['Water Programs']
['Water Quality', 'Safe Drinking Water']
05/03/2022
...
(a) Analytical requirements. Only the analytical method(s) specified in this paragraph, or otherwise approved by EPA, may be used to demonstrate compliance with �141.71, 141.72 and 141.73. Measurements for pH, turbidity, temperature and residual disinfectant concentrations must be conducted by a person approved by the State. Measurement for total coliforms, fecal coliforms and HPC must be conducted by a laboratory certified by the State or EPA to do such analysis. Until laboratory certification criteria are developed for the analysis of fecal coliforms and HPC, any laboratory certified for total coliforms analysis by the State or EPA is deemed certified for fecal coliforms and HPC analysis. The following procedures shall be conducted in accordance with the publications listed in the following section. This incorporation by reference was approved by the Director of the Federal Register in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies of the methods published in Standard Methods for the Examination of Water and Wastewater may be obtained from the American Public Health Association et al., 1015 Fifteenth Street, NW., Washington, DC 20005; copies of the Minimal Medium ONPG-MUG Method as set forth in the article �National Field Evaluation of a Defined Substrate Method for the Simultaneous Enumeration of Total Coliforms and Esherichia coli from Drinking Water: Comparison with the Standard Multiple Tube Fermentation Method� (Edberg et al.), Applied and Environmental Microbiology, Volume 54, pp. 1595-1601, June 1988 (as amended under Erratum, Applied and Environmental Microbiology, Volume 54, p. 3197, December, 1988), may be obtained from the American Water Works Association Research Foundation, 6666 West Quincy Avenue, Denver, Colorado, 80235; and copies of the Indigo Method as set forth in the article �Determination of Ozone in Water by the Indigo Method� (Bader and Hoigne), may be obtained from Ozone Science & Engineering, Pergamon Press Ltd., Fairview Park, Elmsford, New York 10523. Copies may be inspected at the U.S. Environmental Protection Agency, Room EB15, 401 M St., SW., Washington, DC 20460 or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
(1) Public water systems must conduct analysis of pH and temperature in accordance with one of the methods listed at �141.23(k)(1). Public water systems must conduct analysis of total coliforms, fecal coliforms, heterotrophic bacteria, and turbidity in accordance with one of the following analytical methods or one of the alternative methods listed in Appendix A to subpart C of this part and by using analytical test procedures contained in Technical Notes on Drinking Water Methods, EPA-600/R-94-173, October 1994. This document is available from the National Service Center for Environmental Publications (NSCEP), P.O. Box 42419, Cincinnati, OH 45242-0419 or http://www.epa.gov/nscep/.
| Organism | Methodology | Citation1 |
| Total Coliform2 | Total Coliform Fermentation Technique3,4,5 | 9221 A, B, C |
| Total Coliform Membrane Filter Technique6 | 9222 A, B, C | |
| ONPG-MUG Test7 | 9223 | |
| Fecal Coliforms2 | Fecal Coliform Procedure8 | 9221 E |
| Fecal Coliform Filter Procedure | 9222 D | |
| Heterotrophic bacteria2 | Pour Plate Method | 9215 B |
| SimPlate11 | ||
| Turbidity13 | Nephelometric Method | 2130 B |
| Nephelometric Method | 180.19 | |
| Great Lakes Instruments | Method 210 | |
| Hach FilterTrak | 1013312 |
The procedures shall be done in accordance with the documents listed below. The incorporation by reference of the following documents listed in footnotes 1, 6, 7 and 9-12 was approved by the Director of the Federal Register in accordance with 5 U.S.C. 552(a) and 1 CFR part 51. Copies of the documents may be obtained from the sources listed below. Information regarding obtaining these documents can be obtained from the Safe Drinking Water Hotline at 800-426-4791. Documents may be inspected at EPA's Drinking Water Docket, 1301 Constitution Avenue, NW., EPA West, Room B102, Washington DC 20460 (Telephone: 202-566-2426); or at the National Archives and Records Administration (NARA). For information on the availability of this material at NARA, call 202-741-6030, or go to: http://www.archives.gov/federal_register/code_of_federal_regulations/ibr_locations.html.
