...
�158.2200 Applicability.
Part 158, subpart W establishes data requirements for any pesticide product that is:
(a) A pesticide that is intended for use as an �antimicrobial pesticide� within the meaning of FIFRA sec. 2(mm)(1)(A), regardless of whether it also meets the criterion of FIFRA sec. 2(mm)(1)(B). That criterion excludes from the definition any antimicrobial product that is intended for a food-use requiring a tolerance or exemption under FFDCA sec. 408 or a food additive regulation or clearance under FFDCA sec. 409. EPA will apply this subpart to all products intended for an antimicrobial use, purpose or function; the exclusion in FIFRA sec. 2(mm)(1)(B) does not exclude products from the data requirements of this subpart.
(b) A product that bears both antimicrobial and non-antimicrobial uses or claims is subject to the data requirements for pesticides in subparts C through O, R, and U or V of this part with respect to its non-antimicrobial uses and claims, and to the requirements of this subpart with respect to its antimicrobial uses and claims.
(c) A wood preservative, including a product that is intended to prevent wood degradation problems due to fungal rot or decay, sapstain, or molds.
(d) An antifoulant, including a product that is intended to kill or repel organisms that can attach to underwater surfaces, such as boat bottoms.
[87 FR 22484, Apr. 15, 2022]
�158.2201 Antimicrobial use patterns.
(a) Antimicrobial use patterns. The 12 general use patterns used in the data tables in this subpart are:
(1) Agricultural premises and equipment.
(2) Food-handling/storage establishments, premises and equipment.
(3) Commercial, institutional and industrial premises and equipment.
(4) Residential and public access premises.
(5) Medical premises and equipment.
(6) Human drinking water systems.
(7) Materials preservatives.
(8) Industrial processes and water systems.
(9) Antifoulant paints and coatings.
(10) Wood preservatives.
(11) Swimming pools.
(12) Aquatic areas.
(b) Use site index. The Pesticide Use Site Index for Antimicrobial Pesticides is a comprehensive list of specific antimicrobial use sites. The Index associates antimicrobial use sites with one or more of the 12 antimicrobial use patterns. It is to be used in conjunction with the data tables in this subpart to determine the applicability of data requirements to specific uses. The Antimicrobial Pesticide Use Site Index, which will be updated periodically, is available from the Agency or may be obtained from the Agency's Web site at http://www.epa.gov/pesticides.
�158.2203 Definitions.
The following terms are defined for the purposes of this subpart:
Disinfectant means a substance, or mixture of substances, that destroys or irreversibly inactivates bacteria, fungi and viruses, but not necessarily bacterial spores, in the inanimate environment.
Fungicide means a substance, or mixture of substances, that destroys fungi (including yeasts) and fungal spores pathogenic to man or other animals in the inanimate environment.
Microbiological water purifier means any unit, water treatment product or system that removes, kills or inactivates all types of disease-causing microorganisms from the water, including bacteria, viruses and protozoan cysts, so as to render the treated water safe for drinking.
Sanitizer means a substance, or mixture of substances, that reduces the bacteria population in the inanimate environment by significant numbers, but does not destroy or eliminate all bacteria. Sanitizers meeting Public Health Ordinances are generally used on food contact surfaces and are termed sanitizing rinses.
Sterilant means a substance, or mixture of substances, that destroys or eliminates all forms of microbial life in the inanimate environment, including all forms of vegetative bacteria, bacterial spores, fungi, fungal spores, and viruses.
Tuberculocide means a substance, or mixture of substances, that destroys or irreversibly inactivates tubercle bacilli in the inanimate environment.
Virucide means a substance, or mixture of substances, that destroys or irreversibly inactivates viruses in the inanimate environment.
�158.2204 Public health and nonpublic health claims.
(a) Public health claim. An antimicrobial pesticide is considered to make a public health claim if the pesticide product bears a claim to control pest microorganisms that pose a threat to human health, and whose presence cannot readily be observed by the user, including but not limited to, microorganisms infectious to man in any area of the inanimate environment. A product makes a public health claim if one or more of the following apply:
(1) A claim is made for control of specific microorganisms that are directly or indirectly infectious or pathogenic to man (or both man and animals). Examples of specific microorganisms include, but are not limited to: Mycobacterium tuberculosis, Pseudomonas aeruginosa, Escherichia coli (E. coli), human immunodeficiency virus (HIV), Streptococcus, and Staphylococcus aureus. Claims for control of microorganisms infectious or pathogenic only to animals (such as canine distemper virus or hog cholera virus) are not considered public health claims.
(2) A claim is made for the pesticide product as a sterilant, disinfectant, virucide, sanitizer, or tuberculocide against microorganisms that are infectious or pathogenic to man.
(3) A claim is made for the pesticide product as a fungicide against fungi infectious or pathogenic to man, or the product does not clearly state that it is intended for use only against nonpublic health fungi.
(4) A claim is made for the pesticide product as a microbiological water purifier or microbial purification system.
(5) A non-specific claim is made that the pesticide product will beneficially impact or affect public health at the site of use or in the environment in which it is applied, and:
(i) The pesticide product contains one or more ingredients that, under the criteria in 40 CFR 153.125(a), is an active ingredient with respect to a public health microorganism and there is no other functional purpose for the ingredient in the product; or
(ii) The pesticide product is similar in composition to a registered pesticide product that makes antimicrobial public health claims.
(b) Nonpublic health claim. An antimicrobial pesticide is considered to make a nonpublic health claim if the pesticide product bears a claim to control microorganisms of economic or aesthetic significance, where the presence of the microorganism would not normally lead to infection or disease in humans. Examples of nonpublic health claims include, but are not limited to: Algaecides, slimicides, preservatives and products for which a pesticidal claim with respect to odor sources is made.
�158.2210 Product chemistry.
The product chemistry data requirements of subpart D of this part apply to antimicrobial products covered by this subpart.
�158.2220 Product performance.
(a) General(1) Product performance requirement for all antimicrobial pesticides. Each applicant must ensure through testing that his product is efficacious when used in accordance with label directions and commonly accepted pest control practices. The Agency may require, on a case-by-case basis, submission of product performance data for any pesticide product registered or proposed for registration or amendment.
(2) Product performance data for each product that bears a public health claim. Each product that bears a public health claim, as described in �158.2204(a), must be supported by product performance data, as listed in the table in paragraph (c) of this section. Product performance data must be submitted with any application for registration or amended registration.
(3) Product performance data for each product that bears a nonpublic health claim. Each product that bears a nonpublic health claim, as described in �158.2204(b), must be supported by product performance data. Each registrant must ensure through testing that his product is efficacious when used in accordance with label directions and commonly accepted practices. The Agency reserves the right to require, on a case-by-case basis, submission of product performance data for any pesticide product registered or proposed for registration or amendment.
(4) Determination of data requirements. Subpart B of this part and �158.2201 describe how to use the table in paragraph (c) of this section to determine the product performance data requirements for antimicrobial pesticide products.
(b) Key. R = Required; EP = End-use product.
(c) Antimicrobial product performance data requirements table. The following table shows the data requirements for antimicrobial product performance.
| Guideline No. | Data requirement | All use patterns | Test substance |
|---|---|---|---|
| 810.2100 | Sterilants - Efficacy Data Recommendations | R | EP |
| 810.2200 | Disinfectants for Use on Hard Surfaces - Efficacy Data Recommendations | R | EP |
| 810.2300 | Sanitizers for Use on Hard Surfaces - Efficacy Data Recommendations | R | EP |
| 810.2400 | Disinfectants and Sanitizers for Use on Fabrics and Textiles - Efficacy Data Recommendations | R | EP |
| 810.2500 | Air Sanitizers - Efficacy Data Recommendations | R | EP |
| 810.2600 | Disinfectants for Use in Water - Efficacy Data Recommendations | R | EP |
�158.2230 Toxicology.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (g) of this section to determine the toxicology data requirements for an antimicrobial pesticide product. Notes that apply to an individual test, including specific conditions, qualifications, or exceptions are listed in paragraph (h) of this section.
