['Toxic Substances Control Act - EPA']
['Toxic Subtances Control Act - EPA', 'Toxic Substances - EPA']
05/13/2024
...
ENVIRONMENTAL PROTECTION AGENCY
40 CFR Part 700
[EPA-HQ-OPPT-2020-0493; FRL-7911-04-OCSPP]
RIN 2070-AK64
Fees for the Administration of the Toxic Substances Control Act (TSCA)
AGENCY: Environmental Protection Agency (EPA).
ACTION: Supplemental notice of proposed rulemaking.
SUMMARY: The Environmental Protection Agency (EPA) is issuing this document to modify and supplement its proposed rule issued on January 11, 2021, in which the Agency proposed updates and adjustments to the 2018 Fee Rule established under the Toxic Substances Control Act (TSCA). With over five years of experience administering the TSCA amendments of 2016, EPA is publishing this document to ensure that the fees charged accurately reflect the level of effort and resources needed to implement TSCA in the manner envisioned by Congress when it reformed the law. Additionally, the purpose of this document is to propose narrowing certain proposed exemptions for entities subject to the EPA-initiated risk evaluation fees and propose exemptions for the test rule fee activities; to propose modifications to the self-identification and reporting requirements for EPA-initiated risk evaluation and test rule fees; to propose a partial refund of fees for premanufacture notices withdrawn at any time after the first 10 business days during the assessment period of the chemical; to propose modifications to EPA's proposed methodology for the production volume-based fee allocation for EPA-initiated risk evaluation fees in any scenario where a consortium is not formed; to propose expanding the fee requirements to companies required to submit information for test orders; to propose modifying the fee payment obligations to require payment by processors subject to test orders and enforceable consent agreements (ECA); to propose extending the timeframe for test order and test rule payments; as well as to propose changes to the fee amounts and the estimate of EPA's total costs for administering TSCA.
DATES: Comments must be received on or before January 17, 2023.
ADDRESSES: Submit your comments, identified by docket identification (ID) number EPA-HQ-OPPT-2020-0493, through the Federal eRulemaking Portal at https://www.regulations.gov. Follow the online instructions for submitting comments. Do not submit electronically any information you consider to be Confidential Business Information (CBI) or other information whose disclosure is restricted by statute. Additional instructions on commenting and visiting the docket, along with more information about dockets generally, is available at https://www.epa.gov/dockets.
FOR FURTHER INFORMATION CONTACT:
For technical information contact: Marc Edmonds, Existing Chemicals Risk Management Division (7404M), Office of Pollution Prevention and Toxics, Environmental Protection Agency, 1200 Pennsylvania Ave. NW, Washington, DC 20460-0001; telephone number: (202) 566-0758; email address: edmonds.marc@epa.gov.
For general information contact: The TSCA-Hotline, ABVI-Goodwill, 422 South Clinton Ave., Rochester, NY 14620; telephone number: (202) 554-1404; email address: TSCA-Hotline@epa.gov.
SUPPLEMENTARY INFORMATION:
I. Executive Summary
A. Does this action apply to me?
You may be affected by this action if you manufacture (including import), process, or distribute in commerce a chemical substance (or any combination of such activities) and are required to submit information to EPA under TSCA sections 4 or 5, or if you manufacture a chemical substance that is the subject of a risk evaluation under TSCA section 6(b).The following list of North American Industry Classification System (NAICS) codes is not intended to be exhaustive, but rather provides a guide to help readers determine whether this document applies to them.
Potentially affected entities may include companies found in major NAICS groups:
- Chemical Manufacturers (NAICS code 325).
- Petroleum and Coal Products (NAICS code 324).
- Chemical, Petroleum and Merchant Wholesalers (NAICS code 424).
If you have any questions regarding the applicability of this action, please consult the technical person listed under FOR FURTHER INFORMATION CONTACT.
B. What is the Agency's authority for taking this action?
TSCA, 15 U.S.C. 2601 et seq., as amended by the Frank R. Lautenberg Chemical Safety for the 21st Century Act of 2016 (Pub. L. 114-182) (Ref. 1), provides EPA with authority to establish fees to defray, or provide payment for, a portion of the costs associated with administering TSCA sections 4, 5, and 6, as amended, as well as the costs of collecting, processing, reviewing, and providing access to and protecting from disclosure as appropriate under TSCA section 14 information on chemical substances under TSCA. EPA is required in TSCA section 26(b)(4)(F) to review and, if necessary, adjust the fees every three years, after consultation with parties potentially subject to fees, to ensure that funds are sufficient to defray part of the cost of administering TSCA. EPA is issuing this supplemental notice of proposed rulemaking under TSCA section 26(b), 15 U.S.C. 2625(b).
C. What action is the Agency taking?
After establishing fees under TSCA section 26(b), TSCA requires EPA to review and, if necessary, adjust the fees every three years, after consultation with parties potentially subject to fees. This document describes proposed changes to 40 CFR part 700, subpart C as promulgated in the 2018 Fee Rule (Ref. 2) and explains the methodology by which these proposed changes to TSCA fees were determined. This supplemental notice of proposed rulemaking adds to and modifies the proposed rulemaking issued on January 11, 2021 (“the 2021 Proposal”) (Ref. 3). EPA is proposing to narrow certain proposed exemptions for entities subject to the EPA-initiated risk evaluation fees and propose exemptions for test rule fee activities; to modify the self-identification and reporting requirements for EPA-initiated risk evaluation and test rule fees; to institute a partial refund of fees for premanufacture notices withdrawn at any time after the first 10 business days during the assessment period of the chemical; to modify EPA's proposed methodology for the production volume-based fee allocation for EPA-initiated risk evaluation fees in any scenario where a consortium is not formed; to expand the fee requirements to companies required to submit information for test orders; to modify the fee payment obligations to require payment by processors subject to test orders and ECA; to extend the timeframe for test order and test rule payments; and to change the fee amounts and the estimate of EPA's total costs for administering TSCA sections 4, 5, 6, and 14.
D. Why is the Agency taking this action?
The fees collected under TSCA are intended to achieve the goals articulated by Congress by providing a sustainable source of funds for EPA to fulfill its legal obligations under TSCA sections 4, 5, and 6 and with respect to information management under TSCA section 14. Information management includes “collecting, processing, reviewing, and providing access to and protecting from disclosure as appropriate under [section 14] information on chemical substances under [TSCA]. In 2021, EPA proposed changes to the TSCA fee requirements established in the 2018 Fee Rule based upon TSCA fee implementation experience and proposed to adjust the fee amounts based on changes to program costs and inflation and to address certain issues related to implementation of the fee requirements (Ref. 3). EPA consulted and met with stakeholders that were potentially subject to fees, including several meetings with individual stakeholders and a public webinar in February 2021. Additional information on stakeholder engagement can be found in the 2021 Proposal Unit III.A.1 (Ref. 3). EPA is hosting another public engagement after the publication of this proposed rule where EPA will hear from stakeholders on the proposed TSCA fees. This engagement and the previous stakeholder outreach will inform EPA's final rule.
This supplemental proposal takes into consideration comments received in response to the 2021 Proposal which EPA plans to respond to, along with comments received on this notice, when EPA finalizes the rule. Based on these comments, adjustments to EPA's cost estimates, and experience implementing the 2018 Fee Rule, EPA is issuing this supplemental notice and is requesting comments on the proposed provisions and primary alternative provisions described herein that would add to or modify the 2021 Proposal. TSCA allows the Agency to collect approximately but not more than 25 percent of its costs for eligible TSCA activities via fees; however, fee revenue has been roughly half of the estimated costs for eligible activities than EPA estimated in the 2018 Fees Rule. The reason for the shortfall was, in part, that EPA used estimates of the costs based on what the Agency had historically spent on implementing TSCA prior to the 2016 amendments, not what it would cost the Agency to implement TSCA in the manner envisioned and directed by Congress in the Lautenberg Amendments. In the first four years following the 2016 law's enactment, EPA also did not conduct a comprehensive budget analysis designed to estimate the actual costs of implementing the amended law until the spring of 2021. In this notice, EPA is proposing to revise its cost estimate to adequately account for the anticipated costs of meeting its statutory mandates, which are based on the comprehensive analysis conducted in 2021. These proposed revisions are designed to ensure fee amounts capture approximately but not more than 25 percent of the costs of administering certain TSCA activities, fees are distributed equitably among fee payers when multiple fee payers are identified by revising the fee allocation methodology for EPA-initiated risk evaluations, and fee payers are identified via a transparent process.
E. What are the estimated incremental impacts of this action?
EPA has evaluated the potential incremental economic impacts of the 2021 Proposal, as modified by this supplemental notice for FY 2023 through FY 2025. The “Economic Analysis of the Supplemental Notice of Proposed Rule for Fees for the Administration of the Toxic Substances Control Act” (Economic Analysis) (Ref. 4) is available in the docket and is briefly summarized here.
- Benefits. The principal benefit of the 2021 Proposal, as modified by this supplemental notice, is to provide EPA a sustainable source of funding necessary to administer certain provisions of TSCA.
- Cost. The annualized fees collected from industry under the proposed cost estimate described in this supplemental notice are approximately $45.47 million (at both 3 percent and 7 percent discount rates [Note: The annualized fee collection is independent of the discount rate.]), excluding fees collected for manufacturer-requested risk evaluations. Total annualized fee collection was calculated by multiplying the estimated number of fee-triggering events anticipated each year by the corresponding fees (Ref. 4). Total annual fee collection for manufacturer-requested risk evaluations is estimated to be $3.01 million for chemicals included in the 2014 TSCA Work Plan (TSCA Work Plan) (based on the assumed potential for two requests over the three-year period) and approximately $2.99 million for chemicals not included in the TSCA Work Plan (based on the assumed potential for one request over the three-year period) (Refs. 4 and 5). EPA analyzed a three-year period because the statute requires EPA to reevaluate and adjust, as necessary, the fees every three years.
- Small entity impact. EPA estimates that 29 percent of section 5 submissions will be from small businesses that are eligible to pay the section 5 small business fee because they meet the definition of “small business concern.” Total annualized fee collection from small businesses submitting notices under section 5 is estimated to be $666,810 (Ref. 4). For sections 4 and 6, reduced fees paid by eligible small businesses and fees paid by non-small businesses may differ because the fee paid by each entity would be dependent on the number of entities identified per fee-triggering event and production volume of that chemical substance. EPA estimates that average annual fee collection from small businesses for fee-triggering events under section 4 and section 6 would be approximately $103,574 and $2,896,351, respectively (Ref. 4). For each of the three years covered by this proposed rule, EPA estimates that total fee revenue collected from small businesses will account for about 6 percent of the approximately $52 million total fee collection, for an annual average total of approximately $3 million.
- Environmental justice. Although not directly impacting environmental justice-related concerns, the fees will enable the Agency to better protect human health and the environment, including in helping minority, low-income, tribal, or indigenous populations in the United States that potentially experience disproportionate environmental harms and risks, and supporting the fair treatment and meaningful involvement of all people regardless of race, color, national origin, or income with respect to the development, implementation and enforcement of environmental laws, regulations and policies involving TSCA. EPA identifies and addresses environmental justice concerns by providing for fair treatment and meaningful involvement in the implementation of the TSCA program and addressing unreasonable risks from chemical substances.
- Effects on State, local, and Tribal governments. The proposed rule would not have any significant or unique effects on small governments, or federalism or tribal implications.
F. What should I consider as I prepare my comments for EPA?
- Submitting CBI. Do not submit CBI information to EPA through https://www.regulations.gov or email. Clearly mark the part or all of the information that you claim to be CBI. For CBI information in a disk or CD-ROM that you mail to EPA, mark the outside of the disk or CD-ROM as CBI and then identify electronically within the disk or CD-ROM the specific information that is claimed as CBI. In addition to one complete version of the comment that includes information claimed as CBI, a copy of the comment that does not contain the information claimed as CBI must be submitted for inclusion in the public docket. Information so marked will not be disclosed except in accordance with procedures set forth in 40 CFR part 2.
- Tips for preparing your comments. When preparing and submitting your comments, see the commenting tips at https://www.epa.gov/dockets/commenting-epa-dockets#tips.
II. Background
TSCA authorizes EPA to establish, by rule, fees for certain fee-triggering activities under TSCA sections 4, 5, and 6. In so doing, the Agency must set lower fees for small business concerns and establish the fees at a level such that they will offset approximately but not more than 25 percent of the Agency's costs to carry out a broader set of activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14. In addition, in the case of manufacturer-requested risk evaluations, the Agency is directed to establish fees sufficient to defray 50 percent of the costs associated with conducting a manufacturer-requested risk evaluation on a chemical substance included in the TSCA Work Plan, and 100 percent of the costs of conducting a manufacturer-requested risk evaluation for all other chemicals. EPA is also required in TSCA section 26(b)(4)(F) to review and adjust, as necessary, the fees every three years.
On January 11, 2021, EPA proposed updates and adjustments to the 2018 Fee Rule (Ref. 2). This included proposed modifications to the TSCA fees and fee categories for fiscal years (FY) 2023, 2024, and 2025, and explained the methodology by which these TSCA fees were determined. EPA proposed to add three new fee categories: a Bona Fide Intent to Manufacture or Import Notice (Bona Fide Notice), a Notice of Commencement of Manufacture or Import (NOC), and an additional fee associated with test orders. In addition, EPA proposed exemptions for entities subject to certain fee triggering activities, including: (1) an exemption for research and development activities; (2) an exemption for entities manufacturing less than 2,500 pounds (lbs) of a chemical subject to an EPA-initiated risk evaluation; (3) an exemption for manufacturers of chemical substances produced as a non-isolated intermediate; and (4) exemptions for manufacturers of a chemical substance subject to an EPA-initiated risk evaluation if the chemical substance is imported in an article, produced as a byproduct, or produced or imported as an impurity. EPA proposed to update its cost estimates for administering TSCA and individual fee calculation methodologies. EPA also proposed a production volume-based fee allocation for EPA-initiated risk evaluation fees in any scenario where a consortium is not formed and proposed to require export-only manufacturers to pay fees for EPA-initiated risk evaluations. EPA also proposed various changes to the timing of certain activities required throughout the fee payment process.
