...
Method 301 - Field Validation of Pollutant Measurement Methods From Various Waste Media
Using Method 301
1.0 What is the purpose of Method 301?
2.0 What approval must I have to use Method 301?
3.0 What does Method 301 include?
4.0 How do I perform Method 301?
Reference Materials
5.0 What reference materials must I use?
Sampling Procedures
6.0 What sampling procedures must I use?
7.0 How do I ensure sample stability?
Determination of Bias and Precision
8.0 What are the requirements for bias?
9.0 What are the requirements for precision?
10.0 What calculations must I perform for isotopic spiking?
11.0 What calculations must I perform for comparison with a validated method?
12.0 What calculations must I perform for analyte spiking?
13.0 How do I conduct tests at similar sources?
Optional Requirements
14.0 How do I use and conduct ruggedness testing?
15.0 How do I determine the Limit of Detection for the candidate test method?
Other Requirements and Information
16.0 How do I apply for approval to use a candidate test method?
17.0 How do I request a waiver?
18.0 Where can I find additional information?
19.0 Tables.
Using Method 301
1.0 What is the purpose of Method 301?
Method 301 provides a set of procedures for the owner or operator of an affected source to validate a candidate test method as an alternative to a required test method based on established precision and bias criteria. These validation procedures are applicable under 40 CFR part 63 or 65 when a test method is proposed as an alternative test method to meet an applicable requirement or in the absence of a validated method. Additionally, the validation procedures of Method 301 are appropriate for demonstration of the suitability of alternative test methods under 40 CFR parts 59, 60, and 61. If, under 40 CFR part 63 or 60, you choose to propose a validation method other than Method 301, you must submit and obtain the Administrator's approval for the candidate validation method.
2.0 What approval must I have to use Method 301?
If you want to use a candidate test method to meet requirements in a subpart of 40 CFR part 59, 60, 61, 63, or 65, you must also request approval to use the candidate test method according to the procedures in Section 16 of this method and the appropriate section of the part (§59.104, §59.406, §60.8(b), §61.13(h)(1)(ii), §63.7(f), or §65.158(a)(2)(iii)). You must receive the Administrator's written approval to use the candidate test method before you use the candidate test method to meet the applicable federal requirements. In some cases, the Administrator may decide to waive the requirement to use Method 301 for a candidate test method to be used to meet a requirement under 40 CFR part 59, 60, 61, 63, or 65 in absence of a validated test method. Section 17 of this method describes the requirements for obtaining a waiver.
3.0 What does Method 301 include?
3.1 Procedures. Method 301 includes minimum procedures to determine and document systematic error (bias) and random error (precision) of measured concentrations from exhaust gases, wastewater, sludge, and other media. Bias is established by comparing the results of sampling and analysis against a reference value. Bias may be adjusted on a source-specific basis using a correction factor and data obtained during the validation test. Precision may be determined using a paired sampling system or quadruplicate sampling system for isotopic spiking. A quadruplicate sampling system is required when establishing precision for analyte spiking or when comparing a candidate test method to a validated method. If such procedures have not been established and verified for the candidate test method, Method 301 contains procedures for ensuring sample stability by developing sample storage procedures and limitations and then testing them. Method 301 also includes procedures for ruggedness testing and determining detection limits. The procedures for ruggedness testing and determining detection limits are required for candidate test methods that are to be applied to multiple sources and optional for candidate test methods that are to be applied at a single source.
3.2 Definitions.
Affected source means an affected source as defined in the relevant part and subpart under Title 40 (e.g., 40 CFR parts 59, 60, 61, 63, and 65).
Candidate test method means the sampling and analytical methodology selected for field validation using the procedures described in Method 301. The candidate test method may be an alternative test method under 40 CFR part 59, 60, 61, 63, or 65.
Paired sampling system means a sampling system capable of obtaining two replicate samples that are collected as closely as possible in sampling time and sampling location (collocated).
Quadruplicate sampling system means a sampling system capable of obtaining four replicate samples (e.g., two pairs of measured data, one pair from each method when comparing a candidate test method against a validated test method, or analyte spiking with two spiked and two unspiked samples) that are collected as close as possible in sampling time and sampling location.
Surrogate compound means a compound that serves as a model for the target compound(s) being measured (i.e., similar chemical structure, properties, behavior). The surrogate compound can be distinguished by the candidate test method from the compounds being analyzed.
4.0 How do I perform Method 301?
First, you use a known concentration of an analyte or compare the candidate test method against a validated test method to determine the bias of the candidate test method. Then, you collect multiple, collocated simultaneous samples to determine the precision of the candidate test method. Additional procedures, including validation testing over a broad range of concentrations over an extended time period are used to expand the applicability of a candidate test method to multiple sources. Sections 5.0 through 17.0 of this method describe the procedures in detail.
Reference Materials
5.0 What reference materials must I use?
You must use reference materials (a material or substance with one or more properties that are sufficiently homogenous to the analyte) that are traceable to a national standards body (e.g., National Institute of Standards and Technology (NIST)) at the level of the applicable emission limitation or standard that the subpart in 40 CFR part 59, 60, 61, 63, or 65 requires. If you want to expand the applicable range of the candidate test method, you must conduct additional test runs using analyte concentrations higher and lower than the applicable emission limitation or the anticipated level of the target analyte. You must obtain information about your analyte according to the procedures in Sections 5.1 through 5.4 of this method.
5.1 Exhaust Gas Test Concentration. You must obtain a known concentration of each analyte from an independent source such as a specialty gas manufacturer, specialty chemical company, or chemical laboratory. You must also obtain the manufacturer's certification of traceability, uncertainty, and stability for the analyte concentration.
5.2 Tests for Other Waste Media. You must obtain the pure liquid components of each analyte from an independent manufacturer. The manufacturer must certify the purity, traceability, uncertainty, and shelf life of the pure liquid components. You must dilute the pure liquid components in the same type medium or matrix as the waste from the affected source.
5.3 Surrogate Analytes. If you demonstrate to the Administrator's satisfaction that a surrogate compound behaves as the analyte does, then you may use surrogate compounds for highly toxic or reactive compounds. A surrogate may be an isotope or compound that contains a unique element (e.g., chlorine) that is not present in the source or a derivation of the toxic or reactive compound if the derivative formation is part of the method's procedure. You may use laboratory experiments or literature data to show behavioral acceptability.
5.4 Isotopically-Labeled Materials. Isotope mixtures may contain the isotope and the natural analyte. The concentration of the isotopically-labeled analyte must be more than five times the concentration of the naturally-occurring analyte.
Sampling Procedures
6.0 What sampling procedures must I use?
You must determine bias and precision by comparison against a validated test method using isotopic spiking or using analyte spiking (or the equivalent). Isotopic spiking can only be used with candidate test methods capable of measuring multiple isotopes simultaneously such as test methods using mass spectrometry or radiological procedures. You must collect samples according to the requirements specified in Table 301-1 of this method. You must perform the sampling according to the procedures in Sections 6.1 through 6.4 of this method.
6.1 Isotopic Spiking. Spike all 12 samples with isotopically-labelled analyte at an analyte mass or concentration level equivalent to the emission limitation or standard specified in the applicable regulation. If there is no applicable emission limitation or standard, spike the analyte at the expected level of the samples. Follow the applicable spiking procedures in Section 6.3 of this method.
6.2 Analyte Spiking. In each quadruplicate set, spike half of the samples (two out of the four samples) with the analyte according to the applicable procedure in Section 6.3 of this method. You should spike at an analyte mass or concentration level equivalent to the emission limitation or standard specified in the applicable regulation. If there is no applicable emission limitation or standard, spike the analyte at the expected level of the samples. Follow the applicable spiking procedures in Section 6.3 of this method.
6.3 Spiking Procedure.
6.3.1 Gaseous Analyte with Sorbent or Impinger Sampling Train. Sample the analyte being spiked (in the laboratory or preferably in the field) at a mass or concentration that is approximately equivalent to the applicable emission limitation or standard (or the expected sample concentration or mass where there is no standard) for the time required by the candidate test method, and then sample the stack gas stream for an equal amount of time. The time for sampling both the analyte and stack gas stream should be equal; however, you must adjust the sampling time to avoid sorbent breakthrough. You may sample the stack gas and the gaseous analyte at the same time. You must introduce the analyte as close to the tip of the sampling probe as possible.
6.3.2 Gaseous Analyte with Sample Container (Bag or Canister). Spike the sample containers after completion of each test run with an analyte mass or concentration to yield a concentration approximately equivalent to the applicable emission limitation or standard (or the expected sample concentration or mass where there is no standard). Thus, the final concentration of the analyte in the sample container would be approximately equal to the analyte concentration in the stack gas plus the equivalent of the applicable emission standard (corrected for spike volume). The volume amount of spiked gas must be less than 10 percent of the sample volume of the container.
6.3.3 Liquid or Solid Analyte with Sorbent or Impinger Trains. Spike the sampling trains with an amount approximately equivalent to the mass or concentration in the applicable emission limitation or standard (or the expected sample concentration or mass where there is no standard) before sampling the stack gas. If possible, do the spiking in the field. If it is not possible to do the spiking in the field, you must spike the sampling trains in the laboratory.
6.3.4 Liquid and Solid Analyte with Sample Container (Bag or Canister). Spike the containers at the completion of each test run with an analyte mass or concentration approximately equivalent to the applicable emission limitation or standard in the subpart (or the expected sample concentration or mass where there is no standard).
6.4 Probe Placement and Arrangement for Stationary Source Stack or Duct Sampling. To sample a stationary source, you must place the paired or quadruplicate probes according to the procedures in this subsection. You must place the probe tips in the same horizontal plane. Section 17.1 of Method 301 describes conditions for waivers. For example, the Administrator may approve a validation request where other paired arrangements for the probe tips or pitot tubes (where required) are used.
6.4.1 Paired Sampling Probes. For paired sampling probes, the first probe tip should be 2.5 centimeters (cm) from the outside edge of the second probe tip, with a pitot tube on the outside of each probe.
6.4.2 Quadruplicate Sampling Probes. For quadruplicate sampling probes, the tips should be in a 6.0 cm × 6.0 cm square area measured from the center line of the opening of the probe tip with a single pitot tube, where required, in the center of the probe tips or two pitot tubes, where required, with their location on either side of the probe tip configuration. Section 17.1 of Method 301 describes conditions for waivers. For example, you must propose an alternative arrangement whenever the cross-sectional area of the probe tip configuration is approximately five percent or more of the stack or duct cross-sectional area.
7.0 How do I ensure sample stability?
7.1 Developing Sample Storage and Threshold Procedures. If the candidate test method includes well-established procedures supported by experimental data for sample storage and the time within which the collected samples must be analyzed, you must store the samples according to the procedures in the candidate test method and you are not required to conduct the procedures specified in Section 7.2 or 7.3 of this method. If the candidate test method does not include such procedures, your candidate method must include procedures for storing and analyzing samples to ensure sample stability. At a minimum, your proposed procedures must meet the requirements in Section 7.2 or 7.3 of this method. The minimum duration between sample collection and storage must be as soon as possible, but no longer than 72 hours after collection of the sample. The maximum storage duration must not be longer than 2 weeks.
7.2 Storage and Sampling Procedures for Stack Test Emissions. You must store and analyze samples of stack test emissions according to Table 301-2 of this method. You may reanalyze the same sample at both the minimum and maximum storage durations for: (1) Samples collected in containers such as bags or canisters that are not subject to dilution or other preparation steps, or (2) impinger samples not subjected to preparation steps that would affect stability of the sample such as extraction or digestion. For candidate test method samples that do not meet either of these criteria, you must analyze one of a pair of replicate samples at the minimum storage duration and the other replicate at the proposed storage duration but no later than 2 weeks of the initial analysis to identify the effect of storage duration on analyte samples. If you are using the isotopic spiking procedure, then you must analyze each sample for the spiked analyte and the native analyte.
7.3 Storage and Sampling Procedures for Testing Other Waste Media (e.g., Soil/Sediment, Solid Waste, Water/Liquid). You must analyze one of each pair of replicate samples (half the total samples) at the minimum storage duration and the other replicate (other half of samples) at the maximum storage duration or within 2 weeks of the initial analysis to identify the effect of storage duration on analyte samples. The minimum time period between collection and storage should be as soon as possible, but no longer than 72 hours after collection of the sample.
7.4 Sample Stability. After you have conducted sampling and analysis according to Section 7.2 or 7.3 of this method, compare the results at the minimum and maximum storage durations. Calculate the difference in the results using Equation 301-1.

Where:
di = Difference between the results of the i th replicate pair of samples.
Rmini = Results from the i th replicate sample pair at the minimum storage duration.
Rmaxi = Results from the i th replicate sample pair at the maximum storage duration.
For single samples that can be reanalyzed for sample stability assessment (e.g., bag or canister samples and impinger samples that do not require digestion or extraction), the values for Rmini and Rmaxi will be obtained from the same sample rather than replicate samples.
7.4.1 Standard Deviation. Determine the standard deviation of the paired samples using Equation 301-2.

Where:
SDd = Standard deviation of the differences of the paired samples.
di = Difference between the results of the i th replicate pair of samples.
dm = Mean of the paired sample differences.
n = Total number of paired samples.
7.4.2 T Test. Test the difference in the results for statistical significance by calculating the t-statistic and determining if the mean of the differences between the results at the minimum storage duration and the results after the maximum storage duration is significant at the 95 percent confidence level and n-1 degrees of freedom. Calculate the value of the t-statistic using Equation 301-3.

Where:
t = t-statistic.
dm = The mean of the paired sample differences.
SDd = Standard deviation of the differences of the paired samples.
n = Total number of paired samples.
Compare the calculated t-statistic with the critical value of the t-statistic from Table 301-3 of this method. If the calculated t-value is less than the critical value, the difference is not statistically significant. Therefore, the sampling, analysis, and sample storage procedures ensure stability, and you may submit a request for validation of the candidate test method. If the calculated t-value is greater than the critical value, the difference is statistically significant, and you must repeat the procedures in Section 7.2 or 7.3 of this method with new samples using a shorter proposed maximum storage duration or improved handling and storage procedures.
Determination of Bias and Precision
8.0 What are the requirements for bias?
You must determine bias by comparing the results of sampling and analysis using the candidate test method against a reference value. The bias must be no more than ±10 percent for the candidate test method to be considered for application to multiple sources. A candidate test method with a bias greater than ±10 percent and less than or equal to ±30 percent can only be applied on a source-specific basis at the facility at which the validation testing was conducted. In this case, you must use a correction factor for all data collected in the future using the candidate test method. If the bias is more than ±30 percent, the candidate test method is unacceptable.
9.0 What are the requirements for precision?
You may use a paired sampling system or a quadruplicate sampling system to establish precision for isotopic spiking. You must use a quadruplicate sampling system to establish precision for analyte spiking or when comparing a candidate test method to a validated method. If you are using analyte spiking or isotopic spiking, the precision, expressed as the relative standard deviation (RSD) of the candidate test method, must be less than or equal to 20 percent. If you are comparing the candidate test method to a validated test method, the candidate test method must be at least as precise as the validated method as determined by an F test (see Section 11.2.2 of this method).
10.0 What calculations must I perform for isotopic spiking?
You must analyze the bias, RSD, precision, and data acceptance for isotopic spiking tests according to the provisions in Sections 10.1 through 10.4 of this method.
10.1 Numerical Bias. Calculate the numerical value of the bias using the results from the analysis of the isotopic spike in the field samples and the calculated value of the spike according to Equation 301-4.

Where:
B = Bias at the spike level.
Sm = Mean of the measured values of the isotopically-labeled analyte in the samples.
CS = Calculated value of the isotopically-labeled spike level.
10.2 Standard Deviation. Calculate the standard deviation of the Si values according to Equation 301-5.

Where:
SD = Standard deviation of the candidate test method.
Si = Measured value of the isotopically-labeled analyte in the i th field sample.
Sm = Mean of the measured values of the isotopically-labeled analyte in the samples.
n = Number of isotopically-spiked samples.
10.3 T Test. Test the bias for statistical significance by calculating the t-statistic using Equation 301-6. Use the standard deviation determined in Section 10.2 of this method and the numerical bias determined in Section 10.1 of this method.

Where:
t = Calculated t-statistic.
B = Bias at the spike level.
SD = Standard deviation of the candidate test method.
n = Number of isotopically spike samples.
Compare the calculated t-value with the critical value of the two-sided t-distribution at the 95 percent confidence level and n-1 degrees of freedom (see Table 301-3 of this method). When you conduct isotopic spiking according to the procedures specified in Sections 6.1 and 6.3 of this method as required, this critical value is 2.201 for 11 degrees of freedom. If the calculated t-value is less than or equal to the critical value, the bias is not statistically significant, and the bias of the candidate test method is acceptable. If the calculated t-value is greater than the critical value, the bias is statistically significant, and you must evaluate the relative magnitude of the bias using Equation 301-7.

Where:
BR = Relative bias.
B = Bias at the spike level.
CS = Calculated value of the spike level.
If the relative bias is less than or equal to 10 percent, the bias of the candidate test method is acceptable for use at multiple sources. If the relative bias is greater than 10 percent but less than or equal to 30 percent, and if you correct all data collected with the candidate test method in the future for bias using the source-specific correction factor determined in Equation 301-8, the candidate test method is acceptable only for application to the source at which the validation testing was conducted and may not be applied to any other sites. If either of the preceding two cases applies, you may continue to evaluate the candidate test method by calculating its precision. If not, the candidate test method does not meet the requirements of Method 301.

Where:
CF = Source-specific bias correction factor.
B = Bias at the spike level.
CS = Calculated value of the spike level.
If the CF is outside the range of 0.70 to 1.30, the data and method are considered unacceptable.
10.4 Precision. Calculate the RSD according to Equation 301-9.

Where:
RSD = Relative standard deviation of the candidate test method.
SD = Standard deviation of the candidate test method calculated in Equation 301-5.
Sm = Mean of the measured values of the spike samples.
The data and candidate test method are unacceptable if the RSD is greater than 20 percent.
11.0 What calculations must I perform for comparison with a validated method?
If you are comparing a candidate test method to a validated method, then you must analyze the data according to the provisions in this section. If the data from the candidate test method fail either the bias or precision test, the data and the candidate test method are unacceptable. If the Administrator determines that the affected source has highly variable emission rates, the Administrator may require additional precision checks.
11.1 Bias Analysis. Test the bias for statistical significance at the 95 percent confidence level by calculating the t-statistic.
11.1.1 Bias. Determine the bias, which is defined as the mean of the differences between the candidate test method and the validated method (dm). Calculate di according to Equation 301-10.

Where:
di = Difference in measured value between the candidate test method and the validated method for each quadruplicate sampling train.
V1i = First measured value with the validated method in the ith quadruplicate sampling train.
V2i = Second measured value with the validated method in the ith quadruplicate sampling train.
P1i = First measured value with the candidate test method in the ith quadruplicate sampling train.
P2i = Second measured value with the candidate test method in the ith quadruplicate sampling train.
Calculate the numerical value of the bias using Equation 301-11.

Where:
B = Numerical bias.
di = Difference between the candidate test method and the validated method for the ith quadruplicate sampling train.
n = Number of quadruplicate sampling trains.
11.1.2 Standard Deviation of the Differences. Calculate the standard deviation of the differences, SDd, using Equation 301-12.

Where:
SDd = Standard deviation of the differences between the candidate test method and the validated method.
di = Difference in measured value between the candidate test method and the validated method for each quadruplicate sampling train.
dm = Mean of the differences, di, between the candidate test method and the validated method.
n = Number of quadruplicate sampling trains.
11.1.3 T Test. Calculate the t-statistic using Equation 301-13.
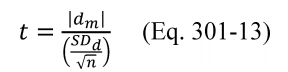
Where:
t = Calculated t-statistic.
dm = The mean of the differences, di, between the candidate test method and the validated method.
SDd = Standard deviation of the differences between the candidate test method and the validated method.
n = Number of quadruplicate sampling trains.
For the procedure comparing a candidate test method to a validated test method listed in Table 301-1 of this method, n equals six. Compare the calculated t-statistic with the critical value of the t-statistic, and determine if the bias is significant at the 95 percent confidence level (see Table 301-3 of this method). When six runs are conducted, as specified in Table 301-1 of this method, the critical value of the t-statistic is 2.571 for five degrees of freedom. If the calculated t-value is less than or equal to the critical value, the bias is not statistically significant and the data are acceptable. If the calculated t-value is greater than the critical value, the bias is statistically significant, and you must evaluate the magnitude of the relative bias using Equation 301-14.

Where:
BR = Relative bias.
B = Bias as calculated in Equation 301-11.
VS = Mean of measured values from the validated method.
If the relative bias is less than or equal to 10 percent, the bias of the candidate test method is acceptable. On a source-specific basis, if the relative bias is greater than 10 percent but less than or equal to 30 percent, and if you correct all data collected in the future with the candidate test method for the bias using the correction factor, CF, determined in Equation 301-8 (using VS for CS), the bias of the candidate test method is acceptable for application to the source at which the validation testing was conducted. If either of the preceding two cases applies, you may continue to evaluate the candidate test method by calculating its precision. If not, the candidate test method does not meet the requirements of Method 301.
11.2 Precision. Compare the estimated variance (or standard deviation) of the candidate test method to that of the validated test method according to Sections 11.2.1 and 11.2.2 of this method. If a significant difference is determined using the F test, the candidate test method and the results are rejected. If the F test does not show a significant difference, then the candidate test method has acceptable precision.
11.2.1 Candidate Test Method Variance. Calculate the estimated variance of the candidate test method according to Equation 301-15.

Where:
p = Estimated variance of the candidate test method.
di = The difference between the i th pair of samples collected with the candidate test method in a single quadruplicate train.
n = Total number of paired samples (quadruplicate trains).
Calculate the estimated variance of the validated test method according to Equation 301-16.

Where:
v = Estimated variance of the validated test method.
di = The difference between the i th pair of samples collected with the validated test method in a single quadruplicate train.
n = Total number of paired samples (quadruplicate trains).
11.2.2 The F test. Determine if the estimated variance of the candidate test method is greater than that of the validated method by calculating the F-value using Equation 301-17.

Where:
F = Calculated F value.
p = The estimated variance of the candidate test method.
v = The estimated variance of the validated method.
Compare the calculated F value with the one-sided confidence level for F from Table 301-4 of this method. The upper one-sided confidence level of 95 percent for F(6,6) is 4.28 when the procedure specified in Table 301-1 of this method for quadruplicate sampling trains is followed. If the calculated F value is greater than the critical F value, the difference in precision is significant, and the data and the candidate test method are unacceptable.
12.0 What calculations must I perform for analyte spiking?
You must analyze the data for analyte spike testing according to this section.
12.1 Bias Analysis. Test the bias for statistical significance at the 95 percent confidence level by calculating the t-statistic.
12.1.1 Bias. Determine the bias, which is defined as the mean of the differences between the spiked samples and the unspiked samples in each quadruplicate sampling train minus the spiked amount, using Equation 301-18.
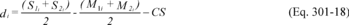
Where:
di = Difference between the spiked samples and unspiked samples in each quadruplicate sampling train minus the spiked amount.
S1i = Measured value of the first spiked sample in the i th quadruplicate sampling train.
S2i = Measured value of the second spiked sample in the i th quadruplicate sampling train.
M1i = Measured value of the first unspiked sample in the i th quadruplicate sampling train.
M2i = Measured value of the second unspiked sample in the i th quadruplicate sampling train.
CS = Calculated value of the spike level.
Calculate the numerical value of the bias using Equation 301-19.

Where:
B = Numerical value of the bias.
di = Difference between the spiked samples and unspiked samples in each quadruplicate sampling train minus the spiked amount.
n = Number of quadruplicate sampling trains.
12.1.2 Standard Deviation of the Differences. Calculate the standard deviation of the differences using Equation 301-20.

Where:
SDd = Standard deviation of the differences of paired samples.
di = Difference between the spiked samples and unspiked samples in each quadruplicate sampling train minus the spiked amount.
dm = The mean of the differences, di, between the spiked samples and unspiked samples.
n = Total number of quadruplicate sampling trains.
12.1.3 T Test. Calculate the t-statistic using Equation 301-21, where n is the total number of test sample differences (di). For the quadruplicate sampling system procedure in Table 301-1 of this method, n equals six.

Where:
t = Calculated t-statistic.
dm = Mean of the difference, di, between the spiked samples and unspiked samples.
SDd = Standard deviation of the differences of paired samples.
n = Number of quadruplicate sampling trains.
Compare the calculated t-statistic with the critical value of the t-statistic, and determine if the bias is significant at the 95 percent confidence level. When six quadruplicate runs are conducted, as specified in Table 301-1 of this method, the 2-sided confidence level critical value is 2.571 for the five degrees of freedom. If the calculated t-value is less than the critical value, the bias is not statistically significant and the data are acceptable. If the calculated t-value is greater than the critical value, the bias is statistically significant and you must evaluate the magnitude of the relative bias using Equation 301-22.

Where:
BR = Relative bias.
B = Bias at the spike level from Equation 301-19.
CS = Calculated value at the spike level.
If the relative bias is less than or equal to 10 percent, the bias of the candidate test method is acceptable. On a source-specific basis, if the relative bias is greater than 10 percent but less than or equal to 30 percent, and if you correct all data collected with the candidate test method in the future for the magnitude of the bias using Equation 301-8, the bias of the candidate test method is acceptable for application to the tested source at which the validation testing was conducted. Proceed to evaluate precision of the candidate test method.
12.2 Precision. Calculate the standard deviation using Equation 301-23.

Where:
SD = Standard deviation of the candidate test method.
Si = Measured value of the analyte in the i th spiked sample.
Sm = Mean of the measured values of the analyte in all the spiked samples.
n = Number of spiked samples.
Calculate the RSD of the candidate test method using Equation 301-9, where SD and Sm are the values from Equation 301-23. The data and candidate test method are unacceptable if the RSD is greater than 20 percent.
13.0 How do I conduct tests at similar sources?
If the Administrator has approved the use of an alternative test method to a test method required in 40 CFR part 59, 60, 61, 63, or 65 for an affected source, and you would like to apply the alternative test method to a similar source, then you must petition the Administrator as described in Section 17.1.1 of this method.
Optional Requirements
14.0 How do I use and conduct ruggedness testing?
Ruggedness testing is an optional requirement for validation of a candidate test method that is intended for the source where the validation testing was conducted. Ruggedness testing is required for validation of a candidate test method intended to be used at multiple sources. If you want to use a validated test method at a concentration that is different from the concentration in the applicable emission limitation under 40 CFR part 59, 60, 61, 63, or 65, or for a source category that is different from the source category that the test method specifies, then you must conduct ruggedness testing according to the procedures in Reference 18.16 of Section 18.0 of this method and submit a request for a waiver for conducting Method 301 at that different source category according to Section 17.1.1 of this method.
Ruggedness testing is a study that can be conducted in the laboratory or the field to determine the sensitivity of a method to parameters such as analyte concentration, sample collection rate, interferent concentration, collection medium temperature, and sample recovery temperature. You conduct ruggedness testing by changing several variables simultaneously instead of changing one variable at a time. For example, you can determine the effect of seven variables in only eight experiments. (W.J. Youden, Statistical Manual of the Association of Official Analytical Chemists, Association of Official Analytical Chemists, Washington, DC, 1975, pp. 33-36).
15.0 How do I determine the Limit of Detection for the candidate test method?
Determination of the Limit of Detection (LOD) as specified in Sections 15.1 and 15.2 of this method is required for source-specific method validation and validation of a candidate test method intended to be used for multiple sources.
15.1 Limit of Detection. The LOD is the minimum concentration of a substance that can be measured and reported with 99 percent confidence that the analyte concentration is greater than zero. For this protocol, the LOD is defined as three times the standard deviation, So, at the blank level.
15.2 Purpose. The LOD establishes the lower detection limit of the candidate test method. You must calculate the LOD using the applicable procedures found in Table 301-5 of this method. For candidate test methods that collect the analyte in a sample matrix prior to an analytical measurement, you must determine the LOD using Procedure I in Table 301-5 of this method by calculating a method detection limit (MDL) as described in 40 CFR part 136, appendix B. For the purposes of this section, the LOD is equivalent to the calculated MDL. For radiochemical methods, use the Multi-Agency Radiological Laboratory Analytical Protocols (MARLAP) Manual (i.e., use the minimum detectable concentration (MDC) and not the LOD) available at https://www.epa.gov/radiation/marlap-manual-and-supporting-documents.
Other Requirements and Information
16.0 How do I apply for approval to use a candidate test method?
16.1 Submitting Requests. You must request to use a candidate test method according to the procedures in §63.7(f) or similar sections of 40 CFR parts 59, 60, 61, and 65 (§59.104, §59.406, §60.8(b), §61.13(h)(1)(ii), or §65.158(a)(2)(iii)). You cannot use a candidate test method to meet any requirement under these parts until the Administrator has approved your request. The request must include a field validation report containing the information in Section 16.2 of this method. You must submit the request to the Group Leader, Measurement Technology Group, U.S. Environmental Protection Agency, E143-02, Research Triangle Park, NC 27711.
16.2 Field Validation Report. The field validation report must contain the information in Sections 16.2.1 through 16.2.8 of this method.
16.2.1 Regulatory objectives for the testing, including a description of the reasons for the test, applicable emission limits, and a description of the source.
16.2.2 Summary of the results and calculations shown in Sections 6.0 through 16.0 of this method, as applicable.
16.2.3 Reference material certification and value(s).
16.2.4 Discussion of laboratory evaluations.
16.2.5 Discussion of field sampling.
16.2.6 Discussion of sample preparation and analysis.
16.2.7 Storage times of samples (and extracts, if applicable).
16.2.8 Reasons for eliminating any results.
17.0 How do I request a waiver?
17.1 Conditions for Waivers. If you meet one of the criteria in Section 17.1.1 or 17.1.2 of this method, the Administrator may waive the requirement to use the procedures in this method to validate an alternative or other candidate test method. In addition, if the EPA currently recognizes an appropriate test method or considers the candidate test method to be satisfactory for a particular source, the Administrator may waive the use of this protocol or may specify a less rigorous validation procedure.
17.1.1 Similar Sources. If the alternative or other candidate test method that you want to use was validated for source-specific application at another source and you can demonstrate to the Administrator's satisfaction that your affected source is similar to that validated source, then the Administrator may waive the requirement for you to validate the alternative or other candidate test method. One procedure you may use to demonstrate the applicability of the method to your affected source is to conduct a ruggedness test as described in Section 14.0 of this method.
17.1.2 Documented Methods. If the bias, precision, LOD, or ruggedness of the alternative or other candidate test method that you are proposing have been demonstrated through laboratory tests or protocols different from this method, and you can demonstrate to the Administrator's satisfaction that the bias, precision, LOD, or ruggedness apply to your application, then the Administrator may waive the requirement to use this method or to use part of this method.
17.2 Submitting Applications for Waivers. You must sign and submit each request for a waiver from the requirements in this method in writing. The request must be submitted to the Group Leader, Measurement Technology Group, U.S. Environmental Protection Agency, E143-02, Research Triangle Park, NC 27711.
17.3 Information Application for Waiver. The request for a waiver must contain a thorough description of the candidate test method, the intended application, and results of any validation or other supporting documents. The request for a waiver must contain, at a minimum, the information in Sections 17.3.1 through 17.3.4 of this method. The Administrator may request additional information if necessary to determine whether this method can be waived for a particular application.
17.3.1 A Clearly Written Test Method. The candidate test method should be written preferably in the format of 40 CFR part 60, appendix A, Test Methods. Additionally, the candidate test must include an applicability statement, concentration range, precision, bias (accuracy), and minimum and maximum storage durations in which samples must be analyzed.
17.3.2 Summaries of Previous Validation Tests or Other Supporting Documents. If you use a different procedure from that described in this method, you must submit documents substantiating the bias and precision values to the Administrator's satisfaction.
17.3.3 Ruggedness Testing Results. You must submit results of ruggedness testing conducted according to Section 14.0 of this method, sample stability conducted according to Section 7.0 of this method, and detection limits conducted according to Section 15.0 of this method, as applicable. For example, you would not need to submit ruggedness testing results if you will be using the method at the same affected source and level at which it was validated.
17.3.4 Applicability Statement and Basis for Waiver Approval. Discussion of the applicability statement and basis for approval of the waiver. This discussion should address as applicable the following: applicable regulation, emission standards, effluent characteristics, and process operations.
18.0 Where can I find additional information?
You can find additional information in the references in Sections 18.1 through 18.18 of this method.
18.1 Albritton, J.R., G.B. Howe, S.B. Tompkins, R.K.M. Jayanty, and C.E. Decker. 1989. Stability of Parts-Per-Million Organic Cylinder Gases and Results of Source Test Analysis Audits, Status Report No. 11. Environmental Protection Agency Contract 68-02-4125. Research Triangle Institute, Research Triangle Park, NC. September.
18.2 ASTM Standard E 1169-89 (current version), “Standard Guide for Conducting Ruggedness Tests,” available from ASTM, 100 Barr Harbor Drive, West Conshohoken, PA 19428.
18.3 DeWees, W.G., P.M. Grohse, K.K. Luk, and F.E. Butler. 1989. Laboratory and Field Evaluation of a Methodology for Speciating Nickel Emissions from Stationary Sources. EPA Contract 68-02-4442. Prepared for Atmospheric Research and Environmental Assessment Laboratory, Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711. January.
18.4 International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use, ICH-Q2A, “Text on Validation of Analytical Procedures,” 60 FR 11260 (March 1995).
18.5 International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use, ICH-Q2b, “Validation of Analytical Procedures: Methodology,” 62 FR 27464 (May 1997).
18.6 Keith, L.H., W. Crummer, J. Deegan Jr., R.A. Libby, J.K. Taylor, and G. Wentler. 1983. Principles of Environmental Analysis. American Chemical Society, Washington, DC.
18.7 Maxwell, E.A. 1974. Estimating variances from one or two measurements on each sample. Amer. Statistician 28:96-97.
18.8 Midgett, M.R. 1977. How EPA Validates NSPS Methodology. Environ. Sci. & Technol. 11(7):655-659.
18.9 Mitchell, W.J., and M.R. Midgett. 1976. Means to evaluate performance of stationary source test methods. Environ. Sci. & Technol. 10:85-88.
18.10 Plackett, R.L., and J.P. Burman. 1946. The design of optimum multifactorial experiments. Biometrika, 33:305.
18.11 Taylor, J.K. 1987. Quality Assurance of Chemical Measurements. Lewis Publishers, Inc., pp. 79-81.
18.12 U.S. Environmental Protection Agency. 1978. Quality Assurance Handbook for Air Pollution Measurement Systems: Volume III. Stationary Source Specific Methods. Publication No. EPA-600/4-77-027b. Office of Research and Development Publications, 26 West St. Clair St., Cincinnati, OH 45268.
18.13 U.S. Environmental Protection Agency. 1981. A Procedure for Establishing Traceability of Gas Mixtures to Certain National Bureau of Standards Standard Reference Materials. Publication No. EPA-600/7-81-010. Available from the U.S. EPA, Quality Assurance Division (MD-77), Research Triangle Park, NC 27711.
18.14 U.S. Environmental Protection Agency. 1991. Protocol for The Field Validation of Emission Concentrations from Stationary Sources. Publication No. 450/4-90-015. Available from the U.S. EPA, Emission Measurement Technical Information Center, Technical Support Division (MD-14), Research Triangle Park, NC 27711.
18.15 Wernimont, G.T., “Use of Statistics to Develop and Evaluate Analytical Methods,” AOAC, 1111 North 19th Street, Suite 210, Arlington, VA 22209, USA, 78-82 (1987).
18.16 Youden, W.J. Statistical techniques for collaborative tests. In: Statistical Manual of the Association of Official Analytical Chemists, Association of Official Analytical Chemists, Washington, DC, 1975, pp. 33-36.
18.17 NIST/SEMATECH (current version), “e-Handbook of Statistical Methods,” available from NIST, http://www.itl.nist.gov/div898/handbook/.
18.18 Statistical Table, http://www.math.usask.ca/∼szafron/Stats244/f_table_0_05.pdf.
19.0 Tables.
If you are . . . | You must collect . . . |
|---|---|
| Comparing the candidate test method against a validated method | A total of 24 samples using a quadruplicate sampling system (a total of six sets of replicate samples). In each quadruplicate sample set, you must use the validated test method to collect and analyze half of the samples. |
| Using isotopic spiking (can only be used with methods capable of measurement of multiple isotopes simultaneously) | A total of 12 samples, all of which are spiked with isotopically-labeled analyte. You may collect the samples either by obtaining six sets of paired samples or three sets of quadruplicate samples. |
| Using analyte spiking | A total of 24 samples using the quadruplicate sampling system (a total of six sets of replicate samples - two spiked and two unspiked). |
If you are . . . | With . . . | Then you must . . . |
|---|---|---|
| Using isotopic or analyte spiking procedures | Sample container (bag or canister) or impinger sampling systems that are not subject to dilution or other preparation steps | Analyze six of the samples within 7 days and then analyze the same six samples at the proposed maximum storage duration or 2 weeks after the initial analysis. |
| Sorbent and impinger sampling systems that require extraction or digestion | Extract or digest six of the samples within 7 days and extract or digest six other samples at the proposed maximum storage duration or 2 weeks after the first extraction or digestion. Analyze an aliquot of the first six extracts (digestates) within 7 days and proposed maximum storage duration or 2 weeks after the initial analysis. This will allow analysis of extract storage impacts. | |
| Sorbent sampling systems that require thermal desorption | Analyze six samples within 7 days. Analyze another set of six samples at the proposed maximum storage time or within 2 weeks of the initial analysis. | |
| Comparing a candidate test method against a validated test method | Sample container (bag or canister) or impinger sampling systems that are not subject to dilution or other preparation steps | Analyze at least six of the candidate test method samples within 7 days and then analyze the same six samples at the proposed maximum storage duration or within 2 weeks of the initial analysis. |
| Sorbent and impinger sampling systems that require extraction or digestion | Extract or digest six of the candidate test method samples within 7 days and extract or digest six other samples at the proposed maximum storage duration or within 2 weeks of the first extraction or digestion. Analyze an aliquot of the first six extracts (digestates) within 7 days and an aliquot at the proposed maximum storage durations or within 2 weeks of the initial analysis. This will allow analysis of extract storage impacts. | |
| Sorbent systems that require thermal desorption | Analyze six samples within 7 days. Analyze another set of six samples at the proposed maximum storage duration or within 2 weeks of the initial analysis. |
| Degrees of freedom | t95 |
|---|---|
| 1 | 12.706 |
| 2 | 4.303 |
| 3 | 3.182 |
| 4 | 2.776 |
| 5 | 2.571 |
| 6 | 2.447 |
| 7 | 2.365 |
| 8 | 2.306 |
| 9 | 2.262 |
| 10 | 2.228 |
| 11 | 2.201 |
| 12 | 2.179 |
| 13 | 2.160 |
| 14 | 2.145 |
| 15 | 2.131 |
| 16 | 2.120 |
| 17 | 2.110 |
| 18 | 2.101 |
| 19 | 2.093 |
| 20 | 2.086 |
| 1 Adapted from Reference 18.17 in section 18.0. | |
| Numerator (k1) and denominator (k2) degrees of freedom | F{F>F.05(k1,k2)} |
|---|---|
| 1,1 | 161.40 |
| 2,2 | 19.00 |
| 3,3 | 9.28 |
| 4,4 | 6.39 |
| 5,5 | 5.05 |
| 6,6 | 4.28 |
| 7,7 | 3.79 |
| 8,8 | 3.44 |
| 9,9 | 3.18 |
| 10,10 | 2.98 |
| 11,11 | 2.82 |
| 12,12 | 2.69 |
| 13,13 | 2.58 |
| 14,14 | 2.48 |
| 15,15 | 2.40 |
| 16,16 | 2.33 |
| 17,17 | 2.27 |
| 18,18 | 2.22 |
| 19,19 | 2.17 |
| 20,20 | 2.12 |
| 1 Adapted from References 18.17 and 18.18 in section 18.0. | |
| If the estimated LOD (LOD1, expected approximate LOD concentration level) is no more than twice the calculated LOD or an analyte in a sample matrix was collected prior to an analytical measurement, use Procedure I as follows | If the estimated LOD (LOD1, expected approximate LOD concentration level) is greater than twice the calculated LOD, use Procedure II as follows. |
| Procedure I: | Procedure II: |
| Determine the LOD by calculating a method detection limit (MDL) as described in 40 CFR part 136, appendix B | Prepare two additional standards (LOD2 and LOD3) at concentration levels lower than the standard used in Procedure I (LOD1). |
| Sample and analyze each of these standards (LOD2 and LOD3) at least seven times. | |
| Calculate the standard deviation (S2 and S3) for each concentration level. | |
| Plot the standard deviations of the three test standards (S1, S2 and S3) as a function of concentration. | |
| Draw a best-fit straight line through the data points and extrapolate to zero concentration. The standard deviation at zero concentration is So. | |
| Calculate the LOD0 (referred to as the calculated LOD) as 3 times So. |
Method 303 - Determination of Visible Emissions From By-Product Coke Oven Batteries
Note:
This method is not inclusive with respect to observer certification. Some material is incorporated by reference from other methods in appendix A to 40 CFR part 60. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of Method 9.
1.0 Scope and Application
1.1 Applicability. This method is applicable for the determination of visible emissions (VE) from the following by-product coke oven battery sources: charging systems during charging; doors, topside port lids, and offtake systems on operating coke ovens; and collecting mains. This method is also applicable for qualifying observers for visually determining the presence of VE. In order for the test method results to be indicative of plant performance, the time of day of the run should vary.
2.0 Summary of Method
2.1 A certified observer visually determines the VE from coke oven battery sources. Certification procedures are presented. This method does not require that opacity of emissions be determined or that magnitude be differentiated.
3.0 Definitions
3.1 Bench means the platform structure in front of the oven doors.
3.2 By-product Coke Oven Battery means a source consisting of a group of ovens connected by common walls, where coal undergoes destructive distillation under positive pressure to produce coke and coke oven gas, from which by-products are recovered.
3.3 Charge or charging period means the period of time that commences when coal begins to flow into an oven through a topside port and ends when the last charging port is recapped.
3.4 Charging system means an apparatus used to charge coal to a coke oven (e.g., a larry car for wet coal charging systems).
3.5 Coke oven door means each end enclosure on the push side and the coking side of an oven. The chuck, or leveler-bar, door is considered part of the push side door. The coke oven door area includes the entire area on the vertical face of a coke oven between the bench and the top of the battery between two adjacent buck stays.
3.6 Coke side means the side of a battery from which the coke is discharged from ovens at the end of the coking cycle.
3.7 Collecting main means any apparatus that is connected to one or more offtake systems and that provides a passage for conveying gases under positive pressure from the by-product coke oven battery to the by-product recovery system.
3.8 Consecutive charges means charges observed successively, excluding any charge during which the observer's view of the charging system or topside ports is obscured.
3.9 Damper-off means to close off the gas passage between the coke oven and the collecting main, with no flow of raw coke oven gas from the collecting main into the oven or into the oven's offtake system(s).
3.10 Decarbonization period means the period of time for combusting oven carbon that commences when the oven lids are removed from an empty oven or when standpipe caps of an oven are opened. The period ends with the initiation of the next charging period for that oven.
3.11 Larry car means an apparatus used to charge coal to a coke oven with a wet coal charging system.
3.12 Log average means logarithmic average as calculated in Section 12.4.
3.13 Offtake system means any individual oven apparatus that is stationary and provides a passage for gases from an oven to a coke oven battery collecting main or to another oven. Offtake system components include the standpipe and standpipe caps, goosenecks, stationary jumper pipes, mini-standpipes, and standpipe and gooseneck connections.
3.14 Operating oven means any oven not out of operation for rebuild or maintenance work extensive enough to require the oven to be skipped in the charging sequence.
3.15 Oven means a chamber in the coke oven battery in which coal undergoes destructive distillation to produce coke.
3.16 Push side means the side of the battery from which the coke is pushed from ovens at the end of the coking cycle.
3.17 Run means the observation of visible emissions from topside port lids, offtake systems, coke oven doors, or the charging of a single oven in accordance with this method.
3.18 Shed means an enclosure that covers the side of the coke oven battery, captures emissions from pushing operations and from leaking coke oven doors on the coke side or push side of the coke oven battery, and routes the emissions to a control device or system.
3.19 Standpipe cap means An apparatus used to cover the opening in the gooseneck of an offtake system.
3.20 Topside port lid means a cover, removed during charging or decarbonizing, that is placed over the opening through which coal can be charged into the oven of a by-product coke oven battery.
3.21 Traverse time means accumulated time for a traverse as measured by a stopwatch. Traverse time includes time to stop and write down oven numbers but excludes time waiting for obstructions of view to clear or for time to walk around obstacles.
3.22 Visible Emissions or VE means any emission seen by the unaided (except for corrective lenses) eye, excluding steam or condensing water.
4.0 Interferences [Reserved]
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to performing this test method.
5.2 Safety Training. Because coke oven batteries have hazardous environments, the training materials and the field training (section 10.0) shall cover the precautions required to address health and safety hazards.
6.0 Equipment and Supplies [Reserved]
7.0 Reagents and Standards [Reserved]
8.0 Sample Collection, Preservation, Transport, and Storage [Reserved]
9.0 Quality Control [Reserved]
10.0 Calibration and Standardization
Observer certification and training requirements are as follows:
10.1 Certification Procedures. This method requires only the determination of whether VE occur and does not require the determination of opacity levels; therefore, observer certification according to Method 9 in appendix A to part 60 of this chapter is not required to obtain certification under this method. However, in order to receive Method 303 observer certification, the first-time observer (trainee) shall have attended the lecture portion of the Method 9 certification course. In addition, the trainee shall successfully complete the Method 303 training course, satisfy the field observation requirement, and demonstrate adequate performance and sufficient knowledge of Method 303. The Method 303 training provider and course shall be approved by the Administrator and shall consist of classroom instruction, field training, and a proficiency test. In order to apply for approval as a Method 303 training provider, an applicant must submit their credentials and the details of their Method 303 training course to Group Leader, Measurement Technology Group (E143-02), Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711. Those details should include, at a minimum:
(a) A detailed list of the provider's credentials.
(b) An outline of the classroom and the field portions of the class.
(c) Copies of the written training and lecture materials, to include:
(1) The classroom audio-visual presentation(s).
(2) A classroom course manual with instructional text, practice questions and problems for each of the elements of the Method 303 inspection (i.e., charging, doors, lids and offtakes, and collecting mains). A copy of Method 303 and any related guidance documents should be included as appendices.
(3) A copy of the Method 303 demonstration video, if not using the one available at: http://www3.epa.gov/ttn/emc/methods/method303trainingvideo.mp4.
(4) Multiple-choice certification tests, with questions sufficient to demonstrate knowledge of the method, as follows: One (1) Initial certification test and three (3) third-year recertification tests (the questions on any one recertification test must be at least 25 percent different from those on the other recertification tests).
(5) A field certification checklist and inspection forms for each of the elements of the Method 303 inspection (i.e., charging, doors, lids and offtakes, and collecting mains).
(6) The criteria used to determine proficiency.
(7) The panel members to be utilized (see Section 10.1.3) along with their qualifications.
(8) An example certificate of successful course completion.
10.1.1 A trainee must verify completion of at least 12 hours of field observation prior to attending the Method 303 certification course. Trainees shall observe the operation of a coke oven battery as it pertains to Method 303, including topside operations, and shall also practice conducting Method 303 or similar methods. During the field observations, trainees unfamiliar with coke battery operations shall receive instruction from an experienced coke oven observer who is familiar with Method 303 or similar methods and with the operation of coke batteries.
10.1.2 The classroom instruction shall familiarize the trainees with Method 303 through lecture, written training materials, and a Method 303 demonstration video. Successful completion of the classroom portion of the Method 303 training course shall be demonstrated by a perfect score on the initial certification test. Those attending the course for third-year recertification must complete one of the recertification tests selected at random.
10.1.3 All trainees must demonstrate proficiency in the application of Method 303 to a panel of three certified Method 303 observers, including an ability to differentiate coke oven emissions from condensing water vapor and smoldering coal. The composition of the panel must be approved by the Administrator as part of the training course approval process. The panel members will be EPA, state or local agency personnel, or industry contractors listed in 59 FR 11960 (March 15, 1994) or qualified as part of the training provider approval process of section 10.1 of this method.
Each panel member shall have at least 120 days experience in reading visible emissions from coke ovens. The visible emissions inspections that will satisfy the experience requirement must be inspections of coke oven battery fugitive emissions from the emission points subject to emission standards under subpart L of this part (i.e., coke oven doors, topside port lids, offtake system(s), and charging operations), using either Method 303 or predecessor state or local test methods. A “day's experience” for a particular inspection is a day on which one complete inspection was performed for that emission point under Method 303 or a predecessor state or local method. A “day's experience” does not mean 8 or 10 hours performing inspections, or any particular time expressed in minutes or hours that may have been spent performing them. Thus, it would be possible for an individual to qualify as a Method 303 panel member for some emission points, but not others (e.g., an individual might satisfy the experience requirement for coke oven doors, but not topside port lids). Until November 15, 1994, the EPA may waive the certification requirement (but not the experience requirement) for panel members. The composition of the panel shall be approved by the EPA.
The panel shall observe the trainee in a series of training runs and a series of certification runs. There shall be a minimum of 1 training run for doors, topside port lids, and offtake systems, and a minimum of 5 training runs (i.e., 5 charges) for charging. During training runs, the panel can advise the trainee on proper procedures. There shall be a minimum of 3 certification runs for doors, topside port lids, and offtake systems, and a minimum of 15 certification runs for charging (i.e., 15 charges). The certification runs shall be unassisted. Following the certification test runs, the panel shall approve or disapprove certification based on the trainee's performance during the certification runs. To obtain certification, the trainee shall demonstrate, to the satisfaction of the panel, a high degree of proficiency in performing Method 303. To aid in evaluating the trainee's performance, a checklist, approved by the EPA, will be used by the panel members.
10.1.4 Those successfully completing the initial certification or third-year recertification requirements shall receive a certificate showing certification as a Method 303 observer and the beginning and ending dates of the certification period.
10.1.5 The training provider will submit to the EPA or its designee the following information for each trainee successfully completing initial certification or third-year recertification training: Name, employer, address, telephone, cell and/or fax numbers, email address, beginning and ending dates of certification, and whether training was for 3-year certification or 1-year recertification. This information must be submitted within 30 days of the course completion.
10.1.6 The training provider will maintain the following records, to be made available to EPA or its designee on request (within 30 days of a request):
(a) A file for each Method 303 observer containing the signed certification checklists, certification forms and test results for their initial certification, and any subsequent third-year recertifications. Initial certification records must also include documentation showing successful completion of the training prerequisites. Testing results from any interim recertifications must also be included, along with any relevant communications.
(b) A searchable master electronic database of all persons for whom initial certification, third-year recertification or interim recertification. Information contained therein must include: The observer's name, employer, address, telephone, cell and fax numbers and email address, along with the beginning and ending dates for each successfully completed initial, third-year and interim recertification.
10.1.7 Failure by the training provider to submit example training course materials and/or requested training records to the Administrator may result in suspension of the approval of the provider and course.
10.2 Observer Certification/Recertification. The coke oven observer certification is valid for 1 year. The observer shall recertify annually by reviewing the training material, viewing the training video and answering all of the questions on the recertification test correctly. Every 3 years, an observer shall be required to pass the proficiency test in section 10.1.3 in order to be certified. The years between proficiency tests are referred to as interim years.
10.3 The EPA (or applicable enforcement agency) shall maintain records reflecting a certified observer's successful completion of the proficiency test, which shall include the completed proficiency test checklists for the certification runs.
10.4 An owner or operator of a coke oven battery subject to subpart L of this part may observe a training and certification program under this section.
11.0 Procedure
11.1 Procedure for Determining VE from Charging Systems During Charging.
11.1.1 Number of Oven Charges. Refer to §63.309(c)(1) of this part for the number of oven charges to observe. The observer shall observe consecutive charges. Charges that are nonconsecutive can only be observed when necessary to replace observations terminated prior to the completion of a charge because of visual interferences. (See Section 11.1.5).
11.1.2 Data Records. Record all the information requested at the top of the charging system inspection sheet (Figure 303-1). For each charge, record the identification number of the oven being charged, the approximate beginning time of the charge, and the identification of the larry car used for the charge.
11.1.3 Observer Position. Stand in an area or move to positions on the topside of the coke oven battery with an unobstructed view of the entire charging system. For wet coal charging systems or non-pipeline coal charging systems, the observer should have an unobstructed view of the emission points of the charging system, including larry car hoppers, drop sleeves, and the topside ports of the oven being charged. Some charging systems are configured so that all emission points can only be seen from a distance of five ovens. For other batteries, distances of 8 to 12 ovens are adequate.
11.1.4 Observation. The charging period begins when coal begins to flow into the oven and ends when the last charging port is recapped. During the charging period, observe all of the potential sources of VE from the entire charging system. For wet coal charging systems or non-pipeline coal charging systems, sources of VE typically include the larry car hoppers, drop sleeves, slide gates, and topside ports on the oven being charged. Any VE from an open standpipe cap on the oven being charged is included as charging VE.
11.1.4.1 Using an accumulative-type stopwatch with unit divisions of at least 0.5 seconds, determine the total time VE are observed as follows. Upon observing any VE emerging from any part of the charging system, start the stopwatch. Stop the watch when VE are no longer observed emerging, and restart the watch when VE reemerges.
11.1.4.2 When VE occur simultaneously from several points during a charge, consider the sources as one. Time overlapping VE as continuous VE. Time single puffs of VE only for the time it takes for the puff to emerge from the charging system. Continue to time VE in this manner for the entire charging period. Record the accumulated time to the nearest 0.5 second under “Visible emissions, seconds” on Figure 303-1.
11.1.5 Visual Interference. If fugitive VE from other sources at the coke oven battery site (e.g., door leaks or condensing water vapor from the coke oven wharf) prevent a clear view of the charging system during a charge, stop the stopwatch and make an appropriate notation under “Comments” on Figure 303-1. Label the observation an observation of an incomplete charge, and observe another charge to fulfill the requirements of Section 11.1.1.
11.1.6 VE Exemptions. Do not time the following VE:
11.1.6.1 The VE from burning or smoldering coal spilled on top of the oven, topside port lid, or larry car surfaces;
Note:
The VE from smoldering coal are generally white or gray. These VE generally have a plume of less than 1 meter long. If the observer cannot safely and with reasonable confidence determine that VE are from charging, do not count them as charging emissions.
11.1.6.2 The VE from the coke oven doors or from the leveler bar; or
11.1.6.3 The VE that drift from the top of a larry car hopper if the emissions had already been timed as VE from the drop sleeve.
Note:
When the slide gate on a larry car hopper closes after the coal has been added to the oven, the seal may not be airtight. On occasions, a puff of smoke observed at the drop sleeves is forced past the slide gate up into the larry car hopper and may drift from the top; time these VE either at the drop sleeves or the hopper. If the larry car hopper does not have a slide gate or the slide gate is left open or partially closed, VE may quickly pass through the larry car hopper without being observed at the drop sleeves and will appear as a strong surge of smoke; time these as charging VE.
11.1.7 Total Time Record. Record the total time that VE were observed for each charging operation in the appropriate column on the charging system inspection sheet.
11.1.8 Determination of Validity of a Set of Observations. Five charging observations (runs) obtained in accordance with this method shall be considered a valid set of observations for that day. No observation of an incomplete charge shall be included in a daily set of observations that is lower than the lowest reading for a complete charge. If both complete and incomplete charges have been observed, the daily set of observations shall include the five highest values observed. Four or three charging observations (runs) obtained in accordance with this method shall be considered a valid set of charging observations only where it is not possible to obtain five charging observations, because visual interferences (see Section 11.1.5) or inclement weather prevent a clear view of the charging system during charging. However, observations from three or four charges that satisfy these requirements shall not be considered a valid set of charging observations if use of such set of observations in a calculation under Section 12.4 would cause the value of A to be less than 145.
11.1.9 Log Average. For each day on which a valid daily set of observations is obtained, calculate the daily 30-day rolling log average of seconds of visible emissions from the charging operation for each battery using these data and the 29 previous valid daily sets of observations, in accordance with Section 12.4.
11.2. Procedure for Determining VE from Coke Oven Door Areas. The intent of this procedure is to determine VE from coke oven door areas by carefully observing the door area from a standard distance while walking at a normal pace.
11.2.1 Number of Runs. Refer to §63.309(c)(1) of this part for the appropriate number of runs.
11.2.2 Battery Traverse. To conduct a battery traverse, walk the length of the battery on the outside of the pusher machine and quench car tracks at a steady, normal walking pace, pausing to make appropriate entries on the door area inspection sheet (Figure 303-2). A single test run consists of two timed traverses, one for the coke side and one for the push side. The walking pace shall be such that the duration of the traverse does not exceed an average of 4 seconds per oven door, excluding time spent moving around stationary obstructions or waiting for other obstructions to move from positions blocking the view of a series of doors. Extra time is allowed for each leak (a maximum of 10 additional seconds for each leaking door) for the observer to make the proper notation. A walking pace of 3 seconds per oven door has been found to be typical. Record the actual traverse time with a stopwatch.
11.2.2.1 Include in the traverse time only the time spent observing the doors and recording door leaks. To measure actual traverse time, use an accumulative-type stopwatch with unit divisions of 0.5 seconds or less. Exclude interruptions to the traverse and time required for the observer to move to positions where the view of the battery is unobstructed, or for obstructions, such as the door machine, to move from positions blocking the view of a series of doors.
11.2.2.2 Various situations may arise that will prevent the observer from viewing a door or a series of doors. Prior to the door inspection, the owner or operator may elect to temporarily suspend charging operations for the duration of the inspection, so that all of the doors can be viewed by the observer. The observer has two options for dealing with obstructions to view: (a) Stop the stopwatch and wait for the equipment to move or the fugitive emissions to dissipate before completing the traverse; or (b) stop the stopwatch, skip the affected ovens, and move to an unobstructed position to continue the traverse. Restart the stopwatch and continue the traverse. After the completion of the traverse, if the equipment has moved or the fugitive emissions have dissipated, inspect the affected doors. If the equipment is still preventing the observer from viewing the doors, then the affected doors may be counted as not observed. If option (b) is used because of doors blocked by machines during charging operations, then, of the affected doors, exclude the door from the most recently charged oven from the inspection. Record the oven numbers and make an appropriate notation under “Comments” on the door area inspection sheet (Figure 303-2).
11.2.2.3 When batteries have sheds to control emissions, conduct the inspection from outside the shed unless the doors cannot be adequately viewed. In this case, conduct the inspection from the bench. Be aware of special safety considerations pertinent to walking on the bench and follow the instructions of company personnel on the required equipment and procedures. If possible, conduct the bench traverse whenever the bench is clear of the door machine and hot coke guide.
11.2.3 Observations. Record all the information requested at the top of the door area inspection sheet (Figure 303-2), including the number of non-operating ovens. Record the clock time at the start of the traverse on each side of the battery. Record which side is being inspected (i.e., coke side or push side). Other information may be recorded at the discretion of the observer, such as the location of the leak (e.g., top of the door, chuck door, etc.), the reason for any interruption of the traverse, or the position of the sun relative to the battery and sky conditions (e.g., overcast, partly sunny, etc.).
11.2.3.1 Begin the test run by starting the stopwatch and traversing either the coke side or the push side of the battery. After completing one side, stop the watch. Complete this procedure on the other side. If inspecting more than one battery, the observer may view the push sides and the coke sides sequentially.
11.2.3.2 During the traverse, look around the entire perimeter of each oven door. The door is considered leaking if VE are detected in the coke oven door area. The coke oven door area includes the entire area on the vertical face of a coke oven between the bench and the top of the battery between two adjacent buck stays (e.g., the oven door, chuck door, between the masonry brick, buck stay or jamb, or other sources). Record the oven number and make the appropriate notation on the door area inspection sheet (Figure 303-2).
Note:
Multiple VE from the same door area (e.g., VE from both the chuck door and the push side door) are counted as only one emitting door, not as multiple emitting doors.
11.2.3.3 Do not record the following sources as door area VE:
11.2.3.3.1 VE from ovens with doors removed. Record the oven number and make an appropriate notation under “Comments;”
11.2.3.3.2 VE from ovens taken out of service. The owner or operator shall notify the observer as to which ovens are out of service. Record the oven number and make an appropriate notation under “Comments;” or
11.2.3.3.3 VE from hot coke that has been spilled on the bench as a result of pushing.
11.2.4 Criteria for Acceptance. After completing the run, calculate the maximum time allowed to observe the ovens using the equation in Section 12.2. If the total traverse time exceeds T, void the run, and conduct another run to satisfy the requirements of §63.309(c)(1) of this part.
11.2.5 Percent Leaking Doors. For each day on which a valid observation is obtained, calculate the daily 30-day rolling average for each battery using these data and the 29 previous valid daily observations, in accordance with Section 12.5.
11.3 Procedure for Determining VE from Topside Port Lids and Offtake Systems.
11.3.1 Number of Runs. Refer to §63.309(c)(1) of this part for the number of runs to be conducted. Simultaneous runs or separate runs for the topside port lids and offtake systems may be conducted.
11.3.2 Battery Traverse. To conduct a topside traverse of the battery, walk the length of the battery at a steady, normal walking pace, pausing only to make appropriate entries on the topside inspection sheet (Figure 303-3). The walking pace shall not exceed an average rate of 4 seconds per oven, excluding time spent moving around stationary obstructions or waiting for other obstructions to move from positions blocking the view. Extra time is allowed for each leak for the observer to make the proper notation. A walking pace of 3 seconds per oven is typical. Record the actual traverse time with a stopwatch.
11.3.3 Topside Port Lid Observations. To observe lids of the ovens involved in the charging operation, the observer shall wait to view the lids until approximately 5 minutes after the completion of the charge. Record all the information requested on the topside inspection sheet (Figure 303-3). Record the clock time when traverses begin and end. If the observer's view is obstructed during the traverse (e.g., steam from the coke wharf, larry car, etc.), follow the guidelines given in Section 11.2.2.2.
11.3.3.1 To perform a test run, conduct a single traverse on the topside of the battery. The observer shall walk near the center of the battery but may deviate from this path to avoid safety hazards (such as open or closed charging ports, luting buckets, lid removal bars, and topside port lids that have been removed) and any other obstacles. Upon noting VE from the topside port lid(s) of an oven, record the oven number and port number, then resume the traverse. If any oven is dampered-off from the collecting main for decarbonization, note this under “Comments” for that particular oven.
Note:
Count the number of topside ports, not the number of points, exhibiting VE, i.e., if a topside port has several points of VE, count this as one port exhibiting VE.
11.3.3.2 Do not count the following as topside port lid VE:
11.3.3.2.1 VE from between the brickwork and oven lid casing or VE from cracks in the oven brickwork. Note these VE under “Comments;”
11.3.3.2.2 VE from topside ports involved in a charging operation. Record the oven number, and make an appropriate notation (e.g., not observed because ports open for charging) under “Comments;”
11.3.3.2.3 Topside ports having maintenance work done. Record the oven number and make an appropriate notation under “Comments;” or
11.3.3.2.4 Condensing water from wet-sealing material. Ports with only visible condensing water from wet-sealing material are counted as observed but not as having VE.
11.3.3.2.5 Visible emissions from the flue inspection ports and caps.
11.3.4 Offtake Systems Observations. To perform a test run, traverse the battery as in Section 11.3.3.1. Look ahead and back two to four ovens to get a clear view of the entire offtake system for each oven. Consider visible emissions from the following points as offtake system VE: (a) the flange between the gooseneck and collecting main (“saddle”), (b) the junction point of the standpipe and oven (“standpipe base”), (c) the other parts of the offtake system (e.g., the standpipe cap), and (d) the junction points with ovens and flanges of jumper pipes.
11.3.4.1 Do not stray from the traverse line in order to get a “closer look” at any part of the offtake system unless it is to distinguish leaks from interferences from other sources or to avoid obstacles.
11.3.4.2 If the centerline does not provide a clear view of the entire offtake system for each oven (e.g., when standpipes are longer than 15 feet), the observer may conduct the traverse farther from (rather than closer to) the offtake systems.
11.3.4.3 Upon noting a leak from an offtake system during a traverse, record the oven number. Resume the traverse. If the oven is dampered-off from the collecting main for decarbonization and VE are observed, note this under “Comments” for that particular oven.
11.3.4.4 If any part or parts of an offtake system have VE, count it as one emitting offtake system. Each stationary jumper pipe is considered a single offtake system.
11.3.4.5 Do not count standpipe caps open for a decarbonization period or standpipes of an oven being charged as source of offtake system VE. Record the oven number and write “Not observed” and the reason (i.e., decarb or charging) under “Comments.”
Note:
VE from open standpipes of an oven being charged count as charging emissions. All VE from closed standpipe caps count as offtake leaks.
11.3.5 Criteria for Acceptance. After completing the run (allow 2 traverses for batteries with double mains), calculate the maximum time allowed to observe the topside port lids and/or offtake systems using the equation in Section 12.3. If the total traverse time exceeds T, void the run and conduct another run to satisfy the requirements of §63.309(c)(1) of this part.
11.3.6 In determining the percent leaking topside port lids and percent leaking offtake systems, do not include topside port lids or offtake systems with VE from the following ovens:
11.3.6.1 Empty ovens, including ovens undergoing maintenance, which are properly dampered off from the main.
11.3.6.2 Ovens being charged or being pushed.
11.3.6.3 Up to 3 full ovens that have been dampered off from the main prior to pushing.
11.3.6.4 Up to 3 additional full ovens in the pushing sequence that have been dampered off from the main for offtake system cleaning, for decarbonization, for safety reasons, or when a charging/pushing schedule involves widely separated ovens (e.g., a Marquard system); or that have been dampered off from the main for maintenance near the end of the coking cycle. Examples of reasons that ovens are dampered off for safety reasons are to avoid exposing workers in areas with insufficient clearance between standpipes and the larry car, or in areas where workers could be exposed to flames or hot gases from open standpipes, and to avoid the potential for removing a door on an oven that is not dampered off from the main.
11.3.7 Percent Leaking Topside Port Lids and Offtake Systems. For each day on which a valid observation is obtained, calculate the daily 30-day rolling average for each battery using these data and the 29 previous valid daily observations, in accordance with Sections 12.6 and 12.7.
11.4 Procedure for Determining VE from Collecting Mains.
11.4.1 Traverse. To perform a test run, traverse both the collecting main catwalk and the battery topside along the side closest to the collecting main. If the battery has a double main, conduct two sets of traverses for each run, i.e., one set for each main.
11.4.2 Data Recording. Upon noting VE from any portion of a collection main, identify the source and approximate location of the source of VE and record the time under “Collecting main” on Figure 303-3; then resume the traverse.
11.4.3 Collecting Main Pressure Check. After the completion of the door traverse, the topside port lids, and offtake systems, compare the collecting main pressure during the inspection to the collecting main pressure during the previous 8 to 24 hours. Record the following: (a) the pressure during inspection, (b) presence of pressure deviation from normal operations, and (c) the explanation for any pressure deviation from normal operations, if any, offered by the operators. The owner or operator of the coke battery shall maintain the pressure recording equipment and conduct the quality assurance/quality control (QA/QC) necessary to ensure reliable pressure readings and shall keep the QA/QC records for at least 6 months. The observer may periodically check the QA/QC records to determine their completeness. The owner or operator shall provide access to the records within 1 hour of an observer's request.
12.0 Data Analysis and Calculations
12.1 Nomenclature.
A = 150 or the number of valid observations (runs). The value of A shall not be less than 145, except for purposes of determinations under §63.306(c) (work practice plan implementation) or §63.306(d) (work practice plan revisions) of this part. No set of observations shall be considered valid for such a recalculation that otherwise would not be considered a valid set of observations for a calculation under this paragraph.
Di = Number of doors on non-operating ovens.
Dno = Number of doors not observed.
Dob = Total number of doors observed on operating ovens.
Dt = Total number of oven doors on the battery.
e = 2.72
J = Number of stationary jumper pipes.
L = Number of doors with VE.
Lb = Yard-equivalent reading.
Ls = Number of doors with VE observed from the bench under sheds.
Ly = Number of doors with VE observed from the yard.
Ly = Number of doors with VE observed from the yard on the push side.
ln = Natural logarithm.
N = Total number of ovens in the battery.
Ni = Total number of inoperable ovens.
PNO = Number of ports not observed.
Povn = Number of ports per oven.
PVE = Number of topside port lids with VE.
PLD = Percent leaking coke oven doors for the test run.
PLL = Percent leaking topside port lids for the run.
PLO = Percent leaking offtake systems.
T = Total time allowed for traverse, seconds.
Tovn = Number of offtake systems (excluding jumper pipes) per oven.
TNO = Number of offtake systems not observed.
TVE = Number of offtake systems with VE.
Xi = Seconds of VE during the ith charge.
Z = Number of topside port lids or offtake systems with VE.
12.2 Criteria for Acceptance for VE Determinations from Coke Oven Door Areas. After completing the run, calculate the maximum time allowed to observe the ovens using the following equation:

12.3 Criteria for Acceptance for VE Determinations from Topside Port Lids and Offtake Systems. After completing the run (allow 2 traverses for batteries with double mains), calculate the maximum time allowed to observe the topside port lids and/or offtake systems by the following equation:

12.4 Average Duration of VE from Charging Operations. Use Equation 303-3 to calculate the daily 30-day rolling log average of seconds of visible emissions from the charging operation for each battery using these current day's observations and the 29 previous valid daily sets of observations.
12.5 Percent Leaking Doors (PLD). Determine the total number of doors for which observations were made on the coke oven battery as follows:

12.5.1 For each test run (one run includes both the coke side and the push side traverses), sum the number of doors with door area VE. For batteries subject to an approved alternative standard under §63.305 of this part, calculate the push side and the coke side PLD separately.
12.5.2 Calculate percent leaking doors by using Equation 303-5:

12.5.3 When traverses are conducted from the bench under sheds, calculate the coke side and the push side separately. Use Equation 303-6 to calculate a yard-equivalent reading:

If Lb is less than zero, use zero for Lb in Equation 303-7 in the calculation of PLD.
12.5.3.1 Use Equation 303-7 to calculate PLD:

Round off PLD to the nearest hundredth of 1 percent and record as the percent leaking coke oven doors for the run.
12.5.3.2 Average Percent Leaking Doors. Use Equation 303-8 to calculate the daily 30-day rolling average percent leaking doors for each battery using these current day's observations and the 29 previous valid daily sets of observations.

12.6 Topside Port Lids. Determine the percent leaking topside port lids for each run as follows:

12.6.1 Round off this percentage to the nearest hundredth of 1 percent and record this percentage as the percent leaking topside port lids for the run.
12.6.2 Average Percent Leaking Topside Port Lids. Use Equation 303-10 to calculate the daily 30-day rolling average percent leaking topside port lids for each battery using these current day's observations and the 29 previous valid daily sets of observations.

12.7 Offtake Systems. Determine the percent leaking offtake systems for the run as follows:

12.7.1 Round off this percentage to the nearest hundredth of 1 percent and record this percentage as the percent leaking offtake systems for the run.
12.7.2 Average Percent Leaking Offtake Systems. Use Equation 303-12 to calculate the daily 30-day rolling average percent leaking offtake systems for each battery using these current day's observations and the 29 previous valid daily sets of observations.

13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References.
1. Missan, R., and A. Stein. Guidelines for Evaluation of Visible Emissions Certification, Field Procedures, Legal Aspects, and Background Material. U.S. Environmental Protection Agency. EPA Publication No. EPA-340/1-75-007. April 1975.
2. Wohlschlegel, P., and D. E. Wagoner. Guideline for Development of a Quality Assurance Program: Volume IX - Visual Determination of Opacity Emission from Stationary Sources. U.S. Environmental Protection Agency. EPA Publication No. EPA-650/4-74-005i. November 1975.
3. U.S. Occupational Safety and Health Administration. Code of Federal Regulations. Title 29, Chapter XVII, Section 1910.1029(g). Washington, D.C. Government Printing Office. July 1, 1990.
4. U.S. Environmental Protection Agency. National Emission Standards for Hazardous Air Pollutants; Coke Oven Emissions from Wet-Coal Charged By-Product Coke Oven Batteries; Proposed Rule and Notice of Public Hearing. Washington, D.C. Federal Register. Vol. 52, No. 78 (13586). April 23, 1987.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
Company name:
Battery no.: ___ Date: ___ Run no.: ___
City, State:
Observer name:
Company representative(s):
| Charge No. |
Oven
No. | Clock time |
Visible
emissions, seconds | Comments |
|---|---|---|---|---|
Figure 303-1. Charging System Inspection
Company name:
Battery no.:
Date:
City, State:
Total no. of ovens in battery:
Observer name:
Certification expiration date:
Inoperable ovens:
Company representative(s):
Traverse time CS:
Traverse time PS:
Valid run (Y or N):
| Time traverse started/completed | PS/CS | Door No. |
Comments
(No. of blocked doors, interruptions to traverse, etc.) |
|---|---|---|---|
Figure 303-2. Door Area Inspection.
Company name:
Battery no.:
Date:
City, State:
Total no. of ovens in battery:
Observer name:
Certification expiration date:
Inoperable ovens:
Company representative(s):
Total no. of lids:
Total no. of offtakes:
Total no. of jumper pipes:
Ovens not observed:
Total traverse time:
Valid run (Y or N):
| Time traverse started/completed |
Type of Inspection
(lids, offtakes, collecting main) |
Location of VE
(Oven #/Port #) | Comments |
|---|---|---|---|
Figure 303-3. Topside Inspection
Method 303A - Determination of Visible Emissions From Nonrecovery Coke Oven Batteries
Note:
This method does not include all of the specifications pertaining to observer certification. Some material is incorporated by reference from other methods in this part and in appendix A to 40 CFR Part 60. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of Method 9 and Method 303.
1.0 Scope and Application
1.1 Applicability. This method is applicable for the determination of visible emissions (VE) from leaking doors at nonrecovery coke oven batteries.
2.0 Summary of Method
2.1 A certified observer visually determines the VE from coke oven battery sources while walking at a normal pace. This method does not require that opacity of emissions be determined or that magnitude be differentiated.
3.0 Definitions
3.1 Bench means the platform structure in front of the oven doors.
3.2 Coke oven door means each end enclosure on the push side and the coking side of an oven.
3.3 Coke side means the side of a battery from which the coke is discharged from ovens at the end of the coking cycle.
3.4 Nonrecovery coke oven battery means a source consisting of a group of ovens connected by common walls and operated as a unit, where coal undergoes destructive distillation under negative pressure to produce coke, and which is designed for the combustion of coke oven gas from which by-products are not recovered.
3.5 Operating oven means any oven not out of operation for rebuild or maintenance work extensive enough to require the oven to be skipped in the charging sequence.
3.6 Oven means a chamber in the coke oven battery in which coal undergoes destructive distillation to produce coke.
3.7 Push side means the side of the battery from which the coke is pushed from ovens at the end of the coking cycle.
3.8 Run means the observation of visible emissions from coke oven doors in accordance with this method.
3.9 Shed means an enclosure that covers the side of the coke oven battery, captures emissions from pushing operations and from leaking coke oven doors on the coke side or push side of the coke oven battery, and routes the emissions to a control device or system.
3.10 Traverse time means accumulated time for a traverse as measured by a stopwatch. Traverse time includes time to stop and write down oven numbers but excludes time waiting for obstructions of view to clear or for time to walk around obstacles.
3.11 Visible Emissions or VE means any emission seen by the unaided (except for corrective lenses) eye, excluding steam or condensing water.
4.0 Interferences [Reserved]
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to performing this test method.
5.2 Safety Training. Because coke oven batteries have hazardous environments, the training materials and the field training (Section 10.0) shall cover the precautions required by the company to address health and safety hazards. Special emphasis shall be given to the Occupational Safety and Health Administration (OSHA) regulations pertaining to exposure of coke oven workers (see Reference 3 in Section 16.0). In general, the regulation requires that special fire-retardant clothing and respirators be worn in certain restricted areas of the coke oven battery. The OSHA regulation also prohibits certain activities, such as chewing gum, smoking, and eating in these areas.
6.0 Equipment and Supplies [Reserved]
7.0 Reagents and Standards [Reserved]
8.0 Sample Collection, Preservation, Transport, and Storage [Reserved]
9.0 Quality Control [Reserved]
10.0 Calibration and Standardization.
10.1 Training. This method requires only the determination of whether VE occur and does not require the determination of opacity levels; therefore, observer certification according to Method 9 in Appendix A to part 60 is not required. However, the first-time observer (trainee) shall have attended the lecture portion of the Method 9 certification course. Furthermore, before conducting any VE observations, an observer shall become familiar with nonrecovery coke oven battery operations and with this test method by observing for a minimum of 4 hours the operation of a nonrecovery coke oven battery in the presence of personnel experienced in performing Method 303 assessments.
11.0 Procedure
The intent of this procedure is to determine VE from coke oven door areas by carefully observing the door area while walking at a normal pace.
11.1 Number of Runs. Refer to §63.309(c)(1) of this part for the appropriate number of runs.
11.2 Battery Traverse. To conduct a battery traverse, walk the length of the battery on the outside of the pusher machine and quench car tracks at a steady, normal walking pace, pausing to make appropriate entries on the door area inspection sheet (Figure 303A-1). The walking pace shall be such that the duration of the traverse does not exceed an average of 4 seconds per oven door, excluding time spent moving around stationary obstructions or waiting for other obstructions to move from positions blocking the view of a series of doors. Extra time is allowed for each leak (a maximum of 10 additional seconds for each leaking door) for the observer to make the proper notation. A walking pace of 3 seconds per oven door has been found to be typical. Record the actual traverse time with a stopwatch. A single test run consists of two timed traverses, one for the coke side and one for the push side.
11.2.1 Various situations may arise that will prevent the observer from viewing a door or a series of doors. The observer has two options for dealing with obstructions to view: (a) Wait for the equipment to move or the fugitive emissions to dissipate before completing the traverse; or (b) skip the affected ovens and move to an unobstructed position to continue the traverse. Continue the traverse. After the completion of the traverse, if the equipment has moved or the fugitive emissions have dissipated, complete the traverse by inspecting the affected doors. Record the oven numbers and make an appropriate notation under “Comments” on the door area inspection sheet (Figure 303A-1).
Note:
Extra time incurred for handling obstructions is not counted in the traverse time.
11.2.2 When batteries have sheds to control pushing emissions, conduct the inspection from outside the shed, if the shed allows such observations, or from the bench. Be aware of special safety considerations pertinent to walking on the bench and follow the instructions of company personnel on the required equipment and operations procedures. If possible, conduct the bench traverse whenever the bench is clear of the door machine and hot coke guide.
11.3 Observations. Record all the information requested at the top of the door area inspection sheet (Figure 303A-1), including the number of non-operating ovens. Record which side is being inspected, i.e., coke side or push side. Other information may be recorded at the discretion of the observer, such as the location of the leak (e.g., top of the door), the reason for any interruption of the traverse, or the position of the sun relative to the battery and sky conditions (e.g., overcast, partly sunny, etc.).
11.3.1 Begin the test run by traversing either the coke side or the push side of the battery. After completing one side, traverse the other side.
11.3.2 During the traverse, look around the entire perimeter of each oven door. The door is considered leaking if VE are detected in the coke oven door area. The coke oven door area includes the entire area on the vertical face of a coke oven between the bench and the top of the battery and the adjacent doors on both sides. Record the oven number and make the appropriate notation on the door area inspection sheet (Figure 303A-1).
11.3.3 Do not record the following sources as door area VE:
11.3.3.1 VE from ovens with doors removed. Record the oven number and make an appropriate notation under “Comments”;
11.3.3.2 VE from ovens where maintenance work is being conducted. Record the oven number and make an appropriate notation under “Comments”; or
11.3.3.3 VE from hot coke that has been spilled on the bench as a result of pushing.
12.0 Data Analysis and Calculations
Same as Method 303, Section 12.1, 12.2, 12.3, 12.4, and 12.5.
13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References
Same as Method 303, Section 16.0.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
Company name:
Battery no.:
Date:
City, State:
Total no. of ovens in battery:
Observer name:
Certification expiration date:
Inoperable ovens:
Company representative(s):
Traverse time CS:
Traverse time PS:
Valid run (Y or N):
| Time traverse started/completed | PS/CS | Door No. |
Comments
(No. of blocked doors, interruptions to traverse, etc.) |
|---|---|---|---|
Figure 303A-1. Door Area Inspection
Method 304A: Determination of Biodegradation Rates of Organic Compounds (Vent Option)
1.0 Scope and Application
1.1 Applicability. This method is applicable for the determination of biodegradation rates of organic compounds in an activated sludge process. The test method is designed to evaluate the ability of an aerobic biological reaction system to degrade or destroy specific components in waste streams. The method may also be used to determine the effects of changes in wastewater composition on operation. The biodegradation rates determined by utilizing this method are not representative of a full-scale system. The rates measured by this method shall be used in conjunction with the procedures listed in appendix C of this part to calculate the fraction emitted to the air versus the fraction biodegraded.
2.0 Summary of Method
2.1 A self-contained benchtop bioreactor system is assembled in the laboratory. A sample of mixed liquor is added and the waste stream is then fed continuously. The benchtop bioreactor is operated under conditions nearly identical to the target full-scale activated sludge process. Bioreactor temperature, dissolved oxygen concentration, average residence time in the reactor, waste composition, biomass concentration, and biomass composition of the full-scale process are the parameters which are duplicated in the benchtop bioreactor. Biomass shall be removed from the target full-scale activated sludge unit and held for no more than 4 hours prior to use in the benchtop bioreactor. If antifoaming agents are used in the full-scale system, they shall also be used in the benchtop bioreactor. The feed flowing into and the effluent exiting the benchtop bioreactor are analyzed to determine the biodegradation rates of the target compounds. The flow rate of the exit vent is used to calculate the concentration of target compounds (utilizing Henry's law) in the exit gas stream. If Henry's law constants for the compounds of interest are not known, this method cannot be used in the determination of the biodegradation rate and Method 304B is the suggested method. The choice of analytical methodology for measuring the compounds of interest at the inlet and outlet to the benchtop bioreactor are left to the discretion of the source, except where validated methods are available.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
5.1 If explosive gases are produced as a byproduct of biodegradation and could realistically pose a hazard, closely monitor headspace concentration of these gases to ensure laboratory safety. Placement of the benchtop bioreactor system inside a laboratory hood is recommended regardless of byproducts produced.
6.0. Equipment and Supplies
Note:
Figure 304A-1 illustrates a typical laboratory apparatus used to measure biodegradation rates. While the following description refers to Figure 304A-1, the EPA recognizes that alternative reactor configurations, such as alternative reactor shapes and locations of probes and the feed inlet, will also meet the intent of this method. Ensure that the benchtop bioreactor system is self-contained and isolated from the atmosphere (except for the exit vent stream) by leak-checking fittings, tubing, etc.
6.1 Benchtop Bioreactor. The biological reaction is conducted in a biological oxidation reactor of at least 6 liters capacity. The benchtop bioreactor is sealed and equipped with internal probes for controlling and monitoring dissolved oxygen and internal temperature. The top of the reactor is equipped for aerators, gas flow ports, and instrumentation (while ensuring that no leaks to the atmosphere exist around the fittings).
6.2 Aeration gas. Aeration gas is added to the benchtop bioreactor through three diffusers, which are glass tubes that extend to the bottom fifth of the reactor depth. A pure oxygen pressurized cylinder is recommended in order to maintain the specified oxygen concentration. Install a blower (e.g., Diaphragm Type, 15 SCFH capacity) to blow the aeration gas into the reactor diffusers. Measure the aeration gas flow rate with a rotameter (e.g., 0-15 SCFH recommended). The aeration gas will rise through the benchtop bioreactor, dissolving oxygen into the mixture in the process. The aeration gas must provide sufficient agitation to keep the solids in suspension. Provide an exit for the aeration gas from the top flange of the benchtop bioreactor through a water-cooled (e.g., Allihn-type) vertical condenser. Install the condenser through a gas-tight fitting in the benchtop bioreactor closure. Install a splitter which directs a portion of the gas to an exit vent and the rest of the gas through an air recycle pump back to the benchtop bioreactor. Monitor and record the flow rate through the exit vent at least 3 times per day throughout the day.
6.3 Wastewater Feed. Supply the wastewater feed to the benchtop bioreactor in a collapsible low-density polyethylene container or collapsible liner in a container (e.g., 20 L) equipped with a spigot cap (collapsible containers or liners of other material may be required due to the permeability of some volatile compounds through polyethylene). Obtain the wastewater feed by sampling the wastewater feed in the target process. A representative sample of wastewater shall be obtained from the piping leading to the aeration tank. This sample may be obtained from existing sampling valves at the discharge of the wastewater feed pump, or collected from a pipe discharging to the aeration tank, or by pumping from a well-mixed equalization tank upstream from the aeration tank. Alternatively, wastewater can be pumped continuously to the laboratory apparatus from a bleed stream taken from the equalization tank of the full-scale treatment system.
6.3.1 Refrigeration System. Keep the wastewater feed cool by ice or by refrigeration to 4°C. If using a bleed stream from the equalization tank, refrigeration is not required if the residence time in the bleed stream is less than five minutes.
6.3.2 Wastewater Feed Pump. The wastewater is pumped from the refrigerated container using a variable-speed peristaltic pump drive equipped with a peristaltic pump head. Add the feed solution to the benchtop bioreactor through a fitting on the top flange. Determine the rate of feed addition to provide a retention time in the benchtop bioreactor that is numerically equivalent to the retention time in the full-scale system. The wastewater shall be fed at a rate sufficient to achieve 90 to 100 percent of the full-scale system residence time.
6.3.3 Treated wastewater feed. The benchtop bioreactor effluent exits at the bottom of the reactor through a tube and proceeds to the clarifier.
6.4 Clarifier. The effluent flows to a separate closed clarifier that allows separation of biomass and effluent (e.g., 2-liter pear-shaped glass separatory funnel, modified by removing the stopcock and adding a 25-mm OD glass tube at the bottom). Benchtop bioreactor effluent enters the clarifier through a tube inserted to a depth of 0.08 m (3 in.) through a stopper at the top of the clarifier. System effluent flows from a tube inserted through the stopper at the top of the clarifier to a drain (or sample bottle when sampling). The underflow from the clarifier leaves from the glass tube at the bottom of the clarifier. Flexible tubing connects this fitting to the sludge recycle pump. This pump is coupled to a variable speed pump drive. The discharge from this pump is returned through a tube inserted in a port on the side of the benchtop bioreactor. An additional port is provided near the bottom of the benchtop bioreactor for sampling the reactor contents. The mixed liquor from the benchtop bioreactor flows into the center of the clarifier. The clarified system effluent separates from the biomass and flows through an exit near the top of the clarifier. There shall be no headspace in the clarifier.
6.5 Temperature Control Apparatus. Capable of maintaining the system at a temperature equal to the temperature of the full-scale system. The average temperature should be maintained within ±2°C of the set point.
6.5.1 Temperature Monitoring Device. A resistance type temperature probe or a thermocouple connected to a temperature readout with a resolution of 0.1°C or better.
6.5.2 Benchtop Bioreactor Heater. The heater is connected to the temperature control device.
6.6 Oxygen Control System. Maintain the dissolved oxygen concentration at the levels present in the full-scale system. Target full-scale activated sludge systems with dissolved oxygen concentration below 2 mg/L are required to maintain the dissolved oxygen concentration in the benchtop ioreactor within 0.5 mg/L of the target dissolved oxygen level. Target full-scale activated sludge systems with dissolved oxygen concentration above 2 mg/L are required to maintain the dissolved oxygen concentration in the benchtop bioreactor within 1.5 mg/L of the target dissolved oxygen concentration; however, for target full-scale activated sludge systems with dissolved oxygen concentrations above 2 mg/L, the dissolved oxygen concentration in the benchtop bioreactor may not drop below 1.5 mg/L. If the benchtop bioreactor is outside the control range, the dissolved oxygen is noted and the reactor operation is adjusted.
6.6.1 Dissolved Oxygen Monitor. Dissolved oxygen is monitored with a polarographic probe (gas permeable membrane) connected to a dissolved oxygen meter (e.g., 0 to 15 mg/L, 0 to 50°C).
6.6.2 Benchtop Bioreactor Pressure Monitor. The benchtop bioreactor pressure is monitored through a port in the top flange of the reactor. This is connected to a gauge control with a span of 13-cm water vacuum to 13-cm water pressure or better. A relay is activated when the vacuum exceeds an adjustable setpoint which opens a solenoid valve (normally closed), admitting oxygen to the system. The vacuum setpoint controlling oxygen addition to the system shall be set at approximately 2.5 ±0.5 cm water and maintained at this setting except during brief periods when the dissolved oxygen concentration is adjusted.
6.7 Connecting Tubing. All connecting tubing shall be Teflon or equivalent in impermeability. The only exception to this specification is the tubing directly inside the pump head of the wastewater feed pump, which may be Viton, Silicone or another type of flexible tubing.
Note:
Mention of trade names or products does not constitute endorsement by the U.S. Environmental Protection Agency.
7.0 Reagents and Standards
7.1 Wastewater. Obtain a representative sample of wastewater at the inlet to the full-scale treatment plant if there is an existing full-scale treatment plant (see section 6.3). If there is no existing full-scale treatment plant, obtain the wastewater sample as close to the point of determination as possible. Collect the sample by pumping the wastewater into the 20-L collapsible container. The loss of volatiles shall be minimized from the wastewater by collapsing the container before filling, by minimizing the time of filling, and by avoiding a headspace in the container after filling. If the wastewater requires the addition of nutrients to support the biomass growth and maintain biomass characteristics, those nutrients are added and mixed with the container contents after the container is filled.
7.2 Biomass. Obtain the biomass or activated sludge used for rate constant determination in the bench-scale process from the existing full-scale process or from a representative biomass culture (e.g., biomass that has been developed for a future full-scale process). This biomass is preferentially obtained from a thickened acclimated mixed liquor sample. Collect the sample either by bailing from the mixed liquor in the aeration tank with a weighted container, or by collecting aeration tank effluent at the effluent overflow weir. Transport the sample to the laboratory within no more than 4 hours of collection. Maintain the biomass concentration in the benchtop bioreactor at the level of the full-scale system + 10 percent throughout the sampling period of the test method.
8.0 Sample Collection, Preservation, Storage, and Transport
8.1 Benchtop Bioreactor Operation. Charge the mixed liquor to the benchtop bioreactor, minimizing headspace over the liquid surface to minimize entrainment of mixed liquor in the circulating gas. Fasten the benchtop bioreactor headplate to the reactor over the liquid surface. Maintain the temperature of the contents of the benchtop bioreactor system at the temperature of the target full-scale system, ±2°C, throughout the testing period. Monitor and record the temperature of the benchtop bioreactor contents at least to the nearest 0.1°C.
8.1.1 Wastewater Storage. Collect the wastewater sample in the 20-L collapsible container. Store the container at 4°C throughout the testing period. Connect the container to the benchtop bioreactor feed pump.
8.1.2 Wastewater Flow Rate.
8.1.2.1 The hydraulic residence time of the aeration tank is calculated as the ratio of the volume of the tank (L) to the flow rate (L/min). At the beginning of a test, the container shall be connected to the feed pump and solution shall be pumped to the benchtop bioreactor at the required flow rate to achieve the calculated hydraulic residence time of wastewater in the aeration tank.

Where:
Qtest = wastewater flow rate (L/min)
Qfs = average flow rate of full-scale process (L/min)
Vfs = volume of full-scale aeration tank (L)
8.1.2.2 The target flow rate in the test apparatus is the same as the flow rate in the target full-scale process multiplied by the ratio of benchtop bioreactor volume (e.g., 6 L) to the volume of the full-scale aeration tank. The hydraulic residence time shall be maintained at 90 to 100 percent of the residence time maintained in the full-scale unit. A nominal flow rate is set on the pump based on a pump calibration. Changes in the elasticity of the tubing in the pump head and the accumulation of material in the tubing affect this calibration. The nominal pumping rate shall be changed as necessary based on volumetric flow measurements. Discharge the benchtop bioreactor effluent to a wastewater storage, treatment, or disposal facility, except during sampling or flow measurement periods.
8.1.3 Sludge Recycle Rate. Set the sludge recycle rate at a rate sufficient to prevent accumulation in the bottom of the clarifier. Set the air circulation rate sufficient to maintain the biomass in suspension.
8.1.4 Benchtop Bioreactor Operation and Maintenance. Temperature, dissolved oxygen concentration, exit vent flow rate, benchtop bioreactor effluent flow rate, and air circulation rate shall be measured and recorded three times throughout each day of benchtop bioreactor operation. If other parameters (such as pH) are measured and maintained in the target full-scale unit, these parameters, where appropriate, shall be monitored and maintained to target full-scale specifications in the benchtop bioreactor. At the beginning of each sampling period (Section 8.2), sample the benchtop bioreactor contents for suspended solids analysis. Take this sample by loosening a clamp on a length of tubing attached to the lower side port. Determine the suspended solids gravimetrically by the Gooch crucible/glass fiber filter method for total suspended solids, in accordance with Standard Methods 3 or equivalent. When necessary, sludge shall be wasted from the lower side port of the benchtop bioreactor, and the volume that is wasted shall be replaced with an equal volume of the reactor effluent. Add thickened activated sludge mixed liquor as necessary to the benchtop bioreactor to increase the suspended solids concentration to the desired level. Pump this mixed liquor to the benchtop bioreactor through the upper side port (Item 24 in Figure 304A-1). Change the membrane on the dissolved oxygen probe before starting the test. Calibrate the oxygen probe immediately before the start of the test and each time the membrane is changed.
8.1.5 Inspection and Correction Procedures. If the feed line tubing becomes clogged, replace with new tubing. If the feed flow rate is not within 5 percent of target flow any time the flow rate is measured, reset pump or check the flow measuring device and measure flow rate again until target flow rate is achieved.
8.2 Test Sampling. At least two and one half hydraulic residence times after the system has reached the targeted specifications shall be permitted to elapse before the first sample is taken. Effluent samples of the clarifier discharge (Item 20 in Figure 304A-1) and the influent wastewater feed are collected in 40-mL septum vials to which two drops of 1:10 hydrochloric acid (HCl) in water have been added. Sample the clarifier discharge directly from the drain line. These samples will be composed of the entire flow from the system for a period of several minutes. Feed samples shall be taken from the feed pump suction line after temporarily stopping the benchtop bioreactor feed, removing a connector, and squeezing the collapsible feed container. Store both influent and effluent samples at 4°C immediately after collection and analyze within 8 hours of collection.
8.2.1 Frequency of Sampling. During the test, sample and analyze the wastewater feed and the clarifier effluent at least six times. The sampling intervals shall be separated by at least 8 hours. During any individual sampling interval, sample the wastewater feed simultaneously with or immediately after the effluent sample. Calculate the relative standard deviation (RSD) of the amount removed (i.e., effluent concentration - wastewater feed concentration). The RSD values shall be <15 percent. If an RSD value is >15 percent, continue sampling and analyzing influent and effluent sets of samples until the RSD values are within specifications.
8.2.2 Sampling After Exposure of System to Atmosphere. If, after starting sampling procedures, the benchtop bioreactor system is exposed to the atmosphere (due to leaks, maintenance, etc.), allow at least one hydraulic residence time to elapse before resuming sampling.
9.0 Quality Control
9.1 Dissolved Oxygen. Fluctuation in dissolved oxygen concentration may occur for numerous reasons, including undetected gas leaks, increases and decreases in mixed liquor suspended solids resulting from cell growth and solids loss in the effluent stream, changes in diffuser performance, cycling of effluent flow rate, and overcorrection due to faulty or sluggish dissolved oxygen probe response. Control the dissolved oxygen concentration in the benchtop bioreactor by changing the proportion of oxygen in the circulating aeration gas. Should the dissolved oxygen concentration drift below the designated experimental condition, bleed a small amount of aeration gas from the system on the pressure side (i.e., immediately upstream of one of the diffusers). This will create a vacuum in the system, triggering the pressure sensitive relay to open the solenoid valve and admit oxygen to the system. Should the dissolved oxygen concentration drift above the designated experimental condition, slow or stop the oxygen input to the system until the dissolved oxygen concentration approaches the correct level.
9.2 Sludge Wasting.
9.2.1 Determine the suspended solids concentration (section 8.1.4) at the beginning of a test, and once per day thereafter during the test. If the test is completed within a two day period, determine the suspended solids concentration after the final sample set is taken. If the suspended solids concentration exceeds the specified concentration, remove a fraction of the sludge from the benchtop bioreactor. The required volume of mixed liquor to remove is determined as follows:

Where:
Vw is the wasted volume (Liters),
Vr is the volume of the benchtop bioreactor (Liters),
Sm is the measured solids (g/L), and
Ss is the specified solids (g/L).
9.2.2 Remove the mixed liquor from the benchtop bioreactor by loosening a clamp on the mixed liquor sampling tube and allowing the required volume to drain to a graduated flask. Clamp the tube when the correct volume has been wasted. Replace the volume of the liquid wasted by pouring the same volume of effluent back into the benchtop bioreactor. Dispose of the waste sludge properly.
9.3 Sludge Makeup. In the event that the suspended solids concentration is lower than the specifications, add makeup sludge back into the benchtop bioreactor. Determine the amount of sludge added by the following equation:

Where:
Vw is the volume of sludge to add (Liters),
Vr is the volume of the benchtop bioreactor (Liters),
Sw is the solids in the makeup sludge (g/L),
Sm is the measured solids (g/L), and Ss is the specified solids (g/L).
10.0 Calibration and Standardization
10.1 Wastewater Pump Calibration. Determine the wastewater flow rate by collecting the system effluent for a time period of at least one hour, and measuring the volume with a graduated cylinder. Record the collection time period and volume collected. Determine flow rate. Adjust the pump speed to deliver the specified flow rate.
10.2 Calibration Standards. Prepare calibration standards from pure certified standards in an aqueous medium. Prepare and analyze three concentrations of calibration standards for each target component (or for a mixture of components) in triplicate daily throughout the analyses of the test samples. At each concentration level, a single calibration shall be within 5 percent of the average of the three calibration results. The low and medium calibration standards shall bracket the expected concentration of the effluent (treated) wastewater. The medium and high standards shall bracket the expected influent concentration.
11.0 Analytical Procedures
11.1 Analysis. If the identity of the compounds of interest in the wastewater is not known, a representative sample of the wastewater shall be analyzed in order to identify all of the compounds of interest present. A gas chromatography/mass spectrometry screening method is recommended.
11.1.1 After identifying the compounds of interest in the wastewater, develop and/or use one or more analytical techniques capable of measuring each of those compounds (more than one analytical technique may be required, depending on the characteristics of the wastewater). Test Method 18, found in appendix A of 40 CFR 60, may be used as a guideline in developing the analytical technique. Purge and trap techniques may be used for analysis providing the target components are sufficiently volatile to make this technique appropriate. The limit of quantitation for each compound shall be determined (see reference 1). If the effluent concentration of any target compound is below the limit of quantitation determined for that compound, the operation of the Method 304 unit may be altered to attempt to increase the effluent concentration above the limit of quantitation. Modifications to the method shall be approved prior to the test. The request should be addressed to Method 304 contact, Emissions Measurement Center, Mail Drop 19, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711.
12.0 Data Analysis and Calculations
12.1 Nomenclature. The following symbols are used in the calculations.
Ci = Average inlet feed concentration for a compound of interest, as analyzed (mg/L)
Co = Average outlet (effluent) concentration for a compound of interest, as analyzed (mg/L)
X = Biomass concentration, mixed liquor suspended solids (g/L)
t = Hydraulic residence time in the benchtop bioreactor (hours)
V = Volume of the benchtop bioreactor (L)
Q = Flow rate of wastewater into the benchtop bioreactor, average (L/hour)
12.2 Residence Time. The hydraulic residence time of the benchtop bioreactor is equal to the ratio of the volume of the benchtop bioreactor (L) to the flow rate (L/h):

12.3 Rate of Biodegradation. Calculate the rate of biodegradation for each component with the following equation:

12.4 First-Order Biorate Constant. Calculate the first-order biorate constant (K1) for each component with the following equation:

12.5 Relative Standard Deviation (RSD). Determine the standard deviation of both the influent and effluent sample concentrations (S) using the following equation:

12.6 Determination of Percent Air Emissions and Percent Biodegraded. Use the results from this test method and follow the applicable procedures in appendix C of 40 CFR part 63, entitled, “Determination of the Fraction Biodegraded (Fbio) in a Biological Treatment Unit” to determine Fbio.
13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References
1. “Guidelines for data acquisition and data quality evaluation in Environmental Chemistry,” Daniel MacDoughal, Analytical Chemistry, Volume 52, p. 2242, 1980.
2. Test Method 18, 40 CFR 60, appendix A.
3. Standard Methods for the Examination of Water and Wastewater, 16th Edition, Method 209C, Total Suspended Solids Dried at 103-105°C, APHA, 1985.
4. Water7, Hazardous Waste Treatment, Storage, and Disposal Facilities (TSDF) - Air Emission Models, U.S. Environmental Protection Agency, EPA-450/3-87-026, Review Draft, November 1989.
5. Chemdat7, Hazardous Waste Treatment, Storage, and Disposal Facilities (TSDF) - Air Emission Models, U.S. Environmental Protection Agency, EPA-450/3-87-026, Review Draft, November 1989.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
Method 304B: Determination of Biodegradation Rates of Organic Compounds (Scrubber Option)
1.0 Scope and Application
1.1 Applicability. This method is applicable for the determination of biodegradation rates of organic compounds in an activated sludge process. The test method is designed to evaluate the ability of an aerobic biological reaction system to degrade or destroy specific components in waste streams. The method may also be used to determine the effects of changes in wastewater composition on operation. The biodegradation rates determined by utilizing this method are not representative of a full-scale system. Full-scale systems embody biodegradation and air emissions in competing reactions. This method measures biodegradation in absence of air emissions. The rates measured by this method shall be used in conjunction with the procedures listed in appendix C of this part to calculate the fraction emitted to the air versus the fraction biodegraded.
2.0 Summary of Method
2.1 A self-contained benchtop bioreactor system is assembled in the laboratory. A sample of mixed liquor is added and the waste stream is then fed continuously. The benchtop bioreactor is operated under conditions nearly identical to the target full-scale activated sludge process, except that air emissions are not a factor. The benchtop bioreactor temperature, dissolved oxygen concentration, average residence time in the reactor, waste composition, biomass concentration, and biomass composition of the target full-scale process are the parameters which are duplicated in the laboratory system. Biomass shall be removed from the target full-scale activated sludge unit and held for no more than 4 hours prior to use in the benchtop bioreactor. If antifoaming agents are used in the full-scale system, they shall also be used in the benchtop bioreactor. The feed flowing into and the effluent exiting the benchtop bioreactor are analyzed to determine the biodegradation rates of the target compounds. The choice of analytical methodology for measuring the compounds of interest at the inlet and outlet to the benchtop bioreactor are left to the discretion of the source, except where validated methods are available.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
5.1 If explosive gases are produced as a byproduct of biodegradation and could realistically pose a hazard, closely monitor headspace concentration of these gases to ensure laboratory safety. Placement of the benchtop bioreactor system inside a laboratory hood is recommended regardless of byproducts produced.
6.0 Equipment and Supplies
Note:
Figure 304B-1 illustrates a typical laboratory apparatus used to measure biodegradation rates. While the following description refers to Figure 304B-1, the EPA recognizes that alternative reactor configurations, such as alternative reactor shapes and locations of probes and the feed inlet, will also meet the intent of this method. Ensure that the benchtop bioreactor system is self-contained and isolated from the atmosphere by leak-checking fittings, tubing, etc.
6.1 Benchtop Bioreactor. The biological reaction is conducted in a biological oxidation reactor of at least 6-liters capacity. The benchtop bioreactor is sealed and equipped with internal probes for controlling and monitoring dissolved oxygen and internal temperature. The top of the benchtop bioreactor is equipped for aerators, gas flow ports, and instrumentation (while ensuring that no leaks to the atmosphere exist around the fittings).
6.2 Aeration gas. Aeration gas is added to the benchtop bioreactor through three diffusers, which are glass tubes that extend to the bottom fifth of the reactor depth. A pure oxygen pressurized cylinder is recommended in order to maintain the specified oxygen concentration. Install a blower (e.g., Diaphragm Type, 15 SCFH capacity) to blow the aeration gas into the benchtop bioreactor diffusers. Measure the aeration gas flow rate with a rotameter (e.g., 0-15 SCFH recommended). The aeration gas will rise through the benchtop bioreactor, dissolving oxygen into the mixture in the process. The aeration gas must provide sufficient agitation to keep the solids in suspension. Provide an exit for the aeration gas from the top flange of the benchtop bioreactor through a water-cooled (e.g., Allihn-type) vertical condenser. Install the condenser through a gas-tight fitting in the benchtop bioreactor closure. Design the system so that at least 10 percent of the gas flows through an alkaline scrubber containing 175 mL of 45 percent by weight solution of potassium hydroxide (KOH) and 5 drops of 0.2 percent alizarin yellow dye. Route the balance of the gas through an adjustable scrubber bypass. Route all of the gas through a 1-L knock-out flask to remove entrained moisture and then to the intake of the blower. The blower recirculates the gas to the benchtop bioreactor.
6.3 Wastewater Feed. Supply the wastewater feed to the benchtop bioreactor in a collapsible low-density polyethylene container or collapsible liner in a container (e.g., 20 L) equipped with a spigot cap (collapsible containers or liners of other material may be required due to the permeability of some volatile compounds through polyethylene). Obtain the wastewater feed by sampling the wastewater feed in the target process. A representative sample of wastewater shall be obtained from the piping leading to the aeration tank. This sample may be obtained from existing sampling valves at the discharge of the wastewater feed pump, or collected from a pipe discharging to the aeration tank, or by pumping from a well-mixed equalization tank upstream from the aeration tank. Alternatively, wastewater can be pumped continuously to the laboratory apparatus from a bleed stream taken from the equalization tank of the full-scale treatment system.
6.3.1 Refrigeration System. Keep the wastewater feed cool by ice or by refrigeration to 4°C. If using a bleed stream from the equalization tank, refrigeration is not required if the residence time in the bleed stream is less than five minutes.
6.3.2 Wastewater Feed Pump. The wastewater is pumped from the refrigerated container using a variable-speed peristaltic pump drive equipped with a peristaltic pump head. Add the feed solution to the benchtop bioreactor through a fitting on the top flange. Determine the rate of feed addition to provide a retention time in the benchtop bioreactor that is numerically equivalent to the retention time in the target full-scale system. The wastewater shall be fed at a rate sufficient to achieve 90 to 100 percent of the target full-scale system residence time.
6.3.3 Treated wastewater feed. The benchtop bioreactor effluent exits at the bottom of the reactor through a tube and proceeds to the clarifier.
6.4 Clarifier. The effluent flows to a separate closed clarifier that allows separation of biomass and effluent (e.g., 2-liter pear-shaped glass separatory funnel, modified by removing the stopcock and adding a 25-mm OD glass tube at the bottom). Benchtop bioreactor effluent enters the clarifier through a tube inserted to a depth of 0.08 m (3 in.) through a stopper at the top of the clarifier. System effluent flows from a tube inserted through the stopper at the top of the clarifier to a drain (or sample bottle when sampling). The underflow from the clarifier leaves from the glass tube at the bottom of the clarifier. Flexible tubing connects this fitting to the sludge recycle pump. This pump is coupled to a variable speed pump drive. The discharge from this pump is returned through a tube inserted in a port on the side of the benchtop bioreactor. An additional port is provided near the bottom of the benchtop bioreactor for sampling the reactor contents. The mixed liquor from the benchtop bioreactor flows into the center of the clarifier. The clarified system effluent separates from the biomass and flows through an exit near the top of the clarifier. There shall be no headspace in the clarifier.
6.5 Temperature Control Apparatus. Capable of maintaining the system at a temperature equal to the temperature of the full-scale system. The average temperature should be maintained within ±2°C of the set point.
6.5.1 Temperature Monitoring Device. A resistance type temperature probe or a thermocouple connected to a temperature readout with a resolution of 0.1°C or better.
6.5.2 Benchtop Bioreactor Heater. The heater is connected to the temperature control device.
6.6 Oxygen Control System. Maintain the dissolved oxygen concentration at the levels present in the full-scale system. Target full-scale activated sludge systems with dissolved oxygen concentration below 2 mg/L are required to maintain the dissolved oxygen concentration in the benchtop bioreactor within 0.5 mg/L of the target dissolved oxygen level. Target full-scale activated sludge systems with dissolved oxygen concentration above 2 mg/L are required to maintain the dissolved oxygen concentration in the benchtop bioreactor within 1.5 mg/L of the target dissolved oxygen concentration; however, for target full-scale activated sludge systems with dissolved oxygen concentrations above 2 mg/L, the dissolved oxygen concentration in the benchtop bioreactor may not drop below 1.5 mg/L. If the benchtop bioreactor is outside the control range, the dissolved oxygen is noted and the reactor operation is adjusted.
6.6.1 Dissolved Oxygen Monitor. Dissolved oxygen is monitored with a polarographic probe (gas permeable membrane) connected to a dissolved oxygen meter (e.g., 0 to 15 mg/L, 0 to 50°C).
6.6.2 Benchtop Bioreactor Pressure Monitor. The benchtop bioreactor pressure is monitored through a port in the top flange of the reactor. This is connected to a gauge control with a span of 13-cm water vacuum to 13-cm water pressure or better. A relay is activated when the vacuum exceeds an adjustable setpoint which opens a solenoid valve (normally closed), admitting oxygen to the system. The vacuum setpoint controlling oxygen addition to the system shall be set at approximately 2.5 ±0.5 cm water and maintained at this setting except during brief periods when the dissolved oxygen concentration is adjusted.
6.7 Connecting Tubing. All connecting tubing shall be Teflon or equivalent in impermeability. The only exception to this specification is the tubing directly inside the pump head of the wastewater feed pump, which may be Viton, Silicone or another type of flexible tubing.
Note:
Mention of trade names or products does not constitute endorsement by the U.S. Environmental Protection Agency.
7.0. Reagents and Standards
7.1 Wastewater. Obtain a representative sample of wastewater at the inlet to the full-scale treatment plant if there is an existing full-scale treatment plant (See Section 6.3). If there is no existing full-scale treatment plant, obtain the wastewater sample as close to the point of determination as possible. Collect the sample by pumping the wastewater into the 20-L collapsible container. The loss of volatiles shall be minimized from the wastewater by collapsing the container before filling, by minimizing the time of filling, and by avoiding a headspace in the container after filling. If the wastewater requires the addition of nutrients to support the biomass growth and maintain biomass characteristics, those nutrients are added and mixed with the container contents after the container is filled.
7.2 Biomass. Obtain the biomass or activated sludge used for rate constant determination in the bench-scale process from the existing full-scale process or from a representative biomass culture (e.g., biomass that has been developed for a future full-scale process). This biomass is preferentially obtained from a thickened acclimated mixed liquor sample. Collect the sample either by bailing from the mixed liquor in the aeration tank with a weighted container, or by collecting aeration tank effluent at the effluent overflow weir. Transport the sample to the laboratory within no more than 4 hours of collection. Maintain the biomass concentration in the benchtop bioreactor at the level of the target full-scale system + 10 percent throughout the sampling period of the test method.
8.0 Sample Collection, Preservation, Storage, and Transport
8.1 Benchtop Bioreactor Operation. Charge the mixed liquor to the benchtop bioreactor, minimizing headspace over the liquid surface to minimize entrainment of mixed liquor in the circulating gas. Fasten the benchtop bioreactor headplate to the reactor over the liquid surface. Maintain the temperature of the contents of the benchtop bioreactor system at the temperature of the target full-scale system, ±2°C, throughout the testing period. Monitor and record the temperature of the reactor contents at least to the nearest 0.1°C.
8.1.1 Wastewater Storage. Collect the wastewater sample in the 20-L collapsible container. Store the container at 4°C throughout the testing period. Connect the container to the benchtop bioreactor feed pump.
8.1.2 Wastewater Flow Rate.
8.1.2.1 The hydraulic residence time of the aeration tank is calculated as the ratio of the volume of the tank (L) to the flow rate (L/min). At the beginning of a test, the container shall be connected to the feed pump and solution shall be pumped to the benchtop bioreactor at the required flow rate to achieve the calculated hydraulic residence time of wastewater in the aeration tank.

