['Toxic and Hazardous Substances - OSHA', 'Hazard Communication']
['Hazard Classifications', 'Hazard Communication', 'Toxic and Hazardous Substances - OSHA']
02/10/2026
...
A.0 General Classification Considerations
A.0.1 Classification
A.0.1.1 The term “hazard classification” is used to indicate that only the intrinsic hazardous properties of chemicals are considered. Hazard classification incorporates three steps:
(a) Identification of relevant data regarding the hazards of a chemical;
(b) Subsequent review of those data to ascertain the hazards associated with the chemical;
(c) Determination of whether the chemical will be classified as hazardous and the degree of hazard.
A.0.1.2 For many hazard classes, the criteria are semi-quantitative or qualitative and expert judgment is required to interpret the data for classification purposes.
A.0.1.3 Where impurities, additives or individual constituents of a substance or mixture have been identified and are themselves classified, they should be taken into account during classification if they exceed the cut-off value/concentration limit for a given hazard class.
A.0.2 Available Data, Test Methods and Test Data Quality
A.0.2.1 There is no requirement for testing chemicals.
A.0.2.2 The criteria for determining health hazards are test method neutral, i.e., they do not specify particular test methods, as long as the methods are scientifically validated.
A.0.2.3 The term “scientifically validated” refers to the process by which the reliability and the relevance of a procedure are established for a particular purpose. Any test that determines hazardous properties, which is conducted according to recognized scientific principles, can be used for purposes of a hazard determination for health hazards. Test conditions need to be standardized so that the results are reproducible with a given substance, and the standardized test yields “valid” data for defining the hazard class of concern.
A.0.2.4 Existing test data are acceptable for classifying chemicals, although expert judgment also may be needed for classification purposes.
A.0.2.5 The effect of a chemical on biological systems is influenced, by the physico-chemical properties of the substance and/or ingredients of the mixture and the way in which ingredient substances are biologically available. A chemical need not be classified when it can be shown by conclusive experimental data from scientifically validated test methods that the chemical is not biologically available.
A.0.2.6 For classification purposes, epidemiological data and experience on the effects of chemicals on humans (e.g., occupational data, data from accident databases) shall be taken into account in the evaluation of human health hazards of a chemical.
A.0.3 Classification Based on Weight of Evidence
A.0.3.1 For some hazard classes, classification results directly when the data satisfy the criteria. For others, classification of a chemical shall be determined on the basis of the total weight of evidence using expert judgment. This means that all available information bearing on the classification of hazard shall be considered together, including the results of valid in vitro tests, relevant animal data, and human experience such as epidemiological and clinical studies and well-documented case reports and observations.
A.0.3.2 The quality and consistency of the data shall be considered. Information on chemicals related to the material being classified shall be considered as appropriate, as well as site of action and mechanism or mode of action study results. Both positive and negative results shall be considered together in a single weight-of-evidence determination.
A.0.3.3 Positive effects which are consistent with the criteria for classification, whether seen in humans or animals, shall normally justify classification. Where evidence is available from both humans and animals and there is a conflict between the findings, the quality and reliability of the evidence from both sources shall be evaluated in order to resolve the question of classification. Reliable, good quality human data shall generally have precedence over other data. However, even well-designed and conducted epidemiological studies may lack a sufficient number of subjects to detect relatively rare but still significant effects, or to assess potentially confounding factors. Therefore, positive results from well-conducted animal studies are not necessarily negated by the lack of positive human experience but require an assessment of the robustness, quality and statistical power of both the human and animal data.
A.0.3.4 Route of exposure, mechanistic information, and metabolism studies are pertinent to determining the relevance of an effect in humans. When such information raises doubt about relevance in humans, a lower classification may be warranted. When there is scientific evidence demonstrating that the mechanism or mode of action is not relevant to humans, the chemical should not be classified.
A.0.3.5 Both positive and negative results are considered together in the weight of evidence determination. However, a single positive study performed according to good scientific principles and with statistically and biologically significant positive results may justify classification.
A.0.4 Considerations for the Classification of Mixtures
A.0.4.1 Except as provided in A.0.4.2, the process of classification of mixtures is based on the following sequence:
(a) Where test data are available for the complete mixture, the classification of the mixture will always be based on those data;
(b) Where test data are not available for the mixture itself, the bridging principles designated in each health hazard chapter of this appendix shall be considered for classification of the mixture;
(c) If test data are not available for the mixture itself, and the available information is not sufficient to allow application of the above-mentioned bridging principles, then the method(s) described in each chapter for estimating the hazards based on the information known will be applied to classify the mixture (e.g., application of cut-off values/concentration limits).
A.0.4.2 An exception to the above order or precedence is made for Carcinogenicity, Germ Cell Mutagenicity, and Reproductive Toxicity. For these three hazard classes, mixtures shall be classified based upon information on the ingredient substances, unless on a case-by-case basis, justification can be provided for classifying based upon the mixture as a whole. See A.5, A.6, and A.7 of this section for further information on case-by-case bases.
A.0.4.3 Use of cut-off values/concentration limits
A.0.4.3.1 When classifying an untested mixture based on the hazards of its ingredients, cut-off values/concentration limits for the classified ingredients of the mixture are used for several hazard classes. While the adopted cut-off values/concentration limits adequately identify the hazard for most mixtures, there may be some that contain hazardous ingredients at lower concentrations than the specified cut-off values/concentration limits that still pose an identifiable hazard. There may also be cases where the cut-off value/concentration limit is considerably lower than the established non-hazardous level for an ingredient.
A.0.4.3.2 If the classifier has information that the hazard of an ingredient will be evident (i.e., it presents a health risk) below the specified cut-off value/concentration limit, the mixture containing that ingredient shall be classified accordingly.
A.0.4.3.3 In exceptional cases, conclusive data may demonstrate that the hazard of an ingredient will not be evident (i.e., it does not present a health risk) when present at a level above the specified cut-off value/concentration limit(s). In these cases the mixture may be classified according to those data. The data must exclude the possibility that the ingredient will behave in the mixture in a manner that would increase the hazard over that of the pure substance. Furthermore, the mixture must not contain ingredients that would affect that determination.
A.0.4.4 Synergistic or antagonistic effects
When performing an assessment in accordance with these requirements, the evaluator must take into account all available information about the potential occurrence of synergistic effects among the ingredients of the mixture. Lowering classification of a mixture to a less hazardous category on the basis of antagonistic effects may be done only if the determination is supported by sufficient data.
A.0.5 Bridging Principles for the Classification of Mixtures Where Test Data Are Not Available for the Complete Mixture
A.0.5.1 Where the mixture itself has not been tested to determine its toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles, subject to any specific provisions for mixtures for each hazard class. These principles ensure that the classification process uses the available data to the greatest extent possible in characterizing the hazards of the mixture.
A.0.5.1.1 Dilution
For mixtures classified in accordance with A.1 through A.10 of this Appendix, if a tested mixture is diluted with a diluent that has an equivalent or lower toxicity classification than the least toxic original ingredient, and which is not expected to affect the toxicity of other ingredients, then:
(a) The new diluted mixture shall be classified as equivalent to the original tested mixture; or
(b) For classification of acute toxicity in accordance with A.1 of this Appendix, paragraph A.1.3.6 (the additivity formula) shall be applied.
A.0.5.1.2 Batching
For mixtures classified in accordance with A.1 through A.10 of this Appendix, the toxicity of a tested production batch of a mixture can be assumed to be substantially equivalent to that of another untested production batch of the same mixture, when produced by or under the control of the same chemical manufacturer, unless there is reason to believe there is significant variation such that the toxicity of the untested batch has changed. If the latter occurs, a new classification is necessary.
A.0.5.1.3 Concentration of mixtures
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, A.9, or A.10 of this Appendix, if a tested mixture is classified in Category 1, and the concentration of the ingredients of the tested mixture that are in Category 1 is increased, the resulting untested mixture shall be classified in Category 1.
A.0.5.1.4 Interpolation within one hazard category
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, A.9, or A.10 of this Appendix, for three mixtures (A, B and C) with identical ingredients, where mixtures A and B have been tested and are in the same hazard category, and where untested mixture C has the same toxicologically active ingredients as mixtures A and B but has concentrations of toxicologically active ingredients intermediate to the concentrations in mixtures A and B, then mixture C is assumed to be in the same hazard category as A and B.
A.0.5.1.5 Substantially similar mixtures
For mixtures classified in accordance with A.1 through A.10 of this Appendix, given the following set of conditions:
(a) Where there are two mixtures:
(i) A + B;
(ii) C + B;
(b) The concentration of ingredient B is essentially the same in both mixtures;
(c) The concentration of ingredient A in mixture (i) equals that of ingredient C in mixture (ii);
(d) And data on toxicity for A and C are available and substantially equivalent; i.e., they are in the same hazard category and are not expected to affect the toxicity of B; then
If mixture (i) or (ii) is already classified based on test data, the other mixture can be assigned the same hazard category.
A.0.5.1.6 Aerosols
For mixtures classified in accordance with A.1, A.2, A.3, A.4, A.8, or A.9 of this Appendix, an aerosol form of a mixture shall be classified in the same hazard category as the tested, non-aerosolized form of the mixture, provided the added propellant does not affect the toxicity of the mixture when spraying.
A.1 Acute Toxicity
A.1.1 Definition
Acute toxicity refers to serious adverse health effects (i.e., lethality) occurring after a single or short-term oral, dermal, or inhalation exposure to a substance or mixture.
A.1.2 Classification Criteria for Substances
A.1.2.1 Substances can be allocated to one of four hazard categories based on acute toxicity by the oral, dermal or inhalation route according to the numeric cut-off criteria as shown in Table A.1.1. Acute toxicity values are expressed as (approximate) LD50 (oral, dermal) or LC 50 (inhalation) values or as acute toxicity estimates (ATE). While some in vivo methods determine LD50/LC50 values directly, other newer in vivo methods (e.g., using fewer animals) consider other indicators of acute toxicity, such as significant clinical signs of toxicity, which are used by reference to assign the hazard category. See the footnotes following Table A.1.1 for further explanation on the application of these values.
| Exposure route | Category 1 | Category 2 | Category 3 | Category 4 |
|---|---|---|---|---|
| Oral (mg/kg bodyweight) see: Note (a) Note (b) | ATE ≤ 5 | >5 ATE ≤ 50 | >50 ATE ≤ 300 | >300 ATE ≤ 2000. |
| Dermal (mg/kg bodyweight) see: Note (a) Note (b) | ATE ≤ 50 | >50 ATE ≤ 200 | >200 ATE ≤ 1000 | >1000 ATE ≤ 2000. |
| Inhalation—Gases (ppmV) see: Note (a) Note (b) Note (c) | ATE ≤ 100 | >100 ATE ≤ 500 | >500 ATE ≤ 2500 | >2500 ATE ≤ 20000. |
| Inhalation—Vapors (mg/l) see: Note (a) Note (b) Note (c) Note (d) | ATE ≤ 0.5 | >0.5 ATE ≤ 2.0 | >2.0 ATE ≤ 10.0 | >10.0 ATE ≤ 20.0. |
| Inhalation—Dusts and Mists (mg/l) see: Note (a) Note (b) Note (c) | ATE ≤ 0.05 | >0.05 ATE ≤ 0.5 | >0.5 ATE ≤ 1.0 | >1.0 ATE ≤ 5.0. |
| Note: Gas concentrations are expressed in parts per million per volume (ppmV). Notes to Table A.1.1: (a) The acute toxicity estimate (ATE) for the classification of a substance is derived using the LD 50 /LC 50 where available. (b) The acute toxicity estimate (ATE) for the classification of a substance or ingredient in a mixture is derived using: (i) the LD 50 /LC 50 where available. Otherwise, (ii) the appropriate conversion value from Table 1.2 that relates to the results of a range test, or (iii) the appropriate conversion value from Table 1.2 that relates to a classification category; (c) Inhalation cut-off values in the table are based on 4 hour testing exposures. Conversion of existing inhalation toxicity data which has been generated according to 1 hour exposure is achieved by dividing by a factor of 2 for gases and vapors and 4 for dusts and mists; (d) For some substances the test atmosphere will be a vapor which consists of a combination of liquid and gaseous phases. For other substances the test atmosphere may consist of a vapor which is nearly all the gaseous phase. In these latter cases, classification is based on ppmV as follows: Category 1 (100 ppmV), Category 2 (500 ppmV), Category 3 (2500 ppmV), Category 4 (20000 ppmV). The terms “dust”, “mist” and “vapor” are defined as follows: (i) Dust: solid particles of a substance or mixture suspended in a gas (usually air); (ii) Mist: liquid droplets of a substance or mixture suspended in a gas (usually air); (iii) Vapor: the gaseous form of a substance or mixture released from its liquid or solid state. | ||||
A.1.2.3 The preferred test species for evaluation of acute toxicity by the oral and inhalation routes is the rat, while the rat or rabbit are preferred for evaluation of acute dermal toxicity. Test data already generated for the classification of chemicals under existing systems should be accepted when reclassifying these chemicals under the harmonized system. When experimental data for acute toxicity are available in several animal species, scientific judgment should be used in selecting the most appropriate LD 50 value from among scientifically validated tests. In cases where data from human experience (i.e., occupational data, data from accident databases, epidemiology studies, clinical reports) is also available, it should be considered in a weight of evidence approach consistent with the principles described in A.0.3.
A.1.2.4 In addition to classification for inhalation toxicity, if data are available that indicates that the mechanism of toxicity was corrosivity of the substance or mixture, the classifier must consider if the chemical is corrosive to the respiratory tract. Corrosion of the respiratory tract is defined as destruction of the respiratory tract tissue after a single, limited period of exposure analogous to skin corrosion; this includes destruction of the mucosa. The corrosivity evaluation could be based on expert judgment using such evidence as: human and animal experience, existing (in vitro) data, Ph values, information from similar substances or any other pertinent data.
A.1.2.4.1 If the classifier determines the chemical is corrosive to the respiratory tract and data are available that indicate that the effect leads to lethality, then in addition to the appropriate acute toxicity pictogram and hazard statement, the chemical must be labelled with the hazard statement “corrosive to the respiratory tract” and the corrosive pictogram.
A.1.2.4.2 If the classifier determines the chemical is corrosive to the respiratory tract and the effect does not lead to lethality, then the chemical must be addressed in the Specific Target Organ Toxicity hazard classes (see A.8). If data is insufficient for classification under STOT, but the classifier determines, based on skin or eye data, that the chemical may be corrosive to the respiratory tract, then the hazard must be addressed using data for classification in the skin corrosion/irritation hazard class (see A.2) or Serious Eye Damage/Eye irritation hazard class (see A.3).
A.1.3 Classification Criteria for Mixtures
A.1.3.1 The approach to classification of mixtures for acute toxicity is tiered, and is dependent upon the amount of information available for the mixture itself and for its ingredients. The flow chart of Figure A.1.1 indicates the process that must be followed:
A.1.1 Figure—1 Tiered Approach to Classification of Mixtures for Acute Toxicity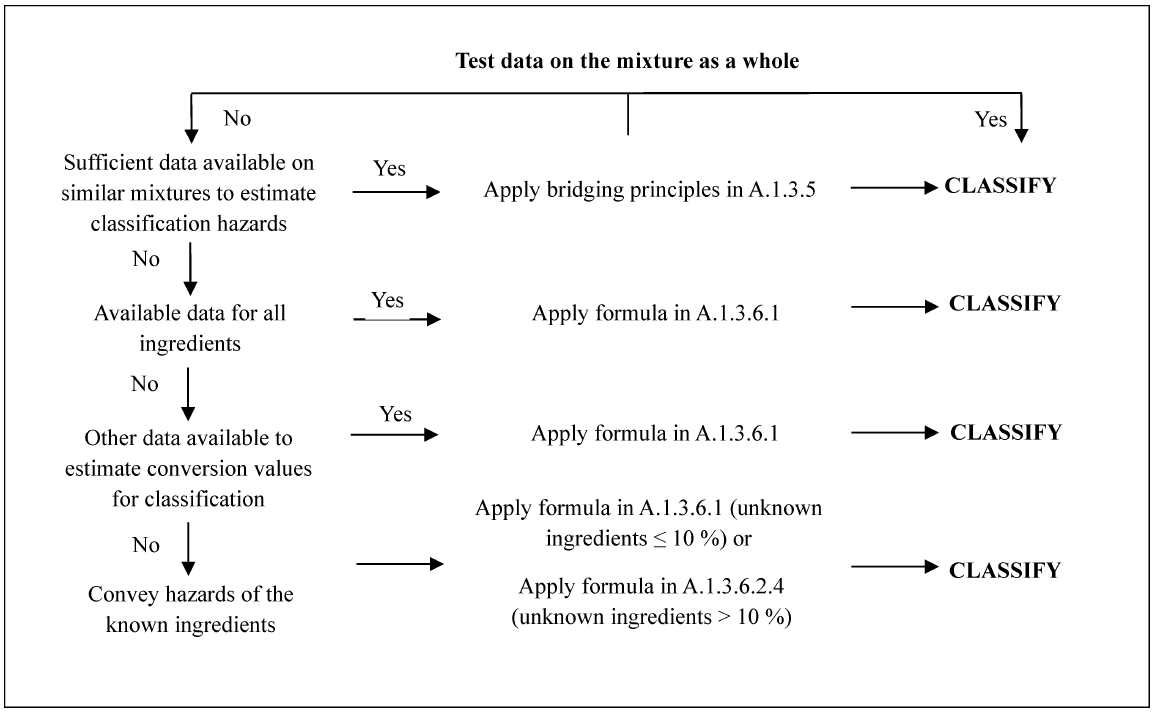
A.1.3.2 Classification of mixtures for acute toxicity may be carried out for each route of exposure, but is only required for one route of exposure as long as this route is followed (estimated or tested) for all ingredients and there is no relevant evidence to suggest acute toxicity by multiple routes. When there is relevant evidence of acute toxicity by multiple routes of exposure, classification is to be conducted for all appropriate routes of exposure. All available information shall be considered. The pictogram and signal word used shall reflect the most severe hazard category; and all relevant hazard statements shall be used.