EPA requested public comments on its proposal through February 25, 2021, and later extended the comment period through March 27, 2021 (86 FR 10918). EPA received a total of 43 comments. Of the 43 submissions, there were two comment submissions and five oral comments associated with a public webinar hosted on February 18, 2021 (Ref. 6) and three requests for a comment period extension. Based on comments received on the proposed rule, stakeholder engagement, and EPA's continued experience in implementing the 2018 Fee Rule (e.g., through collection of fees associated with EPA-initiated risk evaluations for the 20 High Priority Substances (https://www.epa.gov/tsca-fees/tsca-fees-epa-initiated-risk-evaluations), EPA is supplementing its proposal.
III. Proposed Changes
A. Agency Costs for the Administration of TSCA
As explained in Unit I.D. of this document, TSCA allows the Agency to collect approximately but not more than 25 percent of its costs for eligible TSCA activities via fees; however, fee revenue has been approximately half of what was estimated in the 2018 Fees Rule. Therefore, EPA is revising its cost estimates to account for the resources needed for anticipated implementation efforts. The Lautenberg amendments of 2016 were the first major overhaul of the TSCA statute in forty years. The Lautenberg Act promised a broad array of far-reaching improvements to America's chemical safety infrastructure by requiring EPA to use strengthened TSCA authorities to protect human health and the environment more effectively from risks. EPA's early implementation efforts included establishing key rules laying out the framework under which EPA would act in implementing the amendments, initiating the first 10 multi-year risk evaluations of existing chemicals in commerce, developing a process for making required determinations on all TSCA section 5 notices, and refreshing the TSCA inventory of chemicals in commerce. However, EPA faces challenges in TSCA implementation that stem from new requirements established through the 2016 Lautenberg amendments.
The primary reason for these implementation challenges is a lack of resources. Although EPA has the authority to offset approximately but not more than 25 percent of the Agency's costs to carry out a broader set of activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14, the 2018 Fee Rule did not include the collection of any fees for the first 10 TSCA risk evaluations [Note: EPA will not be collecting fees for the first 10 TSCA risk evaluation.] and the baseline cost estimates that drove the fee amounts in that rule were selected by using the costs for implementing TSCA before the law was amended and thus before EPA was required to carry out any of its new responsibilities. In other words, the baseline cost estimates EPA chose were based on what EPA spent on implementing TSCA before it was amended in 2016, not what it would cost the Agency to implement the revised law in the manner envisioned and directed by Congress, resulting in an artificially-low baseline cost estimate. In the first four years following the 2016 law's enactment, EPA also did not conduct a comprehensive budget analysis designed to estimate the actual costs of implementing the amended law until the spring of 2021. Thus, the 2018 Fee Rule, and particularly, the Rule's failure to collect any fees associated with any of the first 10 risk evaluations resulted in collection of roughly half of the (artificially-low) baseline costs EPA has the authority to collect, resulting in additional implementation challenges discussed in the following paragraphs.
Under TSCA section 5, EPA conducts risk assessments and risk management activities for hundreds of new chemical submissions per year to assess the safety of such chemicals before they enter commerce and take action to prevent unreasonable risk. However, due to resource constraints, EPA has a backlog of delayed reviews. The backlog of delayed cases continues to increase and drives competition for Agency resources with new incoming cases. The backlog is due to both a change made by the 2016 amendments, which shifted the Agency's past practice of conducting initial “screening” reviews of chemicals for risk and only making risk determinations on about 20 percent of the new chemical submittals it received to the new statutory requirement to make such determinations on 100 percent of submittals, and the absence of the additional resources required to implement 2016 amendments. This will ensure that new chemicals entering commerce do not present an unreasonable risk to human health and the environment under the conditions of use.
Additional funding collected through TSCA fees will help EPA reduce the backlog of delayed reviews, support additional work for new cases, and provide necessary support to address new chemicals-related, such as those for chemicals like per- and polyfluoroalkyl substances (PFAS) actions.
Under TSCA section 6, EPA is responsible for developing existing chemical risk evaluations, including for chemicals designated as High-Priority Substances through prioritization. TSCA requires evaluations to be completed in three and a half years from the date of initiation of the risk evaluation. EPA experienced significant implementation challenges and missed the statutory deadlines for nine of the first 10 chemical substance risk evaluations, which primarily resulted from the start-up time needed to develop an approach for implementing the Lautenberg Act and scaling up to handle 10 simultaneous risk evaluations. Additionally, as previously noted, no fees were collected for the first 10 risk evaluations, further limiting the resources available to conduct this work. Going forward, EPA has a statutory requirement to ensure that risk evaluations are being conducted on at least 20 High-Priority Substances and an additional number of manufacturer-requested chemicals. Experience has shown that at current funding and staffing levels, 20 risk evaluations will not be completed within the statutory timeframe. Collecting additional resources through TSCA fees will enable EPA to significantly improve on-time performance and quality.
Improved performance (timeliness and quality) in developing risk evaluations is also contingent on obtaining needed data in a timely manner. Increased resources will support issuance of additional TSCA section 4 test orders to close any relevant data gaps identified in the Prioritization process or the Scoping stage of the risk evaluation process for High-Priority Substances or to advance additional information development activities through TSCA section 4, such as the issuance of test order for certain PFAS, as informed by the National PFAS Testing Strategy (Ref. 7). Delivering data that enables the completion of risk evaluations on a timelier basis may also improve EPA's delivery of the risk reduction benefits through earlier development and issuance of risk management actions and may thereby increase benefits to human health and the environment.
Under TSCA section 14, EPA is required to review and make determinations regarding the validity of a significant portion of CBI claims. EPA reviews, processes, and provides access to and/or protects CBI from disclosure, as appropriate, on information reported under TSCA. The CBI review requirements of TSCA section 14 apply to submissions to EPA under TSCA, including sections 4, 5, 6, 8, and 12. Increased resources will ensure EPA continues to establish improved processes, systems, and procedures to enable submitters to provide the information required when making CBI claims and to facilitate EPA's review, where applicable, under TSCA section 14.
To offset approximately but not more than 25 percent of the Agency's costs, and for the various reasons listed throughout this document and in Unit III.B., EPA is proposing to revise its costs estimates to adequately account for the anticipated costs of meeting its statutory mandates, which are based on a comprehensive analysis conducted in 2021. The estimate includes anticipated implementation efforts and resources, which EPA sees as consistent with recommendations and statements made previously by the Office of Inspector General (OIG), the Government Accountability Office (GAO) and Congress. For example, the 2020 EPA OIG report, titled “Lack of Planning Risks EPA's Ability to Meet Toxic Substances Control Act Deadlines,” recommends that EPA include the “anticipated” implementation efforts and financial and staff resources when planning for work conducted under the Lautenberg amendments of 2016, particularly for existing chemicals work (Ref. 8). The GAO, in its 2021 report titled “Dedicated Leadership Needed to Address Limited Progress in Most High-Risk Areas,” acknowledged that a lack of resources has impacted EPA's ability to successfully implement TSCA. The report also stated that EPA needs to conduct planning to make sure it has the resources and plans in place to facilitate progress on risk evaluations and other work implementing TSCA (Ref. 9). In a joint explanatory statement in Congress's FY 2022 omnibus spending bill, Congress reminded the Agency that the Lautenberg Act established a shared responsibility for the taxpayer and industry to contribute their share to support the TSCA program. In addition, Congress encouraged the Agency to properly consider full costs in its deliberations, in line with the Lautenberg Act's intent (Ref. 10).
B. Program Cost Estimates and Activity Assumptions
EPA calculated fees by estimating the total annual costs of carrying out relevant activities under TSCA sections 4, 5, and 6 (excluding the costs of manufacturer-requested risk evaluations) and conducting relevant information management activities under TSCA section 14; identifying the full cost amount to be defrayed by fees under TSCA section 26(b) (i.e., 25 percent of those annual costs); and allocating that amount across the fee-triggering events in TSCA sections 4, 5, and 6. In addition, EPA affords small businesses an approximately 80 percent discount, in accordance with TSCA section 26(b)(4)(A).
The estimated annual Agency costs of carrying out relevant activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14 in the 2021 Proposal were based on cost data from FY 2019 and 2020 which were the first full FY after EPA implemented a time reporting system that tracks employee hours worked on administering TSCA. However, this estimate did not include any costs of TSCA section 6(a) risk management activities that are now required to be underway for the first 10 chemical substances or that will be required for any of the 20 High Priority Substances for which the Agency finds unreasonable risks. Since the proposed rule was published, EPA has developed a more accurate estimate of its anticipated costs to implement TSCA in the manner envisioned by Congress when it amended the law in 2016. The estimate is informed by the Agency's experience administering TSCA since 2016, factors in the Agency's failure to meet the statutory deadlines for 9 of the first 10 existing chemical risk evaluations and consistent challenges meeting the requirements associated with reviewing new chemicals, and thus includes what the Agency believes is a much more reliable estimate of the resources needed for the anticipated implementation efforts than the inaccurate cost estimate that was previously used. Changes to program cost estimates are discussed in the following sections and in more detail in the 2022 TSCA Fees Technical Support Document (TSD) (Ref. 11).
Total Agency costs of carrying out relevant activities under TSCA sections 4, 5, 6 and relevant information management activities under TSCA section 14 are estimated at approximately $181.9 million each year (which differs from the $87.5 million discussed in the 2021 Proposal). Based on the new cost estimates, EPA anticipates collecting approximately 25 percent of that, or $45.5 million each year (which differs from the $22 million discussed in the 2021 Proposal) in fees collected from all fee-triggering events, except manufacturer-requested risk evaluations (MRREs). The increase in costs from the 2021 Proposal is due to multiple factors on top of the lack of a comprehensive analysis of baseline costs until 2021 as has already been discussed in this Unit. For example, estimates in the 2021 Proposal did not include any costs of TSCA section 6(a) risk management activities that are now required to be underway for the first 10 chemical substances or that will be required for any of the 20 High Priority Substances for which the Agency finds unreasonable risks, which resulted in EPA significantly underestimating TSCA section 6 Agency costs. In addition, the estimate from the 2021 Proposal did not include costs for EPA's plan to develop and implement a multi-year collaborative research program under section 5, which is explained in more detail in this Unit.
For new chemical submissions under TSCA section 5, EPA has now formulated a per unit cost estimate that was not included in the 2021 Proposal. The updated estimate provides a more comprehensive accounting of program implementation, which includes, but is not limited to: (1) costs incurred by EPA for multiple rounds of revisions to the risk assessment due to late submission of information or rebuttals by companies, (2) multiple rounds of risk management actions, redactions and posting of final reports to meet transparency commitments while safeguarding CBI, (3) IT infrastructure maintenance and enhancement to ensure the quality and safeguard of data collection, storage and reporting, staffing and contractor support from supporting offices such as the Office of General Counsel (OGC), the Office of Enforcement and Compliance Assurance (OECA), and the Office of Research and Development (ORD), among others, and (4) other operational costs that were not previously captured or fully itemized. The anticipated direct and indirect program costs associated with relevant activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14 for FY 2023 through FY 2025, are listed in Table 1 below.
| Annual costs | |
|---|---|
| TSCA section 4 | $7,383,300 |
| TSCA section 5 | 54,162,600 |
| TSCA section 6 (excluding manufacturer-requested risk evaluations). | 88,251,500 |
| TSCA section 14 | 1,783,800 |
| Agency Indirect Costs | 30,316,200 |
| Total | $181,897,400 |
| Table Note: The indirect cost rate is estimated at 20 percent for the purposes of this analysis. | |
1. Program Costs
To determine the program costs for implementing relevant activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14, the Agency accounted for the direct costs, both intramural and extramural, for those activities.
Intramural costs are those costs related to the efforts exerted by EPA staff and management in operating the program, collecting and processing information and funds, conducting reviews, and related activities. Extramural costs are those costs related to the acquisition of contractors to conduct activities such as analyzing data, developing IT systems, and supporting the TSCA Help Desk.
The Agency then added indirect costs to the direct program cost estimates. The Agency used an indirect cost rate of 20 percent to calculate the indirect costs associated with all direct program cost estimates for TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14 based on EPA's indirect cost methodology as required by Federal Accounting Standards Advisory Board's Statement of Federal Financial Accounting Standards No. 4: Managerial Cost Accounting Standards and Concepts (Ref. 12).
a. TSCA Section 4 Program Costs
TSCA permits the Agency to undertake test rules, test orders, and enforceable consent agreements (ECA). Developing these regulatory actions is a complex, time-consuming, and resource-intensive process involving many scientific and regulatory considerations. EPA must establish what information is required, inventory what reasonably available information EPA has that would address EPA's needs, what testing will provide such information, and what test protocols—such as the Organisation for Economic Co-operation and Development (OECD) test guidelines—can generate such information. Standard globally recognized test guidelines may sometimes be appropriate to inform certain data needs, however, other times, EPA may need to look elsewhere such as at New Approach Methods or even develop new protocols because of the spectrum of data needs and multiple technical considerations that go into determining testing requirements. Additionally, the Agency must satisfy the requirements of the statute to reduce vertebrate testing (i.e., the use of vertebrate animals in testing to generate chemical information to assess risks to health or the environment posed by substances or mixtures), which may involve the use of New Approach Methods. Ultimately, EPA seeks to ensure that the testing required generates useful, high-quality data. For example, depending on the complexity of the chemical substance(s) or mixture(s) that is(are) the subject of a test order, EPA estimates that developing and issuing a test order generally takes a minimum six months of personnel fully allocated (assuming one to two personnel depending on the complexity of the test order and the number of recipients of the test order) and an array of technical personnel from different disciplines partially allocated to doing test order work. The complexity associated with a chemical substance(s) or mixture(s) made the subject of a test order is influenced by EPA's grasp of the scientific and market data on and analytical methods applicable to the chemical(s). Further resources are also needed to administer the test orders after they have been issued (e.g., answering questions related to its requirements, reviewing submissions, etc.); the number of resources needed for such activities varies depending on the complexity of the testing requirements and the number of recipients.