1 Except where noted, all methods refer to Standard Methods for the Examination of Water and Wastewater, 18th edition (1992), 19th edition (1995), or 20th edition (1998), American Public Health Association, 1015 Fifteenth Street, NW., Washington, DC 20005. The cited methods published in any of these three editions may be used. In addition, the following online versions may also be used: 2130 B-01, 9215 B-00, 9221 A, B, C, E-99, 9222 A, B, C, D-97, and 9223 B-97. Standard Methods Online are available at http://www.standardmethods.org. The year in which each method was approved by the Standard Methods Committee is designated by the last two digits in the method number. The methods listed are the only Online versions that may be used.
2 The time from sample collection to initiation of analysis may not exceed 8 hours. Systems must hold samples below 10 �C during transit.
3 Lactose broth, as commercially available, may be used in lieu of lauryl tryptose broth, if the system conducts at least 25 parallel tests between this medium and lauryl tryptose broth using the water normally tested, and this comparison demonstrates that the false-positive rate and false-negative rate for total coliform, using lactose broth, is less than 10 percent.
4 Media should cover inverted tubes at least one-half to two-thirds after the sample is added.
5 No requirement exists to run the completed phase on 10 percent of all total coliform-positive confirmed tubes.
6 MI agar also may be used. Preparation and use of MI agar is set forth in the article, "New medium for the simultaneous detection of total coliform and Escherichia coli in water" by Brenner, K.P., et al., 1993, Appl. Environ. Microbiol. 59:3534-3544. Also available from the Office of Water Resource Center (RC-4100T), 1200 Pennsylvania Avenue, NW., Washington, DC 20460, EPA 600/J-99/225. Verification of colonies is not required.
7 The ONPG-MUG Test is also known as the Autoanalysis Colilert System.
8 A-1 broth may be held up to 7 days in a tightly closed screw cap tube at 4 �C.
9 "Methods for the Determination of Inorganic Substances in Environmental Samples", EPA/600/R-93/100, August 1993. Available at NTIS, PB94-121811.
10 GLI Method 2, �Turbidity,� November 2, 1992, Great Lakes Instruments, Inc., 8855 North 55th Street, Milwaukee, WI 53223.
11 A description of the SimPlate method, �IDEXX SimPlate TM HPC Test Method for Heterotrophs in Water,� November 2000, can be obtained from IDEXX Laboratories, Inc., 1 IDEXX Drive, Westbrook, ME 04092, telephone (800) 321-0207.
12 A description of the Hach FilterTrak Method 10133, �Determination of Turbidity by Laser Nephelometry,� January 2000, Revision 2.0, can be obtained from; Hach Co., P.O. Box 389, Loveland, CO 80539-0389, telephone: 800-227-4224.
13 Styrene divinyl benzene beads (e.g. AMCO-AEPA-1 or equivalent) and stabilized formazin (e.g. Hach StablCal TM or equivalent) are acceptable substitutes for formazin.
(2) Public water systems must measure residual disinfectant concentrations with one of the analytical methods in the following table or one of the alternative methods listed in Appendix A to subpart C of this part. If approved by the State, residual disinfectant concentrations for free chlorine and combined chlorine also may be measured by using DPD colorimetric test kits. In addition States may approve the use of the ITS free chlorine test strip for the determination of free chlorine. Use of the test strips is described in Method D99-003, �Free Chlorine Species (HOCl- and OCl-) by Test Strip,� Revision 3.0, November 21, 2003, available from Industrial Test Systems, Inc., 1875 Langston St., Rock Hill, SC 29730. Free and total chlorine residuals may be measured continuously by adapting a specified chlorine residual method for use with a continuous monitoring instrument provided the chemistry, accuracy, and precision remain the same. Instruments used for continuous monitoring must be calibrated with a grab sample measurement at least every five days, or with a protocol approved by the State.
| Residual | Methodology | SM1 | SM Online2 | Other |
| Free Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D-00 | D1253-033 |
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F-00 | ||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G-00 | ||
| Syringaldazine (FACTS) | 4500-Cl H | 4500-Cl H-00 | ||
| Total Chlorine | Amperometric Titration | 4500-Cl D | 4500-Cl D-00 | D1253-033 |
| Amperometric Titration (low level measurement) | 4500-Cl E | 4500-Cl E-00 | ||
| DPD Ferrous Titrimetric | 4500-Cl F | 4500-Cl F-00 | ||
| DPD Colorimetric | 4500-Cl G | 4500-Cl G-00 | ||
| Iodometric Electrode | 4500-Cl I | 4500-Cl I-00 | ||
| Chlorine Dioxide | Amperometric Titration | 4500-ClO2 C | 4500-ClO2 C-00 | |
| DPD Method | 4500-ClO2 D | |||
| Amperometric Titration | 4500-ClO2 E | 4500-ClO2 E-00 | ||
| Spectrophotometric | 327.0, Revision 1.14 | |||
| Ozone | Indigo Method | 4500-O3 B | 4500-O3 B-97 |
1 All the listed methods are contained in the 18th, 19th, and 20th editions of Standard Methods for the Examination of Water and Wastewater, 1992, 1995, and 1998; the cited methods published in any of these three editions may be used.
