...
§158.200 Experimental use permit data requirements tables.
Sections 158.200 through 158.270 describe how to use these tables to determine the experimental use permit data requirements for a particular pesticide product. Notes that apply to an individual test and include specific conditions, qualifications, or exceptions to the designated test are listed at the end of each table. Refer to 40 CFR part 172 for further information on experimental use permits.
§158.210 Experimental use permit data requirements for product chemistry.
All product chemistry data, as described in §158.310, must be submitted to support a request for an experimental use permit.
§158.220 Experimental use permit data requirements for product performance.
All product performance data, as described in paragraph (c) of this section, must be submitted to support a request for an experimental use permit.
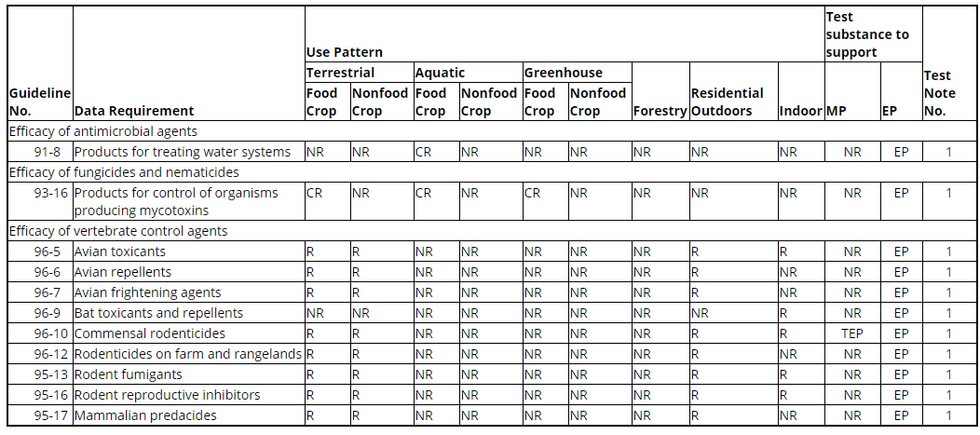
(a) Use patterns. (1) The terrestrial use pattern includes products classified under the general use patterns of terrestrial food crop and terrestrial nonfood crop. The aquatic use pattern includes products classified under the general use patterns of aquatic food crop and aquatic nonfood crop. The greenhouse use pattern includes products classified under the general use patterns of greenhouse food crop and greenhouse nonfood crop. The indoor use pattern includes products classified under the general use patterns of indoor food and indoor nonfood use.
(2) Data are also required for forestry and residential outdoor uses.
(b) Key. CR = Conditionally required; NR = Not required; R = Required; MP = Manufacturing-use product; EP = End-use product; TEP = Typical end-use product.
(c) Table. The following table shows the experimental use data requirements for product performance. The test notes are shown in paragraph (d) of this section.
Table - Experimental Use Permit Data Requirements for Product Performance
(d) Test notes. The following test notes apply to the data requirements in the table to paragraph (c) of this section.
1. The Agency has waived the requirement to submit efficacy data unless the pesticide product bears a claim to control pest microorganisms that pose a threat to human health and whose presence cannot readily be observed by the user including, but not limited to, microorganisms infectious to man in any area of the inanimate environment, or a claim to control vertebrates (such as rodents, birds, bats, canids, and skunks) that may directly or indirectly transmit diseases to humans. However each registrant must ensure through testing that his product is efficacious when used in accordance with label directions and commonly accepted pest control practices. The Agency reserves the right to require, on a case-by-case basis, submission of efficacy data for any pesticide product registered or proposed for registration.
2. [Reserved]
[72 FR 60957, Oct. 26, 2007, as amended at 73 FR 75596, Dec. 12, 2008]
§158.230 Experimental use permit data requirements for toxicology.
All toxicology data, as described in paragraph (c) of this section, must be submitted to support a request for an experimental use permit.