EPA's limited resources have hampered the Agency from effectively exercising those authorities (e.g., in support of the prioritization of the 20 High-priority Substances). In addition, EPA intends to expand the use of Section 4 authorities significantly moving forward to inform prioritization of substances for risk evaluation and develop the most scientifically-sound risk evaluations of those chemical substances. Additional resources will facilitate the Agency's exercise of these authorities under TSCA. Therefore, to estimate the costs associated with TSCA section 4 activities, the Agency relied upon prior experience with the past test orders, test rules and ECAs, and considered anticipated costs to cover future TSCA section 4 activities. Based on past experience and anticipated costs, EPA has calculated the total program costs for TSCA section 4 activities to be approximately $7.38 million annually. More information about EPA's estimated TSCA section 4 costs basis can be found in the TSD (Ref. 11).
b. TSCA Section 5 Program Costs
Under the 2016 amendments to TSCA, EPA must review and make a determination pertaining to all new chemical substances or significant new uses of chemicals submitted under TSCA section 5(a) before they can proceed to the marketplace. Previously, EPA conducted initial reviews of TSCA section 5 notices and determined whether further review was needed, and made an interim finding following the initial review. Before the 2016 amendments, about 80 percent of new chemical reviews were halted at this `interim' stage and were allowed into commerce without further review. Following the 2016 amendments to TSCA, EPA modified its review processes such that all TSCA section 5 notices go through a full risk assessment and receive a risk determination, and therefore the Agency no longer makes interim findings.
EPA estimates that it will receive 210 premanufacture notices (PMNs), significant new use notices (SNUNs), and microbial commercial activity notices (MCANs) per year, and another 290 exemption notices and applications per year. EPA's cost estimates for administering TSCA section 5 include the costs associated with processing and retaining records related to NOC submissions, as well as the costs of pre-notice consultations, processing and reviewing applications, retaining records, and related activities. This estimate is based on a projected 185 full-time equivalents (FTEs) and extramural support needed for these actions. Costs estimates for administering TSCA section 5 activities also include EPA's plan to develop and implement a multi-year collaborative research program to modernize the information used in performing risk assessments for new chemical substances under TSCA and bring innovative science to the review of the new chemicals before they can enter the marketplace. More information related to this research program can be found in the TSD (Ref. 11). These activities and additional funding needs resulted in EPA proposing higher fees for TSCA section 5 activities in this document.
Based on past experience and anticipated costs, EPA has estimated the total program costs for TSCA section 5 activities to be approximately $54.2 million annually in FY 2023 through FY 2025. More information about EPA's estimated TSCA section 5 costs basis can be found in the TSD (Ref. 11).
c. TSCA Section 6 Program Costs
EPA has the authority under TSCA section 26(b) to collect fees to recover costs for TSCA section 6 activities including prioritization, risk evaluations, and risk management rulemaking. TSCA section 6 cost estimates have been informed by the Agency's experience conducting evaluations for the first 10 chemical substances to undergo risk evaluation under amended TSCA, by the Agency's experience prioritizing and developing the scope of the risk evaluations of the 20 chemicals designated as High-Priority Substances in December 2019, and by the Agency's initial and ongoing experience with risk management actions addressing unreasonable risks identified in the first 10 chemical substance risk evaluations. Cost estimates for risk management activities have also been informed by EPA's recent risk management actions on several chemicals under TSCA section 26(l)(4) authority, including development of the proposed rules regarding the use of N-methylpyrrolidone and methylene chloride in paint and coating removal, and the use of trichloroethylene in both commercial vapor and aerosol degreasing and for spot cleaning in dry cleaning facilities, and the development of the final rule regarding methylene chloride in consumer paint and coating removal.
During the public comment period on the 2021 Proposal, EPA received comments stating that EPA underestimated the TSCA section 6 costs. For example, commenters stated that EPA inappropriately relied on narrow, partially completed risk management actions to inform the cost of its current and future risk management actions (Docket Number EPA-HQ-OPPT-2020-0493). Commenters also raised concerns stating that EPA had not reconciled the costs for administering section 6 activities which had been reduced compared to the 2018 Fee Rule despite the increase in risk management workload. Additionally, EPA's estimates did not include any costs of TSCA section 6(a) risk management activities for the first 10 chemical substances or 20 High Priority Substances in the proposal which resulted in EPA underestimating TSCA section 6 Agency costs. Therefore, EPA is proposing to include recent risk management activities into the TSCA section 6 program cost estimates. Although section 6 cost estimates were informed by risk management and risk evaluation activities for the first 10 chemicals, EPA will not be recovering fees for those chemicals. Adding more recent and comprehensive risk management costs and the anticipated increases associated with prioritization and risk evaluation costs, as described previously and in more detail in the TSD, would result in the estimated annual cost to administer TSCA section 6 to be approximately $88 million per year, except the MRREs.
In the case of manufacturer-requested risk evaluations, the Agency is directed to establish fees sufficient to defray 50 percent of the costs associated with conducting a manufacturer-requested risk evaluation on a chemical substance included in the TSCA Work Plan, and 100 percent of the costs of conducting a manufacturer-requested risk evaluation for all other chemicals. EPA is also required in TSCA section 26(b)(4)(F) to review and adjust, as necessary, the fees every three years. The Agency intends to collect fees to recover 50 percent or 100 percent of the actual costs incurred by EPA in conducting chemical risk evaluations requested by manufacturers, depending on whether the chemical substance is included in the TSCA Work Plan. EPA expects the amount collected will be approximately $4.40 million per risk evaluation for chemicals on the TSCA Work Plan and $8.98 million per risk evaluation for chemicals not on the TSCA Work Plan.
d. Costs of Collecting, Processing, Reviewing, and Providing Access to and Protecting From Disclosure as Appropriate Under TSCA Section 14 Information on Chemical Substances
EPA is making minimal changes to estimates of program costs of collecting, processing, reviewing, and providing access to and protecting from disclosure as appropriate under TSCA section 14 information on chemical substances that were previously described in the 2021 Proposal. More information about specific activities considered when developing this estimate for activities under section 14 can be found in the 2021 Proposal (Ref. 3).
The annual cost estimate of collecting, processing, reviewing, and providing access to and protecting from disclosure as appropriate information on chemical substances under section 14 of TSCA, including 8.6 FTE and extramural costs, from FY 2023 through FY 2025 is approximately $1.8 million (Ref. 4).
2. Indirect Costs
Indirect costs are the intramural and extramural costs that are not accounted for in the direct program costs, but are important to capture because of their necessary enabling and supporting nature, and so that EPA's proposed fees will accomplish full cost recovery up to that provided by law. Indirect costs typically include such cost items as accounting, budgeting, payroll preparation, personnel services, purchasing, centralized data processing, and rent.
EPA included indirect costs in its estimate of total Agency costs pursuant to OMB Circular A-25 (Ref. 13) which states that agencies should collect the full costs when setting fees. In addition, section 6(d)(1) explains that full costs include all direct and indirect costs to the Federal Government. EPA describes how an indirect cost rate is determined annually according to EPA's indirect cost methodology and as required by Federal Accounting Standards Advisory Board's Statement of Federal Financial Accounting Standards No. 4: Managerial Cost Accounting Standards and Concepts in the 2021 Proposal. An indirect cost rate of 20 percent was applied to direct program costs of work conducted by EPA's Office of Chemical Safety and Pollution Prevention. Some of the direct program costs included in the estimates for TSCA sections 4, 5, and 6 and collecting, processing, reviewing, and providing access to and protecting from disclosure as appropriate under TSCA section 14 information on chemical substances are for work performed in other Agency offices (e.g., the Office of Research and Development and the Office of General Counsel). Appropriate indirect cost rates were applied to those cost estimates and are based on EPA's existing indirect cost methodology. Indirect cost rates are calculated each year and therefore subject to change. Indirect costs of approximately $30 million were included in the program cost estimates in the previous sections.
3. Total Costs of Fee-Triggering Events
The annual estimated costs for fee categories under TSCA section 4, including both direct and indirect program costs, are shown in Table 2. Note that the costs presented in Tables 2 through 4 include only the costs of fee triggering events and do not include costs associated with activities such as CBI reviews and alternative testing methods development. Costs associated with those activities are part of the overall costs of administering relevant activities under TSCA sections 4, 5, and 6 and relevant information management activities under TSCA section 14 and, as such, are included in the overall cost estimates provided previously in Table 1.
The Agency believes it is reasonable to assume that approximately 75 test orders per year will be initiated between FY 2023 and FY 2025. Approximately 45 of these test orders are expected to be associated with the Agency's actions on PFAS. In addition, the EPA assumed two test rules and two ECAs between FY 2023 and FY 2025.
| $ Total costs | Payroll | $ Non-payroll | FTE | |
|---|---|---|---|---|
| TSCA Section 4 Activities | $7,383,300 | $4,878,000 | $2,505,300 | 27.9 |
| *Table Note: Numbers may not add due to rounding. | ||||
The estimated annual costs for fee categories under TSCA section 5, including both direct and indirect program costs are shown in Table 3. EPA estimates that it will receive 210 PMNs, SNUNs, and MCANs per year, and another 290 exemption applications per year. EPA's cost estimates for administering TSCA section 5 include the costs associated with processing and retaining records related to a NOC submission, as well as the costs of pre-notice consultations, processing and reviewing applications, retaining records, and related activities.
| $ Total costs | Payroll | $ Non-payroll | FTE | |
|---|---|---|---|---|
| TSCA Section 5 Activities | $54,162,600 | $32,370,000 | $21,792,600 | 185.2 |
| *Table Note: Numbers may not add due to rounding. | ||||
The estimated annual costs for fee categories under TSCA section 6, including both program and indirect costs are shown in Table 4. EPA estimates that the EPA's workforce will be involved in at least 3 MRRE and at least 20 EPA‐initiated chemical risk evaluations at all times.
| $ Total costs | Payroll | $ Non-payroll | FTE | |
|---|---|---|---|---|
| TSCA Section 6 | ||||
| TSCA Section 6 Prioritization | $8,820,900 | $6,254,000 | $2,566,900 | 35.9 |
| EPA-initiated Risk Evaluation | 54,877,100 | 28,291,100 | 26,585,900 | 161.40 |
| Manufacturer-requested Risk Evaluation | 7,483,200 | 3,857,900 | 3,625,400 | 22.0 |
| TSCA Section 6 Risk Management | 24,553,500 | 13,536,000 | 11,017,500 | 77.3 |
| Totals | 95,734,700 | 51,939,000 | 43,795,700 | 296.6 |
| *Table Note: Numbers may not add due to rounding. | ||||
C. Fee Amounts
While TSCA allows the Agency to collect approximately but not more than 25 percent of its costs for eligible TSCA activities via fees, to date, EPA has collected roughly half of that amount due to the insufficiencies of the current fees rule. These proposed revisions are designed to ensure fee amounts capture approximately but not more than 25 percent of the costs of TSCA activities, fees are distributed equitably, and fee payers are identified via a transparent process. Although TSCA allows EPA to recover approximately but not more than 25 percent of its costs of implementing certain provisions of TSCA, the percentage applies to the total aggregate cost and does not preclude EPA from recovering an amount above or below 25 percent of the costs for each section of TSCA.
As discussed in the 2021 Proposal, the existing and proposed fee categories are fee-triggering events that result in obligations to pay fees but do not encompass all activities under TSCA sections 4, 5, 6, and 14 that incur costs to the Agency (e.g., costs of administering TSCA section 14, risk management activities under section 6, prioritization of chemicals for evaluation, support for alternative testing and methods development and enhancement). However, costs for all relevant activities are included in the total Agency costs estimate, even those not discussed in this document (e.g., specific TSCA work with other EPA offices). Therefore, EPA is proposing fee amounts to ensure these costs would be captured, not just the costs of the fee-triggering events. EPA is also proposing new fee amounts to capture the higher proportion (in percentage) of the estimated costs of TSCA section 6 activities and ensure EPA fees are set to recover approximately but not more than 25 percent of the total cost for implementing the relevant sections of TSCA.
After estimating the annual costs of administering relevant activities under TSCA sections 4, 5, 6, and relevant information management activities under TSCA section 14, the Agency had to determine how the costs would be allocated over the narrower set of activities under TSCA sections 4, 5, and 6 that trigger a fee. The Agency took an approach to determining fees that tied the payment of fees to individual distinct activity types or “fee-triggering events.”
The proposed fee amounts are described in Table 5.
| Fee category | 2018 Fee rule | Current fees1 | 2022 Supplemental proposed rule |
|---|---|---|---|
| Test order | $9,800 2 | $11,650 | $25,000. |
| Test rule | $29,500 | $35,080 | $50,000. |
| Enforceable consent agreement | $22,800 | $27,110 | $50,000. |
| PMN and consolidated PMN, SNUN, MCAN and consolidated MCAN | $16,000 | $19,020 | $45,000. |
| LoREX, LVE, TME, Tier II exemption, TERA, Film Articles | $4,700 | $5,590 | $13,200. |
| EPA-initiated risk evaluation | $1,350,000 | Two payments resulting in $2,560,000 | Two payments resulting in $5,081,000. |
| Manufacturer-requested risk evaluation on a chemical included in the TSCA Work Plan | Initial payment of $1.25M, with final invoice to recover 50% of actual costs | Two payments of $945,000, with final invoice to recover 50% of actual costs | Two payments of $1,497,000, with final invoice to recover 50% of actual costs. |
| Manufacturer-requested risk evaluation on a chemical not included in the TSCA Work Plan | Initial payment of $2.5M, with final invoice to recover 100% of actual costs | Two payments of $1.89M, with final invoice to recover 100% of actual costs | Two payments of $2,993,000, with final invoice to recover 100% of actual costs. |
| 1The current fees reflect an adjustment for inflation required by TSCA. The adjustment went into effect on January 1, 2022. | |||
| 2In 2018 final rule, the fees for TSCA section 4 test orders and test rules were incorrectly listed as $29,500 for test orders and $9,800 for test rules. The 2021 Proposal proposes to correct this error by changing the fees for TSCA section 4 test orders to $9,800 and TSCA section 4 test rules to $29,500. | |||
1. Fee Amounts for TSCA Section 4 Activities
EPA is proposing changes to the fees associated with TSCA section 4 activities. Additional justification for fee triggering activities associated with each TSCA section is discussed within this Unit. In addition, in the 2021 Proposal, EPA proposed an additional fee category under TSCA section 4 for amended test orders. EPA is proposing to remove this new fee category (discussed in further detail in Unit III.D).
EPA is proposing fees that, based on the expected activity levels of the three fee categories for TSCA section 4 activities, will defray 26.4 percent of the program costs described in the previous paragraphs, or approximately $1.94 million. The proportion (in percentage) of the estimated cost of the activity is slightly higher for fees for TSCA section 4 (26.3 percent) to ensure EPA is recovering the required 25 percent of the total cost for implementing the relevant sections of TSCA in light of collecting less than 25 percent of costs for section 5 activities as explained in Unit III.C.2.
