['Air Programs']
['Air Emissions']
07/11/2024
...
§1065.601 Overview.
(a) This subpart describes how to —-
(1) Use the signals recorded before, during, and after an emission test to calculate brake-specific emissions of each measured exhaust constituent.
(2) Perform calculations for calibrations and performance checks.
(3) Determine statistical values.
(b) You may use data from multiple systems to calculate test results for a single emission test, consistent with good engineering judgment. You may also make multiple measurements from a single batch sample, such as multiple weighings of a PM filter or multiple readings from a bag sample. Although you may use an average of multiple measurements from a single test, you may not use test results from multiple emission tests to report emissions.
(1) We allow weighted means where appropriate.
(2) You may discard statistical outliers, but you must report all results.
(3) For emission measurements related to durability testing, we may allow you to exclude certain test points other than statistical outliers relative to compliance with emission standards, consistent with good engineering judgment and normal measurement variability; however, you must include these results when calculating the deterioration factor. This would allow you to use durability data from an engine that has an intermediate test result above the standard that cannot be discarded as a statistical outlier, as long as good engineering judgment indicates that the test result does not represent the engine's actual emission level. Note that good engineering judgment would preclude you from excluding endpoints. Also, if normal measurement variability causes emission results below zero, include the negative result in calculating the deterioration factor to avoid an upward bias. These provisions related to durability testing are intended to address very stringent standards where measurement variability is large relative to the emission standard.
(c) You may use any of the following calculations instead of the calculations specified in this subpart G:
(1) Mass-based emission calculations prescribed by the International Organization for Standardization (ISO), according to ISO 8178, except the following:
(i) ISO 8178-4 Section 9.1.6, NO X Correction for Humidity and Temperature. See §1065.670 for approved methods for humidity corrections.
(ii) [Reserved]
(2) Other calculations that you show are equivalent to within ±0.1% of the brake-specific emission results determined using the calculations specified in this subpart G.
[70 FR 40516, July 13, 2005, as amended at 73 FR 37324, June 30, 2008; 74 FR 56516, Oct. 30, 2009; 75 FR 23044, Apr. 30, 2010; 79 FR 23778, Apr. 28, 2014; 89 FR 29807, Apr. 22, 2024]
§1065.602 Statistics.
(a) Overview. This section contains equations and example calculations for statistics that are specified in this part. In this section we use the letter “y” to denote a generic measured quantity, the superscript over-bar “-” to denote an arithmetic mean, and the subscript “ref” to denote the reference quantity being measured.
(b) Arithmetic mean. Calculate an arithmetic mean, y , as follows:

Example:
N = 3
y 1 = 10.60
y 2 = 11.91
y N = y 3 = 11.09
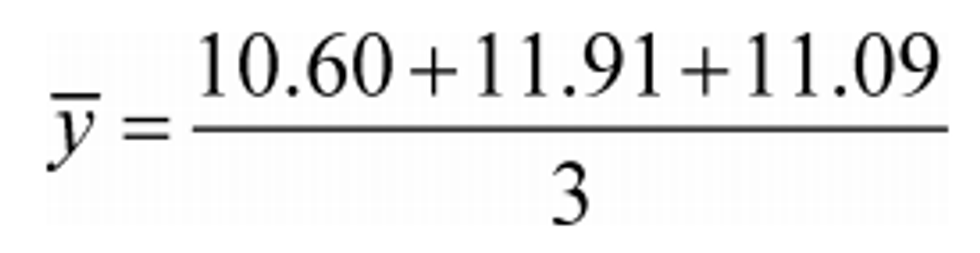
y = 11.20
(c) Standard deviation. Calculate the standard deviation for a non-biased (e.g., N-1) sample, σ, as follows:
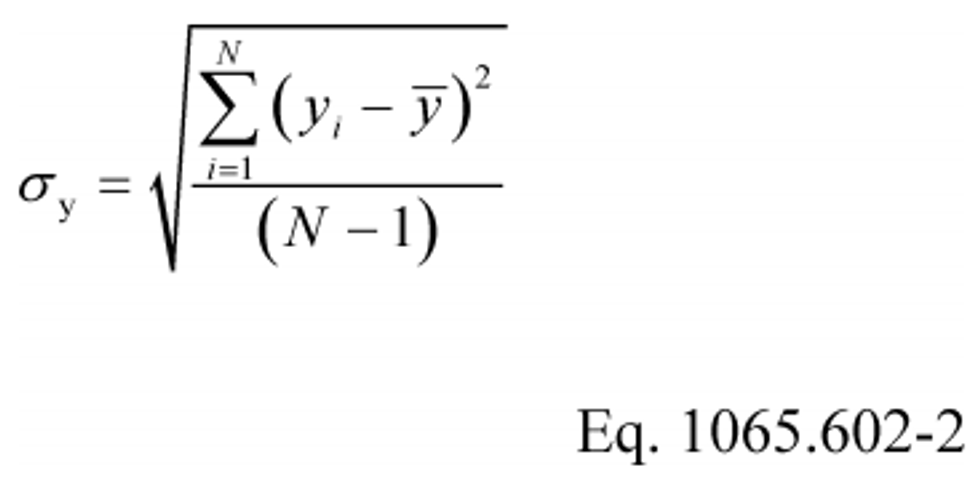
Example:
N = 3
y 1 = 10.60
y 2 = 11.91
y N = y 3 = 11.09
y = 11.20
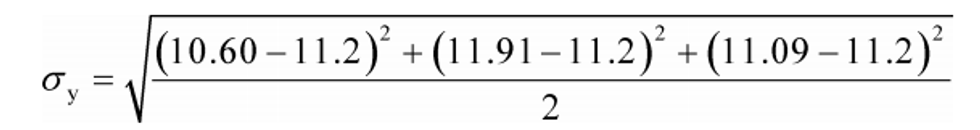
σy = 0.6619
(d) Root mean square. Calculate a root mean square, rms y, as follows:
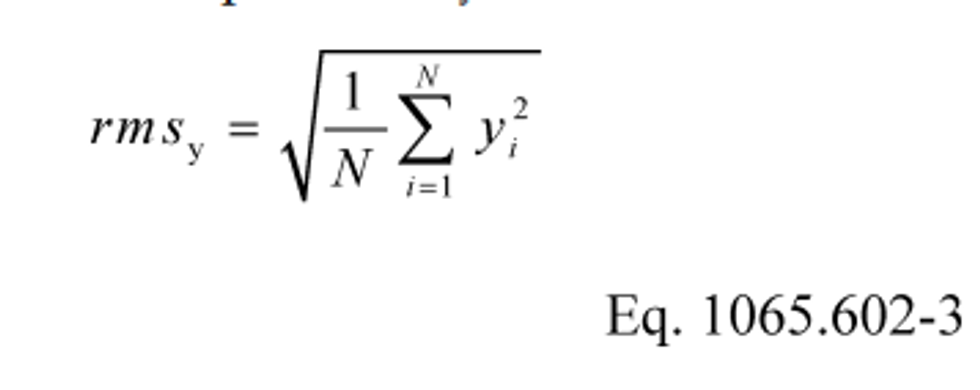
Example:
N = 3
y 1 = 10.60
y 2 = 11.91
y N = y 3 = 11.09
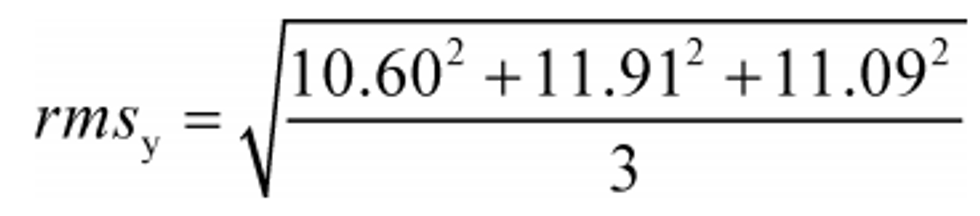
rms y = 11.21
(e) Accuracy. Determine accuracy as described in this paragraph (e). Make multiple measurements of a standard quantity to create a set of observed values, y , and compare each observed value to the known value of the standard quantity. The standard quantity may have a single known value, such as a gas standard, or a set of known values of negligible range, such as a known applied pressure produced by a calibration device during repeated applications. The known value of the standard quantity is represented by y ref . If you use a standard quantity with a single value, y ref would be constant. Calculate an accuracy value as follows:
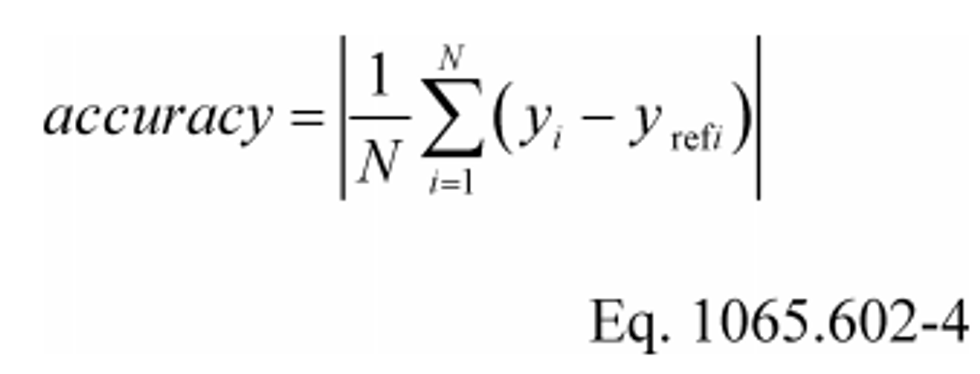
Example:
y ref = 1800.0
N = 3
y 1 = 1806.4
y 2 = 1803.1
y 3 = 1798.9
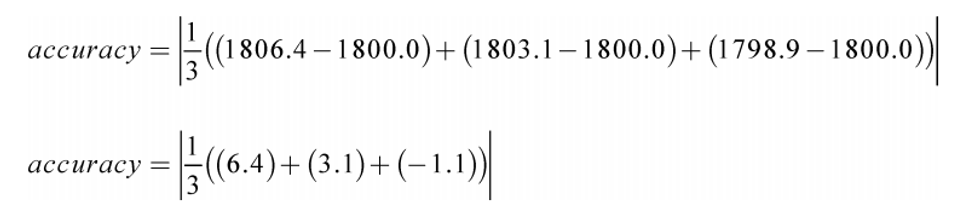
accuracy = 2.8
(f) t-test. Determine if your data passes a t-test by using the following equations and tables:
(1) For an unpaired t-test, calculate the t statistic and its number of degrees of freedom, v, as follows:
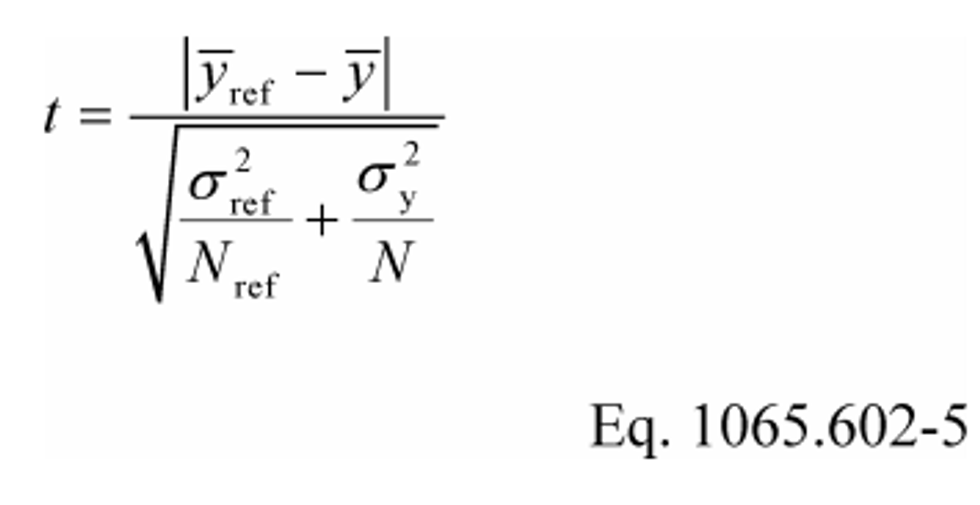
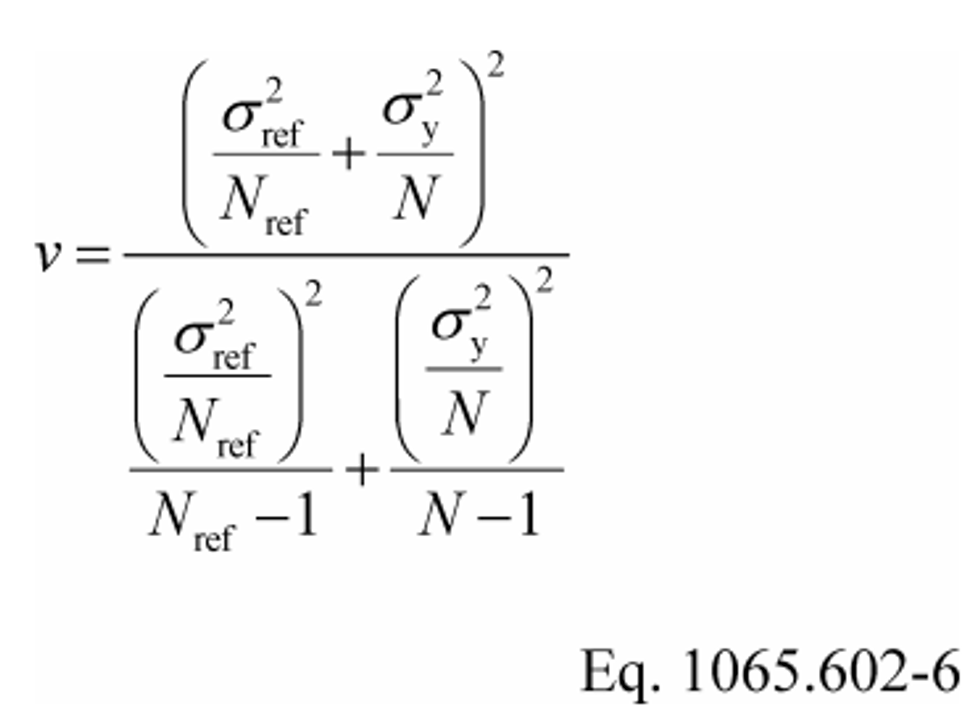
Example:
Y ref = 1205.3
Y = 1123.8
σref = 9.399
σy = 10.583
N ref = 11
N = 7
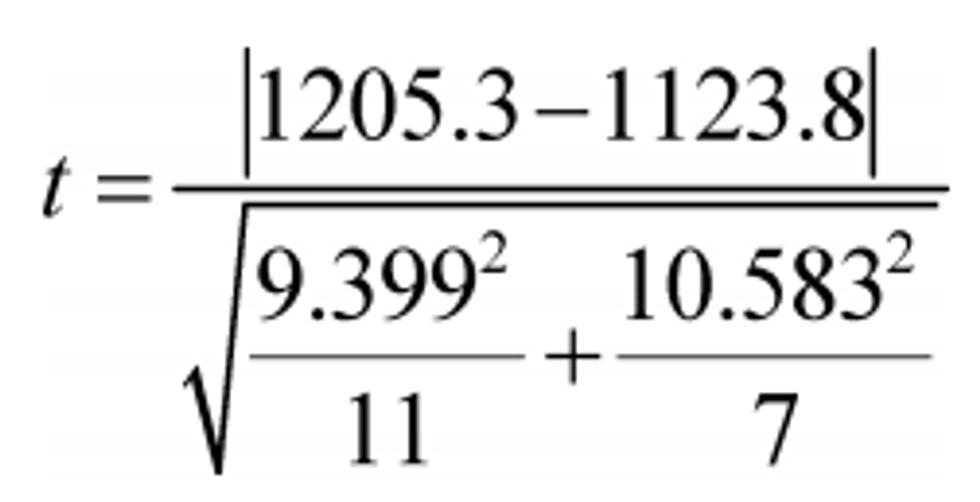
t = 16.63
σref = 9.399
σy = 10.583
N ref = 11
N = 7
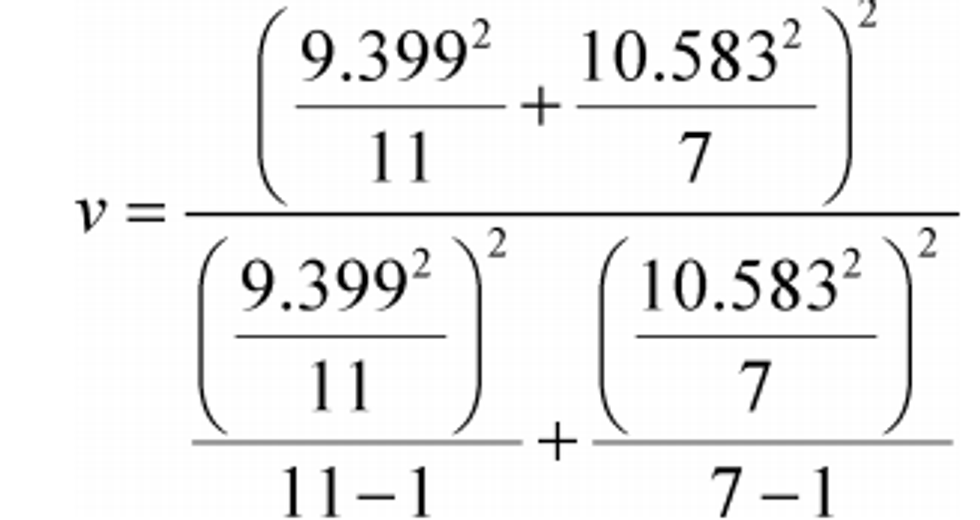
v = 11.76
(2) For a paired t-test, calculate the t statistic and its number of degrees of freedom, v, as follows, noting that the ε are the errors (e.g., differences) between each pair of y ref and y :
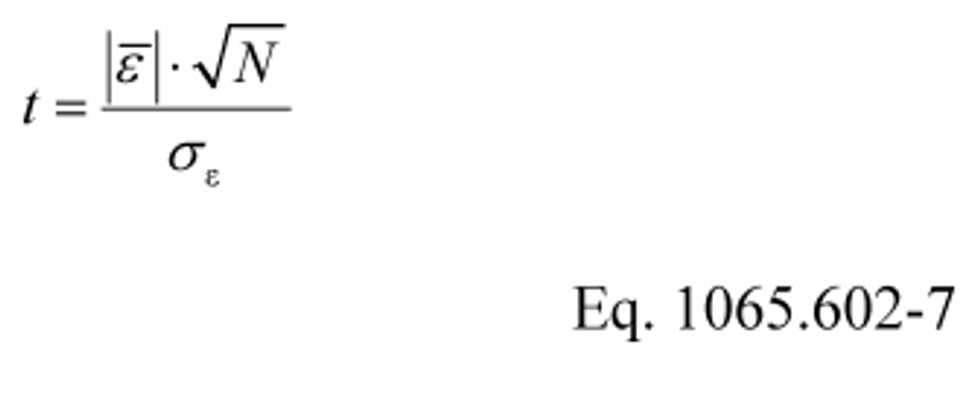
Example 1:
ε = −0.12580
N = 16
σε = 0.04837
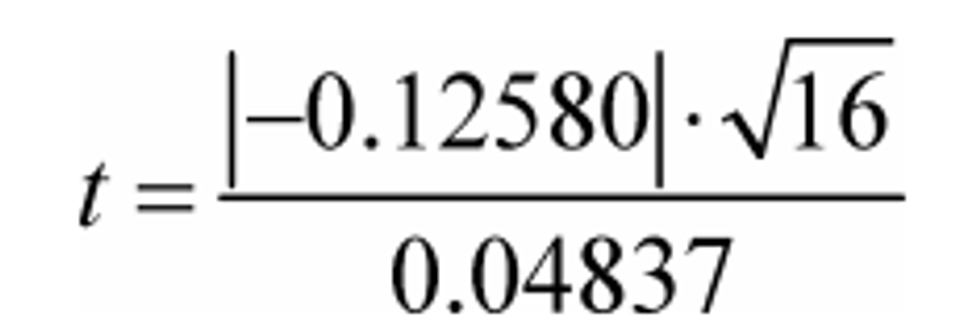
t = 10.403
v = N−1
Example 2:
N = 16
v = 16−1
v = 15
(3) Use Table 1 of this section to compare t to the t crit values tabulated versus the number of degrees of freedom. If t is less than t crit, then t passes the t-test. The Microsoft Excel software has a TINV function that returns results equivalent results and may be used in place of Table 1, which follows:
| v | Confidence | |
|---|---|---|
| 90% | 95% | |
| Use linear interpolation to establish values not shown here. | ||
| 1 | 6.314 | 12.706 |
| 2 | 2.920 | 4.303 |
| 3 | 2.353 | 3.182 |
| 4 | 2.132 | 2.776 |
| 5 | 2.015 | 2.571 |
| 6 | 1.943 | 2.447 |
| 7 | 1.895 | 2.365 |
| 8 | 1.860 | 2.306 |
| 9 | 1.833 | 2.262 |
| 10 | 1.812 | 2.228 |
| 11 | 1.796 | 2.201 |
| 12 | 1.782 | 2.179 |
| 13 | 1.771 | 2.160 |
| 14 | 1.761 | 2.145 |
| 15 | 1.753 | 2.131 |
| 16 | 1.746 | 2.120 |
| 18 | 1.734 | 2.101 |
| 20 | 1.725 | 2.086 |
| 22 | 1.717 | 2.074 |
| 24 | 1.711 | 2.064 |
| 26 | 1.706 | 2.056 |
| 28 | 1.701 | 2.048 |
| 30 | 1.697 | 2.042 |
| 35 | 1.690 | 2.030 |
| 40 | 1.684 | 2.021 |
| 50 | 1.676 | 2.009 |
| 70 | 1.667 | 1.994 |
| 100 | 1.660 | 1.984 |
| 1000+ | 1.645 | 1.960 |
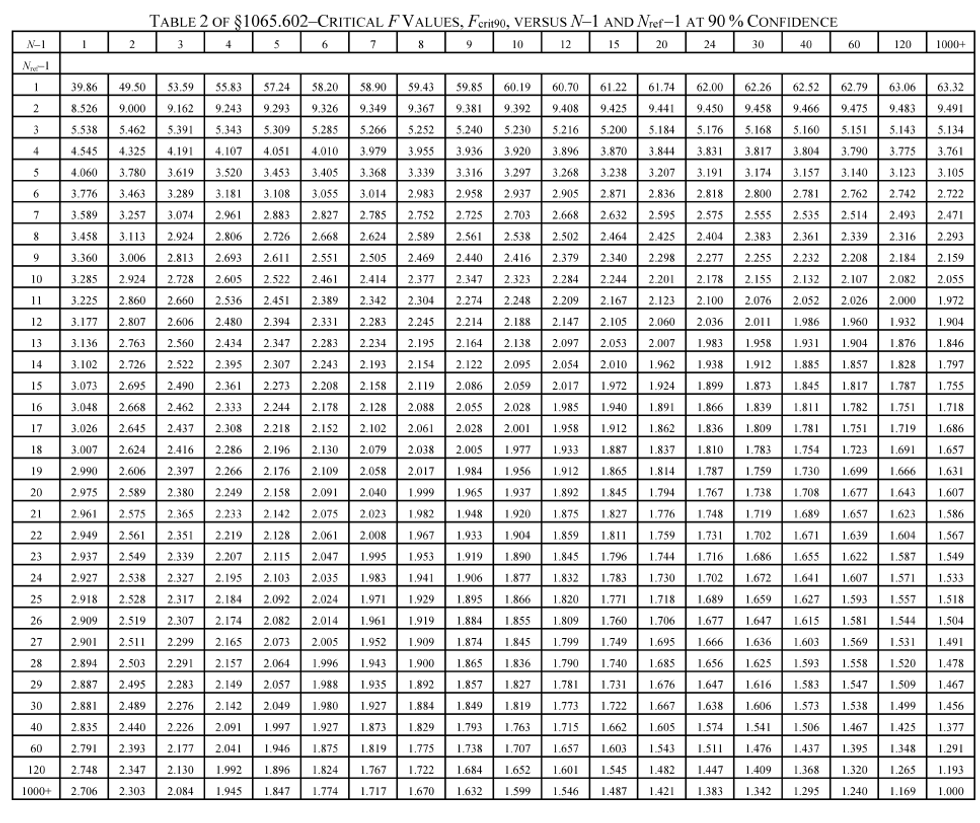
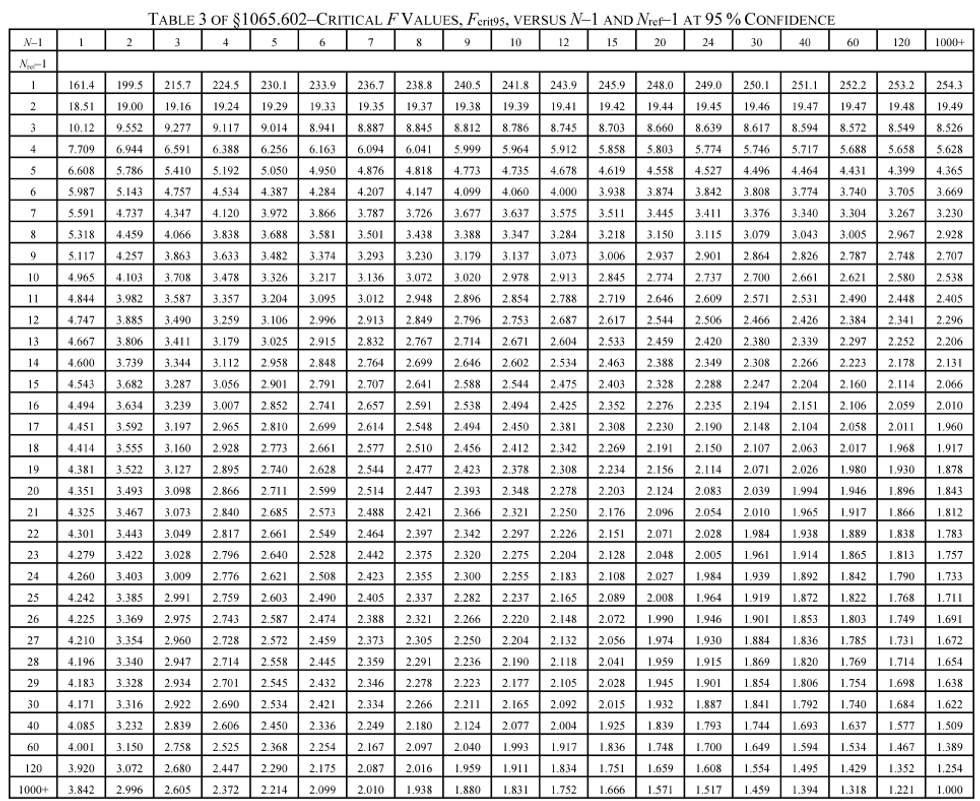
(g) F-test. Calculate the F statistic as follows:

Example:
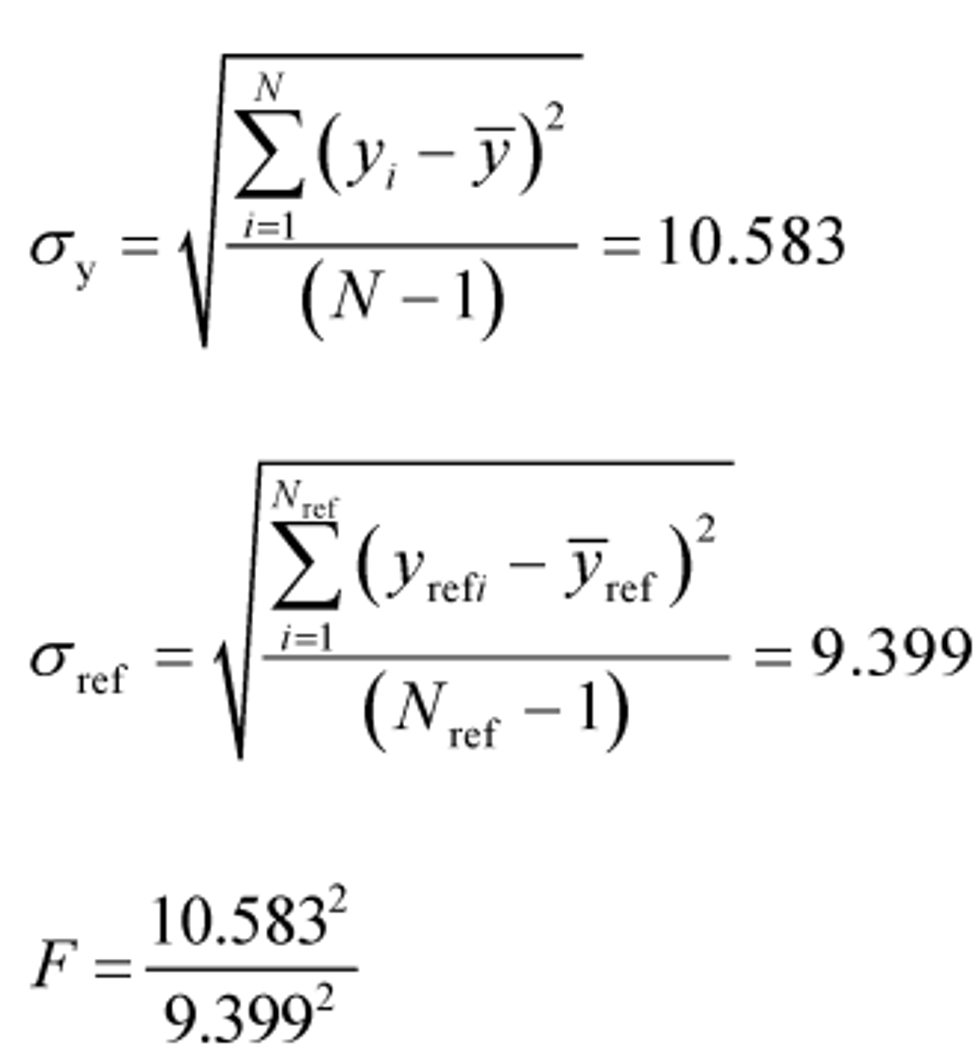
F = 1.268
(1) For a 90% confidence F-test, use the following table to compare F to the F crit90 values tabulated versus (N−1) and (N ref−1). If F is less than F crit90, then F passes the F-test at 90% confidence.
(2) For a 95% confidence F-test, use the following table to compare F to the F crit90 values tabulated versus (N−1) and (N ref−1). If F is less than F crit95, then F passes the F-test at 95% confidence.
(h) Slope. Calculate a least-squares regression slope, a 1y, using one of the following two methods:
(1) If the intercept floats, i.e., is not forced through zero:
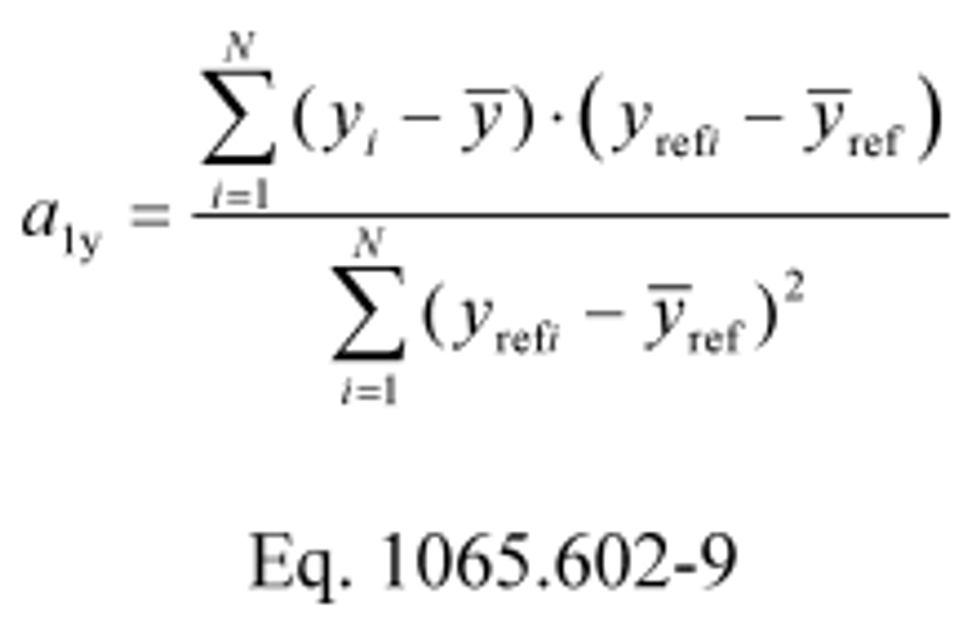
Example:
N = 6000
y 1 = 2045.8
y = 1050.1
y ref1 = 2045.0
y ref = 1055.3
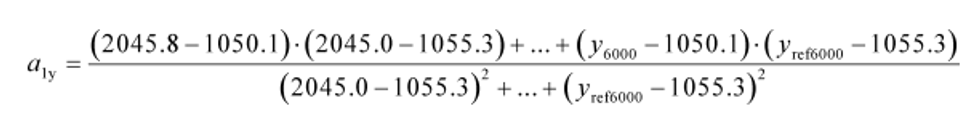
a 1y = 1.0110
(2) If the intercept is forced through zero, such as for verifying proportional sampling:
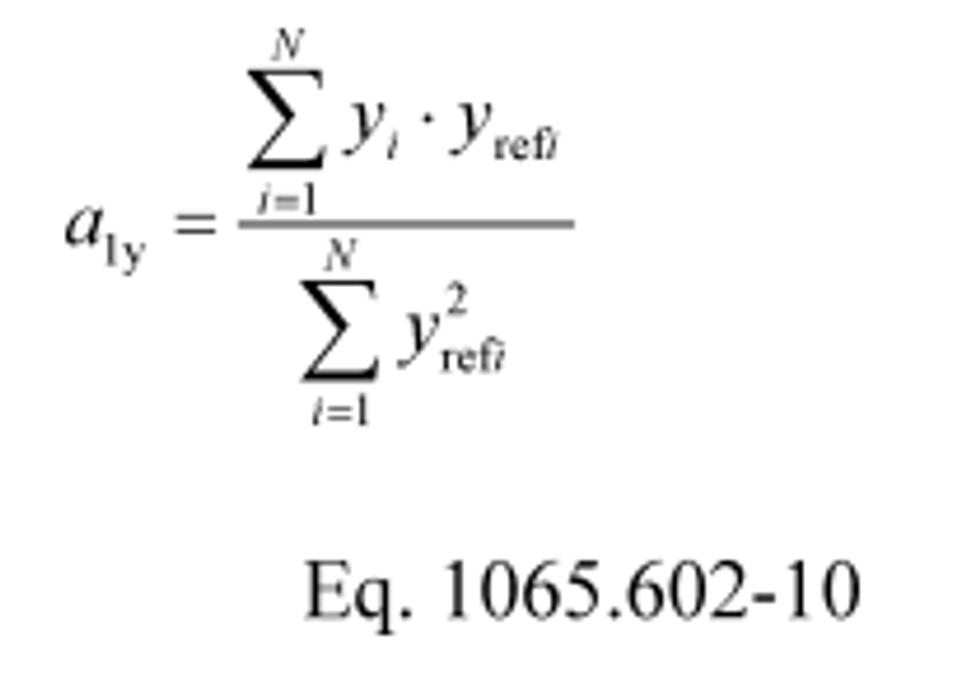
Example:
N = 6000
y 1 = 2045.8
y ref1 = 2045.0
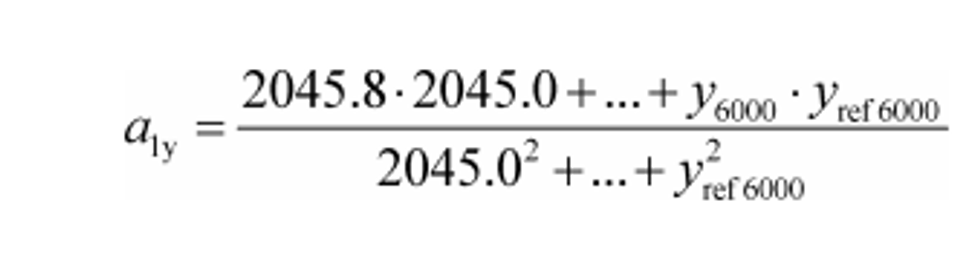
a 1y = 1.0110
(i) Intercept. For a floating intercept, calculate a least-squares regression intercept, a0y, as follows:
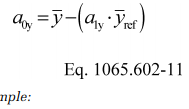
Example:
y = 1050.1
a 1y = 1.0110
y ref = 1055.3
a 0y = 1050.1 − (1.0110 · 1055.3)
a 0y = −16.8083
(j) Standard error of the estimate. Calculate a standard error of the estimate, SEE, using one of the following two methods:
(1) For a floating intercept:
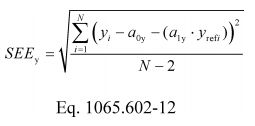
Example:
N = 6000
y 1 = 2045.8
a 0y = −16.8083
a 1y = 1.0110
y ref1 = 2045.0
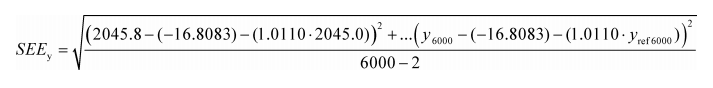
SEE y = 5.348
(2) If the intercept is forced through zero, such as for verifying proportional sampling:
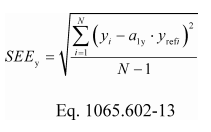
Example:
N = 6000
y 1 = 2045.8
a 1y = 1.0110
y ref1 = 2045.0
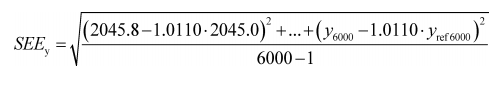
SEE y = 5.347
(k) Coefficient of determination. Calculate a coefficient of determination, r y 2, as follows:
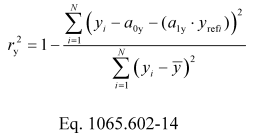
Example:
N = 6000
y 1 = 2045.8
a 0y = −16.8083
a 1y = 1.0110
y ref1 = 2045.0
y = 1480.5
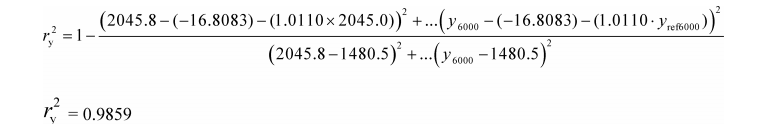
(l) Flow-weighted mean concentration. In some sections of this part, you may need to calculate a flow-weighted mean concentration to determine the applicability of certain provisions. A flow-weighted mean is the mean of a quantity after it is weighted proportional to a corresponding flow rate. For example, if a gas concentration is measured continuously from the raw exhaust of an engine, its flow-weighted mean concentration is the sum of the products of each recorded concentration times its respective exhaust molar flow rate, divided by the sum of the recorded flow rate values. As another example, the bag concentration from a CVS system is the same as the flow-weighted mean concentration because the CVS system itself flow-weights the bag concentration. You might already expect a certain flow-weighted mean concentration of an emission at its standard based on previous testing with similar engines or testing with similar equipment and instruments. If you need to estimate your expected flow-weighted mean concentration of an emission at its standard, we recommend using the following examples as a guide for how to estimate the flow-weighted mean concentration expected at the standard. Note that these examples are not exact and that they contain assumptions that are not always valid. Use good engineering judgment to determine if you can use similar assumptions.
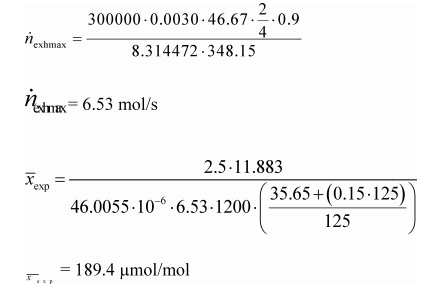
(1) To estimate the flow-weighted mean raw exhaust NOX concentration from a turbocharged heavy-duty compression-ignition engine at a NOX standard of 2.5 g/(kW·hr), you may do the following:
(i) Based on your engine design, approximate a map of maximum torque versus speed and use it with the applicable normalized duty cycle in the standard-setting part to generate a reference duty cycle as described in §1065.610. Calculate the total reference work, W ref, as described in §1065.650. Divide the reference work by the duty cycle's time interval, Δt dutycycle, to determine mean reference power, p ref.
(ii) Based on your engine design, estimate maximum power, P max, the design speed at maximum power, ƒnmax, the design maximum intake manifold boost pressure, P inmax, and temperature, T inmax. Also, estimate a mean fraction of power that is lost due to friction andpumping, Pfrict. Use this information along with the engine displacement volume, V disp, an approximate volumetric efficiency, ηV, and the number of engine strokes per power stroke (two-stroke or four-stroke), N stroke, to estimate the maximum raw exhaust molar flow rate, n exhmax.
(iii) Use your estimated values as described in the following example calculation:
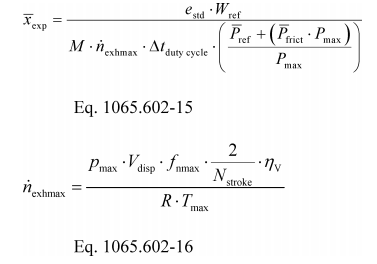
Example:
e NOX = 2.5 g/(kW·hr)
W ref = 11.883 kW·hr
M NOX = 46.0055 g/mol = 46.0055·10−6 g/μmol
Δt dutycycle = 20 min = 1200 s
P ref = 35.65 kW
P frict = 15%
P max = 125 kW
p max = 300 kPa = 300000 Pa
V disp = 3.0 l = 0.0030 m3/r
f nmax = 2800 r/min = 46.67 r/s
N stroke = 4
ηV = 0.9
R = 8.314472 J/(mol·K)
T max = 348.15 K
(2) To estimate the flow-weighted mean NMHC concentration in a CVS from a naturally aspirated nonroad spark-ignition engine at an NMHC standard of 0.5 g/(kW·hr), you may do the following:
(i) Based on your engine design, approximate a map of maximum torque versus speed and use it with the applicable normalized duty cycle in the standard-setting part to generate a reference duty cycle as described in §1065.610. Calculate the total reference work, W ref, as described in §1065.650.
(ii) Multiply your CVS total molar flow rate by the time interval of the duty cycle, Δt dutycycle. The result is the total diluted exhaust flow of the n dexh.
(iii) Use your estimated values as described in the following example calculation:
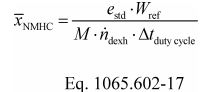
Example:
e NMHC = 1.5 g/(kW·hr)
W ref = 5.389 kW·hr
M NMHC = 13.875389 g/mol = 13.875389·10−6 g/μmol
n dexh = 6.021 mol/s
Δt dutycycle = 30 min = 1800 s
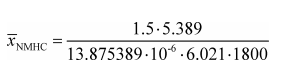
X NMHC = 53.8 µmol/mol
(m) Median. Determine median, M , as described in this paragraph (m). Arrange the data points in the data set in increasing order where the smallest value is ranked 1, the second-smallest value is ranked 2, etc.
(1) For even numbers of data points:
(i) Determine the rank of the data point whose value is used to determine the median as follows:
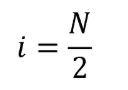
Eq. 1065.602-18
Where:
i = an indexing variable that represents the rank of the data point whose value is used to determine the median.
N = the number of data points in the set.
Example:
N = 4
y1 = 41.515
y2 = 41.780
y3 = 41.861
y4 = 41.902
i = 2
i = 2
(ii) Determine the median as the average of the data point i and the data point i + 1 as follows:

