['Water Programs']
['Water Programs']
03/31/2023
...
ENVIRONMENTAL PROTECTION AGENCY
[EPA-HQ-OW-2022-0114; FRL 8543-01-OW]
RIN 2040-AG18
PFAS National Primary Drinking Water Regulation Rulemaking
AGENCY: Environmental Protection Agency (EPA).
ACTION: Preliminary regulatory determination and proposed rule; request for public comment; notice of public hearing.
SUMMARY: The Environmental Protection Agency (EPA) is committed to using and advancing the best available science to tackle per- and polyfluoroalkyl substances (PFAS) pollution, protect public health, and harmonize policies that strengthen public health protections with infrastructure funding to help communities, especially disadvantaged communities, deliver safe drinking water. In March 2021, EPA issued a final regulatory determination to regulate perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) as contaminants under Safe Drinking Water Act (SDWA). In this notice, EPA is issuing a preliminary regulatory determination to regulate perfluorohexane sulfonic acid (PFHxS), hexafluoropropylene oxide dimer acid (HFPO-DA) and its ammonium salt (also known as a GenX chemicals), perfluorononanoic acid (PFNA), and perfluorobutane sulfonic acid (PFBS), and mixtures of these PFAS as contaminants under SDWA. Through this action, EPA is also proposing a National Primary Drinking Water Regulation (NPDWR) and health-based Maximum Contaminant Level Goals (MCLG) for these four PFAS and their mixtures as well as for PFOA and PFOS. EPA is proposing to set the health-based value, the MCLG, for PFOA and PFOS at zero. Considering feasibility, including currently available analytical methods to measure and treat these chemicals in drinking water, EPA is proposing individual MCLs of 4.0 nanograms per liter (ng/L) or parts per trillion (ppt) for PFOA and PFOS. EPA is proposing to use a Hazard Index (HI) approach to protecting public health from mixtures of PFHxS, HFPO-DA and its ammonium salt, PFNA, and PFBS because of their known and additive toxic effects and occurrence and likely co-occurrence in drinking water. EPA is proposing an HI of 1.0 as the MCLGs for these four PFAS and any mixture containing one or more of them because it represents a level at which no known or anticipated adverse effects on the health of persons is expected to occur and which allows for an adequate margin of safety. EPA has determined it is also feasible to set the MCLs for these four PFAS and for a mixture containing one or more of PFHxS, HFPO-DA and its ammonium salt, PFNA, PFBS as an HI of unitless 1.0. The Agency is requesting comment on this action, including this proposed NPDWR and MCLGs, and have identified specific areas where public input will be helpful for EPA in developing the final rule. In addition to seeking written input, the EPA will be holding a public hearing on May 4, 2023.
DATES: Comments must be received on or before May 30, 2023. Comments on the information collection provisions submitted to the Office of Management and Budget (OMB) under the Paperwork Reduction Act (PRA) are best assured of consideration by OMB if OMB receives a copy of your comments on or before April 28, 2023. Public hearing: EPA will hold a virtual public hearing on May 4, 2023, at https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas. Please refer to the SUPPLEMENTARY INFORMATION section for additional information on the public hearing.
ADDRESSES: You may send comments, identified by Docket ID No. EPA-HQ-OW-2022-0114 by any of the following methods:
• Federal eRulemaking Portal: https://www.regulations.gov/ (our preferred method). Follow the online instructions for submitting comments.
• Mail: U.S. Environmental Protection Agency, EPA Docket Center, Office of Ground Water and Drinking Water Docket, Mail Code 2822IT, 1200 Pennsylvania Avenue NW, Washington, DC 20460.
• Hand Delivery or Courier: EPA Docket Center, WJC West Building, Room 3334, 1301 Constitution Avenue NW, Washington, DC 20004. The Docket Center's hours of operations are 8:30 a.m. to 4:30 p.m., Monday through Friday (except Federal Holidays).
Instructions: All submissions received must include the Docket ID No. for this rulemaking. Comments received may be posted without change to https://www.regulations.gov/, including any personal information provided. For detailed instructions on sending comments and additional information on the rulemaking process, see the “Public Participation” heading of the SUPPLEMENTARY INFORMATION section of this document.
FOR FURTHER INFORMATION CONTACT:
Alexis Lan, Office of Ground Water and Drinking Water, Standards and Risk Management Division (Mail Code 4607M), Environmental Protection Agency, 1200 Pennsylvania Avenue NW, Washington, DC 20460; telephone number 202-564-0841; email address: PFASNPDWR@epa.gov
SUPPLEMENTARY INFORMATION:
Executive Summary
In March 2021, EPA issued a final regulatory determination to regulate perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) as contaminants under Safe Drinking Water Act (SDWA). EPA is issuing a preliminary regulatory determination to regulate perfluorohexane sulfonic acid (PFHxS), hexafluoropropylene oxide dimer acid (HFPO-DA) and its ammonium salt (also known as a GenX chemicals), perfluorononanoic acid (PFNA), and perfluorobutane sulfonic acid (PFBS), and mixtures of these PFAS as contaminants under SDWA (see section III of this preamble for additional discussion on EPA's preliminary regulatory determination). Through this action, EPA is also proposing a National Primary Drinking Water Regulation (NPDWR) and health-based Maximum Contaminant Level Goals (MCLG) for these four PFAS and their mixtures as well as for PFOA and PFOS. Exposure to these PFAS may cause adverse health effects, and all are likely to occur in drinking water.
PFAS are a large family of synthetic chemicals that have been in use since the 1940s. Many of these compounds have unique physical and chemical properties that make them highly stable and resistant to degradation in the environment—colloquially termed “forever chemicals.” People can be exposed to PFAS through certain consumer products, occupational contact, and/or by consuming food and drinking water that contain PFAS (see section II.C of this preamble for additional discussion on PFAS chemistry, production, and uses). Current scientific evidence indicates that consuming water containing the PFAS covered in this proposed regulation above certain levels can result in harmful health effects. Depending on the individual PFAS, health effects can include negative impacts on fetal growth after exposure during pregnancy, on other aspects of development, reproduction, liver, thyroid, immune function, and/or the nervous system; and increased risk of cardiovascular and/or certain types of cancers, and other health impacts (see section II.B and III.B of this preamble for additional discussion on health effects).
This proposed PFAS drinking water regulation contains several key features. Based on a review of the best available health effects data, EPA is proposing MCLGs that address six PFAS. An MCLG is the maximum level of a contaminant in drinking water at which no known or anticipated adverse effect on the health of persons would occur, allowing an adequate margin of safety. A contaminant means any “physical, chemical or biological or radiological substance or matter in water.” This proposal addresses contaminants and certain mixtures of contaminants. Through this action, EPA is also proposing enforceable standards which takes the form of maximum contaminant levels (MCLs) in this proposed regulation. An MCL is the maximum level allowed of a contaminant or a group of contaminants ( i.e., mixture of contaminants) in water which is delivered to any user of a public water system (PWS). The SDWA generally requires EPA to set an MCL “as close as feasible to” the MCLG. EPA has also included monitoring, reporting, and other requirements to ensure regulated drinking water systems, known as a PWS, meet the PFAS limits in the regulation.
Following a systematic review of available human epidemiological and animal toxicity studies, EPA has determined that PFOA and PFOS are likely to cause cancer ( e.g., kidney and liver cancer) and that there is no dose below which either chemical is considered safe (see section IV.A and V.A through B of this preamble for additional discussion). Therefore, EPA is proposing to set the health-based value, the MCLG, for both of these contaminants at zero. Considering feasibility, including currently available analytical methods to measure and treat these chemicals in drinking water, EPA is proposing individual MCLs of 4.0 nanograms per liter (ng/L) or parts per trillion (ppt) for PFOA and PFOS (see sections VI.C and VIII of this preamble for additional discussion on the MCLs and practical quantitation limits [PQLs]).
Due to their widespread use and persistence, many PFAS are known to co-occur in drinking water and the environment—meaning that these compounds are often found together and in different combinations as mixtures (see section III.C and VII of this preamble for additional discussion on occurrence). PFAS disrupt signaling of multiple biological pathways resulting in common adverse effects on several biological systems and functions, including thyroid hormone levels, lipid synthesis and metabolism, development, and immune and liver function. Additionally, EPA's examination of health effects information found that exposure through drinking water to a mixture of PFAS can be assumed to act in a dose-additive manner (see sections III.B and IV.B of this preamble for additional discussion on mixture toxicity). This dose additivity means that low levels of multiple PFAS, that individually would not likely result in adverse health effects, when combined in a mixture are expected to result in adverse health effects. As a result, EPA is proposing to use a Hazard Index (HI) approach to protecting public health from mixtures of four PFAS: PFHxS, HFPO-DA and its ammonium salt (also known as GenX chemicals), PFNA, and PFBS because of their known and additive toxic effects and occurrence and likely co-occurrence in drinking water. PFOA and PFOS are being proposed for separate MCLs and not included in the HI because their individual proposed MCLGs are zero, and the level at which no known or anticipated adverse effects on the health of persons is expected to occur is well below current analytical quantitation levels. Based on our current understanding of health effects, this is not the case for the other covered PFAS. Because of the analytical limitations for PFOA and PFOS, the MCL for these two PFAS is set at the lowest feasible quantitation level and any exceedance of this limit requires action to protect public health, regardless of any mixture in which they are found. As a result, EPA is not proposing to include PFOA or PFOS in the HI.
The HI is a commonly used risk management approach for mixtures of chemicals (USEPA, 1986a; 2000a). In this approach, a ratio called a hazard quotient (HQ) is calculated for each of the four PFAS (PFHxS, HFPO-DA and its ammonium salt (also known as GenX chemicals), PFNA, and PFBS) by dividing an exposure metric, in this case, the measured level of each of the four PFAS in drinking water, by a health reference value for that particular PFAS. For health reference values, in this proposal, EPA is using Health Based Water Concentration (HBWCs) as follows: 9.0 ppt for PFHxS, 10.0 ppt for HFPO-DA; 10.0 ppt for PFNA; and 2000 ppt for PFBS (USEPA, 2023a). The individual PFAS ratios (HQs) are then summed across the mixture to yield the HI. If the resulting HI is greater than one (1.0), then the exposure metric is greater than the health metric and potential risk is indicated. EPA's Science Advisory Board (SAB) opined that where the health endpoints of the chosen compounds are similar, it is reasonable to use an HI as “a reasonable approach for estimating the potential aggregate health hazards associated with the occurrence of chemical mixtures in environmental media.” (USEPA, 2022a). The HI provides an indication of overall potential risk of a mixture as well as individual PFAS that are potential drivers of risk (those PFAS(s) with high(er) ratios of exposure to health metrics) (USEPA, 2000a; see section IV.B and V.C of this preamble for additional discussion on the HI and its derivation). Therefore, EPA is proposing an HI of 1.0 as the MCLGs for these four PFAS and any mixture containing one or more of them because it represents a level at which no known or anticipated adverse effects on the health of persons is expected to occur and which allows for an adequate margin of safety. EPA has determined it is also feasible to set the MCLs for these four PFAS and for a mixture containing one or more of PFHxS, HFPO-DA and its ammonium salt, PFNA, PFBS as an HI of unitless 1.0 (see sections V.C and VI.B of this preamble for discussion of the HI MCLG and MCL, respectively).
Monitoring is a core component of a NPDWR and assures that water systems are providing necessary public health protections (see section IX of this preamble for additional discussion on monitoring and compliance requirements). EPA is therefore proposing requirements for systems to monitor for PFOA, PFOS, PFHxS, HFPO-DA and its ammonium salt, PFNA, and PFBS in drinking water that build upon EPA's Standardized Monitoring Framework (SMF) for Synthetic Organic Compounds (SOCs) where the monitoring frequency for any PWS depends on previous monitoring results. This proposal includes flexibilities related to monitoring, including flexibilities for systems to use certain, previously collected data to satisfy initial monitoring requirements in this proposal as well as reduced monitoring requirements in certain circumstances (see section IX.E of this preamble for additional discussion on monitoring waivers).
In summary, the proposed MCLs for PFOA and PFOS are 4 ng/L (individually), and the proposed MCL of an HI of 1.0 for any mixture containing PFHxS, HFPO-DA and its ammonium salt, PFNA, and/or PFBS. Water systems with PFAS levels that exceed the proposed MCLs would need to take action to provide safe and reliable drinking water. These systems may install water treatment or consider other options such as using a new uncontaminated source water or connecting to an uncontaminated water system. Activated carbon, anion exchange (AIX) and high-pressure membrane technologies have all been demonstrated to remove PFAS, including PFOA, PFOS, PFHxS, HFPO-DA and its ammonium salt, PFNA, and PFBS, from drinking water systems. These treatment technologies can be installed at a water system's treatment plant and are also available through in-home filter options (see section XI of this preamble for additional discussion on available treatment technologies).
As part of its health risk reduction and cost analysis, SDWA requires an evaluation of quantifiable and nonquantifiable health risk reduction benefits and costs. SDWA also requires that EPA considers quantifiable and nonquantifiable health risk reduction benefits from reductions in co-occurring contaminants. The SDWA also requires that EPA determine if the benefits of the proposed rule justify the costs. In accordance with these requirements, the EPA Administrator has determined that the quantified and nonquantifiable benefits of the proposed PFAS NPDWR justify the costs (see section XIII of this preamble for additional discussion on EPA's Health Risk Reduction and Cost Analysis [HRRCA]). Among other things, EPA evaluated which entities which would be affected by the rule, quantified costs using available data and statical models, and described unquantifiable costs. EPA also quantified benefits by estimating reduced cardiovascular events ( e.g., heart attacks and strokes), developmental impacts to fetuses and infants, and reduced cases of kidney cancer. EPA has also quantified benefits by estimating reduced bladder cancer cases caused by reduced disinfection byproduct (DBP) formation in some systems that install treatment to meet the requirements of this rule. EPA has also developed a qualitative summary of benefits expected to result from the removal of regulated PFAS and additional co-removed PFAS contaminants.
To help communities on the frontlines of PFAS contamination, the passage of the Infrastructure Investment and Jobs Act, also referred to as the Bipartisan Infrastructure Law (BIL), invests over $11.7 billion in the Drinking Water State Revolving Fund (SRF); $4 billion to the Drinking Water SRF for Emerging Contaminants; and $5 billion to Small, Underserved, and Disadvantaged Communities Grants. These funds will assist many disadvantaged communities, small systems, and others with the costs of installation of treatment when it might otherwise be cost-challenging.
Public participation and consultations with key stakeholders are critical in developing an implementable and public health protective rule. EPA has engaged with many stakeholders and consulted with entities such as the SAB, and the National Drinking Water Advisory Council (NDWAC) in developing this proposed rule (see section XV of this preamble on EPA's Statutory and Executive Order reviews). The Agency is requesting comment on this action, including this proposed NPDWR and MCLGs, and have identified specific areas where public input will be helpful for EPA in developing the final rule (see section XIV of this preamble on specific topics highlighted for public comment). In addition to seeking written input, EPA will be holding a public hearing on May 4th, 2023.
I. Public Participation
A. Written Comments
Submit your comments, identified by Docket ID No. EPA-HQ-OW-2022-0114, at https://www.regulations.gov (our preferred method), or the other methods identified in the ADDRESSES section. Once submitted, comments cannot be edited or removed from the docket. EPA may publish any comment received to its public docket. Do not submit to EPA's docket at https://www.regulations.gov any information you consider to be Confidential Business Information (CBI), Proprietary Business Information (PBI), or other information whose disclosure is restricted by statute. Multimedia submissions (audio, video, etc.) must be accompanied by a written comment. The written comment is considered the official comment and should include discussion of all points you wish to make. EPA will generally not consider comments or comment contents located outside of the primary submission ( i.e., on the web, cloud, or other file sharing system). Please visit https://www.epa.gov/dockets/commenting-epa-dockets for additional submission methods; the full EPA public comment policy; information about CBI, PBI, or multimedia submissions; and general guidance on making effective comments.
B. Participation in Virtual Public Hearing
EPA will hold a public hearing on May 4th, 2023, to receive public comment and will present the proposed requirements of the draft NPDWR. The hearing will be held virtually from approximately 11 a.m. until 7 p.m. eastern time. EPA will begin registering speakers for the hearing upon publication of this document in the Federal Register (FR). To attend and register to speak at the virtual hearing, please use the online registration form available at https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas. The last day to pre-register to speak at the hearing will be April 28, 2023. On May 3, 2023, EPA will post a general agenda for the hearing that will list pre-registered speakers in approximate order at: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas. The number of online connections available for the hearing is limited and will be offered on a first- come, first-served basis. To submit visual aids to support your oral comment, please contact PFASNPDWR@epa.gov for guidelines and instructions. Registration will remain open for the duration of the hearing itself for those wishing to provide oral comment during unscheduled testimony; however, early registration is strongly encouraged to ensure proper accommodations and adequate timing.
EPA will make every effort to follow the schedule as closely as possible on the day of the hearing; however, please plan for the hearings to run either ahead of schedule or behind schedule. Please note that the public hearing may close early if all business is finished.
EPA encourages commenters to provide EPA with a written copy of their oral testimony electronically by submitting it to the public docket at www.regulations.gov, Docket ID: EPA-HQ-OW-2022-0114. Oral comments will be time limited to allow for maximum participation, which may result in the full statement not being heard. Therefore, EPA also recommends submitting the text of your oral comments as written comments to the rulemaking docket. Any person not making an oral statement may also submit a written statement. Written statements and supporting information submitted during the comment period will be considered with the same weight as oral comments and supporting information presented at the public hearing.
Please note that any updates made to any aspect of the hearing are posted online at https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas. While EPA expects the hearing to go forward as set forth above, please monitor our website or contact PFASNPDWR@epa.gov to determine if there are any updates. EPA does not intend to publish a document in the Federal Register announcing updates.
If you require any accommodations such as language translation, captioning, or other special accommodations for the day of the hearing, please indicate this as a part of your registration and describe your needs by April 28, 2023. EPA may not be able to arrange accommodations without advance notice. Please contact PFASNPDWR@epa.gov with any questions related to the public hearing.
This proposed rule is organized as follows:
I. General Information
A. What is EPA proposing?
B. Does this action apply to me?
II. Background
A. What are PFAS?
B. Definitions
C. Chemistry, Production and Uses
D. Human Health Effects
E. Statutory Authority
F. Statutory Framework and PFAS Regulatory History
G. Bipartisan Infrastructure Law
H. EPA PFAS Strategic Roadmap
III. Preliminary Regulatory Determinations for Additional PFAS
A. Agency Findings
B. Statutory Criterion 1—Adverse Health Effects
C. Statutory Criterion 2—Occurrence
D. Statutory Criterion 3—Meaningful Opportunity
E. EPA's Preliminary Regulatory Determination Summary for PFHxS, HFPO-DA, PFNA, and PFBS
F. Request for Comment on EPA's Preliminary Regulatory Determination for PFHxS, HFPO-DA, PFNA, and PFBS
IV. Approaches to MCLG Derivation
A. Approach to MCLG Derivation for Individual PFAS
B. Approach to MCLG Derivation for a PFAS Mixture
V. Maximum Contaminant Level Goals
A. PFOA
B. PFOS
C. PFAS Hazard Index: PFHxS, HFPO-DA, PFNA, and PFBS
VI. Maximum Contaminant Levels
A. PFOA and PFOS
B. PFAS Hazard Index: PFHxS, HFPO-DA, PFNA, and PFBS
C. Reducing Public Health Risk by Protecting Against Dose Additive Noncancer Health Effects From PFAS
D. Regulatory Alternatives
E. MCL-Specific Requests for Comment
VII. Occurrence
A. UCMR 3
B. State Drinking Water Data
C. Co-Occurrence
D. Occurrence Relative to the Hazard Index
E. Occurrence Model
F. Combining State Data With Model Output To Estimate National Exceedance of Either MCLs or Hazard Index
VIII. Analytical Methods
A. Practical Quantitation Levels (PQLs) for Regulated PFAS
IX. Monitoring and Compliance Requirements
A. What are the monitoring requirements?
B. How are PWS compliance and violations determined?
C. Can systems use previously collected data to satisfy the initial monitoring requirement?
D. Can systems composite samples?
E. Can primacy agencies grant monitoring waivers?
F. When must systems complete initial monitoring?
G. What are the laboratory certification requirements?
X. Safe Drinking Water Act (SDWA) Right to Know Requirements
A. What are the consumer confidence report requirements?
B. What are the public notification (PN) requirements?
XI. Treatment Technologies
A. What are the best available technologies?
B. PFAS Co-Removal
C. Management of Treatment Residuals
D. What are Small System Compliance Technologies (SSCTs)?
XII. Rule Implementation and Enforcement
A. What are the requirements for primacy?
B. What are the primacy agency record keeping requirements?
C. What are the primacy agency reporting requirements?
D. Exemptions and Extensions
XIII. Health Risk Reduction and Cost Analysis
A. Affected Entities and Major Data Sources Used To Develop the Baseline Water System Characterization
B. Overview of the Cost-Benefit Model
C. Method for Estimating Costs
D. Method for Estimating Benefits
E. Nonquantifiable Benefits of PFOA and PFOS Exposure Reduction
F. Nonquantifiable Benefits of Removal of PFAS Included in the Proposed Regulation and Co-Removed PFAS
G. Benefits Resulting From Disinfection By-Product Co-Removal
H. Comparison of Costs and Benefits
I. Quantified Uncertainties in the Economic Analysis
J. Cost-Benefit Determination
XIV. Request for Comment on Proposed Rule
Section III—Regulatory Determinations for Additional PFAS
Section V—Maximum Contaminant Level Goals
Section VI—Maximum Contaminant Levels
Section VII—Occurrence
Section IX—Monitoring and Compliance Requirements
Section X—Safe Drinking Water Right to Know
Section XI—Treatment Technologies
Section XII—Rule Implementation and Enforcement
Section XIII—HRRCA
Section XV—Statutory and Executive Order Reviews
XV. Statutory and Executive Order Reviews
A. Executive Order 12866: Regulatory Planning and Review and Executive Order 13563 Improving Regulation and Regulatory Review
B. Paperwork Reduction Act (PRA)
C. Regulatory Flexibility Act (RFA)
D. Unfunded Mandates Reform Act (UMRA)
E. Executive Order 13132: Federalism
F. Executive Order 13175: Consultation and Coordination With Indian Tribal Governments
G. Executive Order 13045: Protection of Children From Environmental Health and Safety Risks
H. Executive Order 13211: Actions That Significantly Affect Energy Supply, Distribution, or Use
I. National Technology Transfer and Advancement Act of 1995
J. Executive Order 12898: Federal Actions To Address Environmental Justice in Minority Populations and Low-Income Populations
K. Consultations With the Science Advisory Board, National Drinking Water Advisory Council, and the Secretary of Health and Human Services
XVI. References
I. General Information
A. What is EPA proposing?
EPA is proposing for public comment a drinking water regulation that includes six PFAS. EPA is proposing to establish MCLGs and an NPDWR for these PFAS in public drinking water supplies. EPA proposes MCLGs for PFOA and PFOS at zero (0) and an enforceable MCL for PFOA and PFOS in drinking water at 4.0 ppt. Additionally, the Agency is requesting comment on a preliminary determination to regulate additional PFAS to include PFHxS, HFPO-DA 1 (also known as and referred to as “GenX Chemicals” in this proposal), PFNA, and PFBS. Concurrent with this preliminary determination, EPA is proposing an HI of 1.0 as the MCLG and enforceable MCL to address individual and mixtures of these four contaminants where they occur in drinking water. EPA is proposing to calculate the HI as the sum total of component PFAS HQs, calculated by dividing the measured component PFAS concentration in water by the relevant HBWC. In this proposal, EPA is using HBWCs of 9.0 ppt for PFHxS, 10.0 ppt for HFPO-DA; 10.0 ppt for PFNA; and 2000 ppt for PFBS. The proposed approach to calculating the HI for this set of four PFAS compounds is designed to be protective against all adverse effects, not a single outcome/effect, and is a health protective decision aid for use in determining the level at which there are no adverse effects on the health of persons with an adequate margin of safety, thus is appropriate for MCLG development.
1 PFAS may exist in multiple forms, such as acids and organic or metal salts. Each of these forms may be listed as a separate entry in certain databases and have separate Chemical Abstract Service (CAS) Registry numbers. However, PFAS are expected to dissociate in water to their anionic form. For example, the term “GenX Chemicals” acknowledges the “acid” and “ammonium salt” forms of HFPO-DA as two different chemicals. In water, though, these chemicals dissociate and therefore the resulting anion appears as a single analyte for the purposes of detection and quantitation. Please see “definitions” for more information. EPA notes that the chemical HFPO-DA is used in a processing aid technology developed by DuPont to make fluoropolymers without using PFOA. The chemicals associated with this process are commonly known as GenX Chemicals and the term is often used interchangeably for HFPO-DA along with its ammonium salt (USEPA, 2021b).
The requirements in this proposal that apply to (1) PFOA, (2) PFOS, and (3) PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures are distinct and capable of operating independently.
B. Does this action apply to me?
The preliminary regulatory determination to establish drinking water regulations for certain PFAS and their mixtures and the proposed regulation are proposals for public comment and are not requirements or regulations. Instead, this action notifies interested parties of the availability of information supporting the preliminary regulatory determinations for four PFAS and their mixtures, the development of the NPDWR for six PFAS, and proposed rule requirements for public comment. If EPA proceeds to a final regulatory determination and final regulation, once promulgated, this action will potentially affect the following:
| Category | Examples of potentially affected entities |
|---|---|
| Public water systems 2 | Community water systems (CWSs); Non-transient, non-community water systems (NTNCWSs). |
| State and tribal agencies | Agencies responsible for drinking water regulatory development and enforcement. |
This table is not intended to be exhaustive, but rather provides a guide for readers regarding entities that could be affected by this action once promulgated. To determine whether a facility or activities could be affected by this action, this proposed rule should be carefully examined. Questions regarding the applicability of this action to a particular entity may be directed to the person listed in the FOR FURTHER INFORMATION CONTACT section.
2 The term “public water system” means a system for the provision to the public of water for human consumption through pipes or other constructed conveyances, if such system has at least fifteen service connections or regularly serves at least twenty-five individuals. Such term includes (i) any collection, treatment, storage, and distribution facilities under control of the operator of such system and used primarily in connection with such system, and (ii) any collection or pretreatment storage facilities not under such control which are used primarily in connection with such system.
II. Background
A. What are PFAS?
PFAS are a large class of specialized synthetic chemicals that have been in use since the 1940s (USEPA, 2018a). This proposed regulation only applies to certain PFAS: PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS. People may potentially be exposed to these PFAS through certain consumer products such as textiles ( e.g., seat covers, sail covers, weather protection (Janousek et al., 2019)), leather shoes as well as shoe polish/wax (Norden, 2013; Borg and Ivarsson, 2017), along with cooking/baking wares (Blom and Hanssen 2015; KEMI, 2015; Glüge et al., 2020), occupational contact, and/or by consuming food and drinking water that contain PFAS. Due to their widespread use, physicochemical properties, and prolonged persistence, many PFAS co-occur in exposure media ( e.g., air, water, ice, sediment), and bioaccumulate in tissues and blood of aquatic as well as terrestrial organisms, including humans (Domingo and Nadal, 2019; Fromme et al., 2009). Industrial workers who are involved in manufacturing or processing fluoropolymers, or people who live or recreate near fluoropolymer facilities, may encounter greater exposures; particularly of PFOA, PFNA, as well as HFPO-DA. Firefighters as well as people who live near airfields or military bases may have especially higher exposure to PFHxS and PFBS due to the use of aqueous foam forming film as a fire suppressant. Pregnant and lactating women, as well as children, may be more sensitive to the harmful effects of certain PFAS, for example, PFOA, PFOS, PFNA, and PFBS. For example, studies indicate that PFOA and PFOS exposure above certain levels may result in adverse health effects, including developmental effects to fetuses during pregnancy or to breast- or formula-fed infants, cancer, immunological effects, among others (USEPA, 2023b; USEPA, 2023c). Other PFAS are also documented to result in a range of adverse health effects (USEPA, 2021a; USEPA, 2021b; ATSDR, 2021; NASEM 2022).
Although most United States production of PFOS, PFOA, and PFNA, along with other long-chain PFAS, was phased out and then generally replaced by production of PFBS, PFHxS, HFPO-DA and other PFAS, EPA is aware of ongoing use of PFOS, PFOA, PFNA, and other long-chain PFAS. Domestic production and import of PFOA has been phased out in the United States by the companies participating in the 2010/2015 PFOA Stewardship Program. Small quantities of PFOA may be produced, imported, and used by companies not participating in the PFOA Stewardship Program and some uses of PFOS are ongoing (see 40 Code of Federal Regulations (CFR) §721.9582). EPA is also aware of ongoing use of the chemicals available from existing stocks or newly introduced via imports. Additionally, the environmental persistence of these chemicals and formation as degradation products from other compounds may still contribute to their release in the environment.
B. Definitions
The six PFAS proposed for regulation and their relevant Chemical Abstract Service (CAS) registry numbers are:
• PFOA (C8F15CO2-; CAS: 45285-51-6)
• PFOS (C8F17SO3-; CAS: 45298-90-6)
• PFHxS (C6F13SO3-; CAS: 108427-53-8)
• HFPO-DA (C6F11O3-; CAS: 122499-17-6)
• PFNA (C9F17CO2-; CAS: 72007-68-2)
• PFBS (C4F9SO3-; CAS: 45187-15-3)
These PFAS may exist in multiple forms, such as isomers or associated salts and each form may have a separate CAS Registry number or no CAS at all. Additionally, these compounds have various names under different classification systems. However, at environmentally relevant pHs, these PFAS are expected to dissociate in water to their anionic (negatively charged) forms. For instance, International Union of Pure and Applied Chemistry substance 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) propanoate (CAS: 122499-17-6), also known as HFPO-DA, is an anionic molecule which has an ammonium salt (CAS: 62037-80-3), a conjugate acid (CAS: 13252-13-6), a potassium salt (CAS: 67118-55-2), and an acyl fluoride precursor (CAS: 2062-98-8), among other variations. At environmentally relevant pHs these all dissociate into the propanoate/anion form (CAS: 122499-17-6). Each PFAS listed has multiple variants with differing chemical connectivity but the same molecular composition; these are known as isomers. Commonly, the isomeric composition of PFAS is categorized as `linear,' consisting of an unbranched alkyl chain, or `branched,' encompassing a potentially diverse group of molecules including at least one, but potentially more offshoots from the linear molecule. While broadly similar, isomeric molecules may have differences in chemical properties. The proposed regulation covers all salts, isomers and derivatives of the chemicals listed, including derivatives other than the anionic form which might be created or identified.
C. Chemistry, Production and Uses
PFAS are most commonly and widely used to make products resistant to water, heat, and stains. As a result, they are found in industrial and consumer products such as clothing, food packaging, cookware, cosmetics, carpeting, and fire-fighting foam (AAAS, 2020). Facilities associated with PFAS releases into the air, soil, and water include those for manufacturing, chemical as well as well as product production and military installations (USEPA, 2016a; USEPA, 2016b).
The chemical structures of some PFAS cause them to repel water as well as oil, remain chemically and thermally stable, and exhibit surfactant properties. PFAS have strong, stable carbon-fluorine (C-F) bonds, making them resistant to hydrolysis, photolysis, microbial degradation, and metabolism (Ahrens, 2011; Beach et al., 2006; Buck et al., 2011). These properties are what make PFAS useful for commercial and industrial applications and purposes. However, these are also what make some PFAS extremely persistent in the human body and the environment (Calafat et al., 2007, 2019).
PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS belong to a subset of PFAS known as perfluoroalkyl acids (PFAAs), all of which consist of a perfluorinated alkyl chain connected to an acidic headgroup. Humans are exposed to PFAS due to wide-ranging commercial and industrial applications along with long range migration from sources. The structure of these PFAS contribute to their persistence in the environment as well as their resistance to chemical, biological, and physical degradation processes.
PFOA and PFOS are two of the most widely studied and longest used PFAS. These two compounds have been detected in up to 98 percent of human serum samples taken in biomonitoring studies that are representative of the U.S. general population; however, since PFOA and PFOS have been voluntarily phased out in the U.S., serum concentrations have been declining (CDC, 2019). The sole U.S. manufacturer of PFOS agreed to a voluntary phaseout in 2000, and the last reported production was in 2002 (USEPA, 2000b; USEPA, 2018b; USEPA, 2021c). PFOS has been used as a surfactant or emulsifier in firefighting foam, circuit board etching acids, alkaline cleaners, floor polish, and as a pesticide active ingredient for insect bait traps (HSBD, 2016). PFOA has been used as an emulsifier and surfactant in fluoropolymers (such as in the manufacturing of non-stick products like Teflon©), firefighting foams, cosmetics, grease and lubricants, paints, polishes, and adhesives (HSBD, 2016).
PFNA was historically the second most used surfactant for emulsion polymerization (after PFOA) which was its main use (Buck et al., 2012). Fluorinated surfactants improve the physical properties of the polymer as well as improving the polymerization rate (Glüge et al., 2020). Fluoropolymers are used in many applications because of their unique physical properties such as resistance to high and low temperatures, resistance to chemical and environmental degradation, and nonstick characteristics. Fluoropolymers also have dielectric and fire-resistant properties that have a wide range of electrical and electronic applications, including architecture, fabrics, automotive uses, cabling materials, electronics, pharmaceutical and biotech manufacturing, and semiconductor manufacturing (Gardiner, 2014). Although drying processes can release the surfactants when manufacturing is complete, surfactant residues remain in the finished products (KEMI, 2015). Legacy stocks may still be used and products containing PFNA may still be produced internationally and imported to the U.S. (ATSDR, 2021).
The voluntary phase out caused a shift to alternatives such as per- and polyfluoroalkyl ether carboxylic acids (PFECAs). The chemical HFPO-DA is the most prevalent of these and is used in a processing aid technology developed by DuPont to make fluoropolymers without using PFOA. The chemicals associated with this process are commonly known as GenX Chemicals and the term is often used interchangeably for HFPO-DA along with its ammonium salt (USEPA, 2021b). The most common use for GenX Chemicals is for emulsion polymerization.
Another alternative, PFBS, is mainly used as a water and stain repellent protection for leather, textiles, carpets, and porous hard surfaces, representing 25-50 tons/year of PFBS in mixtures (Norwegian Environment Agency, 2017). PFBS and related chemicals are also used in curatives for fluoroelastomers (Glüge et al., 2020). The curatives are used for manufacturing O-rings, seals, linings, protective clothing, cooking wares, and flame retardants (Norwegian Environment Agency, 2017; Blom and Hanssen, 2015).
PFHxS is used in stain-resistant fabrics, fire-fighting foams, flame retardants, insecticides, and as a surfactant in industrial processes (Glüge et al., 2020). Additionally, particle accelerators including the Delphi Detector at Stanford University rely on liquid PFHxS (Glüge et al., 2020). PFHxS production, along with PFOS, was phased out in 2002 nationwide however, production continues in other countries and products containing PFHxS may be imported into the U.S. (USEPA, 2000c). Legacy stocks may also still be used.
D. Human Health Effects
The publicly available landscape of human epidemiological and experimental animal-based exposure-effect data from repeat-dose studies across PFAS derive primarily from linear carboxylic and sulfonic acid species such as PFOA, PFOS, PFHxS, PFNA, and PFBS (ATSDR, 2021). Many other PFAS have preliminary human health effects data (Mahoney et al., 2022) and some PFAS, such as PFBS and HFPO-DA, have sufficient data that has allowed EPA to derive toxicity values and publish toxicity assessments (USEPA, 2021a; USEPA, 2021b). The adverse health effects observed following oral exposure to such PFAS are significant and diverse and include (but are not limited to): cancer and effects on the liver ( e.g., liver cell death), growth and development ( e.g., low birth weight), hormone levels, kidney, immune system, lipid levels ( e.g., high cholesterol), the nervous system, and reproduction. Please see sections III.B, IV, and V of this preamble for additional discussion on health considerations for the six PFAS EPA is proposing to regulate in this document.
E. Statutory Authority
Section 1412(b)(1)(A) of SDWA requires EPA to establish NPDWRs for a contaminant where the Administrator determines that the contaminant: (1) may have an adverse effect on the health of persons; (2) is known to occur or there is a substantial likelihood that the contaminant will occur in PWSs with a frequency and at levels of public health concern; and (3) where in the sole judgment of the Administrator, regulation of such contaminant presents a meaningful opportunity for health risk reduction for persons served by PWSs.
F. Statutory Framework and PFAS Regulatory History
Section 1412(b)(1)(B)(i) of SDWA requires EPA to publish a Contaminant Candidate List (CCL) every five years. The CCL is a list of contaminants that are known or anticipated to occur in PWSs and are not currently subject to any proposed or promulgated NPDWRs. EPA uses the CCL to identify priority contaminants for regulatory decision-making ( i.e., regulatory determinations), and information collection. Contaminants listed on the CCL may require future regulation under SDWA. EPA included PFOA and PFOS on the third and fourth CCLs published in 2009 (USEPA, 2009a) and 2016 (USEPA, 2016c). The Agency published the fifth CCL (CCL 5) earlier this year and it includes PFAS as a chemical group (USEPA, 2022b).
EPA collects data on the CCL contaminants to better understand their potential health effects and to determine the levels at which they occur in PWSs. SDWA 1412(b)(1)(B)(ii) requires that, every five years and after considering public comments on a “preliminary” regulatory determination, EPA issue a final regulatory determination to regulate or not regulate at least five contaminants on each CCL. In addition, Section 1412(b)(1)(B)(ii)(III) authorizes EPA to make a determination to regulate a contaminant not listed on the CCL so long as the contaminant meets the three statutory criteria based on available public health information. SDWA 1412(b)(1)(B)(iii) requires that “each document setting forth the determination for a contaminant under clause (ii) shall be available for public comment at such time as the determination is published.” To implement these requirements, EPA issues preliminary regulatory determinations subject to public comment and then issues a final regulatory determination after consideration of public comment. For any contaminant that EPA determines meets the criteria for regulation under SDWA 1412(b)(1)(A), Section 1412(b)(1)(E) requires that EPA propose a NPDWR within two years and promulgate a final regulation within 18 months of the proposal (which may be extended by 9 additional months).
EPA implements a monitoring program for unregulated contaminants under SDWA 1445(a)(2) which requires that once every five years, EPA issue a list of priority unregulated contaminants to be monitored by PWSs. This monitoring is implemented through the Unregulated Contaminant Monitoring Rule (UCMR), which collects data from CWSs and NTNCWSs. The first four UCMRs collected data from a census of large water systems (serving more than 10,000 people) and from a statistically representative sample of small water systems (serving 10,000 or fewer people). Water system monitoring data for six PFAS were collected during the third UCMR (UCMR3) between 2013 to 2015. The fifth UCMR (UCMR5), published December 2021, requires sample collection and analysis for 29 PFAS to occur between 2023 and 2025 using analytical methods developed by EPA and consensus organizations. Section 2021 of America's Water Infrastructure Act of 2018 (AWIA) (Pub. L. 115-270) amended SDWA and specifies that, subject to the availability of EPA appropriations for such purpose and sufficient laboratory capacity, EPA must require all PWSs serving between 3,300 and 10,000 people to monitor and ensure that a nationally representative sample of systems serving fewer than 3,300 people monitor for the contaminants in UCMR 5 and future UCMR cycles. All large water systems continue to be required to participate in the UCMR program. Section VII of this preamble provides additional discussion on PFAS occurrence. Additionally, while the UCMR 5 information will not be available to inform this proposal, EPA is proposing to consider the UCMR 5 data to support implementation of monitoring requirements under the proposed rule. Section IX of this preamble further discusses monitoring and compliance requirements.
After careful consideration of public comments, EPA issued final regulatory determinations for contaminants on the fourth CCL in March of 2021 (USEPA, 2021d) which included determinations to regulate two contaminants, PFOA and PFOS, in drinking water. EPA found that PFOA and PFOS may have an adverse effect on the health of persons; that these contaminants are known to occur, or that there is a substantial likelihood that they will occur, in PWSs with a frequency and at levels that present a public health concern; and that regulation of PFOA and PFOS presents a meaningful opportunity for health risk reduction for persons served by PWSs. As discussed in the final Regulatory Determinations 4 Notice for CCL 4 contaminants (USEPA, 2021d) and EPA's PFAS Strategic Roadmap (USEPA, 2022c), the Agency has also evaluated additional PFAS chemicals for regulatory consideration as supported by the best available science. The Agency preliminarily finds that additional PFAS compounds also meet SDWA criteria for regulation. EPA's preliminary regulatory determination for these additional PFAS is discussed in section III of this preamble.
Section 1412(b)(1)(E) provides that the Administrator may publish a proposed drinking water regulation concurrent “with a determination to regulate.” This provision authorizes a more expedited process by allowing EPA to make concurrent the regulatory determination and rulemaking processes. As a result, EPA interprets the reference to “determination to regulate” in Section 1412(b)(1)(E) as referring to the regulatory process in 1412(b)(1)(B)(ii) that begins with a preliminary determination. Under this interpretation, Section 1412(b)(1)(E) authorizes EPA to issue a preliminary determination to regulate a contaminant and a proposed NPDWR addressing that contaminant concurrently and request public comment at the same time. This allows EPA to act efficiently to issue a final determination to regulate concurrently with a final NPDWR to avoid delays to address contaminants that meet the statutory criteria. As a result, this proposal contains both a preliminary determination to regulate four PFAS contaminants and proposed regulations for those contaminants as well as the two PFAS contaminants (PFOA and PFOS) for which EPA has already issued a final Regulatory Determination. EPA developed a proposed MCLG and a proposed NPDWR for six PFAS compounds pursuant to the requirements under section 1412(b)(1)(B) of SDWA. The proposed MCLGs and proposed NPDWR are discussed in more detail below.
G. Bipartisan Infrastructure Law
The Agency notes that the passage of the Infrastructure Investment and Jobs Act, also referred to as the BIL, invests over $11.7 billion in the Drinking Water SRF; $4 billion to the Drinking Water SRF for Emerging Contaminants; and $5 billion to Small, Underserved, and Disadvantaged Communities Grants. These funds will assist many disadvantaged communities, small systems, and others with the costs of installation of treatment when it might otherwise be cost-challenging. These funds can also be used to address emerging contaminants like PFAS in drinking water through actions such as technical assistance, water quality testing, and contractor training, which will allow communities supplemental funding to meet their obligations under this proposed regulation and help ensure protection from PFAS contamination of drinking water.
H. EPA PFAS Strategic Roadmap
In October 2021, EPA published the PFAS Strategic Roadmap that outlined the Agency's plan to “further the science and research, to restrict these dangerous chemicals from getting into the environment, and to immediately move to remediate the problem in communities across the country” (USEPA, 2022c). Described in the Roadmap are key commitments the Agency made toward addressing these contaminants in the environment. With this proposal, EPA is delivering on a key commitment in the Roadmap to “establish a National Primary Drinking Water Regulation” for proposal and is working toward promulgating the final NPDWR in Fall of 2023.
III. Preliminary Regulatory Determinations for Additional PFAS
Since 2021 when EPA determined to regulate two PFAS contaminants, PFOA and PFOS, EPA has evaluated additional PFAS compounds for regulatory consideration and has preliminarily determined that an additional four individual PFAS and mixtures of these PFAS meet SDWA criteria for regulation. Section 1401(6) defines the term “contaminant” to mean “any physical, chemical or biological or radiological substance or matter in water.” A mixture of two or more “contaminants” qualifies as a “contaminant” because the mixture itself is “any physical, chemical or biological or radiological substance or matter in water.” (emphasis added). Therefore, pursuant to the provisions outlined in Section 1412(b)(1)(A) and 1412(b)(1)(B) of SDWA, the Agency is making a preliminary determination to regulate PFHxS, HFPO-DA, PFNA, and PFBS in drinking water, and mixtures of these PFAS contaminants. PFHxS, HFPO-DA, PFNA, and PFBS, and mixtures of these PFAS, are known to cause adverse human health effects; there is substantial likelihood that they will occur and co-occur in PWSs with a frequency and at levels of public health concern, particularly when considering them in a mixture; and in the sole judgment of the Administrator, regulation of PFHxS, HFPO-DA, PFNA, PFBS and mixtures of these PFAS present a meaningful opportunity for health risk reductions for people served by PWSs. This section describes the best available science and information used by the Agency to support this preliminary Regulatory Determination. The proposed MCLG and enforceable standard for these four PFAS and mixtures of these PFAS are discussed further in sections V to VI of this preamble.
A. Agency Findings
To support the Agency's preliminary Regulatory Determination, EPA examined health effects information from available peer reviewed human health assessments as well as drinking water monitoring data collected as part of the UCMR 3 and state-led monitoring efforts. EPA finds that oral exposure to PFHxS, HFPO-DA, PFNA, and PFBS may individually and in a mixture each result in adverse health effects, including disrupting multiple biological pathways that result in common adverse effects on several biological systems including the endocrine, cardiovascular, developmental, immune, and hepatic systems (USEPA, 2023a). PFAS, including PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures are anticipated to affect common target organs, tissues, or systems to produce dose-additive effects from co-exposures. Additionally, based on the Agency's evaluation of the best-available science, EPA finds that PFHxS, HFPO-DA, PFNA, and PFBS each have a substantial likelihood to occur in finished drinking water and that these PFAS are also likely to co-occur as mixtures and result in increased exposure above levels of health concern. Therefore, given this high occurrence and co-occurrence likelihood and that adverse health effects arise as a result of both these PFAS individually and as mixtures, the Agency is preliminarily determining that PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures may have adverse human health effects; there is a substantial likelihood that PFHxS, HFPO-DA, PFNA, PFBS and mixtures of these PFAS, will occur and co-occur in PWSs with a frequency and at levels of public health concern; and in the sole judgment of the Administrator, regulation of PFHxS, HFPO-DA, PFNA, and PFBS, and their mixtures, presents a meaningful opportunity for health risk reductions for persons served by PWSs.
B. Statutory Criterion 1—Adverse Health Effects
The Agency finds that PFHxS, HFPO-DA, PFNA, PFBS and their mixtures may have an adverse effect on the health of persons. Discussion related to health effects for each of the four PFAS is below. For this proposal, the Agency is developing HBWCs for PFHxS, HFPO-DA, PFNA and PFBS, defined as a level protective of health effects over a lifetime of exposure, including sensitive populations and life stages. Each of the four HBWCs is used in this proposal to evaluate occurrence data and the likelihood of potential risk to human health to justify the agency's preliminary regulatory determinations for PFHxS, HFPO-DA, PFNA and PFBS. The chemical-specific HBWCs are also used to assess the potential human health risk associated with mixtures of the four PFAS in drinking water using the HI approach. Additional details on the HBWC for PFHxS, HFPO-DA, PFNA and PFBS are found in section IV of this preamble. More information supporting EPA's preliminary regulatory determination relating to adverse health effects for these PFAS and the HI approach for mixtures is available in section V of this preamble.
1. PFHxS
Toxicity studies of oral PFHxS exposure in animals have reported adverse health effects on the liver, thyroid, and development (ATSDR, 2021). EPA has not yet classified the carcinogenicity of PFHxS. For a detailed discussion on adverse effects of oral exposure to PFHxS, please see ATSDR (2021) and USEPA (2023a).
The HBWC for PFHxS is derived using a chronic reference value based on an Agency For Toxic Substances And Disease Registry (ATSDR) intermediate-duration oral Minimal Risk Level, which was based on thyroid effects seen in male rats after oral PFHxS exposure (ATSDR, 2021). The most sensitive non-cancer effect observed was thyroid follicular epithelial hypertrophy/hyperplasia in parental male rats exposed to PFHxS for 42-44 days, identified in the critical developmental toxicity study selected by ATSDR (no observed adverse effect level (NOAEL) of 1 mg/kg/day) (Butenhoff et al., 2009; ATSDR, 2021). To derive the intermediate-duration Minimal Risk Level for PFHxS, ATSDR calculated a human equivalent dose (HED) of 0.0047 mg/kg/day from the NOAEL of 1 mg/kg/day identified in the principal study. Then, ATSDR applied a total uncertainty factor (UF)/modifying factor (MF) of 300X (10X UF for intraspecies variability, 3X UF for interspecies differences, and a 10X MF for database deficiencies) to yield an intermediate-duration oral Minimal Risk Level of 0.00002 mg/kg/day (ATSDR, 2021). Per Agency guidance (USEPA, 2002), to calculate the HBWC, EPA applied an additional UF of 10 to adjust for subchronic-to-chronic duration (UF S ) because the effect was not in a developmental life stage ( i.e., thyroid follicular epithelial hypertrophy/hyperplasia in parental male rats). The resulting chronic reference value was 0.000002 mg/kg/day.
No sensitive population or life stage was identified for bodyweight-adjusted drinking water intake (DWI-BW) selection for PFHxS because the critical effect on which the ATSDR Minimal Risk Level was based (thyroid alterations) was observed in adult male rats. Since this exposure life stage does not correspond to a sensitive population or life stage, a DWI-BW for adults within the general population (0.034 L/kg/day; 90th percentile direct and indirect consumption of community water, consumer-only two-day average, adults 21 years and older) was selected for HBWC derivation (USEPA, 2019a).
EPA calculated the HBWC for PFHxS using a relative source contribution (RSC) of 0.20. This means that 20% of the exposure—equal to the chronic reference value—is allocated to drinking water, and the remaining 80% is attributed to all other potential exposure sources. This was based on EPA's determination that the available data on PFHxS exposure routes and sources did not permit quantitative characterization of PFHxS exposure. In such cases, an RSC of 0.20 is typically used (USEPA, 2000c). See U.S.EPA (2023a) for complete details on the RSC determination for PFHxS.
As further described in USEPA (2023a) and section V of this preamble below, the HBWC for PFHxS is calculated to be 9.0 ppt. This HBWC of 9.0 ppt is also used as the health reference level (HRL) for this preliminary regulatory determination.
2. HFPO-DA
EPA's 2021 Human Health Toxicity Assessment for GenX Chemicals describes potential health effects associated with oral exposure to HFPO-DA (USEPA, 2021b). Toxicity studies in animals indicate that exposures to HFPO-DA may result in adverse health effects, including liver and kidney toxicity and immune system, hematological, reproductive, and developmental effects (USEPA, 2021b). There is Suggestive Evidence of Carcinogenic Potential of oral exposure to HFPO-DA in humans, but the available data are insufficient to derive a cancer risk concentration in water for HFPO-DA. For a detailed discussion on adverse effects of oral exposure to HFPO-DA, please see USEPA (2021b).
EPA's noncancer HBWC for HFPO-DA is derived from a reference dose (RfD) that is based on liver effects observed following oral exposure of mice to HFPO-DA (USEPA, 2021b). The most sensitive noncancer effect observed was a constellation of liver lesions in parental female mice exposed to HFPO-DA by gavage for 53-64 days, identified in the critical reproductive/developmental toxicity study selected by EPA (NOAEL of 0.1 mg/kg/day) (DuPont, 2010; USEPA, 2021b). To develop the chronic RfD for HFPO-DA, EPA derived an HED of 0.01 mg/kg/day from the NOAEL of 0.1 mg/kg/day identified in the principal study. EPA then applied a composite UF of 3,000 ( i.e., 10X for intraspecies variability, 3X for interspecies differences, 10X for extrapolation from a subchronic to a chronic dosing duration, and 10X for database deficiencies) to yield the chronic RfD (USEPA, 2021b).
To select an appropriate DWI-BW for use in derivation of the noncancer HBWC values for HFPO-DA, EPA considered the HFPO-DA exposure interval used in the oral reproductive/developmental toxicity study in mice that was the basis for chronic RfD derivation (the critical study). In this study, parental female mice were dosed from pre-mating through lactation, corresponding to three potentially sensitive human adult life stages that may represent critical windows of exposure for HFPO-DA: women of childbearing age, pregnant women, and lactating women (Table 3-63 in USEPA, 2019a). Of these three, the DWI-BW for lactating women (0.0469 L/kg/day) is anticipated to be protective of the other two sensitive life stages. Therefore, EPA used the DWI-BW for lactating women to calculate the HBWC for the proposed regulation, which is also used for the HRL for the preliminary regulatory determination.
The HBWC value for HFPO-DA was calculated using an RSC of 0.20. This means that 20% of the exposure—equal to the RfD—is allocated to drinking water, and the remaining 80% is attributed to all other potential exposure sources (USEPA, 2022d). Selection of this RSC was based on EPA's determination that the available exposure data for HFPO-DA did not enable a quantitative characterization of relative HFPO-DA exposure sources and routes. In such cases, an RSC of 0.20 is typically used (USEPA, 2000c).
As further described in USEPA (2023a) and USEPA (2022d), the HBWC for HFPO-DA is calculated to be 10.0 ppt. This value is consistent with EPA's 2022 drinking water health advisory for HFPO-DA (USEPA, 2022d), but was derived from EPA's 2021 Human Health Toxicity Assessment for HFPO-DA (USEPA, 2021b). This HBWC of 10 ppt is also used as the HRL for this preliminary Regulatory Determination for HFPO-DA.
3. PFNA
Animal toxicity studies have reported adverse health effects, specifically on development, reproduction, immune function, and the liver, after oral exposure to PFNA (ATSDR, 2021). EPA has not yet classified the carcinogenicity of PFNA. For a detailed discussion on adverse effects of oral exposure to PFNA, please see ATSDR (2021) and USEPA (2023a).
The HBWC for PFNA is derived using a chronic reference value based on an ATSDR intermediate-duration oral Minimal Risk Level, which was based on developmental effects seen in mice after oral PFHxS exposure (ATSDR, 2021). The most sensitive non-cancer effects were decreased body weight (BW) gain and developmental delays ( i.e., delayed eye opening, preputial separation, and vaginal opening) in mice born to mothers that were gavaged with PFNA from gestational days (GD) 1-17, with continued exposure through lactation and monitoring until postnatal day (PND) 287, identified in the critical developmental toxicity study selected by ATSDR (NOAEL of 1 mg/kg/day) (Das et al., 2015; ATSDR, 2021). To derive the intermediate-duration Minimal Risk Level, ATSDR calculated an HED of 0.001 mg/kg/day from the NOAEL of 1 mg/kg/day identified in the principal study. Then, ATSDR applied a total UF/MF of 300X (total UF of 30X and a MF of 10X for database deficiencies) to yield an intermediate-duration Minimal Risk Level of 0.000003 mg/kg/day. EPA did not apply an additional UF to adjust for subchronic-to-chronic duration ( i.e., UF S ) to calculate the chronic reference value because the critical effects were observed during a developmental life stage (USEPA, 2002). The chronic reference value of 0.000003 mg/kg/day was used to derive the HBWC for PFNA.
Based on the life stages of exposure in the principal study from which the intermediate-duration Minimal Risk Level was derived ( i.e., during gestation and lactation), EPA identified three potentially sensitive life stages that may represent critical windows of exposure for PFNA: women of childbearing age (13 to < 50 years), pregnant women, and lactating women (Table 3-63 in USEPA, 2019a). The DWI-BW for lactating women (0.0469 L/kg/day; 90th percentile direct and indirect consumption of community water, consumer-only two-day average) was selected to calculate the HBWC for PFNA because it is the highest of the three DWI-BWs and is anticipated to be protective of the other two sensitive life stages.
EPA calculated the HBWC for PFNA using an RSC of 0.20. This means that 20% of the exposure—equal to the chronic reference value—is allocated to drinking water, and the remaining 80% is attributed to all other potential exposure sources. This was based on EPA's determination that the available data on PFNA exposure routes and sources did not permit quantitative characterization of PFNA exposure. In such cases, an RSC of 0.20 is typically used (USEPA, 2000c). See USEPA (2023a) for complete details on the RSC determination for PFNA.
As further described in USEPA (2023a), the HBWC for PFNA is calculated to be 100 ppt. This HBWC of 10.0 ppt is also used as the HRL for this preliminary Regulatory Determination for PFNA.
4. PFBS
EPA's 2021 PFBS Toxicity Assessmen t describe potential health effects associated with oral PFBS exposure (USEPA, 2021a). Toxicity studies of oral PFBS exposures in animals have reported adverse health effects on development, as well as the thyroid and kidneys (USEPA, 2021a). Human and animal studies evaluated other health effects following PFBS exposure including effects on the immune, reproductive, and hepatic systems and lipid and lipoprotein homeostasis, but the evidence was determined to be equivocal (USEPA, 2021a). No studies evaluating the carcinogenicity of PFBS in humans or animals were identified. EPA concluded that there is “Inadequate Information to Assess Carcinogenic Potential” for PFBS and K PFBS by any route of exposure. For a detailed discussion on adverse effects of oral exposure to PFBS, please see USEPA (2021a).
EPA's noncancer HBWC for PFBS is derived from a chronic RfD that is based on thyroid effects observed following gestational exposure of mice to K PFBS (USEPA, 2021a; USEPA, 2022e). The most sensitive non-cancer effect observed was decreased serum total thyroxine (T4) in newborn (PND 1) mice gestationally exposed to K PFBS from GD 1-20, identified in the critical developmental toxicity study selected by EPA (benchmark dose lower confidence limit HED or BMDLHED) of 0.095 mg/kg/day) (Feng et al., 2017; USEPA, 2021a). To develop the chronic RfD for PFBS, EPA applied a composite UF of 300 ( i.e., 10X for intraspecies uncertainty factor (UF H ), 3X for interspecies uncertainty factor (UF A ), and 10X for database uncertainty factor (UF D )) to yield a value of 0.0003 mg/kg/day (USEPA, 2021a).
To select an appropriate DWI-BW for use in deriving the noncancer HBWC value, EPA considered the PFBS exposure interval used in the developmental toxicity study in mice that was the basis for chronic RfD derivation. In this study, pregnant mice were exposed throughout gestation, which is relevant to two human adult life stages: women of child-bearing age who may be or become pregnant, and pregnant women and their developing embryo or fetus (Table 3-63 in USEPA, 2019a). Of these two, EPA selected the DWI-BW for women of child-bearing age (0.0354 L/kg/day) to derive the noncancer HBWC for PFBS because it was higher and therefore more health-protective (USEPA, 2022e).
The HBWC value for PFBS was calculated using an RSC of 0.20. This means that 20% of the exposure—equal to the RfD—is allocated to drinking water, and the remaining 80% is attributed to all other potential exposure sources (USEPA, 2022e). This was based on EPA's determination that the available data on PFBS exposure routes and sources did not enable a quantitative characterization of PFBS exposure. In such cases, an RSC of 0.20 is typically used (USEPA, 2000c).
As further described in USEPA (2022e), the HBWC for PFBS is calculated to be 2000 ppt. This value is consistent with EPA's 2022 drinking water health advisory for PFBS (USEPA, 2022d), but was derived from EPA's 2021 PFBS Toxicity Assessment (USEPA, 2021a). This HBWC of 2000 ppt is also used as the HRL for this preliminary Regulatory Determination for PFBS.
5. Mixtures of PFHxS, HFPO-DA, PFNA, and PFBS
PFAAs, including PFHxS, HFPO-DA, PFNA, and PFBS, disrupt signaling of multiple biological pathways resulting in common adverse effects on several biological systems including thyroid hormone levels, lipid synthesis and metabolism, as well as on development, and immune and liver function (ATSDR, 2021; EFSA, 2018, 2020; USEPA, 2023a).
Studies with PFAS and other classes of chemicals support the health protective assumption that a mixture of chemicals with similar observed effects should be assumed to also act in a dose additive manner unless data demonstrate otherwise (USEPA, 2023d). Dose additivity means that each of the component chemicals in the mixture (in this case, PFHxS, HFPO-DA, PFNA, and PFBS) behaves as a concentration or dilution of every other chemical in the mixture differing only in relative toxicity (USEPA, 2000a). See additional discussion of PFAS dose additivity in Section V.C of this preamble.
C. Statutory Criterion 2—Occurrence
With this proposal, EPA is preliminarily determining that PFHxS, HFPO-DA, PFNA, and PFBS, both individually and as mixtures of these PFAS, meet SDWA's second statutory criterion for regulatory determination: there is a substantial likelihood that the contaminants will occur and co-occur with a frequency and at levels of public health concern in PWSs based on EPA's evaluation of the best available occurrence information. EPA is seeking public comment on whether additional data or studies exist which EPA should consider that support or do not support this preliminary determination.
EPA has made its preliminary determination based on the most recent, publicly available data, which includes UCMR 3 data and more recent PFAS drinking water data collected by several states. Informed by these data, EPA determined that there is a substantial likelihood PFHxS, HFPO-DA, PFNA, and PFBS will occur and co-occur with a frequency of public health concern. Additionally, when determining that there is a substantial likelihood these PFAS will occur at levels of public health concern, EPA considered both the occurrence concentration levels for each contaminant individually, as well as their collective co-occurrence and corresponding dose additive health effects from co-exposures. Furthermore, the Agency notes that it does not have a bright-line threshold for occurrence in drinking water that triggers whether a contaminant is of public health concern. A determination of public health concern involves consideration of a number of factors, some of which include the level at which the contaminant is found in drinking water, the frequency at which the contaminant is found and at which it co-occurs with other contaminants, whether there is an sustained upward trend that these contaminant will occur at a frequency and at levels of public health concern, the geographic distribution (national, regional, or local occurrence), the impacted population, health effect(s), the potency of the contaminant, other possible sources of exposure, and potential impacts on sensitive populations or lifestages. Given the many possible combinations of factors, a simple threshold is not viable and is a highly contaminant-specific decision that takes into consideration multiple factors.
UCMR 3 monitoring occurred between 2013 and 2015 for PFHxS, PFNA, and PFBS. HFPO-DA were not monitored for as part of the UCMR 3. Under the UCMR 3, 36,972 samples from 4,920 PWSs were analyzed for PFHxS, PFNA, and PFBS. The minimum reporting levels (MRLs) for PFHxS, PFNA, and PFBS were 30 ppt, 20 ppt, and 90 ppt, respectively. EPA notes that these UCMR 3 MRLs are higher than those utilized within the majority of state monitoring data and for the upcoming UCMR 5. A total of 233 samples and 70 systems serving a total population of approximately 6.7 million people had reported detections (greater than or equal to the MRL) of at least one of the three compounds. Moreover, the large majority of these UCMR 3 reported detections were found at concentrations at or above levels of public health concern as described previously in section III.B of this preamble and below within this section. USEPA (2023e) presents sample and system level summaries of the results for the individual contaminants. More information supporting EPA's regulatory determination relating to the occurrence of these PFAS and their mixtures is included in section VII.A. of this preamble.
EPA has also collected more recent finished drinking water data from 23 states who have made their data publicly available as of August 2021 (USEPA, 2023e). EPA used this cutoff date to allow the Agency to conduct thorough analyses of the state information. EPA further refined this dataset based on representativeness and reporting limitations, resulting in detailed technical analyses using a subset of the available state data ( i.e., all 23 states' data were not included within the detailed technical analyses). For example, a few states only reported results as a combination of analytes which was not conducive for analyzing PFAS. In general, the state data which were more recently collected using newer analytical methods that have lower reporting limits than those under UCMR 3 show widespread occurrence of PFOA, PFOS, PFHxS, PFNA, and PFBS in multiple geographic locations. These data also show that there is a substantial likelihood that these PFAS occur at concentrations below UCMR 3 reporting limits. Furthermore, these data include results for more PFAS than were included in the UCMR 3, including HFPO-DA, and show that PFHxS, HFPO-DA, PFNA, and PFBS, and mixtures of these PFAS, occur and co-occur at levels of public health concern as they are measured at concentrations above their respective individual HRLs or, when considering their dose additive impacts, exceed these levels. The Agency notes that the data vary in terms of quantity and coverage, including that some of these available data are from targeted or site-specific sampling efforts ( i.e., monitoring specifically in areas of known or potential contamination) and thus may be expected to have higher detection rates or not be representative of levels found in all PWSs within the state.
Tables 1 and 2 below show the percent of samples with state reported detections of PFHxS, HFPO-DA, PFNA, and PFBS, and the percentage of monitored systems with detections of PFHxS, HFPO-DA, PFNA, and PFBS, respectively, across the non-targeted or non-site specific ( i.e., monitoring not conducted specifically in areas of known or potential contamination) state finished water monitoring data.
EPA notes that different states utilized various reporting thresholds or limits when presenting their data, and for some states there were no clearly defined limits publicly provided. Further, the limits often varied within the data for each state depending on the specific analyte, as well as the laboratory analyzing the data. When conducting data analyses, EPA incorporated individual state-specific reporting limits where possible. In some cases, states reported data at concentrations below EPA's proposed rule trigger level for reduced compliance monitoring frequency and/or PQLs described in sections VIII.A., IX.A., and IX.B of this preamble. However, to present the best available occurrence data, EPA collected and evaluated the data based on the information as reported directly by the states. EPA also notes, and as described in further detail in section VIII.A. of this preamble, some laboratories are able to detect and measure the PFAS addressed in this document at lower concentrations than EPA's proposed rule trigger level and PQLs which account for differences in the capability of laboratories across the country. As such, EPA believes this data can reasonably support EPA's evaluation of PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS occurrence and co-occurrence in drinking water. Specific details on state data reporting thresholds are available in Table 1 within USEPA (2023e).
| State | PFHxS (%) | PFNA (%) | PFBS (%) | HFPO-DA (%) |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| Colorado | 10.8 | 0.9 | 11.0 | 0.2 |
| Illinois | 5.1 | 0.2 | 7.8 | 0.0 |
| Kentucky | 8.6 | 2.5 | 12.3 | 13.6 |
| Massachusetts | 31.9 | 4.6 | 35.5 | 0.0 |
| Michigan | 2.9 | 0.1 | 5.2 | 0.04 |
| New Hampshire | 16.6 | 3.3 | 31.4 | 3.8 |
| New Jersey | 24.7 | 8.0 | 24.9 | N/A |
| North Dakota | 1.6 | 0.0 | 0.0 | 0.0 |
| Ohio | 5.8 | 0.3 | 4.7 | 0.1 |
| South Carolina | 13.5 | 2.1 | 38.3 | 6.0 |
| Vermont | 2.2 | 1.7 | 4.8 | 0.2 |
| State | PFHxS (%) | PFNA (%) | PFBS (%) | HFPO-DA (%) |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| Colorado | 13.4 | 1.0 | 13.4 | 0.3 |
| Illinois | 4.3 | 0.2 | 6.6 | 0.0 |
| Kentucky | 8.6 | 2.5 | 12.3 | 13.6 |
| Massachusetts | 30.2 | 8.4 | 39.4 | 0.0 |
| Michigan | 3.0 | 0.2 | 5.3 | 0.1 |
| New Hampshire | 22.5 | 5.5 | 37.9 | 5.1 |
| New Jersey | 32.6 | 13.3 | 34.0 | N/A |
| North Dakota | 1.6 | 0.0 | 0.0 | 0.0 |
| Ohio | 2.2 | 0.3 | 2.4 | 0.1 |
| South Carolina | 20.0 | 6.1 | 56.0 | 10.9 |
| Vermont | 1.6 | 1.3 | 5.2 | 0.5 |
As shown in Tables 1 and 2, all states except one report sample and system detections for at least three of the four PFAS. For those states that reported detections, the percentage of samples and systems where these PFAS were found ranged from 0.1 to 38.3 percent and 0.1 to 56.0 percent, respectively. While these percentages show occurrence variability across states, several of these states demonstrate a significant number of samples ( e.g., detections of PFHxS in 31.9 percent of Massachusetts samples) and systems ( e.g., detections of HFPO-DA in 13.9 percent of monitored systems in Kentucky) with some or all of the four PFAS, which supports the Agency's preliminary determination that there is a substantial likelihood these PFAS and their mixtures occur and co-occur with a frequency of public health concern. Specific discussion related to occurrence for each of the four PFAS is below.
1. PFHxS
The occurrence data presented above, throughout section VII. of this preamble and discussed in the USEPA (2023e) support the Agency's preliminary determination that there is a substantial likelihood PFHxS occurs with a frequency and at levels of public health concern in drinking water systems across the United States. PFHxS was found under UCMR 3 in approximately 1.1% of systems using an MRL of 30 ppt. All UCMR 3 reported values are greater than the HRL of 9.0 ppt. Additionally, through analysis of available non-targeted state data all states in Tables 1 and 2 had reported detections of PFHxS within 1.6 to 32.6 percent of their systems and reported concentrations ranging from 0.46 to 310 ppt with median sample concentrations ranging from 2.14 to 11.3 ppt. Results from targeted state monitoring data of PFHxS are also consistent with non-targeted state data. For example, California reported 29.2 percent of monitored systems found PFHxS, where concentrations ranged from 1.1 to 140.0 ppt. Therefore, in addition to the UCMR 3 results, these state data reflect PFHxS at frequencies and levels of public health concern. EPA also evaluated PFHxS in a national occurrence model that has been developed and utilized to estimate national-scale PFAS occurrence for four PFAS that were included in UCMR 3 (Cadwallader et al., 2022). The model and results are described in section VII.E of this preamble. Hundreds of systems serving millions of people were estimated to have mean concentrations exceeding the PFHxS HRL (9.0 ppt). Further supporting this preliminary determination, PFAS have dose additive impacts and PFHxS co-occurs in mixtures with other PFAS, including PFOA, PFOS, HFPO-DA, PFNA, and PFBS. More information on PFHxS co-occurrence is available in section VII.C. and VII.D. of this preamble.
2. HFPO-DA
The occurrence data presented above, throughout section VII of this preamble, and discussed in the USEPA (2023e) support the Agency's preliminary determination that there is a substantial likelihood HFPO-DA occur with a frequency and at levels of public health concern in drinking water systems across the United States. Through analysis of available non-targeted state data over half of the states in Tables 1 and 2 had state reported detections of HFPO-DA within 0.1 to 13.6 percent of their systems. State reported sample results were also reported above the HRL of 10.0 ppt with sample results ranging from 1.7 to 29.7 ppt and median sample results ranging from 1.7 to 9.7 ppt. Additionally, targeted state monitoring in North Carolina which conducted sampling across six finished drinking water sites where 438 samples showed HFPO-DA ranging from 9.2 to 1100 ppt, with a median concentration of 40 ppt. Therefore, these state data demonstrate concentrations of HFPO-DA at levels of public health concern. Further supporting this preliminary determination, PFAS have dose additive impacts and HFPO-DA occur in mixtures with other PFAS, including PFOA, PFOS, PFHxS, PFNA, and PFBS. More information on HFPO-DA co-occurrence is available in section VII.C. and VII.D. of this preamble.
3. PFNA
The occurrence data presented above, throughout section VII of this preamble, and discussed in USEPA (2023e) support the Agency's preliminary determination that there is a substantial likelihood PFNA occurs with a frequency and at levels of public health concern in drinking water systems across the United States. PFNA was found under UCMR 3 using an MRL of 20 ppt. Thus, all UCMR 3 reported detections are greater than the HRL of 10.0 ppt. Additionally, through analysis of available non-targeted state data all states except one in Tables 1 and 2 had state reported detections of PFNA within 0.2 to 13.3 percent of their systems, and state reported sample results ranging from 0.25 to 94.2 ppt with median sample results range from 2.1 to 7.46 ppt. Targeted state monitoring data of PFNA are also consistent with non-targeted state data; for example, Pennsylvania reported 5.8 percent of monitored systems found PFNA, where concentrations ranged from 1.8 to 18.1 ppt. Thus, in addition to the UCMR 3 results, these state data also reflect PFNA concentrations at levels of public health concern. Further supporting this preliminary determination, PFAS have dose additive impacts and PFNA co-occurs in mixtures with other PFAS, including PFOA, PFOS, PFHxS, HFPO-DA, and PFBS. More information on PFNA co-occurrence is available in section VII.C. and VII.D. of this preamble.
4. PFBS
The occurrence data presented above, throughout section VII of this preamble, and discussed in USEPA (2023e) support the Agency's preliminary determination that there is a substantial likelihood PFBS occurs with a frequency and at levels of public health concern in drinking water systems across the United States. PFBS was found under UCMR 3 using an MRL of 90 ppt. Additionally, through analysis of available non-targeted state data all states except one in Tables 1 and 2 had state reported detections of PFBS within 2.4 to 56 percent of their systems, with four states finding PFBS in over 34 percent of their systems. Furthermore, PFBS occurred at a greater frequency in all but one state than the other three PFAS. State reported sample results ranged from 1 to 310 ppt with median sample results ranging from 1.99 to 7.26 ppt. Targeted state monitoring data of PFBS are consistent with non-targeted state data. Maryland reported 51.5 percent of monitored systems found PFBS, where concentrations ranged from 1.01 to 21.29 ppt. Further supporting this preliminary determination, PFAS have dose additive impacts and PFBS occurs in mixtures with other PFAS, including PFOA, PFOS, PFHxS, HFPO-DA, and PFNA. Moreover, given the considerable prevalence of PFBS in state data reviewed by EPA and frequency in which it has been shown to have other PFAS co-occurring with it, PFBS may serve as an indicator of broad contamination of other PFAS. Those other PFAS are also likely dose additive to PFBS and other PFAS being proposed for regulation. EPA notes that PFBS concentrations do not exceed their HRL of 2000 ppt when considered in isolation; however, when considering dose additivity and the elevated frequency to which PFBS occurrence has been observed over time, EPA has determined that PFBS is an important component of regulated PFAS mixtures and because of their pervasiveness, there is a substantial likelihood of its occurrence with a frequency and at levels of public health concern. More information on PFBS co-occurrence is available in section VII.C. and VII.D. of this preamble. Based on the occurrence and co-occurrence information above and throughout section VII of this preamble, EPA has preliminarily determined that there is substantial likelihood PFBS occurs with a considerable frequency and at levels of public health concern.
5. Preliminary Occurrence Determination for PFHxS, HFPO-DA, PFNA, and PFBS
Through the information presented within this section and in USEPA (2023e), along with the co-occurrence information presented in section VII.C. and VII.D. of this preamble, EPA's evaluation of the UCMR 3 data and state data collected more recently demonstrates that as analytical methods improved, monitoring has increased, and minimum reporting thresholds are lowered, there is a sustained upward trend that there is a substantial likelihood that these contaminants will occur and co-occur at a frequency and at levels of public health concern. The UCMR 3 results showed there were over 6.5 million people served by PWSs that had reported detections of PFHxS, PFNA, and PFBS, with many of the detections for PFHxS and PFNA above the HRLs. EPA's evaluation of monitoring data from multiple states that was primarily gathered following the UCMR 3 using improved analytical methods that could measure more PFAS at lower concentrations found that there is even greater demonstrated occurrence and co-occurrence of these PFAS, as well as for HFPO-DA, at significantly greater frequencies and at levels of public health concern. EPA anticipates that national monitoring with newer analytical methods capable of quantifying PFAS occurrence to lower levels, significant occurrence and co-occurrence of these PFAS are likely to be observed.
EPA notes that it focused the evaluation of the state data on the non-targeted monitoring efforts from 12 states, given that these types of monitoring efforts are likely to be more representative of PFHxS, HFPO-DA, PFNA, and PFBS occurrence as they are not specifically conducted in areas of known or potential contamination. In these 12 states, there were reported detections of PFHxS, HFPO-DA, PFNA, or PFBS, with nearly all states reporting detections of at least three of these four PFAS. EPA considered the targeted state data separately since a higher rate of detections may occur as a result of specifically looking in areas of suspected or known contamination. For the additional targeted state data that EPA analyzed, EPA also found that these states reported detections at systems serving millions of additional people, as well as at levels of public health concern, particularly when considering PFAS mixtures and dose additive impacts. State data detection frequency and concentration results vary for PFHxS, HFPO-DA, PFNA, and PFBS, both between these four different PFAS and across different states, with some states showing much higher reported detections and concentrations of these PFAS when compared to other states. However, given the overall results, this demonstrates the substantial likelihood that these PFAS and their mixtures will occur at frequencies and levels of public health concern, and where these PFAS have been monitored they are very commonly found. Furthermore, EPA notes that as described in section VII.C.1. of this preamble, when evaluating only a subset of the available state data representing non-targeted monitoring, that one or more of PFHxS, HFPO-DA, PFNA, and PFBS were reported in approximately 13.9 percent of monitored systems; if these results were extrapolated to the nation, one or more of these four PFAS would be detectable in over 9,000 PWSs. Moreover, as shown in section VII.C.2. of this preamble, PFHxS, HFPO-DA, PFNA, and PFBS generally co-occur with each other, as well as with PFOA and PFOS, supporting that there is substantial likelihood that these PFAS will co-occur in mixtures with dose additive impacts. For all of these reasons, EPA has determined that there is sufficient occurrence information available to support this preliminary determination that there is a substantial likelihood that PFHxS, HFPO-DA, PFNA, and PFBS will occur at frequencies and levels of public health concern.
D. Statutory Criterion 3—Meaningful Opportunity
EPA has preliminarily determined that regulation of PFHxS, HFPO-DA, PFNA, and PFBS, both individually and in a mixture, presents a meaningful opportunity for health risk reduction for persons served by PWSs. EPA has made this preliminary determination after evaluating health, occurrence, treatment, and other related information against the three SDWA statutory criteria including consideration of the following for the four PFAS and their mixtures:
• PFHxS, HFPO-DA, PFNA, and PFBS, individually and in a mixture, may cause adverse human health effects on several biological systems including the endocrine, cardiovascular, developmental, immune, and hepatic systems. Additionally, these four PFAS, as well as other PFAS, are likely to produce dose-additive effects from co-exposures.
• The substantial likelihood that PFHxS, HFPO-DA, PFNA, and PFBS, individually occur and co-occur together at frequencies and levels of public health concern in PWSs as discussed in section III of this preamble above and in section VII of this preamble, and the corresponding significant populations served by these water systems.
• PFHxS, HFPO-DA, PFNA, and PFBS, individually and in a mixture, are expected to be environmentally persistent.
• Validated EPA-approved measurement methods are available to measure PFHxS, HFPO-DA, PFNA, and PFBS, individually and in mixtures. See section VIII of this preamble for further discussion.
• Treatment technologies are available to remove PFHxS, HFPO-DA, PFNA, and PFBS, and mixtures of these contaminants, from drinking water. See section XI of this preamble for further discussion.
