...
(a) Performance tests. Initial and repeat performance tests are required for the emissions sources specified in paragraphs (a)(1) and (2) of this section, except for emission sources controlled by a combustion device that is designed and operated as specified in §63.443(d)(3) or (4).
(1) Conduct an initial performance test for all emission sources subject to the limitations in §§63.443, 63.444, 63.445, 63.446, and 63.447.
(2) Conduct repeat performance tests at five-year intervals for all emission sources subject to the limitations in §§63.443, 63.444, and 63.445. The first of the 5-year repeat tests must be conducted by September 7, 2015, and thereafter within 60 months from the date of the previous performance test. Five-year repeat testing is not required for the following:
(i) Knotter or screen systems with HAP emission rates below the criteria specified in §63.443(a)(1)(ii).
(ii) Decker systems using fresh water or paper machine white water, or decker systems using process water with a total HAP concentration less than 400 parts per million by weight as specified in §63.443(a)(1)(iv).
(b) Vent sampling port locations and gas stream properties. For purposes of selecting vent sampling port locations and determining vent gas stream properties, required in §§63.443, 63.444, 63.445, and 63.447, each owner or operator shall comply with the applicable procedures in paragraphs (b)(1) through (b)(6) of this section.
(1) Method 1 or 1A of part 60, appendix A-1, as appropriate, shall be used for selection of the sampling site as follows:
(i) To sample for vent gas concentrations and volumetric flow rates, the sampling site shall be located prior to dilution of the vent gas stream and prior to release to the atmosphere;
(ii) For determining compliance with percent reduction requirements, sampling sites shall be located prior to the inlet of the control device and at the outlet of the control device; measurements shall be performed simultaneously at the two sampling sites; and
(iii) For determining compliance with concentration limits or mass emission rate limits, the sampling site shall be located at the outlet of the control device.
(2) No traverse site selection method is needed for vents smaller than 0.10 meter (4.0 inches) in diameter.
(3) The vent gas volumetric flow rate shall be determined using Method 2, 2A, 2C, or 2D of part 60, appendix A-1, as appropriate.
(4) The moisture content of the vent gas shall be measured using Method 4 of part 60, appendix A-3.
(5) To determine vent gas concentrations, the owner or operator shall conduct a minimum of three test runs that are representative of normal conditions and average the resulting pollutant concentrations using the following procedures.
(i) Method 308 in Appendix A of this part; Method 320 in Appendix A of this part; Method 18 in appendix A-6 of part 60; ASTM D6420-99 (Reapproved 2004) (incorporated by reference in §63.14(b)(28) of subpart A of this part); or ASTM D6348-03 (incorporated by reference in §63.14(b)(54) of subpart A of this part) shall be used to determine the methanol concentration. If ASTM D6348-03 is used, the conditions specified in paragraphs (b)(5)(i)(A) though (b)(5)(i)(B) must be met.
(A) The test plan preparation and implementation in the Annexes to ASTM D6348-03, sections A1 through A8 are required.
(B) In ASTM D6348-03 Annex A5 (Analyte Spiking Technique), the percent (%) R must be determined for each target analyte (Equation A5.5 of ASTM D6348-03). In order for the test data to be acceptable for a compound, %R must be between 70 and 130 percent. If the %R value does not meet this criterion for a target compound, the test data is not acceptable for that compound and the test must be repeated for that analyte following adjustment of the sampling or analytical procedure before the retest. The %R value for each compound must be reported in the test report, and all field measurements must be corrected with the calculated %R value for that compound using the following equation: Reported Result = Measured Concentration in the Stack × 100)/%R.
(ii) Except for the modifications specified in paragraphs (b)(5)(ii)(A) through (b)(5)(ii)(K) of this section, Method 26A of part 60, appendix A-8 shall be used to determine chlorine concentration in the vent stream.
