['Toxic Substances Control Act - EPA']
['Toxic Substances - EPA', 'Toxic Subtances Control Act - EPA']
12/09/2022
...
I. Introduction
The following appendix contains three units. The first unit is the mandatory transmission electron microscopy (TEM) method which all laboratories must follow; it is the minimum requirement for analysis of air samples for asbestos by TEM. The mandatory method contains the essential elements of the TEM method. The second unit contains the complete non- mandatory method. The non-mandatory method supplements the mandatory method by including additional steps to improve the analysis. EPA recommends that the non-mandatory method be employed for analyzing air filters; however, the laboratory may choose to employ the mandatory method. The non-mandatory method contains the same minimum requirements as are outlined in the mandatory method. Hence, laboratories may choose either of the two methods for analyzing air samples by TEM.
The final unit of this Appendix A to Subpart E defines the steps which must be taken to determine completion of response actions. This unit is mandatory.
II. Mandatory Transmission Electron Microscopy Method
A. Definitions of Terms
1. Analytical sensitivity
—Airborne asbestos concentration represented by each fiber counted under the electron microscope. It is determined by the air volume collected and the proportion of the filter examined. This method requires that the analytical sensitivity be no greater than 0.005 structures/ cm3.
2. Asbestiform
—A specific type of mineral fibrosity in which the fibers and fibrils possess high tensile strength and flexibility.
3. Aspect ratio
—A ratio of the length to the width of a particle. Minimum aspect ratio as defined by this method is equal to or greater than 5:1.
4. Bundle
—A structure composed of three or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
5. Clean area
—A controlled environment which is maintained and monitored to assure a low probability of asbestos contamination to materials in that space. Clean areas used in this method have HEPA filtered air under positive pressure and are capable of sustained operation with an open laboratory blank which on subsequent analysis has an average of less than 18 structures/mm2 in an area of 0.057 mm2 (nominally 10 200-mesh grid openings) and a maximum of 53 structures/mm2 for any single preparation for that same area.
6. Cluster
—A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group. Groupings must have more than two intersections.
7. ED
—Electron diffraction.
8. EDXA
—Energy dispersive X-ray analysis.
9. Fiber
—A structure greater than or equal to 0.5 µm in length with an aspect ratio (length to width) of 5:1 or greater and having substantially parallel sides.
10. Grid
—An open structure for mounting on the sample to aid in its examination in the TEM. The term is used here to denote a 200-mesh copper lattice approximately 3 mm in diameter.
11. Intersection
—Nonparallel touching or crossing of fibers, with the projection having an aspect ratio of 5:1 or greater.
12. Laboratory sample coordinator
—That person responsible for the conduct of sample handling and the certification of the testing procedures.
13. Filter background level
—The concentration of structures per square millimeter of filter that is considered indistinguishable from the concentration measured on a blank (filters through which no air has been drawn). For this method the filter background level is defined as 70 structures/mm2.
14. Matrix
—Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
15. NSD
—No structure detected.
16. Operator
—A person responsible for the TEM instrumental analysis of the sample.
17. PCM
—Phase contrast microscopy.
18. SAED
—Selected area electron diffraction.
19. SEM
—Scanning electron microscope.
20. STEM
—Scanning transmission electron microscope.
21. Structure
—a microscopic bundle, cluster, fiber, or matrix which may contain asbestos.
22. S/cm3
—Structures per cubic centimeter.
23. S/mm2
—Structures per square millimeter.
24. TEM
—Transmission electron microscope.
B. Sampling
1. The sampling agency must have written quality control procedures and documents which verify compliance.
2. Sampling operations must be performed by qualified individuals completely independent of the abatement contractor to avoid possible conflict of interest (References 1, 2, 3, and 5 of Unit II.J.).
3. Sampling for airborne asbestos following an abatement action must use commercially available cassettes.
4. Prescreen the loaded cassette collection filters to assure that they do not contain concentrations of asbestos which may interfere with the analysis of the sample. A filter blank average of less than 18 s/mm2 in an area of 0.057 mm2 (nominally 10 200-mesh grid openings) and a single preparation with a maximum of 53 s/mm2 for that same area is acceptable for this method.
5. Use sample collection filters which are either polycarbonate having a pore size less than or equal to 0.4 µm or mixed cellulose ester having a pore size less than or equal to 0.45 µm.
6. Place these filters in series with a 5.0 µm backup filter (to serve as a diffuser) and a support pad. See the following Figure 1:
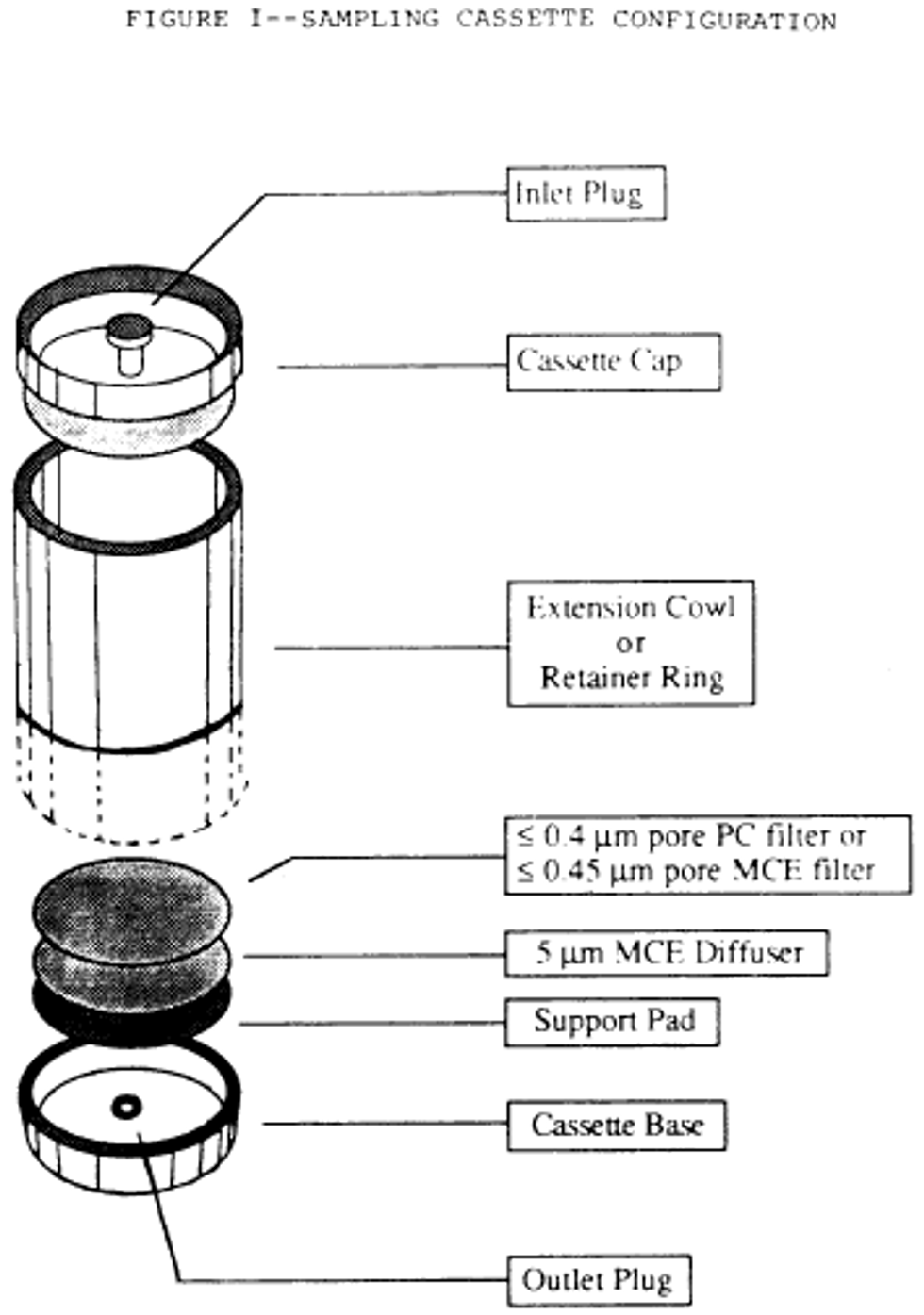
7. Reloading of used cassettes is not permitted.
8. Orient the cassette downward at approximately 45 degrees from the horizontal.
9. Maintain a log of all pertinent sampling information.
10. Calibrate sampling pumps and their flow indicators over the range of their intended use with a recognized standard. Assemble the sampling system with a representative filter (not the filter which will be used in sampling) before and after the sampling operation.
11. Record all calibration information.
12. Ensure that the mechanical vibrations from the pump will be minimized to prevent transferral of vibration to the cassette.
13. Ensure that a continuous smooth flow of negative pressure is delivered by the pump by damping out any pump action fluctuations if necessary.
14. The final plastic barrier around the abatement area remains in place for the sampling period.
15. After the area has passed a thorough visual inspection, use aggressive sampling conditions to dislodge any remaining dust. (See suggested protocol in Unit III.B.7.d.)
16. Select an appropriate flow rate equal to or greater than 1 liter per minute (L/min) or less than 10 L/min for 25 mm cassettes. Larger filters may be operated at proportionally higher flow rates.
17. A minimum of 13 samples are to be collected for each testing site consisting of the following:
a. A minimum of five samples per abatement area.
b. A minimum of five samples per ambient area positioned at locations representative of the air entering the abatement site.
c. Two field blanks are to be taken by removing the cap for not more than 30 seconds and replacing it at the time of sampling before sampling is initiated at the following places:
i. Near the entrance to each abatement area.
ii. At one of the ambient sites. (DO NOT leave the field blanks open during the sampling period.)
d. A sealed blank is to be carried with each sample set. This representative cassette is not to be opened in the field.
18. Perform a leak check of the sampling system at each indoor and outdoor sampling site by activating the pump with the closed sampling cassette in line. Any flow indicates a leak which must be eliminated before initiating the sampling operation.
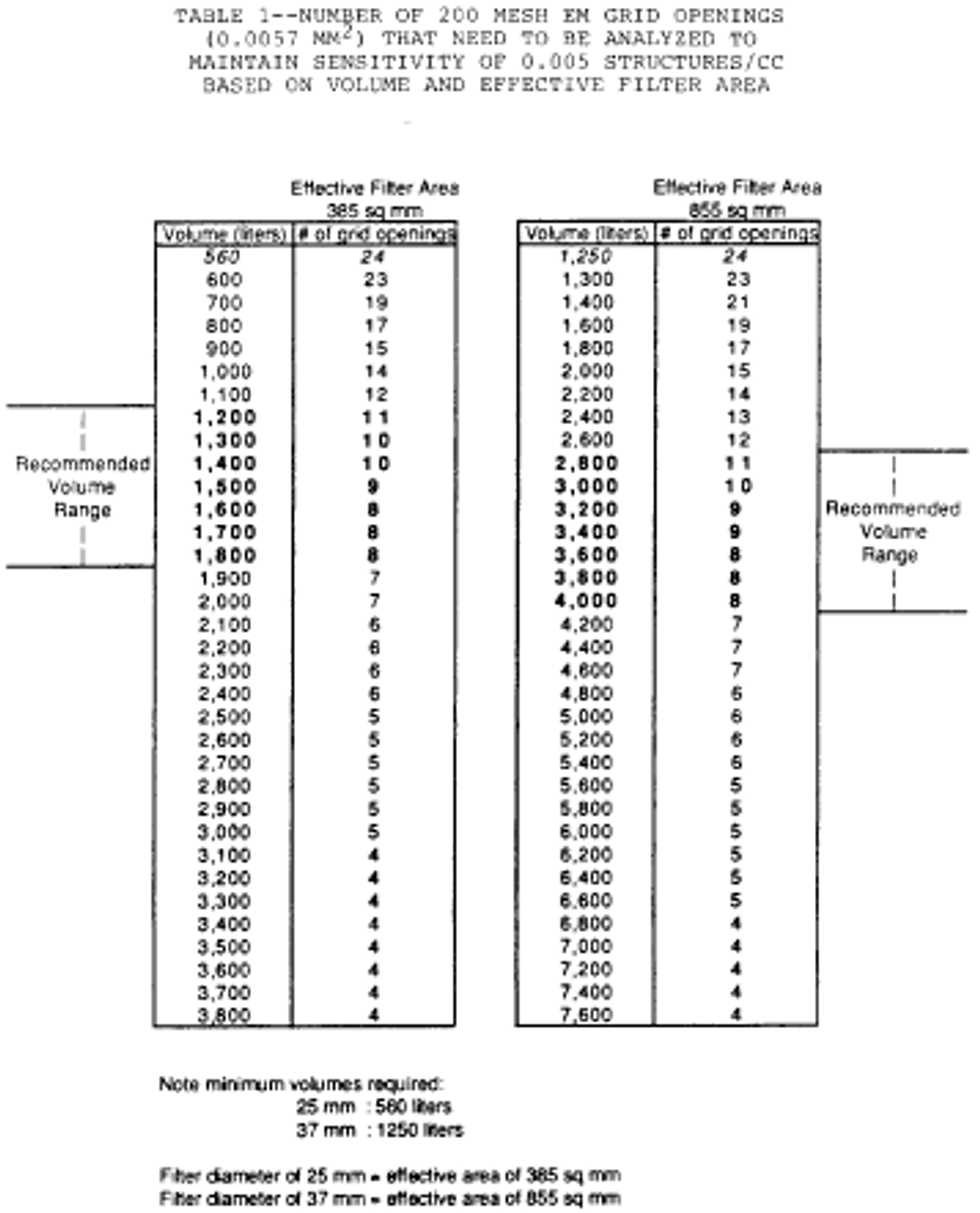
19. The following Table I specifies volume ranges to be used: [Note: Table Omitted]
20. Ensure that the sampler is turned upright before interrupting the pump flow.
21. Check that all samples are clearly labeled and that all pertinent information has been enclosed before transfer of the samples to the laboratory.
22. Ensure that the samples are stored in a secure and representative location.
23. Do not change containers if portions of these filters are taken for other purposes.
24. A summary of Sample Data Quality Objectives is shown in the following Table II:
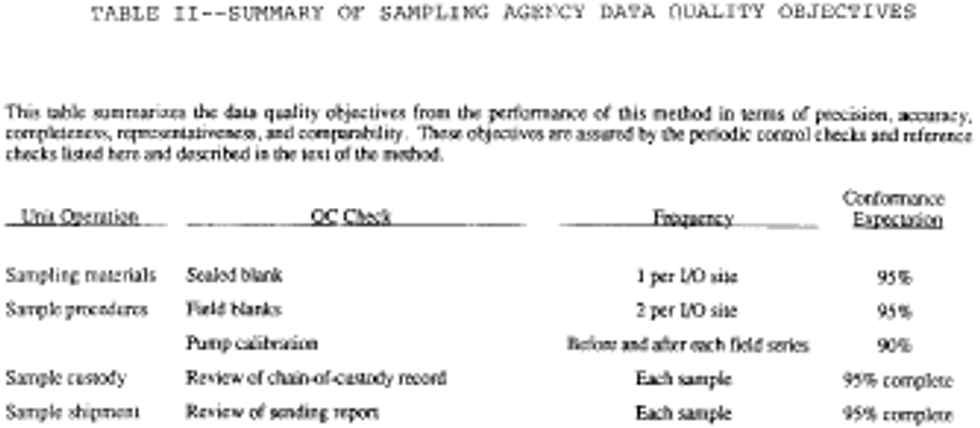
C. Sample Shipment
Ship bulk samples to the analytical laboratory in a separate container from air samples.