2 Standard Methods Online are available at http://www.standardmethods.org. The year in which each method was approved by the Standard Methods Committee is designated by the last two digits in the method number. The methods listed are the only Online versions that may be used.
3 Annual Book of ASTM Standards, Vol. 11.01, 2004 ; ASTM International; any year containing the cited version of the method may be used. Copies of this method may be obtained from ASTM International, 100 Barr Harbor Drive, P.O. Box C700 West Conshohocken, PA 19428-2959.
4 EPA Method 327.0, Revision 1.1, �Determination of Chlorine Dioxide and Chlorite Ion in Drinking Water Using Lissamine Green B and Horseradish Peroxidase with Detection by Visible Spectrophotometry,� USEPA, May 2005, EPA 815-R-05-008. Available online at http://www.epa.gov/safewater/methods/sourcalt.html.
(b) Monitoring requirements for systems that do not provide filtration. A public water system that uses a surface water source and does not provide filtration treatment must begin monitoring, as specified in this paragraph (b), beginning December 31, 1990, unless the State has determined that filtration is required in writing pursuant to �1412(b)(7)(C)(iii), in which case the State may specify alternative monitoring requirements, as appropriate, until filtration is in place. A public water system that uses a ground water source under the direct influence of surface water and does not provide filtration treatment must begin monitoring as specified in this paragraph (b) beginning December 31, 1990, or 6 months after the State determines that the ground water source is under the direct influence of surface water, whichever is later, unless the State has determined that filtration is required in writing pursuant to �1412(b)(7)(C)(iii), in which case the State may specify alternative monitoring requirements, as appropriate, until filtration is in place.
(1) Fecal coliform or total coliform density measurements as required by �141.71(a)(1) must be performed on representative source water samples immediately prior to the first or only point of disinfectant application. The system must sample for fecal or total coliforms at the following minimum frequency each week the system serves water to the public:
| System size (persons served) | Samples/week1 |
| ?500 | 1 |
| 501 to 3,300 | 2 |
| 3,301 to 10,000 | 3 |
| 10,001 to 25,000 | 4 |
| >25,000 | 5 |
1Must be taken on separate days.
Also, one fecal or total coliform density measurement must be made every day the system serves water to the public and the turbidity of the source water exceeds 1 NTU (these samples count towards the weekly coliform sampling requirement) unless the State determines that the system, for logistical reasons outside the system's control, cannot have the sample analyzed within 30 hours of collection.
(2) Turbidity measurements as required by �141.71(a)(2) must be performed on representative grab samples of source water immediately prior to the first or only point of disinfectant application every four hours (or more frequently) that the system serves water to the public. A public water system may substitute continuous turbidity monitoring for grab sample monitoring if it validates the continuous measurement for accuracy on a regular basis using a protocol approved by the State.
(3) The total inactivation ratio for each day that the system is in operation must be determined based on the CT99.9 values in Tables 1.1-1.6, 2.1, and 3.1 of this section, as appropriate. The parameters necessary to determine the total inactivation ratio must be monitored as follows:
(i) The temperature of the disinfected water must be measured at least once per day at each residual disinfectant concentration sampling point.
(ii) If the system uses chlorine, the pH of the disinfected water must be measured at least once per day at each chlorine residual disinfectant concentration sampling point.
(iii) The disinfectant contact time(s) ("T") must be determined for each day during peak hourly flow.
(iv) The residual disinfectant concentration(s) ("C") of the water before or at the first customer must be measured each day during peak hourly flow.
