Under the Toxic Substances Control Act (TSCA) of 1976 (codified at 15 U.S.C. 2601 et seq.), the Environmental Protection Agency (EPA) has broad authority to issue regulations designed to gather health, safety, and exposure information on, require testing of, and control exposure to chemical substances and mixtures. Drugs, cosmetics, foods, food additives, pesticides, and nuclear materials are exempt from TSCA, however.
Chemicals play a vital role
Chemicals — those found in nature and those created in laboratories — are at the heart of the nation’s industrialized, technology-based society. They help protect human health and control pests. Chemicals help clothe, shelter, and feed humans. They are found in innumerable products for homes, businesses, and industry. The chemical industry plays a vital role in the economy of the U.S.
Some chemicals have been found to be harmful
About 42,000 chemical substances are presently manufactured or processed for commercial use in the U.S. with more introduced each year. EPA explains that although most chemicals present no unreasonable risk to the environment or human health when used properly, in the past some chemicals commonly used and widely dispersed have been found to be significantly harmful. An example is the family of chemicals called polychlorinated biphenyls, or PCBs. It was not until after tens of millions of pounds of PCBs were produced and released into the environment that scientists realized how persistent and potentially toxic they were. In fact, scientists have found PCBs in humans and even in the milk of nursing mothers.
Toxic chemical hazards are identified and controlled under TSCA
By enacting TSCA, Congress established a number of new requirements and authorities for identifying and controlling toxic chemical hazards in human health and the environment. Programs now exist under TSCA to gather information about the toxicity of particular chemicals and the extent to which people and the environment are exposed to them, to assess whether they cause unreasonable risks to humans and the environment, and to institute appropriate control actions after weighing their potential risks against their benefits to the nation’s economic and social well-being.
Introduction
- TSCA authorizes EPA to discover and maintain information on all new and existing chemical substances.
- TSCA is divided into six titles and codified into six subchapters of the U.S. Code.
The Toxic Substances Control Act (TSCA) specifically states that it is the policy of the U.S. that:
- Adequate information should be developed with respect to the effect of chemical substances and mixtures on health and the environment and that the development of such information should be the responsibility of those who manufacture and those who process such chemical substances and mixtures;
- Adequate authority should exist to regulate chemical substances and mixtures which present an unreasonable risk of injury to health or the environment, and to take action with respect to chemical substances and mixtures which are imminent hazards; and
- Authority over chemical substances and mixtures should be exercised in such a manner as not to impede unduly or create unnecessary economic barriers to technological innovation while fulfilling the primary purpose of this law to assure that such innovation and commerce in such chemical substances and mixtures do not present an unreasonable risk of injury to health or the environment.
Overview
TSCA was signed into law on October 11, 1976. The Act authorizes the Environmental Protection Agency (EPA) to discover and maintain information on all new and existing chemical substances. EPA must also control any of the substances that are determined to cause an unreasonable risk to public health or the environment.
The Act is divided into six titles and codified into six subchapters of the U.S. Code:
The requirements of TSCA are often referred to by their section number (sections 2 to 601) in the Act. However, because TSCA is also codified in the U.S. Code, TSCA itself is cited as 15 U.S.C. 2601 et seq.
To complicate matters, TSCA authorized EPA to implement federal regulations. These regulations are found in the Code of Federal Regulations at 40 CFR 700 to 799. In addition, the U.S. Customs and Border Protection (CBP) regulations implementing TSCA section 13 for importers can be found at 19 CFR 12.118 to .127.
TSCA Title I Control of Toxic Substances
- Requirements for chemicals regulated under TSCA apply to chemicals manufactured in the U.S. and imported into the U.S.
- TSCA does not allow chemical evaluation and restriction to be delegated to states.
- There are certain noteworthy major sections in Title I of TSCA.
The Toxic Substances Control Act (TSCA) directs the U.S. Environmental Protection Agency (EPA) to evaluate the lifecycle (i.e., manufacture, importation, processing, distribution, use, and disposal) of industrial and commercial chemicals for unreasonable risks and, if warranted, to regulate such chemicals.
TSCA, as amended, requires EPA to gather existing information from chemical manufacturers, processors, and distributors about risks that industrial and commercial chemicals may present to human health or the environment. Prior to introducing a new chemical into commerce, its manufacturer must notify EPA to allow the agency to evaluate the chemical for unreasonable risks. Similar notification requirements apply to existing chemicals proposed for uses determined by EPA to be “significant new uses.”
If EPA has inadequate information about a chemical to determine whether it presents unreasonable risks, the agency may require the manufacturer to develop new information necessary to evaluate risks. TSCA establishes a framework to protect from disclosure submitted information that warrants confidential treatment.
To identify which chemicals may warrant regulation, TSCA requires EPA to systematically prioritize chemicals for risk evaluation. Based on the evaluation, EPA must regulate those chemicals that present unreasonable risks to ensure they no longer do so. Regulatory options available to EPA range from labeling requirements to an outright ban on manufacturing. TSCA directs EPA to take expedited action on chemicals that exhibit characteristics known to present greater risks. Additionally, TSCA authorizes EPA to expedite review of chemicals that present significant risk of serious or widespread harm and initiate enforcement actions against imminently hazardous chemicals.
Requirements for chemicals regulated under TSCA apply to chemicals manufactured in the U.S. and imported into the U.S. TSCA establishes additional procedures for handling imports of chemicals for which EPA has promulgated requirements under the Act. Chemicals marked for export only are subject to recordkeeping and reporting requirements unless EPA has required the development of new information or established a requirement to protect against unreasonable risk.
TSCA authorizes citizen petitions and citizen suits to challenge EPA’s implementation and enforce certain requirements under TSCA through litigation. Additionally, TSCA includes enforcement provisions and establishes civil and criminal penalties for violations.
TSCA does not allow chemical evaluation and restriction to be delegated to states. While states may evaluate and regulate chemicals under their own authorities, TSCA provides an explicit, though limited, preemption of state requirements for chemicals that EPA has evaluated and either determined to present no unreasonable risks or regulated to protect against unreasonable risks. The Act generally preserves long-standing state requirements and allows preemption waivers under certain circumstances.
Congress funds TSCA activities through annual discretionary appropriations. The Act also authorizes EPA to collect fees from chemical manufacturers and processors to partially defray costs that the agency may incur from implementing the statute.
Major sections of TSCA Title I
While the sections in Title I of TSCA number from 2 to 30, the major sections are as follows:
- Section 4 Testing of Chemical Substances and Mixtures — Provides that EPA can issue rules to require companies to generate hazard and exposure information through specific tests or measurements on chemicals in certain circumstances.
- Section 5 Manufacturing and Processing Notices — Prevents future risks through premanufacture screening and regulatory tracking of new chemical products. Significant new use rules can be used to require notice to EPA before new or existing chemical substances and mixtures are used in new ways that might create concerns. EPA can also compile and keep current a list of chemical substances that present or may present an unreasonable risk of injury to human health or the environment.
- Section 6 Prioritization, Risk Evaluation, and Regulation of Hazardous Chemical Substances and Mixtures — Provides for the regulation of hazardous existing chemical substances and mixtures. This includes the control of unreasonable risks already known, risks from polychlorinated biphenyls (PCBs), and risks for existing chemicals that may be discovered in the future.
- Section 8 Reporting and Retention of Information — Requires EPA to gather and disseminate information about chemical production, use, and possible adverse effects to human health and the environment. This section authorizes EPA to maintain the TSCA Inventory.
- Section 9 Relationship to Other Federal Laws — Governs the relationship of TSCA to other federal laws.
- Section 12 Exports — Outlines export notification requirements for any person who exports or intends to export a chemical substance or mixture subject to certain TSCA regulations.
- Section 13 Entry into Customs Territory of the United States — Details import certification requirements that must be met before importing a chemical substance, mixture, or article containing a chemical substance or mixture into the U.S.
- Section 21 Citizens’ Petitions — Allows citizens to petition EPA to take specific regulatory actions on chemicals and mixtures under TSCA.
TSCA Title II Asbestos Hazard Emergency Response
- Title II requires asbestos contractors and analytical laboratories to be certified, and schools to use certified persons for abatement work.
- AHERA authorized EPA to set standards for responding to the presence of asbestos in schools.
Growing public concern about the presence of potentially hazardous asbestos in buildings, especially in schools, led to congressional efforts to address the problem. Toxic Substances Control Act (TSCA) Title II, the Asbestos Hazard Emergency Response Act (AHERA), was enacted in 1986 and amended in July 1988. It required the Environmental Protection Agency (EPA) to set standards by October 1987, for responding to the presence of asbestos in schools.
The standards, set at levels adequate to protect public health and the environment, identify appropriate response actions that depend on the physical condition of asbestos. Schools, in turn, were required to inspect for asbestos-containing material, and to develop and implement a plan for managing any such material. Plans for managing asbestos were to be submitted by schools before May 1989, and implementation was to begin by July 1989. The law contains no deadlines for schools to complete implementation.
Title II also requires asbestos contractors and analytical laboratories to be certified, and schools to use certified persons for abatement work. Training and accreditation requirements also apply to inspectors, contractors, and workers performing asbestos abatement work in all public and commercial buildings. EPA may award training grants to nonprofit organizations for asbestos health and safety programs. However, authorization of appropriations for this grant program expired September 30, 1995. Other Title II requirements (such as mandates that buildings be inspected for asbestos) have not been extended to non-school buildings.
To enforce requirements, TSCA authorizes EPA to take emergency action with respect to schools if school officials do not act to protect children. The Act also authorizes citizen action with respect to asbestos-containing material in a school and to compel action by EPA, either through administrative petition or judicial action. Civil penalties not to exceed $5,000 are authorized for violations such as failing to conduct an inspection or to develop a school management plan.
Concern about how schools would pay for required actions was addressed in separate legislation (the Asbestos School Hazard Abatement Act of 1984, or ASHAA). It established a program offering grants and interest-free loans to schools with serious asbestos problems and demonstrated financial need.
Although EPA for several years did not request funding for this program, Congress appropriated funds. Authorization of appropriations for this program expired September 30, 1995, and Congress has not appropriated funds since fiscal year 1993; a total of $382 million in grant and loan funds were appropriated from fiscal years 1984 through 1993. Repaid ASHAA loans are returned to an Asbestos Trust Fund, established in TSCA Title II, to become a dedicated source of revenues for future asbestos control projects.
TSCA Title III Indoor Radon Abatement
- The basic purpose of Title III is to provide financial and technical assistance to the states that choose to support radon monitoring and control.
- Monitoring nor abatement of radon is required by TSCA.
In October 1988, Congress amended the Toxic Substances Control Act (TSCA) by adding Title III, Indoor Radon Abatement. The basic purpose of Title III is to provide financial and technical assistance to the states that choose to support radon monitoring and control. Neither monitoring nor abatement of radon is required by TSCA.
Title III required the Environmental Protection Agency (EPA) to update its pamphlet “A Citizen’s Guide to Radon,” to develop model construction standards and techniques for controlling radon levels within new buildings, and to provide technical assistance to states. EPA is to provide technical assistance by:
- Establishing an information clearinghouse;Publishing public information materials;
- Establishing a national database of radon levels detected, organized by state;
- Providing information to professional organizations representing private firms involved in building design and construction;
- Submitting to Congress a plan for providing financial and technical assistance to states;
- Operating cooperative projects with states;
- Conducting research to develop, test, and evaluate radon measurement methods and protocols;
- Developing and demonstrating new methods of radon measurement and mitigation, including methods that are suitable for use in nonresidential childcare facilities;
- Operating a voluntary program to rate radon measurement and mitigation devices and methods and the effectiveness of private firms and individuals offering radon-related services; and
- Designing and implementing training seminars.
The proficiency rating program and certification for training programs collect fees for service, and therefore are meant to be self-supporting, but Congress authorized $1.5 million to be appropriated to establish these programs. Congress authorized $3 million to be appropriated for each of three years beginning in 1989 for the other provisions of sections 303, 304, and 305.
A matching grant program was established for the purpose of assisting states in developing and implementing programs for radon assessment and mitigation. For this program, $30 million was authorized to be appropriated over three years, with funds targeted to states or projects that made efforts to ensure adoption of EPA’s model construction standards and techniques for new buildings; gave preference to low-income persons; or addressed serious and extensive radon contamination problems or had the potential to reduce risk or to develop innovative assessment techniques, mitigation measures, or management approaches.
Other sections of Title III require EPA to conduct a study to:
- Determine the extent of radon contamination in schools;
- Identify and list areas of the United States with a high probability of having high levels of indoor radon;
- Make grants or cooperative agreements to establish and operate at least three regional radon training centers; and
- Provide guidance to federal agencies on radon measurement, risk assessment, and remedial measures.
All authorizations for appropriations specific to this title expired September 30, 1991, although appropriations have continued.
TSCA Title IV Lead Exposure Reduction
- Title IV aims to accelerate federal efforts to reduce risks to young children who are exposed daily to lead-based paint in their homes.
- Title IV directs EPA to establish a clearinghouse and hotline to distribute information about the hazards of lead-based paint, how to avoid exposure and reduce risk, and new technologies for removing or immobilizing lead-based paint.
- Title IV authorizes states to propose programs to train and certify inspectors and contractors engaged in the detection or control of lead-based paint hazards.
Congress added Title IV to the Toxic Substances Control Act (TSCA) when it enacted the Residential Lead-Based Paint Hazard Reduction Act of 1992 as Title X in the Housing and Community Development Act of 1992. TSCA Title IV aims to accelerate federal efforts to reduce risks to young children who are exposed daily to lead-based paint in their homes. In addition, it was intended to stimulate development of lead inspection and hazard abatement services in the private sector, while ensuring that the services provided and any products employed are reliable and effective in reducing risk. To these ends, Title IV directs the Environmental Protection Agency (EPA) to:
- Promulgate definitions of lead-contaminated dust, lead-contaminated soil, and lead-based paint hazards;
- Ensure that people engaged in detection and control of lead hazards are properly trained and that contractors are certified;
- Publish requirements for the accreditation of training programs for workers;
- Develop criteria to evaluate the effectiveness of commercial products used to detect or reduce risks associated with lead-based paint;
- Establish protocols, criteria, and minimum performance standards for laboratory analysis of lead in paint films, soil, and dust;
- Establish a program to certify laboratories as qualified to test substances for lead content; and
- Publish and distribute to the public a list of certified or accredited environmental sampling laboratories.
Title IV explicitly applies these requirements to federal facilities and activities that may create a lead hazard. In addition, Congress directed EPA to conduct a study of lead hazards due to renovation and remodeling activities that may incidentally disturb lead-based paint. EPA is required to promulgate guidelines for the renovation and remodeling of buildings or other structures when these activities might create a hazard.
Title IV directs EPA to establish a clearinghouse and hotline to distribute information about the hazards of lead-based paint, how to avoid exposure and reduce risk, and new technologies for removing or immobilizing lead-based paint. In addition, Congress mandated development of a lead hazard information pamphlet; public education and outreach activities for health professionals, the general public, homeowners, landlords, tenants, consumers of home improvement products, the residential real estate industry, and the home renovation industry; and information to be distributed by retailers of home improvement products to provide consumers with practical information related to the hazards of renovation where lead-based paint may be present.
Title IV authorizes states to propose programs to train and certify inspectors and contractors engaged in the detection or control of lead-based paint hazards. States also may develop the required informational pamphlets. TSCA requires EPA to promulgate a model state program that may be adopted by any state. Congress gave EPA the authority to approve or disapprove authorization for state proposals and to provide grants for states to develop and implement authorized programs. A federal program must be established, administered, and enforced by EPA in each state without an authorized program.
The Department of Health and Human Services also has responsibilities under Title IV of TSCA. It mandates a study by the Centers for Disease Prevention and Control (CDC) and the National Institute for Environmental Health Sciences to determine the sources of lead exposure to children who have elevated lead levels in their bodies. The National Institute for Occupational Safety and Health (NIOSH) is directed to study ways of reducing occupational exposure to lead during abatement activities.
The Act established a rulemaking docket to ensure the availability to the general public of all documents submitted to agencies that are relevant to regulatory decisions pursuant to this legislation. The docket is required to include the drafts of all proposed rules submitted by EPA to the White House Office of Management and Budget (OMB), written comments on the drafts, and written responses to comments. EPA must provide an explanation for any major change to a proposed rule that appears in a final rule, and such changes may not be made based on information not filed in the docket. Dockets are required to be established in each EPA regional office.
In addition to amending TSCA, Title X of the Housing and Community Development Act of 1992 authorized:
- Grants to states for risk assessments and lead-based paint removal and immobilization in private housing for low-income residents;
- State training, certification, or accreditation programs for inspectors and abatement contractors; and
- Research at the Department of Housing and Urban Development (HUD).
Authorization for appropriations for these grants expired September 30, 1994, but appropriations have continued. Title X directed HUD to establish guidelines for federally supported work involving risk assessments, inspections, interim controls, and abatement of lead-based paint hazards. In addition, the NIOSH was provided $10 million for training people who remove or immobilize paint.
Finally, Congress authorized to be appropriated “such sums as may be necessary” for TSCA Title IV.
TSCA Title V Healthy High-Performance Schools
- Title V authorizes EPA to establish a state grant program to provide assistance for EPA programs to schools and develop and implement state school environmental health programs.
- The EPA Administrator is directed to prepare an annual report to Congress on activities carried out under Title V authority.
At the end of 2007, Congress added a fifth title to the Toxic Substances Control Act (TSCA), subtitled Healthy High-Performance Schools. Enacted as Title IV, Subtitle E (section 461) of Public Law 110-140, the Energy Independence and Security Act of 2007, TSCA Title V authorizes the Environmental Protection Agency (EPA) to establish a state grant program to provide technical assistance for EPA programs to schools and develop and implement state school environmental health programs.
State programs must include standards for school building design, construction, and renovation, and identify ongoing school building environmental problems and recommended solutions. Environmental problems specifically mentioned in the law include “contaminants, hazardous substances, and pollutant emissions.” EPA’s authority to provide grants expired five years after the date of enactment.
Title V required the EPA Administrator, in consultation with the Secretary of Education and the Secretary of Health and Human Services, to issue voluntary guidelines within 18 months of Title V enactment for selecting sites for schools (presumably new schools). The guidelines are to account for the “special vulnerability of children to hazardous substances or pollution exposures in any case in which the potential for contamination at a potential school site exists,” modes of transportation available to students and staff, efficient use of energy, and potential use of a school at the site as an emergency shelter.
Title V also required the EPA Administrator, in consultation with the Secretary of Education and the Secretary of Health and Human Services, to issue voluntary guidelines within two years of enactment for developing and implementing state environmental health programs for schools. These guidelines had to take into account the findings of federal initiatives established under “relevant federal law with respect to school facilities,” including initiatives related to water and energy conservation authorized by sections 431 through 441, and work related to high performance green buildings authorized by section 492 of Public Law 110-140. In particular, the guidelines had to take into account:
- Environmental problems, contaminants, hazardous substances, and pollutant emissions;
- Natural day lighting;
- Ventilation;
- Heating and cooling;
- Moisture control and mold;
- Maintenance, cleaning, and pest control;
- Acoustics; and
- Other issues relating to the health, comfort, productivity, and performance of occupants of the school facilities.
In addition, Title V required that the guidelines:
- Provide technical assistance on siting, design, management, and operation of school facilities;
- Collaborate with children’s environmental health centers in school environmental investigations;
- Assist states and the public to better understand and improve the environmental health of children; and
- Take into account “the special vulnerability of children in low-income and minority communities to exposures from contaminants, hazardous substances, and pollutant emissions.”
Several provisions in Title V refer to entities established under other sections of the Energy Independence and Security Act of 2007. For example, Title V contains directives for the Federal Director of the Office of Federal High-Performance Green Buildings in the General Services Administration, which was created by section 436(a).
In addition, there is reference to the national high-performance green building clearinghouse established in section 423(1). Title V requires the Federal Director to ensure as much as possible, that the public clearinghouse “receives and makes available information on the exposure of children to environmental hazards in school facilities.” The EPA Administrator is directed to prepare an annual report to Congress on activities carried out under Title V authority, and this report also must be made available to the public through the clearinghouse.
For the purposes of carrying out the provisions of Title V, Congress authorized appropriations of $1.5 million annually through 2013.
TSCA Title VI Formaldehyde Standards for Composite Wood Products
- Title VI mandates specific formaldehyde emission standards for hardwood plywood, medium-density fiberboard, and particleboard that is sold, supplied, offered for sale, or manufactured in the United States.
In July 2010, Congress enacted the Formaldehyde Standards for Composite Wood Products Act, adding a new Title VI to the Toxic Substances Control Act (TSCA). Title VI mandates specific formaldehyde emission standards for hardwood plywood, medium-density fiberboard, and particleboard that is sold, supplied, offered for sale, or manufactured in the United States.
The standards are phased in over two years from enactment and are based on Method ASTM E-1333-96 (2002), the voluntary national formaldehyde emissions standards established by ASTM International (formerly known as the American Society for Testing and Materials).
The standards apply to plywood, particleboard, and medium-density fiberboard in the form of an unfinished panel or incorporated into a finished good. Certain products are excluded, including many forms of lumber and panels used for outdoor applications, such as structural plywood, prefabricated wood I-joists, most windows, antiques or other previously owned goods, and composite wood products used inside automobiles, trucks, rail cars, boats, and aircraft.
The Environmental Protection Agency (EPA) was required to promulgate regulations ensuring compliance with the emission standards and had to include provisions related to:
- Labeling, chain of custody requirements, and sell-through provisions;
- Ultra-low-emitting formaldehyde resins, finished goods, and third-party testing and certification;
- Auditing and reporting of third-party certifiers;
- Recordkeeping;
- Enforcement;
- Laminated products; and
- Exceptions for products and components containing “de minimis amounts” of composite wood products.
The regulations are found at 40 CFR 770.
Title VI prohibits stockpiling of products manufactured before the effective date of the Act for sale after that date. Also prohibited is any requirement for labeling products manufactured prior to the “designated date of manufacture.”
Title VI requires an annual report to Congress on the status of implementation and the extent to which relevant industries have achieved compliance. Finally, the Act directs the Secretary of Housing and Urban Development to update regulations concerning formaldehyde emissions from composite wood in manufactured homes (24 CFR 3280.308) to ensure that the standards established by TSCA Title VI are implemented.
U.S. code sections related to TSCA sections
- Each subchapter of TSCA ties directly to the U.S. Code. This table shows only the major code sections of the Toxic Substances Control Act (TSCA). Facilities will want to consult the official U.S. Code for further details.
| 15 U.S.C. | Section Title | TSCA section (as amended) |
|---|
| Subchapter I Control of Toxic Substances |
| 2601 | Findings, policy, and intent | 2 |
| 2602 | Definitions | 3 |
| 2603 | Testing of chemical substances and mixtures | 4 |
| 2604 | Manufacturing and processing notices | 5 |
| 2605 | Prioritization, risk evaluation, and regulation of hazardous chemical substances and mixtures | 6 |
| 2606 | Imminent hazards | 7 |
| 2607 | Reporting and retention of information | 8 |
| 2608 | Relationship to other Federal laws | 9 |
| 2609 | Research, development, collection, dissemination, and utilization of information | 10 |
| 2610 | Inspections and subpoenas | 11 |
| 2611 | Exports | 12 |
| 2612 | Entry into customs territory of the United States | 13 |
| 2613 | Confidential information | 14 |
| 2614 | Prohibited acts | 15 |
| 2615 | Penalties | 16 |
| 2616 | Specific enforcement and seizure | 17 |
| 2617 | Preemption | 18 |
| 2618 | Judicial review | 19 |
| 2619 | Citizens’ civil actions | 20 |
| 2620 | Citizens’ petitions | 21 |
| 2621 | National defense waiver | 22 |
| 2622 | Employee protection | 23 |
| 2623 | Employment effects | 24 |
| 2624 | Studies | 25 |
| 2625 | Administration | 26 |
| 2626 | Development and evaluation of test methods | 27 |
| 2627 | State programs | 28 |
| 2628 | Authorization of appropriations | 29 |
| 2629 | Annual report | 30 |
| Subchapter II Asbestos Hazard Emergency Response |
| 2641 | Congressional findings and purpose | 201 |
| 2642 | Definitions | 202 |
| 2643 | EPA regulations | 203 |
| 2644 | Requirements if EPA fails to promulgate regulations | 204 |
| 2645 | Submission to State Governor | 205 |
| 2646 | Contractor and laboratory accreditation | 206 |
| 2647 | Enforcement | 207 |
| 2648 | Emergency authority | 208 |
| 2649 | State and Federal Law | 209 |
| 2650 | Asbestos contractors and local educational agencies | 210 |
| 2651 | Public protection | 211 |
| 2652 | Asbestos Ombudsman | 212 |
| 2653 | EPA Study of asbestos-containing material in public buildings | 213 |
| 2654 | Transition rules | 214 |
| 2655 | Worker protection | 215 |
| 2656 | Worker protection | 216 |
| Subchapter III Indoor Radon Abatement |
| 2661 | National goal | 301 |
| 2662 | Definitions | 302 |
| 2663 | EPA’s citizen’s guide | 303 |
| 2664 | Model construction standards and techniques | 304 |
| 2665 | Technical assistance to States for radon programs | 305 |
| 2666 | Grant assistance to States for radon programs | 306 |
| 2667 | Radon in schools | 307 |
| 2668 | Regional radon training centers | 308 |
| 2669 | Study of radon in federal buildings | 309 |
| 2670 | Regulations | 310 |
| 2671 | Additional authorizations | 311 |
| Subchapter IV Lead Exposure Reduction |
| 2681 | Definitions | 401 |
| 2682 | Lead-based paint activities training and certification | 402 |
| 2683 | Identification of dangerous levels of lead | 403 |
| 2684 | Authorized State programs | 404 |
| 2685 | Lead abatement and measurement | 405 |
| 2686 | Lead hazard information pamphlet | 406 |
| 2687 | Regulations | 407 |
| 2688 | Control of lead-based paint at Federal facilities | 408 |
| 2689 | Prohibited acts | 409 |
| 2690 | Relationship to other Federal law | 410 |
| 2691 | General provisions relating to administrative proceedings | 411 |
| 2692 | Authorization of appropriations | 412 |
| Subchapter V Healthy High-Performance Schools |
| 2695 | Grants for healthy school environments | 501 |
| 2695a | Model guidelines for siting of school facilities | 502 |
| 2695b | Public outreach | 503 |
| 2695c | Environmental health program | 504 |
| 2695d | Authorization of appropriations | 505 |
| Subchapter VI Formaldehyde Standards for Composite Wood Products |
| 2697 | Formaldehyde standards | 601 |
Regulations related to TSCA
- Most regulations implementing the TSCA statutes can be found at 40 CFR Parts 700 to 799.
Most regulations implementing the Toxic Substances Control Act (TSCA) statutes can be found at 40 CFR Parts 700 to 799. These regulations are listed below and enforced by the Environmental Protection Agency (EPA):
| CFR | CFR name | Authorized by U.S. Code(s) |
|---|
| 40 CFR 700 | General | 15 U.S.C. 2625 and 2665 and 44 U.S.C. 3504 |
| 40 CFR 702 | General practices and procedures | 15 U.S.C. 2605 and 2619 |
| 40 CFR 703 | Confidentiality claims | 15 U.S.C. 2613 |
| 40 CFR 704 | Reporting and recordkeeping requirements | 15 U.S.C. 2607(a) |
| 40 CFR 705 | Reporting and recordkeeping requirements for certain per- and polyfluoroalkyl substances | 15 U.S.C. 2607(a)(7) |
| 40 CFR 707 | Chemical imports and exports | 15 U.S.C. 2611(b) and 2612 |
| 40 CFR 710 | Compilation of the TSCA chemical substance inventory | 15 U.S.C. 2607(a) and (b) |
| 40 CFR 711 | TSCA chemical data reporting requirements | 15 U.S.C. 2607(a) and (b) |
| 40 CFR 712 | Chemical information rules | 15 U.S.C. 2607(a) and (b) |
| 40 CFR 713 | Reporting requirements for the TSCA inventory of mercury supply, use, and trade | 15 U.S.C. 2607(b)(10)(D) |
| 40 CFR 716 | Health and safety data reporting | 15 U.S.C. 2607(d) |
| 40 CFR 717 | Records and reports of allegations that chemical substances cause significant adverse reactions to health or the environment | 15 U.S.C. 2607(c) |
| 40 CFR 720 | Premanufacture notification | 15 U.S.C. 2604, 2607, and 2613 |
| 40 CFR 721 | Significant new uses of chemical substances | 15 U.S.C. 2604, 2607, and 2625(c) |
| 40 CFR 723 | Premanufacture notification exemptions | 15 U.S.C. 2604 |
| 40 CFR 725 | Reporting requirements and review processes for microorganisms | 15 U.S.C. 2604, 2607, 2613, and 2625 |
| 40 CFR 745 | Lead-based paint poisoning prevention in certain residential structures | 15 U.S.C. 2605, 2607, 2681-2692 and 42 U.S.C. 4852d |
| 40 CFR 747 | Metalworking fluids | 15 U.S.C. 2604 and 2605 |
| 40 CFR 749 | Water treatment chemicals | 15 U.S.C. 2605 and 2607 |
| 40 CFR 750 | Procedures for rulemaking under Section 6 of the Toxic Substances Control Act | 15 U.S.C. 2605 |
| 40 CFR 751 | Regulation of certain chemical substances and mixtures under Section 6 of the Toxic Substances Control Act | 15 U.S.C. 2605 and 2625(I)(4) |
| 40 CFR 761 | Polychlorinated biphenyls (PCBs) manufacturing, processing, distribution in commerce, and use prohibitions | 15 U.S.C. 2605, 2607, 2611, 2614, and 2616 |
| 40 CFR 763 | Asbestos | 15 U.S.C. 2605, 2607(c), 2643, and 2646 |
| 40 CFR 766 | Dibenzo-para-doxins/Dibenzofurans | 15 U.S.C. 2605, 2607(c), 2643, and 2646 |
| 40 CFR 767-769 | Reserved | --- |
| 40 CFR 770 | Formaldehyde standards for composite wood products | 15 U.S.C. 2603 and 2607 |
| 40 CFR 771-789 | Reserved | --- |
| 40 CFR 790 | Procedures governing testing consent agreements and test rules | 15 U.S.C. 2603 |
| 40 CFR 791 | Data reimbursement | 15 U.S.C. 2603 and 2607 |
| 40 CFR 792 | Good laboratory practice standards | 15 U.S.C. 2603 |
| 40 CFR 795 | Provisional test guidelines | 15 U.S.C. 2603 |
| 40 CFR 796 | Chemical fate testing guidelines | 15 U.S.C. 2603 |
| 40 CFR 797 | Environmental effects testing guidelines | 15 U.S.C. 2603 |
| 40 CFR 798 | Health effects testing guidelines | 15 U.S.C. 2603 |
| 40 CFR 799 | Identification of specific chemical substance mixture testing requirements | 15 U.S.C. 2603, 2611, and 2625 |
Other regulations implementing the Toxic Substances Control Act (TSCA) statutes can be found at 40 CFR 2.306, “Special rules governing certain information obtained under the Toxic Substances Control Act.” That section is authorized by 15 U.S.C 2613.
While EPA has import regulations at 40 CFR 707, the U.S. Customs and Border Protection (CBP) also has import regulations implementing TSCA section 13. The CBP regulations are found at:
| CFR | CFR name | Authorized by U.S. Code(s) |
|---|
| 19 CFR 12.118 | Toxic Substances Control Act | 5 U.S.C. 301, 15 U.S.C. 2601 et seq., and 19 U.S.C. 66, 1202 (General Note 3(i), Harmonized Tariff Schedule of the United States (HTSUS)), and 1624 |
| 19 CFR 12.119 | Scope |
| 19 CFR 12.120 | Definitions |
| 19 CFR 12.121 | Reporting requirements |
| 19 CFR 12.122 | Definition of certain shipments |
| 19 CFR 12.123 | Procedure after detention |
| 19 CFR 12.124 | Time limitations and extensions |
| 19 CFR 12.125 | Notice of exportation |
| 19 CFR 12.126 | Notice of abandonment |
| 19 CFR 12.127 | Decision to store or dispose |
TSCA Reporting and Recordkeeping Requirements for Perfluoroalkyl and Polyfluoroalkyl Substances
The EPA has finalized reporting and recordkeeping requirements for Per- and Polyfluoroalkyl Substances (PFAS) under TSCA Section 8(a)(7). In accordance with obligations under TSCA, as amended by the National Defense Authorization Act for Fiscal Year 2020, EPA is requiring any person that manufactures (including import) or has manufactured (including imported) PFAS or PFAS-containing articles in any year since January 1, 2011, to electronically report information regarding PFAS uses, production volumes, disposal, exposures, and hazards. In addition to fulfilling statutory obligations under TSCA, this rule enables EPA to better characterize the sources and quantities of manufactured PFAS in the United States. Any entities, including small entities, that have manufactured (including imported) PFAS in any year since 2011 will need to report their data to EPA through Central Data Exchange (CDX). When the submission period begins, manufacturers will have six months to provide their data. Small manufacturers (as defined at 40 CFR 704.3) whose reporting obligations under this rule are exclusively from imported articles will have an additional six months to report PFAS to EPA.
History
- The CEQ highlighted concerns with risks from metals, metal compounds, and synthetic organic chemicals.
- Five titles have been added to TSCA to address specific concerns.
- The LCSA broadly amended EPA’s information gathering, chemical evaluation, and regulatory authorities under TSCA and provided additional procedures and standards for confidential treatment or disclosure of information submitted to EPA under TSCA.