D. Sample Receiving
1. Designate one individual as sample coordinator at the laboratory. While that individual will normally be available to receive samples, the coordinator may train and supervise others in receiving procedures for those times when he/she is not available.
2. Bulk samples and air samples delivered to the analytical laboratory in the same container shall be rejected.
E. Sample Preparation
1. All sample preparation and analysis shall be performed by a laboratory independent of the abatement contractor.
2. Wet-wipe the exterior of the cassettes to minimize contamination possibilities before taking them into the clean room facility.
3. Perform sample preparation in a well-equipped clean facility.
Note: The clean area is required to have the following minimum characteristics. The area or hood must be capable of maintaining a positive pressure with make-up air being HEPA-filtered. The cumulative analytical blank concentration must average less than 18 s/mm2 in an area of 0.057 mm2 (nominally 10 200-mesh grid openings) and a single preparation with a maximum of 53 s/mm2 for that same area.
4. Preparation areas for air samples must not only be separated from preparation areas for bulk samples, but they must be prepared in separate rooms.
5. Direct preparation techniques are required. The object is to produce an intact film containing the particulates of the filter surface which is sufficiently clear for TEM analysis.
a. TEM Grid Opening Area measurement must be done as follows:
i. The filter portion being used for sample preparation must have the surface collapsed using an acetone vapor technique.
ii. Measure 20 grid openings on each of 20 random 200-mesh copper grids by placing a grid on a glass and examining it under the PCM. Use a calibrated graticule to measure the average field diameters. From the data, calculate the field area for an average grid opening.
iii. Measurements can also be made on the TEM at a properly calibrated low magnification or on an optical microscope at a magnification of approximately 400X by using an eyepiece fitted with a scale that has been calibrated against a stage micrometer. Optical microscopy utilizing manual or automated procedures may be used providing instrument calibration can be verified.
b. TEM specimen preparation from polycarbonate (PC) filters. Procedures as described in Unit III.G. or other equivalent methods may be used.
c. TEM specimen preparation from mixed cellulose ester (MCE) filters.
i. Filter portion being used for sample preparation must have the surface collapsed using an acetone vapor technique or the Burdette procedure (Ref. 7 of Unit II.J.)
ii. Plasma etching of the collapsed filter is required. The microscope slide to which the collapsed filter pieces are attached is placed in a plasma asher. Because plasma ashers vary greatly in their performance, both from unit to unit and between different positions in the asher chamber, it is difficult to specify the conditions that should be used. Insufficient etching will result in a failure to expose embedded filters, and too much etching may result in loss of particulate from the surface. As an interim measure, it is recommended that the time for ashing of a known weight of a collapsed filter be established and that the etching rate be calculated in terms of micrometers per second. The actual etching time used for the particulate asher and operating conditions will then be set such that a 1-2 µm (10 percent) layer of collapsed surface will be removed.
iii. Procedures as described in Unit III. or other equivalent methods may be used to prepare samples.
F. TEM Method
1. An 80-120 kV TEM capable of performing electron diffraction with a fluorescent screen inscribed with calibrated gradations is required. If the TEM is equipped with EDXA it must either have a STEM attachment or be capable of producing a spot less than 250 nm in diameter at crossover. The microscope shall be calibrated routinely for magnification and camera constant.
2. Determination of Camera Constant and ED Pattern Analysis. The camera length of the TEM in ED operating mode must be calibrated before ED patterns on unknown samples are observed. This can be achieved by using a carbon-coated grid on which a thin film of gold has been sputtered or evaporated. A thin film of gold is evaporated on the specimen TEM grid to obtain zone-axis ED patterns superimposed with a ring pattern from the polycrystalline gold film. In practice, it is desirable to optimize the thickness of the gold film so that only one or two sharp rings are obtained on the superimposed ED pattern. Thicker gold film would normally give multiple gold rings, but it will tend to mask weaker diffraction spots from the unknown fibrous particulate. Since the unknown d-spacings of most interest in asbestos analysis are those which lie closest to the transmitted beam, multiple gold rings are unnecessary on zone-axis ED patterns. An average camera constant using multiple gold rings can be determined. The camera constant is one-half the diameter of the rings times the interplanar spacing of the ring being measured.
3. Magnification Calibration. The magnification calibration must be done at the fluorescent screen. The TEM must be calibrated at the grid opening magnification (if used) and also at the magnification used for fiber counting. This is performed with a cross grating replica (e.g., one containing 2,160 lines/mm). Define a field of view on the fluorescent screen either by markings or physical boundaries. The field of view must be measurable or previously inscribed with a scale or concentric circles (all scales should be metric). A logbook must be maintained, and the dates of calibration and the values obtained must be recorded. The frequency of calibration depends on the past history of the particular microscope. After any maintenance of the microscope that involved adjustment of the power supplied to the lenses or the high-voltage system or the mechanical disassembly of the electron optical column apart from filament exchange, the magnification must be recalibrated. Before the TEM calibration is performed, the analyst must ensure that the cross grating replica is placed at the same distance from the objective lens as the specimens are. For instruments that incorporate an eucentric tilting specimen stage, all specimens and the cross grating replica must be placed at the eucentric position.
4. While not required on every microscope in the laboratory, the laboratory must have either one microscope equipped with energy dispersive X-ray analysis or access to an equivalent system on a TEM in another laboratory.
5. Microscope settings: 80-120 kV, grid assessment 250-1,000X, then 15,000- 20,000X screen magnification for analysis.
6. Approximately one-half (0.5) of the predetermined sample area to be analyzed shall be performed on one sample grid preparation and the remaining half on a second sample grid preparation.
7. Individual grid openings with greater than 5 percent openings (holes) or covered with greater than 25 percent particulate matter or obviously having nonuniform loading must not be analyzed.
8. Reject the grid if:
a. Less than 50 percent of the grid openings covered by the replica are intact.
b. The replica is doubled or folded.
c. The replica is too dark because of incomplete dissolution of the filter.
9. Recording Rules.
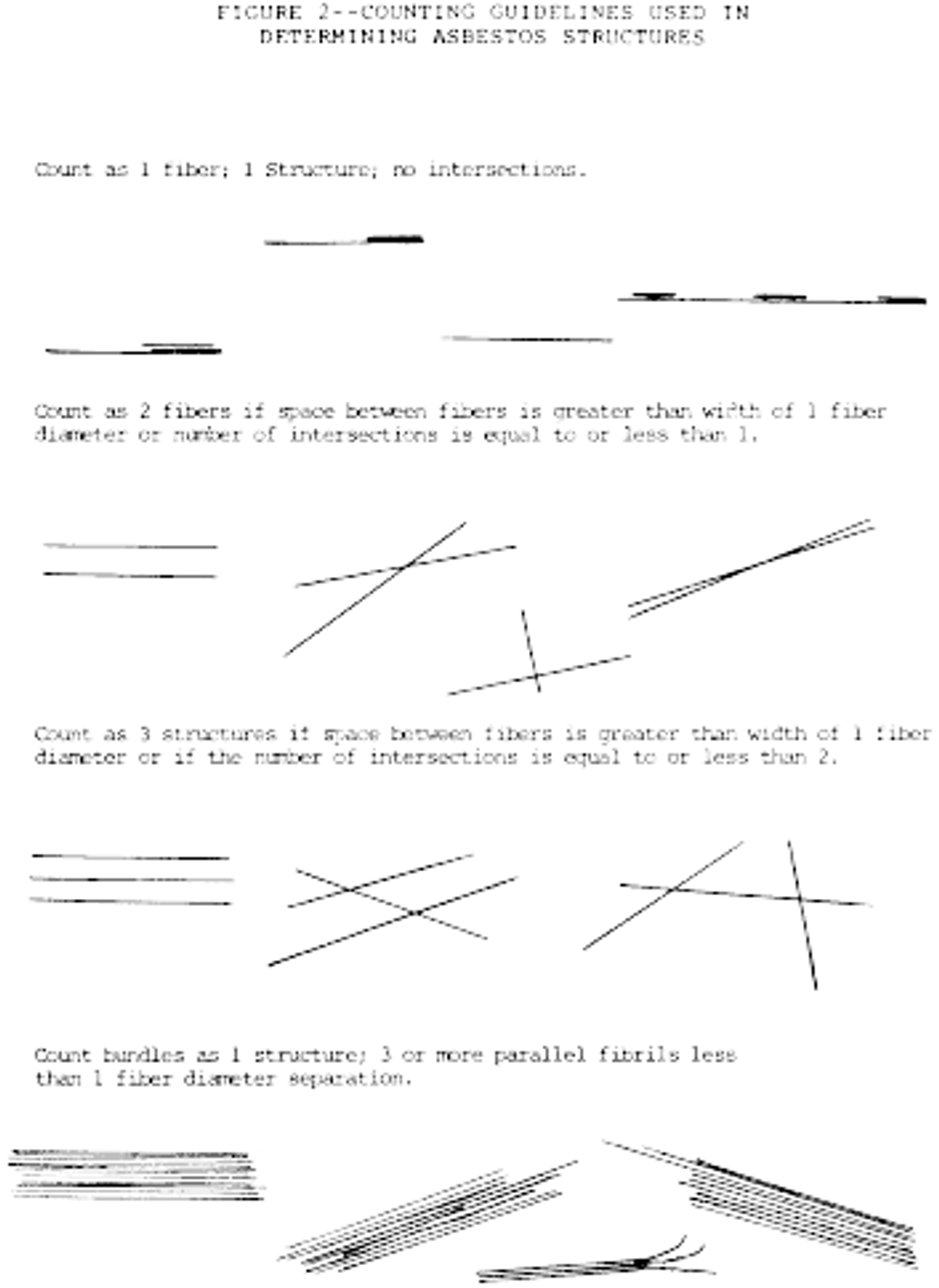
a. Any continuous grouping of particles in which an asbestos fiber with an aspect ratio greater than or equal to 5:1 and a length greater than or equal to 0.5 µm is detected shall be recorded on the count sheet. These will be designated asbestos structures and will be classified as fibers, bundles, clusters, or matrices. Record as individual fibers any contiguous grouping having 0, 1, or 2 definable intersections. Groupings having more than 2 intersections are to be described as cluster or matrix. An intersection is a nonparallel touching or crossing of fibers, with the projection having an aspect ratio of 5:1 or greater. See the following Figure 2:
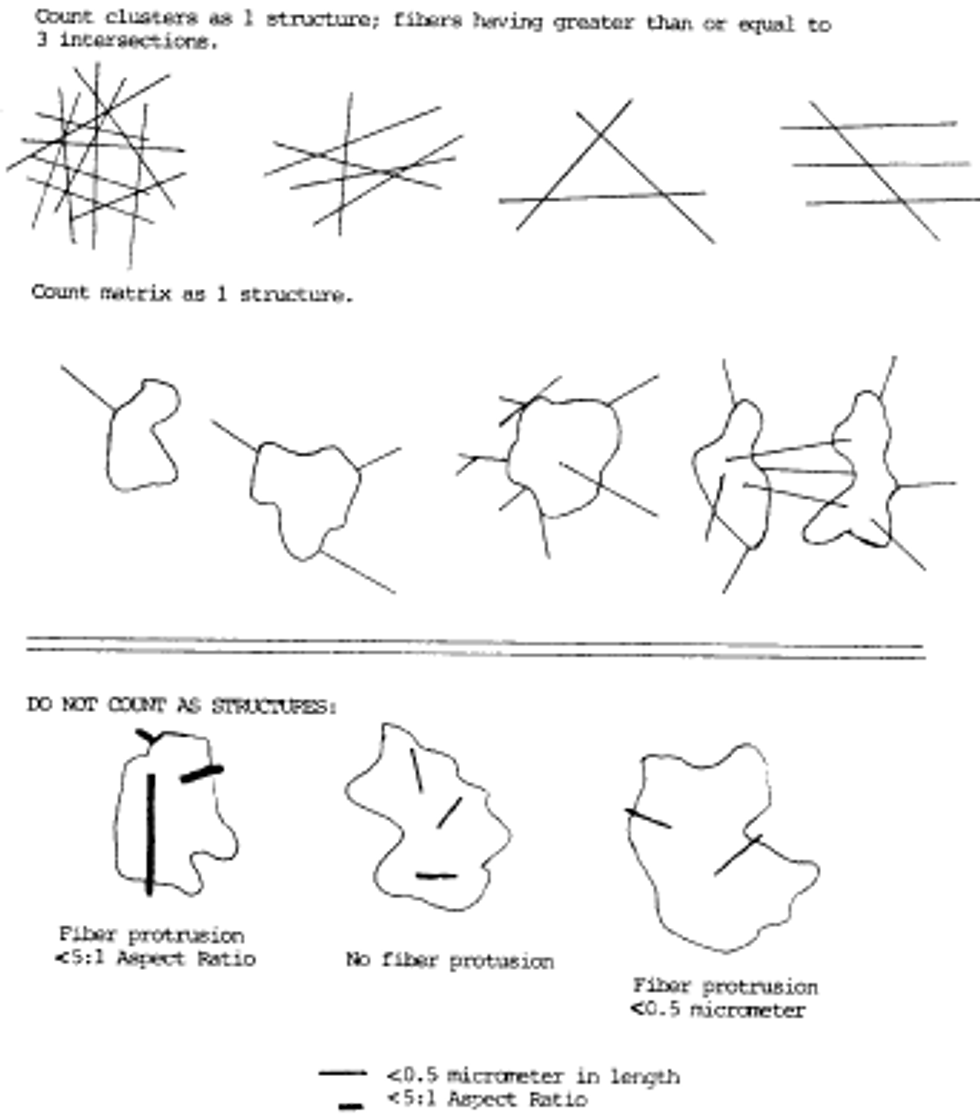
i. Fiber. A structure having a minimum length greater than or equal to 0.5 µm and an aspect ratio (length to width) of 5:1 or greater and substantially parallel sides. Note the appearance of the end of the fiber, i.e., whether it is flat, rounded or dovetailed.
ii. Bundle. A structure composed of three or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
iii. Cluster. A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group. Groupings must have more than two intersections.
iv. Matrix. Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
b. Separate categories will be maintained for fibers less than 5 µm and for fibers equal to or greater than 5 µm in length.
c. Record NSD when no structures are detected in the field.
d. Visual identification of electron diffraction (ED) patterns is required for each asbestos structure counted which would cause the analysis to exceed the 70 s/mm2 concentration. (Generally this means the first four fibers identified as asbestos must exhibit an identifiable diffraction pattern for chrysotile or amphibole.)
e. The micrograph number of the recorded diffraction patterns must be reported to the client and maintained in the laboratory's quality assurance records. In the event that examination of the pattern by a qualified individual indicates that the pattern has been misidentified visually, the client shall be contacted.
f. Energy Dispersive X-ray Analysis (EDXA) is required of all amphiboles which would cause the analysis results to exceed the 70 s/mm2 concentration. (Generally speaking, the first 4 amphiboles would require EDXA.)
g. If the number of fibers in the nonasbestos class would cause the analysis to exceed the 70 s/mm2 concentration, the fact that they are not asbestos must be confirmed by EDXA or measurement of a zone axis diffraction pattern.
h. Fibers classified as chrysotile must be identified by diffraction or X- ray analysis and recorded on a count sheet. X-ray analysis alone can be used only after 70 s/mm2 have been exceeded for a particular sample.
i. Fibers classified as amphiboles must be identified by X-ray analysis and electron diffraction and recorded on the count sheet. (X-ray analysis alone can be used only after 70 s/mm2 have been exceeded for a particular sample.)
j. If a diffraction pattern was recorded on film, record the micrograph number on the count sheet.
k. If an electron diffraction was attempted but no pattern was observed, record N on the count sheet.
l. If an EDXA spectrum was attempted but not observed, record N on the count sheet.
m. If an X-ray analysis spectrum is stored, record the file and disk number on the count sheet.