(b) Uses. The applicant for registration must first determine whether the use is likely to result in pesticide residues in food or water and therefore consult the �Food Use� columns of the table in paragraph (g) of this section. Generally, if the residues of the antimicrobial result from an application to a surface or if incorporated into a material that may come into contact with food or feed, and residues may be expected to transfer to such food or feed, then the �Indirect Food Uses� columns is to be consulted.
(c) Tiering of data requirements. Applicants for registration of antimicrobials may perform tests in a tiered fashion. After the initially required tests are conducted, additional testing may be required if results of the initial tests trigger the need for additional data. Conditions that trigger the need for additional data are given in the test notes in paragraph (h) of this section.
(d) 200 parts per billion (ppb). The 200 ppb level was originally used by the Food and Drug Administration with respect to the concentration of residues in or on food for tiering of data requirements for indirect food use biocides. The Agency has also adopted this same residue level for determining toxicology data requirements for indirect food uses of antimicrobial pesticides. The 200 ppb level is the concentration of antimicrobial residues in or on the food item.
(e) Use of OSHA standards. If EPA determines that industrial standards, such as the workplace standards set by the Occupational Safety and Health Administration (OSHA standards), provide adequate protection for a particular pesticide or a particular use pattern, additional toxicity data may not be required for that pesticide or the use pattern.
(f) Key. R = Required; CR = Conditionally required; NR = Not required; MP = Manufacturing-use product; EP = End-use product; TGAI = Technical grade of the active ingredient; TEP = Typical end-use product; PAI = Pure active ingredient; PAIRA = Pure active ingredient, radiolabeled; Choice = choice of several test substances depending on studies required.
(g) Antimicrobial toxicology data requirements table. The following table shows the data requirements for toxicology. The test notes applicable to the data requirements in this table appear in paragraph (h) of this section.
Table - Antimicrobial Toxicology Data Requirements


(h) Test notes. The following test notes apply to the data requirements in the table to paragraph (g) of this section:
1. Not required if test material is a gas or highly volatile liquid.
2. The six end-use product (EP) acute toxicity studies are required using the product as formulated for sale and distribution. In addition, if the EP label has directions for diluting the product, then, the applicant may also need to conduct certain of the acute toxicity studies using the highest concentration labeled for dilution (i.e., the least diluted product). The end-use dilution testing is in addition to the testing conducted on the EP.
3. Not required if test material is corrosive to skin or has pH less than 2 or greater than 11.5.
4. Data are required when the product consists of, or under conditions of use will result in, a respirable material (e.g., gas, vapor, aerosol or particulates).
5. Data are required if repeated dermal exposure is likely to occur under conditions of use.
6. For indirect food uses ?200 ppb, and all other nonfood uses, data are required if the neurotoxicity screen in the 90-day oral rodent study or other data indicate neurotoxicity.
7. The 90-day dermal toxicity study and/or 90-day inhalation toxicity study are required if the Agency determines that dermal and/or inhalation exposure is the primary route of exposure.
8. All 90-day subchronic studies in the rodent can be designed to simultaneously fulfill the requirements of the 90-day neurotoxicity and/or immunotoxicity studies by adding separate groups of animals for testing of neurotoxicity and/or immunotoxicity parameters.
9. The 90-day study is required in the rodent for hazard characterization (possibly endpoint selection) and dose-setting for the chronic/carcinogenicity study. It is not required in the mouse, but the Agency would encourage the applicant to conduct a 90-day range finding study for the purposes of dose selection for the mouse carcinogenicity study to achieve adequate dosing and an acceptable study.
10. A 1-year non-rodent study (i.e., 1-year dog study) may be required if the Agency finds that a pesticide chemical is highly bioaccumulative and slowly eliminated. EPA may also require the appropriate metabolism and pharmacokinetic studies to evaluate more precisely bioavailability, half life, and steady state to determine if a longer duration dog toxicity study is needed.
11. Although the subchronic toxicity testing guidelines include measurement of neurological endpoints, such screens do not meet the requirement of the 90-day neurotoxicity study. For nonfood uses, if the 90-day study does not include a neurotoxicity screen, then the acute neurotoxicity study will be required.
12. Data are required if all of the following criteria are met:
i. The intended use of the antimicrobial pesticide product is expected to result in repeated dermal human exposure to the product.
ii. Data from a 90-day dermal toxicity study are not available.
iii. The 90-day dermal toxicity study has not been triggered.
13. EP testing is required if the product or any component of the product may increase dermal absorption of the active ingredient(s) or increases its toxic or pharmacologic effects, as determined by testing using the TGAI or based on available information about the toxic effects of the product or its components.
14. Data are required if the active ingredient in the product is known or expected to be metabolized differently by the dermal route of exposure than by the oral route, and a metabolite of the active ingredient is the toxic moiety.
15. A 90-day oral toxicity test is not required for heating, ventilation, air conditioning, and refrigeration systems (collectively referred to as HVAC&R). Instead, two 90-day toxicity tests, one by the dermal route and one by the inhalation route are required.
16. Data are required if there is the likelihood of significant repeated inhalation exposure to the pesticide as a gas, vapor, or aerosol.
17. Based on estimates of the magnitude and duration of human exposure, studies of shorter duration, e.g., 21- or 28-days, may be sufficient to satisfy this requirement. The prime consideration in determining the appropriateness of a shorter duration study is the likely period of time for which humans will be exposed.
18. Based on the positive results of the acute or 90-day neurotoxicity studies, or on other data indicating neurotoxicity, a chronic neurotoxicity study (i.e., a chronic study with additional neurotoxicity evaluations) may be required to provide information about potential neurotoxic effects from long-term exposures.
19. Studies which are designed to simultaneously fulfill the requirements of both the chronic oral and carcinogenicity studies (i.e., a combined study) may be conducted.
20. For indirect food uses ?200 ppb, and all other nonfood uses, data are required if either of the following criteria are met:
i. The use of the pesticide is likely to result in repeated human exposure over a considerable portion of the human lifespan; or
ii. The use requires that a tolerance, tolerance exemption, or food additive regulation or clearance be established.
21. For indirect food uses ?200 ppb, and all other nonfood uses, data are required if any of the following criteria, are met:
i. The use of the pesticide is likely to result in significant human exposure over a considerable portion of the human life span which is significant in terms of frequency, time, duration, and/or magnitude of exposure.
ii. The use requires that a tolerance, tolerance exemption, or food additive regulation or clearance be established.
iii. The active ingredient, metabolite, degradate, or impurity:
A. Is structurally related to a recognized carcinogen;
B. Causes mutagenic effects as demonstrated by in vitro or in vivo testing; or
C. Produces a morphologic effect in any organ (e.g., hyperplasia, metaplasia) in subchronic studies that may lead to a neoplastic change.
22. If the requirement for a carcinogenicity study in any species is modified or waived for any reason, then a subchronic 90-day oral study in the same species may be required.
23. Testing in two species is required for all uses.
24. The oral route, by oral intubation, is preferred, unless the chemical or physical properties of the test substance, or the pattern of human exposure, suggest a more appropriate route of exposure.
25. Additional testing by other routes of exposure may be required if the pesticide is determined to be a prenatal developmental toxicant after oral dosing.
26. The developmental toxicity study in rodents may be combined with the two-generation reproduction study in rodents by using a second mating of the parental animals in either generation. Protocols must be approved by the Agency prior to the initiation of the study.
27. A two-generation reproduction study is required.
28. An information-based approach to testing is preferred, which utilizes the best available knowledge on the chemical (hazard, pharmacokinetic, or mechanistic data) to determine whether a standard guideline study, an enhanced guideline study, or an alternative study should be conducted to assess potential hazard to the developing animal. Applicants must submit any alternative proposed testing protocols and supporting scientific rationale to the Agency. Protocols must be approved by the Agency prior to the initiation of the study.