A.1.3.3 For purposes of classifying the hazards of mixtures in the tiered approach:
(a) The “relevant ingredients” of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases). If there is reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for acute toxicity, that ingredient shall also be considered relevant. Consideration of ingredients present at a concentration <1% is particularly important when classifying untested mixtures which contain ingredients that are classified in Category 1 and Category 2;
(b) Where a classified mixture is used as an ingredient of another mixture, the actual or derived acute toxicity estimate (ATE) for that mixture is used when calculating the classification of the new mixture using the formulas in A.1.3.6.1 and A.1.3.6.2.4.
(c) If the converted acute toxicity point estimates for all ingredients of a mixture are within the same category, then the mixture should be classified in that category.
(d) When only range data (or acute toxicity hazard category information) are available for ingredients in a mixture, they may be converted to point estimates in accordance with Table A.1.2 when calculating the classification of the new mixture using the formulas in A.1.3.6.1 and A.1.3.6.2.4.
A.1.3.4 Classification of mixtures where acute toxicity test data are available for the complete mixture
Where the mixture itself has been tested to determine its acute toxicity, it is classified according to the same criteria as those used for substances, presented in Table A.1.1. If test data for the mixture are not available, the procedures presented below must be followed.
A.1.3.5 Classification of mixtures where acute toxicity test data are not available for the complete mixture: bridging principles
Where the mixture itself has not been tested to determine its acute toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.1.3.6 Classification of mixtures based on ingredients of the mixture (additivity formula)
A.1.3.6.1 Data available for all ingredients.
The acute toxicity estimate (ATE) of ingredients is considered as follows:
(a) Include ingredients with a known acute toxicity, which fall into any of the acute hazard categories, or have an oral or dermal LD 50 greater than 2000 but less than or equal to 5000 mg/kg body weight (or the equivalent dose for inhalation);
(b) Ignore ingredients that are presumed not acutely toxic (e.g., water, sugar);
(c) Ignore ingredients if the data available are from a limit dose test (at the upper threshold for Category 4 for the appropriate route of exposure as provided in Table A.1.1) and do not show acute toxicity.
Ingredients that fall within the scope of this paragraph are considered to be ingredients with a known acute toxicity estimate (ATE). See note (b) to Table A.1.1 and paragraph A.1.3.3 for appropriate application of available data to the equation below, and paragraph A.1.3.6.2.4.
The ATE of the mixture is determined by calculation from the ATE values for all relevant ingredients according to the following formula below for oral, dermal or inhalation toxicity:
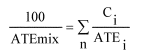
Where:
C i = concentration of ingredient i;
n ingredients and i is running from 1 to n;
ATE i = Acute toxicity estimate of ingredient i;
A.1.3.6.2 Data are not available for one or more ingredients of the mixture.
A.1.3.6.2.1 Where an ATE is not available for an individual ingredient of the mixture, but available information provides a derived conversion value, the formula in A.1.3.6.1 may be applied. This information may include evaluation of:
(a) Extrapolation between oral, dermal and inhalation acute toxicity estimates. Such an evaluation requires appropriate pharmacodynamic and pharmacokinetic data;
(b) Evidence from human exposure that indicates toxic effects but does not provide lethal dose data;
(c) Evidence from any other toxicity tests/assays available on the substance that indicates toxic acute effects but does not necessarily provide lethal dose data; or
(d) Data from closely analogous substances using structure/activity relationships.
A.1.3.6.2.2 This approach requires substantial supplemental technical information, and a highly trained and experienced expert, to reliably estimate acute toxicity. If sufficient information is not available to reliably estimate acute toxicity, proceed to the provisions of A.1.3.6.2.4.
A.1.3.6.2.3 In the event that an ingredient with unknown acute toxicity is used in a mixture at a concentration ≥1%, and the mixture has not been classified based on testing of the mixture as a whole, the mixture cannot be attributed a definitive acute toxicity estimate. In this situation the mixture is classified based on the known ingredients only.
Note: A statement that × percent of the mixture consists of ingredient(s) of unknown acute (oral/dermal/inhalation) toxicity is required on the label and safety data sheet in such cases; see appendix C to this section, Allocation of Label Elements and appendix D to this section, Safety Data Sheets).
A.1.3.6.2.4 If the total concentration of the relevant ingredient(s) with unknown acute toxicity is ≤10% then the formula presented in A.1.3.6.1 must be used. If the total concentration of the relevant ingredient(s) with unknown acute toxicity is >10%, the formula presented in A.1.3.6.1 is corrected to adjust for the percentage of the unknown ingredient(s) as follows:
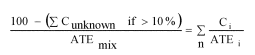
| Exposure routes | Classification category or experimentally obtained acute toxicity range estimate | Converted acute toxicity point estimate |
|---|---|---|
| Oral (mg/kg bodyweight) | 0 < Category 1 ≤ 5 | 0.5 |
| 5 < Category 2 ≤ 50 | 5 | |
| 50 < Category 3 ≤ 300 | 100 | |
| 300 < Category 4 ≤ 2000 | 500 | |
| Dermal (mg/kg bodyweight) | 0 < Category 1 ≤ 50 | 5 |
| 50 < Category 2 ≤ 200 | 50 | |
| 200 < Category 3 ≤ 1000 | 300 | |
| 1000 < Category 4 ≤ 2000 | 1100 | |
| Gases (ppmV) | 0 < Category 1 ≤ 100 | 10 |
| 100 < Category 2 ≤ 500 | 100 | |
| 500 < Category 3 ≤ 2500 | 700 | |
| 2500 < Category 4 ≤ 20000 | 4500 | |
| Vapors (mg/l) | 0 < Category 1 ≤ 0.5 | 0.05 |
| 0.5 < Category 2 ≤ 2.0 | 0.5 | |
| 2.0 < Category 3 ≤ 10.0 | 3 | |
| 10.0 < Category 4 ≤ 20.0 | 11 | |
| Dust/mist (mg/l) | 0 < Category 1 ≤ 0.05 | 0.005 |
| 0.05 < Category 2 ≤ 0.5 | 0.05 | |
| 0.5 < Category 3 ≤ 1.0 | 0.5 | |
| 1.0 < Category 4 ≤ 5.0 | 1.5 | |
| Note: Gas concentrations are expressed in parts per million per volume (ppmV). | ||
A.2 Skin Corrosion/Irritation
A.2.1 Definitions and General Considerations
A.2.1.1 Skin corrosion refers to the production of irreversible damage to the skin; namely, visible necrosis through the epidermis and into the dermis occurring after initial exposure to a substance or mixture.
Skin irritation refers to the production of reversible damage to the skin occurring after initial exposure to a substance or mixture.
A.2.1.2 To classify, all available and relevant information on skin corrosion/irritation is collected and its quality in terms of adequacy and reliability is assessed. Wherever possible classification should be based on data generated using internationally validated and accepted methods, such as OECD Test Guidelines (TG) or equivalent methods. Sections A.2.2.1 to A.2.2.6 provide classification criteria for the different types of information that may be available.
A.2.1.3 A tiered approach (see A.2.2.7) organizes the available information into levels/tiers and provides for decision-making in a structured and sequential manner. Classification results directly when the information consistently satisfies the criteria. However, where the available information gives inconsistent and/or conflicting results within a tier, classification of a substance or a mixture is made on the basis of the weight of evidence within that tier. In some cases when information from different tiers gives inconsistent and/or conflicting results (see A.2.2.7.3) or where data individually are insufficient to conclude on the classification, an overall weight of evidence approach is used (see A.0.3).
A.2.2 Classification Criteria for Substances
Substances shall be allocated to one of the following categories within this hazard class:
(a) Category 1 (Skin Corrosion)
This category may be further divided into up to three sub-categories (1A, 1B, and 1C).
Corrosive substances should be classified in Category 1 where data are not sufficient for sub-categorization.
When data are sufficient, substances may be classified in one of the three sub-categories 1A, 1B, or 1C.
(b) Category 2 (Skin Irritation)
A.2.2.1 Classification Based on Standard Human Data
Existing reliable and good quality human data on skin corrosion/irritation should be given high weight for classification. Existing human data could be derived from single or repeated exposure(s), for example in occupational, consumer, transport or emergency response scenarios and epidemiological and clinical studies in well-documented case reports and observations (see A.0.2.6 and A.0.3). Although human data from accident or poison center databases can provide evidence for classification, absence of incidents is not itself evidence for no classification, as exposures are generally unknown or uncertain.
A.2.2.2 Classification Based on Standard Animal Test Data
OECD TG 404 is the currently available internationally validated and accepted animal test for classification as skin corrosive or irritant (See Table A.2.1 and A.2.2) and is the standard animal test. The current version of OECD TG 404 uses a maximum of 3 animals. Results from animal studies conducted under previous versions of OECD TG 404 that used more than 3 animals are also considered standard animal tests.
A.2.2.2.1 Skin Corrosion
A.2.2.2.1.1 A substance is corrosive to the skin when it produces destruction of skin tissue, namely, visible necrosis through the epidermis and into the dermis, in at least one tested animal after initial exposure up to a 4-hour duration.
A.2.2.2.1.2 Three sub-categories of Category 1 are provided in Table A.2.1, all of which shall be regulated as Category 1.
| Criteria | |
|---|---|
| Category 1 | Destruction of skin tissue, namely, visible necrosis through the epidermis and into the dermis, in at least one tested animal after exposure ≤4 h. |
| Sub-category 1A | Corrosive responses in at least one animal following exposure ≤3 min during an observation period ≤1 h. |
| Sub-category 1B | Corrosive responses in at least one animal following exposure >3 min and ≤1 h and observations ≤14 days. |
| Sub-category 1C | Corrosive responses in at least one animal after exposures >1 h and ≤ 4 h and observations ≤14 days. |
| a The use of human data is discussed in A.2.2.1. | |
A.2.2.2.2 Skin Irritation
A.2.2.2.2.1 A substance is irritant to skin when it produces reversible damage to the skin following its application for up to 4 hours.
A.2.2.2.2.2 A single irritant category (Category 2) is presented in the Table A.2.2. A substance is irritant to skin, when after the first application, it produces reversible damage to the skin following its application for up to 4 hours. An irritation category (Category 2) is provided that:
(a) recognizes that some test substances may lead to effects which persist throughout the length of the test; and
(b) acknowledges that animal responses in a test may be variable.
A.2.2.2.2.3 Reversibility of skin lesions is another consideration in evaluating irritant responses. When inflammation persists to the end of the observation period in two or more test animals, taking into consideration alopecia (limited area), hyperkeratosis, hyperplasia and scaling, then a chemical should be considered to be an irritant.
A.2.2.2.2.4 Animal irritant responses within a test can be quite variable, as they are with corrosion. A separate irritant criterion accommodates cases when there is a significant irritant response but less than the mean score criterion for a positive test. For example, a substance should be designated as an irritant if at least 1 of 3 tested animals shows a very elevated mean score according to test method used throughout the study, including lesions persisting at the end of an observation period of normally 14 days. Other responses should also fulfil this criterion. However, it should be ascertained that the responses are the result of chemical exposure. Addition of this criterion increases the sensitivity of the classification system.
| Criteria | |
|---|---|
| a Grading criteria are understood as described in OECD Test Guideline 404. | |
| Irritant (Category 2) | (1) Mean score of ≥2.3 ≤4.0 for erythema/eschar or for edema in at least 2 of 3 testedanimals from gradings at 24, 48, 72 hours after patch removal or, if reactions are delayed, from grades on 3 consecutive days after the onset of skin reactions; or |
| (2) Inflammation that persists to the end of the observation period normally 14 days in a least 2 animals, particularly taking into account alopecia (limited area), hyperkeratosis, hyperplasia, and scaling; or | |
| (3) In some cases where there is pronounced variability of response among animals, with very definite positive effects related to chemical exposure in a single animal but less than the criteria above. | |
A.2.2.3 Classification Based on In Vitro/Ex Vivo Data
A.2.2.3.1 The currently available individual in vitro/ex vivo test methods address either skin irritation or skin corrosion, but do not address both endpoints in one single test. Therefore, classification based solely on in vitro/ex vivo test results may require data from more than one method.
A.2.2.3.2 Wherever possible classification should be based on data generated using internationally validated and accepted in vitro/ex vivo test methods, and the classification criteria provided in these test methods needs to be applied. In vitro/ex vivo data can only be used for classification when the tested substance is within the applicability domain of the test methods used. Additional limitations described in the published literature should also be taken into consideration.
A.2.2.3.3 Skin corrosion
A.2.2.3.3.1 Where tests have been undertaken in accordance with OECD Test Guidelines (TGs) 430, 431, or 435, a substance is classified for skin corrosion in category 1 (and, where possible and required into sub-categories 1A, 1B, or 1C).
A.2.2.3.3.2 Some in vitro/ex vivo methods do not allow differentiation between sub-categories 1B and 1C. Where existing in vitro/ex vivo data cannot distinguish between the sub-categories, additional information has to be taken into account to differentiate between these two sub-categories. Where no or insufficient additional information is available, category 1 is applied.
A.2.2.3.3.3 A substance identified as not corrosive should be considered for classification as skin irritant.
A.2.2.3.4 Skin irritation
A.2.2.3.4.1 Where a conclusion of corrosivity can be excluded and where tests have been undertaken in accordance with OECD Test Guideline 439, a substance is classified for skin irritation in category 2.
A.2.2.3.4.2 A negative result in an internationally accepted and validated in vitro/ex vivo test for skin irritation, e.g., OECD TG 439, can be used to conclude as not classified for skin irritation.
A.2.2.4 Classification Based on Other, Existing Skin Data in Animals
Other existing skin data in animals may be used for classification, but there may be limitations regarding the conclusions that can be drawn if a substance is highly toxic via the dermal route, an in vivo skin corrosion/irritation study may not have been conducted since the amount of test substance to be applied would considerably exceed the toxic dose and, consequently, would result in the death of the animals. When observations of skin corrosion/irritation in acute toxicity studies are made, these data may be used for classification, provided that the dilutions used and species tested are relevant. Solid substances (powders) may become corrosive or irritant when moistened or in contact with moist skin or mucous membranes. This is generally indicated in the standardized test methods.
A.2.2.5 Classification Based on Chemical Properties
Skin effects may be indicated by pH extremes such as ≤2 and ≥11.5 especially when associated with significant acid/alkaline reserve (buffering capacity). Generally, such substances are expected to produce significant effects on the skin. In the absence of any other information, a substance is considered corrosive (Skin Category 1) if it has a pH ≤2 or a pH ≥11.5. However, if consideration of acid/alkaline reserve suggests the substance may not be corrosive despite the low or high pH, this needs to be confirmed by other data, preferably from an appropriate validated in vitro/ex vivo test. Buffering capacity and pH can be determined by test methods including OECD TG 122.
A.2.2.6 Classification Based on Non-Test Methods
A.2.2.6.1 Classification, including non-classification, can be based on non-test methods, with due consideration of reliability and applicability, on a case-by-case basis. Such methods include computer models predicting qualitative structure-activity relationships (structural alerts, SAR); quantitative structure-activity relationships (QSARs); computer expert systems; and read-across using analogue and category approaches.
A.2.2.6.2 Read-across using analogue or category approaches requires sufficiently reliable test data on similar substance(s) and justification of the similarity of the tested substance(s) with the substance(s) to be classified. Where adequate justification of the read-across approach is provided, it has in general higher weight than (Q)SARs.
A.2.2.6.3 Classification based on (Q)SARs requires sufficient data and validation of the model. The validity of the computer models and the prediction should be assessed using internationally recognized principles for the validation of (Q)SARs. With respect to reliability, lack of alerts in a SAR or expert system is not sufficient evidence for no classification.
A.2.2.7 Classification in a Tiered Approach
A.2.2.7.1 A tiered approach to the evaluation of initial information should be considered, where applicable (Figure A.2.1), recognizing that not all elements may be relevant. However, all available and relevant information of sufficient quality needs to be examined for consistency with respect to the resulting classification.
A.2.2.7.2 In the tiered approach (Figure A.2.1), existing human and animal data form the highest tier, followed by in vitro/ex vivo data, other existing skin data in animals, and then other sources of information. Where information from data within the same tier is inconsistent and/or conflicting, the conclusion from that tier is determined by a weight of evidence approach.
A.2.2.7.3 Where information from several tiers is inconsistent and/or conflicting with respect to the resulting classification, information of sufficient quality from a higher tier is generally given a higher weight than information from a lower tier. However, when information from a lower tier would result in a stricter classification than information from a higher tier and there is concern for misclassification, then classification is determined by an overall weight of evidence approach. The same would apply in the case where there is human data indicating irritation but positive results from an in vitro/ex vivo test for corrosion.