Example:
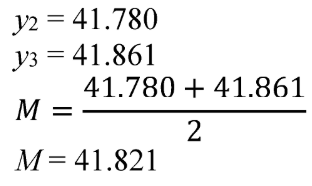
[70 FR 40516, July 13, 2005, as amended at 73 FR 37324, June 30, 2008; 75 FR 23044, Apr. 30, 2010; 76 FR 57452, Sept. 15, 2011; 79 FR 23779, Apr. 28, 2014; 81 FR 74170, Oct. 25, 2016; 86 FR 34548, Jun. 29, 2021; 87 FR 65865, Oct. 26, 2022; 89 FR 29807, Apr. 22, 2024]
§1065.610 Duty cycle generation.
This section describes how to generate duty cycles that are specific to your engine, based on the normalized duty cycles in the standard-setting part. During an emission test, use a duty cycle that is specific to your engine to command engine speed, torque, and power, as applicable, using an engine dynamometer and an engine operator demand. Paragraphs (a) and (b) of this section describe how to “normalize” your engine's map to determine the maximum test speed or torque for your engine. The rest of this section describes how to use these values to “denormalize” the duty cycles in the standard-setting parts, which are all published on a normalized basis. Thus, the term “normalized” in paragraphs (a) and (b) of this section refers to different values than it does in the rest of the section.
(a) Maximum test speed, ƒ . For variable-speed engines, determine ƒ ntest from the torque and power maps, generated according to §1065.510, as follows:
(1) Determine a measured value for ƒ ntest as follows:
(i) Determine maximum power, Pmax, from the engine map generated according to §1065.510 and calculate the value for power equal to 98% of Pmax.
(ii) Determine the lowest and highest engine speeds corresponding to 98% of Pmax, using linear interpolation, and no extrapolation, as appropriate.
(iii) Determine the engine speed corresponding to maximum power, fnPmax, by calculating the average of the two speed values from paragraph (a)(1)(ii) of this section. If there is only one speed where power is equal to 98% of Pmax, take fnPmax as the speed at which Pmax occurs.
(iv) Transform the map into a normalized power-versus-speed map by dividing power terms by Pmax and dividing speed terms by fnPmax. Use the following equation to calculate a quantity representing the sum of squares from the normalized map:
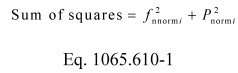
Where:
i = an indexing variable that represents one recorded value of an engine map.
fnnorm = an engine speed normalized by dividing it by fnPmax.
Pnorm = an engine power normalized by dividing it by Pmax.
(v) Determine the maximum value for the sum of the squares from the map and multiply that value by 0.98.

(vi) Determine the lowest and highest engine speeds corresponding to the value calculated in paragraph (a)(1)(v) of this section, using linear interpolation as appropriate. Calculate fntest as the average of these two speed values. If there is only one speed corresponding to the value calculated in paragraph (a)(1)(v) of this section, take fntest as the speed where the maximum of the sum of the squares occurs.
(vii) The following example illustrates a calculation of fntest:
Pmax = 230.0
(fn1 = 2360, P1 = 222.5, fnnorm1 = 1.002, Pnorm1 = 0.9675)
(fn2 = 2364, P2 = 226.8, fnnorm2 = 1.004, Pnorm2 = 0.9859)
(fn3 = 2369, P3 = 228.6, fnnorm3 = 1.006, Pnorm3 = 0.9940)
(fn4 = 2374, P4 = 218.7, fnnorm4 = 1.008, Pnorm4 = 0.9508)
Sum of squares = (1.002 2 + 0.9675 2) = 1.94
Sum of squares = (1.004 2 + 0.9859 2) = 1.98
Sum of squares = (1.006 2 + 0.9940 2) = 2.00
Sum of squares = (1.008 2 + 0.9508 2) = 1.92
(2) For engines with a high-speed governor that will be subject to a reference duty cycle that specifies normalized speeds greater than 100%, calculate an alternate maximum test speed, fntest,alt, as specified in this paragraph (a)(2). If fntest,alt is less than the measured maximum test speed, fntest, determined in paragraph (a)(1) of this section, replace fntest with fntest,alt. In this case, fntest,alt becomes the “maximum test speed” for that engine for all duty cycles. Note that §1065.510 allows you to apply an optional declared maximum test speed to the final measured maximum test speed determined as an outcome of the comparison between fntest, and fntest,alt in this paragraph (a)(2). Determine fntest,alt as follows:
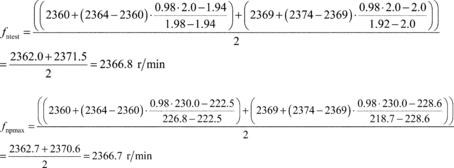

Where:
fntest,alt = alternate maximum test speed
fnhi,idle = warm high-idle speed
fnidle = warm idle speed
% speedmax = maximum normalized speed from duty cycle
Example:
fnhi,idle = 2200 r/min
fnidle = 800 r/min
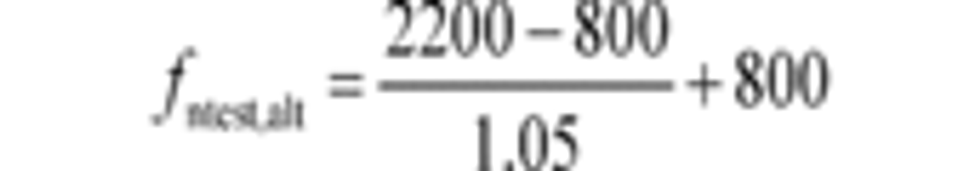
fntest,alt = 2133 r/min
(3) Transform normalized speeds to reference speeds according to paragraph (c) of this section by using the measured maximum test speed determined according to paragraphs (a)(1) and (2) of this section—or use your declared maximum test speed, as allowed in §1065.510.
(b) Maximum test torque,T . For constant-speed engines, determine Ttest from the torque and power-versus-speed maps, generated according to §1065.510, as follows:
(1) For constant speed engines mapped using the methods in §1065.510(d)(5)(i) or (ii), determine a measured value for Ttest as follows:
(i) Determine maximum power, Pmax, from the engine map generated according to §1065.510 and calculate the value for power equal to 98% of Pmax.
(ii) Determine the lowest and highest engine speeds corresponding to 98% of Pmax, using linear interpolation, and no extrapolation, as appropriate.
(iii) Determine the engine speed corresponding to maximum power, fnPmax, by calculating the average of the two speed values from paragraph (a)(1)(ii) of this section. If there is only one speed where power is equal to 98% of Pmax, take fnPmax as the speed at which Pmax occurs.
(iv) Transform the map into a normalized power-versus-speed map by dividing power terms by Pmax and dividing speed terms by fnPmax. Use Eq. 1065.610-1 to calculate a quantity representing the sum of squares from the normalized map.
(v) Determine the maximum value for the sum of the squares from the map and multiply that value by 0.98.
(vi) Determine the lowest and highest engine speeds corresponding to the value calculated in paragraph (a)(1)(v) of this section, using linear interpolation as appropriate. Calculate fntest as the average of these two speed values. If there is only one speed corresponding to the value calculated in paragraph (a)(1)(v) of this section, take fntest as the speed where the maximum of the sum of the squares occurs.
(vii) The measured Ttest is the mapped torque at fntest.
(2) For constant speed engines using the two-point mapping method in §1065.510(d)(5)(iii), you may follow paragraph (a)(1) of this section to determine the measured Ttest , or you may use the measured torque of the second point as the measured Ttest directly.
(3) Transform normalized torques to reference torques according to paragraph (d) of this section by using the measured maximum test torque determined according to paragraph (b)(1) or (2) of this section—or use your declared maximum test torque, as allowed in §1065.510.
(c) Generating reference speed values from normalized duty cycle speeds. Transform normalized speed values to reference values as follows:
(1) % speed. If your normalized duty cycle specifies % speed values, use your warm idle speed and your maximum test speed to transform the duty cycle, as follows:
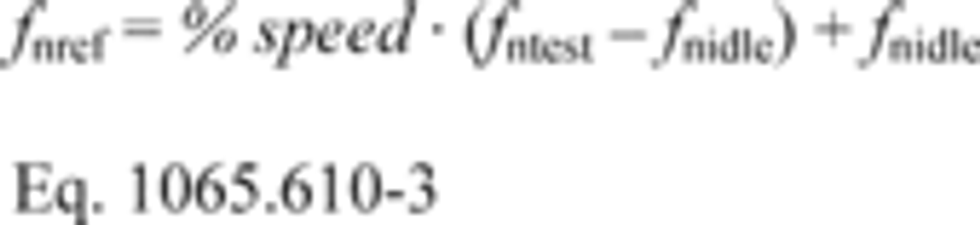
Example:
% speed = 85% = 0.85
fntest = 2364 r/min
fnidle = 650 r/min
fnref = 0.85 (2364−650) + 650
fnref = 2107 r/min
(2) A, B, C, and D speeds. If your normalized duty cycle specifies speeds as A, B, C, or D values, use your power-versus-speed curve to determine the lowest speed below maximum power at which 50% of maximum power occurs. Denote this value as nlo. Take nlo to be warm idle speed if all power points at speeds below the maximum power speed are higher than 50% of maximum power. Also determine the highest speed above maximum power at which 70% of maximum power occurs. Denote this value as nhi. If all power points at speeds above the maximum power speed are higher than 70% of maximum power, take nhi to be the declared maximum safe engine speed or the declared maximum representative engine speed, whichever is lower. Use nhi and nlo to calculate reference values for A, B, C, or D speeds as follows:
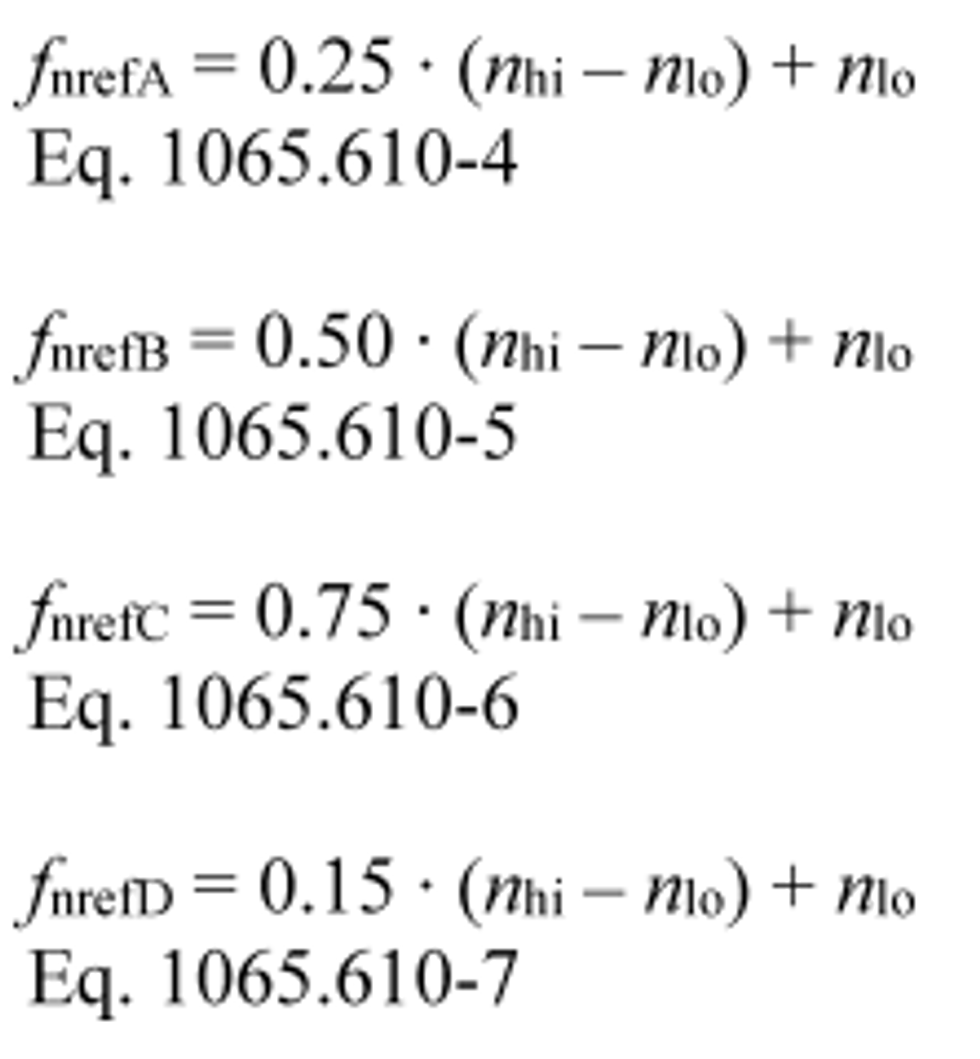
Example:
nlo = 1005 r/min
nhi = 2385 r/min
ƒ nrefA = 0.25 · (2385 − 1005) + 1005
ƒ nrefB = 0.50 · (2385 − 1005) + 1005
ƒ nrefC = 0.75 · (2385 − 1005) + 1005
ƒ nrefD = 0.15 · (2385 − 1005) + 1005
ƒ nrefA = 1350 r/min
ƒ nrefB = 1695 r/min
ƒ nrefC = 2040 r/min
ƒ nrefD = 1212 r/min
(3) Intermediate speed. Based on the map, determine maximum torque, Tmax, and the corresponding speed, fnTmax, calculated as the average of the lowest and highest speeds at which torque is equal to 98% of Tmax. Use linear interpolation between points to determine the speeds where torque is equal to 98% of Tmax. Identify your reference intermediate speed as one of the following values:
(i) fnTmax if it is between (60 and 75) % of maximum test speed.
(ii) 60% of maximum test speed if fnTmax is less than 60% of maximum test speed.
(iii) 75% of maximum test speed if fnTmax is greater than 75% of maximum test speed.
(d) Generating reference torques from normalized duty-cycle torques. Transform normalized torques to reference torques using your map of maximum torque versus speed.
(1) Reference torque for variable-speed engines. For a given speed point, multiply the corresponding % torque by the maximum torque at that speed, according to your map. If your engine is subject to a reference duty cycle that specifies negative torque values (i.e., engine motoring), use negative torque for those motoring points (i.e., the motoring torque). If you map negative torque as allowed under §1065.510(c)(2) and the low-speed governor activates, resulting in positive torques, you may replace those positive motoring mapped torques with negative values between zero and the largest negative motoring torque. For both maximum and motoring torque maps, linearly interpolate mapped torque values to determine torque between mapped speeds. If the reference speed is below the minimum mapped speed (i.e., 95% of idle speed or 95% of lowest required speed, whichever is higher), use the mapped torque at the minimum mapped speed as the reference torque. The result is the reference torque for each speed point.
(2) Reference torque for constant-speed engines. Multiply a % torque value by your maximum test torque. The result is the reference torque for each point.
(3) Required deviations. We require the following deviations for variable-speed engines intended primarily for propulsion of a vehicle with an automatic or manual transmission where that engine is subject to a transient duty cycle that specifies points with normalized reference speed of 0% and normalized reference torque of 0% ( i.e., idle points). These deviations are intended to produce a more representative transient duty cycle for these applications. For steady-state duty cycles or transient duty cycles with no idle operation, the requirements in this paragraph (d)(3) do not apply. Idle points for steady-state duty cycles of such engines are to be run at conditions simulating neutral or park on the transmission. For manual transmissions, set CITT to zero, which results in warm-idle-in-drive speed and torque values being the same as warm-idle-in-neutral values. For the case of a manual transmission where the optional declared idle torque in §1065.510(f)(5)(iii) and the optional declared power in §1065.510(f)(6) are not declared (i.e., idle torque is zero), the required deviations in this paragraph (d)(3) have no impact and may be skipped.
(i) Determine the warm-idle-in-drive speed and torque values with the transmission in drive from the data collected during the engine mapping procedure in §1065.510. The warm-idle-in-drive torque is the sum of CITT and the torques representing loads from vehicle accessories. For example, the sum of the required declared CITT in §1065.510(f)(4), any optional declared torque in §1065.510(f)(5)(iii), and the torque on the primary output shaft from any optional declared power in §1065.510(f)(6).
(ii) Determine the warm-idle-in-neutral speed and torque values with the transmission in neutral from the data collected during the engine mapping procedure in §1065.510. The warm-idle-in-neutral torque is the sum of any optional declared torque in §1065.510(f)(5)(iii) and the torque on the primary output shaft from any optional declared power in §1065.510(f)(6) ( i.e., the sum of the torques representing loads from vehicle accessories).
(iii) Zero-percent speed for denormalization of non-idle points is the warm-idle-in-drive speed.
(iv) For motoring points, make no changes.
(v) If the cycle begins with an idle segment ( i.e., a set of one or more contiguous idle points), set the reference speed and torque values to the warm-idle-in-neutral values for this initial segment. This is to represent idle operation with the transmission in neutral or park at the start of the transient duty cycle, after the engine is started. If the initial idle segment is longer than 24 seconds, change the reference speed and torque values for the remaining idle points in the initial idle segment to the warm-idle-in-drive values ( i.e., change idle points corresponding to 25 seconds to the end of the initial idle segment to warm-idle-in-drive). This is to represent manually shifting the transmission to drive.
(vi) For all other idle segments, set the reference speed and torque values to the warm-idle-in-drive values. This is to represent the transmission operating in drive.
(vii) If the engine is intended primarily for automatic transmissions with a Neutral-When-Stationary feature that automatically shifts the transmission to neutral after the vehicle is stopped for a designated time and automatically shifts back to drive when the operator increases demand ( i.e., pushes the accelerator pedal), reprocess all idle segments. Change reference speed and torque values from the warm-idle-in-drive values to the warm-idle-in-neutral values for idle points in drive after the designated time.
(viii) For all nonidle nonmotoring points with normalized speed at or below zero percent and reference torque from zero to the warm-idle-in-drive torque value, set the reference torque to the warm-idle-in-drive torque value. This is to represent the transmission operating in drive.
(ix) For consecutive nonidle nonmotoring points that immediately follow and precede idle segments, with reference torque values from zero to the warm-idle-in-drive torque value, change their reference torques to the warm-idle-in-drive torque value. This is to represent the transmission operating in drive.
(x) For consecutive nonidle nonmotoring points that immediately follow and precede any point(s) that were modified in paragraph (d)(3)(viii) of this section, with reference torque values from zero to the warm-idle-in-drive torque value, change their reference torques to the warm-idle-in-drive torque value. This is to provide smooth torque transition around these points.
(4) Permissible deviations for any engine. If your engine does not operate below a certain minimum torque under normal in-use conditions, you may use a declared minimum torque as the reference value instead of any value denormalized to be less than the declared value. For example, if your engine is connected to a hydrostatic transmission and it has a minimum torque even when all the driven hydraulic actuators and motors are stationary and the engine is at idle, then you may use this declared minimum torque as a reference torque value instead of any reference torque value generated under paragraph (d)(1) or (2) of this section that is between zero and this declared minimum torque.
(e) Generating reference power values from normalized duty cycle powers. Transform normalized power values to reference speed and power values using your map of maximum power versus speed.
(1) First transform normalized speed values into reference speed values. For a given speed point, multiply the corresponding % power by the mapped power at maximum test speed, fntest, unless specified otherwise by the standard-setting part. The result is the reference power for each speed point, Pref. Convert these reference powers to corresponding torques for operator demand and dynamometer control and for duty cycle validation per 1065.514. Use the reference speed associated with each reference power point for this conversion. As with cycles specified with % torque, linearly interpolate between these reference torque values generated from cycles with % power.
(2) Permissible deviations for any engine. If your engine does not operate below a certain power under normal in-use conditions, you may use a declared minimum power as the reference value instead of any value denormalized to be less than the declared value. For example, if your engine is directly connected to a propeller, it may have a minimum power called idle power. In this case, you may use this declared minimum power as a reference power value instead of any reference power value generated per paragraph (e)(1) of this section that is from zero to this declared minimum power.
[73 FR 37324, June 30, 2008, as amended at 73 FR 59330, Oct. 8, 2008; 75 FR 23045, Apr. 30, 2010; 76 FR 57453, Sept. 15, 2011; 78 FR 36398, June 17, 2013; 79 FR 23783, Apr. 28, 2014; 80 FR 9118, Feb. 19, 2015; 81 FR 74170, Oct. 25, 2016; 86 FR 34555, Jun. 29, 2021; 88 FR 4679, Jan. 24, 2023; 89 FR 29807, Apr. 22, 2024]
§1065.630 Local acceleration of gravity.
(a) The acceleration of Earth's gravity, ag , varies depending on the test location. Determine ag at your location by entering latitude, longitude, and elevation data into the U.S. National Oceanographic and Atmospheric Administration's surface gravity prediction website at https://geodesy.noaa.gov/cgi-bin/grav_pdx.prl.
(b) If the website specified in paragraph (a) of this section is unavailable, or the test location is outside of the continental United States, you may calculate ag for your latitude as follows:
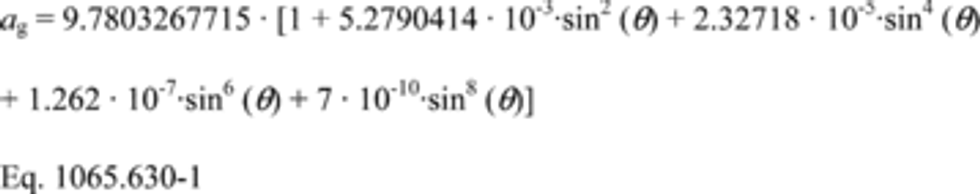
Where:
u = Degrees north or south latitude.
Example:
u = 45°
ag = 9.7803267715 · (1 + 5.2790414 · 10−3 · sin 2 (45) + 2.32718 · 10−5 · sin 4 (45) + 1.262 · 10−7 · sin 6 (45) + 7 · 10−10 · sin 8 (45)
ag = 9.8061992026 m/s 2
[79 FR 23784, Apr. 28, 2014; 88 FR 4680, Jan. 24, 2023]
§1065.640 Flow meter calibration calculations.
This section describes the calculations for calibrating various flow meters. After you calibrate a flow meter using these calculations, use the calculations described in §1065.642 to calculate flow during an emission test. Paragraph (a) of this section first describes how to convert reference flow meter outputs for use in the calibration equations, which are presented on a molar basis. The remaining paragraphs describe the calibration calculations that are specific to certain types of flow meters.
(a) Reference meter conversions. The calibration equations in this section use molar flow rate, n ref, as a reference quantity. If your reference meter outputs a flow rate in a different quantity, such as standard volume rate,V stdref, actual volume rate,V actref, or mass rate, m ref, convert your reference meter output to a molar flow rate using the following equations, noting that while values for volume rate, mass rate, pressure, temperature, and molar mass may change during an emission test, you should ensure that they are as constant as practical for each individual set point during a flow meter calibration:
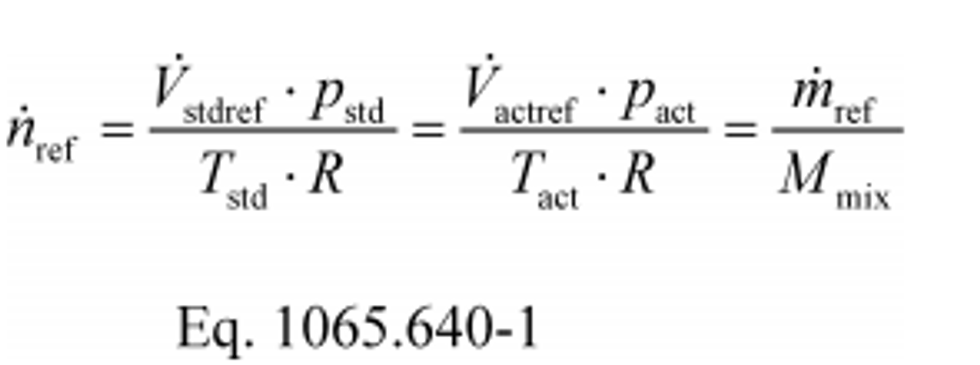
Where:
n ref = reference molar flow rate.
V stdref = reference volume flow rate corrected to a standard pressure and a standard temperature.
V actref = reference volume flow rate at the actual pressure and temperature of the flow rate.
m ref = reference mass flow.
p std = standard pressure.
p act = actual pressure of the flow rate.
T std = standard temperature.
T act = actual temperature of the flow rate.
R = molar gas constant.
M mix = molar mass of the flow rate.
Example 1:
V stdref = 1000.00 ft3/min = 0.471948 m3/s
p std = 29.9213 in Hg @ 32 °F = 101.325 kPa = 101325 Pa = 101325 kg/(m·s2)
T std = 68.0 °F = 293.15 K
R = 8.314472 J/(mol·K) = 8.314472 (m2·kg)/(s2·mol·K)
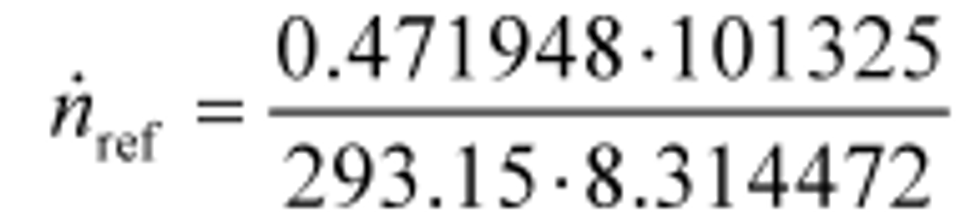
n ref = 19.619 mol/s
Example 2:
m ref = 17.2683 kg/min = 287.805 g/s
M mix = 28.7805 g/mol
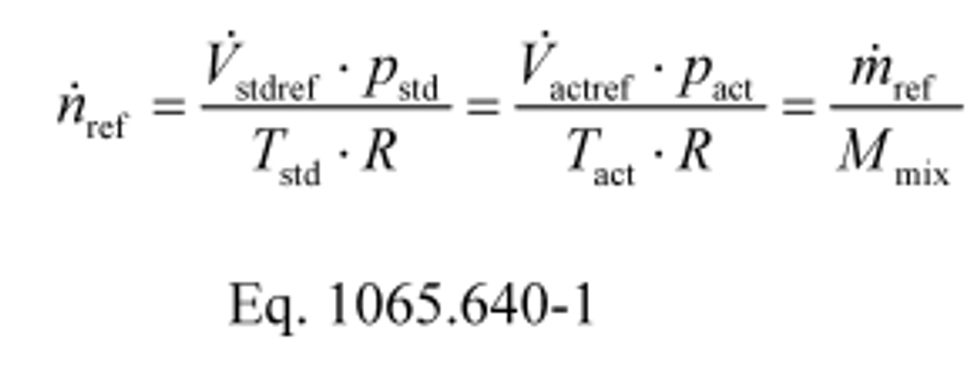
n ref = 10.0000 mol/s
(b) PDP calibration calculations. Perform the following steps to calibrate a PDP flow meter:
(1) Calculate PDP volume pumped per revolution, Vrev, for each restrictor position from the mean values determined in §1065.340 as follows:
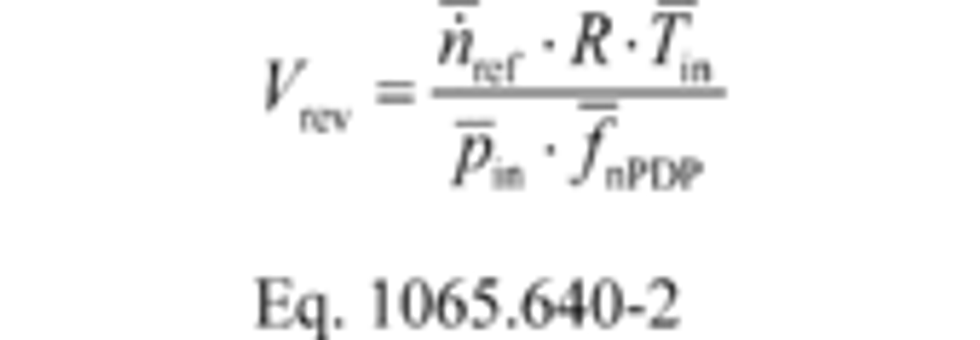
Where:
n ̇ref = mean reference molar flow rate.
R = molar gas constant.
T ͞in = mean temperature at the PDP inlet.
P ͞in = mean static absolute pressure at the PDP inlet.
f ͞nPDP = mean PDP speed.
Example:
n ̇ref = 25.096 mol/s
R = 8.314472 J/(mol·K) = 8.314472 (m 2·kg)/(s 2·mol·K)
T ͞in = 299.5 K
P ͞in = 98.290 kPa = 98290 Pa = 98290 kg/(m·s 2)
f ͞nPDP = 1205.1 r/min = 20.085 r/s
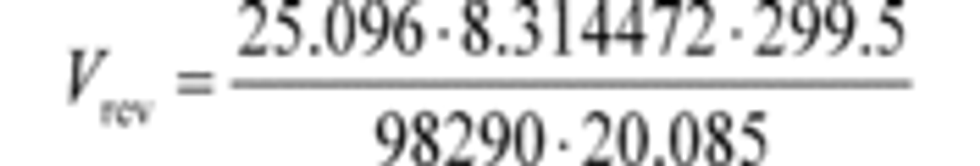
Vrev = 0.03166 m 3/r
(2) Calculate a PDP slip correction factor, Ks, for each restrictor position from the mean values determined in §1065.340 as follows:

Where:
f ͞nPDP = mean PDP speed.
P ͞out = mean static absolute pressure at the PDP outlet.
P ͞in = mean static absolute pressure at the PDP inlet.
Example:
f ͞nPDP = 1205.1 r/min = 20.085 r/s
P ͞out = 100.103 kPa
P ͞in = 98.290 kPa
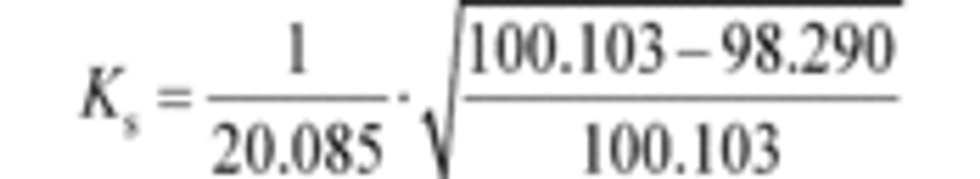
Ks = 0.006700 s/r
(3) Perform a least-squares regression of V rev, versus K s, by calculating slope, a 1, and intercept, a 0, as described for a floating intercept in §1065.602.
(4) Repeat the procedure in paragraphs (b)(1) through (3) of this section for every speed that you run your PDP.
(5) The following table illustrates a range of typical values for different PDP speeds:
| f ͞nPDP (revolution/s) | a1 (m 3/s) | a0 (m 3/revolution) |
|---|---|---|
| 12.6 | 0.841 | 0.056 |
| 16.5 | 0.831 | −0.013 |
| 20.9 | 0.809 | 0.028 |
| 23.4 | 0.788 | −0.061 |
(6) For each speed at which you operate the PDP, use the appropriate regression equation from this paragraph (b) to calculate flow rate during emission testing as described in §1065.642.
(c) Venturi governing equations and permissible assumptions. This section describes the governing equations and permissible assumptions for calibrating a venturi and calculating flow using a venturi. Because a subsonic venturi (SSV) and a critical-flow venturi (CFV) both operate similarly, their governing equations are nearly the same, except for the equation describing their pressure ratio, r (i.e., rSSV versus rCFV). These governing equations assume one-dimensional isentropic inviscid flow of an ideal gas. Paragraph (c)(5) of this section describes other assumptions that may apply. If good engineering judgment dictates that you account for gas compressibility, you may either use an appropriate equation of state to determine values of Z as a function of measured pressure and temperature, or you may develop your own calibration equations based on good engineering judgment. Note that the equation for the flow coefficient, Cf, is based on the ideal gas assumption that the isentropic exponent, g, is equal to the ratio of specific heats, Cp/Cv. If good engineering judgment dictates using a real gas isentropic exponent, you may either use an appropriate equation of state to determine values of γ as a function of measured pressures and temperatures, or you may develop your own calibration equations based on good engineering judgment.
(1) Calculate molar flow rate, n ̇, as follows:
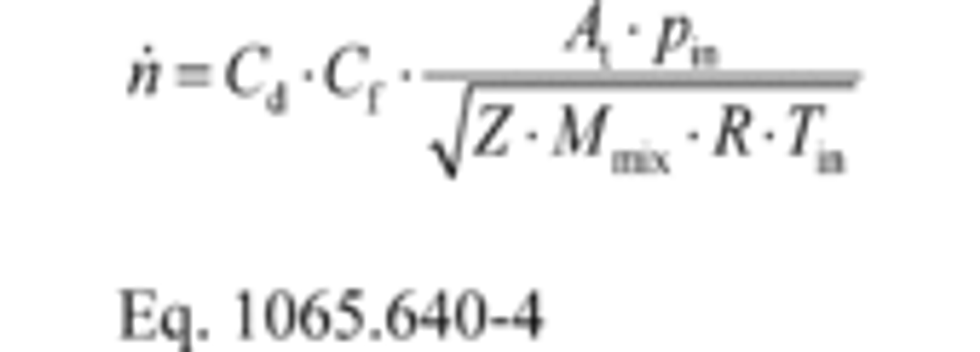
Where:
Cd = discharge coefficient, as determined in paragraph (c)(2) of this section.
Cf = flow coefficient, as determined in paragraph (c)(3) of this section.
At = venturi throat cross-sectional area.
pin = venturi inlet absolute static pressure.
Z = compressibility factor.
Mmix = molar mass of gas mixture.
R = molar gas constant.
Tin = venturi inlet absolute temperature.
(2) Using the data collected in §1065.340, calculate Cd for each flow rate using the following equation:
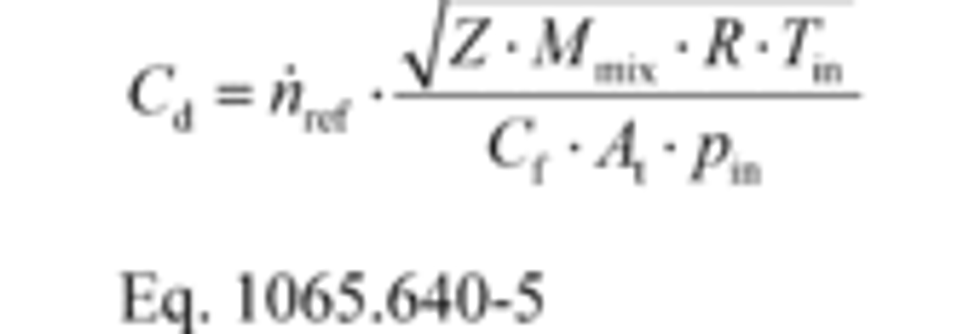
Where:
n ̇ref = a reference molar flow rate.
(3) Determine Cf using one of the following methods:
(i) For CFV flow meters only, determine CfCFV from the following table based on your values for β and γ, using linear interpolation to find intermediate values:
| CfCFV | ||
|---|---|---|
| b | gexh = 385 | gdexh = gair = 399 |
| 0.000 | 0.6822 | 0.6846 |
| 0.400 | 0.6857 | 0.6881 |
| 0.500 | 0.6910 | 0.6934 |
| 0.550 | 0.6953 | 0.6977 |
| 0.600 | 0.7011 | 0.7036 |
| 0.625 | 0.7047 | 0.7072 |
| 0.650 | 0.7089 | 0.7114 |
| 0.675 | 0.7137 | 0.7163 |
| 0.700 | 0.7193 | 0.7219 |
| 0.720 | 0.7245 | 0.7271 |
| 0.740 | 0.7303 | 0.7329 |
| 0.760 | 0.7368 | 0.7395 |
| 0.770 | 0.7404 | 0.7431 |
| 0.780 | 0.7442 | 0.7470 |
| 0.790 | 0.7483 | 0.7511 |
| 0.800 | 0.7527 | 0.7555 |
| 0.810 | 0.7573 | 0.7602 |
| 0.820 | 0.7624 | 0.7652 |
| 0.830 | 0.7677 | 0.7707 |
| 0.840 | 0.7735 | 0.7765 |
| 0.850 | 0.7798 | 0.7828 |
(ii) For any CFV or SSV flow meter, you may use the following equation to calculate Cf for each flow rate:
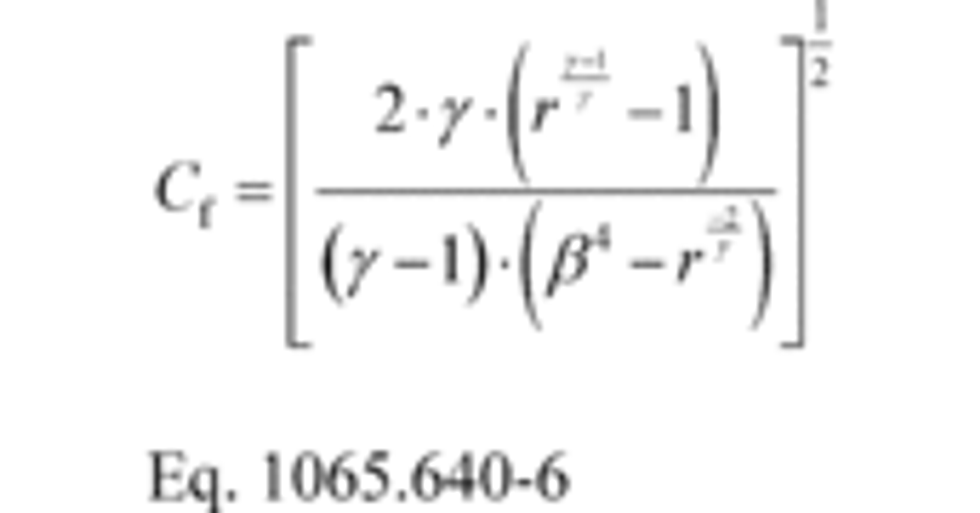
Where:
g = isentropic exponent. For an ideal gas, this is the ratio of specific heats of the gas mixture, Cp/Cv.
r = pressure ratio, as determined in paragraph (c)(4) of this section.
b = ratio of venturi throat to inlet diameters.
(4) Calculate r as follows:
(i) For SSV systems only, calculate rSSV using the following equation:
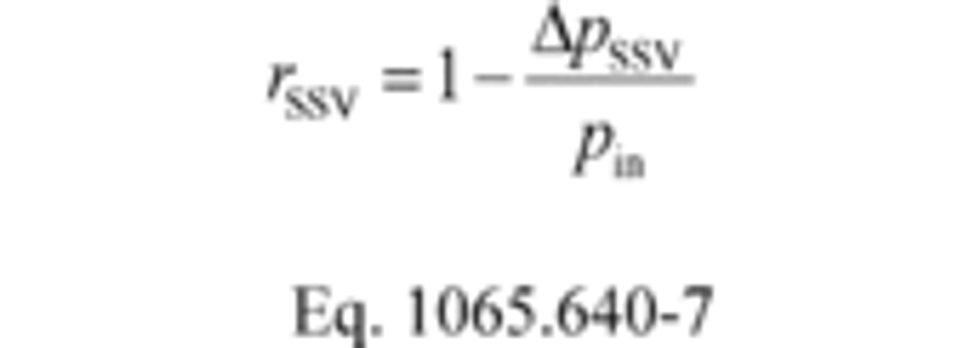
Where:
ΔpSSV = Differential static pressure; venturi inlet minus venturi throat.
(ii) For CFV systems only, calculate rCFV iteratively using the following equation:
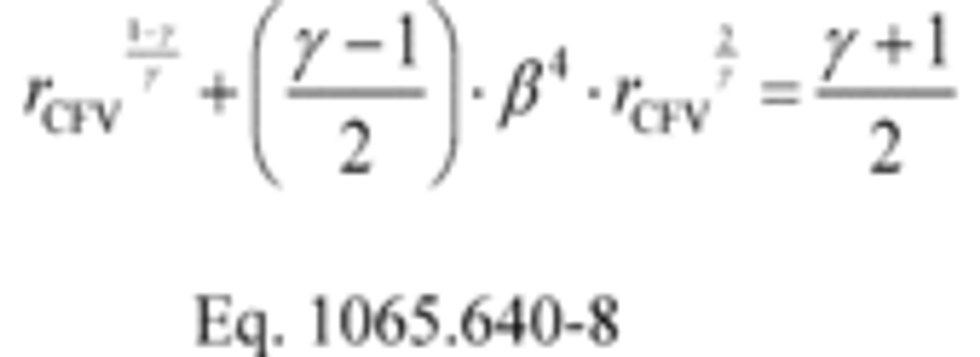
(5) You may apply any of the following simplifying assumptions or develop other values as appropriate for your test configuration, consistent with good engineering judgment:
(i) For raw exhaust, diluted exhaust, and dilution air, you may assume that the gas mixture behaves as an ideal gas: Z = 1.
(ii) For raw exhaust, you may assume g = 1.385.
(iii) For diluted exhaust and dilution air, you may assume g = 1.399.
(iv) For diluted exhaust and dilution air, you may assume the molar mass of the mixture, Mmix, is a function only of the amount of water in the dilution air or calibration air, as follows:
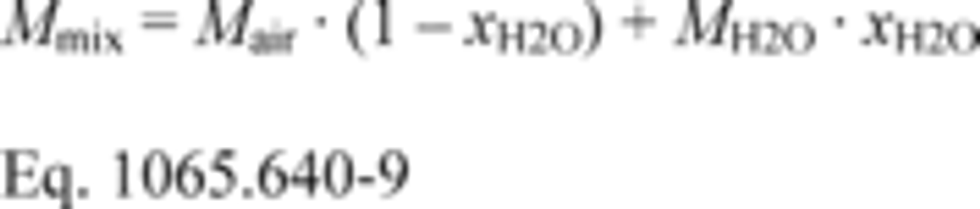
Where:
Mair = molar mass of dry air.
xH2O = amount of H2O in the dilution air or calibration air, determined as described in §1065.645.
MH2O = molar mass of water.
Example:
Mair = 28.96559 g/mol
xH2O = 0.0169 mol/mol
MH2O = 18.01528 g/mol
Mmix = 28.96559 · (1- 0.0169) + 18.01528 · 0.0169
Mmix = 28.7805 g/mol
(v) For diluted exhaust and dilution air, you may assume a constant molar mass of the mixture, Mmix, for all calibration and all testing as long as your assumed molar mass differs no more than ±1% from the estimated minimum and maximum molar mass during calibration and testing.
You may assume this, using good engineering judgment, if you sufficiently control the amount of water in calibration air and in dilution air or if you remove sufficient water from both calibration air and dilution air. The following table gives examples of permissible ranges of dilution air dewpoint versus calibration air dewpoint:
| If calibration Tdew (°C) is . . . | assume the following constant Mmix (g/mol) . . . | for the following ranges of Tdew (°C) during emission tests a |
|---|---|---|
| dry | 28.96559 | dry to 18 |
| 0 | 28.89263 | dry to 21 |
| 5 | 28.86148 | dry to 22 |
| 10 | 28.81911 | dry to 24 |
| 15 | 28.76224 | dry to 26 |
| 20 | 28.68685 | -8 to 28 |
| 25 | 28.58806 | 12 to 31 |
| 30 | 28.46005 | 23 to 34 |
| a Range valid for all calibration and emission testing over the atmospheric pressure range (80.000 to 103.325) kPa. | ||
(6) The following example illustrates the use of the governing equations to calculate Cd of an SSV flow meter at one reference flow meter value. Note that calculating Cd for a CFV flow meter would be similar, except that Cf would be determined from Table 2 of this section or calculated iteratively using values of b and g as described in paragraph (c)(2) of this section.
Example:
n ̇ref = 57.625 mol/s
Z = 1
Mmix = 28.7805 g/mol = 0.0287805 kg/mol
R = 8.314472 J/(mol · K) = 8.314472 (m 2 · kg)/(s 2 · mol · K)
Tin = 298.15 K
At = 0.01824 m 2
pin = 99.132 kPa = 99132.0 Pa = 99132 kg/(m·s 2)
g = 1.399
b = 0.8
Δp = 2.312 kPa
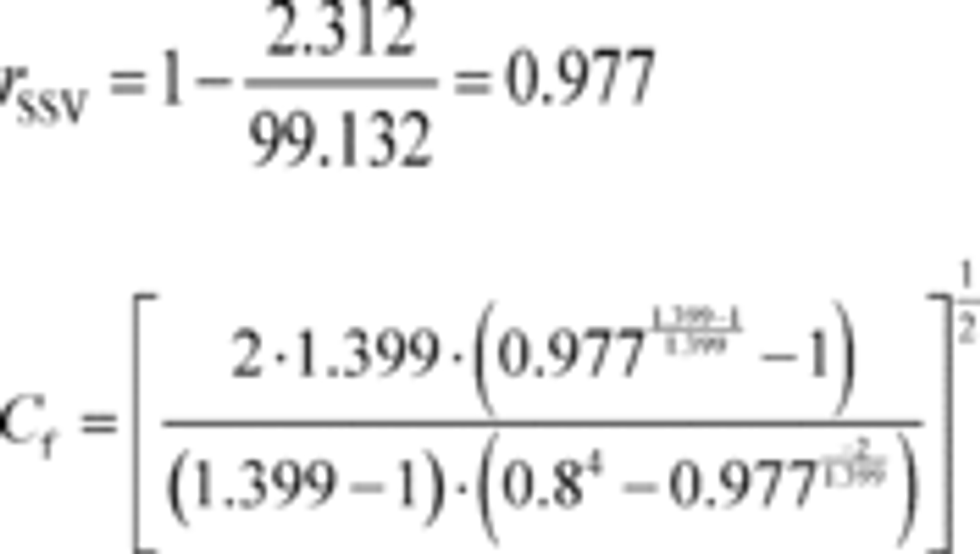
Cf = 0.274

Cd = 0.982
(d) SSV calibration. Perform the following steps to calibrate an SSV flow meter:
(1) Calculate the Reynolds number, Re #, for each reference molar flow rate, n ref, using the throat diameter of the venturi, d t. Because the dynamic viscosity, µ, is needed to compute Re #, you may use your own fluid viscosity model to determine µ for your calibration gas (usually air), using good engineering judgment. Alternatively, you may use the Sutherland three-coefficient viscosity model to approximate µ, as shown in the following sample calculation for Re #:
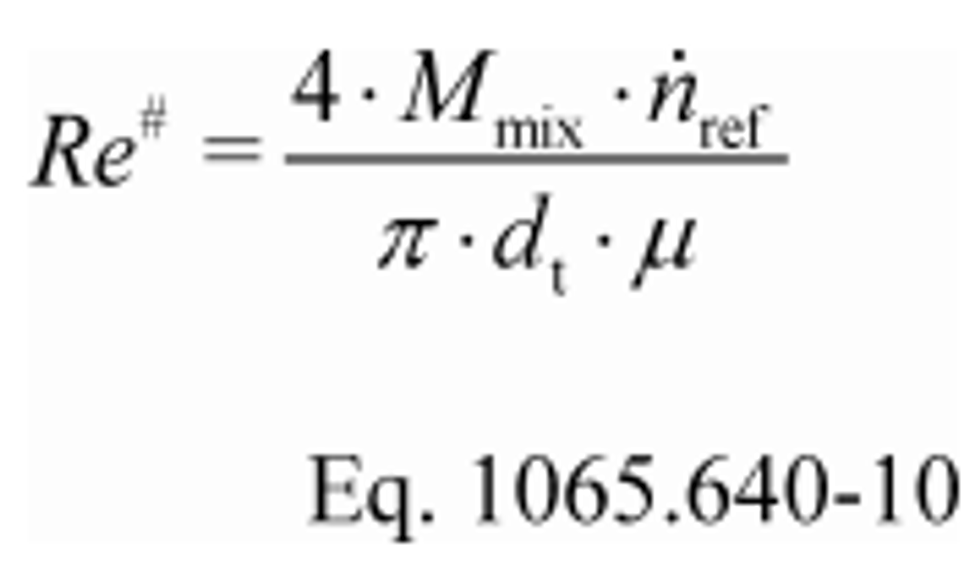
Where, using the Sutherland three-coefficient viscosity model as captured in Table 4 of this section:
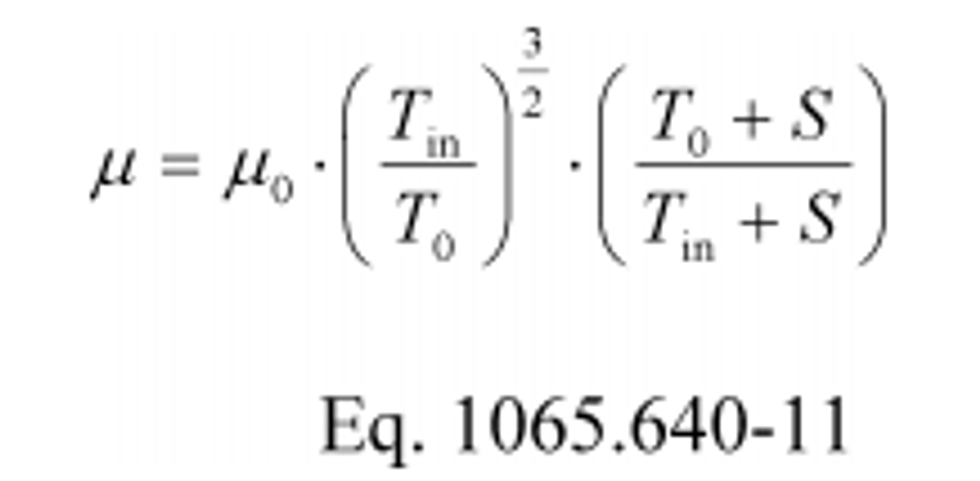
Where:
µ0 = Sutherland reference viscosity.
T 0 = Sutherland reference temperature.
S = Sutherland constant.
| Gas a | µ 0 | 0 | Temperature range within ±2% error b | Pressure limit b | |
|---|---|---|---|---|---|
| (kg/(m·s)) | (K) | (K) | (K) | (kPa) | |
| a Use tabulated parameters only for the pure gases, as listed. Do not combine parameters in calculations to calculate viscosities of gas mixtures. | |||||
| b The model results are valid only for ambient conditions in the specified ranges. | |||||
| Air | 1.716·10−5 | 273 | 111 | 170 to 1900 | ≤1800 |
| CO2 | 1.370·10−5 | 273 | 222 | 190 to 1700 | ≤3600 |
| H2O | 1.12·10−5 | 350 | 1064 | 360 to 1500 | ≤10000 |
| O2 | 1.919·10−5 | 273 | 139 | 190 to 2000 | ≤2500 |
| N2 | 1.663·10−5 | 273 | 107 | 100 to 1500 | ≤1600 |
Example:
µ0 = 1.716·10−5 kg/(m·s)
T 0 = 273 K
S = 111 K
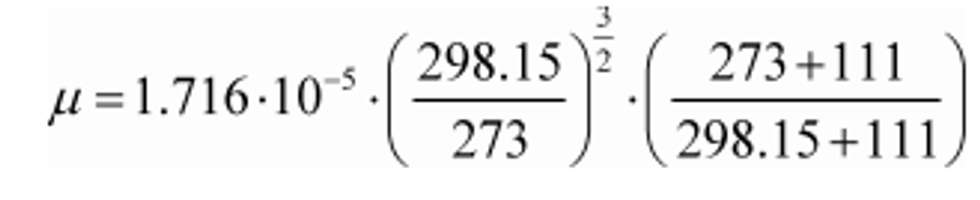
µ = 1.838·10-5 kg/(m·s)
M mix = 28.7805 g/mol = 0.0287805 kg/mol
n ref = 57.625 mol/s
d t = 152.4 mm = 0.1524 m
T in = 298.15 K

Re # = 7.538·105
(2) Create an equation for Cd as a function of Re#, using paired values of the two quantities. The equation may involve any mathematical expression, including a polynomial or a power series. The following equation is an example of a commonly used mathematical expression for relating Cd and Re#:
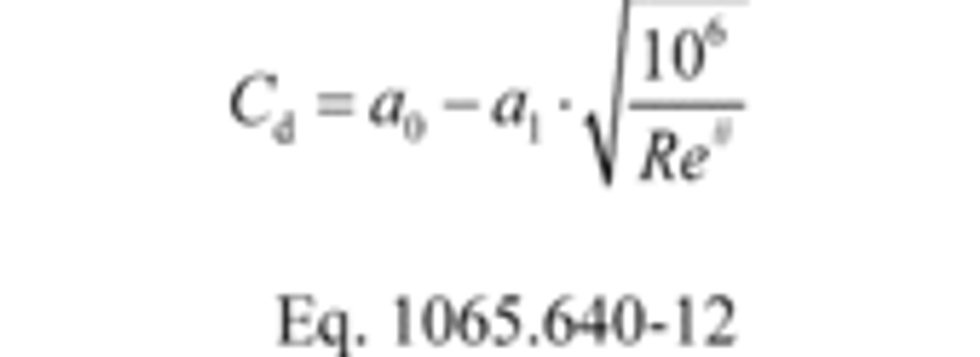
(3) Perform a least-squares regression analysis to determine the best-fit coefficients for the equation and calculate SEE as described in §1065.602. When using Eq. 1065.640-12, treat Cd as y and the radical term as y ref and use Eq. 1065.602-12 to calculate SEE. When using another mathematical expression, use the same approach to substitute that expression into the numerator of Eq. 1065.602-12 and replace the 2 in the denominator with the number of coefficients in the mathematical expression.
(4) If the equation meets the criterion of SEE ≤ 0.5% · Cdmax, you may use the equation for the corresponding range of Re#, as described in §1065.642.
(5) If the equation does not meet the specified statistical criterion, you may use good engineering judgment to omit calibration data points; however you must use at least seven calibration data points to demonstrate that you meet the criterion. For example, this may involve narrowing the range of flow rates for a better curve fit.
(6) Take corrective action if the equation does not meet the specified statistical criterion even after omitting calibration data points. For example, select another mathematical expression for the Cd versus Re# equation, check for leaks, or repeat the calibration process. If you must repeat the calibration process, we recommend applying tighter tolerances to measurements and allowing more time for flows to stabilize.
(7) Once you have an equation that meets the specified statistical criterion, you may use the equation only for the corresponding range of Re#.
(e) CFV calibration. Some CFV flow meters consist of a single venturi and some consist of multiple venturis, where different combinations of venturis are used to meter different flow rates. For CFV flow meters that consist of multiple venturis, either calibrate each venturi independently to determine a separate discharge coefficient, Cd, for each venturi, or calibrate each combination of venturis as one venturi. In the case where you calibrate a combination of venturis, use the sum of the active venturi throat areas as At, the square root of the sum of the squares of the active venturi throat diameters as dt, and the ratio of the venturi throat to inlet diameters as the ratio of the square root of the sum of the active venturi throat diameters (dt) to the diameter of the common entrance to all the venturis. (D). To determine the Cd for a single venturi or a single combination of venturis, perform the following steps:
(1) Use the data collected at each calibration set point to calculate an individual Cd for each point using Eq. 1065.640-4.
(2) Calculate the mean and standard deviation of all the Cd values according to Eqs. 1065.602-1 and 1065.602-2.
(3) If the standard deviation of all the Cd values is less than or equal to 0.3% of the mean Cd, use the mean Cd in Eq. 1065.642-4, and use the CFV only up to the highest venturi pressure ratio, r, measured during calibration using the following equation:
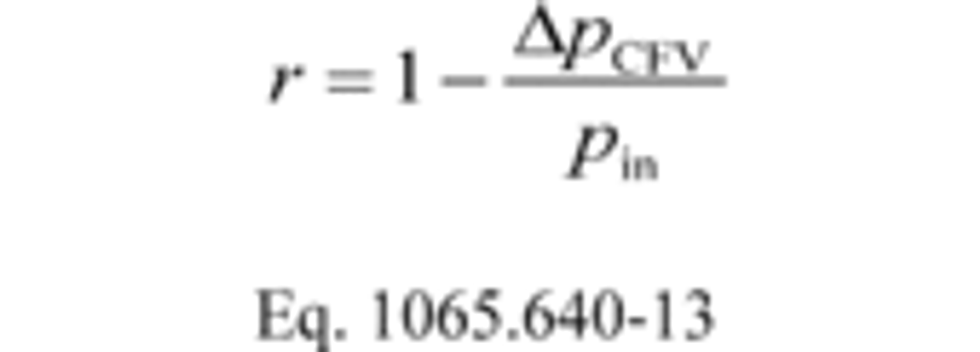
Where:
ΔpCFV = Differential static pressure; venturi inlet minus venturi outlet.
(4) If the standard deviation of all the Cd values exceeds 0.3% of the mean Cd, omit the Cd value corresponding to the data point collected at the highest r measured during calibration.
(5) If the number of remaining data points is less than seven, take corrective action by checking your calibration data or repeating the calibration process. If you repeat the calibration process, we recommend checking for leaks, applying tighter tolerances to measurements and allowing more time for flows to stabilize.
(6) If the number of remaining Cd values is seven or greater, recalculate the mean and standard deviation of the remaining Cd values.
(7) If the standard deviation of the remaining Cd values is less than or equal to 0.3% of the mean of the remaining Cd, use that mean Cd in Eq. 1065.642-4, and use the CFV values only up to the highest r associated with the remaining Cd.
(8) If the standard deviation of the remaining Cd still exceeds 0.3% of the mean of the remaining Cd values, repeat the steps in paragraph (e)(4) through (8) of this section.
[79 FR 23785, Apr. 28, 2014, as amended at 81 FR 74172, Oct. 25, 2016; 86 FR 34556, Jun. 29, 2021]
§1065.642 PDP, SSV, and CFV molar flow rate calculations.
This section describes the equations for calculating molar flow rates from various flow meters. After you calibrate a flow meter according to §1065.640, use the calculations described in this section to calculate flow during an emission test.
(a) PDP molar flow rate. (1) Based on the speed at which you operate the PDP for a test interval, select the corresponding slope, a1, and intercept, a0, as calculated in §1065.640, to calculate PDP molar flow rate,, as follows:
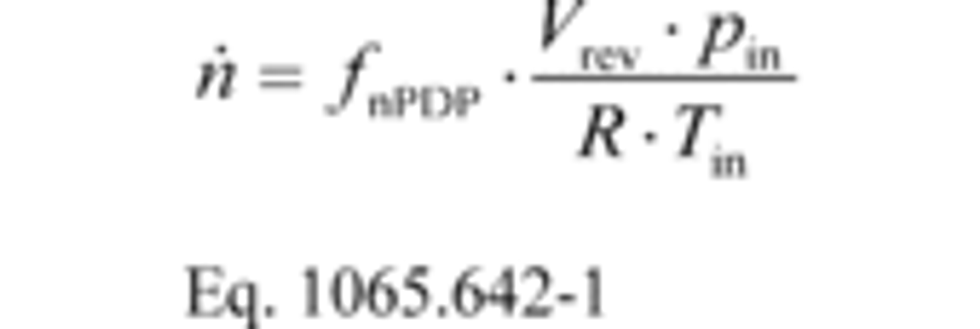
Where:
fnPDP = pump speed.
Vrev = PDP volume pumped per revolution, as determined in paragraph (a)(2) of this section.
pin = static absolute pressure at the PDP inlet.
R = molar gas constant.
Tin = absolute temperature at the PDP inlet.
(2) Calculate Vrev using the following equation:
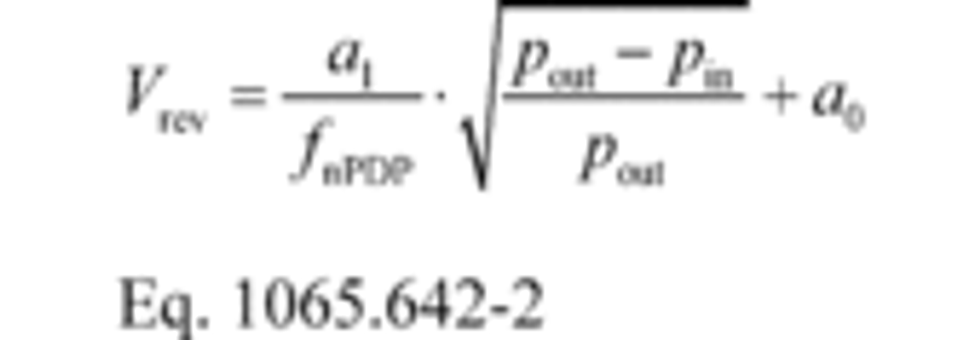
pout = static absolute pressure at the PDP outlet.
Example:
a1 = 0.8405 (m 3/s)
fnPDP = 12.58 r/s
Pout = 99.950 kPa
Pin = 98.575 kPa = 98575 Pa = 98575 kg/(m·s 2)
a0 = 0.056 (m 3/r)
R = 8.314472 J/(mol·K) = 8.314472 (m 2·kg)/(s 2·mol·K)
Tin = 323.5 K
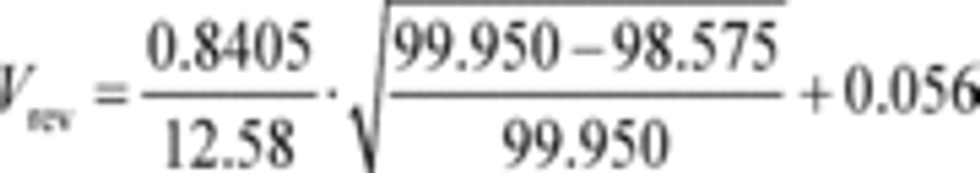
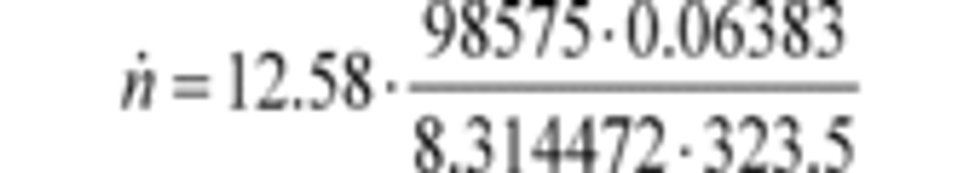
n ̇ = 29.428 mol/s
(b) SSV molar flow rate. Calculate SSV molar flow rate, n , as follows:
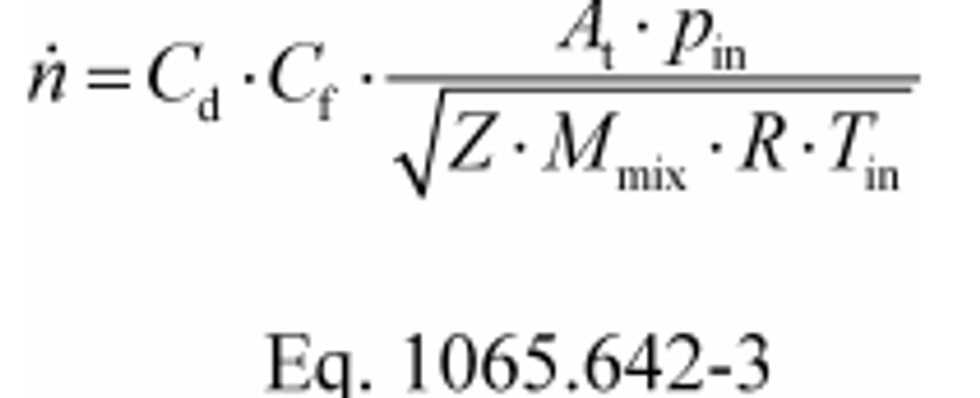
Where:
Cd = discharge coefficient, as determined based on the Cd versus Re# equation in §1065.640(d)(2).
Cf = flow coefficient, as determined in §1065.640(c)(3)(ii).
At = venturi throat cross-sectional area.
pin = static absolute pressure at the venturi inlet.
Z = compressibility factor.
Mmix = molar mass of gas mixture.
R = molar gas constant.
Tin = absolute temperature at the venturi inlet.
Example:
At = 0.01824 m2
pin = 99.132 kPa = 99132 Pa = 99132 kg/(m·s2)
Z = 1
Mmix = 28.7805 g/mol = 0.0287805 kg/mol
R = 8.314472 J/(mol·K) = 8.314472 (m2·kg)/(s2·mol·K)
Tin = 298.15 K
Re# = 7.232·105
γ = 1.399
β = 0.8
Δp = 2.312 kPa
Using Eq. 1065.640-7:
rssv = 0.997
Using Eq. 1065.640-6:
Cf = 0.274
Using Eq. 1065.640-5:
Cd = 0.990

n = 58.173 mol/s
(c) CFV molar flow rate. If you use multiple venturis and you calibrate each venturi independently to determine a separate discharge coefficient, Cd (or calibration coefficient, Kv), for each venturi, calculate the individual molar flow rates through each venturi and sum all their flow rates to determine CFV flow rate, n ̇. If you use multiple venturis and you calibrated venturis in combination, calculate n ̇ using the sum of the active venturi throat areas as At, the square root of the sum of the squares of the active venturi throat diameters as dt, and the ratio of the venturi throat to inlet diameters as the ratio of the square root of the sum of the active venturi throat diameters (dt) to the diameter of the common entrance to all the venturis (D).
(1) To calculate n through one venturi or one combination of venturis, use its respective mean Cd and other constants you determined according to §1065.640 and calculate n as follows:
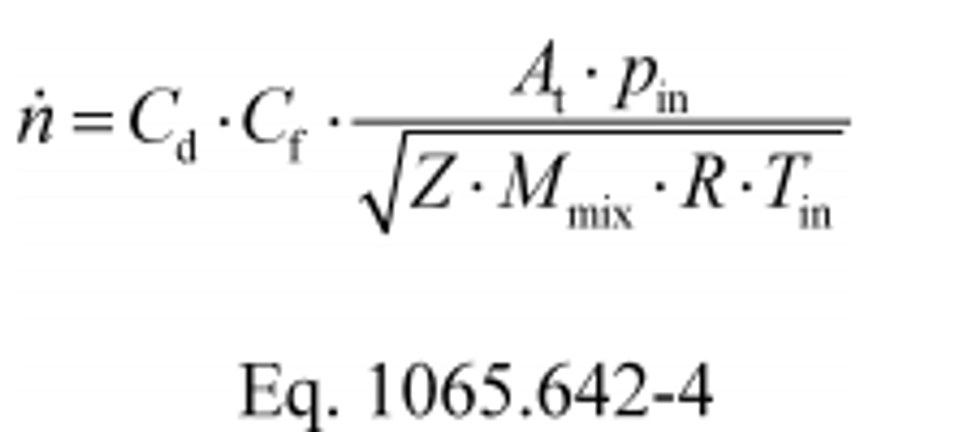
Where:
Cf = flow coefficient, as determined in §1065.640(c)(3).
Example:
Cd = 0.985
Cf = 0.7219
At = 0.00456 m2
pin = 98.836 kPa = 98836 Pa = 98836 kg/(m·s2)
Z = 1
Mmix = 28.7805 g/mol = 0.0287805 kg/mol
R = 8.314472 J/(mol·K) = 8.314472 (m2·kg)/(s2·mol·K)
Tin = 378.15 K
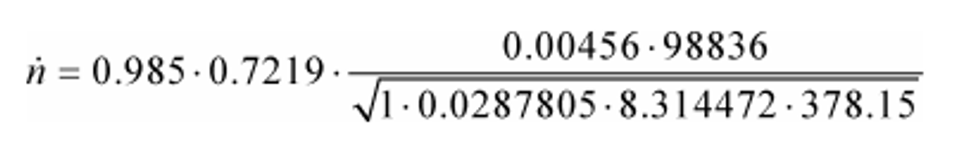
n = 33.690 mol/s
(2) To calculate the molar flow rate through one venturi or a combination of venturis, you may use its respective mean, Kv, and other constants you determined according to §1065.640 and calculate its molar flow rate n ̇ during an emission test. Note that if you follow the permissible ranges of dilution air dewpoint versus calibration air dewpoint in Table 3 of §1065.640, you may set Mmix-cal and Mmix equal to 1. Calculate n ̇ as follows:
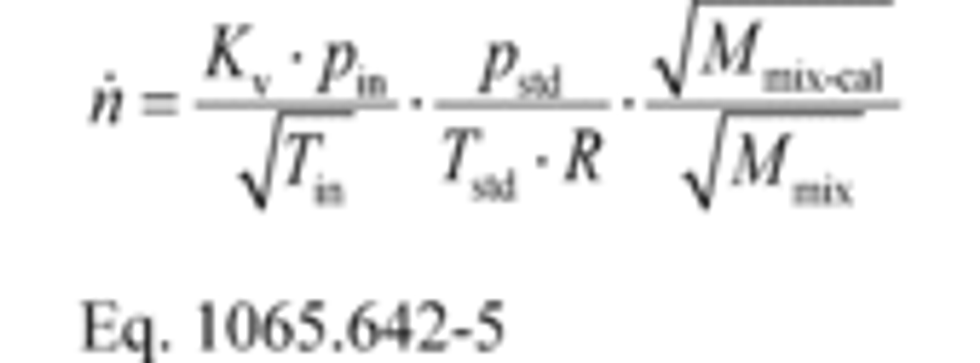
Where:

Vstdref = volume flow rate of the standard at reference conditions of 293.15 K and 101.325 kPa.
Tin-cal = venturi inlet temperature during calibration.
Pin-cal = venturi inlet pressure during calibration.
Mmix-cal = molar mass of gas mixture used during calibration.
Mmix = molar mass of gas mixture during the emission test calculated using Eq. 1065.640-9.
Example:
Vstdref = 0.4895 m 3
Tin-cal = 302.52 K
Pin-cal = 99.654 kPa = 99654 Pa = 99654 kg/(m·s 2)
pin = 98.836 kPa = 98836 Pa = 98836 kg/(m·s 2)
pstd = 101.325 kPa = 101325 Pa = 101325 kg/(m·s 2)
Mmix-cal = 28.9656 g/mol = 0.0289656 kg/mol
Mmix = 28.7805 g/mol = 0.0287805 kg/mol
Tin = 353.15 K
Tstd = 293.15 K
R = 8.314472 J/(mol·K) = 8.314472 (m 2·kg)/(s 2·mol·K)
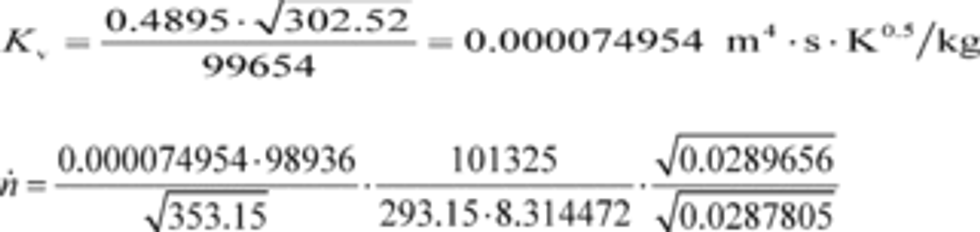
n ̇ = 16.457 mol/s
[81 FR 74177, Oct. 25, 2016; 86 FR 34557, Jun. 29, 2021]
§1065.643 Carbon balance error verification calculations.
This section describes how to calculate quantities used in the carbon balance error verification described in §1065.543. Paragraphs (a) through (c) of this section describe how to calculate the mass of carbon for a test interval from carbon-carrying fluid streams, intake air into the system, and exhaust emissions, respectively. Paragraph (d) of this section describes how to use these carbon masses to calculate four different quantities for evaluating carbon balance error. Use rectangular or trapezoidal integration methods to calculate masses and amounts over a test interval from continuously measured or calculated mass and molar flow rates.
(a) Fuel and other fluids. Determine the mass of fuel, DEF, and other carbon-carrying fluid streams, other than intake air, flowing into the system, mfluidj, for each test interval. Note that §1065.543 allows you to omit all flows other than fuel. You may determine the mass of DEF based on ECM signals for DEF flow rate. You may determine fuel mass during field testing based on ECM signals for fuel flow rate. Calculate the mass of carbon from the combined carbon-carrying fluid streams flowing into the system as follows:
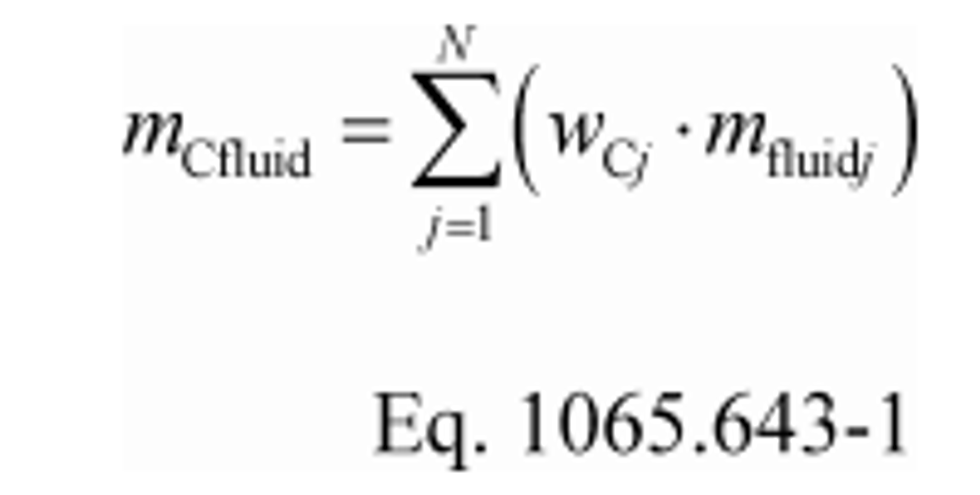
Where:
j = an indexing variable that represents one carbon-carrying fluid stream.
N = total number of carbon-carrying fluid streams into the system over the test interval.
wC = carbon mass fraction of the carbon-carrying fluid stream as determined in §1065.655(d).
mfluid = the mass of the carbon-carrying fluid stream determined over the test interval.
Example:
N = 2
wCfuel = 0.869
wCDEF = 0.065
mfuel = 1119.6 g
mDEF = 36.8 g
mCfluid = 0.869·1119.6 + 0.065·36.8 = 975.3 g
(b) Intake air. Calculate the mass of carbon in the intake air, mCair, for each test interval using one of the methods in this paragraph (b). The methods are listed in order of preference. Use the first method where all the inputs are available for your test configuration. For methods that calculate mCair based on the amount of CO2 per mole of intake air, we recommend measuring intake air concentration, but you may calculate xCO2int using Eq. 1065.655-10 and letting xCO2intdry = 375 µmol/mol.
(1) Calculate mCair, using the following equation if you measure intake air flow:
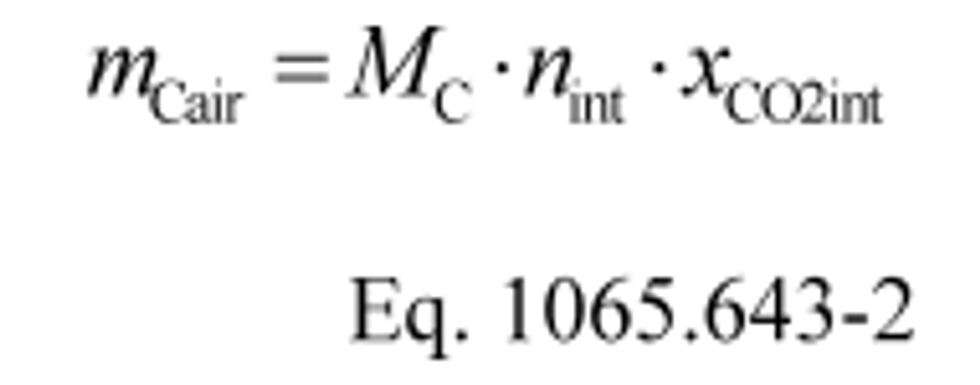
Where:
MC = molar mass of carbon.
nint = measured amount of intake air over the test interval.
xCO2int = amount of intake air CO2 per mole of intake air.
Example:
MC = 12.0107 g/mol
nint = 62862 mol
xCO2int = 369 µmol/mol = 0.000369 mol/mol
mCair = 12.0107·62862·0.000369 = 278.6 g
(2) Calculate mCair, using the following equation if you measure or calculate raw exhaust flow and you calculate chemical balance terms:
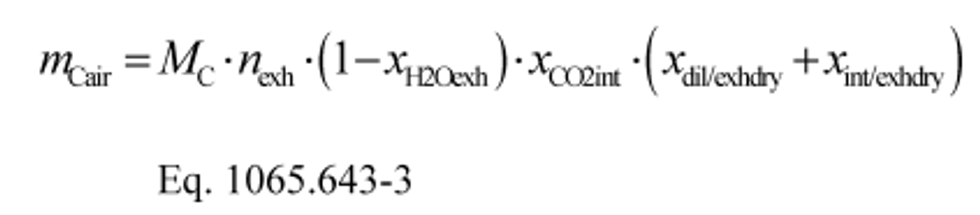
Where:
MC = molar mass of carbon.
nexh = calculated or measured amount of raw exhaust over the test interval.
xH2Oexh = amount of H2O in exhaust per mole of exhaust.
xCO2int = amount of intake air CO2 per mole of intake air.
xdil/exhdry = amount of excess air per mole of dry exhaust. Note that excess air and intake air have the same composition, so xCO2dil = xCO2int and xH2Odil = xH2Oint for the chemical balance calculation for raw exhaust.
xint/exhdry = amount of intake air required to produce actual combustion products per mole of dry exhaust.
Example:
MC = 12.0107 g/mol
nexh = 62862 mol
xH2Oexh = 0.034 mol/mol
xCO2int = 369 µmol/mol = 0.000369 mol/mol
xdil/exhdry = 0.570 mol/mol
xint/exhdry = 0.465 mol/mol
mCair = 12.0107·62862·(1 − 0.034)·0.000369·(0.570 + 0.465) = 278.6 g
(3) Calculate mCair, using the following equation if you measure raw exhaust flow:
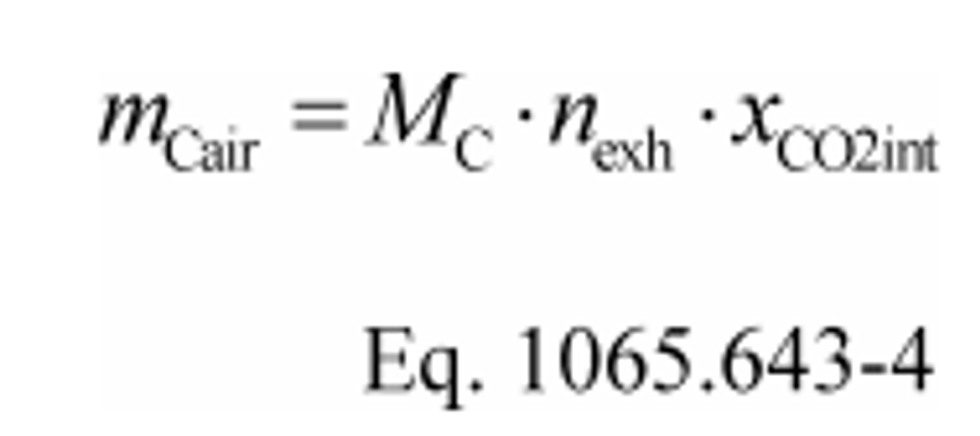
Where:
MC = molar mass of carbon.
nexh = measured amount of raw exhaust over the test interval.
xCO2int = amount of intake air CO2 per mole of intake air.
Example:
MC = 12.0107 g/mol
nexh = 62862 mol
xCO2int = 369 µmol/mol = 0.000369 mol/mol
mCair = 12.0107·62862·0.000369 = 278.6 g
(4) Calculate mCair, using the following equation if you measure diluted exhaust flow and dilution air flow:
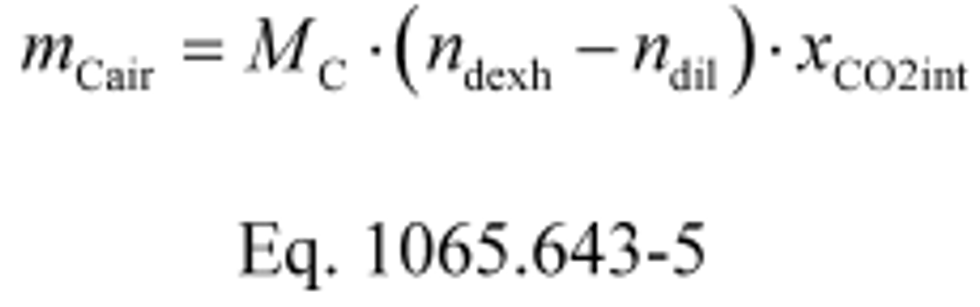
Where:
MC = molar mass of carbon.
ndexh = measured amount of diluted exhaust over the test interval as determined in §1065.642.
ndil = measured amount of dilution air over the test interval as determined in §1065.667(b).
xCO2int = amount of intake air CO2 per mole of intake air.
Example:
MC = 12.0107 g/mol
ndexh = 942930 mol
ndil = 880068 mol
xCO2int = 369 µmol/mol = 0.000369 mol/mol
mCair = 12.0107·(942930 − 880068)·0.000369 = 278.6 g
(5) Determined mCair based on ECM signals for intake air flow as described in paragraph (b)(1) of this section.
(6) If you measure diluted exhaust, determine mCair as described in paragraph (b)(4) of this section using a calculated amount of dilution air over the test interval as determined in §1065.667(d) instead of the measured amount of dilution air.
(c) Exhaust emissions. Calculate the mass of carbon in exhaust emissions, mCexh, for each test interval as follows:
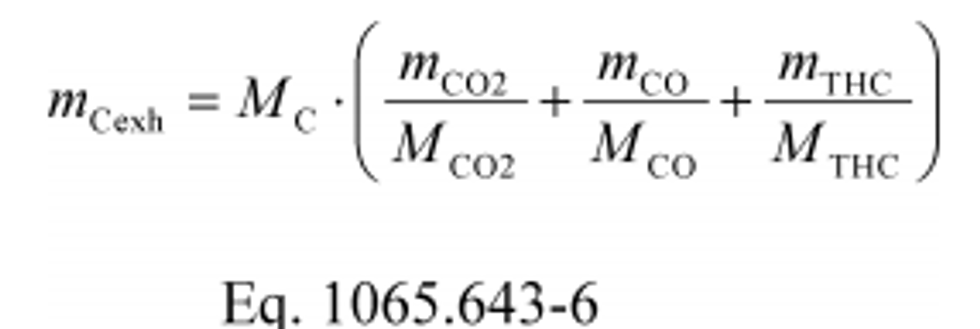
Where:
MC = molar mass of carbon.
mCO2 = mass of CO2 over the test interval as determined in §1065.650(c).
MCO2 = molar mass of carbon dioxide.
mCO = mass of CO over the test interval as determined in §1065.650(c).
MCO = molar mass of carbon monoxide.
mTHC = mass of THC over the test interval as determined in §1065.650(c).
MTHC = effective C1 molar mass of total hydrocarbon as defined in §1065.1005(f)(2).
Example:
MC = 12.0107 g/mol
mCO2 = 4567 g
MCO2 = 44.0095 g/mol
mCO = 0.803 g
MCO = 28.0101 g/mol
mTHC = 0.537 g
MTHC = 13.875389 g/mol
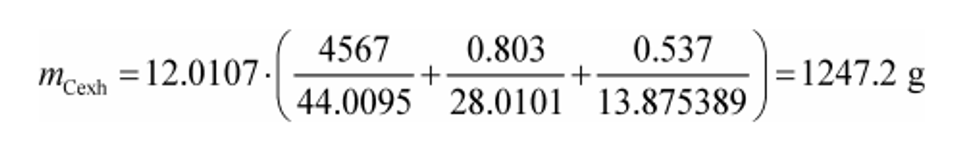
(d) Carbon balance error quantities. Calculate carbon balance error quantities as follows:
(1) Calculate carbon mass absolute error, εaC, for a test interval as follows:
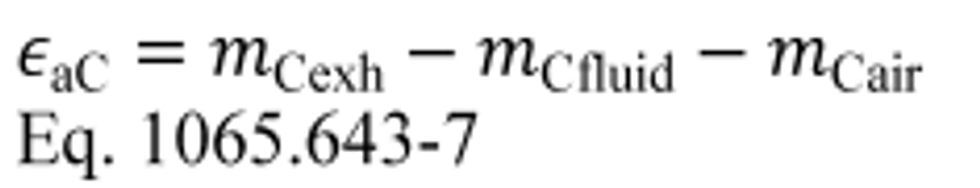
Where:
mCexh = mass of carbon in exhaust emissions over the test interval as determined in paragraph (d) of this section.
mCfluid = mass of carbon in all the carbon-carrying fluid streams flowing into the system over the test interval as determined in paragraph (a) of this section.
mCair = mass of carbon in the intake air flowing into the system over the test interval as determined in paragraph (b) of this section.
Example:
mCexh = 1247.2 g
mCfluid = 975.3 g
mCair = 278.6 g
εaC = 1247.2 − 975.3 − 278.6
εaC = −6.7 g
(2) Calculate carbon mass rate absolute error, εaCrate, for a test interval as follows:
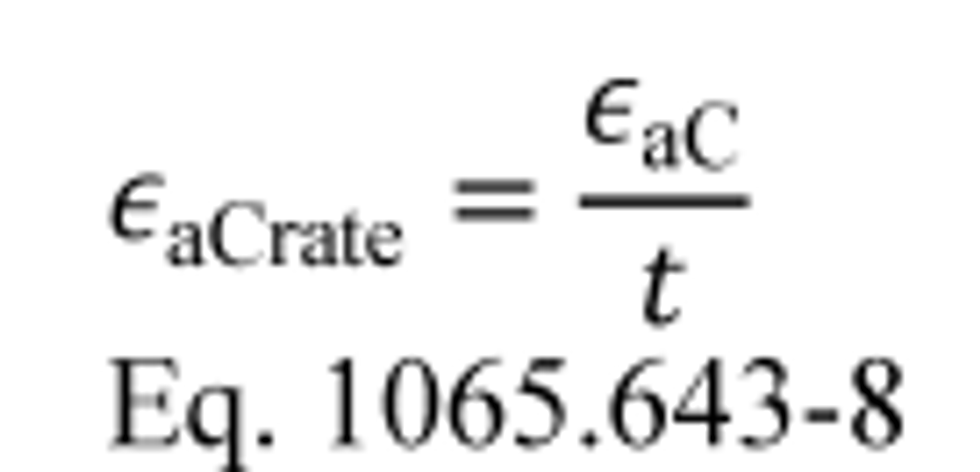
Where:
t = duration of the test interval.
Example:
εaC = −6.7 g
t = 1202.2 s = 0.3339 hr
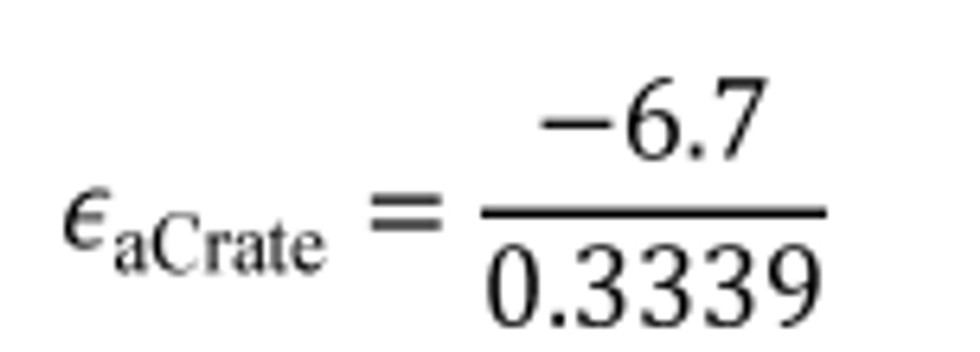
εaCrate = −20.065 g/hr
(3) Calculate carbon mass relative error, εrC, for a test interval as follows:
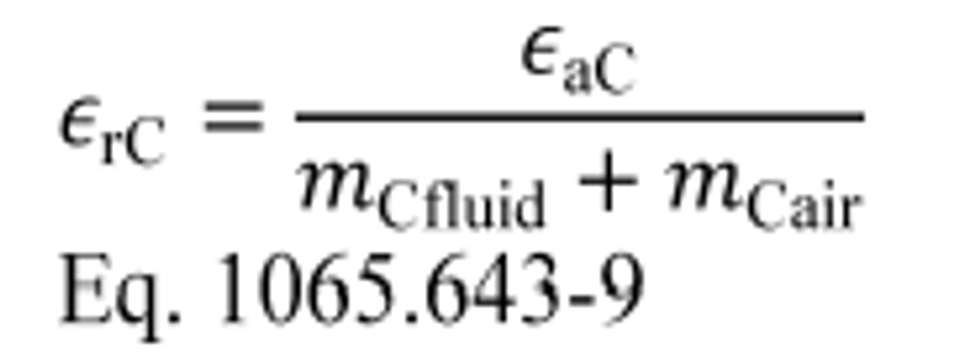
Example:
εaC = −6.7 g
mCfluid = 975.3 g
mCair = 278.6 g
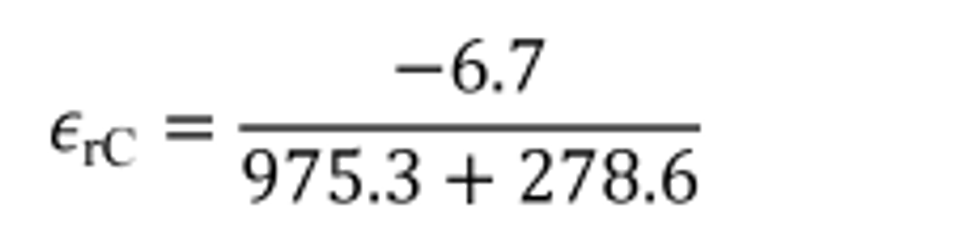
εrC = −0.0053
(4) Calculate composite carbon mass relative error, εrCcomp, for a duty cycle with multiple test intervals as follows:
(i) Calculate εrCcomp using the following equation:
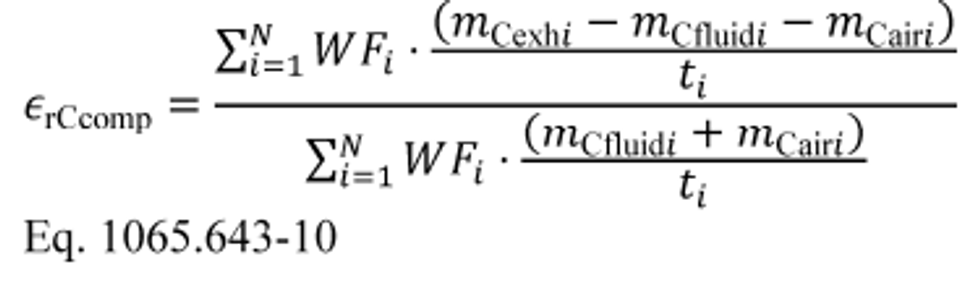
Where:
i = an indexing variable that represents one test interval.
N = number of test intervals.
WF = weighting factor for the test interval as defined in the standard-setting part.
mCexh = mass of carbon in exhaust emissions over the test interval as determined in paragraph (c) of this section.
mCfluid = mass of carbon in all the carbon-carrying fluid streams that flowed into the system over the test interval as determined in paragraph (a) of this section.
mCair = mass of carbon in the intake air that flowed into the system over the test interval as determined in paragraph (b) of this section.
t = duration of the test interval. For duty cycles with multiple test intervals of a prescribed duration, such as cold-start and hot-start transient cycles, set t = 1 for all test intervals. For discrete-mode steady-state duty cycles with multiple test intervals of varying duration, set t equal to the actual duration of each test interval.
(ii) The following example illustrates calculation of εrCcomp, for cold-start and hot-start transient cycles:
N = 2
WF1 = 1/7
WF2 = 6/7
mCexh1 = 1255.3 g
mCexh2 = 1247.2 g
mCfluid1 = 977.8 g
mCfluid2 = 975.3 g
mCair1 = 280.2 g
mCair2 = 278.6 g
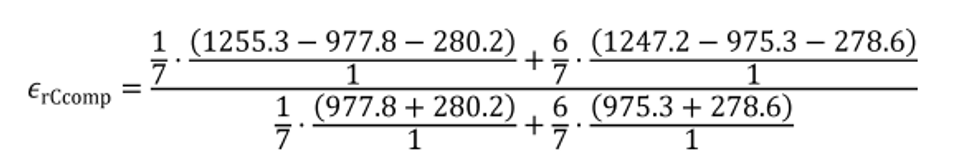
εrCcomp = −0.0049
(iii) The following example illustrates calculation of εrCcomp for multiple test intervals with varying duration, such as discrete-mode steady-state duty cycles:
N = 2
WF1 = 0.85
WF2 = 0.15
mCexh1 = 2.873 g
mCexh2 = 0.125 g
mCfluid1 = 2.864 g
mCfluid2 = 0.095 g
mCair1 = 0.023 g
mCair2 = 0.024 g
t1 = 123 s
t2 = 306 s
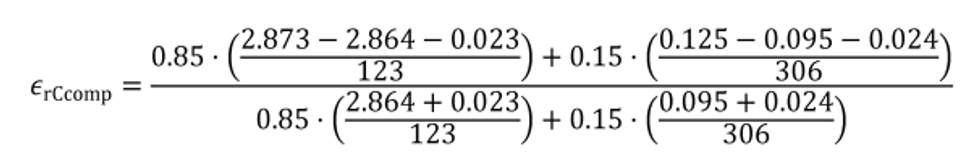
εrCcomp = −0.0047
[86 FR 34557, Jun. 29, 2021; 87 FR 65865, Oct. 26, 2022; 88 FR 4680, Jan. 24, 2023]
§1065.644 Vacuum-decay leak rate.
This section describes how to calculate the leak rate of a vacuum-decay leak verification, which is described in §1065.345(e). Use the following equation to calculate the leak rate, , and compare it to the criterion specified in §1065.345(e):
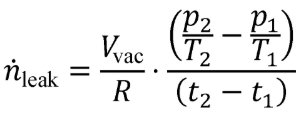
Eq. 1065.644-1
Where:
Vvac = geometric volume of the vacuum-side of the sampling system.
R = molar gas constant.
p2 = vacuum-side absolute pressure at time t 2.
T2 = vacuum-side absolute temperature at time t 2.
p1 = vacuum-side absolute pressure at time t 1.
T1 = vacuum-side absolute temperature at time t 1.
t2 = time at completion of vacuum-decay leak verification test.
t1 = time at start of vacuum-decay leak verification test.
Example:
Vvac = 2.0000 L = 0.00200 m 3
R = 8.314472 J/(mol·K) = 8.314472 (m 2 ·kg)/(s 2 ·mol·K)
p2 = 50.600 kPa = 50600 Pa = 50600 kg/(m·s 2)
T2 = 293.15 K
p1 = 25.300 kPa = 25300 Pa = 25300 kg/(m·s 2)
T1 = 293.15 K
t2 = 10:57:35 a.m.
t1 = 10:56:25 a.m.

[79 FR 23795, Apr. 28, 2014; 89 FR 29808, Apr. 22, 2024]
§1065.645 Amount of water in an ideal gas.
This section describes how to determine the amount of water in an ideal gas, which you need for various performance verifications and emission calculations. Use the equation for the vapor pressure of water in paragraph (a) of this section or another appropriate equation and, depending on whether you measure dewpoint or relative humidity, perform one of the calculations in paragraph (b) or (c) of this section. Paragraph (d) of this section provides an equation for determining dewpoint from relative humidity and dry bulb temperature measurements. The equations for the vapor pressure of water as presented in this section are derived from equations in “Saturation Pressure of Water on the New Kelvin Temperature Scale” (Goff, J.A., Transactions American Society of Heating and Air-Conditioning Engineers, Vol. 63, No. 1607, pages 347-354). Note that the equations were originally published to derive vapor pressure in units of atmospheres and have been modified to derive results in units of kPa by converting the last term in each equation.
(a) Vapor pressure of water. Calculate the vapor pressure of water for a given saturation temperature condition, Tsat, as follows, or use good engineering judgment to use a different relationship of the vapor pressure of water to a given saturation temperature condition:
(1) For humidity measurements made at ambient temperatures from (0 to 100)°C, or for humidity measurements made over super-cooled water at ambient temperatures from (−50 to 0)°C, use the following equation:

(2) For humidity measurements over ice at ambient temperatures from (-100 to 0)°C, use the following equation:

(b) Dewpoint. If you measure humidity as a dewpoint, determine the amount of water in an ideal gas, xH20, as follows:
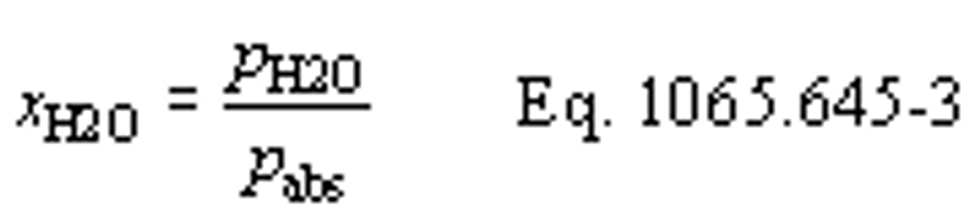
Where:
xH20 = amount of water in an ideal gas.
pH20 = water vapor pressure at the measured dewpoint, Tsat = Tdew.
pabs = wet static absolute pressure at the location of your dewpoint measurement.
Example:
:
pabs = 99.980 kPa
Tsat = Tdew = 9.5°C
Using Eq. 1065.645-1,
pH20 = 1.186581 kPa
xH2O = 1.186581/99.980
xH2O = 0.011868 mol/mol
(c) Relative humidity. If you measure humidity as a relative humidity, RH, determine the amount of water in an ideal gas, xH2O, as follows:

Where:
xH2O = amount of water in an ideal gas.
RH = relative humidity.
pH2O = water vapor pressure at 100% relative humidity at the location of your relative humidity measurement, Tsat = Tamb.
pabs = wet static absolute pressure at the location of your relative humidity measurement.
Example:
RH = 50.77% = 0.5077
pabs = 99.980 kPa
Tsat = Tamb = 20°C
Using Eq. 1065.645-1,
pH2O = 2.3371 kPa
xH2O = (0.5077 · 2.3371)/99.980
xH2O = 0.011868 mol/mol
(d) Dewpoint determination from relative humidity and dry bulb temperature. This paragraph (d) describes how to calculate dewpoint temperature from relative humidity, RH. This is based on “ITS-90 Formulations for Vapor Pressure, Frostpoint Temperature, Dewpoint Temperature, and Enhancement Factors in the Range −100 to + 100°C” (Hardy, B., The Proceedings of the Third International Symposium on Humidity & Moisture, Teddington, London, England, April 1998). Calculate pH20sat as described in paragraph (a) of this section based on setting Tsat equal to Tamb. Calculate pH20scaled by multiplying pH20sat by RH. Calculate the dewpoint, Tdew, from pH20 using the following equation:

Where:
ln(pH2O) = the natural log of pH2Oscaled, which is the water vapor pressure scaled to the relative humidity at the location of the relative humidity measurement, Tsat = Tamb
Example:
RH = 39.61% = 0.3961
Tsat = Tamb = 20.00°C = 293.15K
Using Eq. 1065.645-1,
pH2Osat = 2.3371 kPa
pH2Oscaled = (0.3961 · 2.3371) = 0.925717 kPa = 925.717 Pa
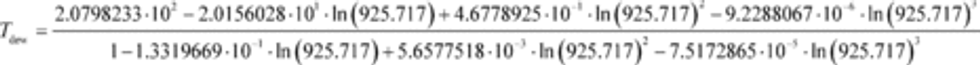
[73 FR 37327, June 30, 2008, as amended at 73 FR 59331, Oct. 8, 2008; 75 FR 23048, Apr. 30, 2010; 76 FR 57456, Sept. 15, 2011;79 FR 23796, Apr. 28, 2014; 81 FR 74179, Oct. 25, 2016]
§1065.650 Emission calculations.
(a) General. Calculate brake-specific emissions over each applicable duty cycle or test interval. For test intervals with zero work (or power), calculate the emission mass (or mass rate), but do not calculate brake-specific emissions. Unless specified otherwise, for the purposes of calculating and reporting emission mass (or mass rate), do not alter any negative values of measured or calculated quantities. You may truncate negative values in chemical balance quantities listed in §1065.655(c) to facilitate convergence. For duty cycles with multiple test intervals, refer to the standard-setting part for calculations you need to determine a composite result, such as a calculation that weights and sums the results of individual test intervals in a duty cycle. If the standard-setting part does not include those calculations, use the equations in paragraph (g) of this section. This section is written based on rectangular integration, where each indexed value (i.e., “ i ”) represents (or approximates) the mean value of the parameter for its respective time interval, delta-t. You may also integrate continuous signals using trapezoidal integration consistent with good engineering judgment.
(b) Brake-specific emissions over a test interval. We specify three alternative ways to calculate brake-specific emissions over a test interval, as follows:
(1) For any testing, you may calculate the total mass of emissions, as described in paragraph (c) of this section, and divide it by the total work generated over the test interval, as described in paragraph (d) of this section, using the following equation:
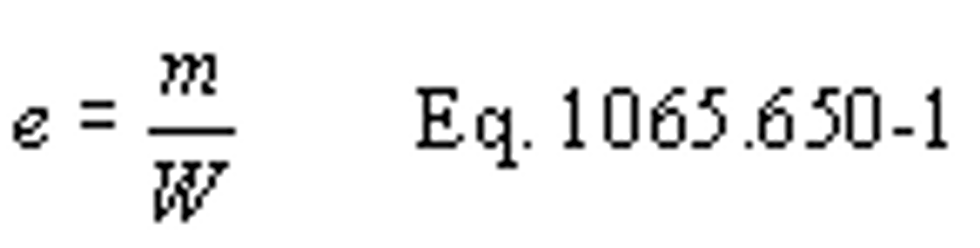
Example:
mNOx = 64.975 g
W = 25.783 kW · hr
eNOx = 64.975/25.783
eNOx = 2.520 g/(kW · hr)
(2) For discrete-mode steady-state testing, you may calculate the brake-specific emissions over a test interval using the ratio of emission mass rate to power, as described in paragraph (e) of this section, using the following equation:
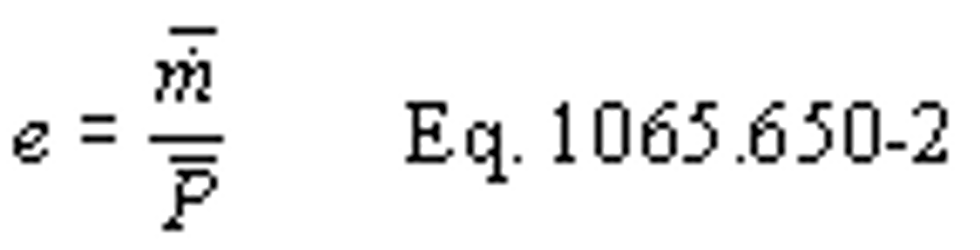
(3) For field testing, you may calculate the ratio of total mass to total work, where these individual values are determined as described in paragraph (f) of this section. You may also use this approach for laboratory testing, consistent with good engineering judgment. Good engineering judgment dictates that this method not be used if there are any work flow paths described in §1065.210 that cross the system boundary, other than the primary output shaft (crankshaft). This is a special case in which you use a signal linearly proportional to raw exhaust molar flow rate to determine a value proportional to total emissions. You then use the same linearly proportional signal to determine total work using a chemical balance of fuel, DEF, intake air, and exhaust as described in §1065.655, plus information about your engine's brake-specific fuel consumption. Under this method, flow meters need not meet accuracy specifications, but they must meet the applicable linearity and repeatability specifications in subpart D or J of this part. The result is a brake-specific emission value calculated as follows:
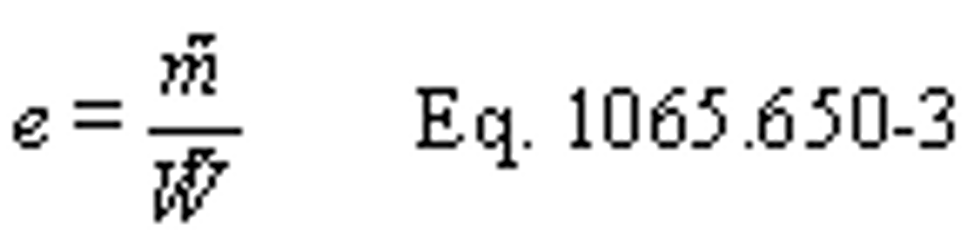
Example:
m ̃ = 805.5 g
W ̃ = 52.102 kW · hr
eCO = 805.5/52.102
eCO = 2.520 g/(kW · hr)
(c) Total mass of emissions over a test interval. To calculate the total mass of an emission, multiply a concentration by its respective flow. For all systems, make preliminary calculations as described in paragraph (c)(1) of this section to correct concentrations. Next, use the method in paragraphs (c)(2) through (4) of this section that is appropriate for your system. Finally, if necessary, calculate the mass of NMHC as described in paragraph (c)(5) of this section for all systems. Calculate the total mass of emissions as follows:
(1) Concentration corrections. Perform the following sequence of preliminary calculations on recorded concentrations:
(i) Use good engineering judgment to time-align flow and concentration data to match transformation time, t50, to within ±1 s.
(ii) Correct all gaseous emission analyzer concentration readings, including continuous readings, sample bag readings, and dilution air background readings, for drift as described in §1065.672. Note that you must omit this step where brake-specific emissions are calculated without the drift correction for performing the drift validation according to §1065.550(b). When applying the initial THC and CH 4 contamination readings according to §1065.520(g), use the same values for both sets of calculations. You may also use as-measured values in the initial set of calculations and corrected values in the drift-corrected set of calculations as described in §1065.520(g)(7).
(iii) Correct all THC and CH4 concentrations for initial contamination as described in §1065.660(a), including continuous readings, sample bags readings, and dilution air background readings.
(iv) Correct all concentrations measured on a “dry” basis to a “wet” basis, including dilution air background concentrations, as described in §1065.659.
(v) Calculate all NMHC and CH4 concentrations, including dilution air background concentrations, as described in §1065.660.
(vi) For emission testing with an oxygenated fuel, calculate any HC concentrations, including dilution air background concentrations, as described in §1065.665. See subpart I of this part for testing with oxygenated fuels.
(vii) Correct all the NOX concentrations, including dilution air background concentrations, for intake-air humidity as described in §1065.670.
(2) Continuous sampling. For continuous sampling, you must frequently record a continuously updated concentration signal. You may measure this concentration from a changing flow rate or a constant flow rate (including discrete-mode steady-state testing), as follows:
(i) Varying flow rate. If you continuously sample from a varying exhaust flow rate, time align and then multiply concentration measurements by the flow rate from which you extracted it. We consider the following to be examples of varying flows that require a continuous multiplication of concentration times molar flow rate: raw exhaust, exhaust diluted with a constant flow rate of dilution air, and CVS dilution with a CVS flow meter that does not have an upstream heat exchanger or electronic flow control. This multiplication results in the flow rate of the emission itself. Integrate the emission flow rate over a test interval to determine the total emission. If the total emission is a molar quantity, convert this quantity to a mass by multiplying it by its molar mass, M. The result is the mass of the emission, m. Calculate m for continuous sampling with variable flow using the following equations:
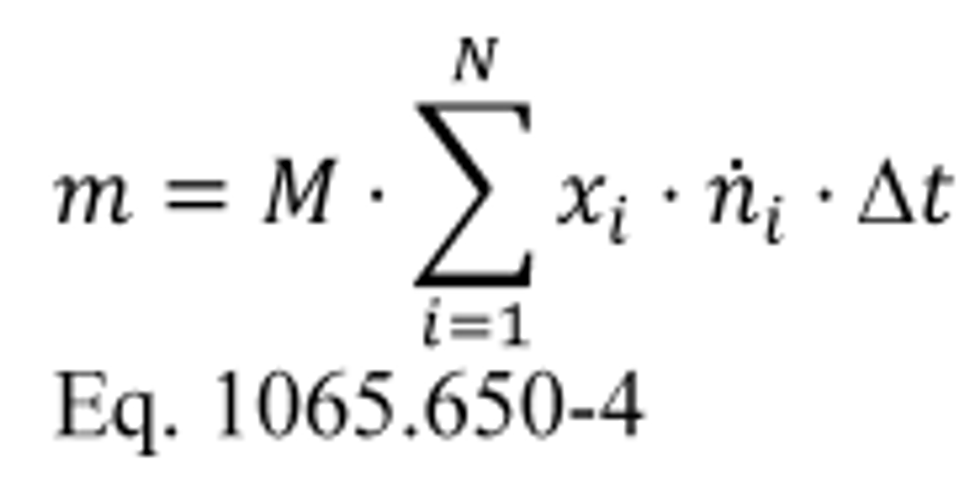
Where:
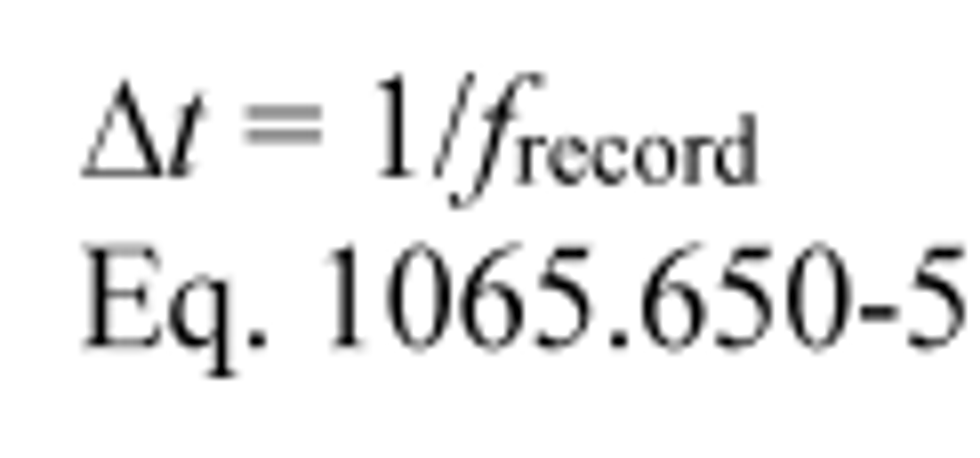
Example:
MNMHC = 13.875389 g/mol
N = 1200
xNMHC1 = 84.5 µmol/mol = 84.5 · 10 −6 mol/mol
xNMHC2 = 86.0 µmol/mol = 86.0 · 10 −6 mol/mol
n exh1 = 2.876 mol/s
n exh2 = 2.224 mol/s
ƒ record = 1 Hz
Using Eq. 1065.650-5,
Δ t = 1/1 = 1 s
mNMHC = 13.875389 · (84.5 · 10 −6 · 2.876 + 86.0 · 10 −6 · 2.224 + . . . + xNMHC1200 · n exh) · 1
mNMHC = 25.23 g
(ii) Constant flow rate. If you continuously sample from a constant exhaust flow rate, use the same emission calculations described in paragraph (c)(2)(i) of this section or calculate the mean or flow-weighted concentration recorded over the test interval and treat the mean as a batch sample, as described in paragraph (c)(3)(ii) of this section. We consider the following to be examples of constant exhaust flows: CVS diluted exhaust with a CVS flowmeter that has either an upstream heat exchanger, electronic flow control, or both.
(3) Batch sampling. For batch sampling, the concentration is a single value from a proportionally extracted batch sample (such as a bag, filter, impinger, or cartridge). In this case, multiply the mean concentration of the batch sample by the total flow from which the sample was extracted. You may calculate total flow by integrating a varying flow rate or by determining the mean of a constant flow rate, as follows:
(i) Varying flow rate. If you collect a batch sample from a varying exhaust flow rate, extract a sample proportional to the varying exhaust flow rate. We consider the following to be examples of varying flows that require proportional sampling: raw exhaust, exhaust diluted with a constant flow rate of dilution air, and CVS dilution with a CVS flow meter that does not have an upstream heat exchanger or electronic flow control. Integrate the flow rate over a test interval to determine the total flow from which you extracted the proportional sample. Multiply the mean concentration of the batch sample by the total flow from which the sample was extracted to determine the total emission. If the total emission is a molar quantity, convert this quantity to a mass by multiplying it by its molar mass, M. The result is the total emission mass, m. In the case of PM emissions, where the mean PM concentration is already in units of mass per mole of exhaust, simply multiply it by the total flow. The result is the total mass of PM, mPM. Calculate m for each constituent as follows:
(A) Calculate m for measuring gaseous emission constituents with sampling that results in a molar concentration, x , using the following equation:
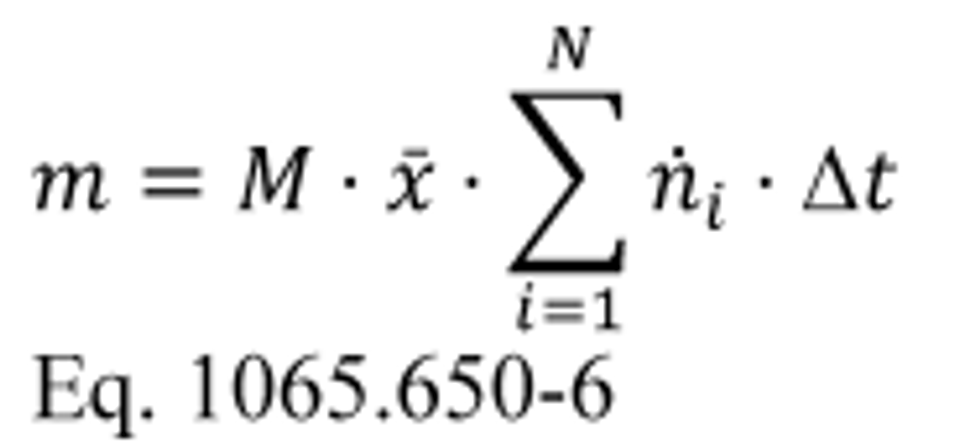
Example:
MNO = 46.0055 g/mol
N = 9000
x = 85.6 µmol/mol = 85.6 · 10 −6 mol/mol
n dexh1 = 25.534 mol/s
n dexh2 = 26.950 mol/s
ƒ record = 5 Hz
Using Eq. 1065.650-5:
Δ t = 1/5 = 0.2 s
mNO 46.0055 · 85.6 · 10 −6 · (25.534 + 26.950+ . . . +
n exh9000) · 0.2
mNO = 4.201 g
(B) Calculate m for sampling PM or any other analysis of a batch sample that yields a mass per mole of exhaust, M , using the following equation:
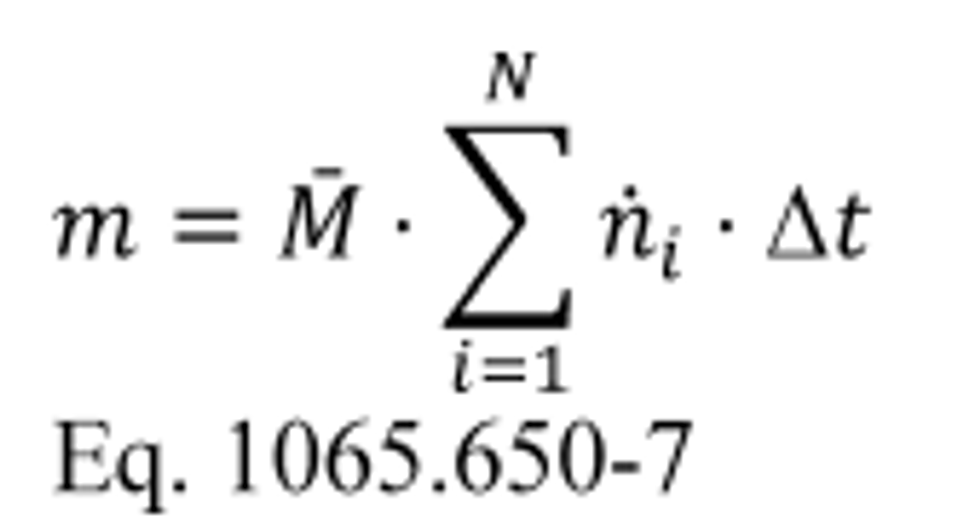
(ii) Proportional or constant flow rate. If you batch sample from a constant exhaust flow rate, extract a sample at a proportional or constant flow rate. We consider the following to be examples of constant exhaust flows: CVS diluted exhaust with a CVS flow meter that has either an upstream heat exchanger, electronic flow control, or both. Determine the mean molar flow rate from which you extracted the sample. Multiply the mean concentration of the batch sample by the mean molar flow rate of the exhaust from which the sample was extracted to determine the total emission and multiply the result by the time of the test interval. If the total emission is a molar quantity, convert this quantity to a mass by multiplying it by its molar mass, M. The result is the total emission mass, m. In the case of PM emissions, where the mean PM concentration is already in units of mass per mole of exhaust, simply multiply it by the total flow, and the result is the total mass of PM, mPM. Calculate m for each constituent as follows:
(A) Calculate m for measuring gaseous emission constituents with sampling that results in a molar concentration, x , using the following equation:
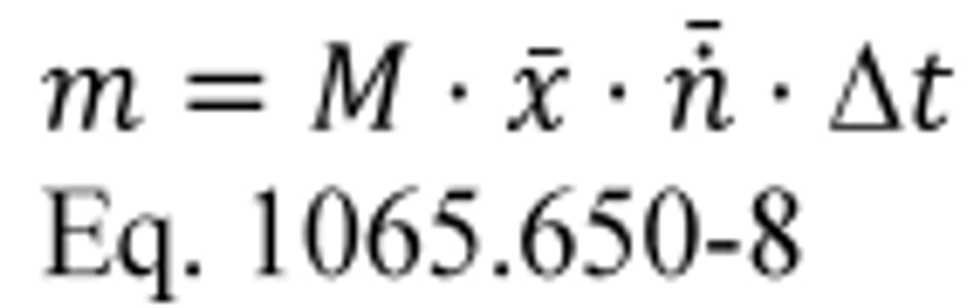
(B) Calculate m for sampling PM or any other analysis of a batch sample that yields a mass per mole of exhaust, M , using the following equation:
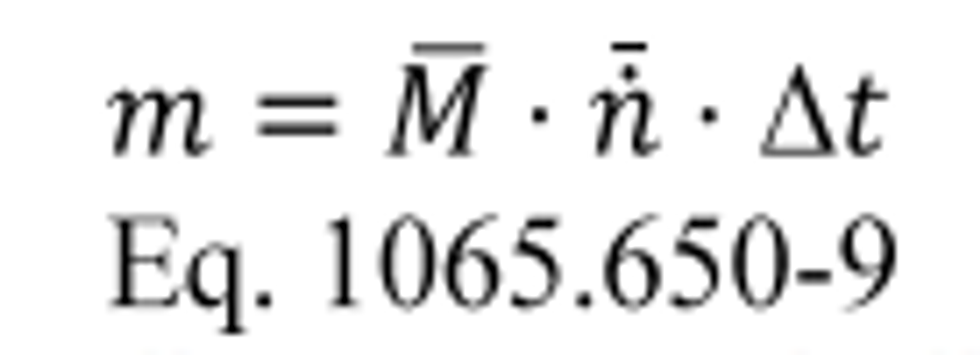
(C) The following example illustrates a calculation of mPM :
M PM = 144.0 µg/mol = 144.0 · 10 −6 g/mol
n dexh = 57.692 mol/s
Δ t = 1200 s
mPM = 144.0 · 10 −6 · 57.692 · 1200
mPM = 9.9692 g
Example:
mPMdil = 6.853 g
DR = 6:1
mPM = 6.853 · 6
mPM = 41.118 g
(4) Additional provisions for diluted exhaust sampling; continuous or batch. The following additional provisions apply for sampling emissions from diluted exhaust: (i) For sampling with a constant dilution ratio, DR, of diluted exhaust versus exhaust flow (e.g., secondary dilution for PM sampling), calculate m using the following equation:
(i) (i) For sampling with a constant dilution ratio, DR, of diluted exhaust versus exhaust flow (e.g., secondary dilution for PM sampling), calculate m using the following equation:
(ii) For continuous or batch sampling, you may measure background emissions in the dilution air. You may then subtract the measured background emissions, as described in § 1065.667.
(5) Mass of NMHC. Compare the corrected mass of NMHC to corrected mass of THC. If the corrected mass of NMHC is greater than 0.98 times the corrected mass of THC, take the corrected mass of NMHC to be 0.98 times the corrected mass of THC. If you omit the NMHC calculations as described in § 1065.660(b)(1), take the corrected mass of NMHC to be 0.98 times the corrected mass of THC.
(6) Mass of NMNEHC. Determine the mass of NMNEHC using one of the following methods:
(i) If the test fuel has less than 0.010 mol/mol of ethane and you omit the NMNEHC calculations as described in §1065.660(c)(1), take the corrected mass of NMNEHC to be 0.95 times the corrected mass of NMHC.
(ii) If the test fuel has at least 0.010 mol/mol of ethane and you omit the NMNEHC calculations as described in §1065.660(c)(1), take the corrected mass of NMNEHC to be 1.0 times the corrected mass of NMHC.
(d) Total work over a test interval. To calculate the total work from the engine over a test interval, add the total work from all the work paths described in §1065.210 that cross the system boundary including electrical energy/work, mechanical shaft work, and fluid pumping work. For all work paths, except the engine's primary output shaft (crankshaft), the total work for the path over the test interval is the integration of the net work flow rate (power) out of the system boundary. When energy/work flows into the system boundary, this work flow rate signal becomes negative; in this case, include these negative work rate values in the integration to calculate total work from that work path. Some work paths may result in a negative total work. Include negative total work values from any work path in the calculated total work from the engine rather than setting the values to zero. The rest of this paragraph (d) describes how to calculate total work from the engine's primary output shaft over a test interval. Before integrating power on the engine's primary output shaft, adjust the speed and torque data for the time alignment used in §1065.514(c). Any advance or delay used on the feedback signals for cycle validation must also be used for calculating work. Account for work of accessories according to §1065.110. Exclude any work during cranking and starting. Exclude work during actual motoring operation (negative feedback torques), unless the engine was connected to one or more energy storage devices. Examples of such energy storage devices include hybrid powertrain batteries and hydraulic accumulators, like the ones illustrated in Figure 1 of §1065.210. Exclude any work during reference zero-load idle periods (0% speed or idle speed with 0 N·m reference torque). Note, that there must be two consecutive reference zero load idle points to establish a period where the zero-load exclusion applies. Include work during idle points with simulated minimum torque such as Curb Idle Transmissions Torque (CITT) for automatic transmissions in “drive”. The work calculation method described in paragraphs (d)(1) though (7) of this section meets the requirements of this paragraph (d) using rectangular integration. You may use other logic that gives equivalent results. For example, you may use a trapezoidal integration method as described in paragraph (d)(8) of this section.
(1) Time align the recorded feedback speed and torque values by the amount used in §1065.514(c).
(2) Calculate shaft power at each point during the test interval by multiplying all the recorded feedback engine speeds by their respective feedback torques.
(3) Adjust (reduce) the shaft power values for accessories according to §1065.110.
(4) Set all power values during any cranking or starting period to zero. See §1065.525 for more information about engine cranking.
(5) Set all negative power values to zero, unless the engine was connected to one or more energy storage devices. If the engine was tested with an energy storage device, leave negative power values unaltered.
(6) Set all power values to zero during idle periods with a corresponding reference torque of 0 N · m.
(7) Integrate the resulting values for power over the test interval. Calculate total work as follows:
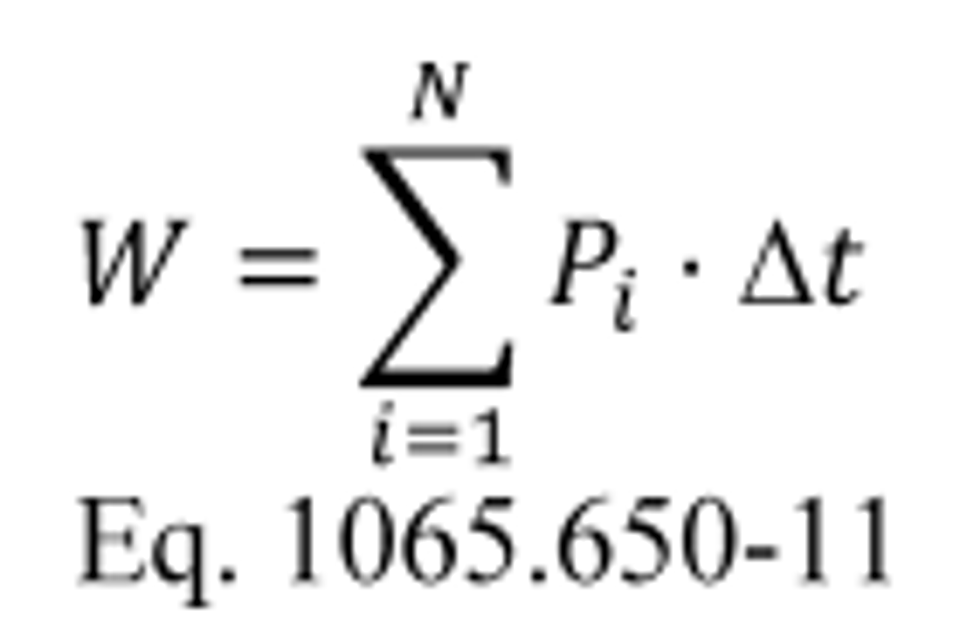
Where:
W = total work from the primary output shaft.
P = instantaneous power from the primary output shaft over an interval i.
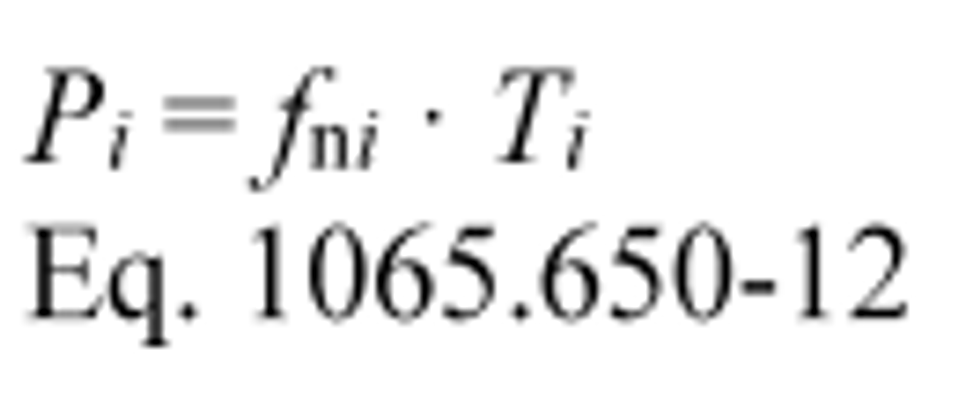
Example:
N = 9000
ƒ n1 = 1800.2 r/min
ƒ n2 = 1805.8 r/min
T1 = 177.23 N·m
T2 = 175.00 N·m
Crev = 2· π rad/r
Ct1 = 60 s/min
Cp = 1000 (N·m·rad/s)/kW
ƒ record = 5 Hz
Ct2 = 3600 s/hr
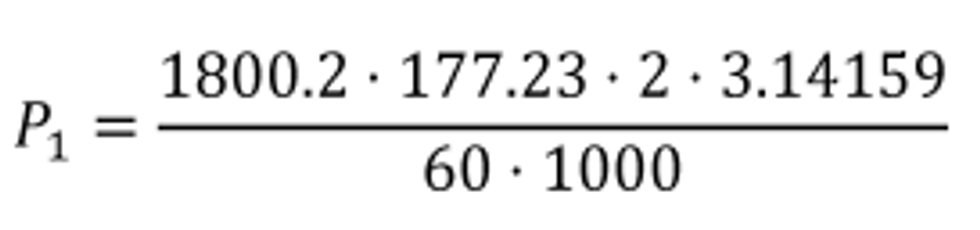
P1 = 33.41 kW
P2 = 33.09 kW
Using Eq. 1065.650-5:
Δ t = 1/5 = 0.2 s
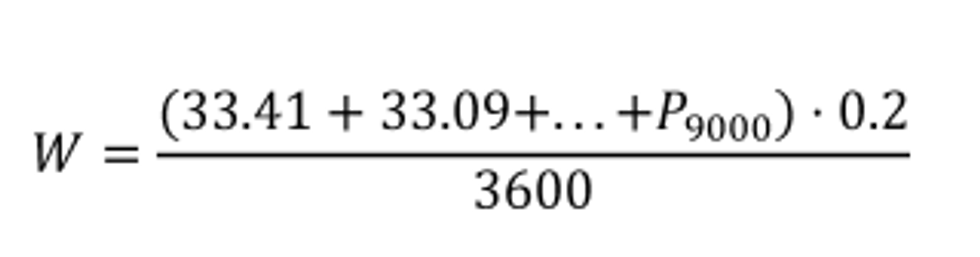
W = 16.875 kW·hr
(8) You may use a trapezoidal integration method instead of the rectangular integration described in this paragraph (d). To do this, you must integrate the fraction of work between points where the torque is positive. You may assume that speed and torque are linear between data points. You may not set negative values to zero before running the integration.
(e) Steady-state mass rate divided by power. To determine steady-state brake-specific emissions for a test interval as described in paragraph (b)(2) of this section, calculate the mean steady-state mass rate of the emission, m ̇̅, and the mean steady-state power, P ͞ as follows:
(1) To calculate, m , multiply its mean concentration, x , by its corresponding mean molar flow rate, n . If the result is a molar flow rate, convert this quantity to a mass rate by multiplying it by its molar mass, M. The result is the mean mass rate of the emission, m . In the case of PM emissions, where the mean PM concentration is already in units of mass per mole of exhaust, simply multiply it by the mean molar flow rate, n . The result is the mass rate of PM, m PM . Calculate m using the following equation:
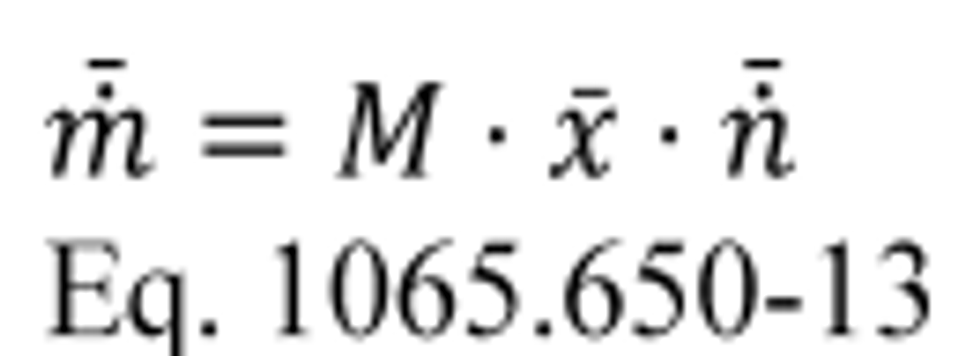
(2) To calculate an engine's mean steady-state total power, P , add the mean steady-state power from all the work paths described in §1065.210 that cross the system boundary including electrical power, mechanical shaft power, and fluid pumping power. For all work paths, except the engine's primary output shaft (crankshaft), the mean steady-state power over the test interval is the integration of the net work flow rate (power) out of the system boundary divided by the period of the test interval. When power flows into the system boundary, the power/work flow rate signal becomes negative; in this case, include these negative power/work rate values in the integration to calculate the mean power from that work path. Some work paths may result in a negative mean power. Include negative mean power values from any work path in the mean total power from the engine rather than setting these values to zero. The rest of this paragraph (e)(2) describes how to calculate the mean power from the engine's primary output shaft. Calculate P using Eq. 1065.650-13, noting that P , f n , and T refer to mean power, mean rotational shaft frequency, and mean torque from the primary output shaft. Account for the power of simulated accessories according to §1065.110 (reducing the mean primary output shaft power or torque by the accessory power or torque). Set the power to zero during actual motoring operation (negative feedback torques), unless the engine was connected to one or more energy storage devices. Examples of such energy storage devices include hybrid powertrain batteries and hydraulic accumulators, like the ones denoted “Acc.” and “Batt.” as illustrated in Figure 1 of §1065.210. Set the power to zero for modes with a zero reference load (0 N·m reference torque or 0 kW reference power). Include power during idle modes with simulated minimum torque or power.
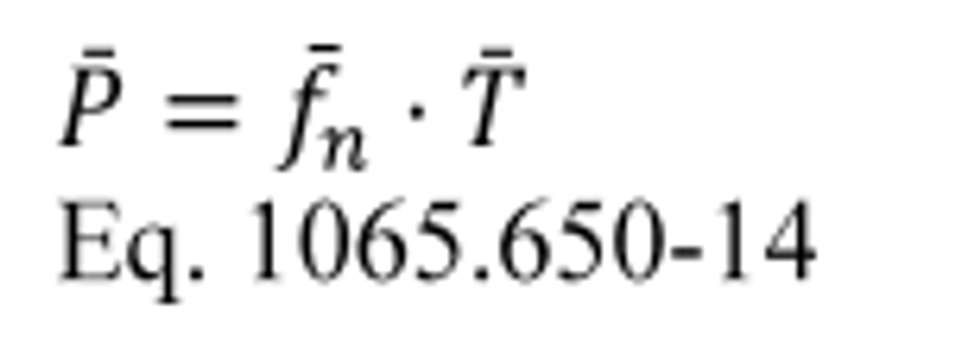
(3) Divide emission mass rate by power to calculate a brake-specific emission result as described in paragraph (b)(2) of this section.
(4) The following example shows how to calculate mass of emissions using mean mass rate and mean power:
MCO = 28.0101 g/mol
x ͞CO = 12.00 mmol/mol = 0.01200 mol/mol
n ̇̅ = 1.530 mol/s
f ͞n = 3584.5 r/min = 375.37 rad/s
T ͞ = 121.50 N · m
m ̇̅ = 28.0101 · 0.01200 · 1.530
m ̇̅ = 0.514 g/s = 1850.4 g/hr
P ͞ = 121.5 · 375.37
P ͞ = 45607 W
P ͞ = 45.607 kW
eCO = 1850.4/45.61
eCO = 40.57 g/(kW · hr)
(f) Ratio of total mass of emissions to total work. To determine brake-specific emissions for a test interval as described in paragraph (b)(3) of this section, calculate a value proportional to the total mass of each emission. Divide each proportional value by a value that is similarly proportional to total work.
(1) Total mass. To determine a value proportional to the total mass of an emission, determine total mass as described in paragraph (c) of this section, except substitute for the molar flow rate, n , or the total flow, n, with a signal that is linearly proportional to molar flow rate, n , or linearly proportional to total flow, n , as follows:
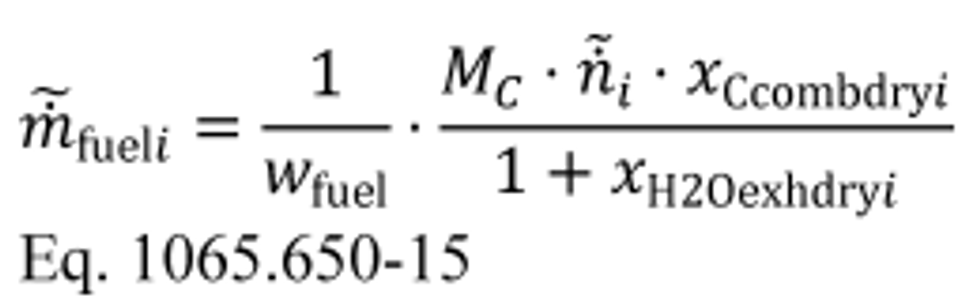
(2) Total work. To calculate a value proportional to total work over a test interval, integrate a value that is proportional to power. Use information about the brake-specific fuel consumption of your engine, efuel, to convert a signal proportional to fuel flow rate to a signal proportional to power. To determine a signal proportional to fuel flow rate, divide a signal that is proportional to the mass rate of carbon products by the fraction of carbon in your fuel, wC. You may use a measured wC or you may use default values for a given fuel as described in §1065.655(e). Calculate the mass rate of carbon from the amount of carbon and water in the exhaust, which you determine with a chemical balance of fuel, DEF, intake air, and exhaust as described in §1065.655. In the chemical balance, you must use concentrations from the flow that generated the signal proportional to molar flow rate, n , in paragraph (e)(1) of this section. Calculate a value proportional to total work as follows:
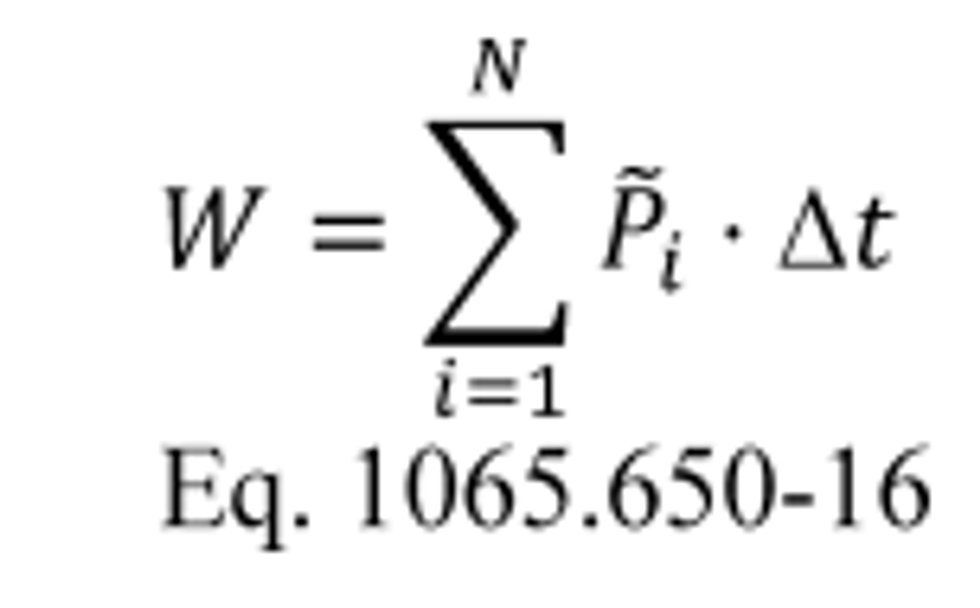
Where:
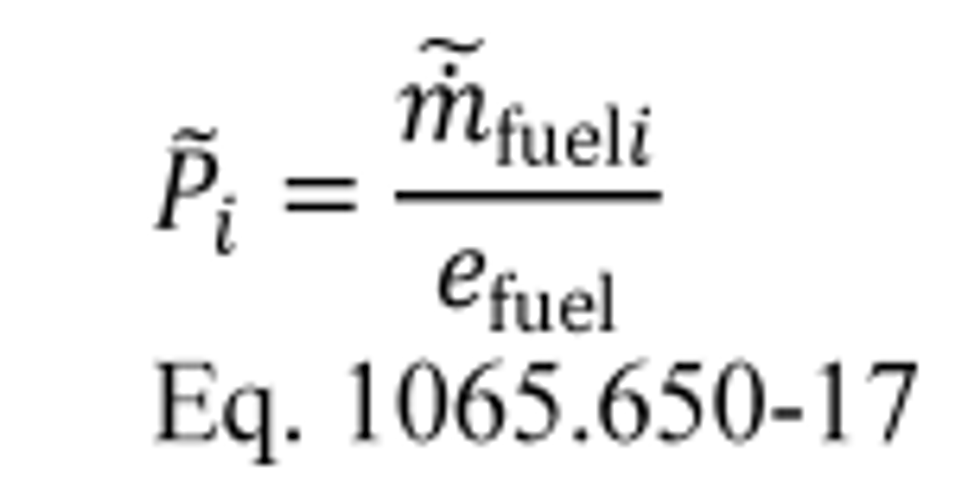
(3) Brake-specific emissions. Divide the value proportional to total mass by the value proportional to total work to determine brake-specific emissions, as described in paragraph (b)(3) of this section.
(4) Example: The following example shows how to calculate mass of emissions using proportional values:
N = 3000
ƒrecord = 5 Hz
efuel = 285 g/(kW·hr)
wfuel = 0.869 g/g
MC = 12.0107 g/mol
n ̇1 = 3.922 mol/s = 14119.2 mol/hr
xCcombdry1 = 91.634 mmol/mol = 0.091634 mol/mol
xH2Oexh1 = 27.21 mmol/mol = 0.02721 mol/mol
Using Eq. 1065.650-5,
Δt = 0.2 s
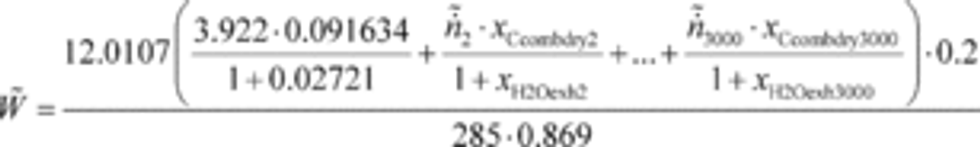
W ̃ = 5.09 (kW·hr)
(g) Brake-specific emissions over a duty cycle with multiple test intervals. The standard-setting part may specify a duty cycle with multiple test intervals, such as with discrete-mode steady-state testing. Unless we specify otherwise, calculate composite brake-specific emissions over the duty cycle as described in this paragraph (g). If a measured mass (or mass rate) is negative, set it to zero for calculating composite brake-specific emissions, but leave it unchanged for drift validation. In the case of calculating composite brake-specific emissions relative to a combined emission standard (such as a NOX + NMHC standard), change any negative mass (or mass rate) values to zero for a particular pollutant before combining the values for the different pollutants.
(1) Use the following equation to calculate composite brake-specific emissions for duty cycles with multiple test intervals all with prescribed durations, such as cold-start and hot-start transient cycles:
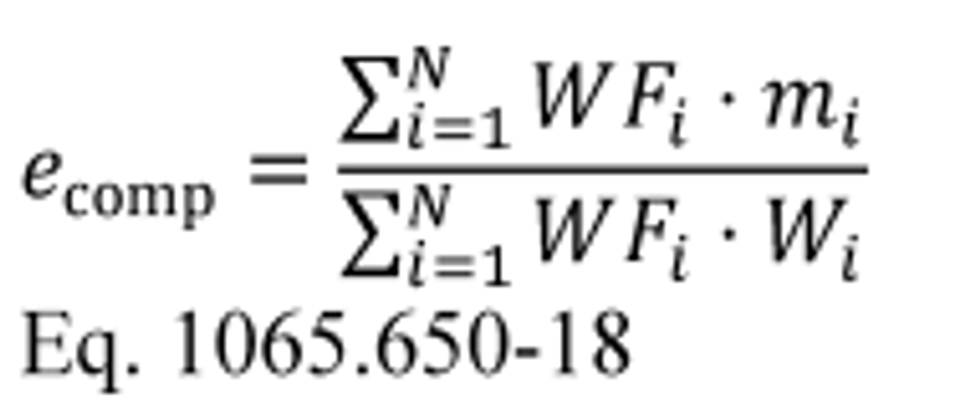
Where:
i = test interval number.
N = number of test intervals.
WF = weighting factor for the test interval as defined in the standard-setting part.
m = mass of emissions over the test interval as determined in paragraph (c) of this section.
W = total work from the engine over the test interval as determined in paragraph (d) of this section.
Example:
N = 2
WF1 = 0.1428
WF2 = 0.8572
m1 = 70.125 g
m2 = 64.975 g
W1 = 25.783 kW·hr
W2 = 25.783 kW·hr
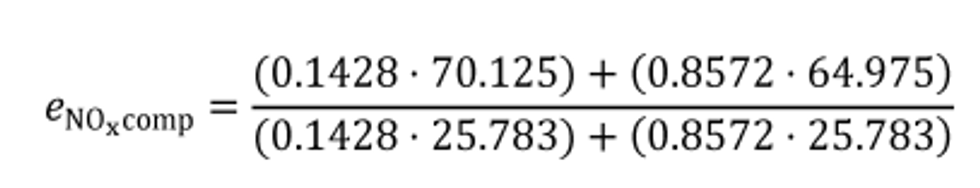
eNOxcomp = 2.548 g/kW·hr
(2) Calculate composite brake-specific emissions for duty cycles with multiple test intervals that allow use of varying duration, such as discrete-mode steady-state duty cycles, as follows:
(i) Use the following equation if you calculate brake-specific emissions over test intervals based on total mass and total work as described in paragraph (b)(1) of this section:
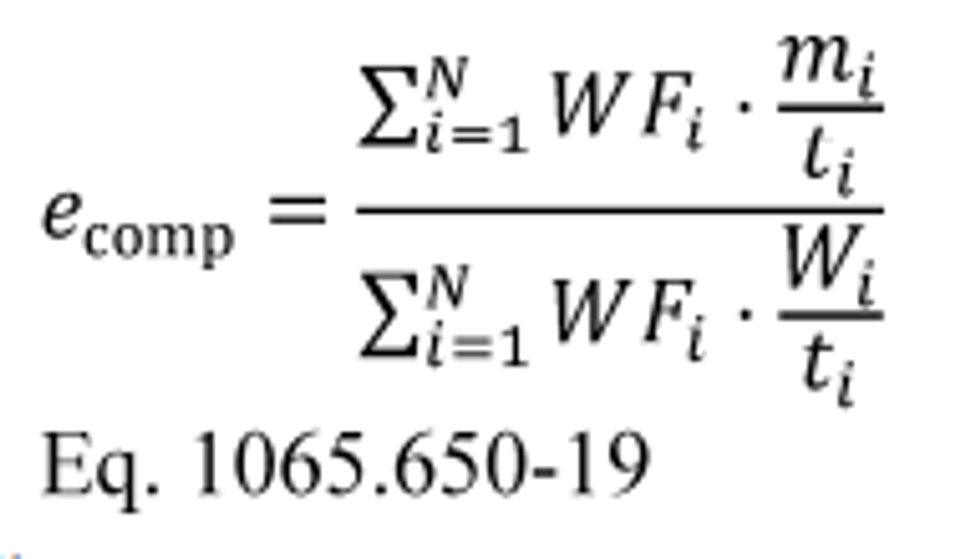
Where:
i = test interval number.
N = number of test intervals.
WF = weighting factor for the test interval as defined in the standard-setting part.
m = mass of emissions over the test interval as determined in paragraph (c) of this section.
W = total work from the engine over the test interval as determined in paragraph (d) of this section.
t = duration of the test interval.
Example:
N = 2
WF1 = 0.85
WF2 = 0.15
m1 = 1.3753 g
m2 = 0.4135 g
t1 = 120 s
t2 = 200 s
W1 = 2.8375 kW · hr
W2 = 0.0 kW · hr
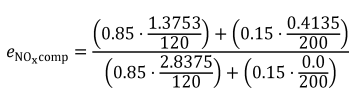
eNOxcomp = 0.5001 g/kW·hr
(ii) Use the following equation if you calculate brake-specific emissions over test intervals based on the ratio of mass rate to power as described in paragraph (b)(2) of this section:
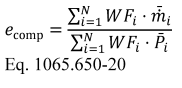
Where:
i = test interval number.
N = number of test intervals.
WF = weighting factor for the test interval as defined in the standard-setting part.
m = mean steady-state mass rate of emissions over the test interval as determined in paragraph (e) of this section.
p = mean steady-state power over the test interval as described in paragraph (e) of this section.
Example:
N = 2
WF1 = 0.85
WF2 = 0.15
m 1 = 2.25842 g/hr
m 2 = 0.063443 g/hr
P 1 = 4.5383 kW
P 2 = 0.0 kW
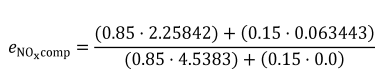
eNOxcomp = 0.5001 g/kW·hr
eNOcomp = 0.5001 g/kW·hr
(h) Rounding. Round the final brake-specific emission values to be compared to the applicable standard only after all calculations are complete (including any drift correction, applicable deterioration factors, adjustment factors, and allowances) and the result is in g/(kW · hr) or units equivalent to the units of the standard, such as g/(hp · hr). See the definition of “Round” in §1065.1001.
[73 FR 37328, June 30, 2008, as amended at 73 FR 59332, Oct. 8, 2008; 75 FR 23048, Apr. 30, 2010; 76 FR 57457, Sept. 15, 2011;79 FR 23799, Apr. 28, 2014; 80 FR 9118, Feb. 19, 2015; 81 FR 74180, Oct. 25, 2016; 86 FR 34560, Jun. 29, 2021; 87 FR 65866, Oct. 26, 2022; 88 FR 4681, Jan. 24, 2023; 89 FR 29808, Apr. 22, 2024]
§1065.655 Carbon-based chemical balances of fuel, DEF, intake air, and exhaust.
(a) General. Chemical balances of fuel, intake air, and exhaust may be used to calculate flows, the amount of water in their flows, and the wet concentration of constituents in their flows. See §1065.520(f) for information about when to use this carbon-based chemical balance procedure. With one flow rate of either fuel, intake air, or exhaust, you may use chemical balances to determine the flows of the other two. For example, you may use chemical balances along with either intake air or fuel flow to determine raw exhaust flow. Note that chemical balance calculations allow measured values for the flow rate of diesel exhaust fluid for engines with urea-based selective catalytic reduction.
(b) Procedures that require chemical balances. We require chemical balances when you determine the following:
(1) A value proportional to total work, W ̃, when you choose to determine brake-specific emissions as described in §1065.650(f).
(2) Raw exhaust molar flow rate either from measured intake air molar flow rate or from fuel mass flow rate as described in paragraph (f) of this section.
(3) Raw exhaust molar flow rate from measured intake air molar flow rate and dilute exhaust molar flow rate, as described in paragraph (g) of this section.
(4) The amount of water in a raw or diluted exhaust flow, xH2Oexh, when you do not measure the amount of water to correct for the amount of water removed by a sampling system. Note that you may not use the water measurement methods in §1065.257 to determine xH2Oexh. Correct for removed water according to §1065.659.
(5) The calculated total dilution air flow when you do not measure dilution air flow to correct for background emissions as described in §1065.667(c) and (d).
(c) Chemical balance procedure. The calculations for a chemical balance involve a system of equations that require iteration. We recommend using a computer to solve this system of equations. You must guess the initial values of up to three quantities: the amount of water in the measured flow, xH2Oexh, fraction of dilution air in diluted exhaust, xdil/exh, and the amount of products on a C 1 basis per dry mole of dry measured flow, xCcombdry. You may use time-weighted mean values of intake air humidity and dilution air humidity in the chemical balance; as long as your intake air and dilution air humidities remain within tolerances of ±0.0025 mol/mol of their respective mean values over the test interval. For each emission concentration, x, and amount of water, xH2Oexh, you must determine their completely dry concentrations, xdry and xH2Oexhdry. You must also use your fuel mixture's atomic hydrogen-to-carbon ratio, α, oxygen-to-carbon ratio, β, sulfur-to-carbon ratio, γ, and nitrogen-to-carbon ratio, δ; you may optionally account for diesel exhaust fluid (or other fluids injected into the exhaust), if applicable. You may calculate α, β, γ, and δ based on measured fuel composition or based on measured fuel and diesel exhaust fluid (or other fluids injected into the exhaust) composition together, as described in paragraph (e) of this section. You may alternatively use any combination of default values and measured values as described in paragraph (e) of this section. Use the following steps to complete a chemical balance:
(1) Convert your measured concentrations such as, xCO2meas, xNOmeas, and xH2Oint, to dry concentrations by dividing them by one minus the amount of water present during their respective measurements; for example: xH2OxCO2meas, xH2OxNOmeas, and xH2Oint. If the amount of water present during a “wet” measurement is the same as the unknown amount of water in the exhaust flow, xH2Oexh, iteratively solve for that value in the system of equations. If you measure only total NOX and not NO and NO2 separately, use good engineering judgment to estimate a split in your total NOX concentration between NO and NO2 for the chemical balances. For example, if you measure emissions from a stoichiometric spark-ignition engine, you may assume all NOX is NO. For a compression-ignition engine, you may assume that your molar concentration of NOX, xNOx, is 75% NO and 25% NO2. For NO2 storage aftertreatment systems, you may assume xNOx is 25% NO and 75% NO2. Note that for calculating the mass of NOX emissions, you must use the molar mass of NO2 for the effective molar mass of all NOX species, regardless of the actual NO2 fraction of NOX.
(2) Enter the equations in paragraph (c)(4) of this section into a computer program to iteratively solve for xH2Oexh, xCcombdry, and xdil/exh. Use good engineering judgment to guess initial values for xH2Oexh, xCcombdry, and xdil/exh. We recommend guessing an initial amount of water that is about twice the amount of water in your intake or dilution air. We recommend guessing an initial value of xCcombdry as the sum of your measured CO2, CO, and THC values. We also recommend guessing an initial xdil/exh between 0.75 and 0.95, such as 0.8. Iterate values in the system of equations until the most recently updated guesses are all within ±1% of their respective most recently calculated values.
(3) Use the following symbols and subscripts in the equations for performing the chemical balance calculations in this paragraph (c):
| xdil/exh | amount of dilution gas or excess air per mole of exhaust. |
| xCcombdry | amount of carbon from fuel and any injected fluids in the exhaust per mole of dry exhaust |
| xH2dry | amount of H2 in exhaust per amount of dry exhaust |
| KH2Ogas | water-gas reaction equilibrium coefficient; you may use 3.5 or calculate your own value using good engineering judgment |
| xH2Oexhdry | amount of H2O in exhaust per dry mole of dry exhaust |
| xprod/intdry | amount of dry stoichiometric products per dry mole of intake air |
| xdil/exhdry | amount of dilution gas and/or excess air per mole of dry exhaust |
| xint/exhdry | amount of intake air required to produce actual combustion products per mole of dry (raw or diluted) exhaust |
| xraw/exhdry | amount of undiluted exhaust, without excess air, per mole of dry (raw or diluted) exhaust |
| xO2int | amount of intake air O2 per mole of intake air |
| xCO2intdry | amount of intake air CO2 per mole of dry intake air; you may use xCO2intdry = 375 µmol/mol, but we recommend measuring the actual concentration in the intake air |
| xH2Ointdry | amount of intake air H2O per mole of dry intake air |
| xCO2int | amount of intake air CO2 per mole of intake air |
| xCO2dil | amount of dilution gas CO2 per mole of dilution gas |
| xCO2dildry | amount of dilution gas CO2 per mole of dry dilution gas; if you use air as diluent, you may use xCO2dildry = 375 µmol/mol, but we recommend measuring the actual concentration in the intake air |
| xH2Odildry | amount of dilution gas H2O per mole of dry dilution gas |
| xH2Odil | amount of dilution gas H2O per mole of dilution gas |
| x[emission]meas | amount of measured emission in the sample at the respective gas analyzer |
| x[emission]dry | amount of emission per dry mole of dry sample |
| xH2O[emission]meas | amount of H2O in sample at emission-detection location; measure or estimate these values according to §1065.145(e)(2) |
| xH2Oint | amount of H2O in the intake air, based on a humidity measurement of intake air |
| α | atomic hydrogen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids |
| β | atomic oxygen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids |
| γ | atomic sulfur-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids |
| δ | atomic nitrogen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids |
(4) Use the following equations to iteratively solve for xdil/exh, xH2Oexh, and xCcombdry:
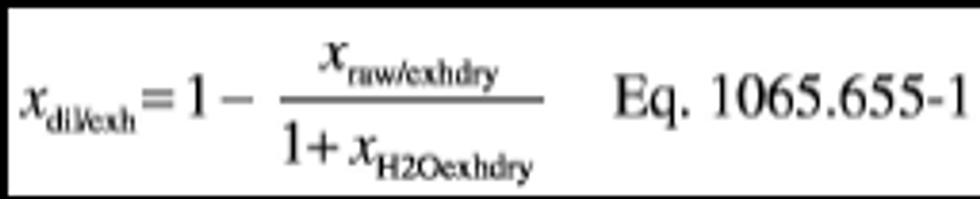
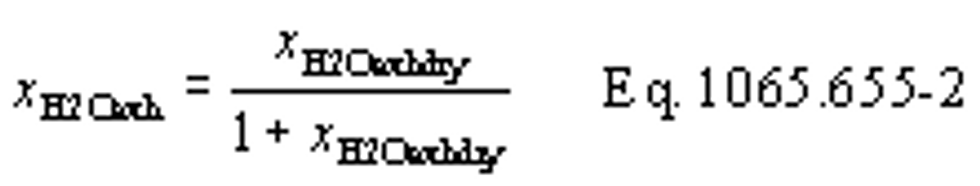
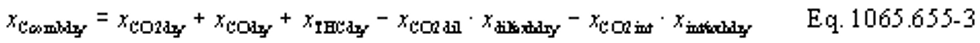
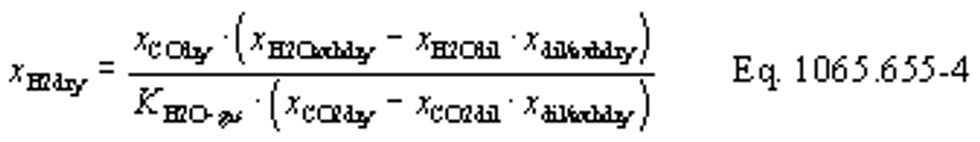
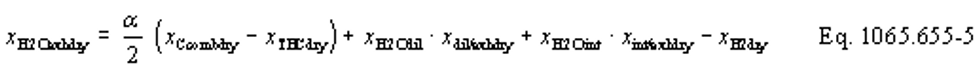
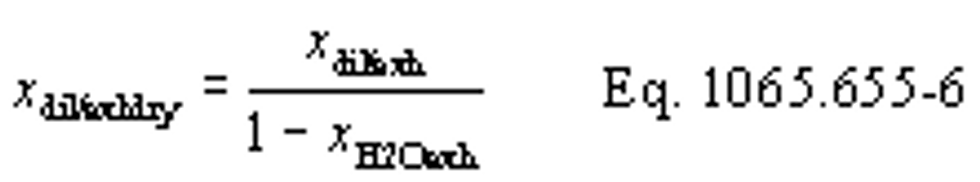
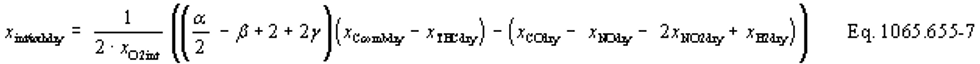
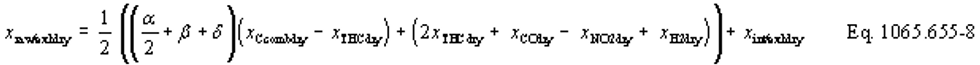
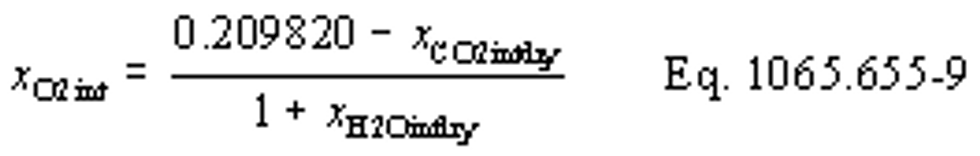