10. Classification Rules.
a. Fiber. A structure having a minimum length greater than or equal to 0.5 µm and an aspect ratio (length to width) of 5:1 or greater and substantially parallel sides. Note the appearance of the end of the fiber, i.e., whether it is flat, rounded or dovetailed.
b. Bundle. A structure composed of three or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
c. Cluster. A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group. Groupings must have more than two intersections.
d. Matrix. Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
11. After finishing with a grid, remove it from the microscope, and replace it in the appropriate grid holder. Sample grids must be stored for a minimum of 1 year from the date of the analysis; the sample cassette must be retained for a minimum of 30 days by the laboratory or returned at the client's request.
G. Sample Analytical Sequence
1. Under the present sampling requirements a minimum of 13 samples is to be collected for the clearance testing of an abatement site. These include five abatement area samples, five ambient samples, two field blanks, and one sealed blank.
2. Carry out visual inspection of work site prior to air monitoring.
3. Collect a minimum of 5 air samples inside the work site and 5 samples outside the work site. The indoor and outdoor samples shall be taken during the same time period.
4. Remaining steps in the analytical sequence are contained in Unit IV of this Appendix.
H. Reporting
1. The following information must be reported to the client for each sample analyzed:
a. Concentration in structures per square millimeter and structures per cubic centimeter.
b. Analytical sensitivity used for the analysis.
c. Number of asbestos structures.
d. Area analyzed.
e. Volume of air sampled (which must be initially supplied to lab by client).
f. Copy of the count sheet must be included with the report.
g. Signature of laboratory official to indicate that the laboratory met specifications of the method.
h. Report form must contain official laboratory identification (e.g., letterhead).
i. Type of asbestos.
I. Quality Control/Quality Assurance Procedures (Data Quality Indicators)
Monitoring the environment for airborne asbestos requires the use of sensitive sampling and analysis procedures. Because the test is sensitive, it may be influenced by a variety of factors. These include the supplies used in the sampling operation, the performance of the sampling, the preparation of the grid from the filter and the actual examination of this grid in the microscope. Each of these unit operations must produce a product of defined quality if the analytical result is to be a reliable and meaningful test result. Accordingly, a series of control checks and reference standards are to be performed along with the sample analysis as indicators that the materials used are adequate and the operations are within acceptable limits. In this way, the quality of the data is defined and the results are of known value. These checks and tests also provide timely and specific warning of any problems which might develop within the sampling and analysis operations. A description of these quality control/quality assurance procedures is summarized in the following Table III:
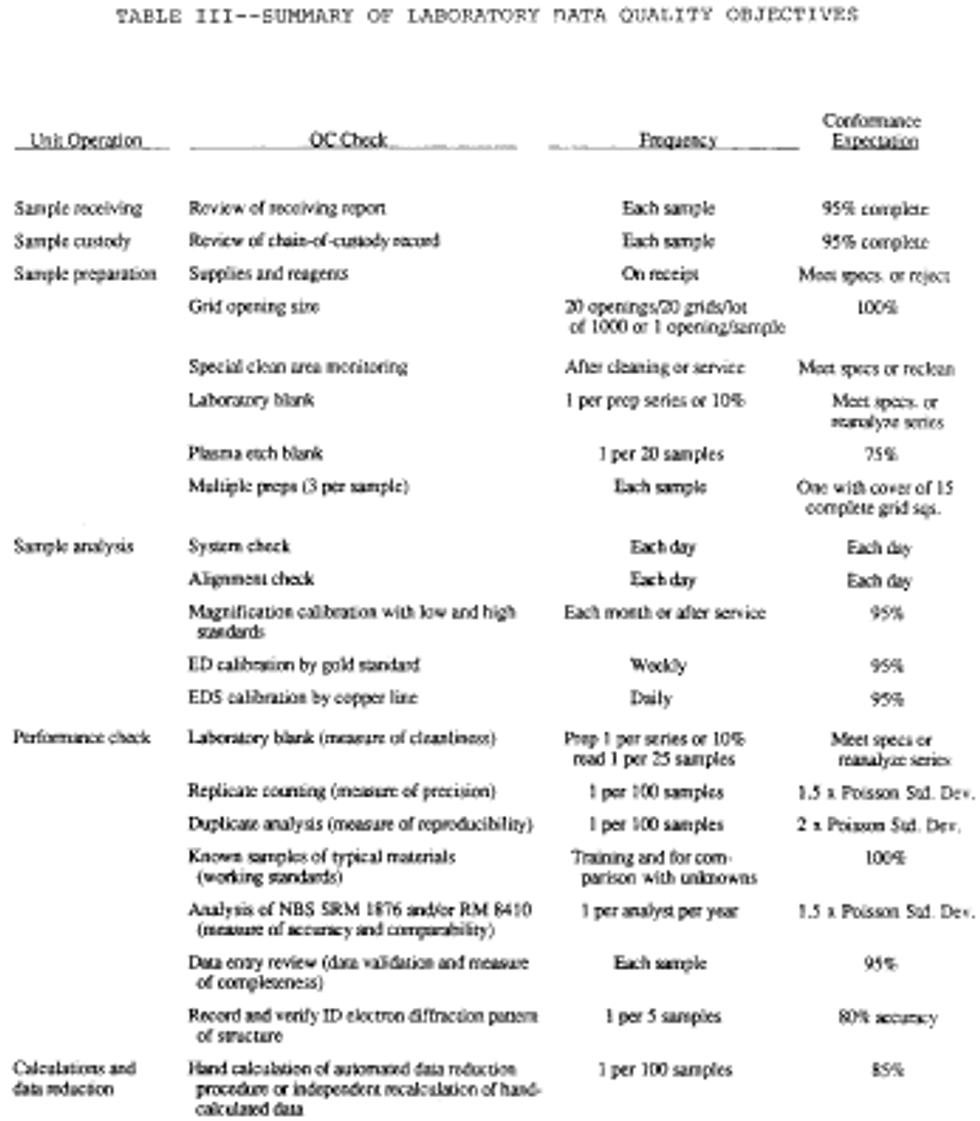
1. When the samples arrive at the laboratory, check the samples and documentation for completeness and requirements before initiating the analysis.
2. Check all laboratory reagents and supplies for acceptable asbestos background levels.
3. Conduct all sample preparation in a clean room environment monitored by laboratory blanks. Testing with blanks must also be done after cleaning or servicing the room.
4. Prepare multiple grids of each sample.
5. Provide laboratory blanks with each sample batch. Maintain a cumulative average of these results. If there are more than 53 fibers/mm2 per 10 200- mesh grid openings, the system must be checked for possible sources of contamination.
6. Perform a system check on the transmission electron microscope daily.
7. Make periodic performance checks of magnification, electron diffraction and energy dispersive X-ray systems as set forth in Table III under Unit II.I.
8. Ensure qualified operator performance by evaluation of replicate analysis and standard sample comparisons as set forth in Table III under Unit II.I.
9. Validate all data entries.
10. Recalculate a percentage of all computations and automatic data reduction steps as specified in Table III under Unit II.I.
11. Record an electron diffraction pattern of one asbestos structure from every five samples that contain asbestos. Verify the identification of the pattern by measurement or comparison of the pattern with patterns collected from standards under the same conditions. The records must also demonstrate that the identification of the pattern has been verified by a qualified individual and that the operator who made the identification is maintaining at least an 80 percent correct visual identification based on his measured patterns.
12. Appropriate logs or records must be maintained by the analytical laboratory verifying that it is in compliance with the mandatory quality assurance procedures.
J. References
For additional background information on this method, the following references should be consulted.
1. "Guidance for Controlling Asbestos-Containing Materials in Buildings," EPA 560/5-85-024, June 1985.
2. "Measuring Airborne Asbestos Following an Abatement Action," USEPA, Office of Toxic Substances, EPA 600/4-85-049, 1985.
3. Small, John and E. Steel. Asbestos Standards: Materials and Analytical Methods. N.B.S. Special Publication 619, 1982.
4. Campbell, W.J., R.L. Blake, L.L. Brown, E.E. Cather, and J.J. Sjoberg. Selected Silicate Minerals and Their Asbestiform Varieties. Information Circular 8751, U.S. Bureau of Mines, 1977.
5. Quality Assurance Handbook for Air Pollution Measurement System. Ambient Air Methods, EPA 600/4-77-027a, USEPA, Office of Research and Development, 1977.
6. Method 2A: Direct Measurement of Gas Volume through Pipes and Small Ducts. 40 CFR Part 60 Appendix A.
7. Burdette, G.J., Health & Safety Exec. Research & Lab. Services Div., London, "Proposed Analytical Method for Determination of Asbestos in Air."
8. Chatfield, E.J., Chatfield Tech. Cons., Ltd., Clark, T., PEI Assoc., "Standard Operating Procedure for Determination of Airborne Asbestos Fibers by Transmission Electron Microscopy Using Polycarbonate Membrane Filters," WERL SOP 87-1, March 5, 1987.
9. NIOSH Method 7402 for Asbestos Fibers, 12-11-86 Draft.
10. Yamate, G., Agarwall, S.C., Gibbons, R.D., IIT Research Institute, "Methodology for the Measurement of Airborne Asbestos by Electron Microscopy," Draft report, USEPA Contract 68-02-3266, July 1984.
11. "Guidance to the Preparation of Quality Assurance Project Plans," USEPA, Office of Toxic Substances, 1984.
III. Nonmandatory Transmission Electron Microscopy Method
A. Definitions of Terms
1.Analytical sensitivity—Airborne asbestos concentration represented by each fiber counted under the electron microscope. It is determined by the air volume collected and the proportion of the filter examined. This method requires that the analytical sensitivity be no greater than 0.005 s/cm3.
2. Asbestiform—A specific type of mineral fibrosity in which the fibers and fibrils possess high tensile strength and flexibility.
3. Aspect ratio—A ratio of the length to the width of a particle. Minimum aspect ratio as defined by this method is equal to or greater than 5:1.
4. Bundle—A structure composed of three or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
5. Clean area—A controlled environment which is maintained and monitored to assure a low probability of asbestos contamination to materials in that space. Clean areas used in this method have HEPA filtered air under positive pressure and are capable of sustained operation with an open laboratory blank which on subsequent analysis has an average of less than 18 structures/mm2 in an area of 0.057 mm2 (nominally 10 200 mesh grid openings) and a maximum of 53 structures/mm2 for no more than one single preparation for that same area.
6. Cluster—A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group. Groupings must have more than two intersections.
7. ED—Electron diffraction.
8. EDXA—Energy dispersive X-ray analysis.
9. Fiber—A structure greater than or equal to 0.5 µm in length with an aspect ratio (length to width) of 5:1 or greater and having substantially parallel sides.
10. Grid—An open structure for mounting on the sample to aid in its examination in the TEM. The term is used here to denote a 200-mesh copper lattice approximately 3 mm in diameter.
11. Intersection—Nonparallel touching or crossing of fibers, with the projection having an aspect ratio of 5:1 or greater.
12. Laboratory sample coordinator—That person responsible for the conduct of sample handling and the certification of the testing procedures.
13.Filter background level—The concentration of structures per square millimeter of filter that is considered indistinguishable from the concentration measured on blanks (filters through which no air has been drawn). For this method the filter background level is defined as 70 structures/mm2.
14. Matrix—Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
15. NSD—No structure detected.
16. Operator—A person responsible for the TEM instrumental analysis of the sample.
17. PCM—Phase contrast microscopy.
18. SAED—Selected area electron diffraction.
19. SEM—Scanning electron microscope.
20. STEM—Scanning transmission electron microscope.
21. Structure—a microscopic bundle, cluster, fiber, or matrix which may contain asbestos.
22. S/cm3 —Structures per cubic centimeter.
23. S/mm2 —Structures per square millimeter.
24. TEM—Transmission electron microscope.
B. Sampling
1. Sampling operations must be performed by qualified individuals completely independent of the abatement contractor to avoid possible conflict of interest (See References 1, 2, and 5 of Unit III.L.) Special precautions should be taken to avoid contamination of the sample. For example, materials that have not been prescreened for their asbestos background content should not be used; also, sample handling procedures which do not take cross contamination possibilities into account should not be used.
2. Material and supply checks for asbestos contamination should be made on all critical supplies, reagents, and procedures before their use in a monitoring study.
3. Quality control and quality assurance steps are needed to identify problem areas and isolate the cause of the contamination (see Reference 5 of Unit III.L.). Control checks shall be permanently recorded to document the quality of the information produced. The sampling firm must have written quality control procedures and documents which verify compliance. Independent audits by a qualified consultant or firm should be performed once a year. All documentation of compliance should be retained indefinitely to provide a guarantee of quality. A summary of Sample Data Quality Objectives is shown in Table II of Unit II.B.