In 1971, the Council on Environmental Quality (CEQ) developed a legislative proposal for coping with the increasing problems of toxic substances. CEQ’s report, “Toxic Substances,” defined a need for comprehensive legislation to identify and control chemicals whose manufacture, processing, distribution, use, and/or disposal was potentially dangerous and not adequately regulated under other environmental statutes.
The CEQ highlighted concerns with risks from metals (e.g., lead, cadmium, mercury, and vanadium), metal compounds, and synthetic organic chemicals (e.g., polychlorinated biphenyls (PCBs), nitrilotriacetic acid, orthonitrochlorobenzene). The Council also noted that pollution control and consumer or occupational safety statutes in effect at the time limited the federal government to controlling pollution at the end of the chemical lifecycle or restricting chemicals that have specific uses (e.g., pesticides and food).
The House and Senate each passed bills in 1972 and 1973, but controversies over the scope of chemical screening prior to commercial production and distribution, level of costs, and the relationship to other regulatory laws stalled final action. However, episodes of environmental contamination — including contamination of the Hudson River and other waterways by PCBs, the threat of stratospheric ozone depletion from chlorofluorocarbon (CFC) emissions, and contamination of agricultural produce by polybrominated biphenyls (PBBs) in the state of Michigan — together with more exact estimates of the costs of imposing toxic substances controls, opened the way for final passage of the legislation.
After five years of public hearings and debate, Congress enacted the Toxic Substances Control Act (TSCA) in the fall of 1976, and President Ford signed it into law on October 11, 1976. The law established new requirements for the Environmental Protection Agency (EPA) to identify and regulate chemicals in U.S. commerce that present an “unreasonable risk of injury to health or the environment” or an imminent hazard. The original legislation included a single title, which has since been designated Title I, Control of Toxic Substances.
Authorization for appropriations for these activities and a state grant program for control of toxic substances in the environment expired on September 30, 1983; although, appropriations for these programs have continued.
Subsequently, five titles have been added to TSCA to address specific concerns — asbestos in 1986 (Title II, Public Law 99-519), radon in 1988 (Title III, Public Law 100-551), lead in 1992 (Title IV, Public Law 102-550), schools in 2007 (Title V, Public Law 110-140), and formaldehyde in 2010 (Title VI, Public Law 111-199). The additional titles did not amend the core chemical evaluation and regulatory program under Title I. Specifically,
- Title II directs EPA to set standards for asbestos mitigation in schools and requires asbestos contractors to be trained and certified.
- Title III directs EPA to provide technical assistance to states that choose to support radon monitoring and control.
- Title IV provides similar assistance with respect to abatement of lead-based paint hazards.
- Title V addresses environmental issues at schools, including energy efficiency.
- Title VI establishes limits on emissions of formaldehyde from composite wood products.
In October 2008, Congress amended TSCA Title I when it enacted the Mercury Export Ban Act of 2008. It prohibits certain activities with respect to elemental mercury.
In June 2016, President Obama signed into law the Frank R. Lautenberg Chemical Safety for the 21st Century Act, or LCSA, which amended Title I of TSCA, due, in part, to long-standing concerns that EPA lacked sufficient authority to obtain information and regulate chemicals that present unreasonable risks. The LCSA broadly amended EPA’s information gathering, chemical evaluation, and regulatory authorities under TSCA and provided additional procedures and standards for confidential treatment or disclosure of information submitted to EPA under TSCA. To supplement funding provided for TSCA implementation, the LCSA expanded EPA’s authority to collect fees from chemical manufacturers and processors to partially defray the costs of conducting risk evaluations.
The table below gives a bird’s eye view of the enactment and amendment of TSCA, which is codified at 15 U.S. Code 2601 to 2697:
| Year | Act | Public Law (P.L.) |
|---|
| 1976 | Toxic Substances Control Act | P.L. 94-469 |
| 1986 | Asbestos Hazard Emergency Response Act | P.L. 99-519 |
| 1988 | Radon Program Development Act | P.L. 100-551 |
| 1990 | Radon Measurement | P.L. 101-508 |
| 1990 | Asbestos School Hazard Abatement Reauthorization Act | P.L. 101-637 |
| 1992 | Residential Lead-Based Paint Hazard Reduction Act | P.L. 102-550 |
| 2007 | Energy Independence and Security Act, subtitle E - Healthy High-Performance Schools | P.L. 110-140 |
| 2008 | Mercury Export Ban Act | P.L. 110-414 |
| 2010 | Formaldehyde Standards for Composite Wood Products Act | P.L. 111-199 |
| 2016 | Frank R. Lautenberg Chemical Safety for the 21st Century Act | P.L. 114-182 |
| 2019 | National Defense Authorization Act for Fiscal Year 2020 | P.L. 116–92 (see section 7351) |
Scope
- TSCA enables EPA to require companies to test selected existing chemicals for toxic effects.
- TSCA also requires EPA to review most new chemicals before they are manufactured.
The Environmental Protection Agency (EPA) has broad authority to issue regulations to gather health/safety and exposure information on, require testing of, and control exposure to chemical substances and mixtures. However, that authority is dictated by the scope of the Toxic Substances Control Act (TSCA). In other words, EPA’s TSCA authority has limits on the activities and chemicals that are covered and the extent to which the agency can regulate them.
To ensure wise and informed decision-making by the government, TSCA gives EPA authority to gather certain kinds of basic information on chemical risks from those who manufacture and process chemicals. The law also enables EPA to require companies to test selected existing chemicals for toxic effects and requires the agency to review most new chemicals before they are manufactured.
To prevent unreasonable risks, EPA may select from a broad range of control actions under TSCA, from requiring hazard-warning labels to outright bans on the manufacture or use of especially hazardous chemicals. EPA may regulate a chemical’s unreasonable risks at any stage of its life-cycle — manufacturing, processing, distribution in commerce, use, or disposal.
Covered and excluded substances
- Congress generally excluded from TSCA regulation groups of chemicals where the risks were considered sufficiently addressed under such other laws.
- Articles (i.e., manufactured items) that contain chemical substances subject to TSCA may be regulated by the Act to the extent that those chemical substances present an unreasonable risk or meet other criteria.
The Toxic Substances Control Act (TSCA) applies to chemical substances, which the statute defines broadly to include substances that have a “particular molecular identity.” This includes:
- Any combination of such substances occurring in whole or in part as a result of a chemical reaction or occurring in nature, and
- Any element or uncombined radical.
It is important to note that the term chemical substance also includes microorganisms. A microorganism is defined as “an organism classified, using the five-kingdom classification system of Whittacker, in the kingdoms Monera (or Procaryotae), Protista, Fungi, and the Chlorophyta and the Rhodophyta of the Plantae, and a virus or virus-like particle.”
Exclusions
To avoid redundancy with other federal pollution control and public health laws, Congress generally excluded from regulation groups of chemicals where the risks were considered sufficiently addressed under such other laws. Congress also specifically excluded the following substances without regard to their regulation under other laws:
- Pesticides when manufactured, processed, or distributed in commerce for use as a pesticide;
- Tobacco and tobacco products;
- Certain radioactive materials;
- Firearms (including pistols and revolvers), shells, cartridges, and their components;
- Food (including poultry, meat, and eggs), food additives (including food contact substances), drugs, cosmetics, and medical devices; and
- Mixtures (see note below).
Since the exclusions are generally framed based on the intended uses of a chemical substance (e.g., a pesticide), the same chemical substance may be subject to TSCA and other federal pollution control and public health laws depending on the intended use. For example, the Environmental Protection Agency (EPA) has examined the risks of bisphenol A under TSCA with regard to its use in manufacturing polycarbonate plastics and epoxy resins. However, the use of bisphenol A as a food contact substance is not covered by TSCA based on the statutory exclusion.
Note on mixtures and articles
Although the definition of a chemical substance excludes mixtures, multiple TSCA provisions apply to mixtures. A mixture is defined as:
- Any combination of two or more chemical substances if the combination does not occur in nature and is not, in whole or in part, the result of a chemical reaction; or
- Any combination which occurs, in whole or in part, as a result of a chemical reaction if none of the chemical substances comprising the combination is a new chemical substance and if the combination could have been manufactured for commercial purposes without a chemical reaction at the time the chemical substances comprising the combination were combined.
Generally, a mixture is subject to requirements under TSCA if the requirements that pertain to its constituent chemical substances will not result in adequate evaluation or control of the risks anticipated from the mixture.
Additionally, as a practical matter, articles (i.e., manufactured items) that contain chemical substances subject to TSCA may be regulated by the Act to the extent that those chemical substances present an unreasonable risk or meet other criteria.
Limitations on authority
- EPA must make an unreasonable risk finding or determination before requiring information on, or regulating, a chemical.
- Even if EPA concludes under TSCA that a chemical presents an unreasonable risk, TSCA provides that other federal laws supersede EPA’s authority under TSCA to address unreasonable risks.
In addition to excluding groups of chemicals from the scope of chemicals covered under the Toxic Substances Control Act (TSCA), Congress generally limited the extent to which the Environmental Protection Agency (EPA) may regulate a chemical.
Unreasonable risk threshold
EPA cannot simply regulate a chemical at will. The agency must make an unreasonable risk finding or determination before requiring information on, or regulating, a chemical. The trouble is TSCA does not explicitly define what constitutes an unreasonable risk. In 1991, the U.S. Court of Appeals for the Fifth Circuit held that the “unreasonable risk” standard as originally set forth in TSCA meant that “[i]n evaluating what is ‘unreasonable,’ the EPA is required to consider the costs of any proposed actions and to ‘carry out this chapter in a reasonable and prudent manner [after considering] the environmental, economic, and social impact of any action.’” This interpretation led the court to vacate parts of the 1989 EPA rule that regulated various asbestos uses.
Though the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) did not amend TSCA to explicitly define unreasonable risk, it prohibited EPA from considering cost or nonrisk factors when evaluating risks. However, EPA must consider “reasonably ascertainable economic consequences” and other nonrisk factors when restricting uses of a chemical. Additionally, the LCSA codified existing agency practice to consider risks for “potentially exposed or susceptible subpopulations” when evaluating the risks of a chemical.
Relationship with other federal laws
Even if EPA concludes under TSCA that a chemical presents an unreasonable risk, TSCA provides that other federal laws supersede EPA’s authority under TSCA to address unreasonable risks. If EPA determines that a risk associated with a chemical may be sufficiently eliminated or reduced by actions taken under other federal laws that the agency administers, section 9(b) of the Act requires the agency to use those authorities to protect against the risk unless the agency determines that it is in the public interest to take action under TSCA.
If EPA determines that a chemical presents an unreasonable risk that may be sufficiently prevented or reduced by action taken under a federal law a different federal agency administers, section 9(a) directs EPA to submit to that other agency a report describing the risk and a request for a response. A federal agency that receives such a report from EPA must respond within 90 days (or a shorter period if specified by EPA). If that federal agency issues an order disagreeing with EPA about the unreasonable risk or initiates action to protect against such risk, then EPA may not regulate the chemical under TSCA.
TSCA inventory
- EPA must compile and keep current an Inventory of Chemical Substances in Commerce, which is a listing of chemical substances manufactured, imported, and processed for commercial purposes in the U.S.
- Chemical substances on the TSCA Inventory include organics, inorganics, polymers, and chemical substances of unknown or variable composition, complex reaction products, and UVCBs.
- EPA’s compilation of the public TSCA Inventory information is updated twice a year to include new and corrected TSCA Inventory chemical listings.
Section 8(b) of the Toxic Substances Control Act (TSCA) requires the Environmental Protection Agency (EPA) to compile and keep current an Inventory of Chemical Substances in Commerce (the TSCA Inventory), which is a published listing of chemical substances manufactured, imported, and processed for commercial purposes in the U.S. Also called “the Inventory,” it plays a central role in the regulation of most industrial chemicals in the U.S.
The initial reporting period by manufacturers, processors, and importers was January to May of 1978 for chemical substances that had been in commerce since January of 1975. The agency subsequently published the first Inventory in 1979. The TSCA Inventory is updated every six months and has continued to grow. It now lists more than 86,000 chemicals.
Since compiling the initial TSCA Inventory, TSCA section 5 requires that any person who proposes to manufacture (which includes import) a “new chemical,” i.e., a chemical not listed on the TSCA Inventory, must provide a premanufacture notice (PMN) or an exemption application to the agency at least 90 days prior to commencing manufacture of that chemical.
The agency regularly adds new chemical substances that have completed new chemical review requirements pursuant to TSCA section 5(a) and that have been manufactured or processed for non-exempt commercial purpose. EPA maintains the TSCA Inventory as the authoritative list of all the chemical substances reported to the agency for inclusion on the Inventory.
What chemicals are on and not on the TSCA Inventory?
TSCA defines a chemical substance as any organic or inorganic substance of a particular molecular identity, including any combination of these substances occurring in whole or in part as a result of a chemical reaction or occurring in nature, and any element or uncombined radical. Chemicals substances on the Inventory include:
- Organics;
- Inorganics;
- Polymers; and
- Chemical substances of unknown or variable composition, complex reaction products, and biological materials (UVCBs).
Chemical substances not on the Inventory are those with uses not regulated under TSCA. The use of these chemical substances is governed by other U.S. statutes on, for example:
- Pesticides
- Foods and food additives
- Drugs
- Cosmetics
- Tobacco and tobacco products
- Nuclear materials
- Munitions
What does it mean for a chemical to be on the Inventory?
For purposes of regulation under TSCA, if a chemical is on the Inventory, the substance is considered an “existing” chemical substance in U.S. commerce. Any chemical that is not on the Inventory is considered a “new chemical substance.”
In addition to defining whether a specific substance is “new” or “existing,” the Inventory also contains “flags” for those existing chemical substances that are subject to manufacturing or use restrictions.
Background on completeness, accuracy, and legal standing
EPA’s compilation of the public TSCA Inventory information is updated twice a year to include new and corrected TSCA Inventory chemical listings, and it contains none of the chemical identities claimed as confidential. Thus, it is not as complete nor current as the information contained in EPA’s TSCA Master Inventory File, which includes the chemical identities claimed as confidential and is updated continuously as new and corrected information is received by EPA.
Consequently, for the purposes of TSCA compliance, the TSCA Master Inventory File maintained by EPA’s Office of Pollution Prevention and Toxics is the only complete and accurate source that can provide authoritative and conclusive information about which chemical substances are currently included in the TSCA Inventory.
Adding chemicals to the Inventory
- Once a complete NOC is received by EPA, the reported substance is considered to be on the Inventory and becomes an “existing chemical.”
After a premanufacture notice (PMN) review has been completed, the company that submitted the PMN must provide a Notice of Commencement of Manufacture or Import (NOC, EPA Form 7710-56) to the Environmental Protection Agency (EPA) within 30 calendar days of the date the substance is first manufactured or imported for nonexempt commercial purposes.
Once a complete NOC is received by EPA, the reported substance is considered to be on the Inventory and becomes an “existing chemical.” The agency receives approximately 400 NOCs each year, thus the TSCA Inventory changes almost daily.
Substances reported through exemption submissions and exempt uses that are not subject to reporting do not require an NOC and are not added to the Inventory. Examples include:
- Low Volume Exemptions (LVEs),
- Low Release/Low Exposure Exemptions (LoREXs),
- Test Market Exemptions (TMEs),
- Substances used for research and development, and
- Polymers that meet the 1995 Polymer Exemption Rule Amendments.
Accessing the inventory
- EPA provides free access to the non-confidential portion of the TSCA Inventory online.
- EPA provides a database management system version and a generic comma-delimited “CSV” text version of the TSCA Inventory.
- The TSCA Inventory can be searched in multiple ways.
- Someone with a valid commercial need for the EPA to verify if a substance is on the inventory can submit a bona fide notice to obtain a written determination from EPA.
Determining if a chemical is on the Inventory is a critical step before beginning to manufacture (which includes importing) a chemical substance. Section 5 of the Toxic Substances Control Act (TSCA) requires anyone who plans to manufacture a new chemical substance for a non-exempt commercial purpose to provide the Environmental Protection Agency (EPA) with a premanufacture notice (PMN) at least 90 days before initiating the activity.
As part of EPA’s commitment to strengthen the management of chemicals and increase information on chemicals, the agency provides free access to the non-confidential portion of the TSCA Inventory online.
Downloading the non-confidential TSCA Inventory
The non-confidential portion of EPA’s TSCA Inventory is updated approximately every six months. EPA provides a database management system version and a generic comma-delimited “CSV” text version of the non-confidential TSCA Inventory for users to download. Facilities that don’t have the database management system that EPA uses will instead use the CSV file.
Both files are compressed “.zip” files. The .zip files contain the actual data files. With either version, the file contains two tables:
- The TSCAINV … file contains non-confidential chemical substance listings on the TSCA Inventory, as identified by Chemical Abstracts Service (CAS) Registry Number and Chemical Abstracts (CA) Index Name.
- The PMNACC … file contains non-confidential data for the confidential chemical substance listings, as identified by EPA accession number and generic chemical name.
For all files, users may need to adjust formatting, including column widths. Use a basic “find” search function and type in a part of a chemical name or CAS Registry Number to find information.
Alternate access of the non-confidential TSCA Inventory
In addition to downloading a database management system version and a generic comma-delimited “CSV” text version, the non-confidential Toxic Substances Control Act (TSCA) Inventory can be searched in multiple ways:
- Use EPA’s Substance Registry Services (SRS) to search the non-confidential TSCA Inventory:
- Go to Substance Registry Services webpage.
- Select the “search by list” option.
- Type “TSCA Inventory” in the List Name field.
- Click the “filter” button and select “TSCA Inventory – TSCA Inv” from the drop-down list.
- Look at other sources of EPA’s non-confidential TSCA Inventory data such as:
- Government Printing Office (GPO) website no longer provides paper copies of the original 1985 TSCA Inventory publication or the 1990 Supplement; however, it can provide expert assistance in finding and using related U.S. government information.
- Several commercial services provide searches for the non-confidential TSCA Inventory for a fee. None of these is connected to or has a specific endorsement from EPA.
Submitting notices of bona fide intent to manufacture
Someone with a valid commercial need for EPA to verify if a substance is on the inventory can submit a Bona Fide Intent to Manufacture or Import Notice (bona fide notice) to obtain a written determination from EPA.
In a bona fide notice, a submitter must provide specific chemical identification data including:
- Chemical Abstracts (CA) Index name. Proper CA Index names can be obtained from the Chemical Abstracts Service’s Inventory Expert Service;
- Information about the manufacture or importation of the substance;
- Letter of support if some information (e.g. specific chemical identity) has been withheld from the submitter by the supplier;
- Certification of intent to manufacture or import the substance for a commercial purpose; and
- All of the other information required in support of a bona fide notice.
EPA will consider the information submitted in a bona fide notice and, if the agency believes that the submitter has demonstrated a genuine intent to manufacture or import, search the full TSCA Inventory master file and provide a written determination to the submitter on the TSCA Inventory status for the chemical substance.
The e-PMN software enables manufacturers (including importers) of TSCA chemical substances to use the Internet, through EPA’s Central Data Exchange (CDX), to submit TSCA section 5 notices to the Agency. These notices include the bona fide notices.
Data fields and flags in the non-confidential TCSA Inventory
- The non-confidential TSCA Inventory files (TSCAINV and PMNACC) have several data fields and special flags.
The non-confidential TSCA Inventory files (TSCAINV and PMNACC) have several data fields and special flags. The following data fields are provided for each non-confidential chemical substance:
| Field ID | Data | Data must be present? | Multiple values possible? |
|---|
| ACTIVITY | Commercial Activity Status ** | Y | N |
| CASRN | Chemical Abstracts Service (CAS) Registry Number | Y | N |
| casregno | CAS Registry Number without “-” [dashes] | Y | N |
| ChemName | Preferred Chemical Abstracts (CA) Index Name * | Y | N |
| DEF | Chemical Substance Definition * | N | N |
| EXP | Expiration Date | N | N |
| FLAG | EPA TSCA Regulatory Flag ** | N | Y *** |
| ID | Record ID Number | Y | N |
| UID | Unique Identifier | N | N |
| UVCB | UVCB Flag | N | N |
* These data can be greater than 256 characters in length.
** Multiple values are separated by a semicolon and space.
*** Information on EPA TSCA regulatory flags is provided below. |
The following non-confidential data fields are provided for each conventional chemical substance:
| Field ID | Data | Data must be present? | Multiple values possible? |
|---|
| ACCNO | EPA Accession Number | Y | N |
| ACTIVITY | Commercial Activity Status ** | Y | N |
| EXP | Expiration Date | N | N |
| FLAG | EPA TSCA Regulatory Flag ** | N | Y *** |
| GenericName | Generic Name * | Y | N |
| ID | Record ID Number | Y | N |
| PMNNO | PMN Number/Form Number | Y | N |
| UID | Unique Identifier | N | N |
* These data can be greater than 256 characters in length.
** Multiple values are separated by a semicolon and space.
*** Information on EPA TSCA regulatory flags is provided below. |
Special flags are used throughout the TSCA Inventory to identify those substances on the Inventory that are the subject of an EPA rule or order promulgated under TSCA, as well as to indicate types of full or partial exemptions from TSCA reporting requirements. The following flags are used:
- 5E — indicates a substance that is the subject of a TSCA section 5(e) order.
- 5F — indicates a substance that is the subject of a TSCA section 5(f) rule.
- 12C — indicates a substance that is prohibited to be exported from the U.S. under TSCA section 12(c).
- FRI — indicates a polymeric substance containing no free-radical initiator in its Inventory name but is considered to cover the designated polymer made with any free-radical initiator regardless of the amount used.
- PE1 — indicates a polymer that has a number-average molecular weight of greater than or equal to 1,000 daltons and less than 10,000 daltons and that is exempt under the 1995 polymer exemption rule. The polymer’s oligomer content must be less than 10 percent by weight below 500 daltons and less than 25 percent by weight below 1,000 daltons.
- PE2 — indicates a polymer that has a number-average molecular weight of greater than or equal to 10,000 daltons and that is exempt under the 1995 polymer exemption rule. The polymer’s oligomer content must be less than 2 percent by weight below 500 daltons and less than 5 percent by weight below 1,000 daltons.
- PE3 — indicates a polymer that is a polyester and that is exempt under the 1995 polymer exemption rule. The polyester is made only from monomers and reactants included in a specified list that comprises one of the eligibility criteria for the 1995 polymer exemption rule.
- PMN — indicates a commenced PMN substance.
- R — indicates a substance that is the subject of a proposed or final TSCA section 6 risk management rule.
- S — indicates a substance that is identified in a final Significant New Use Rule (SNUR).
- SP — indicates a substance that is identified in a proposed SNUR.
- T — indicates a substance that is the subject of a final TSCA section 4 test rule or order.
- TP — indicates a substance that is the subject of a proposed TSCA section 4 test rule or order.
- XU — indicates a substance exempt from reporting under 40 CFR 711, Chemical Data Reporting.
- Y1 — indicates a polymer that has a number-average molecular weight greater than 1,000 and that was exempt under the 1984 polymer exemption rule.
- Y2 — indicates a polymer that is a polyester and that was exempt under the 1984 polymer exemption rule. The polyester is made only from reactants included in a specified list of low-concern reactants that comprises one of the eligibility criteria for the 1984 polymer exemption rule.
Correcting the Inventory
- TSCA Chemical Substance Inventory Reporting Form C provides the chemical industry a method to submit requests to EPA’s OPPT for correcting misreported chemical identities of substances listed on the Inventory.
Shortly after the first Toxic Substances Control Act (TSCA) Inventory was published in 1979, the Environmental Protection Agency (EPA) determined that reported substances may have been unintentionally misidentified as a result of simple typographical errors, the misidentification of substances, or the lack of sufficient technical or analytical capabilities to characterize the exact chemical substances.
TSCA Chemical Substance Inventory Reporting Form C (EPA Form 7710-3C) provides the chemical industry with a method to submit requests to EPA’s Office of Pollution Prevention and Toxics (OPPT) for correcting misreported chemical identities of substances listed on the Inventory. Such requests pertain only to errors discovered in the original submissions to the Inventory when the Inventory was first established in 1979.
The correction mechanism allows the submitter to add the correct substance to the Inventory without having to file a premanufacture notice (PMN) under TSCA section 5. The correction mechanism ensures the accuracy of the Inventory without imposing an unreasonable burden on the chemical industry. Without the Inventory correction mechanism, a submitter would have to file a PMN to place the correct chemical substance on the Inventory whenever finding that the previously reported substance was misidentified.
Historical notes
- TSCA requires EPA to designate chemical substances on the TSCA Chemical Substance Inventory as either “active” or “inactive” in U.S. commerce.
- TSCA requires EPA to establish a rule on CBI claims for specific chemical identities for chemicals reported as “active” in response to the TSCA Inventory Notification Requirements Rule.
The initial reporting period by manufacturers, processors and importers was January to May of 1978 for chemical substances that had been in commerce since January of 1975. The Environmental Protection Agency (EPA) subsequently compiled and published the Toxic Substances Control Act (TSCA) Inventory in 1979. A second version was published in 1982.
Today, the TSCA Inventory is updated every six months, if possible. It is noteworthy that three actions in modern history have shaped the latest TSCA Inventory. They include the:
- TSCA Inventory Notification (Active-Inactive) rule (also called the Inventory Reset rule), August 11, 2017;
- Procedures for Review of Confidential Business Information (CBI) Claims for the Identity of Chemicals on the Toxic Substances Control Act Inventory rule, March 6, 2020;
- EPA Extends Notification Deadline for Updates to Confidential Status of Chemicals on the TSCA Inventory; EPA announcement, May 14, 2021; and
- CBI Claims Under the Toxic Substances Control Act (TSCA) final rule, June 7, 2023.
Inventory reset rule
TSCA, as amended by the Frank R. Lautenberg Chemical Safety for the 21st Century Act, requires EPA to designate chemical substances on the TSCA Chemical Substance Inventory as either “active” or “inactive” in U.S. commerce.
To accomplish that, EPA finalized a rule requiring industry reporting of chemicals manufactured (including imported) or processed in the U.S. over a 10-year period ending on June 21, 2016. This reporting was completed on October 5, 2018, and was used to identify chemical substances on the TSCA Inventory as active or inactive in U.S. commerce.
EPA includes the active and inactive designations on the TSCA Inventory and as part of its regular publications of the Inventory. Starting August 5, 2019, manufacturers and processors were required to notify EPA before reintroducing inactive substances into U.S. commerce. Manufacturers and processors can notify EPA via a Notice of Activity Form B, found in EPA’s Central Data Exchange (CDX). Upon receiving such notification, EPA will change the commercial activity designation of inactive substances to active.
On October 13, 2021, EPA remarked that the Inventory contained 86,607 chemicals of which 41,953 were active in U.S. commerce. Note that the August 2023 update of the Inventory contains 86,718 chemicals, of which 42,242 are active.
Review of CBI claims rule
Section 8(b) of TSCA requires EPA to establish a rule on confidential business information (CBI) claims for specific chemical identities for chemicals reported as “active” in U.S. commerce in response to the TSCA Inventory Notification (Active-Inactive) Requirements Rule.
On March 6, 2020, EPA finalized a rule on the procedures for companies to substantiate their CBI claims for the specific chemical identities of substances on the TSCA inventory, as well as the plan for how the agency will review the claims, the timeframes for EPA to complete reviews, and the annual posting of results.
The final rule also included two additional questions on reverse engineering that manufacturers and processors will be required to answer to substantiate their specific chemical identity CBI claims. These two questions were added as part of the agency’s response to a court-ordered remand of part of the TSCA Inventory Notification (Active-Inactive) Requirements Rule. The rule amended existing sections of 40 CFR 710 and added 40 CFR 710 Subpart C.
Updates to confidential status of chemicals
In April 2021, EPA posted a list of 390 chemicals by accession number expected to lose their confidential chemical identity status and move to the public portion of the TSCA Inventory and gave stakeholders a deadline of May 14, 2021, to notify the agency of any errors on the list. In response to industry stakeholder requests for additional time to review this list, EPA extended the notification deadline to June 30, 2021.
EPA planned to declassify the specific identities of these chemicals because they were reported as non-confidential by one or more manufacturers during the 2012, 2016, and/or 2020 Chemical Data Reporting (CDR) reporting periods — meaning that at least one manufacturer did not request that each of these chemical identities be kept confidential, effectively saying it is not a secret that the chemical is in U.S commerce.
On October 13, 2021, EPA announced that the agency updated the confidential status of 377 chemical identities and would include these chemical identities on the winter 2022 TSCA Inventory. The 377 declassified specific chemical identities were made available with the October 13, 2021, announcement.
Confidential business information claims under TSCA final rule
EPA finalized new and amended requirements on June 7, 2023, concerning the assertion and treatment of CBI claims for information reported to or otherwise obtained by EPA under TSCA. The rule took effect August 7, 2023. Amendments to TSCA in 2016 included many new provisions concerning the assertion, agency review, and treatment of confidentiality claims. This rule:
- Finalized procedures for submitting such claims in TSCA submissions;
- Addressed issues such as substantiation requirements, exemptions, electronic reporting enhancements (including expanding electronic reporting requirements), maintenance or withdrawal of confidentiality claims, and provisions in current rules that are inconsistent with amended TSCA; and
- Addressed EPA procedures for reviewing and communicating with TSCA submitters about confidentiality claims.
New chemicals review
- TSCA requires anyone who plans to manufacture (including import) a new chemical substance for a non-exempt commercial purpose to provide EPA with notice 90 days before initiating the activity.
Section 5 of the Toxic Substances Control Act (TSCA) gives the Environmental Protection Agency (EPA) the authority to require anyone who plans to manufacture (including import) a new chemical substance for a non-exempt commercial purpose to provide EPA with a premanufacture notice (PMN) or an exemption application at least 90 days before initiating the activity.
It should be noted that EPA considers certain genetically engineered microorganisms to be chemical substances for purposes of the notification requirements found in TSCA section 5. However, the 90-day notice for microorganisms is a Microbial Commercial Activity Notice (MCAN).
To determine if a substance is a “new” chemical, consult EPA’s TSCA Chemical Substance Inventory — commonly referred to as the Inventory, which lists “existing” substances. Any chemical that is not on the Inventory is considered a “new chemical substance.” Prior to manufacture (including import) of a new chemical for general commercial use, a notice must be filed with EPA under section 5 of TSCA.
Premanufacture notice (PMN)
- “Existing” chemicals are chemicals that were already in commerce when TSCA was enacted in 1976 or chemicals that have undergone PMN review and are listed on the TSCA Inventory.
Under Toxic Substances Control Act (TSCA) section 5, chemical manufacturers (or importers) must submit a notice to the Environmental Protection Agency (EPA) at least 90 days prior to the initial commercial manufacture (or import) of a new chemical substance, unless the chemical substance meets certain criteria for an exemption from notification. This notification is known as a premanufacture notice (PMN). According to EPA, the agency has received more than 40,000 PMNs since the enactment of TSCA in 1976.
Introduction
Mandated by section 5 of TSCA, EPA’s New Chemicals program helps manage the potential risk to human health and the environment from chemicals new to the marketplace. The program functions as a “gatekeeper” that can identify conditions, up to and including a ban on production, to be placed on the use of a new chemical before it is entered into commerce.
For purposes of regulation under TSCA, if a chemical is on the TSCA Inventory, the substance is considered an “existing” chemical substance in U.S. commerce. “Existing” chemicals are chemicals that were already in commerce when TSCA was enacted in 1976 or chemicals that have undergone PMN review and are listed on the TSCA Inventory.
Who must submit a PMN?
Section 5 of TSCA requires anyone who plans to manufacture (including import) a new chemical substance for a non-exempt commercial purpose to provide EPA with notice before initiating the activity. This notice is known as a PMN. Refer to the chart below that details steps for determining whether a submission is required on a chemical substance.

Exclusions and exemptions
- Excluded product categories from PMN reporting include tobacco and certain tobacco products, nuclear materials, munitions, foods, food additives, drugs, cosmetics, and substances used solely as pesticides.
- Persons may apply for an exemption from the requirements of TSCA section 5 for test-marketing purposes and research and development purposes.
- EPA has promulgated four rules under TSCA section 5(h)(4) for traditional chemical substance exemptions and three rules for exemptions specific to microbial products of biotechnology.
Some new chemical substances are not subject to premanufacture notice (PMN) reporting. These substances are:
- Excluded from Toxic Substances Control Act (TSCA) reporting, or
- Exempt from all or part of PMN reporting because the Environmental Protection Agency (EPA) has determined that they do not warrant review or require only a short review.
Excluded product categories
EPA does not review new substances in the following product categories, which are excluded from TSCA authority at section 3(2)(B):
- Tobacco and certain tobacco products,
- Nuclear materials,
- Munitions,
- Foods, food additives, drugs, cosmetics, and
- Substances used solely as pesticides.
These new substances fall under the jurisdiction of other federal laws and are reviewed by other federal programs. Substances used solely as pesticides are reviewed by a separate EPA Pesticides Program. In addition, the following are excluded from PMN reporting under certain conditions:
- Naturally-occurring materials,
- Products of incidental reactions,
- Products of end-use reactions,
- Mixtures (but not mixture components),
- Impurities,
- By-products,
- Substances manufactured solely for export,
- Nonisolated intermediates, and
- Substances formed during the manufacture of an article.