• Regulating PFHxS, HFPO-DA, PFNA, and PFBS, in addition to PFOA and PFOS, is anticipated to reduce the overall public health risk from all other PFAS that co-occur and are co-removed. Their regulation is anticipated to provide public health protection at the majority of known sites with PFAS-impacted drinking water.
• There are achievable steps to manage drinking water that can be taken to reduce risk.
Due to the environmental persistence of these chemicals, there is potential for toxicity at environmentally relevant concentrations as studies show it can take years for many PFAS to leave the human body (NIEHS, 2020). See section III of this preamble above and section V of this preamble for discussion about the human health effects of PFHxS, HFPO-DA, PFNA, and PFBS.
Data from both the UCMR 3 and state monitoring efforts demonstrates occurrence or likely occurrence and co-occurrence of PFHxS, HFPO-DA, PFNA, and PFBS, and their mixtures, at frequencies and levels of public health concern. Under UCMR 3, 1.4% of systems serving approximately 6.7 million people had reported detections (greater than or equal to their MRLs) of PFHxS, PFNA, and PFBS of at least one of the three compounds. Additionally, based on the available state monitoring data presented earlier in this section, in the 11 states shown in Table 2 that conducted non-targeted sampling of the four PFAS, monitored systems that reported detections of PFHxS, HFPO-DA, PFNA, and PFBS serve approximate populations of 8.3 million, 1.8 million, 2.6 million, and 8.8 million people, respectively. Further, as demonstrated in the UCMR 3 and state data, concentrations of these PFAS, as well as PFOA and PFOS, and their mixtures co-occur at levels of public health concern as described in more detail in section VII.C. and VII.D. of this preamble and USEPA (2023e).
Analytical methods are available to measure PFHxS, HFPO-DA, PFNA, and PFBS in drinking water. EPA has published two multi-laboratory validated drinking water methods for individually measuring PFHxS, HFPO-DA, PFNA, and PFBS: EPA Method 537.1 which measures 18 PFAS and EPA Method 533 which measures 25 PFAS. There are 14 PFAS which overlap between methods and both methods measure PFOA and PFOS). Additional discussion on analytical methods can be found in section VIII of this preamble.
EPA's analysis, summarized in section XI of this preamble, found there are available technologies capable of reducing PFHxS, HFPO-DA, PFNA, and PFBS. These technologies include granular activated carbon (GAC), AIX resins, reverse osmosis (RO), and nanofiltration (NF). See discussion in section XI of this preamble for information about these treatment technologies. Due to the inherent nature of sorptive and high-pressure membrane technologies such as these, treatment technologies that remove PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures also have been documented to co-remove other PFAS (Sörengård et al., 2020; McCleaf et al., 2017; Mastropietro et al., 2021). Furthermore, as described in section VII of this preamble, PFHxS, HFPO-DA, PFNA, and PFBS also co-occur with PFAS for which the Agency is not currently making a preliminary regulatory determination. Many of these other emergent co-occurring PFAS are likely to also pose hazards to public health and the environment (Mahoney et al., 2022). Therefore, based on EPA's findings that PFHxS, HFPO-DA, PFNA, and PFBS have a substantial likelihood to co-occur in drinking water with other PFAS and treating for PFHxS, HFPO-DA, PFNA, and PFBS is anticipated to result in removing these and other PFAS, regulation of PFHxS, HFPO-DA, PFNA, PFBS (as well as PFOA and PFOS) also presents a meaningful opportunity to reduce the overall public health risk from all other PFAS that co-occur and are co-removed with PFHxS, HFPO-DA, PFNA, and PFBS.
With the ability to monitor for PFAS, identify contaminated drinking water sources and contaminated finished drinking water, and reduce PFAS exposure through management of drinking water, EPA has identified meaningful and achievable actions that can be taken to reduce the human health risk of PFAS.
EPA is preliminarily determining that regulation of PFHxS, HFPO-DA, PFNA, and PFBS presents a meaningful opportunity for health risk reduction for persons served by PWSs.
E. EPA's Preliminary Regulatory Determination Summary for PFHxS, HFPO-DA, PFNA, and PFBS
The statute provides EPA significant discretion when making a preliminary determination under Section 1412(b)(1)(A). This decision to make a preliminary regulatory determination for PFHxS, HFPO-DA, PFNA and PFBS and their mixtures is based on consideration of the evidence supporting the factors individually and as a whole.
EPA's preliminary determination that PFHxS, HFPO-DA, PFNA, and PFBS “may have an adverse effect on the health of persons” is strongly supported by numerous studies where multiple health effects are demonstrated following exposure. EPA's preliminary determination regarding occurrence is supported by evidence documenting the trend demonstrated first by the UCMR 3 data and then subsequent state occurrence data that measured occurrence of the four PFAS has increased with more widespread monitoring primarily using EPA approved methods that have, lower reporting thresholds. The statute contemplates that there may be instances where exact occurrence may not be “known” and in these instances EPA need only demonstrate that that it has a basis to determine that there is a “substantial likelihood that the contaminant will occur.” Additional nationwide monitoring data will be conducted between 2023-2025 under the UCMR 5. This data will serve to demonstrate whether the four PFAS are known to occur, however, EPA has sufficient evidence now to support a preliminary determination there is a substantial likelihood that these PFAS will occur frequently and at concentrations where they are likely to exceed their respective HRLs based on the increased occurrence trends documented by available information. This finding is further supported by available dose additive impacts and co-occurrence information that demonstrates that there is a substantial likelihood that these PFAS co-occur in PWSs with a frequency and at levels of public health concern at hundreds of systems serving millions of people. Finally, EPA's preliminary determination that regulating these four PFAS presents a meaningful opportunity for health risks reductions is strongly supported by numerous bases, including the potential adverse human health effects and potential for exposure and co-exposure of these PFAS, and the availability of both analytical methods to measure and treatment technologies to remove these contaminants in drinking water.
After considering these factors individually and together, EPA has preliminarily determined that now is the appropriate time to exercise its discretion under the statute to regulate the four PFAS and their mixtures as contaminants under SDWA. EPA recognizes the public health burden of PFHxS, HFPO-DA, PFNA, and PFBS, as well as PFOA, PFOS, and other PFAS, a public urgency to reduce PFAS concentrations in drinking water, and that the proposed regulation provides a mechanism to reduce these PFAS expeditiously and efficiently for regulated utilities, States, and Tribes. Furthermore, in addition to making this preliminary regulatory determination, EPA is concurrently proposing an NPDWR to include all four of these PFAS, in part to allow utilities to consider these PFAS specifically as they design systems to remove PFAS and to ensure that they are reducing these PFAS in their drinking water as effectively and quickly as feasible, maximizing the protection of drinking water for the American public.
F. Request for Comment on EPA's Preliminary Regulatory Determination for PFHxS, HFPO-DA, PFNA, and PFBS
EPA specifically requests comment on its preliminary regulatory determination for PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures. In particular, EPA requests comment on whether there is additional health information the Agency should consider as to whether PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures may have an adverse effect on the health of persons. EPA also requests comment on whether there are other peer-reviewed health or toxicity assessments for other PFAS the Agency should consider as part of this action. Additionally, EPA requests comment on additional occurrence data the Agency should consider regarding its decision that PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures occur or are substantially likely to occur in PWSs with a frequency and at levels of public health concern. EPA also requests public comment on its evaluation that regulation of PFHxS, HFPO-DA, PFNA, and PFBS and their mixtures, in addition to PFOA and PFOS, will provide protection from PFAS that will not be regulated as part of this proposed PFAS NPDWR.
IV. Approaches to MCLG Derivation
Section 1412(a)(3) of the SDWA requires the Administrator of the EPA to propose a MCLG simultaneously with the NPDWR. The MCLG is set, as defined in Section 1412(b)(4)(A), at “the level at which no known or anticipated adverse effects on the health of persons occur and which allows an adequate margin of safety”. Consistent with SDWA 1412(b)(3)(C)(i)(V), in developing the MCLG, EPA considers “the effects of the contaminant on the general population and on groups within the general population such as infants, children, pregnant women, the elderly, individuals with a history of serious illness, or other subpopulations that are identified as likely to be at greater risk of adverse health effects due to exposure to contaminants in drinking water than the general population.” Other factors considered in determining MCLGs include health effects data on drinking water contaminants and potential sources of exposure other than drinking water. MCLGs are not regulatory levels and are not enforceable.
EPA is proposing individual MCLGs for two PFAS (PFOA and PFOS; see USEPA, 2023b; USEPA, 2023c) and a separate MCLG to account for dose additive noncancer effects for a mixture of four PFAS (PFHxS, HFPO-DA, PFNA, and PFBS; see USEPA, 2023d). The derivation of the proposed MCLG for the mixture is based on an HI approach (USEPA, 2023a).
The SAB, discussed further in section XV.K.1. of this preamble below, supported many of EPA's conclusions presented in the PFOA and PFOS MCLG approaches, mixtures framework, and economics benefits documents including health effects and economic benefits analyses (USEPA, 2022a). Regarding the Proposed Approaches to the Derivation of Draft MCLGs for PFOA and PFOS (USEPA, 2021e; USEPA, 2021f), SAB agreed with the selection of the UFs used in deriving the noncancer RfDs, supported the selection of an RSC of 20%, and agreed with the “likely” designation for PFOA carcinogenicity.
The SAB commented that EPA should “focus on those health outcomes that have been concluded to have the strongest evidence” and “consider multiple human and animal studies for a variety of endpoints in different populations so as to provide convergent evidence that is more reliable than any single study or health endpoint in isolation.” EPA applied these recommendations when deriving points of departure and selecting critical studies used for toxicity value development in the MCLG documents for PFOA and PFOS (USEPA, 2023b; USEPA, 2023c). Specifically, EPA focused on the five health outcomes with the strongest weight of evidence—liver, immune, cardiovascular, developmental, and cancer—during quantitative analyses.
However, the SAB had a number of consensus recommendations and identified “methodological concerns in the draft MCLG documents for PFOA and PFOS.” EPA has addressed these concerns by providing additional clarity and transparency on the systematic literature review process and expanding the systematic review steps included in the health effects assessment. The systematic review protocols, which were developed to be consistent with EPA's Office of Research and Development (ORD) Integrated Risk Information System (IRIS) Staff Handbook (USEPA, 2022f), are available in the Appendices of the MCLG documents for PFOA and PFOS (USEPA, 2023b; USEPA, 2023c). In order to base the MCLG derivation on the best available science, EPA has updated the draft MCLG documents to reflect the results of conducting an update to the literature search and performing new evaluations of models, methods, and data. More information is available in section XV.K.1. of this preamble.
EPA expects to conduct a final literature search update before the final rule is promulgated. The SAB input has made this product more scientifically sound and ensures that it reflects the best available science. The updated supporting information can be found in the MCLG documents for PFOA and PFOS (USEPA, 2023b; USEPA, 2023c).
A. Approach to MCLG Derivation for Individual PFAS
To establish the MCLG, EPA assesses the peer reviewed science examining cancer and noncancer health effects associated with oral exposure to the contaminant. For linear carcinogenic contaminants, where there is a proportional relationship between dose and carcinogenicity at low concentrations, EPA has a long-standing practice of establishing the MCLG at zero (see USEPA, 1998a; USEPA, 2000d; USEPA, 2001). For nonlinear carcinogenic contaminants, contaminants that are suggestive carcinogens, and non-carcinogenic contaminants, EPA typically establishes the MCLG based on an RfD. An RfD is an estimate of a daily exposure to the human population (including sensitive populations) that is likely to be without an appreciable risk of deleterious effects during a lifetime. A nonlinear carcinogen is a chemical agent for which the associated cancer response does not increase in direct proportion to the exposure level and for which there is scientific evidence demonstrating a threshold level of exposure below which there is no appreciable cancer risk.
The MCLG is derived depending on the noncancer and cancer evidence for a particular contaminant. Establishing the MCLG for a chemical has historically been accomplished in one of three ways depending upon a three-category classification approach (USEPA, 1985; USEPA, 1991a). The categories are based on the available evidence of carcinogenicity after exposure via ingestion. The starting point in categorizing a chemical is through assigning a cancer descriptor using EPA's current Guidelines for Carcinogen Risk Assessment (USEPA, 2005). The 2005 Guidelines replaced the prior alphanumeric groupings although the basis for the classifications is similar. In prior rulemakings, the Agency typically placed Group A, B1, and B2 contaminants into Category I, Group C into Category II, and Group D and E into Category III based on the Agency's previous cancer classification guidelines ( i.e., Guidelines for Carcinogen Risk Assessment, published in 51 FR 33992, September 24, 1986 (USEPA, 1986b) and the 1999 draft revised final guidelines (USEPA, 1999):
• Category I chemicals have “strong evidence [of carcinogenicity] considering weight of evidence, pharmacokinetics, and exposure” (USEPA, 1985; USEPA, 1991a). EPA's 2005 Cancer descriptors associated with this category are: “Carcinogenic to Humans” or “Likely to be Carcinogenic to Humans” (USEPA, 2005). EPA's policy under SDWA is to set MCLGs for Category I chemicals at zero, based on the principle that there is no known threshold for carcinogenicity (USEPA, 1985; USEPA, 1991a; USEPA, 2016d). In cases when there is sufficient evidence to determine a nonlinear cancer mode of action (MOA), the MCLG is based on the RfD approach described below.
• Category II chemicals have “limited evidence [of carcinogenicity] considering weight of evidence, pharmacokinetics, and exposure” (USEPA, 1985; USEPA, 1991a). EPA's 2005 Cancer descriptor associated with this category is: “Suggestive Evidence of Carcinogenic Potential” (USEPA, 2005). The MCLG for Category II contaminants is based on noncancer effects (USEPA, 1985; USEPA, 1991a) as described below.
• Category III chemicals have “inadequate or no animal evidence [of carcinogenicity]” (USEPA, 1985; USEPA, 1991a). EPA's 2005 Cancer descriptors associated with this category are: “Inadequate Information to Assess Carcinogenic Potential” and “Not Likely to Be Carcinogenic to Humans” (USEPA, 2005). The MCLG for Category III contaminants is based on noncancer effects as described below.
For chemicals exhibiting a noncancer threshold for toxic effects ( e.g., Category II or III; e.g., see USEPA, 1985 and USEPA, 1991a) and nonlinear carcinogens ( e.g., see USEPA, 2006a), EPA establishes the MCLG based on a toxicity value, typically an RfD, but similar toxicity values may also be used when they represent the best available science ( e.g., ATSDR Minimal Risk Level). A noncancer MCLG is designed to be protective of noncancer effects over a lifetime of exposure with an adequate margin of safety, including for sensitive populations and life stages, consistent with SDWA 1412(b)(3)(C)(i)(V) and 1412(b)(4)(A). The calculation of a noncancer MCLG includes an oral toxicity reference value such as an RfD (or Minimal Risk Level), DWI-BW, and RSC as presented in the equation below:
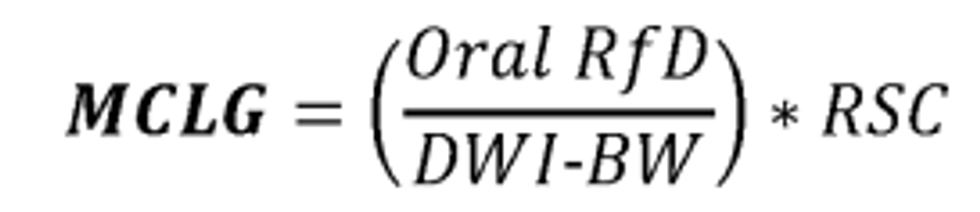
Where:
RfD 3 = reference dose—an estimate (with uncertainty spanning perhaps an order of magnitude) of a daily oral exposure of the human population to a substance that is likely to be without an appreciable risk of deleterious effects during a lifetime. The RfD is equal to a point-of-departure (POD) divided by a composite UF.
3 A reference dose (RfD) is an estimate of the amount of a chemical a person can ingest daily over a lifetime (chronic RfD) or less (subchronic RfD) that is unlikely to lead to adverse health effects in humans.
DWI-BW = An exposure factor in the form of the 90th percentile DWI-BW for the identified population or life stage, in units of liters of water consumed per kilogram BW per day (L/kg/day). The DWI-BW considers both direct and indirect consumption of drinking water (indirect water consumption encompasses water added in the preparation of foods or beverages, such as tea or coffee). Chapter 3 of EPA's Exposure Factors Handbook (USEPA, 2019a) provides DWI-BWs for various populations or life stages within the general population for which there are publicly available, peer-reviewed data such as National Health and Nutrition Examination Survey (NHANES) data.
RSC = relative source contribution—the percentage of the total exposure attributed to drinking water sources (USEPA, 2000c), with the remainder of the exposure allocated to all other routes or sources.
EPA established internal protocols for the systematic review steps of literature search, Population, Exposure, Comparator, and Outcomes (PECO) development, literature screening, study quality evaluation, and data extraction prior to conducting the systematic review for PFOA and PFOS. However, EPA recognizes that while components of the protocols were included in the November 2021 draft Proposed Approaches documents (USEPA, 2021e; USEPA, 2021f), the protocols were only partially described in those documents. EPA has incorporated detailed, transparent, and complete protocols for all steps of the systematic review process into the Proposed MCLG documents (USEPA, 2023b; USEPA, 2023c). Additionally, the protocols and methods have been updated and expanded based on SAB recommendations to improve the transparency of the process used to derive the MCLGs for PFOA and PFOS and to be consistent with the ORD Staff Handbook for Developing IRIS Assessments (USEPA, 2022f). For additional details of EPA's systematic review methods, see USEPA (2023b, 2023c; Chapter 2 and Appendix A).
EPA evaluated strengths and limitations of each study to determine an overall classification of high, medium, low, or uninformative with respect to confidence in the quality and reliability of the study (this was done for each endpoint evaluated in each study). High, medium, and low confidence studies were prioritized for qualitative assessments, while only high and medium confidence studies were prioritized for quantitative assessments. Within each health outcome, the evidence from epidemiology and animal toxicity studies was synthesized. For noncancer health outcomes, the animal toxicological and epidemiological evidence for each health outcome was classified as either robust, moderate, slight, indeterminate, or compelling evidence of no effect. The weight of evidence for each health outcome across all available evidence ( i.e., epidemiology, animal toxicity, and mechanistic studies) was classified as either evidence demonstrates, evidence indicates (likely), evidence suggests, evidence inadequate, or strong evidence supports no effect. To characterize the weight of evidence for cancer effects, EPA followed recommendations of the Guidelines for Carcinogen Risk Assessment (USEPA, 2005). Further description of the methods used to make these determinations for PFOA and PFOS is provided in USEPA (2023b; 2023c). Consistent with the recommendations of the SAB and to ensure that the rule reflects the best available science, EPA continues to evaluate the literature using systematic review methods.
The approach to select the DWI-BW and RSC for MCLG derivation includes a step to identify sensitive population(s) or life stage(s) ( i.e., populations or life stages that may be more susceptible or sensitive to a chemical exposure) by considering the available data for the contaminant, including the adverse health effects reported in the toxicity study on which the RfD was based (known as the critical effect within the critical or principal study). Although data gaps can complicate identification of the most sensitive population ( e.g., not all windows or life stages of exposure or health outcomes may have been assessed in available studies), the critical effect and POD that form the basis for the RfD (or Minimal Risk Level) can provide some information about sensitive populations because the critical effect is typically observed within the low dose range among the available data. Evaluation of the critical study, including the exposure window or interval, may identify a sensitive population or life stage ( e.g., pregnant women, formula-fed infants, lactating women). In such cases, EPA can select the corresponding DWI-BW for that sensitive population or life stage from the Exposure Factors Handbook (USEPA, 2019a) to derive the MCLG. In the absence of information indicating a sensitive population or life stage, the DWI-BW corresponding to the general population may be selected for use in MCLG derivation.
To account for potential aggregate risk from exposures and exposure pathways other than oral ingestion of drinking water, EPA applies an RSC when calculating MCLGs to ensure that total exposure to a contaminant does not exceed the daily exposure associated with the toxicity value, consistent with USEPA (2000c) and long-standing EPA methodology for establishing drinking water MCLGs and NPDWRs. The RSC represents the proportion of an individual's total exposure to a contaminant that is attributed to drinking water ingestion (directly or indirectly in beverages like coffee, tea, or soup, as well as from transfer to dietary items prepared with drinking water) relative to other exposure pathways. The remainder of the exposure equal to the RfD (or Minimal Risk Level) is allocated to other potential exposure sources (USEPA, 2000c). The purpose of the RSC is to ensure that the level of a contaminant ( e.g., MCLG), when combined with other identified potential sources of exposure for the population of concern, will not result in exposures that exceed the RfD (or Minimal Risk Level) (USEPA, 2000c).
To determine the RSC, EPA follows the Exposure Decision Tree for Defining Proposed RfD (or POD/UF) Apportionment in EPA's Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (USEPA, 2000c). EPA considers whether there are significant known or potential uses/sources of the contaminant other than drinking water, the adequacy of data and strength of evidence available for each relevant exposure medium and pathway, and whether adequate information on each exposure source is available to quantitatively characterize the exposure profile. The RSC is developed to reflect the exposure to the general population or a sensitive population within the general population. When exposure data are available for multiple sensitive populations or life stages, the most health-protective RSC is selected. In the absence of adequate data to quantitatively characterize exposure to a contaminant, EPA typically selects an RSC of 20 percent (0.2). When scientific data demonstrating that sources and routes of exposure other than drinking water are not anticipated for a specific pollutant, the RSC can be raised as high as 80 percent based on the available data, thereby allocating the remaining 20 percent to other potential exposure sources (USEPA, 2000c).
B. Approach to MCLG Derivation for a PFAS Mixture
There has been a lot of work evaluating parameters that best inform the combining of PFAS components identified in environmental matrices into mixtures analyses. Indeed, there is currently no consensus on whether or how PFAS should be combined for risk assessment purposes. EPA considered several approaches to account for dose additive noncancer effects associated with PFHxS, HFPO-DA, PFNA, and PFBS in mixtures. PFAS can affect multiple human health endpoints and differ in their impact ( i.e., potency of effect) on target organs/systems. PFAS disrupt signaling of multiple biological pathways resulting in common adverse effects on several biological systems and functions, including thyroid hormone levels, lipid synthesis and metabolism, development, and immune and liver function (ATSDR, 2021; EFSA, 2018, 2020; EPA, 2023d). For example, one PFAS may be most toxic to the liver, and another may be most toxic to the thyroid but both chemicals affect the liver and the thyroid. Other chemicals regulated as groups operate through a common MOA and predominately affect one human health endpoint. This supports a flexible data-driven approach that facilitates the evaluation of multiple health endpoints, such as the HI.
EPA is proposing to establish an MCLG for a mixture of chemicals that are expected to impact multiple endpoints. SDWA requires the agency to establish a health-based MCLG set at, “a level at which no known or anticipated adverse effects on the health of persons occur and which allow for an adequate margin of safety. EPA's SAB opined that where the health endpoints of the chosen compounds are similar, “the HI methodology is a reasonable approach for estimating the potential aggregate health hazards associated with the occurrence of chemical mixtures in environmental media. The HI is an approach based on dose additivity (DA) that has been validated and used by EPA” (USEPA, 2022a). This proposal is based on the Agency's finding that the general HI approach is the most efficient and effective approach for establishing an MCLG for PFAS mixtures consistent with the statutory requirement described above. This finding is based on the level of protection afforded by both the HBWCs for the individual PFAS as components of a mixture and the resulting HI itself, which provides an added margin of safety with respect to potential health hazards of mixtures of these PFAS. An HI greater than 1.0 is generally regarded as an indicator of potential adverse health risks associated with exposure to the mixture (USEPA, 1986a; USEPA, 1991b; USEPA, 2000a). A HI less than or equal to 1.0 is generally regarded as having no appreciable risk (USEPA, 1986a; USEPA, 1991b; USEPA, 2000a). The proposed MCLG is based on using this HI of 1.0, and the HBWCs of each mixture component, which in turn is based on its respective health-based reference value (RfV; RfD or MRL). Because the RfV represents an estimate at which no appreciable risk of deleterious effects exists (USEPA, 1986a, 1991a, 2000a), the use of the HBWCs means that the HI of 1.0 will ensure that there are no known or anticipated effects on the health of persons and allow for an adequate margin of safety. In addition, the resulting HI adds an additional margin of safety for mixtures of the four PFAS, to address the potential for additive toxicity where the contaminants co-occur and the HBWCs for the individual components are less than 1.0. The Agency therefore proposes the general HI approach as the basis for the MCLG, and because treatment to this level is also feasible, the MCL for these PFAS, (see additional discussion in section VI of this preamble) and welcomes public comment on its findings.
EPA considered the two main types of HI approaches: (1) the general HI which allows for component chemicals in the mixture to have different health effects or endpoints as the basis for the component chemical reference values ( e.g., RfDs), and (2) the target-organ specific HI which relies on reference values based on the same organ or organ system ( e.g., liver-, thyroid-, or developmental-specific). The general HI approach is based on the overall RfD which is protective of all effects for a given chemical, and thus is a more health protective indicator of risk. The target-organ specific HI approach produces a less health protective estimate of risk than the general HI when a contaminant impacts multiple organs because the range of potential effects has been scoped to a specific target organ, which may be one of the less potent effects or for which there may be significant currently unquantified effects. Additionally, a target-organ specific HI approach relies on toxicity values aggregated by the “same” target organ endpoint/effect, and the absence of information about a specific endpoint may result in the contaminant not being adequately considered in a target-organ specific approach, and thus, underestimating potential health risk. A target-organ specific HI can only be performed for those PFAS for which a health effect specific RfD is calculated. For example, for some PFAS a given health effect might be poorly characterized or not studied at all, or, as a function of dose may be one of the less(er) potent effects in the profile of toxicity for that particular PFAS. Another limitation is that so many PFAS lack human epidemiological or experimental animal hazard and dose-response information across a broad(er) effect range thus limiting derivation of target-organ specific values. A similar, effect/endpoint-specific method called the relative potency factor (RPF) approach, which represents the relative difference in potency of an effect/endpoint between an index chemical and other members of the mixture, was also considered. (Further background on all of these approaches, plus illustrative examples, and a discussion of the advantages and challenges associated with each approach can be found in Section 5 and 6 in USEPA, 2023d).
EPA also considered setting individual MCLGs instead of and in addition to using a mixtures-based approach for PFHxS, HFPO-DA, PFNA, and/or PFBS in mixtures. EPA ultimately selected the general HI approach for establishing an MCLG for these four PFAS, as described in greater detail below, because it provides the most health protective endpoint for multiple PFAS in a mixture to ensure there would be no known or anticipated adverse effects on the health of persons. EPA also considered a target-specific HI or RPF approach but, because of information gaps, EPA may not be able to ensure that the MCLG is sufficiently health protective. If the Agency only established an individual MCLG, the Agency would not provide any protection against dose-additivity from regulated co-occurring PFAS. EPA is seeking comments on the merits and drawbacks of each of the approaches described above. As discussed later in this proposal, EPA is also seeking comment on whether to set MCLGs for the individual PFAS in addition to or instead of setting them for the mixture.
EPA is proposing use of the general HI approach. Although EPA's SAB opined that it is reasonable to use a HI for evaluation of mixtures of PFAS in drinking water for situations where the profile of health effects of the chosen compounds share similarity in one or more effect domains, the SAB emphasized that using a HI in the context of developing regulations for PFAS should not be directly interpreted as a quantitative estimate of mixture risk. Rather the SAB agreed that the HI can be used as an indicator of potential health risk(s) associated with exposure to mixtures of PFAS; see discussion in USEPA (2023d) and Section V of this preamble for further information. EPA addresses the full range of responses to SAB comments in a response to comment document; that document is included in the docket for this action (USEPA, 2023f).
EPA proposes that the general HI is the most appropriate and justified approach for considering PFAS mixtures in this rulemaking because of the level of protection afforded for the evaluation of chemicals with diverse (but in many cases shared) health endpoints. SDWA requires the agency to establish a MCLG set at, “a level at which no known or anticipated adverse effects on the health of persons occur and which allow for an adequate margin of safety.” In this context, EPA has made a reasonable policy choice for regulating a mixture of chemicals that are expected to adversely impact multiple health endpoints. Because mixture component chemical HBWCs are based on overall lowest RfDs across candidate critical effects, the approach is protective against all health effects across component chemicals and therefore meets the statutory requirements of establishing an MCLG under SDWA. Basing the mixture MCLG on overall RfDs ensures that there are no known or anticipated effects, and using the HI adds an appropriate margin of safety for a class of contaminants that have been shown to co-occur and evidence suggests that they may have dose additive toxicity. Conversely, by definition, a target-organ specific ( e.g., liver-, thyroid-, or developmental-specific) HI or RPF approach would not be protective of all health effects across the four PFAS proposed for regulation with the mixture MCLG.
Use of the general HI approach over the target-organ specific HI for these four PFAS is supported by EPA guidance (EPA, 2000a) and available health assessments and toxicity values (overall RfDs). Target-organ specific reference values and RPFs are not currently available for HFPO-DA, PFBS, PFHxS, and PFNA.
EPA's protocol for MCLG development for the mixture of PFHxS, HFPO-DA, PFNA, and PFBS follows existing Agency guidance, policies, and procedures related to the three key inputs ( i.e., RfD/Minimal Risk Level, DWI-BW, and RSC) and longstanding Agency mixtures guidance (USEPA, 1986a; USEPA, 2000a) to address dose additive health effects. First, EPA identifies or derives a HBWC, calculated using the MCLG equation above, for each of the four individual PFAS in the mixture. More information on HBWCs for PFHxS, HFPO-DA, PFNA, and PFBS is available in section III.B of this preamble. Peer reviewed, publicly available assessments for PFHxS (ATSDR, 2021), HFPO-DA (USEPA, 2021b), PFNA (ATSDR, 2021), and PFBS (USEPA, 2021a) provide the chronic reference values (RfD, adjusted Minimal Risk Level) used to calculate the HBWCs for these four PFAS. The DWI-BW and RSC for each of the four PFAS are determined as described using the processes described for individual PFAS (Section IV.A of this preamble). Briefly, the DWI-BW for each of the four PFAS is selected from the EPA Exposure Factors Handbook (USEPA, 2019a), taking into account the relevant sensitive population(s) or life stage(s). RSCs are determined based on a literature review of potential exposure sources of the four PFAS and using the Exposure Decision Tree approach (USEPA, 2000c).
The HI is based on an assumption of dose addition (DA) among the mixture components (Svendsgaard and Hertzberg, 1994; USEPA, 2000a). An important aspect of the proposed `general HI' approach is that it is based on the availability of a reference value regardless of the critical effect for each mixture component. Unlike a target-organ specific Hazard Index which is typically based on either shared mode-of-action or shared health outcome of mixture components, the general HI is based on a non-cancer reference value (RfD or Minimal Risk Level) for the critical (usually the most sensitive) effect of each component (USEPA, 2000a; USEPA, 1989). Importantly, while many PFAS share some common target organs/health outcomes such as liver toxicity, the potency—and in some cases, even the overall most sensitive target organ—differs among PFAS. As an example, the most sensitive organ to HFPO-DA is the liver while the most sensitive organ to PFBS is the thyroid. Integrating the overall RfDs for each mixture PFAS in the calculation of component HQs and a corresponding mixture HI, regardless of the critical (most sensitive) effect, ensures health protection under an assumption of dose additivity. The alternative may underestimate potential health risk(s) associated with exposure to a PFAS mixture as a given effect-specific HI might entail the use of target-organ specific reference values that are not protective of effects at a given mixture component's corresponding overall RfD. Further, effect-specific RfDs are not typically derived for chemicals beyond the critical effect for the overall RfD which might prohibit the inclusion of a chemical in a target-organ specific HI. Recognizing the various nuances to the HI approach, EPA welcomes public comment.
In the HI approach, an HQ is calculated as the ratio of human exposure (E) to a health-based reference value (RfV) for each mixture component chemical (i) (USEPA, 1986a). The HI involves the use of RfVs for each PFAS mixture component (in this case, PFHxS, HFPO-DA, PFNA, and PFBS), which have been selected based on sensitive health outcomes that are protective of all other adverse health effects observed after exposure to the individual PFAS. Thus, this approach, which protects against all adverse effects, not only a single adverse outcome/effect ( e.g., as would be the case using other mixture approaches such as the target-organ specific HI or RPF approach), is a health protective risk indicator and appropriate for MCLG development. The HI is unitless; in the HI formula, E and the RfV must be in the same units. For example, if E is the oral intake rate (mg/kg/day), then the RfV could be the RfD or Minimal Risk Level, which have the same units. Alternatively, the exposure metric can be a media-specific metric such as a measured water concentration ( e.g., nanograms per liter or ng/L) and the RfV can be an HBWC ( e.g., ng/L). The component chemical HQs are then summed across the mixture to yield the HI. A mixture HI exceeding 1.0 indicates that the exposure metric is greater than the toxicity metric and there is potential concern for a given environmental medium or site, in this case, drinking water served to consumers from a PWS. The HI provides an indication of: (1) concern for the overall mixture and (2) potential driver PFAS ( i.e., those PFAS with high[er] HQs). The HI accounts for differences in toxicity among the mixture component chemicals rather than weighting them all equally. For a detailed discussion of PFAS dose additivity and the HI approach, see the PFAS Mixtures Framework (USEPA, 2023d). The HI is calculated through the following equation:
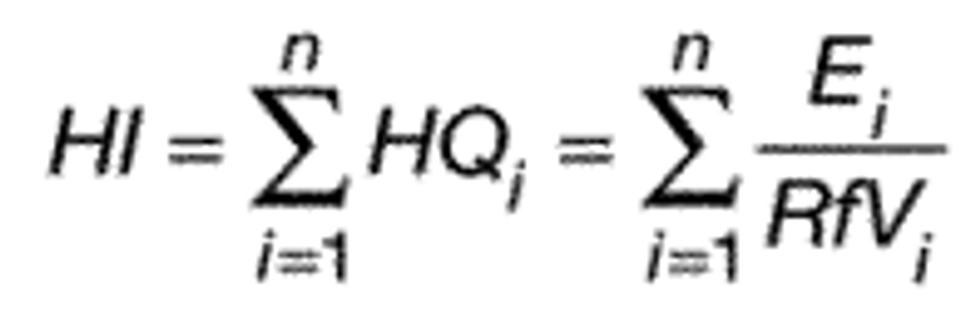
Where:
HI = Hazard Index
HQ i = Hazard Quotient for chemical i
E i = Exposure, i.e., dose (mg/kg/day) or occurrence concentration, such as in drinking water (mg/L), for chemical i
RfV i = Reference value ( e.g., oral RfD or Minimal Risk Level) [mg/kg/day], or corresponding HBWC; e.g., such as an MCLG for chemical i (in milligrams per liter or mg/L)
V. Maximum Contaminant Level Goals
A. PFOA
1. Carcinogenicity Assessment and Cancer Slope Factor (CSF) Derivation
a. Summary of Cancer Health Effects
The carcinogenicity of PFOA has been observed in both human epidemiological and animal toxicity studies. The evidence in high and medium confidence epidemiological studies is primarily based on the incidence of kidney and testicular cancer, as well as some medium quality studies providing limited evidence of breast cancer associated with exposure to PFOA. Other cancer types have been observed in human studies, although the evidence for these is largely from low confidence studies. The evidence of carcinogenicity in animal models was observed in three medium or high quality chronic oral animal studies in adult Sprague-Dawley rats which identified neoplastic lesions in the liver, pancreas, and testes after PFOA exposure.
Since publication of the 2016 PFOA Health Effects Support Document (HESD) (USEPA, 2016e), the evidence supporting the carcinogenicity of PFOA has been strengthened by additional published studies. In particular, the evidence of kidney cancer from highly exposed community studies (Vieira et al., 2013; Barry et al., 2013) is now supported by new evidence of renal cell carcinoma (RCC) from a nested case-control study in the general population (Shearer et al., 2021). In animal models, the evidence of multi-site tumorigenesis reported in two chronic bioassays in rats (Butenhoff et al., 2012a; Biegel et al., 2001) is now supported by new evidence from a third chronic bioassay in rats that also reports multi-site tumorigenesis (NTP, 2020).
The available evidence indicates that PFOA has carcinogenic potential in humans and at least one animal species. A plausible, though not definitively causal, association between human exposure to PFOA and kidney and testicular cancers in the general population and highly exposed populations is supported by the available evidence. As stated in the Guidelines for Carcinogen Risk Assessment (USEPA, 2005), “an inference of causality is strengthened when a pattern of elevated risks is observed across several independent studies.” Two medium confidence studies in independent populations provide evidence of an association between elevated PFOA serum concentrations and kidney cancer (Shearer et al., 2021; Vieira et al., 2013), while two studies from the same cohort provide evidence of an association between testicular cancer and elevated PFOA serum concentrations (Vieira et al., 2013; Barry et al., 2013). A recent National Academies of Science, Engineering, and Mathematics report on PFAS similarly “concluded that there is sufficient evidence for an association between PFAS and kidney cancer” (NASEM, 2022). The evidence of carcinogenicity in animals is from three studies in rats of the same strain. The results from these studies provide evidence of increased incidence of three tumor types (Leydig cell tumors (LCTs), pancreatic acinar cell tumors (PACTs), and hepatocellular adenomas) in males administered diets dosed with PFOA. Importantly, site concordance is not always assumed between humans and animal models; agents observed to produce tumors may do so at the same or different sites in humans and animals, as appears to be the case for PFOA (USEPA, 2005).
b. CSF Derivation
When a chemical is a linear carcinogen, a value that numerically describes the relationship between the dose of a chemical and the risk of cancer, is calculated. This is known as a cancer slope factor (CSF). The CSF is the cancer risk ( i.e., proportion affected) per unit of dose (USEPA, 2005). In addition to reevaluating the CSF previously derived and described in the 2016 HESD (USEPA, 2016e) based on LCTs in male rats observed by Butenhoff et al. (2012a), EPA derived CSFs for combined hepatocellular adenomas and carcinomas and pancreatic acinar cell adenomas in male rats observed by NTP (2020) and kidney cancer in humans reported by Shearer et al. (2021) and Vieira et al. (2013). EPA focused on the CSFs derived from the epidemiological data consistent with the EPA ORD handbook which states “when both laboratory animal data and human data with sufficient information to perform exposure-response modeling are available, human data are generally preferred for the derivation of toxicity values” (USEPA, 2022f).
EPA selected the critical effect of RCCs in human males reported by Shearer et al. (2021) as the basis of the CSF for PFOA. Shearer et al. (2021) is a multi-center case-control epidemiological study nested within the National Cancer Institute's (NCI) Prostate, Lung, Colorectal, and Ovarian Screening Trial (PLCO) with median PFOA levels relevant to the general U.S. population. The PLCO is a randomized clinical trial of the use of serum biomarkers for cancer screening. The cases in Shearer et al. (2021) included all the participants in the screening arm of the PLCO trial who were newly diagnosed with RCC during the follow-up period (N = 326) and all cases were histopathologically confirmed. Controls were selected among participants in the PLCO trial screening arm based on those who had never had RCC and were individually matched to the RCC cases by age at enrollment, sex, race/ethnicity, study center, and year of blood draw. Additionally, analyses conducted by the authors accounted for numerous confounders, including the potential for confounding by other PFAS. Study design advantages of the Shearer et al. (2021) compared with the Vieira et al. (2013) include specificity in the health outcome considered (RCC vs. any kidney cancer), the type of exposure assessment (serum biomarker vs. modeled exposure), source population (multi-center vs. Ohio and West Virginia regions), and study size (324 cases and 324 matched controls vs. 59 cases and 7,585 registry-based controls). The resulting CSF is 0.0293 (ng/kg/day) − 1 .
Selection of RCCs as the critical effect is supported by similar findings from other studies of a highly exposed community (Barry et al., 2013; Vieira et al., 2013), an occupational kidney cancer mortality study (Steenland and Woskie, 2012), as well as a meta-analysis of epidemiological literature that concluded that there was an increased risk of kidney tumors correlated with increased PFOA serum concentrations (Bartell et al., 2021). Further discussion of the rationale for endpoint and study selection and descriptions of the modeling methods are described in USEPA (2023b).
2. Assessment of Noncancer Health Effects and Reference Dose (RfD) Derivation
The Agency has also considered noncancer effects in its assessment of the best available science to derive the MCLG. As described in USEPA (2023b), there is evidence from both human epidemiological and animal toxicological studies that oral PFOA exposure may result in adverse health effects across many health outcomes, including but not limited to: immune, hepatic, developmental, cardiovascular, reproductive, and endocrine outcomes. As recommended by the SAB (USEPA, 2022a), EPA has largely focused its systematic literature review, health outcome synthesis, and toxicity value derivation efforts “on those health outcomes that have been concluded to have the strongest evidence, including the liver disease, immune system dysfunction, serum lipid aberration, impaired fetal growth, and cancer.” Conclusions regarding the four noncancer adverse health outcome categories ( i.e., judgements for human, animal, and integrated evidence streams (USEPA, 2023b)) are described in the subsections below. Descriptions of studies and the basis for conclusions about the non-prioritized health outcomes are described in USEPA (2023b).
a. Summary of Noncancer Health Effects
EPA determined that the evidence indicates that oral PFOA exposure is associated with adverse hepatic effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOA exposure and hepatic outcomes such as elevated serum liver enzymes indicative of hepatic damage. Overall, there is consistent evidence of a positive association between PFOA serum concentrations and alanine aminotransferase (ALT), a liver enzyme marker. The evidence of hepatic effects in humans was supported by robust evidence of hepatic effects resulting from PFOA exposure in animal studies. Several studies provide comprehensive histopathological reports of non-neoplastic hepatic lesions ( e.g., hepatocellular death and necrosis) in PFOA-treated rodents, as well as increases in serum liver enzymes similar to the trends observed in humans.
EPA determined that the evidence indicates that oral PFOA exposure is associated with adverse immunological effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOA and immune outcomes such as immunosuppression. Overall, there is consistent evidence of an association between PFOA serum concentrations and developmental immune effects ( i.e., reduced antibody response to vaccination in children). Associations between PFOA and other immune system effects ( e.g., hypersensitivity and autoimmune disease) were mixed. The evidence for developmental immunological effects in humans was supported by moderate evidence of immunotoxicity resulting from PFOA exposure in animal studies. Studies report varying manifestations of immune system effects including altered immune cell populations and altered spleen and thymus cellularity and weight. PFOA treatment resulted in reduced globulin and immunoglobulin levels in animals that are consistent with the decreased antibody response seen in human populations ( i.e., the observed animal and human study health outcomes are both indicators of immunosuppression).
EPA determined that the evidence indicates that oral PFOA exposure is associated with adverse developmental effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOA and developmental outcomes such as fetal growth. Overall, there is consistent evidence of a relationship between PFOA concentrations and low birth weight. Associations between PFOA and other developmental effects ( e.g., postnatal growth, fetal loss, and birth defects) were mixed. The evidence for developmental effects in humans was supported by robust evidence of developmental toxicity resulting from PFOA exposure in animal studies. Several studies in rodents provide evidence of decreased fetal and pup weight due to gestational PFOA exposure, consistent with the evidence of low birth weight in humans. Other pre- and post-natal effects observed in animal models include decreased offspring survival and developmental delays ( e.g., delayed eye opening).
EPA determined that the evidence indicates that oral PFOA exposure is associated with adverse cardiovascular effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOA and cardiovascular outcomes such as alterations in serum lipids. Overall, there is consistent evidence of positive relationships between PFOA serum concentrations and serum total cholesterol, low-density lipoproteins, and triglycerides. There is also limited evidence of positive associations of PFOA with blood pressure and hypertension among adult populations. The evidence for cardiovascular effects in humans was supported by moderate evidence of cardiovascular effects resulting from PFOA exposure in animal studies. Several studies in rodents provide evidence of alterations in serum total cholesterol and triglycerides, though the effect direction varied with dose. Regardless, these effects indicate a disruption in lipid metabolism resulting from PFOA treatment, consistent with the alterations in serum lipids observed in humans.
b. RfD Derivation
The databases for the four prioritized health outcomes were evaluated further for identification of medium and high confidence studies and endpoints to select for dose-response modeling. EPA prioritized endpoints with the strongest overall weight of evidence based on human and animal evidence for POD derivation. Specifically, EPA focused the dose response assessment on the health outcomes where the evidence indicated that PFOA causes health effects in humans under relevant exposure circumstances. The focus of this Federal Register Notice (FRN) is on epidemiological studies for the four prioritized health outcomes for which studies meeting this consideration were available, as human data are generally preferred “when both laboratory animal data and human data with sufficient information to perform exposure-response modeling are available” (USEPA, 2023b). EPA presents PODs and candidate RfDs for animal studies, as well as other health outcomes determined to have sufficient strength of evidence and studies suitable for dose-response modeling in USEPA (2023b).
EPA identified four candidate critical effects across the four prioritized health outcomes, all of which were represented by several candidate critical studies. These candidate critical effects are decreased antibody production in response to vaccinations (immune), low birth weight (developmental), increased serum total cholesterol (cardiovascular), and elevated ALT (hepatic). As described in the following paragraphs and in further detail in USEPA (2023b), EPA selected studies from each health outcome to proceed with candidate RfD derivation. For all selected candidate RfDs, the composite UF was 10 (10x for intraspecies variability). The candidate RfDs are presented in Table 3.
Two medium confidence studies were considered for POD derivation for the decreased antibody production in response to various vaccinations in children Budtz-Jørgensen and Grandjean (2018); and Timmerman et al. (2021). These candidate studies offer a variety of PFOA exposure measures across various populations and various vaccinations. Budtz-Jørgensen and Grandjean (2018) investigated anti-tetanus and anti-diphtheria responses in Faroese children aged 5-7 and Timmerman et al. (2021) investigated anti-tetanus and anti-diphtheria responses in Greenlandic children aged 7-12. Though the Timmerman et al. (2021) study is also a medium confidence study, the study by Budtz-Jørgensen and Grandjean (2018) has two additional features that strengthen the confidence in this RfD: (1) the response reported by this study was more precise in that it reached statistical significance, and (2) the analysis considered co-exposures of other PFAS. The RfD for anti-tetanus response in 7-year-old Faroese children and anti-diphtheria response in 7-year-old Faroese children, both from Budtz-Jørgensen and Grandjean (2018) were ultimately selected for the immune outcome as they are the same and have no distinguishing characteristics that would facilitate selection of one over the other.
Six high confidence studies (Chu et al., 2020; Govarts et al., 2016; Sagiv et al., 2018; Starling et al., 2017; Wikström et al., 2020; Yao et al., 2021) reported decreased birth weight in infants whose mothers were exposed to PFOA. These candidate studies offer a variety of PFOA exposure measures across the fetal and neonatal window. All six studies reported their exposure metric in units of ng/mL and reported the β coefficients per ng/mL or ln(ng/mL), along with 95% confidence intervals (CIs), estimated from linear regression models. Of the six individual studies, Sagiv et al. (2018) and Wikström et al. (2020) assessed maternal PFOA serum concentrations primarily or exclusively in the first trimester, minimizing concerns surrounding bias due to pregnancy-related hemodynamic effects. Therefore, the RfDs from these two studies were considered further for candidate RfD selection. Both were high confidence prospective cohort studies with many study strengths including sufficient study sensitivity and largely sound methodological approaches, analysis, and design, as well as no evidence of bias. The RfD from Wikström et al. (2020) was ultimately selected for the developmental outcome as it was the lowest candidate RfD from these two studies.
Three medium confidence studies were considered for POD derivation for the cholesterol endpoint (Dong et al., 2019; Lin et al., 2019; Steenland et al., 2009). These candidate studies offer a variety of PFOA exposure measures across various populations. Dong et al. (2019) investigated the NHANES population (2003-2014), while Steenland et al. (2009) investigated effects in a high-exposure community (the C8 Health Project study population). Lin et al. (2019) collected data from prediabetic adults from the Diabetes Prevention Program (DPP) and DPP Outcomes Study at baseline (1996-1999). Of the three studies, Dong et al. (2019) and Steenland et al. (2009) exclude those prescribed cholesterol medication, minimizing concerns surrounding confounding due to the medical intervention altering serum total cholesterol levels. Additionally, Dong et al. (2019) reported measured serum total cholesterol whereas Steenland et al. (2009) reported regression coefficients as the response variable. Since EPA prefers dose response modeling of endpoint data, the RfD from Dong et al. (2019) was selected for the cardiovascular outcome, as there is increased confidence in the modeling results from this study.
Four medium confidence studies were selected as candidates for POD derivation for the ALT endpoint (Gallo et al., 2012; Darrow et al., 2016; Nian et al., 2019; Lin et al., 2010). The two largest studies of PFOA and ALT in adults are Gallo et al. (2012) and Darrow et al. (2016), both conducted in over 30,000 adults from the C8 Study. Gallo et al. (2012) reported measured serum ALT levels, unlike Darrow et al. (2016) which reported a modeled regression coefficient as the response variable. Another difference between the two studies is reflected in exposure assessment: Gallo et al. (2012) includes measured PFOA serum concentrations, while Darrow et al. (2016) based PFOA exposure on modeled PFOA serum levels. Two additional studies (Lin et al., 2010; Nian et al., 2019) were considered by EPA for POD derivation because they reported significant associations in general populations in the U.S and a high exposed population in China, respectively. Nian et al. (2019) examined a large population of adults in Shenyang (one of the largest fluoropolymer manufacturing centers in China) part of the Isomers of C8 Health Project. In an NHANES adult population, Lin et al. (2010) observed elevated ALT levels per log-unit increase in PFOA. While this is a large nationally representative population, several methodological limitations, including lack of clarity about base of logarithmic transformation applied to PFOA concentrations in regression models and the choice to model ALT as an untransformed variable preclude its use for POD derivation. While both Nian et al. (2019) and Gallo et al. (2012) provide measured PFOA serum concentrations and a measure of serum ALT levels, the RfD for increased ALT from Gallo et al. (2012) was ultimately selected for the hepatic outcome as it was conducted in a community exposed predominately to PFOA whereas Nian et al. (2019) was in a community exposed predominately to PFOS, which reduces concerns about confounding from other PFAS.
| Study reference | Measurement of exposure and endpoint | Candidate RfD 1 (mg/kg/day) |
|---|---|---|
| Notes: | ||
| 1 RfDs are rounded to 1 significant digit. | ||
| Bolded values indicate selected health outcome-specific RfDs. | ||
| Immune | ||
| Budtz-Jørgensen and Grandjean, 2018 | PFOA at age five years and anti-tetanus antibody concentrations at age seven years | 3 × 10 − |
| Budtz-Jørgensen and Grandjean, 2018 | PFOA at age five years on anti-diphtheria antibody concentrations at age seven years | 3 × 10 − |
| Timmerman et al., 2021 | PFOA and anti-tetanus antibody concentrations at ages 7-10 years | 3 × 10 − 8 |
| Timmerman et al., 2021 | PFOA and anti-diphtheria antibody concentrations at ages 7-10 years | 2 × 10 − 8 |
| Developmental | ||
| Sagiv et al., 2018 | PFOA in first trimester and decreased birth weight | 1 × 10 − 7 |
| Wikström et al., 2020 | PFOA in first and second trimesters and decreased birth weight | 3 × 10 − |
| Cardiovascular | ||
| Dong et al., 2019 | Increased serum total cholesterol | 3 × 10 − |
| Steenland et al., 2009 | Increased serum total cholesterol | 5 × 10 − 8 |
| Hepatic | ||
| Gallo et al., 2012 | Increased serum ALT | 2 × 10 − |
| Darrow et al., 2016 | Increased serum ALT | 8 × 10 − 7 |
| Nian et al., 2019 | Increased serum ALT | 5 × 10 − 8 |
The available evidence indicates there are effects across immune, developmental, cardiovascular, and hepatic organ systems at the same or approximately the same level of PFOA exposure. Candidate RfDs within the immune, developmental, and cardiovascular outcomes are the same value ( i.e., 3 × 10-8 mg/kg/day). Therefore, EPA has selected an overall RfD for PFOA of 3 × 10-8 mg/kg/day. The immune, developmental and cholesterol RfDs and serve as co-critical effects and are protective of effects that may occur in sensitive populations ( i.e., infants and children), as well as hepatic effects that may result from PFOA exposure.
c. MCLG Derivation
Consistent with the Guidelines for Carcinogen Risk Assessment (USEPA, 2005), EPA reviewed the weight of the evidence and determined that PFOA is Likely to Be Carcinogenic to Humans, as “the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor Carcinogenic to Humans.” This determination is based on the evidence of kidney and testicular cancer in humans and LCTs, pancreatic acinar cell tumors, and hepatocellular adenomas in rats as described in USEPA (2023b).
Consistent with the statutory definition of MCLG, EPA establishes MCLGs of zero for carcinogens classified as Carcinogenic to Humans or Likely to be Carcinogenic to Humans where there is insufficient information to determine that a carcinogen has a threshold dose below which no carcinogenic effects have been observed. In this situation, EPA takes a health protective approach of assuming that there is no such threshold and that carcinogenic effects should therefore be extrapolated linearly to zero. This approach ensures that the MCLG is set at a level where there are no anticipated adverse health effects with a margin of safety. This is the linear default extrapolation approach. Here, EPA has determined that PFOA is Likely to be Carcinogenic to Humans based on sufficient evidence of carcinogenicity in humans and animals and has also determined that a linear default extrapolation approach is appropriate as there is no evidence demonstrating a threshold level of exposure below which there is no appreciable cancer risk (USEPA, 2005) and therefore, it is assumed that there is no known threshold for carcinogenicity (USEPA, 2016d). Based upon a consideration of the best available peer reviewed science and a consideration of an adequate margin of safety, EPA proposes a MCLG of zero for PFOA in drinking water.
EPA is seeking comment on the derivation of the proposed MCLG for PFOA and its determination that PFOA is Likely to be Carcinogenic to Humans and whether the proposed MCLG is set at the level at which there are no adverse effects to the health of persons and which provides an adequate margin of safety. EPA is also seeking comment on its assessment of the noncancer effects associated with exposure to PFOA and the toxicity values described in USEPA (2023b).
B. PFOS
1. Carcinogenicity Assessment and CSF Derivation
a. Summary of Cancer Health Effects
Several medium and high confidence human epidemiological studies and one high confidence animal chronic cancer bioassay comprise the evidence database for the carcinogenicity of PFOS. The available epidemiology studies reported elevated risk of bladder, prostate, kidney, and breast cancers after chronic PFOS exposure. While there are reports of cancer incidence from epidemiological studies, the study designs, analyses, and mixed results preclude a definitive conclusion about the relationship between PFOS exposure and cancer outcomes in humans. The one high confidence animal chronic cancer bioassay study provides evidence of multi-site tumorigenesis in both male and female rats.
While the epidemiological evidence of associations between PFOS and cancer found mixed results across tumor types, the available study findings support a plausible correlation between PFOS exposure and carcinogenicity in humans. The single chronic cancer bioassay performed in rats is positive for multi-site and -sex tumorigenesis (Thomford, 2002; Butenhoff et al., 2012b). In this study, statistically significant increases in the incidences of hepatocellular adenomas or combined hepatocellular adenomas and carcinomas were observed in both male and female rats. There was also a statistically significant dose-response trend of these tumors in both sexes. As described in USEPA (2023c), the available mechanistic evidence is consistent with multiple potential MOAs for this tumor type; therefore, the hepatocellular tumors observed by Thomford (2002)/Butenhoff et al. (2012b) may be relevant to humans. In addition to hepatocellular tumors, Thomford (2002)/Butenhoff et al. (2012b) reported increased incidences of pancreatic islet cell tumors with a statistically significant dose-dependent positive trend, as well as modest increases in the incidence of thyroid follicular cell tumors. The findings of multiple tumor types provide additional support for potential multi-site tumorigenesis resulting from PFOS exposure. Structural similarities between PFOS and PFOA add to the weight of evidence for carcinogenicity of PFOS. Notably, a similar set of noncancer effects have been observed after exposure to either PFOA or PFOS in humans and animal studies including similarities in hepatic, developmental, immunological, cardiovascular, and endocrine effects.
Under the Guidelines for Carcinogen Risk Assessment (USEPA, 2005), EPA reviewed the weight of the evidence and determined that PFOS is Likely to Be Carcinogenic to Humans, as “the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor Carcinogenic to Humans.” As described in USEPA (2023c), EPA determined that the available data for PFOS surpass many of the descriptions for the descriptor of Suggestive Evidence of Carcinogenic Potential.
b. CSF Derivation
The Thomford (2002)/Butenhoff et al. (2012b) chronic cancer study in male and female rats is of high confidence and provides multi-dose tumor incidence findings that are suitable for dose-response modeling and subsequent CSF derivation. As described in USEPA (2023c), EPA derived PODs and candidate CSFs for three endpoints reported by this study: hepatocellular adenomas in male rats; combined hepatocellular adenomas and carcinomas in female rats; and pancreatic islet cell carcinomas in male rats.
EPA selected the hepatocellular adenomas and carcinomas in female rats reported by Thomford (2002)/Butenhoff et al. (2012b) as the basis of the CSF for PFOS because there was a statistically significant increase in tumor incidence in the highest dose group, a trend of increased incidence with increasing PFOS concentrations across dose groups, and it was the most health-protective value. The resulting CSF is 39.5 (mg/kg/day)-1. Selection of hepatocellular adenomas and carcinomas in female rats is supported by statistically significant increases in hepatocellular tumor incidence in the high dose group as well as a statistically significant trend of this response observed in the male rats. The critical effect of pancreatic islet cell carcinomas was not selected as the basis of the CSF because the response of the high dose group was not statistically different from the control group, though the trend of response across dose groups was statistically significant. Further discussion on the rationale for endpoint selection and descriptions of the modeling methods are described in USEPA (2023c).
In support of the selection of hepatocellular tumors as the basis of the CSF for PFOS, a recently published study (Goodrich et al., 2022) reports associations between hepatocellular carcinomas and PFOS serum concentrations in humans. These findings provide further support for both MOA conclusions in USEPA (2023c) and the “Likely to Be Carcinogenic to Humans” designation. This study was published after the systematic literature review cutoff date for the proposed MCLG for PFOS (USEPA, 2023c), therefore EPA requests comment on the Goodrich et al. (2022) study and whether it supports EPA's “Likely to Be Carcinogenic to Humans” designation.
2. Assessment of Noncancer Health Effects and Reference Dose (RfD) Derivation
The Agency has also considered noncancer effects in its assessment of the best available science to derive the MCLG. As described in USEPA (2023c), there is evidence from both human epidemiological and animal toxicological studies that oral PFOS exposure may result in adverse health effects across many health outcomes, including but not limited to immune, hepatic, developmental, cardiovascular, nervous system, and endocrine outcomes. As recommended by the SAB (USEPA, 2022a), EPA has focused its systematic literature review, health outcome synthesis, and toxicity value derivation efforts “on those health outcomes that have been concluded to have the strongest evidence, including the liver disease, immune system dysfunction, serum lipid aberration, impaired fetal growth, and cancer.” Conclusions regarding the four noncancer adverse health outcome categories ( i.e., judgements for human, animal, and integrated evidence streams (USEPA, 2022f)) are described in the subsections below. Descriptions and conclusions about the non-priority health outcomes are described in USEPA (2023c).
a. Summary of Noncancer Health Effects
EPA determined that the evidence indicates that oral PFOS exposure is associated with adverse hepatic effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. Specifically, there is moderate evidence from epidemiological studies supporting an association between PFOS exposure and hepatic outcomes such as elevated serum liver enzymes indicative of hepatic damage. Overall, there is consistent evidence of a positive association between PFOS serum concentrations and ALT, a liver enzyme marker. The evidence of hepatic effects in humans was supported by robust evidence of hepatotoxicity resulting from PFOS exposure in animal studies. Studies in rodents observed several manifestations of hepatic toxicity including histopathological reports of non-neoplastic hepatic lesions ( e.g., hepatic necrosis and inflammation) and increases in serum liver enzymes similar to the trends observed in humans.
EPA determined that the evidence indicates that oral PFOS exposure is associated with adverse immunological effects based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOS and immune outcomes such immunosuppression. Overall, there is generally consistent evidence of an association between PFOS serum concentrations and reduced antibody response to vaccination in children. Associations between PFOS and other immune system effects ( e.g., hypersensitivity and asthma) were mixed. The evidence for immunological effects in humans was supported by moderate evidence of immunotoxicity resulting from PFOS exposure in animal studies. Studies in rodents report immune system effects including altered activity of plaque-forming cells and natural killer cells, altered spleen and thymus cellularity, and bone marrow hypocellularity and extramedullary hematopoiesis. The alterations in plaque-forming and natural killer cells in animals are consistent with the decreased antibody response seen in human populations ( i.e., the observed animal and human study health outcomes are both indicators of immunosuppression).
EPA determined that the evidence indicates that oral PFOS exposure is associated with adverse developmental effects, based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOS and developmental outcomes such as fetal growth and gestational duration. Overall, there is consistent evidence of a relationship between PFOS concentrations and low birth weight, preterm birth, and gestational age. Associations between PFOS and postnatal growth were inconsistent while there was limited evidence for other developmental effects ( e.g., fetal loss and birth defects). The evidence for developmental effects in humans was supported by moderate evidence of developmental toxicity resulting from PFOS exposure in animal studies. Several studies in rodents provide evidence of decreased fetal and pup weight due to gestational PFOS exposure, consistent with the evidence of low birth weight in humans. Decreased maternal BW was also observed. Other pre- and post-natal effects observed in animal models include increased offspring mortality, skeletal and soft tissue effects, and developmental delays ( e.g., delayed eye opening). However, some studies reported no indications of developmental toxicity.
EPA determined that the evidence indicates that oral PFOS exposure is associated with adverse cardiovascular effects, based on the study quality evaluation, evidence synthesis and evidence integration of the relevant human epidemiological and animal toxicity studies. There is moderate evidence from epidemiological studies supporting an association between PFOS and cardiovascular outcomes such as alterations in serum lipids. Overall, there is consistent evidence of positive relationships between PFOS serum concentrations and serum total cholesterol and low-density lipoproteins. There is also evidence of positive associations of PFOS with blood pressure and hypertension in adults. The evidence for cardiovascular effects in humans was supported by moderate evidence of cardiovascular effects resulting from PFOS exposure in animal studies. Several studies in rodents provide evidence of alterations in serum total cholesterol and triglycerides, though the effect direction varied with dose. Regardless, these effects indicate a disruption in lipid metabolism resulting from PFOS treatment, consistent with the alterations in serum lipids observed in humans.
b. RfD Derivation
The databases for the four prioritized health outcomes were evaluated further for identification of medium and high confidence studies and endpoints to select for dose-response modeling. EPA prioritized endpoints with the strongest overall weight of evidence based on human and animal evidence for POD derivation. Specifically, EPA focused the dose response assessment on the health outcomes where the evidence indicated that PFOS causes health effects in humans under relevant exposure circumstances. The focus of this FRN is on epidemiological studies for the four prioritized health outcomes for which studies meeting this consideration were available, as human data are generally preferred “when both laboratory animal data and human data with sufficient information to perform exposure-response modeling are available” (USEPA, 2022f). EPA presents PODs and candidate RfDs for animal studies, as well as other health outcomes determined to have sufficient strength of evidence and studies suitable for dose-response modeling in USEPA (2023c).
EPA identified four candidate critical effects across the four prioritized health outcomes, all of which were represented by several candidate critical studies. These candidate critical effects are decreased antibody production in response to vaccinations (immune), low birth weight (developmental), increased serum total cholesterol (cardiovascular), and elevated ALT (hepatic). As described in the following paragraphs and in further detail in USEPA (2023c), EPA selected studies from each health outcome to proceed with candidate RfD derivation. For all selected candidate RfDs, presented in Table 4, the composite UF was 10 (10x for intraspecies variability).
Two medium confidence studies were considered for POD derivation for the decreased antibody production in response to various vaccinations in children Budtz-Jørgensen and Grandjean (2018) and Timmerman et al. (2021). These candidate studies offer a variety of PFOS exposure measures across various populations and various vaccinations. Budtz-Jørgensen and Grandjean (2018) investigated anti-tetanus and anti-diphtheria responses in Faroese children aged 5-7 and Timmerman et al. (2021) investigated anti-tetanus and anti-diphtheria responses in Greenlandic children aged 7-12. Though the Timmerman et al. (2021) study is also a medium confidence study, the study by Budtz-Jørgensen and Grandjean (2018) has two features that strengthen the results: (1) the response reported by this study reached statistical significance, and (2) the analysis considered co-exposures of other PFAS. The RfD for anti-diphtheria response in 7-year-old Faroese children from Budtz-Jørgensen and Grandjean (2018) was ultimately selected for the immune outcome because the response reported by this study reached statistical significance, this analysis considered co-exposures of other PFAS, and it was the more health-protective of the two vaccine-specific responses reported by Budtz-Jørgensen and Grandjean (2018).
Six high confidence studies (Chu et al., 2020; Sagiv et al., 2018; Starling et al., 2017; Wikström et al., 2020; Darrow et al., 2013; Yao et al., 2021) reported decreased birth weight in infants whose mothers were exposed to PFOS. These candidate studies offer a variety of PFOS exposure measures across the fetal and neonatal window. All six studies reported their exposure metric in units of ng/mL and reported the β coefficients per ng/mL or ln(ng/mL), along with 95% CIs, estimated from linear regression models. Of the six individual studies, Sagiv et al. (2018) and Wikström et al. (2020) assessed maternal PFOS serum concentrations primarily or exclusively in the first trimester, minimizing concerns surrounding bias due to pregnancy-related hemodynamic effects. Therefore, the RfDs from these two studies were considered further for candidate RfD selection. Both were high confidence prospective cohort studies with many study strengths including sufficient study sensitivity and largely sound methodological approaches, analysis, and design, as well as no evidence of bias. The RfD from Wikström et al. (2020) was ultimately selected for the developmental outcome as it was the lowest candidate RfD from these two studies.
Three medium confidence studies were considered for POD derivation for the cholesterol endpoint (Dong et al., 2019; Lin et al., 2019; Steenland et al., 2009). These candidate studies offer a variety of PFOS exposure measures across various populations. Dong et al. (2019) investigated the NHANES population (2003-2014), while Steenland et al. (2009) investigated effects in a high-exposure community (the C8 Health Project study population). Lin et al. (2019) collected data from prediabetic adults from the DPP and DPP Outcomes Study at baseline (1996-1999). Of the three studies, Dong et al. (2019) and Steenland et al. (2009) exclude those prescribed cholesterol medication, minimizing concerns surrounding confounding due to the medical intervention altering serum total cholesterol levels. Additionally, Dong et al. (2019) reported measured serum total cholesterol whereas Steenland et al. (2009) reported modeled regression coefficients as the response variable. Since EPA prefers dose response modeling of measured data, the RfD from Dong et al. (2019) was selected for cardiovascular endpoint as there is increased confidence in the modeling from this study.
Three medium confidence studies were selected as candidates for POD derivation for the ALT endpoint (Gallo et al., 2012; Nian et al., 2019; Lin et al., 2010). The largest study of PFOS and ALT in adults is Gallo et al. (2012), conducted in over 30,000 adults from the C8 Study Project. Two additional studies (Lin et al., 2010; Nian et al., 2019) were considered by EPA for POD derivation because they reported significant associations in general populations in the U.S and a high exposed population in China, respectively. Nian et al. (2019) examined a large population of adults in Shenyang (one of the largest fluoropolymer manufacturing centers in China) part of the Isomers of C8 Health Project. In an NHANES adult population, Lin et al. (2010) observed elevated ALT levels per log-unit increase in PFOS. While this is a large nationally representative population, several methodological limitations, including lack of clarity about base of logarithmic transformation applied to PFOS concentrations in regression models and the choice to model ALT as an untransformed variable preclude its use for POD derivation. The RfD from Nian et al., 2019 was ultimately selected for the hepatic outcome as PFOS was the predominating PFAS in this study which reduces concern about potential confounding by other PFAS.
| Study | Endpoint | Candidate RfD 1 (mg/kg/day) |
|---|---|---|
| Notes: | ||
| 1 RfDs are rounded to 1 significant digit. | ||
| Bolded values indicate selected health outcome-specific RfDs. | ||
| Immune | ||
| Budtz-Jørgensen and Grandjean, 2018 | PFOS at age five years and anti-tetanus antibody concentrations at age seven years | 3 × 10 − 7 |
| Budtz-Jørgensen and Grandjean, 2018 | PFOS at age five years on anti-diphtheria antibody concentrations at age seven years | 2 × 10 − |
| Timmerman et al., 2021 | PFOS and anti-tetanus antibody concentrations at ages 7-10 years | 2 × 10 − 7 |
| Timmerman et al., 2021 | PFOS and anti-diphtheria antibody concentrations at ages 7-10 years | 1 × 10 − 7 |
| Developmental | ||
| Sagiv et al., 2018 | PFOS in first trimester and decreased birth weight | 6 × 10 − 7 |
| Wikström et al., 2020 | PFOS in first and second trimesters and decreased birth weight | 1 × 10 − |
| Cardiovascular | ||
| Dong et al., 2019 | Increased serum total cholesterol | 1 × 10 − |
| Steenland et al., 2009 | Increased serum total cholesterol | 1 × 10 − 7 |
| Hepatic | ||
| Gallo et al., 2012 | Increased serum ALT | 7 × 10 − 7 |
| Nian et al., 2019 | Increased serum ALT | 2 × 10 − |
The available evidence indicates there are effects across immune, developmental, cardiovascular, and hepatic organ systems at the same or approximately the same level of PFOS exposure. Candidate RfDs within the developmental and cardiovascular outcomes are the same value ( i.e., 1 × 10-7 mg/kg/day). Therefore, EPA has selected an overall RfD for PFOS of 1 × 10-7 mg/kg/day. The developmental and cholesterol RfDs serve as co-critical effects and are protective of immune and hepatic effects that may result from PFOS exposure.
c. MCLG Derivation
Consistent with the Guidelines for Carcinogen Risk Assessment (USEPA, 2005), EPA reviewed the weight of the evidence and determined that PFOS is Likely to Be Carcinogenic to Humans, as “the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor Carcinogenic to Humans.” This determination is based on the evidence of hepatocellular tumors in male and female rats, pancreatic islet cell carcinomas in male rats, and mixed but plausible evidence of bladder, prostate, kidney, and breast cancers in humans. As previously noted, the results provided by one chronic cancer bioassay in rats exceeds the descriptor of Suggestive Evidence of Carcinogenic Potential as it provides evidence of multi-site and multi-sex tumorigenesis (Thomford, 2002; Butenhoff et al., 2012b).
Consistent with the statutory definition of MCLG, EPA establishes MCLGs of zero for carcinogens classified as Carcinogenic to Humans or Likely to be Carcinogenic to Humans, described in Section V.A. of this preamble above as the linear default extrapolation approach. EPA has determined that PFOS is Likely to be Carcinogenic to Humans based on sufficient evidence of carcinogenicity in humans and animals and has also determined that a linear default extrapolation approach is appropriate as there is no evidence demonstrating a threshold level of exposure below which there is no appreciable cancer risk (USEPA, 2005) and therefore, it is assumed that there is no known threshold for carcinogenicity (USEPA, 2016d). Based upon a consideration of the best available peer reviewed science and a consideration of an adequate margin of safety, EPA proposes a MCLG of zero for PFOS in drinking water.
EPA is seeking comment on the derivation of the proposed MCLG for PFOS, its determination that PFOS is Likely to be Carcinogenic to Humans and whether the proposed MCLG is set at the level at which there are no adverse effects to the health of persons and which provides an adequate margin of safety. EPA is also seeking comment on its assessment of the noncancer effects associated with exposure to PFOS and the toxicity values described in USEPA (2023c).
C. PFAS Hazard Index: PFHxS, HFPO-DA, PFNA, and PFBS
1. Background
Although it would be optimal to leverage whole mixture data for human health risk assessment, such data for PFAS and other chemicals are extremely rare, particularly at component-chemical ( i.e., individual PFAS) proportions consistent with environmental mixtures. As such, mixtures assessment commonly relies upon integration of toxicity information for the individual component chemicals that co-occur in environmental media. In order to assess the potential health risks associated with PFAS mixtures, EPA has developed a Framework for Estimating Noncancer Health Risks Associated with Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) (“PFAS Mixtures Framework”) (USEPA, 2023d), based on existing EPA mixtures guidelines and guidance (USEPA, 1986a, 2000a). The PFAS Mixtures Framework describes a flexible approach that facilitates practical component-based mixtures evaluation of two or more PFAS based on dose additivity. Studies with PFAS and other classes of chemicals support the assumption that a mixture of chemicals with similar apical effects should be assumed to also act in a dose additive manner unless data demonstrate otherwise. This health protective assumption for PFAS mixture assessment was supported by the SAB in their recent review of the draft PFAS Mixtures Framework (USEPA, 2022a). All of the approaches described in the PFAS Mixtures Framework, including the HI approach (Section III of this preamble), involve integrating dose-response metrics that have been scaled based on the potency of each PFAS in the mixture. As discussed in section XV of this preamble, the SAB has reviewed the PFAS Mixtures Framework, and concluded that the approaches in that document, including the HI approach, are scientifically robust and defensible for assessing dose additive effects from co-occurring PFAS (USEPA, 2022a).
The MOA is considered a key determinant of chemical toxicity. It describes key changes in cellular interaction that may lead to functional or anatomical changes. Toxicants are classified by their type of toxic actions. Yet, because PFAS are an emerging chemical class of note for toxicological evaluations and human health risk assessment, MOA data may be limited or not available at all for many PFAS. Component-based approaches for assessing risks of PFAS mixtures are focused on evaluation of similarity of toxicity endpoint/effect rather than similarity in MOA, consistent with EPA mixtures guidance (USEPA, 2000a). Precedents of prior research conducted on mixtures of various chemical classes with common key events and adverse outcomes support the use of dose additive models for estimating mixture-based effects, even in instances where chemicals with disparate molecular initiating events were included. Thus, in the absence of detailed characterization of molecular mechanisms for most PFAS, it is considered a reasonable health-protective assumption, consistent with the statute's admonition to ensure an adequate margin of safety (1412(b)(4)(A)), that PFAS which can be demonstrated to share one or more key events or adverse outcomes will produce dose-additive effects from co-exposure (USEPA, 2022c, 2023a). This assumption of dose additivity and the HI approach was supported by the SAB in its review of the draft PFAS Mixtures Framework (USEPA, 2022a). For a detailed description of the evidence supporting dose additivity for PFOA, PFOS, and other PFAS, see the revised PFAS Mixtures Framework (USEPA, 2023d).
Following EPA's data-driven approach for component-based mixtures assessment based on dose additivity ( i.e., see Figure 4-1 in USEPA, 2023d), the Agency selected the HI approach for MCLG development to ensure the Agency is protecting against dose additive risk from mixtures of PFHxS, HFPO-DA, PFNA, and PFBS. While a single PFAS may occur in concentrations below where EPA might establish an individual MCLG, PFAS tend to co-occur (see discussion in sections III.C and VII of this preamble). Hence, there are some situations where setting an MCLG while only considering the concentration of an individual PFAS without considering the dose additive effects that would occur from other PFAS that may be present in a mixture may not provide a sufficiently protective MCLG with an adequate margin of safety. For this proposed rule, in addition to the PFOA and PFOS assessments discussed above, peer reviewed, publicly available assessments with final toxicity values ( i.e., RfDs, Minimal Risk Levels) are available for HFPO-DA (USEPA, 2021b), PFBS (USEPA, 2021a), PFNA (ATSDR, 2021), and PFHxS (ATSDR, 2021). These toxicity values (along with DWI-BW and RSC) are used to derive the HBWCs for the HI approach for PFHxS, HFPO-DA, PFNA, and PFBS. EPA is seeking comment on derivation of the HBWCs for each of the four PFAS considered as part of the HI. See discussion in section VI.C of this preamble as to why EPA is not proposing to include PFOA and PFOS in the HI MCLG at this time.
As discussed previously in this document, the Agency is proposing the general HI as the most appropriate and justified approach for considering PFAS mixtures in this rulemaking because of the level of protection afforded for diverse endpoints. SDWA requires the Agency to establish a health-based MCLG set at, “a level at which no known or anticipated adverse effects on the health of persons occur and which allow for an adequate margin of safety.” The Safe Drinking Water Act defines the term “contaminant” very broadly to mean any “physical, chemical, biological, or radiological substance or matter in water (SDWA 1401 4(A)(ii)(C)(6)).” In this context, this proposal addresses contaminants and certain mixtures of contaminants. A mixture of two or more “contaminants” qualifies as a “contaminant” because the mixture itself is “ any physical, chemical or biological or radiological substance or matter in water.” (emphasis added). EPA has a long-standing history of regulating contaminants in this manner ( i.e., as contaminant groups or mixtures). For instance, the TTHM Rule (U.S. EPA, 1979) EPA regulated total trihalomethanes as a group due to their concurrent formation during the chlorination of drinking water; EPA stating that the four regulated THMs were “also indicative of the presence of a host of other halogenated and oxidized, potentially harmful byproducts of the chlorination process that are concurrently formed in even larger quantities but which cannot be characterized chemically” (USEPA, 1979). In the Stage I and II Disinfection Byproduct (DBPs) Rules, EPA regulates a second group of DBPs, in this instance setting regulatory standards for a group of five haloacetic acids (HAA5) (USEPA, 1998a; 2006a). A third example is EPA's regulation of radionuclides, where, among other things, EPA regulates radionuclides mixtures for gross alpha radiation that account for both natural and man-made alpha emitters as a group rather than individually (USEPA, 2000d). In summary, EPA has the statutory authority to regulate groups and/or mixtures of contaminants, EPA has a history of regulating groups and mixtures of contaminants that have improved public health protection, and EPA has made a reasonable policy choice for establishing an MCLG for a mixture of chemicals that are expected to impact multiple endpoints. Because mixture component chemical HBWCs are based on overall ( i.e., not target-organ specific) RfDs, the approach is protective against all health effects across component chemicals and therefore meets the statutory requirements of establishing an MCLG under SDWA. Basing the mixture MCLG on overall RfDs ensures that there are no known or anticipated effects, and using the HI adds an appropriate margin of safety for a class of contaminants that have been shown to co-occur and evidence indicates that they have additive toxicity.