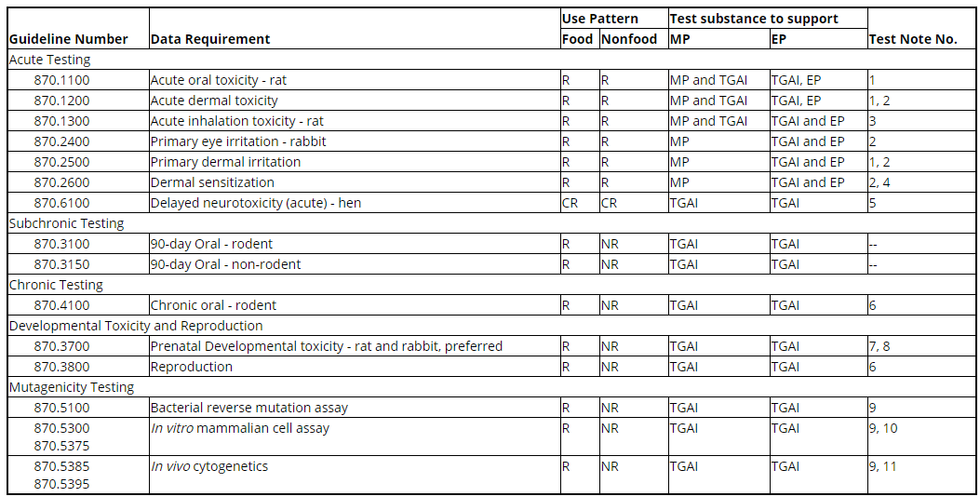
(a) Use patterns. (1) Food use patterns include products classified under the general use patterns of terrestrial food crop use, terrestrial feed crop use, aquatic food crop use, greenhouse food crop use, and indoor food use.
(2) Nonfood use patterns include products classified under the general use patterns of terrestrial nonfood crop use, aquatic nonfood crop use, aquatic nonfood outdoor use, greenhouse nonfood crop use, forestry use, residential outdoor use, indoor nonfood use, and indoor residential use.
(b) Key. CR = Conditionally required; NR = Not required; R = Required; EP = End-use product; MP = Manufacturing-use product; PAIRA = Pure active ingredient radio-labeled; TGAI = Technical grade of the active ingredient.
(c) Table. The following table shows the experimental use data requirements for toxicology. The test notes are shown in paragraph (d) of this section.
Table - Experimental Use Permit Toxicity Data Requirements
(d) Test notes. The following test notes apply to the data requirements in the table to paragraph (c) of this section.
1. Not required if test material is a gas or a highly volatile liquid.
2. Not required if test material is corrosive to skin or has a pH of less than 2 or greater than 11.5.
3. Required if the product consists of, or under conditions of use will result in, a respirable material (e.g., gas, vapor, aerosol, or particulate).
4. Required if repeated dermal exposure is likely to occur under conditions of use.
5. Required if the test material is an organophosphorus substance, which includes uncharged organophosphorus esters, thioesters, or anhydrides of organophosphoric, organophosphonic, or organophosphoramidic acids, or of related phosphorothioic, phosponothioic, or phosphorothioamidic acids, or is structurally related to other substances that may cause the delayed neurotoxicity sometimes seen in this class of chemicals.
6. These studies are seldom required to support EUPs. They may be required if the dietary exposure for these EUPs occupies a large part, e.g., greater than 50%, of the reference dose.
7. The oral route, by oral intubation, is preferred unless the chemical or physical properties of the test substance or the pattern of exposure suggests a more appropriate route of exposure.
8. May be combined with the 2-generation reproduction study in rodents by utilizing a second mating of the parental animals in either generation.
9. At a minimum, an initial battery of mutagenicity tests with possible confirmatory testing is required. Other relevant mutagenicity tests that may have been performed, plus a complete reference list must also be submitted.
10. Choice of assay using either:
i. Mouse lymphoma L5178Y cells, thymidine kinase (tk) gene locus, maximizing assay conditions for small colony expression or detection;
ii. Chinese hamster ovary (CHO) or Chinese hamster lung fibroblast (V79) cells, hypoxanthine-guanine phosphoribosyl transferase (hgprt) gene locus, accompanied by an appropriate in vitro test for clastogenicity; or
iii. CHO cells strains AS52, xanthine-guanine phosphoribosyl transferase (xprt) gene locus.
11. The micronucleus rodent bone marrow assay is preferred; however, rodent bone marrow assays using metaphase analysis (aberrations) are acceptable.