Limited exemptions
Exemptions reduce reporting requirements, thereby providing relief to submitters from the burdens of the full PMN reporting requirements. EPA has limited or no reporting requirements for new chemical substances in the following cases:
- Test marketing exemption — Under TSCA section 5(h)(1), persons may apply for an exemption from the requirements of TSCA section 5 for test-marketing purposes. EPA may grant the exemption if it finds that the test-marketing activities described by the applicant will not present an unreasonable risk of injury to health or the environment including an unreasonable risk to a potentially exposed or susceptible subpopulation identified by the EPA Administrator for the specific conditions of use identified in the application. The applicant must provide the information necessary to make this finding, and EPA must grant or deny the exemption within 45 days. If EPA grants the exemption, it may impose appropriate restrictions on the test-marketing activities.
- Research and development exemption — TSCA section 5(h)(3) exempts from PMN reporting small quantities of chemical substances manufactured only for research and development purposes. Persons using this exemption must have their research overseen by a technically qualified individual and must notify any person involved in the research of any risk. Small quantities of genetically modified microorganisms manufactured solely for research and development purposes are also exempt when additional criteria are met as described in 40 CFR 725.235 (activities conducted inside a structure) and 40 CFR 725.238 and 239 (activities conducted outside a structure).
- TSCA section 5(h)(4) exemptions — TSCA section 5(h)(4) authorizes EPA to exempt any person from the provisions of TSCA section 5 if EPA determines, upon application and by rule, that the chemical substance will not present an unreasonable risk of injury to health or the environment when manufactured, processed, distributed, used, or disposed of under the exemption. To date EPA has promulgated four rules under this section for traditional chemical substance exemptions and three rules for exemptions specific to microbial products of biotechnology:
- Low volume exemption (LVE) — This exemption applies to substances manufactured in quantities of 10,000 kilograms or less per year. Submitters may request that EPA evaluate their exemption at a lower production volume level, to which the submitter would be legally bound.
- Low release / Low exposure (LoREX) — This exemption applies to certain chemical substances that meet strict human exposure and environmental release criteria to ensure that these substances will not present an unreasonable risk.
- Polymer exemption — This exemption applies to polymers that comply with certain chemical characterizations and that therefore will not present an unreasonable risk of injury to health or the environment. Note that TSCA section 5 polymer exemption notices must be submitted electronically, per 40 CFR 723.250.
- Instant photographic film articles exemption — This exemption applies to chemical substances used in or for the manufacture or processing of instant photographic and peel-apart film articles.
- TSCA experimental release application (TERA) — This exemption applies to research and development activities that result in intentional environmental releases of intergeneric microorganisms. EPA may grant the exemption if it finds that the activities described by the applicant will not present an unreasonable risk of injury to health or the environment. The applicant must provide the information necessary to make this finding, and EPA must grant or deny the exemption within 60 days. If EPA grants the exemption, it may impose appropriate restrictions on the activities described in the notice.
- Tier I exemption — This exemption applies to certain microorganisms subject to physical containment and control technologies. EPA has developed specific criteria for the host microorganism, introduced genetic material, and containment technology to ensure that the microorganism will not present an unreasonable risk.
- Tier II exemption — This exemption applies to the same microorganisms subject to a Tier I exemption without specified physical containment and control technologies. EPA may grant the exemption if it finds that the physical containment and control technologies described by the applicant will control releases of and exposure to the microorganism such that the microorganism will not present an unreasonable risk of injury to health or the environment. The applicant must provide the information necessary to make this finding, and EPA must grant or deny the exemption within 45 days. If EPA grants the exemption, it may impose appropriate restrictions on the activities described in the notice.
What about imports and exports?
- Importers of new chemicals must certify that all chemical substances in the shipment comply with all applicable rules or orders under TSCA.
Imports
For the purposes of the Toxic Substances Control Act (TSCA), the term manufacture includes import. This means that imports of new chemicals are subject to TSCA’s section 5(a) requirement to submit a premanufacture notice (PMN) to the Environmental Protection Agency (EPA) at least 90 days before importing a new chemical. In addition, under TSCA section 13, importers of new chemicals must certify that all chemical substances in the shipment comply with all applicable rules or orders under TSCA.
Exports
Under TSCA section 12(b), manufacturers must notify EPA if they intend to export a chemical substance or mixture for which regulatory action has been taken under TSCA sections 4, 5, 6, or 7 (i.e., submission of data is required, an order has been issued, or a rule has been proposed, etc.).
However, according to 40 CFR 720.30(e)(2), any new chemical substance manufactured solely for export is not subject to notification requirements if the manufacturer knows that the person to whom the substance is being distributed intends to export or process it solely for export as defined in 40 CFR 721.3.
PMN submission process
- Submitters must use EPA’s CDX to submit TSCA section 5 notices and supporting documents electronically.
- Six types of submitters may register with CDX.
Submitters must use the Environmental Protection Agency’s (EPA’s) Central Data Exchange (CDX) to submit Toxic Substances Control Act (TSCA) section 5 notices and supporting documents electronically.
Premanufacture notice (PMN) submissions must be made on EPA Form 7710-25 using the electronic PMN software (e-PMN). The e-PMN software enables manufacturers (including importers) of TSCA chemical substances to use the Internet, through EPA’s CDX, to submit TSCA section 5 notices to the EPA. These notices include, but are not limited to:
- PMNs,
- Bona Fide Notices,
- Biotechnology Notices for genetically modified microorganisms;
- Notices of Commencement of Manufacture (including Import) (NOCs), and
- Support documents for section 5 notices (e.g., correspondence, requests for suspensions of the notice review period, amendments, letters of withdrawal, Transfer of Ownership, and test data).
The PMN form should also be used to submit:
- Test Market Exemption applications (TMEAs),
- Low Volume Exemption (LVE) notices and modifications,
- Low Exposure / Low Release (LoREX) exemption notices and modifications, and
- Mock PMNs.
In order to submit documents electronically to EPA, fill out PMN forms using the e-PMN software and submit through the CDX. To access CDX, pre-register with CDX and be approved by EPA. In order to access and use the new e-PMN software, all users must register with CDX for the Chemical Safety and Pesticide Programs (CSPP) program service.
Six types of submitters may register with CDX:
- Primary Authorized Officials,
- Primary Agents/Consultants,
- Primary Supports,
- Secondary Authorized Officials,
- Secondary Agents/Consultants, and
- Secondary Supports.
If there are technical issues or problems regarding the e-PMN software or CDX registration, contact the CDX Help Desk at helpdesk@epacdx.net or (888) 890-1995. International callers who wish to contact the Help Desk should call (970) 494-5500.
Note that the previous version of e-PMN software called eTSCA software can no longer be used to submit directly/electronically via CDX.
What information to submit
- If confidential substances are involved and require a third-party letter of support, then a bona fide notice or PMN submitter must keep in mind that all supporting material must be received by EPA for a bona fide notice or PMN to be considered complete.
- Information frequently requested by the New Chemicals Program for groups of chemicals with common characteristics is identified in the New Chemical Categories document.
The e-PMN software will prompt submitters for the specific information required. In general, premanufacture notice (PMN) submissions require all available data on:
- Chemical identity, structure, and formula;
- Process diagram and description;
- Production volume;
- By-products and impurities;
- Intended use;
- Environmental release;
- Disposal practices; and
- Human exposure.
If the identity of some reactants for substance synthesis, or of the substance itself, is unknown to the manufacturer, a letter of support can be used to provide the Environmental Protection Agency (EPA) with full identity information.
EPA requires that the following information be submitted with the PMN:
- All existing health and environmental data in the possession of the submitter, parent company, or affiliates;
- A description of any existing data known to or reasonably ascertainable by the submitter; and
- Any safety data sheet (SDS) that is already developed for the new chemical substance, including draft SDSs (this requirement would not require submitters to develop an SDS but to submit an already-developed SDS to the extent the SDS is known or reasonably ascertainable by the submitter).
The New Chemicals Program can require submission of any additional data, including development of new data through testing, when the information included with the PMN, coupled with that available to EPA risk reviewers from internal archives, is not adequate to permit a reasoned evaluation of the health and environmental effects of a chemical substance.
Letter of support
Specific chemical identity information is required for Bona Fide Intent to Manufacture (including Import) Notices, PMNs, and other purposes under the Toxic Substances Control Act (TSCA). EPA must be notified of any confidential chemical identity information (e.g., a reactant only known by a trade name is used in the manufacture of a chemical substance that is the subject of a bona fide notice or PMN).
Information that has been withheld from the submitter by a third party should be submitted directly to EPA by that third party (e.g., usually a domestic or foreign supplier or manufacturer). In its letter of support, the third party must provide chemical identity information for the confidential substance as specified in the regulation at 40 CFR 720.45(a).
If confidential substances are involved and require a third-party letter of support, then a bona fide notice or PMN submitter must keep in mind that all supporting material must be received by EPA for a bona fide notice or PMN to be considered complete. A submitter should also have an agreement with its supplier to ensure being informed of any changes in composition that can change the chemical identity of the confidential substance.
Manufacturers and importers whose reportable substances are manufactured with branded materials that have confidential components should take steps to be informed in a timely manner if the branded materials change in composition. EPA does not use brand names in listing substances on the TSCA Inventory, in part because branded materials formulations can change and in part because the TSCA Inventory identifies and lists specific chemical substances and not formulations.
New chemical categories
Information frequently requested by the New Chemicals Program for groups of chemicals with common characteristics is identified in the New Chemical Categories document. All of this information is considered by EPA risk assessors during the notice review process.
Avoiding incomplete PMNs and notifying EPA of new data
- Failure to include the specific, required information may result in EPA declaring the PMN submission incomplete and suspending its review.
- If new information becomes available within five days of the end of the review period, the submitter must inform the EPA contact by telephone.
Avoid an incomplete premanufacture notice (PMN)
Submitters are encouraged to carefully review Environmental Protection Agency (EPA) regulations for new chemical submissions, including 40 CFR 720.45 and 720.50, which provide the information that must be included in the notice form. The accuracy and completeness of the notice form improves timeliness of the new chemical review by reducing or eliminating the need to request additional information and revisions of the risk assessment.
Cases are reviewed for administrative completeness and to ensure that all required technical information concerning chemical identity has been submitted, without errors. In striving to focus attention on timely review of complete and robust notices, EPA has issued regulations at 40 CFR 720.45 and 720.50, which describe the specific information that must be included in the notice form.
EPA will determine if the notice is required, is incomplete and/or contains errors pursuant to regulations at 40 CFR 720.62 and 40 CFR 720.65. Failure to include the specific information required by the regulations may result in EPA declaring the submission incomplete and suspending its review. EPA may declare a submission incomplete at any time during the review period where the circumstances warrant doing so under these regulations. EPA’s Instruction Manual for Premanufacture Notification of New Chemical Substances explains the PMN reporting requirements in detail. The instruction manual information can also be found in the help sections of the e-PMN software.
Notify EPA of newly identified data within ten days of receipt
If, during the new chemical review period, a submitter receives or becomes aware of the existence of reasonably ascertainable test data or other information that adds to or makes more complete the determination of the potential unreasonable risk, the submitter is obliged to send that information to EPA within ten days of its receipt, but not later than five days before the end of the review period. If such information becomes available within five days of the end of the review period, the submitter must inform the EPA contact by telephone. This obligation includes:
- Additional toxicological information;
- Details on manufacture, processing, use, and disposal;
- Likely worker exposures and environmental releases; and
- Facts on innovations and improvements in product chemistry and safety practices.
This requirement is described at 40 CFR 720.40(f). To submit this information, use EPA Form 7710-25 using the electronic PMN (e-PMN) software.
Consolidation of PMN submissions
- EPA will only charge a fee equal to that for a single submission for a consolidated submission of up to six chemical substances.
- Pre-approval before a PMN is submitted is required for a consolidated submission.
When a potential chemical manufacturer wants to submit premanufacture notices (PMNs) on several (up to six) closely similar substances, there are economies for the Environmental Protection Agency (EPA) in reviewing them together. In recognition of these economies and to encourage manufacturers to submit such PMNs together, the agency will only charge a fee equal to that for a single submission for a consolidated submission of up to six chemical substances.
Pre-approval before a PMN is submitted is required for a consolidated submission. The chemical manufacturer should contact the New Chemicals Prenotice Coordinators for consolidation approval. Approval will be given if the substances are adequately similar chemically and toxicologically, if the planned uses are similar enough for combined review, and if intended volumes are not excessively different. Approved consolidations will be given a prenotice communication number, which must be entered on the section 5 submission form. In some cases, a synthetic sequence can be consolidated, as well.
EPA encourages, but has not required, that any single submission be named by Method 1 (see 720.45(a)(3)). The Prenotice Coordinators will not, however, approve any consolidated submission that does not include a Chemical Abstracts Service (CAS) Inventory Expert Service (IES) name for each substance included. Sources other than the IES have, overall, a higher error rate in generating names, and this specifically includes submitters making additional names by analogy to that of one member of an approved consolidation.
When a submission has come in incorrectly named, the process of declaring it incomplete and returning it to the submitter diverts EPA resources from other important work of the New Chemicals Program. The Method 1 requirement for consolidations is not satisfied by simply giving a CAS name and number for substances that have been previously examined by non-IES CAS personnel.
Submitting a PMN for a chemical intermediate
- EPA will charge a reduced fee of $1,000 for the submission of PMN for each chemical intermediate in a synthetic pathway when accompanied by PMN for the final substance on that pathway.
When a potential chemical manufacturer wants to submit premanufacture notices (PMNs) on several substances in a synthetic series leading to a final product, there are several advantages for the Environmental Protection Agency (EPA) in reviewing them together. For example, the agency can make a more complete evaluation of likely emissions and exposure, and there are economies in the review. In recognition of these advantages, and to encourage manufacturers to submit such PMNs together, EPA will charge a reduced fee of $1,000 for the submission of PMN for each chemical intermediate in a synthetic pathway when accompanied by PMN for the final substance on that pathway. The final product is subject to a full user fee.
The fee for a PMN submitted by a “small” manufacturer is reduced to $100 (“small” means less than $40 million in annual sales by the submitter company or it and its parent company together). There is no reduction in fee for intermediate PMNs filed by a “small” company to below the $100 level. It is, however, still helpful to EPA to receive such applications together, and the agency encourages submitters to send them at the same time.
When several parallel synthetic sequences are being considered at once (usually this comes up when a submitter seeks approval of consolidated D, D’, D”, and D”’, final products of the synthetic sequences A-->B-->C-->D, A’-->B’-->C’ -->D’, A’’ B’’ C’’ D’’, and A’’’ B’’’ C’’’ D’’’), then parallel intermediate stages (A, A’, A’’, and A’’’) can be consolidated.
Claiming CBI
- A central point for a CBI claim is consideration of whether the disclosure of the data element, whether alone, or in combination with other information, is likely to cause substantial harm to the business’ competitive position.
- EPA believes that information contained in safety data sheets, with some exceptions, generally will not qualify as CBI.
- Companies communicating with EPA by telephone or fax are advised that the lines are not secure.
There are requirements relating to the submission of confidential business information (CBI), periodic Environmental Protection Agency (EPA) reviews of CBI claims, and expiration of CBI claims. Claims of confidentiality must be made in accordance with the procedures described in 40 CFR 703. Also, see 40 CFR 720 subpart E.
All CBI claims must be substantiated at the time the information claimed as CBI is submitted to EPA, except for those types of information exempt under Toxic Substances Control Act (TSCA) section 14(c)(2). The law requires that the submitter provide a statement concerning the need for the CBI claim and a certification that the statement of need is true and correct. There is also a requirement that when a chemical identity is claimed as CBI, a non-CBI structurally descriptive generic name be provided.
EPA must, with limited exceptions, review all CBI claims for chemical identity, as well as a representative sample of at least 25 percent of other claims. These reviews must occur within 90 days of receipt. Other CBI claims can also be reviewed by the agency based on specific events.
TSCA section 5 filings are included in the universe of submissions requiring review containing CBI claims for information elements other than chemical identity. The following are some pointers that TSCA section 5 submitters should consider when making CBI claims:
- Is the TSCA CBI claim necessary? — The criteria for CBI claims are set forth in 40 CFR 2.208. A central point for a CBI claim is consideration of whether the disclosure of the data element, whether alone, or in combination with other information, is likely to cause substantial harm to the business’ competitive position.
- Is the CBI claim exempt from substantiation? — In a TSCA section 5 filing relating to a chemical substance not yet on the TSCA Inventory many data elements are exempt from substantiation. See TSCA section 14(c)(2) and 40 CFR 703.5(b)(5).
- Is the CBI claim for health and safety data or a health and safety study? — TSCA section 14(b) provides that there are limited CBI protections for these type materials. In general, study names, end points, and similar information may not be claimed as CBI. EPA believes that information contained in safety data sheets, with some exceptions, generally will not qualify as CBI. Also, EPA believes that information related to worker exposure and safety will not generally qualify as CBI. The agency has noticed circumstances where submitted studies have been claimed as CBI in their entirety. However, these “over-redactions” are not authorized under the statute.
- Has the submitter been consistent in its CBI claim for the data element? — EPA has noticed instances where a data element has inconsistently been claimed as CBI by the same submitter. The effect of this is the CBI claim will be found invalid. This is somewhat common where the company name has been claimed as CBI on a premanufacture notice (PMN) form, but the name is not claimed as CBI in an attachment.
- If the specific chemical name is claimed as CBI, is a structurally descriptive generic name provided? — EPA has observed instances of generic names being provided which do not meet this standard. EPA has also observed instances where the provided generic name is exactly the same as the actual name.
E-PMN and CDX allow safe CBI reporting
The e-PMN Software and EPA’s databases are designed for anyone to safely transmit TSCA CBI via EPA’s electronic Chemical Data Exchange (CDX). As a person completes the form and provides attachments, the tool automatically zips everything into one file. The tool then automatically encrypts this file and (after verifying the UserID, Password and the 20-5-1Questionnaire) sends it through CDX to EPA. CDX is unable to open up the submission.
EPA receives a matching decryption key with the submission in a secure environment. Only EPA has the matching decryption key; therefore, the TSCA CBI is fully protected. EPA then decrypts and unzips the submission for further processing. A person should not email any CBI to EPA. The agency’s email system is not secured to protect CBI.
Faxes and telephone calls
Companies communicating with EPA by telephone or fax are advised that the lines are not secure. When someone has a telephone conversation with EPA involving CBI, the person should confirm with the EPA representative (program manager, chemist, pre-notice coordinator, etc.) about authorization to discuss CBI.
If an individual is faxing CBI material to EPA, the person must contact a new chemicals program staff person prior to faxing to ascertain that they are in the office that day and ready to receive the fax AND the fax must be appropriately identified by the submitter as CBI. The fax should include a cover sheet with the name of the EPA recipient and the total number of pages. The New Chemicals Management Branch fax number is (202) 564-9490.
Microbial Commercial Activity Notice (MCAN)
- Persons who wish to commercialize an intergeneric microorganism must submit a MCAN to EPA at least 90 days prior to commercialization.
Toxic Substances Control Act (TSCA) section 5, as interpreted in EPA’s Microbial Products of Biotechnology final rule (April 11, 1997), authorizes EPA to regulate new genetically engineered microorganisms.
According to the rule, new microorganisms are those that, through deliberate human intervention, contain genetic material from dissimilar source organisms. Specifically, intergeneric microorganisms are those formed by either the deliberate combination of genetic material from organisms classified in different taxonomic genera or constructed with synthetic genes that are not identical in DNA that would be derived from the same genus as the recipient microorganism.
Section 5 of TSCA requires the submission of certain information to EPA if a person wishes to:
- Commercialize an intergeneric microorganism; or
- Introduce such microorganisms into the environment for research purposes.
Persons who wish to commercialize an intergeneric microorganism must submit a Microbial Commercial Activity Notice (MCAN) to EPA at least 90 days prior to commercialization. These microorganisms are subject to the same determinations and potential regulatory controls as new chemical substances that undergo the premanufacture notice (PMN) notification and review process.
The e-PMN software enables manufacturers (including importers) of TSCA chemical substances to use the Internet, through EPA’s Central Data Exchange (CDX), to submit TSCA section 5 notices to EPA. There is no required format for biotech submissions in the e-PMN software; therefore, in the “Forms” screen of the e-PMN program, a submitter should choose to create a new form and then select “Biotechnology.” Submitters will be required to enter contact information regarding the submitting company and the technical contact. (EPA offers a sample header sheet, entitled “EPA Biotech Form” for anyone to view.)
MCAN is one of the five biotechnology submission choices. After selecting a submission type, the submitter can enter the submission information in a cover letter and as attachments.
Claims of confidentiality must be made in accordance with the procedures described in 40 CFR 703, except as modified in 40 CFR 725.80.
After PMN or MCAN submission
- EPA’s new chemicals review process for active cases under review includes eight key stages.
- If the substance is determined “not likely to present an unreasonable risk,” the submitter of the PMN or MCAN must provide an NOC to EPA within 30 calendar days of the date the substance is first manufactured or imported for nonexempt commercial purposes.
- The chemical substance is considered to be on the TSCA Inventory as soon as a complete NOC is received by EPA.
After submission of a premanufacture notice (PMN) or Microbial Commercial Activity Notice (MCAN), the notice goes through several stages of review.
- Pre-submission,
- Incoming cases,
- Risk assessment,
- Risk characterization,
- Awaiting submitter information/action,
- Regulatory decision and action development,
- Awaiting submitter signature on consent order, and
- Final determination.
If the Environmental Protection Agency (EPA) fails to make a determination, fees may be refunded; however, nothing relieves EPA of its obligation to make a determination.
Notice of commencement
Under Toxic Substances Control Act (TSCA) section 5(g), when EPA makes a determination that a chemical substance or microorganism is “not likely to present an unreasonable risk,” the submitter of the PMN or MCAN may commence manufacture of the substance, notwithstanding any remaining portion of the applicable review period. If manufacture is commenced, then:
- The submitter of the PMN or MCAN must provide a Notice of Commencement of Manufacture or Import Form (NOC) to EPA within 30 calendar days of the date the substance is first manufactured or imported for nonexempt commercial purposes.
- NOCs must be submitted electronically to EPA.
- If the submitter claims any information on the form as confidential, the claim must be asserted and substantiated in accordance with 40 CFR 703 (along with 40 CFR 720.102, 725.80, and/or 725.190, if applicable) and must be submitted via EPA Form 7710-56.
- The chemical substance is considered to be on the TSCA Inventory as soon as a complete NOC is received by EPA. Actual EPA processing of the NOC to complete the transaction takes about four weeks.
- Excess research and development substances may be used or sold after expiration of the PMN review period and does not require submission of an NOC. The substance will not be placed on the Inventory until an NOC is received; however, an NOC may not be submitted for the substance before commercial manufacture (non-research and development) begins.
New chemicals and microorganisms are added to the TSCA Inventory after the PMN or MCAN review period has completed and the PMN or MCAN submitter has commenced nonexempt commercial manufacture.
Stages of review
- EPA’s 90-day new chemicals review process for active cases under review includes eight key stages, from pre-submission to final determination.
- If EPA fails to make a determination, fees may be refunded.
The PMN Program to review new chemicals under the Toxic Substances Control Act (TSCA) has evolved into an efficient mechanism for identifying those new chemicals which are of greatest concern early on in the 90-day review process. A detailed analysis is focused on these cases with the ultimate goal of identifying and controlling unreasonable risks.
EPA uses an integrated approach that draws on knowledge and experience across disciplinary and organizational lines to identify and evaluate concerns regarding health and environmental effects, exposure and release, and economic impacts. EPA’s new chemicals review process for active cases under review includes the following key stages:
- Pre-submission — Companies are encouraged to contact EPA’s new chemicals program to set up a “pre-notice consultation” to discuss their new chemical submission and understand the agency’s review process. EPA has developed a “Points to Consider When Preparing TSCA New Chemical Notifications” document to assist submitters with the preparation of their submissions. EPA’s predictive tools and models are publicly available and used to estimate releases to the environment, environmental fate, exposures to workers and the general population, and hazards. These models and user manuals explain the procedures and provide the default parameters when notices do not provide specific information.
- Incoming cases — A pre-screen occurs before risk assessment begins. During this step, submissions are reviewed for administrative completeness and to ensure that all required technical information concerning chemical identity has been submitted. EPA has issued regulations at 40 CFR 720.45 and 720.50, which describe the specific information that must be included in the notice form. EPA will check if the notice is required, is incomplete and/or contains errors pursuant to regulations at 720.62 and 720.65. Failure to include the specific information required by the regulations may result in EPA declaring the submission incomplete and suspending its review. EPA may declare a submission incomplete at any time during the review period where the circumstances warrant doing so under these regulations.
- Risk assessment — EPA conducts a full life-cycle risk assessment of the substance. Chemistry, environmental fate, exposure, and hazard (human and ecological) assessments are performed and integrated to determine whether the chemical poses an unreasonable risk to human health or the environment under the conditions of use. For case counting purposes, the submission is included as a “Risk Assessment” case during this step.
- Risk characterization — EPA chemical program managers advise submitters of the initial risk assessment findings. Submitters may elect to provide clarifying information to EPA and/or to amend their PMN if risks have been identified in the risk assessment stage. When this happens, a case may change status from Risk Characterization back to Risk Assessment. EPA uses any additional information provided by submitters, where appropriate, to revise the initial risk assessment. During this step, for case counting purposes, the submission is still included as a “Risk Characterization” case if EPA is working on the assessment. However, the submission is included as an “Awaiting Submitter Information” case if EPA is waiting for submitter action.
- Awaiting submitter information/action — If EPA is waiting for information for more than seven days, the submission will be included as an “Awaiting Submitter Information” case. This category includes, but is not limited to, cases for which the submitter is conducting toxicity testing or fate determinations, clarifying manufacturing or processing information, or conducting analytical measurements.
- Regulatory decision and action development — EPA makes an affirmative determination on whether the new chemical substance or use is likely to present an unreasonable risk to human health or the environment in the regulatory decision and action development stage. The decision is documented in the risk determination document for “Not likely to Present Unreasonable Risk” or an Order for all other cases. This determination is made publicly available. The submission is included as a “Regulatory Decision and Action Development” case during this step. In some instances, submitters may provide additional or clarifying information at this stage that could affect the regulatory decision, and a case may change status from Regulatory Decision and Action Development to Risk Characterization or Risk Assessment.
- Awaiting submitter signature on consent order — EPA generally works with submitters to develop orders that protect human health and the environment and meet the statutory requirements of TSCA.
- Final determination — EPA posts the final determination in the status tables for section 5 notices reviewed under TSCA. These are posted on EPA’s “Premanufacture Notices (PMNs) and Significant New Use Notices (SNUNs) Table” webpage. Possible determinations include:
- Allowed to commercialize without restrictions;
- Allowed to commercialize with restrictions pending information development, if applicable;
- Not allowed to commercialize pending development of information;
- Prohibited from commercializing; or
- Case withdrawn by submitter.
If EPA fails to make a determination, fees may be refunded; however, nothing relieves EPA of its obligation to make a determination.
Existing chemical evaluation, testing, and management
- The three stages of EPA’s process for ensuring the safety of existing chemicals are prioritization, risk evaluation, and risk management.
The Environmental Protection Agency (EPA) evaluates chemicals under the Toxic Substances Control Act (TSCA) to determine whether they meet the unreasonable risk threshold for regulation, and to determine how to regulate chemicals that meet that threshold. Section 6 of TSCA establishes a framework and a timetable for EPA to prioritize which existing chemicals to evaluate for risks.
Overview
TSCA, as amended, requires EPA to evaluate the safety of existing chemicals via a three-stage process. The three stages of EPA’s process for ensuring the safety of existing chemicals are prioritization, risk evaluation, and risk management. The Act also includes a variety of new EPA authorities to drive forward progress on evaluating and ensuring the safety of existing chemicals.
The following flowchart provides an overview of the process for prioritization, risk evaluation, and risk management of existing chemicals, including the key decisions to be made or actions taken at each step:
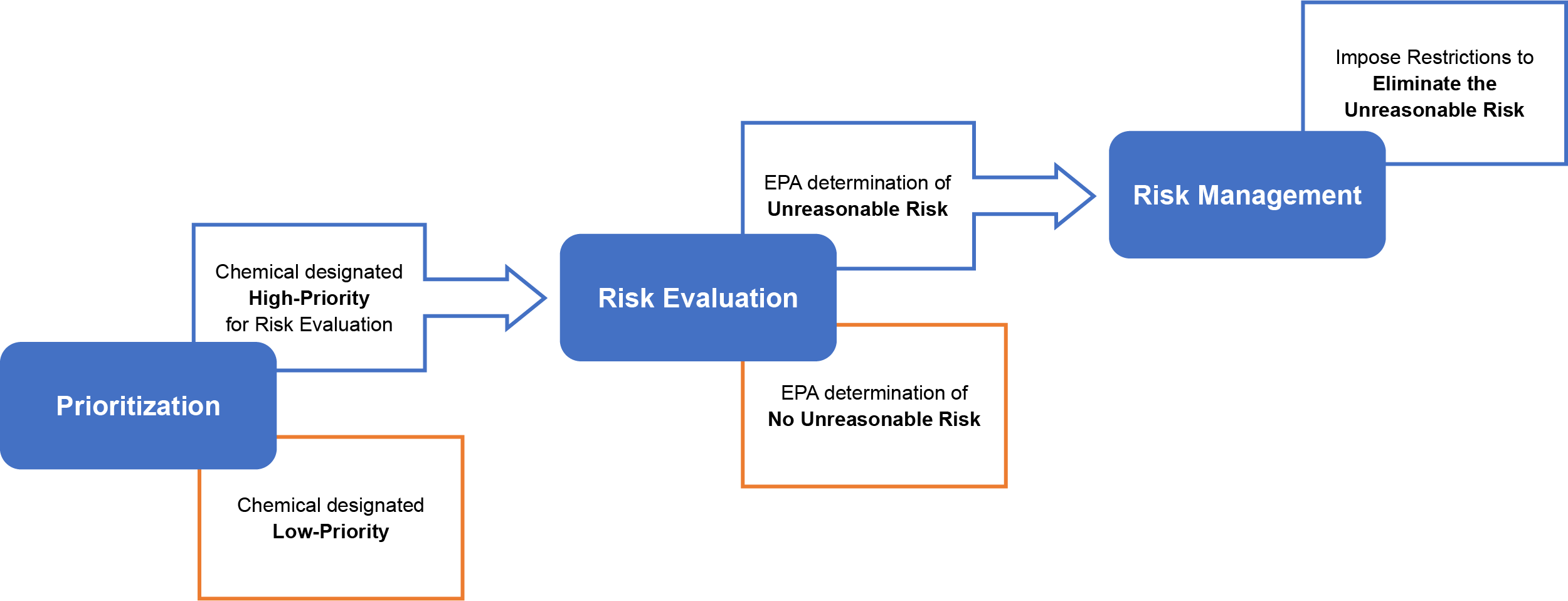
Once a chemical is in the process, which starts with proposed prioritization of a chemical, statutory deadlines are triggered for EPA to complete each stage (prioritization, risk evaluation, and risk management) of the process.
Prioritization
- Prioritization is a risk-based screening process for designating chemical substances as High-Priority Substances for risk evaluation, or Low-Priority Substances for which risk evaluation is not warranted at the time.
The first step in the Environmental Protection Agency’s (EPA’s) process for evaluating the safety of existing chemicals is prioritization. Prioritization is a risk-based screening process for designating chemical substances as either High-Priority Substances for risk evaluation, or Low-Priority Substances for which risk evaluation is not warranted at the time.
The Toxic Substances Control Act (TSCA) requires EPA to give certain preferences to prioritizing chemicals on the 2014 TSCA Work Plan, to consider certain criteria such as hazard/exposure, persistence, and bioaccumulation, but otherwise does not significantly limit EPA’s discretion to choose which chemicals enter the prioritization process. TSCA further prohibits EPA from considering non-risk factors (e.g., costs/benefits) during prioritization.
Once initiated, the process provides stakeholders with ample notice of any EPA risk evaluation activity, as well as two opportunities for the public to submit relevant information to the agency. The process has been designed to ensure that EPA’s limited resources are focused on chemicals with the greatest potential for risk.
Chemical substances with low hazard and/or exposure that meet the definition of Low-Priority Substances are taken out of consideration for further assessment at this time. This gives the public notice of chemical substances for which the hazard and/or exposure potential is anticipated to be low or nonexistent and provides insight into which chemical substances are likely not to need additional evaluation and risk management.