4. Sampling materials.
a. Sample for airborne asbestos following an abatement action using commercially available cassettes.
b. Use either a cowling or a filter-retaining middle piece. Conductive material may reduce the potential for particulates to adhere to the walls of the cowl.
c. Cassettes must be verified as "clean" prior to use in the field. If packaged filters are used for loading or preloaded cassettes are purchased from the manufacturer or a distributor, the manufacturer's name and lot number should be entered on all field data sheets provided to the laboratory, and are required to be listed on all reports from the laboratory.
d. Assemble the cassettes in a clean facility (See definition of clean area under Unit III.A.).
e. Reloading of used cassettes is not permitted.
f. Use sample collection filters which are either polycarbonate having a pore size of less than or equal to 0.4 µm or mixed cellulose ester having a pore size of less than or equal to 0.45 µm.
g. Place these filters in series with a backup filter with a pore size of 5.0 µm (to serve as a diffuser) and a support pad. See the following Figure 1:
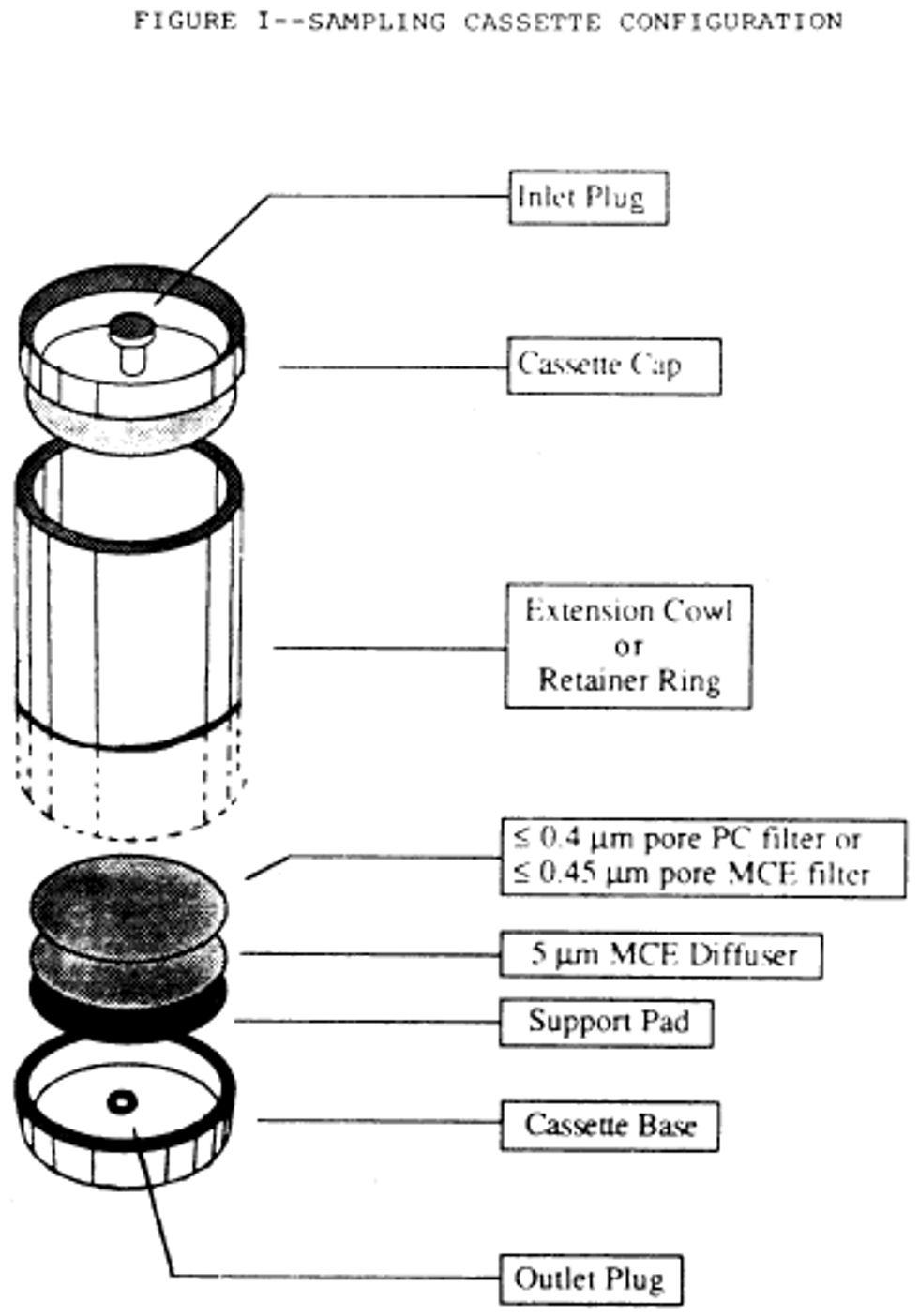
h. When polycarbonate filters are used, position the highly reflective face such that the incoming particulate is received on this surface.
i. Seal the cassettes to prevent leakage around the filter edges or between cassette part joints. A mechanical press may be useful to achieve a reproducible leak-free seal. Shrink fit gel-bands may be used for this purpose and are available from filter manufacturers and their authorized distributors.
j. Use wrinkle-free loaded cassettes in the sampling operation.
5. Pump setup.
a. Calibrate the sampling pump over the range of flow rates and loads anticipated for the monitoring period with this flow measuring device in series. Perform this calibration using guidance from EPA Method 2A each time the unit is sent to the field (See Reference 6 of Unit III.L.).
b. Configure the sampling system to preclude pump vibrations from being transmitted to the cassette by using a sampling stand separate from the pump station and making connections with flexible tubing.
c. Maintain continuous smooth flow conditions by damping out any pump action fluctuations if necessary.
d. Check the sampling system for leaks with the end cap still in place and the pump operating before initiating sample collection. Trace and stop the source of any flow indicated by the flowmeter under these conditions.
e. Select an appropriate flow rate equal to or greater than 1 L/min or less than 10 L/min for 25 mm cassettes. Larger filters may be operated at proportionally higher flow rates.
f. Orient the cassette downward at approximately 45 degrees from the horizontal.
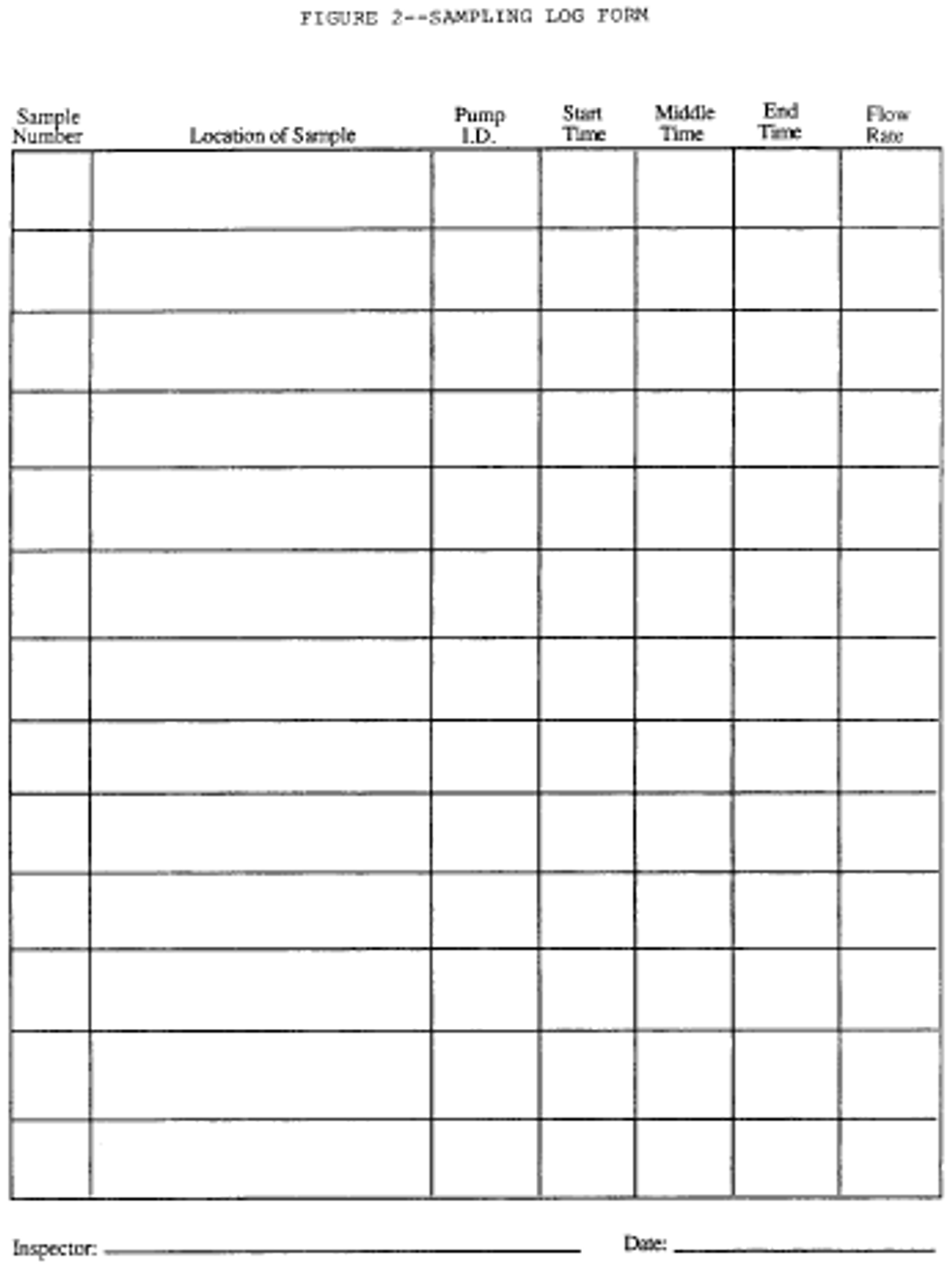
g. Maintain a log of all pertinent sampling information, such as pump identification number, calibration data, sample location, date, sample identification number, flow rates at the beginning, middle, and end, start and stop times, and other useful information or comments. Use of a sampling log form is recommended. See the following Figure 2:
h. Initiate a chain of custody procedure at the start of each sampling, if this is requested by the client.
i. Maintain a close check of all aspects of the sampling operation on a regular basis.
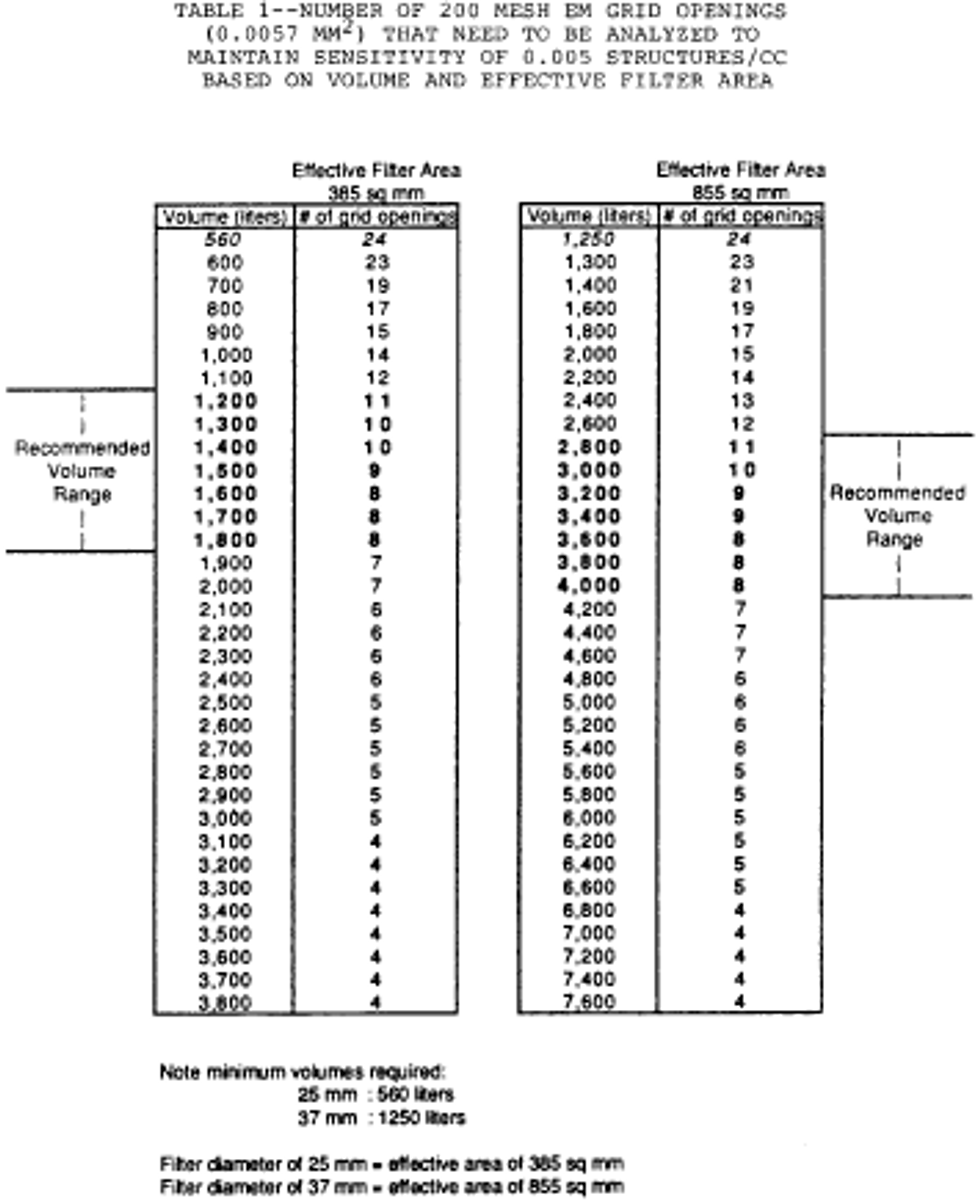
j. Continue sampling until at least the minimum volume is collected, as specified in the following Table I:
k. At the conclusion of sampling, turn the cassette upward before stopping the flow to minimize possible particle loss. If the sampling is resumed, restart the flow before reorienting the cassette downward. Note the condition of the filter at the conclusion of sampling.
l. Double check to see that all information has been recorded on the data collection forms and that the cassette is securely closed and appropriately identified using a waterproof label. Protect cassettes in individual clean resealed polyethylene bags. Bags are to be used for storing cassette caps when they are removed for sampling purposes. Caps and plugs should only be removed or replaced using clean hands or clean disposable plastic gloves.
m. Do not change containers if portions of these filters are taken for other purposes.
6. Minimum sample number per site. A minimum of 13 samples are to be collected for each testing consisting of the following:
a. A minimum of five samples per abatement area.
b. A minimum of five samples per ambient area positioned at locations representative of the air entering the abatement site.
c. Two field blanks are to be taken by removing the cap for not more than 30 sec and replacing it at the time of sampling before sampling is initiated at the following places:
i. Near the entrance to each ambient area.
ii. At one of the ambient sites.
(Note: Do not leave the blank open during the sampling period.)
d. A sealed blank is to be carried with each sample set. This representative cassette is not to be opened in the field.
7. Abatement area sampling.
a. Conduct final clearance sampling only after the primary containment barriers have been removed; the abatement area has been thoroughly dried; and, it has passed visual inspection tests by qualified personnel. (See Reference 1 of Unit III.L.)
b. Containment barriers over windows, doors, and air passageways must remain in place until the TEM clearance sampling and analysis is completed and results meet clearance test criteria. The final plastic barrier remains in place for the sampling period.
c. Select sampling sites in the abatement area on a random basis to provide unbiased and representative samples.
d. After the area has passed a thorough visual inspection, use aggressive sampling conditions to dislodge any remaining dust.
i. Equipment used in aggressive sampling such as a leaf blower and/or fan should be properly cleaned and decontaminated before use.
ii. Air filtration units shall remain on during the air monitoring period.
iii. Prior to air monitoring, floors, ceiling and walls shall be swept with the exhaust of a minimum one (1) horsepower leaf blower.
iv. Stationary fans are placed in locations which will not interfere with air monitoring equipment. Fan air is directed toward the ceiling. One fan shall be used for each 10,000 ft3 of worksite.
v. Monitoring of an abatement work area with high-volume pumps and the use of circulating fans will require electrical power. Electrical outlets in the abatement area may be used if available. If no such outlets are available, the equipment must be supplied with electricity by the use of extension cords and strip plug units. All electrical power supply equipment of this type must be approved Underwriter Laboratory equipment that has not been modified. All wiring must be grounded. Ground fault interrupters should be used. Extreme care must be taken to clean up any residual water and ensure that electrical equipment does not become wet while operational.
vi. Low volume pumps may be carefully wrapped in 6-mil polyethylene to insulate the pump from the air. High volume pumps cannot be sealed in this manner since the heat of the motor may melt the plastic. The pump exhausts should be kept free.
vii. If recleaning is necessary, removal of this equipment from the work area must be handled with care. It is not possible to completely decontaminate the pump motor and parts since these areas cannot be wetted. To minimize any problems in this area, all equipment such as fans and pumps should be carefully wet wiped prior to removal from the abatement area. Wrapping and sealing low volume pumps in 6-mil polyethylene will provide easier decontamination of this equipment. Use of clean water and disposable wipes should be available for this purpose.
e. Pump flow rate equal to or greater than 1 L/min or less than 10 L/min may be used for 25 mm cassettes. The larger cassette diameters may have comparably increased flow.
f. Sample a volume of air sufficient to ensure the minimum quantitation limits. (See Table I of Unit III.B.5.j.)