29. The use of a combined two-generation reproduction/developmental neurotoxicity study that utilizes the two-generation reproduction study in rodents as a basic protocol for the addition of other endpoints or functional assessments in the immature animal is encouraged.
30. A DNT study is required using a weight-of-evidence approach when:
i. The pesticide causes treatment-related neurological effects in adult animal studies (i.e., clinical signs of neurotoxicity, neuropathology, functional or behavioral effects).
ii. The pesticide causes treatment-related neurological effects in developing animals, following pre- or post-natal exposure (i.e., nervous system malformations or neuropathy, brain weight changes in offspring, functional or behavioral changes in the offspring).
iii. The pesticide elicits a causative association between exposures and adverse neurological effects in human epidemiological studies.
iv. The pesticide evokes a mechanism that is associated with adverse effects on the development of the nervous system (i.e., structure-activity-relationship (SAR) to known neurotoxicants, altered neuroreceptor or neurotransmitter responses).
31. To facilitate the weight-of-evidence determination for the pesticide's mutagenicity, in addition to those specifically listed in this table, the Agency requires submission of other mutagenicity test results that may have been performed. A reference list of all studies and papers known to the applicant concerning the mutagenicity of the test chemical must be submitted with the required studies.
32. Due to the nature of antimicrobials, if testing with bacterial strains has not been conducted, then testing using a mammalian cell assay such as the mouse lymphoma TK �assay is preferred. If reverse mutation assay testing with bacterial strains has already been conducted, and the testing was conducted at levels that did not cause toxicity to the bacterial strains tested, then the applicant may submit the study to fulfill this data requirement.
33. For the in vitro mammalian gene mutation study, there is a choice of assays using either mouse lymphoma L5178Y cell thymidine kinase (tk) gene locus, maximizing assay conditions for small colony expression and detection; Chinese hamster ovary (CHO) or Chinese hamster lung fibroblast (v79) cells, hypoxanthine-guanine phosphoribosyl transferase (hgprt) gene locus, accompanied by an appropriate in vitro test for clastogenicity; or CHO cells strains AS52, xanthine-guanine phosphoribosyl transferase (xprt) gene locus.
34. There is a choice of assays, but the micronucleus rodent bone marrow assay is preferred; the rodent bone marrow assays using metaphase analysis (aberrations) are acceptable.
35. Data are required when chronic toxicity or carcinogenicity studies are also required.
36. Data is required if the product label directs that it be applied to domestic animals, such as cats, dogs, cattle, pigs, and horses.
37. In the absence of dermal absorption data or a repeated dose dermal toxicity study, the assumption of 100 percent dermal absorption would be used in a risk assessment to determine if a dermal penetration study is required, and to identify the doses and duration of exposure for which dermal absorption is to be quantified.
38. Required for nonfood uses, if oral exposure could occur.
39. Data may be required if significant adverse effects are seen in available toxicology studies and these effects can be further elucidated by metabolism and pharmacokinetics studies.
�158.2240 Nontarget organisms.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (c) of this section to determine the terrestrial and aquatic nontarget organisms data requirements for a particular antimicrobial pesticide product. Notes that apply to an individual test, including specific conditions, qualifications, or exceptions are listed in paragraph (d) of this section.
(1) Terrestrial and aquatic nontarget organism data are required to support the registration of most end-use and manufacturing-use antimicrobial products.
(2) Data are generally not required to support end-use products of a gas, highly volatile liquid, highly reactive solid, or a highly corrosive material.
(3) Data on transformation/degradation products or leachate residues of the parent compound are also required to support registration, if the transformation/degradation/degradation products or leachate residues meet one of the following criteria:
(i) More toxic, persistent, or bioaccumulative than the parent;
(ii) Have been shown to cause adverse effects in mammalian or aquatic reproductive studies; or
(iii) The moiety of concern (i.e., functional group in the parent chemical molecule that imparts adverse effects) remains intact.
(4) If an antimicrobial may be applied to a field crop, horticultural crop, or turf, then the data requirements in �158.630 apply.
(5) For the purpose of determining data requirements, the all other use patterns category includes the following use patterns:
(i) Agricultural premises and equipment.
(ii) Food-handling/storage establishments, premises, and equipment.
(iii) Commercial, institutional and industrial premises and equipment.
(iv) Residential and public access premises.
(v) Medical premises and equipment.
(vi) Human drinking water systems.
(vii) Materials preservatives.
(viii) Swimming pools.
(b) Key. MP = Manufacturing use product; EP = End-use product; R = Required; CR = Conditionally required; NR = Not required; TGAI = Technical grade of the active ingredient; TEP = Typical end-use product; PAIRA = Pure active ingredient radiolabeled; a.i. = active ingredient.
(c) Antimicrobial nontarget organism data requirements table. The following table shows the data requirements for nontarget organisms. The test notes appear in paragraph (d) of this section.
Table - Antimicrobial Nontarget Organism Data Requirements
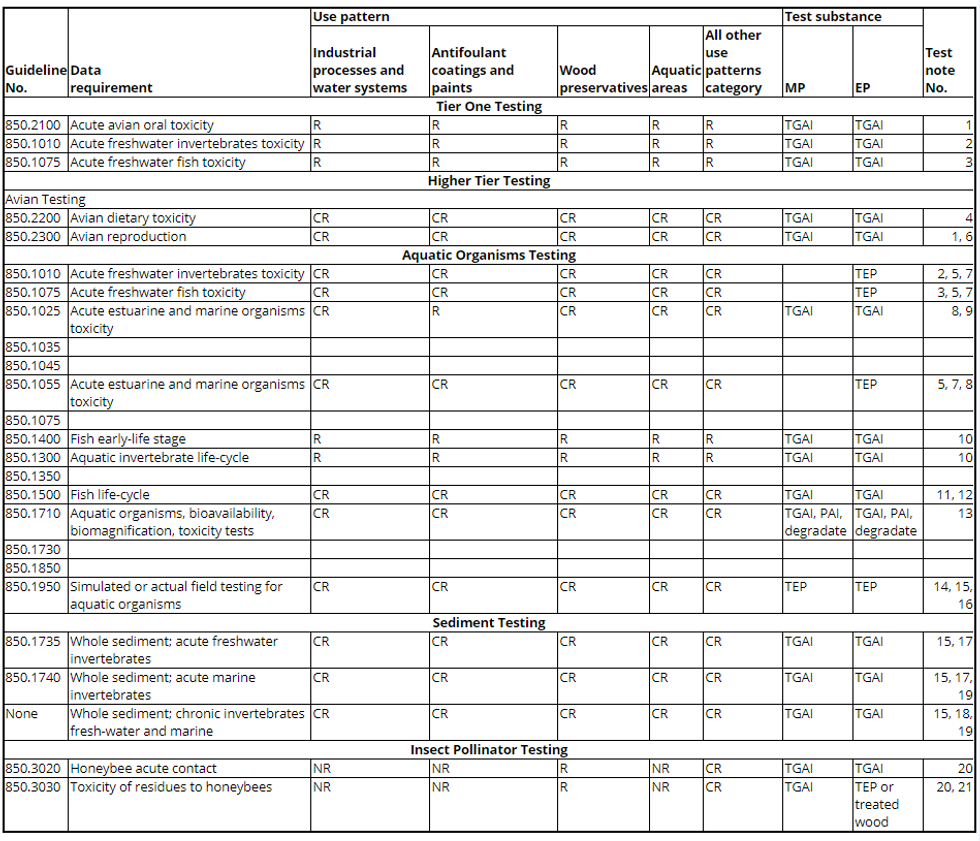
(d) Test notes. The following test notes apply to the data requirements in the table to paragraph (c) of this section:
1. For industrial processes and water systems, antifoulant paints and coatings, wood preservatives, and aquatic areas, data are required for two avian species: one waterfowl species and one upland game bird species. For the all other use patterns category (as specified in �158.2240(a)(5)), data are required for one avian species.
