['Hazard Communication', 'Toxic and Hazardous Substances - OSHA']
['Hazard Classifications', 'Toxic and Hazardous Substances - OSHA', 'Hazard Communication']
01/23/2026
...
B.1 Explosives
B.1.1 Definitions and General Considerations
B.1.1.1 An explosive chemical is a solid or liquid chemical which is in itself capable by chemical reaction of producing gas at such a temperature and pressure and at such a speed as to cause damage to the surroundings. Pyrotechnic chemicals are included even when they do not evolve gases.
A pyrotechnic chemica l is a chemical designed to produce an effect by heat, light, sound, gas or smoke or a combination of these as the result of non-detonative self-sustaining exothermic chemical reactions.
An explosive item is an item containing one or more explosive chemicals.
A pyrotechnic item is an item containing one or more pyrotechnic chemicals.
An unstable explosive is an explosive which is thermally unstable and/or too sensitive for normal handling, transport, or use.
An intentional explosive is a chemical or item which is manufactured with a view to produce a practical explosive or pyrotechnic effect.
B.1.1.2 The class of explosives comprises:
(a) Explosive chemicals;
(b) Explosive items, except devices containing explosive chemicals in such quantity or of such a character that their inadvertent or accidental ignition or initiation shall not cause any effect external to the device either by projection, fire, smoke, heat or loud noise; and
(c) Chemicals and items not included under (a) and (b) of this section which are manufactured with the view to producing a practical explosive or pyrotechnic effect.
B.1.2 Classification Criteria
Chemicals and items of this class shall be classified as unstable explosives or shall be assigned to one of the following six divisions depending on the type of hazard they present:
(a) Division 1.1—Chemicals and items which have a mass explosion hazard (a mass explosion is one which affects almost the entire quantity present virtually instantaneously);
(b) Division 1.2—Chemicals and items which have a projection hazard but not a mass explosion hazard;
(c) Division 1.3—Chemicals and items which have a fire hazard and either a minor blast hazard or a minor projection hazard or both, but not a mass explosion hazard:
(i) Combustion of which gives rise to considerable radiant heat; or
(ii) Which burn one after another, producing minor blast or projection effects or both;
(d) Division 1.4—Chemicals and items which present no significant hazard: chemicals and items which present only a small hazard in theevent of ignition or initiation. The effects are largely confined to the package and no projection of fragments of appreciable size or range is to be expected. An external fire shall not cause virtually instantaneous explosion of almost the entire contents of the package;
(e) Division 1.5—Very insensitive chemicals which have a mass explosion hazard: chemicals which have a mass explosion hazard but are so insensitive that there is very little probability of initiation or of transition from burning to detonation under normal conditions;
(f) Division 1.6—Extremely insensitive items which do not have a mass explosion hazard: items which predominantly contain extremely insensitive detonating chemicals and which demonstrate a negligible probability of accidental initiation or propagation.
B.1.3 Additional Classification Considerations
B.1.3.1 Explosives shall be classified as unstable explosives or shall be assigned to one of the six divisions identified in B.1.2 in accordance with the three step procedure in Part I of UN ST/SG/AC.10 (incorporated by reference, see §1910.6). The first step is to ascertain whether the substance or mixture has explosive effects (Test Series 1). The second step is the acceptance procedure (Test Series 2 to 4) and the third step is the assignment to a hazard division (Test Series 5 to 7). The assessment whether a candidate for “ammonium nitrate emulsion or suspension or gel, intermediate for blasting explosives (ANE)” is insensitive enough for inclusion as an oxidizing liquid (see B.13 of this appendix) or an oxidizing solid (see B.14 of this appendix) is determined by Test Series 8 tests of UN ST/SG/AC.10/.
Note 1: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
Note 2: Some explosive chemicals are wetted with water or alcohols, diluted with other substances or dissolved or suspended in water or other liquid substances to suppress or reduce their explosive properties or sensitivity.
These chemicals shall be classified as desensitized explosives (see Chapter B.17).
Note 3: Chemicals with a positive result in Test Series 2 in Part I, Section 12 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference; see §1910.6) which are exempted from classification as explosives (based on a negative result in Test Series 6 in Part I, Section 16 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference; see §1910.6)), still have explosive properties. The explosive properties of the chemical shall be communicated in Section 2 (Hazard identification) and Section 9 (Physical and chemical properties) of the Safety Data Sheet, as appropriate.
B.1.3.2 Explosive properties are associated with the presence of certain chemical groups in a molecule which can react to produce very rapid increases in temperature or pressure. The screening procedure in B.1.3.1 is aimed at identifying the presence of such reactive groups and the potential for rapid energy release. If the screening procedure identifies the chemical as a potential explosive, the acceptance procedure (see section 10.3 of the UN ST/SG/AC.10 (incorporated by reference; see §1910.6)) is necessary for classification.
Note: Neither a Series 1 type (a) propagation of detonation test nor a Series 2 type (a) test of sensitivity to detonative shock is necessary if the exothermic decomposition energy of organic materials is less than 800 J/g.
B.1.3.3 If a mixture contains any known explosives, the acceptance procedure is necessary for classification.
B.1.3.4 A chemical is not classified as explosive if:
(a) There are no chemical groups associated with explosive properties present in the molecule. Examples of groups which may indicate explosive properties are given in Table A6.1 in Appendix 6 of the UN ST/SG/AC.10 (incorporated by reference; See §1910.6); or
(b) The substance contains chemical groups associated with explosive properties which include oxygen and the calculated oxygen balance is less than −200.