[72 FR 60957, Oct. 26, 2007, as amended at 73 FR 75596, Dec. 12, 2008]
§158.240 Experimental use permit data requirements for ecological effects.
All data for terrestrial nontarget organisms and aquatic nontarget organisms as described in §158.243 must be submitted to support a request for an experimental use permit. No data for nontarget plant protection must be submitted to support a request for an experimental use permit.
§158.243 Experimental use permit data requirements for terrestrial and aquatic nontarget organisms.
All terrestrial and aquatic nontarget organism data, as described in paragraph (c) of this section, must be submitted to support a request for an experimental use permit.
(a) Use patterns. (1) The terrestrial use pattern includes products classified under the general use patterns of terrestrial food crop, terrestrial feed crop, and terrestrial nonfood crop. The aquatic use pattern includes products classified under the general use patterns of aquatic food crop and aquatic nonfood. The greenhouse use pattern includes products classified under the general use patterns of greenhouse food crop and greenhouse nonfood crop. The indoor use pattern includes products classified under the general use patterns of indoor food and indoor nonfood use.
(2) Data are also required for the general use patterns of forestry and residential outdoor use.
(b) Key. CR = Conditionally required; NR = Not required; R = Required; TEP = Typical end-use product; TGAI = Technical grade of the active ingredient; commas between the test substances (e.g. TGAI, TEP) indicate that data may be required on the TGAI or TEP depending on the conditions set forth in the test note.
(c) Table. The following table shows the experimental use data requirements for terrestrial and aquatic nontarget organisms. The test notes are shown in paragraph (d) of this section.
| Guideline No. | Data Requirement | Use Pattern | Test substance | Test Note No. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Terrestrial | Aquatic | Forestry | Residential Outdoor | Greenhouse | Indoor | ||||
| Avian and Mammalian Testing | |||||||||
| 850.2100 | Avian oral toxicity | R | R | R | R | CR | CR | TGAI | 1, 2, 3 |
| 850.2200 | Avian dietary toxicity | R | R | R | R | NR | NR | TGAI | 1, 4 |
| Aquatic Organisms Testing | |||||||||
| 850.1075 | Freshwater fish toxicity | R | R | R | NR | NR | NR | TGAI, TEP | 1, 2, 5, 6, 11 |
| 850.1010 | Acute toxicity freshwater invertebrates | R | R | R | NR | NR | NR | TGAI, TEP | 1, 2, 6, 7, 11 |
| 850.1300 | Aquatic invertebrate life cycle (freshwater) | NR | R | R | NR | NR | NR | TGAI | 1, 7, 8 |
| 850.1400 | Fish early-life stage (freshwater) | NR | R | R | NR | NR | NR | TGAI | 1, 8, 9 |
| Accumulation Study | |||||||||
| 850.1730 | Fish | CR | CR | CR | NR | NR | NR | TGAI or PAIRA | 10 |
| Insect Pollinator Testing | |||||||||
| 850.3020 | Honeybee acute contact toxicity | R | R | R | NR | NR | NR | TGAI | 1 |
(d) Test notes. The following test notes apply to the data requirements in the table to paragraph (c) of this section.
1. Data using the TGAI are required to support all outdoor end-use product uses including, but not limited to, turf. Data are generally not required to support end-use products in the form of a gas, a highly volatile liquid, a highly reactive solid, or a highly corrosive material.
2. For greenhouse and indoor end-use products, data using the TGAI are required to support manufacturing-use products to be reformulated into these same end-use products or to support end-use products when there is no registered manufacturing-use product. Avian acute oral data are not required for liquid formulations for greenhouse and indoor uses. The study is not required if there is no potential for environmental exposure.
3. Data are required on one passerine species and either one waterfowl species or one upland game bird species for terrestrial, aquatic, forestry, and residential outdoor uses. Data are preferred on waterfowl or upland game bird species for indoor and greenhouse uses.
4. Data are required on waterfowl and upland game bird species.
5. Data are required on one coldwater fish and one warmwater fish for terrestrial, aquatic, forestry, and residential outdoor uses. For indoor and greenhouse uses, testing with only one of either fish species is required.