(A) Probe/sampling line. A separate probe is not required. The sampling line shall be an appropriate length of 0.64 cm (0.25 in) OD Teflon ® tubing. The sample inlet end of the sampling line shall be inserted into the stack in such a way as to not entrain liquid condensation from the vent gases. The other end shall be connected to the impingers. The length of the tubing may vary from one sampling site to another, but shall be as short as possible in each situation. If sampling is conducted in sunlight, opaque tubing shall be used. Alternatively, if transparent tubing is used, it shall be covered with opaque tape.
(B) Impinger train. Three 30 milliliter (ml) capacity midget impingers shall be connected in series to the sampling line. The impingers shall have regular tapered stems. Silica gel shall be placed in the third impinger as a desiccant. All impinger train connectors shall be glass and/or Teflon ®.
(C) Critical orifice. The critical orifice shall have a flow rate of 200 to 250 ml/min and shall be followed by a vacuum pump capable of providing a vacuum of 640 millimeters of mercury (mm Hg). A 45 millimeter diameter in-line Teflon 0.8 micrometer filter shall follow the impingers to protect the critical orifice and vacuum pump.
(D) The following are necessary for the analysis apparatus:
(1) Wash bottle filled with deionized water;
(2) 25 or 50 ml graduated burette and stand;
(3) Magnetic stirring apparatus and stir bar;
(4) Calibrated pH Meter;
(5) 150-250 ml beaker or flask; and
(6) A 5 ml pipette.
(E) The procedures listed in paragraphs (b)(5)(ii)(E)(1) through (b)(5)(ii)(E)(7) of this section shall be used to prepare the reagents.
(1) To prepare the 1 molarity (M) potassium dihydrogen phosphate solution, dissolve 13.61 grams (g) of potassium dihydrogen phosphate in water and dilute to 100 ml.
(2) To prepare the 1 M sodium hydroxide solution (NaOH), dissolve 4.0 g of sodium hydroxide in water and dilute to 100 ml.
(3) To prepare the buffered 2 percent potassium iodide solution, dissolve 20 g of potassium iodide in 900 ml water. Add 50 ml of the 1 M potassium dihydrogen phosphate solution and 30 ml of the 1 M sodium hydroxide solution. While stirring solution, measure the pH of solution electrometrically and add the 1 M sodium hydroxide solution to bring pH to between 6.95 and 7.05.
(4) To prepare the 0.1 normality (N) sodium thiosulfate solution, dissolve 25 g of sodium thiosulfate, pentahydrate, in 800 ml of freshly boiled and cooled distilled water in a 1-liter volumetric flask. Dilute to volume. To prepare the 0.01 N sodium thiosulfate solution, add 10.0 ml standardized 0.1 N sodium thiosulfate solution to a 100 ml volumetric flask, and dilute to volume with water.
(5) To standardize the 0.1 N sodium thiosulfate solution, dissolve 3.249 g of anhydrous potassium bi-iodate, primary standard quality, or 3.567 g potassium iodate dried at 103 =/−2 degrees Centigrade for 1 hour, in distilled water and dilute to 1000 ml to yield a 0.1000 N solution. Store in a glass-stoppered bottle. To 80 ml distilled water, add, with constant stirring, 1 ml concentrated sulfuric acid, 10.00 ml 0.1000 N anhydrous potassium bi-iodate, and 1 g potassium iodide. Titrate immediately with 0.1 n sodium thiosulfate titrant until the yellow color of the liberated iodine is almost discharged. Add 1 ml starch indicator solution and continue titrating until the blue color disappears. The normality of the sodium thiosulfate solution is inversely proportional to the ml of sodium thiosulfate solution consumed:
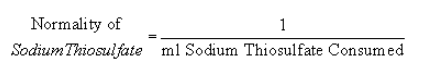
(6) To prepare the starch indicator solution, add a small amount of cold water to 5 g starch and grind in a mortar to obtain a thin paste. Pour paste into 1 L of boiling distilled water, stir, and let settle overnight. Use clear supernate for starch indicator solution.
(7) To prepare the 10 percent sulfuric acid solution, add 10 ml of concentrated sulfuric acid to 80 ml water in a 100 ml volumetric flask. Dilute to volume.