Where:
Qtest = wastewater flow rate (L/min)
Qfs = average flow rate of full-scale process (L/min)
Vfs = volume of full-scale aeration tank (L)
8.1.2.2 The target flow rate in the test apparatus is the same as the flow rate in the target full-scale process multiplied by the ratio of benchtop bioreactor volume (e.g., 6 L) to the volume of the full-scale aeration tank. The hydraulic residence time shall be maintained at 90 to 100 percent of the residence time maintained in the target full-scale unit. A nominal flow rate is set on the pump based on a pump calibration. Changes in the elasticity of the tubing in the pump head and the accumulation of material in the tubing affect this calibration. The nominal pumping rate shall be changed as necessary based on volumetric flow measurements. Discharge the benchtop bioreactor effluent to a wastewater storage, treatment, or disposal facility, except during sampling or flow measurement periods.
8.1.3 Sludge Recycle Rate. Set the sludge recycle rate at a rate sufficient to prevent accumulation in the bottom of the clarifier. Set the air circulation rate sufficient to maintain the biomass in suspension.
8.1.4 Benchtop Bioreactor Operation and Maintenance. Temperature, dissolved oxygen concentration, flow rate, and air circulation rate shall be measured and recorded three times throughout each day of testing. If other parameters (such as pH) are measured and maintained in the target full-scale unit, these parameters shall, where appropriate, be monitored and maintained to full-scale specifications in the benchtop bioreactor. At the beginning of each sampling period (section 8.2), sample the benchtop bioreactor contents for suspended solids analysis. Take this sample by loosening a clamp on a length of tubing attached to the lower side port. Determine the suspended solids gravimetrically by the Gooch crucible/glass fiber filter method for total suspended solids, in accordance with Standard Methods 3 or equivalent. When necessary, sludge shall be wasted from the lower side port of the benchtop bioreactor, and the volume that is wasted shall be replaced with an equal volume of the benchtop bioreactor effluent. Add thickened activated sludge mixed liquor as necessary to the benchtop bioreactor to increase the suspended solids concentration to the desired level. Pump this mixed liquor to the benchtop bioreactor through the upper side port (Item 24 in Figure 304B-1). Change the membrane on the dissolved oxygen probe before starting the test. Calibrate the oxygen probe immediately before the start of the test and each time the membrane is changed. The scrubber solution shall be replaced each weekday with 175 mL 45 percent W/W KOH solution to which five drops of 0.2 percent alizarin yellow indicator in water have been added. The potassium hydroxide solution in the alkaline scrubber shall be changed if the alizarin yellow dye color changes.
8.1.5 Inspection and Correction Procedures. If the feed line tubing becomes clogged, replace with new tubing. If the feed flow rate is not within 5 percent of target flow any time the flow rate is measured, reset pump or check the flow measuring device and measure flow rate again until target flow rate is achieved.
8.2 Test Sampling. At least two and one half hydraulic residence times after the system has reached the targeted specifications shall be permitted to elapse before the first sample is taken. Effluent samples of the clarifier discharge (Item 20 in Figure 304B-1) and the influent wastewater feed are collected in 40-mL septum vials to which two drops of 1:10 hydrochloric acid (HCl) in water have been added. Sample the clarifier discharge directly from the drain line. These samples will be composed of the entire flow from the system for a period of several minutes. Feed samples shall be taken from the feed pump suction line after temporarily stopping the benchtop bioreactor feed, removing a connector, and squeezing the collapsible feed container. Store both influent and effluent samples at 4°C immediately after collection and analyze within 8 hours of collection.
8.2.1 Frequency of Sampling. During the test, sample and analyze the wastewater feed and the clarifier effluent at least six times. The sampling intervals shall be separated by at least 8 hours. During any individual sampling interval, sample the wastewater feed simultaneously with or immediately after the effluent sample. Calculate the RSD of the amount removed (i.e., effluent concentration - wastewater feed concentration). The RSD values shall be <15 percent. If an RSD value is >15 percent, continue sampling and analyzing influent and effluent sets of samples until the RSD values are within specifications.
8.2.2 Sampling After Exposure of System to Atmosphere. If, after starting sampling procedures, the benchtop bioreactor system is exposed to the atmosphere (due to leaks, maintenance, etc.), allow at least one hydraulic residence time to elapse before resuming sampling.
9.0 Quality Control
9.1 Dissolved Oxygen. Fluctuation in dissolved oxygen concentration may occur for numerous reasons, including undetected gas leaks, increases and decreases in mixed liquor suspended solids resulting from cell growth and solids loss in the effluent stream, changes in diffuser performance, cycling of effluent flow rate, and overcorrection due to faulty or sluggish dissolved oxygen probe response. Control the dissolved oxygen concentration in the benchtop bioreactor by changing the proportion of oxygen in the circulating aeration gas. Should the dissolved oxygen concentration drift below the designated experimental condition, bleed a small amount of aeration gas from the system on the pressure side (i.e., immediately upstream of one of the diffusers). This will create a vacuum in the system, triggering the pressure sensitive relay to open the solenoid valve and admit oxygen to the system. Should the dissolved oxygen concentration drift above the designated experimental condition, slow or stop the oxygen input to the system until the dissolved oxygen concentration approaches the correct level.
9.2 Sludge Wasting.
9.2.1 Determine the suspended solids concentration (section 8.1.4) at the beginning of a test, and once per day thereafter during the test. If the test is completed within a two day period, determine the suspended solids concentration after the final sample set is taken. If the suspended solids concentration exceeds the specified concentration, remove a fraction of the sludge from the benchtop bioreactor. The required volume of mixed liquor to remove is determined as follows:

Where:
Vw is the wasted volume (Liters),
Vr is the volume of the benchtop bioreactor (Liters),
Sm is the measured solids (g/L), and
Ss is the specified solids (g/L).
9.2.2 Remove the mixed liquor from the benchtop bioreactor by loosening a clamp on the mixed liquor sampling tube and allowing the required volume to drain to a graduated flask. Clamp the tube when the correct volume has been wasted. Replace the volume of the liquid wasted by pouring the same volume of effluent back into the benchtop bioreactor. Dispose of the waste sludge properly.
9.3 Sludge Makeup. In the event that the suspended solids concentration is lower than the specifications, add makeup sludge back into the benchtop bioreactor. Determine the amount of sludge added by the following equation:

Where:
Vw is the volume of sludge to add (Liters),
Vr is the volume of the benchtop bioreactor (Liters),
Sw is the solids in the makeup sludge (g/L),
Sm is the measured solids (g/L), and
Ss is the specified solids (g/L).
10.0 Calibration and Standardizations
10.1 Wastewater Pump Calibration. Determine the wastewater flow rate by collecting the system effluent for a time period of at least one hour, and measuring the volume with a graduated cylinder. Record the collection time period and volume collected. Determine flow rate. Adjust the pump speed to deliver the specified flow rate.
10.2 Calibration Standards. Prepare calibration standards from pure certified standards in an aqueous medium. Prepare and analyze three concentrations of calibration standards for each target component (or for a mixture of components) in triplicate daily throughout the analyses of the test samples. At each concentration level, a single calibration shall be within 5 percent of the average of the three calibration results. The low and medium calibration standards shall bracket the expected concentration of the effluent (treated) wastewater. The medium and high standards shall bracket the expected influent concentration.
11.0 Analytical Test Procedures
11.1 Analysis. If the identity of the compounds of interest in the wastewater is not known, a representative sample of the wastewater shall be analyzed in order to identify all of the compounds of interest present. A gas chromatography/mass spectrometry screening method is recommended.
11.1.1 After identifying the compounds of interest in the wastewater, develop and/or use one or more analytical technique capable of measuring each of those compounds (more than one analytical technique may be required, depending on the characteristics of the wastewater). Method 18, found in appendix A of 40 CFR 60, may be used as a guideline in developing the analytical technique. Purge and trap techniques may be used for analysis providing the target components are sufficiently volatile to make this technique appropriate. The limit of quantitation for each compound shall be determined. 1 If the effluent concentration of any target compound is below the limit of quantitation determined for that compound, the operation of the Method 304 unit may be altered to attempt to increase the effluent concentration above the limit of quantitation. Modifications to the method shall be approved prior to the test. The request should be addressed to Method 304 contact, Emissions Measurement Center, Mail Drop 19, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711.
12.0 Data Analysis and Calculations
12.1 Nomenclature. The following symbols are used in the calculations.
Ci = Average inlet feed concentration for a compound of interest, as analyzed (mg/L)
Co = Average outlet (effluent) concentration for a compound of interest, as analyzed (mg/L)
X = Biomass concentration, mixed liquor suspended solids (g/L)
t = Hydraulic residence time in the benchtop bioreactor (hours)
V = Volume of the benchtop bioreactor (L)
Q = Flow rate of wastewater into the benchtop bioreactor, average (L/hour)
12.2 Residence Time. The hydraulic residence time of the benchtop bioreactor is equal to the ratio of the volume of the benchtop bioreactor (L) to the flow rate (L/h)

12.3 Rate of Biodegradation. Calculate the rate of biodegradation for each component with the following equation:

12.4 First-Order Biorate Constant. Calculate the first-order biorate constant (K1) for each component with the following equation:

12.5 Relative Standard Deviation (RSD). Determine the standard deviation of both the influent and effluent sample concentrations (S) using the following equation:

12.6 Determination of Percent Air Emissions and Percent Biodegraded. Use the results from this test method and follow the applicable procedures in appendix C of 40 CFR Part 63, entitled, “Determination of the Fraction Biodegraded (Fbio) in a Biological Treatment Unit” to determine Fbio.
13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References
1. “Guidelines for data acquisition and data quality evaluation in Environmental Chemistry”, Daniel MacDoughal, Analytical Chemistry, Volume 52, p. 2242, 1980.
2. Test Method 18, 40 CFR 60, Appendix A.
3. Standard Methods for the Examination of Water and Wastewater, 16th Edition, Method 209C, Total Suspended Solids Dried at 103-105°C, APHA, 1985.
4. Water - 7, Hazardous Waste Treatment, Storage, and Disposal Facilities (TSDF) - Air Emission Models, U.S. Environmental Protection Agency, EPA-450/3-87-026, Review Draft, November 1989.
5. Chemdat7, Hazardous Waste Treatment, Storage, and Disposal Facilities (TSDF) - Air Emission Models, U.S. Environmental Protection Agency, EPA-450/3-87-026, Review Draft, November 1989.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
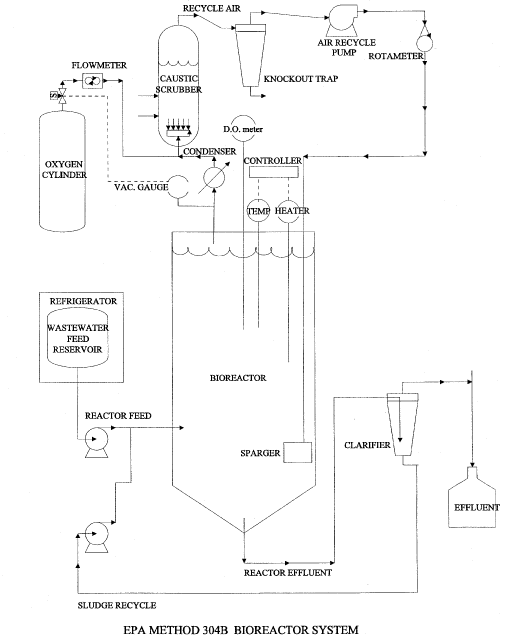
Method 305: Measurement of Emission Potential of Individual Volatile Organic Compounds in Waste
Note:
This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in 40 CFR Part 60, Appendix A. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least Method 25D.
1.0 Scope and Application
1.1 Analyte. Volatile Organics. No CAS No. assigned.
1.2 Applicability. This procedure is used to determine the emission potential of individual volatile organics (VOs) in waste.
1.3 Data Quality Objectives. Adherence to the requirements of this method will enhance the quality of the data obtained from air pollutant sampling methods.
2.0 Summary of Method
2.1 The heated purge conditions established by Method 25D (40 CFR Part 60, Appendix A) are used to remove VOs from a 10 gram sample of waste suspended in a 50/50 solution of polyethylene glycol (PEG) and water. The purged VOs are quantified by using the sample collection and analytical techniques (e.g. gas chromatography) appropriate for the VOs present in the waste. The recovery efficiency of the sample collection and analytical technique is determined for each waste matrix. A correction factor is determined for each compound (if acceptable recovery criteria requirements are met of 70 to 130 percent recovery for every target compound), and the measured waste concentration is corrected with the correction factor for each compound. A minimum of three replicate waste samples shall be analyzed.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
6.0 Equipment and Supplies
6.1 Method 25D Purge Apparatus.
6.1.1 Purge Chamber. The purge chamber shall accommodate the 10 gram sample of waste suspended in a matrix of 50 mL of PEG and 50 mL of deionized, hydrocarbon-free water. Three fittings are used on the glass chamber top. Two #7 Ace-threads are used for the purge gas inlet and outlet connections. A #50 Ace-thread is used to connect the top of the chamber to the base (see Figure 305-1). The base of the chamber has a side-arm equipped with a #22 Sovirel fitting to allow for easy sample introductions into the chamber. The dimensions of the chamber are shown in Figure 305-1.
6.1.2 Flow Distribution Device (FDD). The FDD enhances the gas-to-liquid contact for improved purging efficiency. The FDD is a 6 mm OD (0.2 in) by 30 cm (12 in) long glass tube equipped with four arm bubblers as shown in Figure 305-1. Each arm shall have an opening of 1 mm (0.04 in) in diameter.
6.1.3 Coalescing Filter. The coalescing filter serves to discourage aerosol formation of sample gas once it leaves the purge chamber. The glass filter has a fritted disc mounted 10 cm (3.9 in) from the bottom. Two #7 Ace-threads are used for the inlet and outlet connections. The dimensions of the chamber are shown in Figure 305-2.
6.1.4 Oven. A forced convection airflow oven capable of maintaining the purge chamber and coalescing filter at 75 ±2°C (167 ±3.6°F).
6.1.5 Toggle Valve. An on/off valve constructed from brass or stainless steel rated to 100 psig. This valve is placed in line between the purge nitrogen source and the flow controller.
6.1.6 Flow Controller. High-quality stainless steel flow controller capable of restricting a flow of nitrogen to 6 ±0.06 L/min (0.2 ±0.002 ft 3/min) at 40 psig.
6.1.7 Polyethylene Glycol Cleaning System.
6.1.7.1 Round-Bottom Flask. One liter, three-neck glass round-bottom flask for cleaning PEG. Standard taper 24/40 joints are mounted on each neck.
6.1.7.2 Heating Mantle. Capable of heating contents of the 1-L flask to 120°C (248°F).
6.1.7.3 Nitrogen Bubbler. Teflon ® or glass tube, 0.25 in OD (6.35 mm).
6.1.7.4 Temperature Sensor. Partial immersion glass thermometer.
6.1.7.5 Hose Adapter. Glass with 24/40 standard tapered joint.
6.2 Volatile Organic Recovery System.
6.2.1 Splitter Valve (Optional). Stainless steel cross-pattern valve capable of splitting nominal flow rates from the purge flow of 6 L/min (0.2 ft 3/min). The valve shall be maintained at 75 + 2°C (167 ±3.6°F) in the heated zone and shall be placed downstream of the coalescing filter. It is recommended that 0.125 in OD (3.175 mm) tubing be used to direct the split vent flow from the heated zone. The back pressure caused by the 0.125 in OD (3.175 mm) tubing is critical for maintaining proper split valve operation.
Note:
The splitter valve design is optional; it may be used in cases where the concentration of a pollutant would saturate the adsorbents.
6.2.2 Injection Port. Stainless steel 1/4 in OD (6.35 mm) compression fitting tee with a 6 mm (0.2 in) septum fixed on the top port. The injection port is the point of entry for the recovery study solution. If using a gaseous standard to determine recovery efficiency, connect the gaseous standard to the injection port of the tee.
6.2.3 Knockout Trap (Optional but Recommended). A 25 mL capacity glass reservoir body with a full-stem impinger (to avoid leaks, a modified midget glass impinger with a screw cap and ball/socket clamps on the inlet and outlet is recommended). The empty impinger is placed in an ice water bath between the injection port and the sorbent cartridge. Its purpose is to reduce the water content of the purge gas (saturated at 75°C (167°F)) before the sorbent cartridge.
6.2.4 Insulated Ice Bath. A 350 mL dewar or other type of insulated bath is used to maintain ice water around the knockout trap.
6.2.5 Sorbent Cartridges. Commercially available glass or stainless steel cartridge packed with one or more appropriate sorbents. The amount of adsorbent packed in the cartridge depends on the breakthrough volume of the test compounds but is limited by back pressure caused by the packing (not to exceed 7 psig). More than one sorbent cartridge placed in series may be necessary depending upon the mixture of the measured components.
6.2.6 Volumetric Glassware. Type A glass 10 mL volumetric flasks for measuring a final volume from the water catch in the knockout trap.
6.2.7 Thermal Desorption Unit. A clam-shell type oven, used for the desorption of direct thermal desorption sorbent tubes. The oven shall be capable of increasing the temperature of the desorption tubes rapidly to recommended desorption temperature.
6.2.8 Ultrasonic Bath. Small bath used to agitate sorbent material and desorption solvent. Ice water shall be used in the bath because of heat transfer caused by operation of the bath.
6.2.9 Desorption Vials. Four-dram (15 mL) capacity borosilicate glass vials with Teflon-lined caps.
6.3 Analytical System. A gas chromatograph (GC) is commonly used to separate and quantify compounds from the sample collection and recovery procedure. Method 18 (40 CFR Part 60, Appendix A) may be used as a guideline for determining the appropriate GC column and GC detector based on the test compounds to be determined. Other types of analytical instrumentation may be used (HPLC) in lieu of GC systems as long as the recovery efficiency criteria of this method are met.
6.3.1 Gas Chromatograph (GC). The GC shall be equipped with a constant-temperature liquid injection port or a heated sampling loop/valve system, as appropriate. The GC oven shall be temperature-programmable over the useful range of the GC column. The choice of detectors is based on the test compounds to be determined.
6.3.2 GC Column. Select the appropriate GC column based on (1) literature review or previous experience, (2) polarity of the analytes, (3) capacity of the column, or (4) resolving power (e.g., length, diameter, film thickness) required.
6.3.3 Data System. A programmable electronic integrator for recording, analyzing, and storing the signal generated by the detector.
7.0 Reagents and Standards
7.1 Method 25D Purge Apparatus.
7.1.1 Polyethylene Glycol (PEG). Ninety-eight percent pure organic polymer with an average molecular weight of 400 g/mol. Volatile organics are removed from the PEG prior to use by heating to 120 ±5°C (248 ±9°F) and purging with pure nitrogen at 1 L/min (0.04 ft 3/min) for 2 hours. After purging and heating, the PEG is maintained at room temperature under a nitrogen purge maintained at 1 L/min (0.04 ft 3/min) until used. A typical apparatus used to clean the PEG is shown in Figure 305-3.
7.1.2 Water. Organic-free deionized water is required.
7.1.3 Nitrogen. High-purity nitrogen (less than 0.5 ppm total hydrocarbons) is used to remove test compounds from the purge matrix. The source of nitrogen shall be regulated continuously to 40 psig before the on/off toggle valve.
7.2 Volatile Organic Recovery System.
7.2.1 Water. Organic-free deionized water is required.
7.2.2 Desorption Solvent (when used). Appropriate high-purity (99.99 percent) solvent for desorption shall be used. Analysis shall be performed (utilizing the same analytical technique as that used in the analysis of the waste samples) on each lot to determine purity.
7.3 Analytical System. The gases required for GC operation shall be of the highest obtainable purity (hydrocarbon free). Consult the operating manual for recommended settings.
8.0 Sample Collection, Preservation, Storage, and Transport
8.1 Assemble the glassware and associated fittings (see Figures 305-3 and 305-4, as appropriate) and leak-check the system (approximately 7 psig is the target pressure). After an initial leak check, mark the pressure gauge and use the initial checkpoint to monitor for leaks throughout subsequent analyses. If the pressure in the system drops below the target pressure at any time during analysis, that analysis shall be considered invalid.
8.2 Recovery Efficiency Determination. Determine the individual recovery efficiency (RE) for each of the target compounds in duplicate before the waste samples are analyzed. To determine the RE, generate a water blank (Section 11.1) and use the injection port to introduce a known volume of spike solution (or certified gaseous standard) containing all of the target compounds at the levels expected in the waste sample. Introduce the spike solution immediately after the nitrogen purge has been started (Section 8.3.2). Follow the procedures outlined in Section 8.3.3. Analyze the recovery efficiency samples using the techniques described in Section 11.2. Determine the recovery efficiency (Equation 305-1, Section 12.2) by comparing the amount of compound recovered to the theoretical amount spiked. Determine the RE twice for each compound; the relative standard deviation, (RSD) shall be ≤10 percent for each compound. If the RSD for any compound is not ≤10 percent, modify the sampling/analytical procedure and complete an RE study in duplicate, or continue determining RE until the RSD meets the acceptable criteria. The average RE shall be 0.70 ≤RE ≤1.30 for each compound. If the average RE does not meet these criteria, an alternative sample collection and/or analysis technique shall be developed and the recovery efficiency determination shall be repeated for that compound until the criteria are met for every target compound. Example modifications of the sampling/analytical system include changing the adsorbent material, changing the desorption solvent, utilizing direct thermal desorption of test compounds from the sorbent tubes, utilizing another analytical technique.
8.3 Sample Collection and Recovery.
8.3.1 The sample collection procedure in Method 25D shall be used to collect (into a preweighed vial) 10 g of waste into PEG, cool, and ship to the laboratory. Remove the sample container from the cooler and wipe the exterior to remove any ice or water. Weigh the container and sample to the nearest 0.01 g and record the weight. Pour the sample from the container into the purge flask. Rinse the sample container three times with approximately 6 mL of PEG (or the volume needed to total 50 mL of PEG in the purge flask), transferring the rinses to the purge flask. Add 50 mL of organic-free deionized water to the purge flask. Cap the purge flask tightly in between each rinse and after adding all the components into the flask.
8.3.2 Allow the oven to equilibrate to 75 ±2°C (167 ±3.6°F). Begin the sample recovery process by turning the toggle valve on, thus allowing a 6 L/min flow of pure nitrogen through the purge chamber.
8.3.3 Stop the purge after 30 min. Immediately remove the sorbent tube(s) from the apparatus and cap both ends. Remove the knockout trap and transfer the water catch to a 10 mL volumetric flask. Rinse the trap with organic-free deionized water and transfer the rinse to the volumetric flask. Dilute to the 10 mL mark with water. Transfer the water sample to a sample vial and store at 4°C (39.2°F) with zero headspace. The analysis of the contents of the water knockout trap is optional for this method. If the target compounds are water soluble, analysis of the water is recommended; meeting the recovery efficiency criteria in these cases would be difficult without adding the amount captured in the knockout trap.
9.0 Quality Control
9.1 Miscellaneous Quality Control Measures.
| Section | Quality control measure | Effect |
|---|---|---|
| 8.1 | Sampling equipment leak-check | Ensures accurate measurement of sample volume. |
| 8.2 | Recovery efficiency (RE) determination for each measured compound. | Ensures accurate sample collection and analysis. |
| 8.3 | Calibration of analytical instrument with at least 3 calibration standards. | Ensures linear measurement of compounds over the instrument span. |
10.0 Calibration and Standardization
10.1 The analytical instrument shall be calibrated with a minimum of three levels of standards for each compound whose concentrations bracket the concentration of test compounds from the sorbent tubes. Liquid calibration standards shall be used for calibration in the analysis of the solvent extracts. The liquid calibration standards shall be prepared in the desorption solvent matrix. The calibration standards may be prepared and injected individually or as a mixture. If thermal desorption and focusing (onto another sorbent or cryogen focusing) are used, a certified gaseous mixture or a series of gaseous standards shall be used for calibration of the instrument. The gaseous standards shall be focused and analyzed in the same manner as the samples.
10.2 The analytical system shall be certified free from contaminants before a calibration is performed (see Section 11.1). The calibration standards are used to determine the linearity of the analytical system. Perform an initial calibration and linearity check by analyzing the three calibration standards for each target compound in triplicate starting with the lowest level and continuing to the highest level. If the triplicate analyses do not agree within 5 percent of their average, additional analyses will be needed until the 5 percent criteria is met. Calculate the response factor (Equation 305-3, Section 12.4) from the average area counts of the injections for each concentration level. Average the response factors of the standards for each compound. The linearity of the detector is acceptable if the response factor of each compound at a particular concentration is within 10 percent of the overall mean response factor for that compound. Analyze daily a mid-level calibration standard in duplicate and calculate a new response factor. Compare the daily response factor average to the average response factor calculated for the mid-level calibration during the initial linearity check; repeat the three-level calibration procedure if the daily average response factor differs from the initial linearity check mid-level response factor by more than 10 percent. Otherwise, proceed with the sample analysis.
11.0 Analytical Procedure
11.1 Water Blank Analysis. A water blank shall be analyzed daily to determine the cleanliness of the purge and recovery system. A water blank is generated by adding 60 mL of organic-free deionized water to 50 mL of PEG in the purge chamber. Treat the blank as described in Sections 8.3.2 and 8.3.3. The purpose of the water blank is to insure that no contaminants exist in the sampling and analytical apparatus which would interfere with the quantitation of the target compounds. If contaminants are present, locate the source of contamination, remove it, and repeat the water blank analysis.
11.2 Sample Analysis. Sample analysis in the context of this method refers to techniques to remove the target compounds from the sorbent tubes, separate them using a chromatography technique, and quantify them with an appropriate detector. Two types of sample extraction techniques typically used for sorbents include solvent desorption or direct thermal desorption of test compounds to a secondary focusing unit (either sorbent or cryogen based). The test compounds are then typically transferred to a GC system for analysis. Other analytical systems may be used (e.g., HPLC) in lieu of GC systems as long as the recovery efficiency criteria of this method are met.
11.2.1 Recover the test compounds from the sorbent tubes that require solvent desorption by transferring the adsorbent material to a sample vial containing the desorption solvent. The desorption solvent shall be the same as the solvent used to prepare calibration standards. The volume of solvent depends on the amount of adsorbed material to be desorbed (1.0 mL per 100 mg of adsorbent material) and also on the amount of test compounds present. Final volume adjustment and or dilution can be made so that the concentration of test compounds in the desorption solvent is bracketed by the concentration of the calibration solutions. Ultrasonicate the desorption solvent for 15 min in an ice bath. Allow the sample to sit for a period of time so that the adsorbent material can settle to the bottom of the vial. Transfer the solvent with a pasteur pipet (minimizing the amount of adsorbent material taken) to another vial and store at 4°C (39.2°F).
11.2.2 Analyze the desorption solvent or direct thermal desorption tubes from each sample using the same analytical parameters used for the calibration standard. Calculate the total weight detected for each compound (Equation 305-4, Section 12.5). The slope (area/amount) and y-intercept are calculated from the line bracketed between the two closest calibration points. Correct the concentration of each waste sample with the appropriate recovery efficiency factor and the split flow ratio (if used). The final concentration of each individual test compound is calculated by dividing the corrected measured weight for that compound by the weight of the original sample determined in Section 8.3.1 (Equation 305-5, Section 12.6).
11.2.3 Repeat the analysis for the three samples collected in Section 8.3. Report the corrected concentration of each of the waste samples, average waste concentration, and relative standard deviation (Equation 305-6, Section 12.7).
12.0 Data Analysis and Calculations.
12.1 Nomenclature.
AS = Mean area counts of test compound in standard.
AU = Mean area counts of test compound in sample desorption solvent.
b = Y-intercept of the line formed between the two closest calibration standards that bracket the concentration of the sample.
CT = Amount of test compound (µg) in calibration standard.
CF = Correction for adjusting final amount of sample detected for losses during individual sample runs.
FP = Nitrogen flow through the purge chamber (6 L/min).
FS = Nitrogen split flow directed to the sample recovery system (use 6 L/min if split flow design was not used).
PPM = Final concentration of test compound in waste sample [µg/g (which is equivalent to parts per million by weight (ppmw))].
RE = Recovery efficiency for adjusting final amount of sample detected for losses due to inefficient trapping and desorption techniques.
R.F. = Response factor for test compound, calculated from a calibration standard.
S = Slope of the line (area counts/CT) formed between two closest calibration points that bracket the concentration of the sample.
WC = Weight of test compound expected to be recovered in spike solution based on theoretical amount (µg).
WE = Weight of vial and PEG (g).
WF = Weight of vial, PEG and waste sample (g).
WS = Weight of original waste sample (g).
WT = Corrected weight of test compound measured (µg) in sample.
WX = Weight of test compound measured during analysis of recovery efficiency spike samples (µg).
12.2 Recovery efficiency for determining trapping/desorption efficiency of individual test compounds in the spike solution, decimal value.

12.3 Weight of waste sample (g).

12.4 Response factor for individual test compounds.

12.5 Corrected weight of a test compound in the sample, in µg.

12.6 Final concentration of a test compound in the sample in ppmw.

12.7 Relative standard deviation (RSD) calculation.

13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References [Reserved]
17.0 Tables, Diagrams, Flowcharts, and Validation Data
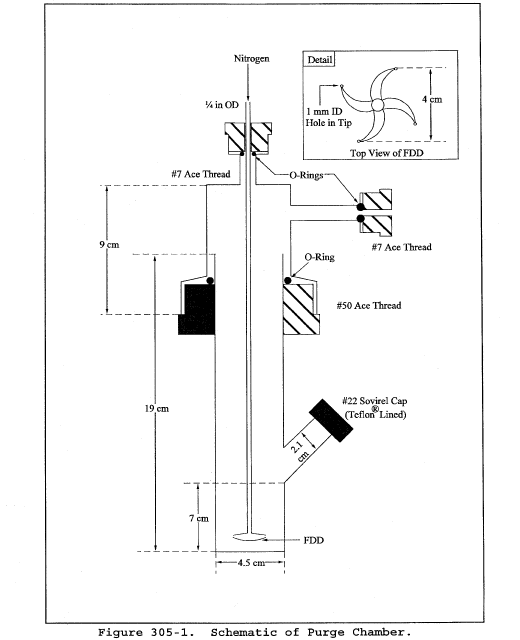
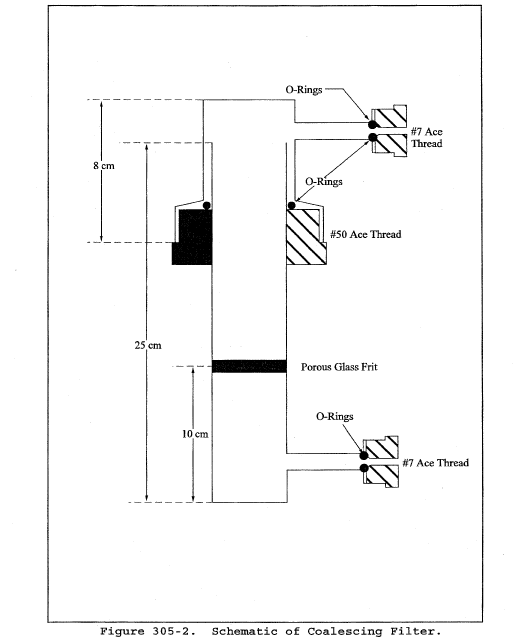
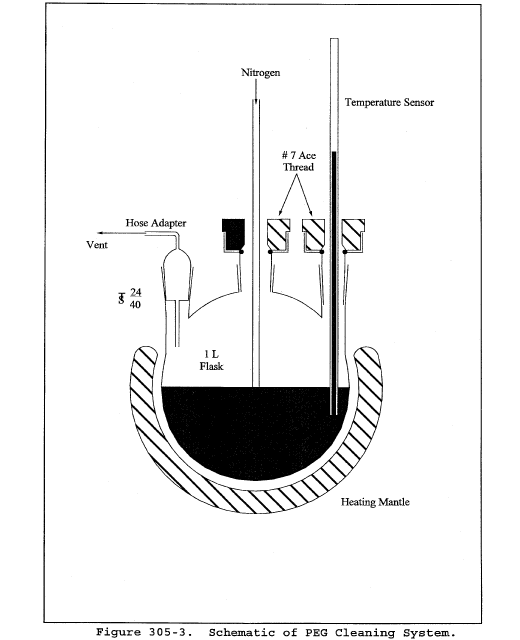
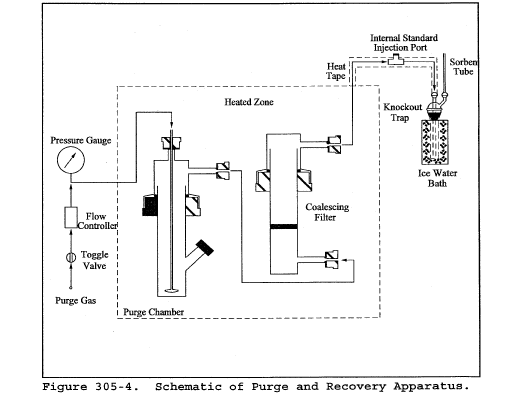
Method 306 - Determination of Chromium Emissions From Decorative and Hard Chromium Electroplating and Chromium Anodizing Operations - Isokinetic Method
Note:
This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in 40 CFR Part 60, Appendix A. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least Method 5.
1.0 Scope and Application
1.1 Analytes.
| Analyte | CAS No. | Sensitivity |
|---|---|---|
| Chromium | 7440-47-3 | See Sec. 13.2. |
1.2 Applicability. This method applies to the determination of chromium (Cr) in emissions from decorative and hard chrome electroplating facilities, chromium anodizing operations, and continuous chromium plating operations at iron and steel facilities.
1.3 Data Quality Objectives. [Reserved]
2.0 Summary of Method
2.1 Sampling. An emission sample is extracted isokinetically from the source using an unheated Method 5 sampling train (40 CFR Part 60, Appendix A), with a glass nozzle and probe liner, but with the filter omitted. The sample time shall be at least two hours. The Cr emissions are collected in an alkaline solution containing 0.1 N sodium hydroxide (NaOH) or 0.1 N sodium bicarbonate (NaHCO3). The collected samples are recovered using an alkaline solution and are then transported to the laboratory for analysis.
2.2 Analysis.
2.2.1 Total chromium samples with high chromium concentrations (≥35 µg/L) may be analyzed using inductively coupled plasma emission spectrometry (ICP) at 267.72 nm.
Note:
The ICP analysis is applicable for this method only when the solution analyzed has a Cr concentration greater than or equal to 35 µg/L or five times the method detection limit as determined according to appendix B in 40 CFR part 136. Similarly, inductively coupled plasma-mass spectroscopy (ICP-MS) may be used for total chromium analysis provided the procedures for ICP-MS analysis described in Method 6020 or 6020A (EPA Office of Solid Waste, publication SW-846) are followed.
2.2.2 Alternatively, when lower total chromium concentrations (<35 µg/L) are encountered, a portion of the alkaline sample solution may be digested with nitric acid and analyzed by graphite furnace atomic absorption spectroscopy (GFAAS) at 357.9 nm.
2.2.3 If it is desirable to determine hexavalent chromium (Cr+6) emissions, the samples may be analyzed using an ion chromatograph equipped with a post-column reactor (IC/PCR) and a visible wavelength detector. To increase sensitivity for trace levels of Cr+6, a preconcentration system may be used in conjunction with the IC/PCR.
3.0 Definitions
3.1 Total Chromium - measured chromium content that includes both major chromium oxidation states (Cr+3, Cr+3).
3.2 May - Implies an optional operation.
3.3 Digestion - The analytical operation involving the complete (or nearly complete) dissolution of the sample in order to ensure the complete solubilization of the element (analyte) to be measured.
3.4 Interferences - Physical, chemical, or spectral phenomena that may produce a high or low bias in the analytical result.
3.5 Analytical System - All components of the analytical process including the sample digestion and measurement apparatus.
3.6 Sample Recovery - The quantitative transfer of sample from the collection apparatus to the sample preparation (digestion, etc.) apparatus. This term should not be confused with analytical recovery.
3.7 Matrix Modifier - A chemical modification to the sample during GFAAS determinations to ensure that the analyte is not lost during the measurement process (prior to the atomization stage)
3.8 Calibration Reference Standards - Quality control standards used to check the accuracy of the instrument calibration curve prior to sample analysis.
3.9 Continuing Check Standard - Quality control standards used to verify that unacceptable drift in the measurement system has not occurred.
3.10 Calibration Blank - A blank used to verify that there has been no unacceptable shift in the baseline either immediately following calibration or during the course of the analytical measurement.
3.11 Interference Check - An analytical/measurement operation that ascertains whether a measurable interference in the sample exists.
3.12 Interelement Correction Factors - Factors used to correct for interfering elements that produce a false signal (high bias).
3.13 Duplicate Sample Analysis - Either the repeat measurement of a single solution or the measurement of duplicate preparations of the same sample. It is important to be aware of which approach is required for a particular type of measurement. For example, no digestion is required for the ICP determination and the duplicate instrument measurement is therefore adequate whereas duplicate digestion/instrument measurements are required for GFAAS.
3.14 Matrix Spiking - Analytical spikes that have been added to the actual sample matrix either before (Section 9.2.5.2) or after (Section 9.1.6). Spikes added to the sample prior to a preparation technique (e.g., digestion) allow for the assessment of an overall method accuracy while those added after only provide for the measurement accuracy determination.
4.0 Interferences
4.1 ICP Interferences.
4.1.1 ICP Spectral Interferences. Spectral interferences are caused by: overlap of a spectral line from another element; unresolved overlap of molecular band spectra; background contribution from continuous or recombination phenomena; and, stray light from the line emission of high-concentrated elements. Spectral overlap may be compensated for by correcting the raw data with a computer and measuring the interfering element. At the 267.72 nm Cr analytical wavelength, iron, manganese, and uranium are potential interfering elements. Background and stray light interferences can usually be compensated for by a background correction adjacent to the analytical line. Unresolved overlap requires the selection of an alternative chromium wavelength. Consult the instrument manufacturer's operation manual for interference correction procedures.
4.1.2 ICP Physical Interferences. High levels of dissolved solids in the samples may cause significant inaccuracies due to salt buildup at the nebulizer and torch tips. This problem can be controlled by diluting the sample or by extending the rinse times between sample analyses. Standards shall be prepared in the same solution matrix as the samples (i.e., 0.1 N NaOH or 0.1 N NaHCO3).
4.1.3 ICP Chemical Interferences. These include molecular compound formation, ionization effects and solute vaporization effects, and are usually not significant in the ICP procedure, especially if the standards and samples are matrix matched.
4.2 GFAAS Interferences.
4.2.1 GFAAS Chemical Interferences. Low concentrations of calcium and/or phosphate may cause interferences; at concentrations above 200 µg/L, calcium's effect is constant and eliminates the effect of phosphate. Calcium nitrate is therefore added to the concentrated analyte to ensure a known constant effect. Other matrix modifiers recommended by the instrument manufacturer may also be considered.
4.2.2 GFAAS Cyanide Band Interferences. Nitrogen should not be used as the purge gas due to cyanide band interference.
4.2.3 GFAAS Spectral Interferences. Background correction may be required because of possible significant levels of nonspecific absorption and scattering at the 357.9 nm analytical wavelength.
4.2.4 GFAAS Background Interferences. Zeeman or Smith-Hieftje background correction is recommended for interferences resulting from high levels of dissolved solids in the alkaline impinger solutions.
4.3 IC/PCR Interferences.
4.3.1 IC/PCR Chemical Interferences. Components in the sample matrix may cause Cr+6 to convert to trivalent chromium (Cr+3) or cause Cr+3 to convert to Cr+6. The chromatographic separation of Cr+6 using ion chromatography reduces the potential for other metals to interfere with the post column reaction. For the IC/PCR analysis, only compounds that coelute with Cr+6 and affect the diphenylcarbazide reaction will cause interference.
4.3.2 IC/PCR Background Interferences. Periodic analyses of reagent water blanks are used to demonstrate that the analytical system is essentially free of contamination. Sample cross-contamination can occur when high-level and low-level samples or standards are analyzed alternately and can be eliminated by thorough purging of the sample loop. Purging of the sample can easily be achieved by increasing the injection volume to ten times the size of the sample loop.
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
5.2 Hexavalent chromium compounds have been listed as carcinogens although chromium (III) compounds show little or no toxicity. Chromium can be a skin and respiratory irritant.
6.0 Equipment and Supplies
6.1 Sampling Train.
6.1.1 A schematic of the sampling train used in this method is shown in Figure 306-1. The train is the same as shown in Method 5, Section 6.0 (40 CFR Part 60, Appendix A) except that the probe liner is unheated, the particulate filter is omitted, and quartz or borosilicate glass must be used for the probe nozzle and liner in place of stainless steel.
6.1.2 Probe fittings of plastic such as Teflon, polypropylene, etc. are recommended over metal fittings to prevent contamination. If desired, a single combined probe nozzle and liner may be used, but such a single glass assembly is not a requirement of this methodology.
6.1.3 Use 0.1 N NaOH or 0.1 N NaHCO3 in the impingers in place of water.
6.1.4 Operating and maintenance procedures for the sampling train are described in APTD-0576 of Method 5. Users should read the APTD-0576 document and adopt the outlined procedures. Alternative mercury-free thermometers may be used if the thermometers are, at a minimum, equivalent in terms of performance or suitably effective for the specific temperature measurement application.
6.1.5 Similar collection systems which have been approved by the Administrator may be used.
6.2 Sample Recovery. Same as Method 5, [40 CFR Part 60, Appendix A], with the following exceptions:
6.2.1 Probe-Liner and Probe-Nozzle Brushes. Brushes are not necessary for sample recovery. If a probe brush is used, it must be non-metallic.
6.2.2 Sample Recovery Solution. Use 0.1 N NaOH or 0.1 N NaHCO3, whichever is used as the impinger absorbing solution, in place of acetone to recover the sample.
6.2.3 Sample Storage Containers. Polyethylene, with leak-free screw cap, 250 mL, 500 mL or 1,000 mL.
6.3 Analysis.
6.3.1 General. For analysis, the following equipment is needed.
6.3.1.1 Phillips Beakers. (Phillips beakers are preferred, but regular beakers may also be used.)
6.3.1.2 Hot Plate.
6.3.1.3 Volumetric Flasks. Class A, various sizes as appropriate.
6.3.1.4 Assorted Pipettes.
6.3.2 Analysis by ICP.
6.3.2.1 ICP Spectrometer. Computer-controlled emission spectrometer with background correction and radio frequency generator.
6.3.2.2 Argon Gas Supply. Welding grade or better.
6.3.3 Analysis by GFAAS.
6.3.3.1 Chromium Hollow Cathode Lamp or Electrodeless Discharge Lamp.
6.3.3.2 Graphite Furnace Atomic Absorption Spectrophotometer.
6.3.3.3 Furnace Autosampler.
6.3.4 Analysis by IC/PCR.
6.3.4.1 IC/PCR System. High performance liquid chromatograph pump, sample injection valve, post-column reagent delivery and mixing system, and a visible detector, capable of operating at 520 nm-540 nm, all with a non-metallic (or inert) flow path. An electronic peak area mode is recommended, but other recording devices and integration techniques are acceptable provided the repeatability criteria and the linearity criteria for the calibration curve described in Section 10.4 can be satisfied. A sample loading system is required if preconcentration is employed.
6.3.4.2 Analytical Column. A high performance ion chromatograph (HPIC) non-metallic column with anion separation characteristics and a high loading capacity designed for separation of metal chelating compounds to prevent metal interference. Resolution described in Section 11.6 must be obtained. A non-metallic guard column with the same ion-exchange material is recommended.
6.3.4.3 Preconcentration Column (for older instruments). An HPIC non-metallic column with acceptable anion retention characteristics and sample loading rates must be used as described in Section 11.6.
6.3.4.4 Filtration Apparatus for IC/PCR.
6.3.4.4.1 Teflon, or equivalent, filter holder to accommodate 0.45-µm acetate, or equivalent, filter, if needed to remove insoluble particulate matter.
6.3.4.4.2 0.45-µm Filter Cartridge. For the removal of insoluble material. To be used just prior to sample injection/analysis.
7.0 Reagents and Standards
Note:
Unless otherwise indicated, all reagents should conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society (ACS reagent grade). Where such specifications are not available, use the best available grade. Reagents should be checked by the appropriate analysis prior to field use to assure that contamination is below the analytical detection limit for the ICP or GFAAS total chromium analysis; and that contamination is below the analytical detection limit for Cr+6 using IC/PCR for direct injection or, if selected, preconcentration.
7.1 Sampling.
7.1.1 Water. Reagent water that conforms to ASTM Specification D1193-77 or 91 Type II (incorporated by reference see §63.14). All references to water in the method refer to reagent water unless otherwise specified. It is recommended that water blanks be checked prior to preparing the sampling reagents to ensure that the Cr content is less than three (3) times the anticipated detection limit of the analytical method.
7.1.2 Sodium Hydroxide (NaOH) Absorbing Solution, 0.1 N. Dissolve 4.0 g of sodium hydroxide in 1 liter of water to obtain a pH of approximately 8.5.
7.1.3 Sodium Bicarbonate (NaHCO3) Absorbing Solution, 0.1 N. Dissolve approximately 8.5 g of sodium bicarbonate in 1 liter of water to obtain a pH of approximately 8.3.
7.1.4 Chromium Contamination.
7.1.4.1 The absorbing solution shall not exceed the QC criteria noted in Section 7.1.1 (≤3 times the instrument detection limit).
7.1.4.2 When the Cr+6 content in the field samples exceeds the blank concentration by at least a factor of ten (10), Cr+6 blank concentrations ≥10 times the detection limit will be allowed.
Note:
At sources with high concentrations of acids and/or SO2, the concentration of NaOH or NaHCO3 should be ≥0.5 N to insure that the pH of the solution remains at or above 8.5 for NaOH and 8.0 for NaHCO3 during and after sampling.
7.1.5 Silica Gel. Same as in Method 5.
7.2 Sample Recovery.
7.2.1 0.1 N NaOH or 0.1 N NaHCO3. Use the same solution for the sample recovery that is used for the impinger absorbing solution.
7.2.2 pH Indicator Strip, for IC/PCR. pH indicator capable of determining the pH of solutions between the pH range of 7 and 12, at 0.5 pH increments.
7.3 Sample Preparation and Analysis.
7.3.1 Nitric Acid (HNO3), Concentrated, for GFAAS. Trace metals grade or better HNO3 must be used for reagent preparation. The ACS reagent grade HNO3 is acceptable for cleaning glassware.
7.3.2 HNO3, 1.0% (v/v), for GFAAS. Prepare, by slowly stirring, 10 mL of concentrated HNO3) into 800 mL of reagent water. Dilute to 1,000 mL with reagent water. The solution shall contain less than 0.001 mg Cr/L.
7.3.3 Calcium Nitrate Ca(NO3)2 Solution (10 µg Ca/mL) for GFAAS analysis. Prepare the solution by weighing 40.9 mg of Ca(NO3)2 into a 1 liter volumetric flask. Dilute with reagent water to 1 liter.
7.3.4 Matrix Modifier, for GFAAS. See instrument manufacturer's manual for suggested matrix modifier.
7.3.5 Chromatographic Eluent, for IC/PCR. The eluent used in the analytical system is ammonium sulfate based.
7.3.5.1 Prepare by adding 6.5 mL of 29 percent ammonium hydroxide (NH4OH) and 33 g of ammonium sulfate ((NH4)2SO4) to 500 mL of reagent water. Dilute to 1 liter with reagent water and mix well.
7.3.5.2 Other combinations of eluents and/or columns may be employed provided peak resolution, repeatability, linearity, and analytical sensitivity as described in Sections 9.3 and 11.6 are acceptable.
7.3.6 Post-Column Reagent, for IC/PCR. An effective post-column reagent for use with the chromatographic eluent described in Section 7.3.5 is a diphenylcarbazide (DPC)-based system. Dissolve 0.5 g of 1,5-diphenylcarbazide in 100 mL of ACS grade methanol. Add 500 mL of reagent water containing 50 mL of 96 percent spectrophotometric grade sulfuric acid. Dilute to 1 liter with reagent water.
7.3.7 Chromium Standard Stock Solution (1000 mg/L). Procure a certified aqueous standard or dissolve 2.829 g of potassium dichromate (K2Cr2O7), in reagent water and dilute to 1 liter.
7.3.8 Calibration Standards for ICP or IC/PCR. Prepare calibration standards for ICP or IC/PCR by diluting the Cr standard stock solution (Section 7.3.7) with 0.1 N NaOH or 0.1 N NaHCO3, whichever is used as the impinger absorbing solution, to achieve a matrix similar to the actual field samples. Suggested levels are 0, 50, 100, and 200 µg Cr/L for ICP, and 0, 1, 5, and 10 µg Cr+6/L for IC/PCR.
7.3.9 Calibration Standards for GFAAS. Chromium solutions for GFAAS calibration shall contain 1.0 percent (v/v) HNO3. The zero standard shall be 1.0 percent (v/v) HNO3. Calibration standards should be prepared daily by diluting the Cr standard stock solution (Section 7.3.7) with 1.0 percent HNO3. Use at least four standards to make the calibration curve. Suggested levels are 0, 10, 50, and 100 µg Cr/L.
7.4 Glassware Cleaning Reagents.
7.4.1 HNO3, Concentrated. ACS reagent grade or equivalent.
7.4.2 Water. Reagent water that conforms to ASTM Specification D1193-77 or 91 Type II.
7.4.3 HNO3, 10 percent (v/v). Add by stirring 500 mL of concentrated HNO3 into a flask containing approximately 4,000 mL of reagent water. Dilute to 5,000 mL with reagent water. Mix well. The reagent shall contain less than 2 µg Cr/L.
8.0 Sample Collection, Preservation, Holding Times, Storage, and Transport
Note:
Prior to sample collection, consideration should be given to the type of analysis (Cr+6 or total Cr) that will be performed. Which analysis option(s) will be performed will determine which sample recovery and storage procedures will be required to process the sample.
8.1 Sample Collection. Same as Method 5 (40 CFR part 60, appendix A), with the following exceptions.
8.1.1 Omit the particulate filter and filter holder from the sampling train. Use a glass nozzle and probe liner instead of stainless steel. Do not heat the probe. Place 100 mL of 0.1 N NaOH or 0.1 N NaHCO3 in each of the first two impingers, and record the data for each run on a data sheet such as shown in Figure 306-2.
8.1.2 Clean all glassware prior to sampling in hot soapy water designed for laboratory cleaning of glassware. Next, rinse the glassware three times with tap water, followed by three additional rinses with reagent water. Then soak the glassware in 10% (v/v) HNO3 solution for a minimum of 4 hours, rinse three times with reagent water, and allow to air dry. Cover all glassware openings where contamination can occur with Parafilm, or equivalent, until the sampling train is assembled for sampling.
8.1.3 Train Operation. Follow the basic procedures outlined in Method 5 in conjunction with the following instructions. Train sampling rate shall not exceed 0.030 m 3/min (1.0 cfm) during a run.
8.2 Sample Recovery. Follow the basic procedures of Method 5, with the exceptions noted.
8.2.1 A particulate filter is not recovered from this train.
8.2.2 Tester shall select either the total Cr or Cr+6 sample recovery option.
8.2.3 Samples to be analyzed for both total Cr and Cr+6, shall be recovered using the Cr+6 sample option (Section 8.2.6).
8.2.4 A field reagent blank shall be collected for either of the Cr or the Cr+6 analysis. If both analyses (Cr and Cr+6) are to be conducted on the samples, collect separate reagent blanks for each analysis.
Note:
Since particulate matter is not usually present at chromium electroplating and/or chromium anodizing operations, it is not necessary to filter the Cr+6 samples unless there is observed sediment in the collected solutions. If it is necessary to filter the Cr+6 solutions, please refer to Method 0061, Determination of Hexavalent Chromium Emissions From Stationary Sources, Section 7.4, Sample Preparation in SW-846 (see Reference 1).
8.2.5 Total Cr Sample Option.
8.2.5.1 Container No. 1. Measure the volume of the liquid in the first, second, and third impingers and quantitatively transfer into a labeled sample container.
8.2.5.2 Use approximately 200 to 300 mL of the 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution to rinse the probe nozzle, probe liner, three impingers, and connecting glassware; add this rinse to Container No. 1.
8.2.6 Cr+6 Sample Option.
8.2.6.1 Container No. 1. Measure and record the pH of the absorbing solution contained in the first impinger at the end of the sampling run using a pH indicator strip. The pH of the solution must be ≥8.5 for NaOH and ≥8.0 for NaHCO3. If it is not, discard the collected sample, increase the normality of the NaOH or NaHCO3 impinger absorbing solution to 0.5 N or to a solution normality approved by the Administrator and collect another air emission sample.
8.2.6.2 After determining the pH of the first impinger solution, combine and measure the volume of the liquid in the first, second, and third impingers and quantitatively transfer into the labeled sample container. Use approximately 200 to 300 mL of the 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution to rinse the probe nozzle, probe liner, three impingers, and connecting glassware; add this rinse to Container No. 1.
8.2.7 Field Reagent Blank.
8.2.7.1 Container No. 2.
8.2.7.2 Place approximately 500 mL of the 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution into a labeled sample container.
8.3 Sample Preservation, Storage, and Transport.
8.3.1 Total Cr Sample Option. Samples to be analyzed for total Cr need not be refrigerated.
8.3.2 Cr+6 Sample Option. Samples to be analyzed for Cr+6 must be shipped and stored at 4°C. Allow Cr+6 samples to return to ambient temperature prior to analysis.
8.4 Sample Holding Times.
8.4.1 Total Cr Sample Option. Samples to be analyzed for total Cr shall be analyzed within 60 days of collection.
8.4.2 Cr+6 Sample Option. Samples to be analyzed for Cr+6 shall be analyzed within 14 days of collection.
9.0 Quality Control
9.1 ICP Quality Control.
9.1.1 ICP Calibration Reference Standards. Prepare a calibration reference standard using the same alkaline matrix as the calibration standards; it should be at least 10 times the instrumental detection limit.
9.1.1.1 This reference standard must be prepared from a different Cr stock solution source than that used for preparation of the calibration curve standards.
9.1.1.2 Prior to sample analysis, analyze at least one reference standard.
9.1.1.3 The calibration reference standard must be measured within 10 percent of it's true value for the curve to be considered valid.
9.1.1.4 The curve must be validated before sample analyses are performed.
9.1.2 ICP Continuing Check Standard.
9.1.2.1 Perform analysis of the check standard with the field samples as described in Section 11.2 (at least after every 10 samples, and at the end of the analytical run).
9.1.2.2 The check standard can either be the mid-range calibration standard or the reference standard. The results of the check standard shall agree within 10 percent of the expected value; if not, terminate the analyses, correct the problem, recalibrate the instrument, and rerun all samples analyzed subsequent to the last acceptable check standard analysis.
9.1.3 ICP Calibration Blank.
9.1.3.1 Perform analysis of the calibration blank with the field samples as described in Section 11.2 (at least after every 10 samples, and at the end of the analytical run).
9.1.3.2 The results of the calibration blank shall agree within three standard deviations of the mean blank value. If not, analyze the calibration blank two more times and average the results. If the average is not within three standard deviations of the background mean, terminate the analyses, correct the problem, recalibrate, and reanalyze all samples analyzed subsequent to the last acceptable calibration blank analysis.
9.1.4 ICP Interference Check. Prepare an interference check solution that contains known concentrations of interfering elements that will provide an adequate test of the correction factors in the event of potential spectral interferences.
9.1.4.1 Two potential interferences, iron and manganese, may be prepared as 1000 µg/mL and 200 µg/mL solutions, respectively. The solutions should be prepared in dilute HNO3 (1-5 percent). Particular care must be used to ensure that the solutions and/or salts used to prepare the solutions are of ICP grade purity (i.e., that no measurable Cr contamination exists in the salts/solutions). Commercially prepared interfering element check standards are available.
9.1.4.2 Verify the interelement correction factors every three months by analyzing the interference check solution. The correction factors are calculated according to the instrument manufacturer's directions. If the interelement correction factors are used properly, no false Cr should be detected.
9.1.4.3 Negative results with an absolute value greater than three (3) times the detection limit are usually the results of the background correction position being set incorrectly. Scan the spectral region to ensure that the correction position has not been placed on an interfering peak.
9.1.5 ICP Duplicate Sample Analysis. Perform one duplicate sample analysis for each compliance sample batch (3 runs).
9.1.5.1 As there is no sample preparation required for the ICP analysis, a duplicate analysis is defined as a repeat analysis of one of the field samples. The selected sample shall be analyzed using the same procedures that were used to analyze the original sample.
9.1.5.2 Duplicate sample analyses shall agree within 10 percent of the original measurement value.
9.1.5.3 Report the original analysis value for the sample and report the duplicate analysis value as the QC check value. If agreement is not achieved, perform the duplicate analysis again. If agreement is not achieved the second time, perform corrective action to identify and correct the problem before analyzing the sample for a third time.
9.1.6 ICP Matrix Spiking. Spiked samples shall be prepared and analyzed daily to ensure that there are no matrix effects, that samples and standards have been matrix-matched, and that the laboratory equipment is operating properly.
9.1.6.1 Spiked sample recovery analyses should indicate a recovery for the Cr spike of between 75 and 125 percent.
9.1.6.2 Cr levels in the spiked sample should provide final solution concentrations that are within the linear portion of the calibration curve, as well as, at a concentration level at least: equal to that of the original sample; and, ten (10) times the detection limit.
9.1.6.3 If the spiked sample concentration meets the stated criteria but exceeds the linear calibration range, the spiked sample must be diluted with the field absorbing solution.
9.1.6.4 If the recoveries for the Cr spiked samples do not meet the specified criteria, perform corrective action to identify and correct the problem prior to reanalyzing the samples.
9.1.7 ICP Field Reagent Blank.
9.1.7.1 Analyze a minimum of one matrix-matched field reagent blank (Section 8.2.4) per sample batch to determine if contamination or memory effects are occurring.
9.1.7.2 If contamination or memory effects are observed, perform corrective action to identify and correct the problem before reanalyzing the samples.
9.2 GFAAS Quality Control.
9.2.1 GFAAS Calibration Reference Standards. The calibration curve must be verified by using at least one calibration reference standard (made from a reference material or other independent standard material) at or near the mid-range of the calibration curve.
9.2.1.1 The calibration curve must be validated before sample analyses are performed.
9.2.1.2 The calibration reference standard must be measured within 10 percent of its true value for the curve to be considered valid.
9.2.2 GFAAS Continuing Check Standard.
9.2.2.1 Perform analysis of the check standard with the field samples as described in Section 11.4 (at least after every 10 samples, and at the end of the analytical run).
9.2.2.2 These standards are analyzed, in part, to monitor the life and performance of the graphite tube. Lack of reproducibility or a significant change in the signal for the check standard may indicate that the graphite tube should be replaced.
9.2.2.3 The check standard may be either the mid-range calibration standard or the reference standard.
9.2.2.4 The results of the check standard shall agree within 10 percent of the expected value.
9.2.2.5 If not, terminate the analyses, correct the problem, recalibrate the instrument, and reanalyze all samples analyzed subsequent to the last acceptable check standard analysis.
9.2.3 GFAAS Calibration Blank.
9.2.3.1 Perform analysis of the calibration blank with the field samples as described in Section 11.4 (at least after every 10 samples, and at the end of the analytical run).
9.2.3.2 The calibration blank is analyzed to monitor the life and performance of the graphite tube as well as the existence of any memory effects. Lack of reproducibility or a significant change in the signal, may indicate that the graphite tube should be replaced.
9.2.3.3 The results of the calibration blank shall agree within three standard deviations of the mean blank value.
9.2.3.4 If not, analyze the calibration blank two more times and average the results. If the average is not within three standard deviations of the background mean, terminate the analyses, correct the problem, recalibrate, and reanalyze all samples analyzed subsequent to the last acceptable calibration blank analysis.
9.2.4 GFAAS Duplicate Sample Analysis. Perform one duplicate sample analysis for each compliance sample batch (3 runs).
9.2.4.1 A digested aliquot of the selected sample is processed and analyzed using the identical procedures that were used for the whole sample preparation and analytical efforts.
9.2.4.2 Duplicate sample analyses results incorporating duplicate digestions shall agree within 20 percent for sample results exceeding ten (10) times the detection limit.
9.2.4.3 Report the original analysis value for the sample and report the duplicate analysis value as the QC check value.
9.2.4.4 If agreement is not achieved, perform the duplicate analysis again. If agreement is not achieved the second time, perform corrective action to identify and correct the problem before analyzing the sample for a third time.
9.2.5 GFAAS Matrix Spiking.
9.2.5.1 Spiked samples shall be prepared and analyzed daily to ensure that (1) correct procedures are being followed, (2) there are no matrix effects and (3) all equipment is operating properly.
9.2.5.2 Cr spikes are added prior to any sample preparation.
9.2.5.3 Cr levels in the spiked sample should provide final solution concentrations that are within the linear portion of the calibration curve, as well as, at a concentration level at least: equal to that of the original sample; and, ten (10) times the detection limit.
9.2.5.4 Spiked sample recovery analyses should indicate a recovery for the Cr spike of between 75 and 125 percent.
9.2.5.5 If the recoveries for the Cr spiked samples do not meet the specified criteria, perform corrective action to identify and correct the problem prior to reanalyzing the samples.
9.2.6 GFAAS Method of Standard Additions.
9.2.6.1 Method of Standard Additions. Perform procedures in Section 5.4 of Method 12 (40 CFR Part 60, Appendix A)
9.2.6.2 Whenever sample matrix problems are suspected and standard/sample matrix matching is not possible or whenever a new sample matrix is being analyzed, perform referenced procedures to determine if the method of standard additions is necessary.
9.2.7 GFAAS Field Reagent Blank.
9.2.7.1 Analyze a minimum of one matrix-matched field reagent blank (Section 8.2.4) per sample batch to determine if contamination or memory effects are occurring.
9.2.7.2 If contamination or memory effects are observed, perform corrective action to identify and correct the problem before reanalyzing the samples.
9.3 IC/PCR Quality Control.
9.3.1 IC/PCR Calibration Reference Standards.
9.3.1.1 Prepare a calibration reference standard at a concentration that is at or near the mid-point of the calibration curve using the same alkaline matrix as the calibration standards. This reference standard should be prepared from a different Cr stock solution than that used to prepare the calibration curve standards. The reference standard is used to verify the accuracy of the calibration curve.
9.3.1.2 The curve must be validated before sample analyses are performed. Prior to sample analysis, analyze at least one reference standard with an expected value within the calibration range.
9.3.1.3 The results of this reference standard analysis must be within 10 percent of the true value of the reference standard for the calibration curve to be considered valid.
9.3.2 IC/PCR Continuing Check Standard and Calibration Blank.
9.3.2.1 Perform analysis of the check standard and the calibration blank with the field samples as described in Section 11.6 (at least after every 10 samples, and at the end of the analytical run).
9.3.2.2 The result from the check standard must be within 10 percent of the expected value.
9.3.2.3 If the 10 percent criteria is exceeded, excessive drift and/or instrument degradation may have occurred, and must be corrected before further analyses can be performed.
9.3.2.4 The results of the calibration blank analyses must agree within three standard deviations of the mean blank value.
9.3.2.5 If not, analyze the calibration blank two more times and average the results.
9.3.2.6 If the average is not within three standard deviations of the background mean, terminate the analyses, correct the problem, recalibrate, and reanalyze all samples analyzed subsequent to the last acceptable calibration blank analysis.
9.3.3 IC/PCR Duplicate Sample Analysis.
9.3.3.1 Perform one duplicate sample analysis for each compliance sample batch (3 runs).
9.3.3.2 An aliquot of the selected sample is prepared and analyzed using procedures identical to those used for the emission samples (for example, filtration and/or, if necessary, preconcentration).
9.3.3.3 Duplicate sample injection results shall agree within 10 percent for sample results exceeding ten (10) times the detection limit.
9.3.3.4 Report the original analysis value for the sample and report the duplicate analysis value as the QC check value.
9.3.3.5 If agreement is not achieved, perform the duplicate analysis again.
9.3.3.6 If agreement is not achieved the second time, perform corrective action to identify and correct the problem prior to analyzing the sample for a third time.
9.3.4 ICP/PCR Matrix Spiking. Spiked samples shall be prepared and analyzed with each sample set to ensure that there are no matrix effects, that samples and standards have been matrix-matched, and that the equipment is operating properly.
9.3.4.1 Spiked sample recovery analysis should indicate a recovery of the Cr+6 spike between 75 and 125 percent.
9.3.4.2 The spiked sample concentration should be within the linear portion of the calibration curve and should be equal to or greater than the concentration of the original sample. In addition, the spiked sample concentration should be at least ten (10) times the detection limit.
9.3.4.3 If the recoveries for the Cr+6 spiked samples do not meet the specified criteria, perform corrective action to identify and correct the problem prior to reanalyzing the samples.
9.3.5 IC/PCR Field Reagent Blank.
9.3.5.1 Analyze a minimum of one matrix-matched field reagent blank (Section 8.2.4) per sample batch to determine if contamination or memory effects are occurring.
9.3.5.2 If contamination or memory effects are observed, perform corrective action to identify and correct the problem before reanalyzing the samples.
10.0 Calibration and Standardization
10.1 Sampling Train Calibration. Perform calibrations described in Method 5, (40 CFR part 60, appendix A). The alternate calibration procedures described in Method 5, may also be used.
10.2 ICP Calibration.
10.2.1 Calibrate the instrument according to the instrument manufacturer's recommended procedures, using a calibration blank and three standards for the initial calibration.
10.2.2 Calibration standards should be prepared fresh daily, as described in Section 7.3.8. Be sure that samples and calibration standards are matrix matched. Flush the system with the calibration blank between each standard.
10.2.3 Use the average intensity of multiple exposures (3 or more) for both standardization and sample analysis to reduce random error.
10.2.4 Employing linear regression, calculate the correlation coefficient .
10.2.5 The correlation coefficient must equal or exceed 0.995.
10.2.6 If linearity is not acceptable, prepare and rerun another set of calibration standards or reduce the range of the calibration standards, as necessary.
10.3 GFAAS Calibration.
10.3.1 For instruments that measure directly in concentration, set the instrument software to display the correct concentration, if applicable.
10.3.2 Curve must be linear in order to correctly perform the method of standard additions which is customarily performed automatically with most instrument computer-based data systems.
10.3.3 The calibration curve (direct calibration or standard additions) must be prepared daily with a minimum of a calibration blank and three standards that are prepared fresh daily.
10.3.4 The calibration curve acceptance criteria must equal or exceed 0.995.
10.3.5 If linearity is not acceptable, prepare and rerun another set of calibration standards or reduce the range of calibration standards, as necessary.
10.4 IC/PCR Calibration.
10.4.1 Prepare a calibration curve using the calibration blank and three calibration standards prepared fresh daily as described in Section 7.3.8.
10.4.2 The calibration curve acceptance criteria must equal or exceed 0.995.
10.4.3 If linearity is not acceptable, remake and/or rerun the calibration standards. If the calibration curve is still unacceptable, reduce the range of the curve.
10.4.4 Analyze the standards with the field samples as described in Section 11.6.
11.0 Analytical Procedures
Note:
The method determines the chromium concentration in µg Cr/mL. It is important that the analyst measure the field sample volume prior to analyzing the sample. This will allow for conversion of µg Cr/mL to µg Cr/sample.
11.1 ICP Sample Preparation.
11.1.1 The ICP analysis is performed directly on the alkaline impinger solution; acid digestion is not necessary, provided the samples and standards are matrix matched.
11.1.2 The ICP analysis should only be employed when the solution analyzed has a Cr concentration greater than 35 µg/L or five times the method detection limit as determined according to Appendix B in 40 CFR Part 136 or by other commonly accepted analytical procedures.
11.2 ICP Sample Analysis.
11.2.1 The ICP analysis is applicable for the determination of total chromium only.
11.2.2 ICP Blanks. Two types of blanks are required for the ICP analysis.
11.2.2.1 Calibration Blank. The calibration blank is used in establishing the calibration curve. For the calibration blank, use either 0.1 N NaOH or 0.1 N NaHCO3, whichever is used for the impinger absorbing solution. The calibration blank can be prepared fresh in the laboratory; it does not have to be prepared from the same batch of solution that was used in the field. A sufficient quantity should be prepared to flush the system between standards and samples.
11.2.2.2 Field Reagent Blank. The field reagent blank is collected in the field during the testing program. The field reagent blank (Section 8.2.4) is an aliquot of the absorbing solution prepared in Section 7.1.2. The reagent blank is used to assess possible contamination resulting from sample processing.
11.2.3 ICP Instrument Adjustment.
11.2.3.1 Adjust the ICP instrument for proper operating parameters including wavelength, background correction settings (if necessary), and interfering element correction settings (if necessary).
11.2.3.2 The instrument must be allowed to become thermally stable before beginning measurements (usually requiring at least 30 min of operation prior to calibration). During this warmup period, the optical calibration and torch position optimization may be performed (consult the operator's manual).
11.2.4 ICP Instrument Calibration.
11.2.4.1 Calibrate the instrument according to the instrument manufacturer's recommended procedures, and the procedures specified in Section 10.2.
11.2.4.2 Prior to analyzing the field samples, reanalyze the highest calibration standard as if it were a sample.
11.2.4.3 Concentration values obtained should not deviate from the actual values or from the established control limits by more than 5 percent, whichever is lower (see Sections 9.1 and 10.2).
11.2.4.4 If they do, follow the recommendations of the instrument manufacturer to correct the problem.
11.2.5 ICP Operational Quality Control Procedures.
11.2.5.1 Flush the system with the calibration blank solution for at least 1 min before the analysis of each sample or standard.
11.2.5.2 Analyze the continuing check standard and the calibration blank after each batch of 10 samples.
11.2.5.3 Use the average intensity of multiple exposures for both standardization and sample analysis to reduce random error.
11.2.6 ICP Sample Dilution.
11.2.6.1 Dilute and reanalyze samples that are more concentrated than the linear calibration limit or use an alternate, less sensitive Cr wavelength for which quality control data have already been established.
11.2.6.2 When dilutions are performed, the appropriate factors must be applied to sample measurement results.
11.2.7 Reporting Analytical Results. All analytical results should be reported in µg Cr/mL using three significant figures. Field sample volumes (mL) must be reported also.
11.3 GFAAS Sample Preparation.
11.3.1 GFAAS Acid Digestion. An acid digestion of the alkaline impinger solution is required for the GFAAS analysis.
11.3.1.1 In a beaker, add 10 mL of concentrated HNO3 to a 100 mL sample aliquot that has been well mixed. Cover the beaker with a watch glass. Place the beaker on a hot plate and reflux the sample to near dryness. Add another 5 mL of concentrated HNO3 to complete the digestion. Again, carefully reflux the sample volume to near dryness. Rinse the beaker walls and watch glass with reagent water.
11.3.1.2 The final concentration of HNO3 in the solution should be 1 percent (v/v).
11.3.1.3 Transfer the digested sample to a 50-mL volumetric flask. Add 0.5 mL of concentrated HNO3 and 1 mL of the 10 µg/mL of Ca(NO3)2. Dilute to 50 mL with reagent water.
11.3.2 HNO3 Concentration. A different final volume may be used based on the expected Cr concentration, but the HNO3 concentration must be maintained at 1 percent (v/v).
11.4 GFAAS Sample Analysis.
11.4.1 The GFAAS analysis is applicable for the determination of total chromium only.
11.4.2 GFAAS Blanks. Two types of blanks are required for the GFAAS analysis.
11.4.2.1 Calibration Blank. The 1.0 percent HNO3 is the calibration blank which is used in establishing the calibration curve.
11.4.2.2 Field Reagent Blank. An aliquot of the 0.1 N NaOH solution or the 0.1 N NaHCO3 prepared in Section 7.1.2 is collected for the field reagent blank. The field reagent blank is used to assess possible contamination resulting from processing the sample.
11.4.2.2.1 The reagent blank must be subjected to the entire series of sample preparation and analytical procedures, including the acid digestion.
11.4.2.2.2 The reagent blank's final solution must contain the same acid concentration as the sample solutions.
11.4.3 GFAAS Instrument Adjustment.
11.4.3.1 The 357.9 nm wavelength line shall be used.
11.4.3.2 Follow the manufacturer's instructions for all other spectrophotometer operating parameters.
11.4.4 Furnace Operational Parameters. Parameters suggested by the manufacturer should be employed as guidelines.
11.4.4.1 Temperature-sensing mechanisms and temperature controllers can vary between instruments and/or with time; the validity of the furnace operating parameters must be periodically confirmed by systematically altering the furnace parameters while analyzing a standard. In this manner, losses of analyte due to higher-than-necessary temperature settings or losses in sensitivity due to less than optimum settings can be minimized.
11.4.4.2 Similar verification of furnace operating parameters may be required for complex sample matrices (consult instrument manual for additional information). Calibrate the GFAAS system following the procedures specified in Section 10.3.
11.4.5 GFAAS Operational Quality Control Procedures.
11.4.5.1 Introduce a measured aliquot of digested sample into the furnace and atomize.
11.4.5.2 If the measured concentration exceeds the calibration range, the sample should be diluted with the calibration blank solution (1.0 percent HNO3) and reanalyzed.
11.4.5.3 Consult the operator's manual for suggested injection volumes. The use of multiple injections can improve accuracy and assist in detecting furnace pipetting errors.
11.4.5.4 Analyze a minimum of one matrix-matched reagent blank per sample batch to determine if contamination or any memory effects are occurring.
11.4.5.5 Analyze a calibration blank and a continuing check standard after approximately every batch of 10 sample injections.
11.4.6 GFAAS Sample Dilution.
11.4.6.1 Dilute and reanalyze samples that are more concentrated than the instrument calibration range.
11.4.6.2 If dilutions are performed, the appropriate factors must be applied to sample measurement results.
11.4.7 Reporting Analytical Results.
11.4.7.1 Calculate the Cr concentrations by the method of standard additions (see operator's manual) or, from direct calibration. All dilution and/or concentration factors must be used when calculating the results.
11.4.7.2 Analytical results should be reported in µg Cr/mL using three significant figures. Field sample volumes (mL) must be reported also.
11.5 IC/PCR Sample Preparation.
11.5.1 Sample pH. Measure and record the sample pH prior to analysis.
11.5.2 Sample Filtration. Prior to preconcentration and/or analysis, filter all field samples through a 0.45-µm filter. The filtration step should be conducted just prior to sample injection/analysis.
11.5.2.1 Use a portion of the sample to rinse the syringe filtration unit and acetate filter and then collect the required volume of filtrate.
11.5.2.2 Retain the filter if total Cr is to be determined also.
11.5.3 Sample Preconcentration (older instruments).
11.5.3.1 For older instruments, a preconcentration system may be used in conjunction with the IC/PCR to increase sensitivity for trace levels of Cr+6.
11.5.3.2 The preconcentration is accomplished by selectively retaining the analyte on a solid absorbent, followed by removal of the analyte from the absorbent (consult instrument manual).
11.5.3.3 For a manual system, position the injection valve so that the eluent displaces the concentrated Cr+6 sample, transferring it from the preconcentration column and onto the IC anion separation column.
11.6 IC/PCR Sample Analyses.
11.6.1 The IC/PCR analysis is applicable for hexavalent chromium measurements only.
11.6.2 IC/PCR Blanks. Two types of blanks are required for the IC/PCR analysis.
11.6.2.1 Calibration Blank. The calibration blank is used in establishing the analytical curve. For the calibration blank, use either 0.1 N NaOH or 0.1 N NaHCO3, whichever is used for the impinger solution. The calibration blank can be prepared fresh in the laboratory; it does not have to be prepared from the same batch of absorbing solution that is used in the field.
11.6.2.2 Field Reagent Blank. An aliquot of the 0.1 N NaOH solution or the 0.1 N NaHCO3 solution prepared in Section 7.1.2 is collected for the field reagent blank. The field reagent blank is used to assess possible contamination resulting from processing the sample.
11.6.3 Stabilized Baseline. Prior to sample analysis, establish a stable baseline with the detector set at the required attenuation by setting the eluent and post-column reagent flow rates according to the manufacturers recommendations.
Note:
As long as the ratio of eluent flow rate to PCR flow rate remains constant, the standard curve should remain linear. Inject a sample of reagent water to ensure that no Cr+6 appears in the water blank.
11.6.4 Sample Injection Loop. Size of injection loop is based on standard/sample concentrations and the selected attenuator setting.
11.6.4.1 A 50-µL loop is normally sufficient for most higher concentrations.
11.6.4.2 The sample volume used to load the injection loop should be at least 10 times the loop size so that all tubing in contact with the sample is thoroughly flushed with the new sample to prevent cross contamination.
11.6.5 IC/PCR Instrument Calibration.
11.6.5.1 First, inject the calibration standards prepared, as described in Section 7.3.8 to correspond to the appropriate concentration range, starting with the lowest standard first.
11.6.5.2 Check the performance of the instrument and verify the calibration using data gathered from analyses of laboratory blanks, calibration standards, and a quality control sample.
11.6.5.3 Verify the calibration by analyzing a calibration reference standard. If the measured concentration exceeds the established value by more than 10 percent, perform a second analysis. If the measured concentration still exceeds the established value by more than 10 percent, terminate the analysis until the problem can be identified and corrected.
11.6.6 IC/PCR Instrument Operation.
11.6.6.1 Inject the calibration reference standard (as described in Section 9.3.1), followed by the field reagent blank (Section 8.2.4), and the field samples.
11.6.6.1.1 Standards (and QC standards) and samples are injected into the sample loop of the desired size (use a larger size loop for greater sensitivity). The Cr+6 is collected on the resin bed of the column.
11.6.6.1.2 After separation from other sample components, the Cr+6 forms a specific complex in the post-column reactor with the DPC reaction solution, and the complex is detected by visible absorbance at a maximum wavelength of 540 nm.
11.6.6.1.3 The amount of absorbance measured is proportional to the concentration of the Cr+6 complex formed.
11.6.6.1.4 The IC retention time and the absorbance of the Cr+6 complex with known Cr+6 standards analyzed under identical conditions must be compared to provide both qualitative and quantitative analyses.
11.6.6.1.5 If a sample peak appears near the expected retention time of the Cr+6 ion, spike the sample according to Section 9.3.4 to verify peak identity.
11.6.7 IC/PCR Operational Quality Control Procedures.
11.6.7.1 Samples should be at a pH ≥8.5 for NaOH and ≥8.0 if using NaHCO3; document any discrepancies.
11.6.7.2 Refrigerated samples should be allowed to equilibrate to ambient temperature prior to preparation and analysis.
11.6.7.3 Repeat the injection of the calibration standards at the end of the analytical run to assess instrument drift. Measure areas or heights of the Cr+6/DPC complex chromatogram peaks.
11.6.7.4 To ensure the precision of the sample injection (manual or autosampler), the response for the second set of injected standards must be within 10 percent of the average response.
11.6.7.5 If the 10 percent criteria duplicate injection cannot be achieved, identify the source of the problem and rerun the calibration standards.
11.6.7.6 Use peak areas or peak heights from the injections of calibration standards to generate a linear calibration curve. From the calibration curve, determine the concentrations of the field samples.
11.6.8 IC/PCR Sample Dilution.
11.6.8.1 Samples having concentrations higher than the established calibration range must be diluted into the calibration range and re-analyzed.
11.6.8.2 If dilutions are performed, the appropriate factors must be applied to sample measurement results.
11.6.9 Reporting Analytical Results. Results should be reported in µg Cr+6/mL using three significant figures. Field sample volumes (mL) must be reported also.
12.0 Data Analysis and Calculations
12.1 Pretest Calculations.
12.1.1 Pretest Protocol (Site Test Plan).
12.1.1.1 The pretest protocol should define and address the test data quality objectives (DQOs), with all assumptions, that will be required by the end user (enforcement authority); what data are needed? why are the data needed? how will the data be used? what are method detection limits? and what are estimated target analyte levels for the following test parameters.
12.1.1.1.1 Estimated source concentration for total chromium and/or Cr+6.
12.1.1.1.2 Estimated minimum sampling time and/or volume required to meet method detection limit requirements (Appendix B 40 CFR Part 136) for measurement of total chromium and/or Cr+6.
12.1.1.1.3 Demonstrate that planned sampling parameters will meet DQOs. The protocol must demonstrate that the planned sampling parameters calculated by the tester will meet the needs of the source and the enforcement authority.
12.1.1.2 The pre-test protocol should include information on equipment, logistics, personnel, process operation, and other resources necessary for an efficient and coordinated test.
12.1.1.3 At a minimum, the pre-test protocol should identify and be approved by the source, the tester, the analytical laboratory, and the regulatory enforcement authority. The tester should not proceed with the compliance testing before obtaining approval from the enforcement authority.
12.1.2 Post Test Calculations.
12.1.2.1 Perform the calculations, retaining one extra decimal figure beyond that of the acquired data. Round off figures after final calculations.
12.1.2.2 Nomenclature.
CS = Concentration of Cr in sample solution, µg Cr/mL.
Ccr = Concentration of Cr in stack gas, dry basis, corrected to standard conditions, mg/dscm.
D = Digestion factor, dimension less.
F = Dilution factor, dimension less.
MCr = Total Cr in each sample, µg.
Vad = Volume of sample aliquot after digestion, mL.
Vaf = Volume of sample aliquot after dilution, mL.
Vbd = Volume of sample aliquot submitted to digestion, mL.
Vbf = Volume of sample aliquot before dilution, mL.
VmL = Volume of impinger contents plus rinses, mL.
Vm(std) = Volume of gas sample measured by the dry gas meter, corrected to standard conditions, dscm.
12.1.2.3 Dilution Factor. The dilution factor is the ratio of the volume of sample aliquot after dilution to the volume before dilution. This ratio is given by the following equation:

12.1.2.4 Digestion Factor. The digestion factor is the ratio of the volume of sample aliquot after digestion to the volume before digestion. This ratio is given by Equation 306-2.

12.1.2.5 Total Cr in Sample. Calculate MCr, the total µg Cr in each sample, using the following equation:

12.1.2.6 Average Dry Gas Meter Temperature and Average Orifice Pressure Drop. Same as Method 5.
12.1.2.7 Dry Gas Volume, Volume of Water Vapor, Moisture Content. Same as Method 5.
12.1.2.8 Cr Emission Concentration (CCr). Calculate CCr, the Cr concentration in the stack gas, in mg/dscm on a dry basis, corrected to standard conditions using the following equation:

12.1.2.9 Isokinetic Variation, Acceptable Results. Same as Method 5.
13.0 Method Performance
13.1 Range. The recommended working range for all of the three analytical techniques starts at five times the analytical detection limit (see also Section 13.2.2). The upper limit of all three techniques can be extended indefinitely by appropriate dilution.
13.2 Sensitivity.
13.2.1 Analytical Sensitivity. The estimated instrumental detection limits listed are provided as a guide for an instrumental limit. The actual method detection limits are sample and instrument dependent and may vary as the sample matrix varies.
13.2.1.2 ICP Analytical Sensitivity. The minimum estimated detection limits for ICP, as reported in Method 6010A and the recently revised Method 6010B of SW-846 (Reference 1), are 7.0 µg Cr/L and 4.7 µg Cr/L, respectively.
13.2.1.3 GFAAS Analytical Sensitivity. The minimum estimated detection limit for GFAAS, as reported in Methods 7000A and 7191 of SW-846 (Reference 1), is 1 µg Cr/L.
13.2.1.4 IC/PCR Analytical Sensitivity. The minimum detection limit for IC/PCR with a preconcentrator, as reported in Methods 0061 and 7199 of SW-846 (Reference 1), is 0.05 µg Cr+6/L.
1.3.2.1.5 Determination of Detection Limits. The laboratory performing the Cr+6 measurements must determine the method detection limit on a quarterly basis using a suitable procedure such as that found in 40 CFR, Part 136, Appendix B. The determination should be made on samples in the appropriate alkaline matrix. Normally this involves the preparation (if applicable) and consecutive measurement of seven (7) separate aliquots of a sample with a concentration <5 times the expected detection limit. The detection limit is 3.14 times the standard deviation of these results.
13.2.2 In-stack Sensitivity. The in-stack sensitivity depends upon the analytical detection limit, the volume of stack gas sampled, the total volume of the impinger absorbing solution plus the rinses, and, in some cases, dilution or concentration factors from sample preparation. Using the analytical detection limits given in Sections 13.2.1.1, 13.2.1.2, and 13.2.1.3; a stack gas sample volume of 1.7 dscm; a total liquid sample volume of 500 mL; and the digestion concentration factor of 1/2 for the GFAAS analysis; the corresponding in-stack detection limits are 0.0014 mg Cr/dscm to 0.0021 mg Cr/dscm for ICP, 0.00015 mg Cr/dscm for GFAAS, and 0.000015 mg Cr+6/dscm for IC/PCR with preconcentration.
Note:
It is recommended that the concentration of Cr in the analytical solutions be at least five times the analytical detection limit to optimize sensitivity in the analyses. Using this guideline and the same assumptions for impinger sample volume, stack gas sample volume, and the digestion concentration factor for the GFAAS analysis (500 mL,1.7 dscm, and 1/2, respectively), the recommended minimum stack concentrations for optimum sensitivity are 0.0068 mg Cr/dscm to 0.0103 mg Cr/dscm for ICP, 0.00074 mg Cr/dscm for GFAAS, and 0.000074 mg Cr+6/dscm for IC/PCR with preconcentration. If required, the in-stack detection limits can be improved by either increasing the stack gas sample volume, further reducing the volume of the digested sample for GFAAS, improving the analytical detection limits, or any combination of the three.
13.3 Precision.
13.3.1 The following precision data have been reported for the three analytical methods. In each case, when the sampling precision is combined with the reported analytical precision, the resulting overall precision may decrease.
13.3.2 Bias data is also reported for GFAAS.
13.4 ICP Precision.
13.4.1 As reported in Method 6010B of SW-846 (Reference 1), in an EPA round-robin Phase 1 study, seven laboratories applied the ICP technique to acid/distilled water matrices that had been spiked with various metal concentrates. For true values of 10, 50, and 150 µg Cr/L; the mean reported values were 10, 50, and 149 µg Cr/L; and the mean percent relative standard deviations were 18, 3.3, and 3.8 percent, respectively.
13.4.2 In another multi laboratory study cited in Method 6010B, a mean relative standard of 8.2 percent was reported for an aqueous sample concentration of approximately 3750 µg Cr/L.
13.5 GFAAS Precision. As reported in Method 7191 of SW-846 (Reference 1), in a single laboratory (EMSL), using Cincinnati, Ohio tap water spiked at concentrations of 19, 48, and 77 µg Cr/L, the standard deviations were ±0.1, ±0.2, and ±0.8, respectively. Recoveries at these levels were 97 percent, 101 percent, and 102 percent, respectively.
13.6 IC/PCR Precision. As reported in Methods 0061 and 7199 of SW-846 (Reference 1), the precision of IC/PCR with sample preconcentration is 5 to 10 percent. The overall precision for sewage sludge incinerators emitting 120 ng/dscm of Cr+6 and 3.5 µg/dscm of total Cr was 25 percent and 9 percent, respectively; and for hazardous waste incinerators emitting 300 ng/dscm of C+6 the precision was 20 percent.
14.0 Pollution Prevention
14.1 The only materials used in this method that could be considered pollutants are the chromium standards used for instrument calibration and acids used in the cleaning of the collection and measurement containers/labware, in the preparation of standards, and in the acid digestion of samples. Both reagents can be stored in the same waste container.
14.2 Cleaning solutions containing acids should be prepared in volumes consistent with use to minimize the disposal of excessive volumes of acid.
14.3 To the extent possible, the containers/vessels used to collect and prepare samples should be cleaned and reused to minimize the generation of solid waste.
15.0 Waste Management
15.1 It is the responsibility of the laboratory and the sampling team to comply with all federal, state, and local regulations governing waste management, particularly the discharge regulations, hazardous waste identification rules, and land disposal restrictions; and to protect the air, water, and land by minimizing and controlling all releases from field operations.
15.2 For further information on waste management, consult The Waste Management Manual for Laboratory Personnel and Less is Better - Laboratory Chemical Management for Waste Reduction, available from the American Chemical Society's Department of Government Relations and Science Policy, 1155 16th Street NW, Washington, DC 20036.
16.0 References
1. “Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846, Third Edition,” as amended by Updates I, II, IIA, IIB, and III. Document No. 955-001-000001. Available from Superintendent of Documents, U.S. Government Printing Office, Washington, DC, November 1986.
2. Cox, X.B., R.W. Linton, and F.E. Butler. Determination of Chromium Speciation in Environmental Particles - A Multi-technique Study of Ferrochrome Smelter Dust. Accepted for publication in Environmental Science and Technology.
3. Same as Section 17.0 of Method 5, References 2, 3, 4, 5, and 7.
4. California Air Resources Board, “Determination of Total Chromium and Hexavalent Chromium Emissions from Stationary Sources.” Method 425, September 12, 1990.
5. The Merck Index. Eleventh Edition. Merck & Co., Inc., 1989.
6. Walpole, R.E., and R.H. Myers. “Probability and Statistics for Scientists and Engineering.” 3rd Edition. MacMillan Publishing Co., NewYork, N.Y., 1985.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
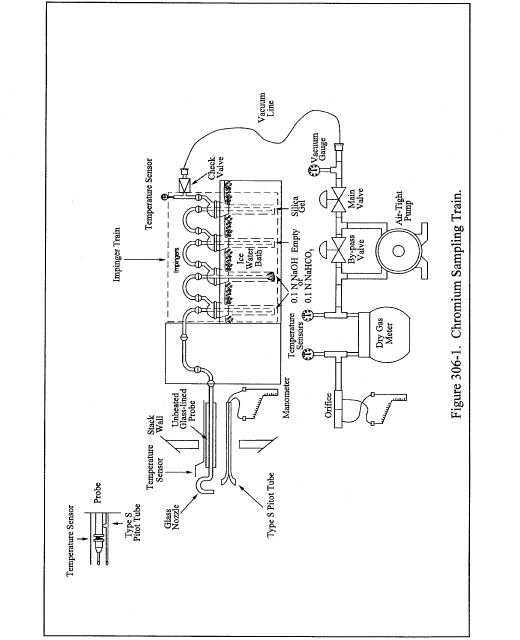
Method 306A - Determination of Chromium Emissions From Decorative and Hard Chromium Electroplating and Chromium Anodizing Operations
Note:
This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in 40 CFR Part 60, Appendix A and in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least Methods 5 and 306.
1.0 Scope and Application
1.1 Analyte. Chromium. CAS Number (7440-47-3).
1.2 Applicability.
1.2.1 This method applies to the determination of chromium (Cr) in emissions from decorative and hard chromium electroplating facilities, chromium anodizing operations, and continuous chromium plating at iron and steel facilities. The method is less expensive and less complex to conduct than Method 306. Correctly applied, the precision and bias of the sample results should be comparable to those obtained with the isokinetic Method 306. This method is applicable for the determination of air emissions under nominal ambient moisture, temperature, and pressure conditions.
1.2.2 The method is also applicable to electroplating and anodizing sources controlled by wet scrubbers.
1.3 Data Quality Objectives.
1.3.1 Pretest Protocol.
1.3.1.1 The pretest protocol should define and address the test data quality objectives (DQOs), with all assumptions, that will be required by the end user (enforcement authority); what data are needed? why are the data needed? how will data be used? what are method detection limits? and what are estimated target analyte levels for the following test parameters.
1.3.1.1.1 Estimated source concentration for total chromium and/or Cr+6.
1.3.1.1.2 Estimated minimum sampling time and/or volume required to meet method detection limit requirements (Appendix B 40 CFR Part 136) for measurement of total chromium and/or Cr+6.
1.3.1.1.3 Demonstrate that planned sampling parameters will meet DQOs. The protocol must demonstrate that the planned sampling parameters calculated by the tester will meet the needs of the source and the enforcement authority.
1.3.1.2 The pre-test protocol should include information on equipment, logistics, personnel, process operation, and other resources necessary for an efficient and coordinated performance test.
1.3.1.3 At a minimum, the pre-test protocol should identify and be approved by the source, the tester, the analytical laboratory, and the regulatory enforcement authority. The tester should not proceed with the compliance testing before obtaining approval from the enforcement authority.
2.0 Summary of Method
2.1 Sampling.
2.1.1 An emission sample is extracted from the source at a constant sampling rate determined by a critical orifice and collected in a sampling train composed of a probe and impingers. The proportional sampling time at the cross sectional traverse points is varied according to the stack gas velocity at each point. The total sample time must be at least two hours.
2.1.2 The chromium emission concentration is determined by the same analytical procedures described in Method 306: inductively-coupled plasma emission spectrometry (ICP), graphite furnace atomic absorption spectrometry (GFAAS), or ion chromatography with a post-column reactor (IC/PCR).
2.1.2.1 Total chromium samples with high chromium concentrations (≥35 µg/L) may be analyzed using inductively coupled plasma emission spectrometry (ICP) at 267.72 nm.
Note:
The ICP analysis is applicable for this method only when the solution analyzed has a Cr concentration greater than or equal to 35 µg/L or five times the method detection limit as determined according to Appendix B in 40 CFR Part 136.
2.1.2.2 Alternatively, when lower total chromium concentrations (<35 µg/L) are encountered, a portion of the alkaline sample solution may be digested with nitric acid and analyzed by graphite furnace atomic absorption spectroscopy (GFAAS) at 357.9 nm.
2.1.2.3 If it is desirable to determine hexavalent chromium (Cr+6) emissions, the samples may be analyzed using an ion chromatograph equipped with a post-column reactor (IC/PCR) and a visible wavelength detector. To increase sensitivity for trace levels of Cr+6, a preconcentration system may be used in conjunction with the IC/PCR.
3.0 Definitions
3.1 Total Chromium - measured chromium content that includes both major chromium oxidation states (Cr + 3, Cr + 6).
3.2 May - Implies an optional operation.
3.3 Digestion - The analytical operation involving the complete (or nearly complete) dissolution of the sample in order to ensure the complete solubilization of the element (analyte) to be measured.
3.4 Interferences - Physical, chemical, or spectral phenomena that may produce a high or low bias in the analytical result.
3.5 Analytical System - All components of the analytical process including the sample digestion and measurement apparatus.
3.6 Sample Recovery - The quantitative transfer of sample from the collection apparatus to the sample preparation (digestion, etc.) apparatus. This term should not be confused with analytical recovery.
4.0 Interferences
4.1 Same as in Method 306, Section 4.0.
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method does not purport to address all of the safety issues associated with its use. It is the responsibility of the user to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
5.2 Chromium and some chromium compounds have been listed as carcinogens although Chromium (III) compounds show little or no toxicity. Chromium is a skin and respiratory irritant.
6.0 Equipment and Supplies
Note:
Mention of trade names or specific products does not constitute endorsement by the Environmental Protection Agency.
6.1 Sampling Train. A schematic of the sampling train is shown in Figure 306A-1. The individual components of the train are available commercially, however, some fabrication and assembly are required.
6.1.1 Probe Nozzle/Tubing and Sheath.
6.1.1.1 Use approximately 6.4-mm ( 1/4-in.) inside diameter (ID) glass or rigid plastic tubing approximately 20 cm (8 in.) in length with a short 90 degree bend at one end to form the sampling nozzle. Grind a slight taper on the nozzle end before making the bend. Attach the nozzle to flexible tubing of sufficient length to enable collection of a sample from the stack.
6.1.1.2 Use a straight piece of larger diameter rigid tubing (such as metal conduit or plastic water pipe) to form a sheath that begins about 2.5 cm (1 in.) from the 90° bend on the nozzle and encases and supports the flexible tubing.
6.1.2 Type S Pitot Tube. Same as Method 2, Section 6.1 (40 CFR Part 60, Appendix A).
6.1.3 Temperature Sensor.
6.1.3.1 A thermocouple, liquid-filled bulb thermometer, bimetallic thermometer, mercury-in-glass thermometer, or other sensor capable of measuring temperature to within 1.5 percent of the minimum absolute stack temperature.
6.1.3.2 The temperature sensor shall either be positioned near the center of the stack, or be attached to the pitot tube as directed in Section 6.3 of Method 2.
6.1.4 Sample Train Connectors.
6.1.4.1 Use thick wall flexible plastic tubing (polyethylene, polypropylene, or polyvinyl chloride) ∼ 6.4-mm ( 1/4-in.) to 9.5-mm ( 3/8-in.) ID to connect the train components.
6.1.4.2 A combination of rigid plastic tubing and thin wall flexible tubing may be used as long as tubing walls do not collapse when leak-checking the train. Metal tubing cannot be used.
6.1.5 Impingers. Three, one-quart capacity, glass canning jars with vacuum seal lids, or three Greenburg-Smith (GS) design impingers connected in series, or equivalent, may be used.
6.1.5.1 One-quart glass canning jar. Three separate jar containers are required: (1) the first jar contains the absorbing solution; (2) the second is empty and is used to collect any reagent carried over from the first container; and (3) the third contains the desiccant drying agent.
6.1.5.2 Canning Jar Connectors. The jar containers are connected by leak-tight inlet and outlet tubes installed in the lids of each container for assembly with the train. The tubes may be made of ∼ 6.4 mm ( 1/4-in.) ID glass or rigid plastic tubing. For the inlet tube of the first impinger, heat the glass or plastic tubing and draw until the tubing separates. Fabricate the necked tip to form an orifice tip that is approximately 2.4 mm ( 3/32-in.) ID.
6.1.5.2.1 When assembling the first container, place the orifice tip end of the tube approximately 4.8 mm ( 3/16-in.) above the inside bottom of the jar.
6.1.5.2.2 For the second container, the inlet tube need not be drawn and sized, but the tip should be approximately 25 mm (1 in.) above the bottom of the jar.
6.1.5.2.3 The inlet tube of the third container should extend to approximately 12.7 mm ( 1/2-in.) above the bottom of the jar.
6.1.5.2.4 Extend the outlet tube for each container approximately 50 mm (2 in.) above the jar lid and downward through the lid, approximately 12.7 mm ( 1/2-in.) beneath the bottom of the lid.
6.1.5.3 Greenburg-Smith Impingers. Three separate impingers of the Greenburg-Smith (GS) design as described in Section 6.0 of Method 5 are required. The first GS impinger shall have a standard tip (orifice/plate), and the second and third GS impingers shall be modified by replacing the orifice/plate tube with a 13 mm ( 1/2-in.) ID glass tube, having an unrestricted opening located 13 mm ( 1/2-in.) from the bottom of the outer flask.
6.1.5.4 Greenburg-Smith Connectors. The GS impingers shall be connected by leak-free ground glass “U” tube connectors or by leak-free non-contaminating flexible tubing. The first impinger shall contain the absorbing solution, the second is empty and the third contains the desiccant drying agent.
6.1.6 Manometer. Inclined/vertical type, or equivalent device, as described in Section 6.2 of Method 2 (40 CFR Part 60, Appendix A).
6.1.7 Critical Orifice. The critical orifice is a small restriction in the sample line that is located upstream of the vacuum pump. The orifice produces a constant sampling flow rate that is approximately 0.021 cubic meters per minute (m3/min) or 0.75 cubic feet per minute (cfm).
6.1.7.1 The critical orifice can be constructed by sealing a 2.4-mm ( 3/32-in.) ID brass tube approximately 14.3 mm ( 9/16-in.) in length inside a second brass tube that is approximately 8 mm ( 5/16-in.) ID and 14.3-mm ( 9/16-in.) in length .
6.1.7.2 Materials other than brass can be used to construct the critical orifice as long as the flow through the sampling train can be maintained at approximately 0.021 cubic meter per minute (0.75) cfm.
6.1.8 Connecting Hardware. Standard pipe and fittings, 9.5-mm ( 3/8-in.), 6.4-mm ( 1/4-in.) or 3.2-mm ( 1/8-in.) ID, may be used to assemble the vacuum pump, dry gas meter and other sampling train components.
6.1.9 Vacuum Gauge. Capable of measuring approximately 760 mm Hg (30 in. Hg) vacuum in 25.4 mm HG (1 in. Hg) increments. Locate vacuum gauge between the critical orifice and the vacuum pump.
6.1.10 Pump Oiler. A glass oil reservoir with a wick mounted at the vacuum pump inlet that lubricates the pump vanes. The oiler should be an in-line type and not vented to the atmosphere. See EMTIC Guideline Document No. GD-041.WPD for additional information.
6.1.11 Vacuum Pump. Gast Model 0522-V103-G18DX, or equivalent, capable of delivering at least 1.5 cfm at 15 in. Hg vacuum.
6.1.12 Oil Trap/Muffler. An empty glass oil reservoir without wick mounted at the pump outlet to control the pump noise and prevent oil from reaching the dry gas meter.
6.1.13 By-pass Fine Adjust Valve (Optional). Needle valve assembly 6.4-mm ( 1/4-in.), Whitey 1 RF 4-A, or equivalent, that allows for adjustment of the train vacuum.
6.1.13.1 A fine-adjustment valve is positioned in the optional pump by-pass system that allows the gas flow to recirculate through the pump. This by-pass system allows the tester to control/reduce the maximum leak-check vacuum pressure produced by the pump.
6.1.13.1.1 The tester must conduct the post test leak check at a vacuum equal to or greater than the maximum vacuum encountered during the sampling run.
6.1.13.1.2 The pump by-pass assembly is not required, but is recommended if the tester intends to leak-check the 306A train at the vacuum experienced during a run.
6.1.14 Dry Gas Meter. An Equimeter Model 110 test meter or, equivalent with temperature sensor(s) installed (inlet/outlet) to monitor the meter temperature. If only one temperature sensor is installed, locate the sensor at the outlet side of the meter. The dry gas meter must be capable of measuring the gaseous volume to within ±2% of the true volume.
Note:
The Method 306 sampling train is also commercially available and may be used to perform the Method 306A tests. The sampling train may be assembled as specified in Method 306A with the sampling rate being operated at the delta H@ specified for the calibrated orifice located in the meter box. The Method 306 train is then operated as described in Method 306A.
6.2 Barometer. Mercury aneroid barometer, or other barometer equivalent, capable of measuring atmospheric pressure to within ±2.5 mm Hg (0.1 in. Hg).
6.2.1 A preliminary check of the barometer shall be made against a mercury-in-glass reference barometer or its equivalent.
6.2.2 Tester may elect to obtain the absolute barometric pressure from a nearby National Weather Service station.
6.2.2.1 The station value (which is the absolute barometric pressure) must be adjusted for elevation differences between the weather station and the sampling location. Either subtract 2.5 mm Hg (0.1 in. Hg) from the station value per 30 m (100 ft) of elevation increase or add the same for an elevation decrease.
6.2.2.2 If the field barometer cannot be adjusted to agree within 0.1 in. Hg of the reference barometric, repair or discard the unit. The barometer pressure measurement shall be recorded on the sampling data sheet.
6.3 Sample Recovery. Same as Method 5, Section 6.2 (40 CFR Part 60, Appendix A), with the following exceptions:
6.3.1 Probe-Liner and Probe-Nozzle Brushes. Brushes are not necessary for sample recovery. If a probe brush is used, it must be non-metallic.
6.3.2 Wash Bottles. Polyethylene wash bottle, for sample recovery absorbing solution.
6.3.3 Sample Recovery Solution. Use 0.1 N NaOH or 0.1 N NaHCO3, whichever is used as the impinger absorbing solution, to replace the acetone.
6.3.4 Sample Storage Containers.
6.3.4.1 Glass Canning Jar. The first canning jar container of the sampling train may serve as the sample shipping container. A new lid and sealing plastic wrap shall be substituted for the container lid assembly.
6.3.4.2 Polyethylene or Glass Containers. Transfer the Greenburg-Smith impinger contents to precleaned polyethylene or glass containers. The samples shall be stored and shipped in 250-mL, 500-mL or 1000-mL polyethylene or glass containers with leak-free, non metal screw caps.
6.3.5 pH Indicator Strip, for Cr +6 Samples. pH indicator strips, or equivalent, capable of determining the pH of solutions between the range of 7 and 12, at 0.5 pH increments.
6.3.6 Plastic Storage Containers. Air tight containers to store silica gel.
6.4 Analysis. Same as Method 306, Section 6.3.
7.0 Reagents and Standards.
Note:
Unless otherwise indicated, all reagents shall conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society (ACS reagent grade). Where such specifications are not available, use the best available grade. It is recommended, but not required, that reagents be checked by the appropriate analysis prior to field use to assure that contamination is below the analytical detection limit for the ICP or GFAAS total chromium analysis; and that contamination is below the analytical detection limit for Cr+6 using IC/PCR for direct injection or, if selected, preconcentration.
7.1 Sampling.
7.1.1 Water. Reagent water that conforms to ASTM Specification D1193 Type II (incorporated by reference see §63.14). All references to water in the method refer to reagent water unless otherwise specified. It is recommended that water blanks be checked prior to preparing the sampling reagents to ensure that the Cr content is less than three (3) times the anticipated detection limit of the analytical method.
7.1.2 Sodium Hydroxide (NaOH) Absorbing Solution, 0.1 N. Dissolve 4.0 g of sodium hydroxide in 1 liter of water to obtain a pH of approximately 8.5.
7.1.3 Sodium Bicarbonate (NaHCO3) Absorbing Solution, 0.1 N. Dissolve approximately 8.5 g of sodium bicarbonate in 1 liter of water to obtain a pH of approximately 8.3.
7.1.4 Chromium Contamination.
7.1.4.1 The absorbing solution shall not exceed the QC criteria noted in Method 306, Section 7.1.1 (≤3 times the instrument detection limit).
7.1.4.2 When the Cr+6 content in the field samples exceeds the blank concentration by at least a factor of ten (10), Cr+6 blank levels ≤10 times the detection limit will be allowed.
Note:
At sources with high concentrations of acids and/or SO2, the concentration of NaOH or NaHCO3 should be ≥0.5 N to insure that the pH of the solution remains at or above 8.5 for NaOH and 8.0 for NaHCO3 during and after sampling.
7.1.3 Desiccant. Silica Gel, 6-16 mesh, indicating type. Alternatively, other types of desiccants may be used, subject to the approval of the Administrator.
7.2 Sample Recovery. Same as Method 306, Section 7.2.
7.3 Sample Preparation and Analysis. Same as Method 306, Section 7.3.
7.4 Glassware Cleaning Reagents. Same as Method 306, Section 7.4.
8.0 Sample Collection, Recovery, Preservation, Holding Times, Storage, and Transport
Note:
Prior to sample collection, consideration should be given as to the type of analysis (Cr+6 or total Cr) that will be performed. Deciding which analysis will be performed will enable the tester to determine which appropriate sample recovery and storage procedures will be required to process the sample.
8.1 Sample Collection.
8.1.1 Pretest Preparation.
8.1.1.1 Selection of Measurement Site. Locate the sampling ports as specified in Section 11.0 of Method 1 (40 CFR Part 60, Appendix A).
8.1.1.2 Location of Traverse Points.
8.1.1.2.1 Locate the traverse points as specified in Section 11.0 of Method 1 (40 CFR Part 60, Appendix A). Use a total of 24 sampling points for round ducts and 24 or 25 points for rectangular ducts. Mark the pitot and sampling probe to identify the sample traversing points.
8.1.1.2.2 For round ducts less than 12 inches in diameter, use a total of 16 points.
8.1.1.3 Velocity Pressure Traverse. Perform an initial velocity traverse before obtaining samples. The Figure 306A-2 data sheet may be used to record velocity traverse data.
8.1.1.3.1 To demonstrate that the flow rate is constant over several days of testing, perform complete traverses at the beginning and end of each day's test effort, and calculate the deviation of the flow rate for each daily period. The beginning and end flow rates are considered constant if the deviation does not exceed 10 percent. If the flow rate exceeds the 10 percent criteria, either correct the inconsistent flow rate problem, or obtain the Administrator's approval for the test results.
8.1.1.3.2 Perform traverses as specified in Section 8.0 of Method 2, but record only the Δp (velocity pressure) values for each sampling point. If a mass emission rate is desired, stack velocity pressures shall be recorded before and after each test, and an average stack velocity pressure determined for the testing period.
8.1.1.4 Verification of Absence of Cyclonic Flow. Check for cyclonic flow during the initial traverse to verify that it does not exist. Perform the cyclonic flow check as specified in Section 11.4 of Method 1 (40 CFR Part 60, Appendix A).
8.1.1.4.1 If cyclonic flow is present, verify that the absolute average angle of the tangential flow does not exceed 20 degrees. If the average value exceeds 20 degrees at the sampling location, the flow condition in the stack is unacceptable for testing.
8.1.1.4.2 Alternative procedures, subject to approval of the Administrator, e.g., installing straightening vanes to eliminate the cyclonic flow, must be implemented prior to conducting the testing.
8.1.1.5 Stack Gas Moisture Measurements. Not required. Measuring the moisture content is optional when a mass emission rate is to be calculated.
8.1.1.5.1 The tester may elect to either measure the actual stack gas moisture during the sampling run or utilize a nominal moisture value of 2 percent.
8.1.1.5.2 For additional information on determining sampling train moisture, please refer to Method 4 (40 CFR Part 60, Appendix A).
8.1.1.6 Stack Temperature Measurements. If a mass emission rate is to be calculated, a temperature sensor must be placed either near the center of the stack, or attached to the pitot tube as described in Section 8.3 of Method 2. Stack temperature measurements, shall be recorded before and after each test, and an average stack temperature determined for the testing period.
8.1.1.7 Point Sampling Times. Since the sampling rate of the train (0.75 cfm) is maintained constant by the critical orifice, it is necessary to calculate specific sampling times for each traverse point in order to obtain a proportional sample.
8.1.1.7.1 If the sampling period (3 runs) is to be completed in a single day, the point sampling times shall be calculated only once.
8.1.1.7.2 If the sampling period is to occur over several days, the sampling times must be calculated daily using the initial velocity pressure data recorded for that day. Determine the average of the Δp values obtained during the velocity traverse (Figure 306A-2).
8.1.1.7.3 If the stack diameter is less than 12 inches, use 7.5 minutes in place of 5 minutes in the equation and 16 sampling points instead of 24 or 25 points. Calculate the sampling times for each traverse point using the following equation:

Where:
n = Sampling point number.
Δp = Average pressure differential across pitot tube, mm H2O (in. H2O).
ΔPavg = Average of Δp values, mm H2O (in. H2O).
Note:
Convert the decimal fractions for minutes to seconds.
8.1.1.8 Pretest Preparation. It is recommended, but not required, that all items which will be in contact with the sample be cleaned prior to performing the testing to avoid possible sample contamination (positive chromium bias). These items include, but are not limited to: Sampling probe, connecting tubing, impingers, and jar containers.
8.1.1.8.1 Sample train components should be: (1) Rinsed with hot tap water; (2) washed with hot soapy water; (3) rinsed with tap water; (4) rinsed with reagent water; (5) soaked in a 10 percent (v/v) nitric acid solution for at least four hours; and (6) rinsed throughly with reagent water before use.
8.1.1.8.2 At a minimum, the tester should, rinse the probe, connecting tubing, and first and second impingers twice with either 0.1 N sodium hydroxide (NaOH) or 0.1 N sodium bicarbonate (NaHCO3) and discard the rinse solution.
8.1.1.8.3 If separate sample shipping containers are to be used, these also should be precleaned using the specified cleaning procedures.
8.1.1.9 Preparation of Sampling Train. Assemble the sampling train as shown in Figure 306A-1. Secure the nozzle-liner assembly to the outer sheath to prevent movement when sampling.
8.1.1.9.1 Place 250 mL of 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution into the first jar container or impinger. The second jar/impinger is to remain empty. Place 6 to 16 mesh indicating silica gel, or equivalent desiccant into the third jar/impinger until the container is half full (∼ 300 to 400 g).
8.1.1.9.2 Place a small cotton ball in the outlet exit tube of the third jar to collect small silica gel particles that may dislodge and impair the pump and/or gas meter.
8.1.1.10 Pretest Leak-Check. A pretest leak-check is recommended, but not required. If the tester opts to conduct the pretest leak-check, the following procedures shall be performed: (1) Place the jar/impinger containers into an ice bath and wait 10 minutes for the ice to cool the containers before performing the leak check and/or start sampling; (2) to perform the leak check, seal the nozzle using a piece of clear plastic wrap placed over the end of a finger and switch on the pump; and (3) the train system leak rate should not exceed 0.02 cfm at a vacuum of 380 mm Hg (15 in. Hg) or greater. If the leak rate does exceed the 0.02 cfm requirement, identify and repair the leak area and perform the leak check again.
Note:
Use caution when releasing the vacuum following the leak check. Always allow air to slowly flow through the nozzle end of the train system while the pump is still operating. Switching off the pump with vacuum on the system may result in the silica gel being pulled into the second jar container.
8.1.1.11 Leak-Checks During Sample Run. If, during the sampling run, a component (e.g., jar container) exchange becomes necessary, a leak-check shall be conducted immediately before the component exchange is made. The leak-check shall be performed according to the procedure outlined in Section 8.1.1.10 of this method. If the leakage rate is found to be ≤0.02 cfm at the maximum operating vacuum, the results are acceptable. If, however, a higher leak rate is obtained, either record the leakage rate and correct the sample volume as shown in Section 12.3 of Method 5 or void the sample and initiate a replacement run. Following the component change, leak-checks are optional, but are recommended as are the pretest leak-checks.
8.1.1.12 Post Test Leak Check. Remove the probe assembly and flexible tubing from the first jar/impinger container. Seal the inlet tube of the first container using clear plastic wrap and switch on the pump. The vacuum in the line between the pump and the critical orifice must be ≥15 in. Hg. Record the vacuum gauge measurement along with the leak rate observed on the train system.
8.1.1.12.1 If the leak rate does not exceed 0.02 cfm, the results are acceptable and no sample volume correction is necessary.
8.1.1.12.2 If, however, a higher leak rate is obtained (>0.02 cfm), the tester shall either record the leakage rate and correct the sample volume as shown in Section 12.3 of Method 5, or void the sampling run and initiate a replacement run. After completing the leak-check, slowly release the vacuum at the first container while the pump is still operating. Afterwards, switch-off the pump.
8.1.2 Sample Train Operation.
8.1.2.1 Data Recording. Record all pertinent process and sampling data on the data sheet (see Figure 306A-3). Ensure that the process operation is suitable for sample collection.
8.1.2.2 Starting the Test. Place the probe/nozzle into the duct at the first sampling point and switch on the pump. Start the sampling using the time interval calculated for the first point. When the first point sampling time has been completed, move to the second point and continue to sample for the time interval calculated for that point; sample each point on the traverse in this manner. Maintain ice around the sample containers during the run.
8.1.2.3 Critical Flow. The sample line between the critical orifice and the pump must operate at a vacuum of ≥380 mm Hg (≥15 in. Hg) in order for critical flow to be maintained. This vacuum must be monitored and documented using the vacuum gauge located between the critical orifice and the pump.
Note:
Theoretically, critical flow for air occurs when the ratio of the orifice outlet absolute pressure to the orifice inlet absolute pressure is less than a factor of 0.53. This means that the system vacuum should be at least ≥356 mm Hg (≥14 in. Hg) at sea level and ∼ 305 mm Hg (∼ 12 in. Hg) at higher elevations.
8.1.2.4 Completion of Test.
8.1.2.4.1 Circular Stacks. Complete the first port traverse and switch off the pump. Testers may opt to perform a leak-check between the port changes to verify the leak rate however, this is not mandatory. Move the sampling train to the next sampling port and repeat the sequence. Be sure to record the final dry gas meter reading after completing the test run. After performing the post test leak check, disconnect the jar/impinger containers from the pump and meter assembly and transport the probe, connecting tubing, and containers to the sample recovery area.
8.1.2.4.2 Rectangle Stacks. Complete each port traverse as per the instructions provided in 8.1.2.4.1.
Note:
If an approximate mass emission rate is to be calculated, measure and record the stack velocity pressure and temperature before and after the test run.
8.2 Sample Recovery. After the train has been transferred to the sample recovery area, disconnect the tubing that connects the jar/impingers. The tester shall select either the total Cr or Cr+6 sample recovery option. Samples to be analyzed for both total Cr and Cr+6 shall be recovered using the Cr+6 sample option (Section 8.2.2).
Note:
Collect a reagent blank sample for each of the total Cr or the Cr+6 analytical options. If both analyses (Cr and Cr+6) are to be conducted on the samples, collect separate reagent blanks for each analysis. Also, since particulate matter is not usually present at chromium electroplating and/or chromium anodizing operations, it is not necessary to filter the Cr+6 samples unless there is observed sediment in the collected solutions. If it is necessary to filter the Cr+6 solutions, please refer to Method 0061, Determination of Hexavalent Chromium Emissions from Stationary Sources, Section 7.4, Sample Preparation in SW-846 (see Reference 1).
8.2.1 Total Cr Sample Option.
8.2.1.1 Shipping Container No. 1. The first jar container may either be used to store and transport the sample, or if GS impingers are used, samples may be stored and shipped in precleaned 250-mL, 500-mL or 1000-mL polyethylene or glass bottles with leak-free, non-metal screw caps.
8.2.1.1.1 Unscrew the lid from the first jar/impinger container.
8.2.1.1.2 Lift the inner tube assembly almost out of the container, and using the wash bottle containing fresh absorbing solution, rinse the outside of the tube that was immersed in the container solution; rinse the inside of the tube as well, by rinsing twice from the top of the tube down through the inner tube into the container.
8.2.1.2 Recover the contents of the second jar/impinger container by removing the lid and pouring any contents into the first shipping container.
8.2.1.2.1 Rinse twice, using fresh absorbing solution, the inner walls of the second container including the inside and outside of the inner tube.
8.2.1.2.2 Rinse the connecting tubing between the first and second sample containers with absorbing solution and place the rinses into the first container.
8.2.1.3 Position the nozzle, probe and connecting plastic tubing in a vertical position so that the tubing forms a “U”.
8.2.1.3.1 Using the wash bottle, partially fill the tubing with fresh absorbing solution. Raise and lower the end of the plastic tubing several times to allow the solution to contact the internal surfaces. Do not allow the solution to overflow or part of the sample will be lost. Place the nozzle end of the probe over the mouth of the first container and elevate the plastic tubing so that the solution flows into the sample container.
8.2.1.3.2 Repeat the probe/tubing sample recovery procedure but allow the solution to flow out the opposite end of the plastic tubing into the sample container. Repeat the entire sample recovery procedure once again.
8.2.1.4 Use approximately 200 to 300 mL of the 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution during the rinsing of the probe nozzle, probe liner, sample containers, and connecting tubing.
8.2.1.5 Place a piece of clear plastic wrap over the mouth of the sample jar to seal the shipping container. Use a standard lid and band assembly to seal and secure the sample in the jar.
8.2.1.5.1 Label the jar clearly to identify its contents, sample number and date.
8.2.1.5.2 Mark the height of the liquid level on the container to identify any losses during shipping and handling.
8.2.1.5.3 Prepare a chain-of-custody sheet to accompany the sample to the laboratory.
8.2.2 Cr+6 Sample Option.
8.2.2.1 Shipping Container No. 1. The first jar container may either be used to store and transport the sample, or if GS impingers are used, samples may be stored and shipped in precleaned 250-mL, 500-mL or 1000-mL polyethylene or glass bottles with leak-free non-metal screw caps.
8.2.2.1.1 Unscrew and remove the lid from the first jar container.
8.2.2.1.2 Measure and record the pH of the solution in the first container by using a pH indicator strip. The pH of the solution must be ≥8.5 for NaOH and ≥8.0 for NaHCO3. If not, discard the collected sample, increase the concentration of the NaOH or NaHCO3 absorbing solution to 0.5 M and collect another air emission sample.
8.2.2.2 After measuring the pH of the first container, follow sample recovery procedures described in Sections 8.2.1.1 through 8.2.1.5.
Note:
Since particulate matter is not usually present at chromium electroplating and/or chromium anodizing facilities, it is not necessary to filter the Cr+6 samples unless there is observed sediment in the collected solutions. If it is necessary to filter the Cr+6 solutions, please refer to the EPA Method 0061, Determination of Hexavalent Chromium Emissions from Stationary Sources, Section 7.4, Sample Preparation in SW-846 (see Reference 5) for procedure.
8.2.3 Silica Gel Container. Observe the color of the indicating silica gel to determine if it has been completely spent and make a notation of its condition/color on the field data sheet. Do not use water or other liquids to remove and transfer the silica gel.
8.2.4 Total Cr and/or Cr+6 Reagent Blank.
8.2.4.1 Shipping Container No. 2. Place approximately 500 mL of the 0.1 N NaOH or 0.1 N NaHCO3 absorbing solution in a precleaned, labeled sample container and include with the field samples for analysis.
8.3 Sample Preservation, Storage, and Transport.
8.3.1 Total Cr Option. Samples that are to be analyzed for total Cr need not be refrigerated.
8.3.2 Cr+6 Option. Samples that are to be analyzed for Cr+6 must be shipped and stored at 4°C (∼40°F).
Note:
Allow Cr+6 samples to return to ambient temperature prior to analysis.
8.4 Sample Holding Times.
8.4.1 Total Cr Option. Samples that are to be analyzed for total chromium must be analyzed within 60 days of collection.
8.4.2 Cr+6 Option. Samples that are to be analyzed for Cr+6 must be analyzed within 14 days of collection.
9.0 Quality Control
9.1 Same as Method 306, Section 9.0.
10.0 Calibration and Standardization
Note:
Tester shall maintain a performance log of all calibration results.
10.1 Pitot Tube. The Type S pitot tube assembly shall be calibrated according to the procedures outlined in Section 10.1 of Method 2.
10.2 Temperature Sensor. Use the procedure in Section 10.3 of Method 2 to calibrate the in-stack temperature sensor.
10.3 Metering System.
10.3.1 Sample Train Dry Gas Meter Calibration. Calibrations may be performed as described in Section 16.2 of Method 5 by either the manufacturer, a firm who provides calibration services, or the tester.
10.3.2 Dry Gas Meter Calibration Coefficient (Ym). The meter calibration coefficient (Ym) must be determined prior to the initial use of the meter, and following each field test program. If the dry gas meter is new, the manufacturer will have specified the Ym value for the meter. This Ym value can be used as the pretest value for the first test. For subsequent tests, the tester must use the Ym value established during the pretest calibration.
10.3.3 Calibration Orifice. The manufacturer may have included a calibration orifice and a summary spreadsheet with the meter that may be used for calibration purposes. The spreadsheet will provide data necessary to determine the calibration for the orifice and meter (standard cubic feet volume, sample time, etc.). These data were produced when the initial Ym value was determined for the meter.
10.3.4 Ym Meter Value Verification or Meter Calibration.
10.3.4.1 The Ym meter value may be determined by replacing the sampling train critical orifice with the calibration orifice. Replace the critical orifice assembly by installing the calibration orifice in the same location. The inlet side of the calibration orifice is to be left open to the atmosphere and is not to be reconnected to the sample train during the calibration procedure.
10.3.4.2 If the vacuum pump is cold, switch on the pump and allow it to operate (become warm) for several minutes prior to starting the calibration. After stopping the pump, record the initial dry gas meter volume and meter temperature.
10.3.4.3 Perform the calibration for the number of minutes specified by the manufacturer's data sheet (usually 5 minutes). Stop the pump and record the final dry gas meter volume and temperature. Subtract the start volume from the stop volume to obtain the Vm and average the meter temperatures (tm).
10.3.5 Ym Value Calculation. Ym is the calculated value for the dry gas meter. Calculate Ym using the following equation:

Where:
Pbar = Barometric pressure at meter, mm Hg, (in. Hg).
Pstd = Standard absolute pressure,
Metric = 760 mm Hg.
English = 29.92 in. Hg.
tm = Average dry gas meter temperature,°C, (°F).
Tm = Absolute average dry gas meter temperature,
Metric°K = 273 + tm (°C).
English°R = 460 + tm (°F).
Tstd = Standard absolute temperature,
Metric = 293°K.
English = 528°R.
Vm = Volume of gas sample as measured (actual) by dry gas meter, dcm,(dcf).
Vm(std),mfg = Volume of gas sample measured by manufacture's calibrated orifice and dry gas meter, corrected to standard conditions (pressure/temperature) dscm (dscf).
Ym = Dry gas meter calibration factor, (dimensionless).
10.3.6 Ym Comparison. Compare the Ym value provided by the manufacturer (Section 10.3.3) or the pretest Ym value to the post test Ym value using the following equation:

10.3.6.1 If this ratio is between 0.95 and 1.05, the designated Ym value for the meter is acceptable for use in later calculations.
10.3.6.1.1 If the value is outside the specified range, the test series shall either be: 1) voided and the samples discarded; or 2) calculations for the test series shall be conducted using whichever meter coefficient value (i.e., manufacturers's/pretest Ym value or post test Ym value) produces the lowest sample volume.
10.3.6.1.2 If the post test dry gas meter Ym value differs by more than 5% as compared to the pretest value, either perform the calibration again to determine acceptability or return the meter to the manufacturer for recalibration.
10.3.6.1.3 The calibration may also be conducted as specified in Section 10.3 or Section 16.0 of Method 5 (40 CFR Part 60, Appendix A), except that it is only necessary to check the calibration at one flow rate of ∼ 0.75 cfm.
10.3.6.1.4 The calibration of the dry gas meter must be verified after each field test program using the same procedures.
Note:
The tester may elect to use the Ym post test value for the next pretest Ym value; e.g., Test 1 post test Ym value and Test 2 pretest Ym value would be the same.
10.4 Barometer. Calibrate against a mercury barometer that has been corrected for temperature and elevation.
10.5 ICP Spectrometer Calibration. Same as Method 306, Section 10.2.
10.6 GFAA Spectrometer Calibration. Same as Method 306, Section 10.3.
10.7 IC/PCR Calibration. Same as Method 306, Section 10.4.
11.0 Analytical Procedures
Note:
The method determines the chromium concentration in µg Cr/mL. It is important that the analyst measure the volume of the field sample prior to analyzing the sample. This will allow for conversion of µg Cr/mL to µg Cr/sample.
11.1 Analysis. Refer to Method 306 for sample preparation and analysis procedures.
12.0 Data Analysis and Calculations
12.1 Calculations. Perform the calculations, retaining one extra decimal point beyond that of the acquired data. When reporting final results, round number of figures consistent with the original data.
12.2 Nomenclature.
A = Cross-sectional area of stack, m2 (ft2).
Bws = Water vapor in gas stream, proportion by volume, dimensionless (assume 2 percent moisture = 0.02).
Cp = Pitot tube coefficient; “S” type pitot coefficient usually 0.840, dimensionless.
CS = Concentration of Cr in sample solution, µg Cr/mL.
CCr = Concentration of Cr in stack gas, dry basis, corrected to standard conditions µg/dscm (gr/dscf).
d = Diameter of stack, m (ft).
D = Digestion factor, dimensionless.
ER = Approximate mass emission rate, mg/hr (lb/hr).
F = Dilution factor, dimensionless.
L = Length of a square or rectangular duct, m (ft).
MCr = Total Cr in each sample, µg (gr).
Ms = Molecular weight of wet stack gas, wet basis, g/g-mole, (lb/lb-mole); in a nominal gas stream at 2% moisture the value is 28.62.
Pbar = Barometric pressure at sampling site, mm Hg (in. Hg).
Ps = Absolute stack gas pressure; in this case, usually the same value as the barometric pressure, mm Hg (in. Hg).
Pstd = Standard absolute pressure:
Metric = 760 mm Hg.
English = 29.92 in. Hg.
Qstd = Average stack gas volumetric flow, dry, corrected to standard conditions, dscm/hr (dscf/hr).
tm = Average dry gas meter temperature,°C (°F).
Tm = Absolute average dry gas meter temperature:
Metric°K = 273 + tm (°C).
English°R = 460 + tm (°F).
ts = Average stack temperature,°C (°F).
Ts = Absolute average stack gas temperature: Metric°K = 273 + ts (°C). English°R = 460 + ts (°F).
Tstd = Standard absolute temperature: Metric = 293°K. English = 528°R.
Vad = Volume of sample aliquot after digestion (mL).
Vaf = Volume of sample aliquot after dilution (mL).
Vbd = Volume of sample aliquot submitted to digestion (mL).
Vbf = Volume of sample aliquot before dilution (mL).
Vm = Volume of gas sample as measured (actual, dry) by dry gas meter, dcm (dcf).
VmL = Volume of impinger contents plus rinses (mL).
Vm(std) = Volume of gas sample measured by the dry gas meter, corrected to standard conditions (temperature/pressure), dscm (dscf).
vs = Stack gas average velocity, calculated by Method 2, Equation 2-9, m/sec (ft/sec).
W = Width of a square or rectangular duct, m (ft).
Ym = Dry gas meter calibration factor, (dimensionless).
Δp = Velocity head measured by the Type S pitot tube, cm H2O (in. H2O).
Δpavg = Average of Δp values, mm H2O (in. H2O).
12.3 Dilution Factor. The dilution factor is the ratio of the volume of sample aliquot after dilution to the volume before dilution. The dilution factor is usually calculated by the laboratory. This ratio is derived by the following equation:

12.4 Digestion Factor. The digestion factor is the ratio of the volume of sample aliquot after digestion to the volume before digestion. The digestion factor is usually calculated by the laboratory. This ratio is derived by the following equation.

