...
Analytical gases must meet the accuracy and purity specifications of this section, unless you can show that other specifications would not affect your ability to show that you comply with all applicable emission standards.
(a) Subparts C, D, F, and J of this part refer to the following gas specifications:
(1) Use purified gases to zero measurement instruments and to blend with calibration gases. Use gases with contamination no higher than the highest of the following values in the gas cylinder or at the outlet of a zero-gas generator:
(i) 2% contamination, measured relative to the flow-weighted mean concentration expected at the standard. For example, if you would expect a flow-weighted CO concentration of 100.0 µmol/mol, then you would be allowed to use a zero gas with CO contamination less than or equal to 2.000 µmol/mol.
(ii) Contamination as specified in the following table:
| Constituent | Purified Air | Purified N2 |
|---|---|---|
| a We do not require these levels of purity to be NIST-traceable. | ||
| b The N 2 O limit applies only if the standard-setting part requires you to report N 2 O or certify to an N 2 O standard. | ||
| c The H 2 limit only applies for testing with H 2 fuel. | ||
| d The NH 3 limit only applies for testing with NH 3 fuel. | ||
| e The H 2 O limit only applies for water measurement according to §1065.257. | ||
| THC (C1 -equivalent) | ≤ 0.05 μmol/mol | ≤ 0.05 μmol/mol |
| CO | ≤ 1 μmol/mol | ≤ 1 μmol/mol |
| CO2 | ≤ 10 μmol/mol | ≤ 10 μmol/mol |
| O2 | 0.205 to 0.215 mol/mol | ≤ 2 μmol/mol |
| NOX | ≤ 0.02 μmol/mol | ≤ 0.02 μmol/mol |
| N2 O b | ≤ 0.02 μmol/mol | ≤ 0.02 μmol/mol |
| H2 c | ≤ 1 μmol/mol | ≤ 1 μmol/mol |
| NH3 d | ≤ 1 μmol/mol | ≤ 1 μmol/mol |
| H2 O e | ≤ 5 μmol/mol | ≤ 5 μmol/mol |
(2) Use the following gases with a FID analyzer:
(i) FID fuel. Use FID fuel with a stated H 2 concentration of (0.39 to 0.41) mol/mol, balance He or N 2 , and a stated total hydrocarbon concentration of 0.05 μmol/mol or less. For GC-FIDs that measure methane (CH 4 using a FID fuel that is balance N 2 , perform the CH 4 measurement as described in SAE J1151 (incorporated by reference, see §1065.1010).
(ii) FID burner air. Use FID burner air that meets the specifications of purified air in paragraph (a)(1) of this section. For field testing, you may use ambient air.
(iii) FID zero gas. Zero flame-ionization detectors with purified gas that meets the specifications in paragraph (a)(1) of this section, except that the purified gas O2 concentration may be any value. Note that FID zero balance gases may be any combination of purified air and purified nitrogen. We recommend FID analyzer zero gases that contain approximately the expected flow-weighted mean concentration of O2 in the exhaust sample during testing.
(iv) FID propane span gas. Span and calibrate THC FID with span concentrations of propane, C3H8. Calibrate on a carbon number basis of one (C1). For example, if you use a C3H8 span gas of concentration 200 µmol/mol, span a FID to respond with a value of 600 µmol/mol. Note that FID span balance gases may be any combination of purified air and purified nitrogen. We recommend FID analyzer span gases that contain approximately the flow-weighted mean concentration of O2 expected during testing. If the expected O2 concentration in the exhaust sample is zero, we recommend using a balance gas of purified nitrogen.
(v) FID CH4span gas. If you always span and calibrate a CH4 FID with a nonmethane cutter, then span and calibrate the FID with span concentrations of CH4. Calibrate on a carbon number basis of one (C1). For example, if you use a CH4 span gas of concentration 200 µmol/mol, span a FID to respond with a value of 200 µmol/mol. Note that FID span balance gases may be any combination of purified air and purified nitrogen. We recommend FID analyzer span gases that contain approximately the expected flow-weighted mean concentration of O2 in the exhaust sample during testing. If the expected O2 concentration in the exhaust sample is zero, we recommend using a balance gas of purified nitrogen.
(3) Use the following gas mixtures, with gases traceable within ±1% of the NIST-accepted gas standard value or other gas standards we approve:
(i) CH4, balance purified air and/or N2 (as applicable).
(ii) C2H6, balance purified air and/or N2 (as applicable).
(iii) C3H8, balance purified air and/or N2 (as applicable).
(iv) CO, balance purified N2.
(v) CO2, balance purified N2.
(vi) NO, balance purified N2.
(vii) NO2, balance purified air.
(viii) O2, balance purified N2.
(ix) C3H8, CO, CO2, NO, balance purified N2.
(x) C3H8, CH4, CO, CO2, NO, balance purified N2.