2. PFAS Mixture MCLG Derivation
To account for dose additive noncancer effects associated with PFHxS, HFPO-DA, PFNA, and PFBS, EPA is proposing an MCLG for the mixture of these four PFAS based on the HI approach (USEPA, 2023a). As described in Section IV of this preamble, a mixture HI can be calculated when HBWCs for a set of PFAS are available or can be calculated. The health effects information including relevant studies mentioned in this section are summarized from USEPA (2023a) and are also described in Section III of this preamble.
There is currently no EPA RfD available for PFHxS; however, EPA's IRIS program is developing a human health toxicity assessment for PFHxS (expected to undergo public comment and external peer review in 2023). The HBWC for PFHxS is derived using an ATSDR intermediate-duration oral Minimal Risk Level based on thyroid effects seen in male rats after oral PFHxS exposure (ATSDR, 2021; USEPA, 2023a). ATSDR calculated an HED of 0.0047 mg/kg/day and applied a combined UF/MF factor of 300X (total UF of 30X and a MF of 10X for database deficiencies) to yield an intermediate-duration oral Minimal Risk Level of 2E-05 mg/kg/day (ATSDR, 2021). To calculate the HBWC, EPA applied an additional UF of 10 to adjust for subchronic-to-chronic duration, per Agency guidance (USEPA, 2002), because the effect is not in a developmental population ( i.e., thyroid follicular epithelial hypertrophy/hyperplasia in parental male rats). The resulting chronic reference value for use in HBWC calculation was 2E-06 mg/kg/day. EPA selected a DWI-BW for adults within the general population (0.034 L/kg/day) and applied an RSC of 20 percent (USEPA, 2022c). The resulting HBWC for PFHxS is 9 ng/L (ppt) (USEPA, 2022c).
Like EPA's drinking water health advisory for HFPO-DA and its ammonium salt (USEPA, 2022d), the HBWC that the agency is using for the HI MCLG was derived from the agency's 2021 human health toxicity assessment, specifically the chronic RfD of 3E-06 mg/kg/day based on liver effects observed following oral exposure of mice to HFPO-DA (USEPA, 2021b). EPA selected a DWI-BW for lactating women (0.0469 L/kg/day) and applied an RSC of 20 percent (USEPA, 2023a) to calculate the HBWC for HFPO-DA. The HBWC for HFPO-DA is 10 ng/L (ppt) (USEPA, 2023a).
There is currently no EPA RfD available for PFNA; however, EPA's IRIS program is developing a human health toxicity assessment for PFNA. The HBWC for PFNA is derived using an ATSDR intermediate-duration oral Minimal Risk Level that was based on developmental effects seen in mice after oral PFNA exposure (ATSDR, 2021; USEPA, 2023a). ATSDR calculated an HED of 0.001 mg/kg/day and applied a combined UF/MF factor of 300X (total UF of 30X and a MF of 10X for database deficiencies) to yield an intermediate-duration oral Minimal Risk Level of 3E-06 mg/kg/day (ATSDR, 2021). EPA did not apply an additional UF to adjust for subchronic-to-chronic duration for PFNA because the critical effects were observed during a developmental life stage (USEPA, 2002). EPA used the chronic reference value of 3E-06 mg/kg/day to calculate the HBWC for PFNA. EPA selected a DWI-BW for lactating women (0.0469 L/kg/day) and applied an RSC of 20 percent (USEPA, 2023a). The resulting HBWC for PFNA is 10 ng/L (ppt) (USEPA, 2023a).
Like EPA's drinking water health advisory for PFBS (USEPA, 2022e), the HBWC that the agency is using for the HI MCLG was derived from the agency's 2021 human health toxicity assessment, specifically the chronic RfD of 3E-04 mg/kg/day based on thyroid effects observed seen in newborn mice born to mothers that had been orally exposed to PFBS throughout gestation (USEPA, 2021a; 2023a). EPA selected a DWI-BW for women of child-bearing age (0.0354 L/kg/day) and applied an RSC of 20 percent (USEPA, 2023a) to calculate the HBWC for PFBS. The HBWC for PFBS is 2,000 ng/L (ppt) (USEPA, 2023a).
As described above, the HBWCs for PFHxS, HFPO-DA, PFNA, and PFBS are 9, 10, 10, and 2000 ppt respectively (see Section III.A of this preamble, as well as in USEPA (2022c)). HQs are calculated by dividing the measured component PFAS concentration in water ( e.g., expressed as ppt) by the relevant HBWC ( e.g., expressed as ppt), as shown in the equation below. Component HQs are then summed across the PFAS mixture to yield the PFAS mixture HI MCLG. Thus, the HI accounts for differences in toxicity among the mixture component chemicals rather than weighting them all equally in the mixture. A PFAS mixture HI greater than 1.0 indicates an exceedance of the health protective level and indicates potential human health risk for noncancer effects from the PFAS mixture in water. For more details on this approach, please see USEPA (2023a). The proposed mixture HI MCLG for PFHxS, HFPO-DA, PFNA, and PFBS is as follows:
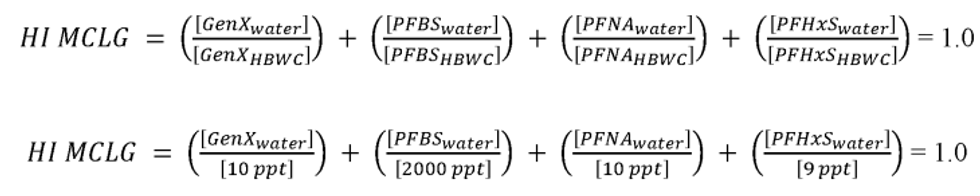
Where:
[PFAS water ] = the measured component PFAS concentration in water and
[PFAS HBWC ] = the HBWC of a component PFAS.
For example, if each of the four PFAS are measured at their respective proposed PQLs described in section VIII.A. of this preamble, the HI calculation would be as follows:
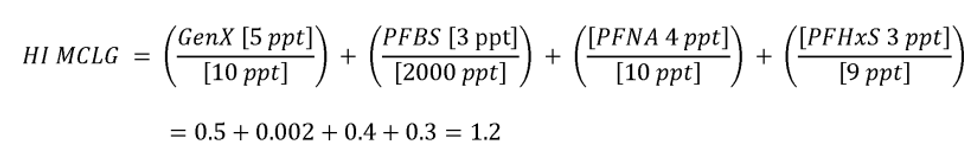
In this scenario, while none of the individual PFAS contaminants exceed their relative HBWC, when considered in the HI, the sum of the four PFAS in the HI exceeds 1.0, and therefore is higher than the MCLG. In the following example, if only PFNA and PFHxS were measured at 8 ppt each, while also below their individual HWBCs, the two would sum to an exceedance of the HI.
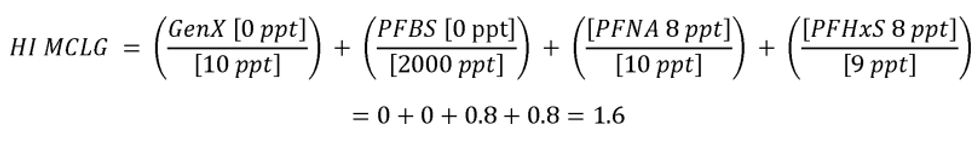
In a final example, if only a single PFAS, PFHxS were reported above its PQL, but that value was 20, this would also result in an HI higher than 1.0.
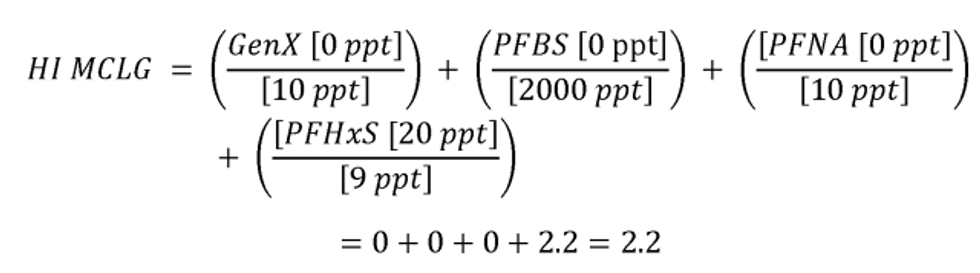
EPA requests comment on significant figure use when calculating both the HI MCLG and the MCL (see discussion in section VI of this preamble). EPA has set the HI MCLG and MCL using two significant figures ( i.e., 1.0). EPA requests comment on the proposed use of two significant figures for the MCLG when considering underlying health information and for the MCL when considering the precision of the analytical methods.
In conclusion, while current weight of evidence suggests that PFAS vary in their precise structure and function, exposure to different PFAS can result in similar health effects. As a result, PFAS exposures are likely to result in dose-additive effects (ATSDR, 2021; USEPA, 2023a) and therefore the assumption of dose-additivity is reasonable. While individual PFAS can pose a potential risk to human health if the exposure level exceeds the chemical-specific toxicity value (RfD or Minimal Risk Level) ( i.e., individual PFAS HQ >1.0), mixtures of PFAS can result in dose additive health effects when lower individual concentrations of PFAS are present in that mixture. For example, if the individual HQs for PFHxS, HFPO-DA, PFNA, and PFBS were each 0.9 that would indicate that the measured concentration of each PFAS in drinking water is below the level of appreciable risk (recall that an RfV, such as an oral RfD, represents an estimate at which no appreciable risk of deleterious effects exists). However, the overall HI for that mixture would be 3.6 ( i.e., sum of four HQs of 0.9). An HI of 3.6 means that the total measured concentration of PFAS is 3.6 times the level associated with potential health risks. Thus, setting an MCLG while only considering the concentration of an individual PFAS without considering the dose additive effects from other PFAS in a mixture would not provide a sufficiently protective MCLG with an adequate margin of safety. In order to account for dose additive noncancer effects associated with co-occurring PFAS and PFAS in mixtures, to protect against health impacts from likely multi-chemical exposures of PFHxS, HFPO-DA, PFNA, and PFBS, with an adequate margin of safety, the Agency is proposing to use of the HI approach, a commonly used component-based mixture risk assessment method, for the MCLG for these four PFAS (USEPA, 2022). Consistent with the statutory requirement under 1412(b)(4)(A), establishing the MCLG for PFHxS, HFPO-DA, PFNA, and PFBS at an HI = 1.0 ensures that MCLG is set at a level where there are no known or anticipated adverse effect on the health of persons and ensuring an adequate margin of safety.
VI. Maximum Contaminant Level
Under section 1412(b)(4)(B) of SDWA, EPA must generally establish an enforceable MCL as close to the MCLG as is feasible, taking costs into consideration. The Agency evaluates feasibility according to several factors including the availability of analytical methods capable of measuring the targeted compounds in drinking water and examining available treatment technologies capable of contaminant removal examined under laboratory and field conditions.
A. PFOA and PFOS
The Agency evaluated available analytical methods to determine the lowest concentration at which PFOA and PFOS can reliably be measured in finished drinking water. There are two analytical methods approved by EPA for analyzing PFAS regulated under this proposed rule, USEPA Methods 537.1 and 533. In this evaluation, EPA determined that 4.0 ppt is the lowest concentration that PFOA and PFOS can be reliably quantified within specific limits of precision and accuracy during routine laboratory operating conditions. EPA has historically called this level the “practical quantitation level,” also known as a PQL (USEPA, 1987). Under UCMR5, EPA published MRLs of 4.0 ppt each for PFOA and PFOS (USEPA, 2022g). As described in the UCMR 5 rulemaking, this reporting level is the minimum quantitation level that, with 95 percent confidence, can be achieved by capable analysts at 75 percent or more of the laboratories using a specified analytical method ( i.e., Method 533 and 537.1, discussed in more detail in section VIII of this preamble). Based on the multi-laboratory data acquired for the UCMR 5 rule, EPA has defined the PQL for PFOA and PFOS to be equal to the UCMR 5 MRL of 0.0000040 mg/L or 4.0 ppt. This quantitation level provides an allowance for the degree of measurement precision and accuracy that EPA estimates can be achieved across laboratories nationwide. Furthermore, the PQLs provide for consistency in data quality from a diverse group of laboratories across the country and provide routine performance goals that many laboratories must strive to achieve. The agency must have a high degree of confidence in the quantified result as it may compel utilities to make potentially costly compliance decisions in order to comply with the MCL. Please see section VIII of this preamble for more information on analytical methods for PFAS and a detailed discussion of the PQL and other levels below this quantitation level that may be appropriate for screening values.
EPA has promulgated and successfully implemented NPDWRs with MCLs equal to the contaminant PQLs. In 1987, EPA finalized the Phase I Volatile Organic Compounds (VOC) rule (USEPA, 1987), where the agency set the MCL at the PQL for benzene, carbon tetrachloride, p-dichlorobenzene, trichloroethylene, vinyl chloride, 1,1,1-trichloroethane, 1,1-dichloroethylene and 1,2-dichloroethane. In that rule, EPA set the PQL at a level consistent with what was then the “general rule of five to ten times the [method detection limit] MDL.” While some commenters at the time stated they believed implementation would be challenging, EPA notes that those rules have been implemented successfully and provided an incentive for laboratories to improve analytical capabilities and reduce method quantitation and detection limits.
EPA requests comment on whether setting the MCL at the PQLs for PFOA and PFOS is similarly implementable and feasible. As in the 1987 rule, EPA recognizes that quantitation of the contaminants can be achieved between the MDL ( e.g., see Method 537.1, section 9.2.8) and the PQL, albeit not necessarily with the same precision and accuracy that is possible at and above the PQL. Measuring PFOA and PFOS results below the PQLs may not be achievable from all laboratories and may not have the same precision as higher-level measurements, nor does EPA believe it is appropriate to make potentially costly compliance decisions based on such lower-level measurements. Nonetheless, the ability to know that PFOA and PFOS may be present within a certain range at these low concentrations ( i.e., below the PQLs) can be used to inform decisions for already installed treatment ( e.g., a utility can evaluate when break though is most likely to occur or is imminent) and to judge appropriate monitoring frequency. In addition, further support for considering measurement levels below PQL, and the demonstrated capability of laboratories to support screening at these lower levels, was found within laboratory calibration standard data submitted as part of the UCMR 5 Laboratory Approval Program. 4 These data revealed that 49 of the 54 laboratories seeking EPA approval included a lowest PFAS calibration standard level at 1 ppt or lower, with the median lowest calibration level among all laboratories at 0.5 ppt. Therefore, for almost all laboratories, the proposed PQLs for PFOA and PFOS of 4.0 ppt are at least 4 times greater than the lowest calibration standard. This suggests the overwhelming majority of laboratories with the necessary instrumentation to support PFAS monitoring have the capability to provide screening measurement results above the proposed trigger level of 1/3 of the MCL ( i.e., 1.3 ppt for PFOS or PFOS). Hence, a utility may use the lower-level measurements as a warning that they may be nearing the PFOA and PFOS MCLs of 4.0 ppt prior to exceeding them and can make informed treatment decisions about managing their systems ( e.g., replacing GAC). For more information on the proposed trigger level, please see sections VIII and IX of this preamble. EPA requests comment on implementation challenges and considerations for setting the MCL at the PQLs for PFOA and PFOS, including on the costs and benefits related to this approach.
4 Instrument calibration for the approved methods is defined by analyzing a set of at least five standard solutions spanning a 20-fold concentration range, in which the lowest concentration must be at or below the quantitation level. Calibration standards below the quantitation level must meet defined precision requirements. The resulting calibration curve is validated by measuring standard solutions of known concentration prepared from commercially available reference materials. Calibration is confirmed at multiple points, including by performing an initial calibration and initial demonstration of capability prior to analysis, through the addition of internal and surrogate standards, and by incorporating continuous calibration check samples into the analysis routine.
Additionally, consistent with EPA's SMF for many drinking water contaminants, EPA is proposing to utilize a running annual average approach to calculate compliance with this proposed rule. As a result, a single occurrence of PFOA or PFOS that is slightly above the proposed MCLs would not result in an MCL violation, assuming other quarterly samples remain below the MCLs. For example, if a system had a sample result of PFOA at 5.0 ppt and the remaining quarter sample results were all 2.0 ppt each, the system would not be violation. In addition, when calculating the running annual averages, if a sample result is less than the PQL for the monitored PFAS, EPA is also proposing to use zero to calculate the average for compliance purposes. For further discussion on monitoring and compliance, please see section IX of this preamble. Hence, while EPA believes utilities should endeavor for all samples to remain below the MCL, the proposed rule allows for temporal fluctuations in concentrations that may occur because of unexpected events such as premature PFOA and PFOS breakthrough or temporary increased source water concentrations. This extra buffer provides the utilities additional operational safety margins in the event of minor management or treatment issues. As an alternative, and as described in more detail in section IX of this preamble, when calculating the running annual averages, rather than using zero for sample results less than the PQL, EPA seeks comment on instead using the proposed rule trigger levels ( i.e., 1.3 ppt for PFOA and PFOS) in the case where PFAS are detected but below their proposed PQLs. This would have the potential to be more protective in the long run than counting sampling results below the PQL as zero and provide PWSs greater forewarning that their results may exceed the MCLs.
EPA anticipates there would not be sufficient laboratory capacity if the quantitation level were set at a level below 4.0 ppt. The rigorous laboratory certification and quality assurance/quality control (QA/QC) procedures could limit the number of laboratories that can achieve lower quantitation levels and many water systems would not be able to secure the services of laboratories that are capable of consistently providing precise and accurate quantitation of concentrations of PFOA and PFOS at levels lower than 4.0 ppt. The Agency has determined that high confidence in the accuracy of analytical results is necessary to demonstrate that any treatment technologies are effectively reducing levels of PFOA and PFOS to the levels as close as feasible to the proposed MCLGs for these contaminants. To achieve this intended purpose, the Agency is proposing to establish the MCLs for PFOA and PFOS at this PQL of 4.0 ppt.
While EPA anticipates potential laboratory capacity issues if the Agency were to propose MCLs below 4.0 ppt, EPA believes there will be sufficient laboratory capacity with the MCLs set at 4.0 ppt. As of September 2022, as a part of the UCMR 5 laboratory approval program, fifty-four (54) laboratories submitted applications to EPA for approval to analyze PFOA and PFOS to quantification limits of 4.0 ppt using EPA Method 533. Each of these 54 laboratories had acquired the analytical equipment necessary to run both EPA Method 533 and 537.1 and laboratories are required to achieve and demonstrate they can meet the PFOA and PFOS PQLs of 4.0 ppt to receive EPA Method 533 approval. EPA received strong interest from a significant number of laboratories seeking UCMR 5 laboratory approval, demonstrating there is effective laboratory capacity to support the program. The commercial market for PFAS analysis is likely to remain strong and, in fact, grow as more laboratories develop the technical capability further enhancing lab capacity to analyze PFAS for drinking water rule compliance purposes. The various State regulatory monitoring programs established in recent years for PFAS incorporate laboratory certification/accreditation programs that further elevate commercial laboratory interest and expand laboratory capacity. Additionally, because EPA is proposing to allow the use of existing PFAS monitoring data to meet the initial monitoring requirements of this proposed rule where available (see section IX of this preamble for further discussion), EPA anticipates the sudden spike in laboratory demands that could otherwise accompany a proposed rule such as this will instead be distributed during the initial rule implementation timeframe. EPA requests comment on the underlying assumptions that sufficient laboratory capacity will be available with the MCLs set at 4.0 ppt; that demand will be sufficiently distributed during rule implementation to allow for laboratory capacity; and on the cost estimates related to these assumptions.
SDWA 1412(b)(4)(d) defines feasibility as, “feasible with the use of the best technology, treatment techniques and other means which the Administrator finds, after examination for efficacy under field conditions and not solely under laboratory conditions, are available (taking cost into consideration).” Further, Section 1412(b)(4)(E) of SDWA requires identification of technologies, referred to as best available technologies (BATs) “which the Administrator finds to be feasible for purposes of meeting [the MCL].” As described in section XI.A. of this preamble, the Agency identifies the BATs as those meeting certain criteria including: (1) The capability of a high removal efficiency; (2) a history of full-scale operation; (3) general geographic applicability; (4) reasonable cost based on large and metropolitan water systems; (5) reasonable service life; (6) compatibility with other water treatment processes; and (7) the ability to bring all the water in a system into compliance. In section XI of this preamble, EPA evaluated treatment technologies for the removal of PFOA and PFOS that would meet these criteria and determined there are multiple technologies ( i.e., GAC, AIX, RO, and NF) that are both available and have reliably demonstrated PFAS removal efficiencies that may exceed >99 percent and can achieve concentrations less than the proposed MCLs for PFOA and PFOS. Based on its evaluation, the Agency proposes to determine that it is feasible to treat PFOA and PFOS to 4.0 ppt because multiple treatment technologies are effective and available and there are methods available to reliably quantify PFOA and PFOS at 4.0 ppt. For more information about treatment technologies, please see section XI of this preamble. For more information about available analytical methods, please see section VIII of this preamble.
For purposes of its proposed feasibility determination, EPA also considered costs when setting the MCLs for PFOA and PFOS at 4.0 ppt and that analysis supports a finding that 4.0 ppt represents the level of what is “feasible” under the standard of Section 1412(b)(4)(D). Based on legislative history (A Legislative History of the Safe Drinking Water Act, Committee Print, 97th Cong., 2d Sess. (1982) at 550), EPA interprets “taking cost into consideration” in Section 1412(b)(4)(D) to be limited to “reasonable cost based on large and metropolitan water systems.” EPA has determined that 4.0 ppt represents what is achievable for BATs given the standard of “reasonable cost based on large and metropolitan water systems.” As discussed in section XII of this preamble, EPA evaluated quantifiable and nonquantifiable costs for MCLs for PFOA and PFOS at 4.0, 5.0, and 10.0 ppt. As part of that evaluation, EPA considered capital, operational, administrative, monitoring, and other costs. In addition to estimating national level costs associated with the proposed rule and potential regulatory alternatives, EPA assessed PWS level costs, costs to small systems, and costs at the household level. For more information about EPA's cost estimates, please see Best Available Technologies and Small System Compliance Technologies Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water (USEPA, 2023g). EPA considered these cost analyses, in addition to analytical methods, quantitation levels, and treatment technologies in coming to its proposed finding that MCLs of 4.0 ppt for PFOA and PFOS represents levels that are as close as feasible to the MCLGs. EPA seeks comment on its PFOA and PFOS evaluation of feasibility for the proposal, including analytical measurement and treatment capability, as well as reasonable costs, as defined by SDWA.
B. PFAS Hazard Index: PFHxS, HFPO-DA, PFNA, and PFBS
To protect against the potential for dose additive health impacts from likely multi-chemical exposures when they occur as mixtures in drinking water, EPA is proposing an MCL for mixtures of PFHxS, HFPO-DA, PFNA, and PFBS expressed as an HI. An HI is the sum of HQs from multiple substances. HQs are the ratio of potential exposure to a substance and the level at which no health effects are expected. EPA is proposing the MCL for mixtures of PFHxS, HFPO-DA, PFNA, and PFBS as equal to the MCLG: as proposed, the HI must be equal to or less than 1.0. SDWA section 1401(3) defines an MCL as the “maximum permissible level of a contaminant in water which is delivered to any user of a public water system.” This approach, as proposed, sets a permissible level for the contaminant mixture ( i.e., a resulting PFAS mixture HI greater than 1.0 indicates an exceedance of the health protective level and indicates potential human health risk for noncancer effects from the PFAS mixture in water). If there is only one contaminant PFAS present, the HI approach in practice also sets a permissible level for the individual contaminant through the use of its respective HBWC (see example and discussion in section V.C2 of this preamble). As discussed below in this section (section VI.D. of this preamble) and in section XIII of this preamble, the Agency is also inviting comment on whether establishing a traditional MCLG and MCL for PFHxS, HFPO-DA, PFNA, and PFBS instead of or in addition to the HI approach would change public health protection, improve clarity for the rule, or change costs.
EPA asked the SAB for advice on using an HI approach as an option for PFAS mixture assessment under an assumption of dose additivity. Consistent with EPA Guidance ( e.g., USEPA, 2000a; USEPA, 1989) the HI is used here as a decision aid, and determination of dose additivity among chemicals is relaxed from the level of common MOA to common target organ(s)/health outcome(s). Per SAB's suggestion, EPA outlines here the validity of, and procedures for, calculating the HI given a mixture such as this one that includes PFAS with varying levels of available information across health outcomes.
Consistent with advice from the SAB, EPA considers it an appropriately health protective approach to assume dose additivity for PFAS co-occurring in mixtures as they share similar profiles of health effect domains ( e.g., liver, thyroid, developmental, etc.). EPA's analysis of finished water monitoring data demonstrates that PFAS often have a substantial likelihood to co-occur in mixtures (see section III.D of this preamble). While PFAS are well documented to co-occur, the exact chemical composition is often site-specific in nature ( i.e., each location of PFAS mixture is influenced by different environmental point and diffuse sources that results in a unique PFAS profile) (Banzhaf et al., 2017). Yet, EPA finds that PFHxS, HFPO-DA, PFNA, and PFBS often co-occur in mixtures in drinking water, including with other PFAS (USEPA, 2023e). To protect against the potential for dose additive health impacts from likely multi-chemical exposures of PFHxS, HFPO-DA, PFNA, and PFBS when they occur as mixtures in drinking water, the Agency is proposing to use the HI approach. Both EPA's recent PFAS mixture's framework (USEPA, 2023d), and SAB's review of the prior draft of this document discuss the strengths and limitations associated with using an HI approach as the basis for evaluating potential health risks associated with exposure to mixtures of PFAS, and consideration as a metric to inform health-based decision-making for regulatory purposes (USEPA, 2022a). For a full discussion of the strengths and limitations identified during SAB's review and how EPA responded, please see USEPA, 2022a and 2023f. The HI approach is used regularly by EPA (and States) to inform potential health risks of chemical mixtures associated with contaminated sites/locations under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA)/the Superfund Amendments and Reauthorization Act (SARA); as such, the application of the HI approach under a regulatory purview is not novel for the Agency though this is the first use of an HI approach for a SDWA National Primary Drinking Water Regulation.
EPA is proposing an MCL based on a HI composed of the four PFAS for which there are validated EPA methods for measurement and treatment, evidence of co-occurrence, the potential for similar health effects, and the availability of finalized peer reviewed toxicity values to use in generating the HI. For this proposal, those PFAS are PFHxS, HFPO-DA, PFNA, and PFBS. The MCL for mixtures of PFHxS, HFPO-DA, PFNA, and PFBS would be an HI = 1.0. In this proposal, the HBWCs that EPA uses to calculate the HI are proposed to be 9.0 ppt for PFHxS; 10.0 ppt for HFPO-DA; 10.0 ppt for PFNA; and 2000 ppt for PFBS (USEPA, 2023a). To calculate the proposed HI, regulated PWSs would be required to monitor to determine the concentrations of PFHxS, HFPO-DA, PFNA, and PFBS in their finished drinking water. See section IX of this preamble for proposed requirements related to monitoring and determining compliance. See equation below for calculation of the PFHxS, HFPO-DA, PFNA, and PFBS HI MCL:
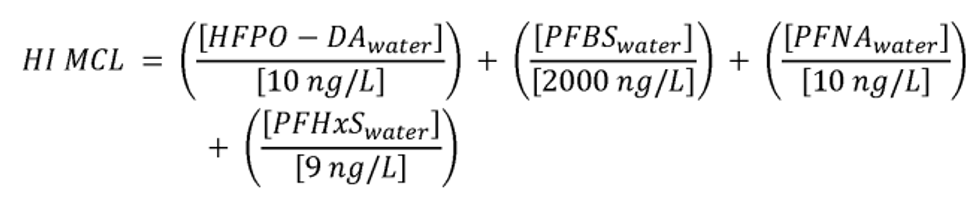
Where:
HFPO-DA water = monitored concentration of HFPO-DA;
PFBS water = monitored concentration of PFBS;
PFNA water = monitored concentration of PFNA; and
PFHxS water = monitored concentration of PFHxS
See discussion in section IV of this preamble above for how EPA derived these values for these contaminants.
As described in section VI.A. of this preamble for PFOA and PFOS, the Agency has similarly considered feasibility as defined by SDWA 1412(b)(4)(D) for PFHxS, HFPO-DA, PFNA, and PFBS. The Agency has determined that there are validated analytical methods that can measure below the HBWC for each of these PFAS. Additionally, as discussed above, the Agency proposes to determine that it is feasible to treat each of these PFAS to below their PQL (between 3.0-5.0 ppt) and it is feasible to treat these PFAS to below their PQLs individually and as a group. When identifying BATs, EPA evaluated the same factors as defined previously in Section VI.A. and in Section XI.A. of this preamble and has found the same technologies identified for PFOA and PFOS are also both available and have reliably demonstrated PFAS removal efficiencies that may exceed >99 percent and achieve concentrations less than the proposed HI MCL for PFHxS, HFPO-DA, PFNA, and PFBS.
As described in section VI.A. of this preamble for PFOA and PFOS, the Agency similarly considered costs as part of its proposed feasibility determination for PFHxS, HFPO-DA, PFNA, and PFBS and setting the HI MCL at 1.0. EPA's analysis supports a finding that an HI of 1.0 is “feasible” under standard of SDWA 1412(b)(4)(D) because it is achievable for BATs given the standard of “reasonable cost based on large and metropolitan water systems.” For more information about EPA's cost estimates, please see Best Available Technologies and Small System Compliance Technologies Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water (USEPA, 2023g; USEPA, 2023h). EPA considered these cost analyses, in addition to analytical methods, quantitation levels, and treatment technologies in coming to its proposal that an HI MCL of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS represents a level that is as close as feasible to the MCLG. EPA seeks comment on its evaluation of feasibility for the proposed HI MCL finding, including analytical measurement and treatment capability, as well as reasonable costs, as defined by SDWA.
C. Reducing Public Health Risk by Protecting Against Dose Additive Noncancer Health Effects From PFAS
As described above, PFOA and PFOS are demonstrated to have the potential for adverse health effects at low levels of exposure. The level at which no known or anticipated adverse effects on the health of persons would occur is well below current analytical quantitation level for PFOA and PFOS. To ensure maximum public health protection for these contaminants, the statute generally requires that exposure be driven to the lowest feasible concentration.
Because of the analytical limitations discussed in the preceding section VI.A of this preamble, EPA is not proposing to include PFOA and PFOS in the HI. The only feasible way to represent PFOA and PFOS in the HI approach would be to only consider values for PFOA and PFOS at or above the PQL of 4.0 ppt. As a result, any measured concentration above 4.0 ppt for PFOA and PFOS would result in an exceedance of the HI of 1.0. Therefore, regulating PFOA and PFOS under a HI approach would not add any meaningful health protection over setting an individual MCL for these PFAS. Additionally, EPA believes that adding PFOA or PFOS to the HI could increase potential compliance challenges with the rule as there could be confusion created by how to consider screening level values above detection but below quantitation (see additional discussion in section VIII of the preamble for discussion on screening and trigger levels). Therefore, EPA is proposing to set MCLs for PFOA and PFOS individually and not part of the HI.
Some PFAS (such as PFHxS, HFPO-DA, PFNA, and PFBS) have HBWCs at thresholds higher than current analytical quantitation levels. As a result of assuming dose-additivity, PFHxS, HFPO-DA, PFNA, and PFBS may have individual detectable or quantifiable concentrations below their individual HBWCs, but their combined concentrations can be above levels of health concern. As proposed, the HI MCL provides a protective approach to avoiding these potential health risks associated with mixtures of PFAS that are below the public health goals individually, yet exceed the PFAS mixture limit ( i.e., HI MCL = 1.0). Separating PFOA and PFOS away from a HI approach is not meant to ignore the potential dose additive health impacts for these compounds in mixtures. As described in the preceding paragraph, EPA is not including PFOA and PFOS as part of the HI approach because the Agency believes doing so would not add meaningful health protection over setting an individual MCL for these PFAS.
EPA recognizes that some PFAS such as PFOA, PFOS, and PFNA have been voluntarily phased out of production and replaced in the United States so their relative concentrations in source waters may decrease over time. However, other PFAS that have been shown to also cause adverse health effects ( e.g., perfluorobutanoic acid [PFBA], PFBS, HFPO-DA) may increase in concentration as their production, use, and discharges into source water continues. The HI framework is designed to inform protection of human health for any source water PFAS, with available human health assessment values, still in production and use. Under the HI approach, additional PFAS can be added over time once more information on health effects, analytics, exposure and/or treatment becomes available, and merits additional regulation as determined by EPA. As such, this approach provides a framework for Federal and State public health agencies to consider using to address other PFAS in the future as needed.
D. Regulatory Alternatives
As discussed in section VI.A of this preamble above, EPA proposes to determine that it is feasible to set MCLs for PFOA and PFOS at 4.0 ppt each and that the level is as close as feasible to the MCLGs. As discussed in Section VI.B of this preamble, EPA proposes to determine it is feasible to set an MCL for mixtures of PFHxS, HFPO-DA, PFNA, and PFBS as a HI = 1.0 which is the same level as the MCLG.
In section XIII of this preamble, the HRRCA section of this proposal, EPA is presenting estimated costs and benefits of regulatory alternatives for PFOA and PFOS of MCLs at 4.0, 5.0 ppt and 10.0 ppt. Quantified costs and benefits for the proposed option and alternative options considered are summarized in section XIII.H of this preamble, specifically tables 66-69. Tables 70-71 summarize the non-quantified benefits and costs and assess the potential impact of non-quantifiable benefits and costs on the overall benefits and costs estimate. Establishing only MCLs at 4.0 ppt for PFOA and PFOS instead of the proposed rule (MCLs at 4.0 ppt for PFOA and PFOS and the HI) would result in a reduction of $16 million in quantified costs and $17 million in quantified benefits at the 3% discount level and $27 million in quantified costs and $13 million in quantified benefits at the 7% discount level. Establishing MCLs at 5.0 ppt for PFOA and PFOS instead of 4.0 ppt would result in a reduction of $145 million in quantified costs and $169 million in quantified benefits at the 3% discount level and $235 million in quantified costs and $122 million in quantified benefits at the 7% discount level. Establishing MCLs at 10.0 ppt for PFOA and PFOS instead of 5.0 ppt would result in a reduction of $318 million in quantified costs and $462 million in quantified benefits at the 3% discount level and $511 million in quantified costs and $337 million in quantified benefits at the 7% discount level. EPA notes that there would also be commensurate reduction in the nonquantifiable benefits and costs among these options. As discussed elsewhere in this proposal, the nonquantifiable benefits are anticipated to be significant. EPA evaluated these regulatory alternatives in its HRRCA, discussed in Section XIII of this preamble below and is requesting comment on these alternatives.
EPA considered an MCL of 5.0 ppt for PFOA and PFOS because it is 25 percent above the PQL of 4.0 ppt. A commenter in EPA's outreach consultations for this regulation suggested the Agency consider a buffer of approximately 20 percent if the MCL is close to the quantitation level because water systems operate with a margin of safety and plan for performance that maintains water quality below quantitation levels. Therefore, in this commenter's opinion, having an increased buffer between the PQL and the MCL may allow utilities to manage treatment technology performance more efficiently because utilities typically aim to achieve lower than the MCL to avoid a violation. With the MCL at the PQL, the commenter believes that utilities would not have the early warning that they may exceed the MCL prior to doing so. EPA disagrees that utilities would not have early warning prior to exceeding the MCL; see discussion above in section VI.A of this preamble for more information. For results between the detection limit and the PQL, EPA has determined that utilities would be able to reliably conclude analyte presence, though this detection is less precise regarding specific concentration. Knowledge regarding the presence of PFOA and PFAS at concentrations below PQLs can inform decisions related to monitoring frequency and existing treatment. EPA requests comment on this approach.
EPA also considered the MCL of 10.0 ppt to evaluate the national costs and benefits and whether the expected reduction in costs would change EPA's determination of the level at which the benefits would justify the costs. See SDWA Section 1412(b)(6)(A). The Agency notes that this regulatory alternative level is consistent with State-enacted MCLs for certain PFAS (NYDOH, 2020). Because there is significant expected occurrence of PFOA and PFOS between 4.0 ppt and 10.0 ppt, raising the MCL from 4.0 to 10.0 would be expected to significantly decrease the number of utilities that must take action to manage PFOA and PFOS concentrations in their finished drinking water. However, it would also result in millions of Americans continuing to be exposed to levels that have the potential for harmful levels of PFOA and PFOS that can feasibly be removed through treatment, thereby decreasing the quantified and non-quantified benefits delivered by this proposed regulation. Furthermore, since EPA has found proposed PFOA and PFOS MCLs of 4.0 ppt to be feasible, the Agency must set the MCL as close to the MCLG as feasible, the Administrator determined the costs were justified by the benefits at a PFOA and PFOS proposed MCL at 4.0 (see discussion in section XIII of this preamble), and setting the PFOA and PFOS MCLs at 10.0 ppt would not reduce PFOA and PFOS exposure risks for millions of Americans to the extent feasible, EPA preliminarily determined that proposing PFOA and PFOS MCLs at 10.0 ppt would not be appropriate or justifiable under the SDWA statutory criteria.
EPA also considered the traditional approach of establishing individual MCLGs and MCLs for PFHxS, HFPO-DA, PFNA, and PFBS in lieu of or in addition to separate rule language for the HI approach. As noted earlier, this action includes a preliminary determination to regulate these additional PFAS and their mixtures. EPA's proposed HI approach addresses both the particular PFAS and their mixtures. If EPA does not finalize a regulatory determination for mixtures of these PFAS, then a more traditional approach may be warranted. Under this alternative, the proposed MCLG and MCL for PFHxS would be 9.0 ppt; for HFPO-DA the MCLG and MCL would be 10 ppt; for PFNA the MCLG and MCL would be 10 ppt; and for PFBS the MCLG and MCL would be 2000 ppt ( i.e., 2.0x103 or 2.0e 3). As discussed in section XIII of this preamble, EPA has not separately presented changes in quantified costs and benefits for these approaches. If EPA adds individual MCLs in addition to using the HI approach, EPA anticipates there will be no change in costs and benefits relative to the proposed rule ( i.e., the same number of systems will incur identical costs to the proposed option and the same benefits will be realized). EPA has not separately quantified the benefits and costs for the approach to regulate PFHxS, PFNA, PFBS, and HFPO-DA with individual MCLs instead of the HI. However, EPA expects both the costs and benefits would be reduced under this approach as fewer systems may be triggered into treatment and its associated costs. Additionally, systems that exceed one or more of the individual MCLs will treat to a less stringent and public health-protective standard. Furthermore, while EPA recognized that regulating these PFAS with individual MCLs and MCLGs might be simpler to implement for some states or operators, if EPA were to regulate these PFAS individually and not under the HI MCL approach, it would not provide equivalent protection against potential dose additive impacts for these PFAS, nor would it establish a framework to consider potential dose additive impacts for future PFAS components or groups as EPA develops a better understanding of the adverse health effects of other PFAS. The Agency is requesting comment on whether establishing a traditional MCLG and MCL for PFHxS, HFPO-DA, PFNA, and PFBS instead of or in addition to the HI approach would change public health protection, improve clarity of the rule, or change costs.
EPA also considered an alternative regulatory construct of establishing both MCLGs and MCLs for these four PFAS in addition to separate rule language for the HI MCL. Hence, these four PFAS would expressly be subject to two MCLs: the individual MCLs and the HI MCL for the mixture. However, this approach has the potential to function the same as the proposed rule because a system cannot have MCL violations of an individually regulated PFAS without also exceeding the HI MCL. EPA considered this approach because it may improve the ability to communicate about PFAS risks with PWSs and the public, while still providing the important benefit of protection against dose additive impacts from these PFAS with the HI approach, as well as building a potential framework for considering future PFAS regulation. Moreover, this approach may improve the ability to communicate about PFAS concentrations and their relative importance with operators and the public although there may be challenges in risk communication with respect to those small number of facilities that would not exceed an individual MCL but would exceed the HI MCL.
While EPA evaluated these regulatory alternatives, EPA proposal is based upon its proposed finding that an MCL of 4.0 ppt for PFOA and PFOS and an HI of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS are feasible because treatment technologies are available that treat to below these levels and there are analytical methods that can reliably quantify at these levels (See discussion above in Section VI.A and Section VIII of this preamble). Additionally, EPA determined that the benefits justify the costs with the current rule's proposed MCLs of 4.0 ppt and an HI of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS.
When proposing an MCL, EPA must publish, and seek public comment on, the HRRCA for the proposed MCL and each alternative standard considered under paragraphs 5 and 6(a) of Section 1412(b) (SDWA Section 1412(b)(3)(C)(i)), including:
• the quantifiable and nonquantifiable health risk reduction benefits attributable to MCL compliance;
• the quantifiable and nonquantifiable health risk reduction benefits of reduced exposure to co-occurring contaminants attributable to MCL compliance;
• the quantifiable and nonquantifiable costs of MCL compliance including monitoring, treatment, and other costs;
• the incremental costs and benefits of each alternative MCL;
• the effects of the contaminant on the general population and sensitive subpopulations likely to be at greater risk of exposure; and
any adverse health risks posed by compliance; and
• other factors such as data quality and uncertainty.
EPA provides this information in section XIII in this preamble. EPA must base its action on the best available, peer-reviewed science and supporting studies, taking into consideration the quality of the information and the uncertainties in the benefit-cost analysis (SDWA Section 1412(b)(3)). The following sections, as well as the health effects discussion in sections IV and V of this preamble document the science and studies that EPA relied upon to develop estimates of benefits and costs and understand the impact of uncertainty on the Agency's analysis.
E. MCL-Specific Requests for Comment
EPA specifically requests comment on its proposal to set MCLs at 4.0 ppt for PFOA and PFOS and whether 4.0 ppt is the lowest PQL that can be achieved by laboratories nationwide. EPA also requests comment on implementation challenges and considerations for setting the MCL at the PQLs for PFOA and PFOS. EPA requests comment on its evaluation of feasibility under SDWA for the proposed PFOA and PFOS MCLs and the proposed HI MCL. EPA also requests comment on using an HI approach for PFHxS, HFPO-DA, PFNA, and PFBS. Additionally, EPA requests comment on its decision to establish stand-alone MCLs for PFOA and PFOS in lieu of including them in the HI approach. Finally, EPA specifically requests comment on whether establishing a traditional MCLG and MCL for each of the following: PFHxS, HFPO-DA, PFNA, and PFBS instead of or in addition to the HI approach would change public health protection or improve clarity of the rule; or change anticipated costs.
VII. Occurrence
EPA relied on multiple data sources, including UCMR 3 and state finished water data to evaluate the occurrence and probability of co-occurrence of PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS. EPA also incorporated both the UCMR 3 and some state data into a Bayesian hierarchical model which supported exposure estimates for select PFAS at lower levels than were measured under UCMR 3. EPA has utilized similar statistical approaches in past regulatory actions to inform its decision making, particularly where a contaminant's occurrence is infrequent or at low concentrations (USEPA, 2006b). The specific modeling framework used to inform this regulatory action is based on the peer-reviewed model published in Cadwallader et al. (2022). Collectively, these data and the occurrence model informed estimates of the number of
water systems (and associated population) expected to be exposed to levels of PFOA and PFOS which would potentially exceed the proposed and alternative MCLs, and to levels of PFHxS, HFPO-DA, PFNA, and PFBS that would potentially exceed the HI.
EPA relied on the UCMR 3 as the primary source of nationwide occurrence data to inform the occurrence model's exposure estimates for four PFAS: PFOA, PFOS, perfluoroheptanoic acid (PFHpA), and PFHxS. Additionally, as described in the final regulatory determination for PFOA and PFOS (USEPA, 2021d), EPA has also considered and evaluated publicly-available state finished water PFAS monitoring data, including data on PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS.
A. UCMR 3
As discussed in section III.B. of this preamble, UCMR 3 monitoring occurred between 2013 and 2015 and is currently the best nationally representative finished water dataset for any PFAS, including PFOA, PFOS, PFNA, PFBS, and PFHxS. Under UCMR 3, 36,972 samples from 4,920 PWSs were analyzed for these five PFAS.
PFOA was found above the UCMR 3 MRL (20 ppt) in 379 samples at 117 systems serving a population of approximately 7.6 million people located in 28 states, tribes, or U.S. territories. PFOS was found in 292 samples at 95 systems above the UCMR 3 MRL (40 ppt). These systems serve a population of approximately 10.4 million people located in 28 states, tribes, or U.S. territories. PFHxS was found above the UCMR 3 MRL (30 ppt) in 207 samples at 55 systems that serve a population of approximately 5.7 million located in 25 states, tribes, and U.S. territories. PFBS was found in 19 samples at 8 systems above the UCMR 3 MRL (90 ppt). These systems serve a population of approximately 350,000 people located in 5 states, tribes, and U.S. territories. Lastly, PFNA was found above the UCMR 3 MRL (20 ppt) in 19 samples at 14 systems serving a population of approximately 526,000 people located in 7 states, tribes, and U.S. territories.
B. State Drinking Water Data
As discussed in section III.B of this preamble, the Agency has supplemented its UCMR 3 data with more recent data collected by states who have made their data publicly available. In general, the large majority of these more recent state data were collected using newer EPA-approved analytical methods and state results reflect lower reporting limits than those in the UCMR 3. State results show continued occurrence of PFOA, PFOS, PFHxS, PFBS, and PFNA in multiple geographic locations. These data also show these PFAS occur at lower concentrations and significantly greater frequencies than were measured under the UCMR 3. Furthermore, these data include results for more PFAS than were included in the UCMR 3, including HFPO-DA.
EPA evaluated publicly available monitoring data from the following 23 states: Alabama, Arizona, California, Colorado, Delaware, George, Illinois, Kentucky, Maine, Massachusetts, Maryland, Michigan, Missouri, New Hampshire, New Mexico, New Jersey, North Carolina, North Dakota, Ohio, Pennsylvania, Rhode Island, South Carolina, and Vermont. The data EPA used in its analyses were collected from public state websites through August 2021, but represent sampling conducted on or before May 2021.
The available data are varied in terms of quantity as well as coverage, and some are from targeted sampling efforts ( i.e., monitoring in areas of known or potential PFAS contamination) so may not be representative of levels found in all PWSs within the state or represent occurrence in other states. EPA further refined this dataset based on representativeness and reporting limitations, resulting in detailed technical analyses using a subset of the available state data ( i.e., all 23 states' data were not included within the detailed technical analyses). USEPA (2023e) presents a comprehensive discussion of all the available state PFAS drinking water occurrence data.
Tables 5 and 6 in this section demonstrate the number and percent of samples with PFOA and PFOS state reported detections, and the number and percent of monitored systems with PFOA and PFOS state reported detections, respectively, for the non-targeted state finished water monitoring data. Section III.B. of this preamble describes the state reported finished water occurrence data for PFHxS, HFPO-DA, PFNA, and PFBS data.
Different states utilized various reporting thresholds when presenting their data, and for some states there were no clearly defined limits. Further, the limits often varied within the data for each state depending on the specific analyte, as well as the laboratory analyzing the data. In some cases, states reported data at concentrations below EPA's proposed rule trigger level and/or PQLs in this document. However, to present the best available occurrence information, EPA collected and evaluated the data based on the information as reported directly by the states. When conducting data analyses, EPA incorporated individual state-specific reporting limits where possible. Specific details on state data reporting thresholds are available in USEPA (2023e).
| State | PFOS samples with state reported detections | PFOS state reported sample percent detection | PFOA samples with state reported detections | PFOA state reported sample percent detections |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| 2 Only reported detections. | ||||
| Alabama 2 | 140 | N/A | 80 | N/A |
| Colorado | 60 | 10.3 | 54 | 9.3 |
| Illinois | 55 | 5.2 | 56 | 5.3 |
| Kentucky | 33 | 40.7 | 24 | 29.6 |
| Massachusetts | 441 | 49.1 | 506 | 66.5 |
| Michigan | 70 | 2.5 | 103 | 3.6 |
| New Hampshire | 495 | 27.1 | 1,010 | 55.3 |
| New Jersey | 3,512 | 37.2 | 4,379 | 46.4 |
| North Dakota | 0 | 0.0 | 0 | 0.0 |
| Ohio | 93 | 4.9 | 93 | 4.9 |
| South Carolina | 88 | 57.9 | 82 | 53.9 |
| Vermont | 87 | 6.9 | 109 | 8.7 |
| State | PFOS monitored systems with state reported detections | PFOS state reported monitored system percent detection | PFOA monitored systems with state reported detections | PFOA state reported monitored system percent detections |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| 2 Only reported detections. | ||||
| Alabama 2 | 49 | N/A | 28 | N/A |
| Colorado | 50 | 12.6 | 45 | 11.3 |
| Illinois | 36 | 5.5 | 32 | 4.9 |
| Kentucky | 33 | 40.7 | 24 | 29.6 |
| Massachusetts | 107 | 47.3 | 126 | 55.5 |
| Michigan | 55 | 2.6 | 82 | 3.8 |
| New Hampshire | 189 | 33.8 | 310 | 55.4 |
| New Jersey | 494 | 45.9 | 564 | 52.4 |
| North Dakota | 0 | 0.0 | 0 | 0.0 |
| Ohio | 29 | 2.0 | 32 | 2.2 |
| South Carolina | 42 | 82.4 | 40 | 78.4 |
| Vermont | 35 | 6.3 | 44 | 7.9 |
As illustrated in Tables 5 and 6, there is a wide range in PFOA and PFOS results between states, however in nearly half of states that conducted non-targeted monitoring, more than 25 percent of the monitored systems found PFOA and/or PFOS. Additionally, considering all states in Tables 5 and 6, PFOA detected concentrations ranged from 0.51 to 153 ppt with a range of median detected concentrations from 1.98 to 9.4 ppt, and PFOS detected concentrations ranged from 0.5 to 350 ppt with a range of median detected concentrations from 3 to 11.9 ppt.
Monitoring data for PFOA and PFOS from states that conducted targeted sampling efforts, including California, Maryland, and Pennsylvania, demonstrate results consistent with the non-targeted state monitoring. For example, in Pennsylvania, 26.3 and 24.9 percent of monitored systems found PFOA and PFOS, respectively, with reported concentrations of PFOA ranging from 1.7 to 59.6 ppt and PFOS ranging from 1.8 to 94 ppt. California reported 26.2 and 29.9 percent of monitored systems found PFOA and PFOS, respectively, including reported concentrations of PFOA ranging from 0.9 to 120 ppt and reported concentrations of PFOS from 0.4 to 250 ppt. In Maryland, PFOA and PFOS were found in 57.6 and 39.4 percent of systems monitored, respectively, with reported concentrations of PFOA ranging from 1.02 to 23.98 ppt and reported concentrations of PFOS ranging from 2.05 to 235 ppt.
As discussed above in section VI of this preamble, EPA is proposing individual MCLs of 4.0 ppt for PFOA and PFOS, and an HI level of 1.0 for PFHxS, PFNA, PFBS, and HFPO-DA. EPA also evaluated occurrence for the regulatory alternatives discussed in section VI of this preamble including alternative MCLs for PFOA and PFOS of 5.0 ppt and 10.0 ppt. Table 7, Table 8, and Table 9 demonstrate, based on available state data, the total state reported number and percentages of monitored systems that exceed these proposed and alternative MCL values across the non-targeted state finished water monitoring data.
| State | PFOS monitored systems with state reported detections | PFOS state reported monitored systems percent detection | PFOA monitored systems with state reported detections | PFOA state reported monitored systems percent detection |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| 2 Only reported detections. | ||||
| Alabama 2 | 37 | N/A | 19 | N/A |
| Colorado | 22 | 5.5 | 18 | 4.5 |
| Illinois | 17 | 2.6 | 16 | 2.5 |
| Kentucky | 4 | 4.9 | 9 | 11.1 |
| Massachusetts | 72 | 31.9 | 90 | 39.6 |
| Michigan | 15 | 0.7 | 24 | 1.1 |
| New Hampshire | 107 | 19.1 | 210 | 37.5 |
| New Jersey | 315 | 29.3 | 411 | 38.2 |
| North Dakota | 0 | 0.0 | 0 | 0.0 |
| Ohio | 29 | 2.0 | 32 | 2.2 |
| South Carolina | 27 | 52.9 | 30 | 58.8 |
| Vermont | 16 | 2.9 | 24 | 4.3 |
| State | PFOS monitored systems with state reported detections | PFOS state reported monitored systems percent detection | PFOA monitored systems with state reported detections | PFOA state reported monitored systems percent detection |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| 2 Only reported detections. | ||||
| Alabama 2 | 31 | N/A | 15 | N/A |
| Colorado | 16 | 4.0 | 14 | 3.5 |
| Illinois | 12 | 1.8 | 11 | 1.7 |
| Kentucky | 3 | 3.7 | 4 | 4.9 |
| Massachusetts | 64 | 28.3 | 83 | 36.6 |
| Michigan | 12 | 0.6 | 17 | 0.8 |
| New Hampshire | 86 | 15.4 | 186 | 33.2 |
| New Jersey | 272 | 25.3 | 363 | 33.7 |
| North Dakota | 0 | 0.0 | 0 | 0.0 |
| Ohio | 29 | 2.0 | 32 | 2.2 |
| South Carolina | 25 | 49.0 | 25 | 49.0 |
| Vermont | 13 | 2.33 | 16 | 2.9 |
| State | PFOS monitored systems with state reported detections | PFOS state reported monitored systems percent detection | PFOA monitored systems with state reported detections | PFOA state reported monitored systems percent detection |
|---|---|---|---|---|
| Notes: | ||||
| 1 Detections determined by individual state reported limits which are not defined consistently across all states. | ||||
| 2 Only reported detections. | ||||
| Alabama 2 | 23 | N/A | 8 | N/A |
| Colorado | 3 | 0.8 | 2 | 0.5 |
| Illinois | 3 | 0.5 | 6 | 0.9 |
| Kentucky | 1 | 1.2 | 1 | 1.2 |
| Massachusetts | 32 | 14.2 | 32 | 14.1 |
| Michigan | 6 | 0.3 | 7 | 0.3 |
| New Hampshire | 39 | 7.0 | 83 | 14.8 |
| New Jersey | 133 | 12.4 | 189 | 17.6 |
| North Dakota | 0 | 0.0 | 0 | 0.0 |
| Ohio | 20 | 1.4 | 15 | 1.0 |
| South Carolina | 3 | 5.9 | 3 | 5.9 |
| Vermont | 4 | 0.7 | 7 | 1.3 |
Based on the available state data evaluated and presented in Table 7, Table 8, and Table 9, within 12 states that conducted non-targeted monitoring there are 661 systems that show exceedances of the proposed PFOS MCL of 4.0 ppt and 883 systems with exceedances of the proposed PFOA MCL of 4.0 ppt. These systems serve populations of approximately 8.8 and 10.5 million people, respectively. As expected, the number of systems exceeding either of the proposed alternative MCLs decreases as the values are higher, however, even at the highest alternative PFOS and PFOA MCL values of 10.0 ppt, would still be 267 and 353 systems with exceedances, serving populations of approximately 3.7 and 4.4 million people, respectively.
Monitoring data for PFOA and PFOS from states that conducted targeted sampling efforts shows additional systems that would exceed the proposed and alternative MCLs. For example, in California, Maine, Maryland, and Pennsylvania, 23.4 percent (25 PWSs), 30.4 percent (7 PWSs), 22.7 percent (15 PWSs), and 19.3 percent (66 PWSs) of monitored systems exceeded the proposed PFOS MCL of 4.0 ppt, respectively, and 20.6 percent (22 PWSs), 21.7 percent (5 PWSs), 25.8 percent (17 PWSs), and 21.1 percent (72 PWSs) of monitored systems exceeded the proposed PFOA MCL of 4.0 ppt, respectively. While these frequencies may be anticipated given the sampling locations, within only these four states that conducted limited, targeted monitoring, the monitored systems exceeding the proposed PFOS MCL and proposed PFOA MCL serve significant populations of approximately 4.6 million people and approximately 4.4 million people, respectively.
C. Co-Occurrence
While the discussions in sections III.B, VII.A. and VII.B of this preamble describe how PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS occur individually, PFAS have been documented to co-occur in finished drinking water (Adamson et al., 2017; Cadwallader et al., 2022; Guelfo and Adamson, 2018). As discussed in section VI of this preamble, EPA is proposing regulation of four PFAS including PFHxS, HFPO-DA, PFNA, and PFBS (collectively referred to as “HI PFAS”) as part of an HI approach. Sampling results in the aggregated state dataset were examined to determine the extent to which the HI PFAS occurred with each other as well as with PFOA and/or PFOS. This involved considering the observed occurrence in terms of grouping ( i.e., groups of HI PFAS and “PFOS or PFOA”) as well as pairwise by means of odds ratios. For the group assessment, the aggregated state dataset was limited to samples from non-targeted monitoring efforts where at least one HI PFAS was analyzed and PFOS and PFOA were analyzed sufficiently to determine whether one was present.
1. Groupwise Chemical Co-Occurrence
Table 10 shows the distribution of systems and samples according to whether states report detections for any HI PFAS (PFHxS, HFPO-DA, PFNA and PFBS) and whether they also reported detections of PFOS or PFOA. USEPA (2023e) provides additional information for this analysis.
| Type | No PFOS or PFOA reported | PFOS or PFOA reported | Total count | ||
| No HI PFAS reported | At least one HI PFAS reported | No HI PFAS reported | At least one HI PFAS reported | ||
| Samples | 12,704 (65.2%) | 357 (1.8%) | 3,380 (17.3%) | 3,041 (15.6%) | 19,482 |
| Systems | 5,560 (78.8%) | 196 (2.8%) | 516 (7.3%) | 784 (11.1%) | 7,056 |
Considering eligible samples and systems within the aggregated state dataset, states reported detections of either PFOS, PFOA, or one or more HI PFAS in 34.8 percent (6,778 of 19,482) of samples and 21.2 percent (1,496 of 7,056) of systems. When any PFAS (among PFOA, PFOS, and the HI PFAS) were reported detected, at least one HI PFAS was also reported in 50.1 percent (3,398 of 6,778) of samples and at 65.5 percent (980 of 1,496) of systems. Further, among samples and systems that reported detections of PFOS or PFOA, at least one HI PFAS was detected in 47.4 percent (3,041 of 6,421) of samples and at 60.3 percent (784 of 1,300) of systems. This demonstrated strong co-occurrence of HI PFAS with PFOA and PFOS and a substantial likelihood (over 50 percent) of at least one HI PFAS being present at systems with reported detections of PFOS or PFOA. Overall, one or more HI PFAS were reported at about 13.9 percent (980 of 7,056) of systems included in the aggregated state dataset of non-targeted monitoring. If this percentage were extrapolated to the nation, one or more HI PFAS would be at detectable levels in over 9,000 systems. Table 11 shows the distribution of systems in a similar manner but provides a breakdown by state and includes only systems that monitored for either three or four of the HI PFAS.
| State | No PFOA/S detected | PFOA/S detected | Total system count | ||
| No HI detected | HI detected | No HI detected | HI detected | ||
| CO | 270 (68.0%) | 26 (6.5%) | 11 (2.8%) | 90 (22.7%) | 397 |
| IL | 582 (89.7%) | 22 (3.4%) | 15 (2.3%) | 30 (4.6%) | 649 |
| KY | 37 (52.9%) | 2 (2.9%) | 16 (22.9%) | 15 (21.4%) | 70 |
| MA | 60 (35.5%) | 2 (1.2%) | 12 (7.1%) | 95 (56.2%) | 169 |
| MI | 1,969 (91.5%) | 82 (3.8%) | 43 (2.0%) | 58 (2.7%) | 2,152 |
| ND | 49 (98%) | 1 (2.0%) | 0 (0.0%) | 0 (0.0%) | 50 |
| NH | 60 (43.2%) | 2 (1.4%) | 34 (24.5%) | 43 (30.9%) | 139 |
| NJ | 225 (36.3%) | 7 (1.1%) | 127 (20.5%) | 261 (42.1%) | 620 |
| OH | 1,397 (94.5%) | 31 (2.1%) | 25 (1.7%) | 26 (1.8%) | 1,479 |
| SC | 10 (22.2%) | 1 (2.2%) | 10 (22.2%) | 24 (53.3%) | 45 |
| VT | 488 (87.6%) | 15 (2.7%) | 31 (5.6%) | 23 (4.1%) | 557 |
The percentage of systems included in Table 11 that reported detections of any HI PFAS ranged from 2.0 to 57.4 percent of systems when broken down by state, with six states exceeding 20 percent of systems. The percentage of systems that reported detections of any PFAS ranged from 2.0 to 77.8 percent. Many systems and/or samples that were included in the aggregated state dataset did not monitor for all four HI PFAS. It is possible that more systems would have detected HI PFAS if they had monitored for all four HI PFAS. Additionally, as demonstrated in Table 11, when PFOA and/or PFOS were reported, at least one of the HI PFAS chemicals were also frequently reported. Table 12 presents system counts for systems where PFOS or PFOA were detected according to (a) how many HI PFAS were monitored and (b) how many HI PFAS were reported to be detected.
| HI analyzed | HI reported present | Total | ||||
| 0 | 1 | 2 | 3 | 4 | ||
| 1 | 143 (70.1%) | 61 (29.9%) | 204 | |||
| 2 | 49 (45.8%) | 41 (38.3%) | 17 (15.9%) | 107 | ||
| 3 | 153 (34.7%) | 95 (21.5%) | 137 (31.1%) | 56 (12.7%) | 441 | |
| 4 | 171 (31.2%) | 135 (24.6%) | 179 (32.7%) | 61 (11.1%) | 2 (0.4%) | 548 |
| Total | 516 | 332 | 333 | 117 | 2 | |
Among systems that reported detections of PFOS and/or PFOA, the fraction of systems that also reported detections of any HI PFAS tended to increase as systems monitored for more of the HI PFAS. At systems monitoring for a single HI PFAS, 29.9 percent reported a detection at some point during sampling. This increased to 68.8 percent of systems reporting detections of at least one HI PFAS when monitoring for all four HI PFAS. Not only did the fraction of systems reporting detections of any HI PFAS increase as the number of HI PFAS increased, so did the number of HI PFAS that were reported. When three or four HI PFAS were monitored, over 40 percent of systems reported detections of two to three of the HI PFAS. Thus, if PFOS or PFOA are reported, there is a reasonable likelihood that multiple HI PFAS would be present as well.
2. Pairwise Chemical Co-Occurrence
In addition to considering the co-occurrence of six PFAS as two groups, EPA conducted a pairwise analysis to further explore co-occurrence relationships. Table 13 shows the calculated system-level odds ratios for every unique pair of PFAS chemicals evaluated. The equation for calculating odds ratios is symmetrical. Because of this, in a given row it does not matter which chemical is “Chemical A” and which is “Chemical B.” Additional information on odds ratios may be found in USEPA (2023e) and a brief explanation is described following Table 13.
| Chem A | Chem B | Chems A and B reported | Only Chem B reported | Only Chem A reported | Neither Chem reported | Odds ratio [95% CI] |
|---|---|---|---|---|---|---|
| HFPO-DA | PFBS | 10 | 452 | 10 | 5,116 | 11.3 [4.8-26.7] |
| HFPO-DA | PFHxS | 2 | 339 | 18 | 5,229 | 1.7 [0.4-6.7] |
| HFPO-DA | PFNA | 2 | 77 | 18 | 5,491 | 7.9 [2.0-31.4] |
| HFPO-DA | PFOA | 16 | 438 | 4 | 5,129 | 46.8 [16.3-134.1] |
| HFPO-DA | PFOS | 14 | 399 | 6 | 5,168 | 30.2 [11.9-76.5] |
| PFBS | PFHxS | 433 | 133 | 261 | 5,501 | 68.6 [54.5-86.5] |
| PFBS | PFNA | 135 | 33 | 560 | 5,601 | 40.9 [27.7-60.4] |
| PFBS | PFOA | 517 | 360 | 178 | 5,273 | 42.5 [34.8-52.0] |
| PFBS | PFOS | 503 | 278 | 192 | 5,355 | 50.5 [41.1-62.0] |
| PFHxS | PFNA | 150 | 38 | 473 | 5,939 | 49.6 [34.3-71.6] |
| PFHxS | PFOA | 510 | 466 | 113 | 5,510 | 53.4 [42.6-66.9] |
| PFHxS | PFOS | 507 | 353 | 116 | 5,623 | 69.6 [55.4-87.6] |
| PFNA | PFOA | 236 | 934 | 15 | 5,871 | 98.9 [58.7-166.5] |
| PFNA | PFOS | 234 | 789 | 17 | 6,016 | 105.0 [64.1-171.9] |
| PFOA | PFOS | 893 | 130 | 277 | 5,756 | 142.7 [114.5-177.9] |
Odds ratios reflect the change in the odds of detecting one chemical ( e.g., Chemical A) given that the second chemical ( e.g., Chemical B) is known to be present compared to the odds of detecting if the second chemical is not present. For example, as shown in Table 13, the point estimate of 142.7 for the odds ratio between PFOA and PFOS indicates that the odds of detecting PFOA after knowing that PFOS has been observed are 142.7 times what the odds would have been if PFOS was not observed, and vice versa. For every pair of chemicals, except for HFPO-DA and PFHxS, both the point estimate and 95 percent CI were above 1, indicating significant increases in the likelihood of detecting one chemical if the other is present. For HFPO-DA and PFHxS, 1 fell within the 95 percent CI, and thus the odds ratio was not determined to be statistically significantly different from 1.
Both as a group and as individual chemicals, the HI PFAS had a higher likelihood of being reported if PFOS or PFOA were present. PFHxS, HFPO-DA, PFNA and PFBS (the individual HI PFAS) are demonstrated to generally co-occur with each other, as well. As such, these data support that there is a substantial likelihood PFHxS, HFPO-DA, PFNA, and PFBS co-occur with a frequency of public health concern in drinking water systems.
D. Occurrence Relative to the Hazard Index
EPA analyzed the available state data in comparison to the proposed HI MCL of 1.0 to evaluate the co-occurrence of PFHxS, HFPO-DA, PFNA, and PFBS. Table 14 presents the total number and percentage of monitored systems that exceeded the proposed HI MCL based on state reported HI PFAS detections for the states that conducted non-targeted monitoring and that sampled all four HI PFAS as a part of their overall monitoring efforts. EPA notes that for equivalent comparison purposes Table 14 only accounts for samples that included reported values (including non-detects) of all four HI PFAS. As shown within the table, the majority of states evaluated had monitored systems exceed the proposed HI MCL, ranging from 0.72 to 7.41 percent of total monitored systems.
| State | Total monitored systems > proposed HI of 1.0 | Percent systems > proposed HI of 1.0 |
|---|---|---|
| Colorado | 5 | 1.26 |
| Illinois | 10 | 1.54 |
| Kentucky | 6 | 7.41 |
| Massachusetts | 8 | 6.40 |
| Michigan | 14 | 0.65 |
| New Hampshire | 4 | 2.99 |
| North Dakota | 0 | 0.00 |
| Ohio | 25 | 1.69 |
| South Carolina | 0 | 0.00 |
| Vermont | 4 | 0.72 |
Further evaluating the available state data related to the proposed HI MCL of 1.0, Table 15 presents the total number of systems and associated populations served that exceed the proposed HI of 1.0 based on state reported HI PFAS detections for the same states shown in Table 15. However, in this case, EPA also analyzed the same non-targeted state data adding in additional samples even if those samples did not contain reported values (including non-detects) for all four HI PFAS ( i.e., exceeding the HI based on only one to three HI PFAS with reported values included within a sample). Moreover, while these states did monitor for all four HI PFAS as a part of their overall monitoring, in a subset of those states some samples did not include reported data on all four HI PFAS ( i.e., values of one or more of the HI PFAS were not reported as non-detect, rather no value was reported). This analysis, presented in Table 15, shows an increase in the number of monitored systems exceeding the proposed HI of 1.0 and demonstrates prevalence of these PFAS at levels of concern, even when all four PFAS may not be included within a sample.
| State | Total monitored systems > proposed HI of 1.0 | Population served |
|---|---|---|
| Colorado | 5 | 5,429 |
| Illinois | 10 | 107,461 |
| Kentucky | 6 | 103,315 |
| Massachusetts | 19 | 302,482 |
| Michigan | 14 | 221,484 |
| New Hampshire | 25 | 36,463 |
| North Dakota | 0 | 0 |
| Ohio | 25 | 234,834 |
| South Carolina | 0 | 0 |
| Vermont | 4 | 410 |
Combining the non-targeted monitoring results shown previously with targeted state monitoring conducted for all four HI PFAS showed at least 917 samples from 157 PWSs in 15 states that exceed the proposed HI of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS. These systems serve approximately 3.08 million people. Additionally, data from New Jersey, which conducted non-targeted monitoring but did not conduct any monitoring that included all four HI PFAS, showed an additional 243 samples within 57 systems serving a total population of approximately 1.43 million people exceeding the proposed HI of 1.0 based solely upon the reported detections of three of the four HI PFAS ( i.e., PFHxS, PFNA, and PFBS). USEPA (2023e) presents a detailed discussion on state PFAS monitoring information. More information on occurrence in state monitoring is available in section III.B. of this preamble.
In summary, the finished water data collected under both non-targeted and targeted state monitoring efforts from 22 states showed there are at least 1,007 PWSs serving a total population of approximately 15.3 million people that have at least one result exceeding the proposed PFOA MCL of 4.0 ppt. In those same 22 states, there are also at least 805 PWSs serving a total population of approximately 13.6 million people that have at least one result exceeding the proposed PFOS MCL of 4.0 ppt. Related to the proposed HI, finished water data collected under both non-targeted and targeted state monitoring efforts in 16 states showed there are at least 214 systems serving a total population of approximately 4.5 million people that exceed the proposed HI value of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS. USEPA (2023e) presents a detailed discussion on state PFAS monitoring information. Additionally, EPA is aware that since the data were collected some of these states may have updated data available and that additional states have or intend to conduct monitoring of finished drinking water, such as New York and Virginia. EPA will consider, and as appropriate, analyze additional data submitted in response to this proposal to inform future regulatory decision making.
E. Occurrence Model
A Bayesian hierarchical occurrence model was developed to explore national occurrence of the four PFAS that were most frequently detected in the UCMR 3: PFOS, PFOA, PFHxS, and PFHpA. While PFNA and PFBS were included in the UCMR 3 as well, they lacked sufficient reported values above the UCMR 3 MRLs to be incorporated into the model. The model has been peer reviewed and is described extensively in Cadwallader et al. (2022). Briefly, inputs to the model include the UCMR 3 dataset as well as subsequent data in publicly available state datasets that were collected at PWSs that took part in the UCMR 3. 23,130 analytical results from state datasets were used to supplement the UCMR 3. These results were derived from 17 state datasets. The objective of the model was to enable national estimates of PFAS occurrence by using available UCMR 3 and state data to inform occurrence distributions both within and across PWSs. Note that while PFHpA was included in the model because of its UCMR 3 occurrence data availability, EPA is not proposing to regulate it in this document.
The model uses Markov chain Monte Carlo (MCMC) and the assumption of lognormality in PFAS chemical occurrence. After log-transforming all available data, system-level means (where each system has a mean concentration for each chemical) were assumed to be distributed multivariate normally. Further, within-system occurrence was assumed to be distributed normally for each chemical. Since system-level means are distributed multivariate normally, correlation between estimated system-level means across chemicals could also be assessed. The assumption of lognormality as well as the incorporation of state data with lower reporting limits allowed the model to generate reasonable estimates for PFAS occurrence at levels below the UCMR 3 MRLs. EPA has used similar hierarchical statistical models to inform regulatory decision making in the past, such as for development of the NPDWR for Arsenic and Cryptosporidium parvum (USEPA, 2006b; USEPA, 2000e).
After the model was fit with available data from PWSs that were included in the UCMR 3, it was used to simulate occurrence at an inventory of active CWS and NTNCWS extracted from the Safe Drinking Water Information System (SDWIS). System-level means for non-UCMR 3 systems were simulated by sampling from the multivariate normal distribution of system-level means that was produced during the model fitting process. For systems that were included in the UCMR 3, the fitted system-level mean was used directly. Using population data retrieved from SDWIS, the total number of systems with system-level mean concentrations of each chemical, as well as their associated population served, could be estimated. The median estimate and the 90 percent credible interval are shown for the systems with system-level means at or above various PFAS concentrations in Table 16 and the population served by those systems in Table 17.
| Concentration (ppt) | PFHxS [90% CI] | PFOA [90% CI] | PFOS [90% CI] |
|---|---|---|---|
| 4.0 | 1,697 [1,053-2,702] | 1,987 [1,338-3,016] | 3,427 [2,326-4,900] |
| 5.0 | 1,232 [745-2,009] | 1,351 [903-2,083] | 2,593 [1,737-3,770] |
| 10.0 | 417 [241-730] | 349 [223-577] | 986 [627-1,531] |
| Concentration (ppt) | PFHxS [90% CI] | PFOA [90% CI] | PFOS [90% CI] |
|---|---|---|---|
| 4.0 | 18,641,000 [15,669,000-21,693,000] | 28,051,000 [24,966,000-33,071,000] | 30,627,000 [27,407,000-35,665,000] |
| 5.0 | 14,092,000 [11,129,000-16,887,000] | 20,844,000 [18,193,000-24,239,000] | 24,405,000 [21,611,000-28,440,000] |
| 10.0 | 4,608,000 [3,432,000-7,262,000] | 7,111,000 [5,566,000-9,335,000] | 10,561,000 [7,858,000-12,866,000] |
For PFOA, PFOS, and PFHxS, thousands of systems were estimated to have mean concentrations over the lowest thresholds ( i.e., 4.0 and 5.0 ppt) presented in Tables 16 and 17 with the total population served estimated to be in the tens of millions. The populations shown here represent the entire populations served by systems estimated to have system-level means over the various thresholds. It is likely that different subpopulations would be exposed to different mean PFAS concentrations if multiple source waters are used.
In addition to the estimates of individual chemical occurrence, the multivariate normal distribution of system-level means allowed the model to provide insight on estimated co-occurrence. Untransformed estimates of system-level means were assessed for correlation across each unique pair of the four modeled chemicals included in the model. Estimates of the Pearson correlation coefficient are shown in Table 18. The Pearson correlation coefficient serves as an indicator of the strength of the linear relationship between two variables and may range from −1 to 1. Positive values indicate a positive relationship ( i.e., as one variable increases, so does the other).
| Chemical pair | Pearson correlation coefficient [90% CI] |
|---|---|
| PFOS-PFOA | 0.71 [0.60-0.79] |
| PFOS-PFHpA | 0.69 [0.57-0.78] |
| PFOS-PFHxS | 0.85 [0.74-0.92] |
| PFOA-PFHpA | 0.85 [0.80-0.89] |
| PFOA-PFHxS | 0.55 [0.41-0.65] |
| PFHpA-PFHxS | 0.62 [0.47-0.72] |
EPA considered a moderate strength correlation as greater than 0.5 and a strong correlation as greater than 0.7. Each point estimate of correlation coefficients between two chemicals was above the threshold for a moderate strength correlation. The carboxylic acids (PFOA-PFHpA) and sulfonic acids (PFOS-PFHxS) had the highest estimated correlation strengths, with both the point estimate and the 90% credible interval above 0.7. PFOS-PFOA and PFOS-PFHpA had similar point estimates and 90% credible interval ranges, spanning the moderate-to-strong correlation range. Both PFOA-PFHxS and PFHpA-PFHxS had the bulk of their posterior distributions fall in the range of a moderate strength correlation. Thus, the model predicted significant positive relationships among system-level means of all four chemicals that were included. These results support the co-occurrence discussion presented in section VII.C of this preamble that indicated extensive co-occurrence of PFOA, PFOS, and the HI PFAS observed in state datasets from both groupwise and pairwise chemical perspectives.
F. Combining State Data With Model Output To Estimate National Exceedance of Either MCLs or Hazard Index
In order to broadly estimate the number of systems that would be impacted by the proposed regulation, including MCLs of 4.0 ppt for PFOA and PFOS alongside an HI of 1.0 for PFHxS, HFPO-DA, PFNA, and PFBS, findings from non-targeted monitoring in state datasets were combined with model estimates. Specific details on the methodology can be found in USEPA (2023e). Briefly, information collected from non-targeted state datasets included the fractions of systems that reported a measurement at or above the UCMR 5 MRL for a given analyte and an empirical cumulative distribution function (eCDF) consisting of system-level maximum observed concentrations of that chemical at these systems. The UCMR 5 MRLs for HFPO-DA, PFNA, and PFBS are equivalent to 5.0 ppt, 4.0 ppt, and 3.0 ppt, respectively (USEPA, 2021e). This applies the assumption that the fraction of systems that observed HFPO-DA, PFNA, and PFBS at or above UCMR 5 MRLs and the maximum concentrations observed at those systems are reasonably representative of the nation.
The model was used to simulate entry point-level concentrations of the four modeled PFAS (PFOA, PFOS, PFHpA, and PFHxS) under the assumption that within-system concentrations are lognormally distributed (a common assumption for drinking water contaminants, see (Cadwallader et al. (2022)) and that variability in concentrations is entirely across entry points (thus a given entry point is assumed to have a constant concentration) For each system, the maximum estimated entry point PFOA or PFOS concentration was selected to determine whether the system exceeded either of the proposed MCLs of 4.0 ppt. The entry point with the maximum concentration is the point that determines whether a system has an entry point that is above an MCL. Estimates of the system-level maximum for PFHxS were also selected for the HI calculation. The maximum value of the sum of the four modeled PFAS at each system was selected and used as a basis for determining which systems would receive superimposed concentrations of the three remaining HI chemicals (HFPO-DA, PFNA, and PFBS). This approach was selected due to the extensive observed co-occurrence of PFAS in the UCMR 3, state data, and modeled estimates.
Multiple methods of system selection were used that reflected different degrees of co-occurrence. The chemical concentration that was applied to selected systems were randomly sampled from the eCDF for each chemical. Based on the model output, this assumes that system-level maximums for HFPO-DA, PFNA, and PFBS would occur at the same location within a system. Substantial co-occurrence among PFAS was observed in the model output, state datasets, and the UCMR 3 dataset. Combination of system-level maximums independently pulled from chemical eCDFs is a reasonable simplifying assumption given this co-occurrence. This is particularly true given that the systems selected for each chemical are not necessarily the same and in most cases were probability-weighted. Estimates of the range of systems impacted were developed by taking Q5 and Q95 estimates for each method. The low end of the range was taken as the lowest Q5 estimate across methods, rounded down, while the high end of the range was taken as the highest Q95 estimate across methods, rounded up. This was also done for the total population served by these systems.