8. Ambient sampling.
a. Position ambient samplers at locations representative of the air entering the abatement site. If makeup air entering the abatement site is drawn from another area of the building which is outside of the abatement area, place the pumps in the building, pumps should be placed out of doors located near the building and away from any obstructions that may influence wind patterns. If construction is in progress immediately outside the enclosure, it may be necessary to select another ambient site. Samples should be representative of any air entering the work site.
b. Locate the ambient samplers at least 3 ft apart and protect them from adverse weather conditions.
c. Sample same volume of air as samples taken inside the abatement site.
C. Sample Shipment
1. Ship bulk samples in a separate container from air samples. Bulk samples and air samples delivered to the analytical laboratory in the same container shall be rejected.
2. Select a rigid shipping container and pack the cassettes upright in a noncontaminating nonfibrous medium such as a bubble pack. The use of resealable polyethylene bags may help to prevent jostling of individual cassettes.
3. Avoid using expanded polystyrene because of its static charge potential. Also avoid using particle-based packaging materials because of possible contamination.
4. Include a shipping bill and a detailed listing of samples shipped, their descriptions and all identifying numbers or marks, sampling data, shipper's name, and contact information. For each sample set, designate which are the ambient samples, which are the abatement area samples, which are the field blanks, and which is the sealed blank if sequential analysis is to be performed.
5. Hand-carry samples to the laboratory in an upright position if possible; otherwise choose that mode of transportation least likely to jar the samples in transit.
6. Address the package to the laboratory sample coordinator by name when known and alert him or her of the package description, shipment mode, and anticipated arrival as part of the chain of custody and sample tracking procedures. This will also help the laboratory schedule timely analysis for the samples when they are received.
D. Quality Control/Quality Assurance Procedures (Data Quality Indicators)
Monitoring the environment for airborne asbestos requires the use of sensitive sampling and analysis procedures. Because the test is sensitive, it may be influenced by a variety of factors. These include the supplies used in the sampling operation, the performance of the sampling, the preparation of the grid from the filter and the actual examination of this grid in the microscope. Each of these unit operations must produce a product of defined quality if the analytical result is to be a reliable and meaningful test result. Accordingly, a series of control checks and reference standards is performed along with the sample analysis as indicators that the materials used are adequate and the operations are within acceptable limits. In this way, the quality of the data is defined, and the results are of known value. These checks and tests also provide timely and specific warning of any problems which might develop within the sampling and analysis operations. A description of these quality control/quality assurance procedures is summarized in the text below.
1. Prescreen the loaded cassette collection filters to assure that they do not contain concentrations of asbestos which may interfere with the analysis of the sample. A filter blank average of less than 18 s/mm2 in an area of 0.057 mm2 (nominally 10 200-mesh grid openings) and a maximum of 53 s/mm2 for that same area for any single preparation is acceptable for this method.
2. Calibrate sampling pumps and their flow indicators over the range of their intended use with a recognized standard. Assemble the sampling system with a representative filter—not the filter which will be used in sampling— before and after the sampling operation.
3. Record all calibration information with the data to be used on a standard sampling form.
4. Ensure that the samples are stored in a secure and representative location.
5. Ensure that mechanical calibrations from the pump will be minimized to prevent transferral of vibration to the cassette.
6. Ensure that a continuous smooth flow of negative pressure is delivered by the pump by installing a damping chamber if necessary.
7. Open a loaded cassette momentarily at one of the indoor sampling sites when sampling is initiated. This sample will serve as an indoor field blank.
8. Open a loaded cassette momentarily at one of the outdoor sampling sites when sampling is initiated. This sample will serve as an outdoor field blank.
9. Carry a sealed blank into the field with each sample series. Do not open this cassette in the field.
10. Perform a leak check of the sampling system at each indoor and outdoor sampling site by activating the pump with the closed sampling cassette in line. Any flow indicates a leak which must be eliminated before initiating the sampling operation.
11. Ensure that the sampler is turned upright before interrupting the pump flow.
12. Check that all samples are clearly labeled and that all pertinent information has been enclosed before transfer of the samples to the laboratory.
E. Sample Receiving
1. Designate one individual as sample coordinator at the laboratory. While that individual will normally be available to receive samples, the coordinator may train and supervise others in receiving procedures for those times when he/she is not available.
2. Adhere to the following procedures to ensure both the continued chain-of-custody and the accountability of all samples passing through the laboratory:
a. Note the condition of the shipping package and data written on it upon receipt.
b. Retain all bills of lading or shipping slips to document the shipper and delivery time.
c. Examine the chain-of-custody seal, if any, and the package for its integrity.
d. If there has been a break in the seal or substantive damage to the package, the sample coordinator shall immediately notify the shipper and a responsible laboratory manager before any action is taken to unpack the shipment.
e. Packages with significant damage shall be accepted only by the responsible laboratory manager after discussions with the client.
3. Unwrap the shipment in a clean, uncluttered facility. The sample coordinator or his or her designee will record the contents, including a description of each item and all identifying numbers or marks. A Sample Receiving Form to document this information is attached for use when necessary. (See the following Figure 3.)

Note: The person breaking the chain-of-custody seal and itemizing the contents assumes responsibility for the shipment and signs documents accordingly.
4. Assign a laboratory number and schedule an analysis sequence.
5. Manage all chain-of-custody samples within the laboratory such that their integrity can be ensured and documented.
F. Sample Preparation
1. Personnel not affiliated with the Abatement Contractor shall be used to prepare samples and conduct TEM analysis. Wet-wipe the exterior of the cassettes to minimize contamination possibilities before taking them to the clean sample preparation facility.
2. Perform sample preparation in a well-equipped clean facility.
Note: The clean area is required to have the following minimum characteristics. The area or hood must be capable of maintaining a positive pressure with make-up air being HEPA filtered. The cumulative analytical blank concentration must average less than 18 s/mm2 in an area of 0.057 s/ mm2 (nominally 10 200-mesh grid openings) with no more than one single preparation to exceed 53 s/mm2 for that same area.
3. Preparation areas for air samples must be separated from preparation areas for bulk samples. Personnel must not prepare air samples if they have previously been preparing bulk samples without performing appropriate personal hygiene procedures, i.e., clothing change, showering, etc.
4. Preparation. Direct preparation techniques are required. The objective is to produce an intact carbon film containing the particulates from the filter surface which is sufficiently clear for TEM analysis. Currently recommended direct preparation procedures for polycarbonate (PC) and mixed cellulose ester (MCE) filters are described in Unit III.F.7. and 8. Sample preparation is a subject requiring additional research. Variation on those steps which do not substantively change the procedure, which improve filter clearing or which reduce contamination problems in a laboratory are permitted.
a. Use only TEM grids that have had grid opening areas measured according to directions in Unit III.J.
b. Remove the inlet and outlet plugs prior to opening the cassette to minimize any pressure differential that may be present.
c. Examples of techniques used to prepare polycarbonate filters are described in Unit III.F.7.
d. Examples of techniques used to prepare mixed cellulose ester filters are described in Unit III.F.8.
e. Prepare multiple grids for each sample.
f. Store the three grids to be measured in appropriately labeled grid holders or polyethylene capsules.
5. Equipment.
a. Clean area.
b. Tweezers. Fine-point tweezers for handling of filters and TEM grids.
c. Scalpel Holder and Curved No. 10 Surgical Blades.
d. Microscope slides.
e. Double-coated adhesive tape.
f. Gummed page reinforcements.
g. Micro-pipet with disposal tips 10 to 100 micro-L variable volume.
h. Vacuum coating unit with facilities for evaporation of carbon. Use of a liquid nitrogen cold trap above the diffusion pump will minimize the possibility of contamination of the filter surface by oil from the pumping system. The vacuum-coating unit can also be used for deposition of a thin film of gold.
i. Carbon rod electrodes. Spectrochemically pure carbon rods are required for use in the vacuum evaporator for carbon coating of filters.
j. Carbon rod sharpener. This is used to sharpen carbon rods to a neck. The use of necked carbon rods (or equivalent) allows the carbon to be applied to the filters with a minimum of heating.
k. Low-temperature plasma asher. This is used to etch the surface of collapsed mixed cellulose ester (MCE) filters. The asher should be supplied with oxygen, and should be modified as necessary to provide a throttle or bleed valve to control the speed of the vacuum to minimize disturbance of the filter. Some early models of ashers admit air too rapidly, which may disturb particulates on the surface of the filter during the etching step.
l. Glass petri dishes, 10 cm in diameter, 1 cm high. For prevention of excessive evaporation of solvent when these are in use, a good seal must be provided between the base and the lid. The seal can be improved by grinding the base and lid together with an abrasive grinding material.
m. Stainless steel mesh.
n. Lens tissue.
o. Copper 200-mesh TEM grids, 3 mm in diameter, or equivalent.
p. Gold 200-mesh TEM grids, 3 mm in diameter, or equivalent.
q. Condensation washer.
r. Carbon-coated, 200-mesh TEM grids, or equivalent.
s. Analytical balance, 0.1 mg sensitivity.
t. Filter paper, 9 cm in diameter.
u. Oven or slide warmer. Must be capable of maintaining a temperature of 65-70°C.
v. Polyurethane foam, 6 mm thickness.
w. Gold wire for evaporation.
6. Reagents.
a. General. A supply of ultra-clean, fiber-free water must be available for washing of all components used in the analysis. Water that has been distilled in glass or filtered or deionized water is satisfactory for this purpose. Reagents must be fiber-free.
b. Polycarbonate preparation method-chloroform.
c. Mixed Cellulose Ester (MCE) preparation method-acetone or the Burdette procedure (Ref. 7 of Unit III.L.).
7. TEM specimen preparation from polycarbonate filters.
a. Specimen preparation laboratory. It is most important to ensure that contamination of TEM specimens by extraneous asbestos fibers is minimized during preparation.
b. Cleaning of sample cassettes. Upon receipt at the analytical laboratory and before they are taken into the clean facility or laminar flow hood, the sample cassettes must be cleaned of any contamination adhering to the outside surfaces.
c. Preparation of the carbon evaporator. If the polycarbonate filter has already been carbon-coated prior to receipt, the carbon coating step will be omitted, unless the analyst believes the carbon film is too thin. If there is a need to apply more carbon, the filter will be treated in the same way as an uncoated filter. Carbon coating must be performed with a high-vacuum coating unit. Units that are based on evaporation of carbon filaments in a vacuum generated only by an oil rotary pump have not been evaluated for this application, and must not be used. The carbon rods should be sharpened by a carbon rod sharpener to necks of about 4 mm long and 1 mm in diameter. The rods are installed in the evaporator in such a manner that the points are approximately 10 to 12 cm from the surface of a microscope slide held in the rotating and tilting device.
d. Selection of filter area for carbon coating. Before preparation of the filters, a 75 mm x 50 mm microscope slide is washed and dried. This slide is used to support strips of filter during the carbon evaporation. Two parallel strips of double-sided adhesive tape are applied along the length of the slide. Polycarbonate filters are easily stretched during handling, and cutting of areas for further preparation must be performed with great care. The filter and the MCE backing filter are removed together from the cassette and placed on a cleaned glass microscope slide. The filter can be cut with a curved scalpel blade by rocking the blade from the point placed in contact with the filter. The process can be repeated to cut a strip approximately 3 mm wide across the diameter of the filter. The strip of polycarbonate filter is separated from the corresponding strip of backing filter and carefully placed so that it bridges the gap between the adhesive tape strips on the microscope slide. The filter strip can be held with fine-point tweezers and supported underneath by the scalpel blade during placement on the microscope slide. The analyst can place several such strips on the same microscope slide, taking care to rinse and wet-wipe the scalpel blade and tweezers before handling a new sample. The filter strips should be identified by etching the glass slide or marking the slide using a marker insoluble in water and solvents. After the filter strip has been cut from each filter, the residual parts of the filter must be returned to the cassette and held in position by reassembly of the cassette. The cassette will then be archived for a period of 30 days or returned to the client upon request.
e. Carbon coating of filter strips. The glass slide holding the filter strips is placed on the rotation-tilting device, and the evaporator chamber is evacuated. The evaporation must be performed in very short bursts, separated by some seconds to allow the electrodes to cool. If evaporation is too rapid, the strips of polycarbonate filter will begin to curl, which will lead to cross-linking of the surface material and make it relatively insoluble in chloroform. An experienced analyst can judge the thickness of carbon film to be applied, and some test should be made first on unused filters. If the film is too thin, large particles will be lost from the TEM specimen, and there will be few complete and undamaged grid openings on the specimen. If the coating is too thick, the filter will tend to curl when exposed to chloroform vapor and the carbon film may not adhere to the support mesh. Too thick a carbon film will also lead to a TEM image that is lacking in contrast, and the ability to obtain ED patterns will be compromised. The carbon film should be as thin as possible and remain intact on most of the grid openings of the TEM specimen intact.
f. Preparation of the Jaffe washer. The precise design of the Jaffe washer is not considered important, so any one of the published designs may be used. A washer consisting of a simple stainless steel bridge is recommended. Several pieces of lens tissue approximately 1.0 cm x 0.5 cm are placed on the stainless steel bridge, and the washer is filled with chloroform to a level where the meniscus contacts the underside of the mesh, which results in saturation of the lens tissue. See References 8 and 10 of Unit III.L.
g. Placing of specimens into the Jaffe washer. The TEM grids are first placed on a piece of lens tissue so that individual grids can be picked up with tweezers. Using a curved scalpel blade, the analyst excises three 3 mm square pieces of the carbon-coated polycarbonate filter from the filter strip. The three squares are selected from the center of the strip and from two points between the outer periphery of the active surface and the center. The piece of filter is placed on a TEM specimen grid with the shiny side of the TEM grid facing upwards, and the whole assembly is placed boldly onto the saturated lens tissue in the Jaffe washer. If carbon-coated grids are used, the filter should be placed carbon-coated side down. The three excised squares of filters are placed on the same piece of lens tissue. Any number of separate pieces of lens tissue may be placed in the same Jaffe washer. The lid is then placed on the Jaffe washer, and the system is allowed to stand for several hours, preferably overnight.
h. Condensation washing. It has been found that many polycarbonate filters will not dissolve completely in the Jaffe washer, even after being exposed to chloroform for as long as 3 days. This problem becomes more serious if the surface of the filter was overheated during the carbon evaporation. The presence of undissolved filter medium on the TEM preparation leads to partial or complete obscuration of areas of the sample, and fibers that may be present in these areas of the specimen will be overlooked; this will lead to a low result. Undissolved filter medium also compromises the ability to obtain ED patterns. Before they are counted, TEM grids must be examined critically to determine whether they are adequately cleared of residual filter medium. It has been found that condensation washing of the grids after the initial Jaffe washer treatment, with chloroform as the solvent, clears all residual filter medium in a period of approximately 1 hour. In practice, the piece of lens tissue supporting the specimen grids is transferred to the cold finger of the condensation washer, and the washer is operated for about 1 hour. If the specimens are cleared satisfactorily by the Jaffe washer alone, the condensation washer step may be unnecessary.