Figure A.2.1—Application of the Tiered Approach for Skin Corrosion and Irritation
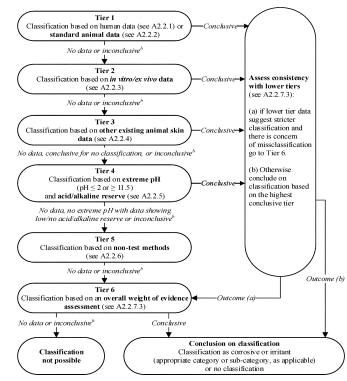
(a) Before applying the approach, the explanatory text in A.2.2.7 should be consulted. Only adequate and reliable data of sufficient quality should be included in applying the tiered approach.
(b) Information may be inconclusive for various reasons, e.g.:
—The available data may be of insufficient quality, or otherwise insufficient/inadequate for the purpose of classification, e.g., due to quality issues related to experimental design and/or reporting.
—The available data may be insufficient to conclude on the classification, e.g., they might be adequate to demonstrate irritancy, but inadequate to demonstrate absence of corrosivity.
—The method used to generate the available data may not be suitable for concluding on no classification (see A.2.2. for details). Specifically, in vitro/ex vivo and non-test methods need to be validated explicitly for this purpose.
A.2.3 Classification Criteria for Mixtures
A.2.3.1 Classification of Mixtures When Data Are Available for the Complete Mixture
A.2.3.1.1 In general, the mixture shall be classified using the criteria for substances, taking into account the tiered approach to evaluate data for this hazard class (as illustrated in Figure A.2.1) and A.2.3.1.2 and A.2.3.1.3. If classification is not possible using the tiered approach, then the approach described in A.2.3.2, or, if that is not applicable A.2.2.3.3 should be followed.
A.2.3.1.2 In vitro/ex vivo data generated from validated test methods may not have been validated using mixtures; although these methods are considered broadly applicable to mixtures, they can only be used for classification of mixtures when all ingredients of the mixture fall within the applicability domain of the test methods used. Specific limitations regarding applicability domains are described in the respective test methods, and should be taken into consideration as well as any further information on the limitations from the published literature. Where there is reason to assume or evidence indicating that the applicability domain of a particular test method is limited, data interpretation should be exercised with caution, or the results should be considered not applicable.
A.2.3.1.3 In the absence of any other information, a mixture is considered corrosive (Skin Category 1) if it has a pH ≤2 or a pH ≥11.5. However, if consideration of acid/alkaline reserve suggests the mixture may not be corrosive despite the low or high pH value, this needs to be confirmed by other data, preferably from an appropriate validated in vitro/ex vivo test.
A.2.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.2.3.2.1 Where the mixture itself has not been tested to determine its skin corrosion/irritation potential, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles, as found in paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.2.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.2.3.3.1 In order to make use of all available data for purposes of classifying the skin corrosion/irritation hazards of mixtures, the following assumption has been made and is applied where appropriate in the tiered approach:
The “relevant ingredients” of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases.). If the classifier has reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for skin corrosion/irritation, that ingredient shall also be considered relevant.
A.2.3.3.2 In general, the approach to classification of mixtures as corrosive or irritant to the skin when data are available on the ingredients, but not on the mixture as a whole, is based on the theory of additivity, such that each corrosive or irritant ingredient contributes to the overall corrosive or irritant properties of the mixture in proportion to its potency and concentration. A weighting factor of 10 is used for corrosive ingredients when they are present at a concentration below the concentration limit for classification with Category 1, but are at a concentration that will contribute to the classification of the mixture as an irritant. The mixture is classified as corrosive or irritant when the sum of the concentrations of such ingredients exceeds a cut-off value/concentration limit.
A.2.3.3.3 Table A.2.3 below provides the cut-off value/concentration limits to be used to determine if the mixture is considered to be corrosive or irritant to the skin.
A.2.3.3.4 Particular care shall be taken when classifying certain types of chemicals such as acids and bases, inorganic salts, aldehydes, phenols, and surfactants. The approach explained in A.2.3.3.1 and A.2.3.3.2 might not work given that many of such substances are corrosive or irritant at concentrations <1%. For mixtures containing strong acids or bases the pH should be used as classification criteria since pH will be a better indicator of corrosion than the concentration limits in Table A.2.3. A mixture containing corrosive or irritant ingredients that cannot be classified based on the additivity approach shown in Table A.2.3, due to chemical characteristics that make this approach unworkable, should be classified as skin corrosion Category 1 if it contains ≥1% of a corrosive ingredient and as skin irritation Category 2 when it contains ≥3% of an irritant ingredient. Classification of mixtures with ingredients for which the approach in Table A.2.3 does not apply is summarized in Table A.2.4 below.
A.2.3.3.5 On occasion, reliable data may show that the skin corrosion/irritation of an ingredient will not be evident when present at a level above the generic cut-off values/concentration limits mentioned in Tables A.2.3 and A.2.4. In these cases the mixture could be classified according to those data (See Use of cut-off values/concentration limits, paragraph A.0.4.3 of this Appendix).
A.2.3.3.6 If there are data showing that (an) ingredient(s) may be corrosive or irritant to skin at a concentration of <1% (corrosive) or <3% (irritant), the mixture shall be classified accordingly (See Use of cut-off values/concentration limits, paragraph A.0.4.3 of this Appendix).
| Sum of ingredients classified as: | Concentration triggering classification of a mixture as: | |
|---|---|---|
| Skin corrosive | Skin irritant | |
| Category 1 | Category 2 | |
| Skin Category 1 | ≥5% | ≥1% but <5% |
| Skin Category 2 | ≥10% | |
| (10 × Skin Category 1) + Skin Category 2 | ≥10% | |
| Note: Where data are available and the sub-categories of skin Category 1 (corrosive) are used, the sum of all ingredients of a mixture classified as sub-category 1A, 1B or 1C respectively, must each be ≥5% in order to classify the mixture as either skin sub-category 1A, 1B or 1C. Where the sum of 1A ingredients is <5% but the sum of 1A + 1B ingredients is ≥5%, the mixture must be classified as sub-category 1B. Similarly, where the sum of 1A + 1B ingredients is <5% but the sum of 1A + 1B + 1C ingredients is ≥5% the mixture must be classified as sub-category 1C. Where at least one relevant ingredient in a mixture is classified as a Category 1 categorization, the mixture must be classified as Category 1 without sub-categorization if the sum of all ingredients corrosive to skin is ≥5%. | ||
| Ingredient | Concentration (percent) | Mixture classified as: Skin |
|---|---|---|
| Acid with pH ≤2 | ≥1 | Category 1. |
| Base with pH ≥11.5 | ≥1 | Category 1. |
| Other corrosive (Category 1) ingredient | ≥1 | Category 1. |
| Other irritant (Category 2) ingredient, including acids and bases | ≥ 3 | Category 2. |
A.3 Serious Eye Damage/Eye Irritation
A.3.1 Definitions and General Considerations
A.3.1.1 Serious eye damage refers to the production of tissue damage in the eye, or serious physical decay of vision, which is not fully reversible, occurring after exposure of the eye to a substance or mixture.
Eye irritation refers to the production of changes in the eye, which are fully reversible, occurring after exposure of the eye to a substance or mixture.
A.3.1.2 Serious eye damage/eye irritation shall be classified using a tiered approach as detailed in Figure A.3.1. Emphasis shall be placed upon existing human data (See A.0.2.6), followed by existing animal data, followed by in vitro data and then other sources of information. Classification results directly when the data satisfy the criteria in this section. In case the criteria cannot be directly applied, classification of a substance or a mixture is made on the basis of the total weight of evidence (See A.0.3.1). This means that all available information bearing on the determination of serious eye damage/eye irritation is considered together, including the results of appropriate scientifically validated in vitro tests, relevant animal data, and human data such as epidemiological and clinical studies and well-documented case reports and observations.
A.3.2 Classification Criteria for Substances
Substances are allocated to one of the categories within this hazard class, Category 1 (serious eye damage) or Category 2 (eye irritation), as follows:
(a) Category 1 (serious eye damage/irreversible effects on the eye): substances that have the potential to seriously damage the eyes (see Table A.3.1).
(b) Category 2 (eye irritation/reversible effects on the eye): substances that have the potential to induce reversible eye irritation (see Table A.3.2).
A.3.2.1 Classification Based on Standard Animal Test Data
A.3.2.1.1 Serious eye damage (Category 1)/Irreversible effects on the eye
A single hazard category is provided in Table A.3.1, for substances that have the potential to seriously damage the eyes. Category 1, irreversible effects on the eye, includes the criteria listed below. These observations include animals with grade 4 cornea lesions and other severe reactions (e.g., destruction of cornea) observed at any time during the test, as well as persistent corneal opacity, discoloration of the cornea by a dye substance, adhesion, pannus, and interference with the function of the iris or other effects that impair sight. In this context, persistent lesions are considered those which are not fully reversible within an observation period of normally 21 days. Category 1 also contains substances fulfilling the criteria of corneal opacity ≥ 3 and/or iritis > 1.5 observed in at least 2 of 3 tested animals detected in a Draize eye test with rabbits, because severe lesions like these usually do not reverse within a 21-day observation period.
| Criteria | |
|---|---|
| Category 1: Serious eye damage/Irreversible effects on the eye | A substance that produces:
calculated as the mean scores following grading at 24, 48 and 72 hours after instillation of the test material. |
| a Grading criteria are understood as described in OECD Test Guideline 405. | |
A.3.2.1.2 Eye irritation (category 2)/reversible effects on the eye
A single Category 2 is provided in Table A.3.2 for substances that have the potential to induce reversible eye irritation.
When data are available, substances may be classified into Category 2A and Category 2B:
(a) For substances inducing eye irritant effects reversing within an observation time of normally 21 days, Category 2A applies.
(b) For substances inducing eye irritant effects reversing within an observation time of 7 days, Category 2B applies.
When a substance is classified as Category 2, without further categorization, the classification criteria are the same as those for 2A.
A.3.2.1.3 For those substances where there is pronounced variability among animal responses this information must be taken into account in determining the classification.
| Criteria | |
|---|---|
| Substances that have the potential to induce reversible eye irritation. | |
| Category 2/2A | Substances that produce in at least 2 of 3 tested animals a positive response of:
calculated as the mean scores following grading at 24, 48 and 72 hours after instillation of the test material, and which fully reverses within an observation period of normally 21 days. |
| Category 2B | Within Category 2A an eye irritant is considered mildly irritating to eyes (Category 2B) when the effects listed above are fully reversible within 7 days of observation. |
| a Grading criteria are understood as described in OECD Test Guideline 405. | |
A.3.2.2 Classification in a Tiered Approach
A.3.2.2.1 A tiered approach to the evaluation of initial information shall be used where applicable, recognizing that all elements may not be relevant in certain cases (Figure A.3.1).
A.3.2.2.2 Existing human and animal data should be the first line of analysis, as they give information directly relevant to effects on the eye. Possible skin corrosion shall be evaluated prior to consideration of any testing for serious eye damage/eye irritation in order to avoid testing for local effects on eyes with skin corrosive substances.
A.3.2.2.3 In vitro alternatives that have been validated and accepted should be used to make classification decisions.
A.3.2.2.4 Likewise, pH extremes like ≤2 and ≥11.5, may indicate serious eye damage, especially when associated with significant acid/alkaline reserve (buffering capacity). Generally, such substances are expected to produce significant effects on the eyes. In the absence of any other information, a substance is considered to cause serious eye damage (Category 1) if it has a pH ≤2 or ≥11.5. However, if consideration of acid/alkaline reserve suggests the substance may not cause serious eye damage despite the low or high pH value, this needs to be confirmed by other data, preferably by data from an appropriate validated in vitro test.
A.3.2.2.5 In some cases sufficient information may be available from structurally related substances to make classification decisions.
A.3.2.2.6 The tiered approach provides guidance on how to organize existing information and to make a weight-of-evidence decision about hazard assessment and hazard classification (ideally without conducting new animal tests). Animal testing with corrosive substances should be avoided wherever possible. Although information might be gained from the evaluation of single parameters within a tier, consideration should be given to the totality of existing information and making an overall weight of evidence determination. This is especially true when there is conflict in information available on some parameters.
A.3.2.2.7 The tiered approach explains how to organize existing information and to make a weight-of-evidence decision about hazard assessment and hazard classification. Although information might be gained from the evaluation of single parameters within a tier, consideration should be given to the totality of existing information and making an overall weight of evidence determination. This is especially true when there is conflict in information available.
Figure A.3.1—Tiered Evaluation for Serious Eye Damage and Eye Irritation (See Also Figure A.2.1)

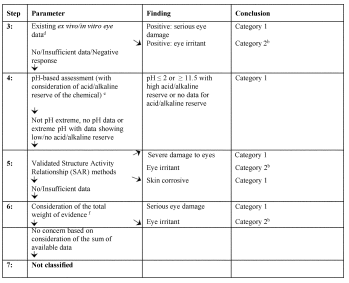
a Existing human or animal data could be derived from single or repeated exposure(s), for example in occupational, consumer, transport, or emergency response scenarios; or from purposely-generated data from animal studies conducted according to validated and internationally accepted test methods. Although human data from accident or poison center databases can provide evidence for classification, absence of incidents is not itself evidence for no classification as exposures are generally unknown or uncertain;
b Classify in the appropriate category as applicable;
c Existing animal data should be carefully reviewed to determine if sufficient serious eye damage/eye irritation evidence is available through other, similar information. It is recognized that not all skin irritants are eye irritants. Expert judgment should be exercised prior to making such a determination;
d Evidence from studies using validated protocols with isolated human/animal tissues or other non-tissue-based, validated protocols should be assessed. Examples of internationally accepted, validated test methods for identifying eye corrosives and severe irritants (i.e., Serious Eye Damage) include OECD Test Guidelines 437 (Bovine Corneal Opacity and Permeability (BCOP)), 438 (Isolated Chicken Eye (ICE) and 460 (Fluorescein leakage (FL)). Presently there are no validated and internationally accepted in vitro test methods for identifying eye irritation. A positive test result from a validated in vitro test on skin corrosion would lead to the conclusion to classify as causing serious eye damage;
e Measurement of pH alone may be adequate, but assessment of acid/alkaline reserve (buffering capacity) would be preferable. Presently, there is no validated and internationally accepted method for assessing this parameter;
f All information that is available on a substance must be considered and an overall determination made on the total weight of evidence. This is especially true when there is conflict in information available on some parameters. The weight of evidence including information on skin irritation may lead to classification for eye irritation. Negative results from applicable validated in vitro tests are considered in the total weight of evidence evaluation.
A.3.3 Classification Criteria for Mixtures
A.3.3.1 Classification of Mixtures When Data Are Available for the Complete Mixture
A.3.3.1.1 The mixture will be classified using the criteria for substances, and taking into account the tiered approach to evaluate data for this hazard class (as illustrated in Figure A.3.1).
A.3.3.1.2 When considering testing of the mixture, chemical manufacturers shall use a tiered approach as included in the criteria for classification of substances for skin corrosion and serious eye damage and eye irritation to help ensure an accurate classification, as well as to avoid unnecessary animal testing. In the absence of any other information, a mixture is considered to cause serious eye damage (Category 1) if it has a pH ≤2 or ≥11.5. However, if consideration of acid/alkaline reserve suggests the mixture may not have the potential to cause serious eye damage despite the low or high pH value, then further evaluation may be necessary.
A.3.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.3.3.2.1 Where the mixture itself has not been tested to determine its skin corrosivity or potential to cause serious eye damage or eye irritation, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles, as found in paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, and Aerosols.
A.3.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.3.3.3.1 For purposes of classifying the serious eye damage/eye irritation hazards of mixtures in the tiered approach:
The “relevant ingredients” of a mixture are those which are present in concentrations ≥1% (weight/weight for solids, liquids, dusts, mists and vapors and volume/volume for gases.) If the classifier has reason to suspect that an ingredient present at a concentration <1% will affect classification of the mixture for serious eye damage/eye irritation, that ingredient shall also be considered relevant.
A.3.3.3.2 In general, the approach to classification of mixtures as seriously damaging to the eye or eye irritant when data are available on the ingredients, but not on the mixture as a whole, is based on the theory of additivity, such that each skin corrosive or serious eye damage/eye irritant ingredient contributes to the overall serious eye damage/eye irritation properties of the mixture in proportion to its potency and concentration. A weighting factor of 10 is used for skin corrosive and serious eye damaging ingredients when they are present at a concentration below the concentration limit for classification with Category 1, but are at a concentration that will contribute to the classification of the mixture as serious eye damaging/eye irritant. The mixture is classified as seriously damaging to the eye or eye irritant when the sum of the concentrations of such ingredients exceeds a threshold cut-off value/concentration limit.
A.3.3.3.3 Table A.3.3 provides the cut-off value/concentration limits to be used to determine if the mixture must be classified as seriously damaging to the eye or an eye irritant.