The resulting range of systems estimated to be impacted by the proposed regulation of an MCL of 4.0 ppt for PFOA and PFOS and an HI of 1.0 for a mixture of PFHxS, HFPO-DA, PFNA, and PFBS was 3,400-6,300 systems serving a total population of 70-94 million people. Among these systems, 100-500 were estimated to be systems exceeding the HI for PFHxS, HFPO-DA, PFNA, and PFBS that had not already exceeded the MCLs for PFOA and/or PFOS. The total population served by these systems was estimated to be 0.6 to 6.3 million people.
In summary, using the MCMC occurrence model, EPA estimated baseline occurrence to derive occurrence and exposure estimates for the proposed MCLs for PFOA and PFOS, as well as alternative MCLs. EPA then used these modeled estimates to inform the costs and benefits determination as described in section XIII of this preamble. Here and in section XIII of this preamble, EPA requests comment on the number of systems estimated to solely exceed the HI (but not the PFOA or PFOS MCLs) according to the approach outlined in USEPA (2023e).
VIII. Analytical Methods
EPA developed the following liquid chromatography/tandem mass spectrometry (LC/MS/MS) analytical methods to quantitatively monitor drinking water for targeted PFAS: EPA Method 533 (USEPA, 2019b) and EPA Method 537.1, Version 2.0 (USEPA, 2009b; USEPA, 2020a). All six PFAS proposed for regulation can be measured by both EPA Methods 533 and 537.1 and both methods are acceptable for meeting the monitoring requirements of this regulation.
EPA Method 533 monitors for 25 select PFAS, including PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS, with published measurement accuracy and precision data for PFOA in reagent water, finished ground water, and finished surface water. For further details about the procedures for this analytical method, please see Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (USEPA, 2019b).
EPA Method 537.1 (an update to EPA Method 537), monitors for 18 select PFAS, including PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS, with published measurement accuracy and precision data for PFOA in reagent water, finished ground water, and finished surface water. For further details about the procedures for this analytical method, please see Method 537.1, Version 2.0, Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) (USEPA, 2020a).
A. Practical Quantitation Levels (PQLs) for Regulated PFAS
As described in section VI of this preamble, a PQL is defined as the “lowest concentration of an analyte that can be reliably measured within specified limits of precision and accuracy during routine laboratory operating conditions” (USEPA, 1985). EPA uses the PQL to estimate or evaluate the minimum, reliable quantitation level that most laboratories can be expected to meet during day-to-day operations. The basis for setting PQLs is (1) quantitation, (2) precision and accuracy, (3) normal operations of a laboratory, and (4) the fundamental need (in the compliance monitoring program) to have a sufficient number of laboratories available to conduct the analyses. For the PFAS regulated in this proposal, EPA is proposing the following PQLs outlined in Table 19:
| Contaminant | PQL (ppt) |
|---|---|
| PFOA | 4.0 |
| PFOS | 4.0 |
| HFPO-DA | 5.0 |
| PFHxS | 3.0 |
| PFNA | 4.0 |
| PFBS | 3.0 |
Drinking water analytical laboratories have different performance capabilities dependent upon their instrumentation (manufacturer, age, usage, routine maintenance, operating configuration, etc.) and analyst experience. Some laboratories will effectively generate accurate, precise, quantifiable results at lower concentrations than others. Organizations that collect data need to establish data quality objectives (DQOs) to meet the needs of their program. These DQOs should consider establishing reasonable quantitation levels that laboratories can routinely meet. Establishing a quantitation level that is too low may result in recurring QC failures that will necessitate repeating sample analyses, increase costs, and potentially reduce laboratory capacity. Establishing a quantitation level that is too high may result in important lower-concentration results not being quantitated.
EPA's approach to establishing DQOs within the UCMR program serves as an example. EPA established MRLs for UCMR 5, finalized in December 2021, and requires laboratories approved to analyze UCMR samples to demonstrate that they can make quality measurements at or below the established MRLs. EPA calculated the UCMR 5 MRLs using quantitation-limit data from multiple laboratories participating in an MRL-setting study. An MRL is set after a statistical determination that 75% of laboratories will be able to meet that level with a 95% CI (USEPA, 2022g). The UCMR 5 MRLs are not intended to represent the lowest achievable measurement level an individual laboratory may achieve. As noted above, these MRLs are derived using the quantitation level results from multiple laboratories participating in an analytical study and account for differences in the capability of laboratories across the country.
For UCMR 5, EPA calculated and published the following multi-laboratory MRLs for the PFAS addressed in this proposed rule: PFOA: 0.004 µg/L (4.0 ppt); PFOS: 0.004 µg/L (4.0 ppt); PFHxS: 0.003 µg/L (3.0 ppt); HFPO-DA: 0.005 µg/L (5.0 ppt); PFNA: 0.004 µg/L (4.0 ppt); PFBS: 0.003 µg/L (3.0 ppt). Based on the multi-laboratory data acquired for the UCMR 5 rule, EPA has defined the PQL for PFAS addressed in this proposed rule to be equal to the UCMR 5 MRL (see Table 19, above).
Some laboratories are capable of measuring the PFAS addressed in this proposed rule at lower concentrations. Indeed, EPA received some public comments prior to developing the final UCMR 5 recommending lower MRLs than those that were ultimately promulgated (USEPA, 2022g). However, after reviewing the data from laboratories that participated in the MRL-setting study for UCMR 5, EPA concluded that the MRLs set in that rule represented “lowest feasible” levels for a national measurement program. Based on laboratory performance in EPA's UCMR 5 Laboratory Approval Program, during 2021-2022, EPA believes that the UCMR 5 MRLs are appropriate for using as PQL for this proposed rulemaking. EPA recognizes that as more laboratories upgrade their instrumentation and gain more experience analyzing drinking water samples for PFAS, more laboratories may become capable of quantitatively measuring PFAS at lower concentrations.
While the values below the PQL will not be used to calculate compliance with the proposed MCLs under this proposed rule (see discussion above in Section VI of this preamble), values lower than the PQL are achievable by individual laboratories, and therefore lower levels can be used for purposes of screening and to determine compliance monitoring frequency. EPA is proposing the use of a rule trigger level for less frequent compliance monitoring under certain circumstances in which systems can demonstrate PFAS concentrations in finished drinking water are below:
• one-third of the MCLs for PFOA and PFOS, i.e., 1.3 ppt; and
• one-third of the HI MCL for the HI PFAS (PFHxS, HFPO-DA, PFNA, and PFBS), i.e., 0.33.
Based on laboratory calibration standard data submitted as part of the UCMR 5 Laboratory Approval Program, described in more detail in section VI.A. of this preamble, EPA maintains that laboratories are capable of screening to this level. For additional discussion on this rule trigger level and monitoring requirements for this proposal, please see sections VI.A. and IX of this preamble.
IX. Monitoring and Compliance Requirements
A. What are the monitoring requirements?
EPA is proposing requirements for CWS and NTNCWSs to monitor for certain PFAS. The Agency is proposing to amend 40 CFR part 141 by adding a new subpart to incorporate the regulated PFAS discussed in this preamble. Under this new subpart, PWSs must sample entry points to the distribution system using a monitoring regime based on EPA's SMF for SOCs. Under the SMF for SOCs, the monitoring frequency for a PWS is dependent on previous monitoring results, among other things (USEPA, 2004). EPA is proposing that, consistent with the SMF for SOCs, groundwater systems serving greater than 10,000 and all surface water systems are initially required to monitor quarterly within a 12-month period for regulated PFAS. To provide additional flexibilities for small groundwater systems, EPA is also proposing and taking comment on a modification to the SMF for SOCs in that groundwater systems serving 10,000 or fewer are initially required to only monitor twice for regulated PFAS within a 12-month period, each sample at least 90 days apart. In this proposal, all systems would be allowed to use previously acquired monitoring data to satisfy the initial monitoring requirements (see subsection (C) of this preamble below for additional details about using previously acquired monitoring data to satisfy initial monitoring requirements). Based on the SMF, EPA is also proposing that based upon the initial monitoring results, primacy agencies would be able to reduce compliance monitoring frequency for a system to once or twice every three years (depending on system size) if the monitoring results are below the rule trigger level (defined below).
EPA is proposing that water systems will conduct compliance monitoring to demonstrate that finished drinking water does not exceed the MCLs for regulated PFAS. Water systems must show the primacy agency that the contaminant is not present in the drinking water supply or, if present, it does not exceed the proposed MCLs for regulated PFAS. For compliance monitoring frequency purposes only, EPA is proposing a rule trigger level of one-third the MCLs (1.3 ppt for PFOA and PFOS and 0.33 for HI PFAS (PFHxS, HFPO-DA, PFNA, and PFBS)). As such, EPA is proposing amendments for a new subpart to include the following term to describe the circumstances in which water systems may be eligible for reduced monitoring for PFOA and PFOS and the HI PFAS if below this:
• Rule Trigger Level: One-third of the MCLs for regulated PFAS, i.e., 1.3 ppt for PFOA and PFOS and 0.33 for PFAS regulated by the HI (PFHxS, HFPO-DA, PFNA, and PFBS).
For more information, including the basis of the rule trigger level, please see sections VI.A. and VIII.A. of this preamble.
EPA notes that for some proposed regulated PFAS, the values used to determine reduced monitoring may be below their PQLs ( e.g., PFOA and PFOS at 1.3 ppt when the PQL is 4.0 ppt). For purposes of screening to determine monitoring frequency, however, EPA has sufficient confidence that while measurements below the PQL may be slightly less precise and accurate, they are achievable by individual laboratories and appropriate for this intended purpose. EPA requests comment on this finding regarding feasibility of the proposed MCLs and more generally on laboratory capacity. As noted earlier, EPA anticipates laboratories will be able to adjust to demand (including possible price effects), which the Agency anticipates will be distributed across the implementation period. Further, at the proposed rule trigger level, the measurement is primarily useful in determining whether the contaminant is present in a sample and for evaluating monitoring flexibilities, rather than to determine its specific concentration. EPA has set these values below the MCLs to allow systems the opportunity to reduce their monitoring schedule and burden, while minimizing the chance of random normal variation resulting in a single sample close to, but below the MCLs, when the “true” annual average value would be above the MCL. For additional discussion on PQL, please see section VII of this preamble. Systems below the rule trigger level would be required to conduct compliance monitoring according to the following schedule:
• Systems that do not detect regulated PFAS in their systems at or above the rule trigger level (1.3 ppt for PFOA and PFOS and 0.33 for the HI PFAS (PFHxS, HFPO-DA, PFNA, and PFBS)), and that serve 3,300 or fewer customers will be required to analyze one sample for all regulated PFAS per three-year compliance period at each entry point to the distribution system (EPTDS) that does not meet or exceed the rule trigger level.
• Systems that do not detect regulated PFAS in their systems at or above the rule trigger level (1.3 ppt for PFOA and PFOS and 0.33 for the HI PFAS (PFHxS, HFPO-DA, PFNA, and PFBS), and that serve a population of greater than 3,300 will be required to analyze two samples for all regulated PFAS at least 90 days apart in one calendar year per three-year compliance period at each EPTDS that does not meet or exceed the rule trigger level.
If a water system is not below the rule trigger level for regulated PFAS at a given EPTDS, it will be required to monitor for all regulated PFAS quarterly at that EPTDS. Systems monitoring less frequently than quarterly whose sample result is at or exceeds the rule trigger level must also begin quarterly sampling at the EPTDS where regulated PFAS were observed at or above the trigger level. In either case, the primacy agency may allow a system to move to a reduced monitoring frequency when the primacy agency determines that the system is below the rule trigger level and reliably and consistently below the MCL. However, primacy agencies cannot determine that the system is below the rule trigger level and reliably and consistently below the MCL until at least four consecutive quarters of quarterly monitoring have occurred. EPA notes that, as described above, systems may have EPTDS within a system on different compliance monitoring schedules depending on monitoring results.
In this document, EPA requests comment on the reduced monitoring approach the Agency is proposing which will save resources for many lower-risk water systems. First, EPA is requesting comment on the allowance of a water system to potentially have each EPTDS on a different compliance monitoring schedule based on specific entry point sampling results ( i.e., some EPTDS being sampled quarterly and other EPTDS sampled only once or twice during each three-year compliance period), and if instead, compliance monitoring frequency should be consistent across all of the system's sampling points. EPA is also requesting comment on establishing the proposed rule trigger level values of 1.3 ppt for PFOA and PFOS and 0.33 for the PFAS regulated by the HI (PFHxS, HFPO-DA, PFNA, and PFBS). EPA is seeking comment on establishing the trigger level at other levels, specifically alternative values of 2.0 ppt for PFOA and PFOS and 0.50 for the HI PFAS. EPA notes that adjusting the trigger levels to 2.0 ppt for PFOA and PFOS and 0.50 for the HI PFAS would result in a considerable number of additional water systems significantly reducing their monitoring frequency from at least four times each year to once or twice every three years. EPA also notes that the higher trigger may provide slightly less assurance of the water systems' current regulated PFAS levels as a result of the more intermittent monitoring. EPA is seeking comment on the merits and drawbacks of these higher trigger levels compared to those proposed in this document.
B. How are PWS compliance and violations determined?
Consistent with existing rules for determining compliance with NPDWRs, EPA is proposing that compliance with this rule will be determined based on the analytical results obtained at each sampling point. For systems monitoring quarterly, compliance with the proposed MCLs for regulated PFAS will be determined by running annual averages at the sampling point. Systems monitoring less frequently whose sample result(s) are at or exceed the rule trigger level must revert to quarterly sampling at each EPTDS where the trigger level is met or exceeded for all regulated PFAS in the next quarter, with the triggered sample result being used for the first quarter of monitoring in calculating the running annual average.
A running annual average is an average of sample analytical results for samples taken at a particular monitoring location during the previous four consecutive quarters. If a system takes more than one compliance sample during each quarter at a particular monitoring location, the system must average all samples taken in the quarter at that location to determine the quarterly averages to be used in calculating the running annual averages. Conversely, if a system does not collect required samples for a quarter, the running annual average will be based on the total number of samples collected for the quarters in which sampling was conducted. A system will not be considered in violation of an MCL until it has completed one year of quarterly sampling, except in the case where, if a quarterly sampling result will cause the running annual averages to exceed an MCL at any sampling point ( i.e., the analytical result is greater than four times the MCL). In that case, the system is out of compliance with the MCL immediately.
When calculating the running annual averages, if a sample result is less than the PQL for the monitored PFAS, EPA is proposing to use zero to calculate the average for compliance purposes. For example, if a system has sample results for PFOA that are 2.0, 1.5, 5.0, and 1.5 ppt for their last four quarters at a sample location, the values used to calculate the running annual average would be 0.0, 0.0, 5.0, and 0.0 with a resulting PFOA running annual average of 1.3 ppt. As described in sections VI and VIII of this preamble, EPA is proposing that values below the PQL will not be used to determine compliance with the proposed MCLs as these PQLs are the lowest concentration of analyte that can be reliably measured within specified limits of precision and accuracy during routine laboratory conditions. As such, quantifying concentrations below the PQL for compliance purposes may decrease the precision and accuracy of the measured value and may not be achievable for some individual laboratories. In this document, EPA is requesting comment on whether EPA should consider an alternative approach when calculating the running annual averages for compliance. Specifically, in the case where a regulated PFAS is detected but below its proposed PQL, that the proposed rule trigger level (1.3 ppt for PFOA and PFOS and 0.33 of each of the HI PFAS PQLs ( i.e., PFHxS=1.0, HFPO-DA=1.7, PFNA=1.3, and PFBS=1.0)) be used as the value in calculating the running annual average for compliance purposes. While this approach may be more complicated to implement than using zero when below the PQL, it is largely consistent with EPA's NPDWRs related to other SOCs and has the potential to slightly increase the public health protection provided by this proposed regulation.
C. Can systems use previously collected data to satisfy the initial monitoring requirement?
As proposed, systems would be allowed to use previously collected monitoring data to satisfy the initial monitoring requirements. In general, a system with appropriate historical monitoring data for each distribution system entry point, collected using EPA Methods 533 or 537.1 as part of UCMR 5 or a state-level or other appropriate monitoring campaign, could use that monitoring data to satisfy initial monitoring requirements.
EPA is proposing that systems with previously acquired monitoring data from UCMR 5 will not be required to conduct separate initial monitoring for regulated PFAS. To satisfy the initial monitoring requirements for these systems using UCMR 5 data, data collected after January 1st, 2023, can be used for entry point samples.
While EPA expects most systems serving 3,300 or greater will have UCMR 5 data, EPA is also proposing that systems with previously acquired monitoring data from outside UCMR 5, including State-led or other appropriate occurrence monitoring using EPA methods 533 or 537.1 will also not be required to conduct separate initial monitoring for regulated PFAS. This addition may allow systems serving fewer than 3,300 to satisfy the initial monitoring requirements. Data collected after January 1st, 2023, can be used for entry point samples. Data collected between January 1st, 2019, and December 31, 2022, may also be used if it is below the proposed rule trigger level of 1.3 ppt for PFOA and PFOS and an HI of 0.33 for PFHxS, HFPO-DA, PFNA, and PFBS. The additional analytical requirement for older data is to ensure the use of these data is adequately representative of current water quality conditions. If systems have multiple years of data, the most recent data must be used.
D. Can systems composite samples?
40 CFR 141.24 subpart C describes instances where primacy agencies may reduce the samples a system must analyze by allowing samples to be composited. Composite sampling is an approach in which equal volumes of water from multiple entry points are combined into a single container and analyzed as a mixture. The reported concentration from the analysis of the composite sample therefore reflects the average of the analyte concentrations from the contributing entry points. Composite sampling can potentially reduce analytical costs because the number of required analyses is reduced by combining multiple samples into one and analyzing the composited sample. However, based on comments EPA received in consulting with state regulators and small business entities (operators of small PWSs), PFAS are ubiquitous in the environment at low concentrations which necessitates robust laboratory analytical precision at these low concentrations. For example, incidental contamination from or adherence to surface laboratory equipment may artificially lower contaminant concentrations or result in false negatives. Additionally, PFAS are demonstrated to be ubiquitous in the environment such that the risk for false positives may increase when combining samples for composite analysis. Based on these potential implementation issues, EPA is proposing a deviation from the SMF for SOCs by not allowing samples to be composited.
E. Can primacy agencies grant monitoring waivers?
40 CFR 141.24 Subpart C describes instances where the primacy agency may grant waivers predicated on proximity of the system to contaminant sources ( i.e., susceptibility to contamination) and previous uses of the contaminant within the watershed (including transport, storage, or disposal). Based on EPA's consultation with state regulators and operators of small PWSs, the Agency believes that due to the ubiquity, environmental persistence, and transport abilities of PFAS, granting waivers based on these conditions would be challenging, therefore EPA is not incorporating this flexibility as a part of these proposed monitoring requirements. However, in this proposal, EPA is considering and taking comment on waivers based on sampling results. Specifically, EPA is requesting comment on whether water systems should be permitted to apply to the primacy agency for a monitoring waiver of up to 9-years (one full compliance cycle) for these proposed PFAS if after at least one year of quarterly sampling the results are below the rule trigger level of one-third of the MCLs, or for systems that may be monitoring less frequently than quarterly if at least two consecutive three year-compliance period sample results are below the rule trigger level. Additionally, EPA is requesting comment on allowing similar monitoring waivers to be granted based on previously acquired monitoring data as described above in subsection (C) of this preamble. In either case, systems with a monitoring waiver would be required to take at least one sample per nine-year compliance cycle in order to maintain or renew an existing waiver. Furthermore, EPA is seeking comment on the identification of possible alternatives to traditional vulnerability assessments that should be considered to identify systems as low risk and potential eligibility for monitoring waivers.
F. When must systems complete initial monitoring?
Pursuant to Section 1412(b)(10), this proposed rule would require compliance three years after promulgation. To satisfy initial monitoring requirements and demonstrate rule compliance, within the three years following rule promulgation, groundwater systems serving a population greater than 10,000 and all surface water systems will be required to demonstrate their baseline concentrations using data from four quarterly samples collected over a one-year period. Groundwater systems serving a population 10,000 or fewer may collect two quarterly samples at least 90 days apart over a one-year period for the purpose of initial monitoring, rather than collecting four quarterly samples. Additionally, as described earlier in this section (subsection C of this preamble), EPA is proposing that systems with appropriate, previously acquired monitoring data from UCMR 5, state-led, or other applicable monitoring programs using EPA Methods 533 or 537.1, will not be required to conduct separate initial monitoring for regulated PFAS. As such, given the advantageous timing of UCMR 5 monitoring data for all systems serving greater than 3,300 and the availability of historical monitoring data that many small systems serving 3,300 or fewer may utilize from state-level monitoring programs, EPA notes this proposed allowance will offer significant burden reduction for these systems and sufficient timing to take necessary actions and ensure rule compliance. For systems that may not have available data and/or choose to conduct additional monitoring, as proposed in this document, EPA would encourage those systems to conduct their initial monitoring as soon as practicable following rule promulgation to allow for actions that may need to be taken based on monitoring results and to certify rule compliance. The Agency seeks comment on EPA's proposed initial monitoring timeframe, particularly for NTNCWS or all systems serving 3,300 or fewer.
G. What are the laboratory certification requirements?
EPA is proposing that laboratories demonstrate their ability to achieve the precision and detection limits necessary to meet the objectives of this regulation. The proposal would require laboratories to analyze performance evaluation (PE) samples every year in order to achieve and maintain certification.
X. Safe Drinking Water Act (SDWA) Right To Know Requirements
A. What are the Consumer Confidence Report requirements?
A CWS must prepare and deliver to its customers an annual Consumer Confidence Report (CCR) in accordance with requirements in 40 CFR 141 Subpart O. A CCR provides customers with information about their local drinking water quality as well as information regarding the water system compliance with drinking water regulations. Under this proposal CWSs would be required to report detected PFAS in their CCR; specifically, PFOA, PFOS, PFHxS, HFPO-DA, PFNA, and PFBS, and the HI for the mixtures of PFHxS, HFPO-DA, PFNA, and PFBS.
B. What are the public notification (PN) requirements?
As part of SDWA, the Public Notification (PN) rule ensures that consumers will know if there is a problem with their drinking water. Notices alert consumers if there is risk to public health. They also notify customers: If the water does not meet drinking water standards; if the water system fails to test its water; if the system has been granted a variance (use of less costly technology); or if the system has been granted an exemption (more time to comply with a new regulation).
All PWSs must give the public notice for all violations of NPDWRs and for other situations. Under this proposal, EPA is proposing that violations of the three MCLs in the proposal would be designated as Tier 2 and as such, PWSs would be required to comply with 40 CFR 141.203. Per 40 CFR 141.203(b)(1), notification of an MCL violation should be provided as soon as practicable but no later than 30 days after the system learns of the violation.
XI. Treatment Technologies
Water systems with PFAS levels that exceed the MCLs proposed would need to take action to provide drinking water which meets the NPDWR by the compliance dates established in the rule when final. For example, systems may install water treatment or consider other options such as source remediation or connecting to an uncontaminated water system. While conventional treatment technologies are unable to remove PFOS, PFOA, PFNA, PFHxS, PFBS, or HFPO-DA to levels protective of public health (McCleaf et al., 2017), there are technologies currently available that effectively remove these and other PFAS.
Section 1412(b)(4)(E) of SDWA requires that the Agency “list the technology, treatment techniques, and other means which the Administrator finds to be feasible for purposes of meeting [the MCL],” which are referred to as BATs. These BATs are used by states to establish conditions for source water variances under Section 1415(a). Section 1412(b)(4)(E)(ii) also requires that the Agency identify small system compliance technologies (SSCTs), which are affordable treatment technologies, or other means that can achieve compliance with the MCL (or treatment technique [TT], where applicable).
A. What are the best available technologies?
The Agency identifies the BATs as those meeting the following criteria: (1) The capability of a high removal efficiency; (2) a history of full-scale operation; (3) general geographic applicability; (4) reasonable cost based on large and metropolitan water systems; (5) reasonable service life; (6) compatibility with other water treatment processes; and (7) the ability to bring all the water in a system into compliance. The Agency is proposing the following technologies as BAT for PFAS removal from drinking water based its review of the treatment and cost literature (USEPA, 2023g):
• GAC
• AIX
• High pressure membranes (RO and NF)
Operationally, GAC and AIX are sorptive processes meaning a process where one substance becomes attached to another. Sorption is typically composed of absorption where one substance is incorporated into another, adsorption where one substance is incorporated onto another, or ion exchange (IX) where an aqueous ion (the contaminant) is traded for a different less dangerous ion (typically chloride in AIX) on an insoluble matrix. Sorptive processes pour feed water through a vessel filled with a sorbent known as a contactor. The operation continues until the sorbent no longer effectively removes the target contaminant; this is when the contaminant “breaks through” the treatment process. At this point, the sorbent must be disposed then replaced or regenerated. The length of time until the sorbent must be replaced or regenerated is known as bed life and is a critical factor in the cost effectiveness of sorptive technology. One bed life measurement is the water volume that can be treated before breakthrough and is measured in bed volumes (BV). BVs are how many times the sorbent ( i.e., media) can be filled in the bed in which the sorbent resides before contaminant breakthrough. EPA estimates GAC treatment will be sufficiently available to support cost-effective compliance with this proposed regulation, and requests comment on whether additional guidance on applicable circumstances for GAC treatment is needed.
High pressure membranes are a separation process where feed water is split into two streams across a membrane. One stream has few contaminants or other solutes left in it and is known as permeate or produced water. The other stream contains the concentrated contaminant and other solutes which is known as concentrate, brine, retentate, or reject water. Membrane flux is how much permeate is produced for a given surface area and time; different system configurations operating at the same flux produce differing quantities of finished water. This means that membrane systems with differing configurations cannot be directly compared based on flux. Flux can be reduced during membrane fouling which is where things accumulate on or in the membrane. Fouling can require membrane cleaning and replacement or operational changes.
There are also non-treatment options which may be used for compliance such as replacing a PFAS-contaminated drinking water source with a new uncontaminated source ( e.g., a new well), or purchasing compliant water from another system. Conventional and most advanced water treatment methods are ineffective at removing PFAS (Rahman et al., 2014). Further information on the proposed BATs is provided below.
1. Granular Activated Carbon
GAC is a separation process where contaminants become attached to specially treated carbon with a high surface area. The GAC manufacturing process can accept any highly carbonaceous material as an input such as bituminous coal, lignite coal, peat, wood, coconut shells, and peach pits. Activation is predominantly a thermal process, although it may also be a chemical process, that creates as well as enlarges pores generating a porous structure with a large surface area per unit mass. Literature suggests that the primary mechanisms of adsorption include both hydrophobic and electrostatic interactions (Ateia et al., 2019). In addition to removing PFAS, GAC can remove contaminants including taste and odor compounds, natural organic matter (NOM), VOCs, SOCs, DBP precursors, and radon. Organic compounds with high molecular weights are also readily adsorbable.
Demonstrated PFAS removal efficiencies can exceed >99 percent and can achieve concentrations less than 4 ng/L (Forrester and Bostardi, 2019; Zeng et al., 2020; Westreich et al., 2018; Belkouteb et al., 2020; Woodard et al., 2017; and Hopkins et al., 2018). During the operation, carbon is removed from the system periodically, for disposal or regeneration, based on treatment objectives. Several factors affect bed life, including the presence of competing contaminants such as nitrate and the carbon type used. Most studies found that natural or dissolved organic matter (NOM/DOM) interferes with PFAS sorption, in general, and its presence dramatically lowers treatment efficacy (McNamara et al., 2018; Pramanik et al., 2015; Yu et al., 2012). The lowered treatment effectiveness was found to be less pronounced for HFPO-DA than for perfluoroalkyl carboxylic acid (PFCA) C7 and above for GAC (Park et al., 2020).
Reactivation is a process that removes organic compounds from adsorption sites on GAC enabling reuse. Although different methods are available for GAC reactivation, the process most commonly involves high temperature thermal treatment in a specialized facility such as a multiple hearth furnace or rotary kiln (Matthis and Carr, 2018; USEPA, 2023g). Reactivated carbon can become totally exhausted with other contaminants not removed during reactivation and must be replaced. However, for GAC, the loss of approximately 10 percent of the media due to abrasion within the reactivation process can result in a somewhat steady state for performance as new GAC is added each time to replace the lost GAC. Systems may decide to dispose of GAC ( i.e., operate on a `throw-away' basis) instead of reactivating the media. GAC can be a cost-effective treatment option despite needing to dispose of contaminated carbon.
2. Anion Exchange
AIX is a separation process where an anion in the aqueous phase is exchanged for an ion attached to an exchange resin. Similar to GAC, AIX uses contactors. These contactors, however, are filled with a bed of beads or gel known as resin instead of carbon. As feed water moves through the resin, an anionic contaminant, such as PFAS exchanges, for an anion, typically chloride, on the resin. For PFAS compounds, vendors generally recommend using PFAS-selective resins (Boodoo, 2018; Boodoo et al., 2019; Lombardo et al., 2018; Woodard et al., 2017). AIX may also have a beneficial effect by removing other undesirable anions from the treated water such as nitrate or sulfate.
Demonstrated PFAS removal efficiencies may be >99 percent and can achieve concentrations less than 4 ng/L (Dixit et al., 2021; Dixit et al., 2020; Zeng et al., 2020; Liu, 2017; Kumarasamy et al., 2020; Arevalo et al., 2014; and Yan et al., 2020). The operation continues until enough of the resin's available IX sites have ions from the feed water and the resin no longer effectively removes the target contaminant, also known as “breaks through.” At this point, the resin must be disposed and replaced or regenerated. The length of time until resin must be replaced or regenerated is known as bed life and is a critical factor in the cost effectiveness of IX as a treatment technology. Several factors affect bed life, including the presence of competing ions such as nitrate and the resin type used.
Conventional regeneration solutions are not generally effective for restoring the capacity of PFAS-selective resins (Liu and Sun, 2021). Regeneration may be possible using organic solvents (Boodoo, 2018; Zaggia et al., 2016) or proprietary methods (Woodard et al., 2017). These alternative regeneration practices are generally practical or cost-effective only with very high influent concentrations, such as in remediation settings. Therefore, in drinking water applications using PFAS-selective resin, vendors recommend a single-use approach where the spent resin is disposed and replaced with fresh resin (Boodoo, 2018; Lombardo et al., 2018). Exhausted resin must be disposed; due to the difficulties mentioned earlier and vendor recommendation, resins are often operated on a `throw-away' basis. This operational mode avoids generating spent regenerant liquid residuals. AIX can be a cost-effective treatment option.
3. High Pressure Membranes (RO and NF)
RO and NF are membrane separation processes where water is forced through a membrane at greater than osmotic pressure. The water that transverses the membrane is known as permeate or produce water, and has few solutes left in it; the remaining water is known as concentrate, brine, retentate, or reject water and forms a waste stream with concentrated solutes. NF has a less dense active layer than RO, which enables lower operating pressures but also makes it less effective at removing contaminants. In drinking water treatment, these membranes are most often used in a spiral-wound configuration that consists of several membrane envelopes, layered with feed spacers, and rolled together in and around a central collection tube. Feed pressures for NF membranes are typically in the range of 50 to 150 pounds per square inch (psi). Feed pressures for RO membranes are in the range of 125 to 300 psi in low pressure applications (such as PFAS removal) but can be as high as 1,200 psi in applications such as seawater desalination (USEPA, 2023d). RO may remove other contaminants including arsenic and chromium-VI.
RO and NF may achieve PFAS removal >99 percent (Lipp et al., 2010; Horst et al., 2018; Liu et al., 2021; Dickenson and Higgins, 2016; Steinle-Darling et al., 2008; Boonya-Atichart et al., 2016; Appleman et al., 2014; Thompson et al., 2011; CDM Smith, 2018; Dickenson and Higgins, 2016; and Dowbiggin et al., 2021). While water quality affects process design ( e.g., recovery rate, cleaning frequency, and antiscalant selection), it has relatively little effect on PFAS removal percent. High pressure membranes generate a relatively large concentrate stream, which will contain PFAS as well as other rejected dissolved species, which will require disposal or additional treatment. The large concentrate stream also means less treated water is available for distribution ( e.g., 70 to 85 percent of source water), which is a disadvantage for systems with limited water supply.
B. PFAS Co-Removal
AIX and GAC are effective at removing PFAS and there is generally a linear relationship between PFAS chain length and removal efficiency shifted by functional group (McCleaf et al., 2017; Sörengård et al., 2020). Perfluoroalkyl sulfonates (PFSA), such as PFOS, are removed with greater efficiency than the corresponding PFCA, such as PFOA, of the same carbon backbone length (Appleman et al., 2014; Du et al., 2014; Eschauzier et al., 2012; Ochoa-Herrera and Sierra-Alvarez, 2008; Zaggia et al., 2016). Generally, for a given water type and concentration, a PFSA is removed about as well as a PFCA which has two more fully perfluorinated carbons in its backbone. For example, PFHxS (six carbon backbone and a sulfonic acid functional group) is removed about as well as PFOA (eight carbon backbone and a carboxylate head) and perfluorohexanoic acid (PFHxA) (six carbon backbone with a carboxylate head) is removed approximately as well as PFBS (four carbon backbone and a sulfonic acid functional group). Additionally, the compounds with longer carbon chain displayed a smaller percentage decrease in average removal efficiency over time (McCleaf et al., 2017).
The three technologies discussed above have all been demonstrated to be effective in removing all six PFAS proposed for regulation as part of this rulemaking. As discussed in section VII.C. of this preamble, PFAS have been shown to co-occur. Hence, where the six PFAS being regulated today occur in concentrations above their respective regulatory standards there is also an increased probability of other unregulated PFAS being present. Further, since these same technologies also remove other long-chain and higher carbon/higher molecular weight PFAS EPA expects this rulemaking will provide additional public health benefits and protection by removing unregulated PFAS that may have adverse health effects. While EPA has not quantified those benefits as part of this rulemaking, the Agency believes these important secondary benefits further enhance public protection offered by this proposed regulation.
C. Management of Treatment Residuals
As part of EPA's BAT evaluation, the Agency assesses the availability of studies of full-scale treatment of residuals that fully characterize residual waste streams and disposal options. At present, the most likely management option for spent material containing PFAS is reactivation for GAC and incineration for spent IX resin. For disposal of RO/NF membrane concentrate, most systems use surface water discharge or discharge to sanitary sewer. The large volume of residuals is a well-known obstacle to adoption of membrane separation technology in general. For more information on current residuals management practices, see Best Available Technologies and Small System Compliance Technologies for Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water (USEPA, 2023g) or Managing and Treating Per- and Polyfluoroalkyl Substances (PFAS) in Membrane Concentrates (Tow et al., 2021).
EPA recognizes that future actions through several statutory authorities other than SDWA may have direct or indirect implications for drinking water treatment facilities and some actions may prevent or reduce PFAS entering drinking water sources. EPA is addressing PFAS through statutory authorities including the CERCLA, Resource Conservation and Recovery Act (RCRA), Toxic Substances Control Act (TSCA), Clean Water Act, Clean Air Act, and Emergency Planning and Community Right-to-Know Act (EPCRA). For example, as part of EPA's PFAS Strategic Roadmap, EPA proposed certain PFAS be designated as CERCLA hazardous substances to require reporting of PFOA and PFOS releases, enhance the availability of data, and ensure agencies can recover cleanup costs (USEPA, 2022c). In the Strategic Roadmap, EPA has also committed to expanding research on and accelerating the deployment of emerging PFAS treatment, remediation, destruction, disposal, and control technologies (USEPA, 2022c). EPA's 2020 Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances outlines the current state of the science on techniques and treatments that may be used to destroy or dispose of PFAS (USEPA, 2020b). In accordance with EPA's PFAS Strategic Roadmap, EPA anticipates releasing an updated version of the Guidance in 2023. As part of this rulemaking, EPA considered that in drinking water treatment, large volumes of spent GAC and ion exchange resin must be removed which does not lend itself to on-site storage over time. The disposal options identified in the Interim Guidance (USEPA, 2020b) are landfill disposal and thermal treatment.
Stakeholders have expressed concern to EPA that a hazardous substance designation for certain PFAS may limit their disposal options for drinking water treatment residuals ( e.g., spent media, concentrated waste streams) and/or potentially increase costs. Although EPA anticipates that designating chemicals as hazardous substances under CERCLA generally should not result in limits on for disposal of PFAS drinking water treatment residuals, EPA has estimated the treatment costs for systems both with the use of hazardous waste disposal and non-hazardous disposal options to assess the effects of potentially increased disposal costs. Specifically, EPA assessed the potential impact on PWS treatment costs associated with hazardous residual management requirements in a sensitivity analysis on the proposed option. Relative to the national analysis for the proposed option assuming non-hazardous disposal, the hazardous waste disposal assumption would increase PWS costs by 4% ($30 million annually) at the 3% discount rate and 5% ($61 million annually) at the 7% discount rate should spent media need to be disposed of as hazardous waste in the future because of separate EPA or State regulatory action. EPA's sensitivity analysis demonstrates that potential hazardous waste disposal requirements may increase PWS treatment costs marginally, however the increase in PWS costs are not significant enough to change the determination that benefits of the rulemaking justify the costs. These estimates are discussed in greater detail in the HRRCA section of this proposed rulemaking and in Appendix N of the Economic Analysis (USEPA, 2023i). These costs are limited to the disposal of the PFAS contaminated residuals and wastes. Results for small systems are presented in Section D of this preamble below. EPA is seeking public input related to PFAS treatment residual disposal in Section XIV of this preamble.
D. What are small system compliance technologies (SSCTs)?
EPA is proposing the SSCTs shown in Table 20. The table shows which of the BATs listed above are also affordable for each small system size category listed in Section 1412(b)(4)(E)(ii) of SDWA. The Agency identified these technologies based on an analysis of treatment effectiveness and affordability.
| System size (population served) | GAC | IX | RO/NF | Point of use (POU) RO/NF 1 |
|---|---|---|---|---|
| Notes: | ||||
| 1 POU RO is not currently listed as a compliance option because the regulatory options under consideration require treatment to concentrations below the current NSF International/American National Standards Institute (NSF/ANSI) certification standard for POU device removal of PFAS. However, POU treatment is reasonably anticipated to become a compliance option for small systems in the future if NSF/ANSI or other independent third-party certification organizations develop a new certification standard that mirrors EPA's proposed regulatory standard. The affordability conclusions presented here reflect the costs of devices certified under the current standard, not a future standard, which may change dependent on future device design. | ||||
| 2 EPA's work breakdown structure (WBS) model for POU treatment does not cover systems larger than 3,300 people (greater than 1 million gallons per day [MGD] design flow), because implementing and maintaining a large-scale POU program is likely to be impractical. | ||||
| 25-500 | Yes | Yes | No | Yes. |
| 501-3,300 | Yes | Yes | No | Yes. |
| 3,301-10,000 | Yes | Yes | Yes | not applicable. 2 |
The operating principle for POU RO devices is the same as centralized RO: Steric exclusion and electrostatic repulsion of ions from the charged membrane surface. In addition to a RO membrane for dissolved ion removal, POU RO devices often have a sediment pre-filter and a carbon filter in front of the RO membrane, a 3- to 5-gallon treated water storage tank, and a carbon filter between the tank and the tap.
EPA identified SSCTs using the affordability criteria methodology developed for drinking water rules (USEPA, 1998b). The analysis method is a comparison of estimated incremental household costs for PFAS treatment to an expenditure margin, which is the difference between baseline household water costs and a threshold equal to 2.5% of median household income (MHI). Table 21 shows the expenditure margins derived for the analysis. These margins show the cap on affordable incremental annual expenditures.
| System size (population served) | MHI 1 | Affordability threshold 2 | Baseline water cost 3 | Expenditure margin |
|---|---|---|---|---|
| Notes: | ||||
| 1 MHI based on U.S. Census Bureau's American Community Survey five-year estimates (United States Census Bureau, 2010) stated in 2010 dollars, adjusted to 2020 dollars using the Consumer Price Index (CPI) (for all items) for areas under 2.5 million persons. | ||||
| 2 Affordability threshold equals 2.5 percent of MHI. | ||||
| 3 Household water costs derived from 2006 Community Water System Survey (USEPA, 2009c), based on residential revenue per connection within each size category, adjusted to 2020 dollars based on the CPI for All Urban Consumers: Water and Sewer and Trash Collection Services in U.S. City Average. | ||||
| A | B = 2.5% × A | C | D = B−C | |
| 25-500 | $55,377 | $1,384 | $507 | $877 |
| 501-3,300 | 53,596 | 1,340 | 587 | 753 |
| 3,301-10,000 | 58,717 | 1,468 | 613 | 855 |
Table 21 shows the estimates of per-household costs by treatment technology and size category generated using the treatment cost method described in section XII.B of this preamble as well as Best Available Technologies and Small System Compliance Technologies for Perchlorate in Drinking Water (USEPA, 2019c) and Technologies and Costs for Treating Perchlorate-Contaminated Waters (USEPA, 2018c). Based on the results presented in Table 22, EPA identified candidate technologies available for which costs do not exceed the corresponding expenditure margin and, therefore, meet the SSCT affordability criterion. As such, EPA has determined that affordable SSCTs are available, and the Agency is not proposing any variance technologies.
| System size (population served) | GAC | IX | RO/NF | POU RO/NF 1 |
|---|---|---|---|---|
| Notes: | ||||
| 1 POU RO is not currently a compliance option because the regulatory options under consideration require treatment to concentrations below the current NSF/ANSI certification standard for POU device removal of PFAS. However, POU treatment is reasonably anticipated to become a compliance option for small systems in the future if NSF/ANSI or other independent third-party certification organizations develop a new certification standard that mirrors EPA's proposed regulatory standard. Costs presented here reflect the costs of devices certified under the current testing standard, not a future standard, which may change dependent on future device design. | ||||
| 2 EPA's WBS model for POU treatment does not cover systems larger than 3,300 people (greater than 1 MGD design flow), because implementing and maintaining a large-scale POU program is likely to be impractical. | ||||
| 25-500 | $395 to $727 | $376 to $645 | $3,711 to $4,676 | $317 to $326. |
| 501-3,300 | $139 to $332 | $133 to $235 | $608 to $1,169 | $299 to $300. |
| 3,301-10,000 | $136 to $329 | $121 to $218 | $326 to $462 | not applicable. 2 |
The results discussed above assume management of spent GAC and spent IX resin using current typical management practices (reactivation for GAC and incineration for resin). EPA is in the process of proposing some PFAS be designated as hazardous substances under CERCLA and listed as hazardous constituents under RCRA. If finalized, neither of these actions should result in limiting disposal options and how PFAS containing waste, including spent GAC or resin, is required to be managed. However, waste management facilities may, at their own discretion, refuse to accept PFAS-containing materials or drinking water treatment operations may choose to send spent GAC and resin containing PFAS to facilities permitted to treat and/or dispose of hazardous wastes. To consider the implications of this possibility, EPA has developed an assessment of the current unit costs for disposing spent treatment materials and the costs associated with their disposal as hazardous waste. Table 23 shows the resulting cost per household if systems dispose of these residuals as hazardous waste. Although costs would increase somewhat compared to if they do not treat the spent media as hazardous waste, those increases are not significant enough to change the conclusions about affordability.
| System size (population served) | GAC | IX |
|---|---|---|
| 25-500 | $417 to $827 | $397 to $678. |
| 501-3,300 | $149 to $368 | $138 to $243. |
| 3,301-10,000 | $146 to $360 | $124 to $222. |
In addition to the required analysis for small system affordability, EPA having received a number of recommendations from the SAB, the NDWAC, and other stakeholders, is exploring the use of alternative expenditure margins and other potential changes to the national level affordability methodology to better understand the cost impacts of new standards on low income and disadvantaged households served by small drinking water systems. The Agency conducted supplemental affordability analyses using alternative metrics suggested to EPA by stakeholders to demonstrate the potential affordability implications of the proposed NPDWR on the determination of affordable technologies for small systems at the national level of analysis.
As required under the 1996 amendments to SDWA, EPA lists treatment technologies for small systems that are affordable and that achieve compliance with the regulatory standard. As part of its affordability analysis for the proposed PFAS rule, EPA determined that there are several affordable treatment technologies for small systems, including GAC, IX, RO, and POU RO. 5 EPA is seeking public comment on the national level analysis of affordability of SSCTs and specifically on the potential methodologies presented. EPA's national small system affordability determination can be found in Section 9.12.1 of the EA. EPA's supplementary affordability analyses can be found in Section 9.12.2 of the EA. EPA is also seeking comment on whether there are additional technologies which are viable for PFAS removal to the proposed MCLs as well as any additional costs which may be associated with non-treatment options such as water rights procurement. Finally, EPA is seeking comment on the benefits from using treatment technologies (such as reverse osmosis and GAC) that have been demonstrated to co-remove other types of contaminants found in drinking water and whether employing these treatment technologies are sound strategies to address PFAS and other regulated or unregulated contaminants that may co-occur in drinking water.
5 POU RO is not currently a compliance option because the regulatory options under consideration require treatment to concentrations below 70 ppt total of PFOA and PFOS, the current certification standard for POU devices. However, POU treatment is anticipated to become a compliance option for small systems in the future should NSF/ANSI or another accredited third-party certification entity develop a new certification standard that mirrors (or is demonstrated to treat to concentrations lower than) EPA's proposed regulatory standard. The affordability conclusions for POU RO should be considered preliminary because they reflect the costs of devices certified under the current standard, not a future standard.
Following finalization of the PFAS NPDWR, EPA will work with primacy agencies to provide assistance to support implementation of the rule. EPA requests comment on the type of assistance that would help small public water systems identify laboratories that can perform the required monitoring, evaluate treatment technologies and determine the most appropriate way to dispose of PFAS contaminated residuals and waste the systems may generate when implementing the rule.
XII. Rule Implementation and Enforcement
A. What are the requirements for primacy?
This section describes the regulations, procedures, and policies primacy entities must adopt, or have in place, to implement the PFAS rule, when it is final. States, Territories, and Tribes must continue to meet all other conditions of primacy in 40 CFR part 142. Section 1413 of SDWA establishes requirements that primacy entities (States or Indian Tribes) must meet to maintain primary enforcement responsibility (primacy) for its PWSs. These include:
• Adopting drinking water regulations that are no less stringent than Federal NPDWRs in effect under sections 1412(a) and 1412(b) of the Act;
• Adopting and implementing adequate procedures for enforcement;
• Keeping records and making reports available on activities that EPA requires by regulations;
• Issuing variances and exemptions (if allowed by the State) under conditions no less stringent than allowed by SDWA Sections 1415 and 1416; and
• Adopting and being capable of implementing an adequate plan for the provision of safe drinking water under emergency situations.
40 CFR part 142 sets out the specific program implementation requirements for States to obtain primacy for the Public Water System Supervision (PWSS) Program, as authorized under 1413 of the Act.
Under 40 CFR 142.12(b), all primacy States/territories/tribes would be required to submit a revised program to EPA for approval within two years of promulgation of any final PFAS NPDWR or could request an extension of up to two years in certain circumstances. To be approved for a program revision, primacy States/territories/tribes would be required to adopt revisions at least as stringent as the revised PFAS-related provisions in 40 CFR 141.6 (Effective Dates); 40 CFR 141.900 subpart Z (Control of Per- and Polyfluoroalkyl Substances); 40 CFR 141.50 (Maximum Contaminant Level Goals for organic contaminants); 40 CFR 141.60 (Maximum Contaminant Levels for organic contaminants); appendix A to subpart O ([Consumer Confidence Report] Regulated contaminants); Appendix A to Subpart Q ((NPDWR violations and other situations requiring public notice); Appendix B to Subpart Q (Standard health effects language for public notification); 40 CFR 142.62 (Variances and exemptions from the MCLs for organic and inorganic contaminants); and 40 CFR 142.16 (Primary Enforcement Responsibility).
B. What are the primacy agency record keeping requirements?
The current regulations in 40 CFR 142.14 require primacy agencies to keep records of analytical results to determine compliance, system inventories, sanitary surveys, state approvals, vulnerability and waiver determinations, monitoring requirements, monitoring frequency decisions, enforcement actions, and the issuance of variances and exemptions. If primacy agencies grant monitoring waivers, they must record monitoring results that are below the rule trigger level in order to ensure systems are eligible for reduced monitoring schedules (for additional discussion on the rule trigger level and monitoring waivers, please see sections VIII and IX of this preamble). The primacy agency record keeping requirements remain unchanged and would apply to PFAS as with any other regulated contaminant.
C. What are the primacy agency reporting requirements?
Currently, primacy agencies must report to EPA information under 40 CFR 142.15 regarding violations, variances and exemptions, enforcement actions, and general operations of State PWS programs. These reporting requirements remain unchanged and would apply to PFAS as with any other regulated contaminant. However, the proposed PFAS MCLs, when final, could result in a greater frequency of reporting by certain primacy agencies. See discussion of PRA compliance in Section XV of this preamble for more information.
D. Exemptions and Extensions
In accordance with SDWA §1412(b)(10), a state or EPA may grant an extension of up to two additional years to comply with an NPDWR's MCL(s) if the state or EPA determines an individual system needs additional time for capital improvements. At this time, EPA does not intend to provide a two-year extension nationwide. However, States may provide such an extension on an individual system basis. Where a State or EPA chooses to provide such an extension, the system would have up to five years from the rule's promulgation date to meet the MCLs. In addition, under SDWA §1416, EPA or primacy Agencies may grant an exemption for systems meeting specified criteria that provides an additional period for compliance not to exceed 3 years beyond the time period provided by Section 1412(b)(10). Under SDWA §1416(a), a State which has primary enforcement responsibility may exempt any public water system within the State's jurisdiction from any requirement respecting a MCL of any applicable NPDWR upon a finding that:
• Due to compelling factors (which may include economic factors, including qualification of the public water system as a system serving a disadvantaged community pursuant to section 300j-12(d) of this title), the public water system is unable to comply with such contaminant level or treatment technique requirement, or to implement measures to develop an alternative source of water supply,
• The public water system was in operation on the effective date of such contaminant level or treatment technique requirement, or, for a system that was not in operation by that date, only if no reasonable alternative source of drinking water is available to such new system,
• The granting of the exemption will not result in an unreasonable risk to health; and
• Management or restructuring changes (or both) cannot reasonably be made that will result in compliance with this subchapter, or if compliance cannot be achieved, improve the quality of the drinking water.
In addition, SDWA §1416(b)(2)(C) also allows for a small system that does not serve a population of more than 3,300 and which needs financial assistance for the necessary improvements to receive up to three additional two-year exemptions, not to exceed a total of six years provided that the system establishes that it is taking all practicable steps to meet the requirements. In total, this means that some systems could potentially exceed the MCLs' numerical standards for up to 14 years after the rule promulgation date (or approximately 2037/2038). EPA is seeking comment as to whether there are specific conditions that should be mandated for systems to be eligible for exemptions under 1416 to ensure that they are only used in rare circumstances where there are no other viable alternatives and what those conditions would be. EPA has established requirements for EPA issuance of these exemptions in 40 CFR 142 subpart F but could consider amending these requirements or establishing requirements for State exemptions.
XIII. Health Risk Reduction and Cost Analysis
This section summarizes the HRRCA for the proposed NPDWR for PFAS, which is written in compliance with SDWA section 1412(b)(3)(C). Section 1412(b)(3)(C)(i) lists the analytical elements required in a HRRCA applicable to a NPDWR that includes an MCL. The prescribed HRRCA elements include:
(1) Quantifiable and nonquantifiable health risk reduction benefits;
(2) quantifiable and nonquantifiable health risk reduction benefits from reductions in co-occurring contaminants;
(3) quantifiable and nonquantifiable costs that are likely to occur solely as a result of compliance;
(4) incremental costs and benefits of rule options;
(5) effects of the contaminant on the general population and sensitive subpopulations including infants, children, pregnant women, the elderly, and individuals with a history of serious illness;
(6) any increased health risks that may occur as a result of compliance, including risks associated with co-occurring contaminants; and
(7) other relevant factors such as uncertainties in the analysis and factors with respect to the degree and nature of the risk.
Based on this analysis and pursuant to Section 1412(b)(4)(C) of SDWA, the Administrator has determined that the quantified and nonquantifiable benefits of the proposed regulation justify the costs. The complete HRRCA for the proposed NPDWR, Economic Analysis for the Proposed PFAS Rule, is hereafter referred to as the “Economic Analysis,” and can be found in the docket at USEPA (2023j).
For purposes of this Economic Analysis, EPA assumes that the NPDWR will be promulgated by the end of 2023. This analysis follows the standard NPDWR compliance schedule with regulatory requirements taking effect three years after the date on which the regulation is promulgated. If EPA issues a final NPDWR for PFAS by the end of 2023, EPA assumes actions to comply with the rule, including installation of treatment technologies, will occur by 2026. Based on an assumed mean human lifespan of 80 years, EPA evaluates costs and benefits under the proposed rule through the year 2104. EPA selected this period of analysis to capture health effects from chronic illnesses that are typically experienced later in life ( i.e., cardiovascular disease [CVD] and cancer). EPA annualized the future estimated streams of costs and benefits symmetrically over this same period of analysis. Capital costs for installation of treatment technologies are spread over the useful life of the technologies. EPA does not capture effects of compliance with the proposed rule after the end of the period of analysis. Costs and benefits discussed in this section are presented as annualized present values in 2021 dollars. EPA determined the present value of these costs using discount rates of 3 and 7 percent, which are discount rates prescribed by the (OMB Circular A-4, 2003).
Estimates of PFAS occurrence used for cost-benefit modeling rely on a Bayesian hierarchical estimation model of national PFAS occurrence in drinking water (Cadwallader et al., 2022) discussed in Section VII.E. of this preamble above. The model was fitted using sample data from systems participating in PFAS sampling under UCMR 3 and included systems serving over 10,000 customers, as well as a subset of 800 smaller systems. A best-fit model was selected using sample data to define occurrence and co-occurrence of PFOA, PFOS, PFHpA, and PFHxS in water systems stratified by system size and incorporating variations within and among systems. Sample data were derived from state-level datasets as well as from UCMR 3. For more information on EPA's occurrence model, please see Section VII.E. of this preamble and USEPA (2023e).
In the Economic Analysis, EPA analyzes the costs and benefits of the proposed rule, as well as several regulatory alternatives. EPA analyzed the costs and benefits of setting individual MCLs for PFOA and PFOS at 4.0 ppt, 5.0 ppt, and 10.0 ppt, referred to as Option 1a, Option 1b, and Option1c, respectively. EPA assessed these options in the Economic Analysis to understand the impact of less stringent PFOA and PFOS MCLs, and the Agency is asking for comment on these assessments in the Economic Analysis. The Agency is also inviting comment on whether establishing a traditional MCLG and MCL for PFHxS, HFPO-DA, PFNA, and PFBS instead of or in addition to the HI approach would change public health protection, improve clarity of the rule, or change costs. EPA has not separately presented changes in quantified costs and benefits for these approaches. If EPA adds individual MCLs in addition to using the HI approach, EPA anticipates there will be no change in costs and benefits relative to the proposed rule ( i.e., the same number of systems will incur identical costs to the proposed option and the same benefits will be realized). EPA has not separately quantified the benefits and costs for the alternative approach to regulate PFHxS, PFNA, PFBS, and HFPO-DA with individual MCLs instead of the HI. However, EPA expects both the costs and benefits would be reduced under this approach as fewer systems may be triggered into treatment and its associated costs. Additionally, systems that exceed one or more of the individual MCLs will treat to a less stringent and public health-protective standard. Furthermore, under the proposed option, PWSs are required to treat based on the combined occurrence of PFAS included in the HI which considers the known and additive toxic effects and occurrence and likely co-occurrence of PFAS compounds in the HI, providing more public health protection compared to an individual MCL approach.
Section A summarizes the entities which would be affected by the rule and provides a list of key data sources used to develop EPA's baseline water system characterization. Section B provides an overview of the cost-benefit model used to estimate the national costs and benefits of the proposed rule. Section C summarizes the methods EPA used to estimate costs associated with the proposed rule. Section D summarizes the methods EPA used to estimate quantified benefits associated with the proposed rule. Section E provides a summary of the nonquantifiable benefits associated with reductions in exposure to both PFOA and PFOS. Section F provides a qualitative summary of benefits expected to result from the removal of PFAS included in the HI component of the proposed regulation and additional co-removed PFAS contaminants. Section G summarizes benefits expected to result from DBPs co-removal. Section H provides a comparison of cost and benefit estimates. Section I summarizes and discusses key uncertainties in the cost and benefit analyses. Quantified costs and benefits for the proposed option and alternative options considered are summarized in section H, specifically Tables 66-69. Tables 70-71 summarizes the non-quantified B-Cs and assess the potential impact of non-quantifiable benefits and costs on the overall B-C estimate. Finally, Section J presents the Administrator's cost-benefit determination for the proposed rule.
A. Affected Entities and Major Data Sources Used To Develop the Baseline Water System Characterization
The entities potentially affected by the proposed PFAS regulation are primacy agencies and PWSs. PWSs subject to the proposed rule requirements are either CWSs or NTNCWSs. These water systems can be publicly or privately owned. PWSs subject to the rule would be required to meet the MCL and comply with monitoring and reporting requirements. Primacy agencies would be required to adopt and enforce the drinking water standard as well as the monitoring and reporting requirements.
Both PWSs and primacy agencies are expected to incur costs, including administrative costs, monitoring and reporting costs, and—in a limited number of cases—anticipated costs to reduce PFAS levels in drinking water to meet this proposed NPDWR using treatment or nontreatment options. Section C of this preamble below summarizes the method EPA used to estimate these costs.
The systems that reduce PFAS concentrations will reduce associated health risks. EPA developed methods to estimate the potential benefits of reduced PFAS exposure among the service populations of systems with PFAS levels exceeding the proposed drinking water standard. Section B of this preamble below summarizes this method used to estimate these benefits.
In its Guidelines for Preparing Economic Analyses, EPA characterizes the “baseline” as a reference point that reflects the world without the proposed regulation (USEPA, 2010). It is the starting point for estimating the potential benefits and costs of the proposed PFAS NPDWR. EPA used a variety of data sources to develop the baseline drinking water system characterization for the regulatory analysis. Table 24 lists the major data sources and the baseline data derived from them. Additional detailed descriptions of these data sources and how they were used in the characterization of baseline conditions can be found in the Chapter 4 of USEPA (2023j).
| Data source | Baseline data derived from the source |
|---|---|
| Notes: | |
| 1 Contains information extracted on January 14, 2022. | |
| SDWIS/Federal version fourth quarter 2021 Q4 “frozen” dataset 1 | • Water System Inventory: PWS inventory, including system unique identifier, population served, number of service connections, source water type, and system type. • Population and Households Served: PWS population served. • Treatment Plant Characterization: Number of unique treatment plant facilities per system, which are used as a proxy for entry points when UCMR 3 sampling site data are not available. |
| UCMR 3 (USEPA, 2017) | • Treatment Plant Characterization: Number of unique entry point sampling sites, which are used as a proxy for entry points. • Treatment Plant Characterization: PFAS concentration data collected as part of UCMR 3. |
| Independent state sampling programs | • Treatment Plant Characterization: PFAS concentration data collected by states. These data supplemented the occurrence modeling for systems included in UCMR 3. |
| Six-Year Review 4 Information Collection Request (SYR4 ICR) Occurrence Dataset (2012-2019) | • Treatment Plant Characterization: Total organic carbon (TOC). |
| Geometries and Characteristics of Public Water Systems (USEPA, 2000f) | • Treatment Plant Characterization: Design and average daily flow per system. |
| 2006 Community Water System Survey (CWSS; USEPA, 2009c) | • Public Water System Labor Rates: PWS labor rates. |
B. Overview of the Cost-Benefit Model
EPA's existing SafeWater Cost Benefit Model (CBX) was designed to calculate the costs and benefits associated with setting a new or revised MCL. Since the proposed PFAS rule simultaneously regulates multiple PFAS contaminants, EPA developed a new model version called the SafeWater Multi-Contaminant Benefit Cost Model (MCBC) to efficiently handle more than one contaminant. SafeWater MCBC, allows for inputs that include differing mixtures of contaminants based on available occurrence data as well as multiple regulatory thresholds. The model structure allows for assignment of compliance technology or technologies that achieve all regulatory requirements and estimates costs and benefits associated with multiple PFAS contaminant reductions. SafeWater MCBC is designed to model co-occurrence, sampling, treatment, and administrative costs, and simultaneous contaminants reductions and resultant benefits. The modifications to the SafeWater model are consistent with the methodology that was developed in the single MCL SafeWater CBX Beta version that was peer reviewed. More detail on the modifications to the SafeWater model can be found in Section 5.2 of EPA's economic analysis.
The costs incurred by a PWS depend on water system characteristics; SDWIS/Fed provides information on PWS characteristics that typically define PWS categories, or strata, for which EPA has develops cost estimates in rulemakings, including system type (CWS, NTNCWS), number of people served by the PWS, the PWS's primary raw water source (ground water or surface water), the PWS's ownership type (public or private), and PWS state.
Because EPA does not have complete PWS-specific data across the approximately 49,000 CWSs and 17,000 NTNCWSs in SDWIS/Fed for many of the baseline and compliance characteristics necessary to estimate costs and benefits, such as design and average daily flow rates, water quality characteristics, treatment in-place, and labor rates, EPA adopted a “model PWS” approach. SafeWater MCBC creates model PWSs by combining the PWS-specific data available in SDWIS/Fed with data on baseline and compliance characteristics available at the PWS category level. In some cases, the categorical data are simple point estimates. In this case, every model PWS in a category is assigned the same value. In other cases, where more robust data representing system variability are available, the category-level data include a distribution of potential values. In the case of distributional information, SafeWater MCBC assigns each model PWS a value sampled from the distribution. These distributions are assumed to be independent.
For a list of PWS characteristics that impact model PWS compliance costs, please see Chapter 5 of USEPA (2023j). These data include inventory data specific to each system and categorical data for which randomly assigned values are based on distributions that vary by category ( e.g., ground water and surface water TOC distributions or compliance forecast distributions that vary by system size category).
Once model PWSs are created and assigned baseline and compliance characteristics, SafeWater MCBC estimates the quantified costs and benefits of compliance for each model PWS under the proposed rule. Because of this model PWS approach, SafeWater MCBC does not output any results at the PWS level. Instead, the outputs are cost and benefit estimates for 36 PWS categories, or strata. Each PWS category is defined by system type (CWS and NTNCWS), primary water source (ground or surface), and size category. Note EPA does not report state specific strata although state location is utilized in the SafeWater MCBC model ( e.g., current state level regulatory limits on PFAS in drinking water). The detailed output across these strata can be found in the Chapter 5 of USEPA (2023j).
For each PWS category, the model then calculates summary statistics that describe the costs and benefits associated with the proposed rule compliance. These summary statistics include total quantified costs of the proposed regulatory requirement, total quantified benefits of the proposed regulatory requirement, the variability in PWS-level costs ( e.g., 5th and 95th percentile system costs), and the variability in household-level costs.
C. Method for Estimating Costs
This section summarizes the cost elements and estimates total cost of compliance for the proposed PFAS NPDWR discounted at 3 and 7 percent. EPA estimated the costs associated with monitoring, administrative requirements, and both treatment and non-treatment compliance actions associated with the proposed rule (USEPA, 2023j).
1. Public Water System (PWS) Costs
a. PWS Treatment and Non-Treatment Compliance Costs
EPA estimated costs associated with engineering, installing, operating, and maintaining PFAS removal treatment technologies, including treatment media replacement and spent media destruction or disposal, as well as non-treatment actions that some PWSs may take in lieu of treatment, such as constructing new wells in an uncontaminated aquifer or interconnecting with and purchasing water from a neighboring PWS. EPA used SafeWater MCBC to apply costs for one of the treatment technologies or non-treatment alternatives at each entry point in a PWS estimated to be out of compliance with the proposed rule. For each affected entry point, SafeWater MCBC selected from among the compliance alternatives using a decision tree procedure, described in more detail in USEPA (2023g) and (2023h). Next, the model estimated the cost of the chosen compliance alternative using outputs from EPA's WBS cost estimating models.
Specifically, EPA used cost equations generated from the following models (USEPA, 2023h):
• the GAC WBS model (USEPA, 2021g);
• the PFAS-selective IX WBS model (USEPA, 2021h);
• the centralized RO/NF WBS model (USEPA, 2021i); and
• the non-treatment WBS model (USEPA, 2021j).
The Technologies and Costs (T&C) document (USEPA, 2023h) provides a comprehensive discussion of each of the treatment technologies, their effectiveness, and the WBS cost models as well as the equations used to calculate treatment costs. In total, there are nearly 3,500 individual cost equations across the categories of capital and operation and maintenance (O&M) cost, water source, component level, flow, bed life (for GAC and IX), residuals management scenarios (for GAC and IX), and design type (for GAC).
b. Decision Tree for Technology Selection
For entry points at which baseline PFAS concentrations exceed regulatory thresholds, the decision tree selects a treatment technology or non-treatment alternative using a two-step process that both:
• Determines whether to include or exclude each alternative from consideration given the entry point's characteristics and the regulatory option selected, and
• Selects from among the alternatives that remain viable based on percentage distributions derived, in part, from data on recent PWS actions in response to PFAS contamination.
Inputs to the decision tree include the following:
• Influent concentrations of individual PFAS contaminants in ppt;
• Entry point design flow in MGD;
• TOC influent to the new treatment process in mg/L.
EPA relied on information from the national PFAS occurrence model to inform influent PFAS concentrations. EPA relied on Geometries and Characteristics of Public Water Supplies (USEPA, 2000f) and SDWIS inventory information to derive entry point design flow. SafeWater MCBC selects influent TOC using the distribution shown below in Table 25.
| Percentile | Surface water | Ground water |
|---|---|---|
| Source: EPA's analysis of TOC concentrations in the SYR4 ICR database. | ||
| 0.05 | 0.65 | 0.35 |
| 0.15 | 1.1 | 0.48 |
| 0.25 | 1.38 | 0.5 |
| 0.35 | 1.6 | 0.5 |
| 0.45 | 1.85 | 0.58 |
| 0.5 | 1.97 | 0.69 |
| 0.55 | 2.14 | 0.75 |
| 0.65 | 2.54 | 1 |
| 0.75 | 3.04 | 1.39 |
| 0.85 | 3.63 | 2.01 |
| 0.95 | 4.81 | 3.8 |
Step 1 of the decision tree uses these inputs to determine whether to include or exclude each treatment alternative from consideration in the compliance forecast. For the treatment technologies (GAC, IX, and RO/NF), this determination is based on estimates of each technology's performance given available data about influent water quality and the regulatory option under consideration.
EPA assumes a small number of PWSs may be able to take non-treatment actions in lieu of treatment. The viability of non-treatment actions is likely to depend on the quantity of water being replaced. Therefore, the decision tree considers non-treatment only for entry points with design flows less than or equal to 3.536 MGD. EPA's WBS model for non-treatment does not generate costs for flows greater than this value, so the decision tree excludes non-treatment actions from consideration above this flow. EPA estimates approximately 2% of systems of this size will develop new wells and approximately 6-7% of systems will elect to interconnect with another system to achieve compliance.
Step 2 of the decision tree selects a compliance alternative for each entry point from among the alternatives that remain in consideration after Step 1. Table 26 shows the initial compliance forecast that is the starting point for this step. The percentages in Table 26 consider data presented in the T&C document (USEPA, 2023h) on actions PWSs have taken in response to PFAS contamination.
To date, the majority of PWSs for which data are available have installed GAC (USEPA, 2023h). The data in USEPA (2023h) suggest that an increasing share of PWSs have selected IX in response to PFAS since the first full-scale system treated with PFAS-selective IX in 2017. EPA expects this trend to continue, so the initial percentages include adjustments to account for this expectation. In addition, the performance of GAC is affected by the presence of TOC, as further described in the cost chapter of the Economic Analysis (USEPA, 2023j). Accordingly, the table includes adjusted distributions for systems with higher influent TOC.
The list of compliance alternatives in Table 26 does not include POU RO for small systems. At this time, EPA is not including POU RO in the national cost estimates because the regulatory options under consideration require treatment to concentrations below 70 ppt PFOA and PFOS summed, the current certification standard for POU devices. Therefore, the decision tree excludes POU RO from consideration and proportionally redistributes the percentages among the other alternatives.
| Source: EPA's analysis of TOC concentrations in the SYR4 ICR database. | ||||||
| Note: EPA is not including POU RO in the national cost estimates for the proposed rule because the regulatory options under consideration require treatment to concentrations below 70 ppt PFOA and PFOS summed, the current certification standard for POU devices. Therefore, the decision tree excludes POU RO from consideration and proportionally redistributes the percentages among the other alternatives. | ||||||
| Compliance alternative | Design flow less than 1 MGD | Design flow 1 to less than 10 MGD | Design flow greater than or equal to 10 MGD | |||
| TOC less than or equal to 1.5 mg/L (%) | TOC greater than 1.5 mg/L (%) | TOC less than or equal to 1.5 mg/L (%) | TOC greater than 1.5 mg/L (%) | TOC less than or equal to 1.5 mg/L (%) | TOC greater than 1.5 mg/L (%) | |
| GAC | 75 | 57 | 77 | 50 | 85 | 50 |
| PFAS-selective IX | 11 | 29 | 10 | 37 | 10 | 45 |
| Central RO/NF | 5 | 5 | 5 | 5 | 5 | 5 |
| Interconnection | 7 | 7 | 6 | 6 | 0 | 0 |
| New Wells | 2 | 2 | 2 | 2 | 0 | 0 |
If all the compliance alternatives remain in consideration after Step 1, the decision tree uses the forecast shown in Table 26 above. If Step 1 eliminated on one or more of the alternatives, the decision tree proportionally redistributes the percentages among the remaining alternatives and uses the redistributed percentages.
EPA's approach to estimating GAC and IX performance under the proposed option and all alternatives considered is discussed in detail within the cost chapter of the Economic Analysis (USEPA, 2023j).
c. Work Breakdown Structure Models
The WBS models are spreadsheet-based engineering models for individual treatment technologies, linked to a central database of component unit costs. EPA developed the WBS model approach as part of an effort to address recommendations made by the Technology Design Panel (TDP), which convened in 1997 to review the Agency's methods for estimating drinking water compliance costs (USEPA, 1997). The TDP consisted of nationally recognized drinking water experts from EPA, water treatment consulting companies, public as well as private water utilities along with suppliers, equipment vendors, and Federal along with State regulators in addition to cost estimating professionals.
In general, the WBS approach involves breaking a process down into discrete components for the purpose of estimating unit costs. The WBS models represent improvements over past cost estimating methods by increasing comprehensiveness, flexibility, and transparency. By adopting a WBS-based approach to identify the components that should be included in a cost analysis, the models produce a more comprehensive assessment of the capital and operating requirements for a treatment system.
Each WBS model contains the work breakdown for a particular treatment process and preprogrammed engineering criteria and equations that estimate equipment requirements for user-specified design requirements ( e.g., system size and influent water quality). Each model also provides unit and total cost information by component ( e.g., individual items of capital equipment) and totals the individual component costs to obtain a direct capital cost. Additionally, the models estimate add-on costs ( e.g., permits and land acquisition), indirect capital costs, and annual O&M costs, thereby producing a complete compliance cost estimate.
Primary inputs common to all the WBS models include design flow and average daily flow in MGD. Each WBS model has default designs (input sets) that correspond to specified categories of flow, but the models can generate designs for many other combinations of flows. To estimate costs for PFAS compliance, EPA fit cost curves to the WBS estimates across a range of flow rates, which is described in Chapter 5 of the Economic Analysis (USEPA, 2023j).
Another input common to all the WBS models is “component level” or “cost level.” This input drives the selection of materials for items of equipment that can be constructed of different materials. For example, a low-cost system might include fiberglass pressure vessels and polyvinyl chloride (PVC) piping. A high-cost system might include stainless steel pressure vessels and stainless-steel piping. The component level input also drives other model assumptions that can affect the total cost of the system, such as building quality and heating and cooling. The component level input has three possible values: low cost, mid cost, and high cost. The components used in each of the estimated component/cost levels provide the treatment efficacy needed to meet the regulatory requirements. Note that the level of component ( e.g., plastic versus resin or stainless-steel piping and vessels) may impact the capital replacement rate but does not interfere with treatment efficacy. EPA estimates the three levels of cost because it has found that the choice of materials associated with the installation of new treatment equipment often varies across drinking water systems. These systems may, for example, choose to balance capital cost with staff familiarity with certain materials and existing treatment infrastructure. Given this experience, EPA models the potential variability in treatment cost based on the three component/cost levels. To estimate costs for PFAS treatment, EPA generated separate cost equations for each of the three component levels, thus creating a range of cost estimates for use in national compliance cost estimates. EPA requests comment on the range of component levels assumed and the range of estimated PFAS treatment costs.
The third input common to all the WBS models is system automation, which allows the design of treatment systems that are operated manually or with varying degrees of automation ( i.e., with control systems that reduce the need for operator intervention). Cost equations for system automation are described in Chapter 5 of the Economic Analysis (USEPA, 2023j).
The WBS models generate cost estimates that include a consistent set of capital, add-on, indirect, and O&M costs. Table 27 below identified these cost elements, which are common to all the WBS models and included in the cost estimates below. As described below and summarized in Tables 28-31 the WBS models also include technology-specific cost elements. The documentation for the WBS models provide more information on the methods and assumptions in the WBS models to estimate the costs for both the technology-specific and common cost elements (USEPA, 2021g; USEPA, 2021h; USEPA, 2021i; and USEPA, 2021j). WBS model accuracy is described in Chapter 5 of the Economic Analysis (USEPA, 2023j).
| Cost category | Components included |
|---|---|
| Direct Capital Costs | • Technology-specific equipment ( e.g., vessels, basins, pumps, treatment media, piping, valves). • Instrumentation and system controls. • Buildings. • Residuals management equipment. |
| Add-on Costs | • Land. • Permits. • Pilot testing. |
| Indirect Capital Costs | • Mobilization and demobilization. • Architectural fees for treatment building. • Equipment delivery, installation, and contractor's overhead and profit. |
| • Sitework. | |
| • Yard piping. | |
| • Geotechnical. | |
| • Standby power. | |
| • Electrical infrastructure. | |
| • Process engineering. | |
| • Contingency. | |
| • Miscellaneous allowance. | |
| • Legal, fiscal, and administrative. | |
| • Sales tax. | |
| • Financing during construction. | |
| • Construction management. | |
| O&M Costs: Technology-specific | • Operator labor for technology-specific tasks ( e.g., managing backwash and media replacement). • Materials for O&M of technology-specific equipment. • Technology-specific chemical usage. • Replacement of technology-specific equipment that occurs on an annual basis ( e.g., treatment media). • Energy for operation of technology-specific equipment ( e.g., mixers). |
| O&M Costs: Labor | • Operator labor for O&M of process equipment. • Operator labor for building maintenance. • Managerial and clerical labor. |
| O&M Costs: Materials | • Materials for maintenance of booster or influent pumps. • Materials for building maintenance. |
| O&M Costs: Energy | • Energy for operation of booster or influent pumps. • Energy for lighting, ventilation, cooling, and heating. |
| O&M Costs: Residuals | • Residuals management operator labor, materials, and energy. • Residuals disposal and discharge costs. |
The GAC model can generate costs for two types of design:
• Pressure designs where the GAC bed is contained in stainless steel, carbon steel, or fiberglass pressure vessel;
• Gravity designs where the GAC bed is contained in open concrete basins.
Table 28 shows the technology-specific capital equipment and O&M requirements included in the GAC model. These items are in addition to the common WBS cost elements listed in the Cost Elements Included in All WBS Models table above.
| Cost category | Major components included |
|---|---|
| Direct Capital Costs | • Booster pumps for influent water. • Contactors (either pressure vessels or concrete basins) that contain the GAC bed. |
| • Tanks and pumps for backwashing the contactors. | |
| • GAC transfer and storage equipment. | |
| • Spent GAC reactivation facilities (if on-site reactivation is selected). | |
| • Associated piping, valves, and instrumentation. | |
| O&M Costs: Labor | • Operator labor for contactor maintenance (for gravity GAC designs). • Operator labor for managing backwash events. |
| • Operator labor for backwash pump maintenance (if backwash occurs weekly or more frequently). | |
| • Operator labor for GAC transfer and replacement. | |
| O&M Costs: Materials | • Materials for contactor maintenance (accounts for vessel relining in pressure designs, because GAC can be corrosive, and for concrete and underdrain maintenance in gravity designs). |
| • Materials for backwash pump maintenance (if backwash occurs weekly or more frequently). | |
| • Replacement virgin GAC (loss replacement only if reactivation is selected). | |
| O&M Costs: Energy | • Operating energy for backwash pumps. |
| O&M Costs: Residuals | • Discharge fees for spent backwash. • Fees for reactivating spent GAC (if off-site reactivation is selected). |
| • Labor, materials, energy, and natural gas for regeneration facility (if on-site reactivation is selected). | |
| • Disposal of spent GAC (if disposal is selected). |
For small systems (less than 1 MGD) using pressure designs, the GAC model assumes the use of package treatment systems that are pre-assembled in a factory, mounted on a skid, and transported to the site. The model estimates costs for package systems by costing all individual equipment line items ( e.g., vessels, interconnecting piping and valves, instrumentation, and system controls) in the same manner as custom-engineered systems. This approach is based on vendor practices of partially engineering these types of package plants for specific systems ( e.g., selecting vessel size to meet flow and treatment criteria). The model applies a variant set of design inputs and assumptions that are intended to simulate the use of a package plant and that reduce the size and cost of the treatment system. USEPA (2021g) provides complete details on the variant design assumptions used for package plants.
To generate the GAC cost equations, EPA used the following key inputs in the GAC model:
• For pressure designs, two vessels in series with a minimum total empty bed contact time (EBCT) of 20 minutes;
• For gravity designs, contactors in parallel with a minimum total EBCT of 20 minutes; and
• Bed life varying over a range from 5,000 to 150,000 BV.
EPA generated separate cost equations for two spent GAC management scenarios:
• Off-site reactivation under current RCRA non-hazardous waste regulations
• Off-site disposal as a hazardous waste and replacement with virgin GAC ( i.e., single use operation).