The oxygen balance is calculated for the chemical reaction:
CxHyOz + [x + (y/4)−(z/2)] O2 → x. CO 2 + (y/2) H 2 O
using the formula: oxygen balance = −1600 [2x + (y/2)−z]/molecular weight; or
(c) The organic substance or a homogenous mixture of organic substances contains chemical groups associated with explosive properties but the exothermic decomposition energy is less than 500 J/g and the onset of exothermic decomposition is below 500°C (932°F). The exothermic decomposition energy may be determined using a suitable calorimetric technique; or
(d) For mixtures of inorganic oxidizing substances with organic material(s), the concentration of the inorganic oxidizing substance is:
(i) less than 15%, by mass, if the oxidizing substance is assigned to Category 1 or 2;
(ii) less than 30%, by mass, if the oxidizing substance is assigned to Category 3.
B.2 Flammable Gases
B.2.1 Definition
Flammable gas means a gas having a flammable range with air at 20°C (68°F) and a standard pressure of 101.3 kPa (14.7 psi).
A pyrophoric gas means a flammable gas that is liable to ignite spontaneously in air at a temperature of 54°C (130°F) or below.
A chemically unstable gas means a flammable gas that is able to react explosively even in the absence of air or oxygen.
B.2.2 Classification Criteria
B.2.2.1 A flammable gas shall be classified in Category 1A, 1B, or 2 in accordance with Table B.2.1:
| Category | Criteria | |
|---|---|---|
| 1A | Flammable gas | Gases, which at 20°C (68°F) and a standard pressure of 101.3 kPa (14.7 psi):
unless data show they meet the criteria for Category 1B. |
| Pyrophoric gas | Flammable gases that ignite spontaneously in air at a temperature of 54°C (130°F) or below. | |
| Chemically unstable gas: | ||
| A | Flammable gases which are chemically unstable at 20°C (68°F) and a standard pressure of 101.3 kPa (14.7 psi). | |
| B | Flammable gases which are chemically unstable at a temperature greater than 20°C (68°F) and/or a pressure greater than 101.3 kPa (14.7 psi). | |
| 1B | Flammable gas | Gases which meet the flammability criteria for Category 1A, but which are not pyrophoric, nor chemically unstable, and which have at least either:
|
| 2 | Flammable gas | Gases, other than those of Category 1A or 1B, which, at 20°C (68°F) and a standard pressure of 101.3 kPa (14.7 psi), have a flammable range while mixed in air. |
Note 1: Aerosols should not be classified as flammable gases. See B.3.
Note 2: In the absence of data allowing classification into Category 1B, a flammable gas that meets the criteria for Category 1A shall be classified by default in Category 1A.
Note 3: Spontaneous ignition for pyrophoric gases is not always immediate, and there may be a delay.
Note 4: In the absence of data on its pyrophoricity, a flammable gas mixture shall be classified as a pyrophoric gas if it contains more than 1% (by volume) of pyrophoric component(s).
B.2.3 Additional Classification Considerations
B.2.3.1 Flammability shall be determined by tests or by calculation in accordance with ISO 10156:1996 or ISO 10156:2017 (incorporated by reference; see §1910.6) and, if using fundamental burning velocity for Category 1B, use Annex C: Method of test for burning velocity measurement of flammable gases of ISO 817:2014(E) (incorporated by reference; see §1910.6). Where insufficient data are available to use this method, equivalent validated methods may be used.
B.2.3.2 Pyrophoricity shall be determined at 130°F (54°C) in accordance with either IEC 60079-20-1 or DIN 51794:2003 (incorporated by reference; see §1910.6).
B.2.3.3 The classification procedure for pyrophoric gases need not be applied when experience in production or handling shows that the substance does not ignite spontaneously on coming into contact with air at a temperature of 130°F (54°C) or below. Flammable gas mixtures, which have not been tested for pyrophoricity and which contain more than one percent pyrophoric components shall be classified as a pyrophoric gas. Expert judgement on the properties and physical hazards of pyrophoric gases and their mixtures should be used in assessing the need for classification of flammable gas mixtures containing one percent or less pyrophoric components. In this case, testing need only be considered if expert judgement indicates a need for additional data to support the classification process.
B.2.3.4 Chemical instability shall be determined in accordance with the method described in Part III of the UN ST/SG/AC.10/11/Rev.6 (incorporated by reference; see §1910.6). If the calculations performed in accordance with ISO 10156:1996 or ISO 10156:2017 (incorporated by reference; see §1910.6) show that a gas mixture is not flammable, no additional testing is required for determining chemical instability for classification purposes.
B.3 Aerosols and Chemicals Under Pressure
B.3.1 Aerosols
B.3.1.1 Definition
Aerosol means any non-refillable receptacle containing a gas compressed, liquefied or dissolved under pressure, and fitted with a release device allowing the contents to be ejected as particles in suspension in a gas, or as a foam, paste, powder, liquid or gas.
B.3.1.2 Classification Criteria
B.3.1.2.1 Aerosols are classified in one of three categories, depending on their flammable properties and their heat of combustion. Aerosols shall be considered for classification in Categories 1 or 2 if they contain more than 1% components (by mass) which are classified as flammable in accordance with this Appendix B, i.e.:
Flammable gases (see B.2);
Flammable liquids (see B.6)
Flammable solids (see B.7)
or if their heat of combustion is at least 20 kJ/g.
B.3.1.2.2 An aerosol shall be classified in one of the three categories for this class in accordance with Table B.3.1.
| Category | Criteria |
|---|---|
| 1 | Contains ≥85% flammable components and the chemical heat of combustion is ≥30 kJ/g; or
|
| 2 | Contains >1% flammable components, or the heat of combustion is ≥20 kJ/g; and
and it does not meet the criteria for Category 1. |
| 3 |
|
Note 1: Flammable components do not include pyrophoric, self-heating or water-reactive chemicals.