(v) If a system uses a disinfectant other than chlorine, the system may demonstrate to the State, through the use of a State-approved protocol for on-site disinfection challenge studies or other information satisfactory to the State, that CT99.9 values other than those specified in Tables 2.1 and 3.1 in this section other operational parameters are adequate to demonstrate that the system is achieving the minimum inactivation rates required by �141.72(a)(1).
| Residual (mg/l) | pH | ||||||
| ?6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ?9.0 | |
| ?0.4 | 137 | 163 | 195 | 237 | 277 | 329 | 390 |
| 0.6 | 141 | 168 | 200 | 239 | 286 | 342 | 407 |
| 0.8 | 145 | 172 | 205 | 246 | 295 | 354 | 422 |
| 1.0 | 148 | 176 | 210 | 253 | 304 | 365 | 437 |
| 1.2 | 152 | 180 | 215 | 259 | 313 | 376 | 451 |
| 1.4 | 155 | 184 | 221 | 266 | 321 | 387 | 464 |
| 1.6 | 157 | 189 | 226 | 273 | 329 | 397 | 477 |
| 1.8 | 162 | 193 | 231 | 279 | 338 | 407 | 489 |
| 2.0 | 165 | 197 | 236 | 286 | 346 | 417 | 500 |
| 2.2 | 169 | 201 | 242 | 297 | 353 | 426 | 511 |
| 2.4 | 172 | 205 | 247 | 298 | 361 | 435 | 522 |
| 2.6 | 175 | 209 | 252 | 304 | 368 | 444 | 533 |
| 2.8 | 178 | 213 | 257 | 310 | 375 | 452 | 543 |
| 3.0 | 181 | 217 | 261 | 316 | 382 | 460 | 552 |
| Free residual (mg/l) | pH | ||||||
| ?6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ?9.0 | |
| ?0.4 | 97 | 117 | 139 | 166 | 198 | 236 | 279 |
| 0.6 | 100 | 120 | 143 | 171 | 204 | 244 | 291 |
| 0.8 | 103 | 122 | 146 | 175 | 210 | 252 | 301 |
| 1.0 | 105 | 125 | 149 | 179 | 216 | 260 | 312 |
| 1.2 | 107 | 127 | 152 | 183 | 221 | 267 | 320 |
| 1.4 | 109 | 130 | 155 | 187 | 227 | 274 | 329 |
| 1.6 | 111 | 132 | 158 | 192 | 232 | 281 | 337 |
| 1.8 | 114 | 135 | 162 | 196 | 238 | 287 | 345 |
| 2.0 | 116 | 138 | 165 | 200 | 243 | 294 | 353 |
| 2.2 | 118 | 140 | 169 | 204 | 248 | 300 | 361 |
| 2.4 | 120 | 143 | 172 | 209 | 253 | 306 | 368 |
| 2.6 | 122 | 146 | 175 | 213 | 258 | 312 | 375 |
| 2.8 | 124 | 148 | 178 | 217 | 263 | 318 | 382 |
| 3.0 | 126 | 151 | 182 | 221 | 268 | 324 | 389 |
1 These CT values achieve greater than a 99.99 percent inactivation of viruses. CT values between the indicated pH values may be determined by linear interpolation. CT values between the indicated temperatures of different tables may be determined by linear interpolation. If no interpolation is used, use the CT99.9 value at the lower temperature, and at the higher pH.
| Free residual (mg/l) | pH | ||||||
| ?6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ?9.0 | |
| ?0.4 | 73 | 88 | 104 | 125 | 149 | 177 | 209 |
| 0.6 | 75 | 90 | 107 | 128 | 153 | 183 | 218 |
| 0.8 | 78 | 92 | 110 | 131 | 158 | 189 | 226 |
| 1.0 | 79 | 94 | 112 | 134 | 162 | 195 | 234 |
| 1.2 | 80 | 95 | 114 | 137 | 166 | 200 | 240 |
| 1.4 | 82 | 98 | 116 | 140 | 170 | 206 | 247 |
| 1.6 | 83 | 99 | 119 | 144 | 174 | 211 | 253 |
| 1.8 | 86 | 101 | 122 | 147 | 179 | 215 | 259 |
| 2.0 | 87 | 104 | 124 | 150 | 182 | 221 | 265 |
| 2.2 | 89 | 105 | 127 | 153 | 186 | 225 | 271 |
| 2.4 | 90 | 107 | 129 | 157 | 190 | 230 | 276 |
| 2.6 | 92 | 110 | 131 | 160 | 194 | 234 | 281 |
| 2.8 | 93 | 111 | 134 | 163 | 197 | 239 | 287 |
| 3.0 | 95 | 113 | 137 | 166 | 201 | 243 | 292 |
1 These CT values achieve greater than a 99.99 percent inactivation of viruses. CT values between the indicated pH values may be determined by linear interpolation. CT values between the indicated temperatures of different tables may be determined by linear interpolation. If no interpolation is used, use the CT99.9 value at the lower temperature, and at the higher pH.