A.3.3.3.4 Particular care must be taken when classifying certain types of chemicals such as acids and bases, inorganic salts, aldehydes, phenols, and surfactants. The approach explained in A.3.3.3.1 and A.3.3.3.2 might not work given that many of such substances are seriously damaging to the eye/eye irritating at concentrations <1%. For mixtures containing strong acids or bases, the pH should be used as classification criteria (See A.3.3.1.2) since pH will be a better indicator of serious eye damage (subject to consideration of acid/alkali reserve) than the concentration limits of Table A.3.3. A mixture containing skin corrosive or serious eye damaging/eye irritating ingredients that cannot be classified based on the additivity approach applied in Table A.3.3 due to chemical characteristics that make this approach unworkable, should be classified as serious eye damage (Category 1) if it contains ≥1% of a skin corrosive or serious eye damaging ingredient and as Eye Irritation (Category 2) when it contains ≥3% of an eye irritant ingredient. Classification of mixtures with ingredients for which the approach in Table A.3.3 does not apply is summarized in Table A.3.4.
A.3.3.3.5 On occasion, reliable data may show that the irreversible/reversible eye effects of an ingredient will not be evident when present at a level above the generic cut-off values/concentration limits mentioned in Tables A.3.3 and A.3.4. In these cases the mixture could be classified according to those data (See also A.0.4.3 Use of cut-off values/concentration limits"). On occasion, when it is expected that the skin corrosion/irritation or the reversible/irreversible eye effects of an ingredient will not be evident when present at a level above the generic concentration/cut-off levels mentioned in Tables A.3.3 and A.3.4, testing of the mixture may be considered. In those cases, the tiered weight of evidence approach should be applied as referred to in section A.3.2, Figure A.3.1 and explained in detail in this chapter.
A.3.3.3.6 If there are data showing that (an) ingredient(s) may be corrosive to the skin or seriously damaging to the eye/eye irritating at a concentration of ≤1% (corrosive to the skin or seriously damaging to the eye) or ≤3% (eye irritant), the mixture shall be classified accordingly (See also paragraph A.0.4.3, Use of cut-off values/concentration limits).
| Sum of ingredients classified as | Concentration triggering classification of a mixture as | |
|---|---|---|
| Serious eye damage | Eye irritation | |
| Category 1 | Category 2/2A | |
| Skin corrosion (Category 1) + Serious eye damage (Category 1) a | ≥3% | ≥1% but <3% |
| Eye irritation (Category 2) | ≥10% b | |
| 10 × (Skin corrosion (Category 1) + Serious eye damage (Category 1)) a + Eye irritation (Category 2) | ≥10% | |
| Notes: a If an ingredient is classified as both skin Category 1 and eye Category 1 its concentration is considered only once in the calculation. b A mixture may be classified as Eye Irritation Category 2B in cases when all relevant ingredients are classified as Eye Irritation Category 2B. | ||
| Ingredient | Concentration (percent) | Mixture classified as |
|---|---|---|
| Acid with pH <2 | ≥1 | Serious eye damage (Category 1). |
| Base with pH ≥11.5 | ≥1 | Serious eye damage (Category 1). |
| Other skin corrosive or serious eye damage (Category 1) ingredients | ≥1 | Serious eye damage (Category 1). |
| Other eye irritant (Category 2) ingredients | ≥3 | Eye irritation (Category 2). |
A.4 Respiratory or Skin Sensitization
A.4.1 Definitions and General Considerations
A.4.1.1 Respiratory sensitization refers to hypersensitivity of the airways occurring after inhalation of a substance or mixture.
Skin sensitization refers to an allergic response occurring after skin contact with a substance or mixture.
A.4.1.2 For the purpose of this chapter, sensitization includes two phases: the first phase is induction of specialized immunological memory in an individual by exposure to an allergen. The second phase is elicitation, i.e., production of a cell-mediated or antibody-mediated allergic response by exposure of a sensitized individual to an allergen.
A.4.1.3 For respiratory sensitization, the pattern of induction followed by elicitation phases is shared in common with skin sensitization. For skin sensitization, an induction phase is required in which the immune system learns to react; clinical symptoms can then arise when subsequent exposure is sufficient to elicit a visible skin reaction (elicitation phase). As a consequence, predictive tests usually follow this pattern in which there is an induction phase, the response to which is measured by a standardized elicitation phase, typically involving a patch test. The local lymph node assay is the exception, directly measuring the induction response. Evidence of skin sensitization in humans normally is assessed by a diagnostic patch test.
A.4.1.4 Usually, for both skin and respiratory sensitization, lower levels are necessary for elicitation than are required for induction.
A.4.1.5 The hazard class “respiratory or skin sensitization” is differentiated into:
(a) Respiratory sensitization; and
(b) Skin sensitization
A.4.2 Classification Criteria for Substances
A.4.2.1 Respiratory Sensitizers
A.4.2.1.1 Hazard Categories
A.4.2.1.1.1 Effects seen in either humans or animals will normally justify classification in a weight of evidence approach for respiratory sensitizers. Substances may be allocated to one of the two sub-categories 1A or 1B using a weight of evidence approach in accordance with the criteria given in Table A.4.1 and on the basis of reliable and good quality evidence from human cases or epidemiological studies and/or observations from appropriate studies in experimental animals.
A.4.2.1.1.2 Where data are not sufficient for sub-categorization, respiratory sensitizers shall be classified in Category 1.
| Category 1 | Respiratory sensitizer |
|---|---|
| A substance is classified as a respiratory sensitizer | |
| (a) if there is evidence in humans that the substance can lead to specific respiratory hypersensitivity and/or | |
| (b) if there are positive results from an appropriate animal test. 1 | |
| Sub-category 1A | Substances showing a high frequency of occurrence in humans; or a probability of occurrence of a high sensitization rate in humans based on animal or other tests. 1 Severity of reaction may also be considered. |
| Sub-category 1B | Substances showing a low to moderate frequency of occurrence in humans; or a probability of occurrence of a low to moderate sensitization rate in humans based on animal or other tests. 1 Severity of reaction may also be considered. |
| 1 As of May 20, 2024, recognized and validated animal models for the testing of respiratory hypersensitivity are not available. Under certain circumstances, data from animal studies may provide valuable information in a weight of evidence assessment. | |
A.4.2.1.2 Human Evidence
A.4.2.1.2.1 Evidence that a substance can lead to specific respiratory hypersensitivity will normally be based on human experience. In this context, hypersensitivity is normally seen as asthma, but other hypersensitivity reactions such as rhinitis/conjunctivitis and alveolitis are also considered. The condition will have the clinical character of an allergic reaction. However, immunological mechanisms do not have to be demonstrated.
A.4.2.1.2.2 When considering the human evidence, it is necessary that in addition to the evidence from the cases, the following be taken into account:
(a) The size of the population exposed;
(b) The extent of exposure.
A.4.2.1.3 The evidence referred to above could be:
(a) Clinical history and data from appropriate lung function tests related to exposure to the substance, confirmed by other supportive evidence which may include:
(i) In vivo immunological test (e.g., skin prick test);
(ii) In vitro immunological test (e.g., serological analysis);
(iii) Studies that may indicate other specific hypersensitivity reactions where immunological mechanisms of action have not been proven, e.g., repeated low-level irritation, pharmacologically mediated effects;
(iv) A chemical structure related to substances known to cause respiratory hypersensitivity;
(b) Data from positive bronchial challenge tests with the substance conducted according to accepted guidelines for the determination of a specific hypersensitivity reaction.
A.4.2.1.2.4 Clinical history should include both medical and occupational history to determine a relationship between exposure to a specific substance and development of respiratory hypersensitivity. Relevant information includes aggravating factors both in the home and workplace, the onset and progress of the disease, family history and medical history of the patient in question. The medical history should also include a note of other allergic or airway disorders from childhood and smoking history.
A.4.2.1.2.5 The results of positive bronchial challenge tests are considered to provide sufficient evidence for classification on their own. It is, however, recognized that in practice many of the examinations listed above will already have been carried out.
A.4.2.1.3 Animal studies
A.4.2.1.2.3 Data from appropriate animal studies 2 which may be indicative of the potential of a substance to cause sensitization by inhalation in humans 3 may include:
2 At this writing, recognized and validated animal models for the testing of respiratory hypersensitivity are not available. Under certain circumstances, data from animal studies may provide valuable information in a weight of evidence assessment.
3 The mechanisms by which substances induce symptoms of asthma are not yet fully known. For preventive measures, these substances are considered respiratory sensitizers. However, if on the basis of the evidence, it can be demonstrated that these substances induce symptoms of asthma by irritation only in people with bronchial hyperactivity, they should not be considered as respiratory sensitizers.
(a) Measurements of Immunoglobulin E (IgE) and other specific immunological parameters, for example in mice
(b) Specific pulmonary responses in guinea pigs.
A.4.2.2 Skin Sensitizers
A.4.2.2.1 Hazard categories
A.4.2.2.1.1 Effects seen in either humans or animals will normally justify classification in a weight of evidence approach for skin sensitizers. Substances may be allocated to one of the two sub-categories 1A or 1B using a weight of evidence approach in accordance with the criteria given in Table A.4.2 and on the basis of reliable and good quality evidence from human cases or epidemiological studies and/or observations from appropriate studies in experimental animals according to the guidance values provided in A.4.2.2.2.1 and A.4.2.2.3.2 for sub-category 1A and in A.4.2.2.2.2 and A.4.2.2.3.3 for sub-category 1B.
A.4.2.2.1.2 Where data are not sufficient for sub-categorization, skin sensitizers shall be classified in Category 1.
| Category 1 | Skin sensitizer |
|---|---|
| A substance is classified as a skin sensitizer (a) if there is evidence in humans that the substance can lead to sensitization by skin contact in a substantial number of persons, or (b) if there are positive results from an appropriate animal test. | |
| Sub-category 1A | Substances showing a high frequency of occurrence in humans and/or a high potency in animals can be presumed to have the potential to produce significant sensitization in humans. Severity of reaction may also be considered. |
| Sub-category 1B | Substances showing a low to moderate frequency of occurrence in humans and/or a low to moderate potency in animals can be presumed to have the potential to produce sensitization in humans. Severity of reaction may also be considered. |
A.4.2.2.2 Human Evidence
A.4.2.2.2.1 Human evidence for sub-category 1A may include:
(a) Positive responses at ≤500 μg/cm2 (Human Repeat Insult Patch Test (HRIPT), Human Maximization Test (HMT)—induction threshold);
(b) Diagnostic patch test data where there is a relatively high and substantial incidence of reactions in a defined population in relation to relatively low exposure;
(c) Other epidemiological evidence where there is a relatively high and substantial incidence of allergic contact dermatitis in relation to relatively low exposure.
A.4.2.2.2.2 Human evidence for sub-category 1B may include:
(a) Positive responses at >500 μg/cm2 (HRIPT, HMT—induction threshold);
(b) Diagnostic patch test data where there is a relatively low but substantial incidence of reactions in a defined population in relation to relatively high exposure;
(c) Other epidemiological evidence where there is a relatively low but substantial incidence of allergic contact dermatitis in relation to relatively high exposure.
A.4.2.2.3 Animal Studies
A.4.2.2.3.1 For Category 1, when an adjuvant type test method for skin sensitization is used, a response of at least 30% of the animals is considered as positive. For a non-adjuvant Guinea pig test method, a response of at least 15% of the animals is considered positive. For Category 1, a stimulation index of three or more is considered a positive response in the local lymph node assay. 4
4 Test methods for skin sensitization are described in OECD Guideline 406 (the Guinea Pig Maximization test and the Buehler guinea pig test) and Guideline 429 (Local Lymph Node Assay). Other methods may be used provided that they are scientifically validated. The Mouse Ear Swelling Test (MEST), appears to be a reliable screening test to detect moderate to strong sensitizers, and can be used, in accordance with professional judgment, as a first stage in the assessment of skin sensitization potential.
A.4.2.2.3.2 Animal test results for sub-category 1A can include data with values indicated in the following Table A.4.3:
| Assay | Criteria |
|---|---|
| Local lymph node assay | EC3 value ≤2%. |
| Guinea pig maximization test | ≥30% responding at ≤0.1% intradermal induction dose or ≥60% responding at >0.1% to ≤1% intradermal induction dose. |
| Buehler assay | ≥15% responding at ≤0.2% topical induction dose or ≥60% responding at >0.2% to ≤20% topical induction dose. |
| Note: EC3 refers to the estimated concentration of test chemical required to induce a stimulation index of 3 in the local lymph node assay. | |
A.4.2.2.3.3 Animal test results for sub-category 1B can include data with values indicated in Table A.4.4 below:
| Assay | Criteria |
|---|---|
| Local lymph node assay | EC3 value >2%. |
| Guinea pig maximization test | ≥30% to <60% responding at >0.1% to ≤1% intradermal induction dose or ≥30% responding at >1% intradermal induction dose. |
| Buehler assay | ≥15% to <60% responding at >0.2% to ≤20% topical induction dose or ≥15% responding at >20% topical induction dose. |
| Note: EC3 refers to the estimated concentration of test chemical required to induce a stimulation index of 3 in the local lymph node assay. | |
A.4.2.2.4 Specific Considerations
A.4.2.2.4.1 For classification of a substance, evidence shall include one or more of the following using a weight of evidence approach:
(a) Positive data from patch testing, normally obtained in more than one dermatology clinic;
(b) Epidemiological studies showing allergic contact dermatitis caused by the substance. Situations in which a high proportion of those exposed exhibit characteristic symptoms are to be looked at with special concern, even if the number of cases is small;
(c) Positive data from appropriate animal studies;
(d) Positive data from experimental studies in humans (See paragraph A.0.2.6 of this Appendix);
(e) Well documented episodes of allergic contact dermatitis, normally obtained in more than one dermatology clinic;
(f) Severity of reaction.
A.4.2.2.4.2 Evidence from animal studies is usually much more reliable than evidence from human exposure. However, in cases where evidence is available from both sources, and there is conflict between the results, the quality and reliability of the evidence from both sources must be assessed in order to resolve the question of classification on a case-by-case basis. Normally, human data are not generated in controlled experiments with volunteers for the purpose of hazard classification but rather as part of risk assessment to confirm lack of effects seen in animal tests. Consequently, positive human data on skin sensitization are usually derived from case-control or other, less defined studies. Evaluation of human data must, therefore, be carried out with caution as the frequency of cases reflect, in addition to the inherent properties of the substances, factors such as the exposure situation, bioavailability, individual predisposition and preventive measures taken. Negative human data should not normally be used to negate positive results from animal studies. For both animal and human data, consideration should be given to the impact of vehicle.
A.4.2.2.4.3 If none of the above-mentioned conditions are met, the substance need not be classified as a skin sensitizer. However, a combination of two or more indicators of skin sensitization, as listed below, may alter the decision. This shall be considered on a case-by-case basis.
(a) Isolated episodes of allergic contact dermatitis;
(b) Epidemiological studies of limited power, e.g., where chance, bias or confounders have not been ruled out fully with reasonable confidence;
(c) Data from animal tests, performed according to existing guidelines, which do not meet the criteria for a positive result described in A.4.2.2.3, but which are sufficiently close to the limit to be considered significant;
(d) Positive data from non-standard methods;
(e) Positive results from close structural analogues.
A.4.2.2.4.4 Immunological contact urticaria
A.4.2.2.4.4.1 Substances meeting the criteria for classification as respiratory sensitizers may, in addition, cause immunological contact urticaria. Consideration shall be given to classifying these substances as skin sensitizers.
A.4.2.2.4.4.2 Substances which cause immunological contact urticaria without meeting the criteria for respiratory sensitizers shall be considered for classification as skin sensitizers.
A.4.2.2.4.4.3 There is no recognized animal model available to identify substances which cause immunological contact urticaria. Therefore, classification will normally be based on human evidence, similar to that for skin sensitization.
A.4.3 Classification Criteria for Mixtures
A.4.3.1 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence, as described in the criteria for substances, from human experience or appropriate studies in experimental animals, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of these data. Care must be exercised in evaluating data on mixtures that the dose used does not render the results inconclusive.
A.4.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.4.3.2.1 Where the mixture itself has not been tested to determine its sensitizing properties, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following agreed bridging principles as found in paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category/subcategory, Substantially similar mixtures, and Aerosols.
A.4.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
The mixture shall be classified as a respiratory or skin sensitizer when at least one ingredient has been classified as a respiratory or skin sensitizer and is present at or above the appropriate cut-off value/concentration limit for the specific endpoint as shown in Table A.4.5.
| Ingredient classified as | Cut-off values/concentration limits triggering classification of a mixture as | ||
|---|---|---|---|
| Respiratory sensitizer Category 1 | Skin sensitizer
Category 1 | ||
| Solid/liquid (%) | Gas (%) | All physical states (%) | |
| Respiratory Sensitizer Category 1 | ≥0.1 | ≥0.1 | |
| Respiratory Sensitizer Sub-category 1A | ≥0.1 | ≥0.1 | |
| Respiratory Sensitizer Sub-category 1B | ≥1.0 | ≥0.2 | |
| Skin Sensitizer Category 1 | ≥0.1 | ||
| Skin Sensitizer Sub-category 1A | ≥0.1 | ||
| Skin Sensitizer Sub-category 1B | ≥1.0 | ||
A.5 Germ Cell Mutagenicity
A.5.1 Definitions and General Considerations
A.5.1.1 Germ cell mutagenicity refers to heritable gene mutations, including heritable structure and numerical chromosome aberrations in germ cells occurring after exposure to a substance or mixture.