The following flowchart provides an overview of EPA’s chemical prioritization process:
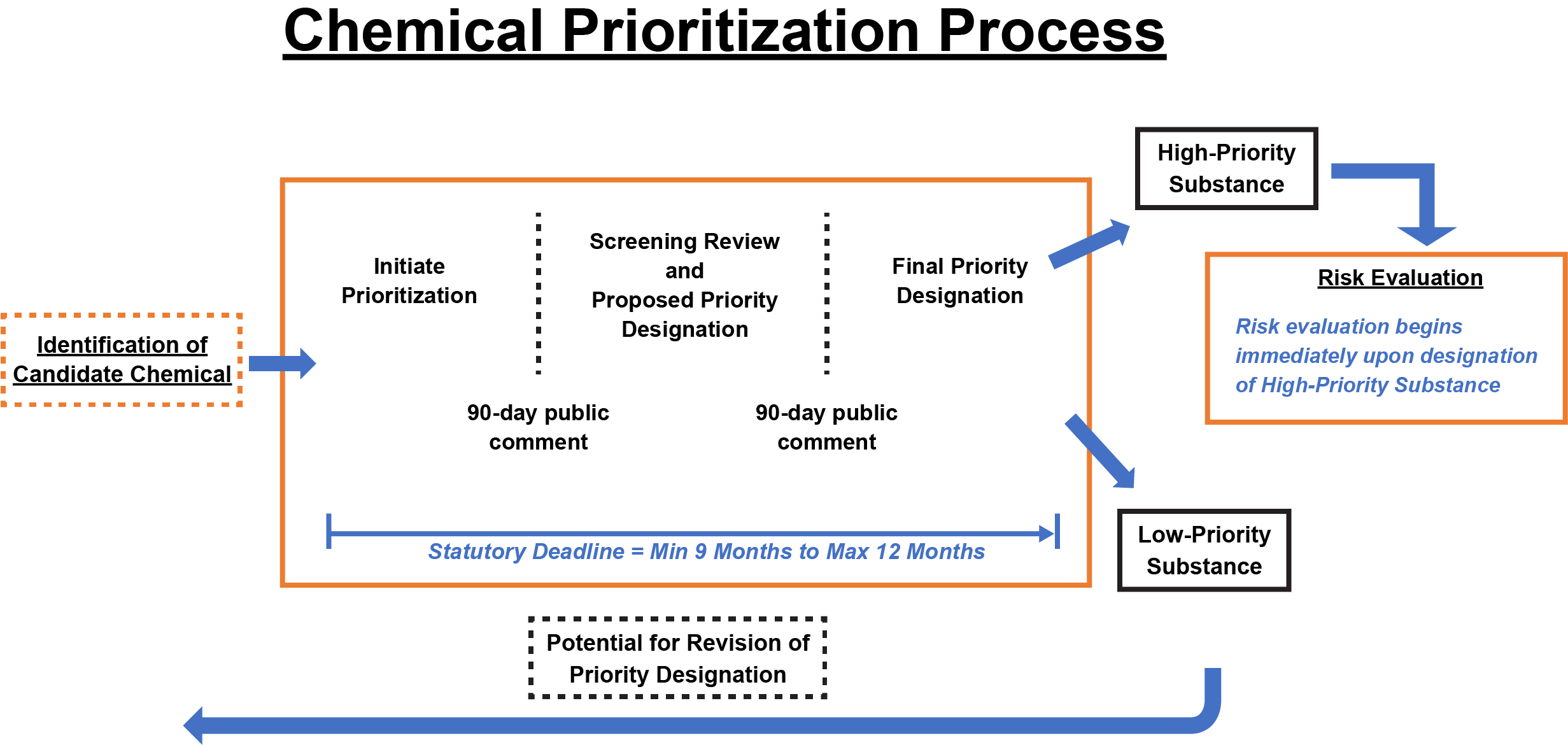
Risk evaluation
- If EPA designates a chemical as a High-Priority Substance, the chemical moves immediately to the risk evaluation phase.
The second step in the Environmental Protection Agency’s (EPA’s) process for evaluating the safety of existing chemicals is risk evaluation. If EPA designates a chemical as a High-Priority Substance, the chemical moves immediately to the risk evaluation phase. At the conclusion of the risk evaluation phase, EPA must use the risk evaluation as a basis to determine whether or not the chemical presents an unreasonable risk to health or the environment under the chemical’s conditions of use.
The Toxic Substances Control Act (TSCA) prohibits EPA from considering non-risk factors (e.g., costs/benefits) during risk evaluation. This includes risks to subpopulations who may be at greater risks than the general population, such as children and workers.
The risk evaluation process has the following components:
- A scope document that provides the public with information on the focus of the risk evaluation;
- Hazard and exposure assessments and a risk characterization to inform the risk determination; and
- A risk determination stating whether or not a chemical substance presents an unreasonable risk to health or the environment under its conditions of use.
In addition to EPA’s prioritization process, TSCA allows manufacturers to request that EPA conduct a risk evaluation on a particular chemical. When this happens, manufacturers are required to provide EPA with the information necessary to conduct a risk evaluation on those conditions of use that are of interest to them. Like the prioritization process, the risk evaluation process affords opportunities for public comment and submission of relevant information.
Overview
- TSCA requires that for each risk evaluation completed on a High-Priority Substance, EPA must begin a new risk evaluation.
- The scope of a risk evaluation will include the hazards, exposures, conditions of use, and the potentially exposed or susceptible subpopulations the EPA expects to consider.
- A draft scope will be published in the Federal Register.
The risk evaluation process is the second step, following Prioritization and before Risk Management, in the Environmental Protection Agency’s (EPA’s) existing chemical process under the Toxic Substances Control Act (TSCA). The purpose of risk evaluation is to determine whether a chemical substance presents an unreasonable risk to health or the environment, under the conditions of use, including an unreasonable risk to a relevant potentially exposed or susceptible subpopulation.
As part of this process, EPA must evaluate both hazard and exposure, exclude consideration of costs or other non-risk factors, use scientific information and approaches in a manner that is consistent with the requirements in TSCA for the best available science, and ensure decisions are based on the weight-of-scientific-evidence. Here is an overview of the steps in EPA’s risk evaluation process for existing chemicals:
A. Initiation of the risk evaluation
A risk evaluation can be initiated by EPA based on the outcome of Prioritization or initiated by EPA based on acceptance of a manufacturer’s request for a risk evaluation:
- EPA-Initiated risk evaluations
- With the passage of the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) in 2016, EPA was required to select the first 10 chemicals to undergo risk evaluations from the 2014 Update to the TSCA Work Plan. Following the first 10 chemicals, EPA has been conducting risk evaluations on chemical substances designated as High-Priority Substances through the Prioritization process.
- TSCA requires that for each risk evaluation completed on a High-Priority Substance, EPA must begin a new risk evaluation. By the end of calendar year 2019, EPA must have had at least 20 chemical risk evaluations ongoing at any given time on High-Priority Substances. The law requires that at least half of all EPA-initiated risk evaluations be drawn from the 2014 Update to the TSCA Work Plan, until that list has been exhausted.
- Manufacturer-requested risk evaluations
- Chemicals may be evaluated following EPA’s approval of a manufacturer’s request for a risk evaluation of a chemical they manufacture. The number of chemicals undergoing risk evaluation as the result of such requests must constitute 25 to 50 percent of the number of EPA-initiated risk evaluations, if EPA receives a sufficient number of compliant requests.
B. Components of a risk evaluation
1. Scope of the risk evaluation
- The scope of a risk evaluation will include the hazards, exposures, conditions of use, and the potentially exposed or susceptible subpopulations EPA expects to consider. The scope will also include:
- A Conceptual Model, which will describe the relationships between the chemical, under the conditions of use, and humans and the environment.
- An Analysis Plan, which will identify the approaches and methods EPA intends to use to assess exposures and hazards.
- Conditions of use under TSCA means “the circumstances, as determined by the [EPA] Administrator, under which a chemical substance is intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used or disposed of.” For purposes of prioritization, the EPA Administrator may determine that certain uses fall outside the definition of conditions of use. During the risk evaluation scoping process, EPA may decide to narrow the scope of the risk evaluation further, potentially excluding conditions of use that present low risk.
- Draft scope
- A draft scope will be published in the Federal Register no later than three months from the initiation of the risk evaluation process.
- 45-day public comment period on the draft scope
- A docket will be opened for no less than 45 days to facilitate public comment on the draft scope.
- Final scope
- A final scope will be published no later than six months after initiation of the risk evaluation, as required by the law.
2. Hazard assessment
- EPA will identify the adverse health or environmental effects caused by exposure to the chemical. Hazards may include, but are not limited to, toxicity with respect to cancer, mutation, reproductive, developmental, respiratory, immune, and cardiovascular impacts, and neurological impairments.
3. Exposure assessment
- EPA will identify, where relevant, the likely duration, intensity, frequency, and number of exposures to a chemical under the conditions of use. This assessment will also include the nature and types of individuals or populations that are exposed to the chemical.
4. Risk characterization
- EPA will integrate and assess the reasonably available information on hazard and exposure and will also include considerations of information quality and alternative interpretations.
5. Risk determination
- EPA will make a draft determination as to whether the chemical substance, under the conditions of use, presents an unreasonable risk to health or the environment. Those conditions of use determined to present an unreasonable risk to health or the environment will move immediately into risk management.
C. Publication of the risk evaluation
1. Draft risk evaluation
- Each draft risk evaluation will be peer reviewed.
- A draft scope will be published in the Federal Register.
- 60-day public comment period on the draft risk evaluation. A docket will be opened for no less than 60 days to facilitate public comment on the draft risk evaluation.
2. Final risk evaluation
- A final risk evaluation will be published no later than three to three-and-a-half years after identification of the chemical as a high priority for risk evaluation.
Use of scientific and technical information
- Under TSCA, EPA relies on scientific and technical information about a chemical to evaluate risks associated with the chemical and determine whether to regulate it.
- LCSA directs EPA to establish an SACC to provide nonbinding, independent scientific and technical advice to the agency regarding implementation of the Act.
The Environmental Protection Agency (EPA) is required to meet the scientific standards in the Toxic Substances Control Act (TSCA) for best available science, utilizing a weight-of-scientific-evidence approach when conducting risk evaluations. The application of these standards will be documented throughout the risk evaluation process and available for public comment.
EPA’s initial work on systematic review was described in the supplemental files for each TSCA scope document, which included the Strategy for Conducting Literature Searches and the Bibliography for each chemical. The agency had been using the Application of Systematic Review in TSCA Risk Evaluations when conducting TSCA chemical risk evaluations.
However, in December 2021, EPA released for public comment a Draft Systematic Review Protocol Supporting TSCA Risk Evaluations for Chemical Substances, that will guide the agency’s review and selection of studies and provide the public with continued transparency regarding how EPA plans to evaluate scientific information.
Lautenberg Act made specifications about use of science
Under TSCA, EPA relies on scientific and technical information about a chemical to evaluate risks associated with the chemical and determine whether to regulate it. The chemical industry and environmental and public health organizations have contested on occasion the quality of scientific and technical information that EPA has relied upon to make regulatory decisions under TSCA. To that end, the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) of 2016 added various provisions specifying how EPA is to use scientific and technical information to carry out the Act.
In determining whether to require development of new information about a chemical or whether to regulate a chemical that presents unreasonable risks, EPA must consider the “best available science” and any applicable factors generally used to assess the quality of scientific information. EPA must also consider “reasonably available information” that relates to chemicals’ conditions of use and make decisions based on the “weight of the scientific evidence.”
More generally, EPA must develop policies, procedures, and guidance necessary to carry out the amendments to TSCA made by the LCSA. EPA must periodically review these policies, procedures, and guidance for their adequacy in carrying out the law and revise them if necessary to reflect new scientific developments or understandings.
Related to the requirements on the use of scientific and technical information, the LCSA directs EPA to establish a Science Advisory Committee on Chemicals (SACC) to provide nonbinding, independent scientific and technical advice to the agency regarding implementation of the Act. SACC held its first in-person meeting in June 2019 to discuss EPA’s draft risk evaluation for C.I. Pigment Violet 29. Since then, SACC has convened multiple times to provide scientific review to other EPA draft risk evaluations.
Minimized animal testing
- LCSA added section 4(h) to TSCA to require EPA to develop a strategic plan to minimize the use of vertebrate animals when requiring the development of new information pertaining to a chemical.
Generally, the development of new information on chemicals relies on animal testing, unless an alternative approach is shown to reliably produce information suitable for evaluating risks. Stakeholders have disagreed about whether animal testing is necessary to develop new information on chemicals, particularly with respect to the usefulness of any new information and whether alternative testing methods can reliably produce information that traditionally has been produced through animal testing.
Lautenberg Act made specifications for minimize animal testing
However, the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) of 2016 added section 4(h) to the Toxic Substances Control Act (TSCA) to require the Environmental Protection Agency (EPA) to develop a strategic plan to minimize, to the extent practicable, the use of vertebrate animals when requiring the development of new information pertaining to a chemical. EPA published the strategic plan in June 2018. The Strategic Plan has three core components:
- Identifying, developing, and integrating New Approach Methodologies (NAMs) for TSCA decisions;
- Building confidence that the NAMs are scientifically reliable and relevant for TSCA decisions; and
- Implementing the reliable and relevant NAMs for TSCA decisions.
TSCA as amended by LCSA, directs EPA to:
- Reduce and replace, to the extent practicable and scientifically justified, the use of vertebrate animals in the testing of chemical substances or mixtures; and
- Promote the development and timely incorporation of alternative test methods or strategies that do not require new vertebrate animal testing.
TSCA section 4(h) also requires EPA to take specified steps to reduce and replace vertebrate animal testing to the extent practicable, scientifically justified, and consistent with policies of Title I of TSCA. Voluntary testing developed for submission under Title I of TSCA, and not pursuant to a request or requirement by EPA, must first be attempted by means of an alternative test method or strategy identified by EPA pursuant to TSCA section 4(h)(2)(C) and for the development of such information before conducting new vertebrate animal testing.
Testing, orders, or consent agreements
- TSCA authorizes EPA to promulgate rules, issue orders, or enter into consent agreements to require manufacturers (including importers) and processors to test chemical substances.
- EPA’s GLPS compliance monitoring program ensures the quality and integrity of test data submitted to the agency in support of section 5 of the TSCA and pursuant to testing consent agreements and test rules issued under section 4 of TSCA.
Under the Toxic Substances Control Act (TSCA), the Environmental Protection Agency (EPA) has broad authority to issue regulations designed to require manufacturers (including importers) or processors to test chemical substances and mixtures. EPA also works with members of the U.S. chemical industry to develop needed data via TSCA section 4 Enforceable Consent Agreements (ECAs).
This testing is required to develop data about health or environmental effects when there is insufficient data for EPA risk assessors to be able to determine whether a chemical substance or mixture presents an unreasonable risk to health or the environment.
TSCA section 4 requires manufacturers and processors of chemical substances and mixtures to conduct testing if EPA finds that the manufacture, distribution, processing, use, or disposal of a chemical substance or a mixture may present an unreasonable risk to human health or the environment. The rationale for establishing the necessity for issuing a TSCA section 4 regulatory action would be presented under the findings and/or statement of need, along with an indication of the underlying evidence, in the specific action.
In addition to the statutory testing authority previously provided under TSCA section 4, the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) of 2016 amended TSCA to explicitly provide authority to issue orders. TSCA section 4(a)(2) now explicitly authorizes EPA to promulgate rules, issue orders, or enter into consent agreements to require testing to develop information that is necessary:
- To review a notice submitted under TSCA section 5;
- To perform a risk evaluation under TSCA section 6(b);
- To implement a requirement imposed in a rule, order, or consent agreement under TSCA section 5(e) or (f), or in a rule promulgated under section 6(a);
- At the request of a federal implementing authority under another federal law, to meet the regulatory testing needs of that authority with regard to toxicity and exposure;
- To determine if a chemical substance or mixture manufactured, processed, or distributed in commerce solely for export presents an unreasonable risk of injury to health or the environment in the U.S., pursuant to TSCA section 12(a)(2); and
- To prioritize a chemical substance under TSCA section 6(b) (subject to certain limitations).
Manufacturers and processors who wish to assert claims of confidentiality must make them in accordance with the procedures described in 40 CFR 703.
Good laboratory practice
EPA 40 CFR 792 prescribes good laboratory practices for conducting studies relating to health effects, environmental effects, and chemical fate testing. Part 792 is intended to ensure the quality and integrity of data submitted pursuant to testing consent agreements and test rules issued under section 4 of the Toxic Substances Control Act (TSCA).
EPA also offers several tools to assist companies submitting data under the Good Laboratory Practice Standards (GLPS). Data obtained through laboratory inspections and data audits is used by the agency to regulate the use of industrial chemicals. EPA’s GLPS compliance monitoring program ensures the quality and integrity of test data submitted to the agency in support of section 5 of the TSCA and pursuant to testing consent agreements and test rules issued under section 4 of TSCA.
Risk management
- If at the end of the risk evaluation process, EPA determines that a chemical presents an unreasonable risk to health or the environment, the chemical must immediately move to risk management action under TSCA.
- EPA is required to implement, via regulation, regulatory restrictions on the manufacture, processing, distribution, use, or disposal of the chemical to eliminate the unreasonable risk.
The third step in the Environmental Protection Agency’s (EPA’s) existing chemicals process is risk management. Generally, risk management is the last step in the process and starts after publication of a chemical’s final risk evaluation showing unreasonable risks. If at the end of the risk evaluation process, the agency determines that a chemical presents an unreasonable risk to health or the environment, the chemical must immediately move to risk management action under the Toxic Substances Control Act (TSCA).
EPA is required to implement, via regulation, regulatory restrictions on the manufacture, processing, distribution, use, or disposal of the chemical to eliminate the unreasonable risk. The agency is given a range of risk management options under TSCA, including labeling, recordkeeping or notice requirements, actions to reduce human exposure or environmental release, and a ban of the chemical or of certain uses.
TSCA requires EPA to issue a:
- Proposed risk management rule under TSCA section 6(a) for the chemical substance no later than one year after the date on which the final risk evaluation regarding the chemical substance is published, and
- Final rule no later than two years after the publication date of the final risk evaluation.
Input from all stakeholders is critical to the risk management process. Like the prioritization and risk evaluation processes, there is an opportunity for public comment on any proposed risk management actions.
EPA’s regulatory options
- Any risk management actions would apply only to the condition(s) of use that EPA found to present an unreasonable risk in the final risk evaluation.
- When EPA grants an exemption, it must publish its analysis of the need for the exemption, and also must establish a time limit on the exemption.
There are several actions the Environmental Protection Agency (EPA) can take to address unreasonable risks. These actions, alone or in combination, may include:
- Prohibiting or otherwise restricting, or limiting the manufacture, processing, or distribution in commerce of the substance or mixture.
- Prohibiting or otherwise restricting, or limiting the manufacture, processing, or distribution in commerce of the substance or mixture for a particular use or above a set concentration for a particular use.
- Requiring adequate minimum warnings and instructions with respect to its use, distribution in commerce, or disposal.
- Requiring recordkeeping, monitoring, or testing by manufacturers and processors.
- Prohibiting or regulating the manner or method of commercial use.
- Prohibiting or regulating the manner or method of disposal.
- Directing manufacturers/processors to give notice of the determination of risk to distributors and users and replacing or repurchasing.
Any risk management actions would apply only to the condition(s) of use that EPA found to present an unreasonable risk in the final risk evaluation. Those that EPA found do not present an unreasonable risk will not be subject to risk management.
Exemptions from rules
As amended by the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) of 2016, section 6(g) of the Toxic Substances Control Act (TSCA) authorizes EPA to grant exemptions from rules promulgated under section 6(a). EPA may grant an exemption if the agency finds that:
- An exemption would be necessary to maintain critical or essential uses of a chemical; or
- An exemption would be necessary to avoid significant disruption to the national economy, security, or critical infrastructure; or
- A specific condition of use provides substantial benefits to health, environment, or public safety despite risks associated with the chemical.
Additionally, EPA must exempt replacement parts for “complex durable goods” and “complex consumer goods” designed prior to a rule’s publication in the Federal Register unless the agency finds that such replacement parts contribute significantly to unreasonable risks identified in a risk evaluation. When EPA grants an exemption, it must publish its analysis of the need for the exemption and establish a time limit on the exemption.
EPA rulemakings
Regarding rulemakings, Section 6 directs EPA to:
- Promulgate one or more of the seven regulatory requirements in accordance with the Administrative Procedure Act’s (APA’s) provisions governing agency rulemaking.
- Adhere to additional procedural requirements, such as considering the chemical’s risks and benefits, “reasonably ascertainable economic consequences” of the requirements on the chemical, and alternative regulatory actions.
- Propose a rule not later than one year after publishing the final risk evaluation and finalize the rule not later than two years after publishing the final risk evaluation.
- Note: EPA may extend these time frames up to two years for chemicals the agency has not already identified as persistent and bioaccumulative.
- Establish mandatory compliance dates that are as soon as practicable but not later than five years after promulgation of the rule, except for uses being exempted.
- Note: EPA may vary the mandatory compliance dates for different affected persons (e.g., chemical manufacturers, processors, and distributors).
- Issue rules that provide for a reasonable transition period before the mandatory compliance dates become effective.
- Note: If EPA determines that the manufacture, processing, distribution, use, or disposal of a chemical is likely to present an unreasonable risk before the effective date, EPA may declare a proposed rule to be effective to protect the public interest, in which case compliance with the proposed requirements is mandatory upon the proposed rule’s publication in the Federal Register until the proposed rule is finalized or revoked.
After the enactment of the LCSA, the first EPA rule to prohibit the manufacture, processing, and distribution of a chemical occurred in March 2019. EPA issued a final rule to prohibit the manufacture, processing, and distribution of methylene chloride in all paint and coating removers for consumer use.
Imminently hazardous chemicals
- The EPA may commence a civil action in U.S. district court to protect against an imminently hazardous chemical through seizure or other relief before promulgating a rule to regulate the chemical.
The Toxic Substances Control Act (TSCA) establishes procedures for addressing imminently hazardous chemicals and chemicals that present significant risk of serious or widespread harm. Section 7(f) defines an “imminently hazardous chemical substance or mixture” as a chemical that “presents an imminent and unreasonable risk of serious or widespread injury to health or the environment.”
Under section 7, the Environmental Protection Agency (EPA) may commence a civil action in U.S. district court to protect against an imminently hazardous chemical through seizure or other relief before promulgating a rule to regulate the chemical. When appropriate, EPA must initiate promulgation of a rule for an imminently hazardous chemical at the same time as commencing the civil action or as soon as practicable thereafter.
Additionally, if EPA receives information indicating a chemical may present significant risk of serious or widespread harm to humans, section 4(f) requires the agency to either initiate an action to prevent or reduce to a sufficient extent such risk or publish a finding that the risk is not unreasonable in the Federal Register within 180 days of receiving the information. EPA may extend the 180-day deadline by an additional 90 days for good cause.
Chemical- and category-specific regulations
- TSCA authorizes EPA to take regulatory action with respect to the risks posed by individual chemicals and specific categories of chemicals.
In addition to regulating individual chemicals that present unreasonable risks, TSCA also authorizes EPA to take regulatory action with respect to the risks posed by specific categories of chemicals. At this time the agency regulates specific chemicals and chemical categories under the following parts of 40 CFR:
- Subpart B to 40 CFR 704 — Chemical-specific reporting and recordkeeping rules [Editor note: This includes asbestos reporting beginning February 24, 2024, and ending May 24, 2024, per the July 25, 2023, final rule on asbestos reporting.]
- Part 705 — Reporting and recordkeeping requirements for certain per- and polyfluoroalkyl substances
- Part 711 — TSCA chemical data reporting requirements
- Part 712 — Chemical information rules
- Part 713 — Reporting requirements for the TSCA Inventory of mercury supply, use, and trade
- Part 716 — Health and safety data reporting
- Part 721 — Significant new uses of chemical substances
- Part 725 — Reporting requirements and review processes for microorganisms
- Part 745 — Lead-based paint poisoning prevention in certain residential structures
- Part 747 — Metalworking fluids
- Part 749 — Water treatment chemicals
- Part 751 — Regulation of certain chemical substances and mixtures under Section 6 of the Toxic Substances Control Act
- Part 761 — Polychlorinated biphenyls (PCBs) manufacturing, processing, distribution in commerce, and use prohibitions
- Part 763 — Asbestos
- Part 766 — Dibenzo-para-dioxins/Dibenzofurans
- Part 770 — Formaldehyde standards for composite wood products
- Part 799 — Identification of specific chemical substance and mixture testing requirements
Noteworthy chemicals regulated under TSCA
- Congress singled out six toxic chemicals for TSCA regulations.
When one thinks of toxic chemicals, certain substances come to mind, such as asbestos and lead. Congress singled out six chemicals for regulations — asbestos, formaldehyde, lead, mercury; persistent, bioaccumulative, and toxic (PBT) chemicals; and polychlorinated biphenyls (PCBs). Two other chemicals have also risen to the Environmental Protection Agency’s (EPA’s) attention (and regulation):
- Methylene chloride, and
- Per- and polyfluoroalkyl substances (PFAS).
All eight chemicals are worthy of further discussion and regulated as shown in the table:
Note that EPA has other chemical-specific regulations under 40 CFR 700 to 799. However, whether flagged as a “noteworthy chemical” or not, facilities must determine if the chemicals or mixtures they manufacture, import, process, distribute, use, or dispose of are regulated and, if so, follow applicable regulations.
Asbestos
- TSCA Title II AHERA program governs the management of asbestos in K-12 schools.
- TSCA section 6 WPR protects state and local government employees who are not protected by the federal OSHA asbestos standards.
- EPA has issued a risk evaluation of asbestos and is working toward a supplemental risk evaluation. The risk management step comes after risk evaluation.
Asbestos is a mineral fiber that occurs in rock and soil. Exposure to asbestos increases risk of developing lung disease. That risk is made worse by smoking. In general, the greater the exposure to asbestos, the greater the chance of developing harmful health effects.
Asbestos fibers may be released into the air by the disturbance of asbestos-containing material during product use, demolition work, building or home maintenance, repair, and remodeling. In general, exposure may occur only when the asbestos-containing material is disturbed or damaged in some way to release particles and fibers into the air. Where asbestos may be found, includes the following:
- Attic and wall insulation containing vermiculite,
- Vinyl floor tiles and the backing on vinyl sheet flooring and adhesives,
- Roofing and siding shingles,
- Textured paint and patching compounds used on walls and ceilings,
- Walls and floors around wood-burning stoves protected with asbestos paper, millboard, or cement sheets,
- Hot water and steam pipes coated with asbestos material or covered with an asbestos blanket or tape,
- Oil and coal furnaces and door gaskets with asbestos insulation,
- Heat-resistant fabrics, and
- Automobile clutches and brakes.
Asbestos in schools
The Toxic Substances Control Act (TSCA) Title II Asbestos Hazard Emergency Response Act (AHERA) program (also called the Asbestos in Schools Program) governs the management of asbestos in Kindergarten through Grade 12 schools. The objective of AHERA compliance monitoring is to ensure regulatory compliance and, thereby, minimize the risk of exposure to asbestos in schools. Implementing regulations are found at 40 CFR 763 and require public school districts and non-profit schools to:
- Perform an original inspection to determine whether asbestos-containing materials are present and then re-inspect asbestos-containing material in each school every three years;
- Develop, maintain, and update an asbestos management plan and keep a copy at the school;
- Provide yearly notification to parent, teacher, and employee organizations on the availability of the school’s asbestos management plan and any asbestos-related actions taken or planned in the school;
- Designate a contact person to ensure the responsibilities of the public school district or the non-profit school are properly implemented;
- Perform periodic surveillance of known or suspected asbestos-containing building material;
- Ensure that trained and licensed professionals perform inspections and take response actions; and Provide custodial staff with asbestos-awareness training.
These legal requirements are founded on the principle of “in-place” management of asbestos-containing material. Removal of these materials is not usually necessary unless the material is severely damaged or will be disturbed by a building demolition or renovation project.
Other TSCA actions related to asbestos
The TSCA section 6 Worker Protection Rule (WPR) protects state and local government employees who are not protected by the federal Occupational Safety and Health Administration (OSHA) asbestos standards.
Other actions to protect the public from exposure to asbestos under the TSCA include the following:
- July 1989 partial ban — In 1989, the Environmental Protection Agency (EPA) attempted to ban most asbestos-containing products by issuing a final rule under section 6 of TSCA. However, most of the original ban on the manufacture, importation, processing, or distribution in commerce for the majority of the asbestos-containing products originally covered in the 1989 final rule was overturned in 1991 by the Fifth Circuit Court of Appeals. As a result, the 1989 asbestos regulation only bans new uses of asbestos in products that would be initiated for the first time after August 25, 1989, and bans five other specific product types.
- April 2019 restrictions on discontinued uses of asbestos rule — This rule aims to ensure that asbestos products that are no longer on the market cannot return to commerce without EPA evaluating them and putting in place any necessary restrictions or prohibiting use. The uses covered under this rule were not already prohibited under TSCA and could have returned to the market at any time. The rule closed that loophole.
- December 2020 final risk evaluation for asbestos, part 1: chrysotile asbestos — This risk evaluation found unreasonable risks to human health for ongoing uses of chrysotile asbestos and no unreasonable risk to the environment from any condition of use. EPA is moving immediately to risk management for the ongoing chrysotile asbestos use where unreasonable risk was found and will work as quickly as possible to propose and finalize actions to protect against the unreasonable risks.
- December 2021 draft scope for risk evaluation for asbestos, part 2: supplemental evaluation including legacy uses and associated disposals of asbestos — The draft scope includes the conditions of use, hazards, exposures, and the potentially exposed or susceptible subpopulations that EPA plans to consider in conducting the risk evaluation for this chemical substance. For part 2 of the risk evaluation for asbestos, EPA:
- Has adopted the definition of asbestos as defined by TSCA Title II Section 202 as the “asbestiform varieties of six fiber types – chrysotile (serpentine), crocidolite (riebeckite), amosite (cummingtonite-grunerite), anthophyllite, tremolite or actinolite.”
- Will consider Libby Amphibole Asbestos (and its tremolite, winchite, and richterite constituents).
- Will assess the relevant conditions of use of asbestos in talc or talc-containing products, because talc has been implicated as a potential source of asbestos exposure.
- July 25, 2023, final rule (with 12/5/2023 final rule correction) to require asbestos reporting and recordkeeping — This rule adds 40 CFR 704.180, as authorized under TSCA section 8(a)(1). Certain persons that manufactured (including imported) or processed asbestos (including as an impurity) in the four years previous to the rulemaking must report certain exposure-related information, including quantities of asbestos manufactured or processed, types of use, and employee data. The rule also covers asbestos-containing articles and where asbestos is a component of a mixture. Information must be reported during the three-month data submission period beginning February 24, 2024, and ending May 24, 2024. This is a one-time reporting requirement, and reported information will be used by EPA and other federal agencies in considering potential actions involving asbestos, including EPA’s TSCA risk evaluation and risk management activities. The final rule also requires a five-year recordkeeping period, beginning on the last date of the submission period. See 704.180 for details. Instructions for how to report using EPA’s CDX-based tool are available on EPA’s “TSCA Section 8(a)(1) Reporting and Recordkeeping Requirements for Asbestos” webpage.
It should be noted that asbestos is also regulated by other EPA, OSHA, Mine Safety and Health Administration (MSHA), and Department of Transportation (DOT) regulations, and these regulations are beyond the scope of TSCA. EPA regulation 40 CFR 61 Subpart M offers a list of these other regulations.
Formaldehyde
- The Formaldehyde Standards for Composite Wood Products Act aims to reduce emissions of formaldehyde from composite wood products by establishing formaldehyde emissions limits.
- All entities along the supply chain, from the manufacture to the sale of composite wood products, are affected by the final rule requirements.
Formaldehyde is a colorless, flammable gas at room temperature and has a strong odor. Formaldehyde can cause irritation of the skin, eyes, nose, and throat. High levels of exposure may cause some types of cancers. The primary way people can be exposed to formaldehyde is by breathing air containing off-gassed formaldehyde. Everyone is exposed to small amounts of formaldehyde in the air that has off-gassed from products, including composite wood products. Formaldehyde is found in:
- Resins used in the manufacture of composite wood products (i.e., hardwood plywood, particleboard, and medium-density fiberboard);
- Building materials and insulation;
- Household products such as glues, permanent press fabrics, paints and coatings, lacquers and finishes, and paper products;
- Preservatives used in some medicines, cosmetics, and other consumer products such as dishwashing liquids and fabric softeners; and
- Fertilizers and pesticides.
TSCA actions related to formaldehyde
The Formaldehyde Standards for Composite Wood Products Act signed into law on July 7, 2010, added Subchapter VI to the Toxic Substances Control Act (TSCA) to reduce emissions of formaldehyde from composite wood products by establishing formaldehyde emissions limits for domestic or imported hardwood plywood, particleboard, and medium-density fiberboard sold, supplied, offered for sale, or manufactured in the U.S., whether in the form of an unfinished panel or incorporated into a finished good.
Effective May 22, 2017, Environmental Protection Agency (EPA) regulation 40 CFR 770, Formaldehyde Standards Composite Wood Products, set limits on how much formaldehyde can be released from composite wood products and established a program in which independent certifying organizations will verify that composite wood panel producers comply with the limits on formaldehyde releases. It should be noted that EPA has amended this regulation several times since it was first promulgated in order to strengthen, relax, increase flexibility with, or delay the effective dates of the provisions. These amendments have also been made to conform to voluntary consensus standards and allow for remote inspections under limited circumstances. Therefore, check out the latest version of Part 770 for your compliance efforts.
All entities along the supply chain, from the manufacture to the sale of composite wood products, are affected by the requirements of Part 770. This includes panel producers, fabricators, third-party certifiers, importers, distributors, retailers, and accreditation bodies. If a business is unsure if it fits into one of these categories, it may wish to refer to the table below. Examples of each category include (but are not limited to) the following:
| Applicable category | Examples |
|---|
| Importers, distributors, and retailers | - Furniture stores or merchant wholesalers
- Lumber, plywood, millwork, and wood panel merchant wholesalers
- Building material and supplies dealers
- Manufactured (mobile) home dealers (also includes trailer homes)
- Recreational vehicle dealers and merchant wholesalers
- Other construction material merchant wholesalers, or wholesale distributors of manufactured homes and/or prefabricated buildings
|
| Panel producers | - Veneer product manufacturing
- Plywood product manufacturing
- Engineered wood product manufacturing
- Laminated product producers (see table note)
|
| Fabricators | - Manufactured (mobile) home manufacturing
- Prefabricated wood building manufacturing
- Motor home and recreational vehicle manufacturing
- Travel trailer and camper home manufacturing
- Furniture and related product manufacturing
- Laminated product producers (see table note)
|
| Third-party certifiers (TPCs) | - Laboratories conducting independent third-party formaldehyde emissions testing of regulated composite wood products
|
| Accreditation bodies (ABs) | - Product ABs
- Laboratory ABs
|
Note: Laminated product producers are fabricators and, beginning March 22, 2024, laminated product producers are also hardwood plywood panel producers except as provided at 770.4. According to 770.2(e):
- Beginning June 1, 2018, laminated product producers must comply with the requirements of Part 770 that are applicable to fabricators.
