['Toxic Substances Control Act - EPA']
['Toxic Subtances Control Act - EPA']
12/09/2022
...
(a) Introduction� (1) Background and purpose. (i) Water is one of the most widely distributed substances in the environment. It covers a large portion of the earth's surface as oceans, rivers, and lakes. The soil also contains water, as does the atmosphere in the form of water vapor. As a result of this ubiquitousness, chemicals introduced into the environment almost always come into contact with aqueous media. Certain classes of these chemicals, upon such contact, can undergo hydrolysis, which is one of the most common reactions controlling chemical stability and is, therefore, one of the main chemical degradation paths of these substances in the environment.
(ii) Since hydrolysis can be such an important degradation path for certain classes of chemicals, it is necessary, in assessing the fate of these chemicals in the environment, to know whether, at what rate, and under what conditions a substance will hydrolyze. Some of these reactions can occur so rapidly that there may be greater concern about the products of the transformation than about the parent compounds. In other cases, a substance will be resistant to hydrolysis under typical environmental conditions, while, in still other instances, the substance may have an intermediate stability that can result in the necessity for an assessment of both the original compound and its transformation products. The importance of transformation of chemicals via hydrolysis in aqueous media in the environment can be determined quantitatively from data on hydrolysis rate constants. This hydrolysis Test Guideline represents a test to allow one to determine rates of hydrolysis at any pH of environmental concern at 25�C.
(a)(2) Definitions and units. (i) "Hydrolysis" is defined as the reaction of an organic chemical with water, such that one or more bonds are broken and the reaction products of the transformation incorporate the elements of water (H2O).
(a)(2)(ii) "Elimination" is defined in this Test Guideline to be a reaction of an organic chemical (RX) in water in which the X group is lost. These reactions generally follow the same type of rate laws that hydrolysis reactions follow and, thus, are also covered in this Test Guideline.
(iii) A "first-order reaction" is defined as a reaction in which the rate of disappearance of the chemical substance being tested is directly proportional to the concentration of the chemical substance and is not a function of the concentrations of any other substances present in the reaction mixture.
(iv) The "half-life" of a chemical is defined as the time required for the concentration of the chemical substance being tested to be reduced to one- half its initial value.
(a)(2)(v) "Hydrolysis" refers to a reaction of an organic chemical with water such that one or more bonds are broken and the reaction products incorporate the elements of water (H2O). This type of transformation often results in the net exchange of a group X, on an organic chemical RX, for the OH group from water. This can be written as:
RX+HOH?ROH+HX.
(A) Another result of hydrolysis can be the incorporation of both H and OH in a single product. An example of this is the hydrolysis of epoxides, which can be represented by
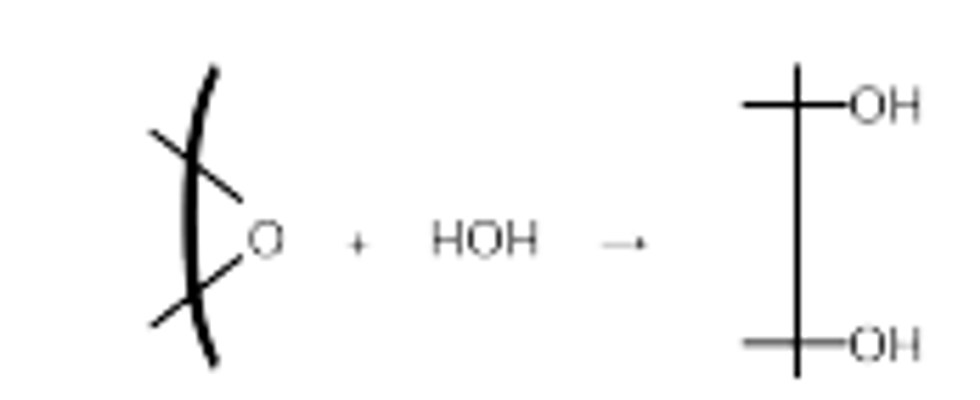
(B) The hydrolysis reaction can be catalyzed by acidic or basic species, including OH- and H3O+ (H=). The promotion of the reaction by H3O- or OH- is called specific acid or specific base catalysis, respectively, as contrasted with general acid or base catalysis encountered with other cationic or anionic species. Usually, the rate law for chemical RX can be written as:
Equation 1
-d[RX]/d= = kh[RX]=kA[H=] [RX] +kB[OH-] [RX]+k'N [H2O] [RX],
where KA, kB and k'N are the second-order rate constants for acid and base catalyzed and neutral water processes, respectively. In dilute solutions, such as are encountered in following this Test Guideline, water is present in great excess and its concentration is, thus, essentially constant during the course of the hydrolysis reaction. At fixed pH, the reaction, therefore, becomes pseudo first-order, and the rate constant (kh) can be written as:
Equation 2
kh=kA [H=]+kB [OH-]+kN,
where kN is the first-order neutral water rate constant. Since this is a pseudo first-order process, the half-life is independent of the concentration and can be written as:
Equation 3
t1/2=0.693/kh.