(F) The procedures specified in paragraphs (b)(5)(ii)(F)(1) through (b)(5)(ii)(F)(5) of this section shall be used to perform the sampling.
(1) Preparation of collection train. Measure 20 ml buffered potassium iodide solution into each of the first two impingers and connect probe, impingers, filter, critical orifice, and pump. The sampling line and the impingers shall be shielded from sunlight.
(2) Leak and flow check procedure. Plug sampling line inlet tip and turn on pump. If a flow of bubbles is visible in either of the liquid impingers, tighten fittings and adjust connections and impingers. A leakage rate not in excess of 2 percent of the sampling rate is acceptable. Carefully remove the plug from the end of the probe. Check the flow rate at the probe inlet with a bubble tube flow meter. The flow should be comparable or slightly less than the flow rate of the critical orifice with the impingers off-line. Record the flow and turn off the pump.
(3) Sample collection. Insert the sampling line into the stack and secure it with the tip slightly lower than the port height. Start the pump, recording the time. End the sampling after 60 minutes, or after yellow color is observed in the second in-line impinger. Record time and remove the tubing from the vent. Recheck flow rate at sampling line inlet and turn off pump. If the flow rate has changed significantly, redo sampling with fresh capture solution. A slight variation (less than 5 percent) in flow may be averaged. With the inlet end of the line elevated above the impingers, add about 5 ml water into the inlet tip to rinse the line into the first impinger.
(4) Sample analysis. Fill the burette with 0.01 N sodium thiosulfate solution to the zero mark. Combine the contents of the impingers in the beaker or flask. Stir the solution and titrate with thiosulfate until the solution is colorless. Record the volume of the first endpoint (TN, ml). Add 5 ml of the 10 percent sulfuric acid solution, and continue the titration until the contents of the flask are again colorless. Record the total volume of titrant required to go through the first and to the second endpoint (TA, ml). If the volume of neutral titer is less than 0.5 ml, repeat the testing for a longer period of time. It is important that sufficient lighting be present to clearly see the endpoints, which are determined when the solution turns from pale yellow to colorless. A lighted stirring plate and a white background are useful for this purpose.
(5) Interferences. Known interfering agents of this method are sulfur dioxide and hydrogen peroxide. Sulfur dioxide, which is used to reduce oxidant residuals in some bleaching systems, reduces formed iodine to iodide in the capture solution. It is therefore a negative interference for chlorine, and in some cases could result in erroneous negative chlorine concentrations. Any agent capable of reducing iodine to iodide could interfere in this manner. A chromium trioxide impregnated filter will capture sulfur dioxide and pass chlorine and chlorine dioxide. Hydrogen peroxide, which is commonly used as a bleaching agent in modern bleaching systems, reacts with iodide to form iodine and thus can cause a positive interference in the chlorine measurement. Due to the chemistry involved, the precision of the chlorine analysis will decrease as the ratio of chlorine dioxide to chlorine increases. Slightly negative calculated concentrations of chlorine may occur when sampling a vent gas with high concentrations of chlorine dioxide and very low concentrations of chlorine.
(G) The following calculation shall be performed to determine the corrected sampling flow rate:
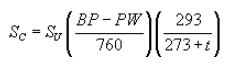
Where:
SC = Corrected (dry standard) sampling flow rate, liters per minute;
SU = Uncorrected sampling flow rate, L/min;
BP = Barometric pressure at time of sampling;
PW = Saturated partial pressure of water vapor, mm Hg at temperature; and
t = Ambient temperature,°C.
(H) The following calculation shall be performed to determine the moles of chlorine in the sample:
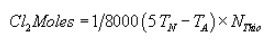
Where:
TN = Volume neutral titer, ml;
TA = Volume acid titer (total), ml; and
NThio = Normality of sodium thiosulfate titrant.
(I) The following calculation shall be performed to determine the concentration of chlorine in the sample:
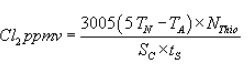
Where:
tS = Time sampled, minutes;
TN = Volume neutral titer, ml;
TA = Volume acid titer (total), ml; and
NThio = Normality of sodium thiosulfate titrant.