- Beginning March 22, 2024, producers of laminated products must comply with the requirements of Part 770 that are applicable to hardwood plywood panel producers (in addition to the requirements of Part 770 that are applicable to fabricators) except as provided at 770.4.
- Beginning March 22, 2024, producers of laminated products that, as provided at 770.4, are exempt from the definition of “hardwood plywood” must comply with the recordkeeping requirements in 770.40(c) and (d) (in addition to the requirements of Part 770 that are applicable to fabricators).
|
Lead
- Title IV of TSCA, along with other authorities in the Residential Lead-Based Paint Hazard Reduction Act of 1992, requires EPA to regulate lead-based paint hazards.
- There are five main programs found in the EPA regulations at 40 CFR 745.
Lead is a naturally occurring element found in small amounts in the earth’s crust. While it has some beneficial uses, it can be toxic to humans and animals, causing negative health effects. Lead can be found in all parts of our environment — air, soil, water, and even inside our homes.
Much of our exposure comes from human activities including the use of fossil fuels like previous use of leaded gasoline, some types of industrial facilities, and previous use of lead-based paint in homes. Lead and lead compounds have been used in a wide variety of products found in and around our homes, including paint, ceramics, pipes and plumbing materials, solders, gasoline, batteries, ammunition, and cosmetics.
Lead is particularly dangerous to children because their growing bodies absorb more lead than adults do and their brains and nervous systems are more sensitive to the damaging effects of lead, including behavior and learning problems, lower intelligence quotient (IQ), hyperactivity, slowed growth, hearing problems, and anemia.
Adults may be exposed to lead by eating and drinking food or water containing lead or from dishes or glasses that contain lead. They may also breath lead dust by spending time in areas where lead-based paint is deteriorating, and during renovation or repair work that disturbs painted surfaces in older homes and buildings. Working in a job or engaging in hobbies where lead is used, such as making stained glass, can increase exposure as can certain folk remedies containing lead.
Adults exposed to lead can suffer from cardiovascular effects, increased blood pressure, and incidence of hypertension; decreased kidney function; and reproductive problems (in both men and women).
TSCA actions related to lead
Title IV of the Toxic Substances Control Act (TSCA), along with other authorities in the Residential Lead-Based Paint Hazard Reduction Act of 1992, requires the Environmental Protection Agency (EPA) to regulate lead-based paint hazards. Five main programs are found in the EPA regulations at 40 CFR 745:
| Program | Related TSCA section | Related CFR | Description |
|---|
| Residential hazard standards for lead in paint, dust, and soil | 403 | 40 CFR 745 Subpart D | This program sets standards for dangerous levels of lead in paint, dust, and soil. |
| Lead renovation, repair, and painting (RRP) program | 402(c)(3) | 40 CFR 745 Subpart E | This program requires renovation firms engaged in renovation activities in homes or child-occupied facilities (COFs) built before 1978 to be trained and certified in lead-safe work practices to guard against lead contamination. The program requires contractors to provide information on lead safety prior to beginning any work. The RRP certification applies to all firms who may disturb paint in pre-1978 residences and COFs. It covers work practice standards, recordkeeping and reporting requirements, individual and firm certification, and enforcement for renovations done in target housing or COFs. It applies to a broad range of firms including renovators, carpenters, painters, electricians, plumbers, handymen, and more. |
| Residential lead-based paint disclosure program | 406* | 40 CFR 745 Subpart F | Buyers and renters of housing built before 1978 must receive certain information about lead and lead hazards in the residence before becoming obligated to buy or rent. Buyers must be given the opportunity to conduct an independent lead inspection.
The disclosure program applies to sellers, agents, managers of rental properties, and landlords, requiring them to disclose any known information concerning potential lead-based paint hazards and available records. They must supply potential buyers and lessees with the lead hazard information pamphlet and must include specific language in the lease or contract related to lead. In addition, sellers must give buyers time to conduct a lead inspection. Most private housing, public housing, federally owned housing, and housing receiving federal assistance built prior to 1978 are covered by the rule. EPA’s lead pamphlet, “Renovate Right: Important Lead Hazard Information for Families, Child Care Providers and Schools,” is required under Subpart F.
Contractors must also provide a copy of the Renovate Right pamphlet to owners and occupants prior to starting work in pre-1978 housing. Contractors must also provide the pamphlet to owners and operators of COFs and schools built prior to 1978 and to parents and guardians of children under age six that attend. |
| Lead abatement program: Training and certification program for lead-based paint activities | 402 and 405 | 40 CFR 745 Subpart L | This program addresses lead removal, risk assessments, lead hazard screens, and inspections in target housing and COFs built before 1978, requiring contractors to be trained and certified in specific practices to ensure accuracy and safety. While EPA’s lead abatement program and the Lead RRP contain many of the same requirements, there are major differences. Lead abatement certification applies to firms who specifically want to work with lead-based paint. This includes abatement firms as well as risk assessment and inspection firms. Abatement is the intentional and permanent elimination of lead-based paint and lead-based paint hazards. According to EPA, “Those who remove lead-based paint simply as a consequence of doing other work do not need lead-based paint abatement certification.” |
| State and Indian tribal programs | 402 and 405 | 40 CFR 745 subpart Q | This program establishes the requirements that state or tribal programs must meet for authorization to administer the standards, regulations, or other requirements established under TSCA section 402 and/or section 406. |
* In addition to section 16 of TSCA, section 1018 of Title X of the Residential Lead-Based Paint Hazard Reduction Act of 1992 (Public Law 102–550) is also an authorizing citation for this program. See the law as codified into the U.S. Code at 42 U.S.C. 4852d.
Lead RRP and contractors
- The Lead RRP Rule establishes requirements for firms and individuals performing renovations, and affects contractors, property managers and others who disturb painted surfaces.
- Firms cannot advertise or perform renovation activities covered by the RRP Rule in homes or child-occupied facilities built before 1978 without firm certification.
The Lead Renovation, Repair, and Painting (RRP) Rule at 40 CFR 745 Subpart E establishes requirements for firms and individuals performing renovations and affects contractors, property managers, and others who disturb painted surfaces. It applies to work in houses, apartments, and child-occupied facilities (such as schools and childcare centers) built before 1978. It includes pre-renovation education requirements as well as training, firm certification, and work practice requirements.
Firms that require certification
In general, anyone who is paid to perform work that disturbs paint in housing and child-occupied facilities built before 1978 must be certified. This includes all firms, even sole proprietorships. Examples of the types of firms covered include:
- Residential rental property owners/managers;
- General contractors; and
- Special trade contractors, including painters, plumbers, carpenters, and electricians.
Firms cannot advertise or perform renovation activities covered by the RRP Rule in homes or child-occupied facilities built before 1978 without firm certification.
Covered activities
In general, any activity that disturbs paint in pre-1978 housing and child-occupied facilities is covered, including:
- Remodeling and repair/maintenance;
- Electrical work;
- Plumbing;
- Painting preparation;
- Carpentry; and
- Window replacement.
However, the following housing or activities are not covered by the rule:
- Housing built in 1978 or later;
- Housing specifically for elderly or disabled persons, unless children under six reside or are expected to reside there;
- “Zero-bedroom” dwellings (studio apartments, dormitories, etc.);
- Housing or components declared lead-free by a certified inspector or risk assessor. Also, a certified renovator may declare specific components lead-free by using a test kit recognized by the Environmental Protection Agency (EPA) or by collecting paint chip samples for analysis by an EPA-recognized laboratory; and
- Minor repair and maintenance activities that disturb six square feet or less of paint per room inside, or 20 square feet or less on the exterior of a home or building. (Note: Window replacement, and partial and full demolition activities, are always covered regardless of square footage. Activities designated as “prohibited” are prohibited regardless of square footage.)
Paint testing
Paint testing is not required by the RRP Rule, but unless a person has documentation that the paint is not lead-based, then the requirements of the RRP Rule apply. If a person chooses to have the paint tested prior to renovation, testing must be done by the appropriate qualified professional on all surfaces to be affected by the work.
| Type of paint testing for renovations | Who can do the testing? |
|---|
| EPA-recognized test kits | Certified renovators |
| X-ray fluorescence instruments | Certified lead-based paint inspectors or risk assessor |
| Paint chip sampling | Certified renovator, inspector, or risk assessor |
Lead abatement
- EPA’s Lead Abatement Program regulations provide a framework for lead abatement, risk assessment, and inspections.
- The federal lead training and certification program establishes five categories or disciplines of lead-based paint professionals.
Environmental Protection Agency’s (EPA’s) Lead Abatement Program regulations at 40 CFR 745 Subpart L provide a framework for lead abatement, risk assessment, and inspections. Subpart L applies only to those individuals, firms, and training providers involved in lead-based paint activities and training related to target housing and child-occupied facilities. However, Subpart L does not cover public or commercial buildings (except child-occupied facilities), superstructures, or bridges.
Those performing these services are required to be trained and certified by EPA or an authorized state. States may, upon approval, receive authorization to carry out their own program in lieu of the federal program. The federal lead training and certification program establishes five categories or disciplines of lead-based paint professionals: supervisors, workers, inspectors, risk assessors, and project designers.
An individual who conducts inspection services must either be a certified inspector or a certified risk assessor. An individual who performs risk assessment services must be a certified risk assessor. The certification requirements for abatement activities depend on the type of work the individuals will be performing. For example, workers and supervisors are required to conduct the actual lead abatement work, while inspectors or risk assessors conduct the clearance testing, and supervisors or project designers prepare occupant protection plans and abatement reports.
The training and certification requirements contained in the regulation ensure that:
- Lead-based paint professionals are properly trained to conduct lead-based paint activities in residential dwellings and facilities regularly occupied by young children, such as day-care centers and pre-schools;
- Lead-based paint inspections, risk assessments, and abatements are conducted reliably, safely, and effectively; and
- Training providers are accredited and capable of providing quality instruction to lead professionals.
Mercury
- MEBA reduces the availability of mercury in domestic and international markets.
- TSCA amendments made it unlawful to export specific mercury compounds after January 1, 2020.
- Persons who manufacture (including import) mercury or mercury-added products, or otherwise intentionally use mercury in a manufacturing process, must report to EPA every three years under the Mercury Inventory Reporting Rule.
Mercury is a naturally occurring chemical element found in rock in the earth’s crust, including in deposits of coal. Elemental or metallic mercury is a shiny, silver-white metal, historically referred to as quicksilver, and is liquid at room temperature. It is used in older thermometers, fluorescent light bulbs, and some electrical switches.
In its inorganic form, mercury occurs abundantly in the environment, primarily as the minerals cinnabar and metacinnabar, and as impurities in other minerals. Mercury can readily combine with chlorine, sulfur, and other elements, and subsequently weather to form inorganic salts. Mercuric chloride is used in photography and as a topical antiseptic and disinfectant, wood preservative, and fungicide. Human exposure to inorganic mercury salts can occur both in occupational and environmental settings.
Mercury exposure at high levels can harm the brain, heart, kidneys, lungs, and immune system of people of all ages. High levels of methylmercury in the bloodstream of babies developing in the womb and young children may harm their developing nervous systems, affecting their ability to think and learn.
TSCA actions related to mercury
Actions to protect the public from exposure to mercury under the Toxic Substances Control Act (TSCA) include the following:
- October 2008 Mercury Export Ban Act (MEBA; Public Law 110-414) reduces the availability of mercury in domestic and international markets. Mercury and mercury compounds may be used to manufacture chemicals and electrical equipment; although, their use has declined due in part to concerns about human health and environmental effects from exposure. Specifically, MEBA:
- Added section 12(c) to TSCA, which prohibits the export of elemental mercury unless the Environmental Protection Agency (EPA) issues an exemption for a specified use as an “essential use” through a petition and rulemaking process, or unless the export involves coal. Essential use exemptions may not exceed three years in duration or 10 metric tons of elemental mercury and are subject to other terms and conditions specified by EPA.
- Added section 6(f) to TSCA to make it unlawful for a federal agency to convey, sell, and distribute elemental mercury under its jurisdiction unless the transfer of elemental mercury facilitates its storage or involves coal. Prior to MEBA’s enactment, the policy of the U.S. Department of Energy (DOE) and Department of Defense (DOD) was to store, not sell, mercury stocks. MEBA codified this existing policy in TSCA and also directed DOE to establish a program for long-term management and storage of elemental mercury generated within the U.S.
- June 2016 Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) amended TSCA section 12(c) to make it unlawful to export specific mercury compounds after January 1, 2020, and to require EPA to report to Congress on the status of mercury compound exports and disposal. The LCSA amended MEBA with regard to the long-term storage of mercury. The LCSA also added section 8(b)(10) to TSCA to direct EPA to gather information regarding the supply, use, and trade of elemental mercury and mercury compounds in the U.S. and periodically publish such information in the Federal Register. In 2017, EPA published its initial report on the inventory of mercury supply, use, and trade in the U.S.
- August 2016 Prohibited Mercury Compounds List identifies five mercury compounds that EPA prohibits from export effective January 1, 2020.
- June 2018 Mercury Inventory Reporting Rule requires reporting from persons who manufacture (including import) mercury or mercury-added products, or otherwise intentionally use mercury in a manufacturing process. Reporting is done by the first of July every three years (2022, 2025, 2028, etc.) using the Mercury Electronic Reporting application. The information collected through the new reporting requirements will be used to develop future inventories of mercury supply, use, and trade in the U.S. Based on the inventory of information collected, EPA will identify any manufacturing processes or products that intentionally add mercury and recommend actions to achieve further reductions in mercury use.
- November 2021 Response to Vacatur of Certain Provisions of the Mercury Inventory Reporting Rule amended the regulations at 40 CFR 713 to expand who must report data to the mercury inventory established under TSCA. The list of exemptions at 713.7(b) was shortened, and now persons who import pre-assembled products that contain a mercury-added component (e.g., a watch that contains a mercury-added battery) are subject to mercury inventory reporting requirements.
Methylene chloride
- The final rule under section 6 of TSCA prohibits the manufacture, processing, and distribution of methylene chloride in all paint and coating removers for consumer use, including distribution to and by retailers.
- Manufacturers, importers, processors, and distributors of methylene chloride for any use must provide downstream notification of the rule’s prohibitions (through a safety data sheet) to companies to whom methylene chloride was shipped.
Methylene chloride is a volatile chemical used in a variety of industries, such as paint and coating removal, plastic processing, metal cleaning and degreasing, adhesive manufacture, and as a heat transfer fluid.
The Environmental Protection Agency (EPA) has found risks to consumers using methylene chloride to be unreasonable due to acute human lethality. Even short-term exposures to the chemical fumes can rapidly cause dizziness, loss of consciousness, and death due to nervous system depression. EPA is particularly concerned about consumers’ risk of death from using methylene chloride in enclosed spaces, e.g., bathrooms.
TSCA actions related to methylene chloride
Actions to protect people and the environment from methylene chloride under the Toxic Substances Control Act (TSCA) are as follows:
- 2019 Consumer Ban on Paint and Coating Removal Products — On March 15, 2019, EPA issued a final rule under section 6 of TSCA to address the agency’s final determination of unreasonable risk of injury to health due to acute human lethality presented by methylene chloride in paint and coating removal for consumer use. (The final rule added regulations to 40 CFR 751.)
- The final rule prohibits the manufacture, processing, and distribution of methylene chloride in all paint and coating removers for consumer use, including distribution to and by retailers. Although EPA had proposed to regulate methylene chloride for other commercial paint and coating removal uses, the agency decided to further evaluate the risks from such uses to inform the development of an appropriate regulatory risk management approach.
- Manufacturers, importers, processors, and distributors of methylene chloride for any use must use a safety data sheet (SDS) to provide downstream notification of the rule’s prohibitions to companies to whom methylene chloride was shipped.
- The recordkeeping requirement under this final rule mandate that each person (excluding retailers of products to consumer end users) who manufactures, processes, or distributes in commerce any methylene chloride must retain in one location at the headquarters of the company, or at the facility for which the records were generated, documentation showing:
* The name, address, contact, and telephone number of companies to whom methylene chloride was shipped;
* A copy of the notification provided to companies to whom the methylene chloride was shipped; and - * The amount of methylene chloride shipped.
- This information must be retained for three years from the date of shipment.
- Managing Risks Found in the 2020 Final Risk Evaluation — EPA selected methylene chloride as one of the first 10 chemicals to undergo risk evaluation to examine risks posed by conditions of use not addressed by the 2019 rule, i.e., commercial methylene chloride in paint and coating removal, including commercial furniture refinishing.
- In June 2020, EPA released the final risk evaluation for methylene chloride, identifying unreasonable risks to workers, occupational non-users, consumers, and bystanders from methylene chloride exposure under 47 out of 53 conditions of use. EPA did not find any unreasonable risks to the environment from use of this chemical.
- The next step in the process required by TSCA is addressing these methylene chloride risks. There are several actions EPA could take to address these risks, including regulations to prohibit or limit the manufacture, processing, distribution in the marketplace, use, or disposal of this chemical substance, as applicable.
Persistent, bioaccumulative, and toxic (PBT) chemical substances
- LCSA includes a provision under TSCA section 6(h) requiring EPA to take expedited action on specific PBT chemicals to address risk and reduce exposures to the extent practicable.
Persistent, bioaccumulative, and toxic (PBT) chemicals are those that build up in the environment over time and can therefore have potential risks for exposed populations, including the general population, consumers and commercial users, and susceptible subpopulations (such as workers, subsistence fishers, tribes, and children).
TSCA actions related to PBT chemical substances
The Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA), enacted on June 22, 2016, includes a provision under Toxic Substances Control Act (TSCA) section 6(h) requiring the Environmental Protection Agency (EPA) to take expedited action on specific PBT chemicals to address risk and reduce exposures to the extent practicable.
EPA identified five PBT chemicals for expedited action in 2016, following criteria outlined in section 6(h) and issued final rules on January 6, 2021, which added regulations to 40 CFR 751 Subpart E. Pursuant to the statute, no risk evaluation was required for these chemicals:
- Decabromodiphenyl ether (DecaBDE)
- Phenol, isopropylated phosphate (3:1) (PIP (3:1))
- 2,4,6-Tris(tert-butyl)phenol (2,4,6-TTBP)
- Hexachlorobutadiene (HCBD)
- Pentachlorothiophenol (PCTP)
However, in September 2021, EPA announced its intent to initiate a new rulemaking and anticipates proposing new rules for all five of the above PBT chemicals that are the subject of final risk management rules under TSCA. EPA explained that it was considering revising all five of the final rules to further reduce exposures, promote environmental justice, and better protect human health and the environment.
EPA took final action on September 17, 2021, to extend the compliance dates for the prohibitions on processing and distribution and the associated recordkeeping requirement of one of these PBT chemicals, phenol, isopropylated phosphate (3:1) (PIP (3:1)) until March 8, 2022. This final rule was effective upon publication. Additionally, on March 8, 2022, EPA issued a final rule further extending the compliance dates until October 31, 2024, for the prohibitions on PIP (3:1) when used in certain articles. This final rule was effective upon publication.
Note that on November 24, 2023, EPA proposed modifications to the regulations of decaBDE and PIP (3:1). The agency explains the proposal, if finalized, addresses risk and reduces exposures to humans and the environment to these PBT chemicals to the extent practicable. In the proposed rule, EPA said that it was not proposing to revise the existing regulations for the other three PBT chemicals (2,4,6-TTBP, HCBD, and PCTP) at that time.
Per- and polyfluoroalkyl substances (PFAS)
- EPA has taken a range of regulatory actions to address PFAS substances in manufacturing and consumer products.
Per- and polyfluoroalkyl substances (PFAS) are a group of human-made chemicals that have been manufactured and used in a variety of industries since the 1940s. PFAS include perfluorooctanoic acid (PFOA), perfluorooctyl sulfonate (PFOS), and many other chemicals.
PFAS are found in a wide range of consumer products that people use daily such as cookware, pizza boxes, and stain repellants. Most people have been exposed to PFAS, and certain PFAS can accumulate and stay in the human body for long periods of time. There is evidence that exposure to PFAS can lead to adverse health outcomes in humans. The most consistent findings from human epidemiology studies are increased cholesterol levels among exposed populations, with more limited findings related to infant birth weights, effects on the immune system, cancer, and thyroid hormone disruption.
TSCA actions related to PFAS
The Environmental Protection Agency (EPA) has taken a range of regulatory actions to address PFAS substances in manufacturing and consumer products as noted below:
- On March 11, 2002, and December 9, 2002, EPA published two significant new use rules (SNURs) to require notification to EPA before any future manufacture (including import) of 88 PFAS chemicals specifically included in the voluntary phase out of PFOS by a chemical company that took place between 2000 and 2002. This SNUR allowed the continuation of a few specifically limited, highly technical uses of these chemicals for which no alternatives were available, and which were characterized by very low volume, low exposure, and low releases. Any other uses of these chemicals would require prior notice to and review by the agency.
- In 2006, EPA invited eight major leading companies in the PFAS industry to join in a global stewardship program.
- On October 9, 2007, EPA finalized a SNUR on 183 PFAS chemicals believed to no longer be manufactured (including imported) or used in the U.S.
- On October 22, 2013, EPA issued a rule requiring companies to report all new uses of certain PFOA-related chemicals as part of carpets, a category of potentially harmful chemicals once used on carpets to impart soil, water, and stain resistance. Companies must now report to EPA their intent to manufacture (including import) these chemical substances intended for use as part of carpets or to treat carpets, as well as import carpets already containing these chemical substances.
- On January 21, 2015, EPA proposed a SNUR to require manufacturers (including importers) of PFOA and PFOA-related chemicals, including as part of articles, and processors of these chemicals to notify EPA at least 90 days before starting or resuming new uses of these chemicals in any products. This notification would allow EPA the opportunity to evaluate the new use and, if necessary, take action to prohibit or limit the activity.
- On June 22, 2016, the Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) significantly amended the Toxic Substances Control Act (TSCA). EPA has issued rules to provide a framework for the implementation of the amended law. The framework rules outline EPA’s path forward for prioritizing, evaluating, and regulating chemicals. It also included a requirement for industry reporting of chemicals manufactured (including imported) or processed in the U.S. over the previous 10 years. (EPA issued this TSCA Inventory Notification (Active-Inactive) rule (also called the Inventory Reset rule) on August 11, 2017.) With regard to PFAS, this reporting will be used to identify which chemical substances are active in U.S. commerce and will help inform the prioritization of chemicals, such as PFAS, for risk evaluation.
- On February 20, 2020, EPA proposed a supplemental SNUR to ensure EPA is notified before anyone begins or resumes the import of long-chain PFAS chemical substances as part of surface coatings on articles.
- In July 2020, EPA issued a final rule strengthening the regulation of PFAS by requiring notice and EPA review before the use of long-chain PFAS that have been phased out in the U.S. could begin again. Additionally, products containing certain long-chain PFAS as a surface coating and carpet containing perfluoroalkyl sulfonate chemical substances can no longer be imported into the U.S. without EPA review. This action means that articles like textiles, carpet, furniture, electronics, and household appliances that could contain certain PFAS chemicals cannot be imported into the U.S. unless EPA reviews and approves the use or puts in place the necessary restrictions to address any unreasonable risks.
- In June 2021, EPA withdrew a compliance guide related to EPA’s July 2020 SNUR on certain long-chain PFAS. The withdrawn compliance guide, which was issued in January 2021, addressed whether certain imported articles were covered by the SNUR. EPA determined the compliance guide inappropriately narrowed the scope and weakened the SNUR. EPA’s July 2020 SNUR continues to be in effect. Articles containing certain long-chain PFAS as a surface coating cannot be imported into the U.S. without EPA review. Importers of articles, but not processors of articles are subject to the SNUR.
- On June 28, 2021, EPA proposed reporting and recordkeeping requirements for PFAS under TSCA section 8(a). In accordance with obligations under TSCA, as amended by the National Defense Authorization Act for Fiscal Year 2020, EPA proposed to require certain persons that manufacture (including import) or have manufactured these chemical substances in any year since January 1, 2011, to electronically report information regarding PFAS uses, production volumes, disposal, exposures, and hazards. The law mandates that EPA issue this data call no later than January 1, 2023. These data will enable EPA to better characterize the sources and quantities of manufactured PFAS in the U.S.
- On October 18, 2021, EPA announced the agency’s comprehensive PFAS Strategic Roadmap: EPA Commitments to Action 2021-2024, which goes over the agency’s approach to confronting PFAS contamination nationwide. Among the strategies, the roadmap calls for:
- A robust review process for new PFAS under TSCA to ensure these substances are safe before they enter commerce;
- A review of existing PFAS under TSCA to: ensure existing PFAS are being used in ways that do not present concerns, and prevent resumed production of legacy PFAS or their use in new ways; and
- Finalize new PFAS reporting under TSCA Section 8 to better characterize the sources and quantities of manufactured PFAS in the U.S.
- Actions from not just EPA’s Office of Chemical Safety and Pollution Prevention but also from the Office of Water, Office of Land and Emergency Management, Office of Air and Radiation, and Office of Research and Development.
- In October 2021, EPA issued the National PFAS Testing Strategy that will help EPA identify and select PFAS for which the agency will require testing using TSCA authorities. EPA’s initial set of test orders for PFAS will be strategically selected from more than 20 different categories of PFAS.
- In March 2022, EPA provided information to manufacturers (including importers), processors, distributors, users, and those that dispose of fluorinated high-density polyethylene (HDPE) containers and similar plastics (i.e., fluorinated polyolefins) about the potential for PFAS to form and migrate from these items.
- In January 2023, EPA proposed a rule that would prevent anyone from starting or resuming, without a complete EPA review and risk determination, the manufacture, processing, or use of an estimated 300 PFAS that have not been made or used for many years, known as “inactive PFAS.” In the past, these chemicals may have been used in many industries, including as binding agents, surfactants, sealants and gaskets, and may also have been released into the environment.
- On June 28, 2023, EPA released a framework for addressing new and new uses of PFAS under TSCA. The framework will ensure that before these chemicals are allowed to enter into commerce, EPA will undertake an extensive evaluation to ensure they pose no harm to human health and the environment.
- In August 2023, EPA finalized its National Enforcement and Compliance Initiatives for 2024-2027, including “Addressing Exposure to PFAS.”
- On October 11, 2023, EPA published a final rule to add 40 CFR 705. The rule will provide EPA, its partners, and the public with the largest-ever dataset of PFAS manufactured and used in the U.S. This final rule will require all manufacturers (including importers) of PFAS and PFAS-containing articles in any year since 2011 to report information to EPA on PFAS uses, production volumes, disposal, exposures, and hazards. Reporting must now be completed by October 13, 2026, for most manufacturers and by April 13, 2027, for small manufacturers reporting exclusively as article importers.. Submissions must be sent electronically to EPA via CDX. See Part 705 for details. Also learn more on EPA’s “TSCA Section 8(a)(7) Reporting and Recordkeeping Requirements for Perfluoroalkyl and Polyfluoroalkyl Substances” webpage.
Polychlorinated biphenyls (PCBs)
- TSCA and the PCB regulations are designed to ban the manufacture of PCBs and ensure the proper disposal of PCBs and PCB Items, while minimizing the risk posed by the storage, use, and handling of the substance.
- The PCB regulations apply to any substance, mixture, or item with a concentration of 50 ppm PCBs or greater or with a concentration below 50 ppm that resulted from dilution.
Polychlorinated biphenyls (PCBs) belong to a broad family of human-made organic chemicals known as chlorinated hydrocarbons. They have no known taste or smell, and range in consistency from an oil to a waxy solid. This chemical has been shown to cause cancer in animals as well as a number of serious non-cancer health effects in animals, including effects on the immune system, reproductive system, nervous system, endocrine system and other health effects. Moreover, PCBs persist and when released into the environment tend to accumulate in tissues of living organisms.
PCBs were domestically manufactured from 1929 until manufacturing was banned in 1979. They have a range of toxicity and vary in consistency from thin, light-colored liquids to yellow or black waxy solids. Due to their non-flammability, chemical stability, high boiling point and electrical insulating properties, PCBs were used in hundreds of industrial and commercial applications including:
- Electrical, heat transfer, and hydraulic equipment;
- Plasticizers in paints, plastics, and rubber products;
- Pigments, dyes, and carbonless copy paper; and
- Other industrial applications.
TSCA and regulatory actions related to PCBs
The Toxic Substances Control Act (TSCA) of 1976 provides the Environmental Protection Agency (EPA) with authority to require reporting, recordkeeping, and testing requirements and restrictions relating to chemical substances and/or mixtures, including PCBs. TSCA addresses the production, importation, use, and disposal of specific chemicals including PCBs.
Current PCB regulations, published pursuant to the TSCA section 6(e), can be found at 40 CFR 761, which has been in place since 1979, as amended. In general, TSCA and the PCB regulations are designed to ban the manufacture of PCBs and ensure the proper disposal of PCBs and PCB Items, while minimizing the risk posed by the storage, use, and handling of the substance.
The PCB regulations generally apply to any substance, mixture, or item with a concentration of 50 parts per million (ppm) PCBs or greater or with a concentration below 50 ppm that resulted from dilution. There are certain exceptions, however. For example, the regulations restrict the marketing and burning of used oil containing any quantifiable PCB level (2 ppm) and prohibit the use of waste oil that contains any detectible concentration of PCBs as a sealant, coating, or dust control agent. However, according to 761.1(b)(3), most provisions in Part 761 apply only if PCBs are present in concentrations above a specified level. Provisions that apply to PCBs at concentrations:
- Less than 50 ppm apply also to contaminated surfaces at PCB concentrations of less than or equal to 10 mg/100 cm2.
- Greater than or equal to 50 to less than 500 ppm apply also to contaminated surfaces at PCB concentrations of greater than 10 mg/100 cm2 to less than 100 mg/100 cm2.
- Greater than or equal to 500 ppm apply also to contaminated surfaces at PCB concentrations of greater than or equal to 100 mg/100 cm2.
Some of the major provisions of the PCB regulations at 40 CFR 761 include:
- General (Subpart A) — In addition to identifying who is regulated, establishing definitions, and listing reference documents, this subpart prescribes the assumed PCB concentrations, for regulatory purposes, of various articles (such as oil filled capacitors) for which the actual PCB concentration is unknown.
- Prohibitions/Authorizations (Subpart B) — There are numerous prohibitions on the use of PCBs or PCB items in a manner other than in a totally enclosed manner; on the manufacture of PCBs for use within the U.S. or for export; and on the processing and distribution of PCBs and PCB items for use within the U.S. or for export. However, the regulations also establish numerous exceptions and authorized activities (e.g., where “non-totally enclosed” activities may be conducted). Such authorizations pertain to the use of PCBs and servicing of PCBs in various PCB equipment, such as transformers, capacitors, natural gas pipelines, and hydraulic systems; the manufacturing of certain products with inadvertent, low-concentration production of PCBs; and the use of sewage sludge with PCBs where such sludge use is regulated by other parts of 40 CFR. Owners of PCB transformers must register the transformers with EPA. Owners of PCB articles may store them for reuse subject to storage area specifications, maximum storage periods, and/or recordkeeping requirements.
- Marking (Subpart C) — Specified items including PCB equipment (e.g., heat transfer systems using PCBs, PCB large low voltage capacitors, and storage areas used to store PCBs/PCB Items) must bear markings warning of PCBs in accordance with prescribed formats. The regulation does not require PCB-contaminated electrical equipment to be marked.
- Storage and disposal (Subpart D) — Regulations govern storage (for reuse or disposal) and disposal of PCBs, PCB waste, and PCB Items, including PCB articles (e.g., transformers, capacitors, and hydraulic machines) and PCB containers. Performance-based cleanup and disposal and cleanup and disposal of PCB wastes caused by an emergency situation are also covered in this subpart. The subpart includes separate sections that set out disposal requirements and allowed disposal methods for PCB remediation waste, PCB bulk product waste, and PCB waste from research and development activities. The regulations exempt PCB household waste from regulatory requirements. The regulations also set out requirements applicable to PCB waste and PCB items in storage for disposal and decontamination of various surfaces. Further regulatory sections specify requirements for each disposal method. PCB disposal and PCB commercial storage facilities must obtain written final approval to operate facilities.
- Exemptions (Subpart E) — This subpart grants exemptions to specific companies or groups of companies for the manufacture, processing, and distribution in commerce of PCBs for specified purposes, including microscopy, research and development, and laboratory sampling and analysis.
- Transboundary shipments of PCBs for disposal (Subpart F) — EPA prohibits the importation of PCBs for disposal without an exemption issued under the authority of TSCA section 6(e)(3). EPA prohibits the exportation of PCBs for disposal at concentrations greater than or equal to 50 ppm. Shipments that leave the U.S. only as part of their transit from one part of the U.S. to another are not considered exports or imports. Shipments passing through from Canada to Mexico or vice versa are not considered exports or imports.
- Recordkeeping/Reporting (Subpart J) — Owners and operators of facilities with PCBs and PCB items in service or projected for disposal, commercial storage facilities of PCB waste, incineration facilities, chemical waste landfill facilities, high efficiency boiler facilities, importers, facilities generating PCBs in excluded manufacturing processes, and facilities that manufacture, import, process, distribute in commerce, or use chemicals containing inadvertently generated PCBs must comply with recordkeeping and reporting requirements. Some types of data for which records may be required to be kept include PCB weights; the identification and numbers of items; storage, transfer, and disposal dates; and the identification of shippers and receivers. Note that EPA Form 6200-025 must be used for the report due July 15 annually per 761.180.