A.5.1.2 A mutation is defined as a permanent change in the amount or structure of the genetic material in a cell. The term mutation applies both to heritable genetic changes that may be manifested at the phenotypic level and to the underlying DNA modifications when known (including, for example, specific base pair changes and chromosomal translocations). The term mutagenic and mutagen will be used for agents giving rise to an increased occurrence of mutations in populations of cells and/or organisms.
A.5.1.3 The more general terms genotoxic and genotoxicity apply to agents or processes which alter the structure, information content, or segregation of DNA, including those which cause DNA damage by interfering with normal replication processes, or which in a non-physiological manner (temporarily) alter its replication. Genotoxicity test results are usually taken as indicators for mutagenic effects.
A.5.1.4 This hazard class is primarily concerned with chemicals that may cause mutations in the germ cells of humans that can be transmitted to the progeny. However, mutagenicity/genotoxicity tests in vitro and in mammalian somatic cells in vivo are also considered in classifying substances and mixtures within this hazard class.
A.5.2 Classification Criteria for Substances
A.5.2.1 The classification system provides for two different categories of germ cell mutagens to accommodate the weight of evidence available. The two-category system is described in the Figure A.5.1.
Figure A.5.1—Hazard Categories for Germ Cell Mutagens
CATEGORY 1: Substances known to induce heritable mutations or to be regarded as if they induce heritable mutations in the germ cells of humans
Category 1A: Substances known to induce heritable mutations in germ cells of humans
Positive evidence from human epidemiological studies.
Category 1B: Substances which should be regarded as if they induce heritable mutations in the germ cells of humans
(a) Positive result(s) from in vivo heritable germ cell mutagenicity tests in mammals; or
(b) Positive result(s) from in vivo somatic cell mutagenicity tests in mammals, in combination with some evidence that the substance has potential to cause mutations to germ cells. This supporting evidence may, for example, be derived from mutagenicity/genotoxic tests in germ cells in vivo, or by demonstrating the ability of the substance or its metabolite(s) to interact with the genetic material of germ cells; or
(c) Positive results from tests showing mutagenic effects in the germ cells of humans, without demonstration of transmission to progeny; for example, an increase in the frequency of aneuploidy in sperm cells of exposed people.
CATEGORY 2: Substances which cause concern for humans owing to the possibility that they may induce heritable mutations in the germ cells of humans
Positive evidence obtained from experiments in mammals and/or in some cases from in vitro experiments, obtained from:
(a) Somatic cell mutagenicity tests in vivo, in mammals; or
(b) Other in vivo somatic cell genotoxicity tests which are supported by positive results from in vitro mutagenicity assays.
Note: Substances which are positive in in vitro mammalian mutagenicity assays, and which also show structure activity relationship to known germ cell mutagens, should be considered for classification as Category 2 mutagens.
A.5.2.2 Specific considerations for classification of substances as germ cell mutagens:
A.5.2.2.1 To arrive at a classification, test results are considered from experiments determining mutagenic and/or genotoxic effects in germ and/or somatic cells of exposed animals. Mutagenic and/or genotoxic effects determined in in vitro tests shall also be considered.
A.5.2.2.2 The system is hazard based, classifying chemicals on the basis of their intrinsic ability to induce mutations in germ cells. The scheme is, therefore, not meant for the (quantitative) risk assessment of chemical substances.
A.5.2.2.3 Classification for heritable effects in human germ cells is made on the basis of scientifically validated tests. Evaluation of the test results shall be done using expert judgment and all the available evidence shall be weighed for classification.
A.5.2.2.4 The classification of substances shall be based on the total weight of evidence available, using expert judgment. In those instances where a single well-conducted test is used for classification, it shall provide clear and unambiguously positive results. The relevance of the route of exposure used in the study of the substance compared to the route of human exposure should also be taken into account.
A.5.3 Classification Criteria for Mixtures 5
5 It should be noted that the classification criteria for health hazards usually include a tiered scheme in which test data available on the complete mixture are considered as the first tier in the evaluation, followed by the applicable bridging principles, and lastly, cut-off values/concentration limits or additivity. However, this approach is not used for Germ Cell Mutagenicity. These criteria for Germ Cell Mutagenicity consider the cut-off values/concentration limits as the primary tier and allow the classification to be modified only on a case-by-case evaluation based on available test data for the mixture as a whole.
A.5.3.1 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.5.3.1.1 Classification of mixtures shall be based on the available test data for the individual ingredients of the mixture using cut-off values/concentration limits for the ingredients classified as germ cell mutagens.
A.5.3.1.2 The mixture will be classified as a mutagen when at least one ingredient has been classified as a Category 1A, Category 1B or Category 2 mutagen and is present at or above the appropriate cut-off value/concentration limit as shown in Table A.5.1 below for Category 1 and 2 respectively.
| Ingredient classified as | Cut-off/concentration limits triggering classification of a mixture as: | |
|---|---|---|
| Category 1 mutagen | Category 2 mutagen | |
| Category 1A/B mutagen | ≥0.1% | |
| Category 2 mutagen | ≥1.0% | |
| Note: The cut-off values/concentration limits in the table above apply to solids and liquids (w/w units) as well as gases (v/v units). | ||
A.5.3.2 Classification of Mixtures When Data Are Available for the Mixture Itself
The classification may be modified on a case-by-case basis based on the available test data for the mixture as a whole. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of germ cell mutagenicity test systems.
A.5.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.5.3.3.1 Where the mixture itself has not been tested to determine its germ cell mutagenicity hazard, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution, Batching, and Substantially similar mixtures.
A.5.4 Examples of Scientifically Validated Test Methods
A.5.4.1 Examples of in vivo heritable germ cell mutagenicity tests are:
(a) Rodent dominant lethal mutation test (OECD 478)
(b) Mouse heritable translocation assay (OECD 485)
(c) Mouse specific locus test
A.5.4.2 Examples of in vivo somatic cell mutagenicity tests are:
(a) Mammalian bone marrow chromosome aberration test (OECD 475)
(b) Mammalian erythrocyte micronucleus test (OECD 474)
A.5.4.3 Examples of mutagenicity/genotoxicity tests in germ cells are:
(a) Mutagenicity tests:
(i) Mammalian spermatogonial chromosome aberration test (OECD 483)
(ii) Spermatid micronucleus assay
(b) Genotoxicity tests:
(i) Sister chromatid exchange analysis in spermatogonia
(ii) Unscheduled DNA synthesis test (UDS) in testicular cells
A.5.4.4 Examples of genotoxicity tests in somatic cells are:
(a) Liver Unscheduled DNA Synthesis (UDS) in vivo (OECD 486)
(b) Mammalian bone marrow Sister Chromatid Exchanges (SCE)
A.5.4.5 Examples of in vitro mutagenicity tests are:
(a) In vitro mammalian chromosome aberration test (OECD 473)
(b) In vitro mammalian cell gene mutation test (OECD 476)
(c) Bacterial reverse mutation tests (OECD 471)
A.5.4.6 As new, scientifically validated tests arise, these may also be used in the total weight of evidence to be considered.
A.6 Carcinogenicity
A.6.1 Definitions
Carcinogenicity refers to the induction of cancer or an increase in the incidence of cancer occurring after exposure to a substance or mixture. Substances and mixtures which have induced benign and malignant tumors in well-performed experimental studies on animals are considered also to be presumed or suspected human carcinogens unless there is strong evidence that the mechanism of tumor formation is not relevant for humans.
Classification of a substance or mixture as posing a carcinogenic hazard is based on its inherent properties and does not provide information on the level of the human cancer risk which the use of the substance or mixture may represent.
A.6.2 Classification Criteria for Substances 6
6 See Non-mandatory appendix F of this section, part A for further guidance regarding hazard classification for carcinogenicity. This appendix is consistent with the GHS and is provided as guidance excerpted from the International Agency for Research on Cancer (IARC) “Monographs on the Evaluation of Carcinogenic Risks to Humans” (2006).
A.6.2.1 For the purpose of classification for carcinogenicity, substances are allocated to one of two categories based on strength of evidence and additional weight of evidence considerations. In certain instances, route-specific classification may be warranted.
Figure A.6.1—Hazard Categories for Carcinogens
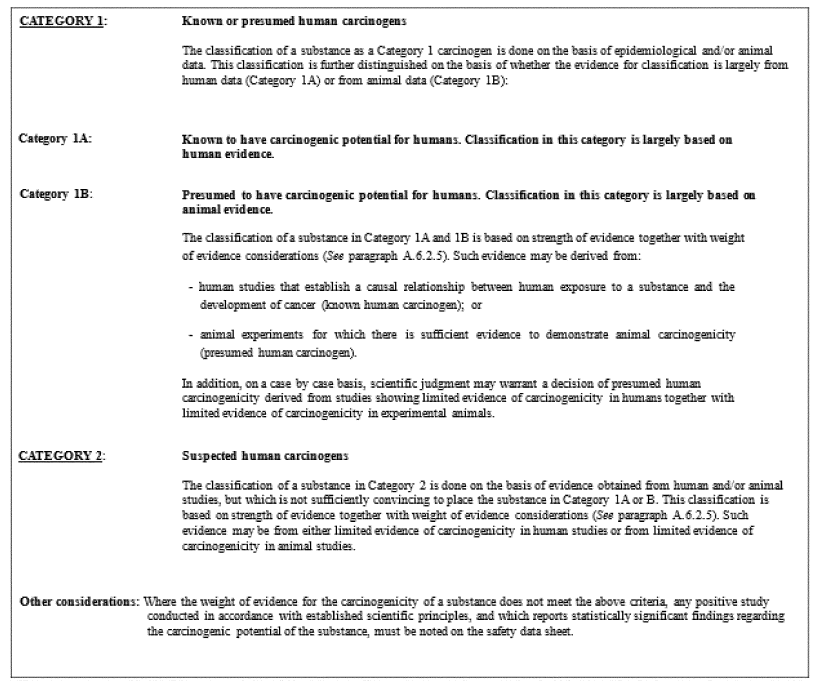
CATEGORY 1: Known or presumed human carcinogens
The placing of a substance in Category 1 is done on the basis of epidemiological and/or animal data. An individual substance may be further distinguished:
Category 1A: Known to have carcinogenic potential for humans; the placing of a substance is largely based on human evidence.
Category 1B: Presumed to have carcinogenic potential for humans; the placing of a substance is largely based on animal evidence.
Based on strength of evidence together with additional considerations, such evidence may be derived from human studies that establish a causal relationship between human exposure to a substance and the development of cancer (known human carcinogen). Alternatively, evidence may be derived from animal experiments for which there is sufficient evidence to demonstrate animal carcinogenicity (presumed human carcinogen). In addition, on a case by case basis, scientific judgement may warrant a decision of presumed human carcinogenicity derived from studies showing limited evidence of carcinogenicity in humans together with limited evidence of carcinogenicity in experimental animals.
Classification: Category 1 (A and B) Carcinogen
CATEGORY 2: Suspected human carcinogens
The placing of a substance in Category 2 is done on the basis of evidence obtained from human and/or animal studies, but which is not sufficiently convincing to place the substance in Category 1. Based on strength of evidence together with additional considerations, such evidence may be from either limited evidence of carcinogenicity in human studies or from limited evidence of carcinogenicity in animal studies.
Classification: Category 2 Carcinogen
A.6.2.2 Classification as a carcinogen is made on the basis of evidence from reliable and acceptable methods, and is intended to be used for substances which have an intrinsic property to produce such toxic effects. The evaluations are to be based on all existing data, peer-reviewed published studies and additional data accepted by regulatory agencies.
A.6.2.3 Carcinogen classification is a one-step, criterion-based process that involves two interrelated determinations: evaluations of strength of evidence and consideration of all other relevant information to place substances with human cancer potential into hazard categories.
A.6.2.4 Strength of evidence involves the enumeration of tumors in human and animal studies and determination of their level of statistical significance. Sufficient human evidence demonstrates causality between human exposure and the development of cancer, whereas sufficient evidence in animals shows a causal relationship between the agent and an increased incidence of tumors. Limited evidence in humans is demonstrated by a positive association between exposure and cancer, but a causal relationship cannot be stated. Limited evidence in animals is provided when data suggest a carcinogenic effect, but are less than sufficient. (Guidance on consideration of important factors in the classification of carcinogenicity and a more detailed description of the terms “limited” and “sufficient” have been developed by the International Agency for Research on Cancer (IARC) and are provided in non-mandatory appendix F of this section.)
A.6.2.5 Weight of evidence: Beyond the determination of the strength of evidence for carcinogenicity, a number of other factors should be considered that influence the overall likelihood that an agent may pose a carcinogenic hazard in humans. The full list of factors that influence this determination is very lengthy, but some of the important ones are considered here.
A.6.2.5.1 These factors can be viewed as either increasing or decreasing the level of concern for human carcinogenicity. The relative emphasis accorded to each factor depends upon the amount and coherence of evidence bearing on each. Generally, there is a requirement for more complete information to decrease than to increase the level of concern. Additional considerations should be used in evaluating the tumor findings and the other factors in a case-by-case manner.
A.6.2.5.2 Some important factors which may be taken into consideration, when assessing the overall level of concern are:
(a) Tumor type and background incidence;
(b) Multisite responses;
(c) Progression of lesions to malignancy;
(d) Reduced tumor latency;
Additional factors which may increase or decrease the level of concern include:
(e) Whether responses are in single or both sexes;
(f) Whether responses are in a single species or several species;
(g) Structural similarity or not to a substance(s) for which there is good evidence of carcinogenicity;
(h) Routes of exposure;
(i) Comparison of absorption, distribution, metabolism and excretion between test animals and humans;
(j) The possibility of a confounding effect of excessive toxicity at test doses; and,
(k) Mode of action and its relevance for humans, such as mutagenicity, cytotoxicity with growth stimulation, mitogenesis, immunosuppression.
Mutagenicity: It is recognized that genetic events are central in the overall process of cancer development. Therefore, evidence of mutagenic activity in vivo may indicate that a substance has a potential for carcinogenic effects.
A.6.2.5.3 A substance that has not been tested for carcinogenicity may in certain instances be classified in Category 1A, Category 1B, or Category 2 based on tumor data from a structural analogue together with substantial support from consideration of other important factors such as formation of common significant metabolites, e.g., for benzidine congener dyes.
A.6.2.5.4 The classification should also take into consideration whether or not the substance is absorbed by a given route(s); or whether there are only local tumors at the site of administration for the tested route(s), and adequate testing by other major route(s) show lack of carcinogenicity.
A.6.2.5.5 It is important that whatever is known of the physico-chemical, toxicokinetic and toxicodynamic properties of the substances, as well as any available relevant information on chemical analogues, i.e., structure activity relationship, is taken into consideration when undertaking classification.
A.6.3 Classification Criteria for Mixtures 7
7 It should be noted that the classification criteria for health hazards usually include a tiered scheme in which test data available on the complete mixture are considered as the first tier i the evaluation, followed by the applicable bridging principles, and lastly, cut-off values/concentration limit or addivity. However, this approach is not used for Carcinogenicity. These criteria for Carcinogenicity consider the cut-off values/concentration limits as the primary tier and allow the classification to be modified only on a case-by-case evaluation based on available test data for the mixture as a whole.
A.6.3.1 The mixture shall be classified as a carcinogen when at least one ingredient has been classified as a Category 1 or Category 2 carcinogen and is present at or above the appropriate cut-off value/concentration limit as shown in Table A.6.1.
| Ingredient classified as | Category 1 carcinogen | Category 2 carcinogen |
| Category 1 carcinogen | ≥0.1% | |
| Category 2 carcinogen | ≥0.1% (note 1) | |
| Note: If a Category 2 carcinogen ingredient is present in the mixture at a concentration between 0.1% and 1%, information is required on the SDS for a product. However, a label warning is optional. If a Category 2 carcinogen ingredient is present in the mixture at a concentration of ≥1%, both an SDS and a label is required and the information must be included on each. | ||
A.6.3.2 Classification of mixtures when data are available for the complete mixture
A mixture may be classified based on the available test data for the mixture as a whole. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of carcinogenicity test systems.
A.6.3.3 Classification of mixtures when data are not available for the complete mixture: bridging principles
Where the mixture itself has not been tested to determine its carcinogenic hazard, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data will be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution; Batching; and Substantially similar mixtures.
A.6.4 Classification of Carcinogenicity 8
8 See Non-mandatory appendix f of this section for further guidance regarding hazard classification for carcinogenicity and how to relate carcinogenicity classification information from IARC and NTP to GHS.
A.6.4.1 Chemical manufacturers, importers and employers evaluating chemicals may treat the following sources as establishing that a substance is a carcinogen or potential carcinogen for hazard communication purposes in lieu of applying the criteria described herein:
A.6.4.1.1 National Toxicology Program (NTP), “Report on Carcinogens” (latest edition);
A.6.4.1.2 International Agency for Research on Cancer (IARC) “Monographs on the Evaluation of Carcinogenic Risks to Humans” (latest editions)
A.6.4.2 Where OSHA has included cancer as a health hazard to be considered by classifiers for a chemical covered by 29 CFR part 1910, subpart Z, chemical manufacturers, importers, and employers shall classify the chemical as a carcinogen.
