLACK/WHITE - provides instructional and directional information. This includes speed limits, one-way traffic, and aisle markings.
Having a standardized color-coding system for safety is effective for alerting employees of workplace hazards - if they can see the colors properly. For those who can’t, employers must ensure these workers understand the hazards and warning signs throughout the workplace.
| Interested in learning more? See our ezExplanation on Color Coding. |
Not only are employers required to ensure workers understand warning signs and colors, but they must also protect workers from becoming color blind. That’s right - color blindness can be acquired. Exposure to lead or carbon disulfide can cause color blindness, even at low levels. Terminal illness and alcohol consumption can also contribute to color blindness, so employers should promote health as part of their safety and health programs.
Color blindness is considered a disability according to the Americans with Disabilities Act (ADA). Employers are required to reasonably accommodate employees with disabilities.
Employers must ensure employees with color blindness are able to understand hazards in the workplace and the meaning of signs and warning labels. The ADA requires employers to make reasonable accommodations for workers with disabilities, including color blindness.
The Environmental Protection Agency (EPA) is proposing a regulation to implement the requirements of the Clean Air Act (CAA) as specified in the Methane Emissions Reduction Program of the Inflation Reduction Act. This program requires the EPA to impose and collect an annual charge on methane emissions that exceed specified waste emissions thresholds from an owner or operator of an applicable facility that reports more than 25,000 metric tons of carbon dioxide equivalent of greenhouse gases emitted per year pursuant to the petroleum and natural gas systems source category requirements of the Greenhouse Gas Reporting Rule. The proposal would implement calculation procedures, flexibilities, and exemptions related to the waste emissions charge and proposes to establish confidentiality determinations for data elements included in waste emissions charge filings.
DATES: This proposed rule is published in the Federal Register January 26, 2024, page 5318.
View proposed rule.
The 150 air-mile exemptions, which are in the regulations at 395.1(e)(1) and (2), allow a driver to use a time record in place of a log, provided that certain conditions are met. While this is possibly the most widely used hours-of-service exemption, it may be the most commonly misused exemption, as well.
To be able to use this logging exemption in 395.1(e)(1), the driver must:
The company must retain the time record and have it available for inspection for six months.
| Need more info? View our ezExplanation on the 150 air-mile exception. |
If the driver cannot meet the terms of the exemption (he or she goes too far or works too many hours), the driver must complete a regular driver’s log for the day as soon as the exemption no longer applies.
If the driver has had to complete a log 8 or fewer days out of the last 30 days, the driver can use a paper log for the day. If the driver had to complete a log more than 8 days out of the last 30 days, the driver needs to use an electronic log for the day (unless one of the ELD exemptions applies, such as operating a vehicle older than model year 2000).
When a property-carrying driver is operating under the 150 air-mile exemption, the driver is also exempt from having to take the required 30-minute break (see 395.3(a)(3)(ii)).
If the driver began the day as a 150 air-mile driver and has driven more than 8 consecutive hours without a break, and something unexpected happens and the driver can no longer use the 150 air-mile exemption, the driver must stop and immediately take the 30-minute break as well as start logging. If the driver went outside of the 150 air-mile area before the driver had 8 hours of driving without a break from driving, the driver would be expected to take the break at the appropriate time.
Here are some of the common myths and misunderstandings about the 150 air-mile exemption:
The 150 air-mile exemption at 395.1(e)(2) only applies to drivers that: Operate property-carrying vehicles that do not require a CDL to operate, and Stay within the 150 air-miles of their work reporting location.
If the driver stays within the 150 air-mile radius of the work reporting location, and returns to the work reporting location within 14 hours on 5 of the last 7 days, and 16 hours on 2 of the last seven days, the driver is allowed to use a time record in place of a log.
If the driver does not meet the terms of the exception, the driver will need to complete a log for the day. If the driver had to log more than 8 days out of the last 30 days, the driver will need to use an electronic log for the day. All of the other issues discussed above would apply to these drivers as well.
If you have drivers that use these exemptions, you will need to check time records to make sure they are complying with the appropriate time limits. You will also need to check movement records to verify that the drivers using these exemptions are staying within the mandated area (within 150 air-miles of the work reporting location for the day).
If a driver is over the hours limit, or has gone too far, you need to verify that the submitted a log for the day, either paper or electronic, depending on how many days the driver had to log out of the previous 30 days.
During an audit, if it is discovered that your drivers are using these exemptions incorrectly, you will be cited for not having drivers’ logs when required. Each day this occurred will be another violation, so the fine could be rather large if you are not managing the use of these exemptions!
When drivers fail a DOT test or engage in other prohibited drug or alcohol behavior, their commercial driving careers are stalled until specific steps in rehabilitation and treatment are completed.
| Read our FAQ: What happens if a DOT return-to-duty or follow-up test is canceled? |
A commercial driver is required to go through the DOT return-to-duty process if:
Actual knowledge occurs when information is provided to the motor carrier indicating a DOT testing violation. This might be learned through:
When a motor carrier learns of a testing violation on a new hire, it must obtain proof that the driver has completed the return-to-duty process. Otherwise, the motor carrier would have to begin the return-to-duty process or pick up where it left off.
To resume a safety-sensitive function, the driver must complete the following return-to-duty steps in Subpart O of Part 40.