12.5 Total Cr in Sample. Calculate MCr, the total µg Cr in each sample, using the following equation:

12.6 Dry Gas Volume. Correct the sample volume measured by the dry gas meter to standard conditions (20°C, 760 mm Hg or 68°F, 29.92 in. Hg) using the following equation:

Where:
K1 = Metric units - 0.3855°K/mm Hg.
English units - 17.64°R/in. Hg.
12.7 Cr Emission Concentration (CCr). Calculate CCr, the Cr concentration in the stack gas, in µg/dscm (µg/dscf) on a dry basis, corrected to standard conditions, using the following equation:

Note:
To convert µg/dscm (µg/dscf) to mg/dscm (mg/dscf), divide by 1000.
12.8 Stack Gas Velocity.
12.8.1 Kp = Velocity equation constant:


12.8.2 Average Stack Gas Velocity.

12.9 Cross sectional area of stack.

12.10 Average Stack Gas Dry Volumetric Flow Rate.
Note:
The emission rate may be based on a nominal stack moisture content of 2 percent (0.02). To calculate an emission rate, the tester may elect to use either the nominal stack gas moisture value or the actual stack gas moisture collected during the sampling run.
Volumetric Flow Rate Equation:

Where:
3600 = Conversion factor, sec/hr.

Note:
To convert Qstd from dscm/hr (dscf/hr) to dscm/min (dscf/min), divide Qstd by 60.
12.11 Mass emission rate, mg/hr (lb/hr):


13.0 Method Performance
13.1 Range. The recommended working range for all of the three analytical techniques starts at five times the analytical detection limit (see also Method 306, Section 13.2.2). The upper limit of all three techniques can be extended indefinitely by appropriate dilution.
13.2 Sensitivity.
13.2.1 Analytical Sensitivity. The estimated instrumental detection limits listed are provided as a guide for an instrumental limit. The actual method detection limits are sample and instrument dependent and may vary as the sample matrix varies.
13.2.1.1 ICP Analytical Sensitivity. The minimum estimated detection limits for ICP, as reported in Method 6010A and the recently revised Method 6010B of SW-846 (Reference 1), are 7.0 µg Cr/L and 4.7 µg Cr/L, respectively.
13.2.1.2 GFAAS Analytical Sensitivity. The minimum estimated detection limit for GFAAS, as reported in Methods 7000A and 7191 of SW-846 (Reference 1), is 1.0 µg Cr/L.
13.2.1.3 IC/PCR Analytical Sensitivity. The minimum detection limit for IC/PCR with a preconcentrator, as reported in Methods 0061 and 7199 of SW-846 (Reference 1), is 0.05 µg Cr+6/L.
13.2.2 In-stack Sensitivity. The in-stack sensitivity depends upon the analytical detection limit, the volume of stack gas sampled, and the total volume of the impinger absorbing solution plus the rinses. Using the analytical detection limits given in Sections 13.2.1.1, 13.2.1.2, and 13.2.1.3; a stack gas sample volume of 1.7 dscm; and a total liquid sample volume of 500 mL; the corresponding in-stack detection limits are 0.0014 mg Cr/dscm to 0.0021 mg Cr/dscm for ICP, 0.00029 mg Cr/dscm for GFAAS, and 0.000015 mg Cr+36/dscm for IC/PCR with preconcentration.
Note:
It is recommended that the concentration of Cr in the analytical solutions be at least five times the analytical detection limit to optimize sensitivity in the analyses. Using this guideline and the same assumptions for impinger sample volume and stack gas sample volume (500 mL and 1.7 dscm, respectively), the recommended minimum stack concentrations for optimum sensitivity are 0.0068 mg Cr/dscm to 0.0103 mg Cr/dscm for ICP, 0.0015 mg Cr/dscm for GFAAS, and 0.000074 mg Cr+6 dscm for IC/PCR with preconcentration. If required, the in-stack detection limits can be improved by either increasing the sampling time, the stack gas sample volume, reducing the volume of the digested sample for GFAAS, improving the analytical detection limits, or any combination of the three.
13.3 Precision.
13.3.1 The following precision data have been reported for the three analytical methods. In each case, when the sampling precision is combined with the reported analytical precision, the resulting overall precision may decrease.
13.3.2 Bias data is also reported for GFAAS.
13.4 ICP Precision.
13.4.1 As reported in Method 6010B of SW-846 (Reference 1), in an EPA round-robin Phase 1 study, seven laboratories applied the ICP technique to acid/distilled water matrices that had been spiked with various metal concentrates. For true values of 10, 50, and 150 µg Cr/L; the mean reported values were 10, 50, and 149 µg Cr/L; and the mean percent relative standard deviations were 18, 3.3, and 3.8 percent, respectively.
13.4.2 In another multilaboratory study cited in Method 6010B, a mean relative standard of 8.2 percent was reported for an aqueous sample concentration of approximately 3750 µg Cr/L.
13.5 GFAAS Precision. As reported in Method 7191 of SW-846 (Reference 1), in a single laboratory (EMSL), using Cincinnati, Ohio tap water spiked at concentrations of 19, 48, and 77 µg Cr/L, the standard deviations were ±0.1, ±0.2, and ±0.8, respectively. Recoveries at these levels were 97 percent, 101 percent, and 102 percent, respectively.
13.6 IC/PCR Precision. As reported in Methods 0061 and 7199 of SW-846 (Reference 1), the precision of IC/PCR with sample preconcentration is 5 to 10 percent; the overall precision for sewage sludge incinerators emitting 120 ng/dscm of Cr+6 and 3.5 µg/dscm of total Cr is 25 percent and 9 percent, respectively; and for hazardous waste incinerators emitting 300 ng/dscm of Cr+6 the precision is 20 percent.
14.0 Pollution Prevention
14.1 The only materials used in this method that could be considered pollutants are the chromium standards used for instrument calibration and acids used in the cleaning of the collection and measurement containers/labware, in the preparation of standards, and in the acid digestion of samples. Both reagents can be stored in the same waste container.
14.2 Cleaning solutions containing acids should be prepared in volumes consistent with use to minimize the disposal of excessive volumes of acid.
14.3 To the extent possible, the containers/vessels used to collect and prepare samples should be cleaned and reused to minimize the generation of solid waste.
15.0 Waste Management
15.1 It is the responsibility of the laboratory and the sampling team to comply with all federal, state, and local regulations governing waste management, particularly the discharge regulations, hazardous waste identification rules, and land disposal restrictions; and to protect the air, water, and land by minimizing and controlling all releases from field operations.
15.2 For further information on waste management, consult The Waste Management Manual for Laboratory Personnel and Less is Better-Laboratory Chemical Management for Waste Reduction, available from the American Chemical Society's Department of Government Relations and Science Policy, 1155 16th Street NW, Washington, DC 20036.
16.0 References
1. F.R. Clay, Memo, Impinger Collection Efficiency - Mason Jars vs. Greenburg-Smith Impingers, Dec. 1989.
2. Segall, R.R., W.G. DeWees, F.R. Clay, and J.W. Brown. Development of Screening Methods for Use in Chromium Emissions Measurement and Regulations Enforcement. In: Proceedings of the 1989 EPA/A&WMA International Symposium-Measurement of Toxic and Related Air Pollutants, A&WMA Publication VIP-13, EPA Report No. 600/9-89-060, p. 785.
3. Clay, F.R., Chromium Sampling Method. In: Proceedings of the 1990 EPA/A&WMA International Symposium-Measurement of Toxic and Related Air Pollutants, A&WMA Publication VIP-17, EPA Report No. 600/9-90-026, p. 576.
4. Clay, F.R., Proposed Sampling Method 306A for the Determination of Hexavalent Chromium Emissions from Electroplating and Anodizing Facilities. In: Proceedings of the 1992 EPA/A&WMA International Symposium-Measurement of Toxic and Related Air Pollutants, A&WMA Publication VIP-25, EPA Report No. 600/R-92/131, p. 209.
5. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846, Third Edition as amended by Updates I, II, IIA, IIB, and III. Document No. 955-001-000001. Available from Superintendent of Documents, U.S. Government Printing Office, Washington, DC, November 1986.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
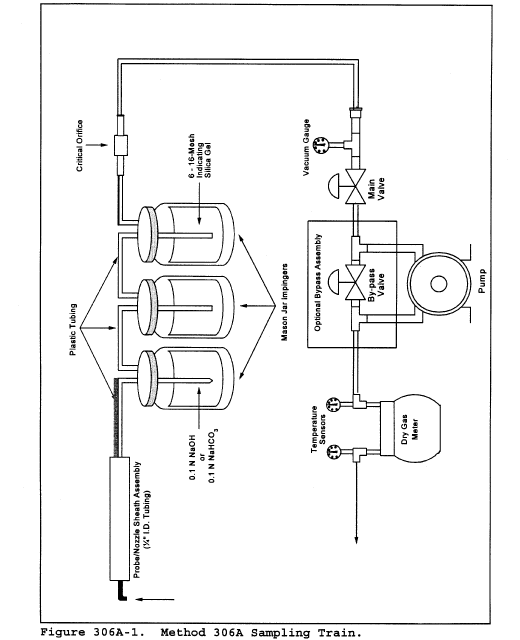


Method 306B - Surface Tension Measurement for Tanks Used at Decorative Chromium Electroplating and Chromium Anodizing Facilities
Note:
This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in 40 CFR Part 60, Appendix A and in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least Methods 5 and 306.
1.0 Scope and Application
1.1 Analyte. Not applicable.
1.2 Applicability. This method is applicable to all chromium electroplating and chromium anodizing operations, and continuous chromium plating at iron and steel facilities where a wetting agent is used in the tank as the primary mechanism for reducing emissions from the surface of the plating solution.
2.0 Summary of Method
2.1 During an electroplating or anodizing operation, gas bubbles generated during the process rise to the surface of the liquid and burst. Upon bursting, tiny droplets of chromic acid become entrained in ambient air. The addition of a wetting agent to the tank bath reduces the surface tension of the liquid and diminishes the formation of these droplets.
2.2 This method determines the surface tension of the bath using a stalagmometer or a tensiometer to confirm that there is sufficient wetting agent present.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method may not address all of the safety problems associated with its use. It is the responsibility of the user to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to performing this test method.
6.0 Equipment and Supplies
6.1 Stalagmometer. Any commercially available stalagmometer or equivalent surface tension measuring device may be used to measure the surface tension of the plating or anodizing tank liquid provided the procedures specified in Section 11.1.2 are followed.
6.2 Tensiometer. A tensiometer may be used to measure the surface tension of the tank liquid provided the procedures specified in ASTM Method D 1331-89, Standard Test Methods for Surface and Interfacial Tension of Solutions of Surface Active Agents (incorporated by reference - see §63.14) are followed.
7.0 Reagents and Standards [Reserved]
8.0 Sample Collection, Sample Recovery, Sample Preservation, Sample Holding Times, Storage, and Transport [Reserved]
9.0 Quality Control [Reserved]
10.0 Calibration and Standardization [Reserved]
11.0 Analytical Procedure
11.1 Procedure. The surface tension of the tank bath may be measured using a tensiometer, stalagmometer, or any other equivalent surface tension measuring device for measuring surface tension in dynes per centimeter.
11.1.1 If a tensiometer is used, the procedures specified in ASTM Method D 1331-89 must be followed.
11.1.2 If a stalagmometer is used, the procedures specified in Sections 11.1.2.1 through 11.1.2.3 must be followed.
11.1.2.1 Check the stalagmometer for visual signs of damage. If the stalagmometer appears to be chipped, cracked, or otherwise in disrepair, the instrument shall not be used.
11.1.2.2 Using distilled or deionized water and following the procedures provided by the manufacturer, count the number of drops corresponding to the distilled/deionized water liquid volume between the upper and lower etched marks on the stalagmometer. If the number of drops for the distilled/deionized water is not within ±1 drop of the number indicated on the instrument, the stalagmometer must be cleaned, using the procedures specified in Section 11.1.3 of this method, before using the instrument to measure the surface tension of the tank liquid.
11.1.2.2.1 If the stalagmometer must be cleaned, as indicated in Section 11.1.2.2, repeat the procedure specified in Section 11.1.2.2 before proceeding.
11.1.2.2.2 If, after cleaning and performing the procedure in Section 11.1.2.2, the number of drops indicated for the distilled/deionized water is not within ±1 drop of the number indicated on the instrument, either use the number of drops corresponding to the distilled/deionized water volume as the reference number of drops, or replace the instrument.
11.1.2.3 Determine the surface tension of the tank liquid using the procedures specified by the manufacturer of the stalagmometer.
11.1.3 Stalagmometer cleaning procedures. The procedures specified in Sections 11.1.3.1 through 11.1.3.10 shall be used for cleaning a stalagmometer, as required by Section 11.1.2.2.
11.1.3.1 Set up the stalagmometer on its stand in a fume hood.
11.1.3.2 Place a clean 150 (mL) beaker underneath the stalagmometer and fill the beaker with reagent grade concentrated nitric acid.
11.1.3.3 Immerse the bottom tip of the stalagmometer (approximately 1 centimeter (0.5 inches)) into the beaker.
11.1.3.4 Squeeze the rubber bulb and pinch at the arrow up (1) position to collapse.
11.1.3.5 Place the bulb end securely on top end of stalagmometer and carefully draw the nitric acid by pinching the arrow up (1) position until the level is above the top etched line.
11.1.3.6 Allow the nitric acid to remain in stalagmometer for 5 minutes, then carefully remove the bulb, allowing the acid to completely drain.
11.1.3.7 Fill a clean 150 mL beaker with distilled or deionized water.
11.1.3.8 Using the rubber bulb per the instructions in Sections 11.1.3.4 and 11.1.3.5, rinse and drain stalagmometer with deionized or distilled water.
11.1.3.9 Fill a clean 150 mL beaker with isopropyl alcohol.
11.1.3.10 Again using the rubber bulb per the instructions in Sections 11.1.3.4 and 11.1.3.5, rinse and drain stalagmometer twice with isopropyl alcohol and allow the stalagmometer to dry completely.
11.2 Frequency of Measurements.
11.2.1 Measurements of the bath surface tension are performed using a progressive system which decreases the frequency of surface tension measurements required when the proper surface tension is maintained.
11.2.1.1 Initially, following the compliance date, surface tension measurements must be conducted once every 4 hours of tank operation for the first 40 hours of tank operation.
11.2.1.2 Once there are no exceedances during a period of 40 hours of tank operation, measurements may be conducted once every 8 hours of tank operation.
11.2.1.3 Once there are no exceedances during a second period of 40 consecutive hours of tank operation, measurements may be conducted once every 40 hours of tank operation on an on-going basis, until an exceedance occurs. The maximum time interval for measurements is once every 40 hours of tank operation.
11.2.2 If a measurement of the surface tension of the solution is above the 40 dynes per centimeter limit when measured using a stalagmometer, above 33 dynes per centimeter when measured using a tensiometer, or above an alternate surface tension limit established during the performance test, the time interval shall revert back to the original monitoring schedule of once every 4 hours. A subsequent decrease in frequency would then be allowed according to Section 11.2.1.
12.0 Data Analysis and Calculations
12.1 Log Book of Surface Tension Measurements and Fume Suppressant Additions.
12.1.1 The surface tension of the plating or anodizing tank bath must be measured as specified in Section 11.2.
12.1.2 The measurements must be recorded in the log book. In addition to the record of surface tension measurements, the frequency of fume suppressant maintenance additions and the amount of fume suppressant added during each maintenance addition must be recorded in the log book.
12.1.3 The log book will be readily available for inspection by regulatory personnel.
12.2 Instructions for Apparatus Used in Measuring Surface Tension.
12.2.1 Included with the log book must be a copy of the instructions for the apparatus used for measuring the surface tension of the plating or anodizing bath.
12.2.2 If a tensiometer is used, a copy of ASTM Method D 1331-89 must be included with the log book.
13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References [Reserved]
17.0 Tables, Diagrams, Flowcharts, and Validation Data [Reserved]
Method 307 - Determination of Emissions From Halogenated Solvent Vapor Cleaning Machines Using a Liquid Level Procedure
1. Applicability and Principle
1.1 Applicability. This method is applicable to the determination of the halogenated solvent emissions from solvent vapor cleaners in the idling mode.
1.2 Principle. The solvent level in the solvent cleaning machine is measured using inclined liquid level indicators. The change in liquid level corresponds directly to the amount of solvent lost from the solvent cleaning machine.
2. Apparatus
Note:
Mention of trade names or specific products does not constitute endorsement by the Environmental Protection Agency.
2.1 Inclined Liquid Level Indicator. A schematic of the inclined liquid level indicators used in this method is shown in figure 307-1; two inclined liquid level indicators having 0.05 centimeters divisions or smaller shall be used. The liquid level indicators shall be made of glass, Teflon, or any similar material that will not react with the solvent being used. A 6-inch by 1-inch slope is recommended; however the slope may vary depending on the size and design of the solvent cleaning machine.
Note:
It is important that the inclined liquid level indicators be constructed with ease of reading in mind. The inclined liquid level indicators should also be mounted so that they can be raised or lowered if necessary to suit the solvent cleaning machine size.
2.2 Horizontal Indicator. Device to check the inclined liquid level indicators orientation relative to horizontal.
2.3 Velocity Meter. Hotwire and vane anemometers, or other devices capable of measuring the flow rates ranging from 0 to 15.2 meters per minute across the solvent cleaning machine.
3. Procedure
3.1 Connection of the Inclined Liquid Level Indicator. Connect one of the inclined liquid level indicators to the boiling sump drain and the other inclined liquid level indicator to the immersion sump drain using Teflon tubing and the appropriate fittings. A schematic diagram is shown in figure 307-2.
3.2 Positioning of Velocity Meter. Position the velocity meter so that it measures the flow rate of the air passing directly across the solvent cleaning machine.
3.3 Level the Inclined Liquid Level Indicators.
3.4 Initial Inclined Liquid Level Indicator Readings. Open the sump drainage valves. Allow the solvent cleaning machine to operate long enough for the vapor zone to form and the system to stabilize (check with manufacturer). Record the inclined liquid level indicators readings and the starting time on the data sheet. A sample data sheet is provided in figure 307-3.
Date
Run
Solvent type
Solvent density, g/m 3 (lb/ft 3)
Length of boiling sump (SB), m (ft)
Width of boiling sump (WB), m (ft)
Length of immersion sump (SI), m (ft)
Width of immersion sump (WI), m (ft)
Length of solvent vapor/air interface (SV), m (ft) ______
Width of solvent vapor/air interface (WV), m (ft) ______
| Clock time | Boiling sump reading | Immersion sump reading | Flow rate reading |
|---|---|---|---|
Figure 307-3. Data sheet.
3.5 Final Inclined Liquid Level Indicator Readings. At the end of the 16-hour test run, check to make sure the inclined liquid level indicators are level; if not, make the necessary adjustments. Record the final inclined liquid level indicators readings and time.
3.6 Determination of Solvent Vapor/Air Interface Area for Each Sump. Determine the area of the solvent/air interface of the individual sumps. Whenever possible, physically measure these dimensions, rather than using factory specifications. A schematic of the dimensions of a solvent cleaning machine is provided in figure 307-4.
4. Calculations
4.1 Nomenclature.
AB = area of boiling sump interface, m 2 (ft 2).
AI = area of immersion sump interface, m 2 (ft 2).
AV = area of solvent/air interface, m 2 (ft 2).
E = emission rate, kg/m 2-hr (lb/ft 2-hr).
K = 100,000 cm . g/m . kg for metric units.
= 12 in./ft for English units.
LBF = final boiling sump inclined liquid level indicators reading, cm (in.).
LBi = initial boiling sump inclined liquid level indicators reading, cm (in.).
LIf = final immersion sump inclined liquid level indicators reading, cm (in.).
LIi = initial immersion sump inclined liquid level indicators reading, cm (in.).
SB = length of the boiling sump, m (ft).
SI = length of the immersion sump, m (ft).
SV = length of the solvent vapor/air interface, m (ft).
WB = width of the boiling sump, m (ft).
WI = width of the immersion sump, m (ft).
WV = width of the solvent vapor/air interface, m (ft).
ρ = density of solvent, g/m3 (lb/ft3).
θ = test time, hr.
4.2 Area of Sump Interfaces. Calculate the areas of the boiling and immersion sump interfaces as follows:
AB = SB WB Eq. 307-1
AI = SI WI Eq. 307-2
4.3 Area of Solvent/Air Interface. Calculate the area of the solvent vapor/air interface as follows:
AV = SV WV Eq. 307-3
4.4 Emission Rate. Calculate the emission rate as follows:

Method 308 - Procedure for Determination of Methanol Emission From Stationary Sources
1.0 Scope and Application
1.1 Analyte. Methanol. Chemical Abstract Service (CAS) No. 67-56-1.
1.2 Applicability. This method applies to the measurement of methanol emissions from specified stationary sources.
2.0 Summary of Method
A gas sample is extracted from the sampling point in the stack. The methanol is collected in deionized distilled water and adsorbed on silica gel. The sample is returned to the laboratory where the methanol in the water fraction is separated from other organic compounds with a gas chromatograph (GC) and is then measured by a flame ionization detector (FID). The fraction adsorbed on silica gel is extracted with deionized distilled water and is then separated and measured by GC/FID.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
5.1 Disclaimer. This method may involve hazardous materials, operations, and equipment. This test method does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and to determine the applicability of regulatory limitations before performing this test method.
5.2 Methanol Characteristics. Methanol is flammable and a dangerous fire and explosion risk. It is moderately toxic by ingestion and inhalation.
6.0 Equipment and Supplies
6.1 Sample Collection. The following items are required for sample collection:
6.1.1 Sampling Train. The sampling train is shown in Figure 308-1 and component parts are discussed below.
6.1.1.1 Probe. Teflon ®, approximately 6-millimeter (mm) (0.24 inch) outside diameter.
6.1.1.2 Impinger. A 30-milliliter (ml) midget impinger. The impinger must be connected with leak-free glass connectors. Silicone grease may not be used to lubricate the connectors.
6.1.1.3 Adsorbent Tube. Glass tubes packed with the required amount of the specified adsorbent.
6.1.1.4 Valve. Needle valve, to regulate sample gas flow rate.
6.1.1.5 Pump. Leak-free diaphragm pump, or equivalent, to pull gas through the sampling train. Install a small surge tank between the pump and rate meter to eliminate the pulsation effect of the diaphragm pump on the rotameter.
6.1.1.6 Rate Meter. Rotameter, or equivalent, capable of measuring flow rate to within 2 percent of the selected flow rate of up to 1000 milliliter per minute (ml/min). Alternatively, the tester may use a critical orifice to set the flow rate.
6.1.1.7 Volume Meter. Dry gas meter (DGM), sufficiently accurate to measure the sample volume to within 2 percent, calibrated at the selected flow rate and conditions actually encountered during sampling, and equipped with a temperature sensor (dial thermometer, or equivalent) capable of measuring temperature accurately to within 3°C (5.4°F).
6.1.1.8 Barometer. Mercury (Hg), aneroid, or other barometer capable of measuring atmospheric pressure to within 2.5 mm (0.1 inch) Hg. See the NOTE in Method 5 (40 CFR part 60, appendix A), section 6.1.2.
6.1.1.9 Vacuum Gauge and Rotameter. At least 760-mm (30-inch) Hg gauge and 0- to 40-ml/min rotameter, to be used for leak-check of the sampling train.
6.2 Sample Recovery. The following items are required for sample recovery:
6.2.1 Wash Bottles. Polyethylene or glass, 500-ml, two.
6.2.2 Sample Vials. Glass, 40-ml, with Teflon ®-lined septa, to store impinger samples (one per sample).
6.2.3 Graduated Cylinder. 100-ml size.
6.3 Analysis. The following are required for analysis:
6.3.1 Gas Chromatograph. GC with an FID, programmable temperature control, and heated liquid injection port.
6.3.2 Pump. Capable of pumping 100 ml/min. For flushing sample loop.
6.3.3 Flow Meter. To monitor accurately sample loop flow rate of 100 ml/min.
6.3.4 Regulators. Two-stage regulators used on gas cylinders for GC and for cylinder standards.
6.3.5 Recorder. To record, integrate, and store chromatograms.
6.3.6 Syringes. 1.0- and 10-microliter (l) size, calibrated, for injecting samples.
6.3.7 Tubing Fittings. Stainless steel, to plumb GC and gas cylinders.
6.3.8 Vials. Two 5.0-ml glass vials with screw caps fitted with Teflon ®-lined septa for each sample.
6.3.9 Pipettes. Volumetric type, assorted sizes for preparing calibration standards.
6.3.10 Volumetric Flasks. Assorted sizes for preparing calibration standards.
6.3.11 Vials. Glass 40-ml with Teflon ®-lined septa, to store calibration standards (one per standard).
7.0 Reagents and Standards
Note:
Unless otherwise indicated, all reagents must conform to the specifications established by the Committee on Analytical Reagents of the American Chemical Society. Where such specifications are not available, use the best available grade.
7.1 Sampling. The following are required for sampling:
7.1.1 Water. Deionized distilled to conform to the American Society for Testing and Materials (ASTM) Specification D 1193-77, Type 3. At the option of the analyst, the potassium permanganate (KMnO4) test for oxidizable organic matter may be omitted when high concentrations of organic matter are not expected to be present.
7.1.2 Silica Gel. Deactivated chromatographic grade 20/40 mesh silica gel packed in glass adsorbent tubes. The silica gel is packed in two sections. The front section contains 520 milligrams (mg) of silica gel, and the back section contains 260 mg.
7.2 Analysis. The following are required for analysis:
7.2.1 Water. Same as specified in section 7.1.1.
7.2.2 [Reserved]
7.2.3 Methanol Stock Standard. Prepare a methanol stock standard by weighing 1 gram of methanol into a 100-ml volumetric flask. Dilute to 100 ml with water.
7.2.3.1 Methanol Working Standard. Prepare a methanol working standard by pipetting 1 ml of the methanol stock standard into a 100-ml volumetric flask. Dilute the solution to 100 ml with water.
7.2.3.2 Methanol Standards For Impinger Samples. Prepare a series of methanol standards by pipetting 1, 2, 5, 10, and 25 ml of methanol working standard solution respectively into five 50-ml volumetric flasks. Dilute the solutions to 50 ml with water. These standards will have 2, 4, 10, 20, and 50 µg/ml of methanol, respectively. After preparation, transfer the solutions to 40-ml glass vials capped with Teflon ® septa and store the vials under refrigeration. Discard any excess solution.
7.2.3.3 Methanol Standards for Adsorbent Tube Samples. Prepare a series of methanol standards by first pipetting 10 ml of the methanol working standard into a 100-ml volumetric flask and diluting the contents to exactly 100 ml with deionized distilled water. This standard will contain 10 µg/ml of methanol. Pipette 5, 15, and 25 ml of this standard, respectively, into three 50-ml volumetric flasks. Dilute each solution to 50 ml with deionized distilled water. These standards will have 1, 3, and 5 µg/ml of methanol, respectively. Transfer all four standards into 40-ml glass vials capped with Teflon®-lined septa and store under refrigeration. Discard any excess solution.
7.2.4 GC Column. Capillary column, 30 meters (100 feet) long with an inside diameter (ID) of 0.53 mm (0.02 inch), coated with DB 624 to a film thickness of 3.0 micrometers, (µm) or an equivalent column. Alternatively, a 30-meter capillary column coated with polyethylene glycol to a film thickness of 1 µm such as AT-WAX or its equivalent.
7.2.5 Helium. Ultra high purity.
7.2.6 Hydrogen. Zero grade.
7.2.7 Oxygen. Zero grade.
8.0 Procedure
8.1 Sampling. The following items are required for sampling:
8.1.1 Preparation of Collection Train. Measure 20 ml of water into the midget impinger. The adsorbent tube must contain 520 mg of silica gel in the front section and 260 mg of silica gel in the backup section. Assemble the train as shown in Figure 308-1. An optional, second impinger that is left empty may be placed in front of the water-containing impinger to act as a condensate trap. Place crushed ice and water around the impinger.
8.1.2 Leak Check. A leak check before and after the sampling run is mandatory. The leak-check procedure is as follows:
Temporarily attach a suitable (e.g., 0- to 40-ml/min) rotameter to the outlet of the DGM, and place a vacuum gauge at or near the probe inlet. Plug the probe inlet, pull a vacuum of at least 250 mm (10 inch) Hg or the highest vacuum experienced during the sampling run, and note the flow rate as indicated by the rotameter. A leakage rate in excess of 2 percent of the average sampling rate is acceptable.
Note:
Carefully release the probe inlet plug before turning off the pump.
8.1.3 Sample Collection. Record the initial DGM reading and barometric pressure. To begin sampling, position the tip of the Teflon ® tubing at the sampling point, connect the tubing to the impinger, and start the pump. Adjust the sample flow to a constant rate between 200 and 1000 ml/min as indicated by the rotameter. Maintain this constant rate (±10 percent) during the entire sampling run. Take readings (DGM, temperatures at DGM and at impinger outlet, and rate meter) at least every 5 minutes. Add more ice during the run to keep the temperature of the gases leaving the last impinger at 20°C (68°F) or less. At the conclusion of each run, turn off the pump, remove the Teflon ® tubing from the stack, and record the final readings. Conduct a leak check as in section 8.1.2. (This leak check is mandatory.) If a leak is found, void the test run or use procedures acceptable to the Administrator to adjust the sample volume for the leakage.
8.2 Sample Recovery. The following items are required for sample recovery:
8.2.1 Impinger. Disconnect the impinger. Pour the contents of the midget impinger into a graduated cylinder. Rinse the midget impinger and the connecting tubes with water, and add the rinses to the graduated cylinder. Record the sample volume. Transfer the sample to a glass vial and cap with a Teflon ® septum. Discard any excess sample. Place the samples in an ice chest for shipment to the laboratory.
8.2.2. Adsorbent Tubes. Seal the silica gel adsorbent tubes and place them in an ice chest for shipment to the laboratory.
9.0 Quality Control
9.1 Miscellaneous Quality Control Measures. The following quality control measures are required:
| Section | Quality control measure | Effect |
|---|---|---|
| 8.1.2, 8.1.3, 10.1 | Sampling equipment leak check and calibration | Ensures accurate measurement of sample volume. |
| 10.2 | GC calibration | Ensures precision of GC analysis. |
| 13.0 | Methanol spike recovery check | Verifies all methanol in stack gas is being captured in impinge/adsorbent tube setup. |
10.0 Calibration and Standardization
10.1 Metering System. The following items are required for the metering system:
10.1.1 Initial Calibration.
10.1.1.1 Before its initial use in the field, first leak-check the metering system (drying tube, needle valve, pump, rotameter, and DGM) as follows: Place a vacuum gauge at the inlet to the drying tube, and pull a vacuum of 250 mm (10 inch) Hg; plug or pinch off the outlet of the flow meter, and then turn off the pump. The vacuum shall remain stable for at least 30 seconds. Carefully release the vacuum gauge before releasing the flow meter end.
10.1.1.2 Next, remove the drying tube, and calibrate the metering system (at the sampling flow rate specified by the method) as follows: Connect an appropriately sized wet test meter (e.g., 1 liter per revolution (0.035 cubic feet per revolution)) to the inlet of the drying tube. Make three independent calibrations runs, using at least five revolutions of the DGM per run. Calculate the calibration factor, Y (wet test meter calibration volume divided by the DGM volume, both volumes adjusted to the same reference temperature and pressure), for each run, and average the results. If any Y-value deviates by more than 2 percent from the average, the metering system is unacceptable for use. Otherwise, use the average as the calibration factor for subsequent test runs.
10.1.2 Posttest Calibration Check. After each field test series, conduct a calibration check as in section 10.1.1 above, except for the following variations: (a) The leak check is not to be conducted, (b) three, or more revolutions of the DGM may be used, and (c) only two independent runs need be made. If the calibration factor does not deviate by more than 5 percent from the initial calibration factor (determined in section 10.1.1), then the DGM volumes obtained during the test series are acceptable. If the calibration factor deviates by more than 5 percent, recalibrate the metering system as in section 10.1.1, and for the calculations, use the calibration factor (initial or recalibration) that yields the lower gas volume for each test run.
10.1.3 Temperature Sensors. Calibrate against mercury-in-glass thermometers. An alternative mercury-free thermometer may be used if the thermometer is, at a minimum, equivalent in terms of performance or suitably effective for the specific temperature measurement application.
10.1.4 Rotameter. The rotameter need not be calibrated, but should be cleaned and maintained according to the manufacturer's instruction.
10.1.5 Barometer. Calibrate against a mercury barometer.
10.2 Gas Chromatograph. The following procedures are required for the gas chromatograph:
10.2.1 Initial Calibration. Inject 1 µl of each of the standards prepared in sections 7.2.3.3 and 7.2.3.4 into the GC and record the response. Repeat the injections for each standard until two successive injections agree within 5 percent. Using the mean response for each calibration standard, prepare a linear least squares equation relating the response to the mass of methanol in the sample. Perform the calibration before analyzing each set of samples.
10.2.2 Continuing Calibration. At the beginning of each day, analyze the mid level calibration standard as described in section 10.5.1. The response from the daily analysis must agree with the response from the initial calibration within 10 percent. If it does not, the initial calibration must be repeated.
11.0 Analytical Procedure
11.1 Gas Chromatograph Operating Conditions. The following operating conditions are required for the GC:
11.1.1 Injector. Configured for capillary column, splitless, 200°C (392°F).
11.1.2 Carrier. Helium at 10 ml/min.
11.1.3 Oven. Initially at 45°C for 3 minutes; then raise by 10°C to 70°C; then raise by 70°C/min to 200°C.
11.2 Impinger Sample. Inject 1 µl of the stored sample into the GC. Repeat the injection and average the results. If the sample response is above that of the highest calibration standard, either dilute the sample until it is in the measurement range of the calibration line or prepare additional calibration standards. If the sample response is below that of the lowest calibration standard, prepare additional calibration standards. If additional calibration standards are prepared, there shall be at least two that bracket the response of the sample. These standards should produce approximately 50 percent and 150 percent of the response of the sample.
11.3 Silica Gel Adsorbent Sample. The following items are required for the silica gel adsorbent samples:
11.3.1 Preparation of Samples. Extract the front and backup sections of the adsorbent tube separately. With a file, score the glass adsorbent tube in front of the first section of silica gel. Break the tube open. Remove and discard the glass wool. Transfer the first section of the silica gel to a 5-ml glass vial and stopper the vial. Remove the spacer between the first and second section of the adsorbent tube and discard it. Transfer the second section of silica gel to a separate 5-ml glass vial and stopper the vial.
11.3.2 Desorption of Samples. Add 3 ml of deionized distilled water to each of the stoppered vials and shake or vibrate the vials for 30 minutes.
11.3.3 Inject a 1-µl aliquot of the diluted sample from each vial into the GC. Repeat the injection and average the results. If the sample response is above that of the highest calibration standard, either dilute the sample until it is in the measurement range of the calibration line or prepare additional calibration standards. If the sample response is below that of the lowest calibration standard, prepare additional calibration standards. If additional calibration standards are prepared, there shall be at least two that bracket the response of the sample. These standards should produce approximately 50 percent and 150 percent of the response of the sample.
12.0 Data Analysis and Calculations
12.1 Nomenclature.
Caf = Concentration of methanol in the front of the adsorbent tube, µg/ml.
Cab = Concentration of methanol in the back of the adsorbent tube, µg/ml.
Ci = Concentration of methanol in the impinger portion of the sample train,µg/ml.
E = Mass emission rate of methanol, µg/hr (lb/hr).
ms = Total mass of compound measured in impinger and on adsorbent with spiked train (mg).
mu = Total mass of compound measured in impinger and on adsorbent with unspiked train (mg).
mv = Mass per volume of spiked compound measured (mg/L).
Mtot = Total mass of methanol collected in the sample train, µg.
Pbar = Barometric pressure at the exit orifice of the DGM, mm Hg (in. Hg).
Pstd = Standard absolute pressure, 760 mm Hg (29.92 in. Hg).
Qstd = Dry volumetric stack gas flow rate corrected to standard conditions, dscm/hr (dscf/hr).
R = fraction of spiked compound recovered
s = theoretical concentration (ppm) of spiked target compound
Tm = Average DGM absolute temperature, degrees K (°R).
Tstd = Standard absolute temperature, 293 degrees K (528°R).
Vaf = Volume of front half adsorbent sample, ml.
Vab = Volume of back half adsorbent sample, ml.
Vi = Volume of impinger sample, ml.
Vm = Dry gas volume as measured by the DGM, dry cubic meters (dcm), dry cubic feet (dcf).
Vm(std) = Dry gas volume measured by the DGM, corrected to standard conditions, dry standard cubic meters (dscm), dry standard cubic feet (dscf).
12.2 Mass of Methanol. Calculate the total mass of methanol collected in the sampling train using Equation 308-1.

12.3 Dry Sample Gas Volume, Corrected to Standard Conditions. Calculate the volume of gas sampled at standard conditions using Equation 308-2.

12.4 Mass Emission Rate of Methanol. Calculate the mass emission rate of methanol using Equation 308-3.

12.5 Recovery Fraction (R)

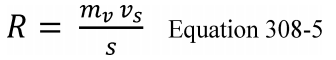
13.0 Method Performance
Since a potential sample may contain a variety of compounds from various sources, a specific precision limit for the analysis of field samples is impractical. Precision in the range of 5 to 10 percent relative standard deviation (RSD) is typical for gas chromatographic techniques, but an experienced GC operator with a reliable instrument can readily achieve 5 percent RSD. For this method, the following combined GC/operator values are required.
(a) Precision. Calibration standards must meet the requirements in section 10.2.1 or 10.2.2 as applicable.
(b) Recovery. After developing an appropriate sampling and analytical system for the pollutants of interest, conduct the following spike recovery procedure at each sampling point where the method is being applied.
i. Methanol Spike. Set up two identical sampling trains. Collocate the two sampling probes in the stack. The probes shall be placed in the same horizontal plane, where the first probe tip is 2.5 cm from the outside edge of the other. One of the sampling trains shall be designated the spiked train and the other the unspiked train. Spike methanol into the impinger, and onto the adsorbent tube in the spiked train prior to sampling. The total mass of methanol shall be 40 to 60 percent of the mass expected to be collected with the unspiked train. Sample the stack gas into the two trains simultaneously. Analyze the impingers and adsorbents from the two trains utilizing identical analytical procedures and instrumentation. Determine the fraction of spiked methanol recovered (R) by combining the amount recovered in the impinger and in the adsorbent tube, using the equations in section 12.5. Recovery values must fall in the range: 0.70 ≤ R ≤ 1.30. Report the R value in the test report.
ii. [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 Bibliography
1. Rom, J.J. “Maintenance, Calibration, and Operation of Isokinetic Source Sampling Equipment.” Office of Air Programs, Environmental Protection Agency. Research Triangle Park, NC. APTD-0576 March 1972.
2. Annual Book of ASTM Standards. Part 31; Water, Atmospheric Analysis. American Society for Testing and Materials. Philadelphia, PA. 1974. pp. 40-42.
3. Westlin, P.R. and R.T. Shigehara. “Procedure for Calibrating and Using Dry Gas Volume Meters as Calibration Standards.” Source Evaluation Society Newsletter. 3 (1) :17-30. February 1978.
4. Yu, K.K. “Evaluation of Moisture Effect on Dry Gas Meter Calibration.” Source Evaluation Society Newsletter. 5 (1) :24-28. February 1980.
5. NIOSH Manual of Analytical Methods, Volume 2. U.S. Department of Health and Human Services National Institute for Occupational Safety and Health. Center for Disease Control. 4676 Columbia Parkway, Cincinnati, OH 45226. (available from the Superintendent of Documents, Government Printing Office, Washington, DC 20402.)
6. Pinkerton, J.E. “Method for Measuring Methanol in Pulp Mill Vent Gases.” National Council of the Pulp and Paper Industry for Air and Stream Improvement, Inc., New York, NY.
17.0 Tables, Diagrams, Flowcharts, and Validation Data [Reserved]
Method 310A - Determination of Residual Hexane Through Gas Chromatography
1.0 Scope and Application
1.1 This method is used to analyze any crumb rubber or water samples for residual hexane content.
1.2 The sample is heated in a sealed bottle with an internal standard and the vapor is analyzed by gas chromatography.
2.0 Summary of Method
2.1 This method, utilizing a capillary column gas chromatograph with a flame ionization detector, determines the concentration of residual hexane in rubber crumb samples.
3.0 Definitions
3.1 The definitions are included in the text as needed.
4.0 Interferences
4.1 There are no known interferences.
5.0 Safety
5.1 It is the responsibility of the user of this procedure to establish safety and health practices applicable to their specific operation.
6.0 Equipment and Supplies
6.1 Gas Chromatograph with a flame ionization detector and data handling station equipped with a capillary column 30 meters long.
6.2 Chromatograph conditions for Sigma 1:
6.2.1 Helium pressure: 50# inlet A, 14# aux
6.2.2 Carrier flow: 25 cc/min
6.2.3 Range switch: 100x
6.2.4 DB: 1 capillary column
6.3 Chromatograph conditions for Hewlett-Packard GC:
6.3.1 Initial temperature: 40°C
6.3.2 Initial time: 8 min
6.3.3 Rate: 0
6.3.4 Range: 2
6.3.5 DB: 1705 capillary column
6.4 Septum bottles and stoppers
6.5 Gas Syringe - 0.5 cc
7.0 Reagents and Standards
7.1 Chloroform, 99.9 + %, A.S.C. HPLC grade
8.0 Sample Collection, Preservation, and Storage
8.1 A representative sample should be caught in a clean 8 oz. container with a secure lid.
8.2 The container should be labeled with sample identification, date and time.
9.0 Quality Control
9.1 The instrument is calibrated by injecting calibration solution (Section 10.2 of this method) five times.
9.2 The retention time for components of interest and relative response of monomer to the internal standard is determined.
9.3 Recovery efficiency must be determined once for each sample type and whenever modifications are made to the method.
9.3.1 Determine the percent hexane in three separate dried rubber crumb samples.
9.3.2 Weigh a portion of each crumb sample into separate sample bottles and add a known amount of hexane (10 microliters) by microliter syringe and 20 microliters of internal standard. Analyze each by the described procedure and calculate the percent recovery of the known added hexane.
9.3.3 Repeat the previous step using twice the hexane level (20 microliters), analyze and calculate the percent recovery of the known added hexane.
9.3.4 Set up two additional sets of samples using 10 microliters and 20 microliters of hexane as before, but add an amount of water equal to the dry crumb used. Analyze and calculate percent recovery to show the effect of free water on the results obtained.
9.3.5 A value of R between 0.70 and 1.30 is acceptable.
9.3.6 R shall be used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type.
10.0 Calibration and Instrument Settings
10.1 Calibrate the chromatograph using a standard made by injecting 10 µl of fresh hexane and 20 µl of chloroform into a sealed septum bottle. This standard will be 0.6 wt.% total hexane based on 1 gram of dry rubber.
10.2 Analyze the hexane used and calculate the percentage of each hexane isomer (2-methylpentane, 3-methylpentane, n-hexane, and methylcyclo-pentane). Enter these percentages into the method calibration table.
10.3 Heat the standard bottle for 30 minutes in a 105°C oven.
10.4 Inject about 0.25 cc of vapor into the gas chromatograph and after the analysis is finished, calibrate according to the procedures described by the instrument manufacturer.
11.0 Procedure
11.1 Using a cold mill set at a wide roller gap (125-150 mm), mill about 250 grams of crumb two times to homogenize the sample.
11.2 Weigh about 2 grams of wet crumb into a septum bottle and cap with a septum ring. Add 20 µl of chloroform with a syringe and place in a 105°C oven for 45 minutes.
11.3 Run the moisture content on a separate portion of the sample and calculate the grams of dry rubber put into the septum bottle.
11.4 Set up the data station on the required method and enter the dry rubber weight in the sample weight field.
11.5 Inject a 0.25 cc vapor sample into the chromatograph and push the start button.
11.6 At the end of the analysis, the data station will print a report listing the concentration of each identified component.
11.7 To analyze water samples, pipet 5 ml of sample into the septum bottle, cap and add 20 µl of chloroform. Place in a 105°C oven for 30 minutes.
11.8 Enter 5 grams into the sample weight field.
11.9 Inject a 0.25 cc vapor sample into the chromatograph and push the start button.
11.10 At the end of the analysis, the data station will print a report listing the concentration of each identified component.
12.0 Data Analysis and Calculation
12.1 For samples that are prepared as in section 11 of this method, ppm n-hexane is read directly from the computer.
12.2 The formulas for calculation of the results are as follows:
ppmhexane = (Ahexane × Rhexane)/(Ais × Ris)
Where:
Ahexane = area of hexane
Rhexane = response of hexane
Ais = area of the internal standard
Ris = response of the internal standard
% hexane in crumb = (ppmhexane/sample amount)100
12.3 Correct the results by the value of R (as determined in sections 9.3.4, 9.3.5, and 9.3.6 of this method).
13.0 Method Performance
13.1 The test has a standard deviation of 0.14 wt% at 0.66 wt% hexane. Spike recovery of 12 samples at two levels of hexane averaged 102.3%. Note: Recovery must be determined for each type of sample. The values given here are meant to be examples of method performance.
14.0 Pollution Prevention
14.1 Waste generation should be minimized where possible. Sample size should be an amount necessary to adequately run the analysis.
15.0 Waste Management
15.1 All waste shall be handled in accordance with federal and state environmental regulations.
16.0 References and Publications
16.1 DSM Copolymer Test Method T-3380.
Method 310B - Determination of Residual Hexane Through Gas Chromatography
1.0 Scope and Application
| Analyte | CAS No. | Matrix | Method sensitivity (5.5g sample size) |
|---|---|---|---|
| Hexane | 110-54-3 | Rubber crumb | .01 wt%. |
| Applicable Termonomer | Rubber crumb | .001 wt%. |
1.1 Data Quality Objectives:
In the production of ethylene-propylene terpolymer crumb rubber, the polymer is recovered from solution by flashing off the solvent with steam and hot water. The resulting water-crumb slurry is then pumped to the finishing units. Certain amounts of solvent (hexane being the most commonly used solvent) and diene monomer remain in the crumb. The analyst uses the following procedure to determine those amounts.
2.0 Summary of Method
2.1 The crumb rubber sample is dissolved in toluene to which heptane has been added as an internal standard. Acetone is then added to this solution to precipitate the crumb, and the supernatant is analyzed for hexane and diene by a gas chromatograph equipped with a flame ionization detector (FID).
3.0 Definitions
3.1 Included in text as needed.
4.0 Interferences
4.1 None known.
4.2 Benzene, introduced as a contaminant in the toluene solvent, elutes between methyl cyclopentane and cyclohexane. However, the benzene peak is completely resolved.
4.3 2,2-dimethyl pentane, a minor component of the hexane used in our process, elutes just prior to methyl cyclopentane. It is included as “hexane” in the analysis whether it is integrated separately or included in the methyl cyclopentane peak.
5.0 Safety
5.1 This procedure does not purport to address all of the safety concerns associated with its use. It is the responsibility of the user of this procedure to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
5.2 Chemicals used in this analysis are flammable and hazardous (see specific toxicity information below). Avoid contact with sources of ignition during sample prep. All handling should be done beneath a hood. Playtex or nitrile gloves recommended.
5.3 Hexane is toxic by ingestion and inhalation. Vapor inhalation causes irritation of nasal and respiratory passages, headache, dizziness, nausea, central nervous system depression. Chronic overexposure can cause severe nerve damage. May cause irritation on contact with skin or eyes. May cause damage to kidneys.
5.4 Termonomer may be harmful by inhalation, ingestion, or skin absorption. Vapor or mist is irritating to the eyes, mucous membranes, and upper respiratory tract. Causes skin irritation.
5.5 Toluene is harmful or fatal if swallowed. Vapor harmful if inhaled. Symptoms: headache, dizziness, hallucinations, distorted perceptions, changes in motor activity, nausea, diarrhea, respiratory irritation, central nervous system depression, unconsciousness, liver, kidney and lung damage. Contact can cause severe eye irritation. May cause skin irritation. Causes irritation of eyes, nose, and throat.
5.6 Acetone, at high concentrations or prolonged overexposure, may cause headache, dizziness, irritation of eyes and respiratory tract, loss of strength, and narcosis. Eye contact causes severe irritation; skin contact may cause mild irritation. Concentrations of 20,000 ppm are immediately dangerous to life and health.
5.7 Heptane is harmful if inhaled or swallowed. May be harmful if absorbed through the skin. Vapor or mist is irritating to the eyes, mucous membranes, and upper respiratory tract. Prolonged or repeated exposure to skin causes defatting and dermatitis.
5.8 The steam oven used to dry the polymer in this procedure is set at 110°C. Wear leather gloves when removing bottles from the oven.
6.0 Equipment and Supplies
6.1 4000-ml volumetric flask
6.2 100-ml volumetric pipette
6.3 1000-ml volumetric flask
6.4 8-oz. French Square sample bottles with plastic-lined caps
6.5 Top-loading balance
6.6 Laboratory shaker
6.7 Laboratory oven set at 110°C (steam oven)
6.8 Gas chromatograph, Hewlett-Packard 5890A, or equivalent, interfaced with HP 7673A (or equivalent) autosampler (equipped with nanoliter adapter and robotic arm), and HP 3396 series II or 3392A (or equivalent) integrator/controller.
6.9 GC column, capillary type, 50m × 0.53mm, methyl silicone, 5 micron film thickness, Quadrex, or equivalent.
6.10 Computerized data acquisition system, such as CIS/CALS
6.11 Crimp-top sample vials and HP p/n 5181-1211 crimp caps, or screw-top autosampler vials and screw tops.
6.12 Glass syringes, 5-ml, with “Luer-lock” fitting
6.13 Filters, PTFE, .45 µm pore size, Gelman Acrodisc or equivalent, to fit on Luer-lock syringes (in 6.12, above).
7.0 Reagents and Standards
7.1 Reagent toluene, EM Science Omnisolv (or equivalent)
Purity Check: Prior to using any bottle of reagent toluene, analyze it according to section 11.2 of this method. Use the bottle only if hexane, heptane, and termonomer peak areas are less than 15 each (note that an area of 15 is equivalent to less than 0.01 wt% in a 10g sample).
7.2 Reagent acetone, EM Science Omnisolv HR-GC (or equivalent)
Purity Check: Prior to using any bottle of reagent acetone, analyze it according to section 11.2 of this method. Use the bottle only if hexane, heptane, and termonomer peak areas are less than 15 each.
7.3 Reagent heptane, Aldrich Chemical Gold Label, Cat #15,487-3 (or equivalent)
Purity Check: Prior to using any bottle of reagent heptane, analyze it according to section 11.2 of this method. Use the bottle only if hexane and termonomer peak areas are less than 5 each.
7.4 Internal standard solution - used as a concentrate for preparation of the more dilute Polymer Dissolving Solution. It contains 12.00g heptane/100ml of solution which is 120.0g per liter.
Preparation of internal standard solution (polymer dissolving stock solution):
| Action | Notes |
|---|---|
| 7.4.1 Tare a clean, dry 1-liter volumetric flask on the balance. Record the weight to three places | If the 1-liter volumetric flask is too tall to fit in the balance case, you can shield the flask from drafts by inverting a paint bucket with a hole cut in the bottom over the balance cover. Allow the neck of the flask to project through the hole in the bucket. |
| 7.4.2 Weigh 120.00 g of n-heptane into the flask. Record the total weight of the flask and heptane as well as the weight of heptane added | Use 99 + % n-heptane from Aldrich or Janssen Chimica. |
| 7.4.3 Fill the flask close to the mark with toluene, about 1 to 2″ below the mark | Use EM Science Omnisolve toluene, Grade TX0737-1, or equivalent. |
| 7.4.4 Shake the flask vigorously to mix the contents | Allow any bubbles to clear before proceeding to the next step. |
| 7.4.5 Top off the flask to the mark with toluene. Shake vigorously, as in section 7.4.4 of this method, to mix well | |
| 7.4.6 Weigh the flask containing the solution on the three place balance record the weight | |
| 7.4.7 Transfer the contents of the flask to a 1 qt Boston round bottle | Discard any excess solution |
| 7.4.8 Label the bottle with the identity of the contents, the weights of heptane and toluene used, the date of preparation and the preparer's name | Be sure to include the words “Hexane in Crumb Polymer Dissolving Stock Solution” on the label. |
| 7.4.9 Refrigerate the completed blend for the use of the routine Technicians |
7.5 Polymer Dissolving Solution (“PDS”) - Heptane (as internal standard) in toluene. This solution contains 0.3g of heptane internal standard per 100 ml of solution.
7.5.1 Preparation of Polymer Dissolving Solution. Fill a 4,000-ml volumetric flask about 3/4 full with toluene.
7.5.2 Add 100 ml of the internal standard solution (section 7.4 of this method) to the flask using the 100ml pipette.
7.5.3 Fill the flask to the mark with toluene. Discard any excess.
7.5.4 Add a large magnetic stirring bar to the flask and mix by stirring.
7.5.5 Transfer the polymer solvent solution to the one-gallon labeled container with 50ml volumetric dispenser attached.
7.5.6 Purity Check: Analyze according to section 11.2. NOTE: You must “precipitate” the sample with an equal part of acetone (thus duplicating actual test conditions - see section 11.1 of this method, sample prep) before analyzing. Analyze the reagent 3 times to quantify the C6 and termonomer interferences. Inspect the results to ensure good agreement among the three runs (within 10%).
7.5.7 Tag the bottle with the following information:
POLYMER DISSOLVING SOLUTION FOR C6 IN CRUMB ANALYSIS
PREPARER'S NAME
DATE
CALS FILE ID'S OF THE THREE ANALYSES FOR PURITY (from section 7.5.6 of this method)
7.6 Quality Control Solution: the quality control solution is prepared by adding specific amounts of mixed hexanes (barge hexane), n-nonane and termonomer to some polymer dissolving solution. Nonane elutes in the same approximate time region as termonomer and is used to quantify in that region because it has a longer shelf life. Termonomer, having a high tendency to polymerize, is used in the QC solution only to ensure that both termonomer isomers elute at the proper time.
First, a concentrated stock solution is prepared; the final QC solution can then be prepared by diluting the stock solution.
7.6.1 In preparation of stock solution, fill a 1-liter volumetric flask partially with polymer dissolving solution (PDS) - see section 7.5 of this method. Add 20.0 ml barge hexane, 5.0 ml n-nonane, and 3 ml termonomer. Finish filling the volumetric to the mark with PDS.
7.6.2 In preparation of quality control solution, dilute the quality control stock solution (above) precisely 1:10 with PDS, i.e. 10 ml of stock solution made up to 100 ml (volumetric flask) with PDS. Pour the solution into a 4 oz. Boston round bottle and store in the refrigerator.
8.0 Sample Collection, Preservation and Storage
8.1 Line up facility to catch crumb samples. The facility is a special facility where the sample is drawn.
8.1.1 Ensure that the cock valve beneath facility is closed.
8.1.2 Line up the system from the slurry line cock valve to the cock valve at the nozzle on the stripper.
8.1.3 Allow the system to flush through facility for a period of 30 seconds.
8.2 Catch a slurry crumb sample.
8.2.1 Simultaneously close the cock valves upstream and downstream of facility.
8.2.2 Close the cock valve beneath the slurry line in service.
8.2.3 Line up the cooling tower water through the sample bomb water jacket to the sewer for a minimum of 30 minutes.
8.2.4 Place the sample catching basket beneath facility and open the cock valve underneath the bomb to retrieve the rubber crumb.
8.2.5 If no rubber falls by gravity into the basket, line up nitrogen to the bleeder upstream of the sample bomb and force the rubber into the basket.
8.2.6 Close the cock valve underneath the sample bomb.
8.3 Fill a plastic “Whirl-pak” sample bag with slurry crumb and send it to the lab immediately.
8.4 Once the sample reaches the lab, it should be prepped as soon as possible to avoid hexane loss through evaporation. Samples which have lain untouched for more than 30 minutes should be discarded.
9.0 Quality Control
Quality control is monitored via a computer program that tracks analyses of a prepared QC sample (from section 7.6.2 of this method). The QC sample result is entered daily into the program, which plots the result as a data point on a statistical chart. If the data point does not satisfy the “in-control” criteria (as defined by the lab quality facilitator), an “out-of-control” flag appears, mandating corrective action.
In addition, the area of the n-heptane peak is monitored so that any errors in making up the polymer dissolving solution will be caught and corrected. Refer to section 12.4 of this method.
9.1 Fill an autosampler vial with the quality control solution (from section 7.6.2 of this method) and analyze on the GC as normal (per section 11 of this method).
9.2 Add the concentrations of the 5 hexane isomers as they appear on the CALS printout. Also include the 2,2-dimethyl-pentane peak just ahead of the methyl cyclopentane (the fourth major isomer) peak in the event that the peak integration split this peak out. Do not include the benzene peak in the sum. Note the nonane concentration. Record both results (total hexane and nonane) in the QC computer program. If out of control, and GC appears to be functioning within normal parameters, reanalyze a fresh control sample. If the fresh QC is not in control, check stock solution for contaminants or make up a new QC sample with the toluene currently in use. If instrument remains out-of-control, more thorough GC troubleshooting may be needed.
Also, verify that the instrument has detected both isomers of termonomer (quantification not necessary - see section 7.0 of this method).
9.3 Recovery efficiency must be determined for high ethylene concentration, low ethylene concentration, E-P terpolymer, or oil extended samples and whenever modifications are made to the method. Recovery shall be between 70 and 130 percent. All test results must be corrected by the recovery efficiency value (R).
9.3.1 Approximately 10 grams of wet EPDM crumb (equivalent to about 5 grams of dry rubber) shall be added to six sample bottles containing 100 ml of hexane in crumb polymer dissolving solution (toluene containing 0.3 gram n-heptane/100 ml solution). The polymer shall be dissolved by agitating the bottles on a shaker for 4 hours. The polymer shall be precipitated using 100 ml acetone.
9.3.2 The supernatant liquid shall be decanted from the polymer. Care shall be taken to remove as much of the liquid phase from the sample as possible to minimize the effect of retained liquid phase upon the next cycle of the analysis. The supernatant liquid shall be analyzed by gas chromatography using an internal standard quantitation method with heptane as the internal standard.
9.3.3 The precipitated polymer from the steps described above shall be redissolved using toluene as the solvent. No heptane shall be added to the sample in the second dissolving step. The toluene solvent and acetone precipitant shall be determined to be free of interfering compounds.
9.3.4 The rubber which was dissolved in the toluene shall be precipitated with acetone as before, and the supernatant liquid decanted from the precipitated polymer. The liquid shall be analyzed by gas chromatography and the rubber phase dried in a steam-oven to determine the final polymer weight.
9.3.5 The ratios of the areas of the hexane peaks and of the heptane internal standard peak shall be calculated for each of the six samples in the two analysis cycles outlined above. The area ratios of the total hexane to heptane (R1) shall be determined for the two analysis cycles of the sample set. The ratio of the values of R1 from the second analysis cycle to the first cycle shall be determined to give a second ratio (R2).
10.0 Calibration and Standardization
The procedure for preparing a Quality Control sample with the internal standard in it is outlined in section 7.6 of this method.
10.1 The relative FID response factors for n-heptane, the internal standard, versus the various hexane isomers and termonomer are relatively constant and should seldom need to be altered. However Baseline construction is a most critical factor in the production of good data. For this reason, close attention should be paid to peak integration. Procedures for handling peak integration will depend upon the data system used.
10.2 If recalibration of the analysis is needed, make up a calibration blend of the internal standard and the analytes as detailed below and analyze it using the analytical method used for the samples.
10.2.1 Weigh 5 g heptane into a tared scintillation vial to five places.
10.2.2 Add 0.2 ml termonomer to the vial and reweigh.
10.2.3 Add 0.5 ml hexane to the vial and reweigh.
10.2.4 Cap, and shake vigorously to mix.
10.2.5 Calculate the weights of termonomer and of hexane added and divide their weights by the weight of the n-heptane added. The result is the known of given value for the calibration.
10.2.6 Add 0.4 ml of this mixture to a mixture of 100 ml toluene and 100 ml of acetone. Cap and shake vigorously to mix.
10.2.7 Analyze the sample.
10.2.8 Divide the termonomer area and the total areas of the hexane peaks by the n-heptane area. This result is the “found” value for the calibration.
10.2.9 Divide the appropriate “known” value from 10.2.5 by the found value from 10.2.8. The result is the response factor for the analyte in question. Previous work has shown that the standard deviation of the calibration method is about 1% relative.
11.0 Procedure
11.1 SAMPLE PREPARATION
11.1.1 Tare an 8oz sample bottle - Tag attached, cap off; record weight and sample ID on tag in pencil.
11.1.2 Place crumb sample in bottle: RLA-3: 10 g (gives a dry wt. of ∼5.5 g).
11.1.3 Dispense 100ml of PDS into each bottle. SAMPLE SHOULD BE PLACED INTO SOLUTION ASAP TO AVOID HEXANE LOSS - Using “Dispensette” pipettor. Before dispensing, “purge” the dispensette (25% of its volume) into a waste bottle to eliminate any voids.
11.1.4 Tightly cap bottles and load samples into shaker.
11.1.5 Insure that “ON-OFF” switch on the shaker itself is “ON.”
11.1.6 Locate shaker timer. Insure that toggle switch atop timer control box is in the middle (“off”) position. If display reads “04:00” (4 hours), move toggle switch to the left position. Shaker should begin operating.
11.1.7 After shaker stops, add 100 ml acetone to each sample to precipitate polymer. Shake minimum of 5 minutes on shaker - Vistalon sample may not have fully dissolved; nevertheless, for purposes of consistency, 4 hours is the agreed-upon dissolving time.
11.1.8 Using a 5-ml glass Luer-lock syringe and Acrodisc filter, filter some of the supernatant liquid into an autosampler vial; crimp the vial and load it into the GC autosampler for analysis (section 11.2 of this method) - The samples are filtered to prevent polymer buildup in the GC. Clean the syringes in toluene.
11.1.9 Decant remaining supernatant into a hydrocarbon waste sink, being careful not to discard any of the polymer. Place bottle of precipitate into the steam oven and dry for six hours - Some grades of Vistalon produce very small particles in the precipitate, thus making complete decanting impossible without discarding some polymer. In this case, decant as much as possible and put into the oven as is, allowing the oven to drive off remaining supernatant (this practice is avoided for environmental reasons). WARNING: OVEN IS HOT - 110°C (230°F).
11.1.10 Cool, weigh and record final weight of bottle.
11.2 GC ANALYSIS
11.2.1 Initiate the CALS computer channel.
11.2.2 Enter the correct instrument method into the GC's integrator.
11.2.3 Load sample vial(s) into autosampler.
11.2.4 Start the integrator.
11.2.5 When analysis is complete, plot CALS run to check baseline skim.
12.0 Data Analysis and Calculations
12.1 Add the concentrations of the hexane peaks as they appear on the CALS printout. Do not include the benzene peak in the sum.
12.2 Subtract any hexane interferences found in the PDS (see section 7.5.6 of this method); record the result.
12.3 Note the termonomer concentration on the CALS printout. Subtract any termonomer interference found in the PDS and record this result in a “% termonomer by GC” column in a logbook.
12.4 Record the area (from CALS printout) of the heptane internal standard peak in a “C7 area” column in the logbook. This helps track instrument performance over the long term.
12.5 After obtaining the final dry weight of polymer used (Section 11.1.10 of this method), record that result in a “dry wt.” column of the logbook (for oil extended polymer, the amount of oil extracted is added to the dry rubber weight).
12.6 Divide the %C6 by the dry weight to obtain the total PHR hexane in crumb. Similarly, divide the % termonomer by the dry weight to obtain the total PHR termonomer in crumb. Note that PHR is an abbreviation for “parts per hundred”. Record both the hexane and termonomer results in the logbook.
12.7 Correct all results by the recovery efficiency value (R).
13.0 Method Performance
13.1 The method has been shown to provide 100% recovery of the hexane analyte. The method was found to give a 6% relative standard deviation when the same six portions of the same sample were carried through the procedure. Note: These values are examples; each sample type, as specified in Section 9.3, must be tested for sample recovery.
14.0 Pollution Prevention
14.1 Dispose of all hydrocarbon liquids in the appropriate disposal sink system; never pour hydrocarbons down a water sink.
14.2 As discussed in section 11.1.9 of this method, the analyst can minimize venting hydrocarbon vapor to the atmosphere by decanting as much hydrocarbon liquid as possible before oven drying.
15.0 Waste Management
15.1 The Technician conducting the analysis should follow the proper waste management practices for their laboratory location.
16.0 References
16.1 Baton Rouge Chemical Plant Analytical Procedure no. BRCP 1302
16.2 Material Safety Data Sheets (from chemical vendors) for hexane, ENB, toluene, acetone, and heptane
Method 310C - Determination of Residual N-Hexane in EPDM Rubber Through Gas Chromatography
1.0 Scope and Application
1.1 This method describes a procedure for the determination of residual hexane in EPDM wet crumb rubber in the 0.01 - 2% range by solvent extraction of the hexane followed by gas chromatographic analysis where the hexane is detected by flame ionization and quantified via an internal standard.
1.2 This method may involve hazardous materials operations and equipment. This method does not purport to address all the safety problems associated with it use, if any. It is the responsibility of the user to consult and establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
2.0 Summary
2.1 Residual hexane contained in wet pieces of EPDM polymer is extracted with MIBK. A known amount of an internal standard (IS) is added to the extract which is subsequently analyzed via gas chromatography where the hexane and IS are separated and detected utilizing a megabore column and flame ionization detection (FID). From the response to the hexane and the IS, the amount of hexane in the EPDM polymer is calculated.
3.0 Definitions
3.1 Hexane - refers to n-hexane
3.2 Heptane - refers to n-heptane
3.3 MIBK - methyl isobutyl ketone (4 methyl 2 - Pentanone)
4.0 Interferences
4.1 Material eluting at or near the hexane and/or the IS will cause erroneous results. Prior to extraction, solvent blanks must be analyzed to confirm the absence of interfering peaks.
5.0 Safety
5.1 Review Material Safety Data Sheets of the chemicals used in this method.
6.0 Equipment and Supplies
6.1 4 oz round glass jar with a wide mouth screw cap lid.
6.2 Vacuum oven.
6.3 50 ml pipettes.
6.4 A gas chromatograph with an auto sampler and a 50 meter, 0.53 ID, methyl silicone column with 5 micron phase thickness.
6.5 Shaker, large enough to hold 10, 4 oz. jars.
6.6 1000 and 4000 ml volumetric flasks.
6.7 Electronic integrator or equivalent data system.
6.8 GC autosampler vials.
6.9 50 uL syringe.
7.0 Reagents and Standards
7.1 Reagent grade Methyl-Iso-Butyl-Ketone (MIBK)
7.2 n-heptane, 99% + purity
7.3 n-hexane, 99% + purity
8.0 Sample Collection
8.1 Trap a sample of the EPDM crumb slurry in the sampling apparatus. Allow the crumb slurry to circulate through the sampling apparatus for 5 minutes; then close off the values at the bottom and top of the sampling apparatus, trapping the crumb slurry. Run cooling water through the water jacket for a minimum of 30 minutes. Expel the cooled crumb slurry into a sample catching basket. If the crumb does not fall by gravity, force it out with demineralized water or nitrogen. Send the crumb slurry to the lab for analysis.
9.0 Quality Control
9.1 The Royalene crumb sample is extracted three times with MIBK containing an internal standard. The hexane from each extraction is added together to obtain a total hexane content. The percent hexane in the first extraction is then calculated and used as the recovery factor for the analysis.
9.2 Follow this test method through section 11.4 of the method. After removing the sample of the first extraction to be run on the gas chromatograph, drain off the remainder of the extraction solvent, retaining the crumb sample in the sample jar. Rinse the crumb with demineralized water to remove any MIBK left on the surface of the crumb. Repeat the extraction procedure with fresh MIBK with internal standard two more times.
9.3 After the third extraction, proceed to section 11.5 of this method and obtain the percent hexane in each extraction. Use the sample weight obtained in section 12.1 of this method to calculate the percent hexane in each of the extracts.
9.4 Add the percent hexane obtained from the three extractions for a total percent hexane in the sample.
9.5 Use the following equations to determine the recovery factor (R):
% Recovery of the first extraction = (% hexane in the first extract/total % hexane) × 100
Recovery Factor (R) = (% Hexane Recovered in the first extract)/100
10.0 Calibration
10.1 Preparation of Internal Standard (IS) solution:
Accuracy weigh 30 grams of n-heptane into a 1000 ml volumetric flask. Dilute to the mark with reagent grade MIBK. Label this Solution “A”. Pipette 100 mls. of Solution A into a 4 liter volumetric flask. Fill the flask to the mark with reagent MIBK. Label this Solution “B”. Solution “B” will have a concentration of 0.75 mg/ml of heptane.
10.2 Preparation of Hexane Standard Solution (HS):
Using a 50 uL syringe, weigh by difference, 20 mg of n-hexane into a 50 ml volumetric flask containing approximately 40 ml of Solution B. Fill the flask to the mark with Solution B and mix well.
10.3 Conditions for GC analysis of standards and samples:
Temperature:
Initial = 40°C
Final = 150°C
Injector = 160°C
Detector = 280°C
Program Rate = 5.0°C/min
Initial Time = 5 minutes Final Time = 6 minutes
Flow Rate = 5.0 ml/min
Sensitivity = detector response must be adjusted to keep the hexane and IS on scale.
10.4 Fill an autosampler vial with the HS, analyze it three times and calculate a Hexane Relative Response Factor (RF) as follows:
RF = (AIS × CHS × PHS)/(AHS × CIS × PIS) (1)
Where:
AIS = Area of IS peak (Heptane)
AHS = Area of peak (Hexane Standard)
CHS = Mg of Hexane/50 ml HS
CIS = Mg of Heptane/50 ml IS Solution B
PIS = Purity of the IS n-heptane
PHS = Purity of the HS n-hexane
11.0 Procedure
11.1 Weight 10 grams of wet crumb into a tared (W1), wide mouth 4 oz. jar.
11.2 Pipette 50 ml of Solution B into the jar with the wet crumb rubber.
11.3 Screw the cap on tightly and place it on a shaker for 4 hours.
11.4 Remove the sample from the shaker and fill an autosampler vial with the MIBK extract.
11.5 Analyze the sample two times.
11.6 Analyze the HS twice, followed by the samples. Inject the HS twice at the end of each 10 samples or at the end of the run.
12.0 Calculations
12.1 Drain off the remainder of the MIBK extract from the polymer in the 4 oz. jar. Retain all the polymer in the jar. Place the uncovered jar and polymer in a heated vacuum oven until the polymer is dry. Reweigh the jar and polymer (W2) and calculate the dried sample weight of the polymer as follows:
Dried SW = W2 - W1 (2)
12.2 Should the polymer be oil extended, pipette 10 ml of the MIBK extract into a tared evaporating dish (W1) and evaporate to dryness on a steam plate.
Reweigh the evaporating dish containing the extracted oil (W2). Calculate the oil content of the polymer as follows:
Gram of oil extracted = 5 (W2 - W1) (3)
% Hexane in polymer = (As × RF × CIS × PIS)/(AIS × SW) (4)
Where:
As = Area of sample hexane sample peak.
AIS = Area of IS peak in sample.
CIS = Concentration of IS in 50 ml.
PIS = Purity of IS.
SW = Weight of dried rubber after extraction. (For oil extended polymer, the amount of oil extracted is added to the dry rubber weight).
% Corrected Hexane = (% Hexane in Polymer)/R (5)
R = Recovery factor determined in section 9 of this method.
13.0 Method Performance
13.1 Performance must be determined for each sample type by following the procedures in section 9 of this method.
14.0 Waste Generation
14.1 Waste generation should be minimized where possible.
15.0 Waste Management
15.1 All waste shall be handled in accordance with Federal and State environmental regulations.
16.0 References [Reserved]
Method 311 - Analysis of Hazardous Air Pollutant Compounds in Paints and Coatings by Direct Injection Into a Gas Chromatograph
1. Scope and Application
1.1 Applicability. This method is applicable for determination of most compounds designated by the U.S. Environmental Protection Agency as volatile hazardous air pollutants (HAP's) (See Reference 1) that are contained in paints and coatings. Styrene, ethyl acrylate, and methyl methacrylate can be measured by ASTM D 4827-03. Formaldehyde can be measured by ASTM D 5910-05 or ASTM D 1979-91. Toluene diisocyanate can be measured in urethane prepolymers by ASTM D 3432-89. Method 311 applies only to those volatile HAP's which are added to the coating when it is manufactured, not to those that may form as the coating cures (reaction products or cure volatiles). A separate or modified test procedure must be used to measure these reaction products or cure volatiles in order to determine the total volatile HAP emissions from a coating. Cure volatiles are a significant component of the total HAP content of some coatings. The term “coating” used in this method shall be understood to mean paints and coatings.
1.2 Principle. The method uses the principle of gas chromatographic separation and quantification using a detector that responds to concentration differences. Because there are many potential analytical systems or sets of operating conditions that may represent useable methods for determining the concentrations of the compounds cited in Section 1.1 in the applicable matrices, all systems that employ this principle, but differ only in details of equipment and operation, may be used as alternative methods, provided that the prescribed quality control, calibration, and method performance requirements are met. Certified product data sheets (CPDS) may also include information relevant to the analysis of the coating sample including, but not limited to, separation column, oven temperature, carrier gas, injection port temperature, extraction solvent, and internal standard.
2. Summary of Method
Whole coating is added to dimethylformamide and a suitable internal standard compound is added. An aliquot of the sample mixture is injected onto a chromatographic column containing a stationary phase that separates the analytes from each other and from other volatile compounds contained in the sample. The concentrations of the analytes are determined by comparing the detector responses for the sample to the responses obtained using known concentrations of the analytes.
3. Definitions [Reserved]
4. Interferences
4.1 Coating samples of unknown composition may contain the compound used as the internal standard. Whether or not this is the case may be determined by following the procedures of Section 11 and deleting the addition of the internal standard specified in Section 11.5.3. If necessary, a different internal standard may be used.
4.2 The GC column and operating conditions developed for one coating formulation may not ensure adequate resolution of target analytes for other coating formulations. Some formulations may contain nontarget analytes that coelute with target analytes. If there is any doubt about the identification or resolution of any gas chromatograph (GC) peak, it may be necessary to analyze the sample using a different GC column or different GC operating conditions.
4.3 Cross-contamination may occur whenever high-level and low-level samples are analyzed sequentially. The order of sample analyses specified in Section 11.7 is designed to minimize this problem.
4.4 Cross-contamination may also occur if the devices used to transfer coating during the sample preparation process or for injecting the sample into the GC are not adequately cleaned between uses. All such devices should be cleaned with acetone or other suitable solvent and checked for plugs or cracks before and after each use.
5. Safety
5.1 Many solvents used in coatings are hazardous. Precautions should be taken to avoid unnecessary inhalation and skin or eye contact. This method may involve hazardous materials, operations, and equipment. This test method does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this test method to establish appropriate safety and health practices and to determine the applicability of regulatory limitations in regards to the performance of this test method.
5.2 Dimethylformamide is harmful if inhaled or absorbed through the skin. The user should obtain relevant health and safety information from the manufacturer. Dimethylformamide should be used only with adequate ventilation. Avoid contact with skin, eyes, and clothing. In case of contact, immediately flush skin or eyes with plenty of water for at least 15 minutes. If eyes are affected, consult a physician. Remove and wash contaminated clothing before reuse.
5.3 User's manuals for the gas chromatograph and other related equipment should be consulted for specific precautions to be taken related to their use.
6. Equipment and Supplies
Note:
Certified product data sheets (CPDS) may also include information relevant to the analysis of the coating sample including, but not limited to, separation column, oven temperature, carrier gas, injection port temperature, extraction solvent, and internal standard.
6.1 Sample Collection.
6.1.1 Sampling Containers. Dual-seal sampling containers, four to eight fluid ounce capacity, should be used to collect the samples. Glass sample bottles or plastic containers with volatile organic compound (VOC) impermeable walls must be used for corrosive substances (e.g., etch primers and certain coating catalysts such as methyl ethyl ketone (MEK) peroxide). Sample containers, caps, and inner seal liners must be inert to the compounds in the sample and must be selected on a case-by-case basis.
6.1.1.1 Other routine sampling supplies needed include waterproof marking pens, tubing, scrappers/spatulas, clean rags, paper towels, cooler/ice, long handle tongs, and mixing/stirring paddles.
6.1.2 Personal safety equipment needed includes eye protection, respiratory protection, a hard hat, gloves, steel toe shoes, etc.
6.1.3 Shipping supplies needed include shipping boxes, packing material, shipping labels, strapping tape, etc.
6.1.4 Data recording forms and labels needed include coating data sheets and sample can labels.
Note:
The actual requirements will depend upon the conditions existing at the source sampled.
6.2 Laboratory Equipment and Supplies.
6.2.1 Gas Chromatograph (GC). Any instrument equipped with a flame ionization detector and capable of being temperature programmed may be used. Optionally, other types of detectors (e.g., a mass spectrometer), and any necessary interfaces, may be used provided that the detector system yields an appropriate and reproducible response to the analytes in the injected sample. Autosampler injection may be used, if available.
6.2.2 Recorder. If available, an electronic data station or integrator may be used to record the gas chromatogram and associated data. If a strip chart recorder is used, it must meet the following criteria: A 1 to 10 millivolt (mV) linear response with a full scale response time of 2 seconds or less and a maximum noise level of ±0.03 percent of full scale. Other types of recorders may be used as appropriate to the specific detector installed provided that the recorder has a full scale response time of 2 seconds or less and a maximum noise level of ±0.03 percent of full scale.
6.2.3 Column. The column must be constructed of materials that do not react with components of the sample (e.g., fused silica, stainless steel, glass). The column should be of appropriate physical dimensions (e.g., length, internal diameter) and contain sufficient suitable stationary phase to allow separation of the analytes. DB-5, DB-Wax, and FFAP columns are commonly used for paint analysis; however, it is the responsibility of each analyst to select appropriate columns and stationary phases.
6.2.4 Tube and Tube Fittings. Supplies to connect the GC and gas cylinders.
6.2.5 Pressure Regulators. Devices used to regulate the pressure between gas cylinders and the GC.
6.2.6 Flow Meter. A device used to determine the carrier gas flow rate through the GC. Either a digital flow meter or a soap film bubble meter may be used to measure gas flow rates.
6.2.7 Septa. Seals on the GC injection port through which liquid or gas samples can be injected using a syringe.
6.2.8 Liquid Charging Devices. Devices used to inject samples into the GC such as clean and graduated 1, 5, and 10 microliter (µl) capacity syringes.
6.2.9 Vials. Containers that can be sealed with a septum in which samples may be prepared or stored. The recommended size is 25 ml capacity. Mininert ® valves have been found satisfactory and are available from Pierce Chemical Company, Rockford, Illinois.
6.2.10 Balance. Device used to determine the weights of standards and samples. An analytical balance capable of accurately weighing to 0.0001 g is required.
7. Reagents and Standards
7.1 Purity of Reagents. Reagent grade chemicals shall be used in all tests. Unless otherwise specified, all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used provided it is first ascertained that the reagent is of sufficient purity to permit its use without lessening the accuracy of determination.
7.2 Carrier Gas. Helium carrier gas shall have a purity of 99.995 percent or higher. High purity nitrogen may also be used. Other carrier gases that are appropriate for the column system and analyte may also be used. Ultra-high purity grade hydrogen gas and zero-grade air shall be used for the flame ionization detector.
7.3 Dimethylformamide (DMF). Solvent for all standards and samples. Some other suitable solvent may be used if DMF is not compatible with the sample or coelutes with a target analyte.
Note:
DMF may coelute with ethylbenzene or p-xylene under the conditions described in the note under Section 6.2.3.
7.4 Internal Standard Materials. The internal standard material is used in the quantitation of the analytes for this method. It shall be gas chromatography spectrophotometric quality or, if this grade is not available, the highest quality available. Obtain the assay for the internal standard material and maintain at that purity during use. The recommended internal standard material is 1-propanol; however, selection of an appropriate internal standard material for the particular coating and GC conditions used is the responsibility of each analyst.
7.5 Reference Standard Materials. The reference standard materials are the chemicals cited in Section 1.1 which are of known identity and purity and which are used to assist in the identification and quantification of the analytes of this method. They shall be the highest quality available. Obtain the assays for the reference standard materials and maintain at those purities during use.
7.6 Stock Reference Standards. Stock reference standards are dilutions of the reference standard materials that may be used on a daily basis to prepare calibration standards, calibration check standards, and quality control check standards. Stock reference standards may be prepared from the reference standard materials or purchased as certified solutions.
7.6.1 Stock reference standards should be prepared in dimethylformamide for each analyte expected in the coating samples to be analyzed. The concentrations of analytes in the stock reference standards are not specified but must be adequate to prepare the calibration standards required in the method. A stock reference standard may contain more than one analyte provided all analytes are chemically compatible and no analytes coelute. The actual concentrations prepared must be known to within 0.1 percent (e.g., 0.1000 ±0.0001 g/g solution). The following procedure is suggested. Place about 35 ml of dimethylformamide into a tared ground-glass stoppered 50 ml volumetric flask. Weigh the flask to the nearest 0.1 mg. Add 12.5 g of the reference standard material and reweigh the flask. Dilute to volume with dimethylformamide and reweigh. Stopper the flask and mix the contents by inverting the flask several times. Calculate the concentration in grams per gram of solution from the net gain in weights, correcting for the assayed purity of the reference standard material.
Note:
Although a glass-stoppered volumetric flask is convenient, any suitable glass container may be used because stock reference standards are prepared by weight.
7.6.2 Transfer the stock reference standard solution into one or more Teflon-sealed screw-cap bottles. Store, with minimal headspace, at −10°C to 0°C and protect from light.
7.6.3 Prepare fresh stock reference standards every six months, or sooner if analysis results from daily calibration check standards indicate a problem. Fresh stock reference standards for very volatile HAP's may have to be prepared more frequently.
7.7 Calibration Standards. Calibration standards are used to determine the response of the detector to known amounts of reference material. Calibration standards must be prepared at a minimum of three concentration levels from the stock reference standards (see Section 7.6). Prepare the calibration standards in dimethylformamide (see Section 7.3). The lowest concentration standard should contain a concentration of analyte equivalent either to a concentration of no more than 0.01% of the analyte in a coating or to a concentration that is lower than the actual concentration of the analyte in the coating, whichever concentration is higher. The highest concentration standard should contain a concentration of analyte equivalent to slightly more than the highest concentration expected for the analyte in a coating. The remaining calibration standard should contain a concentration of analyte roughly at the midpoint of the range defined by the lowest and highest concentration calibration standards. The concentration range of the standards should thus correspond to the expected range of analyte concentrations in the prepared coating samples (see Section 11.5). Each calibration standard should contain each analyte for detection by this method expected in the actual coating samples (e.g., some or all of the compounds listed in Section 1.1 may be included). Each calibration standard should also contain an appropriate amount of internal standard material (response for the internal standard material is within 25 to 75 percent of full scale on the attenuation setting for the particular reference standard concentration level). Calibration Standards should be stored for 1 week only in sealed vials with minimal headspace. If the stock reference standards were prepared as specified in Section 7.6, the calibration standards may be prepared by either weighing each addition of the stock reference standard or by adding known volumes of the stock reference standard and calculating the mass of the standard reference material added. Alternative 1 (Section 7.7.1) specifies the procedure to be followed when the stock reference standard is added by volume. Alternative 2 (Section 7.7.2) specifies the procedure to be followed when the stock reference standard is added by weight.
Note:
To assist with determining the appropriate amount of internal standard to add, as required here and in other sections of this method, the analyst may find it advantageous to prepare a curve showing the area response versus the amount of internal standard injected into the GC.
7.7.1 Preparation Alternative 1. Determine the amount of each stock reference standard and dimethylformamide solvent needed to prepare approximately 25 ml of the specific calibration concentration level desired. To a tared 25 ml vial that can be sealed with a crimp-on or Mininert ® valve, add the total amount of dimethylformamide calculated to be needed. As quickly as practical, add the calculated amount of each stock reference standard using new pipets (or pipet tips) for each stock reference standard. Reweigh the vial and seal it. Using the known weights of the standard reference materials per ml in the stock reference standards, the volumes added, and the total weight of all reagents added to the vial, calculate the weight percent of each standard reference material in the calibration standard prepared. Repeat this process for each calibration standard to be prepared.
7.7.2 Preparation Alternative 2. Determine the amount of each stock reference standard and dimethylformamide solvent needed to prepare approximately 25 ml of the specific calibration concentration level desired. To a tared 25 ml vial that can be sealed with a crimp-on or Mininert ® valve, add the total amount of dimethylformamide calculated to be needed. As quickly as practical, add the calculated amount of a stock reference standard using a new pipet (or pipet tip) and reweigh the vial. Repeat this process for each stock reference standard to be added. Seal the vial after obtaining the final weight. Using the known weight percents of the standard reference materials in the stock reference standards, the weights of the stock reference standards added, and the total weight of all reagents added to the vial, calculate the weight percent of each standard reference material in the calibration standard prepared. Repeat this process for each calibration standard to be prepared.
8. Sample Collection, Preservation, Transport, and Storage
8.1 Copies of material safety data sheets (MSDS's) for each sample should be obtained prior to sampling. The MSDS's contain information on the ingredients, and physical and chemical properties data. The MSDS's also contain recommendations for proper handling or required safety precautions. Certified product data sheets (CPDS) may also include information relevant to the analysis of the coating sample including, but not limited to, separation column, oven temperature, carrier gas, injection port temperature, extraction solvent, and internal standard.
8.2 A copy of the blender's worksheet can be requested to obtain data on the exact coating being sampled. A blank coating data sheet form (see Section 18) may also be used. The manufacturer's formulation information from the product data sheet should also be obtained.
8.3 Prior to sample collection, thoroughly mix the coating to ensure that a representative, homogeneous sample is obtained. It is preferred that this be accomplished using a coating can shaker or similar device; however, when necessary, this may be accomplished using mechanical agitation or circulation systems.
8.3.1 Water-thinned coatings tend to incorporate or entrain air bubbles if stirred too vigorously; mix these types of coatings slowly and only as long as necessary to homogenize.
8.3.2 Each component of multicomponent coatings that harden when mixed must be sampled separately. The component mix ratios must be obtained at the facility at the time of sampling and submitted to the analytical laboratory.
8.4 Sample Collection. Samples must be collected in a manner that prevents or minimizes loss of volatile components and that does not contaminate the coating reservoir. A suggested procedure is as follows. Select a sample collection container which has a capacity at least 25 percent greater than the container in which the sample is to be transported. Make sure both sample containers are clean and dry. Using clean, long-handled tongs, turn the sample collection container upside down and lower it into the coating reservoir. The mouth of the sample collection container should be at approximately the midpoint of the reservoir (do not take the sample from the top surface). Turn the sample collection container over and slowly bring it to the top of the coating reservoir. Rapidly pour the collected coating into the sample container, filling it completely. It is important to fill the sample container completely to avoid any loss of volatiles due to volatilization into the headspace. Return any unused coating to the reservoir or dispose as appropriate.
Note:
If a company requests a set of samples for its own analysis, a separate set of samples, using new sample containers, should be taken at the same time.
8.5 Once the sample is collected, place the sample container on a firm surface and insert the inner seal in the container by placing the seal inside the rim of the container, inverting a screw cap, and pressing down on the screw cap which will evenly force the inner seal into the container for a tight fit. Using clean towels or rags, remove all residual coating material from the outside of the sample container after inserting the inner seal. Screw the cap onto the container.
8.5.1 Affix a sample label (see Section 18) clearly identifying the sample, date collected, and person collecting the sample.
8.5.2 Prepare the sample for transportation to the laboratory. The sample should be maintained at the coating's recommended storage temperature specified on the Material Safety Data Sheet, or, if no temperature is specified, the sample should be maintained within the range of 5°C to 38°C.
8.9 The shipping container should adhere to U.S. Department of Transportation specification DOT 12-B. Coating samples are considered hazardous materials; appropriate shipping procedures should be followed.
9. Quality Control
9.1 Laboratories using this method should operate a formal quality control program. The minimum requirements of the program should consist of an initial demonstration of laboratory capability and an ongoing analysis of blanks and quality control samples to evaluate and document quality data. The laboratory must maintain records to document the quality of the data generated. When results indicate atypical method performance, a quality control check standard (see Section 9.4) must be analyzed to confirm that the measurements were performed in an in-control mode of operation.
9.2 Before processing any samples, the analyst must demonstrate, through analysis of a reagent blank, that there are no interferences from the analytical system, glassware, and reagents that would bias the sample analysis results. Each time a set of analytical samples is processed or there is a change in reagents, a reagent blank should be processed as a safeguard against chronic laboratory contamination. The blank samples should be carried through all stages of the sample preparation and measurement steps.
9.3 Required instrument quality control parameters are found in the following sections:
9.3.1 Baseline stability must be demonstrated to be ≤5 percent of full scale using the procedures given in Section 10.1.
9.3.2 The GC calibration is not valid unless the retention time (RT) for each analyte at each concentration is within ±0.05 min of the retention time measured for that analyte in the stock standard.
9.3.3 The retention time (RT) of any sample analyte must be within ±0.05 min of the average RT of the analyte in the calibration standards for the analyte to be considered tentatively identified.
9.3.4 The GC system must be calibrated as specified in Section 10.2.
9.3.5 A one-point daily calibration check must be performed as specified in Section 10.3.
9.4 To establish the ability to generate results having acceptable accuracy and precision, the analyst must perform the following operations.
9.4.1 Prepare a quality control check standard (QCCS) containing each analyte expected in the coating samples at a concentration expected to result in a response between 25 percent and 75 percent of the limits of the calibration curve when the sample is prepared as described in Section 11.5. The QCCS may be prepared from reference standard materials or purchased as certified solutions. If prepared in the laboratory, the QCCS must be prepared independently from the calibration standards.
9.4.2 Analyze three aliquots of the QCCS according to the method beginning in Section 11.5.3 and calculate the weight percent of each analyte using Equation 1, Section 12.
9.4.3 Calculate the mean weight percent (X͞) for each analyte from the three results obtained in Section 9.4.2.
9.4.4 Calculate the percent accuracy for each analyte using the known concentrations (Ti) in the QCCS using Equation 3, Section 12.
9.4.5 Calculate the percent relative standard deviation (percent RSD) for each analyte using Equation 7, Section 12, substituting the appropriate values for the relative response factors (RRF's) in said equation.
9.4.6 If the percent accuracy (Section 9.4.4) for all analytes is within the range 90 percent to 110 percent and the percent RSD (Section 9.4.5) for all analytes is ≤20 percent, system performance is acceptable and sample analysis may begin. If these criteria are not met for any analyte, then system performance is not acceptable for that analyte and the test must be repeated for those analytes only. Repeated failures indicate a general problem with the measurement system that must be located and corrected. In this case, the entire test, beginning at Section 9.4.1, must be repeated after the problem is corrected.
9.5 Great care must be exercised to maintain the integrity of all standards. It is recommended that all standards be stored at −10°C to 0°C in screw-cap amber glass bottles with Teflon liners.
9.6 Unless otherwise specified, all weights are to be recorded within 0.1 mg.
10. Calibration and Standardization.
10.1 Column Baseline Drift. Before each calibration and series of determinations and before the daily calibration check, condition the column using procedures developed by the laboratory or as specified by the column supplier. Operate the GC at initial (i.e., before sample injection) conditions on the lowest attenuation to be used during sample analysis. Adjust the recorder pen to zero on the chart and obtain a baseline for at least one minute. Initiate the GC operating cycle that would be used for sample analysis. On the recorder chart, mark the pen position at the end of the simulated sample analysis cycle. Baseline drift is defined as the absolute difference in the pen positions at the beginning and end of the cycle in the direction perpendicular to the chart movement. Calculate the percent baseline drift by dividing the baseline drift by the chart width representing full-scale deflection and multiply the result by 100.
10.2 Calibration of GC. Bring all stock standards and calibration standards to room temperature while establishing the GC at the determined operating conditions.
10.2.1 Retention Times (RT's) for Individual Compounds.
Note:
The procedures of this subsection are required only for the initial calibration. However, it is good laboratory practice to follow these procedures for some or all analytes before each calibration. The procedures were written for chromatograms output to a strip chart recorder. More modern instruments (e.g., integrators and electronic data stations) determine and print out or display retention times automatically.
The RT for each analyte should be determined before calibration. This provides a positive identification for each peak observed from the calibration standards. Inject an appropriate volume (see note in Section 11.5.2) of one of the stock reference standards into the gas chromatograph and record on the chart the pen position at the time of the injection (see Section 7.6.1). Dilute an aliquot of the stock reference standard as required in dimethylformamide to achieve a concentration that will result in an on-scale response. Operate the gas chromatograph according to the determined procedures. Select the peak(s) that correspond to the analyte(s) [and internal standard, if used] and measure the retention time(s). If a chart recorder is used, measure the distance(s) on the chart from the injection point to the peak maxima. These distances, divided by the chart speed, are defined as the RT's of the analytes in question. Repeat this process for each of the stock reference standard solutions.
Note:
If gas chromatography with mass spectrometer detection (GC-MS) is used, a stock reference standard may contain a group of analytes, provided all analytes are adequately separated during the analysis. Mass spectral library matching can be used to identify the analyte associated with each peak in the gas chromatogram. The retention time for the analyte then becomes the retention time of its peak in the chromatogram.
10.2.2 Calibration. The GC must be calibrated using a minimum of three concentration levels of each potential analyte. (See Section 7.7 for instructions on preparation of the calibration standards.) Beginning with the lowest concentration level calibration standard, carry out the analysis procedure as described beginning in Section 11.7. Repeat the procedure for each progressively higher concentration level until all calibration standards have been analyzed.
10.2.2.1 Calculate the RT's for the internal standard and for each analyte in the calibration standards at each concentration level as described in Section 10.2.1. The RT's for the internal standard must not vary by more than 0.10 minutes. Identify each analyte by comparison of the RT's for peak maxima to the RT's determined in Section 10.2.1.
10.2.2.2 Compare the retention times (RT's) for each potential analyte in the calibration standards for each concentration level to the retention times determined in Section 10.2.1. The calibration is not valid unless all RT's for all analytes meet the criteria given in Section 9.3.2.
10.2.2.3 Tabulate the area responses and the concentrations for the internal standard and each analyte in the calibration standards. Calculate the response factor for the internal standard (RFis) and the response factor for each compound relative to the internal standard (RRF) for each concentration level using Equations 5 and 6, Section 12.
10.2.2.4 Using the RRF's from the calibration, calculate the percent relative standard deviation (percent RSD) for each analyte in the calibration standard using Equation 7, Section 12. The percent RSD for each individual calibration analyte must be less than 15 percent. This criterion must be met in order for the calibration to be valid. If the criterion is met, the mean RRF's determined above are to be used until the next calibration.
10.3 Daily Calibration Checks. The calibration curve (Section 10.2.2) must be checked and verified at least once each day that samples are analyzed. This is accomplished by analyzing a calibration standard that is at a concentration near the midpoint of the working range and performing the checks in Sections 10.3.1, 10.3.2, and 10.3.3.
10.3.1 For each analyte in the calibration standard, calculate the percent difference in the RRF from the last calibration using Equation 8, Section 12. If the percent difference for each calibration analyte is less than 10 percent, the last calibration curve is assumed to be valid. If the percent difference for any analyte is greater than 5 percent, the analyst should consider this a warning limit. If the percent difference for any one calibration analyte exceeds 10 percent, corrective action must be taken. If no source of the problem can be determined after corrective action has been taken, a new three-point (minimum) calibration must be generated. This criterion must be met before quantitative analysis begins.
10.3.2 If the RFis for the internal standard changes by more than ±20 percent from the last daily calibration check, the system must be inspected for malfunctions and corrections made as appropriate.
10.3.3 The retention times for the internal standard and all calibration check analytes must be evaluated. If the retention time for the internal standard or for any calibration check analyte changes by more than 0.10 min from the last calibration, the system must be inspected for malfunctions and corrections made as required.
11. Procedure
11.1 All samples and standards must be allowed to warm to room temperature before analysis. Observe the given order of ingredient addition to minimize loss of volatiles.
11.2 Bring the GC system to the determined operating conditions and condition the column as described in Section 10.1.
Note:
The temperature of the injection port may be an especially critical parameter. Information about the proper temperature may be found on the CPDS.
11.3 Perform the daily calibration checks as described in Section 10.3. Samples are not to be analyzed until the criteria in Section 10.3 are met.
11.4 Place the as-received coating sample on a paint shaker, or similar device, and shake the sample for a minimum of 5 minutes to achieve homogenization.
11.5 Note: The steps in this section must be performed rapidly and without interruption to avoid loss of volatile organics. These steps must be performed in a laboratory hood free from solvent vapors. All weights must be recorded to the nearest 0.1 mg.
11.5.1 Add 16 g of dimethylformamide to each of two tared vials (A and B) capable of being septum sealed.
11.5.2 To each vial add a weight of coating that will result in the response for the major constituent being in the upper half of the linear range of the calibration curve.
Note:
The magnitude of the response obviously depends on the amount of sample injected into the GC as specified in Section 11.8. This volume must be the same as used for preparation of the calibration curve, otherwise shifts in compound retention times may occur. If a sample is prepared that results in a response outside the limits of the calibration curve, new samples must be prepared; changing the volume injected to bring the response within the calibration curve limits is not permitted.
11.5.3 Add a weight of internal standard to each vial (A and B) that will result in the response for the internal standard being between 25 percent and 75 percent of the linear range of the calibration curve.
11.5.4 Seal the vials with crimp-on or Mininert ® septum seals.
11.6 Shake the vials containing the prepared coating samples for 60 seconds. Allow the vials to stand undisturbed for ten minutes. If solids have not settled out on the bottom after 10 minutes, then centrifuge at 1,000 rpm for 5 minutes. The analyst also has the option of injecting the sample without allowing the solids to settle.
11.7 Analyses should be conducted in the following order: daily calibration check sample, method blank, up to 10 injections from sample vials (i.e., one injection each from up to five pairs of vials, which corresponds to analysis of 5 coating samples).
11.8 Inject the prescribed volume of supernatant from the calibration check sample, the method blank, and the sample vials onto the chromatographic column and record the chromatograms while operating the system under the specified operating conditions.
Note:
The analyst has the option of injecting the unseparated sample.
12. Data Analysis and Calculations
12.1 Qualitative Analysis. An analyte (e.g., those cited in Section 1.1) is considered tentatively identified if two criteria are satisfied: (1) elution of the sample analyte within ±0.05 min of the average GC retention time of the same analyte in the calibration standard; and (2) either (a) confirmation of the identity of the compound by spectral matching on a gas chromatograph equipped with a mass selective detector or (b) elution of the sample analyte within ±0.05 min of the average GC retention time of the same analyte in the calibration standard analyzed on a dissimilar GC column.
12.1.1 The RT of the sample analyte must meet the criteria specified in Section 9.3.3.
12.1.2 When doubt exists as to the identification of a peak or the resolution of two or more components possibly comprising one peak, additional confirmatory techniques (listed in Section 12.1) must be used.
12.2 Quantitative Analysis. When an analyte has been identified, the quantification of that compound will be based on the internal standard technique.
12.2.1 A single analysis consists of one injection from each of two sample vials (A and B) prepared using the same coating. Calculate the concentration of each identified analyte in the sample as follows:

12.2.2 Report results for duplicate analysis (sample vials A and B) without correction.
12.3 Precision Data. Calculate the percent difference between the measured concentrations of each analyte in vials A and B as follows.
12.3.1 Calculate the weight percent of the analyte in each of the two sample vials as described in Section 12.2.1.
12.3.2 Calculate the percent difference for each analyte as:

where Ai and Bi are the measured concentrations of the analyte in vials A and B.
12.4 Calculate the percent accuracy for analytes in the QCCS (See Section 9.4) as follows:

where Xx is the mean measured value and Tx is the known true value of the analyte in the QCCS.
12.5 Obtain retention times (RT's) from data station or integrator or, for chromatograms from a chart recorder, calculate the RT's for analytes in the calibration standards (See Section 10.2.2.2) as follows:

12.6 Calculate the response factor for the internal standard (See Section 10.2.2.3) as follows:

where:
Ais = Area response of the internal standard.
Cis = Weight percent of the internal standard.
12.7 Calculate the relative response factors for analytes in the calibration standards (See Section 10.2.2.3) as follows:
where:

RRFx = Relative response factor for an individual analyte.
Ax = Area response of the analyte being measured.
Cx = Weight percent of the analyte being measured.
12.8 Calculate the percent relative standard deviation of the relative response factors for analytes in the calibration standards (See Section 10.2.2.4) as follows:

12.9 Calculate the percent difference in the relative response factors between the calibration curve and the daily calibration checks (See Section 10.3) as follows:

13. Measurement of Reaction Byproducts That are HAP [Reserved]
14. Method Performance [Reserved]
15. Pollution Prevention [Reserved]
16. Waste Management
16.1 The coating samples and laboratory standards and reagents may contain compounds which require management as hazardous waste. It is the laboratory's responsibility to ensure all wastes are managed in accordance with all applicable laws and regulations.
16.2 To avoid excessive laboratory waste, obtain only enough sample for laboratory analysis.
16.3 It is recommended that discarded waste coating solids, used rags, used paper towels, and other nonglass or nonsharp waste materials be placed in a plastic bag before disposal. A separate container, designated “For Sharp Objects Only,” is recommended for collection of discarded glassware and other sharp-edge items used in the laboratory. It is recommended that unused or excess samples and reagents be placed in a solvent-resistant plastic or metal container with a lid or cover designed for flammable liquids. This container should not be stored in the area where analytical work is performed. It is recommended that a record be kept of all compounds placed in the container for identification of the contents upon disposal.
17. References
1. Clean Air Act Amendments, Public Law 101-549, Titles I-XI, November, 1990.
2. Standard Test Method for Water Content of Water-Reducible Paints by Direct Injection into a Gas Chromatograph. ASTM Designation D3792-79.
3. Standard Practice for Sampling Liquid Paints and Related Pigment Coatings. ASTM Designation D3925-81.
4. Standard Test Method for Determination of Dichloromethane and 1,1,1-Trichloroethane in Paints and Coatings by Direct Injection into a Gas Chromatograph. ASTM Designation D4457-02.
5. Standard Test Method for Determining the Unreacted Monomer Content of Latexes Using Capillary Column Gas Chromatography. ASTM Designation D4827-03.
6. Standard Test Method for Determining Unreacted Monomer Content of Latexes Using Gas-Liquid Chromatography, ASTM Designation D4747-02.
7. Method 301 - “Field Validation of Pollutant Measurement Methods from Various Waste Media,” 40 CFR 63, Appendix A.
8. “Reagent Chemicals, American Chemical Society Specifications,” American Chemical Society, Washington, DC. For suggestions on the testing of reagents not listed by the American Chemical Society, see “Reagent Chemicals and Standards” by Joseph Rosin, D. Van Nostrand Co., Inc., New York, NY and the “United States Pharmacopeia.”
18. Tables, Diagrams, Flowcharts, and Validation Data
Agency:
Inspector:
Date/Time:
Sample ID#:
Source ID:
Coating Name/Type:
Plant Witness:
Type Analysis Required:
Special Handling:
Sample Container Label
Coating Data
Date:
Source:
| Data | Sample ID No. | Sample ID No. |
|---|---|---|
| Coating: | ||
| Supplier Name | ||
| Name and Color of Coating | ||
| Type of Coating (primer, clearcoat, etc.) | ||
| Identification Number for Coating | ||
| Coating Density (lbs/gal) | ||
| Total Volatiles Content (wt percent) | ||
| Water Content (wt percent) | ||
| Exempt Solvents Content (wt percent) | ||
| VOC Content (wt percent) | ||
| Solids Content (vol percent) | ||
| Diluent Properties: | ||
| Name | ||
| Identification Number | ||
| Diluent Solvent Density (lbs/gal) | ||
| VOC Content (wt percent) | ||
| Water Content (wt percent) | ||
| Exempt Solvent Content (wt percent) | ||
| Diluent/Solvent Ratio (gal diluent solvent/gal coating) |
Stock Reference Standard
Name of Reference Material:
Supplier Name:
Lot Number:
Purity:
Name of Solvent Material: Dimethylformamide
Supplier Name:
Lot Number:
Purity:
Date Prepared:
Prepared By:
Notebook/page no.:
| 1. Weight Empty Flask | ____,g |
| 2. Weight Plus DMF | ____,g |
| 3. Weight Plus Reference Material | ____,g |
| 4. Weight After Made to Volume | ____,g |
| 5. Weight DMF (lines 2-1 + 3-4) | ____,g |
| 6. Weight Ref. Material (lines 3-2) | ____,g |
| 7. Corrected Weight of Reference Material (line 6 times purity) | ____,g |
| 8. Fraction Reference Material in Standard (Line 7 ÷ Line 5) soln | ____,g/g |
| 9. Total Volume of Standard Solution | ____, ml |
| 10. Weight Reference Material per ml of Solution (Line 7 ÷ Line 9) | ____,g/ml |
| Laboratory ID No. for this Standard | ____ |
| Expiration Date for this Standard | ____ |
CALIBRATION STANDARD
Date Prepared:
Date Expires:
Prepared By:
Notebook/page:
Calibration Standard Identification No.:
| Final Weight Flask Plus Reagents | ____, g |
| Weight Empty Flask | ____, g |
| Total Weight Of Reagents | ____, g |
| Analyte name a | Stock reference standard ID No. | Amount of stock reference standard added (by volume or by weight) | Calculated weight analyte added, g | Weight percent analyte in calibration standard b | |||
|---|---|---|---|---|---|---|---|
| Volume added, ml | Amount in standard, g/ml | Weight added, g | Amount in standard, g/g soln | ||||
| a Include internal standard(s).
b Weight percent = weight analyte added ÷ total weight of reagents. | |||||||
Quality Control Check Standard
Date Prepared:
Date Expires:
Prepared By:
Notebook/page:
Quality Control Check Standard Identification No.:
| Final Weight Flask Plus Reagents | ____,g |
| Weight Empty Flask | ____,g |
| Total Weight Of Reagents | ____,g |
| Analyte name a | Stock reference standard ID No. | Amount of stock reference standard added (by volume or by weight) | Calculated weight analyte added, g | Weight percent analyte in QCC standard b | |||
|---|---|---|---|---|---|---|---|
| Volume added, ml | Amount in standard, g/ml | Weight added, g | Amount in standard, g/g soln | ||||
| a Include internal Standard(s).
b Weight percent = weight analyte added ÷ total weight of reagents. | |||||||
Quality Control Check Standard Analysis
Date OCCS Analyzed:
OCCS Identification No.
Analyst:
QCC Expiration Date:
| Analyte | Weight percent determined | Mean Wt percent | Percent accuracx | Percent RSD | Meets criteria in Section 9.4.6 | |||
|---|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 |
Percent
accuracy | Percent RSD | ||||
Calibration of Gas Chromatograph
Calibration Date:
Calibrated By:
| Analyte | Stock standard. ID No. | Recorder chart speed | Distance from injection point to peak maximum | Retention time, minutes a | ||
|---|---|---|---|---|---|---|
| Inches/min. | cm/min. | Inches | Centimeters | |||
| a Retention time = distance to peak maxima ÷ chart speed. | ||||||
CALIBRATION OF GAS CHROMATOGRAPH
Calibration Date:
Calibrated By:
| Analyte | Calib. STD ID No. | Calib. STD ID No. | Calib. STD ID No. |
|---|---|---|---|
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT | |||
| Internal Standard Name: | |||
| Conc. in STD | |||
| Area Response | |||
| RT |
Calibration of Gas Chromatograph
Calibration Date:
Calibrated By:
| Analyte | Calib. STD ID | Calib. STD ID | Calib. STD ID | Mean | percent RSD of RF | Is RT within ±0.05 min of RT for stock? (Y/N) | Is percent RSD <30% (Y/N) |
|---|---|---|---|---|---|---|---|
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF | |||||||
| Name: | |||||||
| RT | |||||||
| RF |
Daily Calibration Check
Date:
Analyst:
Calibration Check Standard ID No.:
Expiration Date:
| Analyte | Retention Time (RT) | Response Factor (RF) | ||||
|---|---|---|---|---|---|---|
| Last | This | Difference a | Last | This | Difference b | |
| a Retention time (RT) change (difference) must be less than ±0.10 minutes.
b Response factor (RF) change (difference) must be less than 20 percent for each analyte and for the internal standard. | ||||||
Sample Analysis
Vial A ID No.:
Vial B ID No.:
Analyzed By:
Date:
| Sample preparation information | Vial A (g) | Vial B (g) |
|---|---|---|
| Measured: | ||
| wt empty via | ||
| wt plus DMF | ||
| wt plus sample | ||
| wt plus internal | ||
| standard | ||
| Calculated: | ||
| wt DMF | ||
| wt sample | ||
| wt internal standard |
| Analyte | Area response | RF | Wt percent in sample | |||
|---|---|---|---|---|---|---|
| Vial A | Vial B | Vial A | Vial B | Average | ||
| Internal Standard | ||||||
Method 312A - Determination of Styrene in Latex Styrene-Butadiene Rubber, Through Gas Chromatography
1. Scope and Application
1.1 This method describes a procedure for determining parts per million (ppm) styrene monomer (CAS No. 100-42-5) in aqueous samples, including latex samples and styrene stripper water.
1.2 The sample is separated in a gas chromatograph equipped with a packed column and a flame ionization detector.
2.0 Summary of Method
2.1 This method utilizes a packed column gas chromatograph with a flame ionization detector to determine the concentration of residual styrene in styrene butadiene rubber (SBR) latex samples.
3.0 Definitions
3.1 The definitions are included in the text as needed.
4.0 Interferences
4.1 In order to reduce matrix effects and emulsify the styrene, similar styrene free latex is added to the internal standard. There are no known interferences.
4.2 The operating parameters are selected to obtain resolution necessary to determine styrene monomer concentrations in latex.
5.0 Safety
5.1 It is the responsibility of the user of this procedure to establish appropriate safety and health practices.
6.0 Equipment and Supplies
6.1 Adjustable bottle-top dispenser, set to deliver 3 ml. (for internal standard), Brinkmann Dispensette, or equivalent.
6.2 Pipettor, set to 10 ml., Oxford Macro-set, or equivalent.
6.3 Volumetric flask, 100-ml, with stopper.
6.4 Hewlett Packard Model 5710A dual channel gas chromatograph equipped with flame ionization detector.
6.4.1 11 ft. × 1/8 in. stainless steel column packed with 10% TCEP on 100/120 mesh Chromosorb P, or equivalent.
6.4.2 Perkin Elmer Model 023 strip chart recorder, or equivalent.
6.5 Helium carrier gas, zero grade.
6.6 Liquid syringe, 25-µl.
6.7 Digital MicroVAX 3100 computer with VG Multichrom software, or equivalent data handling system.
6.6 Wire Screens, circular, 70-mm, 80-mesh diamond weave.
6.7 DEHA - (N,N-Diethyl hydroxylamine), 97 + % purity, CAS No. 3710-84-7
6.8 p-Dioxane, CAS No. 123-91-1
7.0 Reagents and Standards
7.1 Internal standard preparation.
7.1.1 Pipette 5 ml p-dioxane into a 1000-ml volumetric flask and fill to the mark with distilled water and mix thoroughly.
7.2 Calibration solution preparation.
7.2.1 Pipette 10 ml styrene-free latex (eg: NBR latex) into a 100-ml volumetric flask.
7.2.2 Add 3 ml internal standard (section 7.1.1 of this method).
7.2.3 Weigh exactly 10 µl fresh styrene and record the weight.
7.2.4 Inject the styrene into the flask and mix well.
7.2.5 Add 2 drops of DEHA, fill to the mark with water and mix well again.
7.2.6 Calculate concentration of the calibration solution as follows:
mg/l styrene = (mg styrene added)/0.1 L
8.0 Sample Collection, Preservation, and Storage
8.1 A representative SBR emulsion sample should be caught in a clean, dry 6-oz. teflon lined glass container. Close it properly to assure no sample leakage.
8.2 The container should be labeled with sample identification, date and time.
9.0 Quality Control
9.1 The instrument is calibrated by injecting calibration solution (Section 7.2 of this method) five times.
9.2 The retention time for components of interest and relative response of monomer to the internal standard is determined.
9.3 Recovery efficiency must be determined once for each sample type and whenever modifications are made to the method.
9.3.1 A set of six latex samples shall be collected. Two samples shall be prepared for analysis from each sample. Each sample shall be analyzed in duplicate.
9.3.2 The second set of six latex samples shall be analyzed in duplicate before spiking each sample with approximately 1000 ppm styrene. The spiked samples shall be analyzed in duplicate.
9.3.3 For each hydrocarbon, calculate the average recovery efficiency (R) using the following equations:
where:
R=Σ(Rn)/6
where:
Rn = (cns−cv)/Sn
n = sample number
cns = concentration of compound measured in spiked sample number n.
cnu = concentration of compound measured in unspiked sample number n.
Sn = theoretical concentration of compound spiked into sample n.
9.3.4 A value of R between 0.70 and 1.30 is acceptable.
9.3.5 R is used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type.
10.0 Calibration and Instrument Settings
10.1 Injection port temperature, 250°C.
10.2 Oven temperature, 110°C, isothermal.
10.3 Carrier gas flow, 25 cc/min.
10.4 Detector temperature, 250°C.
10.5 Range, 1X.
11.0 Procedure
11.1 Turn on recorder and adjust baseline to zero.
11.2 Prepare sample.
11.2.1 For latex samples, add 3 ml Internal Standard (section 7.1 of this method) to a 100-ml volumetric flask. Pipet 10 ml sample into the flask using the Oxford pipettor, dilute to the 100-ml mark with water, and shake well.
11.2.2 For water samples, add 3 ml Internal Standard (section 7.1 of this method) to a 100-ml volumetric flask and fill to the mark with sample. Shake well.
11.3 Flush syringe with sample.
11.4 Carefully inject 2 µl of sample into the gas chromatograph column injection port and press the start button.
11.5 When the run is complete the computer will print a report of the analysis.
12.0 Data Analysis and Calculation
12.1 For samples that are prepared as in section 11.2.1 of this method:
ppm styrene = A × D
Where:
A = “ppm” readout from computer
D = dilution factor (10 for latex samples)
12.2 For samples that are prepared as in section 11.2.2 of this method, ppm styrene is read directly from the computer.
13.0 Method Performance
13.1 This test has a standard deviation (1) of 3.3 ppm at 100 ppm styrene. The average Spike Recovery from six samples at 1000 ppm Styrene was 96.7 percent. The test method was validated using 926 ppm styrene standard. Six analysis of the same standard provided average 97.7 percent recovery. Note: These are example recoveries and do not replace quality assurance procedures in this method.
14.0 Pollution Prevention
14.1 Waste generation should be minimized where possible. Sample size should be an amount necessary to adequately run the analysis.
15.0 Waste Management
15.1 All waste shall be handled in accordance with Federal and State environmental regulations.
16.0 References and Publications
16.1 40 CFR 63 Appendix A - Method 301 Test Methods Field Validation of Pollutant Measurement
16.2 DSM Copolymer Test Method T-3060, dated October 19, 1995, entitled: Determination of Residual Styrene in Latex, Leonard, C.D., Vora, N.M.et al
Method 312B - Determination of Residual Styrene in Styrene-Butadiene (SBR) Rubber Latex by Capillary Gas Chromatography
1.0 Scope
1.1 This method is applicable to SBR latex solutions.
1.2 This method quantitatively determines residual styrene concentrations in SBR latex solutions at levels from 80 to 1200 ppm.
2.0 Principle of Method
2.1 A weighed sample of a latex solution is coagulated with an ethyl alcohol (EtOH) solution containing a specific amount of alpha-methyl styrene (AMS) as the internal standard. The extract of this coagulation is then injected into a gas chromatograph and separated into individual components. Quantification is achieved by the method of internal standardization.
3.0 Definitions
3.1 The definitions are included in the text as needed.
4.0 Interferences [Reserved]
5.0 Safety
5.1 This method may involve hazardous materials, operations, and equipment. This method does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
6.0 Equipment and Supplies
6.1 Analytical balance, 160 g capacity, and 0.1 mg resolution
6.2 Bottles, 2-oz capacity, with poly-cap screw lids
6.3 Mechanical shaker
6.4 Syringe, 10-ul capacity
6.5 Gas chromatograph, Hewlett Packard model 5890A, or equivalent, configured with FID with a megabore jet, splitless injector packed with silanized glass wool.
6.5.1 Establish the following gas chromatographic conditions, and allow the system to thoroughly equilibrate before use.
Injection technique = Splitless
Injector temperature = 225 deg C
Oven temperature = 70 deg C (isothermal)
Detector: temperature = 300 deg C
range = 5
attenuation = 0
Carrier gas: helium = 47 ml/min
Detector gases: hydrogen = 30 ml/min
air = 270 ml/min
make-up = 0 ml/min
Analysis time: = 3.2 min at the specified carrier gas flow rate and column temperature.
6.6 Gas chromatographic column, DB-1, 30 M X 0.53 ID, or equivalent, with a 1.5 micron film thickness.
6.7 Data collection system, Perkin-Elmer/Nelson Series Turbochrom 4 Series 900 Interface, or equivalent.
6.8 Pipet, automatic dispensing, 50-ml capacity, and 2-liter reservoir.
6.9 Flasks, volumetric, class A, 100-ml and 1000-ml capacity.
6.10 Pipet, volumetric delivery, 10-ml capacity, class A.
7.0 Chemicals and Reagents
CHEMICALS:
7.1 Styrene, C8H8, 99 + %, CAS 100-42-5
7.2 Alpha methyl styrene, C9H10, 99%, CAS 98-83-9
7.3 Ethyl alcohol, C2H5OH, denatured formula 2B, CAS 64-17-5
REAGENTS:
7.4 Internal Standard Stock Solution: 5.0 mg/ml AMS in ethyl alcohol.
7.4.1 Into a 100-ml volumetric flask, weigh 0.50 g of AMS to the nearest 0.1 mg.
7.4.2 Dilute to the mark with ethyl alcohol. This solution will contain 5.0 mg/ml AMS in ethyl alcohol and will be labeled the AMS STOCK SOLUTION.
7.5 Internal Standard Working Solution: 2500 ug/50 ml of AMS in ethyl alcohol.
7.5.1 Using a 10 ml volumetric pipet, quantitatively transfer 10.0 ml of the AMS STOCK SOLUTION into a 1000-ml volumetric flask.
7.5.2 Dilute to the mark with ethyl alcohol. This solution will contain 2500 ug/50ml of AMS in ethyl alcohol and will be labeled the AMS WORKING SOLUTION.
7.5.3 Transfer the AMS WORKING SOLUTION to the automatic dispensing pipet reservoir.
7.6 Styrene Stock Solution: 5.0 mg/ml styrene in ethyl alcohol.
7.6.1 Into a 100-ml volumetric flask, weigh 0.50 g of styrene to the nearest 0.1 mg.
7.6.2 Dilute to the mark with ethyl alcohol. This solution will contain 5.0 mg/ml styrene in ethyl alcohol and will be labeled the STYRENE STOCK SOLUTION.
7.7 Styrene Working Solution: 5000 ug/10 ml of styrene in ethyl alcohol.
7.7.1 Using a 10-ml volumetric pipet, quantitatively transfer 10.0 ml of the STYRENE STOCK SOLUTION into a 100-ml volumetric flask.
7.7.2 Dilute to the mark with ethyl alcohol. This solution will contain 5000 ug/10 ml of styrene in ethyl alcohol and will be labeled the STYRENE WORKING SOLUTION.
8.0 Sample Collection, Preservation and Storage
8.1 Label a 2-oz sample poly-cap lid with the identity, date and time of the sample to be obtained.
8.2 At the sample location, open sample valve for at least 15 seconds to ensure that the sampling pipe has been properly flushed with fresh sample.
8.3 Fill the sample jar to the top (no headspace) with sample, then cap it tightly.
8.4 Deliver sample to the Laboratory for testing within one hour of sampling.
8.5 Laboratory testing will be done within two hours of the sampling time.
8.6 No special storage conditions are required unless the storage time exceeds 2 hours in which case refrigeration of the sample is recommended.
9.0 Quality Control
9.1 For each sample type, 12 samples of SBR latex shall be obtained from the process for the recovery study. Half the vials and caps shall be tared, labeled “spiked”, and numbered 1 through 6. The other vials are labeled “unspiked” and need not be tared, but are also numbered 1 through 6.
9.2 The six vials labeled “spiked” shall be spiked with an amount of styrene to approximate 50% of the solution's expected residual styrene level.
9.3 The spiked samples shall be shaken for several hours and allowed to cool to room temperature before analysis.
9.4 The six samples of unspiked solution shall be coagulated and a mean styrene value shall be determined, along with the standard deviation, and the percent relative standard deviation.
9.5 The six samples of the spiked solution shall be coagulated and the results of the analyses shall be determined using the following equations:
Mr = Ms−Mu
R = Mr/S
where:
Mu = Mean value of styrene in the unspiked sample
Ms = Measured amount of styrene in the spiked sample
Mr = Measured amount of the spiked compound
S = Amount of styrene added to the spiked sample
R = Fraction of spiked styrene recovered
9.6 A value of R between 0.70 and 1.30 is acceptable.
9.7 R is used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type.
10.0 Calibration
10.1 Using a 10-ml volumetric pipet, quantitatively transfer 10.0 ml of the STYRENE WORKING SOLUTION (section 7.7.2 of this method) into a 2-oz bottle.
10.2 Using the AMS WORKING SOLUTION equipped with the automatic dispensing pipet (section 7.5.3 of this method), transfer 50.0 ml of the internal standard solution into the 2-oz bottle.
10.3 Cap the 2-oz bottle and swirl. This is the calibration standard, which contains 5000 µg of styrene and 2500 µg of AMS.
10.4 Using the conditions prescribed (section 6.5 of this method), chromatograph 1 µl of the calibration standard.
10.5 Obtain the peak areas and calculate the relative response factor as described in the calculations section (section 12.1 of this method).
11.0 Procedure
11.1 Into a tared 2-oz bottle, weigh 10.0 g of latex to the nearest 0.1 g.
11.2 Using the AMS WORKING SOLUTION equipped with the automatic dispensing pipet (section 7.5.3 of this method), transfer 50.0 ml of the internal standard solution into the 2-oz bottle.
11.3 Cap the bottle. Using a mechanical shaker, shake the bottle for at least one minute or until coagulation of the latex is complete as indicated by a clear solvent.
11.4 Using the conditions prescribed (section 6.5 of this method), chromatograph 1 ul of the liquor.
11.5 Obtain the peak areas and calculate the concentration of styrene in the latex as described in the calculations section (Section 12.2 of this method).
12.0 Calculations
12.1 Calibration:
RF = (Wx × Ais) / (Wis × Ax)
where:
RF = the relative response factor for styrene
Wx = the weight (ug) of styrene
Ais = the area of AMS
Wis = the weight (ug) of AMS
Ax = the area of styrene
12.2 Procedure:
ppmstyrene = (Ax RF × Wis) / (Ais × Ws)
where:
ppmstyrene = parts per million of styrene in the latex
Ax = the area of styrene
RF = the response factor for styrene
Wis = the weight (ug) of AMS
Ais = the area of AMS
Ws = the weight (g) of the latex sample
12.3 Correct for recovery (R) as determined by section 9.0 of this method.
13.0 Precision
13.1 Precision for the method was determined at the 80, 144, 590, and 1160 ppm levels. The standard deviations were 0.8, 1.5, 5 and 9 ppm respectively. The percent relative standard deviations (%RSD) were 1% or less at all levels. Five degrees of freedom were used for all precision data except at the 80 ppm level, where nine degrees of freedom were used. Note: These are example results and do not replace quality assurance procedures in this method.
14.0 Pollution Prevention
14.1 Waste generation should be minimized where possible. Sample size should be an amount necessary to adequately run the analysis.
15.0 Waste Management
15.1 Discard liquid chemical waste into the chemical waste drum.
15.2 Discard latex sample waste into the latex waste drum.
15.3 Discard polymer waste into the polymer waste container.
16.0 References
16.1 This method is based on Goodyear Chemical Division Test Method E-889.
Method 312C - Determination of Residual Styrene in SBR Latex Produced by Emulsion Polymerization
1.0 Scope
1.1 This method is applicable for determining the amount of residual styrene in SBR latex as produced in the emulsion polymerization process.
2.0 Principle of Method
2.1 A weighed sample of latex is coagulated in 2-propanol which contains alpha-methyl styrene as an Internal Standard. The extract from the coagulation will contain the alpha-methyl styrene as the Internal Standard and the residual styrene from the latex. The extract is analyzed by a Gas Chromatograph. Percent styrene is calculated by relating the area of the styrene peak to the area of the Internal Standard peak of known concentration.
3.0 Definitions
3.1 The definitions are included in the text as needed.
4.0 Interferences [Reserved]
5.0 Safety
5.1 When using solvents, avoid contact with skin and eyes. Wear hand and eye protection. Wash thoroughly after use.
5.2 Avoid overexposure to solvent vapors. Handle only in well ventilated areas.
6.0 Equipment and Supplies
6.1 Gas Chromatograph - Hewlett Packard 5890, Series II with flame ionization detector, or equivalent.
Column - HP 19095F-123, 30m × 0.53mm, or equivalent. Substrate HP FFAP (cross-linked) film thickness 1 micrometer. Glass injector port liners with silanized glass wool plug.
Integrator - HP 3396, Series II, or equivalent.
6.2 Wrist action shaker
6.3 Automatic dispenser
6.4 Automatic pipet, calibrated to deliver 5.0 ±0.01 grams of latex
6.5 Four-ounce wide-mouth bottles with foil lined lids
6.6 Crimp cap vials, 2ml, teflon lined septa
6.7 Disposable pipets
6.8 Qualitative filter paper
6.9 Cap crimper
6.10 Analytical balance
6.11 10ml pipette
6.12 Two-inch funnel
7.0 Reagents and Standards
7.1 2-Propanol (HP2C grade)
7.2 Alpha methyl styrene (99 + % purity)
7.3 Styrene (99 + % purity)
7.4 Zero air
7.5 Hydrogen (chromatographic grade)
7.6 Helium
7.7 Internal Standard preparation
7.7.1 Weigh 5.000-5.005 grams of alpha-methyl styrene into a 100ml volumetric flask and bring to mark with 2-propanol to make Stock “A” Solution.
Note:
Shelf life - 6 months.
7.7.2 Pipette 10ml of Stock “A” Solution into a 100ml volumetric flask and bring to mark with 2-propanol to prepare Stock “B” Solution.
7.7.3 Pipette 10ml of the Stock “B” solution to a 1000ml volumetric flask and bring to the mark with 2-propanol. This will be the Internal Standard Solution (0.00005 grams/ml).
7.8 Certification of Internal Standard - Each batch of Stock “B” Solution will be certified to confirm concentration.
7.8.1 Prepare a Standard Styrene Control Solution in 2-propanol by the following method:
7.8.1.1 Weigh 5.000 ±.005g of styrene to a 100ml volumetric flask and fill to mark with 2-propanol to make Styrene Stock “A” Solution.
7.8.1.2 Pipette 10ml of Styrene Stock “A” Solution to a 100ml volumetric flask and fill to mark with 2-propanol to make Styrene Stock “B” Solution.
7.8.1.3 Pipette 10ml of Styrene Stock “B” soluion to a 250ml volumtric flask and fill to mark wtih 2-propanol to make the Certification Solution.
7.8.2 Certify Alpha-Methyl Styrene Stock “B” Solution.
7.8.2.1 Pipette 5ml of the Certification Solution and 25ml of the Alpha Methyl Styrene Internal Standard Solution to a 4-oz. bottle, cap and shake well.
7.8.2.2 Analyze the resulting mixture by GC using the residual styrene method. (11.4-11.6 of this method)
7.8.2.3 Calculate the weight of alpha methyl styrene present in the 25ml aliquat of the new Alpha Methyl Styrene Standard by the following equation:
Wx = Fx xWis(Ax/Ais)
Where
Ax = Peak area of alpha methyl styrene
Ais = Peak area of styrene
Wx = Weight of alpha methyl styrene
Wis = Weight of styrene (.00100)
Fx = Analyzed response factor = 1
The Alpha Methyl Styrene Stock Solution used to prepare the Internal Standard Solution may be considered certified if the weight of alpha methyl styrene analyzed by this method is within the range of .00121g to .00129g.
8.0 Sampling
8.1 Collect a latex sample in a capped container. Cap the bottle and identify the sample as to location and time.
8.2 Deliver sample to Laboratory for testing within one hour.
8.3 Laboratory will test within two hours.
8.4 No special storage conditions are required.
9.0 Quality Control
9.1 The laboratory is required to operate a formal quality control program. This consists of an initial demonstration of the capability of the method as well as ongoing analysis of standards, blanks, and spiked samples to demonstrate continued performance.
9.1.1 When the method is first set up, a calibration is run and the recovery efficiency for each type of sample must be determined.
9.1.2 If new types of samples are being analyzed, then recovery efficiency for each new type of sample must be determined. New type includes any change, such as polymer type, physical form or a significant change in the composition of the matrix.
9.2 Recovery efficiency must be determined once for each sample type and whenever modifications are made to the method.
9.2.1 In determining the recovery efficiency, the quadruplet sampling system shall be used. Six sets of samples (for a total of 24) shall be taken. In each quadruplet set, half of the samples (two out of the four) shall be spiked with styrene.
9.2.2 Prepare the samples as described in section 8 of this method. To the vials labeled “spiked”, add a known amount of styrene that is expected to be present in the latex.
9.2.3 Run the spiked and unspiked samples in the normal manner. Record the concentrations of styrene reported for each pair of spiked and unspiked samples with the same vial number.
9.2.4 For each hydrocarbon, calculate the average recovery efficiency (R) using the following equation:
R=Σ(Rn)/12
Where: n = sample number
Rn = (Ms−Mu)/S
Ms = total mass of compound (styrene) measured in spiked sample (µg)
Mu = total mass of compound (styrene) measured in unspiked sample (µg)
S = theoretical mass of compound (styrene) spiked into sample (µg)
R = fraction of spiked compound (styrene) recovered
9.2.5 A different R value should be obtained for each sample type. A value of R between 0.70 and 1.30 is acceptable.
9.2.6 R is used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type.
10.0 Calibration
A styrene control sample will be tested weekly to confirm the FID response and calibration.
10.1 Using the Styrene Certification Solution prepared in 7.8.1, perform test analysis as described in 7.8.2 using the equation in 7.8.2.3 to calculate results.
10.2 Calculate the weight of styrene in the styrene control sample using the following equation:
Wsty = (Fx xAsty xWis)Ais
The instrument can be considered calibrated if the weight of the styrene analyzed is within range of 0.00097-0.00103gms.
11.0 Procedure
11.1 Using an auto pipet, add 25ml of Internal Standard Solution to a 4 oz. wide-mouth bottle.
11.2 Using a calibrated auto pipet, add 5.0 ±0.01g latex to the bottle containing the 25ml of Internal Standard Solution.
11.3 Cap the bottle and place on the wrist action shaker. Shake the sample for a minimum of five minutes using the timer on the shaker. Remove from shaker.
11.4 Using a disposable pipet, fill the 2ml sample vial with the clear alcohol extract. (If the extract is not clear, it should be filtered using a funnel and filter paper.) Cap and seal the vial.
11.5 Place the sample in the autosampler tray and start the GC and Integrator. The sample will be injected into the GC by the auto-injector, and the Integrator will print the results.
11.6 Gas Chromatograph Conditions
Oven Temp - 70°C
Injector Temp - 225°C
Detector Temp - 275°C
Helium Pressure - 500 KPA
Column Head Pressure - 70 KPA
Makeup Gas - 30 ml/min.
Column - HP 19095F - 123, 30m × 0.53mm Substrate: HP - FFAP (cross-linked) 1 micrometer film thickness
12.0 Calculations
12.1 The integrator is programmed to do the following calculation at the end of the analysis:
%ResidualStyrene = (Ax XWis)/(Ais XWx)XFx X100
Where:
Ax = Peak area of styrene
Ais = Peak area of internal standard
Wx = Weight of sample = 5g
Wis = Weight of internal std. = 0.00125g
Fx = Analyzed response factor = 1.0
12.2 The response factor is determined by analyzing a solution of 0.02g of styrene and 0.02g of alpha methyl styrene in 100ml of 2-propanol. Calculate the factor by the following equation:
Fx = (Wx xAis)/(Wis xAx)
Where:
Wx = Weight of styrene
Ax = Peak area of styrene
Wis = Weight of alpha methyl styrene
Ais = Peak area of alpha methyl styrene
13.0 Method Performance
13.1 Performance must be determined for each sample type by following the procedures in section 9 of this method.
14.0 Waste Generation
14.1 Waste generation should be minimized where possible.
15.0 Waste Management
15.1 All waste shall be handled in accordance with Federal and State environmental regulations.
16.0 References [Reserved]
Method 313A - Determination of Residual Hydrocarbons in Rubber Crumb
1.0 Scope and Application
1.1 This method determines residual toluene and styrene in stripper crumb of the of the following types of rubber: polybutadiene (PBR) and styrene/butadiene rubber (SBR), both derived from solution polymerization processes that utilize toluene as the polymerization solvent.
1.2 The method is applicable to a wide range of concentrations of toluene and styrene provided that calibration standards cover the desired range. It is applicable at least over the range of 0.01 to 10.0 % residual toluene and from 0.1 to 3.0 % residual styrene. It is probably applicable over a wider range, but this must be verified prior to use.
1.3 The method may also be applicable to other process samples as long as they are of a similar composition to stripper crumb. See section 3.1 of this method for a description of stripper crumb.
2.0 Summary of Method
2.1 The wet crumb is placed in a sealed vial and run on a headspace sampler which heats the vial to a specified temperature for a specific time and then injects a known volume of vapor into a capillary GC. The concentration of each component in the vapor is proportional to the level of that component in the crumb sample and does not depend on water content of the crumb.
2.2 Identification of each component is performed by comparing the retention times to those of known standards.
2.3 Results are calculated by the external standard method since injections are all performed in an identical manner. The response for each component is compared with that obtained from dosed samples of crumb.
2.4 Measured results of each compound are corrected by dividing each by the average recovery efficiency determined for the same compound in the same sample type.
3.0 Definitions
3.1 Stripper crumb refers to pieces of rubber resulting from the steam stripping of a toluene solution of the same polymer in a water slurry. The primary component of this will be polymer with lesser amounts of entrained water and residual toluene and other hydrocarbons. The amounts of hydrocarbons present must be such that the crumb is a solid material, generally less that 10 % of the dry rubber weight.
4.0 Interferences
4.1 Contamination is not normally a problem since samples are sealed into vials immediately on sampling.
4.2 Cross contamination in the headspace sampler should not be a problem if the correct sampler settings are used. This should be verified by running a blank sample immediately following a normal or high sample. Settings may be modified if necessary if this proves to be a problem, or a blank sample may be inserted between samples.
4.3 Interferences may occur if volatile hydrocarbons are present which have retention times close to that of the components of interest. Since the solvent makeup of the processes involved are normally fairly well defined this should not be a problem. If it is found to be the case, switching to a different chromatographic column will probably resolve the situation.
5.0 Safety
5.1 The chemicals specified in this method should all be handled according to standard laboratory practices as well as any special precautions that may be listed in the MSDS for that compound.
5.2 Sampling of strippers or other process streams may involve high pressures and temperatures or may have the potential for exposure to chemical fumes. Only personnel who have been trained in the specific sampling procedures required for that process should perform this operation. An understanding of the process involved is necessary. Proper personal protective equipment should be worn. Any sampling devices should be inspected prior to use. A detailed sampling procedure which specifies exactly how to obtain the sample must be written and followed.
6.0 Equipment and Supplies
6.1 Hewlett Packard (HP) 7694 Headspace sampler, or equivalent, with the following conditions:
Times (min.): GC cycle time 6.0 , vial equilibration 30.0 , pressurization 0.25 , loop fill 0.25, loop equilibration 0.05 , inject 0.25
Temperatures (deg C): oven 70, loop 80, transfer line 90
Pressurization gas: He @ 16 psi
6.2 HP 5890 Series II capillary gas chromatograph, or equivalent, with the following conditions:
Column: Supelco SPB-1, or equivalent, 15m × .25mm × .25 µ film
Carrier: He @ 6 psi
Run time: 4 minutes
Oven: 70 deg C isothermal
Injector: 200 deg C split ratio 50:1
Detector: FID @ 220 deg C
6.3 HP Chemstation consisting of computer, printer and Chemstation software, or an equivalent chromatographic data system.
6.4 20 ml headspace vials with caps and septa.
6.5 Headspace vial crimper.
6.6 Microliter pipetting syringes.
6.7 Drying oven at 100 deg C vented into cold trap or other means of trapping hydrocarbons released.
6.8 Laboratory shaker or tumbler suitable for the headspace vials.
6.9 Personal protective equipment required for sampling the process such as rubber gloves and face and eye protection.
7.0 Reagents and Standards
7.1 Toluene, 99.9 + % purity, HPLC grade.
7.2 Styrene, 99.9 + % purity, HPLC grade.
7.3 Dry rubber of same type as the stripper crumb samples.
8.0 Sample Collection, Preservation and Storage
8.1 Collect a sample of crumb in a manner appropriate for the process equipment being sampled.
8.1.1 If conditions permit, this may be done by passing a stream of the crumb slurry through a strainer, thus separating the crumb from the water. Allow the water to drain freely, do not attempt to squeeze any water from the crumb. Results will not depend on the exact water content of the samples. Immediately place several pieces of crumb directly into a headspace vial. This should be done with rubber gloves to protect the hands from both the heat and from contact with residual hydrocarbons. The vial should be between 1/4 and 1/3 full. Results do not depend on sample size as long as there is sufficient sample to reach an equilibrium vapor pressure in the headspace of the vial. Cap and seal the vial. Prepare each sample at least in duplicate. This is to minimize the effect of the variation that naturally occurs in the composition of non homogeneous crumb. The free water is not analyzed by this method and should be disposed of appropriately along with any unused rubber crumb.
8.1.2 Alternatively the process can be sampled in a specially constructed sealed bomb which can then be transported to the laboratory. The bomb is then cooled to ambient temperature by applying a stream of running water. The bomb can then be opened and the crumb separated from the water and the vials filled as described in section 8.1.1 of this method. The bomb may be stored up to 8 hours prior to transferring the crumb into vials.
8.2 The sealed headspace vials may be run immediately or may be stored up to 72 hours prior to running. It is possible that even longer storage times may be acceptable, but this must be verified for the particular type of sample being analyzed (see section 9.2.3 of this method). The main concern here is that some types of rubber eventually may flow, thus compacting the crumb so that the surface area is reduced. This may have some effect on the headspace equilibration.
9.0 Quality Control
9.1 The laboratory is required to operate a formal quality control program. This consists of an initial demonstration of the capability of the method as well as ongoing analysis of standards, blanks and spiked samples to demonstrate continued performance.
9.1.1 When the method is first set up a calibration is run (described in section 10 of this method) and an initial demonstration of method capability is performed (described in section 9.2 of this method). Also recovery efficiency for each type of sample must be determined (see section 9.4 of this method).
9.1.2 It is permissible to modify this method in order to improve separations or make other improvements, provided that all performance specifications are met. Each time a modification to the method is made it is necessary to repeat the calibration (section 10 of this method), the demonstration of method performance (section 9.2 of this method) and the recovery efficiency for each type of sample (section 9.4 of this method).
9.1.3 Ongoing performance should be monitored by running a spiked rubber standard. If this test fails to demonstrate that the analysis is in control, then corrective action must be taken. This method is described in section 9.3 of this method.
9.1.4 If new types of samples are being analyzed then recovery efficiency for each new type of sample must be determined. New type includes any change, such as polymer type, physical form or a significant change in the composition of the matrix.
9.2 Initial demonstration of method capability to establish the accuracy and precision of the method. This is to be run following the calibration described in section 10 of this method.
9.2.1 Prepare a series of identical spiked rubber standards as described in section 9.3 of this method. A sufficient number to determine statistical information on the test should be run. Ten may be a suitable number, depending on the quality control methodology used at the laboratory running the tests. These are run in the same manner as unknown samples (see section 11 of this method).
9.2.2 Determine mean and standard deviation for the results. Use these to determine the capability of the method and to calculate suitable control limits for the ongoing performance check which will utilize the same standards.
9.2.3 Prepare several additional spiked rubber standards and run 2 each day to determine the suitability of storage of the samples for 24, 48 and 72 hours or longer if longer storage times are desired.
9.3 A spiked rubber standard should be run on a regular basis to verify system performance. This would probably be done daily if samples are run daily. This is prepared in the same manner as the calibration standards (section 10.1 of this method), except that only one concentration of toluene and styrene is prepared. Choose concentrations of toluene and styrene that fall in the middle of the range expected in the stripper crumb and then do not change these unless there is a major change in the composition of the unknowns. If it becomes necessary to change the composition of this standard the initial performance demonstration must be repeated with the new standard (section 9.2 of this method).
9.3.1 Each day prepare one spiked rubber standard to be run the following day. The dry rubber may be prepared in bulk and stored for any length of time consistent with the shelf life of the product. The addition of water and hydrocarbons must be performed daily and all the steps described under section 10.1 of this method must be followed.
9.3.2 Run the spiked rubber standard prepared the previous day. Record the results and plot on an appropriate control chart or other means of determining statistical control.
9.3.3 If the results for the standard indicate that the test is out of control then corrective action must be taken. This may include a check on procedures, instrument settings, maintenance or recalibration. Samples may be stored (see section 8.2 of this method) until compliance is demonstrated.
9.4 Recovery efficiency must be determined once for each sample type and whenever modifications are made to the method.
9.4.1 For each sample type collect 12 samples from the process (section 8.1 of this method). This should be done when the process is operating in a normal manner and residual hydrocarbon levels are in the normal range. Half the vials and caps should be tared, labeled “spiked” and numbered 1 through 6. The other vials are labeled “unspiked” and need not be tared but are also numbered 1 through 6. Immediately on sampling, the vials should be capped to prevent loss of volatiles. Allow all the samples to cool completely to ambient temperature. Reweigh each of the vials labeled “spiked” to determine the weight of wet crumb inside.
9.4.2 The dry weight of rubber present in the wet crumb is estimated by multiplying the weight of wet crumb by the fraction of nonvolatiles typical for the sample. If this is not known, an additional quantity of crumb may be sampled, weighed, dried in an oven and reweighed to determine the fraction of volatiles and nonvolatiles prior to starting this procedure.
9.4.3 To the vials labeled “spiked” add an amount of a mixture of toluene and styrene that is between 40 and 60 % of the amount expected in the crumb. This is done by removing the cap, adding the mixture by syringe, touching the tip of the needle to the sample in order to remove the drop and then immediately recapping the vials. The mixture is not added through the septum, because a punctured septum may leak and vent vapors as the vial is heated. The weights of toluene and styrene added may be calculated from the volumes of the mixture added, its composition and density, or may be determined by the weight of the vials and caps prior to and after addition. The exact dry weight of rubber present and the concentration of residual toluene and styrene are not known at this time so an exact calculation of the concentration of hydrocarbons is not possible until the test is completed.
9.4.4 Place all the vials onto a shaker or tumbler for 24 ±2 hours. This is essential in order for the hydrocarbons to be evenly distributed and completely absorbed into the rubber. If this is not followed the toluene and styrene will be mostly at the surface of the rubber and high results will be obtained.
9.4.5 Remove the vials from the shaker and tap them so that all the crumb settles to the bottom of the vials. Allow them to stand for 1 hour prior to analysis to allow any liquid to drain fully to the bottom.
9.4.6 Run the spiked and unspiked samples in the normal manner. Record the concentrations of toluene and styrene reported for each pair of spiked and unspiked samples with the same vial number.
9.4.7 Open each of the vials labeled “spiked”, remove all the rubber crumb and place it into a tarred drying pan. Place in a 100 deg C oven for two hours, cool and reweigh. Subtract the weight of the tare to give the dry weight of rubber in each spiked vial. Calculate the concentration of toluene and styrene spiked into each vial as percent of dry rubber weight. This will be slightly different for each vial since the weights of dry rubber will be different.
9.4.8 For each hydrocarbon calculate the average recovery efficiency (R) using the following equations:
R = R_Σ(Pn)/6 (average of the 6 individual Rn values)
Where:
Rn = (Cns - Cnu) / Sn
Where:
n = vial number
Cns = concentration of compound measured in spiked sample number n.
Cnu = concentration of compound measured in unspiked sample number n.
Sn = theoretical concentration of compound spiked into sample n calculated in step 9.4.7
9.4.9 A different R value should be obtained for each compound (styrene and toluene) and for each sample type.
9.4.10 A value of R between 0.70 and 1.30 is acceptable.
9.4.11 R is used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type (see section 12.2 of this method.)
10.0 Calibration
10.1 Calibration standards are prepared by dosing known amounts of the hydrocarbons of interest into vials containing known amounts of rubber and water.
10.1.1 Cut a sufficient quantity of dry rubber of the same type as will be analyzed into pieces about the same size as that of the crumb. Place these in a single layer on a piece of aluminum foil or other suitable surface and place into a forced air oven at 100°C for four hours. This is to remove any residual hydrocarbons that may be present. This step may be performed in advance.
10.1.2 Into each of a series of vials add 3.0 g of the dry rubber.
10.1.3 Into each vial add 1.0 ml distilled water or an amount that is close to the amount that will be present in the unknowns. The exact amount of water present does not have much effect on the analysis, but it is necessary to have a saturated environment. The water will also aid in the uniform distribution of the spiked hydrocarbons over the surface of the rubber after the vials are placed on the shaker (in step 10.1.5 of this method).
10.1.4 Into each vial add varying amounts of toluene and styrene by microliter syringe and cap the vials immediately to prevent loss. The tip of the needle should be carefully touched to the rubber in order to transfer the last drop to the rubber. Toluene and styrene may first be mixed together in suitable proportions and added together if desired. The weights of toluene and styrene added may be calculated from the volumes of the mixture added, its composition and density, or may be determined by the weight of the vials and caps prior to and after addition. Concentrations of added hydrocarbons are calculated as percent of the dry rubber weight. At least 5 standards should be prepared with the amounts of hydrocarbons added being calculated to cover the entire range possible in the unknowns. Retain two samples with no added hydrocarbons as blanks.
10.1.5 Place all the vials onto a shaker or tumbler for 24 ±2 hours. This is essential in order for the hydrocarbons to be evenly distributed and completely absorbed into the rubber. If this is not followed the toluene and styrene will be mostly at the surface of the rubber and high results will be obtained.
10.1.6 Remove the vials from the shaker and tap them so that all the crumb settles to the bottom of the vials. Allow them to stand for 1 hour prior to analysis to allow any liquid to drain fully to the bottom.
10.2 Run the standards and blanks in the same manner as described for unknowns (section 11 of this method), starting with a blank, then in order of increasing hydrocarbon content and ending with the other blank.
10.3 Verify that the blanks are sufficiently free from toluene and styrene or any interfering hydrocarbons.
10.3.1 It is possible that trace levels may be present even in dry product. If levels are high enough that they will interfere with the calibration then the drying procedure in section 10.1.1 of this method should be reviewed and modified as needed to ensure that suitable standards can be prepared.
10.3.2 It is possible that the final blank is contaminated by the previous standard. If this is the case review and modify the sampler parameters as needed to eliminate this problem. If necessary it is possible to run blank samples between regular samples in order to reduce this problem, though it should not be necessary if the sampler is properly set up.
10.4 Enter the amounts of toluene and styrene added to each of the samples (as calculated in section 10.1.4 of this method) into the calibration table and perform a calibration utilizing the external standard method of analysis.
10.5 At low concentrations the calibration should be close to linear. If a wide range of levels are to be determined it may be desirable to apply a nonlinear calibration to get the best fit.
11.0 Procedure
11.1 Place the vials in the tray of the headspace sampler. Enter the starting and ending positions through the console of the sampler. For unknown samples each is run in duplicate to minimize the effect of variations in crumb composition. If excessive variation is noted it may be desirable to run more than two of each sample.
11.2 Make sure the correct method is loaded on the Chemstation. Turn on the gas flows and light the FID flame.
11.3 Start the sequence on the Chemstation. Press the START button on the headspace unit. The samples will be automatically injected after equilibrating for 30 minutes in the oven. As each sample is completed the Chemstation will calculate and print out the results as percent toluene and styrene in the crumb based on the dry weight of rubber.
12.0 Data Analysis and Calculations
12.1 For each set of duplicate samples calculate the average of the measured concentration of toluene and styrene. If more than two replicates of each sample are run calculate the average over all replicates.
12.2 For each sample correct the measured amounts of toluene and styrene using the following equation:
Corrected Result = Cm/R
Where:
Cm = Average measured concentration for that compound.
R = Recovery efficiency for that compound in the same sample type (see section 9.4 of this method)
12.3 Report the recovery efficiency (R) and the corrected results of toluene and styrene for each sample.
13.0 Method Performance
13.1 This method can be very sensitive and reproducible. The actual performance depends largely on the exact nature of the samples being analyzed. Actual performance must be determined by each laboratory for each sample type.
13.2 The main source of variation is the actual variation in the composition of non homogeneous crumb in a stripping system and the small sample sizes employed here. It therefore is the responsibility of each laboratory to determine the optimum number of replicates of each sample required to obtain accurate results.
14.0 Pollution Prevention
14.1 Samples should be kept sealed when possible in order to prevent evaporation of hydrocarbons.
14.2 When drying of samples is required it should be done in an oven which vents into a suitable device that can trap the hydrocarbons released.
14.3 Dispose of samples as described in section 15.
15.0 Waste Management
15.1 Excess stripper crumb and water as well as the contents of the used sample vials should be properly disposed of in accordance with local and federal regulations.
15.2 Preferably this will be accomplished by having a system of returning unused and spent samples to the process.
16.0 References
16.1 “HP 7694 Headspace Sampler - Operating and Service Manual”, Hewlett-Packard Company, publication number G1290-90310, June 1993.
Method 313B - The Determination of Residual Hydrocarbon in Solution Polymers by Capillary Gas Chromatography
1.0 Scope
1.1 This method is applicable to solution polymerized polybutadiene (PBD).
1.2 This method quantitatively determines n-hexane in wet crumb polymer at levels from 0.08 to 0.15% by weight.
1.3 This method may be extended to the determination of other hydrocarbons in solution produced polymers with proper experimentation and documentation.
2.0 Principle of Method
2.1 A weighed sample of polymer is dissolved in chloroform and the cement is coagulated with an isopropyl alcohol solution containing a specific amount of alpha-methyl styrene (AMS) as the internal standard. The extract of this coagulation is then injected into a gas chromatograph and separated into individual components. Quantification is achieved by the method of internal standardization.
3.0 Definitions
3.1 The definitions are included in the text as needed.
4.0 Interferences [Reserved]
5.0 Safety
5.1 This method may involve hazardous materials, operations, and equipment. This method does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
6.0 Equipment and Supplies
6.1 Analytical balance, 160 g capacity, 0.1 mg resolution
6.2 Bottles, 2-oz capacity with poly-cap screw lids
6.3 Mechanical shaker
6.4 Syringe, 10-ul capacity
6.5 Syringe, 2.5-ml capacity, with 22 gauge 1.25 inch needle, PP/PE material, disposable
6.6 Gas chromatograph, Hewlett-Packard model 5890, or equivalent, configured with FID, split injector packed with silanized glass wool.
6.6.1 Establish the following gas chromatographic conditions, and allow the system to thoroughly equilibrate before use.
6.6.2 Injector parameters:
Injection technique = Split
Injector split flow = 86 ml/min
Injector temperature = 225 deg C
6.6.3 Oven temperature program:
Initial temperature = 40 deg C
Initial time = 6 min
Program rate = 10 deg C/min
Upper limit temperature = 175 deg C
Upper limit interval = 10 min
6.6.4 Detector parameters:
Detector temperature = 300 deg C
Hydrogen flow = 30 ml/min
Air flow = 350 ml/min
Nitrogen make up = 26 ml/min
6.7 Gas chromatographic columns: SE-54 (5%-phenyl) (1%-vinyl)-methylpolysiloxane, 15 M × 0.53 mm ID with a 1.2 micron film thickness, and a Carbowax 20M (polyethylene glycol), 15 M × 0.53 mm ID with a 1.2 micron film thickness.
6.7.1 Column assembly: using a 0.53 mm ID butt connector union, join the 15 M × 0.53 mm SE-54 column to the 15 M × 0.53 mm Carbowax 20M. The SE-54 column will be inserted into the injector and the Carbowax 20M inserted into the detector after they have been joined.
6.7.2 Column parameters:
Helium flow = 2.8 ml/min
Helium headpressure = 2 psig
6.8 Centrifuge
6.9 Data collection system, Hewlett-Packard Model 3396, or equivalent
6.10 Pipet, 25-ml capacity, automatic dispensing, and 2 liter reservoir
6.11 Pipet, 2-ml capacity, volumetric delivery, class A
6.12 Flasks, 100 and 1000-ml capacity, volumetric, class A
6.13 Vial, serum, 50-ml capacity, red rubber septa and crimp ring seals
6.14 Sample collection basket fabricated out of wire mesh to allow for drainage
7.0 Chemicals and Reagents
CHEMICALS:
7.1 alpha-Methyl Styrene, C9H10, 99 + % purity, CAS 98-83-9
7.2 n-Hexane, C6H14, 99 + % purity, CAS 110-54-3
7.3 Isopropyl alcohol, C3H8O 99.5 + % purity, reagent grade, CAS 67-63-0
7.4 Chloroform, CHCl3, 99% min., CAS 67-66-3
REAGENTS:
7.5 Internal Standard Stock Solution: 10 mg/25 ml AMS in isopropyl alcohol.
7.5.1 Into a 25-ml beaker, weigh 0.4 g of AMS to the nearest 0.1 mg.
7.5.2 Quantitatively transfer this AMS into a 1-L volumetric flask. Dilute to the mark with isopropyl alcohol.
7.5.3 Transfer this solution to the automatic dispensing pipet reservoir. This will be labeled the AMS STOCK SOLUTION.
7.6 n-Hexane Stock Solution: 13mg/2ml hexane in isopropyl alcohol.
7.6.1 Into a 100-ml volumetric flask, weigh 0.65 g of n-hexane to the nearest 0.1 mg.
7.6.2 Dilute to the mark with isopropyl alcohol. This solution will be labeled the n-HEXANE STOCK SOLUTION.
8.0 Sample Collection, Preservation and Storage
8.1 A sampling device similar to Figure 1 is used to collect a non-vented crumb rubber sample at a location that is after the stripping operation but before the sample is exposed to the atmosphere.
8.2 The crumb rubber is allowed to cool before opening the sampling device and removing the sample.
8.3 The sampling device is opened and the crumb rubber sample is collected in the sampling basket.
8.4 One pound of crumb rubber sample is placed into a polyethylene bag. The bag is labeled with the time, date and sample location.
8.5 The sample should be delivered to the laboratory for testing within one hour of sampling.
8.6 Laboratory testing will be done within 3 hours of the sampling time.
8.7 No special storage conditions are required unless the storage time exceeds 3 hours in which case refrigeration of the samples is recommended.
9.0 Quality Control
9.1 For each sample type, 12 samples shall be obtained from the process for the recovery study. Half of the vials and caps shall be tared, labeled “spiked”, and numbered 1 through 6. The other vials shall be labeled “unspiked” and need not be tared, but are also numbered 1 through 6.
9.2 Determine the % moisture content of the crumb sample. After determining the % moisture content, the correction factor for calculating the dry crumb weight can be determined by using the equation in section 12.2 of this method.
9.3 Run the spiked and unspiked samples in the normal manner. Record the concentrations of the n-hexane content of the mixed hexane reported for each pair of spiked and unspiked samples.
9.4 For the recovery study, each sample of crumb shall be dissolved in chloroform containing a known amount of mixed hexane solvent.
9.5 For each hydrocarbon, calculate the recovery efficiency (R) using the following equations:
Mr = Ms−Mu
R = Mr/S
Where:
Mu = Measured amount of compound in the unspiked sample
Ms = Measured amount of compound in the spiked sample
Mr = Measured amount of the spiked compound
S = Amount of compound added to the spiked sample
R = Fraction of spiked compound recovered
9.6 Normally a value of R between 0.70 and 1.30 is acceptable.
9.7 R is used to correct all reported results for each compound by dividing the measured results of each compound by the R for that compound for the same sample type.
10.0 Calibration
10.1 Using the AMS STOCK SOLUTION equipped with the automatic dispensing pipet (7.5.3 of this method), transfer 25.0 ml of the internal standard solution into an uncapped 50-ml serum vial.
10.2 Using a 2.0 ml volumetric pipet, quantitatively transfer 2.0 ml of the n-HEXANE STOCK SOLUTION (7.6.2 of this method) into the 50-ml serum vial and cap. This solution will be labeled the CALIBRATION SOLUTION.
10.3 Using the conditions prescribed (6.6 of this method), inject 1 µl of the supernate.
10.4 Obtain the peak areas and calculate the response factor as described in the calculations section (12.1 of this method).
11.0 Procedure
11.1 Determination of Dry Polymer Weight
11.1.1 Remove wet crumb from the polyethylene bag and place on paper towels to absorb excess surface moisture.
11.1.2 Cut small slices or cubes from the center of the crumb sample to improve sample uniformity and further eliminate surface moisture.
11.1.3 A suitable gravimetric measurement should be made on a sample of this wet crumb to determine the correction factor needed to calculate the dry polymer weight.
11.2 Determination of n-Hexane in Wet Crumb
11.2.1 Remove wet crumb from the polyethylene bag and place on paper towels to absorb excess surface moisture.
11.2.2 Cut small slices or cubes from the center of the crumb sample to improve sample uniformity and further eliminate surface moisture.
11.2.3 Into a tared 2 oz bottle, weigh 1.5 g of wet polymer to the nearest 0.1 mg.
11.2.4 Add 25 ml of chloroform to the 2 oz bottle and cap.
11.2.5 Using a mechanical shaker, shake the bottle until the polymer dissolves.
11.2.6 Using the autodispensing pipet, add 25.0 ml of the AMS STOCK SOLUTION (7.5.3 of this method) to the dissolved polymer solution and cap.
11.2.7 Using a mechanical shaker, shake the bottle for 10 minutes to coagulate the dissolved polymer.
11.2.8 Centrifuge the sample for 3 minutes at 2000 rpm.
11.2.9 Using the conditions prescribed (6.6 of this method), chromatograph 1 µl of the supernate.
11.2.10 Obtain the peak areas and calculate the concentration of the component of interest as described in the calculations (12.2 of this method).
12.0 Calculations
12.1 Calibration:
RFx = (Wx × Ais) / (Wis × Ax)
Where:
RFx = the relative response factor for n-hexane
Wx = the weight (g) of n-hexane in the CALIBRATION
SOLUTION
Ais = the area of AMS
Wis = the weight (g) of AMS in the CALIBRATION SOLUTION
Ax = the area of n-hexane
12.2 Procedure:
12.2.1 Correction Factor for calculating dry crumb weight.
F = 1 - (% moisture / 100)
Where:
F = Correction factor for calculating dry crumb weight
% moisture determined by appropriate method
12.2.2 Moisture adjustment for chromatographic determination.
Ws = F × Wc
Where:
Ws = the weight (g) of the dry polymer corrected for moisture
F = Correction factor for calculating dry crumb weight
Wc = the weight (g) of the wet crumb in section 9.6
12.2.3 Concentration (ppm) of hexane in the wet crumb.
ppmx = (Ax * RFx * Wis * 10000) / (Ais * Ws)
Where:
ppmx = parts per million of n-hexane in the polymer
Ax = the area of n-hexane
RFx = the relative response factor for n-hexane
Wis = the weight (g) of AMS in the sample solution
Ais = the area of AMS
Ws = the weight (g) of the dry polymer corrected for moisture
13.0 Method Performance
13.1 Precision for the method was determined at the 0.08% level.
The standard deviation was 0.01 and the percent relative standard deviation (RSD) was 16.3 % with five degrees of freedom.
14.0 Waste Generation
14.1 Waste generation should be minimized where possible.
15.0 Waste Management
15.1 Discard liquid chemical waste into the chemical waste drum.
15.2 Discard polymer waste into the polymer waste container.
16.0 References
16.1 This method is based on Goodyear Chemical Division Test Method E-964.
Method 315 - Determination of Particulate and Methylene Chloride Extractable Matter (MCEM) From Selected Sources at Primary Aluminum Production Facilities
Note:
This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) essential to its performance. Some material is incorporated by reference from other methods in this part. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least the following additional test methods: Method 1, Method 2, Method 3, and Method 5 of 40 CFR part 60, appendix A.
1.0 Scope and Application
1.1 Analytes. Particulate matter (PM). No CAS number assigned. Methylene chloride extractable matter (MCEM). No CAS number assigned.
1.2 Applicability. This method is applicable for the simultaneous determination of PM and MCEM when specified in an applicable regulation. This method was developed by consensus with the Aluminum Association and the U.S. Environmental Protection Agency (EPA) and has limited precision estimates for MCEM; it should have similar precision to Method 5 for PM in 40 CFR part 60, appendix A since the procedures are similar for PM.
1.3 Data quality objectives. Adherence to the requirements of this method will enhance the quality of the data obtained from air pollutant sampling methods.
2.0 Summary of Method
Particulate matter and MCEM are withdrawn isokinetically from the source. PM is collected on a glass fiber filter maintained at a temperature in the range of 120 ±14°C (248 ±25°F) or such other temperature as specified by an applicable subpart of the standards or approved by the Administrator for a particular application. The PM mass, which includes any material that condenses on the probe and is subsequently removed in an acetone rinse or on the filter at or above the filtration temperature, is determined gravimetrically after removal of uncombined water. MCEM is then determined by adding a methylene chloride rinse of the probe and filter holder, extracting the condensable hydrocarbons collected in the impinger water, adding an acetone rinse followed by a methylene chloride rinse of the sampling train components after the filter and before the silica gel impinger, and determining residue gravimetrically after evaporating the solvents.
3.0 Definitions [Reserved]
4.0 Interferences [Reserved]
5.0 Safety
This method may involve hazardous materials, operations, and equipment. This method does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to performing this test method.
6.0 Equipment and Supplies
Note:
Mention of trade names or specific products does not constitute endorsement by the EPA.
6.1 Sample collection. The following items are required for sample collection:
6.1.1 Sampling train. A schematic of the sampling train used in this method is shown in Figure 5-1, Method 5, 40 CFR part 60, appendix A-3. Complete construction details are given in APTD-0581 (Reference 2 in section 17.0 of this method); commercial models of this train are also available. For changes from APTD-0581 and for allowable modifications of the train shown in Figure 5-1, Method 5, 40 CFR part 60, appendix A-3, see the following subsections.
Note:
The operating and maintenance procedures for the sampling train are described in APTD-0576 (Reference 3 in section 17.0 of this method). Since correct usage is important in obtaining valid results, all users should read APTD-0576 and adopt the operating and maintenance procedures outlined in it, unless otherwise specified herein. Alternative mercury-free thermometers may be used if the thermometers are, at a minimum, equivalent in terms of performance or suitably effective for the specific temperature measurement application. The use of grease for sealing sampling train components is not recommended because many greases are soluble in methylene chloride. The sampling train consists of the following components:
6.1.1.1 Probe nozzle. Glass or glass lined with sharp, tapered leading edge. The angle of taper shall be ≤30°, and the taper shall be on the outside to preserve a constant internal diameter. The probe nozzle shall be of the button-hook or elbow design, unless otherwise specified by the Administrator. Other materials of construction may be used, subject to the approval of the Administrator. A range of nozzle sizes suitable for isokinetic sampling should be available. Typical nozzle sizes range from 0.32 to 1.27 cm ( 1/8 to 1/2 in.) inside diameter (ID) in increments of 0.16 cm ( 1/16 in.). Larger nozzle sizes are also available if higher volume sampling trains are used. Each nozzle shall be calibrated according to the procedures outlined in section 10.0 of this method.
6.1.1.2 Probe liner. Borosilicate or quartz glass tubing with a heating system capable of maintaining a probe gas temperature at the exit end during sampling of 120 ±14°C (248 ±25°F), or such other temperature as specified by an applicable subpart of the standards or approved by the Administrator for a particular application. Because the actual temperature at the outlet of the probe is not usually monitored during sampling, probes constructed according to APTD-0581 and using the calibration curves of APTD-0576 (or calibrated according to the procedure outlined in APTD-0576) will be considered acceptable. Either borosilicate or quartz glass probe liners may be used for stack temperatures up to about 480°C (900°F); quartz liners shall be used for temperatures between 480 and 900°C (900 and 1,650°F). Both types of liners may be used at higher temperatures than specified for short periods of time, subject to the approval of the Administrator. The softening temperature for borosilicate glass is 820°C (1,500°F) and for quartz glass it is 1,500°C (2,700°F).
6.1.1.3 Pitot tube. Type S, as described in section 6.1 of Method 2, 40 CFR part 60, appendix A, or other device approved by the Administrator. The pitot tube shall be attached to the probe (as shown in Figure 5-1 of Method 5, 40 CFR part 60, appendix A) to allow constant monitoring of the stack gas velocity. The impact (high pressure) opening plane of the pitot tube shall be even with or above the nozzle entry plane (see Method 2, Figure 2-6b, 40 CFR part 60, appendix A) during sampling. The Type S pitot tube assembly shall have a known coefficient, determined as outlined in section 10.0 of Method 2, 40 CFR part 60, appendix A.
6.1.1.4 Differential pressure gauge. Inclined manometer or equivalent device (two), as described in section 6.2 of Method 2, 40 CFR part 60, appendix A. One manometer shall be used for velocity head (Dp) readings, and the other, for orifice differential pressure readings.
6.1.1.5 Filter holder. Borosilicate glass, with a glass frit filter support and a silicone rubber gasket. The holder design shall provide a positive seal against leakage from the outside or around the filter. The holder shall be attached immediately at the outlet of the probe (or cyclone, if used).
6.1.1.6 Filter heating system. Any heating system capable of maintaining a temperature around the filter holder of 120 ±14°C (248 ±25°F) during sampling, or such other temperature as specified by an applicable subpart of the standards or approved by the Administrator for a particular application. Alternatively, the tester may opt to operate the equipment at a temperature lower than that specified. A temperature gauge capable of measuring temperature to within 3°C (5.4°F) shall be installed so that the temperature around the filter holder can be regulated and monitored during sampling. Heating systems other than the one shown in APTD-0581 may be used.
6.1.1.7 Temperature sensor. A temperature sensor capable of measuring temperature to within ±3°C (5.4°F) shall be installed so that the sensing tip of the temperature sensor is in direct contact with the sample gas, and the temperature around the filter holder can be regulated and monitored during sampling.
6.1.1.8 Condenser. The following system shall be used to determine the stack gas moisture content: four glass impingers connected in series with leak-free ground glass fittings. The first, third, and fourth impingers shall be of the Greenburg-Smith design, modified by replacing the tip with a 1.3 cm (1/2 in.) ID glass tube extending to about 1.3 cm (1/2 in.) from the bottom of the flask. The second impinger shall be of the Greenburg-Smith design with the standard tip. The first and second impingers shall contain known quantities of water (section 8.3.1 of this method), the third shall be empty, and the fourth shall contain a known weight of silica gel or equivalent desiccant. A temperature sensor capable of measuring temperature to within 1°C (2°F) shall be placed at the outlet of the fourth impinger for monitoring.
6.1.1.9 Metering system. Vacuum gauge, leak-free pump, temperature sensors capable of measuring temperature to within 3°C (5.4°F), dry gas meter (DGM) capable of measuring volume to within 2 percent, and related equipment, as shown in Figure 5-1 of Method 5, 40 CFR part 60, appendix A. Other metering systems capable of maintaining sampling rates within 10 percent of isokinetic and of determining sample volumes to within 2 percent may be used, subject to the approval of the Administrator. When the metering system is used in conjunction with a pitot tube, the system shall allow periodic checks of isokinetic rates.
6.1.1.10 Sampling trains using metering systems designed for higher flow rates than that described in APTD-0581 or APTD-0576 may be used provided that the specifications of this method are met.
6.1.2 Barometer. Mercury, aneroid, or other barometer capable of measuring atmospheric pressure to within 2.5 mm (0.1 in.) Hg.
Note:
The barometric reading may be obtained from a nearby National Weather Service station. In this case, the station value (which is the absolute barometric pressure) shall be requested and an adjustment for elevation differences between the weather station and sampling point shall be made at a rate of minus 2.5 mm (0.1 in) Hg per 30 m (100 ft) elevation increase or plus 2.5 mm (0.1 in) Hg per 30 m (100 ft) elevation decrease.
6.1.3 Gas density determination equipment. Temperature sensor and pressure gauge, as described in sections 6.3 and 6.4 of Method 2, 40 CFR part 60, appendix A, and gas analyzer, if necessary, as described in Method 3, 40 CFR part 60, appendix A. The temperature sensor shall, preferably, be permanently attached to the pitot tube or sampling probe in a fixed configuration, such that the tip of the sensor extends beyond the leading edge of the probe sheath and does not touch any metal. Alternatively, the sensor may be attached just prior to use in the field. Note, however, that if the temperature sensor is attached in the field, the sensor must be placed in an interference-free arrangement with respect to the Type S pitot tube openings (see Method 2, Figure 2-4, 40 CFR part 60, appendix A). As a second alternative, if a difference of not more than 1 percent in the average velocity measurement is to be introduced, the temperature sensor need not be attached to the probe or pitot tube. (This alternative is subject to the approval of the Administrator.)
7.0 Reagents and Standards
7.l Sample collection. The following reagents are required for sample collection:
7.1.1 Filters. Glass fiber filters, without organic binder, exhibiting at least 99.95 percent efficiency (<0.05 percent penetration) on 0.3 micron dioctyl phthalate smoke particles. The filter efficiency test shall be conducted in accordance with ASTM Method D 2986-95A (incorporated by reference in §63.841 of this part). Test data from the supplier's quality control program are sufficient for this purpose. In sources containing S02 or S03, the filter material must be of a type that is unreactive to S02 or S03. Reference 10 in section 17.0 of this method may be used to select the appropriate filter.
7.1.2 Silica gel. Indicating type, 6 to l6 mesh. If previously used, dry at l75°C (350°F) for 2 hours. New silica gel may be used as received. Alternatively, other types of desiccants (equivalent or better) may be used, subject to the approval of the Administrator.
7.1.3 Water. When analysis of the material caught in the impingers is required, deionized distilled water shall be used. Run blanks prior to field use to eliminate a high blank on test samples.
7.1.4 Crushed ice.
7.1.5 Stopcock grease. Acetone-insoluble, heat-stable silicone grease. This is not necessary if screw-on connectors with Teflon” sleeves, or similar, are used. Alternatively, other types of stopcock grease may be used, subject to the approval of the Administrator. [Caution: Many stopcock greases are methylene chloride-soluble. Use sparingly and carefully remove prior to recovery to prevent contamination of the MCEM analysis.]
7.2 Sample recovery. The following reagents are required for sample recovery:
7.2.1 Acetone. Acetone with blank values <1 ppm, by weight residue, is required. Acetone blanks may be run prior to field use, and only acetone with low blank values may be used. In no case shall a blank value of greater than 1E-06 of the weight of acetone used be subtracted from the sample weight.
Note:
This is more restrictive than Method 5, 40 CFR part 60, appendix A. At least one vendor (Supelco Incorporated located in Bellefonte, Pennsylvania) lists <1 mg/l as residue for its Environmental Analysis Solvents.
7.2.2 Methylene chloride. Methylene chloride with a blank value <1.5 ppm, by weight, residue. Methylene chloride blanks may be run prior to field use, and only methylene chloride with low blank values may be used. In no case shall a blank value of greater than 1.6E-06 of the weight of methylene chloride used be subtracted from the sample weight.
Note:
A least one vendor quotes <1 mg/l for Environmental Analysis Solvents-grade methylene chloride.
7.3 Sample analysis. The following reagents are required for sample analysis:
7.3.l Acetone. Same as in section 7.2.1 of this method.
7.3.2 Desiccant. Anhydrous calcium sulfate, indicating type. Alternatively, other types of desiccants may be used, subject to the approval of the Administrator.
7.3.3 Methylene chloride. Same as in section 7.2.2 of this method.
8.0 Sample Collection, Preservation, Storage, and Transport
Note:
The complexity of this method is such that, in order to obtain reliable results, testers should be trained and experienced with the test procedures.
8.1 Pretest preparation. It is suggested that sampling equipment be maintained according to the procedures described in APTD-0576. Alternative mercury-free thermometers may be used if the thermometers are at a minimum equivalent in terms of performance or suitably effective for the specific temperature measurement application.
8.1.1 Weigh several 200 g to 300 g portions of silica gel in airtight containers to the nearest 0.5 g. Record on each container the total weight of the silica gel plus container. As an alternative, the silica gel need not be preweighed but may be weighed directly in its impinger or sampling holder just prior to train assembly.
8.1.2 A batch of glass fiber filters, no more than 50 at a time, should placed in a soxhlet extraction apparatus and extracted using methylene chloride for at least 16 hours. After extraction, check filters visually against light for irregularities, flaws, or pinhole leaks. Label the shipping containers (glass or plastic petri dishes), and keep the filters in these containers at all times except during sampling and weighing.
8.1.3 Desiccate the filters at 20 ±5.6°C (68 ±10°F) and ambient pressure for at least 24 hours and weigh at intervals of at least 6 hours to a constant weight, i.e., <0.5 mg change from previous weighing; record results to the nearest 0.1 mg. During each weighing the filter must not be exposed to the laboratory atmosphere for longer than 2 minutes and a relative humidity above 50 percent. Alternatively (unless otherwise specified by the Administrator), the filters may be oven-dried at 104°C (220°F) for 2 to 3 hours, desiccated for 2 hours, and weighed. Procedures other than those described, which account for relative humidity effects, may be used, subject to the approval of the Administrator.
8.2 Preliminary determinations.
8.2.1 Select the sampling site and the minimum number of sampling points according to Method 1, 40 CFR part 60, appendix A or as specified by the Administrator. Determine the stack pressure, temperature, and the range of velocity heads using Method 2, 40 CFR part 60, appendix A; it is recommended that a leak check of the pitot lines (see section 8.1 of Method 2, 40 CFR part 60, appendix A) be performed. Determine the moisture content using Approximation Method 4 (section 1.2 of Method 4, 40 CFR part 60, appendix A) or its alternatives to make isokinetic sampling rate settings. Determine the stack gas dry molecular weight, as described in section 8.6 of Method 2, 40 CFR part 60, appendix A; if integrated Method 3 sampling is used for molecular weight determination, the integrated bag sample shall be taken simultaneously with, and for the same total length of time as, the particulate sample run.
8.2.2 Select a nozzle size based on the range of velocity heads such that it is not necessary to change the nozzle size in order to maintain isokinetic sampling rates. During the run, do not change the nozzle size. Ensure that the proper differential pressure gauge is chosen for the range of velocity heads encountered (see section 8.2 of Method 2, 40 CFR part 60, appendix A).
8.2.3 Select a suitable probe liner and probe length such that all traverse points can be sampled. For large stacks, consider sampling from opposite sides of the stack to reduce the required probe length.
8.2.4 Select a total sampling time greater than or equal to the minimum total sampling time specified in the test procedures for the specific industry such that: (1) The sampling time per point is not less than 2 minutes (or some greater time interval as specified by the Administrator); and (2) the sample volume taken (corrected to standard conditions) will exceed the required minimum total gas sample volume. The latter is based on an approximate average sampling rate.
8.2.5 The sampling time at each point shall be the same. It is recommended that the number of minutes sampled at each point be an integer or an integer plus one-half minute, in order to eliminate timekeeping errors.
8.2.6 In some circumstances (e.g., batch cycles), it may be necessary to sample for shorter times at the traverse points and to obtain smaller gas sample volumes. In these cases, the Administrator's approval must first be obtained.
8.3 Preparation of sampling train.
8.3.1 During preparation and assembly of the sampling train, keep all openings where contamination can occur covered until just prior to assembly or until sampling is about to begin. Place l00 ml of water in each of the first two impingers, leave the third impinger empty, and transfer approximately 200 to 300 g of preweighed silica gel from its container to the fourth impinger. More silica gel may be used, but care should be taken to ensure that it is not entrained and carried out from the impinger during sampling. Place the container in a clean place for later use in the sample recovery. Alternatively, the weight of the silica gel plus impinger may be determined to the nearest 0.5 g and recorded.
8.3.2 Using a tweezer or clean disposable surgical gloves, place a labeled (identified) and weighed filter in the filter holder. Be sure that the filter is properly centered and the gasket properly placed so as to prevent the sample gas stream from circumventing the filter. Check the filter for tears after assembly is completed.
8.3.3 When glass liners are used, install the selected nozzle using a Viton A 0-ring when stack temperatures are less than 260°C (500°F) and an asbestos string gasket when temperatures are higher. See APTD-0576 for details. Mark the probe with heat-resistant tape or by some other method to denote the proper distance into the stack or duct for each sampling point.
8.3.4 Set up the train as in Figure 5-1 of Method 5, 40 CFR part 60, appendix A, using (if necessary) a very light coat of silicone grease on all ground glass joints, greasing only the outer portion (see APTD-0576) to avoid possibility of contamination by the silicone grease. Subject to the approval of the Administrator, a glass cyclone may be used between the probe and filter holder when the total particulate catch is expected to exceed 100 mg or when water droplets are present in the stack gas.
8.3.5 Place crushed ice around the impingers.
8.4 Leak-check procedures.
8.4.1 Leak check of metering system shown in Figure 5-1 of Method 5, 40 CFR part 60, appendix A. That portion of the sampling train from the pump to the orifice meter should be leak-checked prior to initial use and after each shipment. Leakage after the pump will result in less volume being recorded than is actually sampled. The following procedure is suggested (see Figure 5-2 of Method 5, 40 CFR part 60, appendix A): Close the main valve on the meter box. Insert a one-hole rubber stopper with rubber tubing attached into the orifice exhaust pipe. Disconnect and vent the low side of the orifice manometer. Close off the low side orifice tap. Pressurize the system to 13 to 18 cm (5 to 7 in.) water column by blowing into the rubber tubing. Pinch off the tubing, and observe the manometer for 1 minute. A loss of pressure on the manometer indicates a leak in the meter box; leaks, if present, must be corrected.
8.4.2 Pretest leak check. A pretest leak-check is recommended but not required. If the pretest leak-check is conducted, the following procedure should be used.
8.4.2.1 After the sampling train has been assembled, turn on and set the filter and probe heating systems to the desired operating temperatures. Allow time for the temperatures to stabilize. If a Viton A 0-ring or other leak-free connection is used in assembling the probe nozzle to the probe liner, leak-check the train at the sampling site by plugging the nozzle and pulling a 380 mm (15 in.) Hg vacuum.
Note:
A lower vacuum may be used, provided that it is not exceeded during the test.
8.4.2.2 If an asbestos string is used, do not connect the probe to the train during the leak check. Instead, leak-check the train by first plugging the inlet to the filter holder (cyclone, if applicable) and pulling a 380 mm (15 in.) Hg vacuum. (See NOTE in section 8.4.2.1 of this method). Then connect the probe to the train and perform the leak check at approximately 25 mm (1 in.) Hg vacuum; alternatively, the probe may be leak-checked with the rest of the sampling train, in one step, at 380 mm (15 in.) Hg vacuum. Leakage rates in excess of 4 percent of the average sampling rate or 0.00057 m 3/min (0.02 cfm), whichever is less, are unacceptable.
8.4.2.3 The following leak check instructions for the sampling train described in APTD-0576 and APTD-058l may be helpful. Start the pump with the bypass valve fully open and the coarse adjust valve completely closed. Partially open the coarse adjust valve and slowly close the bypass valve until the desired vacuum is reached. Do not reverse the direction of the bypass valve, as this will cause water to back up into the filter holder. If the desired vacuum is exceeded, either leak-check at this higher vacuum or end the leak check as shown below and start over.
8.4.2.4 When the leak check is completed, first slowly remove the plug from the inlet to the probe, filter holder, or cyclone (if applicable) and immediately turn off the vacuum pump. This prevents the water in the impingers from being forced backward into the filter holder and the silica gel from being entrained backward into the third impinger.
8.4.3 Leak checks during sample run. If, during the sampling run, a component (e.g., filter assembly or impinger) change becomes necessary, a leak check shall be conducted immediately before the change is made. The leak check shall be done according to the procedure outlined in section 8.4.2 of this method, except that it shall be done at a vacuum equal to or greater than the maximum value recorded up to that point in the test. If the leakage rate is found to be no greater than 0.00057 m 3/min (0.02 cfm) or 4 percent of the average sampling rate (whichever is less), the results are acceptable, and no correction will need to be applied to the total volume of dry gas metered; if, however, a higher leakage rate is obtained, either record the leakage rate and plan to correct the sample volume as shown in section 12.3 of this method or void the sample run.
Note:
Immediately after component changes, leak checks are optional; if such leak checks are done, the procedure outlined in section 8.4.2 of this method should be used.
8.4.4 Post-test leak check. A leak check is mandatory at the conclusion of each sampling run. The leak check shall be performed in accordance with the procedures outlined in section 8.4.2 of this method, except that it shall be conducted at a vacuum equal to or greater than the maximum value reached during the sampling run. If the leakage rate is found to be no greater than 0.00057 m 3/min (0.02 cfm) or 4 percent of the average sampling rate (whichever is less), the results are acceptable, and no correction need be applied to the total volume of dry gas metered. If, however, a higher leakage rate is obtained, either record the leakage rate and correct the sample volume, as shown in section 12.4 of this method, or void the sampling run.
8.5 Sampling train operation. During the sampling run, maintain an isokinetic sampling rate (within l0 percent of true isokinetic unless otherwise specified by the Administrator) and a temperature around the filter of 120 14°C (248 25°F), or such other temperature as specified by an applicable subpart of the standards or approved by the Administrator.
8.5.1 For each run, record the data required on a data sheet such as the one shown in Figure 5-2 of Method 5, 40 CFR part 60, appendix A. Be sure to record the initial reading. Record the DGM readings at the beginning and end of each sampling time increment, when changes in flow rates are made, before and after each leak-check, and when sampling is halted. Take other readings indicated by Figure 5-2 of Method 5, 40 CFR part 60, appendix A at least once at each sample point during each time increment and additional readings when significant changes (20 percent variation in velocity head readings) necessitate additional adjustments in flow rate. Level and zero the manometer. Because the manometer level and zero may drift due to vibrations and temperature changes, make periodic checks during the traverse.
8.5.2 Clean the portholes prior to the test run to minimize the chance of sampling deposited material. To begin sampling, remove the nozzle cap and verify that the filter and probe heating systems are up to temperature and that the pitot tube and probe are properly positioned. Position the nozzle at the first traverse point with the tip pointing directly into the gas stream. Immediately start the pump and adjust the flow to isokinetic conditions. Nomographs are available, which aid in the rapid adjustment of the isokinetic sampling rate without excessive computations. These nomographs are designed for use when the Type S pitot tube coefficient (Cp) is 0.85 # 0.02 and the stack gas equivalent density (dry molecular weight) is 29 ±4. APTD-0576 details the procedure for using the nomographs. If Cp and Md are outside the above-stated ranges, do not use the nomographs unless appropriate steps (see Reference 7 in section 17.0 of this method) are taken to compensate for the deviations.
8.5.3 When the stack is under significant negative pressure (height of impinger stem), close the coarse adjust valve before inserting the probe into the stack to prevent water from backing into the filter holder. If necessary, the pump may be turned on with the coarse adjust valve closed.
8.5.4 When the probe is in position, block off the openings around the probe and porthole to prevent unrepresentative dilution of the gas stream.
8.5.5 Traverse the stack cross-section, as required by Method 1, 40 CFR part 60, appendix A or as specified by the Administrator, being careful not to bump the probe nozzle into the stack walls when sampling near the walls or when removing or inserting the probe through the portholes; this minimizes the chance of extracting deposited material.
8.5.6 During the test run, make periodic adjustments to keep the temperature around the filter holder at the proper level; add more ice and, if necessary, salt to maintain a temperature of less than 20°C (68°F) at the condenser/silica gel outlet. Also, periodically check the level and zero of the manometer.
8.5.7 If the pressure drop across the filter becomes too high, making isokinetic sampling difficult to maintain, the filter may be replaced in the midst of the sample run. It is recommended that another complete filter assembly be used rather than attempting to change the filter itself. Before a new filter assembly is installed, conduct a leak check (see section 8.4.3 of this method). The total PM weight shall include the summation of the filter assembly catches.
8.5.8 A single train shall be used for the entire sample run, except in cases where simultaneous sampling is required in two or more separate ducts or at two or more different locations within the same duct, or in cases where equipment failure necessitates a change of trains. In all other situations, the use of two or more trains will be subject to the approval of the Administrator.
Note:
When two or more trains are used, separate analyses of the front-half and (if applicable) impinger catches from each train shall be performed, unless identical nozzle sizes were used in all trains, in which case the front-half catches from the individual trains may be combined (as may the impinger catches) and one analysis of the front-half catch and one analysis of the impinger catch may be performed.
8.5.9 At the end of the sample run, turn off the coarse adjust valve, remove the probe and nozzle from the stack, turn off the pump, record the final DGM reading, and then conduct a post-test leak check, as outlined in section 8.4.4 of this method. Also leak-check the pitot lines as described in section 8.1 of Method 2, 40 CFR part 60, appendix A. The lines must pass this leak check in order to validate the velocity head data.
8.6 Calculation of percent isokinetic. Calculate percent isokinetic (see Calculations, section 12.12 of this method) to determine whether a run was valid or another test run should be made. If there was difficulty in maintaining isokinetic rates because of source conditions, consult the Administrator for possible variance on the isokinetic rates.
8.7 Sample recovery.
8.7.1 Proper cleanup procedure begins as soon as the probe is removed from the stack at the end of the sampling period. Allow the probe to cool.
8.7.2 When the probe can be safely handled, wipe off all external PM near the tip of the probe nozzle and place a cap over it to prevent losing or gaining PM. Do not cap off the probe tip tightly while the sampling train is cooling down. This would create a vacuum in the filter holder, thus drawing water from the impingers into the filter holder.
8.7.3 Before moving the sample train to the cleanup site, remove the probe from the sample train, wipe off the silicone grease, and cap the open outlet of the probe. Be careful not to lose any condensate that might be present. Wipe off the silicone grease from the filter inlet where the probe was fastened and cap it. Remove the umbilical cord from the last impinger and cap the impinger. If a flexible line is used between the first impinger or condenser and the filter holder, disconnect the line at the filter holder and let any condensed water or liquid drain into the impingers or condenser. After wiping off the silicone grease, cap off the filter holder outlet and impinger inlet. Ground-glass stoppers, plastic caps, or serum caps may be used to close these openings.
8.7.4 Transfer the probe and filter-impinger assembly to the cleanup area. This area should be clean and protected from the wind so that the chances of contaminating or losing the sample will be minimized.
8.7.5 Save a portion of the acetone and methylene chloride used for cleanup as blanks. Take 200 ml of each solvent directly from the wash bottle being used and place it in glass sample containers labeled “acetone blank” and “methylene chloride blank,” respectively.
8.7.6 Inspect the train prior to and during disassembly and note any abnormal conditions. Treat the samples as follows:
8.7.6.1 Container No. 1. Carefully remove the filter from the filter holder, and place it in its identified petri dish container. Use a pair of tweezers and/or clean disposable surgical gloves to handle the filter. If it is necessary to fold the filter, do so such that the PM cake is inside the fold. Using a dry nylon bristle brush and/or a sharp-edged blade, carefully transfer to the petri dish any PM and/or filter fibers that adhere to the filter holder gasket. Seal the container.
8.7.6.2 Container No. 2. Taking care to see that dust on the outside of the probe or other exterior surfaces does not get into the sample, quantitatively recover PM or any condensate from the probe nozzle, probe fitting, probe liner, and front half of the filter holder by washing these components with acetone and placing the wash in a glass container. Perform the acetone rinse as follows:
8.7.6.2.1 Carefully remove the probe nozzle and clean the inside surface by rinsing with acetone from a wash bottle and brushing with a nylon bristle brush. Brush until the acetone rinse shows no visible particles, after which make a final rinse of the inside surface with acetone.
8.7.6.2.2 Brush and rinse the inside parts of the Swagelok fitting with acetone in a similar way until no visible particles remain.
8.7.6.2.3 Rinse the probe liner with acetone by tilting and rotating the probe while squirting acetone into its upper end so that all inside surfaces are wetted with acetone. Let the acetone drain from the lower end into the sample container. A funnel (glass or polyethylene) may be used to aid in transferring liquid washes to the container. Follow the acetone rinse with a probe brush. Hold the probe in an inclined position, squirt acetone into the upper end as the probe brush is being pushed with a twisting action through the probe, hold a sample container under the lower end of the probe, and catch any acetone and PM that is brushed from the probe. Run the brush through the probe three times or more until no visible PM is carried out with the acetone or until none remains in the probe liner on visual inspection. With stainless steel or other metal probes, run the brush through in the above-described manner at least six times, since metal probes have small crevices in which PM can be entrapped. Rinse the brush with acetone and quantitatively collect these washings in the sample container. After the brushing, make a final acetone rinse of the probe as described above.
8.7.6.2.4 It is recommended that two people clean the probe to minimize sample losses. Between sampling runs, keep brushes clean and protected from contamination.
8.7.6.2.5 After ensuring that all joints have been wiped clean of silicone grease, clean the inside of the front half of the filter holder by rubbing the surfaces with a nylon bristle brush and rinsing with acetone. Rinse each surface three times or more if needed to remove visible particulate. Make a final rinse of the brush and filter holder. Carefully rinse out the glass cyclone also (if applicable).
8.7.6.2.6 After rinsing the nozzle, probe, and front half of the filter holder with acetone, repeat the entire procedure with methylene chloride and save in a separate No. 2M container.
8.7.6.2.7 After acetone and methylene chloride washings and PM have been collected in the proper sample containers, tighten the lid on the sample containers so that acetone and methylene chloride will not leak out when it is shipped to the laboratory. Mark the height of the fluid level to determine whether leakage occurs during transport. Label each container to identify clearly its contents.
8.7.6.3 Container No. 3. Note the color of the indicating silica gel to determine whether it has been completely spent, and make a notation of its condition. Transfer the silica gel from the fourth impinger to its original container and seal the container. A funnel may make it easier to pour the silica gel without spilling. A rubber policeman may be used as an aid in removing the silica gel from the impinger. It is not necessary to remove the small amount of dust particles that may adhere to the impinger wall and are difficult to remove. Since the gain in weight is to be used for moisture calculations, do not use any water or other liquids to transfer the silica gel. If a balance is available in the field, follow the procedure for Container No. 3 in section 11.2.3 of this method.
8.7.6.4 Impinger water. Treat the impingers as follows:
8.7.6.4.1 Make a notation of any color or film in the liquid catch. Measure the liquid that is in the first three impingers to within 1 ml by using a graduated cylinder or by weighing it to within 0.5 g by using a balance (if one is available). Record the volume or weight of liquid present. This information is required to calculate the moisture content of the effluent gas.
8.7.6.4.2 Following the determination of the volume of liquid present, rinse the back half of the train with water, add it to the impinger catch, and store it in a container labeled 3W (water).
8.7.6.4.3 Following the water rinse, rinse the back half of the train with acetone to remove the excess water to enhance subsequent organic recovery with methylene chloride and quantitatively recover to a container labeled 3S (solvent) followed by at least three sequential rinsings with aliquots of methylene chloride. Quantitatively recover to the same container labeled 3S. Record separately the amount of both acetone and methylene chloride used to the nearest 1 ml or 0.5g.
Note:
Because the subsequent analytical finish is gravimetric, it is okay to recover both solvents to the same container. This would not be recommended if other analytical finishes were required.
8.8 Sample transport. Whenever possible, containers should be shipped in such a way that they remain upright at all times.
9.0 Quality Control
9.1 Miscellaneous quality control measures.
| Section | Quality control measure | Effect |
|---|---|---|
| 8.4, 10.1-10.6 | Sampling and equipment leak check and calibration | Ensure accurate measurement of stack gas flow rate, sample volume. |
9.2 Volume metering system checks. The following quality control procedures are suggested to check the volume metering system calibration values at the field test site prior to sample collection. These procedures are optional.
9.2.1 Meter orifice check. Using the calibration data obtained during the calibration procedure described in section 10.3 of this method, determine the ΔHa for the metering system orifice. The ΔHa is the orifice pressure differential in units of in. H20 that correlates to 0.75 cfm of air at 528°R and 29.92 in. Hg. The ΔHa is calculated as follows:

Where
0.0319 = (0.0567 in. Hg/°R)(0.75 cfm) 2;
ΔH = Average pressure differential across the orifice meter, in. H20;
Tm = Absolute average DGM temperature,°R;
Θ = Total sampling time, min;
Pbar = Barometric pressure, in. Hg;
Y = DGM calibration factor, dimensionless;
Vm = Volume of gas sample as measured by DGM, dcf.
9.2.1.1 Before beginning the field test (a set of three runs usually constitutes a field test), operate the metering system (i.e., pump, volume meter, and orifice) at the ΔHa pressure differential for 10 minutes. Record the volume collected, the DGM temperature, and the barometric pressure. Calculate a DGM calibration check value, Yc, as follows:

Where
Yc = DGM calibration check value, dimensionless;
10 = Run time, min.
9.2.1.2 Compare the Yc value with the dry gas meter calibration factor Y to determine that: 0.97 Y <Yc <1.03Y. If the Yc value is not within this range, the volume metering system should be investigated before beginning the test.
9.2.2 Calibrated critical orifice. A calibrated critical orifice, calibrated against a wet test meter or spirometer and designed to be inserted at the inlet of the sampling meter box, may be used as a quality control check by following the procedure of section 16.2 of this method.
10.0 Calibration and Standardization
Note:
Maintain a laboratory log of all calibrations.
10.1 Probe nozzle. Probe nozzles shall be calibrated before their initial use in the field. Using a micrometer, measure the ID of the nozzle to the nearest 0.025 mm (0.001 in.). Make three separate measurements using different diameters each time, and obtain the average of the measurements. The difference between the high and low numbers shall not exceed 0.1 mm (0.004 in.). When nozzles become nicked, dented, or corroded, they shall be reshaped, sharpened, and recalibrated before use. Each nozzle shall be permanently and uniquely identified.
10.2 Pitot tube assembly. The Type S pitot tube assembly shall be calibrated according to the procedure outlined in section 10.1 of Method 2, 40 CFR part 60, appendix A.
10.3 Metering system.
10.3.1 Calibration prior to use. Before its initial use in the field, the metering system shall be calibrated as follows: Connect the metering system inlet to the outlet of a wet test meter that is accurate to within 1 percent. Refer to Figure 5-5 of Method 5, 40 CFR part 60, appendix A. The wet test meter should have a capacity of 30 liters/revolution (1 ft 3/rev). A spirometer of 400 liters (14 ft 3) or more capacity, or equivalent, may be used for this calibration, although a wet test meter is usually more practical. The wet test meter should be periodically calibrated with a spirometer or a liquid displacement meter to ensure the accuracy of the wet test meter. Spirometers or wet test meters of other sizes may be used, provided that the specified accuracies of the procedure are maintained. Run the metering system pump for about 15 minutes with the orifice manometer indicating a median reading, as expected in field use, to allow the pump to warm up and to permit the interior surface of the wet test meter to be thoroughly wetted. Then, at each of a minimum of three orifice manometer settings, pass an exact quantity of gas through the wet test meter and note the gas volume indicated by the DGM. Also note the barometric pressure and the temperatures of the wet test meter, the inlet of the DGM, and the outlet of the DGM. Select the highest and lowest orifice settings to bracket the expected field operating range of the orifice. Use a minimum volume of 0.15 m 3 (5 cf) at all orifice settings. Record all the data on a form similar to Figure 5-6 of Method 5, 40 CFR part 60, appendix A, and calculate Y (the DGM calibration factor) and ΔHa (the orifice calibration factor) at each orifice setting, as shown on Figure 5-6 of Method 5, 40 CFR part 60, appendix A. Allowable tolerances for individual Y and ΔHa values are given in Figure 5-6 of Method 5, 40 CFR part 60, appendix A. Use the average of the Y values in the calculations in section 12 of this method.
10.3.1.1 Before calibrating the metering system, it is suggested that a leak check be conducted. For metering systems having diaphragm pumps, the normal leak check procedure will not detect leakages within the pump. For these cases the following leak check procedure is suggested: make a 10-minute calibration run at 0.00057 m 3/min (0.02 cfm); at the end of the run, take the difference of the measured wet test meter and DGM volumes; divide the difference by 10 to get the leak rate. The leak rate should not exceed 0.00057 m 3/min (0.02 cfm).
10.3.2 Calibration after use. After each field use, the calibration of the metering system shall be checked by performing three calibration runs at a single, intermediate orifice setting (based on the previous field test) with the vacuum set at the maximum value reached during the test series. To adjust the vacuum, insert a valve between the wet test meter and the inlet of the metering system. Calculate the average value of the DGM calibration factor. If the value has changed by more than 5 percent, recalibrate the meter over the full range of orifice settings, as previously detailed.
Note:
Alternative procedures, e.g., rechecking the orifice meter coefficient, may be used, subject to the approval of the Administrator.
10.3.3 Acceptable variation in calibration. If the DGM coefficient values obtained before and after a test series differ by more than 5 percent, either the test series shall be voided or calculations for the test series shall be performed using whichever meter coefficient value (i.e., before or after) gives the lower value of total sample volume.
10.4 Probe heater calibration. Use a heat source to generate air heated to selected temperatures that approximate those expected to occur in the sources to be sampled. Pass this air through the probe at a typical sample flow rate while measuring the probe inlet and outlet temperatures at various probe heater settings. For each air temperature generated, construct a graph of probe heating system setting versus probe outlet temperature. The procedure outlined in APTD-0576 can also be used. Probes constructed according to APTD-0581 need not be calibrated if the calibration curves in APTD-0576 are used. Also, probes with outlet temperature monitoring capabilities do not require calibration.
Note:
The probe heating system shall be calibrated before its initial use in the field.
10.5 Temperature sensors. Use the procedure in Section 10.3 of Method 2, 40 CFR part 60, appendix A-1 to calibrate in-stack temperature sensors. Dial thermometers, such as are used for the DGM and condenser outlet, shall be calibrated against mercury-in-glass thermometers. An alternative mercury-free thermometer may be used if the thermometer is, at a minimum, equivalent in terms of performance or suitably effective for the specific temperature measurement application.
10.6 Barometer. Calibrate against a mercury barometer.
11.0 Analytical Procedure
11.1 Record the data required on a sheet such as the one shown in Figure 315-1 of this method.
11.2 Handle each sample container as follows:
11.2.1 Container No. 1.
11.2.1.1 PM analysis. Leave the contents in the shipping container or transfer the filter and any loose PM from the sample container to a tared glass weighing dish. Desiccate for 24 hours in a desiccator containing anhydrous calcium sulfate. Weigh to a constant weight and report the results to the nearest 0.1 mg. For purposes of this section, the term “constant weight” means a difference of no more than 0.5 mg or 1 percent of total weight less tare weight, whichever is greater, between two consecutive weighings, with no less than 6 hours of desiccation time between weighings (overnight desiccation is a common practice). If a third weighing is required and it agrees within ±0.5 mg, then the results of the second weighing should be used. For quality assurance purposes, record and report each individual weighing; if more than three weighings are required, note this in the results for the subsequent MCEM results.
11.2.1.2 MCEM analysis. Transfer the filter and contents quantitatively into a beaker. Add 100 ml of methylene chloride and cover with aluminum foil. Sonicate for 3 minutes then allow to stand for 20 minutes. Set up the filtration apparatus. Decant the solution into a clean Buchner fritted funnel. Immediately pressure filter the solution through the tube into another clean, dry beaker. Continue decanting and pressure filtration until all the solvent is transferred. Rinse the beaker and filter with 10 to 20 ml methylene chloride, decant into the Buchner fritted funnel and pressure filter. Place the beaker on a low-temperature hot plate (maximum 40°C) and slowly evaporate almost to dryness. Transfer the remaining last few milliliters of solution quantitatively from the beaker (using at least three aliquots of methylene chloride rinse) to a tared clean dry aluminum dish and evaporate to complete dryness. Remove from heat once solvent is evaporated. Reweigh the dish after a 30-minute equilibrium in the balance room and determine the weight to the nearest 0.1 mg. Conduct a methylene chloride blank run in an identical fashion.
11.2.2 Container No. 2.
11.2.2.1 PM analysis. Note the level of liquid in the container, and confirm on the analysis sheet whether leakage occurred during transport. If a noticeable amount of leakage has occurred, either void the sample or use methods, subject to the approval of the Administrator, to correct the final results. Measure the liquid in this container either volumetrically to ±1 ml or gravimetrically to 1 ±0.5 g. Transfer the contents to a tared 250 ml beaker and evaporate to dryness at ambient temperature and pressure. Desiccate for 24 hours, and weigh to a constant weight. Report the results to the nearest 0.1 mg.
11.2.2.2 MCEM analysis. Add 25 ml methylene chloride to the beaker and cover with aluminum foil. Sonicate for 3 minutes then allow to stand for 20 minutes; combine with contents of Container No. 2M and pressure filter and evaporate as described for Container 1 in section 11.2.1.2 of this method.
Notes for MCEM Analysis
1. Light finger pressure only is necessary on 24/40 adaptor. A Chemplast adapter #15055-240 has been found satisfactory.
2. Avoid aluminum dishes made with fluted sides, as these may promote solvent “creep,” resulting in possible sample loss.
3. If multiple samples are being run, rinse the Buchner fritted funnel twice between samples with 5 ml solvent using pressure filtration. After the second rinse, continue the flow of air until the glass frit is completely dry. Clean the Buchner fritted funnels thoroughly after filtering five or six samples.
11.2.3 Container No. 3. Weigh the spent silica gel (or silica gel plus impinger) to the nearest 0.5 g using a balance. This step may be conducted in the field.
11.2.4 Container 3W (impinger water).
11.2.4.1 MCEM analysis. Transfer the solution into a 1,000 ml separatory funnel quantitatively with methylene chloride washes. Add enough solvent to total approximately 50 ml, if necessary. Shake the funnel for 1 minute, allow the phases to separate, and drain the solvent layer into a 250 ml beaker. Repeat the extraction twice. Evaporate with low heat (less than 40°C) until near dryness. Transfer the remaining few milliliters of solvent quantitatively with small solvent washes into a clean, dry, tared aluminum dish and evaporate to dryness. Remove from heat once solvent is evaporated. Reweigh the dish after a 30-minute equilibration in the balance room and determine the weight to the nearest 0.1 mg.
11.2.5 Container 3S (solvent).
11.2.5.1 MCEM analysis. Transfer the mixed solvent to 250 ml beaker(s). Evaporate and weigh following the procedures detailed for container 3W in section 11.2.4 of this method.
11.2.6 Blank containers. Measure the distilled water, acetone, or methylene chloride in each container either volumetrically or gravimetrically. Transfer the “solvent” to a tared 250 ml beaker, and evaporate to dryness at ambient temperature and pressure. (Conduct a solvent blank on the distilled deionized water blank in an identical fashion to that described in section 11.2.4.1 of this method.) Desiccate for 24 hours, and weigh to a constant weight. Report the results to the nearest 0.l mg.
Note:
The contents of Containers No. 2, 3W, and 3M as well as the blank containers may be evaporated at temperatures higher than ambient. If evaporation is done at an elevated temperature, the temperature must be below the boiling point of the solvent; also, to prevent “bumping,” the evaporation process must be closely supervised, and the contents of the beaker must be swirled occasionally to maintain an even temperature. Use extreme care, as acetone and methylene chloride are highly flammable and have a low flash point.
12.0 Data Analysis and Calculations
12.1 Carry out calculations, retaining at least one extra decimal figure beyond that of the acquired data. Round off figures after the final calculation. Other forms of the equations may be used as long as they give equivalent results.
12.2 Nomenclature.
An = Cross-sectional area of nozzle, m 3 (ft 3).
Bws = Water vapor in the gas stream, proportion by volume.
Ca = Acetone blank residue concentration, mg/g.
Cs = Concentration of particulate matter in stack gas, dry basis, corrected to standard conditions, g/dscm (g/dscf).
I = Percent of isokinetic sampling.
La = Maximum acceptable leakage rate for either a pretest leak check or for a leak check following a component change; equal to 0.00057 m 3/min (0.02 cfm) or 4 percent of the average sampling rate, whichever is less.
Li = Individual leakage rate observed during the leak check conducted prior to the “i th” component change (I = l, 2, 3...n), m 3/min (cfm).
Lp = Leakage rate observed during the post-test leak check, m 3/min (cfm).
ma = Mass of residue of acetone after evaporation, mg.
mn = Total amount of particulate matter collected, mg.
Mw = Molecular weight of water, 18.0 g/g-mole (18.0 lb/lb-mole).
Pbar = Barometric pressure at the sampling site, mm Hg (in Hg).
Ps = Absolute stack gas pressure, mm Hg (in. Hg).
Pstd = Standard absolute pressure, 760 mm Hg (29.92 in. Hg).
R = Ideal gas constant, 0.06236 [(mm Hg)(m 3)]/[(°K) (g-mole)] '61' 21.85 [(in. Hg)(ft 3)]/[(°R)(lb-mole)'61' ].
Tm = Absolute average dry gas meter (DGM) temperature (see Figure 5-2 of Method 5, 40 CFR part 60, appendix A),°K (°R).
Ts = Absolute average stack gas temperature (see Figure 5-2 of Method 5, 40 CFR part 60, appendix A),°K(°R).
Tstd = Standard absolute temperature, 293°K (528°R).
Va = Volume of acetone blank, ml.
Vaw = Volume of acetone used in wash, ml.
Vt = Volume of methylene chloride blank, ml.
Vtw = Volume of methylene chloride used in wash, ml.
Vlc = Total volume liquid collected in impingers and silica gel (see Figure 5-3 of Method 5, 40 CFR part 60, appendix A), ml.
Vm = Volume of gas sample as measured by dry gas meter, dcm (dcf).
Vm(std) = Volume of gas sample measured by the dry gas meter, corrected to standard conditions, dscm (dscf).
Vw(std) = Volume of water vapor in the gas sample, corrected to standard conditions, scm (scf).
Vs = Stack gas velocity, calculated by Equation 2-9 in Method 2, 40 CFR part 60, appendix A, using data obtained from Method 5, 40 CFR part 60, appendix A, m/sec (ft/sec).
Wa = Weight of residue in acetone wash, mg.
Y = Dry gas meter calibration factor.
ΔH = Average pressure differential across the orifice meter (see Figure 5-2 of Method 5, 40 CFR part 60, appendix A), mm H2O (in H2O).
ρa = Density of acetone, 785.1 mg/ml (or see label on bottle).
ρw = Density of water, 0.9982 g/ml (0.00220l lb/ml).
ρt = Density of methylene chloride, 1316.8 mg/ml (or see label on bottle).
Θ = Total sampling time, min.
Θ1 = Sampling time interval, from the beginning of a run until the first component change, min.
Θ1 = Sampling time interval, between two successive component changes, beginning with the interval between the first and second changes, min.
Θp = Sampling time interval, from the final (n th) component change until the end of the sampling run, min.
13.6 = Specific gravity of mercury.
60 = Sec/min.
100 = Conversion to percent.
12.3 Average dry gas meter temperature and average orifice pressure drop. See data sheet (Figure 5-2 of Method 5, 40 CFR part 60, appendix A).
12.4 Dry gas volume. Correct the sample volume measured by the dry gas meter to standard conditions (20°C, 760 mm Hg or 68°F, 29.92 in Hg) by using Equation 315-1.

Where
Kl = 0.3858°K/mm Hg for metric units,
= 17.64°R/in Hg for English units.
Note:
Equation 315-1 can be used as written unless the leakage rate observed during any of the mandatory leak checks (i.e., the post-test leak check or leak checks conducted prior to component changes) exceeds La. If Lp or Li exceeds La, Equation 315-1 must be modified as follows:
(a) Case I. No component changes made during sampling run. In this case, replace Vm in Equation 315-1 with the expression:
[Vm - (Lp - La) Θ]
(b) Case II. One or more component changes made during the sampling run. In this case, replace Vm in Equation 315-1 by the expression:

and substitute only for those leakage rates (Li or Lp) which exceed La.
12.5 Volume of water vapor condensed.

Where
K2 = 0.001333 m 3/ml for metric units;
= 0.04706 ft 3/ml for English units.
12.6 Moisture content.

Note:
In saturated or water droplet-laden gas streams, two calculations of the moisture content of the stack gas shall be made, one from the impinger analysis (Equation 315-3), and a second from the assumption of saturated conditions. The lower of the two values of Bws shall be considered correct. The procedure for determining the moisture content based upon assumption of saturated conditions is given in section 4.0 of Method 4, 40 CFR part 60, appendix A. For the purposes of this method, the average stack gas temperature from Figure 5-2 of Method 5, 40 CFR part 60, appendix A may be used to make this determination, provided that the accuracy of the in-stack temperature sensor is ±1°C (2°F).
12.7 Acetone blank concentration.

12.8 Acetone wash blank.
Wa = Ca Vaw ρa Eq. 315-5
12.9 Total particulate weight. Determine the total PM catch from the sum of the weights obtained from Containers l and 2 less the acetone blank associated with these two containers (see Figure 315-1).
Note:
Refer to section 8.5.8 of this method to assist in calculation of results involving two or more filter assemblies or two or more sampling trains.
12.10 Particulate concentration.
cs = K3 mn/Vm(std) Eq. 315-6
where
K = 0.001 g/mg for metric units;
= 0.0154 gr/mg for English units.
12.11 Conversion factors.
| From | To | Multiply by |
|---|---|---|
| ft 3 | m 3 | 0.02832 |
| gr | mg | 64.80004 |
| gr/ft 3 | mg/m 3 | 2288.4 |
| mg | g | 0.001 |
| gr | lb | 1.429 × 10−4 |
12.12 Isokinetic variation.
12.12.1 Calculation from raw data.

where
K4 = 0.003454 [(mm Hg)(m 3)]/[(m1)(°K)] for metric units;
= 0.002669 [(in Hg)(ft 3)]/[(m1)(°R)] for English units.
12.12.2 Calculation from intermediate values.

where
K5 = 4.320 for metric units;
= 0.09450 for English units.
12.12.3 Acceptable results. If 90 percent ≤I ≤110 percent, the results are acceptable. If the PM or MCEM results are low in comparison to the standard, and “I” is over 110 percent or less than 90 percent, the Administrator may opt to accept the results. Reference 4 in the Bibliography may be used to make acceptability judgments. If “I” is judged to be unacceptable, reject the results, and repeat the test.
12.13 Stack gas velocity and volumetric flow rate. Calculate the average stack gas velocity and volumetric flow rate, if needed, using data obtained in this method and the equations in sections 5.2 and 5.3 of Method 2, 40 CFR part 60, appendix A.
12.14 MCEM results. Determine the MCEM concentration from the results from Containers 1, 2, 2M, 3W, and 3S less the acetone, methylene chloride, and filter blanks value as determined in the following equation:
mmcem = Smtotal − wa − wt − fb
13.0 Method Performance [Reserved]
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 Alternative Procedures
16.1 Dry gas meter as a calibration standard. A DGM may be used as a calibration standard for volume measurements in place of the wet test meter specified in section 16.1 of this method, provided that it is calibrated initially and recalibrated periodically as follows:
16.1.1 Standard dry gas meter calibration.
16.1.1.1. The DGM to be calibrated and used as a secondary reference meter should be of high quality and have an appropriately sized capacity, e.g., 3 liters/rev (0.1 ft 3/rev). A spirometer (400 liters or more capacity), or equivalent, may be used for this calibration, although a wet test meter is usually more practical. The wet test meter should have a capacity of 30 liters/rev (1 ft 3/rev) and be capable of measuring volume to within 1.0 percent; wet test meters should be checked against a spirometer or a liquid displacement meter to ensure the accuracy of the wet test meter. Spirometers or wet test meters of other sizes may be used, provided that the specified accuracies of the procedure are maintained.
16.1.1.2 Set up the components as shown in Figure 5-7 of Method 5, 40 CFR part 60, appendix A. A spirometer, or equivalent, may be used in place of the wet test meter in the system. Run the pump for at least 5 minutes at a flow rate of about 10 liters/min (0.35 cfm) to condition the interior surface of the wet test meter. The pressure drop indicated by the manometer at the inlet side of the DGM should be minimized (no greater than 100 mm H2O [4 in. H2O] at a flow rate of 30 liters/min [1 cfm]). This can be accomplished by using large-diameter tubing connections and straight pipe fittings.
16.1.1.3 Collect the data as shown in the example data sheet (see Figure 5-8 of Method 5, 40 CFR part 60, appendix A). Make triplicate runs at each of the flow rates and at no less than five different flow rates. The range of flow rates should be between 10 and 34 liters/min (0.35 and 1.2 cfm) or over the expected operating range.
16.1.1.4 Calculate flow rate, Q, for each run using the wet test meter volume, Vw, and the run time, q. Calculate the DGM coefficient, Yds, for each run. These calculations are as follows:


Where
K1 = 0.3858 for international system of units (SI); 17.64 for English units;
Pbar = Barometric pressure, mm Hg (in Hg);
Vw = Wet test meter volume, liter (ft 3);
tw = Average wet test meter temperature,°C (°F);
tstd = 273°C for SI units; 460°F for English units;
Θ = Run time, min;
tds = Average dry gas meter temperature,°C (°F);
Vds = Dry gas meter volume, liter (ft 3);
Δp = Dry gas meter inlet differential pressure, mm H2O (in H2O).
16.1.1.5 Compare the three Yds values at each of the flow rates and determine the maximum and minimum values. The difference between the maximum and minimum values at each flow rate should be no greater than 0.030. Extra sets of triplicate runs may be made in order to complete this requirement. In addition, the meter coefficients should be between 0.95 and 1.05. If these specifications cannot be met in three sets of successive triplicate runs, the meter is not suitable as a calibration standard and should not be used as such. If these specifications are met, average the three Yds values at each flow rate resulting in five average meter coefficients, Yds.
16.1.1.6 Prepare a curve of meter coefficient, Yds, versus flow rate, Q, for the DGM. This curve shall be used as a reference when the meter is used to calibrate other DGMs and to determine whether recalibration is required.
16.1.2 Standard dry gas meter recalibration.
16.1.2.1 Recalibrate the standard DGM against a wet test meter or spirometer annually or after every 200 hours of operation, whichever comes first. This requirement is valid provided the standard DGM is kept in a laboratory and, if transported, cared for as any other laboratory instrument. Abuse to the standard meter may cause a change in the calibration and will require more frequent recalibrations.
16.1.2.2 As an alternative to full recalibration, a two-point calibration check may be made. Follow the same procedure and equipment arrangement as for a full recalibration, but run the meter at only two flow rates (suggested rates are 14 and 28 liters/min [0.5 and 1.0 cfm]). Calculate the meter coefficients for these two points, and compare the values with the meter calibration curve. If the two coefficients are within 1.5 percent of the calibration curve values at the same flow rates, the meter need not be recalibrated until the next date for a recalibration check.
6.2 Critical orifices as calibration standards. Critical orifices may be used as calibration standards in place of the wet test meter specified in section 10.3 of this method, provided that they are selected, calibrated, and used as follows:
16.2 Sample recovery. The following items are required for sample recovery:
16.2.1 Probe-liner and probe-nozzle brushes. Nylon or Teflon ® bristle brushes with stainless steel wire handles. The probe brush shall have extensions (at least as long as the probe) constructed of stainless steel, nylon, Teflon ®, or similarly inert material. The brushes shall be properly sized and shaped to brush out the probe liner and nozzle.
16.2.2 Wash bottles. Glass wash bottles are recommended. Polyethylene or tetrafluoroethylene (TFE) wash bottles may be used, but they may introduce a positive bias due to contamination from the bottle. It is recommended that acetone not be stored in polyethylene or TFE bottles for longer than a month.
16.2.3 Glass sample storage containers. Chemically resistant, borosilicate glass bottles, for acetone and methylene chloride washes and impinger water, 500 ml or 1,000 ml. Screw-cap liners shall either be rubber-backed Teflon ® or shall be constructed so as to be leak-free and resistant to chemical attack by acetone or methylene chloride. (Narrow-mouth glass bottles have been found to be less prone to leakage.) Alternatively, polyethylene bottles may be used.
16.2.4 Petri dishes. For filter samples, glass, unless otherwise specified by the Administrator.
16.2.5 Graduated cylinder and/or balance. To measure condensed water, acetone wash and methylene chloride wash used during field recovery of the samples, to within 1 ml or 1 g. Graduated cylinders shall have subdivisions no greater than 2 ml. Most laboratory balances are capable of weighing to the nearest 0.5 g or less. Any such balance is suitable for use here and in section 6.3.4 of this method.
16.2.6 Plastic storage containers. Air-tight containers to store silica gel.
16.2.7 Funnel and rubber policeman. To aid in transfer of silica gel to container; not necessary if silica gel is weighed in the field.
16.2.8 Funnel. Glass or polyethylene, to aid in sample recovery.
16.3 Sample analysis. The following equipment is required for sample analysis:
6.3.1 Glass or Teflon ® weighing dishes.
16.3.2 Desiccator. It is recommended that fresh desiccant be used to minimize the chance for positive bias due to absorption of organic material during drying.
16.3.3 Analytical balance. To measure to within 0.l mg.
16.3.4 Balance. To measure to within 0.5 g.
16.3.5 Beakers. 250 ml.
16.3.6 Hygrometer. To measure the relative humidity of the laboratory environment.
16.3.7 Temperature sensor. To measure the temperature of the laboratory environment.
16.3.8 Buchner fritted funnel. 30 ml size, fine (<50 micron)-porosity fritted glass.
16.3.9 Pressure filtration apparatus.
16.3.10 Aluminum dish. Flat bottom, smooth sides, and flanged top, 18 mm deep and with an inside diameter of approximately 60 mm.
16.2.1 Selection of critical orifices.
16.2.1.1 The procedure that follows describes the use of hypodermic needles or stainless steel needle tubing that has been found suitable for use as critical orifices. Other materials and critical orifice designs may be used provided the orifices act as true critical orifices; i.e., a critical vacuum can be obtained, as described in section 7.2.2.2.3 of Method 5, 40 CFR part 60, appendix A. Select five critical orifices that are appropriately sized to cover the range of flow rates between 10 and 34 liters/min or the expected operating range. Two of the critical orifices should bracket the expected operating range. A minimum of three critical orifices will be needed to calibrate a Method 5 DGM; the other two critical orifices can serve as spares and provide better selection for bracketing the range of operating flow rates. The needle sizes and tubing lengths shown in Table 315-1 give the approximate flow rates indicated in the table.
16.2.1.2 These needles can be adapted to a Method 5 type sampling train as follows: Insert a serum bottle stopper, 13 × 20 mm sleeve type, into a 0.5 in Swagelok quick connect. Insert the needle into the stopper as shown in Figure 5-9 of Method 5, 40 CFR part 60, appendix A.
16.2.2 Critical orifice calibration. The procedure described in this section uses the Method 5 meter box configuration with a DGM as described in section 6.1.1.9 of this method to calibrate the critical orifices. Other schemes may be used, subject to the approval of the Administrator.
16.2.2.1 Calibration of meter box. The critical orifices must be calibrated in the same configuration as they will be used; i.e., there should be no connections to the inlet of the orifice.
16.2.2.1.1 Before calibrating the meter box, leak-check the system as follows: Fully open the coarse adjust valve and completely close the bypass valve. Plug the inlet. Then turn on the pump and determine whether there is any leakage. The leakage rate shall be zero; i.e., no detectable movement of the DGM dial shall be seen for 1 minute.
16.2.2.1.2 Check also for leakages in that portion of the sampling train between the pump and the orifice meter. See section 5.6 of Method 5, 40 CFR part 60, appendix A for the procedure; make any corrections, if necessary. If leakage is detected, check for cracked gaskets, loose fittings, worn 0-rings, etc. and make the necessary repairs.
16.2.2.1.3 After determining that the meter box is leakless, calibrate the meter box according to the procedure given in section 5.3 of Method 5, 40 CFR part 60, appendix A. Make sure that the wet test meter meets the requirements stated in section 7.1.1.1 of Method 5, 40 CFR part 60, appendix A. Check the water level in the wet test meter. Record the DGM calibration factor, Y.
16.2.2.2 Calibration of critical orifices. Set up the apparatus as shown in Figure 5-10 of Method 5, 40 CFR part 60, appendix A.
16.2.2.2.1 Allow a warm-up time of 15 minutes. This step is important to equilibrate the temperature conditions through the DGM.
16.2.2.2.2 Leak-check the system as in section 7.2.2.1.1 of Method 5, 40 CFR part 60, appendix A. The leakage rate shall be zero.
16.2.2.2.3 Before calibrating the critical orifice, determine its suitability and the appropriate operating vacuum as follows: turn on the pump, fully open the coarse adjust valve, and adjust the bypass valve to give a vacuum reading corresponding to about half of atmospheric pressure. Observe the meter box orifice manometer reading, DH. Slowly increase the vacuum reading until a stable reading is obtained on the meter box orifice manometer. Record the critical vacuum for each orifice. Orifices that do not reach a critical value shall not be used.
16.2.2.2.4 Obtain the barometric pressure using a barometer as described in section 6.1.2 of this method. Record the barometric pressure, Pbar, in mm Hg (in. Hg).
16.2.2.2.5 Conduct duplicate runs at a vacuum of 25 to 50 mm Hg (1 to 2 in. Hg) above the critical vacuum. The runs shall be at least 5 minutes each. The DGM volume readings shall be in increments of complete revolutions of the DGM. As a guideline, the times should not differ by more than 3.0 seconds (this includes allowance for changes in the DGM temperatures) to achieve ±0.5 percent in K′. Record the information listed in Figure 5-11 of Method 5, 40 CFR part 60, appendix A.
16.2.2.2.6 Calculate K′ using Equation 315-11.