(J) The following calculation shall be performed to determine the moles of chlorine dioxide in the sample:
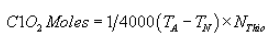
Where:
TA = Volume acid titer (total), ml;
TN = Volume neutral titer, ml; and
NThio = Normality of sodium thiosulfate titrant.
(K) The following calculation shall be performed to determine the concentration of chlorine dioxide in the sample:
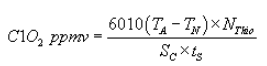
Where:
SC = Corrected (dry standard) sampling flow rate, liters per minute;
tS = Time sampled, minutes;
TA = Volume acid titer (total), ml;
TN = Volume neutral titer, ml; and
NThio = Normality of sodium thiosulfate titrant.
(iii) Any other method that measures the total HAP or methanol concentration that has been demonstrated to the Administrator's satisfaction.
(6) The minimum sampling time for each of the three test runs shall be 1 hour in which either an integrated sample or four grab samples shall be taken. If grab sampling is used, then the samples shall be taken at approximately equal intervals in time, such as 15 minute intervals during the test run.
(c) Liquid sampling locations and properties. For purposes of selecting liquid sampling locations and for determining properties of liquid streams such as wastewaters, process waters, and condensates required in §§63.444, 63.446, and 63.447, the owner or operator shall comply with the following procedures:
(1) Samples shall be collected using the sampling procedures of the test method listed in paragraph (c)(3) of this section selected to determine liquid stream HAP concentrations;
(i) Where feasible, samples shall be taken from an enclosed pipe prior to the liquid stream being exposed to the atmosphere; and
(ii) When sampling from an enclosed pipe is not feasible, samples shall be collected in a manner to minimize exposure of the sample to the atmosphere and loss of HAP compounds prior to sampling.
(2) The volumetric flow rate of the entering and exiting liquid streams shall be determined using the inlet and outlet flow meters or other methods demonstrated to the Administrator's satisfaction. The volumetric flow rate measurements to determine actual mass removal shall be taken at the same time as the concentration measurements.
(3) The owner or operator shall conduct a minimum of three test runs that are representative of normal conditions and average the resulting pollutant concentrations. The minimum sampling time for each test run shall be 1 hour and the grab or composite samples shall be taken at approximately equally spaced intervals over the 1-hour test run period. The owner or operator shall use one of the following procedures to determine total HAP or methanol concentration:
(i) Method 305 in Appendix A of this part, adjusted using the following equation:
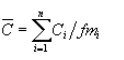
Where:
C͞ = Pollutant concentration for the liquid stream, parts per million by weight.
Ci = Measured concentration of pollutant i in the liquid stream sample determined using Method 305, parts per million by weight.
fmi = Pollutant-specific constant that adjusts concentration measured by Method 305 to actual liquid concentration; the fm for methanol is 0.85. Additional pollutant fm values can be found in table 34, subpart G of this part.
n = Number of individual pollutants, i, summed to calculate total HAP.
(ii) For determining methanol concentrations, NCASI Method DI/MEOH-94.03. This test method is incorporated by reference in §63.14(f)(1) of subpart A of this part.
(iii) Any other method that measures total HAP concentration that has been demonstrated to the Administrator's satisfaction.
(4) To determine soluble BOD 5 in the effluent stream from an open biological treatment unit used to comply with §§63.446(e)(2) and 63.453(j), the owner or operator shall use section B of method 5210 (IBR, see §63.14) with the following modifications:
(i) Filter the sample through the filter paper, into an Erlenmeyer flask by applying a vacuum to the flask sidearm. Minimize the time for which vacuum is applied to prevent stripping of volatile organics from the sample. Replace filter paper as often as needed in order to maintain filter times of less than approximately 30 seconds per filter paper. No rinsing of sample container or filter bowl into the Erlenmeyer flask is allowed.