6. EP or TEP testing is required for any product which meets any of the following conditions:
i. The end-use pesticide will be introduced directly into an aquatic environment (e.g., aquatic herbicides and mosquito larvicides) when used as directed.
ii. The maximum expected environmental concentration (MEEC) or the estimated environmental concentration (EEC) in the aquatic environment is ?one-half the LC50 or EC50 of the TGAI when the EP is used as directed.
iii. An ingredient in the end-use formulation other than the active ingredient is expected to enhance the toxicity of the active ingredient or to cause toxicity to aquatic organisms.
7. Data are required on one freshwater aquatic invertebrate species.
8. Data are generally not required for outdoor residential uses, other than turf, unless data indicate that pesticide residues from the proposed use(s) can potentially enter waterways.
9. Data are required on one freshwater fish species. If the test species is different from the two species used for the freshwater fish acute toxicity tests, a 96 hour LC50 on that species must also be provided.
10. Not required when:
i. The octanol/water partition coefficients of the pesticide and its major degradates are <1,000; or
ii. There are no potential exposures to fish and other nontarget aquatic organisms; or
iii. The hydrolytic half-life is <5 days at pH 5, 7 and 9.
11. The freshwater fish test species for the TEP testing is the most sensitive of the species tested with the TGAI. A freshwater invertebrate must also be tested with the EP or TEP using the same species tested with the TGAI.
[72 FR 60957, Oct. 26, 2007, as amended at 73 FR 75596, Dec. 12, 2008]
§158.250 Experimental use permit data requirements for human exposure.
No data for applicator exposure and post-application exposure must be submitted to support a request for an experimental use permit.
§158.260 Experimental use permit data requirements for environmental fate.
All environmental fate data, as described in paragraph (c) of this section, must be submitted to support a request for an experimental use permit.
(a) Use patterns. (1) The terrestrial use pattern includes products classified under the general use patterns of terrestrial food crop, terrestrial feed crop, and terrestrial nonfood. The aquatic use pattern includes the general use patterns of aquatic food crop, aquatic nonfood residential, and aquatic nonfood outdoors. The greenhouse use pattern includes both food and nonfood uses. The indoor use pattern includes food, nonfood, and residential indoor uses.
(2) Data are also required for the general use patterns of forestry use and residential outdoor use.
(b) Key. CR = Conditionally required; NR = Not required; R = Required; PAIRA = Pure active ingredient radio-labeled; TGAI = Technical grade of the active ingredient.
(c) Table. The following table shows the experimental use data requirements for environmental fate. The test notes are shown in paragraph (d) of this section.
| Guideline No. | Data Requirement | Use Pattern | Test substance | Test Note No. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Terrestrial | Aquatic | Greenhouse | Indoors | Forestry | Residential Outdoors | ||||
| Degradation Study - Laboratory | |||||||||
| 835.2120 | Hydrolysis | R | R | R | NR | R | R | TGAI or PAIRA | 1 |
| Metabolism Studies - Laboratory | |||||||||
| 835.4100 | Aerobic soil | R | CR | NR | NR | R | NR | TGAI or PAIRA | 2 |
| 835.4300 | Aerobic aquatic | NR | R | NR | NR | NR | NR | TGAI or PAIRA | -- |
| Mobility Study | |||||||||
| 835.1230 835.1240 | Leaching and adsorption/desorption | R | NR | NR | NR | R | NR | TGAI or PAIRA | 3 |
(d) Test notes. The following test notes apply to the data requirements in the table to paragraph (c) of this section.
1. Study is required for indoor uses in cases where environmental exposure is likely to occur. Such sites include, but are not limited to, agricultural premises, in or around farm buildings, barnyards, and beehives.
2. Required for aquatic uses for aquatic sites that are intermittently dry. Such sites include, but are not limited to cranberry bogs and rice paddies.
3. Adsorption and desorption using a batch equilibrium method is preferred. However, in some cases, for example, where the pesticide degrades rapidly, soil column leaching with unaged or aged columns may be more appropriate to fully characterize the potential mobility of the parent compound and major transformation products.
[72 FR 60957, Oct. 26, 2007, as amended at 73 FR 75596, Dec. 12, 2008]
§158.270 Experimental use permit data requirements for residue chemistry.
All residue chemistry data, as described in §158.1410, are required for an experimental use permit for which a temporary tolerance under FFDCA section 408(r) is sought. Residue chemistry data are not required for an experimental use permit issued on a crop-destruct basis.