where
K′ = Critical orifice coefficient, [m 3)(°K) 1/2]/[(mm Hg)(min)] [(ft 3)(°R) 1/2)]/[(in. Hg)(min)]
Tamb = Absolute ambient temperature,°K (°R).
16.2.2.2.7 Average the K′ values. The individual K′ values should not differ by more than ±0.5 percent from the average.
16.2.3 Using the critical orifices as calibration standards.
16.2.3.1 Record the barometric pressure.
16.2.3.2 Calibrate the metering system according to the procedure outlined in sections 7.2.2.2.1 to 7.2.2.2.5 of Method 5, 40 CFR part 60, appendix A. Record the information listed in Figure 5-12 of Method 5, 40 CFR part 60, appendix A.
16.2.3.3 Calculate the standard volumes of air passed through the DGM and the critical orifices, and calculate the DGM calibration factor, Y, using the equations below:
Vm(std) = K1 Vm [Pbar + (ΔH/13.6)]/TmEq. 315-12
Vcr(std) = K′ (Pbar Θ)/Tamb1/2Eq. 315-13
Y = Vcr(std)/Vm(std)Eq. 315-14
where
Vcr(std) = Volume of gas sample passed through the critical orifice, corrected to standard conditions, dscm (dscf).
K′ = 0.3858°K/mm Hg for metric units
= 17.64°R/in Hg for English units.
16.2.3.4 Average the DGM calibration values for each of the flow rates. The calibration factor, Y, at each of the flow rates should not differ by more than ±2 percent from the average.
16.2.3.5 To determine the need for recalibrating the critical orifices, compare the DGM Y factors obtained from two adjacent orifices each time a DGM is calibrated; for example, when checking orifice 13/2.5, use orifices 12/10.2 and 13/5.1. If any critical orifice yields a DGM Y factor differing by more than 2 percent from the others, recalibrate the critical orifice according to section 7.2.2.2 of Method 5, 40 CFR part 60, appendix A.
17.0 References
1. Addendum to Specifications for Incinerator Testing at Federal Facilities. PHS, NCAPC. December 6, 1967.
2. Martin, Robert M. Construction Details of Isokinetic Source-Sampling Equipment. Environmental Protection Agency. Research Triangle Park, NC. APTD-0581. April 1971.
3. Rom, Jerome J. Maintenance, Calibration, and Operation of Isokinetic Source Sampling Equipment. Environmental Protection Agency. Research Triangle Park, NC. APTD-0576. March 1972.
4. Smith, W.S., R.T. Shigehara, and W.F. Todd. A Method of Interpreting Stack Sampling Data. Paper Presented at the 63rd Annual Meeting of the Air Pollution Control Association, St. Louis, MO. June 14-19, 1970.
5. Smith, W.S., et al. Stack Gas Sampling Improved and Simplified With New Equipment. APCA Paper No. 67-119. 1967.
6. Specifications for Incinerator Testing at Federal Facilities. PHS, NCAPC. 1967.
7. Shigehara, R.T. Adjustment in the EPA Nomograph for Different Pitot Tube Coefficients and Dry Molecular Weights. Stack Sampling News 2:4-11. October 1974.
8. Vollaro, R.F. A Survey of Commercially Available Instrumentation for the Measurement of Low-Range Gas Velocities. U.S. Environmental Protection Agency, Emission Measurement Branch. Research Triangle Park, NC. November 1976 (unpublished paper).
9. Annual Book of ASTM Standards. Part 26. Gaseous Fuels; Coal and Coke; Atmospheric Analysis. American Society for Testing and Materials. Philadelphia, PA. 1974. pp. 617-622.
10. Felix, L.G., G.I. Clinard, G.E. Lacy, and J.D. McCain. Inertial Cascade Impactor Substrate Media for Flue Gas Sampling. U.S. Environmental Protection Agency. Research Triangle Park, NC 27711. Publication No. EPA-600/7-77-060. June 1977. 83 p.
11. Westlin, P.R., and R.T. Shigehara. Procedure for Calibrating and Using Dry Gas Volume Meters as Calibration Standards. Source Evaluation Society Newsletter. 3 (1):17-30. February 1978.
12. Lodge, J.P., Jr., J.B. Pate, B.E. Ammons, and G.A. Swanson. The Use of Hypodermic Needles as Critical Orifices in Air Sampling. J. Air Pollution Control Association. 16:197-200. 1966.
18.0 Tables, Diagrams, Flowcharts, and Validation Data
|
Gauge/length
(cm) |
Flow rate
(liters/min) |
Gauge/length
(cm) |
Flow rate
(liters/min) |
|---|---|---|---|
| 12/7.6 | 32.56 | 14/2.5 | 19.54 |
| 12/10.2 | 30.02 | 14/5.1 | 17.27 |
| 13/2.5 | 25.77 | 14/7.6 | 16.14 |
| 13/5.1 | 23.50 | 15/3.2 | 14.16 |
| 13/7.6 | 22.37 | 15/7.6 | 11.61 |
| 13/10.2 | 20.67 | 115/10.2 | 10.48 |

|
Final weight
(mg) |
Tare weight
(mg) |
Weight gain
(mg) | |
|---|---|---|---|
| Container No. 1 | |||
| Container No. 2 | |||
| Total | |||
| Less Acetone blank | |||
| Weight of particulate matter | |||
| Final volume (mg) | Initial volume (mg) | Liquid collected (mg) | |
| Moisture Analysis | |||
| Impingers | Note 1 | Note 1 | |
| Silica gel | |||
| Total | |||
| Note 1: Convert volume of water to weight by multiplying by the density of water (1 g/ml). |
| Container No. | Final weight (mg) | Tare of aluminum dish (mg) | Weight gain | Acetone wash volume (ml) | Methylene chloride wash volume (ml) |
|---|---|---|---|---|---|
| MCEM Analysis | |||||
| 1 | |||||
| 2 + 2M | |||||
| 3W | |||||
| 3S | |||||
| Total | ∑mtotal | 0 Vaw | ∑Vtw | ||
| Less acetone wash blank (mg) (not to exceed 1 mg/l of acetone used) | wa = capa ∑Vaw |
| Less methylene chloride wash blank (mg) (not to exceed 1.5 mg/l of methylene chloride used) | wt = ctpt ∑Vtw |
| Less filter blank (mg) (not to exceed . . . (mg/filter) | Fb |
| MCEM weight (mg) | mMCEOM = ∑mtotal − wa − wt− fb |
Method 316 - Sampling and Analysis for Formaldehyde Emissions From Stationary Sources in the Mineral Wool and Wool Fiberglass Industries
1.0 Scope and Application
This method is applicable to the determination of formaldehyde, CAS Registry number 50-00-0, from stationary sources in the mineral wool and wool fiber glass industries. High purity water is used to collect the formaldehyde. The formaldehyde concentrations in the stack samples are determined using the modified pararosaniline method. Formaldehyde can be detected as low as 8.8 × 10−10 lbs/cu ft (11.3 ppbv) or as high as 1.8 × 10−3 lbs/cu ft (23,000,000 ppbv), at standard conditions over a 1-hour sampling period, sampling approximately 30 cu ft.
2.0 Summary of Method
Gaseous and particulate pollutants are withdrawn isokinetically from an emission source and are collected in high purity water. Formaldehyde present in the emissions is highly soluble in high purity water. The high purity water containing formaldehyde is then analyzed using the modified pararosaniline method. Formaldehyde in the sample reacts with acidic pararosaniline, and the sodium sulfite, forming a purple chromophore. The intensity of the purple color, measured spectrophotometrically, provides an accurate and precise measure of the formaldehyde concentration in the sample.
3.0 Definitions
See the definitions in the General Provisions of this Subpart.
4.0 Interferences
Sulfite and cyanide in solution interfere with the pararosaniline method. A procedure to overcome the interference by each compound has been described by Miksch, et al.
5.0 Safety [Reserved]
6.0 Apparatus and Materials
6.1 A schematic of the sampling train is shown in Figure 1. This sampling train configuration is adapted from EPA Method 5, 40 CFR part 60, appendix A, procedures.
The sampling train consists of the following components: probe nozzle, probe liner, pitot tube, differential pressure gauge, impingers, metering system, barometer, and gas density determination equipment.
6.1.1 Probe Nozzle: Quartz, glass, or stainless steel with sharp, tapered (30° angle) leading edge. The taper shall be on the outside to preserve a constant inner diameter. The nozzle shall be buttonhook or elbow design. A range of nozzle sizes suitable for isokinetic sampling should be available in increments of 0.15 cm ( 1/16 in), e.g., 0.32 to 1.27 cm ( 1/8 to 1/2 in), or larger if higher volume sampling trains are used. Each nozzle shall be calibrated according to the procedure outlined in Section 10.1.
6.1.2 Probe Liner: Borosilicate glass or quartz shall be used for the probe liner. The probe shall be maintained at a temperature of 120°C ±14°C (248°F ±25°F).
6.1.3 Pitot Tube: The pitot tube shall be Type S, as described in Section 2.1 of EPA Method 2, 40 CFR part 60, appendix A, or any other appropriate device. The pitot tube shall be attached to the probe to allow constant monitoring of the stack gas velocity. The impact (high pressure) opening plane of the pitot tube shall be even with or above the nozzle entry plane (see Figure 2-6b, EPA Method 2, 40 CFR part 60, appendix A) during sampling. The Type S pitot tube assembly shall have a known coefficient, determined as outlined in Section 4 of EPA Method 2, 40 CFR part 60, appendix A.
6.1.4 Differential Pressure Gauge: The differential pressure gauge shall be an inclined manometer or equivalent device as described in Section 2.2 of EPA Method 2, 40 CFR part 60, appendix A. One manometer shall be used for velocity-head reading and the other for orifice differential pressure readings.
6.1.5 Impingers: The sampling train requires a minimum of four impingers, connected as shown in Figure 1, with ground glass (or equivalent) vacuum-tight fittings. For the first, third, and fourth impingers, use the Greenburg-Smith design, modified by replacing the tip with a 1.3 cm inside diameters ( 1/2 in) glass tube extending to 1.3 cm ( 1/2 in) from the bottom of the flask. For the second impinger, use a Greenburg-Smith impinger with the standard tip. Place a thermometer capable of measuring temperature to within 1°C (2°F) at the outlet of the fourth impinger for monitoring purposes.
6.1.6 Metering System: The necessary components are a vacuum gauge, leak-free pump, thermometers capable of measuring temperatures within 3°C (5.4°F), dry-gas meter capable of measuring volume to within 1 percent, and related equipment as shown in Figure 1. At a minimum, the pump should be capable of 4 cfm free flow, and the dry gas meter should have a recording capacity of 0-999.9 cu ft with a resolution of 0.005 cu ft. Other metering systems may be used which are capable of maintaining sample volumes to within 2 percent. The metering system may be used in conjunction with a pitot tube to enable checks of isokinetic sampling rates.
6.1.7 Barometer: The barometer may be mercury, aneroid, or other barometer capable of measuring atmospheric pressure to within 2.5 mm Hg (0.1 in Hg). In many cases, the barometric reading may be obtained from a nearby National Weather Service Station, in which case the station value (which is the absolute barometric pressure) is requested and an adjustment for elevation differences between the weather station and sampling point is applied at a rate of minus 2.5 mm Hg (0.1 in Hg) per 30 m (100 ft) elevation increase (rate is plus 2.5 mm Hg per 30 m (100 ft) of elevation decrease).
6.1.8 Gas Density Determination Equipment: Temperature sensor and pressure gauge (as described in Sections 2.3 and 2.3 of EPA Method 2, 40 CFR part 60, appendix A), and gas analyzer, if necessary (as described in EPA Method 3, 40 CFR part 60, appendix A). The temperature sensor ideally should be permanently attached to the pitot tube or sampling probe in a fixed configuration such that the top of the sensor extends beyond the leading edge of the probe sheath and does not touch any metal. Alternatively, the sensor may be attached just prior to use in the field. Note, however, that if the temperature sensor is attached in the field, the sensor must be placed in an interference-free arrangement with respect to the Type S pitot openings (see Figure 2-7, EPA Method 2, 40 CFR part 60, appendix A). As a second alternative, if a difference of no more than 1 percent in the average velocity measurement is to be introduced, the temperature gauge need not be attached to the probe or pitot tube.
6.2 Sample Recovery
6.2.1 Probe Liner: Probe nozzle and brushes; bristle brushes with stainless steel wire handles are required. The probe brush shall have extensions of stainless steel, Teflon TM, or inert material at least as long as the probe. The brushes shall be properly sized and shaped to brush out the probe liner, the probe nozzle, and the impingers.
6.2.2 Wash Bottles: One wash bottle is required. Polyethylene, Teflon TM, or glass wash bottles may be used for sample recovery.
6.2.3 Graduated Cylinder and/or Balance: A graduated cylinder or balance is required to measure condensed water to the nearest 1 ml or 1 g. Graduated cylinders shall have division not >2 ml. Laboratory balances capable of weighing to ±0.5 g are required.
6.2.4 Polyethylene Storage Containers: 500 ml wide-mouth polyethylene bottles are required to store impinger water samples.
6.2.5 Rubber Policeman and Funnel: A rubber policeman and funnel are required to aid the transfer of material into and out of containers in the field.
6.3 Sample Analysis
6.3.1 Spectrophotometer - B&L 70, 710, 2000, etc., or equivalent; 1 cm pathlength cuvette holder.
6.3.2 Disposable polystyrene cuvettes, pathlengh 1 cm, volume of about 4.5 ml.
6.3.3 Pipettors - Fixed-volume Oxford pipet (250 µl; 500 µl; 1000 µl); adjustable volume Oxford or equivalent pipettor 1-5 ml model, set to 2.50 ml.
6.3.4 Pipet tips for pipettors above.
6.3.5 Parafilm, 2° wide; cut into about 1” squares.
7.0 Reagents
7.1 High purity water: All references to water in this method refer to high purity water (ASTM Type I water or equivalent). The water purity will dictate the lower limits of formaldehyde quantification.
7.2 Silica Gel: Silica gel shall be indicting type, 6-16 mesh. If the silica gel has been used previously, dry at 175°C (350°F) for 2 hours before using. New silica gel may be used as received. Alternatively, other types of desiccants (equivalent or better) may be used.
7.3 Crushed Ice: Quantities ranging from 10-50 lbs may be necessary during a sampling run, depending upon ambient temperature. Samples which have been taken must be stored and shipped cold; sufficient ice for this purpose must be allowed.
7.4 Quaternary ammonium compound stock solution: Prepare a stock solution of dodecyltrimethylammonium chloride (98 percent minimum assay, reagent grade) by dissolving 1.0 gram in 1000 ml water. This solution contains nominally 1000 µg/ml quaternary ammonium compound, and is used as a biocide for some sources which are prone to microbial contamination.
7.5 Pararosaniline: Weigh 0.16 grams pararosaniline (free base; assay of 95 percent or greater, C.I. 42500; Sigma P7632 has been found to be acceptable) into a 100 ml flask. Exercise care, since pararosaniline is a dye and will stain. Using a wash bottle with high-purity water, rinse the walls of the flask. Add no more than 25 ml water. Then, carefully add 20 ml of concentrated hydrochloric acid to the flask. The flask will become warm after the addition of acid. Add a magnetic stir bar to the flask, cap, and place on a magnetic stirrer for approximately 4 hours. Then, add additional water so the total volume is 100 ml. This solution is stable for several months when stored tightly capped at room temperature.
7.6 Sodium sulfite: Weigh 0.10 grams anhydrous sodium sulfite into a 100 ml flask. Dilute to the mark with high purity water. Invert 15-20 times to mix and dissolve the sodium sulfite. This solution must be prepared fresh every day.
7.7 Formaldehyde standard solution: Pipet exactly 2.70 ml of 37 percent formaldehyde solution into a 1000 ml volumetric flask which contains about 500 ml of high-purity water. Dilute to the mark with high-purity water. This solution contains nominally 1000 µg/ml of formaldehyde, and is used to prepare the working formaldehyde standards. The exact formaldehyde concentration may be determined if needed by suitable modification of the sodium sulfite method (Reference: J.F. Walker, Formaldehyde (Third Edition), 1964.). The 1000 µg/ml formaldehyde stock solution is stable for at least a year if kept tightly closed, with the neck of the flask sealed with Parafilm. Store at room temperature.
7.8 Working formaldehyde standards: Pipet exactly 10.0 ml of the 1000 µg/ml formaldehyde stock solution into a 100 ml volumetric flask which is about half full of high-purity water. Dilute to the mark with high-purity water, and invert 15-20 times to mix thoroughly. This solution contains nominally 100 µg/ml formaldehyde. Prepare the working standards from this 100 µg/ml standard solution and using the Oxford pipets:
| Working standard, µ/mL | µL or 100 µg/mL solution | Volumetric flask volume (dilute to mark with water) |
|---|---|---|
| 0.250 | 250 | 100 |
| 0.500 | 500 | 100 |
| 1.00 | 1000 | 100 |
| 2.00 | 2000 | 100 |
| 3.00 | 1500 | 50 |
The 100 µg/ml stock solution is stable for 4 weeks if kept refrigerated between analyses. The working standards (0.25-3.00 µg/ml) should be prepared fresh every day, consistent with good laboratory practice for trace analysis. If the laboratory water is not of sufficient purity, it may be necessary to prepare the working standards every day. The laboratory must establish that the working standards are stable - DO NOT assume that your working standards are stable for more than a day unless you have verified this by actual testing for several series of working standards.
8.0 Sample Collection
8.1 Because of the complexity of this method, field personnel should be trained in and experienced with the test procedures in order to obtain reliable results.
8.2 Laboratory Preparation
8.2.1 All the components shall be maintained and calibrated according to the procedure described in APTD-0576, unless otherwise specified.
8.2.2 Weigh several 200 to 300 g portions of silica gel in airtight containers to the nearest 0.5 g. Record on each container the total weight of the silica gel plus containers. As an alternative to preweighing the silica gel, it may instead be weighed directly in the impinger or sampling holder just prior to train assembly.
8.3 Preliminary Field Determinations
8.3.1 Select the sampling site and the minimum number of sampling points according to EPA Method 1, 40 CFR part 60, appendix A, or other relevant criteria. Determine the stack pressure, temperature, and range of velocity heads using EPA Method 2, 40 CFR part 60, appendix A. A leak-check of the pitot lines according to Section 3.1 of EPA Method 2, 40 CFR part 60, appendix A, must be performed. Determine the stack gas moisture content using EPA Approximation Method 4,40 CFR part 60, appendix A, or its alternatives to establish estimates of isokinetic sampling rate settings. Determine the stack gas dry molecular weight, as described in EPA Method 2, 40 CFR part 60, appendix A, Section 3.6. If integrated EPA Method 3, 40 CFR part 60, appendix A, sampling is used for molecular weight determination, the integrated bag sample shall be taken simultaneously with, and for the same total length of time as, the sample run.
8.3.2 Select a nozzle size based on the range of velocity heads so that it is not necessary to change the nozzle size in order to maintain isokinetic sampling rates below 28 l/min (1.0 cfm). During the run do not change the nozzle. Ensure that the proper differential pressure gauge is chosen for the range of velocity heads encountered (see Section 2.2 of EPA Method 2, 40 CFR part 60, appendix A).
8.3.3 Select a suitable probe liner and probe length so that all traverse points can be sampled. For large stacks, to reduce the length of the probe, consider sampling from opposite sides of the stack.
8.3.4 A minimum of 30 cu ft of sample volume is suggested for emission sources with stack concentrations not greater than 23,000,000 ppbv. Additional sample volume shall be collected as necessitated by the capacity of the water reagent and analytical detection limit constraint. Reduced sample volume may be collected as long as the final concentration of formaldehyde in the stack sample is greater than 10 (ten) times the detection limit.
8.3.5 Determine the total length of sampling time needed to obtain the identified minimum volume by comparing the anticipated average sampling rate with the volume requirement. Allocate the same time to all traverse points defined by EPA Method 1, 40 CFR part 60, appendix A. To avoid timekeeping errors, the length of time sampled at each traverse point should be an integer or an integer plus 0.5 min.
8.3.6 In some circumstances (e.g., batch cycles) it may be necessary to sample for shorter times at the traverse points and to obtain smaller gas-volume samples. In these cases, careful documentation must be maintained in order to allow accurate calculations of concentrations.
8.4 Preparation of Collection Train
8.4.1 During preparation and assembly of the sampling train, keep all openings where contamination can occur covered with Teflon TM film or aluminum foil until just prior to assembly or until sampling is about to begin.
8.4.2 Place 100 ml of water in each of the first two impingers, and leave the third impinger empty. If additional capacity is required for high expected concentrations of formaldehyde in the stack gas, 200 ml of water per impinger may be used or additional impingers may be used for sampling. Transfer approximately 200 to 300 g of pre-weighed silica gel from its container to the fourth impinger. Care should be taken to ensure that the silica gel is not entrained and carried out from the impinger during sampling. Place the silica gel container in a clean place for later use in the sample recovery. Alternatively, the weight of the silica gel plus impinger may be determined to the nearest 0.5 g and recorded.
8.4.3 With a glass or quartz liner, install the selected nozzle using a Viton-A O-ring when stack temperatures are <260°C (500°F) and a woven glass-fiber gasket when temperatures are higher. See APTD-0576 for details. Other connection systems utilizing either 316 stainless steel or Teflon TM ferrules may be used. Mark the probe with heat-resistant tape or by some other method to denote the proper distance into the stack or duct for each sampling point.
8.4.4 Assemble the train as shown in Figure 1. During assembly, a very light coating of silicone grease may be used on ground-glass joints of the impingers, but the silicone grease should be limited to the outer portion (see APTD-0576) of the ground-glass joints to minimize silicone grease contamination. If necessary, Teflon TM tape may be used to seal leaks. Connect all temperature sensors to an appropriate potentiometer/display unit. Check all temperature sensors at ambient temperatures.
8.4.5 Place crushed ice all around the impingers.
8.4.6 Turn on and set the probe heating system at the desired operating temperature. Allow time for the temperature to stabilize.
8.5 Leak-Check Procedures
8.5.1 Pre-test Leak-check: Recommended, but not required. If the tester elects to conduct the pre-test leak-check, the following procedure shall be used.
8.5.1.1 After the sampling train has been assembled, turn on and set probe heating system at the desired operating temperature. Allow time for the temperature to stabilize. If a Viton-a O-ring or other leak-free connection is used in assembling the probe nozzle to the probe liner, leak-check the train at the sampling site by plugging the nozzle and pulling a 381 mm Hg (15 in Hg) vacuum.
Note:
A lower vacuum may be used, provided that the lower vacuum is not exceeded during the test.
If a woven glass fiber gasket is used, do not connect the probe to the train during the leak-check. Instead, leak-check the train by first attaching a carbon-filled leak-check impinger to the inlet and then plugging the inlet and pulling a 381 mm Hg (15 in Hg) vacuum. (A lower vacuum may be used if this lower vacuum is not exceeded during the test.) Next connect the probe to the train and leak-check at about 25 mm Hg (1 in Hg) vacuum. Alternatively, leak-check the probe with the rest of the sampling train in one step at 381 mm Hg (15 in Hg) vacuum. Leakage rates in excess of (a) 4 percent of the average sampling rate or (b) 0.00057 m 3/min (0.02 cfm), whichever is less, are unacceptable.
8.5.1.2 The following leak-check instructions for the sampling train described in APTD-0576 and APTD-0581 may be helpful. Start the pump with the fine-adjust valve fully open and coarse-valve completely closed. Partially open the coarse-adjust valve and slowly close the fine-adjust valve until the desired vacuum is reached. Do not reverse direction of the fine-adjust valve, as liquid will back up into the train. If the desired vacuum is exceeded, either perform the leak-check at this higher vacuum or end the leak-check, as described below, and start over.
8.5.1.3 When the leak-check is completed, first slowly remove the plug from the inlet to the probe. When the vacuum drops to 127 mm (5 in) Hg or less, immediately close the coarse-adjust valve. Switch off the pumping system and reopen the fine-adjust valve. Do not reopen the fine-adjust valve until the coarse-adjust valve has been closed to prevent the liquid in the impingers from being forced backward in the sampling line and silica gel from being entrained backward into the third impinger.
8.5.2 Leak-checks During Sampling Run:
8.5.2.1 If, during the sampling run, a component change (e.g., impinger) becomes necessary, a leak-check shall be conducted immediately after the interruption of sampling and before the change is made. The leak-check shall be done according to the procedure described in Section 10.3.3, except that it shall be done at a vacuum greater than or equal to the maximum value recorded up to that point in the test. If the leakage rate is found to be no greater than 0.0057 m 3/min (0.02 cfm) or 4 percent of the average sampling rate (whichever is less), the results are acceptable. If a higher leakage rate is obtained, the tester must void the sampling run.
Note:
Any correction of the sample volume by calculation reduces the integrity of the pollutant concentration data generated and must be avoided.
8.5.2.2 Immediately after component changes, leak-checks are optional. If performed, the procedure described in section 8.5.1.1 shall be used.
8.5.3 Post-test Leak-check:
8.5.3.1 A leak-check is mandatory at the conclusion of each sampling run. The leak-check shall be done with the same procedures as the pre-test leak-check, except that the post-test leak-check shall be conducted at a vacuum greater than or equal to the maximum value reached during the sampling run. If the leakage rate is found to be no greater than 0.00057 m 3/min (0.02 cfm) or 4 percent of the average sampling rate (whichever is less), the results are acceptable. If, however, a higher leakage rate is obtained, the tester shall record the leakage rate and void the sampling run.
8.6 Sampling Train Operation
8.6.1 During the sampling run, maintain an isokinetic sampling rate to within 10 percent of true isokinetic, below 28 l/min (1.0 cfm). Maintain a temperature around the probe of 120°C ±14°C (248° ±25°F).
8.6.2 For each run, record the data on a data sheet such as the one shown in Figure 2. Be sure to record the initial dry-gas meter reading. Record the dry-gas meter readings at the beginning and end of each sampling time increment, when changes in flow rates are made, before and after each leak-check, and when sampling is halted. Take other readings required by Figure 2 at least once at each sample point during each time increment and additional readings when significant adjustments (20 percent variation in velocity head readings) necessitate additional adjustments in flow rate. Level and zero the manometer. Because the manometer level and zero may drift due to vibrations and temperature changes, make periodic checks during the traverse.
| Traverse point number |
Sampling time
(e) min. |
Vacuum
mm Hg (in. Hg) |
Stack temperature (T)
°C (°F) |
Velocity head
(ΔP) mm (in) H2O |
Pressure differential across orifice meter
mm H2O (in. H2O) |
Gas sample volume
m 3 (ft 3) | Gas sample temperature at dry gas meter |
Filter holder temperature
°C (°F) |
Temperature of gas leaving condenser or last impinger
°C (°F) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
Inlet
°C (°F) |
Outlet
°C (°F) | |||||||||
| Total | Avg. | Avg. | ||||||||
| Average | Avg. | |||||||||
8.6.3 Clean the stack access ports prior to the test run to eliminate the chance of sampling deposited material. To begin sampling, remove the nozzle cap, verify that the probe heating system are at the specified temperature, and verify that the pitot tube and probe are properly positioned. Position the nozzle at the first traverse point, with the tip pointing directly into the gas stream. Immediately start the pump and adjust the flow to isokinetic conditions. Nomographs, which aid in the rapid adjustment of the isokinetic sampling rate without excessive computations, are available. These nomographs are designed for use when the Type S pitot tube coefficient is 0.84 ±0.02 and the stack gas equivalent density (dry molecular weight) is equal to 29 ±4. APTD-0576 details the procedure for using the nomographs. If the stack gas molecular weight and the pitot tube coefficient are outside the above ranges, do not use the nomographs unless appropriate steps are taken to compensate for the deviations.
8.6.4 When the stack is under significant negative pressure (equivalent to the height of the impinger stem), take care to close the coarse-adjust valve before inserting the probe into the stack in order to prevent liquid from backing up through the train. If necessary, a low vacuum on the train may have to be started prior to entering the stack.
8.6.5 When the probe is in position, block off the openings around the probe and stack access port to prevent unrepresentative dilution of the gas stream.
8.6.6 Traverse the stack cross section, as required by EPA Method 1, 40 CFR part 60, appendix A, being careful not to bump the probe nozzle into the stack walls when sampling near the walls or when removing or inserting the probe through the access port, in order to minimize the chance of extracting deposited material.
8.6.7 During the test run, make periodic adjustments to keep the temperature around the probe at the proper levels. Add more ice and, if necessary, salt, to maintain a temperature of <20°C (68°F) at the silica gel outlet.
8.6.8 A single train shall be used for the entire sampling run, except in cases where simultaneous sampling is required in two or more separate ducts or at two or more different locations within the same duct, or in cases where equipment failure necessitates a change of trains. An additional train or trains may also be used for sampling when the capacity of a single train is exceeded.
8.6.9 When two or more trains are used, separate analyses of components from each train shall be performed. If multiple trains have been used because the capacity of a single train would be exceeded, first impingers from each train may be combined, and second impingers from each train may be combined.
8.6.10 At the end of the sampling run, turn off the coarse-adjust valve, remove the probe and nozzle from the stack, turn off the pump, record the final dry gas meter reading, and conduct a post-test leak-check. Also, check the pitot lines as described in EPA Method 2, 40 CFR part 60, appendix A. The lines must pass this leak-check in order to validate the velocity-head data.
8.6.11 Calculate percent isokineticity (see Method 2) to determine whether the run was valid or another test should be made.
8.7 Sample Preservation and Handling
8.7.1 Samples from most sources applicable to this method have acceptable holding times using normal handling practices (shipping samples iced, storing in refrigerator at 2°C until analysis). However, forming section stacks and other sources using waste water sprays may be subject to microbial contamination. For these sources, a biocide (quaternary ammonium compound solution) may be added to collected samples to improve sample stability and method ruggedness.
8.7.2 Sample holding time: Samples should be analyzed within 14 days of collection. Samples must be refrigerated/kept cold for the entire period preceding analysis. After the samples have been brought to room temperature for analysis, any analyses needed should be performed on the same day. Repeated cycles of warming the samples to room temperature/refrigerating/rewarming, then analyzing again, etc., have not been investigated in depth to evaluate if analyte levels remain stable for all sources.
8.7.3 Additional studies will be performed to evaluate whether longer sample holding times are feasible for this method.
8.8 Sample Recovery
8.8.1 Preparation:
8.8.1.1 Proper cleanup procedure begins as soon as the probe is removed from the stack at the end of the sampling period. Allow the probe to cool. When the probe can be handled safely, wipe off all external particulate matter near the tip of the probe nozzle and place a cap over the tip to prevent losing or gaining particulate matter. Do not cap the probe tightly while the sampling train is cooling because a vacuum will be created, drawing liquid from the impingers back through the sampling train.
8.8.1.2 Before moving the sampling train to the cleanup site, remove the probe from the sampling train and cap the open outlet, being careful not to lose any condensate that might be present. Remove the umbilical cord from the last impinger and cap the impinger. If a flexible line is used, let any condensed water or liquid drain into the impingers. Cap off any open impinger inlets and outlets. Ground glass stoppers, Teflon TM caps, or caps of other inert materials may be used to seal all openings.
8.8.1.3 Transfer the probe and impinger assembly to an area that is clean and protected from wind so that the chances of contaminating or losing the sample are minimized.
8.8.1.4 Inspect the train before and during disassembly, and note any abnormal conditions.
8.8.1.5 Save a portion of the washing solution (high purity water) used for cleanup as a blank.
8.8.2 Sample Containers:
8.8.2.1 Container 1: Probe and Impinger Catches. Using a graduated cylinder, measure to the nearest ml, and record the volume of the solution in the first three impingers. Alternatively, the solution may be weighed to the nearest 0.5 g. Include any condensate in the probe in this determination. Transfer the combined impinger solution from the graduated cylinder into the polyethylene bottle. Taking care that dust on the outside of the probe or other exterior surfaces does not get into the sample, clean all surfaces to which the sample is exposed (including the probe nozzle, probe fitting, probe liner, first three impingers, and impinger connectors) with water. Use less than 400 ml for the entire waste (250 ml would be better, if possible). Add the rinse water to the sample container.
8.8.2.1.1 Carefully remove the probe nozzle and rinse the inside surface with water from a wash bottle. Brush with a bristle brush and rinse until the rinse shows no visible particles, after which make a final rinse of the inside surface. Brush and rinse the inside parts of the Swagelok (or equivalent) fitting with water in a similar way.
8.8.2.1.2 Rinse the probe liner with water. While squirting the water into the upper end of the probe, tilt and rotate the probe so that all inside surfaces will be wetted with water. Let the water drain from the lower end into the sample container. The tester may use a funnel (glass or polyethylene) to aid in transferring the liquid washes to the container. Follow the rinse with a bristle brush. Hold the probe in an inclined position, and squirt water into the upper end as the probe brush is being pushed with a twisting action through the probe. Hold the sample container underneath the lower end of the probe, and catch any water and particulate matter that is brushed from the probe. Run the brush through the probe three times or more. Rinse the brush with water and quantitatively collect these washings in the sample container. After the brushing, make a final rinse of the probe as describe above.
Note:
Two people should clean the probe in order to minimize sample losses. Between sampling runs, brushes must be kept clean and free from contamination.
8.8.2.1.3 Rinse the inside surface of each of the first three impingers (and connecting tubing) three separate times. Use a small portion of water for each rinse, and brush each surface to which the sample is exposed with a bristle brush to ensure recovery of fine particulate matter. Make a final rinse of each surface and of the brush, using water.
8.8.2.1.4 After all water washing and particulate matter have been collected in the sample container, tighten the lid so the sample will not leak out when the container is shipped to the laboratory. Mark the height of the fluid level to determine whether leakage occurs during transport. Label the container clearly to identify its contents.
8.8.2.1.5 If the first two impingers are to be analyzed separately to check for breakthrough, separate the contents and rinses of the two impingers into individual containers. Care must be taken to avoid physical carryover from the first impinger to the second. Any physical carryover of collected moisture into the second impinger will invalidate a breakthrough assessment.
8.8.2.2 Container 2: Sample Blank. Prepare a blank by using a polyethylene container and adding a volume of water equal to the total volume in Container 1. Process the blank in the same manner as Container 1.
8.8.2.3 Container 3: Silica Gel. Note the color of the indicating silica gel to determine whether it has been completely spent and make a notation of its condition. The impinger containing the silica gel may be used as a sample transport container with both ends sealed with tightly fitting caps or plugs. Ground-glass stoppers or Teflon TM caps maybe used. The silica gel impinger should then be labeled, covered with aluminum foil, and packaged on ice for transport to the laboratory. If the silica gel is removed from the impinger, the tester may use a funnel to pour the silica gel and a rubber policeman to remove the silica gel from the impinger. It is not necessary to remove the small amount of dust particles that may adhere to the impinger wall and are difficult to remove. Since the gain in weight is to be used for moisture calculations, do not use water or other liquids to transfer the silica gel. If a balance is available in the field, the spent silica gel (or silica gel plus impinger) may be weighed to the nearest 0.5 g.
8.8.2.4 Sample containers should be placed in a cooler, cooled by (although not in contact with) ice. Putting sample bottles in Zip-Lock TM bags can aid in maintaining the integrity of the sample labels. Sample containers should be placed vertically to avoid leakage during shipment. Samples should be cooled during shipment so they will be received cold at the laboratory. It is critical that samples be chilled immediately after recovery. If the source is susceptible to microbial contamination from wash water (e.g. forming section stack), add biocide as directed in section 8.2.5.
8.8.2.5 A quaternary ammonium compound can be used as a biocide to stabilize samples against microbial degradation following collection. Using the stock quaternary ammonium compound (QAC) solution; add 2.5 ml QAC solution for every 100 ml of recovered sample volume (estimate of volume is satisfactory) immediately after collection. The total volume of QAC solution must be accurately known and recorded, to correct for any dilution caused by the QAC solution addition.
8.8.3 Sample Preparation for Analysis 8.8.3.1 The sample should be refrigerated if the analysis will not be performed on the day of sampling. Allow the sample to warm at room temperature for about two hours (if it has been refrigerated) prior to analyzing.
8.8.3.2 Analyze the sample by the pararosaniline method, as described in Section 11. If the color-developed sample has an absorbance above the highest standard, a suitable dilution in high purity water should be prepared and analyzed.
9.0 Quality Control
9.1 Sampling: See EPA Manual 600/4-77-02b for Method 5 quality control.
9.2 Analysis: The quality assurance program required for this method includes the analysis of the field and method blanks, and procedure validations. The positive identification and quantitation of formaldehyde are dependent on the integrity of the samples received and the precision and accuracy of the analytical methodology. Quality assurance procedures for this method are designed to monitor the performance of the analytical methodology and to provide the required information to take corrective action if problems are observed in laboratory operations or in field sampling activities.
9.2.1 Field Blanks: Field blanks must be submitted with the samples collected at each sampling site. The field blanks include the sample bottles containing aliquots of sample recover water, and water reagent. At a minimum, one complete sampling train will be assembled in the field staging area, taken to the sampling area, and leak-checked at the beginning and end of the testing (or for the same total number of times as the actual sampling train). The probe of the blank train must be heated during the sample test. The train will be recovered as if it were an actual test sample. No gaseous sample will be passed through the blank sampling train.
9.2.2 Blank Correction: The field blank formaldehyde concentrations will be subtracted from the appropriate sample formaldehyde concentrations. Blank formaldehyde concentrations above 0.25 µg/ml should be considered suspect, and subtraction from the sample formaldehyde concentrations should be performed in a manner acceptable to the Administrator.
9.2.3 Method Blanks: A method blank must be prepared for each set of analytical operations, to evaluate contamination and artifacts that can be derived from glassware, reagents, and sample handling in the laboratory.
10 Calibration
10.1 Probe Nozzle: Probe nozzles shall be calibrated before their initial use in the field. Using a micrometer, measure the inside diameter of the nozzle to the nearest 0.025 mm (0.001 in). Make measurements at three separate places across the diameter and obtain the average of the measurements. The difference between the high and low numbers shall not exceed 0.1 mm (0.004 in). When the nozzle becomes nicked or corroded, it shall be repaired and calibrated, or replaced with a calibrated nozzle before use. Each nozzle must be permanently and uniquely identified.
10.2 Pitot Tube: The Type S pitot tube assembly shall be calibrated according to the procedure outlined in Section 4 of EPA Method 2, or assigned a nominal coefficient of 0.84 if it is not visibly nicked or corroded and if it meets design and intercomponent spacing specifications.
10.3 Metering System
10.3.1 Before its initial use in the field, the metering system shall be calibrated according to the procedure outlined in APTD-0576. Instead of physically adjusting the dry-gas meter dial readings to correspond to the wet-test meter readings, calibration factors may be used to correct the gas meter dial readings mathematically to the proper values. Before calibrating the metering system, it is suggested that a leak-check be conducted. For metering systems having diaphragm pumps, the normal leak-check procedure will not delete leakages with the pump. For these cases, the following leak-check procedure will apply: Make a ten-minute calibration run at 0.00057 m 3/min (0.02 cfm). At the end of the run, take the difference of the measured wet-test and dry-gas meter volumes and divide the difference by 10 to get the leak rate. The leak rate should not exceed 0.00057 m 3/min (0.02 cfm).
10.3.2 After each field use, check the calibration of the metering system by performing three calibration runs at a single intermediate orifice setting (based on the previous field test). Set the vacuum at the maximum value reached during the test series. To adjust the vacuum, insert a valve between the wet-test meter and the inlet of the metering system. Calculate the average value of the calibration factor. If the calibration has changed by more than 5 percent, recalibrate the meter over the full range of orifice settings, as outlined in APTD-0576.
10.3.3 Leak-check of metering system: The portion of the sampling train from the pump to the orifice meter (see Figure 1) should be leak-checked prior to initial use and after each shipment. Leakage after the pump will result in less volume being recorded than is actually sampled. Use the following procedure: Close the main valve on the meter box. Insert a one-hole rubber stopper with rubber tubing attached into the orifice exhaust pipe. Disconnect and vent the low side of the orifice manometer. Close off the low side orifice tap. Pressurize the system to 13-18 cm (5-7 in) water column by blowing into the rubber tubing. Pinch off the tubing and observe the manometer for 1 min. A loss of pressure on the manometer indicates a leak in the meter box. Leaks must be corrected.
Note:
If the dry-gas meter coefficient values obtained before and after a test series differ by >5 percent, either the test series must be voided or calculations for test series must be performed using whichever meter coefficient value (i.e., before or after) gives the lower value of total sample volume.
10.4 Probe Heater: The probe heating system must be calibrated before its initial use in the field according to the procedure outlined in APTD-0576. Probes constructed according to APTD-0581 need not be calibrated if the calibration curves in APTD-0576 are used.
10.5 Temperature gauges: Use the procedure in Section 4.3 of EPA Method 2 to calibrate in-stack temperature gauges. Dial thermometers, such as are used for the dry gas meter and condenser outlet, shall be calibrated against mercury-in-glass thermometers. An alternative mercury-free thermometer may be used if the thermometer is, at a minimum, equivalent in terms of performance or suitably effective for the specific temperature measurement application.
10.6 Barometer: Adjust the barometer initially and before each test series to agree to within ±2.5 mm Hg (0.1 in Hg) of the mercury barometer. Alternately, if a National Weather Service Station (NWSS) is located at the same altitude above sea level as the test site, the barometric pressure reported by the NWSS may be used.
10.7 Balance: Calibrate the balance before each test series, using Class S standard weights. The weights must be within ±0.5 percent of the standards, or the balance must be adjusted to meet these limits.
11.0 Procedure for Analysis
The working formaldehyde standards (0.25, 0.50, 1.0, 2.0, and 3.0 µg/ml) are analyzed and a calibration curve is calculated for each day's analysis. The standards should be analyzed first to ensure that the method is working properly prior to analyzing the samples. In addition, a sample of the high-purity water should also be analyzed and used as a “0” formaldehyde standard.
The procedure for analysis of samples and standards is identical: Using the pipet set to 2.50 ml, pipet 2.50 ml of the solution to be analyzed into a polystyrene cuvette. Using the 250 µl pipet, pipet 250 µl of the pararosaniline reagent solution into the cuvette. Seal the top of the cuvette with a Parafilm square and shake at least 30 seconds to ensure the solution in the cuvette is well-mixed. Peel back a corner of the Parafilm so the next reagent can be added. Using the 250 µl pipet, pipet 250 µl of the sodium sulfite reagent solution into the cuvette. Reseal the cuvette with the Parafilm, and again shake for about 30 seconds to mix the solution in the cuvette. Record the time of addition of the sodium sulfite and let the color develop at room temperature for 60 minutes. Set the spectrophotometer to 570 nm and set to read in Absorbance Units. The spectrophotometer should be equipped with a holder for the 1-cm pathlength cuvettes. Place cuvette(s) containing high-purity water in the spectrophotometer and adjust to read 0.000 AU.
After the 60 minutes color development period, read the standard and samples in the spectrophotometer. Record the absorbance reading for each cuvette. The calibration curve is calculated by linear regression, with the formaldehyde concentration as the “x” coordinate of the pair, and the absorbance reading as the “y” coordinate. The procedure is very reproducible, and typically will yield values similar to these for the calibration curve:
Correlation Coefficient: 0.9999
Slope: 0.50
Y-Intercept: 0.090
The formaldehyde concentration of the samples can be found by using the trend-line feature of the calculator or computer program used for the linear regression. For example, the TI-55 calculators use the “X” key (this gives the predicted formaldehyde concentration for the value of the absorbance you key in for the sample). Multiply the formaldehyde concentration from the sample by the dilution factor, if any, for the sample to give the formaldehyde concentration of the original, undiluted, sample (units will be micrograms/ml).
11.1 Notes on the Pararosaniline Procedure
11.1.1 The pararosaniline method is temperature-sensitive. However, the small fluctuations typical of a laboratory will not significantly affect the results.
11.1.2 The calibration curve is linear to beyond 4 “µg/ml” formaldehyde, however, a research-grade spectrophotometer is required to reproducibly read the high absorbance values. Consult your instrument manual to evaluate the capability of the spectrophotometer.
11.1.3 The quality of the laboratory water used to prepare standards and make dilutions is critical. It is important that the cautions given in the Reagents section be observed. This procedure allows quantitation of formaldehyde at very low levels, and thus it is imperative to avoid contamination from other sources of formaldehyde and to exercise the degree of care required for trace analyses.
11.1.4 The analyst should become familiar with the operation of the Oxford or equivalent pipettors before using them for an analysis. Follow the instructions of the manufacturer; one can pipet water into a tared container on any analytical balance to check pipet accuracy and precision. This will also establish if the proper technique is being used. Always use a new tip for each pipetting operation.
11.1.5 This procedure follows the recommendations of ASTM Standard Guide D 3614, reading all solutions versus water in the reference cell. This allows the absorbance of the blank to be tracked on a daily basis. Refer to ASTM D 3614 for more information.
12.0 Calculations
Carry out calculations, retaining at least one extra decimal figure beyond that of the acquired data. Round off figures after final calculations.
12.1 Calculations of Total Formaldehyde
12.1.1 To determine the total formaldehyde in mg, use the following equation if biocide was not used:
Total mg formaldehyde=
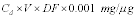
Where:
Cd = measured conc. formaldehyde, µg/ml
V = total volume of stack sample, ml
DF = dilution factor
12.1.2 To determine the total formaldehyde in mg, use the following equation if biocide was used:
Total mg formaldehyde=
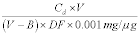
Where:
Cd = measured conc. formaldehyde, µg/ml
V = total volume of stack sample, ml
B = total volume of biocide added to sample, ml
DF = dilution factor
12.2 Formaldehyde concentration (mg/m 3) in stack gas. Determine the formaldehyde concentration (mg/m 3) in the stack gas using the following equation: Formaldehyde concentration (mg/m 3) =
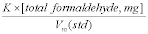
Where:
K = 35.31 cu ft/m 3 for Vm (std) in English units, or
K = 1.00 m 3/m 3 for Vm (std) in metric units
Vm (std) = volume of gas sample measured by a dry gas meter, corrected to standard conditions, dscm (dscf)
12.3 Average dry gas meter temperature and average orifice pressure drop are obtained from the data sheet.
12.4 Dry Gas Volume: Calculate Vm (std) and adjust for leakage, if necessary, using the equation in Section 6.3 of EPA Method 5, 40 CFR part 60, appendix A.
12.5 Volume of Water Vapor and Moisture Content: Calculated the volume of water vapor and moisture content from equations 5-2 and 5-3 of EPA Method 5.
13.0 Method Performance
The precision of this method is estimated to be better than ±5 percent, expressed as ±the percent relative standard deviation.
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 References
R.R. Miksch, et al., Analytical Chemistry, November 1981, 53 pp. 2118-2123.
J.F. Walker, Formaldehyde, Third Edition, 1964.
US EPA 40 CFR, part 60, Appendix A, Test Methods 1-5
Method 318 - Extractive FTIR Method for the Measurement of Emissions From the Mineral Wool and Wool Fiberglass Industries
1.0 Scope and Application
This method has been validated and approved for mineral wool and wool fiberglass sources. This method may not be applied to other source categories without validation and approval by the Administrator according to the procedures in Test Method 301, 40 CFR part 63, appendix A. For sources seeking to apply FTIR to other source categories, Test Method 320 (40 CFR part 63, appendix A) may be utilized.
1.1 Scope. The analytes measured by this method and their CAS numbers are:
Carbon Monoxide 630-08-0
Carbonyl Sulfide 463-58-1
Formaldehyde 50-00-0
Methanol 1455-13-6
Phenol 108-95-2
1.2 Applicability
1.2.1 This method is applicable for the determination of formaldehyde, phenol, methanol, carbonyl sulfide (COS) and carbon monoxide (CO) concentrations in controlled and uncontrolled emissions from manufacturing processes using phenolic resins. The compounds are analyzed in the mid-infrared spectral region (about 400 to 4000 cm−1 or 25 to 2.5 µm). Suggested analytical regions are given below (Table 1). Slight deviations from these recommended regions may be necessary due to variations in moisture content and ammonia concentration from source to source.
| Compound |
Analytical region (cm−1)
FLm − FUm | Potential interferants |
|---|---|---|
| Formaldehyde | 2840.93−2679.83 | Water, Methane. |
| Phenol | 1231.32−1131.47 | Water, Ammonia, Methane. |
| Methanol | 1041.56−1019.95 | Water, Ammonia. |
| COS a | 2028.4−2091.9 | Water, CO2 CO. |
| CO a | 2092.1−2191.8 | Water, CO2, COS. |
| a Suggested analytical regions assume about 15 percent moisture and CO2, and that COS and CO have about the same absorbance (in the range of 10 to 50 ppm). If CO and COS are hundreds of ppm or higher, then CO2 and moisture interference is reduced. If CO or COS is present at high concentration and the other at low concentration, then a shorter cell pathlength may be necessary to measure the high concentration component. | ||
1.2.2 This method does not apply when: (a) Polymerization of formaldehyde occurs, (b) moisture condenses in either the sampling system or the instrumentation, and (c) when moisture content of the gas stream is so high relative to the analyte concentrations that it causes severe spectral interference.
1.3 Method Range and Sensitivity
1.3.1 The analytical range is a function of instrumental design and composition of the gas stream. Theoretical detection limits depend, in part, on (a) the absorption coefficient of the compound in the analytical frequency region, (b) the spectral resolution, (c) interferometer sampling time, (d) detector sensitivity and response, and (e) absorption pathlength.
1.3.2 Practically, there is no upper limit to the range. The practical lower detection limit is usually higher than the theoretical value, and depends on (a) moisture content of the flue gas, (b) presence of interferants, and (c) losses in the sampling system. In general, a 22 meter pathlength cell in a suitable sampling system can achieve practical detection limits of 1.5 ppm for three compounds (formaldehyde, phenol, and methanol) at moisture levels up to 15 percent by volume. Sources with uncontrolled emissions of CO and COS may require a 4 meter pathlength cell due to high concentration levels. For these two compounds, make sure absorbance of highest concentration component is <1.0.
1.4 Data Quality Objectives
1.4.1 In designing or configuring the system, the analyst first sets the data quality objectives, i.e., the desired lower detection limit (DLi) and the desired analytical uncertainty (AUi) for each compound. The instrumental parameters (factors b, c, d, and e in Section 1.3.1) are then chosen to meet these requirements, using Appendix D of the FTIR Protocol.
1.4.2 Data quality for each application is determined, in part, by measuring the RMS (Root Mean Square) noise level in each analytical spectral region (Appendix C of the FTIR Protocol). The RMS noise is defined as the RMSD (Root Mean Square Deviation) of the absorbance values in an analytical region from the mean absorbance value of the region. Appendix D of the FTIR Protocol defines the MAUim (minimum analyte uncertainty of the i th analyte in the m th analytical region). The MAU is the minimum analyte concentration for which the analytical uncertainty limit (AUi) can be maintained: if the measured analyte concentration is less than MAUi, then data quality is unacceptable. Table 2 gives some example DL and AU values along with calculated areas and MAU values using the protocol procedures.
| Protocol value | Form | Phenol | Methanol |
Protocol
appendix |
|---|---|---|---|---|
| Reference concentration a (ppm-meters)/K | 3.016 | 3.017 | 5.064 | |
| Reference Band Area | 8.2544 | 16.6417 | 4.9416 | B |
| DL (ppm-meters)/K | 0.1117 | 0.1117 | 0.1117 | B |
| AU | 0.2 | 0.2 | 0.2 | B |
| CL | 0.02234 | 0.02234 | 0.02234 | B |
| FL | 2679.83 | 1131.47 | 1019.95 | B |
| FU | 2840.93 | 1231.32 | 1041.56 | B |
| FC | 2760.38 | 1181.395 | 1030.755 | B |
| AAI (ppm-meters)/K | 0.18440 | 0.01201 | 0.00132 | B |
| RMSD | 2.28E-03 | 1.21E-03 | 1.07E-03 | C |
| MAU (ppm-meters)/K | 4.45E-02 | 7.26E-03 | 4.68E-03 | D |
| MAU (ppm at 22) | 0.0797 | 0.0130 | 0.0084 | D |
| a Concentration units are: ppm concentration of the reference sample (ASC), times the path length of the FTIR cell used when the reference spectrum was measured (meters), divided by the absolute temperature of the reference sample in Kelvin (K), or (ppm-meters)/K. | ||||
2.0 Summary of Method
2.1 Principle
2.1.1 Molecules are composed of chemically bonded atoms, which are in constant motion. The atomic motions result in bond deformations (bond stretching and bond-angle bending). The number of fundamental (or independent) vibrational motions depends on the number of atoms (N) in the molecule. At typical testing temperatures, most molecules are in the ground-state vibrational state for most of their fundamental vibrational motions. A molecule can undergo a transition from its ground state (for a particular vibration) to the first excited state by absorbing a quantum of light at a frequency characteristic of the molecule and the molecular motion. Molecules also undergo rotational transitions by absorbing energies in the far-infrared or microwave spectral regions. Rotational transition absorbencies are superimposed on the vibrational absorbencies to give a characteristic shape to each rotational-vibrational absorbance “band.”
2.1.2 Most molecules exhibit more than one absorbance band in several frequency regions to produce an infrared spectrum (a characteristic pattern of bands or a “fingerprint”) that is unique to each molecule. The infrared spectrum of a molecule depends on its structure (bond lengths, bond angles, bond strengths, and atomic masses). Even small differences in structure can produce significantly different spectra.
2.1.3 Spectral band intensities vary with the concentration of the absorbing compound. Within constraints, the relationship between absorbance and sample concentration is linear. Sample spectra are compared to reference spectra to determine the species and their concentrations.
2.2 Sampling and Analysis
2.2.1 Flue gas is continuously extracted from the source, and the gas or a portion of the gas is conveyed to the FTIR gas cell, where a spectrum of the flue gas is recorded. Absorbance band intensities are related to sample concentrations by Beer's Law.
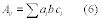
Where:
An = absorbance of the i th component at the given frequency, ν.
a = absorption coefficient of the i th component at the frequency, ν.
b = path length of the cell.
c = concentration of the i th compound in the sample at frequency ν.
2.2.2 After identifying a compound from the infrared spectrum, its concentration is determined by comparing band intensities in the sample spectrum to band intensities in “reference spectra” of the formaldehyde, phenol, methanol, COS and CO. These reference spectra are available in a permanent soft copy from the EPA spectral library on the EMTIC bulletin board. The source may also prepare reference spectra according to Section 4.5 of the FTIR Protocol.
Note:
Reference spectra not prepared according to the FTIR Protocol are not acceptable for use in this test method. Documentation detailing the FTIR Protocol steps used in preparing any non-EPA reference spectra shall be included in each test report submitted by the source.
2.3 Operator Requirements. The analyst must have some knowledge of source sampling and of infrared spectral patterns to operate the sampling system and to choose a suitable instrument configuration. The analyst should also understand FTIR instrument operation well enough to choose an instrument configuration consistent with the data quality objectives.
3.0 Definitions
See Appendix A of the FTIR Protocol.
4.0 Interferences
4.1 Analytical (or Spectral) Interferences. Water vapor. High concentrations of ammonia (hundreds of ppm) may interfere with the analysis of low concentrations of methanol (1 to 5 ppm). For CO, carbon dioxide and water may be interferants. In cases where COS levels are low relative to CO levels, CO and water may be interferants.
4.2 Sampling System Interferences. Water, if it condenses, and ammonia, which reacts with formaldehyde.
5.0 Safety
5.1 Formaldehyde is a suspected carcinogen; therefore, exposure to this compound must be limited. Proper monitoring and safety precautions must be practiced in any atmosphere with potentially high concentrations of CO.
5.2 This method may involve sampling at locations having high positive or negative pressures, high temperatures, elevated heights, high concentrations of hazardous or toxic pollutants, or other diverse sampling conditions. It is the responsibility of the tester(s) to ensure proper safety and health practices, and to determine the applicability of regulatory limitations before performing this test method.
6.0 Equipment and Supplies
The equipment and supplies are based on the schematic of a sampling train shown in Figure 1. Either the evacuated or purged sampling technique may be used with this sampling train. Alternatives may be used, provided that the data quality objectives of this method are met.
6.1 Sampling Probe. Glass, stainless steel, or other appropriate material of sufficient length and physical integrity to sustain heating, prevent adsorption of analytes, and to reach gas sampling point.
6.2 Particulate Filters. A glass wool plug (optional) inserted at the probe tip (for large particulate removal) and a filter rated at 1-micron (e.g., Balston TM) for fine particulate removal, placed immediately after the heated probe.
6.3 Sampling Line/Heating System. Heated (maintained at 250 ±25 degrees F) stainless steel, Teflon TM, or other inert material that does not adsorb the analytes, to transport the sample to analytical system.
6.4 Stainless Steel Tubing. Type 316, e.g., 3/8 in. diameter, and appropriate length for heated connections.
6.5 Gas Regulators. Appropriate for individual gas cylinders.
6.6 Teflon TM Tubing. Diameter (e.g., 3/8 in.) and length suitable to connect cylinder regulators.
6.7 Sample Pump. A leak-free pump (e.g., KNF TM), with by-pass valve, capable of pulling sample through entire sampling system at a rate of about 10 to 20 L/min. If placed before the analytical system, heat the pump and use a pump fabricated from materials non-reactive to the target pollutants. If the pump is located after the instrument, systematically record the sample pressure in the gas cell.
6.8 Gas Sample Manifold. A heated manifold that diverts part of the sample stream to the analyzer, and the rest to the by-pass discharge vent or other analytical instrumentation.
6.9 Rotameter. A calibrated 0 to 20 L/min range rotameter.
6.10 FTIR Analytical System. Spectrometer and detector, capable of measuring formaldehyde, phenol, methanol, COS and CO to the predetermined minimum detectable level. The system shall include a personal computer with compatible software that provides real-time updates of the spectral profile during sample collection and spectral collection.
6.11 FTIR Cell Pump. Required for the evacuated sampling technique, capable of evacuating the FTIR cell volume within 2 minutes. The FTIR cell pump should allow the operator to obtain at least 8 sample spectra in 1 hour.
6.12 Absolute Pressure Gauge. Heatable and capable of measuring pressure from 0 to 1000 mmHg to within ±2.5 mmHg (e.g., Baratron TM).
6.13 Temperature Gauge. Capable of measuring the cell temperature to within ±2°C.
7.0 Reagents and Standards
7.1 Ethylene (Calibration Transfer Standard). Obtain NIST traceable (or Protocol) cylinder gas.
7.2 Nitrogen. Ultra high purity (UHP) grade.
7.3 Reference Spectra. Obtain reference spectra for the target pollutants at concentrations that bracket (in ppm-meter/K) the emission source levels. Also, obtain reference spectra for SF6 and ethylene. Suitable concentrations are 0.0112 to 0.112 (ppm-meter)/K for SF6 and 5.61 (ppm-meter)/K or less for ethylene. The reference spectra shall meet the criteria for acceptance outlined in Section 2.2.2. The optical density (ppm-meters/K) of the reference spectrum must match the optical density of the sample spectrum within (less than) 25 percent.
8.0 Sample Collection, Preservation, and Storage
Sampling should be performed in the following sequence: Collect background, collect CTS spectrum, collect samples, collect post-test CTS spectrum, verify that two copies of all data were stored on separate computer media.
8.1 Pretest Preparations and Evaluations. Using the procedure in Section 4.0 of the FTIR Protocol, determine the optimum sampling system configuration for sampling the target pollutants. Table 2 gives some example values for AU, DL, and MAU. Based on a study (Reference 1), an FTIR system using 1 cm−1 resolution, 22 meter path length, and a broad band MCT detector was suitable for meeting the requirements in Table 2. Other factors that must be determined are:
a. Test requirements: AUi, CMAXi, DLi, OFUi, and tAN for each.
b. Interferants: See Table 1.
c. Sampling system: LS′, Pmin, PS′, TS′, tSS, VSS; fractional error, MIL.
d. Analytical regions: 1 through Nm, FLm, FCm, and FUm, plus interferants, FFUm, FFLm, wavenumber range FNU to FNL. See Tables 1 and 2.
8.1.1 If necessary, sample and acquire an initial spectrum. Then determine the proper operational pathlength of the instrument to obtain non-saturated absorbances of the target analytes.
8.1.2 Set up the sampling train as shown in Figure 1.
8.2 Sampling System Leak-check. Leak-check from the probe tip to pump outlet as follows: Connect a 0- to 250-mL/min rate meter (rotameter or bubble meter) to the outlet of the pump. Close off the inlet to the probe, and note the leakage rate. The leakage rate shall be ≤200 mL/min.
8.3 Analytical System Leak-check.
8.3.1 For the evacuated sample technique, close the valve to the FTIR cell, and evacuate the absorption cell to the minimum absolute pressure Pmin. Close the valve to the pump, and determine the change in pressure ΔPv after 2 minutes.
8.3.2 For both the evacuated sample and purging techniques, pressurize the system to about 100 mmHg above atmospheric pressure. Isolate the pump and determine the change in pressure ΔPp after 2 minutes.
8.3.3 Measure the barometric pressure, Pb in mmHg.
8.3.4 Determine the percent leak volume %VL for the signal integration time tSS and for ΔPmax, i.e., the larger of ΔPv or ΔPp, as follows:
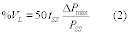
Where:
50 = 100% divided by the leak-check time of 2 minutes.
8.3.5 Leak volumes in excess of 4 percent of the sample system volume VSS are unacceptable.
8.4 Background Spectrum. Evacuate the gas cell to ≤5 mmHg, and fill with dry nitrogen gas to ambient pressure. Verify that no significant amounts of absorbing species (for example water vapor and CO2) are present. Collect a background spectrum, using a signal averaging period equal to or greater than the averaging period for the sample spectra. Assign a unique file name to the background spectrum. Store the spectra of the background interferogram and processed single-beam background spectrum on two separate computer media (one is used as the back-up). If continuous sampling will be used during sample collection, collect the background spectrum with nitrogen gas flowing through the cell at the same pressure and temperature as will be used during sampling.
8.5 Pre-Test Calibration Transfer Standard. Evacuate the gas cell to ≤5 mmHg absolute pressure, and fill the FTIR cell to atmospheric pressure with the CTS gas. Or, purge the cell with 10 cell volumes of CTS gas. Record the spectrum. If continuous sampling will be used during sample collection, collect the CTS spectrum with CTS gas flowing through the cell at the same pressure and temperature as will be used during sampling.
8.6 Samples
8.6.1 Evacuated Samples. Evacuate the absorbance cell to ≤5 mmHg absolute pressure. Fill the cell with flue gas to ambient pressure and record the spectrum. Before taking the next sample, evacuate the cell until no further evidence of absorption exists. Repeat this procedure to collect at least 8 separate spectra (samples) in 1 hour.
8.6.2 Purge Sampling. Purge the FTIR cell with 10 cell volumes of flue gas and at least for about 10 minutes. Discontinue the gas cell purge, isolate the cell, and record the sample spectrum and the pressure. Before taking the next sample, purge the cell with 10 cell volumes of flue gas.
8.6.3 Continuous Sampling. Spectra can be collected continuously while the FTIR cell is being purged. The sample integration time, tss, the sample flow rate through the FTIR gas cell, and the total run time must be chosen so that the collected data consist of at least 10 spectra with each spectrum being of a separate cell volume of flue gas. More spectra can be collected over the run time and the total run time (and number of spectra) can be extended as well.
8.7 Sampling QA, Data Storage and Reporting
8.7.1 Sample integration times should be sufficient to achieve the required signal-to-noise ratios. Obtain an absorbance spectrum by filling the cell with nitrogen. Measure the RMSD in each analytical region in this absorbance spectrum. Verify that the number of scans is sufficient to achieve the target MAU (Table 2).
8.7.2 Identify all sample spectra with unique file names.
8.7.3 Store on two separate computer media a copy of sample interferograms and processed spectra. The data shall be available to the Administrator on request for the length of time specified in the applicable regulation.
8.7.4 For each sample spectrum, document the sampling conditions, the sampling time (while the cell was being filled), the time the spectrum was recorded, the instrumental conditions (path length, temperature, pressure, resolution, integration time), and the spectral file name. Keep a hard copy of these data sheets.
8.8 Signal Transmittance. While sampling, monitor the signal transmittance through the instrumental system. If signal transmittance (relative to the background) drops below 95 percent in any spectral region where the sample does not absorb infrared energy, obtain a new background spectrum.
8.9 Post-run CTS. After each sampling run, record another CTS spectrum.
8.10 Post-test QA
8.10.1 Inspect the sample spectra immediately after the run to verify that the gas matrix composition was close to the expected (assumed) gas matrix.
8.10.2 Verify that the sampling and instrumental parameters were appropriate for the conditions encountered. For example, if the moisture is much greater than anticipated, it will be necessary to use a shorter path length or dilute the sample.
8.10.3 Compare the pre and post-run CTS spectra. They shall agree to within −5 percent. See FTIR Protocol, Appendix E.
9.0 Quality Control
Follow the quality assurance procedures in the method, including the analysis of pre and post-run calibration transfer standards (Sections 8.5 and 8.9) and the post-test quality assurance procedures in Section 8.10.
10.0 Calibration and Standardization
10.1 Signal-to-Noise Ratio (S/N). The S/N shall be sufficient to meet the MAU in each analytical region.
10.2 Absorbance Pathlength. Verify the absorbance path length by comparing CTS spectra to reference spectra of the calibration gas(es). See FTIR Protocol, Appendix E.
10.3 Instrument Resolution. Measure the line width of appropriate CTS band(s) and compare to reference CTS spectra to verify instrumental resolution.
10.4 Apodization Function. Choose appropriate apodization function. Determine any appropriate mathematical transformations that are required to correct instrumental errors by measuring the CTS. Any mathematical transformations must be documented and reproducible.
10.5 FTIR Cell Volume. Evacuate the cell to ≤5 mmHg. Measure the initial absolute temperature (Ti) and absolute pressure (Pi). Connect a wet test meter (or a calibrated dry gas meter), and slowly draw room air into the cell. Measure the meter volume (Vm), meter absolute temperature (Tm), and meter absolute pressure (Pm), and the cell final absolute temperature (Tf) and absolute pressure (Pf). Calculate the FTIR cell volume Vss, including that of the connecting tubing, as follows:
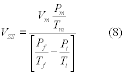
As an alternative to the wet test meter/calibrated dry gas meter procedure, measure the inside dimensions of the cell cylinder and calculate its volume.
11.0 Procedure
Refer to Sections 4.6-4.11, Sections 5, 6, and 7, and the appendices of the FTIR Protocol.
12.0 Data Analysis and Calculations
a. Data analysis is performed using appropriate reference spectra whose concentrations can be verified using CTS spectra. Various analytical programs are available to relate sample absorbance to a concentration standard. Calculated concentrations should be verified by analyzing spectral baselines after mathematically subtracting scaled reference spectra from the sample spectra. A full description of the data analysis and calculations may be found in the FTIR Protocol (Sections 4.0, 5.0, 6.0 and appendices).
b. Correct the calculated concentrations in sample spectra for differences in absorption pathlength between the reference and sample spectra by:
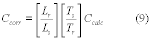
Where:
Ccorr = The pathlength corrected concentration.
Ccalc = The initial calculated concentration (output of the Multicomp program designed for the compound).
Lr = The pathlength associated with the reference spectra.
Ls = The pathlength associated with the sample spectra.
Ts = The absolute temperature (K) of the sample gas.
Tr = The absolute gas temperature (K) at which reference spectra were recorded.
13.0 Reporting and Recordkeeping
All interferograms used in determining source concentration shall be stored for the period of time required in the applicable regulation. The Administrator has the option of requesting the interferograms recorded during the test in electronic form as part of the test report.
14.0 Method Performance
Refer to the FTIR Protocol.
15.0 Pollution Prevention [Reserved]
16.0 Waste Management
Laboratory standards prepared from the formaldehyde and phenol are handled according to the instructions in the materials safety data sheets (MSDS).
17.0 References
(1) “Field Validation Test Using Fourier Transform Infrared (FTIR) Spectrometry To Measure Formaldehyde, Phenol and Methanol at a Wool Fiberglass Production Facility.” Draft. U.S. Environmental Protection Agency Report, Entropy, Inc., EPA Contract No. 68D20163, Work Assignment I-32, December 1994 (docket item II-A-13).
(2) “Method 301 - Field Validation of Pollutant Measurement Methods from Various Waste Media,” 40 CFR part 63, appendix A.
Method 319 - Determination of Filtration Efficiency for Paint Overspray Arrestors
1.0 Scope and Application
1.1 This method applies to the determination of the initial, particle size dependent, filtration efficiency for paint arrestors over the particle diameter range from 0.3 to 10 µm. The method applies to single and multiple stage paint arrestors or paint arrestor media. The method is applicable to efficiency determinations from 0 to 99 percent. Two test aerosols are used - one liquid phase and one solid phase. Oleic acid, a low-volatility liquid (CAS Number 112-80-1), is used to simulate the behavior of wet paint overspray. The solid-phase aerosol is potassium chloride salt (KCl, CAS Number 7447-40-7) and is used to simulate the behavior of a dry overspray. The method is limited to determination of the initial, clean filtration efficiency of the arrestor. Changes in efficiency (either increase or decrease) due to the accumulation of paint overspray on and within the arrestor are not evaluated.
1.2 Efficiency is defined as 1 - Penetration (e.g., 70 percent efficiency is equal to 0.30 penetration). Penetration is based on the ratio of the downstream particle concentration to the upstream concentration. It is often more useful, from a mathematical or statistical point of view, to discuss the upstream and downstream counts in terms of penetration rather than the derived efficiency value. Thus, this document uses both penetration and efficiency as appropriate.
1.3 For a paint arrestor system or subsystem which has been tested by this method, adding additional filtration devices to the system or subsystem shall be assumed to result in an efficiency of at least that of the original system without the requirement for additional testing. (For example, if the final stage of a three-stage paint arrestor system has been tested by itself, then the addition of the other two stages shall be assumed to maintain, as a minimum, the filtration efficiency provided by the final stage alone. Thus, in this example, if the final stage has been shown to meet the filtration requirements of Table 1 of §63.745 of subpart GG, then the final stage in combination with any additional paint arrestor stages also passes the filtration requirements.)
2.0 Summary of Method
2.1 This method applies to the determination of the fractional (i.e., particle-size dependent) aerosol penetration of several types of paint arrestors. Fractional penetration is computed from aerosol concentrations measured upstream and downstream of an arrestor installed in a laboratory test rig. The aerosol concentrations upstream and downstream of the arrestors are measured with an aerosol analyzer that simultaneously counts and sizes the particles in the aerosol stream. The aerosol analyzer covers the particle diameter size range from 0.3 to 10 µm in a minimum of 12 contiguous sizing channels. Each sizing channel covers a narrow range of particle diameters. For example, Channel 1 may cover from 0.3 to 0.4 µm, Channel 2 from 0.4 to 0.5 µm, * * * By taking the ratio of the downstream to upstream counts on a channel by channel basis, the penetration is computed for each of the sizing channels.
2.2 The upstream and downstream aerosol measurements are made while injecting the test aerosol into the air stream upstream of the arrestor (ambient aerosol is removed with HEPA filters on the inlet of the test rig). This test aerosol spans the particle size range from 0.3 to 10 µm and provides sufficient upstream concentration in each of the optical particle counter (OPC) sizing channels to allow accurate calculation of penetration, down to penetrations of approximately 0.01 (i.e., 1 percent penetration; 99 percent efficiency). Results are presented as a graph and a data table showing the aerodynamic particle diameter and the corresponding fractional efficiency.
3.0 Definitions
Aerodynamic Diameter - diameter of a unit density sphere having the same aerodynamic properties as the particle in question.
Efficiency is defined as equal to 1 - Penetration.
Optical Particle Counter (OPC) - an instrument that counts particles by size using light scattering. An OPC gives particle diameters based on size, index of refraction, and shape.
Penetration - the fraction of the aerosol that penetrates the filter at a given particle diameter. Penetration equals the downstream concentration divided by the upstream concentration.
4.0 Interferences
4.1 The influence of the known interferences (particle losses) are negated by correction of the data using blanks.
5.0 Safety
5.1 There are no specific safety precautions for this method above those of good laboratory practice. This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this method to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
6.0 Equipment and Supplies
6.1 Test Facility. A schematic diagram of a test duct used in the development of the method is shown in Figure 319-1.
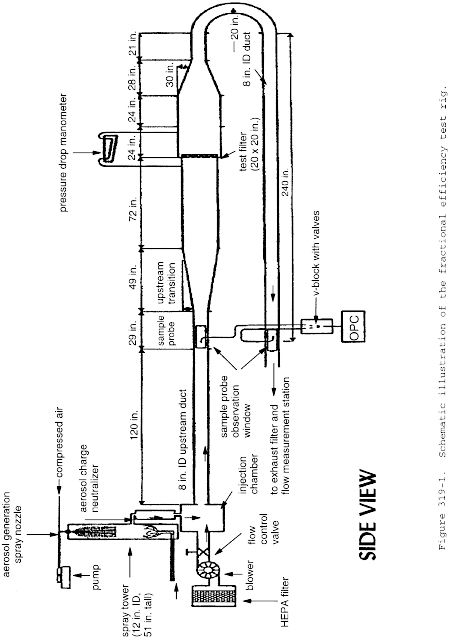
6.1.1 The test section, paint spray section, and attached transitions are constructed of stainless and galvanized steel. The upstream and downstream ducting is 20 cm diameter polyvinyl chloride (PVC). The upstream transition provides a 7° angle of expansion to provide a uniform air flow distribution to the paint arrestors. Aerosol concentration is measured upstream and downstream of the test section to obtain the challenge and penetrating aerosol concentrations, respectively. Because the downstream ducting runs back under the test section, the challenge and penetrating aerosol taps are located physically near each other, thereby facilitating aerosol sampling and reducing sample-line length. The inlet nozzles of the upstream and downstream aerosol probes are designed to yield isokinetic sampling conditions.
6.1.2 The configuration and dimensions of the test duct can deviate from those of Figure 319-1 provided that the following key elements are maintained: the test duct must meet the criteria specified in Table 319-1; the inlet air is HEPA filtered; the blower is on the upstream side of the duct thereby creating a positive pressure in the duct relative to the surrounding room; the challenge air has a temperature between 50° and 100°F and a relative humidity of less than 65 percent; the angle of the upstream transition (if used) to the paint arrestor must not exceed 7°; the angle of the downstream transition (if used) from the paint arrestor must not exceed 30°; the test duct must provide a means for mixing the challenge aerosol with the upstream flow (in lieu of any mixing device, a duct length of 15 duct diameters fulfills this requirement); the test duct must provide a means for mixing any penetrating aerosol with the downstream flow (in lieu of any mixing device, a duct length of 15 duct diameters fulfills this requirement); the test section must provide a secure and leak-free mounting for single and multiple stage arrestors; and the test duct may utilize a 180° bend in the downstream duct.
| Frequency and description | Control limits | |
|---|---|---|
| OPC zero count | Each Test. OPC samples HEPA-filtered air | <50 counts per minute. |
| OPC sizing accuracy check | Daily. Sample aerosolized PSL spheres | Peak of distribution should be in correct OPC channel. |
| Minimum counts per channel for challenge aerosol | Each Test | Minimum total of 500 particle counts per channel. |
| Maximum particle concentration | Each Test. Needed to ensure OPC is not overloaded | <10% of manufacturer's claimed upper limit corresponding to a 10% count error. |
| Standard Deviation of Penetration | Computed for each test based on the CV of the upstream and downstream counts |
<0.10 for 0.3 to 3 µm diameter.
<0.30 for >3 µm diameter. |
| 0% Penetration | Monthly | <0.01. |
| 100% Penetration - KCl | Triplicate tests performed immediately before, during, or after triplicate arrestor tests |
0.3 to 1 µm: 0.90 to 1.10.
1 to 3 µm: 0.75 to 1.25. 3 to 10 µm: 0.50 to 1.50. |
| 100% Penetration - Oleic Acid | Triplicate tests performed immediately before, during, or after triplicate arrestor tests |
0.3 to 1 µm: 0.90 to 1.10.
1 to 3 µm: 0.75 to 1.25. 3 to 10 µm: 0.50 to 1.50. |
6.2 Aerosol Generator. The aerosol generator is used to produce a stable aerosol covering the particle size range from 0.3 to 10 µm diameter. The generator used in the development of this method consists of an air atomizing nozzle positioned at the top of a 0.30-m (12-in.) diameter, 1.3-m (51-in.) tall, acrylic, transparent, spray tower. This tower allows larger sized particles, which would otherwise foul the test duct and sample lines, to fall out of the aerosol. It also adds drying air to ensure that the KCl droplets dry to solid salt particles. After generation, the aerosol passes through an aerosol neutralizer (Kr85 radioactive source) to neutralize any electrostatic charge on the aerosol (electrostatic charge is an unavoidable consequence of most aerosol generation methods). To improve the mixing of the aerosol with the air stream, the aerosol is injected counter to the airflow. Generators of other designs may be used, but they must produce a stable aerosol concentration over the 0.3 to 10 µm diameter size range; provide a means of ensuring the complete drying of the KCl aerosol; and utilize a charge neutralizer to neutralize any electrostatic charge on the aerosol. The resultant challenge aerosol must meet the minimum count per channel and maximum concentration criteria of Table 319-1.
6.3 Installation of Paint Arrestor. The paint arrestor is to be installed in the test duct in a manner that precludes air bypassing the arrestor. Since arrestor media are often sold unmounted, a mounting frame may be used to provide back support for the media in addition to sealing it into the duct. The mounting frame for 20 in. × 20 in. arrestors will have minimum open internal dimensions of 18 in. square. Mounting frames for 24 in. × 24 in. arrestors will have minimum open internal dimensions of 22 in. square. The open internal dimensions of the mounting frame shall not be less than 75 percent of the approach duct dimensions.
6.4 Optical Particle Counter. The upstream and downstream aerosol concentrations are measured with a high-resolution optical particle counter (OPC). To ensure comparability of test results, the OPC shall utilize an optical design based on wide-angle light scattering and provided a minimum of 12 contiguous particle sizing channels from 0.3 to 10 µm diameter (based on response to PSL) where, for each channel, the ratio of the diameter corresponding to the upper channel bound to the lower channel bound must not exceed 1.5.
6.5 Aerosol Sampling System. The upstream and downstream sample lines must be made of rigid electrically-grounded metallic tubing having a smooth inside surface, and they must be rigidly secured to prevent movement during testing. The upstream and downstream sample lines are to be nominally identical in geometry. The use of a short length (100 mm maximum) of straight flexible tubing to make the final connection to the OPC is acceptable. The inlet nozzles of the upstream and downstream probes must be sharp-edged and of appropriate entrance diameter to maintain isokinetic sampling within 20 percent of the air velocity.
6.5.1 The sampling system may be designed to acquire the upstream and downstream samples using (a) sequential upstream-downstream sampling with a single OPC, (b) simultaneous upstream and downstream sampling with two OPC's, or (c) sequential upstream-downstream sampling with two OPC's.
6.5.2 When two particle counters are used to acquire the upstream and downstream counts, they must be closely matched in flowrate and optical design.
6.6 Airflow Monitor. The volumetric airflow through the system shall be measured with a calibrated orifice plate, flow nozzle, or laminar flow element. The measurement device must have an accuracy of 5 percent or better.
7.0 Reagents and Standards
7.1 The liquid test aerosol is reagent grade, 98 percent pure, oleic acid (Table 319-2). The solid test aerosol is KCl aerosolized from a solution of KCl in water. In addition to the test aerosol, a calibration aerosol of monodisperse polystyrene latex (PSL) spheres is used to verify the calibration of the OPC.
| Refractive index |
Density,
g/cm 3 | Shape | |
|---|---|---|---|
| Oleic Acid (liquid-phase challenge aerosol) | 1.46 nonabsorbing | 0.89 | Spherical. |
| KCl (solid-phase challenge aerosol) | 1.49 | 1.98 | Cubic or agglomerated cubes. |
| PSL (calibration aerosol) | 1.59 nonabsorbing | 1.05 | Spherical. |
8.0 Sample Collection, Preservation, and Storage
8.1 In this test, all sampling occurs in real-time, thus no samples are collected that require preservation or storage during the test. The paint arrestors are shipped and stored to avoid structural damage or soiling. Each arrestor may be shipped in its original box from the manufacturer or similar cardboard box. Arrestors are stored at the test site in a location that keeps them clean and dry. Each arrestor is clearly labeled for tracking purposes.
9.0 Quality Control
9.1 Table 319-1 lists the QC control limits.
9.2 The standard deviation (σ) of the penetration (P) for a given test at each of the 15 OPC sizing channels is computed from the coefficient of variation (CV, the standard deviation divided by the mean) of the upstream and downstream measurements as:
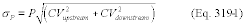
For a properly operating system, the standard deviation of the penetration is <0.10 at particle diameters from 0.3 to 3 µm and less than 0.30 at diameters >3 µm.
9.3 Data Quality Objectives (DQO).
9.3.1 Fractional Penetration. From the triplicate tests of each paint arrestor model, the standard deviation for the penetration measurements at each particle size (i.e., for each sizing channel of the OPC) is computed as:
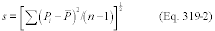
where Pi represents an individual penetration measurement, and P the average of the 3 (n = 3) individual measurements.
9.3.2 Bias of the fractional penetration values is determined from triplicate no-filter and HEPA filter tests. These tests determine the measurement bias at 100 percent penetration and 0 percent penetration, respectively.
9.3.3 PSL-Equivalent Light Scattering Diameter. The precision and bias of the OPC sizing determination are based on sampling a known diameter of PSL and noting whether the particle counts peak in the correct channel of the OPC. This is a pass/fail measurement with no calculations involved.
9.3.4 Airflow. The precision of the measurement must be within 5 percent of the set point.
10.0 Calibration and Standardization
10.1 Optical Particle Counter. The OPC must have an up-to-date factory calibration. Check the OPC zero at the beginning and end of each test by sampling HEPA-filtered air. Verify the sizing accuracy on a daily basis (for days when tests are performed) with 1-size PSL spheres.
10.2 Airflow Measurement. Airflow measurement devices must have an accuracy of 5 percent or better. Manometers used in conjunction with the orifice plate must be inspected prior to use for proper level, zero, and mechanical integrity. Tubing connections to the manometer must be free from kinks and have secure connections.
10.3 Pressure Drop. Measure pressure drop across the paint arrestor with an inclined manometer readable to within 0.01 in. H2O. Prior to use, the level and zero of the manometer, and all tubing connections, must be inspected and adjusted as needed.
11.0 Procedure
11.1 Filtration Efficiency. For both the oleic acid and KCl challenges, this procedure is performed in triplicate using a new arrestor for each test.
11.1.1 General Information and Test Duct Preparation
11.1.1.1 Use the “Test Run Sheet” form (Figure 319-2) to record the test information.
Run Sheet
Part 1. General Information
Date and Time:
Test Operator:
Test #:
Paint Arrestor:
Brand/Model
Arrestor Assigned ID #
Condition of arrestor (i.e., is there any damage? Must be new condition to proceed):
Manometer zero and level confirmed?
Part 2. Clean Efficiency Test
Date and Time:
Optical Particle Counter:
20 min. warm up
Zero count (<50 counts/min)
Daily PSL check
PSL Diam: ___ µm
File name for OPC data:
Test Conditions:
Air Flow: ___
Temp & RH: Temp ___°F RH ___ %
Atm. Pressure: ___in. Hg
(From mercury barometer)
Aerosol Generator: (record all operating parameters)
Test Aerosol:
(Oleic acid or KCl)
Arrestor:
Pressure drop: at start ___ in. H2O
at end ___ in. H2O
Condition of arrestor at end of test (note any physical deterioration):
Figure 319-2. Test Run Sheet
Other report formats which contain the same information are acceptable.
11.1.1.2 Record the date, time, test operator, Test #, paint arrestor brand/model and its assigned ID number. For tests with no arrestor, record none.
11.1.1.3 Ensure that the arrestor is undamaged and is in “new” condition.
11.1.1.4 Mount the arrestor in the appropriate frame. Inspect for any airflow leak paths.
11.1.1.5 Install frame-mounted arrestor in the test duct. Examine the installed arrestor to verify that it is sealed in the duct. For tests with no arrestor, install the empty frame.
11.1.1.6 Visually confirm the manometer zero and level. Adjust as needed.
11.1.2 Clean Efficiency Test.
11.1.2.1 Record the date and time upon beginning this section.
11.1.2.2 Optical Particle Counter.
11.1.2.2.1 General: Operate the OPC per the manufacturer's instructions allowing a minimum of 20 minutes warm up before making any measurements.
11.1.2.2.2 Overload: The OPC will yield inaccurate data if the aerosol concentration it is attempting to measure exceeds its operating limit. To ensure reliable measurements, the maximum aerosol concentration will not exceed 10 percent of the manufacturer's claimed upper concentration limit corresponding to a 10 percent count error. If this value is exceeded, reduce the aerosol concentration until the acceptable conditions are met.
11.1.2.2.3 Zero Count: Connect a HEPA capsule to the inlet of the OPC and obtain printouts for three samples (each a minimum of 1-minute each). Record maximum cumulative zero count. If the count rate exceeds 50 counts per minute, the OPC requires servicing before continuing.
11.1.2.2.4 PSL Check of OPC Calibration: Confirm the calibration of the OPC by sampling a known size PSL aerosol. Aerosolize the PSL using an appropriate nebulizer. Record whether the peak count is observed in the proper channel. If the peak is not seen in the appropriate channel, have the OPC recalibrated.
11.1.2.3 Test Conditions:
11.1.2.3.1 Airflow: The test airflow corresponds to a nominal face velocity of 120 FPM through the arrestor. For arrestors having nominal 20 in. × 20 in. face dimensions, this measurement corresponds to an airflow of 333 cfm. For arrestors having nominal face dimensions of 24 in. × 24 in., this measurement corresponds to an airflow of 480 cfm.
11.1.2.3.2 Temperature and Relative Humidity: The temperature and relative humidity of the challenge air stream will be measured to within an accuracy of ±2°F and ±10 percent RH. To protect the probe from fouling, it may be removed during periods of aerosol generation.
11.1.2.3.3 Barometric Pressure: Use a mercury barometer. Record the atmospheric pressure.
11.1.2.4 Upstream and Downstream Background Counts.
11.1.2.4.1 With the arrestor installed in the test duct and the airflow set at the proper value, turn on the data acquisition computer and bring up the data acquisition program.
11.1.2.4.2 Set the OPC settings for the appropriate test sample duration with output for both printer and computer data collection.
11.1.2.4.3 Obtain one set of upstream-downstream background measurements.
11.1.2.4.4 After obtaining the upstream-downstream measurements, stop data acquisition.
11.1.2.5 Efficiency Measurements:
11.1.2.5.1 Record the arrestor pressure drop.
11.1.2.5.2 Turn on the Aerosol Generator. Begin aerosol generation and record the operating parameters.
11.1.2.5.3 Monitor the particle counts. Allow a minimum of 5 minutes for the generator to stabilize.
11.1.2.5.4 Confirm that the total particle count does not exceed the predetermined upper limit. Adjust generator as needed.
11.1.2.5.5 Confirm that a minimum of 50 particle counts are measured in the upstream sample in each of the OPC channels per sample. (A minimum of 50 counts per channel per sample will yield the required minimum 500 counts per channel total for the 10 upstream samples as specified in Table 319-1.) Adjust generator or sample time as needed.
11.1.2.5.6 If you are unable to obtain a stable concentration within the concentration limit and with the 50 count minimum per channel, adjust the aerosol generator.
11.1.2.5.7 When the counts are stable, perform repeated upstream-downstream sampling until 10 upstream-downstream measurements are obtained.
11.1.2.5.8 After collection of the 10 upstream-downstream samples, stop data acquisition and allow 2 more minutes for final purging of generator.
11.1.2.5.9 Obtain one additional set of upstream-downstream background samples.
11.1.2.5.10 After obtaining the upstream-downstream background samples, stop data acquisition.
11.1.2.5.11 Record the arrestor pressure drop.
11.1.2.5.12 Turn off blower.
11.1.2.5.13 Remove the paint arrestor assembly from the test duct. Note any signs of physical deterioration.
11.1.2.5.14 Remove the arrestor from the frame and place the arrestor in an appropriate storage bag.
11.2 Control Test: 100 Percent Penetration Test. A 100 percent penetration test must be performed immediately before each individual paint arrestor test using the same challenge aerosol substance (i.e., oleic acid or KCl) as to be used in the arrestor test. These tests are performed with no arrestor installed in the test housing. This test is a relatively stringent test of the adequacy of the overall duct, sampling, measurement, and aerosol generation system. The test is performed as a normal penetration test except the paint arrestor is not used. A perfect system would yield a measured penetration of 1 at all particle sizes. Deviations from 1 can occur due to particle losses in the duct, differences in the degree of aerosol uniformity (i.e., mixing) at the upstream and downstream probes, and differences in particle transport efficiency in the upstream and downstream sampling lines.
11.3 Control Test: 0 Percent Penetration. One 0 percent penetration test must be performed at least monthly during testing. The test is performed by using a HEPA filter rather than a paint arrestor. This test assesses the adequacy of the instrument response time and sample line lag.
12.0 Data Analysis and Calculations
12.1 Analysis. The analytical procedures for the fractional penetration and flow velocity measurements are described in Section 11. Note that the primary measurements, those of the upstream and downstream aerosol concentrations, are performed with the OPC which acquires the sample and analyzes it in real time. Because all the test data are collected in real time, there are no analytical procedures performed subsequent to the actual test, only data analysis.
12.2 Calculations.
12.2.1 Penetration.
Nomenclature
U = Upstream particle count
D = Downstream particle count
Ub = Upstream background count
Db = Downstream background count
P100 = 100 percent penetration value determined immediately prior to the arrestor test computed for each channel as:
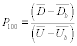
P = Penetration of the arrestor corrected for P100
ρ= sample standard deviation
CV = coefficient of variation = ρ/mean
E = Efficiency.
Overbar denotes arithmetic mean of quantity.
Analysis of each test involves the following quantities:
P100 value for each sizing channel from the 100 percent penetration control test,
2 upstream background values,
2 downstream background values,
10 upstream values with aerosol generator on, and
10 downstream values with aerosol generator on.
Using the values associated with each sizing channel, the penetration associated with each particle-sizing channel is calculated as:
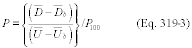

Most often, the background levels are small compared to the values when the aerosol generator is on.
12.3 The relationship between the physical diameter (DPhysical) as measured by the OPC to the aerodynamic diameter (DAero) is given by:
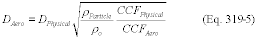
Where:
pO = unit density of 1 g/cm 3.
pParticle = the density of the particle, 0.89 g/cm 3 for oleic acid.
CCFPhysical = the Cunningham Correction Factor at DPhysical.
CCFAero = the Cunningham Correction Factor at D Aero.
12.4 Presentation of Results. For a given arrestor, results will be presented for:
Triplicate arrestor tests with the liquid-phase challenge aerosol,
Triplicate arrestor tests with the solid-phase challenge aerosol,
Triplicate 100 percent penetration tests with the liquid-phase challenge aerosol,
Triplicate 100 percent penetration tests with the solid-phase challenge aerosol, and
One 0 percent filter test (using either the liquid-phase or solid-phase aerosol and performed at least monthly).
12.4.1 Results for the paint arrestor test must be presented in both graphical and tabular form. The X-axis of the graph will be a logarithmic scale of aerodynamic diameter from 0.1 to 100 µm. The Y-axis will be efficiency (%) on a linear scale from 0 to 100. Plots for each individual run and a plot of the average of triplicate solid-phase and of the average triplicate liquid-phase tests must be prepared. All plots are to be based on point-to-point plotting (i.e., no curve fitting is to be used). The data are to be plotted based on the geometric mean diameter of each of the OPC's sizing channels.
12.4.2 Tabulated data from each test must be provided. The data must include the upper and lower diameter bound and geometric mean diameter of each of the OPC sizing channels, the background particle counts for each channel for each sample, the upstream particle counts for each channel for each sample, the downstream particle counts for each channel for each sample, the 100 percent penetration values computed for each channel, and the 0 percent penetration values computed for each channel.
13.0 Pollution Prevention
13.1 The quantities of materials to be aerosolized should be prepared in accord with the amount needed for the current tests so as to prevent wasteful excess.
14.0 Waste Management
14.1 Paint arrestors may be returned to originator, if requested, or disposed of with regular laboratory waste.
15.0 References
1. Hanley, J.T., D.D. Smith and L. Cox. “Fractional Penetration of Paint Overspray Arrestors, Draft Final Report,” EPA Cooperative Agreement CR-817083-01-0, January 1994.
2. Hanley, J.T., D.D. Smith, and D.S. Ensor. “Define a Fractional Efficiency Test Method that is Compatible with Particulate Removal Air Cleaners Used in General Ventilation,” Final Report, 671-RP, American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc., December 1993.
3. “Project Work and Quality Assurance Plan: Fractional Penetration of Paint Overspray Arrestors, Category II,” EPA Cooperative Agreement No. CR-817083, July 1994.
Test Method 320 - Measurement of Vapor Phase Organic and Inorganic Emissions by Extractive Fourier Transform Infrared (FTIR) Spectroscopy
1.0 Introduction
Persons unfamiliar with basic elements of FTIR spectroscopy should not attempt to use this method. This method describes sampling and analytical procedures for extractive emission measurements using Fourier transform infrared (FTIR) spectroscopy. Detailed analytical procedures for interpreting infrared spectra are described in the “Protocol for the Use of Extractive Fourier Transform Infrared (FTIR) Spectrometry in Analyses of Gaseous Emissions from Stationary Sources,” hereafter referred to as the “Protocol.” Definitions not given in this method are given in appendix A of the Protocol. References to specific sections in the Protocol are made throughout this Method. For additional information refer to references 1 and 2, and other EPA reports, which describe the use of FTIR spectrometry in specific field measurement applications and validation tests. The sampling procedure described here is extractive. Flue gas is extracted through a heated gas transport and handling system. For some sources, sample conditioning systems may be applicable. Some examples are given in this method.
Note:
Sample conditioning systems may be used providing the method validation requirements in Sections 9.2 and 13.0 of this method are met.
1.1 Scope and Applicability.
1.1.1 Analytes. Analytes include hazardous air pollutants (HAPs) for which EPA reference spectra have been developed. Other compounds can also be measured with this method if reference spectra are prepared according to section 4.6 of the protocol.
1.1.2 Applicability. This method applies to the analysis of vapor phase organic or inorganic compounds which absorb energy in the mid-infrared spectral region, about 400 to 4000 cm−1 (25 to 2.5 µm). This method is used to determine compound-specific concentrations in a multi-component vapor phase sample, which is contained in a closed-path gas cell. Spectra of samples are collected using double beam infrared absorption spectroscopy. A computer program is used to analyze spectra and report compound concentrations.
1.2 Method Range and Sensitivity. Analytical range and sensitivity depend on the frequency-dependent analyte absorptivity, instrument configuration, data collection parameters, and gas stream composition. Instrument factors include: (a) spectral resolution, (b) interferometer signal averaging time, (c) detector sensitivity and response, and (d) absorption path length.
1.2.1 For any optical configuration the analytical range is between the absorbance values of about .01 (infrared transmittance relative to the background = 0.98) and 1.0
(T = 0.1). (For absorbance >1.0 the relation between absorbance and concentration may not be linear.)
1.2.2 The concentrations associated with this absorbance range depend primarily on the cell path length and the sample temperature. An analyte absorbance greater than 1.0, can be lowered by decreasing the optical path length. Analyte absorbance increases with a longer path length. Analyte detection also depends on the presence of other species exhibiting absorbance in the same analytical region. Additionally, the estimated lower absorbance (A) limit
(A = 0.01) depends on the root mean square deviation (RMSD) noise in the analytical region.
1.2.3 The concentration range of this method is determined by the choice of optical configuration.
1.2.3.1 The absorbance for a given concentration can be decreased by decreasing the path length or by diluting the sample. There is no practical upper limit to the measurement range.
1.2.3.2 The analyte absorbance for a given concentration may be increased by increasing the cell path length or (to some extent) using a higher resolution. Both modifications also cause a corresponding increased absorbance for all compounds in the sample, and a decrease in the signal throughput. For this reason the practical lower detection range (quantitation limit) usually depends on sample characteristics such as moisture content of the gas, the presence of other interferants, and losses in the sampling system.
1.3 Sensitivity. The limit of sensitivity for an optical configuration and integration time is determined using appendix D of the Protocol: Minimum Analyte Uncertainty, (MAU). The MAU depends on the RMSD noise in an analytical region, and on the absorptivity of the analyte in the same region.
1.4 Data Quality. Data quality shall be determined by executing Protocol pre-test procedures in appendices B to H of the protocol and post-test procedures in appendices I and J of the protocol.
1.4.1 Measurement objectives shall be established by the choice of detection limit (DLi) and analytical uncertainty (AUi) for each analyte.
1.4.2 An instrumental configuration shall be selected. An estimate of gas composition shall be made based on previous test data, data from a similar source or information gathered in a pre-test site survey. Spectral interferants shall be identified using the selected DLi and AUi and band areas from reference spectra and interferant spectra. The baseline noise of the system shall be measured in each analytical region to determine the MAU of the instrument configuration for each analyte and interferant (MIUi).
1.4.3 Data quality for the application shall be determined, in part, by measuring the RMS (root mean square) noise level in each analytical spectral region (appendix C of the Protocol). The RMS noise is defined as the RMSD of the absorbance values in an analytical region from the mean absorbance value in the region.
1.4.4 The MAU is the minimum analyte concentration for which the AUi can be maintained; if the measured analyte concentration is less than MAUi then data quality are unacceptable.
2.0 Summary of Method
2.1 Principle. References 4 through 7 provide background material on infrared spectroscopy and quantitative analysis. A summary is given in this section.
2.1.1 Infrared absorption spectroscopy is performed by directing an infrared beam through a sample to a detector. The frequency-dependent infrared absorbance of the sample is measured by comparing this detector signal (single beam spectrum) to a signal obtained without a sample in the beam path (background).
2.1.2 Most molecules absorb infrared radiation and the absorbance occurs in a characteristic and reproducible pattern. The infrared spectrum measures fundamental molecular properties and a compound can be identified from its infrared spectrum alone.
2.1.3 Within constraints, there is a linear relationship between infrared absorption and compound concentration. If this frequency dependent relationship (absorptivity) is known (measured), it can be used to determine compound concentration in a sample mixture.
2.1.4 Absorptivity is measured by preparing, in the laboratory, standard samples of compounds at known concentrations and measuring the FTIR “reference spectra” of these standard samples. These “reference spectra” are then used in sample analysis: (1) Compounds are detected by matching sample absorbance bands with bands in reference spectra, and (2) concentrations are measured by comparing sample band intensities with reference band intensities.
2.1.5 This method is self-validating provided that the results meet the performance requirement of the QA spike in sections 8.6.2 and 9.0 of this method, and results from a previous method validation study support the use of this method in the application.
2.2 Sampling and Analysis. In extractive sampling a probe assembly and pump are used to extract gas from the exhaust of the affected source and transport the sample to the FTIR gas cell. Typically, the sampling apparatus is similar to that used for single-component continuous emission monitor (CEM) measurements.
2.2.1 The digitized infrared spectrum of the sample in the FTIR gas cell is measured and stored on a computer. Absorbance band intensities in the spectrum are related to sample concentrations by what is commonly referred to as Beer's Law.
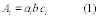
Where:
Ai = absorbance at a given frequency of the ith sample component.
ai = absorption coefficient (absorptivity) of the ith sample component.
b = path length of the cell.
ci = concentration of the ith sample component.
2.2.2 Analyte spiking is used for quality assurance (QA). In this procedure (section 8.6.2 of this method) an analyte is spiked into the gas stream at the back end of the sample probe. Analyte concentrations in the spiked samples are compared to analyte concentrations in unspiked samples. Since the concentration of the spike is known, this procedure can be used to determine if the sampling system is removing the spiked analyte(s) from the sample stream.
2.3 Reference Spectra Availability. Reference spectra of over 100 HAPs are available in the EPA FTIR spectral library on the EMTIC (Emission Measurement Technical Information Center) computer bulletin board service and at internet address http://info.arnold.af.mil/epa/welcome.htm. Reference spectra for HAPs, or other analytes, may also be prepared according to section 4.6 of the Protocol.
2.4 Operator Requirements. The FTIR analyst shall be trained in setting up the instrumentation, verifying the instrument is functioning properly, and performing routine maintenance. The analyst must evaluate the initial sample spectra to determine if the sample matrix is consistent with pre-test assumptions and if the instrument configuration is suitable. The analyst must be able to modify the instrument configuration, if necessary.
2.4.1 The spectral analysis shall be supervised by someone familiar with EPA FTIR Protocol procedures.
2.4.2 A technician trained in instrumental test methods is qualified to install and operate the sampling system. This includes installing the probe and heated line assembly, operating the analyte spike system, and performing moisture and flow measurements.
3.0 Definitions
See appendix A of the Protocol for definitions relating to infrared spectroscopy. Additional definitions are given in sections 3.1 through 3.29.
3.1 Analyte. A compound that this method is used to measure. The term “target analyte” is also used. This method is multi-component and a number of analytes can be targeted for a test.
3.2 Reference Spectrum. Infrared spectrum of an analyte prepared under controlled, documented, and reproducible laboratory conditions according to procedures in section 4.6 of the Protocol. A library of reference spectra is used to measure analytes in gas samples.
3.3 Standard Spectrum. A spectrum that has been prepared from a reference spectrum through a (documented) mathematical operation. A common example is de-resolving of reference spectra to lower-resolution standard spectra (Protocol, appendix K to the addendum of this method). Standard spectra, prepared by approved, and documented, procedures can be used as reference spectra for analysis.
3.4 Concentration. In this method concentration is expressed as a molar concentration, in ppm-meters, or in (ppm-meters)/K, where K is the absolute temperature (Kelvin). The latter units allow the direct comparison of concentrations from systems using different optical configurations or sampling temperatures.
3.5 Interferant. A compound in the sample matrix whose infrared spectrum overlaps with part of an analyte spectrum. The most accurate analyte measurements are achieved when reference spectra of interferants are used in the quantitative analysis with the analyte reference spectra. The presence of an interferant can increase the analytical uncertainty in the measured analyte concentration.
3.6 Gas Cell. A gas containment cell that can be evacuated. It is equipped with the optical components to pass the infrared beam through the sample to the detector. Important cell features include: path length (or range if variable), temperature range, materials of construction, and total gas volume.
3.7 Sampling System. Equipment used to extract the sample from the test location and transport the sample gas to the FTIR analyzer. This includes sample conditioning systems.
3.8 Sample Analysis. The process of interpreting the infrared spectra to obtain sample analyte concentrations. This process is usually automated using a software routine employing a classical least squares (cls), partial least squares (pls), or K- or P-matrix method.
3.9 One hundred percent line. A double beam transmittance spectrum obtained by combining two background single beam spectra. Ideally, this line is equal to 100 percent transmittance (or zero absorbance) at every frequency in the spectrum. Practically, a zero absorbance line is used to measure the baseline noise in the spectrum.
3.10 Background Deviation. A deviation from 100 percent transmittance in any region of the 100 percent line. Deviations greater than ±5 percent in an analytical region are unacceptable (absorbance of 0.021 to −0.022). Such deviations indicate a change in the instrument throughput relative to the background single beam.
3.11 Batch Sampling. A procedure where spectra of discreet, static samples are collected. The gas cell is filled with sample and the cell is isolated. The spectrum is collected. Finally, the cell is evacuated to prepare for the next sample.
3.12 Continuous Sampling. A procedure where spectra are collected while sample gas is flowing through the cell at a measured rate.
3.13 Sampling resolution. The spectral resolution used to collect sample spectra.
3.14 Truncation. Limiting the number of interferogram data points by deleting points farthest from the center burst (zero path difference, ZPD).
3.15 Zero filling. The addition of points to the interferogram. The position of each added point is interpolated from neighboring real data points. Zero filling adds no information to the interferogram, but affects line shapes in the absorbance spectrum (and possibly analytical results).
3.16 Reference CTS. Calibration Transfer Standard spectra that were collected with reference spectra.
3.17 CTS Standard. CTS spectrum produced by applying a de-resolution procedure to a reference CTS.
3.18 Test CTS. CTS spectra collected at the sampling resolution using the same optical configuration as for sample spectra. Test spectra help verify the resolution, temperature and path length of the FTIR system.
3.19 RMSD. Root Mean Square Difference, defined in EPA FTIR Protocol, appendix A.
3.20 Sensitivity. The noise-limited compound-dependent detection limit for the FTIR system configuration. This is estimated by the MAU. It depends on the RMSD in an analytical region of a zero absorbance line.
3.21 Quantitation Limit. The lower limit of detection for the FTIR system configuration in the sample spectra. This is estimated by mathematically subtracting scaled reference spectra of analytes and interferences from sample spectra, then measuring the RMSD in an analytical region of the subtracted spectrum. Since the noise in subtracted sample spectra may be much greater than in a zero absorbance spectrum, the quantitation limit is generally much higher than the sensitivity. Removing spectral interferences from the sample or improving the spectral subtraction can lower the quantitation limit toward (but not below) the sensitivity.
3.22 Independent Sample. A unique volume of sample gas; there is no mixing of gas between two consecutive independent samples. In continuous sampling two independent samples are separated by at least 5 cell volumes. The interval between independent measurements depends on the cell volume and the sample flow rate (through the cell).
3.23 Measurement. A single spectrum of flue gas contained in the FTIR cell.
3.24 Run. A run consists of a series of measurements. At a minimum a run includes 8 independent measurements spaced over 1 hour.
3.25 Validation. Validation of FTIR measurements is described in sections 13.0 through 13.4 of this method. Validation is used to verify the test procedures for measuring specific analytes at a source. Validation provides proof that the method works under certain test conditions.
3.26 Validation Run. A validation run consists of at least 24 measurements of independent samples. Half of the samples are spiked and half are not spiked. The length of the run is determined by the interval between independent samples.
3.27 Screening. Screening is used when there is little or no available information about a source. The purpose of screening is to determine what analytes are emitted and to obtain information about important sample characteristics such as moisture, temperature, and interferences. Screening results are semi-quantitative (estimated concentrations) or qualitative (identification only). Various optical and sampling configurations may be used. Sample conditioning systems may be evaluated for their effectiveness in removing interferences. It is unnecessary to perform a complete run under any set of sampling conditions. Spiking is not necessary, but spiking can be a useful screening tool for evaluating the sampling system, especially if a reactive or soluble analyte is used for the spike.
3.28 Emissions Test. An FTIR emissions test is performed according specific sampling and analytical procedures. These procedures, for the target analytes and the source, are based on previous screening and validation results. Emission results are quantitative. A QA spike (sections 8.6.2 and 9.2 of this method) is performed under each set of sampling conditions using a representative analyte. Flow, gas temperature and diluent data are recorded concurrently with the FTIR measurements to provide mass emission rates for detected compounds.
3.29 Surrogate. A surrogate is a compound that is used in a QA spike procedure (section 8.6.2 of this method) to represent other compounds. The chemical and physical properties of a surrogate shall be similar to the compounds it is chosen to represent. Under given sampling conditions, usually a single sampling factor is of primary concern for measuring the target analytes: for example, the surrogate spike results can be representative for analytes that are more reactive, more soluble, have a lower absorptivity, or have a lower vapor pressure than the surrogate itself.
4.0 Interferences
Interferences are divided into two classifications: analytical and sampling.
4.1 Analytical Interferences. An analytical interference is a spectral feature that complicates (in extreme cases may prevent) the analysis of an analyte. Analytical interferences are classified as background or spectral interference.
4.1.1 Background Interference. This results from a change in throughput relative to the single beam background. It is corrected by collecting a new background and proceeding with the test. In severe instances the cause must be identified and corrected. Potential causes include: (1) Deposits on reflective surfaces or transmitting windows, (2) changes in detector sensitivity, (3) a change in the infrared source output, or (4) failure in the instrument electronics. In routine sampling throughput may degrade over several hours. Periodically a new background must be collected, but no other corrective action will be required.
4.1.2 Spectral Interference. This results from the presence of interfering compound(s) (interferant) in the sample. Interferant spectral features overlap analyte spectral features. Any compound with an infrared spectrum, including analytes, can potentially be an interferant. The Protocol measures absorbance band overlap in each analytical region to determine if potential interferants shall be classified as known interferants (FTIR Protocol, section 4.9 and appendix B). Water vapor and CO2 are common spectral interferants. Both of these compounds have strong infrared spectra and are present in many sample matrices at high concentrations relative to analytes. The extent of interference depends on the (1) interferant concentration, (2) analyte concentration, and (3) the degree of band overlap. Choosing an alternate analytical region can minimize or avoid the spectral interference. For example, CO2 interferes with the analysis of the 670 cm−1 benzene band. However, benzene can also be measured near 3000 cm−1 (with less sensitivity).
4.2 Sampling System Interferences. These prevent analytes from reaching the instrument. The analyte spike procedure is designed to measure sampling system interference, if any.
4.2.1 Temperature. A temperature that is too low causes condensation of analytes or water vapor. The materials of the sampling system and the FTIR gas cell usually set the upper limit of temperature.
4.2.2 Reactive Species. Anything that reacts with analytes. Some analytes, like formaldehyde, polymerize at lower temperatures.
4.2.3 Materials. Poor choice of material for probe, or sampling line may remove some analytes. For example, HF reacts with glass components.
4.2.4 Moisture. In addition to being a spectral interferant, condensed moisture removes soluble compounds.
5.0 Safety
The hazards of performing this method are those associated with any stack sampling method and the same precautions shall be followed. Many HAPs are suspected carcinogens or present other serious health risks. Exposure to these compounds should be avoided in all circumstances. For instructions on the safe handling of any particular compound, refer to its material safety data sheet. When using analyte standards, always ensure that gases are properly vented and that the gas handling system is leak free. (Always perform a leak check with the system under maximum vacuum and, again, with the system at greater than ambient pressure.) Refer to section 8.2 of this method for leak check procedures. This method does not address all of the potential safety risks associated with its use. Anyone performing this method must follow safety and health practices consistent with applicable legal requirements and with prudent practice for each application.
6.0 Equipment and Supplies
Note:
Mention of trade names or specific products does not constitute endorsement by the Environmental Protection Agency.
The equipment and supplies are based on the schematic of a sampling system shown in Figure 1. Either the batch or continuous sampling procedures may be used with this sampling system. Alternative sampling configurations may also be used, provided that the data quality objectives are met as determined in the post-analysis evaluation. Other equipment or supplies may be necessary, depending on the design of the sampling system or the specific target analytes.
6.1 Sampling Probe. Glass, stainless steel, or other appropriate material of sufficient length and physical integrity to sustain heating, prevent adsorption of analytes, and to transport analytes to the infrared gas cell. Special materials or configurations may be required in some applications. For instance, high stack sample temperatures may require special steel or cooling the probe. For very high moisture sources it may be desirable to use a dilution probe.
6.2 Particulate Filters. A glass wool plug (optional) inserted at the probe tip (for large particulate removal) and a filter (required) rated for 99 percent removal efficiency at 1-micron (e.g., Balston”) connected at the outlet of the heated probe.
6.3 Sampling Line/Heating System. Heated (sufficient to prevent condensation) stainless steel, polytetrafluoroethane, or other material inert to the analytes.
6.4 Gas Distribution Manifold. A heated manifold allowing the operator to control flows of gas standards and samples directly to the FTIR system or through sample conditioning systems. Usually includes heated flow meter, heated valve for selecting and sending sample to the analyzer, and a by-pass vent. This is typically constructed of stainless steel tubing and fittings, and high-temperature valves.
6.5 Stainless Steel Tubing. Type 316, appropriate diameter (e.g., 3/8 in.) and length for heated connections. Higher grade stainless may be desirable in some applications.
6.6 Calibration/Analyte Spike Assembly. A three way valve assembly (or equivalent) to introduce analyte or surrogate spikes into the sampling system at the outlet of the probe upstream of the out-of-stack particulate filter and the FTIR analytical system.
6.7 Mass Flow Meter (MFM). These are used for measuring analyte spike flow. The MFM shall be calibrated in the range of 0 to 5 L/min and be accurate to ±2 percent (or better) of the flow meter span.
6.8 Gas Regulators. Appropriate for individual gas standards.
6.9 Polytetrafluoroethane Tubing. Diameter (e.g., 3/8 in.) and length suitable to connect cylinder regulators to gas standard manifold.
6.10 Sample Pump. A leak-free pump (e.g., KNF TM), with by-pass valve, capable of producing a sample flow rate of at least 10 L/min through 100 ft of sample line. If the pump is positioned upstream of the distribution manifold and FTIR system, use a heated pump that is constructed from materials non-reactive to the analytes. If the pump is located downstream of the FTIR system, the gas cell sample pressure will be lower than ambient pressure and it must be recorded at regular intervals.
6.11 Gas Sample Manifold. Secondary manifold to control sample flow at the inlet to the FTIR manifold. This is optional, but includes a by-pass vent and heated rotameter.
6.12 Rotameter. A 0 to 20 L/min rotameter. This meter need not be calibrated.
6.13 FTIR Analytical System. Spectrometer and detector, capable of measuring the analytes to the chosen detection limit. The system shall include a personal computer with compatible software allowing automated collection of spectra.
6.14 FTIR Cell Pump. Required for the batch sampling technique, capable of evacuating the FTIR cell volume within 2 minutes. The pumping speed shall allow the operator to obtain 8 sample spectra in 1 hour.
6.15 Absolute Pressure Gauge. Capable of measuring pressure from 0 to 1000 mmHg to within ±2.5 mmHg (e.g., Baratron TM).
6.16 Temperature Gauge. Capable of measuring the cell temperature to within ±2°C.
6.17 Sample Conditioning. One option is a condenser system, which is used for moisture removal. This can be helpful in the measurement of some analytes. Other sample conditioning procedures may be devised for the removal of moisture or other interfering species.
6.17.1 The analyte spike procedure of section 9.2 of this method, the QA spike procedure of section 8.6.2 of this method, and the validation procedure of section 13 of this method demonstrate whether the sample conditioning affects analyte concentrations. Alternatively, measurements can be made with two parallel FTIR systems; one measuring conditioned sample, the other measuring unconditioned sample.
6.17.2 Another option is sample dilution. The dilution factor measurement must be documented and accounted for in the reported concentrations. An alternative to dilution is to lower the sensitivity of the FTIR system by decreasing the cell path length, or to use a short-path cell in conjunction with a long path cell to measure more than one concentration range.
7.0 Reagents and Standards
7.1 Analyte(s) and Tracer Gas. Obtain a certified gas cylinder mixture containing all of the analyte(s) at concentrations within ±2 percent of the emission source levels (expressed in ppm-meter/K). If practical, the analyte standard cylinder shall also contain the tracer gas at a concentration which gives a measurable absorbance at a dilution factor of at least 10:1. Two ppm SF6 is sufficient for a path length of 22 meters at 250°F.
7.2 Calibration Transfer Standard(s). Select the calibration transfer standards (CTS) according to section 4.5 of the FTIR Protocol. Obtain a National Institute of Standards and Technology (NIST) traceable gravimetric standard of the CTS (±2 percent).
7.3 Reference Spectra. Obtain reference spectra for each analyte, interferant, surrogate, CTS, and tracer. If EPA reference spectra are not available, use reference spectra prepared according to procedures in section 4.6 of the EPA FTIR Protocol.
8.0 Sampling and Analysis Procedure
Three types of testing can be performed: (1) Screening, (2) emissions test, and (3) validation. Each is defined in section 3 of this method. Determine the purpose(s) of the FTIR test. Test requirements include: (a) AUi, DLi, overall fractional uncertainty, OFUi, maximum expected concentration (CMAXi), and tAN for each, (b) potential interferants, (c) sampling system factors, e.g., minimum absolute cell pressure, (Pmin), FTIR cell volume (VSS), estimated sample absorption pathlength, LS′, estimated sample pressure, PS′, TS′, signal integration time (tSS), minimum instrumental linewidth, MIL, fractional error, and (d) analytical regions, e.g., m = 1 to M, lower wavenumber position, FLm, center wavenumber position, FCm, and upper wavenumber position, FUm, plus interferants, upper wavenumber position of the CTS absorption band, FFUm, lower wavenumber position of the CTS absorption band, FFLm, wavenumber range FNU to FNL. If necessary, sample and acquire an initial spectrum. From analysis of this preliminary spectrum determine a suitable operational path length. Set up the sampling train as shown in Figure 1 or use an appropriate alternative configuration. Sections 8.1 through 8.11 of this method provide guidance on pre-test calculations in the EPA protocol, sampling and analytical procedures, and post-test protocol calculations.
8.1 Pretest Preparations and Evaluations. Using the procedure in section 4.0 of the FTIR Protocol, determine the optimum sampling system configuration for measuring the target analytes. Use available information to make reasonable assumptions about moisture content and other interferences.
8.1.1 Analytes. Select the required detection limit (DLi) and the maximum permissible analytical uncertainty (AUi) for each analyte (labeled from 1 to i). Estimate, if possible, the maximum expected concentration for each analyte, CMAXi. The expected measurement range is fixed by DLi and CMAXi for each analyte (i).
8.1.2 Potential Interferants. List the potential interferants. This usually includes water vapor and CO2, but may also include some analytes and other compounds.
8.1.3. Optical Configuration. Choose an optical configuration that can measure all of the analytes within the absorbance range of .01 to 1.0 (this may require more than one path length). Use Protocol sections 4.3 to 4.8 for guidance in choosing a configuration and measuring CTS.
8.1.4 Fractional Reproducibility Uncertainty (FRUi). The FRU is determined for each analyte by comparing CTS spectra taken before and after the reference spectra were measured. The EPA para-xylene reference spectra were collected on 10/31/91 and 11/01/91 with corresponding CTS spectra “cts1031a,” and “cts1101b.” The CTS spectra are used to estimate the reproducibility (FRU) in the system that was used to collect the references. The FRU must be <AU. Appendix E of the protocol is used to calculate the FRU from CTS spectra. Figure 2 plots results for 0.25 cm−1 CTS spectra in EPA reference library: S3 (cts1101b−cts1031a), and S4 [(cts1101b + cts1031a)/2]. The RMSD (SRMS) is calculated in the subtracted baseline, S3, in the corresponding CTS region from 850 to 1065 cm−1. The area (BAV) is calculated in the same region of the averaged CTS spectrum, S4.
8.1.5 Known Interferants. Use appendix B of the EPA FTIR Protocol.
8.1.6 Calculate the Minimum Analyte Uncertainty, MAU (section 1.3 of this method discusses MAU and protocol appendix D gives the MAU procedure). The MAU for each analyte, i, and each analytical region, m, depends on the RMS noise.
8.1.7 Analytical Program. See FTIR Protocol, section 4.10. Prepare computer program based on the chosen analytical technique. Use as input reference spectra of all target analytes and expected interferants. Reference spectra of additional compounds shall also be included in the program if their presence (even if transient) in the samples is considered possible. The program output shall be in ppm (or ppb) and shall be corrected for differences between the reference path length, LR, temperature, TR, and pressure, PR, and the conditions used for collecting the sample spectra. If sampling is performed at ambient pressure, then any pressure correction is usually small relative to corrections for path length and temperature, and may be neglected.
8.2 Leak-Check
8.2.1 Sampling System. A typical FTIR extractive sampling train is shown in Figure 1. Leak check from the probe tip to pump outlet as follows: Connect a 0-to 250-mL/min rate meter (rotameter or bubble meter) to the outlet of the pump. Close off the inlet to the probe, and record the leak rate. The leak rate shall be ≤200 mL/min.
8.2.2 Analytical System Leak check. Leak check the FTIR cell under vacuum and under pressure (greater than ambient). Leak check connecting tubing and inlet manifold under pressure.
8.2.2.1 For the evacuated sample technique, close the valve to the FTIR cell, and evacuate the absorption cell to the minimum absolute pressure Pmin. Close the valve to the pump, and determine the change in pressure ΔPv after 2 minutes.
8.2.2.2 For both the evacuated sample and purging techniques, pressurize the system to about 100 mmHg above atmospheric pressure. Isolate the pump and determine the change in pressure ΔPp after 2 minutes.
8.2.2.3 Measure the barometric pressure, Pb in mmHg.
8.2.2.4 Determine the percent leak volume %VL for the signal integration time tSS and for ΔPmax, i.e., the larger of ΔPv or ΔPp, as follows:
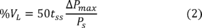
Where:
50 = 100% divided by the leak-check time of 2 minutes.
8.2.2.5 Leak volumes in excess of 4 percent of the FTIR system volume VSS are unacceptable.
8.3 Detector Linearity. Once an optical configuration is chosen, use one of the procedures of sections 8.3.1 through 8.3.3 to verify that the detector response is linear. If the detector response is not linear, decrease the aperture, or attenuate the infrared beam. After a change in the instrument configuration perform a linearity check until it is demonstrated that the detector response is linear.
8.3.1 Vary the power incident on the detector by modifying the aperture setting. Measure the background and CTS at three instrument aperture settings: (1) at the aperture setting to be used in the testing, (2) at one half this aperture and (3) at twice the proposed testing aperture. Compare the three CTS spectra. CTS band areas shall agree to within the uncertainty of the cylinder standard and the RMSD noise in the system. If test aperture is the maximum aperture, collect CTS spectrum at maximum aperture, then close the aperture to reduce the IR throughput by half. Collect a second background and CTS at the smaller aperture setting and compare the spectra again.
8.3.2 Use neutral density filters to attenuate the infrared beam. Set up the FTIR system as it will be used in the test measurements. Collect a CTS spectrum. Use a neutral density filter to attenuate the infrared beam (either immediately after the source or the interferometer) to approximately 1/2 its original intensity. Collect a second CTS spectrum. Use another filter to attenuate the infrared beam to approximately 1/4 its original intensity. Collect a third background and CTS spectrum. Compare the CTS spectra. CTS band areas shall agree to within the uncertainty of the cylinder standard and the RMSD noise in the system.
8.3.3 Observe the single beam instrument response in a frequency region where the detector response is known to be zero. Verify that the detector response is “flat” and equal to zero in these regions.
8.4 Data Storage Requirements. All field test spectra shall be stored on a computer disk and a second backup copy must stored on a separate disk. The stored information includes sample interferograms, processed absorbance spectra, background interferograms, CTS sample interferograms and CTS absorbance spectra. Additionally, documentation of all sample conditions, instrument settings, and test records must be recorded on hard copy or on computer medium. Table 1 gives a sample presentation of documentation.
8.5 Background Spectrum. Evacuate the gas cell to ≤5 mmHg, and fill with dry nitrogen gas to ambient pressure (or purge the cell with 10 volumes of dry nitrogen). Verify that no significant amounts of absorbing species (for example water vapor and CO2) are present. Collect a background spectrum, using a signal averaging period equal to or greater than the averaging period for the sample spectra. Assign a unique file name to the background spectrum. Store two copies of the background interferogram and processed single-beam spectrum on separate computer disks (one copy is the back-up).
8.5.1 Interference Spectra. If possible, collect spectra of known and suspected major interferences using the same optical system that will be used in the field measurements. This can be done on-site or earlier. A number of gases, e.g. CO2, SO2, CO, NH3, are readily available from cylinder gas suppliers.
8.5.2 Water vapor spectra can be prepared by the following procedure. Fill a sample tube with distilled water. Evacuate above the sample and remove dissolved gasses by alternately freezing and thawing the water while evacuating. Allow water vapor into the FTIR cell, then dilute to atmospheric pressure with nitrogen or dry air. If quantitative water spectra are required, follow the reference spectrum procedure for neat samples (protocol, section 4.6). Often, interference spectra need not be quantitative, but for best results the absorbance must be comparable to the interference absorbance in the sample spectra.
8.6 Pre-Test Calibrations
8.6.1 Calibration Transfer Standard. Evacuate the gas cell to ≤5 mmHg absolute pressure, and fill the FTIR cell to atmospheric pressure with the CTS gas. Alternatively, purge the cell with 10 cell volumes of CTS gas. (If purge is used, verify that the CTS concentration in the cell is stable by collecting two spectra 2 minutes apart as the CTS gas continues to flow. If the absorbance in the second spectrum is no greater than in the first, within the uncertainty of the gas standard, then this can be used as the CTS spectrum.) Record the spectrum.
8.6.2 QA Spike. This procedure assumes that the method has been validated for at least some of the target analytes at the source. For emissions testing perform a QA spike. Use a certified standard, if possible, of an analyte, which has been validated at the source. One analyte standard can serve as a QA surrogate for other analytes which are less reactive or less soluble than the standard. Perform the spike procedure of section 9.2 of this method. Record spectra of at least three independent (section 3.22 of this method) spiked samples. Calculate the spiked component of the analyte concentration. If the average spiked concentration is within 0.7 to 1.3 times the expected concentration, then proceed with the testing. If applicable, apply the correction factor from the Method 301 of this appendix validation test (not the result from the QA spike).
8.7 Sampling. If analyte concentrations vary rapidly with time, continuous sampling is preferable using the smallest cell volume, fastest sampling rate and fastest spectra collection rate possible. Continuous sampling requires the least operator intervention even without an automated sampling system. For continuous monitoring at one location over long periods, Continuous sampling is preferred. Batch sampling and continuous static sampling are used for screening and performing test runs of finite duration. Either technique is preferred for sampling several locations in a matter of days. Batch sampling gives reasonably good time resolution and ensures that each spectrum measures a discreet (and unique) sample volume. Continuous static (and continuous) sampling provide a very stable background over long periods. Like batch sampling, continuous static sampling also ensures that each spectrum measures a unique sample volume. It is essential that the leak check procedure under vacuum (section 8.2 of this method) is passed if the batch sampling procedure is used. It is essential that the leak check procedure under positive pressure is passed if the continuous static or continuous sampling procedures are used. The sampling techniques are described in sections 8.7.1 through 8.7.2 of this method.
8.7.1 Batch Sampling. Evacuate the absorbance cell to ≤5 mmHg absolute pressure. Fill the cell with exhaust gas to ambient pressure, isolate the cell, and record the spectrum. Before taking the next sample, evacuate the cell until no spectral evidence of sample absorption remains. Repeat this procedure to collect eight spectra of separate samples in 1 hour.
8.7.2 Continuous Static Sampling. Purge the FTIR cell with 10 cell volumes of sample gas. Isolate the cell, collect the spectrum of the static sample and record the pressure. Before measuring the next sample, purge the cell with 10 more cell volumes of sample gas.
8.8 Sampling QA and Reporting
8.8.1 Sample integration times shall be sufficient to achieve the required signal-to-noise ratio. Obtain an absorbance spectrum by filling the cell with N2. Measure the RMSD in each analytical region in this absorbance spectrum. Verify that the number of scans used is sufficient to achieve the target MAU.
8.8.2 Assign a unique file name to each spectrum.
8.8.3 Store two copies of sample interferograms and processed spectra on separate computer disks.
8.8.4 For each sample spectrum, document the sampling conditions, the sampling time (while the cell was being filled), the time the spectrum was recorded, the instrumental conditions (path length, temperature, pressure, resolution, signal integration time), and the spectral file name. Keep a hard copy of these data sheets.
8.9 Signal Transmittance. While sampling, monitor the signal transmittance. If signal transmittance (relative to the background) changes by 5 percent or more (absorbance = -.02 to .02) in any analytical spectral region, obtain a new background spectrum.
8.10 Post-test CTS. After the sampling run, record another CTS spectrum.
8.11 Post-test QA
8.11.1 Inspect the sample spectra immediately after the run to verify that the gas matrix composition was close to the expected (assumed) gas matrix.
8.11.2 Verify that the sampling and instrumental parameters were appropriate for the conditions encountered. For example, if the moisture is much greater than anticipated, it may be necessary to use a shorter path length or dilute the sample.
8.11.3 Compare the pre- and post-test CTS spectra. The peak absorbance in pre- and post-test CTS must be ±5 percent of the mean value. See appendix E of the FTIR Protocol.
9.0 Quality Control
Use analyte spiking (sections 8.6.2, 9.2 and 13.0 of this method) to verify that the sampling system can transport the analytes from the probe to the FTIR system.
9.1 Spike Materials. Use a certified standard (accurate to ±2 percent) of the target analyte, if one can be obtained. If a certified standard cannot be obtained, follow the procedures in section 4.6.2.2 of the FTIR Protocol.
9.2 Spiking Procedure. QA spiking (section 8.6.2 of this method) is a calibration procedure used before testing. QA spiking involves following the spike procedure of sections 9.2.1 through 9.2.3 of this method to obtain at least three spiked samples. The analyte concentrations in the spiked samples shall be compared to the expected spike concentration to verify that the sampling/analytical system is working properly. Usually, when QA spiking is used, the method has already been validated at a similar source for the analyte in question. The QA spike demonstrates that the validated sampling/analytical conditions are being duplicated. If the QA spike fails then the sampling/analytical system shall be repaired before testing proceeds. The method validation procedure (section 13.0 of this method) involves a more extensive use of the analyte spike procedure of sections 9.2.1 through 9.2.3 of this method. Spectra of at least 12 independent spiked and 12 independent unspiked samples are recorded. The concentration results are analyzed statistically to determine if there is a systematic bias in the method for measuring a particular analyte. If there is a systematic bias, within the limits allowed by Method 301 of this appendix, then a correction factor shall be applied to the analytical results. If the systematic bias is greater than the allowed limits, this method is not valid and cannot be used.
9.2.1 Introduce the spike/tracer gas at a constant flow rate of ≤10 percent of the total sample flow, when possible.
Note:
Use the rotameter at the end of the sampling train to estimate the required spike/tracer gas flow rate.
Use a flow device, e.g., mass flow meter (# 2 percent), to monitor the spike flow rate. Record the spike flow rate every 10 minutes.
9.2.2 Determine the response time (RT) of the system by continuously collecting spectra of the spiked effluent until the spectrum of the spiked component is constant for 5 minutes. The RT is the interval from the first measurement until the spike becomes constant. Wait for twice the duration of the RT, then collect spectra of two independent spiked gas samples. Duplicate analyses of the spiked concentration shall be within 5 percent of the mean of the two measurements.
9.2.3 Calculate the dilution ratio using the tracer gas as follows:


DF = Dilution factor of the spike gas; this value shall be ≥10.
SF6(dir) = SF6 (or tracer gas) concentration measured directly in undiluted spike gas.
SF6(spk) = Diluted SF6 (or tracer gas) concentration measured in a spiked sample.
Spikedir = Concentration of the analyte in the spike standard measured by filling the FTIR cell directly.
CS = Expected concentration of the spiked samples.
Unspike = Native concentration of analytes in unspiked samples.
10.0 Calibration and Standardization
10.1 Signal-to-Noise Ratio (S/N). The RMSD in the noise must be less than one tenth of the minimum analyte peak absorbance in each analytical region. For example if the minimum peak absorbance is 0.01 at the required DL, then RMSD measured over the entire analytical region must be ≤0.001.
10.2 Absorbance Path length. Verify the absorbance path length by comparing reference CTS spectra to test CTS spectra. See appendix E of the FTIR Protocol.
10.3 Instrument Resolution. Measure the line width of appropriate test CTS band(s) to verify instrument resolution. Alternatively, compare CTS spectra to a reference CTS spectrum, if available, measured at the nominal resolution.
10.4 Apodization Function.In transforming the sample interferograms to absorbance spectra use the same apodization function that was used in transforming the reference spectra.
10.5 FTIR Cell Volume. Evacuate the cell to ≤5 mmHg. Measure the initial absolute temperature (Ti) and absolute pressure (Pi). Connect a wet test meter (or a calibrated dry gas meter), and slowly draw room air into the cell. Measure the meter volume (Vm), meter absolute temperature (Tm), and meter absolute pressure (Pm); and the cell final absolute temperature (Tf) and absolute pressure (Pf). Calculate the FTIR cell volume VSS, including that of the connecting tubing, as follows:
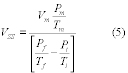
11.0 Data Analysis and Calculations
Analyte concentrations shall be measured using reference spectra from the EPA FTIR spectral library. When EPA library spectra are not available, the procedures in section 4.6 of the Protocol shall be followed to prepare reference spectra of all the target analytes.
11.1 Spectral De-resolution. Reference spectra can be converted to lower resolution standard spectra (section 3.3 of this method) by truncating the original reference sample and background interferograms. Appendix K of the FTIR Protocol gives specific deresolution procedures. Deresolved spectra shall be transformed using the same apodization function and level of zero filling as the sample spectra. Additionally, pre-test FTIR protocol calculations (e.g., FRU, MAU, FCU) shall be performed using the de-resolved standard spectra.
11.2 Data Analysis. Various analytical programs are available for relating sample absorbance to a concentration standard. Calculated concentrations shall be verified by analyzing residual baselines after mathematically subtracting scaled reference spectra from the sample spectra. A full description of the data analysis and calculations is contained in the FTIR Protocol (sections 4.0, 5.0, 6.0 and appendices). Correct the calculated concentrations in the sample spectra for differences in absorption path length and temperature between the reference and sample spectra using equation 6,
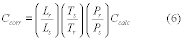
Where:
Ccorr = Concentration, corrected for path length.
Ccalc = Concentration, initial calculation (output of the analytical program designed for the compound).
Lr = Reference spectra path length.
Ls = Sample spectra path length.
Ts = Absolute temperature of the sample gas, K.
Tr = Absolute gas temperature of reference spectra, K.
Ps = Sample cell pressure.
Pr = Reference spectrum sample pressure.
12.0 Method Performance
12.1 Spectral Quality. Refer to the FTIR Protocol appendices for analytical requirements, evaluation of data quality, and analysis of uncertainty.
12.2 Sampling QA/QC. The analyte spike procedure of section 9 of this method, the QA spike of section 8.6.2 of this method, and the validation procedure of section 13 of this method are used to evaluate the performance of the sampling system and to quantify sampling system effects, if any, on the measured concentrations. This method is self-validating provided that the results meet the performance requirement of the QA spike in sections 9.0 and 8.6.2 of this method and results from a previous method validation study support the use of this method in the application. Several factors can contribute to uncertainty in the measurement of spiked samples. Factors which can be controlled to provide better accuracy in the spiking procedure are listed in sections 12.2.1 through 12.2.4 of this method.
12.2.1 Flow meter. An accurate mass flow meter is accurate to ±1 percent of its span. If a flow of 1 L/min is monitored with such a MFM, which is calibrated in the range of 0-5 L/min, the flow measurement has an uncertainty of 5 percent. This may be improved by re-calibrating the meter at the specific flow rate to be used.
12.2.2 Calibration gas. Usually the calibration standard is certified to within ±2 percent. With reactive analytes, such as HCl, the certified accuracy in a commercially available standard may be no better than ±5 percent.
12.2.3 Temperature. Temperature measurements of the cell shall be quite accurate. If practical, it is preferable to measure sample temperature directly, by inserting a thermocouple into the cell chamber instead of monitoring the cell outer wall temperature.
12.2.4 Pressure. Accuracy depends on the accuracy of the barometer, but fluctuations in pressure throughout a day may be as much as 2.5 percent due to weather variations.
13.0 Method Validation Procedure
This validation procedure, which is based on EPA Method 301 (40 CFR part 63, appendix (A), may be used to validate this method for the analytes in a gas matrix. Validation at one source may also apply to another type of source, if it can be shown that the exhaust gas characteristics are similar at both sources.
13.1 Section 6.0 of Method 301 (40 CFR part 63, appendix A), the Analyte Spike procedure, is used with these modifications. The statistical analysis of the results follows section 12.0 of EPA Method 301. Section 3 of this method defines terms that are not defined in Method 301.
13.1.1 The analyte spike is performed dynamically. This means the spike flow is continuous and constant as spiked samples are measured.
13.1.2 The spike gas is introduced at the back of the sample probe.
13.1.3 Spiked effluent is carried through all sampling components downstream of the probe.
13.1.4 A single FTIR system (or more) may be used to collect and analyze spectra (not quadruplicate integrated sampling trains).
13.1.5 All of the validation measurements are performed sequentially in a single “run” (section 3.26 of this method).
13.1.6 The measurements analyzed statistically are each independent (section 3.22 of this method).
13.1.7 A validation data set can consist of more than 12 spiked and 12 unspiked measurements.
13.2 Batch Sampling. The procedure in sections 13.2.1 through 13.2.2 may be used for stable processes. If process emissions are highly variable, the procedure in section 13.2.3 shall be used.
13.2.1 With a single FTIR instrument and sampling system, begin by collecting spectra of two unspiked samples. Introduce the spike flow into the sampling system and allow 10 cell volumes to purge the sampling system and FTIR cell. Collect spectra of two spiked samples. Turn off the spike and allow 10 cell volumes of unspiked sample to purge the FTIR cell. Repeat this procedure until the 24 (or more) samples are collected.
13.2.2 In batch sampling, collect spectra of 24 distinct samples. (Each distinct sample consists of filling the cell to ambient pressure after the cell has been evacuated.)
13.2.3 Alternatively, a separate probe assembly, line, and sample pump can be used for spiked sample. Verify and document that sampling conditions are the same in both the spiked and the unspiked sampling systems. This can be done by wrapping both sample lines in the same heated bundle. Keep the same flow rate in both sample lines. Measure samples in sequence in pairs. After two spiked samples are measured, evacuate the FTIR cell, and turn the manifold valve so that spiked sample flows to the FTIR cell. Allow the connecting line from the manifold to the FTIR cell to purge thoroughly (the time depends on the line length and flow rate). Collect a pair of spiked samples. Repeat the procedure until at least 24 measurements are completed.
13.3 Simultaneous Measurements With Two FTIR Systems. If unspiked effluent concentrations of the target analyte(s) vary significantly with time, it may be desirable to perform synchronized measurements of spiked and unspiked sample. Use two FTIR systems, each with its own cell and sampling system to perform simultaneous spiked and unspiked measurements. The optical configurations shall be similar, if possible. The sampling configurations shall be the same. One sampling system and FTIR analyzer shall be used to measure spiked effluent. The other sampling system and FTIR analyzer shall be used to measure unspiked flue gas. Both systems shall use the same sampling procedure (i.e., batch or continuous).
13.3.1 If batch sampling is used, synchronize the cell evacuation, cell filling, and collection of spectra. Fill both cells at the same rate (in cell volumes per unit time).
13.3.2 If continuous sampling is used, adjust the sample flow through each gas cell so that the same number of cell volumes pass through each cell in a given time (i.e. TC1 = TC2).
13.4 Statistical Treatment. The statistical procedure of EPA Method 301 of this appendix, section 12.0 is used to evaluate the bias and precision. For FTIR testing a validation “run” is defined as spectra of 24 independent samples, 12 of which are spiked with the analyte(s) and 12 of which are not spiked.
13.4.1 Bias. Determine the bias (defined by EPA Method 301 of this appendix, section 12.1.1) using equation 7:
B=Sm − CS
Where:
B = Bias at spike level.
Sm = Mean concentration of the analyte spiked samples.
CS = Expected concentration of the spiked samples.
13.4.2 Correction Factor. Use section 6.3.2.2 of Method 301 of this appendix to evaluate the statistical significance of the bias. If it is determined that the bias is significant, then use section 6.3.3 of Method 301 to calculate a correction factor (CF). Analytical results of the test method are multiplied by the correction factor, if 0.7 ≤CF ≤1.3. If is determined that the bias is significant and CF >±30 percent, then the test method is considered to “not valid.”
13.4.3 If measurements do not pass validation, evaluate the sampling system, instrument configuration, and analytical system to determine if improper set-up or a malfunction was the cause. If so, repair the system and repeat the validation.
14.0 Pollution Prevention
The extracted sample gas is vented outside the enclosure containing the FTIR system and gas manifold after the analysis. In typical method applications the vented sample volume is a small fraction of the source volumetric flow and its composition is identical to that emitted from the source. When analyte spiking is used, spiked pollutants are vented with the extracted sample gas. Approximately 1.6 × 10−4 to 3.2 × 10−4 lbs of a single HAP may be vented to the atmosphere in a typical validation run of 3 hours. (This assumes a molar mass of 50 to 100 g, spike rate of 1.0 L/min, and a standard concentration of 100 ppm). Minimize emissions by keeping the spike flow off when not in use.
15.0 Waste Management
Small volumes of laboratory gas standards can be vented through a laboratory hood. Neat samples must be packed and disposed according to applicable regulations. Surplus materials may be returned to supplier for disposal.
16.0 References
1. “Field Validation Test Using Fourier Transform Infrared (FTIR) Spectrometry To Measure Formaldehyde, Phenol and Methanol at a Wool Fiberglass Production Facility.” Draft. U.S. Environmental Protection Agency Report, EPA Contract No. 68D20163, Work Assignment I-32, September 1994.
2. “FTIR Method Validation at a Coal-Fired Boiler”. Prepared for U.S. Environmental Protection Agency, Research Triangle Park, NC. Publication No.: EPA-454/R95-004, NTIS No.: PB95-193199. July, 1993.
3. “Method 301 - Field Validation of Pollutant Measurement Methods from Various Waste Media,” 40 CFR part 63, appendix A.
4. “Molecular Vibrations; The Theory of Infrared and Raman Vibrational Spectra,” E. Bright Wilson, J.C. Decius, and P.C. Cross, Dover Publications, Inc., 1980. For a less intensive treatment of molecular rotational-vibrational spectra see, for example, “Physical Chemistry,” G.M. Barrow, chapters 12, 13, and 14, McGraw Hill, Inc., 1979.
5. “Fourier Transform Infrared Spectrometry,” Peter R. Griffiths and James de Haseth, Chemical Analysis, 83, 16-25,(1986), P.J. Elving, J.;D. Winefordner and I.M. Kolthoff (ed.), John Wiley and Sons.
6. “Computer-Assisted Quantitative Infrared Spectroscopy,” Gregory L. McClure (ed.), ASTM Special Publication 934 (ASTM), 1987.
7. “Multivariate Least-Squares Methods Applied to the Quantitative Spectral Analysis of Multicomponent Mixtures,” Applied Spectroscopy, 39(10), 73-84, 1985.
| Sample time | Spectrum file name | Background file name | Sample conditioning | Process condition |
|---|---|---|---|---|
| Sample time | Spectrum file | Interferogram | Resolution | Scans | Apodization | Gain | CTS Spectrum |
|---|---|---|---|---|---|---|---|
Addendum to Test Method 320 - Protocol for the Use of Extractive Fourier Transform Infrared (FTIR) Spectrometry for the Analyses of Gaseous Emissions from Stationary Sources
1.0 Introduction
The purpose of this addendum is to set general guidelines for the use of modern FTIR spectroscopic methods for the analysis of gas samples extracted from the effluent of stationary emission sources. This addendum outlines techniques for developing and evaluating such methods and sets basic requirements for reporting and quality assurance procedures.
1.1 Nomenclature
1.1.1 Appendix A to this addendum lists definitions of the symbols and terms used in this Protocol, many of which have been taken directly from American Society for Testing and Materials (ASTM) publication E 131-90a, entitled “Terminology Relating to Molecular Spectroscopy.”
1.1.2 Except in the case of background spectra or where otherwise noted, the term “spectrum” refers to a double-beam spectrum in units of absorbance vs. wavenumber (cm−1).
1.1.3 The term “Study” in this addendum refers to a publication that has been subjected to EPA- or peer-review.
2.0 Applicability and Analytical Principle
2.1 Applicability. This Protocol applies to the determination of compound-specific concentrations in single- and multiple-component gas phase samples using double-beam absorption spectroscopy in the mid-infrared band. It does not specifically address other FTIR applications, such as single-beam spectroscopy, analysis of open-path (non-enclosed) samples, and continuous measurement techniques. If multiple spectrometers, absorption cells, or instrumental linewidths are used in such analyses, each distinct operational configuration of the system must be evaluated separately according to this Protocol.
2.2 Analytical Principle
2.2.1 In the mid-infrared band, most molecules exhibit characteristic gas phase absorption spectra that may be recorded by FTIR systems. Such systems consist of a source of mid-infrared radiation, an interferometer, an enclosed sample cell of known absorption pathlength, an infrared detector, optical elements for the transfer of infrared radiation between components, and gas flow control and measurement components. Adjunct and integral computer systems are used for controlling the instrument, processing the signal, and for performing both Fourier transforms and quantitative analyses of spectral data.
2.2.2 The absorption spectra of pure gases and of mixtures of gases are described by a linear absorbance theory referred to as Beer's Law. Using this law, modern FTIR systems use computerized analytical programs to quantify compounds by comparing the absorption spectra of known (reference) gas samples to the absorption spectrum of the sample gas. Some standard mathematical techniques used for comparisons are classical least squares, inverse least squares, cross-correlation, factor analysis, and partial least squares. Reference A describes several of these techniques, as well as additional techniques, such as differentiation methods, linear baseline corrections, and non-linear absorbance corrections.
3.0 General Principles of Protocol Requirements
The characteristics that distinguish FTIR systems from gas analyzers used in instrumental gas analysis methods (e.g., Methods 6C and 7E of appendix A to part 60 of this chapter) are: (1) Computers are necessary to obtain and analyze data; (2) chemical concentrations can be quantified using previously recorded infrared reference spectra; and (3) analytical assumptions and results, including possible effects of interfering compounds, can be evaluated after the quantitative analysis. The following general principles and requirements of this Protocol are based on these characteristics.
3.1 Verifiability and Reproducibility of Results. Store all data and document data analysis techniques sufficient to allow an independent agent to reproduce the analytical results from the raw interferometric data.
3.2 Transfer of Reference Spectra. To determine whether reference spectra recorded under one set of conditions (e.g., optical bench, instrumental linewidth, absorption pathlength, detector performance, pressure, and temperature) can be used to analyze sample spectra taken under a different set of conditions, quantitatively compare “calibration transfer standards” (CTS) and reference spectra as described in this Protocol.
Note:
The CTS may, but need not, include analytes of interest). To effect this, record the absorption spectra of the CTS (a) immediately before and immediately after recording reference spectra and (b) immediately after recording sample spectra.
3.3 Evaluation of FTIR Analyses. The applicability, accuracy, and precision of FTIR measurements are influenced by a number of interrelated factors, which may be divided into two classes:
3.3.1 Sample-Independent Factors. Examples are system configuration and performance (e.g., detector sensitivity and infrared source output), quality and applicability of reference absorption spectra, and type of mathematical analyses of the spectra. These factors define the fundamental limitations of FTIR measurements for a given system configuration. These limitations may be estimated from evaluations of the system before samples are available. For example, the detection limit for the absorbing compound under a given set of conditions may be estimated from the system noise level and the strength of a particular absorption band. Similarly, the accuracy of measurements may be estimated from the analysis of the reference spectra.
3.3.2 Sample-Dependent Factors. Examples are spectral interferants (e.g., water vapor and CO2) or the overlap of spectral features of different compounds and contamination deposits on reflective surfaces or transmitting windows. To maximize the effectiveness of the mathematical techniques used in spectral analysis, identification of interferants (a standard initial step) and analysis of samples (includes effect of other analytical errors) are necessary. Thus, the Protocol requires post-analysis calculation of measurement concentration uncertainties for the detection of these potential sources of measurement error.
4.0 Pre-Test Preparations and Evaluations
Before testing, demonstrate the suitability of FTIR spectrometry for the desired application according to the procedures of this section.
4.1 Identify Test Requirements. Identify and record the test requirements described in sections 4.1.1 through 4.1.4 of this addendum. These values set the desired or required goals of the proposed analysis; the description of methods for determining whether these goals are actually met during the analysis comprises the majority of this Protocol.
4.1.1 Analytes (specific chemical species) of interest. Label the analytes from i = 1 to I.
4.1.2 Analytical uncertainty limit (AUi). The AUi is the maximum permissible fractional uncertainty of analysis for the i th analyte concentration, expressed as a fraction of the analyte concentration in the sample.
4.1.3 Required detection limit for each analyte (DLi, ppm). The detection limit is the lowest concentration of an analyte for which its overall fractional uncertainty (OFUi) is required to be less than its analytical uncertainty limit (AUi).
4.1.4 Maximum expected concentration of each analyte (CMAXi, ppm).
4.2 Identify Potential Interferants. Considering the chemistry of the process or results of previous studies, identify potential interferants, i.e., the major effluent constituents and any relatively minor effluent constituents that possess either strong absorption characteristics or strong structural similarities to any analyte of interest. Label them 1 through Nj, where the subscript “j” pertains to potential interferants. Estimate the concentrations of these compounds in the effluent (CPOTj, ppm).
4.3 Select and Evaluate the Sampling System. Considering the source, e.g., temperature and pressure profiles, moisture content, analyte characteristics, and particulate concentration), select the equipment for extracting gas samples. Recommended are a particulate filter, heating system to maintain sample temperature above the dew point for all sample constituents at all points within the sampling system (including the filter), and sample conditioning system (e.g., coolers, water-permeable membranes that remove water or other compounds from the sample, and dilution devices) to remove spectral interferants or to protect the sampling and analytical components. Determine the minimum absolute sample system pressure (Pmin, mmHg) and the infrared absorption cell volume (VSS, liter). Select the techniques and/or equipment for the measurement of sample pressures and temperatures.
4.4 Select Spectroscopic System. Select a spectroscopic configuration for the application. Approximate the absorption pathlength (LS′, meter), sample pressure (PS′, kPa), absolute sample temperature TS′, and signal integration period (tSS, seconds) for the analysis. Specify the nominal minimum instrumental linewidth (MIL) of the system. Verify that the fractional error at the approximate values PS′ and TS′ is less than one half the smallest value AUi (see section 4.1.2 of this addendum).
4.5 Select Calibration Transfer Standards (CTS's). Select CTS's that meet the criteria listed in sections 4.5.1, 4.5.2, and 4.5.3 of this addendum.
Note:
It may be necessary to choose preliminary analytical regions (see section 4.7 of this addendum), identify the minimum analyte linewidths, or estimate the system noise level (see section 4.12 of this addendum) before selecting the CTS. More than one compound may be needed to meet the criteria; if so, obtain separate cylinders for each compound.
4.5.1 The central wavenumber position of each analytical region shall lie within 25 percent of the wavenumber position of at least one CTS absorption band.
4.5.2 The absorption bands in section 4.5.1 of this addendum shall exhibit peak absorbances greater than ten times the value RMSEST (see section 4.12 of this addendum) but less than 1.5 absorbance units.
4.5.3 At least one absorption CTS band within the operating range of the FTIR instrument shall have an instrument-independent linewidth no greater than the narrowest analyte absorption band. Perform and document measurements or cite Studies to determine analyte and CTS compound linewidths.
4.5.4 For each analytical region, specify the upper and lower wavenumber positions (FFUm and FFLm, respectively) that bracket the CTS absorption band or bands for the associated analytical region. Specify the wavenumber range, FNU to FNL, containing the absorption band that meets the criterion of section 4.5.3 of this addendum.
4.5.5 Associate, whenever possible, a single set of CTS gas cylinders with a set of reference spectra. Replacement CTS gas cylinders shall contain the same compounds at concentrations within 5 percent of that of the original CTS cylinders; the entire absorption spectra (not individual spectral segments) of the replacement gas shall be scaled by a factor between 0.95 and 1.05 to match the original CTS spectra.
4.6 Prepare Reference Spectra
Note:
Reference spectra are available in a permanent soft copy from the EPA spectral library on the EMTIC (Emission Measurement Technical Information Center) computer bulletin board; they may be used if applicable.
4.6.1 Select the reference absorption pathlength (LR) of the cell.
4.6.2 Obtain or prepare a set of chemical standards for each analyte, potential and known spectral interferants, and CTS. Select the concentrations of the chemical standards to correspond to the top of the desired range.
4.6.2.1 Commercially-Prepared Chemical Standards. Chemical standards for many compounds may be obtained from independent sources, such as a specialty gas manufacturer, chemical company, or commercial laboratory. These standards (accurate to within ±2 percent) shall be prepared according to EPA Traceability Protocol (see Reference D) or shall be traceable to NIST standards. Obtain from the supplier an estimate of the stability of the analyte concentration. Obtain and follow all of the supplier's recommendations for recertifying the analyte concentration.
4.6.2.2 Self-Prepared Chemical Standards. Chemical standards may be prepared by diluting certified commercially prepared chemical gases or pure analytes with ultra-pure carrier (UPC) grade nitrogen according to the barometric and volumetric techniques generally described in Reference A, section A4.6.
4.6.3 Record a set of the absorption spectra of the CTS {R1}, then a set of the reference spectra at two or more concentrations in duplicate over the desired range (the top of the range must be less than 10 times that of the bottom), followed by a second set of CTS spectra {R2}. (If self-prepared standards are used, see section 4.6.5 of this addendum before disposing of any of the standards.) The maximum accepted standard concentration-pathlength product (ASCPP) for each compound shall be higher than the maximum estimated concentration-pathlength products for both analytes and known interferants in the effluent gas. For each analyte, the minimum ASCPP shall be no greater than ten times the concentration-pathlength product of that analyte at its required detection limit.
4.6.4 Permanently store the background and interferograms in digitized form. Document details of the mathematical process for generating the spectra from these interferograms. Record the sample pressure (PR), sample temperature (TR), reference absorption pathlength (LR), and interferogram signal integration period (tSR). Signal integration periods for the background interferograms shall be ≥tSR. Values of PR, LR, and tSR shall not deviate by more than ±1 percent from the time of recording [R1] to that of recording [R2].
4.6.5 If self-prepared chemical standards are employed and spectra of only two concentrations are recorded for one or more compounds, verify the accuracy of the dilution technique by analyzing the prepared standards for those compounds with a secondary (non-FTIR) technique in accordance with sections 4.6.5.1 through 4.6.5.4 of this addendum.
4.6.5.1 Record the response of the secondary technique to each of the four standards prepared.
4.6.5.2 Perform a linear regression of the response values (dependant variable) versus the accepted standard concentration (ASC) values (independent variable), with the regression constrained to pass through the zero-response, zero ASC point.
4.6.5.3 Calculate the average fractional difference between the actual response values and the regression-predicted values (those calculated from the regression line using the four ASC values as the independent variable).
4.6.5.4 If the average fractional difference value calculated in section 4.6.5.3 of this addendum is larger for any compound than the corresponding AUi, the dilution technique is not sufficiently accurate and the reference spectra prepared are not valid for the analysis.
4.7 Select Analytical Regions. Using the general considerations in section 7 of Reference A and the spectral characteristics of the analytes and interferants, select the analytical regions for the application. Label them m = 1 to M. Specify the lower, center and upper wavenumber positions of each analytical region (FLm, FCm, and FUm, respectively). Specify the analytes and interferants which exhibit absorption in each region.
4.8 Determine Fractional Reproducibility Uncertainties. Using appendix E of this addendum, calculate the fractional reproducibility uncertainty for each analyte (FRUi) from a comparison of [R1] and [R2]. If FRUi >AUi for any analyte, the reference spectra generated in accordance with section 4.6 of this addendum are not valid for the application.
4.9 Identify Known Interferants. Using appendix B of this addendum, determine which potential interferants affect the analyte concentration determinations. Relabel these potential interferant as “known” interferants, and designate these compounds from k = 1 to K. Appendix B to this addendum also provides criteria for determining whether the selected analytical regions are suitable.
4.10 Prepare Computerized Analytical Programs
4.10.1 Choose or devise mathematical techniques (e.g, classical least squares, inverse least squares, cross-correlation, and factor analysis) based on equation 4 of Reference A that are appropriate for analyzing spectral data by comparison with reference spectra.
4.10.2 Following the general recommendations of Reference A, prepare a computer program or set of programs that analyzes all of the analytes and known interferants, based on the selected analytical regions (section 4.7 of this addendum) and the prepared reference spectra (section 4.6 of this addendum). Specify the baseline correction technique (e.g., determining the slope and intercept of a linear baseline contribution in each analytical region) for each analytical region, including all relevant wavenumber positions.
4.10.3 Use programs that provide as output [at the reference absorption pathlength (LR), reference gas temperature (TR), and reference gas pressure (PR)] the analyte concentrations, the known interferant concentrations, and the baseline slope and intercept values. If the sample absorption pathlength (LS), sample gas temperature (TS), or sample gas pressure (PS) during the actual sample analyses differ from LR, TR, and PR, use a program or set of programs that applies multiplicative corrections to the derived concentrations to account for these variations, and that provides as output both the corrected and uncorrected values. Include in the report of the analysis (see section 7.0 of this addendum) the details of any transformations applied to the original reference spectra (e.g., differentiation), in such a fashion that all analytical results may be verified by an independent agent from the reference spectra and data spectra alone.
4.11 Determine the Fractional Calibration Uncertainty. Calculate the fractional calibration uncertainty for each analyte (FCUi) according to appendix F of this addendum, and compare these values to the fractional uncertainty limits (AUi; see section 4.1.2 of this addendum). If FCUi >AUi, either the reference spectra or analytical programs for that analyte are unsuitable.
4.12 Verify System Configuration Suitability. Using appendix C of this addendum, measure or obtain estimates of the noise level (RMSEST, absorbance) of the FTIR system. Alternatively, construct the complete spectrometer system and determine the values RMSSm using appendix G of this addendum. Estimate the minimum measurement uncertainty for each analyte (MAUi, ppm) and known interferant (MIUk, ppm) using appendix D of this addendum. Verify that (a) MAUi <(AUi)(DLi), FRUi <AUi, and FCUi <AUi for each analyte and that (b) the CTS chosen meets the requirements listed in sections 4.5.1 through 4.5.5 of this addendum.
5.0 Sampling and Analysis Procedure
5.1 Analysis System Assembly and Leak-Test. Assemble the analysis system. Allow sufficient time for all system components to reach the desired temperature. Then, determine the leak-rate (LR) and leak volume (VL), where VL = LR tSS. Leak volumes shall be ≤4 percent of VSS.
5.2 Verify Instrumental Performance. Measure the noise level of the system in each analytical region using the procedure of appendix G of this addendum. If any noise level is higher than that estimated for the system in section 4.12 of this addendum, repeat the calculations of appendix D of this addendum and verify that the requirements of section 4.12 of this addendum are met; if they are not, adjust or repair the instrument and repeat this section.
5.3 Determine the Sample Absorption Pathlength
Record a background spectrum. Then, fill the absorption cell with CTS at the pressure PR and record a set of CTS spectra [R3]. Store the background and unscaled CTS single beam interferograms and spectra. Using appendix H of this addendum, calculate the sample absorption pathlength (LS) for each analytical region. The values LS shall not differ from the approximated sample pathlength LS′ (see section 4.4 of this addendum) by more than 5 percent.
5.4 Record Sample Spectrum. Connect the sample line to the source. Either evacuate the absorption cell to an absolute pressure below 5 mmHg before extracting a sample from the effluent stream into the absorption cell, or pump at least ten cell volumes of sample through the cell before obtaining a sample. Record the sample pressure PS. Generate the absorbance spectrum of the sample. Store the background and sample single beam interferograms, and document the process by which the absorbance spectra are generated from these data. (If necessary, apply the spectral transformations developed in section 5.6.2 of this addendum). The resulting sample spectrum is referred to below as SS.
Note:
Multiple sample spectra may be recorded according to the procedures of section 5.4 of this addendum before performing sections 5.5 and 5.6 of this addendum.
5.5 Quantify Analyte Concentrations. Calculate the unscaled analyte concentrations RUAi and unscaled interferant concentrations RUIK using the programs developed in section 4 of this addendum. To correct for pathlength and pressure variations between the reference and sample spectra, calculate the scaling factor, RLPS using equation A.1,
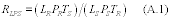
Calculate the final analyte and interferant concentrations RSAi and RSIk using equations A.2 and A.3,
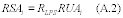
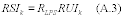
5.6 Determine Fractional Analysis Uncertainty. Fill the absorption cell with CTS at the pressure PS. Record a set of CTS spectra [R4]. Store the background and CTS single beam interferograms. Using appendix H of this addendum, calculate the fractional analysis uncertainty (FAU) for each analytical region. If the FAU indicated for any analytical region is greater than the required accuracy requirements determined in sections 4.1.1 through 4.1.4 of this addendum, then comparisons to previously recorded reference spectra are invalid in that analytical region, and the analyst shall perform one or both of the procedures of sections 5.6.1 through 5.6.2 of this addendum.
5.6.1 Perform instrumental checks and adjust the instrument to restore its performance to acceptable levels. If adjustments are made, repeat sections 5.3, 5.4 (except for the recording of a sample spectrum), and 5.5 of this addendum to demonstrate that acceptable uncertainties are obtained in all analytical regions.
5.6.2 Apply appropriate mathematical transformations (e.g., frequency shifting, zero-filling, apodization, smoothing) to the spectra (or to the interferograms upon which the spectra are based) generated during the performance of the procedures of section 5.3 of this addendum. Document these transformations and their reproducibility. Do not apply multiplicative scaling of the spectra, or any set of transformations that is mathematically equivalent to multiplicative scaling. Different transformations may be applied to different analytical regions. Frequency shifts shall be less than one-half the minimum instrumental linewidth, and must be applied to all spectral data points in an analytical region. The mathematical transformations may be retained for the analysis if they are also applied to the appropriate analytical regions of all sample spectra recorded, and if all original sample spectra are digitally stored. Repeat sections 5.3, 5.4 (except the recording of a sample spectrum), and 5.5 of this addendum to demonstrate that these transformations lead to acceptable calculated concentration uncertainties in all analytical regions.
6.0 Post-Analysis Evaluations
Estimate the overall accuracy of the analyses performed in accordance with sections 5.1 through 5.6 of this addendum using the procedures of sections 6.1 through 6.3 of this addendum.
6.1 Qualitatively Confirm the Assumed Matrix. Examine each analytical region of the sample spectrum for spectral evidence of unexpected or unidentified interferants. If found, identify the interfering compounds (see Reference C for guidance) and add them to the list of known interferants. Repeat the procedures of section 4 of this addendum to include the interferants in the uncertainty calculations and analysis procedures. Verify that the MAU and FCU values do not increase beyond acceptable levels for the application requirements. Re-calculate the analyte concentrations (section 5.5 of this addendum) in the affected analytical regions.
6.2 Quantitatively Evaluate Fractional Model Uncertainty (FMU). Perform the procedures of either section 6.2.1 or 6.2.2 of this addendum:
6.2.1 Using appendix I of this addendum, determine the fractional model error (FMU) for each analyte.
6.2.2 Provide statistically determined uncertainties FMU for each analyte which are equivalent to two standard deviations at the 95 percent confidence level. Such determinations, if employed, must be based on mathematical examinations of the pertinent sample spectra (not the reference spectra alone). Include in the report of the analysis (see section 7.0 of this addendum) a complete description of the determination of the concentration uncertainties.
6.3 Estimate Overall Concentration Uncertainty (OCU). Using appendix J of this addendum, determine the overall concentration uncertainty (OCU) for each analyte. If the OCU is larger than the required accuracy for any analyte, repeat sections 4 and 6 of this addendum.
7.0 Reporting Requirements
[Documentation pertaining to virtually all the procedures of sections 4, 5, and 6 will be required. Software copies of reference spectra and sample spectra will be retained for some minimum time following the actual testing.]
8.0 References
(A) Standard Practices for General Techniques of Infrared Quantitative Analysis (American Society for Testing and Materials, Designation E 168-88).
(B) The Coblentz Society Specifications for Evaluation of Research Quality Analytical Infrared Reference Spectra (Class II); Anal. Chemistry 47, 945A (1975); Appl. Spectroscopy 444, pp. 211-215, 1990.
(C) Standard Practices for General Techniques for Qualitative Infrared Analysis, American Society for Testing and Materials, Designation E 1252-88.
(D) “EPA Traceability Protocol for Assay and Certification of Gaseous Calibration Standards,” U.S. Environmental Protection Agency Publication No. EPA/600/R-93/224, December 1993.
Appendix A to Addendum to Method 320 - Definitions of Terms and Symbols
A.1 Definitions of Terms. All terms used in this method that are not defined below have the meaning given to them in the CAA and in subpart A of this part.
Absorption band means a contiguous wavenumber region of a spectrum (equivalently, a contiguous set of absorbance spectrum data points) in which the absorbance passes through a maximum or a series of maxima.
Absorption pathlength means the distance in a spectrophotometer, measured in the direction of propagation of the beam of radiant energy, between the surface of the specimen on which the radiant energy is incident and the surface of the specimen from which it is emergent.
Analytical region means a contiguous wavenumber region (equivalently, a contiguous set of absorbance spectrum data points) used in the quantitative analysis for one or more analytes.
Note:
The quantitative result for a single analyte may be based on data from more than one analytical region.
Apodization means modification of the ILS function by multiplying the interferogram by a weighing function whose magnitude varies with retardation.
Background spectrum means the single beam spectrum obtained with all system components without sample present.
Baseline means any line drawn on an absorption spectrum to establish a reference point that represents a function of the radiant power incident on a sample at a given wavelength.
Beers's law means the direct proportionality of the absorbance of a compound in a homogeneous sample to its concentration.
Calibration transfer standard (CTS) gas means a gas standard of a compound used to achieve and/or demonstrate suitable quantitative agreement between sample spectra and the reference spectra; see section 4.5.1 of this addendum.
Compound means a substance possessing a distinct, unique molecular structure.
Concentration (c) means the quantity of a compound contained in a unit quantity of sample. The unit “ppm” (number, or mole, basis) is recommended.
Concentration-pathlength product means the mathematical product of concentration of the species and absorption pathlength. For reference spectra, this is a known quantity; for sample spectra, it is the quantity directly determined from Beer's law. The units “centimeters-ppm” or “meters-ppm” are recommended.
Derivative absorption spectrum means a plot of rate of change of absorbance or of any function of absorbance with respect to wavelength or any function of wavelength.
Double beam spectrum means a transmission or absorbance spectrum derived by dividing the sample single beam spectrum by the background spectrum.
Note:
The term “double-beam” is used elsewhere to denote a spectrum in which the sample and background interferograms are collected simultaneously along physically distinct absorption paths. Here, the term denotes a spectrum in which the sample and background interferograms are collected at different times along the same absorption path.
Fast Fourier transform (FFT) means a method of speeding up the computation of a discrete FT by factoring the data into sparse matrices containing mostly zeros.
Flyback means interferometer motion during which no data are recorded.
Fourier transform (FT) means the mathematical process for converting an amplitude-time spectrum to an amplitude-frequency spectrum, or vice versa.
Fourier transform infrared (FTIR) spectrometer means an analytical system that employs a source of mid-infrared radiation, an interferometer, an enclosed sample cell of known absorption pathlength, an infrared detector, optical elements that transfer infrared radiation between components, and a computer system. The time-domain detector response (interferogram) is processed by a Fourier transform to yield a representation of the detector response vs. infrared frequency.
Note:
When FTIR spectrometers are interfaced with other instruments, a slash should be used to denote the interface; e.g., GC/FTIR; HPCL/FTIR, and the use of FTIR should be explicit; i.e., FTIR not IR.
Frequency, v means the number of cycles per unit time.
Infrared means the portion of the electromagnetic spectrum containing wavelengths from approximately 0.78 to 800 microns.
Interferogram, I(σ) means record of the modulated component of the interference signal measured as a function of retardation by the detector.
Interferometer means device that divides a beam of radiant energy into two or more paths, generates an optical path difference between the beams, and recombines them in order to produce repetitive interference maxima and minima as the optical retardation is varied.
Linewidth means the full width at half maximum of an absorption band in units of wavenumbers (cm−1).
Mid-infrared means the region of the electromagnetic spectrum from approximately 400 to 5000 cm−1.
Reference spectra means absorption spectra of gases with known chemical compositions, recorded at a known absorption pathlength, which are used in the quantitative analysis of gas samples.
Retardation, σ means optical path difference between two beams in an interferometer; also known as “optical path difference” or “optical retardation.”
Scan means digital representation of the detector output obtained during one complete motion of the interferometer's moving assembly or assemblies.
Scaling means application of a multiplicative factor to the absorbance values in a spectrum.
Single beam spectrum means Fourier-transformed interferogram, representing the detector response vs. wavenumber.
Note:
The term “single-beam” is used elsewhere to denote any spectrum in which the sample and background interferograms are recorded on the same physical absorption path; such usage differentiates such spectra from those generated using interferograms recorded along two physically distinct absorption paths (see “double-beam spectrum” above). Here, the term applies (for example) to the two spectra used directly in the calculation of transmission and absorbance spectra of a sample.
Standard reference material means a reference material, the composition or properties of which are certified by a recognized standardizing agency or group.
Note:
The equivalent ISO term is “certified reference material.”
Transmittance, T means the ratio of radiant power transmitted by the sample to the radiant power incident on the sample. Estimated in FTIR spectroscopy by forming the ratio of the single-beam sample and background spectra.
Wavenumber, v͞ means the number of waves per unit length.
Note:
The usual unit of wavenumber is the reciprocal centimeter, cm−1. The wavenumber is the reciprocal of the wavelength, λ, when λ is expressed in centimeters.
Zero-filling means the addition of zero-valued points to the end of a measured interferogram.
Note:
Performing the FT of a zero-filled interferogram results in correctly interpolated points in the computed spectrum.
A.2 Definitions of Mathematical Symbols. The symbols used in equations in this protocol are defined as follows:
(1) A, absorbance = the logarithm to the base 10 of the reciprocal of the transmittance (T).
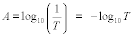
(2) AAIim = band area of the i th analyte in the m th analytical region, at the concentration (CLi) corresponding to the product of its required detection limit (DLi) and analytical uncertainty limit (AUi) .
(3) AAVim = average absorbance of the i th analyte in the m th analytical region, at the concentration (CLi) corresponding to the product of its required detection limit (DLi) and analytical uncertainty limit (AUi).
(4) ASC, accepted standard concentration = the concentration value assigned to a chemical standard.
(5) ASCPP, accepted standard concentration-pathlength product = for a chemical standard, the product of the ASC and the sample absorption pathlength. The units “centimeters-ppm” or “meters-ppm” are recommended.
(6) AUi, analytical uncertainty limit = the maximum permissible fractional uncertainty of analysis for the i th analyte concentration, expressed as a fraction of the analyte concentration determined in the analysis.
(7) AVTm = average estimated total absorbance in the m th analytical region.
(8) CKWNk = estimated concentration of the k th known interferant.
(9) CMAXi = estimated maximum concentration of the i th analyte.
(10) CPOTj = estimated concentration of the j th potential interferant.
(11) DLi, required detection limit = for the i th analyte, the lowest concentration of the analyte for which its overall fractional uncertainty (OFUi) is required to be less than the analytical uncertainty limit (AUi).
(12) FCm = center wavenumber position of the m th analytical region.
(13) FAUi, fractional analytical uncertainty = calculated uncertainty in the measured concentration of the i th analyte because of errors in the mathematical comparison of reference and sample spectra.
(14) FCUi, fractional calibration uncertainty = calculated uncertainty in the measured concentration of the i th analyte because of errors in Beer's law modeling of the reference spectra concentrations.
(15) FFLm = lower wavenumber position of the CTS absorption band associated with the m th analytical region.
(16) FFUm = upper wavenumber position of the CTS absorption band associated with the m th analytical region.
(17) FLm = lower wavenumber position of the m th analytical region.
(18) FMUi, fractional model uncertainty = calculated uncertainty in the measured concentration of the i th analyte because of errors in the absorption model employed.
(19) FNL = lower wavenumber position of the CTS spectrum containing an absorption band at least as narrow as the analyte absorption bands.
(20) FNU = upper wavenumber position of the CTS spectrum containing an absorption band at least as narrow as the analyte absorption bands.
(21) FRUi, fractional reproducibility uncertainty = calculated uncertainty in the measured concentration of the i th analyte because of errors in the reproducibility of spectra from the FTIR system.
(22) FUm = upper wavenumber position of the m th analytical region.
(23) IAIjm = band area of the j th potential interferant in the m th analytical region, at its expected concentration (CPOTj).
(24) IAVim = average absorbance of the i th analyte in the m th analytical region, at its expected concentration (CPOTj).
(25) ISCi or k, indicated standard concentration = the concentration from the computerized analytical program for a single-compound reference spectrum for the i th analyte or k th known interferant.
(26) kPa = kilo-Pascal (see Pascal).
(27) LS′ = estimated sample absorption pathlength.
(28) LR = reference absorption pathlength.
(29) LS = actual sample absorption pathlength.
(30) MAUi = mean of the MAUim over the appropriate analytical regions.
(31) MAUim, minimum analyte uncertainty = the calculated minimum concentration for which the analytical uncertainty limit (AUi) in the measurement of the i th analyte, based on spectral data in the m th analytical region, can be maintained.
(32) MIUj = mean of the MIUjm over the appropriate analytical regions.
(33) MIUjm, minimum interferant uncertainty = the calculated minimum concentration for which the analytical uncertainty limit CPOTj/20 in the measurement of the j th interferant, based on spectral data in the m th analytical region, can be maintained.
(34) MIL, minimum instrumental linewidth = the minimum linewidth from the FTIR system, in wavenumbers.
Note:
The MIL of a system may be determined by observing an absorption band known (through higher resolution examinations) to be narrower than indicated by the system. The MIL is fundamentally limited by the retardation of the interferometer, but is also affected by other operational parameters (e.g., the choice of apodization).
(35) Ni = number of analytes.
(36) Nj = number of potential interferants.
(37) Nk = number of known interferants.
(38) Nscan = the number of scans averaged to obtain an interferogram.
(39) OFUi = the overall fractional uncertainty in an analyte concentration determined in the analysis (OFUi = MAX[FRUi, FCUi, FAUi, FMUi]).
(40) Pascal (Pa) = metric unit of static pressure, equal to one Newton per square meter; one atmosphere is equal to 101,325 Pa; 1/760 atmosphere (one Torr, or one millimeter Hg) is equal to 133.322 Pa.
(41) Pmin = minimum pressure of the sampling system during the sampling procedure.
(42) PS′ = estimated sample pressure.
(43) PR = reference pressure.
(44) PS = actual sample pressure.
(45) RMSSm = measured noise level of the FTIR system in the m th analytical region.
(46) RMSD, root mean square difference = a measure of accuracy determined by the following equation:
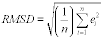
Where:
n = the number of observations for which the accuracy is determined.
ei = the difference between a measured value of a property and its mean value over the n observations.
Note:
The RMSD value “between a set of n contiguous absorbance values (Ai) and the mean of the values” (AM) is defined as
(47) RSAi = the (calculated) final concentration of the i th analyte.
(48) RSIk = the (calculated) final concentration of the k th known interferant.
(49) tscan, scan time = time used to acquire a single scan, not including flyback.
(50) tS, signal integration period = the period of time over which an interferogram is averaged by addition and scaling of individual scans. In terms of the number of scans Nscan and scan time tscan, tS = Nscantscan.
(51) tSR = signal integration period used in recording reference spectra.
(52) tSS = signal integration period used in recording sample spectra.
(53) TR = absolute temperature of gases used in recording reference spectra.
(54) TS = absolute temperature of sample gas as sample spectra are recorded.
(55) TP, Throughput = manufacturer's estimate of the fraction of the total infrared power transmitted by the absorption cell and transfer optics from the interferometer to the detector.
(56) VSS = volume of the infrared absorption cell, including parts of attached tubing.
(57) Wik = weight used to average over analytical regions k for quantities related to the analyte i; see appendix D of this addendum.
Appendix B to Addendum to Method 320 - Identifying Spectral Interferants
B.1 General
B.1.1 Assume a fixed absorption pathlength equal to the value LS′.
B.1.2 Use band area calculations to compare the relative absorption strengths of the analytes and potential interferants. In the m th analytical region (FLm to FUm), use either rectangular or trapezoidal approximations to determine the band areas described below (see Reference A, sections A.3.1 through A.3.3). Document any baseline corrections applied to the spectra.
B.1.3 Use the average total absorbance of the analytes and potential interferants in each analytical region to determine whether the analytical region is suitable for analyte concentration determinations.
Note:
The average absorbance in an analytical region is the band area divided by the width of the analytical region in wavenumbers. The average total absorbance in an analytical region is the sum of the average absorbances of all analytes and potential interferants.
B.2 Calculations
B.2.1 Prepare spectral representations of each analyte at the concentration CLi = (DLi)(AUi), where DLi is the required detection limit and AUi is the maximum permissible analytical uncertainty. For the m th analytical region, calculate the band area (AAIim) and average absorbance (AAVim) from these scaled analyte spectra.
B.2.2 Prepare spectral representations of each potential interferant at its expected concentration (CPOTj). For the m th analytical region, calculate the band area (IAIjm) and average absorbance (IAVjm) from these scaled potential interferant spectra.
B.2.3 Repeat the calculation for each analytical region, and record the band area results in matrix form as indicated in Figure B.1.
B.2.4 If the band area of any potential interferant in an analytical region is greater than the one-half the band area of any analyte (i.e., IAIjm >0.5 AAIim for any pair ij and any m), classify the potential interferant as a known interferant. Label the known interferants k = 1 to K. Record the results in matrix form as indicated in Figure B.2.
B.2.5 Calculate the average total absorbance (AVTm) for each analytical region and record the values in the last row of the matrix described in Figure B.2. Any analytical region where AVTm >2.0 is unsuitable.
Appendix C to Addendum to Method 320 - Estimating Noise Levels
C.1 General
C.1.1 The root-mean-square (RMS) noise level is the standard measure of noise in this addendum. The RMS noise level of a contiguous segment of a spectrum is defined as the RMS difference (RMSD) between the absorbance values which form the segment and the mean value of that segment (see appendix A of this addendum).
C.1.2 The RMS noise value in double-beam absorbance spectra is assumed to be inversely proportional to: (a) the square root of the signal integration period of the sample single beam spectra from which it is formed, and (b) the total infrared power transmitted through the interferometer and absorption cell.
C.1.3 Practically, the assumption of C.1.2 allows the RMS noise level of a complete system to be estimated from the quantities described in sections C.1.3.1 through C.1.3.4:
C.1.3.1 RMSMAN, the noise level of the system (in absorbance units), without the absorption cell and transfer optics, under those conditions necessary to yield the specified minimum instrumental linewidth, e.g., Jacquinot stop size.
C.1.3.2 tMAN, the manufacturer's signal integration time used to determine RMSMAN.
C.1.3.3 tSS, the signal integration time for the analyses.
C.1.3.4 TP, the manufacturer's estimate of the fraction of the total infrared power transmitted by the absorption cell and transfer optics from the interferometer to the detector.
C.2 Calculations
C.2.1 Obtain the values of RMSMAN, tMAN, and TP from the manufacturers of the equipment, or determine the noise level by direct measurements with the completely constructed system proposed in section 4 of this addendum.
C.2.2 Calculate the noise value of the system (RMSEST) using equation C.1.

Appendix D to Addendum to Method 320 - Estimating Minimum Concentration Measurement Uncertainties (MAU and MIU)
D.1 General
Estimate the minimum concentration measurement uncertainties for the i th analyte (MAUi) and j th interferant (MIUj) based on the spectral data in the m th analytical region by comparing the analyte band area in the analytical region (AAIim) and estimating or measuring the noise level of the system (RMSEST or RMSSM).
Note:
For a single analytical region, the MAU or MIU value is the concentration of the analyte or interferant for which the band area is equal to the product of the analytical region width (in wavenumbers) and the noise level of the system (in absorbance units). If data from more than one analytical region are used in the determination of an analyte concentration, the MAU or MIU is the mean of the separate MAU or MIU values calculated for each analytical region.
D.2 Calculations
D.2.1 For each analytical region, set RMS = RMSSM if measured (appendix G of this addendum), or set RMS = RMSEST if estimated (appendix C of this addendum).
D.2.2 For each analyte associated with the analytical region, calculate MAUim using equation D.1,
D.2.3 If only the m th analytical region is used to calculate the concentration of the i th analyte, set MAUi = MAUim.
D.2.4 If more than one analytical region is used to calculate the concentration of the i th analyte, set MAUi equal to the weighted mean of the appropriate MAUim values calculated above; the weight for each term in the mean is equal to the fraction of the total wavenumber range used for the calculation represented by each analytical region. Mathematically, if the set of analytical regions employed is [m′], then the MAU for each analytical region is given by equation D.2.
where the weight Wik is defined for each term in the sum as
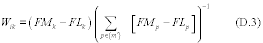
D.2.5 Repeat sections D.2.1 through D.2.4 of this appendix to calculate the analogous values MIUj for the interferants j = 1 to J. Replace the value (AUi) (DLi) in equation D.1 with CPOTj/20; replace the value AAIim in equation D.1 with IAIjm.
Appendix E to Addendum to Method 320 - Determining Fractional Reproducibility Uncertainties (FRU)
E.1 General
To estimate the reproducibility of the spectroscopic results of the system, compare the CTS spectra recorded before and after preparing the reference spectra. Compare the difference between the spectra to their average band area. Perform the calculation for each analytical region on the portions of the CTS spectra associated with that analytical region.
E.2 Calculations
E.2.1 The CTS spectra {R1} consist of N spectra, denoted by S1i, i = 1, N. Similarly, the CTS spectra {R2} consist of N spectra, denoted by S2i, i = 1, N. Each Ski is the spectrum of a single compound, where i denotes the compound and k denotes the set {} of which Ski is a member. Form the spectra S3 according to S3i = S2i−S1i for each i. Form the spectra S4 according to S4i = [S2i + S1i]/2 for each i.
E.2.2 Each analytical region m is associated with a portion of the CTS spectra S2i and S1i, for a particular i, with lower and upper wavenumber limits FFLm and FFUm, respectively.
E.2.3 For each m and the associated i, calculate the band area of S4i in the wavenumber range FFUm to FFLm. Follow the guidelines of section B.1.2 of this addendum for this band area calculation. Denote the result by BAVm.
E.2.4 For each m and the associated i, calculate the RMSD of S3i between the absorbance values and their mean in the wavenumber range FFUm to FFLm. Denote the result by SRMSm.
E.2.5 For each analytical region m, calculate FMm using equation E.1,
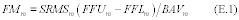
E.2.6 If only the m th analytical region is used to calculate the concentration of the i th analyte, set FRUi = FMm.
E.2.7 If a number pi of analytical regions are used to calculate the concentration of the i th analyte, set FRUi equal to the weighted mean of the appropriate FMm values calculated according to section E.2.5. Mathematically, if the set of analytical regions employed is {m′}, then FRUi is given by equation E.2,
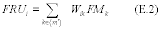
where the Wik are calculated as described in appendix D of this addendum.
Appendix F of Addendum to Method 320 - Determining Fractional Calibration Uncertainties (FCU)
F.1 General
F.1.1 The concentrations yielded by the computerized analytical program applied to each single-compound reference spectrum are defined as the indicated standard concentrations (ISC's). The ISC values for a single compound spectrum should ideally equal the accepted standard concentration (ASC) for one analyte or interferant, and should ideally be zero for all other compounds. Variations from these results are caused by errors in the ASC values, variations from the Beer's law (or modified Beer's law) model used to determine the concentrations, and noise in the spectra. When the first two effects dominate, the systematic nature of the errors is often apparent and the analyst shall take steps to correct them.
F.1.2 When the calibration error appears non-systematic, apply the procedures of sections F.2.1 through F.2.3 of this appendix to estimate the fractional calibration uncertainty (FCU) for each compound. The FCU is defined as the mean fractional error between the ASC and the ISC for all reference spectra with non-zero ASC for that compound. The FCU for each compound shall be less than the required fractional uncertainty specified in section 4.1 of this addendum.
F.1.3 The computerized analytical programs shall also be required to yield acceptably low concentrations for compounds with ISC = 0 when applied to the reference spectra. The ISC of each reference spectrum for each analyte or interferant shall not exceed that compound's minimum measurement uncertainty (MAU or MIU).
F.2 Calculations
F.2.1 Apply each analytical program to each reference spectrum. Prepare a similar table to that in Figure F.1 to present the ISC and ASC values for each analyte and interferant in each reference spectrum. Maintain the order of reference file names and compounds employed in preparing Figure F.1.
F.2.2 For all reference spectra in Figure F.1, verify that the absolute values of the ISC's are less than the compound's MAU (for analytes) or MIU (for interferants).
F.2.3 For each analyte reference spectrum, calculate the quantity (ASC-ISC)/ASC. For each analyte, calculate the mean of these values (the FCUi for the i th analyte) over all reference spectra. Prepare a similar table to that in Figure F.2 to present the FCUi and analytical uncertainty limit (AUi) for each analyte.
| Compound name | Reference spectrum file name | ASC (ppm) | ISC (ppm) | |||||
| Analytes Interferants | ||||||||
| i = 1 I | ||||||||
| j = 1 J | ||||||||
| Analyte name | FCU (%) | AU (%) |
|---|---|---|
Appendix G to Addendum to Method 320 - Measuring Noise Levels
G.1 General
The root-mean-square (RMS) noise level is the standard measure of noise. The RMS noise level of a contiguous segment of a spectrum is the RMSD between the absorbance values that form the segment and the mean value of the segment (see appendix A of this addendum).
G.2 Calculations
G.2.1 Evacuate the absorption cell or fill it with UPC grade nitrogen at approximately one atmosphere total pressure.
G.2.2 Record two single beam spectra of signal integration period tSS.
G.2.3 Form the double beam absorption spectrum from these two single beam spectra, and calculate the noise level RMSSm in the M analytical regions.
Appendix H of Addendum to Method 320 - Determining Sample Absorption Pathlength (LS) and Fractional Analytical Uncertainty (FAU)
H.1 General
Reference spectra recorded at absorption pathlength (LR), gas pressure (PR), and gas absolute temperature (TR) may be used to determine analyte concentrations in samples whose spectra are recorded at conditions different from that of the reference spectra, i.e., at absorption pathlength (LS), absolute temperature (TS), and pressure (PS). This appendix describes the calculations for estimating the fractional uncertainty (FAU) of this practice. It also describes the calculations for determining the sample absorption pathlength from comparison of CTS spectra, and for preparing spectra for further instrumental and procedural checks.
H.1.1 Before sampling, determine the sample absorption pathlength using least squares analysis. Determine the ratio LS/LR by comparing the spectral sets {R1} and {R3}, which are recorded using the same CTS at LS and LR, and TS and TR, but both at PR.
H.1.2 Determine the fractional analysis uncertainty (FAU) for each analyte by comparing a scaled CTS spectral set, recorded at LS, TS, and PS, to the CTS reference spectra of the same gas, recorded at LR, TR, and PR. Perform the quantitative comparison after recording the sample spectra, based on band areas of the spectra in the CTS absorbance band associated with each analyte.
H.2 Calculations
H.2.1 Absorption Pathlength Determination. Perform and document separate linear baseline corrections to each analytical region in the spectral sets {R1} and {R3}. Form a one-dimensional array AR containing the absorbance values from all segments of {R1} that are associated with the analytical regions; the members of the array are ARi, i = 1, n. Form a similar one-dimensional array AS from the absorbance values in the spectral set {R3}; the members of the array are ASi, i = 1, n. Based on the model AS = rAR + E, determine the least-squares estimate of r, the value of r which minimizes the square error E 2. Calculate the sample absorption pathlength, LS, using equation H.1,
H.2.2 Fractional Analysis Uncertainty. Perform and document separate linear baseline corrections to each analytical region in the spectral sets {R1} and {R4}. Form the arrays AS and AR as described in section H.2.1 of this appendix, using values from {R1} to form AR, and values from {R4} to form AS. Calculate NRMSE and IAAV using equations H.2 and H.3,
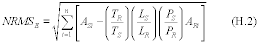
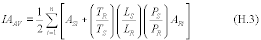
The fractional analytical uncertainty, FAU, is given by equation H.4,
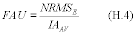
Appendix I to Addendum to Method 320 - Determining Fractional Model Uncertainties (FMU)
I.1 General
To prepare analytical programs for FTIR analyses, the sample constituents must first be assumed. The calculations in this appendix, based upon a simulation of the sample spectrum, shall be used to verify the appropriateness of these assumptions. The simulated spectra consist of the sum of single compound reference spectra scaled to represent their contributions to the sample absorbance spectrum; scaling factors are based on the indicated standard concentrations (ISC) and measured (sample) analyte and interferant concentrations, the sample and reference absorption pathlengths, and the sample and reference gas pressures. No band-shape correction for differences in the temperature of the sample and reference spectra gases is made; such errors are included in the FMU estimate. The actual and simulated sample spectra are quantitatively compared to determine the fractional model uncertainty; this comparison uses the reference spectra band areas and residuals in the difference spectrum formed from the actual and simulated sample spectra.
I.2 Calculations
I.2.1 For each analyte (with scaled concentration RSAi), select a reference spectrum SAi with indicated standard concentration ISCi. Calculate the scaling factors, RAi, using equation I.1,
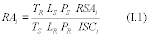
Form the spectra SACi by scaling each SAi by the factor RAi.
I.2.2 For each interferant, select a reference spectrum SIk with indicated standard concentration ISCk. Calculate the scaling factors, RIk, using equation I.2,
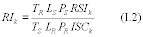
Form the spectra SICk by scaling each SIk by the factor RIk.
I.2.3 For each analytical region, determine by visual inspection which of the spectra SACi and SICk exhibit absorbance bands within the analytical region. Subtract each spectrum SACi and SICk exhibiting absorbance from the sample spectrum SS to form the spectrum SUBS. To save analysis time and to avoid the introduction of unwanted noise into the subtracted spectrum, it is recommended that the calculation be made (1) only for those spectral data points within the analytical regions, and (2) for each analytical region separately using the original spectrum SS.
I.2.4 For each analytical region m, calculate the RMSD of SUBS between the absorbance values and their mean in the region FFUm to FFLm. Denote the result by RMSSm.
I.2.5 For each analyte i, calculate FMm, using equation I.3,
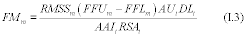
for each analytical region associated with the analyte.
I.2.6 If only the m th analytical region is used to calculate the concentration of the i th analyte, set FMUi = FMm.
I.2.7 If a number of analytical regions are used to calculate the concentration of the i th analyte, set FMi equal to the weighted mean of the appropriate FMm values calculated using equation I-3. Mathematically, if the set of analytical regions employed is {m′}, then the fractional model uncertainty, FMU, is given by equation I.4,
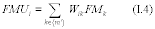
where Wik is calculated as described in appendix D of this addendum.
Appendix J of Addendum to Method 320 - Determining Overall Concentration Uncertainties (OCU)
The calculations in this addendum estimate the measurement uncertainties for various FTIR measurements. The lowest possible overall concentration uncertainty (OCU) for an analyte is its MAU value, which is an estimate of the absolute concentration uncertainty when spectral noise dominates the measurement error. However, if the product of the largest fractional concentration uncertainty (FRU, FCU, FAU, or FMU) and the measured concentration of an analyte exceeds the MAU for the analyte, then the OCU is this product. In mathematical terms, set OFUi = MAX{FRUi, FCUi, FAUi, FMUi} and OCUi = MAX{RSAi*OFUi, MAUi}.
Test Method 321 - Measurement of Gaseous Hydrogen Chloride Emissions At Portland Cement Kilns by Fourier Transform Infrared (FTIR) Spectroscopy
1.0 Introduction
This method should be performed by those persons familiar with the operation of Fourier Transform Infrared (FTIR) instrumentation in the application to source sampling. This document describes the sampling procedures for use in the application of FTIR spectrometry for the determination of vapor phase hydrogen chloride (HCl) concentrations both before and after particulate matter control devices installed at portland cement kilns. A procedure for analyte spiking is included for quality assurance. This method is considered to be self validating provided that the requirements listed in section 9 of this method are followed. The analytical procedures for interpreting infrared spectra from emission measurements are described in the “Protocol For The Use of Extractive Fourier Transform Infrared (FTIR) Spectrometry in Analyses of Gaseous Emissions From Stationary Industrial Sources”, included as an addendum to proposed Method 320 of this appendix (hereafter referred to as the “FTIR Protocol)”. References 1 and 2 describe the use of FTIR spectrometry in field measurements. Sample transport presents the principal difficulty in directly measuring HCl emissions. This identical problem must be overcome by any extractive measurement method. HCl is reactive and water soluble. The sampling system must be adequately designed to prevent sample condensation in the system.
1.1 Scope and Application
This method is specifically designed for the application of FTIR Spectrometry in extractive measurements of gaseous HCl concentrations in portland cement kiln emissions.
1.2 Applicability
This method applies to the measurement of HCl [CAS No. 7647-01-0]. This method can be applied to the determination of HCl concentrations both before and after particulate matter control devices installed at portland cement manufacturing facilities. This method applies to either continuous flow through measurement (with isolated sample analysis) or grab sampling (batch analysis). HCl is measured using the mid-infrared spectral region for analysis (about 400 to 4000 cm−1 or 25 to 2.5 µm). Table 1 lists the suggested analytical region for quantification of HCl taking the interference from water vapor into consideration.
| Compound |
Analytical
region (cm−1) |
Potential
interferants |
|---|---|---|
| Hydrogen chloride | 2679-2840 | Water. |
1.3 Method Range and Sensitivity
1.3.1 The analytical range is determined by the instrumental design and the composition of the gas stream. For practical purposes there is no upper limit to the range because the pathlength may be reduced or the sample may be diluted. The lower detection range depends on (1) the absorption coefficient of the compound in the analytical frequency region, (2) the spectral resolution, (3) the interferometer sampling time, (4) the detector sensitivity and response, and (5) the absorption pathlength.
1.3.2 The practical lower quantification range is usually higher than that indicated by the instrument performance in the laboratory, and is dependent upon (1) the presence of interfering species in the exhaust gas (notably H2O), (2) the optical alignment of the gas cell and transfer optics, and (3) the quality of the reflective surfaces in the cell (cell throughput). Under typical test conditions (moisture content of up to 30 percent, 10 meter absorption path length, liquid nitrogen-cooled IR detector, 0.5 cm−1 resolution, and an interferometer sampling time of 60 seconds) a typical lower quantification range for HCl is 0.1 to 1.0 ppm.
1.4 Data Quality Objectives
1.4.1 In designing or configuring the analytical system, data quality is determined by measuring of the root mean square deviation (RMSD) of the absorbance values within a chosen spectral (analytical) region. The RMSD provides an indication of the signal-to-noise ratio (S/N) of the spectral baseline. Appendix D of the FTIR Protocol (the addendum to Method 320 of this appendix) presents a discussion of the relationship between the RMSD, lower detection limit, DLi, and analytical uncertainty, AUi. It is important to consider the target analyte quantification limit when performing testing with FTIR instrumentation, and to optimize the system to achieve the desired detection limit.
1.4.2 Data quality is determined by measuring the root mean square (RMS) noise level in each analytical spectral region (appendix C of the FTIR Protocol). The RMS noise is defined as the root mean square deviation (RMSD) of the absorbance values in an analytical region from the mean absorbance value in the same region. Appendix D of the FTIR Protocol defines the minimum analyte uncertainty (MAU), and how the RMSD is used to calculate the MAU. The MAUim is the minimum concentration of the ith analyte in the mth analytical region for which the analytical uncertainty limit can be maintained. Table 2 presents example values of AU and MAU using the analytical region presented in Table 1.
| HCl | |
|---|---|
| Reference concentration (ppm-meters)/K | 11.2 |
| Reference Band area | 2.881 |
| DL (ppm-meters)/K | 0.1117 |
| AU | 0.2 |
| CL (DL × AU) | 0.02234 |
| FL (cm−1) | 2679.83 |
| FU (cm−1) | 2840.93 |
| FC (cm−1) | 2760.38 |
| AAI (ppm-meters)/K | 0.06435 |
| RMSD | 2.28E-03 |
| MAU (ppm-meters)/K | 1.28E-01 |
| MAU ppm at 22 meters and 250°F | .0.2284 |
2.0 Summary of Method
2.1 Principle
See Method 320 of this appendix. HCl can also undergo rotation transitions by absorbing energy in the far-infrared spectral region. The rotational transitions are superimposed on the vibrational fundamental to give a series of lines centered at the fundamental vibrational frequency, 2885 cm- 1. The frequencies of absorbance and the pattern of rotational/vibrational lines are unique to HCl. When this distinct pattern is observed in an infrared spectrum of an unknown sample, it unequivocally identifies HCl as a component of the mixture. The infrared spectrum of HCl is very distinctive and cannot be confused with the spectrum of any other compound. See Reference 6.
2.2 Sampling and Analysis. See Method 320 of this appendix.
2.3 Operator Requirements. The analyst must have knowledge of spectral patterns to choose an appropriate absorption path length or determine if sample dilution is necessary. The analyst should also understand FTIR instrument operation well enough to choose instrument settings that are consistent with the objectives of the analysis.
3.0 Definitions
See appendix A of the FTIR Protocol.
4.0 Interferences
This method will not measure HCl under conditions: (1) where the sample gas stream can condense in the sampling system or the instrumentation, or (2) where a high moisture content sample relative to the analyte concentrations imparts spectral interference due to the water vapor absorbance bands. For measuring HCl the first (sampling) consideration is more critical. Spectral interference from water vapor is not a significant problem except at very high moisture levels and low HCl concentrations.
4.1 Analytical Interferences. See Method 320 of this appendix.
4.1.1 Background Interferences. See Method 320 of this appendix.
4.1.2 Spectral interferences. Water vapor can present spectral interference for FTIR gas analysis of HCl. Therefore, the water vapor in the spectra of kiln gas samples must be accounted for. This means preparing at least one spectrum of a water vapor sample where the moisture concentration is close to that in the kiln gas.
4.2 Sampling System Interferences. The principal sampling system interferant for measuring HCl is water vapor. Steps must be taken to ensure that no condensation forms anywhere in the probe assembly, sample lines, or analytical instrumentation. Cold spots anywhere in the sampling system must be avoided. The extent of sampling system bias in the FTIR analysis of HCl depends on concentrations of potential interferants, moisture content of the gas stream, temperature of the gas stream, temperature of sampling system components, sample flow rate, and reactivity of HCl with other species in the gas stream (e.g., ammonia). For measuring HCl in a wet gas stream the temperatures of the gas stream, sampling components, and the sample flow rate are of primary importance. Analyte spiking with HCl is performed to demonstrate the integrity of the sampling system for transporting HCl vapor in the flue gas to the FTIR instrument. See section 9 of this method for a complete description of analyte spiking.
5.0 Safety
5.1 Hydrogen chloride vapor is corrosive and can cause irritation or severe damage to respiratory system, eyes and skin. Exposure to this compound should be avoided.
5.2 This method may involve sampling at locations having high positive or negative pressures, or high concentrations of hazardous or toxic pollutants, and can not address all safety problems encountered under these diverse sampling conditions. It is the responsibility of the tester(s) to ensure proper safety and health practices, and to determine the applicability of regulatory limitations before performing this test method. Leak-check procedures are outlined in section 8.2 of Method 320 of this appendix.
6.0 Equipment and Supplies
Note:
Mention of trade names or specific products does not constitute endorsement by the Environmental Protection Agency.
6.1 FTIR Spectrometer and Detector. An FTIR Spectrometer system (interferometer, transfer optics, gas cell and detector) having the capability of measuring HCl to the predetermined minimum detectable level required (see section 4.1.3 of the FTIR Protocol). The system must also include an accurate means to control and/or measure the temperature of the FTIR gas analysis cell, and a personal computer with compatible software that provides real-time updates of the spectral profile during sample and spectral collection.
6.2 Pump. Capable of evacuating the FTIR cell volume to 1 Torr (133.3 Pascals) within two minutes (for batch sample analysis).
6.3 Mass Flow Meters/Controllers. To accurately measure analyte spike flow rate, having the appropriate calibrated range and a stated accuracy of ±2 percent of the absolute measurement value. This device must be calibrated with the major component of the calibration/spike gas (e.g., nitrogen) using an NIST traceable bubble meter or equivalent. Single point calibration checks should be performed daily in the field. When spiking HCl, the mass flow meter/controller should be thoroughly purged before and after introduction of the gas to prevent corrosion of the interior parts.
6.4 Polytetrafluoroethane tubing. Diameter and length suitable to connect cylinder regulators.
6.5 Stainless Steel tubing. Type 316 of appropriate length and diameter for heated connections.
6.6 Gas Regulators. Purgeable HCl regulator.
6.7 Pressure Gauge. Capable of measuring pressure from 0 to 1000 Torr (133.3 Pa = 1 Torr) within ±5 percent.
6.8 Sampling Probe. Glass, stainless steel or other appropriate material of sufficient length and physical integrity to sustain heating, prevent adsorption of analytes and capable of reaching gas sampling point.
6.9 Sampling Line. Heated 180°C (360°F) and fabricated of either stainless steel, polytetrafluoroethane or other material that prevents adsorption of HCl and transports effluent to analytical instrumentation. The extractive sample line must have the capability to transport sample gas to the analytical components as well as direct heated calibration spike gas to the calibration assembly located at the sample probe. It is important to minimize the length of heated sample line.
6.10 Particulate Filters. A sintered stainless steel filter rated at 20 microns or greater may be placed at the inlet of the probe (for removal of large particulate matter). A heated filter (Balston or equivalent) rated at 1 micron is necessary for primary particulate matter removal, and shall be placed immediately after the heated probe. The filter/filter holder temperature should be maintained at 180°C (360°F).
6.11 Calibration/Analyte Spike Assembly. A heated three-way valve assembly (or equivalent) to introduce surrogate spikes into the sampling system at the outlet of the probe before the primary particulate filter.
6.12 Sample Extraction Pump. A leak-free heated head pump (KNF Neuberger or equivalent) capable of extracting sample effluent through entire sampling system at a rate which prevents analyte losses and minimizes analyzer response time. The pump should have a heated by-pass and may be placed either before the FTIR instrument or after. If the sample pump is located upstream of the FTIR instrument, it must be fabricated from materials non-reactive to HCl. The sampling system and FTIR measurement system shall allow the operator to obtain at least six sample spectra during a one-hour period.
6.13 Barometer. For measurement of barometric pressure.
6.14 Gas Sample Manifold. A distribution manifold having the capabilities listed in sections 6.14.1 through 6.14.4;
6.14.1 Delivery of calibration gas directly to the analytical instrumentation;
6.14.2 Delivery of calibration gas to the sample probe (system calibration or analyte spike) via a heated traced sample line;
6.14.3 Delivery of sample gas (kiln gas, spiked kiln gas, or system calibrations) to the analytical instrumentation;
6.14.4 Delivery (optional) of a humidified nitrogen sample stream.
6.15 Flow Measurement Device. Type S Pitot tube (or equivalent) and Magnahelic set for measurement of volumetric flow rate.
7.0 Reagents and Standards
HCl can be purchased in a standard compressed gas cylinder. The most stable HCl cylinder mixture available has a concentration certified at ±5 percent. Such a cylinder is suitable for performing analyte spiking because it will provide reproducible samples. The stability of the cylinder can be monitored over time by periodically performing direct FTIR analysis of cylinder samples. It is recommended that a 10-50 ppm cylinder of HCl be prepared having from 2-5 ppm SF6 as a tracer compound. (See sections 7.1 through 7.3 of Method 320 of this appendix for a complete description of the use of existing HCl reference spectra. See section 9.1 of Method 320 of this appendix for a complete discussion of standard concentration selection.)
8.0 Sample Collection, Preservation and Storage
See also Method 320 of this appendix.
8.1 Pretest. A screening test is ideal for obtaining proper data that can be used for preparing analytical program files. Information from literature surveys and source personnel is also acceptable. Information about the sampling location and gas stream composition is required to determine the optimum sampling system configuration for measuring HCl. Determine the percent moisture of the kiln gas by Method 4 of appendix A to part 60 of this chapter or by performing a wet bulb/dry bulb measurement. Perform a preliminary traverse of the sample duct or stack and select the sampling point(s). Acquire an initial spectrum and determine the optimum operational pathlength of the instrument.
8.2 Leak-Check. See Method 320 of this appendix, section 8.2 for direction on performing leak-checks.
8.3 Background Spectrum. See Method 320 of this appendix, section 8.5 for direction in background spectral acquisition.
8.4 Pre-Test Calibration Transfer Standard (Direct Instrument Calibration). See Method 320 of this appendix, section 8.3 for direction in CTS spectral acquisition.
8.5 Pre-Test System Calibration. See Method 320 of this appendix, sections 8.6.1 through 8.6.2 for direction in performing system calibration.
8.6 Sampling
8.6.1 Extractive System. An extractive system maintained at 180°C (360°F) or higher which is capable of directing a total flow of at least 12 L/min to the sample cell is required (References 1 and 2). Insert the probe into the duct or stack at a point representing the average volumetric flow rate and 25 percent of the cross sectional area. Co-locate an appropriate flow monitoring device with the sample probe so that the flow rate is recorded at specified time intervals during emission testing (e.g., differential pressure measurements taken every 10 minutes during each run).
8.6.2 Batch Samples. Evacuate the absorbance cell to 5 Torr (or less) absolute pressure before taking first sample. Fill the cell with kiln gas to ambient pressure and record the infrared spectrum, then evacuate the cell until there is no further evidence of infrared absorption. Repeat this procedure, collecting a total of six separate sample spectra within a 1-hour period.
8.6.3 Continuous Flow Through Sampling. Purge the FTIR cell with kiln gas for a time period sufficient to equilibrate the entire sampling system and FTIR gas cell. The time required is a function of the mechanical response time of the system (determined by performing the system calibration with the CTS gas or equivalent), and by the chemical reactivity of the target analytes. If the effluent target analyte concentration is not variable, observation of the spectral up-date of the flowing gas sample should be performed until equilibration of the sample is achieved. Isolate the gas cell from the sample flow by directing the purge flow to vent. Record the spectrum and pressure of the sample gas. After spectral acquisition, allow the sample gas to purge the cell with at least three volumes of kiln gas. The time required to adequately purge the cell with the required volume of gas is a function of (1) cell volume, (2) flow rate through the cell, and (3) cell design. It is important that the gas introduction and vent for the FTIR cell provides a complete purge through the cell.
8.6.4 Continuous Sampling. In some cases it is possible to collect spectra continuously while the FTIR cell is purged with sample gas. The sample integration time, tss, the sample flow rate through the gas cell, and the sample integration time must be chosen so that the collected data consist of at least 10 spectra with each spectrum being of a separate cell volume of flue gas. Sampling in this manner may only be performed if the native source analyte concentrations do not affect the test results.
8.7 Sample Conditioning
8.7.1 High Moisture Sampling. Kiln gas emitted from wet process cement kilns may contain 3- to 40 percent moisture. Zinc selenide windows or the equivalent should be used when attempting to analyze hot/wet kiln gas under these conditions to prevent dissolution of water soluble window materials (e.g., KBr).
8.7.2 Sample Dilution. The sample may be diluted using an in-stack dilution probe, or an external dilution device provided that the sample is not diluted below the instrument's quantification range. As an alternative to using a dilution probe, nitrogen may be dynamically spiked into the effluent stream in the same manner as analyte spiking. A constant dilution rate shall be maintained throughout the measurement process. It is critical to measure and verify the exact dilution ratio when using a dilution probe or the nitrogen spiking approach. Calibrating the system with a calibration gas containing an appropriate tracer compound will allow determination of the dilution ratio for most measurement systems. The tester shall specify the procedures used to determine the dilution ratio, and include these calibration results in the report.
8.8 Sampling QA, Data Storage and Reporting. See the FTIR Protocol. Sample integration times shall be sufficient to achieve the required signal-to-noise ratio, and all sample spectra should have unique file names. Two copies of sample interferograms and processed spectra will be stored on separate computer media. For each sample spectrum the analyst must document the sampling conditions, the sampling time (while the cell was being filled), the time the spectrum was recorded, the instrumental conditions (path length, temperature, pressure, resolution, integration time), and the spectral file name. A hard copy of these data must be maintained until the test results are accepted.
8.9 Signal Transmittance. Monitor the signal transmittance through the instrumental system. If signal transmittance (relative to the background) drops below 95 percent in any spectral region where the sample does not absorb infrared energy, then a new background spectrum must be obtained.
8.10 Post-test CTS. After the sampling run completion, record the CTS spectrum. Analysis of the spectral band area used for quantification from pre- and post-test CTS spectra should agree to within ±5 percent or corrective action must be taken.
8.11 Post-test QA. The sample spectra shall be inspected immediately after the run to verify that the gas matrix composition was close to the assumed gas matrix, (this is necessary to account for the concentrations of the interferants for use in the analytical analysis programs), and to confirm that the sampling and instrumental parameters were appropriate for the conditions encountered.
9.0 Quality Control
Use analyte spiking to verify the effectiveness of the sampling system for the target compounds in the actual kiln gas matrix. QA spiking shall be performed before and after each sample run. QA spiking shall be performed after the pre- and post-test CTS direct and system calibrations. The system biases calculated from the pre- and post-test dynamic analyte spiking shall be within ±30 percent for the spiked surrogate analytes for the measurements to be considered valid. See sections 9.3.1 through 9.3.2 for the requisite calculations. Measurement of the undiluted spike (direct-to-cell measurement) involves sending dry, spike gas to the FTIR cell, filling the cell to 1 atmosphere and obtaining the spectrum of this sample. The direct-to-cell measurement should be performed before each analyte spike so that the recovery of the dynamically spiked analytes may be calculated. Analyte spiking is only effective for assessing the integrity of the sampling system when the concentration of HCl in the source does not vary substantially. Any attempt to quantify an analyte recovery in a variable concentration matrix will result in errors in the expected concentration of the spiked sample. If the kiln gas target analyte concentrations vary by more than ±5 percent (or 5 ppm, whichever is greater) in the time required to acquire a sample spectrum, it may be necessary to: (1) Use a dual sample probe approach, (2) use two independent FTIR measurement systems, (3) use alternate QA/QC procedures, or (4) postpone testing until stable emission concentrations are achieved. (See section 9.2.3 of this method). It is recommended that a laboratory evaluation be performed before attempting to employ this method under actual field conditions. The laboratory evaluation shall include (1) performance of all applicable calculations in section 4 of the FTIR Protocol; (2) simulated analyte spiking experiments in dry (ambient) and humidified sample matrices using HCl; and (3) performance of bias (recovery) calculations from analyte spiking experiments. It is not necessary to perform a laboratory evaluation before every field test. The purpose of the laboratory study is to demonstrate that the actual instrument and sampling system configuration used in field testing meets the requirements set forth in this method.
9.1 Spike Materials. Perform analyte spiking with an HCl standard to demonstrate the integrity of the sampling system.
9.1.1 An HCl standard of approximately 50 ppm in a balance of ultra pure nitrogen is recommended. The SF6 (tracer) concentration shall be 2 to 5 ppm depending upon the measurement pathlength. The spike ratio (spike flow/total flow) shall be no greater than 1:10, and an ideal spike concentration should approximate the native effluent concentration.
9.1.2 The ideal spike concentration may not be achieved because the target concentration cannot be accurately predicted prior to the field test, and limited calibration standards will be available during testing. Therefore, practical constraints must be applied that allow the tester to spike at an anticipated concentration. For these tests, the analyte concentration contributed by the HCl standard spike should be 1 to 5 ppm or should more closely approximate the native concentration if it is greater.
9.2 Spike Procedure
9.2.1 A spiking/sampling apparatus is shown in Figure 2. Introduce the spike/tracer gas mixture at a constant flow (±2 percent) rate at approximately 10 percent of the total sample flow. (For example, introduce the surrogate spike at 1 L/min 20 cc/min, into a total sample flow rate of 10 L/min). The spike must be pre-heated before introduction into the sample matrix to prevent a localized condensation of the gas stream at the spike introduction point. A heated sample transport line(s) containing multiple transport tubes within the heated bundle may be used to spike gas up through the sampling system to the spike introduction point. Use a calibrated flow device (e.g., mass flow meter/controller), to monitor the spike flow as indicated by a calibrated flow meter or controller, or alternately, the SF6 tracer ratio may be calculated from the direct measurement and the diluted measurement. It is often desirable to use the tracer approach in calculating the spike/total flow ratio because of the difficulty in accurately measuring hot/wet total flow. The tracer technique has been successfully used in past validation efforts (Reference 1).
9.2.2 Perform a direct-to-cell measurement of the dry, undiluted spike gas. Introduce the spike directly to the FTIR cell, bypassing the sampling system. Fill cell to 1 atmosphere and collect the spectrum of this sample. Ensure that the spike gas has equilibrated to the temperature of the measurement cell before acquisition of the spectra. Inspect the spectrum and verify that the gas is dry and contains negligible CO2. Repeat the process to obtain a second direct-to-cell measurement. Analysis of spectral band areas for HCl from these duplicate measurements should agree to within ±5 percent of the mean.
9.2.3 Analyte Spiking. Determine whether the kiln gas contains native concentrations of HCl by examination of preliminary spectra. Determine whether the concentration varies significantly with time by observing a continuously up-dated spectrum of sample gas in the flow-through sampling mode. If the concentration varies by more than ±5 percent during the period of time required to acquire a spectra, then an alternate approach should be used. One alternate approach uses two sampling lines to convey sample to the gas distribution manifold. One of the sample lines is used to continuously extract unspiked kiln gas from the source. The other sample line serves as the analyte spike line. One FTIR system can be used in this arrangement. Spiked or unspiked sample gas may be directed to the FTIR system from the gas distribution manifold, with the need to purge only the components between the manifold and the FTIR system. This approach minimizes the time required to acquire an equilibrated sample of spiked or unspiked kiln gas. If the source varies by more than ±5 percent (or 5 ppm, whichever is greater) in the time it takes to switch from the unspiked sample line to the spiked sample line, then analyte spiking may not be a feasible means to determine the effectiveness of the sampling system for the HCl in the sample matrix. A second alternative is to use two completely independent FTIR measurement systems. One system would measure unspiked samples while the other system would measure the spiked samples. As a last option, (where no other alternatives can be used) a humidified nitrogen stream may be generated in the field which approximates the moisture content of the kiln gas. Analyte spiking into this humidified stream can be employed to assure that the sampling system is adequate for transporting the HCl to the FTIR instrumentation.
9.2.3.1 Adjust the spike flow rate to approximately 10 percent of the total flow by metering spike gas through a calibrated mass flowmeter or controller. Allow spike flow to equilibrate within the sampling system before analyzing the first spiked kiln gas samples. A minimum of two consecutive spikes are required. Analysis of the spectral band area used for quantification should agree to within ±5 percent or corrective action must be taken.
9.2.3.2 After QA spiking is completed, the sampling system components shall be purged with nitrogen or dry air to eliminate traces of the HCl compound from the sampling system components. Acquire a sample spectra of the nitrogen purge to verify the absence of the calibration mixture.
9.2.3.3 Analyte spiking procedures must be carefully executed to ensure that meaningful measurements are achieved. The requirements of sections 9.2.3.3.1 through 9.2.3.3.4 shall be met.
9.2.3.3.1 The spike must be in the vapor phase, dry, and heated to (or above) the kiln gas temperature before it is introduced to the kiln gas stream.
9.2.3.3.2 The spike flow rate must be constant and accurately measured.
9.2.3.3.3 The total flow must also be measured continuously and reliably or the dilution ratio must otherwise be verified before and after a run by introducing a spike of a non-reactive, stable compound (i.e., tracer).
9.2.3.3.4 The tracer must be inert to the sampling system components, not contained in the effluent gas, and readily detected by the analytical instrumentation. Sulfur hexafluoride (SF6) has been used successfully (References 1 and 2) for this purpose.
9.3 Calculations
9.3.1 Recovery. Calculate the percent recovery of the spiked analytes using equations 1 and 2.
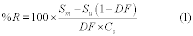
Sm = Mean concentration of the analyte spiked effluent samples (observed).
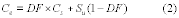
Ce = Expected concentration of the spiked samples (theoretical).
DF = Dilution Factor (Total flow/Spike flow). Total flow = spike flow plus effluent flow.
Cs = cylinder concentration of spike gas.
Su = native concentration of analytes in unspiked samples.
The spike dilution factor may be confirmed by measuring the total flow and the spike flow directly. Alternately, the spike dilution can be verified by comparing the concentration of the tracer compound in the spiked samples (diluted) to the tracer concentration in the direct (undiluted) measurement of the spike gas.
If SF6 is the tracer gas, then
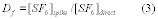
[SF6]spike = the diluted SF6 concentration measured in a spiked sample.
[SF6]direct = the SF6 concentration measured directly.
9.3.2 Bias. The bias may be determined by the difference between the observed spike value and the expected response (i.e., the equivalent concentration of the spiked material plus the analyte concentration adjusted for spike dilution). Bias is defined by section 6.3.1 of EPA Method 301 of this appendix (Reference 8) as,
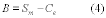
Where:
B = Bias at spike level.
Sm = Mean concentration of the analyte spiked samples.
Ce = Expected concentration of the analyte in spiked samples.
Acceptable recoveries for analyte spiking are ±30 percent. Application of correction factors to the data based upon bias and recovery calculations is subject to the approval of the Administrator.
10.0 Calibration and Standardization
10.1 Calibration transfer standards (CTS). The EPA Traceability Protocol gases or NIST traceable standards, with a minimum accuracy of ±2 percent shall be used. For other requirements of the CTS, see the FTIR Protocol section 4.5.
10.2 Signal-to-Noise Ratio (S/N). The S/N shall be less than the minimum acceptable measurement uncertainty in the analytical regions to be used for measuring HCl.
10.3 Absorbance Pathlength. Verify the absorbance path length by comparing CTS spectra to reference spectra of the calibration gas(es).
10.4 Instrument Resolution. Measure the line width of appropriate CTS band(s) to verify instrumental resolution.
10.5 Apodization Function. Choose the appropriate apodization function. Determine any appropriate mathematical transformations that are required to correct instrumental errors by measuring the CTS. Any mathematical transformations must be documented and reproducible. Reference 9 provides additional information about FTIR instrumentation.
11.0 Analytical Procedure
A full description of the analytical procedures is given in sections 4.6-4.11, sections 5, 6, and 7, and the appendices of the FTIR Protocol. Additional description of quantitative spectral analysis is provided in References 10 and 11.
12.0 Data Analysis and Calculations
Data analysis is performed using appropriate reference spectra whose concentrations can be verified using CTS spectra. Various analytical programs (References 10 and 11) are available to relate sample absorbance to a concentration standard. Calculated concentrations should be verified by analyzing spectral baselines after mathematically subtracting scaled reference spectra from the sample spectra. A full description of the data analysis and calculations may be found in the FTIR Protocol (sections 4.0, 5.0, 6.0 and appendices).
12.1 Calculated concentrations in sample spectra are corrected for differences in absorption pathlength between the reference and sample spectra by
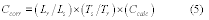
Where:
Ccorr = The pathlength corrected concentration.
Ccalc = The initial calculated concentration (output of the multicomponent analysis program designed for the compound).
Lr = The pathlength associated with the reference spectra.
Ls = The pathlength associated with the sample spectra.
Ts = The absolute temperature (K) of the sample gas.
Tr = The absolute temperature (K) at which reference spectra were recorded.
12.2 The temperature correction in equation 5 is a volumetric correction. It does not account for temperature dependence of rotational-vibrational relative line intensities. Whenever possible, the reference spectra used in the analysis should be collected at a temperature near the temperature of the FTIR cell used in the test to minimize the calculated error in the measurement (FTIR Protocol, appendix D). Additionally, the analytical region chosen for the analysis should be sufficiently broad to minimize errors caused by small differences in relative line intensities between reference spectra and the sample spectra.
13.0 Method Performance
A description of the method performance may be found in the FTIR Protocol. This method is self validating provided the results meet the performance specification of the QA spike in sections 9.0 through 9.3 of this method.
14.0 Pollution Prevention
This is a gas phase measurement. Gas is extracted from the source, analyzed by the instrumentation, and discharged through the instrument vent.
15.0 Waste Management
Gas standards of HCl are handled according to the instructions enclosed with the material safety data sheet.
16.0 References
1. “Laboratory and Field Evaluation of a Methodology for Determination of Hydrogen Chloride Emissions From Municipal and Hazardous Waste Incinerators,” S.C. Steinsberger and J.H. Margeson. Prepared for U.S. Environmental Protection Agency, Research Triangle Park, NC. NTIS Report No. PB89-220586. (1989).
2. “Evaluation of HCl Measurement Techniques at Municipal and Hazardous Waste Incinerators,” S.A. Shanklin, S.C. Steinsberger, and L. Cone, Entropy, Inc. Prepared for U.S. Environmental Protection Agency, Research Triangle Park, NC. NTIS Report No. PB90-221896. (1989).
3. “Fourier Transform Infrared (FTIR) Method Validation at a Coal Fired-Boiler,” Entropy, Inc. Prepared for U.S. Environmental Protection Agency, Research Triangle Park, NC. EPA Publication No. EPA-454/R95-004. NTIS Report No. PB95-193199. (1993).
4. “Field Validation Test Using Fourier Transform Infrared (FTIR) Spectrometry To Measure Formaldehyde, Phenol and Methanol at a Wool Fiberglass Production Facility.” Draft. U.S. Environmental Protection Agency Report, Entropy, Inc., EPA Contract No. 68D20163, Work Assignment I-32.
5. Kinner, L.L., Geyer, T.G., Plummer, G.W., Dunder, T.A., Entropy, Inc. “Application of FTIR as a Continuous Emission Monitoring System.” Presentation at 1994 International Incineration Conference, Houston, TX. May 10, 1994.
6. “Molecular Vibrations; The Theory of Infrared and Raman Vibrational Spectra,” E. Bright Wilson, J.C. Decius, and P.C. Cross, Dover Publications, Inc., 1980. For a less intensive treatment of molecular rotational-vibrational spectra see, for example, “Physical Chemistry,” G.M. Barrow, chapters 12, 13, and 14, McGraw Hill, Inc., 1979.
7. “Laboratory and Field Evaluations of Ammonium Chloride Interference in Method 26,” U.S. Environmental Protection Agency Report, Entropy, Inc., EPA Contract No. 68D20163, Work Assignment No. I-45.
8. 40 CFR 63, appendix A. Method 301 - Field Validation of Pollutant Measurement Methods from Various Waste Media.
9. “Fourier Transform Infrared Spectrometry,” Peter R. Griffiths and James de Haseth, Chemical Analysis, 83, 16-25, (1986), P.J. Elving, J.D. Winefordner and I.M. Kolthoff (ed.), John Wiley and Sons.
10. “Computer-Assisted Quantitative Infrared Spectroscopy,” Gregory L. McClure (ed.), ASTM Special Publication 934 (ASTM), 1987.
11. “Multivariate Least-Squares Methods Applied to the Quantitative Spectral Analysis of Multicomponent Mixtures,” Applied Spectroscopy, 39(10), 73-84, 1985.
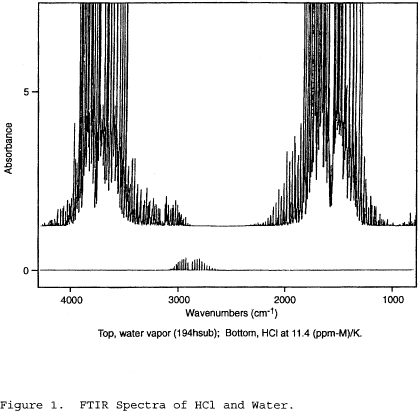
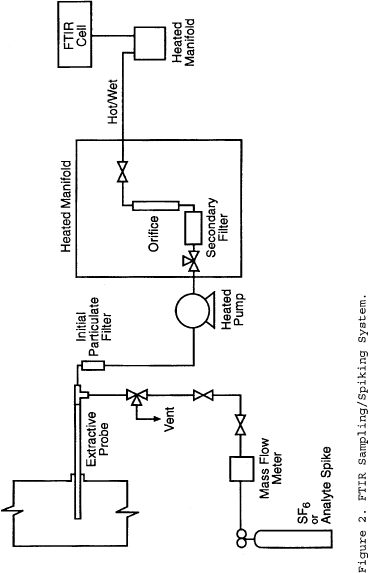
Method 323 - Measurement of Formaldehyde Emissions From Natural Gas-Fired Stationary Sources—Acetyl Acetone Derivatization Method
1.0 Introduction. This method describes the sampling and analysis procedures of the acetyl acetone colorimetric method for measuring formaldehyde emissions in the exhaust of natural gas-fired, stationary combustion sources. This method, which was prepared by the Gas Research Institute (GRI), is based on the Chilled Impinger Train Method for Methanol, Acetone, Acetaldehyde, Methyl Ethyl Ketone, and Formaldehyde (Technical Bulletin No. 684) developed and published by the National Council of the Paper Industry for Air and Stream Improvement, Inc. (NCASI). However, this method has been prepared specifically for formaldehyde and does not include specifications (e.g., equipment and supplies) and procedures (e.g., sampling and analytical) for methanol, acetone, acetaldehyde, and methyl ethyl ketone. To obtain reliable results, persons using this method should have a thorough knowledge of at least Methods 1 and 2 of 40 CFR part 60, appendix A-1; Method 3 of 40 CFR part 60, appendix A-2; and Method 4 of 40 CFR part 60, appendix A-3.
1.1 Scope and Application
1.1.1 Analytes. The only analyte measured by this method is formaldehyde (CAS Number 50-00-0).
1.1.2 Applicability. This method is for analyzing formaldehyde emissions from uncontrolled and controlled natural gas-fired, stationary combustion sources.
1.1.3 Data Quality Objectives. If you adhere to the quality control and quality assurance requirements of this method, then you and future users of your data will be able to assess the quality of the data you obtain and estimate the uncertainty in the measurements.
2.0 Summary of Method. An emission sample from the combustion exhaust is drawn through a midget impinger train containing chilled reagent water to absorb formaldehyde. The formaldehyde concentration in the impinger is determined by reaction with acetyl acetone to form a colored derivative which is measured colorimetrically.
3.0 Definitions
[Reserved]
4.0 Interferences. The presence of acetaldehyde, amines, polymers of formaldehyde, periodate, and sulfites can cause interferences with the acetyl acetone procedure which is used to determine the formaldehyde concentration. However, based on experience gained from extensive testing of natural gas-fired combustion sources using FTIR to measure a variety of compounds, GRI expects only acetaldehyde to be potentially present when combusting natural gas. Acetaldehyde has been reported to be a significant interference only when present at concentrations above 50 ppmv. However, GRI reports that the concentration of acetaldehyde from gas-fired sources is very low (typically below the FTIR detection limit of around 0.5 ppmv); therefore, the potential positive bias due to acetaldehyde interference is expected to be negligible.
5.0 Safety
5.1 Prior to applying the method in the field, a site-specific Health and Safety Plan should be prepared. General safety precautions include the use of steel-toed boots, safety glasses, hard hats, and work gloves. In certain cases, facility policy may require the use of fire-resistant clothing while on-site. Since the method involves testing at high-temperature sampling locations, precautions must be taken to limit the potential for exposure to high-temperature gases and surfaces while inserting or removing the sample probe. In warm locations, precautions must also be taken to avoid dehydration.
5.2 Potential chemical hazards associated with sampling include formaldehyde, nitrogen oxides (NOX), and carbon monoxide (CO). Formalin solution, used for field spiking, is an aqueous solution containing formaldehyde and methanol. Formaldehyde is a skin, eye, and respiratory irritant and a carcinogen, and should be handled accordingly. Eye and skin contact and inhalation of formaldehyde vapors should be avoided. Natural gas-fired combustion sources can potentially emit CO at toxic concentrations. Care should be taken to minimize exposure to the sample gas while inserting or removing the sample probe. If the work area is enclosed, personal CO monitors should be used to insure that the concentration of CO in the work area is maintained at safe levels.
5.3 Potential chemical hazards associated with the analytical procedures include acetyl acetone and glacial acetic acid. Acetyl acetone is an irritant to the skin and respiratory system, as well as being moderately toxic. Glacial acetic acid is highly corrosive and is an irritant to the skin, eyes, and respiratory system. Eye and skin contact and inhalation of vapors should be avoided. Acetyl acetone and glacial acetic acid have flash points of 41°C (105.8°F) and 43°C (109.4°F), respectively. Exposure to heat or flame should be avoided.
6.0 Equipment and Supplies
6.1 Sampling Probe. Quartz glass probe with stainless steel sheath or stainless steel probe.
6.2 Teflon Tubing. Teflon tubing to connect the sample probe to the impinger train. A heated sample line is not needed since the sample transfer system is rinsed to recover condensed formaldehyde and the rinsate combined with the impinger contents prior to sample analysis.
6.3 Midget Impingers. Three midget impingers are required for sample collection. The first impinger serves as a moisture knockout, the second impinger contains 20 mL of reagent water, and the third impinger contains silica gel to remove residual moisture from the sample prior to the dry gas meter.
6.4 Vacuum Pump. Vacuum pump capable of delivering a controlled extraction flow rate between 0.2 and 0.4 L/min.
6.5 Flow Measurement Device. A rotameter or other flow measurement device is required to indicate consistent sample flow.
6.6 Dry Gas Meter. A dry gas meter is used to measure the total sample volume collected. The dry gas meter must be sufficiently accurate to measure the sample volume to within 2 percent, calibrated at the selected flow rate and conditions actually encountered during sampling, and equipped with a temperature sensor (dial thermometer, or equivalent) capable of measuring temperature accurately to within 3°C (5.4°F).
6.7 Spectrophotometer. A spectrophotometer is required for formaldehyde analysis, and must be capable of measuring absorbance at 412 nm.
7.0 Reagents and Standards
7.1 Sampling Reagents
7.1.1 Reagent water. Deionized, distilled, organic-free water. This water is used as the capture solution, for rinsing the sample probe, sample line, and impingers at the completion of the sampling run, in reagent dilutions, and in blanks.
7.1.2 Ice. Ice is necessary to pack around the impingers during sampling in order to keep the impingers cold. Ice is also needed for sample transport and storage.
7.2 Analysis
7.2.1 Acetyl acetone Reagent. Prepare the acetyl acetone reagent by dissolving 15.4 g of ammonium acetate in 50 mL of reagent water in a 100-mL volumetric flask. To this solution, add 0.20 mL of acetyl acetone and 0.30 mL of glacial acetic acid. Mix the solution thoroughly, then dilute to 100 mL with reagent water. The solution can be stored in a brown glass bottle in the refrigerator, and is stable for at least two weeks.
7.2.2 Formaldehyde. Reagent grade.
7.2.3 Ammonium Acetate
7.2.4 Glacial Acetic Acid
8.0 Sample Collection, Preservation, Storage, and Transport
8.1 Pre-test
8.1.1 Collect information about the site characteristics such as exhaust pipe diameter, gas flow rates, port location, access to ports, and safety requirements during a pre-test site survey. You should then decide the sample collection period per run and the target sample flow rate based on your best estimate of the formaldehyde concentration likely to be present. You want to assure that sufficient formaldehyde is captured in the impinger solution so that it can be measured precisely by the spectrophotometer. You may use Equation 323-1 to design your test program. As a guideline for optimum performance, if you can, design your test so that the liquid concentration (Cl) is approximately 10 times the assumed spectrophotometer detection limit of 0.2 µg/mL. However, since actual detection limits are instrument specific, we also suggest that you confirm that the laboratory equipment can meet or exceed this detection limit.
8.1.2 Prepare and then weigh the midget impingers prior to configuring the sampling train. The first impinger is initially dry. The second impinger contains 20 mL of reagent water, and the third impinger contains silica gel that is added before weighing the impinger. Each prepared impinger is weighed and the pre-sampling weight is recorded to the nearest 0.5 gm.
8.1.3 Assemble the sampling train (see Figure 1). Ice is packed around the impingers in order to keep them cold during sample collection. A small amount of water may be added to the ice to improve thermal transfer.
8.1.4 Perform a sampling system leak check (from the probe tip to the pump outlet) as follows: Connect a rotameter to the outlet of the pump. Close off the inlet to the probe and observe the leak rate. The leak rate must be less than 2 percent of the planned sampling rate of 0.2 or 0.4 L/min.
8.1.5 Source gas temperature and static pressure should also be considered prior to field sampling to ensure adequate safety precautions during sampling.
8.2 Sample Collection
8.2.1 Set the sample flow rate between 0.2-0.4 L/min, depending upon the anticipated concentration of formaldehyde in the engine exhaust. (You may have to refer to published data for anticipated concentration levels - see References 5 and 6.) If no information is available for the anticipated levels of formaldehyde, use the higher sampling rate of 0.4 L/min.
8.2.2 Record the sampling flow rate every 5 to 10 minutes during the sample collection period.
Note:
It is critical that you do not sample at a flow rate higher than 0.4 L/min. Sampling at higher flow rates may reduce formaldehyde collection efficiency resulting in measured formaldehyde concentrations that are less than the actual concentrations.
8.2.3 Monitor the amount of ice surrounding the impingers and add ice as necessary to maintain the proper impinger temperature. Remove excess water as needed to maintain an adequate amount of ice.
8.2.4 Record measured leak rate, beginning and ending times and dry gas meter readings for each sampling run, impinger weights before and after sampling, and sampling flow rates and dry gas meter exhaust temperature every 5 to 10 minutes during the run, in a signed and dated notebook.
8.2.5 If possible, monitor and record the fuel flow rate to the engine and the exhaust oxygen concentration during the sampling period. This data can be used to estimate the engine exhaust flow rate based on the Method 19 approach. This approach, if accurate fuel flow rates can be determined, is preferred for reciprocating IC engine exhaust flow rate estimation due to the pulsating nature of the engine exhaust. The F-Factor procedures described in Method 19 may be used based on measurement of fuel flow rate and exhaust oxygen concentration. One example equation is Equation 323-2.
8.3 Post-test. Perform a sampling system leak-check (from the probe tip to pump outlet). Connect a rotameter to the outlet of the pump. Close off the inlet to the probe and observe the leak rate. The leak rate must be less than 2 percent of the sampling rate. Weigh and record each impinger immediately after sampling to determine the moisture weight gain. The impinger weights are measured before transferring the impinger contents, and before rinsing the sample probe and sample line. The moisture content of the exhaust gas is determined by measuring the weight gain of the impinger solutions and volume of gas sampled as described in Method 4. Rinse the sample probe and sample line with reagent water. Transfer the impinger catch to an amber 40-mL VOA bottle with a Teflon-lined cap. If there is a small amount of liquid in the dropout impinger (<10 mL), the impinger catches can be combined in one 40 mL VOA bottle. If there is a larger amount of liquid in the dropout impinger, use a larger VOA bottle to combine the impinger catches. Rinse the impingers and combine the rinsings from the sample probe, sample line, and impingers with the impinger catch. In general, combined rinse volumes should not exceed 10 mL. However, in cases where a long, flexible extension line must be used to connect the sample probe to the sample box, sufficient water must be used to rinse the connecting line to insure that any sample that may have collected there is recovered. The volume of the rinses during sample recovery should not be excessive as this may result in your having to use a larger VOA bottle. This in turn would raise the detection limit of the method since after combining the rinses with the impinger catches in the VOA bottle, the bottle should be filled with reagent water to eliminate the headspace in the sample vial. Keep the sample bottles over ice until analyzed on-site or received at the laboratory. Samples should be analyzed as soon as possible to minimize possible sample degradation. Based on a limited number of previous analyses, samples held in refrigerated conditions showed some sample degradation over time.
8.4 Quality Control Samples
8.4.1 Field Duplicates. During at least one run, a pair of samples should be collected concurrently and analyzed as separate samples. Results of the field duplicate samples should be identified and reported with the sample results. The percent difference in exhaust (stack) concentration indicated by field duplicates should be within 20 percent of their mean concentration. Data are to be flagged as suspect if the duplicates do not meet the acceptance criteria.
8.4.2 Spiked Samples. An aliquot of one sample from each source sample set should be spiked at 2 to 3 times the formaldehyde level found in the unspiked sample. It is also recommended that a second aliquot of the same sample be spiked at around half the level of the first spike; however, the second spike is not mandatory. The results are acceptable if the measured spike recovery is 80 to 120 percent. Use Equation 323-4. Data are to be flagged as suspect if the spike recovery do not meet the acceptance criteria.
8.4.3 Field Blank. A field blank consisting of reagent water placed in a clean impinger train, taken to the test site but not sampled, then recovered and analyzed in the same manner as the other samples, should be collected with each set of source samples. The field blank results should be less than 50 percent of the lowest calibration standard used in the sample analysis. If this criteria is not met, the data should be flagged as suspect.
9.0 Quality Control
| QA/QC | Acceptance | Frequency | Corrective action |
|---|---|---|---|
| Leak-check - Sections 8.1.4, 8.3 | <2% of Sampling rate | Pre- and Post-sampling |
Pre-sampling: Repair leak and recheck
Post-sampling: Flag data and repeat run if for regulatory compliance. |
| Sample flow rate | Between 0.2 and 0.4 L/min | Throughout sampling | Adjust. |
| VOA vial headspace | No headspace | After sample recovery | Flag data. |
| Sample preservation | Maintain on ice | After sample recovery | Flag data. |
| Sample hold time | 14 day maximum | After sample recovery | Flag data. |
| Field Duplicates - Section 8.4.1 | Within 20% of mean of original and duplicate sample | One duplicate per source sample set | Flag data. |
| Spiked Sample - Section 8.4.2 | Recovery between 80 and 120% | One spike per source sample set | Flag data. |
| Field Blank - Section 8.4.3 | <50% of the lowest calibration standard | One blank per source sample set | Flag data. |
| Calibration Linearity - Section 10.1 | Correlation coefficient of 0.99 or higher | Per source sample set | Repeat calibration procedures. |
| Calibration Check Standard - Section 10.3 | Within 10% of theoretical value | One calibration check per source sample set | Repeat check, remake standard and repeat, repeat calibration. |
| Lab Duplicates - Section 11.2.1 | Within 10% of mean of original and duplicate sample analysis | One duplicate per 10 samples | Flag data. |
| Analytical Blanks - Section 11.2.2 | <50% of the lowest calibration standard | One blank per source sample set | Clean glassware/analytical equipment and repeat. |
10.0 Calibration and Standardization
10.1 Spectrophotometer Calibration. Prepare a stock solution of 10 µg/mL formaldehyde. Prepare a series of calibration standards from the stock solution corresponding to 0.0, 0.5, 1.5, 3.5, 5.0, and 7.5 µg/mL formaldehyde. Mix 2.0 ml of each calibration standard with 2.0 mL of acetyl acetone reagent in screw cap vials, thoroughly mix the solution, and place the vials in a water bath (or heating block) at 60 °C for 10 minutes. Remove the vials and allow to cool to room temperature. Transfer each solution to a cuvette and measure the absorbance at 412 nm using the spectrophotometer. Develop a calibration curve (response vs. concentration) from the analytical results of these standards. The acceptance criteria for the spectrophotometer calibration is a correlation coefficient of 0.99 or higher. If this criterion is not met, the calibration procedures should be repeated.
10.2 Spectrophotometer Zero. The spectrophotometer should be zeroed with reagent water when analyzing each set of samples.
10.3 Calibration Checks. Calibration checks consisting of analyzing a mid-range standard separately prepared with each batch of samples. The calibration check standard must be prepared independent of the calibration stock solution. The result of the check standard must be within 10 percent of the theoretical value to be acceptable. If the acceptance criteria are not met, the standard must be reanalyzed. If still unacceptable, a new calibration curve must be prepared using freshly prepared standards.
11.0 Analytical Procedure
11.1 Sample Analysis. A 2.0-mL aliquot of the impinger catch/rinsate is transferred to a screw-capped vial. Two mL of the acetyl acetone reagent are added and the solution is thoroughly mixed. Once mixed, the vial is placed in a water bath (or heating block) at 60°C for 10 minutes. Remove the vial and allow to cool to room temperature. Transfer the solution to a cuvette and measure the absorbance using the spectrophotometer at 412 nm. The quantity of formaldehyde present is determined by comparing the sample response to the calibration curve. Use Equation 323-5. If the sample response is out of the calibration range, the sample must be diluted and reanalyzed. Such dilutions must be performed on another aliquot of the original sample before the addition of the acetyl acetone reagent. The full procedure is repeated with the diluted sample.
11.2 Analytical Quality Control
11.2.1 Laboratory Duplicates. Two aliquots of one sample from each source sample set should be prepared and analyzed (with a minimum of one pair of aliquots for every 10 samples). The percent difference between aliquot analysis should be within 10 percent of their mean. Use Equation 323-3. Data are flagged if the laboratory duplicates do not meet this criteria.
11.2.2 Analytical blanks. Blank samples (reagent water) should be incorporated into each sample set to evaluate the possible presence of any cross-contamination. The acceptance criteria for the analytical blank is less than 50 percent of the lowest calibration standard. If the analytical blank does not meet this criteria, the glassware/analytical equipment should be cleaned and the analytical blank repeated.
12.0 Calculations and Data Analysis
12.1 Nomenclature
A = measured absorbance of 2 mL aliquot
B = estimated sampling rate, Lpm
b = the intercept of the calibration curve at zero concentration.
Cl = target concentration in liquid, µg/mL
D = estimated stack formaldehyde concentration (ppmv)
E = estimated liquid volume, normally 40 mL (the size of the VOA used)
cform = formaldehyde concentration in gas stream, ppmvd
cform @15%02 = formaldehyde concentration in gas stream corrected to 15% oxygen, ppmvd
Csm = measured concentration of formaldehyde in the spiked aliquot
Cu = measured concentration of formaldehyde in the unspiked aliquot of the same sample
Cs = calculated concentration of formaldehyde spiking solution added to the spiked aliquot
F = dilution factor, 1 unless dilution of the sample was needed to reduce the absorbance into the calibration range
Fd = dry basis F-factor from Method 19, dscf per million btu GCVg = Gross calorific value (or higher heating value), btu per scf
K c = spectrophotometer calibration factor, slope of the least square regression line, absorbance/(µg/mL) ( Note: Most spreadsheets are capable of calculating a least squares line, including slope, intercept, and correlation coefficient).
K1 = 0.3855°K/mm Hg for metric units, (17.65°R/in.Hg for English units.)
MW = molecular weight, 30 g/g-mole, for formaldehyde 24.05 = mole specific volume constant, liters per g-mole
m = mass of formaldehyde in liquid sample, mg
Pstd = Standard pressure, 760 mm Hg (29.92 in.Hg)
Pbar = Barometric pressure, mm Hg (in.Hg)
PD = Percent Difference
Qe = exhaust flow rate, dscf per minute
Qg = natural gas fuel flow rate, scf per minute
Tm = Average DGM absolute temperature,°K (°R).
Tstd = Standard absolute temperature, 293°K (528°R).
t = sample time (minutes)
Vm = Dry gas volume as measured by the DGM, dcm (dcf).
Vm(std) = Dry gas volume measured by the DGM, corrected to standard conditions of 1 atmosphere and 20°C, dscm (dscf).
Vt = actual total volume of impinger catch/rinsate, mL
Va = volume (2.0) of aliquot analyzed, mL
X1 = first value
X2 = second value
O2d = oxygen concentration measured, percent by volume, dry basis
%R = percent recovery of spike
Zu = volume fraction of unspiked (native) sample contained in the final spiked aliquot [e.g., Vu/(Vu + Vs), where Vu + Vs should = 2.0 mL]
Zs = volume fraction of spike solution contained in the final spiked aliquot [e.g., Vs/(Vu + Vs)]
R = 0.02405 dscm per g-mole, for metric units at standard conditions of 1 atmosphere and 20°C
Y = Dry Gas Meter calibration factor
12.6 Mass of Formaldehyde in Liquid Sample
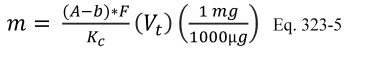
12.2 Pretest Design