(xi) N2O, balance purified air and/or N2 (as applicable).
(xii) CH4, C2H6, balance purified air and/or N2 (as applicable).
(xiii) CH 4 , CH 2 O 2 , C 2 H 2 , C 2 H 4 , C 2 H 4 O, C 2 H 6 , C 3 H 8 , C 3 H 6 , CH 4 O, and C 4 H 10 . You may omit individual gas constituents from this gas mixture. If your gas mixture contains oxygenated hydrocarbons, your gas mixture must be in balance purified N 2 , otherwise you may use balance purified air.
(4) You may use gases for species other than those listed in paragraph (a)(3) of this section (such as methanol in air, which you may use to determine response factors), as long as they are traceable to within ±3% of the NIST-accepted value or other similar standards we approve, and meet the stability requirements of paragraph (b) of this section.
(5) You may generate your own calibration gases using a precision blending device, such as a gas divider, to dilute gases with purified N2 or purified air. If your gas divider meets the specifications in §1065.248, and the gases being blended meet the requirements of paragraphs (a)(1) and (3) of this section, the resulting blends are considered to meet the requirements of this paragraph (a).
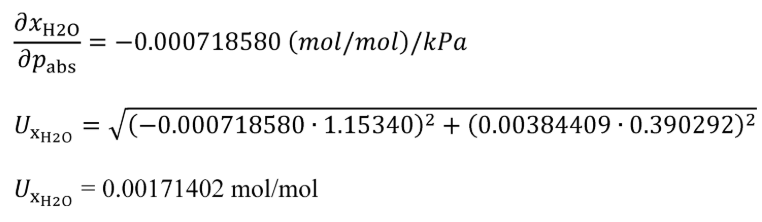
(6) If you measure H 2 O using an FTIR analyzer, generate H 2 O calibration gases with a humidity generator using one of the options in this paragraph (a)(6). Use good engineering judgment to prevent condensation in the transfer lines, fittings, or valves from the humidity generator to the FTIR analyzer. Design your system so the wall temperatures in the transfer lines, fittings, and valves from the point where the mole fraction of H 2 O in the humidified calibration gas, xH2Oref , is measured to the analyzer are at a temperature of (110 to 202) °C. Calibrate the humidity generator upon initial installation, within 370 days before verifying the H 2 O measurement of the FTIR, and after major maintenance. Use the uncertainties from the calibration of the humidity generator's measurements and follow NIST Technical Note 1297 (incorporated by reference, see §1065.1010) to verify that the amount of H 2 O in the calibration gas, xH2Oref , is determined within ±3% uncertainty, UxH2O . If the humidity generator requires assembly before use, after assembly follow the instrument manufacturer's instructions to check for leaks. You may generate the H 2 O calibration gas using one of the following options:
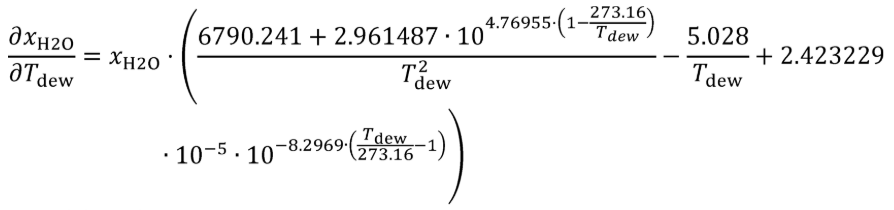
(i) Bubble gas that meets the requirements of paragraph (a)(1) of this section through distilled H 2 O in a sealed vessel. Adjust the amount of H 2 O in the calibration gas by changing the temperature of the H 2 O in the sealed vessel. Determine absolute pressure, pabs , and dewpoint, Tdew , of the humidified gas leaving the sealed vessel. Calculate the amount of H 2 O in the calibration gas as described in §1065.645(a) and (b). Calculate the uncertainty of the amount of H 2 O in the calibration gas, UxH2O , using the following equations:
Eq. 1065.750-1
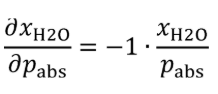
Eq. 1065.750-2
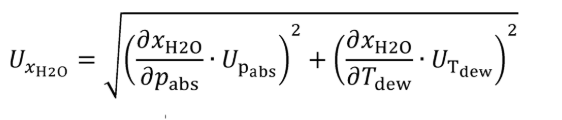
Eq. 1065.750-3
Where:
Tdew = saturation temperature of water at measured conditions.
UT = expanded uncertainty (k = 2) of the measured saturation temperature of water at measured conditions.
pabs = wet static absolute pressure at the location of the dewpoint measurement.
UPabs = expanded uncertainty (k = 2) of the wet static absolute pressure at the location of the dewpoint measurement.