Note 2: Aerosols do not fall additionally within the scope of flammable gases, gases under pressure, flammable liquids, or flammable solids. However, depending on their contents, aerosols may fall within the scope of other hazard classes.
Note 3: Aerosols containing more than 1% flammable components or with a heat of combustion of at least 20 kJ/g, which are not submitted to the flammability classification procedures in this Appendix shall be classified as Category 1.
B.3.2 Chemicals Under Pressure
B.3.2.1 Definition
Chemicals under pressure are liquids or solids (e.g., pastes or powders), pressurized with a gas at a pressure of 200 kPa (gauge) or more at 20°C in pressure receptacles other than aerosol dispensers and which are not classified as gases under pressure.
Note: Chemicals under pressure typically contain 50% or more by mass of liquids or solids whereas mixtures containing more than 50% gases are typically considered as gases under pressure.
B.3.2.2 Classification Criteria
B.3.2.2.1 Chemicals under pressure are classified in one of three categories of this hazard class, in accordance with Table B.3.2, depending on their content of flammable components and their heat of combustion
B.3.2.2.2 Flammable components are components which are classified as flammable in accordance with the GHS criteria, i.e.:
—Flammable gases (see B..2 of this section);
—Flammable liquids (see B.6 of this section);
—Flammable solids (see B.7 of this section).
| Category | Criteria |
|---|---|
| 1 | Any chemical under pressure that:
|
| 2 | Any chemical under pressure that:
or that:
|
| 3 | Any chemical under pressure that:
|
Note 1: The flammable components in a chemical under pressure do not include pyrophoric, self-heating or water-reactive, substances and mixtures because such components are not allowed in chemicals under pressure in accordance with the UN Model Regulations.
Note 2: Chemicals under pressure do not fall additionally within the scope of section B.3.1 (aerosols), B.2.2 (flammable gases), B.2.5 (gases under pressure), B.2.6 (flammable liquids) and B.2.7 (flammable solids). Depending on their contents, chemicals under pressure may however fall within the scope of other hazard classes, including their labelling elements.
B.3.3 Additional Classification Considerations
B.3.3.1 To classify an aerosol, data on its flammable components, on its chemical heat of combustion and, if applicable, the results of the aerosol foam flammability test (for foam aerosols) and of the ignition distance test and enclosed space test (for spray aerosols) are necessary.
B.3.3.2 The chemical heat of combustion (ΔHc), in kilojoules per gram (kJ/g), is the product of the theoretical heat of combustion (ΔHcomb), and a combustion efficiency, usually less than 1.0 (a typical combustion efficiency is 0.95 or 95%).
For a composite formulation, the chemical heat of combustion is the summation of the weighted heats of combustion for the individual components, as follows:
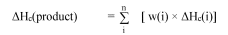
where:
ΔH c (product) = specific heat of combustion (kJ/g) of the product;
ΔH c (i) = specific heat of combustion (kJ/g) of component i in the product;
w(i) = mass fraction of component i in the product;
n = total number of components in the product.
B.3.3.3 The chemical heats of combustion shall be found in literature, calculated or determined by tests: (see ASTM D240; Sections 86.1 to 86.3 of ISO 13943; and NFPA 30B (incorporated by reference; see §1910.6)).
B.3.3.4 The Ignition Distance Test, Enclosed Space Ignition Test and Aerosol Foam Flammability Test shall be performed in accordance with sub-sections 31.4, 31.5 and 31.6 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6).
B.4 Oxidizing Gases
B.4.1 Definition
Oxidizing gas means any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does.
Note: “Gases which cause or contribute to the combustion of other material more than air does” means pure gases or gas mixtures with an oxidizing power greater than 23.5% (as determined by a method specified in ISO 10156:1996, ISO 10156:2017 or 10156-2:2005 (incorporated by reference; see §1910.6) or an equivalent testing method).
B.4.2 Classification Criteria
An oxidizing gas shall be classified in a single category for this class in accordance with Table B.4.1:
| Category | Criteria |
|---|---|
| 1 | Any gas which may, generally by providing oxygen, cause or contribute to the combustion of other material more than air does. |
B.4.3 Additional Classification Considerations
Classification shall be in accordance with tests or calculation methods as described in ISO 10156:1996, ISO 10156:2017 or 10156-2:2005 (incorporated by reference; see §1910.6).
B.5 Gases Under Pressure
B.5.1 Definition
Gases under pressure are gases which are contained in a receptacle at a pressure of 200 kPa (29 psi) (gauge) or more at 20°C (68°F), or which are liquefied or liquefied and refrigerated.
They comprise compressed gases, liquefied gases, dissolved gases and refrigerated liquefied gases.
B.5.2 Classification Criteria
Gases under pressure shall be classified in one of four groups in accordance with Table B.5.1:
| Group | Criteria |
|---|---|
| Compressed gas | A gas which when under pressure is entirely gaseous at −50°C (−58°F), including all gases with a critical temperature 1 ≤−50°C (−58°F) |
| Liquefied gas | A gas which when under pressure, is partially liquid at temperatures above −50°C (−58°F) A distinction is made between:
|
| Refrigerated liquefied gas | A gas which is made partially liquid becuase of its low temperature. |
| Dissolved gas | A gas which when under pressure is dissolved in a liquid phase solvent. |
| 1 The critical temperature is the temperature above which a pure gas cannot be liquefied, regardless of the degree of compression. | |
Note: Aerosols should not be classified as gases under pressure. See Appendix B.3 of this section.