- PCB waste disposal records and reports (Subpart K) — Some generators and all transporters, storers, and disposers of PCB wastes must notify EPA that they are engaging in such activity and obtain an identification number from EPA. When a PCB waste generator sends such wastes offsite, the generator, transporter, and disposer must prepare and maintain manifests identifying the waste and tracking the dates and parties involved in the disposal process. The disposal facility must prepare a Certificate of Disposal and send it to the generator identified on the manifest. The subpart also includes recordkeeping requirements and procedures for cases in which manifests or Certificate of Disposal are not prepared by one of the parties in a transaction. Note that EPA issued final rules on September 6, 2012, July 2, 2015, and August 29, 2023, to update and clarify several sections of the PCB regulations at 40 CFR 761 associated with manifesting requirements.
- Sampling and decontamination procedures for wastes and surfaces (Subparts M through T) — These subparts set out recommended procedures for sampling PCBs in various wastes and surfaces, including sample site and size selection, sample collection, analytical requirements, and interpretation of results. The regulations also set out a method for decontaminating non-porous surfaces and requirements for studies of new decontamination solvents.
Recent rulemaking activity relating to Part 761 includes the August 29, 2023, final rule to address several issues related to implementing the PCB cleanup and disposal. Specifically, among the changes, EPA:
- Expanded the options for extraction and determinative methods used to characterize and verify the cleanup of PCB waste;
- Added more flexible provisions to facilitate cleanup and protective disposal of waste generated by spills that occur during emergency situations (e.g., hurricanes or floods);
- Amended the performance-based disposal option for PCB remediation waste by adding explicit cleanup provisions, including the requirement to notify EPA and follow specific sampling protocols; and
- Removed the provision that had allowed PCB bulk product waste to be disposed of as roadbed material.
Significant new use rules and notices
- EPA determines significant new uses through rulemakings on a chemical-by-chemical basis after considering all relevant factors.
- Persons who intend to export a substance identified in a proposed or final SNUR are subject to the export notification provisions of TSCA section 12(b).
Chemical manufacturers (including importers) and processors must submit a notice to the Environmental Protection Agency (EPA) before manufacturing or processing a chemical for a use that EPA has determined to be significant and new. EPA determines significant new uses through rulemakings on a chemical-by-chemical basis after considering all relevant factors, including:
- Projected volume of manufacturing and processing of a chemical substance;
- Extent to which a use changes the type or form of exposure of humans or the environment to a chemical substance;
- Extent to which a use increases the magnitude and duration of exposure of humans or the environment to a chemical substance; and
- Reasonably anticipated manner and methods of manufacturing, processing, distribution in commerce, and disposal of a chemical substance.
Although most significant new use determinations are associated with new chemicals, EPA has determined that certain non-ongoing uses (i.e., discontinued uses) are new uses for specific existing chemicals. EPA generally determines that a discontinued use of an existing chemical is a significant new use for which notification is required when a manufacturer or processor seeks to reintroduce such discontinued use of the existing chemical into commerce. This notification is intended to give the agency an opportunity to prevent the reintroduction of a discontinued use of an existing chemical if such a reintroduction would pose unreasonable risks. As an example, in April 2019, EPA determined that “any discontinued uses of asbestos cannot re-enter the marketplace without EPA review.”
Rulemaking prompts notifications
When EPA determines significant new uses, the rulemakings the agency issues are known as significant new use rules (SNURs). SNURs create regulations that appear at 40 CFR 721. This prompts three types of notifications:
- Significant new use notice (SNUN) — The notifications submitted by chemical manufacturers, importers, and processors under a SNUR are known as significant new use notices (SNUNs). Toxic Substances Control Act (TSCA) section 5 directs EPA to review a SNUN within 90 days of receipt to determine if regulatory action is warranted. EPA may extend the review period up to 90 days with appropriate justification. EPA promulgates multiple SNURs per year, but the agency does not receive, and generally does not expect, many SNUN submissions per year, based on prior experience. Promulgation of a SNUR does not imply EPA would approve the significant new uses of a chemical reported in a SNUN if the agency were to receive one. EPA does not predetermine the outcome of a SNUN review when promulgating a SNUR.
- Customer notification — Manufacturers (including importers) and processors of chemical substances subject to SNURs must also notify recipients of such chemicals of the SNUR or verify that knowledge of the SNUR has been otherwise acquired by recipients, or that the recipients are unable to engage in significant new uses. Since it is not expected that all such entities will have complete knowledge of all uses of any products subject to a SNUR, and because filing a SNUN could require significantly more burden, it is assumed that manufacturers (including importers) and processors will most often choose to notify their customers of SNUR regulatory activities. Notification may be accomplished by simply annotating and sending a safety data sheet (SDS).
- Export notification — Persons who intend to export a substance identified in a proposed or final SNUR are subject to the export notification provisions of TSCA section 12(b), and regulations that interpret TSCA section 12(b) appear at 40 CFR 707.
Determining if a chemical is subject to a SNUR
- If a facility’s chemical substance is subject to a SNUR and the facility’s intended manufacture, processing, or use of the substance is a significant new use, the facility would be required to submit a SNUN 90 days prior to the manufacture of that substance.
To facilitate determining whether a substance is subject to a significant new use rule (SNUR), substances on the Toxic Substances Control Act (TSCA) Inventory that are subject to SNUR requirements are designated as such by an “S” flag in the Inventory listing. If a facility’s chemical substance is subject to a SNUR and the facility’s intended manufacture, processing, or use of the substance is a significant new use, the facility would be required to submit a significant new use notice (SNUN) 90 days prior to the manufacture of that substance.
Note that, according to 40 CFR 721.63, any facility subject to an “applicable” SNUR must determine and use appropriate engineering and administrative controls BEFORE using personal protective equipment (PPE) for worker protection. It is considered a significant new use NOT to implement this hierarchy of controls to protect workers when required. Where engineering and administrative controls are not feasible or are insufficient to protect exposed workers, facilities that are subject to an applicable SNUR must follow any applicable PPE requirements or submit a SNUN to the Environmental Protection Agency (EPA ).
Similarly, according to 40 CFR 721.72, any facility subject to an “applicable” SNUR must establish a written hazard communication program. It is considered a significant new use NOT to establish this written program when required. Where a hazard communication program is not established, facilities subject to an applicable SNUR must submit a SNUN to EPA.
Other significant new uses of chemical substances, which are identified in 40 CFR 721 subpart E, are described in 40 CFR 721 subpart B. These significant new uses relate to industrial, commercial, and consumer activities; disposal; and releases to water. The provisions of subpart B apply only when referenced as applying to a chemical substance identified in subpart E of 40 CFR 721.
Several steps should be followed to ascertain the TSCA Inventory/SNUR status of a chemical substance. Information on non-confidential chemical substances can be found in the TSCA Inventory. Because the chemical identities of the chemical substances can be claimed to be Confidential Business Information (CBI), the Environmental Protection Agency (EPA) maintains a CBI version of the TSCA Inventory. If an intended manufacturer submits a premanufacture notice or a notice of bona fide intent to manufacture (pursuant to the procedures at 40 CFR 720.25 or 721.11) on a substance that has a listing on the Confidential Inventory, the agency will notify the submitter of the existence of the SNUR and identify any confidential significant new use designations.
It is always the obligation of the manufacturer or processor selling a chemical substance to notify the user of the SNUR status of that substance. Buyers of a chemical substance whose identity is confidential, and thus not disclosed to them, should seek certification from the sellers that their intended use is not a significant new use.
SNUN filing and exemptions
- Receipt of a SNUN triggers EPA review of the risks associated with the SNUN and a determination of whether such risks warrant control.
- SNUNs are reported using the standard e-PMN form and are subject to a 90-day review process similar to that for a PMN.
If the Environmental Protection Agency (EPA) promulgates a significant new use rule (SNUR) under 40 CFR 721, the Toxic Substances Control Act (TSCA) requires persons to submit a significant new use notice (SNUN) to EPA at least 90 days before they manufacture (including import) or process the substance for that use. Information submitted in a SNUN must include, insofar as it is known to or reasonably ascertainable by the submitter, information described in TSCA section 8(a)(2) (i.e., chemical identity, use, and exposure data), as well as test data, and descriptions of other data related to the effects on health and the environment of the manufacture, processing, use, distribution in commerce, and disposal of the chemical substance (TSCA section 5(d)).
Receipt of a SNUN triggers EPA review of the risks associated with the SNUN and a determination of whether such risks warrant control. EPA reviews specific significant new uses proposed in a SNUN on a case-by-case basis before such uses of the chemical enter commerce. The notification gives EPA the opportunity to evaluate the new use and:
- Make an affirmative determination on the safety of the significant new use; or
- Take action to prohibit or limit the activity to address any unreasonable risks identified.
EPA review of a SNUN puts the agency in the position of reviewing the risks of specific significant new uses only when introduction into commerce is imminent.
EPA recommends that submitters consult with the agency prior to submitting a SNUN to discuss what data may be useful in evaluating a significant new use. Discussions with the agency prior to submission can afford ample time to conduct any tests that might be helpful in evaluating risks posed by the substance.
Joint SNUN submissions
Potential SNUN submitters should be aware of 40 CFR 721.25(b), which authorizes joint SNUNs by two or more persons -- for example, a manufacturer and several processors. In many cases, EPA will need to respond to a SNUN by amending the SNUR to allow companies other than the SNUN submitter (such as the submitter’s processor customers) to engage in the newly approved use(s). Note that before EPA amends the SNUR, even after a manufacturer submits a SNUN and the review period expires, processors (and other manufacturers) of the substance are still legally required to submit their own SNUN before engaging in the significant new use.
Electronic submission
SNUNs are reported using the standard e-PMN form and are subject to a 90-day review process similar to that for a premanufacture notice (PMN). When submitting a SNUN, the submitter should include a cover letter that provides the Code of Federal Regulations citation of the SNUR and identifies the specific significant new use(s) for which the SNUN is being submitted.
Exemptions
Under TSCA section 5(h), EPA may exempt a chemical manufacturer or processor from submitting a SNUN when the agency determines that the use of a chemical substance would not pose an unreasonable risk, or when the agency already has information about the chemical substance. In some circumstances, EPA may not grant an exemption unless an entity applies for one. For exemptions that require an application, EPA must grant or deny the exemption within 45 days of receiving the application.
Regulation of significant new uses
- If EPA issues a proposed rule under TSCA section 5(f), the requirements in the proposed rule become effective upon its publication in the Federal Register.
If the Environmental Protection Agency (EPA) finds that a significant new use of a chemical substance subject to a significant new use notice (SNUN) presents an unreasonable risk, section 5(f) of the Toxic Substances Control Act (TSCA) directs the agency to either:
- Propose a rule imposing one or more of the requirements specified in section 6(a) to the extent necessary to protect against such risk, or
- Issue an order to prohibit or limit the manufacture, processing, or distribution of the chemical.
If EPA issues a proposed rule under section 5(f), the requirements in the proposed rule become effective upon its publication in the Federal Register, and EPA must, as expeditiously as possible, either finalize the rule (with or without modification) or revoke it. An issued order becomes effective at the end of the review period, which is 90 days unless extended to 180 days with good cause.
If EPA finds that available information is insufficient to evaluate risks of a new chemical substance or significant new use, section 5(e) requires EPA to issue an administrative order to prohibit or otherwise restrict manufacture, processing, distribution, use, or disposal to the extent necessary to protect against unreasonable risks pending the development of further information. An issued order becomes effective at the end of the review period. The submitter of the SNUN must comply with the order while the required information is being developed. For significant new uses for which information is insufficient to evaluate risks while section 5(e) orders apply to the SNUN submitter, EPA may in practice also promulgate a significant new use rule (SNUR) to apply the restrictions outlined in an order to other manufacturers and processors.
For substances subject to SNUN restrictions that EPA subsequently finds are not likely to present unreasonable risk; the submitter of the notice may commence manufacture of the substance for the uses described in the notice after the agency publishes a statement of its finding.
Orders related to importing
TSCA section 5(e) orders may include restrictions on the amount of the chemical allowed to be manufactured (including imported), as well as other restrictions. (The import/production limits often serve as triggers for toxicity or related testing requirements.) To comply with these requirements when applicable, chemical substances must:
- Not be imported for any prohibited use;
- Satisfy all applicable labeling and safety data requirements;
- Not exceed any specified restrictions on permissible import volume;
- Not be imported for any designated significant new use; and
- Comply with any other applicable requirements.
Other reporting and recordkeeping
- TSCA section 8 directs EPA to promulgate rules that require chemical manufacturers and processors to maintain records pertaining to the chemicals they use.
- EPA requires electronic reporting of certain information submitted to the agency under the TSCA.
When the Toxic Substances Control Act (TSCA) was enacted, Congress recognized that very little was known about chemicals in the environment. It was not even known how many chemicals there were, in what quantities they were produced and where, what their by-products were, and who was exposed to them and under what conditions. This information was available only for a handful of existing chemicals.
Therefore, Congress gave the Environmental Protection Agency (EPA) authority under TSCA section 8 to require the submission of reports and the retention of information so the agency could develop information on these basic questions.
TSCA section 8 overview
TSCA section 8 directs EPA to promulgate rules that require chemical manufacturers and processors (other than small manufacturers and processors) to maintain records pertaining to the chemicals they use. EPA may require these records to include information such as chemical identity, uses, volumes produced, by-products, health and environmental effects, exposure, and disposal methods.
EPA may also require chemical manufacturers and processors to report such records to the agency. For small manufacturers and processors, EPA may promulgate recordkeeping and reporting requirements only for chemicals for which the agency has already required the development of new information or that are already regulated under the Act.
Based on information gathered by EPA, the agency must maintain a list of chemical substances manufactured or processed for commercial purposes in the U.S. EPA refers to this list as the TSCA Inventory. Refer to the TSCA Inventory section.
Section 8 also requires chemical manufacturers or processors to report to EPA any information that indicates a chemical presents a “substantial risk” of injury to human health or the environment with regard to a chemical. The agency may require the submission of health and safety studies which are known or available to those who manufacture, process, or distribute in commerce specified chemicals.
Additionally, section 8 authorizes EPA to require reporting of information documenting “significant adverse reactions” to human health or the environment alleged to have been caused by a chemical, as well as lists and copies of available health and safety studies.
In accordance with TSCA section 8(a), EPA has also issued several chemical-specific regulations that call for manufacturers and processors or potential manufacturers or processors of the chemicals to maintain records and submit reports as EPA requires. Note that EPA requires certain reports to be submitted electronically.
Electronic reporting
EPA requires electronic reporting of certain information submitted to the agency under TSCA. Electronic reporting is intended to save time, improve data quality, and increase efficiency. Submitters will be required to use EPA’s Central Data Exchange (CDX), the agency’s electronic reporting site.
Specifically, EPA requires electronic submission of data and other TSCA section 8 information for:
- TSCA section 8(a) Preliminary Assessment Information Rule (PAIR) submissions;
- TSCA 8(a) Chemical data reporting; and
- TSCA section 8(d) health and safety studies.
Outside of our TSCA section 8 discussion, it should be noted that electronic submission of data and other information is also required for:
- TSCA section 4 test rules;
- TSCA section 5 premanufacture notices; and
- TSCA section 5 Notices of Commencement of Manufacture or Import (NOCs) and support documents relating to section 5 notices sent to EPA before April 6, 2010.
Preliminary assessment information rule (PAIR)
- Under PAIR, producers and importers of a listed chemical are required to report certain site-specific information.
Under TSCA, which covers the production, distribution, use, and disposal of chemical substances, EPA’s Office of Pollution Prevention and Toxics is charged with the responsibility for assuring that chemicals made available for sale and use in the U.S. do not pose any unreasonable adverse risks to human health or to the environment. To carry out this mandate, EPA has broad authority to issue regulations designed to gather health/safety and exposure information on, require testing of, and control exposure to chemical substances and mixtures.
TSCA section 8(a) requirements
TSCA section 8(a), Reporting and Retention of Information, gives EPA the authority to promulgate rules under which manufacturers (which by statute includes importers) and processors of chemical substances must maintain records and/or report such data as EPA may reasonably require in order to carry out the TSCA mandates. Examples of information that can be required to be reported under TSCA section 8(a) include:
- Chemical or mixture identity;
- Categories of use;
- Quantity manufactured or processed;
- By-product description;
- Health and environmental effects information;
- Number of individuals exposed; and
- Method(s) of disposal.
Section 8(a) regulations can be tailored to meet unique information needs (e.g., via chemical-specific rules) or information can be obtained via the use of “model” or standardized reporting rules.
Preliminary Assessment Information Rule (PAIR)
The PAIR requirements at 40 CFR 712 is one example of a model TSCA section 8(a) reporting rule. Under PAIR, producers and importers of a listed chemical are required to report the following site-specific information:
- Quantity of chemical produced and/or imported;
- Amount of chemical lost to the environment during production or importation;
- Quantity of enclosed, controlled, and open releases of the chemical; and
- Per release, the number of workers exposed and the number of hours exposed.
Respondents are only required to report information that is known or reasonably ascertainable by them. Extensive file searches are not required. The PAIR reporting requirements are included in the PAIR form (EPA Form 7710-35) and instructions.
Exemptions for such reporting are as follows:
- Production or importation for the sole purpose of research and development;
- Production or importation of less than 500 kilograms during the reporting period at a single plant site;
- Companies whose total annual sales from all sites owned by the domestic or foreign parent company are below $30 million for the reporting period and who produced or imported less than 45,400 kilograms of the chemical; and
- Production or importation of the listed chemical solely as an impurity, a non-isolated intermediate, and under certain circumstances as a by-product.
EPA uses PAIR to collect information to help identify, assess, and manage human health and environmental risks caused by the manufacture or importation of identified chemical substances, mixtures, and categories. PAIR is also available to EPA for the purpose of informing the prioritization and risk evaluation activities.
EPA or other federal agencies (e.g., the agencies that are part of the Interagency Testing Committee (ITC) as authorized under TSCA section 4(e)) can identify chemicals for a TSCA section 8(a) PAIR expediated rulemaking that have a justifiable need for production, use, or exposure-related data.
Substantial risk notice
- EPA’s guidance states that Substantial Risk Notifications under TSCA section 8(e) should be submitted within 30 calendar days of obtaining substantial risk information.
- EPA allows commercial establishments to assume responsibility for submitting substantial risk information obtained by individual employees and officials.
- The FYI classification was created by EPA to capture chemical risk submissions by persons or organizations not subject to the TSCA section 8(e) reporting requirements.
Section 8(e) of the Toxic Substances Control Act (TSCA) requires U.S. chemical manufacturers (including importers), processors, and distributors to notify the Environmental Protection Agency (EPA) immediately of information that reasonably supports the conclusion that their substances or mixtures present a substantial risk of injury to health or the environment. EPA’s guidance states that Substantial Risk Notifications under TSCA section 8(e) should be submitted within 30 calendar days of obtaining substantial risk information.
Section 8(e) continues to be an important and useful tool for early warning and identification of potential substantial risk situations allowing EPA and others to focus their limited resources on chemicals or mixtures of highest concern. The submission of section 8(e) information makes it possible for the agency and others to learn quickly about potential new chemical hazards/risks posed by exposure to chemical substances, to conduct more complete assessments and, if needed, to take effective action to eliminate or reduce such risks in a timely manner.
Specifically, section 8(e) of TSCA states, “any person who manufactures, [imports,] processes, or distributes in commerce a chemical substance or mixture and who obtains information which reasonably supports the conclusion that such substance or mixture presents a substantial risk of injury to health or the environment shall immediately inform the [EPA] Administrator of such information unless such person has actual knowledge that the Administrator has been adequately informed of such information.”
What is substantial risk?
EPA’s TSCA section 8(e) guidance (in the June 3, 2003, Federal Register 68 FR 33129) states that a “substantial risk of injury to health or the environment” is:
- A risk of considerable concern because of the seriousness of the effect, and
- The fact or probability of its occurrence.
These two criteria are differentially weighted for different types of effects. Certain human health effects are so serious that relatively little weight is given to exposure. The mere fact that the implicated chemical is in commerce constitutes sufficient evidence of exposure. In contrast, the remaining human health effects as well as environmental effects must involve or be accompanied by the potential for significant levels of exposure.
Who is required to report?
Section 8(e) of TSCA requires manufacturers (including importers), processors, and distributors of chemicals to notify the EPA immediately of information that reasonably supports the conclusion that their substances or mixtures present a substantial risk of injury to health of the environment. TSCA section 8(e) does not provide exemptions for small businesses, small production or importation volumes, or commercial activities such as manufacture for export only or research and development.
EPA allows commercial establishments to assume responsibility for submitting substantial risk information obtained by individual employees and officials. EPA’s TSCA section 8(e) policy statement explains that individual officers/employees are viewed as having discharged their individual TSCA section 8(e) responsibilities once they notify a designated supervisor or official in full about pertinent information, provided the employing entity has an established, internally publicized and affirmatively implemented procedure governing such Substantial Risk Notifications. The agency’s TSCA section 8(e) policy statement specifies that such procedures, at a minimum, must:
- Specify the information that must be reported;
- Indicate how the reports are to be prepared and submitted internally;
- Note the federal, civil, and criminal penalties for failure to report substantial risk information; and
- Provide a mechanism for the timely notification of officers and employees who submitted reports about the disposition of those reports. Such notification should inform the reporting employee/officer as to whether or not the information was submitted to EPA, and if not, inform the employee or officer of their protected right (under TSCA section 23) to report the information directly to EPA.
It is important to note that those employees and officers who are responsible for actual management of the organization’s TSCA section 8(e) reporting obligations retain personal, civil, and/or criminal liability for ensuring that substantial risk information is submitted to EPA. In the absence of established internal procedures, all employees and officers retain their individual responsibilities and liabilities for ensuring that substantial risk information is reported to the agency.
It should be noted that trade associations and industry consortia can submit substantial risk notification on behalf of member companies covered under the reporting requirement.
Voluntary submissions
EPA welcomes human health and environmental risk-related information submitted by persons not covered by the TSCA section 8(e) reporting requirement. The agency established a classification system to distinguish voluntary “For Your Information” (FYI) submissions from Substantial Risk Notifications submitted formally to EPA under TSCA section 8(e), discussed above. The FYI classification was created by EPA to capture chemical risk submissions by persons or organizations not subject to the TSCA section 8(e) reporting requirements.
Sometimes FYIs are also submitted when a manufacturer, importer, or processor is not sure its information supports a conclusion of substantial risk. Often, they are submitted when a person or company not required to submit, nevertheless, would like to bring risk information on a chemical to EPA’s attention. Chemical companies, trade associations, public interest groups, and academic institutions are among those who submit FYIs.
When and how to submit a substantial risk notice
- There is no required collection instrument or reporting form on which section 8(e) information must be submitted to EPA.
- Submissions may be filed using EPA’s electronic Central Data Exchange (CDX).
Under Toxic Substances Control Act (TSCA) section 8(e), persons covered under the reporting requirement should report the new information to the Environmental Protection Agency (EPA) within 30 calendar days of obtaining it. The exceptions are:
- Emergency Incidents of Environmental Contamination, which should be reported immediately by telephone to the National Response Center at (800) 424-8802 or to the EPA Administrator or EPA Regional Administrator, and
- Non-emergency Incidents of Environmental Contamination, which should be reported to EPA under TSCA within 90 calendar days of obtaining it, unless reported within 90 days to another EPA office or federal or state regulatory agency, as described in section VII (c) through (f) of the section 8(e) guidance. See page 33139 of the June 3, 2003, Federal Register, and look for section VII (Information Which Need Not Be Reported).
There is no required collection instrument or reporting form on which section 8(e) information must be submitted to EPA; however, the section 8(e) policy statement requires all respondents to ensure that a written section 8(e) notice:
- Is sent to EPA by a method verifying the agency’s receipt;
- States that it is being submitted under section 8(e) of TSCA;
- Contains the name, address, job title, phone number, and signature of the person reporting, and the name and address of the establishment with which the person is associated;
- Identifies the chemical substance(s) or mixture including, if known, the Chemical Abstracts Service (CAS) Registry Number(s);
- Summarizes adverse health/environmental effects being reported including a description of the nature and extent of the risk; and
- Contains the specific source/summary of the supporting data.
Submitters of Substantial Risk Notifications pursuant to TSCA section 8(e) and voluntary “For Your Information” (FYI) submissions have the option to file electronically, rather than by paper. These submissions may be filed using EPA’s electronic document submission system, the Central Data Exchange (CDX).
EPA encourages stakeholders to use CDX to send TSCA section 8(e) notices, versus submitting them by paper. However, EPA will continue to send paper letters acknowledging receipt of notices that were submitted by paper.
The steps to reporting electronically are as follows:
- Register to use EPA’s agency-wide CDX portal for submitting information in a secure manner.
- Once registered and in CDX, select the Chemical Safety and Pesticide Programs (CSPP) section.
- From this section, access the Chemical Information Submission System (CISS), a web-based TSCA reporting tool.
- Data submissions pursuant to TSCA sections 8 and FYI are available under the CISS reporting tool.
This reporting tool is compatible with Windows, Mac, Linux, and UNIX based computers, and uses “Extensible Markup Language” (XML) specifications for efficient data transmission across the Internet.
The CISS Web reporting tool has several important features:
- Provides user-friendly navigation;
- Works with CDX to secure online communication;
- Creates a completed Portable Document Format (PDF) for review prior to submission; and
- Enables data, reports, and other information to be submitted easily as PDF attachments or in other electronic formats, such as XML.
In order to facilitate the efficiency in communications and cost savings in submissions and correspondence for both EPA and respondents, EPA has incorporated the following data elements into the reporting tool:
- Submission Type — Identifies the submission, including the type of submission and whether it is the initial submission, a follow-up, or a final report.
- Summary of Attachment — Allows the respondent to provide a summary or abstract of the attached study or report, any internal company tracking number, an EPA tracking number, and an indication of the number of studies submitted.
- Chemical Identification — Identifies the chemical(s) addressed in the submission.
- Title of Attachment — Identifies the title of the attached study or report.
- Indexing Terms — Allows the respondent to identify the proper terms to use for indexing purposes, which facilitates the search and retrieval of the information.
- Submitter Information — Identifies the submitter and/or technical contact, including name, title, company, mailing address, phone, and email address.
- Comments — Allows the submitter to provide any additional comments, so as to avoid the need for or use of a separate cover letter.
EPA is also now able to electronically acknowledge receipt of substantial risk notices submitted via the CDX. The communication service is similar to how some electronic banking messaging systems work. It will deliver a secure message to the authorized official’s (AO) CDX account and will deliver a second generic message to the AO’s email account, stating a communication has been delivered to their CDX account. The email will also provide directions for retrieving the communication from CDX.
Health and safety studies and data reporting
- Manufacturers and processors of the chemical substances and mixtures subject to a TSCA section 8(d) rulemaking must submit lists and copies of health and safety studies relating to the health and/or environmental effects of the chemical substances and mixtures.
- All studies submitted to EPA will be verified and the contents of the submissions recorded and inspected for the inclusion of CBI.
- EPA will make non-confidential versions of the health and safety studies available via its publicly available ChemView online database.
Toxic Substances Control Act (TSCA) section 8(d) authorizes the Environmental Protection Agency (EPA) to promulgate rules requiring certain persons who manufacture, process, or distribute in commerce (or propose to manufacture, process, or distribute in commerce) chemical substances and mixtures, to submit to EPA lists and copies of health and safety studies in their possession with respect to such chemical substances and mixtures.
Health and safety study is intended to be interpreted broadly and means “any study of any effect of a chemical substance or mixture on health or the environment or on both,” including but not limited to:
- Epidemiological or clinical studies,
- Studies of occupational exposure,
- In vivo and in vitro toxicological studies, and
- Ecotoxicological studies.
Data items
These rules, which are codified in 40 CFR 716, require the manufacturers and processors of the chemical substances and mixtures subject to a TSCA section 8(d) rulemaking to submit lists and copies of health and safety studies relating to the health and/or environmental effects of the chemical substances and mixtures. All submitted studies must be accompanied by a cover letter that contains the following data:
- Name;
- Job title;
- Address;
- Telephone numbers of the submitting official;
- Name and address of the manufacturing or processing establishment on whose behalf the submission was made;
- Identity of any impurity or additive known to have been present in the substance or listed mixtures as studied, unless so noted in the study; and
- Indication that the study is being submitted under Part 716.
List of studies shall include:
- Ongoing health and safety studies conducted by or initiated by respondents — For these studies, the list should include the following data: beginning date of the study, purpose of the study, types of data to be collected, anticipated date of completion, and name and address of the laboratory conducting the study.
- Studies respondents know about but do not have copies of — For these studies, the list should include the name and address of a person known to them that possess a copy of the study.
- Studies that have been sent to another federal agency with no claims of confidentiality — For these studies, the list should include the following data: title of the study, name and address of the person to whom the study was sent, and month and year in which the study was submitted.
Compliance steps
EPA amends the list of subject chemicals in 40 CFR 716 periodically to add chemical substances and mixtures. The listed chemical substances and mixtures include chemicals recommended for testing under TSCA section 4 by the Interagency Testing Committee (ITC) and other chemical substances that EPA, or other federal agencies, choose to assess for health or environmental effects.
Reporting of information is only required when the subject matter information is available. Availability of study reports on the list may occur after the established reporting period for the list and must still be submitted when they become available. Studies previously submitted to EPA are exempt.
To comply, respondents must search their records to identify any health and safety studies in their possession, copy and process relevant studies, list studies that are currently in progress, and submit this information to EPA. Specifically, a representative respondent would engage in the following activities in order to produce the lists of studies and required data:
- Determine whether the firm may be required to report. If so, review the rule in more detail;
- Conduct a corporate review to identify which firm sites must be searched to locate the appropriate health and safety studies;
- Search the files at appropriate sites to locate relevant studies;
- Compile and transcribe lists of studies being submitted, ongoing studies, newly initiated studies, studies known to exist but not known to be in the respondent’s possession, and studies previously submitted to other federal agencies without confidentiality claims;
- Photocopy or prepare electronic versions of the studies;
- Voluntarily prepare robust summaries of the studies;
- Review the responses for possible confidential business information and prepare information to substantiate a claim of confidentiality; and
- Submit the studies to EPA electronically, and, after initial study submissions, notify EPA when other studies are initiated; submit studies completed after the reporting period.
Reporting is mandatory. However, respondents may claim all or part of a response confidential. EPA will disclose information that is covered by a claim of confidentiality only to the extent permitted by, and in accordance with, the procedures in TSCA section 14 and 40 CFR 2.
What happens to the submitted data?
All studies submitted to EPA will be verified and the contents of the submissions recorded and inspected for the inclusion of confidential business information (CBI). EPA will disclose information that is covered by a claim of confidentiality only to the extent permitted by, and in accordance with, the procedures in TSCA section 14, 40 CFR 2, and 40 CFR 703.
Copies of the documents will then be prepared and distributed, based on the associated chemical identity, to program offices at EPA and/or to other federal agencies for scientific analysis. A coding form will be completed to capture certain descriptive information such as identity, document control number, confidentiality indicator, document title, document date, receipt date, and chemical identity. The document will be stored electronically for archival purposes. In addition, EPA will make non-confidential versions of the health and safety studies available via its publicly available ChemView online database.
EPA uses this information to support its investigation of the risks posed by the chemical substance in question and construct a complete picture of the known effects of the chemical substance. This will support the agency’s decisions on whether to require additional test data be submitted under TSCA section 4.
Significant adverse reaction
- TSCA section 8(c) requires that allegations of adverse reactions caused by any chemical substance or mixture to the health of employees be kept for 30 years, and all other allegations be kept for five years.
- Respondents subject to 40 CFR 717 must submit copies of allegation records when required by the EPA.
- Firms subject to 40 CFR 717 must keep their TSCA section 8(c) records at company headquarters or at a site central to their chemical operations.
Toxic Substances Control Act (TSCA) section 8(c) requires that allegations of adverse reactions to the health of employees be kept for 30 years, and all other allegations be kept for five years.
The Environmental Protection Agency (EPA) promulgated 40 CFR 717, on August 22, 1983. The regulation requires manufacturers (including importers) and processors of chemical substances and mixtures to keep records of “significant adverse reactions” alleged to have been caused by such substances or mixtures. The rule also prescribes the conditions under which a firm must submit or make records available to a duly designated representative of EPA.
Required records
Records maintained pursuant to 40 CFR 717 must consist of the following:
- The original allegation as received.
- An abstract of the allegation and other pertinent information as follows:
- The name and address of the plant site that received the allegation.
- The date the allegation was received at that site.
- The implicated substance, mixture, article, company process or operation, or site discharge.
- A description of the alleger (e.g., employee or neighbor), including age and sex, if ascertainable.
- A description of the health effects, including explanation of how the effects became known and the route of exposure, if explained in the allegation.
- The results of any self-initiated investigation with respect to an allegation. (EPA does not require such investigation under TSCA section 8(c).)
- Copies of any further required records relating to the allegation, e.g., records required under the Occupational Safety and Health Administration (OSHA).
Required compliance activities
Respondents subject to 40 CFR 717 must:
- Maintain records of allegations of significant adverse reactions — Entities subject to the rule must record significant reactions alleged to have been caused by substances or mixtures that they manufacture, import, or process. These firms must establish a recordkeeping system for such allegations and monitor incoming complaints to determine if they meet the criteria for filing. Allegations that are filed must be retained for 30 years if they are employee-related and for five years for all other types/sources of allegations.