2. Fee Amounts for TSCA Section 5 Activities
EPA currently sets two fee amounts for TSCA section 5 activities—one for notices (PMNs, SNUNs, and MCANs), and one for exemptions which include low exposure/low release exemptions (LoREXs), low volume exemptions (LVEs), test-marketing exemptions (TMEs), certain microorganism Tier II exemptions (Tier II), and TSCA experimental release applications (TERAs). In the 2021 Proposal, EPA proposed two additional fee categories under TSCA section 5, one for Bona Fide Notices and the other for NOCs. EPA is proposing to remove those two new fee categories (discussed in further detail in Unit III.D), as well as proposing to increase the fee amounts under TSCA section 5 activities. Specifically, EPA is proposing an increase to the fees for PMNs, consolidated PMNs, SNUNs, MCANs, consolidated MCANs, LoREXs, LVEs, TMEs, Tier II, TERAs, and film article exemptions.
Additional funding collected through TSCA section 5 fees will help EPA reduce the backlog of delayed reviews and support additional work for new cases. As previously noted, these delays result from a years-long absence of the additional resources required to implement the 2016 amendments, which shifted the Agency's past practice of making risk determinations on about 20 percent of the new chemical submittals it received to a requirement to make such determinations on 100 percent of submittals. The fee increases for TSCA section 5 activities, if finalized as proposed in this document, would also shift costs for administering TSCA section 5 away from fees for TSCA section 6 actions. EPA proposed to increase TSCA section 6 fees to recover costs for TSCA section 5 activities in the 2021 Proposal. As newly proposed, the fees for TSCA section 5 activities amount to approximately 18 percent of the estimated costs of the activities and are described in Table 5. EPA is proposing to collect less than 25 percent of the costs for section 5 activities to lessen the impact due to the increase in section 5 fee amounts since 2018. For example, before the 2018 Fee Rule the fee for a PMN was $2,500. The fee was increased to $16,000 in the 2018 Fee Rule and will be increased further to $45,000 under this proposal. Due to the significant increase since 2018, is proposing to reduce the impact of increased section 5 fees by collecting less than 25 percent of the implementation costs for section 5. EPA is requesting comment on its proposal to recover less than 25 percent of the costs for implementing TSCA section 5.
EPA also accounted for full (100 percent) refunds that may be provided when estimating the total fees collected and in setting the fee amounts. Full refunds may be provided for notices or exemptions when EPA determines a submission is not a new chemical substance, new microorganism, or significant new use, or when the Agency fails to make a determination on a notice by the end of the applicable notice review period. In addition, EPA is proposing to refund 20 percent of the user fee to the submitter if a notice is withdrawn after 10 business days after the beginning of the applicable review period, but prior to EPA initiating risk management on the chemical substance. The 20 percent refund is based on the allocation of resources needed for risk assessment and risk management of chemical substances under TSCA section 5 where 80 percent of costs are associated with risk assessment and 20 percent with risk management. Based on the number of PMNs withdrawn during FY 2020 and 2021, EPA estimates that approximately 23 percent of PMNs are withdrawn during review (discussed in further detail in Unit III.E).
3. Fee Amounts for TSCA Section 6 Activities
EPA collects one fee amount for EPA-initiated risk evaluations. Based on the expected activity levels of this fee category, this will defray 38.4 percent of the estimated program costs. As explained in Unit III.C.2, EPA is collecting under 25 percent of the costs for section 5 activities. For this reason and to ensure EPA is recovering the required 25 percent of the total cost for implementing the relevant sections of TSCA, the proportion (in percentage) of the estimated cost of EPA-initiated risk evaluations that are recovered by fees is higher (38.4 percent) than the other fee triggering activities. EPA takes an actual cost approach for manufacturer-requested risk evaluations, whereby the requesting manufacturer (or requesting consortia of manufacturers) would be obligated to pay either 50 percent or 100 percent of the actual costs of the activity, depending on whether the chemical was listed on the TSCA Work Plan or not, respectively.
Based on additional cost estimates for risk management and anticipated increases associated with prioritization and risk evaluation costs, as described in Unit III.B.1.a., estimated Agency costs for TSCA section 6 activities have increased to $88,251,500 per year with fee collections of $33,890,270 for EPA-initiated risk evaluations. EPA is proposing to increase the EPA-initiated risk evaluation fees from the 2021 Proposal of $2,560,000 to $5,081,000 (or from $1.35 million in the 2018 Fee Rule). This payment would be collected over two installments, the first payment of 50 percent to be due 180 days after EPA publishes the final scope of a chemical risk evaluation and the second payment due not later than 545 days after EPA publishes the final scope of a chemical risk evaluation, as proposed in the 2021 Fee Proposal.
As stated previously, EPA takes an actual cost approach for manufacturer-requested risk evaluations. In addition, EPA proposed in the 2021 Proposal to separate the manufacturer-requested risk evaluation payments into three installments with the total fee paid reflecting the actual cost. Based on that proposed installment plan and the estimated costs of these risk evaluations, two payments of $1,497,000 then invoiced for the remainder is being proposed for chemicals on the TSCA Work Plan and two payments of $2,993,000 with final invoice for the remainder is being proposed for chemicals not listed on the TSCA Work Plan.
D. Fee Categories
Under the 2018 Fee Rule, EPA has eight distinct fee categories: (1) test orders, (2) test rules, and (3) Enforceable Consent Agreements (ECAs), all under TSCA section 4; (4) notices and (5) exemptions, both under TSCA section 5; and (6) EPA-initiated risk evaluations; (7) manufacturer-requested risk evaluations for chemicals on the TSCA Work Plan; and (8) manufacturer-requested risk evaluations for chemicals not on the TSCA Work Plan, all under TSCA section 6. The activities in these categories are fee-triggering events (other than the first 10 risk evaluations) that result in obligations to pay fees under the 2018 Fee Rule.
In the 2021 Proposal, EPA proposed two additional fee categories under TSCA section 5, Bona Fide Notices and NOCs, and one additional fee category for TSCA section 4 amended test orders. After considering public comments received on the 2021 Proposal, and in an effort to keep the fee structure simple by reducing the number of fee categories, EPA is proposing not to finalize the new fee categories for Bona Fide Notices, NOCs, and amended test orders.
The cost associated with NOCs will continue to be captured with those of PMNs, MCANs, and SNUNs, as they were under the 2018 Fee Rule. EPA believes these fees are better captured under the proposed fee increase for existing TSCA section 5 categories. In addition, while EPA envisioned the additional fee for amended test orders to create an incentive for manufacturers to submit facially complete data outlined under TSCA section 4, in order to simplify the TSCA section 4 fee structure EPA is proposing to remove the amended test order fees. Because the costs incurred by EPA to review resubmitted data are included in the Agency's total program cost estimate, these costs will be captured under other fees.
E. Refund for Withdrawal During Review
In addition to increasing the TSCA section 5 fees for PMNs, SNUNs, and MCANs, EPA is proposing to refund 20 percent of the user fee to the submitter if a notice is withdrawn after 10 business days after the beginning of the applicable review period, but prior to EPA initiating risk management on the chemical substance. In the 2018 Fee Rule, EPA established a partial refund (i.e., 75 percent of the fee amount) for TSCA section 5 submissions withdrawn during the first 10 business days after the beginning of the applicable review period (83 FR 52694, October 17, 2019). EPA is proposing an amendment to add a partial refund of 20 percent for TSCA section 5 submissions withdrawn after the first 10 business days during the assessment period of the chemical but before EPA begins any necessary risk management. This newly proposed refund is in addition to the already existing refund of 75% for notices withdrawn in the first 10 business days established under the 2018 Fee Rule. After EPA concludes the risk assessment for a TSCA section 5 submission, the Agency will provide the submitter notice that the risk assessment has been completed and the submitter will then have five business days to withdraw their notice for a partial refund of 20 percent. After 5 business days from receiving the notice that the risk assessment has been completed, if the company wishes to withdraw a notice, no refund will be given.
When EPA's review leads to a determination that one or more conditions of use may present an unreasonable risk and EPA lacks sufficient information to permit a reasoned evaluation of the health and environmental effects of the PMN substance, or on the basis of insufficient information alone, the Agency will issue a section 5(e) order to address potential risks and may require testing for additional information. After learning of the Agency's determination and risk management actions, a submitter may no longer wish to pursue the commercialization of the chemical substance, depending on the potential risks identified and any risk mitigation likely required to address those risks.
EPA's proposal to refund 20 percent of the fee is based on the allocation of resources needed for risk assessment and risk management of chemical substances under TSCA section 5. EPA's cost estimates for administering TSCA section 5 include the costs of processing, reviewing, and making determinations, and the Agency's costs of taking any regulatory action such issuing an order and a TSCA section 5 significant new use rule (SNUR). Approximately 80 percent of the cost associated with reviewing a new chemical substance is due to activities associated with risk assessment, while approximately 20 percent of the cost is associated with risk management activities. EPA is not able to issue refunds for the entire fee amount because significant work begins as soon as EPA receives the PMN. As described in the 2018 Proposed Fee Rule (83 FR 8212; February 26, 2018), up to three significant milestones of the PMN review process can take place within 10 business days (Ref. 14). The Chemical Review/Search Strategy Meeting occurs between Day 8 and 12; the Structure Activity Team Meeting occurs between Day 9 and 13; and Development of Exposure/Release Assessments occurs between Day 10 and 19. Due to concerns with administrative burden and potential delays in issuing refunds, EPA will not calculate and refund a unique amount for each withdrawn submission. By adding this option for a refund of 20 percent, submitters will be able to recoup part of the cost associated with submitting a notice for chemicals they decide to withdraw during the review period. Based on the cases withdrawn during FY 2020 and 2021, EPA estimates that approximately 23 percent of cases are withdrawn during review. However, EPA anticipates this percentage could be much higher if submitters had the opportunity to obtain a partial refund when risk assessment results and likely risk management actions are known. Withdrawals and refunds provided under such circumstances would prevent the need for EPA to conduct risk assessment rework and executing unneeded risk management actions. Risk assessment rework requires EPA to re-analyze some or all the information supporting a risk assessment in order to factor in new information, causing substantial delay to the review process for that substance and delays staff from initiating or completing risk assessment work on other new chemical substances. The Agency requests comment on this new partial refund process for the review of TSCA section 5 notices.
F. Methodology for Calculating Fees for EPA-Initiated Risk Evaluations
In 2018, the TSCA Fee Rule established a methodology for allocating fees to manufacturers of chemicals subject to EPA-initiated risk evaluations in which EPA distributes the fees evenly among manufacturers, while giving an 80 percent discount for manufacturers that qualify as a small business concern. In January 2021, EPA proposed a production volume-based approach for fee allocation for EPA-initiated risk evaluations under TSCA section 6. Specifically, EPA proposed to reallocate the remaining fee, after allocating the fees for small businesses, across the remaining manufacturers, based on their percentage of total volume produced of that chemical minus the amount produced by the small businesses. EPA continues to believe that using production volume in calculating TSCA section 6 fee allocations will result in a more equitable distribution of fees and better account for the wide variation in production volume sometimes associated with a particular chemical substance, but is proposing modifications to the methodology included in the 2021 Proposal as described in the following section.
1. Description of the Proposed Regulatory Action
While 10 commenters supported EPA's proposed volume-based fee allocation methodology, nine commenters did not support the proposed methodology or expressed concern over unintentional disclosure of CBI under the proposed methodology, stating that collecting and reporting production volumes to EPA could force companies to involuntarily disclose CBI. In response to these comments, EPA is proposing to modify the proposed fee allocation methodology to protect potential submissions of CBI. The modified approach includes ranking the fee-payers that do not qualify as a small business concern by their reported production volume, then assigning fees based on those rankings. The non-small business manufacturers in the top 20th percentile ranking would pay 80 percent of the total fee, distributed evenly among these manufacturers. EPA believes this methodology is equitable, accounts for various fee payer scenarios, protects CBI, and ensures EPA is collecting approximately but not more than 25 percent of applicable program costs. These proposed changes would ensure that the manufacturers of the largest quantity of production volume for a chemical undergoing risk evaluation pay the majority of the obligated fee. In addition, this proposed approach reflects EPA's review of the distribution of production volume data reported across individual producers for the 20 High-Priority Substances and the first 10 chemical substances, and EPA believes it is consistent with the distribution of fee payers expected for any one EPA-initiated risk evaluation expected in the future. EPA is requesting comment on the methodology outlined below, including whether the approach is a more equitable way of distributing fees.
In any scenario where all manufacturers of the chemical substance undergoing the EPA-initiated risk evaluation do not form a single consortium, EPA would take the following steps to allocate fees:
Step 1: Count the total number of manufacturers, including the number of manufacturers within any consortia.
Step 2: Divide the total fee amount by the total number of manufacturers to generate a base fee.
Step 3: Provide all small businesses who are either (a) not associated with a consortium, or (b) associated with an all-small business consortium, with an 80 percent discount from the base fee.
Step 4: Calculate the total remaining fee amount and the total number of remaining manufacturers that will share the fee by subtracting out the discounted fees and the number of small businesses identified.
Step 5: Place remaining manufacturers in ascending order (from lowest to highest production volume based on their average annual production volume from the three calendar years prior to the publication of the preliminary list).
Step 6: Assign each remaining manufacturer a number with 1 for lowest production volume, 2 for second lowest production volume, etc.
Step 7: Multiply the total number of remaining manufacturers by 0.8.
Step 8: Determine the manufacturer(s) in the top 20th percentile spot by comparing the number derived from Step 7 to the manufacturer(s) with the assigned number derived in Step 5. Manufacturers with an assigned number under Step 6 that is equal to or larger than the number in Step 7 are in the top 20th percentile.
Step 9: Reallocate 80 percent of the remaining fee evenly across manufacturers in the top 20th percentile determined in Step 8, counting each manufacturer in a consortium as one person.
Step 10: Reallocate the remaining fee evenly across the remaining manufacturers, counting each manufacturer in a consortium as one person.
In addition, EPA is proposing to require reporting of average production volume over the past three years instead of four years as stated in the 2021 Proposal (Ref. 3). This proposed change would alleviate additional concerns over potential CBI disclosure by further separating the production volume submissions under this rule from other potentially public production volume reporting (e.g., CDR) which could be used in conjunction with data reported under this proposal to estimate a manufacturer's production volume. The reduction to 3-year production volume average would address multiple commenters' concerns that collecting and reporting production volume is burdensome. In addition, EPA is proposing that the production volume calculation be based on the three previous calendar years prior to the publication of the preliminary list, instead of the year self-identification and/or certification was made. This change is being made to alleviate potential confusion that may arise due to inconsistencies with other timeframe provisions in this rulemaking (additional discussion on those timeframes can be found in Unit III.G). If finalized as proposed, applicable manufacturers would be required to report their average production volume using the past three calendar years of production volume data.