A.7 Reproductive Toxicity
A.7.1 Definitions and General Considerations
A.7.1.1 Reproductive toxicity refers to adverse effects on sexual function and fertility in adult males and females, as well as developmental toxicity in the offspring, occurring after exposure to a substance or mixture. Some reproductive toxic effects cannot be clearly assigned to either impairment of sexual function and fertility or to developmental toxicity. Nonetheless, substances and mixtures with these effects shall be classified as reproductive toxicants. For classification purposes, the known induction of genetically based inheritable effects in the offspring is addressed in Germ cell mutagenicity (See A.5).
A.7.1.2 Adverse effects on sexual function and fertility means any effect of chemicals that interferes with reproductive ability or sexual capacity. This includes, but is not limited to, alterations to the female and male reproductive system, adverse effects on onset of puberty, gamete production and transport, reproductive cycle normality, sexual behavior, fertility, parturition, pregnancy outcomes, premature reproductive senescence, or modifications in other functions that are dependent on the integrity of the reproductive systems.
A.7.1.3 Adverse effects on development of the offspring means any effect of chemicals which interferes with normal development of the conceptus either before or after birth, which is induced during pregnancy or results from parental exposure. These effects can be manifested at any point in the life span of the organism. The major manifestations of developmental toxicity include death of the developing organism, structural abnormality, altered growth and functional deficiency.
A.7.1.4 Adverse effects on or via lactation are also included in reproductive toxicity, but for classification purposes, such effects are treated separately (See A.7.2.1).
A.7.2 Classification Criteria for Substances
A.7.2.1 For the purpose of classification for reproductive toxicity, substances shall be classified in one of two categories in accordance with Figure A.7.1(a). Effects on sexual function and fertility, and on development, shall be considered. In addition, effects on or via lactation shall be classified in a separate hazard category in accordance with Figure A.7.1(b).
Figure A.7.1(a)—Hazard Categories for Reproductive Toxicants

CATEGORY 1: Known or presumed human reproductive toxicant
This category includes substances which are known to have produced an adverse effect on sexual function and fertility or on development in humans or for which there is evidence from animal studies, possibly supplemented with other information, to provide a strong presumption that the substance has the capacity to interfere with reproduction in humans. For regulatory purposes, a substance can be further distinguished on the basis of whether the evidence for classification is primarily from human data (Category 1A) or from animal data (Category 1B).
CATEGORY 1A: Known human reproductive toxicant
The placing of the substance in this category is largely based on evidence from humans.
CATEGORY 1B: Presumed human reproductive toxicant
The placing of the substance in this category is largely based on evidence from experimental animals. Data from animal studies should provide clear evidence of an adverse effect on sexual function and fertility or on development in the absence of other toxic effects, or if occurring together with other toxic effects the adverse effect on reproduction is considered not to be a secondary non-specific consequence of other toxic effects. However, when there is mechanistic information that raises doubt about the relevance of the effect for humans, classification in Category 2 may be more appropriate.
CATEGORY 2: Suspected human reproductive toxicant
This category includes substances for which there is some evidence from humans or experimental animals, possibly supplemented with other information, of an adverse effect on sexual function and fertility, or on development, in the absence of other toxic effects, or if occurring together with other toxic effects the adverse effect on reproduction is considered not to be a secondary non-specific consequence of the other toxic effects, and where the evidence is not sufficiently convincing to place the substance in Category 1. For instance, deficiencies in the study may make the quality of evidence less convincing, and in view of this Category 2 could be the more appropriate classification.
Figure A.7.1(b)—Hazard Category for Effects on or Via Lactation
Effects on or via lactation are allocated to a separate category. It is appreciated that for many substances there is no information on the potential to cause adverse effects on the offspring via lactation. However, substances which are absorbed by women and have been shown to interfere with lactation, or which may be present (including metabolites) in breast milk in amounts sufficient to cause concern for the health of a breastfed child, should be classified to indicate this property.
Classification for effects via lactation shall be assigned on the basis of:
(a) absorption, metabolism, distribution and excretion studies that would indicate the likelihood the substance would be present in potentially toxic levels in breast milk; and/or
(b) results of one or two generation studies in animals which provide clear evidence of adverse effect in the offspring due to transfer in the milk or adverse effect on the quality of the milk; and/or
(c) human evidence indicating a hazard to babies during the lactation period.
A.7.2.2 Basis of Classification
A.7.2.2.1 Classification is made on the basis of the criteria, outlined above, an assessment of the total weight of evidence, and the use of expert judgment. Classification as a reproductive toxicant is intended to be used for substances which have an intrinsic, specific property to produce an adverse effect on reproduction and substances should not be so classified if such an effect is produced solely as a non-specific secondary consequence of other toxic effects.
A.7.2.2.2 In the evaluation of toxic effects on the developing offspring, it is important to consider the possible influence of maternal toxicity.
A.7.2.2.3 For human evidence to provide the primary basis for a Category 1A classification there must be reliable evidence of an adverse effect on reproduction in humans. Evidence used for classification shall be from well conducted epidemiological studies, if available, which include the use of appropriate controls, balanced assessment, and due consideration of bias or confounding factors. Less rigorous data from studies in humans may be sufficient for a Category 1A classification if supplemented with adequate data from studies in experimental animals, but classification in Category 1B may also be considered.
A.7.2.3 Weight of Evidence
A.7.2.3.1 Classification as a reproductive toxicant is made on the basis of an assessment of the total weight of evidence using expert judgment. This means that all available information that bears on the determination of reproductive toxicity is considered together. Included is information such as epidemiological studies and case reports in humans and specific reproduction studies along with sub-chronic, chronic and special study results in animals that provide relevant information regarding toxicity to reproductive and related endocrine organs. Evaluation of substances chemically related to the material under study may also be included, particularly when information on the material is scarce. The weight given to the available evidence will be influenced by factors such as the quality of the studies, consistency of results, nature and severity of effects, level of statistical significance for intergroup differences, number of endpoints affected, relevance of route of administration to humans and freedom from bias. Both positive and negative results are considered together in a weight of evidence determination. However, a single, positive study performed according to good scientific principles and with statistically or biologically significant positive results may justify classification (See also A.7.2.2.3).
A.7.2.3.2 Toxicokinetic studies in animals and humans, site of action and mechanism or mode of action study results may provide relevant information, which could reduce or increase concerns about the hazard to human health. If it is conclusively demonstrated that the clearly identified mechanism or mode of action has no relevance for humans or when the toxicokinetic differences are so marked that it is certain that the hazardous property will not be expressed in humans then a chemical which produces an adverse effect on reproduction in experimental animals should not be classified.
A.7.2.3.3 In some reproductive toxicity studies in experimental animals the only effects recorded may be considered of low or minimal toxicological significance and classification may not necessarily be the outcome. These effects include, for example, small changes in semen parameters or in the incidence of spontaneous defects in the fetus, small changes in the proportions of common fetal variants such as are observed in skeletal examinations, or in fetal weights, or small differences in postnatal developmental assessments.
A.7.2.3.4 Data from animal studies shall provide sufficient evidence of specific reproductive toxicity in the absence of other systemic toxic effects. However, if developmental toxicity occurs together with other toxic effects in the dam (mother), the potential influence of the generalized adverse effects should be assessed to the extent possible. The preferred approach is to consider adverse effects in the embryo/fetus first, and then evaluate maternal toxicity, along with any other factors which are likely to have influenced these effects, as part of the weight of evidence. In general, developmental effects that are observed at maternally toxic doses should not be automatically discounted. Discounting developmental effects that are observed at maternally toxic doses can only be done on a case-by-case basis when a causal relationship is established or refuted.
A.7.2.3.5 If appropriate information is available it is important to try to determine whether developmental toxicity is due to a specific maternally mediated mechanism or to a non-specific secondary mechanism, like maternal stress and the disruption of homeostasis. Generally, the presence of maternal toxicity should not be used to negate findings of embryo/fetal effects, unless it can be clearly demonstrated that the effects are secondary non-specific effects. This is especially the case when the effects in the offspring are significant, e.g., irreversible effects such as structural malformations. In some situations it is reasonable to assume that reproductive toxicity is due to a secondary consequence of maternal toxicity and discount the effects, for example if the chemical is so toxic that dams fail to thrive and there is severe inanition; they are incapable of nursing pups; or they are prostrate or dying.
A.7.2.4 Maternal Toxicity
A.7.2.4.1 Development of the offspring throughout gestation and during the early postnatal stages can be influenced by toxic effects in the mother either through non-specific mechanisms related to stress and the disruption of maternal homeostasis, or by specific maternally-mediated mechanisms. So, in the interpretation of the developmental outcome to decide classification for developmental effects it is important to consider the possible influence of maternal toxicity. This is a complex issue because of uncertainties surrounding the relationship between maternal toxicity and developmental outcome. Expert judgment and a weight of evidence approach, using all available studies, shall be used to determine the degree of influence to be attributed to maternal toxicity when interpreting the criteria for classification for developmental effects. The adverse effects in the embryo/fetus shall be first considered, and then maternal toxicity, along with any other factors which are likely to have influenced these effects, as weight of evidence, to help reach a conclusion about classification.
A.7.2.4.2 Based on pragmatic observation, it is believed that maternal toxicity may, depending on severity, influence development via non-specific secondary mechanisms, producing effects such as depressed fetal weight, retarded ossification, and possibly resorptions and certain malformations in some strains of certain species. However, the limited numbers of studies which have investigated the relationship between developmental effects and general maternal toxicity have failed to demonstrate a consistent, reproducible relationship across species. Developmental effects which occur even in the presence of maternal toxicity are considered to be evidence of developmental toxicity, unless it can be unequivocally demonstrated on a case by case basis that the developmental effects are secondary to maternal toxicity. Moreover, classification shall be considered where there is a significant toxic effect in the offspring, e.g., irreversible effects such as structural malformations, embryo/fetal lethality, or significant post-natal functional deficiencies.
A.7.2.4.3 Classification shall not automatically be discounted for chemicals that produce developmental toxicity only in association with maternal toxicity, even if a specific maternally-mediated mechanism has been demonstrated. In such a case, classification in Category 2 may be considered more appropriate than Category 1. However, when a chemical is so toxic that maternal death or severe inanition results, or the dams (mothers) are prostrate and incapable of nursing the pups, it is reasonable to assume that developmental toxicity is produced solely as a secondary consequence of maternal toxicity and discount the developmental effects. Classification is not necessarily the outcome in the case of minor developmental changes, e.g., a small reduction in fetal/pup body weight or retardation of ossification when seen in association with maternal toxicity.
A.7.2.4.4 Some of the endpoints used to assess maternal toxicity are provided below. Data on these endpoints, if available, shall be evaluated in light of their statistical or biological significance and dose-response relationship.
(a) Maternal mortality: An increased incidence of mortality among the treated dams over the controls shall be considered evidence of maternal toxicity if the increase occurs in a dose-related manner and can be attributed to the systemic toxicity of the test material. Maternal mortality greater than 10% is considered excessive and the data for that dose level shall not normally be considered to need further evaluation.
(b) Mating index (Number of animals with seminal plugs or sperm/Number of mated × 100)
(c) Fertility index (Number of animals with implants/Number of matings × 100)
(d) Gestation length (If allowed to deliver)
(e) Body weight and body weight change: Consideration of the maternal body weight change and/or adjusted (corrected) maternal body weight shall be included in the evaluation of maternal toxicity whenever such data are available. The calculation of an adjusted (corrected) mean maternal body weight change, which is the difference between the initial and terminal body weight minus the gravid uterine weight (or alternatively, the sum of the weights of the fetuses), may indicate whether the effect is maternal or intrauterine. In rabbits, the body weight gain may not be a useful indicator of maternal toxicity because of normal fluctuations in body weight during pregnancy.
(f) Food and water consumption (if relevant): The observation of a significant decrease in the average food or water consumption in treated dams (mothers) compared to the control group may be useful in evaluating maternal toxicity, particularly when the test material is administered in the diet or drinking water. Changes in food or water consumption must be evaluated in conjunction with maternal body weights when determining if the effects noted are reflective of maternal toxicity or more simply, unpalatability of the test material in feed or water.
(g) Clinical evaluations (including clinical signs, markers, and hematology and clinical chemistry studies): The observation of increased incidence of significant clinical signs of toxicity in treated dams (mothers) relative to the control group is useful in evaluating maternal toxicity. If this is to be used as the basis for the assessment of maternal toxicity, the types, incidence, degree and duration of clinical signs shall be reported in the study. Clinical signs of maternal intoxication include, but are not limited to: coma, prostration, hyperactivity, loss of righting reflex, ataxia, or labored breathing.
(h) Post-mortem data: Increased incidence and/or severity of post-mortem findings may be indicative of maternal toxicity. This can include gross or microscopic pathological findings or organ weight data, including absolute organ weight, organ-to-body weight ratio, or organ-to-brain weight ratio. When supported by findings of adverse histopathological effects in the affected organ(s), the observation of a significant change in the average weight of suspected target organ(s) of treated dams (mothers), compared to those in the control group, may be considered evidence of maternal toxicity.
A.7.2.5 Animal and Experimental Data
A.7.2.5.1 A number of scientifically validated test methods are available, including methods for developmental toxicity testing (e.g., OECD Test Guideline 414, ICH Guideline S5A, 1993), methods for peri- and post-natal toxicity testing (e.g., ICH S5B, 1995), and methods for one or two-generation toxicity testing (e.g., OECD Test Guidelines 415, 416, 443).
A.7.2.5.2 Results obtained from screening tests (e.g., OECD Guidelines 421—Reproduction/Developmental Toxicity Screening Test, and 422—Combined Repeated Dose Toxicity Study with Reproduction/Development Toxicity Screening Test) can also be used to justify classification, although the quality of this evidence is less reliable than that obtained through full studies.
A.7.2.5.3 Adverse effects or changes, seen in short- or long-term repeated dose toxicity studies, which are judged likely to impair reproductive function and which occur in the absence of significant generalized toxicity, may be used as a basis for classification, e.g., histopathological changes in the gonads.
A.7.2.5.4 Evidence from in vitro assays, or non-mammalian tests, and from analogous substances using structure-activity relationship (SAR), can contribute to the procedure for classification. In all cases of this nature, expert judgment must be used to assess the adequacy of the data. Inadequate data shall not be used as a primary support for classification.
A.7.2.5.5 It is preferable that animal studies are conducted using appropriate routes of administration which relate to the potential route of human exposure. However, in practice reproductive toxicity studies are commonly conducted using the oral route, and such studies will normally be suitable for evaluating the hazardous properties of the substance with respect to reproductive toxicity. However, if it can be conclusively demonstrated that the clearly identified mechanism or mode of action has no relevance for humans or when the toxicokinetic differences are so marked that it is certain that the hazardous property will not be expressed in humans then a substance which produces an adverse effect on reproduction in experimental animals should not be classified.
A.7.2.5.6 Studies involving routes of administration such as intravenous or intraperitoneal injection, which may result in exposure of the reproductive organs to unrealistically high levels of the test substance, or elicit local damage to the reproductive organs, e.g., by irritation, must be interpreted with extreme caution and on their own are not normally the basis for classification.
A.7.2.5.7 There is general agreement about the concept of a limit dose, above which the production of an adverse effect may be considered to be outside the criteria which lead to classification. Some test guidelines specify a limit dose, other test guidelines qualify the limit dose with a statement that higher doses may be necessary if anticipated human exposure is sufficiently high that an adequate margin of exposure would not be achieved. Also, due to species differences in toxicokinetics, establishing a specific limit dose may not be adequate for situations where humans are more sensitive than the animal model.
A.7.2.5.8 In principle, adverse effects on reproduction seen only at very high dose levels in animal studies (for example doses that induce prostration, severe inappetence, excessive mortality) do not normally lead to classification, unless other information is available, for example, toxicokinetics information indicating that humans may be more susceptible than animals, to suggest that classification is appropriate.
A.7.2.5.9 However, specification of the actual “limit dose” will depend upon the test method that has been employed to provide the test results.
A.7.3 Classification Criteria for Mixtures 9
9 It should be noted that the classification criteria for health hazards usually include a tiered scheme in which test data available on the complete mixture are considered as the first tier in the evaluation, followed by the applicable bridging principles, and lastly, cut-off values/concentration limits or additivity. However, this approach is not used for Reproductive Toxicity. These criteria for Reproductive Toxicity consider the cut-off values/concentration limits as the primary tier and allow the classification to be modified only on a case-by-case evaluation based on available test data for the mixture as a whole.
A.7.3.1 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.7.3.1.1 The mixture shall be classified as a reproductive toxicant when at least one ingredient has been classified as a Category 1 or Category 2 reproductive toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.7.1 for Category 1 and 2, respectively.
A.7.3.1.2 The mixture shall be classified for effects on or via lactation when at least one ingredient has been classified for effects on or via lactation and is present at or above the appropriate cut-off value/concentration limit specified in Table A.7.1 for the additional category for effects on or via lactation.
| Ingredient classified as | Cut-off values/concentration limits triggering classification of a mixture as | ||
|---|---|---|---|
| Category 1 reproductive toxicant | Category 2 reproductive toxicant | Additional category for effects on or via lactation | |
| Category 1 reproductive toxicant | ≥0.1% | ||
| Category 2 reproductive toxicant | ≥0.1% | ||
| Additional category for effects on or via lactation | ≥0.1% | ||
A.7.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
Available test data for the mixture as a whole may be used for classification on a case-by-case basis. In such cases, the test results for the mixture as a whole must be shown to be conclusive taking into account dose and other factors such as duration, observations and analysis (e.g., statistical analysis, test sensitivity) of reproduction test systems.