8. TEM specimen preparation from MCE filters.
a. This method of preparing TEM specimens from MCE filters is similar to that specified in NIOSH Method 7402. See References 7, 8, and 9 of Unit III.L.
b. Upon receipt at the analytical laboratory, the sample cassettes must be cleaned of any contamination adhering to the outside surfaces before entering the clean sample preparation area.
c. Remove a section from any quadrant of the sample and blank filters.
d. Place the section on a clean microscope slide. Affix the filter section to the slide with a gummed paged reinforcement or other suitable means. Label the slide with a water and solvent-proof marking pen.
e. Place the slide in a petri dish which contains several paper filters soaked with 2 to 3 mL acetone. Cover the dish. Wait 2 to 4 minutes for the sample filter to fuse and clear.
f. Plasma etching of the collapsed filter is required.
i. The microscope slide to which the collapsed filter pieces are attached is placed in a plasma asher. Because plasma ashers vary greatly in their performance, both from unit to unit and between different positions in the asher chamber, it is difficult to specify the conditions that should be used. This is one area of the method that requires further evaluation. Insufficient etching will result in a failure to expose embedded filters, and too much etching may result in loss of particulate from the surface. As an interim measure, it is recommended that the time for ashing of a known weight of a collapsed filter be established and that the etching rate be calculated in terms of micrometers per second. The actual etching time used for a particular asher and operating conditions will then be set such that a 1-2 µm (10 percent) layer of collapsed surface will be removed.
ii. Place the slide containing the collapsed filters into a low-temperature plasma asher, and etch the filter.
g. Transfer the slide to a rotating stage inside the bell jar of a vacuum evaporator. Evaporate a 1 mm x 5 mm section of graphite rod onto the cleared filter. Remove the slide to a clean, dry, covered petri dish.
h. Prepare a second petri dish as a Jaffe washer with the wicking substrate prepared from filter or lens paper placed on top of a 6 mm thick disk of clean spongy polyurethane foam. Cut a V-notch on the edge of the foam and filter paper. Use the V-notch as a reservoir for adding solvent. The wicking substrate should be thin enough to fit into the petri dish without touching the lid.
i. Place carbon-coated TEM grids face up on the filter or lens paper. Label the grids by marking with a pencil on the filter paper or by putting registration marks on the petri dish lid and marking with a waterproof marker on the dish lid. In a fume hood, fill the dish with acetone until the wicking substrate is saturated. The level of acetone should be just high enough to saturate the filter paper without creating puddles.
j. Remove about a quarter section of the carbon-coated filter samples from the glass slides using a surgical knife and tweezers. Carefully place the section of the filter, carbon side down, on the appropriately labeled grid in the acetone-saturated petri dish. When all filter sections have been transferred, slowly add more solvent to the wedge-shaped trough to bring the acetone level up to the highest possible level without disturbing the sample preparations. Cover the petri dish. Elevate one side of the petri dish by placing a slide under it. This allows drops of condensed solvent vapors to form near the edge rather than in the center where they would drip onto the grid preparation.
G. TEM Method
1. Instrumentation.
a. Use an 80-120 kV TEM capable of performing electron diffraction with a fluorescent screen inscribed with calibrated gradations. If the TEM is equipped with EDXA it must either have a STEM attachment or be capable of producing a spot less than 250 nm in diameter at crossover. The microscope shall be calibrated routinely (see Unit III.J.) for magnification and camera constant.
b. While not required on every microscope in the laboratory, the laboratory must have either one microscope equipped with energy dispersive X-ray analysis or access to an equivalent system on a TEM in another laboratory. This must be an Energy Dispersive X-ray Detector mounted on TEM column and associated hardware/software to collect, save, and read out spectral information. Calibration of Multi-Channel Analyzer shall be checked regularly for A1 at 1.48 KeV and Cu at 8.04 KeV, as well as the manufacturer's procedures.
i. Standard replica grating may be used to determine magnification (e.g., 2160 lines/mm).
ii. Gold standard may be used to determine camera constant.
c. Use a specimen holder with single tilt and/or double tilt capabilities.
2. Procedure.
a. Start a new Count Sheet for each sample to be analyzed. Record on count sheet: analyst's initials and date; lab sample number; client sample number microscope identification; magnification for analysis; number of predetermined grid openings to be analyzed; and grid identification. See the following Figure 4:
b. Check that the microscope is properly aligned and calibrated according to the manufacturer's specifications and instructions.
c. Microscope settings: 80-120 kV, grid assessment 250-1000X, then 15,000- 20,000X screen magnification for analysis.
d. Approximately one-half (0.5) of the predetermined sample area to be analyzed shall be performed on one sample grid preparation and the remaining half on a second sample grid preparation.
e. Determine the suitability of the grid.
i. Individual grid openings with greater than 5 percent openings (holes) or covered with greater than 25 percent particulate matter or obviously having nonuniform loading shall not be analyzed.
ii. Examine the grid at low magnification (<1000X) to determine its suitability for detailed study at higher magnifications.
iii. Reject the grid if:
(1) Less than 50 percent of the grid openings covered by the replica are intact.
(2) It is doubled or folded.
(3) It is too dark because of incomplete dissolution of the filter.
iv. If the grid is rejected, load the next sample grid.
v. If the grid is acceptable, continue on to Step 6 if mapping is to be used; otherwise proceed to Step 7.
f. Grid Map (Optional).
i. Set the TEM to the low magnification mode.
ii. Use flat edge or finder grids for mapping.
iii. Index the grid openings (fields) to be counted by marking the acceptable fields for one-half (0.5) of the area needed for analysis on each of the two grids to be analyzed. These may be marked just before examining each grid opening (field), if desired.
iv. Draw in any details which will allow the grid to be properly oriented if it is reloaded into the microscope and a particular field is to be reliably identified.
g. Scan the grid.
i. Select a field to start the examination.
ii. Choose the appropriate magnification (15,000 to 20,000X screen magnification).
iii. Scan the grid as follows.
(1) At the selected magnification, make a series of parallel traverses across the field. On reaching the end of one traverse, move the image one window and reverse the traverse.
Note: A slight overlap should be used so as not to miss any part of the grid opening (field).
(2) Make parallel traverses until the entire grid opening (field) has been scanned.
h. Identify each structure for appearance and size.
i. Appearance and size: Any continuous grouping of particles in which an asbestos fiber within aspect ratio greater than or equal to 5:1 and a length greater than or equal to 0.5 µm is detected shall be recorded on the count sheet. These will be designated asbestos structures and will be classified as fibers, bundles, clusters, or matrices. Record as individual fibers any contiguous grouping having 0, 1, or 2 definable intersections. Groupings having more than 2 intersections are to be described as cluster or matrix. See the following Figure 5:
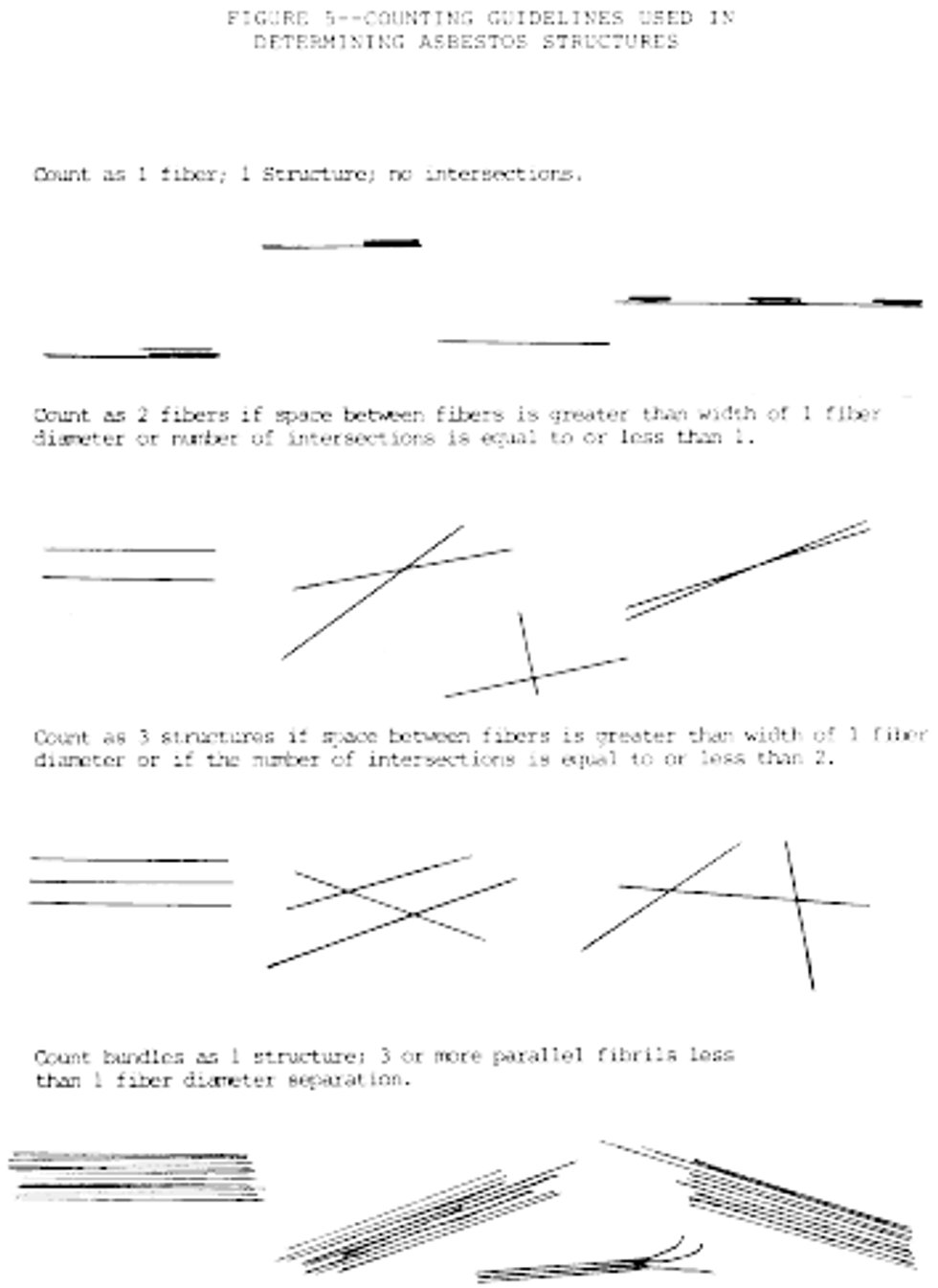
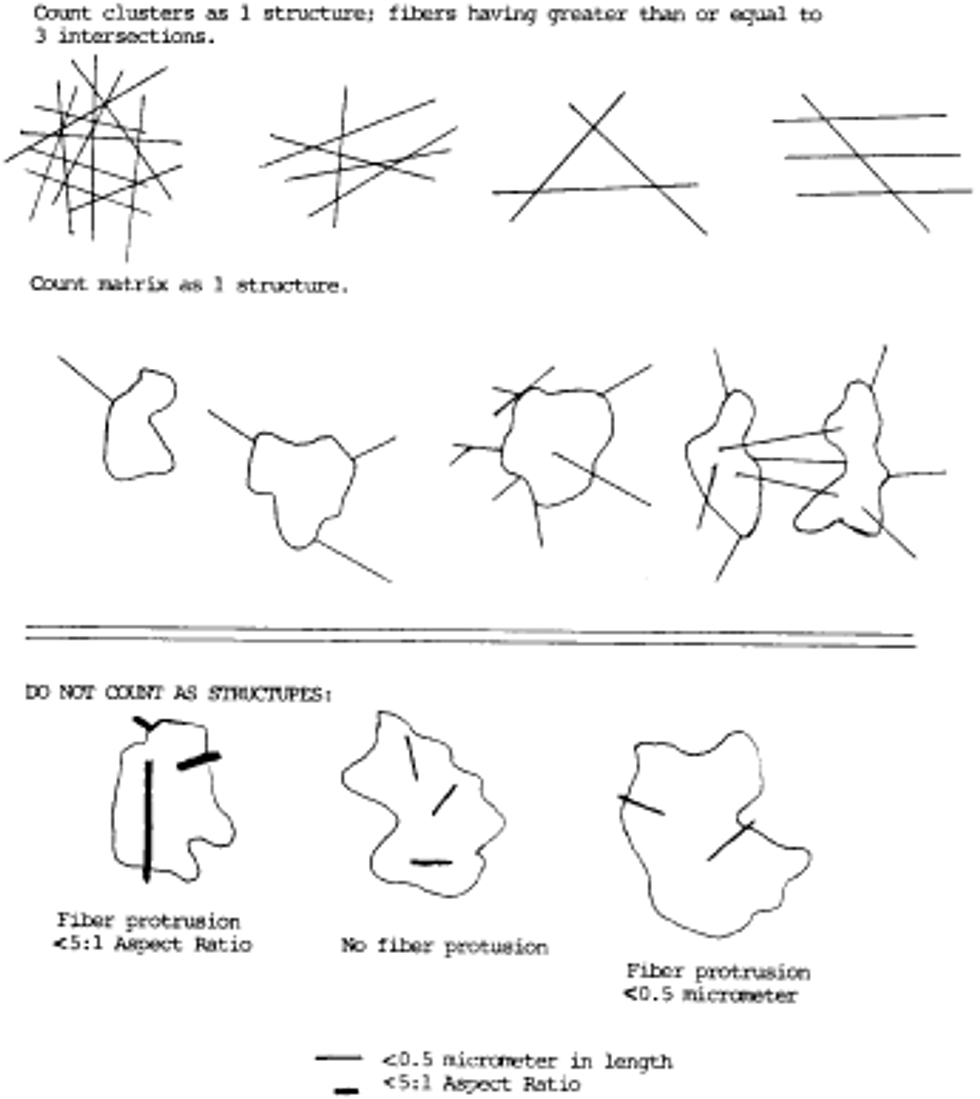
An intersection is a non-parallel touching or crossing of fibers, with the projection having an aspect ratio of 5:1 or greater. Combinations such as a matrix and cluster, matrix and bundle, or bundle and cluster are categorized by the dominant fiber quality-cluster, bundle, and matrix, respectively. Separate categories will be maintained for fibers less than 5 µm and for fibers greater than or equal to 5 µm in length. Not required, but useful, may be to record the fiber length in 1 µm intervals. (Identify each structure morphologically and analyze it as it enters the "window".)
(1) Fiber. A structure having a minimum length greater than 0.5 µm and an aspect ratio (length to width) of 5:1 or greater and substantially parallel sides. Note the appearance of the end of the fiber, i.e., whether it is flat, rounded or dovetailed, no intersections.
(2) Bundle. A structure composed of 3 or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
(3) Cluster. A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group; groupings must have more than 2 intersections.