B.6 Flammable Liquids
B.6.1 Definition
Flammable liquid means a liquid having a flash point of not more than 93°C (199.4°F).
Flash point means the minimum temperature at which a liquid gives off vapor in sufficient concentration to form an ignitable mixture with air near the surface of the liquid, as determined by a method identified in Section B.6.3 of this appendix.
B.6.2 Classification Criteria
A flammable liquid shall be classified in one of four categories in accordance with Table B.6.1 of this appendix:
| Category | Criteria |
|---|---|
| 1 | Flash point <23°C (73.4°F) and initial boiling point ≤35°C (95°F). |
| 2 | Flash point <23°C (73.4°F) and initial boiling point >35°C (95°F). |
| 3 | Flash point ≥23°C (73.4°F) and ≤60°C (140°F). |
| 4 | Flash point >60°C (140°F) and ≤93°C (199.4°F). |
Note: Aerosols should not be classified as flammable liquids. See Appendix B.3 of this section.
B.6.3 Additional Classification Considerations
The flash point shall be determined in accordance with ASTM D56-05, ASTM D3278, ASTM D3828, ASTM D93-08 (incorporated by reference, see §1910.6), or any method specified in 29 CFR 1910.106(a)(14). It may also be determined by any other method specified in GHS Revision 7, Chapter 2.6.
The initial boiling point shall be determined in accordance with ASTM D86- 07a or ASTM D1078 (incorporated by reference; see §1910.6). 9
9 To determine the appropriate flammable liquid storage container size and type, the boiling point shall be determined by §1910.106(a)(5). In addition, the manufacturer, importer, and distributor shall clearly note in sections 7 and 9 of the SDS if an alternate calculation was used for storage purposes and the classification for storage differs from the classification listed in Section 2 of the SDS.
B.7 Flammable Solids
B.71 Definitions
Flammable solid means a solid which is a readily combustible solid, or which may cause or contribute to fire through friction.
Readily combustible solids are powdered, granular, or pasty chemicals which are dangerous if they can be easily ignited by brief contact with an ignition source, such as a burning match, and if the flame spreads rapidly.
B.7.2 Classification Criteria
B.7.2.1 Powdered, granular or pasty chemicals shall be classified as flammable solids when the time of burning of one or more of the test runs, performed in accordance with the test method described in Part III, sub-section 33.2.1 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6), is less than 45 s or the rate of burning is more than 2.2 mm/s (0.0866 in/s).
B.7.2.2 Powders of metals or metal alloys shall be classified as flammable solids when they can be ignited and the reaction spreads over the whole length of the sample in 10 min or less.
B.7.2.3 Solids which may cause fire through friction shall be classified in this class by analogy with existing entries (e.g., matches) until definitive criteria are established.
B.7.2.4 A flammable solid shall be classified in one of the two categories for this class using Method N.1 as described in Part III, sub-section 33.2.1 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6), in accordance with Table B.7.1:
| Category | Criteria |
|---|---|
| 1 | Burning rate test:
|
| 2 | Burning rate test:
|
Note 1: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
Note 2: Aerosols should not be classified as flammable solids. See Appendix B.3.
B.8 Self-Reactive Chemicals
B.8.1 Definitions
Self-reactive chemicals are thermally unstable liquid or solid chemicals liable to undergo a strongly exothermic decomposition even without participation of oxygen (air). This definition excludes chemicals classified under this section as explosives, organic peroxides, oxidizing liquids or oxidizing solids.
A self-reactive chemical is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement.
B.8.2 Classification Criteria
B.8.2.1 A self-reactive chemical shall be considered for classification in this class unless:
(a) It is classified as an explosive according to B.1 of this appendix;
(b) It is classified as an oxidizing liquid or an oxidizing solid according to B.13 or B.14 of this appendix, except that a mixture of oxidizing substances which contains 5% or more of combustible organic substances shall be classified as a self-reactive chemical according to the procedure defined in B.8.2.2;
(c) It is classified as an organic peroxide according to B.15 of this appendix;
(d) Its heat of decomposition is less than 300 J/g; or
(e) Its self-accelerating decomposition temperature (SADT) is greater than 75° C (167°F) for a 50 kg (110 lb) package.
B.8.2.2 Mixtures of oxidizing substances, meeting the criteria for classification as oxidizing liquids or oxidizing solids, which contain 5% or more of combustible organic substances and which do not meet the criteria mentioned in B.8.2.1(a), (c), (d) or (e), shall be subjected to the self-reactive chemicals classification procedure in B.8.2.3. Such a mixture showing the properties of a self-reactive chemical type B to F shall be classified as a self-reactive chemical.
B.8.2.3 Self-reactive chemicals shall be classified in one of the seven categories of “types A to G” for this class, according to the following principles:
(a) Any self-reactive chemical which can detonate or deflagrate rapidly, as packaged, will be defined as self-reactive chemical TYPE A;
(b) Any self-reactive chemical possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package will be defined as self-reactive chemical TYPE B;
(c) Any self-reactive chemical possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion will be defined as self-reactive chemical TYPE C;
(d) Any self-reactive chemical which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) will be defined as self-reactive chemical TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement;
(e) Any self-reactive chemical which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement will be defined as self-reactive chemical TYPE E;
(f) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power will be defined as self-reactive chemical TYPE F;
(g) Any self-reactive chemical which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self- accelerating decomposition temperature is 60°C (140°F) to 75° C (167°F) for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point greater than or equal to 150°C (302°F) is used for desensitization will be defined as self-reactive chemical TYPE G. If the mixture is not thermally stable or a diluent having a boiling point less than 150°C (302°F) is used for desensitization, the mixture shall be defined as self-reactive chemical TYPE F.