These proposed changes would eliminate all expected potential disclosure of production volume that may be claimed as CBI. However, in the rare event of multiple fee payers submitting under the same parent company and asserting a CBI claim for production volume, and/or multiple companies reporting the exact same amount of a competitor, EPA would mask the company names on the final list for that chemical to protect disclosure.
EPA is not proposing these calculation and methodology changes for the fee allocations under TSCA section 4 activities. Fees for section 4 activities are significantly lower than those for a risk evaluation and, therefore, less burdensome, obviating the need to allocate the fees based on production volume. As described in steps one through three previously in this Unit, EPA is also not proposing the production volume-based methodology for manufacturers of a chemical substance undergoing an EPA-initiated risk evaluation that qualify as a small business concern. These entities would be provided an 80 percent discount from the “base fee” calculated as described in the 2018 Fee Rule (40 CFR 700.45(f)).
2. Description of the Primary Alternative Regulatory Action Considered
Commenters have expressed concerns over the burden of calculating and reporting production volume in order to comply with the self-identification and recordkeeping requirements in the 2021 Proposal. As a primary alternative regulatory action, EPA is considering the use of the ranking methodologies as described previously but requiring reporting of production volume ranges instead of averages. These ranges would be consistent with those ranges used to show aggregate national production volume of a chemical under EPA's Chemical Data Reporting (CDR). EPA believes reporting these ranges would be easier for industry to calculate and would ensure CBI is always protected since only ranges would be used. However, these ranges are large, which could result in many manufacturers paying the same share of the fees, negating the point of creating a production volume-based fee to improve distribution of fees and to make fees more equitable. EPA is requesting comment on this alternative and on whether ranges narrower than the ones used for CDR would be feasible or appropriate to use under the described circumstances.
G. Exemptions for Fees Associated With EPA-Initiated Risk Evaluations
In the 2021 Proposal, EPA proposed six fee exemptions for manufacturers of chemical substances undergoing EPA-initiated risk evaluation. These proposed exemptions would apply to: (1) Importers of articles containing a chemical substance; (2) Producers of a chemical substance as a byproduct; (3) Manufacturers (including importers) of a chemical substance as an impurity; (4) Producers of a chemical as a non-isolated intermediate; (5) Manufacturers (including importers) of small quantities of a chemical substance solely for research and development; and (6) Manufacturers (including importers) of chemical substances with production volume less than 2,500 lbs. EPA proposed that the volume threshold exemption would not apply when all manufacturers of that chemical substance manufacture in quantities below 2,500 lbs (See 40 CFR 700.45(a)(3)(vi) of the 2021 Proposal). EPA is proposing modifications to the exemptions included in the 2021 Proposal as described in the following section.
Twenty-seven industry commenters supported one or more of EPA's proposed exemptions for EPA-initiated risk evaluation fees for byproducts, impurities, and non-isolated intermediates and many also suggested that EPA use existing TSCA definitions to identify those that are subject to exemptions (e.g., conform the byproducts definition to match other TSCA programs and use 40 CFR 720.30(g) or 720.30(h)(2)) (EPA-HQ-OPPT-2020-0493). EPA is proposing regulatory action aimed to narrow one of the six proposed exemptions (producers of a chemical substance as a byproduct) and to include self-identification requirements for manufacturers (including importers) of chemical substances with production volume less than 2,500 lbs. EPA is proposing to modify the byproduct exemption to, “producers of a chemical substance as a byproduct that is not later used for commercial purposes or distributed for commercial use.” By narrowing the byproduct exemption to include only manufacturers of byproducts that are not later used for commercial purposes or distributed for commercial use, EPA could still collect fees from producers of chemicals that are then sold or used for commercial purposes. In addition, EPA believes those producers of byproducts that are later used in commerce or distributed for commercial use by that manufacturer will not encounter the same issues and concerns with the self-identification requirements as described in EPA's memorandum issued on March 18, 2020 (Ref. 15) previously discussed in the 2021 Proposal since those producers knowingly produce the byproduct before it is introduced into the market (86 FR 1899) (Ref. 3). The byproduct exemption, with these proposed changes, would address challenges with self-identification raised by stakeholders as it relates to identifying and tracking byproducts that are unintentionally or coincidentally produced (40 CFR 700.45(b)(5)).
Twelve industry commenters specifically supported the 2,500 lbs production volume exemption for EPA-initiated risk evaluation fees. However, three of those commenters requested additional clarification or modification of the provision where the exemption would not apply for the EPA-initiated risk evaluation fee for that chemical substance because all manufacturers are low-volume manufacturers (described in the proposed regulations at 40 CFR 700.45(a)(3)(vi)) (EPA-HQ-OPPT-2020-0493). Specifically, one commenter requested clarification of whether, in this case, additional time to make fee payments would be granted to low-volume manufacturers that would otherwise have qualified for this exemption. The commenter asked if low-volume producers would be subject to reduced fees considering the financial burden risk evaluation fees would impose on low-volume manufacturers. Finally, the commenter sought clarification of the procedural steps that will occur and how manufacturers would be notified if they are all low-volume manufacturers (EPA-HQ-OPPT-2020-0493-0034). Another commenter requested that EPA clarify the timeframes associated with the 2,500 lb exemption, specifically on the proposed provision where all identified manufacturers meet the exemption criteria (EPA-HQ-OPPT-2020-0493-0059).
In response to these comments, EPA is proposing self-identification requirements for manufacturers (including importers) of chemical substances with production volume less than 2,500 lbs. Specifically, EPA is proposing to require manufacturers that qualify for the 2,500 lb exemption to self-identify, as described in the 2021 Proposal at 40 CFR 700.45 (b)(5), to report the average annual production volume from the three calendar years prior to the publication of the preliminary list. Requiring self-identification of those manufacturers that qualify for the 2,500 lbs exemption would allow EPA to allocate fees based on production volume and collect fees in a timely manner in the situation where all fee payers have production volumes below 2,500 lbs. In this situation, as described in the 2021 Proposal and not affected by this document, the exemption would not apply for the fee for that chemical substance (described in the proposed regulations at 40 CFR 700.45(a)(3)(vi)). EPA would mask the company names on the final list for that chemical to protect disclosure of potential CBI and notify subject manufacturers of their obligation to pay fees prior to the 90-day consortium deadline (see 40 CFR 700.45(f)(2) and (3) of the 2021 Proposal). For EPA-initiated risk evaluations, the applicable fee would be paid in two installments, with the first payment due 180 days after publishing the final scope of a risk evaluation (see 40 CFR 700.45(g)(3) of the 2021 Proposal). Additional discussion on how these exemptions would apply to test rules is in the following section, Unit III.H.
In addition, EPA recognizes that requiring reporting of a three-year production volume average (discussed in Unit III.F) differs from the timeframes associated with this exemption for low volume producers in new 40 CFR part 700.45(a) which requires a manufacturer to meet the exemption for the five-year period preceding publication of the preliminary list and the successive five years. In response to comments on the timeframe and to avoid confusion, EPA has made changes to the definition of production volume in new 40 CFR 700.43, as discussed in Unit III.F. EPA has also provided clarification on how to determine if the exemption criteria is met in the following paragraph.
To calculate whether a manufacturer produces low enough amounts of a chemical substance to qualify for the exemption, manufacturers would determine their annual production volume for the five calendar years prior to the publication of the preliminary list and their annual projected production volume for the successive five years (as described in new 40 CFR 700.45(a)). To qualify for the exemption for low volume producers, manufacturers would need to produce below 2,500 lbs. for EPA-initiated risk evaluations and below 1,100 lbs. for test rules (see Unit III.H for more details) for those applicable years. If finalized as proposed, manufacturers would not qualify if they produce 2,500 lbs. or 1,100 lbs. or above for EPA-initiated risk evaluations and test orders respectively for any of the applicable years.
EPA is not proposing a reduced fee amount for test rules and/or fees for EPA-initiated risk evaluations for manufacturers reporting a production volume less than 2,500 lbs or 1,100 lbs, respectively, in the event the exemption does not apply. However, EPA is proposing to utilize the production volume-based fee allocation for EPA-initiated risk evaluation fees. The 80% discount for manufacturers that qualify as a small business concern still applies to both test rules and the EPA-initiated risk evaluation fees. EPA requests comments on the proposed changes, as well as the procedural steps EPA plans to take in implementing this provision.
EPA is requesting comment on all six exemptions, including whether any modifications to the exemptions are warranted and whether any additional CBI concerns are present given EPA's proposed approach. EPA is also requesting comment on whether the exemptions, as described in the proposed, new 40 CFR 700.45(a), should be modified based on other TSCA programs like CDR.
H. Exemptions for Fees Associated With TSCA Section 4 Test Rules
The 2018 Fee Rule and the 2021 Proposal did not establish any exemptions related to TSCA section 4 test rules. Currently, manufacturers subject to test rules (and thereby required to pay fees for such rules) are identified using the same process for identifying fee payers for TSCA section 6 EPA-initiated risk evaluations, which involves publishing preliminary and final lists of manufacturers. Including exemptions for TSCA section 4 rules would prevent similar challenges experienced with the self-identification requirements associated with EPA-initiated risk evaluation fees (Refs. 2 and 3).
1. Description of the Proposed Regulatory Action
Based on comments received during the public comment period for the 2021 Proposal, EPA is proposing and requesting comment on applying the EPA-initiated Risk Evaluation fee exemptions to fees for TSCA section 4 test rules. EPA is proposing this change to TSCA section 4 test rules to reduce confusion and prevent challenges regarding the self-identification requirements which apply to fees for both test rules and EPA-initiated risk evaluations. The self-identification requirements do not apply to test orders or ECA's. For this reason, the exemptions will not be applied to those actions. The exemptions outlined earlier in this Unit will remain the same for test rule fees except the annual production volume threshold will change to 1,100 lbs. Manufacturers with an annual production volume of less than 1,100 lbs will qualify for the exemption for the TSCA section 4 test rule fee. This change is necessary to conform to the regulations at 40 CFR 790.42 (a)(4) which specifies a potential annual production volume threshold exemption of less than 1,100 lbs for chemicals subject to TSCA section 4 test rules. EPA is conforming the regulations to avoid possible confusion by manufacturers regarding the TSCA section 4 test rule requirements.
The proposed exemptions for TSCA section 4 test rule fees include: (1) importers of articles containing a chemical substance; (2) producers of a chemical substance as a byproduct; (3) manufacturers (including importers) of a chemical substance as an impurity; (4) producers of a chemical as a non-isolated intermediate; (5) manufacturers (including importers) of small quantities of a chemical substance solely for research and development and; (6) manufacturers (including importers) of chemical substances with production volume less than 1,100 lbs of a chemical subject to a TSCA section 4 test rule. EPA believes these exemptions will provide greater consistency and fairness between TSCA section 4 and TSCA section 6 fees. Including such exemptions for TSCA section 4 will also prevent challenges regarding the self-identification requirements associated with risk evaluation fees for manufacturers similar to what occurred in March 2020 (Ref. 15).
Under these proposed exemptions, appropriate record keeping must be conducted by affected manufacturers as it relates to each listed exemption. Accordingly, EPA is proposing that these manufacturers must maintain production volume records and ordinary business records related to compliance with the six proposed exemptions as outlined in 40 CFR 700.45 (b)(10)(i)-(iv).
2. Description of the Primary Alternative Regulatory Action Considered
The primary alternative to the proposed regulatory action above is to finalize the 2021 Proposal, which did not establish any exemptions related to TSCA section 4 test rules.
I. Expansion of Fee Requirements To Include Companies Required To Submit Information Under TSCA Section 4
The 2018 Fee Rule does not reflect all circumstances in which a manufacturer subject to a TSCA section 4 test order could be required to pay fees. Specifically, fees are required for manufacturers that conduct testing. However, TSCA section 26(b)(1) provides for the collection of fees “from any person required to submit information” under TSCA section 4. There are circumstances in which a manufacturer subject to information development requirements under TSCA section 4 may not need to conduct any testing. For instance, a manufacturer may have already conducted the testing prior to the issuance of a TSCA section 4 test order, in which case the manufacturer may submit the information they have already produced. As explained in greater detail in Unit III.B.1, developing test orders is a complex, time-consuming, and resource-intensive process involving many scientific and regulatory considerations. EPA must establish what information is required, what testing will provide such information, and what test protocols can inform the generation of such information. Further resources are also needed to administer the test orders after they have been issued; the amount of resources needed for such activities varies depending on the complexity of the testing requirements and the number of recipients.
Regardless of whether a manufacturer conducts testing to comply with a test order, EPA incurs costs for developing the test order and administering the test order after it has been issued, including reviewing the data submitted by test order recipients. To ensure that a portion of these costs will be recovered, EPA proposes to require payment from manufacturers subject to TSCA section 4 test order fees that submit information under TSCA section 4 that do not need to conduct any testing.
1. Description of the Proposed Regulatory Action
EPA is proposing and requesting comment on revising the 2018 Fee Rule language under 40 CFR 700.45(a)(2) to refer to manufacturers required to submit information rather than manufacturers “required to test.” Making this change would extend fee obligations to manufacturers who collect and submit existing data. This proposed change would include all manufacturers of a certain chemical regardless of when data was procured, and would create a more equitable fee allocation. Without this proposed change, in situations where test orders are issued to manufacturers which have already completed testing and procured data, those manufacturers would not be subject to fees despite their submission of data to EPA under that test order and despite the costs incurred by EPA for the resource intensive process of developing and administering a test order as explained further in Unit III.B.1.
2. Description of the Primary Alternative Regulatory Action Considered
The primary alternative action to the proposed regulatory action above is to retain the 2018 Fee Rule language under 40 CFR 700.45(a)(2).
J. Payment by Processors Subject to Test Orders and ECAs
The 2018 Fee Rule established that only manufacturers are required to pay fees for TSCA section 4 test orders and ECAs. As a result, in the event that no manufacturers are identified as recipients, EPA would be required to absorb the entire cost of administering TSCA section 4 test orders and ECAs. As an example, in the TSCA section 4 test order issued in January 2021 for o-dichlorobenzene, because only processors were responsible for submitting information, EPA did not collect fees to support the administration of the test order.