(ii) Perform method 5210B on the filtrate obtained in paragraph (c)(4) of this section. Dilution water shall be seeded with 1 milliliter of final effluent per liter of dilution water. Dilution ratios may require adjustment to reflect the lower oxygen demand of the filtered sample in comparison to 7the total BOD 5 . Three BOD bottles and different dilutions shall be used for each sample.
(5) If the test method used to determine HAP concentration indicates that a specific HAP is not detectable, the value determined as the minimum measurement level (MML) of the selected test method for the specific HAP shall be used in the compliance demonstration calculations. To determine the MML for a specific HAP using one of the test methods specified in paragraph (c)(3) of this section, one of the procedures specified in paragraphs (c)(5)(i) and (ii) of this section shall be performed. The MML for a particular HAP must be determined only if the HAP is not detected in the normal working range of the method.
(i) To determine the MML for a specific HAP, the following procedures shall be performed each time the method is set up. Set up is defined as the first time the analytical apparatus is placed in operation, after any shut down of 6 months or more, or any time a major component of the analytical apparatus is replaced.
(A) Select a concentration value for the specific HAP in question to represent the MML. The value of the MML selected shall not be below the calibration standard of the selected test method.
(B) Measure the concentration of the specific HAP in a minimum of three replicate samples using the selected test method. All replicate samples shall be run through the entire analytical procedure. The samples must contain the specific HAP at the selected MML concentration and should be representative of the liquid streams to be analyzed in the compliance demonstration. Spiking of the liquid samples with a known concentration of the target HAP may be necessary to ensure that the HAP concentration in the three replicate samples is at the selected MML. The concentration of the HAP in the spiked sample must be within 50 percent of the proposed MML for the demonstration to be valid. As an alternative to spiking, a field sample above the MML may be diluted to produce a HAP concentration at the MML. To be a valid demonstration, the diluted sample must have a HAP concentration within 20 percent of the proposed MML, and the field sample must not be diluted by more than a factor of five.
(C) Calculate the relative standard deviation (RSD) and the upper confidence limit at the 95 percent confidence level using the measured HAP concentrations determined in paragraph (c)(5)(i)(B) of this section. If the upper confidence limit of the RSD is less than 30 percent, then the selected MML is acceptable. If the upper confidence limit of the RSD is greater than or equal to 30 percent, then the selected MML is too low, and the procedures specified in paragraphs (c)(5)(i)(A) through (C) of this section must be repeated.
(ii) Provide for the Administrator's approval the selected value of the MML for a specific HAP and the rationale for selecting the MML including all data and calculations used to determine the MML. The approved MML must be used in all applicable compliance demonstration calculations.
(6) When using the MML determined using the procedures in paragraph (c)(5)(ii) of this section or when using the MML determined using the procedures in paragraph (c)(5)(i), except during set up, the analytical laboratory conducting the analysis must perform and meet the following quality assurance procedures each time a set of samples is analyzed to determine compliance.
(i) Using the selected test method, analyze in triplicate the concentration of the specific HAP in a representative sample. The sample must contain the specific HAP at a concentration that is within a factor of two of the MML. If there are no samples in the set being analyzed that contain the specific HAP at an appropriate concentration, then a sample below the MML may be spiked to produce the appropriate concentration, or a sample at a higher level may be diluted. After spiking, the sample must contain the specific HAP within 50 percent of the MML. If dilution is used instead, the diluted sample must contain the specific HAP within 20 percent of the MML and must not be diluted by more than a factor of five.
(ii) Calculate the RSD using the measured HAP concentrations determined in paragraph (c)(6)(i) of this section. If the RSD is less than 20 percent, then the laboratory is performing acceptably.
(d) Detectable leak procedures. To measure detectable leaks for closed-vent systems as specified in §63.450 or for pulping process wastewater collection systems as specified in §63.446(d)(2)(i), the owner or operator shall comply with the following:
(1) Method 21, of part 60, appendix A-7; and
(2) The instrument specified in Method 21 shall be calibrated before use according to the procedures specified in Method 21 on each day that leak checks are performed. The following calibration gases shall be used:
(i) Zero air (less than 10 parts per million by volume of hydrocarbon in air); and
(ii) A mixture of methane or n-hexane and air at a concentration of approximately, but less than, 10,000 parts per million by volume methane or n-hexane.