When drivers engage in prohibited drug or alcohol behavior, they must be immediately removed from performing all safety-sensitive functions. If on a dispatch, a driver must be told of the test result and instructed to park the vehicle. This notification often involves making arrangements to get the driver home and continue the run with different driver.
The employer must present the driver with a list of substance abuse professionals (SAPs) who have the appropriate credentials and DOT training to perform driver evaluations. The list must be given without a fee and made available to the driver (or driver applicant) whether or not the carrier retains the driver.
If the motor carrier does not have another face-to-face meeting with the driver, this list may be mailed or emailed to the driver.
For drug test results, the medical review officer (MRO) will report the violation to the Clearinghouse. This includes shy bladder scenarios without a valid medical explanation.
Failed alcohol tests (.04 or greater BAC), actual knowledge, and certain refusal to test scenarios are reported by the motor carrier to the Clearinghouse.
After the SAP list is given to a driver, the motor carrier cannot force a driver to begin the process. Nevertheless, to resume safety-sensitive functions, the driver must seek a face-to-face evaluation from a qualified SAP as a first step.
Payment of the evaluation is not required of the employer. Instead, it is based on labor-management agreements and healthcare benefits.
The SAP’s referral to an education and/or treatment program is based on a clinical evaluation of the driver during the face-to-face meeting. The SAP should have a working knowledge of what programs and counselors are available.
The SAP may take into consideration the driver’s ability to pay and insurance coverage. Once a SAP-approved provider has been agreed upon with the driver, the SAP will facilitate the referral and provide the program with the diagnostic determinations that led to the treatment plan. Programs range from outpatient treatment to partial or full in-patient resources.
Once the treatment plan has ended, the SAP will determine if it was a success. This decision is based on information provided by the education and/or treatment program and another face-to-face evaluation with the driver.
This second evaluation will result in one of three determinations:
If the SAP is satisfied with the driver’s ability to return to driving, the SAP will issue a report to the designated employer representative (DER). This report will list any continuing treatment and education, if required, and the number of DOT follow-up drug and/or alcohol tests required in a given time frame. The driver will be required to have a minimum of six unannounced follow-up tests in the first 12 months following the return to a safety-sensitive function. The SAP may require follow-up testing for up to five years.
For all Part 382 violations occurring since January 6, 2020, the SAP is required to report the successful completion of the evaluation and treatment to the Clearinghouse, provided the driver has designated the SAP in the driver’s personal Clearinghouse account.
The DER must wait for the go-ahead in the SAP report before sending the driver for the return-to-duty drug and/or alcohol test. All return-to-duty drug tests are performed under direct observation. The motor carrier must report a negative return-to-duty test to the Clearinghouse. In order for the “prohibited” status to be lifted from the driver’s record, both the SAP and motor carrier submission must be entered onto the driver’s record.
Once the Clearinghouse is no longer showing an unresolved testing violation, the driver can return to a safety-sensitive function.
Editor’s Note: Violations occurring prior to January 6, 2020, are not tracked in the Clearinghouse. Instead, the motor carrier would use the SAP report as a green light to perform the return-to-duty test, and once the negative test result is received, the driver can resume a safety-sensitive function.
After the driver returns to safety-sensitive functions, the motor carrier must carry out the unannounced follow-up tests under direct observation as prescribed in the SAP report. The DER must ensure that the tests do not have any discernible pattern.
The follow-up tests are in addition to any other DOT-required tests (e.g., random, post-accident). For instance, you cannot use a follow-up test as a substitute for a random test or vice versa.
If the driver leaves the motor carrier prior to the completion of the very last follow-up test, the next employer(s) must pick up where the process left off.
When the follow-up program is complete, the motor carrier under whose program the last test was performed must report this to the Clearinghouse. If the violation predates the Clearinghouse, the employer does not report the completed follow-up program to anyone.
If the motor carrier fails to begin or continue with a driver’s DOT return-to-duty process and follow-up testing, it is an acute violation that could cost the company up to $15,876. Allowing this driver to operate a CMV puts the carrier at risk of negligent entrustment claims if there is a crash.
The FMCSA is planning to test the effects of letting commercial truck drivers “pause” their 14-hour on-duty limit by up to 3 hours per day.
The agency is hoping to enlist up to 400 drivers to participate in its three-year “Split Duty Period Pilot Program.” Participants would be allowed to use one off-duty break of between 30 minutes and 3 hours to pause the 14-hour driving window, as long as they take 10 consecutive hours off duty at the end of the day. The pause should enable drivers to reduce fatigue, avoid congestion, reduce the pressure to speed, and be more productive, the FMCSA says.
Normally, short breaks taken during a driver’s day must be subtracted from the driver’s 14-consecutive-hour window during which driving is allowed.
| For more information, see our ezExplanation on the 14-hour on-duty rule. |
Under new rules in effect on September 29, 2020, some truck drivers can pause their 14-hour limit with a break of 2 hours or more, but only if they also spend at least 7 hours in a sleeper berth (see below). Under the pilot program, drivers could pause the clock with off-duty time alone, without the need for a sleeper berth. This idea was proposed back in 2019 but didn’t find its way into the recent rule changes because the FMCSA didn’t have enough data to justify it.