At constant pH, Equation 1 can be integrated to yield the first order rate expression
Equation 4
log10 C=-(kht/2.303)+log10 Co,
where C is the concentration of the test chemical at time t and Co is the initial chemical concentration (t=0).
(C) At a given pH, Equation 2 under paragraph (a)(2)(v)(B) of this section contains three unknowns, kA, kB, and kN. Therefore, three equations (i.e., measurements at three different pH's at a fixed temperature) are required if one wishes to solve for these quantities. Making suitable approximations for quantities that are negligible, the expressions for kA, kB, and kN using values of kh measured at pH 3, 7, and 11 are:
Equation 5
kA=103 [kh (3)-kh (7)+10-4 kh (11)]
kB=103 [kh (11)-kh (7)+10-4 kh (3)]
kN=kh (7)-10-4 [kh (3)+kh (11)]
The calculated rate constants from equation 5 under this paragraph can be employed in equation 2 under paragraph (a)(2)(v)(B) of this section to calculate the hydrolysis rate of a chemical at any pH of environmental concern.
(D) The equations under paragraph (a)(2) of this section apply whether the test chemical has one or more hydrolyzable groups. In the latter case, the rate may be written as:
Equation 6
-d[RX]/dt= [RX]+k2 [RX]+ . . . . +kn
[RX] =(k1+k2+ . . . . . kn) [RX]=kh [RX].
Equation 6 applies to the hydrolysis rate of a molecule having n hydrolyzable groups, each of which follows first-order reaction kinetics. The measured kh is now the sum of the individual reaction rates and is the only rate constant required in this section.
(a)(3) Principle of the test method. Procedures described in this section enable sponsors to obtain quantitative information on hydrolysis rates through a determination of hydrolysis rate constants and half-lives of chemicals at pH 3.00, 7.00, and 11.00 at 25�C. The three measured rate constants are used to determine the acidic, basic, and neutral rate constants associated with a hydrolytic reaction. The latter constants can then be employed in determining the hydrolysis rates of chemicals at any pH of environmental concern at 25�C.
(a)(4) Applicability and specificity. There are several different common classes of organic chemicals that are subject to hydrolysis transformation, including esters, amides, lactones, carbamates, organophosphates, and alkyl halides. Processes other than nucleophilic displacement by water can also take place. Among these are elimination reactions that exhibit behavior similar to hydrolysis and, therefore, are also covered in this section.
(b) Test procedures�(1) Test conditions�(i) Special laboratory equipment. (A) A thermostatic bath that can be maintained at a temperature of 25�1�C.
(B) A pH meter that can resolve differences of 0.05 pH units or less.
(C) Stoppered volumetric flasks (no grease) or glass ampoules that can be sealed.
(b)(1)(ii) Purity of water. Reagent-grade water (e.g., water meeting ASTM Type IIA standards or an equivalent grade) shall be used to minimize biodegradation. ASTM Type IIA water is described in ASTM D 1193-77 (Reapproved 1983), "Standard Specification for Reagent Water." ASTM D 1193-77 (Reapproved 1983) is available for inspection at the Office of the Federal Register, Rm. 8301, 1100 L St., NW., Washington, DC. This incorporation by reference was approved by the Director of the Office of the Federal Register. This material is incorporated as it exists on the date of approval and a notice of any change in this material will be published in the Federal Register. Copies of the incorporated material may be obtained from the Director, Environmental Assistance Division (7408), Office of Pollution Prevention and Toxics, Environmental Protection Agency, Room E�543B, 1200 Pennsylvania Ave. NW., Washington, DC 20460�0001, or from the American Society for Testing and Materials (ASTM), 1916 Race Street, Philadelphia, PA 19103.
(b)(1)(iii) Sterilization. All glassware shall be sterilized. Aseptic conditions shall be used in the preparation of all solutions and in carrying out all hydrolysis experiments to eliminate or minimize biodegradation. Glassware can be sterilized in an autoclave or by any other suitable method.
(b)(1)(iv) Precautions for volatility. If the chemical is volatile the reaction vessels shall be almost completely filled and sealed.
(b)(1)(v) Temperature controls. All hydrolysis reactions shall be carried out at 25�C (�1�C) and with the temperature controlled to �0.1�C.
(b)(1)(vi) pH conditions. It is recommended that all hydrolysis experiments be performed at pH 3.00, 7.00, and 11.00 � 0.05 using the appropriate buffers described in paragraph (b)(2)(i)(A) of this section.