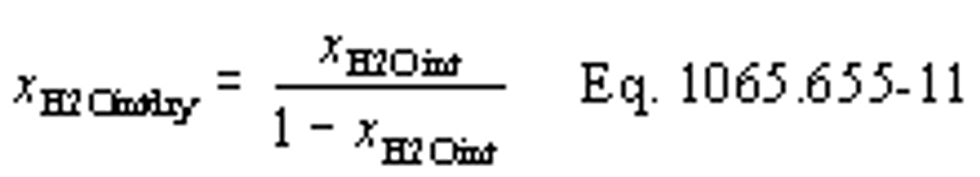
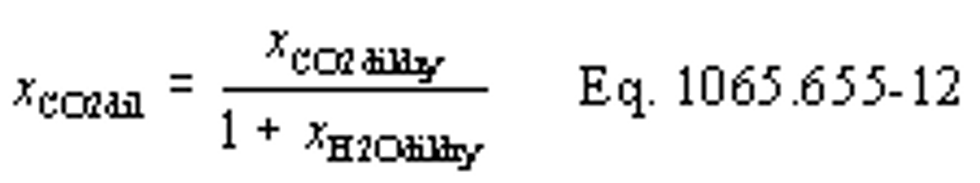
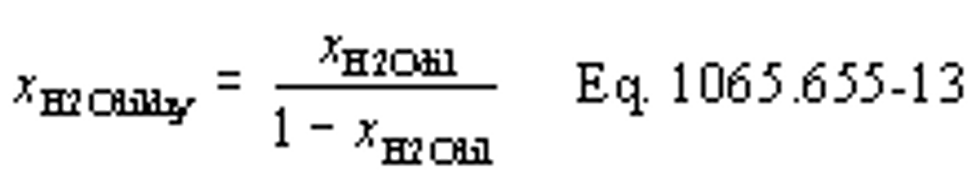
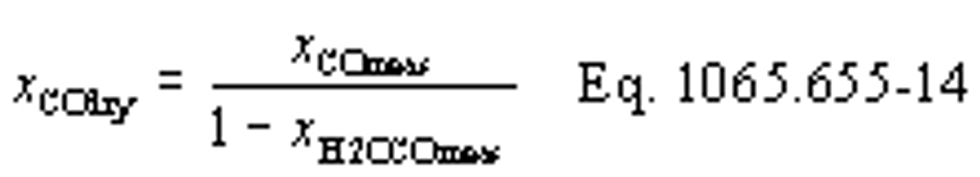
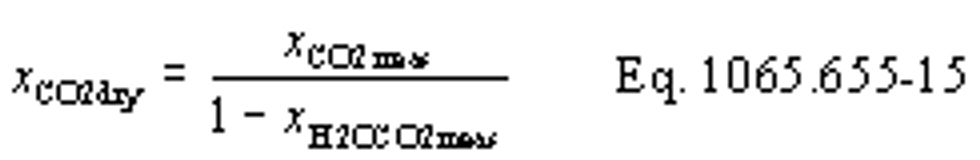
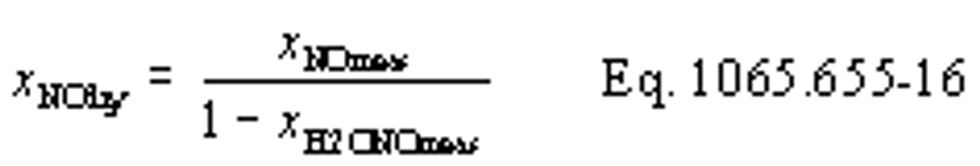
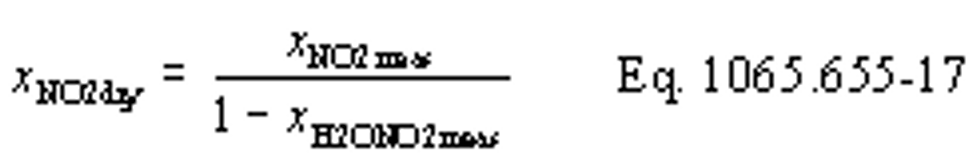
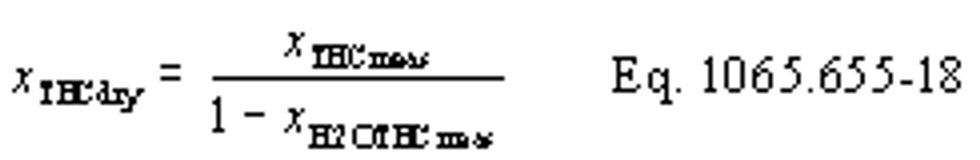
(5) The following example is a solution for xdil/exh,x, xH2Oexh, and xCcombdry using the equations in paragraph (c)(4) of this section:

a = 1.8
b = 0.05
g = 0.0003
d = 0.0001
(d) Carbon mass fraction of fuel. Determine carbon mass fraction of fuel, wC, based on the fuel properties as determined in paragraph (e) of this section, optionally accounting for diesel exhaust fluid's contribution to α, β, γ, and δ, or other fluids injected into the exhaust, if applicable (for example, the engine is equipped with an emission control system that utilizes DEF). Calculate wC using the following equation:
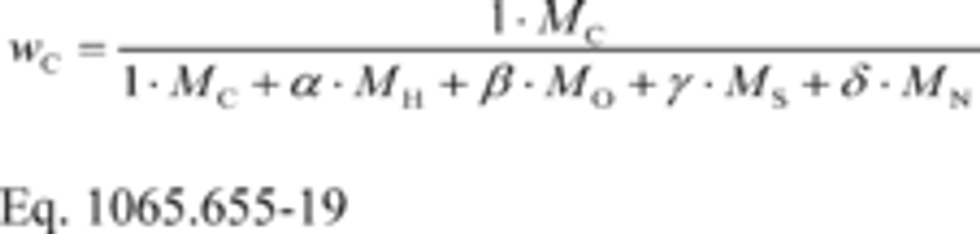
Where:
wC = carbon mass fraction of the fuel (or mixture of test fuels) and any injected fluids.
MC = molar mass of carbon.
a = atomic hydrogen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids.
MH = molar mass of hydrogen.
b = atomic oxygen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids.
MO = molar mass of oxygen.
g = atomic sulfur-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids.
MS = molar mass of sulfur.
d = atomic nitrogen-to-carbon ratio of the fuel (or mixture of test fuels) and any injected fluids.
MN = molar mass of nitrogen.
Example:
a = 1.8
b = 0.05
g = 0.0003
d = 0.0001
MC = 12.0107
MH = 1.00794
MO = 15.9994
MS = 32.065
MN = 14.0067
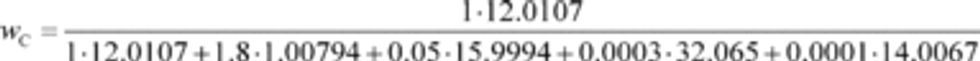
wC = 0.8206
(e) Fuel and diesel exhaust fluid composition. Determine fuel and diesel exhaust fluid composition represented by α, β, γ, and δ as described in this paragraph (e). When using measured fuel or diesel exhaust fluid properties, you must determine values for α and β in all cases. If you determine compositions based on measured values and the default value listed in Table 2 of this section is zero, you may set γ and δ to zero; otherwise determine γ and δ (along with α and β) based on measured values. Determine elemental mass fractions and values for α, β, γ, and δ as follows:
(1) For liquid fuels, use the default values for α, β, γ, and δ in table 2 of this section or determine mass fractions of liquid fuels for calculation of α, β, γ, and δ as follows:
(i) Determine the carbon and hydrogen mass fractions according to ASTM D5291 (incorporated by reference, see §1065.1010). When using ASTM D5291 to determine carbon and hydrogen mass fractions of gasoline (with or without blended ethanol), use good engineering judgment to adapt the method as appropriate. This may include consulting with the instrument manufacturer on how to test high-volatility fuels. Allow the weight of volatile fuel samples to stabilize for 20 minutes before starting the analysis; if the weight still drifts after 20 minutes, prepare a new sample). Retest the sample if the carbon, hydrogen, oxygen, sulfur, and nitrogen mass fractions do not add up to a total mass of 100 ±0.5%; you may assume oxygen has a zero mass contribution for this specification for diesel fuel and neat (E0) gasoline. You may also assume that sulfur and nitrogen have a zero mass contribution for this specification for all fuels except residual fuel blends.
(ii) Determine oxygen mass fraction of gasoline (with or without blended ethanol) according to ASTM D5599 (incorporated by reference, see §1065.1010). For all other liquid fuels, determine the oxygen mass fraction using good engineering judgment.
(iii) Determine the nitrogen mass fraction according to ASTM D4629 or ASTM D5762 (incorporated by reference, see §1065.1010) for all liquid fuels. Select the correct method based on the expected nitrogen content.
(iv) Determine the sulfur mass fraction according to subpart H of this part.
(2) For gaseous fuels and diesel exhaust fluid, use the default values for α, β, γ, and δ in Table 2 of this section, or use good engineering judgment to determine those values based on measurement.
(3) For nonconstant fuel mixtures, you must account for the varying proportions of the different fuels. This paragraph (e)(3) generally applies for dual-fuel and flexible-fuel engines, but it also applies if diesel exhaust fluid is injected in a way that is not strictly proportional to fuel flow. Account for these varying concentrations either with a batch measurement that provides averaged values to represent the test interval, or by analyzing data from continuous mass rate measurements. Application of average values from a batch measurement generally applies to situations where one fluid is a minor component of the total fuel mixture, for example dual-fuel and flexible-fuel engines with diesel pilot injection, where the diesel pilot fuel mass is less than 5% of the total fuel mass and diesel exhaust fluid injection; consistent with good engineering judgment.
(4) Calculate α, β, γ, and δ as described in this paragraph (e)(4). If your fuel mixture contains fuels other than carbon-containing fuels, then calculate those fuels' mass fractions wC , wH , wO , wS , and wN as described in §1065.656(d). Calculate α, β, γ, and δ using the following equations:
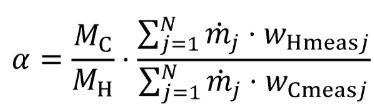
Eq. 1065.655-20
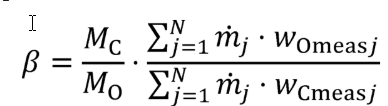
Eq. 1065.655-21
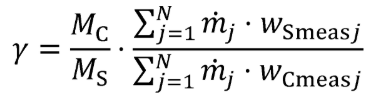
Eq. 1065.655-22
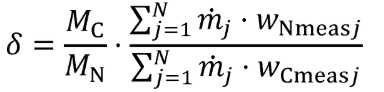
Eq. 1065.655-23
Where:
N = total number of fuels and injected fluids over the duty cycle.
j = an indexing variable that represents one fuel or injected fluid, starting with j = 1.
m j = the mass flow rate of the fuel or any injected fluid j. For applications using a single fuel and no DEF fluid, set this value to 1. For batch measurements, divide the total mass of fuel over the test interval duration to determine a mass rate.
wHmeas = hydrogen mass fraction of fuel or any injected fluid j.
wCmeas = carbon mass fraction of fuel or any injected fluid j.
wOmeas = oxygen mass fraction of fuel or any injected fluid j.
wSmeas = sulfur mass fraction of fuel or any injected fluid j.
wNmeas = nitrogen mass fraction of fuel or any injected fluid j.
Example:
N = 1
j = 1
m 1 = 1
wHmeas1 = 0.1239
wCmeas1 = 0.8206
wOmeas1 = 0.0547
wSmeas1 = 0.00066
wNmeas1 = 0.000095
MC = 12.0107 g/mol
MH = 1.00794 g/mol
MO = 15.9994 g/mol
MS = 32.065 g/mol
MN = 14.0067

(f) Calculated raw exhaust molar flow rate from measured intake air molar flow rate or fuel mass flow rate. You may calculate the raw exhaust molar flow rate from which you sampled emissions, n ̇exh, based on the measured intake air molar flow rate, n ̇int, or the measured fuel mass flow rate, m ̇fuel, and the values calculated using the chemical balance in paragraph (c) of this section. The chemical balance must be based on raw exhaust gas concentrations. Solve for the chemical balance in paragraph (c) of this section at the same frequency that you update and record or n ̇int or m ̇fuel. For laboratory tests, calculating raw exhaust molar flow rate using measured fuel mass flow rate is valid only for steady-state testing. See §1065.915(d)(5)(iv) for application to field testing.
(1) Crankcase flow rate. If engines are not subject to crankcase controls under the standard-setting part, you may calculate raw exhaust flow based on n ̇int or m ̇fuel using one of the following:
(i) You may measure flow rate through the crankcase vent and subtract it from the calculated exhaust flow.
(ii) You may estimate flow rate through the crankcase vent by engineering analysis as long as the uncertainty in your calculation does not adversely affect your ability to show that your engines comply with applicable emission standards.
(iii) You may assume your crankcase vent flow rate is zero.
(2) Intake air molar flow rate calculation. Calculate n ̇exh based on n ̇int using the following equation:

Where:
n ̇exh = raw exhaust molar flow rate from which you measured emissions.
n ̇int = intake air molar flow rate including humidity in intake air.
Example:
n ̇int = 3.780 mol/s
xint/exhdry = 0.69021 mol/mol
xraw/exhdry = 1.10764 mol/mol
xH20exhdry = 107.64 mmol/mol = 0.10764 mol/mol

(3) Fluid mass flow rate calculation. This calculation may be used only for steady-state laboratory testing. You may not use this calculation if the standard-setting part requires carbon balance error verification as described in §1065.543. See §1065.915(d)(5)(iv) for application to field testing. Calculate based on using the following equation:
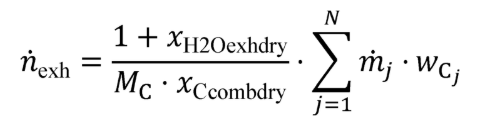
Eq. 1065.655-25
Where:
n exh = raw exhaust molar flow rate from which you measured emissions.
j = an indexing variable that represents one fuel or injected fluid, starting with j = 1.
N = total number of fuels and injected fluids over the duty cycle.
m j = the mass flow rate of the fuel or any injected fluid j.
wC = carbon mass fraction of the fuel and any injected fluid j , as determined in paragraph (d) of this section.
Example:
N = 1
j = 1
m 1 = 7.559 g/s
wC1 = 0.869 g/g
MC = 12.0107 g/mol
xCcombdry1 = 99.87 mmol/mol = 0.09987 mol/mol
xH20exhdry1 = 107.64 mmol/mol = 0.10764 mol/mol
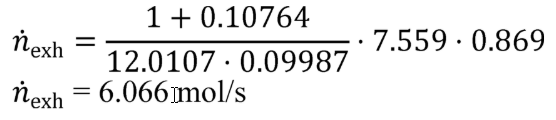
(g) Calculated raw exhaust molar flow rate from measured intake air molar flow rate, dilute exhaust molar flow rate, and dilute chemical balance. You may calculate the raw exhaust molar flow rate, n ̇exh, based on the measured intake air molar flow rate, n ̇int, the measured dilute exhaust molar flow rate, n ̇dexh, and the values calculated using the chemical balance in paragraph (c) of this section. Note that the chemical balance must be based on dilute exhaust gas concentrations. For continuous-flow calculations, solve for the chemical balance in paragraph (c) of this section at the same frequency that you update and record n ̇int and n ̇dexh. This calculated n ̇exh may be used for the PM dilution ratio verification in §1065.546; the calculation of dilution air molar flow rate in the background correction in §1065.667; and the calculation of mass of emissions in §1065.650(c) for species that are measured in the raw exhaust.
(1) Crankcase flow rate. If engines are not subject to crankcase controls under the standard-setting part, calculate raw exhaust flow as described in paragraph (e)(1) of this section.
(2) Dilute exhaust and intake air molar flow rate calculation. Calculate n ̇exh as follows:
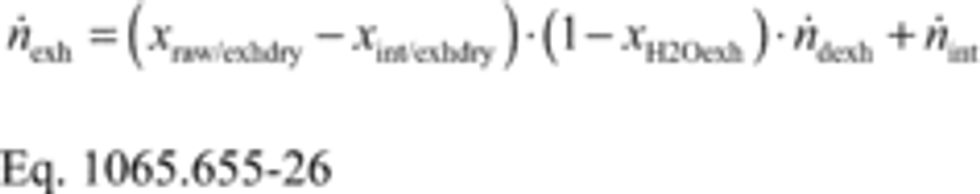
Example:
n ̇int = 7.930 mol/s
xraw/exhdry = 0.1544 mol/mol
xint/exhdry = 0.1451 mol/mol
xH20/exh = 32.46 mmol/mol = 0.03246 mol/mol
n ̇dexh = 49.02 mol/s
n ̇exh = (0.1544 − 0.1451) · (1 − 0.03246) · 49.02 + 7.930 = 0.4411 + 7.930 = 8.371 mol/s
[73 FR 37331, June 30, 2008, as amended at 73 FR 59334, Oct. 8, 2008; 75 FR 23051, Apr. 30, 2010; 76 FR 57458, Sept. 15, 2011; 79 FR 23799, Apr. 28, 2014; 81 FR 74182, Oct. 25, 2016; 86 FR 34563, Jun. 29, 2021; 87 FR 65866, Oct. 26, 2022; 88 FR 4684, Jan. 24, 2023; 89 FR 29808, Apr. 22, 2024]
§1065.656 Hydrogen-based chemical balances of fuel, DEF, intake air, and exhaust.
(a) General. Chemical balances of fuel, DEF, intake air, and exhaust may be used to calculate flows, the amount of water in their flows, and the wet concentration of constituents in their flows. See §1065.520(f) for information about when to use this hydrogen-based chemical balance procedure. With one flow rate of either fuel, intake air, or exhaust, you may use chemical balances to determine the flows of the other two. For example, you may use chemical balances along with either intake air or fuel flow to determine raw exhaust flow. Note that chemical balance calculations allow measured values for the flow rate of diesel exhaust fluid for engines with urea-based selective catalytic reduction.
(b) Procedures that require chemical balances. We require chemical balances when you determine the following:
(1) A value proportional to total work, when you choose to determine brake-specific emissions as described in §1065.650(f).
(2) Raw exhaust molar flow rate either from measured intake air molar flow rate or from fuel mass flow rate as described in paragraph (f) of this section.
(3) Raw exhaust molar flow rate from measured intake air molar flow rate and dilute exhaust molar flow rate as described in paragraph (g) of this section.
(4) The amount of water in a raw or diluted exhaust flow, xH2Oexh , when you do not measure the amount of water to correct for the amount of water removed by a sampling system. Correct for removed water according to §1065.659.
(5) The calculated total dilution air flow when you do not measure dilution air flow to correct for background emissions as described in §1065.667(c) and (d).
(c) Chemical balance procedure. The calculations for a chemical balance involve a system of equations that require iteration. We recommend using a computer to solve this system of equations. You must guess the initial values of two of the following quantities: the amount of hydrogen in the measured flow, xH2exhdry , the fraction of dilution air in diluted exhaust, xdil/exhdry , and the amount of intake air required to produce actual combustion products per mole of dry exhaust, xint/exhdry . You may use time-weighted mean values of intake air humidity and dilution air humidity in the chemical balance; as long as your intake air and dilution air humidities remain within tolerances of ±0.0025 mol/mol of their respective mean values over the test interval. For each emission concentration, x, and amount of water, xH2Oexh , you must determine their completely dry concentrations, xdry and xH2Oexhdry . You must also use your fuel mixture's carbon mass fraction, wC , hydrogen mass fraction, wH , oxygen mass fraction, wO , sulfur mass fraction, wS , and nitrogen mass fraction, wN ; you may optionally account for diesel exhaust fluid (or other fluids injected into the exhaust), if applicable. Calculate wC , w H , wO , w S , and wN as described in paragraphs (d) and (e) of this section. You may alternatively use any combination of default values and measured values as described in paragraphs (d) and (e) of this section. Use the following steps to complete a chemical balance:
(1) Convert your measured concentrations such as xH2meas , x NH3meas , xCO2meas , xCOmeas , xTHCmeas , xO2meas , xH2meas , xNOmeas , xNO2meas , and xH2Oint , to dry concentrations by dividing them by one minus the amount of water present during their respective measurements; for example: xH2Omeas , xH2OxO2meas , xH2OxNOmeas , and xH2Oint . If the amount of water present during a “wet” measurement is the same as an unknown amount of water in the exhaust flow, xH2Oexh , iteratively solve for that value in the system of equations. If you measure only total NO X and not NO and NO 2 separately, use good engineering judgment to estimate a split in your total NO X concentration between NO and NO 2 for the chemical balances. For example, if you measure emissions from a stoichiometric combustion engine, you may assume all NO X is NO. For a lean-burn combustion engine, you may assume that your molar concentration of NO X , xNOx , is 75% NO and 25% NO 2 . For NO 2 storage aftertreatment systems, you may assume xNOx is 25% NO and 75% NO 2. Note that for calculating the mass of NO X emissions, you must use the molar mass of NO 2 for the effective molar mass of all NO X species, regardless of the actual NO 2 fraction of NO X .
(2) Enter the equations in paragraph (c)(5) of this section into a computer program to iteratively solve for xH2exhdry , xdil/exhdry , and xint/exhdry . Use good engineering judgment to guess initial values for xH2exhdry , xdil/exhdry , and xint/exhdry . We recommend guessing an initial amount of hydrogen of 0 mol/mol. We recommend guessing an initial xint/exhdry of 1 mol/mol. We also recommend guessing an initial xdil/exhdry of 0.8 mol/mol. Iterate values in the system of equations until the most recently updated guesses are all within ±1% or ±1 µmol/mol, whichever is larger, of their respective most recently calculated values.
(3) Use the following symbols and subscripts in the equations for performing the chemical balance calculations in this paragraph (c):
| x[emission]meas | Amount of measured emission in the sample at the respective gas analyzer. |
|---|---|
| x[emission]exh | Amount of emission per dry mole of exhaust. |
| x[emission]exhdry | Amount of emission per dry mole of dry exhaust. |
| xH2O[emission]meas | Amount of H 2 O in sample at emission-detection location; measure or estimate these values according to §1065.145(e)(2). |
| xCcombdry | Amount of carbon from fuel and any injected fluids in the exhaust per mole of dry exhaust. |
| xHcombdry | Amount of hydrogen from fuel and any injected fluids in the exhaust per mole of dry exhaust. |
| xdil/exh | Amount of dilution gas or excess air per mole of exhaust. |
| xdil/exhdry | amount of dilution gas and/or excess air per mole of dry exhaust. |
| xHcombdry | Amount of hydrogen from fuel and any injected fluids in the exhaust per mole of dry exhaust. |
| xint/exhdry | Amount of intake air required to produce actual combustion products per mole of dry (raw or diluted) exhaust. |
| xraw/exhdry | Amount of undiluted exhaust, without excess air, per mole of dry (raw or diluted) exhaust. |
| xCO2int | Amount of intake air CO 2 per mole of intake air. |
| xCO2intdry | amount of intake air CO 2 per mole of dry intake air; you may use xCO2intdry = 375 µmol/mol, but we recommend measuring the actual concentration in the intake air. |
| xH2Oint | Amount of H 2 O in the intake air, based on a humidity measurement of intake air. |
| xH2Ointdry | Amount of intake air H 2 O per mole of dry intake air. |
| xO2int | Amount of intake air O 2 per mole of intake air. |
| xCO2dil | Amount of dilution gas CO 2 per mole of dilution gas. |
| xCO2dildry | Amount of dilution gas CO 2 per mole of dry dilution gas; if you use air as diluent, you may use xCO2dildry = 375 µmol/mol, but we recommend measuring the actual concentration in the dilution gas. |
| xH2Odil | Amount of dilution gas H 2 O per mole of dilution gas. |
| xH2Odildry | Amount of dilution gas H 2 O per mole of dry dilution gas. |
| τ | Effective carbon content of the fuel and any injected fluids. |
| χ | Effective hydrogen content of the fuel and any injected fluids. |
| ϕ | Effective oxygen content of the fuel and any injected fluids. |
| ξ | Effective sulfur content of the fuel and any injected fluids. |
| ω | Effective nitrogen content of the fuel and any injected fluids. |
| wC | Carbon mass fraction of the fuel (or mixture of test fuels) and any injected fluids. |
| wH | Hydrogen mass fraction of the fuel (or mixture of test fuels) and any injected fluids. |
| wO | Oxygen mass fraction of the fuel (or mixture of test fuels) and any injected fluids. |
| wS | Sulfur mass fraction of the fuel (or mixture of test fuels) and any injected fluids. |
| wN | Nitrogen mass fraction of the fuel (or mixture of test fuels) and any injected fluids. |
(4) Use the equations specified in this section to iteratively solve for xint/exhdry , xdil/exhdry , and xH2exhdry . The following exceptions apply:
(i) For xH2exhdry multiple equations are provided, see table 2 to paragraph (c)(6) of this section to determine for which cases the equations apply.
(ii) The calculation of xO2exhdry is only required when xO2meas is measured.
(iii) The calculation of xNH3exhdry is only required for engines that use ammonia as fuel and engines that are subject to NH 3 measurement under the standard setting part, for all other engines xNH3exhdry may be set to zero.
(iv) The calculation of xCO2exhdry is only required for engines that use carbon-containing fuels or fluids, either as single fuel or as part of the fuel mixture, and for engines that are subject to CO 2 measurement under the standard setting part, for all other engines xCO2exhdry may be set to a value that yields for xCcombdry a value of zero. (v) The calculation of xCOexhdry and xTHCexhdry is only required for engines that use carbon-containing fuels and for engines that are subject to CO and THC measurement under the standard setting part, for all other engines xCOexhdry and xTHCexhdry may be set to zero. (vi) The calculation of xN2Oexhdry is only required for engines that are subject to N 2 O measurement under the standard setting part, for all other engines xN2Oexhdry may be set to zero.
(5) The chemical balance equations are as follows:
x Ccombdry = xco2exhdry + xcoexhdry + xTHCexhdry − xco2dil · xdil/exhdry − xco2int · xint/exhdry
Eq. 1065.656-1
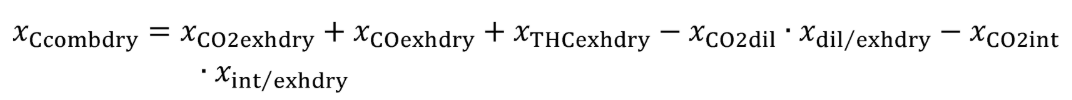
Eq. 1065.656-2
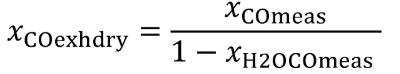
Eq. 1065.656-3
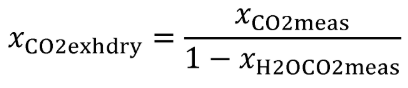
Eq. 1065.656-4
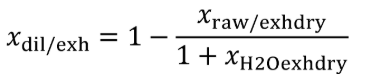
Eq. 1065.656-5
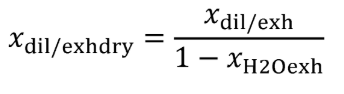
Eq. 1065.656-6 (see table 2 of this section)
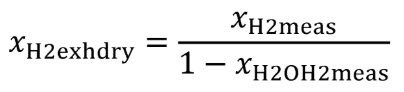
Eq. 1065.656-7 (see table 2 of this section)
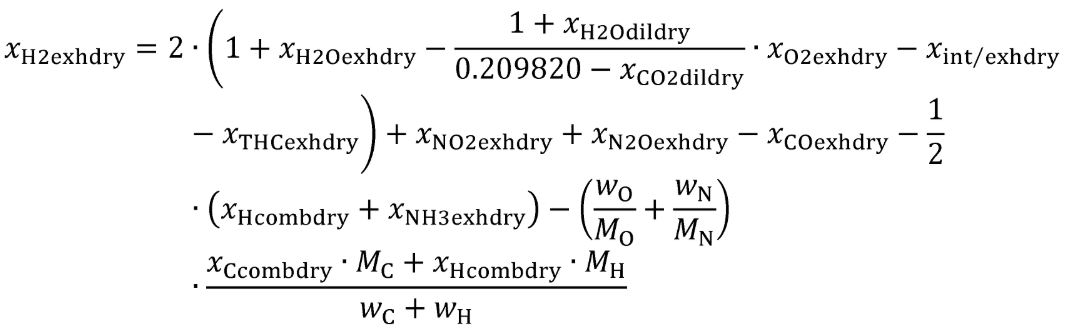
Eq. 1065.656-8
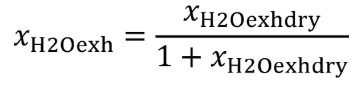
Eq. 1065.656-9
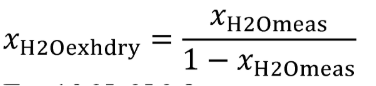
Eq. 1065.656-10
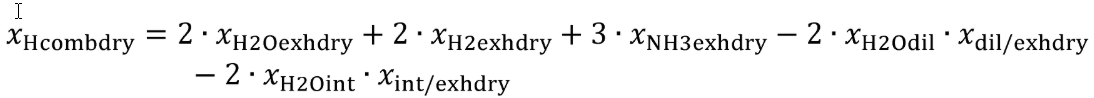
Eq. 1065.656-11