As required by law, the FMCSA is gathering public input on the proposal until November 2nd. It will then decide whether to implement the program. After the program concludes, the agency will need to report to Congress on its findings before it could proceed with any changes to the hours-of-service regulations.
Participation in the pilot program would be limited to between 200 and 400 commercial driver’s license (CDL) holders from companies of all sizes, with each driver participating for up to one year. Motor carriers that want to enroll in the program will need to apply via an FMCSA website which could be available late this year. Comments on the proposal may be submitted online at www.regulations.gov under docket number FMCSA-2020-0098.
Truck drivers who fall under the federal hours-of-service rules can already pause their 14-hour clock with a short rest break, as of September 29, 2020 (see log image). This is known as the “split sleeper-berth” option, and it works like this:
Key to remember: The FMCSA plans to test the safety of allowing truck drivers to pause their 14-hour clock with a rest break of up to 3 hours, even if they don’t have a sleeper berth. The pilot program could open later this year.
As of January 1, 2023, heavy-duty trucks and buses with engines from model years 2007 to 2009 operating in California must be either:
This is the final step in the phase-in of the Truck and Bus regulation. Covered vehicles originally built with engines older than model year 2006 must have already been replaced or retrofitted.
| Need more on CMV maintenance? See our ezExplanation Inspection and Maintenance. |
One issue that has come up is new vehicles are not available for delivery before January 1, 2023. This means fleets that operate in California that cannot get a replacement vehicle will be faced with the choice of either retiring their 2007 to 2009 engine vehicles without a replacement or operating in violation as of January 1, 2023.
However, the California Air Resources Board (CARB), the agency that oversees the Truck and Bus program, is aware of the situation and has provided an exemption. If the company has a written contract to purchase a new vehicle to replace a vehicle with a 2007 to 2009 engine in place before September 1, 2023, the existing vehicle can be operated until the replacement is placed in service.
To use this exemption, the company must register in CARB’s Truck Regulation Upload, Compliance, and Reporting System (TRUCRS) and report the use of the exemption. This is especially important to California-based companies as vehicle registration is tied to compliance with the Truck and Bus regulations requirements.
As of January 1, 2023, California will be placing roadside emissions monitoring devices (REMD) at locations around the state. These devices check emissions on any vehicle that passes it. If the device determines a vehicle may be a high emitter, the owner will receive a Notice to Submit to Testing. This will require the vehicle to be brought in to a “referee” location where the emissions and emissions components can be inspected.
These Truck and Bus requirements go into effect on January 1, 2023, and only apply to vehicles operating in California.
Most motor carriers review their roadside inspection reports for the obvious reasons: fixing mechanical defects and identifying unsafe or noncompliant driver behavior.
Some violations are easy to decipher, such as a burned-out light bulb or exceeding the speed limit by a specific range. Others take a little more to figure out, such as doing the math to determine when and how a driver exceeded hours-of-service (HOS) limits. Then there are all those 392.2 violations with suffixes. Some count against a carrier’s Compliance, Safety, Accountability (CSA) scores, while others do not, depending on whether they contribute to causing a crash.
One that often baffles motor carriers is 392.2C.
Section 392.2C is enforcement’s code for “failure to obey traffic control device.” The C stands for control.
The citation appears in the severity table for the Unsafe Driving BASIC (Behavior Analysis and Safety Improvement Category). The violation has been assigned a value of 5 out 10, with 10 being the most severe. The violation is used when calculating both the carrier’s and driver’s Unsafe Driving BASIC scores.
In most instances, the traffic control device is not a signal light or stop or yield sign. Rather, it is the sign that instructs the driver to pull into a weigh station.
| View our Weigh Stations ezExplanation for additional information. |
The vehicles that must stop at scales and inspection locations vary from state to state and even from location to location within a state. The “weigh scale ahead” or similar sign should be the driver’s guide.
If the sign reads:
Often those who operate commercial vehicles not requiring a commercial driver’s license, such as a large pickup truck or small box truck, mistakenly believe weigh scale inspections are just for larger rigs.
If a driver goes past a weigh station without pulling in as directed by a traffic control device, enforcement will pursue and pull over the driver. The officer will then escort the driver back to the weigh station for a roadside inspection.
Even if the driver was honestly confused whether the sign applied to the vehicle, it is too late. And more than likely enforcement’s interest has been piqued. It is highly unlikely the driver will be waived through at this point, and 392.2C will be entered on the roadside inspection report.
CSA’s enforcement model suggests finding the root cause of roadside inspection violations to prevent future occurrences and ultimately improve BASIC scores.
A violation of 392.2C may have one of several root causes, such as:
Whatever the reason, it must be addressed with the driver. Corrective actions range from refresher training to termination. If the driver was trying to avoid enforcement for other reasons (drugs, alcohol, over HOS limits), these other violations need to be addressed accordingly.
Key to remember: Failing to obey a traffic control device will be used in calculation of the CSA Unsafe Driving BASIC scores. Motor carriers should address the root cause of the violation so it does not recur.
A workplace safety definition for “safety-sensitive position” may lead some motor carriers to mistakenly put employees who don’t qualify in their DOT drug and alcohol testing program.
The Federal Motor Carrier Safety Administration (FMCSA) clearly defines a safety-sensitive position.