A.7.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.7.3.1.1 Where the mixture itself has not been tested to determine its reproductive toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution, Batching, and Substantially similar mixtures.
A.8 Specific Target Organ Toxicity Single Exposure
A.8.1 Definitions and General Considerations
A.8.1.1 Specific target organ toxicity—single exposure, (STOT-SE) refers to specific, non-lethal toxic effects on target organs occurring after a single exposure to a substance or mixture. All significant health effects that can impair function, both reversible and irreversible, immediate and/or delayed and not specifically addressed in A.1 to A.7 and A.10 of this Appendix are included. Specific target organ toxicity following repeated exposure is classified in accordance with SPECIFIC TARGET ORGAN TOXICITY—REPEATED EXPOSURE (A.9 of this Appendix) and is therefore not included here.
A.8.1.2 Classification identifies the chemical as being a specific target organ toxicant and, as such, it presents a potential for adverse health effects in people who are exposed to it.
A.8.1.3 The adverse health effects produced by a single exposure include consistent and identifiable toxic effects in humans; or, in experimental animals, toxicologically significant changes which have affected the function or morphology of a tissue/organ, or have produced serious changes to the biochemistry or hematology of the organism, and these changes are relevant for human health. Human data is the primary source of evidence for this hazard class.
A.8.1.4 Assessment shall take into consideration not only significant changes in a single organ or biological system but also generalized changes of a less severe nature involving several organs.
A.8.1.5 Specific target organ toxicity can occur by any route that is relevant for humans, i.e., principally oral, dermal or inhalation.
A.8.1.6 The classification criteria for specific target organ toxicity—single exposure are organized as criteria for substances Categories 1 and 2 (See A.8.2.1), criteria for substances Category 3 (See A.8.2.2) and criteria for mixtures (See A.8.3). See also Figure A.8.1.
A.8.2 Classification Criteria for Substances
A.8.2.1 Substances of Category 1 and Category 2
A.8.2.1.1 Substances shall be classified for immediate or delayed effects separately, by the use of expert judgment on the basis of the weight of all evidence available, including the use of recommended guidance values (See A.8.2.1.9). Substances shall then be classified in Category 1 or 2, depending upon the nature and severity of the effect(s) observed, in accordance with Figure A.8.1.
Figure A.8.1—Hazard Categories for Specific Target Organ Toxicity Following Single Exposure

CATEGORY 1: Substances that have produced significant toxicity in humans, or that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to produce significant toxicity in humans following single exposure
Placing a substance in Category 1 is done on the basis of:
(a) reliable and good quality evidence from human cases or epidemiological studies; or
(b) observations from appropriate studies in experimental animals in which significant and/or severe toxic effects of relevance to human health were produced at generally low exposure concentrations. Guidance dose/concentration values are provided below (see 3.8.2.1.9) to be used as part of weight-of-evidence evaluation.
CATEGORY 2: Substances that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to be harmful to human health following single exposure
Placing a substance in Category 2 is done on the basis of observations from appropriate studies in experimental animals in which significant toxic effects, of relevance to human health, were produced at generally moderate exposure concentrations. Guidance dose/concentration values are provided below (see 3.8.2.1.9) in order to help in classification.
In exceptional cases, human evidence can also be used to place a substance in Category 2 (see 3.8.2.1.9).
CATEGORY 3: Transient target organ effects
There are target organ effects for which a substance/mixture may not meet the criteria to be classified in Categories 1 or 2 indicated above. These are effects which adversely alter human function for a short duration after exposure and from which humans may recover in a reasonable period without leaving significant alteration of structure or function. This category only includes narcotic effects and respiratory tract irritation. Substances/mixtures may be classified specifically for these effects as discussed in 3.8.2.2.
Note: For these categories the specific target organ/system that has been primarily affected by the classified substance may be identified, or the substance may be identified as a general toxicant. Attempts should be made to determine the primary target organ/system of toxicity and classify for that purpose, e.g., hepatotoxicants, neurotoxicants. One should carefully evaluate the data and, where possible, not include secondary effects, e.g., a hepatotoxicant can produce secondary effects in the nervous or gastro-intestinal systems.
A.8.2.1.2 The relevant route(s) of exposure by which the classified substance produces damage shall be identified.
A.8.2.1.3 Classification is determined by expert judgment, on the basis of the weight of all evidence available including the guidance presented below.
A.8.2.1.4 Weight of evidence of all available data, including human incidents, epidemiology, and studies conducted in experimental animals is used to substantiate specific target organ toxic effects that merit classification.
A.8.2.1.5 The information required to evaluate specific target organ toxicity comes either from single exposure in humans (e.g., exposure at home, in the workplace or environmentally), or from studies conducted in experimental animals. The standard animal studies in rats or mice that provide this information are acute toxicity studies which can include clinical observations and detailed macroscopic and microscopic examination to enable the toxic effects on target tissues/organs to be identified. Results of acute toxicity studies conducted in other species may also provide relevant information.
A.8.2.1.6 In exceptional cases, based on expert judgment, it may be appropriate to place certain substances with human evidence of target organ toxicity in Category 2: (a) when the weight of human evidence is not sufficiently convincing to warrant Category 1 classification, and/or (b) based on the nature and severity of effects. Dose/concentration levels in humans shall not be considered in the classification and any available evidence from animal studies shall be consistent with the Category 2 classification. In other words, if there are also animal data available on the substance that warrant Category 1 classification, the chemical shall be classified as Category 1.
A.8.2.1.7 Effects Considered To Support Classification for Category 1 and 2
A.8.2.1.7.1 Classification is supported by evidence associating single exposure to the substance with a consistent and identifiable toxic effect.
A.8.2.1.7.2 Evidence from human experience/incidents is usually restricted to reports of adverse health consequences, often with uncertainty about exposure conditions, and may not provide the scientific detail that can be obtained from well-conducted studies in experimental animals.
A.8.2.1.7.3 Evidence from appropriate studies in experimental animals can furnish much more detail, in the form of clinical observations, and macroscopic and microscopic pathological examination and this can often reveal hazards that may not be life-threatening but could indicate functional impairment. Consequently, all available evidence, and relevance to human health, must be taken into consideration in the classification process. Relevant toxic effects in humans and/or animals include, but are not limited to:
(a) Morbidity resulting from single exposure;
(b) Significant functional changes, more than transient in nature, in the respiratory system, central or peripheral nervous systems, other organs or other organ systems, including signs of central nervous system depression and effects on special senses (e.g., sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but provide clear evidence of marked organ dysfunction; and,
(g) Evidence of appreciable cell death (including cell degeneration and reduced cell number) in vital organs incapable of regeneration.
A.8.2.1.8 Effects Considered Not To Support Classification for Category 1 and 2
Effects may be seen in humans and/or animals that do not justify classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain, food consumption or water intake that may have some toxicological importance but that do not, by themselves, indicate “significant” toxicity;
(b) Small changes in clinical biochemistry, hematology or urinalysis parameters and/or transient effects, when such changes or effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ dysfunction;
(d) Adaptive responses that are not considered toxicologically relevant; and,
(e) Substance-induced species-specific mechanisms of toxicity, i.e., demonstrated with reasonable certainty to be not relevant for human health, shall not justify classification.
A.8.2.1.9 Guidance Values To Assist With Classification Based on the Results Obtained From Studies Conducted in Experimental Animals for Category 1 and 2
A.8.2.1.9.1 In order to help reach a decision about whether a substance shall be classified or not, and to what degree it shall be classified (Category 1 vs. Category 2), dose/concentration “guidance values” are provided for consideration of the dose/concentration which has been shown to produce significant health effects. The principal argument for proposing such guidance values is that all chemicals are potentially toxic and there has to be a reasonable dose/concentration above which a degree of toxic effect is acknowledged.
A.8.2.1.9.2 Thus, in animal studies, when significant toxic effects are observed that indicate classification, consideration of the dose/concentration at which these effects were seen, in relation to the suggested guidance values, provides useful information to help assess the need to classify (since the toxic effects are a consequence of the hazardous property(ies) and also the dose/concentration).
A.8.2.1.9.3 The guidance value (C) ranges for single-dose exposure which has produced a significant non-lethal toxic effect are those applicable to acute toxicity testing, as indicated in Table A.8.1.
| Route of exposure | Units | Guidance value ranges for: | ||
|---|---|---|---|---|
| Category 1 | Category 2 | Category 3 | ||
| Oral (rat) | mg/kg body weight | C ≤ 300 | 2,000 ≥ C > 300 | Guidance values do not apply. |
| Dermal (rat or rabbit) | mg/kg body weight | C ≤ 1,000 | 2,000 ≥ C > 1,000 | |
| Inhalation (rat) gas | ppmV/4h | C ≤ 2,500 | 20,000 ≥ C > 2,500 | |
| Inhalation (rat) vapor | mg/1/4h | C ≤ 10 | 20 ≥ C > 10 | |
| Inhalation (rat) dust/mist/fume | mg/l/4h | C ≤ 1.0 | 5.0 ≥ C > 1.0 | |
A.8.2.1.9.4 The guidance values and ranges mentioned in Table A.8.1 are intended only for guidance purposes, i.e., to be used as part of the weight of evidence approach, and to assist with decisions about classification. They are not intended as strict demarcation values. Guidance values are not provided for Category 3 since this classification is primarily based on human data; animal data may be included in the weight of evidence evaluation.
A.8.2.1.9.5 Thus, it is feasible that a specific profile of toxicity occurs at a dose/concentration below the guidance value, e.g., <2,000 mg/kg body weight by the oral route, however the nature of the effect may result in the decision not to classify. Conversely, a specific profile of toxicity may be seen in animal studies occurring at above a guidance value, e.g., ≥2,000 mg/kg body weight by the oral route, and in addition there is supplementary information from other sources, e.g., other single dose studies, or human case experience, which supports a conclusion that, in view of the weight of evidence, classification is the prudent action to take.
A.8.2.1.10 Other Considerations
A.8.2.1.10.1 When a substance is characterized only by use of animal data the classification process includes reference to dose/concentration guidance values as one of the elements that contribute to the weight of evidence approach.
A.8.2.1.10.2 When well-substantiated human data are available showing a specific target organ toxic effect that can be reliably attributed to single exposure to a substance, the substance shall be classified. Positive human data, regardless of probable dose, predominates over animal data. Thus, if a substance is unclassified because specific target organ toxicity observed was considered not relevant or significant to humans, if subsequent human incident data become available showing a specific target organ toxic effect, the substance shall be classified.
A.8.2.1.10.3 A substance that has not been tested for specific target organ toxicity shall, where appropriate, be classified on the basis of data from a scientifically validated structure activity relationship and expert judgment-based extrapolation from a structural analogue that has previously been classified together with substantial support from consideration of other important factors such as formation of common significant metabolites.
A.8.2.2 Substances of Category 3
A.8.2.2.1 Criteria for respiratory tract irritation
The criteria for classifying substances as Category 3 for respiratory tract irritation are:
(a) Respiratory irritant effects (characterized by localized redness, edema, pruritis and/or pain) that impair function with symptoms such as cough, pain, choking, and breathing difficulties are included. It is recognized that this evaluation is based primarily on human data;
(b) Subjective human observations supported by objective measurements of clear respiratory tract irritation (RTI) (e.g., electrophysiological responses, biomarkers of inflammation in nasal or bronchoalveolar lavage fluids);
(c) The symptoms observed in humans shall also be typical of those that would be produced in the exposed population rather than being an isolated idiosyncratic reaction or response triggered only in individuals with hypersensitive airways. Ambiguous reports simply of “irritation” should be excluded as this term is commonly used to describe a wide range of sensations including those such as smell, unpleasant taste, a tickling sensation, and dryness, which are outside the scope of classification for respiratory tract irritation;
(d) There are currently no scientifically validated animal tests that deal specifically with RTI; however, useful information may be obtained from the single and repeated inhalation toxicity tests. For example, animal studies may provide useful information in terms of clinical signs of toxicity (dyspnoea, rhinitis etc.) and histopathology (e.g., hyperemia, edema, minimal inflammation, thickened mucous layer) which are reversible and may be reflective of the characteristic clinical symptoms described above. Such animal studies can be used as part of weight of evidence evaluation; and,
(e) This special classification will occur only when more severe organ effects including the respiratory system are not observed as those effects would require a higher classification.
A.8.2.2.2 Criteria for Narcotic Effects
The criteria for classifying substances in Category 3 for narcotic effects are:
(a) Central nervous system depression including narcotic effects in humans such as drowsiness, narcosis, reduced alertness, loss of reflexes, lack of coordination, and vertigo are included. These effects can also be manifested as severe headache or nausea, and can lead to reduced judgment, dizziness, irritability, fatigue, impaired memory function, deficits in perception and coordination, reaction time, or sleepiness; and,
(b) Narcotic effects observed in animal studies may include lethargy, lack of coordination righting reflex, narcosis, and ataxia. If these effects are not transient in nature, then they shall be considered for classification as Category 1 or 2.
A.8.3 Classification Criteria for Mixtures
A.8.3.1 Mixtures are classified using the same criteria as for substances, or alternatively as described below. As with substances, mixtures may be classified for specific target organ toxicity following single exposure, repeated exposure, or both.
A.8.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence from human experience or appropriate studies in experimental animals, as described in the criteria for substances, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of this data. Care shall be exercised in evaluating data on mixtures, that the dose, duration, observation or analysis, do not render the results inconclusive.
A.8.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.8.3.3.1 Where the mixture itself has not been tested to determine its specific target organ toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution, Batching, Concentration of mixtures, Interpolation within one hazard category, Substantially similar mixtures, or Aerosols.
A.8.3.4 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.8.3.4.1 Where there is no reliable evidence or test data for the specific mixture itself, and the bridging principles cannot be used to enable classification, then classification of the mixture is based on the classification of the ingredient substances. In this case, the mixture shall be classified as a specific target organ toxicant (specific organ specified), following single exposure, repeated exposure, or both when at least one ingredient has been classified as a Category 1 or Category 2 specific target organ toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.8.2 for Categories 1 and 2, respectively.
| Ingredient classified as | Cut-off values/concentration limits triggering classification of a mixture as | |
|---|---|---|
| Category 1 | Category 2 | |
| Category 1 Target organ toxicant | ≥1.0% | |
| Category 2 Target organ toxicant | ≥1.0% | |
A.8.3.4.2 These cut-off values and consequent classifications shall be applied equally and appropriately to both single- and repeated-dose target organ toxicants.
A.8.3.4.3 Mixtures shall be classified for either or both single and repeated dose toxicity independently.
A.8.3.4.4 Care shall be exercised when toxicants affecting more than one organ system are combined that the potentiation or synergistic interactions are considered, because certain substances can cause target organ toxicity at <1% concentration when other ingredients in the mixture are known to potentiate its toxic effect.
A.8.3.4.5 Care shall be exercised when extrapolating the toxicity of a mixture that contains Category 3 ingredient(s). A cut-off value/concentration limit of 20%, considered as an additive of all Category 3 ingredients for each hazard endpoint, is appropriate; however, this cut-off value/concentration limit may be higher or lower depending on the Category 3 ingredient(s) involved and the fact that some effects such as respiratory tract irritation may not occur below a certain concentration while other effects such as narcotic effects may occur below this 20% value. Expert judgment shall be exercised. Respiratory tract irritation and narcotic effects are to be evaluated separately in accordance with the criteria given in A.8.2.2. When conducting classifications for these hazards, the contribution of each ingredient should be considered additive, unless there is evidence that the effects are not additive.
A.8.3.4.6 In cases where the additivity approach is used for Category 3 ingredients, the “relevant ingredients” of a mixture are those which are present in concentrations ≥1% (w/w for solids, liquids, dusts, mists, and vapours and v/v for gases), unless there is a reason to suspect that an ingredient present at a concentration <1% is still relevant when classifying the mixture for respiratory tract irritation or narcotic effects.
A.9 Specific Target Organ Toxicity—Repeated or Prolonged Exposure
A.9.1 Definitions and General Considerations
A.9.1.1 Specific target organ toxicity—repeated exposure (STOT-RE) refers to specific toxic effects on target organs occurring after repeated exposure to a substance or mixture. All significant health effects that can impair function, both reversible and irreversible, immediate and/or delayed and not specifically addressed in A.1 to A.7 and A.10 of this Appendix are included. Specific target organ toxicity following a single-event exposure is classified in accordance with SPECIFIC TARGET ORGAN TOXICITY—SINGLE EXPOSURE (A.8 of this Appendix) and is therefore not included here.
A.9.1.2 Classification identifies the substance or mixture as being a specific target organ toxicant and, as such, it may present a potential for adverse health effects in people who are exposed to it.
A.9.1.3 These adverse health effects produced by repeated exposure include consistent and identifiable toxic effects in humans, or, in experimental animals, toxicologically significant changes which have affected the function or morphology of a tissue/organ, or have produced serious changes to the biochemistry or hematology of the organism and these changes are relevant for human health. Human data will be the primary source of evidence for this hazard class.
A.9.1.4 Assessment shall take into consideration not only significant changes in a single organ or biological system but also generalized changes of a less severe nature involving several organs.
A.9.1.5 Specific target organ toxicity can occur by any route that is relevant for humans, e.g., principally oral, dermal or inhalation.