Eq. 1065.656-12
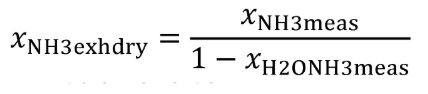
Eq. 1065.656-13
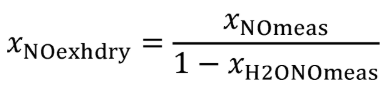
Eq. 1065.656-14
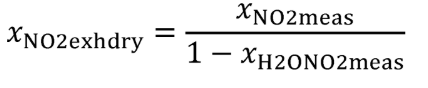
Eq. 1065.656-15
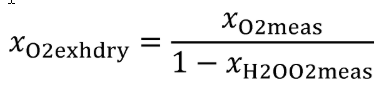
Eq. 1065.656-16 (see table 2 of this section)
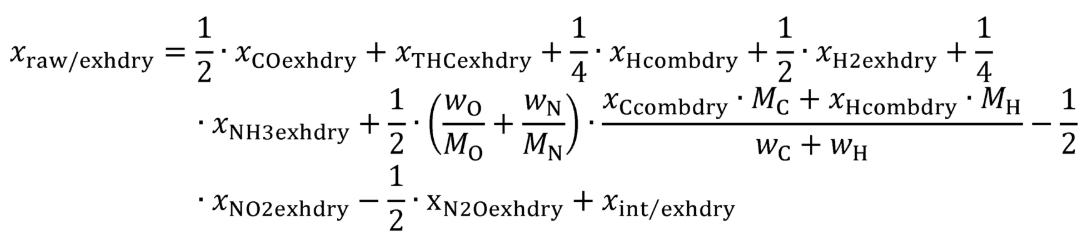
Eq. 1065.656-17
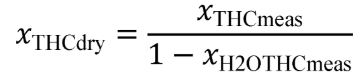
Eq. 1065.656-18
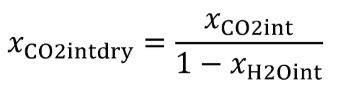
Eq. 1065.656-19
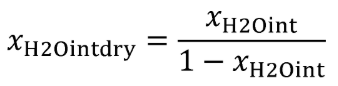
Eq. 1065.656-20
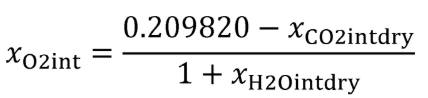
Eq. 1065.656-21
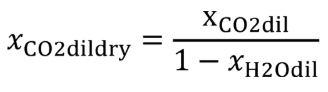
Eq. 1065.656-22
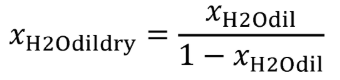
Eq. 1065.656-23
(6) Depending on your measurements, use the equations and guess the quantities specified in the following table:
| When measuring | Guess . . . | Calculate . . . |
|---|---|---|
| (i) xO2meas | xint/exhdry and xH2exhdry | (A) xH2exhdry using Eq. 1065.656-7. |
| (B) xO2exhdry using Eq. 1065.656-16. | ||
| (ii) xH2meas | xint/exhdry and xdil/exhdry | (A) xH2exhdry using Eq. 1065.656-6. |
| (B) [Reserved]. |
(7) The following example is a solution for int/exhdry,dil/exhdry, and HOexhdry using the equations in paragraph (c)(5) of this section:

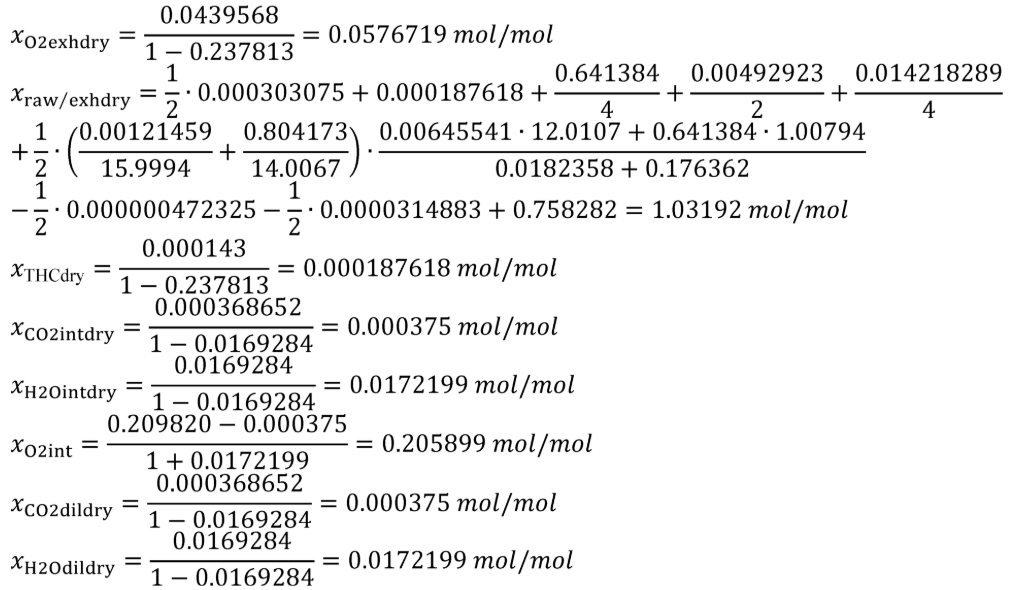
(d) Mass fractions of fuel. (1) For fuels other than carbon-containing fuels determine the mass fractions of fuel WC , WH , WO , WS , and WN, based on the fuel properties as determined in paragraph (e) of this section. Calculate WC , WH , WO , WS , and WN using the following equations:
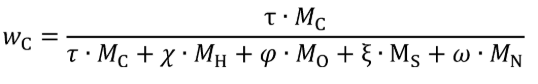
Eq. 1065.656-24
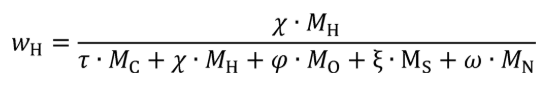
Eq. 1065.656-25
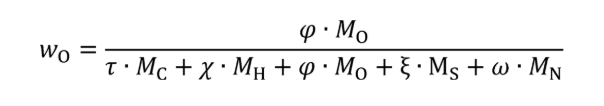
Eq. 1065.656-26
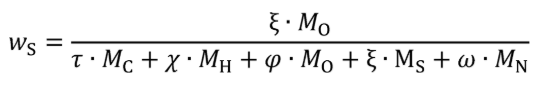
Eq. 1065.656-27
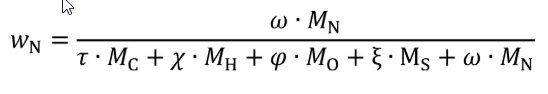
Eq. 1065.656-28
Where:
wC = carbon mass fraction of the fuel and any injected fluids.
wH = hydrogen mass fraction of the fuel and any injected fluids.
wO = oxygen mass fraction of the fuel and any injected fluids.
wS = sulfur mass fraction of the fuel and any injected fluids.
wN = nitrogen mass fraction of the fuel and any injected fluids.
τ = effective carbon content of the fuel and any injected fluids.
MC = molar mass of carbon.
χ = effective hydrogen content of the fuel and any injected fluids.
MH = molar mass of hydrogen.
ϕ = effective oxygen content of the fuel and any injected fluids.
MO = molar mass of oxygen.
ξ = effective sulfur content of the fuel and any injected fluids.
MS = molar mass of nitrogen.
ω = effective nitrogen content of the fuel and any injected fluids.
MN = molar mass of nitrogen.
Example for NH fuel:
τ = 0
χ = 3
ϕ = 0
ξ = 0
ω = 1
MC = 12.0107 g/mol
MH = 1.00794 g/mol
MO = 15.9994 g/mol
MS = 32.065 g/mol
MN = 14.0067 g/mol
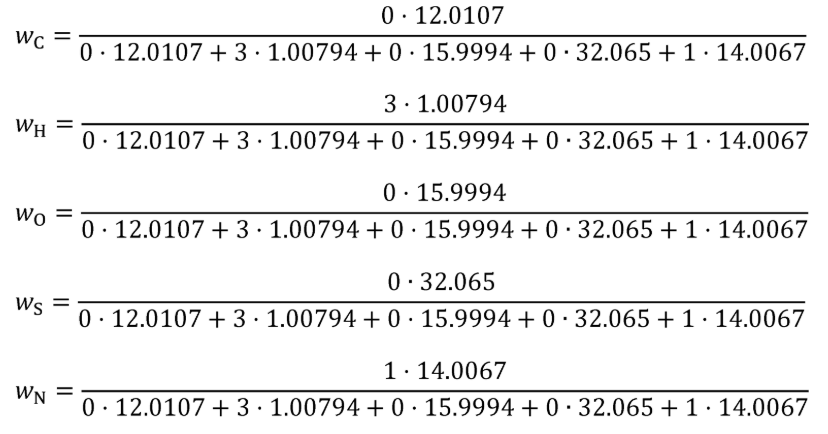
wC = 0 g/g
wH = 0.1775530 g/g
wO = 0 g/g
wS = 0 g/g
wN = 0.8224470 g/g
(2) For carbon-containing fuels and diesel exhaust fluid determine the mass fractions of fuel, WC , WH , WO , WS , and WN , based on properties determined according to §1065.655(d). Calculate WC , WH , WO , WS , and WN using the following equations:
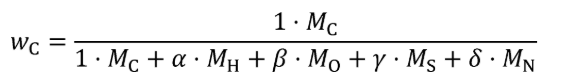
Eq. 1065.656-29
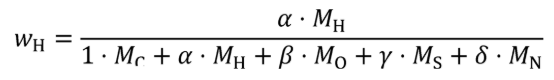
Eq. 1065.656-30
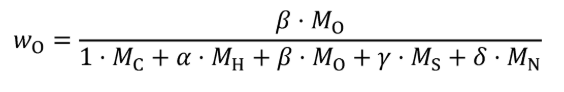
Eq. 1065.656-31
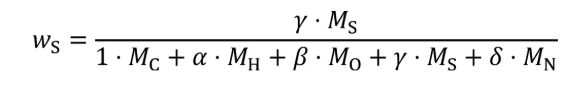
Eq. 1065.656-32
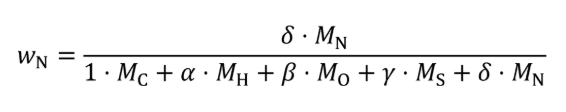
Eq. 1065.656-33
Where:
wC = carbon mass fraction of the fuel and any injected fluids.
wH = hydrogen mass fraction of the fuel and any injected fluids.
wO = oxygen mass fraction of the fuel and any injected fluids.
wS = sulfur mass fraction of the fuel and any injected fluids.
wN = nitrogen mass fraction of the fuel and any injected fluids.
MC = molar mass of carbon.
α = atomic hydrogen-to-carbon ratio of the fuel and any injected fluids.
MH = molar mass of hydrogen.
β = atomic oxygen-to-carbon ratio of the fuel and any injected fluids.
MO = molar mass of oxygen.
γ = atomic sulfur-to-carbon ratio of the fuel and any injected fluids.
MS = molar mass of sulfur.
δ = atomic nitrogen-to-carbon ratio of the fuel and any injected fluids.
MN = molar mass of nitrogen.
Example:
α = 1.8
β = 0.05
γ = 0.0003
δ = 0.0001
MC = 12.0107
MH = 1.00794
MO = 15.9994
MS = 32.065
MN = 14.0067

(3) For nonconstant fuel mixtures, you must account for the varying proportions of the different fuels. This paragraph (d)(3) generally applies for dual-fuel and flexible-fuel engines, but optionally it may also be applied if diesel exhaust fluid or other fluids injected into the exhaust are injected in a way that is not strictly proportional to fuel flow. Account for these varying concentrations either with a batch measurement that provides averaged values to represent the test interval, or by analyzing data from continuous mass rate measurements. Application of average values from a batch measurement generally applies to situations where one fluid is a minor component of the total fuel mixture; consistent with good engineering judgment. Calculate WC , WH , WO , WS , and WN of the fuel mixture using the following equations:
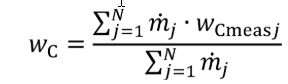
Eq. 1065.656-34
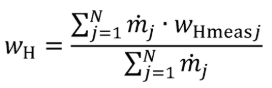
Eq. 1065.656-35
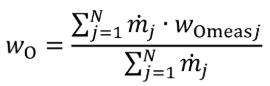
Eq. 1065.656-36
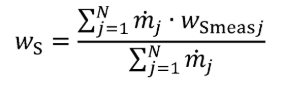
Eq. 1065.656-37
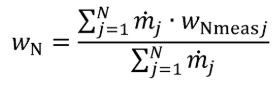
Eq. 1065.656-38
Where:
wC = carbon mass fraction of the mixture of test fuels and any injected fluids.
wH = hydrogen mass fraction of the mixture of test fuels and any injected fluids.
wO = oxygen mass fraction of the mixture of test fuels and any injected fluids.
wS = sulfur mass fraction of the mixture of test fuels and any injected fluids.
wN = nitrogen mass fraction of the mixture of test fuels and any injected fluids.
N = total number of fuels and injected fluids over the duty cycle.
j = an indexing variable that represents one fuel or injected fluid, starting with j = 1.
m = the mass flow rate of the fuel or any injected fluid j. For batch measurements, divide the total mass of fuel over the test interval duration to determine a mass rate.
wCmeasj = carbon mass fraction of fuel or any injected fluid j.
wHmeasj = hydrogen mass fraction of fuel or any injected fluid j.
wOmeasj = oxygen mass fraction of fuel or any injected fluid j.
wSmeasj = sulfur mass fraction of fuel or any injected fluid j.
wNmeasj = nitrogen mass fraction of fuel or any injected fluid j.
Example for a mixture of diesel and NH3fuel where diesel represents 15% of energy:
N = 2
m 1 = 0.5352 g/s
m 2 = 7.024 g/s
wCmeas1 = 0.820628 g/g
wHmeas1 = 0.123961 g/g
wOmeas1 = 0.0546578 g/g
wSmeas1 = 0.00065725 g/g
wNmeas1 = 0.0000957004 g/g
wCmeas2 = 0 g/g
wHmeas2 = 0.177553 g/g
wOmeas2 = 0 g/g
wSmeas2 = 0 g/g
wNmeas2 = 0.822447 g/g

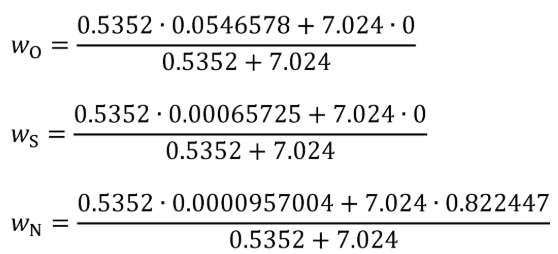
wC = 0.0581014 g/g
wH = 0.1737586 g/g
wO = 0.00386983 g/g
wS = 0.0000465341 g/g
wN = 0.76422359 g/g
(e) Fuel and diesel exhaust fluid composition. (1) For carbon-containing fuels and diesel exhaust fluid determine the composition represented by α, β, γ, and δ, as described in §1065.655(e).
(2) For fuels other than carbon-containing fuels use the default values for τ, χ, ϕ, ξ, and ω in table 3 to this section, or use good engineering judgment to determine those values based on measurement. If you determine compositions based on measured values and the default value listed in table 3 to this section is zero, you may set τ, ϕ, ξ, and ω to zero; otherwise determine τ, ϕ, ξ, and ω (along with χ) based on measured values.
(3) If your fuel mixture contains carbon-containing fuels and your testing requires fuel composition values referencing carbon, calculate α, β, γ, and δ for the fuel mixture as described in §1065.655(e)(4).
(4) Table 3 to this paragraph (e)(4) follows:
| Fuel | Atomic carbon, oxygen, and nitrogen-to-hydrogen ratios C ,H ,O ,S ,N |
|---|---|
| Hydrogen | C 0 H 2 O o S o N o . |
| Ammonia | C 0 H 3 O o S o N 1 . |
(f) Calculated raw exhaust molar flow rate from measured intake air molar flow rate or fuel mass flow rate. You may calculate the raw exhaust molar flow rate from which you sampled emissions, , based on the measured intake air molar flow rate, , or the measured fuel mass flow rate, , and the values calculated using the chemical balance in paragraph (c) of this section. The chemical balance must be based on raw exhaust gas concentrations. Solve for the chemical balance in paragraph (c) of this section at the same frequency that you update and record or . For laboratory tests, calculating raw exhaust molar flow rate using measured fuel mass flow rate is valid only for steady-state testing. See §1065.915(d)(5)(iv) for application to field testing.
(1) Crankcase flow rate. If engines are not subject to crankcase controls under the standard-setting part, you may calculate raw exhaust flow based on or using one of the following:
(i) You may measure flow rate through the crankcase vent and subtract it from the calculated exhaust flow.
(ii) You may estimate flow rate through the crankcase vent by engineering analysis as long as the uncertainty in your calculation does not adversely affect your ability to show that your engines comply with applicable emission standards.
(iii) You may assume your crankcase vent flow rate is zero.
(2) Intake air molar flow rate calculation. Calculate n based on using the following equation:
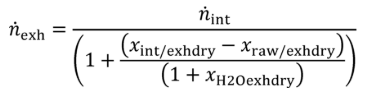
Eq. 1065.656-39
Where:
n exh = raw exhaust molar flow rate from which you measured emissions.
n int = intake air molar flow rate including humidity in intake air.
Example:
n int = 3.780 mol/s
xint/exhdry = 0.69021 mol/mol
xraw/exhdry = 1.10764 mol/mol
xH20exhdry = 107.64 mmol/mol = 0.10764 mol/mol
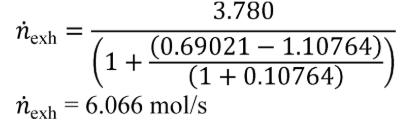
(3) Fluid mass flow rate calculation. This calculation may be used only for steady-state laboratory testing. See §1065.915(d)(5)(iv) for application to field testing. Calculate based on using the following equation:
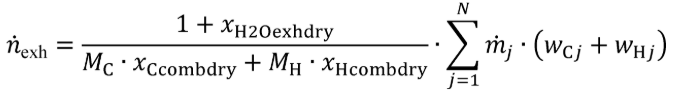
Eq. 1065.656-40
Where:
n exh = raw exhaust molar flow rate from which you measured emissions.
j = an indexing variable that represents one fuel or injected fluid, starting with j = 1.
N = total number of fuels and injected fluids over the duty cycle.
m j = the mass flow rate of the fuel or any injected fluid j.
wCj = carbon mass fraction of the fuel (or mixture of test fuels) and any injected fluid j.
wHj = hydrogen mass fraction of the fuel (or mixture of test fuels) and any injected fluid j.
Example:
xH20exhdry1 = 312.013 mmol/mol = 0.10764 mol/mol
MC = 12.0107 g/mol
MH = 1.00794 g/mol
xCcombdry1 = 6.45541 mmol/mol = 0.00645541 mol/mol
xHcombdry1 = 641.384 mmol/mol = 0.641384 mol/mol
m 1 = 0.167974 g/s
m 2 = 7.39103 g/s
wC1 = 0.820628 g/g
wC2 = 0 g/g
wH1 = 0.123961 g/g
wH2 = 0.177553 g/g
N = 2

(g) Calculated raw exhaust molar flow rate from measured intake air molar flow rate, dilute exhaust molar flow rate, and dilute chemical balance. You may calculate the raw exhaust molar flow rate, n exh , based on the measured intake air molar flow rate, n int , the measured dilute exhaust molar flow rate, n dexh , and the values calculated using the chemical balance in paragraph (c) of this section. Note that the chemical balance must be based on dilute exhaust gas concentrations. For continuous-flow calculations, solve for the chemical balance in paragraph (c) of this section at the same frequency that you update and record n int and n dexh . This calculated n dexh may be used for the PM dilution ratio verification in §1065.546; the calculation of dilution air molar flow rate in the background correction in §1065.667; and the calculation of mass of emissions in §1065.650(c) for species that are measured in the raw exhaust.
(1) Crankcase flow rate. If engines are not subject to crankcase controls under the standard-setting part, calculate raw exhaust flow as described in paragraph (f)(1) of this section.
(2) Dilute exhaust and intake air molar flow rate calculation. Calculate as follows:
n exh = ( xraw/exhdry − xint/exhdry) · (1 − xH20exh) · n dexh + n int
Eq. 1065.656-41
Example:
n int = 7.930 mol/s
xraw/exhdry = 0.1544 mol/mol
xint/exhdry = 0.1451 mol/mol
xH20exhdry = 32.46 mmol/mol = 0.03246 mol/mol
n dexh = 49.02 mol/s
n exh = (0.1544 − 0.1451) · (1 − 0.03246) · 49.02 + 7.930 = 0.4411 + 7.930 = 8.371 mol/s
[73 FR 37335, June 30, 2008, as amended at 76 FR 57462, Sept. 15, 2011; 79 FR 23804, Apr. 28, 2014; 86 FR 34566, Jun. 29, 2021; 89 FR 29810, Apr. 22, 2024]
§1065.659 Removed water correction.
(a) If you remove water upstream of a concentration measurement, x, correct for the removed water. Perform this correction based on the amount of water at the concentration measurement, xH2O[emission]meas, and at the flow meter, xH2Oexh, whose flow is used to determine the mass emission rate or total mass over a test interval. For continuous analyzers downstream of a sample dryer for transient and ramped-modal cycles, you must apply this correction on a continuous basis over the test interval, even if you use one of the options in §1065.145(e)(2) that results in a constant value for xH2O[emission]meas because xH2Oexh varies over the test interval. For batch analyzers, determine the flow-weighted average based on the continuous xH2Oexh values determined as described in paragraph (c) of this section. For batch analyzers, you may determine the flow-weighted average xH2Oexh based on a single value of xH2Oexh determined as described in paragraphs (c)(2) and (3) of this section, using flow-weighted average or batch concentration inputs.
(b) Determine the amount of water remaining downstream of a sample dryer and at the concentration measurement using one of the methods described in §1065.145(e)(2). If you use a sample dryer upstream of an analyzer and if the calculated amount of water remaining downstream of the sample dryer and at the concentration measurement, xH2O[emission]meas, is higher than the amount of water at the flow meter, xH2Oexh, set xH2O[emission]meas equal to xH2Oexh. If you use a sample dryer upstream of storage media, you must be able to demonstrate that the sample dryer is removing water continuously (i.e., xH2Oexh is higher than xH2O[emission]meas throughout the test interval).
(c) For a concentration measurement where you did not remove water, you may set xH2O[emission]meas equal to xH2Oexh. You may determine the amount of water at the flow meter, xH2Oexh, using any of the following methods:
(1) Measure the dewpoint and absolute pressure and calculate the amount of water as described in §1065.645.
(2) If the measurement comes from raw exhaust, you may determine the amount of water based on intake-air humidity, plus a chemical balance of fuel, DEF, intake air, and exhaust as described in §1065.655.
(3) If the measurement comes from diluted exhaust, you may determine the amount of water based on intake-air humidity, dilution air humidity, and a chemical balance of fuel, DEF, intake air, and exhaust as described in §1065.655.
(d) Perform a removed water correction to the concentration measurement using the following equation:
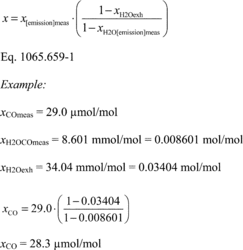
[73 FR 37335, June 30, 2008, as amended at 76 FR 57462, Sept. 15, 2011; 79 FR 23804, Apr. 28, 2014; 86 FR 34566, Jun. 29, 2021]
§1065.660 THC, NMHC, NMNEHC, CH4, and C2H6 determination.
(a) THC determination and initial THC/CH4contamination corrections. (1) If we require you to determine THC emissions, calculate THC[THC-FID]cor using the initial THC contamination concentration THC[THC-FID]init from §1065.520 as follows:
THC[THC-FID]cor = THC[THC-FID]uncor − THC[THC-FID]init
Eq. 1065.660-1
Example:
THCuncor = 150.3 µmol/mol
THCinit = 1.1 µmol/mol
THCcor = 150.3—1.1
THCcor = 149.2 µmol/mol
(2) For the NMHC determination described in paragraph (b) of this section, correct THC[THC-FID] for initial THC contamination using Eq. 1065.660-1. You may correct THC[NMC-FID] for initial contamination of the CH 4 sample train using Eq. 1065.660-1, substituting in CH 4 concentrations for THC.
(3) For the NMNEHC determination described in paragraph (c) of this section, correct THC[THC-FID] for initial THC contamination using Eq. 1065.660-1. You may correct THC[NMC-FID] for initial contamination of the CH 4 sample train using Eq. 1065.660-1, substituting in CH 4 concentrations for THC.
(4) For the CH 4 determination described in paragraph (d) of this section, you may correct THC[NMC-FID] for initial THC contamination of the CH 4 sample train using Eq. 1065.660-1, substituting in CH 4 concentrations for THC.
(5) You may calculate THC as the sum of NMHC and CH 4 if you determine CH 4 with an FTIR as described in paragraph (d)(2) of this section and NMHC with an FTIR using the additive method from paragraph (b)(4) of this section.
(6) You may calculate THC as the sum of NMNEHC, C 2 H 6 , and CH 4 if you determine CH 4 with an FTIR as described in paragraph (d)(2) of this section, C 2 H 6 with an FTIR as described in paragraph (e) of this section, and NMNEHC with an FTIR using the additive method from paragraph (c)(3) of this section.
(b) NMHC determination. Use one of the following to determine NMHC concentration, NMHC :
(1) If you do not measure CH 4 , you may omit the calculation of NMHC concentrations and calculate the mass of NMHC as described in §1065.650(c)(5).
(2) For an NMC, calculate NMHC using the NMC's penetration fractions, response factors, and/or combined penetration fractions and response factors as described in §1065.365, the THC FID's CH 4 response factor, RFCH4[THC-FID], from §1065.360, the initial THC contamination and dry-to-wet corrected THC concentration, THC[THC-FID]cor, as determined in paragraph (a) of this section, and the dry-to-wet corrected CH 4 concentration, THC[NMC-FID]cor, optionally corrected for initial THC contamination as determined in paragraph (a) of this section.
(i) Use the following equation for an NMC configured as described in §1065.365(d):
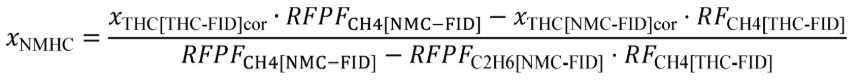
Eq. 1065.660-2
Where:
NMHC = concentration of NMHC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the NMC FID during sampling through the NMC.
RFCH4[THC-FID] = response factor of THC FID to CH 4, according to §1065.360(d).
RFPFC2H6[NMC-FID] = NMC combined C 2 H 6 response factor and penetration fraction, according to §1065.365(d).
RFPFCH4[NMC-FID] = NMC combined CH 4 response factor and penetration fraction, according to §1065.365(d).
Example:
THC[THC-FID]cor = 150.3 µmol/mol
THC[NMC-FID]cor = 20.5 µmol/mol
RFPFC2H6[NMC-FID] = 0.019
RFPFCH4[NMC-FID] = 1.000
RFCH4[THC-FID] = 1.05
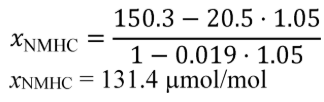
(ii) Use the following equation for penetration fractions determined using an NMC configuration as outlined in §1065.365(e):
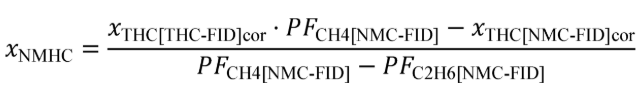
Eq. 1065.660-3
Where:
NMHC = concentration of NMHC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
PFCH4[NMC-FID] = NMC CH 4 penetration fraction, according to §1065.365(e).
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the THC FID during sampling through the NMC.
PFC2H6[NMC-FID] = NMC C 2 H 6 penetration fraction, according to §1065.365(e).
Example:
THC[THC-FID]cor = 150.3 µmol/mol
PFCH4[NMC-FID] = 0.990
THC[NMC-FID]cor = 20.5 µmol/mol
PFC2H6[NMC-FID] = 0.020