Example:
Tdew = 39.5 °C = 312.65 K
UT = 0.390292 K
pabs = 99.980 kPa
UPabs = 1.15340 kPa
Using Eq. 1065.645-1,
xH2O = 0.0718436 mol/mol
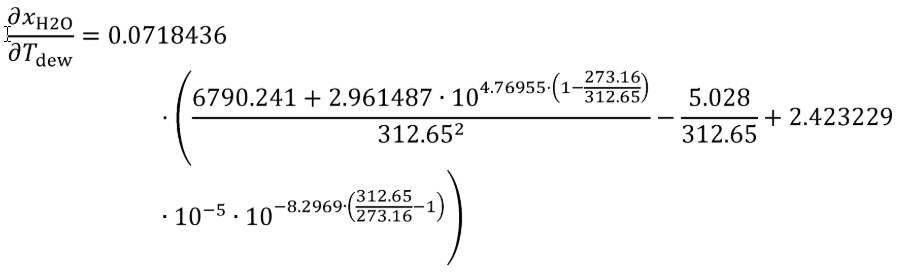

(ii) Use a device that introduces a measured flow of distilled H 2 O as vapor into a measured flow of gas that meets the requirements of paragraph (a)(1) of this section. Determine the molar flows of gas and H 2 O that are mixed to generate the calibration gas.
(A) Calculate the amount of H 2 O in the calibration gas as follows:
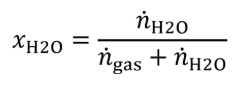
Eq. 1065.750-4
(B) Calculate the uncertainty of the amount of H 2 O in the generated calibration gas, UxH2O, using the following equations:
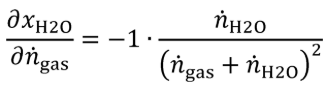
Eq. 1065.750-5
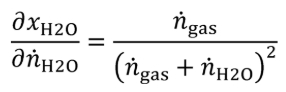
Eq. 1065.750-6
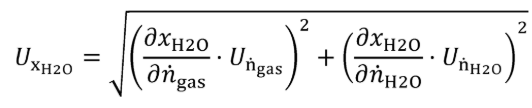
Eq. 1065.750-7
Where:
ngas = molar flow of gas entering the humidity generator.
Un = expanded uncertainty (k=2) of the molar flow of gas entering the humidity generator.
nH2O = molar flow of H 2 O entering the humidity generator, mol/s.
Un = expanded uncertainty (k=2) of the molar flow of H2O entering the humidity generator.
xH2O = amount of H 2 O in the calibration gas.
Ux = expanded uncertainty (k=2) of the amount of H 2 O in the generated calibration gas.
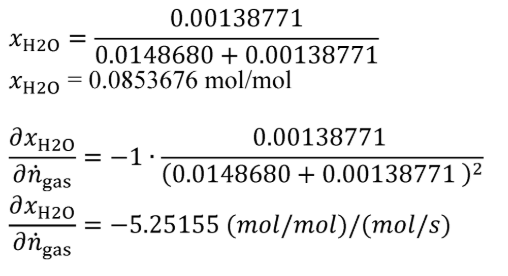
(C) The following example is a solution for using the equations in paragraph (a)(6)(ii)(B) of this section:
nH2O = 0.00138771 mol/s
Un = 0.000226137 mol/s
ngas = 0.0148680 mol/s
U n = 0.0000207436 mol/s

(b) Record the concentration of any calibration gas standard and its expiration date specified by the gas supplier.
(1) Do not use any calibration gas standard after its expiration date, except as allowed by paragraph (b)(2) of this section.
(2) Calibration gases may be relabeled and used after their expiration date as follows:
(i) Alcohol/carbonyl calibration gases used to determine response factors according to subpart I of this part may be relabeled as specified in subpart I of this part.
(ii) Other gases may be relabeled and used after the expiration date only if we approve it in advance.
(c) Transfer gases from their source to analyzers using components that are dedicated to controlling and transferring only those gases. For example, do not use a regulator, valve, or transfer line for zero gas if those components were previously used to transfer a different gas mixture. We recommend that you label regulators, valves, and transfer lines to prevent contamination. Note that even small traces of a gas mixture in the dead volume of a regulator, valve, or transfer line can diffuse upstream into a high-pressure volume of gas, which would contaminate the entire high-pressure gas source, such as a compressed-gas cylinder.
(d) To maintain stability and purity of gas standards, use good engineering judgment and follow the gas standard supplier's recommendations for storing and handling zero, span, and calibration gases. For example, it may be necessary to store bottles of condensable gases in a heated environment.
[70 FR 40516, July 13, 2005, as amended at 73 FR 37343, June 30, 2008; 74 FR 56518, Oct. 30, 2009; 75 FR 68465, Nov. 8, 2010; 76 FR 57467, Sept. 15, 2011; 79 FR 23811, Apr. 28, 2014; 81 FR 74191, Oct. 25, 2016; 86 FR 34574, Jun. 29, 2021; 89 FR 29823, Apr. 22, 2024]