2. Data are required on one freshwater aquatic invertebrate species.
3. For the industrial processes and water systems, antifoulant paints and coatings, wood preservatives, and aquatic use pattern areas, data are required on two species of fish, one cold water species and one warm water species. For the all other use patterns category (as specified in �158.2240(a)(5)), data are required on one species of fish, either one cold water species or one warm water species. Testing on a second species is required if the active ingredient or principal transformation products are stable in the environment and the LC50 in the first species is less than or equal to 1 ppm or 1 mg/L.
4. Data are required on one avian species, either one waterfowl species or one upland game bird species, if the avian acute oral LD50 (TGAI testing) is less than or equal to 100 mg/a.i./kg and a.i. residues or its principal transformation products are likely to occur in avian feed items. Data on the second species are required if the avian dietary LC50 in the first species tested is less than or equal to 500 ppm a.i. in the diet.
5. If TEP testing cannot be conducted due to the physical characteristics of the test substance (for example, a paint), then the applicant should request a waiver.
6. Data are required if one or more of the following criteria are met:
i. Birds may be subjected to repeated or continued exposure to the pesticide or any of its transformation products, especially preceding or during the breeding season.
ii. The pesticide or any of its major metabolites or degradation products are stable in the environment to the extent that a potentially toxic amount may persist in avian feed.
iii. The pesticide or any of its major metabolites or degradation products are stored or accumulated in plant or animal tissues, as indicated by the octanol/water partition coefficient (Kow is greater than or equal to 1,000), accumulation studies, metabolic release and retention studies, or as indicated by structural similarity to known bioaccumulative chemicals.
iv. Any other information, such as that derived from mammalian reproduction studies, indicates that reproduction in terrestrial vertebrates may be adversely affected by the anticipated use of the pesticide product.
7. TEP testing is required for any product which meets one or more of the following conditions:
i. When based on deterministic modeling results: If the Estimated Environmental Concentration (EEC) in the aquatic environment is equal to or greater than one-half the LC50/EC50 of the TGAI.
ii. When based on probabilistic modeling results: If the estimated 10th percentile 7Q10 Surface Water Concentration exceeds the acute concentration of concern (i.e., one-half the LC50/EC50).
iii. If an ingredient in the end-use product other than the active ingredient is expected to enhance the toxicity of the active ingredient or to cause toxicity to aquatic organisms.
iv. The end-use antimicrobial product will be applied directly into an aquatic environment.
8. Data are required on one estuarine/marine mollusk, one other estuarine/marine invertebrate, and one estuarine/marine fish species.
9. For the all other use patterns category (as specified in �158.2240(a)(5)), industrial processes and water systems, wood preservatives, and aquatic areas, data are required if the pesticide residues from the parent compound and/or transformation products are likely to enter the estuarine/marine environment.
10. Testing must be conducted with the most sensitive organism (either freshwater or estuarine/marine vertebrates, or freshwater or estuarine/marine invertebrates), as determined from the results of the acute toxicity tests (acute EC50 freshwater invertebrates; acute LC50/EC50 estuarine and marine organisms; acute freshwater fish LC50).
11. Data are required on estuarine/marine species if the product is intended for direct application to the estuarine or marine environment, or the product is expected to enter this environment in significant concentrations because of its expected use or mobility patterns.
12. Data are required on freshwater species if the end-use product is intended to be applied directly to water, or is expected to be transported to water from the intended use site, and when one or more of the following conditions apply:
i. When based on deterministic modeling results: If the Estimated Environmental Concentration (EEC) in water is equal to or greater than 0.1 of the no-observed-adverse-effect concentration or no-observed-adverse-effect level (NOAEC/NOAEL) in the fish early-life stage or invertebrate life cycle tests.
ii. When based on probabilistic modeling results: If the estimated 10th percentile 7Q10 Surface Water Concentration based on probabilistic modeling exceeds for 20 days or more the chronic concentration of concern (i.e., one-tenth the NOAEC or NOAEL) determined in the fish early-life stage or invertebrate life cycle tests.
iii. If studies of other organisms indicate that the reproductive physiology of fish may be affected.
13. Not required when:
i. The octanol/water partition coefficients of the pesticide and its major degradates are less than 1,000;
ii. There are no potential exposures to fish and other nontarget aquatic organisms; or
iii. The hydrolytic half-life is less than 5 days at pH 5, 7, and 9.
14. Environmental chemistry methods used to generate data associated with this study must include results of a successful confirmatory method trial by an independent laboratory. Test standards and procedures for independent laboratory validation are available as addenda to the guideline for this test requirement.
15. Protocols must be approved by the Agency prior to the initiation of the study.
16. Data are required if the intended use pattern, and the physical/chemical properties and environmental fate characteristics of the antimicrobial indicate significant potential exposure, and, based on the results of the acute and chronic aquatic organism testing, significant impairment of nontarget aquatic organisms could result.
17. Data are required if the half-life of the pesticide in the sediment is equal to or less than 10 days in either the aerobic soil or aquatic metabolism studies, and if one or more of the following conditions are met:
i. The soil partition coefficient (Kd) is equal to or greater than 50 L/kg.
ii. The log Kow is equal to or greater than 3.
iii. The Koc is equal to or greater than 1,000.
18. Data are required if the EEC in sediment is greater than 0.1 of the acute LC50/EC50 values and if one or more of the following conditions are met:
i. The soil partition coefficient (Kd) is equal to or greater than 50 L/kg.
ii. The log Kow is equal to or greater than 3.
iii. The Koc is equal to or greater than 1,000.
19. Sediment testing with estuarine/marine test species is required if the product is intended for direct application to the estuarine or marine environment or the product is expected to enter this environment in significant concentrations either by runoff or erosion, because of its expected use or mobility pattern.
20. For the all other use patterns category (as specified in �158.2240(a)(5)), data are required only for beehive applications when the beehive (empty or occupied) may be treated.
21. A study similar to �Honey Bee Toxicity of Residues on Foliage� is required using treated wood instead of the foliage. Protocols must be approved by the Agency prior to the initiation of the study.
�158.2250 Nontarget plant protection.
(a) Subpart B of this part and �158.2201 describe how to use the table in paragraph (f) of this section to determine the nontarget plant protection data requirements for a particular antimicrobial pesticide product. Notes that apply to an individual test including specific conditions, qualifications, or exceptions are listed in paragraph (g) of this section.
(b) Data on transformation/degradation products or leachate residues of the parent compound are also required to support registration, if the transformation/degradation products or leachate residues meet one of the following criteria:
(1) More toxic, persistent, or bioaccumulative than the parent;
(2) Have been shown to cause adverse effects in mammalian or aquatic reproductive studies; or
(3) The moiety of concern (i.e., functional group in the parent chemical molecule that imparts adverse effects) remains intact.
(c) For the purpose of determining data requirements, the all other use patterns category includes the following use patterns:
(1) Agricultural premises and equipment.
(2) Food-handling/storage establishments, premises, and equipment.
(3) Commercial, institutional and industrial premises and equipment.
(4) Residential and public access premises.
(5) Medical premises and equipment.
(6) Human drinking water systems.
(7) Materials preservatives.
(8) Swimming pools.
(d) If an antimicrobial may be applied to a field crop, horticultural crop, or turf, then the data requirements in �158.660 apply.
(e) Key. MP = Manufacturing use product; EP = End-use product; R = Required; CR = Conditionally required; NR = Not required; TGAI = Technical grade of the active ingredient; TEP = Typical end-use product.
(f) Nontarget plant protection data requirements table. The following table shows the data requirements for nontarget plant protection. The test notes appear in paragraph (g) of this section.
Table - Nontarget Plant Protection Data Requirements

(g) Test notes. The following test notes apply to the data requirements in the table to paragraph (f) of this section:
1. Data on only one plant species (rice, Oryza sativa) are required.
2. Data are required if the risk quotient from any aquatic plant growth Tier II study exceeds a level of concern for aquatic plants.