(iii) Use the following equation for an NMC configured as described in §1065.365(f):
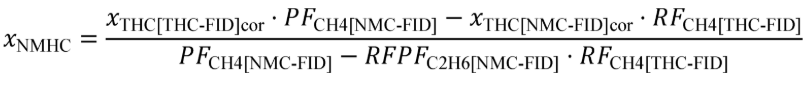
Eq. 1065.660-4
Where:
NMHC = concentration of NMHC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
PFCH4[NMC-FID] = NMC CH 4 penetration fraction, according to §1065.365(f).
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the THC FID during sampling through the NMC.
RFPFC2H6[NMC-FID] = NMC combined C 2 H 6 response factor and penetration fraction, according to §1065.365(f).
RFCH4[THC-FID] = response factor of THC FID to CH 4, according to §1065.360(d).
Example:
THC[THC-FID]cor = 150.3 µmol/mol
PFCH4[NMC-FID] = 0.990
THC[NMC-FID]cor = 20.5 µmol/mol
RFPFC2H6[NMC-FID] = 0.019
RFCH4[THC-FID] = 0.980
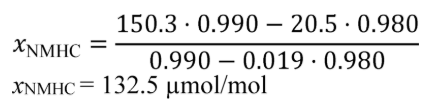
(3) For a GC-FID or FTIR, calculate NMHC using the THC analyzer's CH 4 response factor, RFCH4[THC-FID], from §1065.360, and the initial THC contamination and dry-to-wet corrected THC concentration, THC[THC-FID]cor, as determined in paragraph (a) of this section as follows:
χ NMHC = χ THC[THC-FID]cor − RFCH4[THC-FID] · χ CH4
Eq. 1065.660-5
Where:
NMHC = concentration of NMHC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID.
RFCH4[THC-FID] = response factor of THC-FID to CH 4.
CH4 = concentration of CH 4, dry-to-wet corrected, as measured by the GC-FID or FTIR.
Example:
THC[THC-FID]cor = 145.6 µmol/mol
RFCH4[THC-FID] = 0.970
CH4 = 18.9 µmol/mol
NMHC = 145.6—0.970 · 18.9
NMHC = 127.3 µmol/mol
(4) For an FTIR, calculate NMHC by summing the hydrocarbon species listed in §1065.266(c) as follows:
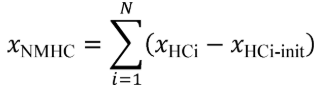
Eq. 1065.660-6
Where:
NMHC = concentration of NMHC.
HCi = the C 1 -equivalent concentration of hydrocarbon species i as measured by the FTIR, not corrected for initial contamination.
HCi-init = the C 1 -equivalent concentration of the initial system contamination (optional) of hydrocarbon species i, dry-to-wet corrected, as measured by the FTIR.
Example:
C2H6 = 4.9 µmol/mol
C2H4 = 0.9 µmol/mol
C2H2 = 0.8 µmol/mol
C3H8 = 0.4 µmol/mol
C3H6 = 0.5 µmol/mol
C4H10 = 0.3 µmol/mol
CH2O = 0.8 µmol/mol
C2H4O = 0.3 µmol/mol
CH2O2 = 0.1 µmol/mol
CH4O = 0.1 µmol/mol
NMHC = 4.9 + 0.9 + 0.8 + 0.4 + 0.5 + 0.3 + 0.8 + 0.3 + 0.1 + 0.1
NMHC = 9.1 µmol/mol
(c) NMNEHC determination. Use one of the following methods to determine NMNEHC concentration, NMNEHC :
(1) Calculate NMNEHC based on the test fuel's ethane content as follows:
(i) If the content of your test fuel contains less than 0.010 mol/mol of ethane, you may omit the calculation of NMNEHC concentration and calculate the mass of NMNEHC as described in §1065.650(c)(6)(i).
(ii) If the content of your fuel test contains at least 0.010 mol/mol of C 2 H 6 , you may omit the calculation of NMNEHC concentration and calculate the mass of NMNEHC as described in §1065.650(c)(6)(ii).
(2) For a GC-FID, NMC FID, or FTIR, calculate NMNEHC using the THC analyzer's CH 4 response factor, RFCH4[THC-FID], and C 2 H 6 response factor, RFC2H6[THC-FID], from §1065.360, the initial contamination and dry-to-wet corrected THC concentration, THC[THC-FID]cor, as determined in paragraph (a) of this section, the dry-to-wet corrected CH 4 concentration, CH4, as determined in paragraph (d) of this section, and the dry-to-wet corrected C 2 H 6 concentration, C2H6, as determined in paragraph (e) of this section as follows:
NMNEHC = THC[THC-FID}cor − RF CH4{THC-FID} . CH4 − RF C2H6{THC-FID] . C2H6
Eq. 1065.660-7
Where:
NMNEHC = concentration of NMNEHC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID.
RFCH4[THC-FID] = response factor of THC-FID to CH 4.
CH4 = concentration of CH 4, dry-to-wet corrected, as measured by the GC-FID, NMC FID, or FTIR.
RFC2H6[THC-FID] = response factor of THC-FID to C 2 H 6.
C2H6 = the C 1 -equivalent concentration of C 2 H 6, dry-to-wet corrected, as measured by the GC-FID or FTIR.
Example:
THC[THC-FID]cor = 145.6 µmol/mol
RFCH4[THC-FID] = 0.970
CH4 = 18.9 µmol/mol
RFC2H6[THC-FID] = 1.02
C2H6 = 10.6 µmol/mol
NMNEHC = 145.6 − 0.970 · 18.9 − 1.02 · 10.6
NMNEHC = 116.5 µmol/mol
(3) For an FTIR, calculate xNMNEHC by summing the hydrocarbon species listed in §1065.266(c) as follows:
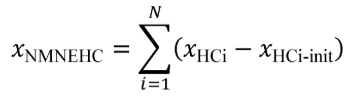
Eq. 1065.660-8
Where:
NMNEHC = concentration of NMNEHC.
HC = the C 1 -equivalent concentration of hydrocarbon species i as measured by the FTIR, not corrected for initial contamination.
HC-init = the C 1 -equivalent concentration of the initial system contamination (optional) of hydrocarbon species i, dry-to-wet corrected, as measured by the FTIR.
Example:
C2H4 = 0.9 µmol/mol
C2H2 = 0.8 µmol/mol
C3H8 = 0.4 µmol/mol
C3H6 = 0.5 µmol/mol
C4H10 = 0.3 µmol/mol
CH2O = 0.8 µmol/mol
C2H4O = 0.3 µmol/mol
CH2O2 = 0.1 µmol/mol
CH4O = 0.1 µmol/mol
NMNEHC = 0.9 + 0.8 + 0.4 + 0.5 + 0.3 + 0.8 + 0.3 + 0.1 + 0.1
xNMNEHC = 4.2 µmol/mol
(d) CH4 determination. Use one of the following methods to determine methane (CH 4) concentration, CH4 :
(1) For an NMC, calculate xCH4 using the NMC's penetration fractions, response factors, and/or combined penetration fractions and response factors as described in §1065.365, the THC FID's CH 4 response factor, RFCH4[THC-FID], from §1065.360, the initial THC contamination and dry-to-wet corrected THC concentration, THC[THC-FID]cor, as determined in paragraph (a) of this section, and the dry-to-wet corrected CH 4 concentration, THC[NMC-FID]cor, optionally corrected for initial THC contamination as determined in paragraph (a) of this section.
(i) Use the following equation for an NMC configured as described in §1065.365(d):
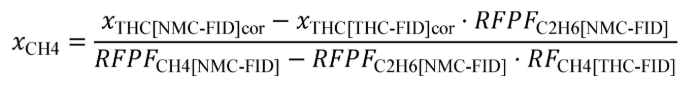
Eq. 1065.660-9
Where:
CH4 = concentration of CH 4 .
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the NMC FID during sampling through the NMC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
RFPFC2H6[NMC-FID] = NMC combined C 2 H 6 response factor and penetration fraction, according to §1065.365(d).
RFCH4[THC-FID] = response factor of THC FID to CH 4, according to §1065.360(d).
RFPFCH4[NMC-FID] = NMC combined CH 4 response factor and penetration fraction, according to §1065.365(d).
Example:
THC[NMC-FID]cor = 10.4 µmol/mol
THC[THC-FID]cor = 150.3 µmol/mol
RFPFC2H6[NMC-FID] = 0.019
RFPFCH4[NMC-FID] = 1.000
RFCH4[THC-FID] = 1.05
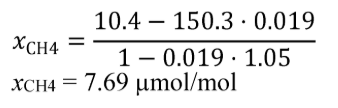
(ii) Use the following equation for an NMC configured as described in §1065.365(e):
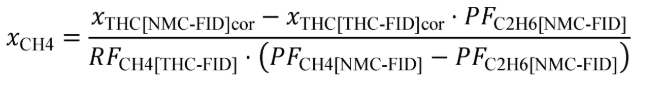
Eq. 1065.660-10
Where:
xCH4 = concentration of CH 4.
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the NMC FID during sampling through the NMC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
PFC2H6[NMC-FID] = NMC C 2 H 6 penetration fraction, according to §1065.365(e).
RFCH4[THC-FID] = response factor of THC FID to CH 4, according to §1065.360(d).
PFCH4[NMC-FID] = NMC CH 4 penetration fraction, according to §1065.365(e).
Example:
THC[NMC-FID]cor = 10.4 µmol/mol
THC[THC-FID]cor = 150.3 µmol/mol
PFC2H6[NMC-FID] = 0.020
RFCH4[THC-FID] = 1.05
PFCH4[NMC-FID] = 0.990
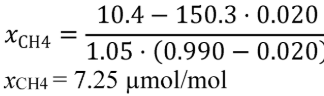
(iii) Use the following equation for an NMC configured as described in §1065.365(f):
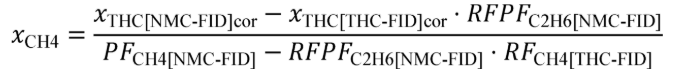
Eq. 1065.660-11
Where:
CH4 = concentration of CH 4.
THC[NMC-FID]cor = concentration of THC, initial THC contamination (optional) and dry-to-wet corrected, as measured by the NMC FID during sampling through the NMC.
THC[THC-FID]cor = concentration of THC, initial THC contamination and dry-to-wet corrected, as measured by the THC FID during sampling while bypassing the NMC.
RFPFC2H6[NMC-FID] = the combined C 2 H 6 response factor and penetration fraction of the NMC, according to §1065.365(f).
PFCH4[NMC-FID] = NMC CH 4 penetration fraction, according to §1065.365(f).
RFCH4[THC-FID] = response factor of THC FID to CH 4, according to §1065.360(d).
Example:
THC[NMC-FID]cor = 10.4 µmol/mol
THC[THC-FID]cor = 150.3 µmol/mol
RFPFC2H6[NMC-FID] = 0.019
PFCH4[NMC-FID] = 0.990
RFCH4[THC-FID] = 1.05
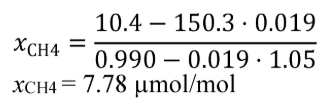
(2) For a GC-FID or FTIR, xCH4 is the actual dry-to-wet corrected CH 4 concentration as measured by the analyzer.
(e) C2 H 6 determination. For a GC-FID or FTIR, C2H6 is the C 1 -equivalent, dry-to-wet corrected C 2 H 6 concentration as measured by the analyzer.
[76 FR 57462, Sept. 15, 2011, as amended at 81 FR 74184, Oct. 25, 2016; 86 FR 34566, Jun. 29, 2021; 88 FR 4686, Jan. 24, 2023; 89 FR 29819, Apr. 22, 2024]
§1065.665 THC, NMHC, NMNEHC, CH 4 , and C 2 H 6 determination.
(a) If you measured an oxygenated hydrocarbon's mass concentration, first calculate its molar concentration in the exhaust sample stream from which the sample was taken (raw or diluted exhaust), and convert this into a C1-equivalent molar concentration. Add these C1-equivalent molar concentrations to the molar concentration of non-oxygenated total hydrocarbon (NOTHC). The result is the molar concentration of total hydrocarbon equivalent (THCE). Calculate THCE concentration using the following equations, noting that Eq. 1065.665-3 is required only if you need to convert your oxygenated hydrocarbon (OHC) concentration from mass to moles:
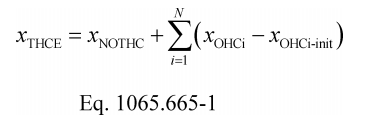
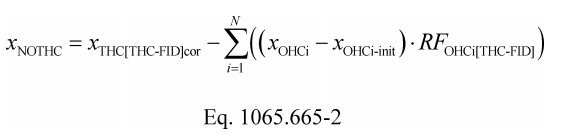
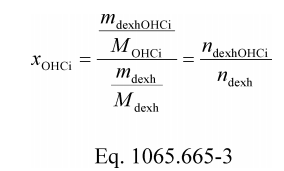
Where:
χTHCE = the sum of the C1-equivalent concentrations of non-oxygenated hydrocarbon, alcohols, and aldehydes.
χNOTHC = the sum of the C1-equivalent concentrations of NOTHC.
χOHCi = the C1-equivalent concentration of oxygenated species i in diluted exhaust, not corrected for initial contamination.
χOHCi-init = the C1-equivalent concentration of the initial system contamination (optional) of oxygenated species i, dry-to-wet corrected.
χTHC[THC-FID]cor = the C1-equivalent response to NOTHC and all OHC in diluted exhaust, HC contamination and dry-to-wet corrected, as measured by the THC-FID.
RFOHCi[THC-FID] = the response factor of the FID to species i relative to propane on a C1-equivalent basis.
Mdexh = the molar mass of diluted exhaust as determine in §1065.340.
mdexhOHCi = the mass of oxygenated species i in dilute exhaust.
MOHCi = the C1-equivalent molecular weight of oxygenated species i.
mdexh = the mass of diluted exhaust.
ndexhOHCi = the number of moles of oxygenated species i in total diluted exhaust flow.
ndexh = the total diluted exhaust flow.
(b) If we require you to determine nonmethane hydrocarbon equivalent (NMHCE), use the following equation:
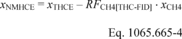
Where:
xNMHCE = the sum of the C1-equivalent concentrations of nonoxygenated nonmethane hydrocarbon (NONMHC), alcohols, and aldehydes.
RFCH4[THC-FID] = the response factor of THC-FID to CH4.
xCH4 = concentration of CH4, HC contamination (optional) and dry-to-wet corrected, as measured by the gas chromatograph FID.
(c) The following example shows how to determine NMHCE emissions based on ethanol (C2H5OH), methanol (CH3OH), acetaldehyde (C2H4O), and formaldehyde (CH2O) as C1-equivalent molar concentrations:
xTHC[THC-FID]cor = 145.6 µmol/mol
xCH4 = 18.9 µmol/mol
xC2H5OH = 100.8 µmol/mol
xCH3OH = 1.1 µmol/mol
xC2H4O = 19.1 µmol/mol
xCH2O = 1.3 µmol/mol
RFCH4[THC-FID] = 1.07
RFC2H5OH[THC-FID] = 0.76
RFCH3OH[THC-FID] = 0.74
RFH2H4O[THC-FID] = 0.50
RFCH2O[THC-FID] = 0.0
xNMHCE = xTHC[THC-FID]cor − (xC2H5OH · RFC2H5OH[THC-FID] + xCH3OH · RFCH3OH[THC-FID] + xC2H4O · RFC2H4O[THC-FID] + xCH2O · RFCH2O[THC-FID]) + xC2H5OH + xCH3OH + xC2H4O + xCH2O − (RFCH4[THC-FID] · xCH4)
xNMHCE = 145.6 − (100.8 · 0.76 + 1.1 · 0.74 + 19.1 · 0.50 + 1.3 · 0) + 100.8 + 1.1 + 19.1 + 1.3 − (1.07 · 18.9)
xNMHCE = 160.71 µmol/mol
[79 FR 23805, Apr. 28, 2014, as amended at 81 FR 74187, Oct. 25, 2016; 86 FR 34567, Jun. 29, 2021]
§1065.667 Dilution air background emission correction.
(a) To determine the mass of background emissions to subtract from a diluted exhaust sample, first determine the total flow of dilution air, ndil, over the test interval. This may be a measured quantity or a calculated quantity. Multiply the total flow of dilution air by the mean mole fraction (i.e., concentration) of a background emission. This may be a time-weighted mean or a flow-weighted mean (e.g., a proportionally sampled background). Finally, multiply by the molar mass, M, of the associated gaseous emission constituent. The product of ndil and the mean molar concentration of a background emission and its molar mass, M, is the total background emission mass, m. In the case of PM, where the mean PM concentration is already in units of mass per mole of exhaust, multiply it by the total amount of dilution air flow, and the result is the total background mass of PM, mPM. Subtract total background mass from total mass to correct for background emissions.
(b) You may determine the total flow of dilution air by a direct flow measurement.
(c) You may determine the total flow of dilution air by subtracting the calculated raw exhaust molar flow as described in §1065.655(g) from the measured dilute exhaust flow. This may be done by totaling continuous calculations or by using batch results.
(d) You may determine the total flow of dilution air from the measured dilute exhaust flow and a chemical balance of the fuel, DEF, intake air, and dilute exhaust as described in §1065.655. For this paragraph (d), the molar flow of dilution air is calculated by multiplying the dilute exhaust flow by the mole fraction of dilution gas to dilute exhaust, χdil/ex, from the dilute chemical balance. This may be done by totaling continuous calculations or by using batch results. For example, to use batch results, the total flow of dilution air is calculated by multiplying the total flow of diluted exhaust, ndexh, by the flow-weighted mean mole fraction of dilution air in diluted exhaust, χ dil/exh. Calculate χ dil/exh using flow-weighted mean concentrations of emissions in the chemical balance, as described in §1065.655. The chemical balance in §1065.655 assumes that your engine operates stoichiometrically, even if it is a lean-burn engine, such as a compression-ignition engine. Note that for lean-burn engines this assumption could result in an error in emission calculations. This error could occur because the chemical balance in §1065.655 treats excess air passing through a lean-burn engine as if it was dilution air. If an emission concentration expected at the standard is about 100 times its dilution air background concentration, this error is negligible. However, if an emission concentration expected at the standard is similar to its background concentration, this error could be significant. If this error might affect your ability to show that your engines comply with applicable standards in this chapter, we recommend that you either determine the total flow of dilution air using one of the more accurate methods in paragraph (b) or (c) of this section, or remove background emissions from dilution air by HEPA filtration, chemical adsorption, or catalytic scrubbing. You might also consider using a partial-flow dilution technique such as a bag mini-diluter, which uses purified air as the dilution air.
(e) The following is an example of using the flow-weighted mean fraction of dilution air in diluted exhaust, x ͞dil/exh, and the total mass of background emissions calculated using the total flow of diluted exhaust, ndexh, as described in §1065.650(c):
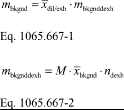
Example:
MNOx = 46.0055 g/mol
x ͞bkgnd = 0.05 µmol/mol = 0.05⋅10−6 mol/mol
ndexh = 23280.5 mol
x ͞dil/exh = 0.843 mol/mol
mbkgndNOxdexh = 46.0055⋅0.05⋅10−6⋅23280.5
mbkgndNOxdexh = 0.0536 g
mbkgndNOx = 0.843 ⋅ 0.0536
mbkgndNOx = 0.0452 g
(f) The following is an example of using the fraction of dilution air in diluted exhaust, xdil/exh, and the mass rate of background emissions calculated using the flow rate of diluted exhaust, n ̇dexh, as described in §1065.650(c):
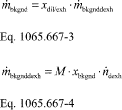
Example:
MNOx = 46.0055 g/mol
xbkgnd = 0.05 µmol/mol = 0.05⋅10−6 mol/mol
n ̇dexh = 23280.5 mol/s
xdil/exh = 0.843 mol/mol
m ̇bkgndNOxdexh = 46.0055⋅0.05⋅10−6⋅23280.5
m ̇bkgndNOxdexh = 0.0536 g/hr
m ̇bkgndNOx = 0.843 ⋅ 0.0536
m ̇bkgndNOx = 0.0452 g/hr
[76 FR 57465, Sept. 15, 2011, as amended at 81 FR 74188, Oct. 25, 2016; 86 FR 34567, Jun. 29, 2021; 88 FR 4686, Jan. 24, 2023]
§1065.670 NOX intake-air humidity and temperature corrections.
See the standard-setting part to determine if you may correct NO X emissions for the effects of intake-air humidity or temperature. Use the NO X intake-air humidity and temperature corrections specified in the standard-setting part instead of the NO X intake-air humidity correction specified in this part 1065. If the standard-setting part does not prohibit correcting NO X emissions for intake-air humidity according to this part 1065, correct NO X concentrations for intake-air humidity as described in this section. See §1065.650(c)(1) for the proper sequence for applying the NO X intake-air humidity and temperature corrections. You may use a time-weighted mean intake air humidity to calculate this correction if your intake air humidity remains within a tolerance of ±0.0025 mol/mol of the mean value over the test interval. For intake-air humidity correction, use one of the following approaches:
(a) For compression-ignition engines operating on carbon-containing fuels and lean-burn combustion engines operating on fuels other than carbon-containing fuels, correct for intake-air humidity using the following equation:<
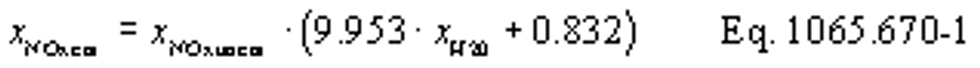
Example:
xNOxuncor = 700.5 µmol/mol
xH2O = 0.022 mol/mol
xNOxcor = 700.5 · (9.953 · 0.022 + 0.832)
xNOxcor = 736.2 µmol/mol
(b) For spark-ignition engines operating on carbon-containing fuels and stoichiometric combustion engines operating on fuels other than carbon-containing fuels, correct for intake-air humidity using the following equation:
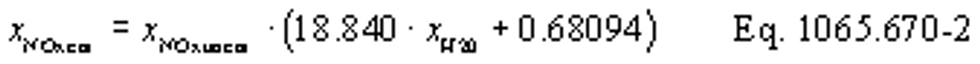
Example:
xNOxuncor = 154.7 µmol/mol
xH2O = 0.022 mol/mol
xNOxcor = 154.7 · (18.840 · 0.022 + 0.68094)
xNOxcor = 169.5 µmol/mol
(c) Develop your own correction, based on good engineering judgment.
[75 FR 23056, Apr. 30, 2010, as amended at 76 FR 57466, Sept. 15, 2011; 88 FR 4686, Jan. 24, 2023; 89 FR 29823, Apr. 22, 2024]
§1065.672 Drift correction.
(a) Scope and frequency. Perform the calculations in this section to determine if gas analyzer drift invalidates the results of a test interval. If drift does not invalidate the results of a test interval, correct that test interval's gas analyzer responses for drift according to this section. Use the drift-corrected gas analyzer responses in all subsequent emission calculations. Note that the acceptable threshold for gas analyzer drift over a test interval is specified in §1065.550 for both laboratory testing and field testing.
(b) Correction principles. The calculations in this section utilize a gas analyzer's responses to reference zero and span concentrations of analytical gases, as determined sometime before and after a test interval. The calculations correct the gas analyzer's responses that were recorded during a test interval. The correction is based on an analyzer's mean responses to reference zero and span gases, and it is based on the reference concentrations of the zero and span gases themselves. Validate and correct for drift as follows:
(c) Drift validation. After applying all the other corrections—except drift correction—to all the gas analyzer signals, calculate emissions according to §1065.650. Then correct all gas analyzer signals for drift according to this section. Recalculate emissions using all of the drift-corrected gas analyzer signals. Validate and report the emission results before and after drift correction according to §1065.550.
(d) Drift correction. Correct all gas analyzer signals as follows:
(1) Correct each recorded concentration, xi, for continuous sampling or for batch sampling, x ͞.
(2) Correct for drift using the following equation:
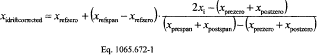
Where:
xidriftcorrected = concentration corrected for drift.
xrefzero = reference concentration of the zero gas, which is usually zero unless known to be otherwise.
xrefspan = reference concentration of the span gas.
xprespan = pre-test interval gas analyzer response to the span gas concentration.
xpostspan = post-test interval gas analyzer response to the span gas concentration.
xi or x ͞ = concentration recorded during test, before drift correction.
xprezero = pre-test interval gas analyzer response to the zero gas concentration.
xpostzero = post-test interval gas analyzer response to the zero gas concentration.
Example:
xrefzero = 0 µmol/mol
xrefspan = 1800.0 µmol/mol
xprespan = 1800.5 µmol/mol
xpostspan = 1695.8 µmol/mol
xi or x ͞ = 435.5 µmol/mol
xprezero = 0.6 µmol/mol
xpostzero = −5.2 µmol/mol
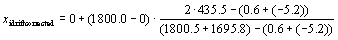
xidriftcorrected = 450.2 µmol/mol
(3) For any pre-test interval concentrations, use the last concentration determined before the test interval. For some test intervals, the last pre-zero or pre-span might have occurred before one or more earlier test intervals.
(4) For any post-test interval concentrations, use the first concentration determined after the test interval. For some test intervals, the first post-zero or post-span might occur after one or more later test intervals.
(5) If you do not record any pre-test interval analyzer response to the span gas concentration, xprespan, set xprespan equal to the reference concentration of the span gas:
xprespan = xrefspan.
(6) If you do not record any pre-test interval analyzer response to the zero gas concentration, xprezero, set xprezero equal to the reference concentration of the zero gas:
xprezero = xrefzero.
(7) Usually the reference concentration of the zero gas, xrefzero, is zero: xrefzero = 0 µmol/mol. However, in some cases you might know that xrefzero has a non-zero concentration. For example, if you zero a CO2 analyzer using ambient air, you may use the default ambient air concentration of CO2, which is 375 µmol/mol. In this case, xrefzero = 375 µmol/mol. Note that when you zero an analyzer using a non-zero xrefzero, you must set the analyzer to output the actual xrefzero concentration. For example, if xrefzero = 375 µmol/mol, set the analyzer to output a value of 375 µmol/mol when the zero gas is flowing to the analyzer.
[70 FR 40516, July 13, 2005, as amended at 74 FR 8427, Feb. 24, 2009; 75 FR 23056, Apr. 30, 2010; 88 FR 4686, Jan. 24, 2023]
§1065.675 CLD quench verification calculations.
Perform CLD quench-check calculations as follows:
(a) Perform a CLD analyzer quench verification test as described in §1065.370.
(b) Estimate the maximum expected mole fraction of water during emission testing, xH2Oexp. Make this estimate where the humidified NO span gas was introduced in §1065.370(e)(6). When estimating the maximum expected mole fraction of water, consider the maximum expected water content in intake air, fuel combustion products, and dilution air (if applicable). If you introduced the humidified NO span gas into the sample system upstream of a sample dryer during the verification test, you need not estimate the maximum expected mole fraction of water and you must set xH2Oexp equal to xH2Omeas.
(c) Estimate the maximum expected CO2 concentration during emission testing, xCO2exp. Make this estimate at the sample system location where the blended NO and CO2 span gases are introduced according to §1065.370(d)(10). When estimating the maximum expected CO2 concentration, consider the maximum expected CO2 content in fuel combustion products and dilution air.
(d) Calculate quench as follows:
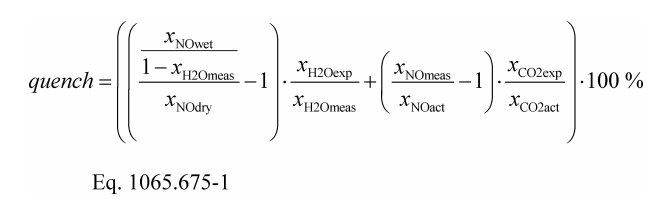
Where:
quench = amount of CLD quench.
χNOdry = concentration of NO upstream of a humidity generator, according to §1065.370(e)(4).
χNOwet = measured concentration of NO downstream of a humidity generator, according to §1065.370(e)(9).
χH2Oexp = maximum expected mole fraction of water during emission testing, according to paragraph (b) of this section.
χH2Omeas = measured mole fraction of water during the quench verification, according to §1065.370(e)(7).
χNOmeas = measured concentration of NO when NO span gas is blended with CO2 span gas, according to §1065.370(d)(10).
χNOact = actual concentration of NO when NO span gas is blended with CO2 span gas, according to §1065.370(d)(11) and calculated according to Eq. 1065.675-2.
χCO2exp = maximum expected concentration of CO2 during emission testing, according to paragraph (c) of this section.
χCO2act = actual concentration of CO2 when NO span gas is blended with CO2 span gas, according to §1065.370(d)(9).
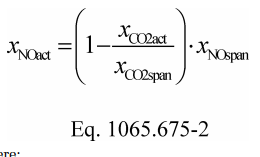
Where:
χNOspan = the NO span gas concentration input to the gas divider, according to §1065.370(d)(5).
χCO2span = the CO2 span gas concentration input to the gas divider, according to §1065.370(d)(4).
Example:
χNOdry = 1800.0 μmol/mol
χNOwet = 1739.6 μmol/mol
χH2Oexp = 0.030 mol/mol
χH2Omeas = 0.030 mol/mol
χNOmeas = 1515.2 μmol/mol
χNOspan = 3001.6 μmol/mol
χCO2exp = 3.2%
χCO2span = 6.1%
χCO2act = 2.98%

quench = (−0.0036655−0.014020171) · 100% = −1.7685671%
[73 FR 59340, Oct. 8, 2008, as amended at 76 FR 57466, Sept. 15, 2011; 81 FR 74188, Oct. 25, 2016; 86 FR 34568, Jun. 29, 2021; 88 FR 4686, Jan. 24, 2023]
§1065.680 Adjusting emission levels to account for infrequently regenerating aftertreatment devices.
This section describes how to calculate and apply emission adjustment factors for engines using aftertreatment technology with infrequent regeneration events that may occur during testing. These adjustment factors are typically calculated based on measurements conducted for the purposes of engine certification, and then used to adjust the results of testing related to demonstrating compliance with emission standards. For this section, “regeneration” means an intended event during which emission levels change while the system restores aftertreatment performance. For example, exhaust gas temperatures may increase temporarily to remove sulfur from an adsorber or SCR catalyst or to oxidize accumulated particulate matter in a trap. The duration of this event extends until the aftertreatment performance and emission levels have returned to normal baseline levels. Also, “infrequent” refers to regeneration events that are expected to occur on average less than once over a transient or ramped-modal duty cycle, or on average less than once per mode in a discrete-mode test.
(a) Apply adjustment factors based on whether there is active regeneration during a test segment. The test segment may be a test interval or a full duty cycle, as described in paragraph (b) of this section. For engines subject to standards over more than one duty cycle, you must develop adjustment factors under this section for each separate duty cycle. You must be able to identify active regeneration in a way that is readily apparent during all testing. All adjustment factors for regeneration are additive.
(1) If active regeneration does not occur during a test segment, apply an upward adjustment factor, UAF, that will be added to the measured emission rate for that test segment. Use the following equation to calculate UAF:
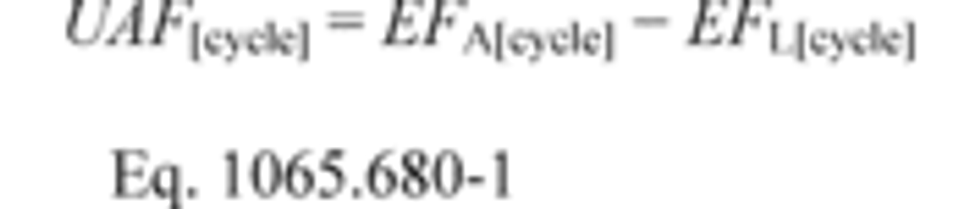
Where:
EFA[cycle] = the average emission factor over the test segment as determined in paragraph (a)(4) of this section.
EFL[cycle] = measured emissions over a complete test segment in which active regeneration does not occur.
Example:
EFARMC = 0.15 g/kW·hr
EFLRMC = 0.11 g/kW·hr
UAFRMC = 0.15 − 0.11 = 0.04 g/kW·hr
(2) If active regeneration occurs or starts to occur during a test segment, apply a downward adjustment factor, DAF, that will be subtracted from the measured emission rate for that test segment. Use the following equation to calculate DAF:
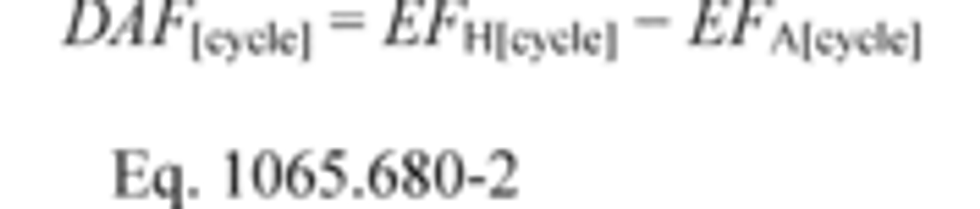
Where:
EFH[cycle] = measured emissions over the test segment from a complete regeneration event, or the average emission rate over multiple complete test segments with regeneration if the complete regeneration event lasts longer than one test segment.
Example:
EFARMC = 0.15 g/kW·hr
EFHRMC = 0.50 g/kW·hr
DAFRMC = 0.50 − 0.15 = 0.35 g/kW·hr
(3) Note that emissions for a given pollutant may be lower during regeneration, in which case EFL would be greater than EFH, and both UAF and DAF would be negative.
(4) Calculate the average emission factor, EFA, as follows:
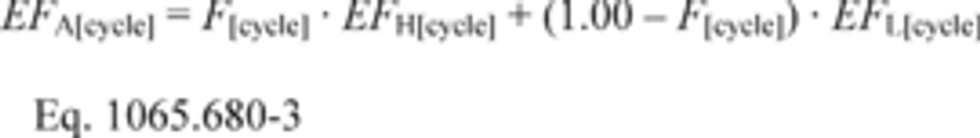
Where:
F[cycle] = the frequency of the regeneration event during the test segment, expressed in terms of the fraction of equivalent test segments during which active regeneration occurs, as described in paragraph (a)(5) of this section.
Example:
FRMC = 0.10
EFARMC = 0.10 · 0.50 + (1.00 − 0.10) · 0.11 = 0.15 g/kW·hr
(5) The frequency of regeneration, F, generally characterizes how often a regeneration event occurs within a series of test segments. Determine F using the following equation, subject to the provisions of paragraph (a)(6) of this section:
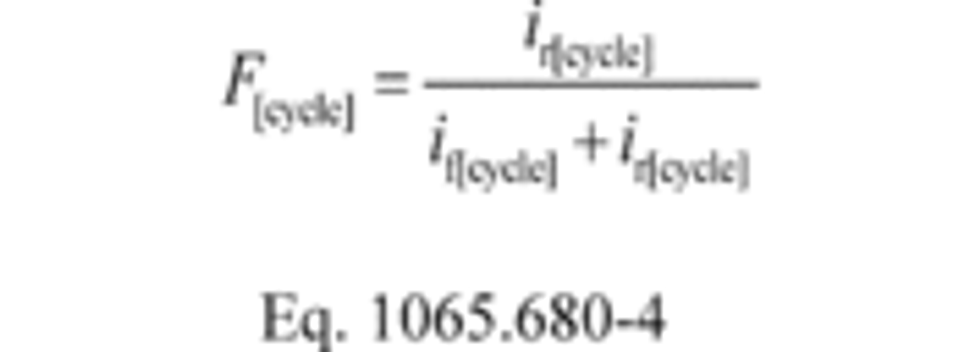
Where:
ir[cycle] = the number of successive test segments required to complete an active regeneration, rounded up to the next whole number.
if[cycle] = the number of test segments from the end of one complete regeneration event to the start of the next active regeneration, without rounding.
Example:
irRMC = 2
ifRMC = 17.86
(6) Use good engineering judgment to determine ir and if, as follows:
(i) For engines that are programmed to regenerate after a specific time interval, you may determine the duration of a regeneration event and the time between regeneration events based on the engine's design parameters. For other engines, determine these values based on measurements from in-use operation or from running repetitive duty cycles in a laboratory.
(ii) For engines subject to standards over multiple duty cycles, such as for transient and steady-state testing, apply this same calculation to determine a value of F for each duty cycle.
(iii) Consider an example for an engine that is designed to regenerate its PM filter 500 minutes after the end of the last regeneration event, with the regeneration event lasting 30 minutes. If the RMC takes 28 minutes, irRMC = 2 (30 ÷ 28 = 1.07, which rounds up to 2), and ifRMC = 500 ÷ 28 = 17.86.
(b) Develop adjustment factors for different types of testing as follows:
(1) Discrete-mode testing. Develop separate adjustment factors for each test mode (test interval) of a discrete-mode test. When measuring EFH, if a regeneration event has started but is not complete when you reach the end of the sampling time for a test interval, extend the sampling period for that test interval until the regeneration event is complete.
(2) Ramped-modal and transient testing. Develop a separate set of adjustment factors for an entire ramped-modal cycle or transient duty cycle. When measuring EFH, if a regeneration event has started but is not complete when you reach the end of the duty cycle, start the next repeat test as soon as possible, allowing for the time needed to complete emission measurement and installation of new filters for PM measurement; in that case EFH is the average emission level for the test segments that included regeneration.
(3) Accounting for cold-start measurements. For engines subject to cold-start testing requirements, incorporate cold-start operation into your analysis as follows:
(i) Determine the frequency of regeneration, F, in a way that incorporates the impact of cold-start operation in proportion to the cold-start weighting factor specified in the standard-setting part. You may use good engineering judgment to determine the effect of cold-start operation analytically.
(ii) Treat cold-start testing and hot-start testing together as a single test segment for adjusting measured emission results under this section. Apply the adjustment factor to the composite emission result.
(iii) You may apply the adjustment factor only to the hot-start test result if your aftertreatment technology does not regenerate during cold operation as represented by the cold-start transient duty cycle. If we ask for it, you must demonstrate this by engineering analysis or by test data.
(c) If an engine has multiple regeneration strategies, determine and apply adjustment factors under this section separately for each type of regeneration.
[81 FR 74189, Oct. 25, 2016; 88 FR 4686, Jan. 24, 2023]
§1065.690 Buoyancy correction for PM sample media.
(a) General. Correct PM sample media for their buoyancy in air if you weigh them on a balance. The buoyancy correction depends on the sample media density, the density of air, and the density of the calibration weight used to calibrate the balance. The buoyancy correction does not account for the buoyancy of the PM itself, because the mass of PM typically accounts for only (0.01 to 0.10)% of the total weight. A correction to this small fraction of mass would be at the most 0.010%.
(b) PM sample media density. Different PM sample media have different densities. Use the known density of your sample media, or use one of the densities for some common sampling media, as follows:
(1) For PTFE-coated borosilicate glass, use a sample media density of 2300 kg/m 3.
(2) For PTFE membrane (film) media with an integral support ring of polymethylpentene that accounts for 95% of the media mass, use a sample media density of 920 kg/m 3.
(3) For PTFE membrane (film) media with an integral support ring of PTFE, use a sample media density of 2144 kg/m 3.
(c) Air density. Because a PM balance environment must be tightly controlled to an ambient temperature of (22 ±1)°C and humidity has an insignificant effect on buoyancy correction, air density is primarily a function of atmospheric pressure. Therefore you may use nominal constant values for temperature and humidity when determining the air density of the balance environment in Eq. 1065.690-2.
(d) Calibration weight density. Use the stated density of the material of your metal calibration weight. The example calculation in this section uses a density of 8000 kg/m 3, but you should know the density of your weight from the calibration weight supplier or the balance manufacturer if it is an internal weight.
(e) Correction calculation. Correct the PM sample media for buoyancy using the following equations:
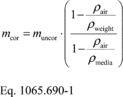
Where:
mcor = PM mass corrected for buoyancy.
muncor = PM mass uncorrected for buoyancy.
rair = density of air in balance environment.
rweight = density of calibration weight used to span balance.
rmedia = density of PM sample media, such as a filter.
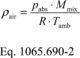
Where:
pabs = absolute pressure in balance environment.
Mmix = molar mass of air in balance environment.
R = molar gas constant.
Tamb = absolute ambient temperature of balance environment.


[70 FR 40516, July 13, 2005, as amended at 73 FR 37339, June 30, 2008; 75 FR 23056, Apr. 30, 2010; 79 FR 23805, Apr. 28, 2014; 81 FR 74191, Oct. 25, 2016]
§1065.695 Data requirements.
(a) To determine the information we require from engine tests, refer to the standard-setting part and request from your EPA Program Officer the format used to apply for certification or demonstrate compliance. We may require different information for different purposes, such as for certification applications, approval requests for alternate procedures, selective enforcement audits, laboratory audits, production-line test reports, and field-test reports.
(b) See the standard-setting part and §1065.25 regarding recordkeeping.
(c) We may ask you the following about your testing, and we may ask you for other information as allowed under the Act:
(1) What approved alternate procedures did you use? For example:
(i) Partial-flow dilution for proportional PM.
(ii) CARB test procedures.
(iii) ISO test procedures.
(2) What laboratory equipment did you use? For example, the make, model, and description of the following:
(i) Engine dynamometer and operator demand.
(ii) Probes, dilution, transfer lines, and sample preconditioning components.
(iii) Batch storage media (such as the bag material or PM filter material).
(3) What measurement instruments did you use? For example, the make, model, and description of the following:
(i) Speed and torque instruments.
(ii) Flow meters.
(iii) Gas analyzers.
(iv) PM balance.
(4) When did you conduct calibrations and performance checks and what were the results? For example, the dates and results of the following:
(i) Linearity verification.
(ii) Interference checks.
(iii) Response checks.
(iv) Leak checks.
(v) Flow meter checks.
(5) What engine did you test? For example, the following:
(i) Manufacturer.
(ii) Family name on engine label.
(iii) Model.
(iv) Model year.
(v) Identification number.
(6) How did you prepare and configure your engine for testing? Consider the following examples:
(i) Dates, hours, duty cycle and fuel used for service accumulation.
(ii) Dates and description of scheduled and unscheduled maintenance.
(iii) Allowable pressure range of intake restriction.
(iv) Allowable pressure range of exhaust restriction.
(v) Charge air cooler volume.
(vi) Charge air cooler outlet temperature, specified engine conditions and location of temperature measurement.
(vii) Fuel temperature and location of measurement.
(viii) Any aftertreatment system configuration and description.
(ix) Any crankcase ventilation configuration and description (e.g., open, closed, PCV, crankcase scavenged).
(x) Number and type of preconditioning cycles.
(7) How did you test your engine? For example:
(i) Constant speed or variable speed.
(ii) Mapping procedure (step or sweep).
(iii) Continuous or batch sampling for each emission.
(iv) Raw or dilute sampling; any dilution-air background sampling.
(v) Duty cycle and test intervals.
(vi) Cold-start, hot-start, warmed-up running.
(vii) Absolute pressure, temperature, and dewpoint of intake and dilution air.
(viii) Simulated engine loads, curb idle transmission torque value.
(ix) Warm-idle speed value.
(x) Simulated vehicle signals applied during testing.
(xi) Bypassed governor controls during testing.
(xii) Date, time, and location of test (e.g., dynamometer laboratory identification).
(xiii) Cooling medium for engine and charge air.
(xiv) Operating temperatures of coolant, head, and block.
(xv) Natural or forced cool-down and cool-down time.
(xvi) Canister loading.
(8) How did you validate your testing? For example, results from the following:
(i) Duty cycle regression statistics for each test interval.
(ii) Proportional sampling.
(iii) Drift.
(iv) Reference PM sample media in PM-stabilization environment.
(v) Carbon balance error verification, if performed.
(9) How did you calculate results? For example, results from the following:
(i) Drift correction.
(ii) Noise correction.
(iii) “Dry-to-wet” correction.
(iv) NMHC, CH4, and contamination correction.
(v) Chemical balance method—carbon-based or hydrogen-based chemical balance method.
(vi) NOX humidity correction.
(vii) Brake-specific emission formulation - total mass divided by total work, mass rate divided by power, or ratio of mass to work.
(viii) Rounding emission results.
(10) What were the results of your testing? For example:
(i) Maximum mapped power and speed at maximum power.
(ii) Maximum mapped torque and speed at maximum torque.
(iii) For constant-speed engines: no-load governed speed.
(iv) For constant-speed engines: test torque.
(v) For variable-speed engines: maximum test speed.
(vi) Speed versus torque map.
(vii) Speed versus power map.
(viii) Brake-specific emissions over the duty cycle and each test interval.
(ix) Brake-specific fuel consumption.
(11) What fuel did you use? For example:
(i) Fuel that met specifications of subpart H of this part.
(ii) Alternate fuel.
(iii) Oxygenated fuel.
(12) How did you field test your engine? For example:
(i) Data from paragraphs (c)(1), (3), (4), (5), and (9) of this section.
(ii) Probes, dilution, transfer lines, and sample preconditioning components.
(iii) Batch storage media (such as the bag material or PM filter material).
(iv) Continuous or batch sampling for each emission.
(v) Raw or dilute sampling; any dilution air background sampling.
(vi) Cold-start, hot-start, warmed-up running.
(vii) Intake and dilution air absolute pressure, temperature, dewpoint.
(viii) Curb idle transmission torque value.
(ix) Warm idle speed value, any enhanced-idle speed value.
(x) Date, time, and location of test (e.g., identify the testing laboratory).
(xi) Proportional sampling validation.
(xii) Drift validation.
(xiii) Operating temperatures of coolant, head, and block.
(xiv) Vehicle make, model, model year, identification number.
[70 FR 40516, July 13, 2005, as amended at 73 FR 37339, June 30, 2008; 79 FR 23807, Apr. 28, 2014; 86 FR 34568, Jun. 29, 2021; 88 FR 4687, Jan. 24, 2023; 89 FR 29823, Apr. 22, 2024]
['Air Programs']
['Air Emissions']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