B.8.3 Additional Classification Considerations
B.8.3.1 For purposes of classification, the properties of self-reactive chemicals shall be determined in accordance with test series A to H as described in Part II of UN ST/SG/AC.10 (incorporated by reference; see §1910.6).
B.8.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with Part II, section 28 of UN ST/SG/AC.10, (incorporated by reference; see §1910.6).
B.8.3.3 The classification procedures for self-reactive substances and mixtures need not be applied if:
(a) There are no chemical groups present in the molecule associated with explosive or self-reactive properties; examples of such groups are given in Tables A6.1 and A6.2 in the Appendix 6 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6); or
(b) For a single organic substance or a homogeneous mixture of organic substances, the estimated SADT is greater than 75°C (167°F) or the exothermic decomposition energy is less than 300 J/g. The onset temperature and decomposition energy may be estimated using a suitable calorimetric technique (See 20.3.3.3 in Part II of UN ST/SG/AC.10 (incorporated by reference; see §1910.6)).
B.9 Pyrophoric Liquids
B.9.1 Definition
Pyrophoric liquid means a liquid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air.
B.9.2 Classification Criteria
A pyrophoric liquid shall be classified in a single category for this class using test N.3 in Part III, sub-section 33.3.1.5 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6), in accordance with Table B.9.1 of this appendix:
| Category | Criteria |
|---|---|
| 1 | The liquid ignites within 5 min when added to an inert carrier and exposed to air, or it ignites or chars a filter paper on contact with air within 5 min. |
B.9.3 Additional Classification Considerations
The classification procedure for pyrophoric liquids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the substance is known to be stable at room temperature for prolonged periods of time (days)).
B.10 Pyrophoric Solids
B.10.1 Definition
Pyrophoric solid means a solid which, even in small quantities, is liable to ignite within five minutes after coming into contact with air.
B.10.2 Classification Criteria
A pyrophoric solid shall be classified in a single category for this class using test N.2 in Part III, sub-section 33.3.1.4 of UN ST/SG/AC.10 (incorporated by reference; see §1910.6), in accordance with Table B.10.1 of this appendix:
| Category | Criteria |
|---|---|
| 1 | The solid ignites within 5 min of coming into contact with air. |
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.10.3 Additional Classification Considerations
The classification procedure for pyrophoric solids need not be applied when experience in production or handling shows that the chemical does not ignite spontaneously on coming into contact with air at normal temperatures (i.e., the chemical is known to be stable at room temperature for prolonged periods of time (days)).
B.11—Self-Heating Chemicals
B.11.1 Definition
A self-heating chemical is a solid or liquid chemical, other than a pyrophoric liquid or solid, which, by reaction with air and without energy supply, is liable to self-heat; this chemical differs from a pyrophoric liquid or solid in that it will ignite only when in large amounts (kilograms) and after long periods of time (hours or days).
Note: Self-heating of a substance or mixture is a process where the gradual reaction of that substance or mixture with oxygen (in air) generates heat. If the rate of heat production exceeds the rate of heat loss, then the temperature of the substance or mixture will rise which, after an induction time, may lead to self-ignition and combustion.
B.11.2 Classification Criteria
B.11.2.1 A self-heating chemical shall be classified in one of the two categories for this class if, in tests performed in accordance with test method N.4 in Part III, sub-section 33.3.1.6 of UN ST/SG/AC.10 (incorporated by reference, see §1910.6), the result meets the criteria shown in Table B.11.1.
| Category | Criteria |
|---|---|
| 1 | A positive result is obtained in a test using a 25 mm sample cube at 140° C (284° F). |
| 2 | A negative result is obtained in a test using a 25 mm cube sample at 140° C (284° F), a positive result is obtained in a test using a 100 mm sample cube at 140° C (284° F), and:
|
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.11.2.2 Chemicals with a temperature of spontaneous combustion higher than 50° C (122° F) for a volume of 27 m3 shall not be classified as self-heating chemicals.
B.11.2.3 Chemicals with a spontaneous ignition temperature higher than 50° C (122° F) for a volume of 450 liters shall not be classified in Category 1 of this class.
B.11.3 Additional Classification Considerations
B.11.3.1 The classification procedure for self-heating chemicals need not be applied if the results of a screening test can be adequately correlated with the classification test and an appropriate safety margin is applied.
B.11.3.2 Examples of screening tests are:
(a) The Grewer Oven test (VDI guideline 2263, part 1, 1990, Test methods for the Determination of the Safety Characteristics of Dusts) with an onset temperature 80°K above the reference temperature for a volume of 1 l;
(b) The Bulk Powder Screening Test (Gibson, N. Harper, D. J. Rogers, R. Evaluation of the fire and explosion risks in drying powders, Plant Operations Progress, 4 (3), 181-189, 1985) with an onset temperature 60°K above the reference temperature for a volume of 1 l.
B.12 Chemicals Which, in Contact With Water, Emit Flammable Gases
B.12.1 Definition
Chemicals which, in contact with water, emit flammable gases are solid or liquid chemicals which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities.