It is one where the employee is expected to operate a commercial motor vehicle (CMV) requiring a commercial driver’s license (CDL). Only these drivers can be placed in the motor carrier’s DOT drug and alcohol testing program under 49 CFR Part 382.
As a result, a carrier would not classify a forklift operator, driver helper, and other positions as safety sensitive for purposes of testing under Part
A driver who operates an FMCSA-regulated vehicle that does not require a CDL fits within the scope of workplace safety-sensitive duties, but not FMCSA.
For property-carrying vehicles, a non-CDL CMV is one that is:
For passenger carriers, a non-CDL CMV is designed to transport 9-15 passengers, including the driver, for compensation.
Even though the above vehicles and drivers are subject to the bulk of FMCSA’s safety regulations, the vehicles (and subsequently the drivers) do not qualify for CDL licensing or FMCSA testing.
If the driver happens to hold CDL, it still does not qualify as a safety-sensitive position. Applicability is always based on whether the employee is assigned to operate a CDL CMV.
Non-CDL CMV drivers are prohibited from operating while impaired under 49 CFR 392.4 and 392.5, but there is no testing mechanism under DOT authority. Testing would be best practice (non-DOT) and managed under the workplace drug program.
If a motor carrier mistakenly uses the workplace criteria for its DOT testing, the number and types of positions placed in the random pool far exceed commercial drivers.
For the general workforce, the term “safety sensitive” has been tossed around, but never clearly defined by OSHA (Occupational Safety and Health Administration). Many safety professionals tie the term to OSHA’s General Duty Clause (GDC), which requires that employers provide all workers with a safe and healthful workplace.
Specifically, the GDC requires employers to recognize hazards that cause or likely will cause death or serious physical harm. Any job title that is likely to cause death or serious harm to someone — including the employee, coworkers, or the general public — is usually put on a list of safety-sensitive positions.
The employer must look at each job’s hazards and decide if the position is classified by its organization as safety sensitive. Examples may include:
Even someone who works as a roofer may be considered a safety-sensitive position because the employee could trip and fall from a high elevation, causing serious personal harm.
Key to remember: When assembling the list of names for your DOT testing program, only include those individuals who are expected to operate a CDL CMV.
Wage overpayment errors happen for many reasons — from clerical mistakes to payroll system snafus.
Regardless of the reason, employees are not necessarily entitled to keep the extra money, and employers need to know their obligations for recouping it.
Under the federal Fair Labor Standards Act (FLSA), employers don’t need an employee’s permission to recoup wage overpayments. The extra money is seen as a loan or a wage advancement to the employee.
Because of this, employers are generally free to recoup the overpayment from the next paycheck — even if such a deduction cuts into the minimum wage or overtime pay due the employee under the FLSA.
State laws, however, may have greater restrictions. For example, New York employers may only make deductions from an employee’s wages for “an overpayment of wages where such overpayment is due to a mathematical or other clerical error by the employer.”
There are also limitations on the timing and duration, frequency, and method and amount of recovery.
The bottom line is, employers should try to avoid getting themselves into an overpayment situation in the first place. Supervisors should closely review their direct reports’ timesheets to catch errors before paychecks are issued
Also, employees should be encouraged to review their pay stubs for accuracy to help catch mistakes sooner rather than later.
If a mistake happens, employers should do their due diligence to communicate with the affected employees and make a reasonable plan to recoup the funds (provided it’s allowed under state law) so as not to cause any unnecessary financial harm to employees.
Wage overpayment errors can and will occur. Employers need to know their obligations under both federal and state laws before recouping money.
With the labor market still tight, employers might choose to hang onto employees even if they’re underperforming. But what about when complaints are rolling in from different angles? Take, for example, a lackluster supervisor who’s annoying employees and disappointing customers.
An employer could be hesitant to let the supervisor go, especially if there’s no documentation backing up claims of misconduct. The employer must weigh their options to decide if putting the supervisor on a performance improvement plan (PIP) or moving right to termination is the ideal choice.
At-will employment
For starters, in most states employers may terminate an employee at-will, meaning they can fire employees for pretty much any reason as long as it doesn’t discriminate against someone in a protected class based on sex, age, race, religion, etc. Employers also cannot terminate in retaliation for an employee making a claim of harassment, discrimination, or safety concerns.
Aside from these limits, employers can terminate employees for good cause, bad cause, or no cause at all.
PIP or terminate
Deciding whether to put an employee on a PIP or terminate must be decided on a case-by-case basis.
A PIP is usually for job performance issues (hence, performance improvement plan). This could mean anything from not making enough sales to being inept at the job’s essential functions. If job performance doesn’t improve under the PIP, termination may be the end result depending on company policies and practices.
Even if an employee has job performance issues, the employer can terminate without going through the PIP process first, unless the usual process is to implement a PIP with employees who have had similar problems. In that case, not doing a PIP could be seen as discrimination against an employee, especially if the person falls into a protected class.
Workplace misconduct, however, is another situation altogether. This could be anything from a one-off poor joke to pervasive harassment. Snapping at customers or coworkers (or worse), for example, is a conduct issue. An employer could issue a warning or move right to termination if the behavior is clearly illegal or a serious threat to workplace safety.
| Read more: ezExplanation on discharging employees |
Termination tips
If an employer decides to terminate, they should treat the employee as respectfully as possible during the termination process. Also, an employer should carefully and clearly communicate the job-related reasons for the termination to avoid any hint of discrimination. Lastly, an employer should document the reasons and reiterate the steps taken leading up to the termination and keep those records handy in case the employee files a wrongful termination lawsuit.