(e) Negative pressure procedures. To demonstrate negative pressure at process equipment enclosure openings as specified in §63.450(b), the owner or operator shall use one of the following procedures:
(1) An anemometer to demonstrate flow into the enclosure opening;
(2) Measure the static pressure across the opening;
(3) Smoke tubes to demonstrate flow into the enclosure opening; or
(4) Any other industrial ventilation test method demonstrated to the Administrator's satisfaction.
(f) HAP concentration measurements. For purposes of complying with the requirements in §§63.443, 63.444, and 63.447, the owner or operator shall measure the total HAP concentration as one of the following:
(1) As the sum of all individual HAPs; or
(2) As methanol.
(g) Condensate HAP concentration measurement. For purposes of complying with the kraft pulping condensate requirements in §63.446, the owner or operator shall measure the total HAP concentration as methanol. For biological treatment systems complying with §63.446(e)(2), the owner or operator shall measure total HAP as acetaldehyde, methanol, methyl ethyl ketone, and propionaldehyde and follow the procedures in §63.457(l)(1) or (2).
(h) Bleaching HAP concentration measurement. For purposes of complying with the bleaching system requirements in §63.445, the owner or operator shall measure the total HAP concentration as the sum of all individual chlorinated HAPs or as chlorine.
(i) Vent gas stream calculations. To demonstrate compliance with the mass emission rate, mass emission rate per megagram of ODP, and percent reduction requirements for vent gas streams specified in §§63.443, 63.444, 63.445, and 63.447, the owner or operator shall use the following:
(1) The total HAP mass emission rate shall be calculated using the following equation:
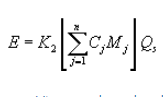
Where:
E = Mass emission rate of total HAP from the sampled vent, kilograms per hour.
K2 = Constant, 2.494 × 10−6 (parts per million by volume)−1 (gram-mole per standard cubic meter) (kilogram/gram) (minutes/hour), where standard temperature for (gram-mole per standard cubic meter) is 20°C.
Cj = Concentration on a dry basis of pollutant j in parts per million by volume as measured by the test methods specified in paragraph (b) of this section.
Mj = Molecular weight of pollutant j, gram/gram-mole.
Qs = Vent gas stream flow rate (dry standard cubic meter per minute) at a temperature of 20°C as indicated in paragraph (b) of this section.
n = Number of individual pollutants, i, summed to calculate total HAP.
(2) The total HAP mass emission rate per megagram of ODP shall be calculated using the following equation:
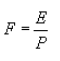
Where:
F = Mass emission rate of total HAP from the sampled vent, in kilograms per megagram of ODP.
E = Mass emission rate of total HAP from the sampled vent, in kilograms per hour determined as specified in paragraph (i)(1) of this section.
P = The production rate of pulp during the sampling period, in megagrams of ODP per hour.
(3) The total HAP percent reduction shall be calculated using the following equation:
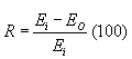
Where:
R = Efficiency of control device, percent.
Ei = Inlet mass emission rate of total HAP from the sampled vent, in kilograms of pollutant per hour, determined as specified in paragraph (i)(1) of this section.
Eo = Outlet mass emission rate of total HAP from the sampled vent, in kilograms of pollutant per hour, determined as specified in paragraph (i)(1) of this section.
(j) Liquid stream calculations. To demonstrate compliance with the mass flow rate, mass per megagram of ODP, and percent reduction requirements for liquid streams specified in §63.446, the owner or operator shall use the following:
(1) The mass flow rates of total HAP or methanol entering and exiting the treatment process shall be calculated using the following equations:
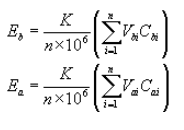
Where:
Eb = Mass flow rate of total HAP or methanol in the liquid stream entering the treatment process, kilograms per hour.
Ea = Mass flow rate of total HAP or methanol in the liquid exiting the treatment process, kilograms per hour.