12.3 Exhaust Flow Rate

12.4 Percent Difference - (Applicable to Field and Lab Duplicates)

12.5 Percent Recovery of Spike

12.6 Mass of Formaldehyde in Liquid Sample
12.7 Dry Gas Sample Volume Corrected to Standard Conditions

12.8 Formaldehyde Concentration in gas Stream

12.9 Formaldehyde Concentration Corrected to 15% Oxygen
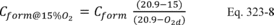
13.0 Method Performance
13.1 Precision. Based on a Method 301 validation using quad train arrangement with post sampling spiking study of the method at a natural gas-fired IC engine, the relative standard deviation of six pairs of unspiked samples was 11.2 percent at a mean stack gas concentration of 16.7 ppmvd.
13.2 Bias. No bias correction is allowed. The single Method 301 validation study of the method at a natural gas-fired IC engine, indicated a bias correction factor of 0.91 for that set of data. An earlier spiking study got similar average percent spike recovery when spiking into a blank sample. This data set is too limited to justify using a bias correction factor for future tests at other sources.
13.3 Range. The range of this method for formaldehyde is 0.2 to 7.5 µg/mL in the liquid phase. (This corresponds to a range of 0.27 to 10 ppmv in the engine exhaust if sampling at a rate of 0.4 Lpm for 60 minutes and using a 40-mL VOA bottle.) If the liquid sample concentration is above this range, perform the appropriate dilution for accurate measurement. Any dilutions must be taken from new aliquots of the original sample before reanalysis.
13.4 Sample Stability. Based on a sample stability study conducted in conjunction with the method validation, sample degradation for 7- and 14-day hold times does not exceed 2.3 and 4.6 percent, respectively, based on a 95 percent level of confidence. Therefore, the recommended maximum sample holding time for the underivatized impinger catch/rinsings is 14 days, where projected sample degradation is below 5 percent.
14.0 Pollution Prevention
Sample gas from the combustion source exhaust is vented to the atmosphere after passing through the chilled impinger sampling train. Reagent solutions and samples should be collected for disposal as aqueous waste.
15.0 Waste Management
Standards of formaldehyde and the analytical reagents should be handled according to the Material Safety Data Sheets.
16.0 References
1. National Council of the Paper Industry for Air and Stream Improvement, Inc. “Volatile Organic Emissions from Pulp and Paper Mill Sources, Part X - Test Methods, Quality Assurance/Quality Control Procedures, and Data Analysis Protocols.” Technical Bulletin No. 684, December 1994.
2. National Council of the Paper Industry for Air and Stream Improvement, Inc., “Field Validation of a Source Sampling Method for Formaldehyde, Methanol, and Phenol at Wood Products Mills.” 1997 TAPPI International Environmental Conference.
3. Roy F. Weston, Inc. “Formaldehyde Sampling Method Field Evaluation and Emission Test Report for Georgia-Pacific Resins, Inc., Russellville, South Carolina.” August 1996.
4. Hoechst Celanese Method CL 8-4. “Standard Test Method for Free Formaldehyde in Air Using Acetyl Acetone.” Revision 0, September 1986.
5. Shareef, G.S., et al. “Measurement of Air Toxic Emissions from Natural Gas-Fired Internal Combustion Engines at Natural Gas Transmission and Storage Facilities.” Report No. GRI-96/0009.1, Gas Research Institute, Chicago, Illinois, February 1996.
6. Gundappa, M., et al. “Characteristics of Formaldehyde Emissions from Natural Gas-Fired Reciprocating Internal Combustion Engines in Gas Transmission. Volume I: Phase I Predictive Model for Estimating Formaldehyde Emissions from 2-Stroke Engines.” Report No. GRI-97/0376.1, Gas Research Institute, Chicago, Illinois, September 1997.
17.0 Tables, Diagrams, Flowcharts, and Validation Data
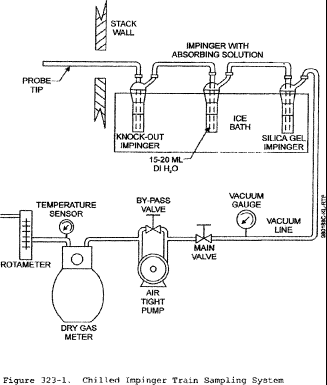
Method 325A - Volatile Organic Compounds from Fugitive and Area Sources:
Sampler Deployment and VOC Sample Collection
1.0 Scope and Application
1.1 This method describes collection of volatile organic compounds (VOCs) at or inside a facility property boundary or from fugitive and area emission sources using passive (diffusive) tube samplers (PS). The concentration of airborne VOCs at or near these potential fugitive- or area-emission sources may be determined using this method in combination with Method 325B. Companion Method 325B (Sampler Preparation and Analysis) describes preparation of sampling tubes, shipment and storage of exposed sampling tubes, and analysis of sampling tubes collected using either this passive sampling procedure or alternative active (pumped) sampling methods.
1.2 This method may be used to determine the average concentration of the select VOCs using the corresponding uptake rates listed in Method 325B, Table 12.1. Additional compounds or alternative sorbents must be evaluated as described in Addendum A of Method 325B or by one of the following national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14), or reported in the peer-reviewed open literature.
1.3 Methods 325A and 325B are valid for the measurement of benzene. Supporting literature (References 1-8) indicates that benzene can be measured by flame ionization detection or mass spectrometry over a concentration range of approximately 0.5 micrograms per cubic meter (µg/m 3) to at least 500 µg/m 3 when industry standard (3.5 inch long × 0.25 inch outside diameter (o.d.) × 5 mm inner diameter (i.d.)) inert-coated stainless steel sorbent tubes packed with Carbograph TM 1 TD, Carbopack TM B, or Carbopack TM X or equivalent are used and when samples are accumulated over a period of 14 days.
1.4 This method may be applied to screening average airborne VOC concentrations at facility property boundaries or monitoring perimeters over an extended period of time using multiple sampling periods (e.g., 26 × 14-day sampling periods). The duration of each sampling period is normally 14 days.
1.5 This method requires the collection of local meteorological data (wind speed and direction, temperature, and barometric pressure). Although local meteorology is a component of this method, non-regulatory applications of this method may use regional meteorological data. Such applications risk that the results may not identify the precise source of the emissions.
2.0 Summary of the Method
2.1 Principle of the Method
The diffusive passive sampler collects VOC from air for a measured time period at a rate that is proportional to the concentration of vapor in the air at that location.
2.1.1 This method describes the deployment of prepared passive samplers, including determination of the number of passive samplers needed for each survey and placement of samplers along or inside the facility property boundary depending on the size and shape of the site or linear length of the boundary.
2.1.2 The rate of sampling is specific to each compound and depends on the diffusion constants of that VOC and the sampler dimensions/characteristics as determined by prior calibration in a standard atmosphere (Reference 1).
2.1.3 The gaseous VOC target compounds migrate through a constant diffusion barrier (e.g., an air gap of fixed dimensions) at the sampling end of the diffusion sampling tube and adsorb onto the sorbent.
2.1.4 Heat and a flow of inert carrier gas are then used to extract (desorb) the retained VOCs back from the sampling end of the tube and transport/transfer them to a gas chromatograph (GC) equipped with a chromatographic column to separate the VOCs and a detector to determine the quantity of target VOCs.
2.1.5 Gaseous or liquid calibration standards loaded onto the sampling ends of clean sorbent tubes must be used to calibrate the analytical equipment.
2.1.6 This method requires the use of field blanks to ensure sample integrity associated with shipment, collection, and storage of the passive samples. It also requires the use of field duplicates to validate the sampling process.
2.1.7 At the end of each sampling period, the passive samples are collected, sealed, and shipped to a laboratory for analysis of target VOCs by thermal desorption gas chromatography, as described in Method 325B.
2.2 Application of Diffusive Sampling
2.2.1 This method requires deployment of passive sampling tubes on a monitoring perimeter encompassing all known emission sources at a facility and collection of local meteorological data. It may be used to determine average concentration of VOC at a facility's “fenceline” using time integrated passive sampling (Reference 2).
2.2.2 Collecting samples and meteorological data at progressively higher frequencies may be employed to resolve shorter term concentration fluctuations and wind conditions that could introduce interfering emissions from other sources.
2.2.3 This passive sampling method provides a low cost approach to screening of fugitive or area emissions compared to active sampling methods that are based on pumped sorbent tubes or time weighted average canister sampling.
2.2.3.1 Additional passive sampling tubes may be deployed at different distances from the facility property boundary or from the geometric center of the fugitive emission source.
2.2.3.2 Additional meteorological measurements may also be collected as needed to perform preliminary gradient-based assessment of the extent of the pollution plume at ground level and the effect of “background” sources contributing to airborne VOC concentrations at the location.
2.2.4 Time-resolved concentration measurements coupled with time-resolved meteorological monitoring may be used to generate data needed for source apportionment procedures and mass flux calculations.
3.0 Definitions
(See also Section 3.0 of Method 325B.)
3.1 Fenceline means the property boundary of a facility or internal monitoring perimeter established in accordance with the requirements in Section 8.2 of this method.
3.2 Passive sampler (PS) means a specific type of sorbent tube (defined in this method) that has a fixed dimension air (diffusion) gap at the sampling end and is sealed at the other end.
3.3 Passive sampling refers to the activity of quantitatively collecting VOC on sorbent tubes using the process of diffusion.
3.4 PSi is the annual average for all PS concentration results from location i.
3.5 PSi3 is the set of annual average concentration results for PSi and two sorbent tubes nearest to the PS location i.
3.6 PSip is the concentration from the sorbent tube at location i for the test period or episode p.
3.7 Sampling period is the length of time each passive sampler is exposed during field monitoring. The sampling period for this method is 14 days.
3.8 Sorbent tube (Also referred to as tube, PS tube, adsorbent tube, and sampling tube) is an inert coated stainless steel tube. Standard PS tube dimensions for this method are 3.5-inch (89 mm) long × 0.25-inch (6.4 mm) o.d. with an i.d. of 5 mm, a cross-sectional area of 19.6 mm 2 and an air gap of 15 mm. The central portion of the tube is packed with solid adsorbent material contained between 2 × 100-mesh stainless steel gauzes and terminated with a diffusion cap at the sampling end of the tube. These axial passive samplers are installed under a protective hood during field deployment.
Note:
Glass and glass- (or fused silica-) lined stainless steel sorbent tubes (typically 4 mm i.d.) are also available in various lengths to suit different makes of thermal desorption equipment, but these are rarely used for passive sampling because it is more difficult to adequately define the diffusive air gap in glass or glass-line tubing. Such tubes are not recommended for this method.
4.0 Sampling Interferences
4.1 General Interferences
Passive tube samplers should be sited at a distance beyond the influence of possible obstructions such as trees, walls, or buildings at the monitoring site. Complex topography and physical site obstructions, such as bodies of water, hills, buildings, and other structures that may prevent access to a planned PS location must be taken into consideration. You must document and report siting interference with the results of this method.
4.2 Background Interference
Nearby or upwind sources of target emissions outside the facility being tested can contribute to background concentrations. Moreover, because passive samplers measure continuously, changes in wind direction can cause variation in the level of background concentrations from interfering sources during the monitoring period. This is why local meteorological information, particularly wind direction and speed, is required to be collected throughout the monitoring period. Interfering sources can include neighboring industrial facilities, transportation facilities, fueling operations, combustion sources, short-term transient sources, residential sources, and nearby highways or roads. As PS data are evaluated, the location of potential interferences with respect to PS locations and local wind conditions should be considered, especially when high PS concentration values are observed.
4.3 Tube Handling
You must protect the PS tubes from gross external contamination during field sampling. Analytical thermal desorption equipment used to analyze PS tubes must desorb organic compounds from the interior of PS tubes and exclude contamination from external sampler surfaces in the analytical/sample flow path. If the analytical equipment does not comply with this requirement, you must wear clean, white, cotton or powder-free nitrile gloves to handle sampling tubes to prevent contamination of the external sampler surfaces. Sampling tubes must be capped with two-piece, brass, 0.25 inch, long-term storage caps fitted with combined polytetrafluoroethylene ferrules (see Section 6.1 and Method 325B) to prevent ingress of airborne contaminants outside the sampling period. When not being used for field monitoring, the capped tubes must be stored in a clean, air-tight, shipping container to prevent the collection of VOCs (see Section 6.4.2 of Method 325B).
4.4 Local Weather Conditions and Airborne Particulates
Although air speeds are a constraint for many forms of passive samplers, axial tube PS devices have such a slow inherent uptake rate that they are largely immune to these effects (References 4,5). Passive samplers must nevertheless be deployed under non-emitting weatherproof hoods to moderate the effect of local weather conditions such as solar heating and rain. The cover must not impede the ingress of ambient air. Sampling tubes should also be orientated vertically and pointing downwards, to minimize accumulation of particulates.
4.5 Temperature
The normal working range for field sampling for sorbent packing is 0-40°C (References 6,7). Note that most published passive uptake rate data for sorbent tubes is quoted at 20°C. Note also that, as a rough guide, an increase in temperature of 10°C will reduce the collection capacity for a given analyte on a given sorbent packing by a factor of 2, but the uptake rate will not change significantly (Reference 4).
5.0 Safety
This method does not purport to include all safety issues or procedures needed when deploying or collecting passive sampling tubes. Precautions typical of field air sampling projects are required. Tripping, falling, electrical, and weather safety considerations must all be included in plans to deploy and collect passive sampling tubes.
6.0 Sampling Equipment and Supplies, and Pre-Deployment Planning
This section describes the equipment and supplies needed to deploy passive sampling monitoring equipment at a facility property boundary. Details of the passive sampling tubes themselves and equipment required for subsequent analysis are described in Method 325B.
6.1 Passive Sampling Tubes
The industry standard PS tubes used in this method must meet the specific configuration and preparation requirements described in Section 3.0 of this method and Section 6.1 of Method 325B.
Note:
The use of PS tubes packed with various sorbent materials for monitoring a wide variety of organic compounds in ambient air has been documented in the literature (References 4-10). Other sorbents may be used in standard passive sampling tubes for monitoring additional target compound(s) once their uptake rate and performance has been demonstrated following procedures in Addendum A to Method 325B. Guidance on sorbent selection can also be obtained from relevant national and international standard methods such as ASTM D6196-03 (Reapproved 2009) (Reference 14) and ISO 16017-2:2003(E) (Reference 13) (both incorporated by reference - see §63.14).
6.2 Passive or Diffusive Sampling Cap
One diffusive sampling cap is required per PS tube. The cap fits onto the sampling end of the tube during air monitoring. The other end of the tube remains sealed with the long-term storage cap. Each diffusive sampling cap is fitted with a stainless steel gauze, which defines the outer limit of the diffusion air gap.
6.3 Sorbent Tube Protection Cover
A simple weatherproof hood, suitable for protecting passive sampling tubes from the worst of the weather (see Section 4.4) consists of an inverted cone/funnel constructed of an inert, non-outgassing material that fits over the diffusive tube, with the open (sampling) end of the tube projecting just below the cone opening. An example is shown in Figure 6.1 (Adapted from Reference 13).
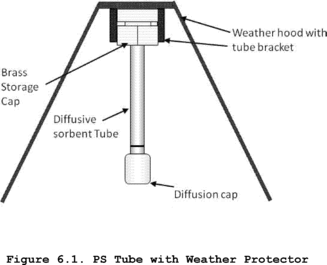
6.4 Thermal Desorption Apparatus
If the analytical thermal desorber that will subsequently be used to analyze the passive sampling tubes does not meet the requirement to exclude outer surface contaminants from the sample flow path (see Section 6.6 of Method 325B), then clean, white, cotton or powder-free nitrile gloves must be used for handling the passive sampling tubes during field deployment.
6.5 Sorbent Selection
Sorbent tube configurations, sorbents or other VOC not listed in this method must be evaluated according to Method 325B, Addendum A or ISO 16017-2:2003(E) (Reference 13) (incorporated by reference - see §63.14). The supporting evaluation and verification data described in Method 325B, Addendum A for configurations or compounds different from the ones described in this method must meet the performance requirements of Method 325A/B and must be submitted with the test plan for your measurement program.
7.0 Reagents and Standards
No reagents or standards are needed for the field deployment and collection of passive sampling tubes. Specifications for sorbents, gas and liquid phase standards, preloaded standard tubes, and carrier gases are covered in Section 7 of Method 325B.
8.0 Sample Deployment, Recovery, and Storage
Pre-deployment and planning steps are required before field deployment of passive sampling tubes. These activities include but are not limited to conducting a site visit, determining suitable and required monitoring locations, and determining the monitoring frequency to be used.
8.1 Conducting the Site Visit
8.1.1 Determine the size and shape of the facility footprint in order to determine the required number of monitoring locations.
8.1.2 Identify obstacles or obstructions (buildings, roads, fences), hills and other terrain issues (e.g., bodies of water or swamp land) that could interfere with air parcel flow to the sampler or that prevent reasonable access to the location. You may use the general guidance in Section 4.1 of this method during the site visit to identify sampling locations. You must evaluate the placement of each passive sampler to determine if the conditions in this section are met.
8.1.3 Identify to the extent possible and record potential off-site source interferences (e.g., neighboring industrial facilities, transportation facilities, fueling operations, combustion sources, short-term transient sources, residential sources, nearby highways).
8.1.4 Identify the closest available meteorological station. Identify potential locations for one or more on-site or near-site meteorological station(s) following the guidance in EPA-454/B-08-002 (Reference 11) (incorporated by reference - see §63.14).
8.2 Determining Sampling Locations (References 2, 3)
8.2.1 The number and placement of the passive samplers depends on the size, the shape of the facility footprint or the linear distance around the facility, and the proximity of emission sources near the property boundaries. Aerial photographs or site maps may be used to determine the size (acreage) and shape of the facility or the length of the monitoring perimeter. Place passive samplers on an internal monitoring perimeter on or inside the facility boundary encompassing all emission sources at the facility at different angles circling the geometric center of the facility or at different distances based on the monitoring perimeter length of the facility.
Note:
In some instances, permanent air monitoring stations may already be located in close proximity to the facility. These stations may be operated and maintained by the site, or local or state regulatory agencies. If access to the station is possible, a PS may be deployed adjacent to other air monitoring instrumentation. A comparison of the pollutant concentrations measured with the PS to concentrations measured by site instrumentation may be used as an optional data quality indicator to assess the accuracy of PS results.
8.2.1.1 The monitoring perimeter may be located between the property boundary and any potential emission source near the property boundary, as long as the distance from the source to the monitoring perimeter is at least 50 meters (162 feet). If a potential emissions source is within 50 meters (162 feet) of the property boundary, the property boundary shall be used as the monitoring perimeter near that source.
8.2.1.2 Samplers need only be placed around the monitoring perimeter and not along internal roads or other right of ways that may bisect the facility.
8.2.1.3 An extra sampler must be placed near known sources of VOCs if potential emission sources are within 50 meters (162 feet) of the boundary and the source or sources are located between two monitors. Measure the distance (x) between the two monitors and place another monitor approximately halfway between (x/2 ±10 percent) the two monitors. Only one extra sampler is required between two monitors to account for known sources of VOCs. For example, in Figure 8.1, the facility added three additional monitors (i.e., light shaded sampler locations), and in Figure 8.2, the facility added two additional monitors to provide sufficient coverage of all area sources.
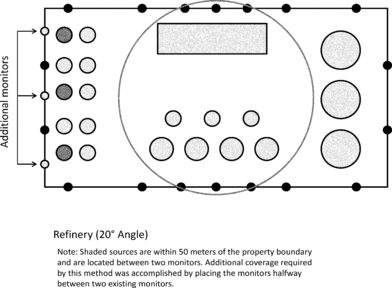
Figure 8.1. Facility with a Regular Shape Between 750 and 1,500 Acres in Area
8.2.2 Option 1 for Determining Sampling Locations.
8.2.2.1 For facilities with a regular (circular, triangular, rectangular, or square) shape, determine the geographic center of the facility.
8.2.2.1.1 For facilities with an area of less than or equal to 750 acres, measure angles of 30 degrees from the center point for a total of twelve 30 degree measurements evenly spaced (±1 degree).
8.2.2.1.2 For facilities covering an area greater than 750 acres but less than or equal to 1,500 acres, measure angles of 20 degrees from the center point for a total of eighteen 20 degree measurements evenly spaced (±1 degree). Figure 8.1 shows the monitor placement around the property boundary of a facility with an area between 750 and 1,500 acres. Monitor placements are represented with black dots along the property boundary.
8.2.2.1.3 For facilities covering an area greater than 1,500 acres, measure angles of 15 degrees from the center point for a total of twenty-four 15 degree measurements evenly spaced (±1 degree).
8.2.2.1.4 Locate each sampling point where the measured angle intersects the outer monitoring perimeter.
8.2.2.2 For irregularly shaped facilities, divide the area into a set of connecting subarea circles, triangles or rectangles to determine sampling locations. The subareas must be defined such that a circle can reasonably encompass the subarea. Then determine the geometric center point of each of the subareas.
8.2.2.2.1 If a subarea is less than or equal to 750 acres (e.g., Figure 8.3), measure angles of 30 degrees from the center point for a total of twelve 30 degree measurements (±1 degree).
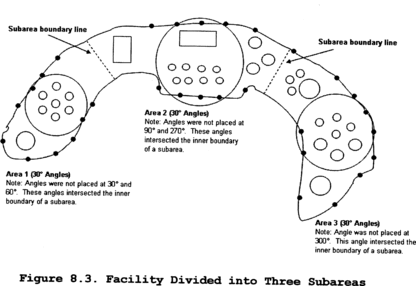
8.2.2.2.2 If a subarea is greater than 750 acres but less than or equal to 1,500 acres (e.g., Figure 8.4), measure angles of 20 degrees from the center point for a total of eighteen 20 degree measurements (±1 degree).
8.2.2.2.3 If a subarea is greater than 1,500 acres, measure angles of 15 degrees from the center for a total of twenty-four 15 degree measurements (±1 degree).
8.2.2.2.4 Locate each sampling point where the measured angle intersects the outer monitoring perimeter. Sampling points need not be placed closer than 152 meters (500 feet) apart (or 76 meters (250 feet) if known sources are within 50 meters (162 feet) of the monitoring perimeter), as long as a minimum of 3 monitoring locations are used for each subarea.
8.2.2.2.5 Sampling sites are not needed at the intersection of an inner boundary with an adjacent subarea. The sampling location must be sited where the measured angle intersects the subarea's outer monitoring perimeter.
8.2.3 Option 2 for Determining Sampling Locations.
8.2.3.1 For facilities with a monitoring perimeter length of less than 7,315 meters (24,000 feet), a minimum of twelve sampling locations evenly spaced ±10 percent of the location interval is required.
8.2.3.2 For facilities with a monitoring perimeter length greater than or equal to 7,315 meters (24,000 feet), sampling locations are spaced 610 ± 76 meters (2,000 ± 250 feet) apart.
8.2.3.3 Unless otherwise specified in an applicable regulation, permit or other requirement, for small disconnected subareas with known sources within 50 meters (162 feet) of the monitoring perimeter, sampling points need not be placed closer than 152 meters (500 feet) apart as long as a minimum of 3 monitoring locations are used for each subarea.
8.3 Siting a Meteorological Station
A meteorological station is required at or near the facility you are monitoring. A number of commercially available meteorological stations can be used. Information on meteorological instruments can be found in EPA-454/R-99-005 (Reference 11) (incorporated by reference - see §63.14). Some important considerations for siting of meteorological stations are detailed below.
8.3.1 Place meteorological stations in locations that represent conditions affecting the transport and dispersion of pollutants in the area of interest. Complex terrain may require the use of more than one meteorological station.
8.3.2 Deploy wind instruments over level, open terrain at a height of 10 meters (33 feet). If possible, locate wind instruments at a distance away from nearby structures that is equal to at least 10 times the height of the structure.
8.3.3 Protect meteorological instruments from thermal radiation and adequately ventilate them using aspirated shields. The temperature sensor must be located at a distance away from any nearby structures that is equal to at least four times the height of the structure. Temperature sensors must be located at least 30 meters (98 feet) from large paved areas.
8.3.4 Collect and record meteorological data, including wind speed, wind direction, temperature and barometric pressure on an hourly basis. Calculate average unit vector wind direction, sigma theta, temperature and barometric pressure per sampling period to enable calculation of concentrations at standard conditions. Supply this information to the laboratory.
8.3.5 Identify and record the location of the meteorological station by its GPS coordinate.
8.4 Monitoring Frequency
8.4.1 Sample collection may be performed for periods up to 14 days.
8.4.2 A site screening protocol that meets method requirements may be performed by collecting samples for a year where each PS accumulates VOC for a 14-day sampling period. Study results are accumulated for the sampling periods (typically 26) over the course of one calendar year. To the extent practical, sampling tubes should be changed at approximately the same time of day at each of the monitoring sites.
8.4.3 When extenuating circumstances do not permit safe deployment or retrieval of passive samplers (e.g., extreme weather, power failure), sampler placement or retrieval earlier or later than the prescribed schedule is allowed but must occur as soon as safe access to sampling sites is possible.
8.5 Passive Sampler Deployment
8.5.1 Clean (conditioned) sorbent tubes must be prepared and packaged by the laboratory as described in Method 325B and must be deployed for sampling within 30 days of conditioning.
8.5.2 Allow the tubes to equilibrate with ambient temperature (approximately 30 minutes to 1 hour) at the monitoring location before removing them from their storage/shipping container for sample collection.
8.5.3 If there is any risk that the analytical equipment will not meet the requirement to exclude contamination on outer tube surfaces from the sample flow path (see Section 6.6 of Method 325B), sample handlers must wear clean, white, cotton or powder-free nitrile gloves during PS deployment and collection and throughout any other tube handling operations.
8.5.4 Inspect the sampling tubes immediately prior to deployment. Ensure that they are intact, securely capped, and in good condition. Any suspect tubes (e.g., tubes that appear to have leaked sorbent) should be removed from the sampling set.
8.5.5 Secure passive samplers so the bottom of the diffusive sampling cap is 1.5 to 3 meters (4.9 to 9.8 feet) above ground using a pole or other secure structure at each sampling location. Orient the PS vertically and with the sampling end pointing downward to avoid ingress of particulates.
Note:
Duplicate sampling assemblies must be deployed in at least one monitoring location for every 10 monitoring locations during each field monitoring period.
8.5.6 Protect the PS from rain and excessive wind velocity by placing them under the type of protective hood described in Section 6.1.3 or equivalent.
8.5.7 Remove the storage cap on the sampling end of the tube and replace it with a diffusive sampling cap at the start of the sampling period. Make sure the diffusion cap is properly seated and store the removed storage caps in the empty tube shipping container.
8.5.8 Record the start time and location details for each sampler on the field sample data sheet (see example in Section 17.0.).
8.5.9 Expose the sampling tubes for the required sampling period-normally 14-days.
8.5.10 Field blank tubes (see Section 9.3 of Method 325B) are stored outside the shipping container at representative sampling locations around the site, but with both long-term storage caps kept in place throughout the monitoring exercise. Collect at least two field blanks sorbent samples per sampling period to ensure sample integrity associated with shipment, collection, and storage.
8.6 Sorbent Tube Recovery and Meteorological Data Collection
Recover deployed sampling tubes and field blanks as follows:
8.6.1 After the sampling period is complete, immediately replace the diffusion end cap on each sampled tube with a long-term storage end cap. Tighten the seal securely by hand and then tighten an additional quarter turn with an appropriate tool. Record the stop date and time and any additional relevant information on the sample data sheet.
8.6.2 Place the sampled tubes, together with the field blanks, in the storage/shipping container. Label the storage container, but do not use paints, markers, or adhesive labels to identify the tubes. TD-compatible electronic (radio frequency identification (RFID)) tube labels are available commercially and are compatible with some brands of thermal desorber. If used, these may be programmed with relevant tube and sample information, which can be read and automatically transcribed into the sequence report by the TD system.
Note:
Sampled tubes must not be placed in the same shipping container as clean conditioned sampling tubes.
8.6.3 Sampled tubes may be shipped at ambient temperature to a laboratory for sample analysis.
8.6.4 Specify whether the tubes are field blanks or were used for sampling and document relevant information for each tube using a Chain of Custody form (see example in Section 17.0) that accompanies the samples from preparation of the tubes through receipt for analysis, including the following information: Unique tube identification numbers for each sampled tube; the date, time, and location code for each PS placement; the date, time, and location code for each PS recovery; the GPS reference for each sampling location; the unique identification number of the duplicate sample (if applicable); and problems or anomalies encountered.
8.6.5 If the sorbent tubes are supplied with electronic (e.g., RFID) tags, it is also possible to allocate a sample identifier to each PS tube. In this case, the recommended format for the identification number of each sampled tube is AA-BB-CC-DD-VOC, where:
AA = Sequence number of placement on route (01, 02, 03 . . .)
BB = Sampling location code (01, 02, 03 . . .)
CC = 14-day sample period number (01 to 26)
DD = Sample code (SA = sample, DU = duplicate, FB = field blank)
VOC = 3-letter code for target compound(s) (e.g., BNZ for benzene or BTX for benzene, toluene, and xylenes)
Note:
Sampling start and end times/dates can also be logged using RFID tube tags.
9.0 Quality Control
9.1 Most quality control checks are carried out by the laboratory and associated requirements are in Section 9.0 of Method 325B, including requirements for laboratory blanks, field blanks, and duplicate samples.
9.2 Evaluate for potential outliers the laboratory results for neighboring sampling tubes collected over the same time period. A potential outlier is a result for which one or more PS tube does not agree with the trend in results shown by neighboring PS tubes - particularly when data from those locations have been more consistent during previous sampling periods. Accidental contamination by the sample handler must be documented before any result can be eliminated as an outlier. Rare but possible examples of contamination include loose or missing storage caps or contaminated storage/shipping containers. Review data from the same and neighboring monitoring locations for the subsequent sampling periods. If the anomalous result is not repeated for that monitoring location, the episode can be ascribed to transient contamination and the data in question must be flagged for potential elimination from the dataset.
9.3 Duplicates and Field Blanks
9.3.1 Collect at least one co-located/duplicate sample for every 10 field samples to determine precision of the measurements.
9.3.2 Collect at least two field blanks sorbent samples per sampling period to ensure sample integrity associated with shipment, collection, and storage. You must use the entire sampling apparatus for field blanks including unopened sorbent tubes mounted in protective sampling hoods. The tube closures must not be removed. Field blanks must be placed in two different quadrants (e.g., 90° and 270°) and remain at the sampling location for the sampling period.
10.0 Calibration and Standardization
Follow the calibration and standardization procedures for meteorological measurements in EPA-454/B-08-002 March 2008 (Reference 11) (incorporated by reference - see §63.14). Refer to Method 325B for calibration and standardization procedures for analysis of the passive sampling tubes.
11.0 Analytical Procedures
Refer to Method 325B, which provides details for the preparation and analysis of sampled passive monitoring tubes (preparation of sampling tubes, shipment and storage of exposed sampling tubes, and analysis of sampling tubes).
12.0 Data Analysis, Calculations and Documentation
12.1 Calculate Annual Average Fenceline Concentration.
After a year's worth of sampling at the facility fenceline (for example, 26 14-day samples), the average (PSi) may be calculated for any specified period at each PS location using Equation 12.1.

Where:
PSi = Annual average for location i.
PSip = Sampling period specific concentration from Method 325B.
i = Location of passive sampler (0 to 360°).
p = The sampling period.
N = The number of sampling periods in the year (e.g., for 14-day sampling periods, from 1 to 26).
Note:
PSip is a function of sampling location-specific factors such as the contribution from facility sources, unusual localized meteorological conditions, contribution from nearby interfering sources, the background caused by integrated far-field sources and measurement error due to deployment, handling, siting, or analytical errors.
12.2 Identify Sampling Locations of Interest
If data from neighboring sampling locations are significantly different, then you may add extra sampling points to isolate background contributions or identify facility-specific “hot spots.”
12.3 Evaluate Trends
You may evaluate trends and patterns in the PS data over multiple sampling periods to determine if elevated concentrations of target compounds are due to operations on the facility or if contributions from background sources are significant.
12.3.1 Obtain meteorological data including wind speed and wind direction or unit vector wind data from the on-site meteorological station. Use this meteorological data to determine the prevailing wind direction and speed during the periods of elevated concentrations.
12.3.2 As an option you may perform preliminary back trajectory calculations (http://ready.arl.noaa.gov/HYSPLIT.php) to aid in identifying the source of the background contribution to elevated target compound concentrations.
12.3.3 Information on published or documented events on- and off-site may also be included in the associated sampling period report to explain elevated concentrations if relevant. For example, you would describe if there was a chemical spill on site, or an accident on an adjacent road.
12.3.4 Additional monitoring for shorter periods (See section 8.4) may be necessary to allow better discrimination/resolution of contributing emission sources if the measured trends and associated meteorology do not provide a clear assessment of facility contribution to the measured fenceline concentration.
12.3.5 Additional records necessary to calculate sampling period average target compound concentration can be found in Section 12.1 of Method 325B.
13.0 Method Performance
Method performance requirements are described in Method 325B.
14.0 Pollution Prevention
[Reserved]
15.0 Waste Management
[Reserved]
16.0 References
1. Ambient air quality - Standard method for measurement of benzene concentrations - Part 4: Diffusive sampling followed by thermal desorption and gas chromatography, BS EN 14662-4:2005.
2. Thoma, E.D., Miller, C.M., Chung, K.C., Parsons, N.L. and Shine, B.C. Facility Fence Line Monitoring using Passive Samplers, J. Air & Waste Mange. Assoc. 2011, 61:834-842.
3. Quality Assurance Handbook for Air Pollution C Systems, Volume II: Ambient Air Quality Monitoring Program, EPA-454/B-13-003, May 2013. Available at http://www.epa.gov/ttnamti1/files/ambient/pm25/qa/QA-Handbook-Vol-II.pdf.
4. Brown, R.H., Charlton, J. and Saunders, K.J.: The development of an improved diffusive sampler. Am. Ind. Hyg. Assoc. J. 1981, 42(12): 865-869.
5. Brown, R. H. Environmental use of diffusive samplers: evaluation of reliable diffusive uptake rates for benzene, toluene and xylene. J. Environ. Monit. 1999, 1 (1), 115-116.
6. Ballach, J.; Greuter, B.; Schultz, E.; Jaeschke, W. Variations of uptake rates in benzene diffusive sampling as a function of ambient conditions. Sci. Total Environ. 1999, 244, 203-217.
7. Brown, R. H. Monitoring the ambient environment with diffusive samplers: theory and practical considerations. J Environ. Monit. 2000, 2 (1), 1-9.
8. Buzica, D.; Gerboles, M.; Plaisance, H. The equivalence of diffusive samplers to reference methods for monitoring O3, benzene and NO2 in ambient air. J. Environ. Monit. 2008, 10 (9), 1052-1059.
9. Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, (16), 2685-94.
10. Pfeffer, H. U.; Breuer, L. BTX measurements with diffusive samplers in the vicinity of a cokery: Comparison between ORSA-type samplers and pumped sampling. J. Environ. Monit. 2000, 2 (5), 483-486.
11. US EPA. 2000. Meteorological Monitoring Guidance for Regulatory Modeling Applications. EPA-454/R-99-005. Office of Air Quality Planning and Standards, Research Triangle Park, NC. February 2000. Available at http://www.epa.gov/scram001/guidance/met/mmgrma.pdf.
12. Quality Assurance Handbook for Air Pollution Measurement Systems. Volume IV: Meteorological Measurements Version 2.0 Final, EPA-454/B-08-002 March 2008. Available at http://www.epa.gov/ttnamti1/files/ambient/met/
Volume%20IV_Meteorological_
Measurements.pdf.
13. ISO 16017-2:2003(E), Indoor, ambient and workplace air - Sampling and analysis of volatile organic compounds by sorbent tube/thermal desorption/capillary gas chromatography. Part 2: Diffusive sampling.
14. ASTM D6196-03 (Reapproved 2009): Standard practice for selection of sorbents, sampling, and thermal desorption analysis procedures for volatile organic compounds in air.
17.0 Tables, Diagrams, Flowcharts and Validation Data

Method 325B - Volatile Organic Compounds from Fugitive and Area Sources:
Sampler Preparation and Analysis
1.0 Scope and Application
1.1 This method describes thermal desorption/gas chromatography (TD/GC) analysis of volatile organic compounds (VOCs) from fugitive and area emission sources collected onto sorbent tubes using passive sampling. It could also be applied to the TD/GC analysis of VOCs collected using active (pumped) sampling onto sorbent tubes. The concentration of airborne VOCs at or near potential fugitive- or area-emission sources may be determined using this method in combination with Method 325A. Companion Method 325A (Sampler Deployment and VOC Sample Collection) describes procedures for deploying the sorbent tubes and passively collecting VOCs.
1.2 The preferred GC detector for this method is a mass spectrometer (MS), but flame ionization detectors (FID) may also be used. Other conventional GC detectors such as electron capture (ECD), photoionization (PID), or flame photometric (FPD) may also be used if they are selective and sensitive to the target compound(s) and if they meet the method performance criteria provided in this method.
1.3 There are 97 VOCs listed as hazardous air pollutants in Title III of the Clean Air Act Amendments of 1990. Many of these VOC are candidate compounds for this method. Compounds with known uptake rates for Carbograph TM 1 TD, Carbopack TM B, or Carbopack TM X are listed in Table 12.1. This method provides performance criteria to demonstrate acceptable performance of the method (or modifications of the method) for monitoring one or more of the compounds listed Table 12.1. If standard passive sampling tubes are packed with other sorbents or used for other analytes than those listed in Table 12.1, then method performance and relevant uptake rates should be verified according to Addendum A to this method or by one of the following national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14), or reported in the peer-reviewed open literature.
1.4 The analytical approach using TD/GC/MS is based on previously published EPA guidance in Compendium Method TO-17 (http://www.epa.gov/ttnamti1/airtox.html#compendium) (Reference 1), which describes active (pumped) sampling of VOCs from ambient air onto tubes packed with thermally stable adsorbents.
1.5 Inorganic gases not suitable for analysis by this method include oxides of carbon, nitrogen and sulfur, ozone (O3), and other diatomic permanent gases. Other pollutants not suitable for this analysis method include particulate pollutants, (i.e., fumes, aerosols, and dusts), compounds too labile (reactive) for conventional GC analysis, and VOCs that are more volatile than propane.
2.0 Summary of Method
2.1 This method provides procedures for the preparation, conditioning, blanking, and shipping of sorbent tubes prior to sample collection.
2.2 Laboratory and field personnel must have experience of sampling trace-level VOCs using sorbent tubes (References 2,5) and must have experience operating thermal desorption/GC/multi-detector instrumentation.
2.3 Key steps of this method as implemented for each sample tube include: Stringent leak testing under stop flow, recording ambient temperature conditions, adding internal standards, purging the tube, thermally desorbing the sampling tube, refocusing on a focusing trap, desorbing and transferring/injecting the VOCs from the secondary trap into the capillary GC column for separation and analysis.
2.4 Water management steps incorporated into this method include: (a) Selection of hydrophobic sorbents in the sampling tube; (b) optional dry purging of sample tubes prior to analysis; and (c) additional selective elimination of water during primary (tube) desorption (if required) by selecting trapping sorbents and temperatures such that target compounds are quantitatively retained while water is purged to vent.
3.0 Definitions
(See also Section 3.0 of Method 325A).
3.1 Blanking is the desorption and confirmatory analysis of conditioned sorbent tubes before they are sent for field sampling.
3.2 Breakthrough volume and associated relation to passive sampling. Breakthrough volumes, as applied to active sorbent tube sampling, equate to the volume of air containing a constant concentration of analyte that may be passed through a sorbent tube at a given temperature before a detectable level (5 percent) of the input analyte concentration elutes from the tube. Although breakthrough volumes are directly related to active rather than passive sampling, they provide a measure of the strength of the sorbent-sorbate interaction and therefore also relate to the efficiency of the passive sampling process. The best direct measure of passive sampling efficiency is the stability of the uptake rate. Quantitative passive sampling is compromised when the sorbent no longer acts as a perfect sink - i.e., when the concentration of a target analyte immediately above the sorbent sampling surface no longer approximates to zero. This causes a reduction in the uptake rate over time. If the uptake rate for a given analyte on a given sorbent tube remains relatively constant - i.e., if the uptake rate determined for 48 hours is similar to that determined for 7 or 14 days - the user can be confident that passive sampling is occurring at a constant rate. As a general rule of thumb, such ideal passive sampling conditions typically exist for analyte:sorbent combinations where the breakthrough volume exceeds 100 L (Reference 4).
3.3 Continuing calibration verification sample (CCV). Single level calibration samples run periodically to confirm that the analytical system continues to generate sample results within acceptable agreement to the current calibration curve.
3.4 Focusing trap is a cooled, secondary sorbent trap integrated into the analytical thermal desorber. It typically has a smaller i.d. and lower thermal mass than the original sample tube allowing it to effectively refocus desorbed analytes and then heat rapidly to ensure efficient transfer/injection into the capillary GC analytical column.
3.5 High Resolution Capillary Column Chromatography uses fused silica capillary columns with an inner diameter of 320 µm or less and with a stationary phase film thickness of 5 µm or less.
3.6 h is time in hours.
3.7 i.d. is inner diameter.
3.8 min is time in minutes.
3.9 Method Detection Limit is the lowest level of analyte that can be detected in the sample matrix with 99% confidence.
3.10 MS-SCAN is the mode of operation of a GC quadrupole mass spectrometer detector that measures all ions over a given mass range over a given period of time.
3.11 MS-SIM is the mode of operation of a GC quadrupole mass spectrometer detector that measures only a single ion or a selected number of discrete ions for each analyte.
3.12 o.d. is outer diameter.
3.13 ppbv is parts per billion by volume.
3.14 Thermal desorption is the use of heat and a flow of inert (carrier) gas to extract volatiles from a solid matrix. No solvent is required.
3.15 Total ion chromatogram is the chromatogram produced from a mass spectrometer detector collecting full spectral information.
3.16 Two-stage thermal desorption is the process of thermally desorbing analytes from a sorbent tube, reconcentrating them on a focusing trap (see Section 3.4), which is then itself rapidly heated to “inject” the concentrated compounds into the GC analyzer.
3.17 VOC is volatile organic compound.
4.0 Analytical Interferences
4.1 Interference from Sorbent Artifacts. Artifacts may include target analytes as well as other VOC that co-elute chromatographically with the compounds of interest or otherwise interfere with the identification or quantitation of target analytes.
4.1.1 Sorbent decomposition artifacts are VOCs that form when sorbents degenerate, e.g., when exposed to reactive species during sampling. For example, benzaldehyde, phenol, and acetophenone artifacts are reported to be formed via oxidation of the polymeric sorbent Tenax® when sampling high concentration (100-500 ppb) ozone atmospheres (Reference 5).
4.1.2 Preparation and storage artifacts are VOCs that were not completely cleaned from the sorbent tube during conditioning or that are an inherent feature of that sorbent at a given temperature.
4.2 Humidity. Moisture captured during sampling can interfere with VOC analysis. Passive sampling using tubes packed with hydrophobic sorbents, like those described in this method, minimizes water retention. However, if water interference is found to be an issue under extreme conditions, one or more of the water management steps described in Section 2.4 can be applied.
4.3 Contamination from Sample Handling. The type of analytical thermal desorption equipment selected should exclude the possibility of outer tube surface contamination entering the sample flow path (see Section 6.6). If the available system does not meet this requirement, sampling tubes and caps must be handled only while wearing clean, white cotton or powder free nitrile gloves to prevent contamination with body oils, hand lotions, perfumes, etc.
5.0 Safety
5.1 This method does not address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate field and laboratory safety and health practices prior to use.
5.2 Laboratory analysts must exercise extreme care in working with high-pressure gas cylinders.
5.3 Due to the high temperatures involved, operators must use caution when conditioning and analyzing tubes.
6.0 Equipment and Supplies
6.1 Tube Dimensions and Materials. The sampling tubes for this method are 3.5-inches (89 mm) long, 1/4 inch (6.4 mm) o.d., and 5 mm i.d. passive sampling tubes (see Figure 6.1). The tubes are made of inert-coated stainless steel with the central section (up to 60 mm) packed with sorbent, typically supported between two 100 mesh stainless steel gauze. The tubes have a cross sectional area of 19.6 square mm (5 mm i.d.). When used for passive sampling, these tubes have an internal diffusion (air) gap (DG) of 1.5 cm between the sorbent retaining gauze at the sampling end of the tube, and the gauze in the diffusion cap.
6.2 Tube Conditioning Apparatus
6.2.1 Freshly packed or newly purchased tubes must be conditioned as described in Section 9 using an appropriate dedicated tube conditioning unit or the thermal desorber. Note that the analytical TD system should be used for tube conditioning if it supports a dedicated tube conditioning mode in which effluent from contaminated tubes is directed to vent without passing through key parts of the sample flow path such as the focusing trap.
6.2.2 Dedicated tube conditioning units must be leak-tight to prevent air ingress, allow precise and reproducible temperature selection (±5°C), offer a temperature range at least as great as that of the thermal desorber, and support inert gas flows in the range up to 100 mL/min.
Note:
For safety and to avoid laboratory contamination, effluent gases from freshly packed or highly contaminated tubes should be passed through a charcoal filter during the conditioning process to prevent desorbed VOCs from polluting the laboratory atmosphere.
6.3 Tube Labeling
6.3.1 Label the sample tubes with a unique permanent identification number and an indication of the sampling end of the tube. Labeling options include etching and TD-compatible electronic (radio frequency identification (RFID)) tube labels.
6.3.2 To avoid contamination, do not make ink markings of any kind on clean sorbent tubes or apply adhesive labels.
Note:
TD-compatible electronic (RFID) tube labels are available commercially and are compatible with some brands of thermal desorber. If used, these may be programmed with relevant tube and sample information, which can be read and automatically transcribed into the sequence report by the TD system (see Section 8.6 of Method 325A).
6.4 Blank and Sampled Tube Storage Apparatus
6.4.1 Long-term storage caps. Seal clean, blank and sampled sorbent tubes using inert, long-term tube storage caps comprising non-greased, 2-piece, 0.25-inch, metal SwageLok®-type screw caps fitted with combined polytetrafluoroethylene ferrules.
6.4.2 Storage and transportation containers. Use clean glass jars, metal cans or rigid, non-emitting polymer boxes.
Note:
You may add a small packet of new activated charcoal or charcoal/silica gel to the shipping container for storage and transportation of batches of conditioned sorbent tubes prior to use. Coolers without ice packs make suitable shipping boxes for containers of tubes because the coolers help to insulate the samples from extreme temperatures (e.g., if left in a parked vehicle).
6.5 Unheated GC Injection Unit for Loading Standards Onto Blank Tubes
A suitable device has a simple push fit or finger-tightening connector for attaching the sampling end of blank sorbent tubes without damaging the tube. It also has a means of controlling carrier gas flow through the injector and attached sorbent tube at 50-100 mL/min and includes a low emission septum cap that allows the introduction of gas or liquid standards via appropriate syringes. Reproducible and quantitative transfer of higher boiling compounds in liquid standards is facilitated if the injection unit allows the tip of the syringe to just touch the sorbent retaining gauze inside the tube.
6.6 Thermal Desorption Apparatus
The manual or automated thermal desorption system must heat sorbent tubes while a controlled flow of inert (carrier) gas passes through the tube and out of the sampling end. The apparatus must also incorporate a focusing trap to quantitatively refocus compounds desorbed from the tube. Secondary desorption of the focusing trap should be fast/efficient enough to transfer the compounds into the high resolution capillary GC column without band broadening and without any need for further pre- or on-column focusing. Typical TD focusing traps comprise small sorbent traps (Reference 16) that are electrically-cooled using multistage Peltier cells (References 17, 18). The direction of gas flow during trap desorption should be the reverse of that used for focusing to extend the compatible analyte volatility range. Closed cycle coolers offer another cryogen-free trap cooling option. Other TD system requirements and operational stages are described in Section 11 and in Figures 17-2 through 17-4.
6.7 Thermal Desorber - GC Interface
6.7.1 The interface between the thermal desorber and the GC must be heated uniformly and the connection between the transfer line insert and the capillary GC analytical column itself must be leak tight.
6.7.2 A portion of capillary column can alternatively be threaded through the heated transfer line/TD interface and connected directly to the thermal desorber.
Note:
Use of a metal syringe-type needle or unheated length of fused silica pushed through the septum of a conventional GC injector is not permitted as a means of interfacing the thermal desorber to the chromatograph. Such connections result in cold spots, cause band broadening and are prone to leaks.
6.8 GC/MS Analytical Components
6.8.1 The GC system must be capable of temperature programming and operation of a high resolution capillary column. Depending on the choice of column (e.g., film thickness) and the volatility of the target compounds, it may be necessary to cool the GC oven to subambient temperatures (e.g., −50°C) at the start of the run to allow resolution of very volatile organic compounds.
6.8.2 All carrier gas lines supplying the GC must be constructed from clean stainless steel or copper tubing. Non-polytetrafluoroethylene thread sealants. Flow controllers, cylinder regulators, or other pneumatic components fitted with rubber components are not suitable.
6.9 Chromatographic Columns
High-resolution, fused silica or equivalent capillary columns that provide adequate separation of sample components to permit identification and quantitation of target compounds must be used.
Note:
100-percent methyl silicone or 5-percent phenyl, 95-percent methyl silicone fused silica capillary columns of 0.25- to 0.32-mm i.d. of varying lengths and with varying thicknesses of stationary phase have been used successfully for non-polar and moderately polar compounds. However, given the diversity of potential target lists, GC column choice is left to the operator, subject to the performance criteria of this method.
6.10 Mass Spectrometer
Linear quadrupole, magnetic sector, ion trap or time-of-flight mass spectrometers may be used provided they meet specified performance criteria. The mass detector must be capable of collecting data from 35 to 300 atomic mass units (amu) every 1 second or less, utilizing 70 volts (nominal) electron energy in the electron ionization mode, and producing a mass spectrum that meets all the instrument performance acceptance criteria in Section 9 when 50 ηg or less of p-bromofluorobenzene is analyzed.
7.0 Reagents and Standards
7.1 Sorbent Selection
7.1.1 Use commercially packed tubes meeting the requirements of this method or prepare tubes in the laboratory using sieved sorbents of particle size in the range 20 to 80 mesh that meet the retention and quality control requirements of this method.
7.1.2 This passive air monitoring method can be used without the evaluation specified in Addendum A if the type of tubes described in Section 6.1 are packed with 4-6 cm (typically 400-650 mg) of the sorbents listed in Table 12.1 and used for the respective target analytes.
Note:
Although Carbopack TM X is the optimum sorbent choice for passive sampling of 1,3-butadiene, recovery of compounds with vapor pressure lower than benzene may be difficult to achieve without exceeding sorbent maximum temperature limitations (see Table 8.1). See ISO 16017-2:2003(E) or ASTM D6196-03 (Reapproved 2009) (both incorporated by reference - see §63.14) for more details on sorbent choice for air monitoring using passive sampling tubes.
7.1.3 If standard passive sampling tubes are packed with other sorbents or used for analytes other than those tabulated in Section 12.0, method performance and relevant uptake rates should be verified according to Addendum A to this method or by following the techniques described in one of the following national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14) - or reported in the peer-reviewed open literature. A summary table and the supporting evaluation data demonstrating the selected sorbent meets the requirements in Addendum A to this method must be submitted to the regulatory authority as part of a request to use an alternative sorbent.
7.1.4 Passive (diffusive) sampling and thermal desorption methods that have been evaluated at relatively high atmospheric concentrations (i.e., mid-ppb to ppm) and published for use in workplace air and industrial/mobile source emissions testing (References 9-20) may be applied to this procedure. However, the validity of any shorter term uptake rates must be verified and adjusted if necessary for the longer monitoring periods required by this method by following procedures described in Addendum A to this method or those presented in national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference-see §63.14).
7.1.5 Suitable sorbents for passive sampling must have breakthrough volumes of at least 20 L (preferably >100 L) for the compounds of interest and must quantitatively release the analytes during desorption without exceeding maximum temperatures for the sorbent or instrumentation.
7.1.6 Repack/replace the sorbent tubes or demonstrate tube performance following the requirements in Addendum A to this method at least every 2 years or every 50 uses, whichever occurs first.
7.2 Gas Phase Standards
7.2.1 Static or dynamic standard atmospheres may be used to prepare calibration tubes and/or to validate passive sampling uptake rates and can be generated from pure chemicals or by diluting concentrated gas standards. The standard atmosphere must be stable at ambient pressure and accurate to ±10 percent of the target gas concentration. It must be possible to maintain standard atmosphere concentrations at the same or lower levels than the target compound concentration objectives of the test. Test atmospheres used for validation of uptake rates must also contain at least 35 percent relative humidity.
Note:
Accurate, low-(ppb-) level gas-phase VOC standards are difficult to generate from pure materials and may be unstable depending on analyte polarity and volatility. Parallel monitoring of vapor concentrations with alternative methods, such as pumped sorbent tubes or sensitive/selective on-line detectors, may be necessary to minimize uncertainty. For these reasons, standard atmospheres are rarely used for routine calibration.
7.2.2 Concentrated, pressurized gas phase standards. Accurate (±5 percent or better), concentrated gas phase standards supplied in pressurized cylinders may also be used for calibration. The concentration of the standard should be such that a 0.5-5.0 mL volume contains approximately the same mass of analytes as will be collected from a typical air sample.
7.2.3 Follow manufacturer's guidelines concerning storage conditions and recertification of the concentrated gas phase standard. Gas standards must be recertified a minimum of once every 12 months.
7.3 Liquid Standards
Target analytes can also be introduced to the sampling end of sorbent tubes in the form of liquid calibration standards.
7.3.1 The concentration of liquid standards must be such that an injection of 0.5-2 µl of the solution introduces the same mass of target analyte that is expected to be collected during the passive air sampling period.
7.3.2 Solvent Selection. The solvent selected for the liquid standard must be pure (contaminants <10 percent of minimum analyte levels) and must not interfere chromatographically with the compounds of interest.
7.3.3 If liquid standards are sourced commercially, follow manufacturer's guidelines concerning storage conditions and shelf life of unopened and opened liquid stock standards.
Note:
Commercial VOC standards are typically supplied in volatile or non-interfering solvents such as methanol.
7.3.4 Working standards must be stored at 6°C or less and used or discarded within two weeks of preparation.
7.4 Gas Phase Internal Standards
7.4.1 Gas-phase deuterated or fluorinated organic compounds may be used as internal standards for MS-based systems.
7.4.2 Typical compounds include deuterated toluene, perfluorobenzene and perfluorotoluene.
7.4.3 Use multiple internal standards to cover the volatility range of the target analytes.
7.4.4 Gas-phase standards must be obtained in pressurized cylinders and containing vendor certified gas concentrations accurate to ±5 percent. The concentration should be such that the mass of internal standard components introduced is similar to those of the target analytes collected during field monitoring.
7.5 Preloaded Standard Tubes
Certified, preloaded standard tubes, accurate within ±5 percent for each analyte at the microgram level and ±10 percent at the nanogram level, are available commercially and may be used for auditing and quality control purposes. (See Section 9.5 for audit accuracy evaluation criteria.) Certified preloaded tubes may also be used for routine calibration.
Note:
Proficiency testing schemes are also available for TD/GC/MS analysis of sorbent tubes preloaded with common analytes such as benzene, toluene, and xylene.
7.6 Carrier Gases
Use inert, 99.999-percent or higher purity helium as carrier gas. Oxygen and organic filters must be installed in the carrier gas lines supplying the analytical system according to the manufacturer's instructions. Keep records of filter and oxygen scrubber replacement.
8.0 Sorbent Tube Handling (Before and After Sampling)
8.1 Sample Tube Conditioning
8.1.1 Sampling tubes must be conditioned using the apparatus described in Section 6.2.
8.1.2 New tubes should be conditioned for 2 hours to supplement the vendor's conditioning procedure. Recommended temperatures for tube conditioning are given in Table 8.1.
8.1.3 After conditioning, the blank must be verified on each new sorbent tube and on 10 percent of each batch of reconditioned tubes. See Section 9.0 for acceptance criteria.
| Sampling sorbent |
Maximum
temperature (°C) |
Conditioning temperature
(°C) | Carrier gas flow rate |
|---|---|---|---|
| Carbotrap® C | >400 | 350 | 100 mL/min |
| Carbopack TM C | |||
| Anasorb® GCB2 | |||
| Carbograph TM 1 TD | |||
| Carbotrap® | |||
| Carbopack TM B | |||
| Anasorb® GCB1 | |||
|
Tenax® TA
Carbopack TM X | 350 | 330 | 100 mL/min |
8.2 Capping, Storage and Shipment of Conditioned Tubes
8.2.1 Conditioned tubes must be sealed using long-term storage caps (see Section 6.4) pushed fully down onto both ends of the PS sorbent tube, tightened by hand and then tighten an additional quarter turn using an appropriate tool.
8.2.2 The capped tubes must be kept in appropriate containers for storage and transportation (see Section 6.4.2). Containers of sorbent tubes may be stored and shipped at ambient temperature and must be kept in a clean environment.
8.2.3 You must keep batches of capped tubes in their shipping boxes or wrap them in uncoated aluminum foil before placing them in their storage container, especially before air freight, because the packaging helps hold caps in position if the tubes get very cold.
8.3 Calculating the Number of Tubes Required for a Monitoring Exercise
8.3.1 Follow guidance given in Method 325A to determine the number of tubes required for site monitoring.
8.3.2 The following additional samples will also be required: Laboratory blanks as specified in Section 9.1.2 (one per analytical sequence minimum), field blanks as specified in Section 9.3.2 (two per sampling period minimum), CCV tubes as specified in Section 10.9.4. (at least one per analysis sequence or every 24 hours), and duplicate samples as specified in Section 9.4 (at least one duplicate sample is required for every 10 sampling locations during each monitoring period).
8.4 Sample Collection
8.4.1 Allow the tubes to equilibrate with ambient temperature (approximately 30 minutes to 1 hour) at the monitoring location before removing them from their storage/shipping container for sample collection.
8.4.2 Tubes must be used for sampling within 30 days of conditioning (Reference 4).
8.4.3 During field monitoring, the long-term storage cap at the sampling end of the tube is replaced with a diffusion cap and the whole assembly is arranged vertically, with the sampling end pointing downward, under a protective hood or shield - See Section 6.1 of Method 325A for more details.
8.5 Sample Storage
8.5.1 After sampling, tubes must be immediately resealed with long-term storage caps and placed back inside the type of storage container described in Section 6.4.2.
8.5.2 Exposed tubes may not be placed in the same container as clean tubes. They should not be taken back out of the container until ready for analysis and after they have had time to equilibrate with ambient temperature in the laboratory.
8.5.3 Sampled tubes must be inspected before analysis to identify problems such as loose or missing caps, damaged tubes, tubes that appear to be leaking sorbent or container contamination. Any and all such problems must be documented together with the unique identification number of the tube or tubes concerned. Affected tubes must not be analyzed but must be set aside.
8.5.4 Intact tubes must be analyzed within 30 days of the end of sample collection (within one week for limonene, carene, bis-chloromethyl ether, labile sulfur or nitrogen-containing compounds, and other reactive VOCs).
Note:
Ensure ambient temperatures stay below 23°C during transportation and storage. Refrigeration is not normally required unless the samples contain reactive compounds or cannot be analyzed within 30 days. If refrigeration is used, the atmosphere inside the refrigerator must be clean and free of organic solvents.
9.0 Quality Control
9.1 Laboratory Blank
The analytical system must be demonstrated to be contaminant free by performing a blank analysis at the beginning of each analytical sequence to demonstrate that the secondary trap and TD/GC/MS analytical equipment are free of any significant interferents.
9.1.1 Laboratory blank tubes must be prepared from tubes that are identical to those used for field sampling.
9.1.2 Analysis of at least one laboratory blank is required per analytical sequence. The laboratory blank must be stored in the laboratory under clean, controlled ambient temperature conditions.
9.1.3 Laboratory blank/artifact levels must meet the requirements of Section 9.2.2 (see also Table 17.1). If the laboratory blank does not meet requirements, stop and perform corrective actions and then re-analyze laboratory blank to ensure it meets requirements.
9.2 Tube Conditioning
9.2.1 Conditioned tubes must be demonstrated to be free of contaminants and interference by running 10 percent of the blank tubes selected at random from each conditioned batch under standard sample analysis conditions (see Section 8.1).
9.2.2 Confirm that artifacts and background contamination are ≤ 0.2 ppbv or less than three times the detection limit of the procedure or less than 10 percent of the target compound(s) mass that would be collected if airborne concentrations were at the regulated limit value, whichever is larger. Only tubes that meet these criteria can be used for field monitoring, field or laboratory blanks, or for system calibration.
9.2.3 If unacceptable levels of VOCs are observed in the tube blanks, then the processes of tube conditioning and checking the blanks must be repeated.
9.3 Field Blanks
9.3.1 Field blank tubes must be prepared from tubes that are identical to those used for field sampling - i.e., they should be from the same batch, have a similar history, and be conditioned at the same time.
9.3.2 Field blanks must be shipped to the monitoring site with the sampling tubes and must be stored at the sampling location throughout the monitoring exercise. The field blanks must be installed under a protective hood/cover at the sampling location, but the long-term storage caps must remain in place throughout the monitoring period (see Method 325A). The field blanks are then shipped back to the laboratory in the same container as the sampled tubes. Collect at least two field blank samples per sampling period to ensure sample integrity associated with shipment, collection, and storage.
9.3.3 Field blanks must contain no greater than one-third of the measured target analyte or compliance limit for field samples (see Table 17.1). If either field blank fails, flag all data that do not meet this criterion with a note that the associated results are estimated and likely to be biased high due to field blank background.
9.4 Duplicate Samples
Duplicate (co-located) samples collected must be analyzed and reported as part of method quality control. They are used to evaluate sampling and analysis precision. Relevant performance criteria are given in Section 9.9.
9.5 Method Performance Criteria
Unless otherwise noted, monitoring method performance specifications must be demonstrated for the target compounds using the procedures described in Addendum A to this method and the statistical approach presented in Method 301.
9.6 Method Detection Limit
Determine the method detection limit under the analytical conditions selected (see Section 11.3) using the procedure in Section 15 of Method 301. The method detection limit is defined for each system by making seven replicate measurements of a concentration of the compound of interest within a factor of five of the detection limit. Compute the standard deviation for the seven replicate concentrations, and multiply this value by three. The results should demonstrate that the method is able to detect analytes such as benzene at concentrations as low as 50 ppt or 1/3rd (preferably 1/10th) of the lowest concentration of interest, whichever is larger.
Note:
Determining the detection limit may be an iterative process as described in 40 CFR part 136, Appendix B.
9.7 Analytical Bias
Analytical bias must be demonstrated to be within ±30 percent using Equation 9.1. Analytical bias must be demonstrated during initial setup of this method and as part of the CCV carried out with every sequence of 10 samples or less (see Section 9.14). Calibration standard tubes (see Section 10.0) may be used for this purpose.
Eq. 9.1
Where:
Spiked Value = A known mass of VOCs added to the tube.
Measured Value = Mass determined from analysis of the tube.
9.8 Analytical Precision
Demonstrate an analytical precision within ±20 percent using Equation 9.2. Analytical precision must be demonstrated during initial setup of this method and at least once per year. Calibration standard tubes may be used (see Section 10.0) and data from CCV may also be applied for this purpose.
Eq. 9.2
Where:
A1 = A measurement value taken from one spiked tube.
A2 = A measurement value taken from a second spiked tube.
A = The average of A1 and A2.
9.9 Field Replicate Precision
Use Equation 9.3 to determine and report replicate precision for duplicate field samples (see Section 9.4). The level of agreement between duplicate field samples is a measure of the precision achievable for the entire sampling and analysis procedure. Flag data sets for which the duplicate samples do not agree within 30 percent.
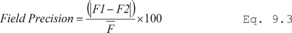
Eq. 9.3
Where:
F1 = A measurement value (mass) taken from one of the two field replicate tubes used in sampling.
F2 = A measurement value (mass) taken from the second of two field replicate tubes used in sampling.
F = The average of F1 and F2.
9.10 Desorption Efficiency and Compound Recovery
The efficiency of the thermal desorption method must be determined.
9.10.1 Quantitative (>95 percent) compound recovery must be demonstrated by repeat analyses on a same standard tube.
9.10.2 Compound recovery through the TD system can also be demonstrated by comparing the calibration check sample response factor obtained from direct GC injection of liquid standards with that obtained from thermal desorption analysis response factor using the same column under identical conditions.
9.10.3 If the relative response factors obtained for one or more target compounds introduced to the column via thermal desorption fail to meet the criteria in Section 9.10.1, you must adjust the TD parameters to meet the criteria and repeat the experiment. Once the thermal desorption conditions have been optimized, you must repeat this test each time the analytical system is recalibrated to demonstrate continued method performance.
9.11 Audit Samples
Certified reference standard samples must be used to audit this procedure (if available). Accuracy within 30 percent must be demonstrated for relevant ambient air concentrations (0.5 to 25 ppb).
9.12 Mass Spectrometer Tuning Criteria
Tune the mass spectrometer (if used) according to manufacturer's specifications. Verify the instrument performance by analyzing a 50 ηg injection of bromofluorobenzene. Prior to the beginning of each analytical sequence or every 24 hours during continuous GC/MS operation for this method demonstrate that the bromofluorobenzene tuning performance criteria in Table 9.1 have been met.
| Target mass | Rel. to mass | Lower limit % | Upper limit % |
|---|---|---|---|
| 50 | 95 | 8 | 40 |
| 75 | 95 | 30 | 66 |
| 95 | 95 | 100 | 100 |
| 96 | 95 | 5 | 9 |
| 173 | 174 | 0 | 2 |
| 174 | 95 | 50 | 120 |
| 175 | 174 | 4 | 9 |
| 176 | 174 | 93 | 101 |
| 177 | 176 | 5 | 9 |
| 1 All ion abundances must be normalized to m/z 95, the nominal base peak, even though the ion abundance of m/z 174 may be up to 120 percent that of m/z 95. | |||
9.13 Routine CCV at the Start of a Sequence
Run CCV before each sequence of analyses and after every tenth sample to ensure that the previous multi-level calibration (see section 10.0) is still valid.
9.13.1 The sample concentration used for the CCV should be near the mid-point of the multi-level calibration range.
9.13.2 Quantitation software must be updated with response factors determined from the CCV standard. The percent deviation between the initial calibration and the CCV for all compounds must be within 30 percent.
9.14 CCV at the End of a Sequence
Run another CCV after running each sequence of samples. The initial CCV for a subsequent set of samples may be used as the final CCV for a previous analytical sequence, provided the same analytical method is used and the subsequent set of samples is analyzed immediately (within 4 hours) after the last CCV.
9.15 Additional Verification
Use a calibration check standard from a second, separate source to verify the original calibration at least once every three months.
9.16 Integration Method
Document the procedure used for integration of analytical data including field samples, calibration standards and blanks.
9.17 QC Records
Maintain all QC reports/records for each TD/GC/MS analytical system used for application of this method. Routine quality control requirements for this method are listed below and summarized in Table 17.1.
10.0 Calibration and Standardization
10.1 Calibrate the analytical system using standards covering the range of analyte masses expected from field samples.
10.2 Analytical results for field samples must fall within the calibrated range of the analytical system to be valid.
10.3 Calibration standard preparation must be fully traceable to primary standards of mass and/or volume, and/or be confirmed using an independent certified reference method.
10.3.1 Preparation of calibration standard tubes from standard atmospheres.
10.3.1.1 Subject to the requirements in Section 7.2.1, low-level standard atmospheres may be introduced to clean, conditioned sorbent tubes in order to produce calibration standards.
10.3.1.2 The standard atmosphere generator or system must be capable of producing sufficient flow at a constant rate to allow the required analyte mass to be introduced within a reasonable time frame and without affecting the concentration of the standard atmosphere itself.
10.3.1.3 The sampling manifold may be heated to minimize risk of condensation but the temperature of the gas delivered to the sorbent tubes may not exceed 100°F.
10.3.1.4 The flow rates passed through the tube should be in the order of 50-100 mL/min and the volume of standard atmosphere sampled from the manifold or chamber must not exceed the breakthrough volume of the sorbent at the given temperature.
10.4 Preparation of calibration standard tubes from concentrated gas standards.
10.4.1 If a suitable concentrated gas standard (see Section 7.2.2) can be obtained, follow the manufacturer's recommendations relating to suitable storage conditions and product lifetime.
10.4.2 Introduce precise 0.5 to 500.0 mL aliquots of the standard to the sampling end of conditioned sorbent tubes in a 50-100 mL/min flow of pure carrier gas.
Note:
This can be achieved by connecting the sampling end of the tube to an unheated GC injector (see Section 6.6) and introducing the aliquot of gas using a suitable gas syringe. Gas sample valves could alternatively be used to meter the standard gas volume.
10.4.3 Each sorbent tube should be left connected to the flow of gas for 2 minutes after standard introduction. As soon as each spiked tube is removed from the injection unit, seal it with long-term storage caps and place it in an appropriate tube storage/transportation container if it is not to be analyzed within 24 hours.
10.5 Preparation of calibration standard tubes from liquid standards.
10.5.1 Suitable standards are described in Section 7.3.
10.5.2 Introduce precise 0.5 to 2 µl aliquots of liquid standards to the sampling end of sorbent tubes in a flow (50-100 mL/min) of carrier gas using a precision syringe and an unheated injector (Section 6.5). The flow of gas should be sufficient to completely vaporize the liquid standard.
Note:
If the analytes of interest are higher boiling than n-decane, reproducible analyte transfer to the sorbent bed is optimized by allowing the tip of the syringe to gently touch the sorbent retaining gauze at the sampling end of the tube.
10.5.3 Each sorbent tube is left connected to the flow of gas for 5 minutes after liquid standard introduction.
10.5.3.1 As soon as each spiked tube is removed from the injection unit, seal it with long-term storage caps and place it in an appropriate tube storage container if it is not to be analyzed within 24 hours.
Note:
In cases where it is possible to selectively purge the solvent from the tube while all target analytes are quantitatively retained, a larger 2 µL injection may be made for optimum accuracy. However, if the solvent cannot be selectively purged and will be present during analysis, the injection volume should be as small as possible (e.g., 0.5 µL) to minimize solvent interference.
Note:
This standard preparation technique requires the entire liquid plug including the tip volume be brought into the syringe barrel. The volume in the barrel is recorded, the syringe is inserted into the septum of the spiking apparatus. The liquid is then quickly injected. Any remaining liquid in the syringe tip is brought back into the syringe barrel. The volume in the barrel is recorded and the amount spiked onto the tube is the difference between the before spiking volume and the after spiking volume. A bias occurs with this method when sample is drawn continuously up into the syringe to the specified volume and the calibration solution in the syringe tip is ignored.
10.6 Preparation of calibration standard tubes from multiple standards.
10.6.1 If it is not possible to prepare one standard containing all the compounds of interest (e.g., because of chemical reactivity or the breadth of the volatility range), standard tubes can be prepared from multiple gas or liquid standards.
10.6.2 Follow the procedures described in Sections 10.4 and 10.5, respectively, for introducing each gas and/or liquid standard to the tube and load those containing the highest boiling compounds of interest first and the lightest species last.
10.7 Additional requirements for preparation of calibration tubes.
10.7.1 Storage of Calibration Standard Tubes
10.7.1.1 Seal tubes with long-term storage caps immediately after they have been disconnected from the standard loading manifold or injection apparatus.
10.7.1.2 Calibration standard tubes may be stored for no longer than 30 days and should be refrigerated if there is any risk of chemical interaction or degradation. Audit standards (see section 9.11) are exempt from this criteria and may be stored for the shelf-life specified on their certificates.
10.8 Keep records for calibration standard tubes to include the following:
10.8.1 The stock number of any commercial liquid or gas standards used.
10.8.2 A chromatogram of the most recent blank for each tube used as a calibration standard together with the associated analytical conditions and date of cleaning.
10.8.3 Date of standard loading.
10.8.4 List of standard components, approximate masses and associated confidence levels.
10.8.5 Example analysis of an identical standard with associated analytical conditions.
10.8.6 A brief description of the method used for standard preparation.
10.8.7 The standard's expiration date.
10.9 TD/GC/MS using standard tubes to calibrate system response.
10.9.1 Verify that the TD/GC/MS analytical system meets the instrument performance criteria given in Section 9.1.
10.9.2 The prepared calibration standard tubes must be analyzed using the analytical conditions applied to field samples (see Section 11.0) and must be selected to ensure quantitative transfer and adequate chromatographic resolution of target compounds, surrogates, and internal standards in order to enable reliable identification and quantitation of compounds of interest. The analytical conditions should also be sufficiently stringent to prevent buildup of higher boiling, non-target contaminants that may be collected on the tubes during field monitoring.
10.9.3 Calibration range. Each TD/GC/MS system must be calibrated at five concentrations that span the monitoring range of interest before being used for sample analysis. This initial multi-level calibration determines instrument sensitivity under the analytical conditions selected and the linearity of GC/MS response for the target compounds. One of the calibration points must be within a factor of five of the detection limit for the compounds of interest.
10.9.4 One of the calibration points from the initial calibration curve must be at the same concentration as the daily CCV standard (e.g., the mass collected when sampling air at typical concentrations).
10.9.5 Calibration frequency. Each GC/MS system must be recalibrated with a full 5-point calibration curve following corrective action (e.g., ion source cleaning or repair, column replacement) or if the instrument fails the daily calibration acceptance criteria.
10.9.5.1 CCV checks must be carried out on a regular routine basis as described in Section 9.14.
10.9.5.2 Quantitation ions for the target compounds are shown in Table 10.1. Use the primary ion unless interferences are present, in which case you should use a secondary ion.
| Compound | CAS No. |
BP
(°C) |
Vapor
pressure (mmHg) a | MW b | Characteristic ion(s) | |
|---|---|---|---|---|---|---|
| Primary | Secondary | |||||
| 1,1-Dichloroethene | 75-35-4 | 32 | 500 | 96.9 | 61 | 96 |
| 3-Chloropropene | 107-05-1 | 44.5 | 340 | 76.5 | 76 | 41, 39, 78 |
| 1,1,2-Trichloro-1,2,2-trifluoroethane-1,1-Dichloroethane | 75-34-3 | 57.0 | 230 | 99 | 63 | 65, 83, 85, 98, 100 |
| 1,2-Dichloroethane | 107-06-2 | 83.5 | 61.5 | 99 | 62 | 98 |
| 1,1,1-Trichloroethane | 71-55-6 | 74.1 | 100 | 133.4 | 97 | 99, 61 |
| Benzene | 71-43-2 | 80.1 | 76.0 | 78 | 78 | |
| Carbon tetrachloride | 56-23-5 | 76.7 | 90.0 | 153.8 | 117 | 119 |
| 1,2-Dichloropropane | 78-87-5 | 97.0 | 42.0 | 113 | 63 | 112 |
| Trichloroethene | 79-01-6 | 87.0 | 20.0 | 131.4 | 95 | 97, 130, 132 |
| 1,1,2-Trichloroethane | 79-00-5 | 114 | 19.0 | 133.4 | 83 | 97, 85 |
| Toluene | 108-88-3 | 111 | 22.0 | 92 | 92 | 91 |
| Tetrachloroethene | 127-18-4 | 121 | 14.0 | 165.8 | 164 | 129, 131, 166 |
| Chlorobenzene | 108-90-7 | 132 | 8.8 | 112.6 | 112 | 77, 114 |
| Ethylbenzene | 100-41-4 | 136 | 7.0 | 106 | 91 | 106 |
| m,p-Xylene | 108-38-3, 106-42-3 | 138 | 6.5 | 106.2 | 106 | 91 |
| Styrene | 100-42-5 | 145 | 6.6 | 104 | 104 | 78 |
| o-Xylene | 95-47-6 | 144 | 5.0 | 106.2 | 106 | 91 |
| p-Dichlorobenzene | 106-46-7 | 173 | 0.60 | 147 | 146 | 111, 148 |
| a Pressure in millimeters of mercury.
b Molecular weight. | ||||||
11.0 Analytical Procedure
11.1 Preparation for Sample Analysis
11.1.1 Each sequence of analyses must be ordered as follows:
11.1.1.1 CCV.
11.1.1.2 A laboratory blank.
11.1.1.3 Field blank.
11.1.1.4 Sample(s).
11.1.1.5 Field blank.
11.1.1.6 CCV after 10 field samples.
11.1.1.7 CCV at the end of the sample batch.
11.2 Pre-desorption System Checks and Procedures
11.2.1 Ensure all sample tubes and field blanks are at ambient temperature before removing them from the storage container.
11.2.2 If using an automated TD/GC/MS analyzer, remove the long-term storage caps from the tubes, replace them with appropriate analytical caps, and load them into the system in the sequence described in Section 11.1. Alternatively, if using a manual system, uncap and analyze each tube, one at a time, in the sequence described in Section 11.1.
11.2.3 The following thermal desorption system integrity checks and procedures are required before each tube is analyzed.
Note:
Commercial thermal desorbers should implement these steps automatically.
11.2.3.1 Tube leak test: Each tube must be leak tested as soon as it is loaded into the carrier gas flow path before analysis to ensure data integrity.
11.2.3.2 Conduct the leak test at the GC carrier gas pressure, without heat or gas flow applied. Tubes that fail the leak test should not be analyzed, but should be resealed and stored intact. On automated systems, the instrument should continue to leak test and analyze subsequent tubes after a given tube has failed. Automated systems must also store and record which tubes in a sequence have failed the leak test. Information on failed tubes should be downloaded with the batch of sequence information from the analytical system.
11.2.3.3 Leak test the sample flow path. Leak check the sample flow path of the thermal desorber before each analysis without heat or gas flow applied to the sample tube. Stop the automatic sequence of tube desorption and GC analysis if any leak is detected in the main sample flow path. This process may be carried out as a separate step or as part of Section 11.2.3.2.
11.2.4 Optional Dry Purge
11.2.4.1 Tubes may be dry purged with a flow of pure dry gas passing into the tube from the sampling end, to remove water vapor and other very volatile interferents if required.
11.2.5 Internal Standard (IS) Addition
11.2.5.1 Use the internal standard addition function of the automated thermal desorber (if available) to introduce a precise aliquot of the internal standard to the sampling end of each tube after the leak test and shortly before primary (tube) desorption).
Note:
This step can be combined with dry purging the tube (Section 11.2.4) if required.
11.2.5.2 If the analyzer does not have a facility for automatic IS addition, gas or liquid internal standard can be manually introduced to the sampling end of tubes in a flow of carrier gas using the types of procedure described in Sections 10.3 and 10.4, respectively.
11.2.6 Pre-purge. Each tube should be purged to vent with carrier gas flowing in the desorption direction (i.e., flowing into the tube from the non-sampling end) to remove oxygen before heat is applied. This is to prevent analyte and sorbent oxidation and to prevent deterioration of key analyzer components such as the GC column and mass spectrometer (if applicable). A series of schematics illustrating these steps is presented in Figures 17.2 and 17.3.
11.3 Analytical Procedure
11.3.1 Steps Required for Thermal Desorption
11.3.1.1 Ensure that the pressure and purity of purge and carrier gases supplying the TD/GC/MS system, meet manufacturer specifications and the requirements of this method.
11.3.1.2 Ensure also that the analytical method selected meets the QC requirements of this method (Section 9) and that all the analytical parameters are at set point.
11.3.1.3 Conduct predesorption system checks (see Section 11.2).
11.3.1.4 Desorb the sorbent tube under conditions demonstrated to achieve >95 percent recovery of target compounds (see Section 9.5.2).
Note:
Typical tube desorption conditions range from 280-350°C for 5-15 minutes with a carrier gas flow of 30-100 mL/min passing through the tube from the non-sampling end such that analytes are flushed out of the tube from the sampling end. Desorbed VOCs are concentrated (refocused) on a secondary, cooled sorbent trap integrated into the analytical equipment (see Figure 17.4). The focusing trap is typically maintained at a temperature between −30 and +30°C during focusing. Selection of hydrophobic sorbents for focusing and setting a trapping temperature of +25 to 27°C aid analysis of humid samples because these settings allow selective elimination of any residual water from the system, prior to GC/MS analysis.
Note:
The transfer of analytes from the tube to the focusing trap during primary (tube) desorption can be carried out splitless or under controlled split conditions (see Figure 17.4) depending on the masses of target compounds sampled and the requirements of the system - sensitivity, required calibration range, column overload limitations, etc. Instrument controlled sample splits must be demonstrated by showing the reproducibility using calibration standards. Field and laboratory blank samples must be analyzed at the same split as the lowest calibration standard. During secondary (trap) desorption the focusing trap is heated rapidly (typically at rates >40°C/s) with inert (carrier) gas flowing through the trap (3-100 mL/min) in the reverse direction to that used during focusing.
11.3.1.5 The split conditions selected for optimum field sample analysis must also be demonstrated on representative standards.
Note:
Typical trap desorption temperatures are in the range 250-360°C, with a “hold” time of 1-3 minutes at the highest temperature. Trap desorption automatically triggers the start of GC analysis. The trap desorption can also be carried out under splitless conditions (i.e., with everything desorbed from the trap being transferred to the analytical column and GC detector) or, more commonly, under controlled split conditions (see Figure 17.4). The selected split ratio depends on the masses of target compounds sampled and the requirements of the system - sensitivity, required calibration range, column overload limitations, etc. If a split is selected during both primary (trap) desorption and secondary (trap) desorption, the overall split ratio is the product of the two. Such `double' split capability gives optimum flexibility for accommodating concentrated samples as well as trace-level samples on the TD/GC/MS analytical system. High resolution capillary columns and most GC/MS detectors tend to work best with approximately 20-200 ng per compound per tube to avoid saturation. The overall split ratio must be adjusted such that, when it is applied to the sample mass that is expected to be collected during field monitoring, the amount reaching the column will be attenuated to fall within this range. As a rule of thumb this means that ∼20 ng samples will require splitless or very low split analysis, ∼2 µg samples will require a split ratio in the order of ∼50:1 and 200 µg samples will require a double split method with an overall split ratio in the order of 2,000:1.
11.3.1.6 Analyzed tubes must be resealed with long-term storage caps immediately after analysis (manual systems) or after completion of a sequence (automated systems). This prevents contamination, minimizing the extent of tube reconditioning required before subsequent reuse.
11.3.2 GC/MS Analytical Procedure
11.3.2.1 Heat/cool the GC oven to its starting set point.
11.3.2.2 If using a GC/MS system, it can be operated in either MS-Scan or MS-SIM mode (depending on required sensitivity levels and the type of mass spectrometer selected). As soon as trap desorption and transfer of analytes into the GC column triggers the start of the GC/MS analysis, collect mass spectral data over a range of masses from 35 to 300 amu. Collect at least 10 data points per eluting chromatographic peak in order to adequately integrate and quantify target compounds.
11.3.2.3 Use secondary ion quantitation only when there are sample matrix interferences with the primary ion. If secondary ion quantitation is performed, flag the data and document the reasons for the alternative quantitation procedure.
11.3.2.4 Data reduction is performed by the instruments post processing program that is automatically accessed after data acquisition is completed at the end of the GC run. The concentration of each target compound is calculated using the previously established response factors for the CCV analyzed in Section 11.1.1.6.
11.3.2.5 Whenever the thermal desorption - GC/MS analytical method is changed or major equipment maintenance is performed, you must conduct a new five-level calibration (see section 10.0). System calibration remains valid as long as results from subsequent CCV are within 30 percent of the most recent 5-point calibration (see section 9.13). Include relevant CCV data in the supporting information in the data report for each set of samples.
11.3.2.6 Document, flag and explain all sample results that exceed the calibration range. Report flags and provide documentation in the analytical results for the affected sample(s).
12.0 Data Analysis, Calculations, and Reporting
12.1 Recordkeeping Procedures for Sorbent Tubes
12.1.1 Label sample tubes with a unique identification number as described in Section 6.3.
12.1.2 Keep records of the tube numbers and sorbent lots used for each sampling period.
12.1.3 Keep records of sorbent tube packing if tubes are manually prepared in the laboratory and not supplied commercially. These records must include the masses and/or bed lengths of sorbent(s) contained in each tube, the maximum allowable temperature for that tube and the date each tube was packed. If a tube is repacked at any stage, record the date of tube repacking and any other relevant information required in Section 12.1.
12.1.4 Keep records of the conditioning and blanking of tubes. These records must include, but are not limited to, the unique identification number and measured background resulting from the tube conditioning.
12.1.5 Record the location, dates, tube identification and times associated with each sample collection. Record this information on a Chain of Custody form that is sent to the analytical laboratory.
12.1.6 Field sampling personnel must complete and send a Chain of Custody to the analysis laboratory (see Section 8.6.4 of Method 325A for what information to include and Section 17.0 of this method for an example form). Duplicate copies of the Chain of Custody must be included with the sample report and stored with the field test data archive.
12.1.7 Field sampling personnel must also keep records of the unit vector wind direction, sigma theta, temperature and barometric pressure averages for the sampling period. See Section 8.3.4 of Method 325A.
12.1.8 Laboratory personnel must record the sample receipt date, and analysis date.
12.1.9 Laboratory personnel must maintain records of the analytical method and sample results in electronic or hardcopy in sufficient detail to reconstruct the calibration, sample, and quality control results from each sampling period.
12.2 Calculations
12.2.1 Complete the calculations in this section to determine compliance with calibration quality control criteria (see also Table 17.1).
12.2.1.1 Response factor (RF). Calculate the RF using Equation 12.1:

Where:
As = Peak area for the characteristic ion of the analyte.
Ais = Peak area for the characteristic ion of the internal standard.
Ms = Mass of the analyte.
Mis = Mass of the internal standard.
12.2.1.2 Standard deviation of the response factors (SDRF). Calculate the SDRF using Equation 12.2:

Where:
RFi = RF for each of the calibration compounds.
RF = Mean RF for each compound from the initial calibration.
n = Number of calibration standards.
12.2.1.3 Percent deviation (%DEV). Calculate the %DEV using Equation 12.3:
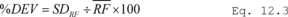
Where:
SDRF = Standard deviation.
RF = Mean RF for each compound from the initial calibration.
12.2.1.4 Relative percent difference (RPD). Calculate the RPD using Equation 12.4:
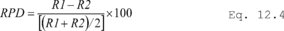
Where:
R1, R2 = Values that are being compared (i.e., response factors in CCV).
12.2.2 Determine the equivalent concentrations of compounds in atmospheres as follows. Correct target compound concentrations determined at the sampling site temperature and atmospheric pressure to standard conditions (25°C and 760 mm mercury) using Equation 12.5.
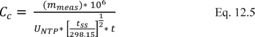
Where:
mmeas = The mass of the compound as measured in the sorbent tube (µg).
t = The exposure time (minutes).
tss = The average temperature during the collection period at the sampling site (K).
UNTP = The method defined diffusive uptake rate (sampling rate) (mL/min).
Note: Diffusive uptake rates (UNTP) for common VOCs, using carbon sorbents packed into sorbent tubes of the dimensions specified in section 6.1, are listed in Table 12.1. Adjust analytical conditions to keep expected sampled masses within range (see sections 11.3.1.3 to 11.3.1.5). Best possible method detection limits are typically in the order of 0.1 ppb for 1,3-butadiene and 0.05 ppb for volatile aromatics such as benzene for 14-day monitoring. However, actual detection limits will depend upon the analytical conditions selected.
| Compound |
Carbopack
TM X a |
Carbograph
TM 1
TD |
Carbopack
TM B |
|---|---|---|---|
| 1,1-Dichloroethene | 0.57 ±0.14 | not available | not available. |
| 3-Chloropropene | 0.51 ±0.3 | not available | not available. |
| 1,1-Dichloroethane | 0.57 ±0.1 | not available | not available. |
| 1,2-Dichloroethane | 0.57 ±0.08 | not available | not available. |
| 1,1,1-Trichloroethane | 0.51 ±0.1 | not available | not available. |
| Benzene | 0.67 ±0.06 | 0.63 ±0.07 b | 0.63 ±0.07 b. |
| Carbon tetrachloride | 0.51 ±0.06 | not available | not available. |
| 1,2-Dichloropropane | 0.52 ±0.1 | not available | not available. |
| Trichloroethene | 0.5 ±0.05 | not available | not available. |
| 1,1,2-Trichloroethane | 0.49 ±0.13 | not available | not available. |
| Toluene | 0.52 ±0.14 | 0.56 ±0.06 c | 0.56 ±0.06 c. |
| Tetrachloroethene | 0.48 ±0.05 | not available | not available. |
| Chlorobenzene | 0.51 ±0.06 | not available | not available. |
| Ethylbenzene | 0.46 ±0.07 | not available | 0.50 c. |
| m,p-Xylene | 0.46 ±0.09 | 0.47 ±0.04 c | 0.47 ±0.04 c. |
| Styrene | 0.5 ±0.14 | not available | not available. |
| o-Xylene | 0.46 ±0.12 | 0.47 ±0.04 c | 0.47 ±0.04 c. |
| p-Dichlorobenzene | 0.45 ±0.05 | not available | not available. |
| a Reference 3, McClenny, J. Environ. Monit. 7:248-256. Based on 24-hour duration.
b Reference 24, BS EN 14662-4:2005 (incorporated by reference - see §63.14). Based on 14-day duration. c Reference 25, ISO 16017-2:2003(E) (incorporated by reference - see §63.14). Based on 14-day duration. | |||
13.0 Method Performance
The performance of this procedure for VOC not listed in Table 12.1 is determined using the procedure in Addendum A of this Method or by one of the following national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14).
13.1 The valid range for measurement of VOC is approximately 0.5 µg/m 3 to 5 mg/m 3 in air, collected over a 14-day sampling period. The upper limit of the useful range depends on the split ratio selected (Section 11.3.1) and the dynamic range of the analytical system. The lower limit of the useful range depends on the noise from the analytical instrument detector and on the blank level of target compounds or interfering compounds on the sorbent tube (see Section 13.3).
13.2 Diffusive sorbent tubes compatible with passive sampling and thermal desorption methods have been evaluated at relatively high atmospheric concentrations (i.e., mid-ppb to ppm) and published for use in workplace air and industrial/mobile source emissions (References 15-16, 21-22).
13.3 Best possible detection limits and maximum quantifiable concentrations of air pollutants range from sub-part-per-trillion (sub-ppt) for halogenated species such as CCl4 and the freons using an electron capture detector (ECD), SIM Mode GC/MS, triple quad MS or GC/TOF MS to sub-ppb for volatile hydrocarbons collected over 72 hours followed by analysis using GC with quadrupole MS operated in the full SCAN mode.
13.3.1 Actual detection limits for atmospheric monitoring vary depending on several key factors. These factors are:
Minimum artifact levels.
GC detector selection.
Time of exposure for passive sorbent tubes.
Selected analytical conditions, particularly column resolution and split ratio.
14.0 Pollution Prevention
This method involves the use of ambient concentrations of gaseous compounds that post little or no danger of pollution to the environment.
15.0 Waste Management
Dispose of expired calibration solutions as hazardous materials. Exercise standard laboratory environmental practices to minimize the use and disposal of laboratory solvents.
16.0 References
1. Winberry, W. T. Jr., et al., Determination of Volatile Organic Compounds in Ambient Air Using Active Sampling onto Sorbent Tubes: Method TO-17r, Second Edition, U.S. Environmental Protection Agency, Research Triangle Park, NC 27711, January 1999. http://www.epa.gov/ttnamti1/airtox.html#compendium
2. Ciccioli, P., Brancaleoni, E., Cecinato, A., Sparapini, R., and Frattoni, M., “Identification and Determination of Biogenic and Anthropogenic VOCs in Forest Areas of Northern and Southern Europe and a Remote Site of the Himalaya Region by High-resolution GC-MS,” J. of Chrom., 643, pp 55-69, 1993.
3. McClenny, W.A., K.D. Oliver, H.H. Jacumin, Jr., E.H. Daughtrey, Jr., D.A. Whitaker. 2005. 24 h diffusive sampling of toxic VOCs in air onto Carbopack TM X solid adsorbent followed by thermal desorption/GC/MS analysis - laboratory studies. J. Environ. Monit. 7:248-256.
4. Markes International (www.markes.com/publications): Thermal desorption Technical Support Note 2: Prediction of uptake rates for diffusive tubes.
5. Ciccioli, P., Brancaleoni, E., Cecinato, A., DiPalo, C., Brachetti, A., and Liberti, A., “GC Evaluation of the Organic Components Present in the Atmosphere at Trace Levels with the Aid of CarbopackTM B for Preconcentration of the Sample,” J. of Chrom., 351, pp 433-449, 1986.
6. Broadway, G. M., and Trewern, T., “Design Considerations for the Optimization of a Packed Thermal Desorption Cold Trap for Capillary Gas Chromatography,” Proc. 13th Int'l Symposium on Capil. Chrom., Baltimore, MD, pp 310-320, 1991.
7. Broadway, G. M., “An Automated System for use Without Liquid Cryogen for the Determination of VOC's in Ambient Air,” Proc. 14th Int'l. Symposium on Capil. Chrom., Baltimore, MD, 1992.
8. Gibitch, J., Ogle, L., and Radenheimer, P., “Analysis of Ozone Precursor Compounds in Houston, Texas Using Automated Continuous GCs,” in Proceedings of the Air and Waste Management Association Conference: Measurement of Toxic and Related Air Pollutants, Air and Waste Management Association, Pittsburgh, PA, May 1995.
9. Vandendriessche, S., and Griepink, B., “The Certification of Benzene, Toluene and m-Xylene Sorbed on Tenax® TA in Tubes,” CRM-112 CEC, BCR, EUR12308 EN, 1989.
10. MDHS 2 (Acrylonitrile in Air), “Laboratory Method Using Porous Polymer Adsorption Tubes, and Thermal Desorption with Gas Chromatographic Analysis,” Methods for the Determination of Hazardous Substances (MDHS), UK Health and Safety Executive, Sheffield, UK.
11. MDHS 22 (Benzene in Air), “Laboratory Method Using Porous Polymer Adsorbent Tubes, Thermal Desorption and Gas Chromatography,” Method for the Determination of Hazardous Substances (MDHS), UK Health and Safety Executive, Sheffield, UK.
12. MDHS 23 (Glycol Ether and Glycol Acetate Vapors in Air), “Laboratory Method Using Tenax® Sorbent Tubes, Thermal Desorption and Gas Chromatography,” Method for the Determination of Hazardous Substances (MDHS), UK Health and Safety Executive, Sheffield, UK.
13. MDHS 40 (Toluene in air), “Laboratory Method Using Pumped Porous Polymer Adsorbent Tubes, Thermal Desorption and Gas Chromatography,” Method for the Determination of Hazardous Substances (MDHS), UK Health and Safety Executive, Sheffield, UK.
14. MDHS 60 (Mixed Hydrocarbons (C to C) in Air), “Laboratory Method Using Pumped Porous Polymer 3 10 and Carbon Sorbent Tubes, Thermal Desorption and Gas Chromatography,” Method for the Determination of Hazardous Substances (MDHS), UK Health and Safety Executive, Sheffield, UK.
15. Price, J. A., and Saunders, K. J., “Determination of Airborne Methyl tert-Butyl Ether in Gasoline Atmospheres,” Analyst, Vol. 109, pp. 829-834, July 1984.
16. Coker, D. T., van den Hoed, N., Saunders, K. J., and Tindle, P. E., “A Monitoring Method for Gasoline Vapour Giving Detailed Composition,” Ann. Occup, Hyg., Vol 33, No. 11, pp 15-26, 1989.
17. DFG, “Analytische Methoden zur prufing gesundheitsschadlicher Arbeistsstoffe,” Deutsche Forschungsgemeinschaft, Verlag Chemie, Weinheim FRG, 1985.
18. NNI, “Methods in NVN Series (Luchtkwaliteit; Werkplekatmasfeer),” Nederlands Normailsatie - Institut, Delft, The Netherlands, 1986-88.
19. “Sampling by Solid Adsorption Techniques,” Standards Association of Australia Organic Vapours, Australian Standard 2976, 1987.
20. Woolfenden, E. A., “Monitoring VOCs in Air Using Pumped Sampling onto Sorbent Tubes Followed by Thermal Desorption-capillary GC Analysis: Summary of Reported Data and Practical Guidelines for Successful Application,” J. Air & Waste Manage. Assoc., Vol. 47, 1997, pp. 20-36.
21. Validation Guidelines for Air Sampling Methods Utilizing Chromatographic Analysis, OSHA T-005, Version 3.0, May 2010, http://www.osha.gov/dts/sltc/methods/chromguide/chromguide.pdf.
22. ASTM D4597-10, Standard Practice for Sampling Workplace Atmospheres to collect Gases or Vapors with Solid Sorbent Diffusive Samplers.
23. Martin, http://www.hsl.gov.uk/media/1619/issue14.pdf.
24. BS EN 14662-4:2005, Ambient air quality - Standard method for the measurement of benzene concentrations - Part 4: Diffusive sampling followed by thermal desorption and gas chromatography.
25. ISO 16017-2:2003(E): Indoor, ambient and workplace air - Sampling and analysis of volatile organic compounds by sorbent tube/thermal desorption/capillary gas chromatography - Part 2: Diffusive sampling.
17.0 Tables, Diagrams, Flowcharts and Validation Data >
| Parameter | Frequency | Acceptance criteria | Corrective action |
|---|---|---|---|
| Bromofluorobenzene Instrument Tune Performance Check | Daily a prior to sample analysis | Evaluation criteria presented in Section 9.5 and Table 9.2 |
(1) Retune and or
(2) Perform Maintenance. |
| Five point calibration bracketing the expected sample concentration | Following any major change, repair or maintenance or if daily CCV does not meet method requirements. Recalibration not to exceed three months |
(1) Percent Deviation (%DEV) of response factors ±30%
(2) Relative Retention Times (RRTs) for target peaks ±0.06 units from mean RRT |
(1) Repeat calibration sample analysis.
(2) Repeat linearity check. (3) Prepare new calibration standards as necessary and repeat analysis. |
| Calibration Verification (CCV Second source calibration verification check) | Following the calibration curve | The response factor ±30% DEV from calibration curve average response factor |
(1) Repeat calibration check.
(2) Repeat calibration curve. |
| Laboratory Blank Analysis | Daily a following bromofluoro benzene and calibration check; prior to sample analysis |
(1) ≤0.2 ppbv per analyte or ≤3 times the LOD, whichever is greater
(2) Internal Standard (IS) area response ±40% and IS Retention Time (RT) ±0.33 min. of most recent calibration check |
(1) Repeat analysis with new blank tube.
(2) Check system for leaks, contamination. (3) Analyze additional blank. |
| Blank Sorbent Tube Certification | One tube analyzed for each batch of tubes cleaned or 10 percent of tubes whichever is greater | <0.2 ppbv per VOC targeted compound or 3 times the LOD, whichever is greater | Re-clean all tubes in batch and reanalyze. |
| Samples - Internal Standards | All samples | IS area response ±40% and IS RT ±0.33 min. of most recent calibration validation | Flag Data for possible invalidation. |
| Field Blanks | Two per sampling period | No greater than one-third of the measured target analyte or compliance limit | Flag Data for possible invalidation due to high blank bias. |
| a Every 24 hours. | |||
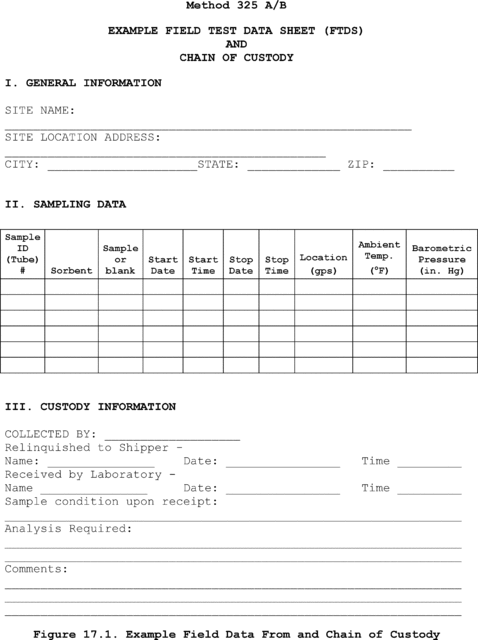
ADDENDUM A to Method 325B - Method 325 Performance Evaluation
A.1 Scope and Application
A.1.1 To be measured by Methods 325A and 325B, each new target volatile organic compound (VOC) or sorbent that is not listed in Table 12.1 must be evaluated by exposing the selected sorbent tube to a known concentration of the target compound(s) in an exposure chamber following the procedure in this Addendum or by following the procedures in the national/international standard methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14), or reported in peer-reviewed open literature.
A.1.2 You must determine the uptake rate and the relative standard deviation compared to the theoretical concentration of volatile material in the exposure chamber for each of the tests required in this method. If data that meet the requirement of this Addendum are available in the peer reviewed open literature for VOCs of interest collected on your passive sorbent tube configuration, then such data may be submitted in lieu of the testing required in this Addendum.
A.1.3 You must expose sorbent tubes in a test chamber to parts per trillion by volume (pptv) and low parts per billion by volume (ppbv) concentrations of VOCs in humid atmospheres to determine the sorbent tube uptake rate and to confirm compound capture and recovery.
A.2 Summary of Method
Note:
The technique described here is one approach for determining uptake rates for new sorbent/sorbate pairs. It is equally valid to follow the techniques described in any one of the following national/international standards methods: ISO 16017-2:2003(E), ASTM D6196-03 (Reapproved 2009), or BS EN 14662-4:2005 (all incorporated by reference - see §63.14).
A.2.1 Known concentrations of VOC are metered into an exposure chamber containing sorbent tubes filled with media selected to capture the volatile organic compounds of interest (see Figure A.1 and A.2 for an example of the exposure chamber and sorbent tube retaining rack). VOC are diluted with humid air and the chamber is allowed to equilibrate for 6 hours. Clean passive sampling devices are placed into the chamber and exposed for a measured period of time. The passive uptake rate of the passive sampling devices is determined using the standard and dilution gas flow rates. Chamber concentrations are confirmed with whole gas sample collection and analysis or direct interface volatile organic compound measurement methods.
A.2.2 An exposure chamber and known gas concentrations must be used to challenge and evaluate the collection and recovery of target compounds from the sorbent and tube selected to perform passive measurements of VOC in atmospheres.
A.3 Definitions
A.3.1 cc is cubic centimeter.
A.3.2 ECD is electron capture detector.
A.3.3 FID is flame ionization detector.
A.3.4 LED is light-emitting diode.
A.3.5 MFC is mass flow controller.
A.3.6 MFM is mass flow meter.
A.3.7 min is minute.
A.3.8 ppbv is parts per billion by volume.
A.3.9 ppmv is parts per million by volume.
A.3.10 PSD is passive sampling device.
A.3.11 psig is pounds per square inch gauge.
A.3.12 RH is relative humidity.
A.3.13 VOC is volatile organic compound.
A.4 Interferences
A.4.1 VOC contaminants in water can contribute interference or bias results high. Use only distilled, organic-free water for dilution gas humidification.
A.4.2 Solvents and other VOC-containing liquids can contaminate the exposure chamber. Store and use solvents and other VOC-containing liquids in the exhaust hood when exposure experiments are in progress to prevent the possibility of contamination of VOCs into the chamber through the chamber's exhaust vent.
Note:
Whenever possible, passive sorbent evaluation should be performed in a VOC free laboratory.
A.4.3 PSDs should be handled by personnel wearing only clean, white cotton or powder free nitrile gloves to prevent contamination of the PSDs with oils from the hands.
A.4.4 This performance evaluation procedure is applicable to only volatile materials that can be measured accurately with direct interface gas chromatography or whole gas sample collection, concentration and analysis. Alternative methods to confirm the concentration of volatile materials in exposure chambers are subject to Administrator approval.
A.5 Safety
A.5.1 This procedure does not address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate field and laboratory safety and health practices and determine the applicability of regulatory limitations prior to use.
A.5.2 Laboratory analysts must exercise appropriate care in working with high-pressure gas cylinders.
A.6 Equipment and Supplies
A.6.1 You must use an exposure chamber of sufficient size to simultaneously expose a minimum of eight sorbent tubes.
A.6.2 Your exposure chamber must not contain VOC that interfere with the compound under evaluation. Chambers made of glass and/or stainless steel have been used successfully for measurement of known concentration of selected VOC compounds.
A.6.3 The following equipment and supplies are needed:
Clean, white cotton or nitrile gloves;
Conditioned passive sampling device tubes and diffusion caps; and
NIST traceable high resolution digital gas mass flow meters (MFMs) or flow controllers (MFCs).
A.7 Reagents and Standards
A.7.1 You must generate an exposure gas that contains between 35 and 75 percent relative humidity and a concentration of target compound(s) within 2 to 5 times the concentration to be measured in the field.
A.7.2 Target gas concentrations must be generated with certified gas standards and diluted with humid clean air. Dilution to reach the desired concentration must be done with zero grade air or better.
A.7.3 The following reagents and standards are needed:
Distilled water for the humidification;
VOC standards mixtures in high-pressure cylinder certified by the supplier (Note: The accuracy of the certified standards has a direct bearing on the accuracy of the measurement results. Typical vendor accuracy is ±5 percent accuracy but some VOC may only be available at lower accuracy (e.g., acrolein at 10 percent)); and
Purified dilution air containing less than 0.2 ppbv of the target VOC.
A.8 Sample Collection, Preservation and Storage
A.8.1 You must use certified gas standards diluted with humid air. Generate humidified air by adding distilled organic free water to purified or zero grade air. Humidification may be accomplished by quantitative addition of water to the air dilution gas stream in a heated chamber or by passing purified air through a humidifying bubbler. You must control the relative humidity in the test gas throughout the period of passive sampler exposure.
Note:
The RH in the exposure chamber is directly proportional to the fraction of the purified air that passes through the water in the bubbler before entering the exposure chamber. Achieving uniform humidification in the proper range is a trial-and-error process with a humidifying bubbler. You may need to heat the bubbler to achieve sufficient humidity. An equilibration period of approximately 15 minutes is required following each adjustment of the air flow through the humidifier. Several adjustments or equilibration cycles may be required to achieve the desired RH level.
Note:
You will need to determine both the dilution rate and the humidification rate for your design of the exposure chamber by trial and error before performing method evaluation tests.
A.8.2 Prepare and condition sorbent tubes following the procedures in Method 325B Section 7.0.
A.8.3 You must verify that the exposure chamber does not leak.
A.8.4 You must complete two evaluation tests using a minimum of eight passive sampling tubes in each test with less than 5-percent depletion of test analyte by the samplers.
A.8.4.1 Perform at least one evaluation at two to five times the estimated analytical detection limit or less.
A.8.4.2 Perform second evaluation at a concentration equivalent to the middle of the analysis calibration range.
A.8.5 You must evaluate the samplers in the test chamber operating between 35 percent and 75 percent RH, and at 25 ±5°C. Allow the exposure chamber to equilibrate for 6 hours before starting an evaluation.
A.8.6 The flow rate through the chamber must be ≤0.5 meter per second face velocity across the sampler face.
A.8.7 Place clean, ready to use sorbent tubes into the exposure chamber for predetermined amounts of time to evaluate collection and recovery from the tubes. The exposure time depends on the concentration of volatile test material in the chamber and the detection limit required for the sorbent tube sampling application. Exposure time should match sample collection time. The sorbent tube exposure chamber time may not be less than 24 hours and should not be longer than 2 weeks.
A.8.7.1 To start the exposure, place the clean PSDs equipped with diffusion caps on the tube inlet into a retaining rack.
A.8.7.2 Place the entire retaining rack inside the exposure chamber with the diffusive sampling end of the tubes facing into the chamber flow. Seal the chamber and record the exposure start time, chamber RH, chamber temperature, PSD types and numbers, orientation of PSDs, and volatile material mixture composition (see Figure A.2).
A.8.7.3 Diluted, humidified target gas must be continuously fed into the exposure chamber during cartridge exposure. Measure the flow rate of target compound standard gas and dilution air to an accuracy of 5 percent.
A.8.7.4 Record the time, temperature, and RH at the beginning, middle, and end of the exposure time.
A.8.7.5 At the end of the exposure time, remove the PSDs from the exposure chamber. Record the exposure end time, chamber RH, and temperature.
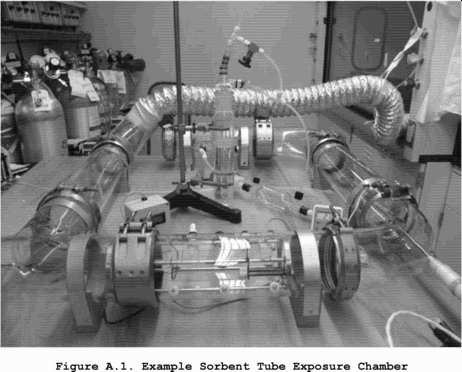
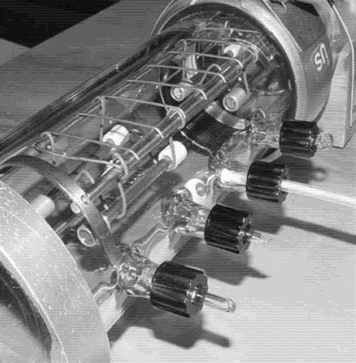
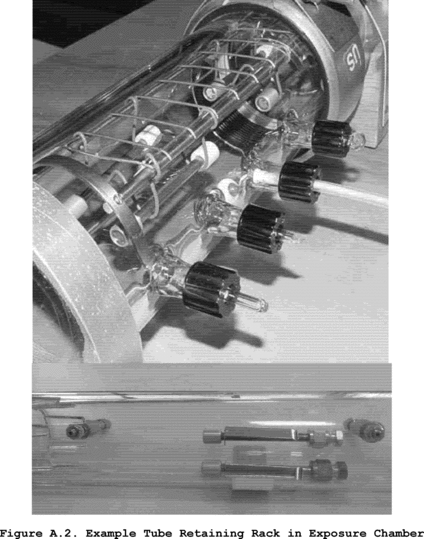
A.9 Quality Control
A.9.1 Monitor and record the exposure chamber temperature and RH during PSD exposures.
A.9.2 Measure the flow rates of standards and purified humified air immediately following PSD exposures.
A.10 Calibration and Standardization
A.10.1 Follow the procedures described in Method 325B Section 10.0 for calibration.
A.10.2 Verify chamber concentration by direct injection into a gas chromatograph calibrated for the target compound(s) or by collection of an integrated SUMMA canister followed by analysis using a preconcentration gas chromatographic method such as EPA Compendium Method TO-15, Determination of VOCs in Air Collected in Specially-Prepared Canisters and Analyzed By GC/MS.
A.10.2.1 To use direct injection gas chromatography to verify the exposure chamber concentration, follow the procedures in Method 18 of 40 CFR part 60, Appendix A-6. The method ASTM D6420-99 (Reapproved 2010) (incorporated by reference - see §63.14) is an acceptable alternative to EPA Method 18 of 40 CFR part 60).
Note:
Direct injection gas chromatography may not be sufficiently sensitive for all compounds. Therefore, the whole gas preconcentration sample and analysis method may be required to measure at low concentrations.
A.10.2.2 To verify exposure chamber concentrations using SUMMA canisters, prepare clean canister(s) and measure the concentration of VOC collected in an integrated SUMMA canister over the period used for the evaluation (minimum 24 hours). Analyze the TO-15 canister sample following EPA Compendium Method TO-15.
A.10.2.3 Compare the theoretical concentration of volatile material added to the test chamber to the measured concentration to confirm the chamber operation. Theoretical concentration must agree with the measured concentration within 30 percent.
A.11 Analysis Procedure
Analyze the sorbent tubes following the procedures described in Section 11.0 of Method 325B.
A.12 Recordkeeping Procedures for Sorbent Tube Evaluation
Keep records for the sorbent tube evaluation to include at a minimum the following information:
A.12.1 Sorbent tube description and specifications.
A.12.2 Sorbent material description and specifications.
A.12.3 Volatile analytes used in the sampler test.
A.12.4 Chamber conditions including flow rate, temperature, and relative humidity.
A.12.5 Relative standard deviation of the sampler results at the conditions tested.
A.12.6 95 percent confidence limit on the sampler overall accuracy.
A.12.7 The relative accuracy of the sorbent tube results compared to the direct chamber measurement by direct gas chromatography or SUMMA canister analysis.
A.13 Method Performance
A.13.1 Sorbent tube performance is acceptable if the relative accuracy of the passive sorbent sampler agrees with the active measurement method by ±10 percent at the 95 percent confidence limit and the uptake ratio is equal to greater than 0.5 mL/min (1 ng/ppm-min).
Note:
For example, there is a maximum deviation comparing Perkin-Elmer passive type sorbent tubes packed with Carbopack TM X of 1.3 to 10 percent compared to active sampling using the following uptake rates.
|
1,3-butadiene
uptake rate mL/min |
Estimated
detection limit (2 week) |
Benzene
uptake rates mL/min |
Estimated
detection limit (2 week) | |
|---|---|---|---|---|
| Carbopack TM X (2 week) | 0.61 ±0.11 a | 0.1 ppbv | 0.67 a | 0.05 ppbv |
| a McClenny, W.A., K.D. Oliver, H.H. Jacumin, Jr., E.H. Daughtrey, Jr., D.A. Whitaker. 2005. 24 h diffusive sampling of toxic VOCs in air onto Carbopack TM X solid adsorbent followed by thermal desorption/GC/MS analysis - laboratory studies. J. Environ. Monit. 7:248-256. | ||||
A13.2 Data Analysis and Calculations for Method Evaluation
A.13.2.1 Calculate the theoretical concentration of VOC standards using Equation A.1.

Where:
Cf = The final concentration of standard in the exposure chamber (ppbv).
FRi = The flow rate of the target compound I (mL/min).
FRt = The flow rate of all target compounds from separate if multiple cylinders are used (mL/min).
FRa = The flow rate of dilution air plus moisture (mL/min).
Cs = The concentration of target compound in the standard cylinder (parts per million by volume).
A.13.2.3 Determine the uptake rate of the target gas being evaluated using Equation A.2.

Where:
MX = The mass of analyte measured on the sampling tube (ηg).
Ce = The theoretical exposure chamber concentration (ηg/mL).
Tt = The exposure time (minutes).
A.13.2.4 Estimate the variance (relative standard deviation (RSD)) of the inter-sampler results at each condition tested using Equation A.3. RSD for the sampler is estimated by pooling the variance estimates from each test run.

Where:
Xi = The measured mass of analyte found on sorbent tube i.
Xi = The mean value of all Xi.
n = The number of measurements of the analyte.
A.13.2.4 Determine the percent relative standard deviation of the inter-sampler results using Equation A.4.