EPA is proposing and requesting comment on modifying the fee payment obligations in 40 CFR 700.45(a) to require payment by processors identified in the TSCA section 4 test orders and ECAs who submit information. In the event that there are no manufacturers receiving a test order or ECA, requiring fee payments by processors would allow EPA to recoup the costs of administering such test orders and ECAs. This proposed change would expand the universe of fee payers for these section 4 actions to include both manufacturers and, in some circumstances, processors subject to TSCA section 4 test orders and ECAs. Increasing the scope of fee payers included in TSCA section 4 test orders and ECAs would prevent situations where no manufacturer was identified, thus leaving EPA responsible for the entire cost of administering the test order or ECA.
K. Timeframe for Test Order and Test Rule Payments
The 2018 Fee Rule established a 120-day timeline for TSCA section 4 test order and test rule payments. This 120-day timeline has been found to be too short for the creation of invoice payments and other Agency work related to allocating such payments before any fees are assessed for entities submitting data. It is difficult to calculate such assessed fees quickly under the current timeline which includes various steps such as allocating fees across a number of different manufacturers, issuing invoices, and notifying consortia of those fees within 120 days.
EPA is proposing and requesting comment on extending the timeframe for test order and test rule payments to 180 days after the effective date of the order or rule. This timeframe aligns with the proposed timeframe for the initial fee payment associated with EPA-initiated risk evaluations under section 6, which is also 180 days. The change would provide EPA with sufficient time to review fee payments, identify and allocate fees across a number of different entities, and issue invoices.
L. Requests for Comment
EPA is issuing this supplemental notice and is requesting comments on the proposed provisions and primary alternative provisions described herein that would add to or modify the 2021 Proposal. In addition to the areas on which EPA has specifically requested comment, EPA requests comment on all other aspects of this proposed rule. This includes feedback on potential flexibilities to address small business concerns especially with regard to their ability to pay.
EPA is proposing to refund 20 percent of the user fee to the submitter if a notice is withdrawn after 10 business days after the beginning of the applicable review period, but prior to EPA initiating risk management on the chemical substance. The Agency requests comment on this new partial refund process for the review of TSCA section 5 notices. EPA is also requesting comment on its proposal to recover less than 25 percent of the costs for implementing TSCA section 5.
EPA is proposing a new approach to allocating fees for EPA-initiated risk evaluations, as discussed in Unit III.F. EPA is requesting comment on the methodology outlined below, including whether the approach is a more equitable way of distributing fees. EPA also considered an alternative approach to allocating those fees using production volume ranges. EPA is requesting comment on this alternative and on whether ranges narrower than the ones used for CDR would be feasible or appropriate to use under the described circumstances.
EPA is proposing to require manufacturers that qualify for the 2,500 lb exemption to self-identify, as described in the 2021 Proposal at 40 CFR 700.45 (b)(5), to report the average annual production volume from the three calendar years prior to the publication of the preliminary list. Unit III.G also outlines steps EPA will take to implement this provision while protecting CBI disclosure. EPA requests comments on the proposed changes, as well as the procedural steps EPA plans to take in implementing this provision.
EPA is requesting comment on all six exemptions, including whether any modifications to the exemptions are warranted and whether any additional CBI concerns are present given EPA's proposed approach. EPA is also requesting comment on whether the exemptions, as described in the proposed, new 40 CFR 700.45(a), should be modified based on other TSCA programs like CDR, as well as whether the EPA-initiated Risk Evaluation fee exemptions should apply to fees for TSCA section 4 test rules.
Lastly, EPA is proposing and requesting comment on revising the 2018 Fee Rule language under 40 CFR 700.45(a)(2) to refer to manufacturers required to submit information rather than manufacturers “required to test,” as well as extending the timeframe for test order and test rule payments to 180 days after the effective date of the order or rule.
IV. References
The following is a listing of the documents that are specifically referenced in this document. The docket includes these documents and other information considered by EPA, including documents that are referenced within the documents that are included in the docket, even if the referenced document is not physically located in the docket. For assistance in locating these other documents, please consult the technical person listed under FOR FURTHER INFORMATION CONTACT.
- The Frank R. Lautenberg Chemical Safety for the 21st Century Act. June 22, 2016. Public Law 114-182.
- EPA. Final Rule; Fees for the Administration of the Toxic Substances Control Act. Federal Register. 83 FR 52694, October 17, 2018 (FRL-9984-41).
- EPA. Proposed Rule; Fees for the Administration of the Toxic Substances Control Act. Federal Register. 86 FR 1890, January 11, 2021 (FRL-10018-40).
- EPA. Economic Analysis of the Supplemental Notice of Proposed Rulemaking for Fees for the Administration of the Toxic Substances Control Act. October 2022.
- EPA. TSCA Work Plan Chemicals: Methods Document. February 2012. https://www.epa.gov/sites/production/files/2014-03/documents/work_plan_methods_document_web_final.pdf.
- EPA. Outreach for the TSCA Administration Fees Rule. February 2021. https://www.epa.gov/tsca-fees/outreach-tsca-administration-fees-rule.
- EPA. National PFAS Testing Strategy: Identification of Candidate Per- and Poly- fluoroalkyl Substances (PFAS) for Testing. October 2021. https://www.epa.gov/system/files/documents/2021-10/pfas-natl-test-strategy.pdf.
- EPA. Office of Inspector General (OIG). Lack of Planning Risks EPA's Ability to Meet Toxic Substances Control Act Deadlines (No. 20-P-0247). August 2020. https://www.epa.gov/sites/default/files/2020-08/documents/_epaoig_20200817-20-p-0247.pdf.
- U.S. Government Accountability Office (GAO). Report to Congressional Committees. High-Risk Series: Dedicated Leadership Needed to Address Limited Progress in Most High-Risk Areas. https://www.gao.gov/assets/gao-21-119sp.pdf.
- Joint Explanatory Statement from the House and Division G—Department of Interior, Environment, and Related Agencies Appropriations Act, 2022, https://docs.house.gov/billsthisweek/20220307/BILLS-117RCP35-JES-DIVISION-G.pdf.
- EPA. Technical Background Document for TSCA Fees. October 2022.
- EPA. Interagency Agreement and Oil Indirect Cost Rates for FY 2022 and Beyond. November 2021.
- OMB. Circular A-25 (Revised). July 8, 1993. https://www.whitehouse.gov/wp-content/uploads/2017/11/Circular-025.pdf.
- EPA. Proposed Rule; User Fees for the Administration of the Toxic Substances Control Act. Federal Register. 83 FR 8212, February 26, 2018 (FRL-9974-31).
- EPA. Request for No Action Assurance Regarding Self-Identification Requirement for Certain “Manufacturers” Subject to the TSCA Fees Rule. March 2020. https://www.epa.gov/sites/production/files/2020-03/documents/tsca_fees_-_naa_request_final.pdf.
- EPA. Information Collection Request (ICR) Supporting Statement under the Paperwork Reduction Act entitled: “Reporting Requirements Associated with the Payment of Fees under Section 26(b) of the Toxic Substances Control Act (TSCA); Supplemental Proposed Rule (RIN 2070-AK64).” EPA ICR No. 2569.05; OMB Control No. 2070-0208. October 20, 2022.
- EPA. Information Collection Request (ICR) Supporting Statement under the Paperwork Reduction Act entitled: “User Fees for the Administration of the Toxic Substances Control Act (TSCA); Proposed Rule (RIN 2070-AK64).” EPA ICR No. 2569.05; OMB Control No. 2070-0208. Submitted January 31, 2021.
- OMB. Notice of Office of Management and Budget Action under the Paperwork Reduction Act on ICR entitled: “User Fees for the Administration of the Toxic Substances Control Act (TSCA) (Proposed Rule).” EPA ICR No. 2569.03; OMB Control No. 2070-0208; OMB ICR Reference No. 202101-2070-002. April 5, 2021. https://www.reginfo.gov/public/do/PRAViewICR?ref_nbr=202101-2070-002#.
V. Statutory and Executive Order Reviews
Additional information about these statutes and Executive Orders can be found at https://www.epa.gov/laws-regulations/laws-and-executive-orders.
A. Executive Order 12866: Regulatory Planning and Review and Executive Order 13563: Improving Regulation and Regulatory Review
This action is a significant regulatory action that was submitted to the Office of Management and Budget (OMB) for review under Executive Orders 12866 (58 FR 51735, October 4, 1993) and 13563 (76 FR 3821, January 21, 2011). Any changes made in response to OMB recommendations have been documented in the docket for this action as required by section 6(a)(3)(E) of Executive Order 12866.
EPA prepared an economic analysis of the potential costs and benefits associated with this action (Ref. 4). A copy of this economic analysis is available in the docket.
B. Paperwork Reduction Act (PRA)
The information collection activities in this supplemental proposed rule have been submitted to OMB under the PRA, 44 U.S.C. 3501 et seq. The Information Collection Request (ICR) that EPA prepared for this supplemental proposed rule has been assigned EPA ICR No. 2569.05 (Ref. 16). EPA also prepared and submitted an ICR for the 2021 proposed rule (Ref. 17), and on April 5, 2021, the Notice of OMB Action was issued on that submission that identified the OIRA Conclusion Action as “Comment filed on proposed rule and continue” (Ref. 18). EPA intends for the final rule ICR to amend an existing ICR that is currently approved under OMB Control No. 2070-0208 through February 28, 2025. You can find a copy of the ICR for this supplemental proposal (Ref. 16) in the docket for this action, and it is briefly summarized here.
The information collection activities associated with the supplemental proposed rule include familiarization with the regulation; reduced fee eligibility determination; CDX registration; formation, management and notification to EPA of participation in consortia; self-identification and certification; and electronic payment of fees through Pay.gov.
Respondents/affected entities: Persons who manufacture or process a chemical substance (or any combination of such activities) and are required to submit information to EPA under TSCA sections 4 or 5, or manufacture a chemical substance that is the subject of a risk evaluation under TSCA section 6(b).
Respondent's obligation to respond: Mandatory. TSCA section 26(b).
Estimated number of respondents: 960.
Frequency of response: On occasion.
Total estimated burden: 496 hours (per year). Burden is defined at 5 CFR 1320.3(b).
Total estimated cost: $31,046 (per year), includes $0 annualized capital or operation and maintenance costs.
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number. The OMB control numbers for EPA's regulations in 40 CFR part 700 are listed in 40 CFR part 9.
Submit your comments on the Agency's need for this information, the accuracy of the provided burden estimates and any suggested methods for minimizing respondent burden to the EPA using the docket identified at the beginning of this rule. You may also send your ICR-related comments to OMB's Office of Information and Regulatory Affairs using the interface at https://www.reginfo.gov/public/do/PRAMain. Find this particular information collection by selecting “Currently under Review—Open for Public Comments” or by using the search function.
If you wish to comment on the information collection requirements in this supplemental proposed rule, please note that OMB is required to make a decision concerning the collection of information contained in this supplemental proposed rule between 30 and 60 days after publication of this supplemental proposed rule in the Federal Register.
EPA will respond to ICR-related comments received on the 2021 proposed rule and on this supplemental proposed rule in the context of the final rule.
C. Regulatory Flexibility Act (RFA)
I certify that this action will not have a significant economic impact on a substantial number of small entities under the RFA, 5 U.S.C. 601 et seq. The small entities expected to be subject to the requirements of this action are small chemical manufacturers and processors, small petroleum refineries, and small chemical and petroleum wholesalers. There may be some potentially affected firms within other sectors, but not all firms within those sectors will be potentially affected firms. 306 small businesses, including 256 processors and 50 manufacturers, may be affected annually by TSCA section 4 actions; 149 small businesses may be affected by section 5 actions; and 31 small businesses may be affected by section 6 actions. EPA estimates the annual revenue distribution using U.S. Census data for small businesses likely to be affected by TSCA sections 4, 5, and 6 actions, with the following properties: 92% of parent firms have an annual revenue greater than $152,800, 7% have an annual revenue between $152,800 and $50,933, and 1% have revenue less than $50,933. The average annual incremental cost per affected small business is expected to be about $392 for TSCA section 4; $2,477 for TSCA section 5, and $44,559 for TSCA section 6. As a result, EPA estimates that, of the 485 small businesses paying fees every year, 451 will have impacts under 1%, 19 will have impacts between 1% and 3%, and 16 will have impacts greater than 3%.
D. Unfunded Mandates Reform Act (UMRA)
This action does not contain an unfunded mandate of $100 million or more as described in UMRA, 2 U.S.C. 1531-1538, and does not significantly or uniquely affect small governments. The rule is not expected to result in expenditures by state, local, and tribal governments, in the aggregate, or by the private sector, of $100 million or more (when adjusted annually for inflation) in any one year. Accordingly, this proposed rule is not subject to the requirements of sections 202, 203, or 205 of UMRA. The total quantified annualized social costs for this supplemental proposal are approximately $85,014 (at both 3% and 7% discount rate), which does not exceed the inflation-adjusted unfunded mandate threshold of $160 million.
E. Executive Order 13132: Federalism
This action does not have federalism implications as specified in Executive Order 13132 (64 FR 43255, August 10, 1999). It will not have substantial direct effects on the states, on the relationship between the national government and the states, or on the distribution of and responsibilities among the various levels of government.
F. Executive Order 13175: Consultation and Coordination With Indian Tribal Governments
This action does not have tribal implications as specified in Executive Order 13175 (65 FR 67249, November 9, 2000). This rule will not impose substantial direct compliance costs on Indian Tribal Governments. Thus, Executive Order 13175 does not apply to this action.
G. Executive Order 13045: Protection of Children From Environmental Health and Safety Risks
EPA interprets Executive Order 13045 (62 FR 19885, April 23, 1997), as applying only to those regulatory actions that concern environmental health or safety risks that the EPA has reason to believe may disproportionately affect children, per the definition of “covered regulatory action” in section 2-202 of the Executive Order. This action is not subject to Executive Order 13045 because it does not establish an environmental standard intended to mitigate environmental health risks or safety risks.
H. Executive Order 13211: Actions Concerning Regulations That Significantly Affect Energy Supply, Distribution, or Use
This action is not a “significant energy action” as specified in Executive Order 13211 (66 FR 28355, May 22, 2001) because it is not likely to have a significant adverse effect on the supply, distribution or use of energy and has not otherwise been designated by the Administrator of the Office of Information and Regulatory Affairs as a significant energy action.