K = Density of the liquid stream, kilograms per cubic meter.
Vbi = Volumetric flow rate of liquid stream entering the treatment process during each run i, cubic meters per hour, determined as specified in paragraph (c) of this section.
Vai = Volumetric flow rate of liquid stream exiting the treatment process during each run i, cubic meters per hour, determined as specified in paragraph (c) of this section.
Cbi = Concentration of total HAP or methanol in the stream entering the treatment process during each run i, parts per million by weight, determined as specified in paragraph (c) of this section.
Cai = Concentration of total HAP or methanol in the stream exiting the treatment process during each run i, parts per million by weight, determined as specified in paragraph (c) of this section.
n = Number of runs.
(2) The mass of total HAP or methanol per megagram ODP shall be calculated using the following equation:
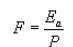
Where:
F = Mass loading of total HAP or methanol in the sample, in kilograms per megagram of ODP.
Ea = Mass flow rate of total HAP or methanol in the wastewater stream in kilograms per hour as determined using the procedures in paragraph (j)(1) of this section.
P = The production rate of pulp during the sampling period in megagrams of ODP per hour.
(3) The percent reduction of total HAP across the applicable treatment process shall be calculated using the following equation:
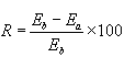
Where:
R = Control efficiency of the treatment process, percent.
Eb = Mass flow rate of total HAP in the stream entering the treatment process, kilograms per hour, as determined in paragraph (j)(1) of this section.
Ea = Mass flow rate of total HAP in the stream exiting the treatment process, kilograms per hour, as determined in paragraph (j)(1) of this section.
(4) Compounds that meet the requirements specified in paragraphs (j)(4)(i) or (4)(ii) of this section are not required to be included in the mass flow rate, mass per megagram of ODP, or the mass percent reduction determinations.
(i) Compounds with concentrations at the point of determination that are below 1 part per million by weight; or
(ii) Compounds with concentrations at the point of determination that are below the lower detection limit where the lower detection limit is greater than 1 part per million by weight.
(k) Oxygen concentration correction procedures. To demonstrate compliance with the total HAP concentration limit of 20 ppmv in §63.443(d)(2), the concentration measured using the methods specified in paragraph (b)(5) of this section shall be corrected to 10 percent oxygen using the following procedures:
(1) The emission rate correction factor and excess air integrated sampling and analysis procedures of Methods 3A or 3B of part 60, appendix A-2 shall be used to determine the oxygen concentration. The samples shall be taken at the same time that the HAP samples are taken. As an alternative to Method 3B, ASME PTC 19.10-1981 [Part 10] may be used (incorporated by reference, see §63.14(i)(1)).
(2) The concentration corrected to 10 percent oxygen shall be computed using the following equation:
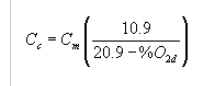
Where:
Cc = Concentration of total HAP corrected to 10 percent oxygen, dry basis, parts per million by volume.
Cm = Concentration of total HAP dry basis, parts per million by volume, as specified in paragraph (b) of this section.
%02d = Concentration of oxygen, dry basis, percent by volume.
(l) Biological treatment system percent reduction and mass removal calculations. To demonstrate compliance with the condensate treatment standards specified in §63.446(e)(2) and the monitoring requirements specified in §63.453(j)(3) using a biological treatment system, the owner or operator shall use one of the procedures specified in paragraphs (1)(1) and (2) of this section. Owners or operators using a nonthoroughly mixed open biological treatment system shall also comply with paragraph (1)(3) of this section.
(1) Percent reduction methanol procedure. For the purposes of complying with the condensate treatment requirements specified in §63.446(e)(2) and (3), the methanol percent reduction shall be calculated using the following equations:
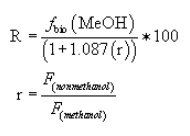
Where:
R = Percent destruction.
fbio(MeOH) = The fraction of methanol removed in the biological treatment system. The site-specific biorate constants shall be determined using the appropriate procedures specified in appendix C of this part.
r = Ratio of the sum of acetaldehyde, methyl ethyl ketone, and propionaldehyde mass to methanol mass.
