Measles vaccines and treatment
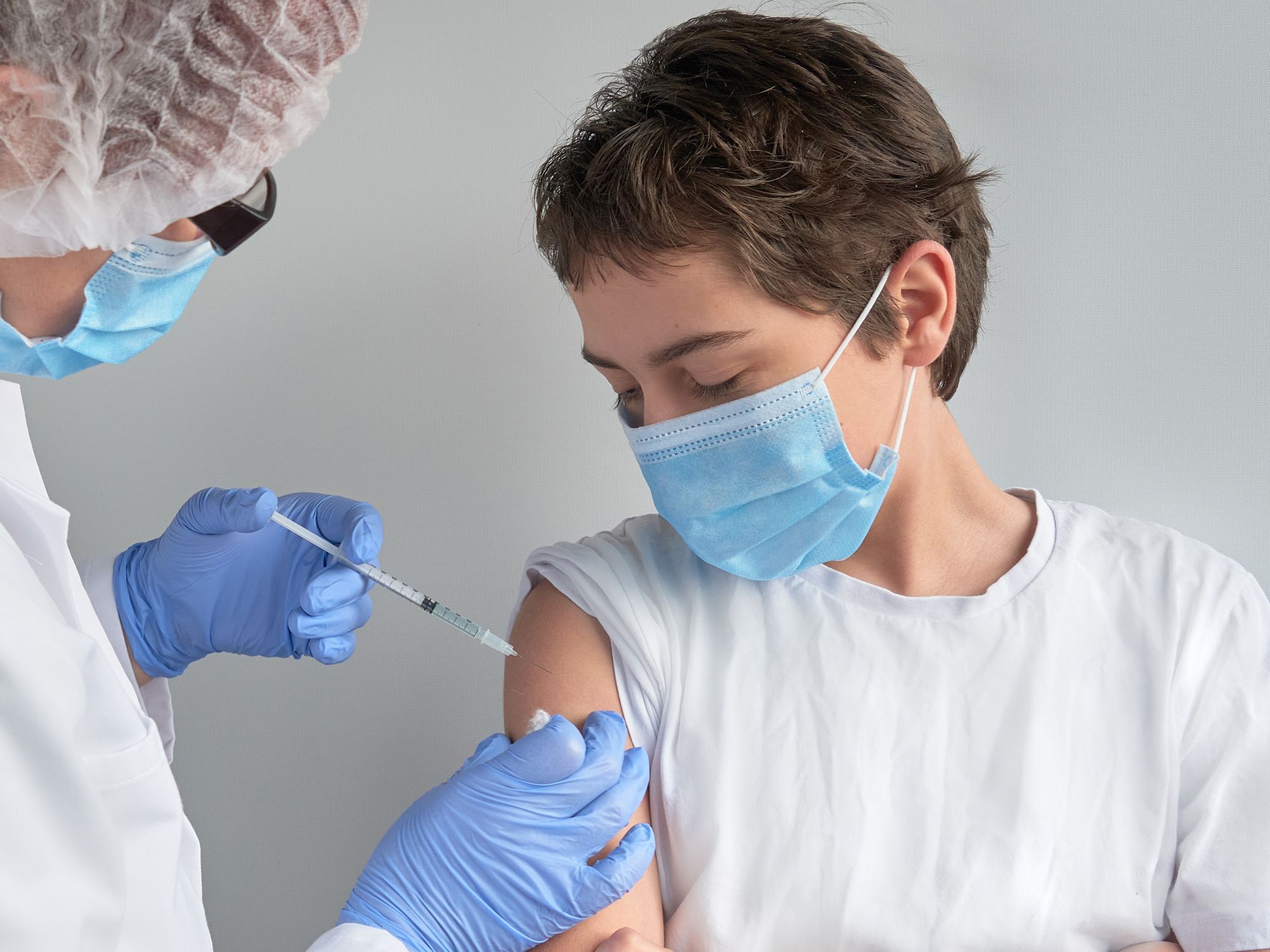
- The CDC says the MMR vaccine for measles is very safe and effective. Adults who did not receive the vaccine as children may still receive it.
- The MMR vaccine is also effective at preventing measles when administered within 72 hours of an exposure.
Most people born in the United States since the early 1970s have been vaccinated for measles, as the Centers for Disease Control and Prevention (CDC) recommends children get two doses of the measles, mumps, and rubella (MMR) vaccine, with the first dose at 12–15 months of age and the second dose at four to six years of age. Adults who did not receive the vaccine as children can still be vaccinated.
According to the CDC, the MMR vaccine is very safe and effective. Two doses of the MMR vaccine are about 97 percent effective at providing immunity to measles (i.e., immunogenicity); one dose is about 93 percent effective. Because measles is highly contagious, about 90 percent of unvaccinated people exposed to measles will get the disease.
Vaccine-related complications are typically infrequent and, when they do occur, mild (e.g., fever, rash). The CDC has found no evidence that any vaccine causes autism or autism spectrum disorder (ASD). By definition of ASD as a developmental disability under the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM-5), adults, including those who receive the MMR or any other vaccine, do not develop autism later in life; cases of ASD diagnosed in adults have been present since childhood but not recognized until later in life.
The MMR vaccine is also effective at preventing measles when administered to a susceptible person within 72 hours following exposure. Because of the short period after exposure during which the vaccine is effective, healthcare providers may opt to give exposed individuals who may not be immune to the virus an additional dose of vaccine rather than waiting for laboratory testing to determine immunity. Unless otherwise contraindicated, extra doses of MMR are not harmful.
Immunoglobulin (IG) may prevent or lessen the severity of measles disease in susceptible people when given within six days following exposure and may be recommended for people who have contraindications for receiving the vaccine. (Note: healthcare providers should consult CDC recommendations for post-exposure prophylaxis, including guidelines for administering the MMR vaccine or IG, but not both.)
Testing for measles
The CDC recommends that healthcare professionals obtain a throat swab (specifically, from the nasopharynx) and blood specimen from all patients with clinical features compatible with measles (i.e., symptoms of the disease).
Healthcare professionals can contact their state and/or local health department to determine where to submit specimens and how to ship them. For information on sending specimens to the CDC from within the United States, please visit the CDC page on specimen collection, storage, and shipment.