| Free residual (mg/l) | pH | ||||||
| ?6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ?9.0 | |
| ?0.4 | 49 | 59 | 70 | 83 | 99 | 118 | 140 |
| 0.6 | 50 | 60 | 72 | 86 | 102 | 122 | 146 |
| 0.8 | 52 | 61 | 73 | 88 | 105 | 126 | 151 |
| 1.0 | 53 | 63 | 75 | 90 | 108 | 130 | 156 |
| 1.2 | 54 | 64 | 76 | 92 | 111 | 134 | 160 |
| 1.4 | 55 | 65 | 78 | 94 | 114 | 137 | 165 |
| 1.6 | 56 | 66 | 79 | 96 | 116 | 141 | 169 |
| 1.8 | 57 | 68 | 81 | 98 | 119 | 144 | 173 |
| 2.0 | 58 | 69 | 83 | 100 | 122 | 147 | 177 |
| 2.2 | 59 | 70 | 85 | 102 | 124 | 150 | 181 |
| 2.4 | 60 | 72 | 86 | 105 | 127 | 153 | 184 |
| 2.6 | 61 | 73 | 88 | 107 | 129 | 156 | 188 |
| 2.8 | 62 | 74 | 89 | 109 | 132 | 159 | 191 |
| 3.0 | 63 | 76 | 91 | 111 | 134 | 162 | 195 |
| Free residual (mg/l) | pH | ||||||
| ? 6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ?9.0 | |
| ? 0.4 | 36 | 44 | 52 | 62 | 74 | 89 | 105 |
| 0.6 | 38 | 45 | 54 | 64 | 77 | 92 | 109 |
| 0.8 | 39 | 46 | 55 | 66 | 79 | 95 | 113 |
| 1.0 | 39 | 47 | 56 | 67 | 81 | 98 | 117 |
| 1.2 | 39 | 48 | 57 | 69 | 83 | 100 | 120 |
| 1.4 | 40 | 49 | 58 | 70 | 85 | 103 | 123 |
| 1.6 | 41 | 50 | 59 | 72 | 87 | 105 | 126 |
| 1.8 | 42 | 51 | 61 | 74 | 89 | 108 | 129 |
| 2.0 | 43 | 52 | 62 | 75 | 91 | 110 | 132 |
| 2.2 | 44 | 53 | 63 | 77 | 93 | 113 | 135 |
| 2.4 | 45 | 54 | 65 | 78 | 95 | 115 | 138 |
| 2.6 | 46 | 55 | 66 | 80 | 97 | 117 | 141 |
| 2.8 | 47 | 56 | 67 | 81 | 99 | 119 | 143 |
| 3.0 | 47 | 57 | 68 | 83 | 101 | 122 | 146 |
1 These CT values achieve greater than a 99.99 percent inactivation of viruses. CT values between the indicated pH values may be determined by linear interpolation. CT values between the indicated temperatures of different tables may be determined by linear interpolation. If no interpolation is used, use the CT99.9 value at the lower temperature, and at the higher pH.