B.12.2 Classification Criteria
B.12.2.1 A chemical which, in contact with water, emits flammable gases shall be classified in one of the three categories for this class, using test N.5 in Part III, sub-section 33.4.1.4 of UN ST/SG/AC.10 (incorporated by reference, see §1910.6), in accordance with Table B.12.1 of this appendix:
| Category | Criteria |
|---|---|
| Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form. | |
| 1 | Any chemical which reacts vigorously with water at ambient temperatures and demonstrates generally a tendency for the gas produced to ignite spontaneously, or which reacts readily with water at ambient temperatures such that the rate of evolution of flammable gas is equal to or greater than 10 liters per kilogram of chemical over any one minute. |
| 2 | Any chemical which reacts readily with water at ambient temperatures such that the maximum rate of evolution of flammable gas is greater than 20 liters per kilogram of chemical per hour, and which does not meet the criteria for Category 1. |
| 3 | Any chemical which reacts slowly with water at ambient temperatures such that the maximum rate of evolution of flammable gas is greater than 1 liter per kilogram of chemical per hour, and which does not meet the criteria for Categories 1 and 2. |
Note: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.12.2.2 A chemical is classified as a chemical which, in contact with water, emits flammable gases if spontaneous ignition takes place in any step of the test procedure.
B.12.3 Additional Classification Considerations
The classification procedure for this class need not be applied if:
(a) The chemical structure of the chemical does not contain metals or metalloids;
(b) Experience in production or handling shows that the chemical does not react with water, (e.g., the chemical is manufactured with water or washed with water); or
(c) The chemical is known to be soluble in water to form a stable mixture.
B.13 Oxidizing Liquids
B.13.1 Definition
Oxidizing liquid means a liquid which, while in itself not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material.
B.13.2 Classification Criteria
An oxidizing liquid shall be classified in one of the three categories for this class using test O.2 in Part III, sub-section 34.4.2 of UN ST/SG/AC.10 (incorporated by reference, see §1910.6), in accordance with Table B.13.1:
| Category | Criteria |
|---|---|
| 1 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, spontaneously ignites; or the mean pressure rise time of a 1:1 mixture, by mass, of chemical and cellulose is less than that of a 1:1 mixture, by mass, of 50% perchloric acid and cellulose; |
| 2 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 40% aqueous sodium chlorate solution and cellulose; and the criteria for Category 1 are not met; |
| 3 | Any chemical which, in the 1:1 mixture, by mass, of chemical and cellulose tested, exhibits a mean pressure rise time less than or equal to the mean pressure rise time of a 1:1 mixture, by mass, of 65% aqueous nitric acid and cellulose; and the criteria for Categories 1 and 2 are not met. |
B.13.3 Additional Classification Considerations
B.13.3.1 For organic chemicals, the classification procedure for this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine; or
(b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen.
B.13.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms.
B.13.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgments based on known experience shall take precedence over test results.
B.13.3.4 In cases where chemicals generate a pressure rise (too high or too low), caused by chemical reactions not characterizing the oxidizing properties of the chemical, the test described in Part III, sub-section 34.4.2 of UN ST/SG/AC.10 (incorporated by reference, see §1910.6) shall be repeated with an inert substance (e.g., diatomite (kieselguhr)) in place of the cellulose in order to clarify the nature of the reaction.
B.14 Oxidizing Solids
B.14.1 Definition
Oxidizing solid means a solid which, while in itself is not necessarily combustible, may, generally by yielding oxygen, cause, or contribute to, the combustion of other material.
B.14.2 Classification Criteria
An oxidizing solid shall be classified in one of the three categories for this class using test O.1 in Part III, sub-section 34.4.1, of UN ST/SG/AC.10 (incorporated by reference, see §1910.6) or test O.3 in Part III, sub-section 34.4.3 of UN ST/SG/AC.10/11 (incorporated by reference, see §1910.6), in accordance with Table B.14.1:
| Category | Criteria using test O.1 | Criteria using test O.3 |
|---|---|---|
| 1 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time less than the mean burning time of a 3:2 mixture, (by mass), of potassium bromate and cellulose | Any chemical which, in the 4:1 or 1:1 sample-to- cellulose ratio (by mass) tested, exhibits a mean burning rate greater than the mean burning rate of a 3:1 mixture (by mass) of calcium peroxide and cellulose. |
| 2 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 2:3 mixture (by mass) of potassium bromate and cellulose and the criteria for Category 1 are not met | Any chemical which, in the 4:1 or 1:1 sample-to- cellulose ratio (by mass) tested, exhibits a mean burning rate equal to or greater than the mean burning rate of a 1:1 mixture (by mass) of calcium peroxide and cellulose and the criteria for Category 1 are not met. |
| 3 | Any chemical which, in the 4:1 or 1:1 sample-to-cellulose ratio (by mass) tested, exhibits a mean burning time equal to or less than the mean burning time of a 3:7 mixture (by mass) of potassium bromate and cellulose and the criteria for Categories 1 and 2 are not met | Any chemical which, in the 4:1 or 1:1 sample-to- cellulose ratio (by mass) tested, exhibits a mean burning rate equal to or greater than the mean burning rate of a 1:2 mixture (by mass) of calcium peroxide and cellulose and the criteria for Categories 1 and 2 are not met. |
Note 1: Some oxidizing solids may present explosion hazards under certain conditions (e.g., when stored in large quantities). For example, some types of ammonium nitrate may give rise to an explosion hazard under extreme conditions and the “Resistance to detonation test” (International Maritime Solid Bulk Cargoes Code, IMO (IMSBC), Appendix 2, Section 5) may be used to assess this hazard. When information indicates that an oxidizing solid may present an explosion hazard, it shall be indicated on the Safety Data Sheet.
Note 2: Classification of solid chemicals shall be based on tests performed on the chemical as presented. If, for example, for the purposes of supply or transport, the same chemical is to be presented in a physical form different from that which was tested and which is considered likely to materially alter its performance in a classification test, classification must be based on testing of the chemical in the new form.
B.14.3 Additional Classification Considerations
B.14.3.1 For organic chemicals, the classification procedure for this class shall not be applied if:
(a) The chemical does not contain oxygen, fluorine or chlorine; or
(b) The chemical contains oxygen, fluorine or chlorine and these elements are chemically bonded only to carbon or hydrogen.