3. Not required when:
i. There are no potential exposures to plants;
ii. The hydrolytic half-life is less than 5 days at pH 5, 7, and 9; or
iii. The results of a biodegradation study indicate that the active ingredient or principal degradation products are not biodegradable in 28 days, i.e., the biodegradation curve has not reached a plateau for at least three determinations within the 28 days.
4. For TEP testing, data are required for the applicant's end-use product if an ingredient in the end-use product, other than the active ingredient, is expected to enhance the toxicity of the active ingredient.
5. One Tier II (dose response) study, conducted with Selenastrum capricornutum, is required for the all other use patterns category (as specified in �158.2250(c)). If the results of this study exhibit detrimental effects (EC50 less than 1.0 ppm or mg/L), then additional Tier II (dose response) studies are required on three species (Anabaena flos-aquae, Navicula pelliculosa, and Skeletonema costatum).
6. For industrial processes and water systems, antifoulant coatings and paints, wood preservatives, and aquatic areas, Tier II (dose response) studies are required on four species (Anabaena flos-aquae, Navicula pelliculosa, Skeletonema costatum, and Selenastrum capricornutum).
7. Environmental chemistry methods used to generate data must include the results of a successful confirmatory method trial by an independent laboratory.
8. Tests are required on a case-by-case basis based on the results of lower tier plant protection studies, adverse incident reports, intended use pattern, and environmental fate characteristics that indicate potential exposure.
9. Protocols must be approved by the Agency prior to the initiation of the study.
10. For the all other use patterns category (as specified in �158.2250(c)), data are required if the aquatic (algal) plant growth Tier II study demonstrates detrimental effects at less than 1.0 ppm or mg/L.
�158.2260 Applicator exposure.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (d) of this section to determine the applicator exposure data requirements for antimicrobial pesticide products. Notes that apply to an individual test including specific conditions, qualifications, or exceptions are listed in paragraph (e) of this section.
(1) The Agency may accept surrogate exposure data estimations and/or modeling estimations from other sources to satisfy exposure data requirements. The surrogate data must meet the basic quality assurance, quality control, good laboratory practice, and other scientific requirements set by EPA. To be acceptable, the Agency must find that the surrogate exposure data estimations have adequate information to address the applicable exposure data requirements and contain adequate monitoring events of acceptable quality. The data must reflect the specific use prescribed on the label and the activity of concern, including formulation type, application methods and rates, type of activity, and other pertinent information.
(2) Occupational uses include not only handlers, mixers, loaders, and applicators, but also commercial applications to residential sites. Residential uses are limited to non-occupational, i.e., non-professional, antimicrobial applications. Both occupational and residential applicator data may be required for the same product.
(b) Criteria for testing. Applicator exposure data described in the table to paragraph (d) of this section are required based on toxicity and exposure criteria. Data are required if at least one of the toxicity criteria in paragraph (b)(1) of this section, and at least one of the exposure criteria in paragraph (b)(2) of this section are met.
(1) Toxicity criteria.(i) Evidence of potentially significant adverse effects have been observed in any applicable toxicity studies.
(ii) Scientifically sound epidemiological or poisoning incident data with a clear cause-effect relationship indicating that adverse health effects may have resulted from exposure to the pesticide.
(2) Exposure criteria.(i) Dermal exposure may occur during product use.
(ii) Respiratory exposure may occur during product use.
(c) Key. R = Required; CR = Conditionally required; TEP = Typical end-use product.
(d) Antimicrobial applicator exposure data requirements table. The following table shows the data requirements for applicator exposure. The test notes appear in paragraph (e) of this section.
| Guideline No. | Data requirements | Use sites | Test substance | Test note No. | |
|---|---|---|---|---|---|
| Occupational | Residential | ||||
| 875.1100 | Dermal exposure | R | R | TEP | 1, 2, 3, 4 |
| 875.1200 | |||||
| 875.1300 | Inhalation exposure | R | R | TEP | 1, 2, 3, 4 |
| 875.1400 | |||||
| 875.1500 | Biological monitoring | CR | CR | TEP | 1, 2, 3 |
| 875.1600 | Data reporting and calculations | R | R | TEP | 5 |
| 875.1700 | Product use information | R | R | TEP | |
(e) Test notes. The following test notes apply to the data requirements in the table to paragraph (d) of this section:
1. Prior to initiation of the study, protocols involving intentional exposure of human subjects must be submitted for review by EPA and then the Human Studies Review Board (HSRB) according to 40 CFR 26.1125. Examples of proposed human study research can be found in various reviews provided by the Human Studies Review Board (http://www.epa.gov/osa/hsrb/index.htm).
2. Biological monitoring data may be submitted in addition to, or in lieu of, dermal and inhalation passive dosimetry exposure data, provided the human pharmacokinetics of the pesticide or metabolite/analog compounds (i.e., whichever method is selected as an indicator of body burden or internal dose) allow for the back calculation to the total internal dose.
3. For products with both indoor and outdoor uses, and similar conditions of use, data are generally required for the indoor applications only. However, data for outdoor uses are required if the Agency expects outdoor uses to result in greater exposure than indoor uses (e.g., higher use rates and application frequency, or longer exposure duration, or application methods/equipment create potential for increased dermal or inhalation exposure in outdoor versus indoor use sites). In certain cases, when a pesticide may be used both indoors and outdoors under dissimilar conditions of use, the Agency may require submission of applicator exposure data for both use patterns.
4. EPA will consider waiving this data requirement for antimicrobials applied via closed loading systems if the antimicrobial has a low vapor pressure.
5. Data reporting and calculations are required only if handler exposure data are required.
�158.2270 Post-application exposure.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (d) of this section to determine the post-application exposure data requirements for antimicrobial pesticide products. The data generated during these studies are used to determine the quantity of pesticide to which people may be exposed after application. Notes that apply to an individual test, including specific conditions, qualifications, or exceptions to the designated test, are listed in paragraph (e) of this section.
(1) Post-application exposure data are required when certain toxicity criteria are met and the human activities associated with the pesticide's use pattern can lead to potential adverse exposures.
(2) The Agency may accept surrogate exposure data estimations and/or modeling estimations from other sources to satisfy exposure data requirements. The surrogate data must meet the basic quality assurance, quality control, good laboratory practice, and other scientific requirements set by EPA. To be acceptable, the Agency must find that the surrogate exposure data estimations have adequate information to address the applicable exposure data requirements and contain adequate monitoring events of acceptable quality. The data must reflect the specific use prescribed on the label and the activity of concern, including formulation type, application methods and rates, type of activity, and other pertinent information.
(b) Criteria for testing. Post-application exposure data described in the table to paragraph (d) of this section are required based on toxicity and exposure criteria. Data are required if at least one of the toxicity criteria in paragraph (b)(1) of this section, and at least one of the exposure criteria in paragraph (b)(2) of this section are met.
(1) Toxicity criteria.(i) Evidence of potentially significant adverse effects have been observed in any applicable toxicity studies.
(ii) Scientifically sound epidemiological or poisoning incident data with a clear cause-effect relationship indicating that adverse health effects may have resulted from exposure to the pesticide.
(2) Exposure criteria(i) Outdoor uses.(A) Occupational human post-application or bystander exposure to residues of antimicrobial pesticides could occur as the result of, but is not limited to, worker reentry into treatment sites, clean-up and equipment maintenance tasks, handling wood preservative-treated wood, or other work-related activity.
(B) Residential human post-application or bystander exposure to residues of antimicrobial pesticides could occur following the application of antimicrobial pesticides to outdoor areas and spaces at residential sites, such as, but not limited to homes, daycare centers, and other public buildings.
(ii) Indoor uses.(A) Occupational human post-application or bystander exposure to pesticide residues could occur following the application of the antimicrobial pesticide to indoor spaces or surfaces.