| Free residual (mg/l) | pH | ||||||
| ? 6.0 | 6.5 | 7.0 | 7.5 | 8.0 | 8.5 | ? 9.0 | |
| ? 0.4 | 24 | 29 | 35 | 42 | 50 | 59 | 70 |
| 0.6 | 25 | 30 | 36 | 43 | 51 | 61 | 73 |
| 0.8 | 26 | 31 | 37 | 44 | 53 | 63 | 75 |
| 1.0 | 26 | 31 | 37 | 45 | 54 | 65 | 78 |
| 1.2 | 27 | 32 | 38 | 46 | 55 | 67 | 80 |
| 1.4 | 27 | 33 | 39 | 47 | 57 | 69 | 82 |
| 1.6 | 28 | 33 | 40 | 48 | 58 | 70 | 84 |
| 1.8 | 29 | 34 | 41 | 49 | 60 | 72 | 86 |
| 2.0 | 29 | 35 | 41 | 50 | 61 | 74 | 88 |
| 2.2 | 30 | 35 | 42 | 51 | 62 | 75 | 90 |
| 2.4 | 30 | 36 | 43 | 52 | 63 | 77 | 92 |
| 2.6 | 31 | 37 | 44 | 53 | 65 | 78 | 94 |
| 2.8 | 31 | 37 | 45 | 54 | 66 | 80 | 96 |
| 3.0 | 32 | 38 | 46 | 55 | 67 | 81 | 97 |
| Temperature | ||||||
| < 1�C | 5 �C | 10 �C | 15 �C | 20 �C | ? 25�C | |
| Chlorine dioxide | 63 | 26 | 23 | 19 | 15 | 11 |
| Ozone | 2.9 | 1.9 | 1.4 | 0.95 | 0.72 | 0.48 |
1 These CT values achieve greater than 99.99 percent inactivation of viruses. CT values between the indicated temperatures may be determined by linear interpolation. If no interpolation is used, use the CT99.9 value at the lower temperature for determining CT99.9 values between indicated temperatures.
| Temperature | ||||
| < 1�C 5 �C | 10�C | 15�C | 20�C | 25 �C |
| 3,800 2,200 | 1,850 | 1,500 | 1,100 | 750 |
1 These values are for pH values of 6 to 9. These CT values may be assumed to achieve greater than 99.99 percent inactivation of viruses only if chlorine is added and mixed in the water prior to the addition of ammonia. If this condition is not met, the system must demonstrate, based on on-site studies or other information, as approved by the State, that the system is achieving at least 99.99 percent inactivation of viruses. CT values between the indicated temperatures may be determined by linear interpolation. If no interpolation is used, use the CT99.9 value at the lower temperature for determining CT99.9 values between indicated temperatures.
(4) The total inactivation ratio must be calculated as follows:
(i) If the system uses only one point of disinfectant application, the system may determine the total inactivation ratio based on either of the following two methods:
(A) One inactivation ratio (CTcalc/CT99.9) is determined before or at the first customer during peak hourly flow and if the CTcalc/CT99.9 ?1.0, the 99.9 percent Giardia lamblia inactivation requirement has been achieved; or
(B) Successive CTcalc/CT99.9 values, representing sequential inactivation ratios, are determined between the point of disinfectant application and a point before or at the first customer during peak hourly flow. Under this alternative, the following method must be used to calculate the total inactivation ratio:
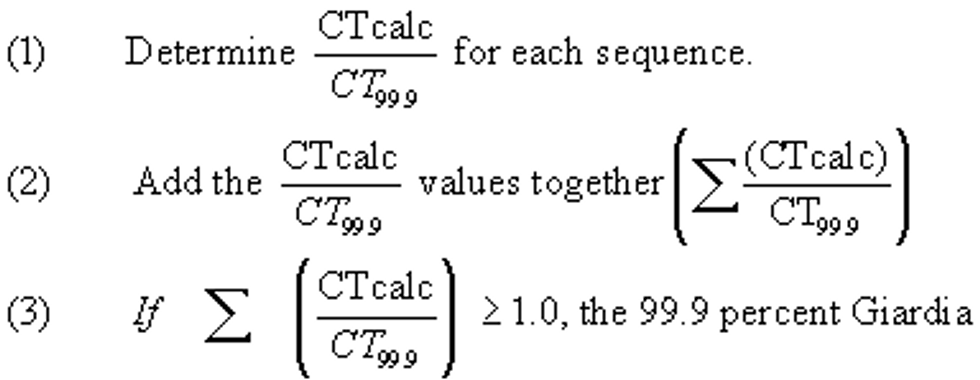
lamblia inactivation requirement has been achieved.