(b)(1)(vii) Concentration of solutions of chemical substances. The concentration of the test chesical shall be less than one-half the chemical's solubility in water but not greater than 10-3M.
(b)(1)(viii) Effect of acidic and basic groups. Complications can arise upon measuring the rate of hydrolysis of chemicals that reversibly ionize or are protonated in the pH range 3.00 to 11.00. Therefore, for these chemicals, it is recommended that these hydrolysis tests be performed at pH 5.00, 7.00, and 900�0.05 using the appropriate buffers described in paragraphs (b)(2)(i)(A) and (B) of this section. If a test chemical reversibly ionizes or protonates in the pH range 5.00 to 9.00, then it is recommended that additional hydrolysis tests should be carried out at pH 6.00 and 8.00�0.05 using the buffers described in paragraph (b)(2)(i)(B) of this section.
(b)(1)(ix) Buffer catalysis. For certain chemicals, buffers may catalyze the hydrolysis reaction. If this is suspected, hydrolysis rate determination shall be carried out with the appropriate buffers and the same experiments repeated at buffer concentrations lowered by at least a factor of five. If the hydrolysis reaction produces a change of greater than 0.05 pH units in the lower concentration buffers at the end of the measurement time, the test chemical concentrations also shall be lowered by at least a factor of five. Alternatively, test chemical concentrations and buffer concentrations may both be lowered simultaneously by a factor of five. A sufficient criterion for minimization of buffer catalysis is an observed equality in the hydrolysis rate constant for two different solutions differing in buffer or test chemical concentration by a factor of five.
(b)(1)(x) Photosensitive chemicals. The solution absorption spectrum can be employed to determine whether a particular chemical is potentially subject to photolytic transformation upon exposure to light. For chemicals that absorb light of wavelengths greater than 290 nm, the hydrolysis experiment shall be carried out in the dark, under amber or red safelights, in amber or red glassware, or employing other suitable methods for preventing photolysis. The absorption spectrum of the chemical in aqueous solution can be measured under �796.1050.
(b)(1)(xi) Chemical analysis of solutions. In determining the concentrations of the test chemicals in solution, any suitable analytical method may be employed, although methods which are specific for the compound to be tested are preferred. Chromatographic methods are recommended because of their compound specificity in analyzing the parent chemical without interferences from impurities. Whenever practicable, the chosen analytical method should have a precision within �5 percent.
(b)(2) Preparation�(i) Reagents and solutions�(A) Buffer solutions. Prepare buffer solutions using reagent-grade chemicals and reagent-grade water as follows:
(1) pH 3.00: use 250 mL of 0.100M potassium hydrogen phthalate; 111 mL of 0.100M hydrochloric acid; and adjust volume to 500 mL with reagent-grade water.
(2) pH 7.00: use 250 mL of 0.100M potassium dihydrogen phosphate; 145 mL of 0.100M sodium hydroxide; and adjust volume to 500 mL with reagent-grade water.
(3) pH 11.00: use 250 mL of 0.0500M sodium bicarbonate; 113 mL of 0.100M sodium hydroxide; and adjust volume to 500 mL with reagent-grade water.
(B) Additional buffer solutions. For chemicals that ionize or are protonated as discussed in paragraph (b)(1)(viii) of this section, prepare buffers using reagent-grade water and reagent-grade chemicals as follows:
(1) pH 5.00: use 250 mL of 0.100M potassium hydrogen phthalate; 113 mL of 0.100M sodium hydroxide; and adjust volume to 500 mL with reagent-grade water.
(2) pH 6.00: use 250 mL of 0.100M potassium dihydrogen phosphate; 28 mL of 0.100M sodium hydroxide; and adjust volume to 500 mL with reagent-grade water.
(3) pH 8.00: use 250 mL of 0.100M potassium dihydrogen phosphate; 234 mL of 0.100M sodium hydroxide; and adjust volume to 500 mL with reagent-grade water.
(4) pH 9.00: use 250 mL of 0.0250M borax (Na2B4O7); 23 mL of 0.100M hydrochloric aid; and adjust volume to 500 mL with reagent-grade water.
(b)(4)(i)(C) Adjustment of buffer concentrations. (1) The concentrations of all the above buffer solutions are the maximum concentration to be employed in carrying out hydrolysis measurements. If the initial concentration of the test chemical is less than 10-3M, the buffer concentration shall be lowered by a corresponding amount; e.g., if the initial test chemical concentration is 10-4M, the concentration of the above buffers shall be reduced by a factor of 10. In addition, for those reactions in which an acid or base is not a reaction product, the minimum buffer concentration necessary for maintaining the pH within +0.05 units shall be employed.