(B) Residential human post-application or bystander exposure to pesticide residues could occur following the application of the antimicrobial pesticide to indoor spaces or surfaces at residential sites, such as, but not limited to homes, daycare centers, hospitals, schools, and other public buildings.
(c) Key. R = Required; CR = Conditionally required; NR = Not required; TEP = Typical end-use product.
(d) Antimicrobial post-application exposure data requirements table. The following table shows the data requirements for post-application exposure. The test notes appear in paragraph (e) of this section.
| Guideline No. | Data requirement | Use sites | Test substance | Test note No. | |
|---|---|---|---|---|---|
| Occupational | Residential | ||||
| 875.2200 | Soil residue dissipation | CR | CR | TEP | 2, 3 |
| 875.2300 | Indoor surface residue dissipation | CR | R | TEP | 3, 4, 5, 6 |
| 875.2400 | Dermal exposure | CR | CR | TEP | 1, 7, 8 |
| 875.2500 | Inhalation exposure | CR | CR | TEP | 1,7, 8, 9 |
| 875.2600 | Biological monitoring | CR | CR | TEP | 1, 8 |
| 875.2700 | Product use information | R | R | TEP | |
| 875.2800 | Description of human activity | R | R | TEP | |
| 875.2900 | Data reporting and calculations | R | R | TEP | 10 |
(e) Test notes. The following test notes apply to the data requirements in the table to paragraph (d) of this section:
1. Prior to initiation of the study, protocols involving intentional exposure of human subjects must be submitted for review by EPA and then the Human Studies Review Board (HSRB) according to 40 CFR 26.1125. Examples of proposed human study research can be found in various reviews provided by the Human Studies Review Board (HSRB) (http://www.epa.gov/osa/hsrb/index.htm).
2. For residential wood preservative uses, data may be required if soil has the potential to be an important exposure pathway, and soil is in contact with or adjacent to treated wood, including but not limited to decks, play sets, and gazebos,
3. Protocols must be approved by the Agency prior to the initiation of the study.
4. For wood preservatives, data are required for treated wood surfaces where post-application contact with treated wood is anticipated.
5. For occupational uses, data are required if the pesticide may be applied to or around surfaces, and if the human activity data indicate that workers are likely to have post-application dermal contact with treated surfaces while participating in typical activities.
6. Data are required for residential use sites, schools, and daycare institutions. This includes but is not limited to the following: Residential and public access premises; material preservatives (including those used in residential products, including but not limited to clothing and plastic toys) and wood preservatives (when contact with treated wood is likely to occur).
7. Data are required for occupational and residential uses if the human activity data indicate the potential for post-application dermal and/or inhalation exposures while participating in typical activities and no acceptable modeling options are available.
8. Biological monitoring data may be submitted in addition to, or in lieu of, dermal and inhalation passive dosimetry exposure data provided the human pharmacokinetics of the pesticide or metabolite/analog compounds (i.e., whichever method is selected as an indicator of body burden or internal dose) allow for a back-calculation to the total internal dose.
9. Data are required for occupational and residential uses if there is the potential for bystander exposure and the pesticide use could result in respirable and/or inhalable material (e.g., gas, vapor, aerosol, or particulates).
10. Data reporting and calculations are required only if post-application exposure data are required.
�158.2280 Environmental fate.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (c) of this section to determine the environmental fate data requirements for antimicrobial pesticide products. Notes that apply to an individual test including specific conditions, qualifications, or exceptions are listed in paragraph (d) of this section.
(1) Environmental fate data are required to support the registrations of all end-use and manufacturing-use antimicrobial products.
(2) Data on transformation/degradation products or leachate residues of the parent compound are also required to support registration, if the transformation/degradation products or leachate residues meet one of the following criteria:
(i) More toxic, persistent, or bioaccumulative than the parent;
(ii) Have been shown to cause adverse effects in mammalian or aquatic reproductive studies; or
(iii) The moiety of concern (i.e., functional group in the parent chemical molecule that imparts adverse effects) remains intact.
(3) For the purpose of determining data requirements, the all other use patterns category includes the following use patterns:
(i) Agricultural premises and equipment.
(ii) Food-handling/storage establishments, premises, and equipment.
(iii) Commercial, institutional and industrial premises and equipment.
(iv) Residential and public access premises.
(v) Medical premises and equipment.
(vi) Human drinking water systems.
(vii) Materials preservatives.
(viii) Swimming pools.
(b) Key. MP = Manufacturing use product; EP = End-use product; R = Required; CR = Conditionally required; NR = Not required; TGAI = Technical grade of the active ingredient; TEP = Typical end-use product; PAIRA = Pure active ingredient radiolabeled; ROC = residue of concern.
(c) Antimicrobial environmental fate data requirements table. The following table shows the data requirements for environmental fate. The test notes appear in paragraph (d) of this section.
Table - Antimicrobial Environmental Fate Data Requirements
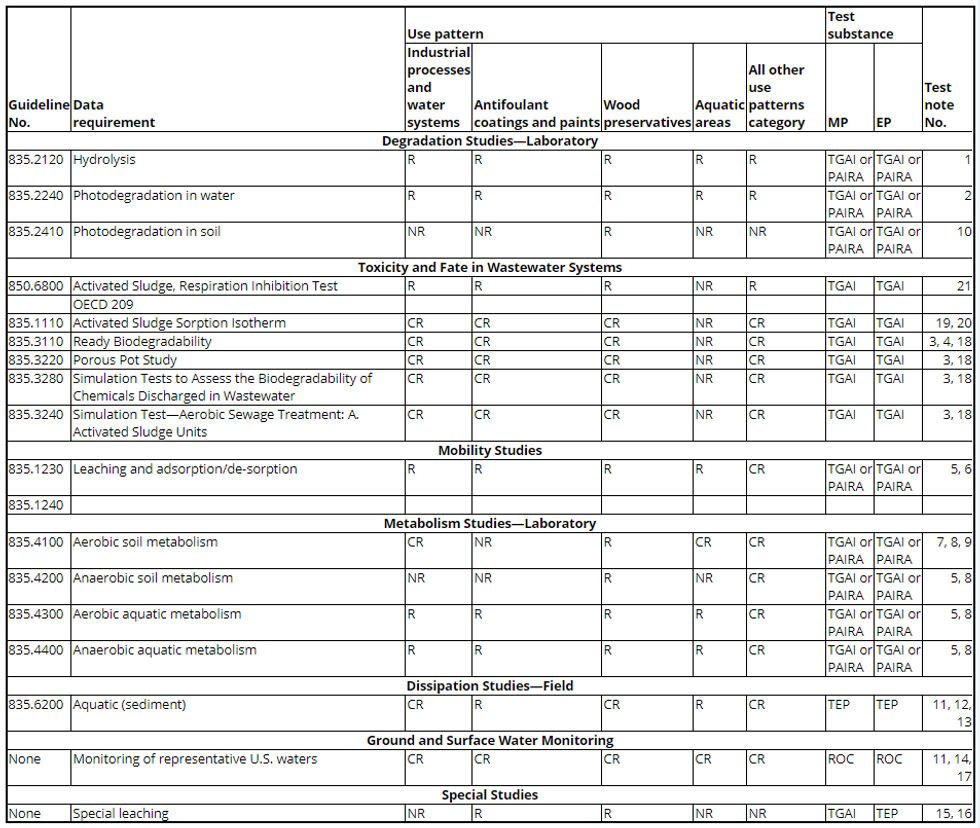
(d) Test notes. The following test notes apply to the data requirements in the table in paragraph (c) of this section:
1. For testing antifoulant paints and coatings, testing is to be performed separately with both sterile buffered distilled water and sterile synthetic seawater at pHs 5, 7, and 9.
2. Not required if:
i. The electronic absorption spectra, measured at pHs 5, 7 and 9, of the chemical and its hydrolytic products, if any, show no absorption or tailing between 290 and 800 nm, inclusive; or
ii. The results of the hydrolysis study at all three pHs (5, 7, and 9) demonstrates a half-life of less than 30 days.