A.13.2.5 Determine the 95 percent confidence interval for the sampler results using Equation A.5. The confidence interval is determined based on the number of test runs performed to evaluate the sorbent tube and sorbent combination. For the minimum test requirement of eight samplers tested at two concentrations, the number of tests is 16 and the degrees of freedom are 15.
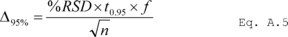
Where:
Δ95% = 95 percent confidence interval.
%RSD = percent relative standard deviation.
t0.95 = The Students t statistic for f degrees of freedom at 95 percent confidence.
f = The number of degrees of freedom.
n = Number of samples.
A.13.2.6 Determine the relative accuracy of the sorbent tube combination compared to the active sampling results using Equation A.6.
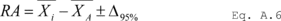
Where:
RA = Relative accuracy.
Xi = The mean value of all Xi.
Xi = The average concentration of analyte measured by the active measurement method.
Δ95% = 95 percent confidence interval.
A.14 Pollution Prevention
This method involves the use of ambient concentrations of gaseous compounds that post little or no pollution to the environment.
A.15 Waste Management
Expired calibration solutions should be disposed of as hazardous materials.
A.16 References
1. ISO TC 146/SC 02 N 361 Workplace atmospheres - Protocol for evaluating the performance of diffusive samplers.
Method 326 - Method for Determination of Isocyanates in Stationary Source Emissions
1.0 Scope and Application
This method is applicable to the collection and analysis of isocyanate compounds from the emissions associated with manufacturing processes. This method is not inclusive with respect to specifications (e.g., equipment and supplies) and sampling procedures essential to its performance. Some material is incorporated by reference from other EPA methods. Therefore, to obtain reliable results, persons using this method should have a thorough knowledge of at least Method 1, Method 2, Method 3, and Method 5 found in Appendices A-1, A-2, and A-3 in Part 60 of this title.
1.1 Analytes. This method is designed to determine the mass emission of isocyanates being emitted from manufacturing processes. The following is a table (Table 1-1) of the isocyanates and the manufacturing process at which the method has been evaluated:
| Compound's name | CAS No. | Detection limit (ng/m 3) a | Manufacturing process |
|---|---|---|---|
| 2,4-Toluene Diisocyanate (TDI) | 584-84-9 | 106 | Flexible Foam Production. |
| 1,6-Hexamethylene Diisocyanate (HDI) | 822-06-0 | 396 | Paint Spray Booth. |
| Methylene Diphenyl Diisocyanate (MDI) | 101-68-8 | 112 | Pressed Board Production. |
| Methyl Isocyanate (MI) | 624-83-0 | 228 | Not used in production. |
| a Estimated detection limits are based on a sample volume of 1 m 3 and a 10-ml sample extraction volume. | |||
1.2 Applicability. Method 326 is a method designed for determining compliance with National Emission Standards for Hazardous Air Pollutants (NESHAP). Method 326 may also be specified by New Source Performance Standards (NSPS), State Implementation Plans (SIPs), and operating permits that require measurement of isocyanates in stationary source emissions, to determine compliance with an applicable emission standard or limit.
1.3 Data Quality Objectives (DQO). The principal objective is to ensure the accuracy of the data at the actual emissions levels and in the actual emissions matrix encountered. To meet this objective, method performance tests are required and NIST-traceable calibration standards must be used.
2.0 Summary of Method
2.1 Gaseous and/or aerosol isocyanates are withdrawn from an emission source at an isokinetic sampling rate and are collected in a multicomponent sampling train. The primary components of the train include a heated probe, three impingers containing derivatizing reagent in toluene, an empty impinger, an impinger containing charcoal, and an impinger containing silica gel.
2.2 The liquid impinger contents are recovered, concentrated to dryness under vacuum, brought to volume with acetonitrile (ACN) and analyzed with a high pressure liquid chromatograph (HPLC).
3.0 Definitions [Reserved]
4.0 Interferences
4.1 The greatest potential for interference comes from an impurity in the derivatizing reagent, 1-(2-pyridyl)piperazine (1,2-PP). This compound may interfere with the resolution of MI from the peak attributed to unreacted 1,2-PP.
4.2 Other interferences that could result in positive or negative bias are (1) alcohols that could compete with the 1,2-PP for reaction with an isocyanate and (2) other compounds that may co-elute with one or more of the derivatized isocyanates.
4.3 Method interferences may be caused by contaminants in solvents, reagents, glassware, and other sample processing hardware. All these materials must be routinely shown to be free from interferences under conditions of the analysis by preparing and analyzing laboratory method (or reagent) blanks.
4.3.1 Glassware must be cleaned thoroughly before using. The glassware should be washed with laboratory detergent in hot water followed by rinsing with tap water and distilled water. The glassware may be dried by baking in a glassware oven at 400°C for at least one hour. After the glassware has cooled, it should be rinsed three times with methylene chloride and three times with acetonitrile. Volumetric glassware should not be heated to 400°C. Instead, after washing and rinsing, volumetric glassware may be rinsed with acetonitrile followed by methylene chloride and allowed to dry in air.
4.3.2 The use of high purity reagents and solvents helps to reduce interference problems in sample analysis.
5.0 Safety
5.1 Organizations performing this method are responsible for maintaining a current awareness file of Occupational Safety and Health Administration (OSHA) regulations regarding safe handling of the chemicals specified in this method. A reference file of material safety data sheets should also be made available to all personnel involved in performing the method. Additional references to laboratory safety are available.
6.0 Equipment and Supplies
6.1 Sample Collection. A schematic of the sampling train used in this method is shown in Figure 207-1. This sampling train configuration is adapted from Method 5 procedures, and, as such, most of the required equipment is identical to that used in Method 5 determinations. The only new component required is a condenser.
6.1.1 Probe Nozzle. Borosilicate or quartz glass; constructed and calibrated according to Method 5, sections 6.1.1.1 and 10.1, and coupled to the probe liner using a Teflon union; a stainless steel nut is recommended for this union. When the stack temperature exceeds 210°C (410°F), a one-piece glass nozzle/liner assembly must be used.
6.1.2 Probe Liner. Same as Method 5, section 6.1.1.2, except metal liners shall not be used. Water-cooling of the stainless steel sheath is recommended at temperatures exceeding 500°C (932°F). Teflon may be used in limited applications where the minimum stack temperature exceeds 120°C (250°F) but never exceeds the temperature where Teflon is estimated to become unstable [approximately 210°C (410°F)].
6.1.3 Pitot Tube, Differential Pressure Gauge, Filter Heating System, Metering System, Barometer, Gas Density Determination Equipment. Same as Method 5, sections 6.1.1.3, 6.1.1.4, 6.1.1.6, 6.1.1.9, 6.1.2, and 6.1.3.
6.1.4 Impinger Train. Glass impingers are connected in series with leak-free ground-glass joints following immediately after the heated probe. The first impinger shall be of the Greenburg-Smith design with the standard tip. The remaining five impingers shall be of the modified Greenburg-Smith design, modified by replacing the tip with a 1.3-cm ( 1/2-in.) I.D. glass tube extending about 1.3 cm ( 1/2 in.) from the bottom of the outer cylinder. A water-jacketed condenser is placed between the outlet of the first impinger and the inlet to the second impinger to reduce the evaporation of toluene from the first impinger.
6.1.5 Moisture Measurement. For the purpose of calculating volumetric flow rate and isokinetic sampling, you must also collect either Method 4 in Appendix A-3 to this part or other moisture measurement methods approved by the Administrator concurrent with each Method 326 test run.
6.2 Sample Recovery
6.2.1 Probe and Nozzle Brushes; Polytetrafluoroethylene (PTFE) bristle brushes with stainless steel wire or PTFE handles are required. The probe brush shall have extensions constructed of stainless steel, PTFE, or inert material at least as long as the probe. The brushes shall be properly sized and shaped to brush out the probe liner and the probe nozzle.
6.2.2 Wash Bottles. Three. PTFE or glass wash bottles are recommended; polyethylene wash bottles must not be used because organic contaminants may be extracted by exposure to organic solvents used for sample recovery.
6.2.3 Glass Sample Storage Containers. Chemically resistant, borosilicate amber glass bottles, 500-mL or 1,000-mL. Bottles should be tinted to prevent the action of light on the sample. Screw-cap liners shall be either PTFE or constructed to be leak-free and resistant to chemical attack by organic recovery solvents. Narrow-mouth glass bottles have been found to leak less frequently.
6.2.4 Graduated Cylinder. To measure impinger contents to the nearest 1 ml or 1 g. Graduated cylinders shall have subdivisions not >2 mL.
6.2.5 Plastic Storage Containers. Screw-cap polypropylene or polyethylene containers to store silica gel and charcoal.
6.2.6 Funnel and Rubber Policeman. To aid in transfer of silica gel or charcoal to container (not necessary if silica gel is weighed in field).
6.2.7 Funnels. Glass, to aid in sample recovery.
6.3 Sample Preparation and Analysis.
The following items are required for sample analysis.
6.3.1 Rotary Evaporator. Buchii Model EL-130 or equivalent.
6.3.2 1000 ml Round Bottom Flask for use with a rotary evaporator.
6.3.3 Separatory Funnel. 500-ml or larger, with PTFE stopcock.
6.3.4 Glass Funnel. Short-stemmed or equivalent.
6.3.5 Vials. 15-ml capacity with PTFE lined caps.
6.3.6 Class A Volumetric Flasks. 10-ml for bringing samples to volume after concentration.
6.3.7 Filter Paper. Qualitative grade or equivalent.
6.3.8 Buchner Funnel. Porcelain with 100 mm ID or equivalent.
6.3.9 Erlenmeyer Flask. 500-ml with side arm and vacuum source.
6.3.10 HPLC with at least a binary pumping system capable of a programmed gradient.
6.3.11 Column Systems Column systems used to measure isocyanates must be capable of achieving separation of the target compounds from the nearest eluting compound or interferents with no more than 10 percent peak overlap.
6.3.12 Detector. UV detector at 254 nm. A fluorescence detector (FD) with an excitation of 240 nm and an emission at 370 nm may be also used to allow the detection of low concentrations of isocyanates in samples.
6.3.13 Data system for measuring peak areas and retention times.
7.0 Reagents and Standards
7.1 Sample Collection Reagents.
7.1.1 Charcoal. Activated, 6-16 mesh. Used to absorb toluene vapors and prevent them from entering the metering device. Use once with each train and discard.
7.1.2 Silica Gel and Crushed Ice. Same as Method 5, sections 7.1.2 and 7.1.4 respectively
7.1.3 Impinger Solution. The impinger solution is prepared by mixing a known amount of 1-(2-pyridyl) piperazine (purity 99.5+%) in toluene (HPLC grade or equivalent). The actual concentration of 1,2-PP should be approximately four times the amount needed to ensure that the capacity of the derivatizing solution is not exceeded. This amount shall be calculated from the stoichiometric relationship between 1,2-PP and the isocyanate of interest and preliminary information about the concentration of the isocyanate in the stack emissions. A concentration of 130 µg/ml of 1,2-PP in toluene can be used as a reference point. This solution shall be prepared, stored in a refrigerated area away from light, and used within ten days of preparation.
7.2 Sample Recovery Reagents.
7.2.1 Toluene. HPLC grade is required for sample recovery and cleanup (see Note to 7.2.2 below).
7.2.2 Acetonitrile. HPLC grade is required for sample recovery and cleanup. Note: Organic solvents stored in metal containers may have a high residue blank and should not be used. Sometimes suppliers transfer solvents from metal to glass bottles; thus blanks shall be run before field use and only solvents with a low blank value should be used.
7.3 Analysis Reagents. Reagent grade chemicals should be used in all tests. All reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available.
7.3.1 Toluene, C6H5CH3. HPLC Grade or equivalent.
7.3.2 Acetonitrile, CH3CN (ACN). HPLC Grade or equivalent.
7.3.3 Methylene Chloride, CH2Cl2. HPLC Grade or equivalent.
7.3.4 Hexane, C6H14. HPLC Grade or equivalent.
7.3.5 Water, H2O. HPLC Grade or equivalent.
7.3.6 Ammonium Acetate, CH3CO2NH4.
7.3.7 Acetic Acid (glacial), CH3CO2H.
7.3.8 1-(2-Pyridyl)piperazine, (1,2-PP), ≥99.5% or equivalent.
7.3.9 Absorption Solution. Prepare a solution of 1-(2-pyridyl)piperazine in toluene at a concentration of 40 mg/300 ml. This solution is used for method blanks and method spikes.
7.3.10 Ammonium Acetate Buffer Solution (AAB). Prepare a solution of ammonium acetate in water at a concentration of 0.1 M by transferring 7.705 g of ammonium acetate to a 1,000 ml volumetric flask and diluting to volume with HPLC Grade water. Adjust pH to 6.2 with glacial acetic acid.
8.0 Sample Collection, Storage and Transport
Note:
Because of the complexity of this method, field personnel should be trained in and experienced with the test procedures in order to obtain reliable results.
8.1 Sampling
8.1.1 Preliminary Field Determinations. Same as Method 5, section 8.2.
8.1.2 Preparation of Sampling Train. Follow the general procedure given in Method 5, section 8.3.1, except for the following variations: Place 300 ml of the impinger absorbing solution in the first impinger and 200 ml each in the second and third impingers. The fourth impinger shall remain empty. The fifth and sixth impingers shall have 400 g of charcoal and 200-300 g of silica gel, respectively. Alternatively, the charcoal and silica gel may be combined in the fifth impinger. Set-up the train as in Figure 326-1. During assembly, do not use any silicone grease on ground-glass joints.
Note:
During preparation and assembly of the sampling train, keep all openings where contamination can occur covered with PTFE film or aluminum foil until just before assembly or until sampling is about to begin.
8.1.3 Leak-Check Procedures. Follow the leak-check procedures given in Method 5, sections 8.4.2 (Pretest Leak-Check), 8.4.3 (Leak-Checks During the Sample Run), and 8.4.4 (Post-Test Leak-Check), with the exception that the pre-test leak-check is mandatory
8.1.4 Sampling Train Operation. Follow the general procedures given in Method 5, section 8.5. Turn on the condenser coil coolant recirculating pump and monitor the gas entry temperature. Ensure proper gas entry temperature before proceeding and again before any sampling is initiated. It is important that the gas entry temperature not exceed 50°C (122°F), thus reducing the loss of toluene from the first impinger. For each run, record the data required on a data sheet such as the one shown in Method 5, Figure 5-3.
8.2 Sample Recovery. Allow the probe to cool. When the probe can be handled safely, wipe off all external particulate matter near the tip of the probe nozzle and place a cap over the tip to prevent losing or gaining particulate matter. Do not cap the probe tip tightly while the sampling train is cooling down because this will create a vacuum in the train. Before moving the sample train to the cleanup site, remove the probe from the sample train and cap the opening to the probe, being careful not to lose any condensate that might be present. Cap the impingers and transfer the probe and the impinger/condenser assembly to the cleanup area. This area should be clean and protected from the weather to reduce sample contamination or loss. Inspect the train prior to and during disassembly and record any abnormal conditions. It is not necessary to measure the volume of the impingers for the purpose of moisture determination as the method is not validated for moisture determination. Treat samples as follows:
8.2.1 Container No. 1, Probe and Impinger Numbers 1 and 2. Rinse and brush the probe/nozzle first with toluene twice and then twice again with acetonitrile and place the wash into a glass container labeled with the test run identification and “Container No. 1.” When using these solvents ensure that proper ventilation is available. Quantitatively transfer the liquid from the first two impingers and the condenser into Container No. 1. Rinse the impingers and all connecting glassware twice with toluene and then twice again with acetonitrile and transfer the rinses into Container No. 1. After all components have been collected in the container, seal the container, and mark the liquid level on the bottle.
8.2.2 Container No. 2, Impingers 3 and 4. Quantitatively transfer the liquid from each impinger into a glass container labeled with the test run identification and “Container No. 2.” Rinse each impinger and all connecting glassware twice with toluene and twice again with acetonitrile and transfer the rinses into Container No. 2. After all components have been collected in the container, seal the container, and mark the liquid level on the bottle.
Note:
The contents of the fifth and sixth impinger (silica gel) can be discarded.
8.2.3 Container No. 3, Reagent Blank. Save a portion of both washing solutions (toluene/acetonitrile) used for the cleanup as a blank. Transfer 200 ml of each solution directly from the wash bottle being used and combine in a glass sample container with the test identification and “Container No. 3.” Seal the container, and mark the liquid level on the bottle and add the proper label.
8.2.4 Field Train Proof Blanks. To demonstrate the cleanliness of sampling train glassware, you must prepare a full sampling train to serve as a field train proof blank just as it would be prepared for sampling. At a minimum, one complete sampling train will be assembled in the field staging area, taken to the sampling area, and leak-checked. The probe of the blank train shall be heated during and the train will be recovered as if it were an actual test sample. No gaseous sample will be passed through the sampling train. Field blanks are recovered in the same manner as described in sections 8.2.1 and 8.2.2 and must be submitted with the field samples collected at each sampling site.
8.2.5 Field Train Spike. To demonstrate the effectiveness of the sampling train, field handling, and recovery procedures you must prepare a full sampling train to serve as a field train spike just as it would be prepared for sampling. The field spike is performed in the same manner as the field train proof blank with the additional step of adding the Field Spike Solution to the first impinger after the initial leak check. The train will be recovered as if it were an actual test sample. No gaseous sample will be passed through the sampling train. Field train spikes are recovered in the same manner as described in sections 8.2.1 and 8.2.2 and must be submitted with the samples collected for each test program.
8.3 Sample Transport Procedures. Containers must remain in an upright position at all times during shipment. Samples must also be stored at <4°C between the time of sampling and concentration. Each sample should be extracted and concentrated within 30 days after collection and analyzed within 30 days after extraction. The extracted sample must be stored at 4°C.
8.4 Sample Custody. Proper procedures and documentation for sample chain of custody are critical to ensuring data integrity. The chain of custody procedures in ASTM D4840-99 (Reapproved 2018) e “Standard Guide for Sampling Chain-of-Custody Procedures” (incorporated by reference, see §63.14) shall be followed for all samples (including field samples and blanks).
9.0 Quality Control
9.1 Sampling. Sampling Operations. The sampling quality control procedures and acceptance criteria are listed in Table 326-2 below; see also section 9.0 of Method 5.
9.2 Analysis. The analytical quality control procedures required for this method includes the analysis of the field train proof blank, field train spike, and reagent and method blanks. Analytical quality control procedures and acceptance criteria are listed in Table 326-3 below.
9.2.1 Check for Breakthrough. Recover and determine the isocyanate(s) concentration of the last two impingers separately from the first two impingers.
9.2.2 Field Train Proof Blank. Field blanks must be submitted with the samples collected at each sampling site.
9.2.3 Reagent Blank and Field Train Spike. At least one reagent blank and a field train spike must be submitted with the samples collected for each test program.
9.2.4 Determination of Method Detection Limit. Based on your instrument's sensitivity and linearity, determine the calibration concentrations or masses that make up a representative low level calibration range. The MDL must be determined at least annually for the analytical system using an MDL study such as that found in section 15.0 to Method 301 of appendix A to part 63 of this chapter.
| QA/QC criteria | Acceptance criteria | Frequency | Consequence if not met |
|---|---|---|---|
| Sampling Equipment Leak Checks | ≤0.00057 m3/min (0.020 cfm) or 4% of sampling rate, whichever is less | Prior to, during (optional) and at the completion to sampling | Prior to: Repair and repeat calibration. During/Completion: None, testing should be considered invalid. |
| Dry Gas Meter Calibration - Pre-Test (individual correction factor - Yi) | within ±2% of average factor (individual) | Pre-test | Repeat calibration point. |
| Dry Gas Meter Calibration - Pre-Test (average correction factor - Yc) | 1.00 ±1% | Pre-test | Adjust the dry gas meter and recalibrate. |
| Dry Gas Meter Calibration - Post-test | Average dry gas meter calibration factor agrees with ±5% Yc | Each Test | Adjust sample volumes using the factor that gives the smallest volume. |
| Temperature sensor calibration | Absolute temperature measures by sensor within ±1.5% of a reference sensor | Prior to initial use and before each test thereafter | Recalibrate; sensor may not be used until specification is met. |
| Barometer calibration | Absolute pressure measured by instrument within ±10 mm Hg of reading with a mercury barometer or NIST traceable barometer | Prior to initial use and before each test thereafter | Recalibrate; instrument may not be used until specification is met. |
| QA/QC criteria | Acceptance criteria | Frequency | Consequence if not met |
|---|---|---|---|
| Calibration - Method Blanks | <5% level of expected analyte | Each analytical method blank | Locate source of contamination; reanalyze. |
| Calibration - Calibration Points | At least six calibration point bracketing the expected range of analysis | Each analytical batch | Incorporate additional calibration points to meet criteria. |
| Calibration - Linearity | Correlation coefficient >0.995 | Each analytical batch | Verify integration, reintegrate. If necessary, recalibrate. |
| Calibration - secondary standard verification | Within ±10% of true value | After each calibration | Repeat secondary standard verification, recalibrate if necessary. |
| Calibration - continual calibration verification | Within ±10% of true value | Daily and after every ten samples | Invalidate previous ten sample analysis, recalibrate and repeat calibration, reanalyze samples until successful. |
| Sample Analysis | Within the valid calibration range | Each sample | Invalidate the sample if greater than the calibration range and dilute the sample so that it is within the calibration range. Appropriately flag any value below the calibration range. |
| Replicate Samples | Within ±10% of RPD | Each sample | Evaluate integrations and repeat sample analysis as necessary. |
| Field Train Proof Blank | ≤10% level of expected analyte | Each test program | Evaluate source of contamination. |
| Field Train Spike | Within ±30% of true value | Each test program | Evaluate performance of the method and consider invalidating results. |
| Breakthrough | Final two impingers Mass collected is >5% of the total mass or >20% of the total mass when the measured results are 20% of the applicable standard. Alternatively, there is no breakthrough requirement when the measured results are 10% of the applicable standard | Each test run | Invalidate test run. |
10.0 Calibration and Standardization
Note:
Maintain a laboratory log of all calibrations.
10.1 Probe Nozzle, Pitot Tube Assembly, Dry Gas Metering System, Probe Heater, Temperature Sensors, Leak-Check of Metering System, and Barometer. Same as Method 5, sections 10.1, 10.2, 10.3, 10.4, 10.5, 8.4.1, and 10.6, respectively.
10.2 High Performance Liquid Chromatograph. Establish the retention times for the isocyanates of interest; retention times will depend on the chromatographic conditions. The retention times provided in Table 10-1 are provided as a guide to relative retention times when using a C18, 250 mm x 4.6 mm ID, 5µm particle size column, a 2 ml/min flow rate of a 1:9 to 6:4 Acetonitrile/Ammonium Acetate Buffer, a 50 µl sample loop, and a UV detector set at 254 nm.
| Retention times | |
|---|---|
| Compound |
Retention
time (minutes) |
| MI | 10.0 |
| 1,6-HDI | 19.9 |
| 2,4-TDI | 27.1 |
| MDI | 27.3 |
10.3 Preparation of Isocyanate Derivatives.
10.3.1 HDI, TDI, MDI. Dissolve 500 mg of each isocyanate in individual 100 ml aliquots of methylene chloride (MeCl2), except MDI which requires 250 ml of MeCl2. Transfer a 5-ml aliquot of 1,2-PP (see section 7.3.8) to each solution, stir and allow to stand overnight at room temperature. Transfer 150 ml aliquots of hexane to each solution to precipitate the isocyanate-urea derivative. Using a Buchner funnel, vacuum filter the solid-isocyanate-urea derivative and rinse with 50 ml of hexane. Dissolve the precipitate in a minimum aliquot of MeCl2. Repeat the hexane precipitation and filtration twice. After the third filtration, dry the crystals at 50°C and transfer to bottles for storage. The crystals are stable for at least 21 months when stored at room temperature in a closed container.
10.3.2 MI. Prepare a 200 µg/ml stock solution of methyl isocyanate-urea, transfer 60 mg of 1,2-PP to a 100-ml volumetric flask containing 50 ml of MeCl2. Carefully transfer 20 mg of methyl isocyanate to the volumetric flask and shake for 2 minutes. Dilute the solution to volume with MeCl2 and transfer to a bottle for storage. Methyl isocyanate does not produce a solid derivative and standards must be prepared from this stock solution.
10.4 Preparation of calibration standards. Prepare a 100 µg/ml stock solution of the isocyanates of interest from the individual isocyanate-urea derivative as prepared in sections 10.3.1 and 10.3.2. This is accomplished by dissolving 1 mg of each isocyanate-urea derivative in 10 ml of Acetonitrile. Calibration standards are prepared from this stock solution by making appropriate dilutions of aliquots of the stock into Acetonitrile.
10.5 Preparation of Method Blanks. Prepare a method blank for each test program (up to twenty samples) by transferring 300 ml of the absorption solution to a 1,000-ml round bottom flask and concentrate as outlined in section 11.2.
10.6 Preparation of Field Spike Solution. Prepare a field spike solution for every test program in the same manner as calibration standards (see Section 10.4). The mass of the target isocyanate in the volume of the spike solution for the field spike train shall be equivalent to that estimated to be captured from the source concentration for each compound; alternatively, you may also prepare a solution that represents half the applicable standard.
10.7 HPLC Calibrations. See Section 11.1.
11.0 Analytical Procedure
11.1 Analytical Calibration. Perform a multipoint calibration of the instrument at six or more upscale points over the desired quantitative range (multiple calibration ranges shall be calibrated, if necessary). The field samples analyzed must fall within at least one of the calibrated quantitative ranges and meet the performance criteria specified below. The lowest point in your calibration curve must be at least 5, and preferably 10, times the MDL. For each calibration curve, the value of the square of the linear correlation coefficient, i.e., r 2, must be ≥0.995, and the analyzer response must be within ±10 percent of the reference value at each upscale calibration point. Calibrations must be performed on each day of the analysis, before analyzing any of the samples. Following calibration, a secondary standard shall be analyzed. A continual calibration verification (CCV) must also be performed prior to any sample and after every ten samples. The measured value of this independently prepared standard must be within ±10 percent of the expected value. Report the results for each calibration standard secondary standard, and CCV as well as the conditions of the HPLC. The reports should include at least the peak area, height, and retention time for each isocyanate compound measured as well as a chromatogram for each standard.
11.2 Concentration of Samples. Transfer each sample to a 1,000-ml round bottom flask. Attach the flask to a rotary evaporator and gently evaporate to dryness under vacuum in a 65°C water bath. Rinse the round bottom flask three times each with 2 ml of acetonitrile and transfer the rinse to a 10-ml volumetric flask. Dilute the sample to volume with acetonitrile and transfer to a 15-ml vial and seal with a PTFE lined lid. Store the vial ≤4°C until analysis.
11.3 Analysis. Analyze replicative samples by HPLC, using the appropriate conditions established in section 10.2. The width of the retention time window used to make identifications should be based upon measurements of actual retention time variations of standards over the course of a day. Three times the standard deviation of a retention time for a compound can be used to calculate a suggested window size; however, the experience of the analyst should weigh heavily in the interpretation of the chromatograms. If the peak area exceeds the linear range of the calibration curve, the sample must be diluted with acetonitrile and reanalyzed. Average the replicate results for each run. For each sample you must report the same information required for analytical calibrations (Section 11.1). For non-detect or values below the detection limit of the method, you shall report the value as “<” numerical detection limit.
12.0 Data Analysis and Calculations
Nomenclature and calculations, same as in Method 5, section 6, with the following additions below.
12.1 Nomenclature.
AS = Response of the sample, area counts.
b = Y-intercept of the linear regression line, area counts.
BR = Percent Breakthrough
CA = Concentration of a specific isocyanate compound in the initial sample, µg/ml.
CB = Concentration of a specific isocyanate compound in the replicate sample, µg/ml.
CI = Concentration of a specific isocyanate compound in the sample, µg/ml.
Crec = Concentration recovered from spike train, µg/ml.
CS = Concentration of isocyanate compound in the stack gas, µg/dscm
CT = Concentration of a specific isocyanate compound (Impingers 1-4), µg/dscm
Cspike = Concentration spiked, µg/ml.
C4 = Concentration of a specific isocyanate compound (Impingers 14), µg/dscm
FIm = Mass of Free Isocyanate
FTSrec = Field Train Spike Recovery
Im = Mass of the Isocyanate
Imw = MW of the Isocyanate
IUm = Mass of Isocyanate-urea derivative
IUmw = MW of the isocyanate-urea
M = Slope of the linear regression line, area counts-ml/µg.
mI = Mass of isocyanate in the total sample
MW = Molecular weight
RPD = Relative Percent Difference
VF = Final volume of concentrated sample, typically 10 ml.
Vmstd = Volume of gas sample measured by the dry-gas meter, corrected to standard conditions, dscm (dscf).
12.2 Conversion from Isocyanate to the Isocyanate-urea derivative. The equation for converting the amount of free isocyanate to the corresponding amount of isocyanate-urea derivative is as follows:
12.2 Conversion from Isocyanate to the Isocyanate-urea derivative. The equation for converting the amount of free isocyante to the corresponding amount of isocyante-urea derivative is as follows:
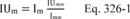
The equation for converting the amount of IU derivative to the corresponding amount of FLm is as follows:
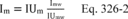
12.3 Calculate the correlation coefficient, slope, and intercepts for the calibration data using the least squares method for linear regression. Concentrations are expressed as the x-variable and response is expressed as the y-variable.
12.4 Calculate the concentration of isocyanate in the sample:

12.5 Calculate the total amount collected in the sample by multiplying the concentration (µg/ml) times the final volume of acetonitrile (10 ml).
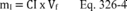
12.6 Calculate the concentration of isocyanate (µg/dscm) in the stack gas.
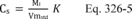
12.7 Calculate Relative Percent Difference (RPD) for each replicative sample
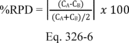
12.8 Calculate Field Train Spike Recovery
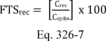
12.9 Calculate Percent Breakthrough
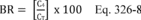
Where:
K = 35.314 ft 3/m 3 if Vm(std) is expressed in English units. = 1.00 m 3/m 3 if Vm(std) is expressed in metric units.
13.0 Method Performance
Evaluation of sampling and analytical procedures for a selected series of compounds must meet the quality control criteria (See Section 9) for each associated analytical determination. The sampling and analytical procedures must be challenged by the test compounds spiked at appropriate levels and carried through the procedures.
14.0 Pollution Prevention [Reserved]
15.0 Waste Management [Reserved]
16.0 Alternative Procedures [Reserved]
17.0 References
1. Martin, R.M., Construction Details of Isokinetic Source-Sampling Equipment, Research Triangle Park, NC, U.S. Environmental Protection Agency, April 1971, PB-203 060/BE, APTD-0581, 35 pp.
2. Rom, J.J., Maintenance, Calibration, and Operation of Isokinetic Source Sampling Equipment, Research Triangle Park, NC, U.S. Environmental Protection Agency, March 1972, PB-209 022/BE, APTD-0576, 39 pp.
3. Schlickenrieder, L.M., Adams, J.W., and Thrun, K.E., Modified Method 5 Train and Source Assessment Sampling System: Operator's Manual, U.S. Environmental Protection Agency, EPA/600/8-85/003/1985).
4. Shigehara, R.T., Adjustments in the EPA Nomograph for Different Pitot Tube Coefficients and Dry Molecular Weights, Stack Sampling News, 2:4-11 (October 1974).
5. U.S. Environmental Protection Agency, 40 CFR part 60, Appendices A-1, A-2, and A-3, Methods 1-5.
6. Vollaro, R.F., A Survey of Commercially Available Instrumentation for the Measurement of Low-Range Gas Velocities, Research Triangle Park, NC, U.S. Environmental Protection Agency, Emissions Measurement Branch, November 1976 (unpublished paper).
18.0 Diagrams
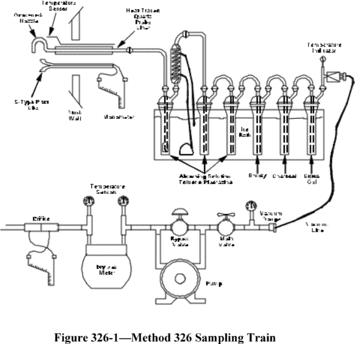
Editorial Note: For Federal Register citations affecting appendix A to part 63, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and at www.govinfo.gov.
Method 327—Fugitive and Area Source Measurement of Selected Volatile Organic Hazardous Air Pollutants Using Specially Prepared Canisters
1.0 Scope and Application
1.1 This method describes the sampling and analysis of emissions from fugitive and area sources collected using specially prepared canisters and analyzed using a gas chromatograph (GC) coupled with a low- or high-resolution mass spectrometer (MS) for the determination of the airborne concentration of selected volatile organic hazardous air pollutants (oHAPs) such as ethylene oxide or vinyl chloride.
1.2 Applicability. The use of this method is strictly intended for determining airborne concentrations of selected speciated oHAPs to determine compliance with a fenceline emission standard and/or work practices when specified by the applicable regulation. This method includes data quality objectives (DQOs) specific to the measurement of airborne concentrations of speciated oHAPs and must not be used for other compliance purposes (i.e., measurements from ducted sources).
1.3 The analytical approach for this method uses a GC coupled with a low- or high-resolution MS, which may consist of a linear quadrupole, ion trap, or time-of-flight (TOF) system. Speciated oHAPs are identified by a combination of the retention times (RTs) and the associated mass spectra by comparing observed fragmentation patterns to reference spectral patterns and relative ion abundances established during calibration. For the speciated oHAPs, the intensity of the observed quantitation ion in the unknown sample is compared with the system response to the same ion for known amounts of the compound.
1.4 The sampling and analytical approach included in this method is based on previously published EPA guidance in Compendium Method TO-15A, which describes the sampling and analytical procedures for measuring volatile organic compounds (VOCs) in ambient air.
2.0 Summary of Method
2.1 In this method, a whole air sample is collected through a particulate filter with a flow control device into an evacuated, specially prepared canister for a length of time specified by the applicable regulation, typically 24 hours. After the air sample is collected, the canister valve is closed, the canister pressure is measured, and the canister is transported to the laboratory for analysis. Upon receipt at the laboratory, the sample collection information is verified, the canister pressure is measured, and the canister is stored at ambient laboratory temperature until analysis. For analysis, a known volume of the sample is directed from the canister into a preconcentrator to collect speciated oHAPs from the sample aliquot and to allow the majority of bulk gases (e.g., nitrogen, oxygen, argon, and carbon dioxide) and water vapor to be vented.
2.2 The laboratory, field laboratory, and field personnel must have experience with sampling trace-level oHAPs using specially prepared canisters and with operating preconcentrator/GC/multidetector instrumentation (e.g., MS) for trace-level analysis.
2.3 This method is performance-based and includes a description of the equipment, instruments, operations, and acceptance and performance criteria. EPA developed these criteria to ensure the collection of high-quality data. Laboratories must develop their own standard operating procedure (SOP) documents describing the equipment, equipment management, targeted compounds, procedures, and quality assurance (QA) activities specific to that laboratory, instrumentation, and potentially specific for the targeted analyte.
2.4 The key steps of this method required for the collection of each sample include stringent leak testing under stop flow, using certified and clean canisters, using certified sampling devices, collecting accurate field data, and collecting field blanks and duplicates. The key steps of this method required for sample analysis include the analysis of blanks, use of high-quality reference standards, and initial and ongoing calibration checks of the instruments used.
3.0 Definitions
3.1 Absolute pressure means the pressure measured with reference to absolute zero pressure, usually expressed in units of kilopascal (kPa) absolute or pounds per square inch absolute (psia).
3.2 Analytical batch means the batch of samples analyzed over a 24-hour period beginning with the daily instrument tune performance check.
3.2 Collocated precision means the precision determined from the analyzed concentrations of samples collected simultaneously from the same air mass using two discrete canisters and collected through two separate sampling devices with separate inlets. This determines the precision of the method including the sampling and analysis processes. Collocated precision is determined by calculating the absolute relative percent difference (RPD) for the collocated measurements (the absolute value of the difference between the two collocated sample results divided by their average value and expressed as a percentage).
3.3 Continuing calibration verification sample (CCV) means single level calibration samples run conducted periodically to confirm that the analytical system continues to generate sample results within acceptable agreement to the current calibration curve.
3.4 Cryogen means a refrigerant used to obtain sub-ambient temperatures in the preconcentrator and/or the GC oven. Typical cryogens are liquid nitrogen (boiling point [BP] −195.8 °C), liquid argon (BP −185.7 °C), and liquid carbon dioxide (BP −79.5 °C).
3.5 Deionized water means ASTM Type I water or equivalent.
3.6 Diluent gas means hydrocarbon-free (HCF) synthetic “zero” air.
3.7 Dynamic dilution means a technique for preparing calibration mixtures in which standard gas(es) from pressurized cylinders are continuously blended with a diluent gas (such as humidified HCF zero air) in a mixing chamber or manifold so that a flowing stream of calibration mixture is created.
3.8 Gauge pressure means the pressure measured with reference to the surrounding atmospheric pressure, usually expressed in units of kPa or inches of mercury (Hg). Gauge pressure is zero-referenced against ambient air pressure; zero is equal to the local atmospheric (barometric) pressure, which is nominally 101.3 kPa (29.92 in. Hg or 14.7 psia) at sea level.
3.9 Mass spectrometer means an instrument that ionizes molecules and atoms (typically into electrically charged fragments), separates these ions according to their mass-to-charge ratio (m/z or m/e), and responds to the impact of the ions based on their population. MS systems suitable for this method include quadrupole, ion trap, and TOF detectors. Quadrupole and ion trap MS operating modes (i.e., full-scan, selected ion monitoring [SIM], and selected ion storage [SIS] modes) can be selected to optimize the ion mass collection range.
3.10 Mechanical Flow Controlling Device (MFCD) means a device that is used to ensure constant flow to an evacuated canister to near ambient pressure. MCFD are designed to maintain a constant pressure drop (and thus a constant flow rate) across a restrictive orifice by allowing a constant leak rate of sample into the canister as the canister vacuum decreases to near ambient pressure without power.
3.11 Nominal concentration means a requested, target, or named concentration that approximates the true, reference, or certified concentration. For example, a nominal 200 parts per trillion by volume (pptv) standard may have an actual certified concentration of 206 pptv.
3.12 Preconcentrator means a device used to concentrate the target compound(s) while the bulk gases are effectively removed. The target compound(s) are then desorbed and injected into a GC-MS system.
3.13 Quantitative accuracy means the degree of measurement accuracy required to measure the concentration of an identified compound, within a given tolerance of uncertainty, with an analytical system.
3.14 Replicate precision means the precision determined from repeated analysis of a gas sample from one canister, which may be evaluated by calculating the absolute RPD for pairwise measurements (N = 2) or by determining the relative standard deviation (RSD) for replicate measurements where N ≥ 3. Replicate analyses are used to determine precision of the analysis processes and do not provide information on sampling precision.
3.15 Second Source Calibration Verification (SSCV) Standard means a humidified calibration standard prepared from a calibration stock gas procured from a separate supplier. An SSCV can only be prepared with a calibration stock from the same supplier if it is unavailable from another supplier and is prepared from a different lot of source material as the primary calibration stock.
3.16 Static dilution means a technique for preparing calibration mixtures in which standard and diluent gases are added to a fixed-volume vessel or chamber at a known ratio. Standard and diluent gas amounts may be measured gravimetrically, by volume, and/or by pressure differential from pressurized cylinders or as neat materials and blended with a known amount of diluent gas (such as humidified zero air) in a mixing chamber or manifold.
3.17 Target concentration means desired, estimated, or approximate concentration (see “nominal concentration” above).
3.18 Theoretical concentration means a reference concentration derived by applying measurements performed with calibrated instruments with known tolerances to a certified reference standard concentration value. Measurements of the target compound(s) concentrations are to be determined using a calibration that is developed based on theoretical concentrations.
3.19 Time-of-flight (TOF) mass spectrometry means a MS method that determines the ion's mass-to-charge ratio by measuring the time the ion takes to reach the detector.
3.20 Wetted surfaces mean the surfaces of the flow path, canister, valving, pumps, etc., that contact the gas undergoing collection, mixing, transfer, or analysis.
4.0 Interferences
4.1 Sample Collection. There are potential physical interferents which could impact the ability to properly time-integrate the sampling, such as leaks of the sampling system or introduction of foreign material (e.g., particulate matter [PM], insect nests, spider webs). These interferences are mitigated by closely following the sampling protocols included in this method (e.g., leak check procedures and sampling system requirements).
4.2 Canister Sampling Media Interferences. Each canister will have its own specific performance characteristics and appropriate cleaning, sampling, and handling procedures are required for attainment of acceptable initial and ongoing method performance. Failure to adhere to the cleaning and certification requirements included in this method may lead to the following interference issues:
(1) Incomplete deactivation of canister interior surfaces (e.g., canister welds) may result in active sites for adsorption or surfaces that facilitate the decomposition of labile VOCs to form other VOCs within the canister. Other potential sources of active sites include canister valves, valve stems, and ferrules. Damage to the canister interior that exposes untreated surfaces may also result in active sites.
(2) Entrained PM deposited in the canister sampling pathway can adsorb VOCs making them unavailable in the canister gas phase which interferes with collected samples. Such trapped VOCs can potentially desorb later and result in the inability to achieve canister cleanliness performance specifications and/or contaminate subsequent canister sampling events. Additionally, organic PM can react with co-sampled ozone or other oxidative species to form target VOCs. PM can also clog tiny openings in critical or restrictive orifices, which impacts collection flow rates.
(3) Under certain conditions, the composition of an air sample may change upon its introduction into the canister and over time such that the air in the canister no longer represents the air sampled. Such changes may be caused by interactions of the VOCs with the interior canister surface or between chemicals in the air matrix. The activity of the interior canister surface is unique to each canister and is based on several factors, including variability in canister manufacturing defects, differences in canister surface deactivation treatments, the presence of PM and co-collected moisture in the canister, and artifacts from reactions of VOCs on the canister walls.
(4) Condensed water within the canister can result in corrosion of the interior surface of canisters with weak or deficient coatings and can result in the partitioning of hydrophilic polar VOCs to liquid water. Under such circumstances, concentrations of these analytes in the gas phase will be biased low until the condensation is eliminated by reduction of the canister pressure below the vapor saturation pressure of water.
4.3 Analytical Interferences. Contamination within the analytical system may come from several sources including, but not limited to, off-gassing of materials within the sample introduction or preconcentrator flow path, carryover from high-concentration samples or standards, and solvent vapors within the laboratory.
(1) Active sites within the sample introduction or preconcentration flow path are often caused by use of improper materials or degradation of deactivated surfaces.
(2) Impurities in source materials or diluent gases for internal standard (IS) gas mixtures may result in contamination of target VOCs.
(3) Water and the delivery systems used to humidify canisters or diluent gas streams may contaminate the canister contents or humidified gases.
(4) Moisture in the sample gas may interfere with VOC analysis by GC-MS. Poor or inconsistent water management during preconcentration can cause peak broadening and RT shifts that can result in peak misidentification, particularly for hydrophilic polar compounds. Water management systems that use semipermeable fluoropolymer membranes remove oxygenated and polar VOCs from the sample matrix and exhibit memory effects for several VOCs. VOCs entrained in the fluoropolymer membrane can convert to ketones and alcohols, which are transported across the membrane bidirectionally such that these ketones and alcohols can contaminate the sample stream and VOCs in the sample stream can be adsorbed into the fluoropolymer and removed from the sample stream.
(5) Carbon dioxide in the collected sample can coelute with more volatile VOCs eluting early in the GC-MS run and interfere with their quantitation.
(6) Artifacts in chromatograms, such as silanol compounds formed from the breakdown of silicon-ceramic linings of canisters and siloxane compounds from the breakdown of the stationary phase in an analytical column, can interfere with identification and quantitation of less volatile VOCs.
(7) Be cognizant of compounds that interfere with target analytes when operating in MS modes that do not provide full-scan ion spectra (i.e., selected ion monitoring [SIM] and selected ion storage [SIS]). Such interfering coeluting compounds may share common ions, may have similar mass spectra, and may be difficult or impossible to separate from target VOCs.
5.0 Safety
This method does not address all the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate field and laboratory safety and health practices prior to use.
6.0 Equipment and Supplies
6.1 Specially Prepared Canisters. You must use specially prepared canisters at least 6 liters in volume that are suitable for trace gas analysis of the target compounds, such that they meet the requirements in Section 8.3 of this method. Canisters must be able to withstand numerous cycles of evacuation to high vacuum of 0.0067 kPa (0.002 in. Hg) and pressurization to 377 kPa (40 pounds per square inch gauge, psig).
6.2 Valves. You must use canisters with valves that are designed specifically for trace level measurements. The wetted portions of the valve must, at a minimum, be constructed of chromatographic-grade stainless steel (preferably type 316), and the valve seal surfaces must be metal to metal to minimize absorption and off-gassing of VOCs and other potential contaminants. It is recommended that valve designs have minimal internal volume and surface area to minimize the risk of contamination.
6.3 Canister Cleaning System. You must use a canister cleaning system that includes the following components.
6.3.1 Manifold constructed of chromatographic-grade stainless-steel tubing and connections for multiple canisters.
6.3.2 Oil-free vacuum pump capable of achieving vacuum of approximately 3.4 kPa absolute (1 in. Hg absolute or 0.5 psia).
6.3.3 High-vacuum pump for achieving a final canister vacuum of approximately 0.0067 kPa (0.002 in. Hg) or less.
6.3.4 Heating oven that can contain the canister and allow heating of the valve. The oven is also used to bake sampling system components.
6.3.5 Humidification system, such as humidifier impinger or bubbler, capable of achieving relative humidity (RH) of at least 50% in the cannister.
6.3.6 Programmable controller for selecting temperature and cycle time and for manually or automatically switching between evacuation and pressurization.
6.3.7 A pressure release valve to minimize the likelihood of system over pressurization.
6.3.8 Tubing and connections constructed of borosilicate glass, quartz glass, or chromatographic-grade stainless-steel (minimum type 316 or silicon-ceramic coated) to minimize dead volume of the system. You must not use butyl rubber or perfluoroalkoxy (PFA) materials. If needed for connections or seals, minimize the use of Viton and Teflon to avoid adsorption and/or off-gassing of compounds of interest or introduction of other potential interferences.
6.3.9 Purge gas such as HCF zero air or ultra-high purity (UHP)-grade cylinder nitrogen or liquid nitrogen dewar headspace.
6.3.10 Charcoal scrubber, catalytic oxidizer, or other systems for eliminating trace contaminants from the purge gas.
6.4 Sampling Device. The sampling device consists of the following equipment and for the purpose of this method, the sampling device consists of the aggregation of equipment in this section. The sampling device must be individually named with an alpha-numeric serial number that is unique.
6.4.1 A stainless-steel particulate filter with pore size of 2 to 7 micrometers (μm) installed on the sampling device inlet.
6.4.2 Sampling Probe. The internal volume of the sample probe must be less than 1% of the volume collected by the sample container with an inverted inlet (e.g., sampling cane to prevent the entry of water droplets) consisting of only chromatographic-grade stainless steel (including silicon-ceramic lined steel) placed 1.5 to 3 meters (4.9 to 9.8 feet) above the ground.
6.4.3 You must use an MFCD to regulate the flow at a constant flow rate over the 24-hour collection period into an evacuated stainless-steel canister.
6.4.4 Canister Sampling Timers (Optional). A device with an inert valve that can be programmed to automatically start and stop canister sampling periods
6.5 Vacuum/Pressure Gauges.
6.5.1 Field Pressure Measurement Gauge. A vacuum/pressure gauge or pressure transducer with an accuracy of ±0.25% full scale calibrated over the range of use for the application with sufficient resolution to permit precise measurement of pressure differentials must be used for field sampling purposes. The accuracy of the vacuum gauge must be measured verified on an annual basis against a National Institute of Standards and Technology (NIST)-certified standard.
6.5.2 Laboratory Canister Pressure Measurement Gauge. A vacuum/pressure gauge or pressure transducer with an accuracy of ±0.1% full scale or 0.13 kPa, whichever is smaller, calibrated over the range of use for the application with sufficient resolution to permit precise measurement of canister pressure must be used for pressurizing field samples with HCF zero air or ultrapure nitrogen for analysis. The accuracy of the vacuum gauge must be measured verified on an annual basis against a NIST-certified standard for analysis.
6.6 Gas Regulators. Regulators for high-pressure cylinders of dilution gas, stock standard gases, and internal standard gases must be constructed of non-reactive material, such as high-purity stainless steel, and may be lined with an appropriate material that is inert to the targeted VOC (e.g., silicon-ceramic). Do not use regulators that contain PFA materials (e.g., for seals and diaphragms) and avoid using regulators that contain Teflon products such as polytetr-rafluoroethylene (PTFE) and flouroethylenepropylene(FEP), where possible, to minimize memory effects. All regulators must be rated for the pressure and flow expected during use. Regulators must be dedicated to a specific task and labeled for use (e.g., do not use the same regulator on a high-concentration stock VOC standard cylinder and a low-concentration stock VOC cylinder).
Note:
Some new regulators (e.g., stainless steel regulators) should be sufficiently passivated prior to use to prevent potential sample loss.
6.7 Reference Flow Meters.
6.7.1 A flow meter (e.g., a calibrated mass flow meter (MFM), a volumetric reference standard, or other similar measurement device) calibrated to measurement range appropriate to measure continuous flow must be used. The flow meter must not interfere with the flow measurement (i.e., the pressure drop across the flow meter may affect the flow being measured).
6.7.2 Reference flow meters must be calibrated on an annual basis and be able to measure within ±2% of the predicted values (e.g., cubic centimeters per minute) against a NIST-traceable volumetric standard.
6.8 Tubing and Fittings. Connecting tubing and fittings for dilution and standard gases must be constructed of chromatographic-grade stainless steel (e.g., 316 type), which includes silicon-ceramic-treated stainless steel. Connections must be metal to metal. Lines may need to be heated to ensure that there is no condensation. You must not use PTFE thread sealants or Buna-N rubber components on any wetted surface in a sampling and analytical system.
6.9 Analytical Instrumentation. Conduct analyses under this method using any combination of preconcentrator, GC, and MS provided the equipment meets the performance specifications of this method.
6.9.1 Gas Chromatographic-Mass Spectrometric (GC-MS) System.
6.9.1.1 Gas Chromatograph. The GC used for analysis under this method must allow temperature programming with quick and accurate temperature ramping. If needed for separation of very light VOCs from the targeted oHAPs, the GC must be capable of sub-ambient cooling (e.g., −50 °C). Carrier gas connections must be constructed of stainless-steel or copper tubing.
6.9.1.2 Chromatographic Column. The capillary chromatographic column must be capable of achieving separation of target compounds and any potential interferences per Section 4 and maintaining retention time stability as required in Section 9.
6.9.1.3 Mass Spectrometer. The MS may be a linear quadrupole, ion trap, or TOF unit, and must have minimum resolution of 1 atomic mass unit (amu) or less. The MS must be capable of analyzing the desired mass range every 1 second or less and operate with an acquisition rate such that at least 12 measurements are performed over a typical chromatographic peak. Quadrupole and ion trap systems employing electron impact (EI) ionization mode must provide nominal 70 volt (V) electron energy in EI mode to produce a bromofluorobenzene (BFB) mass spectrum that meets all the instrument performance acceptance criteria as specified in this method.
6.10 Calibration Gas Standard Preparation Equipment. This section discusses the equipment needed to prepare working-level standards for calibrating the GC-MS by dilution of a higher concentration stock standard gas.
6.10.1 Dynamic Dilution System Instrumentation.
6.10.1.1 The dynamic dilution system must include, at a minimum, calibrated electronic mass flow controllers (MFCs) for the diluent gas and each standard gas to be diluted, a humidifier for the diluent gas, and a manifold or mixing chamber where the diluent and standard gases can be sufficiently combined before introduction to the preconcentrator or canister. The gas dilution system must produce calibration gases whose measured values are within ±2% of the predicted values. The predicted values are calculated based on the certified concentration of the supply gas (protocol gases, when available, are recommended for their accuracy) and the gas flow rates (or dilution ratios) through the gas dilution system.
6.10.1.2 Connection tubing for the dynamic dilution system must be constructed of chromatographic-grade or silicon-ceramic-coated stainless steel. Mixing chambers or manifolds must be constructed of chromatographic-grade or silicon-ceramic-coated stainless steel, borosilicate, or quartz glass.
6.10.1.3 The gas dilution system must be recalibrated at least once per two calendar years using NIST-traceable primary flow standards with an uncertainty ≤0.25%. You must report the results of the calibration whenever the dilution system is used, listing the date of the most recent calibration, the due date for the next calibration, calibration point, reference flow device (device identification [ID], serial number [SN], and acceptance criteria.
6.10.1.4 The gas dilution system must be verified to be non-biasing under HFC zero air and known standards at least one per calendar year for each reactive target compounds (e.g., ethylene oxide and vinyl chloride). Zero air must be flowed through all applicable MFCs, tubing, and manifold used and verified to not be detectable for the target compounds. Additionally, a known standard within the calibration range of the analytical system for each target compound must be flowed through all applicable MFCs, tubing, and manifold to allow equilibration and verified to not bias the standard by ±15% of the concentrations in the reference sample. The equilibration time for the bias verification must be used at a minimum for the development of standards.
6.10.1.5 The gas dilution system MFCs used must be verified quarterly, at a minimum, per Section 3.2 of Method 205 using any available protocol gas and corresponding reference method.
6.10.2 Static Dilution System Instrumentation.
6.10.2.1 The static dilution system must include, at a minimum, a calibrated pressure transducer or pressure gauge to measure the partial pressures of each standard gas to be diluted and the balance gas, and a manifold to introduce the gases into the working standard canister or vessel. Pressure transducer(s) or pressure gauge(s) used for static dilution must have an accuracy of ±0.1% full scale or 0.13 kPa, whichever is smaller, calibrated over the range of use for the application with sufficient resolution to permit precise measurement of pressure differentials.
6.10.2.2 Connection tubing for the static dilution system must be constructed of chromatographic-grade or silicon-ceramic-coated stainless steel. Manifolds must be constructed of chromatographic-grade or silicon-ceramic-coated stainless steel, borosilicate, or quartz glass.
6.10.2.3 The static gas dilution system must be recalibrated once per calendar year using NIST-traceable primary pressure gauge with an uncertainty ≤0.1%. You must report the results of the calibration whenever the dilution system is used, listing the date of the most recent calibration, the due date for the next calibration, calibration point, reference flow device (ID, S:N ratio), and acceptance criteria.
6.10.2.4 The gas dilution system must be verified to be non-biasing under HFC zero air and known standards at least one per calendar year for each reactive target compounds (e.g., ethylene oxide and vinyl chloride). Zero air must be flowed through all applicable tubing and manifold used and verified to not be detectable for the target compounds. Additionally, a known standard within the calibration range of the analytical system for each target compound must be flowed through all applicable tubing and manifold into the standard canister or vessel and verified to not bias the standard by ±15% of the concentrations in the reference sample.
6.11 Calibrated Hygrometer.
6.11.1 The calibrated hygrometer must be capable of a 1% RH resolution with a yearly calibration to a NIST-traceable accuracy of ±3% RH within the range of 20% to 80% RH.
6.11.2 The calibration hydrometer calibration must be verified weekly or per use (whichever is less stringent) at a single point that is approximatively 40 to 50% RH to within ±5% using a second calibrated hygrometer or a saturated salt solution.
7.0 Reagent and Standards
7.1 You must use only NIST-certified or NIST-traceable calibration standards, standard reference materials, and reagents that are stable through certification and recertification for the tests and procedures required by this method. You must use standards and reagents within their expiration period and evaluate working-level standards prepared in canisters within 30 days of preparation. The concentrations of the target compounds in the mixture must be commensurate with the anticipated dilution factor achievable by the laboratory needed to dilute the mixture to the desired working range. You must retain and report the gas standard certificates of analysis.
7.2 Carrier Gas. Use helium, hydrogen, or nitrogen as the carrier gas in the GC. Carrier gas must be ultrapure (99.999% pure or better).
7.3 HCF Zero Air. Purchase HCF zero air in high-pressure cylinders from reputable gas vendors or prepare HCF zero air by passing ambient air through molecular sieves, catalytic oxidizers, and subsequent charcoal filters or similar substrate. HFC zero air must contain impurities less than 20 pptv or undetected (whichever is more stringent) per compound of interest.
7.5 Nitrogen. Use ultrapure (99.999% pure or better) nitrogen from cylinders procured from commercial gas vendors or from the headspace gas from a liquid nitrogen dewar.
7.6 Cryogens. Cryogens (e.g., liquid nitrogen, liquid argon, and liquid carbon dioxide) specified by the instrument manufacturer, if needed.
7.7 Water for Canister Humidification. ASTM Type I (resistivity ≥18 megaohm-centimeter [MΩ·cm]) or equivalent.
8.0 Sample Collection and Preparation
This section presents the sample collection and handling procedures of this method with the initial and ongoing performance evaluation of materials used in sampling and analysis. This method allows the user to choose the materials used for sampling. You must record the exact materials used when conducting this method and include that information in any report associated with sampling according to this method.
8.1 Sampling Device Performance Tests. Prior to initial field deployment and as directed in this section, you must verify that all equipment used to conduct this method meets the performance criteria specified in this section. The primary objectives of the performance tests in this section are to characterize the sampling system and to verify that the sampling system used meets the criteria in the method. The sampling system performance tests include the following:
(a) Flow control verification test,
(b) Flow control flow check,
(c) Sampling device leak check,
(d) Sampling device bias check, and
(e) Sampling device standard check.
8.1.1 Flow Control Verification Test. Prior to initial field deployment and at least every twelve months, you must verify that the sampling device's ability to control flow to the canister is acceptable. Assemble an evacuated canister with the sampling device including filter connected to a certified flow meter. Figure 1 of Section 17 of this method provides an illustration of the apparatus for characterizing the flow control device.
8.1.1.1 Open the evacuated canister, monitor and record (manually or electronically) the canister pressure downstream of the flow control device and the flow upstream of the flow control device on an hourly basis over the period of 24-hours.
8.1.1.2 The flow control verification test is considered acceptable when the sampling apparatus maintains a constant flow rate for 24-hours and until at least 75% of the canister volume is collected, which is equivalent to approximately 28 kPa (7 in. Hg or 4 psia) below atmospheric pressure.
8.1.1.3 Record the average flow rate during this test. This value will be the reference flow rate for the sampling device until the next verification test. Maintain the results as part of a laboratory record associated with the sampling device.
8.1.2 Flow Control Flow Check. Prior to and after each field sampling event, establish or verify the flow rate of the sampling apparatus. This verification must occur in the field prior to and after each field event.
8.1.2.1 Assemble an evacuated canister and the sampling device connected to a certified flow meter in the same manner used for the flow control verification test discussed above.
8.1.2.2 Open the evacuated canister, allow sufficient time for the system to stabilize, and record the flowrate upstream of the flow control device. Collect two additional flow rate measurements.
8.1.2.3 Calculate the average flowrate. The flow control flow check is considered valid if within ±10% of the reference flow rate.
8.1.2.4 If the flow rate has changed and is outside the desired range, you must either adjust or replace the controller and repeat the flow check.
8.1.3 Sampling Device Leak Check. You must demonstrate the sampling device and sampling system are leak-free immediately before you begin sampling.
8.1.3.1 Install the sampling device on an evacuated canister equipped with a MFCD and tightly cap the inlet to the sampling device.
8.1.3.2 Open the canister valve fully, and then re-close the valve and observe the vacuum/pressure gauge for a minimum of 2 minutes.
8.1.3.3 If you observe an increase in pressure, the sampling device does not qualify as leak-free. If no changes are observed, record the data and time of the leak check on the Field Data Page (see Figure 4 in Section 17 of this method for an example).
8.1.4 Sampling Device Bias Check. You must demonstrate that sampling device is non-biasing under zero-air and known-standard conditions. For the procedures in Sections 8.1.5 and 8.1.6 of this method, you must use only canisters that have been qualified as specified in Section 8.3 of this method.
8.1.5 Sampling Device Zero-Air Challenge. You must conduct the sampler bias test at least every twelve months, and after cleaning, replacement of components, or collection of potentially contaminating samples. The volume of air analyzed for the zero-air and reference standard gas must be consistent with the laboratory's typical canister sample injection volume or nominal volume.
8.1.5.1 Provide humidified (>40% RH) HCF zero air through the sampling device into the canister, and then analyze the sample according to Section 11 of this method and record the concentration measurement and maintain the results as part of a laboratory record associated with the sampling device.
8.1.5.2 The results must show that the concentration of the target compounds in the zero-air challenge sample collected through the sampling unit is not greater than 20 pptv higher than the native concentration of the target compounds in the reference sample (sample of zero-air collected upstream of the sampling device) or not detected at 22.1 psi absolute (152 kPa absolute) whichever is more stringent. If a sampling device does not meet this performance criteria, take action to remove the contamination attributable to the sampling unit (e.g., purging with humidified HCF zero air overnight or longer) and repeat the zero-air challenge. You must not use a sampling device that has not met the standards in this section.
Note:
If extended purging durations are not adequate to eliminate contaminants, then disassemble and clean according to Section 8.4 of this method. If the unit cannot be cleaned to meet the specifications, retire the unit from use or repurpose for source sampling.
8.1.6 Sampling Device Standard Check. You must conduct the sampling device standard check prior to initial use and at least every twelve months, and after replacement of components, or collection of potentially contaminating samples. For the procedures specified below, you must use only canisters that have been qualified as specified in Section 8.3 of this method.
8.1.6.1 Collect a humidified (>40% RH) known-standard challenge gas through the sampling device and into a canister. The challenge gas must contain the target oHAPs at 100 to 500 pptv each and you must choose the selected challenge concentration considering the expected measured concentration at the deployment location(s).
8.1.6.2 Analyze the sample according to Section 11 of this method and record the concentration measurement and maintain the results as part of a laboratory record associated with the sampling device. The results must demonstrate that each oHAP in the sample collected through the sampling device must be within ±15% of the concentrations in the reference sample. For compounds exceeding this criterion, you must take steps to eliminate the bias (e.g., cleaning as specified in Section 8.6.1 of this method or replacement of compromised parts) and repeat the known-standard challenge.
8.1.6.3 Following successful completion of the known-standard challenge, flush the sampling device or system with humidified (>50% RH) HCF zero-air or ultrapure nitrogen until the device meets the criteria specified in Section 8.1.5.2 prior field deployment.
8.2 Qualification of Analytical Instrumentation. Prior to initial use and as directed in this section, you must verify that the analytical equipment used in performing this method meets the performance criteria in this section. The primary objectives of these performance tests are to characterize the analytical instrumentation and verify that the analytical instrumentation meets the criteria in this method. The analytical instrumentation performance tests consist of the following:
(a) Analytical zero-air verification,
(b) Analytical known-standard challenge for analytical instrumentation, and
(c) Autosampler verification.
8.2.1 Analytical Zero-Air Verification. Prior to initial use and as part of an instrument's annual calibration, you must demonstrate that the analytical instrumentation (preconcentrator, GC-MS system, and all connections) is non-biasing under zero-air. The volume of air analyzed must be consistent with the laboratory's nominal injection volume.
8.2.1.1 Use the analytical instrumentation to analyze humidified (40 to 50% RH) HCF zero air from a known clean source (e.g., certified clean canister, clean cylinder gas, zero-air generator) at the installation prior to initial use of the instrument.
8.2.1.2 Examine chromatograms for interferences and other chromatographic artifacts such as nontarget peak responses, large peaks or rises in the chromatogram due to undifferentiated compounds, and baseline anomalies. The analysis must show that the concentration of any detected target compounds in the zero-air challenge sample is <20 pptv or undetected (whichever is more stringent) per compound of interest.
8.2.1.3 If you identify exceedances of target compounds in the zero-air challenge, take steps (e.g., analyzing replicates of humidified clean gas until the contamination is eliminated) to remove the contamination attributable to the analytical instrumentation by following the manufacturer's instructions.
8.2.1.4 You must repeat the analytical zero-air verification to ensure that you have mitigated any problems before using the analytical instrumentation.
8.2.2 Analytical Known-Standard Challenge for Analytical Instrumentation. Prior to initial use and as part of an instrument's annual calibration, you must demonstrate that the analytical instrumentation (preconcentrator, GC-MS system, and all connections) is non-biasing under known standards. The volume of air analyzed must be consistent with the laboratory's nominal canister sample injection volume.
8.2.2.1 Analyze a humidified (40 to 50% RH) reference standard in duplicate containing all target compounds at approximately 100 to 500 pptv each, chosen in consideration of the expected concentration at the deployment locations.
8.2.2.2 The results must demonstrate that the target compounds in the sample collected through the sampling device are within ±15% of the expected concentrations in the sample.
8.2.2.3 Compounds demonstrating poor response as indicated by peak absence or minimal peak area may be a result of active sites in the analytical system, cold spots in transfer lines, gas impurities, improper choice of preconcentrator sorbent traps or GC columns, system leaks, and/or poor moisture management. If you identify problems, consult the instrument manufacturer to determine the necessary steps to eliminate the bias.
8.2.3 Autosampler Verification. Prior to initial use and as part of an instrument's annual calibration, you must demonstrate that the auto sampling equipment is non-biasing under zero-air.
8.2.3.1 If you use an autosampler to facilitate analysis of multiple canisters, you must test all ports, transfer lines, and connections of the autosampler after you have calibrated the analytical system and prior to conducting the canister, sampling device and system qualifications, or upon replacement of transfer lines or after analysis of potentially contaminating samples.
8.2.3.2 Connect humidified (40 to 50% RH) HCF zero air to each port and verify that the concentration for each target compound is <20 pptv or undetected (whichever is more stringent) per compound of interest using the procedures in Section 11 of this method.
8.2.3.3 After the zero-air test, challenge each port of the autosampler with a reference standard (approximately 100 to 500 pptv) to verify that the autosampler is not causing bias using the procedures in Section 11 of this method). The concentration of each target compound must be within ±15% of the theoretical concentration of the reference standard.
8.3 Qualification of Canisters. Prior to initial use and as directed in this section, sampling canisters must meet the performance criteria in this section. The primary objectives of these performance tests are to ensure canisters are well characterized and to verify they are non-biasing. The performance criteria in this section are specific to the application of fenceline measurements for regulatory purposes at stationary sources. The performance test consists of the following:
(a) Canister design,
(b) Canister leak check,
(c) Canister zero-air verification, and
(d) Canister known-standard verification.
8.3.1 Canister Design.
8.3.1.1 You must use specially prepared canisters at least 6-liters volume in size that are suitable for trace gas analysis of the target compounds. The canister must include a fixed on/off valve made from chromatographic-grade stainless with metal valve seal surfaces. Each canister must also include a permanent alpha-numeric serial number for identification purposes. Alternative canister volumes may be used, subject to approval by the Administrator.
Note:
Specially prepared canisters are commercially available with a modest range of options for surface preparation of the canister interior surfaces, valves, and connections. Currently, canister interior surfaces are typically passivated by electropolishing or coating with a silicon-ceramic film. EPA does not require a specific treatment or design and any canister type may be used for this method contingent on meeting the performance criteria in this section; however, silicon-ceramic coated canisters have demonstrated superior performance when used to sample reactive compounds, (e.g., ethylene oxide).
8.3.1.2 Canisters should be handled with care to ensure that the interior canister surface is not compromised, the valve-to-canister connection remains intact, and weld integrity is maintained. Excessive torque on unbraced canister valve stems when making connections may cause damage and potentially leaks in the valve stem weld or at the ferrule sealing the canister valve and canister stem. Shocks resulting in dents to the surface of the canister may damage welds or create small cracks in the interior canister surface that may expose active sites. You must not use any canister with dents or compromised welds.
8.3.1.3 You must maintain a record of the results for all canisters used for this method. It is recommended that you evaluate the results for any potential trends that could result in erroneous data.
8.3.2 Canister Leak Check. You must qualify each canister as being acceptably leak-tight to ensure sample validity. Qualify new canisters before initial use and qualify all canisters used for sampling at least annually.
8.3.2.1 Leak Check. In conducting the canister leak check, you can either evacuate the canister to high vacuum ≤0.0067 kPa absolute (0.002 in. Hg or 0.001 psia) or pressurize the canister with clean fill gas to >203 kPa absolute (60 in. Hg or 29.4 psia).
8.3.2.2 After establishing the target pressure in the canister, close the valve and attach a vacuum/pressure gauge.
8.3.2.3 Open the valve and record the initial pressure reading.
8.3.2.4 Close the valve, remove the vacuum/pressure gauge, and loosely cap the canister using a cap fitting to ensure that leakage through the valve is accurately assessed while avoiding potential entry of debris into the valve during storage.
8.3.2.5 After a minimum of two days in storage, reinstall the vacuum/pressure gauge, open the valve, and record the canister pressure reading.
8.3.2.6 Determine the pressure decay rate as the absolute value of the difference between the initial and post-storage canister pressures. You must remove the canister from service if the pressure decay rate exceeds 0.69 kPa/storage day (0.2 in. Hg or 0.1 psia/storage day).
8.3.3 Canister Zero-Air Verification. You must qualify each canister as being acceptably non-biasing under zero-air conditions to ensure sample validity. Qualify new canisters before initial use and qualify all canisters used for sampling at least once every 18 months.
8.3.3.1 Pressurize the clean evacuated canister with humidified (>50% RH) HCF zero air to 152 kPa absolute (22.1 psia). Do not use ultrapure nitrogen to pressurize the canister because the inert nitrogen atmosphere does not permit reactions within the canister that may occur under sampling conditions.
Note:
Canister Zero-Air Verifications must also be performed after canister disassembly and/or replacement of components. Also, more frequent zero-air verifications may be appropriate when canisters are used in areas with higher ambient VOC concentrations or for collection of potentially contaminating samples.
8.3.3.2 Allow the canister to equilibrate for a minimum of 24 hours.
8.3.3.3 After the equilibration period, conduct an initial cleanliness analysis as specified in Section 8.4 of this method.
8.3.3.4 Store the canister for a holding period equal to or exceeding the typical laboratory holding time, nominally 8 days from the canister fill date.
8.3.3.5 After the holding period, conduct a subsequent cleanliness analysis as specified in Section 8.5 of this method.
8.3.3.6 The results of both the initial and subsequent cleanliness analysis must meet the cleanliness criteria specified in Section 8.5 of this method to be used for sampling. You must reclean and retest canisters that fail the zero-air challenge.
Note:
If necessary, use more aggressive cleaning techniques such as water rinses or other rinses as specified by manufacturers. If a canister continues to fail the zero-air challenges, remove the canister from service.
8.3.4 Canister Known-Standard Verification. You must qualify each canister as being acceptably non-biasing under known-standard conditions to ensure sample validity. Qualify new canisters before initial use and qualify all canisters used for sampling at least every 18 months.
8.3.4.1 Fill the clean evacuated canister with a humidified (40 to 50% RH) standard gas in HCF zero air with each target compound at approximately 100 to 500 pptv. Choose the selected challenge concentration based on the concentration expected to be measured during the sampling event.
8.3.4.2 Allow the canister to equilibrate for a minimum of 24 hours.
8.3.4.3 After the equilibration period, conduct an initial analysis according to Section 11 of this method.
8.3.4.4 Store the canister for a holding period equal to or exceeding the typical laboratory holding time, nominally 8 days from the canister fill date.
8.3.4.5 After the holding period, conduct a subsequent analysis.
8.3.4.6 The results of both the initial and subsequent analysis must show that the measured concentrations of the target analytes are within ±30% of the theoretical spiked concentration for each target compound. You must reclean and retest canisters that fail the known-standard verification.
8.4 Canister Cleaning. Clean canisters using repeated cycles of evacuation and pressurization. Table 1 in Section 17 of this method summarizes the canister cleaning procedures.
8.4.1 Gas Source for Canister Cleaning, Pressurization, and Flushing.
8.4.1.1 Verify, by direct analysis, the cleanliness of the purge gas upon initial setup. The analysis must show that the concentration of the individual target compounds is ≤20 pptv or undetected (whichever is more stringent) per compound of interest at 101.3 kPa absolute (29.92 in. Hg or 14.7 psia).
8.4.1.2 Humidify the purge gas to >50% RH and measure the humidity by placing a calibrated hygrometer probe in the humidified gas stream.
8.4.1.3 If using a bubbler-type humidifier, ensure that the downstream pressure is lower than the humidifier upstream pressure to avoid backflow of the water.
8.4.2 Pre-Evacuation of Canisters. You may need to repeat the pre-evacuation process for canisters that contain VOCs at higher concentrations.
8.4.2.1 Pre-evacuate canisters to be cleaned prior to connection to the canister cleaning system. To reduce the potential for contamination of the system, attach the canisters to an oil-free roughing pump and evacuate to approximately 7 kPa absolute (28 in. Hg vacuum or 1.0 psia) with the exhaust of the pump directed to a fume hood or passed through a charcoal trap.
8.4.2.2 Refill canisters to ambient pressure with HCF zero air.
8.4.2.3 Attach the canisters to the cleaning system after completing the pre-evacuation and refilling steps.
8.4.3 Canister Heating During Cleaning.
8.4.3.1 Heat canisters by placing them in an enclosed oven during cleaning to facilitate removal of compounds. Do not use heat bands or heating jackets.
8.4.3.2 Table 1 of Section 17 of this method specifies the temperatures to use for canister cleaning procedures.
8.4.4 Canister Evacuation and Pressurization Cycling.
8.4.4.1 Evacuate canisters to minimally 7 kPa absolute (28 in. Hg vacuum or 1 psia) and maintain this vacuum for a at least 1 minute.
8.4.4.2 Pressurize canisters to 414kPa absolute (≤30 psig) with humidified (>50% RH) HCF zero air and maintain this pressure for a minimum of 1 minute.
8.4.4.3 Repeat the cycle of canister evacuation and pressurization specified in Sections 8.4.4.1 and 8.4.4.2 of this method at least 5 times. You may need to perform 10 to 20 cycle repetitions or use other ancillary procedures to remove stubborn interferents or oxygenated compounds such as ketones, alcohols, and aldehydes (U.S. EPA, 2016b).
8.5 Verification of Canister Cleanliness Prior to Sample Collection.
8.5.1 After cleaning, pressurize each canister from the batch with humidified HCF zero air and maintain that pressure for at least 24 hours.
8.5.2 Connect each canister to the analytical system and measure the concentration of each target compound according to the procedures in Section 11 of this method.
8.5.3 The canister background concentration for each target compound must be ≤20 pptv (0.02 ppbv) or undetected (whichever is more stringent) when a canister is filled to 22.1 psi absolute (152 kPa absolute).
8.5.4 Canisters that meet the blank criteria are suitable to be evacuated for use. If a canister fails to meet the criteria, you must not use that canister until it has been re-cleaned and has met the requirements in Section 8.5.3 of this method.
8.5.5 Prior to field deployment, evacuate canisters to ≤0.0067 kPa (≤0.002 in. Hg or 0.001 psia).
8.6 Cleaning of Sampling Components.
8.6.1 Follow the manufacturer's instructions for cleaning components such as flow controllers and sampling unit parts, when necessary.
Note:
Disassembly of such instruments may void warranties or calibrations.
8.6.1.1 Flush the sampling units with humidified HCF zero air to remove contamination for at least 15 minutes.
8.6.1.2 Disassemble sampling components and visually inspect for cracks, abrasions, and residue prior to sonicating in deionized water for at least 30 minutes.
8.6.1.3 After flushing/sonication, rinse the components with clean deionized water and dry the components in an enclosed oven set to at least 50 °C for a minimum of 12 hours.
8.6.1.4 Following drying, reinspect components for defects, reassemble, and flush the sampler with humidified HCF zero air or ultrapure nitrogen for at least 12 hours.
Note:
To avoid damage to deactivated stainless-steel components due to oxidation in the presence of oxygen-containing atmospheres (e.g., HCF zero air), you should not heat components treated with silicon-ceramic coatings above 80 °C unless evacuated or under an inert atmosphere (e.g., nitrogen).
8.7 Sample Collection. Persons collecting field samples should be familiar with all aspects of this sampling protocol. It is suggested that those collecting these measurements for regulatory purposes develop site-specific SOPs to ensure samples are collected consistently and those doing the sampling are sufficiently trained on this method and the SOP.
8.7.1 Pre-Sampling Activities.
8.7.1.1 Clean canisters and verify that the canisters meet cleanliness and vacuum criteria specified in Sections 8.3 through 8.5 of this method.
8.7.1.2 If canisters were previously cleaned and stored under pressure while awaiting use, you must evacuate the canisters prior to field deployment. If canisters were stored under vacuum, you must verify that the canisters continue to meet vacuum threshold requirements.
8.7.1.3 Clean and verify the cleanliness and flowrates of sample devices that you will use for sampling and ensure that a clean particulate filter is placed in the inlet of the sampling device.
8.7.1.4 Establish sample codes (unique identifiers) and develop field data page and/or chain of custody (COC)/sample collection data form(s).
8.7.1.5 If shipping equipment into the field, make sure you have the proper number of canisters and sampling devices for the number of samples required for the sampling location and QC samples, allowing for sufficient timing for samples to arrive at the site.
8.7.1.6 Develop a unique sampling location ID. The sampling location must meet any requirements set in the applicable regulation and be in a secure location that protects the canister and sampling inlet from unwanted tampering or damage. The sampling location must also be located away from the immediate vicinity of any biasing sources (e.g., outdoor smoking areas; vehicle exhaust; heating, ventilation, and air conditioning units/building exhaust; outdoor fuel storage areas; shelter roofing materials; or exhaust from other sample collection devices). In general, horizontal distances should be >10 meters (m) from biasing sources.
8.7.2 Sample Setup Activities.
8.7.2.1 You must place the canister in a location that protects the canister and sampling inlet from unwanted tampering, damage, or theft.
8.7.2.2 Protect the canister and sampling inlets by placing the canister under shelter, if possible. Do not restrict air flow around inlets and do not locate inlets under building overhangs.
8.7.2.3 Do not place the canister near vegetation or structures that block or significantly restrict air flow to the MFCD inlet or manifold. Ensure that rain cannot be drawn directly into the MFCD, and the inlet heights must be approximately 1.5 to 3 m above ground level.
8.7.3 Sample Setup and Deployment. Perform the following steps at the time of sample setup and deployment.
8.7.3.1 Based on the applicable standard, determine the appropriate number and placement of sampling locations at the fenceline. The applicable standard will define the sampling schedule (e.g., one sampling event over a 5-day period) and the sampling period. All sampling locations must initiate sampling within 60 minutes of each other.
8.7.3.2 You must document all activities associated with sampling on the field data page. (See Figure 4 in Section 17 of this method for an example field data page.) You may choose to use this field data page as the COC, or you may choose to establish a separate COC form. The chain of custody will accompany the canisters during shipment and collection to document sample handling and transport.
8.7.3.4 Verify that each canister has been blanked within the last 30 days. Label each canister with a sample ID code and record the canister and sample ID on the field data page. You must not use a use a canister for sampling that has not been blanked within 30 days of sampling.
8.7.3.5 Verify the sample device is in working order and calibrate/verify the flow rate setting, if applicable, with a reference flow meter. Record the sample device ID, expected flowrate, and the reference flowrate if calibrated/verified in the field, including the reference flow meter if applicable.
8.7.3.6 Attach the sampling device to the canister and locate at the appropriate sampling location. Record the sampling location ID, latitude, longitude, date, and time that you installed the canister on the field data page.
8.7.3.7 Measure and record the canister vacuum using the field pressure measurement gauge, and verify that the canister has not leaked and has sufficient vacuum to collect the sample. You must replace the canister if the initial pressure is not within −1 in. Hg absolute zero (−3.39 kPa or 0.5 psi).
8.7.3.8 Conduct leak checks as specified in Section 8.3.2 of this method and record the results on the field data page.
8.7.3.9 Open the canister valve. Record the date and time that you opened the valve as the start time, and record the initial canister vacuum/pressure and any other comments such as unusual events or conditions that may impact sample results on the field data page.
8.7.3.10 Sample for the period as defined in the applicable standard (e.g., 24 hours +/−1 hour).
8.7.3.11 At the end of the sampling period, close the valve. Record the date and time that you closed the valve as the end time.
8.7.3.12 Remove the sampling device and attach the field pressure measurement gauge.
8.7.3.13 Open the canister valve and measure and record the final canister vacuum/pressure and any other comments such as unusual events or conditions that may impact sample results on the field data page. Flag any canisters with a final pressure greater than −3 in. Hg gauge pressure (10.2 kPag or −1.5 psig).
8.7.3.14 Disconnect the field canister pressure measurement gauge and replace with a cap.
8.7.3.15 If applicable, verify the sample device is still in working order and verify the flow rate setting with a reference flow meter. Record the final flowrate on the field data page.
8.7.4 Field Duplicates. For each sampling day, you must include the collection of a separate co-located sample for at least one sampling location. The collocated duplicate must be sampled using a discrete MFCD. The collection of the field duplicates must follow the same procedure and occur at the same time as the co-located field sample.
8.7.5 Canister Field Blanks. For each sample day, you must collect canister field blanks. A canister blank is prepared by filling a canister with humidified clean diluent gas (prepared in the same manner as the method blank (MB) described in Section 9.3.2 of this method) to approximately 15 in. Hg ± 1 in. Hg . Record the pressure and transport to the field site(s) to accompany field-collected canisters. Canister field blanks are to be treated identically to field-collected samples in the field and laboratory including pressure checks, MFCD leak checks, etc. The field blanks are analyzed by interspersing them among the field samples.
8.7.6 Canister Field Spike. For each sample day, you must collect a canister field spike. A canister field spike is prepared by filling a canister with humidified standard gas at a concentration in the lower third of the calibration curve for the target compound to approximately −15 in. Hg ± 1 in. Hg. The field spike canister is transported to the field site to accompany field-collected canisters and treated identically to field-collected samples in the field and laboratory, including pressure checks, MFCD leak checks, etc. The field spikes are analyzed by interspersing them among the field samples. Field spike acceptance criteria should be within ±30% of the theoretical spiked concentrations.
8.7.7 Prepare and secure the canisters for transport. You must ship canisters in protective hard-shell boxes and/or sturdy cardboard boxes to ensure canister longevity. Do not use boxes that have lost integrity or rigidity.
8.8 Method Detection Limit (MDL) Determination. Determine the MDL under the analytical conditions selected (see Section 11 of this method) using the procedures in this section.
8.8.1 Prepare at least seven blank samples according to the procedures Section 9.3.2 of this method using sampling media (i.e., canisters) that have been deployed in the field, and cleaned per Section 8.4 of this method. The blank samples must be prepared in at least three batches on three separate calendar dates and analyzed on three separate calendar dates according to the procedures in Section 11 of this method.
8.8.2 Prepare at least seven spike samples according to the procedures in either Section 10.2 or 10.3 of this method, at a concentration of the target compound within a factor of five of the expected detection limits. The spike samples must be prepared in at least three batches on three separate calendar dates and analyzed on three separate calendar dates according to the procedures in Section 11 of this method.
8.8.3 Compute the standard deviation for the replicate blank samples concentrations and multiply this value by 3.14 to determine the blank MDL (MDLb).
8.8.4 Compute the standard deviation for the replicate spike sample concentrations and multiply this value by 3.14 to determine the spike MDL (MDLs).
8.8.5 Select the greater of MDLb or MDLs as the MDL for the compound of interest. The results must demonstrate that the method is able to detect analytes such as ethylene oxide at concentrations less than 20 pptv and at least 1/10th of the lowest concentration of interest (i.e., action-level), whichever is larger. If the MDL does not meet the concentration requirement, perform corrective action and repeat the MDL determination.
8.8.6 MDL determinations must be repeated at least annually or whenever significant changes have been made to the sampling or analytical system.
Note:
The MDL calculation is based on single-tailed 99th percentile t static at six degrees of freedom. Additional blank or spike samples would increase the degrees of freedom.
9.0 Quality Control
Table 9-1 in this section lists the quality control (QC) parameters and performance specifications for this method.
9.1 Second Source Calibration Verification (SSCV) Standard.
9.1.1 Prepare a humidified SSCV standard in a canister at a concentration in the lower third of the calibration curve. The SSCV standard must contain all compounds in the calibration mixture. The SSCV standard must be prepared independently from the calibration standards using a certified secondary source calibration standard.
9.1.2 Analyze the SSCV after the initial calibration (ICAL). Recovery of each target oHAP in the SSCV standard must be within ±30% of the theoretical concentration.
9.2 Continuing Calibration Verification (CCV) Standard. Prepare a humidified CCV standard as a dilution of a certified standard in a canister at a concentration in the lower third of the calibration curve. This certified standard must be prepared from the same standard used for the ICAL standards.
9.2.1 Analyze a CCV for each target oHAP prior to analyzing samples, after every 10 sample injections, and at the end of the analytical sequence. Prepare a humidified CCV standard as a dilution of a certified standard in a canister at a concentration in the lower third of the calibration curve. This certified standard must be prepared from the cylinder used for the ICAL standards.
9.2.2 The internal standard (IS) area responses for each CCV standard must meet the criteria outlined in Section 10.8.1.5 of this method, and the quantitated concentrations of the target compounds for each CCV standard must be within ±30% of the theoretical concentrations as determined using Equation 4 in Section 12 of this method.
9.2.3 If the CCV is not within specifications, you must invalidate any results after the last successful CCV. You must investigate and address CCV failures and initiate corrective actions, including, for example, reanalyzing the CCV, preparing and analyzing a new CCV or standard canister, and performing a new ICAL.
9.3 Blank Analyses. Analysis of all blanks must demonstrate each target compound is <20 pptv 14.7 psia or undetected (whichever is more stringent) per compound of interest. Unless otherwise stated, the volume used for analysis of blanks must match the volume of sample to be analyzed.
9.3.1 Instrument Blanks (IB). Analyze an IB at the beginning of the sequence and prior to analysis of the ICAL standard and daily CCV standard.
9.3.2 Method Blanks (MB). Analyze a laboratory MB prior to and following the ICAL in an ICAL sequence and prior to analyzing the CCV standard. The MB consists of a canister filled with humidified (40 to 50% RH) clean diluent gas and is analyzed via the same instrument method as the standards and field samples in the analytical sequence. Your MB must be the same diluent used for sample dilution.
9.3.3 Canister Field Blank. Analyze the canister field blank as part of the same analytical sequence as the accompanying field samples.
9.3.4 Calibration Blank (CB). Analyze the CB when the ICAL is established and when preparing any new CCV standard using the same instrument method that was used for standards and field samples when establishing the ICAL. The CB consists of a canister filled with the humidified (40 to 50% RH) clean diluent gas sourced through the dilution system employed to prepare standards. For laboratories that do not employ a dynamic or automated static dilution system, the CB consists of a humidified (40 to 50% RH) canister of the gas used to dilute the calibration standard.
9.4 Duplicate samples must be analyzed and reported as part of this method. They are used to evaluate sampling and/or analytical precision.
9.4.1 Field Duplicate. The level of agreement between duplicate field samples is a measure of the precision achievable for the entire sampling and analysis. Analyze the field duplicate during the same analytical sequence as the accompanying field sample. The RPD of the precision measurements should agree within ±30% when both measurements are ≥5 times the MDL. Flag associated results to indicate if the RPD indicates poor method precision.
9.4.2 Replicate Analysis. The level of agreement between replicate samples is a measure of precision achievable for the analysis. Analyze at least one replicate analysis for each set of field-collected samples. The RPD of the precision measurements should agree within ±25% when both measurements are ≥5 times the MDL. Flag associated results to indicate if the RPD indicates poor method precision.
| Parameter | Description and details | Required frequency | Acceptance criteria | Corrective action |
|---|---|---|---|---|
| Analytical zero-air verification | Test of instrumentation to demonstrate cleanliness (positive bias) by analyzing humidified zero air; performed by connecting the clean humidified gas sample to the pre concentrator to verify that the analytical instrument and all connections are sufficiently clean. | At installation prior to initial use of the instrument. | Analysis must show that any detected target compounds in the zero-air challenge sample are at response levels that are expected to be <20 pptv or not detected. | Take steps to remove contamination attributable to the analytical instrumentation by following the manufacturer's instructions (e.g., analyzing replicates of humidified clean gas). |
| Analytical known-standard challenge for analytical instrumentation | Test to demonstrate that the analytical instrumentation (preconcentrator and GC-MS system) is not causing loss of compounds (negative bias). | At installation prior to initial use of the instrument and with instrument's annual calibration. | Verifies that all target compounds are detected by the system, that they respond consistently upon repeated injection, and that they exhibit sufficient response to be quantifiable at low concentrations (see Section 8.2.2 of this method). | Check for cold spots in transfer line, gas impurities, sorbent traps, GC column, system leaks, and/or poor moisture management. Consult instrument manufacturer for steps to eliminate bias, as necessary. |
| Zero-air challenge of autosamplers associated with analytical instrument systems | After establishing the ICAL, each port of the autosampler is tested to demonstrate cleanliness (positive bias) by analyzing humidified zero air; performed by connecting the clean humidified gas sample to the port to verify that transfer lines and all connections are sufficiently clean. | Prior to initial use, upon replacement of transfer lines, or after analysis of potentially contaminating samples. | Each target VOC's concentration must be <20 pptv or preferably not detected (see Section 8.2.3 of this method). | (1) Heat and purge any lines, and/or (2) Rinse with deionized water, dry, and purge any lines that fail. |
| Known-standard challenge of autosamplers associated with analytical instrument systems | After establishing the ICAL, each port of the autosampler is tested with a reference standard (approximately 100 to 500 pptv) to demonstrate that the autosampler is not causing bias (typically loss of compounds or negative bias). | Prior to initial use and upon replacement of transfer lines. | Each target VOC's concentration within ±15% of theoretical concentration (see Section 8.2.3 of this method). | (1) Heat and purge any lines, and/or (2) Rinse with deionized water, dry, and purge any lines that fail. |
| Canister leak check | Verification that canisters are leak-free by performing a pressure decay test of a canister pressurized to approximately 203 kPa absolute (29.4 psia) over the course of two days. | Prior to initial use and annually thereafter. | A pressure change ≥0.69 kPa/day (see Section 8.3.2 of this method). | (1) Remove from service, and (2) Repair canister connections and/or valve. |
| Canister zero-air verification | Test of canisters to determine that they are and remain acceptably clean (show acceptably low positive bias) over the course of 7 days, by filling with humidified zero air (not nitrogen). | Initially upon receipt in the laboratory and every 18 months thereafter. | Upon initial analysis after a minimum of 24 hours and after 7 days, each target VOC's concentration ≤20 pptv at 152 kPa absolute (22.1 psia). | (1) Clean and retest canisters that fail the zero-air verification. (2) Remove canisters from service that cannot pass the zero-air verification after the cleaning process. |
| Known-standard challenge of canisters for qualification | Test of canisters to determine bias by filling with a known reference standard (approximately 100 to 500 pptv) prepared in humidified zero air (not nitrogen) and analyzing. | Initially upon receipt in the laboratory and every 18 months thereafter. | Upon initial analysis after a minimum of 24 hours and subsequent analysis at 30 days or typical laboratory holding time, each target VOC's concentration must remain within ±30% of theoretical concentration (see Section 8.3.4 of this method). | (1) Clean and retest canisters that fail the zero-air verification. (2) Remove canisters from service that cannot pass the zero-air verification after the cleaning process. |
| Zero-air challenge of sampling devices | Assessment of positive bias of sampling system by collecting humidified zero air through the sampling device/system and comparing it to the reference sample collected upstream of the sampling device/system. | Prior to initial field deployment and every twelve months thereafter, following maintenance (component replacement), or after collection of potentially contaminating samples. | Analysis must show that the target compounds in the zero-air challenge sample collected through the sampling unit are not >20 pptv higher than the concentration in the reference sample (see Section 8.1.5 of this method). | (1) Take steps to remove the contamination attributable to the sampling unit (e.g., purging with HCF zero air overnight or longer). (2) Disassemble and clean. See Section 8.6 of this method. |
| Flow control flow check | Verification of the mechanical flow control device (MFCD) flow rate. | Prior to and after each field sampling event | Flow measurement must demonstrate that the MFCD flow rate is within ±10% of the calibrated flow setting. | (1) Recalibrate or use a different MFCD for the sampling event as appropriate. (2) Flag any sample(s) collected with a failing post-flow control flow check. |
| Known-standard challenge of sampling devices/systems | Assessment of bias of sampling system by collecting a known reference standard (approximately 100 to 500 pptv) through the sampling device/system and comparing it to the reference standard collected upstream of the sampling device/system. | Prior to initial field deployment and at least every twelve months thereafter, following maintenance (component replacement), or after collection of potentially contaminating samples or damaging sample matrices that may impact the activity of the flow path surfaces. | Each target VOC's concentration within ±15% of concentrations in the reference sample. | (1) Take steps to remove the contamination attributable to the sampling unit (e.g., purging with HCF zero air overnight or longer). (2) Disassemble and clean. See Section 8.6 of this method. |
| Purge gas check | Analysis of canister cleaning purge gas to ensure contaminants are acceptably low. | Verified upon initial setup and in the event of changes in gas sourcing or after the replacement of scrubbers such as hydrocarbon traps and moisture traps, or following maintenance of zero-air generator. | Each target VOC's concentration <20 pptv (see Section 8.4.1 of this method). | Replace hydrocarbon trap, catalytic oxidizer, contaminated tubing, etc. |
| Canister cleaning blank check | Analysis of a sample of humidified diluent gas in a canister after cleaning to ensure acceptably low levels of VOCs in the cleaned canisters. | Every canister from each batch of cleaned canisters. | Upon analysis 24 hours after filling, each target VOC's concentration must meet the canister blank acceptance criterion .(i.e., ≤20 pptv at 152 kPa absolute, 22.1 psia) (see Section 8.5 of this method). | (1) Reclean canister, and/or (2) Disassemble and clean the components according to Section 8.6 of this method. |
| Holding time | Duration from end of sample collection or canister preparation to analysis. | Each field-collected or laboratory QC (standard or blank) canister. | ≤8 days | (1) Reprepare any lab standard or blank. (2) Flag the results of any sample analyzed outside of holding time. |
| Bromofluorobenzene instrument tune performance check | Injection of 1 to 2 nanograms (ng) BFB for tune verification of quadrupole or ion trap MS detector. | Prior to ICAL and prior to analysis of each day's analytical batch. | Abundance criteria for BFB listed in Table 5 in Section 17 of this method (see Section 10.7.2 of this method) | (1) Retune, and/or (2) Perform maintenance. |
| Retention time (RT) | RT of each IS and target compound. | All qualitatively identified compounds and internal standards. | IS compounds and target oHAP within ±2 seconds of most recent calibration check. | Flag data for possible invalidation. |
| Samples—internal standards (IS) | Deuterated or other compounds not typically found in ambient air co-analyzed with samples to monitor instrument response and assess matrix effects. | All laboratory QC samples, and field-collected samples. | Area response for each IS compound must be within ±30% of the average response as determined from the most recent calibration check. | Flag data for possible invalidation. |
| Initial calibration (ICAL) | Analysis of a minimum of five calibration levels covering approximately 20 to 5000 pptv. | Before sample analysis, following failed BFB tune check (as applicable), failed IS criteria, or failed CCV criteria; annually, or when changes/maintenance to the instrument affect calibration response. | Average Relative Response Factor (RRF) ≤30% RSD and each calibration level within ±30% of theoretical concentration; Relative Retention Times (RRTs) for target peaks within 0.06 units from mean RRT. | (1) Repeat calibration standard analysis. (2) Repeat linearity check. (3) Prepare new calibration standards as necessary and repeat analysis. |
| Second source calibration verification (SSCV) | Analysis of a secondary source standard in the lower third of the calibration curve to verify ICAL accuracy for each target analyte. | Immediately after each ICAL. | Measured concentrations of VOCs must be within ±30% of theoretical concentration (see Section 9.1 of this method). | (1) Repeat SSCV analysis. (2) Reprepare and reanalyze SSCV standard. |
| Continuing calibration verification (CCV) | Analysis of a known standard in the lower third of the calibration curve to verify ongoing instrument calibration for each target analyte. | Prior to analyzing samples in an analytical sequence and at the end of a sequence, unless the sequence begins with an ICAL; and after every 10 sample injections. | Measured concentrations of VOCs within ±30% of theoretical concentration (see Section 9.2 of this method). | (1) Repeat CCV analysis. (2) Repeat ICAL. |
| Instrument blank (IB) | Analysis of an injection where no sample or standard is introduced to the preconcentrator to preliminarily demonstrate the carrier gas and instrument are sufficiently clean to begin analysis. | Prior to ICAL and at the beginning of an analytical sequence. | Each target VOC's concentration must be <20 pptv (see Section 9.3.1 of this method). | (1) Repeat IB analysis. (2) Bakeout preconcentrator system and repeat IB analysis. (3) Replace contaminated tubing/traps as needed. |
| Method blank (MB) | Canister filled with clean, humidified diluent gas; indicates that target VOCs and potential interferences are at acceptably low levels in the system as a whole; the MB is to help assess overall quality of the data. | Prior to and following the ICAL and daily following the IB/BFB and prior to the initial daily CCV/SSCV. | This must demonstrate acceptably low carryover in the analytical system prior to analysis of samples; each target VOC's concentration must be <20 pptv (see Section 9.3.2 of this method). | (1) Repeat analysis. (2) Reprepare the MB canister and reanalyze. (3) Check the system for leaks. |
| Calibration blank (CB) | Canister filled with clean, humidified diluent gas sourced through the standard preparation dilution system; indicates that diluent gas and dilution apparatus do not contribute target VOCs, imparting a positive bias to the ICAL | Prepare one CB with each set of calibration standard canisters and analyze with each ICAL | CB must be sufficiently clean such that little or no positive bias is imparted to the calibration (see Section 9.3.3 of this method). | (1) Reanalyze CB. (2) Reprepare CB and ICAL canister standards. |
| Method precision | Duplicate samples: precision is determined from the analyzed concentrations of collocated samples. | Applicable to the collection of samples: one per sampling day. | Precision ≤30% RPD of target VOCs in the compared samples when both measurements are ≥ fivefold MDL (see Section 9.4 of this method). | (1) Check for preconcentrator volume measurement error. (2) Reanalyze primary sample and collocated duplicate. (3) Flag data for possible invalidation. |
| Instrument precision | Precision is determined from repeated analyses of a sample from a single canister; replicate analyses are used to determine precision of the analysis processes and do not provide information on sampling precision. | One replicate analysis to be performed with each sampling day. | Precision ≤25% RPD for target VOCs when both measurements are ≥ fivefold MDL (see Section 9.4 of this method). | (1) Check for preconcentrator volume measurement error. (2) Reanalyze primary sample and collocated duplicate. (3) Flag data for possible invalidation. |
| Preconcentrator leak check | Pressurize or evacuate the canister connection to verify as leak-free. | Each canister connected to the instrument prior to analysis. | <3.4 kPa (0.5 psi) change per minute or as recommended by the manufacturer (see Section 11.4.2 of this method). | Check the tightness of all fittings and recheck. |
10.0 Calibration and Standardization
10.1 Humidification of Canisters.
10.1.1 Calculate the volume of water you must add to standard and blank canisters to achieve 40 to 50% RH at ambient laboratory temperature. (See Equation 6 in Section 12 of this method).
10.1.2 Use a bubbler or impinger within the dilution gas stream, add water to the canister, or use a combination of these two methods to add the calculated volume of deionized water to the canister necessary to achieve internal RH of approximately 40 to 50% at ambient laboratory temperature. For direct injection of water into a canister with a syringe, install a high-pressure PTFE-sealed septum on the canister. For canisters that are to be connected to a gas source for pressurization via a dynamic or static dilution system, you can add the deionized water to the valve opening of the evacuated canister prior to connecting to the dilution system. Do not add water to the canister using a syringe via rubber septum or other materials that may introduce target or interfering compounds.
10.2 Dynamic Dilution.
10.2.1 Gas Dilution System. The gas dilution system must produce calibration gases whose measured concentration values are within ±2% of the predicted values. The predicted values are calculated based on the certified concentration of the supply gas (Protocol gases, when available, are recommended for their accuracy) and the gas flow rates (or dilution ratios) through the gas dilution system.
10.2.2 The gas dilution system must be calibrated and verified per Section 6.10.1 of this method.
10.2.3 Standards Preparation by Dynamic Dilution.
10.2.3.1 Prior to use, power on the dynamic dilution system and allow the diluent and stock gases to flow through the respective MFC at operating flow rates. Allow gases to flow for at least the minimum time used during the yearly bias check in Section 6.10.1.3 of this method, to ensure the concentrations of the oHAPs in the blended gas are stable prior to transferring to the humidified canister (or directly to the preconcentrator).
10.2.3.2 You must prepare humidified (40 to 50% RH) standards in canisters from low concentration to high concentration. When changing stock gas flow rate(s) to prepare a different concentration, allow the calibration gas sufficient time to flow through the system prior to preparation of the working calibration canister (or delivering the working standard directly to the preconcentrator).
10.2.3.3 The final pressure of the calibration standard canister must not exceed the maximum pressure permitted by the preconcentrator.
10.2.2.4 Calculate the final concentration of the diluted standard using Equation 7 in Section 12 of this method.
10.3 Static Dilution.
10.3.1 Static Gas Dilution System. The gas dilution system shall produce calibration gases whose measured values are within ±2% of the predicted values. The predicted values are calculated based on the certified concentration of the supply gases (Protocol gases, when available, are recommended for their accuracy) and their partial pressure measurements (or dilution ratios) in the prepared standard canister.
10.3.2 Static Dilution by Addition of Partial Pressures into a Canister.
10.3.2.1 Connect a pressure transducer or gauge to an evacuated canister to monitor the canister pressure as you add gases. The pressure transducer or gauge must meet the requirements in Section 6.5 of this method.
10.3.2.2 Add stock and diluent gases separately through a manifold or by direct connection of the gas to the standard canister or vessel.
10.3.2.3 Measure the canister pressure before and after standard and diluent gases are bled into the canister and input these pressures into the calculation of the dilution factor and final concentrations.
10.3.2.4 Calculate the final concentration of each target compound in the diluted standard using Equation 8 in Section 12 of this method.
10.4 Storage of Standards. Standards prepared in canisters at ambient laboratory conditions must be stored in locations that are free of potential contaminants for up to 7 days.
10.5 Pre-Concentration System Operation. Condition preconcentrator traps when first installed to eliminate contaminants that act as interferences or chromatographic artifacts, per manufacturer recommendation. After the recommended conditioning procedure is completed, analyze the IBs and MBs to verify the preconcentrator system meets the method criteria.
Note:
For preconcentrator traps that contain multiple types of sorbent beds, the oven temperature must not exceed the lowest conditioning temperature of the sorbents contained in the trap.
10.6 GC-MS System. Optimize GC conditions for compound separation and sensitivity as indicated by baseline separation for the targeted compounds by establishing GC carrier gas flow rates, oven temperature program, and instrument run time based on the manufacturer's recommendations and customize, as needed, to separate the desired target oHAPs.
10.7 MS Tuning/Optimizing and Verification.
10.7.1 General. Tune/optimize the MS (quadrupole, ion trap, or TOF MS) to demonstrate acceptable performance across the selected ion mass range according to the manufacturer's specifications upon initial installation of the instrument and following significant preventive maintenance or repair activities that impact the performance of the GC-MS system (e.g., cleaning the ion source or analyzer; trimming or replacing the capillary column; and adjusting MS tune or optimization parameters).
10.7.2 BFB Tuning Check. Before the ICAL and at least once during every 24-hour period of analyzing samples, blanks, or calibration standards thereafter, you must conduct a BFB tuning check for linear quadrupole or ion trap MS instruments. The BFB tuning check may be combined with the IB.
10.7.2.1 Introduce 1 to 2 ng of BFB into the preconcentrator and analyze the standard using the preconcentrator parameters established and used for the analysis of calibration standards, QC samples, and field samples. You must also use the method integration and analysis parameters employed for routine analysis of standards, QC samples, and field samples.
10.7.2.2 The BFB tuning check must show that the GC-MS system meets the mass spectral ion abundance criteria listed in Table 2 in Section 17 of this method for the target compounds before you can use the system for any analysis. If the GC-MS system cannot meet the BFB tuning criteria, adjust the tuning of the MS or take corrective actions. You must not use this system until the abundance criteria has been met.
10.8 Internal Standards and Calibration.
Method users must meet acceptance criteria for the calibration and QC listed in the following section for the suite of target compounds.
10.8.1 Selection and Use of Internal Standards (IS).
10.8.1.1 Select IS compound(s) to be used for oHAP analysis. At a minimum, you must use a single IS compound. IS compounds must have similar retention times to the compounds being detected. Typical IS compounds include bromochloromethane; 1,4-difluorobenzene; chlorobenzene-d5; 1,2-dichloroethane-d4; hexane-d14; toluene-d8; and 1,2-dichlorobenzene-d4.
10.8.1.2 If using purchased IS stock gases, evaluate the IS upon receipt for the presence of contaminants that may interfere with the quantitation of target compounds by analyzing increasing volumes of the IS (e.g., 25, 50, 100, 250 milliliters [mL]) and examining the results for compound contaminants whose responses increase proportionally with the increasing volume of IS analyzed. Do not use IS gas standards that fail the MB acceptance criteria.
10.8.1.3 You must add the IS through a dedicated non-sample port in the preconcentrator at the same concentration for each injection (e.g., standard, sample, blank) to monitor instrument sensitivity and assess potential matrix effects. Choose the concentration of IS added to each injection such that the peak area response for the IS compound approximates the area responses for target compounds in the lower half of the calibration curve range, but that minimally provides a peak that is on scale and does not exceed the area response of the highest calibration standard.
10.8.1.4 Internal Standard Retention Time (RT). Each IS compound in each sample injection must be within ±2 seconds of the RT for each IS compound in the most recent calibration.
10.8.1.5 Internal Standard Response. The area response for each IS compound in each injection (e.g., calibration standard, field sample, blank, CCV) must be within ±30% of the mean area response of the IS compound determined from the ICAL determined using Equation 10 in Section 12 of this method or most recent calibration check, whichever is most appropriate.
10.8.1.6 Choose the quantitation ion for each IS compound as the most abundant ion (base peak) unless there is a spectral interference from a coeluting or nearby compound or interference that impacts the quantitation of the base peak. In such cases, select another abundant ion that is distinguishable from the other compounds for quantitation.
10.8.1.7 You must invalidate then reanalyze any samples for which the IS area response differs by more than 30% from the mean IS area response.
10.8.2 Establishing Calibration. Calibrate the GC-MS initially, annually, whenever CCV standards exceed acceptance criteria, or when the system is out of control as indicated by IS responses. Prior to calibration, analyze a sufficient number of humidified (40 to 50% RH) HCF zero air blanks or humidified check standards to verify that instrument sensitivity is stable, as indicated by IS response.
10.8.2.1 Preparation for Calibration.
10.8.2.1.1 Prepare the calibration curve by preparing standards that bracket the expected concentration levels at the sampling location(s).
10.8.2.1.2 You must include at least five levels in the ICAL to approximate concentrations of target oHAPs expected at the deployment location(s), including one level within a factor of five of the detection limits of the compounds of interest, and another level within 10% of the compound specific action-level, as defined in the applicable standard.
Note:
To establish the calibration curve, the theoretical concentrations of the working calibration standards must be calculated using the certified concentration from the gas vendor or neat standard provider. Certificates of analysis for stock standard gas mixtures typically include both a nominal (or “requested”) concentration (e.g., 100 ppbv) for each analyte and a certified concentration (e.g., 108 ppbv), which should be within a specified tolerance (e.g., ±10%). These tolerances may permit the certified concentration to differ from the nominal concentration by 10% to 20%, resulting in final theoretical concentration errors for the working-level standards when the nominal concentration is input into standard concentration calculations instead of the certified concentration. Calibration standards prepared with neat materials must account for the standard purity when calculating the working standard concentrations.
10.8.2.2 Calibration Curve.
10.8.2.2.1 Following analysis of all calibration standards, prepare a calibration curve for each target analyte by determining the relative response factor (RRF) of each concentration level. Following data acquisition for the calibration standards, calculate the RRF of each target compound in each calibration level using Equation 10 in Section 12 of this method.
10.8.2.2.2 Choose the quantitation ion for each target compound as the most abundant ion (base peak) unless there is a spectral interference from a coeluting or nearby compound or interference that impacts the quantitation of the base peak. In such cases, select another abundant ion that is distinguishable from the other compounds for quantitation.
10.8.2.2.3 The %RSD of the RRFs of the ICAL levels for each target compound using Equation 17 in Section 12. The %RSD must be ≤30% for the ICAL to be considered acceptable.
10.8.2.2.4 The calculated concentration for each target compound(s) at each calibration level must be within ±30% of the theoretical concentration when quantitated against the resulting calibration curve.
11.0 Analytical Procedures
11.1 Measurement of Canister Receipt Pressure.
11.1.1 Upon receipt at the laboratory, review the sample collection information documented on the field data page and/or COC form(s) for completeness and accuracy. Compare the canister label with the sample collection data sheet and verify that the canister and sample IDs are correct.
11.1.2 Measure and record the canister pressure using a calibrated vacuum/pressure gauge or transducer. The measured canister absolute pressure must be within ±3.5 kPa (1 in. Hg or 0.5 psi) of that measured upon collection in the field. Pressure differences exceeding this criterion indicate the canister has leaked and you must flag the results as invalid.
11.2 Dilution of Canister Samples. A canister must be pressurized to provide sufficient pressure for removing an aliquot from the canister for analysis. Pressurize the canister with diluent gas to a pressure less than or equal to the final pressure of the standard gas canisters.
Note:
Minimum sample pressures will depend on the size of the canister and the capability of the preconcentrator to remove the desired aliquot of the sample and will be indicated by the instrument manufacturer.
11.2.2 Measure the canister pressure using a calibrated vacuum/pressure gauge or pressure transducer just prior to dilution and immediately following dilution and calculate the canister dilution correction factor (DFC) from the two absolute pressure readings (see Equation 12 in Section 12 of this method).
11.2.3 You must allow diluted canisters to equilibrate for a minimum of 12 hours before analysis.
11.3 Sample Preconcentration. Draw a measured aliquot of the whole air sample (typically 100 to 1000 mL) from the sample canister by vacuum through a preconcentrator to minimize the moisture and bulk atmospheric gases (e.g., oxygen, nitrogen, argon, and carbon dioxide) from the sample aliquot prior to introduction of the target compounds to the GC.
Note:
Preconcentrator instrument manufacturers will typically indicate the optimum factory default settings for the sample aliquot volume, trapping time, trapping temperature, gas flows, and additional preconcentration parameters. Adjust each of these variables as needed for the target compounds.
11.4 Sample Analysis. You must analyze samples using the same acquisition methods you used for establishing calibration (i.e., preconcentrator operation parameters, GC oven program, MS parameters, and integration methods). Field-collected samples and QC samples must be at ambient laboratory temperature for analysis. You must use approximately the same sample aliquot volume for all samples unless dilution is required. Adjustment of this sample aliquot volume requires adjustment of a dilution factor to account for the difference in relative analyzed volume, as discussed in Section 11.4.4 of this method.
11.4.1 Leak Check of Preconcentrator Connections.
11.4.1.1 Prior to beginning an analytical sequence, including an ICAL sequence, verify each canister connection as leak-free through the preconcentrator.
11.4.1.2 During the leak check, connect canisters to the autosampler or sample introduction lines and maintain the canister valves in the closed position.
11.4.1.3 Evacuate each port of the autosampler or sample introduction line and monitor for a change in pressure for 1 minute. The pressure must not change by more than 0.5 psig/minute.
11.4.1.4 If a sample line fails the leak check, implement corrective actions (e.g., rechecking the tightness of all fittings) and then retest. Do not perform analysis using any canister connection that does not pass the leak check.
11.4.1.5 Following the successful leak check, evacuate all autosampler ports or sample introduction lines, open the canister valves, and document the leak check results in the analysis records.
11.4.2 Sample Introduction.
11.4.2.1 Prior to each sample analysis sequence, you must connect each sample canister to the preconcentration unit through a port and verify each canister as having a leak-free connection.
11.4.2.2 Accurately measure the sample aliquot volume for analysis by metering the sample with an MFC or with the combination of a fixed-volume vessel and a pressure transducer. Sample introduction volume measurements must be made by the same device as the calibration standards to ensure that analyzed volumes of samples and standards are consistent.
11.4.3 Analysis of Field Samples. Perform the following steps for readying the system and performing the GC-MS analytical sequence. Once these checks meet criteria (summarized in Table 9-1 of this method), verify the instrument calibration by analysis of a CCV and begin sample analysis.
11.4.3.1 Perform an air/water check of the MS prior to any analyses to ensure that the system is acceptably leak-free.
11.4.3.2 Conduct a thorough system bakeout per the manufacturer's instructions for the preconcentrator and ramp the GC column temperature.
11.4.3.3 Analyze a preliminary IB or perform the BFB instrument tuning check.
11.4.3.4 Analyze a laboratory MB to demonstrate that the system is acceptably clean and that each target compound is <20 pptv or undetected (whichever is more stringent) per compound of interest.
11.4.3.5 Analyze a CCV to verify the instrument calibration.
11.4.3.6 Analyze field samples and additional CCV standards (every 10 samples) and MBs to complete the sequence, ending with a CCV, as discussed in Section 9.2 of this method.
11.4.4 Sample Dilution. If the on-column concentration of any compound in any sample exceeds the calibration range, you must dilute the sample for reanalysis by either reducing the sample aliquot volume for an effective dilution or adding diluent gas to the sample canister to physically dilute the sample.
11.5 Compound Identification.
11.5.1 After completing data acquisition, examine each chromatogram. Chromatographic peaks for the target compounds must be appropriately resolved, and integration must not include peak shoulders or inflections indicative of a coelution. If a peak has not been integrated properly, you may choose to manually integrate the peak. If a peak has been manually integrated, you must flag the results and report how and why the peak was manually integrated.
Note:
Deconvolution techniques may be available to the operator to help resolve compound coelutions, depending on the particular instrument and chromatography software package that is in use.
11.5.2 Identify target compounds qualitatively based on their RT and the relative abundance of their characteristic ions from the MS by satisfying the following four criteria. If any of the four criteria are not met, the compound cannot be positively identified.
Note:
Target compounds detected below the lowest calibration standard are estimated and may not be able to satisfy all four criteria.
11.5.2.1 The RT of the compound must be within the RT window of ±2 seconds of the most recent calibration check.
11.5.2.2 The relative abundance ratio of qualifier ion response to target ion response for at least one qualifier ion must be within ±30% of the average relative abundance ratio from the ICAL.
11.5.2.3 The S:N ratio of the target and qualifier ions must be >3:1.
11.5.2.4 The target and qualifier ion peaks must be co-maximized (i.e., peak apexes within one scan of each other).
11.6 Compound Quantitation. After determining the peak areas, initiate the quantitation process using the software package of choice to provide quantitative results compound using the RRF of the daily CCV for each target compound to quantitate the samples for the analytical batch.
11.6.2 Dilution Correction Factors.
11.6.2.1 Calculate an instrument dilution correction factor (DFI) if you analyzed an aliquot from the sample canister that is different from the typical analysis volume (as described in Section 11.4.4 of this method for performing effective dilution) using Equation 14 in Section 12 of this method.
11.6.2.2 Use Equation 15 in Section 12 of this method to determine the final concentration of each target compound in air by multiplying the instrument-detected concentration by the dilution factor from sample pressurization (DFC) (see Section 11.2 of this method) and the DFI.
Note:
The MDL reported with the final concentration data will be corrected by multiplying the MDL by the DFC and DFI applied to the sample concentrations.
12.0 Data Analysis and Calculations
12.1 Canister Final Air/Nitrogen Volume (V calc).
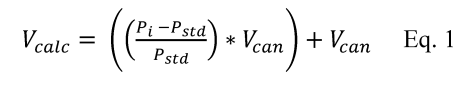
Where:
P clean = absolute pressure of canister cleaning batch blank, kPa absolute.
P std = 152.3 kPa absolute, standard atmospheric pressure.
V can = volume of the canister (mL) at standard conditions (101.3 kPa absolute and 25 °C).
12.2 Acceptable Blank Canister Concentration Criterion (C acc).
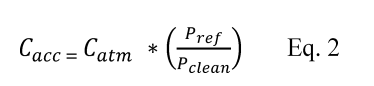
Where:
C acc = acceptance limit concentration at measured canister pressure (pptv).
C atm = 20 pptv, acceptance limit concentration at standard atmospheric pressure.
P ref = 152 kPa absolute, reference pressure.
P clean = absolute pressure of cleaned canister, kPa absolute.
12.3 Percent Difference of the Measured Concentration of Each Target Compound in the CCV Standard (%D CCV) from the Theoretical Concentration.

Where:
%D CCV = percent difference of the measured concentration of each target compound in the CCV standard from the theoretical concentration.
C CCV = measured concentration of the CCV for each target compound (pptv).
C theoretical = theoretical concentration of the CCV for each target compound (pptv).
12.4 Percent Recovery (%Recovery CCV).

Where:
%Recovery CCV = percent recovery of measured versus actual concentration.
C theoretical = theoretical concentration of the CCV for each target compound (pptv).
12.5 Relative Percent Difference (RPD).
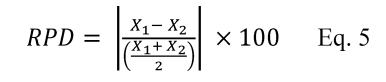
Where:
RPD = relative percent difference.
X 1 = target compound concentration measured in first measurement of the precision pair (pptv).
X 2 = target compound concentration measured in second measurement of the precision pair (pptv).
12.6 Water Volume to Add to Canister (V w).

Where:
D sat = saturation vapor density of water (mg/μL) at ambient laboratory temperature (refer to Table 3 in Section 17 of this method).
RH d = desired RH level expressed as a decimal.
V c = nominal internal volume of canister (L).
P c = final absolute canister pressure (kPa absolute).
P s = standard ambient pressure (101.3 kPa absolute).
D w = density of water (1 mg/μL).
Note:
The equation assumes the density of water to be 1 g/mL and that 100% of the added water to the canister is in the gas phase. The equation does not correct the density of water for the ambient temperature.
12.7 Final Concentration of the Diluted Standard (C f)—Dynamic Dilution.
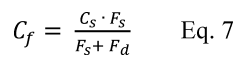
Where:
C f = final concentration of the diluted standard.
C s = certified concentration of stock standard (pptv).
F d = flow of diluent gas (mL/min).
F s = flow of stock standard (mL/min).
Note:
If you combine multiple gas standards for dilution, the equation denominator is the sum of all gas flows combined for preparing the dilution.
12.8 Final Concentration of the Diluted Standard (C f)—Static Dilution.
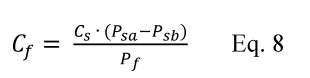
Where:
C s = certified concentration of stock standard (pptv).
P sa = absolute pressure of canister after adding standard gas (kPa).
P sb = absolute pressure of canister before adding standard gas (kPa).
P f = final absolute pressure of canister after adding standard and diluent gases (kPa).
12.9 Average Retention Time ().
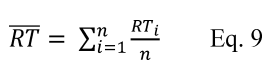
Where:
= average RT for the IS compound (min).
RT i = RT for the IS compound for each calibration level (min).
n = number of units used to generate a sum.
12.10 Relative Response Factor (RRF).
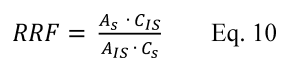
Where:
A s = peak area for quantitation ion of the target compound.
A IS = peak area for quantitation ion of the assigned IS compound.
C s = certified concentration of stock standard (pptv).
C IS = concentration of the assigned IS compound (pptv).
12.11 Average Area Response for the Given IS Compound ().
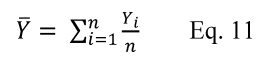
Where:
Y i = area response for an IS compound at calibration concentration i.
n = number of units used to generate a sum.
12.12 Dilution factor for sample pressurization (DF C).

Where:
P d = pressure of the canister following dilution (kPa).
P i = absolute pressure of the canister immediately preceding dilution (kPa).
12.13 Instrument-Detected Analyte Concentration (C D) in pptv.

Where:
C D = instrument-detected analyte concentration (pptv).
A IS = peak area for quantitation ion of the assigned IS compound.
12.14 Instrument Dilution Correction Factor (DF I).
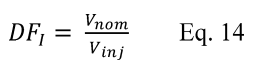
Where:
V nom = The laboratory's typical canister sample injection volume (mL).
V inj = The actual volume of any given sample injection (mL).
12.15 Concentration of the Target Compound in Air (C F).
C = C · DF · DF Eq. 15
Where:
C D = measured concentration of the target compound in the canister as analyzed sample.
12.16 Standard Deviation of the Response Factors (SD RF).
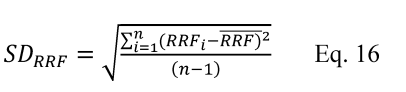
12.17 Percent Relative Deviation (%RSD).
% RSD = SD ÷ × 100 Eq. 17
13.0 Method Performance
Table 9-1 of this method lists the QC parameters and performance specifications for this method. The method performance will be determined by the specific performance of each specific target compound, laboratory, and the associated equipment.
14.0 Pollution Prevention
[Reserved].
15.0 Waste Management
[Reserved].
16.0 References
1. Quality Assurance Handbook for Air Pollution Measurement Systems, Volume II, Ambient Air Quality Monitoring Program, U.S. Environmental Protection Agency, EPA-454/B-17-001, January 2017.
2. Technical Assistance Document for the National Air Toxics Trends Stations Program, Revision 4, U.S. Environmental Protection Agency, July 2022.
3. Clean Air Act Amendments of 1990, U.S. Congress, Washington, DC, November 1990.
4. Method D1356, Standard Terminology Relating to Sampling and Analysis of Atmospheres.
5. Method E355-96, Standard Practice for Gas Chromatography Terms and Relationships.
6. Method D5466, Standard Test Method for Determination of Volatile Organic Compounds in Atmospheres (Canister Sampling Methodology).
7. Agilent Technologies, Inc. (2017, July 11). Innovative Cryogen-Free Ambient Air Monitoring in Compliance with US EPA Method TO-15. Application Note 081, 5991-2829EN. Available at https://www.agilent.com/cs/library/applications/5991-2829EN.pdf (accessed September 21, 2019).
8. ASTM International. (2014). Active Standard ASTM E2655-14: Standard Guide for Reporting Uncertainty of Test Results and Use of the Term Measurement Uncertainty in ASTM Test Methods. West Conshohocken, PA: ASTM International. doi: 10.1520/E2655-14.
9. Boyd, R.K., Basic, C., & Bethem, R.A. (2008). Trace Quantitative Analysis by Mass Spectrometry, Figure 6.7, p. 260. Hoboken, NJ: John Wiley and Sons.
10. Brown, J. (2013, October 22). Choosing the Right Adsorbent for your Thermal Desorption Gas Chromatography Applications. Presented at the Separation Science Webinar for Supelco. Available at https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Supelco/Posters/1/Adsorbent-Selection-TD-GC-Apps.pdf (accessed September 21, 2019).
11. Code of Federal Regulations, 40 CFR part 58 Appendices D and E, Network Design Criteria for Ambient Air Quality Monitoring. Available at https://www.govinfo.gov/app/details/CFR-2012-title40-vol6/CFR-2012-title40-vol6-part58-appD (accessed September 22, 2019).
12. Code of Federal Regulations, 40 CFR part 136 Appendix B, Definition and Procedure for the Determination of the Method Detection Limit, Revision 2. Available at https://www.govinfo.gov/app/search/%7B%22query%22%3A%2240%20CFR%20Part%20136%20Appendix%20B%2C%20Revision%202%22%2C%22offset%22%3A0%7D (accessed September 23, 2019).
13. Code of Federal Regulations, 40 CFR 173.306 (g), Limited quantities of compressed gases. Available at https://www.govinfo.gov/app/search/%7B%22query%22%3A%2249%20CFR%20%C2%A7173.306%20(g)%22%2C%22offset%22%3A0%7D (accessed September 23, 2019).
14. Coutant, R.W. (1992). Theoretical Evaluation of the Stability of Volatile Organic Chemicals and Polar Volatile Organic Chemicals in Canisters. Report EPA/600/R-92/055 prepared under contract 68-DO-0007 for U.S. EPA by Battelle, Columbus, OH.
15. Entech Instruments. (2015, August 25). 3-Stage Preconcentration: Why 3-Stage Preconcentration is Superior for TO-14A and TO-15 Air Methods. Available at http://www.entechinst.com/3-stage-preconcentration-is-superior-for-to-14a-and-to-15-air-methods/# (accessed September 21, 2019).
16. Herrington, J.S. (2013, August 5). TO-15 Canister Relative Humidity: Part II (Examples and Calculations) [Blog post]. Available at https://blog.restek.com/?p=7766 (accessed September 21, 2019).
17. Keith, L.H. (1991). Environmental Sampling and Analysis: A Practical Guide. Boca Raton, FL: CRC Press, pp. 93-119.
18. Kelly T.J., & Holdren, M.W. (1995). Applicability of canisters for sample storage in the determination of hazardous air pollutants. Atmospheric Environment, 29(19), 2595-2608. doi: 10.1016/1352-2310(95)00192-2.
19. McClenny, W.A., Schmidt, S.M., & Kronmiller, K.G. (1999). Variation of the relative humidity of air released from canisters after ambient sampling. Journal of the Air & Waste Management Association, 49(1), 64-69. doi:10.1080/10473289.1999.10463774.
20. Nave, C.R. (2017). Relative Humidity. HyperPhysics website, Department of Physics and Astronomy, Georgia State University, Atlanta, GA. Available at http://hyperphysics.phy-astr.gsu.edu/hbase/Kinetic/relhum.html#c3 (accessed September 21, 2019).
21. Ochiai, N., Daishima, S., & Cardin, D.B. (2003). Long-term measurement of volatile organic compounds in ambient air by canister-based one-week sampling method. Journal of Environmental Monitoring, 5(6), 997-1003. doi:10.1039/b307777m.
22. Ochiai, N., Tsuji, A., Nakamura, N., Daishima, S., & Cardin, D.B. (2002). Stabilities of 58 volatile organic compounds in fused-silica-lined and SUMMA polished canisters under various humidified conditions. Journal of Environmental Monitoring, 4(6), 879-889.
23. Restek. (2010). A Guide to Whole Air Canister Sampling: Equipment Needed and Practical Techniques for Collecting Air Samples. Technical Guide. Literature Catalog # EVTG1073A. Available at http://www.restek.com/pdfs/EVTG1073A.pdf (accessed September 21, 2019).
24. U.S. Environmental Protection Agency (EPA). (2017). Quality Assurance Handbook for Air Pollution Measurement Systems, Volume II, Ambient Air Quality Monitoring Program, EPA-454/B-17-001. Research Triangle Park, NC: EPA Office of Air Quality Planning and Standards, Air Quality Assessment Division. Available at https://www3.epa.gov/ttnamti1/files/ambient/pm25/qa/Final%20Handbook%20Document%201_17.pdf (accessed September 23, 2019).
25. U.S. Environmental Protection Agency (EPA). (2016a). Definition and Procedure for the Determination of the Method Detection Limit, Revision 2. U.S. EPA Office of Water, EPA 821-R-16-006. Available at https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf (accessed September 21, 2019).
26. U.S. Environmental Protection Agency (EPA). (2016b). EPA NATTS Proficiency Testing Results Calendar Year 2016 Quarter 1—Referee Results from EPA Region V. Available from U.S. EPA, Office of Air Quality Planning and Standards (OAQPS), Ambient Air Monitoring Group, Mail Code C304-06, Research Triangle Park, NC 27711.
27. U.S. Environmental Protection Agency (EPA). (2015). OSWER Technical Guide for Assessing and Mitigating the Vapor Intrusion Pathway from Subsurface Vapor Sources to Indoor Air. EPA Office of Solid Waste and Emergency Response (OSWER) Publication 9200.2-154. Available at https://www.epa.gov/vaporintrusion/technical-guide-assessing-and-mitigating-vapor-intrusion-pathway-subsurface-vapor (accessed September 22, 2019).
28. Wang, D.K., & Austin, C.C. (2006). Determination of complex mixtures of volatile organic compounds in ambient air: canister methodology. Analytical and Bioanalytical Chemistry, 386(4), 1099-1120. doi:10.1007/s00216-006-0466-6.
29. Batelle. (2016). Technical Assistance Document for the National Air Toxics Trends Stations Program, Revision 3. Prepared for U.S. EPA by Battelle, Columbus, OH. Available at https://www3.epa.gov/ttn/amtic/files/ambient/airtox/NATTS%20TAD%20Revision%203_FINAL%20October%202016.pdf (accessed September 22, 2019).
30. Daughtrey, E.H. Jr., Oliver, K.D., Jacumin, H.H. Jr., & McClenny, W.A. (2004, April 20-22). Supplement to EPA Compendium Method TO-15—Reduction of Method Detection Limits to Meet Vapor Intrusion Monitoring Needs. Presented at Symposium on Air Quality Measurement Methods and Technology, Research Triangle Park, NC. Available at https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=76137 (accessed September 22, 2019).
31. Entech Instruments. (2019). Articles and documents. Available at https://www.entechinst.com/technical-library/application-notes-applets-chromatograms/ (accessed September 23, 2019).
32. Kelly, T., Gordon, S., Mukund, R., & Hays, M. (1994). Ambient Measurement Methods and Properties of the 189 Clean Air Act Hazardous Air Pollutants. Report EPA/600/R-94/187 prepared under contract 68-DO-0007, work assignment 44, for U.S. EPA by Battelle, Columbus, OH.
33. Kelly, T.J., Callahan, P.J., Pleil, J., & Evans, G.F. (1993). Method development and field measurements for polar volatile organic compounds in ambient air. Environmental Science & Technology, 27(6), 1146-1153.
34. McClenny, W.A., Oliver, K.D., & Daughtrey, E.H. Jr. (1995). Analysis of VOCs in ambient air using multisorbent packings for VOC accumulation and sample drying. Journal of the Air & Waste Management Association, 45(10), 792-800.
35. Morris, C., Berkley, R., & Bumgarner, J. (1983). Preparation of multicomponent volatile organic standards using static dilution bottles. Analytical Letters, 16(20), 1585-1593.
36. Oliver, K.D., Adams, J.R., Daughtrey, E.H., McClenny, W.A., Yoong, M.J., Pardee, M.A., Almasi, E.B., & Kirshen, N.A. (1996). Technique for monitoring toxic VOCs in air: Sorbent preconcentration, closed-cycle cooler cryofocusing, and GC/MS analysis. Environmental Science & Technology, 30(6), 1939-1945.
37. Pleil, J.D., & Lindstrom, A.B. (1995). Collection of a single alveolar exhaled breath for volatile organic compound analysis. American Journal of Industrial Medicine, 28(1), 109-121.
38. Pleil, J.D., McClenny, W.A., Holdren, M.W., Pollack, A.J., & Oliver, K.D. (1993). Spatially resolved monitoring for volatile organic compounds using remote sector sampling. Atmospheric Environment, Part A, 27(5), 739-747.
39. Pollack, A.J., Holdren, M.W., & McClenny, W.A. (1991). Multi-adsorbent preconcentration and gas chromatographic analysis of air toxics with an automated collection/analytical system. Journal of the Air & Waste Management Association, 41(9), 1213-1217.
40. Restek. (2019). Restek Technical Library: Air Sampling. Available at https://www.restek.com/Technical-Resources/Technical-Library/Air-Sampling (accessed September 23, 2019).
41. Stephenson, J., Allen, F., & Slagle, T. (1990). Analysis of volatile organics in air via water methods. In Proceedings of the 1990 EPA/AWMA International Symposium: Measurement of Toxic and Related Air Pollutants, EPA 600/9-90-026. Research Triangle Park, NC: U. S. Environmental Protection Agency.
42. U.S. Environmental Protection Agency (EPA). (1997). Method TO-14A: Determination of volatile organic compounds (VOCs) in ambient air using specially prepared canisters with subsequent analysis by gas chromatography, EPA 600/625/R-96/010b. In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition. Cincinnati, OH: U.S. EPA Center for Environmental Research Information, Office of Research and Development.
43. Whitaker, D.A., Fortmann, R.C., & Lindstrom, A.B. (1995). Development and testing of a whole-air sampler for measurement of personal exposure to volatile organic compounds. Journal of Exposure Analysis & Environmental Epidemiology, 5(1), 89-100.
17.0 Tables, Diagrams, Flow Charts, etc.
| Canister type | Pre-evacuate canister | Suggested maximum canister temperature a | Humidity | Minimum number of pressure/ evacuation cycles | Cycle time |
|---|---|---|---|---|---|
| a Higher purge gas temperatures may be required depending on the canister type—do not exceed the manufacturer's recommended maximum temperatures for component parts such as valves and gauges. | |||||
| All | Yes | 80 °C | 50% | 5 | Varies by system. |
| Mass | Ion abundance criteria a |
|---|---|
| a All ion abundances must be normalized to m/z 95, the nominal base peak, even though the ion abundance of m/z 174 may be up to 120% that of m/z 95. | |
| 50 | 8.0% to 40.0% of m/z 95. |
| 75 | 30.0% to 66.0% of m/z 95. |
| 95 | Base peak, 100% relative abundance. |
| 96 | 5.0% to 9.0% of m/z 95. |
| 173 | <2.0% of m/z 174. |
| 174 | 50.0% to 120.0% of m/z 95. |
| 175 | 4.0% to 9.0% of m/z 174. |
| 176 | 93.0% to 101.0% of m/z 174. |
| 177 | 5.0% to 9.0% of m/z 176. |
| Temperature (°C) | Water saturation vapor density (mg/L) a |
|---|---|
| a Values are generated according to the following formula (Nave, 2017): vapor density (mg/L) = 5.018 + 0.32321 * T + 8.1847 × 10 − 3 * T2 + 3.1243 × 10 − 4 * T3 , where: T = temperature in °C. | |
| 15 | 12.8 |
| 16 | 13.6 |
| 17 | 14.4 |
| 18 | 15.3 |
| 19 | 16.3 |
| 20 | 17.3 |
| 21 | 18.3 |
| 22 | 19.4 |
| 23 | 20.6 |
| 24 | 21.8 |
| 25 | 23.1 |
| 26 | 24.4 |
| 27 | 25.9 |
| 28 | 27.3 |
| 29 | 28.9 |
| 30 | 30.5 |
| 31 | 32.2 |
| 32 | 34.0 |
| 33 | 35.8 |
Figure 1. Apparatus for Characterizing the Flow Control Device
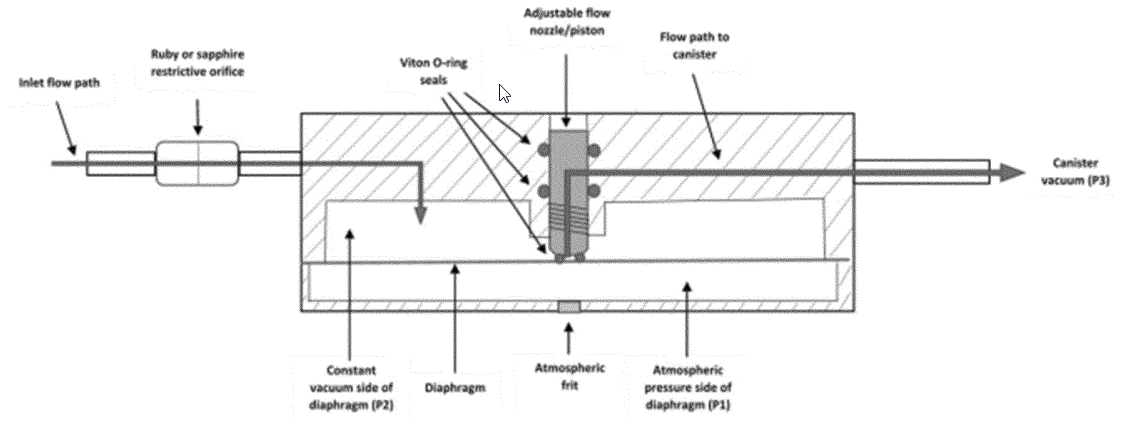
Figure 2. Mechanical Flow Control Device
Figure 3. Method 327 Sampler

Figure 4. Example Field Data Page
[57 FR 61992, Dec. 29, 1992; 85 FR 63419, Oct. 7, 2020; 88 FR 18422, March 29, 2023]