(4) Matrix. Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
(5) NSD. Record NSD when no structures are detected in the field.
(6) Intersection. Non-parallel touching or crossing of fibers, with the projection having an aspect ratio 5:1 or greater.
ii. Structure Measurement.
(1) Recognize the structure that is to be sized.
(2) Memorize its location in the "window" relative to the sides, inscribed square and to other particulates in the field so this exact location can be found again when scanning is resumed.
(3) Measure the structure using the scale on the screen.
(4) Record the length category and structure type classification on the count sheet after the field number and fiber number.
(5) Return the fiber to its original location in the window and scan the rest of the field for other fibers; if the direction of travel is not remembered, return to the right side of the field and begin the traverse again.
i. Visual identification of Electron Diffraction (ED) patterns is required for each asbestos structure counted which would cause the analysis to exceed the 70 s/mm2 concentration. (Generally this means the first four fibers identified as asbestos must exhibit an identifiable diffraction pattern for chrysotile or amphibole.)
i. Center the structure, focus, and obtain an ED pattern. (See Microscope Instruction Manual for more detailed instructions.)
ii. From a visual examination of the ED pattern, obtained with a short camera length, classify the observed structure as belonging to one of the following classifications: chrysotile, amphibole, or nonasbestos.
(1) Chrysotile: The chrysotile asbestos pattern has characteristic streaks on the layer lines other than the central line and some streaking also on the central line. There will be spots of normal sharpness on the central layer line and on alternate lines (2nd, 4th, etc.). The repeat distance between layer lines is 0.53 nm and the center doublet is at 0.73 nm. The pattern should display (002), (110), (130) diffraction maxima; distances and geometry should match a chrysotile pattern and be measured semiquantitatively.
(2) Amphibole Group [includes grunerite (amosite), crocidolite, anthophyllite, tremolite, and actinolite]: Amphibole asbestos fiber patterns show layer lines formed by very closely spaced dots, and the repeat distance between layer lines is also about 0.53 nm. Streaking in layer lines is occasionally present due to crystal structure defects.
(3) Nonasbestos: Incomplete or unobtainable ED patterns, a nonasbestos EDXA, or a nonasbestos morphology.
iii. The micrograph number of the recorded diffraction patterns must be reported to the client and maintained in the laboratory's quality assurance records. The records must also demonstrate that the identification of the pattern has been verified by a qualified individual and that the operator who made the identification is maintaining at least an 80 percent correct visual identification based on his measured patterns. In the event that examination of the pattern by the qualified individual indicates that the pattern had been misidentified visually, the client shall be contacted. If the pattern is a suspected chrysotile, take a photograph of the diffraction pattern at 0 degrees tilt. If the structure is suspected to be amphibole, the sample may have to be tilted to obtain a simple geometric array of spots.
j. Energy Dispersive X-Ray Analysis (EDXA).
i. Required of all amphiboles which would cause the analysis results to exceed the 70 s/mm2 concentration. (Generally speaking, the first 4 amphiboles would require EDXA.)
ii. Can be used alone to confirm chrysotile after the 70 s/mm2 concentration has been exceeded.
iii. Can be used alone to confirm all nonasbestos.
iv. Compare spectrum profiles with profiles obtained from asbestos standards. The closest match identifies and categorizes the structure.
v. If the EDXA is used for confirmation, record the properly labeled spectrum on a computer disk, or if a hard copy, file with analysis data.
vi. If the number of fibers in the nonasbestos class would cause the analysis to exceed the 70 s/mm2 concentration, their identities must be confirmed by EDXA or measurement of a zone axis diffraction pattern to establish that the particles are nonasbestos.
k. Stopping Rules.
i. If more than 50 asbestiform structures are counted in a particular grid opening, the analysis may be terminated.
ii. After having counted 50 asbestiform structures in a minimum of 4 grid openings, the analysis may be terminated. The grid opening in which the 50th fiber was counted must be completed.
iii. For blank samples, the analysis is always continued until 10 grid openings have been analyzed.
iv. In all other samples the analysis shall be continued until an analytical sensitivity of 0.005 s/cm3 is reached.
l. Recording Rules. The count sheet should contain the following information:
i. Field (grid opening): List field number.
ii. Record "NSD" if no structures are detected.
iii. Structure information.
(1) If fibers, bundles, clusters, and/or matrices are found, list them in consecutive numerical order, starting over with each field.
(2) Length. Record length category of asbestos fibers examined. Indicate if less than 5 µm or greater than or equal to 5 µm.
(3) Structure Type. Positive identification of asbestos fibers is required by the method. At least one diffraction pattern of each fiber type from every five samples must be recorded and compared with a standard diffraction pattern. For each asbestos fiber reported, both a morphological descriptor and an identification descriptor shall be specified on the count sheet.
(4) Fibers classified as chrysotile must be identified by diffraction and/ or X-ray analysis and recorded on the count sheet. X-ray analysis alone can be used as sole identification only after 70s/mm2 have been exceeded for a particular sample.
(5) Fibers classified as amphiboles must be identified by X-ray analysis and electron diffraction and recorded on the count sheet. (X-ray analysis alone can be used as sole identification only after 70s/mm2 have been exceeded for a particular sample.)
(6) If a diffraction pattern was recorded on film, the micrograph number must be indicated on the count sheet.
(7) If an electron diffraction was attempted and an appropriate spectra is not observed, N should be recorded on the count sheet.
(8) If an X-ray analysis is attempted but not observed, N should be recorded on the count sheet.
(9) If an X-ray analysis spectrum is stored, the file and disk number must be recorded on the count sheet.
m. Classification Rules.
i. Fiber. A structure having a minimum length greater than or equal to 0.5 µm and an aspect ratio (length to width) of 5:1 or greater and substantially parallel sides. Note the appearance of the end of the fiber, i.e., whether it is flat, rounded or dovetailed.
ii. Bundle. A structure composed of three or more fibers in a parallel arrangement with each fiber closer than one fiber diameter.
iii. Cluster. A structure with fibers in a random arrangement such that all fibers are intermixed and no single fiber is isolated from the group. Groupings must have more than two intersections.
iv. Matrix. Fiber or fibers with one end free and the other end embedded in or hidden by a particulate. The exposed fiber must meet the fiber definition.
v. NSD. Record NSD when no structures are detected in the field.
n. After all necessary analyses of a particle structure have been completed, return the goniometer stage to 0 degrees, and return the structure to its original location by recall of the original location.
o. Continue scanning until all the structures are identified, classified and sized in the field.
p. Select additional fields (grid openings) at low magnification; scan at a chosen magnification (15,000 to 20,000X screen magnification); and analyze until the stopping rule becomes applicable.
q. Carefully record all data as they are being collected, and check for accuracy.
r. After finishing with a grid, remove it from the microscope, and replace it in the appropriate grid hold. Sample grids must be stored for a minimum of 1 year from the date of the analysis; the sample cassette must be retained for a minimum of 30 days by the laboratory or returned at the client's request.
H. Sample Analytical Sequence
1. Carry out visual inspection of work site prior to air monitoring.
2. Collect a minimum of five air samples inside the work site and five samples outside the work site. The indoor and outdoor samples shall be taken during the same time period.
3. Analyze the abatement area samples according to this protocol. The analysis must meet the 0.005 s/cm3 analytical sensitivity.
4. Remaining steps in the analytical sequence are contained in Unit IV. of this Appendix.
I. Reporting
The following information must be reported to the client. See the following Table II:
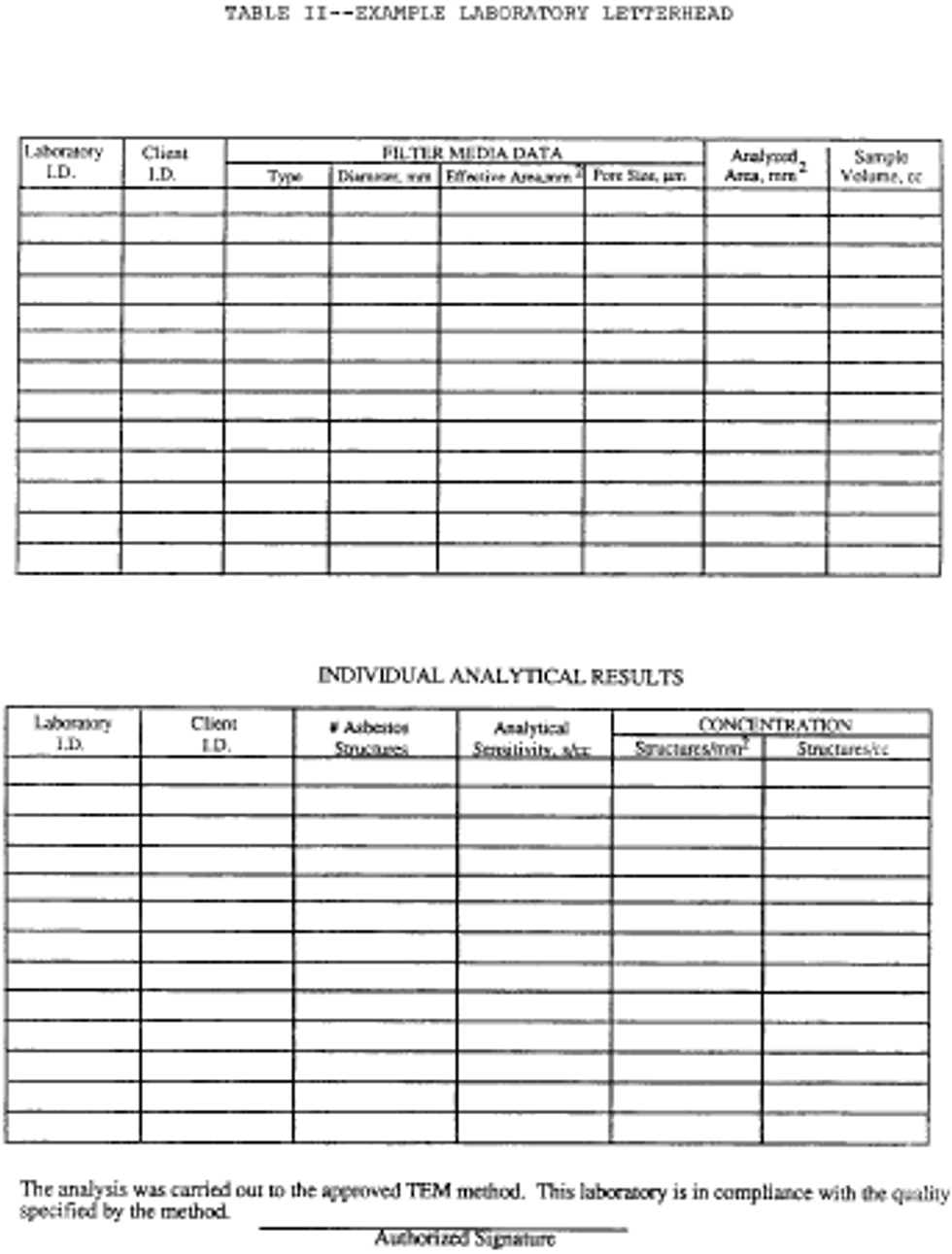
1. Concentration in structures per square millimeter and structures per cubic centimeter.
2. Analytical sensitivity used for the analysis.
3. Number of asbestos structures.
4. Area analyzed.
5. Volume of air samples (which was initially provided by client).
6. Average grid size opening.
7. Number of grids analyzed.
8. Copy of the count sheet must be included with the report.
9. Signature of laboratory official to indicate that the laboratory met specifications of the AHERA method.
10. Report form must contain official laboratory identification (e.g., letterhead).
11. Type of asbestos.
J. Calibration Methodology
Note: Appropriate implementation of the method requires a person knowledgeable in electron diffraction and mineral identification by ED and EDXA. Those inexperienced laboratories wishing to develop capabilities may acquire necessary knowledge through analysis of appropriate standards and by following detailed methods as described in References 8 and 10 of Unit III.L.
1. Equipment Calibration. In this method, calibration is required for the air-sampling equipment and the transmission electron microscope (TEM).
a. TEM Magnification. The magnification at the fluorescent screen of the TEM must be calibrated at the grid opening magnification (if used) and also at the magnification used for fiber counting. This is performed with a cross grating replica. A logbook must be maintained, and the dates of calibration depend on the past history of the particular microscope; no frequency is specified. After any maintenance of the microscope that involved adjustment of the power supplied to the lenses or the high-voltage system or the mechanical disassembly of the electron optical column apart from filament exchange, the magnification must be recalibrated. Before the TEM calibration is performed, the analyst must ensure that the cross grating replica is placed at the same distance from the objective lens as the specimens are. For instruments that incorporate an eucentric tilting specimen stage, all specimens and the cross grating replica must be placed at the eucentric position.
b. Determination of the TEM magnification on the fluorescent screen.
i. Define a field of view on the fluorescent screen either by markings or physical boundaries. The field of view must be measurable or previously inscribed with a scale or concentric circles (all scales should be metric).
ii. Insert a diffraction grating replica (for example a grating containing 2,160 lines/mm) into the specimen holder and place into the microscope. Orient the replica so that the grating lines fall perpendicular to the scale on the TEM fluorescent screen. Ensure that the goniometer stage tilt is 0 degrees.
iii. Adjust microscope magnification to 10,000X or 20,000X. Measure the distance (mm) between two widely separated lines on the grating replica. Note the number of spaces between the lines. Take care to measure between the same relative positions on the lines (e.g., between left edges of lines).
Note: The more spaces included in the measurement, the more accurate the final calculation. On most microscopes, however, the magnification is substantially constant only within the central 8-10 cm diameter region of the fluorescent screen.
iv. Calculate the true magnification (M) on the fluorescent screen:
m=XG/Y
where:
X=total distance (mm) between the designated grating lines;
G=calibration constant of the grating replica (lines/mm):
Y=number of grating replica spaces counted along X.
c. Calibration of the EDXA System. Initially, the EDXA system must be calibrated by using two reference elements to calibrate the energy scale of the instrument. When this has been completed in accordance with the manufacturer's instructions, calibration in terms of the different types of asbestos can proceed. The EDXA detectors vary in both solid angle of detection and in window thickness. Therefore, at a particular accelerating voltage in use on the TEM, the count rate obtained from specific dimensions of fiber will vary both in absolute X-ray count rate and in the relative X-ray peak heights for different elements. Only a few minerals are relevant for asbestos abatement work, and in this procedure the calibration is specified in terms of a "fingerprint" technique. The EDXA spectra must be recorded from individual fibers of the relevant minerals, and identifications are made on the basis of semiquantitative comparisons with these reference spectra.
d. Calibration of Grid Openings.
i. Measure 20 grid openings on each of 20 random 200-mesh copper grids by placing a grid on a glass slide and examining it under the PCM. Use a calibrated graticule to measure the average field diameter and use this number to calculate the field area for an average grid opening. Grids are to be randomly selected from batches up to 1,000.