- Submit copies of these allegation records when required by EPA — EPA will notify those responsible for reporting by letter or will announce any such requirements by notice in the Federal Register. When required to report, firms must submit copies of records via CDX https://cdx.epa.gov/ using the EPA-provided electronic reporting application.
Claims of confidentiality must be made in accordance with the procedures described in 40 CFR 703.
Firms subject to 40 CFR 717 must:
- Keep their TSCA section 8(c) records at company headquarters or at a site central to their chemical operations — A multi-site company will usually require the responsible official at the individual plant site to forward potentially recordable TSCA section 8(c) allegations to a designated TSCA coordinator at their operations headquarters. Depending on the size of the company, such allegations will be reviewed by a committee to determine if the allegations relate to the company’s product, operations, or discharges. If so, the effects cited in the allegation are compared against the rule’s definition and examples of “significant adverse reaction.” If the allegation meets this test, it is recorded. The actual allegation record is to be comprised of an abstract of the allegation along with a record of any company-initiated investigation and other pertinent documents. The rule does not require further investigation. EPA requires that allegations be filed so that they may be readily retrievable by the alleged “cause” of the reaction. EPA does not, however, require a specific form under this rule.
- Maintain an awareness of their reporting requirements — A reporting requirement will take the form of a letter directed to selected respondents or it will be a notice in the Federal Register. Respondents are responsible for monitoring the Federal Register for such notices. Whenever feasible, EPA will also notify those companies that can be identified with the production, importation, or processing of a substance or mixture in question. Respondents then must determine if they manufacture or process the chemical substance or mixture. If so, they must conduct a search of their TSCA section 8(c) files to determine if there are any relevant records of significant adverse reactions alleged to have been caused by the substance or mixture. When required to report, firms must submit copies of records via CDX https://cdx.epa.gov/ using the EPA-provided electronic reporting application. The firm should note that it has submitted such records to EPA so that future duplicative reporting will not occur.
Chemical data reporting (CDR)
- The CDR regulation requires manufacturers (including importers) to provide EPA with information on the production and use of chemicals in commerce.
- Information is collected every four years from manufacturers (including importers) of certain chemicals in commerce generally when production volumes for the chemical are 25,000 pounds or greater for a specific reporting year.
Chemical Data Reporting (CDR) data collection provides chemical manufacture, processing, and use information that helps the Environmental Protection Agency (EPA) identify what chemicals, from those listed on the Toxic Substances Control Act (TSCA) Inventory, the public may be exposed to as consumers or in commercial and industrial settings. The data also help EPA assess routes of potential exposure to those chemicals.
Overview
The CDR regulation at 40 CFR 711, as authorized by section 8(a) of TSCA, requires manufacturers (including importers) to provide EPA with information on the production and use of chemicals in commerce. The CDR rule was formerly known as the Inventory Update Rule (IUR).
Under Part 711, EPA collects basic exposure-related information including information on the types, quantities and uses of chemical substances produced domestically and imported into the U.S. The CDR database constitutes the most comprehensive source of basic screening-level, exposure-related information on chemicals available to EPA and is used to protect the public from potential chemical risks.
The information is collected every four years from manufacturers (including importers) of certain chemicals in commerce generally when production volumes for the chemical are 25,000 pounds or greater for a specific reporting year. However, a reduced reporting threshold (2,500 pounds) applies to chemical substances subject to certain TSCA actions. Collecting the information every four years assures that EPA and (for non-confidential data) the public have access to up-to-date information on chemicals.
How does EPA use CDR data?
EPA uses CDR data to support risk screening, risk assessment, chemical prioritization, risk evaluation, and risk management activities, among other activities. This information allows EPA to develop an understanding of the types, amount, end uses, and possible exposure to chemicals in commerce.
The data include information on the manufacture (including import), industrial processing and use, and consumer and commercial use of certain chemicals currently listed on the TSCA Inventory, a list of chemicals that are manufactured (including imported) or processed in the U.S. Processing and use information helps EPA screen and prioritize chemicals for the purpose of identifying potential human health and environmental effects.
Who is required to report?
- Manufacturers (including importers) are required to report if they meet certain production volume thresholds for a chemical substance at any single site.
- Generally, water and naturally occurring substances are exempt from CDR requirements.
Submission periods are from June 1 to September 30 at 4-year intervals. The 2024 submission period is scheduled for June 1 to September 30, 2024. Manufacturers (including importers) are required to report if they meet certain production volume thresholds for a chemical substance at any single site:
- Reporting is triggered if the annual reporting threshold at a manufacturing (including importing) site is met during any of the calendar years since the last principal reporting year. For example, for the 2024 submission period, the need to report is based on the production volume for the calendar years 2020-2023.
- In general, the annual reporting threshold is 25,000 pounds per site. However, a reduced reporting threshold (2,500 pounds) applies to chemical substances subject to certain Toxic Substances Control Act (TSCA) actions.
- For chemical substances that trigger reporting, total annual production volume must be reported for each calendar year since the last principal reporting year.
- The reporting threshold for processing and use information is the same as the reporting threshold for Chemical Data Reporting (CDR) generally (25,000 pounds or 2,500 pounds), depending on the existence of certain TSCA actions.
- Certain TSCA actions may have one or more of the following effects for specific chemical substances:
- Reduction in the threshold production volume that triggers reporting requirements.
- Limitation on certain full or partial exemptions from reporting requirements.
- Limitation on use of the small manufacturer exemption.
- Only one reporting threshold (either 25,000 pounds or 2,500 pounds) will apply to a chemical substance for the CDR.
Comparison of effects of TSCA actions on different CDR requirements or exemptions:
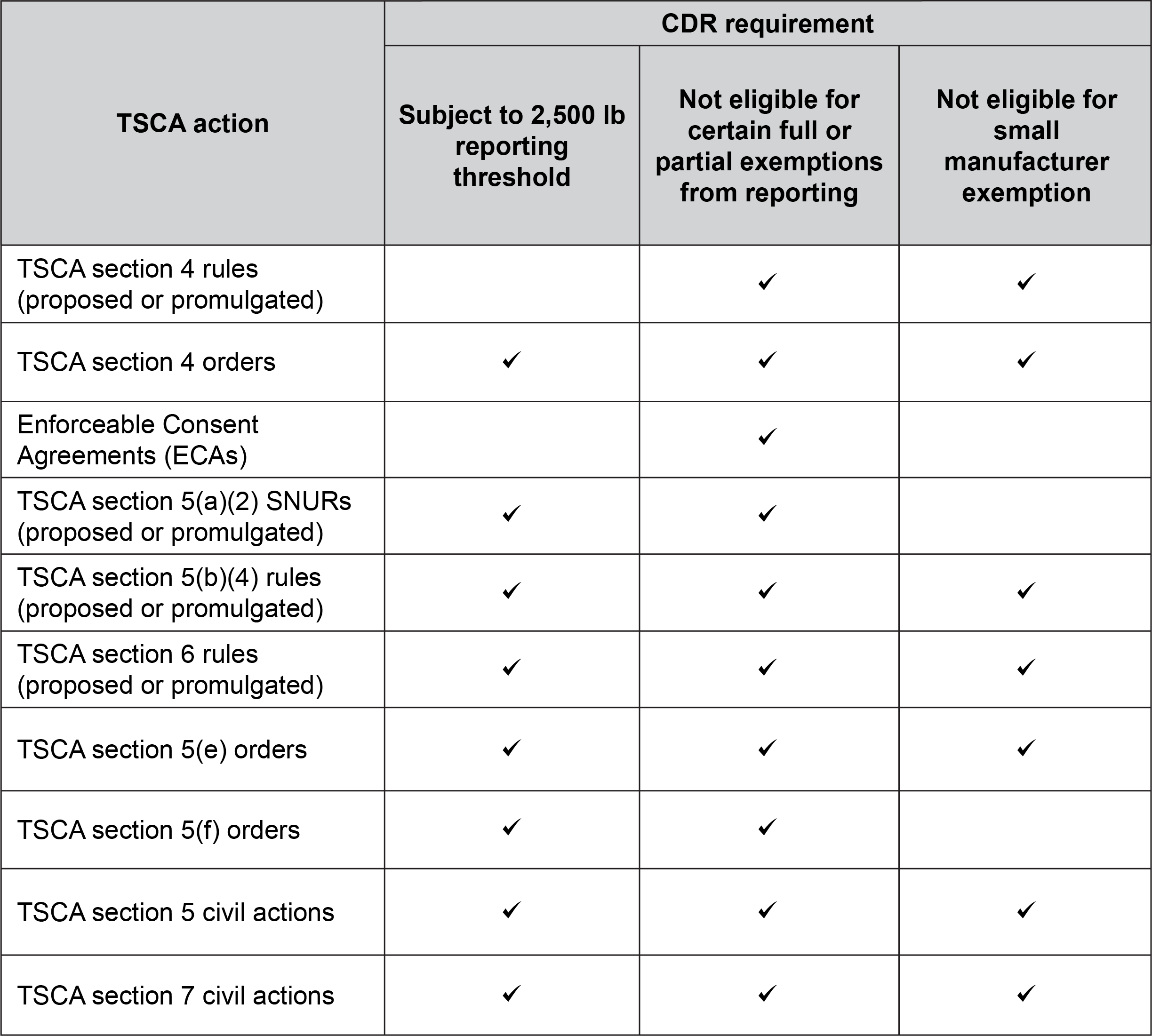
Chemicals exempt from reporting
Manufacturers (including importers) may not be required to report information on certain chemicals to CDR because of the type of chemical or because of the manner of manufacture (including import) or use of the chemical:
- Chemicals manufactured (including imported) for non-TSCA uses are not required to be reported (e.g., pesticides are exempt from reporting under CDR by TSCA). If a portion of a manufacturer’s (including importer’s) production is not subject to TSCA (for example, if the use is regulated by the Food and Drug Administration), then the production associated with the non-TSCA second use will not be reported to CDR. Note that manufacturers may report downstream non-TSCA uses for their chemical.
- Generally, water and naturally occurring substances are exempt from CDR requirements. Three other groups of chemicals (polymers, microorganisms, and certain forms of natural gas) are also generally exempt from CDR requirements. It is important to note that a particular polymer, microorganism, or form of natural gas may not be exempt if the chemical becomes the subject of certain TSCA actions, such as an enforceable consent agreement.
- Chemicals that are non-isolated intermediates, imported as part of an article, impurities, or by-products destined for certain commercial uses are exempt from reporting.
Small manufacturers exempt from reporting
The Environmental Protection Agency (EPA) has finalized amendments to the definition of small manufacturer in accordance with TSCA section 8(a)(3)(C). A submitter meeting the definition of small manufacturer at 40 CFR 704.3 would be generally exempt from CDR reporting.
Note that it is possible to qualify as a small manufacturer with respect to some chemical substances and not others or with respect to some sites and not others. Also, for purposes of the definition of a small manufacturer, total annual sales include all sales of the company, not just the total sales of a given chemical substance.
However, the exemption for small businesses does not apply to persons who manufacture (including import) a chemical substance that is the subject of a rule proposed or promulgated under Section 4, 5(b)(4), or 6 of TSCA; or is the subject of an order in effect under Section 4 or 5(e) of TSCA; or is the subject of relief that has been granted under a civil action under Section 5 or 7 of TSCA (40 CFR 711.9). In such circumstances, the volume thresholds for reporting found in 40 CFR 711.8 apply.
Reporting thresholds and how to report
- CDR is triggered by the amount of a chemical manufactured (including imported), rather than the hazard or potential exposures associated with a chemical.
- To submit Form U electronically to EPA, a person must register with the CDX and be approved by EPA.
Reporting thresholds
Chemical Data Reporting (CDR) is triggered by the amount of a chemical manufactured (including imported), rather than the hazard or potential exposures associated with a chemical. Understanding reporting thresholds of manufactured (including imported) chemicals that trigger reporting is important when using and interpreting CDR data. The thresholds are as follows:
- 25,000 pounds or greater — Reporting for 2016 and future submission periods is triggered based on whether site-specific production volume meets or exceeds 25,000 pounds during any calendar year since the last principal reporting year. For the 2024 submission, the last principal reporting year was 2019 and therefore the submitter considers annual production from 2020 to 2023.
- 2,500 pounds or greater — For chemicals subject to certain Toxic Substances Control Act (TSCA) actions, the production volume reporting threshold was lower, and for those chemicals the submitter considers whether the annual production volume meets or exceeds 2,500 pounds.
- For all reportable chemicals, manufacturers (including importers) are required to report the full manufacturing data for the principal reporting year (e.g., calendar year 2019 for 2020 CDR and calendar year 2023 for 2024 CDR) and production volume for each calendar year since the previous principal reporting year (e.g., 2016 to 2019 for 2020 CDR and 2020 to 2023 for 2024 CDR).
Due to these reporting thresholds, totals of CDR production volumes reported may underestimate the actual total amount manufactured and imported in the U.S., particularly if there are a substantial number of sites that manufacture (including import) the chemical in quantities less than 25,000 pounds per year or are otherwise exempted from reporting (such as small manufacturers). When comparing changes in production over time at a site, particularly on regional or national levels, it is important to take into account regulatory changes in the reporting thresholds across the years.
How to report
To submit Form U electronically to the Environmental Protection Agency (EPA), a person must register with the Central Data Exchange (CDX) and be approved by EPA.
- Register on CDX — To register for CDR reporting, go to the CDX website and register under the program “Submissions for Chemical Safety and Pesticide Programs (CSPP).” If previously registered on CDX for e-TSCA, e-PMN, or TRI, an individual is then able to add the CDR reporting flow to the current registration.
- Access e-CDRweb — An individual can access this agency-provided electronic reporting tool used to complete Form U after registering in CDX. Access e-CDRweb through the CSPP portal.
- Complete and submit the completed electronic Form U.
If there are technical issues or problems regarding CDX registration, the individual should contact the CDX Help Desk at helpdesk@epacdx.net or (888) 890-1995. International callers who wish to contact the Help Desk should call (970) 494-5500.
Confidential business information (CBI)
- Processing and use data elements could be claimed as CBI if a submitter believed that the release of information would reveal trade secrets or confidential commercial or financial information.
It is important for users of the Chemical Data Reporting (CDR) public database to understand what data submitters are permitted to claim as confidential business information (CBI) and the way in which the public database has been aggregated and masked to protect CBI. Submitters could designate individual CDR data elements as CBI when they report information. However, chemical identity may only be claimed confidential if the chemical is listed on the confidential portion of the Toxic Substances Control Act (TSCA) Inventory.
Processing and use data elements may be claimed as CBI if a submitter believes that the release of information would reveal trade secrets or confidential commercial or financial information. Submitters are required to substantiate confidentiality claims for chemical identity, site identification, processing and use information, and other non-exempt data elements by answering a series of questions. A blank response or a response that was designated as “not known or reasonably ascertainable” may not be claimed as confidential. Production volume could also be claimed as CBI.
In preparing the CDR public database, the Environmental Protection Agency (EPA) takes care to avoid releasing CBI while also publishing as much information as possible. Users examining individual records will notice CBI-protected entries in some data fields.
If all of the production volumes for a chemical were not claimed as CBI, then the public CDR database would include specific values for individual and aggregated production volumes for that chemical. However, if some or all of the reported production volumes for a given chemical substance were claimed as CBI, then some or all of the individual CBI production volumes were not published and aggregated production volumes were published as a range.
Chemical imports
- Imports of chemical substances, mixtures, or articles that contain a chemical substance or mixture must comply with TSCA in order to enter the U.S.
- A certification must be signed and filed electronically or in writing with CBP by the importer or an authorized agent of the importer.
- No certification is required for chemicals that are a part of articles, unless required by a specific rule under TSCA and tobacco or tobacco products.
The Toxic Substances Control Act (TSCA) places requirements on those importing chemicals, mixtures, or articles containing a chemical substance or mixture into the customs territory of the U.S. If they fail to comply with any TSCA rule or otherwise violate TSCA, they are not allowed into the customs territory of the U.S.
Import certification
Imports of chemical substances, mixtures, or articles that contain a chemical substance or mixture must comply with TSCA in order to enter the U.S. Importers must certify that imported chemicals either comply with TSCA (positive certification) or, if not otherwise clearly identified as a chemical excluded from TSCA, are not subject to TSCA (negative certification).
The requirements are described in section 13 of TSCA (codified at 15 U.S.C. 2612) and in implementing regulations developed by the U.S. Customs and Border Protection (CBP), in consultation with the Environmental Protection Agency (EPA), at 19 CFR 12.118 through 12.127. EPA itself has a companion chemical importing regulation at 40 CFR 707.
Who must certify?
A certification must be signed and filed electronically or in writing with CBP by the importer or an authorized agent of the importer. A certification must also include the certifier’s name, email address, and telephone number. Certification is required for substances that are imported and are received by mail or commercial carrier, including those intended for research and development. Certifications filed electronically must be filed in the Automated Commercial Environment (ACE).
For paper certification, the statement must be typed, preprinted on the invoice, or otherwise included in the entry documentation and must be filed with the director of the port of entry before release of the shipment.
Certification statements
An importer’s statement must certify either that the chemical shipment is:
- Subject to TSCA and complies with all applicable rules and orders (positive certification); or
- That the chemical shipment is not subject to TSCA (negative certification).
Positive certification statement
The following is a positive certification statement: “I certify that all chemical substances in this shipment comply with all applicable rules or orders under TSCA and that I am not offering a chemical substance for entry in violation of TSCA or any applicable rule or order thereunder.”
A positive certification means that the chemical substance complies with all applicable TSCA regulations, including:
- Section 5 premanufacture notification rules,
- Section 5 significant new use rules,
- Section 5(e) orders,
- Section 5(f) rules and orders,
- Section 6 rules and orders,
- Section 7 judicial actions, and
- Title IV rules and orders.
Note that TSCA sections 4 and 8 rules do not pertain to section 13 import certification requirements. Although importers must satisfy all applicable requirements of sections 4 and 8 rules, compliance with those provisions is not related to individual chemical shipments and therefore does not affect import certification.
Negative certification statement
The following is a negative certification statement: “I certify that all chemicals in this shipment are not subject to TSCA.”
A negative certification is required for the following products when not clearly identified:
- Any pesticide;
- Any food, food additive, drug, cosmetic, or device;
- Source material, special nuclear material, or by-product material; and
- Firearms and ammunitions as defined in section 3 of TSCA.
Note that these products may be considered clearly identified when they are associated with another relevant agency’s entry documentation or electronic entry filing requirements (e.g., Notice of Arrival for pesticides or applicable entry documentation for FDA regulated products).
No certification is required for the following:
- Chemicals that are a part of articles, unless required by a specific rule under TSCA; and
- Tobacco or tobacco products.
More information
For further guidance, refer to:
- Compliance Guide for the Chemical Import Requirements of the Toxic Substances Control Act; EPA publication EPA 305-B-08-001; June 2008; and
- TSCA Section 13 Import Compliance Checklist; EPA publication EPA 740-B-08-001; April 2008.
Importing specific chemicals and imminent hazards
- The 1989 Partial Ban prohibits import of some asbestos-containing products.
- TSCA prohibits import of PCBs.
Importing specific chemicals
Section 6 of the Toxic Substances Control Act (TSCA) contains import requirements applicable to some specific chemicals:
- PCBs — Section 6(e) of TSCA prohibits import of polychlorinated biphenyls (PCBs). See 40 CFR Part 761 for regulations on the import of PCBs. In certain limited circumstances, the Environmental Protection Agency (EPA) may grant exemptions to allow import of PCBs provided statutory and regulatory requirements are met.
- Asbestos — The 1989 Partial Ban prohibits import of some asbestos-containing products, including corrugated paper, rollboard, commercial paper, specialty paper, and flooring felt. EPA also banned new uses of asbestos which prevent new asbestos products from entering the marketplace after August 25, 1989.
- Formaldehyde — The TSCA Title VI import certification requirement applies to composite wood products (i.e., panels of hardwood plywood, particleboard, medium density fiberboard, and thin-medium density fiberboard), component parts containing such composite wood products, and finished goods containing such composite wood products that are imported into the U.S. beginning March 22, 2019. Under TSCA Title VI, importers must also take reasonable precautions by retaining, for three years, bills of lading, invoices, or comparable documents that include a written statement from the supplier that the composite wood products (or component parts/finished goods) are TSCA Title VI compliant. When EPA requests, the importer must make available within 30 days records identifying (1) the panel producer and the date the composite wood products were produced; and (2) records identifying the supplier, if different, and the date the composite wood products (or component parts/finished goods) were purchased. Refer to 40 CFR 770.
In addition to the chemicals listed above, import requirements apply to the following two types of chemicals covered by TSCA section 6 regulations:
- Certain chemical substances that may be used in metalworking fluids and that are regulated under 40 CFR 747; and
- Certain hexavalent-chromium-based water treatment chemicals that are regulated under 40 CFR 749.68.
Imminent hazards
Because imports are required to comply with any judicial orders that may be issued under section 7 of TSCA, importers need to be aware of section 7 requirements. Section 7 authorizes EPA to commence a judicial action for seizure of a chemical substance, mixture, or article containing such a chemical substance or mixture, which EPA has determined is imminently hazardous, and/or for other relief against any person who manufactures (imports), processes, distributes in commerce, uses, or disposes of an imminently hazardous chemical substance or mixture or any article containing such a substance or mixture.
Importing new chemicals and uses
- Because imports are included in the definition of manufacture, importing chemical substances can trigger premanufacture notices, significant new use notices, and other requirements.
Section 3 of the Toxic Substances Control Act (TSCA) defines the term manufacture to include import. This means that the section 5(a)(1)(B) requirement to submit a premanufacture notice (PMN) to EPA at least 90-days before commencing non-exempt commercial manufacture of a new chemical substance in the U.S. applies to the import of new chemicals, as does the section 5(a)(2) significant new use notice (SNUN) requirement. Thus, the intended import of chemical substances can trigger the following provisions:
- Premanufacture notice provisions for new chemicals in 40 CFR 720;
- Significant new use notice provisions in 40 CFR 721;
- Premanufacture notice exemptions for new chemicals in 40 CFR 723; and
- Reporting requirements for inter-generic microorganisms in 40 CFR 725.
Import requirements in TSCA section 5(e) orders and section 5(a)(2) significant new use rules
When appropriate, EPA issues section 5 regulatory requirements on new chemicals or significant new uses of chemicals via a TSCA section 5(e) order or section 5(a)(2) significant new use rule (SNUR, 40 CFR Part 721 or 725 Subparts L and M).
TSCA section 5(e) orders may include use prohibitions, labeling and safety data sheet (SDS) requirements, restrictions on the amount of the chemical allowed to be manufactured (including imported), as well as other restrictions. (The import/production limits often serve as triggers for toxicity or related testing requirements.) SNURs require notifying EPA at least 90 days before manufacture (including import), or processing for uses/activities designated by EPA as a significant new use.
To comply with these requirements when applicable, chemical substances must:
- Not be imported for any prohibited use,
- Satisfy all applicable labeling and SDS requirements,
- Not exceed any specified restrictions on permissible import volume,
- Not be imported for any designated significant new use, and
- Comply with any other applicable requirements.
Testing
- EPA has the authority to require manufacturers (including importers) and processors of chemical substances and mixtures to conduct testing on the health and environmental effects of chemical substances and mixtures.
Under section 4 of the Toxic Substances Control Act (TSCA), the Environmental Protection Agency (EPA) has the authority to require manufacturers (including importers) and processors of chemical substances and mixtures to conduct testing on the health and environmental effects of chemical substances and mixtures.
A person who imports or intends to import a chemical substance or mixture subject to a test rule under section 4 must comply with section 4 requirements unless the importation qualifies for an exemption included in the regulations at 40 CFR 790.42, or under a specific test rule listed under 40 CFR 766 or 799.
Following promulgation of a test rule under section 4, the responsibility to comply with the rule continues for a period of five years from the date the data from all required tests have been submitted or an amount of time equal to that which was required to develop the test data, whichever is longer. Importers therefore have a continuing responsibility to determine whether a chemical substance or mixture which they import or intend to import is subject to a test rule.
Reporting and recordkeeping
- Specific TSCA section 8 rules apply to importers.
Section 8 of the Toxic Substances Control Act (TSCA) authorizes the Environmental Protection Agency (EPA) to require persons that manufacture (includes import), process, and distribute in commerce TSCA-covered chemical substances and mixtures to keep certain records and report certain information to EPA. Specific TSCA section 8 rules (and implementing policy documents in the case of section 8(e)) that apply to importers include:
- TSCA Section 8(a) Chemical Data Reporting (CDR) – See 40 CFR 711.
- TSCA Section 8(a) Preliminary Assessment Information Reporting (PAIR) Rule – See 40 CFR 712.
- TSCA Section 8(a) Chemical Specific Recordkeeping and Reporting Rules – See 40 CFR 704 Subpart B. This subpart includes several chemicals. Asbestos, found at 704.180, has a data submission period beginning February 24, 2024, and ending May 24, 2024.
- TSCA Section 8(a)(7) Perfluoroalkyl or Polyfluoroalkyl Substance (PFAS) Data – See 40 CFR 705. For all reporters submitting information pursuant to 40 CFR 705.15 and 705.18(b) (research and development), the submission period begins on April 13, 2026, and lasts for six months: April 13, 2026, through October 13, 2026. For any reporter who is reporting pursuant to 40 CFR 705.18(a) (article importers), and is also considered a small manufacturer , the submission period begins on April 13, 2026, and lasts for 12 months: April 13, 2026, through April 13, 2027.
- TSCA Section 8(b)(10)(D) Mercury Reporting – See 40 CFR 713. Reporting is done by July 1 every three years (specifically, 2022, 2025, 2028, etc.)
- TSCA Section 8(c) Allegations of Significant Adverse Reactions Recordkeeping and Reporting Rule – See 40 CFR 717.
- TSCA Section 8(d) Unpublished Health and Safety Data Reporting Rule – See 40 CFR 716.
- TSCA Section 8(e) Substantial Risk Information Reporting Requirement (Statutory Provision) – See implementing Policy Statement (43 FR 11110, March 16, 1978), as well as the TSCA Section 8(e) Reporting Guide dated June 1991 (available in hard copy from the TSCA Hotline).
Chemical exports
- Any person who exports or intends to export a chemical substance or mixture that is subject to certain TSCA regulations is required to notify EPA.
- Export notices sent to EPA must be postmarked within seven days of forming the intent to export or on the date of export, whichever is earlier.
The Toxic Substances Control Act (TSCA) places requirements on those exporting chemicals out of the customs territory of the U.S.
Export notification Under TSCA section 12(b), any person who exports or intends to export a chemical substance or mixture that is subject to certain TSCA regulations is required to notify the Environmental Protection Agency (EPA). EPA, in turn, provides information about the exported chemical and EPA’s related regulatory actions, to the importing government. See EPA’s implementing regulations at 40 CFR 707 Subpart D.
TSCA export notification requirements
An exporter is defined as any person who, as the principal party in interest in the export transaction, has the power and responsibility for determining and controlling the sending of the chemical substance or mixture to a destination outside the customs territory of the U.S.
Exporters must notify EPA if they export or intend to export a chemical substance or mixture for which:
- The submission of data is required under TSCA sections 4 or 5(b);
- An order has been issued under TSCA section 5;
- A rule has been proposed or promulgated under TSCA sections 5 or 6; or
- An action is pending, or relief has been granted under TSCA sections 5 or 7.
Regarding section 4 of TSCA, only those chemical substances or mixtures listed in final TSCA section 4 test rules and TSCA section 4 Enforceable Consent Agreements (ECAs) are subject to the export notice requirements under TSCA section 12(b). Chemical substances subject to TSCA section 4 testing requirements “sunset” after a specific period of time. Be aware that a section 4 chemical that has “sunset” may also be the subject of another TSCA action triggering export notice requirements so that an export notice may still be required.
Notification of export is generally not required for articles per 40 CFR 707.60(b) and for specified de minimis concentrations per 40 CFR 707.60(c).
Timing of the exporter’s notice
For substances or mixtures subject to TSCA section 5(f), 6, or 7 actions, exporters must notify EPA of the first export within each calendar year of export per subject chemical per country of import. For substances or mixtures subject to TSCA section 4, 5(a)(2), 5(b), or 5(e) actions, the exporter must submit a notice to EPA only for the first export to a particular country; notice of export to a particular country is not required if an exporter previously submitted to EPA a notice of export to that country prior to January 6, 2007.
Export notices sent to EPA must be postmarked within seven days of forming the intent to export or on the date of export, whichever is earlier. A notice of intent to export must be based on a definite contractual obligation, or an equivalent intra-company agreement, to export the regulated chemical.
Content and submission methods for exporter’s notice
The notice can be submitted using the Central Data Exchange (CDX) or by letter to EPA and include the following information:
- Name and address of the exporter,
- Name of the chemical substance or mixture,[1]
- Date(s) of export or intended export,
- Country or countries of import, and
- Section of TSCA (4, 5, 6, or 7) under which EPA has taken action.
[1] For substances on the confidential portion of the TSCA Inventory, the substance must be identified by generic name and accession number, or by any other nonconfidential identifier under which it is listed on the TSCA section 12(b) reporting list maintained by EPA and available in the TSCA section 12(b) Export Notification Application described in 707.65(c).
Notices shall be marked “TSCA Section 12(b) Notice” and sent to EPA by mail or delivered by hand or courier (see 40 CFR 707.65).
EPA’s notice to receiving countries EPA must send a notice to the government of the importing country no later than five working days after receipt of the first annual notification from any exporter for each substance or mixture that is subject to TSCA section 4, 5(a)(2), 5(b), 5(e), 5(f), 6, or 7 actions. The EPA notice to the importing government includes the following information:
- Identification of the regulated chemical;
- Summary of the EPA regulatory action taken, or an indication of the availability of data under TSCA sections 4 or 5(b);
- EPA official to contact for further information; and
- Copy of the pertinent Federal Register notice.
Exporting specific chemicals
- TSCA prohibits export of PCBs.
- Export of metallic mercury is also prohibited.
Section 6 of the Toxic Substances Control Act (TSCA) contains export requirements applicable to some specific chemicals:
- PCBs — Section 6(e) of TSCA prohibits export of polychlorinated biphenyls (PCBs). See 40 CFR 761 for regulations on the export of PCBs. In certain limited circumstances, the Environmental Protection Agency (EPA) may grant exemptions to allow export of PCBs, provided statutory and regulatory requirements are met.
- Elemental (metallic) mercury — The Mercury Export Ban Act was signed into law on October 14, 2008. The law intends to reduce the availability of elemental (metallic) mercury in domestic and international markets. By reducing the supply of metallic mercury in commerce, the Act aims to reduce the use of mercury for commercial purposes globally. The Act’s three main provisions are the following:
- Federal agencies are prohibited from conveying, selling, or distributing metallic mercury that is under their control or jurisdiction. This includes stockpiles held by the Departments of Energy (DOE) and Defense (DOD).
- Export of metallic mercury is prohibited from the U.S. beginning January 1, 2013.
- DOE shall designate one or more DOE facilities for long-term management and storage of metallic mercury generated within the U.S. This designation began on January 1, 2010.
- Any person residing in the U.S. may petition EPA for an exemption from the prohibition on export of metallic mercury.
In addition to the chemicals listed above, export requirements or prohibitions apply to the following types of chemicals covered by TSCA section 6 regulations:
- Certain chemical substances that may be used in metalworking fluids and that are regulated under 40 CFR 747.
- Certain hexavalent-chromium-based water treatment chemicals that are regulated under 40 CFR 749.68.
- Certain asbestos-containing products that are regulated under 40 CFR 763.
Manufacturing new chemicals solely for export
- A new chemical substance is exempt from the PMN requirement if it is manufactured solely for export and certain conditions are met.
Under section 12(a) of the Toxic Substances Control Act (TSCA) and 40 CFR 720.30(e), a new chemical substance is exempt from the premanufacture notice (PMN) requirement if it is manufactured solely for export and if, when the substance is distributed in commerce:
- The substance is labeled “for export” in accordance with section 12(a)(1)(B) of the Act; and
- The manufacturer knows that the person to whom the substance is being distributed intends to export it or process it solely for export as defined in 40 CFR 721.3.
It should be noted that chemical substances, mixtures, or articles can be covered by TSCA if EPA finds that the substance will present an unreasonable risk within the U.S. For example, EPA may require that the substance be tested.
Confidential business information
- TSCA imposes requirements that submitters must meet when claiming information as CBI.
Because information that the Environmental Protection Agency (EPA) obtains may contain material that, if disclosed, would harm commercial interests, the Toxic Substances Control Act (TSCA) prohibits the disclosure of submitted information for which the submitter has claimed and justified the need for confidentiality. EPA regulations establish procedures to protect such confidential business information (CBI) from public disclosure.
Overview
Some of the information chemical manufacturers and processors submit to EPA under TSCA is proprietary. CBI is broadly defined as proprietary information, considered confidential to the submitter, the release of which would cause substantial business injury to the owner. Companies generally make CBI claims for confidential proprietary information believed to give other companies an advantage in the marketplace, such as details of their manufacturing processes and formulas.
To balance the objectives of protecting sensitive or proprietary information from public disclosure and maintaining the public’s right of access to information about EPA’s activities, section 14 of TSCA protects information from disclosure under certain conditions. Specifically, TSCA builds upon protections from disclosure set forth in the Freedom of Information Act (FOIA).