Key to remember: Employers sometimes struggle when making termination decisions. Having a process in place and documenting steps along the way can help if a case lands in court.
When an employee is on leave under the Family and Medical Leave Act (FMLA), the employer must maintain benefits under the company’s group health plan.
Thus, employees generally must continue paying their share of the health insurance premiums.
But how do employees pay their share of the premiums when FMLA leave is unpaid? Employers may offer three payment options:
Employers may allow a combination of these options, such as pre-pay for part of the leave and catch-up for the remainder. Below is a breakdown of the three available payment options.
When unpaid FMLA leave is foreseeable, employers may allow employees to pre-pay their premiums. For example, if an employee is adopting a child and requests several weeks for bonding time but does not have enough vacation to cover the entire absence, an employer could allow the employee to pre-pay his or her premiums for the portion of the leave that would be unpaid.
Employers may not require an employee to pre-pay, so this cannot be the only option offered.
If an employee chooses this option, however, employers may collect premiums on a pre-tax basis – with one exception. If the absence will extend into the next tax year (such as leave from December through January), only the premiums for the current tax year may be pre-paid with pre-tax income. The IRS does not allow employees to defer untaxed income from one year to the next.
In this example, the premiums for January could either be pre-paid with after-tax income, or the employee could elect one of the other options (pay-as-you-go or catch-up).
Under the pay-as-you-go option, employees pay their share of the premiums based upon the agreed terms made between the employer and employee. These payments are usually made on an after-tax basis.
For example, the employee might mail in a personal check every two weeks. If the employee fails to send in the checks, or otherwise fails to make payments using the agreed-upon system, the FMLA does allow employers to drop coverage after giving specified notices of non-payment.
Dropping coverage would likely cause some administrative headaches, and some insurers may refuse to do this because the employee would have to be reinstated to the health plan upon return from FMLA leave.
Therefore, employers may prefer to continue coverage by paying the employee’s share of the premiums, then use the catch-up option once the employee returns to work. Some insurance carriers recommend this as an alternative to dropping coverage.
Under the catch-up option, the employer and employee agree that the employee will not pay premiums until he or she returns from leave.
This option might be used when the need for FMLA leave was not foreseeable, such as having to care for a parent who was unexpectedly hospitalized.
To use this option, the employer and employee must agree in advance that:
When the employee returns, the employer collects the current premiums plus any catch-up payments, perhaps taking double premiums, until caught up. Contributions under the catch-up option may be taken on a pre-tax basis.
The IRS regulations indicate that, if the employee chooses the pay-as-you-go option, but fails to make the required payments, you may change to the catch-up option even without the employee’s prior agreement.
Employees on unpaid FMLA leave must still pay their share of health insurance premiums by either pre-paying, paying as they go, or making catch-up contributions upon returning to work.
Employers sometimes get tripped up on how to calculate the 1,250 hours worked eligibility criterion when employees need leave under the Family and Medical Leave Act (FMLA).
Does working overtime count toward the 1,250?
Recently, someone asked if overtime hours counted toward the 1,250 hours worked requirement (it does).
All hours actually worked apply to the 1,250, whether overtime or regular time, even if the overtime is not mandatory.
The 1,250 hours is calculated in relation to when the leave will begin, not when the employee puts an employer on notice of the need for leave.
Whether an employee is allowed to work overtime, however, is generally up to company policy. As far as pay goes, remember, if the employee is nonexempt (“hourly”) and works any overtime (mandatory or voluntary) the employee must be paid time and one-half for all hours worked over 40 within the workweek.
More about FMLA leave requirements
To be eligible to take FMLA leave, employees must:
Whether an employee has worked the minimum 1,250 hours is calculated based on determining compensable hours or work under the Fair Labor Standards Act (FLSA).
Calculating the 1,250 hours worked
When it comes to figuring out if an employee has worked at least 1,250 hours, it can get tricky. As was mentioned above, all hours worked, regular and overtime, must be counted.
Hours not worked should not be counted. The “not worked hours” include such time off as vacation time, sick leave, paid or unpaid holidays, or any other time in which an employee isn’t actually working — which can include disability, bereavement, FMLA and other forms of leave.
Once an employee meets the three eligibility criteria, including the 1,250 hours worked, for a particular leave reason, the employee remains eligible for the duration of the 12-month leave year period.
If the employee needs leave for another, different reason, eligibility would be recalculated.
Key to remember: All hours worked must be included in the 1,250 hours criterion when determining whether an employee is eligible for FMLA leave. Hours that aren’t worked (like vacation) are not included.
A new year often begins a new round of employee performance reviews. Since the Family and Medical Leave Act (FMLA) allows eligible employees to take up to 12 (or 26) weeks of leave, many events can occur during an employee’s leave, including the employee’s pre-scheduled performance review. Such reviews might take place on an annual or other scheduled basis. How you treat the timing of those reviews should include some thought.