(ii) If the system uses more than one point of disinfectant application before or at the first customer, the system must determine the CT value of each disinfection sequence immediately prior to the next point of disinfectant application during peak hourly flow. The CTcalc/CT99.9 value of each sequence and
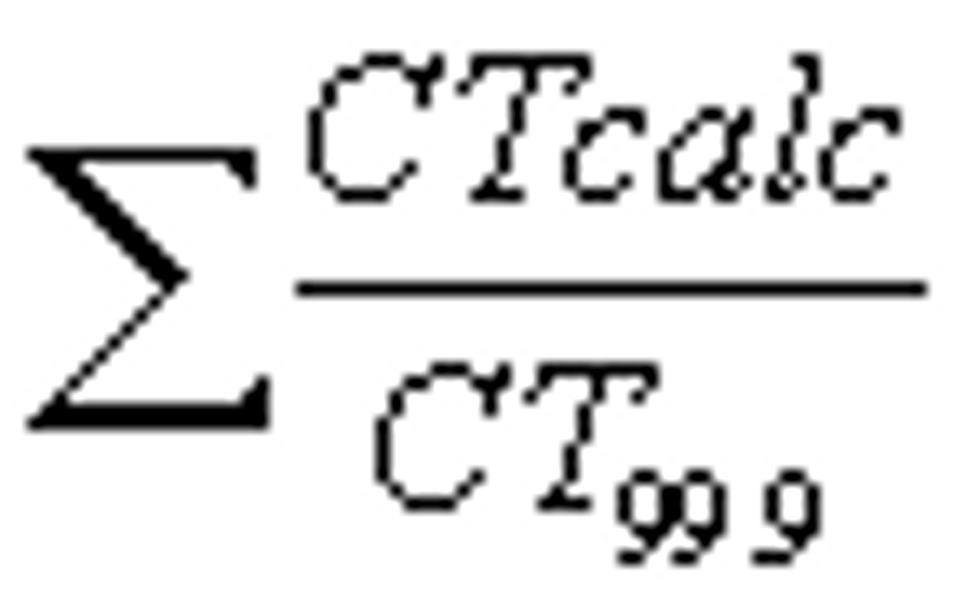
must be calculated using the method in paragraph (b)(4)(i)(B) of this section to determine if the system is in compliance with �141.72(a).
(iii) Although not required, the total percent inactivation for a system with one or more points of residual disinfectant concentration monitoring may be calculated by solving the following equation:
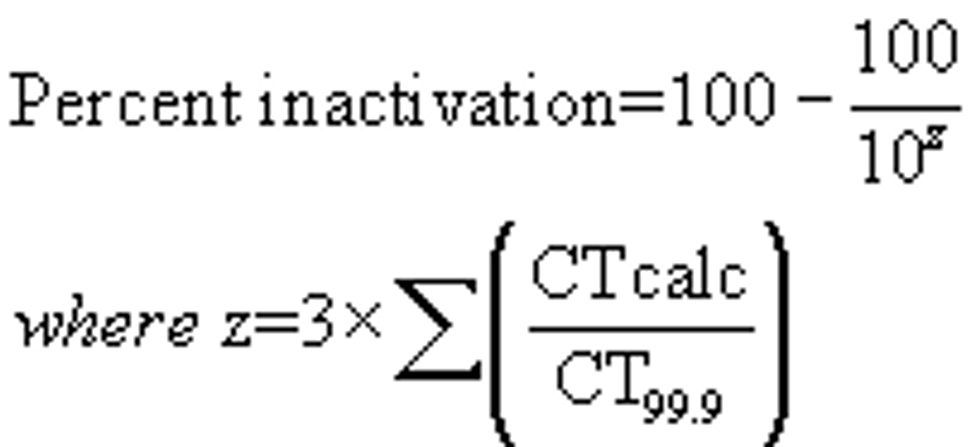
(5) The residual disinfectant concentration of the water entering the distribution system must be monitored continuously, and the lowest value must be recorded each day, except that if there is a failure in the continuous monitoring equipment, grab sampling every 4 hours may be conducted in lieu of continuous monitoring, but for no more than 5 working days following the failure of the equipment, and systems serving 3,300 or fewer persons may take grab samples in lieu of providing continuous monitoring on an ongoing basis at the frequencies prescribed below:
| System size by population | Samples/day1 |
| <500 | 1 |
| 501 to 1,000 | 2 |
| 1,001 to 2,500 | 3 |
| 2,501 to 3,300 | 4 |
1 The day's samples cannot be taken at the same time. The sampling intervals are subject to State review and approval.
If at any time the residual disinfectant concentration falls below 0.2 mg/l in a system using grab sampling in lieu of continuous monitoring, the system must take a grab sample every 4 hours until the residual concentration is equal to or greater than 0.2 mg/l.