The T&C document (USEPA, 2023h) provides a comprehensive discussion of these and other key inputs and assumptions.
Table 29 shows the technology-specific capital equipment and O&M requirements included in the PFAS selective IX model. These items are in addition to the common WBS cost elements listed in the Cost Elements Included in All WBS Models table above.
| Cost category | Major components included |
|---|---|
| Direct Capital Costs | • Booster pumps for influent water. |
| • Pre-treatment cartridge filters. | |
| • Pressure vessels that contain the resin bed. | |
| • Tanks and pumps for initial rinse and (optionally) backwash of the resin bed. | |
| • Tanks (with secondary containment), pumps and mixers for delivering sodium hydroxide for use in post-treatment corrosion control (optional). | |
| • Associated piping, valves, and instrumentation. | |
| O&M Costs: Labor | • Operator labor for pre-treatment filters. |
| • Operator labor for managing backwash/rinse events. | |
| • Operator labor for backwash pump maintenance (only if backwash occurs weekly or more frequently). | |
| • Operator labor for resin replacement. | |
| O&M Costs: Materials | • Replacement cartridges for pre-treatment filters. |
| • Materials for backwash pump maintenance (only if backwash occurs weekly or more frequently). | |
| • Chemical usage (if post-treatment corrosion control is selected). | |
| • Replacement virgin PFAS-selective resin. | |
| O&M Costs: Energy | • Operating energy for backwash/rinse pumps. |
| O&M Costs: Residuals | • Disposal of spent cartridge filters. |
| • Discharge fees for spent backwash/rinse. | |
| • Disposal of spent resin. |
For small systems (less than 1 MGD), the PFAS-selective IX model assumes the use of package treatment systems that are pre-assembled in a factory, mounted on a skid, and transported to the site. The IX model estimates costs for package systems using an approach similar to that described for the GAC model, applying a variant set of inputs and assumptions that reduce the size and cost of the treatment system. USEPA (2021j) provides complete details on the variant design assumptions used for IX package plants.
To generate the IX cost equations, EPA used the following key inputs in the PFAS-selective IX model:
• Two vessels in series with a minimum total EBCT of 6 minutes.
• Bed life varying over a range from 20,000 to 440,000 BV.
EPA generated separate cost equations for two spent resin management scenarios:
• Spent resin managed as non-hazardous and sent off-site for incineration.
• Spent resin managed as hazardous and sent off-site for incineration.
The T&C document (USEPA, 2023h) provides a comprehensive discussion of these and other key inputs and assumptions.
Table 30 shows the technology-specific capital equipment and O&M requirements included in the model for RO/NF (USEPA, 2021i). These items are in addition to the common WBS cost elements listed in listed in the Cost Elements Included in All WBS Models table above.
| Cost category | Major components included |
|---|---|
| Direct Capital Costs | • High-pressure pumps for influent water and (optionally) interstage pressure boost. |
| • Pre-treatment cartridge filters. | |
| • Tanks, pumps, and mixers for pretreatment chemicals. | |
| • Pressure vessels, membrane elements, piping, connectors, and steel structure for the membrane racks. | |
| • Valves for concentrate control and (optionally) per-stage throttle. | |
| • Tanks, pumps, screens, cartridge filters, and heaters for membrane cleaning. | |
| • Equipment, including dedicated concentrate discharge piping, for managing RO/NF concentrate and spent cleaning chemicals. | |
| • Associated pipes, valves, and instrumentation. | |
| O&M Costs: Labor | • Operator labor for pre-treatment filters. |
| • Operator labor for routine O&M of membrane units. | |
| • Operator labor to maintain membrane cleaning equipment. | |
| O&M Costs: Materials | • Replacement cartridges for pre-treatment filters. |
| • Chemical usage for pretreatment. | |
| • Maintenance materials for pre-treatment, membrane process, and cleaning equipment. | |
| • Replacement membrane elements. | |
| • Chemical usage for cleaning. | |
| O&M Costs: Energy | • Energy for high-pressure pumping. |
| O&M Costs: Residuals | • Disposal costs for spent cartridge filters and membrane elements. |
The RO/NF model includes three default ground waters and three default surface waters, ranging from high to low quality ( i.e., from low to high total dissolved solids and scaling potential). To generate the cost equations, EPA used the model's default high-quality influent water parameters to reflect the incremental cost of removing PFAS from otherwise potable water. EPA used the following additional key inputs and assumptions:
• For systems larger than approximately 0.5 MGD, target recovery rates of 80 percent for ground water and 85 percent for surface water.
• Target recovery rates of 70 to 75 percent for smaller systems.
• Flux rates of 19 gallons per square foot per day (gfd) for ground water and 15 to 16 gfd for surface water.
• Direct discharge of RO/NF concentrate to a permitted outfall on a non-potable water body ( e.g., ocean or brackish estuary) via 10,000 feet of buried dedicated piping.
The T&C document (USEPA, 2023h) provides a comprehensive discussion of these and other key inputs and assumptions.
USEPA (2021j) provides a complete description of the engineering design process used by the WBS model for nontreatment actions. The model can estimate costs for two nontreatment alternatives: interconnection with another system and drilling new wells to replace a contaminated source. Table 31 below shows the technology-specific capital equipment and O&M requirements included in the model for each alternative.
| Cost category | Major components included for interconnection | Major components included for new wells |
|---|---|---|
| Direct Capital Costs | • Booster pumps or pressure reducing valves (depending on pressure at supply source) • Concrete vaults (buried) for booster pumps or pressure reducing valves • Interconnecting piping (buried) and valves | • Well casing, screens, and plugs. • Well installation costs including drilling, development, gravel pack, and surface seals. • Well pumps. • Piping (buried) and valves to connect the new well to the system. |
| O&M Costs: Labor | • Operator labor for O&M of booster pumps or pressure reducing valves (depending on pressure at supply source) and interconnecting valves | • Operator labor for operating and maintaining well pumps and valves. |
| O&M Costs: Materials | • Cost of purchased water • Materials for maintaining booster pumps (if required by pressure at supply source) | • Materials for maintaining well pumps. |
| O&M Costs: Energy | • Energy for operating booster pumps (if required by pressure at supply source) | • Energy for operating well pumps. |
To generate the cost equations, EPA used the following key inputs in the non-treatment model for interconnection:
• An interconnection distance of 10,000 feet;
• Minimal differences in pressure between the supplier and the purchasing system, so that neither booster pumps nor pressure reducing valves are needed;
• An average cost of purchased water of $3.00 per thousand gallons in 2020 dollars.
For new wells, EPA used the following key inputs:
• A maximum well capacity of 500 gallons per minute (gpm), such that one new well is installed per 500 gpm of water production capacity required;
• A well depth of 250 feet;
• 500 feet of distance between the new wells and the distribution system.
The T&C document (USEPA, 2023h) provides a comprehensive discussion of these and other key inputs and assumptions.
d. Incremental Treatment Costs
EPA has estimated the national level costs of the proposed rule associated with PFOA, PFOS, and PFHxS. Given the available occurrence data for the other compounds in the proposed rule (PFNA, HFPO-DA, and PFBS) and the regulatory thresholds under consideration, EPA did not model national costs associated with potential HI exceedances as a direct result of these compounds. To assess the potential impact of these compounds, EPA conducted an analysis of the additional, or incremental, system level impact that occurrence of these compounds would have on treatment costs. To do so, EPA used a model system approach. For further detail on the assumptions and findings of EPA's analysis of incremental costs, please see Chapter 5 in USEPA (2023j) and Appendix N in USEPA (2023i).
e. PWS Implementation Administration Costs
EPA estimated PWS costs associated with one-time actions to begin implementation of the rule including reading and understanding the rule and attending training provided by primacy agencies. EPA assumes that systems will conduct these activities during years one through three of the period of analysis. Table 32 lists the data elements and corresponding values associated with calculating the costs of these one-time implementation administration actions.
| Data element description | Data element value |
|---|---|
| The labor rate per hour for systems | $35.48 (systems ≤3,300). $37.84 (systems 3,301-10,000). $39.94 (systems 10,001-50,000). $41.70 (systems 50,001-100,000). $48.74 (systems >100,000). |
| The average hours per system to read and adopt the rule | 4 hours per system. |
| The average hours per system to attend one-time training provided by primacy agencies | 16 hours per system (systems ≤3,300). 32 hours per system (systems >3,300). |
Estimated national annualized PWS implementation and administration startup costs for the proposed option are $1.71 million (3% discount rate) and $3.52 million (7% discount rate). National annualized PWS cost estimates are further summarized in Table 37.
f. PWS Monitoring Costs
EPA assumes that the proposed rule will require initial and long-term monitoring. As Table 33 shows, surface and ground water systems serving 10,000 or more people will collect one sample each quarter, at each entry point, during the initial 12-month monitoring period. Surface water systems serving 10,000 or fewer people are also required to collect a quarterly sample at each entry point during the initial 12-month period. Ground water systems that serve 10,000 or fewer people will be required to sample once at each entry point on a semi-annual basis for the first 12-month monitoring period.
Long-term monitoring requirements differ based on two factors: (1) system size, and (2) whether a system can demonstrate during the initial monitoring period that they are “reliably and consistently” below the proposed MCLs for PFAS. EPA has set the PWS size threshold at systems serving 3,300 or fewer people. The threshold for systems to demonstrate that they are “reliably and consistently” below the proposed MCLs is set at a trigger level of one-third the MCLs for PFOA or PFOS (1.3 ppt) or the HI (0.33). For systems below the trigger level values during the initial 12-month monitoring period and in future long-term monitoring periods may conduct triennial monitoring. Systems serving 3,300 or fewer people will collect one triennial sample per entry point. Systems providing water for more than 3,300 people will take one sample in two consecutive quarters at each entry point, totaling two samples in each triennial period. For systems with concentration values at or above the trigger level regardless of system size, a quarterly sample must be taken at each entry point.
For any samples that have a detection, the system will analyze the field reagent blank samples collected at the same time as the monitoring sample. Systems that have an MCL exceedance will collect one additional sample from the relevant entry point to confirm the results.
| Initial monitoring system size category | Initial 12-month monitoring period | Long-term monitoring system size category | Long-Term monitoring: a PFAS detection <1.3 ppt (PFOA or PFOS) or HI <0.33 | Long-term monitoring: 1 PFAS detection ≥1.3 ppt (PFOA or PFOS) or HI ≥0.33 |
|---|---|---|---|---|
| Notes: | ||||
| 1 EPA used the following thresholds to distinguish whether PFAS concentrations are reliably and consistently below the MCL: PFOA and PFOS—one-third the MCL for each option; PFHxS—one-third the health benchmark of 9 ppt or 3 ppt. | ||||
| ≤10,000 | Surface Water: 1 sample every quarter Ground Water: 1 sample every 6-month period | ≤3,300 | 1 triennial sample | 1 sample every quarter. |
| >10,000 | Surface Water and Ground Water: 1 sample every quarter | >3,300 | 2 triennial samples (1 sample in two consecutive quarters) | 1 sample every quarter. |
For the national cost analysis, EPA assumes that systems with either UCMR 5 data or monitoring data in the State PFAS Database (see Section 3.1.4 in USEPA, 2023j) will not need to conduct the initial year of monitoring. As a simplifying assumption for the cost analysis, EPA assumes all systems serving a population of greater than 3,300 have UCMR 5 data and those with 3,300 or less do not. For the State PFAS Database, EPA relied on the PWSIDs stored in the database and exempted those systems from the first year of monitoring in the cost analysis. Note these simplifying assumptions may result in a small underestimate of initial monitoring costs. Under UCMR 5, individual water systems would be able to request the full release of data from the labs for use in determining their compliance monitoring frequency. PWSs may be able to use these lab analyses to demonstrate a “below trigger level” concentration using the UCMR 5 analyses by following up with the lab for a more detailed results report. EPA requests comment on these underlying assumptions.
EPA used system-level distributions, as described in Cadwallader et al. (2022), to simulate entry point concentrations and estimate PFAS occurrence relative to the proposed option MCLs and trigger levels. Based on these occurrence distributions, EPA estimates that the large majority of water systems subject to the proposed rule (approx. 52,000) will have EPs with concentrations below the proposed trigger level and would conduct reduced monitoring on a triennial basis. EPA estimates that the remainder of water systems subject to the proposed rule (approx. 14,000) will have at least one or more EPs exceed the proposed trigger level and therefore would be required to conduct quarterly monitoring. EPA requests comment on these estimates and the underlying assumptions.
EPA assumes that systems with an MCL exceedance will implement actions to comply with the MCL by the compliance date. EPA assumes a treatment target, for systems required to treat for PFAS, that includes a margin of safety so finished water PFAS levels at these systems are 80 percent of the MCL or HI. This target is insufficient to meet the triennial monitoring threshold. Therefore, systems implementing treatment will continue with quarterly monitoring. All other systems that do not have PFAS concentrations at or below the trigger level threshold will also continue quarterly monitoring.
For all systems, the activities associated with the sample collection in the initial 12-month monitoring period are the labor burden and cost for the sample collection and analysis, as well as a review of the sample results. Table 34 presents the data elements and corresponding values associated with calculating sampling costs during the implementation monitoring period.
| Data element description | Data element value |
|---|---|
| Notes: | |
| 1 Systems greater than 3,300 will rely on UCMR 5 data and a subset of other systems will rely on data in the State PFAS Monitoring Database discussed in USEPA, 2023j. | |
| 2 This incremental sample cost applies to all samples that exceed MDLs. EPA used the Method 537.1 detection limits to apply this cost because Method 533 does not include detection limits. | |
| The labor rate per hour for systems | $35.48 (systems ≤3,300). |
| $37.84 (systems 3,301-10,000). | |
| $39.94 (systems 10,001-50,000). | |
| $41.70 (systems 50,001-100,000). | |
| $48.74 (systems >100,000). | |
| The number of samples per entry point per monitoring round for the initial monitoring in Year 1 | 2 samples (Ground Water systems ≤10,000). 4 samples (all systems) 1 . |
| The number of samples per entry point per long-term monitoring year for entry points that exceed the triennial monitoring threshold | 4 samples (all other systems). |
| The number of samples per entry point per long-term monitoring round for entry points that meet the triennial threshold | 1 sample (systems ≤3,300). 2 samples (systems >3,300). |
| The hours per sample to travel to sampling locations, collect samples, record any additional information, submit samples to a laboratory, and review results | 1 hour. |
| The laboratory analysis cost per sample for EPA Method 533 | $376. |
| The laboratory analysis cost per sample for EPA Method 537.1 | $302. |
| The laboratory analysis cost per sample for field reagent blank under EPA Method 533 | $327. 2 |
| The laboratory analysis cost per sample for the field reagent blank under EPA Method 537.1 | $266. 2 |
Estimated national annualized PWS sampling costs for the proposed option are $90.32 million (3 discount rate) and $92.97 million (7% discount rate). National annualized PWS cost estimates are further summarized in Table 37.
g. Treatment Administration Costs
Any system with an MCL exceedance adopts either a treatment or non-treatment alternative to comply with the proposed rule. The majority of systems are anticipated to install treatment technologies while a subset of systems will choose alternative methods. EPA assumes that systems will bear administrative costs associated with these treatment or non-treatment compliance actions ( i.e., permitting costs). EPA assumes that systems will install treatment in the fourth year of the period of analysis. Table 35 presents the data elements and corresponding values associated with calculating treatment administration costs.
| Data element description | Data element value |
|---|---|
| Notes: | |
| 1 EPA applied the cost per entry point for this economic analysis because the notification, consultation, and permitting process occurs for individual entry points. | |
| The labor rate per hour for systems | $35.48 (systems ≤3,300). |
| $37.84 (systems 3,301-10,000). | |
| $39.94 (systems 10,001-50,000). | |
| $41.70 (systems 50,001-100,000). | |
| $48.74 (systems >100,000). | |
| The hours per entry point for a system to notify, consult, and submit a permit request for treatment installation a | 3 hours (systems ≤100) 5 hours (systems 101-500). |
| 7 hours (systems 501-1,000). | |
| 12 hours (systems 1,001-3,300). | |
| 22 hours (systems 3,301-50,000). | |
| 42 hours (systems >50,000). | |
| The hours per entry point for a system to notify, consult, and submit a permit request for source water change or alternative method 1 | 6 hours. |
h. Public Notification (PN) Costs
EPA's cost analysis assumes full compliance with the rule throughout the period of analysis and, as a result, EPA does not estimate costs for the PN requirements in the proposed rule for systems with certain violations. The proposed rule designates MCL violations for PFAS as Tier 2, which requires systems to provide PN as soon as practical, but no later than 30 days after the system learns of the violation. The system must repeat notice every three months if the violation or situation persists unless the primacy agency determines otherwise. At a minimum, systems must give repeat notice at least once per year. The proposed rule also designates monitoring and testing procedure violations as Tier 3, which requires systems to provide public notice not later than one year after the system learns of the violation. The system must repeat the notice annually for as long as the violation persists. For approximate estimates of the potential burden associated with Tier 2 and 3 PNs, please see USEPA (2023j).
i. Primacy Agency Costs
EPA assumes that primacy agencies will have upfront implementation costs as well as costs associated with system actions related to sampling and treatment. The activities that primacy agencies are expected to carry out under the proposed rule include:
• Reading and understanding the rule and adopting regulatory requirements,
• Providing primacy agency officials training for the rule implementation,
• Providing systems with training and technical assistance during the rule implementation,
• Reporting to EPA on an ongoing basis any PFAS-specific information under 40 CFR 142.15 regarding violations as well as enforcement actions and general operations of PWS programs,
• Reviewing the sample results during the implementation monitoring period and the SMF period, and
• Reviewing and consulting with systems on the installation of treatment technology or alternative methods, including source water change.
With the exception of the first four activities listed above, the primary agency burdens are incurred in response to action taken by PWSs; for instance, the cost to primacy agencies of reviewing sample results depends on the number of samples taken at each entry point by each system under an Agency's jurisdiction. Table 36 presents the data elements and corresponding values associated with calculating primacy agency costs.
| Data element description | Data element value |
|---|---|
| Notes: | |
| 1 In USBLS (2022), State employee wage rate of $33.91 from National Occupational Employment and Wage Estimates, United States, BLS SOC Code 19-2041, “State Government, excluding schools and hospitals—Environmental Scientists and Specialists, Including Health,” hourly mean wage rate. May 2020 data (published in March 2021): https://www.bls.gov/oes/current/oes192041.htm. Wages are loaded using a factor of 62.2 from the Bureau of Labor Statistics (BLS) Employer Costs for Employee Compensation report, Table 3, March 2020. Percent of total compensation—Wages and Salaries—All Workers—State and Local Government Workers ( https://www.bls.gov/news.release/archives/ecec_06182020.pdf ). See worksheet BLS Table 3. The final loaded wage is adjusted for inflation. | |
| 2 EPA assumes that the proposed PFAS rule will have no discernable incremental burden for quarterly or annual reports to SDWIS/Fed. | |
| The labor rate per hour for primacy agencies 1 | $58.14. |
| The average hours per primacy Agency to read and understand the rule, as well as adopt regulatory requirements | 416 hours per primacy Agency. |
| The average hours per primacy Agency to provide initial training to internal staff | 250 hours per primacy Agency. |
| The average hours per primacy Agency to provide initial training and technical assistance to systems | 2,080 hours per primacy Agency. |
| The average hours per primacy Agency to report annually to EPA information under 40 CFR 142.15 regarding violations, variances and exemptions, enforcement actions and general operations of State PWS programs | 0. |
| The hours per sample for a primacy Agency to review sample results | 1 hour. |
| The hours per entry point for a primacy agency to review and consult on installation of a TT 2 | 3 hours (systems ≤100). 5 hours (systems 101-500). 7 hours (systems 501-1,000). |
| 12 hours (systems 1,001-3,300). | |
| 22 hours (systems 3,301-50,000). | |
| 42 hours (systems >50,000). | |
| The hours per entry point for a primacy agency to review and consult on a source water change 2 | 4 hours. |
Estimated national annualized primacy agency costs for the proposed option are $7.96 million (3% discount rate) and $8.76 million (7% discount rate). National annualized cost estimates are further summarized in Table 37.
In addition to the costs described above, a primacy agency may also have to review the certification of any Tier 2 or 3 PNs sent out by systems. EPA assumes full compliance with the proposed rule and therefore does not include this cost in national estimated cost totals but provides a brief discussion of the possible primacy agency burden associated with this component in USEPA (2023j).
In Table 37, EPA summarizes the total annualized quantified cost of the proposed option at both a 3 percent and 7 percent discount rate expressed in millions of 2021 dollars. The first three rows show the annualized PWS sampling costs, the annualized PWS implementation and administrative costs, and the annualized PWS treatment costs. The fourth row shows the sum of the annualized PWS costs. At a 3 percent discount rate, the expected annualized PWS costs are $769 million. The uncertainty range for annualized PWS costs are $699 million to $862 million. Finally, annualized primacy agency implementation and administrative costs are added to the annualized PWS costs to calculate the total annualized cost of the proposed option. At a 3 percent discount rate, the expected total annualized cost of the proposed rule is $777 million. The uncertainty range for the total annualized costs of the proposed rule is $706 million to $872 million. At a 7 percent discount rate, the expected total annualized cost of the proposed option is $1.211 billion, while the uncertainty range for the total annualized costs of the proposed option is $1.103 billion to $1.353 billion. Note as described in section j. Data Limitations and Uncertainties in the Cost Analysis below, given the available occurrence data for the other compounds in the proposed rule (PFNA, HFPO-DA, and PFBS) and the regulatory thresholds under consideration, EPA did not model national costs associated with potential HI exceedances as a direct result of these compounds; therefore, the additional treatment cost, from co-occurrence of PFNA, HFPO-DA, PFBS or other PFAS, at systems already required to treat because of PFOA, PFOS, or PFHxS MCL and HI exceedances are not quantitatively assessed in the national cost estimates. Nor are treatment costs for systems that exceed the HI based on the combined occurrence of PFNA, HFPO-DA, PFBS, and PFHxS (where PFHxS itself does not exceed 9 ppt) included in the national monetized cost estimates. These potential additional costs are described in Section 5.3.1.4 of USEPA (2023j) and Appendix N of USEPA (2023i).
In these sections of the Economic Analysis, EPA uses a model system approach to explore the potential costs of treatment at a system that: (1) has no detections of PFOA, PFOS, or PFHxS (modeled in the national analysis), but has occurrence of all the other PFAS included in the HI (HFPO-DA, PFBS, and PFNA), and (2) has occurrence of PFOA, PFOS, and PFHxS identical to the national model but also has occurrence of all the other PFAS included in the HI (HFPO-DA, PFBS, and PFNA). The first type of system represents additional systems that are not currently captured in the national costs but would incur treatment costs under the HI. The second type of system illustrates a range of potential incremental treatment costs for systems that are already treating to remove PFOA, PFOS, and/or PFHxS in the national cost analysis. EPA analyzed system costs for GAC, IX, and OR for two scenarios: high occurrence of the three PFAS not included in the national analysis and medium occurrence of those PFAS. The model system analysis found for IX and RO/NF that costs were slightly less or the same as modeled system treatment costs under a national cost scenario across both types of systems defined above, the medium and high PFAS scenarios, and across model system size categories. The assessment of GAC produced more variability in results. For systems that are not currently captured in the national costs but would incur treatment costs under the HI, EPA found under the medium PFAS concentrations cost would be the same or slightly less than a model system treating for the PFAS included in the national analysis. The systems representing the potential incremental treatment costs for systems that are already treating to remove PFOA, PFOS, and/or PFHxS in the national cost analysis, the model system analysis under the medium scenario found that costs of treatment would increase by 1-9 percent, depending on system size and other cost assumptions associated with bed life changes as a result of TOC assumptions. Under the high PFAS scenario across both types of systems GAC treatment costs were found to range from 0 to 77% higher than treatment of national PFAS values depending on system size and other costing assumptions like bed life. This high-end cost increase of 77 percent is unlikely to occur at a large number of systems given the assumed high levels of PFAS and the assumed high levels of TOC at 2 mg/L. It is also likely that systems facing these GAC treatment cost will select IX or RO/NF as lower cost alternative treatments and therefore national cost estimates are unlikely to be substantially underestimated. EPA requests comment on these estimated impacts and the assumption that HI exceedances resulting from these additional compounds will not significantly impact overall compliance costs.
The national annualized costs below do not reflect costs of hazardous waste disposal for GAC and IX media. As a general matter, EPA notes that such wastes are not currently regulated under Federal law as a hazardous waste. To address stakeholder concerns, including those raised during the SBREFA process, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. As part of this analysis, EPA generated a second full set of unit cost curves that are identical to the curves used for the national cost analysis with the exception that spent GAC and spent IX resin are considered hazardous. EPA acknowledges that if Federal authorities later determine that PFAS-contaminated wastes require handling as hazardous wastes, the residuals management costs are expected to be higher. See Appendix N.2 of USEPA (2023j) for a sensitivity analysis describing the potential increase in costs associated with hazardous waste disposal (USEPA, 2023i).
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. Percentiles cannot be summed because cost components are not perfectly correlated. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71. This range does not include the uncertainty described in Table 41. | ||||||
| 2 Total quantified national cost values do not include the incremental treatment costs associated with the co-occurrence of HFPO-DA, PFBS, and PFNA at systems required to treat for PFOA, PFOS, and PFHxS. The total quantified national cost values do not include treatment costs for systems that would be required to treat based on HI exceedances apart from systems required to treat because of PFHxS occurrence alone. See Appendix N, Section 3 of the Economic Analysis (USEPA, 2023i) for additional detail on co-occurrence incremental treatment costs and additional treatment costs at systems with HI exceedances. | ||||||
| 3 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 4 See Table 70 for a list of the nonquantifiable costs, and the potential direction of impact these costs would have on the estimated monetized total annualized costs in this table. | ||||||
| 3% Discount rate | 7% Discount rate | |||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Annualized PWS Sampling Costs | $76.12 | $90.32 | $106.95 | $78.54 | $92.97 | $109.19 |
| Annualized PWS Implementation and Administration Costs | 1.71 | 1.71 | 1.71 | 3.52 | 3.52 | 3.52 |
| Annualized PWS Treatment Costs | 617.05 | 676.56 | 762.05 | 1,008.88 | 1,105.66 | 1,232.92 |
| Total Annualized PWS Costs 2 3 4 | 698.90 | 768.57 | 861.78 | 1,096.29 | 1,202.09 | 1,341.19 |
| Primacy Agency Rule Implementation and Administration Cost | 6.86 | 7.96 | 9.18 | 7.67 | 8.76 | 10.04 |
| Total Annualized Rule Costs 2 3 4 | 705.85 | 776.54 | 871.50 | 1,102.71 | 1,210.91 | 1,352.71 |
In Table 38, Table 39, and Table 40, EPA summarizes the total annualized quantified cost of options 1a, 1b, and 1c, respectively.
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. Percentiles cannot be summed because cost components are not perfectly correlated. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71. This range does not include the uncertainty described in Table 41. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable costs, and the potential direction of impact these costs would have on the estimated monetized total annualized costs in this table. | ||||||
| 3% Discount rate | 7% Discount rate | |||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Annualized PWS Sampling Costs | $75.54 | $89.45 | $105.44 | $77.76 | $92.10 | $108.29 |
| Annualized PWS Implementation and Administration Costs | 1.71 | 1.71 | 1.71 | 3.52 | 3.52 | 3.52 |
| Annualized PWS Treatment Costs | 601.03 | 661.40 | 745.31 | 984.54 | 1,079.05 | 1,205.22 |
| Total Annualized PWS Costs 2 3 | 680.76 | 752.56 | 848.52 | 1,066.70 | 1,174.69 | 1,314.49 |
| Primacy Agency Rule Implementation and Administration Cost | 6.83 | 7.89 | 9.12 | 7.59 | 8.69 | 9.96 |
| Total Annualized Rule Costs 2 3 | 687.54 | 760.45 | 857.04 | 1,078.01 | 1,183.41 | 1,324.41 |
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. Percentiles cannot be summed because cost components are not perfectly correlated. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71. This range does not include the uncertainty described in Table 41. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable costs, and the potential direction of impact these costs would have on the estimated monetized total annualized costs in this table. | ||||||
| 3% Discount rate | 7% Discount rate | |||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Annualized PWS Sampling Costs | $66.40 | $78.38 | $93.04 | $68.77 | $80.92 | $95.70 |
| Annualized PWS Implementation and Administration Costs | 1.71 | 1.71 | 1.71 | 3.52 | 3.52 | 3.52 |
| Annualized PWS Treatment Costs | 479.50 | 527.00 | 597.91 | 778.40 | 853.94 | 960.05 |
| Total Annualized PWS Costs 2 3 | 549.52 | 607.08 | 686.67 | 854.64 | 938.38 | 1,052.52 |
| Primacy Agency Rule Implementation and Administration Cost | 6.03 | 6.94 | 8.03 | 6.74 | 7.69 | 8.84 |
| Total Annualized Rule Costs 2 3 | 555.94 | 614.03 | 694.18 | 860.01 | 946.07 | 1,064.56 |
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. Percentiles cannot be summed because cost components are not perfectly correlated. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71. This range does not include the uncertainty described in Table 41. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable costs, and the potential direction of impact these costs would have on the estimated monetized total annualized costs in this table. | ||||||
| 3% Discount rate | 7% Discount rate | |||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th percentile 1 | |
| Annualized PWS Sampling Costs | $46.19 | $52.84 | $64.34 | $48.33 | $55.14 | $66.82 |
| Annualized PWS Implementation and Administration Costs | 1.71 | 1.71 | 1.71 | 3.52 | 3.52 | 3.52 |
| Annualized PWS Treatment Costs | 214.02 | 233.87 | 257.12 | 336.54 | 367.40 | 404.42 |
| Total Annualized PWS Costs 2 3 | 264.49 | 288.43 | 317.66 | 390.39 | 426.06 | 468.83 |
| Primacy Agency Rule Implementation and Administration Cost | 4.28 | 4.76 | 5.65 | 4.91 | 5.40 | 6.28 |
| Total Annualized Rule Costs 2 3 | 269.11 | 293.19 | 323.45 | 395.35 | 431.46 | 474.75 |
j. Data Limitations and Uncertainties in the Cost Analysis
Table 41 lists data limitations and characterizes the impact on the quantitative cost analysis. EPA notes that in most cases it is not possible to judge the extent to which a particular limitation or uncertainty could affect the cost analysis. EPA provides the potential direction of the impact on the cost estimates when possible but does not prioritize the entries with respect to the impact magnitude.
| Uncertainty/assumption | Effect on quantitative analysis | Notes |
|---|---|---|
| Notes: | ||
| 1 EPA Office of Land and Emergency Management's Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances can be found at https://www.epa.gov/system/files/documents/2021-11/epa-hq-olem-2020-0527-0002_content.pdf. | ||
| WBS engineering cost model assumptions and component costs | Uncertain | The WBS engineering cost models require many design and operating assumptions to estimate treatment process equipment and operating needs. Chapter 5 of the Economic Analysis (USEPA, 2023j) addressed the bed life assumption. The Technologies and Costs document (USEPA, 2023h) and individual WBS models in the rule docket provide additional information. The component-level costs approximate national average costs, which can over- or under-estimate costs at systems affected by the proposed rule. |
| Compliance forecast | Uncertain | The forecast probabilities are based on historical full-scale compliance actions. Site-specific water quality conditions, changes in technology, and changes in market conditions can result in future technology selections that differ from the compliance forecast. |
| TOC concentration | Uncertain | The randomly assigned values from the two national distributions are based on a limited dataset. Actual TOC concentrations at systems affected by the proposed rule can be higher or lower than the assigned values. |
| Insufficient UCMR 3 data for PFBS and PFNA and no UCMR 3 data for HFPO-DA were available to incorporate into the Bayesian hierarchical occurrence model | Underestimate | The HI in the proposed option would regulate PFBS, PFNA, and HFPO-DA in addition to the modeled PFAS. In instances when concentrations of PFBS, PFNA, and/or HFPO-DA are high enough to cause a HI exceedance, the modeled costs may be underestimated. If these PFAS occur in isolation at levels that affect treatment decisions, or if they occur in sufficient concentration to result in an exceedance when the concentration of PFHxS alone would be below the HI, then costs would be underestimated. Note that EPA has conducted an analysis of the potential changes in system level treatment cost associated with the occurrence of PFBS, PFNA, and HFPO-DA using a model system approach which is discussed in detail in Chapter 5 and Appendix N of the Economic Analysis (USEPA, 2023j; USEPA, 2023i). |
| POU not included in compliance forecast | Overestimate | If POU devices can be certified to meet concentrations that satisfy the proposed rule, then small systems may be able to reduce costs by using a POU compliance option instead of centralized treatment or source water changes. |
| Process wastes not classified as hazardous | Underestimate | The national cost analysis reflects the assumption that PFAS-contaminated wastes are not considered hazardous wastes. As a general matter, EPA notes that such wastes are not currently regulated under Federal law as a hazardous waste. To address stakeholder concerns, including those raised during the SBREFA process, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. As part of this analysis, EPA generated a second full set of unit cost curves that are identical to the curves used for the national cost analysis with the exception that spent GAC and spent IX resin are considered hazardous. EPA acknowledges that if Federal authorities later determine that PFAS-contaminated wastes require handling as hazardous wastes, the residuals management costs in the WBS treatment cost models are expected to be higher. See Appendix N of the Economic Analysis (USEPA, 2023j; USEPA, 2023i) for a sensitivity analysis describing the potential increase in costs associated with hazardous waste disposal at 100% of systems treating for PFAS. The costs estimated in Appendix N are consistent with EPA OLEM's “Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances.” 1 |
D. Method for Estimating Benefits
EPA's quantification of health benefits resulting from reduced PFAS exposure in drinking water was driven by PFAS occurrence estimates, pharmacokinetic (PK) model availability, information on exposure-response relationships, and available information to monetize avoided cases of illness. In the Economic Analysis, EPA either quantitatively assesses or qualitatively discusses health endpoints associated with exposure to PFAS. EPA assesses potential benefits quantitatively if evidence of exposure and health effects is likely, it is possible to link the outcome to risk of a health effect, and there is no overlap in effect with another quantified endpoint in the same outcome group. Particularly, the most consistent epidemiological associations with PFOA and PFOS include decreased immune system response, decreased birthweight, increased serum lipids, and increased liver enzymes (particularly ALT). The available evidence indicates effects across immune, developmental, cardiovascular, and hepatic organ systems at the same or approximately the same level of exposure.
Table 42 presents an overview of the categories of health benefits expected to result from the implementation of treatment that reduces PFAS levels in drinking water. Of the PFAS compounds included in the proposed rule, EPA quantifies some of the adverse health effects associated with PFOA and PFOS. EPA also quantifies one adverse health effect of PFNA in a sensitivity analysis only. These compounds have likely evidence linking exposure to a particular health endpoint and have reliable PK models connecting the compound to PFAS blood serum. PK models describe the distribution of chemicals in the body and pharmacodynamic relation between blood concentration and clinical effects. Benefits from avoided adverse health effects of HFPO-DA, PFHxS and PFBS are discussed qualitatively in this section.
As Table 42 demonstrates, only a subset of the avoided morbidity and mortality stemming from reduced PFAS levels in drinking water can be quantified and monetized. The monetized benefits evaluated in the Economic Analysis for the proposed rule include changes in human health risks associated with CVD and infant birth weight from reduced exposure to PFOA and PFOS in drinking water and RCC from reduced exposure to PFOA. EPA also quantified benefits from reducing bladder cancer risk due to the co-removal of non-PFAS pollutants via the installation of drinking water treatment, discussed in greater detail in USEPA (2023j).
EPA was not able to quantify or monetize other benefits, including those related to other reported health effects including immune, liver, endocrine, metabolic, reproductive, musculoskeletal, other cancers. EPA discusses these benefits qualitatively in more detail below, as well as in Section 6.2 of USEPA (2023j).
| Health outcome | PFAS Compound 1 2 3 | Benefits analysis 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Endpoint | PFOA | PFOS | PFNA | PFHxS | PFBS | HFPO-DA | Discussed quantitatively | Discussed qualitatively |
| Notes: | |||||||||
| 1 Fields marked with “X” indicate the PFAS compound for which there is evidence of an association with a given health outcome in epidemiological studies. | |||||||||
| 2 Fields marked with “•” indicate the PFAS compound for which there is evidence of an association with a given health outcome only in toxicological studies. | |||||||||
| 3 Note that only PFOA and PFOS effects were modeled in the assessment of benefits under the proposed rule. PFNA was modeled only in sensitivity analyses of birth weight benefits (See Economic Analysis Appendix K in USEPA (2023i)). | |||||||||
| 4 Outcomes with likely evidence of an association between a PFAS compound and a health outcome are assessed quantitatively unless (1) there is an overlap within the same outcome group ( e.g., LDLC overlaps with total cholesterol, and SGA overlaps with low birth weight), or (2) it is not possible to link the outcome to the risk of the health effect ( e.g., evidence is inconclusive regarding the relationship between PFOS exposure and leptin levels and associated health outcomes). Such health outcomes are discussed qualitatively. | |||||||||
| 5 Evidence of the relationship between the PFAS compound and the health outcome is not conclusive. Note that EPA sought comments from the EPA SAB on the CVD exposure-response approach (USEPA, 2023j). The SAB recommended that EPA evaluate how the inclusion of HDLC effects would influence results. EPA evaluated the inclusion of HDLC effects in a sensitivity analysis, described in Appendix K. | |||||||||
| Lipids | Total cholesterol | X | X | e X | X | ||||
| High-density lipoprotein cholesterol (HDLC) | 5 X | 5 X | X | ||||||
| Low-density lipoprotein cholesterol (LDLC) | X | X | 5 X | X | |||||
| CVD | Blood pressure | X | X | ||||||
| Developmental | Birth weight | X | X | X | 5 X | • | 5 • | X | |
| Small for gestational age (SGA), non-birth weight developmental | X | 5 X | X | • | X | ||||
| Endocrine | Thyroid hormone disruption | • | • | • | • | X | |||
| Hepatic | ALT | X | X | 5 X | X | • | X | ||
| Immune | Antibody response (tetanus, diphtheria) | X | X | 5 X | X | • | X | ||
| Metabolic | Leptin | X | X | ||||||
| Renal | Organ weight | • | • | X | |||||
| Musculoskeletal | Osteoarthritis, bone mineral density | X | 5 X | X | |||||
| Hematologic | Vitamin D levels, hemoglobin levels, albumin levels | • | X | ||||||
| Cancer | RCC | X | X | ||||||
| Testicular | X | X | |||||||
| Other | 5 • | ||||||||
EPA developed PK models to evaluate blood serum PFAS levels in adults resulting from exposure to PFAS via drinking water. To date, EPA has developed PK models for PFOA and PFOS. EPA used baseline and regulatory alternative PFOA/PFOS drinking water concentrations as inputs to its PK model to estimate blood serum PFOA/PFOS concentrations for adult males and females. For further detail on the PK model and its application in EPA's benefits analysis, please see EPA's Proposed MCLG documents (USEPA, 2023b; USEPA, 2023c) and Section 6.3 of USEPA (2023j).
1. Quantified Developmental Effects
Research indicates that exposure to PFOA and PFOS is associated with developmental effects, including infant birth weight (Verner et al., 2015; USEPA, 2016e; USEPA, 2016f; USEPA, 2023b; USEPA, 2023c; Negri et al., 2017; ATSDR, 2021; Waterfield et al., 2020). The route through which the embryo and fetus are exposed prenatally to PFOA and PFOS is maternal blood serum via the placenta. Most studies of the association between maternal serum PFOA/PFOS and birth weight report negative relationships (Verner et al., 2015; Negri et al., 2017; Dzierlenga et al., 2020). EPA's PK model assumes that mothers were exposed to PFOA/PFOS from birth to the year in which pregnancy occurred.
EPA quantified and valued changes in birth weight-related risks associated with reductions in exposure to PFOA and PFOS in drinking water. Entry point-specific time series of the differences between serum PFOA/PFOS concentrations under baseline and regulatory alternatives are inputs into this analysis. For each entry point, evaluation of the changes in birth weight impacts involves the following key steps:
1. Estimating the changes in birth weight based on modeled changes in serum PFOA/PFOS levels and exposure-response functions for the effect of serum PFOA/PFOS on birth weight;
2. Estimating the difference in infant mortality probability between the baseline and regulatory alternatives based on changes in birth weight under the regulatory alternatives and the association between birth weight and mortality;
3. Identifying the infant population affected by reduced exposure to PFOA/PFOS in drinking water under the regulatory alternatives;
4. Estimating the changes in the expected number of infant deaths under the regulatory alternatives based on the difference in infant mortality rates and the population of surviving infants affected by increases in birth weight due to reduced PFOA/PFOS exposure; and
5. Estimating the economic value of reducing infant mortality based on the Value of a Statistical Life and infant morbidity based on reductions in medical costs associated with changes in birth weight for the surviving infants based on the cost of illness.
EPA also considered the potential benefits from reduced exposure to PFNA that may be realized as a direct result of the proposed rule. The Agency explored the birth weight impacts of PFNA in a sensitivity analysis, using a unit PFNA reduction scenario ( i.e., 1.0 ppt change) and Lu and Bartell (2020) to estimate PFNA blood serum levels resulting from PFNA exposures in drinking water. To estimate blood serum PFNA based on its drinking water concentration, EPA used a first-order single-compartment model whose behavior was previously demonstrated to be consistent with PFOA PKs in humans (Bartell et al., 2010). In addition to the PFOA-birth weight and PFOS-birth weight effects analyzed in the Economic Analysis, EPA examined the effect of inclusion of PFNA-birth weight effects using estimates from two studies (Lenters et al., 2016; Valvi et al., 2017). EPA found that inclusion of a 1.0 ppt PFNA reduction could increase annualized birth weight benefits 5.4-7.7-fold, relative to the scenario that quantifies a 1.0 ppt reduction in PFOA and a 1.0 ppt reduction in PFOS only. The range of estimated PFNA-related increases in benefits is driven by the exposure-response, with smaller estimates produced using the slope factors from Lenters et al. (2016), followed by Valvi et al. (2017). EPA notes that the PFNA slope factor estimates are orders of magnitude larger than the slope factor estimates used to evaluate the impacts of PFOA/PFOS reductions. EPA also notes that the PFNA slope factor estimates are not precise, with 95% CIs covering wide ranges that include zero ( i.e., serum PFNA slope factor estimates are not statistically significant at 5% level). Caution should be exercised in making judgements about the potential magnitude of change in the national benefits estimates based on the results of these sensitivity analyses, although conclusions about the directionality of these effects can be inferred. EPA did not include PFNA effects in the national benefits estimates for the proposed rulemaking because of limitations associated with the UCMR 3 PFNA occurrence data and the slope factor estimates are less precise. For more information, see Appendix K of USEPA (2023j).
To estimate changes in birth weight resulting from reduced exposure to PFOA and PFOS under the regulatory alternatives, EPA relied on the estimated time series of changes in serum PFOA/PFOS concentrations specific to women of childbearing age and serum-birth weight exposure-response functions provided in recently published meta-analyses. For more detail on the evaluation of the studies used in these meta-analyses, please see EPA's Proposed Maximum Contaminant Level Goal for PFOA and PFOS in Drinking Water (USEPA, 2023b; USEPA, 2023c) and Section 6.4 of USEPA (2023j).
Changes in serum PFOA and PFOS concentrations are calculated for each PWS entry point during each year in the analysis period. EPA assumes that, given long half-lives of PFOS and PFOA, any one-time measurement during or near pregnancy is reflective of a critical window and not subject to considerable error. The mean change in birth weight per increment in long-term PFOA and PFOS exposure is calculated by multiplying each annual change in PFOA and PFOS serum concentration (ng/mL serum) by the PFOA and PFOS serum-birth weight exposure-response slope factors (g birth weight per ng/mL serum) provided in Table 43, respectively. The mean annual change in birth weight attributable to changes in both PFOA and PFOS exposure is the sum of the annual PFOA- and PFOS-birth weight change estimates. Additional detail on the derivation of the exposure-response functions can be found in Appendix D in USEPA (2023i). Appendix K in USEPA (2023i) presents an analysis of birth weight risk reduction considering slope factors specific to the first trimester.
| Compound | g/ng/mL serum (95% CI) |
|---|---|
| Notes: | |
| 1 The serum-birth weight slope factor for PFOA is based on the main random effects estimate from Negri et al. (2017); Steenland et al. (2018). | |
| 2b The serum-birth weight slope factor for PFOS is based on an EPA reanalysis of Dzierlenga et al. (2020). | |
| PFOA 1 | −10.5 (−16.7, −4.4) |
| PFOS 2 | −3.0 (−4.9, −1.1) |
EPA places a cap on estimated birth weight changes in excess of 200 g, assuming that such changes in birth weight are unreasonable even as a result of large changes in PFOA/PFOS serum concentrations. This cap is based on existing studies that found that changes to environmental exposures result in relatively modest birth weight changes (Windham and Fenster, 2008; Klein and Lynch, 2018; Kamai et al., 2019).
Low birth weight is linked to a number of health effects that may be a source of economic burden to society in the form of medical costs, infant mortality, parental and caregiver costs, labor market productivity loss, and education costs (Chaikind and Corman, 1991; Behrman and Butler, 2007; Behrman and Rosenzweig, 2004; Joyce et al., 2012; Kowlessar et al., 2013; Colaizy et al., 2016; Nicoletti et al., 2018; Klein and Lynch, 2018). Recent literature also linked low birth weight to educational attainment and required remediation to improve students' outcomes, childhood disability, and future earnings (Jelenkovic et al., 2018; Temple et al., 2010; Elder et al., 2020; Hines et al., 2020 Chatterji et al., 2014; Dobson et al., 2018).
EPA's analysis focuses on two categories of birth weight impacts that are amenable to monetization associated with incremental changes in birth weight: (1) medical costs associated with changes in infant birth weight and (2) the value of avoiding infant mortality at various birth weights. The birth weight literature related to other sources of economic burden to society ( e.g., parental and caregiver costs and productivity losses) is limited in geographic coverage, population size, and range of birth weights evaluated and therefore cannot be used in the economic analysis of birth weight effects from exposure to PFOA/PFOS in drinking water (ICF, 2021).
Two studies showed statistically significant relationships between incremental changes in birth weight and infant mortality: Almond et al. (2005) and Ma and Finch (2010). Ma and Finch (2010) used 2001 National Center for Health Statistics (NCHS) linked birth/infant death data for singleton and multiple birth infants among subpopulations defined by sex and race/ethnicity to estimate a regression model assessing the associations between 14 key birth outcome measures, including birth weight, and infant mortality. They found notable variation in the relationship between birth weight and mortality across race/ethnicity subpopulations, with odds ratios for best-fit birth weight-mortality models ranging from 0.8-1 (per 100 g birth weight change). Almond et al. (2005) used 1989-1991 NCHS linked birth/infant death data for multiple birth infants to analyze relationships between birth weight and infant mortality within birth weight increment ranges. For their preferred model, they reported coefficients in deaths per 1,000 births per 1 g increase in birth weight that range from −0.420 to −0.002. However, the data used in these studies (Almond et al., 2005 and Ma, 2010) are outdated (1989-1991 and 2001, respectively). Given the significant decline in infant mortality over the last 30 years (ICF, 2020) and other maternal and birth characteristics that are likely to influence infant mortality ( e.g., average maternal age and rates of maternal smoking), the birth weight-mortality relationship estimates from Almond et al. (2005) and Ma and Fitch (2010) are likely to overestimate the benefits of birth weight changes.
Considering the discernible changes in infant mortality over the last 30 years, EPA developed a regression analysis to estimate the relationship between birth weight and infant mortality using the most recently available Period/Cohort Linked Birth-Infant Death Data Files published by NCHS from the 2017 period/2016 cohort and the 2018 period/2017 cohort (CDC, 2017, 2018). EPA selected variables of interest for the regression analysis, including maternal demographic and socioeconomic characteristics, maternal risk and risk mitigation factors ( e.g., number of prenatal care visits, smoker status), and infant birth characteristics. EPA included several variables used in Ma and Fitch (2010) (maternal age, maternal education, marital status, and others) as well as additional variables to augment the set of covariates included in the analyses. In addition, EPA developed separate models for different race/ethnicity categories (non-Hispanic Black, non-Hispanic White, and Hispanic) and interacted birth weight with categories of gestational age, similar to Ma and Finch (2010). Appendix E to USEPA (2023i) provides details on model development and regression results.
Table 44 presents the resulting odds ratios and marginal effects (in terms of deaths per 1,000 births for every 1 g increase in birth weight) estimated for changes in birth weight among different gestational age categories in the mortality regression models for non-Hispanic Black, non-Hispanic White, and Hispanic race/ethnicity subpopulations. Marginal effects for birth weight among gestational age categories vary across different race/ethnicity subpopulations. The marginal effects for birth weight among different gestational age categories are higher in the non-Hispanic Black model than in the non-Hispanic White and Hispanic models, particularly for extremely and very preterm infants, indicating that low birth weight increases the probability of mortality within the first year more so among non-Hispanic Black infants than among non-Hispanic White and Hispanic infants.
EPA relies on odds ratios estimated using the birth weight-mortality regression model to assess mortality outcomes of reduced exposures to PFOA/PFOS in drinking water under the regulatory alternatives. To obtain odds ratios specific to each race/ethnicity and 100 g birth weight increment considered in the birth weight benefits model, 6 EPA averaged the estimated odds ratios for 1 g increase in birth weight over the gestational age categories using the number of infants (both singleton and multiple birth) that fall into each gestational age category as weights. Separate gestational age category weights were computed for each 100 g birth weight increment and race/ethnicity subpopulation within the 2017 period/2016 cohort and 2018 period/2017 cohort Linked Birth-Infant Death Data Files. The weighted birth weight odds ratios are then used in conjunction with the estimated change in birth weight and baseline infant mortality rates to determine the probability of infant death under the regulatory alternatives, as described further in Section 6.4 of USEPA (2023j).
6 The birth weight risk reduction model evaluates changes in birth weight in response to PFOA/PFOS drinking water level reductions for infants who fall into 100 g birth weight increments ( e.g., birth weight 0-99 g, 100-199 g, 200-299 g. . . 8,000-8,099 g, 8,100-8,165 g).
| Race | Gestational age category 2 | Marginal effect per 1,000 births (95% CI) | Odds ratio (95% CI) |
|---|---|---|---|
| Notes: | |||
| 1 Data based on the 2016/17 and 2017/18 CDC Period Cohort Linked Birth-Infant Death Data Files obtained from NCHS/National Vital Statistics System (NVSS). Marginal effects and odds ratios are estimated using a regression model that also includes covariates representative of infant birth characteristics in addition to birth weight, maternal demographic characteristics, and maternal risk factors. All effects were statistically significant at the 5% level. Additional details are included in Appendix E to the Economic Analysis. | |||
| 2 Gestational age categories defined as extremely preterm (<=28 weeks), very preterm (>28 weeks and <=32 weeks), moderately preterm (>32 weeks and <=37 weeks), and term (>37 weeks). | |||
| Non-Hispanic Black | Extremely Preterm | −0.20400 (−0.21910, −0.18890) | 0.99817 (0.99802, 0.99832) |
| Very Preterm | −0.04580 (−0.04820, −0.04340) | 0.99816 (0.99804, 0.99827) | |
| Moderately Preterm | −0.01030 (−0.01080, −0.009850) | 0.99852 (0.99846, 0.99857) | |
| Term | −0.00453 (−0.00472, −0.00434) | 0.99856 (0.99851, 0.9986) | |
| Non−Hispanic White | Extremely Preterm | −0.12160 (−0.13080, −0.11240) | 0.99866 (0.99855, 0.99878) |
| Very Preterm | −0.03290 (−0.03430, −0.03140) | 0.9985 (0.99842, 0.99858) | |
| Moderately Preterm | −0.00677 (−0.00702, −0.00652) | 0.99867 (0.99863, 0.99872) | |
| Term | −0.00228 (−0.00236, −0.00221) | 0.99865 (0.99861, 0.99868) | |
| Hispanic | Extremely Preterm | −0.15260 (−0.16770, −0.13750) | 0.99835 (0.99817, 0.99853) |
| Very Preterm | −0.03290 (−0.03510, −0.03070) | 0.99846 (0.99835, 0.99858) | |
| Moderately Preterm | −0.00626 (−0.00659, −0.00592) | 0.99856 (0.99849, 0.99862) | |
| Term | −0.00219 (−0.00229, −0.00208) | 0.99849 (0.99844, 0.99855) | |
EPA weighted the race/ethnicity-specific odds ratios in Table 44 by the proportions of the infant populations who fell into each gestational age within a 100 g birth weight increment, based on the 2016/17 and 2017/18 period cohort data, to obtain a weighted odds ratio estimate for each modeled race/ethnicity subpopulation and 100 g birth weight increment.
Based on reduced serum PFOA/PFOS exposures under the regulatory alternatives and the estimated relationship between birth weight and infant mortality, EPA estimates the subsequent change in birth weight for those infants affected by decreases in PFOA/PFOS and changes in the number of infant deaths. EPA evaluated these changes at each PWS entry point affected by the regulatory alternatives and the calculations are performed for each race/ethnicity group, 100 g birth weight category, and year of the analysis. Additional detail on the calculations EPA used to estimate changes in birth weight, the affected population size, and infant deaths avoided, and the number of surviving infants is provided in Chapter 6 of USEPA (2023j).
EPA used the Value of a Statistical Life to estimate the benefits of reducing infant mortality and the cost of illness to estimate the economic value of increasing birth weight in the population of surviving infants born to mothers exposed to PFOA and PFOS in drinking water. EPA's approach to monetizing benefits associated with incremental increases in birth weight resulting from reductions in drinking water PFOA/PFOS levels relies on avoided medical costs associated with various ranges of birth weight. Although the economic burden of treating infants at various birth weights also includes non-medical costs, very few studies to date have quantified such costs (Klein and Lynch, 2018; ICF, 2021). EPA selected the medical cost function from Klein and Lynch (2018) to monetize benefits associated with the estimated changes in infant birth weight resulting from reduced maternal exposure to PFOA/PFOS. 7
7 The Klein and Lynch (2018) report was externally peer reviewed by three experts with qualifications in economics and public health sciences. EPA's charge questions to the peer reviewers sought input on the methodology for developing medical cost estimates associated with changes in birth weight. The Agency's charge questions and peer reviewer responses are available in the docket.
Using the incremental cost changes from Klein and Lynch (2018), EPA calculates the change in medical costs resulting from changes in birth weight among infants in the affected population who survived the first year following birth, provided in Table 45.
| Birth weight 1 2 | Simulated cost changes for birth weight increases, dollars per gram ($2021) 3 | ||
|---|---|---|---|
| 0.04 lb ( 18 g) | 0.11 lb ( 50 g) | 0.22 lb ( 100 g) | |
| Notes: | |||
| 1 Values for birth weight have been converted from lb to g. | |||
| 2 Note that simulated medical costs increase, rather than decrease, in response to increased birth weight changes among high birth weight infants (those greater than 8 lb). Among high birth weight infants, there is a higher risk of birth trauma, metabolic issues, and other health problems (Klein and Lynch, 2018). | |||
| 3 Values scaled from $2010 to $2021 using the medical care CPI (Bureau of Labor Statistics, 2021). | |||
| 2 lb (907 g) | −$126.53 | −$112.87 | −$109.39 |
| 2.5 lb (1,134 g) | −$94.88 | −$84.64 | −$82.03 |
| 3 lb (1,361 g) | −$71.15 | −$63.47 | −$61.51 |
| 3.3 lb (1,497 g) | −$59.86 | −$53.40 | −$51.75 |
| 4 lb (1,814 g) | −$40.00 | −$35.69 | −$34.59 |
| 4.5 lb (2,041 g) | −$30.00 | −$26.76 | −$25.93 |
| 5 lb (2,268 g) | −$22.49 | −$20.07 | −$19.45 |
| 5.5 lb (2,495 g) | −$0.93 | −$0.84 | −$0.84 |
| 6 lb (2,722 g) | −$0.91 | −$0.83 | −$0.83 |
| 7 lb (3,175 g) | −$0.88 | −$0.80 | −$0.80 |
| 8 lb (3,629 g) | −$0.85 | −$0.77 | −$0.77 |
| 9 lb (4,082 g) | $3.15 | $2.87 | $2.89 |
| 10 lb (4,536 g) | $3.54 | $3.23 | $3.26 |
Tables 46 to 49 provide the health effects avoided and valuation associated with birth weight impacts. EPA estimated that, over the evaluation period, the proposed rule will result in an average annual benefit from avoided reductions in birth weight from $139 million ($2021, 7% discount rate) to $178 million ($2021, 3% discount rate).
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Increase in Birth Weight (millions of grams) | 114.2 | 209.3 | 329.7 | 114.2 | 209.3 | 329.7 |
| Number of Birth Weight-Related Deaths Avoided | 676.8 | 1,232.7 | 1,941.0 | 676.8 | 1,232.7 | 1,941.0 |
| Total Annualized Birth Weight Benefits (Million $2021) 2 | $97.36 | $177.66 | $279.49 | $74.62 | $139.01 | $219.43 |
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Increase in Birth Weight (millions of grams) | 111.7 | 206.3 | 326.9 | 111.7 | 206.3 | 326.9 |
| Number of Birth Weight-Related Deaths Avoided | 665.4 | 1,214.7 | 1,915.4 | 665.4 | 1,214.7 | 1,915.4 |
| Total Annualized Birth Weight Benefits (Million $2021) 2 | $95.73 | $175.05 | $276.44 | $74.66 | $136.97 | $217.02 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Increase in Birth Weight (millions of grams) | 97.6 | 181.9 | 292.1 | 97.6 | 181.9 | 292.1 |
| Number of Birth Weight-Related Deaths Avoided | 578.9 | 1,069.5 | 1,707.3 | 578.9 | 1,069.5 | 1,707.3 |
| Total Annualized Birth Weight Benefits (Million $2021) 2 | $83.27 | $154.13 | $246.43 | $64.94 | $120.59 | $193.47 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Increase in Birth Weight (millions of grams) | 51.0 | 109.2 | 195.3 | 51.0 | 109.2 | 195.3 |
| Number of Birth Weight-Related Deaths Avoided | 299.5 | 643.3 | 1,140.5 | 299.5 | 643.3 | 1,140.5 |
| Total Annualized Birth Weight Benefits (Million $2021) 2 | $43.22 | $92.70 | $164.19 | $34.18 | $72.51 | $125.80 |
2. Quantified Cardiovascular Effects
CVD is one of the leading causes of premature mortality in the United States (D'Agostino et al., 2008; Goff et al., 2014; Lloyd-Jones et al., 2017). As discussed in EPA's Proposed Maximum Contaminant Level Goals for PFOA and PFOS in Drinking Water, exposure to PFOA and PFOS through drinking water contributes to increased serum PFOA and PFOS concentrations and potentially elevated levels of total cholesterol and elevated levels of systolic blood pressure (USEPA, 2023b; USEPA, 2023c). Changes in total cholesterol and blood pressure are associated with changes in incidence of CVD events such as myocardial infarction ( i.e., heart attack), ischemic stroke, and cardiovascular mortality occurring in populations without prior CVD event experience (D'Agostino et al., 2008; Goff et al., 2014; Lloyd-Jones et al., 2017).
EPA recognizes that the epidemiologic literature that provides strong support for an effect of PFOA and PFOS on cholesterol and blood pressure does not provide direct support for an effect of PFOA and PFOS on the risk of CVD. Therefore, EPA uses the approach outlined below to link changes in CVD risk biomarkers ( i.e., cholesterol and blood pressure) to changes in CVD risk.
For each entry point, evaluation of the changes in CVD risk involves the following key steps:
1. Estimation of annual changes in total cholesterol and blood pressure levels using exposure-response functions for the potential effects of serum PFOA/PFOS on these biomarkers;
2. Estimation of the annual incidence of fatal and non-fatal first hard CVD events, defined as fatal and non-fatal myocardial infarction, fatal and non-fatal ischemic stroke or other coronary heart disease death occurring in populations without prior CVD event experience (D'Agostino et al., 2008; Goff et al., 2014; Lloyd-Jones et al., 2017), and post-acute CVD mortality corresponding to baseline and regulatory alternative total cholesterol and blood pressure levels in all populations alive during or born after the start of the evaluation period; and
3. Estimation of the economic value of reducing CVD mortality and morbidity from baseline to regulatory alternative levels, using the Value of a Statistical Life and cost of illness measures, respectively.
Given the breadth of evidence linking PFOA and PFOS exposure to effects on total cholesterol and blood pressure in general adult populations, EPA quantified public health impacts of changes in these well-established CVD risk biomarkers (D'Agostino et al., 2008; Goff et al., 2014; Lloyd-Jones et al., 2017) by estimating changes in incidence of several CVD events. Specifically, EPA assumed that PFOA/PFOS-related changes in total cholesterol and blood pressure had the same effect on the CVD risk as the changes unrelated to chemical exposure and used the Pooled Cohort Atherosclerotic Cardiovascular Disease (ASCVD) model (Goff et al., 2014) to evaluate their impacts on the incidence of myocardial infarction, ischemic stroke, and cardiovascular mortality occurring in populations without prior CVD event experience.
The ASCVD model includes total cholesterol as a predictor of first hard CVD events. EPA did not identify any readily available relationships for PFOA or PFOS and total cholesterol that were specifically relevant to the age group of interest (40-89 years, the years for which the ASCVD model estimates the probability of a first hard CVD event). Therefore, the Agency developed a meta-analysis of studies reporting associations between serum PFOA or PFOS and total cholesterol in general populations ( e.g., populations that are not a subset of workers or pregnant women). Statistical analyses that combine the results of multiple studies, such as meta-analyses, are widely applied to investigate the associations between contaminant levels and associated health effects. Such analyses are suitable for economic assessments because they can improve precision and statistical power (Engels et al., 2000; Deeks, 2002; Rücker et al., 2009).
EPA identified 14 studies from which to derive slope estimates for PFOA and PFOS associations with serum total cholesterol levels. Appendix A to USEPA (2023i) provides further detail on the studies selection criteria, meta-data development, meta-analysis results, and discussion of the uncertainty and limitations inherent in EPA's exposure-response analysis.
EPA developed exposure-response relationships between serum PFOA/PFOS and total cholesterol for use in the CVD analysis using the meta-analyses restricted to studies of adults in the general population reporting similar models. When using studies reporting linear associations between total cholesterol and serum PFOA or PFOS, EPA estimated a positive increase in total cholesterol of 1.57 (95% CI: 0.02, 3.13) mg/dL per ng/mL serum PFOA (p-value=0.048), and of 0.08 (95% CI: −0.01, 0.16) mg/dL per ng/mL serum PFOS (p-value=0.064). Based on the systematic review conducted by EPA to develop EPA's Proposed Maximum Contaminant Level Goals for PFOA and PFOS in Drinking Water, the available evidence supports a positive association between PFOS and total cholesterol in the general population. For more information on the systematic review and results, see USEPA, 2023b and USEPA, 2023c.
PFOS exposure has been linked to other cardiovascular outcomes, such as systolic blood pressure and hypertension (Liao et al., 2020; USEPA, 2023c). Because systolic blood pressure is another predictor used by the ASCVD model, EPA included the estimated changes in blood pressure from reduced exposure to PFOS in the CVD analysis. EPA selected the slope from the Liao et al. (2020) study—a high confidence study conducted based on U.S. general population data from NHANES cycles 2003-2012. The evidence on the associations between PFOA and blood pressure is not as consistent as for PFOS. Therefore, EPA is not including effect estimates for the serum PFOA-blood pressure associations in the CVD analysis.
EPA relies on the life table-based approach to estimate CVD risk reductions because (1) changes in serum PFOA/PFOS in response to changes in drinking water PFOA/PFOS occur over multiple years, (2) CVD risk, relying on the ASCVD model, can be modeled only for those older than 40 years without prior CVD history, and (3) individuals who have experienced non-fatal CVD events have elevated mortality implications immediately and within at least five years of the first occurrence. Recurrent life table calculations are used to estimate a PWS entry point-specific annual time series of CVD event incidence for a population cohort characterized by sex, race/ethnicity, birth year, age at the start of the PFOA/PFOS evaluation period ( i.e., 2023), and age- and sex-specific time series of changes in total cholesterol and blood pressure levels obtained by combining serum PFOA/PFOS concentration time series with exposure-response information. Baseline and regulatory alternatives are evaluated separately, with regulatory alternative total cholesterol and blood pressure levels estimated using baseline information on these biomarkers from external statistical data sources and modeled changes in total cholesterol and blood pressure due to conditions under the regulatory alternatives.
EPA estimated the incidence of first hard CVD events based on total cholesterol serum and blood pressure levels using the ASCVD model (Goff et al., 2014), which predicts the 10-year probability of a hard CVD event to be experienced by a person without a prior CVD history. EPA adjusted the modeled population cohort to exclude individuals with pre-existing conditions, as the ASCVD risk model does not apply to these individuals. For blood pressure effects estimation, EPA further restricts the modeled population to those not using antihypertensive medications for consistency with the exposure-response relationship. Modeled first hard CVD events include fatal and non-fatal myocardial infarction, fatal and non-fatal ischemic stroke, and other coronary heart disease mortality. EPA also has estimated the incidence of post-acute CVD mortality among survivors of the first myocardial infarction or ischemic stroke within 6 years of the initial event.
The estimated CVD risk reduction resulting from reducing serum PFOA and serum PFOS concentrations is the difference in annual incidence of CVD events ( i.e., mortality and morbidity associated with first-time CVD events and post-acute CVD mortality) under the baseline and regulatory alternatives. Appendix G to USEPA (2023i) provides detailed information on all CVD model components, computations, and sources of data used in modeling.
EPA uses the Value of a Statistical Life to estimate the benefits of reducing mortality associated with hard CVD events in the population exposed to PFOA and PFOS in drinking water. EPA relies on cost of illness-based valuation that represents the medical costs of treating or mitigating non-fatal first hard CVD events (myocardial infarction, ischemic stroke) during the three years following an event among those without prior CVD history, adjusted for post-acute mortality.
The annual medical expenditure estimates for myocardial infarction and ischemic stroke are based on O'Sullivan et al. (2011). The estimated expenditures do not include long-term institutional and home health care. For non-fatal myocardial infarction, O'Sullivan et al. (2011) estimated medical expenditures are $51,173 ($2021) for the initial event and then $31,871, $14,065, $12,569 annually within 1, 2, and 3 years after the initial event, respectively. For non-fatal ischemic stroke, O'Sullivan et al. (2011) estimated medical expenditures are $15,861 ($2021) for the initial event and then $11,521, $748, $1,796 annually within 1, 2, and 3 years after the initial event, respectively. Annual estimates within 1, 2, and 3 years after the initial event include the incidence of secondary CVD events among survivors of first myocardial infarction and ischemic stroke events.
To estimate the present discounted value of medical expenditures within 3 years of the initial non-fatal myocardial infarction, EPA combined O'Sullivan et al. (2011) myocardial infarction-specific estimates with post-acute survival probabilities based on Thom et al. (2001) (for myocardial infarction survivors aged 40-64) and Li et al. (2019) (for myocardial infarction survivors aged 65 ). To estimate the present discounted value of medical expenditures within 3 years of the initial non-fatal ischemic stroke, EPA combined O'Sullivan et al. (2011) ischemic stroke-specific estimates with post-acute survival probabilities based on Thom et al. (2001) (for ischemic stroke survivors aged 40-64, assuming post-acute myocardial infarction survival probabilities reasonably approximate post-acute ischemic stroke survival probabilities) and Li et al. (2019) (for ischemic stroke survivors aged 65 ). EPA did not identify post-acute ischemic stroke mortality information in this age group, but instead applied post-acute myocardial infarction mortality estimates for ischemic stroke valuation. Table 50 presents the resulting myocardial infarction and ischemic stroke unit values.
| Type of first non-fatal hard CVD event | Age group | Present discounted value of 3-year medical expenditures ($2021) 1 2 , adjusted for post-acute mortality 3 | |
|---|---|---|---|
| 3% discount rate | 7% discount rate | ||
| Notes: | |||
| 1 Estimates of annual medical expenditures are from O'Sullivan et al. (2011); | |||
| 2 Original values from O'Sullivan et al. (2011) were inflated to $2021 using the medical care CPI (Bureau of Labor Statistics, 2021); | |||
| 3 Post-acute myocardial infarction mortality data for those aged 40-64 years is from Thom et al. (2001); probabilities to survive 1 year, 2 years, and 3 years after the initial event are 0.93, 0.92, and 0.90, respectively. EPA applies these mortality values to derive the ischemic stroke value in this age group. Post-acute myocardial infarction mortality data and post-acute IS mortality data for persons aged 65 and older are from Li et al. (2019). For myocardial infarction, probabilities to survive 1 year, 2 years, and 3 years after the initial event are 0.68, 0.57, and 0.49, respectively. For ischemic stroke, probabilities to survive 1 year, 2 years, and 3 years after the initial event are 0.67, 0.57, and 0.48, respectively. | |||
| Myocardial Infarction (MI) | 40-65 years | $105,419 | $104,155 |
| 66 years | 92,658 | 91,881 | |
| Ischemic Stroke (IS) | 40-65 years | 29,154 | 29,017 |
| 66 years | 26,844 | 26,762 | |
Table 51 to Table 54 provide the health effects avoided and valuation associated with CVD. EPA estimated that, over the evaluation period, the proposed option will result in an average annual benefit from avoided CVD cases and deaths from $421 million ($2021, 7% discount rate) to $533 million ($2021, 3% discount rate).
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal MI Cases Avoided | 1,251.5 | 6,081.0 | 11,738.7 | 1,251.5 | 6,081.0 | 11,738.7 |
| Number of Non-Fatal IS Cases Avoided | 1,814.0 | 8,870.8 | 17,388.5 | 1,814.0 | 8,870.8 | 17,388.5 |
| Number of CVD Deaths Avoided | 753.6 | 3,584.6 | 7,030.9 | 753.6 | 3,584.6 | 7,030.9 |
| Total Annualized CVD Benefits (Million $2021) 2 | $111.78 | $533.48 | $1,051.00 | $85.94 | $421.10 | $822.88 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal MI Cases Avoided | 1,248.7 | 5,983.8 | 11,614.9 | 1,248.7 | 5,983.8 | 11,614.9 |
| Number of Non-Fatal IS Cases Avoided | 1,786.4 | 8,729.6 | 17,149.5 | 1,786.4 | 8,729.6 | 17,149.5 |
| Number of CVD Deaths Avoided | 744.6 | 3,527.8 | 6,951.5 | 744.6 | 3,527.8 | 6,951.5 |
| Total Annualized CVD Benefits (Million $2021) 2 | $110.45 | $525.05 | $1,035.36 | $86.32 | $414.45 | $817.79 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal MI Cases Avoided | 1,105.9 | 5,220.7 | 10,215.4 | 1,105.9 | 5,220.7 | 10,215.4 |
| Number of Non-Fatal IS Cases Avoided | 1,609.3 | 7,624.2 | 15,029.5 | 1,609.3 | 7,624.2 | 15,029.5 |
| Number of CVD Deaths Avoided | 645.9 | 3,084.6 | 6,102.2 | 645.9 | 3,084.6 | 6,102.2 |
| Total Annualized CVD Benefits (Million $2021) 2 | $99.73 | $459.09 | $908.82 | $72.72 | $362.42 | $717.85 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal MI Cases Avoided | 619.0 | 3,032.5 | 6,320.7 | 619.0 | 3,032.5 | 6,320.7 |
| Number of Non-Fatal IS Cases Avoided | 878.1 | 4,445.9 | 9,439.4 | 878.1 | 4,445.9 | 9,439.4 |
| Number of CVD Deaths Avoided | 343.8 | 1,806.7 | 3,835.8 | 343.8 | 1,806.7 | 3,835.8 |
| Total Annualized CVD Benefits (Million $2021) 2 | $51.00 | $268.78 | $571.32 | $41.85 | $212.18 | $450.51 |
3. Quantified Kidney Cancer Effects
Data on the association between PFOA exposure and kidney cancer ( i.e., RCC) are limited but suggest a positive association between exposure and increased risk of RCC. Epidemiology studies indicated that exposure to PFOA was associated with an increased risk of RCC (California Environmental Protection Agency, 2021; USEPA, 2016e; ATSDR, 2021; USEPA, 2023b). In the PFOA HESD (USEPA, 2016e), EPA characterized the evidence for PFOA effects on RCC as “probable” based on two occupational population studies (Raleigh et al., 2014; Steenland and Woskie, 2012) and two high-exposure community studies (Vieira et al., 2013; Barry et al., 2013). A recent study of the relationship between PFOA and RCC in U.S. general populations found strong evidence that exposure to PFOA causes RCC in humans (Shearer et al., 2021). As such, EPA selected RCC as a key outcome when assessing the health impacts of reduced PFOA exposures.
EPA quantified and valued the changes in RCC risk associated with reductions in serum PFOA levels that are in turn associated with reductions in drinking water PFOA concentrations under the regulatory alternatives. PWS entry point-specific time series of the differences between serum PFOA concentrations under baseline and regulatory alternatives are inputs into this analysis. For each PWS entry point, evaluation of the changes in RCC impacts involves the following key steps:
1. Estimating the changes in RCC risk based on modeled changes in serum PFOA levels and the exposure-response function for the effect of serum PFOA on RCC;
2. Estimating the annual incidence of RCC cases and excess mortality among those with RCC in all populations corresponding to baseline and regulatory alternative RCC risk levels, as well as estimating the regulatory alternative-specific reduction in cases relative to the baseline, and
3. Estimating the economic value of reducing RCC mortality from baseline to regulatory alternative levels, using the Value of a Statistical Life and cost of illness measures, respectively.
To identify an exposure-response function, EPA reviewed three studies highlighted in the HESD for PFOA (USEPA, 2016e) and a recent study discussed in both the California Environmental Protection Agency's Office of Environmental Health Hazard Assessment (OEHHA) PFOA Public Health Goals report (California Environmental Protection Agency, 2021) and EPA's Proposed Maximum Contaminant Level Goal (MCLG) for PFOA (USEPA, 2023b). Steenland et al. (2015) observed an increase in kidney cancer deaths among workers with high exposures to PFOA. Vieira et al. (2013) found that kidney cancer was positively associated with high and very high PFOA exposures. Barry et al. (2013) found a slight trend in cumulative PFOA serum exposures and kidney cancer among the C8 Health Project population. In a large case-control general population study of the relationship between PFOA and kidney cancer in 10 locations across the U.S., Shearer et al. (2021) found strong evidence that exposure to PFOA causes RCC, the most common form of kidney cancer, in humans.
To evaluate changes between baseline and regulatory alternative RCC risk resulting from reduced exposure to PFOA, EPA relied on the estimated time series of changes in serum PFOA concentrations (Section 6.3) and the serum-RCC exposure-response function provided by Shearer et al. (2021): 0.00178 (ng/mL)-1. The analysis from Shearer et al. (2021) was designed as a case-control study with population controls based on 10 sites within the U.S. population. Shearer et al. (2021) included controls for age, sex, race, ethnicity, study center, year of blood draw, smoking, and hypertension. Results showed a strong and statistically significant association between PFOA and RCC. EPA selected the exposure-response relationship from Shearer et al. (2021) because it included exposure levels typical in the general population and was found to have a low risk of bias based on EPA's Proposed Maximum Contaminant Level Goal for PFOA (USEPA, 2023b).
The linear slope factor based on Shearer et al. (2021) enables estimation of the changes in lifetime RCC risk associated with reduced lifetime serum PFOA levels. Because baseline RCC incidence statistics are not readily available from the NCI public use data, EPA used kidney cancer statistics in conjunction with an assumption that RCC comprises 90% of all kidney cancer cases to estimate baseline lifetime probability of RCC (USEPA, 2023b). EPA estimated the baseline lifetime RCC incidence for males at 1.89% and the baseline lifetime RCC incidence for females at 1.05%. Details of these calculations are provided in Appendix H to USEPA (2023i).
Similar to its approach for estimating of CVD risk reductions, EPA relies on the life table approach to estimate RCC risk reductions. The outputs of the life table calculations are the PWS entry point-specific estimates of the annual change in the number of RCC cases and the annual change in excess RCC population mortality. For more detail on EPA's application of the life table to cancer benefits analyses, please see Appendix H to USEPA (2023j).
Although the change in PFOA exposure likely affects the risk of developing RCC beyond the end of the analysis period (the majority of RCC cases manifest during the latter half of the average individual lifespan; see Appendix H to USEPA (2023j), EPA does not capture effects after the end of the period of analysis, 2104. Individuals alive after the end of the period of analysis likely benefit from lower lifetime exposure to PFOA. Lifetime health risk model data sources include EPA SDWIS, age-, sex-, and race/ethnicity-specific population estimates from the U.S. Census Bureau (2020), the Surveillance, Epidemiology, and End Results (SEER) program database (Surveillance Research Program—National Cancer Institute, 202a; 2020b), and the Centers for Disease Control and Prevention (CDC) NCHS. Appendix H to USEPA (2023i) provides additional detail on the data sources and information used in this analysis as well as baseline kidney cancer statistics. Appendix B to USEPA (2023i) describes estimation of the affected population.
EPA uses the Value of a Statistical Life to estimate the benefits of reducing mortality associated with RCC in the population exposed to PFOA in drinking water. EPA uses the cost of illness-based valuation to estimate the benefits of reducing morbidity associated with RCC.
EPA used the medical cost information from a recent RCC cost-effectiveness study by Ambavane et al. (2020) to develop cost of illness estimates for RCC morbidity. Ambavane et al. (2020) used a discrete event simulation model to estimate the lifetime treatment costs of several RCC treatment sequences, which included first and second line treatment medication costs, medication administration costs, adverse effect management costs, and disease management costs on- and off-treatment. To this end, the authors combined RCC cohort data from CheckMate 214 clinical trial and recent US-based healthcare cost information assembled from multiple sources (see supplementary information from Ambavane et al. (2020)). Ambavane et al. (2020) found that RCC treatment sequences using a combination of two immunotherapy drugs as the first line medications were the most cost-effective.