If, for example, Jo Employee takes 12 weeks of FMLA leave, during which her annual performance review is scheduled, here are some questions to ponder:
Delaying a review
An annual performance review generally takes into consideration a full years’ worth of work. Some employers think it’s best to delay the performance review by the same amount of time an employee took FMLA leave to capture an entire years’ work. This practice, however, might risk running afoul of one of the cornerstones of the FMLA: Returning the employee to his or her position, including the equivalent pay, benefits, and working conditions.
The issues can be particularly concerning if the performance review affects wage increases or other compensation.
What the regulations say
The FMLA regulations indicate that an equivalent position includes equivalent pay, which includes any unconditional pay increases that may have occurred during the FMLA leave period. Equivalent pay also includes bonuses or payments, whether discretionary or non-discretionary. FMLA leave cannot undermine the employee’s right to such pay.
Furthermore, “… employers cannot use the taking of FMLA leave as a negative factor in employment actions, such as hiring, promotions, or disciplinary actions; nor can FMLA leave be counted under no fault attendance policies.” [29 CFR 825.220(c)]
Avoiding a negative factor
Therefore, you would need to look at whether delaying an employee’s performance review could be seen as having a negative factor for the employee.
If, for example, Jo Employee took 12 weeks of leave from April through June, during which she would otherwise have obtained a pay increase in May, but you delayed this increase until September (so you could use a full 12 months of work), you may have violated the equivalent pay provision. If delaying a review creates a new review schedule going forward, the negative impacts could continue.
If, however, a pay increase is conditioned upon seniority, length of service, or work performed, you would grant it in accordance with your policy or practice as applied to other employees on an equivalent leave status for a reason that does not qualify as FMLA leave.
In other words, don’t treat an employee on FMLA leave differently than you would an employee on other forms of leave.
Key to remember: It might be less risky to keep the performance review on schedule and prorate wage increases to account for FMLA leave.
Recent news headlines say many employers are requiring employees to return to the physical office; some after employees have been working remotely for about three years.
Since the long arm of long COVID and other medical conditions (physical or mental) continue to haunt the workplace, employers could find employees asking to keep working remotely because of their condition.
To help with these requests, here are six steps to take when an employee asks to work remotely as a reasonable accommodation under the Americans with Disabilities Act (ADA):
If this process looks familiar, it is because it’s pretty much the same process used for any accommodation request under the ADA. The goal is to find an effective solution for both employer and employee.
Key to remember: Having a process to follow can not only help comply with the ADA, but it can also help employees get their jobs done even while working remotely. Ensure employees are treated fairly, which can go a long way toward retention goals, as well as help avoid discrimination claims.
The COVID-19 outbreak created a shortage of latex and nitrile gloves in many workplaces.
Latex and nitrile gloves are used extensively in health care, and their disposable (single use) nature meant that large quantities were consumed during the peak of the pandemic. The shortage was also worsened because of hoarding by some consumers. In addition, certain businesses and government agencies began using these gloves to protect employees, even if their workers didn’t normally require gloves on the job.
If you have trouble obtaining your staff’s usual gloves, be prepared to identify feasible alternatives. You don’t want to endanger them by having them wear any old gloves they find lying around.
To identify alternatives for workers who rely on latex or nitrile gloves as PPE, you must know which chemicals workers handle or come in contact with. That’s because all glove materials are not suitable for all hazards.
Evaluate which materials offer appropriate protection from the specific chemicals that workers handle to select appropriate alternative gloves.
Here’s a summary of glove types and the protection given to help evaluate alternatives.
Butyl gloves protect against a variety of chemicals such as peroxide, highly corrosive acids, strong bases, alcohols, aldehydes, ketones, esters and nitrocompounds. Butyl gloves also resist oxidation, ozone corrosion and abrasion, and remain flexible at low temperatures. However, they do not perform well with aliphatic and aromatic hydrocarbons and halogenated solvents.
Natural (latex) rubber gloves have good elasticity and temperature resistance, and resist abrasions well. They protect against most water solutions of acids, alkalis, salts, and ketones. Latex gloves may cause allergic reactions and may not be appropriate for all employees. Hypoallergenic gloves, glove liners, and powderless gloves are possible alternatives for employees who are allergic.
Neoprene gloves protect against hydraulic fluids, gasoline, alcohols, organic acids, and alkalis. Their chemical and wear resistance are generally better than gloves of natural rubber.
Nitrile gloves are intended for jobs requiring dexterity, and they stand up even after prolonged exposure to substances that cause other gloves to deteriorate. They offer protection when working with greases, oils, acids, caustics, and alcohols but are not recommended for use with strong oxidizing agents, aromatic solvents, ketones, and acetates.
OSHA’s 1910.151(c) standard, Medical Services and First Aid, requires that employers provide emergency eyewashes when employees may be exposed to injurious corrosive materials during the course of their work. Employers have a wide range of eyewash types available to choose from on the market, including portable units (i.e., eyewash bottles). While many employers use bottles, OSHA says that they can’t be the only eyewash made available to employees, and their use should be limited.
The OSHA standard does not provide a great deal of detail on eyewashes for employers. However, where the regulation is silent, OSHA refers employers to the American National Standards Institute (ANSI) standard Z358.1-2009, “Emergency Eyewash and Shower Equipment,” regarding installation, operation, and maintenance of emergency eyewashes. This includes capacity and flushing requirements. The ANSI standard states that an eyewash must deliver 0.4 gallons of flushing fluid per minute for at least 15 minutes.