I. National Technology Transfer and Advancement Act (NTTAA)
This rulemaking does not involve technical standards. As such, NTTAA section 12(d), 15 U.S.C. 272 note, does not apply to this action.
J. Executive Order 12898: Federal Actions To Address Environmental Justice in Minority Populations and Low-Income Populations and Executive Order 14008: Tackling the Climate Crisis at Home and Abroad
In accordance with Executive Order 12898 (59 FR 7629, February 16, 1994) and Executive Order 14008 (86 FR 7619, January 27, 2021), EPA finds that this action will not result in disproportionately high and adverse human health, environmental, climate-related, or other cumulative impacts on disadvantaged communities. The documentation for this decision is contained in the Economic Analysis (Ref. 4), which is in the docket for this action. Although not directly impacting environmental justice-related concerns, the fees will enable the Agency to better protect human health and the environment, including in low-income and minority communities. The fees also provide for fair treatment and meaningful involvement in the implementation of TSCA.
List of Subjects 40 CFR Part 700
Chemicals, Environmental protection, Hazardous substances, Reporting and recordkeeping requirements, User fees.
Michael S. Regan,
Administrator.
Therefore, for the reasons presented in the preamble, it is proposed that 40 CFR chapter I, subchapter R, be amended as follows:
PART 700—GENERAL [AMENDED]
1. The authority citation for part 700 continues to read as follows:
Authority: 15 U.S.C. 2625 and 2665, 44 U.S.C. 3504.
2. Amend §700.43 by adding in alphabetical order a definition for “Production volume” and “Small quantities solely for research and development” the additions read as follows:
§700.43 Definitions applicable to this subpart.
* * * * *
Production volume means manufactured (including imported) amount in pounds.
* * * * *
Small quantities solely for research and development (or “small quantities solely for purposes of scientific experimentation or analysis or chemical research on, or analysis of, such substance or another substance, including such research or analysis for the development of a product”) means quantities of a chemical substance manufactured (including imported), or processed or proposed to be manufactured (including imported), or processed solely for research and development that are not greater than reasonably necessary for such purposes.
* * * * *
3. Amend §700.45 by:
a. Revising paragraphs (a)(2) and (3).
b. In paragraph (b), by:
i. Revising the introductory text in paragraph (b)(5) and paragraphs (b)(5)(ii) and (iii),
ii. Adding paragraphs (b)(5)(iv) and (v),
iii. Revising paragraph (b)(7), and
v. Adding paragraph (b)(10).
c. In paragraph (c), by:
i. Revising the intro text heading in paragraph (c),
ii. Revising paragraphs (c)(1)(i) through (iii) and (iv) through (viii), and
iii. Revising paragraphs (c)(2)(i) through (iii) and (iv) through (xi).
d. Revising paragraph (d),
e. In paragraph (f), by:
i. Revising paragraphs (f)(2)(i), (3)(i), (4) and (5), and
ii. Adding paragraph (f)(6).
f. In paragraph (g) by:
i. Revising paragraphs (g)(3)(i) and (iv),
ii. Revising paragraph (g)(5), and (6).
g. In paragraph (i), by:
i. Revising paragraphs (i)(1) through (3), and
ii. Adding paragraph (i)(4).
The revisions and additions read as follows.
§700.45 Fee payments.
(a) * * *
(2) Manufacturers and processors of chemical substances and mixtures required to submit information for these chemical substances and mixtures under a TSCA section 4(a) test order or enforceable consent agreement, or manufacturers of chemical substances and mixtures required to submit information for these chemical substance and mixtures under a TSCA section 4(a) test rule, shall remit for each such test rule, order, or enforceable consent agreement the applicable fee identified in paragraph (c) of this section in accordance with the procedures in paragraphs (f) and (g) of this section. Manufacturers of a chemical substance subject to a test rule under section 4(a) of the Act are exempted from fee payment requirements in this section, if they meet one or more of the exemptions under this paragraph (a)(2)(i) through (vi) for the five-year period preceding publication of the preliminary list and do not conduct manufacturing outside of those exemptions during the five-year period preceding publication of the preliminary list; and will meet one or more of the exemptions in paragraph (a)(2)(i) through (vi) of this section in the successive five years and will not conduct manufacturing outside of those exemptions in the successive five years:
(i) import articles containing that chemical substance;
(ii) produce that chemical substance as a byproduct that is not later used for commercial purposes or distributed for commercial use;
(iii) manufacture (including import) that chemical substance as an impurity as defined in §704.3;
(iv) manufacture that chemical substance as a non-isolated intermediate as defined in 40 §704.3;
(v) manufacture (including import) small quantities of that chemical substance solely for research and development, as defined in §700.43; and/or
(vi) manufacture (including import) that chemical substance in quantities below a 1,100 lbs annual production volume as described in §700.43, unless all manufacturers of that chemical substance manufacture that chemical in quantities below a 1,100 lbs annual production volume as described in §700.43, in which case this exemption is not applicable.
(3) Manufacturers of a chemical substance that is subject to a risk evaluation under section 6(b) of the Act, shall remit for each such chemical risk evaluation the applicable fee identified in paragraph (c) of this section in accordance with the procedures in paragraphs (f) and (g) of this section. For the purposes of this section, entities that manufacture a chemical substance subject to a risk evaluation under section 6(b) of the Act solely for export are subject to fee requirements in this section whenever such substance is manufactured, processed, or distributed in commerce by any other entity for any purpose other than export from the United States. Manufacturers of a chemical substance subject to risk evaluation under section 6(b) of the Act are exempted from fee payment requirements in this section, if they meet one or more of the exemptions under this paragraph (a)(3)(i) through (vi) for the five-year period preceding publication of the preliminary list and do not conduct manufacturing outside of those exemptions during the five-year period preceding publication of the preliminary list; and will meet one or more of the exemptions in paragraph (a)(3)(i) through (vi) of this section in the successive five years and will not conduct manufacturing outside of those exemptions in the successive five years:
(i) import articles containing that chemical substance;
(ii) produce that chemical substance as a byproduct that is not later used for commercial purposes or distributed for commercial use;
(iii) manufacture (including import) that chemical substance as an impurity as defined in §704.3;
(iv) manufacture that chemical substance as a non-isolated intermediate as defined in §704.3;
(v) manufacture (including import) small quantities of that chemical substance solely for research and development, as defined in §700.43; and/or
(vi) manufacture (including import) that chemical substance in quantities below a 2,500 lbs annual production volume as described in §700.43, unless all manufacturers of that chemical substance manufacture that chemical in quantities below a 2,500 lbs annual production volume as described in §700.43, in which case this exemption is not applicable.
* * * * *
(b) * * *
* * * * *
(5) Self-identification. All manufacturers other than those listed in paragraph (a)(2)(i) through (iii) and (a)(3)(i) through (iii) of this section who have manufactured (including imported) the chemical substance in the previous five years must submit notice to EPA, irrespective of whether they are included in the preliminary list specified in paragraph (b)(3) of this section. The notice must be submitted electronically via EPA's Central Data Exchange (CDX), the Agency's electronic reporting portal, using the Chemical Information Submission System (CISS) reporting tool, and must contain the following information:
(i) * * *
(ii) Certification of cessation. If a manufacturer has manufactured in the five-year period preceding publication of the preliminary list, but has ceased manufacture prior to the certification cutoff dates identified in paragraph (b)(6) of this section and will not manufacture the substance again in the successive five years, the manufacturer may submit a certification statement attesting to these facts. If EPA receives such a certification statement from a manufacturer, the manufacturer will not be included in the final list of manufacturers described in paragraph (b)(7) of this section and will not be obligated to pay the fee under this section.
(iii) Certification of no manufacture. If a manufacturer is identified on the preliminary list but has not manufactured the chemical in the five-year period preceding publication of the preliminary list, the manufacturer may submit a certification statement attesting to these facts. If EPA receives such a certification statement from a manufacturer, the manufacturer will not be included in the final list of manufacturers described in paragraph (b)(7) of this section and will not be obligated to pay the fee under this section.
(iv) Certification of meeting exemption. If a manufacturer is identified on the preliminary list and exclusively meets one or more of the exemptions in paragraph (a)(2)(i) through (vi) or (a)(3)(i) through (vi) of this section for the five-year period preceding publication of the preliminary list and will exclusively meet one of more of the exemptions in paragraph (a)(2)(i) through (vi) or (a)(3)(i) through (vi) of this section in the successive five years, the manufacturer must submit a certification statement attesting to these facts in order to not be included in the final list of manufacturers described in paragraph (b)(7) of this section. If a manufacturer is not on a preliminary list and exclusively meets one or more of the exemptions in paragraph (a)(2)(i) through (vi) or (a)(3)(i) through (vi) of this section for the five-year period preceding publication of the preliminary list and will exclusively meet one of more of the exemptions in paragraph (a)(2)(i) through (vi) or (a)(3)(i) through (vi) of this section in the successive five years, the manufacturer may submit a certification statement attesting to these facts. If EPA receives such a certification statement from a manufacturer, the manufacturer will not be included in the final list of manufacturers described in paragraph (b)(7) of this section and will not be obligated to pay the fee under this section, unless all manufacturers of that chemical substance meet the exemption as described in (a)(2)(vi) or (a)(3)(vi) of this section.
(v) Production volume. If a manufacturer has not submitted certification of cessation, as described in paragraph (b)(5)(ii) of this section, or certification of no manufacture, as described in paragraph (b)(5)(iii) of this section, for purposes of identifying manufacturers subject to fees for section 6 EPA-initiated risk evaluations and does not meet one or more of the exemptions in paragraph (a)(3)(i) through (v) of this section, the manufacturer must submit their production volume as defined in §700.43 for the applicable substance for the three calendar years prior to publication of the preliminary list. Only production volume reported to EPA prior to the final list being published will be used in determining fees described in §700.45(f).
* * * * *
(7) Publication of final list. EPA expects to publish a final list of manufacturers to identify the specific manufacturers subject to the applicable fee. This list will indicate if additional manufacturers self-identified pursuant to paragraph (b)(5) of this section, if other manufacturers were identified through credible public comment, and if manufacturers submitted certification of cessation, no manufacture, or meeting exemption pursuant to paragraph (b)(5)(ii), (iii), or (iv) of this section. The final list will be published no later than concurrently with the final scope document for risk evaluations initiated by EPA under section 6, and with the final test rule for test rules under section 4.
* * * * *
(10) Recordkeeping. After [date 60 calendar days after the date of publication of the final rule ]:
(i) All manufacturers other than those listed in paragraph (a)(2)(i) through (v) or (a)(3)(i) through (v) of this section must maintain production volume records related to compliance with paragraph (b)(5)(v) of this section. These records must be maintained for a period of five years from the date notice is submitted pursuant to paragraph (b)(5) of this section.
(ii) Those manufacturers that are exempt from fee payment requirements pursuant to paragraph (a)(2)(iv) or (3)(iv) of this section must maintain ordinary manufacturing and other business records related to compliance with the exemption criteria described in paragraph (a)(2)(iv) or (3)(iv) of this section, respectively. These records must be maintained for a period of five years from the date the record is generated.
(iii) Those manufacturers that are exempt from fee payment requirements pursuant to paragraph (a)(2)(v) or (3)(v) of this section must maintain ordinary manufacturing and other business records related to compliance with the exemption criteria described in paragraph (a)(2)(v) or (3)(v) of this section respectively, such as production volume, plans of study, information from research and development notebooks, study reports, or notice solely for research and development use. These records must be maintained for a period of five years from the date the record is generated.
(iv) Those manufacturers that are exempt from fee payment requirements pursuant to paragraph (a)(2)(vi) or (3)(vi) of this section must maintain production volume records related to compliance with the exemption criteria described in paragraph (a)(2)(vi) or (3)(vi) of this section, respectively. These records must be maintained for a period of five years from the date the exemption is claimed.
* * * * *
(c) Fees for the 2023, 2024, and 2025 fiscal years.
(1) * * *
(i) Premanufacture notice and consolidated premanufacture notice. Persons shall remit a fee totaling $7,880 for each premanufacture notice (PMN) or consolidated PMN submitted in accordance with part 720 of this chapter.
(ii) Significant new use notice. Persons shall remit a fee totaling $7,880 for each significant new use notice (SNUN) submitted in accordance with part 721 of this chapter.
(iii) Exemption application. Persons shall remit a fee totaling $2,650 for each of the following exemption requests submitted under section 5 of the Act:
(A) * * *
(iv) Instant photographic film article exemption notice. Persons shall remit a fee totaling $2,650 for each instant photographic film article exemption notice submitted in accordance with §723.175 of this chapter.
(v) Microbial commercial activity notice and consolidated microbial commercial activity notice. Persons shall remit a fee totaling $7,880 for each microbial commercial activity notice (MCAN) or consolidated MCAN submitted in accordance with §§725.25 through 725.36 of this chapter.
(vi) Test rule, test order, or enforceable consent agreement. Persons shall remit a total of twenty percent of the applicable fee under paragraph (c)(2)(vi), (vii) or (viii) of this section for a test rule, test order, or enforceable consent agreement.
(vii) EPA-initiated risk evaluation. Persons shall remit a total fee of twenty percent of the applicable fee under paragraphs (c)(2)(ix) of this section for an EPA-initiated risk evaluation.
(viii) Manufacturer-requested risk evaluation. Persons shall remit the total fee under paragraph (c)(2)(x) or (xi) of this section, as applicable, for a manufacturer-requested risk evaluation.
(2) * * *
(i) PMN and consolidated PMN. Persons shall remit a fee totaling $45,000 for each PMN or consolidated PMN submitted in accordance with part 720 of this chapter.
(ii) SNUN. Persons shall remit a fee totaling $45,000 for each significant new use notice submitted in accordance with part 721 of this chapter.
(iii) Exemption applications. Persons shall remit a fee totaling $13,230 for each of the following exemption requests, and modifications to previous exemption requests, submitted under section 5 of the Act:
(A) * * *
(iv) Instant photographic film article exemption notice. Persons shall remit a fee totaling $13,230 for each exemption notice submitted in accordance with §723.175 of this chapter.
(v) MCAN and consolidated MCAN. Persons shall remit a fee totaling $45,000 for each MCAN or consolidated MCAN submitted in accordance with §§725.25 through 725.36 of this chapter.