A.9.2 Classification Criteria for Substances
A.9.2.1 Substances shall be classified as STOT—RE by expert judgment on the basis of the weight of all evidence available, including the use of recommended guidance values which take into account the duration of exposure and the dose/concentration which produced the effect(s), (See A.9.2.9). Substances shall be placed in one of two categories, depending upon the nature and severity of the effect(s) observed, in accordance with Figure A.9.1.
Figure A.9.1—Hazard Categories for Specific Target Organ Toxicity Following Repeated Exposure
CATEGORY 1: Substances that have produced significant toxicity in humans, or that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to produce significant toxicity in humans following repeated or prolonged exposure
Substances are classified in Category 1 for specific target organ toxicity (repeated exposure) on the basis of:
(a) reliable and good quality evidence from human cases or epidemiological studies; or,
(b) observations from appropriate studies in experimental animals in which significant and/or severe toxic effects, of relevance to human health, were produced at generally low exposure concentrations. Guidance dose/concentration values are provided below (See A.9.2.9) to be used as part of weight-of-evidence evaluation.
CATEGORY 2: Substances that, on the basis of evidence from studies in experimental animals can be presumed to have the potential to be harmful to human health following repeated or prolonged exposure
Substances are classified in Category 2 for specific target organ toxicity (repeated exposure) on the basis of observations from appropriate studies in experimental animals in which significant toxic effects, of relevance to human health, were produced at generally moderate exposure concentrations. Guidance dose/concentration values are provided below (See A.9.2.9) in order to help in classification.
In exceptional cases human evidence can also be used to place a substance in Category 2 (See A.9.2.6).
Note: The primary target organ/system shall be identified where possible, or the substance shall be identified as a general toxicant. The data shall be carefully evaluated and, where possible, shall not include secondary effects (e.g., a hepatotoxicant can produce secondary effects in the nervous or gastro-intestinal systems).
A.9.2.2 The relevant route of exposure by which the classified substance produces damage shall be identified.
A.9.2.3 Classification is determined by expert judgment, on the basis of the weight of all evidence available including the guidance presented below.
A.9.2.4 Weight of evidence of all data, including human incidents, epidemiology, and studies conducted in experimental animals, is used to substantiate specific target organ toxic effects that merit classification.
A.9.2.5 The information required to evaluate specific target organ toxicity comes either from repeated exposure in humans, e.g., exposure at home, in the workplace or environmentally, or from studies conducted in experimental animals. The standard animal studies in rats or mice that provide this information are 28 day, 90 day or lifetime studies (up to 2 years) that include hematological, clinico-chemical and detailed macroscopic and microscopic examination to enable the toxic effects on target tissues/organs to be identified. Data from repeat dose studies performed in other species may also be used. Other long-term exposure studies, e.g., for carcinogenicity, neurotoxicity or reproductive toxicity, may also provide evidence of specific target organ toxicity that could be used in the assessment of classification.
A.9.2.6 In exceptional cases, based on expert judgment, it may be appropriate to place certain substances with human evidence of specific target organ toxicity in Category 2: (a) when the weight of human evidence is not sufficiently convincing to warrant Category 1 classification, and/or (b) based on the nature and severity of effects. Dose/concentration levels in humans shall not be considered in the classification and any available evidence from animal studies shall be consistent with the Category 2 classification. In other words, if there are also animal data available on the substance that warrant Category 1 classification, the substance shall be classified as Category 1.
A.9.2.7 Effects Considered To Support Classification
A.9.2.7.1 Classification is supported by reliable evidence associating repeated exposure to the substance with a consistent and identifiable toxic effect.
A.9.2.7.2 Evidence from human experience/incidents is usually restricted to reports of adverse health consequences, often with uncertainty about exposure conditions, and may not provide the scientific detail that can be obtained from well-conducted studies in experimental animals.
A.9.2.7.3 Evidence from appropriate studies in experimental animals can furnish much more detail, in the form of clinical observations, hematology, clinical chemistry, macroscopic and microscopic pathological examination and this can often reveal hazards that may not be life-threatening but could indicate functional impairment. Consequently, all available evidence, and relevance to human health, must be taken into consideration in the classification process. Relevant toxic effects in humans and/or animals include, but are not limited to:
(a) Morbidity or death resulting from repeated or long-term exposure. Morbidity or death may result from repeated exposure, even to relatively low doses/concentrations, due to bioaccumulation of the substance or its metabolites, or due to the overwhelming of the de-toxification process by repeated exposure;
(b) Significant functional changes in the central or peripheral nervous systems or other organ systems, including signs of central nervous system depression and effects on special senses (e.g., sight, hearing and sense of smell);
(c) Any consistent and significant adverse change in clinical biochemistry, hematology, or urinalysis parameters;
(d) Significant organ damage that may be noted at necropsy and/or subsequently seen or confirmed at microscopic examination;
(e) Multi-focal or diffuse necrosis, fibrosis or granuloma formation in vital organs with regenerative capacity;
(f) Morphological changes that are potentially reversible but provide clear evidence of marked organ dysfunction (e.g., severe fatty change in the liver); and,
(g) Evidence of appreciable cell death (including cell degeneration and reduced cell number) in vital organs incapable of regeneration.
A.9.2.8 Effects Considered Not To Support Classification
Effects may be seen in humans and/or animals that do not justify classification. Such effects include, but are not limited to:
(a) Clinical observations or small changes in bodyweight gain, food consumption or water intake that may have some toxicological importance but that do not, by themselves, indicate “significant” toxicity;
(b) Small changes in clinical biochemistry, hematology or urinalysis parameters and/or transient effects, when such changes or effects are of doubtful or of minimal toxicological importance;
(c) Changes in organ weights with no evidence of organ dysfunction;
(d) Adaptive responses that are not considered toxicologically relevant;
(e) Substance-induced species-specific mechanisms of toxicity, i.e., demonstrated with reasonable certainty to be not relevant for human health, shall not justify classification.
A.9.2.9 Guidance Values To Assist With Classification Based on the Results Obtained From Studies Conducted in Experimental Animals
A.9.2.9.1 In studies conducted in experimental animals, reliance on observation of effects alone, without reference to the duration of experimental exposure and dose/concentration, omits a fundamental concept of toxicology, i.e., all substances are potentially toxic, and what determines the toxicity is a function of the dose/concentration and the duration of exposure. In most studies conducted in experimental animals the test guidelines use an upper limit dose value.
A.9.2.9.2 In order to help reach a decision about whether a substance shall be classified or not, and to what degree it shall be classified (Category 1 vs. Category 2), dose/concentration “guidance values” are provided in Table A.9.1 for consideration of the dose/concentration which has been shown to produce significant health effects. The principal argument for proposing such guidance values is that all chemicals are potentially toxic and there has to be a reasonable dose/concentration above which a degree of toxic effect is acknowledged. Also, repeated-dose studies conducted in experimental animals are designed to produce toxicity at the highest dose used in order to optimize the test objective and so most studies will reveal some toxic effect at least at this highest dose. What is therefore to be decided is not only what effects have been produced, but also at what dose/concentration they were produced and how relevant is that for humans.
A.9.2.9.3 Thus, in animal studies, when significant toxic effects are observed that indicate classification, consideration of the duration of experimental exposure and the dose/concentration at which these effects were seen, in relation to the suggested guidance values, provides useful information to help assess the need to classify (since the toxic effects are a consequence of the hazardous property(ies) and also the duration of exposure and the dose/concentration).
A.9.2.9.4 The decision to classify at all can be influenced by reference to the dose/concentration guidance values at or below which a significant toxic effect has been observed.
A.9.2.9.5 The guidance values refer to effects seen in a standard 90-day toxicity study conducted in rats. They can be used as a basis to extrapolate equivalent guidance values for toxicity studies of greater or lesser duration, using dose/exposure time extrapolation similar to Haber's rule for inhalation, which states essentially that the effective dose is directly proportional to the exposure concentration and the duration of exposure. The assessment should be done on a case- by-case basis; for example, for a 28-day study the guidance values below would be increased by a factor of three.
A.9.2.9.6 Thus for Category 1 classification, significant toxic effects observed in a 90-day repeated-dose study conducted in experimental animals and seen to occur at or below the (suggested) guidance values (C) as indicated in Table A.9.1 would justify classification:
| Route of exposure | Units | Guidance values (dose/concentration) |
|---|---|---|
| Oral (rat) | mg/kg body weight/day | C ≤ 10 |
| Dermal (rat or rabbit) | mg/kg body weight/day | C ≤ 20 |
| Inhalation (rat) gas | ppmV/6h/day | C ≤ 50 |
| Inhalation (rat) vapor | mg/liter/6h/day | C ≤ 0.2 |
| Inhalation (rat) dust/mist/fume | mg/liter/6h/day | C ≤ 0.02 |
A.9.2.9.7 For Category 2 classification, significant toxic effects observed in a 90-day repeated-dose study conducted in experimental animals and seen to occur within the (suggested) guidance value ranges as indicated in Table A.9.2 would justify classification:
| Route of exposure | Units | Guidance value range (dose/concentration) |
|---|---|---|
| Oral (rat) | mg/kg body weight/day | 10 < C ≤ 100 |
| Dermal (rat or rabbit) | mg/kg body weight/day | 20 < C ≤ 200 |
| Inhalation (rat) gas | ppmV/6h/day | 50 < C ≤ 250 |
| Inhalation (rat) vapor | mg/liter/6h/day | 0.2 < C ≤ 1.0 |
| Inhalation (rat) dust/mist/fume | mg/liter/6h/day | 0.02 < C ≤ 0.2 |
A.9.2.9.8 The guidance values and ranges mentioned in A.2.9.9.6 and A.2.9.9.7 are intended only for guidance purposes, i.e., to be used as part of the weight of evidence approach, and to assist with decisions about classification. They are not intended as strict demarcation values.
A.9.2.9.9 Thus, it is possible that a specific profile of toxicity occurs in repeat-dose animal studies at a dose/concentration below the guidance value, e.g., <100 mg/kg body weight/day by the oral route, however the nature of the effect, e.g., nephrotoxicity seen only in male rats of a particular strain known to be susceptible to this effect, may result in the decision not to classify. Conversely, a specific profile of toxicity may be seen in animal studies occurring at above a guidance value, e.g., ≥100 mg/kg body weight/day by the oral route, and in addition there is supplementary information from other sources, e.g., other long-term administration studies, or human case experience, which supports a conclusion that, in view of the weight of evidence, classification is prudent.
A.9.2.10 Other Considerations
A.9.2.10.1 When a substance is characterized only by use of animal data the classification process includes reference to dose/concentration guidance values as one of the elements that contribute to the weight of evidence approach.
A.9.2.10.2 When well-substantiated human data are available showing a specific target organ toxic effect that can be reliably attributed to repeated or prolonged exposure to a substance, the substance shall be classified. Positive human data, regardless of probable dose, predominates over animal data. Thus, if a substance is unclassified because no specific target organ toxicity was seen at or below the dose/concentration guidance value for animal testing, if subsequent human incident data become available showing a specific target organ toxic effect, the substance shall be classified.
A.9.2.10.3 A substance that has not been tested for specific target organ toxicity may in certain instances, where appropriate, be classified on the basis of data from a scientifically validated structure activity relationship and expert judgment-based extrapolation from a structural analogue that has previously been classified together with substantial support from consideration of other important factors such as formation of common significant metabolites.
A.9.3 Classification Criteria for Mixtures
A.9.3.1 Mixtures are classified using the same criteria as for substances, or alternatively as described below. As with substances, mixtures may be classified for specific target organ toxicity following single exposure, repeated exposure, or both.
A.9.3.2 Classification of Mixtures When Data Are Available for the Complete Mixture
When reliable and good quality evidence from human experience or appropriate studies in experimental animals, as described in the criteria for substances, is available for the mixture, then the mixture shall be classified by weight of evidence evaluation of these data. Care shall be exercised in evaluating data on mixtures, that the dose, duration, observation or analysis, do not render the results inconclusive.
A.9.3.3 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.9.3.3.1 Where the mixture itself has not been tested to determine its specific target organ toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution; Batching; Concentration of mixtures; Interpolation within one hazard category; Substantially similar mixtures; and Aerosols.
A.9.3.4 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.9.3.4.1 Where there is no reliable evidence or test data for the specific mixture itself, and the bridging principles cannot be used to enable classification, then classification of the mixture is based on the classification of the ingredient substances. In this case, the mixture shall be classified as a specific target organ toxicant (specific organ specified), following single exposure, repeated exposure, or both when at least one ingredient has been classified as a Category 1 or Category 2 specific target organ toxicant and is present at or above the appropriate cut-off value/concentration limit specified in Table A.9.3 for Category 1 and 2 respectively.
| Ingredient classified as | Cut-off values/concentration limits triggering classification of a mixture as | |
|---|---|---|
| Category 1 | Category 2 | |
| Category 1 Target organ toxicant | ≥1.0% | |
| Category 2 Target organ toxicant | ≥1.0% | |
A.9.3.4.2 These cut-off values and consequent classifications shall be applied equally and appropriately to both single- and repeated-dose target organ toxicants.
A.9.3.4.3 Mixtures shall be classified for either or both single- and repeated-dose toxicity independently.
A.9.3.4.4 Care shall be exercised when toxicants affecting more than one organ system are combined that the potentiation or synergistic interactions are considered, because certain substances can cause specific target organ toxicity at <1% concentration when other ingredients in the mixture are known to potentiate its toxic effect.
A.10 Aspiration Hazard
A.10.1 Definitions and General Considerations
A.10.1.1 Aspiration hazard refers to severe acute effects such as chemical pneumonia, pulmonary injury or death occurring after aspiration of a substance or mixture.
A.10.1.2 Aspiration means the entry of a liquid or solid chemical directly through the oral or nasal cavity, or indirectly from vomiting, into the trachea and lower respiratory system.
A.10.1.3 Aspiration is initiated at the moment of inspiration, in the time required to take one breath, as the causative material lodges at the crossroad of the upper respiratory and digestive tracts in the laryngopharyngeal region.
A.10.1.4 Aspiration of a substance or mixture can occur as it is vomited following ingestion. This may have consequences for labeling, particularly where, due to acute toxicity, a recommendation may be considered to induce vomiting after ingestion. However, if the substance/mixture also presents an aspiration toxicity hazard, the recommendation to induce vomiting may need to be modified.
A.10.1.5 Specific Considerations
A.10.1.5.1 The classification criteria refer to kinematic viscosity. The following provides the conversion between dynamic and kinematic viscosity:
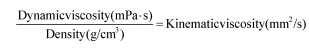
A.10.1.5.2 Although the definition of aspiration in A.10.1.1 includes the entry of solids into the respiratory system, classification according to (b) in table A.10.1 for Category 1 is intended to apply to liquid substances and mixtures only.
A.10.1.5.3 Classification of aerosol/mist products
Aerosol and mist products are usually dispensed in containers such as self- pressurized containers, trigger and pump sprayers. Classification for these products shall be considered if their use may form a pool of product in the mouth, which then may be aspirated. If the mist or aerosol from a pressurized container is fine, a pool may not be formed. On the other hand, if a pressurized container dispenses product in a stream, a pool may be formed that may then be aspirated. Usually, the mist produced by trigger and pump sprayers is coarse and therefore, a pool may be formed that then may be aspirated. When the pump mechanism may be removed and contents are available to be swallowed then the classification of the products should be considered.
Table A.10.1: Criteria for Aspiration Toxicity

A.10.3 Classification Criteria for Mixtures
A.10.3.1 Classification When Data Are Available for the Complete Mixture
A mixture shall be classified in Category 1 based on reliable and good quality human evidence.
A.10.3.2 Classification of Mixtures When Data Are Not Available for the Complete Mixture: Bridging Principles
A.10.3.2.1 Where the mixture itself has not been tested to determine its aspiration toxicity, but there are sufficient data on both the individual ingredients and similar tested mixtures to adequately characterize the hazard of the mixture, these data shall be used in accordance with the following bridging principles as found in paragraph A.0.5 of this Appendix: Dilution; Batching; Concentration of mixtures; Interpolation within one hazard category; and Substantially similar mixtures. For application of the dilution bridging principle, the concentration of aspiration toxicants shall not be less than 10%.
A.10.3.3 Classification of Mixtures When Data Are Available for All Ingredients or Only for Some Ingredients of the Mixture
A.10.3.3.1 The “relevant ingredients” of a mixture are those which are present in concentrations ≥1%.
A.10.3.3.2 Category 1
A.10.3.3.2.1 A mixture is classified as Category 1 when the sum of the concentrations of Category 1 ingredients is ≥10%, and the mixture has a kinematic viscosity of ≤20.5 mm 2 /s, measured at 40°C.
A.10.3.3.2.2 In the case of a mixture which separates into two or more distinct layers, the entire mixture is classified as Category 1 if in any distinct layer the sum of the concentrations of Category 1 ingredients is ≥10%, and it has a kinematic viscosity of ≤20.5 mm 2 /s, measured at 40°C.
[77 FR 17790, March 26, 2012; 78 FR 9313, Feb. 8, 2013; 89 FR 44356, May 20, 2024; 89 FR 81830, Oct. 9, 2024; 91 FR 566, Jan. 8, 2026]
['Toxic and Hazardous Substances - OSHA', 'Hazard Communication']
['Hazard Classifications', 'Hazard Communication', 'Toxic and Hazardous Substances - OSHA']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