FOIA generally requires federal agencies to disclose information requested by any person unless the information falls under one of nine exemptions. Section 14 of TSCA requires EPA to withhold from disclosure information that meets the criteria under FOIA Exemption 4 for which a confidentiality claim has been properly asserted. FOIA Exemption 4 protects “trade secrets and commercial or financial information obtained from a person and privileged or confidential.” Knowing and willful disclosure of such information is subject to a criminal penalty under TSCA.
TSCA requires the agency to review and make determinations regarding the validity of claims based on the criteria in the statute and regulations. TSCA also imposes requirements that submitters must meet when claiming information as CBI. Some of these requirements include:
- Assertions that the submitter must make;
- Substantiation of all CBI claims, except for those on information exempt from substantiation under TSCA section 14(c)(2); and
- A structurally descriptive generic name, if a CBI claim is for a specific chemical identity.
EPA may also review and make determinations on CBI claims in other circumstances, including when information that is claimed as CBI is responsive to a request under FOIA.
Making CBI claims in TSCA submissions
- There are several procedural requirements that must be followed when asserting CBI claims in TSCA submissions.
- If specific chemical identity is claimed as CBI, a structurally descriptive generic name must also be provided.
Under section 14(a) of the Toxic Substances Control Act (TSCA), submitters may claim information submitted to the Environmental Protection Agency (EPA) under TSCA as confidential business information (CBI). CBI claims must be asserted and substantiated concurrently with the submission of the information, except for those types of information exempt under TSCA section 14(c)(2).
Examples of information that may not be protected as CBI
TSCA section 14(b) identifies certain information that may not be protected as CBI, including:
- Health and safety studies and information from health and safety studies where the chemical or mixture has been offered for commercial distribution or for which testing is required under TSCA section 4 or notification is required under TSCA section 5. However, process information and portions of mixture information may be protected;
- Any general information describing manufacturing volumes, expressed as specific aggregated volumes or, if the EPA Administrator determines that disclosure of specific aggregated volumes would reveal confidential information, expressed in ranges; and
- A general description of a process used in the manufacture or processing and industrial, commercial, or consumer functions and uses of a chemical substance, mixture, or article containing a chemical substance or mixture, including information specific to an industry or industry sector that customarily would be shared with the general public or within an industry or industry sector.
How to make CBI claims in TSCA submissions
There are several procedural requirements that must be followed when asserting CBI claims in TSCA submissions. These are described in detail in 40 CFR 703.
TSCA submitters are solely responsible for ensuring that all the requirements for claiming information as CBI are met at the time the information is submitted to EPA. The procedural requirements for making a CBI claim include:
- The required certification statements;
- Information claimed as CBI is clearly identified;
- Substantiation of CBI claims for information that is not exempt from substantiation under section 14(c)(2);
- Sanitized copy provided as required; and
- A structurally descriptive generic name, if a CBI claim is for a specific chemical identity.
The authorized official submitting CBI claims must make several assertions as well as certify that information submitted to substantiate a CBI claim is true and correct, as required by sections 14(c)(1)(B) and 14(c)(5) of TSCA.
EPA has combined these requirements into the certification statement (below) that can be used to satisfy these requirements. See 40 CFR 703.5(a). If specific chemical identity is claimed as CBI, a structurally descriptive generic name must also be provided. See 40 CFR 703.5(d).
Certification statement for CBI claims
I hereby certify to the best of my knowledge and belief that all information entered on this form is complete and accurate. I further certify that, pursuant to 15 U.S.C. 2613(c), for all claims for confidentiality made with this submission, all information submitted to substantiate such claims is true and correct, and that it is true and correct that
- My company has taken reasonable measures to protect the confidentiality of the information;
- I have determined that the information is not required to be disclosed or otherwise made available to the public under any other Federal law;
- I have a reasonable basis to conclude that disclosure of the information is likely to cause substantial harm to the competitive position of my company; and
- I have a reasonable basis to believe that the information is not readily discoverable through reverse engineering.
Any knowing and willful misrepresentation is subject to criminal penalty pursuant to 18 U.S.C. 1001.
Electronic submissions of CBI required
- In most cases, information that may be claimed as CBI includes a checkbox for designating the information as CBI adjacent to the field where the information is to be entered.
- In cases in which the electronic reporting application does not support making a CBI claim adjacent to where the information is entered, the submitter is responsible for indicating what information is being claimed as CBI.
Almost all Toxic Substances Control Act (TSCA) submissions are required by regulation to be submitted electronically. See 40 CFR 703.5(f). The Environmental Protection Agency (EPA) electronic reporting systems within the Central Data Exchange (CDX) prompt users for some required elements when confidential business information (CBI) claims are made.
TSCA electronic reporting tools are available in the Chemical Safety and Pesticide Programs (CSPP) portion of EPA’s CDX. The electronic reporting applications for many TSCA submission types are based on fillable forms where each data element is entered individually. In most cases, information that may be claimed as CBI includes a checkbox for designating the information as CBI adjacent to the field where the information is to be entered.
In cases in which the electronic reporting application does not support making a CBI claim adjacent to where the information is entered (for example, if a submission includes document attachments), the submitter is responsible for indicating what information is being claimed as CBI. For documents created outside of the CDX reporting application that include CBI claims, both the complete document (with information claimed as CBI shown) and a sanitized version (with CBI redacted or otherwise masked) generally must be attached to the submission.
For electronic submissions, the certification statement is incorporated into the reporting application. The official making the submission will be prompted to agree to the certification statement prior to being able to complete the submission.
If a specific chemical identity is claimed as CBI, a structurally descriptive generic name must also be provided. Electronic reporting applications provide a field for inputting a structurally descriptive generic name for all submission types. Substantiation questions are also incorporated into the reporting applications.
Substantiating CBI claims at time of initial submission
- In order for EPA to make a determination regarding the validity of a CBI claim in a particular TSCA submission, information regarding the basis for the CBI claim must be provided.
- EPA has developed substantiation templates that may be used as a starting point in preparing CBI substantiations for uploading into CDX electronic reporting applications or for inclusion with paper submissions.
- Certain information is generally not subject to substantiation requirements including marketing and sales information.
All confidential business information (CBI) claims must be substantiated at the time the information claimed as CBI is submitted to the Environmental Protection Agency (EPA), except for those types of information exempt under Toxic Substances Control Act (TSCA) section 14(c)(2).
How to submit substantiations of CBI claims
EPA’s electronic reporting systems for TSCA submissions have been modified to accept substantiations for CBI claims in submissions filed on or after March 21, 2017. EPA’s electronic tools were further updated to incorporate substantiation and other TSCA CBI procedural requirements to coincide with the June 6, 2023, final rule on CBI claims procedures and 40 CFR 703.
What to include in CBI substantiations
In order for EPA to make a determination regarding the validity of a CBI claim in a particular TSCA submission, information regarding the basis for the CBI claim (i.e., substantiation) must be provided. Submitters may provide to EPA any information they believe supports the validity of their CBI claims. However, at a minimum the information must answer the substantiation questions required in 40 CFR 703.5(b),except as modified in the individual reporting rules, e.g., the Chemical Data Reporting Standard at 40 CFR 711.
When reviewing and making a CBI determination, EPA considers each CBI claim and corresponding substantiation, the provisions of TSCA and applicable regulations, any previously issued determinations which are pertinent, and other relevant materials as appropriate. See also 40 CFR 703.7(f).
In order to assist submitters in substantiating their CBI claims, EPA has developed substantiation templates that may be used as a starting point in preparing their CBI substantiations for uploading into Central Data Exchange (CDX) electronic reporting applications or for inclusion with paper submissions. The templates have largely been superseded by:
- Requirements of the June 6, 2023, final rule on CBI claims procedures and 40 CFR 703; and
- Corresponding development or updates to most TSCA electronic reporting applications, which now incorporate substantiation requirements.
However, TSCA submitters may find the templates useful as guidance when preparing a TSCA submission that includes CBI claims or when providing substantiation for an older TSCA submission that has become the subject of CBI review (e.g., as related to a FOIA request). Find the templates at https://www.epa.gov/tsca-cbi/what-include-cbi-substantiations#substantiationtemplates.
Information exempt from substantiation
TSCA section 14(c)(2) and 40 CFR 703.5(b)(5) identifies certain information that is generally not subject to substantiation requirements. This information includes:
- Specific information describing the processes used in manufacture or processing of a chemical substance, mixture, or article;
- Marketing and sales information;
- Information identifying a supplier or customer;
- In the case of a mixture, details of the full composition of the mixture and the respective percentages of constituents;
- Specific information regarding the use, function, or application of a chemical substance or mixture in a process, mixture, or article;
- Specific production or import volumes of the manufacturer or processor; and
- Prior to the date on which a chemical substance is first offered for commercial distribution, the specific chemical identity of the chemical substance, including the chemical name, molecular formula, Chemical Abstracts Service number, and other information that would identify the specific chemical substance, if the specific chemical identity was claimed as confidential at the time it was submitted in a notice under TSCA section 5.
In addition, individual TSCA reporting applications and specific reporting rules generally indicate which data elements are exempt or may be exempt from upfront substantiation requirements.
EPA review and determination of CBI claims
- EPA evaluates submissions with CBI claims to ensure that the procedural requirements for making a claim are met.
- EPA makes a determination on the validity of each CBI claim in the context of the submission in which it is received.
Toxic Substances Control Act (TSCA) section 14(g)(1) requires that the Environmental Protection Agency (EPA), within 90 days of receipt of the claim:
- Review and make determinations on confidential business information (CBI) claims for chemical identity after the chemical substance has been offered for commercial distribution; and
- Review and make determinations on a representative subset of at least 25 percent of other CBI claims that are not exempt from substantiation and review.
EPA evaluates submissions with CBI claims to ensure that the procedural requirements for making a claim are met. These requirements include:
- The required certification statements;
- Information claimed as CBI is clearly identified;
- Substantiation of CBI claims for information that is not exempt from substantiation under section 14(c)(2);
- Sanitized copy provided as required; and
- A structurally descriptive generic name, if a CBI claim is for specific chemical identity.
EPA considerations for CBI determinations
Once EPA verifies that all the procedural requirements for making a CBI claim have been satisfied, EPA considers each of the following in making a CBI determination:
- A business’ claim and substantiation;
- The provisions of TSCA and applicable regulations;
- Any previously issued determinations which are pertinent; and
- Other materials as appropriate.
See also 40 CFR 703.7(f), the substantive criteria for use in confidentiality determinations.
EPA’s determinations identify the specific information in the case for which the CBI determination applies. In some instances, a determination only covers the specific chemical identity of the chemical substance. In other instances, a CBI determination covers all of the other non-exempt information in a case. Finally, in some instances, a CBI determination is made for both the specific chemical identity of the chemical substance and for all of the other non-exempt information in the case.
While EPA will consider prior confidentiality determinations as one factor in making a determination under TSCA, EPA makes a determination on the validity of each CBI claim in the context of the submission in which it is received. For this reason, EPA advises submitters to substantiate CBI claims by looking carefully at the contents of each submission and providing all the information the submitter feels EPA should consider when making a determination.
CBI claims can be approved, approved in part, denied in part, or denied by EPA as a result of a review. If a claim is denied in part or denied, EPA provides a written statement explaining the reasons for the denial or denial in part according to the provisions of TSCA section 14(g)(2). Submitters whose claims have been asserted according to the requirements in section 14(c) and which have been approved in part and denied in part or denied can appeal the denial in a U.S. District Court according to the requirements of TSCA section 14(g)(2)(D).
Deficient TSCA CBI claims
- One of the most common deficiencies that EPA sees is that submitters substantiate only some of the information claimed as CBI.
- Generic name deficiencies in CBI submissions include having a missing generic name and not having a structurally descriptive name.
Submitters should carefully review the entirety of their confidential business information (CBI) claim submission to avoid deficiencies. A CBI claim is deficient if it meets one or more of the following criteria:
- The claim is not accompanied by a required supporting statement and certification.
- The claim is not accompanied by required substantiation OR asserts an inapplicable substantiation exemption.
- The claim is not accompanied by an appropriate public copy or “sanitized” version.
- The claim is for a specific chemical identity but is not accompanied by an adequate generic name.
Substantiation deficiencies
One of the most common deficiencies that EPA sees is that submitters substantiate only some of the information claimed as CBI. In particular, submitters will often only substantiate claims for specific chemical identity but not for other information that is claimed as CBI. Submitters should carefully review the entirety of their submission, including all studies, correspondence, and other attachments for information that is being claimed as CBI and make sure there is a substantiation for every information element in all the documents.
If a submitter wishes to assert that certain information is exempt from substantiation (in addition to information already identified in the reporting application as exempt), it must be noted in the substantiation section of the submission, citing the exemption and the reason it applies to the specific claimed information. In most reporting applications, the submitter may select the “opt out” box for substantiating the claim and then provide an explanation in the pop-up box. See also 40 CFR 703.5(b)(5).
EPA electronic reporting systems within the Central Data Exchange (CDX) prompt users for required generic name and substantiation when CBI claims are made.
Notices of deficiency
When deficiencies are identified, EPA will provide electronic notice and a 10-business-day period to correct the deficiency. If the deficiency is not remedied during the 10-day period, EPA will proceed with review of the submission and may deny the CBI claim(s). See also 40 CFR 703.5(e).
Submitters may generally correct deficiencies by logging into the submission via CDX, amending it to correct the deficiency (or withdraw the claim), and then resubmitting.
EPA disclosure of data
- Information from “health and safety studies” is not protected, unless disclosure would reveal specific manufacturing and processing information or a mixture’s chemical proportions.
Toxic Substances Control Act (TSCA) section 14(b) provides that certain categories of information are not protected from disclosure. In particular, information from “health and safety studies” is not protected, unless disclosure would reveal specific manufacturing and processing information or a mixture’s chemical proportions. It is presumed that information can be released when it pertains to chemicals that the Environmental Protection Agency (EPA) has banned or phased out.
For specific chemical identities determined to warrant confidential treatment, TSCA section 14 requires the use of unique generic identifiers when disclosing the chemical names may provide information about the structure and identity of a chemical substance.
Even when information warrants confidential treatment, section 14 establishes circumstances in which such information may or must be disclosed. For instance, such information must be disclosed for certain law enforcement purposes, or to states, localities, tribes, and health or environmental professionals if EPA determines that disclosure is necessary to protect health or the environment against an unreasonable risk.
Requesting access to CBI
- TSCA allows EPA, under certain conditions, to disclose CBI to state, tribal, and local governments; environmental, health, and medical professionals; and emergency responders.
Amendments to the Toxic Substances Control Act (TSCA) expanded the categories of people who may now access information claimed as confidential business information (CBI) under TSCA. Information that a business claims as CBI under TSCA is protected from disclosure until the business withdraws the CBI claim, the CBI claim expires, the Environmental Protection Agency (EPA) determines that the claim is not entitled to confidential treatment, or as authorized under TSCA and EPA regulations.
TSCA allows EPA, under certain conditions, to disclose CBI to:
- State, tribal, and local governments;
- Environmental, health, and medical professionals; and
- Emergency responders.
A person may be able to request access to the following types of information that are often claimed as CBI under TSCA:
- Specific chemical identity (e.g., the specific name of the chemical),
- Company name,
- Production figures,
- Manufacturing or processing details,
- Specific uses, and
- Names of individual company or laboratory personnel.
Be sure to review EPA’s guidance documents for accessing CBI under TSCA before submitting a request:
CBI-related historical notes
On June 7, 2023, EPA issued a final rule to update confidential business information (CBI) requirements under Toxic Substances Control Act (TSCA) that increases transparency, modernizes the reporting and review procedures for CBI, and aligns with the 2016 amendments to TSCA. The rule took effect August 7, 2023, and included the following:
- Clarifying changes to ensure the regulations specify precisely where EPA has a statutory obligation to require substantiation and deny claims, which will result in more information being made available.
- Changes to better assure that the scope of a CBI claim is clear and limited to information the submitter views as confidential.
- Amendments to narrow the types of information in health and safety studies that can be claimed as CBI.
- A provision to address overly broad CBI claims in public copies of TSCA submissions, especially health and safety related information, that specifies a process for the submitter to promptly correct those issues early in the CBI review.
- Expanded requirements for electronic reporting and uniform requirements to provide publicly releasable copies of certain documents like scientific studies, which would make more data available to the public more quickly.
- Requirements for electronic communication and maintaining accurate contact information to ensure that required notices of CBI claims are delivered more quickly to the submitters.
- Clarifying language on how EPA will handle information used in the TSCA program but obtained under other statutes that also have valid CBI claims under those statutes. This ensures consistency with the agency’s duty to make information publicly available when it’s legally able to do so.
- Clear, uniform guidance on requirements for asserting and maintaining CBI claims, including a standard set of substantiation questions used to support a CBI claim.
- Requirements for electronic reporting of nearly all CBI claims, with enhancements to reporting tools that will prevent or mitigate common procedural errors.
- A new section of the regulation to centralize and standardize how TSCA CBI claims must be asserted and substantiated.
- A requirement that submitters use an appropriate Organisation for Economic Co-operation and Development harmonized template, when available, when submitting health and safety information
Fees
- Fees for manufacturer-requested risk evaluations are not subject to the 25 percent limitation in TSCA.
- EPA will divide the total fee amongst responsible individual and joint payers.
The 2016 amendments to the Toxic Substances Control Act (TSCA) provided the Environmental Protection Agency (EPA) with expanded authority to collect fees from chemical manufacturers and importers to help defray up to 25 percent of the costs associated with overall TSCA implementation efforts. TSCA further required EPA to establish a fee structure by rulemaking. EPA finalized the Fees for the Administration of TSCA rule on October 16, 2018.
The rule requires payment of fees for eight different categories of activities or fee-triggering events under TSCA sections 4, 5, and 6:
- Test Rules under TSCA section 4;
- Enforceable Consent Agreements under TSCA section 4;
- Test Orders under TSCA section 4;
- New Chemical Notices (Premanufacture Notices (PMNs), Significant New Use Notices (SNUNs), and Microbial Commercial Activity Notices (MCANs)) under TSCA section 5;
- New Chemical Exemption Applications (Low Volume Exemption, Test Marketing Exemption Application, TSCA Environmental Release Application, etc.) under TSCA section 5;
- EPA-Initiated Risk Evaluations under TSCA section 6;
- Manufacturer-Requested Risk Evaluations for Chemicals on the TSCA Work Plan; and
- Manufacturer-Requested Risk Evaluations for Chemicals NOT on the TSCA Work Plan.
The fee amount for each of these categories was developed by estimating the total annual costs of administering TSCA sections 4, 5, and 6 (excluding the costs of manufacturer-requested risk evaluations) and of collecting, processing, reviewing, providing access to and protecting from disclosure as appropriate confidential business information. EPA then allocated 25 percent of those costs (the full amount recoverable under TSCA section 26) across six fee triggering events in sections 4, 5, and 6. Fees for manufacturer-requested risk evaluations are not subject to the 25 percent limitation in TSCA; the final fee amount is a percentage of the actual cost of conducting the evaluation.
As a general matter, most fee responsibilities under the rule are assigned to chemical manufacturers (including importers). In certain circumstances, fees may also apply to chemical processors (e.g., when a processor submits a SNUN under TSCA section 5).
Entities that meet the definition of a “small business concern” as defined in the rule can receive a substantial discount of approximately 80 percent.
Where multiple entities are subject to a fee, the rule allows those entities to pay individually or through a consortium of payers. EPA will divide the total fee amongst responsible individual and joint payers in accordance with a formula and process described in the rule.
As required by law, EPA will evaluate and readjust, if necessary, the fees every three years. Find the latest fees on EPA’s Toxic Substances Control Act (TSCA) Administration Fees webpage.
Enforcement
- EPA can subpoena witnesses, documents, and other information necessary to carry out TSCA.
- Any person who knowingly or willfully violates any provision of section 2614 or 2689 of Title 15 of the U.S. Code, shall, in addition to or in lieu of any civil penalty for such violation, be subject, upon conviction, to a fine of not more than $50,000 for each day of violation, or to imprisonment for not more than one year, or both.
Several Toxic Substances Control Act (TSCA) provisions (sections 11, 15, 16, and 17) relate to enforcement of the statute.
Inspections
The Environmental Protection Agency (EPA) can inspect any establishment in which chemicals or mixtures are manufactured, processed, imported to, stored, or held before or after their distribution in commerce, or any conveyance being used to transport chemicals or mixtures. No inspection shall include financial, sales, pricing, personnel, or research data, unless specified in an inspection notice.
Subpoenas
EPA can subpoena witnesses, documents, and other information necessary to carry out TSCA. Witnesses shall be paid the same fees and mileage that are paid witnesses in the courts of the U.S. Any district court of the U.S. in which venue is proper shall have jurisdiction to order any such person to comply with such subpoena. Any failure to obey such an order of the court is punishable by the court as a contempt thereof.
Civil penalties
Any person who violates a provision of TSCA section 2614 or 2689 shall be liable to the U.S. for a civil penalty in an amount not to exceed the dollar amount specified at 40 CFR 19.4, for each such violation. Each day such a violation continues shall, for purposes of section 2615, constitute a separate violation of section 2614 or 2689.
According to section 2614:
“It shall be unlawful for any person to —
- (1) fail or refuse to comply with any requirement of this subchapter [Subchapter I Control of Toxic Substances] or any rule promulgated, order issued, or consent agreement entered into under this subchapter, or any requirement of subchapter II [Asbestos Hazard Emergency Response] or any rule promulgated or order issued under subchapter II;
- (2) use for commercial purposes a chemical substance or mixture which such person knew or had reason to know was manufactured, processed, or distributed in commerce in violation of section 2604 or 2605 of this title, a rule or order under section 2604 or 2605 of this title, or an order issued in action brought under section 2604 or 2606 of this title;
- (3) fail or refuse to (A) establish or maintain records, (B) submit reports, notices, or other information, or (C) permit access to or copying of records, as required by this chapter or a rule thereunder; or
- (4) fail or refuse to permit entry or inspection as required by section 2610 of this title.”
According to section 2689,
- “It shall be unlawful for any person to fail or refuse to comply with a provision of this subchapter [Subchapter IV Lead Exposure Reduction] or with any rule or order issued under this subchapter.”
Criminal liability
In general, any person who knowingly or willfully violates any provision of section 2614 or 2689 of this title, shall, in addition to or in lieu of any civil penalty for such violation, be subject, upon conviction, to a fine of not more than $50,000 for each day of violation, or to imprisonment for not more than one year, or both.
However, any person who knowingly and willfully violates any provision of section 2614 or 2689 of this title, and who knows at the time of the violation that the violation places an individual in imminent danger of death or serious bodily injury, shall be subject on conviction to a fine of not more than $250,000, or imprisonment for not more than 15 years, or both. Also, an organization that commits a knowing violation as described shall also be subject on conviction to a fine of not more than $1,000,000 for each violation.
Judicial review and citizens actions
- Petitions for judicial review of certain rules and orders under TSCA, and civil actions challenging low-priority designations, must be filed within 60 days after EPA finalizes the action.
- Any person may file a lawsuit against EPA to compel it to perform a nondiscretionary duty or against any other person alleged to be in violation of certain types of TSCA rules or orders.
Toxic Substances Control Act (TSCA) section 19 governs judicial review of various Environmental Protection Agency (EPA) actions under the act; whereas, under TSCA section 20, any person may bring a civil suit to restrain a TSCA violation by any party or to petition EPA to issue, amend, or repeal a rule.
Judicial review
In general, section 19 requires that petitions for judicial review of certain rules and orders under TSCA, and civil actions challenging low-priority designations, be filed within 60 days after EPA finalizes the action.
The federal courts of appeals have exclusive jurisdiction over challenges to rules and orders issued under TSCA. For civil actions challenging low-priority designations, jurisdiction is limited specifically to the U.S. Court of Appeals for the District of Columbia Circuit. Review is presumptively limited to the administrative record.
Courts review EPA rules and orders under TSCA pursuant to a specific standard of review set forth in section 19, rather than the more common (and more deferential) “arbitrary and capricious” standard of review under the Administrative Procedure Act. Specifically, section 19 provides that courts must “hold unlawful and set aside” such actions “if the court finds that the rule is not supported by substantial evidence in the rulemaking record taken as a whole.”
Citizens actions
Under section 20, any person may file a lawsuit (commonly called a citizen suit) against EPA to compel it to perform a nondiscretionary duty or against any other person (including government entities) alleged to be in violation of certain types of TSCA rules or orders. Section 20 generally requires a citizen suit plaintiff to give 60 days’ notice of the claims to the EPA Administrator and the alleged violator, if applicable, prior to filing the suit.
A citizen suit may not proceed if EPA or the Department of Justice is already “diligently prosecuting” an administrative or judicial proceeding against the alleged violator. However, the citizen who has given notice of the claims may intervene as a matter of right in the enforcement proceeding if it is initiated after notice is given. If a citizen suit is successful, the court may require the violator to take actions to correct a violation and may impose civil penalties on the violator.
In addition, any person may petition EPA to issue, amend, or repeal a rule under the testing, reporting, or restriction sections of TSCA. The agency has 90 days to respond to the petition. If no action is taken or a petition is denied, the party has the opportunity for judicial review in the U.S. District Court to compel the agency to undertake the requested action.
Employee protection
- Employees and employers may obtain judicial review in the U.S. Courts of Appeals.
- EPA may investigate allegations of potential effects on employment resulting from a TSCA requirement.
The Toxic Substances Control Act (TSCA) includes provisions intended to protect employees of regulated entities from retaliation for participating in a proceeding under the Act or from potential effects on employment because of economic costs in implementing the Act. No employer may discharge any employee or otherwise discriminate against any employee with respect to the employee’s compensation, terms, conditions, or privileges of employment because the employee (or any person acting pursuant to a request of the employee) has:
- Commenced, caused to be commenced, or is about to commence or cause to be commenced a proceeding under TSCA;
- Testified or is about to testify in any such proceeding; or
- Assisted or participated or is about to assist or participate in any manner in such a proceeding or in any other action to carry out the purposes of TSCA.
TSCA section 23 authorizes the Department of Labor (DOL) to investigate alleged retaliations and provides DOL with administrative and judicial mechanisms to resolve such allegations. If an employee believes that the employer has discriminated against him or her because of the employee’s participation in carrying out TSCA, that employee may file a complaint with the Secretary of Labor. The Labor Secretary shall investigate the alleged discrimination and, if warranted, may order the employer to remedy the effects of any such discriminatory action. Employees and employers may obtain judicial review in the U.S. Courts of Appeals.
TSCA section 24 directs the Environmental Protection Agency (EPA) to investigate allegations of potential effects on employment resulting from a TSCA requirement. In response to a petition by an employee, EPA may hold public hearings concerning job losses or other adverse effects allegedly resulting from a requirement under TSCA. The agency will make public these findings and recommendations.
Relationship to state requirements
- TSCA does not preempt states from requiring the development of new information or regulating a chemical for which the EPA has not taken action under TSCA.
To avoid potential conflict between federal requirements under the Toxic Substances Control Act (TSCA) and state requirements or restrictions on chemicals, TSCA identifies circumstances in which a federal requirement for a specific chemical under the Act would preempt state requirements that apply to the same chemical unless exempted or waived.
On the other hand, TSCA does not preempt states from requiring the development of new information or regulating a chemical for which the Environmental Protection Agency (EPA) has not taken action under TSCA. Additionally, TSCA authorizes EPA to award grants to states to take action to address unreasonable risk associated with a chemical that the agency is unable or not likely to address.
Preemption of state requirements and exemptions to preemption
- TSCA section 18 contains two preemption sections that prohibit different kinds of state or local regulations corresponding to different stages in EPA’s review process under TSCA.
- Section 18 also provides exceptions that, in effect, preserve state chemical requirements that otherwise would be preempted.
Preemption of state requirements
Under the Supremacy Clause of the U.S. Constitution, state law and policy must yield to the exercise of Congress’s powers if Congress so intends. As Congress debated whether to expand EPA authority to regulate chemicals under TSCA leading up to the enactment of the 2016 Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA), federal preemption of state requirements was a key issue.
As enacted in 1976, TSCA preempted state and local requirements only for specific chemicals. Some states and localities enacted their own laws or promulgated regulations pertaining to other chemicals in response to concerns regarding the risks of commercial chemicals and the absence of federal regulatory action. The chemical industry and associated entities (e.g., retailers) expressed concern over regulatory requirements that differed from one state to another.
In response, the LCSA revised TSCA’s preemption provisions. In broad terms, TSCA section 18 prohibits specific state and local actions, and then restricts the reach of those prohibitions through limitations, exceptions, and waivers. Any preemption is chemical-specific, and EPA must take specific actions under TSCA in order to trigger the preemption of state regulation of chemicals. Exceptions to preemption may apply under certain conditions, and EPA may grant preemption waivers by rule.
As amended, section 18 contains two preemption sections that prohibit different kinds of state or local regulations corresponding to different stages in EPA’s review process under TSCA:
- TSCA section 18(a) prohibitions — The prohibitions in section 18(a) apply once EPA implements information requirements, notification of significant new use requirements, or restrictions or prohibitions based on its risk evaluation for the particular chemical. Under section 18(a), once EPA has acted to regulate a chemical, no state may establish or continue to enforce any of the following, subject to exemptions and potential waivers:
- A statute or administrative action that requires the development of information on a chemical that would be “reasonably likely” to be the same as information required under an existing EPA rule, order, or consent agreement under the Act.
- A statute, criminal penalty, or administrative action to prohibit or otherwise restrict the manufacture, processing, or distribution in commerce or use of a chemical for which EPA has either: (1) issued an order finding the chemical not to present an unreasonable risk, or (2) found the chemical to present an unreasonable risk and promulgated a final rule to address the unreasonable risk after the effective date of the rule.
- A statute or administrative action requiring the notification of a use of a chemical for which EPA has already determined that notification as a significant new use is required.
- TSCA section 18(b) prohibitions — The prohibitions in section 18(b) apply while EPA is conducting its risk evaluation of a particular chemical, beginning on the date EPA determines the scope of its risk evaluation and ending either on the deadline for completion of the risk evaluation or when EPA publishes the risk evaluation, whichever is earlier. Section 18(b) imposes a temporary prohibition on states from establishing any new prohibition or restriction on a chemical designated by EPA as a high-priority substance for risk evaluation. States are preempted from regulating only the hazards, exposures, risks, and uses included in the scope of EPA’s risk evaluation.
Exceptions to preemption
Section 18 provides exceptions that, in effect, preserve state chemical requirements that otherwise would be preempted. States and localities may continue to enforce any chemical substance prohibitions or restrictions that were in effect before April 22, 2016. States and localities also may adopt new regulations and other actions pursuant to any laws in effect on August 31, 2003.
TSCA also leaves in place the “cooperative federalism” structure of several other federal environmental laws, under which states administer programs that either meet or exceed minimum federal requirements. Thus, it does not preempt a state from adopting or enforcing any rule, standard of performance, risk evaluation, scientific assessment, or any other protection for public health or the environment that:
- Is adopted under the authority of or to satisfy any other federal law;
- Implements a reporting, monitoring, disclosure, or other information obligation not otherwise required by the EPA under TSCA or required under other federal law; or
- Is generally adopted under a state law related to water quality, air quality, or waste treatment or disposal, with certain exceptions.
In addition to these exemptions from preemption, TSCA sets forth “savings clauses,” providing that the Act’s requirements do not preempt penalties for criminal conduct or common law rights or statutes creating remedies for civil relief — such as damages — under any legal theory of liability. TSCA also provides that determinations, such as risk assessments, made pursuant to the statute are not definitive proof in court for private actions.
In addition to the specific statutory exemptions, section 18 allows EPA the discretion to exempt from preemption a state requirement under certain circumstances. If the state submits an application for the exemption, EPA may grant the exemption if the agency determines that compelling conditions warrant granting the waiver to protect health or the environment, that compliance with the requirement would not unduly burden interstate commerce and would not cause a violation of federal requirements, and that the risk identified by the state is based on sufficiently strong science.
EPA must exempt from preemption new state requirements established during EPA’s risk evaluation period for a chemical if either the:
- State requirement is enacted within 18 months of EPA’s prioritization of the chemical for review; or
- EPA determines that compliance with the requirement would not unduly burden interstate commerce or cause a violation of federal requirements, and that the state’s concern about the chemical is based on peer-reviewed science.
EPA must act on a required exemption application within 110 days after an application is submitted. All waiver applications are subject to public notice and comment requirements, and decisions regarding such applications are final agency actions subject to judicial review.
Co-enforcement and state grants
- Penalties and other sanctions that are applicable to violations under state law must not be more stringent than those available under TSCA.
Co-enforcement
Section 18 of the Toxic Substances Control Act (TSCA) also establishes parameters for states that adopt and enforce requirements under their own laws that are identical to TSCA requirements. Penalties and other sanctions that are applicable to violations under state law must not be more stringent than those available under TSCA.
Additionally, a state may not assess a penalty for a violation under its own law if the Environmental Protection Agency (EPA) has already assessed an adequate penalty for the same violation under TSCA. If a state has already assessed a penalty for a specific violation under state law, TSCA limits the penalty EPA may assess for the same violation so that the combined total penalty amount would not exceed the maximum penalty amount allowed under the Act. Under this scheme, regulated entities may face enforcement by either state or federal regulators.
State grants
TSCA Section 28 authorizes EPA to award grants to states to establish and operate programs intended to prevent or eliminate unreasonable risks associated with chemicals for which the agency “is unable or is not likely to take action” under the Act. Section 28 limits grant awards to 75 percent of the establishment and operation costs of the program.
The 2016 Frank R. Lautenberg Chemical Safety for the 21st Century Act (LCSA) repealed the authorization of appropriations for state grants but not the program authority, and Congress has continued to provide TSCA grant funding to states through annual discretionary appropriations.