(i) Until March 31, 2016, the residual disinfectant concentration must be measured at least at the same points in the distribution system and at the same time as total coliforms are sampled, as specified in �141.21. Beginning April 1, 2016, the residual disinfectant concentration must be measured at least at the same points in the distribution system and at the same time as total coliforms are sampled, as specified in ��141.854 through 141.858. The State may allow a public water system which uses both a surface water source or a ground water source under direct influence of surface water, and a ground water source, to take disinfectant residual samples at points other than the total coliform sampling points if the State determines that such points are more representative of treated (disinfected) water quality within the distribution system. Heterotrophic bacteria, measured as heterotrophic plate count (HPC) as specified in paragraph (a)(1) of this section, may be measured in lieu of residual disinfectant concentration.
(ii) If the State determines, based on site-specific considerations, that a system has no means for having a sample transported and analyzed for HPC by a certified laboratory under the requisite time and temperature conditions specified by paragraph (a)(1) of this section and that the system is providing adequate disinfection in the distribution system, the requirements of paragraph (b)(6)(i) of this section do not apply to that system.
(c) Monitoring requirements for systems using filtration treatment. A public water system that uses a surface water source or a ground water source under the influence of surface water and provides filtration treatment must monitor in accordance with this paragraph (c) beginning June 29, 1993, or when filtration is installed, whichever is later.
(1) Turbidity measurements as required by �141.73 must be performed on representative samples of the system's filtered water every four hours (or more frequently) that the system serves water to the public. A public water system may substitute continuous turbidity monitoring for grab sample monitoring if it validates the continuous measurement for accuracy on a regular basis using a protocol approved by the State. For any systems using slow sand filtration or filtration treatment other than conventional treatment, direct filtration, or diatomaceous earth filtration, the State may reduce the sampling frequency to once per day if it determines that less frequent monitoring is sufficient to indicate effective filtration performance. For systems serving 500 or fewer persons, the State may reduce the turbidity sampling frequency to once per day, regardless of the type of filtration treatment used, if the State determines that less frequent monitoring is sufficient to indicate effective filtration performance.
(2) The residual disinfectant concentration of the water entering the distribution system must be monitored continuously, and the lowest value must be recorded each day, except that if there is a failure in the continuous monitoring equipment, grab sampling every 4 hours may be conducted in lieu of continuous monitoring, but for no more than 5 working days following the failure of the equipment, and systems serving 3,300 or fewer persons may take grab samples in lieu of providing continuous monitoring on an ongoing basis at the frequencies each day prescribed below:
| System size by population | Samples/day 1 |
| �500 | 1 |
| 501 to 1,000 | 2 |
| 1,001 to 2,500 | 3 |
| 2,501 to 3,300 | 4 |
1 The day's samples cannot be taken at the same time. The sampling intervals are subject to State review and approval.
If at any time the residual disinfectant concentration falls below 0.2 mg/l in a system using grab sampling in lieu of continuous monitoring, the system must take a grab sample every 4 hours until the residual disinfectant concentration is equal to or greater than 0.2 mg/l.
(i) Until March 31, 2016, the residual disinfectant concentration must be measured at least at the same points in the distribution system and at the same time as total coliforms are sampled, as specified in �141.21. Beginning April 1, 2016, the residual disinfectant concentration must be measured at least at the same points in the distribution system and at the same time as total coliforms are sampled, as specified in ��141.854 through 141.858. The State may allow a public water system which uses both a surface water source or a ground water source under direct influence of surface water, and a ground water source, to take disinfectant residual samples at points other than the total coliform sampling points if the State determines that such points are more representative of treated (disinfected) water quality within the distribution system. Heterotrophic bacteria, measured as heterotrophic plate count (HPC) as specified in paragraph (a)(1) of this section, may be measured in lieu of residual disinfectant concentration.
(ii) If the State determines, based on site-specific considerations, that a system has no means for having a sample transported and analyzed for HPC by a certified laboratory under the requisite time and temperature conditions specified by paragraph (a)(1) of this section and that the system is providing adequate disinfection in the distribution system, the requirements of paragraph (c)(3)(i) of this section do not apply to that system.
[63 FR 47113 Sept. 3, 1998; 64 FR 72200 Dec. 31, 1999; 66 FR 3496 Jan. 16, 2001; 67 FR 65252 Oct. 23, 2002; 67 FR 65901 Oct. 29, 2002; 69 FR 38855 June 29, 2004; 72 FR 11247, March 12, 2007; 74 FR 30958 June 29, 2009; 78 FR 10347, Feb. 13, 2013]
['Water Programs']
['Water Quality', 'Safe Drinking Water']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