(vi) Test rule. Persons shall remit a fee totaling $50,000 for each test rule.
(vii) Test order. Persons shall remit a fee totaling $25,000 for each test order.
(viii) Enforceable consent agreement. Persons shall remit a fee totaling $50,000 for each enforceable consent agreement.
(ix) EPA-initiated chemical risk evaluation. Persons shall remit a fee totaling $5,081,000.
(x) Manufacturer-requested risk evaluation of a Work Plan Chemical. Persons shall remit an initial fee of $1,497,000, a second payment of $1,497,000, and final payment to total 50% of the actual costs of this activity, in accordance with the procedures in paragraph (g) of this section. The final payment amount will be determined by EPA, and invoice issued to the requesting manufacturer.
(xi) Manufacturer-requested risk evaluation of a non-work plan chemical. Persons shall remit an initial fee of $2,993,000, a second payment of $2,993,000, and final payment to total 100% of the actual costs of the activity, in accordance with the procedures in paragraph (g) of this section. The final payment amount will be determined by EPA, and invoice issued to the requesting manufacturer.
* * * * *
(d) Fees for 2025 fiscal year and beyond.
(1) Fees for the 2025 and later fiscal years will be adjusted on a three-year cycle by multiplying the fees in paragraph (c) of this section by the current PPI index value with a base year of 2023 using the following formula:
FA = F × I
here:
FA = the inflation-adjusted future year fee amount.
F = the fee specified in paragraph (c) of this section.
I = Producer Price Index for Chemicals and Allied Products inflation value with 2023 as a base year.
(2) Updated fee amounts for PMNs, SNUNs, MCANs, exemption notices, exemption applications, and manufacturer-requested risk evaluation requests apply to submissions received by the Agency on or after October 1 of every three-year fee adjustment cycle beginning in fiscal year 2023 (October 1, 2022). Updated fee amounts also apply to test rules, test orders, enforceable consent agreements and EPA-initiated risk evaluations that are “noticed” on or after October 1 of every three-year fee adjustment cycle, beginning in fiscal year 2025.
(3) The Agency will initiate public consultation through notice-and-comment rulemaking prior to making fee adjustments beyond inflation. If it is determined that no additional adjustment is necessary beyond for inflation, EPA will provide public notice of the inflation-adjusted fee amounts through posting to the Agency's web page by the beginning of each three-year fee adjustment cycle (October 1, 2025, October 1, 2028, etc.). If the Agency determines that adjustments beyond inflation are necessary, EPA will provide public notice of that determination and the process to be followed to make those adjustments.
* * * * *
(f) * * *
(2) * * *
(i) The consortium must identify a principal sponsor and provide notification to EPA that a consortium has formed. The notification must be accomplished within 90 days of the publication date of a test rule under section 4 of the Act, or within 90 days of the effective date of a test order under section 4 of the Act, or within 90 days of the signing of an enforceable consent agreement under section 4 of the Act. EPA may permit additional entities to join an existing consortium after the expiration of the notification period if the principal sponsor provides updated notification.
* * * * *
(3) * * *
(i) Notification must be provided to EPA that a consortium has formed. The notification must be accomplished within 90 days of the publication of the final scope of a chemical risk evaluation under section 6(b)(4)(D) of the Act or within 90 days of EPA providing notification to a manufacturer that a manufacturer-requested risk evaluation has been granted. EPA may permit additional entities to join an existing consortium after the expiration of the notification period if the principal sponsor provides updated notification.
* * * * *
(4) If multiple persons are subject to fees triggered by section 4 or 6(b) of the Act and no consortium is formed, EPA will determine the portion of the total applicable fee to be remitted by each person subject to the requirement.
(i) Each person's share of the applicable fees triggered by section 4 of the Act specified in paragraph (c) of this section shall be in proportion to the total number of manufacturers and/or processors of the chemical substance, with lower fees for small businesses:
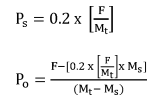
Where:
Ps = the portion of the fee under paragraph (c) of this section that is owed by a person who qualifies as a small business concern under §700.43 of this chapter.
Po = the portion of the fee owed by a person other than a small business concern.
F = the total fee required under paragraph (c) of this section.
Mt = the total number of persons subject to the fee requirement.
Ms = the number of persons subject to the fee requirement who qualify as a small business concern.
(ii) Each person's share of the applicable fees triggered by section 6(b) of the Act specified in paragraph (c) of this section shall be in proportion to the total number of manufacturers and their reported production volume as described in §700.45(b)(v) of the chemical substance, with lower fees for small businesses:
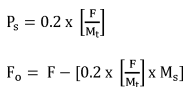
(iii) Remaining manufacturers (i.e., those that do not qualify as a small business concern) are then ranked in ascending order (from lowest to highest) based on reported production volume as described in §700.45(b)(v). Each remaining manufacturer is assigned a number with 1 for lowest production volume, 2 for second lowest production volume, etc.
| Manufacturer(s) | Assigned No. (N) |
|---|---|
| Manufacturer with lowest production volume | 1 |
| Manufacturer with 2nd lowest production volume | 2 |
| Manufacturer with 3rd lowest production volume | 3 |
| * * * etc |
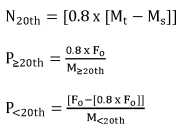
Where:
Ps = the portion of the fee under paragraph (c) of this section that is owed by a person who qualifies as a small business concern under §700.43 of this chapter.
P≥20th = the portion of the fee owed by a person other than a small business concern in the top 20th percentile.
P<20th = the portion of the fee owed by a person other than a small business concern not in the top 20th percentile.
F = the total fee required under paragraph (c) of this section.
Mt = the total number of persons subject to the fee requirement.
Ms = the number of persons subject to the fee requirement who qualify as a small business concern.
N20th = The assigned number as illustrated in Table 1 to the manufacturer(s) with a production volume as described in §700.45(b)(v) at which the manufacturers with production volume greater than or equal to are in the top 20th percentile.
M≥20th = the total number of persons with production volume as described in §700.45(b)(v) greater than or equal to the manufacturer(s) with a production volume as N 20th.
M<20th = the total number of persons with production volume as described in §700.45(b)(v) less than the manufacturer(s) with a production volume as N 20th.
Fo = the total fee required under paragraph (c) of this section by all person(s) other than a small business concern.
(vi) In the event there are three or less manufacturers identified for a chemical substance, EPA will distribute the fee evenly among those three or less fee payers, regardless of production volume.
(v) In the event the number assigned to the top 20th percentile is not an integer, EPA will round to the nearest integer to determine the manufacturer(s) with the reported production volume as described in §700.45(b)(v) greater than or equal to the top 20th percentile.
(vi) In the event multiple manufacturers report the same production volume as described in §700.45(b)(v) and are greater than or equal to the top 20th percentile, EPA will include all manufacturers with that same production volume in the fee calculation for the top 20th percentile group.
(5) If multiple persons are subject to fees triggered by section 4 of the Act and some inform EPA of their intent to form a consortium while others choose not to associate with the consortium, EPA will take the following steps to allocate fee amounts:
(i) Count the total number of manufacturers, including the number of manufacturers within any consortia; divide the total fee amount by the total number of manufacturers; and allocate equally on a per capita basis to generate a base fee;
(ii) Provide all small businesses who are either not associated with a consortium, or associated with an all- small business consortium, with an 80% discount from the base fee referenced previously;
(iii) Calculate the total remaining fee and total number of remaining manufacturers by subtracting out the discounted fees and the number of small businesses identified;
(iv) Reallocate the remaining fee across those remaining individuals and groups in equal amounts, counting each manufacturer in a consortium as one person; and
(v) Inform consortia and individuals of their requisite fee amount. Small businesses in a successfully-formed consortium, other than a consortium of all small businesses, will not be afforded the 80% discount by EPA, but consortia managers are strongly encouraged to provide a discount for small business concerns.
(6) If multiple persons are subject to fees triggered by section 6(b) of the Act and some inform EPA of their intent to form a consortium while others choose not to associate with the consortium, EPA will take the following steps to allocate fee amounts:
(i) Count the total number of manufacturers, including the number of manufacturers within any consortia; divide the total fee amount by the total number of manufacturers; and allocate equally on a per capita basis to generate a base fee;
(ii) Provide all small businesses who are either not associated with a consortium, or associated with an all-small business consortium, with an 80% discount from the base fee referenced previously;
(iii) Calculate the total remaining fee and total number of remaining manufacturers by subtracting out the discounted fees and the number of small businesses identified;
(iv) Place remaining manufacturers in ascending order (from lowest to highest) based on reported production volume as described in §700.45(b)(v). Assign each remaining manufacturer a number with 1 for lowest production volume, 2 for second lowest production volume, etc.;
(v) Determine the manufacturer(s) in the top 20th percentile by multiplying the total number of remaining manufacturers by 0.8. then comparing that number to the manufacturer(s) with that assigned number as described in paragraph (iv) of this section;
(vi) Reallocate 80% of the total remaining fee evenly across that manufacturer(s) with a production volume amount equal to or larger than that manufacturer(s) (the top 20th percentile), counting each manufacturer in a consortium as one person;
(vii) Reallocate the remaining fee evenly across the remaining manufacturers, counting each manufacturer in a consortium as one person; and
(v) Inform consortia and individuals of their requisite fee amount. Small businesses in a successfully-formed consortium, other than a consortium of all small businesses, will not be afforded the 80% discount by EPA, but consortia managers are strongly encouraged to provide a discount for small business concerns.
* * * * *
(g) * * *
(3) * * *
(i) Test orders and test rules. The applicable fee specified in paragraph (c) of this section shall be paid in full not later than 180 days after the effective date of a test rule or test order under section 4 of the Act.
* * * * *
(iv) Risk evaluations. (A) For EPA-initiated risk evaluations, the applicable fee specified in paragraph (c) of this section shall be paid in two installments, with the first payment of 50% due 180 days after publishing the final scope of a risk evaluation and the second payment for the remainder of the fee due 545 days after publishing the final scope of a risk evaluation under section 6(b)(4)(D) of the Act.
(B) For manufacturer-requested risk evaluations under section 6(b)(4)(C)(ii) of the Act, the applicable fees specified in paragraph (c) of this section shall be paid as follows:
(1) The applicable fee specified in paragraph (c) of this section shall be paid in three installments. The first payment shall be due no later than 180 days after EPA provides the submitting manufacture(s) notice that it has granted the request.
(2) The second payment shall be due no later than 545 days after EPA provides the submitting manufacturer(s) notice that it has granted the request.
(3) The final payment shall be due no later than 30 days after EPA publishes the final risk evaluation.
* * * * *
(5) Small business certification. (i) Each person who remits the fee identified in paragraph (c)(1) of this section for a PMN, consolidated PMN, or SNUN shall insert a check mark for the statement, “The company named in part 1, section A is a small business concern under 40 CFR 700.43 and has remitted a fee of $7,880 in accordance with 40 CFR 700.45(c).” under “CERTIFICATION” on page 2 of the Premanufacture Notice for New Chemical Substances (EPA Form 7710-25).
(ii) Each person who remits the fee identified in paragraph (c)(1) of this section for a LVE, LoREX, TERA, TME, or Tier II exemption request under TSCA section 5 shall insert a check mark for the statement, “The company named in part 1, section A is a small business concern under 40 CFR 700.43 and has remitted a fee of $2,650 in accordance with 40 CFR 700.45(c).” in the exemption application.
(iii) Each person who remits the fee identified in paragraph (c)(1) of this section for an exemption notice under §723.175 of this chapter shall include the words, “The company or companies identified in this notice is/are a small business concern under 40 CFR 700.43 and has/have remitted a fee of $2,650 in accordance with 40 CFR 700.45(c).” in the certification required in §723.175(i)(1)(x) of this chapter.
(iv) Each person who remits the fee identified in paragraph (c)(1) of this section for a MCAN or consolidated MCAN for a microorganism shall insert a check mark for the statement, “The company named in part 1, section A is a small business concern under 40 CFR 700.43 and has remitted a fee of $7,880 in accordance with 40 CFR 700.45(c).” in the certification required in §725.25(b) of this chapter.
(6) Payment certification statement. (i) Each person who remits a fee identified in paragraph (c)(2) of this section for a PMN, consolidated PMN, or SNUN shall insert a check mark for the statement, “The company named in part 1, section A has remitted the fee of $45,000 specified in 40 CFR 700.45(c).” under “CERTIFICATION” on page 2 of the Premanufacture Notice for New Chemical Substances (EPA Form 7710-25).
(ii) Each person who remits a fee identified in paragraph (c)(2) of this section for a LVE, LoREX, TERA, TME, or Tier II exemption request under TSCA section 5 shall insert a check mark for the statement, “The company named in part 1, section A has remitted the fee of $13,230 specified in 40 CFR 700.45(c).” in the exemption application.
(iii) Each person who remits the fee identified in paragraph (c)(2) of this section for an exemption notice under §723.175 of this chapter shall include the words, “The company or companies identified in this notice has/have remitted a fee of $13,230 in accordance with 40 CFR 700.45(c).” in the certification required in §723.175(i)(1)(x) of this chapter.
(iv) Each person who remits the fee identified in paragraph (c)(2) of this section for a MCAN for a microorganism shall insert a check mark for the statement, “The company named in part 1, section A has remitted the fee of $45,000 in accordance with 40 CFR 700.45(c).” in the certification required in §725.25(b) of this chapter.
* * * * *
(i) Partial fee refunds.
(1) If a TSCA section 5 notice is withdrawn during the first 10 business days after the beginning of the applicable review period under §720.75(a) of this chapter, the Agency will refund all but 25% of the fee as soon as practicable.
(2) If a TSCA section 5 notice is withdrawn during the period beginning 10 business days after the beginning of the applicable review period under §720.75(a) of this chapter and ending 5 business days after EPA has provided the submitter notice that the risk assessment on the chemical substance(s) has concluded, the Agency will refund all but 80% of the fee as soon as practicable.
(3) Once withdrawn, any future submission related to the TSCA section 5 notice must be submitted as a new notice.
(4) If EPA determines that the initial payment for a manufacturer-requested risk evaluation exceeds the applicable fee in paragraph (c) of this section, EPA will refund the difference.
* * * * *
[FR Doc. 2022-24137 Filed 11-15-22; 8:45 am]
BILLING CODE 6560-50-P
['Toxic Substances Control Act - EPA']
['Toxic Subtances Control Act - EPA', 'Toxic Substances - EPA']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