B.14.3.2 For inorganic chemicals, the classification procedure for this class shall not be applied if the chemical does not contain oxygen or halogen atoms.
B.14.3.3 In the event of divergence between test results and known experience in the handling and use of chemicals which shows them to be oxidizing, judgements based on known experience shall take procedure over test results.
B.15 Organic Peroxides
B.15.1 Definition
B.15.1.1 Organic peroxide means a liquid or solid organic chemical which contains the bivalent -0-0- structure and as such is considered a derivative of hydrogen peroxide, where one or both of the hydrogen atoms have been replaced by organic radicals. The term organic peroxide includes organic peroxide mixtures containing at least one organic peroxide. Organic peroxides are thermally unstable chemicals, which may undergo exothermic self-accelerating decomposition. In addition, they may have one or more of the following properties:
(a) Be liable to explosive decomposition;
(b) Burn rapidly;
(c) Be sensitive to impact or friction;
(d) React dangerously with other substances.
B.15.1.2 An organic peroxide is regarded as possessing explosive properties when in laboratory testing the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement.
B.15.2 Classification Criteria
B.15.2.1 Any organic peroxide shall be considered for classification in this class, unless it contains:
(a) Not more than 1.0% available oxygen from the organic peroxides when containing not more than 1.0% hydrogen peroxide; or
(b) Not more than 0.5% available oxygen from the organic peroxides when containing more than 1.0% but not more than 7.0% hydrogen peroxide.
Note: The available oxygen content (%) of an organic peroxide mixture is given by the formula:
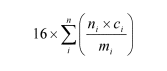
where:
ni = number of peroxygen groups per molecule of organic peroxide i;
ci = concentration (mass %) of organic peroxide i;
mi = molecular mass of organic peroxide i.
B.15.2.2 Organic peroxides shall be classified in one of the seven categories of “Types A to G” for this class, according to the following principles:
(a) Any organic peroxide which, as packaged, can detonate or deflagrate rapidly shall be defined as organic peroxide TYPE A;
(b) Any organic peroxide possessing explosive properties and which, as packaged, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package shall be defined as organic peroxide TYPE B;
(c) Any organic peroxide possessing explosive properties when the chemical as packaged cannot detonate or deflagrate rapidly or undergo a thermal explosion shall be defined as organic peroxide TYPE C;
(d) Any organic peroxide which in laboratory testing meets the criteria in (d)(i), (ii), or (iii) shall be defined as organic peroxide TYPE D:
(i) Detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or
(ii) Does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or
(iii) Does not detonate or deflagrate at all and shows a medium effect when heated under confinement;
(e) Any organic peroxide which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement shall be defined as organic peroxide TYPE E;
(f) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power shall be defined as organic peroxide TYPE F;
(g) Any organic peroxide which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power, provided that it is thermally stable (self-accelerating decomposition temperature is 60° C (140° F) or higher for a 50 kg (110 lb) package), and, for liquid mixtures, a diluent having a boiling point of not less than 150 ;° C (302° F) is used for desensitization, shall be defined as organic peroxide TYPE G. If the organic peroxide is not thermally stable or a diluent having a boiling point less than 150° C (302° F) is used for desensitization, it shall be defined as organic peroxide TYPE F.
B.15.3 Additional Classification Considerations
B.15.3.1 For purposes of classification, the properties of organic peroxides shall be determined in accordance with test series A to H as described in Part II of UN ST/SG/AC.10 (incorporated by reference, see §1910.6).
B.15.3.2 Self-accelerating decomposition temperature (SADT) shall be determined in accordance with UN ST/SG/AC.10 (incorporated by reference, see §1910.6), Part II, section 28.
B.15.3.3 Mixtures of organic peroxides may be classified as the same type of organic peroxide as that of the most dangerous ingredient. However, as two stable ingredients can form a thermally less stable mixture, the SADT of the mixture shall be determined.
B.16 Corrosive to Metals
B.16.1 Definition
A chemical which is corrosive to metals means a chemical which by chemical action will materially damage, or even destroy, metals.
B.16.2 Classification Criteria
A chemical which is corrosive to metals shall be classified in a single category for this class, using the test in Part III, sub-section 37.4 of UN ST/SG/AC.10 (incorporated by reference, see §1910.6), in accordance with Table B.16.1:
| Category | Criteria |
|---|---|
| 1 | Corrosion rate on either steel or aluminum surfaces exceeding 6.25 mm per year at a test temperature of 55° C (131° F) when tested on both materials. |
Note: Where an initial test on either steel or aluminium indicates the chemical being tested is corrosive the follow-up test on the other metal is not necessary.
B.16.3 Additional Classification Considerations
The specimen to be used for the test shall be made of the following materials:
(a) For the purposes of testing steel, steel types S235JR+CR (1.0037 resp. St 37- 2), S275J2G3+CR (1.0144 resp. St 44-3), ISO 3574, Unified Numbering System (UNS) G 10200, or SAE 1020;
(b) For the purposes of testing aluminium: non-clad types 7075-T6 or AZ5GU-T6.
B.17 Desensitized Explosives
B.17.1 Definitions and General Considerations
Desensitized explosives are solid or liquid explosive chemicals which are phlegmatized 10 to suppress their explosive properties in such a manner that they do not mass explode and do not burn too rapidly and therefore may be exempted from the hazard class “Explosives” (Chapter B.1; see also Note 2 of paragraph B.1.3). 11
10 Phlegmatized means that a substance (or “phlegmatizer”) has been added to an explosive to enhance its safety in handling and transport. The phlegmatizer renders the explosive insensitive, or less sensitive, to the following actions: heat, shock, impact, percussion or friction. Typical phlegmatizing agents include, but are not limited to: wax, paper, water, polymers (such as chlorofluoropolymers), alcohol and oils (such as petroleum jelly and paraffin).