As such, ANSI says that an eyewash bottle does not meet these criteria; therefore, it can only be used to support eyewashes that do (i.e., plumbed and self-contained units), but cannot replace them.
The reason for this limitation is that eyewash bottles simply cannot provide the required 15 minutes of flushing. Eyewash bottles typically hold less than a gallon of water, which would supply the user with flushing fluid for approximately 1 minute. Even larger self-contained units (those with bladders) that have a capacity of 5 to 10 gallons would only provide maximum use of about 5 minutes.
In other words, eyewash bottles don’t provide an adequate amount of flushing fluid and cannot be considered a primary means of protection.
For this reason, OSHA warns that the use of eyewash bottles should be limited. In a 1986 memorandum to Regional Administrators, the agency states, “In general, squeeze bottles should not be used except where the hazard severity or distance from plumbed eyewash equipment requires personal equipment at work stations for immediate flushing prior to prolonged flushing at a plumbed or self-contained unit.”
In other words, employers can provide eyewash bottles in instances where plumbed or self-contained units can’t reasonably be provided (e.g., an outside yard) in the immediate work area, but only until they can reach a unit which can provide the amount of flushing fluid necessary to flush the eyes for at least 15 minutes.
OSHA expects the employer to determine the level of the potential risk to employees and provide eyewash (and/or shower) protection accordingly. The severity of the hazard(s) involved is a critical consideration when making this determination. In the past, OSHA has said that 1910.151(c)is meant to cover strong acids and alkalis, and the requirement to provide suitable facilities for quick drenching or flushing depends on the exposure and the strength of the hazardous chemical. Chemicals and materials such as household detergents or cleaners, sawdust, metal filings, etc. would not require emergency eyewash (or shower) under the standard.
If an employer determines that an eyewash is needed, then it must meet the provisions set forth in the American National Standards Institute (ANSI) standard Z358.1. The agency uses the ANSI standard as an enforcement tool. This is clarified in a November 1, 2002, Letter of Interpretation, which says, “If OSHA inspects a workplace and finds unsuitable facilities for quick drenching or flushing of the eyes and body, a citation under 29 CFR 1910.151(c)would be issued. When determining whether the eyewash or shower facilities are suitable given the circumstances of a particular worksite, OSHA may refer to the most recent consensus standard regarding eyewash or shower equipment…”
| Need information on eyewash inspections? See our Institute document on Inspections and Maintenance. |
Without the ANSI standard, employers would find it difficult to demonstrate to OSHA exactly how their eyewash and shower units were suitable exclusive to the regulatory language under 1910.151(c) since it’s limited and vague.
Eyewash bottles don’t meet the requirement under 1910.151(c) to provide “suitable” facilities for quick drenching or flushing of the eyes. They cannot be the only eyewash provided in the workplace.
Each year, the National Fire Protection Agency (NFPA) reminds employers not to prop open fire doors for convenience. Propping open doors has become a common violation of fire codes after the pandemic because workers didn’t want to become exposed to germs on common touchpoints.
I know firsthand this is an issue at construction jobsites and remember telling workers not to prop open fire doors in our clients’ facilities. Workers were doing this out of convenience because they carried things into and out of the existing facility. Propping open a fire door, or wedging it open, are serious fire and safety hazards. Keep fire doors closed to prevent smoke and fire from spreading into the fire evacuation route, like a stairwell. OSHA and NFPA don’t prohibit propping open a fire exit door but caution employers against doing this for safety and security reasons.
Fire doors must remain closed, although some may be designed to automatically close when fire and smoke are sensed by jobsite fire detection equipment. To reduce the need to disinfect frequently touched points, workers can push open fire doors using their sleeves by pushing against the push bar instead of using their hands. You can also increase housekeeping efforts and the frequency that doorknobs, handles, and push bars are cleaned throughout the shift.
One way employers try to handle skyrocketing inflation is to manage first aid supplies. But do OSHA regulations allow employers to lock first aid supplies as a way to control costs?
Our experts are often asked whether OSHA permits locking first aid supplies. In a January 23, 2007, OSHA letter of interpretation (LOI), OSHA confirmed that first aid cabinets can be locked. The LOI stated, however, that first aid supplies must be readily accessible in the event of an emergency. Additionally, 29 CFR 1910.151(b) states: “In the absence of an infirmary, clinic, or hospital in near proximity to the workplace which is used for the treatment of all injured employees, a person or persons shall be adequately trained to render first aid. Adequate first aid supplies shall be readily available.”
OSHA defines “readily available” as accessible within three to five minutes and warns that locking first aid supplies, whether kits or cabinets, may limit employee accessibility per the standard. The agency advises that if an employer was relying on first aid services not provided by a clinic, infirmary, or hospital and adequate first aid supplies were not available when needed, then the employer would be in violation of 1910.151(b).
If you’re concerned with supplies being used in a manner not intended by the company, there are ways to manage supplies. For example, employers could use vending machines that allow employees to scan their badges and get basic supplies or personal protective equipment free of charge. This can help employers manage their supply chain and evaluate by whom and for what supplies are being used.