3. The results of the activated sludge, respiration inhibition (ASRI) test determine which of the following tests are required: Ready biodegradability, porous pot, the biodegradation in activated sludge study as described in the �Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater,� or simulation test - aerobic sewage treatment: A. activated sludge units.
i. If the ASRI test EC50 is equal to or less than 20 mg/L, then the applicant must choose either to:
A. Conduct the biodegradation in activated sludge study as described in the �Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater�;
B. Conduct the porous pot test; or
C. Conduct the simulation test - aerobic sewage treatment: A. activated sludge units.
ii. If the ASRI test EC50 is greater than 20 mg/L, then the applicant must choose either to:
A. Conduct a ready biodegradability study; or
B. Conduct one of the following studies: The biodegradation in activated sludge study as described in the �Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater,� the porous pot test, or the simulation test - aerobic sewage treatment: A. activated sludge units.
4. Pass criteria for the ready biodegradability study are: 70 percent removal of dissolved organic carbon (DOC) and 60 percent removal of theoretical oxygen demand (ThOD) or theoretical carbon dioxide (ThCO2) production for respirometric methods. These pass levels must be reached in a 10-day window within the 28-day period of the test. If the antimicrobial passes the ready biodegradability study, then no further testing is required. If the antimicrobial fails the ready biodegradability study, then the applicant must conduct one of the following studies: The biodegradation in activated sludge study as described in the �Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater,� the porous pot test, or the simulation test - aerobic sewage treatment: A. activated sludge units.
5. For the all other use patterns category (as specified in �158.2280(a)(3)), data are required based on a weight-of-evidence evaluation of the results of the hydrolysis, photodegradation in water, activated sludge sorption isotherm, biodegradability, and activated sludge, respiration inhibition tests.
6. Adsorption and desorption using a batch equilibrium method is preferred. In some cases, as when the antimicrobial pesticide degrades rapidly, soil column leaching with unaged or aged columns may be more appropriate to fully characterize the potential mobility of the parent compound and major transformation products.
7. For industrial processes and water systems, aquatic areas, and the all other use patterns category (as specified in �158.2280(a)(3)), data are required based on a weight-of-evidence evaluation of the results of the hydrolysis, photodegradation in water, activated sludge sorption isotherm, biodegradability, and activated sludge, respiration inhibition tests.
8. The environmental media (soil, water, hydrosoil, and biota) to be utilized in these studies must be collected from areas representative of potential use sites.
9. For industrial processes and water systems, and aquatic areas, data are required for use sites that are intermittently dry.
10. Data are not required if the antimicrobial is an inorganic substance or a metal salt; or if the standardized soil profiles demonstrate that the antimicrobial is likely to readily degrade either microbially or via redox reactions (chemically) and no transformation/degradate/leachate products of concern (as described under �158.2280(a)(2)) are produced.
11. Analytical methods used to generate data associated with this study must include results of a successful confirmatory method trial by an independent laboratory.
12. Protocols must be approved by the Agency prior to the initiation of the study.
13. For industrial processes and water systems, wood preservatives, and the all other use patterns category (as specified in �158.2280(a)(3)), data are required based on the potential for aquatic exposure and if the weight-of-evidence indicates that the active ingredient or principal transformation products are likely to have the potential for persistence, mobility, nontarget aquatic toxicity, or bioaccumulation.
14. Data are required if the weight-of-evidence indicates that the active ingredient or principal transformation products are likely to occur in nontarget freshwater, estuarine, or marine waters such that human or environmental exposures are likely to occur. In making that determination, the Agency takes into account other factors such as the toxicity of the chemical(s), available monitoring data and the vulnerability of the freshwater, estuarine, or marine water resources in the antimicrobial use area.
15. For wood preservatives, an aquatic leaching study is required. A soil leaching study is required if human or environmental exposures are likely to occur from leachates that contain the active ingredient or principal transformation products from wood treated with a preservative product. Protocols must be approved by the Agency prior to the initiation of the study.
16. For antifoulant paints and coatings, a leaching study is required. Protocols must be approved by the Agency prior to the initiation of the study.
17. Protocols, which include the residues of concern (such as parent, degradate/transformation product, and/or leachate residues) that would be monitored, must be approved by the Agency prior to the initiation of the study.
18. A biodegradation study is not required if the antimicrobial meets one or more of the following criteria:
i. Classified as a metal,
ii. Relatively volatile, but not hydrophobic,
iii. Highly reactive,
iv. Both the parent and all transformation/degradate products (as described under �158.2280(a)(2)) have half-lives of less than 3 hours,
v. None of the registered or proposed product uses would result in transport of the parent and its transformation/degradate products (as described under �158.2280(a)(2)) to a wastewater treatment plant.
19. The activated sludge sorption isotherm test is not required if the antimicrobial is:
i. Relatively volatile, but not hydrophobic;
ii. Highly reactive; or
iii. The log Kow is less than 3.0.
20. If the criteria of test note 19 of this paragraph are not met, then the activated sludge sorption isotherm test is required if one or more of the following criteria are also met:
i. The antimicrobial is a metal,
ii. The log Kow is greater than or equal to 3.0,
iii. The antimicrobial is positively charged or polycationic,
iv. The EC50 in the activated sludge, respiration inhibition test is less than or equal to 20 mg/L,
v. The EC50 in the activated sludge, respiration inhibition test is greater than 20 mg/L, and the antimicrobial fails the ready biodegradability study.
21. The activated sludge respiration inhibition study is not required if none of the registered or proposed product uses would result in transport of the parent and its transformation/degradate products (as described under �158.2280(a)(2)) to a wastewater treatment plant.
�158.2290 Residue chemistry.
(a) General. Subpart B of this part and �158.2201 describe how to use the table in paragraph (h) of this section to determine the residue chemistry data requirements for antimicrobial pesticide products. Notes that apply to an individual test including specific conditions, qualifications, or exceptions are listed in paragraph (i) of this section.
(b) Residue chemistry data are required for:
(1) Antimicrobial end-use products with uses that may result in residues in or on food, including but not limited to:
(i) Products that require a tolerance, tolerance exemption, or food additive regulation or clearance.
(ii) Products that may be used to treat livestock or poultry drinking water, for food egg washing, or for fruit and vegetable rinses.
(iii) Products that may be applied to a surface or incorporated into a material that may contact food or feed. Data are required regardless of whether the antimicrobial is applied or impregnated for the purpose of imparting antimicrobial protection to external surfaces of the substance or article, or for the purpose of protecting the substance or article itself.
(iv) Products that may be applied to water that have the potential to result in residues in potable water, or in water used for livestock and poultry drinking water, irrigation of crops, or water containing fish that may be used for human food.
(v) Wood preservative or antifoulant products intended for treating submerged materials that may result in food contact (e.g., lobster pots, fish cages on fish farms).
(2) Each manufacturing-use product bearing directions for formulation into an end-use product bearing uses described in paragraph (b)(1) of this section.
(c) Residue chemistry data are not required under paragraph (b) of this section if no adverse effects (no toxicity endpoints) are associated with dietary exposure to the active ingredient or if theoretical (high-end) dietary exposure estimates combined with the applicable toxicity endpoint result in acute and chronic dietary risks that are below the Agency levels of concern.
(d) For purposes of this section, Magnitude of the Residue Studies include the following: Food-handling, migration studies, potable water, fish, irrigated crops, meat/milk/poultry/eggs, crop field trails, processed food or feed, and anticipated residues.
(e) If the antimicrobial chemical may be applied to a field crop, then the residue chemistry data requirements of �158.1410 apply.
(f) The following term is defined for the purposes of this section: Residue of concern means the parent pesticidal compound and its metabolites, degradates, and impurities of toxicological concern.
(g) Key. R = Required; CR = Conditionally required; NR = Not required; TGAI = Technical grade of the active ingredient; TEP = Typical end-use product; PAI = Pure active ingredient; PAIRA = Pure active ingredient radiolabeled; ROC = Residue of concern.