11 Unstable explosives as defined in Chapter B.1 can also be stabilized by desensitization and consequently may be re-classified as desensitized explosives, provided all criteria of Chapter B.17 are met. In this case, the desensitized explosive should be tested according to Test Series 3 (Part I of UN ST/SG/AC.10/11/Rev. 6 (incorporated by reference, see §1910.6)) because information about its sensitiveness to mechanical stimuli is likely to be important for determining conditions for safe handling and use. The results shall be communicated on the safety data sheet.
B.17.1.1 The class of desensitized explosives comprises:
(a) Solid desensitized explosives: explosive substances or mixtures which are wetted with water or alcohols or are diluted with other substances, to form a homogeneous solid mixture to suppress their explosive properties.
Note: This includes desensitization achieved by formation of hydrates of the substances.
(b) Liquid desensitized explosives: explosive substances or mixtures which are dissolved or suspended in water or other liquid substances, to form a homogeneous liquid mixture to suppress their explosive properties.
B.17.2 Classification Criteria
B.17.2.1 Any explosive which is desensitized shall be considered in this class, unless:
(a) It is intended to produce a practical, explosive or pyrotechnic effect; or
It has a mass explosion hazard according to test series 6 (a) or 6 (b) or its corrected burning rate according to the burning rate test described in part V, subsection 51.4 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference, see §1910.6) is greater than 1200 kg/min; or
(b) Its exothermic decomposition energy is less than 300 J/g.
Note 1: Substances or mixtures which meet the criterion (a) or (b) shall be classified as explosives (see Chapter B.1). Substances or mixtures which meet the criterion (c) may fall within the scope of other physical hazard classes.
Note 2: The exothermic decomposition energy may be estimated using a suitable calorimetric technique (see section 20, sub-section 20.3.3.3 in Part II of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference, see §1910.6).
B.17.2.2 Desensitized explosives shall be classified in one of the four categories of this class depending on the corrected burning rate (Ac) using the test “burning rate test (external fire)” described in Part V, sub-section 51.4 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference, see §1910.6), according to Table B.17.1:
| Category | Criteria |
|---|---|
| 1 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 300 kg/min but not more than 1200 kg/min. |
| 2 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 140 kg/min but less than 300 kg/min. |
| 3 | Desensitized explosives with a corrected burning rate (AC) equal to or greater than 60 kg/min but less than 140 kg/min. |
| 4 | Desensitized explosives with a corrected burning rate (AC) less than 60 kg/min. |
Note 1: Desensitized explosives shall be prepared so that they remain homogeneous and do not separate during normal storage and handling, particularly if desensitized by wetting. The manufacturer, importer, or distributor shall provide information in Section 10 of the safety data sheet about the shelf-life and instructions on verifying desensitization. Under certain conditions the content of desensitizing agent (e.g., phlegmatizer, wetting agent or treatment) may decrease during supply and use, and thus, the hazard potential of the desensitized explosive may increase. In addition, Sections 5 and/or 8 of the safety data sheet shall include advice on avoiding increased fire, blast or protection hazards when the chemical is not sufficiently desensitized.
Note 2: Explosive properties of desensitized explosives shall be determined using data from Test Series 2 of UN ST/SG/ AC.10/11/Rev.6 (incorporated by reference, see §1910.6) and shall be communicated in the safety data sheet. For testing of liquid desensitized explosives, refer to section 32, sub-section 32.3.2 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference, see 1910.6). Testing of solid desensitized explosives is addressed in section 33, sub-section 33.2.3 of UN ST/SG/AC.10/11/Rev.6 (incorporated by reference, see §1910.6).
Note 3: Desensitized explosives do not fall additionally within the scope of chapters B.1 (explosives), B.6 (flammable liquids) and B.7 (flammable solids).
B.17.3 Additional Classification Considerations
B.17.3.1 The classification procedure for desensitized explosives does not apply if:
(a) The substances or mixtures contain no explosives according to the criteria in Chapter B.1; or
(b) The exothermic decomposition energy is less than 300 J/g.
B.17.3.2 The exothermic decomposition energy shall be determined using the explosive already desensitized (i.e., the homogenous solid or liquids mixture formed by the explosive and the substance(s) used to suppress its explosive properties). The exothermic decomposition energy may be estimated using a suitable calorimetric technique (see Section 20, sub-section 20.3.3.3 in Part II of UN ST/SG/AC.10/11/Rev. 6 (incorporated by reference, see §1910.6).
[77 FR 17817, March 26, 2012; 78 FR 9313, Feb. 8, 2013; 89 FR 44356, May 20, 2024; 89 FR 81830, Oct. 9, 2024]
['Hazard Communication', 'Toxic and Hazardous Substances - OSHA']
['Hazard Classifications', 'Toxic and Hazardous Substances - OSHA', 'Hazard Communication']
UPGRADE TO CONTINUE READING
Load More
J. J. Keller is the trusted source for DOT / Transportation, OSHA / Workplace Safety, Human Resources, Construction Safety and Hazmat / Hazardous Materials regulation compliance products and services. J. J. Keller helps you increase safety awareness, reduce risk, follow best practices, improve safety training, and stay current with changing regulations.
Copyright 2026 J. J. Keller & Associate, Inc. For re-use options please contact copyright@jjkeller.com or call 800-558-5011.