If opting to lock your first aid supplies, remember to make supplies readily accessible (within three to five minutes). This may require that additional keys for locks be made available to multiple personnel at all times when workers are present.
Although their recommendations are non-mandatory, OSHA suggests using the American National Standards Institute (ANSI) for reference to determine what supplies you need to have. The contents for Class A kits listed in the ANSI standard should be adequate for small worksites. Class B kits are designed with a broader range and quantity of supplies to deal with injuries in more complex or high-risk environments (for example, larger operations or multiple operations conducted at the same location).
It’s important to note that although OSHA is still citing ANSI’s 1998 standard, an updated version of the standard, ANSI/International Safety Equipment Association (ISEA) Z308.1-2021, was approved on April 15, 2022, and went into effect on October 15, 2022. Major changes to the standard included:
Determining what first aid supplies should be accessible depends on the workplace hazards and potential injuries. A great place to begin is by assessing your Form 300 injury logs to see the types of injuries already reported. Most employers perform risk assessments, beginning with a review of the Form 300 logs, to drive their decisions. OSHA also provides guidance to employers in 1910.151 Appendix A.
Employers must understand the accessibility risks associated with locking first aid cabinets even though OSHA and ANSI do not prohibit this practice. First aid supplies must be readily accessible (within three to five minutes) in the event of an emergency.
Hi everyone! Welcome to the monthly news roundup video, where we’ll review the most impactful environmental, health, and safety news. Please view the content links in the transcript for more information about the topics I’ll be covering today. With that said, let’s get started!
For the 13th year in a row, fall protection for construction topped OSHA’s list of violations. In fiscal year 2023, there were over 7,000 recorded violations, up from 5,250 in fiscal year 2022.
Workers who are exposed to lead in industries such as painting, battery manufacturing, and building renovation risk bringing lead home on their clothing and personal items. Take-home lead can contaminate a worker’s car and home, posing an exposure risk to family members. A new NIOSH publication outlines steps workers can take to minimize this risk.
Private industry employers reported 2.8 million nonfatal workplace injuries and illnesses in 2022. This is a 7.5 percent increase over 2021. The increase is due to a rise in both illnesses, which were up 26.1 percent, and injuries, which were up 4.5 percent. Respiratory illness cases drove the spike in reported illnesses.
OSHA faces significant challenges in ensuring worker safety, particularly in high-risk industries. This is according to a report from the Office of Inspector General, or OIG. Among OSHA’s top challenges are verifying timely hazard abatement, employer reporting, completing inspections, workplace violence, and protecting workers from crystalline silica.
And finally, turning to environmental news, a recent EPA rule requires covered facilities to include all quantities of per- and polyfluoroalkyl substances, or PFAS, on their Toxics Release Inventory reports. The rule also mandates that suppliers notify product users of the presence of any chemicals of special concern contained in their mixtures and products.
Thanks for tuning in to the monthly news roundup. We’ll see you next month!
Employers must select a North American Industry Classification System (NAICS) code for every establishment, which usually means a single business location. This code determines whether the establishment is exempt from keeping an OSHA 300 Log. For locations that must keep a 300 Log, the code determines whether the establishment must submit injury data to OSHA by March 2nd.
The NAICS codes get updated every five years, with 2022 as the most current. Adding confusion, different OSHA regulations use different versions of the codes. For example:
Searching codes online may default to the 2022 version, and some codes changed. For example, the 1904.41 appendix lists 4529 for “Other general merchandise stores” which covers retail outlets like dollar stores and variety stores. However, searching that code in the 2022 list shows “no result” since that number changed. The 2022 NAICS code for general merchandise stores is 4552, but that code does not appear in OSHA’s appendix. Employers using the 2022 NAICS codes may incorrectly believe their establishment is not on OSHA’s list.
Employers can search older versions of the NAICS codes at https://www.census.gov/naics/ which also indicates whether a particular code has changed in more recent versions.
In addition to using the NAICS list for the correct year, employers must choose the correct code for each establishment. If a location engages in more than one type of business activity, employers must choose only one NAICS code for OSHA recordkeeping. OSHA says to choose the code for the activity that generates the most revenue or has the most employees.
In some cases, employers can divide a single physical location into more than one “establishment” as defined in 1904.46. To split a single location into multiple establishments, all of the following must apply:
For example, OSHA noted that if an employer operates a construction company at the same physical location as a lumber yard, the employer may consider each business as a separate establishment.
For employers with multiple establishments, the NAICS code for each location should reflect the primary business activity at each establishment. For example, a manufacturing company might have a separate administration office. Using a manufacturing code for the office might not be appropriate, even though it supports the other manufacturing locations. However, NAICS codes starting 5511 for “Management of Companies and Enterprises” might apply.
For example, code 551114 gives examples as follows:
That might better describe a corporate administrative office, if the location does not have any warehousing or manufacturing operations. In fact, codes starting 5511 appear on OSHA’s list of establishments under 1904.2 that are exempt from keeping a 300 Log, so applying the correct code could mean that office doesn’t need a 300 Log at all.
Finally, counting employees gets confusing because some OSHA regulations require counting all employees in the company (combining all locations) and others require counting only the employees at each establishment.
Key to remember: NAICS codes update every five years, and employers must use the correct list, which may differ in various regulations.