Table 55 summarizes RCC morbidity cost of illness estimates derived by EPA using Ambavane et al. (2020)-reported disease management costs on- and off-treatment along with medication, administration, and adverse effect management costs for the first line treatment that initiated the most cost-effective treatment sequences as identified by Ambavane et al. (2020), i.e., the nivolumab/ipilimumab drug combination. This is a forward-looking valuation approach in that it assumes that the clinical practice would follow the treatment recommendations in Ambavane et al. (2020) and other recent studies cited therein. EPA notes that the second line treatment costs are not reflected in EPA's cost of illness estimates, because Ambavane et al. (2020) did not report information on the expected durations of the treatment-free interval (between the first line treatment discontinuation and the second line treatment initiation) and the second line treatment phase, conditional on survival beyond discontinuation of the second line treatment. As such, EPA valued RCC morbidity at $251,007 ($2021) during year 1 of the diagnosis, $190,969 ($2021) during year 2 of the diagnosis, and $1,596 ($2021) starting from year 3 of the diagnosis. Additionally, EPA assumed that for individuals with RCC who die during the specific year, the entire year-specific cancer treatment regimen is applied prior to the death event. This may overestimate benefits if a person does not survive the entire year.
| Time interval | First line medication ($2018) 1 | First line administration ($2018) 1 | First line adverse effect management ($2018) 1 3 | Disease management ($2018) 1 | Total ($2018) | Total ($2021) 4 |
|---|---|---|---|---|---|---|
| Notes: | ||||||
| 1 Ambavane et al. (2020) Table 1. | ||||||
| 2 Ambavane et al. (2020) p. 41, a maximum treatment duration assumption of 2 years. | ||||||
| 3 The adverse effect management costs of $1,868 in Ambavane et al. (2020) Table 1 were reported for the treatment duration. EPA used the treatment duration of 24 months ( i.e., 2 years) to derive monthly costs of $77.83. | ||||||
| 4 To adjust for inflation, EPA used U.S. BLS CPI for All Urban Consumers: Medical Care Services in U.S. (City Average). | ||||||
| 5 First line treatment induction. | ||||||
| 6 First line treatment maintenance. | ||||||
| 7 Treatment-free interval. | ||||||
| Monthly cost, month 1-3 from diagnosis 1 5 | 32,485 | 516 | 78 | 73 | 33,152 | 35,927 |
| Monthly cost, month 4-24 from diagnosis 2 6 | 13,887 | 647 | 78 | 73 | 14,685 | 15,914 |
| Monthly cost, month 25 from diagnosis 7 | 123 | 123 | 133 | |||
| Annual cost, year 1 from diagnosis | 222,438 | 7,371 | 934 | 878 | 231,621 | 251,007 |
| Annual cost, year 2 from diagnosis | 166,644 | 7,764 | 934 | 878 | 176,220 | 190,969 |
| Annual cost, year 3 from diagnosis | 1,473 | 1,473 | 1,596 | |||
Tables 56 to 59 provide the health effects avoided and valuation associated with RCC. EPA estimated that, over the evaluation period, the proposed rule will result in an average annual benefit from avoided RCC cases and deaths from $217 million ($2021, 7% discount rate) to $301 million ($2021, 3% discount rate).
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal RCC Cases Avoided | 1,313.6 | 6,872.0 | 17,387.8 | 1,313.6 | 6,872.0 | 17,387.8 |
| Number of RCC-Related Deaths Avoided | 308.7 | 1,927.8 | 5,049.3 | 308.7 | 1,927.8 | 5,049.3 |
| Total Annualized RCC Benefits (Million $2021) 2 | $54.23 | $300.56 | $758.03 | $45.36 | $217.37 | $515.89 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal RCC Cases Avoided | 1,289.6 | 6,753.3 | 17,147.8 | 1,289.6 | 6,753.3 | 17,147.8 |
| Number of RCC-Related Deaths Avoided | 300.5 | 1,895.2 | 4,960.4 | 300.5 | 1,895.2 | 4,960.4 |
| Total Annualized RCC Benefits (Million $2021) 2 | $52.92 | $295.53 | $744.64 | $45.09 | $213.78 | $508.56 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal RCC Cases Avoided | 1,017.6 | 5,681.7 | 14,962.1 | 1,017.6 | 5,681.7 | 14,962.1 |
| Number of RCC-Related Deaths Avoided | 235.9 | 1,602.1 | 4,317.6 | 235.9 | 1,602.1 | 4,317.6 |
| Total Annualized RCC Benefits (Million $2021) 2 | $42.28 | $250.60 | $643.71 | $36.32 | $182.24 | $446.80 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized total annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal RCC Cases Avoided | 433.5 | 2,903.0 | 8,205.4 | 433.5 | 2,903.0 | 8,205.4 |
| Number of RCC-Related Deaths Avoided | 101.1 | 831.8 | 2,406.2 | 101.1 | 831.8 | 2,406.2 |
| Total Annualized RCC Benefits (Million $2021) 2 | $18.58 | $131.44 | $367.38 | $17.34 | $97.30 | $260.54 |
4. Key Limitations and Uncertainties in the Benefits Analysis
The section below discusses the uncertainty information incorporated in the quantitative benefits analysis. There are additional sources of uncertainty and limitations that could not be modeled quantitatively as part of the national benefits analysis. These sources of uncertainty are characterized in detail in Section 6.8 of USEPA (2023j). This summary includes uncertainties that are specific to application of PK models for blood serum PFAS concentration estimation, developmental effects ( i.e., infant birth weight) modeling, CVD impacts modeling, RCC impacts modeling, and modeling of bladder cancer impacts from GAC treatment-related reductions in the sum of four trihalomethanes (THM4). Table 60 below presents the key limitations and uncertainties that apply to the benefits analysis for the proposed rule. EPA notes that in most cases it is not possible to judge the extent to which a particular limitation or uncertainty could affect the magnitude of the estimated benefits. Therefore, in each table below, EPA notes the potential direction of the impact on the quantified benefits ( e.g., a source of uncertainty that tends to underestimate quantified benefits indicates expectation for larger quantified benefits) but does not prioritize the entries with respect to the impact magnitude.
| Uncertainty/assumption | Effect on benefits estimate | Notes |
|---|---|---|
| EPA quantified benefits for three health endpoints for PFOA and PFOS | Underestimate | For various reasons, EPA has not quantified the benefit of removing PFOA and PFOS from drinking water for most of the health endpoints PFOA and PFOS are expected to impact. See discussion in section C for more information about these non quantifiable benefits. |
| EPA has only quantified benefits for one co-removed contaminant group (THM4) | Underestimate | Treatment technologies installed to remove PFAS can also removes numerous other contaminants, including other unregulated PFAS, additional regulated and unregulated DBPs, heavy metals, organic contaminants, pesticides, among others. These co-removal benefits may be significant, depending on co-occurrence, how many facilities install treatment and which treatment option they select. |
| EPA has not quantified benefits for any health endpoint for PFHxS, PFNA, PFBS, and HFPO-DA | Underestimate | PFHxS, PFNA, PFBS, and HFPO-DA each have substantial health impacts on multiple health endpoints. See discussion in section D for more information about these nonquantifiable benefits. |
| The analysis considers PFOA/PFOS concentrations from NTNCWSs | Overestimate | Some SDWIS population served estimates for NTNCWSs represent the both the population that has regular exposure to the NTNCWS' drinking water ( e.g., the employees at a location) and the peak day transient population ( e.g., customers) who have infrequent exposure to the NTNCWS' drinking water. Estimating the demographic distribution and the share of daily drinking water consumption for these two types of NTNCWS populations would be difficult across many of the industries which operate NTNCWSs. The inclusion of NTNCWS results is an overestimate of benefits because daily drinking water consumption for these populations is also modeled at their residential CWS. |
| EPA assumes that the effects of PFOA and PFOS exposures are independent | Uncertain | The exposure-response functions used in benefits analyses assume that the effects of serum PFOA/PFOS on the health outcomes considered are independent and therefore additive. Due to limited evidence, EPA does not consider synergies or antagonisms in PFOA/PFOS exposure-response. |
| The derivation of PFOA/PFOS exposure-response functions for the relationship between PFOA/PFOS serum and associated health outcomes assumes that there are no threshold serum concentrations below which effects do not occur | Overestimate | The new data and EPA's proposed MCLGs indicate that the levels at which adverse health effects could occur are much lower than previously understood when EPA issued the 2016 health advisories for PFOA and PFOS (70 parts per trillion or ppt)—including near zero for certain health effects. Therefore, the exposure-response functions used in benefits analyses assume that there are no threshold serum concentrations below which effects do not occur. This could result in a slight overestimate of benefits for certain health endpoints. |
| The exposure-response functions used to estimate risk assume causality | Overestimate | Analyses evaluating the evidence on the associations between PFAS exposure and health outcomes are ongoing and EPA has not conclusively determined causality. As described in Section 6.2, EPA modeled health risks from PFOA/PFOS exposure for endpoints for which the evidence of association was found to be likely. These endpoints include birth weight, total cholesterol, and RCC. While the evidence supporting causality between DBP exposure and bladder cancer has increased since EPA's Stage 2 DBP Rule (NTP, 2021; Weisman et al., 2022), causality has not yet been conclusively determined (Regli et al., 2015). |
| The analysis assumes that quantified benefits categories are additive | Uncertain | EPA did not model birth weight, CVD, RCC, and bladder cancer benefits jointly, in a competing risk framework. Therefore, reductions in health risk in a specific benefits category do not influence health risk reductions in another benefits category. For example, lower risk of CVD and associated mortality implies a larger population that could benefit from cancer risk reductions, because cancer incidence grows considerably later in life. |
| The analysis does not take into account population growth and other changes in long-term trends | Underestimate | The benefits analysis does not reflect the effects of growing population that may benefit from reduction in PFOA/PFOS exposure. Furthermore, EPA uses present-day information on life expectancy, disease, environmental exposure, and other factors, which are likely to change in the future. |
| For PWSs with multiple entry points, the analysis assumes a uniform population distribution across the entry points | Uncertain | Data on the populations served by each entry point are not available and EPA therefore uniformly distributes system population across entry points. Effects of the regulatory alternative may be greater or smaller than estimated, depending on actual populations served by affected entry points. For one large system serving more than one million customers EPA has sufficient data on entry point flow to proportionally assign effected populations. |
| EPA does not characterize uncertainty associated with the Value of Statistical Life (VSL) reference value or VSL elasticity | Uncertain | EPA did not quantitatively characterize the uncertainty for the VSL reference value and income elasticity. Because the economic value of avoided premature mortality comprises the majority of the overall benefits estimate, not considering uncertainty surrounding the VSL is a limitation. |
E. Nonquantifiable Benefits of PFOA and PFOS Exposure Reduction
In this section EPA qualitatively discusses the potential health benefits resulting from reduced exposure to PFOA and PFOS in drinking water. These nonquantifiable benefits are expected to be realized as avoided adverse health effects as a result of the proposed NPDWR, in addition to the benefits that EPA has quantified. EPA anticipates additional benefits associated with developmental, cardiovascular, liver, immune, endocrine, metabolic, reproductive, musculoskeletal, and carcinogenic effects beyond those benefits associated with decreased PFOA and PFOS that EPA has quantified. The evidence for these adverse health effects is briefly summarized below.
EPA identified a wide range of potential health effects associated with exposure to PFOA and PFOS using five comprehensive Federal government documents that summarize the recent literature on PFAS (mainly PFOA and PFOS) exposure and its health impacts: EPA's Health Effects Support Documents for PFOA and PFOS, hereafter referred to as EPA HESDs (USEPA, 2016e; USEPA, 2016f); EPA's Proposed Maximum Contaminant Level Goals for PFOA and PFOS in Drinking Water (USEPA, 2023b; USEPA, 2023c); and the U.S. Department of Health and Human Services Agency for Toxic Substances and Disease Registry's (ATSDR) Toxicological Profile for Perfluoroalkyls (ATSDR, 2021). Each source presents comprehensive literature reviews on adverse health effects associated with PFOA and PFOS. EPA notes that the National Academies of Science, Engineering, and Medicine also published a report which includes a review of the adverse health effects for numerous PFAS (NASEM 2022). That document is included in the docket for this proposed rulemaking.
The most recent literature reviews on PFAS exposures and health impacts, which are included in EPA's Proposed Maximum Contaminant Level Goal for PFOA and PFOS in Drinking Water (USEPA, 2023b; USEPA, 2023c), discuss the weight of evidence supporting associations between PFOA or PFOS exposure with health outcomes as indicative (likely), inadequate, or suggestive. For the purposes of the reviews conducted to develop the proposed MCLGs, an association is deemed indicative when findings are consistent and supported by substantial evidence. The association is inadequate if there is a lack of information or an inability to interpret the available evidence ( e.g., findings across studies). The association is suggestive if findings are consistent but supported by a limited number of studies or analyses, or only observed in certain populations or species. Note that these determinations are based on information available as of February 2022.
Developmental effects: Exposure to PFOA and PFOS during developmental life stages is linked to developmental effects including but not limited to the infant birth weight effects that EPA quantified. Other developmental effects include SGA, birth length, head circumference at birth, and other effects (Verner et al., 2015; USEPA, 2016e; USEPA, 2016f; Negri et al., 2017; ATSDR, 2021; Waterfield et al., 2020; USEPA, 2023b; USEPA, 2023c). SGA is a developmental health outcome of interest when studying potential effects of PFOA/PFOS exposure because SGA infants have increased health risks during pregnancy and delivery as well as post-delivery (Osuchukwu and Reed, 2022). Epidemiology evidence related to PFOA/PFOS exposure was mixed; some studies reported increased risk of SGA with PFOA/PFOS exposure, while other studies observed null results (USEPA, 2023b; USEPA, 2023c). For instance, some studies suggested a potentially positive association between PFOA exposure and SGA (Govarts et al., 2018; Lauritzen et al., 2017; Y. Wang et al., 2016; USEPA, 2023b). For PFOS, few patterns were discernible, and overall confidence of an association between the two factors was low (USEPA, 2023c). Similarly, ATSDR found no strong associations between PFOA or PFOS exposure and increases in risk of SGA infants (ATSDR, 2021). Toxicology studies on PFOS exposures in rodents reported effects on multiple developmental toxicity endpoints (including increased mortality, decreased BW and BW change, skeletal and soft tissue effects, and delayed eye-opening) (USEPA, 2023c). For additional details on developmental studies and their individual outcomes, see Chapter 3.4.1 (Developmental) in USEPA (2023b) and USEPA (2023c).
Cardiovascular effects: In addition to the CVD effects that EPA quantified associated with changes in total cholesterol and blood pressure from exposure to PFOA or PFOS (see Section 6.2 of USEPA (2023j)), available evidence suggests an association between exposure to PFOA or PFOS and increased LDLC (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). High levels of LDLC lead to the buildup of cholesterol in the arteries, which can raise the risk of heart disease and stroke. Epidemiology studies showed a positive association between PFOA or PFOS exposure and LDLC levels in children (USEPA, 2023b; USEPA, 2023c). In particular, the evidence suggested positive associations between serum PFOA and PFOS levels and LDLC levels in adolescents ages 12-18, while positive associations between serum levels and LDLC levels in younger children were observed only for PFOA (ATSDR, 2021). Studies conducted on PFOS showed evidence of an association between exposure and LDLC levels in adults. For instance, all five epidemiology studies evaluated in EPA's Proposed MCLGs for PFOA and PFOS in Drinking Water reported positive associations, although the association was only statistically significant in obese women. Available evidence regarding the impact of PFOA and PFOS exposure on pregnant women was too limited for EPA to determine an association (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). For additional details on LDLC studies and their individual outcomes, see Chapter 3.4.4 (Cardiovascular) in USEPA (2023b) and USEPA (2023c).
Liver effects: Several biomarkers can be used clinically to diagnose liver diseases, including the ALT. High levels of serum ALT may indicate liver damage. Epidemiology data provides consistent evidence of a positive association between PFOS/PFOA exposure and ALT levels in adults (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). Studies of adults showed consistent evidence of a positive association between PFOA exposure and elevated ALT levels at both high exposure levels and exposure levels typical of the general population (USEPA, 2023b). There is also consistent epidemiology evidence of associations between PFOS and elevated ALT levels, although the associations observed were not large in magnitude. Study results showed inconsistent evidence on whether the observed changes led to changes in specific liver disease (USEPA, 2023c).
Associations between PFOS/PFOA exposure and ALT levels in children were less consistent than in adults (USEPA, 2023b; USEPA, 2023c), and PFOA toxicology studies showed increases in ALT and other liver enzymes across multiple species, sexes, and exposure paradigms (USEPA, 2023b). Toxicology studies on the impact of PFOS exposure on ALT in rodents also reported increases in ALT and other liver enzyme levels in rodents, though these increases were modest (USEPA, 2023c). For additional details on the ALT studies and their individual outcomes, see Section 3.4.2 (Hepatic) in USEPA (2023b) and USEPA (2023c).
Immune effects: Proper antibody response helps maintain the immune system by recognizing and responding to antigens. Some evidence suggests a relationship between PFOA exposure and immunosuppression; epidemiology studies showed suppression of at least one measure of the antibody response for tetanus and diphtheria among people with higher prenatal, childhood, and adult serum concentrations of PFOA (USEPA, 2023b). It is less clear whether PFOA exposure impacts antibody response to vaccinations other than tetanus and diphtheria (ATSDR, 2021; USEPA, 2023b). Epidemiology evidence suggests that children with preexisting immunological conditions are particularly susceptible to immunosuppression associated with PFOA exposure (USEPA, 2023b). Available studies supported an association between PFOS exposure and immunosuppression in children, where increased PFOS serum levels were associated with decreased antibody production (USEPA, 2023c). However, the association between PFOS exposure and immunosuppression was not apparent in adults (USEPA, 2023c). 8 Other potential associations with PFOS exposure with a high degree of uncertainty included asthma and infectious diseases ( e.g., the common cold, lower respiratory tract infections, pneumonia, bronchitis, ear infections) (USEPA, 2023c). Animal toxicology study evidence suggested that PFOA or PFOS exposure results in effects similarly indicating immune suppression, such as reduced response of immune cells ( e.g., natural killer cell activity and immunoglobulin production) (USEPA, 2023b; USEPA, 2023c). For additional details on antibody studies and their individual outcomes, see Section 3.4.3 (Immune) in USEPA (2023b) and USEPA (2023c).
8 This may be due to the lack of high-quality data at present.
Endocrine effects: Elevated thyroid hormone levels can accelerate metabolism and cause irregular heartbeat; low levels of thyroid hormone can cause neurodevelopmental effects, tiredness, weight gain, and increased susceptibility to the common cold. There is suggestive evidence of a positive association between PFOA/PFOS exposure and thyroid hormone disruption (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). Epidemiology studies reported inconsistent evidence regarding associations between PFOA or PFOS exposure and general endocrine outcomes, such as thyroid disease, hypothyroidism, and hypothyroxinemia (USEPA, 2023b; USEPA, 2023c). However, studies reported suggestive evidence of positive associations for thyroid stimulating hormone (TSH) in adults, and the thyroid hormone thyroxine (T4) in children (USEPA, 2023b; USEPA, 2023c). Toxicology studies indicated that PFOA and PFOS exposure leads to decreases in thyroid hormone levels 9 and adverse effects to the endocrine system (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). Despite uncertainty around the applicability of animal studies in this area, changes in thyroid hormone levels in animals did indicate adverse effects after PFOS and PFOA exposure that is relevant to humans (USEPA, 2023b; USEPA, 2023c). For additional details on endocrine effects studies and their individual outcomes, see Chapter C.2 (Endocrine) in USEPA (2023k) and USEPA (2023l).
9 Decreased thyroid hormone levels are associated with effects such as changes in thyroid and adrenal gland weight, hormone fluctuations, and organ histopathology (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c).
Metabolic effects: Leptin is a hormone that controls hunger, and high leptin levels are associated with obesity, overeating, and inflammation ( e.g., of adipose tissue, the hypothalamus, blood vessels, and other areas). Evidence suggests a direct association between PFOA exposure and leptin levels in the general adult population (ATSDR, 2021; USEPA, 2023b). Based on a review of 69 human epidemiology studies, evidence of associations between PFOS and metabolic outcomes appears inconsistent, but in some studies, suggestive evidence was observed between PFOS exposure and leptin levels (USEPA, 2023c). Studies examining newborn leptin levels did not find associations with maternal PFOA levels (ATSDR, 2021). Maternal PFOS levels were also not associated with alterations in leptin levels (ATSDR, 2021). For additional details on metabolic effect studies and their individual outcomes, see Chapter C.3 (Metabolic/Systemic) in USEPA (2023k) and USEPA (2023l).
Reproductive effects: Studies of the reproductive effects from PFOA/PFOS exposure have focused on associations between exposure to these pollutants and increased risk of gestational hypertension and preeclampsia in pregnant women (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). Gestational hypertension (high blood pressure during pregnancy) can lead to fetal health outcomes such as poor growth and stillbirth. Preeclampsia—instances of gestational hypertension where the mother also has increased levels of protein in her urine—can similarly lead to fetal problems and maternal complications. The epidemiology evidence yields mixed (positive and non-significant) associations, with some suggestive evidence supporting positive associations between PFOA/PFOS exposure and both preeclampsia and gestational hypertension (ATSDR, 2021; USEPA, 2023b; USEPA, 2023c). For additional details on reproductive effects studies and their individual outcomes, see Chapter C.1 (Reproductive) in USEPA (2023k) and USEPA (2023l).
Musculoskeletal effects: Adverse musculoskeletal effects such as osteoarthritis and decreased bone mineral density impact bone integrity and cause bones to become brittle and more prone to fracture. There is limited evidence from studies pointing to effects of PFOS on skeletal size (height), lean body mass, and osteoarthritis (USEPA, 2023c). Epidemiology evidence suggested that PFOA exposure may be linked to decreased bone mineral density, bone mineral density relative to bone area, height in adolescence, osteoporosis, and osteoarthritis (ATSDR, 2021; USEPA, 2023b). Evidence from four PFOS studies suggests that PFOS exposure has a harmful effect on bone health, particularly measures of bone mineral density, with greater statistically significance of effects occurring among females (USEPA, 2023c). Some studies found that PFOA/PFOS exposure was linked to osteoarthritis, in particular among women under 50 years of age (ATSDR, 2021). However, other reviews reported mixed findings on the effects of PFOS exposure including decreased risk of osteoarthritis, increased risk for some demographic subgroups, or no association (ATSDR, 2021). For additional details on musculoskeletal effects studies and their individual outcomes, see Chapter C.8 (Musculoskeletal) in USEPA (2023k) and USEPA (2023l).
Cancer Effects: In EPA's Proposed Maximum Contaminant Level Goal for PFOA in Drinking Water, the Agency evaluates the evidence for carcinogenicity of PFOA that has been documented in both epidemiological and animal toxicity studies (USEPA, 2023b). The evidence in epidemiological studies is primarily based on the incidence of kidney and testicular cancer, as well as some evidence of breast cancer, which is most consistent in genetically susceptible subpopulations. Other cancer types have been observed in humans, although the evidence for these is generally limited to low confidence studies. The evidence of carcinogenicity in animal models is provided in three chronic oral animal bioassays in Sprague-Dawley rats which identified neoplastic lesions of the liver, pancreas, and testes (USEPA, 2023b). EPA determined that PFOA is Likely to Be Carcinogenic to Humans, as “the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor Carcinogenic to Humans.” This determination is based on the evidence of kidney and testicular cancer in humans and LCTs, PACTs, and hepatocellular adenomas in rats (USEPA, 2023b). EPA's benefits analysis for avoided RCC cases from reduced PFOA exposure is discussed in Section XII.D of this preamble and in Section 6.6 of USEPA (2023j).
In EPA's Proposed Maximum Contaminant Level Goal for PFOS in Drinking Water, the Agency evaluates the evidence for carcinogenicity of PFOS and concluded that several epidemiological studies and a single chronic cancer bioassay comprise the evidence database for the carcinogenicity of PFOS (USEPA, 2023c). The available epidemiology studies report elevated risk of bladder, prostate, kidney, and breast cancers after chronic PFOS exposure. However, in developing this proposal, EPA did not identify information to quantify the benefits that reducing PFOS would have on reducing various cancers in humans. The sole animal chronic cancer bioassay study provide support for multi-site tumorigenesis in male and female rats. EPA reviewed the weight of the evidence and determined that PFOS is Likely to Be Carcinogenic to Humans, as “the evidence is adequate to demonstrate carcinogenic potential to humans but does not reach the weight of evidence for the descriptor Carcinogenic to Humans.”
EPA anticipates there are additional nonquantifiable benefits related to potential testicular, bladder, prostate, kidney, and breast carcinogenic effects summarized above. For additional details on cancer studies and their individual outcomes, see Chapter 3.5 (Cancer) in USEPA (2023b) and USEPA (2023c).
After assessing the available health and economic information, EPA was unable to quantify the benefits of avoided health effects discussed above. The Agency prioritized health endpoints with the strongest weight of evidence conclusions for this assessment and readily available data for monetization, namely cardiovascular effects, developmental effects, and carcinogenic effects. Several other health endpoints that had indicative evidence of associations with exposure to PFOA or PFOS have not been selected for the Economic Analysis for the reasons below.
• While immune effects had indicative evidence of associations with exposure to PFOA or PFOS, EPA did not identify the necessary information to connect the measured biomarker responses ( i.e., decrease in antibodies) to a clinical effect that could be valued in the Economic Analysis;
• Evidence indicates associations between PFOA and PFOS exposure and hepatic effects, such as increases in ALT. However, EPA is not able to model this health endpoint because ALT is a non-specific biomarker. Similar challenges with non-specificity of the biomarkers representing metabolic effects ( i.e., leptin) and musculoskeletal effects ( i.e., bone density) prevented economic analysis of these endpoints;
• There is indicative evidence of association with exposure to PFOA for testicular cancer; however, the available slope factor implied small changes in the risk of this endpoint. Furthermore, testicular cancer is rarely fatal which implies low expected economic value of reducing this risk because Value of Statistical Life is the driver of economic benefits evaluated in the Economic Analysis;
• Finally, other health endpoints, such as SGA and LDLC effects, were not modeled in the Economic Analysis because they overlap with effects that EPA did model. For example, infants that are considered SGA are often born at low birth weight or receive similar care to infants born at low birth weight. LDLC is a component of total cholesterol and could not be modeled separately as EPA used total cholesterol as an input to the ASCVD model to estimate CVD outcomes.
F. Nonquantifiable Benefits of Removal of PFAS Included in the Proposed Regulation and Co-Removed PFAS
EPA also qualitatively summarized the potential health benefits resulting from reduced exposure to PFAS other than PFOA and PFOS in drinking water. The proposed option and all regulatory alternatives are expected to result in benefits that have not been quantified. Treatment responses implemented to reduce PFOA and PFOS exposure under the proposed option and Options 1a-c are likely to remove some amount of additional PFAS contaminants where they co-occur. Co-occurrence among PFAS compounds has been observed frequently as discussed in Section VII of this preamble and USEPA (2023e). The proposed option will require reduced exposure to PFHxS, HFPO-DA, PFNA, and PFBS to below their respective HBWCs. EPA also expects that compliance actions taken under the proposed rule will remove additional unregulated co-occurring PFAS contaminants where present because the BATs have been demonstrated to co-remove additional PFAS (see Section XI of this preamble for more information). EPA identified a wide range of potential health effects associated with exposure to PFAS compounds other than PFOA and PFOS using documents that summarize the recent literature on exposure and associated health impacts: ATSDR's Toxicology Profile for Perfluoroalkyls (ATSDR, 2021); EPA's summary of HFPO-DA toxicity (USEPA, 2021b); publicly available draft IRIS assessments for PFBA, and PFHxA (USEPA, 2021k; USEPA, 2022h); a human health assessment for PFBS (USEPA, 2021a); and the recent National Academies of Sciences, Engineering, and Medicine Guidance on PFAS Exposure, Testing, and Clinical Follow-up (NASEM, 2022). Note that the determinations of associations between PFAS compounds and associated health effects are based on information available as of May 2022, and that the finalization of the IRIS assessments may result in slight changes to the discussion of evidence. Additional discussion of the evidence from epidemiology and toxicology studies for associations between different categories of health effects and exposure to additional PFAS can be found in Section 6.2 of USEPA (2023j).
Developmental effects: Toxicology and/or epidemiology studies observed evidence of associations with decreased birth weight and/or other developmental effects and exposure to PFBA, perfluorodecanoic acid (PFDA), PFHxS, HFPO-DA, PFNA, and PFBS. Specifically, data from animal toxicological studies support this association for PFBS, PFBA, and HFPO-DA while both animal toxicological and epidemiological studies support this association for PFDA and PFNA (ATSDR 2021) although some mixed results have been found for birth outcomes, particularly birth weight. In general, epidemiological studies did not find associations between perfluoroalkyl exposure and adverse pregnancy outcomes (miscarriage, preterm birth, or gestational age) for PFHxS, PFNA, PFDA, or perfluoroundecanoic acid (PFUnA) (ATSDR, 2021; NASEM, 2022).
Cardiovascular effects: Epidemiology and toxicology studies observed evidence of associations between PFNA or PFDA exposures and total cholesterol, LDLC, and HDLC. Evidence for associations between PFNA exposure and serum lipids levels in epidemiology studies was mixed; associations have been observed between serum PFNA levels and total cholesterol in general populations of adults but not in pregnant women, and evidence in children is inconsistent (ATSDR, 2021). Most epidemiology studies did not observe associations between PFNA and LDLC or HDLC (ATSDR, 2021).
Similarly inconsistent evidence was observed for PFDA (ATSDR, 2021). Other PFAS for which lipid outcomes were examined in toxicology or epidemiology studies observed limited to no evidence of associations. Studies have examined possible associations between various PFAS and blood pressure in humans or heart histopathology in animals. However, studies did not find suggestive or likely evidence for any PFAS in this summary except for PFOS.
Hepatic effects: Toxicology studies reported associations between exposure to PFAS compounds (PFBA, PFDA, PFHxA, PFHxS, HFPO-DA, and PFBS) and hepatotoxicity following inhalation, oral, and dermal exposure in animals. The results of these studies provide strong evidence that the liver is a sensitive target of PFHxS, PFNA, PFDA, PFUnA, PFBS, PFBA, perfluorododecanoic acid (PFDoDA), and PFHxA toxicity. Observed effects in rodents include increases in liver weight, hepatocellular hypertrophy, hyperplasia, and necrosis (ATSDR, 2021; USEPA, 2021b; USEPA, 2022h). Increases in serum enzymes (such as ALT) and decreases in serum bilirubin were observed in one epidemiologic study of PFHxS, and mixed effects were observed for epidemiologic studies for PFNA (ATSDR, 2021).
Immune effects: Epidemiology studies have reported evidence of associations between PFDA and PFHxS exposure and antibody response to tetanus or diphtheria. There is also some limited evidence for decreased antibody response for PFNA, PFUnA, and PFDoDA, although many of the studies did not find associations for these compounds. There is limited evidence for associations between PFHxS, PFNA, PFDA, PFBS, and PFDoDA and increased risk of asthma due to the small number of studies evaluating the outcome and/or conflicting study results. The small number of studies investigating immunotoxicity in humans following exposure to PFHpA and PFHxA did not find associations (ATSDR, 2021). Toxicology studies have reported evidence of associations between HFPO-DA and immune-related endpoints in animals (USEPA, 2021b). No laboratory animal studies were identified for PFUnA, PFHpA, PFDoDA, or perfluorooctane sulfonamide (FOSA). A small number of toxicology studies evaluated the immunotoxicity of other perfluoroalkyls and most did not evaluate immune function. No alterations in spleen or thymus organ weights or morphology were observed in studies on PFHxS, PFBA, and PFDA. A study on PFNA found decreases in spleen and thymus weights and alterations in splenic lymphocyte phenotypes (ATSDR, 2021).
Endocrine effects: Epidemiology studies have observed associations between serum PFHxS, PFNA, PFDA, and PFUnA and TSH, triiodothyronine (T3), or thyroxine (T4) levels or thyroid disease, however the results are not consistent across studies and a large number of studies have not found associations (ATSDR, 2021; NASEM, 2022). Toxicology studies have reported associations with thyroid hormone disruption in animals for PFBA, PFHxA, and PFBS (USEPA, 2021a; 2021k; USEPA, 2022h).
Metabolic effects: Epidemiology and toxicology studies have examined possible associations between various PFAS and metabolic effects, including leptin, BW, or body fat in humans or animals (ATSDR, 2021). However, evidence of associations was not suggestive or likely for any PFAS in this summary except for PFOA. Evidence did not include changes such as BW gain, pup BW, or other developmentally focused weight outcomes (ATSDR, 2021; NASEM, 2022).
Renal effects: A small number of epidemiology studies with inconsistent results evaluated possible associations between PFHxS, PFNA, PFDA, PFBS, PFDoDA, or PFHxA and renal functions (including estimated glomerular filtration rate and increases in uric acid levels) (ATSDR, 2021; NASEM 2022). Toxicology studies have not observed impaired renal function or morphological damage following exposure to PFHxS, PFDA, PFUnA, PFBS, PFBA, PFDoDA, or PFHxA. Associations with kidney weight in animals were observed for HFPO-DA and PFBS (ATSDR, 2021; USEPA, 2021b; USEPA, 2021a).
Reproductive effects: A small number of epidemiology studies with inconsistent results evaluated possible associations between PFHxS, PFNA, PFUnA, PFDoDA, or PFHxA exposure and reproductive hormone levels (ATSDR, 2021). Some associations between PFAS (PFHxS, PFNA, or PFDA) exposures and sperm parameters have been observed. While there is suggestive evidence of an association between PFHxS or PFNA exposure and an increased risk of early menopause, this may be due to reverse causation since an earlier onset of menopause would result in a decrease in the removal of PFAS via menstrual blood. Epidemiological studies provide mixed evidence of impaired fertility (increased risks of longer time to pregnancy and infertility), with some evidence for PFHxS, PFNA, PFHpA, and PFBS but the results are inconsistent across studies or were only based on one study (ATSDR, 2021). Toxicology studies have evaluated the potential histological alterations in reproductive tissues, alterations in reproductive hormones, and impaired reproductive functions. No effect on fertility was observed for PFBS, PFHxS or PFDoDA, and no histological alterations were observed for PFBS, PFHxS and PFBA. One study found alterations in sperm parameters and decreases in fertility in mice exposed to PFNA, and one study for PFDoDA observed ultrastructural alterations in the testes (ATSDR, 2021).
Musculoskeletal effects: Epidemiology studies observed evidence of associations between PFNA or PFHxS and musculoskeletal effects including osteoarthritis and bone mineral density, but data are limited to two studies (ATSDR, 2021). Epidemiology studies reported limited to no evidence of associations between exposure to PFDA and musculoskeletal effects. Toxicology studies reported no morphological alterations in bone or skeletal muscle in animals exposed to PFBA, PFHxA, PFHxS, or PFBS (ATSDR, 2021).
Hematological effects: A single epidemiologic study reported on blood counts in pregnant Chinese women exposed to PFHxA and observed no correlations with any of the hematological parameters evaluated (total white blood cell counts, red blood cell (RBC) counts, and hemoglobin) (USEPA, 2022h). Epidemiological data were not identified for the other PFAS (ATSDR, 2021). A limited number of toxicology studies observed alterations in hematological indices following exposure to higher doses of PFHxS, PFDA, PFUnA, PFBS, PFBA, PFDoDA, or PFHxA (ATSDR, 2021). Toxicology studies observed evidence of association between HFPO-DA exposure and hematological effects including decreases in RBC number, hemoglobin, and percentage of RBCs in the blood (USEPA, 2021b).
Other non-cancer effects: A limited number of epidemiology and toxicology studies have examined possible associations between other PFAS and dermal, ocular, and other non-cancer effects. However, evidence of associations was not considered to be suggestive or likely for any PFAS compound in this summary except for PFOA and PFOS (ATSDR, 2021; USEPA, 2021a; USEPA, 2021k; USEPA, 2022h).
Cancer effects: A small number of epidemiology studies reported limited associations between exposure to multiple PFAS ( i.e., PFHxS, PFDA, PFUnA, and FOSA) and cancer effects. No consistent associations were observed for breast cancer risk for PFHxS, PFNA, PFHpA, or PFDoDA; increased breast cancer risks were observed for PFDA and FOSA, but this was based on a single study (Bonefeld-Jørgensen et al., 2014). No associations between exposure to PFHxS, PFNA, PFDA, or PFUnA, individually and prostate cancer risk were observed. However, among men with a first-degree relative with prostate cancer, associations were observed for PFHxS, PFDA, and PFUnA, but not for PFNA (ATSDR, 2021). Epidemiological studies examining potential cancer effects were not identified for PFBS, PFBA, or PFHxA (ATSDR, 2021). Aside from a study that suggested an increased incidence of liver tumors in rats exposed to high doses of HFPO-DA, toxicology studies reported no evidence of associations between exposure to PFDA or PFHxA and risk of cancer (ATSDR, 2021; USEPA, 2021b).
Coronavirus Disease 2019 (COVID-19): A cross-sectional study in Denmark (Grandjean et al., 2020) showed that PFBA exposure was associated with increasing severity of COVID-19, with an OR of 1.77 [95% Confidence Interval (CI): 1.09, 2.87] after adjustment for age, sex, sampling site, and interval between blood sampling and diagnosis. However, the study design does not allow for causal determinations.
A case-control study showed increased risk for COVID-19 infection with high urinary PFAS (including PFOA, PFOS, PFHxA, PFHpA, PFHxS, PFNA, PFBS, PFDA, PFUnA, PFDoDA, perfluorotridecanoic acid [PFTrDA], and perfluorotetradecanoic acid [PFTeDA]) levels (Ji et al., 2021). Adjusted odds ratios were 1.94 (95% CI: 1.39, 2.96) for PFOS, 2.73 (95% CI: 1.71, 4.55) for PFOA, and 2.82 (95% CI: 1.97, 3.51) for sum PFAS, while other PFAS were not significantly associated with COVID-19 susceptibility after adjusting for confounders.
In a spatial ecological analysis, Catelan et al. (2021) showed higher mortality risk for COVID-19 in a population heavily exposed to PFAS (including PFOA, PFOS, PFHxS, PFBS, PFBA, perfluoropentanoic acid [PFPeA], PFHxA, and PFHpA) via drinking water in Veneto, Italy. Overall, results may indicate a general immunosuppressive effect of PFAS and/or increased COVID-19 respiratory toxicity due to a concentration of PFBA in the lungs, however the study design precludes causal determinations.
Although these studies provide a suggestion of possible associations, the body of evidence does not permit any conclusions about the relationship between COVID-19 infection, severity, or mortality, and exposures to PFAS.
G. Benefits Resulting From Disinfection By-Product Co-Removal
As part of its health risk reduction and cost analysis, EPA is directed by SDWA to evaluate quantifiable and nonquantifiable health risk reduction benefits for which there is a factual basis in the rulemaking record to conclude that such benefits are likely to occur from reductions in co-occurring contaminants that may be attributed solely to compliance with the MCL (SDWA 1412(b)(3)(C)(II)). These co-occurring contaminants are expected to include additional PFAS contaminants not directly regulated by the proposed PFAS NPDWR, co-occurring chemical contaminants such as SOCs, VOCs, and DBP precursors. In this section, EPA presents a quantified estimate of the reductions in DBP formation potential that are likely to occur as a result of compliance with the proposed PFAS NPDWR. The methodology detailed below and in Section 6.7.1 of USEPA (2023j) to estimate DBP reductions was externally peer reviewed by three experts in GAC treatment for PFAS removal and DBP formation potential (USEPA, 2023m). The external peer reviewers supported EPA's approach and edits based on their recommendations for clarity and completeness are reflected in the following analysis and discussion. Some peer reviewer comments suggested EPA provide additional baseline data summaries for TOC and THM4 occurrence information. EPA intends to evaluate and potentially include these additional summaries in the EA for the final rule.
DBPs are formed when disinfectants react with naturally occurring materials in water. There is a substantial body of literature on DBP precursor occurrence and THM4 formation mechanisms in drinking water treatment. EPA regulates 11 individual DBPs from three subgroups: THM4, five haloacetic acids (HAA5), and two inorganic compounds (bromate and chlorite) under the Stage 2 Disinfectants and Disinfection Byproducts Rule (USEPA, 2006a). The formation of THM4 in a particular drinking water treatment plant is a function of several factors including disinfectant type, disinfectant dose, bromide concentration, organic material type and concentration, temperature, pH, and system residence times. Epidemiology studies have shown that THM4 exposure, a surrogate for chlorinated drinking water, is associated with an increased risk of bladder cancer, among other diseases (Cantor et al., 1998; Cantor et al., 2010; Costet et al., 2011; Beane Freeman et al., 2017; King and Marrett, 1996; Regli et al., 2015; USEPA, 2019d; Villanueva et al., 2004; Villanueva et al., 2006; Villanueva et al., 2007). These studies considered THM4 as surrogate measures for DBPs formed from the use of chlorination that may co-occur. The relationships between exposure to DBPs, specifically THM4 and other halogenated compounds resulting from water chlorination, and bladder cancer are further discussed in Section 6.7 of USEPA (2023j). Reductions in exposure to THM4 is expected to yield public health benefits, including a decrease in bladder cancer incidence (Regli et al., 2015). Among other things, Weisman et al. (2022) found that there is even a stronger weight of evidence linking DBPs and bladder cancer since the promulgation of the 2006 Stage 2 DBP regulations and publication of Regli et al. (2015). While not the regulated contaminant for this rulemaking, the expected reduction of DBP precursors and subsequent DBPs that result from this rulemaking are anticipated to reduce cancer risk in the U.S. population.
GAC adsorption has been used to remove SOCs, taste and odor compounds, and NOM during drinking water treatment (Chowdhury et al., 2013). Recently, many water utilities have installed or are considering installing GAC and/or other advanced technologies as a protective or mitigation measure to remove various contaminants of emerging concern, such as PFAS (Dickenson and Higgins, 2016). Because NOM often exists in a much higher concentration (in mg/L) than trace organics (in μg/L or ppt) in water, NOM, often measured as TOC, can interfere with the adsorption of trace organics by outcompeting the contaminants for adsorption sites and by general fouling (blockage of adsorption pores) of the GAC.
NOM and inorganic matter are precursors for the formation of trihalomethanes (THMs) and other DBPs when water is disinfected using chlorine and other disinfectants to control microbial contaminants in finished drinking water. Removal of DBP precursors through adsorption onto GAC has been included as a treatment technology for compliance with the existing DBP Rules and is a BAT for the Stage 2 DBP Rule. DOM can be removed by GAC through adsorption and biodegradation (Crittenden et al., 1993; Kim et al., 1997; Yapsakli et al., 2010). GAC is well-established for removal of THM and haloacetic acid precursors (Cheng et al., 2005; Dastgheib et al., 2004; Iriarte-Velasco et al., 2008; Summers et al., 2013; Cuthbertson et al., 2019; L. Wang et al., 2019). In addition to removal of organic DBPs, GAC also exhibits some capacity for removal of inorganic DBPs such as bromate and chlorite (Kirisits et al., 2000; Sorlini et al., 2005) and removal of preformed organic DBPs via adsorption and biodegradation (Jiang, et al., 2017; Terry and Summers, 2018). Further, GAC may offer limited removal of dissolved organic nitrogen (Chili et al., 2012).
Based on an extensive review of published literature in sampling studies where both contaminant groups (PFAS and DBPs) were sampled, there is limited information about PFAS removal and co-occurring reductions in DBPs, specifically THMs. To help inform its Economic Analysis, EPA relied on the DBP Information Collection Rule Treatment Study Database and DBP formation studies to estimate reductions in THM4 (ΔTHM4) that may occur when GAC is used to remove PFAS. Subsequently, these results were compared to THM4 data from PWSs that have detected PFAS and have indicated use of GAC.
The objective of EPA's co-removal benefits analysis was to determine the reduction in bladder cancer cases associated with the decrease of regulated THM4 in treatment plants due to the installation of GAC for PFAS removal. Evaluation of the expected reductions in bladder cancer risk resulting from treatment of PFAS in drinking water involves five steps:
1. Estimating the number of systems expected to install GAC treatment in compliance with the proposed PFAS NPDWR and affected population size;
2. Estimating changes in THM4 levels that may occur when GAC is installed for PFAS removal based on influent TOC levels;
3. Estimating changes in the cumulative risk of bladder cancer using an exposure-response function linking lifetime risk of bladder cancer to THM4 concentrations in residential water supply (Regli et al., 2015);
4. Estimating annual changes in the number of bladder cancer cases and excess mortality in the bladder cancer population corresponding to changes in THM4 levels under the regulatory alternative in all populations alive during or born after the start of the evaluation period; and
5. Estimating the economic value of reducing bladder cancer mortality from baseline to regulatory alternative levels, using the Value of a Statistical Life and cost of illness measures, respectively.
EPA expects PWSs that exceed the PFAS MCLs to consider both treatment and non-treatment options to achieve compliance with the drinking water standard. EPA assumes that the populations served by systems with entry points expected to install GAC based on the compliance forecast detailed in Section 5.3 of USEPA (2023j) will receive the DBP exposure reduction benefits. EPA notes that other compliance actions included in the compliance forecast could result in DBP exposure reductions, including installation of RO. However, these compliance actions are not included in the DBP benefits analysis because this DBP exposure reduction function is specific to GAC. Switching water sources may or may not result in DBP exposure reductions, therefore EPA assumed no additional DBP benefits for an estimated percentage of systems that elect this compliance option. Lastly, EPA assumed no change in DBP exposure at water systems that install IX, as that treatment technology is not expected to remove a substantial amount of DBP precursors. EPA also assumes that the PWSs in this analysis use chlorine only for disinfection and have conventional treatment in place prior to installation of GAC technology.
EPA used the relationship between median raw water TOC levels and changes in THM4 levels estimated in the 1998 DBP Information Collection Rule to estimate changes in THM4 concentrations in the finished water of PWSs fitted with GAC treatment. For more detail on the approach EPA used to apply changes in THM4 levels to PWSs treating for PFAS under the proposed rule, please see Section 6.7 of USEPA (2023j).
EPA models a scenario where reduced exposures to THM4 begin in 2026. Therefore, EPA assumed that the population affected by reduced THM4 levels resulting from implementation of GAC treatment is exposed to baseline THM4 levels prior to actions to comply with the rule ( i.e., prior to 2026) and to reduced THM4 levels from 2026 through 2104. Rather than modeling individual locations, EPA evaluates changes in bladder cancer cases among the aggregate population per treatment scenario and source water type that is expected to install GAC treatment to reduce PFAS levels. Because of this aggregate modeling approach, EPA used national-level population estimates to distribute the SDWIS populations based on single-year age and sex and to grow the age- and sex-specific populations to future years. Appendix B to USEPA (2023j) provides additional details on estimation of the affected population.
Regli et al. (2015) analyzed the potential lifetime bladder cancer risks associated with increased bromide levels in surface source water resulting in increased THM4 levels in finished water. To account for variable levels of uncertainty across the range of THM4 exposures from the pooled analysis of Villanueva et al. (2004), they derived a weighted mean slope factor from the odds ratios reported in Villanueva et al. (2004). They showed that, while the original analysis deviated from linearity, particularly at low concentrations, the overall pooled exposure-response relationship for THM4 could be well-approximated by a linear slope factor that predicted an incremental lifetime cancer risk of 1 in ten thousand exposed individuals (10-4) per 1 µg/L increase in THM4. The linear slope factor developed by Regli et al. (2015) enables estimation of the changes in the lifetime bladder cancer risk associated with lifetime exposures to reduced THM4 levels. Weisman et al. (2022) applied the dose-response information from Regli et al. (2015) and developed a robust, national-level risk assessment of DBP impacts, where the authors estimated that approximately 8,000 of 79,000 annual U.S. bladder cancer cases are attributable to chlorination DBPs, specifically associated with THM4 concentrations.
EPA estimated changes in annual bladder cancer cases and annual excess mortality in the bladder cancer population due to estimated reductions in lifetime THM4 exposure using a life table-based approach. This approach was used because (1) annual risk of new bladder cancer should be quantified only among those not already experiencing this chronic condition, and (2) bladder cancer has elevated mortality implications.
EPA used recurrent life table calculations to estimate a water source type-specific time series of bladder cancer incidence for a population cohort characterized by sex, birth year, and age at the beginning of the PFOA/PFOS evaluation period under the baseline scenario and the GAC regulatory alternative. The estimated risk reduction from lower exposure to DBPs in drinking water is calculated based on changes in THM4 levels used as inputs to the Regli et al. (2015)-based health impact function, described in more detail in Section 6.7 of USEPA (2023j). The life table analysis accounts for the gradual changes in lifetime exposures to THM4 following implementation of GAC treatment under the regulatory alternative compared to the baseline. The outputs of the life table calculations are the water source type-specific estimates of the annual change in the number of bladder cancer cases and the annual change in excess bladder cancer population mortality.
EPA uses the Value of a Statistical Life to estimate the benefits of reducing mortality associated with bladder cancer in the affected population. EPA uses the cost of illness-based valuation to estimate the benefits of reducing morbidity associated with bladder cancer. Specifically, EPA used bladder cancer treatment-related medical care and opportunity cost estimates from Greco et al. (2019). Table 61 shows the original cost of illness estimates from Greco et al. (2019), along with the values updated to $2021 used in this analysis.
| Notes: | |||||
| 1 The estimates for non-invasive bladder cancer subtype were used to value local, regional, and unstaged bladder cancer morbidity reductions, while the estimates for the invasive bladder cancer subtype were used to value distant bladder cancer morbidity reductions. | |||||
| 2 The estimates come from Greco et al. (2019). | |||||
| 3 To adjust for inflation, EPA used U.S. BLS CPI for All Urban Consumers: Medical Care Services in U.S. (City Average). | |||||
| Bladder cancer subtype 1 | Type of cost | Cost in first year ($2010) 2 | Cost in subsequent years ($2010) 2 | Cost in first year ($2021) c | Cost in subsequent years ($2021) 3 |
| Non-invasive | Medical care | 9,133 | 916 | 12,350 | 1,239 |
| Total cost | 13,705 | 941 | 18,272 | 1,270 | |
| Invasive | Medical care | 26,951 | 2,455 | 36,445 | 3,320 |
| Opportunity cost | 10,513 | 77 | 13,616 | 100 | |
| Total cost | 37,463 | 2,532 | 50,061 | 3,420 | |
Table 62 to 65 presents the estimated changes in bladder cancer cases and excess bladder cancer mortality from exposure to THM4 due to implementation of GAC treatment by option. EPA estimated that, over the evaluation period, the proposed rule will result in an average annual benefit from avoided bladder cancer cases and deaths from $131 million ($2021, 7% discount rate) to $221 million ($2021, 3% discount rate).
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal Bladder Cancer Cases Avoided | 4,079.1 | 5,238.6 | 6,475.3 | 4,079.1 | 5,238.6 | 6,475.3 |
| Number of Bladder Cancer-Related Deaths Avoided | 1,436.0 | 1,844.4 | 2,280.0 | 1,436.0 | 1,844.4 | 2,280.0 |
| Total Annualized Bladder Cancer Benefits (Million $2021) 2 | $173.09 | $221.30 | $273.62 | $102.08 | $130.63 | $161.56 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal Bladder Cancer Cases Avoided | 4,066.1 | 5,219.4 | 6,488.8 | 4,066.1 | 5,219.4 | 6,488.8 |
| Number of Bladder Cancer-Related Deaths Avoided | 1,431.5 | 1,837.6 | 2,284.9 | 1,431.5 | 1,837.6 | 2,284.9 |
| Total Annualized Bladder Cancer Benefits (Million $2021) 2 | $171.72 | $220.48 | $274.24 | $101.34 | $130.15 | $161.56 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal Bladder Cancer Cases Avoided | 3,342.7 | 4,334.3 | 5,382.5 | 3,342.7 | 4,334.3 | 5,482.5 |
| Number of Bladder Cancer-Related Deaths Avoided | 1,176.8 | 1,526.0 | 1,895.3 | 1,176.8 | 1,526.0 | 1,895.3 |
| Total Annualized Bladder Cancer Benefits (Million $2021) 2 | $141.17 | $183.10 | $227.85 | $83.31 | $108.08 | $135.37 |
| Benefits category | 3% Discount rate | 7% Discount rate | ||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 72. This range does not include the uncertainty described in Table 60. | ||||||
| 2 See Table 70 for a list of the nonquantifiable benefits, and the potential direction of impact these benefits would have on the estimated monetized annualized benefits in this table. | ||||||
| 5th Percentile 1 | Expected benefits | 95th Percentile 1 | 5th Percentile 1 | Expected benefits | 95th Percentile 1 | |
| Number of Non-Fatal Bladder Cancer Cases Avoided | 1,615.9 | 2,175.5 | 2,807.4 | 1,615.9 | 2,175.5 | 2,807.4 |
| Number of Bladder Cancer-Related Deaths Avoided | 568.9 | 766.0 | 988.6 | 568.9 | 766.0 | 988.6 |
| Total Annualized Bladder Cancer Benefits (Million $2021) 2 | $68.26 | $91.90 | $118.64 | $40.29 | $54.25 | $70.10 |
H. Comparison of Costs and Benefits
This section provides a comparison of the costs and benefits of the proposed rule, as described in Chapter 7 of the Economic Analysis. Included here are estimates of total quantified annualized costs and benefits for the proposed option and regulatory alternatives considered, as well as considerations for the nonquantifiable costs and benefits. EPA notes that it cannot make determinations as to whether the costs are justified by the benefits based on quantified costs and benefits alone, as SDWA 1412(b)(3)(C)(I) and (II) mandates that the Agency must consider nonquantifiable benefits.
The incremental cost is the difference between quantified costs that will be incurred if the proposed rule is enacted over and above current baseline conditions. Incremental benefits reflect the avoided future adverse health outcomes attributable to PFAS reductions and co-removal of additional contaminants due to actions undertaken to comply with the proposed rule.
Table 66 provides the incremental quantified costs and benefits of the proposed option at both a 3 percent and a 7 percent discount rate in 2021 dollars. The top row shows total monetized annualized costs including total PWS costs and primacy agency costs. The second row shows total monetized annualized benefits including all endpoints that could be quantified and valued. For both, the estimates are the expected (mean) values and the 5th percentile and 95th percentile estimates from the uncertainty distribution. These percentile estimates come from the distributions of annualized costs and annualized benefits generated by the 4,000 iterations of SafeWater MCBC. Therefore, these distributions reflect the joint effect of the multiple sources of variability and uncertainty for costs, benefits, and PFAS occurrence, as detailed in Sections 5.1.2, 6.1.2, and Chapter 4 of the Economic Analysis, respectively (USEPA, 2023j). For further discussion of the quantified uncertainties in the Economic Analysis, see Section G of this preamble below.
The third row shows net benefits (benefits minus costs). At a 3 percent discount rate, the net annual incremental benefits are $461 million. The uncertainty range for net benefits is a negative $45 million to $1,141 million. At a 7 percent discount rate, the net annual incremental quantified benefits are a negative $297 million. The uncertainty range for net benefits is a negative $628 million to $141 million.
| 3% Discount rate | 7% Discount rate | |||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71 and Table 72. This range does not include the uncertainty described in Table 41 for costs and Table 60 for benefits. | ||||||
| 2 Total quantified national cost values do not include the incremental treatment costs associated with the cooccurrence of HFPO-DA, PFBS, and PFNA at systems required to treat for PFOA, PFOS, and PFHxS. The total quantified national cost values do not include treatment costs for systems that would be required to treat based on HI exceedances apart from systems required to treat because of PFHxS occurrence alone. See Appendix N, Section 3 of the Economic Analysis (USEPA, 2023i) for additional detail on co-occurrence incremental treatment costs and additional treatment costs at systems with HI exceedances. | ||||||
| 3 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 4 See Table 70 for a list of the nonquantifiable benefits and costs, and the potential direction of impact these benefits and costs would have on the estimated monetized total annualized benefits and costs in this table. | ||||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Total Annualized Rule Costs 2 3 4 | $704.53 | $771.77 | $850.40 | $1,106.01 | $1,204.61 | $1,321.01 |
| Total Annualized Rule Benefits 4 | 659.91 | 1,232.98 | 1,991.51 | 477.69 | 908.11 | 1,462.43 |
| Total Net Benefits | −44.62 | 461.21 | 1,141.11 | −628.31 | −296.50 | 141.42 |
Tables 67 to 69 summarize the total annual costs and benefits for Options 1a, 1b, and 1c, respectively.
| 3% Discount rate | 7% Discount rate | |||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71 and Table 72. This range does not include the uncertainty described in Table 41 for costs and Table 60 for benefits. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable benefits and costs, and the potential direction of impact these benefits and costs would have on the estimated monetized total annualized benefits and costs in this table. | ||||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Total Annualized Rule Costs 2 3 | $688.09 | $755.82 | $833.48 | $1,078.51 | $1,177.31 | $1,292.01 |
| Total Annualized Rule Benefits 3 | 651.19 | 1,216.08 | 1,971.01 | 471.53 | 895.36 | 1,456.23 |
| Total Net Benefits | −36.90 | 460.26 | 1,137.53 | −606.97 | −281.95 | 164.22 |
| 3% Discount rate | 7% Discount rate | |||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71 and Table 72. This range does not include the uncertainty described in Table 41 for costs and Table 60 for benefits. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable benefits and costs, and the potential direction of impact these benefits and costs would have on the estimated monetized total annualized benefits and costs in this table. | ||||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Total Annualized Rule Costs 2 3 | $558.71 | $611.01 | $674.32 | $864.74 | $942.28 | $1,035.56 |
| Total Annualized Rule Benefits 3 | 553.37 | 1,046.91 | 1,706.81 | 398.21 | 773.33 | 1,292.96 |
| Total Net Benefits | −5.34 | 435.90 | 1,032.49 | −466.53 | −168.95 | 257.40 |
| 3% Discount rate | 7% Discount rate | |||||
|---|---|---|---|---|---|---|
| Notes: | ||||||
| Detail may not add exactly to total due to independent rounding. | ||||||
| 1 The 5th and 95th percentile range is based on modeled variability and uncertainty described in section XIII.I of this preamble and Table 71 and Table 72. This range does not include the uncertainty described in Table 41 for costs and Table 60 for benefits. | ||||||
| 2 PFAS-contaminated wastes are not considered hazardous wastes at this time and therefore total costs reported in this table do not include costs associated with hazardous waste disposal of spent filtration materials. To address stakeholder concerns about potential costs for disposing PFAS-contaminated wastes as hazardous should they be regulated as such in the future, EPA conducted a sensitivity analysis with an assumption of hazardous waste disposal for illustrative purposes only. See Appendix N, Section 2 of the Economic Analysis (USEPA, 2023i) for additional detail. | ||||||
| 3 See Table 70 for a list of the nonquantifiable benefits and costs, and the potential direction of impact these benefits and costs would have on the estimated monetized total annualized benefits and costs in this table. | ||||||
| 5th Percentile 1 | Expected value | 95th Percentile 1 | 5th Percentile 1 | Expected value | 95th Percentile 1 | |
| Total Annualized Rule Costs 2 3 | $269.36 | $292.57 | $320.76 | $396.22 | $430.87 | $472.20 |
| Total Annualized Rule Benefits 3 | 280.42 | 584.80 | 1,030.56 | 208.71 | 436.24 | 784.59 |
| Total Net Benefits | 11.06 | 292.23 | 709.80 | −187.51 | 5.36 | 312.39 |
The benefit-cost analysis reported dollar figures presented above reflect benefits and costs that could be quantified for each regulatory alternative given the best available scientific data. EPA notes that the quantified benefit-cost results above are not representative of all benefits and costs anticipated under the proposed NPDWR. Due to occurrence, health, and economic data limitations, there are several adverse health effects associated with PFAS exposure and costs associated with treatment that EPA could not estimate in a quantitative manner.
PFAS exposure is associated with a wide range of adverse health effects including reproductive effects such as decreased fertility; increased high blood pressure in pregnant women; developmental effects or delays in children, including low birth weight, accelerated puberty, bone variations, or behavioral changes; increased risk of some cancers, including prostate, kidney, and testicular cancers; reduced ability of the body's immune system to fight infections, including reduced vaccine response; interference with the body's natural hormones; and increased cholesterol levels and/or risk of obesity. Based on the available data, EPA is only able to quantify three PFOA- and PFOS-related health endpoints in this analysis. All regulatory alternatives are expected to produce substantial benefits that have not been quantified. Treatment responses implemented to remove PFOA and PFOS under Options 1a-c are likely to remove some amount of additional PFAS contaminants where they co-occur. Co-occurrence among PFAS compounds has been observed frequently as discussed in the PFAS Occurrence Technical Support Document (USEPA, 2023e). The proposed option is expected to produce the greatest reduction in exposure to PFAS compounds because it includes PFHxS, HFPO-DA, PFNA, and PFBS in the regulation. Inclusion of the HI will trigger more systems into treatment (as shown in Section 4.4.4 of the Economic Analysis) and provides enhanced public health protection by ensuring reductions of these additional compounds when present above the HI of 1.0. EPA conducted a sensitivity analysis to evaluate the additional benefits anticipated due to regulating PFAS compounds beyond PFOA and PFOS. Specifically, EPA's sensitivity analysis demonstrates the potential significant quantified benefits associated with infant birth weight expected to result from reductions in PFNA under the proposed rule. For further discussion of the quantitative and qualitative benefits associated with the proposed rule, see Section 6.2 of the Economic Analysis.
EPA also expects that the proposed option will result in additional nonquantifiable costs in comparison to Options 1a-c. As noted above, the HI is expected to trigger more systems into more frequent monitoring and treatment. Due to occurrence data limitations, EPA has quantified the national treatment and monitoring costs associated with the HI for PFHxS only and has not quantified the cost impacts associated with HI exceedances resulting from HFPO-DA, PFNA, and PFBS. In instances when concentrations of HFPO-DA, PFNA, and PFBS are high enough to cause or contribute to an HI exceedance when the concentrations of PFOA, PFOS, and PFHxS would not have already otherwise triggered treatment, the modeled costs may be underestimated. If these PFAS occur in isolation at levels that affect treatment decisions, or if these PFAS occur in combination with PFHxS when PFHxS concentrations were otherwise below the HI in isolation ( i.e., <9.0 ppt) then the quantified costs underestimate the impacts of the proposed rule. As such, EPA conducted a semi-quantitative analysis of the anticipated incremental costs associated with regulating HFPO-DA, PFNA, and PFBS (for additional detail, please see USEPA (2023i)).
Table 70 provides a summary of the likely impact of nonquantifiable benefit-cost categories. In each case, EPA notes the potential direction of the impact on costs and/or benefits. For example, benefits are underestimated if the PFOA and PFOS reductions result in avoided adverse health outcomes that cannot be quantified and valued. Sections 5.7 and 6.8 of the Economic Analysis identify the key methodological limitations and the potential effect on the cost or benefit estimates, respectively. Additionally, Table 71 summarizes benefits and costs that are quantified and nonquantifiable under the proposed rule.
| Source | (Proposed option) | Option 1a | Option 1b | Option 1c |
|---|---|---|---|---|
| Nonquantifiable PFOA and PFOS health endpoints | B: underestimate | B: underestimate | B: underestimate | B: underestimate. |
| Limitations with available occurrence data for HFPO-DA, PFNA, and PFBS | C: underestimate | n/a | n/a | n/a. |
| Nonquantifiable HI (HFPO-DA, PFNA, PFHxS and PFBS) health endpoints | B: underestimate | n/a | n/a | n/a. |
| Limitations with available occurrence data for additional PFAS compounds | B C: underestimate | B C: underestimate | B C: underestimate | B C: underestimate. |
| Removal of co-occurring non-PFAS contaminants | B C: underestimate | B C: underestimate | B C: underestimate | B C: underestimate. |
| POU not in compliance forecast | C: overestimate | C: overestimate | C: overestimate | C: overestimate. |
| Unknown future hazardous waste management requirements for PFAS (including HI) | C: underestimate | C: underestimate | C: underestimate | C: underestimate. |
| Category | Quantified | Non-quantified | Methods (economic analysis report section where analysis is detailed) |
|---|---|---|---|
| Notes: | |||
| 1 Due to occurrence data limitations, EPA quantified the national treatment and monitoring costs associated with the HI for PFHxS only and has not quantified the national cost impacts associated with HI exceedances resulting from PFNA, PFBS, and HFPO-DA. | |||
| Costs: | |||
| PWS treatment costs 1 | X | Section 5.3.1. | |
| PWS sampling costs | X | Section 5.3.2.2. | |
| PWS implementation and administration costs | X | Section 5.3.2.1. | |
| Primacy agency rule implementation and administration costs | X | Section 5.3.2. | |
| Hazardous waste disposal for treatment media | X | Section 5.6. | |
| POU not in compliance forecast | X | Section 5.6. | |
| Benefits: | |||
| PFOA and PFOS birth weight effects | X | Section 6.4. | |
| PFOA and PFOS cardiovascular effects | X | Section 6.5. | |
| PFOA and PFOS RCC | X | Section 6.6. | |
| Health effects associated with disinfection byproducts | X | Section 6.7. | |
| Other PFOA and PFOS health effects | X | Section 6.2.2.2. | |
| Health effects associated with HI compounds (HFPO-DA, PFNA, PFBS, PFHxS) | X | Section 6.2. | |
| Health effects associated with other PFAS | X | Section 6.2. | |
I. Quantified Uncertainties in the Economic Analysis
EPA characterized sources of uncertainty in its estimates of costs expected to result from the proposed PFAS NPDWR. EPA conducted Monte-Carlo based uncertainty analysis as part of SafeWater MCBC. With respect to the cost analysis, EPA modeled the sources of uncertainty in Table 72.
| Source | Description of uncertainty |
|---|---|
| TOC concentration | The TOC value assigned to each system is from a distribution derived from the SYR4 ICR database (see Section 5.3.1.1 in Economic Analysis). |
| Compliance technology unit cost curve selection | Cost curve selection varies with baseline PFAS concentrations and also includes a random selection from a distribution across feasible technologies (see Section 5.3.1.2 in Economic Analysis), and random selection from a triangular distribution of low-, mid-, and high-cost equipment (25%, 50%, and 25%, respectively). |
For each iteration, SafeWater MCBC assigned new values to the four sources of modeled uncertainty as described in Table 72, and then calculated costs for each of the model PWSs. This was repeated 4,000 times to reach an effective sample size for each parameter. At the end of the 4,000 iterations, SafeWater MCBC outputs the expected value as well as the 90% confidence interval for each cost metric ( i.e., bounded by the 5th and 95th percentile estimates for each cost component). Detailed information on the data used to model uncertainty is provided in Appendix L to USEPA (2023i).
Additionally, EPA characterized sources of uncertainty in its analysis of potential benefits resulting from changes in PFAS levels in drinking water. The analysis reports uncertainty bounds for benefits estimated in each health endpoint category modeled for the proposed rule. Each lower (upper) bound value is the 5th (95th) percentile of the category-specific benefits estimate distribution represented by 4,000 Monte Carlo draws.
Table 73 provides an overview of the specific sources of uncertainty that EPA quantified in the benefits analysis. In addition to these sources of uncertainty, reported uncertainty bounds also reflect the following upstream sources of uncertainty: baseline PFAS occurrence, affected population size and demographic composition, and the magnitude of PFAS concentration reductions. These analysis-specific sources of uncertainty are further described in Appendix L to USEPA (2023i).
| Source | Description of uncertainty |
|---|---|
| Note: | |
| 1 The slope factors contributing to the CVD benefits analysis include the relationship between total cholesterol and PFOA and PFOS, the relationship between HDLC and PFOA and PFOS, and the relationship between blood pressure and PFOS. | |
| Health effect-serum PFAS slope factors | The slope factors that express the effects of serum PFOA and serum PFOS on health outcomes (birth weight, CVD, 1 and RCC) are based either on EPA meta-analyses or high-quality studies that provide a central estimate and a confidence interval for the slope factors. EPA assumed that the slope factors would have a normal distribution within their range. |
| RCC risk reduction cap | EPA implemented a cap on the cumulative RCC risk reductions due to reductions in serum PFOA based on the population attributable fraction (PAF) estimates for a range of cancers and environmental contaminants. This parameter is treated as uncertain; its uncertainty is characterized by a log-uniform distribution with a minimum set at the smallest PAF estimate identified in the literature and a maximum set at the largest PAF estimate identified in the literature. The central estimate for the PAF is the mean of this log-uniform distribution. |
J. Cost-Benefit Determination
When proposing an NPDWR, the Administrator shall publish a determination as to whether the benefits of the MCL justify, or do not justify, the costs based on the analysis conducted under paragraph 1412(b)(3)(C). With this proposed rule, the Administrator has determined that the quantified and nonquantifiable benefits of the proposed PFAS NPDWR justify the costs.
Sections XIII.A to XIII.I of this preamble summarize the results of this proposed rule analysis. As indicated in section XIII.H of this preamble, EPA discounted the estimated monetized cost and benefit values using both 3 and 7 percent discount rates. In Federal regulatory analyses, EPA follows OMB Circular A4 (OMB, 2003) guidance which recommends using both 3 percent and 7 percent is intended to account for the different streams of monetized benefits and costs affected by regulation. The 7 percent discount rate represents the estimated rate of return on capital in the U.S. economy, to reflect the opportunity cost of capital when “the main effect of a regulation is to displace or alter the use of capital in the private sector.” Regulatory effects, however, can fall on both capital and private consumption. 10 In 2003, Circular A-4 estimated the rate appropriate for discounting consumption effects at 3 percent. The estimated monetized costs and benefits of this rulemaking result in expected annual net benefits (total monetized annual benefits minus total monetized annual costs) of $461.21 million at a 3 percent discount rate and −$296.50 at a 7 percent discount rate. There are a variety of considerations with respect to the capital displacement in this particular proposal. For example, a meaningful number of PWSs may not be managed as profit-maximizing private sector investments, which could impact the degree to which the rate of return on the use of capital in the private sector applies to PWS costs. Federal funding is expected to defray many such PWS costs; 11 where that occurs, such costs are transferred to the government. Additionally, to the extent that the benefits extend over a long time period into the future, including to future generations, Circular A-4 advises agencies to consider conducting sensitivity analyses using lower discount rates. Regardless, the impacts in this rulemaking are such that costs are expected to occur in the nearer term, and in particular that larger one-time capital investments are expected to occur in the near term; and public health benefits are expected to occur over the much longer term. Discounting across an appropriate range of rates can help explore how sensitive net benefits are to assumptions about whether effects fall more to capital or more to consumption.
10 Private consumption is the consumption of goods and services by households for the direct satisfaction of individual needs (rather than for investment).
11 As noted above in this preamble, “Infrastructure Investment and Jobs Act, also referred to as the Bipartisan Infrastructure Law (BIL), invests over $11.7 billion in the Drinking Water State Revolving Fund (SRF); $4 billion to the Drinking Water SRF for Emerging Contaminants; and $5 billion to Small, Underserved, and Disadvantaged Communities Grants.”
EPA has followed Circular A-4's default recommendations to use 3 and 7 percent rates to represent the range of potential impacts accounting for diversity in stakeholders' time preferences. The Agency views the 3 to 7 percent range of costs and benefits as characterizing a significant portion of the uncertainty in the discount rate and views the quantified endpoint values as demonstrating a range of monetized costs and benefits which encompass a significant portion of the uncertainty associated with discount rates. Material unquantified benefits expected as a result of this proposed rulemaking are discussed in greater detail later in this section.
The quantified analysis is limited in its characterization of uncertainty. In Section XIII.H, Table 66 of this preamble, EPA provides 5th and 95th percentile values associated with the 3 and 7 percent discounted expected values for net benefits. These values represent the quantified, or modeled, potential range in the expected net benefit values associated with the variability in system characteristics and the uncertainty resulting from the following variables; the baseline PFAS occurrence; the affected population size; the compliance technology unit cost curves, which are selected as a function of baseline PFAS concentrations and population size, the distribution of feasible treatment technologies, and the three alternative levels of treatment capital costs; the concentration of TOC in a system's source water which impacts GAC O&M costs; the demographic composition of the systems population; the magnitude of PFAS concentration reductions; the health effect-serum PFOA and PFOS slope factors that quantify the relationship between changes in PFAS serum level and health outcomes for birth weight, CVD, and RCC; and the cap placed on the cumulative RCC risk reductions due to reductions in serum PFOA. These modeled sources of uncertainty are discussed in more detail in section XIII.I of this preamble. What the quantified 5th and 95th percentile values do not include are a number of factors which impact both costs and benefits but for which the Agency did not have sufficient data to include in the quantification of uncertainty. The factors influencing the proposed rule cost estimates that are not quantified in the uncertainty analysis are detailed in section XIII.C.j and Table 41 of this preamble. These uncertainty sources include: the specific design and operating assumptions used in developing treatment unit cost; the use of national average costs that may differ from the geographic distribution of affected systems; the possible future deviation from the compliance technology forecast; and the degree to which actual TOC source water values differ from EPA's estimated distribution. EPA has no information to indicate a directional influence of the estimated costs with regard to these uncertainty sources. To the degree that uncertainty exists across the remaining factors it would most likely influence the estimated 5th and 95th percentile range and not significantly impact the expected value estimate of costs. Section XIII.D and Table 60, of this preamble, discuss the sources of uncertainty affecting the estimated benefits not captured in the estimated 5th and 95th reported values. The modeled values do not capture the uncertainty in: the exposure that results from daily population changes at NTNCWSs or routine population shifting between PWSs, for example spending working hours at a NTNCWS or CWS and home hours at a different CWS; the exposure-response functions used in benefits analyses assume that the effects of serum PFOA/PFOS on the health outcomes considered are independent, additive, and that there are no threshold serum concentrations below which effects do not occur; the distribution of population by size and demographics across entry points within modeled systems and future population size and demographic changes; and the Value of Statistical Life reference value or income elasticity used to update the VSL. Given information available to the Agency four of the listed uncertainty sources would not affect the benefits expected value but the dispersion around that estimate. They are the unmodeled movements of populations between PWS which potentially differing PFAS concentrations; the independence and additivity assumptions with regard to the effects of serum PFOA/PFOS on the health outcomes; the uncertainty in the population and demographic distributions among entry points within individual systems; and the VSL value and the income elasticity measures. Two of the areas of uncertainty not captured in the analysis would tend to indicate that the quantified benefits numbers are overestimates. First, the data available to EPA with regard to population size at NTNCWs while likely capturing peaks in populations utilizing the systems does not account for the variation in use and population and would tend to overestimate the exposed population. The second uncertainty, which definitionally would indicate overestimates in the quantified benefits values is the assumption that there are no threshold serum concentrations below which health effects do not occur. One factor not accounted for in the quantified analysis associated with the underestimation of benefits is the impact of general population growth over the extended period of analysis.
In addition to the quantified cost and benefit expected values, the modeled uncertainty associated within the 5th and 95th percentile values, and the un-modeled uncertainty associated with a number of factors listed above, there are also significant nonquantifiable costs and benefits which are important to the overall weighing of costs and benefits. Table 70 provides a summary of these nonquantifiable cost and benefit categories along with an indication of the directional impact each category would have on total costs and benefit. Tables 41 and 60 also provide additional information on a number of these nonquantifiable categories.
On the nonquantifiable costs side of the equation EPA had insufficient nationally representative data to precisely characterize occurrence of HFPO-DA, PFNA, and PFBS at the national level and therefore could not include complete treatment costs associated with; the co-occurrence of these PFAS at systems already required to treat as a result of estimated PFOA, PFOS, or PFHxS levels, which would shorten the filtration media life and therefore increase operation costs; and the occurrence of HFPO-DA, PFNA, and/or PFBS at levels high enough to cause systems to exceed the HI and have to install PFAS treatment. EPA expects that the quantified national costs are marginally underestimated as a result of this lack of sufficient nationally representative occurrence data for purposes of model integration. In an effort to better understand the costs associated with treatment of potentially co-occurring HFPO-DA, PFNA, and PFBS at systems already required to treat and the potential costs resulting from an HI exceedance associated with the same chemicals EPA estimated the potential unit treatment costs for model systems under both scenarios for differing assumed HI PFAS concentrations. The analysis is discussed in section 5.3.1.4 and Appendix N of the Economic Analysis (USEPA, 2023j; USEPA, 2023i). Two additional nonquantifiable cost impacts stemming from insufficient co-occurrence data could also potentially shorten filtration media life and increase operation costs. The co-occurrence of other PFAS and other non-PFAS contaminants not regulated in the proposed rule could both increase costs to the extent that they reduce media life. EPA did not include POU treatment in the compliance technology forecast because current POU units are not certified to remove PFAS to the standards required in the proposed rule. Once certified this technology may be a low-cost treatment alternative for some subset of small systems. Not including POU treatment in this analysis has resulted in a likely overestimate of cost values. Appendix N of the Economic Analysis (USEPA, 2023j; USEPA, 2023i) contains a sensitivity analysis that estimates there may be a national annual costs of $30 to $61 million, discounted at 3 and 7 percent, respectively, which would accrue to systems if the waste filtration media from GAC and IX were handled as hazardous waste. This sensitivity analysis includes only disposal costs and does not consider other potential environmental costs associated with the disposal of the waste filtration media.
There are significant nonquantifiable sources of benefits that were not captured in the quantified benefits estimated for the proposed rule. While EPA was able to monetize some of the PFOA and PFOS benefits related to CVD, infant birthweight, and RCC effects, the Agency was unable to quantify additional negative health impacts. EPA did not quantify PFOA and PFOS benefits related to health endpoints including developmental, cardiovascular, hepatic, immune, endocrine, metabolic, reproductive, musculoskeletal, and other types of carcinogenic effects. See Section XIII.E, of this preamble, for additional information on the nonquantifiable impacts of PFOA and PFOS. Further, the Agency did not quantify any health endpoint benefits associated with the potential reductions in HI PFAS, which include PFHxS, HFPO-DA, PFNA, and PFBS, or other co-occurring non-regulated PFAS which would be removed by the installation of required filtration technology at those systems with PFOA, PFOS, or HI exceedances. The nonquantifiable benefits impact categories associated with PFHxS, HFPO-DA, and PFBS include developmental, cardiovascular, immune, hepatic, endocrine, metabolic, reproductive, musculoskeletal, and carcinogenic effects. In addition, EPA did not quantify the potential developmental, cardiovascular, immune, hepatic, endocrine, metabolic, reproductive, musculoskeletal, and carcinogenic impacts related to the removal of other co-occuring non-regulated PFAS. See Section XIII.F, of this preamble, for additional information on the nonquantifiable impacts of PFHxS, HFPO-DA, PFNA, and PFBS and other non-regulated co-occurring PFAS.