F(nonmethanol) = The sum of acetaldehyde, methyl ethyl ketone, and propionaldehyde mass flow rates (kg/Mg ODP) entering the biological treatment system determined using the procedures in paragraph (j)(2) of this section.
F(methanol) = The mass flow rate (kg/Mg ODP) of methanol entering the system determined using the procedures in paragraph (j)(2) of this section.
(2) Mass removal methanol procedure. For the purposes of complying with the condensate treatment requirements specified in §63.446(e)(2) and (4), or §63.446(e)(2) and (5), the methanol mass removal shall be calculated using the following equation:
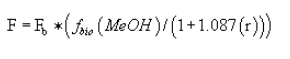
Where:
F = Methanol mass removal (kg/Mg ODP).
Fb = Inlet mass flow rate of methanol (kg/Mg ODP) determined using the procedures in paragraph (j)(2) of this section.
fbio(MeOH) = The fraction of methanol removed in the biological treatment system. The site-specific biorate constants shall be determined using the appropriate procedures specified in appendix C of this part.
r = Ratio of the sum of acetaldehyde, methyl ethyl ketone, and propionaldehyde mass to methanol mass determined using the procedures in paragraph (1) of this section.
(3) The owner or operator of a nonthoroughly mixed open biological treatment system using the monitoring requirements specified in §63.453(p)(3) shall follow the procedures specified in section III.B.1 of appendix E of this part to determine the borate constant, Ks, and characterize the open biological treatment system during the initial and any subsequent performance tests.
(m) Condensate segregation procedures. The following procedures shall be used to demonstrate compliance with the condensate segregation requirements specified in §63.446(c).
(1) To demonstrate compliance with the percent mass requirements specified in §63.446(c)(2), the procedures specified in paragraphs (m)(1)(i) through (iii) of this section shall be performed.
(i) Determine the total HAP mass of all condensates from each equipment system listed in §63.446 (b)(1) through (b)(3) using the procedures specified in paragraphs (c) and (j) of this section.
(ii) Multiply the total HAP mass determined in paragraph (m)(1)(i) of this section by 0.65 to determine the target HAP mass for the high-HAP fraction condensate stream or streams.
(iii) Compliance with the segregation requirements specified in §63.446(c)(2) is demonstrated if the condensate stream or streams from each equipment system listed in §63.446(b)(1) through (3) being treated as specified in §63.446(e) contain at least as much total HAP mass as the target total HAP mass determined in paragraph (m)(1)(ii) of this section.
(2) To demonstrate compliance with the percent mass requirements specified in §63.446(c)(3), the procedures specified in paragraphs (m)(2)(i) through (ii) of this section shall be performed.
(i) Determine the total HAP mass contained in the high-HAP fraction condensates from each equipment system listed in §63.446(b)(1) through (b)(3) and the total condensates streams from the equipment systems listed in §63.446(b)(4) and (b)(5), using the procedures specified in paragraphs (c) and (j) of this section.
(ii) Compliance with the segregation requirements specified in §63.446(c)(3) is demonstrated if the total HAP mass determined in paragraph (m)(2)(i) of this section is equal to or greater than the appropriate mass requirements specified in §63.446(c)(3).
(n) Open biological treatment system monitoring sampling storage. The inlet and outlet grab samples required to be collected in §63.453(j)(1)(ii) shall be stored at 4°C (40°F) to minimize the biodegradation of the organic compounds in the samples.
(o) Performance tests shall be conducted under such conditions as the Administrator specifies to the owner or operator based on representative performance of the affected source for the period being tested. Upon request, the owner or operator shall make available to the Administrator such records as may be necessary to determine the conditions of performance tests.
[63 FR 18617, Apr. 15, 1998, as amended at 64 FR 17564, Apr. 12, 1999; 65 FR 80763, Dec. 22, 2000; 66 FR 24269, May 14, 2001; 77 FR 55712, Sept. 11, 2012; 88 FR 18412, March 29, 2023]