(h) Antimicrobial residue chemistry data requirements table. The following table shows the data requirements for residue chemistry. The test notes appear in paragraph (i) of this section.
| Guideline No. | Data requirement | Uses | Test
substance | Test note No. | |||
|---|---|---|---|---|---|---|---|
| Agricultural
premise | Indirect food | Direct food | Aquatic | ||||
| Supporting Information | |||||||
| 860.1100 | Chemical identity | R | R | R | R | TGAI | |
| 860.1200 | Directions for use | R | R | R | R | ||
| 860.1550 | Proposed tolerance/tolerance exemption | R | R | R | R | 1 | |
| 860.1560 | Reasonable grounds in support of petition | R | R | R | R | 1 | |
| 860.1650 | Submittal of analytical reference standards | R | R | R | R | PAI/ROC | 2 |
| Food-Contact Surfaces or Impregnated Materials | |||||||
| 860.1460 | Food-handling | CR | CR | CR | CR | TEP | 3 |
| None | Nature of residue on surfaces | CR | CR | CR | CR | PAIRA or TGAI | 4 |
| None | Migration studies | CR | CR | CR | CR | TEP | 5 |
| 860.1340 | Residue analytical method for data collection | CR | CR | CR | CR | ROC | 6 |
| 860.1380 | Storage stability | R | R | R | R | TEP or ROC | 7 |
| Higher tiered | |||||||
| 860.1300 | Nature of the residue in plants | CR | CR | CR | CR | PAIRA | 8 |
| 860.1300 | Nature of the residue in livestock | CR | CR | CR | CR | PAIRA | 9 |
| 860.1340 | Residue analytical methods for tolerance/tolerance exemption enforcement | CR | CR | CR | CR | ROC | 10 |
| 860.1360 | Multiresidue method testing | CR | CR | CR | CR | ROC | 11 |
| 860.1400 | Potable water | CR | CR | CR | CR | TEP | 12 |
| 860.1400 | Fish | CR | CR | CR | CR | TEP | 13 |
| 860.1400 | Irrigated crops | CR | CR | CR | CR | TEP | 14 |
| 860.1480 | Meat/milk/poultry/eggs | CR | CR | CR | CR | TGAI or ROC | 15 |
| 860.1500 | Crop field trials | CR | CR | CR | CR | TEP | 16 |
| 860.1520 | Processed food or feed | CR | CR | CR | CR | TEP | 17 |
| None | Anticipated residues | CR | CR | CR | CR | ROC | 18 |
(i) Test notes. The following test notes apply to the data requirements in the table to paragraph (h) of this section:
1. A petition proposing a numerical tolerance or a tolerance exemption is required for any food or feed use subject to section 408 of FFDCA if the use is not covered by an existing tolerance or tolerance exemption. If the use is subject to FFDCA section 409, the applicant must identify to EPA an applicable section 409 food additive regulation or clearance, or submit a copy of a petition to FDA requesting a section 409 food additive regulation or clearance for the food or feed use.
2. An analytical reference standard is required for any food or feed use requiring a numeric tolerance or exemption. Material safety data sheets as specified by the Occupational Safety and Health Administration in 29 CFR 1910.1200 must accompany analytical standards.
3. Data are required if a pesticide may be used in a food-handling establishment unless data including, but not limited to, theoretical (high-end) estimates, radiolabeled laboratory data, or the nature of the residue on surfaces study show that residues will not occur in food or feed.
4. If an antimicrobial pesticide may be applied to a food-contact surface or impregnated into a food-contact material and if theoretical (high-end) estimates of exposure exceed EPA's risk level of concern, then the nature of the residue on surfaces study is required. Protocols must be approved by the Agency prior to the initiation of the study.
5. Based on the results of the nature of the residue on surfaces study, if residues of concern are identified, then the migration study will be required. Protocols must be approved by the Agency prior to the initiation of the study.
6. If a magnitude of the residue study, as specified in �158.2290(d), is required, then a residue analytical method suitable for collecting data is also required. The method must be capable of determining all residues of concern, to permit calculation of dietary risk or to establish a tolerance or tolerance exemption.
7. If a magnitude of the residue study, as specified in �158.2290(d), is required, then storage stability data are also required, unless analytical samples are stored for 30 days or less. If, during hazard characterization, a residue has been identified as �of concern� and is known to be volatile or labile, then storage stability data are required regardless of sample storage time.
8. If crop plants or metabolically active raw agricultural commodities of food crops may be directly or indirectly exposed to an antimicrobial, plant metabolism studies are required to determine the transformation products that may enter the human diet. Such exposure could include, but is not limited to:
i. Treatment of storage or shipping containers,
ii. Postharvest fruit and vegetable treatment prior to shipping or storage,
iii. Use of antimicrobial-treated water for irrigation, and
iv. Any direct food contact use.
9. If livestock may be exposed to an antimicrobial, then hen and ruminant metabolism studies are required to determine the identities of residues of concern that may enter the human diet from consumption of livestock commodities. Livestock may be exposed via the oral, dermal, or inhalation route following treatment or contamination of sites including, but not limited to, livestock premises, feed, and drinking water. Shell eggs and other metabolically active livestock products may also be treated. If livestock may be exposed to one or more residues of concern differing from those found in animals, then one or more additional livestock metabolism studies involving dosing with these residues may be required.
10. If there is a numerical tolerance or tolerance exemption level to enforce, then a residue analytical method suitable for enforcement purposes is required. The method must be supported by an independent laboratory validation.
11. If there is a numerical tolerance or tolerance exemption level to enforce, then testing is required to determine whether the Food and Drug Administration/United States Department of Agriculture multiresidue methodology would detect and identify the antimicrobial and its residues of concern, as part of programs to monitor pesticides in the U.S. food supply.
12. Data are required if an antimicrobial may be applied directly to water or if there is the potential that the antimicrobial-treated water could be used directly for drinking water purposes by humans or animals or that contaminated water could run-off, leach, or be discharged from treated sites or materials and make its way into potable water.
13. Data are required if an antimicrobial may be applied directly to water inhabited by fish or that will be inhabited by fish or if contaminated water could run-off, leach, or be discharged from treated sites or materials and make its way into bodies of water containing fish that may be used for human consumption.
14. Data are required if an antimicrobial may be applied directly to water used for irrigation of food crops or such that contaminated water could run-off, leach, or be discharged from treated sites or materials to make its way into water used for irrigation of food crops.
15. If the antimicrobial may be applied directly to livestock, metabolically-active livestock commodities (e.g., eggs), livestock feed or drinking water, or livestock premises, or a livestock metabolism study indicates that residues of the antimicrobial may result in livestock commodities, studies are required to determine the magnitude of the residues of concern in fat, meat, meat by-products, milk, poultry, and eggs that may be consumed by humans. These studies, however, may not be required in cases where the livestock metabolism studies indicate that transfer of pesticide residues of concern to tissues, milk, and eggs is not expected to occur at the maximum expected exposure level for the animals.
16. If food crops or raw agricultural commodities of food crops may be exposed to an antimicrobial, then residue studies are required to determine the magnitude of the residues of concern that may enter the human diet. Such exposures include, but are not limited to, postharvest fruit and vegetable treatments and application of antimicrobial chemicals to field crops, mushroom houses, empty or occupied beehives, or wood used to construct beehives.
17. Data on the nature and magnitude of residues in processed food or feed are required if antimicrobial residues could potentially concentrate on processing. If so, the establishment of a separate tolerance higher than that in the raw agricultural commodity may be required.
18. Data are required when dietary exposure values at the tolerance level or screening-level (high-end) result in estimates of dietary or aggregate risk that meet or exceed the Agency's level of concern. These data may include, but are not limited to, washing, cooking, processing, or degradation studies as well as market basket surveys for a more realistic residue determination. Protocols must be approved by the Agency prior to the initiation of the study.