The treatment technologies installed to remove PFAS can also remove numerous other non-PFAS drinking water contaminants which have negative health impacts including additional regulated and unregulated DBPs (the quantified benefits assessment does estimate benefits associated with THM4), heavy metals, organic contaminants, and pesticides, among others. The removal of these co-occurring non-PFAS contaminants could have significant positive health benefits. In total these nonquantifiable benefits are anticipated to be significant and are discussed qualitatively in Section 6.2 of the Economic Analysis (USEPA, 2023j).
To fully weigh the costs and benefits of the action the Agency considered the totality of the monetized values, the potential impacts of the unquantified uncertainties described above, and the nonquantifiable costs and benefits. The Administrator has determined that the benefits of this proposed regulation justify the costs.
XIV. Request for Comment on Proposed Rule
The Agency is requesting comment on this proposed NPDWR for PFAS. In the proposal, the Agency highlighted numerous areas where specific public comment will be helpful for EPA in developing a final rule. EPA specifically requests comment on the following topics within each section of this preamble.
Section III—Regulatory Determinations for Additional PFAS
• EPA requests comment on its preliminary regulatory determination for PFHxS and its evaluation of the statutory criteria that supports the finding. EPA also requests comment on if there are additional data or studies EPA should consider that support or do not support the Agency's preliminary regulatory determination for PFHxS, including additional health information and occurrence data.
• EPA requests comment on its preliminary regulatory determination for HFPO-DA and its evaluation of the statutory criteria that supports the finding. EPA also requests comment on if there are additional data or studies EPA should consider that support or do not support the Agency's preliminary regulatory determination for HFPO-DA, including additional health information and occurrence data.
• EPA requests comment on its preliminary regulatory determination for PFNA and its evaluation of the statutory criteria that supports the finding. EPA also requests comment on if there are additional data or studies EPA should consider that support or do not support the Agency's preliminary regulatory determination for PFNA, including additional health information and occurrence data.
• EPA requests comment on its preliminary regulatory determination for PFBS and its evaluation of the statutory criteria that supports the finding. EPA also requests comment on if there are additional data or studies EPA should consider that support or do not support the Agency's preliminary regulatory determination for PFBS, including additional health information and occurrence data.
• EPA requests comment on whether there are other peer-reviewed health or toxicity assessments for other PFAS the Agency should consider as a part of this action.
• EPA requests comment on its evaluation that regulation of PFHxS, HFPO-DA, PFNA, PFBS, and their mixtures, in addition to PFOA and PFOS, will provide protection from PFAS that will not be regulated under this proposed rule.
Section V—Maximum Contaminant Level Goal
• EPA requests comment on the derivation of the proposed MCLG for PFOA and its determination that PFOA is Likely to be Carcinogenic to Humans and whether the proposed MCLG is set at the level at which there are no adverse effects to the health of persons and which provides an adequate margin of safety. EPA is also seeking comment on its assessment of the noncancer effects associated with exposure to PFOA and the toxicity values described in the support document on the proposed MCLG for PFOA.
• EPA requests comment on the derivation of the proposed MCLG for PFOS, its determination that PFOS is Likely to be Carcinogenic to Humans and whether the proposed MCLG is set at the level at which there are no adverse effects to the health of persons and which provides an adequate margin of safety. EPA is also seeking comment on its assessment of the noncancer effects associated with exposure to PFOS and the toxicity values described in the support document on the proposed MCLG for PFOS.
• EPA requests comment on the general HI approach for the mixture of four PFAS.
• EPA requests comment on the merits and drawbacks of the target-specific HI or RPF approach.
• EPA requests comment on significant figure use when calculating both the HI MCLG and the MCL. EPA has set the HI MCLG and MCL using two significant figures ( i.e., 1.0). EPA requests comment on the proposed use of two significant figures for the MCLG when considering underlying health information and for the MCL when considering the precision of the analytical methods.
• EPA requests comment on the derivation of the HBWCs for each of the four PFAS considered as part of the HI.
• EPA requests comment on whether the HBWCs should instead be proposed as stand-alone MCLGs in addition to or in lieu of the mixture MCLGs.
Section VI—Maximum Contaminant Level
• EPA requests comment on its proposed determination to set MCLs at 4.0 ppt for PFOA and PFOS and whether 4.0 ppt is the lowest PQL that can be achieved by laboratories nationwide.
• EPA seeks comment on its PFOA and PFOS evaluation of feasibility for the proposal, including analytical measurement and treatment capability, as well as reasonable costs, as defined by SDWA.
• EPA seeks comment on its evaluation of feasibility for the proposed HI MCL finding, including analytical measurement and treatment capability, as well as reasonable costs, as defined by SDWA.
• EPA requests comment on implementation challenges and considerations for setting the MCL at the PQLs for PFOA and PFOS, including on the costs and benefits related to this approach.
• EPA requests comment on the underlying assumptions that sufficient laboratory capacity will be available with the proposed MCLs; that demand will be sufficiently distributed during rule implementation to allow for laboratory capacity; and on the cost estimates related to these assumptions.
• EPA requests comment on its proposal of using an HI approach for PFHxS, HFPO-DA, PFNA, and PFBS, including whether it can be clearly implemented and achieves the goal of protecting against dose additive noncancer health effects.
• EPA requests comment on its proposed decision to establish stand-alone MCLs for PFOA and PFOS in lieu of including them in the HI approach.
• EPA requests comment on whether establishing a traditional MCLG and MCL for PFHxS, HFPO-DA, PFNA, and PFBS instead of, or in addition to, the HI approach would change public health protection, improve clarity of the rule, or change costs.
Section VII—Occurrence
• EPA requests comment on the number of systems estimated to solely exceed the HI (but not the PFOA or PFOS MCLs) according to the approach outlined in USEPA (2023e).
Section IX—Monitoring and Compliance Requirements
• EPA requests comment on the proposed monitoring flexibility for groundwater systems serving 10,000 or fewer to only collect two samples at each EPTDS to satisfy initial monitoring requirements.
• EPA requests comment on monitoring-related flexibilities that should be considered to further reduce burden while also maintaining public health protection including a rule trigger level at different values than the currently proposed values of 1.3 ppt for PFOA and PFOS and 0.33 for the HI PFAS (PFHxS, HFPO-DA, PFNA, and PFBS), specifically alternative values of 2.0 ppt for PFOA and PFOS and 0.50 for the HI PFAS. EPA also requests comment other monitoring flexibilities identified by commenters.
• EPA requests comment on the proposed allowance of a water system to potentially have each EPTDS on a different compliance monitoring schedule based on specific entry point sampling results ( i.e., some EPTDS being sampled quarterly and other EPTDS sampled only once or twice during each three-year compliance period), or if compliance monitoring frequency should be consistent across all of the system's sampling points.
• EPA requests comments on whether water systems should be permitted to apply to the primacy agency for monitoring waivers. Specifically, EPA is requesting comment on the allowance of monitoring waivers of up to nine years if after at least one year of sampling results are below the proposed rule trigger level. Similarly, EPA also requests comment on whether allowance of monitoring waivers of up to nine years should be permitted based on previously acquired monitoring data results that are below the proposed rule trigger level. Additionally, EPA is also requesting comment on the identification of possible alternatives to traditional vulnerability assessments that should be considered to identify systems as low risk and potentially eligible for monitoring waivers.
• EPA requests comment on if all water systems, regardless of system size, be allowed to collect and analyze one sample per three-year compliance period if the system does not detect regulated PFAS in their system at or above the rule trigger level.
• EPA requests comment on its proposal to allow the use of previously acquired monitoring data to satisfy initial monitoring requirements including the data collection timeframe requirements and if other QA requirements should be considered.
• EPA requests comment on whether EPA should consider an alternative approach to what is currently proposed when calculating compliance with proposed MCLs. Specifically, in the case where a regulated PFAS is detected but below its proposed PQL, rather than using zero for the measurement value of the specific PFAS in the running annual average compliance calculation, that the proposed rule trigger levels (1.3 ppt for PFOA and PFOS and 0.33 of each of the HI PFAS PQLs ( i.e., PFHxS=1.0, HFPO-DA=1.7, PFNA=1.3, and PFBS=1.0)) be used as the values in calculating the running annual average for compliance purposes.
• EPA requests comment on other monitoring related considerations including laboratory capacity and QA/QC of drinking water sampling.
• EPA seeks comment on the Agency's proposed initial monitoring timeframe, particularly for NTNCWS or all systems serving 3,300 or fewer.
Section X—Safe Drinking Water Right to Know
• EPA requests comment on its proposal to designate violations of the proposed MCLs as Tier 2.
• EPA requests comment on what may be needed for water systems to effectively communicate information about the PFAS NPDWR to the public.
Section XI—Treatment Technologies
• EPA requests comment on whether PWSs can feasibly treat to 4.0 ppt or below.
• EPA requests additional information on PFAS removal treatment technologies not identified in the proposed rule that have been shown to reduce levels of PFAS to the proposed regulatory standard.
• EPA requests comment on the co-removal of the HI chemicals (PFHxS, PFBS, PFNA, and HFPO-DA) when GAC, IX, or RO are used in the treatment of PFOA and/or PFOS.
• EPA requests comment on whether there are additional technologies which are viable for PFAS removal to the proposed MCLs as well as any additional costs which may be associated with non-treatment options such as water rights procurement.
• EPA estimates GAC treatment will be sufficiently available to support cost-effective compliance with this proposed regulation, and requests comment on whether additional guidance on applicable circumstances for GAC treatment is needed.
• EPA is seeking comment on the benefits from using treatment technologies (such as reverse osmosis and GAC) that have been demonstrated to co-remove other types of contaminants found in drinking water and whether employing these treatment technologies are sound strategies to address PFAS and other regulated or unregulated contaminants that may co-occur in drinking water.
• EPA requests comment on the estimates for disposing of drinking water treatment residuals or regenerating drinking water treatment media including assumptions related to the transport distance to disposal sites and other costs that arise out of disposal of PFAS contaminated drinking water treatment residuals.
• EPA requests comment on the availability of facilities to dispose of or regenerate drinking water treatment media that contains PFAS. EPA requests comment on whether there will be sufficient capacity to address the increased demand for disposal of drinking water treatment residuals or to regenerate media for reuse by drinking water treatment facilities.
• EPA requests comment on the impacts that the disposal of PFAS contaminated treatment residuals may have in communities adjacent to the disposal facilities.
• EPA requests comment on the type of assistance that would help small public water systems identify laboratories that can perform the required monitoring, evaluate treatment technologies and determine the most appropriate way to dispose of PFAS contaminated residuals and waste the systems may generate when implementing the rule.
Section XII—Rule Implementation and Enforcement
• EPA is seeking comment as to whether there are specific conditions that should be mandated for systems to be eligible for exemptions under 1416 to ensure that they are only used in rare circumstances where there are no other viable alternatives and what those conditions would be.
Section XIII—HRRCA
• EPA requests comment on all components of the HRRCA for the proposed NPDWR.
• In the Economic Analysis, EPA presented estimated costs and benefits of regulatory alternatives for PFOA and PFOS if setting MCLs at 5.0 ppt and 10.0 ppt. EPA is requesting comment on its evaluation of these alternatives within the Economic Analysis.
• EPA requests comment on the methodology used to estimate national costs for the proposed rule and regulatory alternatives. EPA's cost analysis can be found in Chapter 5 of the Economic Analysis.
• EPA is requesting comment on the WBS models, including the range of component levels assumed in the input to the models, and the range of cost estimates for GAC, IX, and centralized RO.
• EPA requests comment on Table 26 which provides the initial treatment technology compliance forecast, presented in percentages of systems adopting GAC, PFAS-selective IX, centralized RO, system interconnection, and use new wells across system design flows and TOC levels. This information is used in EPA's cost and benefit modeling. Please also comment on the potential for point-of-use devices, including those using RO or activated carbon as a compliance option.
• EPA requests comment on the cost of treatment when additional co-occurring but not targeted PFAS chemicals are found in source water.
• EPA requests comment generally on its estimation of sampling costs. The Agency is also specifically requesting comment on the ability of systems to demonstrate they are reliably and consistently below 1.3 ppt for PFOA and PFOS and 0.33 ppt for PFAS regulated by the HI in order to qualify for reduced monitoring.
• EPA requests comment on the underlying assumptions that, under UCMR 5, individual water systems would be able to request the full release of data from the labs for use in determining their compliance monitoring frequency and that PWSs may be able to use these lab analyses to demonstrate a “below trigger level” concentration using the UCMR 5 analyses by following up with the lab for a more detailed results report.
• EPA requests comment on the costs associated with the storage, transportation and underground injection of the brine concentrate residuals from the RO/NF process.
• EPA requests comment on the small system affordability analysis, including both the national affordability determination using EPA's existing 2.5% of MHI methodology and the supplemental analyses using use of alternative metrics ( i.e., expenditure margins at 1% of MHI and 2.5% of lowest quintile income). EPA's national small system affordability determination can be found in Section 9.12.1 of the Economic Analysis. EPA's supplementary affordability analyses can be found in Section 9.12.2 of the Economic Analysis.
• EPA requests comment on the discussion of estimated PN costs provided in the proposed rule.
• EPA requests comment on the assumption that exceedances of HI PFAS not included in the national cost analyses (HFPO-DA, PFBS, and PFNA) will not significantly impact overall compliance costs and national costs estimates are, therefore, unlikely to be substantially underestimated.
• EPA requests comments on the approaches we used to estimate each of the health impacts of exposure to the PFAS chemicals covered in this proposed rule, including the transparency of the assumptions we made and the impact of these assumptions on the magnitude of the risks avoided by the proposed regulatory action.
• EPA requests comment on whether factors such as anticipated Federal funding, the structure of PWSs relative to private enterprises, or the nature of the public health benefits should be further explored in the final rule analysis, including as it relates to the estimated range of impacts under the applied discount rates.
Section XV—Statutory and Executive Order Reviews
• EPA requests comment on all aspects of its EJ analysis, particularly its choice of comparison groups to determine potential demographic disparities in anticipated PFAS exposure and its use of thresholds against which to examine anticipated exposures. For more information, please see section XV.J of this preamble.
XV. Statutory and Executive Order Reviews
Additional information about these statutes and Executive Orders can be found at https://www.epa.gov/laws-regulations/laws-and-executive-orders.
A. Executive Order 12866: Regulatory Planning and Review and Executive Order 13563: Improving Regulation and Regulatory Review
This action is an economically significant regulatory action that was submitted to the Office of Management and Budget (OMB) for review. Any changes made in response to OMB recommendations have been documented in the docket. EPA prepared an analysis of the potential costs and benefits associated with this action. This analysis, the Economic Analysis (USEPA, 2023j), is available in the docket and is summarized in section XIII of this preamble.
B. Paperwork Reduction Act (PRA)
The information collection activities in this proposed rule have been submitted for approval to the Office of Management and Budget (OMB) under the PRA. The Information Collection Request (ICR) document that EPA prepared has been assigned EPA ICR number 2732.01. You can find a copy of the ICR in the docket for this rule at https://www.regulations.gov/docket/EPA-HQ-OW-2022-0114, and it is briefly summarized here.
The monitoring information collected as a result of the proposed rule should allow primacy agencies and EPA to determine appropriate requirements for specific systems and evaluate compliance with the proposed rule. For the first three-year period following rule promulgation, the major information requirements concern primacy agency activities to implement the rule including adopting the NPDWR into state regulations, providing training to state and PWS employees, updating their monitoring data systems, and reviewing system monitoring data and other requests. Compliance actions for drinking water systems (including monitoring, administration, and treatment costs) would not begin until after three years due to the proposed effective date of this rule. More information on these actions is described in Section XII of this preamble and in Chapter 9 from the Economic Analysis of the Proposed PFAS NPDWR (USEPA, 2023j).
The respondents/affected entities are PWSs and primacy agencies. The collection requirements are mandatory under SDWA (42 U.S.C. 300g-7). For the first three years after publication of the rule in the FR, information requirements apply to an average of 38,089 respondents annually, including 38,033 PWSs and 56 primacy agencies. The burden associated with the proposed rule over the three years covered by the ICR is 3.8 million hours, for an average of 1.3 million hours per year. The total costs over the three-year period is $142.6 million, for an average of $47.5 million per year (simple average over three years). The average burden per response ( i.e., the amount of time needed for each activity that requires a collection of information) is 6.6 hours for PWSs and 1.1 hours for primacy agencies; the average cost per response is $234.41 for PWSs and $60.89 for primacy agencies. Details on the calculation of the proposed rule information collection burden and costs can be found in the ICR for the proposed rule.
Burden is defined at 5 CFR 1320.3(b) and means the total time, effort, and financial resources required to generate, maintain, retain, disclose, or provide information to or for a Federal agency. This includes the time needed to review instructions; develop, acquire, install, and utilize technology and systems for the purposes of collecting, validating, and verifying information, processing and maintaining information, and disclosing and providing information; adjust the existing ways to comply with any previously applicable instructions and requirements; train personnel to be able to respond to a collection of information; search data sources; complete and review the collection of information; and transmit or otherwise disclose the information.
An agency may not conduct or sponsor, and a person is not required to respond to, a collected for information unless it displays a currently valid OMB control number. The OMB control numbers for EPA's regulations in 40 CFR are listed in 40 CFR part 9.
Submit your comments on the Agency's need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden to EPA using the docket identified at the beginning of this proposed rule. EPA will respond to any ICR-related comments in the final rule. You may also send your ICR-related comments to OMB's Office of Information and Regulatory Affairs using the interface at www.reginfo.gov/public/do/PRAMain. Find this particular information collected by selected “Currently under Review—Open for Public Comments” or by using the search function. OMB must receive comments no later than May 30, 2023.
C. Regulatory Flexibility Act (RFA)
Pursuant to section 603 of the Regulatory Flexibility Act (RFA), EPA prepared an initial regulatory flexibility analysis (IRFA) that examines the impact of the proposed rule on small entities along with regulatory alternatives that could minimize the impact. The complete IRFA is available in Section 9.3 of the Economic Analysis in the docket and is summarized here.
For purposes of assessing the impacts of this proposed rule on small entities, EPA considered small entities to be water systems serving 10,000 people or fewer. This is the threshold specified by Congress in the 1996 Amendments to SDWA for small water system flexibility provisions. As required by the RFA, EPA proposed using this alternative definition in the FR (USEPA, 1998c), sought public comment, consulted with the Small Business Administration (SBA), and finalized the small water system threshold in the Agency's Consumer Confidence Report Regulation (USEPA, 1998d). As stated in the document, the alternative definition would apply to all future drinking water regulations.
The SDWA is the core statute addressing drinking water at the Federal level. Under the SDWA, EPA sets public health goals and enforceable standards for drinking water quality. As previously described, the proposed PFAS NPDWR requires water systems to minimize certain PFAS in drinking water. EPA is proposing to regulate PFAS in drinking water to improve public health protection by reducing drinking water exposure to PFAS in drinking water.
The proposed rule contains provisions that would affect approximately 62,000 small PWSs. A small PWS serves between 25 and 10,000 people. These water systems include approximately 45,000 CWSs that serve the year-round residents and approximately 17,000 NTNCWSs that serve the same persons over six months per year ( e.g., a PWS that is an office park or school). The proposed PFAS NPDWR includes development of legally enforceable regulatory standards with requirements for monitoring, PN, and treatment or non-treatment options for water systems exceeding the regulatory standard. This proposed rule also include reporting, recordkeeping, and other administrative requirements. States are required to implement operator certification (and recertification) programs per SDWA Section 1419 to ensure operators of CWSs and NTNCWSs, including small water system operators, have the appropriate level of certification.
Under the proposed rule requirements, small CWSs and NTNCWs serving 10,000 or fewer people are required to conduct initial monitoring or demonstrate recent, previously collected monitoring data to determine the level of certain PFAS in their water system. Based on these initial monitoring results, systems will be required to conduct ongoing monitoring at least every three years or as often as four times per year. Systems that exceed the drinking water standard will be required to choose between treatment and non-treatment as the compliance option. Under the proposed rule, EPA estimates that approximately 18,000 small CWSs (40 percent of small CWSs) could incur annual total PFAS NPDWR related costs of more than one percent of revenues, and that approximately 10,000 small CWSs (22 percent of small CWSs) could incur annual total costs of three percent or greater of revenue. See Section 9.3 of the proposed PFAS NPDWR Economic Analysis for more information on the characterization of the impacts under the proposed rule.
As required by section 609 (b) of the RFA, EPA also convened a Small Business Advocacy Review (SBAR) Panel to obtain advice and recommendations from small entity representatives (SERs) that potentially would be subject to the rule's requirements. On May 24, 2022, EPA's Small Business Advocacy Chairperson convened the Panel, which consisted of the Chairperson, the Director of the Standards and Risk Management Division within EPA's Office of Ground Water and Drinking Water, the Administrator of the Office of Information and Regulatory Affairs within OMB, and the Chief Counsel for Advocacy of the SBA. Prior to convening the Panel, EPA conducted outreach with SERs that will potentially be affected by this regulation and solicited comments from them. Additionally, after the Panel was convened, the Panel provided additional information to the SERs and requested their input. In light of the SERs' comments, the Panel considered the regulatory flexibility issues and elements of the IRFA specified by RFA/Small Business Regulatory Enforcement Fairness Act (SBREFA) and developed the findings and discussion summarized in the SBAR report. For example, the SBAR Panel recommended several flexibilities in monitoring requirements for small systems, including the use of existing monitoring data (such as the UCMR 5) for initial monitoring purposes; as well as reduced compliance monitoring requirements specifically for small groundwater systems. EPA is including these flexibilities as a part of the proposed rule requirements. The report includes a number of other observations and recommendations to meet the statutory obligations for achieving small-system compliance through flexible regulatory compliance options. The report was finalized on August 1, 2022 and transmitted to the EPA Administrator for consideration. A copy of the full SBAR Panel Report is available in the rulemaking docket (USEPA, 2022a).
D. Unfunded Mandates Reform Act (UMRA)
This action contains a Federal mandate under the Unfunded Mandates Reform Act (UMRA), 2 U.S.C. 1531-1538 that may result in expenditures of $100 million or more for state, local, and tribal governments, in the aggregate, or the private sector in any one year. Accordingly, EPA has prepared a written statement required under section 202 of UMRA that is included in the docket for this action (see Chapter 9 of the Economic Analysis for the Proposed PFAS NPDWR) and briefly summarized here.
Consistent with UMRA section 205, EPA identified and analyzed a reasonable number of regulatory alternatives to determine the MCL requirement in the proposed rule. Sections VI, IX, X, and XII of this preamble describe the proposed options. See section XIII of this preamble and Chapter 9 of the Economic Analysis for the Proposed PFAS NPDWR (USEPA, 2023j) for alternative options that were considered.
Consistent with the intergovernmental consultation provisions of UMRA section 204, EPA consulted with governmental entities affected by this rule. EPA describes the government-to-government dialogue and comments from state, local, and tribal governments in section XV.E Executive Order 13132: Federalism and section XV.F Executive Order 13175: Consultation and Coordination with Indian Tribal Governments of this document.
Consistent with UMRA section 205, EPA identified and analyzed a reasonable number of regulatory alternatives to determine the regulatory requirements in the proposed PFAS NPDWR. Section VI of this preamble describes the proposed option. See section XIII of this preamble and Section 9.4 in the Economic Analysis of the Proposed PFAS NPDWR (USEPA, 2023j) for alternative options that were considered.
This action may significantly or uniquely affect small governments. EPA consulted with small governments concerning the regulatory requirements that might significantly or uniquely affect them. EPA describes this consultation above in the RFA, section XV.C of this preamble.
E. Executive Order 13132: Federalism
EPA has concluded that this action has federalism implications because it imposes substantial direct compliance costs on state or local governments, and the Federal government will not provide the funds necessary to pay those costs. However, EPA notes that the Federal government will provide a potential source of funds necessary to offset some of those direct compliance costs through the BIL. EPA estimates that the net change in primacy agency related cost for state, local, and tribal governments in the aggregate is estimated to be $8 million (3 percent discount rate) or $9 million (7 percent discount rate).
EPA provides the following federalism summary impact statement. EPA consulted with State and local governments early in the process of developing the proposed action to allow them to provide meaningful and timely input into its development. EPA held a federalism consultation on February 24, 2022. EPA invited the following national organizations representing State and local elected officials to a virtual meeting on February 24, 2022: The National Governors' Association, the National Conference of State Legislatures, the Council of State Governments, the National League of Cities, the U.S. Conference of Mayors, the National Association of Counties, the International City/County Management Association, the National Association of Towns and Townships, the County Executives of America, and the Environmental Council of States. Additionally, EPA invited the Association of State Drinking Water Administrators, the Association of Metropolitan Water Agencies, the National Rural Water Association, the American Water Works Association, the American Public Works Association, the Western Governors' Association, the Association of State and Territorial Health Officials, the National Association of Country and City Health Officials, and other organizations to participate in the meeting. In addition to input received during the meeting, EPA provided an opportunity to receive written input within 60 days after the initial meeting. A summary report of the views expressed during federalism consultations is available in the Docket (EPA-HQ-OW-2022-0114).
In addition to the federalism consultation, regarding state engagement more specifically, EPA notes there were multiple meetings held by the Association of State Drinking Water Administrators where EPA gathered input from state officials related to the considerations for the development of the proposed rule. EPA utilized this state input to inform this rule proposal.
F. Executive Order 13175: Consultation and Coordination With Indian Tribal Governments
This action has tribal implications, it imposes direct compliance costs on tribal governments, and the Federal government will not provide funds necessary to pay those direct compliance costs. However, EPA notes that the Federal government will provide a potential source of funds necessary to offset some of those direct compliance costs through the BIL.
EPA has identified 998 PWSs serving tribal communities, 84 of which are federally owned. EPA estimates that tribal governments will incur PWS compliance costs of $5 million per year attributable to monitoring, treatment or non-treatment actions to reduce PFAS in drinking water, and administrative costs, and that these estimated impacts will not fall evenly across all tribal systems. The proposed PFAS NPDWR does offer regulatory relief by providing flexibilities for all water systems to potentially utilize pre-existing monitoring data in lieu of initial monitoring requirements and for groundwater CWSs and NTNCWSs serving 10,000 or fewer to reduce initial monitoring from quarterly monitoring during a consecutive 12-month period to only monitoring twice during a consecutive 12-month period. These flexibilities may result in implementation cost savings for many tribal systems since 98 percent of tribal CWSs and 94 percent of NTNCWs serve 10,000 or fewer people.
Accordingly, EPA provides the following Tribal summary impact statement as required by section 5(b) of Executive Order 13175. Consistent with EPA Policy on Consultation and Coordination with Indian Tribes (May 4, 2011), EPA consulted with Tribal officials and their representatives early in the process of developing this proposed regulation to permit them to have meaningful and timely input into its development. EPA conducted consultation with Indian Tribes beginning on February 7, 2022 and ending on April 16, 2022. The consultation included two national webinars with interested tribes on February 23, 2022, and March 8, 2022, where EPA provided proposed rulemaking information and requested input. A total of approximately 35 tribal representatives participated in the two webinars. Updates on the consultation process were provided to the National Tribal Water Council and EPA Region 6's Regional Tribal Operations Committee upon request at regularly scheduled monthly meetings during the consultation process. Additionally, EPA received written comments from the following Tribes and Tribal organizations: Little Traverse Bay Bands of Odawa Indians, Sault Ste. Marie Tribe of Chippewa Indians, and National Tribal Water Council. A summary report of the webinars and views expressed during the consultation is available in the Docket (EPA-HQ-OW-2022-0114).
G. Executive Order 13045: Protection of Children From Environmental Health and Safety Risks
This action is subject to Executive Order 13045 because it is an economically significant regulatory action as defined by Executive Order 12866, and EPA believes that the environmental health or safety risk addressed by this action has a disproportionate effect on children. Additionally, the Agency's 2021 Policy on Children's Health ( https://www.epa.gov/children/epas-policy-evaluating-risk-children ) is to protect children from environmental exposures by consistently and explicitly considering early life exposures (from conception, infancy, early childhood and through adolescence) and lifelong health in all human health decisions through identifying and integrating data when conducting risk assessments of children's health. Accordingly, EPA has evaluated the environmental health or safety effects of PFAS found in drinking water on children and estimated the risk reduction and health endpoint impacts to children associated with adoption of treatment or non-treatment options to reduce PFAS in drinking water. The results of these evaluations are contained in the Economic Analysis of the Proposed PFAS NPDWR (USEPA, 2023j) and described in section XIII of this preamble. Copies of the Economic Analysis of the Proposed PFAS NPDWR and supporting information are available in the Docket (EPA-HQ-OW-2022-0114).
H. Executive Order 13211: Actions That Significantly Affect Energy Supply, Distribution, or Use
This action is not a “significant energy action” because it is not likely to have a significant adverse effect on the supply, distribution or use of energy. The public and private water systems affected by this action do not, as a rule, generate power. This action does not regulate any aspect of energy distribution as the water systems that are proposed to be regulated by this rule already have electrical service. Finally, EPA has determined that the incremental energy used to implement the identified treatment technologies at drinking water systems in response to the proposed regulatory requirements is minimal. As such, EPA does not anticipate that this rule will have a significant adverse effect on the supply, distribution, or use of energy.
I. National Technology Transfer and Advancement Act of 1995
The proposed rule could involve voluntary consensus standards in that it would require monitoring for PFAS and analysis of the samples obtained from monitoring based on required methods. EPA proposed two analytical methods for the identification and quantification of PFAS in drinking water. EPA methods 533 and 537.1 incorporate QC criteria which allow accurate quantitation of PFAS. Additional information about the analytical methods is available in section VIII of this preamble. EPA has made, and will continue to make, these documents generally available through www.regulations.gov and at the U.S. Environmental Protection Agency Drinking Water Docket, William Jefferson Clinton West Building, 1301 Constitution Ave. NW, Room 3334, Washington, DC 20460, call (202) 566-2426.
EPA's monitoring and sampling protocols generally include voluntary consensus standards developed by agencies such as ASTM International, Standard Methods and other such bodies wherever EPA deems these methodologies appropriate for compliance monitoring. EPA welcomes comments on this aspect of the proposed rulemaking and, specifically, invites the public to identify potentially-applicable voluntary consensus standards and to explain why such standards should be used in this regulation. The Director of the FR approved the voluntary consensus standards incorporated by reference in §141.23 of the proposed regulatory text as of April 11, 2007.
J. Executive Order 12898: Federal Actions To Address Environmental Justice in Minority Populations and Low-Income Populations
EPA believes that this action does not have disproportionately high and adverse human health or environmental effects on minority populations or low-income populations, as specified in Executive Order 12898 (USEPA, 1994). The proposed rule is anticipated to increase the level of public health protection for all affected populations without having any disproportionately high and adverse human health or environmental effects on any population, including any minority population. Additionally, EPA has determined that the proposed rule is anticipated to mitigate the disproportionate impacts of baseline PFAS exposure. The documentation for this decision, including additional detail on the methodology, results, and conclusions of EPA's EJ analysis, is contained in Chapter 8 of USEPA (2023j) and is available in the public docket for this action.
Consistent with the Agency's Technical Guidance for Assessing Environmental Justice in Regulatory Analysis (USEPA, 2016g), EPA conducted an EJ analysis to assess the demographic distribution of baseline PFAS drinking water exposure and impacts anticipated to result from the proposed PFAS NPDWR. EPA conducted two separate analyses: an EJ exposure analysis using EJScreen, the Agency's Environmental Justice Screening and Mapping Tool (USEPA, 2019e), and an analysis of EPA's proposed regulatory option and alternatives using SafeWater MCBC (detailed in Section XIII of this preamble). EPA's analyses examine EJ impacts on a subset of PWSs across the country, based on availability of PFAS occurrence data and information on PWS' service area boundaries. In EPA's analysis, results for income, race, and ethnicity groups are generally summarized separately due to how underlying American Community Survey (ACS) statistics are aggregated at the census block group level; for more information, please see: https://www.census.gov/data/developers/data-sets/acs-5year.html (United States Census Bureau, 2022). Additional information on both analyses can be found in Chapter 8 of USEPA (2023j).
EPA's EJ exposure analysis using EJScreen utilized hypothetical regulatory scenarios, which differ from EPA's proposed option and regulatory alternatives (for additional detail, please see Chapter 8 of USEPA (2023j). EPA's EJ exposure analysis demonstrated that across hypothetical regulatory scenarios evaluated, elevated baseline PFAS drinking water exposures, and thus greater anticipated reductions in exposure, are estimated to occur in communities of color and/or low-income populations. For the exposure analysis, EPA examined individuals served by PWSs with modeled PFAS exposure above baseline concentration thresholds or a specific alternative policy threshold. EPA also summarized population-weighted average concentrations in the baseline as well as reductions that would accrue to each demographic group from hypothetical regulatory scenarios. In this analysis, EPA presents the total affected population as a possible metric of comparison, noting however that each affected demographic group is reflected also within the total affected population. For the purpose of evaluating potential EJ concerns, a commonly used demographic category is “people of color,” which includes those who identify as a race other than White and/or as Hispanic. It is possible that EPA understates the magnitude of disproportionate baseline exposure to PFAS for people of color because the total affected population includes some portion of the specific populations of concern. For this reason, EPA included information for non-Hispanic White populations in all tables of Section 8.3 in Chapter 8 of USEPA (2023j). EPA also described differences in potential disproportionate impact when comparison is drawn from population groups of concern to the non-Hispanic White population instead of the total population across all demographic groups. EPA requests comment on all aspects of the EJ analysis, including its choice of comparison groups to help identify potential demographic disparities in anticipated PFAS exposure.
Additionally, EPA's analysis in SafeWater MCBC evaluated the demographic distribution of health benefits and incremental household costs anticipated to result from the proposed PFAS NPDWR. EPA's proposed option and all regulatory alternatives are anticipated to provide benefits across all health endpoint categories for all race/ethnicity groups. Across all health endpoints, communities of color are anticipated to experience the greatest quantified benefits associated with EPA's proposed option.
EPA's analysis in SafeWater MCMC also demonstrated that communities of color are anticipated to bear elevated incremental household costs associated with the rule. Although the incremental household cost differences across race/ethnicity groups are minimal, for communities already facing underlying EJ concerns, the impact of these incremental cost increases are likely to impose a higher cost burden. In general, incremental household costs to all race/ethnicity groups decrease as system size increases, an expected result due to economies of scale. Due to the overlap in vulnerabilities demonstrated by slightly elevated household costs anticipated for particular race/ethnicity groups and consistently elevated household costs for households served by small systems, communities of color served by small systems are anticipated to face compounding burdens. To alleviate potential cost disparities identified by EPA's analysis, there may be an opportunity for some communities to utilize funding from national legislation, including BIL (Public Law 117-58), funds allocated to the Low-Income Household Water Assistance Program (LIHWAP) by the American Rescue Plan (Public Law 117-2), and funding from other sources, to provide financial assistance for addressing emerging contaminants. BIL funding has specific allocations for both disadvantaged and/or small communities and emerging contaminants, including PFAS.
Additionally, on March 2, 2022, and April 5, 2022, EPA held public meetings related to EJ and the development of the proposed NPDWR. The meetings provided an opportunity for EPA to share information and for communities to offer input on EJ considerations related to the development of the proposed rule. During the meeting and in subsequent written comments EPA received public comment on topics including establishing an MCL for PFAS, affordability of PFAS abatement options, limiting industrial discharge of PFAS, and EPA's relationship with community groups. For more information on the public meetings, please refer to the Environmental Justice Considerations for the Development of the Proposed PFAS Drinking Water Regulation Public Meeting Summary for each of the meeting dates in the public docket at https://www.regulations.gov/docket/EPA-HQ-OW-2022-0114. Additionally, the written public comments are included within the public docket.
K. Consultations With the Science Advisory Board, National Drinking Water Advisory Council, and the Secretary of Health and Human Services
In accordance with sections 1412(d) and 1412(e) of the SDWA, the Agency consulted with the NDWAC (or the Council); the Secretary of Health and Human Services; and with the EPA SAB.
1. SAB
The SAB PFAS Review Panel met virtually via a video meeting platform on December 16, 2021, and then at three (3) subsequent meetings on January 4, 6 and 7, 2022 to deliberate on the Agency's charge questions. Another virtual meeting was held on May 3, 2022, to discuss their draft report. Oral and written public comments were considered throughout the advisory process. EPA sought guidance from the EPA SAB on how best to consider and interpret life stage information, epidemiological and biomonitoring data, the Agency's physiologically-based pharmacokinetic (PBPK) analyses, and the totality of PFAS health information to derive a MCLG for PFOA and PFOS, combined toxicity framework, and CVD. The documents sent to SAB were EPA's Proposed Approaches to the Derivation of a Draft Maximum Contaminant Level Goal for Perfluorooctanoic Acid (PFOA) (CASRN 335-67-1) in Drinking Water (USEPA, 2021e); EPA's Proposed Approaches to the Derivation of a Draft Maximum Contaminant Level Goal for Perfluorooctane Sulfonic Acid (PFOS) (CASRN 1763-23-1) in Drinking Water (USEPA, 2021f); EPA's Framework for Estimating Noncancer Health Risks Associated with Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) (USEPA, 2023d); and EPA's Analysis of Cardiovascular Disease Risk Reduction as a Result of Reduced PFOA and PFOS Exposure in Drinking Water. On May 3 and July 20, 2022, EPA received input from SAB, summarized in the report, Review of EPA's Analyses to Support EPA's National Primary Drinking Water Rulemaking for PFAS (USEPA, 2022a).
In response to EPA's request that the SAB review EPA's four draft documents listed above, the SAB identified subject matter experts to augment the SAB Chemical Assessment Advisory Committee (CAAC) and assembled the SAB PFAS Review Panel to conduct the review.
In general, the SAB recognized the time constraints for completing the rule-making process and was supportive of EPA's efforts to the utilize the latest scientific finding to inform their decisions. The SAB applauded the Agency's efforts to develop new approaches for assessing the risk of PFAS mixtures and the benefits arising from reducing exposure to these chemicals as adopted by EPA in the HI approach in this proposed rule. In general, the SAB agreed with many of the conclusions presented in the assessments, framework, and analysis. The SAB also identified many areas that would benefit from further clarification to enhance their transparency and increase their utility. The SAB provided numerous recommendations which can be found in the SAB's final report (USEPA, 2022a) and some highlights are outlined below.
a. Approaches to the Derivation of Draft MCLGs for PFOA and PFOS
The primary purpose of the Proposed Approaches to the Derivation of Draft MCLGs for PFOA and PFOS (USEPA, 2021e; USEPA, 2021f) was to develop MCLGs based on the best available health effects information for PFOA and PFOS. Each MCLG draft document includes derivation of an updated chronic oral RfD, CSF when relevant data were available, and an RSC for SAB review. The health effects information used to derive these toxicity values and RSC values built upon the information in the 2016 PFOA and PFOS HESDs (USEPA, 2016e; USEPA, 2016f) and Health Advisories (USEPA, 2016a; USEPA, 2016b), respectively. EPA has considered all SAB consensus advice in the development of the proposed values derived in this health effects assessment and subsequently derived MCLGs for the NPDWRs for PFOA and PFOS based on the best available science and EPA guidance and precedent. Please see section IV and V of this preamble for discussions on the process for derivation of the MCLGs and the resulting proposed MCLG values for this proposed action.
The SAB charge questions for the MCLG draft documents addressed the systematic review study identification and inclusion, non-cancer hazard identification, cancer hazard identification and slope factor, toxicokinetic modeling, RfD derivation, and RSC. The complete list of charge questions was included in EPA's documents prepared for the SAB (USEPA, 2022a). The SAB provided numerous specific recommendations to consider alternative approaches, expand the systematic review steps for the health effects assessment, and to develop additional analyses in order to improve the rigor and transparency of EPA's documents. The complete list of SAB consensus advice is described in their final report (USEPA, 2022a).
In general, the SAB agreed with many of the conclusions presented in the assessments, framework, and analyses. The SAB recognized the time constraints for completing the rule-making process and supported EPA's efforts to use the latest scientific information to inform their decisions. The SAB applauded the Agency's efforts to develop new approaches for assessing the risk of PFAS mixtures and the benefits arising from reducing exposure to PFAS.
The SAB also identified areas that would benefit from further clarification, expansion, and transparency. The SAB provided written comments and responses to EPA's charge questions (USEPA, 2022a) and the following is a summary of their recommendations and EPA's associated revisions.
Regarding the approaches to deriving MCLG draft documents, the SAB stated that the systematic review methods could be more transparent and complete. Specifically, study identification and criteria for inclusion could be improved. EPA made revisions to the systematic review description and process by updating and expanding the scope of the literature search; providing greater transparency regarding the study inclusion criteria; and adding additional systematic review steps and transparently describing each of these steps in the PFOA and PFOS systematic review protocols.
In the charge questions, EPA sought advice on the noncancer health assessment, and the SAB recommended that EPA separate hazard and dose-response assessment systematic review steps. In response, EPA made revisions to the noncancer hazard identification by expanding systematic review steps beyond study quality evaluation to include evidence integration to address the need to separate hazard identification and dose-response assessment and to ensure consistent hazard decisions; and strengthening rationales for selection of points of departure for the noncancer health outcomes. Additionally, the SAB advised EPA to focus on the health endpoints with the strongest evidence ( i.e., liver, immune, serum lipids, development, and cancer).
EPA consulted with the SAB on the cancer risk assessment. On the cancer HI and CSF, the SAB agreed that PFOA was a “likely” designation but recommended undertaking and describing a more structured and transparent discussion of the “weight of evidence” for both PFOA and PFOS. EPA revised this assessment by following the structured approach in the EPA cancer guidelines (USEPA, 2005) to develop a weight of evidence narrative for cancer, to consider the data for selecting the cancer classification, evaluating and integrating mechanistic information, and strengthening the rationales for decisions.
For the toxicokinetics model that EPA sought advice on, SAB requested more details on the toxicokinetic modeling including model code and parameters and recommended that EPA consider expressing the RfD in water concentration equivalents to better account for possible life-stage specific differences in exposure rates and toxicokinetics. EPA considered the alternate approach suggested by SAB and made revisions by evaluating alternative toxicokinetic models and further validating the selected model.
EPA also sought advice on the draft RfD derivation. The SAB advised that EPA consider multiple human and animal studies for a variety of endpoints and populations. The SAB also stated a need for stronger and more transparent justification of benchmark response selections and asked EPA to consider adopting a probabilistic framework to calculate risk-specific doses. SAB also recommended that EPA clearly state that RfDs apply to both short-term and chronic exposure. EPA made revisions based on these recommendations by providing additional descriptions and rationale for the selected modeling approaches and conducting new dose-response analyses of additional studies and endpoints.
On the RSC charge question, SAB supported the selection of a 20% RSC, but asked that EPA provide clarity and rationale to support the value. To address this recommendation, EPA added clarifying language related to the RSC determination from EPA guidance (USEPA, 2000c), including the relevance of drinking water exposures and the relationship between the RfD and the RSC.
b. Combined Toxicity Framework
EPA sought advice from an external SAB on the Draft Framework for Estimating Noncancer Health Risks Associated with Mixtures of PFAS document (USEPA, 2023d). The main purpose of this document was to provide a data-driven framework for estimating human health risks associated with oral exposures to mixtures of PFAS. The charge questions for the SAB pertaining to the framework draft documents included whether EPA provided clear support for the assumption of dose additivity, and application of the HI, RPF, and mixtures benchmark dose (BMD) approaches for the evaluation of mixtures of PFAS. The full list of charge questions was included in EPA's documents prepared for the SAB (USEPA, 2022a). The SAB agreed in general with the assumption of dose additivity at the level of common health effect, and application of the HI, RPF and mixture BMD approaches for the evaluation of mixtures of PFAS. The SAB identified instances in which the communication of the analyses and approaches in EPA's framework document could be improved to be clearer.
On EPA's charge question for dose additivity, the SAB agreed with the use of the dose additivity default assumption when evaluating PFAS mixtures that have similar effects and concluded that this assumption was health protective. SAB recommended a more thoroughly and clearly presented list of the uncertainties associated with this approach along with information supporting this approach. EPA made revisions that added clarity to the text by expanding upon the uncertainties and including additional support for using dose additivity.
The SAB panel agreed with the use of the HI as a screening method and decision-making tool. SAB advised that EPA should consider using a menu-based framework to support selection of fit-for-purpose approaches, rather than a tiered approach as described in the draft mixtures document. Based on this feedback, EPA has since reorganized the approach to provide a data-driven “menu of options” to remove the tiered logic flow and is adding text to clarify the flexibility in implementation.
EPA sought SAB's opinion on the RPF approach for estimating health risks associated with PFAS mixtures and the SAB panel considered the RPF approach to be a reasonable methodology for assessing mixtures. On the mixture BMD, the SAB agreed that the mixture BMD approach was a reasonable methodology for estimating a mixture-based POD. For both the RPF and mixture BMD approach, SAB recommended that EPA's approach would be strengthened by the use of PODs from animal studies that are based on HEDs rather than administered doses. SAB also requested clarification as to the similarities and differences among the RPF and mixture BMD approaches. SAB also asked EPA to provide additional information on how the proposed mixtures BMD approach would be applied in practice. To address these concerns, EPA made revisions to provide better context and delineation about the applicability of the data across these approaches.
c. CVD Analysis
EPA consulted with the SAB on the Agency's methodology to determine the avoided cases of CVD events ( e.g., heart attack, stroke, death from coronary heart disease) associated with reductions in exposure to PFOA and PFOS in drinking water to support a benefits analysis. Specifically, EPA sought SAB comment on the extent to which the approach to estimating reductions in CVD risk is scientifically supported and clearly described. EPA posed specific charge questions on the exposure-response information used in the analysis, the risk model and approach used to estimate the avoided cases of CVD events, and EPA's discussion of limitations and uncertainties of the analysis. Overall, the SAB supported EPA's approach to estimating reductions in CVD risk associated with reductions in exposure to PFOA and PFOS in drinking water. The SAB provided feedback on several areas of the analysis; main points of their feedback and EPA's responses are discussed below.
The SAB noted a discrepancy between the draft CVD document's focus on CVD risk, and the draft MCLG documents' conclusions that the evidence of CVD was not sufficient to form the basis of a RfD. Based on SAB feedback on the draft MCLG document's assessment of CVD related risks, EPA has developed an RfD for total cholesterol (For more information see USEPA, 2023b; USEPA, 2023c). The derivation of an RfD for this endpoint addresses the SAB's concerns about inconsistency between the two documents. The SAB also recommended that EPA ensure that recommendations for the draft MCLG documents relating to evidence identification and synthesis are applied to the CVD endpoint. All studies in EPA's CVD benefits analysis were evaluated for risk of bias, selective reporting, and sensitivity as applied in EPA's Public Comment Draft—Toxicity Assessment and Proposed MCLGs for PFOA and PFOS in Drinking Water (USEPA, 2023b; USEPA, 2023c).
The SAB recommended that EPA provide more discussion as to the rationale for selecting CVD for risk reduction analysis and that the approach follows the pathway that links cholesterol to cardiovascular events rather than looking at the reported effects of PFAS directly on CVD. The SAB also recommended that EPA consider risk reduction analyses for other endpoints. In Section 6.5 of the Economic Analysis, EPA discusses the rationale for quantifying CVD and analytical assumptions. Sections 6.4 and 6.6 discusses the Agency's quantified risk reduction analyses for other adverse health effects, including infant birthweight effects and RCC, respectively. In Section 6.2.2 EPA assesses the qualitative benefits of other adverse health effects of PFAS.
Although the SAB generally agreed with the meta-analysis, life table and risk estimation methods, the SAB recommended that EPA provide additional clarity as to the application of these approaches and conduct additional sensitivity analyses. In response to these comments, EPA expanded documentation and conducted additional sensitivity analyses to evaluate the impact of inclusion or exclusion of certain studies in the meta-analyses of exposure-response estimates. Further, EPA expanded documentation and conducted additional sensitivity analyses to assess the effects of using a key single study approach versus the meta-analysis approach to inform the exposure-response estimates. EPA identified two suitable key studies for use in the single study approach. EPA found that the single study approach resulted in increased benefits, and this trend was driven by the larger estimates of PFAS-total cholesterol slope factors and inverse associations in the HDLC effect for one or both contaminants in the key single studies. EPA elected to retain the meta-analysis approach in the benefits analysis because the Agency identified several studies on adults in the general population with large numbers of participants and low risk of bias, and in this case the meta-analytical approach offers an increased statistical power over the single study approach. While the single study approach is common for RfD derivations, the meta-analysis pooled estimate provides a slope factor that represents the average response across a larger number of studies, which is useful in evaluating benefits resulting from changes in CVD risk on a national scale.
The SAB also recommended that EPA evaluate how inclusion of HDLC effects would influence the results and provide further justification for the inclusion or exclusion of HDLC and blood pressure effects. EPA found that, as expected, inclusion of HDLC effects decreases annualized CVD benefits and inclusion of blood pressure effects slightly increases annualized CVD benefits. Because HDLC was shown to have a stronger effect than blood pressure on annualized CVD benefits, inclusion of blood pressure and HDLC effects together decreases annualized CVD benefits. For more information see sensitivity analyses evaluating these effects in Appendix K of the EA. Inclusion of HDLC effects into the national analysis would reduce national benefits estimates but would not change EPA's bottom-line conclusion that the quantifiable and nonquantifiable benefits of the rule justify the quantifiable and nonquantifiable costs. After further examination of the evidence for HDLC and blood pressure effects, EPA elected to include blood pressure effects because the findings from a single high confidence study and several medium confidence studies conducted among the general population provided consistent evidence of an association between PFOS exposure and blood pressure. EPA did not include HDLC effects in the national benefits analysis because available evidence of associations between PFOS exposures and HDLC levels is inconsistent and there is no evidence of an association between PFOA exposures and HDLC levels.
Finally, the SAB noted that while the ASCVD model is a reasonable choice for estimating the probability of first time CVD events, it is not without limitations. The panel recommended that EPA include more discussion of the accuracy of its predictions, particularly for sub-populations. EPA expanded its evaluation of the ASCVD model's limitations, including a comparison of the ASCVD model predictions with race/ethnicity and sex-specific CVD incidence from CDC's public health surveys (See Section 6.5.3.2 and Appendix G of the Economic Analysis for details). Results show that the ASCVD model coefficients for the non-Hispanic Black model are more consistent with data on CVD prevalence and mortality for Hispanic and non-Hispanic other race subpopulations than the ASCVD model coefficients for the non-Hispanic White model.
2. NDWAC
The Agency consulted with NDWAC during the Council's April 19, 2022, virtual meeting. A summary of the NDWAC recommendations is available in the National Drinking Water Advisory Council, Fall 2022 Meeting Summary Report (NDWAC, 2022 https://www.federalregister.gov/documents/2022/03/29/2022-06576/meeting-of-the-national-drinking-water-advisory-council ) and the docket for this proposed rule. EPA carefully considered NDWAC recommendations during the development of a proposed drinking water rule for PFAS, including PFOA and PFOS.
3. HHS
On September 28, 2022, EPA consulted with the Department of Health and Human Services (HHS). EPA provided information to HHS officials on the draft proposed NPDWR and considered HHS input as part of the interagency review.
XVI. References
Adamson, D.T., E.A. Piña, A.E. Cartwright, S.R. Rauch, R.H. Anderson, T. Mohr, and J.A. Connor. 2017. 1,4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Science of the Total Environment vol. 596:236-245.
Ahrens, L. 2011. Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. Journal of Environmental Monitoring, 13(1):20-31. https://doi.org/10.1039/c0em00373e.
Almond, D., Chay, K.Y., and Lee, D.S. 2005. The Costs of Low Birth Weight. The Quarterly Journal of Economics, 120(3):1031-1083. https://doi.org/10.1093/qje/120.3.1031
Ambavane, A., Yang, S., Atkins, M.B., Rao, S., Shah, A., Regan, M.M., McDermott, D.F., and Michaelson, M.D. 2020. Clinical and Economic Outcomes of Treatment Sequences for Intermediate- to Poor-Risk Advanced Renal Cell Carcinoma. Immunotherapy, 12(1):37-51. https://doi.org/10.2217/imt-2019-0199.
American Association for the Advancement of Science (AAAS). 2020. Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water. Available on the internet at: https://www.aaas.org/epi-center/pfas.
Appleman, T.D., Higgins, C.P., Quiñones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C., and Dickenson, E.R. 2014. Treatment of Poly-and Perfluoroalkyl Substances in US Full-scale Water Treatment Systems. Water Research, 51(15):246-255. https://doi.org/10.1016/j.watres.2013.10.067.
Arevalo E., Strynar, M., Lindstorm, A., McMillan, L., and Knappe, D. 2014. Removal of Perfluorinated Compounds by Anion Exchange Resins: Identifying Effective Resin Regeneration Strategies. AWWA Annual Conference and Exposition. Boston, MA.
Ateia, M., Maroli, A., Tharayil, N., and Karanfil, T. 2019. The Overlooked Short- and Ultrashort-Chain Poly- and Perfluorinated Substances: A Review. Chemosphere, 220(2019):866-882. https://doi.org/10.1016/j.chemosphere.2018.12.186. Agency for Toxic Substances and Disease Registry (ATSDR). 2021. Toxicological Profile for Perfluoroalkyls. Available on the internet at: https://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf.
Banzhaf, S., Filipovic, M., Lewis, J., Sparrenbom, C.J., Barthel, R. 2017. A Review of Contamination in Surface-, Ground-, and Drinking Water in Sweden by Perfluoroalkyl Substances (PFAS). Ambio, 45: 335-346. https://doi.org/10.1007/s13280-016-0848-8.
Barry, V., Winquist, A., and Steenland, K. 2013. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living Near a Chemical Plant. Environmental Health Perspectives, 121(11-12):1313-1318. https://doi.org/10.1289/ehp.1306615.
Bartell, S.M., Calafat, A.M., Lyu, C., Kato, K., Ryan, P.B., and Steenland, K. 2010. Rate of Decline in Serum PFOA Concentrations after Granular Activated Carbon Filtration at Two Public Water Systems in Ohio and West Virginia. Environmental Health Perspectives, 118 (2):222-228. https://doi.org/10.1289/ehp.0901252.
Bartell, S.M., and Vieira, V.M. 2021. Critical Review on PFOA, Kidney Cancer, and Testicular Cancer. Journal of the Air & Waste Management Association, 71(6):663-679. https://doi.org/10.1080/10962247.2021.1909668.
Beach, S.A., Newsted, J.L., Coady, K., and Giesy, J.P. 2006. Ecotoxicological Evaluation of Perfluorooctanesulfonate (PFOS). Reviews of Environmental Contamination and Toxicology, 186:133-174. https://doi.org/10.1007/0-387-32883-1_5.
Beane Freeman, L.E., Cantor, K.P., Baris, D., Nuckols, J.R., Johnson, A., Colt, J.S., Schwenn, M., Ward, M.H., Lubin, J.H., Waddell, R., Hosain, G.M., Paulu, C., McCoy, R., Moore, L.E., Huang, A., Rothman, N., Karagas, M.R., and Silverman, D.T. 2017. Bladder Cancer and Water Disinfection By-product Exposures through Multiple Routes: A Population-Based Case-Control Study (New England, USA). Environmental Health Perspectives, 125(6). https://doi.org/10.1289/EHP89.
Behrman, J.R., and Rosenzweig, M.R. 2004. Returns to Birthweight. The Review of Economics and Statistics, 86(2):586-601. https://doi.org/10.1162/003465304323031139.
Behrman, R.E., and Butler, A.S. 2007. Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. https://doi.org/10.17226/11622.
Belkouteb, N., Franke, V., McCleaf, P., Kohler, S., and Ahrens, L. 2020. Removal of Per- and Polyfluoroalkyl Substances (PFASs) in a Full-scale Drinking Water Treatment Plant: Long-term Performance of Granular Activated Carbon (GAC) and Influence of Flow-Rate. Water Research, 182(2020):115913. https://doi.org/10.1016/j.watres.2020.115913.
Biegel, L.B., Hurtt, M.E., Frame, S.R., O'Connor, J.C., and Cook, J.C. 2001. Mechanisms of Extrahepatic Tumor Induction by Peroxisome Proliferators in Male CD Rats. Toxicology Sciences, 60:44-55. https://doi.org/10.1093/toxsci/60.1.44.
Blom, C., and Hanssen, L. 2015. Analysis of per- and polyfluorinated substances in articles. Nordiske Arbejdspapirer, 2015:911. https://doi.org/10.6027/NA2015-911.
Boodoo, F. 2018. Short and Long Chain PFAS Removal with Single-Use Selective Ion Exchange Resin. Presentation by Purolite Corporation. Available on the internet at: https://docs.house.gov/meetings/-IF/IF18/20180906/108649/HHRG-115-IF18-20180906-SD027.pdf.
Boodoo, F., Begg, T., Funk, T., Kessler, T., Shaw, E., and Pickel, M. 2019. Polishing PFAS to Non-Detect Levels Using PFAS-Selective Resin. Water Online, February 13. Available on the internet at: https://www.wateronline.com/doc/polishing-pfas-to-non-detect-levels-using-pfas-selective-resin-0001.
Boonya-Atichart, A., Boontanon, S.K., and Boontanon, N. 2016. Removal of perfluorooctanoic acid (PFOA) in groundwater by nanofiltration membrane. Water Science and Technology, 74 (11), 2627-2633. https://doi.org/10.2166/wst.2016.434.
Borg, D., and Ivarsson, J. 2017. Analysis of PFASs and TOF in products. Available on the internet at: http://urn.kb.se/resolve?urn=urn:nbn:se:norden:org:diva-4901.
Buck, R.C., Franklin, J., Berger, U., Conder, J.M., Cousins, I.T., de Voogt, P., Jensen, A.A., Kannan, K., Mabury, S.A., and van Leeuwen, S.P. 2011. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integrated Environmental Assessment and Management, 7(4):513-541. https://doi.org/10.1002/ieam.258.
Buck, R.C., Murphy, P.M., and Pabon, M. 2012. Chemistry, Properties, and Uses of Commercial Fluorinated Surfactants. In: Knepper, T., Lange, F. (eds). Polyfluorinated Chemicals and Transformation Products. The Handbook of Environmental Chemistry, v. 17. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-21872-9_1.
Budtz-Jørgensen, E., and Grandjean, P. 2018. Application of Benchmark Analysis for Mixed Contaminant Exposures: Mutual Adjustment of Perfluoroalkylate Substances Associated with Immunotoxicity. PLoS ONE, 13:e0205388. https://doi.org/10.1371/journal.pone.0205388.
Butenhoff, J.L., Chang, S., Ehresman, D.J., and York, R.G. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reproductive toxicology, 27:331-341. https://doi.org/10.1016/j.reprotox.2009.01.004.
Butenhoff, J.L., Kennedy, G.L., Chang, S.C., and Olsen, G.W. 2012a. Chronic Dietary Toxicity and Carcinogenicity Study with Ammonium Perfluorooctanoate in Sprague-Dawley Rats. Toxicology, 298: 1-13. https://doi.org/10.1016/j.tox.2012.04.001.
Butenhoff, J.L., Chang, S.C., Olsen, G.W., and Thomford, P.J. 2012b. Chronic Dietary Toxicity and Carcinogenicity Study with Potassium Perfluorooctane Sulfonate in Sprague Dawley Rats. Toxicology, 293:1-15. https://doi.org/10.1016/j.tox.2012.01.003.
Cadwallader, A., Greene, A., Holsinger, H., Lan, A., Messner, M., Simic, M., and Albert, R. 2022. A Bayesian Hierarchical Model for Estimating National PFAS Drinking Water Occurrence. AWWA Water Science, 4(3):1284. https://doi.org/10.1002/aws2.1284.
Calafat, A.M., Wong, L.-Y., Kuklenyik, Z., Reidy, J.A., and Needham, L.L. 2007. Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and Comparisons with NHANES 1999-2000. Environmental Health Perspectives, 115(11):1596-1602. https://doi.org/10.1289/ehp.10598.
Calafat, A.M., Kato, K., Hubbard, K., Jia, T., Botelho, J.C., and Wong, L.Y. 2019. Legacy and Alternative Per and Polyfluoroalkyl Substances in the U.S. General Population: Paired Serum-urine Data from the 2013-2014 National Health and Nutrition Examination Survey. Environment International, 131(2019):105048. https://doi.org/10.1016/j.envint.2019.105048.
California Environmental Protection Agency. 2021. Proposed Public Health Goals for Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid in Drinking Water. Available on the internet at: https://oehha.ca.gov/media/downloads/crnr/pfoapfosphgdraft061021.pdf.
Cantor, K.P., Lynch, C.F., Hildesheim, M.E., Dosemeci, M., Lubin, J., Alavanja, M., and Craun, G. 1998. Drinking Water Source and Chlorination Byproducts. I. Risk of Bladder Cancer. Epidemiology, 9(1):21-28. http://dx.doi.org/10.1097/00001648-199801000-00007.
Cantor, K.P., Villanueva, C.M., Silverman, D.T., Figueroa, J.D., Real, F.X., Garcia-Closas, M., Malats, N., Chanock, S., Yeager, M., Tardon, A., Garcia-Closas, R., Serra, C., Carrato, A., Castaño-Vinyals, G., Samanic, C., Rothman, N., and Kogevinas, M. 2010. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, Disinfection By-products, and Risk of Bladder Cancer in Spain. Environmental Health Perspectives, 118(11):1545-1550. https://doi.org/10.1289/ehp.1002206.
Catelan, D., Biggeri, A., Russo, F., Gregori, D., Pitter, G., Da Re, F., Fletcher, T., and Canova, C. 2021. Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy). International Journal of Environmental Research and Public Health, 18(5):2734. https://doi.org/10.3390/ijerph18052734.
CDM Smith. 2018. Advanced Treatment Options for the Northwest Water Treatment Plant. Final Report. Prepared for Brunswick County Public Utilities.
Centers for Disease Control and Prevention (CDC). 2017. User Guide to the 2017 Period/2016 Cohort Linked Birth/Infant Death Public Use File. Available on the internet at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/period-cohort-linked/17PE16CO_linkedUG.pdf.
CDC. 2018. User Guide to the 2018 Period/2017 Cohort Linked Birth/Infant Death Public Use File. Available on the internet at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/period-cohort-linked/18PE17CO_linkedUG.pdf.
CDC. 2019. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019, Volume 1. Department of Health and Human Services, Centers for Disease Control and Prevention. Available on the internet at: https://doi.org/10.15620/cdc75822.
Chaikind, S., and Corman, H. 1991. The Impact of Low Birthweight on Special Education Costs. Journal of Health Economics, 10(3):291-311. https://doi.org/10.1016/0167-6296(91)90031-H.
Chatterji, P., Kim, D., and Lahiri, K. 2014. Birth Weight and Academic Achievement in Childhood. Health Economics, 23(9):1013-1035. https://doi.org/10.1002/hec.3074.
Cheng, W., Dastgheib, S.A. and Karanfil, T., 2005. Adsorption of dissolved natural organic matter by modified activated carbons. Water Research , 39(11):2281-2290.
Chili, C.A., Westerhoff, P., and Ghosh, A. 2012. GAC removal of organic nitrogen and other DBP precursors. Journal‐American Water Works Association, 104(7):E406-E415.
Chowdhury, Z.Z., Zain, S.M., Rashid, A.K., Rafique, R.F., and Khalid, K. 2013. Breakthrough Curve Analysis for Column Dynamics Sorption of Mn(II) Ions from Wastewater by Using Mangostana garcinia Peel-Based Granular-Activated Carbon. Journal of Chemistry, 2013:959761. https://doi.org/10.1155/2013/959761.
Chu, C., Zhou, Y., Li, Q.Q., Bloom, M.S., Lin, S., Yu, Y.J., Chen, D., Yu, H.Y., Hu, L.W., Yang, B.Y., Zeng, X.W., and Dong, G.H. 2020. Are Perfluorooctane Sulfonate alternatives Safer? New Insights from a Birth Cohort Study. Environment International, 135:105365. https://doi.org/10.1016/j.envint.2019.105365.
Colaizy, T.T., Bartick, M.C., Jegier, B.J., Green, B.D., Reinhold, A.G., Schaefer, A.J., Bogen, D.L., Schwarz, E.B., Stuebe, A.M., Jobe, A.H., and Oh, W. 2016. Impact of Optimized Breastfeeding on the Costs of Necrotizing Enterocolitis in Extremely Low Birthweight Infants. The Journal of Pediatrics, 175:100-105. https://doi.org/10.1016/j.jpeds.2016.03.040.
Costet, N., Villanueva, C.M., Jaakkola, J.J.K., Kogevinas, M., Cantor, K.P., King, W.D., Lynch, C.F., Nieuwenhuijsen, M.J., and Cordier, S. 2011. Water Disinfection By-products and Bladder Cancer: is there a European Specificity? A Pooled and Meta-analysis of European Case-control Studies. Occupational & Environmental Medicine, 68(5):379-385. https://doi.org/10.1136/oem.2010.062703.
Cuthbertson, A.A., Kimura, S.Y., Liberatore, H.K., Summers, R.S., Knappe, D.R., Stanford, B.D., Maness, J.C., Mulhern, R.E., Selbes, M., and Richardson, S.D. 2019. Does granular activated carbon with chlorination produce safer drinking water? From disinfection byproducts and total organic halogen to calculated toxicity. Environmental Science & Technology , 53(10):5987-5999.
Crittenden, J.C., Vaitheeswaran, K., Hand, D.W., Howe, E.W., Aieta, E.M., Tate, C.H., McGuire, M.J., and Davis, M.K. 1993. Removal of Dissolved Organic Carbon using Granular Activated Carbon. Water Research, 27(4):715-721. https://doi.org/10.1016/0043-1354(93)90181-G.
D'Agostino, R.B., Vasan, R.S., Pencina, M.J., Wolf, P.A., Cobain, M., Massaro, J.M., and Kannel, W.B. 2008. General Cardiovascular Risk Profile for Use in Primary Care. Circulation, 117(6):743-753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
Darrow, L.A., Stein, C.R., and Steenland, K. 2013. Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonate Concentrations in Relation to Birth Outcomes in the Mid-Ohio Valley, 2005-2010. Environmental Health Perspectives, 121:1207-1213. https://doi.org/10.1289%2Fehp.1206372.
Darrow, L.A., Groth, A.C., Winquist, A., Shin, H.M., Bartell, S.M., and Steenland, K. 2016. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a mid-Ohio Valley Community. Environmental Health Perspectives, 124:1227-1233. https://doi.org/10.1289/ehp.1510391.
Das, K.P., Grey, B.E., Rosen, M.B., Wood, C.R., Tatum-Gibbs, K.R., Zehr, R.D., Strynar, M.J., Lindstrom, A.B., and Lau, C. 2015. Developmental toxicity of perfluorononanoic acid in mice. Reproductive toxicology, 51:133-144. 10.1016/j.reprotox.2014.12.012.
Dastgheib, S.A., Karanfil, T., and Cheng, W. 2004. Tailoring activated carbons for enhanced removal of natural organic matter from natural waters. Carbon , 42(3):547-557.
Deeks, J.J. 2002. Issues in the Selection of a Summary statistic for Meta‐analysis of Clinical Trials with Binary Outcomes. Statistics in Medicine, 21(11):1575-1600. https://doi.org/10.1002/sim.1188.
Du, Z., Deng, S., Bei, Y., Huang, Q., Wang, B., Huang, J., and Yu, G. 2014. Adsorption Behavior and Mechanism of Perfluorinated Compounds on Various Adsorbents—A Review. Journal of Hazardous Materials, 274(2014):443-454. https://doi.org/10.1016/j.jhazmat.2014.04.038.
Dickenson, E., and Higgins, C. 2016. Treatment Mitigation Strategies for Poly- and Perfluorinated Chemicals. Water Research Foundation. Available on the internet at: https://www.waterrf.org/research/projects/treatment-mitigation-strategies-poly-and-perfluorinated-chemicals.
Dixit, F., Barbeau, B., Mostafavi, S.G., and Mohseni, M. 2020. Efficient Removal of GenX Chemicals (HFPO-DA) and Other Perfluorinated Ether Acids from Drinking and Recycled Waters using Anion Exchange Resins. Journal of Hazardous Materials, 384(2020):121261. https://doi.org/10.1016/j.jhazmat.2019.121261.
Dixit, F., Dutta R., Barbeau B., Berube P., and Mohseni M. 2021. PFAS Removal by Ion Exchange Resins: A review. Chemosphere, 272(2021):129777. https://doi.org/10.1016/j.chemosphere.2021.129777.
Dobson, K.G., Ferro, M.A., Boyle, M.H., Schmidt, L.A., Saigal, S., and Van Lieshout, R.J. 2018. How do Childhood Intelligence and Early Psychosocial Adversity Influence Income Attainment Among Adult Extremely Low Birth Weight Survivors? A Test of the Cognitive Reserve Hypothesis. Development and Psychopathology, 30(4):1421-1434. https://doi.org/10.1017/S0954579417001651.
Domingo, J.L., and Nadal, M. 2019. Human Exposure to Per- and Polyfluoroalkyl Substances (PFAS) through Drinking Water: A Review of the Recent Scientific Literature. Environmental Research, 177(2019):108648. https://doi.org/10.1016/j.envres.2019.108648.
Dong, Z., Wang, H., Yu, Y.Y., Li, Y.B., Naidu, R., and Liu, Y. 2019. Using 2003-2014 U.S. NHANES Data to Determine the Associations between Per- and Polyfluoroalkyl Substances and Cholesterol: Trend and Implications. Ecotoxicology & Environmental Safety, 173:461-468. https://doi.org/10.1016/j.ecoenv.2019.02.061.
Dowbiggin, B., Treadway, J., Nichols, J., and Walker G. 2021. Exploring Treatment Options for PFAS Removal in Brunswick County, North Carolina. Journal AWWA, 113(4), 10-19. https://doi.org/10.1002/awwa.1705.
DuPont. 2010. H-28548: Subchronic Toxicity 90-Day Gavage Study in Mice. OECD Test Guideline 408. Study conducted by E.I. du Pont de Nemours and Company (Study Completion Date: February 19, 2010), Newark, DE.
Dzierlenga, M., Crawford, L., and Longnecker, M. 2020. Birth Weight and Perfluorooctane Sulfonic Acid: a Random-effects Meta-regression Analysis. Environmental Epidemiology, 4(3):e095. https://doi.org/10.1097/EE9.0000000000000095.
Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P., and De Voogt, P. 2012. Impact of Treatment Processes on the Removal of Perfluoroalkyl Acids from the Drinking Water Production Chain. Environmental Science & Technology, 46(3):1708-1715. https://doi.org/10.1021/es201662b.
Elder, T., Figlio, D., Imberman, S., and Persico, C. 2020. The Role of Neonatal Health in the Incidence of Childhood Disability. American Journal of Health Economics, 6(2):216-250. https://doi.org/10.1086/707833.
Engels, E.A., Schmid, C.H., Terrin, N., Olkin, I., and Lau, J. 2000. Heterogeneity and Statistical Significance in Meta‐analysis: an Empirical Study of 125 Meta‐analyses. Statistics in Medicine, 19(13):1707-1728. https://doi.org/10.1002/1097-0258(20000715)19:13<1707::aid-sim491>3.0.co;2-p.
European Food Safety Authority (ESFA), Knutsen, H.K., Alexander, J., Barregard, L., Bignami, M., Bruschweiler, B., Ceccatelli, S., Cottrill, B., Dinovi, M., Edler, L., GraslKraupp, B., Hogstrand, C., Hoogenboom, L.R., Nebbia, C.S., Oswald, I.P., Petersen, A., Rose, M., Roudot, A.C., Vleminckx, C., Vollmer, G., Wallace, H., Bodin, L., Cravedi, J.P., Halldorsson, T.I., Haug, L.S., Johansson, N., van Loveren, H., Gergelova, P., Mackay, K., Levorato, S., van Manen, M., and Schwerdtle, T. 2018. Risk to Human Health Related to the Presence of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid in Food. EFSA Journal, 16(12):e05194. https://doi.org/10.2903/j.efsa.2018.5194.
ESFA, Schrenk, D., Bignami, M., Bodin, L., Chipman, J.K., Del Mazo, J., Grasl-Kraupp, B., Hogstrang, C., Hoogenboom, L.R., Leblanc, J.C., Nebbia, C.S., Nielsen, E., Ntzani, E., Petersen, A., Sand, S., Vleminckx, C., Wallace, H., Barregard, L., Ceccatelli, S., Cravedi, J.P., Halldorsson, T.I., Haug, L.S., Johansson, N., Knutsen, H.K., Rose, M., Roudot, A.C., Van Loveren, H., Vollmer, G., Mackay, K., Riolo, F., and Schwerdtle, T. 2020. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA Journal, 18(9):e06223. https://doi.org/10.2903/j.efsa.2020.6223.
Feng, X., Cao, X., Zhao, S., Wang, X., Hua, X., Chen, L., and Chen, L. 2017. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicological Sciences, 155: 409-419. http://dx.doi.org/10.1093/toxsci/kfw219.
Forrester, E., and Bostardi, C. 2019. PFAS Removal: GAC & IX. Calgon Carbon Corporation. Webinar. April 23, 2019.
Fromme, H., Tittlemier, S.A., Volkel, W., Wilhelm, M., and Twardella, D. 2009. Perfluorinated Compounds—Exposure Assessment for the General Population in Western Countries. International Journal of Hygiene and Environmental Health, 212:239-270. https://doi.org/10.1016/j.ijheh.2008.04.007.
Gallo, V., Leonardi, G., Genser, B., Lopez-Espinosa, M.J., Frisbee, S.J., Karlsson, L., Ducatman, A.M., and Fletcher, T. 2012. Serum Perfluorooctanoate (PFOA) and Perfluorooctane Sulfonate (PFOS) Concentrations and Liver Function Biomarkers in a Population with Elevated PFOA Exposure. Environmental Health Perspectives, 120:655-660. http://dx.doi.org/10.1289/ehp.1104436.
Gardiner, J., 2014. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Australian Journal of Chemistry. 68(1):13. https://doi.org/10.1071/CH14165.
Glüge, J., Scheringer, M., Cousins, I.T., DeWitt, J.C., Goldenman, G., Herzke, D., Lohmann, R., Ng., C.A., Trier, X., and Wang, Z. 2020. An Overview of the Uses of Per-and Polyfluoroalkyl Substances (PFAS)-Electronic Supplementary Information. Environmental Science: Processes & Impacts, 12(2020):2345. https://doi.org/10.1039/D0EM00291G.
Goff, DC, Lloyd-Jones, D.M., Bennett, G., Coady, S., D'Agostino, R., Gibbons, R., Greenland, P., Lackland, D.T., Levy, D., O'donnell, C.J. Robinson, J.G., Schwartz, J.S., Shero, S.T., Smith, S.C., Sorlie, P., Stone, N., and Wilson, P.W.F. 2014. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 129(25 supplemental 2):49-73. https://doi.org/10.1161/01.cir.0000437741.48606.98.
Goodrich, J.A., Walker, D., Lin, X., Wang, H., Lim, T., McConnell, R., Conti, D.V., Chatzi, L., and Setiawan, V.W. 2022. Exposure to Perfluoroalkyl Substances and Risk of Hepatocellular Carcinoma in a Multiethnic Cohort. JHEP Reports. 100550. https://doi.org/10.1016/j.jhepr.2022.100550.
Govarts, E., Remy, S., Bruckers, L., Den Hond, E., Sioen, I., Nelen, V., Baeyens, W., Nawrot, T.S., Loots, I., Van Larebeke, N., and Schoeters, G. 2016. Combined Effects of Prenatal Exposures to Environmental Chemicals on Birth Weight. International Journal of Environmental Research and Public Health, 13(5):495. https://doi.org/10.3390%2Fijerph13050495.
Govarts, E., Iszatt, N., Trnovec, T., De Cock, M., Eggesbø, M., Murinova, L. P., van de Bor, M., Guxens, M., Chevrier, C., Koppen, G. and Lamoree, M. 2018. Prenatal exposure to en-docrine disrupting chemicals and risk of being born small for gestational age: Pooled analysis of seven European birth cohorts. Environment International, 115, 267-278. https://doi:10.1016/j.envint.2018.03.01 .
Grandjean, P., Timmermann, C.A.G., Kruse, M., Nielsen, F., Vinholt, P.J., Boding, L., Heilmann, C., and Mølbak, K. 2020. Severity of COVID-19 at Elevated Exposure to Perfluorinated Alkylates. PLoS One, 15(12):e0244815. https://doi.org/10.1371/journal.pone.0244815.
Greco, S.L., Belova, A., Haskell, J., and Backer, L. 2019. Estimated Burden of Disease from Arsenic in Drinking Water Supplied by Domestic Wells in the United States. Journal of Water and Health, 17(5):801-812. https://doi.org/10.2166/wh.2019.216.
Guelfo, J.L. and D.T. Adamson. 2018. Evaluation of a national data set for insights into sources, composition, and concentrations of per- and polyfluoroalkyl substances (PFASs) in U.S. drinking water. Environmental Pollution, 236, 505-513. https://doi.org/10.1016/j.envpol.2018.01.066.
Hazardous Substances Data Bank (HSDB). 2016. Profile for Perfluorooctanoic acid. Available on the internet at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Last revision date October 25, 2016.
Hines, C.T., Padilla, C.M., and Ryan, R.M. 2020. The Effect of Birth Weight on Child Development Prior to School Entry. Child Development, 91(3):724-732. https://doi.org/10.1111/cdev.13355.
Hopkins, Z. R., Sun, M., DeWitt, J. C., and Knappe, D. R. 2018. Recently detected drinking water contaminants: GenX and other per‐and polyfluoroalkyl ether acids. Journal‐American Water Works Association, 110(7), 13-28. https://doi.org/10.1002/awwa.1073.
Horst, J., McDonough, J., Ross, I., Dickson, M., Miles, J., Hurst, J., and Storch, P. 2018. Water Treatment Technologies for PFAS: The Next Generation. Groundwater Monitoring & Remediation, 38(2), 13-23. https://doi.org/10.1111/gwmr.12281.