Note: A grid opening is considered as one field.
ii. The mean grid opening area must be measured for the type of specimen grids in use. This can be accomplished on the TEM at a properly calibrated low magnification or on an optical microscope at a magnification of approximately 400X by using an eyepiece fitted with a scale that has been calibrated against a stage micrometer. Optical microscopy utilizing manual or automated procedures may be used providing instrument calibration can be verified.
e. Determination of Camera Constant and ED Pattern Analysis.
i. The camera length of the TEM in ED operating mode must be calibrated before ED patterns on unknown samples are observed. This can be achieved by using a carbon-coated grid on which a thin film of gold has been sputtered or evaporated. A thin film of gold is evaporated on the specimen TEM grid to obtain zone-axis ED patterns superimposed with a ring pattern from the polycrystalline gold film.
ii. In practice, it is desirable to optimize the thickness of the gold film so that only one or two sharp rings are obtained on the superimposed ED pattern. Thicker gold film would normally give multiple gold rings, but it will tend to mask weaker diffraction spots from the unknown fibrous particulates. Since the unknown d-spacings of most interest in asbestos analysis are those which lie closest to the transmitted beam, mulitiple gold rings are unnecessary on zone-axis ED patterns. An average camera constant using multiple gold rings can be determined. The camera constant is one-half the diameter, D, of the rings times the interplanar spacing, d, of the ring being measured.
K. Quality Control/Quality Assurance Procedures (Data Quality Indicators)
Monitoring the environment for airborne asbestos requires the use of sensitive sampling and analysis procedures. Because the test is sensitive, it may be influenced by a variety of factors. These include the supplies used in the sampling operation, the performance of the sampling, the preparation of the grid from the filter and the actual examination of this grid in the microscope. Each of these unit operations must produce a product of defined quality if the analytical result is to be a reliable and meaningful test result. Accordingly, a series of control checks and reference standards is performed along with the sample analysis as indicators that the materials used are adequate and the operations are within acceptable limits. In this way, the quality of the data is defined and the results are of known value. These checks and tests also provide timely and specific warning of any problems which might develop within the sampling and analysis operations. A description of these quality control/quality assurance procedures is summarized in the following Table III:
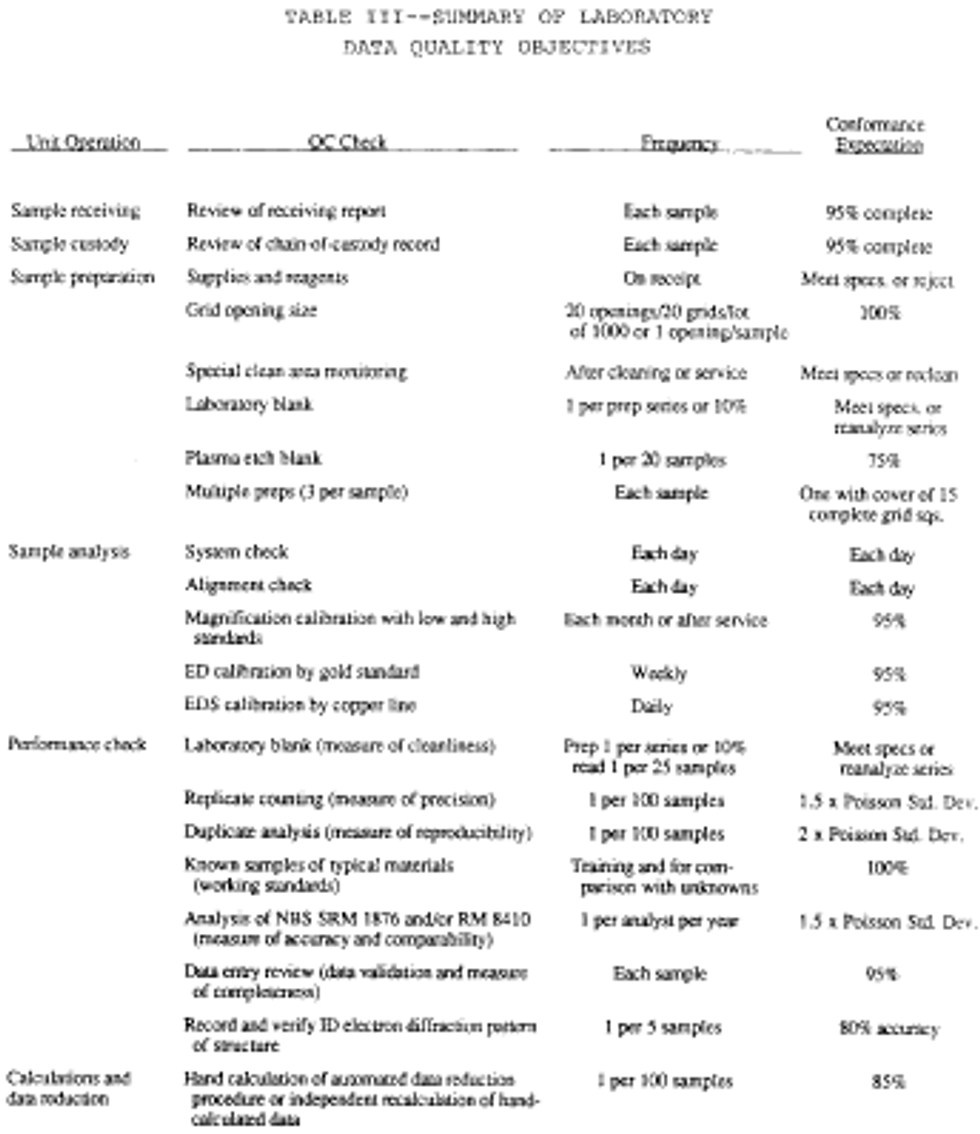
1. When the samples arrive at the laboratory, check the samples and documentation for completeness and requirements before initiating the analysis.
2. Check all laboratory reagents and supplies for acceptable asbestos background levels.
3. Conduct all sample preparation in a clean room environment monitored by laboratory blanks and special testing after cleaning or servicing the room.
4. Prepare multiple grids of each sample.
5. Provide laboratory blanks with each sample batch. Maintain a cumulative average of these results. If this average is greater than 53 f/mm2 per 10 200-mesh grid openings, check the system for possible sources of contamination.
6. Check for recovery of asbestos from cellulose ester filters submitted to plasma asher.
7. Check for asbestos carryover in the plasma asher by including a blank alongside the positive control sample.
8. Perform a systems check on the transmission electron microscope daily.
9. Make periodic performance checks of magnification, electron diffraction and energy dispersive X-ray systems as set forth in Table III of Unit III.K.
10. Ensure qualified operator performance by evaluation of replicate counting, duplicate analysis, and standard sample comparisons as set forth in Table III of Unit III.K.
11. Validate all data entries.
12. Recalculate a percentage of all computations and automatic data reduction steps as specified in Table III.
13. Record an electron diffraction pattern of one asbestos structure from every five samples that contain asbestos. Verify the identification of the pattern by measurement or comparison of the pattern with patterns collected from standards under the same conditions.
The outline of quality control procedures presented above is viewed as the minimum required to assure that quality data is produced for clearance testing of an asbestos abated area. Additional information may be gained by other control tests. Specifics on those control procedures and options available for environmental testing can be obtained by consulting References 6, 7, and 11 of Unit III.L.
L. References
For additional background information on this method the following references should be consulted.
1. "Guidelines for Controlling Asbestos-Containing Materials in Buildings," EPA 560/5-85-024, June 1985.
2. "Measuring Airborne Asbestos Following an Abatement Action," USEPA/ Office of Toxic Substances, EPA 600/4-85-049, 1985.
3. Small, John and E. Steel. Asbestos Standards: Materials and Analytical Methods. N.B.S. Special Publication 619, 1982.
4. Campbell, W.J., R.L. Blake, L.L. Brown, E.E. Cather, and J.J. Sjoberg. Selected Silicate Minerals and Their Asbestiform Varieties. Information Circular 8751, U.S. Bureau of Mines, 1977.
5. Quality Assurance Handbook for Air Pollution Measurement System. Ambient Air Methods, EPA 600/4-77-027a, USEPA, Office of Research and Development, 1977.
6. Method 2A: Direct Measurement of Gas Volume Through Pipes and Small Ducts. 40 CFR Part 60 Appendix A.
7. Burdette, G.J. Health & Safety Exec., Research & Lab. Services Div. , London, "Proposed Analytical Method for Determination of Asbestos in Air."
8. Chatfield, E.J., Chatfield Tech. Cons., Ltd., Clark, T., PEI Assoc. "Standard Operating Procedure for Determination of Airborne Asbestos Fibers by Transmission Electron Microscopy Using Polycarbonate Membrane Filters." WERL SOP 87-1, March 5, 1987.
9. NIOSH. Method 7402 for Asbestos Fibers, December 11, 1986 Draft.
10. Yamate, G., S.C. Agarwall, R.D. Gibbons, IIT Research Institute, "Methodology for the Measurement of Airborne Asbestos by Electron Microscopy." Draft report, USEPA Contract 68-02-3266, July 1984.
11. Guidance to the Preparation of Quality Assurance Project Plans. USEPA, Office of Toxic Substances, 1984.
IV. Mandatory Interpretation of Transmission Electron Microscopy Results to Determine Completion of Response Actions
A. Introduction
A response action is determined to be completed by TEM when the abatement area has been cleaned and the airborne asbestos concentration inside the abatement area is no higher than concentrations at locations outside the abatement area. "Outside" means outside the abatement area, but not necessarily outside the building. EPA reasons that an asbestos removal contractor cannot be expected to clean an abatement area to an airborne asbestos concentration that is lower than the concentration of air entering the abatement area from outdoors or from other parts of the building. After the abatement area has passed a thorough visual inspection, and before the outer containment barrier is removed, a minimum of five air samples inside the abatement area and a minimum of five air samples outside the abatement area must be collected. Hence, the response action is determined to be completed when the average airborne asbestos concentration measured inside the abatement area is not statistically different from the average airborne asbestos concentration measured outside the abatement area.
The inside and outside concentrations are compared by the Z-test, a statistical test that takes into account the variability in the measurement process. A minimum of five samples inside the abatement area and five samples outside the abatement area are required to control the false negative error rate, i.e., the probability of declaring the removal complete when, in fact, the air concentration inside the abatement area is significantly higher than outside the abatement area. Additional quality control is provided by requiring three blanks (filters through which no air has been drawn) to be analyzed to check for unusually high filter contamination that would distort the test results.
When volumes greater than or equal to 1,199 L for a 25 mm filter and 2,799 L for a 37 mm filter have been collected and the average number of asbestos structures on samples inside the abatement area is no greater than 70 s/ mm2 of filter, the response action may be considered complete without comparing the inside samples to the outside samples. EPA is permitting this initial screening test to save analysis costs in situations where the airborne asbestos concentration is sufficiently low so that it cannot be distinguished from the filter contamination/background level (fibers deposited on the filter that are unrelated to the air being sampled). The screening test cannot be used when volumes of less than 1,199 L for 25 mm filter or 2,799 L for a 37 mm filter are collected because the ability to distinguish levels significantly different from filter background is reduced at low volumes.
The initial screening test is expressed in structures per square millimeter of filter because filter background levels come from sources other than the air being sampled and cannot be meaningfully expressed as a concentration per cubic centimeter of air. The value of 70 s/mm2 is based on the experience of the panel of microscopists who consider one structure in 10 grid openings (each grid opening with an area of 0.0057 mm2) to be comparable with contamination/background levels of blank filters. The decision is based, in part, on Poisson statistics which indicate that four structures must be counted on a filter before the fiber count is statistically distinguishable from the count for one structure. As more information on the performance of the method is collected, this criterion may be modified. Since different combinations of the number and size of grid openings are permitted under the TEM protocol, the criterion is expressed in structures per square millimeter of filter to be consistent across all combinations. Four structures per 10 grid openings corresponds to approximately 70 s/mm2.
B. Sample Collection and Analysis
1. A minimum of 13 samples is required: five samples collected inside the abatement area, five samples collected outside the abatement area, two field blanks, and one sealed blank.
2. Sampling and TEM analysis must be done according to either the mandatory or nonmandatory protocols in Appendix A. At least 0.057 mm2 of filter must be examined on blank filters.
C. Interpretation of Results
1. The response action shall be considered complete if either:
a. Each sample collected inside the abatement area consists of at least 1,199 L of air for a 25 mm filter, or 2,799 L of air for a 37 mm filter, and the arithmetic mean of their asbestos structure concentrations per square millimeter of filter is less than or equal to 70 s/mm2; or
b. The three blank samples have an arithmetic mean of the asbestos structure concentration on the blank filters that is less than or equal to 70 s/mm2 and the average airborne asbestos concentration measured inside the abatement area is not statistically higher than the average airborne asbestos concentration measured outside the abatement area as determined by the Z-test. The Z-test is carried out by calculating
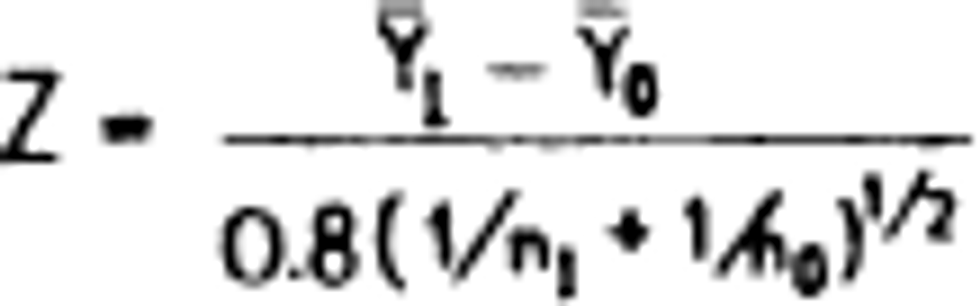
where YI is the average of the natural logarithms of the inside samples and YO is the average of the natural logarithms of the outside samples, nI is the number of inside samples and nO is the number of outside samples. The response action is considered complete if Z is less than or equal to 1.65.
Note: When no fibers are counted, the calculated detection limit for that analysis is inserted for the concentration.
2. If the abatement site does not satisfy either (1) or (2) of this Section C, the site must be recleaned and a new set of samples collected.
D. Sequence for Analyzing Samples
It is possible to determine completion of the response action without analyzing all samples. Also, at any point in the process, a decision may be made to terminate the analysis of existing samples, reclean the abatement site, and collect a new set of samples. The following sequence is outlined to minimize the number of analyses needed to reach a decision.
1. Analyze the inside samples.
2. If at least 1,199 L of air for a 25 mm filter or 2,799 L of air for a 37 mm filter is collected for each inside sample and the arithmetic mean concentration of structures per square millimeter of filter is less than or equal to 70 s/mm2, the response action is complete and no further analysis is needed.
3. If less than 1,199 L of air for a 25 mm filter or 2,799 L of air for a 37 mm filter is collected for any of the inside samples, or the arithmetic mean concentration of structures per square millimeter of filter is greater than 70 s/mm2, analyze the three blanks.
4. If the arithmetic mean concentration of structures per square millimeter on the blank filters is greater than 70 s/mm2, terminate the analysis, identify and correct the source of blank contamination, and collect a new set of samples.
5. If the arithmetic mean concentration of structures per square millimeter on the blank filters is less than or equal to 70 s/mm2, analyze the outside samples and perform the Z-test.
6. If the Z-statistic is less than or equal to 1.65, the response action is complete. If the Z-statistic is greater than 1.65, reclean the abatement site and collect a new set of samples.
['Toxic Substances Control Act - EPA']
['Toxic Substances - EPA', 'Toxic Subtances Control Act - EPA']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