(2) Check the pH of all buffer solutions with a pH meter at 25�C and adjust the pH to the proper value, if necessary.
(b)(2)(i)(D) Preparation of test solution. (1) If the test chemical is readily soluble in water, prepare an aqueous solution of the chemical in the appropriate buffer and determine the concentration of the chemical. Alternatively, a solution of the chemical in water may be prepared and added to an appropriate buffer solution and the concentration of the chemical then determined. In the latter case, the aliquot shall be small enough so that the concentration of the buffer in the final solution and the pH of the solution remain essentially unchanged. Do not employ heat in dissolving the chemical. The final concentration shall not be greater than one-half the chemical's solubility in water and not greater than 10-3M.
(2) If the test chemical is too insoluble in pure water to permit reasonable handling and analytical procedures, it is recommended that the chemical be dissolved in reagent-grade acetonitrile and buffer solution and then added to an aliquot of the acetonitrile solution. Do not employ heat to dissolve the chemical in acetonitrile. The final concentration of the test chemical shall not be greater than one-half the chemical's solubility in water and not greater than 10-3M. In addition, the final concentration of the acetonitrile shall be one volume percent or less.
(b)(3) Performance of the test. Carry out all hydrolysis experiments by employing one of the procedures described in this paragraph. Prepare the test solutions as described in paragraph (b)(2)(i) of this section at pH 3.00, 7.00, and 11.00�0.05, and determine the initial test chemical concentration (Co) in triplicate. Analyze each reaction mixture in triplicate at regular intervals, employing one of the following procedures:
(b)(3)(i) Procedure 1. Analyze each test solution at regular intervals to provide a minimum of six measurements with the extent of hydrolysis between 20 to 70 percent. Rates should be rapid enough so that 60 to 70 percent of the chemical is hydrolyzed in 672 hours.
(b)(3)(ii) Procedure 2. If the reaction is too slow to conveniently follow hydrolysis to high conversion in 672 hours but still rapid enough to attain at least 20 percent conversion, take 15 to 20 time points at regular intervals after 10 percent conversion is attained.
(b)(3)(iii) Procedure 3. (A) If chemical hydrolysis is less than 20 percent after 672 hours, determine the concentration (C) after this time period.
(B) If the pH at the end of concentration measurements employing any of the above three procedures has changed by more than 0.05 units from the initial pH, repeat the experiment using a solution having a test chemical concentration lowered sufficiently to keep the pH variation within 0.05 pH units.
(b)(3)(iv) Analytical methodology. Select an analytical method that is most applicable to the analysis of the specific chemical being tested under paragraph (b)(1)(xi) of this section.
(c) Data and reporting�(1) Treatment of results. (i) If Procedure 1 or 2 were employed in making concentration measurements, use a linear regression analysis with Equation 4 under paragraph (a)(2)(v)(B) of this section to calculate kh at 25�C for each pH employed in the hydrolysis experiments. Calculate the coefficient of determination (R2) for each rate constant. Use Equation 3 under paragraph (a)(2)(v)(B) of this section to calculate the hydrolysis half-life using kh.
(ii) If Procedure 3 was employed in making rate measurements, use the mean initial concentration (Co) and the mean concentration of chemical (C) in Equation 4 under paragraph (a)(2)(v)(B) of this section to calculate kh for each pH used in the experiments. Calculate the hydrolysis half-life using kh in Equation 3 under paragraph (a)(2)(v)(B) of this section.
(iii) For each set of three concentration replicates, calculate the mean value of C and the standard deviation.
(iv) For test chemicals that are not ionized or protonated between pH 3 and 11, calculate kA, kB, and kN using Equation 5.
(2) Specific analytical and recovery procedures. (i) Provide a detailed description or reference for the analytical procedure used, including the calibration data and precision.
(ii) If extraction methods were used to separate the solute from the aqueous solution, provide a description of the extraction method as well as the recovery data.
(c)(3) Test data report. (i) For Procedures 1 and 2, report kh, the hydrolysis half-life (t1/2), and the coefficient of determination (R2) for each pH employed in the rate measurements. In addition, report the individual values, the mean value, and the standard deviation for each set of replicate concentration measurements. Finally, report kA, kB, and kN.
(ii) For Procedure 3, report kh and the half-life for each pH employed in the rate measurements. In addition, report the individual values, the mean value, and the standard deviation for each set of replicate concentration measurements. Finally, report kA, kB, and kN.
(iii) If, after 672 hours, the concentration (C) is the same as the initial concentration (Co) within experimental error, then kh cannot be calculated and the chemical can be reported as being persistent with respect to hydrolysis.
[77 FR 46293, Aug. 3, 2012]
['Toxic Substances Control Act - EPA']
['Toxic Subtances Control Act - EPA']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2025 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