ICF. 2021. Memorandum re: Summary of Literature Review of Non-Medical Effects and Economic Burden Related to Low Birth Weight Infants. Submitted to U.S. Environmental Protection Agency Office of Groundwater and Drinking Water, October 5, 2021.
ICF. 2020. Memorandum re: Trends in Infant Mortality by Birth Weight Categories, EPA Contract No. 68HE0C18D0001, TO 68HERC20F0107. Submitted to U.S. Environmental Protection Agency Office of Groundwater and Drinking Water July 30, 2020.
Iriarte-Velasco, U., I. Álvarez-Uriarte, J., Chimeno-Alanis, N. and R. González-Velasco, J., 2008. Natural organic matter adsorption onto granular activated carbons: implications in the molecular weight and disinfection byproducts formation. Industrial & Engineering Chemistry Research, 47(20):7868-7876.
Janousek, R.M., Lebertz, S., and Knepper, T.P. 2019. Previously Unidentified Sources of Perfluoroalkyl and Polyfluoroalkyl Substances from Building Materials and Industrial Fabrics. Environmental Science: Processes & Impacts, 11(2019). https://doi.org/10.1039/c9em00091g.
Jelenkovic, A., Mikkonen, J., Martikainen, P., Latvala, A., Yokoyama, Y., Sund, R., Vuoksimaa, E., Rebato, E., Sung, J., Kim, J., Lee, J., Lee, S., Stazi, M.A., Fagnani, C., Brescianini, S., Derom, C.A., Vlietinck, R.F., Loos, R.J.F., Krueger, R.F., McGue, M., Pahlen, S., Nelson, T.L., Whitfield, K.E., Brandt, I., Nilsen, T.S., Harris, J.R., Cutler, T.L., Hopper, J.L., Tarnoki, A.D., Tarnoki, D.L., Sørensen, T.I.A., Kaprio, J., and Silventoinen, K. 2018. Association Between Birth Weight and Educational Attainment: an Individual-based Pooled Analysis of Nine Twin Cohorts. Journal of Epidemiology & Community Health, 72(9):832-837. https://doi.org/10.1136/jech-2017-210403.
Ji, J., Song, L., Wang, J., Yang, Z., Yan, H., Li, T., Yu, L., Jian, L., Jiang, F., Li, J. Zheng, J., and Li, K. 2021. Association Between Urinary Per-and Poly-Fluoroalkyl Substances and COVID-19 Susceptibility. Environment International, 153(2021):106524. https://doi.org/10.1016/j.envint.2021.106524.
Jiang, J., Zhang, X., Zhu, X. and Li, Y. 2017. Removal of intermediate aromatic halogenated DBPs by activated carbon adsorption: a new approach to controlling halogenated DBPs in chlorinated drinking water. Environmental Science & Technology, 51(6): 3435-3444.
Joyce, C., Goodman-Bryan, M., and Hardin, A. 2012. Preterm Birth and Low Birth Weight. Available on the internet at: http://www.urbanchildinstitute.org/sites/all/files/2010-10-01-PTB-and-LBW.pdf.
Kamai, E.M., McElrath, T.F., and Ferguson, K.K. 2019. Fetal Growth in Environmental Epidemiology: Mechanisms, Limitations, and a Review of Associations with Biomarkers of Non-persistent Chemical Exposures during Pregnancy. Environmental Health, 18(1):43. https://doi.org/10.1186/s12940-019-0480-8.
KEMI Swedish Chemical Agency, Occurrence and Use of Highly Fluorinated Substances and Alternatives. 2015. Available on the internet at: https://www.kemi.se/en/publications/reports/2015/report-7-15-occurrence-and-use-of-highly-fluorinated-substances-and-alternatives.
Kim, W. H., Nishijima, W., Shoto, E., and Okada, M. 1997. Competitive Removal of Dissolved Organic Carbon by Adsorption and Biodegradation on Biological Activated Carbon. Water Science and Technology, 35(7):147-153. https://doi.org/10.1016/S0273-1223(97)00125-X.
King, W.D., and Marrett, L.D. 1996. Case-control Study of Bladder Cancer and Chlorination By-products in Treated Water (Ontario, Canada). Cancer Causes & Control, 7(6):596-604. https://doi.org/10.1007/BF00051702.
Klein, R., and Lynch, M. 2018. Development of Medical Cost Estimates for Adverse Birth Outcomes. Prepared for U.S. EPA National Center for Environmental Economics.
Kowlessar, N.M., Jiang, H.J., and Steiner, C. 2013. Hospital Stays for Newborns, 2011: Statistical Brief# 163. Available on the internet at: https://pubmed.ncbi.nlm.nih.gov/24308074/.
Kirisits, M.J., Snoeyink, V.L. and Kruithof, J.C. 2000. The reduction of bromate by granular activated carbon. Water Research, 34(17):4250-4260.
Kumarasamy, E., Manning, I.M., Collins, L.B., Coronell, O., and Leibfarth, F.A. 2020. Ionic Fluorogels for Remediation of Per-and Polyfluorinated Alkyl Substances from Water. ACS Central Science, 2020(6):487-492. https://doi.org/10.1021/acscentsci.9b01224.
Lauritzen, H. B., Larose, T. L., Øien, T., Sandanger, T. M., Odland, J. O., van der Bor, M., and Jacobsen, G. W. 2017. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatric research, 81(1), 33-42. http://doi:10.1038/pr.2016.187.
Lenters, V., Portengen, L., Rignell-Hydbom, A., Jonsson, B.A.G., Lindh, C.H., Piersma, A.H., Toft, G., Bonde, J.P., Heederik, D., Rylander, L., and Vermeulen, R. 2016. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environmental Health Perspectives, 124:365-372. https://doi.org/10.1289/ehp.1408933.
Li, S., Peng, Y., Wang, X., Qian, Y., Xiang, P., Wade, SW, Guo, H., Lopez, J.A.G., Herzog, C.A., and Handelsman, Y. 2019. Cardiovascular Events and Death after Myocardial Infarction or Ischemic Stroke in an Older Medicare Population. Clinical Cardiology, 42(3):391-399. https://doi.org/10.1002/clc.23160.
Liao, S., Yao, W., Cheang, I., Tang, X., Yin, T., Lu, X., Zhou, Y., Zhang, H., and Li, X. 2020. Association between Perfluoroalkyl Acids and the Prevalence of Hypertension among US Adults. Ecotoxicology and Environmental Safety, 196(2020):110589. https://doi.org/10.1016/j.ecoenv.2020.110589.
Lin, C.Y., Lin, L.Y., Chiang, C.K., Wang, W.J., Su, Y.N., Hung, K.Y., and Chen, P.C. 2010. Investigation of the Associations Between Low-Dose Serum Perfluorinated Chemicals and Liver Enzymes in US Adults. American Journal of Gastroenterology, 105:1354-1363. https://doi.org/10.1038/ajg.2009.707.
Lin, P., Cardenas, A., Hauser, R., Gold, D.R., Kleinman, K., Hivert, M.F., Fleisch, A.F., Calafat, A.M., Webster, T.F., Horton, E.S., and Oken, E. 2019. Per- and Polyfluoroalkyl Substances and Blood Lipid Levels in Pre-diabetic Adults-Longitudinal Analysis of the Diabetes Prevention Program Outcomes Study. Environment International, 129: 343-353. https://doi.org/10.1016/j.envint.2019.05.027.
Lipp, P., Sacher, F., and Baldauf, G. 2010. Removal of organic micro-pollutants during drinking water treatment by nanofiltration and reverse osmosis. Desalination and Water Treatment, 13(1-3), 226-237. http://doi.org/10.5004/dwt.2010.1063.
Liu, C. 2017. Removal of Perfluorinated Compounds in Drinking Water Treatment: A Study of Ion Exchange Resins and Magnetic Nanoparticles. Doctoral Dissertation, University of Waterloo. Available on the internet at: http://hdl.handle.net/10012/12660.
Liu, C.J., Strathmann, T.J. and Bellona, C. 2021. Rejection of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam by high-pressure membranes. Water Research, 188(2021), 116546. https://doi.org/10.1016/j.watres.2020.116546.
Liu, Y. L., and Sun, M. 2021. Ion exchange removal and resin regeneration to treat per-and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Research, 207, 117781. https://doi.org/10.1016/j.watres.2021.117781.
Lloyd-Jones, D.M., Huffman, M.D., Karmali, K.N., Sanghavi, D.M., Wright, J.S., Pelser, C., Gulati, M., Masoudi, F.A., and Goff, Jr., D.C. 2017. Estimating Longitudinal Risks and Benefits from Cardiovascular Preventive Therapies among Medicare Patients: the Million Hearts Longitudinal ASCVD Risk Assessment Tool: a Special Report from the American Heart Association and American College of Cardiology. Circulation, 135(13):e793-e813. https://doi.org/10.1016/j.jacc.2016.10.018.
Lombardo, J., Berretta, C., Redding, A., Swanson, C., and Mallmann, T. 2018. Carbon and Resin Solutions for PFAS Removal. Evoqua Water Technologies Webinar. March 6. Available on the internet at: https://www.evoqua.com/en/webinars/pfas-removal-3-case-studies2/#video-modal.
Lu, S., and Bartell, S.M. 2020. Serum PFAS Calculator for Adults. Available on the internet at: www.ics.uci.edu/~sbartell/pfascalc.html.
Ma, S., and Finch, B.K. 2010. Birth Outcome Measures and Infant Mortality. Population Research and Policy Review, 29(6):865-891. https://doi.org/10.1007/s11113-009-9172-3.
Mahoney, H., Xie, Y., Brinkmann, M., and Giesy, J.P. 2022. Next Generation Per-and Poly-Fluoroalkyl Substances: Status and Trends, Aquatic Toxicity, and Risk Assessment. Eco-Environment & Health, 1(2):117-131. https://doi.org/10.1016/j.eehl.2022.05.002.
Mastropietro, T.F., Bruno, R., Pardo, E., and Armentano, D. 2021. Reverse Osmosis and Nanofiltration Membranes for Highly Efficient PFASs Removal: Overview, Challenges and Future Perspectives. Dalton Transactions, 50(16):5398-5410. https://doi.org/10.1039/d1dt00360g.
Matthis, J., and Carr, S. 2018. Reactivation of Spent Activated Carbon Used for PFAS Adsorption. In Kempisty, D.M, Xing, Y., and Racz, L. (Eds.), Perfluoroalkyl Substances in the Environment: Theory, Practice, and Innovation (pp. 303-323). Taylor & Francis. https://doi.org/10.1201/9780429487125.
McCleaf, P., Englund, S., Östlund, A., Lindegren, K., Wiberg, K., and Ahrens, L. 2017. Removal Efficiency of Multiple Poly-and Perfluoroalkyl Substances (PFASs) in Drinking Water using Granular Activated Carbon (GAC) and Anion Exchange (AE) Column Tests. Water Research, 120(2017):77-87. https://doi.org/10.1016/j.watres.2017.04.057.
McNamara, J.D., Franco, R., Mimna R., and Zappa, L. 2018. Comparison of Activated Carbons for Removal of Perfluorinated Compounds from Drinking Water. Journal-American Water Works Association, 110(1):E2-E14. https://doi.org/10.5942/jawwa.2018.110.0003.
National Academies of Sciences, Engineering, and Medicine (NASEM). 2022. Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington, DC: The National Academies Press. https://doi.org/10.17226/26156.
National Institute of Environmental Health Services (NIEHS). 2020. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). Available on the internet at: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm.
National Drinking Water Advisory Council (NDWAC). 2022. National Drinking Water Advisory Council Fall 2022 Meeting Summary Report https://www.federalregister.gov/documents/2022/03/29/2022-06576/meeting-of-the-national-drinking-water-advisory-council.
Negri, E., Metruccio, F., Guercio, V., Tosti, L., Benfenati, E., Bonzi, R., La Vecchia, C., and Moretto, A. 2017. Exposure to PFOA and PFOS and Fetal Growth: a Critical Merging of Toxicological and Epidemiological Data. Critical Reviews in Toxicology, 47(6):489-515. https://doi.org/10.1080/10408444.2016.1271972.
Nian, M., Li, Q.Q., Bloom, M., Qian, Z.M., Syberg, K.M., Vaughn, M.G., Wang, S.Q., Wei, Q., Zeeshan, M., Gurram, N., Chu, C., Wang, J., Tian, Y.P., Hu, L.W., Liu, K.K., Yang, B.Y., Liu, R.Q., Feng, D., Zeng, X.W., and Dong, GH. 2019. Liver Function Biomarkers Disorder is Associated with Exposure to Perfluoroalkyl Acids in Adults: Isomers of C8 Health Project in China. Environmental Research, 172:81-88. https://doi.org/10.1016/j.envres.2019.02.013.
Nicoletti, C., Salvanes, K.G., and Tominey, E. 2018. Response of Parental Investments to Child's Health Endowment at Birth. In Health Econometrics: Emerald Publishing Limited. https://doi.org/10.1108/S0573-855520180000294009.
Norden. 2013. Per- and Polyfluorinated Substances in the Nordic Countries—Use, Occurrence and Toxicology. Available on the internet at: https://www.norden.org/en/publication/and-polyfluorinated-substances-nordic-countries.
Norwegian Environment Agency. 2017. Investigation of Sources of PFBS into the Environment (M-759). Available on the internet at: https://www.miljodirektoratet.no/globalassets/publikasjoner/M759/M759.pdf.
National Toxicology Program (NTP). 2020. NTP technical report on the toxicology and carcinogenesis studies of perfluorooctanoic acid (CASRN 335-67-1) administered in feed to Sprague Dawley (Hsd: Sprague Dawley SD) rats [NTP]. (Technical Report 598). Research Triangle Park, NC. Available on the internet at: https://www.ncbi.nlm.nih.gov/books/NBK560147/.
NTP. 2021. Report on Carcinogens, Fifteenth Edition.; Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service.
New York State Department of Health (NYDOH). 2020. Public Water Systems and NYS Drinking Water Standards for PFAS and Other Emerging Contaminants. Center for Environmental Health. Available on the internet at: https://www.health.ny.gov/environmental/water/drinking/docs/water_supplier_fact_sheet_new_mcls.pdf.
Ochoa-Herrera, V., and Sierra-Alvarez, R. 2008. Removal of Perfluorinated Surfactants by Sorption onto Granular Activated Carbon, Zeolite and Sludge. Chemosphere, 72(10):1588-1593. https://doi.org/10.1016/j.chemosphere.2008.04.029.
Office of Management and Budget (OMB). 2003. Circular A-4: Regulatory Analysis. Washington, DC: OMB. Available on the internet at: https://obamawhitehouse.archives.gov/omb/circulars_a004_a-4/.
Osuchukwu, O., and Reed, D. 2022. Small for Gestational Age. StatPearls.
O'Sullivan, A.K., Rubin, J., Nyambose, J., Kuznik, A., Cohen, D.J., and Thompson, D. 2011. Cost Estimation of Cardiovascular Disease Events in the US. Pharmacoeconomics, 29(8);693-704. https://doi.org/10.2165/11584620-000000000-00000.
Park, M., Wu, S., Lopez, I.J., Chang, J.Y., Karanfil, T., and Snyder, S.A. 2020. Adsorption of Perfluoroalkyl Substances (PFAS) in Groundwater by Granular Activated Carbons: Roles of Hydrophobicity of PFAS and Carbon Characteristics. Water Research, 170(2020):115364. https://doi.org/10.1016/j.watres.2019.115364.
Pramanik, B.K., Pramanik, S.K., and Suja, F. 2015. A Comparative Study of Coagulation, Granular-and Powdered-Activated Carbon for the Removal of Perfluorooctane Sulfonate and Perfluorooctanoate in Drinking Water Treatment. Environmental Technology, 36(20):2610-2617. https://doi.org/10.1080/09593330.2015.1040079.
Rahman, M.F., Peldszus, S., and Anderson, W.B. 2014. Behaviour and Fate of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Drinking Water Treatment: a Review. Water Research, 50:318-340. https://doi.org/10.1016/j.watres.2013.10.045.
Raleigh, K.K., Alexander, B.H., Olsen, G.W., Ramachandran, G., Morey, S.Z., Church, T.R., Loga, P.W., Scott, L.L.F., and Allen, E.M. 2014. Mortality and Cancer Incidence in Ammonium Perfluorooctanoate Production Workers. Occupational Environmental Medicine, 71:500-506. https://doi.org/10.1136/oemed-2014-102109.
Regli, S., Chen, J., Messner, M., Elovitz, M.S., Letkiewicz, F.J., Pegram, R.A., Pepping, T.J., Richardson, S.D., and Wright, J.M. 2015. Estimating Potential Increased Bladder Cancer Risk Due to Increased Bromide Concentrations in Sources of Disinfected Drinking Waters. Environmental Science & Technology, 49(22):13094-13102. https://doi.org/10.1021/acs.est.5b03547.
Rücker, G., Schwarzer, G., Carpenter, J., and Olkin, I. 2009. Why Add Anything to Nothing? The Arcsine Difference as a Measure of Treatment Effect in Meta-analysis with Zero Cells. Statistics in Medicine, 28(5):721-738. https://doi.org/10.1002/sim.3511.
Sagiv, S.K., Rifas-Shiman, S.L., Fleisch, A.F., Webster, T.F., Calafat, A.M., Ye, X., Gillman, M.W., and Oken, E. 2018. Early Pregnancy Perfluoroalkyl Substance Plasma Concentrations and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? American Journal of Epidemiology, 187:793-802. https://doi.org/10.1093%2Faje%2Fkwx332.
Shearer, J.J., Callahan, C.L., Calafat, A.M., Huang, W.Y., Jones, R.R., Sabbisetti, V.S., Freedman, N.D., Sampson, J.N., Silverman, D.T., Purdue, M.P., and Hofmann, J.N. 2021. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. Journal of the National Cancer Institute, 113(5):580-587. https://doi.org/10.1093/jnci/djaa143.
Sorlini, S. and Collivignarelli, C. 2005. Chlorite removal with granular activated carbon. Desalination, 176(1-3):255-265.
Sörengård, M., Östblom, E., Köhler, S., and Ahrens, L. 2020. Adsorption Behavior of Per-and Polyfluoralkyl Substances (PFASs) to 44 Inorganic and Organic Sorbents and Use of Dyes as Proxies for PFAS Sorption. Journal of Environmental Chemical Engineering, 8(3):103744. https://doi.org/10.1016/j.jece.2020.103744.
Starling, A.P., Adgate, J.L., Hamman, R.F., Kechris, K., Calafat, A.M., Ye, X., and Dabelea, D. 2017. Perfluoroalkyl Substances during Pregnancy and Offspring Weight and Adiposity at Birth: Examining Mediation by Maternal Fasting Glucose in the Healthy Start Study. Environmental Health Perspectives, 125:067016. https://doi.org/10.1289/ehp641.
Steenland, K., Tinker, S., Frisbee, S., Ducatman A., and Vaccarino, V. 2009. Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate with Serum Lipids among Adults Living Near a Chemical Plant. American Journal of Epidemiology, 170:1269-1278. https://doi.org/10.1093/aje/kwp279.
Steenland, K., and Woskie, S. 2012. Cohort Mortality Study of Workers Exposed to Perfluorooctanoic Acid. American Journal of Epidemiology, 176:909-917. https://doi.org/10.1093/aje/kws171.
Steenland, K., Zhao, L., and Winquist, A. 2015. A Cohort Incidence Study of Workers Exposed to Perfluorooctanoic Acid (PFOA). Occupational & Environmental Medicine, 72:373-380. http://dx.doi.org/10.1136/oemed-2014-102364.
Steenland, K., Barry, V., and Savitz, D. 2018. An Updated Meta-Analysis of the Association of Serum PFOA and Birthweight, with an Evaluation of Potential Biases. Paper presented at the ISEE Conference Abstracts.
Steinle-Darling, E., and Reinhard, M. 2008. Nanofiltration for Trace Organic Contaminant Removal: Structure, Solution, and Membrane Fouling Effects on the Rejection of Perfluorochemicals. J. Environ. Sciences, 42, 5292-5297. https://doi.org/10.1021/es703207s.
Summers, R.S., Kim, S.M., Shimabuku, K., Chae, S.H. and Corwin, C.J. 2013. Granular activated carbon adsorption of MIB in the presence of dissolved organic matter. Water Research, 47(10):3507-3513.
Surveillance Research Program, National Cancer Institute. 2020a. SEER*Stat software Incidence—SEER Research Limited-Field Data, 21 Registries, Nov 2020 Sub (2000-2018). Available on the internet at: seer.cancer.gov/seerstat.
Surveillance Research Program, National Cancer Institute. 2020b. SEER*Stat software Incidence-Based Mortality—SEER Research Data, 18 Registries, Nov 2020 Sub (2000-2018). Available on the internet at: seer.cancer.gov/seerstat.
Svendsgaard, D.J., and Hertzberg, R.C. 1994. Statistical Methods for the Toxicological Evaluation of the Additivity Assumption as used in the Environmental Protection Agency Chemical Mixture Risk Assessment Guidelines. In Toxicology of chemical mixtures: Case studies, mechanisms, and novel approaches. Academic Press, San Diego, CA.
Temple, J.A., Reynolds, A.J., and Arteaga, I. 2010. Low Birth Weight, Preschool Education, and School Remediation. Education and Urban Society, 42(6):705-729. https://doi.org/10.1177/0013124510370946.
Terry, L.G. and Summers, R.S. 2018. Biodegradable organic matter and rapid-rate biofilter performance: A review. Water Research, 128:234-245.
Thom, T., Kannel, W., Silbershatz, H., and D'Agostino, R. 2001. Cardiovascular Diseases in the United States and Prevention Approaches. Hurst's the heart, 1, 3-17.
Thomford, P. 2002. 104-Week Dietary Chronic Toxicity and Carcinogenicity Study with Perfluorooctane Sulfonic Acid Potassium Salt (PFOS; T-6295) in Rats (pp. 1-216). 3M. Available on the internet at: https://www.ag.state.mn.us/Office/Cases/3M/docs/PTX/PTX2805.pdf.
Thompson, J., Eaglesham, G., Reungoat, J., Poussade, Y., Bartkowf, M., Lawrence, M. and Mueller, J.F. 2011. Removal of PFOS, PFOA and other perfluoroalkyl acids at water reclamation plants in South East Queensland Australia. Chemosphere, 82(2011):9-17. https://doi.org/10.1016/j.chemosphere.2010.10.040.
Timmermann, C.A.G., Pedersen, H.S., Weihe, P., Bjerregaard, P., Nielsen, F., Heilmann, C., and Grandjean, P. 2021. Concentrations of Tetanus and Diphtheria Antibodies in Vaccinated Greenlandic Children Aged 7-12 years Exposed to Marine Pollutants, a Cross Sectional Study. Environmental Research, 203:111712. https://doi.org/10.1016/j.envres.2021.111712.
Tow, E.W., Ersan, M.S., Kum, S., Lee, T., Speth, T.F., Owen, C. Bellona, C., Nadagouda, M.N., Mikelonis, A.M., Westerhoff, P., Mysore, C., Frenkel, V.S., DeSilva, V., Walker, SW, Safulko, A.K., and Ladner, D.A. 2021. Managing and treating per- and polyfluoroalkyl substances (PFAS) in membrane concentrates. AWWA Water Science, 3(5), e1233. https://doi.org/10.1002/aws2.1233.
United States Bureau of Labor Statistics (USBLS). 2021. Medical care in U.S. city average, all urban consumers, not seasonally adjusted 1980-2021 CUUR0000SAM. Available on the internet at: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths.
USBLS. 2022. Table 1. Employment Cost Index for total compensation, by occupational group and industry. Employment Cost Index Historical Listing—Volume III. Retrieved from https://www.bls.gov/web/eci/eci-current-nominal-.dollar.pdf.
United States Census Bureau. 2010. American Community Survey Five-Year Estimates. American Community Survey Demographic and Housing Estimates. Retrieved from https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes.2010.html.
United States Census Bureau. 2020. Annual County Resident Population Estimates by Age, Sex, Race, and Hispanic Origin: April 1, 2010 to July 1, 2019, downloaded at https://www2.census.gov/programs-surveys/popest/ on May 3, 2022.
United States Census Bureau. 2022. American Community Survey 5-Year Data (2009-2021). Retrieved from https://www.census.gov/data/developers/data-sets/acs-5year.html.
United States Environmental Protection Agency (USEPA). 1979. National Primary Drinking Water Regulations: Control of Trihalomethanes in Drinking Water; Final Rule. 44 FR 68624-68707. November 29, 1979.
USEPA. 1985. National Primary Drinking Water Regulations; Volatile Synthetic Organic Chemicals; Inorganic Chemicals and Microorganisms. Federal Register . 50 FR 46936-46906. November 13, 1985.
USEPA. 1986a. Guidelines for the Health Risk Assessment of Chemical Mixtures. EPA 630-R-98-002. Available on the internet at: https://www.epa.gov/risk/guidelines-health-risk-assessment-chemical-mixtures.
USEPA. 1986b. Guidelines for Carcinogen Risk Assessment. Federal Register . 51 FR 33992. September 24, 1986.
USEPA. 1987. National Primary Drinking Water Regulations—Synthetic Organic Chemicals; Monitoring for Unregulated Contaminants; Final Rule. Federal Register . 52 FR 25690, July 8, 1987.
USEPA. 1989. Risk assessment guidance for Superfund. Vol. 1. Human Health Evaluation Manual (Part A). EPA/540/1-89/002.
USEPA. 1991a. National Primary Drinking Water Regulations—Synthetic Organic Chemicals and Inorganic Chemicals; Monitoring for Unregulated Contaminants; National Primary Drinking Water Regulations Implementation; National Secondary Drinking Water Regulations. Federal Register . 56 FR 3526. January 30, 1991.
USEPA. 1991b. Risk Assessment Guidance for Superfund, Vol 1: Human Health Evaluation Manual (Part B, Development of Risk-Based Preliminary Remediation Goals). EPA/540/R-92/003. EPA, Emergency and Remedial Response, Washington, DC. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-b.
USEPA. 1994. Executive Order 12898—Federal Actions to Address Environmental Justice in Minority Populations and Low-Income Populations. Federal Register . 59 FR 7629, February 16, 1994.
USEPA. 1997. Discussion Summary: EPA Technology Design Workshop. Available on the internet at: https://downloads.regulations.gov/EPA-HQ-OW-2018-0780-0197/content.pdf.
USEPA. 1998a. National Primary Drinking Water Regulations: Disinfectants and Disinfection Byproducts. Federal Register . 63 FR 69390-69476. December 16, 1998.
USEPA. 1998b. Announcement of Small System Compliance Technology Lists for Existing National Primary Drinking Water Regulation and Findings Concerning Variance Technologies. Federal Register . 63 FR 42032. August 6, 1998.
USEPA. 1998c. National Primary Drinking Water Regulations: Consumer Confidence; Proposed Rule. Federal Register . 63 FR 7620, February 13, 1998.
USEPA. 1998d. National Primary Drinking Water Regulations: Consumer Confidence Reports. Federal Register . 63 FR 44524, August 19, 1998.
USEPA. 1999. Guidelines for Carcinogen Risk Assessment (review draft). Risk Assessment Forum, Washington, DC. NCEA-F-0644.
USEPA. 2000a. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA 630-R-00-002. Available on the internet at: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=20533.
USEPA. 2000b. EPA and 3M Announce Phase Out of PFOS. News Release. May 16, 2000. Available on the internet at: https://archive.epa.gov/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4.html.
USEPA. 2000c. Methodology for Deriving Ambient Water Quality Criteria for the Protection of Human Health (2000). EPA 822-B-00-004. Available on the internet at: https://www.epa.gov/sites/default/files/2018-10/documents/methodology-wqc-protection-hh-2000.pdf.
USEPA. 2000d. National Primary Drinking Water Regulations; Radionuclides; Final Rule. Federal Register . 65 FR 76708. December 7, 2000.
USEPA. 2000e. Arsenic Occurrence in Public Drinking Water Supplies. EPA 815-R-00-023.
USEPA. 2000f. Geometries and Characteristics of Public Water Systems. EPA-815-R-00-24.
USEPA. 2001. National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring. Federal Register . FR 28342. July 19, 2001.
USEPA. 2002. A Review of the Reference Dose and Reference Concentration Process. EPA 630-P-02-002F. Available on the internet at: https://www.epa.gov/sites/default/files/2014-12/documents/rfd-final.pdf.
USEPA. 2004. The Standardized Monitoring Framework: A Quick Reference Guide. EPA 816-F-04-010. Available on the internet at: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=3000667K.txt.
USEPA. 2005. Guidelines for Carcinogen Risk Assessment. EPA/630/P-03/001F. Available on the internet at: https://www.epa.gov/sites/default/files/2013-09/documents/cancer_guidelines_final_3-25-05.pdf.
USEPA. 2006a. National Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule; Final Rule. [EPA-HQ-OW-2002-0043; FRL-8012-1]. https://www.govinfo.gov/content/pkg/FR-2006-01-04/pdf/06-3.pdf.
USEPA. 2006b. National Primary Drinking Water Regulations: Long Term 2 Enhanced Surface Water Treatment Rule. Federal Register . 71 FR 654. January 5, 2006.
USEPA. 2009a. Drinking Water Contaminant Candidate List 3-Final. Federal Register . 74 FR 51850. October 8, 2009.
USEPA. 2009b. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). EPA/600/R-18/352. Available on the internet at: https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL.
USEPA. 2009c. Community Water System Survey, Volume II: Detailed Tables and Survey Methodology. EPA 815-R-09-002. Available on the internet at: http://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P1009USA.txt.
USEPA. 2010. Guidelines for Preparing Economic Analyses. Available on the internet at: https://www.epa.gov/sites/default/files/2017-08/documents/ee-0568-50.pdf.
USEPA. 2016a. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). EPA 822-R-16-004. Available on the internet at: https://www.epa.gov/sites/default/files/2016-05/documents/pfos_health_advisory_final-plain.pdf.
USEPA. 2016b. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA). EPA 822-R-16-005. Available on the internet at: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final-plain.pdf.
USEPA. 2016c. Drinking Water Contaminant Candidate List 4-Final. Federal Register . 81 FR 81099. November 17, 2016.
USEPA. 2016d. Six-Year Review 3—Health Effects Assessment for Existing Chemical and Radionuclide National Primary Drinking Water Regulations—Summary Report. EPA 822-R-16-008. Available on the internet at: https://www.epa.gov/sites/default/files/2016-12/documents/822r16008.pdf.
USEPA. 2016e. Health Effects Support Document for Perfluorooctanoic Acid (PFOA). EPA 822-R-16-003. Available on the internet at: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_hesd_final-plain.pdf.
USEPA. 2016f. Health Effects Support Document for Perfluorooctane Sulfonate (PFOS). EPA 822-R-16-002. Available on the internet at: https://www.epa.gov/sites/default/files/2016-05/documents/pfos_hesd_final_508.pdf.
USEPA. 2016g. Technical Guidance for Assessing Environmental Justice in Regulatory Analysis. Available on the internet at: https://www.epa.gov/sites/production/files/2016-06/documents/ejtg_5_6_16_v5.1.pdf.
USEPA. 2017. Third Unregulated Contaminant Monitoring Rule: Occurrence Data. Available on the internet at: https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule#3.
USEPA. 2018a. Basic Information on PFAS. Available on the internet at: https://19january2021snapshot.epa.gov/pfas/basic-information-pfas_.html.
USEPA. 2018b. Fact Sheet: 2010/2015 PFOA Stewardship Program. Available on the internet at: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program. Last updated August 9, 2018.
USEPA. 2018c. Technologies and Costs for Treating Perchlorate-Contaminated Waters. EPA 816-R-19-005. Available on the internet at: https://nepis.epa.gov/Exe/ZyPDF.cgi/P101340O.PDF?Dockey=P101340O.PDF.
USEPA. 2019a. Update for Chapter 3 of the Exposure Factors Handbook: Ingestion of Water and Other Select Liquids. EPA 600-R-18-259F. Available on the internet at: https://www.epa.gov/sites/default/files/2019-02/documents/efh_-_chapter_3_update.pdf.
USEPA. 2019b. Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry. EPA 815-B-19-020. Available on the internet at: https://www.epa.gov/sites/default/files/2019-12/documents/method-533-815b19020.pdf.
USEPA. 2019c. Best Available Technologies and Small System Compliance Technologies for Perchlorate in Drinking Water. EPA 816-R-19-006.
USEPA. 2019d. Supplemental Environmental Assessment for Proposed Revisions to Effluent Limitations Guidelines and Standards for the Steam Electric Power Generating Point Source Category (EA).
USEPA. 2019e. EJSCREEN Technical Documentation. Available on the internet at: https://www.epa.gov/sites/default/files/2021-04/documents/ejscreen_technical_document.pdf.
USEPA. 2020a. Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). EPA 600-R-20-006, Version 2.0, March 2020. Available on the internet at: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=348508.
USEPA, 2020b. Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances. EPA-HQ-OLEM-2020-0527-0002. Available on the internet at: https://www.epa.gov/system/files/documents/2021-11/epa-hq-olem-2020-0527-0002_content.pdf.
USEPA. 2021a. Human Health Toxicity Values for Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). EPA/600/R-20/345F. Available on the internet at: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=350888.
USEPA. 2021b. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3). Also Known as “GenX Chemicals.” EPA/822/R-21/010. Available on the internet at: https://www.epa.gov/system/files/documents/2021-10/genx-chemicals-toxicity-assessment_tech-edited_oct-21-508.pdf.
USEPA. 2021c. Assessing and Managing Chemicals under TSCA: Fact Sheet PFOA Stewardship Program. https://www.epa.gov/assessing-andmanaging-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program.
USEPA. 2021d. Announcement of Final Regulatory Determinations for Contaminants on the Fourth Drinking Water Contaminant Candidate List. Federal Register . 86 FR 12272, March 3, 2021.
USEPA. 2021e. Proposed Approaches to the Derivation of a Draft Maximum Contaminant Level Goal for Perfluorooctanoic Acid (PFOA)(CASRN 335-67-1) in Drinking Water. EPA 822-D-21-001. Available on the internet at: https://sab.epa.gov/ords/sab/f?p=100:18:16490947993:::RP,18:P18_ID:2601.
USEPA. 2021f. Proposed Approaches to the Derivation of a Draft Maximum Contaminant Level Goal for Perfluorooctane Sulfonic Acid (PFOS) (CASRN 1763-23-1) in Drinking Water. EPA 822-D-21-002. Available on the internet at: https://sab.epa.gov/ords/sab/f?p=100:18:16490947993:::RP,18:P18_ID:2601.
USEPA. 2021g. Work Breakdown Structure-Based Cost Model for Granular Activated Carbon Drinking Water Treatment. Available on the internet at: https://www.epa.gov/system/files/documents/2022-03/gac-documentation-.pdf_0.pdf.
USEPA. 2021h. Work Breakdown Structure-Based Cost Model for Ion Exchange Treatment of Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water.
USEPA. 2021i. Work Breakdown Structure-Based Cost Model for Reverse Osmosis/Nanofiltration Drinking Water Treatment. Available on the internet at: https://www.epa.gov/system/files/documents/2022-03/ronf-documentation-.pdf.pdf.
USEPA. 2021j. Work Breakdown Structure-Based Cost Model for Nontreatment Options for Drinking Water Compliance. Available on the internet at: https://www.epa.gov/sites/default/files/2019-07/documents/wbs-nontreatment-documentation-june-2019.pdf.
USEPA. 2021k. IRIS Toxicological Review of Perfluorobutanoic Acid (PFBA) and Related Salts (Public Comment and External Review Draft, 2021). EPA/635/R-20/424. Retrieved from https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=350051.
USEPA. 2022a. Transmittal of the Science Advisory Board Report titled, “Review of EPA's Analyses to Support EPA's National Primary Drinking Water Rulemaking for PFAS.” EPA-22-008. Available on the internet at: https://sab.epa.gov/ords/sab/f?p=114:12:15255596377846.
USEPA. 2022b. Drinking Water Contaminant Candidate List 5—Final. Federal Register . 87 FR 68060, November 14, 2022.
USEPA. 2022c. PFAS Strategic Roadmap: EPA's Commitments to Action 2021-2024. Available on the internet at: https://www.epa.gov/pfas/pfas-strategic-roadmap-epas-commitments-action-2021-2024.
USEPA. 2022d. Drinking Water Health Advisory: Hexafluoropropylene Oxide (HFPO) Dimer Acid (CASRN 13252-13-6) and HFPO Dimer Acid Ammonium Salt (CASRN 62037-80-3), Also Known as “GenX Chemicals.” EPA/822/R-22/005. Available on the internet at: https://www.epa.gov/system/files/documents/2022-06/drinking-water-genx-2022.pdf.
USEPA. 2022e. Drinking Water Health Advisory: Perfluorobutane Sulfonic Acid (CASRN 375-73-5) and Related Compound Potassium Perfluorobutane Sulfonate (CASRN 29420-49-3). EPA/822/R-22/006. Available on the internet at: https://www.epa.gov/system/files/documents/2022-06/drinking-water-pfbs-2022.pdf.
USEPA. 2022f. ORD Staff Handbook for Developing IRIS Assessments. EPA 600/R-22/268. Available on the internet at: https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=356370.
USEPA. 2022g. Fifth Unregulated Contaminant Monitoring Rule. Available on the internet at: https://www.epa.gov/dwucmr/fifth-unregulated-contaminant-monitoring-rule.
USEPA, 2022h. IRIS Toxicological Review of Perfluorohexanoic Acid (PFHxA) and Related Salts (Public Comment and External Review Draft). EPA/635/R-21/312a. Retrieved from https://cfpub.epa.gov/ncea/iris_drafts/recordisplay.cfm?deid=352767.
USEPA. 2023a. Maximum Contaminant Level Goal (MCLG) Summary Document for a Mixture of Four Per- and Polyfluoroalkyl Substances (PFAS): GenX Chemicals, PFBS, PFNA and PFHxS. EPA-822-P-23-004.
USEPA. 2023b. Public Comment Draft—Toxicity Assessment and Proposed Maximum Contaminant Level Goal (MCLG) for Perfluorooctanoic Acid (PFOA) (CASRN 335-67-1) in Drinking Water. EPA-822-P-23-005.
USEPA. 2023c. Public Comment Draft—Toxicity Assessment and Proposed Maximum Contaminant Level Goal (MCLG) for Perfluorooctane Sulfonic Acid (PFOS) (CASRN 1763-23-1) in Drinking Water. EPA-822-P-23-007.
USEPA. 2023d. Framework for Estimating Noncancer Health Risks Associated with Mixtures of Per- and Polyfluoroalkyl Substances (PFAS). EPA-822-P-23-003.
USEPA. 2023e. PFAS Occurrence and Contaminant Background Support Document. EPA-822-P-23-010.
USEPA. 2023f. EPA Response to Final Science Advisory Board Recommendations (August 2022) on Four Draft Support Documents for the EPA's Proposed PFAS National Primary Drinking Water Regulation. EPA-822-D-23-001.
USEPA. 2023g. Best Available Technologies and Small System Compliance Technologies Per- and Polyfluoroalkyl Substances (PFAS) in Drinking Water. EPA-822-P-23-009.
USEPA. 2023h. Technologies and Costs for Removing Per- and Polyfluoroalkyl Substances from Drinking Water. EPA-822-P-23-011.
USEPA. 2023i. Appendix: Economic Analysis of the Proposed National Primary Drinking Water Regulation for Per- and Polyfluoroalkyl Substances. EPA-822-P-23-002.
USEPA. 2023j. Economic Analysis of the Proposed National Primary Drinking Water Regulation for Per- and Polyfluoroalkyl Substances. EPA-822-P-23-001.
USEPA. 2023k. Public Comment Draft—Appendix: Toxicity Assessment and Proposed Maximum Contaminant Level Goal for Perfluorooctane Sulfonic acid (PFOS) (CASRN 1763-23-1) in Drinking Water. Washington, DC EPA-822-P-23-008.
USEPA. 2023l. Public Comment Draft—Appendix: Toxicity Assessment and Proposed Maximum Contaminant Level Goal for Perfluorooctanoic Acid (PFOA) (CASRN 335-67-1) in Drinking Water. Washington, DC EPA-822-P23-006.
USEPA. 2023m. EPA Response to Letter of Peer Review for Disinfectant Byproduct Reduction as a Result of Granular Activated Carbon Treatment for PFOA and PFOS in Drinking Water: Benefits Analysis Related to Bladder Cancer. EPA-815-B23-001.
Valvi, D., Oulhote, Y., Weihe, P., Dalgård, C., Bjerve, K.S., Steuerwald, U., and Grandjean, P. 2017. Gestational Diabetes and Offspring Birth Size at Elevated Environmental Pollutant Exposures. Environment International, 107:205-215. https://doi.org/10.1016/j.envint.2017.07.016.
Verner, M.A., Loccisano, A.E., Morken, N.H., Yoon, M., Wu, H., McDougall, R., Maisonet, M., Marcus, M., Kishi, R., Miyashita, C., Chen., M.-H, Hsieh, W.-S, Andersen, M.E., Clewell III, H.J., and Longnecker, M.P. 2015. Associations of Perfluoroalkyl Substances (PFAS) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK). Environmental Health Perspectives, 123(12):1317-1324. https://doi.org/10.1289/ehp.1408837.
Vieira, V.M., Hoffman, K., Shin, H., Weinberg, J.M., Webster, T.F., and Fletcher, T. 2013. Perfluorooctanoic Acid Exposure and Cancer Outcomes in a Contaminated Community: A Geographic Analysis. Environmental Health Perspectives, 121(3):318-323. https://doi.org/10.1289/ehp.1205829.
Villanueva, C.M., Cantor, K.P., Cordier, S., Jaakkola, J.J.K., King, W.D., Lynch, C.F., Porru, S., and Kogevinas, M. 2004. Disinfection Byproducts and Bladder Cancer: a Pooled Analysis. Epidemiology, 15(3):357-367. https://doi.org/10.1097/01.ede.0000121380.02594.fc.
Villanueva, C.M., Cantor, K.P., King, W.D., Jaakkola, J.J., Cordier, S., Lynch, C.F., Porru, S., and Kogevinas, M. 2006. Total and Specific Fluid Consumption as Determinants of Bladder Cancer Risk. International Journal of Cancer, 118(8):2040-2047. https://doi.org/10.1002/ijc.21587.
Villanueva, C.M., Cantor, K.P., Grimalt, J.O., Malats, N., Silverman, D., Tardon, A., Garcia-Closas, R., Serra, C., Carrato., A., Castano-Vinyals, G., Marcos, R., Rothman, N., Real, F.X., Dosemeci, M., and Kogevinas, M. 2007. Bladder Cancer and Exposure to Water Disinfection By-products through Ingestion, Bathing, Showering, and Swimming in Pools. American Journal of Epidemiology, 156(2):148-156. https://doi.org/10.1093/aje/kwj364.
Wang, Y., Adgent, M., Su, P.-H., Chen, H.-Y., Chen, P.-C., Hsiung, C. A., and Wang, S.-L. 2016. Prenatal exposure to perfluorocarboxylic acids (PFCAs) and fetal and postnatal growth in the Taiwan Maternal and Infant Cohort Study. Environmental health perspectives, 124(11), 1794-1800.
Wang, L., Vacs Renwick, D., and Regli, S. 2019. Re-assessing effects of bromide and granular activated carbon on disinfection byproduct formation. AWWA Water Science, 1 (4).
Waterfield, G., Rogers, M., Grandjean, P., Auffhammer, M., and Sunding, D. 2020. Reducing Exposure to High Levels of Perfluorinated Compounds in Drinking Water Improves Reproductive Outcomes: Evidence from an Intervention in Minnesota. Environmental Health, 19:1-11. https://doi.org/10.1186/s12940-020-00591-0.
Weisman, R.J., Heinrich, A., Letkiewicz, F., Messner, M., Studer, K., Wang, L., and Regli, S. 2022. Estimating National Exposures and Potential Bladder Cancer Cases Associated with Chlorination DBPs in US Drinking Water. Environmental Health Perspectives, 130(8):087002. https://doi.org/10.1289/EHP9985.
Westreich, P., Mimna, R., Brewer, J., and Forrester, F. 2018. The Removal of Short‐chain and Long‐chain Perfluoroalkyl Acids and Sulfonates via Granular Activated Carbons: A Comparative Column Study. Remediation, 29(1)19-26. https://doi.org/10.1002/rem.21579.
Wikström, S., Lin, P.I., Lindh, C.H., Shu, H., and Bornehag, C.G. 2020. Maternal Serum Levels of Perfluoroalkyl Substances in Early Pregnancy and Offspring Birth Weight. Pediatric Research, 87(6):1093-1099. https://doi.org/10.1038/s41390-019-0720-1.
Windham, G., and Fenster, L. 2008. Environmental Contaminants and pregnancy Outcomes. Fertility and Sterility, 89(2):e111-e116. https://doi.org/10.1016/j.fertnstert.2007.12.041.
Woodard, S., Berry, J., and Newman, B. 2017. Ion Exchange Resin for PFAS Removal and Pilot Test Comparison to GAC. Remediation, 27:19-27. https://doi.org/10.1002/rem.21515.
Yan, B., Munoz, G., Sauvé, S., and Liu, J. 2020. Molecular Mechanisms of Per- and Polyfluoroalkyl Substances on a Modified Clay: a Combined Experimental and Molecular Simulation Study. Water Research, 184(2020):116166. https://doi.org/10.1016/j.watres.2020.116166.
Yao, Q., Gao, Y., Zhang, Y., Qin, K., Liew, Z., and Tian, Y. 2021. Associations of Paternal and Maternal Per- and Polyfluoroalkyl Substances Exposure with Cord Serum Reproductive Hormones, Placental Steroidogenic Enzyme and Birth Weight. Chemosphere, 285:131521. https://doi.org/10.1016/j.chemosphere.2021.131521.
Yapsakli, K., and Çeçen, F. 2010. Effect of Type of Granular Activated Carbon on DOC Biodegradation in Biological Activated Carbon Filters. Process Biochemistry, 45(3):355-362. https://doi.org/10.1016/j.procbio.2009.10.005.
Yu, J., Lv, L., Lan P., Zhang, S., Pan, B., and Zhang, W. 2012. Effect of Effluent Organic atter on the Adsorption of Perfluorinated Compounds onto Activated Carbon. Journal of Hazardous Materials, 225:99-106. https://doi.org/10.1016/j.jhazmat.2012.04.073.
Zaggia, A., Conte, L., Falletti, L., Fant, M., and Chiorboli, A. 2016. Use of Strong Anion Exchange Resins for the Removal of Perfluoroalkylated Substances from Contaminated Drinking Water in Batch and Continuous Pilot Plants. Water Research, 91:137-146. https://doi.org/10.1016/j.watres.2015.12.039.
Zeng, C., Atkinson, A., Sharma, N., Ashani, H., Hjelmstad, A., Venkatesh, K., and Westerhoff, P. 2020. Removing Per-and Polyfluoroalkyl Substances from Groundwaters using Activated Carbon and Ion Exchange Resin Packed Columns. AWWA Water Science, 2(1):e1172. https://doi.org/10.1002/aws2.1172.
List of Subjects
40 CFR Part 141
Environmental protection, Indians—lands, Intergovernmental relations, National Primary Drinking Water Regulation, PFAS, Monitoring and analytical requirements, Reporting and recordkeeping requirements, Water supply, Incorporation by reference.
40 CFR Part 142
Environmental protection, Administrative practice and procedure, Indians—lands, Intergovernmental relations, National Primary Drinking Water Regulation, PFAS, Monitoring and analytical requirements, Reporting and recordkeeping requirements, Water supply.
Michael S. Regan,
Administrator.
For the reasons stated in the preamble, the Environmental Protection Agency proposes to amend 40 CFR parts 141 and 142 as follows:
PART 141—NATIONAL PRIMARY DRINKING WATER REGULATIONS
1. The authority citation for part 141 continues to read as follows:
Authority:
42 U.S.C. 300f, 300g-1, 300g-2, 300g-3, 300g-4, 300g-5, 300g-6, 300j-4, 300j-9, and 300j-11.
2. Amend §141.2 by adding in alphabetical order definitions for “Hazard Index (HI)”, “Hazard Quotient (HQ)”, “Health-based water concentration (HBWC)”, “HFPO-DA or GenX chemicals”, “PFBS”, “PFHxS”, “PFNA”, “PFOA”, and “PFOS” to read as follows:
§141.2 Definitions.
* * * * *
Hazard index (HI) is the sum of component hazard quotients (HQs), which are calculated by dividing the measured regulated PFAS component contaminant concentration in water ( e.g., expressed as ppt or ng/l) by the associated Health-Based Water Concentration ( e.g., HBWC expressed as ppt). For PFAS, a mixture HI greater than 1.0 (unitless) is an exceedance of the MCL.
Hazard quotient (HQ) are the ratio of potential exposure to a substance and the level at which no health effects are expected.
Health-based water concentration (HBWC) are levels protective of health effects over a lifetime of exposure, including sensitive populations and life stages.
HFPO-DA or GenX chemicals means Chemical Abstract Service registration number 122499-17-6, chemical formula C6F11O3-, International Union of Pure and Applied Chemistry preferred name 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
* * * * *
PFBS means Chemical Abstract Service registration number 45187-15-3, chemical formula C4F9SO3-, perfluorobutane sulfonate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
PFHxS means Chemical Abstract Service registration number 108427-53-8, chemical formula C6F13SO3-, perfluorohexane sulfonate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
PFNA means Chemical Abstract Service registration number 72007-68-2, chemical formula C9F17O2-, perfluorononanoate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
PFOA means Chemical Abstract Service registration number 45285-51-6, chemical formula C8F15CO2-, perfluorooctanoate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
PFOS means Chemical Abstract Service registration number 45298-90-6, chemical formula C8F17SO3-, perfluorooctanesulfonate, along with its conjugate acid and any salts, derivatives, isomers or combinations thereof.
* * * * *
3. Amend §141.6 by revising paragraph (a) and adding paragraph (l) to read as follows:
§141.6 Effective dates.
(a) Except as provided in paragraphs (b) through (1) of this section the regulations set forth in this part shall take effect on June 24, 1977.
* * * * *
(l) The regulations contained in the revision to §§141.50, 141.60, 141.61, 141.154, 141.151 through 141.155; and 141.201 through 141.211 are effective for the purposes of compliance on [DATE THREE YEARS AFTER DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ]
4. Amend §141.28 by revising paragraph (a) to read as follows:
§141.28 Certified laboratories.
(a) For the purpose of determining compliance with §141.21 through 141.27, 141.30, 141.40, 141.74, 141.89, 141.402, and 141.900 through 141.905, samples may be considered only if they have been analyzed by a laboratory certified by the State except that measurements of alkalinity, disinfectant residual, orthophosphate, pH, silica, temperature, and turbidity may be performed by any person acceptable to the State.
* * * * *
5. Amend §141.50 by adding paragraphs (a)(24) and (25) and in the table in paragraph (b), revising the heading for the second column and adding an entry for “(34)” and footnote 1 to read as follows:
§141.50 Maximum contaminant level goals for organic contaminants.
(a) * * *
(24) PFOA
(25) PFOS
(b) * * *
| Contaminant | MCLG in mg/l (unless otherwise noted) |
| * * * * * * * | |
| (34) Hazard Index PFAS (PFNA, HFPO-DA, PFHxS, and PFBS) | 1.0 (unitless). 1 |
| 1 The PFAS Mixture HI MCLG is the sum of component hazard quotients (HQs), which are calculated by dividing the measured component PFAS concentration in water ( e.g., expressed as ppt or ng/l) by the corresponding contaminant's Health-Based Water Concentration ( e.g., HBWC expressed as ppt). The HBWC for PFHxS is 9.0 ppt; the HBWC for HFPO-DA is 10.0 ppt; the HBWC for PFNA is 10 ppt; the HBWC for PFBS is 2000.0 ppt. A PFAS Mixture HI MCLG greater than 1.0 (unitless) indicates an exceedance of the health protective level and indicates potential human health risk from the PFAS mixture in drinking water. HI MCLG = ([GenXwater]/[10 ppt]) ([PFBSwater]/[2000 ppt]) ([PFNAwater]/[10 ppt]]) ([PFHxSwater]/[9.0 ppt]). | |
6. Amend §141.60 by adding paragraph (a)(4) to read as follows:
§141.60 Effective dates.
(a) * * *
(4) The effective date for paragraphs (c)(34) through (36) is [DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ].
* * * * *
7. Amend §141.61:
a. In the table in paragraph (b) by adding entries for “45285-51-6”, “45298-90-6”, and “108427-53-8; 122499-17-6; 72007-68-2; 45187-15-3” at the end of the table; and
b. In the table in paragraph (c) by revising the heading for the third column, adding entries for “(34)”, “(35)”, and “(36)” at the end of the table, and adding footnote 1.
The additions read as follows:
§141.61 Maximum contaminant levels for organic contaminants.
(b) * * *
| CAS. No. | Contaminant | GAC | PTA | OX |
| * * * * * * * | ||||
| 45285-51-6 | PFOA | X | ||
| 45298-90-6 | PFOS | X | ||
| 108427-53-8; 122499-17-6; 72007-68-2; 45187-15-3 | Hazard Index PFAS (PFNA, HFPO-DA, PFHxS, and PFBS | X | ||
(c) * * *
| CAS. No. | Contaminant | MCL (mg/L) (unless otherwise noted) |
| * * * * * * * | ||
| (34) 45285-51-6 | PFOA | 0.0000040. |
| (35) 45298-90-6 | PFOS | 0.0000040. |
| (36) 108427-53-8; 122499-17-6; 72007-68-2; 45187-15-3 | Hazard Index PFAS (PFNA, HFPO-DA, PFHxS, and PFBS | 1.0 (unitless). 1 |
| 1 The PFAS Mixture HI MCL is the sum of component hazard quotients (HQs), which are calculated by dividing the measured component PFAS concentration in water ( e.g., expressed as ppt) by the relevant Health-Based Water Concentration ( e.g., HBWC expressed as ppt. The HBWC for PFHxS is 9.0 ppt; the HBWC for HFPO-DA is 10.0 ppt; the HBWC for PFNA is 10.0 ppt the HBWC for PFBS is 2000.0 ppt. A PFAS Mixture HI MCL greater than 1.0 is an MCL violation. HI MCL = ([GenXwater]/[10 ppt]) ([PFBSwater]/[2000 ppt]) ([PFNAwater]/[10 ppt]) ([PFHxSwater]/[9.0 ppt]). | ||
8. Amend §141.151 by revising paragraph (d) to read as follows:
§141.151 Purpose and applicability of this subpart
* * * * *
(d) For the purpose of this subpart, detected means: at or above the levels prescribed by §141.23(a)(4) for inorganic contaminants, at or above the levels prescribed by §141.24(f)(7) for the contaminants listed in §141.61(a), at or above the levels prescribed by §141.24(h)(18) for the contaminants listed in §141.61(c), at or above the levels prescribed by §141.131(b)(2)(iv) for the contaminants or contaminant groups listed in §141.64, at or above the levels prescribed by §141.25(c) for radioactive contaminants, and at or above the levels prescribed §141.902(a)(9) for PFAS listed in §141.61(c).
* * * * *
9. Amend §141.154 by adding paragraph (g) to read as follows:
§141.154 Required additional health information.
* * * * *
(g) Community water systems that detect any PFAS above the MCL in §141.61(c), as monitored and calculated under the provisions of subpart Z of this part must include health effects language for PFAS prescribed by appendix A to subpart O of this part.
10. Amend appendix A to subpart O by adding entries for “PFOA”, “PFOS”, and “Hazard Index PFAS (PFHxS, HFPO-DA, PFNA, and PFBS)” at the end of the table and adding footnote 2 immediately after footnote 1 to read as follows:
Appendix A to Subpart O of Part 141—Regulated Contaminants
| * * * * * * * | ||||||
| 2 Subpart A of §141.2. | ||||||
| Contaminant (units) | Traditional MCL in mg/L | To convert for CCR, multiply by | MCL in CCR units | MCLG | Major sources in drinking water | Health effects language |
| * * * * * * * | ||||||
| PFOA | 0.0000040 | 1,000,000 | 4.0 ppt | 0 | Discharge from manufacturing and industrial chemical facilities, and certain firefighting activities | Some people who drink water containing PFOA in excess of the MCL could develop immune health effects, fetal growth effects after exposure during pregnancy, certain types of cancers, or an increased risk of cardiovascular disease or liver disease. |
| Hazard Index PFAS (PFHxS, HFPO-DA, PFNA, and PFBS) | 1.0 (unitless) | No conversion | No conversion | 2 1.0 | Discharge from manufacturing and industrial chemical facilities, and certain firefighting activities | Some people who drink water containing PFHxS, HFPO-DA, PFNA, and PFBS in excess of the Hazard Index MCL could develop thyroid, liver, or developmental health effects. |
* * * * *
11. Amend appendix A to subpart Q under the Contaminant heading “D. Synthetic Organic Chemicals (SOCs)” by adding entries for “31”, “32”, and “33” in numerical to read as follows:
Appendix A to Subpart Q of Part 141—NPDWR Violations and Other Situations Requiring Public Notice 1
| Contaminant | MCL/MRDL/TT violations 2 | Monitoring & testing procedure violations | ||
| Tier of public notice required | Citation | Tier of public notice required | Citation | |
| * * * * * * * | ||||
| 31 | 2 | 141.61(c) | 3 | 141.XX |
| 32 | 2 | 141.61(c) | 3 | 141.XX |
| 33 | 2 | 141.61(c) | 3 | 141.XX |
| * * * * * * * | ||||
| * * * * * * * | ||||
| 1 Violations and other situations not listed in this table ( e.g., failure to prepare Consumer Confidence Reports), do not require notice, unless otherwise determined by the primacy agency. Primacy agencies may, at their option, also require a more stringent public notice tier ( e.g., Tier 1 instead of Tier 2 or Tier 2 instead of Tier 3) for specific violations and situations listed in this appendix, as authorized under §141.202(a) and §141.203(a). | ||||
| 2 MCL—Maximum contaminant level, MRDL—Maximum residual disinfectant level, TT—Treatment technique. | ||||
* * * * *
12. Amend appendix B to subpart Q by adding entries for “PFOA”, “PFOS”, and “Hazard Index PFAS (PFHxS, HFPO-DA, PFNA, and PFBS)” at the end of the table under new heading “J. PFAS” and adding footnote 24 to read as follows:
Appendix B to Subpart Q of Part 141—Standard Health Effects Language for Public Notification
| 1 MCLG—Maximum contaminant level goal. | |||
| 2 MCL—Maximum contaminant level. | |||
| * * * * * * * | |||
| 24 Subpart A of §141.2. | |||
| * * * * * * * | |||
| Contaminant | MCLG 1 mg/L | MCL 2 mg/L | Standard health effects language for public notification |
| * * * * * * * | |||
| J. PFAS | |||
| PFOA | 0 | 0.0000040 | Some people, including children, who drink water containing PFOA in excess of the MCL could develop immune health effects, fetal growth effects after exposure during pregnancy, certain types of cancers, or an increased risk of cardiovascular disease or liver disease. |
| Hazard Index PFAS (PFHxS, HFPO-DA, PFNA, and PFBS) | 1.0 (unitless) | 1.0 (unitless) 24 | Some people who drink water containing PFHxS, HFPO-DA, PFNA, and PFBS in excess of the Hazard Index MCL could develop thyroid, liver, or developmental health effects. |
13. Amend appendix C to subpart Q by adding in alphabetical order the acronyms “HI” and “PFAS” to read as follows:
Appendix C to Subpart Q of Part 141—List of Acronyms Used in Public Notification Regulation
* * * * *
HI Hazard Index
* * * * *
PFAS Per- and Polyfluoroalkyl Substances
* * * * *
14. Subpart Z is added to read as follows:
Subpart Z—Control of Per- and Polyfluoroalkyl Substances (PFAS)
Sec.
141.900 General requirements.
141.901 Analytical requirements.
141.902 Monitoring requirements.
141.903 Compliance requirements.
141.904 Reporting and recordkeeping requirements.
141.905 Violations.
Subpart Z—Control of Per- and Polyfluoroalkyl Substances (PFAS)
§141.900 General requirements.
(a) The requirements of this subpart constitute national primary drinking water regulations. These regulations establish criteria under which control of certain PFAS is required for community water systems (CWS) and non-transient, non-community water systems (NTNCWS). Each CWS and NTNCWS must comply with the maximum contaminant levels for certain PFAS as outlined in this subpart.
(b) Compliance dates.
(c) CWS and NTNCWS, unless otherwise noted, must comply with the requirement of this subpart.
§141.901 Analytical requirements.
(a) General. (1) Systems must use only the analytical methods specified in this section to demonstrate compliance with the requirement of this subpart.
(2) The following documents are incorporated by reference. This incorporation by reference was approved by the Director of the Federal Register in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies may be inspected at EPA's Drinking Water Docket, 1301 Constitution Avenue NW, EPA West, Room 3334, Washington, DC 20460 (Telephone: 202-566-2426); or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
(i) EPA method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry, (December 2019, 815-B-19-020). https://www.epa.gov/dwanalyticalmethods/method-533-determination-and-polyfluoroalkyl-substances-drinking-water-isotope;
(ii) Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) (November 2018, EPA/600/R-18/352). https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=343042&Lab=NERL.
(b) PFAS —(1) Analytical methods. Systems must measure regulated PFAS by the methods listed in the following table:
| Contaminant | EPA method |
|---|---|
| Perfluorooctanesulfonic acid (PFOS) | 533, 537.1 |
| Perfluorooctanoic acid (PFOA) | 533, 537.1 |
| Hazard Index PFAS (PFNA, HFPO-DA, PFHxS, and PFBS) | 533, 537.1 |
(2) Laboratory certification. Analyses under this section for regulated PFAS must be conducted by laboratories that have received certification by the State.
(i) Beginning [DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ], report quantitative data for concentrations at least as low as the ones listed in the following table for all PFAS samples analyzed for compliance with §141.902 (Monitoring Requirements).
(ii) [Reserved]
(iii) To receive certification to conduct analyses for the regulated PFAS contaminants, the laboratory must:
(A) Analyze Performance Evaluation (PE) samples that are acceptable to the State at least once during each consecutive 12-month period by each method for which the laboratory desires certification.
(B) Beginning [DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ], the laboratory must achieve quantitative results on the PE sample analyses that are within the following acceptance limits:
| Contaminant | Acceptance limits (percent of true value) |
|---|---|
| Perfluorooctanesulfonic acid (PFOS) | 70-130 |
| Perfluorooctanoic acid (PFOA) | 70-130 |
| Hazard Index PFAS—PFNA | 70-130 |
| Hazard Index PFAS—HFPO-DA | 70-130 |
| Hazard Index PFAS—PFHxS | 70-130 |
| Hazard Index PFAS—PFBS | 70-130 |
§141.XX. Monitoring requirements.
(a) General requirements. (1) Systems must take all samples during normal operating conditions at all entry points to the distribution system.
(2) If the system draws water from more than one source and the sources are combined before distribution, the system must sample at an entry point to the distribution system during periods of representative operating conditions.
(3) Failure to monitor in accordance with the monitoring requirements required under paragraph (b) of this section is a monitoring violation.
(4) If a system fails to collect the required number of samples, compliance will be based on the total number of samples collected.
(5) Systems must only use data collected under the provisions of this subpart to qualify for reduced monitoring.
(6) All new systems that begin operation after, or systems that use a new source of water after, [DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ] must demonstrate compliance with the MCLs within a period of time specified by the State. The system must also comply with initial sampling frequencies required by the State to ensure that the system can demonstrate compliance with the MCLs. Routine and increased monitoring frequencies must be conducted in accordance with the requirements in this section.
(7) For purposes of this section, the trigger level is defined as 1.3 ppt for PFOA and PFOS and a Hazard Index of 0.33 for PFAS.
(8) Based on initial monitoring results, for each sampling point at which a contaminant listed in §141.61(c) is detected at a level greater than or equal to the trigger level, the system must monitor quarterly for all regulated PFAS beginning in the next quarter, in accordance with §141.902(a).
(9) For purposes of this section, a reportable detection means at or above one-third of the levels described in the table outlined in §141.903(f)(1)(i)(3).
(b) Monitoring requirements for PFAS —(1) Initial compliance period. (i) Groundwater CWS and NTNCWS serving greater than 10,000 and all surface water CWS and NTNCWS must take four consecutive quarterly samples for each contaminant listed in §141.61(c).
(ii) All groundwater CWS and NTNCWS serving 10,000 or fewer shall take two samples for each contaminant listed in 141.61(c) at least ninety days apart within a 12-month period.
(iii) All groundwater under the direct influence (GWUDI) CWS and NTNCWS shall follow the surface water CWS and NTNCWS monitoring schedule based on system size, though a State may require more frequent monitoring on a system-specific basis.
(iv) Systems must monitor at a frequency indicated in the following table:
| Type of system | Minimum monitoring frequency | Sample location |
|---|---|---|
| Groundwater CWS and NTNCWS serving greater than 10,000 persons and all surface water CWS and NTNCWS | Four consecutive quarters of samples per entry point to the distribution system (EPTDS). Samples must be taken at least ninety days apart | EPTDS. |
| Groundwater CWS and NTNCWS serving 10,000 or fewer persons | In a consecutive 12-month period, two samples per each EPTDS. Samples must be acquired at least ninety days apart | EPTDS. |
(v) To satisfy initial compliance period monitoring requirements a State may accept data that has been previously acquired by a water system to count toward the initial monitoring requirements listed in table 1 to paragraph (b)(1)(iv) of this section. Such data may only be used if it was collected in accordance with §141.40 and that such samples were collected starting on or after January 1, 2023. Data collected between January 1, 2019, and December 31, 2022, may also be used if it is below the rule trigger level of 1.3 ppt for PFOA and PFOS and below an HI of 0.33.
(vi) If systems have multiple years of data, the most recent data must be used. If a system has fewer than the number of samples required for initial monitoring as listed in the table, then all surface water systems, GWUDI systems, and groundwater systems serving greater than 10,000 must collect at least one sample in each quarter of a calendar year that was not acquired, and groundwater systems serving 10,000 or fewer must collect one sample in a different quarter of the calendar year than the one in which the previous sample was acquired. This must be completed by [DATE THREE YEARS AFTER DATE OF PUBLICATION OF FINAL RULE IN THE FEDERAL REGISTER ].
(2) Compliance monitoring. (i) Based on initial monitoring results, or on compliance monitoring results after the initial monitoring period, systems may reduce monitoring at each sampling point at which the rule trigger level was not met or exceeded in accordance with the following table, except as otherwise provided by the State.
| If you are a . . . | You may reduce monitoring if your . . . | To this level |
|---|---|---|
| CWS and NTNCWS serving more than 3,300 persons | Averages from initial monitoring period or compliance monitoring running annual averages for PFOA and PFOS are each <1.3 ppt and HI <0.33 | In a consecutive 12-month period, two samples per each EPTDS during each three-year compliance period. Samples must be acquired at least ninety days apart. |
| CWS and NTNCWS serving 3,300 or fewer persons | Averages from initial monitoring period or compliance monitoring running annual averages for PFOA and PFOS are each <1.3 ppt and HI <0.33 | One sample at each EPTDS during each three-year compliance period for a total of one sample per three-year compliance period. |
(ii) If a system is monitoring less frequently than quarterly and if a contaminant listed in §141.61(c) is detected at a level exceeding the trigger level of 1.3 ppt for either PFOS or PFOA, or a Hazard Index of 0.33 for PFNA, PFHxS, HFPO-DA, and PFBS in any sample, then the system must monitor quarterly beginning in the next quarter at each sampling point which resulted in a detection in accordance with §141.902(a). The triggering sample must be used as the first quarter of monitoring for the running annual average calculation.
(iii) Systems that are at or exceed the trigger level of 1.3 ppt for either PFOS or PFOA, or a Hazard Index of 0.33 for PFNA, PFHxS, HFPO-DA, and PFBS must conduct quarterly monitoring for regulated PFAS for at least four consecutive quarters. If after four consecutive quarters of quarterly monitoring, the running annual average is less than the trigger level, then the State may determine that the system is reliably and consistently below the MCL for regulated PFAS and allow the system to return to reduced monitoring as shown in table 2 to paragraph (b)(2)(i) of this section.
(iv) The State may require a confirmation sample for any sampling result. If a confirmation sample is required by the State, the result must be averaged with the first sampling result and the average must be used for the compliance determination as specified by §141.903. States may delete results of obvious sampling errors from this calculation.
(v) The State may increase the required monitoring frequency, where necessary, to detect variations within the system ( e.g., fluctuations in concentration due to seasonal use, changes in water source).
(vi) Each public water system shall monitor at the time designated by the State within each compliance period.
§141.903 Compliance requirements.
(a) Compliance with §141.61(c) shall be determined based on the analytical results obtained at each sampling point. If one sampling point is in violation of an MCL, the system is in violation of the MCL.
(b) For systems monitoring more than once per year, compliance with the MCL is determined by a running annual average at each sampling point.
(c) If a system fails to collect the required number of samples, compliance will be based on the total number of samples collected.
(d) Systems monitoring triennially whose sample result equals or exceeds the trigger level of 1.3 ppt for either PFOS or PFOA, or a Hazard Index of 0.33 for PFNA, PFHxS, HFPO-DA, and PFBS must begin quarterly sampling. If the sample result exceeds an MCL, the system will not be considered in violation of the MCL until it has completed one year of quarterly sampling with the triggering sample used as the first quarter of monitoring for the running annual average calculation.
(e) If any sample result will cause the running annual average to exceed the MCL at any sampling point, the system is out of compliance with the MCL immediately.
(f) Systems must calculate compliance using the following method:
(1) For each PFAS regulated by an individual MCL:
(i) For systems monitoring quarterly, divide the sum of the measured concentrations for each analyte by the number of samples collected for that analyte during the consecutive quarters. If more than one compliance sample for that analyte is available in the quarter, systems must average all the results in a quarter then average the quarterly averages. If the value calculated exceeds the MCL, the system is not in compliance with the MCL requirements.
(ii) For systems monitoring less frequently than quarterly, report the results of each sampling event:
(A) For systems taking one sample during each three-year compliance period, if more than one compliance sample is available systems must average all the results to determine compliance. If the value calculated exceeds the MCL, the system is required to initiate quarterly monitoring with the sampling result used as the first quarter of monitoring for the running annual average calculation.
(B) For systems taking two samples during each three-year compliance period, divide the sum of the measured concentrations for each analyte by the number of samples collected during the three-year compliance period. If more than one compliance sample is available for a quarter, systems must average all of the results of that quarter then average the two quarterly averages. If the value calculated exceeds the MCL, the system is required to initiate quarterly monitoring, with the sample result used as the first quarter of monitoring for the running annual average calculation.
(iii) If a sample result is less than the practical quantitation limit for a regulated PFAS, in accordance with the following table, zero will be used for that analyte to calculate the annual average.
| Contaminant | PQL (ppt) |
|---|---|
| PFOA | 4.0 |
| PFOS | 4.0 |
| HFPO-DA | 5.0 |
| PFHxS | 3.0 |
| PFNA | 4.0 |
| PFBS | 3.0 |
(2) For each PFAS regulated under the Hazard Index:
(i) For systems monitoring quarterly, divide observed sample analytical results by the corresponding HBWC listed in §141.61(c) to obtain a Hazard Quotient for each sampling event at each EPTDS. Sum the resulting Hazard Quotients together to determine the Hazard Index. If more than one compliance sample is available for an analyte in a quarter, systems must average all the results for that analyte in that quarter and then determine the Hazard Quotient(s) from those average values. If the Hazard Index exceeds the MCL, the system is not in compliance with the Hazard Index MCL requirements.
(ii) For systems monitoring less frequently, divide the observed sample analytical results by the corresponding HBWC listed in §141.61(c) to obtain a Hazard Quotient. Sum the resulting Hazard Quotients together to determine the Hazard Index.
(A) For systems taking one sample during each three-year compliance period, if more than one compliance sample is available for an analyte, systems must average all the results for that analyte to determine the Hazard Quotient and the Hazard Index. If the Hazard Index exceeds the MCL, the system is required to initiate quarterly monitoring with the Hazard Index sampling result used as the first quarter of monitoring for the running annual average calculation.
(B) For systems taking two samples during each three-year compliance period, if more than one sample is available for an analyte, systems must average all the results for that analyte to determine the Hazard Quotient(s) and the Hazard Index for that quarter. Average the two Hazard Indices calculated during the compliance period. If the average of the Hazard Indices exceeds the MCL, the system is required to initiate quarterly monitoring with the Hazard Index average sampling result used as the first quarter of monitoring for the running annual average calculation.
(iii) If a sample result is less than the practical quantitation limit for a regulated PFAS, in accordance with the table in paragraph (f)(1)(i)(C) of this section, zero will be used for that analyte to calculate the annual average.
§141.904 Reporting and recordkeeping requirements.
Systems required to sample must report to the State according to the timeframes and provisions of §141.31. Systems must report the information specified in the following table:
| If you are a . . . | You must report . . . |
|---|---|
| System monitoring for regulated PFAS under the requirements of §141.902 on a quarterly basis | 1. All sample results, including the location, number of samples taken at each location, date, and result during the previous quarter. |
| 2. The running annual average at each sampling point of all samples taken in the last four quarters. | |
| 3. Whether, based on §141.902, the MCL was violated. | |
| 4. Whether, based on §141.902, the trigger level was met or exceeded. | |
| System monitoring for regulated PFAS under the requirements of §141.902 less frequently than quarterly | 1. The location, date, and result of each sample taken during the last monitoring period. |
| 2. The running annual average at each sampling point of all samples taken in the last twelve months. | |
| 3. Whether, based on §141.902, the trigger level was met or exceeded. |
§141.905 Violations.
(a) PFAS MCL violations, both for PFOA and PFOS MCLs as well as the Hazard Index MCL are based on a running annual average under §141.XX.d. Failure to monitor in accordance with the requirements under §141.XX.c (monitoring requirements) of this section is a monitoring violation.
(b) Compliance with §141.61(c) must be determined based on the analytical results obtained at each sampling point. If one sampling point is in violation of an MCL, the system is in violation of the MCL.
(1) For systems monitoring quarterly, compliance with the MCL is determined by a running annual average at each sampling point.
(2) Systems monitoring triennially whose sample result is at or exceeds the trigger level as defined by §141.902(a)(7) of this section must begin quarterly sampling. The system will not be considered in violation of the MCL until it has completed one year of quarterly sampling.
(i) If any sample result will cause the running annual average to exceed the MCL at any sampling point, the system is out of compliance with the MCL immediately.
(ii) If a system fails to collect the required number of samples, compliance will be based on the total number of samples collected.
(iii) If a sample result is less than the practical quantitation limit for regulated PFAS as shown in §141.903(f)(1)(i)(C), zero will be used to calculate the annual average.
PART 142—NATIONAL PRIMARY DRINKING WATER REGULATIONS IMPLEMENTATION
15. The authority citation for part 142 continues to read as follows:
Authority:
42 U.S.C. 300f, 300g-1, 300g-2, 300g-3, 300g-4, 300g-5, 300g-6, 300j-4, 300j-9, and 300j-11.
16. Amend §142.16 by adding paragraph (r) to read as follows:
§142.16 Special primacy requirements.
* * * * *
(r) Requirements for States to adopt 40 CFR part 141, subpart Z. In addition to the general primacy requirements elsewhere in this part, including the requirements that State regulations be at least as stringent as Federal requirements, an application for approval of a State program revision that adopts 40 CFR part 141, subpart Z, must contain the following:
(1) The States procedures for use of pre-existing data to meet the initial monitoring requirements specified in §141.902, including the criteria that will be used to determine if the data is acceptable.
(2) The States procedures for ensuring all systems complete the initial monitoring period requirements that will result in a high degree of monitoring compliance by the regulatory deadlines.
(i) The initial monitoring plan must describe how systems will be scheduled during the initial monitoring period and demonstrate that the analytical workload on certified laboratories has been taken into account.
(ii) The State will update the initial monitoring plan as necessary and must demonstrate that the monitoring plan is enforceable under State law.
(3) After the initial monitoring period, States establish the initial monitoring requirements for new systems and new sources. States must explain their initial monitoring schedules and how these monitoring schedules ensure that public water systems and sources comply with MCL's and monitoring requirements. States must also specify the time frame in which new systems will demonstrate compliance with the MCLs.
17. Amend §142.62 by revising paragraph (a) to read as follows:
§142.62 Variances and exemptions from the maximum contaminant levels for organic and inorganic chemicals.
(a) The Administrator, pursuant to section 1415(a)(1)(A) of the Act, hereby identifies the following as the best available technology, treatment techniques, or other means available for achieving compliance with the maximum contaminant levels for the PFAS listed in §141.61(c) of this chapter, for the purposes of issuing variances and exemptions, as shown in tables 1 and 2 to this paragraph (a).
| Contaminant | BAT |
|---|---|
| PFOA | Ion exchange, reverse osmosis, GAC, nanofiltration. |
| PFOS | Ion exchange, reverse osmosis, GAC, nanofiltration. |
| Hazard Index PFAS (PFHxS, HFPO-DA, PFNA, PFBS) | Ion exchange, reverse osmosis, GAC, nanofiltration. |
| Unit technologies | Limitations a b c | Operator skill level required | Raw water quality range and considerations |
|---|---|---|---|
| a Mostly operated as a single use. The regeneration solution contains high concentrations of the organic solvents not typically used in the regeneration of resins contaminated with other pollutants. Disposal options should be considered before choosing this technology. | |||
| b Waste media may contain high concentrations of the contaminant. Disposal options should be considered before choosing this technology. | |||
| c Point of use is not currently accepted as a small system compliance technology, however POU treatment is reasonably anticipated to become a compliance option for small systems in the future if third-party certification organizations develop a new certification standard that meets or requires treatment to concentrations lower than EPA's proposed MCLs. | |||
| Ion Exchange | a, b | Basic to Intermediate | All ground waters. |
| GAC | B | Basic to Intermediate | All waters. |
* * * * *
[FR Doc. 2023-05471 Filed 3-23-23; 11:15 am]
BILLING CODE 6560-50-P
['Water Programs']
['Water Programs']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
